A
Clinician’s Pearls & Myths in Rheumatology
Second Edition
Editor
John H. Stone
Massachusetts General Hospital
Harvard University Massachusetts General Hospital
Boston, MA, USA
ISBN 978-3-031-23487-3 ISBN 978-3-031-23488-0 (eBook) https://doi.org/10.1007/978-3-031-23488-0
© Springer Nature Switzerland AG 2009, 2023
This work is subject to copyright. All rights are solely and exclusively licensed by the Publisher, whether the whole or part of the material is concerned, specifcally the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microflms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed.
The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specifc statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use.
The publisher, the authors, and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. Neither the publisher nor the authors or the editors give a warranty, expressed or implied, with respect to the material contained herein or for any errors or omissions that may have been made. The publisher remains neutral with regard to jurisdictional claims in published maps and institutional affliations.
This Springer imprint is published by the registered company Springer Nature Switzerland AG The registered company address is: Gewerbestrasse 11, 6330 Cham, Switzerland
To Sarah Lucretia (“Lu”) Stone (1936–1991)
A mother holds her children’s hands for a short time, and their hearts forever.
Preface
When I was a child in the second grade, my mother developed an acutely painful index fnger on her right hand. After several sleepless nights with unrelenting pain that was disguised from me, she was admitted to the hospital. The surgeon suspected a glomus tumor—a benign growth that sometimes develops in the nailbed. But no tumor was found at surgery. Instead, the surgeon shared an astonishing observation with my father: “When I removed her fngernail, she didn’t bleed.”
And at that point, the nature of her disease began to dawn on her doctors and on my father, who was himself a physician. My mother had developed Raynaud’s phenomenon as a teenager. When she and my father were newlyweds and he was a medical student, they had mused together at the oddity of her intermittently pale white fngers, which developed sometimes even in the summer. Around the time I was born, she suffered from a skin problem that I learned—years later—was dermatitis herpetiformis. As a young boy, I remember her scratching her intensely itchy legs. As I grew up, I became aware of the subtle scars left on her face, usually well-hidden with makeup.
The active skin infammation faded after a few years, but other aspects of the autoimmune kaleidoscope came into sharper focus. The ischemic fnger in the context of her preceding issues led to the diagnosis of scleroderma. In that light, most of the medical events in my mother’s life make sense now. Her fnger hadn’t bled at surgery because of the severity of vasoconstriction. The shapes of her fngers that I can still see and remember dearly, including the curved fngernail that never grew back normally, were the result of her disease. The calcinoses and digital pitting that developed in her fngers were also consequences of her slowmoving but relentless illness, as were the intermittent bouts of dysphagia caused by esophageal dysmotility. For a time, during my teens, my mother often had to excuse herself from the dinner table because she just couldn’t swallow her food. A painful memory for me.
Despite that, Mom led a full life and it seemed to most that nothing slowed her down: raising two rambunctious boys; being a loving wife and returning to graduate school; becoming a devoted frst-grade teacher; using her hands constantly in spite of sensitive fngers on sewing, knitting, cooking, and playing the piano; enjoying lifelong friends; attending our soccer games with mittens on to counter Raynaud’s attacks; and delighting in family travel.
It all came to a halt far too early. When I was a medical intern, the hospital operator paged me to return a call from my father. Mom had developed an acute bowel obstruction because of scleroderma gut and had been taken to surgery that morning. I few home, to her bedside in the intensive care unit. There, far more fragile that anyone had realized, she died twelve days later of adult respiratory distress syndrome. The real cause, of course, was scleroderma. She was 54 years old.
It is little consolation that traumatic health events in one’s family make a physician a better doctor. Yet it is true. My mother’s nearly lifelong struggle with scleroderma—ironically regarded as “limited”—helps me understand the uncertainties, frustrations, and tears of my patients and their families. Mom’s illness, of course, contributed to my becoming a rheumatologist and dedicating my career to helping patients address diseases like hers. It is only ftting, then, that this book is dedicated to her.
So much has happened since the First Edition was published in 2009. New rheumatic diseases have been identifed. (IgG4-related disease was scarcely mentioned in the First Edition!) Biomedical science has witnessed important advances in assessment and diagnosis, some of which have already altered approaches to patient care. Creative new therapies have been conceived and studied. Some in fact have worked, been approved, and are already improving patients’ lives. The world has endured (and still persists in) a viral pandemic that has underscored the importance of vaccines and public health and forced us to re-think how we apply many of our existing treatments. Though many challenges remain, my inherent optimism - a trait inherited from my mother - inspires confdence that the coming years will mark growing progress for the patients we treat.
The judgment of inspired clinicians will remain fundamental to inspiring advances and maintaining safe speeds on the paths ahead. It is in this spirit that this book is written: by clinicians, for clinicians. I am grateful to the more than 200 contributors who offer guidance here from their own approaches to our art, sharing their hard-earned and frequently elegant clinical wisdom.
Boston, MA, USA
John H. Stone
1 Rheumatoid Arthritis
Kevin D. Deane, Daniel Aletaha, Joan M. Bathon, Paul Emery, George E. Fragoulis, V. Michael Holers, T. W. J. Huizinga, Jason R. Kolfenbach, James R. O’Dell, Duane W. Pearson, Elizabeth Park, Josef Smolen, Yoshiya Tanaka, Peter C. Taylor, Annette van der Helm-van Mil, Ronald F. van Vollenhoven, and E. William St. Clair
2 Rheumatoid Vasculitis
Ashima Makol, Tanaz Kermani, Kenneth Warrington, John H. Stone, and Eric L. Matteson
3 Adult-Onset Still’s Disease .
Yutong Su and Chengde Yang
4 Juvenile Idiopathic Arthritis
Randy Q. Cron, Sangeeta Sule, Jordan T. Jones, Tristan A. Kerr, Kimberly A. Morishita, Ross E. Petty, and Carol B. Lindsley
5 Monogenic Autoinflammatory Syndromes
Ivona Aksentijevich and Eldad Ben-Chetrit
6 Juvenile Spondyloarthritis
Dax G. Rumsey, David A. Cabral, and Shirley M. L. Tse
7 Axial Spondyloarthritis
Xenofon Baraliakos, Dennis McGonagle, and Jürgen Braun
8 Psoriatic Arthritis
Philip Helliwell, Laura C. Coates, and Dafna Gladman
9 Reactive Arthritis
John H. Stone
10 Systemic Sclerosis (Scleroderma) and Raynaud’s Phenomenon
Ami A. Shah, Janet E. Pope, Dinesh Khanna, Maureen Mayes, Virginia Steen, and Christopher Denton
11 Sjögren’s Disease
Alan Baer, Vatinee Bunya, Ava Wu, Xavier Mariette, and Frederick Vivino
12 Systemic Lupus Erythematosus 167
Michelle Petri, Martin Aringer, Isabelle Ayoub, Salem Almaani, Hermine Brunner, Maria Dall’Era, Mengdi Jiang, Richard Furie, Jessica Greco, Fiona Goldblatt, Jennifer Huggins, T. W. J. Huizinga, David Isenberg, Nicholas L. Li, R. C. Monahan, Samir V. Parikh, David Pisetsky, Abin P. Puravath, Brad Rovin, Daniel Wallace, Xuan Zhang, and Lidan Zhao
13 Childhood-Onset SLE and Neonatal Lupus Erythematosus
Deborah M. Levy, Jill Buyon, and Earl D. Silverman
14 The Antiphospholipid Syndrome 225
David P. D’Cruz, Jason S. Knight, Lisa Sammaritano, Jane Salmon, Ricard Cervera, and Munther Khamashta
15 Reproductive Health in the Rheumatic Diseases 241
Julia Sun, Laura Andreoli, Jane Salmon, Meghan Clowse, Caroline Gordon, Jill Buyon, Rosalind Ramsay-Goldman, and Lisa Sammaritano
16 Inflammatory Myopathies
Chester Oddis, Vidya Limaye, Frederick Miller, and Lisa Christopher-Stine
17 Juvenile Dermatomyositis 275
Lauren M. Pachman, Sarah Tansley, Ann M. Reed, Clarissa M. Pilkington, Brian M. Feldman, and Lisa G. Rider
18 Vasculitic Neuropathy 287
John H. Stone
19 Pediatric Vasculitis
Seza Ozen, Despina Eleftheriou, Anne Rowley, and Paul Brogan
20 Behçet Syndrome
Johannes Nowatzky, Gulen Hatemi, Vedat Hamuryudan, Hasan Yazici, and Yusuf Yazici
21 Eosinophilic Granulomatosis with Polyangiitis
John H. Stone
22 Granulomatosis with Polyangiitis 335
John H. Stone
23 Microscopic Polyangiitis 357
Duvuru Geetha and John H. Stone
24 Oral Manifestations Associated with Rheumatic Diseases 369
Sonia Marino, Sook-Bin Woo, Roberta Gualtierotti, John A. G. Buchanan, Shaiba Shandu, Francesco Spadari, and Massimo Cugno
25 Cryoglobulinemia 395
Franco Dammacco, Patrice Cacoub, John H. Stone, and David Saadoun
26 Polyarteritis Nodosa
John H. Stone
27 Giant Cell Arteritis and Polymyalgia Rheumatica
Peter M. Villiger, Lisa Christ, Luca Seitz, Godehard Scholz, Christoph Tappeiner, Francesco Muratore, Carlo Salvarani, Sue Mollan, Vanessa Quick, Christian Dejaco, Michael Lee, Neil Basu, Neil Miller, and John H. Stone
28 Takayasu’s Arteritis 447
Kaitlin A. Quinn, Durga P. Misra, Aman Sharma, Andrew Porter, Justin Mason, and Peter C. Grayson
