Savanna Woody Plants and Large Herbivores
Edited by Peter Frank Scogings
University of KwaZulu-Natal
Pietermaritzburg
South Africa and Mahesh Sankaran
National Centre for Biological Sciences
Tata Institute of Fundamental Research
Bangalore, Karnataka
India and School of Biology
Faculty of Biological Sciences
University of Leeds
Leeds, UK
This edition first published 2020 © 2020 John Wiley & Sons Ltd
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by law. Advice on how to obtain permission to reuse material from this title is available at http://www.wiley.com/go/permissions.
The right of Peter Frank Scogings and Mahesh Sankaran to be identified as the Editors of the editorial material in this work has been asserted in accordance with law.
Registered Office(s)
John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, USA
John Wiley & Sons Ltd, The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK
Editorial Office
The Atrium, Southern Gate, Chichester, West Sussex, PO19 8SQ, UK
For details of our global editorial offices, customer services, and more information about Wiley products visit us at www.wiley.com.
Wiley also publishes its books in a variety of electronic formats and by print‐on‐demand. Some content that appears in standard print versions of this book may not be available in other formats.
Limit of Liability/Disclaimer of Warranty
While the publisher and authors have used their best efforts in preparing this work, they make no representations or warranties with respect to the accuracy or completeness of the contents of this work and specifically disclaim all warranties, including without limitation any implied warranties of merchantability or fitness for a particular purpose. No warranty may be created or extended by sales representatives, written sales materials or promotional statements for this work. The fact that an organization, website, or product is referred to in this work as a citation and/or potential source of further information does not mean that the publisher and authors endorse the information or services the organization, website, or product may provide or recommendations it may make. This work is sold with the understanding that the publisher is not engaged in rendering professional services. The advice and strategies contained herein may not be suitable for your situation. You should consult with a specialist where appropriate. Further, readers should be aware that websites listed in this work may have changed or disappeared between when this work was written and when it is read. Neither the publisher nor authors shall be liable for any loss of profit or any other commercial damages, including but not limited to special, incidental, consequential, or other damages.
Library of Congress
Cataloging‐in‐Publication Data
Names: Scogings, Peter Frank, editor. | Sankaran, Mahesh, editor.
Title: Savanna woody plants and large herbivores / edited by Professor Peter Frank Scogings (University of KwaZulu-Natal, Pietermaritzburg, South Africa) and Dr. Mahesh Sankaran (National Centre for Biological Sciences, Tata Institute of Fundamental Research, Bangalore, Karnataka, India, and School of Biology, Faculty of Biological Sciences, University of Leeds, Leeds, UK).
Description: Hoboken, NJ : Wiley, 2019. | Includes bibliographical references and index. | Identifiers: LCCN 2019008174 (print) | LCCN 2019009247 (ebook) | ISBN 9781119081128 (Adobe PDF) | ISBN 9781119081135 (ePub) | ISBN 9781119081104 (hardcover)
Subjects: LCSH: Savanna plants. | Woody plants. | Savanna ecology. | Savanna animals. | Herbivores. Classification: LCC QK938.P7 (ebook) | LCC QK938.P7 S275 2019 (print) | DDC 581.7/48–dc23
LC record available at https://lccn.loc.gov/2019008174
Cover Design: Wiley
Cover Image: © Peter Frank Scogings
Set in 10/12pt Warnock by SPi Global, Pondicherry, India
Contents
List of Contributors xv
Preface xix
Part I Introduction 1
1 Distribution and Determinants of Savannas 3
Sally Archibald, William J. Bond, William Hoffmann, Caroline Lehmann, Carla Staver, and Nicola Stevens
1.1 Introduction 3
1.2 Evolutionary History of Savanna Vegetation and Fauna 4
1.3 Defining Savannas 7
1.3.1 Are Savannas Tropical Systems? 7
1.3.2 Distinguishing Savannas from Grasslands 7
1.3.3 Distinguishing Savannas from Forests 8
1.4 Global Determinants of Savannas 9
1.4.1 Mesic Transition: Points of Contention 10
1.4.1.1 The Role of Nutrients 10
1.4.1.2 Rainfall Seasonality 10
1.4.2 Mesic Transition: Toward Resolution 11
1.4.3 Mesic Transition: Unresolved Ideas 12
1.4.4 Arid Transition 12
1.4.5 Arid Transition: Toward Resolution 13
1.4.6 Determinants of Temperate Savannas 14
1.5 Functional Differences Between Savannas 14
1.5.1 Temperate vs Tropical Savannas 14
1.5.2 Functional Differences Within Tropical Savannas 15
1.6 Conclusions and the Future of Savanna Ecosystems 17 References 17
2 African and Asian Savannas: Comparisons of Vegetation Composition and Drivers of Vegetation Structure and Function 25
Jayashree Ratnam, Chintan Sheth, and Mahesh Sankaran
2.1 Introduction 25
2.2 Climate and Vegetation Formations 27
2.3 Fine‐Leaved and Broad‐Leaved Savannas: Vegetation Structure, Composition, and Geographic Distribution 30
2.4 Role of Bottom‐Up Drivers in Regulating Vegetation Structure: Climate and Soil Nutrients 33
2.5 Role of Top‐Down Forces: Fire and Herbivory 36
2.6 African and Asian Savannas in the Anthropocene 40 References 42
3 Savannas of Australia and New Guinea: Vegetation and the Functional Role of Extant and Extinct Fauna 51
