TiO2 Nanoparticles
ApplicationsinNanobiotechnologyandNanomedicine
EditedbyAiguoWuandWenzhiRen
Editors
Prof.AiguoWu
CixiInstituteofBiomedicalEngineering NingboInstituteofMaterials TechnologyandEngineering ChineseAcademyofSciences No.1219ZhongguanWestRoad 315201Ningbo,Zhejiang China
Dr.WenzhiRen
CixiInstituteofBiomedicalEngineering
NingboInstituteofMaterials TechnologyandEngineering ChineseAcademyofSciences No.1219ZhongguanWestRoad 315201Ningbo China
Cover: Streamingbloodcells:Shutterstock ID2008770/SebastianKaulitzki
Microscopicviewofpancreaticcancer Cells:StocktrekImages/GettyImages 506837559
Bacteriaillustration:Eraxion/ iStockphoto8493122
Allbookspublishedby Wiley-VCH arecarefullyproduced.Nevertheless, authors,editors,andpublisherdonot warranttheinformationcontainedin thesebooks,includingthisbook,to befreeoferrors.Readersareadvised tokeepinmindthatstatements,data, illustrations,proceduraldetailsorother itemsmayinadvertentlybeinaccurate.
LibraryofCongressCardNo.: appliedfor
BritishLibraryCataloguing-in-Publication Data
Acataloguerecordforthisbookis availablefromtheBritishLibrary.
Bibliographicinformationpublishedby theDeutscheNationalbibliothek TheDeutscheNationalbibliotheklists thispublicationintheDeutsche Nationalbibliografie;detailed bibliographicdataareavailableonthe Internetat <http://dnb.d-nb.de>
©2020Wiley-VCHVerlagGmbH& Co.KGaA,Boschstr.12,69469 Weinheim,Germany
Allrightsreserved(includingthoseof translationintootherlanguages).No partofthisbookmaybereproducedin anyform–byphotoprinting, microfilm,oranyothermeans–nor transmittedortranslatedintoa machinelanguagewithoutwritten permissionfromthepublishers. Registerednames,trademarks,etc.used inthisbook,evenwhennotspecifically markedassuch,arenottobe consideredunprotectedbylaw.
PrintISBN: 978-3-527-34724-7
ePDFISBN: 978-3-527-82545-5
ePubISBN: 978-3-527-82544-8
oBookISBN: 978-3-527-82543-1
CoverDesignFormgeber,Mannheim, Germany
Typesetting SPiGlobal,Chennai,India PrintingandBinding
Printedonacid-freepaper 10987654321
Contents
Preface ix
1TiO2 Nanoparticles:PropertiesandApplications 1 OziomaU.Akakuru,ZubairM.Iqbal,andAiguoWu
1.1Introduction 1
1.2PropertiesofTiO2 Nanoparticles 2
1.2.1CrystalProperties 2
1.2.2OpticalProperties 3
1.2.3ElectrochemicalProperties 5
1.3SynthesisofTiO2 Nanoparticles 6
1.3.1TheHydrothermalMethod 6
1.3.2Sol–GelMethod 8
1.3.3SolvothermalMethod 10
1.3.4ChemicalandPhysicalVaporDepositionMethod 11
1.3.5ThermalDecompositionMethod 12
1.3.6OxidationMethod 13
1.4ApplicationsofTiO2 Nanoparticles 14
1.4.1Nanobiotechnology 14
1.4.2Nanomedicine 17
1.4.3WastewaterTreatment 21
1.4.4AirTreatment 26
1.4.5EnergyDevices 29
1.4.6WaterSplittingforHydrogenProduction 30
1.4.7FoodandCosmetics 34
1.4.8SoilRemediation 35
1.4.9PesticidesRemoval 36
1.4.10PaintandPaperProductions 40
1.5Conclusion 41 Acknowledgments 42 References 42
2ToxicityofTiO2 Nanoparticles 67
GoharI.Dar,MadihaSaeed,andAiguoWu
2.1Introduction 67
2.2ModesofExposure,Biodistribution,Clearance,andFateofTiO2 NPs 70
2.2.1Inhalation 70
2.2.2OralRoute 71
2.2.3Injection 72
2.2.4DermalRoute 73
2.3CellDeathPathwaysInducedbyTiO2 NPs 74
2.3.1Apoptosis 74
2.3.2Autophagy 76
2.3.3CrosstalkBetweenApoptosisandAutophagy 76
2.3.4Necrosis 77
2.4ToxicityofTiO2 77
2.4.1CellularUptake 78
2.4.2OxidativeStressEffectuatesbyTiO2 78
2.4.3Genotoxicity 79
2.4.4ReproductiveandDevelopmentalToxicity 81
2.4.5Carcinogenicity 81
2.4.6ImmunotoxicityofTiO2 NPs 83
2.4.7NeurotoxicityofTiO2 NPs 83
2.4.8AcuteToxicityofTiO2 84
2.4.9Sub-acuteToxicityofTiO2 86
2.4.10Sub-chronicToxicityofTiO2 NPs 87
2.5AlternativePerspective 88
2.6Conclusion 89 Acknowledgments 89 References 89
3AntibacterialApplicationsofTiO2 Nanoparticles 105 ChenXu,JianjunZheng,andAiguoWu
3.1Introduction 105
3.2AntibacterialEffectofTiO2 Nanoparticle 107
3.3Ion-DopedTiO2 NanoparticlesandTheirAntibacterialEffect 113
3.4AntibacterialAgent-DopedTiO2 NanoparticlesandTheir AntibacterialEffect 120
3.4.1Ag-DopedTiO2 Nanoparticles 120
3.4.2Zn-DopedTiO2 Nanoparticles 123
3.4.3Cu-DopedTiO2 Nanoparticles 123
3.5AntibacterialApplicationsofTiO2 -BasedNanoparticles 124
3.5.1MedicalApplication 124
3.5.2EnvironmentalApplication 125
3.5.3FoodSafetyApplication 126
3.6ConclusionandOutlook 126
Acknowledgments 126 References 127
4Surface-EnhancedRamanSpectrumofTiO2 Nanoparticlefor Biosensing(TiO2 NanoparticleServedasSERSSensing Substrate) 133
JieLinandAiguoWu
4.1Introduction 133
4.2SERSEffectofNanomaterialSubstrate 135
4.2.1MechanismofNon-metalSERSPlatforms 135
4.2.2AdvantagesforTiO2 SERSSubstrate 136
4.2.3SERSMechanismofTiO2 SERSSubstrate 137
4.2.4NovelMethodstoImproveTiO2 SERSEnhancement 139
4.3TiO2 SERSSubstrateAppliedinBiosensing 144
4.3.1CellCaptureonTiO2 NanorodArrays 144
4.3.2CellCapturePerformanceofTiO2 Coatings 147
4.4ConclusionsandFuturePerspectives 150
Acknowledgments 151 References 151
5CancerTheranosticsofWhiteTiO2 Nanomaterials 153
YangGao,SijiaSun,ZhibinYin,YanhongLiu,AiguoWu,andLeyongZeng
5.1Introduction 153
5.2TiO2 asInorganicPhotosensitizerforPhotodynamicTherapy 154
5.2.1PhotodynamicTherapyPrinciple 154
5.2.2UltravioletLightResponsivePhotodynamicTherapy 155
5.2.3980nmNear-InfraredLightResponsivePhotodynamicTherapy 156
5.2.4808nmNear-InfraredLightResponsivePhotodynamicTherapy 158
5.2.5Organic/InorganicDual-ModePhotodynamicTherapy 161
5.3TiO2 asSonosensitizerforSonodynamicTherapy 162
5.3.1SonodynamicTherapyPrinciple 162
5.3.2SonodynamicTherapy 162
5.3.3HighIntensity-FocusedUltrasoundTherapy 166
5.4TiO2 asMultifunctionalNanoprobesforVisualizedTheranostics 167
5.4.1MagneticResonanceImagingandPhototherapy 167
5.4.2FluorescentImagingandPhototherapy 168
5.4.3Multi-modeImagingandPhototherapy 169
5.4.4DrugDeliveryandSynergisticTherapy 170
5.5ConclusionsandFuturePerspectives 172 Acknowledgments 173 References 173
6CancerTheranosticsofBlackTiO2 Nanoparticles 185 TingDai,WenzhiRen,andAiguoWu
6.1Introduction 185
6.1.1LimitationsofWhiteTiO2 NanoparticlesinCancerTreatment 185
6.1.2AdvantagesofBlackTiO2 NanoparticlesinCancerTreatment 186
6.2PreparationsandPropertiesofBlackTiO2 Nanomaterials 187
6.2.1HydrogenationReductionMethod 187
6.2.2HydrogenPlasmaMethod 187
6.2.3ChemicalReductionMethod 188
6.2.4PropertiesofBlackTiO2 Nanomaterials 188
6.3BlackTiO2 forPhototherapy 189
6.3.1BlackTiO2 forPhotothermalTherapy 190
6.3.2BlackTiO2 forPhotodynamicTherapy 194
6.3.3BlackTiO2 forSynergisticPhotothermalandPhotodynamic Therapy 194
6.4BlackTiO2 forImaging-GuidedPhototherapy 195
6.5BlackTiO2 forSynergisticChemo-phototherapy 199
6.6BlackTiO2 x forSynergisticofSonodynamic–Photothermal Therapy 202
6.7OverviewandFurtherPerspective 205 Acknowledgments 207 References 207
7NeurodegenerativeDiseaseDiagnosticsandTherapyof TiO2 -BasedNanoparticles 217 XiangGao,JielingQin,ZhenqiJiang,JuanLi,andAiguoWu
7.1Introduction 217
7.2TheSymptomsofNeurodegenerativeDisease 219
7.3TraditionalDiagnosisandTreatmentforNDs 220
7.3.1CurrentMethodsfortheDiagnosisofNDs 220
7.3.2CurrentMedicinefortheTreatmentofNDs 220
7.4NanoparticlesintheDiagnosisandTreatmentofNDs 221
7.4.1TheDiagnosisofNDUsingNPs 221
7.4.2TheTreatmentofNDUsingNPsasDrugDelivery 222
7.5TheDiagnosisofNDsUsingTiO2 222
7.6TheTherapyofNDsUsingTiO2 225
7.6.1TheTherapyofNDsUsingTiO2 asDrugDeliveryPlatform 225
7.6.2TheTherapyofNDsUsingTiO2 asLight-ResponsiveMaterial 226
7.7TheDilemmaSituationofTiO2 intheNDs 226
7.8Conclusion 228
Acknowledgments 228 References 228
Index 237
Preface
Theauthorsofthisbookhavestudiednanoprobe-baseddiagnosisandtherapy ofdiseasesovertenyears.Accordingtotheconceptoffunctionalmodification ofnanomaterials,theyhavecarriedoutaseriesofresearchonthedesign, construction,andperformanceregulationofTiO2 nanoprobesintheranosticsofcancers.Forexample,theypreparedJanusstructureofTiO2 –Fe3 O4 nanoprobesformagneticresonanceimaging(MRI)-visualizedphotodynamic therapy(PDT)ofbreastcancer,developedTiO2 –upconversionnanoprobes fornear-infrared-triggeredPDT/chemotherapyofbreastcancer,andexplored novelblackTiO2 nanoprobesforphotothermaltherapy(PTT)ofbreastcancer andMRI-visualizedPTTofpancreaticcancerstemcells,actingassubstrateof surface-enhancedRamanspectroscopy(SERS)forthedetectionofdrug-resistant cancercells.BasedontheirstudyofTiO2 nanoprobesinthepasttenyears,and referencetotherelatedworksofpeers,theauthorsdecidetowritethisbookon theapplicationofTiO2 nanoparticlesinnanobiotechnology,andnanomedicine. ThisbooknotonlysummarizestheresearchprogressofTiO2 butalsopoints outtheexistentproblemsinTiO2 biomedicalapplicationsandthepotential developmentdirectionsinfuture.
