MXeneMembranesforSeparations
HaihuiWang YanyingWei
LiDing
Prof.HaihuiWang
SouthChinaUniversityofTechnology SchoolofChemistry&Chemical Engineering Building16,SouthChinaUniTech No.381,WushanRoad TianheDistrict 510640Guangzhou China
Prof.YanyingWei
SouthChinaUniversityofTechnology Chemistry&ChemicalEngineering No.381,WuShanRoad TianheDistrict 510640Guangzhou China
Dr.LiDing
SouthChinaUniversityofTechnology ChemistryandChemicalEngineering No.381,WushanRoad,Bldg.16 TianheDistrict 510640Guangzhou China
Cover: shutterstock.com
Allbookspublishedby WILEY-VCH arecarefully produced.Nevertheless,authors,editors,and publisherdonotwarranttheinformation containedinthesebooks,includingthisbook, tobefreeoferrors.Readersareadvisedtokeep inmindthatstatements,data,illustrations, proceduraldetailsorotheritemsmay inadvertentlybeinaccurate.
LibraryofCongressCardNo.: appliedfor
BritishLibraryCataloguing-in-PublicationData Acataloguerecordforthisbookisavailable fromtheBritishLibrary.
Bibliographicinformationpublishedby theDeutscheNationalbibliothek TheDeutscheNationalbibliotheklists thispublicationintheDeutsche Nationalbibliografie;detailedbibliographic dataareavailableontheInternetat <http://dnb.d-nb.de>.
©2022WILEY-VCHGmbH,Boschstr.12, 69469Weinheim,Germany
Allrightsreserved(includingthoseof translationintootherlanguages).Nopartof thisbookmaybereproducedinanyform–by photoprinting,microfilm,oranyother means–nortransmittedortranslatedintoa machinelanguagewithoutwrittenpermission fromthepublishers.Registerednames, trademarks,etc.usedinthisbook,evenwhen notspecificallymarkedassuch,arenottobe consideredunprotectedbylaw.
PrintISBN: 978-3-527-34794-0
ePDFISBN: 978-3-527-82883-8
ePubISBN: 978-3-527-82885-2
oBookISBN: 978-3-527-82884-5
CoverDesign ADAMDESIGN,Weinheim, Germany
Typesetting Straive,Chennai,India
PrintingandBinding
Printedonacid-freepaper 10987654321
Contents
Preface ix
AbouttheAuthors xi
Acknowledgment xiii
AbbreviationsandSymbols xv
1Introduction 1
1.1MembraneDevelopmentataGlance 1
1.2Two-DimensionalMembranes 1
1.3SeparationMechanismsof2DMembranes 2
1.4FabricationMethodsfor2DMembranes 4
1.5Applicationsof2DMembranes 6
References 6
2Typesof2DMaterial-BasedMembranes 9
2.1PorousTwo-DimensionalNanosheet-BasedMembranes 9
2.1.1Zeolite2DMembranes 9
2.1.2Metal–OrganicFramework2DMembranes 10
2.1.3CovalentOrganicFramework(COF)2DMembranes 11
2.1.4GraphiticCarbonNitride(g-C3 N4 )Membranes 12
2.2Nonporous2DNanosheet-BasedMembranes 13
2.2.1Graphene-BasedMembranes 13
2.2.2LayeredDoubleHydroxide(LDH)Membranes 15
2.2.3TransitionMetalDichalcogenide(TMD)Membranes 15
2.2.4MXeneMembranes(TypicallyTi3 C2 Tx ) 16
References 18
3MXeneNanosheetsandMembranes 25
3.1PreparationandCharacterizationofMXeneNanosheets 25
3.1.1Top-downSynthesis 25
3.1.2Bottom-upSynthesis 31
3.2PreparationandCharacterizationofMXeneMembranes 34 References 40
4MXeneMembranesforNanofiltration 43
4.1Introduction 43
4.2SeparationPerformanceofMXene-BasedNanofiltrationMembranes 44
4.3Summary 57 References 57
5MXeneMembranesfortheIsolationofAntibiotics 61
5.1Introduction 61
5.2PhysicalAdsorption 62
5.3AdvancedOxidation 68
5.4MembraneSeparation 72
5.5Summary 84 References 84
6MXene-BasedMembranesforGasSeparation 89
6.1Introduction 89
6.2GasSeparationPerformanceofMXene-BasedMembranes 90
6.3Summary 101 References 102
7MXeneMembranesforIonSeparation 105
7.1Introduction 105
7.2Self-cross-linkedMXeneMembranesforMonovalentMetalIon Sieving 106
7.2.1PreparationofSelf-cross-linkedMXeneMembranes 106
7.2.2MonovalentMetalIon-sievingPerformanceofSelf-cross-linkedMXene Membranes 107
7.2.3CharacterizationofSelf-cross-linkedMXeneMembranes 109
7.3ThermallyCross-LinkedMXeneMembranesforHeavyMetalIon SeparationbyaVoltage-supportedProcess 112
7.3.1Mixed-ionSievingandExclusionoftheHeavyMetalIonPb2+ 113
7.3.2CharacterizationofThermallyCross-LinkedMXeneMembranes 115
7.4UltrathinMXene-DerivedMembranesbySinter-cross-linkingwith TunableInterlayerSpacing 117
7.4.1PropertiesandIon-rejectionPerformanceofSinter-cross-linkedMXene Membranes 117
7.4.2CharacterizationofSinter-cross-linkedMXeneMembranesandMXene NanosheetsatDifferentSinteringTemperatures 120
7.5Al3+ -cross-linkedMXeneMembranes 121
7.5.1PreparationandCharacterizationofAl3+ -cross-linkedMXene Membranes 122
7.5.2Ion-sievingPerformanceofAl3+ -cross-linkedMXeneMembranes 123
7.6Summary 126 References 126
8MXeneMembraneforOil/WaterEmulsionSeparation 129
8.1Introduction 129
8.2FunctionalPolymerLayeronSupport 130
8.3Low-DimensionalMaterials 134
8.4Summary 151 References 151
9MXeneMembranesforSalinityGradientEnergy Conversion 157
9.1Introduction 157
9.2PerformanceofMXeneMembranesforSalinityGradientEnergy Conversion 158
9.3Summary 169 References 170
10Scale-UpofMXeneMembranes 175
10.1Introduction 175
10.2Scale-Upof2DMembranes 176
10.2.1SpinCoating 177
10.2.2SprayCoating 177
10.2.3DropCoatingandDipCoating 179
10.2.4DoctorBladeMethod 179
10.2.5ElectrophoreticDeposition(EPD) 181
10.3Summary 191 References 192
11Perspectives 197
11.1FurtherApplicationsofMXeneNanosheets 197
11.2ChallengesandOutlookforMXeneMembranes 198
11.2.1StabilityofMXeneNanosheetsMustBeImproved 198
11.2.2ScalableFabricationofMXeneMembraneswithSuitableInterlayer ChannelsIsRequired 198
11.2.3OperatingTimeofMXeneMembranesunderRealisticOperation ConditionsMustBeExtended 199
11.2.4FundamentalsofMassTransportMechanismsinConfined Nanochannels/Sub-nanochannelsWithinMXeneMembranesHastoBe Studied 199 References 200
Index 203
Preface
Membrane-basedseparationtechnologieshavereceivedincreasingattention attributingtolotsofadvantagessuchaslowenergyconsumption,easyoperation,andenvironmentalfriendliness.Inrecentyears,theemergenceofnovel two-dimensional(2D)materials,suchasgraphene,providesanewopportunityfor membranedevelopment.EmergingMXene-basedmembranesplaysignificantroles inthecompetitivemembrane-separationfield.ThedevelopmentoftheseMXene membranesisofbothscientificandtechnologicalinterest.