Garry D. Cook, William J. Bond, Edmund C. February, and Richard J. Williams
3.1 Introduction 51
3.2 The Biota of Australia’s and New Guinea’s Savannas 51
3.3 Climate, Landforms, and Fire 53
3.4 Human History and Impacts 54
3.5 Are Native Mammals Irrelevant? 55
3.6 Was Ecosystem Functioning Different Prior to Human Dispersal to Australia? 57
3.7 Critique of the “Nutrient Poverty/Intense Fire” Theory 58
3.8 Australia’s Lost Megafauna 61
3.9 Habitat Variation and the Pleistocene Megafauna 64
3.10 Impacts of Herbivores in Australian Savannas 64
3.11 Toward a New Hypothesis of Plant–Animal Interactions in Australian Savannas 66 References 67
4 South American Savannas 77
Fabian Borghetti, Eduardo Barbosa, Leandro Ribeiro, José Felipe Ribeiro, and Bruno Machado Teles Walter
4.1 Introduction 77
4.2 Origin of South American Savannas 77
4.3 Distribution and Diversity of South American Savannas 78
4.4 Northern Savannas 80
4.4.1 Colombo–Venezuelan Llanos 80
4.4.1.1 Orinoco Llanos 80
4.4.1.2 Llanos Orientales 84
4.4.2 Gran Sabana 85
4.4.3 Rio Branco–Rupununi Savannas 85
4.4.3.1 Rio Branco Savannas 86
4.4.3.2 Rupununi Savannas 86
4.4.4 Savannas of Amapá 87
4.5 Southern Savannas 87
4.5.1 Savannas of Humaitá 87
4.5.2 Savannas of Pará 87
4.5.3 Beni Savannas 88
4.5.4 Cerrado 89
4.5.4.1 Cerrado (Sensu Stricto) 91
4.5.4.2 Cerrado Park 92
4.5.4.3 Palm Groves 92
4.5.4.4 Vereda 92
4.5.4.5 Campo Limpo (“Open Grassland”) 92
4.5.4.6 Campo Sujo (“Dense Grassland”) 92
4.5.4.7 Campo Rupestre (“Rocky Field”) 96
4.5.5 Pantanal 96
4.5.6 Chaco 97
4.6 Effects of Water Deficit, Herbivory, and Fire on Vegetation Dynamics 102
4.6.1 Water Deficit 102
4.6.2 Herbivory 103
4.6.3 Fire 104
4.7 Climate Change, Anthropogenic Pressure, and the Future 106
4.8 Concluding Remarks 109
4.9 Acknowledgments 109 References 110
5 Savannas of North America 123
Norma L. Fowler and Brian Beckage
5.1 Introduction 123
5.1.1 Definitions 123
5.1.2 Climatic Patterns 126
5.2 Fire 127
5.3 Grazing 128
5.4 Biodiversity 129
5.5 Conservation 129
5.6 Oak Savannas 130
5.6.1 Central US, South‐Central Canada, Northern Sierra Madre (Mexico) Oak Savannas 130
5.6.2 California Oak Savannas 132
5.6.3 South‐West (Arizona, New Mexico, Northern Mexico) Oak Savannas 132
5.6.4 Pacific Northwest Oak Savannas 132
5.6.5 East‐Central US: Glades, Barrens, and Other Forest Openings 132
5.6.6 Oak‐Dominated Shrub Savannas 133
5.7 Pine Savannas 133
5.7.1 South‐Eastern US Pine Savannas 133
5.7.2 Rocky Mountains Pine Savannas 134
5.8 Juniper Savannas 135
5.8.1 Juniper Savannas in the Western Mountains 135
5.8.2 Eastern Red Cedar Savannas 138
5.8.3 South‐Central US and Northern Sierra Madre Oriental Juniper Savannas 138
5.9 Mesquite Savannas 138
5.10 Northern and High‐Elevation Savannas 140
5.11 Shrub Savannas 140
5.12 Conclusions 141
5.13 Acknowledgments 141 References 141
6 Socioeconomic Value of Savannas 151 Wayne Twine
6.1 Introduction 151
6.2 Land Tenure and Land Use 153
6.3 Livestock Farming 155
6.3.1 Overview 155
6.3.2 Commercial Livestock Farming 157
6.3.3 Subsistence Livestock Farming 157
6.4 Wildlife Industry 159
6.4.1 Overview 159
6.4.2 Ecotourism 161
6.4.3 Hunting 162
6.4.4 Animal Products 163
6.4.5 Game Breeding and Live Sales 164
6.5 Commercial Timber 164
6.6 Non‐timber Products 164
6.6.1 Uses 164
6.6.2 Economic Value 166
6.6.2.1 Non‐monetary Income 166
6.6.2.2 Cash Income 167
6.6.2.3 Environmental Income 168
6.7 Conclusion 169 References 170 Part II Herbivores 181
7 Ecology of Smaller Animals Associated with Savanna Woody Plants: The Value of the Finer Details 183 Colleen Seymour and Grant Joseph
7.1 Introduction 183
7.2 Woody Plant Seed Herbivory 184
7.2.1 Seed Herbivores 184
7.3 Woody Plant Seed and Fruit Dispersal 187
7.3.1 Diplochory 187
7.3.1.1 Seed Dispersal by Birds 188
7.3.1.2 Invertebrate Seed Dispersal 189
7.3.2 Fruit Dispersal 189
7.4 Woody Plant Seedling Establishment 190
7.5 Leaves and Herbivory 191
7.6 Pollination and Nectarivory 193
7.7 Nutrient Cycling 195
7.8 Conclusions 199 References 201
8 Evolution of Large Mammal Herbivores in Savannas 213 Daryl Codron 213
8.1 Introduction 213
8.2 Herbivore Dietary Niches 215
8.3 Diversification of Browsers and Grazers 220
8.4 Effects of Vegetation Change 223
8.5 Herbivore Body Size 226
8.6 Pleistocene Extinctions and Contemporary Herbivore Diversity 228
8.7 Summary 233 References 234
9 Browser Population–Woody Vegetation Relationships in Savannas: From Bites to Landscapes 245
Melissa H. Schmitt and Adrian M. Shrader
9.1 Introduction 245
9.2 Factors Influencing Diet Selection 246
9.2.1 Browser Traits that Influence Foraging 247
9.2.1.1 Body Size 247
9.2.1.2 Gut Morphology 248
9.2.2 Woody Plant Traits that Influence Browsers 248
9.2.2.1 Seasonality 248
9.2.2.2 High Nutrient Levels (Positive) 249
9.2.2.3 Chemical Defenses (Negative) 250
9.2.2.4 Physical Defenses 252
9.2.2.5 Mutualisms 253
9.2.3 Herbivore Coping Mechanisms 253
9.3 Browser Impacts on Vegetation 255
9.3.1 Biomass Removal (Small and Large) 255
9.3.2 Impacts on Seeds 256
9.4 Feedback from Browsed Plants to Browsers 257
9.4.1 Lowered Food Availability 257
9.4.2 Habitat Changes 259
9.4.3 Change in Landscapes of Fear 260
9.4.4 New Growth 261
9.4.5 Nutrient Hot Spots 261
9.4.6 Browsing Lawns 261
9.5 Scaling from Bites to Browser Population Dynamics 262
9.5.1 Population Dynamics 263
9.5.2 Intake and Population Size 263
9.5.3 Food Availability, Food Quality, and Population Dynamics 264
9.5.4 Future Research 265
9.6 Conclusions 265 References 265
