Visit to download the full and correct content document: https://ebookmass.com/product/nanocolloids-for-petroleum-engineering-fundamentals -and-practices-baghir-suleimanov/
More products digital (pdf, epub, mobi) instant download maybe you interests ...
Formulas And Calculations For Petroleum Engineering 1st Edition Edition Cenk Temizel
https://ebookmass.com/product/formulas-and-calculations-forpetroleum-engineering-1st-edition-edition-cenk-temizel/
Environmental Engineering Principles and Practices (Water Supply Engineering) N. S. Varandani
https://ebookmass.com/product/environmental-engineeringprinciples-and-practices-water-supply-engineering-n-s-varandani/
Methods and Applications in Petroleum and Mineral Exploration and Engineering Geology Said Gaci
https://ebookmass.com/product/methods-and-applications-inpetroleum-and-mineral-exploration-and-engineering-geology-saidgaci/
Fundamentals of Nuclear Science and Engineering 3rd Edition
https://ebookmass.com/product/fundamentals-of-nuclear-scienceand-engineering-3rd-edition/
Practical Petroleum Geochemistry for Exploration and Production 1st Edition Edition Harry Dembicki (Auth.)
https://ebookmass.com/product/practical-petroleum-geochemistryfor-exploration-and-production-1st-edition-edition-harrydembicki-auth/
Fundamentals of Enhanced Oil Recovery Methods for Unconventional Oil Reservoirs (Developments in Petroleum Science, Volume 67) 1st Edition Dheiaa Alfarge
https://ebookmass.com/product/fundamentals-of-enhanced-oilrecovery-methods-for-unconventional-oil-reservoirs-developmentsin-petroleum-science-volume-67-1st-edition-dheiaa-alfarge/
Engineering Fundamentals: An Introduction to Engineering, SI Edition Saeed Moaveni
https://ebookmass.com/product/engineering-fundamentals-anintroduction-to-engineering-si-edition-saeed-moaveni/
Engineering Fundamentals: An Introduction to Engineering, 6th Edition Moaveni Saeed
https://ebookmass.com/product/engineering-fundamentals-anintroduction-to-engineering-6th-edition-moaveni-saeed/
Surveying fundamentals and practices 6th Edition Jerry A. Nathanson M.S. P.E.
https://ebookmass.com/product/surveying-fundamentals-andpractices-6th-edition-jerry-a-nathanson-m-s-p-e/
NanocolloidsforPetroleum Engineering
NanocolloidsforPetroleumEngineering FundamentalsandPractices
BaghirA.Suleimanov
OilandGasScientificResearchProjectInstitute StateOilCompanyofAzerbaijanRepublic(SOCAR) Baku,Azerbaijan
ElchinF.Veliyev
OilandGasScientificResearchProjectInstitute StateOilCompanyofAzerbaijanRepublic(SOCAR) Baku,Azerbaijan
VladimirVishnyakov UniversityofHuddersfield Huddersfield,UK
Thiseditionfirstpublished2022 ©2022byJohnWiley&SonsLtd.Allrightsreserved.
Allrightsreserved.Nopartofthispublicationmaybereproduced,storedinaretrievalsystem,ortransmitted, inanyformorbyanymeans,electronic,mechanical,photocopying,recordingorotherwise,exceptas permittedbylaw.Adviceonhowtoobtainpermissiontoreusematerialfromthistitleisavailableat http://www.wiley.com/go/permissions.
TherightofBaghirA.Suleimanov,ElchinF.VeliyevandVladimirVishnyakovtobeidentifiedastheauthors ofthisworkhasbeenassertedinaccordancewithlaw.
RegisteredOffices
JohnWiley&Sons,Inc.,111RiverStreet,Hoboken,NJ07030,USA
JohnWiley&SonsLtd,TheAtrium,SouthernGate,Chichester,WestSussex,PO198SQ,UK
EditorialOffice
TheAtrium,SouthernGate,Chichester,WestSussex,PO198SQ,UK
Fordetailsofourglobaleditorialoffices,customerservices,andmoreinformationaboutWileyproductsvisit usatwww.wiley.com.Wileyalsopublishesitsbooksinavarietyofelectronicformatsandbyprint-ondemand.Somecontentthatappearsinstandardprintversionsofthisbookmaynotbeavailableinother formats.
LimitofLiability/DisclaimerofWarranty
Inviewofongoingresearch,equipmentmodifications,changesingovernmentalregulations,andthe constantflowofinformationrelatingtotheuseofexperimentalreagents,equipment,anddevices,thereader isurgedtoreviewandevaluatetheinformationprovidedinthepackageinsertorinstructionsforeach chemical,pieceofequipment,reagent,ordevicefor,amongotherthings,anychangesintheinstructionsor indicationofusageandforaddedwarningsandprecautions.Whilethepublisherandauthorshaveusedtheir besteffortsinpreparingthiswork,theymakenorepresentationsorwarrantieswithrespecttotheaccuracyor completenessofthecontentsofthisworkandspecificallydisclaimallwarranties,includingwithout limitationanyimpliedwarrantiesofmerchantabilityorfitnessforaparticularpurpose.Nowarrantymaybe createdorextendedbysalesrepresentatives,writtensalesmaterialsorpromotionalstatementsforthiswork. Thefactthatanorganization,website,orproductisreferredtointhisworkasacitationand/orpotential sourceoffurtherinformationdoesnotmeanthatthepublisherandauthorsendorsetheinformationor servicestheorganization,website,orproductmayprovideorrecommendationsitmaymake.Thisworkis soldwiththeunderstandingthatthepublisherisnotengagedinrenderingprofessionalservices.Theadvice andstrategiescontainedhereinmaynotbesuitableforyoursituation.Youshouldconsultwithaspecialist whereappropriate.Further,readersshouldbeawarethatwebsiteslistedinthisworkmayhavechangedor disappearedbetweenwhenthisworkwaswrittenandwhenitisread.Neitherthepublishernorauthorsshall beliableforanylossofprofitoranyothercommercialdamages,includingbutnotlimitedtospecial, incidental,consequential,orotherdamages.
Forgeneralinformationonourotherproductsandservicesorfortechnicalsupport,pleasecontactour CustomerCareDepartmentwithintheUnitedStatesat(800)762-2974,outsidetheUnitedStatesat(317)5723993orfax(317)572-4002.
Wileyalsopublishesitsbooksinavarietyofelectronicformats.Somecontentthatappearsinprintmay notbeavailableinelectronicformats.FormoreinformationaboutWileyproducts,visitourwebsiteat ww.wiley.com.