Theauthorhasbeendevotedtothedevelopmentofinorganicmembranedesign andnovelmembranematerialssince1998whenhewasaPhDcandidatein Prof.WeishenYang’sgroupintheChineseAcademyofSciences.From2006to 2020,heworkedinSouthChinaUniversityofTechnologyandbuiltupthegroupof “MembraneScienceandEnergyMaterials”(http://www.scut.hhwang.ycym.com/ scut).Themainresearchfieldofthisgroupconcernsthefundamentalresearch oninorganicmembranesforcleanenergyandenvironment.Theyhavecarried outmanymeaningfulresearchworkssuchasinorganicmembraneseparation, membranecatalysisreactors,andnewenergymaterials.Themainresearchorientationsinthegroupincludeinorganicmembraneandmembraneprocess,new energymaterials,andenergydevices.Thefeaturesandvisionofsuchresearch grouparefocusingonthefundamentalresearch,aimingatthescientificfrontier andorientingsignificantdemandfromsociety,toobtaininnovationalachievement, anddevoteourselvestotakeupthechallengeswithhumanbeing’sdevelopment, energy,andenvironmentalissues.HeiscurrentlyaprofessoratTsinghuaUniversity (http://www.chemeng.tsinghua.edu.cn/info/1165/2592.htm).
Thisbookisthefirstonethatcomprehensivelyandsystematicallyintroduces MXenemembraneswithvariousapplicationsforseparation.Thecontentsrange fromtheMXenemembranesforgasseparation,ionrejection,nanofiltrationfor dyesandantibiotics,etc.,aswellasthescale-upprospectsof2DMXenemembranes.Eachchapterisindependent,inwhichthedesignconcept,fabrication strategyandmethods,separationperformanceofMXenemembraneswithdifferent structures,andconclusionsareclearlydescribed.Livelyschematicillustrations andpicturesthroughoutthetexthelpmakethetheoryandtechnologiesmore accessibletoreaders.Theauthorsincerelyhopesthatthisbookwillbeavaluable
x Preface referenceworkfordesigningandpreparingMXene-basedmembranesforvarious separationpurposes.
Thebookiscomposedof11chapters.InChapter1,abriefintroductionof2D membranesasemergingnovelmembranesareoutlined,includingtheseparation mechanism,preparationmethods,andseparationapplications.InChapter2,two typesof2Dmaterials-basedmembranesareintroduced,includingtheporousand nonporous2Dnanosheet-composedmembranes.InChapter3,thepreparationand characterizationoftheMXenenanosheetsandMXenemembranesaredescribed indetail.InChapters4–8,differentMXenemembraneswithstructuresdesigned inparticularappliedfornanofiltration,isolationofantibiotics,gasseparation,ion separation,andoil/wateremulsionseparationareintroduced,especially.Andin Chapter7,twotypesofMXenemembranesforionseparationareintroduced,such asself-cross-linkedMXenemembranesandAl3+ cross-linkedMXenemembranes. InChapter9,theMXenemembranesusedforsalinitygradientenergyconversion aredescribed.InChapter10,thepromisingscale-upfabricationtechnologiesof MXenemembranesarepresent,includingspincoating,spraycoating,dropcoating, dipcoating,doctorblademethod,andelectrophoreticdeposition,etc.Finally, perspectivesonthedevelopmentofMXenenanosheetsandMXenemembranesare giveninChapter11.
Mostofthecontentsinthisbookarethefreshachievementsoftheauthor’s grouponMXenemembranesinrecentyears;ofcourse,manyotherrelated worksarealsodiscussed.Theauthorgratefullyacknowledgesthefinancial supportforthecontinuousstudyofMXenemembranesfromtheNaturalScienceFoundationofChina(22022805,21861132013,22078107,and 22008077),GuangdongNaturalScienceFundsforDistinguishedYoungScholar (2017A030306002),GuangdongBasicandAppliedBasicResearchFoundation (2019A1515110958),ChinaPostdoctoralScienceFoundation(2019TQ0101and 2019M662920).
TheauthorisverygratefultoProf.CaroJuergenatLeibnizUniversityof Hannover,GermanyandProf.YuryGogotsiattheDepartmentofMaterialsScience andEngineering,DrexelUniversity,USA.Theauthorwouldliketothankallhis formerandcurrentstudentswhocontributedtothestudyonMXenemembranes, especiallyDr.ZhongkunLi,MasterZongLu,JunjieDeng,WeiWang,andChunjie Chen,fortheirhardworkandcreativeresearchondevelopingMXenemembranes withvariousstructures.
Finally,theauthorisdeeplyindebtedtohisfamilymembers,especiallyhiswife, FanZhangandhisson,SunuoWang,fortheirlove,encouragement,andsupport.
Guangzhou,China April2021
HaihuiWang
AbouttheAuthors
Prof.HaihuiWang receivedhisPhDdegreefrom theChineseAcademyofSciencesin2003.Aftera one-and-a-half-yearstayasanAlexandervonHumboldt ResearchFellowintheInstituteofPhysicalChemistry andElectrochemistry,LeibnizUniversityofHannover, Germany,hecontinuedhisresearchthereasapostdoctoral fellowfrom2005.From2007to2020,heworkedattheSouth ChinaUniversityofTechnology.FromNovember2020,heis afullprofessoratTsinghuaUniversity,China.Hisinterests focusoninorganicmembranes,membranereactors,and energymaterials.Hehaspublishedover240peer-reviewedjournalpapersat Nat. Energy, Nat.Sustain., Sci.Adv.,etc.,withcitationofmorethan16000,andheld28 authorizedpatents.

