CatalyticProcessesforWaterand WastewaterTreatment
Editor
JohnVakros
MDPI • Basel • Beijing • Wuhan • Barcelona • Belgrade • Manchester • Tokyo • Cluj • Tianjin
Editor
JohnVakros
ChemicalEngineering UniversityofPatras Patras
Greece
EditorialOffice
MDPI
St.Alban-Anlage66 4052Basel,Switzerland
ThisisareprintofarticlesfromtheSpecialIssuepublishedonlineintheopenaccessjournal Catalysts (ISSN2073-4344)(availableat:www.mdpi.com/journal/catalysts/special issues/catalytic wastewater treatment).
Forcitationpurposes,citeeacharticleindependentlyasindicatedonthearticlepageonlineandas indicatedbelow:
LastName,A.A.;LastName,B.B.;LastName,C.C.ArticleTitle. JournalName Year, VolumeNumber, PageRange.
ISBN978-3-0365-7299-4(Hbk) ISBN978-3-0365-7298-7(PDF)
©2023bytheauthors.ArticlesinthisbookareOpenAccessanddistributedundertheCreative CommonsAttribution(CCBY)license,whichallowsuserstodownload,copyandbuildupon publishedarticles,aslongastheauthorandpublisherareproperlycredited,whichensuresmaximum disseminationandawiderimpactofourpublications.
ThebookasawholeisdistributedbyMDPIunderthetermsandconditionsoftheCreativeCommons licenseCCBY-NC-ND.
Contents
AbouttheEditor vii
Prefaceto”CatalyticProcessesforWaterandWastewaterTreatment” ............... ix
JohnVakros
CatalyticProcessesforWaterandWastewaterTreatment
Reprintedfrom: Catalysts 2023, 13,677,doi:10.3390/catal13040677.................. 1
GururajM.Neelgund,SanjuanaFabiolaAguilar,EricaA.JimenezandRamL.Ray AdsorptionEfficiencyandPhotocatalyticActivityofSilverSulfideNanoparticlesDepositedon CarbonNanotubes
Reprintedfrom: Catalysts 2023, 13,476,doi:10.3390/catal13030476.................. 7
ShakeelKhan,AwalNoor,IdreesKhan,MianMuhammad,MuhammadSadiqandNiaz Muhammad
PhotocatalyticDegradationofOrganicDyesContaminatedAqueousSolutionUsingBinary CdTiO2 andTernaryNiCdTiO2 Nanocomposites
Reprintedfrom: Catalysts 2022, 13,44,doi:10.3390/catal13010044.................. 27
CristinaPeiYingKong,NurulAmaninaA.Suhaimi,NurulizzatulNingshehM.Shahri, Jun-WeiLim,MuhammadNurandJonathanHobleyetal. AuramineOUVPhotocatalyticDegradationonTiO2 NanoparticlesinaHeterogeneous AqueousSolution
Reprintedfrom: Catalysts 2022, 12,975,doi:10.3390/catal12090975.................. 47
NurulAmaninaA.Suhaimi,CristinaPeiYingKong,NurulizzatulNingshehM.Shahri, MuhammadNur,JonathanHobleyandAnwarUsman
DynamicsofDiffusion-andImmobilization-LimitedPhotocatalyticDegradationofDyesby MetalOxideNanoparticlesinBinaryorTernarySolutions
Reprintedfrom: Catalysts 2022, 12,1254,doi:10.3390/catal12101254................. 63
JishuRawal,UroojKamran,MiraPark,BishweshwarPantandSoo-JinPark NitrogenandSulfurCo-DopedGrapheneQuantumDotsAnchoredTiO2 Nanocompositesfor EnhancedPhotocatalyticActivity
Reprintedfrom: Catalysts 2022, 12,548,doi:10.3390/catal12050548.................. 79
SourayaGoumri-SaidandMohammedBenaliKanoun InsightintotheEffectofAnionic–AnionicCo-DopingonBaTiO3 forVisibleLightPhotocatalytic WaterSplitting:AFirst-PrinciplesHybridComputationalStudy
Reprintedfrom: Catalysts 2022, 12,1672,doi:10.3390/catal12121672................. 99
LuminitaIsac,CristinaCazan,LuminitaAndronicandAlexandruEnesca CuS-BasedNanostructuresasCatalystsforOrganicPollutantsPhotodegradation
Reprintedfrom: Catalysts 2022, 12,1135,doi:10.3390/catal12101135................. 111
MarieLePivert,Aur´eliePiebourg,St´ephaneBastide,MyriamDucandYaminLeprince-Wang DirectOne-StepSeedlessHydrothermalGrowthofZnONanostructuresonZinc:Primary StudyforPhotocatalyticRoofDevelopmentforRainwaterPurification
Reprintedfrom: Catalysts 2022, 12,1231,doi:10.3390/catal12101231................. 129
AkbarMehdizadeh,ZahraDerakhshan,FaribaAbbasi,MohammadRezaSamaei, MohammadAliBaghapourandMohammadHoseinietal.
TheEffectofArseniconthePhotocatalyticRemovalofMethylTetButylEther(MTBE)Using Fe2O3/MgOCatalyst,Modeling,andProcessOptimization
Reprintedfrom: Catalysts 2022, 12,927,doi:10.3390/catal12080927.................. 143
MariaAntonopoulou,MariaPapadaki,IlaeiraRaptiandIoannisKonstantinou PhotocatalyticDegradationofPharmaceuticalAmisulprideUsingg-C3N4 CatalystandUV-A Irradiation
Reprintedfrom: Catalysts 2023, 13,226,doi:10.3390/catal13020226.................. 155
IlaeiraRapti,VasilikiBoti,TriantafyllosAlbanisandIoannisKonstantinou PhotocatalyticDegradationofPsychiatricPharmaceuticalsinHospitalWWTPSecondary EffluentsUsingg-C3N4 andg-C3N4/MoS2 CatalystsinLaboratory-ScalePilot
Reprintedfrom: Catalysts 2023, 13,252,doi:10.3390/catal13020252.................. 169
LalitGoswami,AnamikaKushwaha,SarojRajKafleandBeom-SooKim SurfaceModificationofBiocharforDyeRemovalfromWastewater
Reprintedfrom: Catalysts 2022, 12,817,doi:10.3390/catal12080817.................. 183
SpyridonGiannakopoulos,JohnVakros,ZachariasFrontistis,IoannisD.Manariotis,Danae VenieriandStavrosG.Poulopoulosetal.
BiocharfromLemonStalks:AHighlyActiveandSelectiveCarbocatalystfortheOxidationof SulfamethoxazolewithPersulfate
Reprintedfrom: Catalysts 2023, 13,233,doi:10.3390/catal13020233.................. 211
KonstantinosKouvelis,AdamantiaA.Kampioti,AthanasiaPetalaandZachariasFrontistis DegradationofSulfamethoxazoleUsingaHybridCuOx–BiVO4/SPS/SolarSystem
Reprintedfrom: Catalysts 2022, 12,882,doi:10.3390/catal12080882.................. 231
AbouttheEditor
JohnVakros
JohnVakrosservesasresearcherintheDepartmentofChemicalEngineeringatUniversityof Patras.Histeachingactivitiescovergeneralchemistry,physicalchemistry,catalysisandinstrumental chemicalanalysis.Hisresearchactivitiesareinthefieldofheterogeneouscatalysis,preparation ofcatalysts,preparationandcharacterizationofbiocharsandapplicationofnovelmaterialsfor environmentalprotection.
CatalyticProcessesforWaterandWastewaterTreatment
JohnVakros 1,2
1 DepartmentofChemicalEngineering,UniversityofPatras,Caratheodory1,UniversityCampus, GR-26504Patras,Greece;vakros@chemistry.upatras.gr
2 SchoolofSciencesandEngineering,UniversityofNicosia,2417Nicosia,Cyprus
Citation: Vakros,J.Catalytic ProcessesforWaterandWastewater Treatment. Catalysts 2023, 13,677. https://doi.org/10.3390/catal13040677
Received:27March2023
Revised:28March2023
Accepted:29March2023
Published:30March2023
Copyright: ©2023bytheauthor. LicenseeMDPI,Basel,Switzerland. Thisarticleisanopenaccessarticle distributedunderthetermsand conditionsoftheCreativeCommons Attribution(CCBY)license(https:// creativecommons.org/licenses/by/ 4.0/).
Waterandwastewatertreatmentstillfacesignificantchallengestoday.Duetoclimate change,watershortagesarealreadynoticeableinmanyregionsoftheworld.Toovercometheseproblems,alternativewatersourcesmustbegeneratedandused,forexample, treatedwastewater,rainwater,surfacewater,etc.Sincethedevelopmentofhighlysensitive methodsrevealsthepresenceofhazardouscompoundsinlowconcentrations,thesewater sourcesmustbetreatedbeforeuse.Thedetectedcompoundsincludepharmaceuticals, whichhaveaseriousinfluenceonhumanhealth.Generally,theconcentrationsofthese compoundsislow.However,recentstatisticshaverevealedthatthepercapitaconsumption ofmedicineswithintheEuropeanUnionfortheperiodof2000–2014hasalmosttripled, reflectingtheexcessiveandperhapsrecklessuseofsimilarproducts.Inparticular,there arenumerousdifferentwaysthatremediesmaypermeateintosurfacerecipients,suchas landfillleachates,excessiveconsumptionbypeoplethemselves,highvolumesoforganic hospitaleffluents,andtheundeniablyincorrectrejectionofmedicinesthathaveexpiredor werenotused.Duetotheincompletebiodegradationofcomplexorganicmoleculesinconventionalwastewatertreatmentplants,theaccumulationoforganicpollutantsonsurface watersendangersbiodiversitythroughitspotentialtoxicity.Thus,thesepollutantsshould beremoved.Manyprocesseshavebeenproposedfortheirdegradation/removal,including catalyticmethods.Thesemethods,inwhichcatalystsareinasolidforminparticular, exhibitsignificantadvantagessuchaseasyseparation,betterstability,higheractivity,anda lowercostoftheprocess.Catalyticoxidationorreductionprocessesareamongthemost efficientprocesses.Theseincludephotocatalysisandsulfate-andhydroxyl-radical-based advancedoxidationprocesses.
