2nd Edition Richard J. Martin Visit to download the full and correct content document: https://ebookmass.com/product/thermal-systems-design-fundamentals-and-projects-2 nd-edition-richard-j-martin/
More products digital (pdf, epub, mobi) instant download maybe you interests ...
Design of Thermal Energy Systems Pradip Majumdar
https://ebookmass.com/product/design-of-thermal-energy-systemspradip-majumdar/
Fanaroff & Martin’s Neonatal-Perinatal Medicine 11th Edition Richard J. Martin
https://ebookmass.com/product/fanaroff-martins-neonatalperinatal-medicine-11th-edition-richard-j-martin/
Thermal system design and simulation Dhar
https://ebookmass.com/product/thermal-system-design-andsimulation-dhar/
Earth's Oldest Rocks 2nd Edition Martin J. Van Kranendonk
https://ebookmass.com/product/earths-oldest-rocks-2nd-editionmartin-j-van-kranendonk/
Fundamentals of Thermal-Fluid Sciences 6th Edition
Cengel
https://ebookmass.com/product/fundamentals-of-thermal-fluidsciences-6th-edition-cengel/
Fundamentals of Thermal and Nuclear Power Generation
Yasuo Koizumi (Editor)
https://ebookmass.com/product/fundamentals-of-thermal-andnuclear-power-generation-yasuo-koizumi-editor/
Fundamentals of Thermal-Fluid Sciences 6th Edition
Yunus A. Cengel
https://ebookmass.com/product/fundamentals-of-thermal-fluidsciences-6th-edition-yunus-a-cengel/
Fundamentals of Thermal-Fluid Sciences 5th Edition
Yunus A. Çengel
https://ebookmass.com/product/fundamentals-of-thermal-fluidsciences-5th-edition-yunus-a-cengel/
micro:bit Projects with Python and Single Board
Computers: Building STEAM Projects with Code Club and Kids' Maker Groups Martin Tan
https://ebookmass.com/product/microbit-projects-with-python-andsingle-board-computers-building-steam-projects-with-code-cluband-kids-maker-groups-martin-tan/
Thermal Systems Design Fundamentals and Projects Richard J. Martin
ThermalSystemsDesign
ThermalSystemsDesign FundamentalsandProjects
SecondEdition
RichardJ.Martin MartinThermalEngineering,Inc.
CaliforniaPolytechnicStateUniversity,SanLuisObispo SantaClaraUniversity,SantaClara California,USA
Thiseditionfirstpublished2022 ©2022JohnWiley&Sons,Inc.
Allrightsreserved.Nopartofthispublicationmaybereproduced,storedinaretrievalsystem,ortransmitted,inany formorbyanymeans,electronic,mechanical,photocopying,recordingorotherwise,exceptaspermittedbylaw. Adviceonhowtoobtainpermissiontoreusematerialfromthistitleisavailableathttp://www.wiley.com/go/ permissions.
TherightofRichardJ.Martintobeidentifiedastheauthorofthisworkhasbeenassertedinaccordancewithlaw.
RegisteredOffice
JohnWiley&Sons,Inc.,111RiverStreet,Hoboken,NJ07030,USA
EditorialOffice 111RiverStreet,Hoboken,NJ07030,USA
Fordetailsofourglobaleditorialoffices,customerservices,andmoreinformationaboutWileyproductsvisitusat www.wiley.com.
Wileyalsopublishesitsbooksinavarietyofelectronicformatsandbyprint-on-demand.Somecontentthatappearsin standardprintversionsofthisbookmaynotbeavailableinotherformats.
LimitofLiability/DisclaimerofWarranty
Whilethepublisherandauthorshaveusedtheirbesteffortsinpreparingthiswork,theymakenorepresentationsor warrantieswithrespecttotheaccuracyorcompletenessofthecontentsofthisworkandspecificallydisclaimall warranties,includingwithoutlimitationanyimpliedwarrantiesofmerchantabilityorfitnessforaparticularpurpose. Nowarrantymaybecreatedorextendedbysalesrepresentatives,writtensalesmaterials,orpromotionalstatements forthiswork.Thefactthatanorganization,website,orproductisreferredtointhisworkasacitationand/orpotential sourceoffurtherinformationdoesnotmeanthatthepublisherandauthorsendorsetheinformationorservicesthe organization,website,orproductmayprovideorrecommendationsitmaymake.Thisworkissoldwiththe understandingthatthepublisherisnotengagedinrenderingprofessionalservices.Theadviceandstrategiescontained hereinmaynotbesuitableforyoursituation.Youshouldconsultwithaspecialistwhereappropriate.Further,readers shouldbeawarethatwebsiteslistedinthisworkmayhavechangedordisappearedbetweenwhenthisworkwas writtenandwhenitisread.Neitherthepublishernorauthorsshallbeliableforanylossofprofitoranyother commercialdamages,includingbutnotlimitedtospecial,incidental,consequential,orotherdamages.
