SmartStimuli-ResponsivePolymers, Films,andGels
Editedby LiangHu YongfengGao MichaelJ.Serpe
Editors
Prof.LiangHu SoochowUniversity RAD-X 199Ren’aiRoad 215123Suzhou China
Dr.YongfengGao UniversityofAlberta DepartmentofChemistry 11227SaskatchewanDrive
T6G2G2NK Canada
Prof.MichaelJ.Serpe UniversityofAlberta DepartmentofChemistry 11227SaskatchewanDrive
T6G2G2NK Canada
CoverImage:CourtesyofLiangHu
Allbookspublishedby WILEY-VCH arecarefully produced.Nevertheless,authors,editors,and publisherdonotwarranttheinformation containedinthesebooks,includingthisbook, tobefreeoferrors.Readersareadvisedtokeep inmindthatstatements,data,illustrations, proceduraldetailsorotheritemsmay inadvertentlybeinaccurate.
LibraryofCongressCardNo.: appliedfor BritishLibraryCataloguing-in-PublicationData Acataloguerecordforthisbookisavailable fromtheBritishLibrary.
Bibliographicinformationpublishedby theDeutscheNationalbibliothek TheDeutscheNationalbibliotheklists thispublicationintheDeutsche Nationalbibliografie;detailedbibliographic dataareavailableontheInternetat <http://dnb.d-nb.de>
©2022WILEY-VCHGmbH,Boschstraße12, 69469Weinheim,Germany
Allrightsreserved(includingthoseof translationintootherlanguages).Nopartof thisbookmaybereproducedinanyform–by photoprinting,microfilm,oranyother means–nortransmittedortranslatedintoa machinelanguagewithoutwrittenpermission fromthepublishers.Registerednames, trademarks,etc.usedinthisbook,evenwhen notspecificallymarkedassuch,arenottobe consideredunprotectedbylaw.
PrintISBN: 978-3-527-34901-2
ePDFISBN: 978-3-527-83237-8
ePubISBN: 978-3-527-83239-2
oBookISBN: 978-3-527-83238-5
CoverDesign:ADAMDESIGN,Weinheim, Germany
Typesetting Straive,Chennai,India
Contents
Preface xi
1FromMechanochemistrytoMechanoresponsiveMaterials 1 LasithS.Kariyawasam,ConnorFilbin,CameronLocke,andYingYang
1.1Introduction 1
1.2MechanochemistryinBiologicalSystems 2
1.2.1AStressfulEnvironmentDuringHeartDevelopment 3
1.2.2ProteinUnfoldingbyForce 4
1.2.3StressMitigationbyTissue 6
1.2.4SensingbyIonChannelOpening 6
1.3MechanisticViewofMechanochemistry 7
1.4PolymerCovalentMechanochemistry 15
1.4.1Pyran-BasedMechanochromophores 16
1.4.2Retro-Cycloadditions 19
1.4.3Ladderenes 23
1.4.4StableRadicalSystems 24
1.4.5OtherTypesofMechanophores 27
1.5PolymerNoncovalentMechanochemistry 29
1.5.1MechanoresponsesofMetal–LigandBonds 30
1.5.2MechanochemistryofOtherNoncovalentInteractionsandTheir ApplicationsinFunctionalPolymers 37
1.6Conclusions 41 References 42
2PhotoresponsivePolymers 53
HosseinRoghani-MamaqaniandZeinabTajmoradi
2.1Introduction 53
2.2PhotoresponsivePolymers 55
2.2.1PhotoluminescentPolymers 55
2.2.1.1FluorescentPolymers 56
2.2.1.2PhosphorescentPolymers 58
2.2.2PhotochromicPolymers 62
2.2.3PhotocleavablePolymers 71
2.2.4PhotodimerizablePolymers 74
2.2.5PhotoadaptablePolymers 76
2.3ApplicationsofPhotoresponsivePolymers 83
2.3.1SmartPolymericInks 83
2.3.2PolymerSensors 89
2.3.3Photolithography 93
2.3.4SurfaceActiveAgents 95
2.3.5PhotorheologicalPolymers 98
2.3.6Self-HealingPolymers 103
2.3.7Shape-ChangingPolymers 106
2.3.8PhotoconductivePolymers 109
2.3.9DrugDelivery 111
2.3.10Membranes,Films,andTextiles 112
2.4SummaryandtheFuture 115
2.4.1WaterContactAngleVariation 116
2.4.2ViscosityVariation 116
2.4.3ColorChangeandEmission 117
2.4.4Sol–GelTransition 117
Abbreviations 118 References 119
3PolymerSystemsforIonizingRadiationDosimetryand Radiotherapy 135
LiJiang,ChengfangZhang,RenshengWang,andLiangHu
3.1Introduction 135
3.2InteractionofRadiationwithMatter 136
3.2.1 α-Particles 136
3.2.2Electrons 137
3.2.3Photons 137
3.3PolymerSystemsforIonizingRadiationDosimetry 139
3.3.1Polymer-BasedDosimeters 139
3.3.2Polymer/DyeDosimeters 139
3.3.3FluorescentPolymerDosimeters 141
3.3.4Polymer/MetalNanomaterialsDosimeters 143
3.4IonizingRadiation-ResponsivePolymerSystemsforTherapy 146
3.5Conclusion 149 Acknowledgments 151 References 151
4ShrinkandWrinkle–ThermallyResponsiveSubstratesfor Thin-FilmStructuring 157
EduardoGonzález-MartínezandJoseMoran-Mirabal
4.1StructuredThinFilms 157
4.2MeasuringtheMechanicalPropertiesofThinFilmsUsingThermal Wrinkling 159
4.2.1ThermallyStructuredThinFilmsforCellCulture 162
4.2.2WrinkledConductiveThinFilmsforWearableElectronics 167
4.2.3WrinkledElectrochemicalSensors 173
4.2.4CurrentChallengesandFuturePerspectivesfortheUseofWrinkled ThinFilms 175 References 176
5DesignofNanocompositeMicrogelsPreparedbySeeded EmulsionPolymerizationinthePresenceofMicrogels 181 TakumiWatanabeandDaisukeSuzuki
5.1BackgroundonCompositeHydrogels 181
5.2BackgroundonCompositeMicrogels 182
5.3ConventionalEmulsionPolymerizationandSEP 184
5.4NanocompositeMicrogelsPreparedbySEPinthePresenceof Microgels 186
5.5DesignoftheInternalStructureoftheNanocompositeMicrogels 188
5.6SynthesisofMulti-layeredNanocompositeMicrogels 189
5.7CharacterizationofNanocompositeMicrogels 190
5.8ApplicationsofNanocompositeMicrogels 193
5.9SummaryandPerspective 195 References 196
6CompressibleMicrogelsinConcentratedSuspensions:Phase Behavior,FlowProperties,andScatteringTechniquestoProbe TheirStructureandDynamics 203
A.Scotti,U.Gasser,B.Zhou,A.Arenas-Gullo,A.delaCotte,J.RojoGonzález, andA.Fernandez-Nieves
6.1Introduction 203
6.2SwellingThermodynamics 207
6.2.1Polymer/SolventMixing 208
6.2.2Elasticity 208
6.2.3IonicEffects 209
6.2.4EquationofState 210
6.3ExperimentalTechniques 210
6.3.1DynamicLightScattering 211
6.3.1.1Auto-correlationExperiments 213
6.3.1.2Cross-correlationand3D-DLSExperiments 214
6.3.2Small-angleNeutron-scattering 216
6.3.2.1SANSSetup 218
6.3.2.2ScatteringTheory 218
6.3.2.3FormFactorandStructureFactor 220
6.3.2.4ContrastVariation 222
6.4SuspensionPhaseBehavior 228
6.5FlowProperties 231
6.6FinalRemarks 235 References 236
7StructureandPropertiesofSmartMicro-andNanogels Determinedby(Neutron)ScatteringMethods 241 JulianOberdisseandThomasHellweg
7.1Introduction 241
7.2ScatteringTechniquesAppliedtoMicrogels 242
7.2.1StaticandDynamicLightScatteringAppliedtoMicrogels 242
7.2.1.1StaticLightScattering(SLS) 243
7.2.2DynamicLightScattering(DLS/PCS) 245
7.2.3Small-AngleNeutronandX-RayScatteringAppliedtoMicrogels 247