Theapplicationofasolidcatalystinaremovalexperimentcombinesnotonlythe oxidationreactionbutalsotheadsorptionofthecontaminantonthecatalystsurface.The adsorptionisanecessarystepforthecatalyticprocess,andhydrophilicityofthesurface isrequiredinsuchcases.Byitself,adsorptionveryusefulforremovingheavymetalions. Heavymetalsarehighlysolubleinwaterandaretoxic,carcinogenic,andnon-degradable. InthestudybyNeelgundetal.[1],ananocompositeCNTs-Ag2Swaspreparedthroughthe faciledepositionofAg2Snanoparticlesontooxidizedcarbonnanotubes(CNTs),usingthe hydrothermalmethod.Atfirst,thehydrophobicCNTsweretreatedwithconcentratednitric andsulfuricacidsunderreflux.Themodificationincreasedthehydrophilicnatureofthe CNTsbyintroducingoxygenspeciesontothesurface.TheAg2Snanoparticleswerethen depositedontothemodifiedCNTs.TheCNTs-Ag2SismoreefficientthanCNTsandAg2S andcancompletelyadsorbCd(II)within80min.Theadsorptionfollowspseudo-secondorderkinetics,whileintraparticlediffusionandtheboundarylayereffectcontributetothe removalofCd(II)[1].TheCNTs-Ag2Scanalsoactasaphotocatalystforthedegradation ofalizarinyellowR(AYR),adyeusedinthetextileindustry.Withtheirradiationofthe materialwithnaturalsunlight,completedegradationofAYRisachieved.Thedegradation followspseudo-first-orderkinetics.Differentreactivespeciessuchaselectrons,holes, hydrogenperoxide,andROSaretheactivespeciesforthedegradation,andtheCNTs-Ag2S wasfoundtobestableforthreecycles[1].

Photocatalysisisprobablythemostcommonmethodusedforthedegradationof organiccompoundsinwatermatrices,andtitania-basedmaterialsarewidelyusedas photocatalysts.InthestudybyKhanetal.,[2]binaryCdTiO2 andternaryNiCdTiO2 werecomparedwithbareTiO2 forthephotocatalyticdegradationoforganicdyessuch asmethyleneblue(MB)andmethylgreen(MG).Itwasfoundthatthephotocatalytic degradationofTiO2,CdTiO2,andNiCdTiO2 forMBwas77,82,and86%,whileforMG thebinaryandternarymaterialswereevenbetter,achievingadegradationof63.5,88,and 97.5%,respectively.Inallthreematerials,theTiO2 wasintheformofanatase,whilethe depositionofCdandNicausedaredshiftintheabsorbancespectrabycouplingwithNi andCd.Thisshiftcanbeconsideredresponsiblefortheenhancedphotocatalyticactivity. Konget.al.[3]usedanataseTiO2 todegradeAuramineO(AO)under365nmlight irradiation.Thetitaniananoparticleshadameandiameterof100nm.Thedegradation processfollowedapseudo-firstorderreaction,withk=0.048 ± 0.002min 1.Thisvalueis significantlylowerthanthecorrespondingvalueforthedegradationofmethyleneblue (0.173 ± 0.019min 1);thisisduetothenonplanarstructureofAO.Thehigherphotocatalyticefficiencywasfoundtobe96%,anditdecreasednonlinearlywithanincreaseinthe initialconcentrationandcatalystdosage.Finally,theauthorsconfirmedthesuperiorityof titaniananoparticlescomparedtotheuseofmicro-sizedpowders.
Thedegradationprocessismorecomplicatedwhenmorethanonedyeispresentinthe solution;however,thisscenarioisalsomorerealistic.Therefore,therearenumerousstudies inwhichbinaryorternarysolutionsofdyesareusedinphotocatalyticdegradation.In thisrecentreview[4],thephotocatalyticbehaviorofmethylene,rhodamineB,andmethyl orangeintheirbinaryorternarysolutionswassummarized.Inthisreview,theimportance ofdiffusionwasdiscussed.Itwasrevealedthatsmallerdyeswithaplanarconformation areeasiertodegradeanddominateinphotocatalyticdegradationintheirbinaryorternary solutions.
Althoughtitaniaislikelythebetterphotocatalyst,ithassomelimitations,among whichisitslimitedopticaladsorptioninthevisiblelightspectrum.Manymethodshave beenusedtofurtherexpandtheopticalUV–VisabsorptionregionofTiO2 andtoenhance theefficientlight-inducedchargeseparation.ThebestoptionreportedistocombineTiO2 withtypicalnarrow-bandgapquantumdots(QDs)ofdifferentsemiconductorsandcarbon quantumdots(CQDs).IntheworkofRawalet.al.,[5]nitrogen(N)andsulfur(S)co-doped graphenequantumdots(GQDs)wereused.ThismaterialhasanarrowedEgvaluethat improvesphotogeneratedelectrontransferdueto π-conjugation.Theactivitywasfound tobe2.3–3timeshigherthanthatofTiO2.Thephotocatalystwasstableforatleastthree cycles,andtheformationofC-O-Tibondsprovidedachargetransferpathway.
BaTiO3 isanotherpromisingmaterial,especiallywhenitiscombinedwiththedoubleholecouplingofanion–anioncombinations.Bariumtitanatehasaperovskitestructure andlargebandgapof3.0–3.3eV;ithastheappropriatebandpositionstosplitwater intohydrogenandoxygen,althoughthebandgapshouldberegulatedinlowervaluesto providetheabilitytoabsorbmorevisiblelight.Ithasalsobeenreportedthatbysubstituting ametaldopantataBaorTisite,adirecteffectonthebandgapenergy(Eg),thestability, andtheformationofoxygenvacanciescanbeachieved.Onthisbasis,theelectronic propertiesandopticalabsorptioncharacteristicsofBaTiO3,withthedouble-holecoupling ofanion–anioncombinationsusingfirst-principlesmethods,werestudied.Itwasfound thattheN–Nco-dopedBaTiO3 exhibitedamorefavorableformationenergyunderan O-poorcondition,andalltheco-dopingconfigurationsreducedthevalueofEg.Thetuning oftheEgmakesthephotocatalystapromisingcandidateforvisible-lightwatersplitting[6].
Duetotheimportanceofphotocatalysis,asitisoneofthemostinnovativeadvanced oxidationtechniquesused,manymaterialshavebeentestedfortheirphotocatalyticactivity. Aphotocatalyticprocessshouldpresentahighefficiency,lowcost,andavoidtheproductionofintermediateproductswithahightoxicity.Theselectionofaphotocatalystisvery important,asitmustbecheap,stable,environmentallyfriendly,andhavehighactivity. Usually,aphotocatalystisasemiconductorwithahighdegreeofabsorptionoflight,prefer-
ablysolarlight,andhighrateofphotogeneratedchargecarriers.Onereviewsummarized theapplicationofCuSasaphotocatalyst[7].CuSisap-typesemiconductorwithdifferent nanostructures,suchasnanoparticlesandquantumdotsorheterojunctionswithcarbon materials,organicmaterials,ormetaloxides.Therecentdevelopmentsinorganicpollutant photodegradationwithaCuSascatalystarediscussedonthebasisofdifferentsynthesis parameters(Cu:Smolarratios,surfactantconcentrationetc.)andproperties(particlesize, morphology,bandgapenergy,andsurfaceproperties)
InthestudybyLePivertet.al.,[8],ZnOnanoparticlesweresynthesizedontoZn andusedasroofforthephotocatalyticremovaloforganiccontaminantsinrainwater. TheauthorspreparedtheZnOnanoparticlesontheZnroofwithahydrothermalgrowth methodin2h.ThepresenceofZn(II)ionsandthenativeoxidefilmontheZnsurface actedasactivesitesforthegrowthofZnOnanoparticles.Thephotocatalyticactivitywas determinedusingMethyleneBlueunderUVirradiationandAcidRed14withsolarlight. TheremovalofMethylTertButylEther(MTBE)usingFe2O3/MgOasacatalystwas studiedinthepresenceofarsenicunderUVirradiation[9].Thecatalystcontained33.06% Fe2O3 and45.06%MgO.Themainparametersofthedegradationprocess,includingthe effectofarsenicandMTBEconcentrations,catalystmass,pH,etc.,werestudied.Itwas foundthattheconcentrationofMTBEinfluencedthemostthephotocatalyticprocess. ThehigherperformanceintheremovalofMTBEwasachievedatpH=5andaninitial concentrationofMTBeequalto37.5mg/L,withacatalystdoseof1.58mg/Lwithout thepresenceofAs.Thepresenceofarsenicdecreasedtheremovalefficiencyremarkably. Therefore,pretreatmentfortheremovalofarsenicandmoredetailsofthisinterference effectaresuggested.
Psychiatricdrugs,includingamisulpride,atypicalantipsychoticdrug,areaclass ofpharmaceuticalsthatareoftenprescribedandusedinawiderangeofmentalhealth problems.Theiruseisincreasingworldwide.Graphiticcarbonnitride(g-C3N4)canbeused asphotocatalystforthedegradationofthistypeofdrug.IntheworkofAntonopoulouet. al.[10],theapplicationofg-C3N4 catalystsforthedegradationofamisulpridewasstudied. Particularly,inthedegradationprocessofamisulprideunderUV-Airradiationwiththe applicationofg-C3N4,thetransformationproductsandtheecotoxicologicalassessment wereinvestigated.Itwasfoundthatthedegradationpathwaysofamisulprideincluded mainlyoxidation,dealkylation,andthecleavageofthemethoxygroup.Themainreactive specieswerefoundtobeh+ andO2 •−.Inultrapurewater,thetransformationproductshad alowtoxicity,whileanincreasedtoxicitywasobservedwhenwastewaterwasusedasa matrix,especiallyatthebeginningoftheprocess.
g-C3N4 isaninterestingmaterialwithagraphene-likestructure,chemicalandthermal stability,andalowcost.Isagoodvisiblelightabsorberandcanbeusedinawidevariety ofphotocatalyticapplications.Ithasanarrowbandgapof~2.7eV,butitexhibitsahigh recombinationprocessandthuslimitedactivity.Thisdrawbackcanbeovercomewiththe combinationofg-C3N4 andMoS2,sinceheterostructuringcandecreasetherecombination effectsbetweenelectronsandholes.Thephotocatalyticremovalofpsychiatricdrugs insecondaryeffluentsofhospitalwastewaterwithg-C3N4 or1%MoS2/g-C3N4 was studied[11]inalaboratory-scalepilotplantandinasolarsimulatorapparatus.Theresults showedthatthe1%MoS2/g-C3N4 wasmoreactivethantheg-C3N4,with54%and30% removal,respectively.Thedegradationfollowedfirstorderkinetics,andsixtransformation productsweregeneratedduringdegradation;theseweretotallydegradedattheend ofthetreatment.