Prof.YanyingWei receivedherPhDdegreeunderthe supervisionofProf.HaihuiWangattheSouthChina UniversityofTechnologyin2013.During2013–2015,she workedasaHumboldtresearchfellowinProf.Juergen Caro’sgroupatLeibnizUniversityofHannover.SheiscurrentlyaprofessorintheSchoolofChemistry&Chemical EngineeringattheSouthChinaUniversityofTechnology. Herresearchfocusesonnovel2Dnanosheetmembranes,MOFmembranesforvariousseparations,and catalyticmembranereactors.Shehaspublishedover70 peer-reviewedjournalpapersat Nat.Sustain., Nat.Commun., Sci.Adv., JACS, Angew.Chem.Int.Ed.,etc.,2co-authoredmonographsandheld39patents.

Dr.LiDing isapostdoctoralresearchfellowintheSchool ofChemistryandChemicalEngineeringattheSouthChina UniversityofTechnology,workingwithProf.HaihuiWang. HereceivedhisPhDdegreeinchemicalengineeringat theSouthChinaUniversityofTechnologyin2019,under thesupervisionofProf.HaihuiWang.Hiscurrentresearch focusesonMXene-basedmembranesforefficientseparationapplicationsandmasstransportinnanochannels.He haspublishedover13peer-reviewedjournalpapersat Nat. Sustain.,Nat.Commun.,Angew.Chem.Int.Ed.,etc.,with citationmorethan1200.
Acknowledgment
WegratefullyacknowledgethefundingsfromtheNaturalScienceFoundationof China(22022805,21861132013,and22078107),andGuangdongNaturalScience FundsforDistinguishedYoungScholar(2017A030306002).
AbbreviationsandSymbols
Abbreviations
2DTwo-Dimensional
AAOAnodicAluminumOxide
ACActivatedCarbon
AEPPS N -AminoethylPiperazinePropaneSulfonate
AFMAtomicForceMicroscopy
AgNPsSilverNanoparticles
AHAzithromycin
ANFKevlarNanofiber
APTESAminopropyltriethoxysilane
BBBrilliantBlue
BCNCarbonBN
BEBindingEnergy
BETBrunauer,Emett,Teller
BNBoronNitride
BNNSBNNanoSheets
BPABisphenolA
BSABovineSerumAlbumin
CACalciumAlginate,andCephalexin
CAPChloramphenicol
CFXCiprofloxacin
CMPCarbamazepine
CMCo3 O4 /MXeneComposite
C3 N4 CarbonNitride
CNCCelluloseNanocrystal
CNFsCelluloseNanofibers
CNTCarbonNanotube
CNTCg-C3 N4 /Ti3 C2
COFCovalentOrganicFramework
COFsCovalentOrganicFrameworks
CPClindamycinPhosphate
xvi AbbreviationsandSymbols
C-PVDFCommercialPolyvinylideneFluoride
CTXCefotaxime
Cyt.cCytochromec
DDiffusionCoefficient
DCDoxycycline
DCFDiclofenac
DFTDensityFunctionalTheory
DIDeionizedWater
DLSDynamicLightScattering
DSCDifferentialScanningCalorimetry
EBEvansBlue
EDLElectricalDoubleLayer
EDS/EDX/EDXEnergyDispersiveX-raySpectroscopy
EE217-α-Ethynylestradiol
EPDElectrophoreticDeposition
FFTFastFourierTransform
FOForwardOsmosis
FTIRFourierTransformInfraredSpectroscopy
FTOFluorinatedTinOxide
GAGallicAcid
g-C3 N4
GraphiticCarbonNitride
GMGrapheneOxide/MXeneComposite
g-MoS2
Graphene-likeLayeredMolybdenumDisulfide
GOGrapheneOxide
GPUGasPermeationUnit
HAADFHigh-AngleAnnularDark-Field
HRTEMHigh-ResolutionTransmissionElectronMicroscope
HTHydrotalcite
ICIonChromatography
ICP-OESInductivelyCoupledPlasmaOpticalEmissionSpectroscopy
IEPIsoelectricPoint
IFFTInverseFastFourierTransform
IPAIsopropanol
ITOIndiumTinOxide
LBLangmuir–Blodgett
LbLLayer-by-Layer
LDHLayeredDoubleHydroxide
LSLangmuir–Schäfer
MAXTi3 AlC2
MAXMn+1 AXn
MBMethylBlue
MBCNMesoporousBoronNitride-Carbon
MCEMixedCelluloseEster
MDMolecularDynamicsandMembraneDistillation
AbbreviationsandSymbols xvii
MFIMobileFiveRing
MGMethylGreen
MMMsMixedMatrixMembranes
MnBMethyleneBlue
MOFsMetal–OrganicFrameworks
MPC-co-AEMAPoly(2-methacryloyloxyethyl-phosphorylcholine-co-2aminoethyl-methacrylatehydrochloride)
MRMethylRed
MWCOMolecularWeightCutoff
MXMAl3+ -intercalatedMXeneMembrane
MXM-REDMXeneMembranes-BasedReverseElectrodialysis
NFNanofiltration
NMRNuclearMagneticResonance
N-MXMNegativelyChargedMXeneMembrane
NORNorfloxacin
NPGNanoporousGraphene
OFLOfloxacin
OTCOxytetracycline
PPermeability
PAAPolyacrylicAcid
PAA-g-PVDEPoly(acrylicacid)-graftedPVDF
PAMPolyacrylamide
PAN-HPolyacrylonitrile-Hydrolysis
p-BNPorousHexagonalBoronNitride
PCPropyleneCarbonateandProanthocyanidins
PDAPolydopamine
PDMSPolydimethylsiloxane
PEGPolyethyleneGlycol
PEIPolyetherimide
PEIPolyethyleneimine
PESPolyethersulfone
PFTSPerfluorode-cyltriethoxysilane
PGPPolymer@GO@polyacrylonitrile
PIPolyimide
PIMsPolymersofIntrinsicMicroporosity
PKPolyketone
PM2.5 ParticulateMatter,particlesthathavediameterlessthan 2.5 μm
PMFPotentialofMeanForce
PMMPristineMXeneMembrane
PMSPeroxymonosulfate
P-MXMPositivelyChargedMXeneMembrane
PNPPoissonandNernst–Planck
PROPressure-RetardedOsmosis
PTFEPolytetrafluoroethylene
xviii AbbreviationsandSymbols
PVDEPolyvinylideneFluoride
PVDFPolyvinylideneDifluoride
PXRDPowderX-rayDiffraction
REDReverseElectrodialysis
rGOReducedGrapheneOxide
RhBRhodamineB
RosBRoseBengal
ROXRoxithromycin
RTRoomTemperature
SSolubility
SAEDSelectedAreaElectronDiffraction
