Novel Mammogram-Based Predictors of Breast Cancer Risk John L Hopper
Visit to download the full and correct content document: https://ebookmass.com/product/going-beyond-conventional-mammographic-density-t o-discover-novel-mammogram-based-predictors-of-breast-cancer-risk-john-l-hopper/

More products digital (pdf, epub, mobi) instant download maybe you interests ...

Breast Cancer Sourcebook, 6th Edition Williams
https://ebookmass.com/product/breast-cancer-sourcebook-6thedition-williams/

Breast Cancer and Gynecologic Cancer Rehabilitation 1st Edition Adrian Cristian Md (Editor)
https://ebookmass.com/product/breast-cancer-and-gynecologiccancer-rehabilitation-1st-edition-adrian-cristian-md-editor/

Combinational Therapy in Triple Negative Breast Cancer Manzoor Ahmad Mir
https://ebookmass.com/product/combinational-therapy-in-triplenegative-breast-cancer-manzoor-ahmad-mir/

Breast Cancer Sourcebook: Health Reference Series 6th Edition Angela Williams
https://ebookmass.com/product/breast-cancer-sourcebook-healthreference-series-6th-edition-angela-williams/

Discovery and Development of Anti-Breast Cancer Agents from Natural Products
Goutam Brahmachari
https://ebookmass.com/product/discovery-and-development-of-antibreast-cancer-agents-from-natural-products-goutam-brahmachari/

Role of Tumor Microenvironment in Breast Cancer and Targeted Therapies Manzoor Ahmad Mir
https://ebookmass.com/product/role-of-tumor-microenvironment-inbreast-cancer-and-targeted-therapies-manzoor-ahmad-mir/

Atlas of Reconstructive Breast Surgery 1st Edition Lee
L. Q. Pu
https://ebookmass.com/product/atlas-of-reconstructive-breastsurgery-1st-edition-lee-l-q-pu-2/

Universal Life: An Inside Look Behind the Race to Discover Life Beyond Earth Alan Boss
https://ebookmass.com/product/universal-life-an-inside-lookbehind-the-race-to-discover-life-beyond-earth-alan-boss/

Electronic Devices, Conventional Current Version 10th Edition Thomas L. Floyd
https://ebookmass.com/product/electronic-devices-conventionalcurrent-version-10th-edition-thomas-l-floyd/

Commentary GoingBeyondConventionalMammographicDensity toDiscoverNovelMammogram-BasedPredictorsof BreastCancerRisk
JohnLHopper*,TuongLNguyen,DanielFSchmidt,EnesMakalic ,Yun-MiSong, JoohonSung,GillianSDite ,JamesGDowtyandShuaiLi
CentreforEpidemiology&Biostatistics/MelbourneSchoolofPopulation&GlobalHealth,FacultyofMedicine, Dentistry&HealthSciences,TheUniversityofMelbourne,Victoria3010,Australia; nguk@unimelb.edu.au(T.L.N.);daniel.schmidt@monash.edu(D.F.S.);emakalic@unimelb.edu.au(E.M.); yunmisong@skku.edu(Y.-M.S.);jsung@snu.ac.kr(J.S.);g.dite@unimelb.edu.au(G.S.D.); jdowty@unimelb.edu.au(J.G.D.);shuai.li@unimelb.edu.au(S.L.)
* Correspondence:j.hopper@unimelb.edu.au
Received:31January2020;Accepted:17February2020;Published:26February2020
Abstract: Thiscommentaryisaboutpredictingawoman’sbreastcancerriskfromhermammogram, buildingontheworkofWolfe,BoydandYaffeonmammographicdensity.Wesummariseourefforts atfindingnewmammogram-basedriskpredictors,andhowtheycombinewiththeconventional mammographicdensity,inpredictingriskforintervalcancersandscreen-detectedbreastcancers acrossdifferentagesatdiagnosisandforbothCaucasianandAsianwomen.UsingtheOPERA (oddsratioperadjustedstandarddeviation)concept,inwhichtheriskgradientismeasuredon anappropriatescalethattakesintoaccountotherfactorsadjustedforbydesignoranalysis,we showthatournewmammogram-basedmeasuresarethestrongestofallcurrentlyknownbreast cancerriskfactorsintermsofriskdiscriminationonapopulation-basis.Wesummariseourfindings graphicallyusingapathdiagraminwhichconventionalmammographicdensitypredictsinterval cancerduetoitsroleinmasking,whilethenewmammogram-basedriskmeasurescouldhavea causaleffectonbothintervalandscreen-detectedbreastcancer.Wediscussattemptsbyothersto pursuethislineofinvestigation,themeasurementchallengethatallowsdifferentmeasurestobe comparedinanopenandtransparentmanneronthesamedatasets,aswellasthebiologicaland publichealthconsequences.
Keywords: breastcancer;cumulus;cirrocumulus;cirrus;mammogram-basedrisk;mammographic density;OPERA
1.Introduction
ThisSpecialIssuecallsformanuscriptsthatwillhelpadvanceourunderstandingofmammographic densitytoimprovethedetectionandpreventionofbreastcancer.Buttheissueismuchlargerthan mammographicdensity,atleastasithasbeendefinedconventionallyasthe‘whiteorbrightareas onamammogram’.Thelastfewdecadeshadseenmanyeffortstomeasurethishistoricalconcept, includingautomatedmeasuresnowthatdigitalmammographyiswidespread,buildingontheseminal workofBoydandYaffewhocreatedasemi-automatedmethod[1].Butthoseeffortshadmisseda fundamentalpoint.Theaimshouldn’tbetobettermeasuremammographicdensity perse,buttobetter predictrisk.
Thiscommentaryisaboutfindingwaystopredictawoman’sbreastcancerriskfromhermammogram, buildingontheworkofWolfe,BoydandYaffeonwhathasbecomeknownasmammographicdensity. Wesummariseoureffortsatfindingnewmammogram-basedriskpredictors,andhowtheycombine
J.Clin.Med. 2020, 9,627;doi:10.3390/jcm9030627
www.mdpi.com/journal/jcm
withtheconventionalmeasureofmammographicdensityinpredictingbreastcancerriskforinterval cancersandscreen-detectedcancers,aswellasacrossdifferentagesatdiagnosisandforbothCaucasian andAsianwomen.Wealsodiscussmorerecentattemptsbyotherstopursuethislineofinvestigation, theMeasurementChallengethatallowsdifferentmeasurestobecomparedinanopenandtransparent manneronthesamedatasets,aswellasthebiologicalandpublichealthconsequences.
Beforewedoso,wediscussthepivotalconceptofOPERA(oddsratioperadjustedstandard deviation)whichshouldnotberelegatedtoanAppendixorSupplementaryMaterial.Itisfundamental tounderstanding,andputtingintoproperperspective,therelativestrengthsofriskfactorswithone another,andwithotherriskfactors.Itisparticularlyrelevanttomammographicdensityduetoits negativeconfoundingwiththemajorbreastcancerriskfactorofageand,forsome,anotherbreast cancerriskfactorinbodymassindex.Thereadermightwanttofirstskipthissectionandgostraight tothesectiononWolfeandbeyond,butitisessentialtounderstandingthearea.
