Biomass-DerivedCarbonMaterials
ProductionandApplications
Editedby
AlagarsamyPandikumar,PerumalRameshkumar,and PitchaimaniVeerakumar
Editors
Dr.AlagarsamyPandikumar ElectroOrganicandMaterials ElectrochemisryDivision (CSIR)-CentralElectrochemical ResearchInstitute Karaikudi-630003,TamilNadu India
Dr.PerumalRameshkumar DepartmentofChemistry KalasalingamAcademyofResearchand Education,Krishnankoil-626126 TamilNadu India
Dr.PitchaimaniVeerakumar InstituteofAtomicandMolecular SciencesAcademiaSinica(IAMS) NationalTaiwanUniversity,10617 Taipei Taiwan
Cover:©From“RecentAdvanceson PorousCarbonMaterialsfor ElectrochemicalEnergyStorage”by LibinWangandXianluoHu,Chem. AsianJ.10.1002/asia.201800553, CopyrightWiley-VCHGmbH. Reproducedwithpermission.
Allbookspublishedby WILEY-VCH arecarefully produced.Nevertheless,authors,editors,and publisherdonotwarranttheinformation containedinthesebooks,includingthisbook, tobefreeoferrors.Readersareadvisedtokeep inmindthatstatements,data,illustrations, proceduraldetails,orotheritemsmay inadvertentlybeinaccurate.
LibraryofCongressCardNo.: appliedfor
BritishLibraryCataloguing-in-PublicationData Acataloguerecordforthisbookisavailable fromtheBritishLibrary.
Bibliographicinformationpublishedby theDeutscheNationalbibliothek TheDeutscheNationalbibliotheklists thispublicationintheDeutsche Nationalbibliografie;detailedbibliographic dataareavailableontheInternetat <http://dnb.d-nb.de>.
©2023WILEY-VCHGmbH,Boschstraße12, 69469Weinheim,Germany
Allrightsreserved(includingthoseof translationintootherlanguages).Nopartof thisbookmaybereproducedinanyform–by photoprinting,microfilm,oranyother means–nortransmittedortranslatedintoa machinelanguagewithoutwrittenpermission fromthepublishers.Registerednames, trademarks,etc.,usedinthisbook,evenwhen notspecificallymarkedassuch,arenottobe consideredunprotectedbylaw.
PrintISBN: 978-3-527-34926-5
ePDFISBN: 978-3-527-83289-7
ePubISBN: 978-3-527-83291-0
oBookISBN: 978-3-527-83290-3
Typesetting Straive,Chennai,India
Contents
Preface xi
Acknowledgments xiii
1IntroductiontoBiomass-DerivedCarbonMaterials 1 A.Sivakami,R.Sarankumar,S.Vinodha,andL.Vidhya
1.1Introduction 1
1.2BiomassResourcesandComposition 3
1.2.1Plant-BasedBiomass 4
1.2.2Fruit-BasedBiomass 5
1.2.3Microorganism-BasedBiomass 7
1.2.4Animal-BasedBiomass 7
1.3ConditionforPrecursorSelectionofBiomass-DerivedCarbon 8
1.4ProductionMethodsofBiomass-DerivedCarbon 8
1.4.1Carbonization 9
1.4.1.1HydrothermalCarbonization 9
1.4.1.2Pyrolysis 10
1.5Biomass-DerivedCarbons(B-d-CMs)ActivationMethods 11
1.5.1PhysicalActivation 11
1.5.2ChemicalActivation 13
1.5.3CombinationofPhysicalandChemicalActivation 14
1.5.4ModificationandStructuralControlofB-d-CMs 14
1.5.4.1SurfaceModificationandHeteroatomDopingofB-d-CMs 15
1.5.4.2B-d-CMsSurfaceLoadingofMetalOxidesorHydroxides 15
1.5.4.3SurfaceIncorporationwithDifferentNanostructures 17
1.6ProductionProcessDescription 17
1.7CostAnalysis 19
1.8Summary 19 References 20
2IntroductiontoBiowaste-DerivedMaterials 27 ThangaveluKokulnathan,BalasubramanianSriram,Sabarison Pandiyarajan,SubramanianRamanathan,andThangaveluSakthiPriya
2.1Introduction 27
2.2Synthesis 28
2.2.1ActivationMechanismofBW-ACbyPhysicalActivation 28
2.2.2ActivationMechanismofBW-ACsbyChemicalActivation 29
2.2.2.1InfluenceofAlkalineActivatingAgents 30
2.2.2.2InfluenceofAcidicActivatingAgents 31
2.2.2.3InfluenceofNeutralActivatingAgents 31
2.2.2.4InfluenceofSelf-ActivatingAgents 32
2.3Characterization 32
2.3.1ElectronMicroscopes 32
2.3.2HR-TEMAnalysis 34
2.3.3FTIRSpectroscopy 35
2.3.4RamanSpectroscopy 36
2.3.5XPSAnalysis 38
2.3.6XRDPatterns 39
2.3.7BETAnalysis 41
2.4Properties 43
2.4.1SurfaceDefectsinBW-AC 43
2.4.2CharacterizationsofCarbonDefects 46
2.4.3IntrinsicCarbonDefectsActivity 47
2.4.4HeteroatomDopingDefects(or)ExtrinsicCarbonDefectsActivity 48
2.4.5ElectronicBandStructureProperties 48
2.5Summary 50 References 50
3Biomass-derivedCarbon-basedMaterialsforMicrobicidal Applications 63 SelvamuthuPreethi,ArunachalamArulraj,RamalingaViswanathan Mangalaraja,VelayuthamRavichandran,andNatesanSubramanian
3.1Introduction 63
3.2BiomassMaterials 64
3.2.1CarbonandItsDerivatives 65
3.3Microbicidal 66
3.3.1MechanismofAction 67
3.3.2MicrobicidalResistance 68
3.3.3FactorsAffectingMicrobicidalResistance 68
3.4MicrobicidalPerformanceofBiomass-DerivedCarbonaceous Materials 69
3.4.1RoleofMaterialPhysicochemicalProperties 70
3.4.1.1StructuralDestruction 70
3.4.1.2OxidativeStress 73
3.4.1.3WrappingEffect 76
3.4.1.4PhotothermalEffect 77
3.4.1.5ExtractionofLipid 78
3.4.1.6MetabolicInhibitoryEffect 79
3.5BioengineeringProspectiveTowardCarbonaceousMaterials 79
3.5.1WoundDressing 80
3.5.2SurfaceModifications(Coating)onMedicalDevices 81
3.5.3NanoantibioticFormulations 82
3.6Biosafety 83
3.7ConclusionandFuturePerspectives 84 Acknowledgment 85 References 85
4Carbon-BasedNanomaterialsPreparedfromBiomassfor Catalysis 93
A.Rajeswari,E.JackcinaStobelChristy,andAnithaPius
4.1Introduction 93
4.2PreparationofBiomass-DerivedCarbon-BasedNanomaterials 94
4.3Graphene 95
4.3.1PreparationofGraphene 95
4.3.2GraphenefromDifferentSources 95
4.4CarbonNanotubes(CNTs) 99
4.4.1SynthesisofCNTs 99
4.4.2SynthesisofCNTsUsingBiomassMaterials 99
4.5CarbonQuantumDots(CQDs) 102
4.5.1CQDsfromBiomass 102
4.6CatalyticApplicationsofCarbon-BasedNanomaterials 104
4.6.1PotentialAdvantagesinUsingCarbon-BasedNanomaterialsfor AdvancedCatalysts 104
4.6.2Photocatalysts 105
4.6.3ElectroCatalysts 107
4.7Conclusions,FutureOutlook,andChallenges 107 Acknowledgments 107 References 108
5Biomass-DerivedCarbonQuantumDotsforFluorescence Sensors 113
SomasundaramAnbuAnjugamVandarkuzhali,JeyabalanShanmugapriya, ChinnaAyyaSwamyP,SubramanianSingaravadivel,andGandhiSivaraman
5.1Introduction 113
5.2CharacterizationofCDs 114
5.3OpticalProperties 115
5.3.1Absorbance 115
5.3.2Fluorescence 115
5.4MethodsfortheSynthesisofCDs 115
5.4.1HydrothermalCarbonizationMethod 116
5.4.2MicrowaveMethod 116
5.4.3ChemicalOxidationMethod 116
5.4.4Pyrolysis 117
5.5ApplicationofCDs 117
5.5.1MetalIonSensing 117
5.5.1.1Mercury(Hg2+ )Sensor 118
5.5.1.2Iron(Fe3+ )Sensor 119
5.5.1.3Lead(Pb2+ )Sensor 120
5.5.1.4Copper(Cu2+ )Sensor 120
5.5.1.5MiscellaneousMetalIons 122
5.5.2AnionSensors 122
5.5.3MiscellaneousMolecules 123
5.6ConclusionandFuturePerspectives 123 References 124
6Biomass-DerivedMesoporousCarbonNanomaterialsforDrug DeliveryandImagingApplications 129 BalajiMaddiboyina,RamyaKrishnaNakkala,andGandhiSivaraman
6.1Introduction 129
6.2DrugDeliverySystemsBasedonMCNs 130
6.2.1Immediate-releaseDDS 130
6.2.2Sustained-releaseDDS 130
6.2.3Controlled/TargetedDDS 131
6.3PhotothermalTherapy 131
6.3.1SynergisticTherapy 135
6.3.2CellLabeling 135
6.3.3RemovalofToxicSubstances 139
6.3.4TransmembraneDelivery 139
6.3.5PhotoacousticImaging 139
6.3.6TherapeuticBiomoleculeDelivery 140
6.3.7Biosensing 140
6.3.8MagneticResonance(MR)Imaging 142
6.4ConclusionandFuturePerspectives 143 References 143
7MesoporousCarbonSynthesizedfromBiomassasAdsorbent forToxicChemicalRemoval 147 BabuCadiamMohan,SrinivasanVinjuVasudevan,RamkumarVanaraj, SundaravelBalachandran,andSelvamaniArumugam
7.1Introduction 147
7.2SynthesizedMethodsofMesoporousCarbonsfromBiowasteor Biomass 148
7.3ApplicationofMesoporousActivatedCarbons 149
7.3.1RemovalofDyes 149
7.3.1.1GWACasanAdsorbentforMethyleneBlueandMetanilYellow 150
7.3.1.2RiceHusk(RH)-DerivedMesoporousActivatedCarbon(AC)for MethyleneBlue(MB)DyeRemoval 151
