Copper,Brass, andBronzeSurfaces
AGuidetoAlloys,Finishes, Fabrication,andMaintenance
inArchitectureandArt
L.WilliamZahner
Coverimage:©wepix/GettyImages
Coverdesign:Wiley
Thisbookisprintedonacid-freepaper.
Copyright©2020byJohnWiley&Sons,Inc.Allrightsreserved.
PublishedbyJohnWiley&Sons,Inc.,Hoboken,NewJersey.
PublishedsimultaneouslyinCanada.
Nopartofthispublicationmaybereproduced,storedinaretrievalsystem,ortransmittedinanyformorbyany means,electronic,mechanical,photocopying,recording,scanning,orotherwise,exceptaspermittedunderSection 107or108ofthe1976UnitedStatesCopyrightAct,withouteitherthepriorwrittenpermissionofthePublisher,or authorizationthroughpaymentoftheappropriateper-copyfeetotheCopyrightClearanceCenter,222Rosewood Drive,Danvers,MA01923,(978)750-8400,fax(978)646-8600,oronthewebatwww.copyright.com.Requeststo thePublisherforpermissionshouldbeaddressedtothePermissionsDepartment,JohnWiley&Sons,Inc.,111 RiverStreet,Hoboken,NJ07030,(201)748-6011,fax(201)748-6008,oronlineatwww.wiley.com/go/permissions.
LimitofLiability/DisclaimerofWarranty:Whilethepublisherandauthorhaveusedtheirbesteffortsinpreparing thisbook,theymakenorepresentationsorwarrantieswithrespecttotheaccuracyorcompletenessofthecontents ofthisbookandspecificallydisclaimanyimpliedwarrantiesofmerchantabilityorfitnessforaparticularpurpose. Nowarrantymaybecreatedorextendedbysalesrepresentativesorwrittensalesmaterials.Theadviceand strategiescontainedhereinmaynotbesuitableforyoursituation.Youshouldconsultwithaprofessionalwhere appropriate.Neitherthepublishernortheauthorshallbeliablefordamagesarisingherefrom.
Forgeneralinformationaboutourotherproductsandservices,pleasecontactourCustomerCareDepartment withintheUnitedStatesat(800)762-2974,outsidetheUnitedStatesat(317)572-3993orfax(317)572-4002.
Wileypublishesinavarietyofprintandelectronicformatsandbyprint-on-demand.Somematerialincludedwith standardprintversionsofthisbookmaynotbeincludedine-booksorinprint-on-demand.Ifthisbookrefersto mediasuchasaCDorDVDthatisnotincludedintheversionyoupurchased,youmaydownloadthismaterialat http://booksupport.wiley.com.FormoreinformationaboutWileyproducts,visitwww.wiley.com.
LibraryofCongressCataloging-in-PublicationData:
Names:Zahner,L.William,author.
Title:Copper,brass,andbronzesurfaces:aguidetoalloys,finishes, fabrication,andmaintenanceinarchitectureandart/L.WilliamZahner.
Description:Hoboken,NewJersey:Wiley,2020.|Series:Zahner’s architecturalmetalsseries|Includesbibliographicalreferencesand index.
Identifiers:LCCN2019045098(print)|LCCN2019045099(ebook)| ISBN9781119541660(paperback)|ISBN9781119541677(adobepdf)| ISBN9781119541684(epub)
Subjects:LCSH:Copper.|Brass.|Bronze.|Architecturalmetal—work.| Artmetal—work.
Classification:LCCTS620.Z342020(print)|LCCTS620(ebook)|DDC 739—dc23
LCrecordavailableathttps://lccn.loc.gov/2019045098
LCebookrecordavailableathttps://lccn.loc.gov/2019045099
PrintedintheUnitedStatesofAmerica 10987654321
ThisbookisinhonorofSalvatoreOrlando. Hewasagoodfriendandadvocateoftheredmetal.
