NanocarbonElectrochemistry
Editedby NianjunYang InstituteofMaterialsEngineering UniversityofSiegen Germany
GuohuaZhao SchoolofChemicalScience&Engineering TongjiUniversity Shanghai,China
JohnS.Foord DepartmentofChemistry UniversityofOxford UK
Thiseditionfirstpublished2020 ©2020JohnWileyandSonsLtd
Allrightsreserved.Nopartofthispublicationmaybereproduced,storedinaretrievalsystem,or transmitted,inanyformorbyanymeans,electronic,mechanical,photocopying,recordingorotherwise, exceptaspermittedbylaw.Adviceonhowtoobtainpermissiontoreusematerialfromthistitleisavailable athttp://www.wiley.com/go/permissions.
TherightofNianjunYang,GuohuaZhao,andJohnS.Foordtobeidentifiedastheauthorsoftheeditorial materialinthisworkhasbeenassertedinaccordancewithlaw.
RegisteredOffices
JohnWiley&Sons,Inc.,111RiverStreet,Hoboken,NJ07030,USA
JohnWiley&SonsLtd.,TheAtrium,SouthernGate,Chichester,WestSussex,PO198SQ,UK
EditorialOffice
TheAtrium,SouthernGate,Chichester,WestSussex,PO198SQ,UK
Fordetailsofourglobaleditorialoffices,customerservices,andmoreinformationaboutWileyproducts visitusatwww.wiley.com.
Wileyalsopublishesitsbooksinavarietyofelectronicformatsandbyprint-on-demand.Somecontentthat appearsinstandardprintversionsofthisbookmaynotbeavailableinotherformats.
LimitofLiability/DisclaimerofWarranty
Inviewofongoingresearch,equipmentmodifications,changesingovernmentalregulations,andthe constantflowofinformationrelatingtotheuseofexperimentalreagents,equipment,anddevices,thereader isurgedtoreviewandevaluatetheinformationprovidedinthepackageinsertorinstructionsforeach chemical,pieceofequipment,reagent,ordevicefor,amongotherthings,anychangesintheinstructionsor indicationofusageandforaddedwarningsandprecautions.Whilethepublisherandauthorshaveused theirbesteffortsinpreparingthiswork,theymakenorepresentationsorwarrantieswithrespecttothe accuracyorcompletenessofthecontentsofthisworkandspecificallydisclaimallwarranties,including withoutlimitationanyimpliedwarrantiesofmerchantabilityorfitnessforaparticularpurpose.Nowarranty maybecreatedorextendedbysalesrepresentatives,writtensalesmaterialsorpromotionalstatementsfor thiswork.Thefactthatanorganization,website,orproductisreferredtointhisworkasacitationand/or potentialsourceoffurtherinformationdoesnotmeanthatthepublisherandauthorsendorsethe informationorservicestheorganization,website,orproductmayprovideorrecommendationsitmaymake. Thisworkissoldwiththeunderstandingthatthepublisherisnotengagedinrenderingprofessionalservices. Theadviceandstrategiescontainedhereinmaynotbesuitableforyoursituation.Youshouldconsultwitha specialistwhereappropriate.Further,readersshouldbeawarethatwebsiteslistedinthisworkmayhave changedordisappearedbetweenwhenthisworkwaswrittenandwhenitisread.Neitherthepublishernor authorsshallbeliableforanylossofprofitoranyothercommercialdamages,includingbutnotlimitedto special,incidental,consequential,orotherdamages.
LibraryofCongressCataloging-in-Publicationdataappliedfor 9781119468233
CoverDesign:Wiley CoverImage:©LAGUNADESIGN/GettyImages
Setin10/12ptWarnockProbySPiGlobal,Chennai,India 10987654321
Contents
ListofContributors xi
SeriesPreface xv
Preface xvii
1NanoelectrochemistryofAdsorption-CoupledElectronTransferat CarbonElectrodes 1
ShigeruAmemiya
1.1Introduction 1
1.2OverviewofAdsorption-CoupledET 2
1.3CleanCarbonElectrodes 4
1.4SECM-BasedNanogapVoltammetry 7
1.5Adsorption-CoupledOuter-SphereET 13
1.6Self-InhibitionofOuter-SphereET 16
1.7CouplingBetweenOuter-andInner-SphereET 19
1.8ResolvingOuter-andInner-SphereET 23
1.9SummaryandPerspectives 26
Acknowledgments 26 References 26
2TheCapacitanceofGraphene:FromModelSystemstoLarge-Scale Devices 33
PawinIamprasertkunandRobertA.W.Dryfe
2.1GrapheneOverview 33
2.2IntroductiontoCapacitance 34
2.2.1CapacitanceModel 34
2.2.2SpaceChargeCapacitance 36
2.2.3QuantumCapacitance 37
2.3CapacitanceofGraphene 39
2.4FormationofHeterostructures:GrapheneandOther2DMaterials 43
2.4.1TransitionMetalDichalcogenides(TMDCs) 43
2.4.22DNanocrystalorMXenes 44
2.4.3HexagonalBoronNitride(h-BN) 46
2.4.4Phosphorene 47
2.5Formulationof3DGrapheneArchitectures 49
2.5.1GrapheneSponges 49
2.5.2Template-AssistedGraphene 49
2.5.3GrapheneAerogels 51
2.5.4PillaredGrapheneFrameworks(PGFs) 54
2.5.5CarbonComposites 56
2.6TheInfluenceofHeteroatomDopingonGraphene 56
2.6.1Oxygen-DopedGraphene 57
2.6.2Nitrogen-DopedGraphene 58
2.6.3Boron-DopedGraphene 61
2.6.4UseofOtherElementstoDopeGraphene 61
2.6.5Co-dopedGraphene 63
2.6.6Multi-elementDopingofGraphene 64
2.7ApplicationofGrapheneinLarge-ScaleDevices 65
2.7.1GeneralPrinciplesofSupercapacitors 65
2.7.2Graphene-BasedSupercapacitorsandNovelCellDesign 68
2.7.3Li/NaIonCapacitors 70
2.8SummaryandFutureOutlook 71 References 75
3GrapheneandRelatedMaterialsasAnodeMaterialsinLiIonBatteries: ScienceandPracticality 85 SandeepKumarMarka,VeeraVenkataHarishPeruswamula,andVenkataSatyaSiva SrikanthVadali
3.1Introduction 85
3.2GraphiteasanAnodeMaterialinLiIonBatteries 86
3.3GrapheneandRelatedMaterialsasAnodeMaterialinLiIonBatteries 89
3.3.1GrapheneandRelatedMaterialsasAnodeMaterialinLIBs-Scienceand Practicality 90