LibraryofCongressCataloging-in-PublicationDataappliedfor:
ISBN:9781119803478
CoverdesignbyWiley
Coverimages:©AnitaVDB/GettyImages;©Photographee.eu/Shutterstock;©DerekPinkston/Getty;© leungchopan/Shutterstock;©momente/Shutterstock;©MintImages/Getty;©GrantFaint/Getty;©Mechanical Engineering/Shutterstock;©AndriiStepaniuk/Shutterstock;©artpartner-images/Getty;©mikulas1/Getty
Setin9.5/12.5ptSTIXTwoTextbyStraive,Pondicherry,India 10987654321
Contents
PrefacetotheFirstEdition(AMostPracticalGuidebook) xi
Acknowledgments xi
PrefacetotheSecondEdition(FundamentalsandProjects) xiii
Acknowledgments xv
AbouttheAuthorandtheTextbook xvii
AbouttheCompanionWebsite xix
1Thermodynamics 1
1.1UnitsofMeasure 1
1.2Mass/ForceUnitConversion 2
1.3StandardTemperatureandPressure 3
1.4ControlMass,ControlVolume 3
1.5LawsofThermodynamics 5
1.6ConservationLaws 6
1.7ThermodynamicVariableCategories 7
1.8IdealGasLaw 10
1.9HistoryofTemperature 11
1.10ThermodynamicStates 12
1.11InternalEnergy,Enthalpy,Entropy 13
1.12Availability(Exergy) 15
1.13HomeworkProblems 16 CitedReferences 17
2FluidMechanics 19
2.1Viscosity,Shear,Velocity 19
2.2Hydrostatics,Buoyancy 20
2.3TheContinuityEquation 21
2.4Mass,Volume,MoleFlows 22
2.5ReynoldsNumber,VelocityProfiles 23
2.6TheMomentumEquation 27
2.7Bernoulli’sEquation 27
2.8Stagnation,Static,DynamicPressure 28
2.9FrictionFactor,HydraulicDiameter 29
2.10MoodyChart,ChenEquation 31
2.11ModifiedBernoulliEquation 33
2.12AlternateMoodyCharts 33
2.13EntryEffects,MinorLosses 35
2.14PorousMediaPressureDrop 36
2.15HomeworkProblems 37 CitedReferences 38
3HeatTransfer 41
3.1Fourier’sLaw 41
3.2Newton’sLawofCooling 43
3.3TheStefan–BoltzmannLaw 43
3.4TheEnergyEquation 44
3.5TheEntropyEquation 45
3.6ElectricityAnalogyforHeat 45
3.7Film,MeanTemperature 47
3.8Nusselt,PrandtlNumbers 48
3.9FlowsAcrossTubeBanks 49
3.10 “Gotcha” Variables 52
3.11RadiationandNaturalConvection 53
3.12RadiantExchange 54
3.13TypesofHeatExchangers 58
3.14HeatExchangerFundamentals 59
3.15OverallHeatTransferCoefficient 59
3.16LMTDMethod 60
3.17Effectiveness-NTUMethod 61
3.18PorousMediaHeatTransfer 63
3.19ExternalConvectiontoIndividualSpheresandCylinders 65
3.20HomeworkProblems 67 CitedReferences 68
4IntroductiontoCombustion 71
4.1FuelsforCombustion 71
4.2AirforCombustion 72
4.3AtomicandMolarMass 73
4.4BalancingChemicalEquations 73
4.5StoichiometryandEquivalenceRatio 74
4.6TheAtomEquations 76
4.7SensibleandChemicalEnthalpies 78
4.8ThermochemicalPropertyTables 82
4.9EnthalpyofCombustion 83
4.10EnthalpyDatumStates 85
4.11AdiabaticCombustionTemperature 86
4.12EquilibriumandKinetics 88
4.13PollutantFormationandControl 93
4.14CombustionSafetyFundamentals 95
4.15OtherTopicsinCombustion 96
4.16HomeworkProblems 97 CitedReferences 98
5ProcessFlowDiagrams 101
5.1IntelligentCAD 101
5.2Equipment 102
5.3ProcessLines 105
5.4ValvesandInstruments 105
5.5NonengineeringItems 105
5.6HeatandMaterialBalance 106
5.7PFDTechniques 107
5.8HomeworkProblems 111 CitedReferences 113
6AdvancedThermodynamics 115
6.1EquationsofState 115
6.2ThermodynamicPropertyDiagrams 117
6.3Gibbs,Helmholtz,andMaxwell 118
6.4EquationsofState 121
6.5BoilingandCondensation 124
6.6Psychrometry 126
6.7Liquid –VaporEquilibriumfor NH3 + H2O Mixtures 133
6.8EfficiencyvsEffectiveness 137
6.9SpacevsTime 139
6.10HomeworkProblems 141 CitedReferences 142
7BurnersandHeatRecovery 145
7.1Burners 145
7.2CombustionSafeguarding 147
7.3ThermalOxidizers 149
7.4DestructionEfficiency 151
7.5RecuperatorsandRegenerators 152
7.6Packed-bedHeatStorage 156
7.7HeatExchangerDiscretization 157
7.8ThermalDestructionofAirbornePathogens 159
7.9SpecialAtmosphereHeatTreating 160
7.10BurnerandHeatExchangerFailures 161
7.11HomeworkProblems 163 References 166
8BoilersandPowerCycles 169
8.1RankineCycle 169
8.2BoilerTerminology 171
8.3EfficiencyImprovement 174
8.4ControlsandSafeguards 177
8.5BlowdownandWaterTreatment 179
8.6AirPollutantReduction 181
8.7OrganicRankineCycle 185
8.8BoilerFailureAnalysis 186
8.9HomeworkProblems 189 CitedReferences 191
9CombustionTurbines 193
9.1Turbomachinery 193
9.2BraytonCycle 194
9.3PolytropicProcesses 196
9.4IsentropicEfficiency 197
9.5GasPropertyRelationships 200
9.6Reheating,Intercooling 201
9.7Recuperation 202
9.8HomeworkProblems 204 CitedReferences 206
10RefrigerationandHeatPumps 207
10.1VaporRefrigerationCycle 207
10.2GasRefrigerationCycle 210
10.3HeatPumpEfficiency 211
10.4SizingandEnergyUsage 212
10.5Refrigerants 214
10.6Compressors 217
10.7AirHandlers 219
10.8RefrigerationControl 222
10.9CoilDefrost 224
10.10CompressorlessRefrigeration 225
10.11ThermoelectricCoolers 234
10.12RefrigerationSystemFailures 235
10.13HomeworkProblems 238 CitedReferences 242
11OtherThermalSystems 245
11.1SolarFluidHeating 245
11.2FluidHeaters 248
11.3EvaporativeCooling 251
11.4GeothermalHeatSink 252
11.5ThermalEnergyStorage 254
11.6Thick-layerProductDehydration 257
11.7Desalination 259
11.8SteamSterilization 261
11.9EspressoMachine 262
11.10HotAirBalloon 266
11.11HomeworkProblems 269 CitedReferences 272
12PipeandFluidMoverAnalysis 275
12.1FluidMoverCategories 275
12.2ConveyingMeansCategories 277
12.3LeakPrevention 278
12.4PressureRiseandDrop 279
12.5ElectricityAnalogyforFlow 280
12.6PipingNetworkRules 282
12.7BlowerandSystemCurves 283
12.8PumpandBlowerWork 287
12.9CompressibilityinLongPipes 291
12.10ChimneyEffect 292
12.11HomeworkProblems 295
CitedReferences 297
13ThermalProtection 299
13.1RefractoryCeramics 299
13.2RefractoryMetals 301
13.3ThermalInsulation 301
13.4Radiative-ConvectiveInsulationSystems 304
13.5SkinContactBurns 304
13.6ProtectionAgainstThermalExpansion 305
13.7ProtectionAgainstThermalShock 308
13.8HomeworkProblems 309 CitedReferences 310
14PipingandInstrumentationDiagrams 311
14.1DesignPackages 311
14.2TemperatureSensors 313
14.3PressureSensors 315
14.4FlowSensors 317
14.5LevelSensors 319
14.6ExhaustGasAnalyzers 321
14.7CombustionSafetyInstruments 323
14.8ValvesandActuators 325
14.9ISATagGlossary 329
14.10P&IDTechniques 331
14.11HomeworkProblems 332 CitedReferences 333
15ControlofThermalSystems 335
15.1ControlNomenclature 335
15.2ThermostaticControl 335
15.3PIDControl 338
15.4SafetyControlsandInterlocks 341
15.5SequencingControl 342
15.6LadderLogic 343
15.7HomeworkProblems 345 CitedReferences 346
Contents
16ProcessSafety 347
16.1SafetyTerminology 347
16.2SafetyHierarchy 349
16.3SafeguardsandWarnings 350
16.4HistoryofSafetyStandards 351
16.5ProcessHazardAnalysis 352
16.6HomeworkProblems 354 CitedReferences 355
17ProcessQualityMethods 357
17.1QualityTerminology 357
17.2AdvancedStatisticalMethodsforQualityinThermalProcesses 358
17.3ManagementofChangeforQuality,Stewardship,andSafety 362
17.4HomeworkProblems 364 CitedReferences 366
18Procurement,Operation,andMaintenance 367
18.1EngineeringDesignDeliverable 367
18.2EngineeringDataSheets 367
18.3ConstructionandCommissioning 368
18.4Inspection,Maintenance,andTraining 371
18.5OperationandMaintenanceManual 373
18.6HomeworkProblems 375 CitedReferences 375
AppendixAPropertyTables 377 AppendixBExcel(VBA)CustomFunctions 449 Index 511
PrefacetotheFirstEdition(AMostPracticalGuidebook) Thethemeandstructureofthistextbookarosefromtheauthor’s16semestersinstructingmechanicalandchemicalengineeringstudentsattheUniversityofSouthernCalifornia(USC),andmuchof thespecializedcontentincorporatedherearosefrominvestigationstheauthorperformedforclients ofMartinThermalEngineering,Inc.Theauthor(inparallelwithseveralcolleagues)considered numerouspublishedtextsandfoundthatnonecontainedthefocusorbreadthnecessaryforacomprehensiveclassinthermalsystemsdesign – hence,theneedforthistomewasclear.