2.OPERA:AMeasureoftheAbilityofaRiskFactortoIdentifyFutureCasesona PopulationBasis
Considertheissueofhowtorankriskfactorsintermsoftheirabilitytopredictdiseaseriskona population-basis;namelytheextenttowhichtheydifferentiatecasesfromcontrols.Forsimplicity,we considerriskfactorsmeasuredonacontinuousscale,thoughthisisnotanessentialassumption.Thekey issueistorecognisethatdiseaseriskalmostinvariablydependsonage,andoftensex(dramaticallyso forbreastcancer),aswellasmanyotherfactors.Someofthesefactorsaremeasuredandadjusted forinanalysiswhileotherscanbecontrolledfor,atleasttosomeextent,bydesign.Consequently, studiesofbreastcancerandmammographymeasurestypicallyconsiderwomenonly,andusecases andcontrolswhoaresimilar,orevenmatchedfor,age.Somefamily-baseddesignscontrolforfamilial factors—geneticandenvironmental.Theresultisanestimateofrelative,notabsolute,risk—thelatter canbedeterminedbyappropriateuseofpopulationage-specificincidences.
Thepointthatisoftennotappreciatedisthattheresultingriskestimateappliestoachangeinthe riskfactorofconcern whileholdingallotherfactorsconstant.Therefore,theriskscaleisnotthatofthe unadjustedriskfactor.Expressingtheriskgradientintermsofunitsofthe unadjusted riskfactoris inappropriate,yetstandardpracticethroughoutepidemiologydespiteitgivingmisleadingresults.
Formammogram-basedriskmeasuresinparticular,theseconsiderationsareparticularlyimportant. Conventionalmammographicdensitydeclineswithageinthedecadesaroundthemenopausalyears. Percentmammographicdensityhasameanofaround25–35%beforeage40years,thendecreasesover thenexttwodecadestoplateauatabout15–25%inlaterlifeforCaucasianwomen[2].Asimilarpattern withagehasbeenobservedforAsianwomen[3].Afteradjustingforageandbodymassindex,percent densityhasastandarddeviationof~10%andishighly,thoughnotcompletely,stable(correlation betweenmeasures10yearsapart~0.9)[2].
Thecommonlyusedconceptofpercentmammographicdensity(absolutedensitydividedby thetotalbreastsize)isalsomoderately,andnegatively,correlatedwithbodymassindex.But breastcancerriskincreasessubstantiallywithage,andpost-menopause,withbodymassindex. Intriguingly,bodymassindexhasanegative(i.e.,protective)associationwithbreastcancerriskpriorto menopause[4].Consequently,almostallstudiesofmammogram-basedriskmeasuresusecase-control samplesofsimilarages,andadjustforage,bodymassindex,andoftenothermeasuredriskfactors thoughthelatterdon’texplainmuchvariance[5].Thefactthattheadjustmenthasbeenmadeinthe analysisistypicallydenotedinthefootnotestoTablesofresultsbutrarelycommentedoninthetext. Majorproblemsarisewhentheestimatedriskgradientispresentedintermsoftheso-called ‘changeinriskperstandarddeviation’specificallyastheincrementinriskforaone-unitchangeofthe standarddeviationoftheunadjusted,rawmeasures[6].These‘perstandarddeviation’risksarenot meaningful(theydonotrepresentwhathasbeenestimated)andshouldnotbeusedtocomparerisk factorsacrossmeasures.
Theconsiderationsabovewhenappliedtomammogram-basedriskfactorswerethedrivingforce inusdevelopingtheconceptofOPERA(oddsratioperadjustedstandarddeviation)[7].Theword “adjusted”referstotheuseofthestandarddeviationoftheriskfactor(forthepopulationaboutwhich inferenceisbeingmade)afterithasbeen adjusted forotherfactorsbeingtakenintoconsiderationby thedesign and analysis.
OPERAisthecorrectwaytointerpretriskestimatesintermsofriskdiscriminationonapopulation basisbecauseitrecognisesthatariskfactorisineffecttheriskmeasureadjustedforcovariates,not therawmeasureitself.Inaddition,ifitisassumedthattheriskmeasureisapproximatelynormally distributed(typicallyresearchershavetransformedmammographicdensitymeasurestoachieve this)andthatriskincreasesmultiplicativelyacrosstheriskspectrum(whichempiricallyappears tobeareasonableassumption),thenthelog(OPERA)isapproximatelyequaltothedifferencein means(intermsofstandarddeviationforthepopulation)betweencasesandcontrols,andsois easilyinterpreted.
Themeasurelog(OPERA),therefore,isanappropriatescaleonwhichtodiscussandcompare riskdiscrimination.Thereisamathematicalrelationshipbetweenlog(OPERA)andtheareaunderthe receiveroperatingcharacteristiccurve(AUC)whichisapproximatelylinearatleastforAUCbetween 0.5to0.7(OPERAbetween1and2).Undertheseassumptions,theinter-quartileriskratio(IQRR)is approximatelyOPERA2.5 (seeSupplementaryMaterial[8]).
WhenOPERAisused,itemergesthatconventionalmammographicdensityisoneofthestronger riskfactorsforfemalebreastcancer.TheOPERAistypicallyaround1.4,substantiallymorethanfor theclassicepidemiologicalriskfactors,asdiscussedpreviously[7].Theriskdiscriminationfrom conventionalmammographicdensitycomparesfavourablywiththatachievedbythelatestpolygenic riskscoresforwhichtheOPERAisabout1.6[9].Ournewmammogram-basedriskmeasureshave OPERAsof1.6orhigher[8,10–14]andwewillnowdiscusshowthiscameabout.
3.WolfeandBeyond
Inthe1970s,JohnWolfedemonstratedthatcertainaspectsofamammogramwerepredicting,for womendeemedatamammographicscreentonothaveabreastcancer,whichofthemweremorelikely tobediagnosedwithbreastcanceratsubsequentscreens[15].Anadvocateofearlymammographic screening,Wolfedevelopedaclassificationschemethatinitiallyapparentlyfailedtogainadoptionby radiologistsduetolackofreplicationinmeasurementandinconsistentresults[16],thoughthelatter mighthavebeenaconsequenceoftheformer.Itwasrecognised,however,thatthedenseregionsof mammogramswereimplicatedinamajorweaknessofmammographicscreening-thefailuretodetect cancershidden,or‘masked’,bythewhiteorbrightregionsthataremammographically‘dense’.
TheseminalworkofBoydandYaffe,whocreatedasemi-automatedwaytomeasure mammographicdensitycalledCUMULUS[17]andappliedittoprospectivestudiesoflargescreening populations,consistentlyfoundevidencethatpercentmammographicdensity—adjustedbydesign and/oranalysisforageandbodymassindex—waspredictingbothintervalandscreen-detectedcancers inthelong-term[18].UseoftheOPERAconceptconfirmedthatthisriskgradientwasstrongerfor intervalcancer[19],consistentwitharoleofmasking.Forexample,Krishnanandcolleagues[20] foundthatforpercentdensityadjustedforageandbodymassindex,theOPERAwas2.05(95% confidenceinterval(CI)1.65to2.56)forintervalcancercomparedwith1.37(95%CI1.21to1.56)for screen-detectedcancer(p fordifference = 0.002).Moreover,percentmammographicdensitywasthe bestpredictorofwhetherawomanwastodevelopintervalversusscreen-detectedcancer,withan OPERAof1.51(95%CI1.23to1.86; p = 0.00005).
Therefore,percentmammographicdensitywasclearlypredictingsomethingspecifictointerval cancers,presumablymasking,andwasalsoassociatedwithintrinsicbreastcancerrisk,buttoafar lesserextent.Whileitsassociationwithintervalcancerwasbyitsnaturelikelytobecausal,inthesense thatchangingpercentdensitycouldleadtoareductioninrisk,itwasstilluncleariftheconventional measureofdensity,orevendensityperse,iscausalforbreastcancer.Itispossiblethattheconventional
conceptofdensityasariskfactorsiscorrelatedwithothermammogram-orlifestyle-relatedcausalrisk factors.Toexaminethesehypotheses,wetooktwoapproachestodiscoveringnewmammogram-based measuresofrisk,andimportantly,consideredtheirriskpredictionsconcurrentlywiththosearising fromconventionalmammographicdensitywhenfittedtogether.