3.3.2Intercalation-based 91
3.3.2.1rGO-TiO2 System 91
3.3.2.2rGO-Li4 Ti5 O12 System 91
3.3.2.3rGO-VanadiumOxidesSystem 92
3.3.3Conversion-based 92
3.3.3.1MMoO4 (i.e.,M = Fe,Co,Ni,Ca,Mn,Zn,andCu) 92
3.3.3.2Mo-ClusterOxysalts(i.e.,A2 Mo3 O8 Type,A = Fe,Co,Mn,andZnor LiHoMo3 O8 ) 95
3.3.4Alloying-based 97
3.3.4.1rGO-SiSystem 97
3.3.4.2rGO-GeSystem 99
3.3.4.3rGO-SnO2 System 110 References 114
4NanocarbonMaterialsTowardTextile-BasedElectrochemicalEnergy StorageDevices 123 QiyaoHuang,DongruiWang,andZijianZheng
4.1Introduction 123
4.2NanocarbonMaterialsforTEESDs 125
4.2.1NanocarbonasActiveMaterialforSCs 125
4.2.2NanocarbonasFunctionalMaterialforLIBs 127
4.3FabricationofNanocarbon-BasedElectrodesforTEESDs 127
4.3.1DirectCoatingonExistingTextileFibers,YarnsandFabrics 128
4.4In-SituGrowthonTextileSurfaces 130
4.4.1DirectSpinningofNanocarbonFibers 133
4.5ConclusionandPerspective 136 References 137
51Dand2DFlexibleCarbonMatrixMaterialsforLithium–Sulfur Batteries 145
TianyiWang,YushuLiu,DaweiSu,andGuoxiuWang
5.1Introduction 145
5.2TheWorkingMechanismandChallengesofLi–SBatteries 145
5.3FlexibleCathodeHostsforLithium–SulfurBatteries 146
5.4ElectrolyteMembranesforFlexibleLi–SBatteries 155
5.5SolidPolymerElectrolytesforFlexibleLi–SBatteries 157
5.6GelPolymerElectrolytesforFlexibleLi–SBatteries 159
5.7CompositePolymerElectrolytesforFlexibleLi–SBatteries 159
5.8SeparatorforFlexibleLi–SBatteries 161
5.9Summary 165 References 165
6ConductiveDiamondforElectrochemicalEnergyApplications 171
SiyuYu,NianjunYang,XinJiang,WenjunZhang,andShetianLiu
6.1Introduction 171
6.2ElectrochemicalEnergyStorage 172
6.2.1Supercapacitor 172
6.2.1.1DiamondEDLCs 173
6.2.1.2DiamondPC 177
6.2.1.3SupercapacitorDevice 179
6.2.2Battery 180
6.3ElectrochemicalEnergyConversion 183
6.3.1FuelCell 183
6.3.2SolarCell 186
6.4ElectrocatalysisforCO2 Conversion 187
6.5SummaryandOutlook 191 Acknowledgments 192 References 192
7ElectrocatalysisatNanocarbons:ModelSystemsandApplicationsin EnergyConversion 201
CarlotaDomínguez,JamesA.Behan,andPaulaE.Colavita
7.1Introduction 201
7.2High-PerformingNanocarbonElectrocatalysts 203
7.2.1Zero-Dimensional(0D)CarbonMaterials 204
7.2.1.1CarbonDots 205
7.2.1.2CarbonNano-Onions 205
7.2.1.3CarbonBlacksandActivatedCarbons 207
7.2.2HighAspectRatio(1D)Nanocarbons 208
7.2.2.1Nanohorns 209
7.2.2.2CarbonNanotubesandNanofibers 211
7.2.3Two-Dimensional(2D)CarbonMaterials 216
7.2.3.1GrapheneandGrapheneNanoribbons 216
7.2.3.2CarbonNanobeltsandThinFilms 221
7.2.4Three-Dimensional(3D)CarbonMaterials 221
7.2.4.1Bottom-UpSynthesisof3DNetworks 222
7.2.4.2Templated3DSuperstructures 224
7.3CarbonModelSystems 225
7.3.1HOPG 229
7.3.2Graphene 233
7.3.3AmorphousCarbon 236
7.4ConcludingRemarksandOutlook 239
Acknowledgments 240
References 240
8Metal-OrganicFrameworksBasedPorousCarbonsforOxygen ReductionReactionElectrocatalystsforFuelCellApplications 251 ShaofangFu,JunhuaSong,ChengzhouZhu,DanDu,andYueheLin
8.1Introduction 251
8.2MOF-DerivedPorousCarbonCatalysts 253
8.2.1HeteroatomsDopantEffectsonMOF-BasedPorousCarbonCatalysts 254
8.2.2MOF-DerivedCarbonComposites 257
8.3MetalIncorporatedMOF-DerivedPorousCarbonCatalysts 259
8.3.1ImpactofMetallicCompositiononORRActivity 260
8.3.2HeteroatomDopantEffectonIncorporatedMetalandSingleAtoms 266
8.3.3MorphologicalInfluenceontheCatalyticActivity 268
8.4ChallengesandPerspective 274 References 276
9DiamondElectrodesforElectrogeneratedChemiluminescence 285 AndreaFiorani,Irkham,GiovanniValenti,YasuakiEinaga,andFrancescoPaolucci
9.1Introduction 285
9.2FundamentalsofElectrogeneratedChemiluminescence 285
9.3Coreactants 287
9.4ECLLuminophores 289
9.5ElectrochemiluminescenceatDiamondElectrodes 289
9.6TPrA 290
9.7Oxalate 295
9.8HydroxylRadical 299
9.9Persulfate 303
9.10Luminol 306
9.11Conclusions 312 References 312
10DecorationofAdvancedCarbonMaterialswithMetalOxidesfor PhotoelectrochemicalApplications 323 Ya-nanZhang,HuijieShi,YuqingChen,RongrongCui,andGuohuaZhao
10.1Introduction 323
10.2BDDanditsApplicationinElectro-Analysis,EC,andPECOxidationof EnvironmentalPollutants 324
10.2.1DetectionofPollutantsonBDD 324
10.2.2ECOxidationofPollutantsonBDD 330
10.2.3PECOxidationofPollutantsonBDD 333
10.3DecorationofCAwithMetalOxidesandtheirPhotoelectrochemical Applications 337
10.3.1FabricationandStructuresofCA 337
10.3.2DecorationofCAwithMetalOxidesforEnvironmentalApplication 341
10.3.2.1EnhancedElectrocatalyticOxidationofOrganicPollutants 341
10.3.2.2Electro-FentonandPhoto–Electro–FentonOxidationofPollutants 342
10.3.2.3EfficientElectrosorption-PromotedPhotoelectrochemicalOxidationof Wastewater 344
10.4Summary 346 Acknowledgments 347 References 347 Index 357
ListofContributors
ShigeruAmemiya DepartmentofChemistry UniversityofPittsburgh Pennsylvania,USA
JamesA.Behan SchoolofChemistry,CRANNand AMBERResearchCentres TrinityCollegeDublin Ireland
YuqingChen SchoolofChemicalScienceand Engineering,ShanghaiKeyLabof ChemicalAssessmentandSustainability TongjiUniversity Shanghai,China
PaulaE.Colavita SchoolofChemistry,CRANNand AMBERResearchCentres TrinityCollegeDublin Ireland