7.3.1.3ActivatedCarbonfromRattanWasteforMethyleneBlue(MB) Removal 152
7.3.1.4ActivatedCarbonfromCattailBiomass(CAC)forMalachiteGreen(MG) Removal 152
7.3.1.5WoodSawdustWasteActivatedCarbon(WACF-P)forXylenolOrange (XO)Removal 152
7.3.1.6MesoporousActivatedCarbonfromAgriculturalWasteforMethylene BlueRemoval 153
7.3.1.7MesoporousActivatedCarbonfromEdibleFungiResidue(EFR-AC)for ReactiveBlack5Removal 153
7.3.1.8MesoporousActivatedCarbonfromPlantWastesforMethyleneBlue (MB)Removal 153
7.3.1.9MesoporousActivatedCarbonfrom Corozooleifera ShellforMethylene Blue(MB)Removal 154
7.3.1.10MesoporousActivatedCarbonfromCoconutCoirDustforMethylene Blue(MB)andRemazolYellow(RY)Removal 154
7.3.1.11MesoporousActivatedCarbonfromMacadamiaNutShell(MNS)Waste forMethyleneBlue(MB)Removal 155
7.3.1.12MesoporousActivatedCarbonfromNeobalanocarpusHeimiiWood Sawdust(WSAC)forMethyleneBlue(MB)Removal 155
7.3.2RemovalofMetalIons 155
7.3.2.1UseofChickenFeatherandEggshelltoSynthesizeaNovelMagnetized ActivatedCarbonforSorptionofHeavyMetalIons 157
7.3.2.2Meso/micropore-ControlledHierarchicalPorousCarbonDerivedfrom ActivatedBiocharasaHigh-PerformanceAdsorbentforCopper Removal 158
7.3.3RemovalofPhenolicCompounds 158
7.4ConclusionandFutureOutlooks 165 References 165
8Biomass-derivedCarbonasElectrodeMaterialsfor Batteries 171
P.Vengatesh,C.KarthikKumar,T.S.Shyju,andM.Paulraj
8.1Introduction 171
8.1.1Batteries 172
8.1.2ClassificationofBatteries 172
8.1.3CharacteristicsofBatteries 172
8.2RoleofCarbonwithMechanismofRechargeableBatteries(RBs) 174
8.2.1Li-IonBatteries(LIBs) 174
8.2.2Li-SBatteries(Li-S) 175
8.2.3Na-IonBatteries(SIBs) 176
8.2.4Zn-AirBatteries(ZABs) 178
8.3Biomass-derivedCarbonaceousMaterials 179
8.4ElectrochemicalPerformancesofRBsusingBiomass-derivedCarbon Electrodes 181
8.4.1Li-IonBatteries(LIBs) 181
8.4.1.1Biomass-derivedUndopedCarbonElectrodes 181
8.4.1.2MetalOxides@Biomass-derivedCarbonNanocomposite Electrodes 186
8.4.1.3MetalSulfides@Biomass-derivedCarbonNanocomposite Electrodes 188
8.4.2Na-IonBatteries(SIBs) 189
8.4.2.1Biomass-derivedUndopedCarbonElectrodes 190
8.4.3Li-Sbatteries 195
8.4.3.1Biomass-derivedCarbonHosts 198
8.4.4Zn-AirBatteries 199
8.5Biomass-derivedHeteroatom-DopedCarbonElectrodesforRBs 201
8.5.1Single-Heteroatom-DopedCarbonElectrodes 202
8.5.2Dual-Heteroatom-DopedCarbonElectrodes 204
8.6SummaryandFutureProspectives 206 References 207
9RecentAdvancesinBio-derivedNanostructuredCarbon-based MaterialsforElectrochemicalSensorApplications 215 AkshatMathur,JayashankarDas,andSushmaDave
9.1Introduction 215
9.2ConclusionandFuturePerspectives 224 References 225
10PorousCarbonDerivedFromBiomassforFuelCells 229
A.Sivakami,AristatilGanesan,P.Sakthivel,KishoreSridharan, SabarinathanVenkatachalam,andSudhagarPitchaimuthu
10.1Introduction 229
10.2FuelCells–TheoryandFundamentals 233
10.3CatalystSupportMaterials 234
10.3.1AsaCatalyst 236
10.3.2SynthesisMethodsofPorousCarbonfromBiomass 236
10.4PorousCarbonSynthesisfromDifferentBiomass 237
10.4.1OxygenReductionReaction(ORR) 237
10.5SynthesisofBiomass-DerivedORRCatalystforFuelCell 238
10.6FutureOutlook 245
10.7Summary 245 References 246
11Biomass-DerivedCarbon-BasedMaterialsforSupercapacitor Applications 253
G.Murugadoss,M.Rajaboopathi,M.RajeshKumar,and A.M.KamalanKirubaharan
11.1Introduction 253
11.1.1Capacitor 253
11.1.2Battery 254
11.2Supercapacitor 255
11.2.1TypesofSupercapacitors 255
11.2.2ElectricalDouble-LayerCapacitors(EDLC) 256
11.2.3Pseudocapacitor 257
11.2.4HybridCapacitors 258
11.3ActivatedCarbonObtainedfromBiomassforSupercapacitor Application 259
11.3.1EssentialforCarbon-basedElectrodes 259
11.4ElectrochemicalMeasurements 262
11.5StructuralDiversitiesofBiomass-DerivedCarbonforSupercapacitor Applications 262
11.5.1SphericalStructure 263
11.5.2FibrousStructure 263
11.5.3TubularStructure 263
11.5.4SheetStructure 263
11.5.5PorousStructure 265
11.5.6MesocrystalStructure 268
11.6ConclusionandFuturePerspectives 269 References 269
12Biomass-DerivedCarbonforDye-SensitizedandPerovskite SolarCells 275
N.Santhosh,P.Vijayakumar,M.SenthilPandian,andP.Ramasamy
12.1Introduction 275
12.2DSSCWorkingPrinciple 276
12.3DSSCComponents 277
12.3.1TransparentConductingSubstrate(TCO) 277
12.3.2Photoanode 277
12.3.3DyeSensitizer 277
12.3.4Electrolyte 278
12.3.5CounterElectrode 278
12.4PerovskiteSolarCells 278
12.5TunabilityofBandgapEnergy 280
12.6DevelopmentofPerovskiteSolarCellsfromDye-SensitizedSolar Cells 280
12.6.1WorkingPrincipleofPSC 281
12.6.2PerovskiteSolarCellsArchitecture 281
12.6.3HoleTransportMaterial 282
12.7Biomass-DerivedCarbonCounterElectrodeforDSSC 283
12.7.1PerformanceofDSSCwithCounterElectrodeviaBio-derived Carbon 284
12.7.2Biomass-DerivedCarbonasaCounterElectrodeforPerovskite SolarCells 285
12.8ConclusionandFuturePerspectives 287 References 287
x Contents
13RecentAdvancesofBiomass-DerivedPorousCarbonMaterials inCatalyticConversionofOrganicCompounds 293 N.MahendarReddy,D.Saritha,NaveenK.Dandu,Ch.G.Chandaluri,and GubbalaV.Ramesh
13.1Introduction 293
13.2SynthesisProcedures 295
13.2.1Carbonization 295
13.2.1.1HydrothermalCarbonization(HTC) 296
13.2.1.2Pyrolysis 297
13.2.2Activation 297
13.2.2.1PhysicalActivation 297
13.2.2.2ChemicalActivation 298
13.2.3PhysicochemicalActivation 299
13.2.4Microwave-basedsynthesis 299
13.2.5Functionalization/Doping/CompositesofACs 300
13.3Applications 302
13.3.1HeterogeneousCatalysis 302
13.4ConclusionandFutureChallenges 308 References 309
14SummaryonPropertiesofBio-DerivedCarbonMaterialsand theirRelationwithApplications 317
S.Vinodha,L.Vidhya,andT.Ramya
14.1RemovalofToxicChemicals 321
14.2ElectrodeMaterialsforBatteries 322
14.3ElectrochemicalSensorApplications 323
14.4FuelCellApplications 324 References 329
Index 331
Preface
Biomass-derivedcarbon-basedmaterialsareofgreatinterestbecauseofabundant andeasyavailabilityofbio-precursors,andthematerialswithlowdimensions possesslargesurfaceareaandporositythatallowforavarietyofapplications. Biomass-derivedcarbonisincreasinglypopularinmakingcompositematerials becauseofitscontinuity,interconnection,andporousandhierarchicalstructure. Todate,awidevarietyofcompositematerialsinvolvingbiomass-derivedcarbon havebeenpreparedandusedforinterestingapplications.Thisbookaimstoprovide adeepinsightonthepreparationandactivationprocessesofbiomass-derived carbon,synthesisofcomposites,andfutureopportunitiesontheexplorationof thesematerials.Theintroductorychaptersdealthepossiblesources,synthesis, properties,characterization,activation,andcostanalysisofbiomass-derived carbon-basedmaterials.Theremainingchapterselaboratelydiscusstheapplicationsofbiomass-derivedcarbon-basedmaterials,includingcatalysis,sensors, microbicidalactivity,toxicchemicalsremoval,drugdelivery,andelectrochemical energyconversionandstorageapplications.Thefinalchaptersgiveanoverviewof propertiesofbiomass-derivedcarbonmaterialsandtheirrelationwithapplications. Hence,thisbookgathersandreviewsmultidisciplinaryaspectsofbiomassderivedcarbon-basedmaterialsresearchperformedbychemists,physicists, materialsscientists,biologists,andengineers.Readerscaneasilyunderstand thefundamentalsofbiomass-derivedcarbonmaterialssynthesis,activation processes,properties,characteristics,andtheirroleinthecurrentscenarioof application-orientedresearchanddevelopment.Thisbookwillbehelpfulfor researcherstoestablishtheirownresearchintheareaofbiomass-derivedcarbon materials.
