NanowireEnergyStorageDevices
Synthesis,Characterization,andApplications
EditedbyLiqiangMai
TheEditor
Prof.LiqiangMai WuhanUniversityofTechnology 122LuoshiRoad
Wuhan China
430070
CoverImage: ©MF3d/GettyImages
Allbookspublishedby WILEY-VCH arecarefully produced.Nevertheless,authors,editors,and publisherdonotwarranttheinformation containedinthesebooks,includingthisbook, tobefreeoferrors.Readersareadvisedtokeep inmindthatstatements,data,illustrations, proceduraldetailsorotheritemsmay inadvertentlybeinaccurate.
LibraryofCongressCardNo.: appliedfor
BritishLibraryCataloguing-in-PublicationData Acataloguerecordforthisbookisavailable fromtheBritishLibrary.
Bibliographicinformationpublishedby theDeutscheNationalbibliothek TheDeutscheNationalbibliotheklists thispublicationintheDeutsche Nationalbibliografie;detailedbibliographic dataareavailableontheInternetat <http://dnb.d-nb.de>
©2024WILEY-VCHGmbH,Boschstraße12, 69469Weinheim,Germany
Allrightsreserved(includingthoseof translationintootherlanguages).Nopartof thisbookmaybereproducedinanyform–by photoprinting,microfilm,oranyother means–nortransmittedortranslatedintoa machinelanguagewithoutwrittenpermission fromthepublishers.Registerednames, trademarks,etc.usedinthisbook,evenwhen notspecificallymarkedassuch,arenottobe consideredunprotectedbylaw.
PrintISBN: 978-3-527-34917-3
ePDFISBN: 978-3-527-83245-3 ePubISBN: 978-3-527-83247-7
oBookISBN: 978-3-527-83246-0
Typesetting Straive,Chennai,India
Contents
Preface xi
1NanowireEnergyStorageDevices:Synthesis, Characterization,andApplications 1
1.1Introduction 1
1.1.1One-DimensionalNanomaterials 1
1.1.1.1Nanorods 3
1.1.1.2CarbonNanofibers 3
1.1.1.3Nanotubes 3
1.1.1.4Nanobelts 5
1.1.1.5Nanocables 6
1.1.2EnergyStorageScienceandTechnology 6
1.1.2.1MechanicalEnergyStorage 7
1.1.2.2ElectromagneticEnergyStorage 9
1.1.2.3ElectrochemicalEnergyStorage 9
1.1.3OverviewofNanowireEnergyStorageMaterialsandDevices 13
1.1.3.1SiNanowires 15
1.1.3.2ZnONanowires 17
1.1.3.3SingleNanowireElectrochemicalEnergyStorageDevice 18 References 19
2FundamentalsofNanowireEnergyStorage 27
2.1PhysicalandChemicalPropertiesofNanowires 27
2.1.1ElectronicStructure 27
2.1.2ThermalProperties 29
2.1.2.1MeltingPoint 29
2.1.2.2ThermalConduction 30
2.1.3MechanicalProperties 31
2.1.4AdsorptionandSurfaceActivity 32
2.1.4.1Adsorption 33
2.1.4.2SurfaceActivity 33
2.2ThermodynamicsandKineticsofNanowiresElectrodeMaterials 34
2.2.1Thermodynamics 34
2.2.2Kinetics 34
2.3BasicPerformanceParametersofNanowiresElectrochemicalEnergy StorageDevices 35
2.3.1ElectromotiveForce 36
2.3.2OperatingVoltage 36
2.3.3CapacityandSpecificCapacity 36
2.3.4EnergyandSpecificEnergy 37
2.3.5CurrentDensityandCharge–DischargeRate 37
2.3.6PowerandSpecificPower 38
2.3.7CoulombicEfficiency 38
2.3.8CycleLife 38
2.4InterfacialPropertiesofNanowiresElectrodeMaterials 38
2.4.1InterfaceBetweenNanowireElectrodeMaterialsandElectrolytes 38
2.4.2HeterogeneousInterfacesinNanowireElectrodeMaterials 40
2.5OptimizationMechanismofElectrochemicalPropertiesofNanowires ElectrodeMaterials 42
2.5.1MechanismofElectron/IonBicontinuousTransport 42
2.5.2Self-BufferingMechanism 44
2.6TheoreticalCalculationofNanowiresElectrodeMaterials 44
2.7SummaryandOutlook 48 References 49
3DesignandSynthesisofNanowires 51
3.1ConventionalNanowires 51
3.1.1WetChemicalMethods 51
3.1.1.1Hydrothermal/SolvothermalMethod 52
3.1.1.2Sol–GelMethod 53
3.1.1.3CoprecipitationMethod 54
3.1.1.4UltrasonicSprayPyrolysisMethod 55
3.1.1.5ElectrospinningMethod 55
3.1.2DryChemicalMethod 57
3.1.2.1High-TemperatureSolid-StateMethod 57
3.1.2.2ChemicalVaporDepositionMethod 58
3.1.3PhysicalMethod 59
3.2PorousNanowires 60
3.2.1TemplateMethod 60
3.2.1.1TemplatebyNanoconfinement 60
3.2.1.2TemplatebyOrientationInduction 62
3.2.2Self-AssemblyMethod 63
3.2.3ChemicalEtchingMethod 64
3.3HierarchicalNanowires 65
3.3.1Self-AssemblyMethod 65
3.3.2SecondaryNucleationGrowthMethod 68
3.4HeterogeneousNanowires 69
3.4.1HeterogeneousNucleation 69
3.4.2SecondaryModification 71
3.5HollowNanowires 73
3.5.1WetChemicalMethod 73
3.5.2TemplateMethod 73
3.5.3GradientElectrospinning 76
3.6NanowireArrays 79
3.6.1TemplateMethod 79
3.6.2WetChemicalMethod 81
3.6.3ChemicalVaporDeposition 83
3.7SummaryandOutlook 86
References 88
4NanowiresforInSituCharacterization 95
4.1InSituElectronMicroscopyCharacterization 95
4.1.1InSituScanningElectronMicroscopy(SEM)Characterization 95
4.1.2InSituTransmissionElectronMicroscope(TEM)Characterization 97
4.2InSituSpectroscopyCharacterization 101
4.2.1InSituX-rayDiffraction 101
4.2.2InSituRamanSpectroscopy 106
4.2.3InSituX-rayPhotoelectronSpectroscopy 108
4.2.4InSituXASCharacterization 108
4.3InSituCharacterizationofNanowireDevices 111
4.3.1NanowireDevice 111
4.3.2NanowireDeviceCharacterizationExample 111
4.4OtherInSituCharacterization 115
4.4.1InSituAtomicForceMicroscopyCharacterization 115
4.4.2InSituNuclearMagneticResonance 117
4.4.3InSituNeutronDiffraction 119
4.4.4InSituTime-of-FlightMassSpectrometry 121
4.5SummaryandOutlook 123 References 124
5NanowiresforLithium-ionBatteries 131
5.1Electrochemistry,Advantages,andIssuesofLIBsBatteries 131
5.1.1HistoryofLithium-ionBatteries 131
5.1.2ElectrochemistryofLithium-ionBatteries 132
5.1.2.1TheoreticalOperationPotential 133
5.1.2.2TheoreticalSpecificCapacityofElectrodeMaterialsandCells 133
5.1.2.3TheoreticalSpecificEnergyDensityofanElectrochemicalCell 134
5.1.3KeyMaterialsforLithium-ionBatteries 134
5.1.3.1Cathode 134
5.1.3.2Anode 135
5.1.3.3Electrolyte 135
5.1.3.4Separator 136
5.1.4AdvantagesandIssuesofLithium-ionBatteries 137
5.2UniqueCharacteristicofNanowiresforLIBs 138
5.2.1EnhancingtheDiffusionDynamicsofCarriers 138
5.2.2EnhancingStructuralStabilityofMaterials 138
5.2.3BefittingtheInSituCharacterizationofElectrochemicalProcess 139
5.2.4EnablingtheConstructionofFlexibleDevices 139
5.3NanowiresasAnodesinLIBs 139
5.3.1Alloy-TypeAnodeMaterials(Si,Ge,andSn) 139
5.3.1.1LithiumStorageinSiNanowires 139
5.3.1.2LithiumStorageinGeNanowires 142
5.3.1.3LithiumStorageinSnNanowires 145
5.3.2MetalOxideNanowires 146
5.3.3CarbonaceousAnodeMaterials 148
5.4NanowiresasCathodesinLIBs 151
5.4.1TransitionMetalOxides 151
5.4.2VanadiumOxideNanowires 153
5.4.3IronCompoundsIncludingOxidesandPhosphates 157
5.5Nanowires-BasedSeparatorsinLIBs 160
5.6Nanowires-BasedSolid-StateElectrolytesinLIBs 163
5.7Nanowires-BasedElectrodesforFlexibleLIBs 168