Theintendedaudienceismechanicalorchemicalengineeringstudentsseekingcapstonedesign guidanceforthermal-fluidsystems,includingheating,drying,boiling,refrigeration,air-conditioning,compression,expansion,combustion,andpowergeneration.Practitionersofthermalengineeringdesignmayalsofindthistobeahelpfulreferencework – onethatoffersbreadth, clarity,andsimplicity.
Theoverarchinggoalofthistextbookistohelpstudentsvisualizethelandscapeofathermalsystemdesignprojectandtoequiptheirintellectual “toolkits” withawidevarietyoftechniquesfor applyingsolidengineeringtheorytowardausefulandsuccessfuldesign,whileexposingthemto predictablestumblingblocksthatwillrequireingenuitytoovercome.
Thermalsystemsdesignstudentsshouldenterthiscoursehavingsuccessfullycompletedmath andscienceprerequisitessuchasadvancedcalculus,differentialequations,chemistry,andphysics, aswellasintermediateengineeringcoursesinthermodynamics,fluidmechanics,andheattransfer. Priorstudyofcombustionishelpful,butnotrequired.Thebookadoptsuseofatechniquecalled “ThinkStop,” whichtriggersapauseforreflectionwhenevertheauthorseesanopportunityfor “extracurricularlearning” aboutasubject.
Thecoursecanbetaughtina15-weeksemesterwith3-lecturehoursperweekorina10-week quarterwith4-lecturehoursperweek.Expectationsforstudentprojectsandhomeworkshouldbe reducedabitforthe10-weekcourse.Chapters16–18maybeexcludedwithoutalossofcontinuity, butstudentsshouldbeencouragedtodigestthiscontentintheirsparetime.
Acknowledgments Inspirationformuchofthematerialdevelopedherewouldnotbepossiblewithoutcontributions frommycolleaguesatUSCandelsewhere.IamindebtedtoLarryRedekoppandGeoffSpeddingfor bringingmeintothefoldoftheAerospace&MechanicalEngineeringDepartmentasapart-time facultymemberandfornumerousdiscussionsaboutteachingphilosophyandtechnique.Ialso thankmycolleaguesFokionEgolfopoulosandPaulRonneyfortheirencouragementandadvice aboutsubjectmatterforwhichourfondnessiskindred.Itiswithdeepgratitudethat IacknowledgemyclassroomcolleaguesMannyDekermenjianandLeslieKing,withoutwhom
theteachingandlearningelementsthatembodythesoulofthisbookwouldnothavebeenpossible. TheoriginalillustrationswereexpertlycraftedbyEdThielen,oneoftheworld’sfinestcreatorsof clearandcompellingcourtroomdemonstrativeexhibits.TheeditorwasAlisonMartin,arisingstar inthefieldofcomposition,rhetoric,andnewsanalysis.ThedelightfulcoverartwascreatedbyNik HallinandCaraKoenigofMotionSquaredDesign.BrianMartin,JohnTaber,JohnMcArthur,and ZuhairIbrahimattendedlecturesandprovidedhelpfulcomments,asdidnumerousstudentsfrom myFall2017class,whostudiedfromadraftversionofthisbook.Finally,toDawnMartin,myCFO, mycheerleader,andmymore-than-equalpartnerinallthingsnonengineering,IgiveaTomHanksto-MegRyansmileofgratitudeandlove.
PrefacetotheSecondEdition(FundamentalsandProjects) Wheretheprioreditionclaimedtobe “mostpractical,” thecurrenteditionattemptstoearnrecognitionforhavingthe “coolest” (andthe “hottest”)projects.Theprincipalchangesinthisedition relatetotheinclusionofmanynewdesignprojectsforthestudentteams,butitalsoincludes newanalyticaltoolsforstudentstoemployastheyundertaketheirdesignprojects.Wecontinue ourpriorthemesofmotivatinggoodengineeringhabitsbyapplyingthelawsofthermodynamics, fluidmechanics,andheattransfertocreateafunctionaldesign – whilealsoinsistingstudentsdemonstrateahigherlevelofdesignacumenthanrequiredbymostothertextbooksinthedesigncategory.Thisdistinctionappearsintheprojectsbytheinclusionofphasechange(vaporization, condensation,humidity),chemistry(combustion,multiphasethermochemistry),and/orflowin porousmedia – togobeyondthemoreelementary p-T-h analysesfoundinless-thoroughworks.
Withthisedition,theauthorhasadoptedanaugmentedmission:toconvincestudentsthatthey cananalyzethermalsystemswithoutspendingtensofthousandsofdollarsperyeartolicensea process/flowsimulationsoftwarepackage.Theoutcomeweseekisforstudentstofirstunderstand thefundamentalsandthenapproachtheirdesignprojectswithconfidenceandcreativity.Ifour instructionissuccessful,studentswillbegintheircareerswithanunsurpassedbreadthofknowledgeandastoutcollectionofengineeringdesigntoolsintheirtoolkits.
