TransitionMetalOxidesforElectrochemical EnergyStorage
EditedbyJagjitNandaandVeronicaAugustyn
Editors
Dr.JagjitNanda
OakRidgeNationalLaboratory ChemicalSciencesDivision 1BethelValleyRoad 37831OakRidgeTN UnitedStates
Prof.VeronicaAugustyn NorthCarolinaStateUniversity DepartmentofMaterialsScience& Engineering 911PartnersWay 27606RaleighNC UnitedStates
CoverImages: ©Antoine2K/Shutterstock; SmileFight/Shutterstock
Allbookspublishedby WILEY-VCH arecarefully produced.Nevertheless,authors,editors,and publisherdonotwarranttheinformation containedinthesebooks,includingthisbook, tobefreeoferrors.Readersareadvisedtokeep inmindthatstatements,data,illustrations, proceduraldetailsorotheritemsmay inadvertentlybeinaccurate.
LibraryofCongressCardNo.: appliedfor BritishLibraryCataloguing-in-PublicationData Acataloguerecordforthisbookisavailable fromtheBritishLibrary.
Bibliographicinformationpublishedby theDeutscheNationalbibliothek TheDeutscheNationalbibliotheklists thispublicationintheDeutsche Nationalbibliografie;detailedbibliographic dataareavailableontheInternetat <http://dnb.d-nb.de>.
©2022WILEY-VCHGmbH,Boschstr.12, 69469Weinheim,Germany
Allrightsreserved(includingthoseof translationintootherlanguages).Nopartof thisbookmaybereproducedinanyform–by photoprinting,microfilm,oranyother means–nortransmittedortranslatedintoa machinelanguagewithoutwrittenpermission fromthepublishers.Registerednames, trademarks,etc.usedinthisbook,evenwhen notspecificallymarkedassuch,arenottobe consideredunprotectedbylaw.
PrintISBN: 978-3-527-34493-2
ePDFISBN: 978-3-527-81722-1
ePubISBN: 978-3-527-81724-5
oBookISBN: 978-3-527-81725-2
Typesetting Straive,Chennai,India
Printedonacid-freepaper 10987654321
Contents
Foreword xiii
1AnOverviewofTransitionMetalOxidesforElectrochemical EnergyStorage 1 EthanC.Self,DevendrasinhDarbar,VeronicaAugustyn,andJagjitNanda
1.1FundamentalsofElectrochemicalCells 1
1.2Li-IonBatteries:BasicPrinciplesandTMOElectrodes 3
1.3BriefHistoryofLithium-IonBatteries 4
1.4TheRoleofAdvancedCharacterizationandComputingResources 4
1.5BeyondLithium-IonBatteries 5 Acknowledgments 6 References 6
2Metal–Ion-CoupledElectronTransferKineticsin Intercalation-BasedTransitionMetalOxides 9 VictoriaA.NikitinaandKeithJ.Stevenson
2.1Introduction 9
2.2ThermodynamicControl 11
2.3DiffusionalControl 14
2.4KineticControl 16
2.5EffectofSurfaceLayersonIonTransferKinetics 20
2.6SlowDesolvationasaLimitingIntercalationStep 24
2.7ConcludingRemarks 28 References 28
3Next-GenerationCobalt-FreeCathodes–AProspective SolutiontotheBatteryIndustry’sCobaltProblem 33 NitinMuralidharan,EthanC.Self,JagjitNanda,andIliasBelharouak
3.1Introduction 33
3.2PotentialofCobalt-FreeCathodeMaterials 35
3.3LayeredCathodes 35
3.3.1ConventionalLayeredCathodes 35
3.3.2BinaryLayeredNi-RichCathodeMaterials 36
3.3.3TernaryLayeredNi-RichCathodeMaterials 39
3.4SpinelandOlivineCathodes 41
3.5DisorderedRocksalt(DRX)Cathodes 43
3.6ChallengesinCommercialAdoptionofNewCobalt-Free Chemistries 45
3.6.1SynthesisofCathodePrecursors 46
3.6.2SynthesisofFinalCathodePowders 47
3.6.3ElectrodeFabrication 47
3.6.4BatteryAssembly 47
3.7SummaryandPerspective 48 Acknowledgments 49 ConflictofInterest 49 References 49
4TransitionMetalOxideAnodesforElectrochemicalEnergy StorageinLithium-andSodium-IonBatteries 55 ShanFang,DominicBresser,andStefanoPasserini
4.1Introduction 55
4.2PotentialAdvantagesandChallengesoftheConversion Mechanism 58
4.3TransitionMetalOxidesasAnodeMaterials 61
4.3.1IronOxide(Fe3 O4 ,Fe2 O3 ) 61
4.3.2CobaltOxide(CoO,Co3 O4 ) 67
4.3.3ManganeseOxide(MnO,Mn3 O4 ,MnO2 ) 71
4.3.4CopperOxide(Cu2 O,CuO) 78
4.3.5NickelOxide(NiO) 82
4.3.6RutheniumOxide(RuO2 ) 86
4.3.7OtherTransitionMetalOxides 88
4.4SummaryandOutlook 88 References 90
5LayeredNa-IonTransition-MetalOxideElectrodesfor Sodium-IonBatteries 101 BaskarSenthilkumar,ChristopherS.Johnson,andPremkumarSenguttuvan
5.1Introduction 101
5.2LayeredTransition-MetalOxides 102
5.2.1StructuralClassification 102
5.2.2SingleTransition-Metal-BasedLayeredTransition-MetalOxides 103
5.2.3Mixed-Metal-BasedLayeredTransition-MetalOxides 107
5.2.4AnionicRedoxActivityforHighCapacity 110
5.3SummaryandOutlook 112 References 114
6AnionicRedoxReactioninLi-ExcessHigh-Capacity Transition-MetalOxides 121 NaoakiYabuuchi
6.1StoichiometricLayeredOxidesforRechargeableLithium Batteries 121
6.2Li-ExcessRocksaltOxidesasHigh-CapacityPositiveElectrode Materials 123
6.3ReversibleandIrreversibleAnionicRedoxforLi3 NbO4 -and Li2 TiO3 -BasedOxides 126
6.4ActivationofAnionicRedoxbyChemicalBondswithHighIonic Characters 130
6.5Li4 MoO5 asaHostStructureforLithium-ExcessOxides 131
6.6ExtremelyReversibleAnionicRedoxforLi2 RuO3 System 133
6.7AnionicRedoxforSodium-StorageApplications 135
6.8FuturePerspectivesofAnionicRedoxforEnergy-Storage Applications 138 References 139
7TransitionMetalOxidesinAqueousElectrolytes 145 XiaoqiangShanandXiaoweiTeng
7.1Introduction:OpportunitiesandChallengesofAqueousBatteries 145
7.2ElectrochemistryofAqueousBatteries 146
7.2.1PotentialWindow 146
7.2.2DiverseChargeTransferandStorageProcessesinAqueous Batteries 148
7.2.2.1OverviewofVariousStorageMechanisms 148
7.2.2.2Semi-quantitativeAnalysisofStorageMechanismfromSweeping VoltammetryAnalysis 151
7.2.2.3StorageMechanismsinElectrolytewithDifferentpHValues 152
7.3TransitionMetalOxidesforAqueousEES 156
7.3.1ManganeseCompounds 157
7.3.1.1CrystalStructuresofManganeseOxidesforAqueousStorage 157
7.3.1.2CompositingManganeseOxideswithOtherAdditives 161
7.3.1.3SurfaceEngineeringCrystalFacets,EdgeSites,andBulk/NanoSize Domain 161
7.3.1.4DopingandDefectChemistry 162
7.3.1.5Pre-intercalatedSpecies 163
7.3.2NiCompounds 165
7.3.3VanadiumCompounds 167
7.3.3.1LiorNaVanadates 169
7.3.4IronCompounds 171
7.3.4.1Fe/Fe3 O4 171
7.3.4.2Fe2 O3 /FeOOH 172
7.4Conclusion 173 Acknowledgments 174 References 174
8NanostructuredTransitionMetalOxidesforElectrochemical EnergyStorage 183
SimonFleischmann,IshitaKamboj,andVeronicaAugustyn
8.1FundamentalElectrochemistryofNanostructuredTMOs 183
8.1.1ThermodynamicsofChargeStorageinNanostructuredTMOs 183
8.1.2KineticsofChargeStorageinNanostructuredTMOs 186
8.2EmergingNanostructuredTMOs 189
8.2.1NanostructuredTMOCathodesforLIBs 189
8.2.2NanostructuredBinaryTMOsforConversion-TypeCharge Storage 193
8.2.3NanostructuredBinaryTMOsforIntercalation-TypeCharge Storage 195
8.3ImplementationofNanostructuredTMOsinElectrode Architectures 198
8.3.1One-DimensionalandTwo-DimensionalArchitectures 201
8.3.1.1NanowiresandNanotubes 201
8.3.2Three-DimensionalArchitectures 203