RongrongCui SchoolofChemicalScienceand Engineering,ShanghaiKeyLabof ChemicalAssessmentandSustainability TongjiUniversity Shanghai,China
CarlotaDomínguez SchoolofChemistry,CRANNand AMBERResearchCentres TrinityCollegeDublin Ireland
RobertA.W.Dryfe SchoolofChemistry UniversityofManchester UnitedKingdom
DanDu SchoolofMechanicalandMaterials Engineering
WashingtonStateUniversity WA,USA
YasuakiEinaga DepartmentofChemistry
KeioUniversity Yokohama,Japan
AndreaFiorani DepartmentofChemistry
KeioUniversity Yokohama
Japan
ShaofangFu SchoolofMechanicalandMaterials
Engineering
WashingtonStateUniversity WA,USA
QiyaoHuang LaboratoryforAdvancedInterfacial MaterialsandDevices,Instituteof TextilesandClothing TheHongKongPolytechnicUniversity China
PawinIamprasertkun SchoolofChemistry UniversityofManchester UnitedKingdom Irkham DepartmentofChemistry KeioUniversity Yokohama Japan
XinJiang InstituteofMaterialsEngineering UniversityofSiegen Germany
YueheLin SchoolofMechanicalandMaterials Engineering
WashingtonStateUniversity WA,USA
ShetianLiu SchoolofChemistryandChemical Engineering SouthwestUniversity Chongqing,P.R.China
YushuLiu UniversityofTechnologySydney,School ofMathematicalandPhysicalSciences ResearchCentreforCleanEnergy Technology
Ultimo,NSW Australia
SandeepKumarMarka SchoolofEngineeringSciencesand Technology(SEST) UniversityofHyderabad Telangana,India
FrancescoPaolucci DepartmentofChemistry“G.Ciamician” UniversityofBologna Italy
VeeraVenkataHarishPeruswamula SchoolofEngineeringSciencesand Technology(SEST) UniversityofHyderabad Telangana,India
HuijieShi SchoolofChemicalScienceand Engineering,ShanghaiKeyLabof ChemicalAssessmentandSustainability TongjiUniversity Shanghai,China
JunhuaSong SchoolofMechanicalandMaterials Engineering WashingtonStateUniversity WA,USA
DaweiSu UniversityofTechnologySydney SchoolofMathematicalandPhysical Sciences,ResearchCentreforClean EnergyTechnology Ultimo,NSW Australia
VenkataSatyaSivaSrikanthVadali SchoolofEngineeringSciencesand Technology(SEST) UniversityofHyderabad Telangana,India
GiovanniValenti DepartmentofChemistry“G.Ciamician” UniversityofBologna Italy
GuoxiuWang UniversityofTechnologySydney SchoolofMathematicalandPhysical Sciences,ResearchCentreforClean EnergyTechnology Ultimo,NSW Australia
TianyiWang UniversityofTechnologySydney SchoolofMathematicalandPhysical Sciences,ResearchCentreforClean EnergyTechnology Ultimo,NSW Australia
NianjunYang InstituteofMaterialsEngineering UniversityofSiegen Germany
SiyuYu SchoolofChemistryandChemical Engineering SouthwestUniversity Chongqing,P.R.China
WenjunZhang DepartmentofMaterialsScienceand Engineering CityUniversityofHongKong China
Ya-nanZhang SchoolofChemicalScienceand Engineering,ShanghaiKeyLabof ChemicalAssessmentandSustainability TongjiUniversity Shanghai,China
GuohuaZhao SchoolofChemicalScienceand Engineering,ShanghaiKeyLabof ChemicalAssessmentandSustainability TongjiUniversity Shanghai,China
ZijianZheng LaboratoryforAdvancedInterfacial MaterialsandDevices,Instituteof TextilesandClothing TheHongKongPolytechnicUniversity China
ChengzhouZhu SchoolofMechanicalandMaterials Engineering WashingtonStateUniversity WA,USA
SeriesPreface
Carbon,the6thelementintheperiodictable,isextraordinary.Itformsavarietyofmaterialsbecauseofitsabilitytocovalentlybondwithdifferentorbitalhybridizations.For millennia,therewereonlytwoknownsubstancesofpurecarbonatoms:graphiteand diamond.Inthemid-1980s,asoccer-ball-shapedbuckminsterfullerene,namelyanew carbonallotropeC60,wasdiscovered.Togetherwithfullerene-structures(C70,C84) foundlater,thenanocarbonresearcherwasspawned.Intheearly1990s,carbonnanotubeswerediscovered.Theyaredirectdescendantsoffullerenesandcappedstructures composedof5-and6-memberedrings.Thiswasthenextmajoradvanceinnanocarbon research.Duetotheirground-breakingworkonthesefullerenematerials,Curl,Kroto, andSmalleywereawardedthe1996NobelPrizeinChemistry.Inthebeginningofthe 2000s,graphenewaspreparedusingScotchtape.Itisasinglesheetofcarbonatoms packedintoahexagonallatticewithabonddistanceof0.142nm.Fortheirseminalwork withthisnewnanocarbonmaterial,GeimandNovoselovwereawardedthe2010Nobel PrizeinPhysics.
Asnewmembers,carbonnanoparticles,suchasdiamondnanoparticles,carbondots, andgraphene(quantum)dots,haveemergedinthefamilyofnanocarbonmaterials. Althoughallthesematerialsonlyconsistofthesamecarbonatoms,theirphysical, chemical,andengineeringfeaturesaredifferent,whicharefullydependentontheir structures.
Thepurposeofthisseriesistobringtogetherup-to-dateaccountsofrecentdevelopmentsandnewfindingsinthefieldofnanocarbonchemistryandinterfaces,oneofthe mostimportantaspectsofnanocarbonresearch.Thecarbonmaterialscoveredinthis seriesincludediamond,diamondnanoparticles,graphene,graphene-oxide,graphene (quantum)dots,carbonnanotubes,carbonfibers,fullerenes,carbondots,carboncomposites,andtheirhybrids.Theformation,structure,properties,andapplicationsofthese carbonmaterialsaresummarized.Theirrelevantapplicationsinthefieldsofelectroanalysis,biosensing,catalysis,electrosynthesis,energystorageandconversion,environment sensingandprotection,biology,andmedicinearehighlightedindifferentbooks.