Thisbookincludessevenchapters.Chapter1brieflyintroducestheproperties, preparationmethods,andapplicationsofTiO2 nanoparticlesinnanomedicine andnanobiotechnology.ThepropertiesofTiO2 nanoparticlesaretheintrinsic reasonfortheirapplications.Understandingthepreparationmethodswillhelp peopletoimprovetheperformancesofTiO2 nanoparticles.Chapter2summarizesthepathway,distribution,clearance,ultimatefateofTiO2 products invivo, andthedose-dependentpotentialtoxicity,aswellastheinteractionsbetween TiO2 andcells,whichwillhelppeopletoimprovematerialdesignandreduce theirtoxicity.Chapter3reviewsthelatestresearchprogressofTiO2 -based antimicrobialapplication,includinghowtoimprovethephotocatalyticactivity ofTiO2 bynon-metaldopingsuchasnitrogenandcarbon,aswellasmetal dopingwithnickelandneodymium,whichachievesvisiblelight-triggeredhigh efficiencyofantimicrobialactivity.Chapter4introducestheapplicationof TiO2 asSERSsubstrateinbiosensorsandfocusesontheSERSenhancement mechanismofTiO2 nanoparticles.Chapter5mainlyreviewswhiteTiO2 nanoparticlesandtheirnanocompositeswithnovelstructuresandpropertiesin cancertheranostics,suchasultravioletornear-infraredlight-stimulatedPDT, ultrasound-triggeredsonodynamictherapy(SDT),anddrugdelivery.Chapter6
x Preface summarizestheproperties,preparation,andresearchprogressofblackTiO2 nanoparticlesincancertheranostics,includingblackTiO2 withthefunctionsof PTT,PDT,SDT,drugcarriers,andphotothermalandphotoacousticimaging. Chapter7reviewstheapplicationofTiO2 nanoparticlesinneurodegenerative diseases.
Theauthorshopethatthepublicationofthisbookwillhelpreaderstofully understandthepreparation,performanceregulation,latestresearchprogressin thefieldofnanomedicine,andtheproblemshinderingthefurtherapplication ofTiO2 nanoparticles.Inthisregard,itwillencouragereaderstofocuson andsolvetheseproblemsinfuture,promotetheclinicalapplicationofTiO2 in nanomedicine,andprovidemoreefficientsafetreatmentsforpatients.Finally, theeditorsofthisbookwouldliketothankalltheauthorsineachchapterfor theirhardworkindiscussionandwritingthisbook,andtheyappreciatethe supportoftalentpoliciesandthefundingofvariousscientificandtechnological projects.Theyalsothanktheauthorsofthereferenceswhosefiguresarereused inthisbook.
September20,2019
AiguoWu
CixiInstituteofBiomedicalEngineering CASKeyLaboratoryofMagnetic MaterialsandDevices &KeyLaboratoryofAdditiveManufacturing MaterialsofZhejiangProvince NingboInstituteofMaterials TechnologyandEngineering ChineseAcademyofSciences Ningbo315201,P.R.China
TiO2 Nanoparticles:PropertiesandApplications
OziomaU.Akakuru 1 ,ZubairM.Iqbal 1,2∗ ,andAiguoWu 1
1 CixiInstituteofBiomedicalEngineering,CASKeyLaboratoryofMagneticMaterialsandDevices,&Key LaboratoryofAdditiveManufacturingMaterialsofZhejiangProvince,NingboInstituteofMaterialsTechnology andEngineering,ChineseAcademyofSciences,No.1219ZhongguanWestRoad,Ningbo315201,P.R.China
2 SchoolofMaterialsScienceandEngineering,ZhejiangSci-TechUniversity,No.2RoadofXiasha,Hangzhou, 310018,P.R.China
CHAPTERMENU
Introduction,1 PropertiesofTiO2 Nanoparticles,2 SynthesisofTiO2 Nanoparticles,6 ApplicationsofTiO2 Nanoparticles,14 Conclusion,41
1.1Introduction
Theimportantdiscoveryofultraviolet(UV)light-mediatedwatersplittingon titaniumdioxide(TiO2 )surfacebyFujishimaandHonda[1]haspromoted immenseresearchonotherapplicationsofTiO2 nanoparticles,particularlyin nanobiotechnologyandnanomedicine[2].Undoubtedly,thepastfewdecades havewitnessedanexponentialgrowthinnanoscienceandnanotechnology researchactivities[3–5].Nanoparticleshavegainedimmenseinterestforboth academicandindustrialapplicationsowingtothefactthattheuseofthese nanoparticleswithothermaterialshasimprovedscientificdiscoveriesand breakthroughs[6].Theseadvanceshavebeenaidedbythefactthatnewchemical andphysicalpropertiesofmaterialsemergeonreductionoftheirsizestothe nanometerrangeandvaryingtheirshapes[7].Ithasbeenreportedthatthe specificsurfaceareaandtheratioofthesurfacetothevolumeofnanomaterials dramaticallyincreaseastheirsizesdecrease[8].Interestingly,highsurfacearea, aconsequenceofsmallparticlesize,isofbenefittoTiO2 nanoparticles,asthe surfacearea-dependentinteraction/reactionofTiO2 nanoparticlesdevicesand thoseofthecontactmediabasicallyoccursatthesurfacesorinterfaces[7].
*Correspondingauthor:zubair@nimte.ac.cn,zubair@zstu.edu.cn
TiO2 Nanoparticles:ApplicationsinNanobiotechnologyandNanomedicine, FirstEdition.EditedbyAiguoWuandWenzhiRen. ©2020Wiley-VCHVerlagGmbH&Co.KGaA.Published2020byWiley-VCHVerlagGmbH&Co.KGaA.
Interestingly,TiO2 nanoparticleshavebeenextensivelystudiedowingto theirlowproductioncosts,mechanicalandchemicalstabilities,thinfilm transparency,bio-andchemicalinertness,hydrophilicity,highlightconversionefficiency,andcorrosionresistance[9,10].Basedontheseexceptional properties,TiO2 nanoparticleshavevastarrayofapplicationsthatinclude nanomedicine,nanobiotechnology,solarandelectrochemicalcells,wastewater treatment,food,soilremediation,gassensing,cosmetics,plastics,paintand paperproductions,hydrogenfuelgeneration,antisepticsandantibacterialcompositions,self-cleaningdevices,andprintinginks[10,11].Extensiveresearchhas beenrecentlyconductedonthenanomedicalapplicationofTiO2 nanoparticles inthedomainsofcancertherapyandimaging.Theserecentprogressesin nanomedicinedonotonlydependontheTiO2 nanoparticlesthemselvesbut alsoontheirfunctionalizationwithotherinorganicororganiccompounds[12]. Specifically,TiO2 nanoparticlescombinedwithmagneticnanoparticleshave beenusedasmagneticresonanceimaging(MRI)contrastagentsandinorganic photosensitizersforphotodynamictherapy[13],nanocarriersinchemotherapy [12],andintherecentlydiscoveredhydrogenatedblackTiO2 nanoparticlesas efficientcancerphotothermaltherapeuticagents[14].Theircancertherapeutic efficacyhasbeenlinkedtotheirgoodbiocompatibility,lowcytotoxicity,and uniquephotocatalyticproperties[15].
Someresearchersrecentlyopinedthattherearemanyothernewapplications forTiO2 thatareeitherunderwayorpresentlyinpilotproduction[16].These newapplicationsmaybeuncoveredsoonerthanexpected,duetotheUSFDA approvalofTiO2 tobefreelyincorporatedintonumerousdomesticproducts (dentalpastes,non-parenteralmedicines,tablets,andoralcapsules),thereby dramaticallyincreasingtheproductionandavailabilityofTiO2 forvarious applications[17].Thischapterwillthereforediscusstheproperties,synthesis, andapplicationsofTiO2 nanoparticleswithrespecttonanobiotechnologyand nanomedicine.
1.2PropertiesofTiO2 Nanoparticles
ThefascinatingpropertiesofTiO2 nanoparticleshavedramaticallyenhanced theirapplicationsinvariousaspects[18,19].Forinstance,theirhigh light-conversionefficiencieshavebeenexploitedforthefabricationofenergy devices[20].Theirchemicalstability,thinfilmtransparency,andlowproduction costsareresponsiblefortheirutilityasphotocatalystsforvariousenvironmental remediationstrategiessuchaswastewatertreatment,airpollution,andsoil viabilityimprovement[9].Recently,TiO2 nanoparticleswereappliedforcancer photothermaltherapy(PTT),exploitingtheirnon-radiativerecombinationability[14].Inthefollowingsubsections,specificpropertiesofTiO2 nanoparticles willbediscussedinmoredetail.
