CHAPTER1 Introduction
Thischapterisanattempttointroducehydrates,withoutmuchbackgroundmaterial.Manyofthewordsandprincipleswillbebetterdefinedin subsequentchaptersofthisbook.However,theyareneededheretopresent thebasicintroductoryconcepts.Ifyouarealittleconfusedasyoureadthis chapter,hopefullythingswillbecomeclearerasyouprogressthroughthe book.
Let’sbeginwiththemainfocusofthebook,hydrates.Initsmost generalsense,ahydrateisacompoundcontainingwater.Forexample, thereisaclassofinorganiccompoundscalled “solidhydrates.” Theseare ionicsolidswheretheionsaresurroundedbywatermoleculesandform crystallinesolids.However,asusedinthisbook,andcommonlyinthe naturalgasindustry,ahydrateissolidphasecomposedofacombinationof certainsmallmoleculesandwater.
Hydratesarecrystallinesolidcompoundsformedfromwaterandsmall molecules,withoutwatertherearenohydratesandwithoutthesmall moleculesthatstabilizethestructuretherearenohydrates.Theyarea subsetofcompoundsknownasclathratesorinclusioncompounds. Aclathratecompoundisonewhereamoleculeofonesubstanceisenclosed inastructurebuiltupfrommoleculesofanothersubstance.Onetypeof moleculeisliterallytrappedinacagecomposedofthemoleculesofa differentsubstance.Herewaterbuildsupthestructureandtheother moleculeresideswithin.Thesizeoftheothermoleculemustbesuchthatit can fitwithinthewaterstructures.Moredetailsofthenatureofthese structuresformedbywaterandthemoleculeswithinarepresentedin Chapter2ofthisbook.
Althoughtheclathratesofwater,theso-calledhydrates,arethefocusof thiswork,theyarenottheonlyclathratecompounds.Forexample,urea formsinterestinginclusioncompoundsaswell.
Althoughhydrateswereprobablyencounteredbyothersearlier,credit fortheirdiscoveryisusuallygiventothefamousEnglishchemist,Sir HumphreyDavy.Hereportedofthehydrateofchlorineintheearly19th century.Inparticular,henoted(1)thattheice-likesolidformedat
NaturalGasHydrates
ISBN978-0-12-821771-9
https://doi.org/10.1016/B978-0-12-821771-9.00001-X
temperaturesgreaterthanthefreezingpointofwaterand(2)thatthesolid wascomposedofmorethanjustwater.Whenmelted,thehydrateof chlorinereleasedchlorinegas.
Davy’sequallyfamousassistant,MichaelFaraday,alsostudiedthehydrateofchlorine.In1823,Faradayreportedthecompositionofthe chlorinehydrate.Althoughhisresultwasinaccurate,itwasthe firsttimethe compositionofahydratewasmeasured.
Throughoutthe19thcentury,hydratesremainedbasicallyanintellectualcuriosity.Earlyeffortsfocusedon findingwhichcompoundsformed hydratesandunderwhattemperaturesandpressurestheywouldform. Manyoftheimportanthydrateformerswerediscoveredduringthisera.
Amongthe19th-century,hydrateresearcheswhodeservementionare theFrenchchemistsVillardanddeForcrand.Theymeasuredthehydrate conditionsforawiderangeofsubstances,includinghydrogensulfide.
The firstcrystallographicstudiesofgashydrateswerepublishedbyvon StackelbergfromtheUniversityofBonninGermanyinthe1940sand50s. VonStackelbergandhisgroupestablishedthatthereweretwodistinct typesofhydratecrystalstructures.Wewilldiscussthesehydratetypesin Chapter2.
However,itwouldnotbeuntilthe20thcenturythattheindustrial importanceofgashydrateswouldbeestablished,especiallyforthenatural gasindustry(Hammerschmidt,1934).
Overtheyears,therehavebeenmany,manyexperimentalstudiesof hydrateformation.Theseincludethehydratesforsinglecomponents, binarymixtures,andmulticomponentmixtures.Someofthesestudiesare discussedinthechaptersthatfollow.Ifthereaderhasdoubtsaboutmethods usedinthework,theyshouldconsulttheliterature.Theymaynot findthe exactdatafortheirsituation,buttheymay finddatawhichareusefulfor testingthemodelstheychosetoemploy.
1.1Whatiswater?
Thismayseemlikeastrangequestion,butitismorecomplicatedthanyou mightthink.Manyofthetermsusedinthisbookwillnodoubtcause confusion,anditmightbesurprisingthatwaterisoneofthem.Someofthe confusionarisesfromtheEnglishlanguageandour,sometimesmy,useof it.Forexample,wecandefinewateras “acolorless,transparent,odorless liquid,” whichisprobablywhatmostpeoplethinkwhentheyhearthe wordandatypicaldictionarydefinition.Butitcouldbedefinedas
“achemicalcompoundmadeupofhydrogenandoxygenwiththeformula H2O(regardlessofthephaseitisin),” whichisamoregeneraldefinition butsoundsmorelikeachemist.InthisbookIwillusethetermaqueous liquidtomeanwaterintheliquidphaseandhopefullyavoidthisconfusion. Inaddition,inthisbook,thetermwaterwillbeusedinthechemicalsense, thatis,acompoundmadefromtwohydrogenatomsandoneoxygenatom.
Ifthewaterispureornearlysoandexistsinthevaporphase,wecallthis “steam.” Ifthegascontainswaterbutthewaterisdilute,suchasintheair, weusuallyrefertothisas “moisture.” Thisfurtherleadstotermslike “moisturecontent,” howmuchwaterisinthegas, “moisturemeasurement,” andmoistureanalyzer,adeviceusedtomeasurethemoisture content.
Wealsohavethetermshumidity,whichreferstotheamountofwater inagas,againtypicallytheair,andrelativehumidity.Soiftheairis50% relativehumidity,itcontainsonlyhalfthewateritcanhold.Therelative humidityisafunctionofthetemperatureandthepressure.