7.3MulticompartmentandMulti-Stimuli-ResponsiveMicrogels 254
7.4Time-ResolvedSmall-AngleScattering 263
7.5CrowdedMicrogelSystems 266
7.6ConclusionandOutlook 270 Appendix:AbsoluteIntensityforFuzzySphereFormFactors 270 References 271
8Stimuli-ResponsiveFluorescentPolymericHydrogels 281 WeiLu,ShuxinWei,andTaoChen
8.1Introduction 281
8.2StrategiesforPreparingFluorescentPolymericHydrogels(FPHs) 282
8.2.1PhysicallyIncorporatingFluorogensintoPolymericHydrogels 282
8.2.2CovalentlyBondingFluorogensintoPolymericHydrogels 284
8.2.3SupramolecularPolymerizing/CrosslinkingMonomeric Fluorogens 286
8.2.4ComparisonofDifferentSyntheticStrategies 290
8.3PromisingApplications 290
8.3.1OpticalSensingandBio-imaging 290
8.3.2InformationEncodingandEncryption 293
8.3.3BioinspiredMechanosensingSystemsandSoftActuators/Robotics 294
8.4Conclusions 297 References 298
9TheFabricationandApplicationsofBioinspiredHydrogel Actuators 301
BaoyiWu,JiaweiZhang,andTaoChen
9.1Introduction 301
9.2TheClassificationofHydrogelActuators 302
9.2.1AdditionofActiveIngredient 302
9.2.2Pneumatic/HydraulicActuators 305
9.2.3Stimuli-ResponsiveHydrogelActuatorDerivedfromAsymmetric Swelling 306
9.2.3.1Single-Stimulus-ResponsiveHydrogelActuators 307
9.2.3.2Multi-stimuli-ResponsiveHydrogelActuators 308
9.3AnisotropicStructures 310
9.3.11D/2DAnisotropicStructures 310
9.3.1.1Bilayer 310
9.3.1.2Oriented 311
9.3.1.3Gradient 312
9.3.1.4Patterned 315
9.3.23DAnisotropicStructures 315
9.4MethodstoFabricateAnisotropicStructures 318
9.4.1TraditionalTechnology 318
9.4.1.1StepwisePolymerization 318
9.4.1.23DPrinting 320
9.4.1.3MacromolecularAssembly 322
9.4.2InnovativeTechnology 322
9.5Applications 325
9.5.1SoftRobots 325
9.5.2ArtificialMuscles 327
9.5.3BiomimeticDevices 329
9.5.4InformationStorageMaterials 329
9.6Conclusion 332 ConflictofInterest 333 Acknowledgments 333 References 333
10Hydrogels-BasedElectronicDevicesforBiosensing Applications 339 QuanduoLiang,YuyuanLu,andQiangZhang
10.1Introduction 339
10.2FlexibleHydrogel-BasedSensors 342
10.2.1PrinciplesofConductiveHydrogelSensors 343
10.2.2ImprovedMechanicalPropertiesofHydrogel-BasedSensors 347
10.2.3ProlongedLongevityofHydrogelSensors 351
10.2.4ExpandedUsageScenariosofHydrogel-BasedSensors 353
10.2.5MultifunctionalizationandExpandingApplicationofHydrogel Sensor 354
10.3Tissue–MachineInterfaces 356
10.3.1DesignandMechanismoftheNeuralInterfaces 356
10.3.2MultifunctionalApplicationsofBiointerfaces 360
10.4TheProspectsofHydrogelBioelectronicDevices 363 Acknowledgments 364 References 364
Index 375
Preface
Innature,manylivingsystemscanreacttochangesintheirexternalenvironment. Inspiredbythisnaturalresponsivity,scientistshaveendeavoredtounderstand stimuli-responsivepolymers,whichareabletochangetheirsolubility,volume, and/orconformationinresponsetoexternalstimuli.Overthepastfewdecades, interestinthese“smart”responsivepolymer-basedsystemshasbeenincreasing,and henceabookonthistopiciswarranted,tocapturerecenttrendsinthisburgeoning area.
Thisbookwasassembledprimarilywiththeneedsofbothjuniorandsenior chemistsandmaterialsscientistsinmind.Wefirstwishtoacknowledgethecontributionofallauthorsfromallovertheworld.Withoutallyoursupport,this bookwouldnothavebeenpublished.Thisbookisdividedinto10chapters. Chapters1–3discussthemechanoresponsive,photoresponsive,andionizingradiation-responsivepolymers.Chapter4highlightsthermalresponsivefilm. Chapters5–10discussthestimuli-responsivegelsfromfundamentalsynthesis strategytoscatteringtechniquescharacterizationandapplications.
ThisbookwaspublishedbyWiley,whosesupportandhelpwegratefullyacknowledge,especiallythewarmwelcomeandcontinuedaidgiventousbyoureditors, KatherineWong,AnneBrennführer,andElkeMaase.
14April2022
LiangHu SoochowUniversity,China
YongfengGao,MichaelJ.Serpe UniversityofAlberta,Canada
FromMechanochemistrytoMechanoresponsiveMaterials
LasithS.Kariyawasam,ConnorFilbin,CameronLocke,andYingYang
UniversityofNevada,DepartmentofChemistry,VirginiaStreet,Reno,NV89557,USA
1.1Introduction
Ourskincansensethetouchbyaseriesofmechanotransductionmechanisms. Kneadingbreaddoughuncoilsglutenproteins,creatinganelasticmacromolecular networkthatgivesthedoughtoughness.Stretchingorscratchingapieceofplasticis likelytobreakcovalentbonds.Theseprocessesinvolvereactionsthatareactivated bymechanicalenergy,whichareprevalentinourdailylives.However,theyare lesscommonlydiscussedcomparedtothermochemical,photochemical,orelectrochemicalreactions.Mechanoactivatedreactionshavebeenreporteddatingbackto 315BCE.Theseearlyaccountsdescribegrindingnativecinnabarinacoppermortar withacopperpestleinthepresenceofvinegartoyieldthereductionproduct, mercury.However,itwasnotuntilthenineteenthcenturythatsystematicstudies wereconducted[1].In1860s,CareyLeashowedthatgrindingmercuryandsilver halidesinapestleandmortaratroomtemperaturefavorsdecomposition,whereas heatingonlyleadstomeltingorsublimationwithoutanydecomposition[2].This discoveryprovidedclearevidencethatmechanochemicalreactionsaredistinctively differentfromthermalones.Mechanochemistrywas,therefore,classifiedasthe fourthtypeofchemicalreactionbyOstwaldin1919[3].
Thefirstwidelyaccepteddefinitionformechanochemistrywasformulatedby Heinickein1984[1],thatmechanochemistryisabranchofchemistry,whichis concernedwithchemicalandphysicochemicaltransformationsofsubstancesinall statesofaggregationproducedbytheeffectofmechanicalenergy.IUPACdefines itasthechemicalreactionthatisinducedbythedirectabsorptionofmechanical energy[4].Infact,thedefinitionisstillunderextensivedebate.Molecularmotors, whichconvertchemicalenergyintomechanicalwork,certainlydonotfitinto thesedefinitions.However,themotionsgeneratedbymolecularmotorscanapply forcetothesurroundingmoleculestoinduceacascadeofsubsequentreactions. Suchtopicisofgreatinteresttochemistsandengineersworkinginthefieldof mechanochemistry.Thelackofunificationandslowprogresssincetheestablishmentofmechanochemistryinthenineteenthcenturyreflectsthecomplexityand
SmartStimuli-ResponsivePolymers,Films,andGels,FirstEdition. EditedbyLiangHu,YongfengGao,andMichaelJ.Serpe.
