Two-Dimensional-Materials-Based Membranes: Preparation, Characterization, and Applications
Gongping Liu
Visit to download the full and correct content document: https://ebookmass.com/product/two-dimensional-materials-based-membranes-prepar ation-characterization-and-applications-gongping-liu/
More products digital (pdf, epub, mobi) instant download maybe you interests ...
Two-Dimensional Materials for Nonlinear Optics: Fundamentals, Preparation Methods, and Applications
Qiang Wang
https://ebookmass.com/product/two-dimensional-materials-fornonlinear-optics-fundamentals-preparation-methods-andapplications-qiang-wang/
Defects in Two-Dimensional Materials Rafik Addou
https://ebookmass.com/product/defects-in-two-dimensionalmaterials-rafik-addou/
Starch-based materials in food packaging processing, characterization and applications Barbosa
https://ebookmass.com/product/starch-based-materials-in-foodpackaging-processing-characterization-and-applications-barbosa/
Amorphous
Nanomaterials: Preparation, Characterization and Applications Lin Guo
https://ebookmass.com/product/amorphous-nanomaterialspreparation-characterization-and-applications-lin-guo/
Hybrid
Nanofluids: Preparation, Characterization and Applications Zafar Said
https://ebookmass.com/product/hybrid-nanofluids-preparationcharacterization-and-applications-zafar-said/
Spintronic 2D Materials: Fundamentals and Applications (Materials Today) Wenqing Liu (Editor)
https://ebookmass.com/product/spintronic-2d-materialsfundamentals-and-applications-materials-today-wenqing-liu-editor/
Nanomaterial and Polymer Membranes. Synthesis, Characterization, and Applications 1st Edition Tawfik Abdo Saleh
https://ebookmass.com/product/nanomaterial-and-polymer-membranessynthesis-characterization-and-applications-1st-edition-tawfikabdo-saleh/
Two-Dimensional (2D) NMR Methods Konstantin
Ivanov
https://ebookmass.com/product/two-dimensional-2d-nmr-methodskonstantin-ivanov/
Calculations and Simulations of Low-Dimensional Materials : Tailoring Properties for Applications 1st Edition Ying Dai
https://ebookmass.com/product/calculations-and-simulations-oflow-dimensional-materials-tailoring-properties-forapplications-1st-edition-ying-dai/
Two-Dimensional-Materials-BasedMembranes
Two-Dimensional-Materials-Based Membranes
Preparation,Characterization,andApplications
EditedbyGongpingLiuandWanqinJin
Editors
Prof.GongpingLiu
NanjingTechUniversity CollegeofChemicalEngineering 30PuzhuRoad(S) 211816Nanjing China
Prof.WanqinJin
NanjingTechUniversity CollegeofChemicalEngineering 30PuzhuRoad(S) 211816Nanjing China
CoverImage: ©LAGUNADESIGN/Getty
Images
Allbookspublishedby WILEY-VCH arecarefully produced.Nevertheless,authors,editors,and publisherdonotwarranttheinformation containedinthesebooks,includingthisbook, tobefreeoferrors.Readersareadvisedtokeep inmindthatstatements,data,illustrations, proceduraldetailsorotheritemsmay inadvertentlybeinaccurate.
LibraryofCongressCardNo.: appliedfor BritishLibraryCataloguing-in-PublicationData Acataloguerecordforthisbookisavailable fromtheBritishLibrary.
Bibliographicinformationpublishedby theDeutscheNationalbibliothek TheDeutscheNationalbibliotheklists thispublicationintheDeutsche Nationalbibliografie;detailedbibliographic dataareavailableontheInternetat <http://dnb.d-nb.de>
©2022WILEY-VCHGmbH,Boschstraße12, 69469Weinheim,Germany
Allrightsreserved(includingthoseof translationintootherlanguages).Nopartof thisbookmaybereproducedinanyform–by photoprinting,microfilm,oranyother means–nortransmittedortranslatedintoa machinelanguagewithoutwrittenpermission fromthepublishers.Registerednames, trademarks,etc.usedinthisbook,evenwhen notspecificallymarkedassuch,arenottobe consideredunprotectedbylaw.
PrintISBN: 978-3-527-34848-0
ePDFISBN: 978-3-527-82983-5
ePubISBN: 978-3-527-82984-2
oBookISBN: 978-3-527-82985-9
Typesetting Straive,Chennai,India
Contents
Preface xiii
1Introduction 1 GongpingLiuandWanqinJin
References 2
2FabricationMethodsfor2DMembranes 3 LongCheng,GongpingLiu,andWanqinJin
2.1Introduction 3
2.2SynthesisofNanosheets 4
2.2.1Top-DownMethod 4
2.2.1.1Mechanical-ForceExfoliation 4
2.2.1.2Ion-IntercalationExfoliation 6
2.2.1.3Oxidation-AssistedExfoliation 7
2.2.1.4Selective-EtchingMethod 8
2.2.2Bottom-UpMethod 9
2.2.2.1ChemicalVaporDeposition 9
2.2.2.2Hydro/SolvothermalSynthesis 10
2.2.2.3InterfacialSynthesis 10
2.3MembraneStructuresandFabricationMethods 11
2.3.1Two-Dimensional-MaterialNanosheetMembranes 11
2.3.1.1ZeoliteMembrane 11
2.3.1.2MOFMembrane 12
2.3.1.3PorousGrapheneMembrane 13
2.3.2Two-Dimensional-MaterialLaminarMembranes 15
2.3.2.1AssemblyStrategiesofLaminates 15
2.3.2.2NanostructureControllingofLaminarMembranes 18
2.3.3Two-Dimensional-Materials-BasedMixed-MatrixMembranes (MMMs) 24
2.3.3.1FabricationMethodsofMMMs 24
2.3.3.2EffectofPhysicochemicalPropertiesof2DFillers 25
2.3.4OtherHybridMembranes 27
2.4SummaryandOutlook 29
References 30
3NanoporousSingle-LayerGrapheneMembranesforGas Separation 35
LucBondaz,ShiqiHuang,MojtabaRezaei,ShaoxianLi,and KumarVaroonAgrawal
3.1Introduction 35
3.2Gas-SeparationPotentialofN-SLGMembranes 37
3.3EngineeringGas-SelectiveVacancyDefects 48
3.3.1Bottom-UpSynthesisofN-SLG 49
3.3.2PostsyntheticEtchingofSLG 51
3.3.2.1PhysicalEtchingMethods 52
3.3.2.2ChemicalEtchingTechniques 55
3.4FabricationofLarge-AreaN-SLGMembranes 66
3.5SummaryandOutlook 71 References 72
4Graphene-BasedMembranesforWaterSeparation 83
YuanKangandXiwangZhang
4.1Introduction 83
4.2WaterTransportMechanismsinGraphene-BasedMembranes 84
4.2.1Internal-Geometry-MediatedTransport 84
4.2.1.1SizeEffects 85
4.2.1.2LengthEffects 87
4.2.2Surface-Chemistry-MediatedTransport 88
4.2.3External-Environment-MediatedTransport 91
4.2.3.1SolutionChemistryEffects 91
4.2.3.2AppliedPressureEffects 93
4.2.3.3AppliedPotentialEffects 93
4.2.4Guest-Material-MediatedTransport 94
4.2.4.1StabilizingEffects 94
4.2.4.2Size-ControllingEffects 96
4.2.4.3Surface-Chemistry-ModifyingEffects 96
4.2.4.4SmartGatingEffects 97
4.3Graphene-basedMembraneWaterSeparationApplications 100
4.3.1Nanofiltration 100
4.3.2ReverseOsmosis 105
4.3.3ForwardOsmosis 105
4.4ConclusionsandPerspectives 107 References 108
5Graphene-BasedMembranesforIonsSeparation 113
GuokeZhaoandHongweiZhu
5.1Introduction 113
5.2Single-LayerGraphene 114
5.2.1TheoreticalCalculations 115
5.2.2ExperimentalValidations 118
5.3GrapheneOxideMembranes 120
5.3.1StructureofGrapheneOxideandGrapheneOxideMembranes 121
5.3.2UltrafastWaterPermeability 121
5.3.3IonSelectivity 123
5.3.4MicrostructureOptimizationforDesalination 124
5.3.5InterlayerSpacingControlforDesalination 126
5.3.5.1Cross-Linking 126
5.3.5.2Reduction 130
5.3.5.3ExternalPressure 131
5.3.6ChargeModificationforDesalination 132
5.3.7ExternalFieldModulatedIonTransport 134
5.3.8IonTransportThroughPlanarGOLaminates 135
5.4SummaryandPerspective 137 References 139
6Graphene-BasedMembranesforPervaporation 145 JingZhaoandWanqinJin