Dr.AlagarsamyPandikumar Scientist
ElectroOrganicandMaterialsElectrochemisryDivision CSIR-CentralElectrochemicalResearchInstitute Karaikudi-630003,TamilNadu,India
xii Preface
Dr.PerumalRameshkumar AssistantProfessor DepartmentofChemistry SchoolofAdvancedSciences KalasalingamAcademyofResearchandEducation Krishnankoil-626126,TamilNadu,India and Dr.PitchaimaniVeerakumar DepartmentofChemistry InstituteofAtomicandMolecularSciencesAcademiaSinica(IAMS) NationalTaiwanUniversity Taipei-10617,Taiwan
Acknowledgments
Wearegratefultoalltheauthorswhocontributedtheirchapterstomakethisa valuablebookandforthesuccessfulcompletionoftheprocess.Wearethankful tothepublishingeditor,Wiley-VCH,foracceptingourproposalandgivingusan opportunitytoeditthisbook,andtheirhelptowardthesuccessfulcompletionofthe workisgreatlyacknowledged.
IntroductiontoBiomass-DerivedCarbonMaterials
Sources,Production,Activation,andCostAnalysis
A.Sivakami 1 ,R.Sarankumar 2 ,S.Vinodha 3 ,andL.Vidhya 3
1 MallaReddyUniversity,SchoolofSciences,DepartmentofPhysics, Maisammaguda,Hyderabad,500100,India
2 QISInstituteofTechnology,DepartmentofElectronicsandCommunicationEngineering, Ongole,523272,India
3 SethuInstituteofTechnology,DepartmentofChemicalEngineering, Virudhunagar,626115,TamilNadu,India
1.1Introduction
Thetransformationinclimateanddemandforenergyencouragesustoconcentrate andcultivateanewtechniqueforgeneratingandstoringenergyviacleanandgreen technologies.Inordertofulfillourrequirementsforbothenergygenerationandstorage,itisnecessarytoproducehigh-performancematerials[1–4].Duetotheirless cost,comparativelysimplepreparation,richness,eco-friendlycredentials,decent conductivity,andstabilityinchemicalandthermalenvironments,carbonmaterials areplacedatthetopmostpositioninthelistamongothermaterials.Thesupplyof differentwastebiomassesisgrowingglobally,andthedisposalofthisbiomassisan issue.Itismostlydisposedofbyburningmaterialsinopenspaces,which,byreleasinggreenhousegases,directlyaffectsouratmosphere.Theconversionofbiomass intousefulmaterialsisabeneficialalternativeforwastemanagement.
Becauseofthewastetorichnessprinciples,thecarbonsextractedfrombiomass attractedadditionalinterest.Inaddition,thecarbonmaterialsderivedfrom biomassdemonstratedbeneficialpropertiesthatbroadenedtheirapplications.At present,byenhancingbenefitoftheirphysicalandfunctionaldiversity,carbon materials,suchasgraphene,carbonnanotubes,activatedcarbon(AC),andporous carbon,areusedintheenergystoringarea.However,demandsforgreenand renewableenergystoragematerialshavebeenspurredbythegrowthofadvanced scienceandtechnology.Asasourceofelectrodematerials,biomass-derivedcarbon gainedconsiderableattentionduetoitsstructuraldiversity,modifiablephysical/ chemicalproperties,environmentalfriendliness,andsignificantworthintradeand industry[5–10].
Sincenatureaddsbizarremicrostructurestobiomass,thecarbonmaterials derivedfrombiomassalsoexhibitnaturalstructuraldiversities,suchas0Dspherical,1Dfibrous,2Dlamellar,and3Dspatialstructures.Inaddition,itisimportantto Biomass-DerivedCarbonMaterials:ProductionandApplications,FirstEdition. EditedbyAlagarsamyPandikumar,PerumalRameshkumar,andPitchaimaniVeerakumar. ©2023WILEY-VCHGmbH.Published2023byWILEY-VCHGmbH.
1IntroductiontoBiomass-DerivedCarbonMaterials
moldthecharacteristicsofthebio-derivedcarbonmaterials(B-d-CMs)according totheirapplication.Differentapproachesformanufacturingandmodifyingcarbon materialsarepursued.
Thereareseveralgeneralpropertiesofcarbonmaterialsthatmakethemattractiveindifferentapplications.Carbonisacommoncommodity,indicatingthatit haslongbeenusedbyindividuals.Graphite,carbonblack(CB),andACmaterials areincludedintheselong-usedtraditionalcarbonmaterials[11–14].Newcarbon materialswithcustomizedpropertieshavebeendevelopedinthepastcentury.This includedcarbonfibers,graphitethatwasstronglyfocused,andseveralothers.
Muchmoresophisticatednanosizedornanostructuredcarbonmaterialshave beendevelopedinrecentdecades.Carbonmaterialsarecurrentlybeingintensively researched,inparticularthenewestnanocarbons,butalsomacroscopiccarbons suchascarbonfibers[15,16].Duetotheirdistinguishedphysicochemicalproperties,innovativecarbonmaterialsnamelygrapheneanditsderivatives,fullerene, andcarbonnanotubeshavegainedsignificantinterestintheareaofenergystoring nowadays.Theyhavegoodconductivity,outstandingchemicalstability,porosity thatcanbetuned,largespecificsurfacearea,andenrichedelectroactivesites enriched.Porouscarbonnanomaterialshavegainedsignificantattentionbecause oftheirphysiochemicalpropertiesandhighsurfacearea.Thecarbonwithdifferent
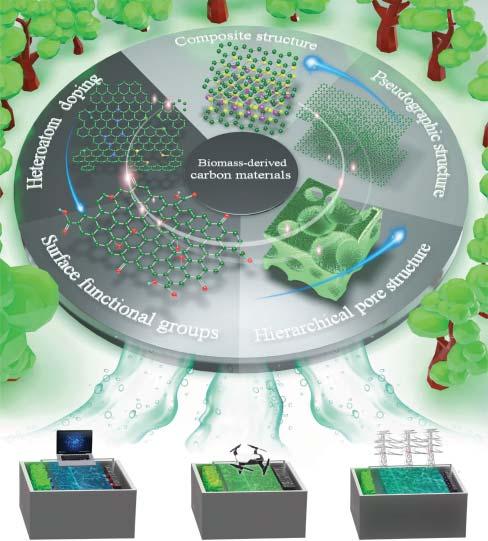
Figure1.1 B-d-CMsstructurestrategiesfordifferentEESapplications.Source:Ref.[19]/ AmericanAssociationfortheAdvancementofScience/CCBY4.0.
1.2BiomassResourcesandComposition 3 poresizeshasattractedsignificantattentionforhighlyefficientelectrochemical storageapplications[17,18].