©2022WILEY-VCHGmbH.Published2022byWILEY-VCHGmbH.
1FromMechanochemistrytoMechanoresponsiveMaterials
thelackofunderstandingofthescopeandmechanismsforsuchreactions.Recently, mechanochemicalresearchhasintensifiedthroughtheuseofnewtoolsdevelopedformechanisticstudiesandtheopportunitiestocreatefunctionalmaterials [5–12].Asmechanochemicalreactionsoccurforbothsmallandmacromolecules, ongoingresearchcanbeclarifiedinfoursub-areas:developingnovelandscalable mechanochemicalsynthesistomakeusefulchemicalsviaenvironmental-friendly solvent-freeprocesses;understandingbiomechanochemistry,suchasmotor proteins,mechanosensing,andmechanotransductionmechanisms;creating mechanoresponsivepolymericmaterialsforwhichmechanicalforcesbecomeconstructivefortechnologicaladvances;andinvestigatingthemolecularmechanisms throughsimulationsandsingle-molecularforceexperiments.
Inthischapter,wewillfocusonthefundamentalaspectsofpolymer mechanochemistrythatarethekeyelementsindesigningmechanoresponsive materials.Wewillstartwithabriefintroductionoftheroleofmechanochemistryinbiologicalsystemsasaspringboardforinspiration.Themechanistic aspectsofmechanochemistryingeneralterms,fromsmallmoleculestopolymer mechanochemistry,willthenbediscussedtoshowtheuniquebond-activation mechanisms.Theforce-responsivemolecules,namedmechanophores,candepend onthecleavageofeithercovalentornoncovalentbonds.Theactivationenergy, dynamics,andreversibilitycanbetunedviavariousstructuralproperties.Therefore,thechemistryofthesetwoclassesofmechanophoreswillbecoveredindetail. Therearedifferentmechanicalsourcesforgeneratingmechanicalenergy,suchas shearing,stretching,grinding,ballmilling,andsonication.Thesemethodsdifferin thedirectionofforce,frequency,andheatformation,leadingtodifferenteffectson moleculardistortionandkinetics.However,thischapterwillfocusonthechemistry ofmechanoresponsivebondswithinpolymermaterialsregardlessofthetypeof appliedforce.
1.2MechanochemistryinBiologicalSystems
Apowerfulsourceofinspirationforimprovingthedesignofpolymermaterials isNatureasmechanochemicalsystemsareubiquitousinorganisms.Awealth ofknowledgecanbegainedbecausebiologicalsystemshaveevolvedelegant mechanoresponsivearrangementsthatarecriticalforsupportingandmaintaininglife.Theyofteninvolvecomplicatedprocessesviacoherentlyorganized biopolymernetworks.Acell,forexample,isconstantlyundermechanicalstress, includingtension,osmosis,compression,andshearforces.Uponmechanical deformation,feedbackfromproteinsinthecellcytoskeletonactivatesavarietyof mechanosensorsthatworkinunisontocreatearesponseinthecellnucleusvia multiplemechanotransductionevents[13].AsshowninFigure1.1,themechanism beginsbytransducingforcethroughthecellmembranetomicrofilamentsand microtubulesofthecytoskeletoninthecytoplasm.Subsequently,cytoskeletal changesdirectlyaffectnucleoskeletalproteinscalledlamina.Thishasanexplicit effectonthespatialarrangementoflamin-boundintermediatefilamentsaswellas
Membrane
Mechanotransduction (signaling pathways)
Figure1.1 Intra-andextracellularforcesstimulateacellinaninterconnectedsystemof reactionscausingcompletechangeofstructureandresultingcellfunction.Source:Adapted withpermissionfromTsimbouri[13].Copyright2015MDPI.
chromosomes,astheyareanchoredtonuclearlamina[14].Shiftsinchromosome packingaffectgeneexpression,whichallowskeybiologicalfunctionsinresponse toforce,suchassurvival,motility,reproduction,anddifferentiation.Theseevents incellsplayimportantrolesinmaintaininghomeostasisandpreventingdisease inthebody.Althoughunderstandingofmanyofthesebiologicalpathwaysisstill limited,wewilldiscussafewchemistriesknowntobeinvolvedintheseprocesses asinspirationsformaterialdesign.
1.2.1AStressfulEnvironmentDuringHeartDevelopment
Inavertebrateembryo,theheartfirststartsasatubecomposedofprimarilyearly cardiomyocytes,thecardiacmusclecellsthatdrivetheheartcontraction.Itquickly differentiatesintodifferentpartsandmorphologies.Astheembryogrows,thecontractilecapacityofthecardiomyocytesincreasestoprovidegreaterdrivingforcesto pumpmoreblood.Meanwhile,theextracellularmatrix(ECM)surroundingthecardiomyocytesmustincreaseinitsstiffnessparallellytokeepapropertissuemechanicalintegritywiththeincreasingcontractilestress.Cellsinconnectivetissue,called fibroblasts,secretecollagenandothermatrixproteinstomaintainthestructural framework.Therefore,duringthedevelopmentoftheheart,abalancebetweencardiacfibroblastandcardiomyocytecellpopulationsmustbeestablishedtomaintain musclecontractionalongwithasignificantcollagenousmatrix.Thereismounting evidencesuggestingthatmechanicalstressitselfplaysimportantroleindirecting tissuegrowthwithmechanochemicalfeedbackloopsforgeneandproteinexpression[15].Incomparison,thebraintissueinalow-stressenvironmentdoesnotshow thesamedevelopmentinstiffness,althougharecentstudyfoundstrongmechanicalinteractionsofthesynapses[16]whichmaybeacriticalmechanisminbrain
1FromMechanochemistrytoMechanoresponsiveMaterials functions,indicatingthebroadinvolvementsofmechanochemistryandmechanotransductioninnumerousbioprocesses.
Duringembryonicheartdevelopment,manymechanosensitivepathwayshave beenlinkedtotheproperfunctionsofthemyocyteandfibroblastcells.Majkutetal. surveyedliteratureevidenceandproposedthenetworkmodelforunderstanding howcontractionagainsttissuestiffnessaffordsafunctionalequilibriumbetween thecelltypes[15].Ononehand,cardiomyocytesproducecontractilestressthat promotestheexpressionofmatrixstructuralproteinsbyfibroblasts.Ontheother hand,ascontractionmusteffectivelystrainthehearttissue,itispostulatedthatthe proliferationoffibroblastsislimitedbythestiffnessoftheirenvironmentandthus collagenousmatrixdensity.Additionally,themodelsuggeststhatstabilizingmatrix collagenanddegradationofmotorproteinsunderstrainedconditionsarealso importantinregulatingtissuestiffness.Thestabilizationmayberelatedtoinhibited proteasebindingtocollagenfibersorkinasebindingtomyosinminifilaments whentheyareundertension,thuspreventingtheirdissociationanddigestion.A modelofdynamiccell–matrixinteractionisalsoextendedtonuclearmechanics becauseduringdevelopmenttherearevariationsinlaminlevelsthatappearto correlatewithECMmechanics[15].Aspreviouslydiscussed,mechanicalsignals fromtheextracellularenvironmentcanbephysicallytransmittedbythecontractile cytoskeletontothenucleusbyconnectionsthroughthenuclearmembranetothe nuclearlamina.Laminacaninteractwithchromatinandvariousproteinsthat regulatetranscription.Therefore,laminexpressionisalsoregulatedandaffects tissuematuration.