Aspreviouslynoted,researchonnewmaterialsisalwayschallenging.Thesematerials shouldbeactive,selective,andofalowcost.Itisalsodesirablethattheyareecofriendly, and,ifpossible,theyshouldoriginatefromthevalorizationofwaste.Biocharissucha materials.Itcanbeproducedfromanykindofbiomassthroughapyrolysisprocessunder alimited-orno-oxygenatmosphere.Generally,biocharhasamoderateorhighspecific surfacearea,ahierarchicalporestructure,andactivesurfacegroups.Theutilization ofbiocharandbiochar-basednanocompositesfordyeremovalfromwastewaterwas
reviewed[12].Theremovalofdyesfromwastewatervianaturalandmodifiedbiochar followsnumerousmechanisms,suchasprecipitation,surfacecomplexation,ionexchange, cation–π interactions,andelectrostaticattraction.Thepreparationparametersandthepost treatmentofbiocharcanalterthesurfacespeciation,andthemodifiedbiocharcanexhibit betterperformanceintheremovalofdyes.Aframeworkforartificialneuralnetworking andmachinelearningtomodelthedyeremovalefficiencyofbiocharfromwastewaterwas alsoproposed.
Inadditiontotheexcellentadsorptionpropertiesofthebiochar,itcanalsoactas acatalystfortheactivationofpersulfateionsandtheoxidationoforganiccompounds. Biocharfromlemonstalks,pyrolyzedat850 ◦Cunderalimitedoxygenatmosphere, producedahighlyactiveandselectivebiocharfortheoxidationofsulfamethoxazole(SMX), usingsodiumpersulfateasoxidant[13].Thebiocharhadasignificantamountofcarbonates andcouldcompletelydegrade0.5mgL 1 SMXwithin20minusing500mgL 1 sodium persulfate(SPS)and100mgL 1 biocharinultrapurewater(UPW).Thedegradation processisfavoredatalowpHandinthepresenceofchlorides.Thecomplexityofthewater matricesusuallyhasanegativeimpactonthedegradation.Themechanismfollowsradicals withhydroxylradicalsasthemainactivespeciesandnon-radicalpathways;electron transferpathwaywasprovenwithelectrochemicalcharacterization.Thebiocharfrom lemonstalksisstableandactive.
Thecombinationofmorethanoneadvancedoxidationprocessoftenleadstosynergisticeffectsandhasprovedtobeamoreefficientstrategy.Inthisrespect,thedegradation ofSMXwasstudiedusingaCuOx–BiVO4 catalystaseitheraphotocatalystorpersulfate (sodiumpersulfate,SPS)activator[14].Thishybridprocess(catalyst/SPS/SolarSystem) wasfoundtobesynergeticforcopper-promotedBiVO4 photocatalysts.Specifically,differentloadingsofCuOx(0.75–10%wt.)weredepositedonBiVO4 andtestedundersolar irradiationforthedegradationofSMX.Themostactivecatalystwasfoundtobethe 0.75CuOx–BiVO4,whileahigherloadingofCuOxdelayedthedegradationprocess.The complexityofthewatermatrix(fromultrapuretobottled,BW,andwastewater,WW)also hasanegativeeffectonthedegradationprocess.TheadditionofSPSisbeneficialforthe degradationprocess,andthetwoprocessesexhibitasynergisticeffect.Specifically,ina BWmatrix,theapplicationofSPSresultsincompleteeliminationafter60min,whileinthe absenceofSPS,anSMXdegradationofonly40%tookplacein120minundersolarlight. FortheWW,~37%,45%,and66%degreesofsymmetryweredeterminedusing0.75,3.0, and10.0CuOx–BiVO4,respectively.
Catalyticprocessesforwaterandwastewatertreatmentareapromisingapproach toimprovingthequalityofthewater.Althoughphotocatalysisisthemostpopularand attractiveprocessduetoitssimplicityandutilizationoftheabundantsolarlight,thecombinationofotheradvancedoxidationprocessescanleadtoeffectivesystems.Interestingly, thepresentedexperimentalresultshighlightthatitisdifficulttodecidewhichcatalyst orprocessisbetter,andthedeterminationoftheactivitymustbeperformedinawide windowofoperatingparameters.
ConflictsofInterest: Theauthordeclaresnoconflictofinterest.
References
1. Neelgund,G.M.;Aguilar,S.F.;Jimenez,E.A.;Ray,R.L.AdsorptionEfficiencyandPhotocatalyticActivityofSilverSulfide NanoparticlesDepositedonCarbonNanotubes. Catalysts 2023, 13,476.[CrossRef]
2. Khan,S.;Noor,A.;Khan,I.;Muhammad,M.;Sadiq,M.;Muhammad,N.PhotocatalyticDegradationofOrganicDyesContaminatedAqueousSolutionUsingBinaryCdTiO2 andTernaryNiCdTiO2 Nanocomposites. Catalysts 2023, 13,44.[CrossRef]
3. Kong,C.P.Y.;Suhaimi,N.A.A.;Shahri,N.N.M.;Lim,J.-W.;Nur,M.;Hobley,J.;Usman,A.AuramineOUVPhotocatalytic DegradationonTiO2 NanoparticlesinaHeterogeneousAqueousSolution. Catalysts 2022, 12,975.[CrossRef]
4. Suhaimi,N.A.A.;Kong,C.P.Y.;Shahri,N.N.M.;Nur,M.;Hobley,J.;Usman,A.DynamicsofDiffusion-andImmobilization-Limited PhotocatalyticDegradationofDyesbyMetalOxideNanoparticlesinBinaryorTernarySolutions. Catalysts 2022, 12,1254.[CrossRef]
5. Rawal,J.;Kamran,U.;Park,M.;Pant,B.;Park,S.-J.NitrogenandSulfurCo-DopedGrapheneQuantumDotsAnchoredTiO2 NanocompositesforEnhancedPhotocatalyticActivity. Catalysts 2022, 12,548.[CrossRef]
2023, 13,677
6. Goumri-Said,S.;Kanoun,M.B.InsightintotheEffectofAnionic–AnionicCo-DopingonBaTiO3 forVisibleLightPhotocatalytic WaterSplitting:AFirst-PrinciplesHybridComputationalStudy. Catalysts 2022, 12,1672.[CrossRef]
7. Isac,L.;Cazan,C.;Andronic,L.;Enesca,A.CuS-BasedNanostructuresasCatalystsforOrganicPollutantsPhotodegradation. Catalysts 2022, 12,1135.[CrossRef]
8. LePivert,M.;Piebourg,A.;Bastide,S.;Duc,M.;Leprince-Wang,Y.DirectOne-StepSeedlessHydrothermalGrowthofZnO NanostructuresonZinc:PrimaryStudyforPhotocatalyticRoofDevelopmentforRainwaterPurification. Catalysts 2022, 12,1231. [CrossRef]
9. Mehdizadeh,A.;Derakhshan,Z.;Abbasi,F.;Samaei,M.R.;Baghapour,M.A.;Hoseini,M.;Lima,E.C.;Bilal,M.TheEffectof ArseniconthePhotocatalyticRemovalofMethylTetButylEther(MTBE)UsingFe2O3/MgOCatalyst,Modeling,andProcess Optimization. Catalysts 2022, 12,927.[CrossRef]
10. Antonopoulou,M.;Papadaki,M.;Rapti,I.;Konstantinou,I.PhotocatalyticDegradationofPharmaceuticalAmisulprideUsing g-C3N4 CatalystandUV-AIrradiation. Catalysts 2023, 13,226.[CrossRef]
11. Rapti,I.;Boti,V.;Albanis,T.;Konstantinou,I.PhotocatalyticDegradationofPsychiatricPharmaceuticalsinHospitalWWTP SecondaryEffluentsUsingg-C3N4 andg-C3N4/MoS2 CatalystsinLaboratory-ScalePilot. Catalysts 2023, 13,252.[CrossRef]
12. Goswami,L.;Kushwaha,A.;Kafle,S.R.;Kim,B.-S.SurfaceModificationofBiocharforDyeRemovalfromWastewater. Catalysts 2022, 12,817.[CrossRef]
13. Giannakopoulos,S.;Vakros,J.;Frontistis,Z.;Manariotis,I.D.;Venieri,D.;Poulopoulos,S.G.;Mantzavinos,D.Biocharfrom LemonStalks:AHighlyActiveandSelectiveCarbocatalystfortheOxidationofSulfamethoxazolewithPersulfate. Catalysts 2023, 13,233.[CrossRef]
14. Kouvelis,K.;Kampioti,A.A.;Petala,A.;Frontistis,Z.DegradationofSulfamethoxazoleUsingaHybridCuOx–BiVO4/SPS/Solar System. Catalysts 2022, 12,882.[CrossRef]
Disclaimer/Publisher’sNote: Thestatements,opinionsanddatacontainedinallpublicationsaresolelythoseoftheindividual author(s)andcontributor(s)andnotofMDPIand/ortheeditor(s).MDPIand/ortheeditor(s)disclaimresponsibilityforanyinjuryto peopleorpropertyresultingfromanyideas,methods,instructionsorproductsreferredtointhecontent.
catalysts
Article
AdsorptionEfficiencyandPhotocatalyticActivityofSilver SulfideNanoparticlesDepositedonCarbonNanotubes
GururajM.Neelgund 1,* ,SanjuanaFabiolaAguilar 1,EricaA.Jimenez 1 andRamL.Ray 2
1 DepartmentofChemistry,PrairieViewA&MUniversity,PrairieView,TX77446,USA 2 CollegeofAgricultureandHumanSciences,PrairieViewA&MUniversity,PrairieView,TX77446,USA * Correspondence:gmneelgund@pvamu.edu
Abstract: Amultimode,dualfunctionalnanomaterial,CNTs-Ag2S,comprisedofcarbonnanotubes (CNTs)andsilversulfide(Ag2S)nanoparticles,waspreparedthroughthefacilehydrothermalprocess. BeforethedepositionofAg2Snanoparticles,hydrophobicCNTsweremodifiedtobecomehydrophilic throughrefluxingwithamixtureofconcentratednitricandsulfuricacids.TheoxidizedCNTswere employedtodeposittheAg2Snanoparticlesfortheirefficientimmobilizationandhomogenous distribution.TheCNTs-Ag2ScouldadsorbtoxicCd(II)andcompletelydegradethehazardous AlizarinyellowRpresentinwater.TheadsorptionefficiencyofCNTs-Ag2Swasevaluatedby estimatingtheCd(II)adsorptionatdifferentconcentrationsandcontacttimes.TheCNTs-Ag2Scould adsorbCd(II)entirelywithin80minofthecontacttime,whileCNTsandAg2Scouldnotpursueit.The Cd(II)adsorptionfollowedthepseudo-second-order,andchemisorptionwastherate-determining stepintheadsorptionprocess.TheWeber Morrisintraparticleporediffusionmodelrevealedthat intraparticlediffusionwasnotthesolerate-controllingstepintheCd(II)adsorption.Instead,itwas contributedbytheboundarylayereffect.Inaddition,CNTs-Ag2Scouldcompletelydegradealizarin yellowRinwaterundertheilluminationofnaturalsunlight.TheLangmuir-Hinshelwood(L-H) modelshowedthatthedegradationofalizarinyellowRproceededwithpseudo-first-orderkinetics. Overall,CNTs-Ag2Sperformedasanefficientadsorbentandacompetentphotocatalyst.
