FlowerDevelopment
Methods and Protocols
Second Edition
Edited by José Luis Riechmann
Centre for Research in Agricultural Genomics CSIC-IRTA-UAB-UB, Barcelona, Spain; Institució Catalana de Recerca i Estudis Avancats (ICREA), Barcelona, Spain
Cristina Ferrándiz
Instituto de Biología Molecular y Celular de Plantas CSIC-UPV, Valencia, Spain
Editors
Jose ´ Luis Riechmann
Centre for Research in Agricultural Genomics CSIC-IRTA-UAB-UB
Barcelona, Spain
Cristina Ferra ´ ndiz
Instituto de Biologı´a Molecular y Celular de Plantas CSIC-UPV
Valencia, Spain
Institucio ´ Catalana de Recerca i Estudis Avancats (ICREA)
Barcelona, Spain
ISSN 1064-3745ISSN 1940-6029 (electronic)
Methods in Molecular Biology
ISBN 978-1-0716-3298-7ISBN 978-1-0716-3299-4 (eBook) https://doi.org/10.1007/978-1-0716-3299-4
© Springer Science+Business Media, LLC, part of Springer Nature 2023
This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed.
The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use.
The publisher, the authors, and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. Neither the publisher nor the authors or the editors give a warranty, expressed or implied, with respect to the material contained herein or for any errors or omissions that may have been made. The publisher remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This Humana imprint is published by the registered company Springer Science+Business Media, LLC, part of Springer Nature.
The registered company address is: 1 New York Plaza, New York, NY 10004, U.S.A.
Preface
Over the past 35 years, detailed insights into the genetic and molecular mechanisms that control flower development in different angiosperm species have been obtained, making great progress in the identification of key regulators of flower morphogenesis, in elucidating their organization in pathways and networks, and in characterizing the developmental process at multiple levels, from organismal to single cells. These advances have relied on the continuous development of methodologies and techniques, from molecular genetics to advanced microscopy or single-cell biology, and their implementation into plant research. To facilitate further progress in the field of flower development, this book provides a collection of protocols for many of the experimental approaches that are currently used to study the formation of flowers, from genetic methods and phenotypic analyses, to genomewide experiments, modeling, and system-wide approaches. An effort has been made to facilitate the incorporation of non-model species that can be useful to study specific developmental processes or the origin of evolutionary innovations. In addition, several introductory chapters provide an overview of the current status on the field of flower development, also highlighting open questions and future directions. Methods chapters are organized in three major sections: genetic and phenotypic analyses; experimental systems; and molecular biology, genomics, and systems biology. Each chapter contains a brief introduction, step-bystep methods, a list of necessary materials, and a Notes section with tips on troubleshooting, as well as extensive reference lists. Comprehensive and up to date, we hope that this book on flower development will become an essential guide for plant developmental biologists, from the more novice to the experienced researcher, and for those considering venturing into the field.
Barcelona, SpainJose´ Luis
SpainCristina
Riechmann Valencia,
Ferra ´ ndiz
PART IREVIEW AND OVERVIEW CHAPTERS
1 Flower Development in Arabidopsis
Hicham Chahtane, Xuelei Lai, Gabrielle Tichtinsky, Philippe Rieu, Moı¨ra Arnoux-Courseaux, Coralie Cance´, Claudius Marondedze, and Franc¸ois Parcy
2 Flower Development in the Solanaceae
Marie Monniaux and Michiel Vandenbussche
3 The ABC of Flower Development in Monocots: The Model of Rice Spikelet
Ludovico Dreni
4 Model Species to Investigate the Origin of Flowers
Charles P. Scutt
5 Hormones and Flower Development in Arabidopsis
Victor M. Zu ´ niga-Mayo, Yolanda Dura ´ n-Medina, Nayelli Marsch-Martı ´ nez, and Stefan de Folter
6 Genetic Screens for Floral Mutants in Arabidopsis thaliana: Enhancers and Suppressors 131 Zhigang Huang, Thanh Theresa Dinh, Elizabeth Luscher, Shaofang Li, Xigang Liu, So Youn Won, and Xuemei Chen
7 Genetic and Phenotypic Analysis of Shoot Apical and Floral Meristem Development 163 Mona M. Monfared, Thai Q. Dao, and Jennifer C. Fletcher
8 Cell Biological Analyses of Anther Morphogenesis and Pollen Viability in Arabidopsis and Rice
Fang Chang, Shuangshuang Wang, Zesen Lai, Zaibao Zhang, Yue Jin, and Hong Ma
199
9 Isolation of Meiocytes and Cytological Analyses of Male Meiotic Chromosomes in Soybean, Lettuce, and Maize 219 Cong Wang, Xiang Li, Jiyue Huang, Hong Ma, Chung-Ju Rachel Wang, and Yingxiang Wang
10 Genetic and Phenotypic Analyses of Carpel Development in Arabidopsis 241 Vicente Balanza ` , Patricia Ballester, Monica Colombo, Chloe´ Fourquin, Irene Martı ´ nez-Ferna ´ ndez, Clara I. Ortiz-Ramı ´ rez, and Cristina Ferra ´ ndiz
11 Genetic and Phenotypic Analysis of Ovule Development in Arabidopsis
Dayton C. Bird, Chao Ma, Sara Pinto, Weng Herng Leong, and Matthew R. Tucker
PART III EXPERIMENTAL SYSTEMS
12 Floral Induction Systems for the Study of Arabidopsis Flower Development
Diarmuid O ´ ’Maoile´idigh, Bennett Thomson, and Frank Wellmer
13 Protoplasting and Fluorescence-Activated Cell Sorting of the Shoot
Apical Meristem Cell Types
G. Venugopala Reddy
14 Protoplast Isolation for Plant Single-Cell RNA-seq .
Shulin Ren and Ying Wang
15 Plant Nuclei Isolation for Single-Nucleus RNA Sequencing .
Xu Xin, Fei Du, and Yuling Jiao
16 Isolation of Nuclei Tagged in Specific Cell Types (INTACT) in Arabidopsis
Ruben M. Benstein, Markus Schmid, and Yuan You
PART IV MOLECULAR BIOLOGY,GENOMICS, AND SYSTEMS BIOLOGY
17 RNA In Situ Hybridization on Plant Tissue Sections: Expression Analysis at Cellular Resolution
Vladislav Gramma and Vanessa Wahl
18 The GUS Reporter System in Flower Development Studies
Janaki S. Mudunkothge, C. Nathan Hancock, and Beth A. Krizek
19 Expression and Functional Studies of Leaf, Floral, and Fruit Developmental Genes in Non-model Species
Natalia Pabon-Mora, Harold Sua ´ rez-Baron, Yesenia Madrigal, Juan F. Alzate, and Favio Gonza ´ lez
20 Gene Expression Analysis by Quantitative Real-Time PCR for Floral Tissues .
Raquel A ´ lvarez-Urdiola, Mariana Bustamante, Joana Ribes, and Jose´ Luis Riechmann
21 Misexpression Approaches for the Manipulation of Flower Development . . . . . . 429
Yifeng Xu, Eng-Seng Gan, and Toshiro Ito
22 Genomic Approaches for the Study of Flower Development in Floriculture Crops 453
Tomas Hasing and Aureliano Bombarely
23 Multi-Omics Methods Applied to Flower Development 495
Raquel A ´ lvarez-Urdiola, Jose´ Toma ´ s Matus, and Jose´ Luis Riechmann
24 Peptidomics Methods Applied to the Study of Flower Development 509
Raquel A ´ lvarez-Urdiola, Eva Borra ` s, Federico Valverde, Jose´ Toma ´ s Matus, Eduard Sabido, and Jose´ Luis Riechmann