8.3.2.1Assemblies 203
8.3.2.2Foams 205
8.3.2.3Aerogels 205
8.4Conclusions 206 References 206
9InterfacesinOxide-BasedLiMetalBatteries 213 MoranBalaish,KunJoongKim,MasakiWadaguchi,LingpingKong, andJenniferL.M.Rupp
9.1Introduction 213
9.2SolidOxideElectrolytes 215
9.3Cathode:TowardTrueSolid 216
9.3.1OriginofInterfacialImpedanceandCurrentPressingIssuesat Cathode/SolidElectrolyteInterfaces 217
9.3.1.1InterfacialReactionDuringCellFabrication 220
9.3.1.2ElectrochemicalOxidationandChemicalReactionDuringCycle 222
9.3.1.3Chemo-mechanicalDegradationDuringCycling 223
9.3.2StrategiesandApproachesTowardEnhancedStabilityand Performance 224
9.3.2.1CathodeCoating 224
9.3.2.2GeometricArrangementConcernsandStrategiesTowardMaximizing ReactionSites 226
9.3.2.3ConductiveAdditivesinSolid-StateCathode 229
9.4Anode:AdoptingLithiumMetalintheSolid 229
9.4.1Li/Solid–ElectrolyteInterface:Chemical,Electrochemical,and MechanicalConsiderations,IncludingMitigationStrategies 230
9.4.2LiDendriteFormationandPropagationinSolidElectrolytes: ChallengesandStrategies 237
9.5OutlookandPerspective 242 Acknowledgments 244 Contributions 244 EthicsDeclarations 244 References 244
10DegradationandLifePerformanceofTransitionMetalOxide CathodesusedinLithium-IonBatteries 257 SatishB.Chikkannanavar,JongH.Kim,andWangmoJung
10.1Introduction 257
10.2DegradationTrends 257
10.3TransitionMetalOxideCathodes 260
10.3.1SpinelCathodes 260
10.3.2NCMSystemofCathodes 262
10.3.3NCMACathodes 265
10.4DegradationMechanism 266
10.5ConcludingRemarks 268 References 269
11MechanicalBehaviorofTransitionMetalOxide-BasedBattery Materials 273
TruongCai,JungHwiCho,andBrianW.Sheldon
11.1Introduction 273
11.2MechanicalResponsestoCompositionalChanges 274
11.2.1VolumeChangesandDeformationinElectrodeParticles 274
11.2.2ParticleFracture 277
11.3ImpactofStrainEnergyonChemicalPhenomena 280
11.3.1Thermodynamics 280
11.3.2Two-PhaseEquilibrium 283
11.4SolidElectrolytes 284
11.4.1Electrode/ElectrolyteInterfaces 284
11.4.2ElectrolyteFracture 288
11.5Summary 293 References 294
12Solid-StateNMRandEPRCharacterizationofTransition-Metal OxidesforElectrochemicalEnergyStorage 299
XiangLi,MichaelDeck,andYan-YanHu
12.1Introduction 299
12.2BriefIntroductionofNMRBasics 301
12.2.1NuclearSpins 301
x Contents
12.2.2NMRSpinInteractions 301
12.2.3ParamagneticInteractionsandExperimentalApproachestoAchieve HighSpectralResolution 302
12.3MultinuclearNMRStudiesofTransition-metal-oxideCathodes 305
12.3.1LiExtractionandInsertionDynamics 305
12.3.2OEvolution 312
12.4EPRStudies 314
12.5Summary 316 References 316
13 InSitu and InOperando NeutronDiffractionofTransitionMetal OxidesforElectrochemicalStorage 319 ChristopheDidier,ZaipingGuo,BohangSong,AshfiaHuq,and VanessaK.Peterson
13.1Introduction 319
13.1.1NeutronDiffractionandTransitionMetalOxides 319
13.1.1.1NeutronReflectometry 321
13.1.1.2Small-AngleNeutronScattering 322
13.1.1.3QuasielasticandInelasticNeutronScattering 322
13.1.2NeutronDiffractionInstrumentation 323
13.1.3 InSitu and InOperando NeutronDiffraction 325
13.2DeviceOperation 326
13.2.1ExperimentalDesignandApproachtotheReal-TimeAnalysisof BatteryMaterials 326
13.2.2AdvancementsinUnderstandingElectrodeStructureDuring BatteryOperation 327
13.3GasandTemperatureStudies 330
13.3.1ExperimentalDesignandApproachtothe InSitu StudyofSolid OxideFuel-Cell(SOFC)Electrodes 330
13.3.2AdvancementsinUnderstandingSolidOxideFuel-CellElectrode Function 331
13.4MaterialsFormationandSynthesis 332
13.5Short-RangeStructure 333
13.6Outlook 334 Acknowledgments 335 References 335
14SynchrotronX-raySpectroscopyandImagingforMetalOxide IntercalationCathodeChemistry 343 ChixiaTianandFengLin
14.1Introduction 343
14.2X-rayAbsorptionSpectroscopy 345
14.2.1SoftX-rayAbsorptionSpectroscopy 345
14.2.2HardX-rayAbsorptionSpectroscopy 352
14.3Real-SpaceX-raySpectroscopicImaging 358
14.3.12DFull-FieldX-rayImaging 358
14.3.2X-rayTomographicImaging 362
14.4Conclusion 368 References 369
15Atomic-ScaleSimulationsoftheSolidElectrolyte Li7 La3 Zr2 O12 375 SeunghoYuandDonaldJ.Siegel
15.1Introduction 375
15.1.1Motivation 375
15.1.2SolidElectrolytes 376
15.1.3Li7 La3 Zr2 O12 (LLZO) 376
15.1.4Challenges 377
15.2ElasticPropertiesofLi7 La3 Zr2 O12 377
15.3PotentialFailureModesArisingfromLLZOMicrostructure 381
15.4Conclusions 386
Acknowledgements 387 References 387
16Machine-LearningandData-IntensiveMethodsfor AcceleratingtheDevelopmentofRechargeableBattery Chemistries:AReview 393
AustinD.Sendek,EkinD.Cubuk,BrandiRansom,JagjitNanda,and EvanJ.Reed
16.1Introduction 393
16.2Machine-LearningMethodsandAlgorithms 396
16.3Lithium-Ion-ConductingSolidElectrolytes 399
16.4LiquidElectrolytes 402
16.5CathodeDesign 402
16.5.1Anodes 403
16.6BeyondLithium 403
16.7ElectrochemicalCapacitors 404
16.8ApplicationofMLinLifeCycleDegradation 404
16.9ConclusionandFutureOutlook 405
Acknowledgments 405 References 405
Index 411
Foreword
Sincetheirintroductionintoportableelectronicdevicesmorethan30yearsago, lithium-ionbatterieshavegrowntodominatetheenergystoragesectorandare nowthetechnologyofchoiceforpoweringportabledevices,electricvehicles,and increasingly,smartgrids.Attheheartoflithium-ionbatterytechnologyisthemechanismoflithium-ionintercalationintosolid-statehostmaterialsforboththecathode andtheanode.Intercalationreactionsintotransitionmetalcompoundswereof greatinterestinthe1970sbecauseofthepossibilityofcontrolledstructuraland electronicmodification.OurteamatExxonmadeseminaldiscoveriesintothe mechanismofelectrochemicalLi+ intercalationformingthebasisofLi-ionbatteries.Thesestudiesdevelopedtheimportantdistinctionbetweenintercalationvs. conversionordecompositionreactions,andshowedtheimportanceofintercalation reactionsforextendedhigh-capacitycycling.Thisworkledtothefirstgeneration oflithium-ionbatteries(ExxonandMoli-Energy),andidentifiedthechallengesof workingwithlithiummetalanodes.
Followingtheseminalstudiesoflithiumintercalationintotransitionmetal chalcogenides,attentionturnedtotransitionmetaloxidesduetotheirhigherintercalationpotentialsinanumberoflaboratories.JohnGoodenough’sgroupidentified thelayeredoxidessuchasLiCoO2 asoutstandingreplacementsforthelayered sulfides.Today,transitionmetaloxidesarethepreferredmaterialsforlithium-ion batterycathodesbecauseoftheirhighredoxpotentialsandgoodlithium-ionintercalationcapacity.BesidestheiruseinexistingLi-ionbatterytechnology,transition metaloxidesalsoformthebasisofemergingbeyondLi-ionchemistriessuchas Na-ion,wheretheyhavemuchhighercapacitiesthanthesulfides.