29 Central Nervous System Vasculitis and Reversible Cerebral Vasoconstriction Syndrome
Rula A. Hajj-Ali, David S. Younger, and Leonard H. Calabrese
30 Thromboangiitis Obliterans
Ashima Makol, Leslie T. Cooper, John H. Stone, and Eric L. Matteson
31 Less Common Vasculitides
Eric L. Matteson and John H. Stone
32 Relapsing Polychondritis 491
Jeremie Dion, Guillaume Moulis, and Nathalie Costedoat
33 Fibromyalgia .
Daniel Clauw
34 Clinical Features of Gout
Robert Terkeltaub, Nicola Dalbeth, Naomi Schlesinger, Brian Mandell, and Michael Pillinger
35 Epidemiology of Gout
Hyon K. Choi and John H. Stone
36 Treatment of Gout
Nicola Dalbeth, Michael Pillinger, Naomi Schlesinger, Brian Mandell, and Robert Terkeltaub
37 CPPD and Other Microcrystalline Disorders
Ann K. Rosenthal, Mariano Andres, Abhishek Abhishek, and Robert Terkeltaub
38 Inflammatory Eye Disease 545
Bart Chwalisz, Michael Lee, Lucia Sobrin, and Suzanne K. Freitag
39 Immune-Mediated Inner Ear Disease 569 Soumyajit Das and Steven Rauch
40 Osteoporosis: Epidemiology and Assessment
Mary Beth Humphrey, Bita Zahedi, Amy Warriner, Sarah Morgan, Benjamin Z. Leder, Ken Saag, and Elaine W. Yu
41 Osteoporosis Prevention and Treatment 587
Mary Beth Humphrey, Bita Zahedi, Amy Warriner, Sarah Morgan, Benjamin Z. Leder, Ken Saag, and Elaine W. Yu
42 Paget’s Disease of Bone
Ian R. Reid and Margaret Seton
43 Lyme
Sheila L. Arvikar, John J. Halperin, and Allen C. Steere 44 Osteoarthritis
Lauren King, Ian Stanaitis, and Gillian Hawker
45 Regional Musculoskeletal Problems
Atul Deodhar and John H. Stone
46 Low Back and Neck Pain
Rajiv K. Dixit, Daniel Tobert, and Joseph H. Schwab
47 The Ehlers-Danlos Syndromes
Marlies Colman, Anne De Paepe, and Fransiska Malfait
48 Sarcoidosis
Marc A. Judson, Elyse E. Lower, Edward S. Chen, Jeffrey A. Sparks, Jocelyn R. Farmer, and Robert P. Baughman
49 Amyloidosis 687
Andrew Staron, Morie Gertz, and Giampaolo Merlini
50 IgG4-Related Disease 701
Mitsuhiro Kawano, Yoh Zen, Takako Saeki, Lingli Dong, Wen Zhang, Emanuel
Della-Torre, Philip A. Hart, Judith A. Ferry, and John H. Stone
51 Castleman Disease 727
Luke Chen and David C. Fajgenbaum
52 Erdheim-Chester Disease 737
Matthew J. Koster
53 Kikuchi-Fujimoto Disease
Guillaume Dumas and Olivier Fain
54 Whipple’s Disease
Rima N. El-Abassi, Daniel Raines, and J. D. England
743
Contributors
Abhishek Abhishek University of Nottingham, Nottingham, UK
Ivona Aksentijevich Infammatory Disease Section, National Human Genome Research Institute, Bethesda, MD, USA
Daniel Aletaha Division of Rheumatology, Department of Medicine 3, Medical University of Vienna, Vienna, Austria
Salem Almaani Department of Internal Medicine, The Ohio State Wexner Medical Center, Columbus, OH, USA
Laura Andreoli Department of Clinical and Experimental Sciences, University of Brescia, Brescia, Italy
Mariano Andres Sección de Reumatología, Hospital General Universitario de Alicante, Departamento de Medicina Clínica, Universidad Miguel Hernández, and ISABIAL, Elche, Spain
Martin Aringer Division of Rheumatology, Department of Medicine III, University Medical Center and Faculty of Medicine Carl Gustav Carus, Dresden, Germany
Sheila L. Arvikar Center for Immunology and Infammatory Diseases, Division of Rheumatology, Allergy, and Immunology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
Isabelle Ayoub Department of Medicine, Division of Nephrology, The Ohio State University Wexner Medical Center, Columbus, OH, USA
Alan Baer Division of Rheumatology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Xenofon Baraliakos Rheumazentrum Ruhrgebiet, Herne, Ruhr-University Bochum, Herne, Germany
Neil Basu Institute of Infection, Immunity, and Infammation, University of Glasgow, Glasgow, UK
Joan M. Bathon Columbia University Irving Medical Center, New York Presbyterian Hospital, New York, NY, USA
Robert P. Baughman Department of Medicine, University of Cincinnati Medical Center, Cincinnati, OH, USA
Eldad Ben-Chetrit Rheumatology Unit, Hadassah-Hebrew University Medical Center, Jerusalem, Israel
Jürgen Braun Rheumazentrum Ruhrgebiet, Herne, Germany
Paul Brogan Great Ormond Street Hospital for Children NHS Foundation Trust, University College London Great Ormond Street Institute of Child Health, London, UK
Hermine Brunner Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, OH, USA
John A. G. Buchanan Centre for Education and Innovation, Dental Institute, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, UK
Vatinee Bunya Scheie Eye Institute, University of Pennsylvania, Philadelphia, PA, USA
Jill Buyon New York University School of Medicine, New York, NY, USA
David A. Cabral Department of Pediatrics, British Columbia Children’s Hospital, Vancouver, BC, Canada
Patrice Cacoub AP-HP, Groupe Hospitalier Pitié-Salpêtrière, Department of Internal Medicine and Clinical Immunology, Sorbonne Université, Paris, France
Leonard H. Calabrese Cleveland Clinic Center for Vasculitis Care and Research, Cleveland, OH, USA
Ricard Cervera Department of Autoimmune Diseases, Hospital Clinic, Barcelona, Spain
Edward S. Chen Division of Pulmonary and Critical Care Medicine, Department of Medicine, The Johns Hopkins University, Baltimore, MD, USA
Luke Chen Division of Hematology, The University of British Columbia, Vancouver, BC, Canada
Hyon K. Choi Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
Lisa Christ Department of Rheumatology and Immunology, Inselspital, Bern University Hospital, Bern, Switzerland
Lisa Christopher-Stine Division of Rheumatology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Johns Hopkins Myositis Precision Medicine Center of Excellence, Baltimore, MD, USA
Bart Chwalisz Harvard Medical School, Boston, MA, USA
Division of Neuroimmunology and Neuroinfectious Disease, Massachusetts General Hospital, Boston, MA, USA
Department of Ophthalmology, Massachusetts Eye and Ear Infrmary, Boston, MA, USA
Daniel Clauw Division of Rheumatology, University of Michigan, Ann Arbor, MI, USA
Meghan Clowse Division of Rheumatology and Immunology, Duke University Medical Center, Durham, NC, USA
Laura C. Coates Nuffeld Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, Oxford, UK
Marlies Colman Center for Medical Genetics, Department of Biomolecular Medicine, Ghent University and Ghent University Hospital, Ghent, Belgium
Leslie T. Cooper Department of Cardiology, Mayo Clinic, Jacksonville, FL, USA
Nathalie Costedoat UMR 1027 Inserm, University of Toulouse, Toulouse, France
Service de Médecine Interne, Hôpital Cochin, Paris, France
Randy Q. Cron University of Alabama at Birmingham, Birmingham, AL, USA
Massimo Cugno Department of Pathophysiology and Transplantation, Internal Medicine, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Università degli Studi di Milano, Milan, Italy
David P. D’Cruz Louise Coote Lupus Unit, Guy’s Hospital, London, UK
Nicola Dalbeth Department of Medicine, University of Auckland, Auckland, New Zealand
Maria Dall’Era School of Medicine, University of California, San Francisco, CA, USA
Franco Dammacco Department of Biological Sciences and Human Oncology, Section of Internal Medicine, University of Bari “Aldo Moro” Medical School, Bari, Italy
Soumyajit Das Department of ENT, All India Institute of Medical Sciences, Mangalagiri, Andhra Pradesh, India
Kevin D. Deane Division of Rheumatology, University of Colorado Denver Anschutz Medical Campus, Aurora, CO, USA
Christian Dejaco Rheumatology, Medical University Graz, Graz, Austria
Rheumatology, Hospital of Bruneck, Bruneck, Italy
Emanuel Della-Torre IRCCS San Raffaele Scientifc Institute, Università Vita-Salute San Raffaele, Milan, Italy
Unit of Immunology, Rheumatology, Allergy and Rare Diseases (UnIRAR), IRCCS San Raffaele Scientifc Institute, Milan, Italy
Christopher Denton CL Division of Medicine, University College London, London, UK
UCL Centre for Rheumatology and Connective Tissue Diseases, Royal Free Hospital, London, UK
Atul Deodhar Division of Arthritis and Rheumatic Diseases, Oregon Health and Science University, Portland, OR, USA
Anne De Paepe Center for Medical Genetics, Department of Biomolecular Medicine, Ghent University and Ghent University Hospital, Ghent, Belgium
Jeremie Dion National Referral Center for Rare Systemic Autoimmune Diseases, Hôpital Cochin, AP-HP, Université Paris Descartes, Paris, France
Rajiv K. Dixit Northern California Arthritis Center, Walnut Creek, CA, USA
Lingli Dong Department of Rheumatology and Immunology, Tongji Medical College, Tongji Hospital, Huazhong University of Science and Technology, Wuhan, China
Guillaume Dumas APHP, Service de médecine intensive et de réanimation, hôpital SaintLouis, Paris, France