1.2.2ProteinUnfoldingbyForce
Proteinsarefoldedintovariousthree-dimensionalstructurestoperformbiological functions.Manyofthemarehighlyresistanttounfoldingundermechanicalstressto avoiddenaturation.However,ithasbeenobservedthatcertainproteinscontaining hiddenbindingsites(crypticsites)thatrelyonforceforaconformationalchange viaunfoldingtoenableproteinactivityandinitiationofsignalingpathways[17–19]. Oneofthemostextensivelystudiedmechanicallyresponsiveproteinsisthevon Willebrandfactor(vWF),whichplaysacrucialroleinbloodclotting.Whenvascular systemsareruptured,adecreaseinpressuretriggershydrodynamicforcesfrom changingvelocityintheblood.Thesehydrodynamicforcesinduceashearforce onvWFintheblood,causingaconformationchange.Atacriticalshearstressof approximately50dyn/cm2 ,theglobularproteinissignificantlyelongated[20–22]. Figure1.2showsthedomainstructuresandtheforce-inducedconformational change.ThisleadstoexposureandactivationoftheA1domaintobindtoplatelets andA3domaintoattachtothecollagenproteinonthesurfaceofvasculardamage toinitiaterepairofthevesselinjury[23].TheproteinunfoldingoccursattheA2 domainandissuggestedtoinvolvemechanoactivatedcleavageofdisulfidebondsin combinationwithhydrophobicinteractions.ThevWFcontainsaneight-membered ringlinkedbyadisulfidebondfromvicinalcysteines(Cys1669 –Cys1670 ),whichis thelowestenergybarriertoinitiateforce-inducedunfoldingoftheA2domain
Folded cis-Pro1645
1.2MechanochemistryinBiologicalSystems 5
Unfolded trans-Pro1645
Shear forces
Shear forces Water contained in hydrophilic region
Disulfide bond breaks, water enters hydrophobic core
Repulsion of water from hydrophobic core results in isomerization of Pro1645 and subsequent unfolding
Figure1.2 ModelofvWFproteinmechanochemicalunfoldingfromshearforces.When vWFproteinisintheunfoldedconformationtheA1domainbindsplatelets(green),A2 domainelongates(pink),andA3domainbindscollagen(blue).Source:Adaptedwith permissionfromCrawleyetal.[21].Copyright2011AmericanSocietyofHematology.
Figure1.3 Schematicofmarinemusselcuticledemonstratinghighdensityofdopa-Fe3+ crosslinksingranulecomparedtomatrix.Thisresultsintheformationofmicrocracks followingincreasedstrain,preventingcompletematerialfracture.Source:Adaptedwith permissionfromHolten-Andersenetal.[24].
(Figure1.3).Whenappliedforceistransducedthroughtheprotein,thedisulfide bondismechanicallycleavedbyshearforces.Itisspeculatedthataftercleavage, buriedwatermoleculesareadmittedtothehydrophobiccoreofthevWFprotein commencingunfolding[25].Additionally,theunfoldedstateoftheproteinisstabilizedbyaforce-inducedisomerizationofaprolineresidue(Pro1645 ).Thepeptide cis-Pro1645 intheA2domainisstabilizedinacisconformationbyhydrogenbondingwithresidueArg1618 whenthevWFproteinisinthefoldedconformation.After initialunfolding,thestabilizationfromhydrogenbondingislostandtheproline residueundergoesisomerizationtotrans.Conversionto trans-Pro1645 notablydelays A2domainrefoldingandallowsunfoldedvWFtocontinuetheprocessofblood vesselcoagulationandrepair[23].Theforce-inducedunfoldingofvWFshowsan
Mussel foot
Byssal threads
1FromMechanochemistrytoMechanoresponsiveMaterials organizedprocessinvolvingmechanoscissionsofcovalentandnoncovalentbonds, hydrophobicinteractions,andsubsequentisomerization.
1.2.3StressMitigationbyTissue
Biologicaltissuehasshowntoundergostressmitigationtoavoidcompletematerialfailurethroughtheuseofnoncovalentinteractions.Onespecificexampleisthe metal–ligandinteractionsintheoutercoatingofbyssalthreadsinmarinemussels [24,26].Thesebyssalthreadsaresecretedasbundlesof50–100individualthreads andarecoveredinaproteinaceousoutercoating,calledthecuticle,whichisfive timesstrongerthantheinternalthreadsthemselves.Musselsusebyssalthreadsto attachtorocks;thecuticle’stoughnesspreventsthebyssalthreadsfromdisconnectingfollowingappliedforce.Theprimarycuticleproteinisamodifiedtyrosine,which includesthemolecule3,4-dihydroxyphenylalanine(dopa).Includedamongtheproteinsarenaturallyexistingmetalions,suchasironandcalcium.Uptothreedopa ligandscancrosslinktotheiron(III)ions(Fe3+ ),resultinginanincreasedhardness (Figure1.3).Granulesareareasofhigh-concentrationdopa-Fe3+ crosslinkingwithin thecuticle.Lighterdopa-Fecrosslinkingoccursinthematrixsurroundingthegranules.Followingincreasedstrain(>30%),thegranulesdeformslightly,whereasthe surroundingmatrixcrosslinksdissociate,formingmicrocracks.Thesemicrocracks allowthecuticletoabsorbmechanicalforcewithoutcompletefracture.Following removalofstrain,thegranulesinstantlyregaintheiroriginalshape,whilethemicrocrackcrosslinksslowlyself-healovertime.
1.2.4SensingbyIonChannelOpening
Mechanotransductionisimportantforsignalinginsensorysystems.Haircellsinthe earcalledstereociliaaremechanosensorybiologicalstructuresthatconvertmechanicalenergyfromsoundwavesintoelectrochemicalsignalsthatcanbeprocessed bythenervoussystemashearing[19].Stereociliaarelinkedtogetherattheirtips intobundlesbyproteinscalledtiplinks.Thesetiplinksareanchoredintothecell membraneconnectinganionchannelofonestereociliatothetipofanother[27]. Vibrationalperturbationsfromsounddeflectstereociliatipscauseopeningandclosingofionchannels(Figure1.4).Openingandclosingofthechannelsresultina changeofionfluxleadingtoanelectricpotentialthatcanstimulatetheauditory nerve[28].
Anotherfascinatingexampleofmechanotransductioninhumansensesisfound inthemechanismoftouch.Touchisessentialforlifeastactileinputguidesbehavior.Discriminativetouchallowsuniquelyhumanactivitieswherefinesseisneeded. Delicatetouchisallowedbythemanysensoryreceptorsimbuedintheskin.Adiversityofsomatosensoryneuronsintheskinallowsforavarietyofsensationsbytakingadvantageofvaryingmechanicalthresholdsindifferentproteins.Forexample, lighttouchismediatedbyAβ afferentsthathavealowmechanicalthresholdcomparedtothehighmechanicalthresholdofnociceptorsthatmediatepainfultouch [29].Moreover,anassortmentofmechanoreceptorsundertheskindetectsstretches,
0.5 μm
Figure1.4 Illustrationshowingsensorycellscalledstereocilialocatedintheinnerearof mammals.Thestereociliadeflectbackandforthuponmechanicalstimulationbysound waves.Source:Hoffmannetal.[27]/withpermissionofRoyalSocietyofChemistry.
vibrations,slip,andmotionthatenableustodetermineanobject’sshapeandtexture.Littleisknownaboutthemechanismsformechanotransductionintouch;however,therecentdiscoveryofpiezoproteinsmayhelpbetterunderstandhowproteins areactivatedbymembranedeformation.Piezoproteinsareionchannelsmechanicallyactivatedbytouch,suction,andshearstress[30].Highconcentrationsofpiezo proteinsaroundreceptorsandneuronsimplicatetheirresponsibilityforencoding transductionchannelsintouchreceptors.
Ascanbeseenfromtheseexamples,mechanoresponsivenessinbiological systemsreliesonmechanotransduction,whichtransformsmechanicalenergyinto abiochemicalsignalthatinducesspecificcellularresponses,whichfrequently takesadvantageofnoncovalentinteractions[19].Alsocommonlyfoundinliving systemsarekineticallytrappedstatesthatarestructureslockedfarawayfrom thermodynamicequilibriuminahigh-energyconformation,suchasfoldedproteinsorprestressedcellmembranes[31].Organismstakeadvantageofthese structurestoenablesophisticateddynamicresponsestoforce.Preciselymimicking biologicalstructuresandmechanismsischallengingandunnecessarybecauseof thecomplexity.However,similarmechanoresponsivebondingdomainsutilizing thechemistryandstructurecharacteristicslearnedfrombiomacromoleculescanbe employed,andliving-likefunctionariescanbecreatedthroughthedesignprinciples ofsyntheticmaterials.
