Sodium-IonCapacitors
Mechanisms,Materials,andTechnologies
EditedbyGuoqiangZou,XiaoboJi,andHongshuaiHou
Editors
Prof.GuoqiangZou CentralSouthUniversity CollegeofChemistryandChemical Engineering No.932,LushanSouthRoad YueLuDistrict Changsha410083 China
Prof.XiaoboJi CentralSouthUniversity CollegeofChemistryandChemical Engineering No.932,LushanSouthRoad YueLuDistrict Changsha410083 China
Prof.HongshuaiHou CentralSouthUniversity CollegeofChemistryandChemical Engineering No.932,LushanSouthRoad YueLuDistrict Changsha410083 China
CoverImage: ©AndreiKuzmik/ Shutterstock
Allbookspublishedby WILEY-VCH arecarefully produced.Nevertheless,authors,editors,and publisherdonotwarranttheinformation containedinthesebooks,includingthisbook, tobefreeoferrors.Readersareadvisedtokeep inmindthatstatements,data,illustrations, proceduraldetailsorotheritemsmay inadvertentlybeinaccurate.
LibraryofCongressCardNo.: appliedfor
BritishLibraryCataloguing-in-PublicationData Acataloguerecordforthisbookisavailable fromtheBritishLibrary.
Bibliographicinformationpublishedby theDeutscheNationalbibliothek TheDeutscheNationalbibliotheklists thispublicationintheDeutsche Nationalbibliografie;detailedbibliographic dataareavailableontheInternetat <http://dnb.d-nb.de>
©2024WILEY-VCHGmbH,Boschstraße12, 69469Weinheim,Germany
Allrightsreserved(includingthoseof translationintootherlanguages).Nopartof thisbookmaybereproducedinanyform–by photoprinting,microfilm,oranyother means–nortransmittedortranslatedintoa machinelanguagewithoutwrittenpermission fromthepublishers.Registerednames, trademarks,etc.usedinthisbook,evenwhen notspecificallymarkedassuch,arenottobe consideredunprotectedbylaw.
PrintISBN: 978-3-527-35037-7
ePDFISBN: 978-3-527-83734-2
ePubISBN: 978-3-527-83735-9
oBookISBN: 978-3-527-83736-6
Typesetting: Straive,Chennai,India
Contents
Preface ix
1Introduction 1
PengCai,WentaoDeng,HongshuaiHou,GuoqiangZou,andXiaoboJi
1.1ABriefDevelopmentofSICs 1
1.2ComparisonBetweenDifferentHybrid-IonCapacitors 4
1.3SICsEnergyStorageMechanismIntroduction 16
1.4KeyTechnologiesofSICs 17
References 21
2CharacteristicsofSodium-IonCapacitorDevices 27
PengCai,WentaoDeng,HongshuaiHou,GuoqiangZou,andXiaoboJi
2.1BasicFeatures 27
2.2WorkingPrinciple 30
2.3Equations 32
References 42
3FundamentalUnderstandingofSodium-IonCapacitors Mechanism 45
PengCai,WentaoDeng,HongshuaiHou,GuoqiangZou,andXiaoboJi
3.1EDLC-TypeMechanismofSCsandBattery-TypeMechanism ofSIBs 45
3.2PseudocapacitanceMechanism 46
3.2.1MotivationfortheSearchforPseudocapacitance 46
3.2.2DefinitionandTypesofPseudocapacitance 47
3.2.3EnergyStorageMechanismofPseudocapacitors 50
3.2.3.1AdsorptionPseudocapacitance 50
3.2.3.2RedoxPseudocapacitance 50
3.2.3.3IntercalationPseudocapacitance 50
3.2.4PseudocapacitanceElectrodeMaterials 51
3.2.4.1ConductivePolymer 51
3.2.4.2MXene 52
3.2.4.3PseudocapacitiveMaterialsforComparison 53
3.2.5EvolutionofPseudocapacitance 56
3.2.6ElectrochemicalFeaturesofPseudocapacitance 57 References 59
4ClassificationofSodium-IonCapacitorsCell Configurations 63 PengCai,WentaoDeng,GuoqiangZou,HongshuaiHou,andXiaoboJi
4.1Battery-TypeAnodeandEDLCCathodeSICsCellConfigurations 63
4.2Battery-TypeAnodeandPseudocapacitiveCathodeSICsCell Configurations 64
4.3EDLCAnodeandBattery-TypeCathodeSICsCellConfigurations 66
4.4PseudocapacitiveAnodeandBattery-TypeCathodeSICsCell Configurations 66
4.5CapacitiveAnodeandHybridCathodeSICsCellConfigurations 67
4.6Summary 69 References 73
5CathodeMaterialsforSodium-IonCapacitors 75 XiongZhang,WenjieLiu,LeiWang,ChenLi,andYanweiMa
5.1Introduction 75
5.2EDLCCathodeMaterials 77
5.2.10DCarbonaceousCathodes 78
5.2.21DCarbonaceousCathodes 78
5.2.2.1CarbonNanotubes 78
5.2.2.2CarbonNanofibers 79
5.2.32DCarbonaceousCathodes 81
5.2.3.1ReducedGrapheneOxide 81
5.2.3.2CarbonNanosheets 83
5.2.43DCarbonaceousCathodes 84
5.2.4.1HollowCarbonMicrospheres 84
5.2.4.2ActivatedHardCarbon 85
5.2.4.3DisorderCarbon 86
5.2.4.4FoldedCarbon 89
5.3PseudocapacitiveCathodeMaterials 90
5.3.1AdsorptionPseudocapacitiveMaterials 93
5.3.2RedoxPseudocapacitiveMaterials 95
5.3.2.1ConductivePolymers 95
5.3.2.2Vanadium-BasedMaterials 96
5.3.3IntercalationPseudocapacitiveMaterials 98
5.4Battery-TypeCathodeMaterials 102
5.4.1NaMn1/3 Co1/3 Ni1/3 PO4 Cathodes 102
5.4.2Na3 V2 (PO4 )3 Cathodes 104
5.4.3Na3 V2 O2 (PO4 )2 FCathodes 105
5.4.4SodiumTransitionMetalOxidesCathodes 107
5.4.4.1Na0.67 (Mn0.75 Al0.25 )O2 107
5.4.4.2Na0.67 Co0.5 Mn0.5 O2 108
5.4.4.3Na0.5 Mn0.5 Co0.48 Mg0.02 O2 109
5.4.4.4Na0.66 Mn0.54 Ni0.13 Co0.13 O2 110 References 110
6AnodeMaterialsforSodium-IonCapacitors 115 KangyuZou,WentaoDeng,HongshuaiHou,XiaoboJi,andGuoqiangZou
6.1EDLCAnodeMaterials 120
6.2PseudocapacitiveAnodeMaterials 123
6.3Battery-TypeAnodeMaterials 128
6.3.1IntercalationMaterials 128
6.3.1.1CarbonaceousAnode 128
6.3.1.2Titanium-BasedCompound 142
6.3.1.3Niobium-BasedCompound 148
6.3.1.4Vanadium-BasedOxide 152
6.3.1.5OtherNewIntercalationAnodes 153
6.3.2ConversionMaterials 154
6.3.2.1MetalOxides 154
6.3.2.2MetalSulfides 157
6.3.2.3MetalSelenides 162
6.3.3AlloyingMaterials 165
6.3.3.1Sn-BasedAnode 165
6.3.3.2SbAnode 168
6.3.3.3BiAnode 169
6.4OtherNovelMaterials 169 References 176
7FlexibleSodium-IonCapacitorDevices 183 TaoqiuZhangandHuanwenWang
7.1FlexibleSICsDevices 183
7.1.1FlexibleBattery-TypeAnodeandCapacitiveCathodeSICsCell Configurations 185
7.1.1.1FlexibleElectrodesBasedonCarbonNanofiber 186
7.1.1.2FlexibleElectrodesBasedonGrapheneSubstrates 201
7.1.1.3FlexibleElectrodesBasedonCarbonCloth 205
7.1.1.4FlexibleElectrodesBasedonMXenes 206
7.1.1.5FlexibleElectrodesBasedonMetalFoil 208
7.2FlexibleCapacitiveAnodeandBattery-TypeCathodeSICsCell Configurations 211
7.3ElectrolytesinFlexibleSICsDevices 217 References 222
8Pre-sodiationTechnologies 225 ZiruiSong,ChangLiu,andXiaoboJi
8.1Introduction 225
8.2Pre-lithiationinLithium-IonBatteries 226
8.2.1OperationwithLiMetal 227
8.2.2UsageofLi-BasedAlternatives 229
8.2.3SupplyofExtraAdditives 232
8.3Pre-sodiationinSodium-IonBatteries 236
8.3.1OperationwithNaMetal 236
8.3.2UsageofNa-BasedAlternatives 237
8.3.3SupplyofExtraAdditives 237
8.4Pre-sodiationinSodium-IonCapacitors 238
8.4.1ElectrochemicalMethod 239
8.4.2AlternativesMethod 239
8.4.3SacrificialAdditivesMethod 241 References 245
9ConclusionsandFuturePerspective 249 KangyuZou,WentaoDeng,HongshuaiHou,GuoqiangZou,andXiaoboJi
9.1DefinitionsandMechanisms 249
9.2Configurations 250
9.3ElectrodeMaterials 251
9.4KeyTechnologies 251
9.5FuturePerspective 252
Index 259
Preface
Standingatthepointwhereglobalwarmingandclimatechangerisksareappearingextremelyprominent,thetransformationoftheglobalenergyconfigurationto renewableenergysystemisaninevitabletrendinordertoachievethegoalsinthe ParisAgreementandaccomplishthereductionofcarbonemission.Theutilization ofrenewableenergyisexpectedtobewieldedinalargerscaletoreducethedependenceontraditionalfossilfuels(coal,oil,etc.)andbecomethemajorsourceofpower generation.Significantly,thematurityofrenewableenergytechnologyandtherapid expansionofscalehavegreatlyreducedtherelevantcostsinrecentyears,forminga strongcompetitionwithtraditionalfossilenergyintermsofprice.