4.RedefiningMammographicDensityinTermsofBrightness: Cumulus,Altocumulus and Cirrocumulus
Initially,mammographicdensitywasdefinedasthelightorbrightareaandcalculatedusing theCUMULUSsoftware[17].Wecalltheresultantmeasure Cumulus inhonourofthemethodand itscreators,andbecauseitalignswiththemeteorologicaluseofthiswordtodescribecloudsoften describedaspuffy,cotton-likeorfluffyandbasedontheLatinword“cumulo”,meaningheaporpile. Wechallengedthisconventionbydefiningmammographicdensityat,ineffect,twosubstantially higherpixel-brightnessthresholdsasthebrighter(Altocumulus)andbrightest(Cirrocumulus)regions. WeusedtheCUMULUSsoftwarebutchosethebrightregionswithinthewhiteareastodefine Altocumulus,andthebrighterregionsamongthebrightareastodefine Cirrocumulus.Thesewords alsoalignwiththeirmeteorologicalusebymatchingpixelintensitytoheight(theLatinword“alus” meanshigh).Altocumulusaremiddlealtitudecloudstypicallyhigherthancumuluscloudsandhave elementsthatarelargerthanthoseofcirrocumuluscloudswhichtypicallyappearatanevenhigher altitude—theLatinword“cirro”meanscurlandisusedtodescribeahighcloud.
OperatorsofCUMULUShavetraditionallybeentrainedtobehighlyrepeatableintheirmeasures of Cumulus,bothwiththemselvesandwithotheroperators.Wesimilarlytrainedoperatorstoensure measuresof Altocumulus and Cirrocumulus werehighlyrepeatable.
WefoundfrommultiplestudiesofAustralianandKoreanwomen[10–14]thatthenewmeasures ofmammographicdensity, Altocumulus and Cirrocumulus,didbetterthan Cumulus atpredictingrisk. Onenotableexceptionisriskofintervalbreastcancer[14].
Tables 1 and 2 showthattheriskgradientsfor Altocumulus and/or Cirrocumulus werecomparable tothoseforthegeneticriskscoresdevelopedfromgenome-wideassociationstudies(OPERA = 1.6;[9]) andoftenbetterthanfor Cumulus (OPERA = 1.4).Theriskestimatesfor Altocumulus wereoftenlittle differenttothosefor Cumulus,buttheriskestimatesfor Cirrocumulus inparticularwereingeneral stronger,especiallyforKoreanwomen(whoarediagnosedonaverageadecadeorsoearlierthan Australianwomen)[10]andforAustralianwomendiagnosedatayoungerage[11].Thesefindings appliedtofilmandwellasdigitalmammograms,andforthelatter,appearedtodependonthe manufacturer[12].
Table1. Oddsratioperunadjustedstandarddeviation(OPERA1)fordenseareamammographic densitymeasuresinpredictingyoungerageatdiagnosisbreastcancerforwomenwithafamilyhistory ofbreastcancer(Nguyenetal.(2017))[11].
UnivariableMultivariable
Cirrocumulus 1.73(1.52–1.97)1.41(1.21–1.65)1.44(1.20–1.72)
Altocumulus 1.74(1.53–1.99)1.43(1.22–1.68)1.48(1.21–1.81) Cumulus 1.62(1.42–1.83)–0.95(0.76–1.18)
1 standarddeviationiscalculatedforeachmeasureadjustedforageandbodymassindexusingthecontrolssample and,forthemultivariableanalysis,ineffectadjustedfortheothermeasure.
Table2. Oddsratioperunadjustedstandarddeviation(OPERA1)fordenseareamammographic densitymeasuresinpredictingscreen-detectedbreastcancer(Nguyenetal.(2018))[13].
UnivariableMultivariable
Cirrocumulus 1.32(1.18–1.48)1.19(1.06–1.34)1.19(1.04–1.36) Altocumulus 1.25(1.12–1.40)–0.99(0.85–1.14) Cumulus 1.25(1.12–1.40)0.98(0.87–1.10)0.99(0.85–1.15)
1 standarddeviationiscalculatedforeachmeasureadjustedforageandbodymassindexusingthecontrolssample and,forthemultivariableanalysis,ineffectadjustedfortheothermeasure.
5.RevisitingTextureusingAgnosticMachineLearning: Cirrus
Wolfeoriginallydescribed‘parenchymalpatterns’usingverbaldescriptionsandsubjective measuresoftexturalfeatures[15].Weinsteadusedmachinelearningtotrytofindagnosticallyif therearetexturalpatterns(definedmathematically)thatpredictbreastcancerrisk[8].Westudiedfilm mammogramsfromtwosamplesofAustralianwomenandfromonesampleofAmerican-Hawaiian womenlivinginHawaii.Wemeasured20texturalfeaturesandfoundthatthemajorprincipal componentwasbasedon11ofthesefeatures.Riskmeasurescreatedfromthese11featuresandlearnt ononesampledidwellinpredictingbreastcancerinanothersample(OPERAs~1.7),evenwhen learntonthesmallsampleofJapanesewomenandappliedtoAustralianwomen.
Wecalltheriskmeasurebasedonfittingthese11featurestothecombinedsample Cirrus.These featureswerehighlycorrelatedwithoneanother,andthebestpredictingindividualfeaturewas ‘homogeneity’,ameasureofthedegreeof“scatteredness”ofthetexturewithinanimage;imageswith largeareasofsimilarintensitypixelshaveahigherdegreeofhomogeneitythanthosecomposedofa largenumberofsmalldissimilarregions.
6.WhatHappensWhentheNewMeasuresareFittedTogetherwiththeConventionalMeasure?
Wenowconsiderwhathappenswhenthenewmeasuresarefittedwithandwithouttakinginto accounttheconventionalmeasure,and viceversa.Thisallowsinferencetobemadeabouttheextent towhichassociationsareduetoconfoundingwithoneanotherfromexaminingwhathappensto theestimates.
6.1.CirrocumulusandAltocumulus
Exceptforintervalcancer,whenthenewmeasures Cirrocumulus and Altocumulus werefittedwith theconventionalmeasure Cumulus,the Cumulus riskestimateattenuatedtowardsthenullandwas oftennolongerstatisticallysignificant.Incontrast,thenewmeasuresgenerallyremainedsignificant. Thishappenedconsistently,despitethesemeasuresallbeingcorrelatedwithoneanother(e.g.,~0.8for Cumulus and Altocumulus;~0.6for Cumulus and Cirrocumulus).Thesegeneralfindingshavebeen replicated[21].
Table 1 showsanexampleinwhichthedifferentdensitymeasures(inabsoluteterms)were consideredtogetheraspredictorsof,onaverage,youngerageatdiagnosisbreastcancerforwomen withafamilyhistoryofbreastcancer[11].Allthreemeasuresgavestrongandsomewhatsimilarrisk predictions,withOPERAsofabout1.6–1.7,whenconsideredalone(univariableanalyses).Whenfitted together,the Altocumulus and Cirrocumulus measuresbothattenuatedtobeabout1.4butremained highlysignificant.(TheaverageofthesetwomeasureshadanOPERAofabout1.8).The Cumulus measure,however,wenttothenullandwasnolongersignificant.Thishappenedforeachcombination thatincluded Cumulus.Thedenseareameasuresgaveabetterfitthanthepercentmammographic densityareasforwhichasimilarpatternofresultswereobserved(theunivariableassociationfor Cumulus asapercentagehadasmallerOPERAof1.52(95%CI1.34–1.73)).
