MaleandSperm FactorsthatMaximize IVFSuccess
Editedby R.JohnAitken
UniversityofNewcastle,NewSouthWales
DavidMortimer
OozoaBiomedicalInc.,Vancouver
GaborT.Kovacs
EpworthHealthCare,Melbourne
UniversityPrintingHouse,CambridgeCB28BS,UnitedKingdom OneLibertyPlaza,20thFloor,NewYork,NY10006,USA
477WilliamstownRoad,PortMelbourne,VIC3207,Australia
314–321,3rdFloor,Plot3,SplendorForum,JasolaDistrictCentre,NewDelhi – 110025,India 79AnsonRoad,#06–04/06,Singapore079906
CambridgeUniversityPressispartoftheUniversityofCambridge.
ItfurtherstheUniversity’smissionbydisseminatingknowledgeinthepursuitof education,learning,andresearchatthehighestinternationallevelsofexcellence.
www.cambridge.org
Informationonthistitle: www.cambridge.org/9781108708319
DOI: 10.1017/9781108762571
©JohnAitken,DavidMortimerandGaborKovacs2020
Thispublicationisincopyright.Subjecttostatutoryexception andtotheprovisionsofrelevantcollectivelicensingagreements, noreproductionofanypartmaytakeplacewithoutthewritten permissionofCambridgeUniversityPress.
Firstpublished2020
PrintedintheUnitedKingdombyTJInternationalLtd,PadstowCornwall AcataloguerecordforthispublicationisavailablefromtheBritishLibrary.
ISBN978-1-108-70831-9Paperback
CambridgeUniversityPresshasnoresponsibilityforthepersistenceoraccuracyof URLsforexternalorthird-partyinternetwebsitesreferredtointhispublication anddoesnotguaranteethatanycontentonsuchwebsitesis,orwillremain, accurateorappropriate.
Everyefforthasbeenmadeinpreparingthisbooktoprovideaccurateandup-to-dateinformationthat isinaccordwithacceptedstandardsandpracticeatthetimeofpublication.Althoughcasehistoriesare drawnfromactualcases,everyefforthasbeenmadetodisguisetheidentitiesoftheindividualsinvolved. Nevertheless,theauthors,editors,andpublisherscanmakenowarrantiesthattheinformation containedhereinistotallyfreefromerror,notleastbecauseclinicalstandardsareconstantlychanging throughresearchandregulation.Theauthors,editors,andpublishersthereforedisclaimallliabilityfor directorconsequentialdamagesresultingfromtheuseofmaterialcontainedinthisbook.Readersare stronglyadvisedtopaycarefulattentiontoinformationprovidedbythemanufacturerofanydrugsor equipmentthattheyplantouse.
1 SpermSelectionforART Success 1 R.JohnAitken
2 NewHorizonsinMaleSubfertility andInfertility 15
BrettNixonandElizabethG.Bromfield
3 ChromosomeAbnormalities andtheInfertileMale 28
CsillaKrauszandViktóriaRosta
4 TheEffectofEndocrineDisruptors andEnvironmentalandLifestyle FactorsontheSperm Epigenome 41
VivianeSantana,AlbertSalas-Huetos, EmmaR.James,andDouglasT.Carrell
5 LifestyleFactorsandSperm Quality 59 CiaraWright
6 TheEffectofAgeonMaleFertility andtheHealthofOffspring 73 AllanPaceyandSarahMartinsdaSilva
7 TheAssessmentandRoleof Anti-spermAntibodies 83 GaryN.Clarke
8 FSHTreatmentinMale Infertility 95
CsillaKrausz,ViktóriaRosta, andAlbertoFerlin
9 AntioxidantstoImproveSperm Quality 106
ElenaMartínezHolguín,Enrique LledóGarcía,ÁngelRebolloRomán, JavierGonzálezGarcía,JoséJara Rascón,andCarlosHernández Fernández
10 TheHistoryofUtilizationofIVFfor MaleFactorSubfertility 121 GaborT.Kovacs
11 TheCaseAgainstIntracytoplasmic SpermInjectionforAll 130 DavidMortimerandSharonT. Mortimer
12 PerinatalOutcomesfromIVFand ICSI 141 MichaelDavies
13 ArtificialInseminationwithPartner’s SpermforMaleSubfertility 154 WillemOmbelet
14 ObstructiveAzoospermia:IsThere aPlaceforMicrosurgicalTesticular SpermExtraction? 166
AhmadAboukhshaba,RussellP. Hayden,andPeterN.Schlegel
15 ShouldVaricoceleBeOperatedon BeforeIVF? 176
ShannonH.K.KimandVictoria Nisenblat
RussellP.Hayden,Ahmad Aboukhshaba,andPeterN.Schlegel
Contributors
AhmadAboukhshaba
WeillCornellMedicine,Departmentof Urology,NewYork,NY,USA
RachelAgnew
ReproductiveandDevelopmental Biology,DivisionofSystems Medicine,SchoolofMedicine, NinewellsHospitaland MedicalSchool,UniversityofDundee, Dundee,UK
R.JohnAitken
PriorityResearchCentrefor ReproductiveScience,Universityof Newcastle,Callaghan,NSW Australia
ChristopherL.R.Barratt
ReproductiveandDevelopmental Biology,DivisionofSystemsMedicine, SchoolofMedicine,NinewellsHospital andMedicalSchool,UniversityofDundee, Dundee,UK
ElizabethG.Bromfield TheUniversityofNewcastle PriorityResearchCentreforReproductive Science,SchoolofEnvironmental andLifeSciences,Universityof Newcastle,Callaghan,NSW Australia
DouglasT.Carrell SurgeryandHumanGenetics,University ofUtahSchoolofMedicine,SaltLakeCity, UT,USA
GaryN.Clarke
AndrologyUnit,RoyalWomen’s&Royal Children’sHospitals,Melbourne,Victoria, Australia
MichaelDavies
Professor,TheRobinsonResearch Institute,UniversityofAdelaide,South Australia,Australia
AlbertoFerlin DepartmentofClinicalandExperimental Sciences,UnitofEndocrinologyand Metabolism,UniversityofBrescia,Brescia, Italy
JavierGonzálezGarcía UrologyServiceUniversityGeneral HospitalGregorioMarañón,Madrid, Spain
RussellP.Hayden WeillCornellMedicine,Departmentof Urology,NewYork,NY,USA
EleanorHeighton
ReproductiveandDevelopmental Biology,DivisionofSystemsMedicine, SchoolofMedicine,NinewellsHospital andMedicalSchool,UniversityofDundee, Dundee,UK
CarlosHernández-Fernández UrologyService,UniversityGeneral HospitalGregorioMarañónand DepartmentofSurgery,Complutense UniversityofMadrid,Madrid,Spain
EmmaR.James AndrologyandIVFLaboratories, DepartmentofSurgery,UniversityofUtah SchoolofMedicine,SaltLakeCity,UT,USA
JoséJara-Rascón UrologyService,UniversityGeneral HospitalGregorioMarañónand DepartmentofSurgery,Complutense UniversityofMadrid,Madrid,Spain
ShannonHeeKyungKim UniversityofNewSouthWales,Royal HospitalforWomen,Randwick,andIVF Australia,Sydney,NSW,Australia
GaborT.Kovacs MonashUniversity,Clayton,andEpworth HealthCare,Richmond,Victoria,Australia
CsillaKrausz DepartmentofBiomedical, ExperimentalandClinicalSciences “ MarioSerio, ” UniversityofFlorence, Florence,Italy
EnriqueLledóGarcía DepartmentofUrology,University GeneralHospitalGregorioMarañón, Madrid,SpainandComplutense UniversityofMadrid,Madrid,Spain
ElenaMartínezHolguín UrologyService,UniversityGeneral HospitalGregorioMarañón,Madrid,Spain
SarahMartinsdaSilva ReproductiveMedicine,Ninewells HospitalandMedicalSchool,Universityof Dundee,Dundee,UK
DavidMortimer OozoaBiomedicalInc.,West Vancouver,BC,Canada
SharonT.Mortimer
OozoaBiomedicalInc.,WestVancouver andAdjunctProfessor,Divisionof EndocrinologyandInfertility, DepartmentofObstetricsand Gynaecology,FacultyofMedicine, UniversityofBritishColumbia, Vancouver,BC,Canada
VictoriaNisenblat RobinsonResearchInstitute,Schoolof
MedicineDepartmentO&G,Universityof Adelaide,Adelaide,Australia
BrettNixon PriorityResearchCentreforReproductive Science,SchoolofEnvironmentalandLife Sciences,UniversityofNewcastle, CallaghanNSW,Australia,andHunter MedicalResearchInstitute,NewLambton Heights,NSW,Australia
WillemOmbelet
GenkInstituteforFertilityTechnology, DepartmentofObstetricsand Gynaecology,GenkandHasselt University,DepartmentofPhysiology, Hasselt,Belgium
AllanPacey
DepartmentofOncologyandMetabolism, UniversityofSheffield,Sheffield,UK
ÁngelRebolloRomán
UnitofClinicalManagement, EndocrinologyandNutrition,ReinaSofía UniversityHospital,Córdoba,Spain
ViktóriaRosta
DepartmentofBiomedical,Experimental andClinicalSciences “MarioSerio,” UniversityofFlorence,Florence,Italy
AlbertSalas-Huetos
AndrologyandIVFLaboratories, DepartmentofSurgery,UniversityofUtah SchoolofMedicine,SaltLakeCity,UT, USA
VivianeSantana
AndrologyandIVFLaboratories, DepartmentofSurgery,Universityof UtahSchoolofMedicine,SaltLakeCity, UT,USAandDepartmentofGynecology andObstetrics,RibeiraoPretoMedical School,UniversityofSaoPaulo,Sao Paulo,Brazil
PeterN.Schlegel
E.DarracottVaughanSeniorAssociate DeanforClinicalAffairs,WeillCornell Medicine,Urologist-in-Chief,NewYork Presbyterian/WeillCornell,NewYork, NY,USA
CiaraWright GlenvilleNutritionIreland,Rathgar, Dublin,Ireland
R.JohnAitken
1.2PrinciplesofSpermIsolation:PreventingLeukocytic Attack
Humansemenisanextremelycomplexcellularmixturecontainingliveanddeadspermatozoa,leukocytes(largelyneutrophils),precursorgermcells,bacteriaandcellulardetritus originatingfromthesecondarysexualglands.Theclinicalchallengeistoextractthehighqualityspermatozoafromthiscomplexmélange,withoutinducinganyiatrogenicdamage. Inthiscontext,seminalplasmaisourfriendbecauseitisrichlyendowedwithantioxidants specificallydesignedtoprotectthespermatozoaduringtheirshortjourneyfromthemale reproductivetractintothefemale.TheseantioxidantsincludesmallmolecularmassscavengerssuchasvitaminE,glutathione,uricacidandtaurineaswellashighlyspecialized antioxidantenzymesincludingcatalase,glutathioneperoxidaseandsuperoxidedismutase [5].Thiscomplexantioxidantmixturehasevolvedbecausethespermatozoaarevery vulnerabletooxidativeattackduringtheinseminationprocess – andthisattackmay comefromavarietyofsources.Inthiscontextitisimportanttorememberthatspermatozoahavebeenmaturedandstoredintheepididymallumen,whichisessentiallyfreeof phagocyticleukocytescapableofgeneratingreactiveoxygenspecies(ROS).Then,atthe momentofejaculation,thespermatozoaaresuddenlyexposedtomacrophagesandneutrophilsthathaveinfiltratedtheejaculateviathesecondarysexualglandsandurethra.These phagocytesareinanactivatedfree-radical-generatingstateandwouldcauseconsiderable damagetothespermplasmamembraneandotherstructuresifitwerenotforthe antioxidantsinseminalplasmaprovidingahighleveloffreeradicalscavengingprotection (Figure1.1)[6].
