Structural Adhesive Joints Design,
Analysis and Testing
Edited by K.L. Mittal and S. K. Panigrahi
This edition first published 2020 by John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, USA and Scrivener Publishing LLC, 100 Cummings Center, Suite 541J, Beverly, MA 01915, USA © 2020 Scrivener Publishing LLC
For more information about Scrivener publications please visit www.scrivenerpublishing.com.
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, except as permitted by law. Advice on how to obtain permission to reuse material from this title is available at http://www.wiley.com/go/permissions.
Wiley Global Headquarters
111 River Street, Hoboken, NJ 07030, USA
For details of our global editorial offices, customer services, and more information about Wiley products visit us at www.wiley.com.
Limit of Liability/Disclaimer of Warranty
While the publisher and authors have used their best efforts in preparing this work, they make no representations or warranties with respect to the accuracy or completeness of the contents of this work and specifically disclaim all warranties, including without limitation any implied warranties of merchantability or fitness for a particular purpose. No warranty may be created or extended by sales representatives, written sales materials, or promotional statements for this work. The fact that an organization, website, or product is referred to in this work as a citation and/or potential source of further information does not mean that the publisher and authors endorse the information or services the organization, website, or product may provide or recommendations it may make. This work is sold with the understanding that the publisher is not engaged in rendering professional services. The advice and strategies contained herein may not be suitable for your situation. You should consult with a specialist where appropriate. Neither the publisher nor authors shall be liable for any loss of profit or any other commercial damages, including but not limited to special, incidental, consequential, or other damages. Further, readers should be aware that websites listed in this work may have changed or disappeared between when this work was written and when it is read.
Library of Congress Cataloging-in-Publication Data
ISBN 978-1-119-73643-1
Cover image: Pixabay.Com
Cover design by Russell Richardson
Set in size of 11pt and Minion Pro by Manila Typesetting Company, Makati, Philippines
6.2
6.2.1
6.4
6.2.2
6.3.1
6.3.3
6.4.1
6.4.2
6.4.3
6.2.2.1
6.2.2.2
7
6.5
8
Rashmi Ranjan Das
7.1
7.2
8.5.1
8.5.2
8.5.3
9
10.8 Study of Crack Growth Arrest Mechanisms with Z-Fibre Pins in Composite Laminated Joints
10.9 Modelling and Analysis of Skin-Stiffener Joints with Z-Fiber Pins at Embedded Delamination
10.9.1 Estimation of Crack Growth Arrest (a) with Single Row of Z-Fiber Pins Reinforcement (b) with Multiple Rows of Z-Fiber Pins Reinforcement (c) Influence of Diameter and Space in between the Reinforced Pins on Fracture Toughness of the Composite Laminated Joint
Preface
Among the myriad techniques available for mating/joining similar and dissimilar materials (metals, plastics, glass, ceramics and composites) the adhesive bonding technology is preferred and widely used because it offers many advantages vis-a-vis the other methods of joining, e.g., mechanical fastening, riveting, nailing, brazing and welding.
Adhesive bonding is used for mundane (gluing of toys) to highly sophisticated applications. Most structures are comprised of a number of individual parts or components which have to be connected to form a system with integral load transmission path. The structural adhesive bonding represents one of the most enabling technologies to fabricate most complex structural configurations involving advanced materials (e.g., composites) for load-bearing applications.
Even a cursory look at the literature will evince that there is a brisk activity in all relevant aspects to enhance the performance and durability of structural adhesive joints. Recently there has been activity in harnessing nanotechnology (use of nanomaterials) in ameliorating the existing or devising better performing structural adhesives. It should be emphasized that proper (adequate) surface preparation is sine qua non for high joint strength. Concomitantly, depending on the surfaces to be bonded, there is much interest in coming up with environmentally-benign (green) surface cleaning and modification techniques. Apropos, surface contaminants are a bête noire to an adhesive bond. Also, there is much interest in modeling and simulation of structural adhesive joints.