Themostpracticalwayweattempttofurtherthismissioniswithanewappendixthatcontains VBAscriptsforcustomizedExcelfunctionsthatcomputevaluesforthermodynamicpropertiesof fluidsandothercomplexengineeringequations.Ifreaders(i.e.studentsorengineeringpractitioners)investasmallamountoftimecopyingthescriptsandpastingthemintoVBAmodules withinMicrosoftExcel,theirtoolkitswillbeaugmentedwithpowerfultoolsthatwouldcostasmall fortuneelsewhere.Inaddition,thepropertytablesfromthefirsteditionwererelocatedtoaseparate appendixandnewpropertytablesforpropane,ammonia,andammonia/watermixtures wereadded.
Eightprojectswerepresentedinthefirstedition(asend-of-chapterproblemsinChapters5and 14),andtheyremainprominentinthesecondedition,evenasnewprojectsarepresentedvianew homeworkquestions.Thenewprojectsinclude:ahotairballoon,anexothermicgasgenerator,a tenter-framedryingoven,anespressomachine,anammonia/water/hydrogenabsorptionrefrigerationsystemwithnomovingparts,andathermallyassistedairfiltrationsystemfordestructionof biohazardparticles.
Thecompanionwebsiteforthisbookincludesasubstantialcollectionofsupplementarytoolsto assistinstructorsandstudents.IncludedinthisresourcecachearePowerPointslideswithcontent organizedfora15-weeklecturecourse, “customerspecifications” thatprovidenecessarysizingand operatingparametersthatformthebasesfortheprojectdesigns,andsolutionkeysformostofthe homeworkproblems.Thesolutionkeysalsoarerepositoriesforseveralimportantderivationsand projectexamplesthatweretoolongforthepublishedtext.
xiv PrefacetotheSecondEdition(FundamentalsandProjects)
Thenewprojectsareintendedtobeexecutedinthesamemannerastheoldprojects.First,studentscreateaschematicofthesystemwithmajorequipment(unitoperations)connectedbylines (processstreams).Thentheycomputethermodynamicdetailsateachstateandprepareaprocess flowdiagram(PFD)withstreamtable.Next,theydeterminesizesforpipes/ductsandmajorequipmentusingapplicablerulesforheattransfer,fluidmechanics,phasechange,andcombustion.And finally,theycompletetheoverallprocessdesignbyselectingsensors(instruments)andvalves(final controlelements)andaddingfeedbackcontrolloopsandsafetyinterlocks – allofwhicharecommunicatedviaapipingandinstrumentationdiagram(P&ID).IfthePFDandP&IDdrawingsare preparedusingcommercialsoftware,thoseexercisescanprovidea “laboratory” experiencefor designstudents.
Theapproachtakenhereisforstudentstocompletea processdesign package,nota mechanical design package.Consequently,noemphasisisplacedondetailedstructuralormechanicaldesign ofequipmentbeyondbasicsizing(D, L,# tubes)ofpiping,vessels,andheatexchangers,alongwith sizing/selectionofcommodityitemssuchasblowers,pumps,andburners.Economicanalysesand costoptimizationmaybeaddedbyinstructors,butthesetopicsareexcludedfromthechaptercontentheretohelpensurethecoverageremainsmanageablefora45-lecture-hoursemesterora40lecture-hourquarter.
Inadditiontothenewprojects,extensivenewcontentisprovidedinseveralchapters:Chapter4 hasanewanalyticalmethodforcomputingequilibriuminfuel-richcombustionandanimproved methodofestimatingdestructionefficiencyforthermaloxidationbasedonVOCproperties;Chapters6and10provideathoroughdiscussionofdewpointsandbubblepointsfortwo-component refrigerantmixtures.Chapter13isbroadenedtoincludeengineeringmethodsforprotectionof materialsagainstthermalshockandthermalexpansion;Chapter17containsamorerigorous developmentofstatisticalmethodsforqualityandefficiency.
Finally,wewishtocallattentiontoaconcernwehaveencounteredregardingthenomenclature ofconservation,andwhyweelectedtoinventapedagogicallypreferredsymbology – eventhoughit modestlydisrespectsscientificorthodoxy.AsdescribedinChapters1–4,weuseanomenclature shortcuttoilluminatethefourelementsofconservationinacontrolvolumeforanyarbitraryproperty B:production P B ,inflow Bin ,outflow Bout ,andstorage S B
Strictlyspeaking,thesuperdotsymbolshouldapplyonlytothetwoflowterms,andtheproductionandstoragetermsshouldbeexpressedastimederivatives(withoutanysuperdot).Certainly, thisorthodoxyofferstheonlyvalidmathematicalwaytoconstructaconservationequationandthis bookcarefullyembracestheseconventionsinChapters2–4.Theorthodoxmathisvalidbecause (i)itissenselesstothinkofinflowsandoutflowsasbeingderivativesofsomethingelse(hence theneedforasuperdotsymboltodenotequantityflowingperunittime)and(ii)itiscompletely sensibletoapplydifferentiation(withrespecttotime)toextensivefluidpropertiessuchasmass, momentum,andenergy – becausetheycananddochangewithtime.
Despitetheobviousvalidityofthelong-acceptedorthodoxy,weintroduceourunorthodoxsuperdotsymbologyforproductionandstorageinChapter1foritsdescriptivesimplicity,andwebelieve thisapproachgreatlyhelpsstudentsunderstandthecontrastingconceptsof production and storage.Thisapproachprotectstheintegrityofthephysicsbyemployingthemathematicalrigorofthe Reynoldstransporttheoremwhenpresentingthedetailedconservationequations,whileitalso highlightsthebrightlinedistinguishingproductionandstorageinasimpleway.
Acknowledgments ManythanksareduetoProfessorsBettaFisher(CornellUniversity),MahboobeMahdavi(Gannon University),andZuhairIbrahim(UniversityofSouthernCalifornia)foridentifyingminorerrorsin thefirsteditionandsuggestingsectionswhereadditionalclaritywasneeded.Specialthanksto BrianKaiserforlengthydiscussionsaboutprinciplesofmulticomponent,multiphasemixtures, toJayHudsonforfluidheatersizingandsafetyknow-how,toCraigSchulerforhelpfuldiscussions oniterationtechniques,andtoGabrielGundlingforatutorialonballooningtechnologiesandpractices.SpecialthanksaredueagaintoEdThielenforcompellingnewartworkandtoDawnMartin forside-by-sideassistancewithtableprepandthekeywordindex.
TheauthorisindebtedtotheWileyteam,especiallyLaurenPoplawski,GabbyRobles,Jenny Seward,AmudhapriyaSivamurthy,andBeckyCowan,foradoptingthissecondeditiontextbook astheirprojectandfornumeroushelpfuleditorialandpedagogicdiscussionsalongtheway.