Advancedsecondaryionbatteries,astherepresentationaltechnologyofenergy storage,playapivotalrolefordecarbonization.Triumphingtowardcleanenergy conversioninthenextfewdecades,lithium-ionbatteries(LIBs)havebeen developedinthedirectionofhigh-energydensity.However,thelimitedoutput performanceathigh-powerdensityseriouslyrestrictsthecorrespondingwide application.Importantly,well-balancedtrade-offsbetweenenergydensityand powerdensityhavebeenpresentedbymetal-ioncapacitors,inwhichcapacitor-type electrodewithadsorption/desorptionbehaviorandbattery-typeelectrodewith intercalation/conversion/alloyingmechanismareselectedascathodeandanode, respectively.Owingtotheoverwhelmingadvantageofmuchmoreabundant contentofsodiumsourcesinthecrustthanlithium,sodium-ioncapacitors(SICs) haveattractedextensiveinterestinconstructingnext-generationenergystorage systems.
SICsarejustlikeRubik’scubeswithcharmingdiversities;thisbookcomprehensivelyandsystematicallydescribestechnology,relevantmaterials,anddevelopment trendsofSICs,includingcharacteristicofSICsdevices,fundamentalunderstanding ofSICsmechanism,classificationofSICscellconfigurations,cathodematerialsfor SICs,anodematerialsforSICs,flexibleSICsdevices,andpre-sodiationtechnologies.Meanwhile,thisbookoffersguidelinestoconstructadvancedSICs,whichis suitableforscientificandtechnologicalworkersinthefieldofenergystoragesystems,aswellasseniorundergraduates,graduatestudents,andteachersofrelated majorsincollegesanduniversities.
x Preface
ThisbookiseditedbyProf.Zou,Prof.Ji,andProf.HouoftheCentralSouth UniversityofChina.Thespecificchapterwritersdividedtheworkasfollows: Chapters1–4aremainlywrittenbyProfessorGuoqiangZou,XiaoboJi,and HongshuaiHouofCentralSouthUniversity;Chapter5ismainlywrittenby ProfessorXiongZhang,andYanweiMaofInstituteofElectricalEngineering, ChineseAcademyofSciences;Chapter6ismainlywrittenbyProfessorGuoqiang Zou,XiaoboJi,andHongshuaiHouofCentralSouthUniversity;Chapter7ismainly writtenbyProfessorHuanwenWangofFacultyofMaterialandChemistry,China UniversityofGeosciences;Chapter8ismainlywrittenbyProfessorChangLiuof SchoolofChemistryandChemicalEngineering,HunanInstituteofEngineering; Chapter9ismainlywrittenbyProfessorGuoqiangZou,XiaoboJi,andHongshuai HouofCentralSouthUniversity.
Duetothewiderangeofcontentinvolvedinthisbook,coupledwiththelimited timeandability,ifthereareomissionsandinadequacies,pleasecriticizeandinform ustocorrect!
7November2022 CentralSouthUniversity Changsha,China
GuoqiangZou,XiaoboJi,andHongshuaiHou
Introduction
PengCai,WentaoDeng,HongshuaiHou,GuoqiangZou,andXiaoboJi
CentralSouthUniversity,CollegeofChemistryandChemicalEngineering,No.932,LushanSouthRoad, YueluDistrict,Changsha410083,China
1.1ABriefDevelopmentofSICs
Nowadays,withtherapiddevelopmentofdailyhouseholdappliances,portable instruments,datastoragesystems,andaerospacefacilities,itisevenmorenecessary todevelopnewenergystoragedeviceswithhighenergydensity,highpowerdensity, andgoodcyclestability.Inthepast,manyresearchershavebeencommittedto designingexcellentenergystoragedevicesthattakeintoaccounthighenergy densityandhighpowerdensity,suchasrechargeablebatteriesandelectrochemical supercapacitors(SCs)[1–13].However,fortheworld,theconfigurationofelectrochemicalenergystoragedevicesthatprovidebothhighenergydensityandhigh powerhasbecomeanurgentneed[14].
Thesuccessfulcommercializationoflithium-ionbatteries(LIBs)in1991has receivedextensiveattentionfromresearchers[15–18].LIBsarecharacterizedby highworkingvoltage,highenergydensity,andwideworkingvoltagewindowbut poorrateperformance[19–22].Incomparison,SCshavehigherpowerdensity andcyclestability,buttheirapplicationislimitedduetotheirlowenergydensity defects[23,24].Therefore,inresponsetothisdefect,theconceptofhybrid-ion capacitorswasbroughtup[25–32].In2001,Amatucciusedactivatedcarbon(AC) toconstructthefirstlithiumhybridcapacitorasthecathodeandnanostructured Li4 Ti5 O12 (LTO)astheanode[17,33,34].Theenergydensityofthecontaineris twicethatoftraditionalcarbon-basedSCs,andatthesametimeitpresentsabright magnificationprospect.Sincethen,aftertheapplicationoflithiumhybridcapacitorsinassemblingavarietyofdevices,manyresearchersarestillexploringand payingattentiontothecostandfuturereservesoflithium,especiallyrelatedtothe applicationoflarge-scaleenergydevicesandsmartgrids,andtheyhavegradually proposedtheideaofreplacinglithiumwithsodium[32,35–47].Inaddition,there isanotherkindofalkalimetalioncapacitor–potassiumioncapacitor(PIC),which isrichinresources,butitsrelatedtechnologyresearchisstillinthepreliminary explorationstage.Therearefivemainfactorsrestrictingthedevelopmentof
Sodium-IonCapacitors:Mechanisms,Materials,andTechnologies,FirstEdition. EditedbyGuoqiangZou,XiaoboJi,andHongshuaiHou. ©2024WILEY-VCHGmbH.Published2024byWILEY-VCHGmbH.
PICs:(i)lowiondiffusionrateinsolidelectrodesandpoorpotassiumionreaction kinetics;(ii)largevolumechangesduringpotassiuminsertion/depotassization;(iii) serioussidereactionsandelectrolyteconsumption;(iv)dendritegrowthandsafety hazards;and(v)limitedenergydensity/powerdensitycausedbytherelatively highatomicmassof K .Inaddition,someaqueousmetal-ioncapacitors(zinc-ion capacitors)havealsoreceivedwidespreadattention.However,duetotheirlower energydensityandlongercyclelife,aqueousmetal-ioncapacitorsaremoresuitable forapplicationsinbiologicalsystems.
Fromthedevelopmentoflithium-ioncapacitors(LICs)andotherhybrid-ion capacitorsabove,itisnotdifficulttoseethatmasteringthedevelopmentofLICs isverymeaningfulforresearcherstoexplorethedevelopmentofsodium-ion capacitors(SICs).Therefore,inordertobetterunderstandthedevelopmentfeatures andadvantagesofSICs,othertypesofhybrid-ioncapacitorswillbeintroduced inSection1.2.Otherhybrid-ioncapacitorswillbeintroducedinthecategoriesof monovalenthybrid-ioncapacitorsandmultivalenthybrid-ioncapacitors.Taking themonovalenthybrid-ioncapacitorasanexample,thePICsarefirstlyintroduced. Then,abriefintroductiontomultivalenthybrid-ioncapacitorsisgiven.For example:magnesium-ionhybridcapacitors(MICs),calcium-ionbatteries(CIBs), zinc-ionhybridcapacitors(ZICs),andaluminum-ionhybridcapacitors(AICs). Comparedwithhighpowerdensity,thecoreproblemsoftheseemergingsystems maylieinotheraspects.Therefore,thesesystemsmaynotbefullydevelopedin thefieldofhybrid-ioncapacitors.Somebasicthinking,attempts,andexplorations ofhybrid-ioncapacitorsintheseemergingdevelopmentfieldswillalsobebriefly covered.ThischapterhopestoinspirereaderstofullyunderstandandmasterSICs bycoveringavarietyofbackgroundsandintroductions.Table1.1andFigure1.1 demonstratethedifferentcharacteristicaspectsofthechargecarriersofLi+ ,Na+ , K+ ,Mg2+ ,Ca2+ ,Zn2+ ,andAl3+ fortheirrespectiveenergystoragesystems. Asrepresentativeofmonovalenthybrid-ioncapacitors,LICs,theirdevelopment historyandsuccessfulcommercializationpathareworthdiscussing.In1984, Dr.YamabeofKyotoUniversitycooperatedwithDr.YataofKaneboCo.,Ltd. tosynthesizeanewtypeofcarbonaceousmaterial,namelypolyacenesemiconductor(PAS),bypyrolysisofphenolicresin[49].In1987,Yataetal.reported theintercalation/deintercalationpropertiesoflithiumionsinPAS,andresearch activitiesonLICshavereceivedmuchattentionsincethen[50].In2005,FujiHeavy IndustrieswasthefirsttocommercializeLICbasedonaporouscarboncathode andapre-lithiatedPASanodewithastackedstructure[51].Sincethen,different companieshaveattemptedtocommercializeLICsbasedonvarioustechnologies, andafewpromisingcommercialproductsaredescribedinTable1.2.Recently commercializedLICscanachievegravimetricenergyandpowerdensitiesof 20Whkg 1 and7.5kWkg 1 ,respectively,withlifetimesrangingfrom100000to 800000[52].
AmongthemanyLICs,themostclassicisthepioneeringworkofAmatuccietal. Asmentionedbefore,Amatuccietal.werethefirsttoreportLICsviahybridization ofEDLCsandLIBs[33].In2001,AC(+)//Li4 Ti5 O12 ( )LICusing1MLiPF6 in ethylenecarbonate(EC)/dimethylcarbonate(DMC)(2:1,v/v)wasfirstreported
Table1.1 CharacteristicphysicalpropertiesofLi+ ,Na+ ,K+ ,Mg2+ ,Ca2+ ,Zn2+ ,andAl3+ ion carriersforhybrid-ioncapacitors.
