1 Introduction to Immunology Chapter Outline
■ Objectives—LeveL i
After this chapter, the student should be able to:
1. Define immunology, immunity, antigen, humoral, serum, and plasma.
2. Give examples of immunity that occurs in simpler species.
3. Compare and contrast the external and internal innate defense systems.
4. Compare and contrast innate immunity and acquired immunity.
5. List factors that affect the innate immune system and describe the resulting effect.
6. Describe the cellular appearance in terms of relative size, nucleus shape, associated CD marker, color when Wright stained, and presence or absence of granules for each of the following cell types: neutrophils, eosinophils, basophils, mast cells, monocytes, macrophages, and dendritic cells.
7. Describe the function(s) of the cell types in (6).
8. Describe the composition of the white blood cells in blood in terms of percentages, and list which cells are found predominately in tissues, not in blood.
9. List the different names for macrophages as they reside in different tissues.
10. Describe phagocytosis and list cells that perform it.
11. Define phagosome, lysosome, chemotaxin, and opsonin.
12. Describe acute phase proteins and give examples.
13. Describe C-reactive protein, alpha-1 acid glycoprotein, haptoglobin, fibrinogen, and serum amyloid A.
14. Describe cytokines and complement proteins as well as autocrine, paracrine, and endocrine.
15. Describe lymphocytes in terms of amount of cytoplasm, nucleus, product, and antigen specificity.
16. Differentiate between primary and secondary lymphoid organs in terms of functions and organs involved in each.
17. Describe a bursa.
18. Describe the function and architecture of a lymph node, spleen, SALT, and MALT.
19. Diagram lymphatic circulation.
20. Describe the size of a thymus from fetal development to adulthood.
21. Compare and contrast a follicle and a germinal center.
22. Discuss the role of the thymus in T cell maturation.
23. Describe where differentiation and maturation of a B cell occurs from the pre-B cell to a mature B cell and from a B cell to a plasma cell.
24. Explain what a CD marker is, and list CD markers that are on B cells.
25. Identify and discuss the function of the following key antigens on T cells: CD2, CD3, CD4, and CD8.
Objectives—Level I and II . . 1
Key terms . . . . . . . . . . . . . . . . 2
Introduction . . . . . . . . . . . . . . 3
The innate immune system . . . . . . . . . . . . . . . . . . 4
The innate immune system components of the external/ internal interface . . . . . . . . . . 4
The innate immune system internal components . . . . . . 5
The acquired immune system . . . . . . . . . . . . . . . . . 12
The cells of the acquired immune system . . . . . . . . 12
Antibody introduction— General structure . . . . . 12
The cellular arm . . . . . . . . 13
The lymphoid organs . . . . . . 14
The primary lymphatic organs . . . . . . . . . . . . . . . 14
The secondary lymphatic organs . . . . . . . . . . . . . . . 16
Associated laboratory method . . . . . . . . . . . . . . . . . 17
Review
■ Objectives—LeveL i (continued )
26. Compare and contrast the T-cell receptor on a T cell and the surface immunoglobulin on a B cell.
27. Define human leukocyte antigen (HLA) and major histocompatibility complex (MHC)
28. Differentiate T cell subsets on the basis of antigenic structure and function.
29. Explain how natural killer cells differ from cytotoxic T cells.
30. Discuss the principles involved in the analysis of cells by flow cytometry.
31. Analyze data obtained using a flow cytometer.
32. Interpret the use of gating in a particular flow cytometer analysis.
33. Apply the information in this chapter concerning CD markers on particular cell types, and evaluate the significance of flow scattergram data.
34. Define apoptosis.
■ Objectives—LeveL ii
After this chapter, the student should be able to:
1. Name seven types of pathogen-associated molecular patterns that trigger the innate immune response.
2. Describe pathogen recognition receptors.
3. Describe Toll-like receptors.
4. Describe antimicrobial peptides, and name two families.
Key terms
acquired immune system
acute phase proteins
acute phase reactants
adaptive immune system
alpha-1 acid glycoprotein
antigen antigen presentation antimicrobial peptides
autocrine response
basophil
bone marrow
C-reactive protein (CrP)
cathelicidins
chemotactic factors
complement proteins
complement system
cytokine defensins
dendritic cells
diapedesis
endocrine response
eosinophil
fibrinogen
flow cytometry
haptoglobin
humoral
immune system
immunity immunology
inflammation
innate immune system lectins
macrophages
mast cells
natural immune system
natural killer (nK) cells
neutrophils
opsonin
paracrine response
pathogen-associated molecular patterns (PamPs)
pattern recognition receptors (Prrs) phagocytosis
plasma
polymorphonuclear leukocyte
serum
serum amyloid a t cells
toll-like receptor (tLr)
intrOductiOn
When you registered for a course in immunology or decided to read a book about immunology, you may have thought that you would be studying a lot about vaccines. As you opened this book and perused the chapter titles, you probably realized that there is much more to immunology than vaccines, and I can wholeheartedly agree with this conclusion. The immune system is a complicated and wondrous thing to learn about. The knowledge that we have gained about the immune system not only helps us prevent diseases with vaccines, but also helps us diagnose disease and develop drugs to thwart it. New inroads to understanding the immune system continue to help improve health. I hope you enjoy your journey through this book and all your studies of immunology and serology.
Immunology is the study of the reaction when the host encounters a foreign substance. The foreign substance responded to is called an antigen. Immunity is the discrimination between self and nonself and the subsequent protection from nonself. The system in an individual that is related to this response is called the immune system.
Because every living thing on this planet can be invaded, vertebrates and invertebrates alike needed to develop defense mechanisms. An early evolutionary example of the presence of an immune system includes the fact that some bacteria add a methyl group to their DNA, and if they are invaded by the injection of DNA from a bacteriophage (a virus that attacks bacteria), bacterial enzymes will destroy the nonmethylated DNA (Figure 1.1 ■). Another example of a primitive immune system was discovered as a result of an experiment in which a blue sponge and a red sponge were placed in a blender to dissociate them into single cells. These cells were then poured back into an aquarium. If there had been no recognition of the difference between self and nonself, the formation of a purple sponge or a mosaic sponge would have been expected. However, because of self-recognition, the actual result was the formation of a blue sponge and a separate red sponge (Figure 1.2 ■). The self-recognition in the sponges involves surface carbohydrates and lectin interaction. Lectins are molecules that specifically bind to carbohydrates. Many
Bacterial enzyme specific for nonmethylated DNA
other examples of immunity in earlier species exist, and it is important to realize that many of the primitive mechanisms of self-recognition and protection have been maintained in vertebrates where they have been augmented with additional protective mechanisms.
The study of the immune system begins with its separation into the innate or natural immune system and the acquired immune system. The hallmarks of the innate immune system are that it is available quickly and is not specific for the pathogen in question. Thus, an innate immune response to the measles virus could also work against the influenza virus. The hallmarks of the acquired or adaptive immune system are that it is specific, has a large scope, can discriminate, and has a memory. The specificity engenders a reaction to a particular pathogen without reaction to nonrelated structures. Scope involves the fact that the acquired or adaptive immune system is diverse enough that it can react to many different pathogens and molecules, including pathogens that
■ Figure 1.1 Bacterial immunity. Bacterial enzymes destroying nonself DNA which entered in an attack by a bacteriophage virus.
Bacteriophage
Viral DNA not methylated
Methylated bacterial DNA
■ Figure 1.2 When a red sponge and a blue sponge are blended together, they reaggregate, not as a purple or mosaic sponge but as a red sponge and a blue sponge because the individual cells recognize self-surface molecules and aggregate.
✪ Table 1.1
The innate and the Acquired immune response
Type of response
Specificity
Innate Specific to certain patterns recognized in many pathogens called pathogenassociated molecular patterns (PAMPs)
Acquired Highly specific; recognizes epitopes with an exact fit
Molecules involved in recognitions
Pattern recognition receptors (PRRs) can be cell surface or in solution; an important cell surface receptor family is called the Toll-like receptor family
Antibody in solution and on the surface of B cells
T-cell receptors on the surface of T cells
Cells involved
Neutrophils, dendritic cells, monocytes, macrophages, mast cells, basophils, eosinophils, and NK cells
Lymphocytes
B cells and T cells with antigen presenting cells
have not yet evolved. The concept that the acquired immune system can discriminate is based on the fact that the immune system will produce a response only to those molecules that are not present naturally in the individual; that is, there is discrimination between what is self and what is nonself. The acquired immune response improves with subsequent exposure to the pathogen, and this memory of the response is the basis of the success of vaccines (1, 2, 3, 4, 5, 6, 7). Table 1.1 ✪ summarizes differences between the innate and the acquired immune response.