SCMM-80Self-Cross-linkedMXeneMembranesobtainedat80 ∘ C
SCMM-120Self-Cross-linkedMXeneMembranesobtainedat120 ∘ C
SCMM-180Self-Cross-linkedMXeneMembranesobtainedat180 ∘ C
SCMMsSelf-Cross-linkingMXeneMembranes
SDZSulfadiazine
SEMScanningElectronMicroscope
SMXSulfamethoxazole
SSStainlessSteel
SSFSinteredSilicaFiber
SSMStainlessSteelMeshes
STEMScanningTransmissionElectronMicroscopy
STPStandardTemperatureandPressure
SWCNTSingle-WalledCarbonNanotube
TCTetracycline
TCGTiO2 /Co3 O4 /GOHeterojunction
TCHTetracyclineHydrochloride
TEMTransmissionElectronMicroscopy
TFNThin-FilmNanocomposite
TGAThermogravimetricAnalysis
TMAOHTetramethylammoniumHydroxide
TMCTrimesoylChloride
T/MCTiO2 /MXene(Ti3 C2 Tx )
TMDsTransitionMetalDichalcogenides
TMNTransition-MetalNitride
TMPyP
α,β,γ,δ-Tetrakis(1-methylpyridinium-4-yl)porphyrin
TOBTobramycin
VAFVacuum-AssistedFiltration
VCVitaminC
VFVacuumFiltration
VFBVanadiumFlowBattery
XPSX-rayPhotoelectronSpectroscopy
XRDX-RayDiffraction
ZIFZeoliticImidazolateFrameworks
AbbreviationsandSymbols xix
Symbols
Chapter1
d Effectivedistancebetweentheplatelets
DA GasdiffusivityinpathwayA
DB GasdiffusivityinpathwayB
h Membranethickness
K A GasadsorptionequilibriumconstantinpathwayA
K B GasadsorptionequilibriumconstantinpathwayB
l Diffusionlength
L Lateraldimensionoftheplatelet
P Membranepermeability
W Thicknessoftheplatelet
��A PorosityforporesofpathwayA
��B PorosityforporesofpathwayB
�� A TortuosityforporesofpathwayA
�� B TortuosityforporesofpathwayB
Chapter5
F Faradayconstant
R Idealgasconstant
�� Permittivity
�� Zetapotential
�� Debyelength
�� SurfacechargedensityoftheTi3 C2 T x membrane
Chapter6
A EffectiveareaoftheMXenemembrane
C Constant
d Spacingbetweenthediffractionplanes
dv/ dt Volumetricdisplacementrateinthebubbleflowmeter
Eact Apparentactivationenergy
Ediff Diffusionactivationenergy
L Thicknessofthemembrane
n Aninteger(1,2,3, )
P Permeability
Patm Atmosphericpressure
Pi Permeabilityof i component
Pj Permeabilityof j component
xx AbbreviationsandSymbols
R Idealgasconstant
T Temperature
x i
x j
yi
yj
Volumetricfractionsofthe i componentinthefeedside
Volumetricfractionsofthe j componentinthefeedside
Volumetricfractionsofthe i componentinthepermeateside
Volumetricfractionsofthe i componentinthepermeateside
�� Selectivityoftwocomponentsinthesinglegaspermeation
�� i/j
Selectivityoftwocomponentsinthemixedgaspermeation
ΔA Pre-exponentialfactor
ΔH ads Heatofadsorption
Δp Transmembranepressure
�� Incidentangle
�� WavelengthoftheX-ray
Chapter7
A Effectiveareaofthemembrane
C Concentration
Cd Saltconcentrationinpermeateside
Cf Saltconcentrationinfeedside
J i Ionpermeationrate
J r Saltrejection
J w Waterflux
t Permeationtime
V Effectivevolume
Δt Timeevolved
ΔV Volumeofliquidchange
�� Measuredconductivity
Λm Molarconductivity
Chapter8
aDLS
Peakpositionattheprimaryintensityparticlesize
I SC Short-circuitcurrent
L Meansheetsize
Pout Outputpowerdensity
RL Loadresistance
RM Internalresistance
V OC Open-circuitvoltage
AbbreviationsandSymbols xxi
Chapter9
I SC Shortcircuitcurrent
Pout Outputpowerdensity
RL Loadresistance
RM Internalresistance
V OC Open-circuitvoltage
Chapter10
A Surfaceareaofanelectrode
C Particleconcentrationinsuspension
Cd Saltconcentrationinpermeateside
Cf Saltconcentrationinfeedside
E Strengthofelectricfield
f Efficiencycoefficientofdeposition
J r Saltrejection
S1 Surfaceareaofnanosheet
Sr Resistancearea
t Depositiontime
v Driftvelocityofthemovingsheets
w(t)Massofdepositforacolloidalsolution
H Viscositycoefficientofthesolvent
K Constantfactor
�� Electrophoreticmobilityofaparticle
�� Surfacechargedensity
Introduction
Inthischapter,thestate-of-the-artdevelopmentofmembranesisbrieflyhighlighted.Two-dimensional(2D)membranesandtheirseparationmechanisms andfabricationprocessesareintroducedtoemphasizethehighpotentialof2D membranesforpermeationseparation.Finally,someapplicationsof2Dmembranes arebrieflydiscussed.
1.1MembraneDevelopmentataGlance
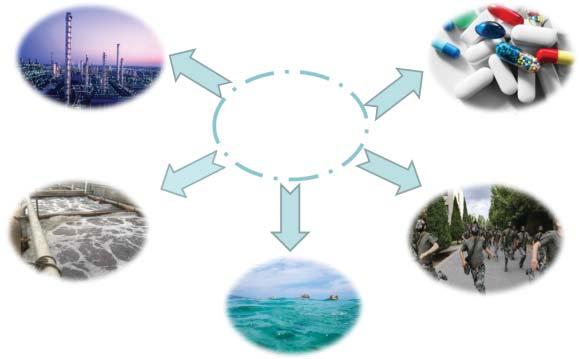
Separationprocessesareimportantforindustrialdevelopmentandalsoinour dailylife.Theyhavebeenimplementedforvariouspurposes(Figure1.1)suchas thetreatmentofenvironmentalpollutants(includingparticulatematter(PM)2.5 rejection,wastewatertreatment,andoilremovalfromseawater),drinkingwater production(includingresidentialwatersofteningandwaterpurificationanddesalinationofseawater),foodproduction(ethanoldehydrationforliquorpurification andproductionofmilkanddrinks),drugdeliveryinbiomedicine,gasseparation, chemicalseparationinpetrochemicalandchemicalindustries,aswellasmilitary anddefense.Mostseparationprocessesinvolvedistillationbasedonheating,which accountsfor10–15%oftheworld’senergyconsumption[1,2].