1.3MechanisticViewofMechanochemistry
Oneofthemostinterestingaspectsofmechanochemistryisthatityieldsproducts thataredifferentfromthermal-andphoto-reactionpathways.Thisphenomenonhas beenobservedinbothinorganicandorganiccompounds.Here,wewilluseanextensivelystudiedmechanochemicalreaction,thepericyclicreaction,asanexample
8 1FromMechanochemistrytoMechanoresponsiveMaterials
(a) Thermal activation
cis-isomer
(b) Photo activation
E, E-isomer E, Z-isomer E, E-isomer
(c) Mechanical activation
cis-isomer
E, Z-isomer
E, E-isomer trans-isomer trans-isomer trans-isomer
Figure1.5 Ringopeningof1,2-disubstitutedbenzocyclobutenes(BCB)underdifferent energyinputs.Source:Hickenbothetal.[32]/withpermissionofSpringerNature.
todiscussitsmechanisticorigin.Thermalactivationof1,2-disubstitutedbenzocyclobutene(BCB)inducesconrotatory(con)ringopenings,sothatthecisandtrans isomersgivedifferentring-openingproducts(Figure1.5a).Whenactivatedbylight, disrotatory(dis)ringopeningisfavoredinsteadofconforbothcisandtransisomers(Figure1.5b).Thisstereospecificityunderthermalandphoto-activationsis describedbythewell-knownWoodward–Hoffmann(WH)rules.However,when BCBisplacedwithinlongpolymerchains,themechanoproductsdonotfollowWH rules[32].Mechanicalforcesinduceaformaldisrotatoryringopeninginthecisisomerandaformalconrotatoryringopeninginthetransisomer,yieldingthesame E,E-isomer(Figure1.5c).Thelackofselectivitywasalsoobservedformechanoactivationof gem-dihalocyclopropanes[33–35].Iftheforceisviewedaspullinginoppositedirectionsatthetwomolecularanchoringpoints,thereactionpathwayfavors bondbreakingtoincreasethedistancebetweenthepullingpoints.
Thesestunningmechanochemicalphenomenaintriguedthestudyforthe underlyingmechanisms.Photoirradiationactivatesthermallyforbiddenpathways bypromotingthereactantstoelectronicallyexcitedstates.Force,however,does notdirectlyaltertheenergyoftheelectrons.Thus,howdoesmechanochemical reactionovercometheWHrule?Thisisdiscussedingreatdetailinseveralstudies viaquantumchemicalmethods[36–39].Theresultsshowthatappliedstresses donotaltertheelectronicstructure.Instead,theylowertheactivationbarrierfor WH-forbiddenpathways.Figure1.6showstheminimumenergypathwaysfor mechanicalringopeningofBCBundervariousforcesfromquantumchemical calculations[36].Fordirectionalpullingofthecisisomer,beyondacriticalforce of1.5nN,thebarrierforWH-forbiddendisrotatorypathwaydropsbelowthe conrotatorypathway,anddisrotatorybecomesthemechanicallyfavoredpathway. Forthetransisomer,conrotatoryisfavored.Therefore,thetwoisomersyieldthe samering-openingproductsasshowninFigure1.5c.Sincemechanicalworkalters thepotentialenergysurface(PES),loweringtheactivationbarrierwithoutchanging electronicstructures,electronicconsiderations,suchasWHrules,isnotdirectly applicable.
Thenextquestionishowmuchforceittakestobreakachemicalbond.Mechanicalstrengthofmacroscopicmaterialscanbecharacterizedbyaruptureforce.This holdstrueforsinglechemicalbondsonlyiftheydonotundergoanyvibrations. Thebondswillrupturewhendissociationforceexceedsthebondstrength.However,bondsundergothermalfluctuations.Combinedwithapullingforce,theyled toafarmorecomplexdependenceonforceconditions.Developedtodescribethe strengthofcelladhesion,thewell-knownBell’smodelprovidesasimpleexplanation fortheeffectsofstressonbondrupture[40].Whenabondispulled,themechanical forcedeformsthePES,reducingtheactivationenergyforbondrupture,whichcan beovercomebyadditionalenergyfromthermalfluctuations.Asaresult,molecular bondshavenosingleruptureforce,whichisnotsointuitive.Mathematically,Bell’s modelisdescribedas
�� = ��0 exp[(E0 �� f)∕kT]
where �� isthelifetimeofabond,whichisthereciprocalofoscillationfrequency,and �� 0 isthebondlifetimewithoutload.Thechangeofactivationenergyisexpressedby E0 �� f ,where E0 isthebondenergyatzeroforce, f istheappliedforce,and �� isa structuralparameterthataccountsforthedistancetothetransitionstatealongthe reactioncoordinate.Thus,theactivationbarrierchangeslinearlywithforce,andthe bondlifetimedecreasesexponentiallywithincreasingforceandtemperature.Based onthisequation,itwasproposedthatforceimpulseisthehighestwhen F = kT /�� at whichforcetransductionismaximized[41].AlimitationinapplyingBell’stheory tocomputationalcalculationsistheselectionofthestructuralparameter �� .Since �� itselfisforcedependent,themodelfailstoaccountforthemechanicallyinduced distortionoftransition-stategeometry,resultinginanoverestimateofthebondruptureratewhenthereisconsiderableelongationofthescissilebond.Manyextensions havebeendevelopedtoimproveBell’stheory.Theextendedmodelsalongwithother quantumchemicaltreatmentstounderstandtherelationsofgeometries,energies,
10 1FromMechanochemistrytoMechanoresponsiveMaterials
andtransitionstatesinmechanochemistryaresummarizedinareviewarticleby StauchandDreuw[42].
Experimentalstudiesevolvedparallellywiththetheoreticalpredictionsto measuretheruptureforceofasinglebond.Inlate1990s,theuseofatomicforce microscopy(AFM)madeitpossibletoprobethemechanicalresponsesofcovalent andnoncovalentbondsinasinglemacromolecule[43–48].Forceresponsesare probedonananoscalewithforcesonnanoNewton(nN)tosub-nNlevels.Ina force-probeAFMexperiment,themoleculeisanchoredbetweenasurfaceand anAFMtip.Itisthenstretchedataconstantforcerateuntilrupture[48].A force–extensioncurveforasinglechemicalbondisthusgenerated,whichreveals conformationalchanges,supramolecularrearrangements,alongwithbondrupture steps.BondruptureoftheBCB,whichisshowninFigure1.6,ismeasuredusing single-moleculeforcemicroscopy[49].Itprovidesthefirstexperimentalevidence

Figure1.6 Minimumenergy pathwaysfordisrotatoryand conrotatoryringopeningofBCB undervariousforcemagnitudes calculatedviasteered moleculardynamicscombined with abinitio steeredmolecular dynamics(AISMD).Source:Ong etal.[36]/withpermissionof AmericanChemicalSociety.
1.3MechanisticViewofMechanochemistry
thatthesymmetry-forbiddenconrotatoryringopeningcanbemechanicallyaccelerated,requiringa130pNlessforcecomparedtodisrotatorypathwayata ∼0.1second experimentaltimescale.