1.2.1CrystalProperties
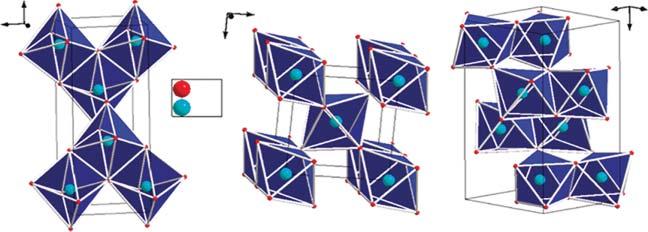
NanocrystallineTiO2 existsinthreemajorpolymorphicforms,whichinclude rutile,anatase,andbrookite,basedontheconditionsoffabricationandpost
Figure1.1 Thecrystalstructuresofanatase,rutile,brookiteTiO2 phases.Source:Adaptedwith permissionfromDambournetetal.2010[27].CopyrightAmericanChemicalSociety.
fabricationheattreatment[21].ThefourthpolymorphicformTiO2 (B)isquite uncommon[22].Asidethesefourpolymorphs,someresearchershavereported thesuccessfulsynthesisoftwohigh-pressurephasesfromthatofrutile:the TiO2 (II)thathasthePbO2 -likestructure[23]andtheTiO2 (H)thatstructure looksmorelikeahollandite[24].
Boththeanataseandrutilephasespossesstetragonalcrystalstructures eventhoughtheydonotbelongtothesamephasegroups,whilebrookitehas anorthorhombicstructureandtheuncommonTiO2 (B)phaseismonoclinic [22,25,26].AsshowninFigure1.1,thedistortionofanatasephaseoctahedral structureisslightlylargerthanthatofrutile[27].Ithasbeenreportedthateven thoughtherutilephaseislessstablethantheanatasephaseat0K,corresponding energydifferencebetweenthesephasesisrathersmall(about2–10kJ/mol). Withrespecttosolarcellapplication,anatasephaseTiO2 ischosenoverother phasesasaresultofitslowdensity,highelectronmobility,andlowdielectric constant[22].Itisalsoattractivethatinananatasecrystal,thereactivityofits (101)facetsismuchlowerthanthatofits(001)facets[28].
Asaconsequenceoftheanatasephaselowdensity,iteasilyundergoestransitiontotherutilephaseathightemperatures(usuallyaround450–1200 ∘ C) [29].Thisobservedtransformationisnotonlytemperaturedependentbutisalso affectedbysomeotherfactorssuchasdopantconcentration,initialphase,and particlesize[30].Ithasalsobeenobservedthatboththebrookiteandanatase phasesusuallytransformtotherutilephaseatpre-determinedparticlesizes, whereintherutilephasegainshigherstabilityagainsttheanatasephaseatparticlessizesthataregreaterthan14nm[31].Additionally,ithasbeenreported thatwhenevertherutilephaseisformed,itgrowsquickerincomparisontothe anatasephase[22].ThecrystalpropertiesofTiO2 aresummarizedinTable1.1.
1.2.2OpticalProperties
TheextensiveuseofTiO2 nanoparticlesinopticaldevicesareattributedtotheir excellentmechanicaldurability,hightransparencyinthevisibleregion,and chemicalstabilityinaqueousmedium[18].Severalotherstructuralparameters
1TiO2 Nanoparticles:PropertiesandApplications
Table1.1 ThecrystalpropertiesofTiO2 [20–22].
PropertiesAnataseRutileBrookite CrystalstructureTetragonalTetragonalOrthorhombic Density(g/cm2 )3.8944.2504.120
SpacegroupI41 /amdP42 /mnmPbca Molecule(cell)224
suchasphasecomposition,bandgap,crystallinequality,sizedistribution, morphology,porosity,andparticlesizehavebeenreportedtoinfluencethe opticalactivitiesofTiO2 nanoparticles[32].Remarkably,decreasingtheparticle sizeofTiO2 nanoparticlesfrom200nmtosmallermaterialsofabout10nm orlesschangestheopticalpropertiesofthesenanoparticlesfromopaqueto transparentinthevisibleregionofthelightspectrumandsubsequentlyto intriguingUVlightblockers[33].
InpureTiO2 ,theanatasephaseshowssuperiorcatalyticabilityandelectron mobilitythaneithertherutileorbrookitephases,apropertythatisbeneficialfor photovoltaicandphotocatalyticapplications[20,34].Theincreasedphotoreactivityoftheanatasephasehasbeenlinkedtoitslowoxygenadsorptioncapacity, increasedhydroxylationdegree,andslightlyhigherFermilevel[20].Ontheother hand,therutilephaseexhibitshighrefractiveindexandhighopticalabsorptivity, whichareresponsibleforitsapplicationinopticalcommunicationdevicessuch asmodulators,switches,andisolators[34].
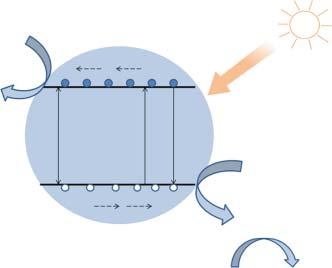
OnexposureofTiO2 nanoparticlestoUVlight,theelectronsintheirvalence bands(VB)gainenergyandassuchundergoexcitationtocorrespondingconductionbands(CBs),thusgeneratingholes(h+ )onthoseVBs(Figure1.2).At thisinstance,theexcitedelectrons(e )arepurelyin3dstates,andduetodissimilarparity,theprobabilityfore transitionisreduced,withattendantdecrease inthee /h+ recombination[22].Inthisrespect,theanatasephaseisregarded astheactivephotocatalyticcomponentthatisalsoattributabletoitschemical propertiesandthegenerationofchargecarriers(e andh+ )thatarisefromits abilitytoabsorbUVlight,whichisincorrespondencewithitsbandgap[35].The rutilephaseisgenerallyregardedasapoorphotocatalyst,duetothefactthat thebulkrecombinationofh+ ande takesplace,wherebyonlytheh+ thatare quiteneartothesurfacearewithheldandsubsequentlyundergosurfacetransferbytheupwardbandbending[36].Theseh+ subsequentlyinteractwiththe
Figure1.2 Illustrationofthe generalmechanismofTiO2 insolar photocatalysisprocess.
moleculesofH2 O,therebyforminghydroxylradicals(OH• )thatinsynergywith theh+ effectivelyoxidizetheorganiccompoundsintheirvicinityonthesurface oftheparticles[35].Also,thee intheCBinteractwithairmolecularoxygenfollowingareductionreactionprocess,therebyproducingsuperoxideradicalanions (O2 •− )[36].
Itisworthyofnotethatthechargecouple(e /h+ )redoxpotentialofanideal photocatalystisexpectedtofallwithinthedomainofitsbandgap[37].Additionally,thereductionactivityofphotoelectronsisdeterminedbyexistenceofenergy levelonthebottomoftheCBofthephotocatalyst,whiletheoxidationactivityof photogeneratedh+ isafunctionoftheenergylevelontopofthephotocatalystVB [22].Basedontheseproperties,TiO2 nanoparticlesareconsideredasnear-ideal photocatalystsastheirh+ areredoxselectiveandstronglyoxidized,dramatically increasingmoreresearchintonano-sizedTiO2 -basedphotocatalysts[37].
1.2.3ElectrochemicalProperties
Thechemicalandphysicalpropertiesofnano-TiO2 arealteredbytheirinherent electronicstructure,size,shape,surfaceproperties,andorganization[38].The electronicpropertiesofTiO2 nanoparticleshavebeenreportedashugecontributorstotheirparticle-andcrystal-sizedistributions[33].TiO2 initspureform isawidebandgapn-typesemiconductorthatpossessesindirectenergyband gapsoftherutile,anatase,andbrookitephasesof3.02,3.2,and2.96eV,respectively[29,39,40].IthasalsobeenreportedthatrutileFermilevelislowerthan theanataseby ∼0.1eV[20].Additionally,anatasehasasmallerelectroneffective massthanrutile,resultingtoanincreaseinmobilityforthechargecarriersin anatase,acharacteristicthatishighlyfavorableforoptoelectronicdevicesproduction[20].TheTiO2 CBconsistsof3dorbitalsoftitanium,whereasitsVBhas 2poxygenorbitalsthatformhybridsalongside3dtitaniumorbitals[22].
ThelatticeoxygensitesinTiO2 areveryimportantwithrespecttotheobserved superhydrophilicityofTiO2 nanoparticles,originatingfromthechemicalconformationchangesoftheirsurfaces[41].Thisisbecausesomeamountsofthe trappedh+ onthelatticesitesofoxygenmightinteractwiththeTiO2 specifically, therebyweakeningtheoxygenionsandlatticetitaniumbonds.Asaconsequence, H2 Omoleculescaninterruptthesebondsresultinginthegenerationofnew-OH
1TiO2 Nanoparticles:PropertiesandApplications
groups.Thesesinglylinked-OHgroupsarethermodynamicallyunstableand possesshighsurfaceenergy,whichleadstothegenerationofTiO2 surfacewith superhydrophilicity[36,41].
OneoftheobserveddisadvantagesfortheuseofTiO2 nanoparticlesinphotoelectrochemicaldevicesisthelargedensityofstatesthatareinvolvedine /h+ recombinationandelectrontransferreactionsattheelectrolyte–oxideinterface, iflocatedinthebandgap[42].Ontheotherhand,thisisanadvantageforthe adsorptionofredox-activecompoundsonthesurfacesofTiO2 nanoparticles thatisofspecialinterestindentalimplantapplications[43].Forinstance,serum proteinssuchasfibrinogenarereportedlychemisorbedbyanelectrontransferprocessonthesurfaceofTiO2 nanoparticles[42].Itwasdemonstratedthat theelectronicbehavioroftheTiO2 nanoparticlesaffectsthethrombogenicityof thefibrinogenduetothesemiconductingpropertyoffibrinogen,whichiscrucialinthebloodcoagulationpathway.Itthenfollowsthatanalterationinthe Fermienergyplacementand/orbandsizeoftheTiO2 nanoparticlescouldtrigger achangeintheadsorptionandsubsequentdecompositionoftheprotein,since thebandstructureofthefibrinogenfitsintothatoftheTiO2 nanoparticles[42].