Thegeneraltermforsolidwaterinthepurestateisice.However,ifyou thinkaboutit,wehavemanytermsforsolidwaterdependinguponits physicalnature,suchasfrost,snow,hail,glacier,etc.
Hydrateisnotice,butitisice-like,similarinappearanceandphysical properties.Butahydrateisasolutioncomposedofwaterandother components,whereasiceispurewater.
However,thetermfrostpointisthetemperaturewhereasolid first appearswhetheritisiceorhydrateorsomeothersolid.Soifonecoolsagas streamisobaricallyuntilasolidforms,thatisthefrostpointtemperature regardlessofwhetherornotthesolidisiceorhydrate.
1.2Naturalgas
Althoughallterrestrialgases(air,volcanicemissions,swampgas,etc.)are natural,theterm “naturalgas” iscustomarilyreservedforthemineralgases foundinsubsurfacerockreservoirs.Thesegasesareoftenassociatedwith crudeoil.Naturalgasisamixtureofhydrocarbons(suchasmethane, ethane,propane,etc.)andafewnonhydrocarbons(hydrogensulfide, carbondioxide,nitrogen,etc.)andwater.
Thelighthydrocarbonsinnaturalgashavevalueasfuelsandasfeedstockforpetrochemicalplants.Asafuel,theyareusedforheatingand cookinginprivatehomes,togenerateelectricity,andincreasinglyasfuelfor motorvehicles.Inthechemicalplants,theyareconvertedtoahostof
consumerproducts,everythingfromindustrialchemicals,suchasmethanol, toplastics,suchaspolyethylene.
Thenonhydrocarbonstendtobelessvaluable.However,depending uponthemarketsituation,hydrogensulfidehassomevalueasaprecursor tosulfur.Sulfurinturnhasseveralapplications,themostimportantof whichisprobablytheproductionofchemicalfertilizer.Carbondioxideand nitrogenhavenoheatingvalueandthusareuselessasfuels.
Naturalgasthatcontainssignificantamountsofsulfurcompounds,and hydrogensulfideinparticular,isreferredtoas “ sour. ” Incontrast,natural gaswithonlyminuteamountsofsulfurcompoundsiscalled “sweet.” Unfortunately,thereisnostrictdefiningsulfurcontentthatseparatessour gasfromsweetgas.Aswehavenoted,salesgastypicallycontainslessthan about15ppmandisindeedsweet,butforotherapplicationsthereareother definitions.Forexample,intermsofcorrosion,thesweetgasmaycontain moresulfurcompoundsandnotrequirespecialmaterials.
Strictlyspeaking,gasthatcontainscarbondioxidebutnosulfurcompoundsisnotsour.However,gasthatcontainscarbondioxidesharesmany characteristicswithsourgasandisoftenhandledinthesameway.Probably, themostsignificantdifferencebetweencarbondioxideandhydrogen sulfidearethephysiologicalpropertiesandthisiswhatreallyseparatesthe two.Hydrogensulfideishighlytoxic,whereascarbondioxideisessentially nontoxic,exceptatveryhighconcentrations.Furthermore,hydrogen sulfidehasanobnoxiousodorwhilecarbondioxideisodorless.
1.2.1Salesgas
Anarrangementismadebetweenthecompanyproducingthenaturalgas andthepipelinecompanyforthequalityofthegasthepurchaserwill accept.Limitsareplacedontheamountsofimpurities,heatingvalue, hydrocarbondewpoint,andotherconditions.Thisarrangementiswhat defines “salesgas.”
Amongtheimpuritiesthatarelimitedinthesalesgasiswater.Oneof thereasonswhywatermustberemovedfromnaturalgasistohelpprevent hydrateformation.
Intermsofwatercontent,atypicalsalesgasspecificationwouldbeless thanapproximately10lbofwaterpermillionstandardcubicfeetofgas (10lb/MMCF).IntheUnitedStates,thevalueisusually7lb/MMCF, whereasinCanadaitis4lb/MMCFandotherjurisdictionshaveother values.ForthosewhopreferSIunits,10lb/MMCFisequalto0.16grams
perstandardcubicmeter(0.16g/Sm3)or160milligramsperstandardcubic meter(160mg/Sm3).Morediscussionofunitsandstandardconditionsis presentedlaterinthischapter.
Thereareseveralotherrestrictionsonthecompositionofsalesgas.For example,thereisalimitontheamountofhydrogensul fidepresent (typicallyontheorderofabout10partspermillionor10ppm)andthe amountofcarbondioxide(typicallyaround2molepercent).Thesetoo varyfromjurisdictiontojurisdiction,contracttocontract.
1.2.2Hydrates
Incombinationwithwater,manyofthecomponentscommonlyfoundin naturalgasformhydrates.Oneoftheproblemsintheproduction,processing,andtransportationofnaturalgasandliquidsderivedfromnatural gasistheformationofhydrates.Hydratescostthenaturalgasindustry millionsofdollarsannually.Infact,individualincidentscancost$1,000,000 ormoredependinguponthedamageinflicted.Thereisalsoahumanprice tobepaidbecauseofhydrates.Sadly,therehavebeendeathseitherdirectly orindirectlyassociatedwithhydrateandtheirmishandling.
However,theimportanceofnaturalgashydrateswasnotapparentin theearlyeraofthegasbusiness.Intheearlyeraofthenaturalgasbusiness, gaswasproducedanddeliveredatrelativelylowpressure.Thus,hydrates wereneverencountered.Inthe20thcentury,withtheexpansionofthe naturalgasindustry,theproduction,processing,anddistributionofgas becamehigh-pressureoperations.Underpressure,itwasdiscoveredthat pipelinesandprocessingequipmentwerebecomingpluggedwithwhat appearedtobeice,excepttheconditionsweretoowarmforicetoform.It wasnotuntilthe1930sthat Hammerschmidt(1934) clearlydemonstrated thatthe “ice” wasactuallygashydrates.Andthatthehydrateswerea mixtureofwaterandthecomponentsofnaturalgas.