Keywords: CNTs;Ag2S;cadmium;adsorption;alizarinyellowR
Citation: Neelgund,G.M.;Aguilar, S.F.;Jimenez,E.A.;Ray,R.L. AdsorptionEfficiencyand PhotocatalyticActivityofSilver SulfideNanoparticlesDepositedon CarbonNanotubes. Catalysts 2023, 13, 476. https://doi.org/10.3390/ catal13030476
AcademicEditor:JohnVakros
Received:31December2022
Revised:21February2023
Accepted:21February2023
Published:26February2023
Copyright: ©2023bytheauthors. LicenseeMDPI,Basel,Switzerland. Thisarticleisanopenaccessarticle distributedunderthetermsand conditionsoftheCreativeCommons Attribution(CCBY)license(https:// creativecommons.org/licenses/by/ 4.0/).
1.Introduction
Waterpollutionengrossedbyheavymetalsisaprimaryenvironmental,ecological, andpublichealthconcern[1].Heavymetalsarehighlysolubleinwaterandaretoxic, carcinogenic,andnon-degradable[2,3].Rapidindustrializationandtechnologicaldevelopmenthaveresultedinthedischargeofheavymetal-containingeffluentsintosurfaceand groundwater[4].Thedischargedheavymetalsareadsorbedbysoilandenterthehuman bodythroughthefoodchain.Heavymetalsaccumulateinvariousorgansandbodytissues andcancauseirreparabledamage,includingdeath[5].Amongtheheavymetals,cadmium isenormouslyusedandhighlytoxic[6].Thesourcefordischargingthecadmiumintothe environmentisindustrialactivities,suchaselectroplating,smelting,alloymanufacturing, pigments,plastic,battery,fertilizers,pesticides,pigments,dyes,textileoperations,and refining[7].Inwater,cadmiumexistsasbivalent,Cd(II),whichisresponsibleforseveral adverseeffects,suchaskidneydysfunction,nephritis,hypertension,renaldysfunction, nervoussystemdysfunction,bonelesions,digestivesystemdysfunction,cancer,andreproductiveorgandamage[8–10].Theitai-itaidisease,causedbyCd(II)-contaminatedwater fromtheJinzuriverinJapan,hasinstigatedseverepain,bonefractures,proteinuria,and osteomalacia[11].TheCd2+ ionshaveahighaffinityforbindingtosulfhydryl(-SH)groups ofproteinsinbiologicalsystems[12].ThepresenceofCd(II)inwatercanleadtosevere healthandenvironmentalproblems.Therefore,itseliminationiscriticallyneeded.The Cd(II)presentinwatercouldbeeradicatedthroughchemicalprecipitation,ionexchange,

solventextraction,membraneseparation,electrochemicalremoval,coagulation,andadsorption[13–21].Inthesetechniques,adsorptionissuperiorbecauseofitshighefficiency, relativesimplicityindesign,easyoperation,andlowoperationalcost[20,21].Becauseof this,manyadsorbentshavebeendevelopedandtestedfortheirefficiency[20–22].
Anotherclassofpollutantsthatalsocausesmajorenvironmentalproblems,suchas heavymetals,isazodyes.Azodyeshaveattractivecolorsandareenormouslyusedin industriesduetotheiravailabilityandstability[23].Thesedyescontainazobonds(-N=N-) andsubstitutedaromaticrings[24].Thecomplexmolecularstructureofazodyeshasmade themrecalcitrantandresistanttobiodegradation[25].Azodyesaremutagenic,teratogenic, andcarcinogenic[25].Moreover,azodyescandecomposeintopotentiallycarcinogenic polychlorinatednaphthalenes,benzidine,andamines[26,27].Thetoxicityofazodyescan resultinlungcancer,heartdiseases,chromosomalaberrations,neurotoxicity,skindisease, andrespiratoryproblemsinhumans[25].Thesedyescancausedisordersofthecentral nervoussystemandtheinactivationofenzymaticactivities[28].Beyondtoxicity,azodyes areabundantlyusedinindustriesbecauseoftheircostandadvancementincolorsthat covertheentirespectrum.Aftertheiruseinindustries,about10-15%oftheazodyesaredischargedintotheenvironmentthroughwatereffluents[29,30].Releasingazodyescontent watercanresultinseveralenvironmentalproblemsincludingreducedlightpenetrationin waterbodies,whichleadstodiminishedphotosyntheticactivities,lessenedgrowthand reproductionofaquaticcreatures,andaestheticdamage[30,31].Theconsequencesofthe xenobioticandrecalcitrantazodyesimpacttheecosystem’sstructureandfunctioning. Consideringtheadverseeffects,itisessentialtocompletelyobliterateazodyestoprevent severedamageandprotecthealthandtheenvironment.Differenttechniqueshavebeen developedtoremovetheazodyesinwater,viz.,coagulation/flocculation,ultrafiltration andmembraneprocessing,chemicalprecipitation,electrochemicaldegradation,andozonation[32–37].Incomparison,photocatalysisisanexcellentmethodforeradicatingazodyes fromwaterinanenvironmentallyfriendlyapproachastheendproductsofthisprocessare harmless[38,39].Duetoitsefficiencyindegradingazodyes,manyphotocatalystshave beendevelopedtoeliminatedyes[37–45].Amongazodyes,alizarinyellowR(AYR)is aprominentlyuseddyeinindustries.Itisahighlywater-solubleanionicdyethatcontainspolycyclicaromatichydrocarbons[25].AYRisaderivativeofsalicylicacidandwas preparedin1887byRudolfNietzkithroughthereactionof m-nitroanilineandsalicylic acid[46].AYRisanindustriallyimportantdyeandisvastlyusedinthetextile,leather, plastics,paints,andlacquerindustries[24].Furthermore,itisusedasanacid-baseindicator andemployedinhistology,stains,andnutrientmediapreparations[47].
ConsideringthehealthandenvironmentalproblemsassociatedwithCd(II)andAYR, wedesignedthedualapplicablenanocomposite,CNTs-Ag2SforefficientCd(II)adsorption andphotodegradationofAYR.ThemultimodeCNTs-Ag2Swasproducedthroughthe faciledepositionprocessofAg2Snanoparticlesoveroxidizedcarbonnanotubes(CNTs) usingthehydrothermalmethod.TheadsorptionefficiencyofCNTs-Ag2Swasestimated byevaluatingtheadsorptionrateofCd(II)fromwater.Thedynamicsandcontrolling mechanismsofCd(II)adsorptionwereassessedbypseudo-first-andsecond-orderkinetic models.Thereactionpathwaysandtherate-controllingstepunderlyingCd(II)adsorption wereevaluatedusingtheWeber Morrisintraparticleporediffusionmodel.Theadsorption equilibriumwasdeterminedbyfittingtheexperimentalresultswithLangmuir,Freundlich, andTemkinisothermmodels.Furthermore,thecatalyticactivityofCNTs-Ag2Swas determinedthroughthedegradationofAYRunderexposuretonaturalsunlight.The photocatalyticactivityofCNTs-Ag2SwasquantifiedusingtheLangmuir-Hinshelwood (L-H)model.