Verysoonafterinsemination,thebestqualityspermatozoawiththehighestlevelsof progressivemotilityleavetheprotectionprovidedbyseminalplasmaandcolonizethe cervix.Inthislocationthespermatozoaaresafe – forawhile.Withinafewhoursthe presenceofspermatozoaandsemeninthecervixandvaginastimulatesaleukocytic infiltration,largelyneutrophils,designedtophagocytoseanydeadormoribundspermatozoaremainingatthesiteofinsemination.Thepost-inseminationphagocytosisofnonviablespermatozoaisgenerally “silent” inthesensethatnoROSorpro-inflammatory cytokinesaregenerated.Thesilentphagocytosisofsenescentspermatozoaisthoughttobe aresponsetomarkers,suchasphosphatidylserine(PS),whichareexpressedonthesurface ofspermatozoaastheyengageintheintrinsicapoptoticcascade.Thisconcepthasarisenby analogytosilentphagocytosisinsomaticsystems,inwhichtheexpressionofapoptotic markerssuchasPSonthesurfaceofphagocytosedcellsinformsthephagocytethat engulfmentmust not beaccompaniedbyanoxidativeburst.Suchsilentphagocytosisis widespreadinbiologyandcanbeseen,forexample,whensenescentneutrophilsarebeing removedbymacrophagesfromsitesoftissuerepair[7].Thesilentnatureofthisphagocytic processensuresthatundernormalphysiologicalcircumstance,thespermatozoadonot havetocontendwithadditionaloxidativestresswhiletheyascendthefemalereproductive tract.However,ifsurfaceexpressionofPSdoesnotoccurbecausethespermatozoahave diedanecroticdeathmediatedbyperoxynitrite[8]orattackbyspermicidaldetergentssuch asnonoxynol-9,thentheymaywellbeexposedtoapost-inseminationoxidativeattack,only thistimetheywillnotbeabletorelyontheantioxidantpropertiesofseminalplasmato protectthem.
Theprotectiveroleofseminalplasmaisanimportantfundamentalconceptthathas helpeddesignoptimizedspermisolationstrategies.Whateverspermpreparationapproach
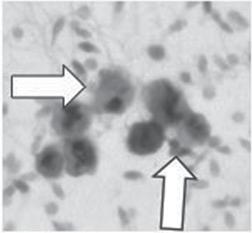
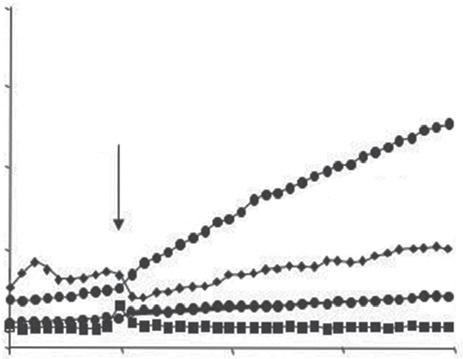
Figure1.1 Humansemensamplesareinvariablycontaminatedwithleukocytes,particularlyneutrophils,whichare capableofgeneratingreactiveoxygenspecies(ROS)anddamagingthespermatozoa.(A)Leukocytesinsemen (arrowed)stainedwithananti-CD45antibody.(B)Theconcentrationofleukocytesinhumansemencorrelatesvery closelywithROSgenerationbytheunfractionatedejaculate,indicatingthatasignificantproportionoftheseminal leukocytepopulationisinanactivatedstate.(C) UsingopsonizedzymosantoinvestigatethepresenceofROSgeneratingphagocytesinspermsamplespreparedforIVFrevealsthatelectrophoreticallyseparatedsperm suspensions(E-separated)possesslowerlevelsofleukocytecontaminationthanthosepreparedbydiscontinuous gradientcentrifugation(DGC)(Percoll).Followingelectrophoresis,mostoftheleukocytesremaintrappedinthe inoculationchamber(Residual)[6].
isused,itisimportantthatseminalleukocytesandspermatozoaarenotallowedtocome intocontactwitheachotherintheabsenceofseminalplasma.Whenthisispermittedto occur,aswhenspermatozoaarepreparedbyrepeatedcyclesofwashingandcentrifugation orareswumupfromawashedpellet,thenspermqualityisinvariablycompromised[9]. Thiscanoccurinadvertently,ifthespermpreparationprocedurehasnotbeensuccessfulin removingallofthecontaminatingleukocytesfromthesuspensionusedforIVF.Thus,in onestudyinvolvingtheuseofdiscontinuousgradientcentrifugation(DGC)tocarefully purifyspermatozoaforanIVFprogram,leukocytecontaminationcouldstillbedemonstratedin28.5%ofthespermpreparations.Furthermore,thepresenceofthesecontaminatingcellswasassociatedwithelevatedlevelsofspontaneousROSproduction,impaired
Zymosan
Residual Percoll Control E-Separated
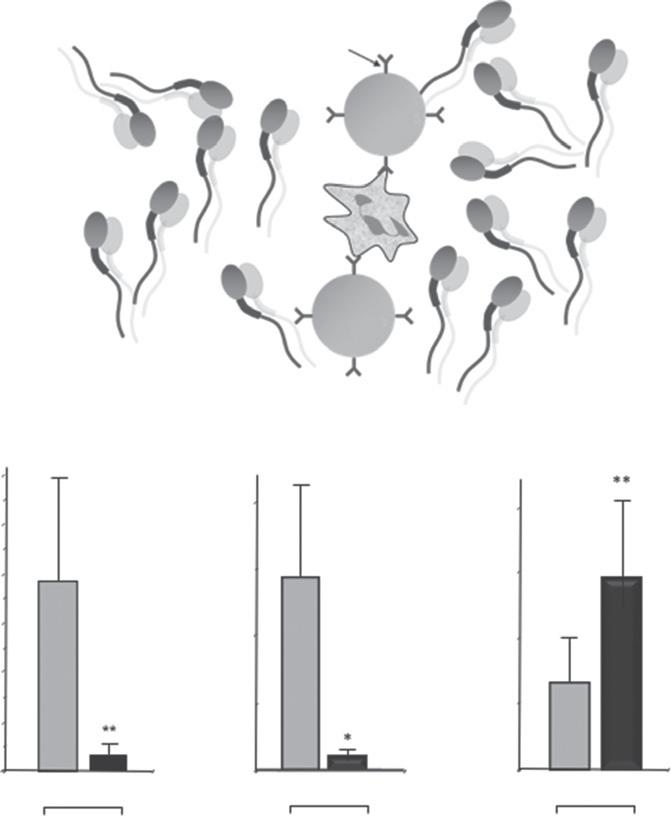
Figure1.2 Whenleukocytecontaminationisobservedinhumanspermsuspensions,thesecellscanberemoved, resultinginareductioninoxidativestressandanincreaseinspermfunction.(A) Principlebehindtheleukocyte separationprocedureusingmagneticbeadscoatedwithantibodiesagainstthecommonleukocyteantigen(CD45). (B) Thetreatmentisextremelyeffectiveinremovingresidualleukocytesfromspermsuspensions.(C) Theremovalof contaminatingleukocytesresultsinamassivereductioninoxidativestressasdeterminedbythechemiluminescent measurementofROSgeneration.(D) Leucocyteremovalresultsinanincreaseinlevelsofsperm–oocytefusionas observedwiththehamsteroocytepenetrationassay[11].
movement,andasignificantlyreducedcapacityforfertilization invitro [10].Treatmentof suchhumanspermsuspensionswithmagneticbeadsorferrofluidscoatedwithantibodies againstthecommonleukocyteantigen(CD45)hasbeenfoundtosuccessfullyremovethese cellularcontaminantsand,bysodoing,significantlyenhancethefertilizingcapacityofthe remainingspermatozoa(Figure1.2)[11].