Dr.RichardJ.Martinplayedmajorrolesinthedesign,commissioning,operation,andtestingof combustionandheattransferequipmentinthe1980sand1990s.Duringthistimehebecamea namedinventoron24utilitypatents.Inthetwodecadesofthecurrentcentury,heinvestigated hundredsoffailures(e.g.fires,explosions,thermalequipmentfailures) – themajorityofwhichoriginatedwithinthermalsystemsemployedinmanyfieldsofcommerce.Hehasbeenavolunteer leaderoftechnicalcommitteesthatwritesafetystandardsforindustrialheatingequipment.
InadditiontoteachingthermalsystemsdesignandheattransferatUniversityofSouthern California,hehasalsotaughtcoursesinairpollution,fluidmechanics,heattransfer,programming applicationsinengineering,andthermalsystemsdesignattheCaliforniaPolytechnicState University(PomonaandSanLuisObispo).Dr.MartinwillbestartinganewengagementatSanta ClaraUniversityshortlyafterthepublicationofthistextbook,wherehewillteachHeatTransfer andThermalSystemsDesign.
Theprimarypurposeofthistextbookistoencourageandexemplifyhighquality,accurate,wellcommunicated,engineeringdesign.Adesignmaybeamazing,butifitispoorlycommunicatedto thosewhobuildanduseit,horribleconsequencescouldensue.Similarly,adesignthatiscommunicatedwithaccuracyandclaritymaybeequallyproblematicifitwasproducedusingerroneous principlesorinsufficientforethought.
Anotherimportantpurposeofthistextbookistomotivateengineeringstudentstoenhancetheir: (i) knowledge ofscientificfundamentalsthatgovernhumaninteractionswiththeenvironment; (ii) habits ofobserving,investigating,andanalyzingengineeringsuccessesandfailures;and (iii) desire toapplyengineeringknow-howtotheserviceofhumankind.
Theauthor’suniquebackground,comprisingequaltenuresininnovativedesignandtechnology failureinvestigation,givesthistextbookaperspectivedifferentfrommostothers.Notonlyarestudentsgivenvalidtoolsandmethodstosolvereal-worldengineeringdesignproblems,buttheyare alsocautionedaboutnumerouswaysthetoolsmaybemisunderstoodoraccidentallymisapplied. Bydetailingboth “right and wrong” approaches,studentsarebetterequippedtoadopttheright, whilerejectingthewrong.
Theauthorencouragesfeedbackfromreadersifanypartofthistextbookcontainsinaccurateor confusinginformationorcouldbenefitfromadditionaltechnicalcontentthatwasomitted.
AbouttheCompanionWebsite Thisbookisaccompaniedbyacompanionwebsite:
www.wiley.com\go\Martin\ThermalSystemsDesign2
Thiswebsiteincludes:
Instructorsite
• HomeworkSolutions
• AllfiguresfromtheprintbookdownloadableincolorinPowerPoint
• AlltablesfromtheprintbookdownloadableinPowerPoint
• Courseschedulesforinstructors
• Projectspecifications
Studentsite
• AllfiguresfromtheprintbookavailableincolorinPDF
Thermodynamics Thermodynamicsisapredictivesciencethatdescribesinterrelationsbetweenpropertiesofmattersuch astemperature,pressure,density,andentropyandhowthesepropertiesareaffectedbytransfersof mechanicalandthermalenergy(i.e.workandheat)intoandoutofthesystemandbyinterconversions ofenergyforms(e.g.kinetic,potential,electrical,chemical,thermal,andmechanical)withinasystem.
Theprimaryfocusofengineeringthermodynamicsistocharacterizethe “states” ofsubstances (typicallyfluids)participatinginathermodynamiccycleandhowthosestatesarealteredbyequipment(e.g.heatexchangers,compressors)incorporatedintothecycle.Thermodynamiccyclesare oftenassumedtobesteady,inthesensethatstatevariablesareinlocalequilibriumandstatesare unchangingintime,buttheyvaryspatially.Transientconditions,suchasstartupandshutdown, aretreatedseparately.
Thischaptercoversbasicthermodynamictopicssuchasunitsofmeasure,thermodynamiclaws, controlmasscontrolvolume,idealgaslaw,thermodynamicvariables,andavailability.Combustion,whichisaveryimportantbranchofthermochemistry,iscoveredinChapter4.Several advancedthermodynamictopicsarecoveredinChapter6.
1.1UnitsofMeasure Studentsmustnotundervaluetheimportanceofknowingandtracking “unitsofmeasure” when performinganengineeringcalculation.Inmanycases,erroneousresultsofanalysisordesignproblem-solvingcanbetracedbacktoanengineer’sfailuretoensurethatproperunitsandproperunit conversionsareincorporatedintoamathematicalrepresentationofaphysicalorchemicalprocess.
ThinkStop.Studentsareencouragedtodeveloparuthlesshabitoflabelingallnumericalvalues withthecorrectunitsandensuringthattheunitsontheleftsideofanequationareidenticaltothe unitsontherightside.AsimpleexampleofthisisseeninEquation(1.1),thegeneralized1Dheat conductionequation:
Thistextbookutilizesauniquesymbolconstitutingan “equals” signsuperimposedwitha “breve” or “crescent” diacriticalmark = tomean “hasunitsof.” Forexample,thephrase “heatfluxhas unitsofwattspersquaremeter” wouldbewrittensymbolicallyas q = W m2 .
ThermalSystemsDesign:FundamentalsandProjects,SecondEdition.RichardJ.Martin. ©2022JohnWiley&Sons,Inc.Published2022byJohnWiley&Sons,Inc. Companionwebsite:www.wiley.com\go\Martin\ThermalSystemsDesign2
Becausethermalprocessesofteninvolvechemicalreactions,studentsmustalsohavefullcommandoftheabilitytointerrelatemolarunitsandmassunits.Forexample,themolarmass(M , historicallycalledmolecularweight)ofcarbondioxide(CO2),whichiscomprisedofonecarbon atom(atomicmass, AC =12.0096 amu)andtwooxygenatoms(atomicmass, AO =15.99903 amu each),is M CO2 =44 00766=1×12 0096+2×15 99903 kg kmol.
ThinkStop.Studentsshouldindividuallyvalidatethatthefollowingsixexpressionsareequivalentrepresentationsofidenticalquantitiesofcarbondioxidemolecules:
1.2Mass/ForceUnitConversion TheuseoftraditionalEnglishunitsisintentionallykepttoaminimuminthistextbookbecausethe authorpreferstouse SI unitswhereverpossible,predominantlytoavoiderrorsassociatedwithnondecimalEnglish-to-Englishunitconversions(e.g.1.0 ft =12.0 in,or1.0 mi =5280 ft).Despitethe SI favoritismexpressedhere,engineeringstudentsarenonethelessencouragedtodevelopskillsfor workinginbothsetsofunits.Inkeepingwiththistheme,aminorityoftheexamplesandproblems givenhereareexpressedintheless-preferredunits.