The book comprising 10 chapters written by eminent researchers is divided into two parts: Part 1: General Topics and Part 2: Analysis and Testing. The topics covered include: surface preparation for structural adhesive joints; use of nanoparticles in enhancing performance of structural adhesive joints; optimization of structural adhesive joints; durability aspects of structural adhesive joints; debonding of structural adhesive joints; fracture mechanics of structural adhesive joints; failure analysis of structural adhesive joints; damage behavior in functionally graded
structural adhesive joints; impact shock and vibration characteristics of composites for structural adhesive joints; and delamination arrest methods in structural adhesive joints. It should be recorded here that all chapters were rigorously reviewed and all were suitably revised (some twice or thrice). So the material presented in this book is of archival value and meets the highest standard of publication.
The book consolidates in an easily accessible volume the current state of structural adhesive joints and reflects the cumulative wisdom of a number of researchers actively engaged in the arena of structural adhesive joints. The book is profusely referenced and copiously illustrated. The book should be of immense interest to those involved in the mechanics of adhesive joints, adhesive bonding, and structural adhesive joints. Also the book should be of much use to adhesionists, materials scientists, polymer scientists and those working in construction, railway, automotive, aviation, bridge, and ship industries. As more advanced adhesives and adherend materials become available, the applications and use of structural adhesive joints will proliferate.
Now it is our pleasant task to thank all those who were instrumental in making this book possible. Obviously, first and foremost our sincere and heartfelt thanks go to the authors for their keen interest, sustained enthusiasm, unwavering cooperation and sharing their valuable research experience in the form of written accounts without which this book would not have seen the light of day. We will be remiss if we fail to extend our thanks to Martin Scrivener (publisher) for his steadfast interest in and wholehearted support for this book endeavor.
Kash Mittal P.O. Box 1280 Hopewell Jct., NY 12533
E-mail: ushaRmittal@gmail.com
S. K. Panigrahi Defence Institute of Advanced Technology Pune, India
4 Structural Adhesive Joints
1.1 Introduction
Structural adhesives can be considered as high strength glues, used to hold two substrate surfaces with load-bearing permanent bonds [1]. The structural adhesives offer high modulus, high strength, and flexible bonds to produce lightweight materials [2] capable of maintaining structural integrity even under high stress. This cost-effective method of fabricating stress-bearing materials also provides the advantage of protection against corrosion [3]. The structural adhesives help in maintaining structural integrity of the materials by their ability of transmitting structural stress across the interface when subjected to high loads. The bonding of substrate surfaces using structural adhesives may result due to van der Waals forces, electrostatic attraction, or chemical bonding arising between the surfaces and adhesives (Figure 1.1).
The structural adhesives are generally in liquid form during application between the surfaces, giving wettability to the surfaces followed by curing, thus resulting in rigid, high molecular weight and crosslinked attachment between adhesive and adherend. The structural adhesives are used in aircraft, ships, medical/dental, automotive, domestic appliances, electronics, and construction applications because they provide excellent loadbearing capability [4, 5]. The most widely used structural adhesives include silicones, epoxies, acrylics, urethanes, etc. [5]. The substrates that can be joined using structural adhesives include metals, composites, rubber, plastics, biomaterials (wood and honeycomb panels), etc. [6]. The binding and fabrication of materials using structural adhesives depends on a variety of factors including selection of suitable adhesive, surface preparation of adherend, quality control, proper joining design, etc. Out of all these requirements, surface preparation of the materials to be joined is an important aspect in the field of adhesive bonding. The surface preparation
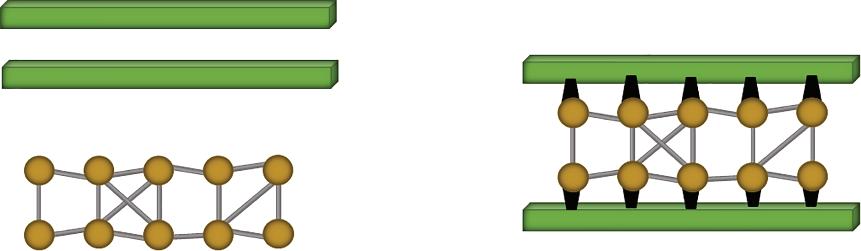
Figure 1.1 Interaction between adhesive and substrates.
of materials prior to the application of structural adhesives is preferred because of the following reasons [6]:
• To ensure the formation of strong layer on the surface;
• To provide the maximum substrate surface for optimum molecular interaction between the adhesive and adherend;
• To ensure achievement of significant joint strength for better stress transmittance across the interface;
• To create specific voids/cavities/microstructures or to alter the chemical structure of surface in order to alter the surface free energy for proper adhesive bonding.