Thisbookprovidesatimelyreferenceintothesolid-statechemistry,characterization,andmodelingoftransitionmetaloxidesforelectrochemicalenergystorage writtenbyinternationalexperts.Chaptersaredevotedtomechanismsofenergy storage(suchascoupledcation/electrontransferreactions;Na+ intercalation; conversionreactions;andanionredox),characterization(suchasnuclearmagnetic resonanceandX-raytechniques),andmodeling.Emerginghottopics,suchasthe roleofartificialintelligenceandmachinelearningmethodsinpredictingproperties
xiv Foreword ofenergystoragematerialsandcobalt-freetransitionmetaloxidescathodesfor Li-ion,arealsocovered.Overall,thebookprovidesagreatreferencefornovicesand expertstogainadeeperunderstandingintothesolid-statechemistryoftransition metaloxidesofpotentialuseforenergystorage.
Vestal,NY
8September2021
M.StanleyWhittingham 2019NobelLaureateinChemistry
AnOverviewofTransitionMetalOxidesforElectrochemical EnergyStorage
EthanC.Self 1 ,DevendrasinhDarbar 1 ,VeronicaAugustyn 2 ,andJagjitNanda 1
1 OakRidgeNationalLaboratory,ChemicalSciencesDivision,OakRidge,TN37831,USA
2 NorthCarolinaStateUniversity,DepartmentofMaterialsScienceandEngineering,Raleigh,NC27695,USA
Transitionmetaloxides(TMOs)areusedinmanycommercialandresearchapplications,includingcatalysis,electrochemicalenergystorage/conversion,electronics, andthermoelectrics.ThisbookfocusesonTMOsforelectrochemicalenergystorage deviceswithparticularemphasisonintercalation-basedsecondary(rechargeable) batteries.Thisintroductorychapterprovidesabroadoverviewofsuchapplications, anddetailedtreatmentsofspecificsubjectsaregiveninChapters2–16.
1.1FundamentalsofElectrochemicalCells
Anelectrochemicalcellconsistsoftwoelectrodes(denotedascathode/anodeor positive/negative)separatedbyanionicallyconductive,electronicallyinsulating electrolyte.Batteriesconvertchemicalenergyintoelectricalenergythrough Faradaicchargetransferprocesseswhere:(i)oxidation/reductionreactionsoccur withinanode/cathodeactivematerialsand(ii)electronsaretransportedthrough anexternalcircuittomaintainchargeneutralityateachelectrode.Thesereactions areirreversibleinprimarybatteries(e.g.Zn–MnO2 andLi–MnO2 )designedfor single-useapplications.Ontheotherhand,secondarybatteries(e.g.lead-acid, nickel-metalhydride,andLi-ion)leveragereversibleredoxprocessesandcanbe repeatedlycharged/discharged,arequirementformanyend-useapplications(e.g. electricvehicles).
Electrochemicalcapacitorsareanotherformofenergystoragedeviceswhichprovidespecificenergyandpowerbetweenthatofdielectriccapacitorsandrechargeablebatteries.Supercapacitorsstore/deliverenergythroughnon-Faradaicprocesses whereionsarestoredintheelectrochemicaldoublelayerneartheelectrodesurfaces. Ontheotherhand,pseudocapacitivematerialsstoreenergythroughchargetransfer reactionswhichmayinclude:(i)oxidation/reductionoftheelectrodesurfaceand/or (ii)intercalationofionsintoahostactivematerial.Hybridconfigurationsutilizing pseudocapacitivematerialsapproachthespecificenergyofrechargeablebatteries.
TransitionMetalOxidesforElectrochemicalEnergyStorage, FirstEdition. EditedbyJagjitNandaandVeronicaAugustyn. ©2022WILEY-VCHGmbH.Published2022byWILEY-VCHGmbH.
Figure1.1 Ragoneplotshowingthespecificenergyandpowerofseveralelectrochemical energystoragesystems.Source:ImagereproducedfromHayneretal.[1],International EnergyAgency,TechnologyRoadmaps:ElectricandPlug-inHybridElectricVehicles,2009, p.12.(Originalsource:JohnsonControl–SAFT2005and2007.)
Twoimportantperformancemetricsofenergystoragedevicesarethespecific energy(Whkg 1 )andspecificpower(Wkg 1 ),whichdescribe howmuch and how quickly energycanbestored/delivered,respectively.Analogousquantitiesnormalizedtosystemvolume(i.e.energy/powerdensitieswithunitsofWhL 1 andWL 1 ) arealsocommonlyused.Ragoneplots(Figure1.1)summarizetheseenergy/power relationshipsandareusefultoassesstheviabilityofdifferentenergystorage platformsforagivenapplication.Figure1.1showsafundamentaltradeoffbetween asystem’sspecificenergyandpower.Forexample,supercapacitorsexhibit:(i)high specificpowerduetorapidionadsorption/desorptionnearelectrodesurfacesbut (ii)lowspecificenergysincechargestorageonlyoccurswithintheelectrochemical doublelayer.Ontheotherhand,batteriesstoreenergywithinthebulkstructureof activematerials,enablinghighspecificenergy.Therateofenergystorage/delivery inbatteriesisgenerallylimitedbysolid-statediffusionorphasenucleationkinetics intheactivematerial,resultinginlowerspecificpowerthansupercapacitors.With thesetrendsinmind,itshouldbeemphasizedthattheenergy/powercharacteristics ofanelectrochemicaldevicearealsohighlydependentondesignfactorssuchas materialselection,cellformat,andelectrodearchitecture.
Sections1.2–1.5providebasicoverviewsofelectrochemicalenergystoragedevices whereTMOsplaycriticalrolesindeviceoperation.Theimportanceofadvanced characterizationandcomputingresourcesonguidingmaterialdevelopment,understandingdegradationmechanisms,andoptimizingsystemperformanceisalsohighlighted.
1.2Li-IonBatteries:BasicPrinciplesandTMO Electrodes
Overthelastfourdecades,Li-ionbatterieshavesuccessfullytransitionedfrom researchanddevelopmenttocommercialapplications,includingportableelectronics,electricvehicles,andgridstorage.Thefoundationofthistechnologyisbasedon cationintercalationreactionswhereinLi+ isstoredinTMOcathodesandgraphite anodes[2].Theseintercalationreactionsarehighlyreversible,andtoday’sLi-ion batteriescanundergohundredsorthousandsofcycleswithminimalchemical and/orstructuralchangestotheactivematerial(seeChapters9and10fordetailed discussionondegradationmechanismsofLi-ionbatteries).
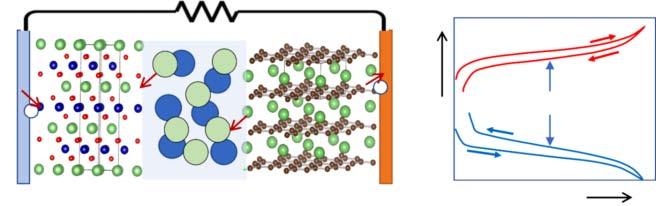
TheworkingprinciplesofLi-ionbatteriesareillustratedinFigure1.2.During charge,Li+ deintercalatefromtheTMOcathode(e.g.LiCoO2 ),transportthrough theelectrolyte,andintercalateintotheanodeactivematerial(e.g.graphite). Tomaintainchargeneutrality,electronsaresimultaneouslyextractedfromthe cathode(typicallyviatransitionmetaloxidation),transportedthroughanexternal circuit,andinsertedintheanode(electrochemicalreductionofgraphite).During discharge,theseprocessesarereversed,andLi+ ionsandelectronsaretransported backtothecathode.Figure1.2bshowsqualitativecathode/anodevoltageprofiles asafunctionofcapacity.CommercialLi-ionbatteriestypicallyhavecellvoltages ∼3.6Vandspecificenergies ∼200Whkg 1 ,althoughthesevaluesdependonactive materialselectionandcelldesign.
CommercialLi-ionelectrodesarepreparedbycastingaslurrycontainingactive material(thehostmaterialwhichreversiblystoresLi+ ),electronicallyconductive carbonadditives,andpolymerbinderontoacurrentcollector(typicallyCuforthe anodeandAlforthecathode).Keyelectrochemicalproperties,includingoperatingvoltage,reversiblecapacity,andcyclability,arestronglydependentontheactive material’scrystallographicstructureandtransitionmetalselection.Conventional cathodeactivematerialsinclude:(i)lithiumTMOswithlayeredorspinelcrystallographicstructures(e.g.LiCoO2 andLiMn2 O4 ,respectively)and(ii)olivinestructures containingpolyanionicgroups(e.g.LiFePO4 ).Awiderangeofrelatedcompositions
Figure1.2 (a)Schematicoftheworkingprincipleofalithium-ionbatterycontaininga LiCoO2 cathodeandgraphiteanode.(b)Qualitativevoltageprofilesduringchargeand discharge.Source:Belharouaketal.[3].