Inthepetroleumindustry,theterm “hydrate” isreservedforsubstances thatareusuallygaseousatroomtemperature.Theseincludemethane, ethane,carbondioxide,andhydrogensulfide.Thisleadstotheterm “ gas hydrates” andalsoleadstooneofthepopularmisconceptionsregarding thesecompounds.Itiscommonlybelievedthatnonaqueousliquidsdonot formhydrates.However,liquidsmayalsoformhydrates.Anexampleofa compoundthatisliquidatroomconditions,yetformsahydrate,is dichlorodifluoromethane(Freon12).Butwearegettingaheadofourselves.
Moredetailsaboutwhatcompoundsformhydrateswillbegivenin Chapter2.
Oneoftheiconicimagesofahydrateisthe “iceon fire ” burningofa hydrate.Becausethehydrateiscomposedofbothhost(water)andguest,as thehydratemeltstheguestisreleased.Withamethanehydrateoranatural gashydrate,sufficienthydrocarbonisreleasedsuchthatitcanbeliton fire. Fig.1.1 isaphotographshowingtheiceon fire.Itisimportanttonotethat notallhydratescanbeignited.Onlythosethatcontainasuf ficientamount ofhydrocarboncanbeseton fire.Forexample,tryasyoumayyouwill neverbeabletoigniteacarbondioxidehydrate CO2 doesnotburn.
1.3Thewatermolecule
Manyoftheusualpropertiesofwater(andyes,ifyouarenotawareofit, waterdoeshavesomeunusualproperties)canbeexplainedbythestructure ofthewatermoleculeandtheconsequencesofthisstructure.
Ofparticularinteresttousisthefactthatthestructureofthewater moleculeleadstothepossibilityofhydrateformation.Inthenextsections, itwillbedemonstratedthatwaterdoesindeedhavesomeunusual properties.
Figure1.1 Iconic “IceonFire” pictureshowingamethanehydrateburning. (Credit:J. PinkstonandL.Stern(USGS),USGS.Publicdomain.)
1.3.1Thenormalboilingpointofwater
Asanexampleoftheunusualpropertiesofwater,considertheboiling point.Wewillusesomesimplechemistrytodemonstratethattheboiling pointofwaterisunusuallyhigh.Theboilingpointsusedinthisdiscussion aretakenfrom Dean(1973).
Theperiodictableofelementsisnotjustanicewaytodisplaythe elements.Theoriginaldesignofthetablecamefromaligningelements withsimilarproperties.Thus,elementsintherowsofthetableshavesimilar propertiesoratleastpropertiesthatvaryinaperiodic,predictablemanner. The6Acolumninthetableconsistsofoxygen,sulfur,selenium,and tellurium.Wewouldexpecttheseelementsandtheircompoundstohave similarproperties,oratleasttobehaveinapredictablepattern.
Thehydrogencompoundsofthecolumn6Aelementsarewater (hydrogenoxide),hydrogensulfide,hydrogenselenide,andhydrogen telluride.AllhavethechemicalformulaH2X,whereXrepresentsthe group6Aelement.IfwelookatthenormalboilingpointsofH2S,H2Se, andH2Te,weshouldbeabletopredicttheboilingpointofwater. Fig.1.2
showsaplotofthenormalboilingpointsforthesethreecompounds.Note thatasthesizeofthemoleculeincreases,sodoesthenormalboilingpoint. Althoughitisnotexactlylinear,wecanusealinearapproximationto estimatetheboilingpointofwater.Thisextrapolationyieldsanestimated boilingpointof 74 C!Astheboilingpointofwateris100 C,thisis clearlyaverypoorestimation.Thereisprobablysomethingunusualabout water.
Itisworthnotingthatasimilarplotcouldbeconstructedshowingthe meltingpointsforthesecompounds.Againthepredictedmeltingpointof water,basedontheothersubstances,ismuchtoolow.
Asasecondexample,considerthehomologousseriesofnormalalcohols. Fig.1.3 showsaplotofthenormalboilingpointsofthealcoholsasa functionoftheirmolarmass(molecularweight).Inthiscase,therelationis nearlylinear.Assumethatwateristhesmallestmemberofthisgroupof compounds,andextrapolatethecorrelationtoestimatetheboilingpoint. Thisyields43 Cfortheboilingpoint,whichissignificantlylowerthanthe actualvalue.
Whydoeswaterhavesuchananomalouslylargeboilingpoint?
1.3.2Enthalpyofvaporization
In Table1.1,theenthalpiesofvaporizationofseveralcomponentsattheir boilingpointarelisted.Admittedly,itisasmalllist,butthetableincludes bothpolarandnonpolarsubstances.Fromthistableitcanbeseenthat waterhasafairlylargeenthalpyofvaporization,evenincomparisonto otherpolarsubstances.
Ittakessignificantlymoreenergytoboil1kgofwaterthanitdoesto boilanyofthehydrocarbonslistedinthetable approximately5timesas muchenergy.
Again,wemustaskthequestion,whydoeswaterbehaveso anomalously?
1.3.3Expansionuponfreezing
Anotherunusualpropertyofwateristhatitexpandsuponfreezing.In commonterms,thismeansthatice floatsonwater.Inengineeringterms, thedensityofice(917kg/m3 or57.2lb/ft3)islessthanthatofliquidwater (1000kg/m3 or62.4lb/ft3)atthefreezingpoint.
Table1.1 Theenthalpyofvaporizationofseveralsubstancesattheirnormalboiling point.
CompoundNatureEnthalpyofvaporization(kJ/kg)
WaterPolar2257 MethanolPolar1100
EthanolPolar855
AcetonePolar521
EthyleneglycolPolar800
AmmoniaPolar1369
MethaneNonpolar510 n-pentaneNonpolar357 n-octaneNonpolar306
BenzeneNonpolar394 o-xyleneNonpolar347
CyclohexaneNonpolar358
DatatakenfromDean,J.A.(ed.),1973.Lange’sHandbookofChemistry,eleventhed.McGraw-Hill, NewYork,NY,pp.9 85to9 95.