5.8SummaryandOutlook 174 References 175
6NanowiresforSodium-ionBatteries 185
6.1AdvantagesandChallengesofSodium-ionBatteries 185
6.1.1DevelopmentofSodium-ionBatteries 185
6.1.2CharacteristicofSodium-ionBatteries 186
6.1.2.1TheWorkingPrincipleofSodium-ionBattery 186
6.1.2.2AdvantagesofSodium-ionBatteries 186
6.1.3KeyMaterialsforSodium-ionBatteries 187
6.1.3.1Cathode 188
6.1.3.2Anode 188
6.1.3.3Electrolyte 189
6.1.3.4Separator 189
6.1.4ChallengesforSodium-ionBatteries 191
6.2NanowiresasCathodesinSodium-ionBatteries 193
6.2.1LayeredOxideNanowires 193
6.2.2Tunnel-typeOxideNanowires 195
6.2.3PolyanionicCompoundNanowires 196
6.3NanowiresasAnodesinSodium-ionBatteries 200
6.3.1CarbonaceousMaterialsandPolyanionicCompounds 200
6.3.1.1GraphitizedCarbonMaterials 200
6.3.1.2AmorphousCarbonMaterials 201
6.3.1.3CarbonNanomaterials 201
6.3.2PolyanionicCompounds 203
6.3.3MetalsandMetalOxides 206
6.3.3.1MetalNanowires 206
6.3.3.2TransitionMetalOxideNanowires 207
6.3.4MetalSulfides 215
6.3.4.1MolybdenumSulfideandItsComposites 216
6.3.4.2TungstenSulfideandItsComposites 216
6.3.4.3StannicSulfideandItsComposites 218
6.3.4.4NickelSulfide,FerrousSulfideandTheirComposites 218
6.4Summary 220
References 220
7ApplicationofNanowireMaterialsinMetal-Chalcogenide Battery 229
7.1Lithium–SulfurBattery 230
7.1.1Sulfur–CarbonNanowireCompositeCathodeMaterials 231
7.1.2ConductivePolymerNanowire/SulfurCompositeCathode Materials 236
7.1.3MetalCompoundNanowires/SulfurCompositeCathodeMaterials 237
7.2Sodium–SulfurBatteryandMagnesium–SulfurBattery 243
7.2.1Sodium–SulfurBattery 243
7.2.2Magnesium–SulfurBattery 247
7.3Lithium–SeleniumBattery 249
7.3.1ReactionMechanismofLithium–SeleniumBattery 250
7.3.2Selenium-BasedCathodeMaterials 251
7.3.3ExistingProblemsandPossibleSolutions 256
7.4SummaryandOutlook 257 References 258
8ApplicationofNanowiresinSupercapacitors 263
8.1NanowireElectrodeMaterialforElectrochemicalDouble-Layer Capacitor 265
8.1.1TheApplicationofCarbonNanotubesinEDLCs 266
8.1.2TheApplicationofCarbonNanofibersinEDLCs 267
8.2NanowireElectrodeMaterialsforPseudocapacitive Supercapacitors 269
8.2.1MetalOxideNanowireElectrodeMaterials 269
8.2.2ConductingPolymerNanowireElectrodeMaterials 271
8.3NanowireElectrodeMaterialsofHybridSupercapacitors 272
8.3.1HybridSupercapacitorBasedonAqueousElectrolyte 274
8.3.1.1Carbon/MetalOxide 274
8.3.1.2Carbon/ConductiveNanowirePolymer 276
8.3.2OtherElectrolyteSystemHybridSupercapacitors 277
8.3.2.1OrganicElectrolyteSystem 277
8.3.2.2Redox-ActiveElectrolyteSystem 278
8.3.3SolidElectrolyteorQuasi-Solid-StateHybridSupercapacitor 279
x Contents
8.4SummaryandOutlook 279 References 280
9NanowiresforMultivalent-ionBatteries 285
9.1NanowiresforMagnesium-IonBattery 285
9.1.1Vanadium-BasedNanowiresforMIBs 286
9.1.2Manganese-BasedNanowiresforMIBs 289
9.1.3OtherNanowiresforMIBs 290
9.2NanowiresforCalcium-IonBatteries 292
9.3NanowiresforZinc-IonBatteries 293
9.3.1Vanadium-BasedNanowiresforZIBs 294
9.3.2Manganese-BasedNanowiresforZIBs 295
9.4NanowiresforAluminumIonBatteries 296
9.5SummaryandOutlook 298 References 299
10ConclusionandOutlook 305
10.1StructureDesignandPerformanceOptimizationof1D Nanomaterials 305
10.2AdvancedCharacterizationMethodsfor1DNanomaterials 308
10.3ApplicationsandChallengesofNanowireEnergyStorageDevices 314
10.3.1ApplicationofNanowireStructuresinLithium-ionBatteries 314
10.3.2ApplicationsofNanowireStructuresinNa-ionBattery 315
10.3.3ApplicationsofNanowireStructuresinOtherMonovalent-ion Batteries 316
10.3.4ApplicationofNanowiresinLithium–SulfurBatteries 316
10.3.5Applicationof1DNanomaterialsinSupercapacitors 318
10.3.6NanowiresforOtherEnergyStorageDevices 319
10.3.6.1MetalAirBatteries 319
10.3.6.2Multivalent-ionBattery 320
10.3.6.3MetalSulfurBatteries 320 References 322
Index 327
Preface
Withtheincreasingprominenceofworldwideenergycrisis,materialsscience andnanotechnologieshavebecomethekeytothefieldsofnewenergystorage andutilization.Withthein-depthstudyofnanotechnologies,nanoenergystorage materialsareintherapiddevelopmentstage.Especially,owingtotheirexcellent chemistry,electricity,thermal,mechanical,andotherproperties,nanowirematerialsexhibituniqueelectronandiontransportcharacterization,makingthem distinctiveamongnanomaterials.Fromthisperspective,nanowireenergystorage devices(ESDs)showoutstandingelectrochemicalandenergystoragecharacteristics.Andnanowirematerialsalsohavemoreuniqueadvantagesintheconstruction ofmicroandnanoenergystoragedevicesandtheassemblyofflexibleESDs.Tothis end,nanowirematerialsoffervariousopportunitiestoaddressthechallengesof advancedESD,thusattractingthegreatattentionandstronginterestofthemajority ofscientificresearchers.
Inthisbook,wepresentacomprehensivediscourseonnanowireenergystorage devices(ESDs)fromtheperspectiveofsynthesis,characterization,andapplications. Thispublicationisstructuredinto10chapters,eachwithcoherentconnectionsand distinctfocalpoints.Readerscanreadsystematicallyorselectively,accordingtotheir needs.
The Chapter1 iswrittenby Prof.LiqiangMaiandWenLuo Chapter1 intendsto offeranall-sidedintroductiontoone-dimensionalnanomaterials,energystoragescienceandtechnology,andreviewthenanowireenergystoragematerialsanddevices.
The Chapter2 iswrittenby Prof.LiqiangMaiandChaojiangNiu. Chapter2 summarizesthephysicochemicalcharacteristicsofnanowire,theuniqueadvantagesofnanowiresaselectrodematerials,thebasicperformanceparametersof nanowireelectrochemicalESDs,theoptimizationmechanismofelectrochemical performance,andbrieflyintroducesthetheoreticalcalculationsrelatedtonanowire electrodematerials.
The Chapter3 iswrittenby Prof.LiqiangMaiandLinXu. Chapter3 focuses onthecommonsynthesismethodsandgrowthmechanismsofvarioustypes ofnanowirematerialsbasedontheclassificationofnanowireswithdifferent morphologies.
The Chapter4 iswrittenby Prof.LiqiangMaiandXiaocongTian Chapter4 brieflyintroducesvariouscharacterizationmethodsandsummarizestheprogress inthecharacterizationofnanowiresuptonow.Chapter4willalsodiscussthe
futureofcharacterizationfornanowiresandgiveperspectivesontheopportunities ofnanowiresinpost-lithiumenergystoragesystems.
The Chapter5 iswrittenby Prof.LiqiangMaiandYaYou. Chapter5 elaborates theapplicationofnanowiresinlithium-ionbatteries,discussestheuniquecharacteristicofnanowiresforenergystorage,andgivesperspectivesontheopportunities ofnanowiresinpost-lithiumenergystoragesystems.