Forcedependencyofreactionratecanalsobemeasuredusingsingle-molecule forcespectroscopy.Aconstantforceisapplied,andthedeformationisrecordedas afunctionoftime.Thisapproachiscalledforce-clampAFM.Thetechniquehas beenusedtostudythekineticsofthiol/disulfideexchangereactionswithinaprotein[50].Thereactionisknowntooccurduringproteinunfoldinginmechanically stressedproteinsandiscrucialinregulatingproteinfunction.Theexperimentutilizedproteinengineeredwithapreciselypositioneddisulfidebond.Disulfidereductioneventscanthusbecorrelatedwithasignatureproteincontourlength,which canbeidentifiedintheextensionexperiment.AsshowninFigure1.7a,mechanical forcefirsttriggerstheunfoldingoftheprotein(unsequesteredunfolding),exposing thedisulfidebond.Inthepresenceofareducingagent,1,4-DL-dithiothreitol(DTT), thedisulfidereductionoccurs.Theratesofthebiomolecularreductionweremeasuredbyfittingtheextension-timecurvesignaturingthedisulfideexchangeunder varyingforcemagnitudes.Theexchangerateshowedanexponentialincreasewith theappliedforce(Figure1.7b).FittingtheexperimentalresultsusingBell-likemodel givesa �� value,thelengtheningofthetransitionstate,of0.34Å.Anenergylandscapeisplottedshowingan8.2kJ/molreductionofactivationenergywhena400pN forceisapplied(Figure1.7c).Thereversibilityofdisulfideexchangewasrecordedby force-clampAFM[51].MechanicalforceenabledthethermodynamicallyunfavorableSN 2substitutionofadisulfidewithweaknucleophilicthiols.Uponremovalof load,thebondingreturnedtotheoriginaldisulfideoflowerenergy.Reversibilityis criticalinmechanical-responsivematerialssothatperturbationscanberepeatedin ahighlydynamicmanner.
GoingbacktoBell’smodel,asabondundergoesconstantthermalfluctuations, howfasttheforceisappliedrelativetothethermalfluctuationaffectsthebreaking force.Therefore,bond-breakingforceisdependentontheforce-loadingrate (df /dt).Thisdependencycanbecharacterizedintothreeregimes,thespontaneous, force-assisted,andactivationlessregimes,asshowninFigure1.8[52,53].When theloadingrateislowrelativetobondthermaldissociationrate,bondsbreak spontaneouslyduetothermalfluctuations.Thisiscalledaspontaneousregime.In thisregion,thebreakingforceislowandindependentoftheforcerate.Bondswith rapidthermaloscillationsandshortbondlifetime,suchashost–guestinteractions, fallwithinthisregime[54].Asforcerateincreases,itbecomessufficientlyfastthat thebondisstretchedtosomeextentbeforebreaking.Thisiscalledtheforce-assisted regime,andthebreakingforceincreasesrapidlywithforcerate.Thishasbeen observedforavidin–biotincomplex[55],H-bondedcarboxylicacidgroups[56],and gold–octanedithiol–goldlinks[57].Intheactivationlessregimeunderhigh-loading rate,theenergybarriertobreakingisreducedtozeroasthebondismaximally stretched,andbondbreakssolelybytheappliedforce.Inthisregion,thebreaking forcereachesthehighestandbecomesconstantagain.Whilesingle-moleculeexperimentsallowustovalidatethetheoreticalpredictions,cautionmustbeusedwhen comparingtheruptureforcevalues,whicharehighlydependentonexperimental

Figure1.7 (a)Unfoldinganddisulfide(SS)reductionforanengineeredproteinwitha preciselypositioneddisulfidebond.(b)Rateofreductionofthedisulfidebondasafunction ofappliedforcemeasuredbyforce-clampAFM.Concentrationofthereducingagent,DTT,is keptconstantat12.5mM.(c)Calculatedenergylandscapewithandwithoutforces.A 400pNforcereducestheactivationenergyofdisulfidereductionby8.2kJ/mol.Source: Wiitaetal.[50]/withpermissionofNationalAcademyofSciences.
conditions.AcomprehensivereviewbyRibas-ArinoandMarxsummarizedthe single-moleculespectroscopyandthetheoreticaltreatmentsingreatdetail[58].
Inthecontextofbulkpolymers,sensitivityofthemechanoresponsivebonds becomesdependentonforcetransductionandheterogeneitiesofforcedistribution. Forasinglechain,theweakerbondsbreakfirstfollowedbythestrongerbonds.The ruptureforcesforcommoncovalentbondsarewithinafewnN(Figure1.9)[59]. Fornoncovalentinteractions,theruptureforcesareinamuchlowerrange,around 10–100pN[58].Whenmechanocleavablebondsareplacedwithinapolymerstrand, however,theforcetransductioncanbefarmorecomplicated.Anoften-askedquestioniswhichbondismostlikelytorupturewithinapolymericmaterialunder mechanicaldeformation.Thisisdeterminedbyboththebondstrengthandthe

Figure1.8 Dependenceofbond-breakingforceonforcerate.(a)Host-guestcomplex representativeofspontaneousbreaking.(b)Bindingsbetweenbiomoleculessuchasavidin andbiotinrepresentativeofforce-assistedbreaking.(c)Hydrogenbondingbetween carboxylicacidsfallswithinthetransitionregimefromspontaneoustoforce-assisted breaking.(d)Gold-octanedithiol-goldinteractionfallswithinthetransitionregimefrom force-assistedtoactivationlessbreaking.(e)breakingofthebondbetweentwogoldatoms atroomtemperaturespansacrosstheentirespectrum.Source:Pobelovetal.[52]/with permissionofSpringerNature.
Figure1.9 Bondruptureprobabilitydensities(nN 1 )asafunctionofforce F (nN) calculatedbydensityfunctionaltheory.Force-loadingrateis10nN/s.Source:Beyer [59]/withpermissionofAmericanInstituteofPhysics.
forcedistribution.Theformercanbepredictedormeasured,thelatterisanisotropic withinapolymericmaterial.Onestudyincorporatedcoumarindimer(CD)into specificlocationsofwell-definedpolymersasameanstodeterminetheroleof mechanophorespatialdistributionontheefficiencyofmechanoactivation[60]. Theexperimentwascarriedoutinsolutionunderultrasonication.WhenCDwas incorporatednearthecenterofachain,selectivebreakingoftheCDdimmer
Figure1.10 Color-changingmechanoresponsivefibersconfirmthestresspatterns predictedbycontinuumsimulationsforthetrefoilknot(a)andthefigure-of-eightknot (b)duringthetighteningprocess.ThecoloriscodedintheCIE1931XYZcolorspace. Approximatelyredendofthespectrumcorrespondstolowstrainandtheblueend correspondstohighstrain.Source:ReproducedwithpermissionfromPatiletal. [63]/AmericanAssociationfortheAdvancementofScience AAAS.
occurred.However,whentheCDwaslocatedawayfromthechaincenter,random cleavagesofbackbonebondsweredominant.Inthesolidstate,forcetransduction isfurthercomplicatedbyentanglements,knots,andotherdefects.Earlystudies suggestedthatthehigheststressisformedattheentrancepositiontotheknot[61]. However,thechainsconsideredinthissimulationarerelativelyshort;thus,itis possiblethatbeingclosetothechainendalsocontributestothehighstressatthe immediatevicinityoftheknot.Whenpolyethyleneof40carbonatomsissimulated forend-to-endstretching,thestressisconcentratedatthetorsionsaroundthe curvedpartoftheknot[62].Amechanisticstudyoftyingropesintodifferent topologies[63]mayshedsomelightonthisdisagreement.Figure1.10a,bshowsa color-changingphotonicfiberwithtwodifferentknots.Thefiberiscoatedwitha periodiccladding.Whentheknotistightened,thicknessofthecladdingchanges uponelongationorbending,leadingtocolorvariations.Notethisisastructural coloranddoesnotinvolveanymechanochemistry.Movingfromtheredtothe blueendofthecolorspectrum,bothstrainandstressincrease,andthestressis localizedatthecurvedpartsofthetightenedknot.Thestimulationresultsshowa similarstressdistributionpattern.Interestingly,thestudyshowedthatthestress localizationwithintheknotcanbemoresubstantialforcertainknotsthanothers underthesamepullingforce.Ifwedrawananalogyofentangledpolymerchains withknottedropes,onecanclearlyseetheextentofanisotropyinstressdistribution.Additionally,cumulativeforcesofnumerousintermolecularinteractions withcertainconfigurationscanshieldaweakbondfrommechanicalactivation. Crystallinedomains,covalentcrosslinks,physicalcrosslinks,andtopological defects,suchasloops,canfurthercontributetotheheterogeneities.Theeffectsof sidegroups,backbonerigidity,backbonecreep,andrelaxationcannotbeneglected either.Furthermore,aninterestingbehavioristhatthecleavablebondsdonothave todirectlyalignwiththepullingaxis.ThisisfurtherdiscussedinSection1.4ofthis
1.4PolymerCovalentMechanochemistry 15 chapter.Therefore,thecomplexandrichbehaviorsofpolymermechanochemistry areanareatobefurtherexplored.