Oneareawherethechoiceofunitscanbepotentiallyconfoundingisthedeterminationofweight (unitsofforce)bymultiplyingmasstimestheaccelerationofgravity.Theauthorhasfoundithelpfultoutilizetheconceptofa “gravitationalconversionconstant” (perNewton)asfollows: F w = mg gc ,where F w = N istheweightforce, m = kg isthemass, g = m s2 istheaccelerationduetogravity,and gc = kg m N s2 isthegravitationalconversionconstant.Thevalue ofthegravitationalconversionconstantcanbewritteninmanydifferentsetsofunits,threeof whicharegiveninEquations(1.2):
ThevaluesinEquations(1.2)demonstratethatthestandard(earth)accelerationofgravityacting on1.0 kg massgivesrisetoaweightforceof9.80665 N,whereasthesamegravityactingon1.0 lbm givesrisetoaweightforceof1.0 lbf.Bythislogic,itiseasytoseethata slug ofmaterialisequivalent to32.174 lbm ofthesamematerial.
Thegravitationalconversionconstantbecomesvitallyimportantinfluidproblemswherethe densityofafluid(massperunitvolume)affectsthecomputationofpressure(whichisforceper unitarea).Intheseproblems, lbm mustbeappropriatelyconvertedto lbf toobtainunitsofpressure (in psi or psf).The SI calculationsaremorestraightforwardinpartbecausemassisexpressedin kg andforceisexpressedin N,sotheambiguitybetween lbm and lbf isavoidedentirely.
1.4ControlMass,ControlVolume 3
Table1.1 Preferredconversionfactorsandreferencevaluesforfundamentalconstants.
Source: MartinThermalEngineeringInc.
Asetofpreferredconversionfactorsandfundamentalconstantsaregivenwithmoderatelyhigh precisioninTable1.1,wherereferencevalues(fromwhichallotherfactorsarederived)are indicatedwithanasterisk.Constantsnotdisplayedhere(e.g.enthalpyofformation)canbefound intheappendices.
1.3StandardTemperatureandPressure Thermodynamicsystemsinvariablyrequireadesignationofthe “standard” stateofmatter.Chemiststypicallyusethecondition Tref, chem =273.15 K (=0.0 C)and pref =1.00 atm.Otherpractitionersusedifferentreferencetemperatures(e.g. Tref, other1 =288.15 K or Tref, other2 =293.15 K),and recently,adifferentreferencepressure(e.g. pref, other =0.100 MPa)hasbeenutilizedbytheNational InstituteofStandardsandTechnology(ChaseJr.1998).
Forpurposesofthistextbook, StandardTemperatureandPressure(STP) isdefinedinharmonywiththe1971EditionoftheJointArmyNavyAirForce(JANAF)Thermochemical Tables(StullandProphet1971): T STP =298 15 K =25 0 C and pSTP =101 325 kPa .
1.4ControlMass,ControlVolume Thermodynamiclawsareappliedto systems.Sometextbooksexclusivelyequatetheterm system withtheuniquephysicalconstructknownasacontrolmass.Thetreatmenthereaffordsmoreflexibilitytotheterm system suchthatitcanapplytoeitheracontrolmassoracontrolvolume.
ControlMass.Systemswithaknownquantity(massormoles)ofmaterialinaknownlocation areusuallytreatedwithacontrolmassapproach.Inacontrolmass,thequantityofthematerialis notexplicitlydeterminedbyitsvolumeunlessthedensityisalsoknown.Variationsinthestateofa controlmassoccurasafunctionoftimeandtypicallyinvolveworkorheatcrossingthesystem boundarytoalterthesystem’sstate.Thestateofacontrolmasscanalsovarywhenchemicalreactionsoccurinsidethesystem,causingvariationsinthemolecularcompositionofthemassand interconversionsbetweenchemicalandthermalenergy.
ControlVolume.Systemsthatcontainworkingfluidsflowingthroughpipesanddifferentpieces ofequipmentareusuallytreatedwithacontrolvolumeapproach.Frequently,thepiecesofequipmentareconnectedtoeachotherinathermodynamic cycle.Theterm cycle isusedbecausethe workingfluidpassesthroughalltheequipmentinadesignatedsequenceandthenreturnstoits startingpointtobeginanothercycle.
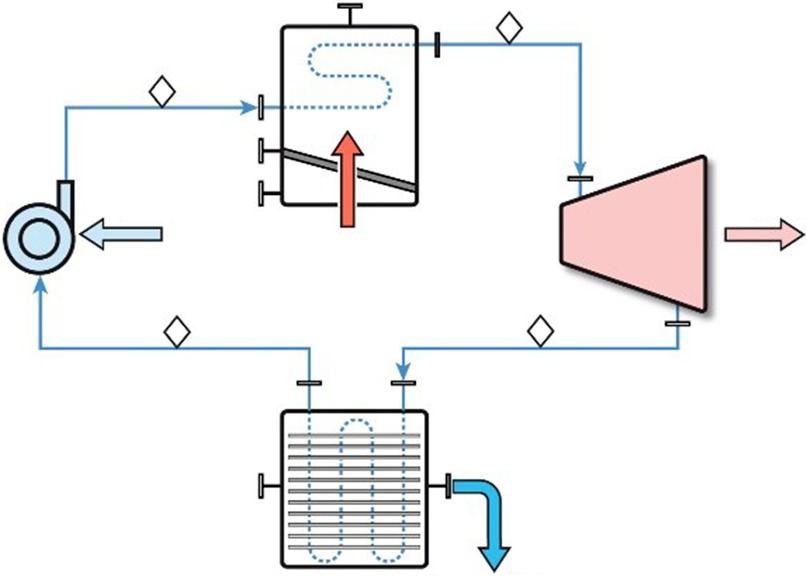
Aschematicversionoftheclassic “Rankinecycle” isshowninFigure1.1.
Analysisofathermodynamiccycletypicallyrequiresananalysisofuniquecontrolvolumessurroundingoneormorepiecesofequipmenttodeterminethethermodynamicstateofthefluidatthe variouslocationsorstates(1,2,3,4asshown).Controlvolumeanalysisalsocanbeusedtocompute theflowsofenergyandmassacrossacontrolvolumeboundary.Forexample,ifthepumpin Figure1.1 isknowntoproduceapressureriseof Δppump =250 psi,thenthepressureatState2 willbe250 psi higherthanthepressureatState1.Acontrolvolumedrawnaroundthepumpwould revealthreeimportantboundarycrossings – low-pressureliquidwaterenteringatState1,highpressureliquidwaterexitingatState2,andmechanicalpower(topressurizeandmovetheliquid water)enteringthroughthepumpshaft.