1.3SynthesisofTiO2 Nanoparticles
NumeroustypesofTiO2 nanoparticleshavebeensynthesizedintheformof nanotubes,nanofibers,nanosheets,nanorods,andinterconnectedarchitectures [36,44,45].Theregularlyemployedsynthesismethodsarethehydrothermal, sol–gel,solvothermal,vapordeposition,oxidation,andthethermaldecompositionmethods.Othermethodsthathavebeenusedforthesuccessfulsynthesis ofvariousformsofTiO2 nanoparticlesincludebutnotlimitedtosol,electrodeposition,sonochemicalandmicrowave-assisted,andmicelleandinversemicellar methods.Forinstance,inthemicellarsyntheticstrategy,TiO2 nanoparticleswere preparedviathetetraisopropyltitanatehydrolysisinthepresenceofsodiumbis (2-ethylhexyl)sulfosuccinatereversemicellestoobtainnanoparticleswhoseaveragesizerangedfrom20to200nm[21].Basedonthemicellarwaterpool,various TiO2 phaseswereproduced,whereinlargepoolsaffordedanataseandsmallpools affordedtheamorphousnanoparticles.Inthefollowingsubsections,somecommonlyusedstrategiesforsynthesizingTiO2 nanoparticlesarediscussedinmore detail.
1.3.1TheHydrothermalMethod
Thehydrothermalsyntheticmethodisusuallyconductedinsteelpressurevessels, i.e.autoclaveswiththeTeflonlinersunderregulatedpressureandtemperature. Thistemperaturecanbeincreasedfromtheboilingpointofwateruptosaturationvaporpressure,butthepressureproducedisalsodependentontheamount ofaddedsolution[7].Hydrothermalmethodhasthereforebeenutilizedbymany researchersforthesynthesisofTiO2 nanoparticles[46–48].Inthisregard,Dawsonetal.synthesizedTiO2 nanoparticlesbythehydrothermalmethod,wherein
1.3SynthesisofTiO2 Nanoparticles 7
theysubjectedvariouscompositionsandparticlesizesofTiO2 mixedpowders tohydrothermalreactioninthepresenceofNaOH[49].At140 ∘ C,trititanate nanotubeseasilyformedfromtheanatasephaseofthestartingmaterialandat 170 ∘ C,trititanatebeltsandplatesformedfromtherutilephasecomponent. IncreasingthereactiontimetosevendaysexclusivelyconvertedalltheTiO2 to trititanatenanoplatesandbelts,withouttheformationofnanotubes.Hitherto, thereisnocleardescriptionofthestructureofTiO2 nanotubesbutresearchers haveassumedtheexistenceofhydrogentitanatewherethetubesareseparated byhydrogenions[2].
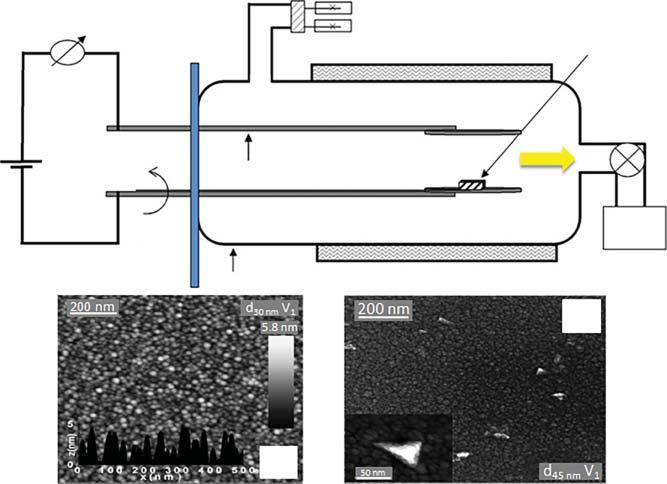
Inarelatedexperiment,purerutilenanotubeswithaveragediameterslessthan 20nmweresynthesizedfromrutile–anataseTiO2 particlesusingthehydrothermalmethodinNaOHwater–ethanolsolution[50].Itwasobservedthatthestructureandmorphologyoftheproductsdependedonthetypeofalcohol,aswellas thealcohol–waterratio.Thiswasexplainedbythevariationinoptoelectronic behaviorsoftherutilenanotubesandrawTiO2 .Thehydrothermalmethodwas alsousedtosynthesizetitanatenanotubesforthedegradationofAcidRed18 [51].Theseresearchersfurthercalcinedthenanotubesatvarioustemperatures (400–700 ∘ C)andobservedthatthenanotubescalcinedat600 ∘ Cweremost activetowardtheAcidRed18decomposition.ItwasreportedthatthemechanismfortheformationofTiO2 nanotubesinvolvedmultilayerednanosheetwrapping,ratherthanthewrappingorscrollingofsingle-layerednanosheets,whichis usuallyaccompaniedbythecrystallizationofsuccessivelayers[52].Nano-sized TiO2 hasalsobeensynthesizedfromcommercialTiO2 onafluorine-dopedtin oxide(FTO)substrateasshowninFigure1.3a[53].Thereactionwascarriedout for24hoursat135 ∘ CinasolutionofNaOH,resultinginTiO2 nanotubeswith averagediameterof10–12nm(Figure1.3b,c).
Inanotherstudy,nano-TiO2 wereobtainedfromtitanium(IV)alkoxidein anacidifiedwater–ethanolsolution[47].Thedropwiseadditionoftitanium tetraisopropoxide(TTIP)intothewater–ethanolsolutionatpH0.7,followed byafourhourreactionat240 ∘ C,yieldedtheTiO2 nanoparticlesdominated bytheanatasephase.Itwasobservedthattuningthesolventsystemandthe Tiprecursorwasabletocontrolthesizeoftheas-synthesizednanoparticlesto about7–25nm.NanowiresofTiO2 havealsobeenobtainedbythehydrothermal methodusingwhiteTiO2 powdersin10–15MNaOHattemperaturesof about150–200 ∘ Cwithoutstirringinanautoclavefor24–72hours[54].Zhang andGaotreatedTiCl4 solutionwithanacidororganicsaltfor12hoursat 33–423 ∘ CtoobtainTiO2 nanorods[55].Theyalsoreportedthatachangein thesurfactantorthesolventcompositioncouldaffectthemorphologyofthe synthesizednanorods.ThehydrothermalmethodwasalsoemployedbyChong etal.tosynthesizedTiO2 nanofiberswithaveragethicknessof40–100nm [56].Thehydrothermalsyntheticmethodwasconductedinthepresenceof NaOHandanion-exchangepost-synthesisinasolutionofHCl.Specifically, theresearchersreacted3ganataseTiO2 in10MNaOH(80ml),followedby autoclavingfor48hoursat180 ∘ Cinapoly-tetrafluoroethylenecontainer.They obtainedasodiumtitanatenanofiberprecipitatethatunderwentH+ exchange andcalcinationforthreehoursat700 ∘ CtoobtaintheTiO2 nanofibers.
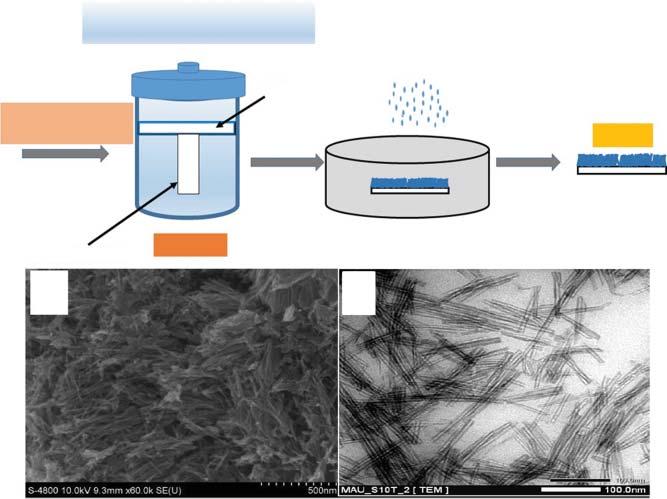
Figure1.3 (a)IllustrationofthesynthesisprocessofTiO2 nanotubesonanFTOsubstratevia thehydrothermalmethod,(b)fieldemissionscanningelectronmicroscopy(FESEM),and (c)transmissionelectronmicroscopy(TEM)imagesofthesynthesizedTiO2 nanotubes. Source:ReprintedwithpermissionfromVietetal.2016[53].CopyrightAIMSPress.
1.3.2Sol–GelMethod
Thesol–gelsyntheticstrategyisgenerallyemployedtosynthesizecrystallineor amorphousstructuresoforganicorinorganicmaterialsthatofferstheadvantageofobtainingbetterpurityandhomogeneityofthesynthesizednanoparticles atlowtemperatures[45].Utilizingthesol–gelstrategy,nano-TiO2 weresynthesizedfollowinghydrolysisofaTiO2 precursorasshowninFigure1.4[57].This usuallyproceedsbyacid-mediatedtitanium(IV)alkoxidehydrolysis,whichis followedbyacondensationreaction[7].TheformationofthechainsofTi–O–Ti hasbeenreportedlyencouragedbyslowhydrolysisrates,highamountoftitanium(IV)alkoxide,andsmallamountofwater[58,59].Ontheotherhand,it ispossibletoobtaindifferentshapesandsizesofanataseTiO2 nanoparticles withhighcrystallinitybythetetramethylammoniumhydroxide-mediatedtitanium(IV)alkoxidepolycondensation[58].Typically,thetitanium(IV)alkoxide couldbecomplementedwithNaOHandanalcoholat2 ∘ Candheatedfor13days at50–60 ∘ Corsixhoursatanelevatedtemperature(90–100 ∘ C).Forthepurpose ofimprovingthecrystalnatureoftheas-synthesizednanoparticle,asecondary heattreatment(175and200 ∘ C)inanautoclavecanbeperformed[7].Furthermore,fortheavoidanceofagglomerationduringthecrystallizationprocessof thenanoparticles,aprolongedheattreatmentcanbeperformedattemperatures below100 ∘ C[58].