Table 2.showsanotherexample,thistimeforscreen-detectedbreastcancer[13].Allthree measurespredictedriskontheirown,withtheriskgradientfor Cirrocumulus being24%greateronthe log(OPERA)scale.When Cirrocumulus wasfitted,the Cumulus measurewenttothenullandwasno longersignificantwhetherornot Altocumulus wasalsofitted.
Table 3 showsanexampleforintervalbreastcancer[13].Thistimethepercentmammographic densitymeasuresgavebetterfitsthandidthedenseareameasures.Allthreemeasurespredicted riskontheirown,withtheriskgradientfor Cumulus beingthegreatest.Contrarytotheprevious examples,itwasthe Cumulus measurethatremainedsignificantinthemultivariableanalyseswhereas both Altocumulus and Cirrocumulus werenolongersignificantonce Cumulus wasalsofitted.
Table3. Oddsratioperunadjustedstandarddeviation(OPERA1)forpercentmammographicdensity measuresinpredictingintervalbreastcancer(Nguyenetal.(2018))[13].
UnivariableMultivariable Cirrocumulus 2.06(1.67–2.54)–1.10(0.90–1.36) Altocumulus 1.92(1.55–2.36)0.88(0.72–1.07)Cumulus 2.33(1.85–2.92)1.67(1.36–2.07)1.48(1.19–1.95)
1 standarddeviationiscalculatedforeachmeasureadjustedforageandbodymassindexusingthecontrolssample and,forthemultivariableanalysis,ineffectadjustedfortheothermeasure.
6.2.Cirrus
For Cirrus,Table 4 showswhathappenedwhen Cirrus wasfittedtogetherwithabsoluteand percentage Cumulus measures[8].The Cirrus riskestimateremainedhighandattenuatedbyonly16% onthelog(OPERA)scale,whilethe Cumulus riskestimateattenuatedbymorethan50%.Mostofthe datausedtheseanalysescamefromaprospectivecohortstudythatinvolvedabout70%screen-detected and30%intervalcancers.Notethattheapproachusedtocreate Cirrus onlyusedrelativebrightness betweenpixels,nottheabsolutebrightnessofpixelsasdoestheCUMULUS-basedmeasuresabove.
Table4. Oddsratioperunadjustedstandarddeviation(OPERA1)fromSchmidtetal.combineddata setcombineddataset[8].
UnivariableMultivariable
Cirrus 1.90(1.73–2.09)1.76(1.59–1.95)1.74(1.57–1.93)
Cumulus (absolute)1.34(1.25–1.43)1.16(1.08–1.24)–Cumulus (percent)1.38(1.29–1.48)–1.16(1.07–1.25)
1 standarddeviationiscalculatedforeachmeasureadjustedforageandbodymassindexusingthecontrolssample and,forthemultivariableanalysis,ineffectadjustedfortheothermeasure.
7.SomeOtherNewMammogram-BasedMeasuresofBreastCancerRisk
Otherresearchershavealsobeentryingtofindaspectswithinamammogram,otherthan mammographic density,thatcontaininformationaboutriskofbreastcancer.Butrarelyhavethese researchersconsideredtheirnewmammogram-basedmeasuretogetherwiththeconventionalmeasure ofmammographicdensityaswehavedone.
OnerecentexampleisbyDembrowerandcolleagues[22]whodevelopedanewriskmeasure usingdeeplearning,whichtheycallDL.TheirDLmeasurewas“moderately”correlatedwith conventionalmammographicdensitymeasures(Spearmancorrelationsbetween0.25and0.42).Using aprospectivecohortdesign,theycombinedintervalandscreen-detectedcancersasoutcome(278cases and2005controlsnotmatchedforage,average3.6yearsfollow-up,meanageatbaselinemammogram 55years)andcalculatedtheORperunadjustedstandarddeviation;seeTable 5.TheyusedLIBRA automatedsoftware[23]tocalculateconventionaldenseareaandpercentagedensityandadjustedthe mammogram-basedmeasuresforage(butnotforbodymassindex,whichmightexplainthepoor predictiveperformanceoftheirpercentmammographicdensitymeasure,asispredictedbytheearlier discussionabouttheOPERAconcept).
Table5. Oddsratioperunadjustedstandarddeviation1 fromDembroweretal.[22]. UnivariableMultivariable
DL1.56(1.48–1.64)1.52(1.42–1.59)1.55(1.47–1.64) Densearea1.31(1.24–1.38)1.15(1.09–1.22)Percentdensity1.18(1.11–1.25)-1.02(0.95–1.08)
1 standarddeviationiscalculatedforeachmeasureadjustedforageandbodymassindexusingthecontrolssample and,forthemultivariableanalysis,ineffectadjustedfortheothermeasure.
Table 5 showsthattheriskgradientfortheDLmeasurewasunchangedbyadjustingforthe conventionalmammographicdensitymeasures.Theriskgradientfortheabsolutedenseareameasure halvedafteradjustingfortheDLmeasure.Wandersandcolleaguesalsoconsideredtogethera conventionalmammographicdensitymeasureandatexturemethodassessmentmethodtheyhad developed[24],buttheyadjustedthetexturemeasureformammographicdensity(butnotviceversa). Theriskgradientforthetexturemeasureattenuatedbyabout15–35%,andremainedhighlysignificant. Unfortunately,theydidnotreportwhathappenedtothemammographicdensityriskafteradjustment fortexture.
8.Interpretation
Figure 1 showsaninterpretation,intermsofapathdiagram,ofthegeneralfindingsabove. Unmeasuredcausesareshownascirclesandthearrowsrepresentcausesinagivendirection. Theconventionalandnewmeasuresaremoderatelycorrelated,sotheremustbecausalfactorsspecific tobothmeasures(orevenacausalrelationshipbetweentheconventionalandnewmeasures,though wehaven’tshownthatonthefigure).

Figure1. Pathdiagramindicatingcausalpathwaysimplicatedinconventionalandnewmammogram-based predictorsofintervalandscreen-detectedbreastcancers.
Becausethereisnoconvincingevidencethatconventionalmammographicdensitypredicts screen-detectedbreastcanceroncetheothermammogram-basedriskfactorsaretakenintoaccount, itappearstobeapredictorofintervalcancersdueonly(ormostly)toitsroleinmasking.Thenew mammogram-basedriskmeasuresappeartobepredictingintervalcancerthroughtheirassociation withconventionalmammographicdensity,particularlytheothermammographicdensitymeasures definedbyhigherpixelbrightnessthresholdswhichattenuateoncetheconventionalmeasureisfitted (seeTable 3).Butthenewmeasurescouldalsobehavingadirecteffectgiventhattheirriskassociation isstillevidentevenafteradjustmentforconventionalmammographicdensity(seeTables 1 and 2 and Tables 4 and 5).Thedataareconsistentwiththenewmammogram-basedriskmeasures(ratherthan conventionalmammographicdensity)havingadirectcausaleffectonbothintervalandscreen-detected breastcancer,andthishypothesisnowneedstobeaddressed.
9.ImportanceofMammogram-BasedRiskFactorsasBreastCancerRiskPredictors
FollowingtheconceptofOPERA,Figure 2 showsthatthenewmammogram-basedmeasuresare thestrongestofthecurrentlyknownriskfactorsintermsofriskdiscriminationonapopulation-basis. ThisFigureisanupdateofTable 1 intheoriginalpaperonOPERA[7].Thenextbestriskfactoris derivedfrommulti-generationalfamilyhistoryusingthetoolsBOADICEAandBRCAPRO;theOPERA inFigure 2 pertainstoriskofbreastcancerdiagnosedbeforeage50yearsforwomenundertheageof 50years[25].Givenfamilialrisksarehighlydependentonageatdiagnosis,andageatrisk,thisis
likelytobegreaterthanitwouldbeforbreastcancerdiagnosedatanyage.TheOPERAforpolygenic riskscoresisbasedonthelatestpublicationfromtheBreastCancerAssociationConsortium[9].