Center of Epidemiology and Biostatistics, INSERM, Université de Paris, UMR 1153, Paris, France
Rima N. El-Abassi Department of Neurology, School of Medicine, Louisiana State University Health Sciences Center, New Orleans, LA, USA
Despina Eleftheriou Infection, Immunity and Infammation Programme Research and Teaching Department, UCL Great Ormond Street Institute of Child Health, University College London, London, UK
Rheumatology, Great Ormond Street Hospital, London, UK
Paul Emery Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Chapel Allerton Hospital, Leeds, UK
NIHR Leeds Musculoskeletal Biomedical Research Centre, Leeds Teaching Hospitals NHS Trust, Leeds, UK
J. D. England Department of Neurology, School of Medicine, Louisiana State University Health Sciences Center, New Orleans, LA, USA
Olivier Fain AP-HP, service de médecine interne, Hôpital Saint Antoine, Sorbonne Université, Paris, France
David C. Fajgenbaum Center for Cytokine Storm Treatment and Laboratory & Division of Translational Medicine and Human Genetics, Department of Medicine, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
Jocelyn R. Farmer Department of Allergy and Immunology, Massachusetts General Hospital, Boston, MA, USA
Brian M. Feldman Departments of Pediatrics and Medicine, IHPME, University of Toronto, Toronto, ON, Canada
Division of Rheumatology, Hospital for Sick Children, Toronto, ON, Canada
Judith A. Ferry Department of Pathology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, USA
George E. Fragoulis Institute of Infection, Immunity and Infammation, University of Glasgow, Glasgow, UK
Suzanne K. Freitag Ophthalmic Plastic Surgery Service, Massachusetts Eye and Ear Infrmary, Harvard Medical School, Boston, MA, USA
Richard Furie Division of Rheumatology, Northwell Health and Zucker School of Medicine at Hofstra/Northwell, Great Neck, NY, USA
Duvuru Geetha Division of Nephrology, Johns Hopkins University, Baltimore, MD, USA
Morie Gertz Division of Hematology, Mayo Clinic, Rochester, MN, USA
Dafna Gladman Department of Medicine, Krembil Research Institute, University of Toronto, Toronto Western Hospital, Toronto, ON, Canada
Fiona Goldblatt Department of Rheumatology, The Repatriation General Hospital, Adelaide, SA, Australia
Caroline Gordon University of Birmingham, Birmingham, UK
Peter C. Grayson, MD, Mac National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD, USA
Jessica Greco Department of Internal Medicine, Davis Heart and Lung Research Institute, Ohio State University Medical Center, Columbus, OH, USA
Roberta Gualtierotti Department of Pathophysiology and Transplantation, Internal Medicine, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Università degli Studi di Milano, Milan, Italy
Rula A. Hajj-Ali Department of Rheumatology/Immunology, Cleveland Clinic, Cleveland, OH, USA
John J. Halperin Atlantic Health System, Summit, NJ, USA
Vedat Hamuryudan Division of Rheumatology, Department of Internal Medicine, School of Medicine, Behçet’s Disease Research Centre, Istanbul University-Cerrahpasa, Istanbul, Turkey
Philip A. Hart Division of Gastroenterology, Hepatology, and Nutrition, The Ohio State University Wexner Medical Center, Columbus, OH, USA
Gulen Hatemi Division of Rheumatology, Department of Internal Medicine, School of Medicine, Behçet’s Disease Research Centre, Istanbul University-Cerrahpasa, Istanbul, Turkey
Gillian Hawker Department of Medicine, University of Toronto, Toronto, ON, Canada
Women’s College Research Institute, Women’s College Hospital, Toronto, ON, Canada
Philip Helliwell Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, UK
Leeds Musculoskeletal Biomedical Research Unit, Leeds Teaching Hospitals NHS Trust, Leeds, UK
V. Michael Holers Division of Rheumatology, University of Colorado Denver Anschutz Medical Campus, Aurora, CO, USA
Jennifer Huggins Cincinnati Children’s Hospital Medical Center and University of Cincinnati College of Medicine, Cincinnati, OH, USA
T. W. J. Huizinga Leiden University, Leiden, The Netherlands
Mary Beth Humphrey University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA
David Isenberg Centre for Rheumatology, Division of Medicine, University College London, London, UK
Department of Rheumatology, University College London Hospitals NHS Foundation Trust, London, UK
Mengdi Jiang Department of Nephrology, Ruijin Hospital, School of Medicine, Shanghai
Jiao Tong University, Shanghai, China
Jordan T. Jones University of Kansas City Medical Center, Kansas City, KS, USA
Marc A. Judson Division of Pulmonary and Critical Care Medicine, Albany Medical Center, Albany, NY, USA
Mitsuhiro Kawano Department of Rheumatology, Graduate School of Medical Science, Kanazawa University, Kanazawa, Japan
Tanaz Kermani Division of Rheumatology, University of California, Los Angeles, Los Angeles, CA, USA
Tristan A. Kerr Division of Pediatric Rheumatology, BC Children’s Hospital, Vancouver, BC, Canada
Munther Khamashta Lupus Research Unit, The Rayne Institute, St Thomas’ Hospital, King’s College University, London, UK
Dinesh Khanna University of Michigan, Ann Arbor, MI, USA
Lauren King University of Toronto, Toronto, ON, Canada
Women’s College Research Institute, Women’s College Hospital, Toronto, ON, Canada
Jason S. Knight Division of Rheumatology, University of Michigan, Ann Arbor, MI, USA
Jason R. Kolfenbach Division of Rheumatology, University of Colorado Denver Anschutz Medical Campus, Aurora, CO, USA
Matthew J. Koster Division of Rheumatology, Mayo Clinic, Rochester, MN, USA
Benjamin Z. Leder, MD Department of Medicine, Endocrine Unit, Massachusetts General Hospital, Boston, MA, USA
Michael Lee Department of Ophthalmology and Visual Neurosciences, University of Minnesota, Minneapolis, MN, USA
Department of Neurology, University of Minnesota, Minneapolis, MN, USA
Department of Neurosurgery, University of Minnesota, Minneapolis, MN, USA
Deborah M. Levy Division of Rheumatology, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada
Vidya Limaye Discipline of Medicine, School of Medicine, University of Adelaide, Adelaide, SA, Australia
Carol B. Lindsley University of Kansas City Medical Center, Kansas City, KS, USA
Nicholas L. Li Department of Internal Medicine, Division of Nephrology, The Ohio State University, Columbus, OH, USA
Elyse E. Lower Department of Medicine, University of Cincinnati Medical Center, Cincinnati, OH, USA
Ashima Makol Division of Rheumatology, Department of Internal Medicine, Mayo Clinic, Rochester, MN, USA
Fransiska Malfait Center for Medical Genetics, Department of Biomolecular Medicine, Ghent University and Ghent University Hospital, Ghent, Belgium
Brian Mandell Department of Rheumatic and Immunologic Diseases, The Cleveland Clinic, Cleveland, OH, USA
Xavier Mariette Université Paris-Saclay, INSERM, CEA, Centre de recherche en Immunologie des infections virales et des maladies auto-immunes, Paris, France
AP-HP, Université Paris-Saclay, Hôpital Bicêtre, Rheumatology Department, Le Kremlin Bicêtre, Paris, France
Sonia Marino Department of Biomedical Surgical and Dental Sciences, Maxillo-Facial and Odontostomatology Unit, Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Università degli Studi di Milano, Milan, Italy
Justin Mason Vascular Sciences and Rheumatology, Imperial Centre for Translational and Experimental Medicine, National Heart and Lung Institute, Imperial College London, Hammersmith Hospital, London, UK
Eric L. Matteson Division of Rheumatology and Department of Health Sciences Research, Mayo Clinic, Rochester, MN, USA
Maureen Mayes Division of Rheumatology and Clinical Immunogenetics, University of Texas McGovern Medical School, Houston, TX, USA
Dennis McGonagle LTHT, Leeds NIHR Biomedical Research Centre, Leeds, UK Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Leeds, UK
Giampaolo Merlini Amyloidosis Research and Treatment Centre, Fondazione Istituto di Ricovero e Cura a Carattere Scientifco Policlinico San Matteo, Pavia, Italy Department of Molecular Medicine, University of Pavia, Pavia, Italy
Frederick Miller Clinical Research Branch, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC, USA
Neil Miller Johns Hopkins University School of Medicine, Baltimore, MD, USA
Durga P. Misra Department of Clinical Immunology and Rheumatology, Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, India
Sue Mollan Metabolic Neurology, Institute of Metabolism and Systems Research, University of Birmingham, Birmingham, UK
R. C. Monahan Department of Rheumatology, Leiden University Medical Center, Leiden, The Netherlands
Sarah Morgan Division of Clinical Immunology and Rheumatology, The University of Alabama at Birmingham, Birmingham, AL, USA