Aswillbeillustratedseveraltimesinlaterchapters,acycleanalysiscanonlyproceedtocompletionwhenenoughinformationisknown(orestimated)aboutthevariouscontrolvolumeboundary crossingstodeterminealltheunknownstatesandflowparametersalgebraically.InFigure1.1, uniquecontrolvolumesmaybeestablishedaroundtheboiler,turbine,andcondensertoevaluate
Figure1.1 ClassicRankinecyclewithwaterasworkingfluid. Source: MartinThermalEngineeringInc.
statevariablesupstreamordownstreamofeachofthosepiecesofequipment.Acontrolvolume aroundtheentiresystemmaybeusefultodetermineoneoftheenergyflowsiftheothersare known.Initially,acontrolvolumeanalysismightinvolveatrial-and-errormethodologytonarrow thefieldofpossibleequipmentchoices.
AsindicatedintheForeword,thistrial-and-errorprocess(placingequipmentontoablankschematicandbeginningthermodynamicanalyseswithlittleotherguidance)isoftenthemostdaunting aspectofathermalsystemdesigneffort.Overtime,studentswillgainanintuitivefeelforwhereto startananalysisandhowtoapplycontrolvolumesinthemostproductivemanner.Whenstudents learnto “embracethestruggle” (especiallywhenhelpedbyjudiciousquestionsfromtheinstructor), theirtenacity,engineeringknow-how,andhandybagoftoolswillpropelthemforwardtomany successfuloutcomes.
1.5LawsofThermodynamics Macroscopicsystems(unlikequantumsystems,whichonlyobeythesamelawsasmacroscopicsystemswhentheyareaggregatedtogetherinlargeensemblesofquantumparticles)obeyfourlawsof thermodynamics:
• The Zerothlaw statesthatwhentwobodiesaresimultaneouslyinthermalequilibriumwitha thirdbody,theymustbeinthermalequilibriumwitheachother,andallareatthesame temperature.
• The Firstlaw statesthatthetotalenergyofanisolatedsystemdoesnotchange,althoughthe energycanbetransformedfromonetypetoanother(e.g.kineticenergy,potentialenergy, mechanicalenergy,thermalenergy,andchemicalenergy).
• The Secondlaw statesthatthetotalentropyofanisolatedsystemmustincreaseorremainconstant(itmaynotdecrease).
• The Thirdlaw statesthatwhenapuresubstanceissolidifiedasaperfectcrystal,ithaszero entropyatanabsolutetemperatureofzero(0 K or0 R).
TheFirstandSecondlawsofthermodynamicsarequintessential conservationequations as coveredinthenextsection.Energyandentropycanflowacrossthesystemboundaryinoneormore ofthefollowingforms – shaftwork,heattransfer,flowsofmatterthatcarryenergyorentropy withthem.
Intheidealsituation,anincrementofshaftwork δW is reversibly deliveredtoorextractedfrom thefluidinthesystem,whichmeansthatnoentropyis produced byirreversibilitiesassociatedwith theshaftandimpellermotion.Conversely,theidealsituationforheatenteringorexitingasystem meansthattheheatflowisdeliveredreversiblyandthatzeroentropyis produced byirreversibilitiesassociatedwiththemolecularcollisionsatthesystemboundary.Nevertheless,whenanincrementofheat δQ crossesthesystem’sboundaryin(orout),regardlessofwhetheritisdelivered reversibly,theworkingfluid’sentropywillincrease(ordecrease)byanamount dS thatisexactly equaltotheincrementofheattransferred(δQ)dividedbythetemperatureattheboundary(T):
Theconceptof production isbestillustratedbycombiningitwiththeLatinphrase exnihilo, whichmeans “outofnothing.” Byusingthephrase production,exnihilo,studentscandifferentiatebetween production and storage,whichareaddressedforseveralconservedvariablesinthe contextoftheReynoldstransporttheoreminChapters2–4.
Toclarifyhere, storage simplyconnotesanaccumulationofaconservedquantity(e.g.massor energy)withinacontrolvolume,whereas production connotessomethingthatbasicallyappears outof(ordisappearsinto)thinair.Withinthiscontext,theReynoldstransporttheorem(see Chapter2)demonstrateshowachangein stored momentumorentropycanbecaused:(i)bya net inflow or outflow acrossthesystem’sboundaryor(ii)by production,whichisessentiallycreationordestruction exnihilo. Themostcommon irreversibilities inthermalsystemsare:
• Friction
• Heattransferacrossafinite(i.e.notinfinitesimal)temperaturedifference
• Masstransferbetweenregionsoffinite(i.e.notinfinitesimal)concentrationdifference
• Rapid(i.e.notinfinitesimallyslow)compression/expansionofafluidtoadifferentpressure
• Rapid(i.e.notinfinitesimallyslow)accelerationsanddecelerationsofafluid
Irreversibilitiesrelatedtoshaftworkinflowingsystemsareanalyticallyaddressablewithafactor calledisentropicefficiency(seeChapter9).Themathematicsofthisaresimple,becausethereversiblecaseforshaftworkisfullyisentropic.Onthecontrary,irreversibilitiescausedbyotherfactors (heat,mixing,orfriction)involvechangesinsystementropyeveniftheyareperformedreversibly, sotheconceptofisentropicefficiencyisnothelpful.Theconceptofexergyispresentedlaterin thischapter.Itsrelationshiptoisentropicefficiencyisindirectatbest,butitdoesprovideananalytic methodforaddressingirreversibilitiesinasystemthatcantransferheatandworktothe environment,whichisalsocalledthedeadstate.
1.6ConservationLaws “Conservationlaws” arevitaltotheanalysisofthermalsystems.TheFirstlawisanexampleofa conservationlawwhereinenergycanbetransformedamongdifferenttypes(e.g.thermal,chemical, andmechanical),butthetotalenergyofanisolatedsystemmustbeconserved.
ConservationlawscanbewrittenintheformofEquation(1.3),asfollows:
B + Bin = Bout + B 1 3 where B istheconservedquantity(e.g.mass,momentum,energy,entropy,andatoms)and:
B istherateof production of B (exnihilo)withinthesystem.
Bin istherateof inflow of B acrossthesystemboundary.
Bout istherateof outflow of B acrossthesystemboundary.
B isthetimerateofchangein storage of B insidethesystem.
Aswillbeseeninlaterchapters,theproductionratesofmass m ,energy e ,andatoms ie g C ,H ,O,N arealwayszeroforconventionalthermalsystems(i.e.whennuclearreactions aredisallowed),buttheproductionratesofmomentum p ,entropy s ,andspecies
1.7ThermodynamicVariableCategories
ke g H 2 O,CO2 ,O2 areroutinelynotzero.Itisalsoimportanttorememberthatmass,energy, entropy,species,andatomsarenonnegativescalarquantities,butmomentumisavectorquantity andcanthereforetakeonnegativeorpositivevalues.Angularmomentumisalsoavectorquantity thatobeysitsownconservationequation,however,thisbookexcludesdetailedcoverageofthis phenomenon.