ItfollowsfromtheabovethatanystrategyforisolatingspermatozoaforARTpurposes shouldtakeitsleadfromnatureandisolatethecellsdirectlyfromsemenratherthan awashedspermsuspension.Giventhatthisisthecase,therearealimitednumberof strategiesthatcanbepursuedinordertoachievetheeffectiveisolationofhigh-quality spermatozoafromtheejaculatebasedonspermmotility,density,chargeandothersurface
(B)(C)(D) Leucocytes
Anti-CD-45 monoclonal
Magnetic Dynabeads
Magnetic Dynabeads
characteristics.Inthesectionsthatfollow,weshallconsidertheeffectivenessofthese approachesandtheirpotentialforapplicationinaclinicalsetting.
1.3IntrinsicMotility
Becauseourspeciesisavaginalinseminator,wegeneratespermatozoathatarecapableof progressivelinearmovementcapableofpenetratingthedenseextracellularmatricesthat characterizethefemalereproductivetract,beginningwithcervicalmucus.Ifwewishto emulatenatureindevelopingoptimizedmethodsforisolatinghigh-qualityspermatozoa,we coulddoworsethandevelopamediumresemblingcervicalmucusforthespermatozoato colonize.Inthiscontext,cervicalmucusitself,whetherofhumanorbovineorigin,istoo logisticallydifficulttoobtainandtoodifficulttostandardizeforroutinespermisolation purposes.Recognizingthis,scientistsintheearly1990sstartedtoexperimentwithcervical mucussubstitutesfortheisolationofspermatozoaanddiscoveredthathyaluronatepolymerswereextremelyeffectiveinthisregard[12].Furthermore,spermatozoathathavebeen isolatedusinghyaluronatesolutionshavebeenshowntobefunctionallynormalasjudged bytheirmotilityandabilitytoundergobothcapacitationandtheacrosomereaction[13]. Swim-upproceduresinvolvingthelayeringofahyaluronatesolutionoverhumansemen, followedbyanincubationperiodof50–60minutesat37ºCinanatmosphereof5%CO2 in air,havebeenreportedtogeneratespermsuspensionsthatareofsignificantlyhigherquality thanthosepreparedbyswim-upfollowingcentrifugation[14].Asasimpleinexpensive techniqueforspermisolation,theself-migrationofspermatozoadirectlyfromsemeninto mediumthathasbeenmodifiedbytheadditionofsodiumhyaluronateinordertoincrease itsviscosityandlimitcontaminationofthesamplewithseminalplasmaconstituents,has muchtocommendit.Avariationonthisthemehasbeenadouble-uptechniqueinwhich spermatozoaareswumupfromapelletpreviouslypreparedbyDGC[15].Suchatechnique generatesspermsuspensionsofhighpurityandquality,althoughpreparationtimeis prolongedandsuccessultimatelydependsontheintrinsicqualityoftheoriginalsemen sampleintermsofmotilityandcount.
1.4MicrofluidicDevices
Theintrinsicmotilityofspermatozoahasalsobeenexploitedinthegenerationofmicrofluidicsystemsforspermisolationthatemploymediabasedonhyaluronateoroccasionally methylcellulosefortheinitialisolationofthespermatozoa[16].Avarietyofsuchmicrofluidicsystemshavebeendevelopedthatattempttoreplicatethemechanismsbywhich spermatozoaareisolated invivo. Mostofthesedevicesfeaturecarefullyengineeredmicrochannelsthatspermatozoacolonizeinthesamewayastheycolonizecervicalmucusby virtueoftheirownmotility.Orientationofthespermatozoainsuchchannelshasbeen achievedbyavarietyofdifferentmechanisms,dependingonsuchpropertiesasrheotaxis, chemotaxisorthermotaxis,allofwhichspermatozoadisplay.
Rheotaxis,forexample,referstotheorientationofspermatozoaina fluid flow.Insome respects,spermatozoaareliketrout,inthatfacedwithamild flowintheextracellular environment,theywillorientateupstream.Ofcourse,unliketrout,spermatozoadonot possessalaterallinetodetectthe flowbutratherdependoncomplexhydrodynamic interactionstoachievetheupstreamorientation[16].Therateof flowiscriticalbecauseif itbecomestoostrongthespermatozoawillsimplybesweptaway.However,acarefully engineered flowofaround15–100µm/scanfacilitatethemigrationofspermatozoathrough
thedevice.Buildingonthesefundamentals,aningeniousmicrofluidiccorrallingsystemhas recentlybeendevelopedforisolatingsubpopulationsofhighlymotilehumanspermatozoa[17].
Chemotaxisisanotherpotentialmechanismfororientatingspermatozoa,particularly formicrofluidicssystemsthataimtonotonlyisolatethespermatozoabutalsofertilizethe oocyteandculturetheembryo.Somecandidatesforhumanspermchemotaxishavealready beenidentified,notablyprogesterone,andusedasthebasisforcreatingaspermisolation system.Instudieswiththisdevice,spermatozoawereinitiallyseparatedonPercollgradients andtheninoculatedintoasystemthatmimickedthedimensionsofthehumanfemaletract [18].Afteraprolonged150-minincubationat37ºC,spermatozoaattractedbythepresence ofaprogesteronegradientwereshowntohavesignificantlybettermorphologyandsignificantlyreducedlevelsofDNAdamagecomparedwithspermatozoaisolatedbyvirtueof theirmotilityalone.Althoughtheincubationtimeusedinthisstudywasprotractedandthe recoveryefficiencylow,thegeneralprincipleofusingchemotaxisasanaidtosperm isolationcertainlyhasmerit.Clearly,speciesexhibitinganinternalmodeoffertilization exhibitnothinglikethestrongchemotaxisevidentinaquaticspecieswherefertilizationis externalandconspecificgameteshavetoquickly findeachotherbeforebecomingdilutedto infinityinthewatercolumn.Nevertheless,thereisstrongevidencefortheinvolvementof chemotaxisinhumanfertilizationassuggestedbythesynthesisandinsertionofolfactory receptorsinthespermplasmamembrane[19].Althoughprogesteroneisevidentlyaplayer inthiscontext,thereareotherpossiblymorepowerfulchemotacticfactorselaboratedbythe oocytethatstillawaitdefinitivecharacterization[20].Furtherresolutionofsuchfactorsmay greatlyassistinthedevelopmentofspermisolationsystemsthatreflectthe invivo situation.
Alongsimilarlines,microfluidicdeviceshavealsobeenconstructedbasedonthe principleofthermotaxisandexploitingthefactthatspermatozoamovealong atemperaturegradientastheyascendthefemalereproductivetract.Althoughthemechanismsthatunpinthisthermotaxisarecurrentlyunresolved,thecapacityofthesecellsto discriminatechangesintemperaturehasbeenanalysedindetailwithsomeastonishing results.AccordingtoBahatandcolleagues[21],humanspermatozoacansenseandrespond toatemperaturedifferenceoflessthan0.0006°C!Usingthisprinciple,anovelmicrofluidics devicehasbeenconstructedthatappearstobeeffectiveintrappingspermatozoathathave migratedintoregionsofelevatedtemperature[22].
Todate,therearenoreportsofhowsuchmicrofluidicdevicesperformwithpathological samplesunderreal-lifeclinicallaboratoryconditions.Theimplementationofsuchstudies willbeimportantnotjusttodeterminewhethermicrofluidicssystemshaveanypractical potentialinaclinicalcontextbutalsotodeterminewhethersuchsystemscouldbeusedas theinitialcomponentofamicrofluidicssystemthatwillnotonlycapturehigh-quality spermatozoabutalsosupportfertilizationandtheearlystagesofembryodevelopment –IVFonachip.Usingdonatedfrozen-thawedhumanembryos,microfluidicdeviceshave beenassessedthatappeartoprovidethesamelevelofsupportforpreimplantationembryonicdevelopmentasconventionalembryoculturemethodologies[23].Thisisclearlyan extremelyactiveareaofresearchatthepresenttime,whichenvisionsdevelopmentof asinglemicrofluidicsdevicethatcanachieveisolationofthehighestqualityspermatozoa, fertilizationandthedetailedmonitoringofpreimplantationdevelopmentwithaminimal degreeofinvolvementonbehalfoftheembryologistoverseeingtheprocess.Onecouldeven imaginesystemswheretheembryoculture fluidisautomaticallysampledandscannedfor markersthatwillconfirmtheireuploidstatuspriortobeingselectedfortransfer[24].
ThedifficultieswithsuchahighlytechnicalautomatedapproachtoIVFarecost,time and,ultimately,feasibility.Thecapacitytotroubleshootanyunexpectedproblemsthatarise duringthespermisolation–fertilization–developmentcontinuummaybecurtailedifthe processbecomestooautomatedand,attheendoftheday,theembryowillstillhavetobe recoveredandmanuallytransferredintotheuterinecavity – andthiscomprisesoneofthe mostdifficultriskyphasesoftheentireARTprocess.Furthermore,thespermselection componentofsuchdevicesdependsforitssuccessontheintrinsicmotilityofthespermatozoa.InanIVFprogramwheremanyofthesemensamplesthatarebeingprocessedfor treatmentwillbeofpoorqualityandfrequentlysufferingfromlowlevelsofmotility,the abilityofspermatozoatoself-selectmaybeimpaired.Inordertocompensateforthepoor intrinsicmotilityofpathologicalspermsamples,alternativespermseparationstrategies havebeendevelopedthatactivelyrecruitthespermatozoaratherthanrelyingontheir capacitytoself-select.Thetwoprinciplepropertiesonwhichsuchselectionshavebeen basedarespermdensityandspermcharge.
1.5IsolationAccordingtoDensity
Oneofthefeaturesofmammalianspermatozoaisthattheyhavearelativelyhighdensityby virtueoftheirlackofcytoplasmicspace.Hence,ifthesecellsareplacedinacontinuous densitygradientandcentrifuged,thehighestqualitycellswiththegreatestisopycnic densitieswillbecarriedtothedensestregionofthegradient[25].SuchDGCtechniques havebeenprogressivelysimplifiedwiththepassageoftimeand,inminimaltwo-stepmode, haveprovenhighlyeffectiveinisolatingmotilespermatozoaexhibitinghighlevelsof fertilizingpotentialcapableofestablishingviablepregnanciesfollowingIVF[26].