ATin–BronzeAlloy76
Nickel–SilverAlloys77
AManganese–BronzeAlloy78
CHAPTER3SurfaceFinishes79
Introduction79
MillSurfaces80
MechanicalFinishesandTemporaryProtection81
ColorfromOxidationandChemicalReactions91
Textures123
Tin-CoatedCopper135
MeltedCopperAlloySurfacing136
CopperandGlass137
ProtectingtheSurface138
CHAPTER4ExpectationsoftheVisualSurface139
Introduction139
Intent:AnUnchangedSurfaceAppearance143
Intent:ASurfaceAppearanceThatChangesNaturally154
Flatness155
TexturingtheSurface160
InitialOxidationonCopperAlloys161
InSituPatination163
Prepatination164
TheEffectofSealants166
TheCastSurface167 ArrivingattheBestPossibleOutcome167
CHAPTER5DesigningwiththeAvailableForms173
ABriefHistory173 WroughtForms176
TheCastForm204
CHAPTER6FabricationProcessesandTechniques211
Introduction211
Forming213 V-cutting215
CuttingCopperAlloys220
Machining227
Soldering,Brazing,andWelding231
Casting241
CHAPTER8MaintainingtheCopperAlloySurface295
APPENDIXAComparativeAttributesofMetalsUsedinArtandArchitecture337
APPENDIXBHardwareFinishCodesandDescriptions341 APPENDIXCNumberingSystemsUsedforCopperAlloys345
Preface
Thepassageoftimeisreflectedinthecolorofcopper.
Ofallthemetalsusedinartandarchitecture,copperisthemostengaging.
Throughouthistory,mankindhashadaspecialrelationshipwithcopper.Copperhasaweight andfeelingofsubstance.Itcanbeshapedandformedintousefulobjects,andmoreimportantly, ithasanappearanceasnaturalasthecolorsofanoakforestinthefall:acolorthatshowsvalueand thepassageoftime.
Powerandforceareneededtoshapetheothermetalsusedinartandarchitecture.Copper,on theotherhand,shapesandmovesundertheblowsofhandheldchasingtools.Itcanbeeasilyfolded, curved,stretched,andembossed.Onegetsclosetothemetalwhenworkingwithcopper.
Throughouthumantimecopperandcopperalloyshaveplayeddifferentandexpandingroles. Bronzesculpturesofancientdeitiesandheroeshaveoutlivedtheircivilizations,evenwhilerestingundertheseaforthousandsofyears.Copperhasbeenminedbyeverymajorcivilizationand convertedandcastintobothusefultoolsanddecorativestatues.
Themaritimeworldembracedcopperalloys,particularlybrass.Alloyswithnamessuchas AdmiraltyBronzeandNavalBrassrecallatimewhenthismetalservedasbiocladdingontheundersideofashipandornamentationonthetop.Considerthatthemilitaryterm“brass”relatesto someoneofhighrank:theonewiththebrassmetalsorthebrass-adornedhat.
Copperalloys,boththosewithnew,untarnishedsurfacesandthosewithcolorfulpatinas,offer theartistandthedesigneranamazingpaletteofcolortochoosefromanddesignwith.Oxidecolors willbepredictabletoapoint,butbeyondthatitisnaturethatwilltakeoverthedesign.Thesecolors willalsoactaspotentinhibitorsofcorrosion.Butnotethatbothnatural,untarnishedsurfacesand beautifulpatinasonartandarchitectureformswillrequiresomethingadditional—eitherinthe formofacoatingorintheformofenergyappliedfromanelbow—tokeepthemlookinggood.
Ihaveworkedwithcopperandmanyofitsalloysfordecades.Ihavehammeredit,cutit,welded it,andshapeditintobeautifulpiecesofart.Ihaveexperimentedwithcreatingcoloronthesurface ofthemetalwithchemicalinteraction,heat,andselectiveelectroplating.Ihaveworkedwithfriends tocastitandIhaveformeditssheetstocreateincrediblesurfaces.
Copperisspecialforitsamazingabilitytobeshapedandstretchedandforitsabilitytoreact withotherelementsandcompoundstoachieveauniqueandbeautifulsurface.
Coppercanbecastintoglass,andtheglassacceptsit.Itcanbeseverelyshaped,andityieldsto takethenewshape.Itcanwithstandtheattackofpowerfulacidsandbases,allthewhileforminga naturalmineralsurfacethatslowsfurtherreactionwhilegivingabeautifulpatina.
CHAPTER 1 Introduction—Element29
CopperandCopperAlloys
INTRODUCTION
Ofthemetals,onlycopperandgoldpossesscolorsotherthangrayorsilverintheirnaturalforms. Whencopperiscombinedwithdifferentelements,elegantcolorsandtonescanbeproducedboth naturallyandartificially.Thisisthereasonwhyourancientancestorsfirstworkedwiththismetal: theycouldidentifyitamongrocksandstonesmoreeasilyanditwasmoreabundantthangold. Thebrightlycoloredoresofcopper,malachite,andazuritesurelyattractedtheattentionofearly humans.Thesewerenotyournormalrocks.