However,thesecarbonmaterialsdependonprecursorsbasedonfossilfuelsusing energy-consumingsyntheticmethods(e.g.chemicalvapordeposition,discharge ofelectricarcs,andlaserablation)thataretoxicandexpensivetotheatmosphere. Whilethesesyntheticmethodsareadvancedtechnologyonabenchscale,due tocomplexsyntheticprocesses,theyarenotyetreadyforcommercialization. Consequently,theestablishmentofmoreeffective,environmentallysustainable andeconomicapproachestotheprocessingofcarbonmaterialsisimportant.
B-d-CMsareshowinggreatimportance,andeffortshavebeendevotedfor enhancingtheperformanceofelectrochemicalstorageapplications[19].Itis essentiallytoknowhowthestructuredesignanddiffusionkineticsofB-d-CMsare affectingtheperformanceofelectrochemicalenergystorage(EES)devices.Itis showninFigure1.1.
1.2BiomassResourcesandComposition
Biomassreferstoanimal-andplant-basedmaterialsorby-productsthatmayserveas apotentialenergysource.Protein,carbohydrates,starch,lignin,andlipidsconstitute biomassandaresuchcomponentsthatdifferdependentonthegeographicsituation andsource.Proximateandfinalstudieshaveshownthatbiomassisabundantin carbon,hydrogen,oxygen,andnitrogen,andtracesofchlorineandsulfurarealso shown.Biomass-derivedcarbonhasmanycrucialadvantagesassociatedwithadditionalelectrodematerialsforenergyandecologicalapplications,suchascheapand plentifulsupply,environmentallysafe,insitunanoporousstructureestablishment, andprocessingelasticity[20–24].Asthesourceaffectsthefinishingcarbonreturn anditsstructuralfeatures,whicharemandatoryforenergystoringandecological applications,theoptionofabiomassprecursoriscrucial.
Agro-residuesfromcropproduction,solidwastefrommunicipal,andfurther agro-basedmanufacturingunitsarekeysourcesofprecursors.Owingtothelarge availabilityandlesscost,theseprecursorshavegainedalotofattention.They do,however,havevariouschemicalfunctionalities,creatingthemanidealchoice forawiderangeofmorphologiesfortheproposalofcarbonmaterials.Biomass derivativecarbonsaredeliberatedtobefavorableelectrodematerialsfordifferent formsofelectrochemicalenergystoringandtransformationsystemsduetothe aforementionedadvantages,includinglithiumbatteries,supercapacitors,potassiumbatteries,sodiumbatteries,andfuelcells[25–29].Giventhequickgrowthin thissector,athoroughanalysisandcomparisonoftheirmanufacturingapproaches, features,applications,andperformanceintheseelectrochemicalenergystoring applicationsarenotonlynecessarybutalsourgent.
Thecarbonpowderthatcompriseseggwhite,bacterialcellulose,mushrooms, peelsoforange,humanhair,dryelmsamara,chitin,catkin,etc.hasbeenused tomanufactureawidevarietyofbiomaterials.Thesebiomassproducts,however, canbeclassifiedintofourmaingroupings,i.e.biomassbasedonmicroorganisms,
1IntroductiontoBiomass-DerivedCarbonMaterials
Table1.1 Differencebetweenbiochar,activatedcarbon,andcarbonblack.
BiocharActivatedcarbonsCarbonblack PrecursorsBiomassCoal,asphalt,and biomass.
Carbon content
structural features
Preparation method
Petroleum,coaltar, andasphalt.
40–90%80–95% >95%
Amorphousand porouscarbonwith enrichedsurface functionalities
Mediumtemperature pyrolysis(400–600 ∘ C), followedbyphysicalor chemicalactivations
Amorphouscarbonand highlyporous
High-temperature carbonization (700–1000 ∘ C)with physicalorchemical treatments
Source:Ref.[30]/withpermissionofAmericanChemicalSociety.
Microcrystalor amorphouscarbon particles
Combustion processwithlittle orwithoutair
animalbased,plantbased,andfoodbased.Itbecomesmoredifficulttopredictthe concludingarrangementandconstructionofaderivedbiochar.Generallyspeaking, itisdifficulttounderstandtheawarenessoftheelementalandchemicalcompositionofbiomassastheresponsescanhappeninthephasesofcarbonizationand stimulation.Themorphologyandstructureofresultingcarboncouldeventuallybe changedbytheseinducedreactions.Table1.1liststhedifferencebetweenactivated, biochar,andCB[30].
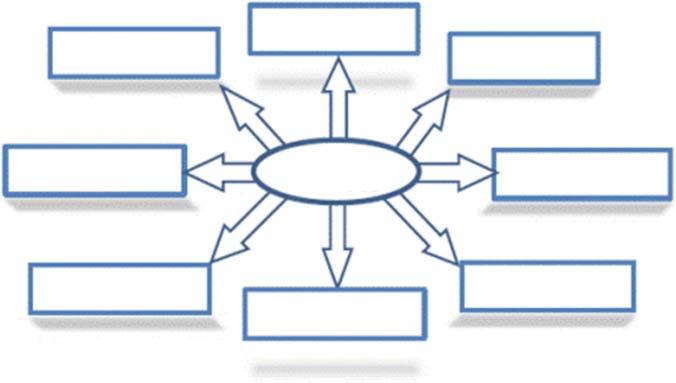
Significantresearchstudiesaredoneinpursuitofbiomassthatatthesametime receivesfunctionalgroupscontainingoxygenornitrogen,interrelatedmicroor mesoporousarrangements,andsimilarlyhasalargecarboncontentproductionthat amplifiestheapplicationoftheenvironmentandresources.Mostadvancedprecursors,however,endupwithlowyields.Thebiocharisaderivativeofwillowcatkins, forexample,provedexcellentpresentationinperformance,capacitance,andcycling asenergystoragedevices.Thefinalcarbonoutcomewassignificantlylesser(5.5% wt.)relativetosimilarbiomassprecursorsincludingricestraw,consideringthese benefits[31–34].Thebiocharoutcome,heteroatom,anddopingofthebiochar dependgreatlyontheprecursors’basiccompositionandchemicalstructure.Itis thusimportanttodiscoverandunderstanddifferentprecursor-relatedpropertiesin ordertoenhancebiocharoutcometocreateitappropriatelyinenergystoringand ecologicalapplications.ThedifferentbiomassresourcesaregiveninFigure1.2.
1.2.1Plant-BasedBiomass
Thenumericalchemicalcompositionofplant-basedbiomassvariesaccording togeographicfactors,categorizationsoforganisms,andorgandependency.Still, cellulose,lignin,hemicellulose,andextractivesconsistofthequalitativechemicalconfigurationsofplant-basedbiomass[35,36].Forinstance,seedshells, palm,areca,etc.consistofsubstantialligninand83%ofcellulose.Whereas,for
Agricultural crops and residues
Marine processing wastes
Animal processing wastes
Biodiesel production waste
Biomass sources
Forestry crops and residues
Municipal solid and sewage waste
Agro/food industrial wastes
Fermentation process waste
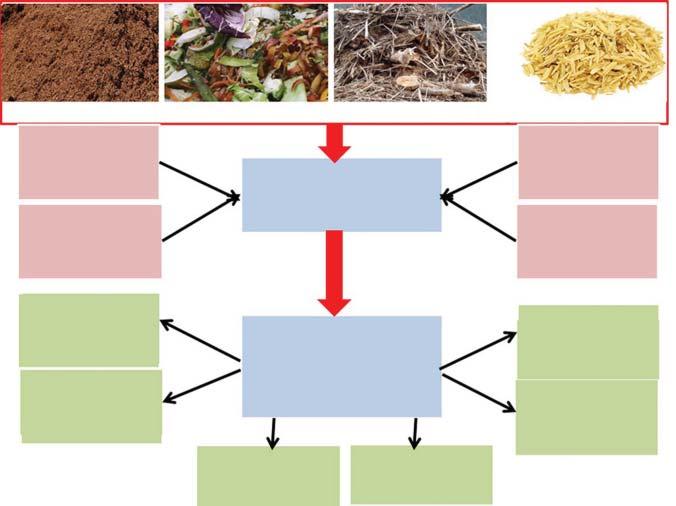
Figure1.2 Popularbiomassresources.