The basic surface preparation methods employed prior to the application of structural adhesive include degreasing, mechanical abrasion, and chemical treatments. The degreasing of joint surfaces works by removing the traces of oil and grease contaminants and providing clean and contaminant-free surface to be joined. The degreasing of the joint surfaces can be done via multiple ways such as vapor degreasing, ultrasonic degreasing, and immersion/ spray/solvent wipe degreasing. Another surface preparation method with the advantages of simplicity and ease of performance is the mechanical abrasion. Mechanical abrasion methods (like grit blasting) remove most of the surface deposits (metal deposits such as tarnish, rust and mill scale) and provide joint materials with rough surfaces in order to enable the formation of strong bonds. The mechanical abrasion method is always followed by degreasing to remove the loose materials produced on the abraded material. The chemical treatment or electrolytic pretreatment is another surface preparation method which results in the joint surfaces with optimum strength, reproducibility and resistant to damage. Chemical treatment methods like acid etching work by altering the chemical morphology and composition of the joint surfaces which results in the elevation of the surface free energy, a favorable condition for the formation of high-strength bonds. Chemical treatment of joint surfaces also raises the probability of chemical bonds (van der Waals, covalent, hydrogen, polar, etc.) formation at the substrate – adhesive interface. The alternative surface preparation methods to the chemical treatment provide the same surface modifications but produce less harmful waste materials are corona, plasma and flame treatment methods. These surface treatment methods are always preferred and performed prior to the application of structural adhesives to obtain the optimum load-bearing and high strength bonds [6, 7]. The surface preparation method for the treatment of adherend surface is highly dependent on the nature of adhesive as well as adherend.
6 Structural Adhesive Joints
The surface preparation methods used for the metals include degreasing, grit blasting, acid etching, anodization, etc. The surface preparation methods for the plastics are corona treatment, flame treatment, chemical etching, etc. and for the fluoroplastics, the chemical etching and grit blasting are generally performed. This chapter aims to describe distinct theories of adhesion (mechanical interlocking, electrostatic, diffusion, wetting, chemical bonding, and weak boundary layer), surface preparation methods that include degreasing (vapor degreasing, ultrasonic vapor degreasing, ultrasonication/ immersion/spray/wiping), chemical treatment (acid etching, anodization, etc.), mechanical abrasion (grit blasting), and other physical methods (flame treatment, corona treatment, plasma treatment). This is followed by various surface evaluation tests like dyne test, water-break test, contact angle test, etc. The chapter also covers a wide range of applications and future scope of structural adhesives.
1.2 Theories of Adhesion
The phenomenon of adhesion has been described by various theories, explaining the mode of action by which adhesives bond adherends. These theories of adhesions include mechanical theory (mechanical interlocking), diffusion theory, electrostatic (electronic) theory, and adsorption/ surface reaction theory (wetting/chemical bond/acid - base interactions), etc. [8, 9]. These theories of adhesion are discussed as follows:
1.2.1 Mechanical Interlocking
The mechanical interlocking theory postulates that the adhesion occurs due to the weaving of adhesive into the microstructures (pores, valleys, cavities, etc.) present on the surfaces of adherends. These irregularities arise by mechanical abrasion of joint surfaces which enhances the bond strength formed between two joint surfaces as shown in Figure 1.2.
Figure 1.2 Mechanical interlocking mechanism on an abraded surface.
The mechanical interlocking theory is supported by the fact that a better adhesion is achieved on the abraded/roughened surfaces as compared to adhesion between polished/smooth surfaces. This improved adhesive bonding after abrasion may also occur due to the increased contact area at the interface or formation of highly reactive surface after abrasion. But, this theory of adhesion fails because of the fact that good adhesion can also be achieved between smooth adherends. Also, the application of mechanical abrasion is not suitable on ductile material surfaces because it deteriorates the strong bond formation and makes material more susceptible to damage under unfavorable environmental conditions [10–13].