LibraryofCongressCataloging-in-PublicationDataappliedfor: HardbackISBN:9781119889595
CoverDesign:Wiley
CoverImage:©sharply_done
Setin9.5/12.5ptSTIXTwoTextbyStraive,Pondicherry,India
Contents
Acknowledgments ix
Introduction xi
PartANanocolloids – AnOverview 1
1NanocolloidClassification 3
1.1WhatisaColloid? 3
1.1.1ColloidClassification 3
1.1.2ColloidEvaluation 4
1.2WhatisaNanocolloid? 5
2NanocolloidProperties 7
2.1DifferentKindsofInteractionsinNanocolloids 7
2.1.1VanderWaalsInteractions 7
2.1.2ElectrostaticInteraction 7
2.1.3Elastic–StericInteraction 8
2.1.4HydrophobicInteraction 8
2.1.5SolvationInteraction 8
2.1.6DepletionInteraction 8
2.1.7MagneticDipole–DipoleInteraction 8
2.1.8OsmoticRepulsion 9
2.2TheStabilityofNanocolloids 9
2.3RheologyofNanocolloids 10
2.3.1EffectofNanoparticleInteractionontheColloidRheology 10
2.3.2EffectofNanoparticleMigrationontheColloidRheology 14
2.4SurfaceTension.Wettability 21
2.4.1WettabilityAlteration 21
2.4.2SurfaceTension 23
Nomenclature 25 References 27
PartBReservoirDevelopment 33
3ReservoirConditionsforNanocolloidFormation 35
3.1In-SituFormationofNanogasEmulsions 35
3.1.1StabilityoftheSubcriticalGasNuclei 35
3.2In-SituFormationofNanoaerosoles 38
3.2.1StabilityoftheSubcriticalLiquidNuclei 39
4NanogasEmulsionsinOilFieldDevelopment 41
4.1HydrodynamicsofNanogasEmulsions 41
4.1.1FlowMechanismofGasifiedNewtonianLiquids 41
4.1.2FlowofGasifiedNewtonianLiquidsinPorousMediaatReservoirConditions 52
4.2HydrodynamicsofNanogasEmulsionsinHeavyOilReservoirs 60
4.2.1FlowMechanismofGasifiedNon-NewtonianLiquids 60
4.2.2FlowofGasifiedNon-NewtonianLiquidsinPorousMediaatReservoir Conditions 66
4.3FieldValidationofSlippagePhenomena 73
4.3.1Steady-StateRadialFlow 73
4.3.2UnsteadyStateFlow 86
4.3.3ViscosityAnomalyNeartothePhaseTransitionPoint 95
5NanoaerosolesinGasCondensateFieldDevelopment 107
5.1StudyoftheGasCondensateFlowinaPorousMedium 107
5.2MechanismoftheGasCondensateMixtureFlow 111
5.2.1RheologyMechanismoftheGasCondensateMixtureDuringSteady-State Flow 112
5.2.2MechanismofPorousMediumWettabilityInfluenceontheSteady-StateGas CondensateFlow 120
5.2.3MechanismofPressureBuild-UpattheUnsteady-StateFlowoftheGas Condensate 121
5.2.4ConcludingRemarks 124
Nomenclature 125 References 127
PartCProductionOperations 131
6AnOverviewofNanocolloidApplicationsinProductionOperations 133
7NanosolforWellCompletion 137
7.1TheInfluenceoftheSpecificSurfaceAreaandDistributionofParticlesonthe CementStoneStrength 139
7.2TheInfluenceofNano-SiO2 andNano-TiO2 ontheCementStoneStrength 140
7.3RegressionEquation 141
7.4ConcludingRemarks 142
8NanogasEmulsionforSandControl 145
8.1FluidizationbyGasifiedFluids 145
8.1.1CarbonDioxideGasifiedWaterasFluidizingAgent 146
8.1.2NaturalGasorAirGasifiedWaterasFluidizingAgent 149
8.2ChemicalAdditivesImpactontheFluidizationProcess 151
8.2.1Water–AirMixtureswithSurfactantAdditivesasFluidizingAgent 151
8.2.2FluidizationbyPolymerCompositions 152
8.3MechanismofObservedPhenomena 153
9VibrowaveStimulationImpactonNanogasEmulsionFlow 157
9.1ExactSolution 158
9.2ApproximateSolution 161
9.3ConcludingRemarks 162
Nomenclature 165 References 167
PartDEnhancedOilRecovery 171
10AnOverviewofNanocolloidApplicationsforEOR 173
10.1CoreFloodingExperimentsFocusedonDispersionPhaseProperties 174
10.2CoreFloodingExperimentsFocusedonDispersionMediumProperties 175
11Surfactant-BasedNanofluid 177
11.1NanoparticleInfluenceonSurfaceTensioninaSurfactantSolution 177
11.2NanoparticleInfluenceontheSurfactantAdsorptionProcess 178
11.3NanoparticleInfluenceonOilWettability 179
11.4NanoparticleInfluenceonOpticalSpectroscopyResults 179
11.5NanoparticleInfluenceontheRheologicalPropertiesofNanosuspension 182
11.6NanoparticleInfluenceontheProcessesofNewtonianOilDisplacementin HomogeneousandHeterogeneousPorousMediums 183
11.7ConcludingRemarks 186
12NanofluidsforDeepFluidDiversion 187
12.1Pre-formedParticleNanogels 187
12.1.1NanogelStrengthEvaluation 189
12.1.2KineticMechanismofGelation 191
12.1.3CoreFloodingExperiments 192
12.1.4ConcludingRemarks 197
12.2ColloidalDispersionNanogels 197
12.2.1Rheology 198
12.2.2AgingEffect 200
12.2.3InterfacialTension 200
12.2.4ZetaPotential 202
12.2.5ParticleSizeDistribution 203
Contents
12.2.6ResistanceFactor/ResidualResistanceFactor 204
12.2.7ConcludingRemarks 206
13NanogasEmulsionsasaDisplacementAgent 207
13.1OilDisplacementbyaNewtonianGasifiedFluid 207
13.2OilDisplacementbyaNon-NewtonianGasifiedFluid 208
13.3MechanismofObservedPhenomena 210
13.4FieldApplication 213
Nomenclature 217
References 219
14MetalStringComplexMicroandNanoFluids 227
14.1WhatareMetalStringComplexes? 227
14.2ThermalConductivityEnhancementofMicrofluidswithNi3(μ3-ppza)4Cl2 Metal StringComplexParticles 228
14.2.1MicroparticlesofMSCNi3(μ3-ppza)4Cl2 229
14.2.2Ni3 Microfluid 230
14.2.3FluidStability 230
14.2.4ThermalConductivity 232
14.2.5Rheology 235
14.2.6SurfaceTension 235
14.2.7FreezingPoints 236
14.2.8ConcludingRemarks 237
14.3ThermophysicalPropertiesofNano-andMicrofluidswithNi5(μ5-pppmda)4Cl2 MetalStringComplexParticles 237
14.3.1MicroparticlesoftheMetalStringComplexNi5(μ5-pppmda)4Cl2 238
14.3.2Micro-andNanofluidPreparations 239
14.3.3FluidStability 240
14.3.4ThermalConductivity 242
14.3.5Rheology 245
14.3.6SurfaceTension 245
14.3.7FreezingPoints 249
14.3.8ConcludingRemarks 249
Nomenclature 251 References 253
AppendixADeterminationofDispersed-PhaseParticleInteractionInfluenceon theRheologicalBehavior 259
AppendixBDeterminationofInflectionPoints 263 References 267 Index 269
Acknowledgments Theauthorswouldliketorecognizethecontributioninexperimentalstudiesofthe followingpeople:Dr.ElhanM.Abbasov,Dr.HakimF.Abbasov,Dr.OlegA.Dyshin, andDr.RayyetH.Ismayilov.
Theauthorsalsothanktheirfamiliesforthesupport,patience,andunderstandingthey haveshownduringthepreparationofthisbook.
Introduction Incontemptofgrowinginvestmentsinrenewableenergy,theoilindustrystillstaysthe mainsourceofenergyintheworld.Itiswellknownthatthegreatestpartofoilresources isstillmarkedasunrecoverableduetothelimitationofconventionaloilrecoverymethods. Thenumberofexploredoilfieldsalreadyovertakethefieldstobeexplored.Inthisregard, theincrementinoilproductionofmatureoilfieldsisverycrucial.Theoilindustrytodayis standinginthefrontierofnewpioneeringachievements.Withahighprobabilityitcanbe arguedthattheseachievementswillbemadeandhavealreadybeencommittedtolaboratories.Anoilpricedecreasehasalreadyreducedeconomicbenefitsofenhancedoilrecovery (EOR)methodsduetoCAPEXincrements.Thesamechallengesarelyingaheadofall upstreamtechnologies,suchascompletion,workover,sandcontrol,etc.Theapplication ofnewnano-basedmaterialscouldopennewopportunitiesandfindnewdecisionsforconventionalproblems.