6.1Introduction 145
6.2Mass-TransportMechanism 146
6.2.1MassTransportinPervaporationProcess 146
6.2.2MassTransportinGOMembrane 147
6.3ProgressesinGOMembranesforPervaporation 150
6.3.1ControllingSelf-AssemblyCondition 151
6.3.2DesigningGrapheneOxide-Framework(GOF)Membrane 152
6.3.3AssemblywithPolymers 157
6.3.4IntercalatingNanomaterials 162
6.3.5TuningSurfaceStructure 163
6.4SummaryandPerspective 165 References 167
7Two-Dimensional-MaterialsMembranesforGas Separations 173 YunpanYing,HadiRouhani,DechaoWang,andDanZhao
7.1Introduction 173
7.22D-MaterialsMembranes 174
7.2.1Zeolites 174
7.2.2Graphene-BasedMaterials 176
7.2.2.1NanoporousGraphene 176
7.2.2.2GrapheneOxide 179
7.2.3MOFs 181
7.2.4COFs 183
7.2.5g-C3 N4 185
7.2.6MXenes 185
7.2.7Other2DMaterials 188
7.3Preparationof2DNanosheets 190
7.3.1Top-DownMethod 190
7.3.2Bottom-UpMethod 194
7.4Preparationof2D-MaterialsMembranes 196
7.4.1Top-DownMethod 196
7.4.1.1Filtration-AssistedAssembly 196
7.4.1.2Coating 198
7.4.1.3Layer-by-LayerAssembly 199
7.4.2Bottom-UpMethod 199
7.5GasSeparations 201
7.5.1H2 /CO2 ,H2 /N2 ,andH2 /CH4 Separations 201
7.5.2CO2 /N2 andCO2 /CH4 Separations 201
7.5.3OtherGas/VaporSeparations 210
7.6ConclusionsandPerspectives 210 References 211
8LayeredDoubleHydroxideMembranesforVersatile SeparationApplications 221 YiLiu
8.1IntroductiononLDHsandLDH-BasedMembranes 221
8.2StrategyforLDH-BasedMembranePreparation 223
8.2.1Solution-Based InSitu Growth 223
8.2.2Post-SyntheticDeposition 224
8.2.3BlendingwithPolymers 226
8.3ResearchProgressonLDH-BasedMembranes 226
8.3.1InterlayerGallery-BasedSeparation 226
8.3.1.1PristineInterlayerGallery-BasedSeparation 226
8.3.1.2RegeneratedInterlayerGallery-BasedSeparation 229
8.3.2GeometricShape-BasedSeparation 232
8.3.2.1GeometricShape-BasedGasSeparation 232
8.3.2.2GeometricShape-BasedLiquidSeparation 234
8.3.2.3GeometricShape-BasedParticulateMatterCapture 236
8.3.2.4GeometricShape-BasedSacrificingTemplates 236
8.3.3UnusualThermalBehavior-BasedSeparation 236
8.3.4PhotocatalyticActivity-BasedSeparation 238
8.3.5PositiveSurfaceCharge-BasedSeparation 238
8.3.5.1PositiveSurfaceCharge-BasedUltrafiltration 239
8.3.5.2PositiveSurfaceCharge-BasedNanofiltration 239
8.3.5.3PositiveSurfaceCharge-BasedReverseOsmosis 240
8.3.5.4PositiveSurfaceCharge-BasedForwardOsmosis 241
8.3.5.5PositiveSurfaceCharge-BasedNanocompositeMembranes 241
8.3.6Hydrophilicity-BasedWaterTreatment 242
8.3.6.1Hydrophilicity-BasedMicrofiltration 243
8.3.6.2Hydrophilicity-BasedUltrafiltration 243
8.3.6.3Hydrophilicity-BasedNanofiltration 243
8.3.6.4Hydrophilicity-BasedReverseOsmosis 244
8.3.6.5Hydrophilicity-BasedForwardOsmosis 244
8.4SummaryandOutlook 245 References 246
9MXene:ANovelTwo-DimensionalMembraneMaterialfor MolecularSeparation 253 GuozhenLiu,GongpingLiu,andWanqinJin
9.1Introduction 253
9.2SynthesisandProcessing 254
9.2.1SynthesisofMultilayeredMXenePhases 254
9.2.2FabricationofSingleMXeneFlakes 255
9.2.3SurfacePropertiesofMXeneFlakes 257
9.2.4PreparationofMXene-BasedMembranes 257
9.2.4.1Drop-Coating 258
9.2.4.2SprayingorSpinningCoating 258
9.2.4.3Pressure-AssistedFiltration 258
9.3MXene-BasedMembranesforMolecularSeparation 258
9.3.1LiquidSeparation 258
9.3.1.1Desalination 259
9.3.1.2OrganicSolventNanofiltration 261
9.3.1.3PervaporationSolventDehydration 263
9.3.1.4DyesandNaturalOrganicMatterRejection 265
9.3.1.5Oil–WaterSeparation 267
9.3.2GasSeparation 268
9.4ConclusionsandPerspective 271 References 271
102D-MaterialsMixed-MatrixMembranes 279 ZitingZhu,ShaoyuWang,FushengPan,andZhongyiJiang
10.1Introduction 279
10.2Two-DimensionalMaterialsasDispersedPhaseofMMMs 281
10.2.1GrapheneOxide(GO) 282
10.2.1.1IncreasingMolecularTransportChannels 282
10.2.1.2ReducingNonselectiveDefects 283
10.2.1.3IntroducingtheFunctionalSitesforFacilitatedTransport 284
10.2.2Metal–OrganicFrameworks(MOFs) 285
10.2.2.1IncreasingMolecularTransportChannels 286
10.2.2.2EnhancingtheInterfacialCompatibilityBetweenNanomaterialsand Polymers 287
10.2.3CovalentOrganicFrameworks(COFs) 287
10.2.3.1IncreasingMoleculeTransportChannels 288
x Contents
10.2.3.2IntroducingFacilely-TailoredFunctionality 289
10.2.3.3ConstructingHierarchicalStructuresinMMMs 290
10.2.4Other2DMaterials 292
10.2.4.1Transition-MetalDichalcogenides(TMDs) 292
10.2.4.2GraphiticCarbonNitride(g-C3 N4 ) 293
10.2.4.3MXenes 294
10.3Two-DimensionalMaterialasContinuousPhaseofMMMs 295
10.3.1GrapheneOxide(GO) 295
10.3.1.1ControllingInterlayerSpacing 296
10.3.1.2ModulatingthePhysical/ChemicalMicroenvironment 296
10.3.2Metal–OrganicFramework(MOF) 297
10.3.2.1EnhancingProcessabilityandStabilityofMOFs 299
10.3.2.2ModulatingthePhysical/ChemicalMicroenvironment 299
10.3.3CovalentOrganicFrameworks(COFs) 300
10.3.3.1RegulatingthePhysical/ChemicalMicroenvironment 301
10.3.3.2ModulatingCrystallinity,Porosity,MechanicalProperties 302
10.4ConclusionandOutlook 303 References 306
11TransportMechanismof2DMembranes 313
TaeHoonLeeandHoBumPark
11.1Introduction 313
11.2FundamentalsofMassTransportThroughMembranes 316
11.2.1TransportMechanisminPorousMembranes 317
11.2.2TransportMechanisminNonporousMembranes 319
11.2.3TransportMechanisminChargedMembranes 321
11.2.4Permeability–SelectivityTrade-OffforPolymers 324
11.3NanofluidicTransportThroughConfinedDimensions 326
11.3.1ConfinementArchitecturesforArtificialNanofluidicSystems 328
11.3.2ContinuumModelingofNanofluidicTransportinConfined Channels 330
11.3.3MechanismsofNanofluidicTransportinAtomicallyThin Nanopores 333
11.3.4EffectsofElectricalDoubleLayerinNanofluidicIonTransport 335
11.3.5VariousConfinementEffectsinNanofluidicTransportatthe SubnanometerScale 338
11.3.5.1MolecularRearrangement 338
11.3.5.2PartialDehydrationorDesolvation 339
11.3.5.3ElectricalEffects 340
11.3.5.4QuantumEffects 341
11.4UniqueMass-TransportPropertiesin2DMembranes:Structural Aspects 341
11.4.1NanoporousAtomicallyThin2DMembranes(NATMs) 342
11.4.2Staked2DMembraneswithLaminarStructure 345
11.4.32DMaterials-EmbeddedMixed-MatrixMembranes(MMMs) 350
11.5SummaryandOutlook 355 References 356
12ConclusionsandPerspectives 365
GongpingLiuandWanqinJin
Index 369
Preface
Syntheticmembraneshavebeenusedinvariousprocesses,fromwatertreatment, gaspurificationtoenergyconversion.Theconventionalmembraneprocesses, includingmicrofiltration,ultrafiltration,andreverseosmosis,havebeencommercializedandwidelyusedinfood,chemical,andmedicalindustries.However,it remainschallengingtorealizethepreciseseparationofthemolecules/ionswith subnanometersizes,suchassalinewater,organicsolventsolution,andgasmixtures.Asweknow,thereisageneraltrade-offbetweenpermeabilityandselectivity forpolymericmembranes.Althoughinorganicmembranescouldachieveboth highpermeabilityandselectivity,thehighcostofmembranefabricationrestricts theirdevelopment.Thekeyliesinthedesignandfabricationofhigh-performance (highpermeability,selectivity,andstability)membranesforefficientmolecular separation.