The Chapter6 iswrittenby Prof.LiqiangMaiandTingZhu. Chapter6 summarizestheapplicationofnanowirecathodeandanodematerialsinsodium-ion batteries.Thesynthesis,characterization,andreactionmechanismofthenanowires arecompletelydemonstrated.Theperspectivesonthedevelopmentofnanowiresin sodium-ionbatteriesarealsodiscussedinthischapter.
The Chapter7 iswrittenby Prof.LiqiangMaiandXuXu. Chapter7 expounds theprinciplesandapplicationsofvariousnewbatteriesfromtheperspectiveof nanowireelectrodematerialsandexplainsthecharacteristicsandadvantagesof nanowireelectrodematerialsinnewbatteries.
The Chapter8 iswrittenby Prof.LiqiangMaiandLiangZhou Chapter8 focuses onone-dimensionalnanomaterialsappliedtothreetypicalcapacitors,including Electricdouble-layercapacitors(EDLCs),pseudocapacitivesupercapacitors,and hybridsupercapacitors.Therelationshipbetweenone-dimensionalstructureand performanceofcapacitorsisexplored,whichcouldprovidetheoreticalguidancefor thedeeperimprovementofnanowire-basedsupercapacitors.
The Chapter9 iswrittenby Prof.LiqiangMaiandQinyouAn Chapter9 introducesabroaderapplicationofnanowireESDsinthevariousfieldsofmultivalent-ion batteries,suchasmagnesium-ionbattery,calcium-ionbattery,zinc-ionbattery,and aluminum-ionbatteries.Inthechapter,wealsogiveasimplesummaryandoutlook onnanowiresformultivalent-ionbatteries.
Thelastchapteriswrittenby Prof.LiqiangMaiandWenLuo. Chapter10 provides asystematicconclusionandoutlookonnanowireESDandgivesperspectiveson thestructuredesign,performanceoptimization,andadvancedcharacterization methodsof1Dnanomaterials.Moreover,inthischapter,wealsointendtopresenta broadpictureofmicro-nanodevicestructureoptimizationandfabricationprocess improvementfordifferentapplications.
Thisbookisavailableforinstitutionsofhigherlearningandscientificresearch unitsengagedinthedevelopmentofnanomaterialsandESDsresearchrelated researchersandpractitionerstouse,alsocanbeusedasinstitutionsofhigher learningmaterials,chemicalenergy,physicalandrelatedprofessionalteachers, graduate,andseniorundergraduateprofessionalreferencebook.Overall,despite thebesteffortsoftheauthor,duetothelimitationsoflevelandtime,itisinevitable thattherewillbesomemistakesandomissionsinthebook.Wesincerelyhopethat thereaderswillcontactuswithvaluablesuggestionsforitscontinuedimprovement.
Finally,Iwouldliketothankallthecontributorstothisbook.Wethank thesupportoftheNationalKeyResearchandDevelopmentProgramofChina (2020YFA0715000),theNationalNaturalScienceFoundationofChina(51832004, 52127816,51802239,52172233).
February2023
1.1Introduction
1.1.1One-DimensionalNanomaterials
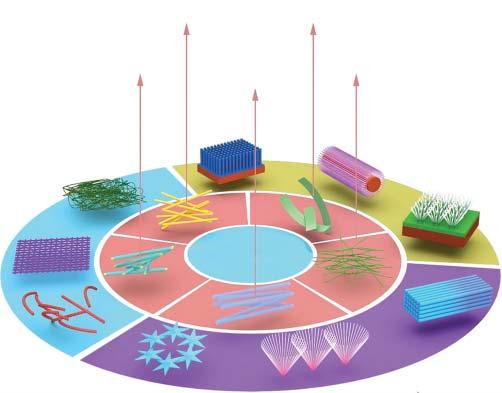
Asanimportantmemberofthelargefamilyofnanomaterials,one-dimensional nanowire(NW)materials,includingnanorods,nanotubes,nanobelts,and nanocables,havegraduallyreceivedattentionfromresearchers(Figure1.1).ANW canbedefinedasaone-dimensionalstructurethatislessthan100nminthelateral direction(thereisnolimitationinthelongitudinaldirection).Theaspectratioofa partofaNWismorethan1000[1].Accordingtotheirdifferentcompositions,NWs canbedividedintodifferenttypes,includingmetalNWs(suchasNi,Pt,andAu), semiconductorNWs(suchasInP,Si,andGaN),andinsulatorNWs(suchasSiO2 andTiO2 ).NWmaterialshaveimportantimplicationsfortheoreticalresearchand technicalapplications.Thiskindofmaterialhaspeculiarphysicalandchemical properties,suchasthetransitionfrommetaltoinsulator,supermechanicalstrength, highluminousefficiency,lowerlaserthreshold,andenhancedthermoelectric coefficient[2].
TheresearchonNWscanbetracedtotheearly1960s.In1964,Wagnerand Ellis[3]usedvapor–liquid–solid(VLS)growthtoepitaxiallygrowSisinglecrystalwhiskersonasinglecrystalSi(111)substrate,creatingaprecedentforSiNW research.In1975,Givargizov[4]conductedasystematicstudyontheprocessofVLS growthandgaveareasonableVLSgrowthmechanismforNWs.In1998,Morales[5] andZhangetal.[6]usedlaserablationdeposition(LAD)tosuccessfullygrowSi NWs.NWmaterialsexhibituniqueoptical,electrical,andmagneticpropertiesthat manybulkmaterialsdonothave.Therefore,theyhaveanextremelyimportant positioninthefieldofproducingnanodevices,varioussensors,microtools,microelectrodes,device-integratedconnectionlines,andnext-generationELdisplay devices.Currentresearchresultsshowthatmanyone-dimensionalnanomaterials havedemonstratedessentialapplications.Forexample,Huangetal.[7]grewZnO NWarraywithadiameterof20–150nmandalengthofabout10 μmthrough avapor-phasetransportprocessonasapphiresubstrate,successfullypreparing
NanowireEnergyStorageDevices:Synthesis,Characterization,andApplications,FirstEdition. EditedbyLiqiangMai. ©2024WILEY-VCHGmbH.Published2024byWILEY-VCHGmbH.
Figure1.1 Schematicdiagramofdifferenttypesofnanowiresandtheirsecondary structures.
nanolasersin2001.In2007,Prof.LieberfromHarvardUniversityandhisteam developedthefirstsingleNWsolarcell[8].Theyreportedasolarcellpreparedby asinglecoaxialsiliconnanowire(SiNW),whichadoptsap–i–ncoaxialstructure. ThesynthesisandapplicationofNWshavebecomehotspotsforscientistsinrecent yearsbecauseoftheiruniqueadvantages.Prof.MaifromWuhanUniversityof TechnologyandhisteamdesignedandassembledthefirstsingleNWelectrochemicalenergystoragedevicetorevealtheintrinsicmechanismofelectrochemical performancedegradationin2010[9].Inthesameyear,Huangetal.[10]usedin situTEMtoobservethelithiationphenomenonofasingleNWofSnO2 forthe firsttime.In2012,KouwenhovenfromDelftUniversityofTechnologyandhis teamverifiedthehypothesisthatMajoranafermionsinNWsarecoupledwith superconductors[11].In2014,Prof.ChengfromMonashUniversityandhisteam builtawearablepressuresensorusinggoldnanowires[12].In2019,scientists fromCambridgeUniversity,King’sCollegeLondon,PekingUniversity,Zhejiang University,ShanghaiJiaoTongUniversity,andotheruniversitiesdevelopedthe firstsingleNWspectrometerintheworld[13].In2020,researchersfromChinese AcademyofSciencesdemonstratedanewflexibledual-NWstructureconsisting ofaGeSnlayerwithSncontentof10%heteroepitaxiallygrownonthesidewall ofaGeNWbymolecularbeamepitaxy(MBE),whicheffectivelysuppressed theformationofdefectsattheGeSn/Geinterfaceandgreatlyreducedthedark currentandstaticpowerconsumptionofthephotodetectorwithGeSn/Gedual-NW structure[14].NWsanddeviceswithvariousstructureshavebeencontinuously developed,andtheirapplicationsinscientificresearchandindustrializationhave becomeincreasinglyextensivewiththecontinuousprogressofnanotechnology.
1.1Introduction 3
TheimportantdevelopmentprocessisshowninFigure1.2.Thisbookfocuseson theintroductionofNWmaterialsinelectrochemicalenergystorage.