IcertainlywanttoexpressmysincerethankstoMissSarahHigginbotham,Jenny Cossham,EmmaStrickland,andLesleyJebarajfromWiley’sOxfordoffice.Withouttheir efficienthelporvaluablesuggestionsduringthisbookproject,thepublicationofthis bookserieswouldnotbepossible.Last,butnotleast,Iwanttothankmyfamily,especiallymywife,Dr.XiaoxiaWang,andmychildrenZimoandChuqianLuisa,fortheir
xvi SeriesPreface constantandstrongsupportaswellasfortheirpatienceinlettingmefinalizesucha bookseries.
Siegen,Germany April2019
NianjunYang
Preface
Electrochemistryisanextensivelyutilizedfieldofchemistrythatintegrateschemicals andelectricfields.Awell-designedelectrodematerialisthekeyofelectrochemistry. Theconnectionofelectrochemistrywithcarbonmaterialssuchasgraphite,diamond, andcarbonfibershashadalonghistory.Thediscoveriesofnewcarbonmaterialssuch asfullerene,graphene,carbonnanotubes,graphenenanoribbon,carbondots,and graphdiyneinpastdecadeshavetriggeredmoreresearchadvanceswithrespecttotheir electrochemicalpreparation,characterization,andapplications.
Thepurposeofthisvolumeisthustobringtogetherup-to-dateaccountsofrecent progress,developments,andachievementsintheelectrochemistryofdifferentcarbon materials,focusingontheiruniquepropertiesandvariousapplications.Webeginwith achapterconcerningthestudiesofheterogeneouselectrontransferatvariouscarbon electrodeswhenredox-activemoleculesarereversiblyandspecificallyadsorbedonthe carbonelectrodesurfaces,followedbyelectrochemicalenergystorageapplicationsof variouscarbonmaterials,particularlytheconstructionandperformanceofsupercapacitorsandbatteriesbyuseofgrapheneandrelatedmaterials.Thethirdpartofthis volumeisconcentratedonelectrochemicalenergyconversionapplicationswhereelectrocatalysisat0D,1D,2D,and3Dcarbonmaterialsnanocarbonmaterialsishighlighted. Thisvolumeisthenclosedwithconsiderationofelectrogeneratedchemiluminescence andphotoelectrochemicalpollutantdegradationbyuseofdiamondandrelatedcarbon materials.
Itistheinvaluableeffortsofdistinguishedresearchersfromninedifferentcountries withthirteendifferentaffiliationsthathavehelpedusbuildsuchacomprehensivevolumecoveringthevariousperspectivesofnanocarbonelectrochemistry.Thechapters coverthefundamentalpropertiesofdifferentcarbonmaterialsandtheirapplications acrossawiderangeofareas.Sufficientbackgroundregardingdifferentapplicationshas beenprovidedineachchapter.Byincludinginformationwithsuchawiderange,we hopethisvolumecancontributetotheunderstandingofnon-specialistsandspecialistsalike.Thusitshouldbeofinterestandusetostudents,researchers,andindustrial partnersworkingonmanydiversefieldsofelectrochemistry,whethertheyalreadymake frequentuseofcarbonelectrodesinoneformoranotherorwhethertheyarelooking forelectrodesfornewapplications.
xviii Preface
Wecertainlywanttoexpressoursincerethankstoallcolleaguesfromourpublisher, Wiley,especiallytoLesleyJebarajandEmmaStrickland.Theirefficienthelpandvaluable suggestionsmadethisvolumeproceedsmoothlytopublication.Last,butnotleast,we wanttothankourfamilymembersfortheirconstantandstrongsupportinlettingus finalizesuchavolume.
NianjunYang Siegen,Germany GuohuaZhao Shanghai,China
JohnS.Foord Oxford,UnitedKingdom
NanoelectrochemistryofAdsorption-CoupledElectronTransfer atCarbonElectrodes
ShigeruAmemiya
DepartmentofChemistry,UniversityofPittsburgh,USA
1.1Introduction
Thischapterisconcernedwithstudiesofheterogeneouselectrontransfer(ET)atcarbon electrodeswhenredox-activemoleculescanbereversiblyandspecificallyadsorbedon theelectrodesurfaces[1].Thesestudiesofadsorption-coupledETcomplementseminalstudiesofredox-activemoleculesirreversiblyattachedtotheelectrodesurfaces [2,3].Adsorption-coupledETisubiquitousinappliedelectrochemistryandplayscrucialrolesinelectrocatalysis[4],photocatalysis[4],electrodeposition[5],electropolymerization[6],electrochemicalremediation[7],andelectroanalysis[8].Fortheseapplications,carbonmaterialsarehighlyattractiveowingtotheirwell-knownpropensity toadsorbmolecules[9,10].Infact,significantattentionhasbeengivennotonlyto traditionalcarbonmaterials,butalsotoemergingcarbonnanomaterialsasimportant electrodematerials[11]fordiverseapplications,especiallyinenergyconversionand storage,e.g.fuelcells[12],batteries[13],solarcells[14],andsupercapacitors[15].This propensity,however,hampersafundamentalunderstandingofadsorption-coupledET atcarbonelectrodes,whicharereadilycontaminated[9,16,17].Theadventitiouscontaminationofcarbonsurfaceshasbeenwellrecognized[16,18],buthasnotbeenpreventedeffectivelydespiteahugebodyofworkoncarbonelectrochemistry[11,19].It hasbeendifficulttoreliablycharacterizetheintrinsicelectrochemicalreactivityand adsorptionpropertiesofcarbonelectrodes[20].
Inthischapter,weintroducerecentprogressintheoryofadsorption-coupled ETanditsexperimentalassessmentatcarbonelectrodes.Theoreticalprogress wasmaderecentlybyAmatoreandcoworkers[21,22],whorefinedaquantitative modelofadsorption-coupledETdevelopedbyLaviron[1,23]byconsideringouterandinner-sphereETandsurfaceadsorptionaselementaryelectrodesteps[24]. Experimentalprogressweremadebythedevelopmentandnanoelectrochemicalcharacterizationofcleancarbonelectrodesasrequisitestoreliablyexaminetheoriesfor adsorption-coupledET.Inparticular,nanoscalescanningelectrochemicalmicroscopy (SECM)[25,26]servesasanemergingpowerfulmethodtoquantitativelyinvestigate carbonelectrochemistry[27]andbeyond.