Thisbookaimstocoveratheoreticalandpracticalbackgroundrelatedtonanocolloid applicationexperiencegainedoverthelastdecades.Nanocolloidsareadmittedbythe majorityofresearchersasanewperspectiveandapromisinginvestigationtopic.Thehigh surfaceareaofdispersionphasecausesamorereactivebehaviorcomparedtoconventional counterpartsandsignificantlychangesthepropertiesofcolloidsystems.Forinstance,the propagationabilityofnanogelsconsiderablyincreasesanopeningofnewopportunitiesfor in-depthfluiddiversiontechniquesaswellasgeldurabilityinreservoirconditions.The bookconsistsoffivepartsdividedintochapterstomaketheexperienceofreadingthebook morereader-friendly.Theparagraphsthatfollowbrieflydescribeeachpartandhighlight themaindiscussedtopics.
PartA.Nanocolloids – AnOverview consistsoftwochaptersdevotedtoabrief introductionandclassificationofcolloidsystems.Itwaspresentedandexplainedtheterm “nanocolloid. ” Thechaptersdescribethemainpropertiesofnanocolloidscrucialfor practicalapplicationsinpetroleumengineering:forexample,stability,rheological behavior,surfacetension,andwettability.
PartB.ReservoirDevelopment consistsofthreechaptersdevotedtonanocolloid applicationsinreservoirengineering.Thechaptersdescribereservoirconditionsnecessary fornanocolloidformation.Nanogasemulsionhydrodynamicsatreservoirconditionshave beendescribedindetail.Fieldvalidationresultsoftheproposedkineticmechanisms accompaniedbytechnicalrecommendationsforsuccessfulimplementationwerealso presented.
PartC.ProductionOperations consistsoffourchaptersdevotedtonanocolloid applicationsinproductionoperations.Thechaptersdescribethemechanismofthe nanoscaledispersionphaseimpactonphysicalpropertiesofconventionalsubstances utilizedinupstreamprocesses.Particularly,themechanismofPortlandcementreinforcementinthepresenceofnanoparticleswasdescribedandverified.Nanogasemulsionswere investigatedintermsofsandcontrolapplicationsbasedonfluidizationphenomena.The vibrowavestimulationimpactonnano-gasemulsionflowwasalsoreported.
PartD.EnhancedOilRecovery consistsoffourchaptersdevotedtonanocolloidapplicationsforEOR.Thechaptersdescribetheimpactofnanoparticlesonconventionaldisplacementagents.Forinstance,theadditionofnanoparticlessignificantlychanges surfaceandrheologicalbehaviorinsurfactantaqueoussolutions.Themechanismofthe observedphenomenahasbeenexplainedandspecified.Deepflowdiversionagentsdemonstrateimprovedstabilityandenhancedphysicalpropertiesintheexistenceofnanoparticles inthedispersionphase.Enhancedflowbehaviorofnanogelsimprovesconformancecontrol duetodeeperin-situpropagationandincreasedthermalstability.Fieldapplicationresults encouragestatingnanogasemulsionsaseffectiveandprofitabledisplacementagentsforoil recovery.Explainedmechanismsandreporteddatareferringtoobservedphenomenacould beagoodtheoreticalandpracticalbasisforfutureinvestigations.
PartE.NovelPerspectiveNanocolloids describesnewperspectivematerialsfor petroleumengineering.Metalstringcomplexesduetotheexistenceofextrametalions andpaddlewheelgeometryhavelargevarietiesofmetal–metalbondsthatleadtosignificant changesinphysicalproperties.Colloidstabilityandthermalconductivityhavebeen sustainablyimprovedinthepresenceofmetalstringcomplexes.Theoverallreportedresults allowthesecompoundstobeshownasapromisingareaforfuturestudiesandapplications inthepetroleumindustry.
Thebookisbasedonmaterialsfrommanysources,includingacademicpapers,publicationsfromtheindexedpetroleumjournals,academicinstitutionslaboratoryreportdata,as wellastheexperienceofServiceandNationalOilCompaniesgainedoverthelastthree decades.Tomakethisbookausefulandeffectiveguideintothenanocolloids,applied andtheoreticalbasisofthedetailedobservedresultswereprovided.
NanocolloidClassification 1.1WhatisaColloid? Acolloid(colloidalsystemormixture)isaheterogeneousmatterinwhichonephaseplaysa hostrole(dispersionmedia)andanotherphaseispresentasastabledistinguishabledispersed(dispersion)entity(wecanalsosayparticlesifthedispersionisfromasolidphase). Thedispersionphasecanhavesizesbetween1and1000nm[1].Theshapecanvaryovera very broadrange.Itisassumedthatacolloidshouldbethermodynamicallystableandthat thedispersedphase(entities)remainsevenlydistributedthroughoutthecolloid. Ifthedispersedentitiesaretoosmall,itwouldnotbepossibletodefinethemasaphase. At the otherextreme,iftheyaretoobigthenthesystemwouldnotbestable. Infact,twocommonmethodsexisttodeterminewhetheramixtureisacolloidornot:
1) The Tyndalleffect.Thisisbasedonlightscatteringbyparticlesinacolloidoraveryfine suspension.Thelightpasseseasilythroughatruesolutionbutincolloidsthedispersed phasescattersitinalldirections,makingithardlytransparent.
2) Filtrationofthecolloidthroughasemipermeablemembrane.Infact,thedispersion phasecannotpassthroughthemembraneandisfilteredoutofadispersionmedium.
Therearethreemainclassificationsofcolloidsaccordingtodifferentpropertiesofthe dispersed phaseandmedium[2,3]
1.1.1ColloidClassification 1) Classificationbasedonthephysicalstateofthedispersionmediumandofthe dispersedphase. Usingthesecriteria,colloidscanbedividedasshowninTable1.1.
2) Classificationbasedonthenatureoftheinteractionbetweenthedispersion mediumandthedispersedphase. Usingthiscriterion,colloidscanbeclassifiedas eitherlyophilicorlyophobic.
• Lyophilic(intrinsiccolloids ).Ahighforceofattractionexistsbetweenadispersed phaseandthedispersionmedium.Thesetypesofcolloidsareverystableanddo notrequirespecialmixingrequirements.Examplesarestarch,rubber,protein,etc.
4 1NanocolloidClassification
Table1.1 Themaintypesofnanocolloid.
DispersedphaseDispersedmediumNameofcolloidalsolutionCommonexamples
Solid Gas Solidaerosol
Smoke,dust
Solid Liquid Sol,gel,suspensionPaints,inks,jellies
Solid Solid Solidsol Alloys,opals
Liquid Gas Liquidaerosol Dust,smog,clouds
Liquid Liquid Emulsion Butter,cream,milk
Liquid Solid Solidemulsion(gel)Butter,cheese,curd
Gas Liquid Gasemulsion,foamWhippedcream,suds
Gas Solid Solidfoam Cake,marshmallow,lava
• Lyophobic(extrinsiccolloids).Averyweakforceofattractionexistsbetweenthe dispersedphaseandthedispersionmedium.Thesetypesofcolloidsareunstablewithouttheapplicationofstabilizingagentsandrequirespecialmixingprocedures.Examplesaresolsofmetalslikesilverandgold.