1AnOverviewofTransitionMetalOxidesforElectrochemicalEnergyStorage containingtransitionmetalsubstitutions(e.g.LiNix Mny Co1 x y O2 )havebeendevelopedtomaximizetheenergydensityandcyclingstabilityofTMOcathodes.While mostcommercialLi-ionbatteriescontaingraphiteanodes,somesystemsalsoutilize TMOanodessuchasLi4 Ti5 O12 .ThevastcompositionallandscapeofTMOsexplored asLi-ionactivematerialsisdetailedinChapters2,3,5,7,and16.
1.3BriefHistoryofLithium-IonBatteries
Inthe1960s,intercalationchemistrywasaprominentmethodusedtoaltermaterials’electronicandopticalproperties[4,5].Forinstance,theelectronicconductivity ofWO3 canbevariedseveralordersofmagnitudebyintercalatingmonovalent cationsintothestructure[6].Rechargeablebatterieswhichutilizeionintercalation reactionswerefirstdemonstratedinthe1970sbyWhittingham[7]atExxon Corporation.TheseprototypecellscontainedalayeredTiS2 cathode,lithiummetal anode,andliquidelectrolyte(e.g.LiClO4 dissolvedinamixtureofdimethoxyethane andtetrahydrofuran)[7].OnelimitationofthissystemwastheLimetalanode whichformsdendritesthatcanpenetratetheseparatorandinternallyshort-circuit thecell.Asanalternativeanode,YazamidemonstratedreversibleLi+ intercalation ingraphiticcarbonsusingapolymerelectrolyteinthe1980s[8].However,inliquid electrolytestheseintercalationanodeswerehinderedbysolventco-intercalation whichresultedingraphiteexfoliationandelectrodedegradationduringcycling. In1985,agroupledbyYoshinoatAsahiKaseiCorporation(Japan)identified petroleumcokeanodes[9]whichwerestableduringLi+ insertion/removal,and theseanodeswereincorporatedintoLi-ionfullcellscontainingliquidelectrolyte. ThesediscoveriesultimatelyledtothefirstcommercialLi-ionbatteriesintroduced bySonyin1991.Ataroundthesametime,suitableelectrolytesolvents(e.g.ethylene carbonate)whichdonotco-intercalateingraphitewerealsoidentified.Compared todisorderedcarbonsderivedfrompetroleumcoke,graphiticanodesoperateat morenegativepotentialswhichenableshighercellvoltagesandenergydensities.
Inadditiontocarbonaceousanodes,the1980salsowitnessedadvancementsin Li-ionintercalationcathodes.NotableexamplesincludeLiCoO2 (Goodenoughand coworkers)[10],LiMn2 O4 (Thackerayandcoworkers)[11],andpolyanioniccathodessuchasLiy Fe2 (XO4 )3 ,X = S,Mo,andW(Manthiramandcoworkers)[12].
ThesescientificbreakthroughsenabledLi-ionbatteriestodominatetherechargeablebatterymarketfordecades,andthesesystemsarenowubiquitousincommercialdevicesrangingfromportableelectronicstoelectricvehicles.The2019Nobel PrizeinChemistrycelebratedthisachievementwithanawardtoProfs.JohnGoodenough,StanleyWhittingham,andAkiraYoshino.
1.4TheRoleofAdvancedCharacterization andComputingResources
TherichhistoryofLi-ionbatteriesdemonstratesthattranslatingscientificdiscoveriesintoviableenergystoragesystemsrequiresdecadesofresearchanddevelopment.
1.5BeyondLithium-IonBatteries 5
Centraltothisdevelopmentprocessarefundamentalscientificdiscoveries(e.g. identifyingstructure/propertycorrelationsandunderstandingdegradationmechanisms)neededtooptimizematerialselectionandsystemperformance.Such effortsoftenrequirecombiningroutinematerialcharacterization(e.g.electroanalyticalmethods,vibrationalspectroscopy,andlab-sourceX-raydiffraction)with world-classinstrumentation(e.g.X-ray/neutronbeamlines).Severalchaptersin thisbookdescribeadvancedcharacterizationmethodsthatenabledcriticalinsights onTMOsforelectrochemicalenergystorage,andexamplesinclude:(i)solid-state nuclearmagneticresonancespectroscopy(NMR)andelectronparamagneticresonance(EPR)toprobelocalbondingstructure(Chapter11),(ii) insitu and operando neutrondiffractiontoidentifyphasetransformationsduringmaterialsynthesisand batteryoperation(Chapter12),and(iii)X-rayspectroscopyandimagingtoprobe transitionmetalvalencestates(Chapter13).Inadditiontotheseexperimental efforts,advancedcomputingresourcesandmachinelearning(Chapters14and15) haveacceleratedmaterialdiscoveryandprovidedmechanisticinsightintoprocesses occurringatlength/timescaleswhicharedifficulttoprobeexperimentally.
1.5BeyondLithium-IonBatteries
TheperformanceofLi-ionbatteriesislargelydictatedbytheactivematerials,and replacingthegraphiteanodewithLimetalcanenablebatterieswithspecificenergies >400Whkg 1 (comparedto ∼200Whkg 1 fortoday’scommercialLi-ionbatteries). Aspreviouslymentioned,formationofLidendritesduringchargingpresentsmajor safetyandreliabilityconcerns,andtodate,liquidelectrolyteshavebeenunableto effectivelysuppressLidendriteformation.TocombatthisissueandstabilizetheLi metalanode,thereisgrowinginterestinsolid-statebatteries(SSBs)containingsolid electrolyteseparators.Representativeclassesofsolid-stateLi+ conductorsinclude garnetoxides(e.g.Li7 La3 Zr2 O12 ),sulfides(e.g. β-Li3 PS4 andLi6 PS5 Cl),polymers (e.g.poly(ethyleneoxide)containingaLi+ salt),andinorganicglasses(e.g.Lipon). TheopportunitiesforLimetalaretremendous,butenablingpracticalSSBsrequires overcomingmajorchallengesrelatedtomaterialsynthesis,processing,andinterfacialstabilizationasdiscussedinChapter8.
Energystoragecostsareaprimaryfactorindeterminingtheviabilityofbattery technologiesforgridstorageapplications.WhilethecostofLi-ionbatterieshas decreasedsubstantiallyinthepastdecade(e.g.from ∼US$1000/kWhin2008to ∼US$130/kWhin2020),furthercostreductionsareneededtoenablewidespread adoptionforlarge-scalestationarystorage.Recentdevelopmentsonlow-cost rechargeablebatteriesdiscussedinthisbookinclude:(i)aqueouselectrolytesfor Li-ionsystems(Chapter6)and(ii)Na-basedchemistrieswhichutilizeactivematerialhoststhatundergointercalation,conversion,oralloyingreactions(Chapters3 and4).
Developmentofnext-generationenergystoragesystemsiscriticaltocombat climatechangeinthecomingdecades.Globalenergyconsumptionrates[13]are
1AnOverviewofTransitionMetalOxidesforElectrochemicalEnergyStorage expectedtoincreaseto40.8TWby2050,andelectrochemicalenergystoragesystems representoneofthemostpromisingplatformstobridgethesupply/demandgap forintermittentrenewableresources(e.g.solarandwind).Inthisregard,TMOs areexpectedtocontinueplayingcriticalrolesascomponentsforlow-cost,reliable energystoragetoenableasustainablefuture.
Acknowledgments
TheauthorsacknowledgesupportfromEnergyEfficiencyandRenewableEnergy (EERE),VehicleTechnologiesOffice(VTO),DepartmentofEnergy.
ThischapterhasbeenauthoredinpartbyUT-Battelle,LLC,undercontract DE-AC05-00OR22725withtheUSDepartmentofEnergy(DOE).TheUSgovernmentretainsandthepublisher,byacceptingthearticleforpublication, acknowledgesthattheUSgovernmentretainsanonexclusive,paid-up,irrevocable, worldwidelicensetopublishorreproducethepublishedformofthismanuscript,or allowotherstodoso,forUSgovernmentpurposes.DOEwillprovidepublicaccess totheseresultsoffederallysponsoredresearchinaccordancewiththeDOEPublic AccessPlan(http://energy.gov/downloads/doe-public-access-plan).