Two-dimensional(2D)materials(e.g.nanosheetsofgraphenefamilymaterials, zeolites,metal–organicframeworks,orlayereddoublehydroxides)havebecome excellentnanobuildingblockstodevelopnext-generationseparationmembranes featuringuniquenanoporesand/ornanochannels.Recently,2Dmaterials-based membraneshavebeenappearingasaveryhottopicbothintheoreticaland experimentalstudiesandhaveattractedahugesurgeofinterest.Importantnew resultsdemonstratedthatthefascinating2D-materialmembranesshowedextraordinarypermeationproperties,openingthedoortoultrafastandhighlyselective membranesforwaterpurification,desalination,gasseparation,andbioseparation. Uptonow,bookslackedaninstructiveviewontheimportantcontemporarytopic “2Dmaterialsmembranes.”Thereisanurgentneedforabookthatbringsalive thenewresults,andcurrentsignificancesandchallengesconcerning2D-material membranes.Inthisbook,weaimtogivecomprehensiveinformationondesign, fabrication,andapplicationof2D-materialmembranesthatareusedformolecular separation.
Variousmembranestructuresbasedondifferent2Dmaterialsareinvolved inthebook,includingporousnanosheetmembranes,2Dlaminarmembranes, andmixed-matrixmembranes(MMMs)with2Dmaterialsservingasnanofillers. Fabricationstrategies,physicochemicalproperties,morphologies,host–guest interactions,transportcharacteristics,andmechanismsof2D-materialmembranes willbethoroughlydiscussedbasedonadvancedcharacterizationtechniquesand
xiv Preface theoreticalcalculations.Meanwhile,thebookcontainsseveraltypicalapplications of2D-materialmembranesinfields,suchaswaterdesalination,ionseparation, solventdehydration,andgasseparation.Inaddition,milestonesinthelarge-scale fabricationof2D-materialmembranesforwideimplementationarebrieflyintroduced.Finally,weconcludethisbookwithanoverviewofremainingchallenges andnewopportunitiesthathaveopenedfor2D-materialmembranesinmolecular separation.
Atlast,wewouldliketoexpressourappreciationandgratitudetothechapter authors:Dr.KumarVaroonAgrawalfromÉcolePolytechniqueFédéraledeLausanne(EPFL),Dr.XiwangZhangfromMonashUniversity,Dr.HongweiZhufrom TsinghuaUniversity,Dr.DanZhaofromNationalUniversityofSingapore,Dr.YiLiu fromDalianUniversityofTechnology,Dr.FushengPanfromTianjinUniversity,and HoBumParkfromHanyangUniversity.
ProfessorinChemicalEngineering
NanjingTechUniversity
Nanjing,China
10January2022
GongpingLiu WanqinJin
Introduction
GongpingLiuandWanqinJin
NanjingTechUniversity,CollegeofChemicalEngineering,StateKeyLaboratoryofMaterials-Oriented ChemicalEngineering,30PuzhuSouthRoad,Nanjing,211816,P.R.China
Two-dimensional(2D)materialshavebeenemergingstarsincondensedmatterphysics,materialsscience,andchemistrysincethesuccessfulexfoliationof graphenebyNovoselovandGeimin2004[1–3].Theatomicthicknessandmicrometerlateraldimensionsendow2Dmaterialswithgreatpotentialinmembrane separation.Syntheticmembranesareusedwidelyinmanyseparationprocesses, fromindustrial-scaleprocesses,suchasremovingsaltfromseawaterandseparatingatmosphericgases,tosmaller-scaleprocessesinchemicalsynthesisand purification[4,5].Themembranesfunctionbyformingaselectivebarrierbetween thetwophases,restrictingthemovementofsomemoleculeswhilelettingothers penetrate.Ononehand,themembranesassembledfromultrathinnanosheets canminimizethetransportresistanceandmaximizethemass-transferrate.On theotherhand,theintrinsicorartificialnanoporesandinterlayergalleriescan provideexcellentmolecularsievingproperties[6,7].Hence,2Dmaterials,including graphene-family,zeolites,metal–organicframeworks(MOFs),covalent–organic frameworks(COFs),metalcarbidesandnitrides(MXenes),andlayereddouble hydroxides(LDHs),havebeendemonstratedasexcellentbuildingblocksfor high-performancemembranes[8,9].
2Dmaterialsareeitherporousornonporousbasedontheiratomicstructure.The intrinsicnanoporesinzeolitesandMOFsorthedrillednanoporesingraphenecan providemolecular/ionictransportpathway.Incontrast,thenonporousnanosheets (e.g.grapheneoxideandMXene)mustbeassembledintolaminateswithinterlayer channelsformasstransfer,whichisdrivenbyexternalforces,suchaspressure difference,centrifugalforce,andmolecularinteraction[10].Hence,accordingtothe differenceinmembranestructures,2D-material-basedmembranescanbecategorizedintothreetypes—(i)porousnanosheetmembranes,(ii)laminarmembranes, and(iii)2D-material-basedmixed-matrixmembranes(MMMs).Thefabrication methodsanduniquepropertiesofthesemembraneswillbedetailedlydiscussed inthefollowingchapters.Bythepreciseconstructionandmanipulationofthe in-planenanopores/slitsandinterlayerchannels,2D-material-basedmembranes
Two-Dimensional-Materials-BasedMembranes:Preparation,Characterization,andApplications, FirstEdition.EditedbyGongpingLiuandWanqinJin. ©2022WILEY-VCHGmbH.Published2022byWILEY-VCHGmbH.
haveexhibitedoutstandingmolecularseparationpropertiesinvariousmembrane processes,suchasultrafiltration,nanofiltration,reverseosmosis,forwardosmosis, pervaporation,andgasseparation[9].
Withtherapiddevelopmentof2D-material-basedmembranes,acomprehensive summaryofthebreakthroughsandmilestonesforthesemembranesisurgently neededtoguidenewcomersandinspireinnovationsinthisfield.Thefollowing chapterssummarizetherecentprogressof2D-material-basedmembranesformolecularseparation,focusingonmembranepreparation,characterization,andapplication.Chapter2introducesthefabricationmethodsfor2Dmaterialsandmembranes. Chapter3presentsthedevelopmentofporousgraphene-basednanosheetmembranes.Chapters4–6describethedesignandapplicationofgraphene-based membranesforwaterseparation,ionsseparation,andpervaporation,respectively. Chapter7showsthedevelopmentof2D-materialmembranesforgasseparation. Chapter8introducestheadvancein2D-LDHsmembranes.Chapter9demonstrates thedesignofMXeneandotheremerging2D-materialmembranes.Chapter10 summarizesthemilestonesfor2D-materialmixed-matrixmembranes.Chapter11 revealsthetransportmechanismof2D-materialmembranes.Finally,Chapter12 givestheconclusionsandperspectivesofthisresearchtopic.
References
1 Geim,A.K.andGrigorieva,I.V.(2013).VanderWaalsheterostructures. Nature 499(7459):419–425.
2 Koltonow,A.R.andHuang,J.(2016).Two-dimensionalnanofluidics. Science 351(6280):1395–1396.
3 Shen,J.,Liu,G.,Han,Y.etal.(2021).Artificialchannelsforconfinedmass transportatthesub-nanometrescale. Nat.Rev.Mater. 6(4):294–312.
4 Gin,D.L.andNoble,R.D.(2011).Designingthenextgenerationofchemical separationmembranes. Science 332(6030):674–676.
5 Koros,W.J.andZhang,C.(2017).Materialsfornext-generationmolecularly selectivesyntheticmembranes. Nat.Mater. 16(3):289–297.
6 Cheng,L.,Liu,G.,andJin,W.(2019).Recentprogressintwo-dimensionalmaterialmembranesforgasseparation. ActaPhys.Chim.Sin. 35:351090–351098.
7 Liu,G.,Jin,W.,andXu,N.(2015).Graphene-basedmembranes. Chem.Soc.Rev. 44(15):5016–5030.
8 Liu,G.,Jin,W.,andXu,N.(2016).Two-dimensional-materialmembranes:a newfamilyofhigh-performanceseparationmembranes. Angew.Chem.Int.Ed. 55(43):13384–13397.
9 Cheng,L.,Liu,G.,Zhao,J.etal.(2021).Two-dimensional-materialmembranes: manipulatingthetransportpathwayformolecularseparation. AccountsMater. Res. 2(2):114–128.
10 Wang,S.,Yang,L.,He,G.etal.(2020).Two-dimensionalnanochannelmembranesformolecularandionicseparations. Chem.Soc.Rev. 49(4):1071–1089.