PropertiesLi+ Na+ K+ Mg2+ Ca2+ Zn2+ Al3+
Relativeatomicmass6.946.949.1024.3140.0865.3826.58
Mass-to-chargeratio6.946.949.1012.1520.0432.698.86
Theoreticalgravimetric capacityofACoO2 (mAhg 1 )
Theoreticalvolumetric capacityofACoO2 (mAhcm 3 )
274235206260242–268
Shannon’sionicradii(Å)0.761.021.380.7210.740.535
StokesradiiinH2 O(Å)2.381.841.253.473.103.494.39
StokesradiiinPC(Å)4.84.63.6————
Molarionicconductivity inPC(Scm2 mol 1 ) 8.39.115.2————
Molarionicconductivity inAClO4 /PC (Scm2 mol 1 )
DesolvationenergyinPC (kJmol 1 )
Meltingpoint(∘ C)180.597.863.4650842419660
Coordinationpreference (O = octahedral, T = tetrahedral, P = prismatic)
Source:Naskaretal.[48]/withpermissionofJohnWiley&Sons.
byAmatuccietal.TheLICsdisplayedslopingcharge/dischargecurvesinthe 1.5–3.0Vwindowwith90%capacityutilizationat10Crateand10–15%capacity lossafter5000cycles.Intheirreports,theLi4 Ti5 O12 anodesexhibitalmostzero volumechangeuponLi+ ionintercalation/deintercalationandshowaterminal lithiationvoltageof1.55V.Inafollow-uppublicationin2004,typicaldevices with40Whkg 1 largeenergydensityand4000Wkg 1 highpowerdensitywere developedbyusingnanostructuredLi4 Ti5 O12 anodeandAC/LiCoO2 composite cathodein2MLiBF4 /acetonitrile(AN)electrolyte[53].Highdurabilityisachieved inthisdeviceduetotheutilizationofACEDLcathodematerialandLi4 Ti5 O12 nanostructuredanodematerial.Thecapacitylossafter9000cyclesatfulldepthof dischargeis20%,whichisquitesuperiorcomparedtoconventionalLIBs.
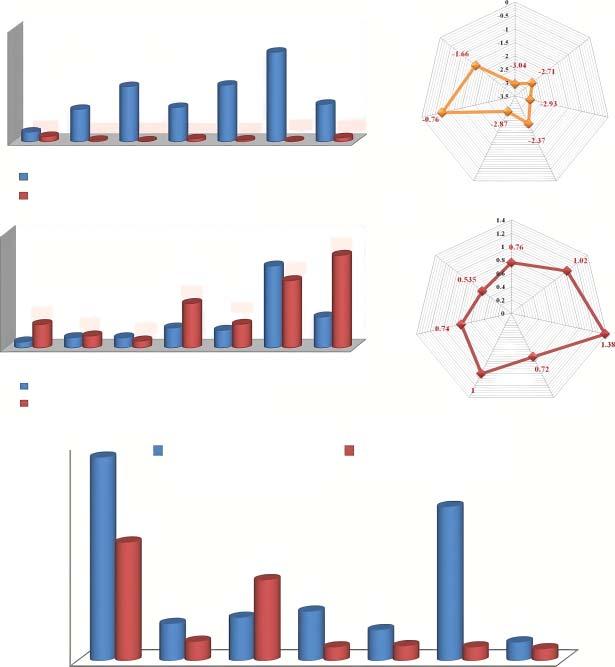
Figure1.1 (a)Relativeatomicmassandgravimetricspecificcapacityofmetal.(b)Density andvolumetricspecificcapacityofmetal.(c)Standardelectrodepotential(Vvs.SHE). (d)Shannon’sionicradii(Å)ofdifferentchargecarriersforrechargeablebatteries,and (e)Relativeabundance-rankandcostofdifferentchargecarriers(Li+ ,Na+ ,K+ ,Mg2+ ,Ca2+ , Zn2+ ,andAl3+ )forrechargeablebatteries.Source:ReproducedwithpermissionofNaskar etal.[48]Copyright2021,Wiley-VCHGmbH.
1.2ComparisonBetweenDifferentHybrid-Ion Capacitors
Similarly,amongmonovalenthybrid-ioncapacitors,PICshaverecentlydrawn attentionaspromisingnext-generationenergystoragesystemsduetotheirrelativelyabundantpotassiumreservesandlowcost.ThedevelopmentofPICsis accompaniedbythedevelopmentofpotassium-ionbatteries(PIBs).Since2015,an increasingnumberofscientificpublicationsonPIBsandPICs,canbeobserved.PICs exhibitsomeadvantages[54].Forexample,potassiumdoesnotthermodynamically formAl–Kintermetallics,cheaperaluminumfoilscanbeutilizedasanodecurrent
Table1.2 Productcharacteristicsofsomeofthemostadvancedcommerciallithium-ion capacitors(LICs).
CompanyDevicetype Potential (V)
Energy density (Whkg 1 )Cycles
JMEnergy Corporation Prismatic2.2–3.8330013300000
GeneralCapacitor Intl,Inc. Laminate2.2–3.8300018100000
TaiyoYudenCylinder2.2–3.820015100000
VinaTechnologyCylinder2.2–3.8270——
AoweiTechnologyModule2.2–3.89000 >20 >30000
Greenway217002.0–4.033310.750000
LRNETLaminate415000—50000
AsahiKaseiFDK energy Module1560012Wh—
ACTLaminate2.0–4.0500015—
NECTokinLaminate2.2–3.810008.0— MSRMicroLaminate/ Prismatic 2.2–3.892–825range——
PuriXel,SouthKoreaLaminate2.25–3.00——100000
Source:AdaptedfromNaoietal.[52].
collectorsforPICs.Moreover,thestandardelectrodepotentialofK( 2.936Vvs. K+ /K)islowerthanthatofNa( 2.714Vvs.Na+ /Na),whichmayresultinawider voltagewindow,indicatinghigherenergydensitythanSICs.Furthermore,dueto theweakLewisacidityofK+ ,K+ canformsmallersolvatedions(3.6Å)thanLi+ (4.8Å)andNa+ (4.6Å)inmonovalenthybrid-ioncapacitors.Thus,PICsachieve fastionicdiffusionratesandhighelectricalconductivityinpropylenecarbonate (PC)solvent.Furthermore,unlikeSICs,commerciallyavailablegraphitecanbe usedasanodematerialsforPICsbyformingintercalationcompounds(KC8 )with theoreticalcapacitiesof279mAhg 1 .Anothersignificantadvantagecomparedto SICsisthat,formostanodematerials,theintercalationpotentialofKionsisabout 0.2VrelativetoK+ /K,whichreducesthepossibilityofmetalpotassiumplatingand effectivelyavoidstheriskofdendriteformationduringcharging.Forexample,for hardcarbon(HC)inSICs,thesodiumpotentialisabout0.05VrelativetoNa+ /Na, whileforK+ /KinPICs,thepotassiumpotentialis0.2V.However,PICsalsosuffer frommorelimitedcyclingstability.Duetothelargeionicradiusofpotassiumions (1.38Å),amongmanyelectrodematerials,especiallytheanodeswouldshowlarge volumeexpansionduringchargeanddischarge,resultinginashortlifespanin mostPICs(usually <500cycles).Consequently,morelimitedcyclingstabilitiesare alsogreatchallengesforimprovingthepowerdensitiesofPICs.Therefore,PICsare stillinthedevelopmentalstages.