Monodispersed sol
Figure1.4 Illustratingthesol–gelsyntheticmechanisminthepresenceofan(a)acid,(b)base, and(c)P123-templated,basicconditionindimethylformamide.Source:Adaptedwith permissionfromCaoetal.2011[57].CopyrightTheRoyalSocietyofChemistry.
Althoughthesol–gelmethodhasbeenregardedasaconventionallyaccepted methodforthesynthesisofTiO2 nanoparticles,severaldisadvantagesofthis methodhasinevitablycausedresearcherstoexploreothersynthesisstrategies. Amongthesedisadvantagesisthefactthatthecalcinationprocessobviously causesthegraingrowthandlowspecificsurfaceareaandinducesphase transformations[60].Additionally,comparativelypoorcrystallinityandparticle sizeuniformityoftencharacterizethesynthesizednanoparticles[32].These drawbacksnotwithstanding,someresearchershavesuccessfullyappliedthis strategyforthesynthesisofTiO2 nanoparticlesforpracticalapplications.For instance,TiO2 nanorodsweresuccessfullypreparedbysol–gelsyntheticstrategy bydippingporousanodicaluminamembrane(denotedasAAM)templateina boiledsolofTiO2 ,accompaniedwiththeusualproceduresofdryingandheating [61].Theseresearchersobservedthattuningthecalcinationtemperaturecould controlthenanorodcrystalphases,demonstratedbytheformationofanatase andrutilenanorodsatlowandhighcalcinationtemperatures,respectively. Inanotherstudy,TiO2 –zincphthalocyaninenanoparticleswereobtainedby thesol–gelstrategyandappliedasphotosensitizersforcancerphotodynamic therapy[62].Additionally,thehydrolysisandcondensationoftitaniumbutoxide withgammaaminebutyricacidandmineralacidsastheprecursorsyielded amine-,phosphate-,andsulfate-functionalizedTiO2 nanoparticlesinatypical sol–gelsyntheticmethod[63].Thesefunctionalizednanoparticlesdemonstrated goodpotentialascoppercomplexdrugcarriersforcancerchemotherapy.
UtilizingtheelectrophoreticdepositionofthecolloidalsuspensionsofTiO2 intoAAMpores,Linetal.obtainedorderedarraysofTiO2 nanowires[64]. TheyemployedplatinumanodeandtheAAMwithagoldsubstratefastened
(1) Polymeric sol(2) Gelation(3) Form gel
1TiO2 Nanoparticles:PropertiesandApplications tocopperasthecathode.Makinguseofavoltageofabout2–5V,aTiO2 sol wassuccessfullydepositedintotheAAMpores.A5%NaOHdissolutionof theAAMtemplateyieldedtheTiO2 nanowires,anditwasobservedthatan AAMwithlongporeswasdesirableforthepreparationofnanowiresinfavor ofnanotubes.Inarelatedstudy,nanotubesofTiO2 weresynthesizedwithan AAMtemplateandotherorganiccompounds[65].Typically,theAAMwas dippedintoasolutionofTTIPandlaterremovedandkeptinvacuumtoallow theTTIPvolumetopullthroughthetemplate.Then,watervaporhydrolysisin thepresenceofHCl(24hours),airdryingatambienttemperature,calcination fortwohoursat673KanddissolutionoftheAAMin6MNaOHwereconductedtoobtainpureTiO2 nanotubes.Inanotherstudy,TiO2 nanotubeswere synthesizedwithadiluteTiF4 coatingoftheAAMmembranesattimeperiods of12–48hoursat60 ∘ C[66].TheAAMmembraneswerethenremovedafterthe observedformationofthenanotubes.InanotherexperimentbySugimotoetal., TTIPwasmixedwithtriethanolamine(TEOA)inasol–gelsyntheticstrategy, followedbyagingforonedayat100 ∘ Candthreedaysat140 ∘ C[67].These researchersemployedamines(TEOA,diethylenetriamine,ethylenediamine, trimethylamine,andtriethylamine)assurfactantsandshapecontrollersforthe TiO2 nanoparticlesandobservedamorphologychangeatabovepHvaluesof11 and9.5inthepresenceofTEOAandtheotheramines,respectively.Thiscontrol overshapewasattributedtotheadsorptionoftheamineshapecontrollerson thecrystalplanesoftheTiO2 nanoparticles.Mahshidetal.preparedbiphase TiO2 nanoparticlesbythesol–gelmethodthatyieldedcrystallitesizesbetween 27and107nm[68].Hussainetal.alsosynthesizednovelnano-TiO2 whosesize rangedfrom10to20nmbythesol–geltechnique[69].
Inanotherstudy,Chongetal.synthesizedTiO2 nanoparticlesbythesol–gel method[70].TheyhydrolyzedtitaniumIVbutoxideprecursorwithethanol, followedbyacondensationreaction.Throughthisfirststep,itwaspossibleto controlthehomogeneityandmicrostructuresoftheeventuallyformedTiO2 crystals.Controlofthehydrolysisextentisverycrucialinobtainingnano-sized TiO2 .Forthispurpose,theseresearcherscarriedoutthehydrolysisintwo stages,wheretheethanoladditiontothetitaniumIVbutoxideprecursorwas utilizedtotargetthepartiallyhydrolyzedstate.Thispartiallyhydrolyzedstate ensuresanimprovedmolecularhomogeneityandnon-hydrolyzableTiligands forenhancedgrowthoftheTiO2 [71].Thecontrolofthehydrolysisreaction extentwasachievedbyanacid-mediatedhydrolysiswithnitricacid,owingto thelowrangeofethanolfunctionalityinthetitaniumIVbutoxideprecursor hydrolysis.
1.3.3SolvothermalMethod
Thesolvothermalsyntheticstrategyiscloselyrelatedtothehydrothermaltechniquebutfortheuseofnon-aqueoussolventsintheformer[58].Duetotheuseof awiderangeoforganicsolventsofappreciablyhighboilingpointsintheformer, theworkingtemperaturescanbeelevatedwaymuchhigherthanisattainable inthelater[7].Moreover,thesolvothermalmethodoffersintriguingadvantages includinganimprovedtuningofmorphology,particlesize,crystallinityofthe
1.3SynthesisofTiO2 Nanoparticles 11 synthesizednanoparticles,andtemperatureandpressurecontrolsofthesolvents usedforthesynthesis[72].Additionally,thismethodprovidesaversatileroute tothesynthesisofTiO2 nanoparticleswithimproveddispersityandnarrowsize distribution[7].
ThesolvothermalsyntheticstrategywasusedforthepreparationofTiO2 nanorodsandnanoparticleswithorwithoutsurfactantmediation[58,73].In thelightofthis,anhydroustoluenewasemployedat250 ∘ Cfor20hoursto dissolveTTIP,usingoleicacidasasurfactant[74].Inacontrolledhydrolyzation reaction,redispersibleTiO2 nanorodsandnanoparticlesweresynthesizedwith Ti(OC4 H9 )4 inthepresenceoflinoleicacid.Thisreactionwascarriedoutwith acatalyst(triethylamine)toaidtheinorganicTi–O–Ticondensationinorderto obtainthecrystalswithconsistentmorphology[73].
1.3.4ChemicalandPhysicalVaporDepositionMethod
Vapordepositioncanbereferredtoasthecondensationofmaterialsinthevapor phasetosolidphase[58].Intheabsenceofachemicalreactioninthevacuum chamber,theprocessistermedphysicalvapordeposition(PVD),otherwiseit istermedchemicalvapordeposition(CVD)[75].InatypicalCVDprocess,the depositionreactionisdrivenbythermalenergythatheatsthegasespresentin thecoatingcompartment[7].EmployingTTIPpyrolysisinanatmosphereof oxygen/heliumutilizingaliquidprecursor,TiO2 nanoparticleswithaveragesizes below10nmweresuccessfullysynthesized[76].
Nevertheless,otherCVDapproachesexist,whichincludediffusionflame pyrolysis,ultrasonicspraypyrolysis,ultrasonic-assistedhydrolysis,electrostatic sprayhydrolysis,laser-inducedpyrolysis,andplasma-enhancedCVD(PECVD) [58].InatypicalPECVD,theamorphousTiO2 nanoparticlesareplacedonthe coldcompartmentsofthereactorattemperatureslessthan90 ∘ Casshownin Figure1.5a[77].TheobtainednanoparticlesfromPECVDafterannealingsame athightemperaturesusuallypossesshighsurfaceareas(Figure1.5b,c)[78].
Inanotherexperimentemployingcatalyst-andtemplate-freemetalorganic CVD(MOCVD),WuandYusuccessfullygrewTiO2 nanorodsonfusedsilica substrates[79].Typically,O2 /N2 flowwasusedtoconveytitaniumacetylacetonatethatwasvaporizinginthelow-temperatureregionofthefurnace (200–230 ∘ C)tothehigh-temperatureregion(500–700 ∘ C),resultinginthe growthoftheTiO2 nanorodsonthesubstratesdirectly.Themorphologyand phaseofthenanorodsweredemonstratedtobetunablewiththereaction conditions.Toillustratethis,atapressureof5Torr,single-crystallineanatase andrutilenanorodswereobtainedat560and630 ∘ C,respectively,whileat 3.6Torr,anatasenanowallscomprisingofwell-alignednanorodswereobtained at535 ∘ C.TheMOCVDmethodhasalsobeenappliedusingaWC–Cosubstrate andaTTIPprecursorforthesuccessfulsynthesisofTiO2 nanorodsasreported inastudybyPradhanetal.[80].