References
1 Hayner,C.M.,Zhao,X.,andKung,H.H.(2012).Materialsforrechargeable lithium-ionbatteries. AnnualReviewofChemicalandBiomolecularEngineering 3:445–471.
2 Dunn,B.,Kamath,H.,andTarascon,J.M.(2011).Electricalenergystoragefor thegrid:abatteryofchoices. Science 334(6058):928–935.
3 Belharouak,I.,Nanda,J.,Self,E.C., etal.(2020).Operation,manufacturing,and supplychainoflithium-ionbatteriesforelectricvehicles.https://doi.org/10.2172/ 1737479.
4 Gamble,F.R.,Osiecki,J.H.,Cais,M.etal.(1971).Intercalationcomplexesof Lewisbasesandlayeredsulfides:alargeclassofnewsuperconductors. Science 174:493–497.
5 Aronson,S.,Salzano,F.J.,andBellafiore,D.(1968).Thermodynamicproperties ofthepotassium-graphitelamellarcompoundsfromsolid-stateemfmeasurements. JournalofChemicalPhysics 49(1):434–439.
6 Whittingham,M.S.,Michael,S.,andJacobson,A.J.(1982). IntercalationChemistry.NewYork:AcademicPress.
7 Whittingham,M.S.(1976).Electricalenergystorageandintercalationchemistry. Science 192:1126–1127.
8 Yazami,R.andTouzain,P.(1983).Areversiblegraphite-lithiumnegativeelectrodeforelectrochemicalgenerators. JournalofPowerSources 9:365–371.
9 Yoshino,A.,Sanechika,K.,Nakajima,T.(1987).Secondarybattery.USPatent 4,668,595,filed9May1986andissued26May1987,https://patents.google.com/ patent/US4668595A/en.
10 Mizushima,K.,Jones,P.C.,Wiseman,P.J.,andGoodenough,J.B.(1980).Lix CoO2 (0<x ≤1):anewcathodematerialforbatteriesofhighenergydensity. Materials ResearchBulletin 15:783–789.
11 Thackeray,M.M.,David,W.I.F.,Bruce,P.G.,andGoodenough,J.B.(1983). Lithiuminsertionintomanganesespinels. MaterialsResearchBulletin 18: 461–472.
12 Manthiram,A.(2020).Areflectiononlithium-ionbatterycathodechemistry. NatureCommunications 11(1):1550.
13 Lewis,N.S.andNocera,D.G.(2006).Poweringtheplanet:chemicalchallenges insolarenergyutilization. ProceedingsoftheNationalAcademyofSciencesofthe UnitedStatesofAmerica 103(43):15729–15735.
Metal–Ion-CoupledElectronTransferKinetics inIntercalation-BasedTransitionMetalOxides*
VictoriaA.NikitinaandKeithJ.Stevenson
SkolkovoInstituteofScienceandTechnology,CenterforEnergyScienceandTechnology,NobelStraße3, 121205Moscow,Russia
2.1Introduction
Intensiveresearchinthefieldoflithiumionintercalatingsystemsoverthelastseveraldecadesresultedinthedesignofhundredsofactivematerialandelectrolyte systemsforpracticalbatteryapplications[1–3].Giventhehighpriorityofachieving maximumcapacity,energydensity,andratecapabilitycharacteristicsoftheLi-ion batteries,thefocusofthemajorityofstudiesonthecoulombicefficiency,cyclability,andcharge–discharge(C-rate)characteristicsoftheactivematerialsisnot surprising.Still,adetailedmechanisticunderstandingonthemetal–ion-coupled electrontransfer(ET)kineticandthermodynamicpropertiesisneededtorealize improvedperformancefornext-generationenergystoragesuchasfortransportation andgrid-scaleapplications[4–6].
Mostofthemodernelectrochemicalcharacterizationmethodsintheassessmentofmetal–ionbatterykineticsandthermodynamicprocessesfocusonthe analysisofcharge/dischargecurvesperformedundergalvanostaticconditions orthedifferentialcapacity(derivativeofthecharge Q withrespecttovoltage V ) dQ/dV vs. V curves,whichresultsfromasimpledifferentiationoftheexperimental charge/dischargecurves[7].Yet,classicalelectrochemicalkineticmodels,which offeravarietyoftechniquestotreattheelectrochemicaldataforredoxprocessesin solution,foradsorbedreactants,andaswellasforverycomplexcasesofmultistep processes[8,9],arerarelyappliedforaquantitativeanalysisoftheintercalationprocesses,whichoccuratthesolidelectrode/electrolyteinterface.Inthischapter,we reviewtheprocesses,whichinvolveredoxreactionsintransitionmetaloxideelectrodescoupledwithmetal–ionintercalationsteps,withemphasisonthespecifics ofmetal–ion-coupledelectrontransferkineticsinmultiparticlecompositesystems, whichareindeedthekeyelectrodearchitecturesinmetal–ionbatteryresearch.We aimtobroadenthewell-knownclassicalelectrochemicalconceptsconcerningthe
*ThischapterwasoriginallypublishedinAdvancedEnergyMaterials,2021,Volume10,Issue22; DOI:10.1002/aenm.201903933.ReproducedwithpermissionofWILEY-VCHGmbH.
TransitionMetalOxidesforElectrochemicalEnergyStorage, FirstEdition. EditedbyJagjitNandaandVeronicaAugustyn. ©2022WILEY-VCHGmbH.Published2022byWILEY-VCHGmbH.
2Metal–Ion-CoupledElectronTransferKineticsinIntercalation-BasedTransitionMetalOxides
reaction-limitingstepstoencompassionintercalationprocessesaswellasdiscuss theconditionsrequiredforagivenslowsteptomanifestitself.Thepointofinterest inthisdiscussionisthecriteria,whichshouldallowforfacileandreliablediagnosticsoftheintercalationratecontrolregimefornewmaterialsorforunconventional solvent/metal–ionsaltsystems,astheknowledgeoftherate-determiningfactors isessentialforchoosingtheoptimalstrategytoenhancetheratecapabilityand capacitycharacteristicsofametal–ionbattery(e.g.bydecreasingtheparticlesize, tuningthesurfacelayerscompositionandstructure,andthesolvatingproperties ofthemedium).Thisdiscussionalsofocusesspecificallyon“battery-type”intercalationmetaloxidesystemsandnot“pseudocapactive-type”metaloxidematerials. Severalrecentreviewsthatprovideformaldefinitionanddistinctionbetweenthese twotypesofenergystoragemechanismscanbefoundhere[10–12].
Atitsmostbasiclevel,anintercalationprocesscanberegardedascomprisingtwo steps:electrontransfer(ET)fromthecurrentcollectortotheredox-activematerial (faradaic),whichresultsinthechangeofstructureduetothetransitionmetaloxidation/reduction,andiontransfer(IT)intothemetaloxidecrystallatticefromthe bulkelectrolyte.ThesecondprocessofITalsoinvolvesseveralstages:(i)iondiffusioninthesolventbulk;(ii)transitionthroughsurfacefilms(generallyregarded assolidelectrolyteinterphase[SEI]layersinconventionalcarbonate-basedelectrolytes);(iii)chargetransferatthesolution/electrodeinterfaceorSEI/electrode interface;(iv)iondiffusionintheelectrodematerial.Apartfromthesesimpleststeps, thestepofionicdesolvationinthevicinityoftheelectrode/solutionorelectrode/SEI interfaceshouldgenerallyoccur,asinmostcasestheionentersthecrystallattice withoutitssolvationshell.Morecomplexcasesalsoinvolvethestepofnucleating anewphase,whichistypicalformaterialswithawidemiscibilitygap(suchas LiFePO4 ),wheretherateofinitiatingthephasetransitionturnsouttobeslower thanthecharge-transferkineticsorionicdiffusion[13].Asthecomplexnucleation dynamicsshouldbeconsideredinthiscasetogetherwiththecharge-transferand diffusionequations,inthiscontributionwelimitourselvestotheanalysisofthe metaloxideelectrodematerialswithwidersingle-phaseregions,wheretheevents ofnucleationdonotdeterminetheintercalationkinetics.
Weprimarilyaddressthevoltammetricandelectrochemicalimpedanceresponses oftheoxideelectrodematerialsduetotherelativesimplicityofthedataanalysisand highlyinformativeshapeoftheelectrochemicalresponses,whichallowsforboth theinitialqualitative-levelrate-limitingstepdiagnosticsaswellasfortheadvanced quantitativeestimationofthekeykineticparameters,whichcharacterizeboththe transportandthekineticlimitationsinanintercalationmaterial/electrolytesystem. However,wenotethatevenforaqualitative-levelanalysisthebasicrequirements fortheexperimentalsetuparetobefulfilledtoensurethereproducibilityandthe reliabilityoftheelectrochemicaldataobtained.