3) Classification based onthetypesofparticlesofthedispersedphasecolloids. Usingthesecriteria,colloidscanbedividedinto:
• Multimolecularcolloids. Theseareformedbyacolloidalrangeaggregationof atomsorsmallmoleculesthathaveaparticlesizelessthanthecolloidalrange(i.e. withadiameterlessthan1nm)inadispersionmedium.Forexample,goldconsists ofvarioussizedparticlescomposedofseveralatomsofgold.
• Macromolecularcolloids. Thesearecomposedofmacromoleculeshavingstrong chemicalbondsbetweenmoleculesandofasizeinthecolloidalrange.Theyarevery stable.Examplesarestarch,proteins,cellulose,etc.
• Associatedcolloids. Thesearesubstancesthatbehaveaselectrolytesatlowconcentrations,butshowcolloidalpropertiesathighconcentrationsdueto a micelle formation.
1.1.2ColloidEvaluation
Colloidsareusuallyevaluatedbythefollowingdispersioncharacteristics[4]:
• Particleaggregate. Aninter-particleforcecausestheaggregationofthedispersion phaseinsmallspecies,wheretwotypesofaggregatesoccur.:
a) Nanoparticleagglomerationindrypowderform. It isveryhardtosegregate them,evenwithultrasonication.
b) Nanoparticlesincolloidsformalargecluster. Thistypeofclusterrequiresmore thanoneproceduretodispersethem.
1.2WhatisaNanocolloid? 5
Table1.2 Stabilityofsuspensionswithrelationtothezetapotential[5].
Zetapotential
This is anelectrokineticvalueforadispersedphase.Thepotentialis stronglyrelatedtothecolloidstability.
Stabilitycharacteristics
Maximumagglomerationandprecipitation
Averagezeta potentialinmV
0to+3
Rangeofstrongagglomerationandprecipitation +5to 5
Thresholdofagglomeration 10to 15
Thresholdofdelicatedispersion 16to 30
Moderatestability 31to 40
Fairlygoodstability 41to 60
Verygoodstability 61to 80
Extremelygoodstability 81to 100
• Particlestructure(sizeandshape). Dispersionphaseparticledimensionsandshapes haveahugeimpactoncolloidsproperties.Examplearethermophysicalproperties,rheologicalbehavior,etc.
• Polydispersity. Ingeneral,acolloidwouldhavevarioussizedparticlesactingasthedispersionphase.Apolydispersityindexisameasureoftheparticlesizevariationandranges from0to1.Accordingtointernationalstandards,thevalueoftheindexbelow0.05is characteristicofmonodisperseddistribution(allparticleshavethesamesize),while theindexvaluesabove0.7indicateabroadlypolydispersesystem.
• Zetapotential. Thisisanelectrokineticvalueforadispersedphase.Thepotentialis stronglyrelatedtothecolloidstability(seeTable1.2).
1.2WhatisaNanocolloid? Thetermnanocolloidisrelativelynewandconsidersacolloidthathasnanoparticlesasa dispersionphase[4,6].Nanocolloidshavethefollowingmainfeatures:
– Thedispersionphaseisestablishedbycompoundsintheamorphousorcrystallinestate, either organic orinorganic,andmaydemonstratecollectivebehaviorandconsistsofparticlesinthe1–100nmrange.
– Repulsionforcesactasthemainforcetopreventamacroscopicphaseseparation.
Mean while itshouldbementionedthatthedispersionmediumisalsoaverycriticalfactoraswellasaninterfaciallayercoveringthedispersionmedium.Thestabilityofthe obtainedcolloidsdependsstronglyonthefactorsmentionedabove.Thiswillbediscussed andillustratedfurtherlaterinthebook.
Table1.3 showsthemaintypesofnanocolloidoftenfoundinpetroleumengineering.
6 1NanocolloidClassification
Table1.3 Themaintypesofnanocolloidoftenfoundinpetroleumengineering.
Dispersed phase Dispersed mediumNameofcolloidalsolution
Applicationsinpetroleum engineering
SolidLiquidNanogel,nanosuspension (nanofluid) [6–49]
SolidSolid Nanosol [12,50,51]
LiquidGas Nanoaerosol [12]
LiquidLiquidNanoemulsion [6,12,52–54]
Gas LiquidNanogasemulsion,nanofoam[6]
NanocolloidProperties 2.1DifferentKindsofInteractionsinNanocolloids
Asmentionedearlier,theaggregationofnano-sizedparticlesisthemainchallengeinadiscussionofcolloidstability.Inparticular,flocculationismostoftenobservedasakindof aggregation.Inthisregard,dominationofattractiveforcesisdefinedasundesirable,while repulsionisfoundtobepositiveforcolloidstability.However,collectivepropertiesofnanoparticlesthatarenotpresentedinindividualparticlesshouldalsobetakenintoaccount[4].
2.1.1VanderWaalsInteractions VanderWaalsforcesareintermolecularforcesthatgenerallydemonstrateattractivebehavior.Insomecases,however,theycoulddisplayrepulsivebehaviorbetweendissimilarmaterialsinathirdmedium.Inhydrocarbonliquids(i.e.inertpolar),uncoatednanoparticles favortheformationofaggregates.
TheVanderWaalsinteractionpotentialbetweentwoparticlescanbecalculatedusingthe HamakertheorysimplifiedbytheDerjaguinapproximation:
where c = R1 + R2 + h andisthecenter-to-centerdistance, h isthedistanceofseparation, and A istheHamakerconstant.ParticlesthathaveahighHamakerconstantvalueshowa strongeraggregationbehaviorincomparisonwithparticlesthathavealowervalueof Hamakerconstant.
2.1.2ElectrostaticInteraction Electrostaticinteractionsaretheattractiveorrepulsiveinteractionsbetweenchargedmoleculesandparticles.Therearevariousmechanismsdefiningthesurfacecharginginliquids byadsorption,ionization,etc.[55].
Tocalculatetheelectrostaticinteractionpotential,eitherDerjaguinapproximation(DA) orlinear-superpositionapproximation(LSA)isusuallyused.Theenvironmentalconditions shouldbetakenintoaccountforeverycaseasthesolutionpH,electrolytemedia,andionic strengthhaveastrongimpactonthesetypesofinteraction.
NanocolloidsforPetroleumEngineering:FundamentalsandPractices,FirstEdition. BaghirA.Suleimanov,ElchinF.Veliyev,andVladimirVishnyakov. ©2022JohnWiley&SonsLtd.Published2022byJohnWiley&SonsLtd.
2.1.3Elastic
–StericInteraction Stericstabilizationistheprocessbywhichadsorbednonionicsurfactantsorpolymersproducestrongrepulsionbetweenparticlesinadispersion[56].Basedonthismechanism,polymerchainsbecomeadsorbedontothesurfaceofcolloidalparticlesinordertoavoidthe flocculationdrivenbyVanderWaalsforces.
2.1.4HydrophobicInteraction
Hydrophobicinteractionsincolloidsaredefinedasinteractionsthatoccurbetweenwater andlowwater-solublemolecules(i.e.hydrophobes).Thisprocessismainlyassociatedwith anentropydecreaseintheliquidduetosolubilizationofnonpolarmolecules.However,it shouldbenotedthathydrophobicinteractionsarestronglydependentonelectrostatic forces(i.e.thepresenceofions,chargeoftheinterface,etc.)[55].
2.1.5SolvationInteraction
Theprocessofattractionandassociationofsolventmoleculeswithsolutemolecules/ions involvesbondingandVanderWaalsforces.Inthecaseofwatertheprocessiscalledhydration.Solvationforcesdependonphysicochemicalproperties,suchasthewettabilityangle, surfacemorphology,etc.[57].