Asdiscussedbefore,multivalenthybrid-ioncapacitorsmayhaveadifferent focusthanthecoreissuesofmonovalenthybrid-ioncapacitors.Tounderstand thefeaturesofMICs,abriefoverviewoftheelectrodesandelectrolytesofMICs isrequired.Asadivalentcation,energysystemsbasedonMgionsencounter sluggishkineticsduetothestrongelectrostaticinteractionsofMgionswithanions inthehostframeworkinthecathode.Therefore,thedesignofthecathodeactive materialisakeyfactor.Chevrelphases(Mgx Mo6 T8 ,T = S,Se,Te)anddisulfides (MoS2 ,WSe2 ,etc.)arepreferredaspromisingMg-ioncathodeactivematerials [55–59];sinceinthesehostmaterials,theelectrostaticinteractionbetweenMgions andanionsislow.Inaddition,sufficientchannelsizeinthecrystalstructurealso facilitatesthefacileintercalation/deintercalationofMgions.Ontheotherhand, moderatelypolaranions(S2 ,Se2 ,Te2 ,etc.)inelectrodematerialscanleadto weakerbondstrengthsbetweentransitionmetalcations,resultinginrelativelylow redoxpotentialsfortransitionmetals[60].Therefore,Chevrelphasesanddisulfide showredoxpotentialsbelow2V(vs.Mg2+ /Mg).Thisdiscussionsuggeststhat highervoltages(i.e.higherenergies)andfastkinetics(i.e.higherpower)arenot easilyachievedsimultaneouslyinMICs.BesidesthesematerialsV2 O5 [61],MnO2 [62],MoO3 [63],MgCo2 O4 [64],TiS2 [65],TiO2 [66],sulfur[67],iodine[68],and polyanion-basedmaterials(MgMnSiO4 ,MgFeSiO4 ,etc.)[69,70]havealsobeen reportedascathodeactivematerialsforMICs.Tobetterunderstandtheproperties ofMICs,somecharacteristicsofmetallicmagnesiumanodesinmagnesium-ion batteries(MIBs)arealsointroduced.Fromtheanodepointofview,metallicMg electrodeisagoodchoicefornonaqueousMIBsbecauseofitshighnegative voltage( 2.37Vvs.SHE)andhightheoreticalcapacity(2205mAhkg 1 ).However, complexreactionsarepronetooccurbetweenthemetalmagnesiumanodesandthe electrolytes.Hence,thediscussionofanodesandelectrolytesneedstobecovered. DuetothechemicalactivityofMginconventionalelectrolytes,itencounters seriousproblems:DuetothepresenceofMg-ionsaltsinpolaraproticsolvents,the Mgsurfacetendstoformahardpassivationlayerthatinhibitsionpathwaysand accompanyingelectrochemicalreactions.Tocircumventthisproblem,researchers areturningtoalloyed/dealloyedorMginsertion/deintercalationtypesofanodes. However,suchmaterialsalsosufferfromslowkineticsandpulverizationdueto excessivevolumechangesduringcharge/discharge.Amongalloying/dealloying materials,Bi,Sb,Bi–Sb,Ge,Si,Sn,andSn-basedbinaryalloys(Cu–Sn,Pb–Sn,and In–Sn)havebeenreportedintheliterature[71–74].Inaddition,thedevelopment ofnon-MgmetalanodeshaspromotedthefurtherdevelopmentofMICstoa certainextent[75,76].In2Dmaterials,theanodeofMICscanalsousedefectivegrapheneandgrapheneallotropemoieties[77],blackphosphorus[78],and Li4 Ti5 O12 [79].Inorganicsystems,thedevelopmentofMICsismainlylimitedby electrolytes.Furthermore,adiscussionofelectrolytesinMIBsisessentialwhenit comestoelectrolytesforMICs.IntheworkofAurbachetal.,anunconventional electrolytesystembasedonorganohaloaluminatemagnesiumsaltsintetrahydrofuran(THF)andpolyethersoftheglymewasdeveloped,inwhichmetallic magnesiumelectrodesworkreversiblywithrelativelyfastkinetics[80].Grignard reagents(RMgX;R:alkyloraryl,X:ClorBr)aselectrolytesforMIBshavealso
1.2ComparisonBetweenDifferentHybrid-IonCapacitors 7 beenreportedintheliteratureforpassivation-freemetalMgelectrodes,buttheir strongreducibilitylimitstheoxidativestabilityofcathodes[81].Anotherelectrolyte system,namelyorganoborate(magnesiumdibutyldiphenylMg(BPh2 Bu2 )2 and tributylphenylMg(BPhBu3 )2 )inTHF(>0.4mol),canrealizeanodereversibleMg stripping/platingaswellascathodereversibleMgioninsertion/extraction[82]. Inrecentyears,MIBsofaqueouselectrolyteshaveattractedattentionduetotheir safeandcost-effectiveproperties.Forexample,in2019,Zhangetal.constructed devicesbasedon δ-MnO2 @carbonmolecularsievecompositeascathodeand nanowireVO2 asanode[83].In2017,Zhangetal.reportedanaqueousanode withcarbon-coatedFeVO4 andatodorokite-typemagnesiumoctahedralmolecular sieve(Mg-OMS-1)cathode[84].Nametal.proposedsmartmaterialengineering byintroducingcrystalH2 OintothelayeredstructureofthebruciteMnO2 cathode toefficientlyscreenelectrostaticinteractionsbetweenMg2+ andhostframework anions[85].Thegroupalsodemonstratedlowerdesolvationenergiesatthecathode andelectrolyteinterfacesbyaddingH2 Otothenonaqueouselectrolytesolution. ThisisbecausehydratedMgionsareallowedtointercalateintheirhydratedform, therebyminimizingdesolvationenergyloss.TheintercalatedhydratedMgionsin thehostframeworkfurtherminimizetheelectrostaticinteractionsbetweenMg ionsandhostanions[86].Therefore,thebirnessiteMnO2 cathodeinpureaqueous electrolyteexhibitsalargereversiblecapacityof231.1mAhg 1 at2.8V.
However,theaboveoverviewofaqueous/nonaqueouselectrodematerialsisused tocomplementthebasicbackgroundofMICs.In2014,Yooetal.developedthefirst MICsutilizingACclothandmagnesiummetalascathodesandanodes,respectively. Topreventhardpassivationfilmsanddendritegrowthonthemetalanodes,0.25M organohalidemagnesiumaluminatecomplexes(Mg2 Cl3 + –Ph2 AlCl2 )wereutilized inTHFelectrolytes[87].Inthiselectrolyte,theMgelectrodeworksreversiblyfor thousandsofcycleswithaCEofapproximately100%.Ontheotherhand,theporesin thecathodesaresaturatedwithlargeions(bulkyMgandAl-basedioniccomplexes consistingofCl,alkylorarylgroups,andTHFligands)beforethepotentiallimitis reached.Surprisingly,theintroductionof0.5MLiClsolvesthisproblem,assmall ionicsubstanceswillbepresentintheelectrolytes.Thefull-celldeviceexhibiteda specificcapacitanceof90Fg 1 at5mAg 1 within0.9–2.4Vandmaintained79%of theinitialcapacitanceafter4500charge/dischargecycles.Inthefollowingyears, severalresearchersscreenedsuitablematerialsandelectrolytesystemsforadvanced MICs.BreakthroughsinMICsaremorefocusedonaqueouselectrolytesduetothe significantchallengesinorganicsystems.Sunetal.andMaitraetal.reportedMnO2 nanowiresinMgSO4 /Mg(NO3 )2 electrolytesandMgNiO2 /Mg(ClO4 )2 electrolytes forlow-costaqueousMICs,respectively[88].In2017,Zhangetal.demonstrated aqueousMICswith(+)Mg-OMS-2/graphene//0.5MMg(NO3 )2 (aq)//AC( )configuration[89].Thecryptomelane-typemanganeseoxideoctahedralmolecular sieves(OMS-2)areauniqueelectrodewith2 × 2and1 × 1tunnelingstructures ofMnO2 inMICs,whichiswidelyusedasanactivematerialinmagnesiumion batteries.Besides,thelowelectronicconductivityofOMS-2canbemitigatedby thepreparationofcompositescontainingcarbonmaterial.Thefullcellexhibited ahighenergydensityof46.9Whkg 1 (100mAg 1 )andexcellentcyclingstability
(95.8%capacityretentionat100mAg 1 after500cycles)at0–2V.Caoetal.reported theutilizationofMn3 O4 andACastheactivematerialsincathodeandanode, respectively,usingaqueouselectrolytesof2MMgSO4 [90].TheMICsshowedan energydensityof20.2Whkg 1 (125Wkg 1 )andexcellentcyclingstability(95% capacityretentionafter6000cyclesat0.5Ag 1 )withthepotentialof0–2V.In addition,thescaled-upflexiblepackagingdepicts80%capacityretentionafter 500cyclesatacurrentdensityof0.5Ag 1 .Tianetal.reportedMg2+ ioninsertion/detachmentinneutralaqueousMgSO4 electrolytesforVNanodes[91].By increasingthescanratefrom1to200mVs 1 ,therectangular-likeCVandthesmall polarizedredoxpeaksindicatethefastreactionkineticsduetoasurface-controlled process.InordertounderstandthepseudocapacitancemechanismofVNduring charging/discharging,bothXPSandCVcanconfirmthattheV(III)toV(II)transitionisthekeytotheVNcharge/dischargereaction.Quasi-solidaqueousMICs witha(+)MnO2 @C/MgSO4 gel/VN( )configurationshowabulkenergydensity of13.10mWhcm 3 (72mWcm 3 ),andabulkpowerdensityof440mWcm 3 (10.35mWhcm 3 )andexcellentcyclingstabilities(5000cyclesat16mAcm 2 )in therangeof0–2.2V.Inaddition,thedevicewasfurtherdevelopedasaflexible solar-chargingintegratedunitbasedonscreen-printedmicro-supercapacitors. However,thenumberofpromisingMICsystemsisindeedlimitedfromthepointof viewoflaboratoryprototypesorpracticaldevices.