25 Quantifying Gene Expression Domains in Plant Shoot Apical Meristems . .
Pau Formosa-Jordan and Benoit Landrein
26 A NanoLuc-Based Transactivation Assay in Plants .
Rosa Esmeralda Becerra-Garcı ´ a, Jose´ Erik Cruz-Valderrama, Vincent E. Cerbantez-Bueno, Nayelli Marsch-Martı ´ nez, and Stefan de Folter
553
27 A Hands-On Guide to Generate Spatial Gene Expression Profiles by Integrating scRNA-seq and 3D-Reconstructed Microscope-Based Plant Structures 567
Manuel Neumann and Jose M. Muino
Index
Contributors
RAQUEL A ´ LVAREZ-URDIOLA • Centre for Research in Agricultural Genomics (CRAG) CSICIRTA-UAB-UB, Edifici CRAG, Campus UAB, Cerdanyola del Valle`s, Barcelona, Spain
JUAN F. ALZATE • Centro Nacional de Secuenciacion Genomica–CNSG, Sede de Investigacion Universitaria–SIU, Medellı ´ n, Antioquia, Colombia
MOIRA ARNOUX-COURSEAUX • CNRS, Universite´ Grenoble Alpes, CEA, INRAE, IRIG, BIG-LPCV, Grenoble, France
VICENTE BALANZA ` • Instituto de Biologı ´ a Molecular y Celular de Plantas CSIC-UPV, Campus de la Universidad Polite´cnica de Valencia, Valencia, Spain
PATRICIA BALLESTER • Instituto de Biologı ´ a Molecular y Celular de Plantas CSIC-UPV, Campus de la Universidad Polite´cnica de Valencia, Valencia, Spain
ROSA ESMERALDA BECERRA-GARCI ´ A • Unidad de Genomica Avanzada (UGA-LANGEBIO), Centro de Investigacion y de Estudios Avanzados del Instituto Polite´cnico Nacional (CINVESTAV-IPN), Irapuato, Guanajuato, Mexico; Departamento de Biotecnologı ´ ay Bioquı ´ mica, Unidad Irapuato, CINVESTAV-IPN, Irapuato, Guanajuato, Mexico
RUBEN M. BENSTEIN • Umea ˚ Plant Science Centre, Department of Plant Physiology, Umea ˚ University, Umea ˚ , Sweden
DAYTON C. BIRD • School of Agriculture, Food and Wine, University of Adelaide, Waite Campus, Urrbrae, SA, Australia
AURELIANO BOMBARELY • Instituto de Biologı ´ a Molecular y Celular de Plantas (IBMCP) (UPV-CSIC), Valencia, Spain
EVA BORRA ` S • Centre for Genomic Regulation (CRG), Barcelona Institute of Science and Technology, Barcelona, Spain; Universitat Pompeu Fabra, Barcelona, Spain
MARIANA BUSTAMANTE • Centre for Research in Agricultural Genomics (CRAG) CSICIRTA-UAB-UB, Edifici CRAG, Campus UAB, Cerdanyola del Valle`s, Barcelona, Spain; Roche Pharma Research and Early Development, Roche Innovation Center Basel, F. Hoffmann-La Roche Ltd., Basel, Switzerland
CORALIE CANCE ´ • CNRS, Universite´ Grenoble Alpes, CEA, INRAE, IRIG, BIG-LPCV, Grenoble, France
VINCENT E. CERBANTEZ-BUENO • Unidad de Genomica Avanzada (UGA-LANGEBIO), Centro de Investigacion y de Estudios Avanzados del Instituto Polite´cnico Nacional (CINVESTAV-IPN), Irapuato, Guanajuato, Mexico
HICHAM CHAHTANE • CNRS, Universite´ Grenoble Alpes, CEA, INRAE, IRIG, BIG-LPCV, Grenoble, France; Institut de Recherche Pierre Fabre, Green Mission Pierre Fabre, Conservatoire Botanique Pierre Fabre, Soual, France
FANG CHANG • State Key Laboratory of Genetic Engineering, Ministry of Education Key Laboratory of Biodiversity Sciences and Ecological Engineering and Institute of Biodiversity Sciences, Institute of Plant Biology, School of Life Sciences, Fudan University, Shanghai, China
XUEMEI CHEN • Department of Botany and Plant Sciences, University of California, Riverside, CA, USA
MONICA COLOMBO • Instituto de Biologı ´ a Molecular y Celular de Plantas CSIC-UPV, Campus de la Universidad Polite´cnica de Valencia, Valencia, Spain; CREA Research Centre for Genomics and Bioinformatics, Fiorenzuola d’Arda, Italy
JOSE ´ ERIK CRUZ-VALDERRAMA • Unidad de Genomica Avanzada (UGA-LANGEBIO), Centro de Investigacion y de Estudios Avanzados del Instituto Polite´cnico Nacional (CINVESTAV-IPN), Irapuato, Guanajuato, Mexico
THAI Q. DAO • Plant Gene Expression Center, USDA-ARS/UC Berkeley, Albany, CA, USA; Department of Plant and Microbial Biology, University of California, Berkeley, CA, USA; Department of Botany and Plant Pathology, Oregon State University, Corvallis, OR, USA
STEFAN DE FOLTER • Unidad de Genomica Avanzada (UGA-LANGEBIO), Centro de Investigacion y de Estudios Avanzados del Instituto Polite´cnico Nacional (CINVESTAVIPN), Irapuato, Guanajuato, Mexico
THANH THERESA DINH • Department of Botany and Plant Sciences, University of California, Riverside, CA, USA
LUDOVICO DRENI • Instituto de Biologı ´ a Molecular y Celular de Plantas (IBMCP), Consejo Superior de Investigaciones Cientı ´ ficas-Universidad Polite´cnica de Valencia, Valencia, Spain
FEI DU • State Key Laboratory of Plant Genomics, Institute of Genetics and Developmental Biology, The Innovative Academy of Seed Design, Chinese Academy of Sciences, Beijing, China
YOLANDA DURA ´ N-MEDINA • Departamento de Biotecnologı ´ a y Bioquı ´ mica, Centro de Investigacion y de Estudios Avanzados del Instituto Polite´cnico Nacional (CINVESTAVIPN), Irapuato, Guanajuato, Mexico
CRISTINA FERRA ´ NDIZ • Instituto de Biologı ´ a Molecular y Celular de Plantas CSIC-UPV, Campus de la Universidad Polite´cnica de Valencia, Valencia, Spain
JENNIFER C. FLETCHER • Plant Gene Expression Center, USDA-ARS/UC Berkeley, Albany, CA, USA; Department of Plant and Microbial Biology, University of California, Berkeley, CA, USA
PAU FORMOSA-JORDAN • Sainsbury Laboratory, University of Cambridge, Cambridge, UK; Department of Plant Developmental Biology, Max Planck Institute for Plant Breeding Research, Cologne, Germany; Cluster of Excellence on Plant Science (CEPLAS), Max Planck Institute for Plant Breeding Research, Cologne, Germany
CHLOE ´ FOURQUIN • Instituto de Biologı ´ a Molecular y Celular de Plantas CSIC-UPV, Campus de la Universidad Polite´cnica de Valencia, Valencia, Spain
ENG-SENG GAN • Republic Polytechnic, School of Applied Science (SAS), Singapore, Singapore
FAVIO GONZA ´ LEZ • Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Sede Bogota ´ , Bogota ´ , Colombia
VLADISLAV GRAMMA • Max Planck Institute of Plant Physiology, Potsdam, Germany
C. NATHAN HANCOCK • Department of Biology and Geology, University of South Carolina Aiken, Aiken, SC, USA
TOMAS HASING • ELO Life Systems, Durham, NC, USA
JIYUE HUANG • College of Life Sciences, South China Agricultural University, Guangzhou, China; Guangdong Laboratory for Lingnan Modern Agriculture, Guangzhou, China
ZHIGANG HUANG • Hunan Provincial Key Laboratory of Phytohormones and Growth Development, Hunan Agricultural University, Changsha, China
TOSHIRO ITO • Nara Institute of Science and Technology, Biological Sciences, Plant Stem Cell Regulation and Floral Patterning Laboratory, Ikoma, Nara, Japan
YULING JIAO • State Key Laboratory of Plant Genomics, Institute of Genetics and Developmental Biology, The Innovative Academy of Seed Design, Chinese Academy of Sciences, Beijing, China; State Key Laboratory of Protein and Plant Gene Research, Peking-
Contributorsxiii
TsinghuaCenterfor PekingUniversity,Be
Life Sciences, Center for Quantitative Biology, School of Life Sciences, ijing, China
YUE JIN • State Key Laboratory of Genetic Engineering, Ministry of Education Key Laboratory of Biodiversity Sciences and Ecological Engineering and Institute of Biodiversity Sciences, Institute of Plant Biology, School of Life Sciences, Fudan University, Shanghai, China