1.4PolymerCovalentMechanochemistry
Inthissectionofthechapter,wewillconsidermechanicalactivationofnumerous mechanophoresbasedoncovalentbondswithinpolymericmaterials.Suchlabile covalentbondsincludeC—C,C-heteroatom(e.g.C—O,C—S,C—N,C—Cl, andC—Br),andheteroatom–heteroatom(e.g.O—O,S—S,andSe—Se)bonds. Thecovalentmechanophoresdescribedinthissectionareclassifiedaspyrans, retro-cycloadditions,ladderenes(LDEs),stableradicalsystems,andothertypes.The mechanochemicalrupturecangenerallyoccurviapericyclic(e.g.electrocyclicring openingofpyransandretro-cycloadditions),homolytic(e.g.radicalformations),or heterolytic(e.g.benzoxazole[Bz’s]esterbondcleavage)reactions.Theoccurrences ofmechanochemistryareoftenassociatedwithdistinctivechangesinmaterials’ propertieswhichcanbeutilizedtoperformcertainfunctions.Forexample, colorchangeandfluorescentemissionsareinvolvedinmechanoactivationof pyran-basedcompounds.Suchmechanophoresaretermedmechanochromophores andmechanofluorophores,andtheyenabledthedetectionandmappingofstresses inpolymermaterials[64–67].Mechanoradicalsaregeneratedviahomolyticcleavageofcovalentbondsunderforceandhavebeenemployedinpolymerizationsto enableself-healingproperties[68].Ultrasonication-inducedselectivecleavageof disulfide-centeredpolymersgavethiol-terminatedpolymerchains,whichcould thenundergothia-MichaeladditiontoDiels–Alderadductsoffuran-functionalized drugsandacetylenedicarboxylates,followedbyaretro-Diels–Alderreactionin whichtheliberationofasmallmoleculardrugoccurred[69].Likewise,another studyreportedonareleaseofanalkaloid-typeanticancerdrugbyanintramolecular cyclizationofmechanochemicallygeneratedthiol-terminatedpolymers[70].
Constrained-geometry-simulatingexternalforce(CoGEF)[59]thatisbasedon densityfunctionaltheory(DFT)hasbeencarriedouttocomputationallydetermine thethresholdforcesrequiredtoactivatecovalentmechanophores.InthisCoGEF method,thesystembeginswithanunstrainedstateofthemechanophore.Two anchoringpointsfromtheoppositesidesofthemechanophorearethenselected,the distancebetweenthesetwopointsisincreasedinsmallsteps,andthegeometrywith theminimumenergyisidentifiedforeachelongationstep.Aplotofrelativeenergy versusequilibriumdisplacementissubsequentlyconstructed,andthethreshold forceisextractedfromtheslopeoftheplotjustbeforethebondcleavageoccurs. Thethresholdactivationforcesfornumerousmechanophoreshavebeenassessed viaCoGEFcalculations,andthepredictivepowerofthismethodforpolymer mechanochemistryhasbeendemonstrated[71].Forarangeofmechanophores,the CoGEFpredictionsareingoodagreementwiththeexperimentalvaluesobtained fromthesingle-moleculeforcespectroscopymeasurements.Inthissection,we willfocusondiscussingthechemistriesinvolvedandthetunabilityofthebond dynamics.
1.4.1Pyran-BasedMechanochromophores
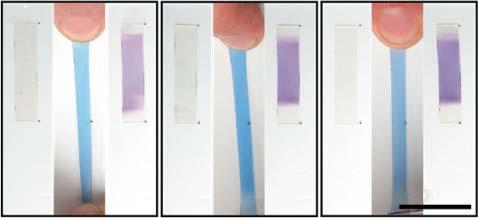
Spiropyran(SP)isawell-knownphotochromicmolecule.UnderUVradiation,the colorlessandnonfluorescentSPundergoes6π electrocyclicringopeningtoform merocyanine(MC)species(Scheme1.1).TheincreaseinconjugationinMCshifts theabsorptiontolongerwavelengths,givingrisetovisiblecolorandfluorescence [72].RingopeningofSPcanalsobemechanicallyactivatedwhenincorporatedinto polymerbackbonesbytetheringitstwoends.Underuniaxialstress,theconversionofSPtoMCresultedinacolorchangeofthepolymerfromyellowtopurple andtoredoncefailed[72].SP-functionalizedpolymericmaterialshavealsoshown mechanochromisminducedbygrinding,ultrasonication[73],andshearstress[74]. EventhoughSPincorporatedpolymersaretypicallycolorlessoryellow,theirMC formscanshowdifferentcolors,suchasblue,purple,orred,underdifferentstrains. AsshowninFigure1.11,thepolymerturnedbluewhenstretched.Uponrelease, itscolorchangedtopurpleviatheisomerizationofthemethinebridgethatlinks thetwocycliccomponents[75].Thecolorlessformisrestoredafterradiationwith visiblelight.
Itiscrucialthatthetensilestressisappliedacrossthespiro-junctiontopull aparttheindolineandbenzopyranmoietiestoexclusivelycleavetheCspiro —O bond.Thiscanbeachievedbytetheringthepolymerchainstotheopposingsides ofthespiro-junction,suchaspositions7or8ofthebenzopyranand5′ or6′ of theindoline[72].FunctionalizingSPatotherpositions,suchas1′ and5′ ofthe indolinecomponent,preventsthemechanicalforcetransductionacrossthereactive
Electrocyclicringopeningofspiropyrantoformmerocyanine.
Figure1.11 Originalspiropyransampleiscolorless(a)andturnsbluewhenstretched(b). Oncethesampleisrelaxed,itturnspurple(c).Source:Reproducedwithpermissionfrom Gossweileretal.[75]/AmericanChemicalSociety.
Spiropyran (SP)Merocyanine (MC)
Scheme1.1
Cspiro —Obond,althoughSPtoMCtransformationcanstilltakeplacebyheating orUVradiation[76].Regiochemicaleffectsonmechanicalactivationhavebeen quantifiedbyusingtwoSPregioisomersviasingle-moleculeforcespectroscopy measurements[77].Inoneisomerthepolymerchainsweretetheredtopositions5′ and8,whileinthesecondisomerthepolymerchainswereattachedtopositions8 andindole’sNatom,andthethresholdforcesforactivationweredeterminedtobe 260and240pN,respectively.Therateofmechanochromismisgovernedbyseveral factorsthatincludethenatureofthematerials(e.g.elastomericpolymershave demonstratedfasterdecayofthecolorchangethanthoseofglassyones)[72],local environment(e.g.temperatureandplasticizers)[78],andsubstituentsontheSP (i.e.thehighertheelectrondensityonthearomaticringofbenzopyran,thefaster theringclosure)[79].
Spirothiopyran(STP),anSPanalog,displaysbothmechanochromismand stress-inducedadditionreactionsrenderingitaversatilemechanophore.Ring openingofthethiopyranringformsthecorrespondingthiomerocyanine(TMC) initsthiophenolateform(Scheme1.2),whichcantakepartinthiol-eneclick reactionsinthepresenceofolefinicdoublebonds.Ultrasonication-activated polyester-functionalizedSTPshowedyellowtogreencolorchangebecauseof theformationoftheTMCform;however,thegreencolorquicklyfadedinthe presenceof N -methylmaleimideduetothethiol-eneclickreaction[80].When N -methylmaleimideisreplacedwiththebifunctional1,6-bismaleimidehexnae crosslinker,mechanical-inducedcrosslinkingofthelinearpolymerswasachieved, givinginsolublenetworks.