PossessingthehighestShannon’sionicradiiofallmultivalentchargecarriers (Table1.1),Ca2+ ionsexhibitfasterelectrochemicalreactionkineticsthanMg2+ , Zn2+ ,andAl3+ ions,duetolowpolarization.However,asmentionedabove, thepromisingclassesofelectrodematerialsforCICsaresimilartoMICs.Differentexperimentalandsimulationstudieshaveshownthat3Dtunnelingand layeredstructures,suchasCaMn2 O4 ,V2 O5 ,graphite,etc.,aresuitableforCa2+ intercalation/de-intercalation.Therefore,thesematerialscanbeconsideredas anodematerialsforCICs.InspiredbytheMg2+ ionsystem,asimilarChevral phase(CaMo6 T8 [T = S,Se,Te])istheoreticallyenvisagedfortheCa2+ energy storagesystems[92].TheoperatingvoltageofCaMo6 S8 ispredictedtobe1.4V (vs.Ca/Ca2+ ).However,thediffusionofCa2+ isslowerinCaMo6 S8 thanthatof Mg2+ .ThediffusionenergiesofMg2+ andCa2+ inCaMo6 Se8 arebothlowerthan thoseofCaMo6 S8 .ThediffusionenergiesofCa2+ inMo6 S8 andMo6 Se8 are780 and520meV,respectively,whicharerelativelyhigherthanthediffusionenergies ofMg2+ (270and180meVforMo6 S8 andMo6 Se8 ,respectively).Basedonthe quantitativediffusionbarrierlimitsforcelloperation,thenanostructuredMo6 Se8 couldbepromisingasasuitableanodeforCICswithanaveragevoltageof1.25V. However,noexperimentaldataontheinsertion/deinsertionofCa2+ ionsbased ontheChevralphaseareavailableintheliteraturetodate.Cubicframework structuresofPrussianblueanalogues(Ax MFe(CN)6 ⋅yH2 O[whereA = Li,Na,K, Mg,Ca,etc.,andM = Ba,Ti,Mn,Fe,Co,orNi])havealsobeeninvestigatedas insertionelectrodesforCICs,buttheircapacitiesarenotuptothemark[93].While CametalanodesmaybeattractiveforCICsduetotheirhighbulkandweight capacities,surfacepassivationandsubsequenthardSEIsformationinconventional electrolyteshindersreversibleCa2+ stripping/plating.Therefore,inconventional
1.2ComparisonBetweenDifferentHybrid-IonCapacitors 9 electrolytes(containingCaionsaltsinpolarnon-protonicsolvents),anefficient Caionenergystoragesystemcannotbeachievedusingmetalanodes[94].On theotherhand,alloyedmaterialssuchasCa–SnandCa–Sianodescanexhibit reversiblealloying/dealloyingelectrochemicalreactionswithappreciablecapacity byavoidingtheformationofhardpassivationfilms[93,95].In1991,Aurbach etal.demonstratedtheelectrochemicalbehaviorofCa-metalelectrodesinseveral organicelectrolytes,suchasCa(ClO4 )2 ,Ca(BF4 )2 ,LiAsF6 ,andtetrabutylammoniumsalts(BF4 andClO4 )inTHF,PC,AN,and γ-butyrolactonesolvents[96]. Duringtheelectrochemicalreductionofelectrolytesolutions,CaCl2 (inClO4 salt solutions),Ca(OH)2 ,CaCO3 ,andcalciumalcoholsaltsformpassivationfilmswhich limitthereversibledeposition/dissolutionofelectrodesinCa-basednonaqueous electrolytes.In2016,Ponrouchetal.reportedthatsaltsinorganicelectrolytes mixedwithcarbonatesolvents(PCandEC)containingCa(ClO4 )2 ,Ca(BF4 )2 ,and Ca(TFSI)2 exhibitedawideelectrochemicalstabilitywindowathightemperatures (e.g. 0.5to3.5Vvs.Ca/Ca2+ at100 ∘ C)[94].Ultimately,theseelectrolytesalso showirreversibilityofCa2+ stripping/plating.In2017,Wangetal.developeda THF-basedelectrolytecontainingCa(BF4 )2 saltthatcanbeoperatedatroomtemperature[97].Inthiselectrolyte,thereversiblestripping/platingofCa2+ wasvery satisfactory,butwithloweranodicstability(3Vvs.Ca/Ca2+ )andlowerCE.Unlike thereductionproductsthatformhardSEIsinconventionalelectrolytes,CaH2 was identifiedastheSEIscomponentintheTHF-basedelectrolytes.Unfortunately, theSEIfilmswereunstableandformedcontinuouslyduringcyclingduetoCaH2 deposition,resultinginaCElowerthanthatrequiredforpracticalapplications (99.98%).In2019,Shyamsunderetal.synthesizedanewfluorinatedalkoxyborate (Ca(B(Ohfip)4 )2 ⋅4DME),whichisbasedonthehexafluoroisopropoxy(Ohfip ) ligand[98].Itshowsreversiblestripping/platingofCa2+ in1,2-dimethoxyethane (DME)solventat25 ∘ Cwithlowpolarization(170mV).Thissaltalsoexhibits higheranodicstabilityupto4.1and4.9VinDMEand N,N -dimethyltriflamide, respectively.In2019,Lietal.reportedasimilarelectrolytesystem,calcium tetrakis(hexafluoroisopropoxy)borate(Ca[B(hfip)4 ]2 )inDME,showingreversible stripping/platingofCa2+ atroomtemperaturewithhighoxidativestabilityupto 4.5Vvs.Ca/Ca2+ andhighionicconductivity(>8mScm 1 )[99].Nevertheless, theseelectrolyteshavesomelimitationsandthereforerequireextensiveresearch onthenon-aqueousandaqueouselectrolytes.Taetal.andLeeetal.report computationalsimulationstudiesofCaelectrodepositionandspeciesformation processesinnonaqueouselectrolytesandmodulatethehydrationnumberofCa2+ ionsbyvaryingtheelectrolyteconcentration,respectively[100,101].Different theoreticalandexperimentalstudiesonelectrodes/electrolytesareessentialin ordertogainamorecomprehensiveknowledgeonthefeasibilityofpracticalCICs. In2019,Wuetal.reportedthefirstCICdevicesbasedonACcathodes,Snfoil anodes,anda0.8MCa(PF6 )2 electrolytesolutioninamixedcarbonatesolvent (EC,PC,DMC,andEMC)[102].DuringthechargingofCICs,PF6 anionswere adsorbedtotheACsurfaceandCa2+ weremigratedtowardtheSnanodes,forming theCa7 Sn6 alloy.DuringthedischargeofCICs,theoppositeprocessoccurred. Basedontheabovemechanism,thefulldeviceexhibitedanoperatingvoltageof
1.5–4.8V.Moreover,theCVcurvesatdifferentscanrates(10–100mVs 1 )indicated thatthefull-celldevicedeliversgoodCICsperformances.Reversiblecapacitiesof 92mAhg 1 (0.1Ag 1 )and82mAhg 1 (0.4Ag 1 )andacapacityretentionrateof 84%over1000cyclesat0.2Ag 1 wereachieved.Toourknowledge,thisistheonly CICdevicereportedtodatethatshowspromisingelectrochemicalperformancesat roomtemperature.
ZICsaredevelopedfromZIBs.ZIBshavereceivedalotofattentionfortheir lowcost,highsafety,andenvironmentfriendliness.Typically,ZIBsconsistofzinc metalanodes,aqueouselectrolytessuchasZnSO4 (aq),andcathodesforZn2+ intercalation/de-intercalation.Unliketheextremelyactivelithium,sodium,and potassiummetalelectrodes,zincmetalelectrodesarestableinairandcanbeused directlyastheanodesinZIBs.Atthesametime,metallicZnelectrodeshaveahigh weightcapacityof823mAhg 1 (correspondingtoanultra-high-volumecapacityof 5845Ahl 1 ,muchhigherthan2046Ahl 1 forlithiumelectrodesand3833Ahl 1 forMgmetalelectrodes)andalowredoxpotentialof 0.76Vcomparedtostandard hydrogenelectrodes[103].Inaddition,thehighionicconductivityoftheaqueous Zn2+ -containingelectrolyteoftheZIBsfacilitatesfastcharge/dischargerates. Therefore,ZICsinherittheexcellentadvantagesofZIBs.Asmentionedabove, thepowerdensityoftheZICsishighlycompetitivecomparedtootherhybrid-ion capacitors.ThetwoelectrodesofZICs(ACandZn)arealsoverystable(inthis neutral/lightsystem)andensureexcellentcyclingstabilities[104].Mostimportantly,ZICshavethepotentialtobeahybridioncapacitordevicewithgoodenergy density,powerdensity,andexcellentlong-termstabilities.ZICstypicallyutilize porouscarbon(orAC)asthecathodesandzincmetalastheanodes.Thehighly reversiblechargestoragemechanismoftheporouscarboncathodegivesZICsan extremelylongservicelife.Zincanodesstorechargebyplatingandstripping,thus providinghighcapacityforZICs.Giventhelowredoxpotentialofthezincanodes, aqueousZICswithcarbon-basedcathodescanprovidehighervoltages(≈1.6–1.8V) thansymmetricalC//CSCs(≈1V)[105].Thankstothehybridconfiguration,ZICs canbridgetheenergydensitygapbetweenSCsandrechargeablebatteries.In addition,ZICsoffermorestablecyclingperformancesandhigherpowerdensities thantheirZIBcounterparts.Basedonthelowcostofcarbonmaterialsandzinc metal,ZICsholdpromiseforlow-costandlarge-scaleapplications,especiallyfor thoserequiringhighpower.
ResearchworkonZICshasonlyrecentlybegun.In2016,Wangandcoworkers reportedthefirstZICsassembledfromoxidizedcarbonnanotubecathodesand zincanodesinaqueousZnSO4 electrolytes[106].TheZICsshowedalowspecific capacitanceof53Fg 1 ,whichwasattributedtothelowspecificsurfacearea(SSA) oftheoCNTcathode(211m2 g 1 ).In2017,Tangandcoworkersusedporous carbonwithahighSSA(3384m2 g 1 )asZICcathodes[107].SuchZICsprovide acapacitanceof170Fg 1 atacurrentdensityof0.1Ag 1 ,correspondingtoan energydensityof52.7Whkg 1 at1725Wkg 1 .Inordertoincreasetheenergy densityofZICs,researchworkhasattemptedtoexpandthevoltagewindow byoptimizingtheelectrolyte.Typically,aqueousZnSO4 electrolyte-basedZICs haveavoltagewindowof0.2–1.8V.In2018,Wangetal.developedanultra-high
1.2ComparisonBetweenDifferentHybrid-IonCapacitors 11 concentrationwater-in-salt(WIS)electrolyte[108].TheWISelectrolyteconsisted of20M(molkg 1 )lithiumbis(trifluoromethanesulfonyl)imide(LiTFSI)and1M Zn(TFSI)2 .TheWISelectrolyteprovidedawidevoltagewindowwithvoltagesup to2.1VsupportedbyahighlystableWISelectrolyte,andachievedahighaverage CEof99.7%.In2019,LuadcoworkersassembledZICswithhighenergydensityby employingnitrogen-dopedgradedporouscarbonascathodes[109].Thenitrogen dopantwasshowntoobtainpseudocapacitancebyloweringtheenergybarrierfor theformationofC—O—Znbonding.In2019,Zapienandcoworkersfabricated thefirstflexibleZICsusingpolyacrylamidehydrogelelectrolytes[110].In2020, BimboandcoworkersintroducedTi3 C2 MXeneasananodematerialforZICs [111].Ti3 C2 -basedZICsexhibitedahighcapacityof189mAhg 1 withcapacity retentionof96%after1000cycles.ZhiandcoworkersZICswerefabricatedusing phospheneasthecathodes[112].Phosphenecathodesshowedhighcapacitanceof over300Fg 1 .Itwasworthnotingthatpseudocapacitancescanbeusedtoincrease theenergydensityofZICs.QuandcoworkersimprovedthecapacitanceofZICsby manipulatingtheZICsinanoxygenreductionreaction[113].Duetotheoxygen reductionreaction,thedischargecapacitiesofZICsweremuchhigherthantheir chargingcapacity.Alshareefetal.utilizedhydrogenandoxygenpseudocapacitance toincreasethecapacitancesofZICs.Thesepseudocapacitancestudiesprovidea newapproachtothedesignofaqueousZICswithhighenergydensities[114].