Kimberly A. Morishita Department of Paediatrics, The University of British Columbia, Vancouver, BC, Canada
Division of Rheumatology, British Columbia Children’s Hospital, Vancouver, BC, Canada
Guillaume Moulis Department of Internal Medicine, Toulouse University Hospital (CHU de Toulouse), Toulouse, France
CIC 1436, Toulouse University Hospital (CHU de Toulouse), Toulouse, France
Francesco Muratore Rheumatology Unit, Azienda Unità Sanitaria Locale IRCCS, Reggio Emilia, Italy
Johannes Nowatzky Division of Rheumatology, Department of Medicine, NYU Langone Behçet’s Disease Program, NYU Langone Ocular Rheumatology Program, New York University Grossman School of Medicine, New York, NY, USA
James R. O’Dell Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE, USA
Chester Oddis Division of Rheumatology and Clinical Immunology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
Seza Ozen Department of Paediatrics, Hacettepe University, Ankara, Turkey
Lauren M. Pachman Feinberg School of Medicine, Chicago, IL, USA
Northwestern University, Chicago, IL, USA
Department of Pediatrics, Division of Rheumatology, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL, USA
Cure JM Center of Excellence in JM Research and Care, Chicago, LI, USA
Samir V. Parikh Division of Nephrology, Department of Internal Medicine, The Ohio State University Wexner Medical Center, Columbus, OH, USA
Elizabeth Park Columbia University Irving Medical Center and New York Presbyterian Hospital, New York, NY, USA
Duane W. Pearson Division of Rheumatology, University of Colorado Denver Anschutz Medical Campus, Aurora, CO, USA
Michelle Petri Division of Rheumatology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Division of Rheumatology, Department of Medicine III, University Medical Center and Faculty of Medicine Carl Gustav Carus, Dresden, Germany
Ross E. Petty Department of Paediatrics, The University of British Columbia, Vancouver, BC, Canada
Division of Rheumatology, University of British Columbia, Vancouver, BC, Canada
Clarissa M. Pilkington Adolescent and Pediatric Rheumatology, University College and Great Ormond Street Hospital, London, UK
Michael Pillinger The Division of Rheumatology, New York University Grossman School of Medicine, New York, NY, USA
The Section of Rheumatology, New York Harbor Health Care System, New York Campus of the U.S. Department of Veterans Affairs, New York, NY, USA
David Pisetsky Departments of Medicine and Immunology, Duke University Medical Center and Medical Research Service, Veterans Administration Medical Center, Durham, NC, USA
Janet E. Pope Division of Rheumatology, St Joseph’s Hospital, Western University, London, ON, Canada
Andrew Porter Rheumatology Department, Imperial College Healthcare NHS Trust, Hammersmith Hospital, London, UK
Abin P. Puravath Division of Rheumatology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Vanessa Quick Rheumatology, Luton and Dunstable University Hospital NHS Foundation Trust, Luton, UK
Kaitlin A. Quinn, MD, MHS National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD, USA
Daniel Raines Section of Gastroenterology, Department of Medicine, Louisiana State University Health Sciences Center, New Orleans, LA, USA
Rosalind Ramsay-Goldman Department of Medicine, Rheumatology Division, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA
Steven Rauch Department of Otolaryngology-Head and Neck Surgery, Massachusetts Eye and Ear, Boston, MA, USA
Department of Otolaryngology-Head and Neck Surgery, Harvard Medical School, Boston, MA, USA
Ann M. Reed Cure JM Center of Excellence in JM Research and Care, Durham, NC, USA
Department of Pediatrics, Duke University Medical School, Durham, NC, USA
Ian R. Reid Department of Medicine, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand
Auckland District Health Board, Auckland, New Zealand
Lisa G. Rider Cure JM Center of Excellence in JM Research and Care, Washington, DC, USA
Environmental Autoimmunity Group, Clinical Research Branch, National Institute of Environmental Health Sciences, National Institutes of Health, Bethesda, MD, USA
Ann K. Rosenthal Department of Medicine, Division of Rheumatology, Medical College of Wisconsin, Milwaukee, WI, USA
Brad Rovin Department of Internal Medicine, The Ohio State Wexner Medical Center, Columbus, OH, USA
Anne Rowley Department of Pediatrics, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
Department of Microbiology-Immunology, Northwestern University Feinberg School of Medicine, Chicago, IL, USA
Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL, USA
Dax G. Rumsey Department of Pediatrics, Division of Rheumatology, University of Alberta, Edmonton, AB, Canada
David Saadoun AP-HP, Groupe Hospitalier Pitié-Salpêtrière, Department of Internal Medicine and Clinical Immunology, Sorbonne Universités, Paris, France
Ken Saag University of Alabama at Birmingham, Birmingham, AL, USA
Takako Saeki Nagaoka Red Cross Hospital, Nagaoka, Japan
Jane Salmon Division of Rheumatology, Department of Medicine, Hospital for Special Surgery and Weill Cornell Medicine, New York, NY, USA
Carlo Salvarani Rheumatology Service, Arcispedale S. Maria Nuova, Reggio Emilia, Italy
Lisa Sammaritano Weill Cornell Medicine, Hospital for Special Surgery, New York, NY, USA
Naomi Schlesinger Division of Rheumatology, Rutgers—Robert Wood Johnson Medical School, New Brunswick, NJ, USA
Godehard Scholz Department of Rheumatology, Immunology and Allergology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
Joseph H. Schwab Massachusetts General Hospital, Boston, MA, USA
Luca Seitz Department of Rheumatology, Immunology and Allergology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
Margaret Seton Boston, MA, USA
Ami A. Shah Division of Rheumatology, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Shaiba Shandu Department of Oral Medicine Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA
Aman Sharma Clinical Immunology and Rheumatology Services, Department of Internal Medicine, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India
Earl D. Silverman Division of Rheumatology, The Hospital for Sick Children, University of Toronto, Toronto, ON, Canada
Josef Smolen Division of Rheumatology, Department of Medicine 3, Medical University of Vienna, Vienna, Austria
Lucia Sobrin Retina Service, Department of Ophthalmology, Massachusetts Eye and Ear, Boston, MA, USA
Francesco Spadari Department of Biomedical Surgical and Dental Sciences, Maxillo-Facial and Odontostomatology Unit, Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Università degli Studi di Milano, Milan, Italy
Jeffrey A. Sparks Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
E. William St. Clair Duke University Medical Center, Durham, NC, USA
Ian Stanaitis Women’s College Research Institute, Women’s College Hospital, Toronto, ON, Canada
Andrew Staron Amyloidosis Center, Boston University Chobanian & Avedisian School of Medicine, Boston Medical Center, Boston, MA, USA
Virginia Steen Georgetown University Medical Center, Washington, DC, USA
Allen C. Steere Center for Immunology and Infammatory Diseases, Division of Rheumatology, Allergy, and Immunology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
John H. Stone Division of Rheumatology, Allergy, and Immunology, Massachusetts General Hospital, Harvard University, Boston, MA, USA
Division of Rheumatology, Allergy, and Immunology, Massachusetts General Hospital, Harvard University, Boston, MA, USA
Sangeeta Sule Children’s National Hospital, Washington, DC, USA
Julia Sun Department of Medicine, Rheumatology Division, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA
Yutong Su Department of Rheumatology and Immunology, Ruijin Hospital, Shanghai Jiao
Tong University School of Medicine, Shanghai, China
Yoshiya Tanaka The First Department of Internal Medicine, School of Medicine, University of Occupational and Environmental Health, Kitakyushu, Japan
Sarah Tansley Department of Life Sciences, University of Bath, Bath, UK
Christoph Tappeiner Department of Ophthalmology, Inselspital, University of Bern, Bern, Switzerland
Peter C. Taylor Botnar Research Centre, University of Oxford, Oxford, UK
Robert Terkeltaub Rheumatology, Allergy-Immunology Section, San Diego VA Medical Center, University of California at San Diego, San Diego, CA, USA
Daniel Tobert Department of Orthopedic Surgery, Orthopedic Spine Center and Orthopedic Oncology Service, Massachusetts General Hospital, Boston, MA, USA
Shirley M. L. Tse Division of Rheumatology, University of Toronto/SickKids, Toronto, ON, Canada
Annette van der Helm-van Mil Department of Rheumatology, Leiden University Medical Center, Leiden, The Netherlands