(1)Theelectrochemicalmeasurementsshouldbecarriedoutinathree-electrode cellwithareliablereferenceelectrodewithahighexchangecurrentdensityto ensureitsreversibility.WhileaLimetalreferenceelectrodegenerallydemonstratesratherfastET/ITkinetics[14],NaandKmetalelectrodesduetotheir higherreactivitywiththeelectrolytecannotbeusedasreferenceelectrodes
2.2ThermodynamicControl 11
inathree-electrodesetupinorganiccarbonateelectrolytes[15].Alternative redoxelectrodesarerecommendedforaccurateelectrochemicalkineticstudies ofNa+ andK+ intercalation(e.g.Ag+ /Agelectrodesorreferenceelectrodes basedonthepartiallychargedphasetransformingmaterialswithawide miscibilitygap[16]).
(2)Theresponsesofinhomogeneousthickporouselectrodescannotbeanalyzed quantitativelywithintheframeworkofsimplemodels[17].Hence,themass loadingoftheelectrodematerialpowdershouldbeminimizedto1–0.5mgcm 2 ofthecurrentcollector.
(3)Minimizationoftheelectrolyte/materialratio(i.e.usingsmallamountsof electrolyte)isessentialincaseofreductive/oxidativeelectrolytedecomposition withtheSEIlayersformationtostabilizetheelectrochemicalinterfaces,aslow volumeoftheelectrolyteminimizesthedissolutionoftheformedprotective surfacelayers.
Inthisdiscussion,werelyonthephenomenologicalelectrochemicalkinetics theoryofintercalationmaterials,asformulatedinearlierworksofLeviandAurbach [18,19],asthisapproachprovidesatransparentwaytoestimatethekeykinetic parametersofanintercalationreaction,suchasdiffusioncoefficients,apparent rateconstants,andion–hostinteractionparameters.Amoreadvancedtreatment byM.Bazantisbasedonthephase-fieldtheoryofelectrochemicalkinetics,which generalizestheclassicalButler–VolmerandMarcusequations[20,21].Thelatter approachallowstodescribethekineticandthermodynamiceventsinthecourse ofintercalationwithintheframeworkofhighlyaccurateandphysicallyadequate formulism,whichpossessesahighexplanatorypower.However,theapplicationof thisapproachtotreatexperimentalkineticdataforintercalationsystemsrequires knowledgeofalargenumberofparameters,suchasreorganizationenergies,activitycoefficients,strainenergies,forreactants,products,andintermediates,which canhardlybeestimatedquantitatively.Thephenomenologicaltreatmentdoesnot grantinformationonthephysicaloriginofchargetransferactivationbarriers,but allowsfortheelucidationofthenatureoftheintercalationrate-limitingstepand comparativeanalysisofthekineticparametersfordifferention–hostsystems,which areessentialfortherationalcontrolofintercalationkineticsinenergystorageand conversiondevices.
2.2ThermodynamicControl
Thesimplestcaseinthemetal–ion-coupledelectrontransferkineticsiswhen boththerateofdiffusionandtherateofiontransferareveryfastanddonot affecttheshapeofthecyclicvoltammograms(CVs),charge/dischargecurves,or currenttransients.Inthiscase,thecurrent(underpotentiostaticconditions)or thepotential(undergalvanostaticconditions)aredeterminedbytheshapeofthe intercalationisotherm,i.e.bythedependenceofthehostlatticefreeenergyonthe amountoftheintercalatedmetalions,whichischaracterizedbytheintercalation
2Metal–Ion-CoupledElectronTransferKineticsinIntercalation-BasedTransitionMetalOxides
level �� (theratioofthenumberoftheionsinthehostlatticetothenumberofthe availableintercalationsites).Themostdirectanalogybetweentheintercalation andtheadsorptionprocessesreliesontheNernstequationfortheintercalation ofanioninsideanemptysiteinthehostlattice:M+ + e + [] = [M],whichcan bemodifiedtoaccountfortherepulsiveorattractiveinteractionsbyintroducing aphenomenologicalparameter“g”(negativefortheattractionbetweenionsin thehostandpositiveforrepulsion).TheresultingequationrepresentsaFrumkin intercalationisotherm,asintroducedbyLeviandAurbach[18]:
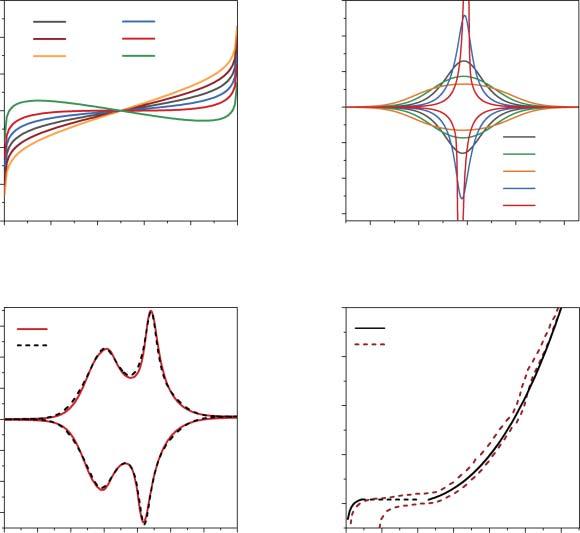
where Eeq isthestandardredoxpotential(V)(theequilibriumpotentialofarealor ahypotheticalphasehavingacompositionof �� = 0.5); g –dimensionlessconstant, whichcorrespondstotheinteractionparameterintheFrumkinisothermformulism.
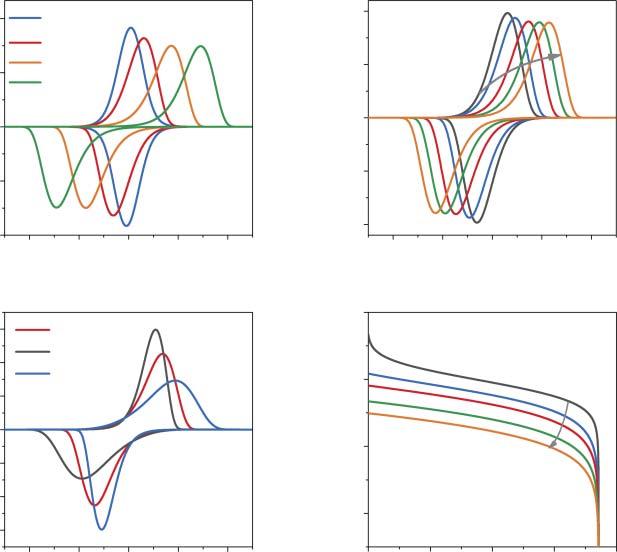
Thevariationinthevalueofthe g parameterinduceschangesintheslopeofthe galvanostaticcharge/dischargeprofiles(Figure2.1a).Thelimitingcaseof g =−4 resultsinatypicalplateau,whichmarksthelossofsystemstabilityinasingle-phase intercalationprocess.Withintheframeworkofthephenomenologicalapproaches, theattractiveinteractionsinthesystembecomestrongenoughtoresultintoseparationofthesystemintocation-richandcation-poorphases.Indeed,furtherincrease inthenegative g valueresultsinanS-shapedisotherm,whichclearlyimpliesthe coexistenceoftwophasesinsomeintervalof �� values.Thecorrespondingcyclic voltammetrycurves(Figure2.1b)calculatedfordifferent g valuesshowbroad peaksfortherepulsiveinteractions(g = 0,2,4)andsharperpeaksfortheattractive interactions,whichissofarcompletelyanalogoustotheadsorptionprocesses,and canbetreatedusingLavironanalysisforsurfaceconfinedredox-activespecies[22] (theequationsforthecurrent/potentialcurvescalculationcanbefoundin[18]). Anexperimentalexampleofasystemwithoutanysignificantchargetransferor diffusionallimitations(thiscaseisdesignatedas“thermodynamiccontrol”)isthe slightlyoverlithiatedlithium-manganeseoxideLi1+x Mn2 O4 [14,23].Atlowscan rates(<0.1mVs 1 ),theexperimentalCVsshowtwopairsofpeaks,centeredat4.0 and4.13V(vs.Li+ /Li)correspondingtotheconsecutivede/insertionof0.5Liper f.u.(Figure2.1c).Thesymmetryofthecathodicandanodicpeaksandnegligible peak-to-peakseparationimpliesthethermodynamiccontrolofLi-ionintercalation. Indeed,thebasicfeaturesofexperimentalcurvescanbereproducedusing g values of1.1and 0.88forthebroaderandthesharperpeaks,respectively.