PrimaryPVDmethodsincludeionplating,sputtering,lasersurfacealloying, thermaldeposition,andlaservaporization[7].ThePVDmethodorthermal depositionhasbeenemployedforthesynthesisofTiO2 nanowires[81].In atypicalexperimentconductedinatubefurnace,atitaniumsource(pure
Figure1.5 (a)SchematicdiagramofatypicalPECVD.Source:Baghgaretal.2009[77]. ReproducedwithpermissionofInstituteofPhysics.(b)Atomicforce.Source:Adaptedwith permissionfromBorrasetal.2009[78].CopyrightAmericanChemicalSociety.(c)Scanning electronmicroscopy(SEM)imagesofTiO2 grownbyPECVD.
Timetal)wasloadedonaquartzboat,andunderanargonatmosphere,the temperaturewasraisedto850 ∘ C.Thiswasfollowedbypumpingdownofthe furnacechambertoabout300Torrwiththeargonflowrateof100sccmforthree hours,toobtainfineTiO2 nanowires.Inanalternativeroute,Wuetal.employed AuasacatalystanddepositedananopowderlayerofTionthesubstrate,prior tothegrowthoftheTiO2 nanowires[82].
1.3.5ThermalDecompositionMethod
Thethermaldecompositionmethodhasbeenemployedinthepastdecadeby variousresearchersforthesynthesisofTiO2 nanoparticles[83–85].Forinstance, thethermaldegradationofTTIPvaporat300 ∘ C,yieldedTiO2 nanoparticlesin astudybyMoravecetal.[84].Theysaturatedthecarriergas(dry,particlefree, anddeoxidizednitrogen)withtheTTIPvaporinasaturatorthatwasexternally heated.ItwasobservedthattheformationoffineTiO2 nanoparticleswasinitiatedbytheheterogeneousdecompositionoftheTTIP.Inanotherexperiment, TiO2 nanoparticleswereobtainedbythermaldecompositionoftitanium(IV) n-butoxidein1,4-butanediol.Thereactionwasalsoconductedinanautoclave at300 ∘ Cfortwohoursat25 ∘ C.Theobtainednanoparticleswerefurthercalcinedfortwohoursat500 ∘ Cinaboxfurnaceandwereobtainedascrystalsof anataseTiO2 nanoparticleswithaveragediameterof15nm.
Furthermore,TiO2 nanoparticleswithimprovedphotocatalyticabilitywere successfullysynthesizedusingthethermaldecompositionmethodbytreating theprecursortitanylsulfatewithperoxocompoundssuchashydrogenperoxide, ureahydrogenperoxide,andammoniumpersulfate[86].Annealingtemperaturesof600,850,and700 ∘ Cwereutilizedinairat60minutesforthehydrogen peroxide,ureahydrogenperoxide,andammoniumpersulfateperoxo-containing phases,respectively,toobtaintheanatasephaseTiO2 nanoparticles.Inadifferent study,Imetal.synthesized10nm-TiO2 nanoparticlesbythermallydecomposing metal–organicframeworks(denotedasMIL-125andMIL-125-NH2 )thatcontaintitanium,forsixhoursat350 ∘ Cinair[85].Theyobservedarandomaggregationofthenanoparticleswithinthecrystallineparticlesofthemetal–organic frameworkprecursors.Thesenanoparticlesshowedcapacityforeffectivedecompositionof4-chlorophenolbyphotocatalysis.
1.3.6OxidationMethod
Thismethodinvolvesthedirectoxidationoftheelementtitaniumbytheuse ofoxidantsoranodicreactionstoproducethenanoparticles[58].Forinstance, usingavoltageof10–20Vand0.5%hydrogenfluoride,Vargheseetal.successfullypreparedwell-alignedTiO2 nanotubes[87].Annealingtheanodizedplateof titaniumat500 ∘ Cforsixhoursinthepresenceofoxygenwasnecessaryinobtainingthenanotubes.Itwasobservedthatthediameterandlengthofthenanotubes couldbecontrolledbytuningtheappliedvoltage.
Thehydrogenperoxide-inducedoxidationoftheelementtitaniumwas reportedtobeeffectiveforthesynthesisofTiO2 nanotubesbyplacingthe titaniummetalin50mlhydrogenperoxidesolution(30wt%)for72hoursat 353K[88].IntheoxidativesynthesisofTiO2 nanoparticles,thephaseofthe productswerecontrolledbyaddingspecificinorganicsaltsinthepreparation process,particularlyduringthedissolutionprecipitation[89].Forinstance, NaClisusuallyaddedforthegenerationofrutilephase,andNaForNa2 SO4 arerequiredtoobtaintheanatasephaseTiO2 nanoparticles[90].Acetonehas alsobeenemployedasanoxygensourceat850 ∘ Cfor90minutestoobtain well-alignedandhighlydenseTiO2 nanorodarrays[91].
Furthermore,TiO2 nanoparticlescanbesynthesizedbyeitherthewetordry processes.Inthedryprocess,thevaporphaseoxidationofTiCl4 isconducted, whichleadstoformationofamorphousTiO2 nanoparticlesasshowninEq.(1.1). ThisisusuallyfollowedbyannealingofthesynthesizedamorphousTiO2 nanoparticlesatdifferenttemperaturestoobtaindesiredcrystallinephasesof anataseorrutile[21].
Inthewetprocess,homogenousproductsandnanocompositeswithcomplex shapescanbeobtained,andthereisalsothepossibilityofcontrollingthe stoichiometry.However,thepresenceofcarbon(animpurityinthiscase),long processingtimes,andexpensiveprecursorsarethemajordrawbacksofthis process[22].
1.4ApplicationsofTiO2 Nanoparticles
Duetotheirfascinatingproperties,TiO2 nanoparticleshavefoundvastarray ofapplicationssuchasinnanobiotechnology[92],nanomedicine[14],energy devices[93],soilremediation[94],food[95],healthcareandcosmeticproducts [96],wastewatertreatment[97],andpaint[98],paper[99],andplasticsproductions[100].SomeofthesegeneralapplicationsofTiO2 nanoparticleswillbe brieflydiscussedinthefollowingsubsections.
1.4.1Nanobiotechnology
Owingtotheincreasingdifficultyintreatingmultidrugresistantstrains (Pseudomonasaeruginosa),thereisanurgentneedtofabricatenewclassof antibacterialagentswithtotallydifferentmodeofactionandstructurefrom conventionalagents[19].Additionally,multidrugresistantstrainshavebeen linkedtocomplicationsinpresent-daymedicine[101].Thesestrainshavealso beenidentifiedworldwideasmajornosocomialpathogens[102].Thelastdecade haswitnessedvariousfailuresinthetreatmentofanimalandhumanbacterial diseasesthathavebeengenerallyattributedtothestrongresistanceofbacterial pathogenstoconventionaldrugs[103,104].Thisismainlyduetotheabilityof thesebacterialpathogenstorapidlyadapttonewenvironmentalterrainssuchas antimicrobialmoleculespresence,consequentlyincreasingthebacterialresistancefollowingtheuseofantimicrobials[105].Inrecentyears,agoodnumberof nanoparticleshavedemonstratedencouragingbiologicalactivities,particularly towardbacterialtreatment[106].Amongthesenanoparticles,nano-TiO2 has gainedattentionbasedonitshydrolysisandoxidativeproperties[92].Ithasalso beenobservedthatthebiocidalactionofnano-TiO2 ispartlyaconsequenceof chargecarrier(electron-hole)modulationattheexternalsurfaceinterfaceofthe nanoparticles,givingrisetoappreciableantimicrobialcapabilitiesbyoptimizing theinorganic–organicinterfacialcontactandinorganicphasedispersions [107–110].Additionally,nano-TiO2 hasbeentippedtooutperformmetal-based systemsandotherchemicalssuchasH2 O2 ,NO,andothersmallmoleculesdue totheintrinsicallyenvironmentalfriendlinessandnon-contactantimicrobial actionofTiO2 –polymernanocomposites[111,112].Assuch,noreleaseoftoxic nanoparticlestothesurroundingmedia(withadversehealtheffects)occursin ordertoobtainthedisinfectionefficacies[113,114].
Nano-TiO2 hasalsobeenusedforthedisinfectionofabroadmicroorganism spectrum[92,115],buthitherto,themechanismbywhichthesenanoparticles inducemicrobialdeathispoorlyunderstood[116].Rather,moststudieshave focusedonthealterationofenzymeactivitiesthatdependoncoenzyme-A[117], hydroxylradicals-inducedDNAdamage[118],andthepopularoxidativeattack bynano-TiO2 ontheinnerandoutercellmembranesofmicroorganisms[119, 120].Asregardsantibacterialcoatings,highdensityofnano-TiO2 onthesurfaceenhancesthegenerationofreactiveoxygenspecies(ROS)withresultant increaseinantibacterialefficacy[121].Notwithstanding,severalotherantimicrobialapplicationsbasedonthegenerationofROSasamechanismofaction
1.4ApplicationsofTiO2 Nanoparticles 15 havebeendocumented[115,122].Theseincludedisinfectantsorbiocidessuchas potassiumpermanganate,ozone,hydrogenperoxide,pancreaticacid,etc.[123]. Someresearchershaveincorporatedthesebiocidesintothenanocompositesof polymers,therebygeneratingvastantimicrobialmaterialswithwiderangeof applicationssuchasinbiomedicine,packaging,etc.[116,124–128].Unfortunately,kineticandthermodynamicbarriersreducethedispersalofsuchinorganic (oftenhydrophilic)nanoparticles(NPs)inthematricesofhydrophobicpolymers, therebyposingseriouschallengestothedevelopmentofsuchmaterials[116]. However,otherapproachesthatareseeminglymoretargetedcanbeconsidered inordertoenhancethegenerationofROS,suchasthephotodynamicinactivation(PDI).AtypicalPDIentailstheexcitationofphotosensitizerdyestothe long-livedtripletstatesthatthenundergosomereactionsinvolvingenergytransferstoeitherproducesingletoxygenorthehydroxylradicals[129,130].PDI hasthereforebeenappliedwithphotosensitizersinsolutionorheterogeneous dyes,whereinthedyesareconjugatedtopolymerfilmsorothersolidsupports [131,132].Forinstance,PDIwasexploitedusingthecommonphotosensitizer (methyleneblue)thatwasexcitedat660nm(redlight)andpotentiatedwithan inorganicnontoxicsalt(potassiumiodide)[133].Thisgaverisetoanincrease inthekillingofGram-positiveandGram-negativebacteriaby2-logs.However, arecentreporthasdemonstratedthattheantimicrobialphotocatalysisofTiO2 nanoparticlescouldbepotentiatedwithsodiumiodidewitharesultant3-log increaseinantimicrobialefficacy[134].