Thepositioningofspermatozoainsuchdiscontinuousgradientsisafunctionof theirdensityandthus,inversely,theirvolume.High-qualityspermatozoaarethe productofahighlyeffi cientspermatogenicprocessthateff ectivelyremovesmostof theresidualcytoplasmfromthespermatozoajustpriortotheirreleasefromthe germinalepithelium.Anyresidualcytoplasmthensnapsbackintothemidpieceof thecelltocreateacytoplasmicremnantthat,asfarasweareaware,isnotfurther processedbythecell.Theretentionofexcessresidualcytoplasminthismannerisin contrasttomanyothermammalianspecieswhereanycytoplasmremainingwiththe spermatozoonafterspermiationisconcentratedintoaroundedcytoplasmicdroplet whichthenslipsdowntheshaftofthespermtailtotheannulus,whereitisdiscarded orresorbed.Humanspermatozoadonotbehaveinthesameway.Theretentionof excessresidualcytoplasmbyhumanspermatozoaisapathologicalchangethatinversely refl ectsthequalityofthespermatogenicprocessinvolvedintheircreationandthus, ultimately,theirfunctionality(Figure1.3).Themorecytoplasmisretainedbythe spermatozoatheworsetheirmotility,thepoorertheircapacityforacrosomalexocytosis andsperm – oocytefusionandthehigherthelevelsofROSgeneration[ 27].
Exactlywhytheretentionofexcessresidualcytoplasmshouldbesodetrimentaltosperm functionisstillsomethingofamystery.Formostcelltypes,cytoplasmisavirtuous possessionthatencapsulatesmostoftheenzymesandalloftheorganellesneededtosustain life.Thelimiteddistributionandsmallvolumeofspermcytoplasmactuallycreatesvulnerabilitiesforthiscelltype,duetoalackofantioxidantprotectionandDNA-repaircapacity which,insomaticcells,areorchestratedbyenzymesthathavetheiroriginsinthecytoplasm andtheorganelleshousedwithinthisspace.Giventheextremevulnerabilitycreatedbythis
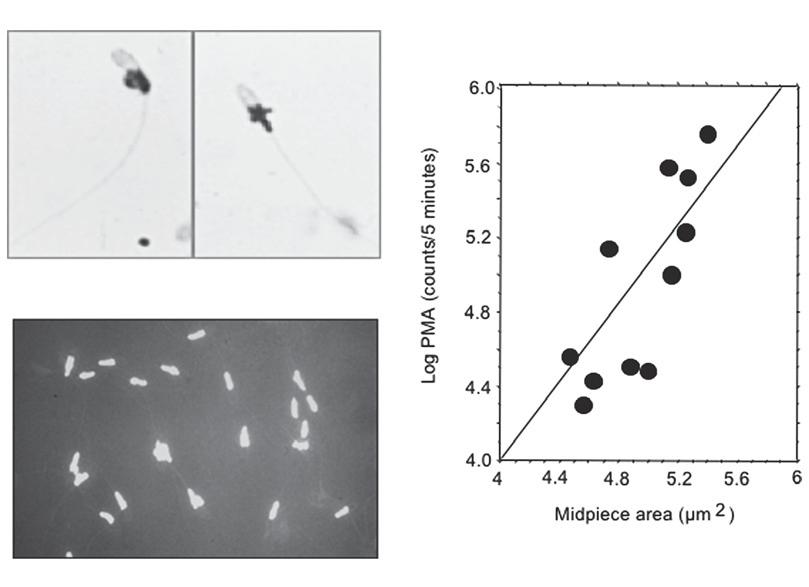
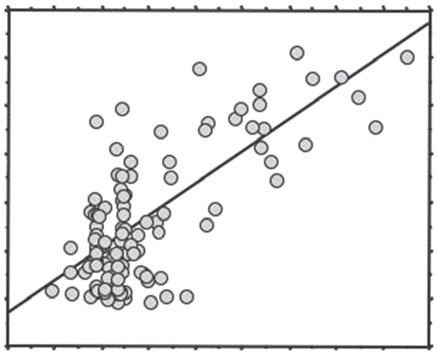
Figure1.3 Thebehaviourofhumanspermatozoaindensitycentrifugationgradientsdependsontheirdensity which,inturn,reflectstheextenttowhichtheyhaveextrudedtheircytoplasm.(A)Anyresidualcytoplasmsnaps backintothemidpieceofthecellscreatinglargecytoplasmicextensions,whichcanbereadilyimagedusingan NADH-nitrobluetetrazoliumreductiontechnique[27].(B)Theareasofnitrobluetetrazoliumreductioncanbereadily imagedandquantifiedtogenerateanobjectivemeasureofhowmuchresidualcytoplasmagivensamplemight haveretained.(C)Theamountofresidualcytoplasmcorrelatesextremelywellwiththecapacityofthepurified spermatozoatogenerateROS,whenallleukocyteshavebeenremoved[27].
strategy,theremustbeaverypowerfulsetofreasonswhyspermatozoagotosuchlengthsto removeamajorityoftheircytoplasmandconfinewhatremainstothemidpieceofthecell.
Ofcourse,forahighlymotilecell,thepresenceofexcesscytoplasmmaysimply representaphysicalencumbrancethatlimitsthecapacityofthesecellstomovequickly thoughaviscousextracellularenvironmentand,ultimately,topenetratethezonapellucida. Inaddition,thedrivetominimizetheamountofcytoplasmretainedbyspermatozoamaybe relatedtotheneedtoprotectthenucleusfromendonucleaseattack.Thus,thehighly specializedarchitectureofhumanspermatozoaresultsinthecytoplasmandmitochondria beingphysicallyseparatedfromthenucleus.Thisconfersadvantagesuponthecellby limitingthevulnerabilityoftheirDNAtofragmentationduringtheearlystagesofapoptosis.Thelatterisusuallyaccomplishedbyendonucleasesactivatedinthecytoplasmor releasedfromthemitochondriathatthenmigrateintothecellnucleusinordertocomplete theapoptoticcascade.Becausethespermnucleusisphysicallyseparatedfromthemitochondriaandamajorityofthecytoplasm,suchendonuclease-mediatedDNAdamagerarely featuresinthesecells[28].Anotherimportantreasontogetridofspermcytoplasmis becauseitspresenceisassociatedwithexcessROSgeneration,whichnotonlydamagesthe capacityofthesecellsformotilityandsperm–egginteractionbutalsodamagestheDNAin thespermnucleusandmitochondria[29].Whyexcesscytoplasmshouldberesponsiblefor
(A)
(B)
(C)
generatingexcessROSmayberelatedtothecapacityofspermatozoatoconcentrate lipoxygenaseinthecytoplasmicspaceandthefactthatthisenzymeisemergingas apossiblecandidateforthegenerationofROSbydefectivehumanspermatozoa[30]. AlternativesuggestionssuchastheinvolvementofcytoplasmicNADPHoxidaseactivity inROSgenerationarecurrentlyunresolved[29].
Whatevertheunderlyingmechanisms,therecanbenodoubtthatDGCisanextremely effectivemeansofisolatinghigh-qualityspermatozoaexhibitinglowlevelsofROSgenerationandhighlevelsoffunctionalityintermsoftheirmotilityandcompetenceforfertilization.Moreover,becausetheenergyrequiredtoeffecttheseparationisachievedthroughthe externalimpositionofagravitationalforce,thistechniqueshouldbemoreeffectivethan self-migrationswim-upprotocolsforisolatingspermatozoafrompoor-qualityejaculates. Ontheotherhand,whileDGCprotocolsaregenerallyhighlyeffectiveingettingridofmost oftheleukocytescontaminatinghumansemensamples,theyarenotperfectinthisregard andspermsuspensionspreparedinthismannercanpossessresidualleukocytesinnumbers sufficienttocompromisethefertilizingcapacityofthespermatozoa[10].
AnotherproblemwithDGCisthatitsapplicationcanbeassociatedwithanelevationin thelevelsofDNAdamageseeninspermatozoa[31].Thereissomeinter-individual variationinthelevelsofDNAdamageinducedinthiscontextthatmayberelatedtothe specificvulnerabilityofindividualsamplestotheoxidativestresscreatedduringcentrifugationasaresultofexposuretotransitionmetalsinthecolloidalsiliconsolutionsusedto createthediscontinuousgradients.Interestingly,caseswhereanincreaseinDNAfragmentationisobservedfollowingDGCexhibitlowerpregnancyratesthansampleswhereDNA damageisnotelevated[32].So,whatevervulnerabilityisbeinghighlightedbythisresponse toDGChasabearingontheoverallcompetenceofthesampletoestablishapregnancy.This makesDGCaninterestingdiagnosticprocedurebutdoesnotnecessarilycommenditasan approachtospermisolation.