Membrane-basedseparationtechnologieshaveattractedincreasingattention inrecentdecadesbecauseoftheirlowenergyconsumption,easyoperation,and environmentalfriendliness.However,conventionalmembranes,suchasorganic membranes,typicallysufferfromatrade-offbetweenselectivityandpermeability, asexpressedthroughtheRobesonplot[3].Thus,novelmembranesthatsurpassthe Robesonupperlimitmustbedeveloped.
1.2Two-DimensionalMembranes
Withtheemergenceofnovel2Dmaterials,suchasgraphene,aneweraofmembrane developmenthasbegun.ThediscoveryofmonocrystallinegraphiticfilmsbyGeim’s groupin2004hasdrawnwidespreadattentionto2Dmaterialsformembraneseparation[4].Inadditiontotheearlieststudiedgraphene,othernovel2Dmaterialssuchas MXeneMembranesforSeparations, FirstEdition.HaihuiWang,YanyingWei,andLiDing. ©2022WILEY-VCHGmbH.Published2022byWILEY-VCHGmbH.
Figure1.1 Separationprocessesinoursociety.
zeolites,metal–organicframeworks(MOFs),covalentorganicframeworks(COFs), grapheneoxide(GO),layereddoublehydroxides(LDHs),transitionmetaldichalcogenides(TMDs),MXenes,andg-C3 N4 havealsobeenusedasnano-buildingblocks toprepare2Dmembranes.Sincetheprecisecontrolofinterlayerchannel/poresize andreductionofmasstransferresistancebecamefeasible,ultrathin2Dnanosheet membranesachievehighpermeabilityandhighselectivity,surpassingtheRobeson upperlimit,thusmakingthesenovel2Dmembranespromisingcandidatesfor permeationseparation.
1.3SeparationMechanismsof2DMembranes
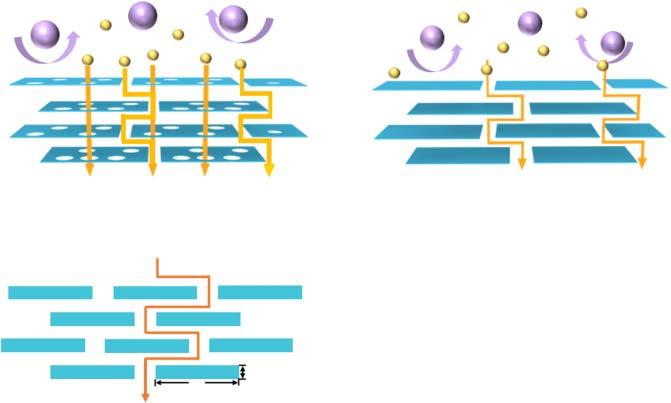
Inmembraneseparation,ions,atoms,ormoleculesselectivelypassthrough amembrane,whichservesastheseparationmedium.2Dmembranescanbe assembledusingtwotypesofnanosheets.Onemembranetypeistheporous 2Dnanosheets,suchaszeolite[5,6],MOF[7–9],COF[10,11],andg-C3 N4 [12,13].In2Dmembranesassembledwithporousnanosheets,theinterlayer nanochannels/sub-nanochannelsareutilizedformasstransportation,andthe poresineachnanosheetcontributetothepermeationflux.Moreimportantly, themasstransportationpathlengthcanbedrasticallyreducedbecauseofthe presenceofnanopores/sub-nanoporesinthenanosheets.Thesmallmolecules tobeseparateddiffuseonlyashorterwaythroughtheporesinthenanosheets, thusavoidingatedious,long,zigzagpathwayasthelargemoleculesthatcannot passtheporeinthenanosheets(Figure1.2a).Theothertypeisthenonporous2D nanosheets,suchasGO[14–16],MXene[17,18],andTMDs[19,20].Inthe2D membranesconstructedbyusingthenonporousnanosheets,thepermeateflux goesonlythroughtheinterlayerspacingbetweenthenanosheets(Figure1.2b).
Pathway BPathway A
Pathway A
Figure1.2 Transportmodelsof2Dmembranes.(a)Porousnanosheets,(b)nonporous nanosheets,and(c)inter-sheetpathwayA.
Ingeneral,therearetwotypesofpathwaysformasstransportationthrough2D membranes:inter-sheetpathwayA(whichreferstotheinterlayerspacingbetween thenanosheets)andintra-sheetpathwayB(whichreferstothein-planepore-based transport).Forgasseparation,themembranepermeabilityPcanbecalculatedusing thefollowingequation[21]:
where h isthemembranethickness; �� and �� aretheporosityandtortuosityforthe poresofAandBpathways,respectively; D and K arethegasdiffusivityandadsorptionequilibriumconstantintheAandBpathways,respectively.Thetortuosity(�� ) of2Dmembranescanbeapproximatelycalculatedastheratioofthelateralsizeto thethicknessofananosheet,whileporosity(��)isrelatedtotheinterlayer d-spacing, thickness,andporosityofthenanosheets.
Forinter-sheetpathwayA,thediffusionlengthcanbeestimatedusingtheNielsen transportmodel[22]:
where l isthediffusionlength, h isthemembranethickness, L and W arethelateral dimensionsandthicknessoftheplatelet,respectively,and d istheeffectivedistance betweentheplatelets(Figure1.2c).
1.4FabricationMethodsfor2DMembranes
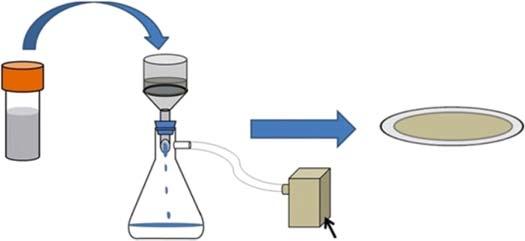
Pressure-assistedfiltration,asthemostwidelyusedmethodtofabricate2Dmembranes,hasdistinctadvantagesoverothermethods,suchassimpleoperationand lowcost.Thenanosheetsarefilteredonaporoussubstratefromananosheetsuspensionwithorwithoutvacuumsupport(Figure1.3)[23].Notably,themembrane thicknesscanbeadjustedbythenanosheetconcentrationinthedispersionandthe durationofdeposition.However,forthelarge-scaleandreproduciblefabrication ofmembranelayerswithappropriatethickness,pressure-assistedfiltrationisnot theoptimalapproach.Asthemembranebecomesthicker,cracksandwrinkles cangenerateonitssurface,resultinginlowseparationperformance.Further,as thefiltercakethickens,thefiltrationresistanceincreases,whichincreasesthe preparationtimeformembraneswithlargerthickness[24].