BETH A. KRIZEK • Department of Biological Sciences, University of South Carolina, Columbia, SC, USA
XUELEI LAI • CNRS, Universite´ Grenoble Alpes, CEA, INRAE, IRIG, BIG-LPCV, Grenoble, France; Huazhong Agricultural University, National Key Laboratory of Crop Genetic Improvement, Hubei Hongshan Laboratory, Wuhan, China
ZESEN LAI • State Key Laboratory of Genetic Engineering, Ministry of Education Key Laboratory of Biodiversity Sciences and Ecological Engineering and Institute of Biodiversity Sciences, Institute of Plant Biology, School of Life Sciences, Fudan University, Shanghai, China
BENOIT LANDREIN • Sainsbury Laboratory, University of Cambridge, Cambridge, UK; Laboratoire Reproduction et De´veloppement des Plantes, Universite´ de Lyon, ENS de Lyon, UCB Lyon 1, CNRS, INRAE, INRIA, Lyon, France
WENG HERNG LEONG • School of Agriculture, Food and Wine, University of Adelaide, Waite Campus, Urrbrae, SA, Australia
SHAOFANG LI • Department of Botany and Plant Sciences, University of California, Riverside, CA, USA
XIANG LI • College of Horticulture, Henan Agricultural University, Zhengzhou, China
XIGANG LIU • Department of Botany and Plant Sciences, University of California, Riverside, CA, USA
ELIZABETH LUSCHER • Department of Botany and Plant Sciences, University of California, Riverside, CA, USA
CHAO MA • School of Agriculture, Food and Wine, University of Adelaide, Waite Campus, Urrbrae, SA, Australia
HONG MA • Department of Biology, The Huck Institutes of the Life Sciences, The Pennsylvania State University, University Park, PA, USA
YESENIA MADRIGAL • Instituto de Biologı ´ a, Universidad de Antioquia, Medellı ´ n, Colombia
CLAUDIUS MARONDEDZE • CNRS, Universite´ Grenoble Alpes, CEA, INRAE, IRIG, BIG-LPCV, Grenoble, France; Department of Biochemistry, Faculty of Medicine, Midlands State University, Senga, Gweru, Zimbabwe
NAYELLI MARSCH-MARTI ´ NEZ • Departamento de Biotecnologı ´ a y Bioquı ´ mica, Centro de Investigacion y de Estudios Avanzados del Instituto Polite´cnico Nacional (CINVESTAVIPN), Irapuato, Guanajuato, Mexico
IRENE MARTI ´ NEZ-FERNA ´ NDEZ • Instituto de Biologı ´ a Molecular y Celular de Plantas CSICUPV, Campus de la Universidad Polite´cnica de Valencia, Valencia, Spain
JOSE ´ TOMA ´ S MATUS • Centre for Research in Agricultural Genomics (CRAG) CSIC-IRTAUAB-UB, Edifici CRAG, Campus UAB, Cerdanyola del Valle`s, Barcelona, Spain; Institute for Integrative Systems Biology (I2SysBio), Universitat de Vale`ncia-CSIC, Paterna, Valencia, Spain
MONA M. MONFARED • Plant Gene Expression Center, USDA-ARS/UC Berkeley, Albany, CA, USA; Department of Plant and Microbial Biology, University of California, Berkeley, CA, USA; Department of Molecular and Cellular Biology, University of California, Davis, CA, USA
MARIE MONNIAUX • Laboratoire Reproduction et De´veloppement des Plantes, Universite´de Lyon, ENS de Lyon, UCB Lyon 1, CNRS, INRAE, Lyon, France
JANAKI S. MUDUNKOTHGE • Department of Biological Sciences, University of South Carolina, Columbia, SC, USA
JOSE M. MUINO • Institute for Biology, Humboldt-Universit € at zu Berlin, Berlin, Germany
MANUEL NEUMANN • Institute for Biology, Humboldt-Universit € at zu Berlin, Berlin, Germany
DIARMUID O ´ ’MAOILE ´ IDIGH • Department of Biology, Maynooth University, Maynooth, County Kildare, Ireland
CLARA I. ORTIZ-RAMI ´ REZ • Instituto de Biologı ´ a Molecular y Celular de Plantas CSIC-UPV, Campus de la Universidad Polite´cnica de Valencia, Valencia, Spain
NATALIA PABO ´ N-MORA • Instituto de Biologı ´ a, Universidad de Antioquia, Medellı ´ n, Colombia
FRANC¸ OIS PARCY • CNRS, Universite´ Grenoble Alpes, CEA, INRAE, IRIG, BIG-LPCV, Grenoble, France
SARA PINTO • LAQV REQUIMTE, Departamento de Biologia, Faculdade de Cieˆncias da Universidade do Porto, Porto, Portugal
G. VENUGOPALA REDDY • Department of Botany and Plant Sciences, Center for Plant Cell Biology (CEPCEB), Institute of Integrative Genome Biology (IIGB), University of California, Riverside, CA, USA
SHULIN REN • College of Life Sciences, University of Chinese Academy of Sciences, Beijing, China
JOANA RIBES • Centre for Research in Agricultural Genomics (CRAG) CSIC-IRTA-UABUB, Edifici CRAG, Campus UAB, Cerdanyola del Valle`s, Barcelona, Spain
JOSE ´ LUIS RIECHMANN • Centre for Research in Agricultural Genomics (CRAG) CSICIRTA-UAB-UB, Edifici CRAG, Campus UAB, Cerdanyola del Valle`s, Barcelona, Spain; Institucio Catalana de Recerca i Estudis Avanc¸ats (ICREA), Barcelona, Spain
PHILIPPE RIEU • CNRS, Universite´ Grenoble Alpes, CEA, INRAE, IRIG, BIG-LPCV, Grenoble, France; Structural Plant Biology Laboratory, Department of Botany and Plant Biology, University of Geneva, Geneva, Switzerland
EDUARD SABIDO ´ • Centre for Genomic Regulation (CRG), Barcelona Institute of Science and Technology, Barcelona, Spain; Universitat Pompeu Fabra, Barcelona, Spain
MARKUS SCHMID • Umea ˚ Plant Science Centre, Department of Plant Physiology, Umea ˚ University, Umea ˚ , Sweden; Department of Plant Biology, Linnean Centre for Plant Biology, Swedish University of Agricultural Sciences, Uppsala, Sweden
CHARLES P. SCUTT • Laboratoire Reproduction et De´veloppement des Plantes, Universite´de Lyon, ENS de Lyon, UCB Lyon-1, CNRS, INRA, Lyon, France
HAROLD SUA ´ REZ-BARON • Instituto de Biologı ´ a, Universidad de Antioquia, Medellı ´ n, Colombia; Departamento de Ciencias Naturales y Matema ´ ticas, Pontificia Universidad Javeriana, Cali, Colombia
BENNETT THOMSON • Smurfit Institute of Genetics, Trinity College Dublin, Dublin, Ireland
GABRIELLE TICHTINSKY • CNRS, Universite´ Grenoble Alpes, CEA, INRAE, IRIG, BIG-LPCV, Grenoble, France
MATTHEW R. TUCKER • School of Agriculture, Food and Wine, University of Adelaide, Waite Campus, Urrbrae, SA, Australia
FEDERICO VALVERDE • Institute for Plant Biochemistry and Photosynthesis CSIC – University of Seville, Seville, Spain
MICHIEL VANDENBUSSCHE • Laboratoire Reproduction et De´veloppement des Plantes, Universite´ de Lyon, ENS de Lyon, UCB Lyon 1, CNRS, INRAE, Lyon, France
VANESSA WAHL • Max Planck Institute of Plant Physiology, Potsdam, Germany; The James Hutton Institute, Dundee, UK
CHUNG-JU RACHEL WANG • Institute of Plant and Microbial Biology, Academia Sinica, Taipei, Taiwan
CONG WANG • College of Life Sciences, South China Agricultural University, Guangzhou, China; Guangdong Laboratory for Lingnan Modern Agriculture, Guangzhou, China
SHUANGSHUANG WANG • State Key Laboratory of Genetic Engineering, Ministry of Education Key Laboratory of Biodiversity Sciences and Ecological Engineering and Institute of Biodiversity Sciences, Institute of Plant Biology, School of Life Sciences, Fudan University, Shanghai, China; School of Life Sciences, East China Normal University, Shanghai, China
YING WANG • College of Life Sciences, University of Chinese Academy of Sciences, Beijing, China
YINGXIANG WANG • College of Life Sciences, South China Agricultural University, Guangzhou, China; Guangdong Laboratory for Lingnan Modern Agriculture, Guangzhou, China