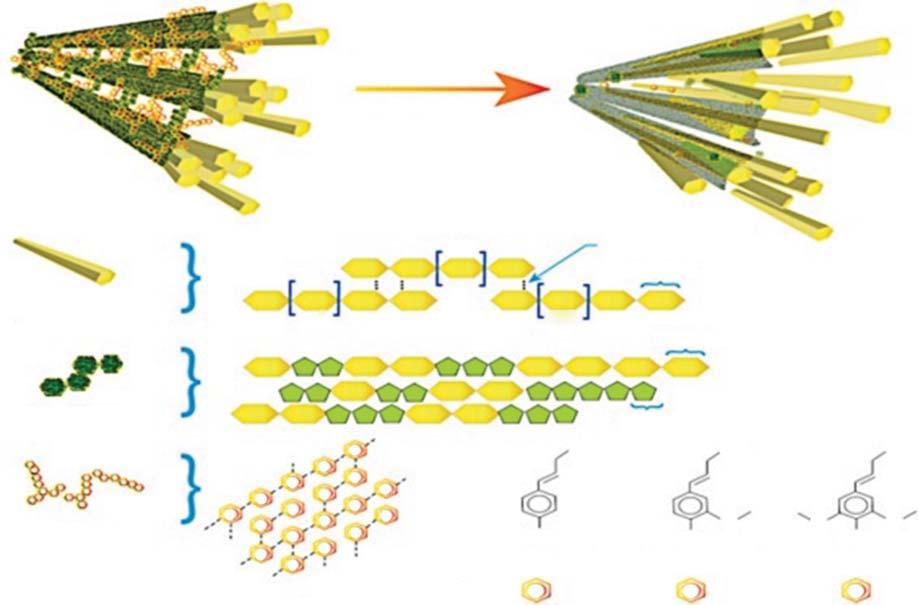
instance,plant’sbastfibersaregoodincellulose,andincotton,jute,andfax,bast comprises67,64,and56%comparativelyhighercellulosewithrespecttoother partsoftheplant.Figure1.3showsthechemicalcompositionoflignin,cellulose, andhemicellulose.Investigationsonthebasiccompositionofprecursors,suchas theinvolvementofmicrostructure,capacitance,andconductivityinprecursorsof oxygenandnitrogencontent.
Highoxygencontentinprecursorshasbeendeterminedtoyieldlowcrystallinity andlargerdefects,andmoreunstablemixesareproducedintheprocessofpyrolysis andthermaldecay;however,higherlevelsofnitrogencontentcouldyieldenhanced electrochemicalfeaturednitrogen-dopedcarbon.
Comparedtolignin,celluloseandhemicelluloseofferlessstabilityinthermal decompositionandleadtoconsiderablylesscarbonyields.Nevertheless,lignin hemicelluloseandcellulosecomponentscontributetobiocharyieldporosity.Itwas notedintheprocessofthermalpyrolysisat500 ∘ Cthatnonoteworthyrelations betweenhemicelluloseandcellulosewereseen,butobviousinteractionsbetween thecomponentsofligninandcellulosewereobserved.
Therefore,inordertogetbiocharoutcomewithworthyconductivity,controllable faults,andahighdegreeofgraphitizationtomakeitsuitableforapplicationin ecologicalandenergystoring,itisimportanttochooseplantbiomassprecursors richinnitrogencontentandlessinoxygencontent,cellulosefraction,andlignin fraction.
1.2.2Fruit-BasedBiomass
Thequantifiableelementalandchemicalcompositionoffruit-basedbiomasscan varywithregardtogeographicalparametersandspecies.Themainfruit-based biomasscomponentsaresugars,lipids,ash,crudeproteins,andfibers.Thelipid quantityinthepeelsandpulpvariesfrom0.7to9.96%and1.4to28.6%,whilethe
High temperature and pressure
Oxidizing agents
Residence time Explosive decompression
Figure1.3 Plant-basedbiomassconstituents.Source:Ref.[36]/withpermissionofElsevier.
Untreated cell wall
Crystalline cellulose
Hemicellulose
Lignin
Hydrogen bond
Glucose
1.2BiomassResourcesandComposition 7 crudeproteinyields3.5to28.6%and5.8to43.4%,respectively.Largerlevelsof proteinsandcrudelipids,however,leadtodisadvantagesintheultimatebiochar outcome,astheseproteinsandcrudelipidsstartdegradingwhentemperatureis low,exemptingunstablecompoundsnamelywatervapors,methylesters,olefins, carbondioxide,andammoniafumes.Ontheotherhand,thepresenceofnitrogen andphosphorouscontentinproteinsandcrudelipidscanleadtoheteroatom-doped carbonproduction[25,37,38].Thecrudefibersoflignin,hemicellulose,and cellulosearethemainproviderstotheprocessingofcarbon.Themassportionsof crudefibers,however,areconsiderablylessandtypicallygoodincellulose,which affectsthegraphicstructureandbiocharyield.
1.2.3Microorganism-BasedBiomass
Newmeasurementsopenuptheprospectofusingmicroorganismsderivedfrom biochar,suchasbacterialcelluloseandfungi.Thefungi,suchasmushroomsand yeasts,haveevidencedtobeimprovedprecursorsofreformativebiomassforbiochar derivation,attributingtheirrapidlyincreasingcapacityandtheireaseofusein environmenttobulk.Carbohydrates,crudeproteins,fibers,andfatsarethemain elementsofmicroorganism-basedbiomass.Plant-andfruit-derivedbiomassare thekeycomponentsfoundinthemicroorganism-basedbiochar.Buttheindividual compoundsandelementsfromthesemodulesareconsiderablydifferent.
Thecarbohydratesexistinginthemicroorganismincludechitinsthatestablish aglucancross-link,servingasthemainsourceofcarbonintheprocessofpyrolysis,whilesucroseandstarcharethesourceofcarbohydratesinnon-cross-linked plantswiththelowestthermalstability.Inmicroorganisms,crudefibersareprimarilymadeofcellulose,whichshowsthesameplantorfruitbiomasscarbonization behavior.
Themainprecursorsaremushroomsbasedonmicroorganism-basedprecursors. Comparedtootherelementssuchasmycelium,thefruitingsectionofthemushroom ismostcommonlyusedforprocessingasitgoesintodepth.Owingtotheexistenceof largenitrogencontent,mushroomsareanattractivechoice.Themushroom’snitrogencontentvariesfrom3to10%and17%nitrogencontentisobservedforsome species.Astheyareabletogeneratecarbonderivedfromnitrogen-dopedbiomass, themushroomprecursorsarepromising[39–41].Geographicalfactors,however, haveamajoreffectonthecompositionoftheelementsandcandifferfromregionto region.
1.2.4Animal-BasedBiomass
Chitinisanalternativepositivebio-precursorforawidespreadvarietyofapplicationsowingtotheexistenceoflargernitrogenconcentrations,chemical stabilityandlargeexistenceintheenvironment.Chitiniscapableofcreating chitin-catecholamineandchitinglucancomplexcross-linknetworksandisableto formintermolecularhydrogenbonds.Comparedwithcellulose,chitinhaslarger thermalstabilityandcarbonoutcome.Chitinextractioncanbeusedforthepopular
8 1IntroductiontoBiomass-DerivedCarbonMaterials
animalspeciescontainingmollusks,pests,andcrustaceans.Thecrustaceans consistofasubstantiallyhighamountofchitincontentrangingfrom17to72%,i.e. Carcinus, Pandalus, Carangon,and Cancer [42,43].
Likewise,thecuticlesandsloughsofmanytypesofinsects,suchas,butterflies Holotrichiaparallela,andsilkworms,haveextraordinarychitincontentconcentrationsrangingfrom18.4to64%.
Chemicaldemineralization,deproteinization,andmechanicalcrushingareused toremovethechitincontentfromthebiomass.Thefinalextractionyielddepends onbiomassprecursors,anditrangesfrom4to40%intheprocessingsystem. Chitin’snitrogencontentisextractedfromanimalbiomassandmatcheswith thatofmicroorganism-basedbiomasswithnitrogenconcentrations,capableofits appropriatenessinenergyandecologicalapplications[44,45].
1.3ConditionforPrecursorSelectionof
Biomass-DerivedCarbon
Thefollowingconditionsmustbeaddressedinordertosynthesizegood-quality biocharbymeansofsuperiorconductivityandporosityandtosatisfytheneedsfor hugeecologicalandenergyapplications.
Theexistenceofnitrogencontentimprovesnitrogen-dopedcarbonproduction withgreaterconductivityandenhancedcyclingstability.Still,itisimportantto selectprecursorshavinglessoxygencontent,orelsethearomaticcarbonformation wouldbeobstructed.Theexistenceofstronglycross-linked,largemolecularweight withthermalstabilitybiomacromoleculesincludinglignin,keratin,andchitin enablestheformationofaromaticcarbonandintheprocessofcarbonization providessuperiorbiochar.Aliphaticcompoundsmustbepreventedbytheexistence oflittlecontentsofnoncrosslinkedandmolecularweight,otherwisetheyhinder aromaticcarbonformations.
1.4ProductionMethodsofBiomass-DerivedCarbon
Numerousactivationandvariousmethodsofcarbonizationcanbeusedtoturn biomassintocarbon.Inordertoturnbiomassintovalue-addedcarbongoods, physical,chemical,andacombinationmaybeused[16].