2.1.6DepletionInteraction
Largeparticlessuspendedindilutesolutionsofsmallersolutesgenerateanattractiveforce calledthedepletionforce[58].Forexample,thesearesolutionsthatcontainnonadsorbing polymers.Thedepletionpotentialcanbecalculatedbythefollowingformula:
where P istheosmoticpressureand LD isthedepletionlayerthickness(roughlytwicethe radiusofgyration).
2.1.7MagneticDipole–DipoleInteraction Magneticdipole–dipoleinteractionisobservedasadirectinteractionbetweentwomagnetic dipoles(e.g.alsocalleddipolarcoupling).Thefollowingformulaexpressesthedipole –dipoleinteractionpotential:
where M isthemagnetizationofthematerial, a theradiusoftheparticles, s thedistance betweenthesurfacesoftwointeractingparticles,and μ0 isthepermeabilityofthe vacuum[59].
2.2TheStabilityofNanocolloids 9
2.1.8OsmoticRepulsion Osmoticrepulsionisbasedonthelocalincreaseofosmoticpressureduetoariseinthe polymerconcentrationwhileoverlappingofpolymerligandsincolloidstakesplace[60].
2.2TheStabilityofNanocolloids Asdescribedpreviously,thestabilityofacolloidstronglydependsoninterparticleinteractions.Tocharacterizeinteractionpotentialbetweenparticlesandevaluateanaggregation tendency,theDerjaguin–Landau–Verwey–Overbeek(DLVO)theoryisthemostwidely used[61].
Thetheorystatesthatcolloidstabilityisdefinedbythetotalpotentialenergyoftheparticles(VT),whichisthesumoftheattractive(i.e.VanderWaals) VA andrepulsive(i.e.electrostatic) VR potentials:
V T = V A + V R
Theexpressionfortheattractivepotentialenergy VA is
V A = Ar 12x
where A istheHamakerconstant, r istheradiusoftheparticles(applicableforthespherical particleorapproximation),and x isthedistancebetweenthesurfaces.
Theexpressionfortherepulsivepotentialenergyis
V R =2
2 e kx where ε isthedielectricconstantofthesolvent, ε0 isthevacuumpermittivity, ζ isthezeta potential,and k isafunctionoftheionicconcentration(while k 1 isthecharacteristic lengthoftheelectricdoublelayer,orEDL).
However,someassumptionsofDLVOtheorythathavebeenfoundtobecriticalfornanocolloidsshouldbenotedandarepresentedbelow[62,63]:
1) Allparticlesconsideredtobesimilarintermsofsurfaceandshapemorphologyare describedasspherical.However,inthecaseofnanoparticlesthisitisnotcorrect.
2) Thedispersionmediumsinsideandoutsideofaparticleareconsideredtobehomogeneous.However,thisdoesnotapplyinthecaseoftheinterfaciallayer.Forthenanosizeddispersionphase,theinterfaciallayercouldbecomparableandevengreaterthan theparticlediameter,whichcouldhaveagreatimpactontheagglomerationproperties ofthenanoparticles.
3) Therepulsionandattractiveforcesarepresumedtobeindependentforces.However,on nanoscaledimensions,thisisnotanaccurateassumption.Forexample,polarizability increasesthecouplingbetweendifferentforces.
4) Electrostaticpropertiesaredistributeduniformlyonthedispersionphasesurfaces.
5) Brownianmotionandelectrostaticforcesgovernthedistributionofions.
Furthermore,Suleimanovetal.hadshownthat,apartfromthepotentialenergyoftotal particles,thegravityandbuoyancyforcesshouldbetakenintoconsideration[22].
Theauthorsdemonstratedthatparticleaggregationsizesandconsequentlythecolloidstabilitystronglydependonequilibriumbetweenthementionedforces.
Thevalueofdispersionphaseparticledensityatwhichcolloidstabilizationisachieved canbedeterminedfromtheconditionofbuoyancywhenthegravityactingontheparticles aggregationisbalancedbythebuoyancy:
where g isgravitationalacceleration, N istheaveragenumberofparticlesintheassembly, rp istheaverageradiusofthemicroparticles, H isthethicknessoftheassociatedsurrounding liquidlayer, ρp isthedensityofthedispersionphase, Ra istheaverageradiusoftheassembly,and ρbf isthedensityofthedispersionmedium.
Thedensityofmicroparticlesiscalculatedasfollows:
Thisshowsthattheparticlesizedistributionandmorphologyofparticlesshouldbeconsideredintermsofnanocolloidstabilization.ItisimportanttonotethattheParticleSize DistributionAnalyzerandScanningElectronMicroscopeshouldbeusedtoobtaintheparametersmentionedintheequations.
2.3RheologyofNanocolloids Innanocolloidswithaliquiddispersionmedium,whereariseinnanoparticleconcentrationleadstoswitchinginrheologyfromNewtoniantonon-Newtonian(e.g.nanofluidsor nanosuspension,gasemulsions,emulsions)behavior,Suleimanovetal.reportedtheflow characteristicalterationofasurfactantaqueoussolutionfromaNewtoniantoanonNewtonianstateinthepresenceofnon-ferrousmetalnanoparticles[49].However,the mechanismbehindthatdeviationhasstillnotbeensufficientlystudied.Thefollowingsubsectiondiscussesthemechanismofnanoparticleinteractionandtheimpactofmigrations ontherheologyofnanocolloids.
2.3.1EffectofNanoparticleInteractionontheColloidRheology
Nonlineareffectsobservedindispersesystemswithtime-dependentrheologicalproperties (e.g.self-oscillationsandrandomoscillationsofrheologicalcharacteristics)couldbe explainedbyspecificfeaturesoftheinteractionbetweentheirstructuralelements.The interactionofdispersedphaseparticlesinpurelyviscousnon-Newtoniansystemsdoes notnotablyaffectthedynamicbehaviorofrheologicalcharacteristicsbutrathermanifests itselfintheirsteady-statevalues.Thereasonsaretherelativelysmallsize(~10 μm)ofthe dispersedphaseparticlesandtheabsenceofanydevelopedthree-dimensionalstructure. Thespecificfeaturesoftherheologicalbehaviorofpurelyviscousnon-Newtoniansystems canbeexplainedbyusingthedataontheiropticalcharacteristicsmeasuredintheprocess offlow.
2.3RheologyofNanocolloids 11
Theinvestigationscouldbeconductedonanexperimentalsetupsimilartothoseusedin references[64] and[65].Lightispassedthroughacylindricallayer(cell)ofthesolutionin question.Thesolutionflowsataconstantspeed,e.g.theshearrate.Theintensityofthe transmittedlight,registeredasavoltagefromaphotomultiplier,isrecorded.Thisallows thetimedependenceofthesolutiontransparencytobeobserved.Thetransparencychanges whenaggregatesofthedispersedphaseparticlesareformedordestroyed.Therefore,itis possibletoestimatethedynamicsoftheinteractionbetweenthedispersedphaseparticles bymeasuringthesystemtransparency.
Example Thesolutionofa2wt.%partiallyhydrolyzedpolyacrylonitrile(PAN)wasinvestigated.Itsflowdependenceat20 CisshowninFigure2.1.Itisapparentfromthefigure thattheflowhasaninitialportionofpseudoplasticcharacter,whichisreplacedbyadilatantflow(e.g.anincreaseofviscositywiththeshearrate)afterthethresholdrate(ataround γ =600s 1)isexceeded.