FabricationMethodsfor2DMembranes
LongCheng,GongpingLiu,andWanqinJin
NanjingTechUniversity,CollegeofChemicalEngineering,StateKeyLaboratoryofMaterials-Oriented ChemicalEngineering,30PuzhuSouthRoad,Nanjing,211816,P.R.China
2.1Introduction
Two-dimensional-materialmembraneshavedemonstratedgreatpotentialin molecularseparation.Thesynthesisofhigh-qualitynanosheetswithintegrated structureisaprerequisiteforfabricatingdefect-freetwo-dimensional-material membranes.Thesyntheticmethodsofnanosheetsaimedatmembraneapplication includemechanical-forceexfoliation,ion-intercalationexfoliation,oxidationassistedexfoliation,selective-etchingmethod,chemicalvapordeposition(CVD), hydro/solvothermalsynthesis,andinterfacialsynthesis.Allofthesemethodscan bedividedintotwocategories,top-downandbottom-upmethods[1],whichare discussedindetail.Afterthenanosheetsaresuccessfullysynthesized,novelmembranefabricationmethodsshouldbeproposedfortuningthein-planeporesand constructinginterlayerchannels.Therearethreemaintypesof2D-materialmembranes—(i)nanosheetmembranes;(ii)laminarmembranes;and(iii)mixed-matrix membranes(MMMs).Thefirsttypeconsistsofamonolayerorafewlayersof 2Dmaterialwithintrinsicallyuniformlysizedpores(e.g.zeolite,metal-organic frameworks(MOFs))ordrillednanopores(graphene)formolecularsieving.The secondtype,laminarmembrane,iscomposedoforderlystackednanosheets withtunableinterlayerchannelsbyvariousassemblystrategies.Thesizeand functionalgroupsoftheinterlayerchannelsplayanimportantroleinselective permeationofsmallmolecules.Thirdly,theadditionof2Dfillersintopolymer matrixcancombinetheuniquetransportpropertyofnanosheetsandtheexcellent processabilityofapolymer,furtherenhancingseparationperformanceandeven mechanicalstrengthofpristinepolymermembrane.Owingtothedifferencein membranestructures,variousfabricationmethodshavebeenproposed.Inthis chapter,weaimtosummarizetheadvancesindesignandfabricationofnanosheets and2D-materialmembranes.Wefocusonthediscussionofdifferentapproachesfor assemblingthenanosheets,andtheprominenteffectoftuningthenanostructures
Two-Dimensional-Materials-BasedMembranes:Preparation,Characterization,andApplications, FirstEdition.EditedbyGongpingLiuandWanqinJin. ©2022WILEY-VCHGmbH.Published2022byWILEY-VCHGmbH.
2FabricationMethodsfor2DMembranes
andphysicochemicalpropertiesoftransportchannelsishighlighted.Opportunities andchallengesconcerningthedevelopmentoffabricationmethodsfor2D-material membranesarediscussedaswell.
2.2SynthesisofNanosheets
Thesyntheticmethodsof2Dnanosheetscanbecategorizedintotop-downand bottom-upmethods.Thetop-downmethod,includingmechanical-forceexfoliation, ion-intercalationexfoliation,oxidation-assistedexfoliation,andselective-etching method,reliesontheexfoliationofbulkcrystalsinto2Dnanosheets.Conversely, thebottom-upmethodreferstotheconstructionof2Dframeworksfrombasicunits. CVD,hydro/solvothermalsynthesis,andinterfacialsynthesisarewell-established bottom-upmethods.Alltheaforementionedmethodsareintroducedanddiscussed, respectively.
2.2.1Top-DownMethod
2.2.1.1Mechanical-ForceExfoliation
Theearlymechanical-forceexfoliationmethodisthemicromechanicalcleavage technique[2],namely“Scotch-tape”method.Themicromechanicalforcecan weakenthevanderWaalsinteractionbetweenthelayersofbulkcrystals,whichis beneficialfortheisolationofneighboringlayers,andsingle-orfew-layernanosheets canbeobtainedafterseveraltimesofcleavage.Thesizesoftheas-prepared nanosheetscanreachafewtotensofmicrometers,andthisnondestructivetechniquecancontributetocleansurfacesandexcellentcrystalquality,makingthem goodcandidatesforinvestigatingtheintrinsicphysicalproperties.Nevertheless,the lowyieldofthismethodrestrictsitspracticalapplication.Therearemoreeasilyscalablestrategies,suchassonication,shearforce,andball-millingexfoliations.Forthe sonicationmethod,thelayeredcrystalswerefirstlydispersedinapropersolvent, followedbyasonicationtreatment(Figure2.1)[3].Torealizeefficientexfoliation, thesurfaceenergyofthechosensolventshouldmatchthatofthebulkcrystal. Colemanandcoworkerspioneeredtheliquidexfoliationofgraphiteintographene inorganicsolvents,whichisascalablemethodtoproducehigh-qualitygraphene nanosheets[4].Theyfurtherextendedthismethodforthesynthesisofother2D nanosheets,suchasMoS2 ,WS2 ,and h-BN[5].Moreover,theadditionofpolymers orsurfactantscantunethesurfacetensionoftheaqueoussolutiontomatchthe surfaceenergyofbulkcrystals.Forinstance,Coleman’sgroupdemonstratedthat theintroductionofsodiumdodecylbenzenesulfonate(SDBS)orsodiumcholate (SC)intotheaqueoussolutioncanlargelyimprovetheexfoliationefficiency, contributingtohighyieldofgraphenenanosheets[6,7].Inaddition,thecontrol ofsonicationparameters,suchasultrasonicpowder,sonicationtime,sonication temperature,andtheshapeofvessels,cantunetheproperties(e.g.concentration, lateralsize,andthickness)ofas-synthesizednanosheets[8].
Solvent molecule (e.g. N-methyl-2-pyrrolidone (NMP))
/ intercalators (so-called surface stabilizers)
liquid-phase exfoliation
Figure2.1 Schematicrepresentationofthesonicationexfoliationprocessofgraphitein theabsence(topright)andpresence(bottomright)ofsurfactantmolecules.Source: CiesielskiandSamori[3]/withpermissionfromRoyalSocietyofChemistry.
Anothermechanicalforceforefficientexfoliationistheshearforce,which canbegeneratedfromthehigh-shearratesortheball-millingimpact.Coleman andcoworkersemployedahigh-shearrotor–statormixertoprovidehigh-shear ratesinliquid,facilitatingtheexfoliationofbulkgraphite[9].Theysuccessfully producedgraphenenanosheetsin1-methyl-2-pyrrolidinone(NMP)solventinthis shear-force-assistedmethod.Besidestherotor–statormixer,acommerciallyavailablekitchenblenderwasprovedtogeneratesufficientshearratesforfabrication ofvariousnanosheets,includinggrapheneandMoS2 [10,11],whichimpliesthe potentialofindustrialrotatingbladestirred-tankreactorsinlarge-scalegraphene production.Ball-millingmethodwasalsoemployedastheshear-force-assisted exfoliation.Therotatingspeedshouldbecarefullycontrolledtoavoidthein-plane defectscausedbyshockstressandensurethedominantshearstress.Chenand coworkersdemonstratedtheexfoliationofgraphitein N,N -dimethylformamide (DMF)bytheball-millingmethod[12].Afterhigh-speedcentrifugation,they obtainedsingle-andfew-layergraphenenanosheetswithathicknessaround 0.8–1.8nm.Themonolayerfractioncouldbefurtherincreasedbyoptimizingthe millingconditions(e.g.thediametersofmillingballs,themillingtime,andthe concentrationofgraphite).Yangandcoworkersproposedasoft-physicalstrategy combiningtheball-millingandsonicationprocessestoexfoliatelayeredZn2 (bim)4 (bim = benzimidazole)crystals[13].Themixtureofmethanolandpropanolis provedtobethemostappropriatefortheexfoliation—smallmethanolmolecules
Graphite
Liquid-phase exfoliation
Surfactant-assisted
Surfactant
Graphene
2FabricationMethodsfor2DMembranes
canentertheinterlayergalleriestopromotetheexfoliation,andpropanolhelps tostabilizetheexfoliatednanosheetsbyadsorbingonthenanosheetswithits hydrophobicalkanetails.Therefore,theselectionofpropersolventsiscritical forsuccessfulexfoliation,whichisuniversallyapplicabletoalmostallexfoliation methods.Ingeneral,themechanicalforceexfoliationcancontributetohighyield andmassiveproductionofnanosheets,butthesonicationorballmillingmaycause somedefectsonthenanosheets,affectingtheseparationperformanceofassembled membranes.
2.2.1.2Ion-IntercalationExfoliation
Theion-intercalationisaccomplishedbyintercalatingcationions(e.g.Li+ ,Na+ , andK+ )intotheinterlayergalleriesofbulkcrystals,whichwouldweakenthevar derWaalsinteractionbetweenneighboringlayersandexpandtheinterlayerspace foreasiercleavage.In1980s,Morrisonandcoworkersemployed n-butyllithium, astheintercalator,tosynthesizeMoS2 nanosheets[14].ThebulkMoS2 wasfirst soakedinhexanecontaining n-butyllithiumforatleasttwodaystointercalateLi intotheMoS2 .ThentheobtainedLi-intercalatedcompoundwastransferredinto water,followedbysonicationtoassistintheexfoliation.ThereactionbetweenintercalatedLiandwatercontinuouslygeneratedhydrogengasandfurtherfacilitated theexfoliationprocess.Inaddition,Lohandcoworkersproposedatwo-stepchemicalion-intercalationmethodtosynthesizelarge-sizednanosheets(Figure2.2)[15]. N2 H4 wasfirstintercalatedintothebulkcrystalsandcouldexpandthevolumeof thecrystalsafteritsdecomposition.Afterthat,theproductwassecondlyintercalatedbymetalnaphthalenide(metal = Li,Na,K)toformintercalatedcompounds. Inthisway,theyieldofMoS2 reached90%,andthelateralsizewasupto400 μm2 Itisworthpointingoutthatorganometalliccompoundsusedinthismethodare
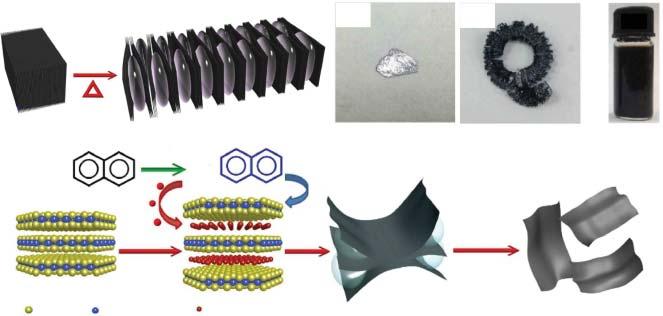
Figure2.2 Schematicillustrationofthetwo-stepchemicalion-intercalationmethodfor thepreparationofMoS2 nanosheets.(a)BulkMoS2 waspre-exfoliatedbythedecomposition productsofN2 H4 .(b)Pre-exfoliatedMoS2 wassecondlyintercalatedbymetal naphthalenide,followedbyexfoliationinwater.Photographsof(c)bulksingle-crystalMoS2 , (d)pre-exfoliatedMoS2 ,and(e)Na-exfoliatedsingle-layerMoS2 dispersioninwater. Source:Zhengetal.[15]/withpermissionfromSpringerNature.