Thesimplereasonforthisexpansionisthatthewateratomsarrange themselvesinanorderedfashionandthemoleculesinthecrystaloccupy morespacethanthoseintheliquidwater.Thereasonforthisbehavioris alsobecauseoftheshapeofthewatermoleculeandsomethingcalledthe hydrogenbond.
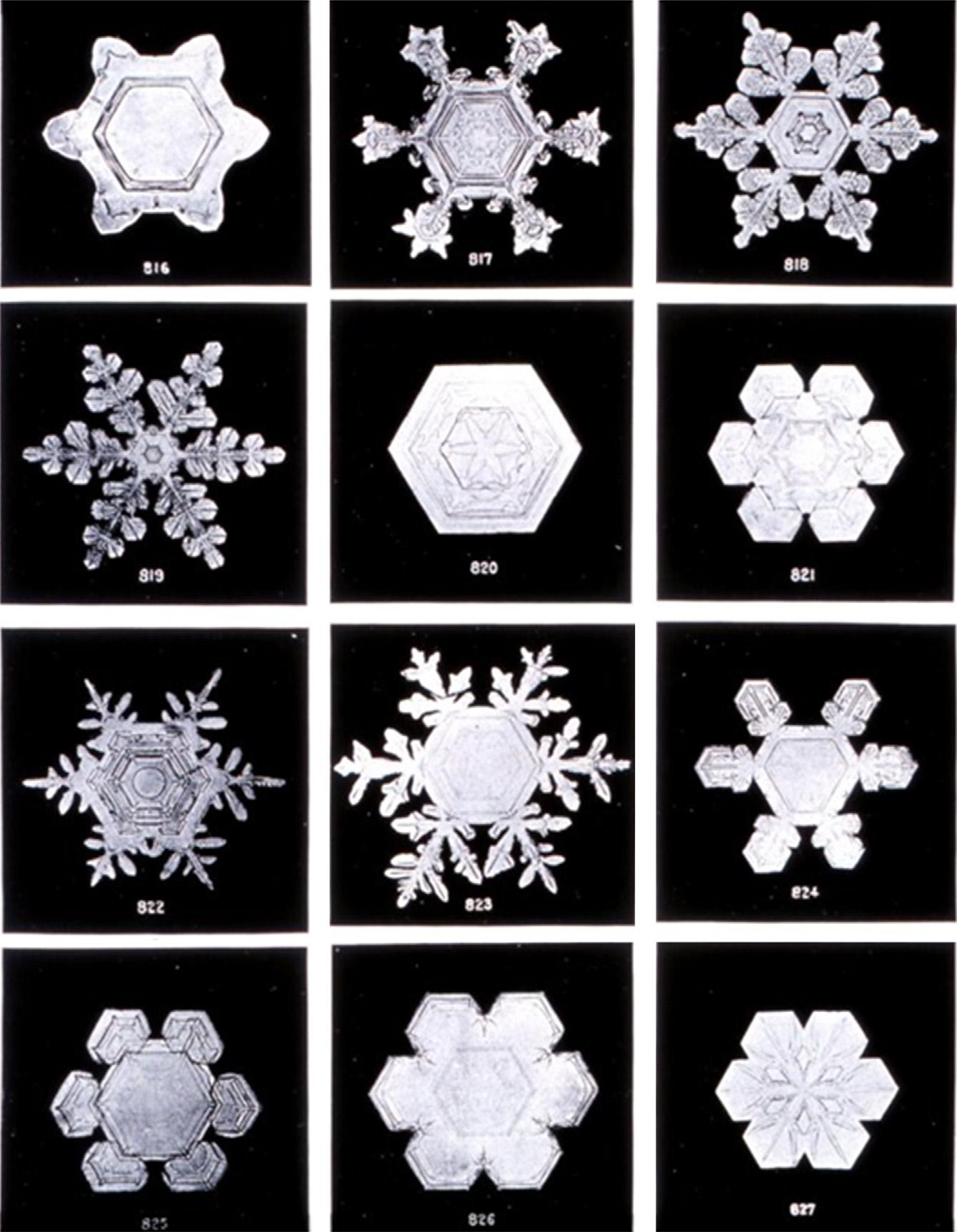
Themoleculesinsolidwaterformahexagonalcrystal.Thisismost obviousinsnowwithitscharacteristicpatternstructures(seeforexample Fig.1.4).
Figure1.4 Microphotographsofssnowflakes. (CourtesyoftheNationalOceanicand AtmosphericAdministration(NOAA),Washington,DC http://www.photolib.noaa.gov OriginalphotographsbyWilsonBentley(1865 1931),whichwerenotcopyright.)
1.3.4Theshapeofthewatermoleculeandthehydrogen bond
Virtuallyalloftheunusualpropertiesofwaternotedearliercanbe explainedbytheshapeofthewatermoleculeandtheinteractionsthat resultfromitsshape.