2.ResultsandDiscussion
TheATR-FTIRspectrumofCNTs-COOH,showninFigure 1a,demonstratedaband at3427cm 1 correspondingtotheO-Hbond.ThebandfortheC=Obondofthe-COOH groupsappearedat1699cm 1,andthebandfortheC=CbondsofCNTswasfoundat

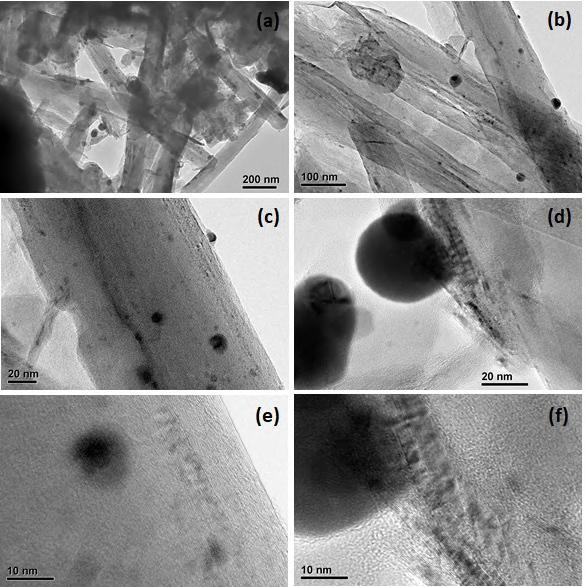
1554cm 1 [48–50].ThespectrumofAg2S(Figure 1b)displayedthepeakoftheAg-S bondat552cm 1 [51].ThespectrumofCNTs-Ag2S(Figure 1c)revealedthecharacteristic absorptionbandsrelatedtoCNTsandAg2S.Theprominentpeaksobservedat1066and 1096cm 1 inFigure 1cwereattributedtoC-Ostretching.Thepeakat3574cm 1,was duetothe OHstretchingvibrationsofadsorbedwatermolecules.ThebandduetoC=O wasobservedat1693cm 1,andthepeak,at1517cm 1,wasattributedtoC-H.Thepeak relatedtoAg-Swassituatedaround550cm 1.TheXRDpatternofCNTs-Ag2S(Figure 2) showedacharacteristic(002)reflectionofhexagonalgraphiteofCNTsat26.1◦ [52]. ItrevealedthereflectionsrelatedtoAg2Sat22.7( 101),25.2( 111),26.5(012), 29.2(111),31.7( 112),33.8(120),34.6( 121),34.9(022),36.8(111),37.0(121), 37.3(013),37.9( 103),40.9(031),43.6(023),44.4( 131),46.4( 123),48.0( 212), 48.9(014),53.0(040),53.5( 213),58.3( 141),58.4( 223),60.2( 105),61.4(015), 62.8(231),63.4(213),63.9( 1,34),and68.0◦ (232).Thesevaluesagreewiththe valuesfoundinthestandardpatternofacanthiteAg2S(JCPDSfile14-0072)[52].TheTEM imagesofCNTs-Ag2SpresentedinFigure 3 exploredthedepositionofspherical-resembling Ag2Snanoparticlesoverthesurfaceoftubular-structuredCNTs.TheproportionofAg2S nanoparticleswaslow,possiblyduetothelowquantityofprecursorAgNO3 usedinthe preparationcomparedtotheratioofCNTs.ThepresenceofAg2Snanoparticlesoverthe surfaceofCNTsisperceptibleinFigure 3a–f.ItisnoticeableinFigure 3athatthefew Ag2Snanoparticleshavecombinedandformedclusters.Figure 3b,drevealtheruptured surfaceofsomeCNTs.ItcouldhavehappenedthroughtheharshtreatmentofCNTswith themixtureofconcentratedHNO3 andH2SO4.Thisprocesswasrequiredtomakethe CNTshydrophilicandprovideasuitableenvironmentfortheeffectivedepositionofAg2S nanoparticles.However,theentiresurfaceoftheCNTswasnotdistractedandthesmooth surfaceoftheCNTisperceptibleinFigure 3e,f.Overall,afewdefectivesiteswereformed inCNTs.Figure 3d-frevealsthestrongadherenceofAg2SnanoparticlestothesmoothsurfacedCNT.Theabsenceofleachedorfree-standingnanoparticlesinthevoidareashows thestrongadherenceofAg2SnanoparticlestothesurfaceofCNTs.ThesizeoftheAg2S nanoparticlepresentinFigure 3d,e,wasaround38and15nm,respectively.Therefore, Ag2Snanoparticleshavebroadsizedistribution.TheaveragesizeofAg2Snanoparticles, calculatedfromXRD,wasaround29nm,whichagreeswiththesizedeterminedfromthe TEMimages.ThecoreoftheAg2Snanoparticles(Figure 3d)appearsdenserthantheedge. TheCNTshaveawidthofaround110nmandalengthofseveralmicrometers. Overall, CNTshaveeffectivelyexfoliated.Further,theEDSofCNTs-Ag2S(FigureS1,Supplementary Materials)demonstratedthepresenceofO,Ag,andSinCNTs-Ag2S.ThepeaksforCuand CawerefoundinFigureS1,thosearebythecoppergridusedintheTEMmeasurement.

Catalysts 2023, 13, 476
Figure 1. ATR‐FTIR spectra of (a) CNTs‐COOH, (b) Ag2S, and (c) CNTs‐Ag2S.
Figure1. ATR-FTIRspectraof(a)CNTs-COOH,(b)Ag2
Catalysts 2023, 13,476
Figure 1. ATR‐FTIR spectra of (a) CNTs‐COOH, (b) Ag2S, and (c) CNTs‐Ag2S.
2023, 13, 476
XRDofCNTs-Ag2S.
2. XRD of CNTs‐Ag2S.
3. (a–f) TEM images of CNTs‐Ag2S.
(a–f)TEMimagesofCNTs-Ag2S.
The TGA pattern of CNTs‐Ag2S (Figure 4) displayed four weight loss steps. The ini‐tial weight loss of 6.5% that occurred before 100 C was attributed to the desorption of physically adsorbed water molecules over the surface of CNTs‐Ag2S. The following weight loss of 15% ensued within the range of 100‐490 C was due to the detachment and decomposition of oxygen‐containing functional groups existing on the surface of the CNTs. The subsequent weight loss of 10.5% transpired within the range of 500‐540 C, was owing to the breaking of the bond between silver and sulfide in Ag2S nanoparticles and releasing sulfur. The successive sharp and significant weight loss of 31.5% taken place within the range of 540‐550 C was by the decomposition of sulfur and the formation of metallic silver and silver oxide. The residual weight remained was about 64%. The broad endothermic peak found in the DTA curve around 80 C was because of the dehydration
TheTGApatternofCNTs-Ag2S(Figure 4)displayedfourweightlosssteps.The initialweightlossof6.5%thatoccurredbefore100 ◦Cwasattributedtothedesorption ofphysicallyadsorbedwatermoleculesoverthesurfaceofCNTs-Ag2S.Thefollowing weightlossof15%ensuedwithintherangeof100–490 ◦Cwasduetothedetachmentand decompositionofoxygen-containingfunctionalgroupsexistingonthesurfaceoftheCNTs. Thesubsequentweightlossof10.5%transpiredwithintherangeof500–540 ◦C,wasowing
Figure
Figure2.
Figure
Figure3.
tothebreakingofthebondbetweensilverandsulfideinAg2Snanoparticlesandreleasing sulfur.Thesuccessivesharpandsignificantweightlossof31.5%takenplacewithinthe rangeof540–550 ◦Cwasbythedecompositionofsulfurandtheformationofmetallicsilver andsilveroxide.Theresidualweightremainedwasabout64%.Thebroadendothermic peakfoundintheDTAcurvearound80 ◦Cwasbecauseofthedehydrationofthesample. Thesmallendothermicpeakfoundat170 ◦Caccountedfortheremovaloffunctionalgroups fromthesurfaceoftheCNTs.Theintenseexothermicpeakat545 ◦Cwasassignedtorelease sulfurbybreakingthebondbetweensilverandsulfide.TheUV-visabsorptionspectrum ofCNTs-Ag2S,showninFigure 5,exhibitedacharacteristicabsorptionbandoftheC=C bondsofCNTsat232nm[52].Inaddition,thebroadabsorptiontailcorrespondingtoAg2S nanoparticleswasobservedinthevisibleregion[52,53].Ifsemiconductornanoparticles areconjugatedtoCNTs,itshowsthecharge-transferband[52,54].However,nosuchband wasobservedinFigure 5,illustratingthattheconjugationofAg2SnanoparticlestoCNTs hasnotalteredtheirenergystates[52,54].
Figure 4. TG/DTA of CNTs‐Ag2S.
TG/DTAofCNTs-Ag2S.
C=C
Wavelength (nm)
Figure5. UV-visabsorptionspectrumofCNTs-Ag2S.
Figure 5. UV‐vis absorption spectrum of CNTs‐Ag2S.
The XPS survey spectrum of CNTs‐Ag2S, shown in Figure 6a, confirmed the presence of C, O, Ag, and S. The high‐resolution spectrum of C1s (Figure 6b) divulged four distinct peaks by Gaussian fitting situated at 284.5, 285.5, 286.4, and 287.8 eV. Among these, peaks at 284.5 and 285.5 eV were assigned to the C‐C bonds of the sp2 and sp3 hybridized carbon
The XPS survey spectrum of CNTs‐Ag2S, shown in Figure 6a, confirmed the presence of C, O, Ag, and S. The high‐resolution spectrum of C1s (Figure 6b) divulged four distinct peaks by Gaussian fitting situated at 284.5, 285.5, 286.4, and 287.8 eV. Among these, peaks at 284.5 and 285.5 eV were assigned to the C‐C bonds of the sp2 and sp3 hybridized carbon atoms of CNTs, respectively [55]. The peaks at 286.4 and 287.8 eV due to the C O
Catalysts
Figure 4. TG/DTA of CNTs‐Ag2S.
Figure 5. UV‐vis absorption spectrum of CNTs‐Ag2S.
Figure4.
presence of Ag 3d3/2 and Ag 3d5/2 peaks and their positions confirm the Ag+ state of silver [57]. The difference in the position of Ag 3d5/2 and Ag 3d3/2 peaks was 6 eV, and no shoulder or satellite peaks were observed between them. The high‐resolution S 2p spec‐trum (Figure 6e) for the spin‐orbit splitting of S2+ was deconvoluted into the 2p3/2 level peak at 161.2 eV, and the 2p1/2 peak at 162.3 eV. These peaks occurred by the Ag‐S bonds of the Ag2S nanoparticles [58]. The additional peak at 163.9 eV is related to the presence of sulfur in CNTs‐Ag2S [58].
TheXPSsurveyspectrumofCNTs-Ag2S,showninFigure 6a,confirmedthepresence ofC,O,Ag,andS.Thehigh-resolutionspectrumofC1s(Figure 6b)divulgedfourdistinct peaksbyGaussianfittingsituatedat284.5,285.5,286.4,and287.8eV.Amongthese,peaks at284.5and285.5eVwereassignedtotheC-Cbondsofthesp2 andsp3 hybridizedcarbon atomsofCNTs,respectively[55].Thepeaksat286.4and287.8eVwereduetotheC-Oand C=ObondsofCNTs,respectively[55].ThedeconvolutedO1speak(Figure 6c)exhibiteda peakat531.3eVforthelatticeoxygenandapeakat532.4eVcorrespondingtothecarbonyl (=C-O)functionalgroupsoftheCNTs[56].Thecore-electronbindingenergyofAg3d3/2 wasfoundat373.9eV,andthatofAg3d5/2wasat367.9eV(Figure 6d).Thepresenceof Ag3d3/2andAg3d5/2peaksandtheirpositionsconfirmtheAg+ stateofsilver[57].The differenceinthepositionofAg3d5/2andAg3d3/2peakswas6eV,andnoshoulderor satellitepeakswereobservedbetweenthem.Thehigh-resolutionS2pspectrum(Figure 6e) forthespin-orbitsplittingofS2+ wasdeconvolutedintothe2p3/2levelpeakat161.2eV, andthe2p1/2peakat162.3eV.ThesepeaksoccurredbytheAg-SbondsoftheAg2S nanoparticles[58].Theadditionalpeakat163.9eVisrelatedtothepresenceofsulfurin CNTs-Ag2S[58].
Figure6. Cont.
Catalysts 2023, 13, 476
Intensity (a.u.)
Intensity (a.u.)
Binding energy (eV)
363366369372375378
Binding energy (eV)
Intensity (a.u.)
Binding energy (eV)
Figure 6. (a) XPS survey spectrum of CNTs‐Ag2S. (b) High‐resolution spectrum of C1s. (c) High‐resolution spectrum of O1s. (d) High‐resolution spectrum of Ag3d. (e) High‐resolution spectrum of S2p.