2.2SynthesisofNanosheets 7 highlyexplosiveandverysensitivetomoistureandoxygen,sotheexperimentneeds tobeoperatedinagloveboxwithextremecaution.Bearingthisinmind,Zhengand coworkersdemonstratedthatsomesaferinorganicsalts,includingNaClandCuCl2 , canserveasintercalatorsforefficientexfoliation[16,17].ThegraphitewasdispersedinsaturatedNaClorCuCl2 solution,whichwasheatedat100 ∘ Ctoevaporate thewaterandobtainthecation-intercalatedgraphite.Theintercalatedcompound wasfurthersonicatedinorganicsolvent(e.g.DMF,ethanol,andNMP)toobtain graphenenanosheets.Moreover,theyextendedthismethodforthesynthesisofother nanosheets,includingMoS2 ,MoSe2 ,WS2 ,andWSe2 [17],demonstratingtheuniversalityofthesaferion-intercalationexfoliation.
2.2.1.3Oxidation-AssistedExfoliation
Oxidation-assistedexfoliationisawell-establishedmethodforthesynthesisof grapheneoxide(GO).ThismethodwasfirstlyproposedbyBrodiein1859,and furtherdevelopedbyStaudenmaierandHummers[18],formingthethreeprimary syntheticroutes.Theexfoliationisbasedontheoxidationofgraphiteintographite oxidebystrongoxidizingagents.Thegeneratedoxygen-containingfunctional groupscangreatlyexpandtheinterlayerspaceoflayeredgraphiteandweakenthe vanderWaalsinteractionbetweenneighboringnanosheets.Aftertheoxidation treatment,thesubsequentsonicationcanfurthertransformthegraphiteoxide intosingle-layerGOnanosheets.BothBrodieandStaudenmaiermethodsuse potassiumchlorateandnitricacidastheoxidizingagent,andthemostcommonly usedHummers’methodemployedthepotassiumpermanganateandconcentrated sulfuricacidtoensuresufficientoxidation.Theadvantageofthismethodliesin thehighyieldofsingle-layerGOnanosheets.However,theoxidationprocesscan causesevereexplosionsatelevatedtemperatures,andahugeamountofwastewatercontainingacidandmetalionscanbeproducedandleadtoenvironmental pollution.Therefore,agreenerandsaferexfoliationmethodbasedonelectrochemicaloxidationprocesswasproposedforthesynthesisofGOnanosheets.Renand coworkersreportedatwo-stepelectrochemicalmethodtofabricateGOnanosheets withahighlevelofoxygencontent(Figure2.3)[19].Inthefirstintercalationstep, thecommercialgraphitepaperservingastheanodewasimmersedin98wt%H2 SO4 atalowvoltageof1.6V,resultinginthebluegraphiteintercalationcompound.The secondstep(rapidoxidationprocess)requiredasuitableconcentrationofH2 SO4 , normallyintherangeof40–60wt%,toachievehighlyoxidizedGOwithC/Oratio of1.5–1.8,surpassingthatofclassicchemicaloxidationroutes(around2.2)[20]. Inaddition,theas-synthesizedGOnanosheetsexhibitedgooddispersibilityand nanoscalethickness,whicharesimilartothosesynthesizedbyHummers’method. Theyattributedtheextensivegraphiteoxidationtotheabundantradicalintermediates(e.g. * OH, * O,and * OOH)fromtheelectrolyticwatersplitting.Inthisway,the oxidationofgraphenelatticewasonlywithinafewseconds,whichismuchfaster thantheHummers’method.Theelectrochemicaloxidationmethodcombinedthe advantagesofsafety,environmentalfriendliness,ultrafastsynthesis,andeasiness toscaleup,whichpavesthewayforindustrialproductionandapplicationofGO sheetsatalowcost.
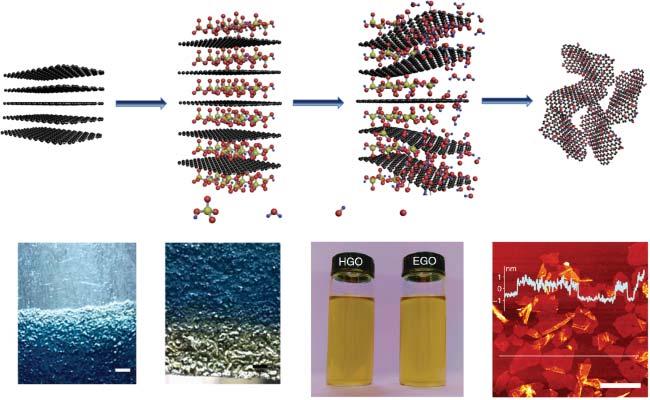
Figure2.3 SynthesisofGOnanosheetsbywaterelectrolyticoxidation.(a)Schematic illustrationofthefabricationprocessofelectrochemicallysynthesizedGO(EGO).Source: (a)Peietal.[19]/SpringerNature/CCBY4.0.(b)Photographofthegraphiteintercalation compoundpaper(GICP)(bluearea)afterthereactionwith98wt%H2 SO4 at1.6Vfor 20minutes.(c)PhotographofGO(yellowarea)resultingfromthewaterelectrolytic oxidationofGICPin50wt%H2 SO4 at5Vfor30seconds.(d)ComparisonofEGOwithGO preparedbyHummers’method(HGO)inwater(1mgml 1 ).(e)AFMimagesofEGO nanosheets.Source:Peietal.[19]/withpermissionfromSpringerNature.
2.2.1.4Selective-EtchingMethod
In2011,Gogotsiandcoworkersproposedtheselective-etchingmethodforthe exfoliationofbulkMAXphasesintothenanosheetsoftransitionmetalcarbides and/orcarbonitrides(MXenes)[21].ThetermMAXphasesreflectthechemical compositionofMn+1 AXn ,where n = 1,2,or3(M2 AX,M3 AX2 ,orM4 AX3 ),“M”is anearlytransitionmetal,“A”isanAgroup(mostlygroups13and14)element,and “X”isCand/orN[22].IntheMAXphases,theMlayersarenearlyclosedpacked withtheXatomsfillingtheoctahedralsites,andtheMn+1 Xn layersareinterleaved withlayersofAatoms.TheM—Xbondhasamixedcovalent/metallic/ionic character,whiletheM—Abondismetallic.Comparedwiththeweakvander Waalsinteractionsingraphiteandtransitionmetaldichalcogenides(TMDs),the metallicbondsaretoostrongtobebrokenbythetraditionalmechanicalforces.To solvethisproblem,GogotsiandcoworkersreportedtheselectiveetchingoftheAl fromTi3 AlC2 byintroducingthehydrogenfluoride(HF)(Figure2.4)[22].Owing tothedifferencesincharacterandrelativestrengthsoftheTi—Albondcompared withtheTi—Cbond,theAllayerscanbeselectivelyetchedwithoutdisruptingthe Ti—Cbond.TheetchedintermediateproductwasfurthersonicatedtoobtainTi3 C2 nanosheets.MXenespreparedbythismethodpossessnumerousterminations(e.g. O,–OH,and–F)onthesurface,whicharefavorableforstabilizingtheminsolution andtheassemblyintomembranes.ItisworthpointingoutthattheetchantHFis
MAX phase are layered ternary carbides, nitrides, and carbonitrides consisting of “M”, “A” , and “X” layers
Selective HF etching only of the “A” layers from the MAX phase
Physically separated 2D MXene sheets after sonication
Figure2.4 SchematicillustrationofthesynthesisprocessofMXenesfromMAXphasesby selective-etchingmethod.Source:Naguibetal.[22].Reproducedwithpermission. Copyright2012,AmericanChemicalSociety.
highlycorrosiveanddangerous.Therefore,Gogotsi’sgroupdevelopedthemixture offluoridesaltsandHClorH2 SO4 asthealternativetothecorrosiveHF[23],which improvedthesafetyoftheselective-etchingmethod.Moreover,morekindsof MXenes,suchasTi2 C,Nb2 C,V2 C,andNb4 C3 ,havebeensuccessfullysynthesized bythismethod[24],demonstratingitsgreatpotentialforwiderapplication.The limitationisthatthemethodisnotapplicabletothesynthesisofothernanosheets, suchasgrapheneorTMDs.