1.1.1.1Nanorods
NanorodsandNWsarewell-knownone-dimensionalnanostructures,whichare frequentlyusednotonlyinnano-electromechanicalsystemsbutalsoinbiomedical treatments,dentistry,productionofenergyfromsolarcells,andhumidity-sensitive analysis[15].Nanorodsconsistofrod-likenanoparticles.Itslengthisusuallymuch largerthanitssizeinthetwo-dimensionaldirectionandcanachieveamacroscopic magnitude.ItshouldbenotedthatthereisnostrictdistinctionbetweenNWsand nanorods.Generally,thosewithalargeslendernessratioarecallednanofibers, andthosewithasmallaspectratioarecallednanorods.Thedividinglineislocated at1 μm.ThepreparationmethodsfornanorodsmainlyadoptVLSgrowthand template-basedsynthesis.NanorodscanalsobeformedbyusingLAD.Panasonic,theU.S.BureauofStandardsandMetrology,andtheUniversityofFlorida havedonealargequantityofworkinthisarea,usingthepulsedlasermethod tosuccessfullyprepareone-dimensionalSiNWsandboronnitridenanotubes. Theone-dimensionalnanomaterialsynthesizedbythismethodhastheadvantages ofhighpurity,largeoutput,anduniformdiameter.ResearchersattheUniversityof MinnesotaandPrincetonUniversitysuccessfullypreparedaquantumdiskin1998. Thisdiskisanano-arraysystemcomposedofmagneticnanorodswithadensityof 109bitcm 2
1.1.1.2CarbonNanofibers
Carbonnanofibers(CNFs)aresp2 -basedlinear,noncontinuousfilamentsthatare differentfromcarbonfibers,whicharecontinuouswithdiameterofseveralmicrometers[16].Theyareanewtypeofquasi-one-dimensionalcarbonmaterialthathas attractedmoreattentioninrecentyears.Itsdiameterisgenerally50–200nm,the lengthis50–100 μm,andtheaspectratiois100–500.Itsstructureandperformance areinthetransitionstatebetweenordinarycarbonfiberandcarbonnanotubes,and areformedbystackingnano-sizedgraphitesheetsatdifferentanglestotheaxial directionofthefiberinspace[17].One-dimensionalCNFshavemanysuperiorproperties,sotheirapplicationprospectsareextensive.CNFshaveporesonmolecular leveloverthesurfaceandalsohaveporesinside,aswellasalargespecificsurface area[18].Therefore,itcanabsorbalargeamountofgasandisapotentialhydrogenstoragematerial.Itcanalsobeusedasahigh-efficiencyadsorbent,catalyst,and catalystcarrier[19].CNFsalsohavehighelectricalconductivityandcanbeused ascathodematerialsforlithium-ionsecondarybatteriesandelectrodesforelectric double-layercapacitors[20].
1.1.1.3Nanotubes
Carbonnanotubes,theone-dimensionalallotropesofcarbon,haveattractedsignificantresearchinteresteversincetheirdiscoveryduetotheiroutstandingmaterial properties.Carbonnanotubesareatypicalrepresentativeofnanotubes.Inthe1970s,
Z. Di et al. demonstrate a flexible GeSn/Ge dualnanowire structure
W. Cheng et al. buil a wearable pressure sensor using gold nanowires
J. Huang et al. observed the lithiation of a single SnO2 nanowire for the first time
Z. Wang et al. developed the first nanowire generator in the world
Z. Wang et al. discovered and synthesized semiconductor oxide nanobelts
R. E. Smalley et al. synthesized singlewalled carbon nanotube bundles arranged in lines
M. Endo developed carbon fiber with a diameter of 7 nm
T. Hasan et al. developed the first single nanowire spectrometer in the world
L. P. Kouwenhoven et al. verified the hypothesis of coupling between Majorana fermions and superconductors of nanowires
L. Mai et al. developed a single nanowire electrochemical device
C. M. Lieber et al. developed a single silicon nanowire solar cell
R. R. Llinás et al. have developed a new method of implanting platinum metal nano-cables into human blood vessels
P. Yang et al. developed the first nanowire laser
R. S. Wagner et al. proposed “solidliquid-gas growth mechanism”
Figure1.2 Importantdevelopmenthistoryofnanowirematerials.Source:Refs.[3–14].
M.EndofromtheUniversityofOrléansinFrancesuccessfullysynthesizedcarbon fiberswithadiameterof7nm,buthedidnotcarefullyevaluateandcharacterize thestructureofthesecarbonfibers[21].Until1991,Iijima[22]oftheJapaneseNEC companydiscoveredcarbonnanotubesforthefirsttimewithahigh-resolutionelectronmicroscope.Sincethen,nanomaterialshaveattractedwidespreadattentionin theglobalscientificresearchfield.In1996,thefamousAmericanNobelPrizewinnerSmalleyandotherssynthesizedsingle-walledcarbonnanotube(SWNT)bundles arrangedinrows,whichsignifiesthatscientistscanpreparebatchesofNWsina controlledmanner[23].Xieandcoworkers[24]fromInstituteofPhysics,Chinese AcademyofSciences,haveachievedthedirectionalgrowthofcarbonnanotubesand successfullysynthesizedultra-long(millimeter-level)carbonnanotubes,reported thesynthesisofstrong,highlyconducting,andtransparentSWNTfilmsin2007. Becausecarbonnanotubeshavetheadvantagesoflargespecificsurfacearea, goodelectricalconductivity,andhighchemicalstability,theyhavealsobeenwidely usedforelectricdouble-layersupercapacitorelectrodesorasconductiveadditives inotherelectrochemicalenergystoragesystems,suchaspseudocapacitorsand metalionbatteries[25].Atthesametime,theinternalandsurfacebondstates ofcarbonnanotubesaredifferent,andthereisaphenomenonofincomplete coordinationofsurfaceatoms,whichleadstoanincreaseinactivesitesonthe surface.Thesecharacteristicsmakecarbonnanotubesoneoftheidealcatalyst supportmaterials[26].Inaddition,carbonnanotubesarealsoexpectedtobeused forfieldemissiontubes,fieldemissionflatpaneldisplays,microwavegenerators, gasdischargetubes,andfluorescentlamps,andtheirnano-scalehollowpipesalso makethemhavegreatpotentialforhydrogenstorage[27].
1.1.1.4Nanobelts
Itisdifficulttomaintainstabilityduringlarge-scaleindustrialproductionofcarbon nanotubes,slowingtheresearchofnanomaterials.In2001,Prof.ZhonglinWangand histeamusedthehigh-temperaturesolidgasphasemethodtodiscoverandsynthesizesemiconductoroxidenanobeltstructuresforthefirsttimeintheworld[28]. Then,theysuccessfullysynthesizedtheribbonlikestructureofbroadbandsemiconductorsystemssuchaszincoxide,tinoxide,indiumoxide,cadmiumoxide,and galliumoxide.Thepurityoftheseribbonlikestructurematerialsisashighas95%, andtheyhavetheadvantagesoflargeoutput,perfectstructure,cleansurface,no internaldefects,andnodislocations.In2007,ZhonglinWangandhisteamdevelopedthefirstNWgeneratorintheworld[29].
Thecrosssectionofthenanobeltisanarrowrectangularstructurewithabandwidthof30–300nm,athicknessof5–10nm,andlengthofuptoseveralmillimeters. Comparedwithcarbonnanotubes,silicon,andcompoundsemiconductorlinear structures,nanobeltsaretheonlybroadbandsemiconductorone-dimensionalribbonstructuresthathavebeenfoundtohaveacontrollablestructure,bedefect-free, andhavemoreuniqueandsuperiorfeaturesofstructureandphysicalproperties thancarbonnanotubes.Althoughnanobeltslackthehighstructuralstrengthof cylindricalnanotubes,theycanensurethenecessarystabilityofelectronicequipmentandtheuniformityofthematerialstructureduringmassproductionbecause
1NanowireEnergyStorageDevices:Synthesis,Characterization,andApplications oftheirsuperiorperformance,whichisveryimportantinnanophysicsresearch andnanodeviceapplications.Thisstructureisanidealsystemforresearchingthe transportprocessesoflight,electricity,andheatinone-dimensionalfunctional andsmartmaterials.Itcanenablescientiststousesingleoxidenanobeltstomake nanometer-sizedgasandliquidsensors,nanofunctions,andsmartoptoelectronic components,layingasolidfoundationfornanooptoelectronics[30].