10 Predator Effects on Herbivore Dynamics and Behavior: What Mechanisms Lead to Trophic Cascades in Savannas? 279 Simon Chamaillé‐Jammes, Marion Valeix, and Joris Cromsigt
10.1 Introduction 279
10.2 Consumptive Effects of Predation 280
10.2.1 Concepts, Theory, and Evidence from Biomes Other than Savanna 280
10.2.1.1 Additive Versus Compensatory Mortality 281
10.2.1.2 Predator Functional Response 282
10.2.1.3 Ecosystem Characteristics 284
10.2.2 Evidence from Savannas 285
10.2.2.1 Additive Versus Compensatory Mortality 286
10.2.2.2 Predator Functional Response 288
10.2.2.3 Ecosystem Characteristics 288
Contents x
10.3 Non‐consumptive Effects of Predation 290
10.3.1 Concepts, Theory, and Evidence from Biomes Other than Savanna 290
10.3.1.1 Landscape Use 290
10.3.1.2 Vigilance and Grouping Strategies 291
10.3.1.3 The Importance of Food–Safety Trade‐Offs 292
10.3.1.4 Demographic Costs of Behavioral Adjustments 293
10.3.2 Evidence from Savannas 293
10.3.2.1 Landscape Use 293
10.3.2.2 Vigilance and Grouping Strategies 295
10.4 Cascading Effects of Consumptive and Non‐consumptive Effects of Predation on Lower Trophic Levels 296
10.5 The Times they Are A‐changin’: Changes in Megaherbivory, Migration Patterns, and Climate 297
References 299
Part III Woody Plants 309
11 Physiological Traits of Savanna Woody Species: Adaptations to Resource Availability 311
Edmund C. February, Corli Coetsee, Garry D. Cook, Jayashree Ratnam, and Benjamin Wigley
11.1 Introduction 311
11.2 Soil Nutrients and Root Responses 314
11.3 Leaf Phenology and Available Water 317
11.4 Competition for Resources 321 References 323
12 Patterns and Determinants of Woody Plant Growth in Savannas 331
Anthony Swemmer and David Ward
12.1 Introduction: The Relevance of Growth Rates 331
12.2 Determinants of Growth Rates 333
12.2.1 Seedlings 334
12.2.2 Saplings 342
12.2.3 Adults 344
12.2.4 Demographic Significance 344
12.2.4.1 Growth Trajectory 345
12.2.4.2 Size or Age of Individuals 345
12.2.4.3 Above vs Below Ground 345
12.2.4.4 Plant Part 347
12.2.4.5 Interacting Factors 347
12.2.4.6 Experimental Conditions 348
12.2.4.7 Individual vs Population Growth 348
12.2.4.8 Time and Size 348
12.2.4.9 Species 348
12.2.5 The Value of Long‐Term Research 349
12.3 Modeling Growth 350
12.3.1 Insights from Published Data 351
12.3.2 Predicting Rates from Environment or Phylogeny 353
12.3.3 Deficiencies in Growth Rate Data 356
12.4 Conclusions 357
12.A Appendix: Growth Rate Data 358 References 428
13 Fire and Browsers in Savannas: Traits, Interactions, and Continent‐Level Patterns 439
Gareth P. Hempson, Sally Archibald, and Carla Staver
13.1 Introduction 439
13.2 Browser and Fire Attributes 440
13.2.1 How do Fire and Browsers Compare as Consumers of Woody Plants? 440
13.2.1.1 Frequency and Seasonality 440
13.2.1.2 Selectivity, Intensity, and Scale 440
13.2.1.3 Elimination Thresholds 442
13.2.2 Plant Responses to Fire and Browsing 442
13.2.2.1 Defense Traits 442
13.2.2.2 Architecture 443
13.2.2.3 Resprouting and Bud Protection 444
13.2.2.4 Fire‐ and Browser‐Traps 445
13.2.2.5 Reproduction and Seedling Recruitment 446
13.3 Fire–Browser Interactions 447
13.3.1 Consequences of Fire for Browsers 447
13.3.1.1 Post‐Fire Environment 448
13.3.1.2 Woody Plant Regeneration 449
13.3.1.3 Decadal Fire Regimes 450
13.3.2 Browser Feedbacks to Fire 451
13.3.2.1 Browser Facilitation of Fire 451
13.3.2.2 Negative Feedbacks of Mixed‐Feeders 451
13.3.3 Fire–Browser Vegetation Impacts 452
13.3.3.1 Sapling Escape 452
13.3.3.2 Elephant Bark Stripping and Canopy Breakage 452
13.4 Biogeography of Fire and Browsing in Africa 453
13.4.1 Continental‐Scale Patterns of Fire and Browsing 455
13.4.2 Fire–Browser Regimes 457
13.4.3 Fine‐ vs Broad‐Leaved Savannas 457
13.5 Synthesis 460 References 460
14 Woody Plant Architecture and Effects on Browsing Herbivores in Savannas 469
Tristan Charles‐Dominique, Jean‐Francois Barczi, and Simon Chamaillé‐Jammes
14.1 Introduction 469
14.2 Factors Limiting Bite Size 471
14.3 Factors Limiting Biting Rate 474
14.4 Simulating Plant–Herbivore Interactions at the Individual Plant Scale 476
14.4.1 Plant Growth Model 477
14.4.2 Virtual Browsing and Consequences for Plant Fitness 478
14.4.3 Virtual Experiment Set‐up 478
14.4.4 Simulation Results 480
14.4.4.1 Effect of Leaf Size 481
14.4.4.2 Effect of Short Shoots 481
14.4.4.3 Effect of Spines 482
14.4.4.4 Effect of Cage Architecture 482
14.4.4.5 Effect of Short Shoot Induction 482
14.4.4.6 Effect of Sprouting 482
14.4.5 Significance of Simulation Results 482
14.5 Future Directions for Modeling Plant–Herbivore Interactions 483 Acknowledgments 483
14.A Appendix 484 References 484
15 Browsing Herbivore–Woody Plant Interactions in Savannas 489
Peter Frank Scogings and Juan H. Gowda
15.1 Introduction 489
15.1.1 The raison d’être 489
15.1.2 Approach 490
15.2 Feedback Between Woody Individuals and Browsing Herbivores 492
15.2.1 Shoot Growth 492
15.2.2 Spinescence 493
15.2.3 Nutrients and Phenolics 494
15.2.4 Is Positive Feedback Widespread? 495
15.3 Selective Browsing and Shifts in Woody Vegetation Composition and Structure 497
15.3.1 Recruitment and Mortality 497
15.3.2 Community Composition and Structure 500
15.4 Linking Responses of Woody Individuals and Communities to Functional Traits 501
15.5 Future Directions 504
15.5.1 Key Gaps 504
15.5.2 Standardizing Methods 505 References 539
16 Mesobrowser Abundance and Effects on Woody Plants in Savannas 551
David J. Augustine, Peter Frank Scogings, and Mahesh Sankaran
16.1 Introduction 551
16.2 Mesobrowser Abundance in Savannas 552
16.3 Mesobrowser Diets in Savannas 559