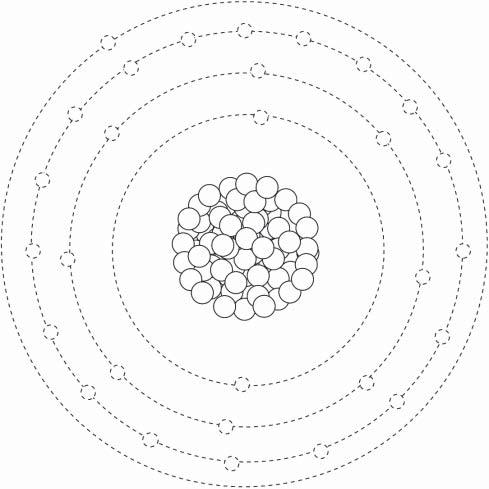
Copperiselement29intheperiodictable(Figure1.1).Itfallsbetweenelement28(nickel)and element30(zinc).Copperisinthesamegroupassilverandgold:metalsthatitmixeswithandthat possesssimilarpropertiesofelectricalandthermalconductivity.Likecopper,goldandsilverwere alsohighlyvaluedbyearlyman.Beingmoreabundant,coppertookontheheaviestworkload.The agenamedforitmarksanadvanceincivilization.
Copperpossessesaface-centeredcubicstructureinitspurestate,butthisstructurechangesas alloyingelementsareadded(Figure1.2).Thisface-centeredstructureissharedwithmanyother metals,suchasaluminumandiron.
Theatomicmakeupofcopperisresponsibleformanyoftheuniqueattributesthismetaloffers (Figure1.3).Theatomicnumberof29meansthatcopperhas29protonsinthenucleuswith29electronsmakingupitsoutershells.Itistheloneelectroninitsfourthorbitalthatgivescopperoneof
Copper’spositionintheperiodictableofelements.
FIGURE1.1
FIGURE1.2 Face-centeredcubicstructureofacoppercrystal.
Outer shell has only one electron
29 Electrons Copper Atom
29 Protons 34 Neutrons
FIGURE1.3 Copperatom.
itsmostimportanttraits.Thiselectronisfreetomoveabout,allowingelectricalcurrenttomove easilyfromatomtoatom.
Thislowerresistancecomingfromasingleelectronintheouterorbitgivescopperitsexceptionalabilitytotransportelectricity.Themetalswhoseatomshavethefewestelectronsintheirouter (orvalence)shelloffertheleastresistancetothemovementofelectricityfromoneatomtothenext. Gold,silver,andcopperaremetalsthathaveonlyoneelectronintheirvalenceshells,whichgives themtheirabilitytoconductelectricalcurrentmoreefficientlythanatomswithmorethanone electron.Thisloneelectronmovesfreelyaroundandthroughthelatticestructureofthemetal, transferringtheelectricalcurrentwithlittleresistance.Aluminumhasthreeelectronsinitsvalence orbit,whilezinc,nickel,iron,andtitaniumhavetwo(Table1.1).
Operatingunderaprinciplesimilartoelectricalconductivity,theconductivityofheathasthe sameorderacrossmetals.Alloyingwillchangetheheatconductivityofaparticularmetal,asit doestheelectricalconductivity.Forinstance,mostbronzealloysofcopperwillnotconductaswell asmanyaluminumalloys.Thealloyingelementsinbronzediminishitsabilitytomoveanelectricalcharge.
Astheyareessentiallyshared,thesevalenceelectronsarefreetoflowinandaroundtheatoms, creatinga“sea”ofchargedparticles.Thisseaofelectronsallowsthechargetomoverapidlyand withlittleresistanceasenergyistransferredfromelectrontoelectronthatcollectivelymakeupthe seaaroundthecopperatoms(Figure1.4).
MostofthecopperfoundontheEarth’ssurfaceisfromhydrothermalactivitythatbroughtthe metaltoornearthesurface.Othersurfacecopperisdriftcopper,depositedbyglacieractivityand randomlysetinrubble.Coppermakesupapproximately0.0068%oftheuppermineralcrustofthe Earthandiswidelydistributedwithconcentrationsinselectregions.