1.6ElectrophoreticIsolation
Analternativetotheuseofspermdensityasacriterionforisolatingspermatozoaistouse spermcharge.Spermatozoaarenegativelychargedcellsthathavebeenknownforseveral decadestomigratetowardstheanodeinanelectric field.Thispropertyhasbeenexploitedto generateadevicefortheisolationofhumanspermatozoaforassistedconceptionpurposes (Figure1.4)[33].Thissystemwasfoundtobeeffectiveingeneratingsuspensionsofhuman spermatozoaexhibitinghighlevelsofmotility,goodmorphologyandlowlevelsofDNA damagethatwereessentiallyfreefromleukocytecontamination.Thespermpreparations aregenerallysimilarinqualitytothosegeneratedbyDGCexceptthatlevelsofspermDNA damagearelower,possiblybecausethismethoddoesnotinvolveexposureofthespermatozoatoanyextraneousreagentscapableoftriggeringROSgeneration.Moreover,oneof themajoradvantagesoftheelectrophoretictechniqueisitsgreatrapidity,generating suspensionscontainingmorethan10millionspermatozoa/mlwithin5minutes(Figure 1.4).Subsequentstudiesconfirmedthattheelectrophoreticallyisolatedspermatozoawere competenttoestablishviablepregnanciesinaclinicalsettingandwereassociatedwith measuresoffertilizationandembryoqualitythatwerenotsignificantlydifferentfrom traditionalmethods[34].Inaddition,itwasestablishedthattheelectrophoreticisolation ofspermatozoadidnotbiasthegenotypeoftheselectedcellstowardseitheranX-orYbearinggenotype.Furthermore,theelectrophoreticmethodwasfoundtobeeffectivewith
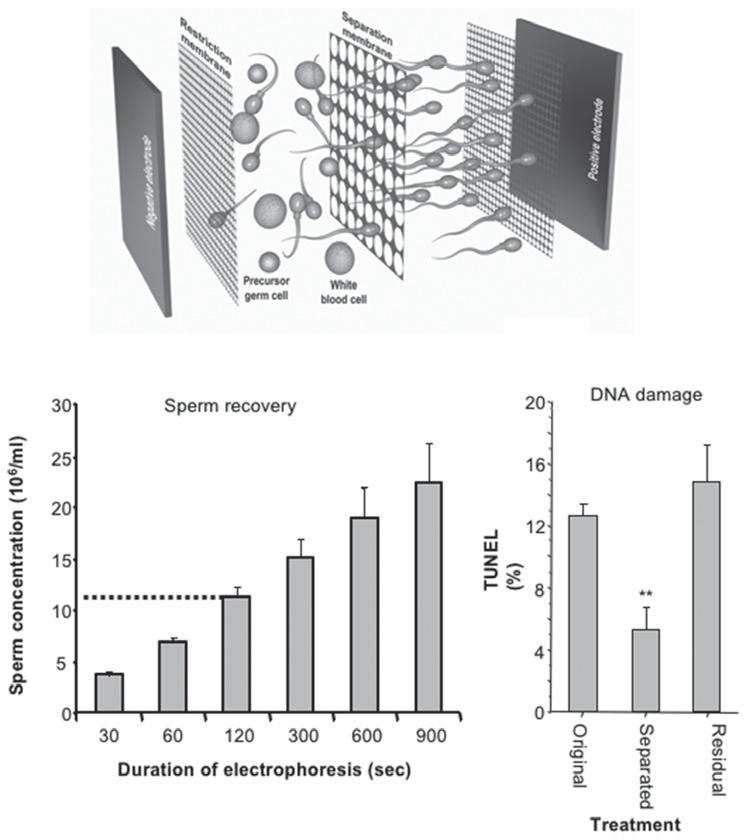
Figure1.4 Electrophoresisrepresentsapromisingapproachtospermisolation.(A) Theprinciplebehindthis approachisthathigh-qualityspermatozoaarenegativelychargedandmovetowardstheanodeinanelectric field. Thecreationofacassettecontainingaseparationmembranewithporesizesofaround5µmallowsspermatozoato bepulledawayfromothercelltypessuchasleukocytesorprecursorgermcellsthatmightalsocarryanetnegative chargebutaretoolargetopassthroughthepores.(B) Thetechniqueisextremelyefficaciousallowingtheisolation ofmorethan10millionhighlymotilespermatozoa/mlwithin5minutes.(C) Theseparatedcellspossesssignificantly lowerlevelsofDNAdamagethantheoriginalspermpopulation[33].
severelycompromisedsamplesincludingtesticularbiopsymaterial,wherethespermatozoa areimmotileanddeeplyburiedwithinanextremelycomplexcellularmixture. Electrophoreticallyisolatedcellswerealsoshowntocapacitatenormallywhenwashed freefromseminaldecapacitationfactorsthatco-migratewiththespermatozoaduring theirelectrophoreticmigration[35].Itwasalsoestablishedinthesestudiesthatthenegative chargeresponsibleforthemigrationofspermatozoainanelectric fieldislargelydetermined bysurfacesialicacidresidues.Thisconclusionwasbasedontheabilityofneuraminidaseto significantlysuppresstheelectrophoreticisolationofspermatozoa,suggestingthatthis associationbetweencellsurfacesialationandspermbehaviourinanelectric fieldhas acausativebasis[35].
(B)(C)
Withthismethod,theapplicationofcurrentforspermisolationpurposeshastobe carefullycontrolledbecausewhile fieldstrengthispositivelycorrelatedwithspermrecovery ratesitisnegativelycorrelatedwithspermmovement,irrespectiveofwhetherthecurrentor thevoltageisheldconstant.Importantly,thelossofmotilityobservedwhentheintensityof theelectric fieldishigh,orthedurationofexposureisprolonged,isnotassociatedwithany increaseinROSgenerationortheinductionofDNAdamage.Indeed,thelevelsofoxidative stressandDNAdamageobservedfollowingexposureofhumanspermatozoatoanelectric fieldaresominimalthatthismayconstituteaneffectivemethodforimmobilizingspermatozoaforICSI[36].
Exploitingthenegativechargeexpressedbyhigh-qualityhumanspermatozoa,others havedevelopedmicroelectrophoresissystemsforthesmall-scaleisolationofindividual spermatozoaforICSI[37].Inanothervariationonthistheme,azetamethodhasbeen developedwhichdependsonthecreationofapositivelychargedsurfacetowhichthe negativelychargedspermatozoacanadhere.Thismethodhasbeenreportedtoresultin adoublingintheratesofhyperactivationandprogressivemotilityandahalvinginthelevels ofDNAdamageobservedintheisolatedpopulationsofspermatozoa[38].Takentogether, theseresultssuggestthattheisolationofhumanspermatozoaonthebasisoftheirchargeis asrapidasitisefficacious.Furthermore,thisapproachissafebecauseitdoesnotcarrywith ittheriskofinadvertentDNAdamageasaconsequenceofenhancedROSgeneration.
1.7ConsequencesofSpermPreparationProtocols
Doesthemethodofspermpreparationmakemuchofadifferencetooverallsuccessrates? Theanswertothisquestiondependsonhowyoumeasuresuccess.Ifthelatterisassessedin termsofpregnancyorlivebirthratethenthemethodofspermpreparationprobablymakes verylittledifference,exceptwhensemenqualityissopoorthatmethodsrelyingonthe intrinsicmotilityofthespermatozoaoradequatespermnumbers(swim-up,forexample) becomeineffective.Forpoor-qualityhumansemensamplesofthetypethatmightbe commonlyencounteredinaninfertilityclinic,methodsthatarelessdependentonsperm motilityornumbersuchasmini-DGC,glasswoolcolumnsorelectrophoresismightbe preferred[40].Outsideoftheextremesofsemenquality,whendirectcomparisonshave beenmadebetweenspermsuspensionspreparedbyrepeatedcentrifugation,swim-upfrom awashedpellet,glasswool filtrationorDGC,thenlittledifferencehasbeenrevealedin termsoftheper-cyclepregnancyrate[39].Similarly,whenpregnancyrateshavebeen comparedbetweenspermatozoapreparedDGCandelectrophoresis,neitherconception ratesnorpregnancyrateswereverydifferentbetweenthetwomethods[34].
However,pregnancyrateisanextremelycrudemethodofdeterminingwhethersperm preparationmethodologieshavemadeadifference.Usingavarietyofmethodsforassessing spermquality,itisclearlypossibletodetecthighlysignificantdifferencesinthequalityof thegametesisolatedasafunctionoftheisolationprocedureusedintheirpreparation.Of particularimportanceistheimpactofspermpreparationtechniquesonthelevelsofDNA damageseeninthespermatozoa[36].SuchdifferencesinDNAintegritymaynothave amajorimpactonpregnancyrates, perse.However,theyhaveeverypossibilityofgeneratingdifferencesinthegenetic/epigeneticmake-upoftheoffspring[40].
Asaprocess,fertilizationappearstoberelativelyforgivingofdifferencesinDNA integrity,particularlywhenICSIisusedastheinseminationstrategy[4].Ifspermatozoa areisolatedusingless-than-optimaltechniques,suchthattheystillcontainsuchdamageor
haveitiatrogenicallyinducedasaconsequenceoftheprocedure,thenthereisariskthat theseDNAlesionswillbecometranslatedintogenetic/epigeneticchangesintheoffspring, followinginefficientoraberrantpost-fertilizationrepairintheoocyte[29].Suchchanges havethepotentialtosignificantlyimpacttheprogeny’slong-termhealthtrajectoryand,if geneticchangesareinvolved,toimpactthewellbeingofprogenyformanygenerationsto come.Whatthe fieldneedsnowarestudiesthatexaminetheimpactofspermpreparation strategy,notonratesoffertilizationorimplantation,butonthelevelsofDNAdamage carriedbythespermatozoaandthegenetic/epigeneticmutationalloadsubsequently imposedupontheoffspring.Techniquesthataresimple,rapidandcapableofeffecting theisolationofspermatozoawithminimalDNAdamagefromarangeofpathological samples,includingthosecharacterizedbylowspermnumbersandlittleintrinsicmotility, willbecomethemethodsofthefuture.InaneradominatedbyICSI,werequirestudiesthat focuslessonthefunctionalattributesofthespermatozoaandmoreonthemutationalload carriedby,anddevelopmentalnormalityof,theoffspring.Thetechniqueswearecurrently usingarealmosthalfacenturyold.Hopefully,someofthenewinsightsdiscussedinthis chapteronthebehaviourofspermatozoain fluid flows,gradientsandelectric fieldswill providethebasisforanewgenerationofsafer,moreeffectivespermisolationmethodologiesinthefuture.