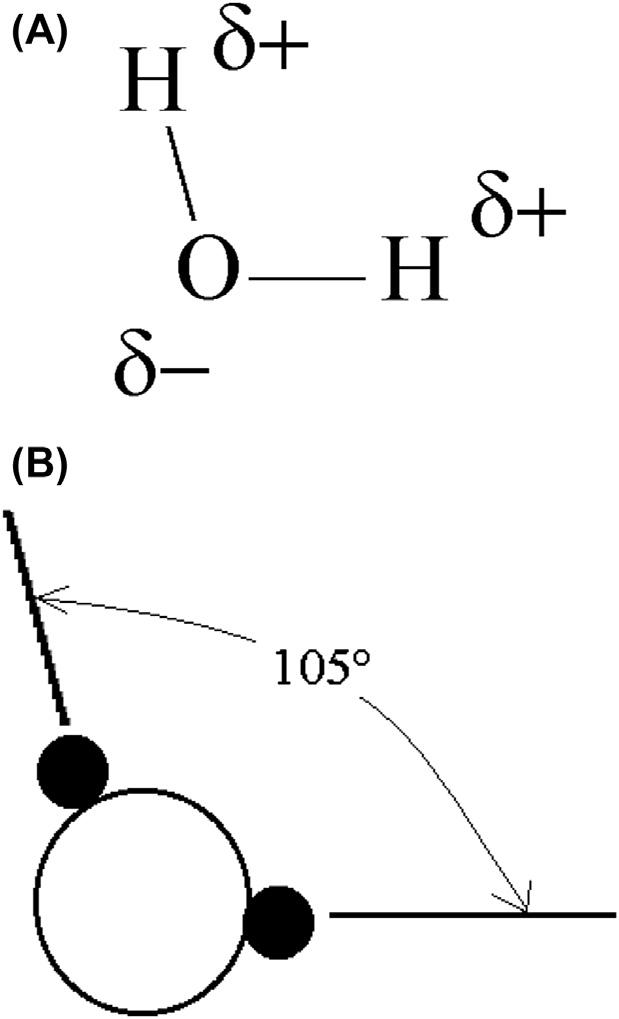
Thewatermoleculeconsistsofasingleatomofoxygenbondedtotwo hydrogenatoms,asdepictedin Fig.1.5.Inthewatermoleculethebond betweentheoxygenandhydrogenatomsisacovalentbond.Acovalent bondisessentiallyasharedpairofelectrons.Theanglebetweenthetwo hydrogenatomsinthewatermoleculeisabout105degrees.
What Fig.1.5 doesnotshowisthattherearetwopairsofunbonded electronsonthe “back” oftheoxygenmolecule.Theseelectronsinduce negativechargesontheoxygenmoleculeandasmallpositivechargeonthe hydrogenatoms.Theinducedelectrostaticchargesonthemolecule (denoted dþ forthepositivechargeand d forthenegative)areshownin Fig.1.5A.Thus,thewatermoleculeswilltendtoalignwithahydrogen moleculeliningupwithanoxygen.
Figure1.5 Theshapeofthewatermolecule.(A)Stickrepresentationshowinginduced charges,whichresultinhydrogenbonding,and(B)ballmodelshowingtheangle betweenthehydrogenmolecules.
Thisaligningofthehydrogenandoxygenatomsiscalleda “hydrogen bond.” Thehydrogenbondisessentiallyanelectrostaticattractionbetween themolecules.Itshouldbenotedthateachwatermoleculehastwopairof unbondelectronsandthushastwohydrogenbonds twowatermolecules “stick” toeachwatermolecule.Thehydrogenbondisonly1/10or1/20as strongasacovalentbond,whichiswhatholdstheoxygenandhydrogen atomstogetherinthewatermolecule,butthisisstillstrongenoughto explainthepropertiesdiscussedearlier.
Thehydrogenbondsareparticularlystronginwater,althoughtheyare presentinothersubstances,suchasthealcoholsdiscussedearlier.Itisforthis reasonthatthenormalboilingpointsofthealcoholsaresignificantlylarger thantheirparaffinanalogues.
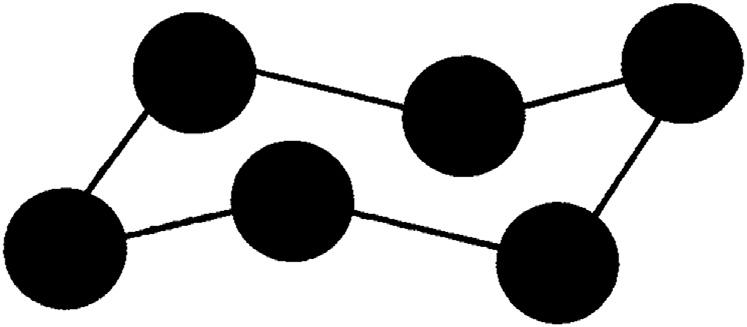
Whenthewatermoleculeslineup,theyformahexagonalpattern.This isthehexagonalcrystalstructurediscussedearlier.Fromelementarygeometry,itiswellknowthattheanglebetweenthesidesofaregular hexagonis120degrees,whichisgreaterthanthe105degreesangleinthe watermolecule.Thisseemingparadoxisovercomebecausethehexagonal patternofthewatermoleculesisnotplanar.Thehexagonalpatternofthe watermoleculesintheicecrystalisshownin Fig.1.6.Inthis figurethe circlesrepresentthewatermoleculesandthelinesthehydrogenbonds.
1.4Hydrates
Itisaresultofthehydrogenbondthatwatercanformhydrates.The hydrogenbondcausesthewatermoleculestoaligninregularorientations. Thepresenceofcertaincompoundscausesthealignedmoleculestostabilize andasolidmixtureprecipitates.
Thewatermoleculesarereferredtoasthe “host” moleculesandthe othercompounds,whichstabilizethecrystal,arecalledthe “guest” molecules.Inthisbooktheguestmoleculesaremoreoftenreferredtoas
Figure1.6 Thethree-dimensionalhexagonalarrangementofthewatermoleculesin anicecrystal.
“formers.” Thehydratecrystalshavecomplex,three-dimensionalstructures wherethewatermoleculesformacageandtheguestmoleculesare entrappedinthecages.
Thestabilizationresultingfromtheguestmoleculeispostulatedtobe duetovanderWaalsforces,whichistheattractionbetweenmoleculesthat isnotasaresultofelectrostaticattraction.Asdescribedearlier,thehydrogen bondisdifferentfromthevanderWaalsforcebecauseitisduetostrong electrostaticattraction,althoughsomeclassifythehydrogenbondasavan derWaalsforce.