Copperhasapoorstrength-to-weightratioascomparedtoothermetalsusedinindustry.However,copperalloys,suchasbrassalloys,haveastrength-to-weightratioequivalenttostainlesssteels.
MetalSiemensm 1
Silver6.30 × 107
× 107
× 107
× 107
× 107
× 107
× 107
× 106
TABLE1.1 Electricalconductivityofvariousmetals insiemensm 1 at20 ∘ C.
FIGURE1.4 Seaofsharedelectronsaroundthemetalatomsofcopper.
Othercharacteristicsofcopperincludeexcellentductility,adeepformingability,highfracture toughness,highelasticity(resiliencyundershockloading),andsoftedges.
Copperisalsonontoxic—althoughcoppersaltsareconsideredecotoxicincertaininstances— andhassuperiorcorrosionresistanceinmanynaturalenvironments.
Copper:Element29
Atomicnumber29
CrystalstructureFace-centeredcube
MainmineralsourceChalcopyriteandchalcocite
ColorSalmonred
OxideBrowntoblack Density8960kg/m 3
Specificgravity8.8
Meltingpoint1083 ∘ C
Thermalconductivity401W/m 1 ∘ C
Coefficientoflinearexpansion16 × 10 6 /∘ C
Electricalconductivity100%IACS
Modulusofelasticity110GPa
FinishesMillspecularandnonspecular Polishedsatinandmirror Glassbead
Coppercanbepaintedbutthisisrarelydone.
Porcelainenamelisanartprocessusedextensivelyoncopper. Platingwithothermetalssuchassilver,nickel,andgoldiseasilyaccomplishedoncopper (continued)
(continued)
ArtificialpatinaGreens,browns,yellows,reds,blacks,andcombinationsare achievableoncopperandcopperalloys;thedevelopmentofchemical patinasonthesurfaceofcopperalloysisunmatchedinanyothermetal BrightappearanceCopperabsorbsandreflectstheredendofthevisiblespectrum;alloys alterthisreflectionbyemittingyellowwavelengthsalongwiththered endofthevisiblespectrum
Reflectance ofultravioletVerygood ofinfraredPoor;copperabsorbsinfraredwavelengths
RelativecostMedium
StrengtheningColdworkingisthemainmethodusedtostrengthencopperand copperalloys
RecyclabilityVeryeasilyrecycled;recycledcopperandcopperalloysretaina highvalue
WeldingandjoiningCopperandcopperalloyscanbewelded,brazed,andsoldered CastingCopperandcopperalloysarefrequentlycastinallcasting methodologies
PlatingCopperandcopperalloyscanbeelectroplated EtchingandmillingCopperandcopperalloyscanbeetchedandchemicallymilled
IACS = InternationalAnnealedCopperStandard;GPa = gigapascal.
COLOR
Anotherattributeofcopperanditsalloysiscolor.Thereareonlytwometalsthatpossessatone otherthangrayorsilver:goldandcopper.
Whenlightfallsonthemetalsurfaceitisintenselyabsorbedbytheatomsatthesurface.The electromagneticwavethatwecalllightonlypenetratesaverysmallfractionaldistance—lessthan awavelength—intothemetal’ssurface.Butthisabsorptionisintenseduetoatomiccharacteristics specifictometalsandtheelectronsthatmakeuptheseathatflowaroundtheatoms.
Thisintenseabsorptionoftheelectromagneticwaveonthesurfacecausesapulseofalternating current,whichthenexcitesthisseaofchargedparticlesandreemitslight.Thisisluster:theintense reflectionfromapolishedmetalsurface.Thesmootherthesurfaceis,thegreaterthereflection.If thesurfaceiscoarse,adiffusereflectionoccurs.Forexample,inthecaseoftarnish—athickened, diffuseoxidethatcandeveloponthesurfaceofcopper—acontrastingdarkersurfaceisapparent, dullingthecopper’sluster.Tarnishisamineralformationthatcapturestheelectronsandmakesthe metalslightlylessconductive.
Thecolorofcopperandgoldisdeterminedbythemakeupoftheiratoms.Whenalightwave strikesacopperorgoldsurface,theportionofthewavelengthfrom600to700nmisstrongly
absorbed,asFigure1.3shows.Inmetals,thisabsorptionleadstoreemissionasreflectedlight. Atthesametime,bothofthesemetalsabsorbthewavelengthsattheblueandvioletendofthe spectrumpoorly.Thisgivescopperareddishcolorandgoldayellowishcolor.Itisthissignificant drop-offinabsorption—strongononeendofthespectrumandweakontheotherend—thatgives thesemetalstheircharacteristiccolor.