References
1.Hourcade,J.D.,Pérez-Crespo,M., Fernández-González,R.,Pintado,B.and Gutiérrez-Adán,A.(2010)Selectionagainst spermatozoawithfragmentedDNAafter postovulatorymatingdependsonthetypeof damage. ReprodBiolEndocrinol 8:9.
2.Twigg,J.P.,Irvine,D.S.andAitken,R.J. (1998)OxidativedamagetoDNAinhuman spermatozoadoesnotprecludepronucleus formationatintracytoplasmicsperm injection. HumReprod 13:1864–1871.
3.Liu,D.Y.,Liu,M.L.,Clarke,G.N.and Baker,H.W.(2007)Hyperactivationof capacitatedhumanspermcorrelateswith thezonapellucida-inducedacrosome reactionofzonapellucida-boundsperm. HumReprod 22:2632–2638.
4.Nishimura,H.,Kim,E.,Nakanishi,T.and Baba,T.(2004)Possiblefunctionofthe ADAM1a/ADAM2Fertilincomplexinthe appearanceofADAM3onthesperm surface. JBiolChem 279:34957–34962.
5.Aitken,R.J.andRoman,S.D.(2008) Antioxidantsystemsandoxidativestressin thetestes. AdvExpMedBiol 636:154–171.
6.Aitken,R.J.andBaker,M.A.(2013) Oxidativestress,spermatozoaand leukocyticinfiltration:relationshipsforged bytheopposingforcesofmicrobial invasionandthesearchforperfection. JReprodImmunol 100:11–19.
7.Leitch,A.E.,Duffin,R.,Haslett,C.and Rossi,A.G.(2008)Relevanceof granulocyteapoptosistoresolutionof inflammationattherespiratorymucosa. MucosalImmunol 1:350–363.
8.Uribe,P.,Cabrillana,M.E.,Fornés,M.W., Treulen,F.,Boguen,R.,Isachenko,V., etal.(2018)Nitrosativestressinhuman spermatozoacausescelldeath characterizedbyinductionof mitochondrialpermeability transition-drivennecrosis. AsianJAndrol 20:600–607.
9.Aitken,R.J.andClarkson,J.S.(1988) Significanceofreactiveoxygenspeciesand antioxidantsindefiningtheefficacyof spermpreparationtechniques. JAndrol 9:367–376.
10.Krausz,C.,Mills,C.,Rogers,S.,Tan,S.L. andAitken,R.J.(1994)Stimulationof oxidantgenerationbyhumansperm suspensionsusingphorbolestersand formylpeptides:relationshipswithmotility andfertilization invitro. FertilSteril 62:599–605.
11.Aitken,R.J.,Buckingham,D.W.,West,K. andBrindle,J.(1996)Ontheuseof
paramagneticbeadsandferrofluidsto assessandeliminatetheleukocytic contributiontooxygenradicalgeneration byhumanspermsuspensions. AmJReprod Immunol 35:541–551.
12.Neuwinger,J.,Cooper,T.G.,Knuth,U.A. andNieschlag,E.(1991)Hyaluronicacidas amediumforhumanspermmigration tests. HumReprod 6:396–400.
13.Perry,R.L.,Barratt,C.L.,Warren,M.A. andCooke,I.D.(1996)Comparativestudy oftheeffectofhumancervicalmucusand acervicalmucussubstitute,Healonid,on capacitationandtheacrosomereactionof humanspermatozoa invitro HumReprod 11:1055–1062.
14.Wikland,M.,Wik,O.,Steen,Y.,Qvist,K., Söderlund,B.andJansonP.O.(1987)A self-migrationmethodforpreparationof spermfor in-vitro fertilization. Hum Reprod 2:191–195.
15.Yamanaka,M.,Tomita,K.,Hashimoto,S., Matsumoto,H.,Satoh,M.,Kato,H.,etal. (2016)Combinationofdensitygradient centrifugationandswim-upmethods effectivelydecreasesmorphologically abnormalsperms. JReprodDev 62:599–606.
16.Suarez,S.S.andWu,M.(2017) Microfluidicdevicesforthestudyofsperm migration. MolHumReprod 23:227–234.
17.Zaferani,M.,Cheong,S.H.and Abbaspourrad,A.(2018)Rheotaxisbasedseparationofspermwith progressivemotilityusingamicro fluidic corralsystem. ProcNatlAcadSciUSA 115:8272–8277.
18.Li,K.,Li,R.,Ni,Y.,Sun,P.,Liu,Y., Zhang,D.andHuang,H.(2018)Novel distance-progesterone-combinedselection approachimproveshumanspermquality. JTranslMed 16:203.
19.Flegel,C.,Vogel,F.,Hofreuter,A., Schreiner,B.S.,Osthold,S.,Veitinger,S., etal.(2016)Characterizationofthe olfactoryreceptorsexpressedinhuman spermatozoa. FrontMolBiosci 2:73.
20.Armon,L.,Ben-Ami,I.,Ron-El,R.and Eisenbach,M.(2014)Human oocyte-derivedspermchemoattractantis
ahydrophobicmoleculeassociatedwith acarrierprotein. FertilSteril 102:885–890.
21.Bahat,A.,Caplan,S.R.andEisenbach,M. (2012)Thermotaxisofhumanspermcells inextraordinarilyshallowtemperature gradientsoverawiderange. PLoSOne 7: e41915.
22.Li,Z.,Liu,W.,Qiu,T.,Xie,L.,Chen,W., Liu,R.,etal.(2014)Theconstructionofan interfacialvalve-basedmicrofluidicchip forthermotaxisevaluationofhuman sperm. Biomicrofluidics 8:024102.
23.Kieslinger,D.C.,Hao,Z.,Vergouw,C.G., Kostelijk,E.H.,Lambalk,C.B.andLe Gac,S.(2015) Invitro developmentof donatedfrozen-thawedhumanembryosin aprototypestaticmicrofluidicdevice: arandomizedcontrolledtrial. FertilSteril 103:680–686.e2.
24.Xu,J.,Fang,R.,Chen,L.,Chen,D., Xiao,J.P.,Yang,W.,etal.(2016). Noninvasivechromosomescreeningof humanembryosbygenomesequencingof embryoculturemediumfor invitro fertilization. ProcNatlAcadSciUSA 113:11907–11912.
25.Gorus,F.K.andPipeleers,D.G.(1981) Arapidmethodforthefractionationof humanspermatozoaaccordingtotheir progressivemotility. FertilSteril 35:662–665.
26.Hyne,R.V.,Stojanoff,A.,Clarke,G.N., Lopata,A.andJohnston,W.I.(1986) Pregnancyfrom invitro fertilizationof humaneggsafterseparationofmotile spermatozoabydensitygradient centrifugation. FertilSteril 45:93–96.
27.Gomez,E.,Buckingham,D.W.,Brindle,J., Lanzafame,F.,Irvine,D.S.and Aitken,R.J.(1996)Developmentofan imageanalysissystemtomonitorthe retentionofresidualcytoplasmbyhuman spermatozoa:correlationwithbiochemical markersofthecytoplasmicspace,oxidative stress,andspermfunction. JAndrol 17:276–287.
28.Koppers,A.J.,Mitchell,L.A.,Wang,P., Lin,M.andAitken,R.J.(2011) Phosphoinositide3-kinasesignalling pathwayinvolvementinatruncated
apoptoticcascadeassociatedwithmotility lossandoxidativeDNAdamageinhuman spermatozoa. BiochemJ 436:687–698.
29.Aitken,R.J.(2018)Noteveryspermis sacred:aperspectiveonmaleinfertility. MolHumReprod 24:287–298.
30.Walters,J.L.H.,DeIuliis,G.N., Dun,M.D.,Aitken,R.J, McLaughlin,E.A.,Nixon,B.,etal.(2018) Pharmacologicalinhibitionof arachidonate15-lipoxygenaseprotects humanspermatozoaagainstoxidative stress. BiolReprod 98:784–794.
31.Aitken,R.J.,Finnie,J.M.,Muscio,L., Whiting,S.,Connaughton,H.S., Kuczera,L.,etal.(2014)Potential importanceoftransitionmetalsinthe inductionofDNAdamagebysperm preparationmedia. HumReprod 29:2136–2147.
32.Muratori,M.,Tarozzi,N.,Cambi,M., Boni,L.,Iorio,A.L.,Passaro,C.,etal. (2016)VariationofDNAFragmentation levelsduringdensitygradientsperm selectionforassistedreproduction techniques:apossiblenewmalepredictive parameterofpregnancy? Medicine (Baltimore) 95:e3624.
33.Ainsworth,C.,Nixon,B.andAitken,R.J. (2005)Developmentofanovel electrophoreticsystemfortheisolationof humanspermatozoa. HumReprod 20:2261–2270.
34.Fleming,S.D.,Ilad,R.S.,Griffin,A.M., Wu,Y.,Ong,K.J.,Smith,H.C.,etal. (2008)Prospectivecontrolledtrialofan
electrophoreticmethodofsperm preparationforassistedreproduction: comparisonwithdensitygradient centrifugation. HumReprod 23:2646–2651.
35.Ainsworth,C.J.,Nixon,B.andAitken,R.J. (2011)Theelectrophoreticseparationof spermatozoa:ananalysisofgenotype, surfacecarbohydratecompositionand potentialforcapacitation. IntJAndrol 34: e422–e434.
36.Aitken,R.J.,Hanson,A.R.andKuczera,L. (2011)Electrophoreticspermisolation: optimizationofelectrophoresisconditions andimpactonoxidativestress. Hum Reprod 26:1955–1964.
37.Simon,L.,Murphy,K.,Aston,K.I., Emery,B.R.,Hotaling,J.M.and Carrell,D.T.(2016)Optimizationof microelectrophoresistoselecthighly negativelychargedsperm. JAssistReprod Genet 33:679–688.