1.2OverviewofAdsorption-CoupledET
Thespecificandreversibleadsorptionofaredoxmoleculefromthesolutiontotheelectrodesurfacecouplesheterogeneousouter-andinner-sphereETasmodeledbyLaviron [23]andAmatore[24].Intheirmodel(Figure1.1),aredoxmolecule,O,istransported fromthesolutiontotheouterHelmholtzplane(OHP)[28]neartheelectrodesurface toparticipateinouter-sphereET,i.e.
Osol + e ⇌ Rsol (1.1)
wherethetransportedmolecule,Osol ,andtheproductofouter-sphereET,Rsol ,canbe adsorbedattheinnerHelmholtzplane(IHP)[28]oftheelectrodesurface,i.e.
⇌ Oads
⇌ Rads
Whenbothformsoftheredoxcoupleareadsorbed,inner-sphereETcanbemediated, i.e.
+ e ⇌ Rads
Inaddition,aheterogeneousself-exchangeETreactionispossibleasgivenby
+ Rsol ⇌ Rads + Osol
AquantitativeunderstandingoftheLaviron–Amatoremodelrequirestheknowledge oftime-dependentconcentrationsoffourspeciesincludingOads ,Rads ,Osol ,andRsol in additiontotime-anddistance-dependentconcentrationsofOandRinadiffusionlayer neartheelectrodesurface,wheretherespectiveconcentrationsaregivenby ΓO , ΓR , C O (0, t ), C R (0, t ), C O (x, t ), C R (x, t )(Figure1.1).Mathematically,adsorption-coupled ETcanbemodeledbysolvingtwoFickiandiffusionequationswithfourindependent equationsattheelectrodesurfaceasboundaryconditions.Forinstance,theequilibriumadsorptionofoxidizedandreducedformsofaredoxcoupleisdescribedbytwo isothermsforcompetitiveadsorption.Inaddition,twoNernstequationsareobtained todescribetheequilibriumofouter-andinner-sphereET.Adsorptionisothermsand Nernstequationsmustbesubstitutedbythecorrespondingkineticexpressionswhen equilibriumadsorptionorreversibleETisnotmaintained,respectively.
Historically,thecouplingbetweenouter-andinner-sphereETofaredoxcouple throughspecificadsorptionandheterogeneousself-exchangeETwasproposedby
Figure1.1 Thereactionschemeofouter-and inner-sphereETofnon-adsorbedandadsorbedredox molecules,respectively.Dashedarrowsindicate heterogeneousself-exchangeET.
NanoelectrochemistryofAdsorption-CoupledElectronTransferatCarbonElectrodes 3
Ansonin1961[29]toaccountfortheanomalousnon-linearconcentration-dependence ofexchangecurrentbasedontheFe3+/2+ coupleatthePtelectrode[30].Anson’swork [29]istheoreticallypioneeringandinsightful,butseemsovershadowedbytheerroneousassignmentofathin-layereffecttoanadsorptioneffect[29],andiswellknown ratherforthefollowingdevelopmentofthin-layerelectrochemistry[31–33].Prior toAnson,LaitinenandRandelsproposedamodelbasedoninner-sphereETof tris(ethylenediamine)complexesofCo3+ andCo2+ adsorbedonthemercuryelectrode withoutconsideringanouter-spherepathway[34].Similarly,othersalsoconsidered onlytheinner-spherepathway[35,36].Later,Ansonattemptedtokineticallydistinguishbetweenouter-andinner-sphereETusingtheMarcustheory[37]andalso experimentallyidentifytheelectrodereactionmechanismofaredoxcoupleaseither outerorinnersphere[38,39].Morerecently,Laviron[23,40–42]andAmatore[21] theoreticallydemonstratedconditionswhereouter-andinner-sphereETofaredox couplearenotdistinguishableelectrochemically,whichwillbediscussedthroughthis chapter.Aredoxcouple,however,isstillconsideredeitherasanouter-orinner-sphere couple[4],evencontroversially[43,44],despitethepossibilitythatbothouter-and inner-spherepathwayscanbemediatedsimultaneouslyandindistinguishablybythe sameredoxcouple.
Aquantitativeunderstandingofadsorption-coupledETishighlychallenging evenunderdiffusion-controlledconditionsowingtotherequirementofadsorption isothermswithmultipleparameters.Forinstance,fourparametersareintroducedwhen theadsorptionofeachformofaredoxcoupleisrepresentedbythesimpleLangmuir isothermforcompetitiveadsorptionofbothformsoftheredoxcouple,toyield[45]
where Γi,s isthesaturatedsurfaceconcentrationofspeciesiand �� i isaparametercharacterizingthemagnitudeofadsorbate–substrateinteraction.Inaddition,twoNernst equationsmustbeconsideredtodescribediffusion-controlledouter-andinner-sphere ET.AconventionalNernstequationisgivenforouter-sphereETby[46]
where n isthenumberoftransferredelectronsand E 0′ OS istheformalpotentialof outer-sphereET.Equation(1.8)canbecombinedwithLangmuirisotherms,Eqs.(1.6) and(1.7),toyieldaNernstequationforinner-sphereETas[47]
Equation(1.10)indicatesthattheequilibriumofinner-sphereETiscontrolledbythe equilibriumofouter-sphereETandsurfaceadsorption,therebyintroducingnoadditionalparameter.Itshouldbenotedthataformalpotentialisdefinedfornon-adsorbed redoxcouplesintheelectrochemistryliterature[48]torepresentouter-sphereET,i.e. E 0′ OS .Bycontrast,interactionsbetweenredoxmoleculesandelectrodeswereconsidered inarecenttheoreticalstudytocalculateformalpotentials[49],whichshouldbemore directlyrelevanttoinner-sphereET,i.e. E 0′ IS ,ratherthanouter-sphereET.
AcomparisonofNernstequationsforouter-andinner-sphereETpredictsthat thesetwopathwaysarethermodynamicallycoupledordecoupleddependingonthe adsorptivitiesofaredoxcouple.Specifically,outer-andinner-sphereETarecoupled anddrivensimultaneouslywhenbothformsofaredoxcoupleareadsorbedsimilarly,i.e. ΓO,s �� O =ΓR,s �� R ,toyield E 0′ IS = E 0′ OS = E 0′ inEq.(1.10).Inthiscase,reduction(oroxidation)isdriventhroughbothouter-andinner-spherepathwayssimultaneouslyat E < E 0′ (or E > E 0′ ),therebyyieldinganelectrochemicalresponsebasedontheconvolution ofbothpathways.Bycontrast,outer-andinner-sphereETaredecoupledwhenO (orR)isadsorbedmorestronglythantheother,i.e. ΓO,s �� O >> ΓR,s �� R (or ΓO,s �� O << ΓR,s �� R ),toyield
).Specifically,thestrongeradsorptionofa reactant,O(orR),thermodynamicallyhampersinner-spherereduction(oroxidation), whichrequiresmorenegative(orpositive)potentialsthanrequiredfortheouter-sphere counterpart.Bycontrast,inner-sphereETisthermodynamicallyfacilitatedtorequire lessextremepotentialswhenaproductismorestronglyadsorbedontheelectrode surface.Thisthermodynamicassessmentrevealsamisconceptioninarecentstudy thattheadsorptionofhard-to-reducemetalions,e.g.Ca2+ ,ontheelectrodesurface facilitatestheirinner-spherereductiontoyieldvoltammetricresponseswithinthe potentialwindow[50].