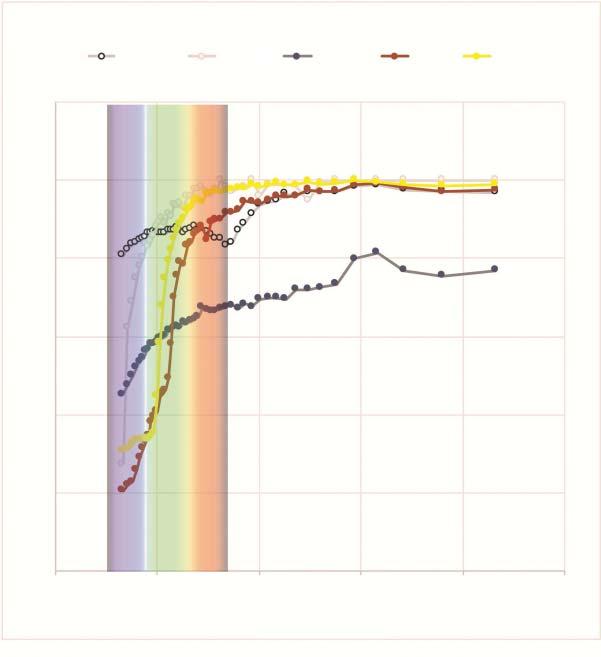
Forexample,ironabsorbsthewavelengthsassociatedwithblueandvioletmuchmorethan copper,butitdoesnotabsorbthewavelengthsassociatedwithred,orange,andyellow.Ithasa fairlyflatabsorptionlineacrossallthewavelengths.1 Stainlesssteelissimilartoiron’sreflectivity, buttheaddedchromiumincreasesthereflectionofportionsofthelightwaveover60%.Stainless steelissaidtoreflect,onaverage,60%ofthelightwave(Figure1.5).
FIGURE1.5 Reflectivityofaluminum,silver,stainlesssteel,copper,andgold.
Source:Dataplottedfrom NASATechnicalNoteD-5353,“SolarAbsorptancesandSpectralReflectancesof 12MetalsforTemperaturesRangingfrom300to500K.”
1 KurtNassau, ThePhysicsandChemistryofColor (NewYork:Wiley,1983),161.
COLORSOFALLOYS
Thecolorofcopperalloysisdeterminedbyseveralfactors.Theadditionofvariousalloyingconstituentsinfluencesthecoloruptothepointatwhichthecrystalstructureofthemetalchanges. Whenthisoccurs,thedensityandformofthecrystalchangesandlightabsorptioncanpotentially changeaswell.
Forexample,aszincisaddedtomoltencopperitdissolvesandintegratesintoasinglecrystal structure,orphase,alongwiththecopperatoms.Thealloybecomesprogressivelymoreyellowin color.Atthepointatwhich35%zincisalloyedwithcopper,themetalreachesasaturationpointand thecolorwillbeyellow.ThisisthealloyC27000,alsoknownasyellowbrass.Aszincisaddedbeyond this35%level,aphasechangeoccursinthecrystalmakeup,theyellowcolorlosesintensity,andthe colorgoesbacktoabronzetone.Thecoppercrystallatticecannolongertakethezincatomsinand twophasesdevelop:analphaphase,withaface-centeredcubicstructure,andabetaphase,witha body-centeredstructure.Forexample,alloyC28000,commonlyknownasMuntzmetal,contains 40%zincandislessyellow.BothalphaandbetagrainsareapparentinalloyC28000(seeFigure1.6 forcomparisonsofthenaturalcolorsofcopperalloys).
Mechanicalcharacteristicsalsochangeasalloyingconstituentsareadded.Aszincisadded, brassalloysgetstronger.However,theircorrosionresistance,particularlytotheconditionknown asdezincification,willdecrease.Oncethedualphaseappearsinthealloy,whichoccursataround 40%zinc,coldworkingabilitydeclines.TheC28000alloyishardertocoldworkthanalloyswith lesszinc.