Ronald F. van Vollenhoven Unit for Clinical Therapy Research, Infammatory Diseases (ClinTRID), Karolinska Institutet, Stockholm, Sweden
Peter M. Villiger Department of Rheumatology, Immunology and Allergology, Bern University Hospital, University of Bern, Bern, Switzerland
Department for BioMedical Research, University of Bern, Bern, Switzerland
Frederick Vivino Division of Rheumatology, Penn Presbyterian Medical Center, Philadelphia, PA, USA
Penn Sjogren’s Syndrome Center, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA
Daniel Wallace Division of Rheumatology, Cedars-Sinai Medical Center, Los Angeles, CA, USA
Amy Warriner Division of Endocrinology, Metabolism and Diabetes, The University of Alabama at Birmingham, Birmingham, AL, USA
Kenneth Warrington Department of Medicine, Mayo Clinic College of Medicine and Science, Rochester, MN, USA
Sook-Bin Woo Department of Oral Medicine Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA
Department of Pathology, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA
Center of Oral Pathology, StrataDx, Lexington, MA, USA
Ava Wu Sjögren’s Syndrome Clinic, University of California, San Francisco, San Francisco, CA, USA
Chengde Yang Department of Rheumatology and Immunology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Hasan Yazici Division of Rheumatology, New York University School of Medicine, New York, NY, USA
Yusuf Yazici Division of Rheumatology, New York University School of Medicine, New York, NY, USA
David S. Younger Department of Neurology, Division of Neuro-Epidemiology, New York University School of Medicine, New York, NY, USA
School of Public Health, City University of New York, New York, NY, USA
Elaine W. Yu, MD, MMSC Division of Endocrinology, Diabetes, and Metabolism, Massachusetts General Hospital, Boston, MA, USA
Bita Zahedi, MD, MA Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA
Division of Endocrinology, Diabetes, and Metabolism, Massachusetts General Hospital, Boston, MA, USA
Yoh Zen Institute of Liver Studies, King’s College Hospital, London, UK
Wen Zhang Peking Union Medical College Hospital, Beijing, China
Xuan Zhang Department of Rheumatology, Beijing Hospital, National Center of Gerontology, Beijing, China
Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, China
Lidan Zhao Departments of Rheumatology, Peking Union Medical College Hospital, Clinical Immunology Center, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China
1
K. D. Deane (*) · V. M. Holers · J. R. Kolfenbach · D. W. Pearson
Division of Rheumatology, University of Colorado Denver
Anschutz Medical Campus, Aurora, CO, USA
e-mail: Kevin.Deane@cuanschutz.edu; michael.holers@cuanschutz.edu; jason.kolfenbach@ucdenver.edu; duane.pearson@cuanschutz.edu
D. Aletaha · J. Smolen
Division of Rheumatology, Department of Medicine 3, Medical University of Vienna, Vienna, Austria e-mail: daniel.aletaha@meduniwien.ac.at; josef.smolen@meduniwien.ac.at
J. M. Bathon
Columbia University Irving Medical Center, New York Presbyterian Hospital, New York, NY, USA
e-mail: jmb2311@mail.cumc.columbia.edu
P. Emery
Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Chapel Allerton Hospital, Leeds, UK
NIHR Leeds Musculoskeletal Biomedical Research Centre, Leeds Teaching Hospitals NHS Trust, Leeds, UK
e-mail: p.emery@leeds.ac.uk
G. E. Fragoulis
Institute of Infection, Immunity and Infammation, University of Glasgow, Glasgow, UK
T. W. J. Huizinga
Leiden University, Leiden, The Netherlands
e-mail: t.w.j.huizinga@lumc.nl
J. R. O’Dell
Department of Internal Medicine, University of Nebraska Medical Center, Omaha, NE, USA
e-mail: jrodell@unmc.edu
E. Park
Columbia University Irving Medical Center and New York Presbyterian Hospital, New York, NY, USA
e-mail: ep2899@cumc.columbia.edu
Y. Tanaka
The First Department of Internal Medicine, School of Medicine, University of Occupational and Environmental Health, Kitakyushu, Japan
e-mail: tanaka@med.uoeh-u.ac.jp
P. C. Taylor
Botnar Research Centre, University of Oxford, Oxford, UK
e-mail: peter.taylor@kennedy.ox.ac.uk
A. van der Helm-van Mil
Department of Rheumatology, Leiden University Medical Center, Leiden, the Netherlands
e-mail: a.h.m.van_der_helm@lumc.nl
R. F. van Vollenhoven
Unit for Clinical Therapy Research, Infammatory Diseases (ClinTRID), Karolinska Institutet, Stockholm, Sweden
e-mail: r.vanvollenhoven@amsterdamumc.nl
E. W. St. Clair
Duke University Medical Center, Durham, NC, USA
e-mail: stcla003@mc.duke.edu
© The Author(s), under exclusive license to Springer Nature Switzerland AG 2023
J. H. Stone (ed.), A Clinician’s Pearls & Myths in Rheumatology, https://doi.org/10.1007/978-3-031-23488-0_1
a migratory oligoarthritis. In other cases, the onset of disease is explosive, with the development of severe symptoms over only a few days.
• If untreated (or undertreated), RA can lead to progressive joint damage and disability.
• Autoantibodies, including rheumatoid factor (RF) and antibodies to cyclic citrullinated protein antigens (ACPA), are present in up to 80% of patients. A growing number of other autoantibodies are now described, including antibodies to carbamylated proteins.
• For patients in whom an infammatory arthritis is present in an RA-like pattern but autoantibodies are not detected, the term “seronegative RA” can be used. In such cases, however, it is important not to overlook other forms of arthritis such as microcrystalline disease, spondyloarthritis, virus-associated arthritis, or paraneoplastic syndromes.
• The principal goals of modern treatment is the prevention of joint damage and to improve quality of life.
• Treatment approaches now emphasize early diagnosis and interventions designed to suppress joint infammation. Treat-to-target approaches in which frequent visits to gauge disease activity and adjust medications accordingly for the purpose of achieving low disease activity or even remission are standard-of-care.
Early Rheumatoid Arthritis
Myth Symmetrical polyarthritis at presentation is the rule for RA.
Reality: RA that has been clinically apparent for a prolonged period of time classically involves a symmetrical pattern of affected joints (Fig. 1.1) (Arnett et al. 1988). This symmetry may not be present in early disease, however. Studies have shown that a signifcant proportion of patients who subsequently meet classifcation criteria for RA have only one or two clinically infamed joints as the initial manifestation (Barra et al. 2013). Presentations with arthritis involving only one joint for many months – e.g., the wrist –are well-described.
A migratory oligoarthritis pattern of joint infammation may also occur early on. In a migratory oligoarthritis, infammatory joint disease affects only one joint (e.g., the shoulder) very briefy, and then migrates to another joint such as a knee, typically not stopping at any joint for more than a day or two. In addition, in some individuals joint infammation may come and go in the same joints; when this occurs, it can be termed ‘palindromic rheumatism’ (Mankia and Emery 2019).
For patients with infammatory joint symptoms that are not the classic symmetrical small-joint polyarthritis, sero-
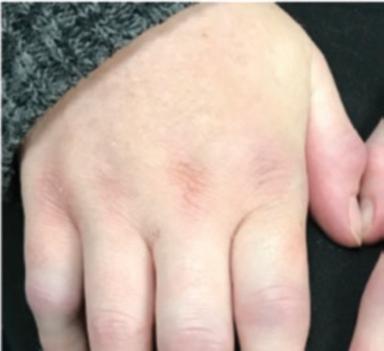
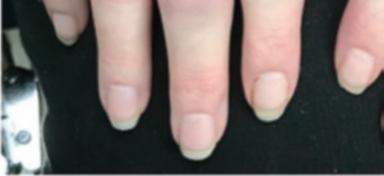
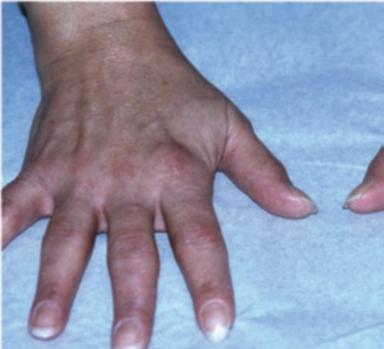
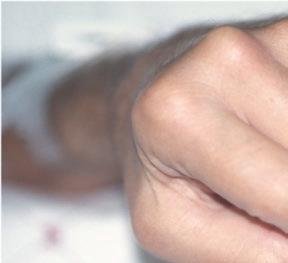
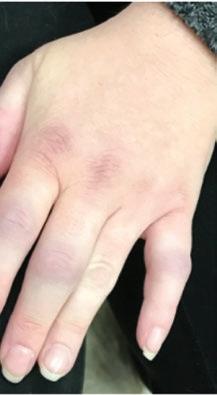
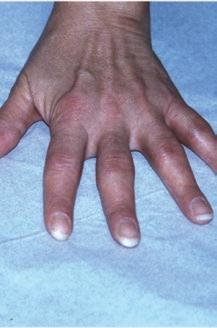
Fig. 1.1 (a–c) Symmetric involvement with infammatory arthritis in rheumatoid arthritis. The patients in (a, b) principally have involvement of the proximal interphalangeal (PIP) joints. Note sparing of the distal interphalangeal joints in both cases. Metacarpophalangeal (MCP) involvement may not be appreciated easily in these images. The patient in (c), however, has newly diagnosed RA with intense synovitis at both the MCP and PIP joints, severely restricting his ability to make a fst and use his hands for activities of daily living
logical fndings of rheumatoid factor (RF) positivity or antibodies to cyclic citrullinated protein antigens (ACPA) have important diagnostic signifcance.
K. D. Deane et
Myth Seropositive RA begins in the joint.
Reality: Although seropositive RA can sometimes present with an explosive clinical onset of arthritis, retrospective studies and an increasing number of prospective studies have demonstrated that RA-related autoantibodies (e.g., ACPA and RF) are often present for many years before the appearance of clinical arthritis (Malmström et al. 2017, Deane and Holers 2021). These observations suggest that the disease process is initiated outside of the joint, perhaps at mucosal sites (e.g., the respiratory mucosa), only targeting the joints and other sites of disease later (Holers et al. 2018).