Theflowcelltransparencymeasurementswereconductedatfourshearrates:inthe regionofpseudoplasticflow(γ =400s 1),attheinflectionpointoftheflowcurve (γ =600s 1),neartheinflectionpointintheregionofthedilatantflow(γ =800s 1), andintheregionofthedevelopeddilatantflow(γ =1200s 1).AFourierspectralanalysis wassubsequentlyperformedforthesemeasurementsandtheresultsarepresentedasthe powerspectraldensity.
Intheregionofthepseudoplasticflow,thetransparencydidnotchangewithtime,i.e.the flowofthedispersionwassteady.Attheinflectionpoint,thesteadyflowisdisturbedand thereareperiodictransparencyoscillations(seeFigure2.2).Furtherinthetransitional regiontothedilatantflow,onecanobservecomplexperiodicvariationsinthetransparency, withthreecharacteristicfrequencies.Intheregionofthedevelopeddilatantflow,thetransparencyvariesinaquasi-randommanner,asindicatedbythepresenceofmanyharmonics inthepowerspectraldensitygraph(Figure2.2c).
Onthedatabasisprovided,onecouldarriveatthefollowingconclusion:whenthesteady flowofthedispersionisdisrupted,thehydraulicresistanceincreasesand,accordingly,the
Powerspectraldensity.(a) γ =600, (b)800,and(c)1200s–1
apparentviscosityofthesystemalsoincreases.Eachnewlevelofinteractionbetweenthe structuralelementsofthesystemischaracterizedbyanewvalueofitsapparentviscosity.
Themajorityofresearchersconsidertheobservedphenomenontobequietrare.Accordingtoreferences[66] and[67],thedilatancyisobservedinsystemswithahighconcentration of thesolidphaseandincoarsedispersions.Dilatantflowforsuchsystemsisexplained onthebasisofthetheoryofan “excluded-volume.” Itisassumedthattheeffectiveconcentrationofthesolventintheprocessofflowconstantlydecreasesduetoitshighermobility, thedryfrictionbetweendispersedphaseparticlesincreasesasaresult,and,hence,the apparentviscosityofthesystemincreases.
Figure2.2
2.3RheologyofNanocolloids
Inreference[68] itwasshownthatstrongdilatancymaybeobservedinacolloidwitha dispersionphasesmallerthan5 μm.Itisexplainedbypreferentialparticlemigrationtothe wallsofthecapillary,whichleadstoanincreaseintheapparentviscosityofthesystem.
Inreference[69],adilatantflowwasobservedinapolymersolutionofpolymethacrylic acid.Theflowbehaviorwasexplainedbythemechanisminvolvingunfoldingofthemacromoleculechainswithanincreaseinshearrateandanincreaseinparticleinteraction.In somecolloidsthetransitionfromalowtoahighshearratewasassociatedwiththetransitionfromapseudoplasticflowtoadilatant(e.g.forpolyvinylchloride,hydrolyzedPAN).
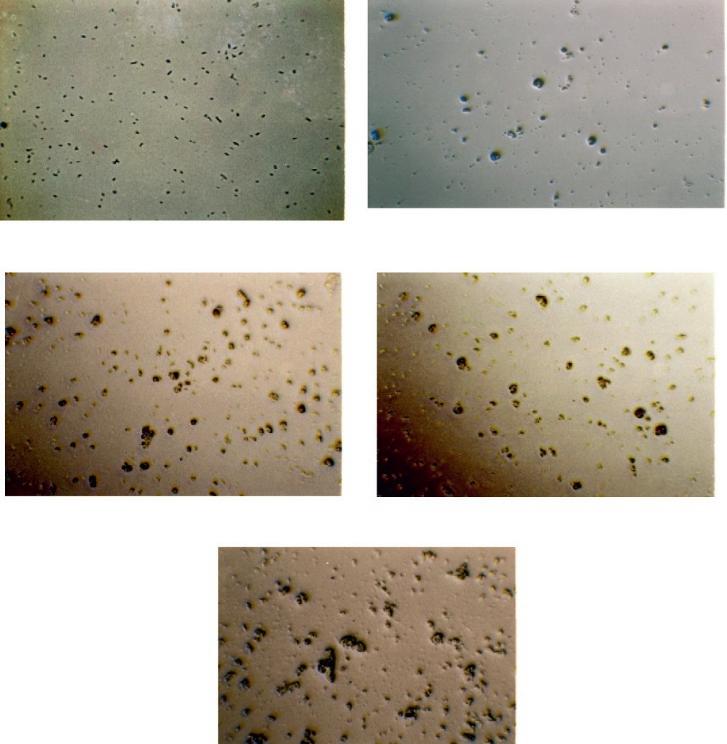
ThesteadypseudoplasticflowofthedispersedphaseisnotdisruptedwhentheconcentrationofthepartiallyhydrolyzedPANintheaqueoussolutionisbelow1wt.%.Therefore, thedisruptionofthesteadyflowisinitiatedbyintensificationofinter-particleinteraction, whichisusuallyobservedwithincreasingconcentrationofthedispersedphaseandmanifestsintheformationofdispersedphaseparticleagglomerates.Toverifythisassumption, thestructureofthedispersionatvariouspolymerconcentrationshasbeeninvestigated usinganopticalmicroscope.Atlowconcentrations(upto0.05wt.%),thedispersedphase consistsofseparatedeformablerandomlyorientedelongated(rod-shaped)formswithasize ofabout100nm.Astheconcentrationincreasestoandabove1wt.%,theagglomerates (mostlycircularoroval)withsizesof5–10 μmareobserved(Figure2.3).Inaddition,the averagetransparencydecreaseswithanincreasingshearrate.
Inordertodeterminetheuniformityofparticledistributionfrommicrophotographs,the dependencesoftheparticlenumber n inacirclewitharbitraryselectedradius r, n(r),was measured.Basedontheobtainedfunction,thefractaldimensionalityofthesystem’sgeometricalstructurewasdeterminedinlinewithreference[70].Figure2.4showsthefractal
dimensionality D functiononthepolymerconcentration.Ascanbeseenfromthefigure,at concentrationsupto1%thegeometricalstructureofthesystemishomogeneousandthe fractaldimensionalitycorrespondstotheEuclideandimensionalityofthearea.Afurther increaseintheconcentrationleadstoadecreaseinthefractaldimensionalityoftheinhomogeneities,butatthe2%concentrationitinsignificantly(by8%)differsfromtheEuclideandimensionalityofthesurface.Atlowpolymerconcentrationsitispossibleinthefirst approximationtoconsiderthespatialdistributionofthedispersedphaseashomogeneous.
Itisclearthattheobservedchangesinthetransparencyofsheareddispersionaredueto processesoftheassociateddestructionandformation.Theabsenceofasteadystateforthese processesisevidentlyattributedtothespecificityoftheeffectofsingleparticlesandassociates onthehydrodynamicstressesthatdevelopinthedispersionwhenundergoingshearflowin theregionofdilatancy.Inordertobuildakineticmodelthatwouldexplaintheobserveddispersiontransparencyoscillations,thenatureofthedilatantbehaviorshouldbestudied.
Theprocessesinconcentrateddispersedsystemsmainlyshowdynamicbehaviorthat leadstoasituationwhenthetheoreticalstudy,withaccountingforcoagulationeffectseven forthesimplestcases,becomesmathematicallyintricate.Theconstructionofsimplified modelsthatprovidesufficientaccuracyforpracticalapplicationisverycritical.
Inreferences[71–79],basedonthekineticapproach,thixotropicprocessesindispersed systems were investigated.Itwasshownthatitispossibletopredicttherheologicalpropertiesofadispersedsolutionwithacceptableaccuracy.However,theauthorsdidnotconsider motiondynamicsofthedispersemediumandtheinfluenceofdispersed-phaseparticle interactionontherheologicalbehavior.SeeAppendix1foranillustrationofasimplemodel thattakesintoaccounttheabove-mentionedissues.