FRANK WELLMER • Smurfit Institute of Genetics, Trinity College Dublin, Dublin, Ireland
SO YOUN WON • Department of Botany and Plant Sciences, University of California, Riverside, CA, USA
XU XIN • State Key Laboratory of Plant Genomics, Institute of Genetics and Developmental Biology, The Innovative Academy of Seed Design, Chinese Academy of Sciences, Beijing, China; College of Advanced Agricultural Sciences, University of Chinese Academy of Sciences, Beijing, China
YIFENG XU • College of Life Sciences, Nanjing Agricultural University, Nanjing, Jiangsu, China
YUAN YOU • Center for Plant Molecular Biology (ZMBP), Department of General Genetics, Eberhard Karls University of Tu¨bingen, Tu¨bingen, Germany; Department of Molecular Life Sciences, Technical University of Munich, Freising, Germany
ZAIBAO ZHANG • State Key Laboratory of Genetic Engineering, Ministry of Education Key Laboratory of Biodiversity Sciences and Ecological Engineering and Institute of Biodiversity Sciences, Institute of Plant Biology, School of Life Sciences, Fudan University, Shanghai, China
VICTOR M. ZU ´ NIGA-MAYO • CONACyT – Postgrado en Fitosanidad-Fitopatologı ´ a, Colegio de Postgraduados, Campus Montecillo, Montecillo, Estado de Me´xico, Mexico
Flower Development in Arabidopsis
Hicham Chahtane, Xuelei Lai, Gabrielle Tichtinsky, Philippe Rieu, Moı ¨ ra Arnoux-Courseaux, Coralie Cance ´ , Claudius Marondedze, and Franc¸ois Parcy
Abstract
Like in other angiosperms, the development of flowers in Arabidopsis starts right after the floral transition, when the shoot apical meristem (SAM) stops producing leaves and makes flowers instead. On the flanks of the SAM emerge the flower meristems (FM) that will soon differentiate into the four main floral organs, sepals, petals, stamens, and pistil, stereotypically arranged in concentric whorls. Each phase of flower development floral transition, floral bud initiation, and floral organ development is under the control of specific gene networks. In this chapter, we describe these different phases and the gene regulatory networks involved, from the floral transition to the floral termination.
Key words Flower development, Arabidopsis, Meristem, MADS-box
1 Part 1: The Floral Transition
The moment at which the floral transition occurs in plants is key for reproductive success and is fine-tuned by external and internal cues. Eventually, the signaling pathways of these cues converge on a handful of genes called floral integrators that make the link between the regulation of flowering time and the triggering of flower development [1]. Among these integrators, the gene FLOWERING LOCUS T (FT) plays a fundamental role [2](see Fig. 1). It encodes a mobile protein produced in the leaves and that travels through the phloem to activate other floral integrators at the SAM [3]. These include LEAFY (LFY), an orphan and plant-specific transcription factor (TF) of the Helix-Loop-Helix class and SUPPRESSOR OF CONSTANS OVEREXPRESSION 1 (SOC1), a TF belonging to the MADS-box TF family [4, 5]. As a result, the onset of expression of the florigen FT greatly influences the passage from the vegetative
Jose ´ Luis Riechmann and Cristina Ferrandiz (eds.), Flower Development: Methods and Protocols, Methods in Molecular Biology, vol. 2686, https://doi.org/10.1007/978-1-0716-3299-4_1, © Springer Science+Business Media, LLC, part of Springer Nature 2023

Fig. 1 Simplified representation of the gene network involved in floral transition. Close up on the vegetative SAM containing leaves (in green) before the floral transition. Internal (grey panel) and external (yellow panel) cues regulate the expression of the florigen FT. Sugar content represented as T6P, and nitrates, are both positive regulators of flowering and activate indirectly FT expression. The age-dependent pathway is under the control of SPL and miRNA156. When the plant ages, SPL accumulates and negatively regulates AP2-like transcription through mir172 to promote FT transcription. Temperature responses are mediated at least by SVP, FLC, and PIF4. Increased ambient temperature induces chromatin accessibility allowing PIF4 to activate FT. Upon cold response, SVP (in complex with FLMβ) represses FT transcription. Vernalization response is mediated by the repressive activity of FLC on FT. In daylight conditions such as in the afternoon, GI promotes CO by modulating CDF activity. PHYA promotes CO stability during the day. During the night, the complex COP1/SPA/ELF3 represses CO activity. Once FT is promoted, the florigen complex FT/FD acts in the SAM to activate several floral integrators such as SOC1, AP1 and indirectly LFY. Accumulation of the floral integrators induces the SAM to switch to the reproductive phase. Please note that the crosstalk between pathways exist and that this simplified representation does not include all the regulation processes discussed in the text
1.1External
Parameters
Influencing
Flowering Temperature and Photoperiod
1.1.1 Temperature
to the reproductive stage. This peculiar gene acts as a hub to integrate several external and internal signals and its regulation is highly controlled by a myriad of mechanisms [6](see Fig. 1). We will mention a non-exhaustive list of the major actors controlling FT expression.
Temperature is a major determinant of flowering and is effective through two distinct mechanisms (see review by Susila, et al. [7] for details). One is a prolonged exposure of seedlings to cold called vernalization, whereas the other results in the internalization of the ambient temperature [8–10].
The central gene in vernalization is FLOWERING LOCUS C (FLC), which encodes a MADS-box TF [11](see Fig. 1). Genetic, transcriptomic and genome-wide binding profiles of FLC revealed its main activities in the direct repression of two major floral integrators, FT and SOC1 [12]. The action of FLC on FT and SOC1 is partially under the control of gibberellic acid (GA) sensitive proteins DELLA, where DELLA and FLC form a repressor complex to downregulate the expression of floral integrators, revealing the crosstalk between vernalization and hormonal pathways [13]. The expression of FLC is strongly and negatively correlated with flowering time and FT expression [11]. The main positive regulator of FLC is FRIGIDA (FRI), which maintains high FLC mRNA level and conditions the plant to remain in the vegetative state. On the other hand, FLC expression is suppressed by the chromatin remodelling protein VERNALIZATION INSENSITIVE3 (VIN3), whose expression is rapidly induced after a cold period [14]. More recently, another mode of regulation of FLC has been discovered, involving post-transcriptional mechanisms (see review for details [15, 16]). It has been shown that cold exposure induces the formation of two types of non-coding RNA variants of FLC, called COOLAIR (for COLD INDUCED LONG ANTISENSE INTRAGENIC RNA) and COLDAIR (for COLD ASSISTED INTRONIC NONCODING RNA)[17, 18]. These two non-coding RNAs repress FLC by either preventing its translation (in the case of COOLAIR) or by promoting epigenetic repression at the locus [18–21].