However,thedevelopmentofpracticalZICsrequiresovercomingmultiple challenges[115].Onelimitationisthelowenergydensity,whichcanbeimproved byincreasingthecapacitancesofthecathodematerialsandbywideningthevoltage windows.ThevoltagewindowsofZICsaremainlydrivenbytheelectrochemical stabilitiesoftheelectrolytes.Inalkalineelectrolytes,thezincmetalinteractswith hydroxideions,resultingintheaccumulationofzinchydroxidematerialsonthe zincanodes.Isolatedzinchydroxidematerialsreducetheoverallconductivity andincreasethepolarizationofthezincanodes.Therefore,alkalineelectrolytes shouldbeavoidedinZICs.Inneutralandweaklyacidicelectrolytes(pHabove3), thevoltagewindowsofZICsarelimitedbyparasiticreactionssuchasHER,OER, andoxidationofporouscarboncathodes.AnotherlimitationofZICsisthecycling stabilitiesofthezincanodes.Comparedtothecarboncathodes,zincanodesexhibit limitedcyclingstabilitiesduetodendritegrowth,HER,andcorrosionreactions.The formationofzincdendritesinvolvesacomplexprocess,whichishighlydependent ontheinhomogeneouselectricfieldsandiondistributions.Duringchargingand discharging,thegrowthofzincdendritesincreasestheSSAofthezincanodes, therebyacceleratingtheHERrates.ThehighHERrateconsumesprotonsinthe aqueouselectrolytes,leadingtotheaccumulationofhydroxideions.Thehydroxide ionsinteractwiththezincanodeandthustriggerthezinccorrosionreactions.Zinc corrosionresultsintheaccumulationofzinchydroxidesubstancesonthesurfaces ofthezincanodes.Thesebyproductsreducetheoverallelectricalconductivityand exacerbatetheinhomogeneouselectricfieldofthezincanodes,therebyincreasing dendritegrowth.Subsequently,theviciouscyclesofzincdendrites,HER,andzinc corrosioncontinue.Besidesmetalliczincanodes,themodificationofporouscarbon electrodesisalsoaconcern.Thecapacitancesofporouscarboncathodeshave
beenimprovedbyporousstructureengineeringandpseudocapacitiveengineering. Advancedcapacitivecathodematerials,suchasMXenes,phosphorene,andTiN, haveprovedtobepromisingcandidatesforcathodematerialsforZICs.Inaddition toelectrodes,thedesignofelectrolytesisalsoacorearea.AqueousZnSO4 solutions arethemostwidelyusedelectrolytesinZICs.ZICelectrolytescontainingZnSO4 aqueoussolutionshavealimitedvoltagewindowof0.2–1.8V.Beyondthisvoltage range,theelectrolyteissubjectedtoanumberofdifferenttypesofelectrodes. Beyondthisvoltagerange,parasiticreactions,suchasHER,canoccuranddamage ZICdevices.Tosuppressparasiticreactionsandexpandthevoltagewindow,the electrolytecomposition,suchassubstance,concentration,andsolvent,canbeoptimized.Inaddition,zincanodesexhibitpoorcyclingstabilitiescomparedtoporous carboncathodes,whichhaveanextremelylongcyclelife.Inthecaseofzincanodes, thegrowthofzincdendrites,iondistributors,andartificialSEIsissuppressedby expandingtheelectrochemicalsurfaceareawitha3Darchitecturaldesign.Theuse oflow-potentialintercalationanodesalsohelpstoavoidzincdendritegrowth.
DespitetheresearchresultsachievedwithZICs,thecurrentresultsarebasedon laboratory-scaledevicesandfocusonspecificcomponentsratherthancomplete devices.InthereportedZICs,thecapacitiesoftheanodes,cathodes,andelectrolytes donotmatch.ForstudiesofZICs,zincanodesandelectrolytesareoftenusedin excess.ThereportedenergydensitiesofZICsareunreliable,asthesevaluesare basedontheactivemassoftheporouscarboncathodes.Furtherresearchshould thereforefocusoncapacitymatchingbetweenanodesandcathodes.Inotherwords, theutilizationofzincshouldbeashighaspossible.Cyclelife,energydensity,and powerdensityareonlymeaningfulifthereisalimiteduseofzinccathodes.From apracticalpointofview,theresourcecostisanimportantparametertoprovethe feasibilityofZICdevicesinpracticalapplications.Estimatingtheactualenergy densityandthecorrespondingcapitalcostofthedeviceisnecessaryforthefurther developmentofZICs.AstheenergydensityofZICscanbefurtherincreasedby variousengineeringstrategies,ZICsarepromisingenergystoragetechnologiesin thefutureenergystoragemarket.Costisoneofthekeyparametersdetermining whetherZICscanbeusedforpracticalapplications.Althoughporouscarbonand ZICshaveagoodindustrialbase,thecostofcommercialAC(US$57–114perkg) ismuchhigherthanthatofZICs(US$2.1perkg).Basedontheaboveestimated data,thecostofelectrodematerialforZICwouldbeUS$365.6perkWh(US$ 7.2perkWhforzincanodesandUS$358.4perkWhforporouscarboncathodes) [116].Thehighcostofporouscarbonisthereforeabottleneckforthepractical applicationofZICs.InordertoreducethecostofZICs,asimplelow-costporous carbonmanufacturingprocessisrequired.Itisbelievedthatmoregreensynthesis processeswillbedevelopedforlow-costporouscarbonmaterials.Thelastfew yearshaveseentremendousprogressintheelectrochemicalperformancesofZICs. Infuture,agreatdealofeffortshouldbeputintothemanufactureofZICsfor practicalapplications.Inthenextfewdecades,therewillbenumerouspotential opportunitiestoscaleupZICswithhighperformances.
Asthree-electronredoxsystems(Al/Al3+ ),energystoragedevicesbasedonaluminumionshaveahighertheoreticalcapacityandhigherenergydensity.However,
13 duetothehigherpolarization,thetrivalentAl3+ suffersfromahigherdesolvation energyandahighersolid-statediffusionenergybarrier.Consideringtheeaseof practicaldevicesbasedondivalentions(Mg2+ ,Ca2+ ,andZn2+ ),trivalentions(Al3+ ) areexpectedtobemoredifficult.However,thefirstreporteduseofaluminum metalasanodesdatesbackto1857,andtheconceptofAIBsforaluminumion batterieswasintroducedin1972[117].Sincethen,researchershavebeenworking todeveloppracticalaluminum-baseddevicesbyfindingsolutionsforscreening suitablecathodeactivematerialswitheasyAl3+ iondiffusion(solidstate),finding suitablecathodeactivematerialsforaqueousandnonaqueousmedia,optimizing theelectrolytesforlowerdesolventizationenergy,andminimizingtheformationof hardSEIsonaluminummetalelectrodes.Therefore,thecorrectchoiceofelectrolyte compositioniskeytothesuccessofAICs.AqueoussaltelectrolyteswithAlCl3 or Al2 (SO4 )3 cannotbeusedforAlanodesbecauseoftheformationofAl2 O3 through surfacepassivationandtheintrinsichydrogenprecipitationreaction.Nonaqueous electrolytescanovercometheselimitations.Historically,binary(NaCl–AlCl3 )and ternary(KCl–NaCl–AlCl3 )moltensaltshavebeenusedaspossibleelectrolytes; however,Al3+ isnotpresent[118].Thischloroaluminatemoltensaltelectrolyte systemisdividedintothreeparts,viz.acidic,neutral,andbasicspecies,basedonthe molarconcentrationofAlCl3 .MolarconcentrationsofAlCl3 oflessthan50%inthe electrolyteprovidethebasiccharacteristicsbycontainingAlCl4 andCl primary anions,whilemorethan50%providesanacidicelectrolytewithAlCl4 andAl2 Cl7 anions.ThereisevidencethatreversibleAlelectrochemicalstripping/platingoccurs onlyinacidiccompositions[119].However,thehighmeltingpointofsuchmolten chloridealuminateelectrolyteslimitstheirpracticalapplication.Ontheother hand,fluorinatedsaltsinorganicsolventsarenotsuitableforaluminum-based systems,butthisisnegligibleforlithium/sodium/potassium-basedsystems.For example,aluminumsaltscontainingfluorideanionsintheelectrolyte(similar toLiPF6 inLICs)formelectronicallyandionicallynonconductivepassivated AlF3 layersonaluminummetalelectrodes,whichhindersthereversibilityof aluminumstripping/plating.Atthesametime,highdesolvationenergylossesat theelectrode/electrolyteinterfaceincreasethepolarizationoftheelectrochemical reactionsintypicalorganicsystems(e.g.ethers).Theseproblemsareresponsible fortheslowkineticsofAICsusingconventionalfluorinatedelectrolytesinorganic media.RecentresearchanddevelopmentshaveshownthatILswithhighionic conductivity,lowvolatility,andhighchemical/electrochemicalstabilitycanbeused aselectrolytesolventsinAICsatroomtemperature[120].CommonIL-basedelectrolytesarepreparedbymixingAlCl3 withimidazoliumchlorideinaspecificratio. Themostcommonlyusedimidazoliumchloridesare1-butyl-3-methylimidazolium chloride([BMIM]Cl)and1-ethyl-3-methylimidazoliumchloride([EMIM]Cl). Herein,theLewisacidityoftheelectrolyteisachievedbymaintainingthemolar ratioofAlCl3 /imidazoliumsaltgreaterthanone.Thereactionmechanismfor aluminumstripping/platingissimilartothatofthemoltenchloroaluminateelectrolytedescribedaboveintermsofthemainanionspeciesandreversiblereactions. Therefore,inthecathodesinAICsdevices,graphite-basedmaterials(3Dgraphite foam,graphenemicroflower,etc.),metalsulfides(Mo6 S8 ,FeS2 ,SnS2 ,Ni3 S2 ,CuS,
Co9 S8 ,etc.),metaloxides(TiO2 ,V2 O5 ,etc.),Prussianblueanalogues(CuHCF), MXene(V2 CTx ),chloroaluminate-dopedconductivepolymers,etc.havebeenwell reported[52].Duetotheirhighbulkdensity,highabundance,andenvironment friendliness,aluminummetalanodesaremostlyacceptedbyconventionalAICs. Notably,afterremovalofthesurfaceoxidefilms,thethinAl2 O3 filmsincommercial aluminumfoilsshowbetterelectrochemicalpropertiescomparedtopurealuminum foils[52].Thedefectivesitesintheoxidefilmsfacilitateefficientpenetrationofthe electrolytesandminimizedendritegrowth.Inaqueousaluminum-basedsystems, surfacemodificationofaluminumfoilsbyionicliquidimpregnationintroduces artificialSEIstominimizehardsurfacepassivationonthealuminumanodes. Salt-packedaqueousorsolid/quasi-solidelectrolytesarealsosuitableforaqueous AICs,whiletheoverpotentialofthehydrogenprecipitationreactionissignificantly increased[52].Interestingly,inadditiontoaluminummetalelectrodes,zincmetal, MoO3 @PPynanotubes,SWCNT/W18 O49 nanowires,etc.havealsobeentriedas anodematerialsinaqueousAICs.