Copperanditsalloyshavetheabilitytocreateamazingcolorswhentheycombinewithnonmetalelementssuchassulfur,chlorine,carbon,andoxygen(Figure1.7).Almosteveryoneisfamiliar withthebeautifulpatinasthatareapparentoncopperroofsbuiltacenturyago.Theseattractive, natural-lookinggreensurfacesdevelopedovertimeandwithexposuretotheatmosphere.Theywere notprecoloredbutallowedtoabsorbthecarbon,sulfur,andchlorinefromtheair.Inarealsense, coppercapturestheindustrialpollutantsofsulfurandcarbondioxidefromtheairandformsthese beautifulsurfacescomposedofcoppersulfate,copperchlorides,andcoppercarbonates.
Forinstance,thegreenpatinaadorningtheroofsofmanycenturies-oldbuildingsincities aroundtheworldisaformofthemineralbrochantite.ThismineralhastheformulaCu4 SO4 (OH)6
FIGURE1.6 Imageofdifferentalloysandcolors.
anddisplaysthecharacteristiccolorofpalegreen.Thecopperbeganasabrightsalmon-redcolor, butasitwasexposedtotheair,naturalhumidity,andrainthecoppercombinedwiththesulfur andformedthemineralonthesurface(Figure1.8).
Thepatinaisthecoppersurfacecorroding,butthedifferenceisthatonceformed,thistightly adheredcompoundprotectstheunderlyingmetal.Therateofcorrosionslowswaydownasthis inert,mineralformofcopperachievesalevelofequilibriumwiththesurroundingenvironment.
TheStatueofLiberty(Figure1.9)initiallyhadthecolorofapennywhendelivered.Itwasnot polishedcopperbuthadtherichcoppercolorofaslightlyagedpenny.Dedicatedin1886,itwas subjecttoyearsofexposuretothepollutedenvironmentofindustrialNewYorkinthelaterpart ofthe1800sandearly1900s.Exposedtoboththechloride-richseasideandpollutionfromheavy industryontheEastCoast,thegreenpatinaweseetodayformed.Astheinertminerallayerof brochantite,antlerite,andatacamitethatmadeupthepatinaformed,itprotectedthecopperplates fromdegradation.2
2 ThomasGraedelandJohnFraney, FormationandCharacteristicsoftheStatueofLibertyPatina (Houston:National AssociationofCorrosionEngineers,1990),101–108.
FIGURE1.7 Colorchangesinbrassalloys.
FIGURE1.8 Anaturallyformedpatina.
FIGURE1.9 TheStatueofLiberty.
PerseuswiththeHeadofMedusa,byBenvenutoCellini.
Intheworldofart,thisnaturaloxidationisasignthatthemetaliscorroding.Bronzestatuesandcopperalloyartifactsmustbeprotected,orthesurfaceswillcorrodetosomedegreeand canbevisuallyaffectedasthesurfacemetalcombinestoformthevariouscorrosionsalts.Sculpture,ifnotmaintained,willoxidizeanddevelopapatina.Inseverecases,particularlyinchloride environments,thecopperalloycanundergoadestructivecorrosionconditionknownas“bronze disease.”However,oneonlyhastolookattheancientsculpturesthathavebeenresidingunder theseaforcenturiestoobservethattheymayhavecorrosionproducts,buttheyarestillintactand recognizable.
Whensomemaintenanceisperformed,bronzesculpturescanlastcenturiesandappearasif theywerecastinrecenttimes.Figure1.10showsthefamoussculpture PerseuswiththeHeadof Medusa,createdbyBenvenutoCelliniaround1550andlocatedintheLoggiadeiLanziinFlorence, Italy.Oneofthemostintricateandbeautifulcastsculpturesinexistence,itstandsat5.2m(approximately18ft.)asmeasuredfromthestonebasetothetopofMedusa’shead.Thisremarkable,nearly 500-year-oldsculptureiswellmaintained.
FIGURE1.10
COPPERMINERALS
Copperandthebrassandbronzealloysofcopperaremetalsthathavebeenwithmankindsince antiquity.Gold,silver,platinum,andcopperaretheonlymetalsfoundintheirnativestate.Copper, beinglessrareandoccasionallyfoundonthesurface,wasmoreavailabletoearlyman.Whenthe natureofthematerialwasdiscoveredanddisseminatedbyourearlyancestors,theStoneAgewas overandtheCopperAgewasuponmankind.Usefultoolsthatcouldbereusedandreshapedoriginatedwiththemetalcopper.