In addition to the effect of temperature through vernalization, the ambient temperature also strongly affects flowering time. This control likely involves a wealth of pathways and mechanisms and research is still ongoing to understand what are the major ones [10]. For example, a recent study described how ambient temperature controls the degree of unsaturation of phosphatidyl glycerol, that in turn affects the retention of the FT protein in leaf cells [22]. Higher temperature reduces unsaturation leading to increased release of FT in phloem companion cells and accelerated flowering [22, 23].
The SHORT VEGETATIVE PHASE (SVP) gene, which also belongs to the MADS-box family, acts as a repressor of flowering, particularly on FT transcription [24, 25]. How ambient temperature regulates SVP has been recently unravelled but is still under debate (see discussion in [26]). Like other MIKC-type MADS box proteins, SVP acts in complex with homologous proteins to repress FT transcription [27]. One of them is FLOWERING LOCUS M (FLM) that is transcribed as several splice variants in a temperaturedependent manner [27, 28]. One splice variant, called FLM-β,is preferentially produced when the temperature is cooler. At low temperature, SVP preferentially interacts with FLM-β, which allows SVP to be translocated to the nucleus and to inhibit suppresses FT expression [27–29](see Fig. 1). In addition to the transcriptional regulation of FT by SVP, the stability of SVP protein is also temperature-dependent: it is degraded via the proteasome when the ambient temperature increases, reducing further the SVP repressive activity on FT level under elevated temperature [30].
PHYTOCHROME INTERACTING FACTOR4 (PIF4) protein has also been proposed to regulate FT expression in a temperature-dependent manner [31]. PIF4 binds directly to a regulatory region of the FT promoter that is only accessible at high temperature (see Fig. 1). More recent shreds of evidence suggest that PIF4 activity is highly dependent on external temperature, mainly due to its interaction with the thermosensor protein PHYTOCHROME B (PHYB) (see review for details [32]). In addition, others temperature-dependent mechanism regulating PIF4 expressions have recently been identified in response to warm temperature. One involved the TF CYCLOIDEA AND PCF TRANSCRIPTION FACTOR 5 (TCP5), which is stabilized under heat stress, and both positively regulates PIF4 expression and modulates its activity to integrate thermomorphogenesis responses [33]. Other mechanisms mediated by the chromatin remodelling POWERDRESS protein participates in the regulation of histone accessibility at the PIF4 locus after elevated temperature and thus is a critical component of the temperature responses [34].
Recently, it has been shown that EARLY FLOWERING3 (ELF3), a member of the evening complex (EC; Consists of ELF3, ELF4 and LUX ARRYTHMO (LUX)) involved in circadian clock regulation, also controls the response to ambient temperature. elf3 mutant display a strong early flowering phenotype, due to ELF3 repressive action on FT expression [35, 36]. The activity of the prion-like domain protein ELF3, and its binding to specific genomic regions is dependent on temperature, suggesting that the EC could act as a sensor of temperature to control flowering [35, 37–40]. Interestingly, several light signaling components such as PHYB and ELF3 bind common genomic regions, suggesting that these two proteins act additively to integrate external cues (temperature and light) to modulate flowering [38].
1.1.2 Photoperiod The onset of flowering is also largely regulated by the duration of light exposure, a mechanism called photoperiod perception, as revealed by the earlier Arabidopsis blooming in long day (LD) relative to short day (SD) conditions (see review [41]). The major actor of the photoperiodic pathway is the CONSTANS (CO) gene, which encodes a B-box TF directly responsible for the transcription of FT. In growth chamber conditions, the CO gene is repressed in the early morning, then its transcription increases at the end of the day and during the night [42, 43]. This rhythmic regulation is controlled by two complexes: CYCLING DOF FACTOR (CDF) and GIGANTEA (GI)/FLAVIN-BINDING KELCH REPEAT-F-BOX 1 (FKF1). During the morning, the CDFs directly represses the transcription of CO while the rest of the day and night the GI/FKF1 complex strongly represses the CDFs transcription, allowing indirect activation of CO [44](see Fig. 1). Other factors such as light quality influence the activity of GI/FKF1 complex and add another layer of complexity to the regulation of CO (see review for details [41, 44]).
Regulation of CO also occurs at the post-transcriptional level. During the day, perception of light involves PHYTOCHROMES (PHY) such as PHYA, which stabilizes CO and thus affects FT regulation [42](see Fig. 1). During the night, CO protein is degraded by the proteasome through the action of an E3 ubiquitin ligase complex involving CONSTITUTIVE PHOTOMORPHOGENESIS1 (COP1) and SUPPRESSOR OF PHYA-105 protein (SPA1) [43]. ELF3 also physically interacts with COP1/SPA complex and is key to negatively affect CO stability [36, 45](see Fig. 1).
Importantly, several studies highlight different genetic responses to flowering when plants were grown in artificially controlled conditions compared to natural conditions. For instance, a morning peak of FT expression is observed only under natural environments but not in lab-controlled conditions [36]. This is probably due to changes in CO stability, where the protein is slowly degraded in natural environments due to different light and circadian clock crosstalk [36]. Another example is illustrated by the light spectrum used in classic lab growth conditions, which most of the time lack ultraviolet-B (UV-B) wavelength. Plants sense UV-B thanks to UV RESISTANT LOCUS 8 (UVR8) photoreceptor and respond by upregulating several genes, including the REPRESSOR OF UV-B PHOTOMORPHOGENESIS 2 (RUP2) (see review for details [46]). Under SD conditions and in response to light containing UV-B, RUP2 is induced and acts as a repressor of flowering by preventing FT expression via physical interaction with CO and inactivation of CO activity [47]. This study reveals an UVR8-dependent flowering pathway (under specific photoperiod conditions) and again challenges the classical use of standardized conditions found in many labs to study flowering time regulation.
1.2 Internal Cues—
Metabolism and AgeDependent Pathways
1.2.1 Sugar and Nitrate
Metabolism
1.2.2 Age-Dependent Pathway
Besides the above-mentioned external factors that play an important role in flowering, plants also use endogenous signals such as metabolic cues to trigger the energy-consuming production of flowers, fruits, and seeds. As a result, internal signals such as energy reserves or the perception of age allow a genetic control of genes involved in flowering.
Trehalose 6-phosphate (T6P) is a disaccharide that acts as a sensor of the plant’s energy reserves [48]. T6P is synthesized by TREHALOSE-6-PHOSPHATE SYNTHASE1 (TPS1), an enzyme presents in leaves and SAM. Mutation in the TPS1 gene leads to the death of the embryo, demonstrating the key function of this gene in the plant life cycle [49]. An ingenious complementation experiment to produce plants with reduced T6P level by driving TPS1 expression under a seed-specific promoter to promote embryogenesis and seedling development shows that those plants are late flowering [49]. This experiment strongly suggests that T6P is an endogenous signal that positively regulates flowering. Further study confirmed the fundamental role of T6P in the positive regulation of FT and also genes involved in the age-dependent pathway (which will be discussed later in the review) [50](see Fig. 1). However, it is still unclear how T6P triggers downstream events leading to gene regulation of FT and other targets, including its perception, integration, and activation of transcriptional targets. Nitrate availability is another key component that positively regulates floral transition, for example, a delayed of flowering is often observed when a suboptimal concentration of nitrate is used [51]. Master regulators of nitrates signaling, the TFs NIN-LIKE PROTEIN 6 and 7 (NLP6 and 7) probably directly regulate SQUAMOSA PROMOTER BINDING PROTEIN-LIKE 3 (SPL3) expression in a nitrate-dependent manner. This leads to the upregulation of SOC1 expression, a key floral integrator in the nitrate response, thus promoting bolting [51](see Fig. 1). Interestingly, crosstalk between nitrate signaling, T6P metabolism and photoperiod pathway exists, highlighting the importance of the metabolic state to flowering regulation [51].