Inthelightofthese,aninvestigationintotheinhibitioneffectivenessofTiO2 nanoparticlesincombinationwithactiveantibioticsofthecellwall(cefotaxime andcefazidime)against P.aeruginosa (amultidrugresistantstrain)wascarried out[19].The P.aeruginosa wasisolatedfromendo-trachealtract,sputum,pus, andbronchoalveolarlavage.ItwasobservedthatuponexposuretoUVlightfor onehour,theTiO2 nanoparticlesdemonstratedantimicrobialeffectonthenosocomialpathogenatnanoparticleconcentrationsof350mg/mlandhigher.Additionally,a6-logincreaseinminimuminhibitoryconcentrationvalueswasgeneratedforthecefotaximeagainstcefazidime.
Inarelatedstudy,thephotocatalyticeffectoflow-doseUVlight-activated TiO2 nanoparticlesonthegenome/proteome-wideexpressionof P.aeruginosa wasinvestigatedtwominutesposttreatment[116].Theyanalyzedandquantifiedtheradicalspeciesreleasedfrom P.aeruginosa biofilmsandestimated thecontributionofsuchradicalsinthedamageandultimatedeathofcells.It wasobservedthatthephotocatalyticactionoftheTiO2 nanoparticlestriggered adeclineintheexpressionofalargecollectionofgenes/proteinsresponsible forgrowthandsignalingfunctionsinparallelwiththesubsequentimpactson coenzyme-independentrespiration,cellwallstructure,andionhomeostasis. Itwasalsoobservedthatthenano-TiO2 -mediatedbiocidalactionorthose ofotherphotoactivenanoparticleswerecounteractedbythecellsasthe cellstriggerednumerousregulatoryresponseswithnosignificantmetabolic functionlosswithinthetimescaleoftheexperiment.Theyalsoestablished clear-cutindicationsofnano-TiO2 selectivecatalysisindistinctcell-critical systems.Theseresearchersthereforeproposedthecombinationofbiological andchemicalmethodstotacklethepresentlimitationsinunderstandingthelink
1TiO2 Nanoparticles:PropertiesandApplications
betweenmicroorganismtransportandmetabolicproperties,andnano-TiO2 photo-inducedbiocidalefficacy.Thiswouldalsobenefittheproperdefinitionof thenatureofphotocatalyzedreactions,thecellsthattakepartintheresponse, andthedegreeatwhichnano-TiO2 biocidalactioncouldbereversed.
TheantimicrobialeffectofTiO2 nanoparticlesonanotherdrug-resistant nosocomialpathogen(methicillin-resistant Staphylococcusaureus,denotedas MRSA)wasdemonstratedinastudybyJeslineetal.[106].TheMRSAwas describedasamajornosocomialpathogenlinkedtoanarrayofinfections suchasthecatheter-relatedandsurgicalsiteinfections,bacteremia,softtissue andskininfection,andpneumonia.Theyattributedthemaincontributory factorforpathogenresistanceofantibioticstotheformationofbiofilms. Additionally,thestrongresistancetodrugsbyMRSAwasdescribedbytheir formationofextracellularpolymericsubstancesconsistingofnucleicacids, lipids,andpolysaccharides.Importantly,asessilebacteriathatisembeddedin anextracellularpolymericsubstanceiscapableofwithstandingtheimmune responsesfromthehostandassuchbecomeslessaccessiblebyantibioticsas suchdrugsexperiencedifficultiesinpenetratingthebiofilm.Theseresearchers monitoredtheproductionofbiofilmsbytheMRSAusingthecultureplate methodandthusreportedthatUV-mediatedtreatmentwithTiO2 nanoparticles significantlysuppressedtheproductionofthesebiofilms.Anearlierreportalso explainsthattheabilityofTiO2 nanoparticlestodecomposeorganiccompounds liesontheirconstantreleaseofsuperoxideionsandhydroxylradicalsthatis efficientforinhibitingMRSAgrowth[135].Furthermore,Chorianopoulosetal. investigatedthephotocatalyticeffectofTiO2 nanoparticlesontheproduction ofbiofilmsbythe Listeriamonocytogenes bacteria[136].Inthatstudy,various TiO2 nanoparticlesweredepositedonstainlesssteelandglasssurfacesina doctor-bladestrategy.ThesesurfaceswereirradiatedwithUVlightafterthe formationofthebiofilms.Theyobservedafastlogreductionofthebiofilm attributabletothepresenceoftheUV-activatednanoparticles,comparedwith thecontroltest.
Ithasalsobeendemonstratedthattheadditionofaninorganicsalt(potassium iodide)significantlyincreasedthephotocatalyticeffectofTiO2 nanoparticleson fungiandGram-positiveandGram-negativebacteriabyapproximatelysixlogs [115].Theantimicrobialeffectwasdependentontheintensityoftheapplied UVlight,andtheconcentrationsofthepotassiumiodideandTiO2 nanoparticles.Theyestablishedthatthemosteffectivepotassiumiodideconcentrationfor theenhancedantimicrobialeffectwas100mM.Theyobservedthatinthereactionmixture,therewasagenerationoflong-livedantimicrobialspeciesthatmay probablybeiodineandhypoiodite,butmorekillingwasproducedbyshort-lived antibacterialreactivespecies.Strategically,theiodidequenchedthefluorescent probesforsingletoxygenandthehydroxylradicals.TheTiO2 –UV–potassium iodideproducedatri-iodide(whosepeakwasobservedat350nmandformed bluecompoundwithstarch)butwasintensivelydecreasedwhentheMRSAcells werepresent.
Nano-TiO2 hasalsobeenusedtokillabroadspectrumofbacteriaincluding Escherichiacoli, S.aureus, Bacteroidesfragilis,P.aeruginosa,and Enterococcushirae,inastudyonorthopedicimplantsinfections[137].Intheclinic,the
1.4ApplicationsofTiO2 Nanoparticles 17 metalpinsusedfortheapplicationofexternalfixationdevicesorskeletaltractionstomanageorthopedicfracturesarehighlysusceptibletopinsiteinfections [138].Thesefracturesmosttimesareassociatedwithboneandwoundinfections [139].Thehighadaptabilityofbacteriaenablingtheircolonizationondamaged tissuecellsor“inert”biomaterialssurfacesremainthemostcriticalcomplication intheexternalfixationstrategyforthemanagementofpintractinfection[140]. Notably,thestabilityofpin–boneinterfacesisreducedbythepintractinfection, whileanunstablepin–bone-fixatorconstructmaycausehalf-pinlooseningand pinsiteinfections[137].Thecolonizationofexternalfixatorswithbacteriasuch as Staphylococcusepidermidis and S.aureus wasreportedinanearlierstudyby Collingeetal.[141].Indevelopedcountries,antibioticsareroutinelyadministeredtoamelioratetheseinfections,whileothercommonpracticesincludesurgicaldebridement,stabilization,andirrigation,whenindicated[138].Duetothe widespreaduseofantibioticsandthebuildupofdrugresistanceinbacteria,the needtodevelopalternativemeansforsterilizationcannotbeoveremphasized. Moreover,theconventionalsterilizationstrategyofwipingisnoteffectiveatthe longrun,timeandstaffintensive,andcannotbestandardized,coupledwiththe challengesassociatedwithusingaggressivechemicalsforsuchpractice[142]. Forthisreason,Tsuangetal.demonstratedthatthecoatingofanorthopedic implantwithTiO2 nanoparticlescouldreducebacteria-relatedcolonizationof suchimplantsandtheassociatedinfections[137].Theyobservedthatalmostall thetestedbacteriawerekilledfollowing50minutesofUVirradiationoftheTiO2 nanoparticles.Additionally,adecreaseintheformationofbacterialcolonieson topofthenano-TiO2 -coatedmetalplates.Itwasalsoestablishedthatthephotokillingofthebacteriafollowedatwo-stepdecaykinetics,whereinarelatively lowerratewasfollowedbyahigherone.However,thiswascontrarytoearlier photokillingkineticreportsthatweredescribedbypseudo-first-orderreactions [143,144].
1.4.2Nanomedicine
ExploitingtheopticalpropertiesofTiO2 nanoparticles,someresearchershave appliedsamefor invitro and invivo cancerPTT[14,15].Arecentstrategic researchdemonstratedtheuseofhydrogenatedTiO2 nanoparticlesincancer PTT,whereinthephotothermalabilityofthenanoparticleswasattributedtotheir dramaticallyimprovednon-radiativerecombination[14].Theyemployedblack TiO2 nanoparticlesforthePTTofcancercellsowingtothelimitationsofwhite TiO2 nanoparticlesasregardsthemutagenicityandshallowpenetrationofUV light.ThehydrogenatedTiO2 nanoparticleswerecoatedwithpolyethyleneglycol (PEG)andwereobservedtopossessgoodbiocompatibilityandlowtoxicity,low costandsimplepreparationstrategy,andexcellentnear-infrared(NIR)effect.A bettercancerPTTabilitytriggeredbyNIRofthehydrogenatedTiO2 nanoparticleswasobservedasagainstphotodynamictherapy(PDT)ofTiO2 nanoparticles inducedbyUVlight.
Inarelatedstudy,anNIR-triggeredmultifunctionalnanoplatformcomprised ofblackTiO2 nanoparticleswassuccessfullyandrationallyconstructedforan effectivecancertheranostics[15].ThesePEGylatednanoparticlesdemonstrated
1TiO2 Nanoparticles:PropertiesandApplications
fascinatinghemo/histocompatibility,wideabsorptionrange,andhighstability anddispersityinaqueoussolution.IrradiatedbyasingleNIRlaser,these novelmultifunctionalnanoparticlesshowedhighPDT/PTTsynergisticcancer therapeuticefficacyunderphotoacoustic/infraredthermalimaging.Notably, theobstaclesassociatedwiththeuseofhighlaserpowerdensity,UVlight, multi-componentnanocomposites,andcombinationcancertherapywere circumventedbytheuseofthesesynthesizednovelmultifunctionaltheranostic nanoparticles.Furthermore,thesenovelmultifunctionalnanoparticlesdemonstratednegligiblesideeffectstomaintissuesandbloodinthreemonthsof investigation.