1.3CleanCarbonElectrodes
Experimentally,acleancarbonsurfaceisrequiredtoreliablystudyadsorption-coupled ET,becausebothheterogeneousET[51–54]andsurfaceadsorption[55]canbe affectedbyadventitioussurfacecontamination.Historically,methodshavebeen wellestablishedtoobtain,maintain,andensurecleansurfacesofmetalelectrodes, especiallyPt[56].Aflame-annealingmethodwaspioneeredbyClavilier[57]andis widelyusedtoreproduciblyyieldcleansurfacesofsingle-crystalmetalelectrodes.This method,however,isnotapplicabletocarbonmaterials,whicharecleanedbysolvents, laserillumination,(vacuum)heattreatment,electrochemicalpolarization,mechanical polishing,plasmatreatment,andchemicaloxidation[58].Thesemethods,however, canbedestructiveforgraphiticmaterials,especiallynanomaterials[58].Moreover, aflame-annealedmetalelectrodemustbeimmediatelyimmersedinultrapurewater topreventairbornecontamination,whichquicklyoccursalsooncarbonsurfaces [17,59–61].Theultrapurewatermustbefreefromorganic,surface-activeimpurities, whichwasoriginallyachievedbypyrocatalyticdistillation[62]toloweratotalorganic carbon(TOC)to3ppb[63].Currently,ultrapurewaterwithTOCatthelevelof1ppb isobtainablebyUVphotooxidationoforganiccontaminants[64]usingcommercial waterpurifiers[65].Finally,thein-situcleanlinessofaPtsurfaceinanelectrolyte solutioncanbequantitativelyevaluatedbystudyingtheelectrosorptionofhydrogen
NanoelectrochemistryofAdsorption-CoupledElectronTransferatCarbonElectrodes 5 [56].Thereis,however,noestablishedmethodtovalidatethein-situcleanlinessofa carbonelectrodesurfaceinanelectrolytesolution.
Recentstudiesshowedthatthepreventionofadventitiousorganiccontaminationon thesurfaceofacarbonelectrodeisstillhighlychallenging.Thesurfaceofhighlyoriented pyrolyticgraphite(HOPG)andgrapheneisquicklycontaminatedbyairbornehydrocarbons,asconfirmedbywatercontactangle(WCA)measurement,attenuatedtotalinternalreflectanceFouriertransforminfraredspectroscopy(ATR-FTIR),andellipsometry [17,60].Theheterogeneousdistributionofdisorderedadsorbatesonthegraphenesurfacewasalsoobservedbyatomicforcemicroscopy(AFM)[66].Traditionally,afreshly exposedHOPGsurfacewaselectrochemicallycharacterizedasquicklyaspossibleto minimizeairbornecontamination[67–69].Thesestudies,however,reportedverydifferentETkinetics[20,68].Moreover,thecontaminationofafreshlyHOPGsurface inwaterwasrevealedbySECM–AFM[70,71],butnotbyscanningelectrochemical cellmicroscopy(SECCM)[68,72],wherethesurfaceofatargetsubstratewasalways exposedtoambientair.
Recently,wedemonstratedthatthecleansurfacesofsp2 carbons,i.e.HOPGand graphene,canbeprotectedfromairbornecontaminationsimplybycondensinga nanometer-thickadlayerofwateronthesurfacesinhumidifiedair[61](Figure1.2a). TheWCAofanunprotectedHOPGsurfacequicklyincreasedfrom ∼60∘ to ∼90∘ in ambientairatroomtemperatureuntilthesurfacewassaturatedwithhydrophobic contaminants(Figure1.2b[61])asdetectedbyATR-FTIR.Bycontrast,theWCAofa HOPGsurfaceincreasedonlyto ∼70∘ in ∼20minuteswhenexfoliatedandstoredin humidifiedairat 20 ∘ Ctocondenseawateradlayer,therebyestimatingthemaximum surfacecoverageof ∼33%withairbornecontaminants.Thewater-protectedHOPG surfaceshowednodamage,asconfirmedbyX-rayphotoelectronspectroscopy(XPS). Moreover,ATR-FTIRoftheprotectedHOPGsurfaceshowedOHbondstretchingata lowerfrequency,whichindicatestheformationofanice-likestructure.Thequantitative analysisofellipsometrydataindicatedtheformationofsubmonolayerwaterwitha thicknessof0.08nmontheHOPGsurface.
Morerecently,wedevelopedanewmethodtoprotectthepristinesurfaceof electron-beam-depositedcarbon(eC)byusingaKCllayer(Figure1.3).Theamorphous eCfilmconsistsofamixtureofsp2 andsp3 hybridizedcarbon[74]incontrastto HOPGandgraphene.ThethineCfilmwasdepositedonametalsupporttoyield sufficientconductivityforelectrochemistryaswellasmolecularelectronics[75–78]. Earlierstudiesshowedthatthelowbackgroundcurrentandsufficienttransparencyof thethineCfilmareadvantageousforconventionalelectrochemistry[74]andoptical spectroscopy[79,80],respectively.Technically,wedepositedalllayersofelectrode materialsincludingaKCllayerinvacuumtoobtainthepristineeCsurface,which wasexposedforthefirsttimewhentheprotectiveKCllayerwasdissolvedinthe aqueouselectrolytesolutionofredoxmolecules.TheexposedeCsurfacewasextremely flat,asrepresentedbyaverageandroot-mean-squareroughnessof0.34 ± 0.01and 0.43 ± 0.02nm,respectively,basedonAFMmeasurements.RamanandX-rayphotoelectronspectroscopieswereemployed[76]todetermineanapproximately70:30 ratioofsp2 andsp3 hybridizedcarbon.Advantageously,KCl-protectedelectrodesare storableandtransportableinambientairwithoutcompromisingsurfacecleanliness.In fact,KCl-coatedeCsampleswerepreparedinEdmontonandexposedtoambientair duringshipmenttoPittsburghtomaintainthecleanlinessoftheunderlyingeCsurface


Figure1.2 (a)Schemeofgraphitesurfaceprotectedfromairbornehydrocarboncontaminantsbya wateradlayer.Yellow,red,white,andgrayballsrepresentsp2 carbon,oxygen,hydrogen,andsp3 carbonatoms.(b)TemporalevolutionoftheWCAmeasuredonfreshlyexfoliatedHOPGstoredat roomtemperatureandlowtemperature.Forthesamplestoredatlowtemperature,itwasonly exposedtoroomtemperatureduringtheWCAmeasurement.Source:Reprintedwithpermissionfrom Lietal.[61].Copyright2016AmericanChemicalSociety.
asensuredbySECM-basednanogapvoltammetry(NV)(seebelow).Potentially,the KCl-basedprotectionmethodwillbeapplicabletoavarietyofcleanelectrodematerials preparedinvacuum.