38.Chan,P.J.,Jacobson,J.D.,Corselli,J.U. andPatton,W.C.(2006)Asimplezeta methodforspermselectionbasedon membranecharge. FertilSteril 85:481–486.
39.Johnson,D.E.,Confino,E.and Jeyendran,R.S.(1996)Glasswoolcolumn filtrationversusmini-Percollgradientfor processingpoorqualitysemensamples. FertilSteril 66:459–462.
40.Aitken,R.J.,Bronson,R.,Smith,T.B.and DeIuliis,G.N.(2013)Thesourceand significanceofDNAdamageinhuman spermatozoa:acommentaryondiagnostic strategiesandstrawmanfallacies. Mol HumReprod 19:475–485.
NewHorizonsinMaleSubfertility andInfertility
BrettNixonandElizabethG.Bromfield
2.1Introduction
Infertilityisadistressinglycommonconditionthata fflictsupto15%ofcouplesof reproductiveage.Malefactorinfertilityisdirectlyimplicatedinapproximately30%of couplesexperiencingproblemswithconceptionandisacontributoryfactorinasmany as50%ofcases[ 1].Inthe40yearssincethe fi rstdescriptionofsuccessfulhuman invitro fertilisation(IVF),substantialimprovementshavebeenmadeintheeffi cacyof treatmentsformalesubfertility.Onesuchadvancehasbeentheintroductionofgamete micromanipulationtechniquessuchasintracytoplasmicsperminjection(ICSI),which havehadatransformativeimpactonthetr eatmentofindividualswithcompromised spermparameters,e ff ectivelydispensingwiththeneedforspermatozoatodisplay progressivemotilityorhavethepotentialtorecogniseanoocyteandcompletean acrosomereaction[2 ].Soprofoundhastheimpactonclinicalandrologybeen,that ICSIhasbecomethefavouredchoiceforfertilisationirrespectiveofthemalefactor, nowfeaturinginasmanyastwo-thirdsofIVFcyclesundertakenincountriessuchas Australia[ 3].However,despitetechnologicaladvances,bothworldwideclinicalpregnancyandlivebirthratesremainrelativelymodestatapproximately26.8%and20% perstartedcycle,respectively[2 ].Notwithstandingconfounders,recentepidemiological datahasdocumentedthepotentialforanelevatedriskofbirthdefectsandperpetuationofdefectivesemenpro fi lesinchildrenconceivedviaICSI[4 , 5].Suchdata encouragecautionintheutilisationofICSIbeyonditsintendedapplicationfor achievingpregnancyincoupleswithseverelycompromisedsemenparametersand highlightapressingneedtoexplorenewhorizonsfordiagnostictoolstoassistwith patientstrati fi cation,spermselectionandtherapeutictreatmentoptionsformales su fferingfromsubfertilityandinfertility.Here,wereviewthecurrentstateoftheart andfuturedirectionsinthe fi eldofmaleinfertilitywiththegoalofaddressingthe long-standingquestionofhowbesttoapproachtheclinicalmanagementofthese individuals.Outofnecessity,wehavealsosoughttodirectthereadertoanumber ofexcellentreviewsthatcriticallyappraiseourcurrentarmouryofdiagnosticand therapeuticstrategies.
2.2DiagnosticAndrology
Withoutquestion,therearemanydiff erentpathologiescapturedundertheumbrellaof maleinfertilityandeachofthesepresentsdi ff erentimplicationsfortheclinical managementofthepatient.Untilrecently,ourapproachtodiagnosticandrologyhas beenbaseduponthetenetthatfertilitycanbede fi nedintermsofaroutinedescriptive
semenprofi lethatplacesemphasisonspermcount,morphologyandmotility. Regrettably,theseconventionalcriteriareveallittleabouttheunderlyingaetiologyof theinfertilityorthefunctionalityofthesperm,haveproventoberelativelyinsensitive indicatorsoffertilisationsuccess,andarethereforeoflimitedutilityinselectingthe optimalclinicalmanagementregimen[ 6].Indeed,wenowappreciatethatstalwartsof andrologicalassessmentsuchasspermnumberrepresentrelativelyweakcriterionfor thepredictionoffertility.Likewise,despiteprogresstowardsautomationofgross morphologyassessmentsandtheadventofultra-highmagnifi cation(i.e.motile spermorganellemorphologyexamination[ MSOME])tostandardisethedetectionof evenverysubtlemorphologicaldefects,limitationsremainintermsoftheabilityto discriminatethosemorphologicalelementsthatde fi nethefunctionalityofagiven spermcell.Thus,whilstthecombinationofMSOMEintandemwithICSI(i.e. intracytoplasmicmorphologicallyselectedsperminjection[IMSI])hasatheoretical potentialtoimprovereproductiveoutcomes,thereiscurrentlylimitedevidencesupportingthepositivee ff ectofIMSIonlivebirthormiscarriagerates,andtheveracityof evidencecitingimprovedclinicalpregnan cyfollowingIMSIremainsquestionable[ 7]. Ithasyettobedeterminedwhethersuchde fi cienciesmaybeovercomethroughthe applicationofselectivestainstohighlightparticularlyimportantattributesofsperm structureandquality,suchasthosere fl ectingspermviability,acrosomalstatus,capacitation,mitochondrialmembranepotential, reactiveoxygenspecies(ROS)generation, lipidperoxidation,apoptoticmarkersandDNAdamage.
Asthe finalcomponentoftheroutinedescriptivesemenprofile,spermmotilityholds promise,particularlywhenundertakenwiththeaidofobjectivecomputer-aidedsemen analysis(CASA)systemstoaccuratelymeasurespermtrajectories[8].Accordingly,positive correlationshavebeenestablishedbetweenthemotilityandfertilityofhumanspermatozoa [9].Regrettably,owingtothefactthatthemotilityprofileofspermisaconstantlychanging entityinfluencedbythedifferingphysiologicalenvironmentsencounteredbythecell,these correlationsareoftenweak.Indeed,duringtheirtransporttothesiteoffertilisation, spermatozoaarevariablycharacterisedbyforwardprogressivemotility,completequiescenceassociatedwiththeformationofastoragereservoirintheisthmicregionofthe fallopiantubes,andtheinductionofhyperactivatedmotilitycharacterisedbyhigh-frequency,high-amplitude,asymmetric flagellarwaves.Whilstthemeasurementofthese variousformsofmovementcanbeachievedbyCASA[8],problemsassociatedwiththe intermittentnatureoftheseprofilesrenderthemdifficultdiagnosticcriteriatoapplyin aclinicalsetting.Moreover,theselectionofspermonthebasisoftheirmotilityusing “swim-up” techniqueshasfailedtodeliversuperiorpregnancyratestothatofthemost widelyadoptedspermpreparationproceduresutilisingcolloidalsilicadensitygradients[10].
2.3ImprovementsinDiagnosticAndrology
Inordertoalleviatethelimitationsandvariabilityinherentincurrentmethodologiesused forfertilitydiagnosisandselectionofspermatozoainassistedreproductivetechnology (ART),newmodalitiesareurgentlyrequired.Intermsofdrivingadvancesinthis field,we havemuchtolearnfromthestudyofthemechanisticbasisofspermdysfunctionand, conversely,thecharacteristicsofthosespermcellsthatareabletoparticipateinthecascade ofeventsthatunderpinnaturalconception.Inthissetting,itisevidentthatonlyrelatively
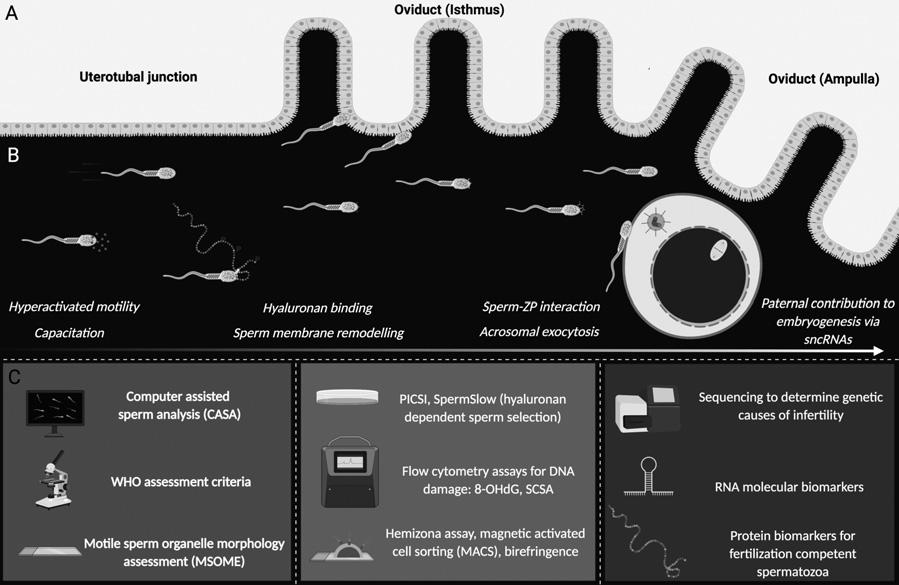
Figure2.1 Toolsunderconsiderationforthediagnosisofmaleinfertility.(A)Asspermsenterthereproductivetract theytransitthroughtheuterus,navigatetheuterotubaljunctionandprogressthroughtheisthmusandampullaof theoviducttocontacttheovulatedoocyte.(B)Toachievefertilisation,spermatozoamust firstundergoanextensive periodofmembraneremodellinginthefemalereproductivetract,termedcapacitation,wheredynamicprotein phosphorylationeventsgiverisetoimportantphysiologicalchangesinmotility(hyperactivatedmotility).These dynamicproteinchangesalsofacilitatethepresentation/unmaskingofkeyreceptorsonthespermsurfacethat facilitatesequentialcumulusmatrix(hyaluronan)andsperm–zonapellucidabindingandpenetration.Spermatozoa arerequiredtoundergoexocytosisoftheiracrosomalcontentsandacrosomalmembraneremodellingeventsprior tocell–cellmembranefusionandfertilisation.Post-fertilisation,thereismountingevidencethatsperm-bornesmall non-codingRNAs(sncRNAs)influencepre-implantationembryogenesis.(C)Currently,routineprotocolsforthe diagnosisofmaleinfertilityandsubfertilityincludebasicandadvancedmorphology,motilityandsperm concentrationassessments(includingcomputer-assistedspermanalysis(CASA),WorldHealthOrganisation (WHO)assessmentcriteriaandmotilespermorganellemorphologyassessment(MSOME).Inaddition,thereare nowseveraltoolsavailabletodeterminecellquality,DNAintegrityandfertilisationcapacity(includinghyaluronanbaseddetectionmethods,high-throughput flowcytometryassaysfortheoxidisedbase8-hydroxyl2-deoxyguanosine(8-OHdG)andthespermchromatinstructureassay(SCSA).Finally,theadvanceofomics technologyispermittingthedevelopmentmolecularbiomarkersthatcanstratifyinfertileandfertilesperm populationsandaidourunderstandingofthegeneticcausesofmaleinfertility.