Anotherinterestingthingaboutgashydratesisthatthereisnobonding betweentheguestandhostmolecules.Theguestmoleculesarefreeto rotateinsidethecagesbuiltupfromthehostmolecules.Thisrotationhas beenmeasuredusingadvancedchemicaltechniquessuchasspectroscopic, diffraction,etc.Therefore,thesecompoundsarebestdescribedasasolid solution.
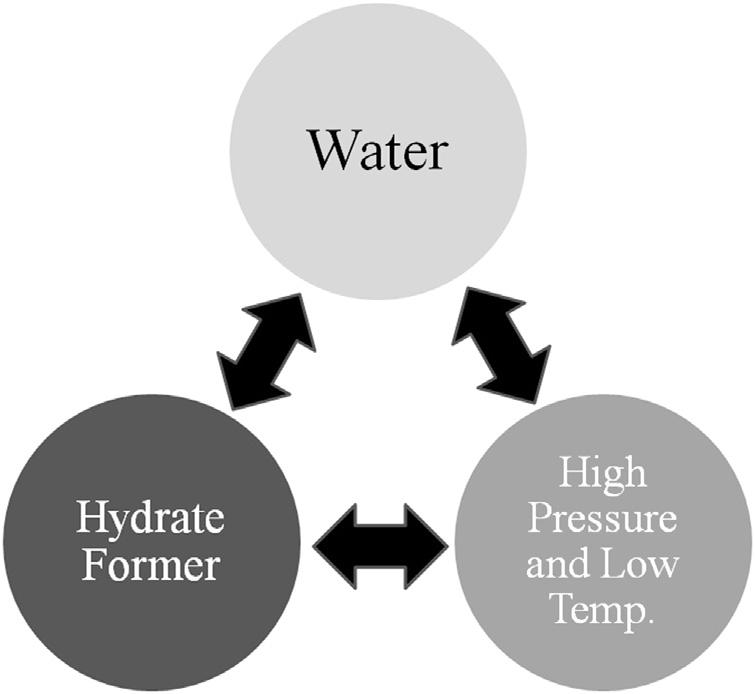
Theformationofahydraterequiresthefollowingthreeconditions:
1. Therightcombinationoftemperatureandpressure.Hydrateformation isfavoredbylowtemperatureandhighpressure.
2. Ahydrateformermustbepresent.Hydrateformersincludemethane, ethane,andcarbondioxide.
3. Asufficientamountofwater nottoomuch,nottoolittle.
Fig.1.7 givesavisualofthethreecriteriaforhydrateformation.The threeareinterconnected violateoneandahydratedoesnotform.
Figure1.7 Simplifieddiagramofthethreecriteriaforhydrateformation.
Althoughthis figuregivesaquickvisualimage,itlacksthedetailprovided bythediscussionpresentedearlier.However,itprovidesausefulvisual.
Thesethreepointswillbeexaminedinsomedetailinsubsequent chapters,buttheydeserveafewcommentsatthispoint.Aswasnoted,low temperatureandhighpressurefavorhydrateformation.Theexacttemperatureandpressuredependsuponthecompositionofthegas.However, hydratesformattemperaturesgreaterthan0 C(32 F),thefreezingpointof water.ThenatureofhydrateformersisdiscussedindetailinChapter2.
Topreventhydrateformation,onemerelyhastoeliminateoneofthe threeconditionsstatedabove.Typicallywecannotremovethehydrate formersfromthemixture.Inthecaseofnaturalgas,itisthehydrateformers thatarethedesiredproduct.Soweattackhydratesbyaddressingtheother twoconsiderations.
Otherphenomenathatenhancehydrateformationincludethe following:
1. Turbulence
a. Highvelocity
Hydrateformationisfavoredinregionswherethe fluidvelocity ishigh.Thismakeschokevalvesparticularlysusceptibletohydrate formation.First,thereisusuallyasignificanttemperaturedrop whennaturalgasischokedthroughavalveduetotheJoule Thomsoneffect.Second,thevelocityishighthroughthenarrowing inthevalve.
b. Agitation
Mixinginapipeline,processvessel,heatexchanger,etc.,enhanceshydrateformation.Themixingmaynotbeduetoanactual mixerbutperhapsatortuousroutingoftheline.
2. Nucleationsites
Ingeneralterms,anucleationsiteisapointwhereaphasetransition isfavored,andinthiscasetheformationofasolidfroma fluidphase.An exampleofnucleationisthedeepfryerusedtomakeFrenchfriesinfastfoodrestaurantsthroughouttheworld.Inthefryertheoilisveryhot butitdoesnotundergothefullrollingboilbecausetherearenosuitable nucleationsites.However,whenthepotatoesareplacedintotheoil,it vigorouslyboils.TheFrenchfriesprovideanexcellentnucleationsite. Goodnucleationsitesforhydrateformationincludeanimperfection inthepipeline,aweldspot,orapipeline fitting(elbow,tee,valve,etc.).
Corrosionby-products,silt,scale,dirt,andsandallmakegoodnucleationsitesaswell.
3. Freewater
No,thisisnotacontradictiontoearlierstatements.Freewaterisnot necessaryforhydrateformation,butthepresenceoffreewatercertainly enhanceshydrateformation.
Thepresenceoffreewateralsoassuresthatthereisplentyofwater present,whichismorelikelytoformaplug.
Inaddition,thewater gasorthewater oilinterfaceisagood nucleationsiteforhydrateformation.
Theitemsintheabovelistenhancetheformationofahydrate,butare notnecessary.Onlythethreeconditionsgivenearlierarenecessaryfor hydrateformation.
Anotherimportantaspectofhydrateformationistheaccumulationof thesolid.Thehydratedoesnotnecessarilyagglomerateinthesamelocation asitisformed.Inapipeline,thehydratecan flowwiththe fluidphase, especiallytheliquid.Itwouldtendtoaccumulateinthesamelocationas theliquiddoes.Usuallyitistheaccumulationsofthehydratesthatcausethe problems.Inamultiphasepipeline,itistheaccumulationsthatblockline andpluganddamageequipment.