During the vegetative growth, the morphology of successive leaves evolves with age, with changes in size, shape, petiole length, and trichome distribution [52]. This is due to a juvenile-to-adult phase transition conditioning flowering (see [53] for details). This transition is mainly under the control of an interplay between microRNAs and TFs. Vegetative growth is controlled by miR156 and its targets, the members of the SPL TF family [54]. SPL protein levels increase with leaf age, whereas the miR156 is abundant during the juvenile phase. In young leaves, high levels of miR156 repress SPL translation [55]. Thus, the miR156/SPL ratio represents a sensor of the age of the plant. Upon miR156 decrease, SPL proteins are
1.3 Integration of the Different Input to Promote Flowering and Flower Formation
produced and induce the synthesis of miR172, which targets several members of the APETALA2 (AP2) TF family [56](see Fig. 1). Among them, TARGET OF EAT 1 binds to CO and inhibits its activity, leading to FT repression [57]. Some SPLs, such as SPL9 or SPL3, also activate flowering by acting directly on the transcription of integrators such as LFY and SOC1 in the SAM [54, 55, 58].
Genetic analyses revealed that the age-dependent pathway controlling flowering is highly dependent on environmental conditions or plant species. For instance, multiple spl mutants flowered similarly later in both LD or SD conditions in Arabidopsis Col-0 ecotype [54], whereas in others plants such as Arabis alpina or Cardamine flexuosa the interplay between the photoperiod or vernalization pathways revealed the importance of the SPL/miRNA156/miR172 pathway on flowering [59, 60].
The different signaling pathways described above converge towards the regulation of FT either in leaves or in the SAM. Signals in favor of blooming result in an accumulation of FT protein in leaves, where it is loaded via the phloem companion cells and transported to the SAM. In association with the bZIP TF FLOWERING LOCUS D (FD), the complex FT/FD activates the transcription of other floral integrators such as SOC1 and APETALA1 (AP1) [2] (see Fig. 1). Using genome-wide analyses, it has been shown that FD binds not only G-boxes (as expected for a bZIP TF) but also non-canonical DNA motifs [61]. Once activated by FT/FD, AP1 and SOC1 (in association with AGL24) directly activate LFY in the SAM to promote flowering [62–64](see Fig. 1).
The FT/FD complex must act transiently to promote the expression of the floral integrators AP1 and LFY since a prolonged FT/FD activity prevents the development of normal flowers [65]. Once the floral integrators accumulate in SAM, the plant starts to produce floral primordia, the earliest recognizable stage of a flower.
2 Part 2: The First Hours of Arabidopsis Flowers
Whether a primordium will become a leaf or a flower, its emergence involves a set of basic regulators including the auxin phytohormone. In both cases, auxin distribution and signaling are critical but the auxin-driven floral primordia emergence requires the action of a few floral regulators, which will confer the floral fate (reviewed in [66]).
2.1 Primordium
Positioning and Auxin
Signaling
Clear evidence from the role of auxin in flower development came from the study of the pin-formed 1 (pin1) and pinoid (pid) mutants that lack floral primordia: they are a phenocopy of auxin polar transport inhibitor application and can be rescued by exogenous
2.2 Auxin
Biosynthesis and Transport
auxin application [67–70]. Auxin indeed controls the regular and predictable arrangement of leaves and flowers around the stem, called phyllotaxis. Several genetic and mathematical evidences showed that the regulation of auxin localization leads to a selforganized pattern involving local auxin accumulation surrounded by auxin depletion, thereby creating the heterogeneity triggering organ initiation [70–73]. This process is made more robust by the interplay between auxin and cytokinin signaling [74]. Deciphering the roles of auxin distribution in flower initiation implies to detail auxin biosynthesis, transport, and signaling.
Local auxin synthesis, in addition to polar auxin transport, also contributes to flower emergence (see [75] for review). The major actors in the tryptophan-dependent auxin biosynthesis are proteins encoded by TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS/YUCCA (YUC) genes. Their role is most obvious in the yuc1 yuc2 yuc4 yuc6 quadruple mutant, which makes almost no floral bud or only rudimentary incomplete flowers [76].
Auxin dynamic transport, named polar auxin transport, is driven at the plasma membrane by the specific influx and efflux carriers, respectively encoded by the AUXIN RESISTANT1 (AUX1) and PIN FORMED1 (PIN1) gene families [77](see Fig. 2). Local auxin accumulation and depletion are mediated by asymmetric localization of PIN proteins, which drive auxin in the epidermis and generate an auxin sink below the primordia after they have formed [70, 78]. PIN1 polarity undergoes a dynamic change during organ initiation due to the action of PID, a serine-threonine protein kinase, and of a PP2A-phosphatase responsible for the phosphorylation status and the asymmetric distribution of PIN1 in the young floral meristem [79, 80]. More recently, D6 PROTEIN KINASE (D6PK) has been shown to also phosphorylate PIN1 proteins and be required for efficient PIN1 distribution in the inflorescence [81]. However, the two kinases have non-redundant roles as their phosphosites on PIN1 are different and d6pk and pid display distinct phenotypes. PIN1 dynamics in the cell also involve the MACCHI-BOU4 (MAB4) gene family, which encode NONPHOTOTROPIC HYPOCOTYL 3-like proteins and regulate PIN1 endocytosis. Multiple mutants for MAB4 genes (such as the triple mab4 mel1 mel2 mutant) have pin-like inflorescences [82].
2.3 Downstream of Auxin Signaling
Cells transcriptionally respond to auxin through the nuclear auxin pathway, which involves AUXIN RESPONSE FACTORs (ARFs), Aux/IAA repressors, and the TIR1/AFB auxin co-receptors [70, 83]. ARF5 (also referred to as MONOPTEROS, MP) is the major ARF acting in the floral meristem initiation, as some mp/arf5 mutant alleles lack floral buds on the stem [84, 85] (see Fig. 2). MP induces floral meristem emergence in various ways, such as

Fig. 2 Key regulators involved in floral meristem initiation. Close up on the nascent FM (stage 2) on the flank of the SAM. Locally produced auxin (for instance IAA) by YUC biosynthesis enzymes is canalized in the epidermis (L1) of the flank of the SAM by the PIN transporters (under the control of PID kinase) to form an auxin maximum. This auxin peak triggers the activation of ARF5/MP TF through degradation of the associated Aux/IAA repressors. ARF5/MP is the main factor between auxin and downstream events. Associated with SYD and BRM chromatin remodelers, it activates the AINT/AIL6 and LFY/RAX1 cascades that control redundantly meristem initiation. ARF5/MP also induces FIL, involved in meristem initiation and in specifying the abaxial territory of the FM. The WUS/CLV meristem homeostasis loop is indicated as central to allow the formation of the floral meristem, but the way it is controlled by upstream actors is still unclear. In collaboration with ARF3 and HDA19, FIL also represses the expression of the SAM marker STM. On the other hand, STM is induced by mechanical stress at the border between the SAM and the FM. This spatial regulation of STM, in collaboration with CUC genes and other partners mentioned in the text, allows the establishment of a clear border between the SAM and the FM
2.4 Boundaries and Polarization Set the Limit of the Floral Primordium
reinforcing local auxin maxima by orienting PIN polarity in a non-cell autonomous manner, possibly via the activation of MAB4 [82, 86].