Myth The earliest joint-related target of the systemic autoimmune disease process that characterizes preclinical RA is the synovium.
Reality: Although clinically one assesses for the presence of synovitis, some studies suggest that the initial manifestation of RA may be interosseous tendonitis and tenosynovitis. This can be apparent magnetic resonance imaging (MRI) or ultrasound studies (Mankia et al. 2019).
Pearl Tenosynovitis is more specifc for RA than synovitis.
Comment: RA is typically regarded as being a disease consisting of synovitis and bony erosions of small joints. This classic picture was derived from comparing the clinical and radiographic characteristics of patients with RA with those of patients with other rheumatic diseases affecting the joints. Advanced imaging modalities now reveal that tenosynovitis at the level of the hand and feet joints is a feature worthy of recognition as another classic trait of RA (Rogier et al. 2019).
The reported prevalence of tenosynovitis depends on the number of tendon sheaths studied. The tendon sheaths targeted for imaging typically include those of the wrist, metacarpophalangeal (MCP) and metatarsophalangeal (MTP) joints. MRI studies in consecutive early RA showed a sensitivity of tenosynovitis of 75%–87%. In contrast, imaging studies in persons from the general population typically show a prevalence of tenosynovitis at small joints ranging from 0% to 3% (Mangnus et al. 2015, 2016). The specifcity of fnding tenosynovitis on imaging is therefore on the order of 97% or higher.
The specifcity in patients with other arthritides as reference is also high. A study at the tendon level of the wrist and MCP joints, comparing consecutive patients with RA and other early arthritis (including psoriatic arthritis), reported a specifcity ranging from 82% to 99% (Krabben et al. 2015). Thus, tenosynovitis at the level of small joints (MCPs, wrist, MTPs) has high sensitivity and specifcity for RA.
Myth Seropositive and seronegative RA have similar genetic origins and likely differ only in the presence or absence of ACPA and RF.
Reality: Genetic and epidemiologic studies suggest that seronegative RA is a distinct illness with unique pathophysiologic characteristics. It generally responds to the same types of medications as the seropositive disease –although there are some exceptions – but differences between “seropositive” and “seronegative” RA are important and need to be acknowledged in the interest of understanding them better (Klareskog et al. 2011). Little is known about the preclinical processes that are present in seronegative patients with RA prior to the onset of clinically apparent arthritis.
An increasing number of autoantibodies are being discovered that in some individuals characterize patients who otherwise lack both ACPA and IgM RF antibodies (Trouw et al. 2017). These can include antibodies to carbamylated or methylated proteins.
Myth There is no good advice to give patients with RA and those at risk for RA about dietary or lifestyle changes that can alter the likelihood of developing future RA or the course of disease once established.
Reality: Epidemiologic studies have suggested that higher levels of omega-3 fatty acid intake decrease the likelihood of future RA development (Gan et al. 2017). Similarly, other environmental and personal lifestyle factors such as absence of smoking, maintenance of a lower BMI and potentially a Mediterranean-style diet are associated with a decreased likelihood of developing RA (Karlson and Deane 2012; Zaccardelli et al. 2019). Although clinical trials have not yet been performed to validate these approaches, these lifestyle and exposure modifcations are not unreasonable to discuss with patients with RA and those at risk for future disease.
Myth Individuals who exhibit a high titer ACPA or RF antibodies and arthralgias but who do not have clinical examination or imaging evidence of synovitis or tenosynovitis should be treated with a disease-modifying antirheumatic drug (DMARD) such as hydroxychloroquine.
Reality: Although there is an increasing tendency to utilize hydroxychloroquine in individuals who are thought to be “on their way” to getting RA (which may be termed ‘preRA’), no clinical trial evidence demonstrates that this is the correct approach. In fact, in preliminary results from interim analyses of the STOPRA trial Deane et al. (2022) showed no beneft of hydroxychloroquine in the prevention of progression to RA in ACPA positive individuals who did not have infammatory arthritis at baseline. However, there are intriguing fndings from the TREAT EARLIER study where methotrexate use in individuals with ‘subclinical arthritis’ (defned as MRI evidence of joint infammation without clinical examination fndings of infammatory arthritis) was associated with the development of a milder form of clinical RA although overall progression to RA was not signifcantly
reduced (Krijbolder et al. 2022). At this time, rather than starting a medication with the intention of preventing evolution to RA, it is likely better to educate patients carefully about the symptoms and signs of RA, to perform a close follow-up and to consider treatment only if or when infammatory arthritis evolves becomes present. However, there are multiple prevention trials in RA underway and we may see a shift to possible pharmacologic preventive interventions in RA in the near future (Deane and Holers 2021).
Myth The absence of a smoking history in some patients who develop seropositive RA suggests that pulmonary exposures do not have a role in the pathogenesis of their disease.
Reality: The association of RA development with a smoking history is most striking in those patients with the HLA-shared epitope alleles, which are only present in ~2/3 of patients with RA. However, other lung exposures such as air pollution and silica dust exposure might account for lungrelated risks that are not directly tied to smoking
In addition, there is increasing understanding that other mucosal sites such as the periodontal region and gut may play some role in the development of RA.
Clinical Features
Myth A patient must have a score of 6 or more out of 10 on the 2010 American College of Rheumatology (ACR)/ European League Against Rheumatism (EULAR) classifcation criteria to be diagnosed as having RA.
Reality: Diagnostic criteria do not exist for RA. The 2010 ACR/EULAR classifcation criteria (as well as the earlier 1987 ACR criteria) were not intended for the purpose of diagnosing individual patients, but rather for defning patient populations that are comparable across different clinical studies (Aletaha et al. 2010; Arnett et al. 1988). The gold standard for the diagnosis of RA continues to be the clinical judgment of a rheumatologist.
The 2010 criteria were designed in part to be able to classify RA patients earlier, and subsequent studies have demonstrated success in that regard. The 2010 criteria diverge from the 1987 criteria in their incorporation of ACPA; the inclusion of acute-phase reactant elevation; the requirement for exclusion of alternative causes for the infammatory arthritis; and reduced emphasis on disease manifestations that are characteristic of later disease stages (bone erosions, rheumatoid nodules (Table 1.1).
Table 1.1 The 1987 ARA (ACR) and 2010 ACR/EULAR RA classifcation criteria
1987 criteria (Arnett et al. 1988) 2010 criteria (Aletaha et al. 2010)
1. Morning stiffness defned as morning stiffness in and around the joints lasting at least 1 hour before maximal improvement
2. Arthritis of three or more joint areas defned as at least three joint areas simultaneously have had soft tissue swelling or fuid (not bony overgrowth alone) observed by a physician. The 14 possible areas are the right or left PIP, MCP, wrist, elbow, knee, ankle, and MTP joints
3. Arthritis of hand joints defned as at least one area is swollen (as defned above) in a wrist, MCP, or PIP joint
4. Symmetric arthritis defned as simultaneous involvement of the same joint areas (as defned in 2) on both sides of the body (bilateral involvement of PIPs, MCPs, or MTPs is acceptable without absolute symmetry)
5. Rheumatoid nodules defned as subcutaneous nodules, over bony prominences, or extensor surfaces, or in juxtaarticular regions, observed by a physician
6. Serum rheumatoid factor defned as the demonstration of abnormal amounts of serum rheumatoid factor by any method for which the result has been positive in <5% of normal control subjects
7. Radiographic changes defned as radiographic changes typical of rheumatoid arthritis on posteroanterior hand and wrist radiographs, which must include erosions or unequivocal bony decalcifcation localized in or most marked adjacent to the involved joints (osteoarthritis changes alone do not qualify)
For classifcation purposes, a patient shall be said to have RA if he/ she has satisfed at least four of the seven of these criteria. Criteria 1 through 4 must have been present for at least 6 weeks. Patients with two clinical diagnoses are not excluded
Target population (Who should be tested?)
Individuals with the following should be included:
1. ≥1 joint with defnite clinical synovitis
2. Synovitis not better explained by another disease
A. Joint Involvementa
1 large joint¶
2–10 large joints
1–3 small joints (with or without involvement of large joints)b 4–10 small joints (with or without involvement of large joints)
>10 joints (at least one small joint)
B. Serology (at Least One Test Result Is Needed for Classifcation)
Negative RF and negative ACPA
Low-positive RF or low-positive ACPA
High-positive RF or high-positive ACPA (>3× upper limit of normal)
C. Acute-Phase Reactants (at Least One Test Result Is Needed for Classifcation)
Normal CRP and normal ESR
Abnormal CRP or abnormal ESR
D. Duration of Symptoms
<6 weeks
≥6 weeks
Classifcation Criteria for RA (Score-Based Algorithm: Add Score of Categories A–D; A Score of ≥6/10 Is Needed for the Classifcation of a Patient as Having Defnite RA)
a Joint involvement refers to any swollen or tender joint on examination. Distal interphalangeal joints, frst carpometacarpal joints, and frst metatarsophalangeal joints are excluded from assessment. “Large joints” refers to the shoulders, elbows, hips, knees, and ankles
b “Small joints” refers to the metacarpophalangeal joints, proximal interphalangeal joints, second through ffth metatarsophalangeal joints, thumb interphalangeal joints, and wrists
K.
Myth Patient with rheumatoid arthritis do not get gout.