2.2.2Bottom-UpMethod
2.2.2.1ChemicalVaporDeposition
ThefirstindustrialapplicationofCVDtechniquewasdatedbackto1897when deLodyguinereducedtungstenhexachloridewithhydrogentodeposittungsten ontothecarbonfilamentoflamps[25].Thenthistechniquewasfurtheremployed fortheproductionofhigh-puritymaterials,includingTi,Ta,Zr,andSi.Overthe pastdecade,CVDhasbeenconsideredasanefficienttechniqueforthemassive productionof2Dnanosheets.Typically,thesubstratesareplacedinafurnace,and gas/vaporprecursorsarecycledthroughthechambertoreactand/ordecompose onthesurfaceofsubstrates.Sometimesthecatalysts,intheshapeofafilm,are neededinthereactionprocess.Thenthenanosheetscanbeobtainedandfurther transferredtothedesiredsubstrates.Somaniandcoworkerspioneeredthesynthesis ofgraphenefilmsfromcamphorpyrolysisonNisubstratesviatheCVDmethod [26].Despitethefilmthicknessofabout30layers,thisworkdemonstratedthe feasibilityoftheCVDmethodforthefabricationofgraphene.Followingthiswork,
MXene sheets
2FabricationMethodsfor2DMembranes
manyeffortshavebeendevotedtooptimizingtheexperimentalconditions,such astheprecursors,substrates,catalysts,temperature,andatmospheres,whichare criticaltothestructuralfeaturesoftheobtainedgrapheneproducts.Pollardetal. demonstratedtheCVDgrowthofsingle-layergrapheneontheNifilmevaporated ontheSiO2 /Sisubstrate[27].ItisworthnotingthattheNifilmservedasthe substrate,whichsupportedthegrowinggrapheneandthecatalystfacilitating thenucleationofprecursorsatthesametime.Inaddition,Lietal.synthesized large-areagraphenefilmwithlateralsizeofcentimetersandsingle-layerrateover 95%byCVDmethodoncopperfoilsubstrate[28].Methaneandhydrogenwere chosenasgassources,andthesubstratewasremovedbythetreatmentwithiron nitritesolutiontoobtainfreestandinggraphenefilms.Similarly,theCVDgrowth methodcanbeappliedforthefabricationofothernanosheets,including h-BN [28,29],MoS2 [30],andsoon.DespitethegreatpotentialofCVDtechniqueinthe massiveproductionofnanosheetswithhighcrystalqualityandpurity,thesynthetic conditions,suchashightemperatures(∼1000 ∘ C)andinertatmosphere,leadto highproductioncostsandrestrictitsindustrialapplication.
2.2.2.2Hydro/SolvothermalSynthesis
Thehydro/solvothermalsynthesis,inwhichwaterororganicsolventservesas thereactionmediuminasealedvesselathightemperatureandpressure,has beenapopularstrategyforpreparingnanosheets,suchas2Dmetaloxides,metal chalcogenides,andMOFs[31].Duringthesynthesis,thekeyissueliesinhowto selectivelyblockthegrowthofnanomaterialsalongonedirectiontoformdesired nanosheets.Theintroductionofstructure-directingagentsisdemonstratedtobe aneffectivestrategy.Tsapatsisandcoworkersproposedthebottom-upsynthesis ofMFInanosheetsviathehydrothermalgrowthofMFIseedswiththeassistance ofbis-1,5(tripropylammonium)pentamethylenediiodide(dC5)[32].ThedC5 wasproventodirectthegrowthofplate-likeMFIwiththethincrystaldimension alongthe b axis,andthestraightmicroporesalignedalongthisdirection,endowing the2.5-unit-cellthick(5nm)MFInanosheetswithgreatpotentialinmembrane separation.Thehydro/solvothermalsynthesismethodisascalablemethodforthe high-yieldproductionofultrathinnanosheets,buttheproductisquitesensitiveto thesynthesisparameters,suchastheconcentrationofprecursors,surfactantand solventtype,andreactiontemperature/time,thusinfluencingthereproducibility ofeachbatch[1].
2.2.2.3InterfacialSynthesis
Theinterfacialsynthesisisanotherbottom-upmethodforthefabricationof2D nanosheets.Duringthesynthesisprocess,themonomersreactattheconfined interfacestoguaranteealowthickness.Thediffusionratesofthemonomerstoward theinterfaceshouldbecarefullycontrolled,whichdeterminesthestructural integrityoftheas-preparednanosheets.Gasconandcoworkersproposeda three-layersynthesisstrategyforthepreparationofMOFnanosheets[33].The systemconsistedofthreeliquidlayerscomposedofmixturesofDMFandasuitable miscibleco-solventinappropriateratios,whichwereverticallyarrangedaccording
2.3MembraneStructuresandFabricationMethods 11 totheirdifferentdensities.ThetopandbottomlayerscontainedCu(NO3 )2 and 1,4-benzenedicarboxylicacid(BDCA),respectively,whiletheintermediateregion actedasahighlydilutedmediumforthegrowthofMOFnanosheets.Theobtained nanosheetsexhibitedlateralsizeof0.5–4 μmandthicknessintherangeof5–25nm. Moreover,themethodcanbeextendedtoavarietyofMOFsbychangingthe metalnodesororganiclinkers,demonstratingtheversatilityofthethree-layer synthesisstrategy.BesidesMOFnanosheets,covalentorganicframework(COF) nanosheetscanbepreparedbytheinterfacialsynthesismethodaswell.Payamyar etal.successfullysynthesizedCOFnanosheetsatthewater/airinterface[34].They designedanamphiphilicmonomerthatcanpreorganizeonthewatersurfaceand realizeface-to-facestackingoftheanthraceneunits.Asaresult,themonomerswere connectedviathephotochemicalanthracene[4+4]-cycloadditiondimerizationto constructCOFnanosheets.Althoughtheinterfacialsynthesismethodcanproduce nanosheetswithintegratedstructure,theyieldisrelativelylowandcannotmeet thedemandforindustrial-scaleproduction.
2.3MembraneStructuresandFabricationMethods
Accordingtothedifferencesinmembranestructures,two-dimensional-material membranescanbecategorizedintothreemaintypes,includingnanosheetmembranes,laminarmembranes,andMMMs,andthefabricationmethodsforeach structuredifferfromoneanother.Inthissection,wefocusonvariousstrategies formembranefabricationandintroducethelatestadvancesindifferenttypesof membranes.Importantly,theeffectofconstructingandtuningthein-planepores orinterlayerchannelsonthemembranestructureandseparationperformance issystematicallysummarized.Inaddition,theprosandconsofthefabrication methodsarebrieflycompared.Inthelastpart,somehybridmembranesbeyondthe mentionedtypesarediscussedaswell.
2.3.1Two-Dimensional-MaterialNanosheetMembranes
2.3.1.1ZeoliteMembrane
Zeolitesaremicroporousmaterialsbasedoncrystallinesilicawithporesizeswithin severalangstromstonanometers.Thethicknessof2Dzeolitenanosheetscanbe reducedasthinasthedimensionofunitcells,whichenablesthenanosheetstobe promisingmaterialforfabricatingultrathinmembraneswithsuperiorseparation performance.Tsapatsisandcoworkershavebeendevotedtothefabricationof high-qualityzeolitenanosheetsandmolecular-sievingmembranes.In2011,they reportedthesynthesisofhighlycrystallineMFInanosheetsviathemelt-blending methodcombinedwithsonication[35].Theobtainednanosheetswere ∼3.4nm, only1.5-unit-cellthickalongthe b axis.Thentheyemployedfiltrationmethodto depositnanosheetsontheporoussupporttoformthenanosheetcoating,whichwas orientedsincetheshortdimensionofnanosheetswasperpendiculartothesupport surface.Nevertheless,nanometer-sizedpinholedefectsexistedinthedeposits,
whichseriouslyaffectedtheseparationperformance.Thesecondarysolvothermal growthmethodwasproposedtoeliminatethedefects,andthepreferredorientationcouldbemaintainedbytheintroductionofappropriatestructure-directing agents.Thethicknessofas-preparedultrathinzeolitemembranescanbereduced to ∼100nm,whichwas10timesthinnerthantypicalzeolitemembranes[36]. Importantly,themembraneshowedexcellentmolecular-sievingpropertyand high-separationperformanceforxyleneisomermixtures.Althoughthezeolite nanosheetssynthesizedinthismethodwereofhighpurity,theyieldwaslow (∼0.01%w/wnanosheetsinthedispersion)andthelateralsizeofnanosheetswas relativelysmall.Bearingthisinmind,theyfurtherdemonstratedthedirectsynthesisofzeolitenanosheetsviahydrothermalgrowthofMFIseeds[32],whichwas previouslydiscussedinSection2.2.2.Theas-synthesizednanosheetscombinedthe lateraldimensionofconventionalMFIcrystals(1.5–2.0 μm)withnanometer-scale thickness.Thelargelateraldimensionswerefavorforloweringthedensityofgrain boundariesinthemembranes,whichwouldleadtononselectivetransportpathways andreducethesievingproperty.Aftergel-freesecondarygrowth,adenselayerwith thicknessrangingbetween250nmand1 μmwasformedontheporoussupport. Thesuccessfulsynthesisofhigh-qualityzeolitenanosheetsendowedthemembrane withexcellent p-xylene/o-xyleneseparationperformance(p-xylenepermeance: 0.56 × 10 6 molPa 1 m 2 s 1 ,separationfactor:2000at150 ∘ C).Themembrane throughputcanbefurtherenhancedifthemembranethicknesscanbereduced to10nm.Furthermore,morezeolitecrystalswithlayeredstructuresshouldbe discoveredforimprovingthediversityofzeolitenanosheets.Theearlyadvancein 2Dzeolitemembraneslaysthefoundationforthefastdevelopmentof2D-material membranes.