Coppermakesuponlyabout0.007%oftheEarth’suppermineralstrata.Likeironandnickelit isadensermetal,soonewouldexpectcoppertobefounddeeperintheEarth,aslighterelements suchassiliconandaluminumwouldbeexpectedtofloatoverheavierelements(Table1.2).
Primarycopperoresarecalled“porphyrydeposits.”Theseoresarethevirgin,nonrecycled sourcesofcopper.Theyflowduringmagmareleasesbuttheyoftencombinewithsulfurtoform heavycopperandironsulfides,suchasthemineralschalcopyrite(CuFeS2 ),amineralcomposedof copper,iron,andsulfur,andbornite(Cu2 FeS4 ).MostcopperismilesbelowthesurfaceoftheEarth. AstheEarthwasformedtheseheaviermetalssank,leavingasurfacewithverylittlecopper.These heavycompoundstendedtosinkdeeperinthefluidflowsandwerethenexpelledinmagmaduring aneruption.3 ThisoccurredintheRockyMountainregionintheUnitedStatesandintheformation oftheAndesinSouthAmerica.
Bothoftheseregionsareareaswherelargedepositsofcoppermineralsarestillbeingmined. Similarregionswherevolcanicactivityovertheeonsmovedmineralsontothesurfaceoftheplanet existaroundtheworld;placessuchasPapuaprovince,wheretheSudirmanMountainsarelocated, andthesouthernCongoarerichinporphyrymineraldeposits.
TABLE1.2 Approximatepercentagesofelementsin theEarth’suppermineralstrata.
3 Geology 66,no.255(2008); Science 319,no.5871(March28,2008);RiceUniversity,“CopperChains:Earth’s Deep-SeatedHoldonCopperRevealed,” ScienceDaily (April5,2012).
TABLE1.3 Mineralformsofcopper.
CommonmineralnameFormulaColor
CupriteCu2 ORedoxide
TenoriteCuOBlack
MalachiteCuCO3 (OH)2
Intensegreenwithbanding
PseudomalachiteCu5 (PO4 )2 (OH) 4 Emeraldgreen
AzuriteCu3 (CO3 )2 (OH)2
BorniteCu5 FeS4
Intenseblue
Darkredwithslightiridescence
ChalcociteCuSBlackorblackgray
BrochantiteCu4 SO4 (OH)6 Green
AntleriteCu3 SO4 (OH)6 Green
NantokiteCuClPalegreen
AtacamiteCu2 (OH)3 ClCrystallinegreen
Themineralformofametalismorestableandslowtochange.Whenexposedtotheatmosphere copperanditsalloyscandevelopanoxidelayerthatapproachesoneofthemorecommonmineral formsofcopper.Cuprite,forinstance,formsonexposedcopperalloysurfaceswhensubjectedto heatandhumidity.Nantokitewithatacamitecanformoncopperalloysexposedtochlorideswhen nearthesea(Table1.3).
Bronzesculpturesthathavebeenexposedtotheenvironmentforalongperiodoftimecan formseveralofthesemineralcompounds,whichcanappearasspotsorstreaksonthesurfaceofthe originalpatinaprovidedbythefoundry(Figure1.11).
HISTORY
Thedistinctivecolorcopperpossessesdifferentiatesitfromothernaturalminerals.Theoccasional purenativeformofthemetalwouldhaveattractedearlymantothisheavy,denserock—arockthat wouldnotfracturebutthatwouldyieldtoblowsandtakeadifferentshape.Itsweightandmalleable naturemadecopperausefuldiscovery,andbecauseoftheseattributesearlymanprobablycollected itwhenhecameacrossit.
Easilyrecognizablebyitscolorandtactilenature,thesubstancewouldhavearousedtheinquisitivenatureofearlyhumans.Thesurfaceofnativecopperallowedearlymantoshapeitbyhammeringitwithstoneorwood.Initialhammeringwasprobablyperformedusingroundstonesorshaped logs,afterwhichthesubstancewashammeredintowoodenforms.Theplasticityofthemetalwould havebeenlikenoothermaterialknown.Formsofcopper,coldhammeredintojewelry,weapons,
Variousmineralsofcopper.
andtools,havebeenfoundinAnatoliadatingbackto9000 BCE.Skillsusedinmakingclaypottery, developedpriortotheCopperAge,wouldhavebeenadaptedtothismetal,althoughmoreforce wouldhavebeenneededandhardermaterialsnecessarytoshapethecopperintoausefulform.