Oftenpiggingissufficienttoremovethehydratefromthepipeline. Piggingistheprocessofinsertingatool(calleda “pig”)intotheline. Modernpigshavemanyfunctions,butthemainoneremainspipeline cleaning.Thepig fitstightlyintothelineandscrapstheinsideofthepipe.It istransportedalongthelinewiththe flowofthe fluidandbydoingsoit removesanysolids(hydrate,wax,dirt,etc.)frominsidetheline.The piggingcanalsobeusedtoremoveaccumulationsofliquids.
However,thepiggingmustbescheduledsuchthattheaccumulationsof hydratesdonotbecomeproblematic.Usuallypiggingisnotusedtoclean hydratesfromaline.Othermeasuresaremorecommonlyusedtodealwith hydratesandthesearedetailedinsubsequentchaptersofthisbook.
Anotherbenefitofpiggingistheremovalofsalt,scale,etc.,whichis importantfortheproperoperationofapipeline.Italsomeansthatpotential nucleationsitesforhydrateformationareremoved.
1.4.1Temperatureandpressure
Aswasnotedearlier,hydrateformationisfavoredbylowtemperatureand highpressure.Foreachgasitispossibletogenerateahydratecurvethat mapstheregioninthepressure temperatureplanewherehydratescan form.Muchoftherestofthisbookisdedicatedtothetoolsusedtopredict
thislocus.Again,withoutgettingtofaraheadofourselves,somepreliminarydiscussionofhydratecurvesisappropriate.
Fig.1.8 showsatypicalhydratecurve(labeled “hydratecurve”).The regiontotheleftandabovethiscurve(highpressure,lowtemperature)is wherehydratescanform.Intheregiontotherightandbelowthehydrate curve,hydratescanneverform inthisregion,the firstcriterionisviolated. Therefore,ifyourprocess,pipeline,well,etc.,operatesintheregion labeled “nohydrates,” thenhydratesarenotaproblem.Ontheotherhand, ifitisintheregionlabeled “hydratesregion,” thensomeremedialactionis requiredtoavoidhydrates.
Itmightseemasthoughwecantreatthetemperatureandpressureas separatevariablesbutwhendiscussinghydrates,theyarelinked.For example,youcannotsay “Ahydratewillnotformat10 Cforthegas mixtureshownin Fig.1.8.” Youmustqualifythiswithapressure.Soat 10 Cand5MPa,theprocessisinthe “hydrateregion,” whereasat10 C and1MPa,theprocessisintheregionwhereahydratewillnotform. Thus,wemusttalkaboutacombinationoftemperatureandpressureand noteachvariableonitsown.
withnohydrates,andasafetymargin.
Figure1.8 Pressure temperaturediagramshowingthehydrateregion,theregion
Furthermore,itiscommontoaddamarginofsafety,eventothebest hydratepredictionmethods.Thismargincanbe3to5Celsiusdegrees(5to 10Fahrenheitdegrees),buttypically3Celsiusdegreesisused.Theauthor typicallyuses3Celsiusdegreesbutthereadermayhavetheirownmargin orperhapsthereisonespecifiedbytheircompany.Amarginofsafetyis shownin Fig.1.8 (“plus3Celsius”)andthebufferzonebetweenthe estimatedhydratecurveandthe þ3Celsiuscurveisnoted.
Throughouttheremainderofthebook,usuallyonlythecalculated hydratecurveswillbeshownbuttheusershouldkeepthissafetymarginin mind.
1.5Waterandnaturalgas
Waterisoftenassociatedwithnaturalgas.Inthereservoirwaterisalways present.Thus,producednaturalgasisalwayssaturatedwithwater.In addition,formationwaterisoccasionallyproducedalongwiththegas.As thetemperatureandpressurechangeduringtheproductionofthegas, watercancondenseout.Methodsforestimatingthewatercontentof naturalgasarepresentedinChapter10.
Inaddition,waterisofteninvolvedintheprocessingofnaturalgas.The processtosweetennaturalgas(i.e.,toremovehydrogensulfideandcarbon dioxide,theso-called “acidgases”)oftenemploysaqueoussolutions.The mostcommonoftheseprocessesinvolveaqueoussolutionsofalkanolamines.Thesweetgas(i.e.,theproductofthesweeteningprocess)from theseprocessesissaturatedwithwater.
Thereareseveralprocessesthataredesignedtoremovewaterfrom naturalgas.ThesewillbediscussedinChapter6ofthisbook.
Thisassociationofwaterandnaturalgasmeansthathydrateswillbe encounteredinallaspectsoftheproductionandprocessingofnaturalgas.
Alargeportionofthisbookisdedicatedtothepredictionofthe conditionsatwhichhydrateswillform.Armedwiththisknowledge,engineersworkinginthenaturalgasindustryknowwhetherornothydrates willbeaproblemintheirapplication.
Onceithasbeendeterminedthathydratesareaproblem,orpotentially aproblem,whatcanbedone?Anotherlargesegmentofthisbookaddresses thistopic.
1.5.1Free-water
Thereisamythinthenaturalgasindustrythat “freewater” (i.e.,an aqueousphase)mustbepresenttoformahydrate.Insubsequentsectionsof thisbook,wewilldemonstratethatthisisnotcorrect.Freewatercertainly increasesthepossibilitythatahydratewillform,butitisnotanecessity.
Astrongargumentdemonstratingthatfreewaterisnotnecessaryfor hydrateformationispresentedinChapter9onphasediagrams.
Anotherargument,theso-called “frostargument,” asksthesimple question:Isitnecessarytohavefreewatertoformice?Theanswerisno. Frostformswithoutliquidwaterforming.Thefrostsublimesfromtheair ontomycaronwinternights.Thewatergoesdirectlyfromtheairtothe solidphasewithoutaliquidbeingencountered.Theair watermixtureisa gas,andthewaterisnotpresentintheairinaliquidform.Ifyouhavean old-fashionedfreezer,i.e.,onethatisnotfrost-free,justlookinside.Alayer offrostbuildswithoutliquidwatereverforming.Hydratescan “frost” via thesamemechanism.