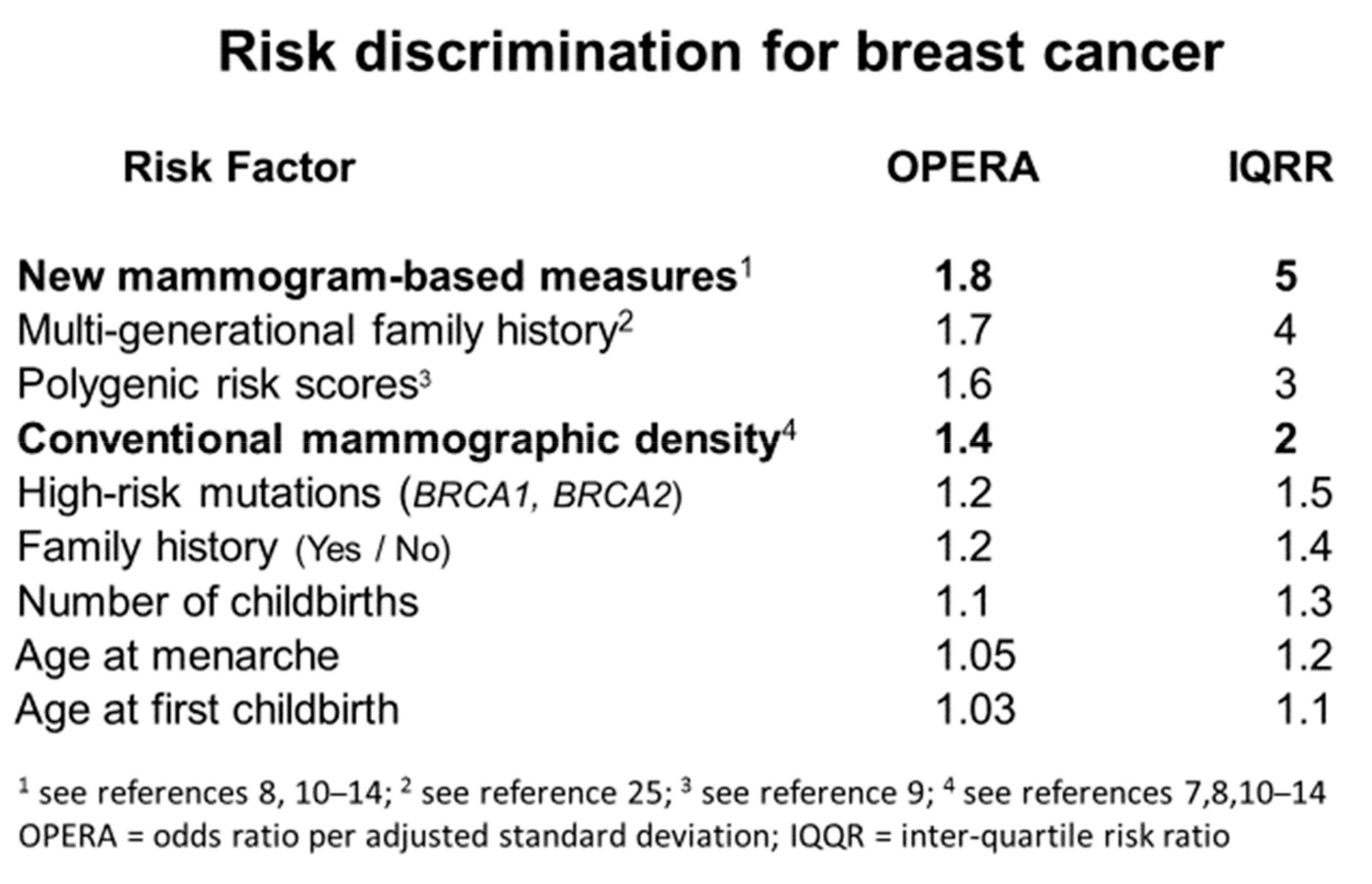
Figure2. Riskdiscriminationforbreastcancerbasedontheoddsratioperadjustedstandarddeviation (OPERA)(see[7])andequivalentinter-quartileriskratio(IQRR).
Conventionalmammographicdensity,adjustedforageandbodymassindex,isatmostweakly associatedwiththeotherknownlifestyle-basedbreastcancerriskfactors[5],asmentionedinSection 1 Familialandgeneticfactorsareperhapsthestrongest;about10–15%oftheincreasedriskofbreast cancerassociatedwithhavingatleastoneaffectedfirst-degreerelativefamilyhistoryisexplainedby thefactthatmammographicdensityadjustedforageandbodymassindexhasacorrelationofabout 0.3infirst-degreerelativesand0.6inmonozygotictwinpairs[5,26,27]Indeed,asimilarproportionof theknowngeneticvariantsassociatedwithbreastcancerriskarealsoimplicatedinexplainingthe varianceofmammographicdensityforageandbodymassindex[28,29].Therelationshipsbetweenthe newmammogram-basedriskfactorsandknownbreastcancerriskfactorshavenotyetbeenpublished.
10.Discussion
Wolfewasright,andwrong.Thereareaspectsofamammographicimagethatpredictriskof breastcancer—butitisn’tallaboutparenchymalpatterns,breastdensity,orthepercentageofthe breastcoveredbywhiteareas.Wolfe’sinitialstrongpredictionswere,onfacevaluecorrect,butthey weremostlyduetothestrongroleofconventionalmammographicdensityinpredictingmasking,not somuchasriskperse.
Riskofintervalcancersisanimportantissue,butitisnotallduetodensityandmasking.Wehave foundthatitcanbegreatlyimprovedbytreatingdensityasacontinuum(notasabinaryconstruct asincurrentclinicalpractice)andbyincludingotherriskfactorssuchasfamilyhistory,andanew measurebasedonbreasttissueageing[14].Itwilllikelyimprovewiththeadditionofpolygenicrisk scoresandtheothermammogram-basedriskfactorsrelatedtorisk perse
Ourworkhassuccessfullyidentifiednewinsightsintobreastcancerrisk,butwewouldnothave beenabletodothiswithouttheenormouscontributionsofothers.Inparticular,ProfessorNorman BoydkeptalivetheideasofWolfebydevelopingthetechnologyandresources,andinspiringthe workforce,topursuethislineofinvestigation.TheCUMULUSsoftwarehasbeenanincisivetoolfor understandingbreastcancerrisk.Wetrainedobservers,notnecessarilyradiologistsorphysicians, touseCUMULUSinnovelwaysandachievedhighrepeatabilityandbetterpredictionofbreastcancer thanthelatestpolygenicriskscoresfoundfromamassiveexpenditureongeneticsappliedtovery large-scaleepidemiologicalstudies.Thesenewmammogram-basedriskfactorsarealsomuchbetter
thantheotherknownepidemiologicalriskfactors(seeFigure 2)andcomplementpedigree-basedrisk modelslikeBOADICEAandBRCAPRO[25].
Whatdifferentiatesourworkoverthelastfewyearshasbeenitsfocusontheaspectsofa mammographicimagethatpredictrisk,ratherthanonmammographicdensityalone.Wehavenot beenaloneinthispursuit,atleastnotrecently,andnowthatdigitalmammographyiswidespread, theprospectoffindingevenstrongerriskpredictorsusingagnosticcomputer-learningapproachesis areality.