Incarbonmaterials,manyfactorsincludingsurfaceproperties,temperature,time, reagents,andavailabilitycauseaneffect.Thekeyprocessesusedtoextractcarbon frombiomassarepyrolysisandhydrothermalcarbonization(HTC).Pyrolysisis performedatadefinedtemperaturelevelinarestrictedoxygenorinertatmosphere environment,whereasathermochemicalmechanismisusedfortransforming biomassintocarbon.Temperature,temperaturerampingrate,catalyst,andparticle sizearetheproductsobtainedfrombiomasspyrolysis.HTCisdonewithorwithout theuseofacatalystinapressurizedaqueousatmosphereatlesstemperaturerange from120to250 ∘ C.Comparedtonaturalbiomasscoalification,theHTCprocedure
(500–1000 °C)
Single or combined with activation
1.4ProductionMethodsofBiomass-DerivedCarbon 9
(120–250 °C)
Single or combined with activation
Activated biochar
(< 500 °C)
Steam (800–1200 °C) Carbon dioxide (800–1200 °C) Chemical activation
H3PO4 (Low SSA)
ZnCl2, FeCl3 (Dehydrating agent)
KOH, NaOH (High SSA)
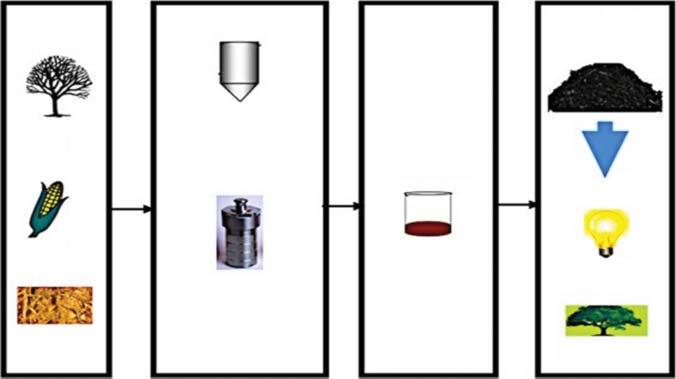
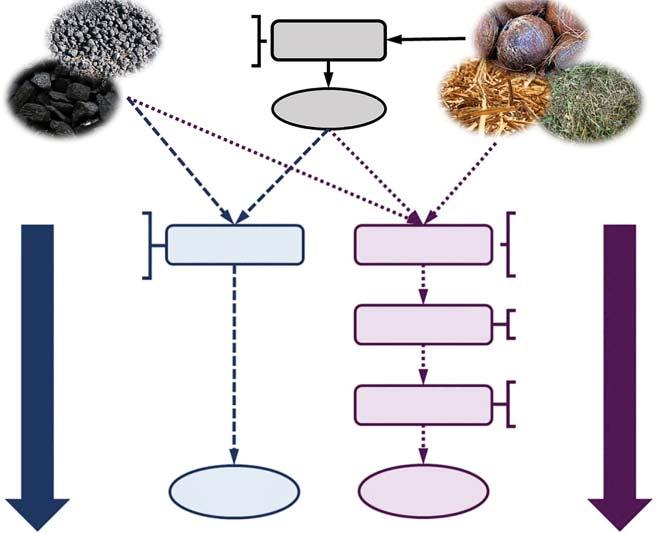
Figure1.4 OverviewofoverallproductionmethodsofB-d-CMs.Source:Ref.[36]/with permissionofElsevier.
isdoneatarateofhigherreactionaddedwithasmallerreactionlength.Various publicationshavestudiedhydrothermalconversioninrecentresearch[46–48].
TheoverviewofdifferentproductionmethodsofB-d-CMsisshowninFigure1.4.
HTCisathermochemicalconversionmethodthatrequiresavarietyof componentsincludingprecursorconcentration,catalyst,residenceperiod,and temperature.Itusessubcriticalwaterstotransformbiomasstocarbonproductsfor successfuldehydrationandhydrolysisofhydrocharprecursorswithhigh-oxygenrichfunctionalgroups.Throughtheuseofadditivesordoping-containing precursors,otherfunctionalgroups,includingnitrogengroups,mayalsobeadded tohydrochars.Recoveredcarbonproductshaveattractedinterestinawiderangeof uses,includingenergyharvesting,catalytic,andtraptechnologies.
ThemethodusedfortransformingcarbonmaterialsintoACisactivation.For activation,chemicalandphysicalmethodsmaybeintroduced.Physicalactivation bypyrolysisisachievedat1200 ∘ Cinthepresenceofcarbondioxide.Inthepresenceofachemicalagent,chemicalactivationtakesplaceattemperaturesbetween 450and900 ∘ C.ThemostusedchemicalactivatorsareNaOH,KOH,K2 CO3 ,FeCl3 , H3 PO4 ,andZnCl2 [48].
1.4.1Carbonization
1.4.1.1HydrothermalCarbonization
ThehydrocharmaterialproducedbythemethodofHTCispartlycarbonized andcontainsoxygengroupsoflargedensity.Thefinalyield,however,depends ontheprecursorfeaturesemployed.Forenergystorageapplications,hydrochars arestraightawayusedaselectrodes.Poorporosityandsmallspecificsurfacearea arenaturallypresentinthehydrocharformedbyHTC.Consequentinitiation orcarbonizationiscompulsorytochangeitschemicalandphysicalproperties.
1IntroductiontoBiomass-DerivedCarbonMaterials
Forsupercapacitorapplications,Zhuandhisteamcreatedelectrodesderivedfrom fungibyhydrothermal-assistedpyrolysis[49].Initially,at120 ∘ C,HTCisdone forsixhours.Withlargeoxygencompositionandlesssurfacearea,theparticles obtaineddifferbetween50and200nm.
Hydrocharisexposedforthreehourstoadditionalpyrolysisat700 ∘ Cinorder toincreaseelectronicconductivityandenhanceporosity.Afixedsurfaceareaof 80m2 g 1 withanoxygencontentof5wt.isthefinalcarbonproductobtained. ComparedwithotherindustrialAC,thecontentdisplaysaparticularcapacitance of196Fg 1 .Theporouscarbonmaterialsdependentonhydrocharexhibitahigh numberofheteroatoms.ViaactivationusingKOH,Salinas-Torresandresearch workgroupestablishedACbydopingwithOandNheteroatoms[50].Atlarge currentdensities,theexistenceofbothnitrogenandoxygengroupsincreasescharge transmissionandcapacitance.UniformoxygenhasbeendevelopedbyWeietal., whichshowsalargespecificsurfaceareaandporousstructure.
ThecompleteoverviewofcarbonizationmethodsofproducingB-d-CMsisshown inFigure1.5.
1.4.1.2Pyrolysis
Inanoxygen-limitedatmosphere,biomasspyrolysisiscarriedoutwhenthetemperaturerangesfrom300to1200 ∘ C.Pyrolysisiscategorizedintofastpyrolysisand slowpyrolysisdependingontherangeofheating.Themethodofslowpyrolysishasa longerresidenceperiodandlowerheatingrates.Itiscarriedoutatthetemperatureof
Figure1.5 Completeoverviewofcarbonizationprocess.Source:Ref.[51]/with permissionofElsevier.
1.5Biomass-DerivedCarbons(B-d-CMs)ActivationMethods 11
400–600 ∘ C,resultinginmaximizedbiocharyieldandlowbio-oilandsyngasproduct yields[52].Quickpyrolysis,ontheotherhand,possesseslargerheatingrateand lowerresidenceperiod,providingthevalueofmaximizedbio-oiloutcomeupto75%. Temperatureisamainparameterinregulatingthepyrolysisprocessandthenaffectingthebehaviorandbiocharoutcome,relativetorateofheating,reactiontime,and particlesizeoffeedstock.
Growingpyrolysistemperaturedecreasestheyieldofbiochar,theabilityofcation exchange,andthequalityofnutrients,butincreasesitsdegreeofaromatization, specificsurfacearea,largerrangeofheatingvalue,andsolutionpH.Inaddition, biochargeneratedatalowertemperatureofpyrolysishasalowerstablefraction ratiothanbiocharproducedathighertemperatures[53].Duetoitslessconductivity, poorerporecharacteristics,andlessspecificsurfacearea,biochardevelopedatlower temperaturepyrolysisisnotappropriateforuseasenergystoringandconversion materials.Therefore,surfacealterationandactivationproceduresarerequiredprior totheirimplementation[54].
1.5Biomass-DerivedCarbons(B-d-CMs)Activation Methods
ThetwobasicmethodsthatareappliedtoachieveACbasedonbiomassare physicalandchemicalactivation.Physicalactivationiseasierandmoreenvironmentallyfriendlythanthechemicalactivation,whichisnormallydoneathigher temperatures.
1.5.1PhysicalActivation
Overall,varioussuitabilityandacceptableactivationmethodscouldbealtered andmaterialfeaturescouldbedifferent.Itisafamousfactthat,asopposedto physicalactivation,chemicalactivationneedslessactivationperiodandtemperature.Chemicalactivation,however,hasshownmanysignificantdrawbacks comprisingofwaterwashingstepafteractivationthatisneededandneededto eradicate≪Reviseas“ thatisneededtoeradicate ”impurities.Normally,physicalactivationdeploysatwo-stepprocedure.Biomasscontentisinitiallypyrolyzed tocreatebiochar,whichisthentriggeredusinggases,namelysteam,CO2 ,air,or theirmixturebycontrolledgasification[23,56].Disorganizedcarbonsaremade intheprocessofpyrolysisfromtardecomposition,blockingbiocharpores,and minimizingtheirparticularsurfacearea.