2.3.2EffectofNanoparticleMigrationontheColloidRheology Experimentsonvariousdispersesystemshaveproventhatrheologicalflowdependences cannotbeuniversallydescribedbytheexponentiallaw.Indeed,inthecaseofflowthrough tubeswithvariablecross-sections,therheologicalfunctiondoesnotobeytheexponential law.Moreover,thefunctionoftheapparentviscosityupontheflowratehasanonmonotoniccharacter.Similareffectsarealsoobservedintubeswithvariablecross-sectionsfor non-Newtonianoils.
Figure2.4 Fractaldimensionofassociatesasafunctionofthepolymerconcentration C insolution.
2.3RheologyofNanocolloids 15
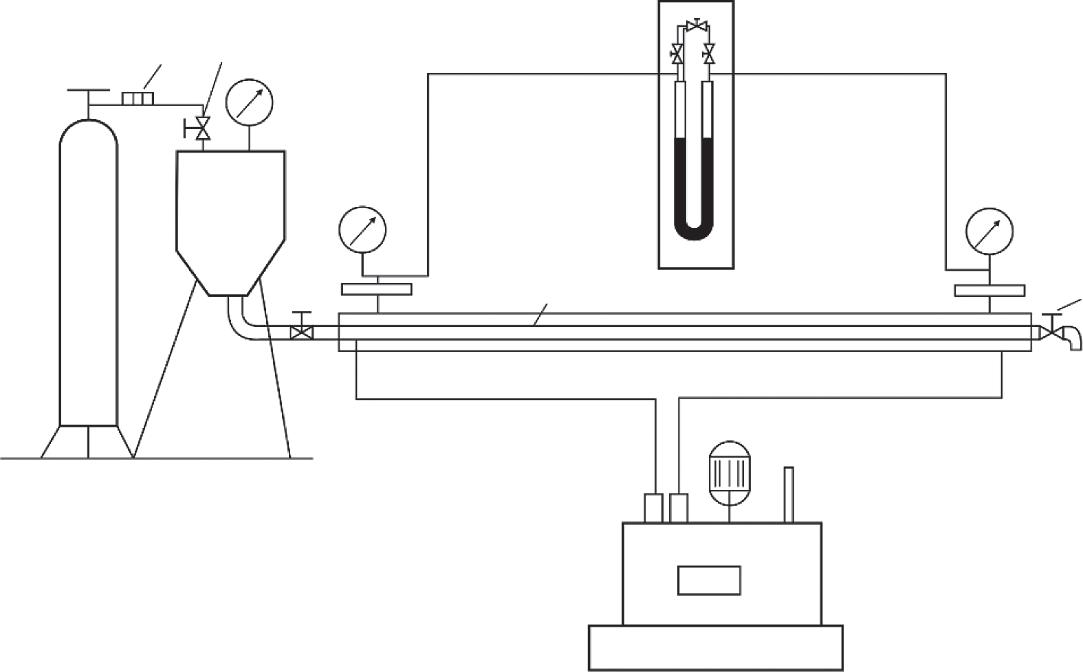
However,fielddataonoiltransportindicatesthattheseeffectscanalsobeobservedin tubeswithconstantdiameters,e.g.duringthetransportofwater–oilemulsions.Moreover, anomalousphenomenaalsooccurwhensuspensionswithsolidparticlesareflowing throughthetubes.Experimentalinvestigationsofnon-Newtonianoilrheologyaswellas suspensionswithsolidparticleswerecarriedoutinordertostudytheseproblemsindetail. Heavygradesofoilwithacomplicatedrheologicalbehaviorwereobtainedfromthe GryazevayaSopkaandBusachidepositsfortheinvestigation.Adispersesystemmodelconsistingofaclay(bentonite)slurrymixedwithaddedquartzsand(densityof2600kg/m3) invariousfractions(withaverageparticlesizeof10 4 m)wasalsoexamined(Table2.1). TheexperimentalsetupshowninFigure2.5wasused.
Table2.1 Basicphysicalcharacteristicsofthesystemsstudied.
Characteristicsandunitsof measurement Oilsamplefromthe GryazevayaSopkadeposit Oilsamplefromthe Busachideposit
Figure2.5 Schematicdiagramoftheexperimentalsetup,where(7)isahigh-pressurevessel,(2)an electricalstirrervessel(forthesystemsunderinvestigation),(3)atubeofconstantdiameter(4or 16×10 3 m)withanexchangeablehorizontal2.7mlongsection,(4)standardpressuregauges,(5)agas pressureregulator,(6)adifferentialpressuregauge,(7)anultra-thermostat,(8)one-wayvalves,(9)a lockingvalve,and(10)separatingcompensators.
Throughoutalltheexperiments,aconstanttemperature(287K)andlaminarflowwere maintained.Therheologicalcurvesortime-dependentflow-ratecharacteristicswere recorded.Figure2.6 showsarheologicalcurvefortheoilsamplesfromtheBusachideposit, which wasusedtoplotthedependenceofthedimensionlessapparentviscosityagainstthe valueoftheshearrateaveragedoverthecross-sectionofthetube(Figure2.7).Ascanbe seenfromthesefigures,therheologicalcurveisS-shaped,whereasthecorresponding dependenceoftheapparentviscosityupontheshearrateisnonmonotonic.Attheinflection point(pointAinFigures2.6and2.7),thedilatantbehaviorbecomespseudo-plastic(inthis case, γ ≈ 160s 1).SimilarresultswereobtainedfortheoilsamplestakenfromtheGryazevayaSopkadeposit.
Thedispersedphaseofoilsystemsisknowntobecomposedmainlyofasphaltenes,which tend to formsupermolecularstructuresofthesize10 6 m.Thesestructuresmaybe deformedundertheinfluenceofexternalforces(forexample,shearstress).Inaccordance withthemicrorheologicalstudydescribedinreference[80],duringthelaminarflowofa suspension, solidparticlesmovepredominantlynearthetubewalls,whereasthedeformed particlesmigratetothecenteroftheflow.Hence,therheologicalcurvesfortheoilswitha non-Newtonianbehaviormaybedescribedbythefollowingkineticmechanism.Ata
Figure2.6 RheologicalcurvefortheoilsamplefromtheBusachideposit,obtainedinexperiments witha tube4×10 3mindiameter.
Figure2.7 Dependenceofthedimensionlessapparentviscosityontheshearrateaveragedoverthe cross-sectionof thetube.
2.3RheologyofNanocolloids 17
relativelylowshearrate,theparticlesofadispersedphasethatarechaoticallydistributedin aliquidarenotdeformed(similartosolidparticles)andaregraduallyconcentrated(predominantlynearthewallsofthecylindricaltube),thusresultinginanincreaseintheapparentviscosityofthesystem.Then,afterattainingacertainthresholdshearrate,theparticles becomedeformed,whichleadstoanadditionalperturbationofthefieldofliquidvelocities, andtheymigratetowardthecenteroftheflow.Thisisreflectedbyadecreaseintheapparentviscosityofthesystem.Infact,ashasbeenshownabove,thecharacterofflowisaltered at γ ≈ 160s 1,whichisconsistentwiththeresultsofstudy[80].Accordingtoit,adispersed particle10 6 minsizestartstobecomedeformedwhentheshearrate γ averagedoverthe cross-sectionofthetubeexceeds150s 1 .