1.2.2 Electrostatic (Electronic) Theory
The electrostatic theory of adhesion is based on the phenomenon of electron transfer between two materials (adhesive and adherend) with different electronic band structures [14–16]. This transfer of charges at the interface results in the formation of an electrical double layer, and the generated electrostatic forces provide the resistance to the separation of joint surfaces (Figure 1.3).
This theory of adhesion also gets support from the observation of discharge when the adhesive is peeled off from the adherend surface [10]. However, the electrostatic interaction is relatively weak and not applicable to all substrates.
1.2.3 Diffusion Theory
The diffusion theory of adhesion is generally applicable to polymeric adherends having sufficiently long polymeric chains with the ability of movement. The diffusion theory assumes that adhesives weaves into the adherend through interdiffusion mechanism. This can be perceived as entanglement of adhesive and adherend chains providing inseparable forces for the joint surfaces as shown in Figure 1.4.
Figure 1.3 Electrical double layer formation across adhesive/adherend interface.
Substrate
Interdi usion
The bond strength depends on the matching of solubility parameters between adhesives and adherends. The higher is the matching of solubility parameters between adhesives and adherends, the higher is the bond strength [10]. The cohesive energy density (CED) can interpret the diffusion bonding. The cohesive energy density (CED) and solubility parameter are given by equations (1.1) and (1.2).
Where, Ecoh = Total energy required to separate molecules
V = Molar Volume
δ = Solubility Parameter
The interdiffusion of adhesives into adherends as well as the bond strength increase at elevated temperature, e.g., the adhesion of polyethylene and polypropylene with butyl rubber increases rapidly above the melting temperatures of both polymers, i.e., 135 °C for polyethylene and 175 °C for polypropylene [17].
1.2.4 Wetting Theory
The wetting theory postulates that a continuous molecular contact between two materials and the resultant forces at the interface provide good adhesive bond strength. An ideal adhesive should have good wetting property which requires lower surface tension of adhesive than the critical surface tension of the joint surfaces. A proper wetting is achieved when the applied adhesive flows into the irregularities like valleys and holes present on joint surfaces and in the case of poor wetting, the adhesive forms air bubbles,
Adhesive
Figure 1.4 Diffusion mechanism of adhesion.
and thus reduces the actual contact area between the adhesive and joint surfaces [8, 10, 18]. Figure 1.5 shows the concept of poor wetting and good wetting across the interface.
The wetting between adhesive and adherend can be improved by some surface preparation techniques, such as application of a primer layer which is a solution of adhesive and organic solvent. Both surface topography and surface chemistry are responsible for the wetting behavior of adherend. There are distinct wetting regimes which describe wetting in different manners. These wetting regimes include Wenzel, Cassie-Baxter, and impregnated Cassie wetting regimes. As per the Wenzel regime, when a liquid drop contacts the adherend rough surface, the liquid completely infuses into the pores and only surface roughness is responsible for the increase in contact angle (for contact angle greater than 90° on a smooth surface). This is described by Wenzel’s equation for the contact angle on a rough surface given by
Where, θ = Contact angle on the rough surface
Rf = Roughness factor (Ratio of actual to projected area)
θY = Young’s contact angle on smooth surface
According to the Cassie-Baxter regime, when liquid does not infuse into the pores, air pockets are formed at the adhesive/adherend interface which further increase the contact angle on the surface given by the Cassie-Baxter equation:
Where, fSL= Fraction of projected area constituting solid-liquid interface. But, when the partial infusion of liquid in the pores occurs then a contact angle between Wenzel and Cassie-Baxter is formed and this is called
Figure 1.5 Wetting behavior of adhesives: (a) good wetting and (b) poor wetting.
impregnating Cassie wetting regime. The contact angle in this case is given by [19–21];
Cos θ = 1 + fSL (Cos θY – 1) (1.5)
The proper wetting is always preferred to impart good adhesion with permanent high strength bonds. This can also be achieved using suitable surface preparation methods.