Mechanochromismcanbeaffectedbybothelectronicandstericeffects.This isshownfornaphthopyran(NP)inScheme1.3.Theactivatedmerocyaninesnot onlyexhibitdifferentcolorsbutalsotheirthresholdforcesvarydependingonthe natureofthesubstituents.NPspecies(Scheme1.3a)formsayellowMC,whereas NPmodifiedwithelectron-donatingpyrrolidine(Scheme1.3b)givesapurpleMC [81].CoGEFcalculationsindicatethatelectron-donatingandbulkypyrrolidine decreasestheactivationforceformechanicalscission[71].Oncethemechanical forceiseliminated,heat-inducedringclosureresultsinthereformationofNPand NP-Pyr.
AsistrueforSPandNP,oxazine(OX)-derivedmechanophoresalsodemonstrate regioisomer-dependentmechanochromism[82].AsshowninScheme1.4,thebulk
Scheme1.3 Electrocyclicringopeningof(a)naphthopyrans(NP)and (b)pyrrolidine-appendednaphthopyrans(NP-Pyr).
Scheme1.4 Electrocyclicringopeningofoxazinetogivezwitterionicindoliumspecies.
polymer-embeddedOXcoreundergoesmechanicalstress-inducedpericyclicring openingtogenerateacolored,zwitterionicindoliumisomer,whichrevertstothe ring-closedformuponremovalofthetensilestresswithinlessthanasecond.This markedlyfastermechanoresponsivenessofOXwithoutanyphaselagorfatiguecomparedtoSPorNPisstriking.ItshouldbenotedthattheringopeningofbothSP andNPisfollowedbyadouble-bondisomerizationprocesstoformthecorresponding trans-MCs,butsuchanisomerizationdoesnottakeplacewithOX,whichmight explainOX’sfastermechanoresponse.IncomparisontoSPorNP,OXmechanophore mayholdbetterpromiseasamolecularforceprobesinceOXhasashortercleavage displacement(e.g.SPandNPframeworksneedtobestretchedaboutthriceasmuch asOXforthebondrupture)aswellasitslesscomplicatedring-openingprocesswithoutthedouble-bondisomerization.
Theabove-mentionedmechanophoresallcontainonepyranringandgivethe samemechano-andphoto-product.Mechanochromicbis(naphthopyran)(BNP) featuringdoublyclosedpyranring(BNPC–C )configurationexhibitsadifferent
Scheme1.5 Electrocyclicringopeningofbis(naphthopyran).UVirradiationleadsto stepwiseringopeningviaBNPO–C ,whilemechanicalforcedirectlytransformsBNPC–C to BNPO–O
mechanochemicalpathway[83].AsshowninScheme1.5,undercontinuousUV irradiation,BNPC–C undergoesringopeningofoneofthepyranringstofirstform anopen–closedBNPO–C MCspecies,followedbyafullyopenMCform(BNPO–O ).In contrasttothissequentialphotochemicalring-openingprocess,undermechanical activation,anequilibriumofBNPC–C ,BNPO–C ,andBNPO–O isreachedbutthrough adifferentmechanism.BNPO–O isformeddirectlyfromBNPC–C bymechanical activation;therefore,itsconcentrationisforcedependent.BNPO–C isproduced predominatelyfromthethermalelectrocyclizationofBNPO–O .Meanwhile,BNPO–C canbemechanicallyactivatedtogenerateBNPO–O again.Therefore,thedistribution ofBNPO–C andBNPO–O varieswiththemagnitudeofforce.SincethetwoMC productshavedistinctlydifferentabsorptionsinthevisiblerange,BNPexhibits gradientmulticolormechanochromism,whichcanbeusedasastresssensor. Systematicstudiesofthesecompoundshavebeenconductedtoillustratethe substituenteffects[43,71],stereochemistry,andregioselectivity[71].Computation andexperimentalstudiesallowustounderstandmechanismsofmechanochemical reactions,asdiscussedinSection1.3.
1.4.2Retro-Cycloadditions
Inacycloadditionreaction,two π reactantsreacttoformacyclicadductthat containstwonew σ bonds.Theadductsaresusceptibleforreversereactions(i.e.
BNPO–C
BNPC–C
BNPO–O
1FromMechanochemistrytoMechanoresponsiveMaterials retro-cycloadditions)undersufficientmechanicalforce.Here,wewillsummarize theuseofretro-cycloadditionsindesigningpolymericmaterialsforsensing,release ofsmallmolecules,gatedringopening,anddegradablematerials.
Anthracene-derivedDiels–Alderadductshavebeenwidelyusedasmechanofluorophoresbecauseoftheirhighfluorescentquantumyields.Anthracene dimers[84],anthracene–maleimideadducts[85–88],aswellas π-extended anthracene–maleimideadducts[89]incorporatedintopolymerscanbemechanicallyactivated.Theliberationoffluorescentanthracenederivativestakesplacevia stress-inducedretroDiels–Alderoftheanthraceneadducts,whichinturnleads tothescissionofthepolymerchains.Usingthisconcept,anthracene–maleimide adductshavebeenemployedasdamagesensorsatheterointerfaces[90,91]. Ananthracene–maleimideadduct-embeddedpolymerchainwasgraftedonto thesurfaceofsilicananoparticles.Here,anthracenespecieswasliberatedvia ultrasound.Thisshowedtheselectiveruptureoractivationofmechanophores undermechanochemicalstressataheterointerface.Themechanochemical activationof π-extendedanthracene–maleimideadducts(Scheme1.6a)isparticularlyinterestingbecausetheygiveafluorescencequantumyieldof0.72,which isabout2ordersofmagnitudehigherthanthosereportedformerocyanines derivedfromspiropyrans[89].Besides,thefluorescenceisstableinthepresenceofexcessoxygenandthesystemisnotsusceptibletothermalreversibility. Theappropriatefunctionalizationofthismechanofluorochromophore(viathe substituentsinanthracene)givestheopportunitytotuneexcitationandemissionwavelengthswhilemaintainingmechanochemicalpropertiesofthesystem. Thishighlysensitive π-extendedanthracene–maleimideadductwasusedasa crosslinkerinapoly(N -isopropylacrylamide)hydrogelnetworktodetectand accuratelylocalizethecovalentbondscissioninducedbyaneedle-puncturing (hand)[92].Anthracene–maleimideadductshavebeenemployedasdamage sensorsinpolymersusingthematheterointerfaces[90,91].Forexample,an anthracene–maleimideadduct-embeddedpolymerchainhasbeenlinkedtoa surfaceofsilicananoparticles,andultrasoundactivationofthemechanophorehas ledtotheliberationoftheanthracenespeciestherebyensuringtheruptureofthe polymerchain.
Mechanochemicalactivationofthemechanophore-centeredpolymerchains notonlyyieldsopticalresponsesbutalsocandisplaychemicalresponsesbythe releaseofsmallmolecularcargos,suchasfurans[93,94],coumarin[95],and phenyltriazolinedione[75].Adifunctionalcrosslinkercomprisingmechanophore oxanorbornadiene,whichisaDiels–Alderadductoffuranandacetylenedicarboxylate,wasincorporatedintoapolymethylacrylate(PMA)matrixinwhich compression-inducedretro-[4 + 2]Diels–Alderreactionliberatedthebenzyl furfurylethersmallmolecules(Scheme1.6b)[93].Inthisexample,thecleaved covalentbondswerenotanintegralpartofthepolymermainchain,which isquitecounterintuitive.Theactivationoccurredbybondbendinginsteadof stretching.Hencetheoverallpolymerarchitectureisretainedintact,whileindeed reinforcingthebondsinthepolymerbackbone.Force-accelerateddissociation ofunloadedbondsalsooccurredforphosphotriesters[96].Inanotherstudy,