Auxin activated ARF5 also regulates the expression of two early acting regulators of floral meristem identity, AINTEGUMENTA (ANT), an AP2/ERF TF, and LFY [87–90]. ARF5 recruits the chromatin remodelers BRAHMA (BRM) or SPLAYED (SYD), which are subunits of the SWI/SNF (SWITCHING DEFECTIVE/SUCROSE NON FERMENTING) complex that enables chromatin opening, thus leading to gene activation of ANT and LFY [91](see Fig. 2). Both ANT and LFY participate in floral primordium emergence, a role that remains cryptic in ant or lfy single mutants but is revealed in mutant combinations such as pin ant ail, lfy pid or lfy pin [92–94]. Moreover, ANT and its homolog AINTEGUMENTA like 6 (AIL6) bind common genomic regions to activate (likely directly) LFY expression in parallel to ARF5 as well as a wide range of genes involved in floral development [95, 96]. The few ARF5 direct targets identified in FM include MAB4 and ARABIDOPSIS HISTIDINE PHOSPHOTRANSFER PROTEIN6 (AHP6), a negative regulator of cytokinin signaling that reinforces the auxin pattern adding robustness to the phyllotaxis [5, 74]. Further efforts such as elucidating the ARF5 binding landscape in floral tissue will shed light on the gene network involved in this step [97].
Genes involved in abaxial/adaxial polarity such as REVOLUTA (REV) and FILAMENTOUS FLOWER (FIL) are also necessary during flower meristem development as attested by floral defects in the corresponding mutants or the fil rev double [98–101]. REV may function by modulating local response to auxin during floral primordium formation [102]. Other important factors involved in early meristem development include several TFs such as LATERAL SUPPRESSOR (LAS), CUP-SHAPED COTYLEDON 1 to 3 (CUC1–3), or REGULATOR OF AXILLARY MERISTEMS 1 (RAX1) [103–107]. Particularly, RAX1 is expressed in the earliest stage of floral primordium and directly activated by LFY [108]. Conversely, the expression of the SHOOT MERISTEMLESS (STM), a SAM marker gene, is repressed at the site of auxin maxima, where the floral bud forms. This repression is important to promote the initiation of floral primordium and is controlled by at least two convergent pathways [109] (see Fig. 2). First, STM expression is downregulated directly by the repressive class B ARF3 TF that binds to STM second intron. In parallel, ARF5 directly activates FIL, which in turn participates in STM repression [91, 109]. Based on protein-protein interactions and genetic data, it has been proposed that FIL/ARF3 complex jointly recruits the histone deacetylase HDA19 to promote chromatin compaction on STM locus
2.5 Establishment of a Transient Stem Cell Identity
and ensure its silencing [109]. This dual function of ARF proteins warrants stable repression of STM in the early floral primordium steps, to allow proper development of floral primordia initiation.
Floral meristem initiation also involves re-establishing a stem cell niche in the flower with a combined expression of CLAVATA3 (CLV3), a stem cell marker gene, and WUSCHEL (WUS), a master regulator of the organizing center [110, 111]. As in the SAM, these two regulators form a minimal genetic circuitry that ensures a transient stem cell niche formation. Models have been proposed on how a combination of signals (hormonal or derived from the L1 layer) should re-establish an organizing center when the flower meristem reaches a certain size [112]. However, the necessary connection to the early FM regulators (MP, LFY, ANT) remains elusive (see Fig. 2).
2.6 Modifications in Cell Wall Composition Trigger Floral Outgrowth
Whereas gene regulatory networks between TFs have been unravelled, few connections have been made with terminal actors bearing the enzymatic activities that shape cells. This gap started to be filled when ANT and AIL6 were shown to promote cell wall polysaccharide modification leading to change in cell growth [113]. The importance of cell wall composition is illustrated by mutations in several GLYCOSYLTRANSFERASES OF THE CELLULOSE SYNTHASE LIKE D (CSLD) genes that strongly affect the number of floral primordia [114]. This likely affects auxin distribution, as revealed by the reduced pattern of the auxin transporter PIN1 in some cell wall cellulose biosynthesis mutant, such as cesa3 [115]. Also, flower primordia express a specific set of glycosyltransferases, suggesting that the FM outgrowth is facilitated by local modification of the physical properties of the cell wall [114]. The regulation of plant cell growth is indeed closely linked to the cell wall structure [116]. Combining use of the naked pin1 stem with chemical and physical treatments, Sassi et al. illustrated how auxin affects cell wall stiffness and microtubule orientation. Before floral outgrowth, cell cortical microtubules are anisotropic thereby promoting vertical growth and inhibiting bulging. An auxin maximum reduces anisotropy and cell wall stiffness, promoting the emergence of the FM [117]. This change in cell wall properties and tissue shape generates local mechanical perturbations that rapidly induces STM in FM boundaries [118](see Fig. 2).
3 Part 3: Determination of the Floral Primordia Identity
3.1 Activation of the Floral Meristem Identity
While mutants affected in floral primordiaoutgrowth show naked stems, other mutants develop lateral structures but they lack a proper floral identity. This is typically the case of lfy, where flowers are usually replaced by inflorescences subtended by leaves or by
3.2 Repression of the Inflorescence Trait
abnormal flowers often bearing bracts. LFY encodes an orphan plant-specific TF which controls FM identity together with the MADS-Box gene AP1 or CAULIFLOWER (CAL), a close homolog of AP1 [5, 119, 120]. They are both expressed early in the FM, LFY from the floral stage 0 on and immediately inducing AP1. LFY and AP1 orchestrate the robust acquisition of floral meristem identity thanks to a feed-forward and positive feedback loop between them and with the class I HD-Zip TF LATE MERISTEM IDENTITY2 (LMI2) [63, 121–123].
In parallel to the direct AP1 activation, LFY also modulates the GA hormone pathway: it induces expression of ELA1, which encodes a gibberellin catabolic enzyme thus leading to reduced GA levels in the FM [124]. This GA reduction frees the AP1 activator SPL9 from the repression by the DELLA proteins. Therefore, multiple activations of AP1 by LFY ensure an irreversible acquisition of floral identity (see Fig. 3).
Several new LFY and AP1 upstream regulators have been recently identified: the DORNRO ¨ SCHEN (DRN), known as a direct target of MP/ARF5 in the embryo [85], and DRNL and PUCHI TFs (all belonging to the AP2/EREBP family) act redundantly with the BTB/POZ proteins BOP1/2 to regulate LFY expression. The quintuple drn drnl puchi bop1 bop2 exhibits such a strong decrease in LFY expression that it phenocopies the lfy mutant [125]. BOP1/2 proteins not only affect LFY expression, but these components of an E3 ligase complex also seem to promote LFY activity through a post-transcriptional mechanism [126, 127].