16.4 Mesobrowser Effects on Woody Plant Communities 561
16.4.1 Hluhluwe‐iMfolozi Park, South Africa 564
16.4.2 Central Laikipia, Kenya 565
16.4.3 Chobe National Park, Botswana 567
16.4.4 Kruger National Park, South Africa 568
16.5 Evidence from Long‐Term Perspectives 569
16.6 The Influence of High Densities of Individual Mesobrowser Species 570
16.7 Water, Nutrients, and Mesobrowsers 571
16.8 Synthesis 573
Acknowledgments 576
References 576
17 Megabrowser Impacts on Woody Vegetation in Savannas 585
Norman Owen‐Smith, Bruce Page, Gabriella Teren, and Dave J. Druce
17.1 Introduction 585
17.2 Use of Woody Plants Versus Grasses and Other Plant Forms 586
17.3 Selection for Size Classes and Woody Plant Parts 589
17.4 Plant Damage Imposed and Mortality 590
17.5 Plant Species Selected 592
17.6 Landscape Transformations Caused by Elephants, Along with Fire 599
17.7 A Cautionary Note 602
17.8 Overview 602
References 604
18 Indirect Effects of Browsing Herbivores in Savannas 613
Corli Coetsee, Dario Fornara, Antoinette Veldtman, and Benjamin Wigley
18.1 Introduction 613
18.2 Indirect Effects of Browsers on Other Fauna 614
18.2.1 Mammals 614
18.2.1.1 Large Herbivore Effects on Rodents 614
18.2.1.2 Mesobrowser Effects on Other Herbivores 615
18.2.1.3 Megaherbivore Effects on Mesoherbivores 616
18.2.1.4 Interactions Among Browsers Where the Type of Browser is Not Apparent 616
18.2.1.5 Interactions Among Megaherbivores 617
18.2.1.6 Summary 617
18.2.2 Birds 617
18.2.2.1 Summary 619
18.2.3 Reptiles and Amphibians 619
18.2.3.1 Summary 619
18.2.4 Invertebrates 619
18.2.4.1 Summary 621
18.3 Effects on Ecosystem Processes 622
18.3.1 Carbon Cycling 622
18.3.1.1 Consumption of Vegetation by Browsers Affects Ecosystem Carbon Pools 622
18.3.1.2 Changes in Litterfall Affect Soil Carbon 625
18.3.1.3 Global Change Can Override the Effects of Herbivory on Soil Carbon 625
18.3.1.4 Summary 626
18.3.2 Soil Nutrient Cycling and Soil Nutrient Pools 626
18.3.2.1 Changes in Litter Quality 626
18.3.2.2 Herbivore Effects on both Litter Quality and Quantity 629
18.3.2.3 Summary 629
18.4 Conclusions 629
References 630
Part IV Synthesis 643
19 Water Limitation, Fire, and Savanna Persistence: A Conceptual Model 645
Brian Beckage, Gabriela Bucini, Louis J. Gross, William J. Platt, Steven I. Higgins, Norma L. Fowler, Matthew G. Slocum, and Caroline Farrior
19.1 Introduction 645
19.2 Conceptual Model 646
19.2.1 Water Limitation 648
19.2.2 Fire 650
19.2.3 Fire Feedbacks 651
19.2.4 Other Processes 652
19.3 Summary 653
Acknowledgments 654 References 654
20 Savanna Ecosystem Models: What Should a Clever Modeler Code? 661
Gregory Kiker and Peter Frank Scogings
20.1 Introduction 661
20.2 Local‐Scale Aspects of Woody Plant–Browser Interactions 662
20.3 Model Designs for Plant–Herbivore Interactions 663
20.3.1 Plant‐Focused Models 666
20.3.2 Herbivore‐Based Models 668
20.3.3 Integrated Models: Adding Complexity into Plant–Herbivore Models 670
20.4 Discussion 672 References 674
21 Woody Plants and Large Herbivores in Savannas: Ancient Past – Uncertain Future 683
Peter Frank Scogings and Mahesh Sankaran
21.1 Introduction 683
21.2 Woody Plants 685
21.3 Large Herbivores 688
21.4 Interactions Between Woody Plants and Browsers 690
21.4.1 Adaptations of Woody Plants to Browsing by Mesobrowsers 690
21.4.2 Woody Community Responses to Mesobrowsers and Megaherbivores 692
21.4.3 Indirect Effects of Browsing 696
21.5 Models 698
21.5.1 General Conceptual (Qualitative) Models 698
21.5.2 Mathematical (Quantitative) Models 700
21.6 The Future 701 References 703
Index 713
List of Contributors
Sally Archibald Centre for African Ecology, School of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg, South Africa
David J. Augustine Rangeland Resources Research Unit, US Department of Agriculture – Agricultural Research Service, Fort Collins, CO, USA
Eduardo Barbosa Departamento Botânica, Universidade de Brasília, Brasília, Distrito Federal, Brazil
Jean‐Francois Barczi CIRAD, UMR AMAP, Montpellier, France
Brian Beckage Departments of Plant Biology and Computer Science, University of Vermont, Burlington, VT, USA
William J. Bond
South African Environmental Observation Network, National Research Foundation, Cape Town, South Africa
Fabian Borghetti Departamento Botânica, Universidade de Brasília, Brasília, Distrito Federal, Brazil
Gabriela Bucini Departments of Plant Biology and Computer Science, University of Vermont, Burlington, VT, USA
Simon Chamaillé‐Jammes Centre d’Ecologie Fonctionnelle et Evolutive, CNRS, Montpellier, France
Tristan Charles‐Dominique Center for Integrative Conservation, Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Menglun, Yunnan, China
Daryl Codron Department of Zoology and Entomology, University of the Free State, Bloemfontein, South Africa
Corli Coetsee SANParks, Scientific Services, Skukuza, South Africa and
School of Natural Resource Management, Nelson Mandela University, George, South Africa
Garry D. Cook
CSIRO Land and Water, Darwin, Northern Territory, Australia
Joris Cromsigt
Department of Wildlife, Fish, and Environmental Studies, Swedish University of Agricultural Sciences, Umeå, Sweden and Centre for African Conservation Ecology, Department of Zoology, Nelson Mandela University, Port Elizabeth, South Africa
Dave J. Druce
Ezemvelo KZN Wildlife, Hluhluwe‐iMfolozi Park, Hluhluwe, South Africa
Caroline Farrior
Department of Integrative Biology, University of Texas at Austin, Austin, TX, USA
Edmund C. February
Department of Biological Sciences, University of Cape Town, Cape Town, South Africa