Consideringthatforanoildispersesystemthedensitiesofthedispersedphaseandthe dispersionmediumarepracticallyequal,thefollowingviscosityfunctionwassuggested[81]:
hc = 1 η c + A r 2 c
where η(c)isthefunctionofviscosityversusconcentration, r isthecylindricalcoordinate, c istheconcentrationofthedispersedphase,and A isaconstant.Inaccordancewithreference[81], A [0, ∞], r [0,1],and c [0,1].Thevalueofthefunction c(r)isdefinedso that h(c)and,accordingly,theflowratearemaximal.
Assumingthatthefunction η(c)isexpressedbytheEinsteinequation,weobtainthefollowingexpression:
= 1
BydifferentiatingEquation(2.1) withrespectto c,thefollowingexpressionscanbe obtained: h c = a 1+ ac 2 + A r 2 h c = 2a2 1+ ac 3
AnanalysisofEquation(2.2) indicatesthatthefunction h(c)acquiresitsmaximumvalue eitherat c =0orat c =1.Determiningthefunction h(c)atthesevaluesof c,
h 1 = 1 1+ a + A r 2 , h 0 1
Sincefunction h(1)decreaseswithincreasing r, cm(r)forthedistributionoftheconcentrationoverthecross-sectionofthetube,thusprovidingthefollowingmaximumflowrate, couldbeexpressedasfollows:
cm r = 1,0< r <1+ a A a 0,1+ a A a < r ≤ 1 2 3
Fortheconcentrationaveragedoverthecross-section,
i.e.at A (0, a/(1+ a)2)and F (0,1/2)thedistributionexpressedbyEquation(2.3) is validandthesystemiscompletelyseparated.
Itshouldbenotedthat,intheconsideredexample,whenthedispersedphaseismoving nearthetubewall,completeseparationisnotobtained.However,accordingtoreference [80],astheparticlesmovefromthecenterofthetubetoitswalls,anincreaseintheconcentrationand,accordingly,intheviscositytakesplace.
Thenextstepisaninvestigationintotheimpactoftheobtaineddistributionontheliquid flowrate.Inthisregard,assumethattwoviscousincompressibleliquidswithdifferentviscositiesaremovinginthefollowingmanner – oneoftheliquidsismovinginanannulusnear thetubewallsandtheotherliquidismovinginthecenteroftheflux.Theflowratesofthese liquidsaredeterminedbythefollowingformula:
where ΔP/l isthepressuregradient, ηi istheviscosityofaliquid,and ai and bi areconstants. Theliquidnearthewallisdenotedbythesubscriptindex1andthecore,thesubscript index2.
SolvingEquation(2.4) undertheboundaryconditions
where R1 isthetuberadiusand R2 istheradiusoftheinterfacebetweenthetwoliquids,we obtainthefollowingexpressionforthetotalflowrate:
where δ isthethicknessofthelayernearthewall(δ =1 R2/R1)and ε = η2/η1.
Ananalysisofrelationship(2.5) indicatesthatat ε >1,i.e.whenalayerwithalowerviscositymovesnearthetubewalls,theflowrateoftheliquidincreaseswiththethicknessof thelayernearthewall,whereasat ε <1,theflowrateoftheliquiddiminisheswiththe increasingthicknessofthislayer.
InFigure2.8,thedependenceoftheflowrateonthetimeisrepresentedataconstant pressuredropof1.1×104Paforaclayslurrywithaddedquartzsand.Itcanbeseenthat theflowrateisadescendingfunction,virtuallyvanishingoveracertainperiodoftime. Thescatterofexperimentaldata,whichisobservedbeforethesteadystateisattained, maybeattributedtoagradualredistributionofthedispersedphase.Notethatasimilarscatterisalsoobservedduringtheflowofrheopecticsuspensions.
2.3RheologyofNanocolloids
Figure2.8 Dependenceoftheclayslurryflowratewithaddedquartzsandontimeataconstant pressuredropof1.1×104Pa,obtainedinexperimentswithatube1.6×10 2m indiameter.
Anincreaseintheinputpressureofupto1.3×104Paresultsincontinuedflowofthe system.Thesuspensionunderinvestigationpossessesareasonablyhighsedimentationstabilityduetothesmallaveragesizeoftheparticlesofthedispersedphaseandtherelatively highviscosityofthedispersionmediumwithacomparativelysmalldifferenceindensities, aswellasduetothepresenceofyieldshearstress.Thiswassupportedbythefactthatthe concentrationofsandintheoutflowoftheslurryremainsvirtuallyconstant.
Thedependencesoftheyieldshearstressandthestructuralviscosityontheconcentration forthesuspensionunderconsiderationhavealsobeenstudied.Ithasbeenestablishedthat theyieldshearstressandstructuralviscosityincreaserapidlywithconcentration.
Thedecreaseobservedintheflowrateafterstoppingtheflowmaybeexplainedasfollows.Afteracertainperiodoftime,inthetubewherethesuspensionhasbeenflowing adistributionofthedispersedphaseisestablishedsothatitsconcentrationincreaseswith proximitytothetubewall.This,inturn,resultsinanincreaseintheyieldshearstressofthe system.
Thenextstepistoobtainaqualitativeconformationofthesuggestedmodel.Abbasov foundthatadensitydifferencebetweenthedispersephaseandthedispersionmediumcannotbeneglected.Then,transformingthefunctionobtainedinreference[81] fortheinstant case,thefollowingexpressionwasachieved:
where ρ(c)isthefunctionofthedensityoverconcentration.Forthefunction c(r),wefinda valuethatresultsinminimizationoftheflowrateoftheliquidafterthefunction h(c)is maximized.Forthefunctionofdensityversusconcentration,thisisassumedtobe
where b istheratioofthedensityofthedispersionmediumtothatofthedispersedphase. Thefunctionofviscosityversusconcentrationisexpressedbytheformula[82]
Thenthefunction h(c) takesthefollowingform: hc = c a b +1 b c 2a + A 1 r 2 c
Differentiatingthiswithrespectto c wasobtainedas h c = 2c a 1 b + b 2a + A 1 r 2 h c = 1 b a 2 6
Ananalysisofthisequationshowsthatfunction h(c) attainsitsmaximumateither c =0 or c =1.Determiningthisfunctionatthesevaluesof c wasobtainedusingthefollowing expressions:
=
Sincefunction h(1)decreaseswith r,[a > b/(1 b)]dr wasobtainedas
r =
Theexpressionfortheaveragedconcentration
1
i.e.at A = a 1 b b 2a , F =(0,1/2)thedistributionaccordingtoEquation(2.7) is observed,andthesystemiscompletelyseparated.Notethat,inpractice,thecompleteseparationofaliquiddoesnotoccur.Therefore,itwouldbenaturaltoassumethatarelatively thinlayernearthewall(δ =0.3 0.5)willexhibitaconsiderablyhigherconcentrationof thesolidphaseand,accordingly,ahigherviscositythanthosewithauniformlydistributed dispersedphase.
Intheliquidflowrateevaluationwehadassumedthattwovisco-plasticliquidsflow throughacylindricaltubeinacircularmode:aviscous-plasticliquidwiththestructural viscosity η1 andyieldshearstress τ 1 isflowingnearthetubewalls,whereasanotherviscous-plasticliquidwith η2 and τ 2 flowsinthecenterofthetube.Inthiscase, η1 > η2 and τ 1 > τ 2.Assumingthattheradiusoftheflowcoreisrelativelysmall,asimplifiedapproach hasbeenapplied,inaccordancetowhichtheequationofmotionfortheviscous-plasticliquidholdsthroughouttheentirerangeof r values.Theflowrateofviscous-plasticliquidsis determinedfromtheknownformula(usingthenotationsintroducedearlier)