1.1.1.5Nanocables
In1997,FrenchscientistsC.Colliexandhisteamfoundasandwich-geometry C–BN–Ctubeintheproductobtainedbyanalyzingthearcdischarge.Becauseits geometryissimilartothatofacoaxialcableandthediameterisatthenanometer level,itiscalledacoaxialnanocable[31].In1998,Zhangetal.[32]usedlaser ablationtosynthesizeacoaxialnanocablewithadiameteroftensofnanometers andalengthof50 μm.TheirexperimentshowsthatifthemixedpowderofBN,C, andSiO2 isusedastherawmaterial,asingle-corenanocablecanbeformedwith β-SiCcorewireinsideandamorphousSiO2 singlewireoutside.IfLi3 Nisaddedto therawmaterials,acoaxialnanocablewithanotherstructurecanbeformed,thatis, thecoreisSiC,themiddlelayerisamorphousSiO2 ,andtheoutermostlayerisBNC withagraphitestructure.Atthesametime,Mengetal.[33]developedanewcoaxial nanocablepreparationmethod,whichissol–gelandcarbothermalreductionwith evaporation–condensation,andsuccessfullysynthesizedacoaxialnanocablewith SiCsemiconductorcoreandSiO2 insulatorontheouterlayer.After2000,people havesuccessfullypreparedhundredsofcoaxialnanocablesusingdifferentsynthesis methodsanddifferentkindsofmaterials,suchasSiGaN/SiOx Ny [34],Fe/C[35], Ag/polypyrrole[36],GaP/ZnS[37],CdSe/TiO2 [38],andthree-layerstructureof Ag/SiO2 /ppy[39]andNd/FM(FM = Fe,Co,Ni)/PA66[40].
Coaxialnanocablesplayacrucialroleinthefieldsofbiomedicine,nano-electronic devices,nano-microprocessing,andtestingtechnology.Intermsofpracticalapplications,thecomponentsintheultrahigh-density-integratedcircuitaremainlyconnectedbynanocablecoupling;hence,thenanocablealsoplaysanimportantrole intheconnectionline.Consideringtheresearchonsolarcells,theUSNational RenewableEnergyLaboratoryhasdevelopedakindofsolarcellwithhigh-energy conversionusingcoaxialnanocable.Respectingmedicalresearch,in2005,scientists intheUnitesStatesandJapansuccessfullydevelopedanewmethodofimplantingplatinummetalnanocableswithadiameterof1%ofhumanhairintohuman bloodvesselsandhopedthatonedayitwouldhelpdoctorstreatcertainhumandiseases[41].Nanocablescanalsobeusedasprobesforminiaturedetectorsonimportantnano-resolutiondetectorssuchasfieldemissiondetectors,biologicaldetectors, andatomicforcemicroscopes[42].Itisbelievedthatwiththecontinuousdiscovery ofnewspecialpropertiesofnanocables,coaxialnanocableswillalsobecomeanew forceinthefieldofNWmaterials.
1.1.2EnergyStorageScienceandTechnology
Thewidespreadapplicationoffossilfuels(oil,coal,andnaturalgas)hasgreatlypromotedtherapiddevelopmentoftheworldeconomy.Nowadays,theimprovement
oftheworldeconomyisstilllargelybasedontheuseoffossilfuels.However,with thecontinuousconsumptionofnonrenewableenergy,traditionalfossilfuelssuch asoil,coal,andnaturalgaswilleventuallyfaceexhaustion.Itispredictedthat thelifespanofcoal,naturalgas,andoilwillbelessthan100yearsonaverageif calculatedatthecurrentrateoffossilfuelconsumption.Theglobaldemandfor energywillreach28TWby2050[43],whichisequivalenttotheenergyproduced byconsuming1010tonsofoileachyear.Obviously,oneofthebiggestissuesof thetwenty-firstcenturyistheenergycrisis.Thedeteriorationoftheenergycrisis willleadtoaworldeconomiccrisis,whichinturnleadstoanintensificationof economicconflicts.Atthesametime,majorproblemssuchasenvironmental pollutionandglobalwarmingcausedbythegrowingconsumptionoffossilfuels poseaseverethreattothesustainabledevelopmentofmankind.Therefore,energy andenvironmentalissueshavebecomeinternationalissuesthatthreatenhuman survivalanddevelopment.Insummary,thedevelopmentofrenewableenergyisthe keytosolvingenergyandenvironmentalproblemsandistheonlychoiceforhuman beingstoachievesustainabledevelopment.Renewableenergymainlyincludes solarenergy,biomassenergy,windenergy,hydropower,geothermalenergy,and tidalenergy.Themostimportantwaytosolveenergyandenvironmentalproblems istovigorouslydeveloprenewableenergy.However,renewableenergysources suchassolarandwindenergyarediscontinuousandhavelarge,unpredictable,and variablecharacteristics.Theiracquisitionandoutputareunstable,andtheirenergy densityislow,whichhasagreatimpactonthereliabilityofthepowergrid.Itis difficulttointegrateintothegrid.Hence,howtoachieveeffectiveconversionand storagebetweenvariousenergyformsisparticularlyimportant.Thedevelopment ofenergystoragetechnologycaneffectivelysolvethisproblemenablingrenewable energytobestoredandappliedinastableform.Inaddition,withenergystorage technologyasthedevelopmentdirectionofthefuturepowergrid,smartgrids canadjustgridpeakusingenergystoragedevices,increasingthecapacityofthe transmissionanddistributionsystemandoptimizingefficiency.Energystorage technologycanbewidelyusedforthegeneration,transmission,distribution,and usageoftheentirepowerindustry.Inthisbook,energystoragemainlyrefersto electricalenergystorage.Itisatechnologyinwhichelectricalenergyisstored inanotherformandreleasedwhenneeded,whichcaneffectivelysolvethe aboveproblems.
Theexistingenergystoragemethodsaremainlydividedintothefollowingcategories:electrochemicalenergystorage(lead-acidbatteries,flowbatteries,alkaline metalsecondarybatteries,multivalentionbatteries,metal-chalcogenidebatteries, supercapacitors,etc.);mechanicalenergystorage(pumpedhydrostorage,flywheel energystorage,compressedairenergystorage,etc.);electromagneticenergystorage (superconductingenergystorage);andlatentheatstorage.
1.1.2.1MechanicalEnergyStorage
Theessenceofmechanicalenergystorageisthatelectricalenergyisconvertedinto mechanicalenergyforstorage.Therearethreemaintypesofmechanicalenergystoragetechnologiesthathavebeenappliedinindustry:pumpedhydrostorage,flywheel energystorage,andcompressedairenergystorage.
PumpedHydroStorage
Pumpedhydrostorageiscurrentlythemostmatureandwidelyusedenergystoragetechnologyintheworld,whichismainlyusedforsystembackupandpeakload andfrequencymodulation.Theenergystoragesystemisequippedwithtwostoragereservoirs:theupperreservoirandthelowerreservoir.Waterispumpedfrom thelowerreservoirtotheupperreservoirwhenstoringelectricity.Waterflowsfrom theupperreservoirtothepumplocationwhenusingelectricity,andthepotential energyofthewaterflowisusedtodrivetheturbinetogenerateelectricity.Pumped hydroenergystoragehasthelongestlifecycle(30–60years),thelargestnumber ofcycles(10000–30000times)andthelargestcapacity(500–8000MWh)compared withotherenergystorage[44].However,thedisadvantageisthatthesiteselection isgreatlyaffectedbythegeographicallocationandtheconstructionperiodislong aswellastheinvestmentcostishigh.
CompressedAirEnergyStorage
Compressedairenergystoragesystem(CAES)usesgasturbineenergytogenerate electricity.Theaircompressionsystemconsumesexcesselectricitytocompressthe airandstoreitinundergroundsaltmines,abandonedquarries,orlargeground storagetankswhenstoringelectricity.Thestoredairisreleasedfromtheairstorage chamberandburnedwithfuelwhenusingelectricity.Theproducedgas,with itshightemperatureandpressure,drivesthegasturbinetogenerateelectricity. Thesystemconstructioninvestmentandpowergenerationcostarelowerthan pumpedhydrostorage.Besides,ithasthefollowingadvantages:longservicelife (20–40years),largecapacity,andlargequantitiesofcharge–dischargecycles.Ithas wideapplicationsinthefieldsofpowerproduction,transportation,andconsumption,includingpeakshavingandvalleyfilling,powerloadbalancing,renewable energyaccess,andbackuppowersupplies[45].Ithasbroadapplicationprospects inconventionalpowersystems,renewableenergy,distributedfunctionsystems, andsmartgrids.However,thenegativeaspectsofCAESareobvious.Tobeginwith, itsenergydensityislow.Tomakemattersworse,itdependsonlargegasstorage chambersandcausesfossilfuelconsumptionandenvironmentalpollution.