Dario Fornara
Agri‐food and Biosciences Institute, Belfast, UK
Norma L. Fowler
Department of Integrative Biology, University of Texas at Austin, Austin, TX, USA
Juan H. Gowda
INIBIOMA, CONICET/Universidad
Nacional del Comahue, Bariloche, Río Negro, Argentina
Louis J. Gross
National Institute for Mathematical and Biological Synthesis, University of Tennessee, Knoxville, TN, USA
Gareth P. Hempson
Centre for African Ecology, School of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg, South Africa and
South African Environmental Observation Network (SAEON), Ndlovu Node, Kruger National Park, Phalaborwa, South Africa
Steven I. Higgins
Department of Botany, University of Otago, Dunedin, New Zealand
William Hoffmann
Department of Plant Biology, North Carolina State University, Raleigh, NC, USA
Grant Joseph
Percy FitzPatrick Institute of African Ornithology, Department of Biological Sciences, University of Cape Town, Rondebosch, South Africa and
Centre for Invasion Biology, School of Mathematical & Natural Sciences, University of Venda, Thohoyandou, South Africa
Gregory Kiker
Agricultural and Biological Engineering Department, University of Florida, Gainesville, FL, USA and
School of Mathematics, Statistics and Computer Science, University of KwaZulu‐Natal, Pietermaritzburg, South Africa
Caroline Lehmann
School of GeoSciences, University of Edinburgh, Edinburgh, UK
Norman Owen‐Smith
Centre for African Ecology, School of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg, South Africa
Bruce Page
School of Life Sciences, University of KwaZulu‐Natal, Westville, South Africa
William J. Platt
Department of Biological Sciences, Louisiana State University, Baton Rouge, LA, USA
Jayashree Ratnam
National Centre for Biological Sciences, Tata Institute of Fundamental Research, Bangalore, Karnataka, India
José Felipe Ribeiro
Núcleo de Recursos Naturais, Embrapa Cerrados, Planaltina, Distrito Federal, Brazil
Leandro Ribeiro
Departamento Botânica, Universidade de Brasília, Brasília, Distrito Federal, Brazil and
Instituto Federal de Educação, Ciência e Tecnologia do Ceará, Fortaleza, Ceará, Brazil
Mahesh Sankaran
National Centre for Biological Sciences, Tata Institute of Fundamental Research, Bangalore, Karnataka, India and
School of Biology, Faculty of Biological Sciences, University of Leeds, Leeds, UK
Melissa H. Schmitt
South African Environmental Observation Network (SAEON),
Ndlovu Node, Kruger National Park, Phalaborwa, South Africa
Peter Frank Scogings
School of Life Sciences, University of KwaZulu‐Natal, Pietermaritzburg, South Africa
Colleen Seymour
South African National Biodiversity Institute, Claremont, South Africa and
Percy FitzPatrick Institute of African Ornithology, Department of Biological Sciences, University of Cape Town, Rondebosch, South Africa
Chintan Sheth
National Centre for Biological Sciences, Tata Institute of Fundamental Research, Bangalore, Karnataka, India
Adrian M. Shrader
Mammal Research Institute, Department of Zoology and Entomology, University of Pretoria, Pretoria, South Africa
Matthew G. Slocum
Department of Biological Sciences, Louisiana State University, Baton Rouge, LA, USA
Carla Staver Ecology and Evolutionary Biology, Yale University, New Haven, CT, USA
Nicola Stevens
Department of Botany and Zoology, University of Stellenbosch, Stellenbosch, South Africa and
School of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg, South Africa
Anthony Swemmer
South African Environmental Observation Network, National Research Foundation, Phalaborwa, South Africa
Gabriella Teren
Centre for African Ecology, School of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg, South Africa
Wayne Twine
School of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg, South Africa
Marion Valeix
Laboratoire de Biométrie et Biologie Evolutive, CNRS, Villeurbanne, France
Antoinette Veldtman
Cape Nature, Jonkershoek, Stellenbosch, South Africa
Bruno Machado Teles Walter Herbário CEN, Embrapa Recursos Genéticos e Biotecnologia, Brasília, Distrito Federal, Brazil
David Ward
Department of Biological Sciences, Kent State University, Kent, OH, USA
Benjamin Wigley
School of Natural Resource Management, Nelson Mandela University, George, South Africa and
National Centre for Biological Sciences, Tata Institute of Fundamental Research, Bangalore, Karnataka, India
Richard J. Williams CSIRO Land and Water, Darwin, Northern Territory, Australia
Preface
“Savanna” has different meanings for different people, and the term has continued to elude a widely agreed definition (Lehmann and Parr 2016). “Savanna” continues to be used extensively to label any vegetation comprising a continuous herbaceous layer and a discontinuous woody layer of variable density and height (e.g. Furley 2016). However, in the past decade, it has become increasingly recognized that savannas are synonymous with “C4 grassy biomes” or “tropical grassy biomes” (e.g. Bond et al. 2008; Parr et al. 2014), i.e. biomes containing herbaceous vegetation dominated by graminoids using the C4 photosynthetic pathway. As such, savannas are largely tropical and sub‐tropical ecosystems, but are also found within warm temperate climates of North America and Asia (see Edwards et al. 2010). Although reference is occasionally made in this book to savannas where C3 grasses dominate the herbaceous layer, and to savannas that are seasonally flooded grasslands, the scope of this book is the majority of global savannas, which are C4 grassy ecosystems with a woody component.