Moreover,maincoatingmethodsforfabricating2Dlamellarmembranesarespin coating,spraycoating,anddropcasting[25].Indropcasting,2Dmembranesare obtainedbydepositingnanosheetsuspensionsinadropwisemannerontoaporous support.However,inspincoating,thenanosheetsuspensionisdroppedonaspin coater,whichpromotessolventevaporationifheated,topreparethemembranelayer duringrotation.Inspraycoating,thenanosheetsolutionisdispersedintosmall dropsandsprayedontothe(heated)substratesurface,whichalsofacilitatessolvent evaporation.Althoughdropcastingandspraycoatinghavemanyadvantagessuch assimpleprocessing,wide-scaleapplicability,andhighspeed,thesemethodshave someproblemsthatneedtobesolved.Forexample,itisdifficulttopreparehomogeneousmembranesonalargescaleusingdropandspraycoating.
Layer-by-layer(LbL)assemblyistheprocessofdepositingmultilayershaving interactionssuchaselectrostaticattraction,hydrogenbonding,andevencovalent bondformation.IntheLbLprocess,themembranethicknesscanbeprecisely controlledbythenumberofdepositioncycles.Inaddition,differentmaterials canbedepositedineachcycletoimprovemembraneperformance.TheLbL assemblymethodissimplebutnotefficientforpracticalapplicationbecauseit
Langmuir–BlodgettLangmuir–Schafer
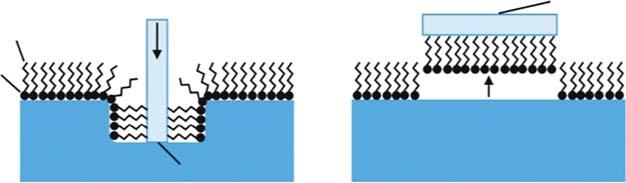
Figure1.4 Comparisonbetween(a)Langmuir–Blodgett(LB)and(b)Langmuir–Schaefer (LS)films.Source:Puzzovioetal.[26].ReproducedwithpermissionofAmericanScientific Publishers.
istime-consuming.TheLangmuir–Schaefer(LS)andLangmuir–Blodgett(LB) methodsaretwotypesoftheLbLassemblymethodandhavebeenusedtoprepare 2Dmembranes.Ananosheetlayerisformedontheliquidsurfaceandthen transferredtothesurfaceofasolidporoussubstrate.ThedifferencebetweentheLB andLSmethodsisthewayoftransferringthenanosheetlayer(Figure1.4).Inthe LBmethod,nanosheetdepositionisachievedbyverticallyliftingaplanarsubstrate, buttubulargeometriesarealsopossible.IntheLSmethod,asubstrateiscontacted face-to-facewithananosheetlayerattheinterface[26].High-qualitymonolayer
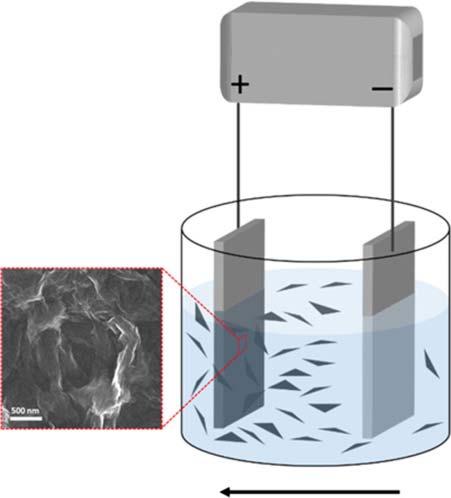
Figure1.5 Schematic illustrationofelectrophoreticdeposition(EPD). Source:Dibaetal.[27]. Reproducedwithpermission ofElsevier.
Power supply
EPD direction (for negatively charged particles)
membranescanbeobtainedbythesemethods.However,thesemethodsrequire specialexperimentalequipmentand,thus,incurhighoperationcost.
Inrecentyears,electrophoreticdeposition(EPD)iswidelyusedasafabrication method,althoughitiseffectiveonlyfortheassemblyofelectricallycharged2D materials.InEPD,electricallychargednanosheetsmovetowardtheelectrodewith oppositechargeinthepresenceofanelectricfield,andthedepositednanosheets directlyformathinmembranelayeronthesubstrate(Figure1.5)[27].Astabledispersionofchargednanosheetsiscrucialforelectrostaticdepositionwithaconcentrationofapproximately0.1gl 1 andaZetapotentialof < 30mVor >+30mV.As thethicknessofthedepositedmembraneincreases,EPDbecomesdifficultbecause thenanosheetsarenonconductorsandmembranethicknessislimited,whichis favorableforthefabricationofthinmembranes[28].Also,thesubstratematerial usedasanelectrodeshouldbeelectricallyconductive,butsputteringthinlayersof metalorcarboncaneasilyprovidethenecessaryconductivity.EPDisatechnique withenormouscommercialpotentialbecauseitissuitableforthepreparationof large-area2Dmembranes.TheapplicabilityofEPDatthemembranescalewillbe discussedindetailinChapter10.
1.5Applicationsof2DMembranes
2Dmembranescanbeusedforgasseparation,ionrejection,dehydrationoforganic solvents,andnanofiltration.Forvarioustypesofseparation,2Dmembraneswith differentpropertiesarerequired,suchasmembraneswithappropriateporesizefor sieving(normallydependingontheinterlayerspacingof2Dmembranes),stability forlong-termseparation,mechanicalstrength,andanti-swellingabilityinliquids. Adetailedintroductionto2Dmembraneswithvariousseparationapplicationsis providedinChapter2.
References
1 OakRidgeNationalLaboratory(ORN)andBCS(2005). MaterialsforSeparation Technologies:EnergyandEmissionReductionOpportunities.OakRidgeNational Laboratory(ORN)andBCS.
2 Humphrey,J.L.andKeller,G.E.(1997). SeparationProcessTechnology. NewYork:MacGraw-Hill.
3 Robeson,L.M.(2008).Theupperboundrevisited. JournalofMembraneScience 320(1–2):390–400.
4 Novoselov,K.S.,Geim,A.K.,Morozov,S.V.etal.(2004).Electricfieldeffectin atomicallythincarbonfilms. Science 306(5696):666–669.
5 Kim,D.,Jeon,M.,Stottrup,B.L.,andTsapatsis,M.(2018). para-Xylene ultra-selectivezeoliteMFImembranesfabricatedfromnanosheetmonolayers attheair-waterinterface. AngewandteChemieInternationalEdition 130(2): 489–494.
6 Cao,Z.,Zeng,S.,Xu,Z.etal.(2018).UltrathinZSM-5zeolitenanosheet laminatedmembraneforhigh-fluxdesalinationofconcentratedbrines. Science Advances 4(11):eaau8634.