Forexample,PerutzandcolleaguescreatedOpenBreast[30],byapplyinglogisticregression analysestomultiplefeaturesaswedidincreating Cirrus,thistimeusingdigitalmammogramsbut withamuchsmallerdataset.TheyrecentlyreportedthatOpenBreasthadanOPERAof2.5,equivalent toanextraordinarilyhighIQRRofalmost10butwithawide95%CI(giventhesmalldataset)for whichthelowerboundwas4[30].FromtheSupplementaryMaterial[30],themostprominent featurecontributingtoOpenBreastwas‘maximumgray-levelvalue’,whichcouldbepickingup theaspectscapturedby Cirrocumulus (theamountofbrightestareas).Thenextmostprominent featurewas‘highgray-levelrun’followedby‘run-percentage’andthenboth‘short-runemphasis’ and‘long-runemphasis’,separatedby‘gray-levelrange’.Therefore,OpenBreastcouldbeutilising aspectsof‘homogeneity’andthemajorprincipalcomponentonwhich Cirrus isbased,combinedwith measuresofmeanandrangeofbrightnessimplicatedin Cirrocumulus.
Itmightbethat,byapplyingmachinelearningtodigitalmammograms,essentiallythesame risk-associatedaspectsofamammogramarebeingdiscovered,butwithgreaterpowerandmore precision.Asimilarapproachtoidentifytexture-basedriskpredictorsagnosticallyalsoperformedwell withanIQQRofabout6,withalowerboundofthe95%CI < 4(againreflectingthesmalldataset)[31].
Nevertheless,thesetworecentstudies,combinedwithourdiscoveriesusingmostlyfilm mammograms,areveryencouragingforfindingverystrong,andeasilyautomated,breastcancerrisk predictionfromapplyingmachineleaningtodigitalmammograms.Ourstudiesweredetectingsignals despiteconsiderablenoisefromdigitisingfilmmammogramsacrossdifferentmachines.Despitethis, weappeartohavebeendetectingtruesignals.Discoveryofmoreandbettersignalsmightbemuch easierusingdigitalmammography.
Anotherattempttoachievethisaimusing‘deeplearning’appliedtoalargedataset[32]failedto usecasesandcontrolsofthesameage,sotheyineffectlearnthowtomeasureagefromamammogram, derivedinflatedestimatesofriskdiscriminationbasedonnaïveuseofAUCstatistics,andmightnot havediscoveredanythingnew.Weknowagepredictsbreastcancerrisk,andiseasilymeasured,sowe wanttoknowwhatitisaboutamammogramthatpredictsriskforwomenofthesameage.Reuseof thatlargedatasettoapplylearningandreplicationusingcase-controlsetsmatchedforagecouldbe worthwhile,especiallyifitwasmadeopenlyavailable.
Tohelpachievetheaimofdeterminingtheoptimalmeasuresofthedifferentaspectofbreast cancerriskfromdifferentmammographicimages,weandothershavecreatedtheMeasurement Challenge[33].Thisisanopeninternationalresourcewhichofferssetsofanonymisedfull-field digitalmammogramimagesforanalysissothatunbiasedandtransparentcomparisonscanbemade betweenmeasures.
Thesenewdiscoverieshaveimportantimplicationsforunderstandingthecausesofbreast cancer.Wehaveappliedanewmethodformakinginferenceaboutcausationbyexaminingfamilial confounding,calledICEFALCON[34],todataontwinpairs.Wefoundnoevidencethatconventional DNAmethylationinbloodishavingacausaleffectonconventionalmammographicdensity(Cumulus), orviceversa[35].Wewillapplythisapproachtostudyournewmammogram-basedriskfactors.
Thatconventionalmammographicdensitymightnotnecessarilybehavingadirectcausaleffect onbreastcancerriskhasseriousimplicationsforbiologicalresearch.Atthisstageitisverydifficultto identifywhichcomponentsofbreasttissueareimplicatedinmammogram-basedrisk,butthiswillbe anareaoflikelyproductiveaetiologicalresearchoncethenewgenerationofriskfactorsemerging fromagnosticapproachestodigitalmammogramsareestablishedandautomated.
Therearealsomajorpublichealthimplications,especiallygiventhemagnitudeofbreastcancer over-diagnosisassociatedwithmammographicscreening[36].ItisinterestingtoreflectonWolfebeing anadvocateofearlymammographicscreening.Thesenewfindingsplaytothatscenariobymaking itpossibletoclarifywithconsiderableprecision,andatayoungadultage,awoman’sriskofbreast cancer[37].
Ifscreeningwastobetailoredtoawoman’sriskusingnewmammogram-basedmeasures,family historyandpolygenicriskscores,largeproportionsofthepopulationwouldbeclassifiedaswell below‘average’riskwhilesmallproportions(butsubstantialnumbersgiventhenumberofwomen screened)ofwomenwithrisksashighas BRCA1 and BRCA2 mutationcouldbereadilyidentified[37]. Newautomatedriskpredictorsbasedonmammograms,improvedgeneticriskpredictionandproper collectionandutilisationoffamilyhistorydatacouldgreatlychangethelandscapeofbreastcancerrisk predictionwithoutneedingtocollectdatafromwomenaboutlifestyleandotherfactorswithsmall impactonriskdiscrimination,makingimplementationfarmorepracticalandrealistic.
Theseadvancesinbreastcancerriskpredictionbasedonaspectsofmammogramscouldalsobe usedtohelpalleviateracialandethnicdisparities.Forexample,intheU.S.,blackwomenaremore likelytobediagnosedwithmoreaggressiveintervalbreastcancer.Asianwomenalsohaveonaverage higherpercentmammographicdensityandareatincreasedriskofthistypeofbreastcancer.Better riskpredictionforintervalbreastcancerusingmultipleriskfactorsasin[14]is,therefore,apriority. Tobeeffective,however,itwillneedtobeautomated,includenewmeasuressuchas Cirrus,andbe validatedforuseinraciallyandethnicallydiversepopulations.
Insummary,ourresearchhasdirectlychallengedthehistoricalparadigmofmammographic densityresearchthatthereisoneconceptofmammographicdensity,definedasthelightofbright regionsonamammogram,andthatthismeasureispredictiveofbothmaskingofexistingtumours andrisk.Wehavedrasticallychangedtheconventionalviewbyfindingevidencethattheconventional densityisimplicatedinbreastcancerrisk—specificallyintervalcancers—bymasking,notdirectly withriskperse.Otheraspectsofamammogram,basedonbrightnessandtexture,appeartobemore directlyimplicatedinrisk,andthisappliestoCaucasianaswellasAsianwomen.Theprospectof automatedmammogram-basedriskmeasures,combinedwithothermajorriskfactors,couldchange populationscreeningtobebasedmoreonrelativeriskthanjustage,makescreeningmoreeffective, reduceitsnegativeaspects,andhopefullysavemorelives.
AuthorContributions: Conceptualization:J.L.H.,T.L.N.,D.F.S.,E.M.,Y.-M.S.,J.S.,J.G.D.,S.L.;methodology: J.L.H.,T.L.N.,D.F.S.,E.M.,Y.-M.S.,J.S.,G.S.D.,J.G.D.,S.L.;software:D.F.S.,E.M.,J.S.;formalanalysis:J.L.H., T.L.N.,D.F.S.,E.M.,G.S.D.,J.S.;resources:J.L.H.,T.L.N.,Y.-M.S.,J.S.;writing—originaldraftpreparation:J.L.H., T.L.N.;writing—reviewandediting:J.L.H.,T.L.N.,D.F.S.,E.M.,Y.-M.S.,J.S.,G.S.D.,J.G.D.,S.L.;visualisation: J.L.H.,T.L.N.;supervision:J.L.H.,T.L.N.;projectadministration:J.L.H.,T.L.N.,Y.-M.S.,J.S.Allauthorshaveread andagreedtothepublishedversionofthemanuscript.