The irreversible switch towards flower identity requires the repression of the inflorescence identity promoted by TERMINAL FLOWER 1 (TFL1) and other genes such as SOC1, SVP, and AGL24. TFL1 belongs to the same family as FT but acts oppositely: while FT promotes flowering, TFL1 represses flowering due to a small difference in 4 residues critical for the protein function [128, 129]. TFL1 plays a double role: it represses LFY and AP1 expression and promotes the vegetative state of the meristem (see [130] for details) (see Fig. 3). As FT, TFL1 (devoid of DNA binding capability) acts in complex with other TFs, such as the bZIP protein FD to regulate gene expression [61, 131–133]. To allow a stable switch to floral identity, AP1 directly binds CArG boxes in a remote 3′ regulatory region of TFL1 and represses its expression. AP1 also represses the vegetative MADS-box TFs, including SOC1, AGL24, and SVP [63, 134] (see Fig. 3). Until recently, LFY was also thought to directly repress TFL1 [135]. However, recent evidence suggests that LFY directly activates TFL1, and only represses it indirectly by activating AP1 [5, 136, 137]. The functional relevance of this incoherent feedback loop between LFY, AP1, and TFL1 is still unclear but it has been proposed that it could act as a sensor of
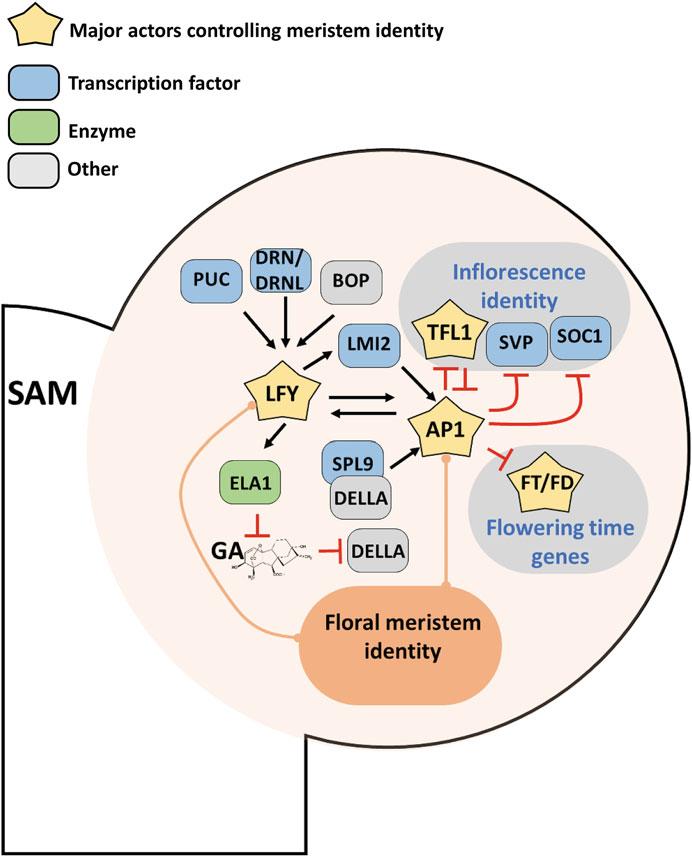
Fig. 3 Gene network specifying floral meristem identity. Close up on the nascent FM (stage 2–3) on the flank of the SAM. Inflorescence and floral genetic identity are antagonistic. Whereas the inflorescence meristem is specified by TFL1, as well as SVP and SOC1, the floral meristem is under the control of LFY and AP1 TFs. LFY is early expressed in the floral anlagen below ARF5/MP (see Fig. 2), but is also controlled by other factors such as PUC, DRN/DRNL, and BOP1/2. LFY induces the expression of AP1 directly, or indirectly through LMI2 and the GA hormonal pathway. AP1 also induces LFY in a positive feedback regulatory loop stabilizing the genetic floral identity. Once induced, AP1 inhibits the flowering time genes and the inflorescence identity genes. Reciprocal inhibition between TFL1 and AP1 is considered as central to maintain exclusive inflorescence and floral identities
floral meristem marker and make sure that floral patterning starts only when the levels of LFY and AP1 are sufficient [136, 138]. A model of the early floral meristem regulatory network has been proposed that captures the different gene expressions observed in wild-type and several mutant backgrounds [139].
3.3
A Common Early Regulatory Network Between LFY and AP1
Despite their completely different DNA binding specificity, LFY and AP1 share several common target genes [140, 141]. Almost 30% (769 genes) of all genomic regions bound by LFY are also bound by AP1. Among them, 25% are both regulated by LFY and AP1. Many of them are linked to flower development, such as genes involved in auxin (MAB4, ARF6, ARF11, and IAA18), the GA catabolism enzymes (ELA1, GA2ox20) and other regulators of floral development (SEP3, RAX1, LMI1, FD, and TFL1) [63, 140, 142, 143]. Another feature shared by LFY and AP1 is their ability to bind to closed chromatin region, a property characteristic of pioneer factors [144–148]. The fact that LFY and AP1 share many bound regions and regulate a common set of target genes suggests that they may act in the same complex. This could be direct physical interaction or indirect association mediated by common co-factors, such as chromatin remodelers BRM and SYD, which are recruited by LFY and to interact with AP1 as shown by mass spectrometry experiments [149, 150]. However, the only MADS-box TF interacting with LFY is SEP3, and available proteomics data do not suggest a direct interaction between LFY and other MADS-box TFs [150, 151].
Another possibility could explain why LFY and AP1 shared common targets. Genes bound by multiple TFs show more dynamic expression during flower development [152]. Thus, genes with multiple different TF binding sites in their regulatory sequence tend to be more rapidly and more efficiently regulated to promote the switch to flower development.
3.4 The Genesis of the Boundary FM-SAM
Emerging flower primordia are separated from the SAM by a group of specialized cells that form a boundary, characterized by reduced growth [153]. This boundary interface separates and influences two territories of different developmental fates, the SAM and the FP. Several TFs belonging to the TALE superfamily contribute redundantly to SAM maintenance, including the KNOX class protein STM, and the BELL class proteins PENNYWISE (PNY) and the closely related POUND-FOOLISH (PNF) [154–156]. Both KNOX and BELL class proteins specify meristems and define boundarieslikely by forming functionalheterodimers [156].Several genes involved in boundary formation are regulated, directly or indirectly by the TALE proteins. Some of them are encoded by NAC family TFs CUP-SHAPED COTYLEDON (CUC1–3). CUC1 and 2 act redundantly to establish boundaries of floral primordia [157, 158]. CUC1 directly activates STM expression in a positive feedback loop to generate the boundary between the SAM and the FP, a function probably shared by the closed relatives CUC2 and CUC3 proteins [104, 105, 159]. Similarly to CUCs, BOP1 and BOP2 are expressed in the boundary and also define boundaries of the floral meristem in addition to their role in floral identity [160]. PNY and PNF downregulate BOP1 and BOP2 to
ensure that their expression domain is restricted to the boundary zone [161]. On the other hand, BOP1, together with the bZIP TF TGACG MOTIF-BINDING FACTOR 4 (TGA4), activate the expression of the BELL class gene ARABIDOPSIS HOMEOBOX 1 (ATH1) in different boundary regions [162]. Other TALE genes are also regulated by BOP and may involve interaction with TGA [163, 164].
Another typical boundary protein is HANABA TARANU (HAN), a GATA-3 type TF expressed in several boundary regions, including in early stage of floral primordium [165, 166]. HAN interacts with proteins specific to the SAM and the boundary, respectively. HAN likely directly regulates both BOP1 and BOP2, but also activates and interacts physically with PINHEAD (PNH), a member of ARGONAUTE family involved in SAM maintenance by sequestering miR166/165 [166, 167]. Boundaries are also characterized by a specific hormonal context that downregulates growth and differentiation. While high CK to low GA promotes SAM maintenance, the opposite hormonal balance (low CK to high GA) prevents differentiation in the boundary zones [168]. HAN reduces CK levels in the boundary by directly activating CYTOKININ OXYDASE 3 (CKK3), a gene involved in cytokinin degradation [166]. Besides, CUC1 and 2 also modulate cytokinin homeostasis in inflorescences, how it acts during early flower development is not yet clear [169].
4 Part 4: Establishment of the Floral Patterning from the Historical ABC to the Revised ABCDE Model
In Arabidopsis, floral organs are arranged into a whorled pattern, with, from outside to inside, four sepals, four petals, six stamens, and two fused carpels. The identity of these organs is specified by the combined actions of floral homeotic genes, namely A-, B-, C-, D-and E-function genes. Their expressions are initiated by floral meristem identity genes, such as LFY and AP1/CAL, and maintained throughout most of the floral organ development. These genes were discovered based on homeotic organ transformation occurring in the respective mutants. The combinatorial action of these genes led to the formulation of the ABC model, later revised to ABCDE model when the role of E-function genes (SEPALLATA1 to 4, SEP1 to SEP4) in all four whorls was discovered [170] (see Fig. 4a). In the ABCDE model, A (AP1 and AP2)-and E-function genes, together, specify sepal formation and development. Genes from class A-, B (APETALA3, AP3; and PISTILLATA, PI)-and E-function genes initiate petal formation and development. Class B-, C (AGAMOUS, AG)-and E-function genes are involved in stamen formation and development. Class