The specific focus of this book is on the ecology of woody plants and associated herbivores in savannas. Advancement of the understanding of interactions between woody plants and browsing mammals in savannas, and the application thereof in models for savanna management, whether for biodiversity conservation or animal production purposes, has been neglected for far too long. Rather, to put it bluntly, it has been commonly assumed that all browsing mammals behave the same and all woody species respond in the same way to browsing, and that forage intake is inhibited by the same factors in both grasses and woody plants. For various reasons, books on savannas have tended to not include anything substantial about the interactions between woody plants and browsing mammals, except for the impacts of elephants on vegetation and how browsing affects the woody–grass interaction. One of these reasons is that, for a long time, the vast majority of understanding of browse–browser interactions was developed in other biomes, for example, boreal forests (Rooke et al. 2004). Hence, books on large herbivore ecology have also tended to contain very little information on browsing in savannas. This is a noteworthy omission from the literature, given the rich diversity of woody species and the abundance of large herbivores, whether domestic or wild, in savannas of the world.
This book is intended to complement, rather than compete with, other contemporary books on either savanna ecology or large herbivore ecology. The feature that distinguishes this book from others is its focus on the woody component of savanna ecosystems and, in particular, the woody plant–large herbivore interactions in savannas. A further defining feature of this book is the contrasts made among different systems: savannas
within continents, savannas on different continents, and, to some extent, savannas compared with other biomes where woody plants and browsing mammals occur. As such, lead authors were encouraged to (i) collaborate across continents to develop global perspectives; (ii) consider how development of concepts in other woody biomes could contribute to developing the understanding of savanna functioning; (iii) consider the roles of soils and climate (traditionally seen as the main determinants); and (iv) consider possible impacts of climate change. Inevitably, the extent to which these “terms of reference” where adhered varies from chapter to chapter; most chapters emphasize some aspects more than others.
Broadly, the first section comprises general chapters that introduce readers to contemporary views and debates about what savannas are, where they are found, and why they are important to understand, while being attentive to the woody component. Among the chapters about each continent, readers will note that each chapter has a different angle or focus, depending on the main direction that research on each continent has taken due to different circumstances among continents. So, for example, the chapter on African and Asian savannas places emphasis on determinants of savannas, including herbivory; the chapter on Australian savannas has a focus on Late Pleistocene human impacts, megafauna extinctions and recent introductions of ungulates (see Williams et al. [2017] for details of climate, soils, and vegetation of Australian savannas); the chapter on South American savannas is detailed in its descriptions of floristic composition; and the chapter on North American savannas places emphasis on the roles of fire and herbivory in recent management of savannas. All the continent‐specific chapters deal with the roles of resources (soils and climate) and fire, as well as human impacts, to greater or lesser extent, but the role of extant native browsers is limited to the chapter on Africa and Asia.
The second section comprises chapters that update readers about the ecology and evolution of animals that are closely associated with woody plants in savannas. The third section comprises chapters that focus on the ecology and evolution of woody plants in relation to browsing mammals in savannas. There is no single chapter devoted to the evolution of woody plants in savannas, as this is touched on in many of the chapters. The final section consists of chapters on modeling savannas for management purposes, and a concluding synthesis. Thus, when taken together, there is scope for inferring impacts of introduced, wild or domestic, browsers on different continents, as well as inferring impacts of browser removal from savannas. Such information is useful for managing conservation programs, as well as managing domestic livestock farming or feral animal control programs.
The expected readership is international and primarily includes advanced students, researchers, and academics in fields such as plant ecology, animal ecology, rangeland ecology, wildlife biology, conservation biology, and natural resource management. General readers might include geographers, evolutionary biologists, and informed members of the general public. For the reader, this book provides comprehensive insights into recent advances in the understanding of global savannas, especially in areas of research that have been neglected in other books, or are emerging. For example, substantial advances have been made in understanding drivers at the boundaries of savannas (Accatino and de Michele 2013; Rosatto et al. 2013). The recognition that savannas on different continents function differently is a significant recent advance (Lehmann et al. 2014). Yet, similarities between savannas and forests in certain aspects of their functioning are also emerging (Scogings et al. 2013; Churski et al. 2017). The
understanding of interactions between browsing mammals and woody plants in savannas is increasing. The important role of small‐ and medium‐sized mammal herbivores has recently been emphasized (Sankaran et al. 2013; O’Kane et al. 2014). Research into these interactions has started to follow new and exciting trajectories. For example, it has been recognized in recent years that better knowledge of the complex mixtures of secondary metabolites in woody plants, and their heritability, is needed to gain better understanding of such interactions (Wallis et al. 2012; de Gabriel et al. 2014). Understanding of the responses of plants to resources in savannas is also increasing (Tomlinson et al. 2013; Barbosa et al. 2014; Vadigi and Ward 2014). While updating the reader comprehensively, future directions for research are highlighted, as well as how concepts developed in one biome may be applicable in another, either as frameworks for future research, or in managing biomes for biodiversity conservation.
We are grateful for the efforts made by numerous reviewers who contributed to improving each chapter: Sally Archibald, David Augustine, Sumanta Bagchi, Daryl Codron, Garry Cook, Ben Cousins, Joris Cromsigt, Kevin Duffy, Johan du Toit, Augusto Franco, Hervé Fritz, Jake Goheen, Iain Gordon, Juan Gowda, Ricardo Holdo, Bill Hoffmann, Lindsay Hutley, Christine Janis, Felicia Keesing, Greg Kiker, Mike Lawes, Norman Owen‐Smith, Adam Pellegrini, Jayashree Ratnam, Christina Skarpe, Lisa Shipley, Julius Tjelele, Kyle Tomlinson, Joe Veldman, and Ben Wigley.
References
Accatino, F. and de Michele, C. (2013). Humid savanna–forest dynamics: a matrix model with vegetation–fire interactions and seasonality. Ecological Modelling 265: 170–179. Barbosa, E.R.M., van Langevelde, F., Tomlinson, K.W. et al. (2014). Tree species from different functional groups respond differently to environmental changes during establishment. Oecologia 174: 1345–1357.