1.2.5 Chemical Bonding Theory
The chemical bonding theory is based on the formation of chemical bonds such as hydrogen bonds, covalent bonds, ionic bonds, van der Waals bonds, and acid-base interactions. Out of all these chemical bonds, covalent bond is the strongest and provides much better adhesion in comparison to the secondary forces/bonds [22]. The acid-base theory of adhesion is based on the polar interaction between Lewis acids (electron-deficient materials) and Lewis bases (electron-rich materials), e.g., interaction between BF3 and NH3 is an acid-base interaction. In BF3, the electronegative fluorine displaces the electrons far from boron, which imparts positive charge on boron and negative charge on fluorine. Similarly, NH3 is a Lewis base and nitrogen has negative charge. Therefore, positive boron and negative nitrogen can interact via acid-base interaction [23, 24].
1.2.6 Weak Boundary Layer Theory
Weak boundary layer assumes that cohesive failure and weak boundary layers are the causes by which bond failure occurs [25]. The weak boundary layers can be formed in adherend as well as in adhesive and the contaminants present on the surfaces are responsible for their formation. There are some materials essentially having weak boundary layer problem, e.g., polyethylene and metal oxides. In polyethylene, the low molecular weight components are distributed in the polymer resulting in low-stress failure when PE is used either as adhesive or adherend. Similar problem is associated with the certain oxides (like aluminum oxide). The weak boundary layers can be formed in adhesive or adherend, and unfavorable bonding environment as well as improper wetting are the causes of their formation. In case of improper wetting at the interface, the air gets trapped in the adherend valley, reducing actual contact area, and thus decreasing the joint strength. The weak boundary layer problem can be overcome via various surface preparation methods [10, 23].
1.3 Surface Preparation Methods
The proper adhesion of adhesive with the joint surfaces requires a variety of surface preparation techniques prior to the application of adhesive. These surface preparation methods remove the contaminants like dirt, oil, grease, paint coatings, rust, tarnish, etc., and thus allow the actual contact area of adherend to interact with the applied adhesive. If these surface preparation methods are not performed then the contaminants may remain attached to adherend surface, resulting in improper wetting, and hence, weak boundary formation may take place which may cause the bond failure during the prolonged service period of the material. As mentioned earlier, there
Table 1.1 Various surface preparation methods for structural adhesives [10].
Surface preparation methods Working principle
Degreasing Vacuum degreasing
Ultrasonic vapor degreasing
Immersion/ wiping/spray
Mechanical abrasion
Chemical treatment
Grit blasting
Acid etching
Using vapors of degreasing solvents which condense on materials, solubilize contaminants and then drip off
Vapor degreasing followed by ultrasonication
Immersing adherend in solvent, or wiping off contaminants by spraying solvent on adherend
Impinging hard grits like Al2O3 on adherend’s surface
Immersing material in acids to obtain cavities and holes
Anodization Formation of coatings using the application of voltage between anode and cathode
Physical methods Corona treatment
Collision of the stream of charged particles driven by electric field with adherend surface
Flame treatment Use of oxidizing flame to treat the surface
Plasma treatment
Collision of plasma obtained by ionizing the air/gas (by applying voltage at the electrode) with adherend
12 Structural Adhesive Joints
are several surface preparation methods (Table 1.1) which are selected depending on the nature of adhesive as well as adherend. In addition to the adhesive-adherend nature, environmental factors as well as processing parameters play an important role in the selection [7]. This section describes different surface preparation methods in detail.
1.3.1 Degreasing
Degreasing is the basic cleaning method applied to the joint surfaces before application of adhesives. There are distinct ways by which degreasing method removes the light as well as heavy contaminants from the adherend surfaces. The degreasing process is generally done before the physical and chemical modifications of the surfaces as this helps in the improvement of bond strength. The degreasing removes dust, grease, oil, paints, particulate matter, etc., and exposes the actual surface and adhesion receptive sites for the adhesive. The main degreasing methods include vapor degreasing, ultrasonic vapor degreasing, and other (immersion/spray/solvent wipe/ ultrasonication) degreasing methods [7, 26]. The SEM image of aluminum degreased with soap is shown in Figure 1.6. These degreasing methods are described as follows.
1.3.1.1 Vapor Degreasing
Vapor degreasing method can be applied to both metallic as well as non-metallic adherends to remove the waxes, oil, grease, soil, etc. In vapor degreasing method, vapors of the cleaning solvent are directed towards the
Figure 1.6 SEM Image of degreased aluminum surface. Reprinted with the permission from [64]. Copyright 2018 Elsevier.