The adsorption ability of CNTs‐Ag2S was determined by estimating the Cd(II) ad‐sorption present in water. The contact time between adsorbent and adsorbate is critical and it controls adsorption. Therefore, the Cd(II) adsorption was determined by allowing for contact with CNTs‐Ag2S at different times and compared with that of CNTs and Ag2S (Figure 7). The plot of the adsorption capacity, q versus t, is presented in Figure S2. Cd(II)
Figure6. (a)XPSsurveyspectrumofCNTs-Ag2S.(b)High-resolutionspectrumofC1s.(c)High-resolution spectrumofO1s.(d)High-resolutionspectrumofAg3d.(e)High-resolutionspectrumofS2p. 13

TheadsorptionabilityofCNTs-Ag2SwasdeterminedbyestimatingtheCd(II)adsorptionpresentinwater.Thecontacttimebetweenadsorbentandadsorbateiscritical anditcontrolsadsorption.Therefore,theCd(II)adsorptionwasdeterminedbyallowing forcontactwithCNTs-Ag2SatdifferenttimesandcomparedwiththatofCNTsandAg2S (Figure 7).Theplotoftheadsorptioncapacity,qt versust,ispresentedinFigureS2.Cd(II) adsorptionistimedependentandoccursasagradientfunctionoftime.Hence,contact timeiscrucialincontrollingtheCd(II)adsorption.ThetendencyinCd(II)adsorptionby CNTs,Ag2S,andCNTs-Ag2Swasidenticalliketheadsorptionwasrapid,thengradually reduced,andfinallyattainedequilibrium.TheinitialrapidCd(II)adsorptioncouldhave occurredduetothedifferenceintheconcentrationofCd2+ ionsinthesolutionandoverthe surfaceoftheCNTs-Ag2S,whichfacilitatedthemovementofCd2+ ionsfromthesolutionto thesurfaceoftheCNTs-Ag2S[8].Also,inthebeginning,manyactivesiteswereavailable onthesurfaceofCNTs-Ag2StooccupybyCd2+ ions[8].Withtheelapsedcontacttime,the activesitesoftheCNTs-Ag2SwerepredominantlyoccupiedbyCd2+ ions.Apart,thereisa possibilityofrepulsionbetweenCd2+ ionsinthesolutionandCd2+ ionsexistoverthesurfaceofCNTs-Ag2S.Owingtothesereasons,theadsorptionratecouldgraduallydecrease andeventuallyattainequilibrium[8].Within80minofthecontacttime,CNTs-Ag2Scould adsorbtheCd(II)completely;however,underidenticalconditions,theadsorptionratesof CNTsandAg2Swere79and53%,respectively.Thus,theadsorptionefficiencyofCNTsand Ag2SwassignificantlyimprovedbytheirconjugationinCNTs-Ag2S.Further,toidentify theadsorptionkineticsofCNTs-Ag2S,thedataweresimulatedwiththepseudo-first-order kineticmodelusingtheexpressionpresentedinEquation(1). ln(qe qt )= lnqe k1t (1) where qe and qt arethequantityofadsorbate(mg/g)atequilibriumandparticulartime, t (min),respectively,and k1 (min 1)isthepseudo-first-orderrateconstant.
CNTs-Ag2S CNTs Ag2S
Figure7. AdsorptionkineticsofCd(II)overCNTs,Ag2S,andCNTs-Ag2S.
Figure 7. Adsorption kinetics of Cd(II) over CNTs, Ag2S, and CNTs‐Ag2S.
Thepseudo-first-orderplotobtainedforCd(II)adsorptionoverCNTs,Ag2S,and CNTs-Ag2SisshowninFigure 8.Thecorrelationcoefficients(R2)perceivedforCNTs,Ag2S, andCNTs-Ag2Swere0.9906,0.9540,and0.9930,respectively.Thek1 andqe werecomputed
The pseudo‐first‐order plot obtained for Cd(II) adsorption over CNTs, Ag2S, and CNTs‐Ag2S is shown in Figure 8. The correlation coefficients (R2) perceived for CNTs, Ag2S, and CNTs‐Ag2S were 0.9906, 0.9540, and 0.9930, respectively. The k1 and qe were computed using the slope and intercept values of the straight lines acquired in Figure 8. The estimated values of k1 and qe is summarized in Table 1. The calculated qe(cal) could not agree with the experimental value qe(exp) for all samples. Thus, the experimental data for the Cd(II) adsorption could not be projected by the pseudo‐first‐order kinetic model.
usingtheslopeandinterceptvaluesofthestraightlinesacquiredinFigure 8.Theestimated valuesofk1 andqe issummarizedinTable 1.Thecalculatedqe(cal)couldnotagreewith theexperimentalvalueqe(exp)forallsamples.Thus,theexperimentaldatafortheCd(II) adsorptioncouldnotbeprojectedbythepseudo-first-orderkineticmodel.Alternatively,it wasanalyzedusingthepseudo-second-orderkineticmodelusingEquation(2).
where k2 [g/(mg.min)]isthepseudo-second-orderrateconstant.
Figure8. Pseudo-first-orderkineticsforCd(II)adsorptionoverCNTs,Ag2S,andCNTs-Ag2S.
Figure 8. Pseudo‐first‐order kinetics for Cd(II) adsorption over CNTs, Ag2S, and CNTs‐Ag2S.
Table1. ParameterscalculatedfortheCd(II)adsorptionusingadsorptionkineticmodels.
Table 1. Parameters calculated for the Cd(II) adsorption using adsorption kinetic models.
Adsorbent qe (exp)mgg 1
Pseudo-First-OrderKineticModelPseudo-Second-OrderKineticModel
Thepseudo-second-ordermodelplotisillustratedinFigure 9,andthedetermined parametersarepresentedinTable 1.TheR2 valuesderivedfromthepseudo-secondorderplotsforCNTs,Ag2S,andCNTs-Ag2Swere0.9963,0.9985,and0.9980,respectively. TheR2 valueofthesecond-orderplotwasrelativelyhigherthanthatfoundforthefirstorderplot,whichwascloseto1.Theqe(cal)ofthesecond-ordermodelmatchedthe qe(exp)forallsamples.Thelinearityofthepseudo-second-orderplotandtheagreeing valuesofqe(exp)andqe(cal)showthattheCd(II)adsorptioncouldbeanalyzedusingthe pseudo-second-orderkineticsratherthanthepseudo-first-orderkinetics.Theagreement ofthesecond-orderkineticsdemonstratesthatchemisorptionwastherate-determining stepintheCd(II)adsorption[9].Moreover,itrepresentstherapidtransferofCd(II) fromasolutionwithalowerinitialconcentrationtothesurfaceoftheCNTs-Ag2Sdueto

The pseudo‐second‐order model plot is illustrated in Figure 9, and the determined parameters are presented in Table 1. The R2 values derived from the pseudo‐second‐order plots for CNTs, Ag2S, and CNTs‐Ag2S were 0.9963, 0.9985, and 0.9980, respectively. The R2 value of the second‐order plot was relatively higher than that found for the first‐order plot, which was close to 1. The qe(cal) of the second‐order model matched the qe(exp) for all samples. The linearity of the pseudo‐second‐order plot and the agreeing values of qe(exp) and qe(cal) show that the Cd(II) adsorption could be analyzed using the pseudo‐second‐order kinetics rather than the pseudo‐first‐order kinetics. The agreement of the second‐order kinetics demonstrates that chemisorption was the rate‐determining step in the Cd(II) adsorption [9]. Moreover, it represents the rapid transfer of Cd(II) from a solu‐tion with a lower initial concentration to the surface of the CNTs‐Ag2S due to the concen‐tration gradient [9]. Further, the experimental data were explored using the Weber Morris
theconcentrationgradient[9].Further,theexperimentaldatawereexploredusingthe Weber Morrisintraparticleporediffusionmodeltoevaluatethediffusionmechanism usingEquation(3).
qt = kid t0.5 + C (3)
where kid (mg/g.min)istheintraparticlediffusionrateconstant,whichcanbecalculated fromthelinearplotof qt versus t0.5.c(mg/g)istheintraparticlediffusionconstant, estimatedfromtheinterceptanddirectlyproportionaltotheboundarylayerthickness.Itis assumedthatthehighertheinterceptvalue,themoresignificantthecontributionofthe surfaceadsorptionintherate-controllingstep.Iftheregressionofthe qt versus t0.5 plot islinearandpassesthroughtheorigin,intraparticlediffusionplaysasignificantrolein controllingthekineticsoftheadsorptionprocess.Ifitdoesnotpassthroughtheorigin, intraparticlediffusionisnottheonlyrate-limitingstep.Instead,itiscontributedbythe boundarylayereffect[59].Theintraparticlediffusionplotof qt versus t0.5 acquiredfor theCd(II)adsorptiononCNTs-Ag2SisdemonstratedinFigure 10.TheplotinFigure 10 doesnotpassthroughtheoriginofthecoordinates(FigureS3).So,intraparticlediffusion isnotthesolerate-limitingstepinCd(II)adsorptionoverCNTs-Ag2S.Theassociation ofthetwostraightlinesintheintraparticlediffusionplot(FigureS3)andtheintercept values(TableS1)supportthattheintraparticlediffusionwasnotonlyarate-controlling stepintheCd(II)adsorption;italsocontributedthroughtheboundarylayereffect[59]. ThemultilinearityoftheplotinFigure 10 (FigureS3)showsthattheCd(II)adsorption transpiresthroughmultiplephases[60].Amongthese,theinitialstageoccurredthrough therapidadsorptionofCd2+ ions,thesecondphasewasowingtothediffusionofCd2+ ions intotheporesofCNTs-Ag2S,andthethirdphasewasduetoequilibriumoftheadsorption thatcausedachemicalreaction/bonding[60].
surface adsorption in the rate‐controlling step. If the regression of the qt versus t0.5 plot is linear and passes through the origin, intraparticle diffusion plays a significant role in con‐trolling the kinetics of the adsorption process. If it does not pass through the origin, intra‐particle diffusion is not the only rate‐limiting step. Instead, it is contributed by the bound‐ary layer effect [59]. The intraparticle diffusion plot of qt versus t0.5 acquired for the Cd(II) adsorption on CNTs‐Ag2S is demonstrated in Figure 10. The plot in Figure 10 does not pass through the origin of the coordinates (Figure S3). So, intraparticle diffusion is not the sole rate‐limiting step in Cd(II) adsorption over CNTs‐Ag2S. The association of the two straight lines in the intraparticle diffusion plot (Figure S3) and the intercept values (Table S1) support that the intraparticle diffusion was not only a rate‐controlling step in the Cd(II) adsorption; it also contributed through the boundary layer effect [59]. The multi‐linearity of the plot in Figure 10 (Figure S3) shows that the Cd(II) adsorption transpires through multiple phases [60]. Among these, the initial stage occurred through the rapid adsorption of Cd2+ ions, the second phase was owing to the diffusion of Cd2+ ions into the pores of CNTs‐Ag2S, and the third phase was due to equilibrium of the adsorption that caused a chemical reaction/bonding [60].