ThecompleteoverviewofdifferentactivationmethodsisshowninFigure1.6. Thesuccessivelyregulatedgasificationisabletopromotefurtherdecomposition oftheas-preparedbiocharandgetafullycreated,usable,andinterrelatedporous structure.Porosityproductionalsoyieldsfromcarboncalcinationandgeneration ofvolatilesubstances,anddependingheavilyonthetriggeringgas.CO2 issafeand easyforusage,soitisalwaysused.Activationperiod,temperature,rateofgasflow, andfurnaceselectioncaninfluencethedegreeofcarboncalcination.Byactivation
Figure1.6 Overviewofcompleteactivationmethods.Source:Ref.[55]/FrontiersMedia S.A./CCBY4.0.
ofCO2 ,Zhangetal.[57]developedcarbonsderivedfrombiomassfromvarious forestryareaandagriculturalresiduetypes.Ingeneral,highersurfaceareasand microporevolumecanbenefitfromthehigheractivationtemperature.Guoetal.[58] researchedtheactivationofcarbon-basedcoconutshellbyCO2 andmethodically observedtheeffectsontheparticularsurfaceregion,microporevolume,andtotal volumeofactivationtemperatureflowrateandtime.
Theirfindingsshowedthatincreasingactivationtemperatureaidedtheformationofpores,expandedporediameter,andincreasedgenerationofmesopores. Inaddition,thegenerationofmicroporesandmesoporeswasfavoredbyincreasing activationtime,butexcessivelylengthyactivationperiodledtoporescollapsingand deteriorating.Taeretal.[59]investigatedtheCO2 activationofcarbonextracted fromrubberwoodandtestedthecapacitiveandelectrochemicalcharacteristicsof theACcontent.Withtheriseinactivationtemperatures,theconductivityandbasic capacitanceoftheirfindingsincreased.Theincreasedspecificsurfaceareainduced bytheincreasingtemperatureofactivationwasstraightwayconnectedtotherise inthevolumeofmicroporesinthecarbonprecursor.Though,poredeformation resultedfromalargertemperatureabove900 ∘ C[60,61].Second,duetoitscheap priceandease,steamisoftenusedinbiomassmaterialsasanactivationagent.
Demiraletal.[62]examinedolivebagassesteamactivation,andtheirfindingsexhibitedthatriseinactivationperiod(30–45minutes)andtemperature (750–900 ∘ C)couldincreasethetotalporevolumeofthespecificsurfaceregion.
1.5Biomass-DerivedCarbons(B-d-CMs)ActivationMethods 13
Still,additionalenhancementinactivationperiod(60minutes)decreasedtotal volumeandtherealsurfaceareaofthepore,asthecreationandexpansionof microporeswerelesssuccessfulthantheworseningofhighporosity.Chang etal.[63]andOkadaetal.[60]publishedsimilarfindings.Theporestructures weremainlycontributedbymicropores,closetoCO2 activation,andtheratioof microporevolumetototalporevolume(Vmicro/VT)wasbetween0.63and0.84. Thus,allmethodsofactivationofCO2 andsteamledtowell-builtmicroporosity. ComparedtoCO2 activation,however,steamactivationfavorsthewideningof microporosityandincreasesthegrowthofmesoporesatthecostofmicroporosity.
1.5.2ChemicalActivation
AiractivationneedslesstemperaturecomparedtotheCO2 andsteamactivation mentionedearlier.Nevertheless,sincetheycommonlygrowahierarchicalporosity, steamandCO2 activationsareoftenused.Researchonbiomassphysicalactivation isstillincomplete,incomparisontochemicalactivation.Thereisaneedforfurther methodicalresearchandevaluationsofCO2 ,air,andsteamactivationsofcarbons derivedfrombiomass,particularlyintheapplicationscenariosinthefieldofelectrochemicalenergystoring.Thermalpreparationofthebiomasscarbonprecursor andthetriggeringagentinthe450–900 ∘ Ctemperaturerangeispartoftheprocess ofchemicalactivation.Incontrasttophysicalactivation,chemicalactivationneeds lesserpyrolysistemperature,improvedcarbonoutcome,carbonyieldedwithlarge surfacearea,andproperlyarrangedanddefinedmicroporousstructure[64].
Thelargesurfaceareaandproperlyarrangedanddefinedmicroporousstructureof thecarbongeneratedshowavitalroleinecologicalandenergystoringapplications.
KOHiswidelyusedasanagentforchemicalactivation,performingasanoxidant andformingoxygenfunctionalgroupsonbiochar.Therefore,notonlyelectrochemicaldouble-layercapacitancebutalsopseudo-capacitancecanbecontributedby KOH-ACs.KOHfirstdehydratesintoK2 Oat400 ∘ Cintheactivationphase.Then, withthedevelopmentofH2 ,carbonreactswithH2 O,followedbyCO2 formation. K2 OreactsandformsK2 CO3 withCO2 .Oncethetemperatureisincreasedbeyond 600 ∘ C,itrespondscompletelytoKOH.K2 CO3 beginstodecomposeabove700 ∘ C andisabsentfromthesystemat800 ∘ C[65].Simultaneously,metallicpotassiumis formed.TogenerateCOathighertemperatures,theCO2 producedwillreactwith carbon.
ThethreekeymechanismsforporosityproductionbyactivationofKOHaregenerallyaccepted.AredoxreactiondecomposesthecarbonmatrixwithKOH,leading totheformationofabundantmicro-andmesopores.TheformationofH2 Oand CO2 helpsintheproductionofporosity.Afterextractingthemetallicpotassium andotherpotassiumsubstancesbyrinsing,asshowninthefigure,expandedcarbon latticesintegratedwithintermediatemetallicKareunabletorestoretheiroriginal structure.Therefore,onthebasisofthesynergisticeffectsofphysicalandchemical activation,microporosity,carbonlatticeexpansion,andhighspecificsurfacearea areformed.Variousprecursorsaredevelopedintoporouscarbonmaterialbymeans ofdistinguishingmorphologicalcharacteristics,poretexture,andsurfacefunctional
Figure1.7 KOHactivationmechanism.Source:Ref.[66]/withpermissionofElsevier. groupsbycontrollingtheactivationparameters,furtherinfluencingthebehaviorof KOH-ACsintheirelectrochemicalenergystoringsystemapplications[66].TheKOH activationmechanismisshowninFigure1.7.
Pengetal.[67]developedcarbonsthroughleftovertealeavesvialargetemperaturecarbonizationandactivationprocesswithKOH.Thecarbonmaterialsdemonstratedaporousstructurewithalargespecificsurfacearearangingfrom2245to 2841,andwhenmeasuredinathree-electrodedeviceina2MKOHelectrolytesolutionitshowedperfectcapacitivebehaviorswithamaximumspecificcapacitance of330Fatacurrentdensityof1A.Toenhancespecificsurfaceareaandporevolumefurther,theKOHactivationmethodiscombinedwithCO2 activation.The arrangementofCO2 gasification,however,leadstoadecreaseinfunctionalgroupsof oxygen,decreasingthecontributionofpseudo-capacitancetotheoverallrealcapacitance(upto30%loss).Therefore,thegroupingofactivationofKOHandCO2 yields verycomplicatedeffectontheelectrochemicalfeaturesofbiomass-derivedcarbon.
1.5.3CombinationofPhysicalandChemicalActivation
ThecombinationofbothphysicalandchemicalactivationshowsbetterporesizedistributionandporositydevelopmentofACthantheindividualone.Thistwo-stage mechanismcomprisesofinitialchemicalactivationusingdifferentagentsthenfollowedbyphysicalactivation.Thehighmesoporecontentandsurface-area-modified ACareobtainedandreportedbyseveralauthors,whichisutilizedinbothchemical andphysicalactivationprocesses[68–70].