Thecleanlinessofamacroscopicelectrodeismaintainableinultrapurewaterwithlow TOCof2–3ppbcontaininghigh-purityinorganicelectrolytes,becauseofslowlinear diffusionoforganicimpuritiestothelargeelectrodesurface,aspredictedtheoretically andconfirmedexperimentallyusingHOPGinourrecentwork[51].Specifically,we appliedamodelbasedonthediffusion-limitedandirreversibleadsorptionoforganic
NanoelectrochemistryofAdsorption-CoupledElectronTransferatCarbonElectrodes
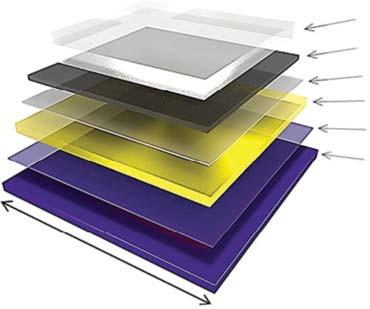
1 μm KCI
10 nm eC
3 nm TiC
30 nm Au
30 nm Cr
Si/SiOx (300 nm)
Figure1.3 SchematicillustrationofdepositedlayerswithindicatedthicknessesonSi/SiOxsubstrate.
Source:ReprintedwithpermissionfromMortezaNajarianetal.[73].Copyright2017American ChemicalSociety.
impurities[65]toaplanarmacroscopicsurfacetoestimatethesurfacecoverageofcontaminants, �� c ,asgivenby[51]
where cc and Dc aretheconcentrationanddiffusioncoefficientoforganicimpurities, respectively, t isthedurationofcontamination, �� sat isasaturationcoverageoftheHOPG surface(= 0.14forbenzene[81]asamodelforthesakeofgenerality[65]),and ��c isthe densityofcarbonatomatthegraphitesurface(= 6.3 × 10 9 molcm 2 ).Equation(1.11) predictsthatultrapurewaterwithTOCvaluesof2–3ppb(e.g.28–42nMbenzene) givesextremelylow �� valuesof0.0066–0.0099with t = 1hour.Asubstantial �� valueof 0.066,however,isexpectedfromEq.(1.11)with t = 1hourwhentheHOPGsurface isimmersedinultrapurewaterwithaTOCvalueof20ppb(cc = 0.28 μMforbenzene),whichistypicallyobtainedusingonlyfilterstoremoveorganicimpurities[63].By contrast,theoriginalmodelwasdevelopedtopredictthatnanoelectrodesarequickly contaminatedeveninultrapurewaterwithaTOCvalueof1ppbowingtofastmass transportoforganicimpuritiestocompromisetheelectroactivityofnanoelectrodes [65],e.g.oxygenreductionatPtnanoelectrodes[82].
1.4SECM-BasedNanogapVoltammetry
In-situcleanlinessofacarbonelectrodesurfacewascheckedexperimentallybyNV basedonSECM.Originally,SECM-basedNVwasdevelopedtoreliablymeasure fastkineticsofnearlyreversibleETbymonitoringbothoxidationandreductionof aredoxcoupleatquasi-steadystatesunderhighmass-transportconditionsacrossa nanometer-widegapbetweenanultramicroelectrode(UME)tipandamacroscopic substrateelectrode[83].Whenanonlyreducedformofaredoxcouple,R,isinitially presentinthesolution,theSECMfeedbackmodeisemployedtoinvestigatethe reductionoftheredoxcoupleonthesubstrate(e.g.eCinFigure1.4a).Specifically,the
Figure1.4 Schemeof(a)feedbackand(b)SG/TCmodesofSECMforNVataneCelectrode. Heterogeneousreductionandoxidationrateconstantsarerepresentedby k red and k ox ,respectively. Source:ReprintedwithpermissionfromMortezaNajarianetal.[73].Copyright2017American ChemicalSociety.SEMimagesofaborosilicate-sealedPtUME(c)beforeand(d)afterelectrowstatic damage.Source:Reprintedwithpermissionfrom Nioradzeetal.[84].Copyright2013American ChemicalSociety.
diffusion-limitedoxidationofthereductantattheSECMtipyieldsanamperometrictip current,whichvarieswiththesubstratepotential.Thetipcurrentisenhancedatsufficientlynegativepotentials,wherethereductantisregeneratedfromthetip-generated oxidant,O,tocompleteefficientredoxcyclingacrossanarrowgapbetweenthetip andthesubstrate.Thispositivefeedbackeffectisreducedtoeventuallydiminish thetipcurrentatpositivepotentials,wherethereductantisoxidativelyconsumed atthesubstrate,i.e.ashieldingeffect[85].Bycontrast,theoxidationofthereductant atthesubstratesurfaceismonitoredinthesubstrategeneration/tipcollection(SG/TC) modebydetectingthesubstrate-generatedspecies,O,atthetip(Figure1.4b).Overall, apairofNVsisobtainedbyplottingtheamperometrictipcurrentagainstthesubstrate potentialinfeedbackandSG/TCmodes.Theresultantvoltammogramsareobtained underquasi-steadystatestoyieldasigmoidalshapewhenthetip–substratedistance issufficientlynarrow.TheNVapproachbasedoncomplementaryoperationmodes ismorereliablethanthemeasurementofsinglesteady-statevoltammogramsbased oneitheroxidationorreductionofaredoxcouple,asoriginallydemonstratedfor ion-transfernanopipetvoltammetry[86,87].ItshouldbenotedthatSECM-basedNV enabledthedeterminationofhigh k 0 valuesofupto25cms 1 [52]byscanningthe potentialofamacroscopicsubstratearoundtheformalpotentialofaredoxcouple underquasi-steady-stateconditions[83].Bycontrast,macroscopicsubstrateshave beenstudiedbythefeedbackmodeofSECMtopredictmeasurableETrateconstants ofupto20cms 1 [88],whichwererecentlymisrepresentedasmeasurable k 0 values [89],i.e.,ETrateconstantsattheformalpotential.ThemeasurableETrateconstants
NanoelectrochemistryofAdsorption-CoupledElectronTransferatCarbonElectrodes 9 correspondtolower k 0 valuesofupto ∼2cms 1 evenwithnanogapconfigurations [90],becausethesubstratepotentialisusuallyfarfromtheformalpotentialinthe feedbackmodeofSECMtoirreversiblyelectrolyzethetip-generatedspeciesatthe substrate,whichisrequiredtoachievesteadystates.