Oneofthereasonswhyitisbelievedthatfreewaterisrequiredfor hydrateformationisthathydratesformedwithoutfreewatermaynotbe problematic.Theinsideofapipemay “frost” withhydrates,butstill functionproperly.Ortheamountofhydratemaybesmallandthusdoes notpluglinesordamageprocessingequipment.Such “frost” hydratescan beeasilycleanedusingthepiggingprocessdiscussedearlier.
Theprocessofgoingdirectlyfromthegastothesolidiscalledsublimationanditisnotthatrare.Forexample,carbondioxidesublimesat atmosphericpressure.SolidCO2,commonlycalled “dryice,” goesdirectly fromthesolidtothevaporwithoutformingaliquid.Atatmospheric pressure,CO2 goesdirectlyfromthesolidtothevaporatatemperatureof about 78 C( 108 F).Anotherexampleofasolidthatsublimesatatmosphericpressureisnaphthalene,themaincomponentofmothballs.The reasonwhyyoucansmellmothballsisbecausethenaphthalenegoes directlyfromthesolidtothevaporanditisthevaporthatyoucansmell.In reality,allpuresubstancessublimeatpressuresbelowtheirtriplepoint pressureandthisincludespurewater.Soitshouldcomeasnosurprisethat hydrates,undertherightsetofconditions,cansublimedirectlyfromthegas phasetothesolidphase.
1.6Heavywater
Deuteriumisanisotopeofhydrogen.Inthesimplehydrogenmolecule, thereisoneproton,oneelectron,andnoneutrons,protons,electrons,and neutronsbeingtheelementaryparticlesthatmakeuptheatom.Deuterium, ontheotherhand,iscomposedofoneproton,oneelectron,andone neutron.Becauseoftheadditionalparticle,deuteriumis “heavier” than normalhydrogen.
Wateriscomposedoftwohydrogenatomsandanoxygenatom.Heavy water,alsocalleddeuteriumoxide,iscomposedoftwodeuteriumatoms andanoxygenatom.
Nowthequestionarises,doesheavywaterformahydrate?Theanswer isyes.Heavywaterstillexhibitshydrogenbonding thekeytohydrate formation.However,itrequiresslightlymorepressuretoformhydratesin heavywaterthaninregularwater(forexample,see Chunetal.,1996).
1.7Thehydratetoolbox
Whatwillbepresentedinthisbookareasetoftoolsforstudyinghydrates, predictingtheconditionsatwhichtheywillform,preventingtheirformation,and finallywhattodowhenoneoccurs.Thesearethetoolsinour hydratetoolbox.
Wefocusontwoaspectsofhydrates:(1)preventionand(2)remediation.Whatcanwedotopreventhydratesfromformingandwhatcanwe doifahazardoushydrateforms.Isitpossibletohaveabenignhydrate?
Theengineermusthaveatoolkittocombathydrates.Thisincludes firmware(suchasheaters),softwaretopredicthydratebehavior,andintelligencetoanalyzethesituation,interpretpredictions,andapplythe correctmeasures.Thisbookwilladdresstheseitemsinmoredetail;infact thisisthemajorthemeofthebook.However,anoverviewisprovided here.
First,theengineershouldhavethetoolstopredicthydrateformation. Herewehavesplitthemintotwobroadcategories:(1)handcalculations and(2)computer-based.Althoughthehandcalculationmethodseems antiquated,theyarestillusedinsomecirclesandthusdeservediscussion,in particularthelimitedaccuracyofthesepredictions.Forthecomputer-based applications,wewilldiscussthebasisofthemodelsaswellasdemonstrate theaccuracyofsomeofthecommercialsoftwarepackages.
Nextarethetoolswecanuse:(1)conditions,(2)chemicals,and(3) dehydration.
Basically,conditionsmeanoperatingattemperaturesandpressuressuch thathydratesdonotform.First,theengineermustusetechniquesdiscussed inthisbooktoestablishtheconditionsatwhichhydrateswillform.The applicationofheaters,heattracing,andinsulationcanbeusedtokeepthe fluidabovethehydratetemperature.
Theinjectionofchemicalsinhibitshydrateformation.Thesecomein twobroadclasses:(1)thermodynamicand(2)lowdosage,whichare subdividedinto(a)kineticand(b)antiagglomerant.Theapplicationofthese chemicalsisalsodiscussed.
Thetoolsareappliedsuchthatengineerscanbuildadesignthatwill minimizetheriskofhydrateformationandhowwecanusethetoolsto dealwiththeunfortunatesituationwherehydratesform.
Thethermodynamicinhibitorscanbemodeledusingthermodynamics, bothsimpleandmorerigorous.Wewillexaminesomeofthesemodelsin laterchaptersofthebook.Therearekineticmodelsavailableforboththe formationanddissociationofhydrates,buthowthesemodelsareappliedto individualinhibitorsisnotexactlyclear.Wewillexamineboththekinetic modelsandtheapplicationofkineticinhibitsinsubsequentchapters.
Table1.2 summarizesthetoolsavailabletotheengineeranddirects themtotheappropriatechapterformorediscussiononthegiventopic.
1.8Additionalreading
Althoughthisbookismeanttoprovidethedesignengineerwithtoolsfor dealingwithhydrates,itisnotmeanttobethedefinitivevolumeonthe subject.ThereaderisreferredtotheworkslistedintheBibliographyatthe endofthechapterforadditionalinformationonthesubjectofgashydrates.
1.9Units
BothAmericanEngineeringUnits(ft-lb-sec)andSIUnits(m-kg-s)are commonlyemployedinthenaturalgasbusinessthroughouttheworld. Dependinguponthelocationonesystemtendstodominateovertheother, butbothareinwidespreaduse.Thus,toreachaswideanaudienceas possible,bothsetsofunitsareusedinthisbook.Usuallytheappropriate conversionsaregiven.