7 Peng,Y.,Li,Y.,Ban,Y.etal.(2014).Metal-organicframeworknanosheetsas buildingblocksformolecularsievingmembranes. Science 346(6215):1356–1359.
8 Peng,Y.,Li,Y.,Ban,Y.,andYang,W.(2017).Two-dimensionalmetal-organic frameworknanosheetsformembrane-basedgasseparation. AngewandteChemie InternationalEdition 56(33):9757–9761.
9 Wang,X.,Chi,C.,Zhang,K.etal.(2017).Reversedthermo-switchablemolecular sievingmembranescomposedoftwo-dimensionalmetal-organicnanosheetsfor gasseparation. NatureCommunications 8:14460.
10 Yang,H.,Yang,L.,Wang,H.etal.(2019).Covalentorganicframework membranesthroughamixed-dimensionalassemblyformolecularseparations. NatureCommunications 10:2101.
11 Ying,Y.,Tong,M.,Ning,S.etal.(2020).Ultrathintwo-dimensionalmembranes assembledbyioniccovalentorganicnanosheetswithreducedaperturesforgas separation. JournaloftheAmericanChemicalSociety 142(9):4472–4480.
12 Wang,Y.,Li,L.,Wei,Y.etal.(2017).Watertransportwithultralowfriction throughpartiallyexfoliatedg-C3 N4 nanosheetmembraneswithself-supporting spacers. AngewandteChemieInternationalEdition 56(31):8974–8980.
13 Ran,J.,Pan,T.,Wu,Y.etal.(2019).Endowingg-C3 N4 membraneswithsuperior permeabilityandstabilitybyusingacidspacers. AngewandteChemieInternationalEdition 58(46):16463–16468.
14 Nie,L.,Goh,K.,Wang,Y.etal.(2020).Realizingsmall-flakegrapheneoxide membranesforultrafastsize-dependentorganicsolventnanofiltration. Science Advances 6(17):eaaz9184.
15 Li,H.,Song,Z.,Zhang,X.etal.(2013).Ultrathin,molecular-sievinggraphene oxidemembranesforselectivehydrogenseparation. Science 342(6154):95–98.
16 Zhang,M.,Guan,K.,Ji,Y.etal.(2019).Controllableiontransportby surface-chargedgrapheneoxidemembrane. NatureCommunications 10:1253.
17 Ding,L.,Wei,Y.,Wang,Y.etal.(2017).Atwo-dimensionallamellarmembrane: MXenenanosheetstacks. AngewandteChemieInternationalEdition 129(7): 1851–1855.
18 Ding,L.,Wei,Y.,Li,L.etal.(2018).MXenemolecularsievingmembranesfor highlyefficientgasseparation. NatureCommunications 9:155.
19 Li,H.,Ko,T.J.,Lee,M.etal.(2019).Experimentalrealizationoffewlayer two-dimensionalMoS2 membranesofnearatomicthicknessforhighefficiency waterdesalination. NanoLetters 19(8):5194–5204.
20 Chen,D.,Wang,W.,Ying,W.etal.(2018).CO2 -philicWS2 laminatedmembraneswithananoconfinedionicliquid. JournalofMaterialsChemistryA 6(34): 16566–16573.
21 Ibrahim,A.andLin,Y.S.(2017).Gaspermeationandseparationpropertiesof large-sheetstackedgrapheneoxidemembranes. JournalofMembraneScience 550:238–245.
1Introduction
22 Nielsen,L.E.(1967).Modelsforthepermeabilityoffilledpolymersystems. JournalofMacromolecularScience:PartA–Chemistry 1(5):929–942.
23 Yang,E.,Kim,C.M.,Song,J.H.etal.(2017).Enhanceddesalinationperformance offorwardosmosismembranesbasedonreducedgrapheneoxidelaminates coatedwithhydrophilicpolydopamine. Carbon 117:293–300.
24 Tsou,C.H.,An,Q.F.,Lo,S.C.etal.(2015).Effectofmicrostructureofgraphene oxidefabricatedthroughdifferentself-assemblytechniqueson1-butanoldehydration. JournalofMembraneScience 477:93–100.
25 Zhao,J.(2019).Solution-processableconductivegraphene-basedmaterialsfor flexibleelectronics.DissertationatUppsalaUniversity,Sweden.
26 Puzzovio,D.,Naim,A.A.,Hague,L.etal.(2012).Technologyplatformfor samplingwaterwithelectrolyte-gatedorganictransistorssensitisedwith Langmuir-depositedcalixarenesurfacelayers. JournalofSurfacesandInterfaces ofMaterials 1:1–6.
27 Diba,M.,Fam,D.W.H.,Boccaccini,A.R.,andShaffer,M.S.P.(2016).Electrophoreticdepositionofgraphene-relatedmaterials:areviewofthefundamentals. ProgressinMaterialsScience 82:83–117.
28 Diba,M.,García-Gallastegui,A.,KluppTaylor,R.N.etal.(2014).Quantitative evaluationofelectrophoreticdepositionkineticsofgrapheneoxide. Carbon 67: 656–661.
Typesof2DMaterial-BasedMembranes
Thischapterprovidesanoverviewoftheadvantagesinthedevelopmentoftwodimensional(2D)material-basedmembranes,including2Dzeolites,metal–organic frameworks(MOFs),covalentorganicframeworks(COFs),andg-C3 N4 , graphene-based,layereddoublehydroxide(LDH),andtransitionmetaldichalcogenide(TMD)membranes.ThedevelopmentprogressofMXenemembranesfor molecular,nano,andmicroseparationwillbeintroducedinChapters4–9.