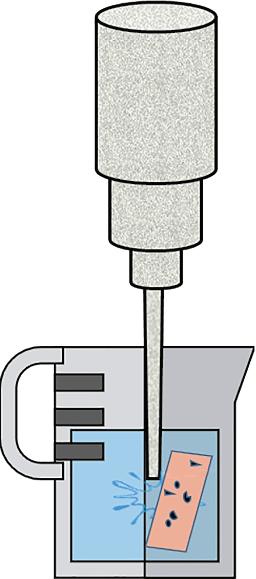
surface to be cleaned. The condensed drops of the solvents then solubilize the contaminants which then drip off with the condensed solvent drops (Figure 1.7). If there is a thick coating of contaminants on adherend surface which is hard to clean using vapor degreasing method then acetone scrubbing is performed first followed by the vapor degreasing method [10]. The vapor degreasing solvents are 1,1,1-trichloroethane (methyl chloroform), dichloromethane (methylene chloride), tetrachloroethylene (perchloroethylene), trichloroethylene, trichloro trifluoroethane, etc. Out of all these degreasing solvents, methyl chloroform and methylene chloride (DCM) are banned due to their high level of toxicity. Tetrachloroethylene and trichloroethylene are the most widely used solvents for the vapor degreasing process [27]. These solvents provide a wide range of boiling temperatures such as DCM has boiling point equivalent to 39°C and perchloroethylene has a boiling point equivalent to 121°C. Taking the toxicity levels of these chemicals into consideration, some ecofriendly degreasing solvents have been synthesized under the trade names of ventrel CMS/ SMT, EnSolv, HFE-7100, etc. [10]
1.3.1.2 Ultrasonic Vapor Degreasing
The ultrasonic vapor degreasing method provides the benefits of vapor degreasing as well as ultrasonication. The surfaces to be joined are first subjected to vapor degreasing followed by submerging in a suitable solvent and
Heating Jacket
Cooling System
Figure 1.7 Vapor degreasing method.
14 Structural Adhesive Joints
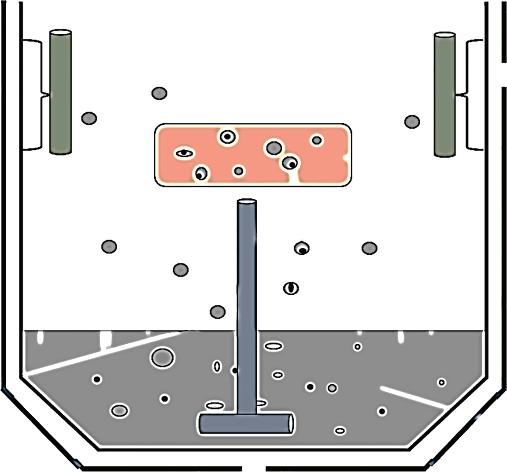
then ultrasonicating it. The ultrasonication transmits the high frequency sonic waves via solvent, thus agitating and causing cavitation (formation and collapse of bubbles in the solvent). This leads to the transmittance of energy to the contaminants present on the surface as shown in Figure 1.8. This method of degreasing is the most effective among all as it has the power of detaching contaminants even from cavities and holes. The frequency to be used for the ultrasonication generally ranges from 20,000 cycles/s to 50,000 cycles/s and the selection of particular frequency depends on various factors including adherend surface, contaminants on adherend as well as ultrasonication solvent [7, 10].
1.3.1.3 Other Degreasing Methods
The other degreasing methods include immersion, solvent wiping, spray, and ultrasonication method. The ultrasonication method involves immersion of the material and then allows the transmittance of sonic waves that provides sufficient energy for the removal of contaminants. This method is less effective than other methods, but still it is suitable and sufficient for the removal of light contaminants. Solvent wiping is the simplest and straightforward among all the degreasing methods. Solvent wiping uses different chemicals which are poured just once (instead of complete immersion) on the tissue/ wipe followed by scrubbing the joint surfaces. This process is repeated a few
Ultrasonicator Probe
Piezoelectric Probe Supplying Sound Energy
Figure 1.8 Ultrasonication surface preparation method.