FlywheelEnergyStorage
Flywheelenergystorageandpowergenerationtechnologyusestheconversion betweenelectricalandkineticenergytostoreandgenerateenergy.Theflywheel isconnectedtothepowergrid,andtheelectricalenergyprovidedbythepower griddrivestheflywheeltorotateatahighspeed,convertingtheelectricalenergy intokineticenergyandstoringitwhenstoringenergy.Therotatingflywheeldrives themotortogenerateelectricity,convertingkineticenergyintoelectricalenergy whenusingenergy.Comparedwithotherenergystoragetechnologies,flywheel energystoragehastheadvantagesofhighenergyconversionefficiency(85–95%), non-polluting,simplemaintenance,freefromgeographicalenvironmentrestrictions,andcontinuousoperation[46].Mainlyusedforuninterruptiblepowersupply (UPS),gridpeakshaving,andfrequencycontrol.However,shortpowergeneration timesandhighequipmentcostsareimportantfactorsrestrictingitsdevelopment.
1.1.2.2ElectromagneticEnergyStorage
Superconductingmagneticenergystorage(SMES)isthemostcommonenergy storagetechnologyforelectromagneticenergystorage.SMESsystemdirectlystores electromagneticenergyinthesuperconductingcoil.Thestoredelectromagnetic energyissentbacktothegridorotherloadswhenelectricityisneeded.SMES systemhasuniquebenefitsthatotherenergystoragetechnologiesdonothave. SMESsystemscanstoreenergyalmostlosslessforalongtime(conversionefficiency exceeds90%)[47],releaseenergyrapidly,haveasmallsize,andareenvironmentallyfriendly.Thelow-frequencypoweroscillationofthegridcanbereducedor eliminated,andtheactiveandreactivepowercanbeadjustedmoreeasilywith theexistenceofSMESsystem.Therefore,SMEScanimprovethestabilityofthe powersystemandenhancethecontrollabilityofpowergenerationwithnewenergy. However,itsshortboardishighincost,complicatedinprocess,andoperating conditions.
1.1.2.3ElectrochemicalEnergyStorage
Theprincipleofelectrochemicalenergystorageisthatelectricalenergyisconverted intochemicalenergyforstorage.Itincludesvarioustypesofbatteries(lead–acidbatteries,nickel-metalhydride(NiMH)batteries,lithium-ionbatteries,etc.)andsupercapacitors.Electrochemicalenergystorageisconvenientandsafe,isnotrestrictedby region,haslessenvironmentalpollution,isnotrestrictedbyCarnotcycleinenergy conversion,andhashighconversionefficiencycomparedwithothermethods[48]. Electrochemicalenergystorageisthemostimportantcomponentinthefieldof energystorage.SinceLeClancyinventedlead-acidbatteriesin1859,variouschemicalbatteriesinvolvingdifferentenergystoragesystemshavebeendevelopingin thedirectionofhighcapacity,highpower,lowpollution,longservicelife,andhigh safetytomeettheneedsofdifferentfields.Atpresent,lithium-ionbatteryhasthe advantagesofhighenergydensity,highconversionefficiency(closeto100%),long cyclelife,andlowself-discharge.Theyhavebeenwidelyusedinvariouselectronics andelectricvehiclesandaregraduallybeingdeployedinthefieldoflarge-scale energystorage.Manylarge-scalelithiumbatteryenergystoragedemonstration systemsandbaseshavebeenbuiltinmostcountries.However,in2018,theU.S.GeologicalSurveyMineralandCommoditySummariesshowedthatthegloballithium reservescouldnotmeettherapiddevelopmentofglobalelectricvehicles[49]. Thus,inordertomeettherapiddevelopmentofelectricvehiclesandtheincreasing demandforenergystoragedevicesinotherfields,othertypesofsecondarybatteries andenergystoragedeviceshavealsobeensignificantlydeveloped,suchasnew alkalinemetalsecondarybatteries(sodiumionbattery,potassiumionbattery), multivalentionbatteries(magnesiumionbattery,zincionbattery,aluminumion battery,etc.),metal-chalcogenidebatteries,metal-oxygenbatteries,andsupercapacitors.Thecommontypesofelectrochemicalenergystorageinvolvedandtheirmain characteristicsareshowninTable1.1.Electrodematerialsarethecorecomponent ofenergystoragedevices.Improvingtheperformanceofchemicalenergystorage equipmentbyexploringanddevelopingnewenergystoragematerialshasbecome aglobalissue,whichhasreceivedgreatattentionandsupportfromallcountries.
1NanowireEnergyStorageDevices:Synthesis,Characterization,andApplications
Table1.1 Commontypesofelectrochemicalenergystorageandtheirmainadvantages anddisadvantages.
TypeofbatteryMechanismofenergystorage
Lead-acid batteries
NiMH batteries
PbO2 + 2H2 SO4 + Pb ↔ 2H2 O + 2PbSO4
Analysisofadvantages anddisadvantages
Pros:maturetechnologyand lowcost
Cons:shortcyclelifeand pollutionproblem
MHPros:withstandoverchargeand overdischarge,strongcapability ofhighratedischarge,safetyand highpowerdensity
Cons:lowvoltageandlowenergy density
Monovalent ionbatteries
Embeddingreaction,alloying reaction,conversionreaction
Multivalent ionbatteries
Embeddingreaction,alloying reaction,conversionreaction
Metalchalcogenide batteries
Metal-air batteries
Theanode(M)ismainlythe depositionanddissolutionreaction ofmetalions,andthe oxidation–reductionreactionofthe chalcogenelement(X)occursinthe cathode.Thereactionoftheentire batterysystemcanbesummarized as:M + X ↔ MX
Catalyticmaterialsareusedto catalyzetheoxygenorpureoxygen intheairasthecathodeactive material,themetalistheanode,and theammoniumchlorideorcaustic solutionisusedastheelectrolyteto participateintheelectrochemical reaction.
Pros:maturetechnologyfor lithium-ionbatteries,small self-discharge,lightweight,small size,highworkingvoltage, environmentallyfriendly,no memoryeffect,chargingrapidly andhighenergycapacity
Cons:internalimpedanceis high,theworkingvoltage changesgreatlyandthecapacity decaysquickly
Pros:thetheoreticalvolumeratio ishigh,andtheanodehasrich metalresources
Cons:multivalentionshavea largeradius,thepolarization phenomenonisserious,and configuringasuitableelectrolyte isdifficult
Pros:highenergydensity
Cons:lowpowerdensity,short cyclelife,immaturesystem,and difficulttoachieve commercialization
Pros:relativelylowcostand stableoutputvoltage
Cons:lowpowerdensity,short cyclelife,andlowoutputvoltage (Continued)
Table1.1 (Continued)
TypeofbatteryMechanismofenergystorage
SupercapacitorsElectricdoublelayer, pseudocapacitance(underpotential deposition,redoxreaction,and embeddedpseudocapacitance)
FlowbatteriesAhigh-performancebatterythat usespositiveandnegative electrolytesisseparatedand circulate,respectively
Lead-AcidBatteries
Analysisofadvantages anddisadvantages
Pros:highpowerdensityand goodcyclestability
Cons:lowenergydensityand lowoutputvoltage
Pros:longcyclelife
Cons:lowenergystoragedensity
Thebasicchemicalcompositionofalltypesoflead-acidbatteryisthesame.Inthe chargedstate,thecathodeisleaddioxide,theanodeismetalliclead,andtheelectrolyteissulfuricacidsolution.Inthedischargestate,themaincomponentsofthe positiveandnegativeelectrodesareleadsulfate.Thegloballead-acidbatteryindustryhasalwaysbeenlargerthanotherbatteriesbecauseofaseriesofadvantagessuch aslowcost,highworkingvoltage,safety,andreliabilitythatotherbatteriescannot replace[50].Lead-acidbatterieshavealonghistoryofdevelopment,andthetechnologyismatureandisnowwidelyusedinmanyfields.Intheautomotiveindustry, lead-acidbatteriesareusedforthestarting,ignition,andlightingofvariouscars, motorcycles,andships.Intheelectricvehicleindustry,lead-acidbatteriesprovide powerforelectriccarsandelectricbicycles.Inaddition,themarketdemandfor lead-acidbatteriesinthecommunicationsindustryishuge.Inaddition,whennew energysourcesgenerateelectricity,italsoneedstobesuppliedwithlead-acidbatteries[51].Lead-acidbatteriescanreplaceexpensivegasandoilturbinegenerators tomeetloadbalancingrequirementsasanalternativepowersupplymethodduring peakelectricityconsumption.