Theauthornotesthatuseofa super-dot symbolaboveeachvariableinEquation(1.3) isasimplisticwayofrepresentingthattheunitsofallfourtermsincludereciprocaltime(e.g. sec 1).This simplificationisintroducedhereasateachingrubricbecauseeventhoughthephysicsofconservationiswellestablished,themathematicalsymbologyisnot.InChapters2–4,wesummarizeseveralexpressionsoftheReynoldstransporttheorem(Reynolds1903)fordifferentconserved variables.Thisclassictreatmentofthedifferentialmathematicsofconservationissimultaneously vitalanddense,andinouropinion,itfailstopersuasivelyreinforcephysicalintuitionaboutthe conceptof production,exnihilo,hencetheneedforEquation(1.3)anditsunorthodoxsymbology.
Foramorecomprehensiveviewofthephysicsofconservationinacontrolvolume,werecommendthatstudentsreviewandcomparethetreatmentsbyWhite(2016)andMoranetal.(2018).We feelstronglythatMoran’schoicetolimitusageoftheterm “conservation” toenergyandmass accountingsonly(andtherebytodisallowtheSecondlawandtheMomentumequationfrombeing classifiedas “conservationequations”)shortchangesstudentsfromasignificantlearningframework.Incontrast,WhitefirstintroducestheReynoldstransportTheoremasbeingfullyapplicable toanyproperty(B)ofaworkingfluidandthenproceedstodeveloptheaccountingequationforthe simplestcase – conservationofmass.
WhereMoran’streatmentofmassandenergyas “conserved” quantitiesissatisfactoryasfarasit goes,White’streatmentissuperiorbecauseitpermitsmomentumandentropyalsotobeviewedas “conserved” quantitiesthatobeytheReynoldstransporttheorem(whichtheymostcertainlydo).In White’sapproach,thereisonemajordistinctionbetweenmassandmomentum – the “production” termisidenticallyzerofortheformer,andonlyoccasionallynonzeroforthelatter.Nonetheless, WhiteusesalongerversionofEquation(1.3) forbothmassandmomentum,whereasMoran excludesmomentum(andentropy,species,etc.)fromtherealmofpropertyconservationentirely, becausehefailstoaddresstheconceptofproduction.
1.7ThermodynamicVariableCategories Studentsaresometimesconfusedaboutthenatureanduseofdifferentthermodynamicvariables. Thefollowingcategoriesanddistinctionsareofferedtohelpcharacterizethesevariablesforgases andsomeliquids.
Forcontrolvolumesystems(e.g.theRankinecycleofFigure1.1),theworkingfluidspatially experiencesdifferentthermodynamicstatesasthefluidpassesthroughdifferentprocessingequipment(e.g.pumps,boilers,turbines,etc.).
Forcontrolmasssystems(e.g.gasolinevapor,air,andcombustionproductsinthecylinderofan automobileengine),thefluidstateisoftenconsideredtobeuniforminspace(i.e.throughoutthe controlmass)andvaryingintime.
Controlvolumesystems,wherethefluidflowrateisunchangingintime,arecalled steady-flow systems.Iftheindividualfluidstatesateachpointinspacedonotvarywithtime,thesystemis called steadystate
Inthistextbook,theterm “statevariable” isappliedtointensivepropertiesofsubstances(e.g.temperatureandpressure)thatdo not dependonthequantityoffluidpresentorrateoffluidflowing throughthesystem.Similarly,theterms “quantityvariable” (e.g.mass)and “flowvariable” (e.g. enthalpyflow)areappliedtoextensivepropertiesthatexpresssomemeasureofthesizeofthesystem. Forexample,adirigiblecontainsabout10000000×asmuchheliumasapartyballoon,andanocean cruiselinerdieselengineproducesabout25000×asmuchshafthorsepowerasachainsawengine. Whenavariablecanbeexpressedinboththeextensiveandintensiveforms(e.g.enthalpy H and specificenthalpy h)thelowercasevariablerepresentstheintensivepropertyandtheuppercasevariablerepresentstheextensiveproperty.Itshouldbenotedthatcertainintensivestatevariables(e.g. temperature, T)historicallyhaveusedtheuppercaseformandcertainquantityvariables(e.g.mass, m)historicallyhaveusedthelowercaseform.Thisbookmakesnoattempttoovercometheseinconsistencieswherehistoricalinertiaisgreat.
StateVariables(ControlMass).Foracontrolmass,theintensivepropertiesofthesystemthat defineitsthermodynamicstatearetemperatureandpressure,withvolumehavingrelevanceas astatevariableifthefluidisinasaturated(two-phase)condition.Atleastonequantityvariable (seelater)isalsorequiredtodefinethestateofacontrolmass.
T = TemperatureK
p = PressurePa = Volumem3
M = Molarmasskg kmol
StateVariables(ControlVolume).Foracontrolvolume,therearenumerousintensiveproperties thatcanbeutilizedinterchangeablytofixorlockthestateofthefluid.Intensivethermodynamic statevariablessuchas h, s,and v aretabulatedforpuresubstances(andsomemixturessuchasair), sothatifyouknowanytwostatevariables,youcanfindtheothers.
T = TemperatureK
p = PressurePa
v = Specificvolumem3 kg
s = SpecificentropykJ kg K
h = SpecificenthalpykJ kg
s = MolarentropykJ kmol K
h = MolarenthalpykJ kmol
M = Molarmasskg kmol
QuantityVariables.Foracontrolmass,thequantityvariablesarenecessarytodefinewhatconstitutesthesystem’sentiretyorextent.Controlvolumesfrequentlydon’tneedtheirquantityvariablesanalyzed,butasignificantcounterexampleofthisisaflowingsystemwhosetransient behaviorisjustasimportantasitssteady-statebehavior.Thetimetoreachsteadystatewilldepend onthetotalmassoftheworkingfluidinallpartsofthesystem.Heat(Q)andwork(W)arequantity variablesforthermalandmechanicalenergythatcrossthecontrolmassboundaryfromthebeginningtoendofatransientprocess.Molefractionsandmassfractionsareintensiveproperties,but theycanbeusedtocomputeproportionsofdifferentconstituentsinthecontrolmass.
m = Masskg W = WorktransferredkJ
n = Moleskmol Q = HeattransferredkJ
H = EnthalpykJ χ i = Molefractionikmoli kmoltot
S = EntropykJ K Y i = Massfractionikgi kgtot