Thepotentialofnano-sizedTiO2 asphotosensitizersforPDThaveindeed beenextensivelyinvestigatedinthelastfewdecades[2,13,62].Tothis end,photosensitizersbasedonTiO2 nanoparticlesforcancerPDTwere preparedbyincorporatingzincphthalocyanine(ZnPc)intotheporousnetworksoftheTiO2 nanoparticlesasreportedinastudybyLopezetal.[62]. Thesephotosensitizersdemonstratednegligiblecytotoxicityfollowingthe 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide(MTT)assayon fourmammaliancelllinesandparasites,namely,humanhepatocellularliver carcinomacells,human-derivedfibroblasts,humanacutemonocyticleukemia cellline,andtheAfricangreenmonkeyepithelialcells.Themechanismforcell necrosisfollowingthePDTwasdeterminedbythepreferentiallocalizationof thephotosensitizersintargetorganellessuchaslysosomesormitochondria. Withoutanexternalimpetus,TiO2 nanoparticlesareexcitedbyUVwavelengths intheregionof320–400nm,whichareharmfultothehumanbodyduetothe factthathemoglobin,melanin,andproteinsarealsoexcitedinthisUVrange [2].Takingcognizanceofthis,Lopezetal.demonstratedthatincorporating theZnPcincreasestheusefulwavelengthoftheTiO2 nanoparticlestothe visibleregion,whereinanintercomponentelectrontransferisachievedinthe molecularsemiconductor–semiconductoroxideofthecouple[62].
Similarly,ZnPc-integratedTiO2 nanoparticleshavealsobeensynthesizedfor invitro cancerPDT[145].ItwasobservedthatthetoxicityofZnPcwasreduced bytheTiO2 nanoparticles,whiletheZnPc–TiO2 nanoparticlesdemonstrated goodPDTpotentialwhentesteddifferentlywithhumanhealthylungcelllines (WI38),hepatocellularcarcinoma(HepG2),andcolorectaladenocarcinoma tumor(HT29).TheZnPc–TiO2 nanoparticleswerefurtherlabeledwith 131 I radionuclideanditwasobservedthatthe 131 I–ZnPc–TiO2 nanoparticles demonstratedgoodpotentialfornuclearimaging.Recently,Yurtetal.synthesizedSubphthalocyanine(SubPc)-integratedTiO2 nanoparticlesthatwerealso labelledwiththe 131 Iradionuclide(denotedas131 I–SubPc–TiO2 )[146].When treateddifferentlywithWI38,HepG2,andHT29,thesenanoparticlesalso demonstratedgoodpotentialasnuclearimagingandPDTagents.
UpconversionTiO2 nanoparticleshavealsobeensynthesizedandapplied inthePDTofcancer[147–149].Theupconversionnanoparticles(UCNPs) ofNaYF4 :Yb3+ andTm3+ @NaGdF4 :Yb3+ wereusedforthesynthesisof UCNP@TiO2 nanoparticlesfortheNIR-inducedphotoactivationofthe TiO2 nanoparticles.TheUCNP@TiO2 nanoparticlesgeneratedROS-induced apoptosisoftumorcellsbyreducingthemitochondrialmembranepotential,
1.4ApplicationsofTiO2 Nanoparticles 19 subsequentlyreleasingcytochromeCfortheactivationofcaspase-3.Tomitigate themultidrugresistanceofbreastcancercells,UCNPsofNaYF4 :Yb/Tm-TiO2 asinorganicphotosensitizersloadedwithananticancerdrugdoxorubicin (DOX)weresuccessfullysynthesizedandappliedfortheNIR-inducedPDTof breastcancer[149].Byexploitingtheupconversionluminescenceabilityofthe NaYF4 :Yb/TmthatconvertsNIRradiationtoUVlight,theTiO2 couldthen generatesufficientROSforeffectivePDTwithobservedlowphoto-damageand deeppenetration.Thecellularuptakeofthesenanoprobeswasenhancedby theirfolicacidfunctionalization,enablingtheacceleratedreleaseofDOXinthe drugresistantMCF-7/ADRandMCF-7cells.
Recently,TiO2 nanoparticlesweregraftedintoandoutsideUCNP@mesoporoussilica(UCNP@mSiO2 )nanovehicle[148].Furthermore,aUV-cleavable derivativelinkerof o-nitrobenzene(TClinker)wasemployedasa“gate”for theencapsulationofDOXinsidethemSiO2 .ItwasreportedthatuponNIR irradiation,theemittedUVlightexcitedtheTiO2 toaffordROS,causedthe photodegradationoftheTClinkerandalsoenhancedDOXreleasefora synergisticPDTandchemotherapy.
Furthermore,TiO2 nanoparticleshavealsobeenappliedasdrugcarriersfor cancerchemotherapy.Inthisregard,thesurfacesofTiO2 nanoparticleswere functionalizedbyphosphate,sulfate,andaminegroupsinasol–gelsynthetic strategywithtitaniumbutoxide[63].ThesefunctionalizedTiO2 nanoparticles werefurtherloadedwithcoppercomplexestoobtainpotentialdrugdelivery systemswithaveragesizesof4–10nm.Interestingly,themorphologyofthe functionalizedTiO2 nanoparticlesandthestructuresofthecoppercomplexes remainedunchangedafterthedrugloading.Additionally,cellviabilitytests usingthesenanoparticlesshowednotlessthana90%survivalforallthecelllines tested.Inanotherstudy,theporosityofTiO2 nanowhiskers(Ws)wereexploited fortheloadingoftheanticancerdrugdaunorubicin(DNR)[150].TheseTiO2 Wsdemonstratedgoodbiocompatibilityandenhancedphotocatalyticability. OnirradiationwithUVlight,efficientPDTwasobservedonhepaticcarcinoma cells(SMMC-7721)astherewasanincreaseintheintracellularconcentrationof theDNRthatobviouslyincreaseditsanti-tumorefficacyasshowninFigure1.6. Moreover,theexcellentdrugdeliverycapacityoftheTiO2 Wshasbeenlinkedto theirlargesurfaceareaandreactivity[2].
NanoparticlesofTiO2 havealsobeeninvestigatedfortheirapplicationinsonodynamiccancertherapyasaresultofthedeeppenetrationcapacityofultrasound radiationenablingthetargetingofdeep-seatedtumorsandotherdiseases[151]. PriortotheemergenceofTiO2 -basedsonosensitizersforsonodynamictherapy, mostsonosensitizersusedforgeneratingROSonultrasonicactivationwere organiccompoundssuchas5-aminolevulinicacid,phthalocyanines,porphyrin, etc.[152,153].ToinvestigatetheutilityofTiO2 nanoparticlesforsonodynamic therapy,Shenetal.synthesizedTiO2 -encapsulatedFe3 O4 nanoparticles,denoted asTiO2 @Fe3 O4 [151].TheTiO2 nanoparticleswereemployedasbothsonosensitizersandascarriersfortheloadingofDOX.Itwasobservedthatthecore–shell TiO2 @Fe3 O4 nanoparticlesdemonstratedapH-dependentloadingofDOXand itssubsequentreleasetoMCF-7andsublineoftheubiquitouskeratin-forming tumor(KB)cells.Additionally,ROSwereefficientlygeneratedbythe

Figure1.6 FluorescencemicroscopicimagesoftheSMMC-7721cancercellsthatweretreated withDNR(a),DNR–TiO2 nanoparticles(b),andDNR–TiO2 Ws(c).(d)Illustrationofthepossible mechanismforthecellularuptakeofTiO2 WsintheSMMC-7721cancercells.Source:Adapted withpermissionfromLietal.2009[150].CopyrightElsevierLimited.
TiO2 @Fe3 O4 nanoparticlesuponultrasoundirradiation.Owingtotheenhanced accumulationofthecore–shellnanoparticlesinthetumorregionandthe long-timeretentioneffect,acombinationalsonodynamicandchemotherapeutic outcomewasobserved,withminimalsideeffects.Toillustratethis,theyreported thatthecombinationtherapyshowedan88.36%inhibitoryeffectasagainst 38.91%and28.36%forsonodynamictherapyandchemotherapy,respectively.
Inanotherstudy,TiO2 –PEGnanoparticlesweresuccessfullysynthesizedand alsoappliedassonosensitizersinacombinationofphotodynamic(PD)andsonodynamictherapies[154].Uponirradiationofthenanoparticleswith5.0mW/cm2 laser,thenanoparticlesdemonstratedaneffectivereductionintheviabilityand membraneintegrityofhumanglioblastomacellsU251.Thegenerationofsinglet oxygenalongsideotherROSaftersonoactivationwasutilizedforsonodynamic therapyusingcore–shellTiO2 –polyallylaminemicellenanoparticlesinastudyby Haradaetal.[155].Theirfindingsareveryimportantandcapableofrevolutionizingimaging-guidedsonodynamictherapyfordiseasessuchastheneoplastic maladies.
Incombinationwithothermacromoleculesandnanomaterials,nano-sized TiO2 hasbeenusedforthesynergistictheranosticsofcertaindiseases [13,151,156,157].Tothisend,Zengetal.synthesizedTiO2 –Fe3 O4 nanocompositesandappliedsameforthetheranosticsofneoplasticdisease[13].The TiO2 nanoparticlesinthenanocompositesservedasexcellentphotosensitizers forPDT,whiletheFe3 O4 providedcontrastenhancementforMRI.Inanother experiment,TiO2 nanoparticleswereusedincombinationwithanotherphotosensitizerchlorinee6toobtainasynergisticallyenhancedPDT,contraryto thoseobtainedfromtheindividualphotosensitizers[156].