NiMHBatteries
NiMHbatteriesareanewgenerationofhigh-energysecondarybatteriesthatreplace Ni–Cdbatteries[52].NiMHbatteriesareakindofgreenbatterycomparedwith lead-acidbatteries.ThecathodeofNiMHbatteryisNi(OH)2 .Theanodematerialis metalhydride(MH).Theelectrolyteisanaqueouspotassiumhydroxidesolution.In thefieldofsmallbatteries,althoughtheenergydensityofNiMHbatteriesishigher thanNi–Cdbatteries,itsenergydensityislowerthanthatoflithium-ionbatteries. Therefore,atpresent,themarketutilizationrateofNiMHbatteriesislowerthan thatoflithium-ionbatteries.TherearealsosomeelectrictoolsthatretainNiMH batteries,likesweepingrobots.Intheautomotiveindustry,NiMHbatteriesare usedaspowerbatteries.NiMHbatterieshaveoutstandingadvantagescompared withlithium-ionbatteriesandhydrogenfuelcells,whichmainlyshowintheir fastcharginganddischargingspeeds,highmass-specificpower,andgoodstability.
1NanowireEnergyStorageDevices:Synthesis,Characterization,andApplications
Inaddition,theenergystoragebatteriescurrentlyusedinsmartgridsmainlyinclude lead-acidbatteries,lithium-ionbatteries,sodium–sulfurbatteries,all-vanadium flowbatteries,andNiMHbatteries.Amongthem,lead-acidbatterieswillproduce leadpollution,andmanyEuropeancountrieshavegraduallybannedlead-acid batteries.Li-ionbatteriesarenotyetmatureinlarge-scaleintegrationtechnology. ThetechnologyofNiMHbatteriesasasmartgridenergystoragesystemisrelatively mature,withgoodsafetyperformanceandlongservicelife[53].
MonovalentIonBatteries
Lithium-ionbatteriesarethemostrepresentativeofmonovalentionbatteries. Itmainlyrealizeschargeanddischargethroughrepeatedinsertionandextraction oflithiumionsbetweenthepositiveandnegativeelectrodes.Lithiumionsare extractedfromthecathodematerialandinsertedintotheanodematerialthrough theelectrolyte,andtheanodematerialisinalithium-richstatewhencharging.The situationisoppositewhendischarging.Beforetheadventoflithium-ionbatteries, NiMHbatterieswerewidelyusedintheportableelectronicequipmentindustry. Aftertheadventoflithium-ionbatteries,theyaresuperiortoNiMHbatteriesin termsofweight,capacity,andpower.Theenergydensityoflithium-ionbatteriesis greaterthanthatofNiMHbatteries,theself-dischargephenomenonisweakened,as wellasthechargingtimeisshorter.Lithium-ionbatteryenergystoragetechnology currentlyoccupiesaleadingpositionintheenergystorageindustryandhasbeen widelyusedinelectricvehicles,smartgrids,cutting-edgedefenseequipment, biomedicine,portabledevices,artificialintelligence,etc.,asshowninFigure1.3. However,large-scaleapplicationsoflithium-ionbatteriesarestilllimited,and securityandhighcostarethemaininfluencingfactors.Thecurrentcommercial cathodematerialsforlithium-ionbatteriesaremainlyLiCoO2 ,LiMn2 O4 ,and LiFePO4 .TheanodematerialsaremainlycarbonmaterialsandLi4 Ti5 O12 [54]. Theelectrolyteisanonaqueousorganicelectrolyte.Inrecentyears,LiFePO4
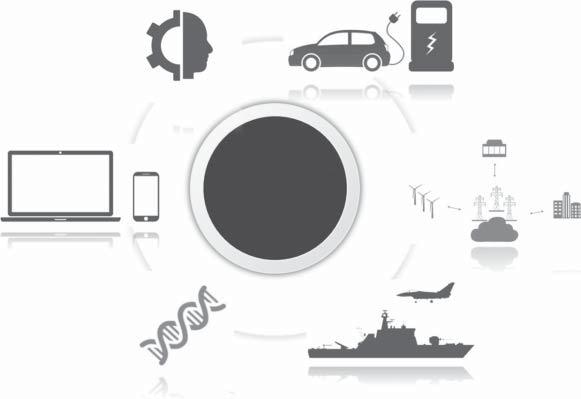
Figure1.3 Mainapplicationareasofhigh-performanceenergystoragedevices.
hasreceivedwidespreadattentionaroundtheworldduetoitsbettersafetyand high-ratedischargeperformance,anditsapplicationinelectricvehiclesandgrid energystoragehasbeenrapidlypromoted[55].
MultivalentIonBatteries
Thereisanincreasingdemandforlithium-ionbatteriesinvariousfieldsduetotheir continuousdevelopment.Theshortageoflithiumresourcesisoneoftheimportant factorshinderingthedevelopmentoflithium-ionbatteries.Inordertoalleviatethis problem,multivalentionbatteries,includingmagnesiumionbatteries,zincionbatteries,aluminumionbatteries,andcalciumionbatteries,havebeenrapidlydeveloped.Multivalentionbatterieshavehighersafetyperformanceandlowercostthan lithiumionbatteries.Moreover,multivalentionbatteriesdirectlyusemetalsasnegativeelectrodematerials,sotheyhavethepotentialtogreatlyincreasetheenergy densityofthebatteriescomparedwithlithiumionbatteryusingcarbonmaterials asnegativeelectrodes.Therefore,ithasgoodapplicationvalueanddevelopment prospectsinthefieldsoflarge-scaleenergystorageandcivilbatteries.Unfortunately, multivalentionbatteriesarestillintheresearchstage,andthestrongpolarization effectandhighrequirementsforelectrolyteareimportantfactorsthatlimitthedevelopmentofmultivalentionbatteries[56].
Supercapacitors
Supercapacitorsaredifferentfromtraditionalbatteries.Theenergystoragemechanismofsupercapacitorswasdividedintophysicalenergystorageandchemical energystorage.Thephysicalmechanismisthatadoublelayerstructureformed bytheinterfaceofelectrode/electrolytewithporouscarbonmaterialwithhigh specificsurfaceareaisusedforenergystorage[57].Thechemicalmechanismis thattherapid,reversibleredoxreactionsbetweenelectrodesandelectrolyteare usedforenergystorage(pseudocapacitance)[58].Therefore,accordingtodifferent energystoragemechanisms,supercapacitorscanbedividedintoelectricdouble layersupercapacitors(EDLC),pseudocapacitors,andhybridcapacitors(both physicalenergystorageandchemicalenergystorage).Itcombinestheadvantages oftraditionalcapacitorsandbatteries,notonlyhastheadvantagesofhighdischarge powerofelectrostaticcapacitors,butalsohasalargechargestoragecapacity, assameasbatteries.Itscapacitycanreachthefaradleveloreventhousandsof faradlevels.Atthesametime,ithastheadvantagesofhighpowerdensity,good cyclestability,strongtemperatureadaptability,andbeingenvironmentallyfriendly. Supercapacitorsarecurrentlywidelyusedindifferentmarketareassuchasauxiliary peakpower,backuppower,storageofrenewableenergy,andalternativepower supplies.Moreimportantly,italsohasverybroadmarketprospectsinindustrial control,windandsolarpowergeneration,transportation,militaryindustry,and otherdirections[59].
1.1.3OverviewofNanowireEnergyStorageMaterialsandDevices
Nanomaterialshaveahighspecificsurfaceareaandexcellentactivity.Whenused asabatteryelectrodematerial,ithasalargecontactareawiththeelectrolyteand
1NanowireEnergyStorageDevices:Synthesis,Characterization,andApplications
ashortiondiffusiondistance,whichcaneffectivelyimprovetheelectricalactivity ofthematerial.Italsohassignificantadvantageswhenusedasahigh-powerbatteryelectrodematerial.Atthesametime,areasonabledesignofnanostructuresis beneficialtoalleviatestressreleaseduringcycling,andisconducivetoimprovingthe stabilityofthestructureinordertoobtainalongercyclelife.Forthisreason,research onlarge-capacity,high-power,long-life,andlow-costelectrochemicalenergystoragetechnologybasedonnewnano-electrodematerialsisoneofthefrontiersand areasoffocusinthelow-carboneconomyera.Lithium-ionbatteries,supercapacitors,andlithium-airbatterieshavebeenextensivelystudiedduetotheirrespective advantages,buttraditionalelectrodematerialsarestilldifficulttomeettheneedsof highcapacityandhighpower.Numerousresearcheshaveshownthattheelectrochemicalpropertiesofelectrodematerialsarecloselyrelatedtotheirscale,internal crystalstructure,andapparentmorphology.NWelectrodematerials,duetotheir uniqueanisotropy,rapidaxialelectrontransmission,andradialiondiffusioncharacteristics,aresuitableforthedesign,integration,andperformancecontrolofalkali metalionbatteries,supercapacitors,transparentflexibleenergystoragedevices,and hybriddevices.