Funding: ThisresearchwassupportedbytheNationalHealthandMedicalResearchCouncil(251533,209057, and504711),theVictorianHealthPromotionFoundation,CancerCouncilVictoria,CancerCouncilNSW,Cancer Australia,andtheNationalBreastCancerFoundation.IthasalsobeensupportedbytheBreastCancerNetwork Australia,theNationalBreastCancerFoundation,VictoriaBreastCancerResearchConsortiumandwasfurther supportedbyinfrastructureprovidedbytheCancerCouncilVictoriaandtheUniversityofMelbourne.Wethank theVictorianCancerRegistry,BreastScreenVictoria,andtheAustralianMammographicDensityResearch Facility.TLNhasbeensupportedbyCureCancerAustraliaFoundationthroughCancerAustraliaPriority-Driven CollaborativeCancerResearchScheme(1159399).TLNandSLhasbeensupportedbyaVictorianCancerCouncil Post-DoctoralFellowshipandagrantfromthePicchiFoundation—VictorianComprehensiveCancerCentre.JLH isaNHMRCSeniorPrincipalResearchFellow.
Acknowledgments: WethanktheparticipantsinourstudiesandtheVictorianCancerRegistry,BreastScreen Victoria,theAustralianMammographicDensityResearchFacility.
ConflictsofInterest: Theauthorshavenoconflictsofinterest.
2020, 9,627
References
1. Byng,J.W.;Yaffe,M.J.;Jong,R.A.;Shumak,R.S.;Lockwood,G.A.;Tritchler,D.L.;Boyd,N.F.Analysis ofmammographicdensityandbreastcancerriskfromdigitizedmammograms. Radiographics 1998, 18, 1587–1598.[CrossRef][PubMed]
2. Krishnan,K.;Baglietto,L.;Stone,J.;Simpson,J.A.;Severi,G.;Evans,C.F.;MacInnis,R.J.;Giles,G.G.; Apicella,C.;Hopper,J.L.LongitudinalStudyofMammographicDensityMeasuresThatPredictBreast CancerRisk. CancerEpidemiol.BiomarkersPrev. 2017, 26,651–660.[CrossRef][PubMed]
3. Shia,W.C.;Wu,H.K.;Huang,Y.L.;Lin,L.S.;Chen,D.R.MammographicDensityDistributionofHealthy TaiwaneseWomenanditsNaturallyDecreasingTrendwithAge. Sci.Rep. 2018, 8,14937.[CrossRef] [PubMed]
4. Hopper,J.L.;Dite,G.S.;MacInnis,R.J.;Liao,Y.;Zeinomar,N.;Knight,J.A.;Southey,M.C.;Milne,R.L.; Chung,W.K.;Giles,G.G.;etal.Age-specificbreastcancerriskbybodymassindexandfamilialrisk: Prospectivefamilystudycohort(ProF-SC). BreastCancerRes. 2018, 20,132.[CrossRef][PubMed]
5. Nguyen,T.L.;Schmidt,D.F.;Makalic,E.;Dite,G.S.;Stone,J.;Apicella,C.;Bui,M.;Macinnis,R.J.;Odefrey,F.; Cawson,J.N.;etal.Explainingvarianceinthecumulusmammographicmeasuresthatpredictbreastcancer risk:Atwinsandsistersstudy. CancerEpidemiol.BiomarkersPrev. 2013, 22,2395–2403.[CrossRef][PubMed]
6. Eng,A.;Gallant,Z.;Shepherd,J.;McCormack,V.;Li,J.;Dowsett,M.;Vinnicombe,S.;Allen,S.; dos-Santos-Silva,I.Digitalmammographicdensityandbreastcancerrisk:Acase-controlstudyofsix alternativedensityassessmentmethods. BreastCancerRes. 2014, 16,439.[CrossRef]
7. Hopper,J.L.Oddsperadjustedstandarddeviation:Comparingstrengthsofassociationsforriskfactors measuredondifferentscalesandacrossdiseasesandpopulations. Am.J.Epidemiol. 2015, 182,863–867. [CrossRef]
8. Schmidt,D.F.;Makalic,E.;Goudey,B.;Dite,G.S.;Stone,J.;Nguyen,T.L.;Dowty,J.G.;Baglietto,L.; Southey,M.C.;Maskarinec,G.;etal.Cirrus:Anautomatedmammography-basedmeasureofbreastcancer riskbasedontexturalfeatures. JNCICancerSpectrum 2018, 2.[CrossRef]
9. Mavaddat,N.;Michailidou,K.;Dennis,J.;Lush,M.;Fachal,L.;Lee,A.;Tyrer,J.P.;Chen,T.H.;Wang,Q.; Bolla,M.K.;etal.PolygenicRiskScoresforPredictionofBreastCancerandBreastCancerSubtypes. Am.J. Hum.Genet 2019, 104,21–34.[CrossRef]
10. Nguyen,T.L.;Aung,Y.K.;Evans,C.F.;Yoon-Ho,C.;Jenkins,M.A.;Sung,J.;Hopper,J.L.;Song,Y.M. Mammographicdensitydefinedbyhigherthanconventionalbrightnessthresholdbetterpredictsbreast cancerriskforfull-fielddigitalmammograms. BreastCancerRes. 2015, 17,142.[CrossRef]
11. Nguyen,T.L.;Aung,Y.K.;Evans,C.F.;Dite,G.S.;Stone,J.;MacInnis,R.J.;Dowty,J.G.;Bickerstaffe,A.; Aujard,K.;Rommens,J.M.;etal.Mammographicdensitydefinedbyhigherthanconventionalbrightness thresholdsbetterpredictsbreastcancerrisk. Int.J.Epidemiol. 2017, 46,652–661.[CrossRef][PubMed]
12. Nguyen,T.L.;Choi,Y.H.;Aung,Y.K.;Evans,C.F.;Trinh,N.H.;Li,S.;Dite,G.S.;Kim,M.S.;Brennan,P.C.; Jenkins,M.A.;etal.Breastcancerriskassociationswithdigitalmammographicdensitybypixelbrightness thresholdandmammographicsystem. Radiology 2018, 286,433–442.[CrossRef][PubMed]
13. Nguyen,T.L.;Aung,Y.K.;Li,S.;Trinh,N.H.;Evans,C.F.;Baglietto,L.;Krishnan,K.;Dite,G.S.;Stone,J.; English,D.R.;etal.Predictingintervalandscreen-detectedbreastcancersfrommammographicdensity definedbydifferentbrightnessthresholds. BreastCancerRes. 2018, 20,152.[CrossRef][PubMed]
14. Nguyen,T.L.;Li,S.;Dite,G.S.;Aung,Y.K.;Evans,C.F.;Trinh,H.N.;Baglietto,L.;Stone,J.;Song,Y.M.; Sung,J.;etal.Intervalbreastcancerriskassociationswithbreastdensity,familyhistoryandbreasttissue aging. Int.J.Cancer 2019.[CrossRef]
15. Wolfe,J.N.Riskforbreastcancerdevelopmentdeterminedbymammographicparenchymalpattern. Cancer 1976, 37,2486–2492.[CrossRef]
16. Rafferty,E.A.Mammographicbreastdensity:FromWolfeandbeyond. Menopause 2014, 21,920–921. [CrossRef][PubMed]
17. Byng,J.W.;Boyd,N.F.;Fishell,E.;Jong,R.A.;Yaffe,M.J.Thequantitativeanalysisofmammographicdensities. Phys.Med.Biol. 1994, 39,1629–1638.[CrossRef]