Figure9. Pseudo-second-orderkineticsforCd(II)adsorptionoverCNTs,Ag2S,andCNTs-Ag2S.
Figure 9. Pseudo‐second‐order kinetics for Cd(II) adsorption over CNTs, Ag2S, and CNTs‐Ag2S.
Figure 10. Weber‐Morris intraparticle diffusion plot for the Cd(II) adsorption over CNTs‐Ag2S.
Figure10. Weber-MorrisintraparticlediffusionplotfortheCd(II)adsorptionoverCNTs-Ag2S.
The experimental equilibrium parameters for Cd(II) adsorption were determined by applying three isotherm models: Langmuir, Freundlich, and Temkin. The Langmuir model predicts that the adsorbed molecules form a monolayer and adsorption emerges at a static number of adsorption sites, each of which is equivalent in efficiency. Moreover, each molecule has a constant enthalpy and adsorption activation energy, it means that all molecules have an affinity equal to entire adsorption sites [61]. With the help of Langmuir isotherm model, it could be find the value of the maximum adsorption capacity of the adsorbent using Equation (4) [62]:
TheexperimentalequilibriumparametersforCd(II)adsorptionweredetermined byapplyingthreeisothermmodels:Langmuir,Freundlich,andTemkin.TheLangmuir modelpredictsthattheadsorbedmoleculesformamonolayerandadsorptionemerges atastaticnumberofadsorptionsites,eachofwhichisequivalentinefficiency.Moreover, eachmoleculehasaconstantenthalpyandadsorptionactivationenergy,itmeansthatall moleculeshaveanaffinityequaltoentireadsorptionsites[61].WiththehelpofLangmuir isothermmodel,itcouldbefindthevalueofthemaximumadsorptioncapacityofthe adsorbentusingEquation(4)[62]:
(4)
where qe (mg/g) is the amount of adsorbed Cd(II) per unit mass of CNTs‐Ag2S; Ce (mg/L) is the concentration of Cd(II) at equilibrium; qm is the maximum amount of the Cd(II) adsorbed per unit mass of CNTs‐Ag2S to form a complete monolayer on the surface‐bound at high Ce KL is the Langmuir adsorption constant related to the free energy of adsorption. The Langmuir plot for Cd(II) adsorption over CNTs‐Ag2S is presented in Figure 11. The estimated parameters are shown in Table 2. The maximum adsorption capacity (qm) cal‐culated for Cd(II) adsorption over CNTs‐Ag2S was 256.4 mg/g.
(4)
where qe (mg/g)istheamountofadsorbedCd(II)perunitmassofCNTs-Ag2S; Ce (mg/L) istheconcentrationofCd(II)atequilibrium; qm isthemaximumamountoftheCd(II) adsorbedperunitmassofCNTs-Ag2Stoformacompletemonolayeronthesurface-bound athigh Ce. KL istheLangmuiradsorptionconstantrelatedtothefreeenergyofadsorption. TheLangmuirplotforCd(II)adsorptionoverCNTs-Ag2SispresentedinFigure 11.The estimatedparametersareshowninTable 2.Themaximumadsorptioncapacity(qm) calculatedforCd(II)adsorptionoverCNTs-Ag2Swas256.4mg/g.
Catalysts 2023, 13, 476 14 of 23
(mg/L)
Figure 11. Langmuir isotherm plot for Cd(II) adsorption over CNTs‐Ag2S.
Table 2. Parameters calculated for Cd(II) adsorption over CNTs‐Ag2S using Langmuir, Freundlich, and Temkin adsorption isotherm models.
Figure11. LangmuirisothermplotforCd(II)adsorptionoverCNTs-Ag2S.
Table2. ParameterscalculatedforCd(II)adsorptionoverCNTs-Ag2SusingLangmuir,Freundlich, andTemkinadsorptionisothermmodels. LangmuirIsothermFreundlichIsothermTemkinIsotherm
TheFreundlichisothermisdevelopedbasedontheassumptionthattheadsorption sitesaredistributedexponentiallyconcerningtheheatofadsorption[62,63].Itprovides therelationshipbetweentheequilibriumofliquidandsolidphasecapacitieswithmultilayeradsorptionpropertiesconsistingoftheheterogeneoussurfaceoftheadsorbent.The FreundlichisothermthatsupportsmultilayeradsorptionagreeswiththeLangmuirmodel overmoderaterangesofconcentrationsbutdiffersatlowandhighconcentrations.The linearformoftheFreundlichisothermcanberepresentedbEquation(5):
e =
where qe (mg/g)istheamountofCd(II)adsorbedatequilibrium. KF andnareFreundlich constants. KF symbolizestheaffinityoftheadsorbent,andnsignifiestheadsorption intensity. Ce (mg/L)istheCd(II)concentrationatequilibrium.TheFreundlichisotherm plotattainedforCd(II)adsorptionisshowninFigureS4,andthecalculatedparametersare includedinTable 2
TheTemkinisothermmodelisbasedonthefactthat,duringadsorption,theheatof allmoleculesdecreaseslinearlywithanincreaseinthecoverageoftheadsorbentsurface andthatadsorptionischaracterizedbytheuniformdistributionofbindingenergiesup tothemaximumbindingenergy[62,64].ThelinearformoftheTemkinisothermmodelis showninEquation(6):
e = BlnA + BlnCe (6)
whereB=RT/KT,KT istheTemkinconstantrelatedtotheheatofadsorption(J/mol);A istheTemkinisothermconstant(L/g),Risthegasconstant(8.314J/molK),andTisthe absolutetemperature(K).TheTemkinisothermfittingplotfortheCd(II)adsorptionis showninFigureS5.ThevaluesestimatedfromFigureS5areillustratedinTable 2.
TheR2 Lan valuefoundfortheLangmuirisothermplotwas0.9972;forFreundlichand Temkinisothermplots,theR2 Fre andR2 Tem valueswere0.3562and0.0019,respectively.The FreundlichandTemkinisothermmodelsprovidedscatteredpointsandfailedtoaccomplish linearity.Therefore,theFreundlichandTemkinisothermmodelswereunsuitablefor explainingCd(II)adsorptionoverCNTs-Ag2S.However,theLangmuirisothermmodel producedlinearity.Therefore,theLangmuirmodelisappropriateforelucidatingtheCd(II) adsorption.ThevalidationoftheLangmuirmodelindicatesthattheCd(II)adsorptionover CNTs-Ag2Sensuedwithmonolayermolecularcoveringandchemisorption,together[65].
Further,thephotocatalyticactivityofCNTs-Ag2SwasinvestigatedthroughthedegradationofAYRunderexposuretosunlight.ThedegradationofAYRbyCNTs-Ag2Sis presentedinFigure 12.TheactivityofCNTs-Ag2SwascomparedwiththatofCNTsand Ag2S.TheCNTs-Ag2ScoulddegradetheAYRcompletelywithin120minofillumination. However,CNTsandAg2Swerecapabletodegrade77.3and41%ofAYR,respectively,in 120min.Hence,theconjugationofCNTsandAg2SwassubstantiallyimprovedthephotocatalyticactivityinCNTs-Ag2S.ThedegradationofAYRwasquantifiedbythereductionin theelectronicabsorptionbandlocatedat373nm(Figure 13).Thehybridnano-architecture ofCNTs-Ag2SwasproficientinadsorbinghighernumberofAYRmoleculesandcapable inabsorptionofsunlight.Inaddition,theeffectualhindranceofrecombiningofelectrons andholesduringphotocatalysisfacilitatedthedegradationprocess.Thephotocatalytic activityofCNTs-Ag2Swasfurtherexploredbyfindingtheapparentrateconstantsusing
lnq
lnKF + lnCe n (5)
q
120 min. Hence, the conjugation of CNTs and Ag2S was substantially improved the pho
tocatalytic activity in CNTs‐Ag2S. The degradation of AYR was quantified by the reduc‐tion in the electronic absorption band located at 373 nm (Figure 13). The hybrid nano‐architecture of CNTs‐Ag2S was proficient in adsorbing higher number of AYR molecules and capable in absorption of sunlight. In addition, the effectual hindrance of recombining of electrons and holes during photocatalysis facilitated the degradation process. The pho‐tocatalytic activity of CNTs‐Ag2S was further explored by finding the apparent rate con‐stants using the Langmuir‐Hinshelwood (L‐H) model and with the help of Equation (7) that could be applicable for low concentrations of dyes [38,39,44,45].
theLangmuir-Hinshelwood(L-H)modelandwiththehelpofEquation(7)thatcouldbe applicableforlowconcentrationsofdyes[38,39,44,45].
ln C0 C = kapp t (7)
where C0 istheinitialconcentrationofAYRand C istheconcentrationataparticulartime ofirradiation. kapp istheapparentrateconstantofthereaction,andtistheirradiationtime.
Ag2S CNTs CNTs-Ag2S
where C0 is the initial concentration of AYR and C is the concentration at a particular time of irradiation. kapp is the apparent rate constant of the reaction, and t is the irradiation time. 020406080100120
Figure12. ThedegradationofalizarinyellowRinthepresenceofCNTs,Ag2S,andCNTs-Ag2S undertheilluminationtosunlight.
Figure 12. The degradation of alizarin yellow R in the presence of CNTs, Ag2S, and CNTs‐Ag2S under the illumination to sunlight.
Figure 13. Reduction in the electronic absorption of alizarin yellow R in the presence of CNTs‐Ag2S under the irradiation to sunlight.
Figure13. ReductionintheelectronicabsorptionofalizarinyellowRinthepresenceofCNTs-Ag2S undertheirradiationtosunlight.