In2016,Yooetal.filedaUSpatentforAICsbypreferentiallyusingaluminum (includingaluminumfoil,aluminumpowder,aluminumfoam,shellparticles withanaluminumcoating,andaluminumalloys)anodes,andhighsurfacearea porouscarbon(includingAC,CNT,andgraphene)cathodes/[EMIM]ClwithAlCl3 electrolytes[121].Accordingtothepatentdescription,inadditiontoaluminum metal,graphite,aluminum-dopedgraphite,carbon,silicon,titaniumdioxide, molybdenumsulfide,andotherintercalation/de-intercalationmaterialscanalso beusedaspotentialanodes.Similarly,conductivepolymers,oxides,sulfides,and nitridesarealsosuitableforpotentialcathodes.Thepatentdescriptionisnotlimited toelectrolytesinionicliquids;italsoincludesorganic/aqueouselectrolytesandeven solidelectrolytes.Tianandhiscolleaguesdevelopedlow-costandhigh-performance aqueousAICsbyusingnanostructuredV2 O5 -impregnatedmesoporouscarbon microspheres(MCM/V2 O5 )ina1MAl2 (SO4 )3 electrolyte.TheAICsoperated throughtheintercalation/de-intercalationofAl3+ andthereduction/oxidationof V5+ /V4+ pairs.TheCVoftheAICsshowedtypicalredoxpropertiesduetothe pseudocapacitancecontributionoftheV5+ /V4+ pair.The b-valueofthecomposite electrodes(closeto1)indicatedthedominantcapacitance/pseudocapacitance contributionintheenergystorage.ThehigherqualityloadingofV2 O5 reducedthe b-value,suggestingthatV2 O5 ledtoadiffusion-controlledmechanism.As-prepared AICsdevicesdeliveredanenergydensityof13.2Whkg 1 (147Wkg 1 ),apower densityof5840Wkg 1 (7Whkg 1 ),andgoodcyclingperformances(capacitance retentionof90%after10000cyclesat1Ag 1 )intherangeof0–1.6V.TheAICs designedbyLeietal.[122]werecomposedofnitrogen-dopedgrapheneasthe cathodematerials,aluminumfoilastheanodematerials,glassfibersasthe separators,and[EMIM]Cl/AlCl3 astheelectrolytes.Inthisdevice,thecapacitive behaviorswerederivedfromtheadsorption/desorptionofAlCl4 ionsinthe nitrogen-dopedgraphene.TherectangularCVcurvesofthehybriddevicesat variousscanratesindicatedthatthenitrogen-dopedgrapheneexhibitedgoodpropertiesinAICs.Inaddition,aweakreductionpeakat1.9Vindicatedthepresenceof AlCl4 intercalation/de-intercalationin/fromthelayerstructures.TheAICsdevice
1.2ComparisonBetweenDifferentHybrid-IonCapacitors 15 showedaninitialdischargecapacitanceof160Fg 1 (95%CEat0.3Ag 1 within 0.3–2.3V)increasingto254Fg 1 (90%CE)after1000cycles.Thenitrogen-doped graphenecathodewasactivatedinthefirst30cycles,afterwhichthecapacitanceshowedaslowincreaseupto1000cycles.Wangetal.reportedAICs witha(+)AC//0.5MAl2 (SO4 )3 (aq)//PPy@MoO3 ( )structures[123].CV curvesforthefulldevicesoftheAICsshowedbroadpeaksassociatedwith theintercalation/de-intercalationofAl3+ into/outofMoO3 @PPy. b values(0.5)also demonstratedthesolid-statediffusionkineticsofMoO3 intheAl2 (SO4 )3 electrolytes. Thedeviceexhibitedanenergydensityof28Whkg 1 (460Wkg 1 ),apowerdensity of2840Wkg 1 (20Whkg 1 ),andgoodcyclingperformances(93%capacitance retentionafter1800cyclesat2Ag 1 )inthe0–1.5V.Considerableelectrochemical performancesareachievedduetothePPycoatingandthenanotubestructureof MoO3 .PPyprovidesaconductivenetworkwithreducedchargetransferresistance andalsoprotectsMoO3 fromacidetching(acidcanbeproducedbyhydrolysis ofAl2 (SO4 )3 ).Thenanotubestructurefacilitatesthepenetrationofelectrolytes andbuffersMoO3 volumechangesduetoAl3+ intercalation/de-intercalation. Furthermore,thefabricated(+)AC//PPy@MoO3 ( )deviceswereabletolightup redLEDs,whichdemonstratedthepracticalityofthedevices.Lietal.reported flexibleAICswith(+)SWCNT/PANI//1MAlCl3 (aq)//SWCNT/W18 O49 ( )[124]. TheCVcurvesofSWCNT/W18 O49 compositesrevealedbroadredoxpeaksdueto thefastpseudocapacitivereactions.Thisuniquenetworkstructurecontributed tofastiontransportandprovidedhighelectrodeconductivity(1626Scm 1 ).In addition,theW18 O49 nanowiresexhibitedawidelatticespacing,highaspectratio, andhomogeneouslaminarstructures,whichfacilitatedtheefficientintercalationof Al3+ ions.Ontheotherhand,theSWCNT/PANIcathodeprovidedacombinationof capacitive/pseudocapacitivemechanisms.Thehybriddeviceshowedabulkenergy densityof19.0mWhcm 3 (295mWcm 3 ),abulkpowerdensityof1278mWcm 3 (14.5mWhcm 3 ),andanexcellentcyclingstability(95.9%capacitanceretention at14mAcm 2 after6000cycles)at0–1.8V.Nevertheless,thenumberofsuccessful AICsreportedintheliteratureisindeedlimited.
NowturnourattentionbacktotheSICs.Bothsodiumandlithiumareelementsof thefirstmaingroup,andtheirchemicalpropertiesaresimilar.Althoughtheradius ofsodiumionsislargerthanthatoflithiumion(0.102vs.0.076nm),theresource reservesoflithiumionsarefarbehindthoseofsodiumions[125–132].Sodiumis oneofthemostabundantelementsintheearth’scrust,about2.74%,rankingsixth. AndwhensodiumionsaredissolvedinH2 O,theradiusisrelativelysmall(compared withpotassiumions),anditdiffusesinH2 Ofasterthanotherions,sotheconductivityofsodiumionsinthesolutionishigher[133].MusashiEnergySolutionsCo., Ltd.pointedoutthatSICscanbeusedinmanyfieldssuchasconstructionmachinery,photovoltaics,windfarms,andmedicalmachinery.Althoughthesolid-state diffusionintheelectrodemateriallatticeoftendeterminestherateoftraditional batteries,sodiumionenergystoragedevicesmaychangethistrendandgenerate betterpowerthanLIBs[134–136].Therefore,inthelongrun,thedevelopmentof sodium-ioncapacitorsisverypromising.Therefore,SICs,asanemergingtechnology,areconsideredtobeasupplementandextensiontoexistingLICs[137,138].
In2012,KurataniandcoworkersdevelopedanSICenergystoragedevicewithhard carbon(HC)astheanodeandACasthecathode.Sincethen,theresearchofSICs hasdevelopedrapidly.Surprisingly,theenergydensityofSICsis20–50timesgreater thanthatoftraditionalcapacitors,andthepowerdensityofSICsis20–40times greaterthanthatoftraditionalsodiumionbatteries(SIBs)[138–146].Therefore, SICscanbridgethegapbetweenSIBsandSCs[147,148].Comparedwithtraditional SCs,theenergydensityofSICsisuptofourtimeshigher,somechanicaldevicesused forregenerativebrakingmaybesignificantlybeneficial.Inthepastfewdecades,differentmaterialshavealsobeenusedtoenhancetheenergystorageperformance ofSIC,andtheseconceptsaregraduallydeviatingfromtheconceptofclassicSIC. Therefore,itisofgreatsignificancetoclassifySICandclarifydifferentenergystoragemechanismsinthecurrentcontext[149–153].Therefore,itisveryimportant tobuildhigh-performanceSICdevicesbasedonanin-depthunderstandingofthe mechanism.