Bond, W.J., Silander, J.A., Ranaivonasy, J., and Ratsirarson, J. (2008). The antiquity of Madagascar’s grasslands and the rise of C₄ grassy biomes. Journal of Biogeography 35: 1743–1758.
Churski, M., Bubnicki, J.W., Jęzdrzejewska, B. et al. (2017). Brown world forests: increased ungulate browsing keeps temperate trees in recruitment bottlenecks in resource hotspots. New Phytologist 214: 158–168.
De Gabriel, J.L., Moore, B.D., Felton, A.M. et al. (2014). Translating nutritional ecology from the laboratory to the field: milestones in linking plant chemistry to population regulation in mammalian browsers. Oikos 123: 298–308.
Edwards, E.J., Osborne, C.P., Strömberg, C.A.E. et al. (2010). The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science 328: 587–591.
Furley, P.A. (2016). Savannas: A Very Short Introduction. Oxford: Oxford University Press. Lehmann, C.E.R., Anderson, T.M., Sankaran, M. et al. (2014). Savanna vegetation‐fire‐climate relationships differ among continents. Science 343: 548–552.
Lehmann, C.E.R. and Parr, C.L. (2016). Tropical grassy biomes: linking ecology, human use and conservation. Philosophical Transactions of the Royal Society B 371: 20160329.
O’Kane, C.A.J., Duffy, K.J., Page, B.R., and Macdonald, D.W. (2014). Model highlights likely long‐term influences of mesobrowsers versus those of elephants on woodland dynamics. African Journal of Ecology 52: 192–208.
Parr, C.L., Lehmann, C.E.R., Bond, W.J. et al. (2014). Tropical grassy biomes: misunderstood, neglected, and under threat. Trends in Ecology and Evolution 29: 205–213.
Rooke, T., Danell, K., Bergström, R. et al. (2004). Defensive traits of savanna trees – the role of shoot exposure to browsers. Oikos 107: 161–171.
Rosatto, D.R., Hoffmann, W.A., de Carvalho Ramos Silva, L. et al. (2013). Seasonal variation in leaf traits between congeneric savanna and forest trees in central Brazil: implications for forest expansion into savanna. Trees 27: 1139–1150.
Sankaran, M., Augustine, D., and Ratnam, J. (2013). Native ungulates of diverse body sizes collectively regulate long‐term woody plant demography and structure of a semi‐arid savanna. Journal of Ecology 101: 1389–1399.
Scogings, P.F., Hjältén, J., and Skarpe, C. (2013). Does large herbivore removal affect secondary metabolites, nutrients and growth in woody species in semi‐arid savannas?
Journal of Arid Environments 88: 4–8.
Tomlinson, K.W., Poorter, L., Sterck, F.J. et al. (2013). Leaf adaptations of evergreen and deciduous trees of semi‐arid and humid savannas on three continents. Journal of Ecology 101: 430–440.
Vadigi, S. and Ward, D. (2014). Herbivory effects on saplings are influenced by nutrients and grass competition in a humid South African savanna. Perspectives in Plant Ecology, Evolution and Systematics 16: 11–20.
Wallis, I.R., Edwards, M.J., Windley, H. et al. (2012). Food for folivores: nutritional explanations linking diets to population density. Oecologia 169: 281–291.
Williams, R.J., Cook, G.D., Liedloff, A.C., and Bond, W.J. (2017). Australia’s tropical savannas: vast, ancient and rich landscapes. In: Australian Vegetation (ed. D. Keith), 368–388. Cambridge: Cambridge University Press.
Part I Introduction
Distribution and Determinants of Savannas
Sally Archibald1, William J. Bond2, William Hoffmann3, Caroline Lehmann4, Carla Staver5, and Nicola Stevens6,7
1 Centre for African Ecology, School of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg, South Africa
2 South African Environmental Observation Network, National Research Foundation, Cape Town, South Africa
3 Department of Plant Biology, North Carolina State University, Raleigh, NC, USA
4 School of GeoSciences, University of Edinburgh, Edinburgh, UK
5 Ecology and Evolutionary Biology, Yale University, New Haven, CT, USA
6 Department of Botany and Zoology, University of Stellenbosch, Stellenbosch, South Africa
7 School of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg, South Africa
1.1 Introduction
Savannas are notoriously difficult to define and map – both because they are very variable in structure, and because their dynamics are influenced by a range of top‐down and bottom‐up processes. White (1983) chose not to use the term at all – preferring terms that described the physiognomy such as “grasslands,” “wooded grasslands,” “grassy woodlands,” and “bushlands.” However, the term “savanna” persists and continues to be used by people trying to understand the global distribution of vegetation, probably because there are some similarities in the functioning and evolutionary origins of these mixed woody–grass systems that make it useful to compare and contrast them. Publications with the term savanna (or savannah) used in them have grown at twice the background rate since the 1990s (Figure 1.1) for two possible reasons: (i) increasing use of this term to describe mixed woody–grass systems, and (ii) increasing interest in these systems as modelers start to try simulating the dynamics of the globe in Earth system models.
Savannas cover large extents of the terrestrial biosphere, and African savannas are the birthplace of modern humans (Chapter 6). Their dynamics (both seasonally and inter‐annually, as well as over longer time scales) are particularly difficult to describe and predict for reasons that will be discussed below. In this chapter we summarize current thinking on the factors affecting the global distribution of savannas and especially the limiting factors at their boundaries. In this regard we aim to highlight points of contention and their possible resolution. We make comparisons across continents and between temperate and tropical savannas, hoping to isolate some general patterns and rules that persist despite their varied histories and climates. We also discuss some of the current
Savanna Woody Plants and Large Herbivores, First Edition. Edited by Peter Frank Scogings and Mahesh Sankaran.
© 2020 John Wiley & Sons Ltd. Published 2020 by John Wiley & Sons
Figure 1.1 Publications using the word savanna (or savannah) have increased rapidly since the 1990s compared with the background rate of increase in publications in all journals in similar fields. Data collated from Web of Science covering 1945 to 2016. Calculated as the percentage of all publications for that year. The background rate was calculated by summing the publications in the five fields where work on savannas is published: ecology, environmental sciences, plant sciences, forestry, and zoology.
conservation and management threats to savannas. Thus, this chapter sets the scene for more detailed descriptions of savannas, and the processes driving them, in subsequent chapters.