Technologically,thedevelopmentofSECM-basedNVrevealedandovercame challengesthatwereunrecognizedpreviously[25].Theformationofananogap requiresaflatregionofasubstrateunderasmalltipwithwell-definedflatgeometry (Figure1.4c),whichwasfoundcompromisedbyelectrostaticdamagefromanexperimenter(Figure1.4d)anda(bi-)potentiostat[84,91,92].Moreover,itwasfoundthatthe maintenanceofananogapwithafixedwidthrequiredtheeliminationofthenanoscale thermaldriftoftipposition[92,93].Theimportanceofpreventingtipdamageand thermaldriftwasoverlookedinearlierstudies[94,95],whereonlythefeedbackmode wasemployedinSECM-basedNVoftipreactionstoobtainunreliableETkineticsas revealedinarecentstudy[96].Itshouldbeemphasizedthatthefindingofelectrostatic damageofsubmicrometer-andnanometer-sizedelectrodesisnewandsignificant, becausethepossibilityofill-definedgeometryofnanometer-sizedelectrodeshadbeen pointedout,butattributedtoimperfectelectrodefabrication[97–100]ratherthanthe damageofwell-fabricatedelectrodes.Moreover,thedamageofnanoelectrodeswas unseenpreviously[101,102],butwasnoticed[103]aftertheproblemwasreported [84].
SECM-basedNVwasusedtoensurethecleanlinessofKCl-protectedeCsurface [73].BothSG/TCandfeedbackmodesofSECMwereemployedtoinvestigatethe oxidationandreductionoftheFcTMA2+/+ couple,respectively,atKCl-protectedand non-protectedeCelectrodes(Figure1.5a,b,respectively).WhentheeCsurfacewasprotectedbyaKCllayer,symmetricpairsofreversibleNVswereobtainedattip–substrate distancesofdownto49nm.Theshortesttip–substratedistancewasusedtoestimate
Mode Non-Protected eC
Figure1.5 Nanogapvoltammogramsof0.3mMFcTMA+ in1MKClateCelectrodespreparedwithout (a)andwith(b)aprotectiveKCllayerobtainedusing1.01and1.04 μm-diameterPttips,respectively. Forwardandreversewavesarerepresentedbysolidanddottedlines,respectively.Tippotentials,0.55 and0.15VvsAg/AgClinfeedbackandSG/TCmodes,respectively.Potentialsweeprate,0.05Vs 1 Circlesrepresenttheoreticalcurveswith E 0′ = 0.371VvsAg/AgClandgapwidthsof44–275nm. DashedlinesinpanelBrepresenttheoreticalreversiblecurvesattheshortesttip substratedistance of49nm.Source:ReprintedwithpermissionfromMortezaNajarianetal.[73].Copyright2017 AmericanChemicalSociety.
Tip Current (nA)
Substrate Potential (V vs Ag/AgCl)
thediffusion-limitedminimum k 0 valueof14cms 1 ,whichisequivalentto k 0 a/D = 10 withatipradius, a,of0.52 μmandadiffusioncoefficient, D,of6.0 × 106 cm2 s 1 .The minimum k 0 valueisoneofthehighest k 0 valuesestimatedforaferrocenederivative atcarbonandmetalelectrodes(Table1.1)asdiscussedfurtherbelow.Bycontrast, NVsatnon-protectedeCsurfaceswerekineticallylimitedtoyieldafinite k 0 valueof 1.45cms 1 ,whichisatleast10timeslowerthanthe k 0 valueattheKCl-protectedeC surface.Importantly,thehigh k 0 valueatthecleaneCsurfaceisattributedtoastandard rateconstantofouter-sphereET, k 0 OS ,becausetheeCsurfaceadsorbsFcTMA+ ,but notFcTMA2+ ,i.e. ΓR,s �� R >> ΓO,s �� O ,tothermodynamicallydecoupleinner-sphere oxidationofadsorbedFcTMA+ tomuchmorepositivepotentials.Theminimum k 0 OS valueof14cms 1 isstilllowerthana k 0 OS valueof100cms 1 predictedforthe FcTMA2+/+ couple[104]byMarcustheory[108]
k 0 OS = Zhet (kex ∕Zbi )1
where k ex isahomogeneousself-exchangerateconstant,and Z het (∼104 cms 1 ) and Z bi (∼1011 M 1 sec 1 )areheterogeneousandbimolecularcollisionfrequencies, respectively.Equation(1.12)assumesadiabaticconditions,whereouter-sphereETis controlledbythereorganizationoftheredoxcoupleandsurroundingsolventstoyield thehighestpossible k 0 OS value.Theadiabatic k 0 OS valueof100cms 1 forFcTMA+ is nearlyidenticaltothehighest k 0 valuethatcanbemeasuredbySECM,whichislimited byelectrontunnelingacrossanarrowgapbetweenthetipandthesubstrate[109].
WeemployedSECM-basedNValsotostudyacleansurfaceofHOPG[104],which wasprotectedbyananometer-thickwateradlayercondensedontheHOPGsurface freshlypeeledinhumidifiedair[61].Thein-situcleanlinessofwater-protectedHOPG wasensuredbyasymmetricpairofNVsfortheFcTMA2+/+ coupleinfeedbackand
Table1.1 StandardETrateconstantsofFcTMA+ andFcCH2 OHatcarbonsandPt.
ElectrodeFerrocene k 0 (cms 1 )MethodRef.
—FcTMA+ 100a) —[104]
eC(KClprotection)FcTMA+ ≥14SECMb) [73]
eCFcTMA+ 1.45 ± 0.06SECMb) [73]
HOPG(waterprotection)FcTMA+ ≥12SECMb) [104]
HOPGFcTMA+ ≥17, ≥13SECMb) [51]
HOPGFcTMA+ 1.7 ± 0.3,0.7 ± 0.3SECM[51]
HOPGFcTMA+ ≥0.1–1.0SECCMc) [105]
SWCNTFcTMA+ 9 ± 2SECCMc) [106]
SWCNT(metallic)FcTMA+ 7 ± 2SECCMc) [107]
SWCNT(semi-conductive)FcTMA+ 4 ± 2SECCMc) [107]
PtnanoelectrodeFcCH2 OH >20SECMb) [96] FcCH2 OH6.8 ± 0.7SECM[94]
a)Marcustheory(Eq.(1.12)).
b)low-TOCwater. c)ambientair.