However,theexperimentalisothermsareveryrarelyassimpleasimposedby Eq.(2.1).Figure2.1dshowstheexperimentalisothermforLiCoO2 inaconventional 1MLiPF6 ethylenecarbonate/dimethylcarbonate(EC/DMC)electrolyte[24].It isestablishedthataphasetransitionfromhexagonaltomonoclinicphaseoccurs uponLi-iondeinsertionfromtheLi�� CoO2 structureat �� ∼ 0.78[25].Thus,two isothermsforthelithiatedandthedelithiatedphasesarerequired(solidlinesin Figure2.1d).WhiletheinitialpartoftheisothermintheLi-richphasecanbe approximatedusingasingle g value,thisisimpossiblefortheexponentialriseof thecurveat0.5 <��< 0.75,whichsimplyreflectstheverystrongdependenceofthe crystallatticeenergyontheamountoftheintercalatedions.Thiscanbeovercome
E
Figure2.1 Chargingcurves(deintercalationisotherms)(a)andCVs(b)calculatedfor differentvaluesoftheinteractionparameter g.CVat0.1mVs 1 andthefitforLiMn2 O4 electrodein1MLiPF6 inEC/DMCelectrolyte(c).Experimental(de)intercalationisotherm forLiCoO2 electrode(dashedlines)andthefitsforLi-richandLi-poorphases(solidlines,d). Source:Vassilievetal.[14]/withpermissionofElsevier.
byintroducingaseriesof g1 …gn values,whichmodifiesEq.(2.1): E(�� )= Eeq + RT F ln
Thefittingparameters gn introduceverylittleuncertaintyintotheexperimentaldata modeling,astheseareevaluateddirectlyfromtheexperimentalcharge/discharge curvesandareusedonlytoconstructananalyticalapproximationoftheexperimentalintercalationisotherm.Forinstance,thedataforLiMn2 O4 electrodescan beaccuratelyreproducedbyapproximatingtheexperimentallyderived E(�� )using three g valuesforeachredoxprocess(processat4.0V: g1 = 1.13, g2 =−0.45, g3 = 2.34; processat4.13V: g1 =−0.81, g2 = 1.57, g3 = 9.58)[14].Thethermodynamiccontrol isalsotypicalforsodium-andpotassium-ionintercalationintoPrussianbluecompounds[26]andNa+ intercalationintoAVPO4 Fmaterials[15,27].Asthediagnostic criteriaforthethermodynamicregime,onecanusethesymmetryoftheanodicand cathodiccurvesandtheproportionalityofthecurrentpeaktothescanratevalue,
2Metal–Ion-CoupledElectronTransferKineticsinIntercalation-BasedTransitionMetalOxides whiletheconclusionsonthetypeofinteractionsbetweentheionsinthehostmatrix canbeformulatedbasedonthepeaks’shape.
2.3DiffusionalControl
Thecationmobilityinsideasolidstructureisoftenthekeyparametertobeaddressed upontheintroductionofanewmetal–ionbatterymaterial.Theapparentdiffusion coefficient D isusedtocharacterizetheionicmobilityinsideasolidstructure:
where l isthediffusionlength(cm)(halfoftheparticlediameter d orthethin-film thickness)and �� isthediffusiontimeconstant(s).Certainspecificsofionintercalatingmaterialsaredeterminedbytheneedtoconsiderthedimensionalityofthe diffusiongeometry:planar(or1D,asinchannelsintheLiFePO4 structure),cylindrical(2D,diffusioninlayersofLiCoO2 andotherlayeredmaterials),spherical(3D, diffusioninLiMn2 O4 ),andthefinitenatureofthediffusioninsideamaterialparticle.Anexcellenttreatiseonthedeterminationofthediffusioncoefficientfrom chronoamperometrydatawithanaccountofthetypicalhinderingexperimentalfactors(suchasslowchargetransferkinetics,uncompensatedohmicdrop,andwide particlesizedistribution)isavailablein[28–30].
ThegeneraltreatmentofthediffusionproblemisbasedonastandardsetofFick’s equationsforagivendiffusiongeometry(e.g.planar)withboundaryconditionsat theparticlesurfaceandatitscenter[31]:
where x istherelativecoordinate(0 ≤ x = 2l/d ≤ 1), E istheelectrodepotential(V), I isthecurrent(μA), F istheFaradayconstant, �� (E)or E(�� )istheintercalation isotherm, S istheworkingareaoftheparticlesurface(cm2 )(onlyplanescontributingtolithiumdiffusionarerelevant),whichiscalculatedaccordingtotheequation:
where n = 2,4,and6forplanar,cylindrical,andsphericaldiffusiongeometries, m isthemassoftheintercalatingmaterialintheelectrode(g), �� isthephasedensity (gcm 3 ).
Asmostintercalationmaterialsundergophasetransformationsinthecourseof charge/discharge,amorecomplexdiffusionproblemshouldbesolvedforthephase boundarymovement,whichisdiscussedindetailinRefs.[14,32].
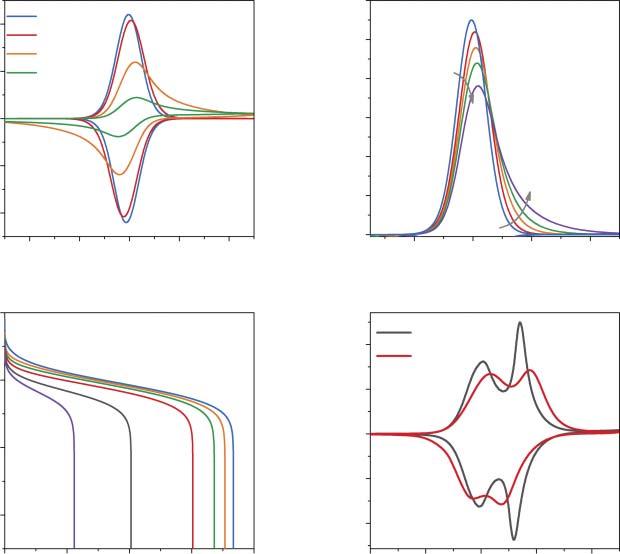
Diffusionalcontrolinintercalationmaterialsisgenerallyunavoidableathigh scanorcharge/dischargeratesduetothefinitesizeofthematerialparticlesand thegenerallylowionicmobilitiesinintercalationmaterials[33].Toillustratethe effectofthediffusioncoefficientvalueontheshapeoftheCVs,asetofsimulated voltammogramsforasimplemodelintercalationprocessisshowninFigure2.2a.
10–11cm2s–1
10–12cm2s–1
10–13cm2s–1
10–14cm2s–1
0.1mVs–1 1mVs–1 E (V)(Li+/Li) C (mAhg–1)
Figure2.2 (a)SimulatedCVsforasimpleintercalationprocess(E eq = 4.0V, g1 = 1, m = 0.5mg, �� = 0.2mVs 1 , d = 1 μm)fordifferent D values(indicatedintheplot).
(b)NormalizedperscanrateCVsforscanratevalues:0.05,0.2,0.4,0.6,1mVs 1 (D = 10 12 cm2 s 1 , g = 1, E eq = 4.0V, m = 0.5mg).(c)Charge/dischargecurvescalculated fordifferent C rates: C /10, C /5,1C ,2C ,5C ,10C .(d)ExperimentalCVsofaLiMn2 O4 electrode in1MLiPF6 EC/DMCelectrolyteat0.1and1mVs 1 .Source:Vassilievetal.[14]/with permissionofElsevier.
Themodelredoxprocessoccursinamaterialwiththekineticparameterssimilarto thoseforthe(de)intercalationstepofLiMn2 O4 (Eeq = 4.0V, g1 = 1, m = 0.5mg,scan rate �� = 0.2mVs 1 , d = 1 μm).Evenforthelargediffusionlengthofamicron-sized particle,completelyreversiblebehavior(i.e.thermodynamiccontrol)isobserved whenthediffusioncoefficientreaches10 11 to10 12 cm2 s 1 .Diffusionallimitations appearwhenthe D valuedropsto10 13 cm2 s 1 (Figure2.2a).Thepeak-to-peak separationsintheCVreachthetypicalvaluefordiffusion-controlledprocesses (59mV),whilethesymmetryofthepeaksisdecreasedandthecharacteristiccurrent “tails”(slowdecaysofcurrentafterthemaxima)appearafterthepeakpotentials. Notethatwhenthediffusioncoefficientisfurtherdecreased,thepeaks’height andthetotalintercalationchargedecrease,whichisaconsequenceofincomplete discharge(lithiation)ofaparticleduringthetimeofthepotentialsweep.Inexperimentswithpolydispersepowderelectrodes,thelong-timecurrentdecayswould alsoreflectthecontributionsoflargerparticleswithhigherdiffusionlengths[14].