1.3SICsEnergyStorageMechanismIntroduction
Inordertoimprovetheperformanceofthecathodeandanode,scholarswill furtherexploreandstudypseudocapacitancematerialsinSICdevices[154].On theonehand,manystudieswillfocusonhowtousepseudocapacitivematerials toreplacethetraditionalEDLC-typecathodesandfurtherassemblethemwith batterymaterialstorealizehybriddevices.SICdevicesinvolvingpseudocapacitorcathodesareinthemiddlepositionbetweenEDLCcathodesandbattery cathodes.Pseudocapacitancecanberegardedasasupplementarymechanismof EDLCbecauseitisnotamechanismofelectrostaticadsorption,butcompared toEDLC,pseudocapacitancehasasimilarCVcurveshapeandcomparableconstantCDCcurves[155].Ontheotherhand,thepseudocapacitancematerialin theanodeisalsothemaininterestofSICsresearch.Withtherapiddevelopment ofanodenanotechnologyandnanoscience,nanomaterialanodeshaveplayedan importantroleinelectrochemicalenergydevicesovertheyears.Nano-scalematerialsaresmallinsizebutlargeinsurfacearea.Inthiscase,itisimpossibletoaccurately distinguishbetweensurfaceandvolume.Therefore,someanodesbasedontheFaradaymechanismusuallyexhibitstrongredoxreactionsinthematrix.Whenthesizeis reducedtonanometers,theybehavelikepseudocapacitivematerials,characterized bythedisappearanceofCVandCDCcurves.Therefore,inrecentyears,theboundarybetweenbatterymaterialsandpseudocapacitivematerialshasbecomeblurred [156].Itshouldbenotedthatthemisunderstandingoftheconfusingelectrochemicalenergystoragemechanismwillleadtotheopaquedevelopmentofcontroversial batteryconfigurations(dual-ionbatteryconfigurationanddual-capacitorconfiguration)orSICdevices(flexibledevicesandprefabricatedtechnology).Therefore, inadditiontothechaoticmechanism,theneglectedcellconfigurationandthe newlydevelopedflexibleequipmentandprefabricatedareaswerecompletely researched.
1.4KeyTechnologiesofSICs
SICsmainlyconsistofcathode/anodematerials,collectors,electrolytes,separators, andmetalshells.Amongotherthings,thedesignofflexibledevicesandthedevelopmentofpre-sodiationmethodsarethefocusoffutureresearchintoSICdevices.On theonehand,flexibleelectronicshaverevolutionizedourlivesandpermeateevery aspectofourdailylife.Flexibleenergystoragedevicesarereceivingincreasedattentionduetotheirenormouspotentialintheemergingflexibleelectronicsmarket, includingroll-updisplays,flexiblemobilephones,skinsensorsforhumanhealth monitoring,andimplantablemedicaldevices.ThedevelopmentofflexibleSICsis importantforthenextgenerationofSICdevices.Ontheotherhand,pre-sodiation methodsareanintegralpartofelectrochemicalenergystoragesystemsandcaneffectivelycompensateforirreversiblecapacitylosses,increasetheoperatingvoltage,and improvetheconcentrationofNa+ intheelectrolytes.Therefore,flexibleSICsdevices andpre-sodiationmethodswillbetwoimportantareasofkeytechnologiesforSICs devices.BothoftheseareasareimportantresearchareasforSICsdevicesandplaya keyroleindrivingthedevelopmentofSICsdevices.
Thecurrentenergystoragedevicesarerigidandbulkyandcannotmeetthebasic requirementsofflexibleenergystorage.Therefore,effortsmustbemadetoachieve structuraloptimization,lightweight,andflexibility.Apromisingresearchdirection isthedevelopmentoflighter,smaller,andthinnermodernflexibledevices,includingsoftelectronics,windingdisplays,andwearableproducts.Thisalsorequires advancesinthecorrespondingenergysupplydevices.Thedevelopmentofflexible electrodeswithlowcost,highperformance,goodstability,safety,andreliabilityisa majorchallengeformanypracticalapplications.Therapiddevelopmentofportable electronicdeviceshascontributedtothedevelopmentofbetterelectrochemical energystoragetechnologies.FlexibleSICdevicesarecomplexdeviceswhose effectiveenergydensitiesandcyclingstabilitiesdependonvariousdesignfactors, includingtheconfigurationandchoiceofelectrolytes.Eachofthesefactorsplaysa keyroleinimprovingelectrochemicalperformance.Inthischapter,severalsimple flexibleSICdevicesareclassifiedaccordingtothedifferentconfigurationsofSICs, whichgivesthereaderastepaheadtofamiliarizethemselveswiththesignificance oftheconfigurationandthesimpledevelopmentofflexibleSICdevices.Forthis chapter,theseintroductionsareimportant.Amoredetailedanalysisanddiscussion canbestudiedinChapter7.
ThefirstkindofflexibleSICdevicepresentedhereconsistsofanEDLC-typecathodeandabattery-typeanode.Forbattery-typeanodes,titanium-basedcomposites, inparticularNa2 Ti3 O7 andNa2 Ti2 O5 x ,havebeenwidelyexploredasattractiveflexiblematerialsduetotheirlowpotentialandgoodelectronicconductivity[157].In theabsenceofbindersormetalcollectors,Zhangandcoworkersdevelopedflexible SICdevicesbasedonNa2 Ti3 O7 nanosheetarrays/CTanodesandflexiblerGOfilm cathodes[158].Byasimplehydrothermalmethodcombinedwithasubsequentcalcinationprocess,theNa2 Ti3 O7 nanosheetarraysweregrowndirectlyontheCTand showedgoodadhesion.Thesynthesizedmaterialcanbebentatarbitraryangles andhasgoodflexibility.ThewholedeviceusingnanostructuredNa2 Ti3 O7 /CT
andrGOfilmsaselectrodesexhibitsexcellentelectrochemicalpropertieswhile maintaininggoodflexibility.TheCDCcurvesoftheflexibleSICsdevicescanbe recycledatdifferentratesoverapotentialrangeof1–3Vfrom0.1to1.5Ag 1 andshowstabilityat0.5Ag 1 (over80.3%ofcapacityafter2500cycles)aswellas highercoulombicefficiency(closeto100%).Inanothercase,Guandcoworkers preparedflexibleNa2 Ti2 O5 x electrodesandbyintroducingoxygenvacancies[159]. TheyassembledflexibleSICs(labeledrGO/AC//Na2 Ti2 O5 x @Ti//rGO/AC)using Na2 Ti2 O5 x @TiastheanodesandrGO/ACasthecathodes.ThehybridSICdevices offerhighcoulombicefficiency(closeto100%)andgoodcyclingstabilities(82.5% capacityretention)at1Ag 1 after5000cycles.TheflexibleSICsdevicesoperateat potentialsof1–3.8Vandhavemaximumenergyandpowerdensitiesof15.6Whl 1 and120Wl 1 ,respectively.
TheflexibleSICdevicespresentedinthisplaceconsistofcell-typecathodesand EDLC-typeanodes.AsfortheEDLC-typeanodes,researchhasmainlyfocused onAC.Inordertoprovidehighpowercharacteristics,Fanetal.designedand fabricatedanoveltypeofN-dopedmesoporouscarbonnanosheets(mp-CNS)consistingofinterconnectedCo/Znmetalorganicframework(MOF)nanosheets[160]. InordertogivetheflexibleSICdeviceshighpower,thepseudocapacitive VO2 @mp-CNSs/CFCmaterialissynthesizedbydepositinginterconnectedVO2 on topofmp-CNSs/CFC.FlexibleSICdevicesareassembledfrompseudocapacitive VO2 @mp-CNSs/CFCanodesandcellularNVP@mp-CNSs/CFCcathodes.At 1Ag 1 ,theflexibleSICachievesacapacityretentionof78%after2000cycles. Furthermore,nosignificantstructuraldamagewasobservedatdifferentbending anglesfrom0∘ to90∘
Theaboveexamplesofflexibleelectrodesareinnovativeorimprovedinthat therearenobindersorcollectors,buttheelectrolyteremainsaconventional liquidorganicelectrolyte.Inaddition,mostoftheliquidorganicelectrolytesusedin hybridflexibleSICdevicesarehighlyvolatileandflammable,posingasafetyhazard. Therefore,effectivesolutionsneedtobeproposed.Duetothelowliteraturecoverageinthisarea,onlyquasi-solidelectrolytesarebrieflydiscussedhere.Fanetal. andYuetal.usedP(VDF-HFP)membranestoavoidtheseproblems.Yuetal.used seaurchin-likeNa2 Ti3 O7 ,peanutshell-derivedcarbon(PSC),andP(VDF-HFP) membranesasanodes,cathodes,andelectrolytestoconstructflexiblequasi-solid SICs[161].FlexibleNa2 Ti3 O7 //PSCquasi-solidSICsprovided86%capacitance retentionandnearly100%coulombicefficiencyafter3000cycles.Theprepared Na2 Ti3 O7 //PSCquasi-solidstateSICsdevicesshowednosignificantcapacityloss underdifferentbendingconditions,confirmingtheirsuperiormechanicalstrength. TheflexibleNa2 Ti3 O7 //PSCquasi-solid-stateSICdevicescanpowerupto50red LEDsandshowbothhighpowerandhighenergycharacteristics.
ForflexibleSICdevices,theconceptoftheconfigurationandthedevelopment oftheelectrolytehavebeenbrieflyoutlined.Thedevelopmentoftherelevant detailswillbeprovidedindetailinSections7.1,7.2,and7.3.Webelievethatthese discussionshavegiventhereaderalotofinsight.InadditiontoflexibleSICdevices, anothertopicthatcannotbeavoidedisthefieldofpre-sodiationmethodsinSICs.In pursuitofthegrowingdemandfornewapplicationswithhigherenergydensities,