2.1PorousTwo-DimensionalNanosheet-Based Membranes
2.1.1Zeolite2DMembranes
Zeolitesareaclassofnanoporousmaterialsbasedoncrystallinesilicatesor aluminumsilicateswithcrystallographicallydeterminedporesizesintherange of0.2nmtomorethan1nm.Inrecentyears,2Dzeolitenanosheets,suchas mobilefivering(MFI)[1–6],mobilcompositionofmatter(MCM-22)[7,8],adsorbent/membranehybrid(AMH-3)[9,10],andJilin–Davy–Faradaylayeredsolid no.1(JDF-L1)[11–13],havebeenassembledintomembranestacksforseparation applications.Amongthese,theMFInanosheetmembraneisthemostwidely studied2Dzeolitemembrane.ItwassuccessfullypreparedbyTsapatsis’groupin 2011,whousedatop-downsynthesismethodtopreparethenanosheets.However, thesefilmsexhibitedlowseparationperformancebecauseofthenonselectivegaps betweentheneighboringMFInanosheets.Tsapatsisandcoworkersattempted developingvariousmethods,suchassecondarygrowth[1],andfilter-coating seeding[2],toimprovetheseparationperformanceoftheMFI-nanosheetmembrane.Yoon’sgroupcontributedtothegel-freesecondarygrowthmethod[6]. Subsequently,theTsapatsisgroupsuccessfullypreparedhigh-aspect-ratioMFI nanosheetsbydirectbottom-upsynthesis.Theyusedthesedirectlysynthesized MFInanosheetstoprepareanMFInanosheetmonolayerbyanovelfloating particlecoatingmethod[4].Then,ahigh-fluxMFImembranewithanexcellent p-xylene/o-xyleneseparationfactor(>10000)withthemaximum p-xylenepermeanceof2.9 × 10 7 molm 2 s 1 Pa 1 wasobtainedduetothegel-freesecondary MXeneMembranesforSeparations, FirstEdition.HaihuiWang,YanyingWei,andLiDing. ©2022WILEY-VCHGmbH.Published2022byWILEY-VCHGmbH.
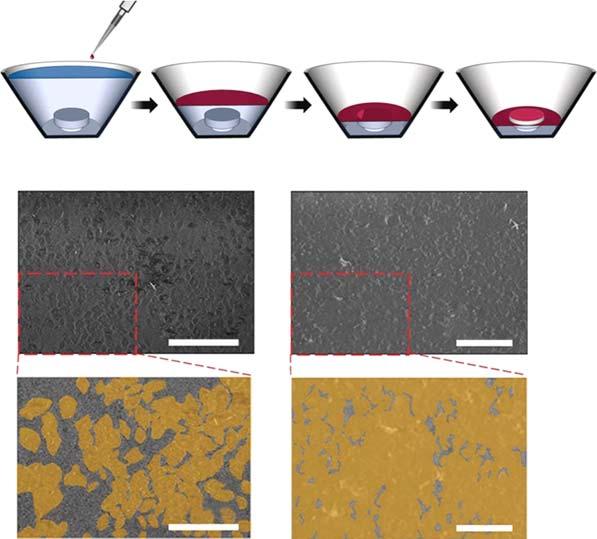
Figure2.1 (a)SchematicofcoatingMFInanosheetseedsbythefloatingparticlecoating method.SEMimagesofMFInanosheetcoatingsonsinteredsilicafiber(SSF)supportsby (b)LS(theprincipleofLScoatingisshowninFigure1.4)and(c)floatingparticlecoating methods.Source:Kimetal.[4].ReproducedwithpermissionofJohnWiley&Sons,Inc.
growthoftheMFInanosheetmonolayerseeds[4].ComparedtotheLSmethod,the floatingparticlecoatingmethodproducesadenselypackedcoatingofnanosheets onthesupport(Figure2.1).
2.1.2Metal–OrganicFramework2DMembranes
TraditionalMOFmaterialsusedformembranespossessthree-dimensional(3D) frameworks.Because2DMOFmembranesareultrathinandhaveahighspecificareaandtuneablemolecularstructure,excessiveattentionhasbeenpaidto explorethepotentialof2DMOFsformembraneseparation.In2014,Pengetal. madeasignificantbreakthroughinthefabricationof2DMOFmembranes[14]. TheysuccessfullypreparedamembranefromseveralnanometerthickZn2 (bim)4 nanosheetsthathadanexcellentH2 /CO2 separationfactorof291andhighH2 permeanceof2700GPUs,surpassingtheRobesonupperlimit.Recently,Zhang andcoworkerssuccessfullypreparedanultrathinAl-MOFmembranewithwater permeanceof2.2molm 2 h 1 bar 1 andarejectionrateofapproximately100% forinorganicions,making2DMOFmembranespromisingcandidatesforion separation(Figure2.2)[15].Inspiredbythiswork,someother2DMOFmaterials
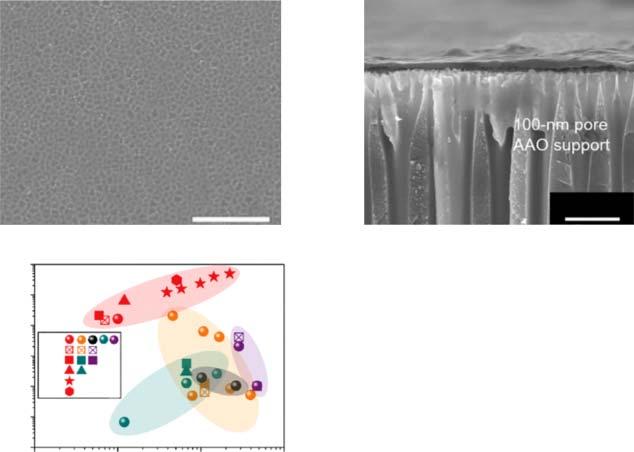
Figure2.2 (a)SEMimageofanultrathinAl-MOFmembraneonanodicaluminumoxide (AAO)substrate.(b)Cross-sectionofanultrathinAl-MOFmembraneonAAOsubstrate. (c)ComparisonofAl-MOFmembrane,usedforionseparation,withotherrepresentative2D lamellarmembranes.Source:Jianetal.[15].ReproducedwithpermissionofAmerican AssociationfortheAdvancementofScience.
suchasZn2(benzimidazole)3(Zn2 (bim)3 )[16],meshadjustablemolecularsieve-1, Ni8(5-bbdc)6(m-OH)4(MAMS-1)[17],zeoliticimidazolateframework-leaf(ZIF-L) [18],andironporphyrincomplex(Zn-TCP(Fe))[19]werealsoassembledasstacks forfabricating2Dmembranes.Inadditiontopure2DMOFmembranes,MOF nanosheetscanbesuccessfullyappliedwithpolymerstoobtainnanocomposite membranescalledMOFnanosheet-basedMixedMatrixMembranes(MMMs), whichcannegatethetrade-offbetweenselectivityandpermeability[20–23].In additiontothepreparationof2DMOFmembranesonporousflat/disc-shaped substrates,Zhang’sgroupprepared2DZIFmembranesonpractice-relevantporous tubesforindustrialapplications[24–26].
2.1.3CovalentOrganicFramework(COF)2DMembranes
Inrecentyears,COFnanosheetshavealsobeenassembledforfabricatingmembranesandsome2DCOFmembraneshaveshownhighmembraneseparation performance[27–30].However,becausetheporesizesof2DCOFmembranesare typicallymuchlargerthanthoseofmostgasmolecules,theapplicationof2DCOF membranesforthesievingofsmallmoleculesischallenging.Thedevelopment of2DCOFmembranesformolecularsievinghasdominatedtheresearchfieldin