2Metal–Ion-CoupledElectronTransferKineticsinIntercalation-BasedTransitionMetalOxides
Naturally,theincreaseinthescanratealsoresultsintheappearanceofasymmetriccurrenttailsandthedecreaseofthepeak’sheight(Figure2.2b).Underthese conditions,thereversiblecapacityonchargeoronthedischargeoftheelectrodes decreaseswiththeincreaseinthe C ratevalue,e.g.foramicron-sizedparticlewith D = 10 12 cm2 s 1 ata5C charge/dischargerate,almosthalfofthereversiblecapacitywillbelost(Figure2.2c).TheexperimentalCVsofaLiMn2 O4 electrodeshowthe transitionfromthermodynamiccontroltodiffusionalcontroluponincreaseofthe scanratefrom0.1to1mVs 1 (Figure2.2d).
Basicfeaturesofdiffusionalcontrol,whichcanbeusedforthekinetic regimediagnosticsare:(i)theproportionalityofthecurrentmaximato �� 1/2 , (ii)59-mVpeak-to-peakseparationbetweentheanodicandthecathodicpeaks,and (iii)decreaseintheintercalationcapacitywithincreaseinthecharge/dischargerate.
2.4KineticControl
Despitebeingmentionedquiteoften,diffusionalcontrolisbyfarnottheonly waytoimposeratelimitationsonanintercalationmaterialcharge/dischargerate. Theintercalationprocessinvolvestheinterfacialtransferofboththeelectron (fromthecurrentcollectortothematerialparticle)andtheion(fromthesolution sidetothesiteinsidethecrystallattice).Bothprocessesinvolvethetransferof chargeacrosstheboundarybetweentwophases,whichcanbephenomenologically describedwithintheframeworkofButler–Volmerkinetics.Itisgenerallynotyet establishedwhetheritisthetransferofanionorofanelectronthatdetermines therateoftheprocess.However,atleastforanumberofwell-studiedmaterials (LiCoO2 andLiMn2 O4 ),changingthesolventresultsinquitepronouncedchanges intheintercalationrate[15,26,28,29,34,35].Itisthusmorenaturaltosuggestthat theintercalationrate(atleastforthestudiedsystems)islimitedbytheinterfacial transferofametalion,asthesolventeffectcanhardlybeexpectedforasolid-state electrontransferreaction.Regardlessofthetypeofspecieswhichtraversesthe interface,theButler–Volmerequationcanbeusedtodescribethedependenceof the(de)intercalationrateontheovervoltage �� = E E0 (�� )[14]:
(E)= SF ⋅ 106 ⋅ �� nM Mr ⋅ ks
where �� isthetransfercoefficient, M r isthemolecularmassofthematerial(gmol 1 ), nM isthenumberofintercalatingM+ ionsperf.u., ks istheapparentheterogeneous rateconstant(cms 1 ),whichincludestheintercalatingmetalionconcentrationin solution cM ,i.e. ks = k0 s c�� M tokeepthestandarddimensionality.
Foramaterialwitharelativelyhighchargetransferrateconstant(10 8 cms 1 )and fastdiffusionaltransport(10 9 cm2 s 1 ),thekineticlimitationswouldnotimpactthe shapeoftheCVresponse(Figure2.3a),whiledecreasingtherateconstantvaluewill resultinanincreaseinthepeak-to-peakseparationsandamoderatedecreaseinthe
Figure2.3 (a)SimulatedCVs(E eq = 4.0V, g1 = 1, m = 0.5mg, �� = 0.1mVs 1 , D = 10 9 cm2 s 1 , �� = 0.5, d = 1 μm)fordifferentvaluesof k s .(b)Theeffectofthescanrate (0.05,0.1,0.3,0.7,1.5mVs 1 )ontheCVshape(currentisnormalizedtothescanrate). (c)TheeffectofthetransfercoefficientvalueontheCVpeakssymmetry(D = 10 9 cm2 s 1 , k s = 10 9 cms 1 ).(d)Charge/dischargecurvesforthecaseofratecontrolbyslowcharge transfer(D = 10 9 cm2 s 1 , k s = 10 9 cms 1 )calculatedfordifferent C rates: C /10,1C ,2C , 5C ,10C
peaks’heights(atlowenoughscanrates).AsetofnormalizedCVcurvesshows thechangesinthedifferentialintercalationcapacity(currentdividedbethescan rate �� )vs.potentialcurvesuponincreasingthescanrateforarateconstantvalueof 10 9 cms 1 ,whichisclosetothetypicalvalueforLi+ (de)intercalationintoLiCoO2 materials(Figure2.3b).Theincreaseinthepotentialdifferencebetweentheanodic andcathodiccurrentmaximaresultsinavisiblehysteresisonboththeCVcurves andcharge/dischargeprofiles.Thetransfercoefficientvalue �� isnotatransparent parameterincaseofiontransferreactions,asincontrasttosimpleouter-sphere redoxprocesses �� shouldnotnecessarilybecloseto0.5atlowoverpotentials[36].
The �� valuedeterminesthesymmetryoftheanodicandcathodicCVbranches, whichisillustratedinFigure2.3c.
Kineticlimitations(giventheabsenceofanydiffusionallimitations)donotresult inapronouncedreversiblecapacitylossathighcharge/dischargerates(Figure2.3d). However,thehysteresisbetweenthechargeanddischargecurvesdecreasesthe
2Metal–Ion-CoupledElectronTransferKineticsinIntercalation-BasedTransitionMetalOxides
averagevoltage(forourmodelcase,from4.05to3.85V),whichdecreasesthe energydensityofthematerial.Correspondingly,ifboththediffusionandkinetic limitationsareoperative,boththecapacityandtheaverageworkingpotential woulddecreasetosomeextent,dependingonthe C-rateand D and ks values.
“Mixed”intercalationratecontrolregimereferstothesituationwhenbothdiffusionandchargetransferkineticsaresufficientlyslowtocontributetotheintercalationrate.Atypicalvoltammogramofamodelmaterialundermixedkineticcontrolis showninFigure2.4a.Thecharacteristicfeaturesofboththekineticanddiffusional casesofcontrolmanifestthemselvesintheincreaseofpeak-to-peakseparations andcurrentdecaytimeswiththeincreaseinscanrate.Anexperimentalexample ofmixedcontrolbychargetransferkineticsandsolid-statediffusionisLi+ intercalationintoLiCoO2 (Figure2.4b),whichiscomplicatedbythephasetransformation proceedingat E = 3.91V(vs.Li+ /Li).Thetreatmentofphaseboundarymovementis
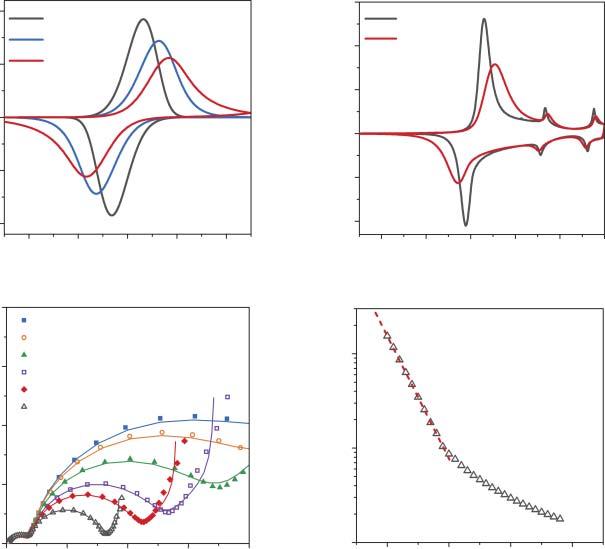
Figure2.4 (a)CalculatedCVsforthecaseof“mixed”reactionratecontrol (k s = 5 × 10 9 cms 1 , D = 10 12 cm2 s 1 ).(b)ExperimentalCVsofLiCoO2 electrodein1M LiPF6 EC/DMCelectrolyteatscanrates0.05and0.2mVs 1 .Source:Reproducedwith permissionfromRef.Levinetal.[34],Copyright(2017),Elsevier.(c)Experimental impedancespectraofLi1 �� CoO2 electrodesin1MLiClO4 propylenecarbonatesolutionata setofpotentials,correspondingto0.003 <��< 0.015.(d) Rct vs. E dependenceforthecaseof ratecontrolbyslowinterfacialchargetransfer.Source:Vassilievetal.[37]/withpermission ofElsevier.