1.5.4ModificationandStructuralControlofB-d-CMs
TheelectrochemicalperformanceofB-d-CMsdependsupontheirconsistencyof surfacefunctionalities.Themicro-,meso-,andmacropore-sizedACsareproduced basedontheirapplicationswithchoosingsuitableactivationprocessthatisalready known.Thedouble-layerpropertiessuchascapacitance,self-chargecharacteristics, electricalconductivity,andwettabilitycanbealteredbysurfacefunctionalitiesof B-d-CMswithoxygen-containingcarboxyl,hydroxyl,andphenolicgroups[71–74]. Thereareseveralmethodsavailabletoimprovetheelectrochemicalperformance ofB-d-CMsviasurfacefunctionalitiesmodification,heteroatomdoping,surface
Resources
Treatment
Categories
Details
Explanations
Carbonization Biomass Activation
Hydrothermal carbonization (HTC)
Properties
Applications
Pyrolysis
120–250 °C300–1200 °C
Partially carbonization with oxygencontaining groups; usually combined with pyrolysis or activation One step or Combined with activation
Enriched specific surface area and porosity; Tunable pore size distribution
Chemical activation Physical activation
KOH, NaOH
ZnCI2, FeCI3 H3PO4 CO 2 Steam Air
1) Relatively low SSA; 2) P-doping: 3) Contributing to pseudo-capacitance; 4) Wider voltage window:
1) Usually one step: 2) Acting as dehydrating agents;
1) The most common chemical activation agent 2) High SSA; 3) Lower degree of graphitization;
Modified surface chemistry and heteroatoms/functional groups
Surface modification
Doping Loading Incorporation
Relatively low temperature (<500 °C)
One of the most useful activating agents (800–1200 °C)
1) The most commonly used physical activation process (800–1000 °C): 2) Relatively higher degree of graphitization in comparison to traditional chemical activation methods;
Controllable morphologies and structures
SupercapacitorsLithium/sodium/potassium batteriesHydrogen storage and fuel cells
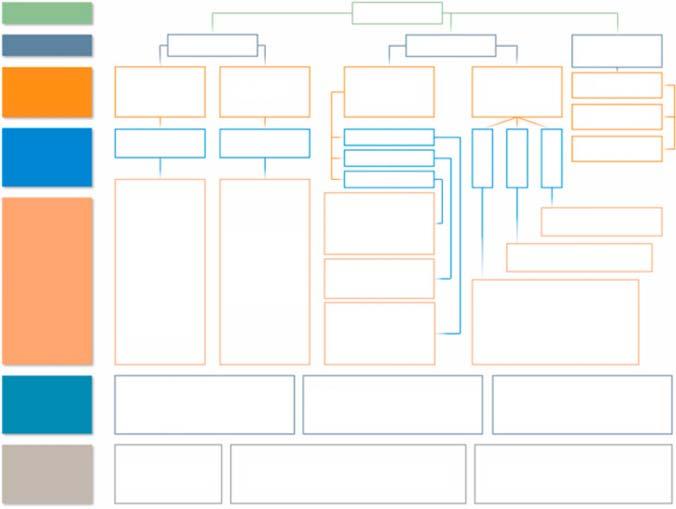
Figure1.8 Completeoverviewofproductionmethodsconvertingbiomassintocarbon materialsforEESdevices.Source:Ref.[66]/withpermissionofElsevier.
incorporationwithnanostructures,andsurfaceloadingwithoxidesincluding physicalandchemicalactivation.Thecompleteproductionmethodsandstructural modificationofB-d-CMsareshowninFigure1.8.
1.5.4.1SurfaceModificationandHeteroatomDopingofB-d-CMs
SeveralauthorsinvestigatedthesurfacemodificationofB-d-CMsusingfunctionalgroupsordopingdifferentheteroatomssuchasnitrogen,phosphorus, oxygen,sulfur,andboron.Theyhavereportedthatthesurfacemodificationand heteroatomdopingB-d-CMsareenhancingthedouble-layerproperties,which includepseudo-capacitance,wettability,effectivesurfacearea,etc.forenergy (supercapacitorelectrodes,fuelcells,andbatteries)andenvironmental(wastewater treatment)applications[75–78].
Insteadofsingleheteroatomdoping,codoping(twoheteroatomswithB-d-CMs) isincreasingtheelectrochemicalperformanceofB-d-CMsbecauseofsynergistic effect.Amongallheteroatoms,thenitrogen-containinggroups(amide,pyrrolic, pyridinic,imide,andlactame)dopedB-d-CMsshowsignificanceandexcellent performanceinCO2 capture,catalyst,electrochemicalstorage,andenvironmental applications[79].
1.5.4.2B-d-CMsSurfaceLoadingofMetalOxidesorHydroxides
Thesurfaceofbio-massderivedcarbon(BM-DCs)isalsomodifiedbyloading differenttransitionmetaloxidesorhydroxidessuchasNiO,MnO2 ,RuO2 ,and
MnO2-CNT-sponge supercapacitor Cell assembly
MnO2-CNTs-sponge
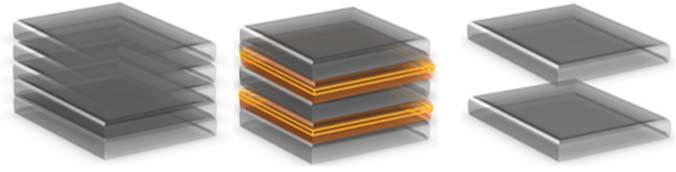
Figure1.9 (a–d)ThecompletehybridMnO2 /CNTspongeelectrodefabrication(Source:FromRef.[83]/withpermissionofAmericanChemicalSociety) and(eandf)representationofthescanningelectronmicroscope(SEM)andhighresolutionscanningelectronmicroscope(HRSEM)imagesshowing flower-likeindividualMnO2 nanoparticles.Source:Ref.[83]/withpermissionofAmericanChemicalSociety.
(a)(b) (e)
Sponge block Cellulose CNT dipping CNTs
CNT-coated sponge
MnO2 deposition
Co3 O4 [80,81].DuetoloadingofmetaloxidesintotheBM-DCs,theagglomeration ofmetalparticlesisreduced,butthesurfaceredoxactivitycanbeenhanced,which isusefultoimprovetheelectrochemicalperformanceofB-d-CMs.Amongallmetal oxides,theNiOisapromisingsignificantelectrodematerialforenergyapplications becauseofitsuniquepropertiesandavailability.
1.5.4.3SurfaceIncorporationwithDifferentNanostructures
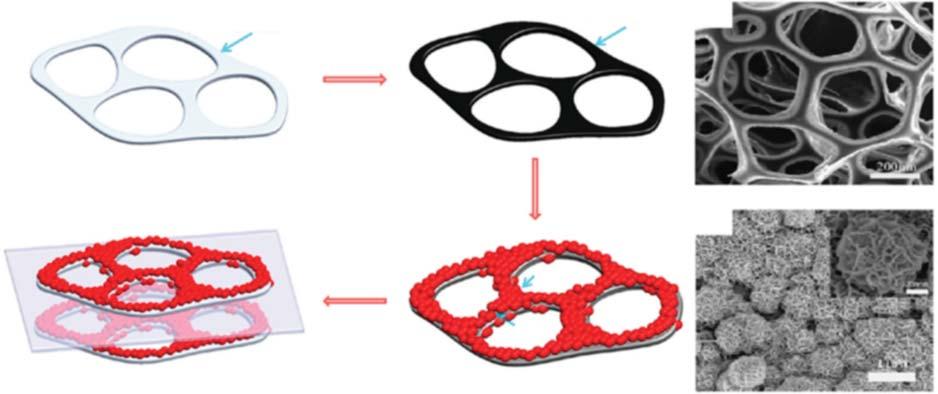
TheB-d-CMsareincorporatedwithdifferentnanostructures,suchascarbon nanotubes(CNTs)andgraphene,whichareimportantelectrodematerialsfor electrochemicalstorageapplications.Dengetal.[82]investigatedcellulose/carbon nanotubenanocompositefibersfollowedbydeacetylationandcarbonization,and as-preparedactivenanofiberswereusedaselectrodesforsupercapacitors.Chen etal.[83]studiedthattheMnO2 nanoparticlesareincorporatedwithCNTsponge hybridelectrodeforsupercapacitorapplications.ThecompletediagrammaticrepresentationoffabricationofhybridMnO2 /CNTelectrodeisusedforsupercapacitor applicationsasshowninFigure1.9.
1.6ProductionProcessDescription
TheabundantamountofAC,carbonaceousmaterialsarepreparedfromvarious biomasswastessuchaspecanshell,coconutshell,ricewaste,bamboocane,and peat[84–86]becausetheseACsaremainlyusedasodors,removalofcolors,and metalions.Ingeneral,theproductioncostofsteam-ACislowerthanthatofacid-AC. TheeconomicestimationofproductionofACprocessdependsuponthevariousraw materialsisconsideredusingtheeconomiccriterianamelyreturnoninvestment,net presentvalue,simplypaybackperiod,andinternalrateofreturn.Theprofitableis attainedinthestand-alonecaseindicatedbyeconomicindicesandmoreattractive intheintegratedplant.TheyfoundthatcostestimationwassensitivetoKOHcost, plantcapacity,andproductsellingprice.
Intermsofproductoutputandactivationroute,ACplanteconomiesaremostly extremelysensitive.Theeconomyisalsosignificantlyimpactedbythepriceof theproduct,basedonitsquality(adsorptioncapacity)andnotquantity(weight). Therawmaterialcostsandplantcapabilityarealsofactoringthataffectthecosts oftherawmaterials.Calculationsshowedthatpetcokecomplieswithallthese requirementsandwillbethebestoptionforthemanufactureofAC[87].
Largecapacitymanufacturingfacilitiesarenecessarytoproduceappealing economicsfortheothermaterialsexaminedandthismightofferchallengesin smallmarkets.Thestudymightstartwithaplantcapacitydeterminedatthe momentofbreakeven.Theconclusionisthatthehighestrawmaterialyieldshould bechosen,achemicalactivationschemeshouldbeadopted,andtheproductprice basedonproduct-surfacedareas(ormoregenerallyonproductadsorptioncapacity ofadsorbate)beconsideredforattractiveinvestmentinACproduction.