Reality: This myth may be an artifact of mid-twentieth century RA treatment regimens that utilized high-dose aspirin, a uricosuric agent, for most RA patients. In fact, in a large cohort of RA patients, 17% had elevated uric acid (>6.8 mg/dl), and 6.1% were diagnosed with gout over time (Chiou et al. 2020). In another study, not only was gout shown to be prevalent in RA compared to controls, 1.61% vs 0.92%; p < 0.001, RA was statistically associated with gout and in a multivariable analysis (OR = 1.72; 95% CI 1.45–2.05) (Merdler-Rabinowicz et al. 2017). These observations confrm that gout and RA actually co-exist frequently.
With the development of more effective treatment regimens for RA that do not involve high-dose aspirin, it is not uncommon to see the two diseases in the same patient (Olaru et al. 2017). Indeed, gout or other crystalline diseases (e.g., CPPD) should be considered in a patient with a diagnosis of RA who has mono- or oligo-articular fares of arthritis. When patients with RA do not respond as expected to diseasemodifying treatment, it is worthwhile to take a step back, consider the possibility of a co-existing crystalline-induced arthritis, and perform appropriate diagnostic studies (i.e., arthrocentesis and synovial fuid analysis).
Myth RA burns out with time in many patients.
Reality: Some clinicians refer to “burned out” RA. Patients to whom this term is applied generally have long-standing disease, severe joint damage, and little evidence of active synovitis on physical examination. Most are on minimal therapy for RA, albeit some have tried multiple agents in the past. Many patients with “burned out” RA may have moderately elevated infammatory parameters and continue to deteriorate radiographically despite having symptoms that are minimal compared to patients with early disease. Moreover, if these patients are treated, their symptoms improve, indicating a response to therapy. Thus, the label of “burned out” RA is probably appropriate only on rare occasions. Strong consideration should be given to treating patients who ft the operative defnition of burned out RA with anti-infammatory therapies.
This concept of burned out RA is distinct from the more formal defnition of disease remission in RA. A patient with RA in remission has little to no discernible disease activity according to the history, examination, laboratory measurements, and imaging. Remission can be defned by various criteria, including the ACR/EULAR remission criteria created for clinical trials in 2011 (Table 1.2) (Felson et al. 2011). It is not yet clear how to select patients in remission who may safely reduce or stop their medications. Drug-free remission, however, is achieved only rarely (Schett et al. 2016; Terslev et al. 2021a, b).
Table 1.2 The 2011 ACR/EULAR defnitions of remission in rheumatoid arthritis clinical trials (Felson et al. 2011)
Boolean-based defnition
At any time point, the patient must satisfy all of the following:
Tender joint count ≤1a
Swollen joint count ≤1a C-reactive protein ≤1 mg/dL
Patient global assessment ≤1 (on a 0–10 scale)b
Index-based defnition
Simplifed Disease Activity Index score of ≤3c
a For tender and swollen joint counts, use of a 28-joint count may miss actively involved joints, especially in the feet and ankles, and it is preferable to include feet and ankles also when evaluating remission
b For the assessment of remission, we suggest the following format and wording for the global assessment questions. Format: a horizontal 10-cm visual analog or Likert scale with the best anchor and lowest score on the left side and the worst anchor and highest score on the right side. Wording of question and anchors: For patient global assessment, “Considering all of the ways your arthritis has affected you, how do you feel your arthritis is today?” (anchors: very well–very poor). For physician/assessor global assessment, “What is your assessment of the patient’s current disease activity?” (anchors: none-extremely active)
c Defned as the simple sum of the tender joint count (using 28 joints), swollen joint count (using 28 joints), patient global assessment (0–10 scale), physician global assessment (0–10 scale), and C-reactive protein level (mg/dl)
Myth Rheumatoid nodules are an important fnding in the early diagnosis of RA.
Comment: The presence of rheumatoid nodules is one of the items in the 1987 classifcation criteria for RA. Rheumatoid nodules are not typically present within the frst few months of the onset of synovitis, and thus their absence at presentation does not preclude the diagnosis of RA. Furthermore, rheumatoid nodules are found in only one-third of patients with established RA. Hence, their presence remains an insensitive marker for disease even in later stages (Turesson and Jacobsson 2004). In rare cases, individuals may have rheumatoid nodules and circulating autoantibodies without appreciable synovitis (Hewitt and Cole 2005).
Myth Troublesome rheumatoid nodules cannot be treated.
Reality: Nodules that are painful or interfere with function require an intervention. In general, DMARDs do not reduce nodules signifcantly. Some case reports, however, suggest that biologic therapies such as rituximab and antiIL6 therapy help decrease nodule size and number (Sautner et al. 2013; De Stefano et al. 2011; Andres et al. 2012). Direct injection of the nodule with a mixture of local anesthetic and glucocorticoids may be benefcial as well (Baan et al. 2006).
The procedure for injecting rheumatoid nodules is as follows. Up to 0.3 mL of methylprednisolone or triamcinolone 40 mg/mL is mixed in 1:1 ratio with 1% lidocaine. Following local anesthesia of the overlying skin, the needle is inserted
directly into the center of the nodule. The medication is injected slowly as the needle is withdrawn. Considerable force on the plunger of the syringe is needed in some cases in order to express the mixture into the nodule. Surgical excision is necessary for some nodules, although recurrence may occur (Riches et al. 2016).
Surgical removal of a nodule from sites other than the skin may be necessary for diagnosis, such as a pulmonary nodule that must be differentiated from a malignancy. Finally, limited data suggest a potential association between methotrexate and the development of rheumatoid nodules, i.e., “rheumatoid nodulosis” (Patatanian and Thompson 2002). Possible mechanisms relate to folate and adenosine pathways (Soukup et al. 2017). If rheumatoid nodulosis is suspected, an alternative to methotrexate may be considered after balancing this potential risk against the benefts of methotrexate in RA.
Myth Rheumatoid nodules frequently occur in the heart and cause conduction defects.
Reality: Although mentioned in most textbooks as an extra-articular complication of RA, this is a surpassingly rare phenomenon (Thery et al. 1974; Ahern et al. 1983).
Rheumatoid nodules are not likely to be the explanation for conduction defects in a patient with RA. When tissue has been available from the unusual cases in which rheumatoid nodules occur in the heart, the atrioventricular node may show a variety of histopathologic fndings, including granulomata (resembling that of a typical rheumatoid nodule), diffuse lymphocytic infammation, or fbrosis (Ben Hamda et al. 2004).
Pearl Rheumatoid pleural effusions can mimic empyemas.
Comment: RA is one of the few causes of a low pH in pleural effusions (Balbir-Gurman et al. 2006; Light 2011). Two other major ones are empyema and esophageal rupture. Pleural effusions in RA are exudates, characterized by high protein and lactate dehydrogenase levels and low glucose concentration. Cholesterol may be elevated in chronic effusions, leading to a milky appearance to the pleural fuid. White blood cell counts are frequently elevated in RA effusions, although often less than 5000/mm3. In contrast to empyemas, lymphocytes tend to predominate over neutrophils unless the fuid collection is tapped early in its course. Pleural effusions in RA should be evaluated carefully to ensure that infection and malignancy are not missed.
Pearl Amyloidosis and rheumatoid vasculitis pose less of a threat than they did before the availability of effective therapy.
Comment: Two potentially lethal complications of RA are the development of secondary amyloidosis and the occurrence of rheumatoid vasculitis. Fortunately, however, the
incidence of both seems to be declining with modern therapies (Nakamura et al. 2019; Myasoedova et al. 2011). Cases of RA-associated amyloidosis and Felty’s syndrome occurring in the absence of active synovitis have been reported in the literature (Owlia et al. 2014). One must maintain a high index of suspicion for RA-related extra-articular disease even if joint disease is not active.
Myth RA does not affect the central nervous system.
Reality: RA can directly affect the central nervous system in multiple ways (Atzeni et al. 2018). These include erosive disease and pannus formation in the C1 and C2 area, leading to spinal cord impingement. Meningeal involvement can also occur in RA, though thankfully it is rare. Meningeal disease may present with clinical fndings of headache, encephalopathy, and cranial nerve defects. Imaging in such cases confrms meningeal thickening, nodularity, and enhancement. Biopsy may be required for diagnosis and to differentiate RA-associated disease from that caused by ANCA-associated vasculitis, IgG4-related disease, malignancy, and other conditions, depending on the individual patient’s circumstances. In some cases, cerebrospinal fuid elevation of RA-related autoantibodies can be seen and aid in the diagnosis, especially if articular disease is absent (McKenna et al. 2019).
Myth Rheumatoid arthritis is associated with severe neutropenia
Comment: Neutropenia is not rare in the context of RA. It occurs in about 5%–10% of patients treated with conventional DMARDs and in 15%–20% of patients treated with biologic DMARDs. The neutropenia is usually is mild, however, and seldom leads to treatment discontinuation. Few RA patients with neutropenia will develop serious infections. In the differential diagnosis of RA patients with neutropenia, Felty’s syndrome and large granulocytic leukemia should be included (Fragoulis et al. 2018).
Myth RA goes into remission during pregnancy.
Reality: Some studies have suggested that RA disease activity improves during pregnancy in a high percentage of cases – approximately 60% – to the extent that treatment reduction is possible in some. Among women with RA whose disease does improve during pregnancy, improvements in disease activity generally start in the frst trimester and last through delivery. In fact, however, remission by defned criteria such as the DAS28-CRP occurs in only about 25% of patients overall (Jethwa et al. 2019; de Man et al. 2014). The universal statement that RA “resolves” temporarily during pregnancy is wishful thinking.