Oncesomeonefoundthatyoucouldsoftenthemetalfurtherbyheatingit,theselumpsofcopper couldbeflattenedtoplatelikeforms,evenblades.Ashammeringisrepeatedonthecopperformit thinsoutasitisbeingshapedandtheworkedmetalhardens,losingsomeofitselasticity.Anedge canbesharpenedoncoarserocks,makingabladeformthatcanactasatooloraweapon.Acopper edgeisshort-lived,buttheutilityitwouldhaveofferedandtheeaseofworkingthemetalwould havemadecopperanimportantearlymaterial.
Everyearlycivilizationusedcopperinartanddecoration.FromtheSumeriansandChaldeans inMesopotamia,acrossEgypt,topresent-dayTurkeyandontoIndia,copperwasapartofearly civilizationsdatingbacknearlyto8000 BC.Weknowthisbythecharacterofthemetalitselfand itscorrosionresistance.Artifactsmadefromcopperexhibitonlyminordecay,evenafterallthese centuries(Figure1.12).
IntheUpperPeninsulaofMichiganontheshoresofLakeSuperior,intheareaknownas Keweenaw,ancientcoppermineshavebeenfoundalongwithcoppertools.4 Thoughttodateback asfaras4000 BC,theseancientminesshowthatpeopledugshallowminesinsearchofcopper.Large copperbouldersandmile-longveinswereintermixedwithgeneralrockandrubbleinthisregion.
4 CharlesWhittlesey, AncientMiningontheShoresofLakeSuperior (Washington,DC:SmithsonianInstitution,1863).
FIGURE1.11
FIGURE1.12 Approximatetimeperiodsofmajorcopperactivityinancienttimes.
High-puritycopperlumpswereintermixedwiththerubbleonornearthesurface.Surfacecopper, sometimesreferredtoas“driftcopper,”wasdepositedbytherecedingglaciers.Theseearlyinhabitantshadnoavailablemeansofcuttingthelargechunksofmetaldown,sotheygatheredthesmaller, moremanageableportionsandleftthelargesectionswithsomeoftheirbrokentools.EarlyNative Americansarebelievedtohaveventuredyearlytothissitetogathercoppertomakeornamentationandotheritems.Oldstonehammersandaxeshavebeenfoundwheretheseearlyinhabitants attemptedtocarveoffsectionstheycouldtransportbacktotheirvillages.Somesectionsweresimplytoomassivetotransport.Youcanseeoneofthelargerbouldersofnearlypurecopper,calledthe OntonagonBoulder,intheSmithsonian’sNationalMuseumofNaturalHistory.Thislargeboulder weighsinat1682kg(3708lb.).TheKeweenawIndiansclaimeditasasacredobjectandtheysought itsreturn.5 Thisboulderwasoneofthe“proofs”givenin1843forstartingthemineralrushtothe UpperPeninsularegionofMichigan(Figure1.13).
ThisregionoftheUnitedStateswasinvaluableinbringingAmericaintotheIndustrialRevolution.ItwasthefirstsiteofamadrushformineralwealthandprecededtheCaliforniaGoldRushby
5 OfficeofRepatriation(2000).ExecutiveSummaryAssessmentofaRequestfortheRepatriationoftheOntonagon BoulderbytheKeweenawBayIndianCommunity.
Source:WikimediaCommons;publicdomain. anumberofyears.Fromtheearly1800sandfornearlythenext150years,copperwasheavilymined inthisregion.Morethan6billionkgaresaidtohavebeenminedduringthistime.Artifactshave beenrecoveredfromtheseandotherancientcivilizationsstillintact,demonstratingthediverseuses thismetalwasputtobyearlymankind.
Theductilenatureofcopperwasoneofthefirstcharacteristicsofthemetalthatearlymanwas abletousetohisadvantage.Copperwashammeredthinandusedincrudewater-pipingsystems inearlyEgypt,theRomansusedthinplatesofcoppertocladtheroofofstructuressuchasthe Pantheon,andhelmetsandshieldswerecreatedbyartisansfamiliarwithworkingwiththispliable metal(Figure1.14).
FIGURE1.13 ImageoftheOntonagoncopperboulder.
FIGURE1.14 Romanhelmetsmadefromcopper.