2.3.1.2MOFMembrane
Owingtotheabundantporousstructureandfunctionalsurfacegroups,MOFs canserveasbuildingblocksformolecular-sievemembranes.Alargeamountof MOFcrystalswithlayeredstructurescanbeexfoliatedintoultrathinnanosheets, demonstratingthegreatpotentialof2DMOFmembranes.However,itischallengingtoretainthemorphologicalandstructuralintegrityofthesynthesized nanosheets,whichisacriticalfactorforfabricatingdefect-freeMOFmembranes. YangandcoworkersdemonstratedthefabricationofultrathinMOFnanosheets andhigh-performancemolecular-sievemembranes[13].TheZn2 (bim)4 nanosheets wereexfoliatedviaasoft-physicalstrategy,whichwasdiscussedinSection2.2.1. Filtrationmethodiscommonlyusedforthepreparationofnanosheetmembranes.Nevertheless,theyfoundthismethodcouldleadtotherestackingof nanosheetsbacktoorderedpristinestructures,whichwouldpartiallyortotally blockthemolecular-sievepores.Ahot-dropcoatingmethodwasproposedto realizethedisorderedstackingofnanosheets.Inatypicalpreparation,acertainamountofnanosheetsuspensionwasdepositeddropwiseontothesurface oftheporoussupport,whichwasheatedonaheatingplate.Asaresult,the MOFnanosheetswereuniformlydepositedonthesupporttoformacontinuous layer.Thetextureoftheunderlyingsupportwasdistinguishable,indicatingan
ultrathinMOFlayer.Inaddition,thedetectionofaluminumsignalfromAl2 O3 supportbyX-rayphotoelectronspectroscopy(XPS)provedthatthemembrane wasonlyseveralnanometersthick.Moreover,thestructure–performancerelationshipwasinvestigatedaswell.Thelow-anglehumpand(002)reflection correspondedtotheexpandedstackingandorderedrestackingofnanosheets, respectively,whichwouldblockthepathwayforH2 andreducedtheseparationperformance.TheultrathinMOFmembrane,consistingofdisordered nanosheets,exhibitedexcellentH2 /CO2 separationperformancewithH2 permeanceofupto2700GPUandselectivityreaching291.Wangetal.proposedthe freeze–thawapproachtoexfoliatethebulkMAMS-1(meshadjustablemolecular sieve,Ni8 (5-bbdc)6 (μ-OH)4 ,5-bbdc = 5-tert-butyl-1,3-benzenedicarboxylate)crystalsinto ∼4nmnanosheets[37].ThentheMAMS-1nanosheetswereassembled intomolecular-sievemembranesviathehot-dropcastingmethod.Theas-prepared membranespossessedporeopeningsparalleltogasconcentrationgradient,which allowedhighgasfluxandhighselectivityatthesametime.Inaddition,theydemonstratedthereversedthermos-switchablepropertyofthegastransportpathways.
TheH2 /CO2 selectivityexhibitedatemperature-dependentbehavior,withthemaximumof245at20 ∘ Candtheminimumofc.5at100 ∘ C.Basedon insitu variable temperaturepowderX-raydiffraction(PXRD)andmoleculardynamicssimulation, thisphenomenonwasattributedtotheflexibilityoftheMAMS-1nanosheets. Hence,theMOFmembranewithreversedthermo-switchablemolecular-sieving propertycouldhavepotentialinthetemperature-relatedgasseparationprocess.
2.3.1.3PorousGrapheneMembrane
Theperfectsingle-layergraphenesheetisimpermeabletogasesassmallashelium becausetheelectrondensityofitsaromaticringsistoosubstantialtoallowthetransportofatomsandmoleculesthroughtheserings[38].Therefore,researcherswere motivatedtodrillholesingraphenesheetstofabricateporousgraphenemembranes, whichholdthepotentialtoachievesuperiorseparationperformanceduetotheir single-atomthinness.Atfirst,theoreticalstudiesonporousgraphenemembranes werereported.Jiangetal.designedporesfunctionalizedwithdifferentatomsonthe graphenenanosheetandinvestigatedthegasseparationperformanceoftheporous graphenemembranebyfirst-principlesdensity-functionaltheorycalculations[39]. Cohen-TanugiandGrossmanreportedthedesalinationperformanceofsingle-layer graphenewithnanometer-scaleporesbasedonclassicalmoleculardynamics[40]. Theyfoundthattheporediameteroftheporousnanosheetdeterminedthesaltrejectionperformanceandthehydrophilicgroups,suchashydroxylgroups,bondedtothe poreedgescontributedtoprominentlyenhancedwaterflux.Inaddition,thewater permeabilityoftheporousgraphenewasseveralordersofmagnitudehigherthan theconventionalreverseosmosismembrane,whichexhibitedthegreatpotentialof porousgrapheneinwaterpurification.
Apartfromthetheoreticalcalculations,experimentalstudiesondrillingporesin graphenesheetswerereported.Theultraviolet-inducedoxidativeetchingmethod wasfirstlyproposedforcreatingporesingraphenemembranes[41].Afterthe introductionofnanopores,thepressurizedblistertestcombinedwithmechanical
2FabricationMethodsfor2DMembranes
resonancewasemployedtomonitorthemoleculartransportthroughnanopores. Theas-fabricatedmembraneexhibitedagaspermeationratecomparabletothat ofcomputationalmodelingandmolecular-sievingproperty,makinganimportant steptowardtherealizationofmacroscopicporousgraphenemembranes.Celebi etal.reportedthephysicallyperforateddouble-layergraphenebythefocused ionbeam[42].Theperforatedgraphenemembranepossessedlotsofnanopores withnarrowlydistributeddiametersrangingfrom <10nmto1 μm,endowingthe membranewithorders-of-magnitudeenhancementingas,water,andwatervapor permeances.Moreover,theO2 plasma-etchingmethodwasemployedtofabricate porousgraphenemembranesforwaterdesalination[43].Themembranewithfinely tunednanoporesshowedfastwatertransportandasaltrejectionrateofnearly100%, provingthefeasibilityofporousgraphenemembranefordesalination.Tocreate nanoporeswithangstromprecisionatahighporedensity,Zhaoetal.developed synergisticdefectnucleationandpore-expansionstrategybycombiningO2 plasma andO3 treatment[44].Ramanspectrawereemployedtodetecttheevolutionof defectsandporosityoftheporousgraphenenanosheet.Asaresult,theyachieved ahighdensity(c.2.1 × 1012 cm 2 )ofH2 -sievingporesandrecord-highH2 /CH4 and H2 /C3 H8 separationperformance.Moreimportantly,thispore-etchingstrategy ishighlyscalableandwillcontributetotherapiddevelopmentofsingle-layer graphene-basedmembranes.
Althoughmanystrategieshavebeenproposedtomakeporesonthegraphene nanosheet,theproblemofstructuralintegrityoftheatomic-thinmembranewill restrictitspracticalapplication.Yuanandcoworkersreportedthefabricationof large-areagraphene-nanomesh/single-walledcarbonnanotube(GNM/SWNT) hybridmembranewithexcellentmechanicalstrength[45].Inatypicalprocess, single-layergraphenewaspreparedonCufoilviatheCVDmethod.Alayerof SWNTmembraneconsistingofinterconnectedSWNTswastransferredontopof thegraphene,andafreestandingmembranewithSWNT-supportedgraphenewas obtainedbyetchingtheCufoil.ThenalayerofmesoporousSiO2 filmwithperpendicularmesoporouschannelswascreatedonthegraphenesurface,servingasthe poroustemplate.O2 plasmawasemployedtodrillporesinthegraphenetoformthe graphenenanomesh,andthemeso-SiO2 layerwasremovedbyhydrofluoricacid etching.Asaresult,theGNM/SWNThybridmembranewasobtained,inwhichthe mechanicallystrongSWNTwebsformedstrong π–π interactionwiththesupported GNMandactedasamicroscopicframeworktosupporttheGNM,ensuringthe structuralintegrityoftheatomicallythinGNMovermacroscopicscale.Moreover, thehigh-densitysubnanometerporesintheGNMallowthefasttransportofwater molecules,whileblockingsoluteions/moleculestoenableselectiveseparation.The as-fabricatedultrathinGNM/SWNThybridmembraneshowhighwaterpermeance andahighrejectionratioforsaltionsororganicmolecules.
Inall,theporousgraphenemembranehasdemonstrateditshugepotentialinthe process,suchaswaterpurificationandgasseparation,butthetechnicalissueofcreatinghigh-densitynanoporeswithnarrowlydistributeddiameterinthemembrane areaofm2 levelremainstobesolved.
2.3.2Two-Dimensional-MaterialLaminarMembranes
2.3.2.1AssemblyStrategiesofLaminates
Forthelaminarmembraneassembledfrom2Dnanosheets,theselectionofproper assemblystrategydeterminesthemicrostructureofmembranesandthusthetransportbehavior.ForGOmembranespreparedbyself-assembly,theintrinsicrepulsive forcebetweenthecarboxylgroupofneighboringnanosheetswillcausethedisorderedstackingofnanosheetsandthegenerationofnonselectivedefects[8],which greatlyinfluencetheseparationperformanceoflaminarmembranes.Hence,variousdrivingforces,includingpressuredifference,shearforce,centrifugalforce,and electric-fieldforce,areemployedtoregulatetheassemblyof2Dnanosheetsand reducetheinterlayerdefect.