Disease fares during pregnancy have actually been reported in 29% of patients with RA. Some of these fares are associated with discontinuation of anti-TNF biologics in
K. D. Deane et
early pregnancy (van den Brandt et al. 2017). One-third of RA patients may have active disease at some point during pregnancy, and active disease during any trimester has a high correlation with preterm delivery (Zbinden et al. 2018). Moreover, 47% of RA patients experience disease fares in the immediate post-partum period.
Pearl Tenosynovitis should not be disregarded.
Reality: Although tendon sheath infammation is not part of the ACR/EULAR criteria for RA, it is recognized as a common feature pf active RA and may be a major source of pain, stiffness, and functional impairment. The fact that it is not part of the classifcation criteria does mean it should be disregarded. On the contrary, tenosynovitis may be one of the earliest fndings (on ultrasound or MRI) of early RA.
Serological Features and Radiology
Pearl High levels of rheumatoid factor (RF) or antibodies to citrullinated protein antigens (ACPA) are of diagnostic and prognostic value.
Comment: Seropositive RA is characterized by the presence of autoantibodies, among which RF was the frst described. The fnding of a serum RF is relatively nonspecifc in the sense that it may be associated with a variety of other conditions that can cause an infammatory arthritis. In particular, connective tissue diseases, cryoglobulinemia, chronic hepatitis, and certain hematologic malignancies (e.g., Waldenstrom’s macroglobulinemia) are often associated with RF positivity. In addition, up to 15% of the healthy elderly population have RF, albeit usually in low levels. A high cut-off point for a positive assay (e.g., 50 IU/ ml) enhances the positive predictive value of RF for RA. Indeed, the 2010 ACR/EULAR classifcation criteria for RA implement this practically by awarding greater weight to the presence of an RF level that is >3 times the upper limit of normal. High levels of RF are a marker for patients at risk for aggressive, destructive joint disease, and extra-articular complications of RA. When it comes to RA-associated autoantibodies, height matters!
Assays for ACPA have a sensitivity similar to that of RF but achieve a higher specifcity for RA. For example, the RA specifcities for a variety of ACPA assays exceed 90% (Whiting et al. 2010). Moreover, ACPA also have prognostic value. They identify a subset of patients more likely to develop erosive disease. Some data indicate that ACPA predict erosive disease more accurately than RF. Most patients with ACPA are also RF-positive, and vice versa; however, the overlap is not perfect, and studies suggest that ~10%–15% of patients with RA have one of these antibodies but not the other. Testing for both ACPA and RF at the initial evalu-
ation in a patient with infammatory arthritis improves the overall sensitivity for detection of disease. Finally, both RF and ACPA can be elevated in individuals prior to the onset of clinically apparently infammatory arthritis, during a period termed “pre-RA” (Deane and Holers 2021).
Pearl In patients with early infammatory arthritis who do not meet RA classifcation criteria, ACPA positivity is an excellent predictor of persistent infammatory arthritis and disease that should be classifed as RA.
Comment: In patients with early undifferentiated arthritis who are ACPA positive, at least 93% have persistent infammatory arthritis and ultimately can be classifed as having RA (by 1987 criteria) within 3 years (van Gaalen et al. 2004).
Myth All ACPA tests provide the same diagnostic accuracy for RA.
Reality: Many commercially available ACPA tests are available for clinical care. Most of these use a cyclic citrullinated peptide (CCP) as the antibody target, although there is also an antibody to mutated citrullinated vimentin. A variety of other antibody tests for specifc citrullinated antigens will be available soon. All have similar sensitivities for the diagnosis of RA, but the assays differ in terms of specifcity, ranging between 92% and 99% (Whiting et al. 2010). These differences are sometimes large enough to be clinically relevant, and clinicians should be aware of which tests are being performed by their local laboratory.
A growing number of autoantibodies are known to be present in RA. These include antibodies to carbamylated proteins (anti-CarP), antibodies to peptidyl arginine deiminases (PADs), and others (van Delft and Huizinga 2020). Some of these are available clinically, but their true value in clinical care remains to be fully determined.
Myth Clinically detectable synovitis and radiographic progression in RA are linked tightly, such that the control of one leads to control of the other.
Reality: This dogma, believed widely for decades, is now recognized as potentially false. Several trials have shown that continued radiographic deterioration can proceed despite excellent control of clinical features of RA activity (e.g., joint tenderness and swelling on examination, patientreported pain, stiffness, and swelling).
As an example of the uncoupling of joint infammation and damage, clinical trials have shown that treatment with denosumab (an anti-RANK-ligand monoclonal antibody) reduces radiographic progression despite achieving little effect on clinical measures of disease activity (Takeuchi et al. 2019). Subclinical disease activity as detected by ultrasound or magnetic resonance imaging may explain why some RA patients show evidence of radiographic progres-
sion of joint damage despite the absence of clinically overt signs of joint infammation (Brown et al. 2008; Conaghan et al. 2009; Terslev et al. 2021a, b). The converse is also true – i.e., patients who show no radiologic evidence of damage can have clinical signs of ongoing synovitis.
It is not clear whether treatment should be altered in a patient whose disease is controlled well by clinical parameters yet has evidence of disease activity detected with advanced imaging.
Risk Factors
Pearl Exposure to tobacco, especially cigarette smoke, is harmful in many aspects of RA.
Comment: Several studies show a signifcant and independent association between smoking and susceptibility to RA. In particular, some studies suggest that tobacco smoke interacts with the HLA-DRB1-shared epitope to heighten RA susceptibility (Ishikawa and Terao 2020). Smoking is also associated with extraarticular manifestations and RF positivity. In addition, smoking increases the risk for infection, cardiovascular disease, progression of lung disease, and cancer among patients with RA. Finally, smoking is associated with increased disease activity and reduced responses to DMARDs (Gianfrancesco et al. 2019; Nyhall-Wahlin et al. 2009; Floris et al. 2021). All of these are reasons to emphasize smoking cessation in patients with RA and in those at risk for future RA.
Pearl Cigarette smoking can worsen RA disease activity.
Comment: Much has been published regarding the association between smoking and risk of developing RA. Less is known regarding the potential of worsening existing RA through smoking. One longitudinal study (Gianfrancesco et al. 2019) demonstrated that smoking was associated with a 0.64 unit increase in patient global score (p = 0.01) and 2.58 more swollen joints (p < 0.001). One cross-sectional study (Hammam and Gheita 2017) reported higher DAS28 in RA patients exposed to second-hand smoking, while another (Sokolove et al. 2016) also reported higher DAS28 in ACPApositive RA patients who were current smokers compared with nonsmokers. Patients should be counselled about the potential detrimental effect that smoking may have on controlling their RA.
The contribution of the shared epitope and cigarette smoking to the risk for RA has been studied in a large, population-based, case-controlled study from Sweden (Hedström et al. 2019). In the ACPA-positive, RF-positive individuals, the shared epitope and cigarette smoking independently conferred increased risk for RA. Moreover, there was also a strong interaction between these two risk factors.
Among the individuals who were ACPA-positive but RF-negative, the shared epitope was associated with an increased risk of RA, but cigarette smoking had a more limited effect. The same strong interaction between the shared epitope and cigarette smoking was found in that group, as well. In contrast, among individuals that were ACPAnegative, the effect of the shared epitope was either marginal (in the ACPA-negative, RF-positive group) or nonexistent (ACPA-negative, RF-negative). The impact of smoking on the development of RA is clearly magnifed in the subset of individuals who are ACPA-positive.
The interplay between the shared epitope and cigarette smoking as a predictor of serum levels of ACPA was recently investigated in Japanese persons with RA. In this study, an interaction between cigarette smoking and serum ACPA levels was found only in individuals with the shared epitope; however, cigarette smoking was independently predictive of RF levels regardless of the shared epitope (Ishikawa et al. 2019). Moreover, these risks declined if cigarette smoking had stopped prior to disease onset.
The sum of studies to date on the relationship between cigarette smoking and RA suggests a model in which cigarette smoking triggers anti-CCP antibodies in patients with RA bearing the shared epitope.
Pearl Obesity may be a risk factor for RA and may also contribute to disease activity.
Comment: Several studies show that obesity is a risk factor for RA (Zaccardelli et al. 2019). This may be due to infammation generated in fat cells, or other factors such as diet and lack of exercise. Furthermore, obesity has been associated with increased disease activity. (Paradoxically, low body weight may also be associated with higher disease activity – perhaps because of cachexia due to poorly controlled infammation.) Addressing obesity through diet and exercise may ultimately be an important part of management in RA.
Disease Assessment
Pearl “Treat-to-target” leads to improved outcomes in RA.
Comment: Prospective data indicate that the adjustment of RA therapies every 8–12 weeks, with the goal of a “treatto-target” approach of improving specifc indices of disease activity, leads to improved outcomes (Stoffer et al. 2016; Smolen et al. 2016). The frequent assessment of disease activity in patients is important in both clinical trials and clinical practice.
Composite disease activity indices are superior to individual variables in the assessment of disease activity (England et al. 2019). Two major factors pose potential barriers to the routine use of validated disease activity indices in
K. D. Deane