fewspermcellssuccessfullynavigatethefemalereproductivetracttoreachthesiteof fertilisationintheoviduct.Thishighlyselectedpopulationnotonlyareendowedwiththe motilityneededtopenetratethroughcervicalmucusandgainentryintotheuterusandthe oviducts,butalsocompletetheprocessofcapacitation,whichprimesthecellsfortheir interactionwiththeoutervestmentsoftheoocyte[11],thecumulusoophorusandzona pellucida.Suchinteractionsarecoordinatedbyspecialisedspermdomainsoverlyingthe anteriorregionofthespermhead.Thesedomainsareformedduringthelatterphasesof spermatogenesisbeforebeingdynamicallymodifieduponpassagethroughboththemale andfemalereproductivetracts[12].Accordingly,thedevelopmentofbioassaysthatseekto imposethestringencyofnaturalspermselectionbarriershavebeenreceivingincreased attention(Figure2.1).
2.3.1HyaluronicAcidBinding
Illustrativeofstrategiesdesignedtomimicphysiologicalspermselectionareprotocols basedonthehyaluronicacid(HA)bindingpropertiesofspermatozoa.Suchtechniques seektoexploittheprinciplethatfunctionallymaturespermatozoaexpresshyaluronicacid bindingsitescapableofadheringto,anddigesting,thehyaluronicacid-richmatrixofthe cumulusoophorus[13].Twosystemsarecurrentlymarketedbasedonhyaluronicacid spermselection,differingwithrespecttotheiruseofeitherimmobilisedHA(i.e.physiologicalintracytoplasmicsperminjection(PICSI))orHAsuspendedinaviscousmedium (i.e.SpermSlow),whichtheoreticallyimmobilisesorretardsmaturespermmovement respectively,andisthuspermissiveoftheirselectionawayfromimmaturecounterparts inpreparationfordownstreamICSI[13].Despitethepromiseofsuchinterventions,metaanalyseshaveconcludedthatcurrentevidenceisinsufficienttosupporttheirutilityinterms ofimprovedclinicalpregnancyrates,miscarriageratesand,perhapsmoreimportantly,in livebirthratesfromART[14].Availableevidenceisalsoinsufficienttoprecludeadverse effectsorestablishdifferencesintheefficacyofthePICSIversusSpermSlowHAbinding systems[14].Bywayofabiologicalexplanationforthelackofaclearconsensusregarding theefficacyofHA-basedspermselection,thereismountingevidencethathumanspermatozoaexperienceadynamic,capacitation-associatedremodellingoftheirsurfacearchitecture[11].Thisprocessischaracterisedbyanapparentreductioninthenumberof spermatozoawithhyaluronidaseenzymes(i.e.spermadhesionmolecule1,(SPAM1)) superficiallyexposedontheirsurface.Conversely,capacitatedpopulationsofhuman spermatozoaarecharacterisedbyanincreaseintheproportionofcellswithzonapellucida (ZP)receptorspresentedontheouterleafletoftheirplasmamembrane[15].Onesuch receptorisarylsulphataseA(ARSA),anenzymewithaffinityforthesulphatedsugarligands thatdecoratetheZP,andwhichisalmostexclusivelyexpressedonthesurfaceofcapacitated spermatozoa.Thus,HAadhesionmayinfactbeselectingthesub-populationofspermatozoathathavefailedtocompletecapacitation,buttheextenttowhichthisisreflectiveof underlyinglesionsincapacitation-associatedsignallingremainstobedetermined.
2.3.2ZonaPellucidaBinding
DownstreamofHAbinding,spermatozoaencountertheZP,anacellularmatrixthat surroundstheovulatedoocyteandservesasatenaciousphysiologicalbarriertofertilisation. Accordingly,afailuretobindandpenetratetheZPisarelativelycommondefectencounteredinthespermatozoaofinfertilepatients.Indeed,approximately10%ofmenwith idiopathicinfertilityexhibitafailureofsperm–ZPrecognition[16].Formostinfertile males,however,failuresofsperm–ZPrecognitionareamatterofdegreeandwhencarefully quantifiedusingahemizonaassay(HZA)provideanaccurateassessmentofoverallfertility, both invivo and invitro [17].Thus,theHZAhashistoricallybeenfoundtoprovidethe highestdiscriminatorypowerforfertilisationsuccess/failureofanyspermparameter assessed[17].Such findingsraisetheprospectthattheZPmaypossesstheabilityto discriminatesuperiorqualityspermatozoa,anotionsupportedbydemonstrationsthat theZPselectivelybindsspermwithnormalmotility,morphologyandhighlevelsofnuclear chromatinDNAintegrity[18].Thisphenomenonmaybeattributed,atleastinpart,tothe factthateachofthesefunctionalparametersarehighlysensitivetophysiologicalinsults, suchasthosearisingfromelevatedlevelsofROS,whichthespermofinfertileindividuals commonlyencounter[19].
Asanextensionoftheseobservations,biologicalselectionofspermforICSIonthebasis oftheirZPbindingaffinityhasbeenshowntoproducehigherqualityembryosand contributetoimprovedimplantationandclinicalpregnancyratecomparedtosperm selectedbyconventionalsubjectiveapproaches[18].Despitethebiologicalimportanceof ZPbinding,thefactthatthisbarriercannowbereadilybreachedthroughICSIhas contributedtoasituationwherebythemolecularbasisofsperm–ZPrecognitionremains poorlycharacterised[12].Whatwedoappreciateisthatthisinteractioninvolvesboth lectin-likeandprotein–proteininteractions,raisingtheprospectofutilisingtheseligandsas uniquemolecularsignatureswithwhichtodiscriminatehigh-quality,fertilisationcompetentspermatozoa.WhilstresourcingandethicallimitationsprohibittheuseofnativeZPfor thispurpose,ithaslongbeenknownthatcomplexcarbohydratesubstratessuchasfucoidan orneoglycoproteinsterminatedwiththesialyl-Lewisx(sLex)sequencecancompetitively inhibithumansperm–ZPbinding[20,21].These findingssupportthehypothesisthat selectivecarbohydratemotifscouldbeemployedforspermselection.Asanextensionof thisprinciple,theprospectofdevelopingadvancedspermselectionstrategiesbasedon surfacepropertiesthatdifferentiateviablematurespermatozoasuchasnegativecharge, exposureofapoptoticmarkers,orbirefringencepropertieshavealsobeentrialledandare brieflydiscussedbelow.Itisimportanttonote,however,thatwhileeachofthesesperm selectionstrategieshasshownpromise,moredetailedrandomisedevaluationoftheir efficacyisneededtosupporttheirwidespreadadoptioninclinicalpractice[14].
2.3.3SpermSurfaceProperties
Aswithallcellsurfaces,thespermatozoonisadornedwithadenseglycocalyxmatrix comprisingaheterogeneousarrayofcomplexcarbohydrates.Thisextracellularcoatis subjectedtosubstantialtemporalremodellingduringthesuccessivephasesofsperm maturation,withanuptakeofglycoproteinsrichinterminalsialicacidresiduesbeing akeyhallmarkofthosecellsthathavecompletedtheirepididymalmaturation[22].Thus, agradientofincreasingsialylationisassociatedwiththeattainmentoffullspermfertilising abilityandhasalsobeenlinkedtothemaskingoftheallogeneicspermcellfromimmune surveillanceuponenteringthefemalereproductivetract[22].However,inadditiontoits protectiverole,theattendantincreaseinelectronegativityassociatedwithsialylationhas beenexploitedasameansbywhichtoelectrophoreticallyseparatematurespermawayfrom contaminatingleucocytes,bacteriaandimmaturegermcells,allofwhichcanexertdeleteriouseffectsonspermfunction,priortotheiruseineitherIVForICSI.Inrecentdevelopments,novelelectrophoreticapproacheshaveseenapplicationfortherapidisolationof populationsofmotile,viable,morphologicallynormalspermatozoaexhibitinghighlevelsof DNAintegrity[23].Clinicalutilityhasalsobeendemonstratedusingarangeofdifficult startingmaterials(biopsies,cryostoredsemenandsnap-frozenspermsuspensions),culminatinginthe firstreportedhumanpregnancyfollowingelectrophoreticspermisolation [23].Inasimilarcontext,electrokineticpropertiessuchasthezetapotential(i.e.thecharge thatexistsacrosstheplasmamembrane,whichinmaturespermatozoais~ 16mVto 20 mV)hasalsobeenusedtoseparatematurespermcellsviaadherencetoapositivelycharged solidsupportsuchasaglassslideorcentrifugetube[24].Suchmethodshavebeenreported toselectforspermatozoawithenhancedlevelsofmaturity,DNAintegrity,normalmorphologyandkinematicparametersthatareeachassociatedwithincreasedfertilisationand pregnancysuccessfollowingART[25].