1.2 Evolutionary History of Savanna Vegetation and Fauna
Grassy ecosystems spread across the globe 11–24 million years ago (Jacobs et al. 1999). The original grass flora used the C3 photosynthetic pathway to fix carbon. However, grass species with the C4 photosynthetic pathway replaced these grasses in warm, seasonallyarid places and expanded further into previously wooded ecosystems about 8 million years ago (Edwards et al. 2010; Keeley and Rundel 2005). Estimates vary, but current grassy systems cover ~25% of the land surface (Ramankutty and Foley 1999), with C4‐dominated grassy systems contributing more than half of this (Figure 1.2). About 21% of global net primary production is estimated to come from C4 grasses alone (Lloyd et al. 2008).
There is no clarity on the conditions that enabled this rapid and fundamental change in the biology of the Earth system (Edwards et al. 2010); both of these shifts (from forest to C3 grass and from C3 grass to C4 grass) were roughly correlated across the globe, but the exact timing on different continents varied by several million years (Jacobs et al. 1999). Moreover, atmospheric CO2 levels were already low and remained low throughout this period of expansion, so low CO2 probably enabled, but did not precipitate, the spread of grasslands (Edwards et al. 2010; Osborne and Beerling 2006). Expansion of C3 grasslands has been associated with both increased and decreased aridity, decreased seasonality, and increased herbivory (Charles‐Dominique et al. 2016; Strömberg 2005) – indicating strong biogeographic contingencies in the drivers of their spread. There is more information on the drivers of the spread of C4 grasslands (Keeley and Rundel

Figure 1.2 Broad distinction between C4 and C3 grassy systems, using classification of Edwards et al. (2010): Orange = C4 grassy vegetation, Yellow = C3 grassy vegetation, Green = forest, Purple = croplands, Gray = sparse vegetation, bare ground, or ice. Note that the extensive spinifex C4 grasslands in central Australia are classified as sparse vegetation in this map; although they can accumulate substantial biomass, they are functionally more similar to arid shrublands than savannas.
Source: Data from International Satellite Land Surface Climatology Project (ISLSCP, http://catalogue. ceda.ac.uk/uuid/5a226b1468ca4fc1ace5e76815a1a4de). (See color plate section for the color representation of this figure.)
2005; Osborne and Beerling 2006). This was associated both with increased aridity (the expansion of certain C4 grass clades into dry environments) and with increased fire and rainfall seasonality (the expansion of other C4 grass clades into wetter environments).
These confusing origins are perhaps to be expected, given that these ecosystems are thought to be non‐deterministic (Bond et al. 2003a; Sankaran et al. 2005; Staver et al. 2011), that is, not clearly defined or controlled by the climatic limits of the dominant life‐forms, but by complex interactions among climate, soils, plants, animals, and fire. Thus, although the most commonly accepted definition of savanna is a discontinuous tree layer with a continuous grassy understory (Scholes and Archer 1997), to which some people add C4 grass physiology as a requirement (see below), closed canopy formations exist and are possible throughout the environmental space occupied by savannas (Lehmann et al. 2011).
Importantly, savannas are a recent phenomenon in evolutionary terms, being ecosystems that arose long after the continents separated. Thus, although structurally similar, savanna ecosystems on different continents are floristically distinct. These floristic differences are most noticeable in the woody layer – totally different families dominate on different continents (Dexter et al. 2015), but the grass layer also varies, as evidenced by the dramatic floristic and functional changes that occur when grasses from one savanna ecosystem invade on other continents (Visser et al. 2016). Common African savanna woody plants are mimosoid legumes from the Caesalpiniodeae clade (previously acacias), broad‐leafed Detariodeae legumes, as well as many Combretaceae (Chapter 2). In South America dominant tree clades are Papillionoideae and Detariodeae legumes, but from very different genera, and the Vochysiaceae family also dominates (Françoso et al. 2016; Chapter 4). In Australian savannas Eucalyptus and other Myrtaceae dominate
(Chapter 3). Recent papers have highlighted how these floristic differences can result in different functional responses and trajectories under global change (Lehmann et al. 2014; Moncrieff et al. 2016) implying that we need more understanding of the varied histories and determinants of savanna ecosystems globally.
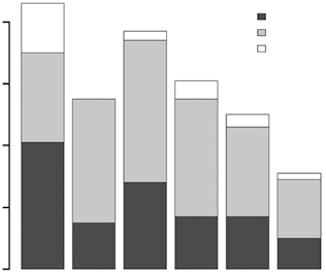
The mammalian herbivore communities with which these systems evolved were also distinct, evolving from very different clades on each continent – for example, equids in North America, bovids in Africa and Eurasia, macropods and other diprotodonts in Australia (Jacobs et al. 1999). A snapshot of herbivore communities before the Pleistocene extinctions ~40 000–10 000 years ago shows that South America had almost double the megaherbivore species (>1000 kg) of any other continent, and fewer small herbivores (Figure 1.3). Moreover, grazers made up 60% of the mammalian herbivore species in Africa, but were only 27–38% of the species on other continents (Figure 1.3). These data give no indication of the abundances of these herbivore species, but certainly indicate that the savanna vegetation on each continent was exposed to very different top‐down stresses. For example, grazers, and browsers impact different plant functional types and have opposite relationships with fire, the other consumer in the system (Archibald and Hempson 2016; Chapter 13).
Information on past fire regimes is much less rich, but to the extent that grassy fuels impact fire one would expect somewhat different fire regimes on the continents based on the traits of their grass communities and based on the changes in fire regimes that occur when non‐native grasses invade other continents (Rossiter et al. 2003; Veldman 2016).
Relative to humans, however, savannas are ancient systems; all the evidence suggests that savannas gave rise to humans, not the converse (Cerling et al. 2011). Nevertheless, human activities can clearly affect the extent and functioning of savannas – both by expanding their extent into tropical forests (Silverio et al. 2013) and by causing encroachment of forest into savanna (Honda and Durigan 2016), not to mention conversion of
Figure 1.3 Large herbivore fauna of the globe before the late Pleistocene extinction wave (Source: Data from Owen‐Smith 2013). (a) Number of grazing vs browsing species by continent. Africa is a clear outlier with a higher grazer: browser ratio than other continents. (b) Number of herbivore species in three size classes by continent. South America had more megaherbivore species and very few small herbivores (10–100 kg), while Australia was the opposite.