18. Boyd,N.F.;Guo,H.;Martin,L.J.;Sun,L.;Stone,J.;Fishell,E.;Jong,R.A.;Hislop,G.;Chiarelli,A.; Minkin,S.;etal.Mammographicdensityandtheriskanddetectionofbreastcancer. N.Engl.J.Med. 2007, 356,227–236.[CrossRef]
19. Baglietto,L.;Krishnan,K.;Stone,J.;Apicella,C.;Southey,M.C.;English,D.R.;Hopper,J.L.;Giles,G.G. Associationsofmammographicdenseandnondenseareasandbodymassindexwithriskofbreastcancer. Am.J.Epidemiol. 2014, 179,475–483.[CrossRef]
20. Krishnan,K.;Baglietto,L.;Apicella,C.;Stone,J.;Southey,M.C.;English,D.R.;Giles,G.G.;Hopper,J.L. Mammographicdensityandriskofbreastcancerbymodeofdetectionandtumorsize:Acase-controlstudy. BreastCancerRes. 2016, 18,63.[CrossRef]
21. Wang,C.;Brentnall,A.R.;Cuzick,J.;Harkness,E.F.;Evans,D.G.;Astley,S.Exploringtheprediction performanceforbreastcancerriskbasedonvolumetricmammographicdensityatdifferentthresholds. BreastCancerRes. 2018, 20,49.[CrossRef][PubMed]
22. Dembrower,K.;Liu,Y.;Azizpour,H.;Eklund,M.;Smith,K.;Lindholm,P.;Strand,F.ComparisonofaDeep LearningRiskScoreandStandardMammographicDensityScoreforBreastCancerRiskPrediction. Radiology 2020, 294,265–272.[CrossRef][PubMed]
23. Keller,B.M.;Chen,J.;Daye,D.;Conant,E.F.;Kontos,D.Preliminaryevaluationofthepubliclyavailable LaboratoryforBreastRadiodensityAssessment(LIBRA)softwaretool:Comparisonoffullyautomatedarea andvolumetricdensitymeasuresinacase-controlstudywithdigitalmammography. BreastCancerRes. 2015, 17,117.[CrossRef][PubMed]
24. Wanders,J.O.P.;vanGils,C.H.;Karssemeijer,N.;Holland,K.;Kallenberg,M.;Peeters,P.H.M.;Nielsen,M.; Lillholm,M.Thecombinedeffectofmammographictextureanddensityonbreastcancerrisk:Acohort study. BreastCancerRes. 2018, 20,36.[CrossRef][PubMed]
25. Dite,G.S.;MacInnis,R.J.;Bickerstaffe,A.;Dowty,J.G.;Allman,R.;Apicella,C.;Milne,R.L.;Tsimiklis,H.; Phillips,K.A.;Giles,G.G.;etal.Breastcancerriskpredictionusingclinicalmodelsand77independent risk-associatedSNPsforwomenagedunder50years:Australianbreastcancerfamilyregistry. CancerEpidemiol.BiomarkersPrev. 2016, 25,359–365.[CrossRef]
26. Boyd,N.F.;Dite,G.S.;Stone,J.;Gunasekara,A.;English,D.R.;McCredie,M.R.;Giles,G.G.;Trichler,D.; Chiarelli,A.;Yaffe,M.J.;etal.Heritabilityofmammographicdensity,ariskfactorforbreastcancer. N.Engl. J.Med. 2002, 347,886–894.[CrossRef]
27. Martin,L.J.;Melnichouk,O.;Guo,H.;Chiarelli,A.M.;Hislop,T.G.;Yaffe,M.J.;Minkin,S.;Hopper,J.L.; Boyd,N.F.Familyhistory,mammographicdensity,andriskofbreastcancer. CancerEpidemuiol.Biomakers Prev. 2010, 19,456–463.[CrossRef]
28. Stone,J.;Thompson,D.J.;DosSantoSilva,I.;Scott,C.;Tamimi,R.M.;Lindstrom,S.;Kraft,P.;Hazra,A.; Li,J.;Eriksson,L.;etal.Novelassocaitionsbetweencommonbreastcancersusceptibilityvariantsand risk-predictingmammographicdensitymeasures. CancerRes. 2015, 75,2457–2467.[CrossRef]
29. Lindstrom,S.;Thompson,D.J.;Paterson,A.D.;Li,J.;Gierach,G.L.;Scott,C.;Stone,J.;Douglas,J.A.; dosSantos-Silva,I.;Fernando-Navarro,O.;etal.Genome-wideassociationstudyidentifiesmultipleloci associatedwithbothmammographicdensityandbreastcancerrisk. Nat.Commun. 2014, 5,5303.[CrossRef]
30. Pertuz,S.;Sassi,A.;Holli-Helenius,K.;Kamarainen,J.;Rinta-Kiikka,I.;Laaperi,A.L.;Arponen,O.Clinical evaluationofafully-automatedparenchymalanalysissoftwareforbreastcancerriskassessment:Apilot studyinaFinnishsample. Eur.J.Radiol. 2019, 121,108710.[CrossRef]
31. Tan,M.;Mariapun,S.;Yip,C.H.;Ng,K.H.;Teo,S.H.Anovelmethodofdeterminingbreastcancerriskusing parenchymaltexturalanalysisofmammographyimagesonanAsiancohort. Phys.Med.Biol. 2019, 64, 035016.[CrossRef][PubMed]
32. Yala,A.;Lehman,C.;Schuster,T.;Portnoi,T.;Barzilay,R.ADeepLearningMammography-basedModelfor ImprovedBreastCancerRiskPrediction. Radiology 2019, 292,60–66.[CrossRef]
33. Dench,E.;Bond-Smith,D.;Darcey,E.;Lee,G.;Aung,Y.K.;Chan,A.;Cuzick,J.;Ding,Z.Y.;Evans,C.F.; Harvey,J.;etal.Measurementchallenge:Protocolforinternationalcase-controlcomparisonof mammographicmeasuresthatpredictbreastcancerrisk. BMJOpen 2019, 9,e031041.[CrossRef][PubMed]
34. Li,S.;Wong,E.M.;Bui,M.;Nguyen,T.L.;Joo,J.E.;Stone,J.;Dite,G.S.;Dugue,P.A.;Milne,R.L.;Giles,G.G.;etal. InferenceaboutcausationbetweenbodymassindexandDNAmethylationinbloodfromatwinfamily study. Int.J.Obes. 2019, 43,243–252.[CrossRef][PubMed]
35. Li,S.;Dugue,P.A.;Baglietto,L.;Severi,G.;Wong,E.M.;Nguyen,T.L.;Stone,J.;English,D.R.;Southey,M.C.; Giles,G.G.;etal.Genome-wideassociationstudyofperipheralbloodDNAmethylationandconventional mammographicdensitymeasures. Int.J.Cancer 2019, 145,1768–1773.[CrossRef]
, 9,627
36. Glasziou,P.P.;Jones,M.A.;Pathirana,T.;Barratt,A.L.;Bell,K.J.Estimatingthemagnitudeofcancer overdiagnosisinAustralia. Med.J.Aust. 2019.[CrossRef]
37. Hopper,J.L.Geneticsforpopulationandpublichealth. Int.J.Epidemiol. 2017, 46,8–11.[CrossRef]

© 2020bytheauthors.LicenseeMDPI,Basel,Switzerland.Thisarticleisanopenaccess articledistributedunderthetermsandconditionsoftheCreativeCommonsAttribution (CCBY)license(http://creativecommons.org/licenses/by/4.0/).