Inrecentyears,thedevelopmentofnanowireshasshowndiversifiedcharacteristics,includingmultilayerNWs,NWclusters,nanotubeclusters,NWclusters,and otherone-dimensionalNWmaterialswithcompositestructuresthathavebeen abletodeveloprapidly.Itisusedforthetipofascanningtunnelingmicroscope, nanodevices,ultralargeintegratedcircuitwires,opticalfibers,micro-drillsin microelectronics,electrodematerialsforenergyhuntingsystems,activematerials forcatalyticsystemssuchasphotocatalysisandelectrocatalysis,andelectrode materialsforenergystoragesystems(suchasmetalionbatteriesandsupercapacitors).Thischapterfocusesontheelectrochemicalenergystoragesystemby discussingthephysicalandchemicalproperties,syntheticchemistry,andpractical applicationsofNWelectrodematerials.
TherearemanywaystosynthesizeNWmaterials,suchashydrothermalmethods[60],electrospinningmethods[61],andtemplatemethods[62].Various directionalcontrolmethodscansynthesizeNWmaterialswithdifferentmorphologies.However,thephysicalandchemicalpropertiesofNWswillbedifferent,and theexternalpropertieswillalsobedifferent.Withtheprogressoftechnology,NW materialshavealsodeveloped,fromconventionalNWstoultra-longNWs[63], mesoporousNWs[64],dendriticNWs[65],etc.Theuniformity,controllability,and electrochemicalpropertiesofNWmaterialshavebeencontinuouslyimprovedwith thecontinuousoptimizationofthestructure.Thespecificpreparationmethodsfor differentNWstructureswillbedescribedindetailinChapter3.
One-dimensionalNWmaterialshavethefollowingadvantagesforelectrochemicalenergystorage.Firstly,comparedtogranularnanomaterials,NWelectrode materialsprovidetransmissionpathswithcontinuousaxialelectronandradial iontransmissionpaths,whichendowNWmaterialswithbetterrateperformance. Secondly,NWmaterialscandirectlygrowonthesubstrateofmetalorcarbon material.Itcanbeusedasaframeworktocompositewithothermaterialswithout theadditionofabinder,constructingacomplexandmulti-elementelectrode
1.1Introduction
structure.Thirdly,NWmaterialshavegeometriccharacteristics,whicharetensof micronsinlengthandtensofnanometersindiameter,makingiteasytomakea singleNWelectrochemicaldeviceforinsituelectronmicroscopyandspectroscopy characterization[66].
Atthesametime,theuseofNWmaterialsforelectrochemicalenergystorage alsohasthefollowingchallenges.Firstly,whensemiconductorNWsareusedfor electrochemicalenergystorage,theirpoorconductivityrequiresadditionalconductiveagentsorconductivematerialcoatingslikecarboncoatings,whichincreases thecomplexityoftheelectrodeconstructionprocess.Besides,thecontinuouscharginganddischargingprocesseswillcausethedeteriorationofthematerialstructure, whichwillaffecttheaxialelectronconductionoftheNWmaterial,thusaffecting thecyclestabilityofthebattery.Additionally,thematerialsintheelectrodenetwork createdbyoverlappingNWsarelargelyinpointcontactwithoneanother,which reducestheconductivityoftheentireelectrodeandraisestheinternalresistanceof thebattery[66].InviewoftheadvantagesandchallengesofusingNWmaterialsfor energystorage,researchershaveproposedavarietyofoptimizationstrategiesand designedandconstructedNWelectrodematerialswithvariousstructures.
Energystoragematerialsanddeviceswithhighenergydensity,goodpower density,andlongservicelivesaremajorrequirementsforthedevelopmentofnew energyvehicles,smartgrids,andcutting-edgenationaldefenseequipment.However,rapidcapacitydecayanddifficultybalancingenergyandpowerdensityare majorbottlenecks,restrictingthedevelopmentofhigh-performanceenergystorage materialsanddevicetechnologies.Inordertosolvetheabovebottlenecks,the authorofthisbookhasbeencommittedtotheresearchofNWenergystoragematerialsanddevicesincombinationwiththeadvantagesofNWmaterialsdescribed above.TheauthorandhisteamfocusedonbasicresearchandapplicationdevelopmentofNWelectrodematerialsinbasictheory,controlledgrowth,performance regulation,deviceassembly,insitucharacterization,andenergystorageapplications.TheydesignedandassembledthefirstsingleNWsolid-stateenergystorage deviceformonitoringcapacitydecayinreal-timeandtooktheleadinrealizingthe large-scalepreparationandapplicationofhigh-performanceNWenergystorage devicesandkeymaterials,achievingmanyinnovativeresearchresults.Themain researchcontentoftheNWenergystoragedevicesinvolvedinthisbookisshown inFigure1.4.
1.1.3.1SiNanowires
Siliconmaterialshavethecharacteristicsofhighspecificcapacity(4200mAhg 1 ) thatothermaterialscannotachieveasnegativeelectrodematerials.Ithasattracted theattentionofresearchersandisthethird-generationnegativeelectrodematerial thatcanreplacegraphitematerials[67].Inaddition,assiliconelementisgenerally nontoxic,abundant,andwidelyavailable,newsystemsmayhavecostandrecyclingbenefits.Theapplicationofsiliconinlithiumbatteriesoriginatedinthe1970s. Nelsonetal.[68]andSharmaetal.[69]reportedthatthephenomenonofreversible lithiationanddelithiationofsiliconathightemperature.Subsequently,researchers suchasWeydanzetal.[70],Gaoetal.[71],Limthongkuletal.[72],Lietal.[73],
Supercapacitor Double layer capacitors
Pseudocapacitors
Figure1.4 Mainresearchcontentsofnanowireenergystoragedevices. andObrovacetal.[74]conductedin-depthresearchandgraduallyimprovedthe reversiblelithiumstoragemechanismofsiliconmaterials.
Nanotechnologyappliedtosiliconmaterialscanresolvethevolumeexpansion ofsilicon-basedanodematerialsduringcycle.Thevolumeexpansionphenomenon willcausestructurecollapseoftheactivematerialsandsheddingofthecurrent collectortoloseelectricalcontactwhenthelithiumionsarereleased,therebyaffectingthecycle’sperformance.Yueetal.[75]usednanosiliconpowderswithdifferent sizes(5,10,and20nm)toprepareanodematerialsandfoundthatsiliconparticles withaparticlesizeof10nmhavethehighestspecificcapacityafterelectrochemicaltests.One-dimensionalNWsandnanotubescanalsoimprovetheproblemof volumeexpansion,exceptforzero-dimensionalnanoparticles.SiNWshavemany excellentpropertiesthataredifferentfromthoseofgeneralsiliconmaterials,such aselectrontransport,fieldemissioncharacteristics,surfaceactivity,andquantum confinement[76].Therefore,ithasawiderangeofapplicationprospectsinthe manufactureoflow-dimensionalnanodevices[77].Atpresent,thepreparationand applicationofnanodevicessuchasSiNWtransistors,sensors,andNWbatterieshave beenrealized.
ThebatterymadewithSiNWsastheanodehasbetterstoragecapacitythanthebatterymadewithtraditionalgraphiteastheanode.Traditionalgraphiteanodesneed sixcarbonatomstostoreoneLi+ ,whileoneSiatomcanstorefourLi+ .However, alargenumberofmovingLiatomswithhighspeedwillbreaktheSiinabattery madeofSithinlayersorSiionsandwillalsodestroythebondbetweenSiandthe substrate,weakeningthepowerstoragecapacity.Cuietal.ofStanfordUniversity preparedSiNWsonastainlesssteelsubstrateandthenmadealithiumionbatterywithSiNWs,whichnotonlyincreasedtheanodecapacityofthebatterybut alsoensuredthattheSiNWsneverfelloffthesubstrateafteraseriesofexpansion
Hybrid capacitors
Chip type
Flexible electrodes and energy storage devices
Fibrous type
Layered type Li+, Na+, K+ monovalent ion batteries