The L‐H plots perceived for the degradation of AYR were linear, as depicted in Fig‐ure 14. The linearity of the L‐H plots reveals that the degradation of AYR ensue with pseudo‐first‐order kinetics. The apparent rate constants determined by the L‐H plots for CNTs, Ag2S, and CNTs‐Ag2S were 0.0108, 0.0035, and 0.0378 min‐1 , respectively. The value determined for CNTs‐Ag2S was about four‐fold higher than CNTs and eleven‐fold greater than Ag2S. Therefore, the conjugation of CNTs and Ag2S has magnificently improved the photocatalytic activity in CNTs‐Ag2S. Due to the conjugated structure, containing aro‐matic, carbonyl, and azo groups, the AYR solution possessed an intense color in water. Accordingly, AYR degradation could happen by breaking the conjugated system in the presence of CNTs‐Ag2S under sunlight irradiation. With this assumption, the possible
TheL-HplotsperceivedforthedegradationofAYRwerelinear,asdepictedinFigure 14 ThelinearityoftheL-HplotsrevealsthatthedegradationofAYRensuewithpseudofirst-orderkinetics.TheapparentrateconstantsdeterminedbytheL-HplotsforCNTs, Ag2S,andCNTs-Ag2Swere0.0108,0.0035,and0.0378min 1,respectively.Thevalue determinedforCNTs-Ag2Swasaboutfour-foldhigherthanCNTsandeleven-foldgreater
thanAg2S.Therefore,theconjugationofCNTsandAg2Shasmagnificentlyimproved thephotocatalyticactivityinCNTs-Ag2S.Duetotheconjugatedstructure,containing aromatic,carbonyl,andazogroups,theAYRsolutionpossessedanintensecolorinwater. Accordingly,AYRdegradationcouldhappenbybreakingtheconjugatedsysteminthe presenceofCNTs-Ag2Sundersunlightirradiation.Withthisassumption,thepossible mechanismfortherapiddegradationofAYRbyCNTs-Ag2Scouldbeexplainedasfollows (Figure 15).
Figure 14. Langmuir‐Hinshelwood plot for the degradation of alizarin yellow R in the presence of CNTs‐Ag2S under illumination to sunlight.
Figure 14. Langmuir‐Hinshelwood plot for the degradation of alizarin yellow R in the presence of CNTs‐Ag2S under illumination to sunlight.
Figure14. Langmuir-HinshelwoodplotforthedegradationofalizarinyellowRinthepresenceof CNTs-Ag2Sunderilluminationtosunlight.
Figure Ag
Figure15. ApossiblemechanismforthedegradationofalizarinyellowRinthepresenceofCNTsAg2Sunderexposuretosunlight.
Further, to find the stability of CNTs‐Ag2S, it was recovered by centrifugation after photocatalysis. It was washed with DI water, dried and used in further two cycles of the photocatalysis. The reused CNTs‐Ag2S was able to degrade AYR completely in further two cycles without any significant reduction in activity (Figure S6). The XRD of CNTs‐Ag2S recorded before and after using in the three degradation cycles of AYR could not show any structural modification or rapture (Figure S7). Consequently, CNTs‐Ag2S is ta‐ble and suitable for reuse.
Figure 15. A possible mechanism for the degradation of alizarin yellow R in the presence of CNTs‐Ag2S under exposure to sunlight.
Further, to find the stability of CNTs‐Ag2S, it was recovered by centrifugation after photocatalysis. It was washed with DI water, dried and used in further two cycles of the photocatalysis. The reused CNTs‐Ag2S was able to degrade AYR completely in further two cycles without any significant reduction in activity (Figure S6). The XRD of CNTs‐
Catalysts 2023, 13, 476
Further,tofindthestabilityofCNTs-Ag2S,itwasrecoveredbycentrifugationafter photocatalysis.ItwaswashedwithDIwater,driedandusedinfurthertwocyclesofthe photocatalysis.ThereusedCNTs-Ag2SwasabletodegradeAYRcompletelyinfurthertwo cycleswithoutanysignificantreductioninactivity(FigureS6).TheXRDofCNTs-Ag2S recordedbeforeandafterusinginthethreedegradationcyclesofAYRcouldnotshow anystructuralmodificationorrapture(FigureS7).Consequently,CNTs-Ag2Sistableand suitableforreuse.
TheCNT/Ag2SnanocompositepreparedbyDietal.andappliedittothedegradation ofrhodamineBrevealeditshigheractivitythanbareAg2Snanoparticlesunderilluminationtovisibleandnear-infrared(NIR)light[57].Inaddition,therecycledCNT/Ag2S nanocompositecouldnotlosttheactivity[57].TheAg2S-CNTnanocomposite,reported byMengetal.[66],efficientlydegradedtexbriteBA-Linpresenceofvisiblelight.Inthis study,theAg2S-CNTnanocompositewasforfourdegradationcyclesoftexbriteBA-L[66]. Further,thephotocatalyticactivityofCNTs-Ag2SinthedegradationofAYRwascompared withdifferentphotocatalystsandpresentedinTable 3 [24,25,46,67–69].
Table3. ComparisonofthedegradationrateofAYRbyCNTs-Ag2Swithreportedphotocatalysts. PhotocatalystSourceofIrradiationDegradationRate(%)Ref CNTs-Ag2SSunlight100Thiswork FenanoparticlesSunlight93.725 H2O2 UV10024 ZincoxideUV92.546 β-MnO2 nanowiresMercurylamp98.069 Mn3O4 Mercurylamp62.069 MnO(OH)nanorodsMercurylamp54.069 Bi2O3@RGOSunlight41.570 ZnOnanoparticlesUV95.071 ZnOnanoparticlesSunlight13.271 ZnOnanoparticlesVisible06.271
3.Experimental
3.1.Materials
ThechemicalsandCNTswerepurchasedfromMilliporeSigmaandusedasreceived. TheaqueoussolutionswerepreparedusingultrapurewaterobtainedbytheMilli-QPlus system(Millipore;Burlington,MA,USA).
3.2.PreparationofCNTs-Ag2S
ThehydrophobicpristineCNTsweremodifiedtohydrophilicthroughtheoxidization byrefluxinginamixtureof1:3(v/v)concentratedHNO3 andH2SO4 at70 ◦Cfor24h.The resultingoxidizedCNTswerecollectedthroughcentrifugationandpurifiedbywashing themwithDIwater[70,71].TheresultingoxidizedCNTsweredriedunderavacuumand usedtodepositAg2Snanoparticles.About40mgofoxidizedCNTs(CNTs-COOH)were dispersedin40mLofDIwaterusingsonicator,andasolutionof0.04mol/LofAgNO3 in 40mLofDIwaterwasadded.Themixturewasallowedtostirinroomtemperaturefor 30min,andafreshlyprepared40mLaqueoussolutionof0.02mol/Lsodiumsulfidewas addedslowly.Thissuspensionwasstirredfor10minandtransferredtoanautoclave.The autoclavewasheatedat180 ◦Cfor6htoyieldCNTs-Ag2S.Thus,theformedCNTs-Ag2S wascollectedbycentrifugationandpurifiedwithwashingwithDIwater.
3.3.PreparationofAg2S
Forcontrolexperiments,Ag2Snanoparticleswerepreparedusingtheproceduresused forCNTs-Ag2SwithoutCNTs.
3.4.AdsorptionExperiments
ThestocksolutionofCd(II),withaconcentrationof1g/L,waspreparedinDI waterusingcadmiumchlorideandwasdilutedtothedesiredconcentrations.Thekinetic Cd(II)adsorptionexperimentswereconductedtofindthecontacttimeneededtoattain equilibrium.Inthetypicalexperiment,100mgofCNTs-Ag2Swasdispersedinto500mLof aCd(II)solutionwithaconcentrationof0.5mg/Landstirredatroomtemperature.After therequiredcontacttime,anadequatesamplewascollected,andthedispersedCNTs-Ag2S wasseparatedbycentrifugation.TheconcentrationofresidualCd(II)inthecollected sampleswasestimatedbyatomicabsorptionspectrometer.TheamountofCd(II),adsorbed byCNTs-Ag2Swasmonitoredasafunctionoftimefor120min.ThequantityofCd(II) adsorbedwascalculatedusingEquation(8).
where qt istheamountofadsorbedCd(II)(mg/g)attimet; C0 istheinitialconcentration oftheCd(II)(mg/L),and Ct istheconcentrationoftheCd(II)(mg/L)attimet;Visthe volumeofthesolution(L),andMistheamountofadsorbent(g).
Further,theefficiencyofCNTs-Ag2SinCd(II)adsorptionwasestimatedusing Equation(9).
Removalefficiency (%) = (C0 Ct ) V C0 × 100(9)
Foradsorptionisothermexperiments,10mgofCNTs-Ag2Swasmixedwith50mL oftheCd(II)solutionandstirredatroomtemperaturefor24htoreachequilibriumin theconcentrationbetween50and140mg/L.AfterseparatingthedispersedCNTs-Ag2S, theconcentrationofCd(II)inthesolutionwasmeasuredusinganatomicabsorption spectrometer.TheamountofCd(II)adsorbedatequilibrium,qe (mg/g),wasdetermined byEquation(10):
where qe istheamountofCd(II)adsorbed(mg/g)atequilibrium.
3.5.PhotocatalyticActivity
Toevaluatethephotocatalyticactivity,10mgofCNTs-Ag2Swasaddedtothe100mL aqueoussolutionofAYRwithaconcentrationof10mg/L.Itwasallowedtostirinthe darkfor30mintoreachadsorption/desorptionequilibriumoftheAYRmoleculesoverthe surfaceofCNTs-Ag2S.Thissuspensionwastransferredtoaphotocatalyticreactorhavinga waterjacketwithawatercirculationsystemtomaintainaconstanttemperature,andthe suspensionwasexposedtosunlight.Attherequiredtime,5mLofthereactionmixture waswithdrawn,andthesuspendedCNTs-Ag2Swasseparatedusingcentrifugation.The concentrationofAYRafterphotocatalysiswasassessedwithUV-visspectrophotometer byrecordingtheabsorbanceat373nm.ThenormalizedconcentrationofAYRafterphotocatalysiswascalculatedasC/C0,whereC0 istheinitialconcentrationofAYRandCis itsconcentrationafterphotocatalysis.Allphotocatalyticexperimentswereconductedin themonthofJune,between1pmand4pm.Theintensityofsunlightmeasuredduring photocatalysiswas800–900W/m2
3.6.Characterization
TheUV-visabsorptionspectrawererecordedusingaJascoV-770UV-vis-NIRspectrophotometer(Easton,MD,USA),andATR-FTIRspectrawerecollectedwithaSmiths ChemIDdiamondattenuatedtotalreflection(DATR)spectrometer(SmithsDetection,Inc., London,UnitedKingdom).TheXRDwasobtainedbyaScintagX-raydiffractometer (Cupertino,CA,USA),modelPADX,equippedwithaCu-Kα photonsource(45kV, 40mA),atascanningrateof3◦/min.Thethermogravimetrydifferentialthermalanalysis