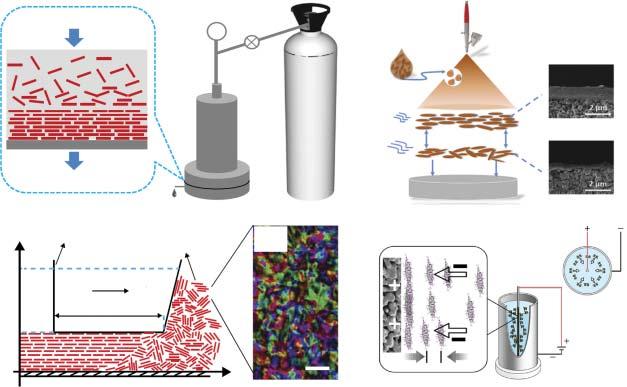
ToconstructcontinuousGOlaminateswithfewdefects,vacuumfiltrationmethod wasemployedtosurpasstherepulsiveforcebetweenneighboringnanosheets.Yu andcoworkersfabricatedultrathin(1.8–18nm)GOmembranesontheanodicaluminumoxide(AAO)supportviafiltrationforhydrogenpurification[46].ThemembranethicknesscouldbeeasilytunedbycontrollingthedepositionamountofGO nanosheets.TheyobservedthatthereducedGOmembranewithnarrowedinterlayerspacingshowedsimilargaspermeancetothepristinemembrane,suggesting theselectivestructuraldefectswithinGOflakesinsteadoftheinterlayerspacing determinedthegastransportbehavior.Theas-preparedGOmembraneexhibited ultrahighH2 /CO2 selectivityof3400andH2 /N2 selectivityof900.Moreover,our groupfirstlyproposedavacuumsuctionmethodfordepositingGOnanosheetson theceramichollowfiber(Figure2.5)[47],whichpossesseshigh-packingdensity andisbeneficialtothescale-upfabricationofGOmembranes.Owingtothepreferentialwater-sorptionabilityandfastwaterdiffusivitythroughtheGOlayer,the as-fabricatedGOmembraneshowedexcellentpervaporationdehydrationperformanceforthedimethylcarbonate(DMC)/watermixture.Similarly,basedonthe drivingforceofpressuredifference,pressurizedfiltrationmethodwasemployedto preparethefreestandingGOmembrane(Figure2.6a)[48].Thefiltrationpressure wasprovedtoinfluencethepackingdensityofGOnanosheetsandthustheinterlayerspacingofGOlaminates.ThefreestandingGOmembranewasappliedinthe pervaporationseparationofwater/ethanolmixture,butthebinary-componentselectivitywasmuchlowerthantheidealwater/ethanolselectivity,whichwasattributed totheswellingofGOlaminatesinaqueoussolution.ThestrategyforstabilizingGO membranesisdiscussedinSection2.3.2.2.
Besidesthepressuredifference,centrifugalforcewasemployedtoassistthe assemblyofGOlaminates.Kimetal.reportedthefabricationoffew-layered GOmembranesviathespin–coatingmethod[52].Theyemployedtwodifferent coatingmethodstoprepareGOmembranes.Inmethodone,theycontactedthe surfaceofthesupporttotheair–liquidinterfaceofaGOsolution,followedby spincoating.Forcomparison,theydirectlyspin-coatedtheGOsolutiononthe supportinmethodtwo.Asaresult,twoGOmembranesexhibitedsimilarsurface morphologybutquitedifferentgastransportbehavior.Therepulsiveedge-to-edge
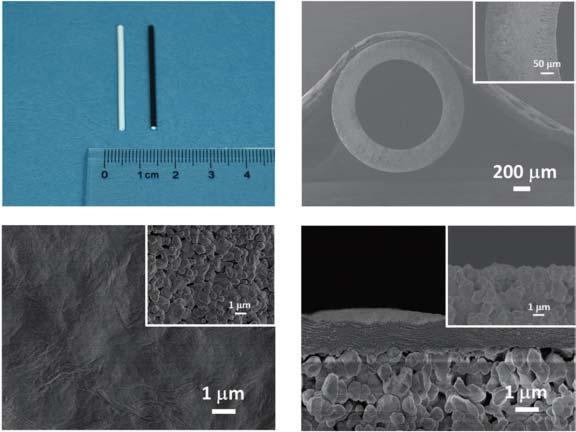
Figure2.5 GOmembranesfabricatedbyvacuumsuctionmethod.(a)Digitalpicturesof blankhollowfiber(white)andGOmembrane(black).SEMimagesof(b)aceramichollow fiber(inset:anenlargedcrosssectionoftheceramichollowfiber),(c)thesurfaceofthe blankhollowfiber(inset)andtheGOmembrane,and(d)thecrosssectionoftheblank hollowfiber(inset)andtheGOmembrane.Source:Huangetal.[47]/withpermissionfrom JohnWiley&Sons,Inc.
interactionfromthecarboxylicgroupsofGOnanosheetsledtoaheterogeneous GOdepositioninmethodone,whiletheface-to-faceattractivecapillaryforces duringspincoatingoverwhelmedtherepulsiveinteractioninmethodtwo,contributingtothedensestackingofGOnanosheets.TheGOmembranepreparedby methodtwoformedhighlyinterlockedstructureandexhibitedexcellentCO2 /N2 separationperformance.Inaddition,wefirstlyproposedtofabricateGOlaminar membranesbythespray–evaporationmethod(Figure2.6b)[49].Sprayingisan efficientcoatingmethodwithhighcontrollabilityforrealizingthehomogeneous coating.Wefoundthatthesprayingtimesandevaporationrateofthecasting solutioncouldbemanipulatedtocontrolthemicrostructureofGOmembraneand thustheH2 /CO2 separationperformance.Itwasdemonstratedthattheheating temperatureandtheethanol/watermassratiointhesolventofGOsolution togetherinfluencedtheevaporationrate.TheoptimizedGOmembraneexhibited superiorH2 /CO2 separationperformanceexceedingtheupperboundofpolymeric membranes.
Topromotetheindustrialapplicationoftwo-dimensional-materialmembranes, thepreconditionisrealizingthefabricationoflarge-areamembranesbasedon ultrathinnanosheets.Majumderandcoworkerssuccessfullyfabricatelarge-area GOmembranes(13 × 14cm2 )viatheshearforce-assistedassembly(Figure2.6c,d) [50].TheyinnovativelyconcentratedtheGOsolutiontotheliquidcrystallinephase (40mgml 1 )byintroducingthehydrophilicpolymerhydrogelbeadsintoaqueous
Figure2.6 (a)Schematicofapressurizedultrafiltrationsystem.Source:Tangetal.[48]/ withpermissionofElsevier.(b)SchematicoftheassemblyofGOlaminatesviaspray–evaporationmethod.Source:Guanetal.[49]/withpermissionfromElsevier.(c)Schematic ofshear-alignmentprocessingofanematicGOtoafilm,and(d)Polarizedlightimagesof fullynematicGOat40mgml 1 (scalebar,1 μm).Source:Akbarietal.[50]/Springer Nature/CCBY4.0.(e)SchematicofGOelectrophoresisdepositiononPSSHFwithacircular electricfield.Source:Qietal.[51]/withSpringerNature/CCBY4.0.
solution.ThenthenematicphaseofGOwasshearalignedtoformcontinuous andhighlyorderedGOlayerbythedoctorblade.TheviscosityoftheGOcasting solution,whichwascontrolledbytheGOconcentrationfrom0.1to60mgml 1 , wasdemonstratedtobethedominantfactorontheimposedshearstressand influencedthemembranestructure.Theyobservedthattheuniformityofthe castfilmincreasedwithincreasingGOconcentration,andtheGOsuspensionof 40and60mgml 1 contributedtothebestuniformityandcontinuity.Moreover, theyemployedagravureprintertomakelarge-areaGOmembranes,verifyingthe proficiencyofthemethodinmembranefabrication.ThereducedGOmembrane, whichwasexposedtothehydrazinevapor,showedhighretention(>90%)for organicmoleculesandmodest(30–40%)retentionofmonovalentanddivalentsalts duringthenanofiltrationprocess.
Besidesthemechanicalforcementionedabove,electric-fieldforcewasutilizedto facilitatetheassemblyofGOnanosheets.Zengandcoworkersproposedtodeposit ultrathinGOlayerontheporousstainlesssteelhollowfiber(PSSHF)viaafacileelectrophoresisdeposition(ED)method[51].Inatypicalfabricationprocess,acircular electricfieldwasformedbetweentheconcentricelectrodesofPSSHFandstainlesssteeltubularcontainerfortheGOsolution,ensuringauniformdrivingforce fromeverydirection(Figure2.6e).Asaresult,theoxygen-containinggroupofGO nanosheetswaspartiallyreducedbythecathode,leadingtothenarrowed2DchannelofGOlaminatesforenhancedmolecularsieving.TheGOmembranefabricated bytheEDmethodexhibitsasharpcutoffbetweenC2(ethaneandethene)andC3