Checkpoint! 1.1
A thorn carrying bacteria and a fungus pierces your skin while you are gardening. Cells come and gobble up (phagocytize) the bacteria and the fungus immediately. From what you just learned about the innate and acquired immune system, were these cells from the innate or acquired immune system?
the innate immune system
A piece of meat that you left out on the counter during a hot day would smell of decay by the time you got home. This piece of meat has neither an innate nor an acquired immune system and, thus, would be rapidly invaded. Because you do have these systems, you can be confident that you will not be invaded by bacteria of decay during a normal day. The parts of the innate immune system involved in this protection are those things that separate the inside of the human body from the outside. The innate immune system has components that function at
Time to response
Memory (enhanced response with next interaction with the same antigen) effector
Minutes to hours No
Days Yes
Molecules
Cytokines
Antimicrobial peptides
Acute phase proteins
Complement Perforins
Antibody
Cytokines Perforins
the interface between the external world and the individual in addition to internal components that protect after entry.
the innate immune system
cOmpOnents Of the externaL/ internaL interface
The external components that prevent entry of pathogens include the physical and chemical barriers formed by skin, mucous, and the associated cilia, earwax, lysozyme in tears, and the acidic pH of sweat, stomach acids, urine, and vaginal fluids (Figure 1.3 ■). The normal bacteria of your skin and gastrointestinal tract are also part of the innate immune system. Skin serves as a barrier, and mucous and earwax serve to entrap pathogens prior to entry. Cilia in the respiratory tract push out the invaders, and the reflex that causes coughing and sneezing help push invaders away from the host (Figure 1.4 ■). Lysozyme is an enzyme present in tears and saliva that digests the cell wall of gram-positive bacteria. The acidic pH of many fluids inhibits the survival and growth of pathogens. When these innate systems are not intact, an opportunity exists for the entrance of pathogens. It is obvious that there is an increased risk of infection when there is a break in the skin, but what about a pH change? Do you think this pH change would allow for pathogen growth? The answer is yes. For example, previous vaginal douches that were at neutral pH caused a decrease in vaginal acidity, which was thought to increase susceptibility to infection. The next time you are in a store that sells vaginal douches, notice that most brands now say “pH adjusted,” “contains
Tonsil
Lymph nodes
Thymus
Spleen
Peyer’s patches in intestine
Lymph nodes
Lymphatics
Bone marrow
Earwax
Lysozyme in tears
Mucous
Sweat
Stomach acid
Normal flora
Vaginal secretions
Urine
Skin
an antibiotic. Because antibiotics work systemically, the bacteria in the throat are not the only ones killed, and the removal of the normal gut flora can result in overgrowth of resistant bacteria and the resultant nausea and diarrhea. We will not talk much more about innate defenses, but their importance should not be forgotten.
the innate immune system internaL cOmpOnents
■ Figure 1.3 (a) Blue arrows (right side): External components of the innate immune system: human body with skin, mucous and the associated cilia, earwax, lysozyme in tears; the acid pH of sweat, stomach acids, urine, and vagina fluids; and the normal bacteria of the skin and gastrointestinal tract labeled. (b) Red arrows (left side): organs of the acquired immune system: tonsil, lymph node, thymus, spleen, Peyer’s patches, lymphatics, bone marrow. Source: “Human Body image” by Joanna Cameron. DK Human Body Books, reprinted by permission of Dorling Kindersley.
vinegar,” or some other expression that indicates that they will not change the protective low pH of the vagina. However, even with this lower pH, vaginal douches still disrupt the mucous layer and the normal flora of the vagina. Perhaps because of this disruption of the innate immune system, vaginal douching has been linked to bacterial vaginosis, pelvic inflammatory disease, cervical cancer, and increased transmission rates of HIV (8).
The normal bacteria that colonize an individual are called their normal flora. Alteration of the normal flora through the use of antibiotics can upset the balance in an area and lead to an infection with a pathogen that normally would not be able to grow. An example of this is the gastrointestinal distress that can result after treatment of strep throat or any infection with
The internal components of the innate immune system include both a cellular and a molecular component that is in the fluid phase of the blood. This fluid phase is called the humoral component of the blood, and when blood has been allowed to clot, the fluid phase is called serum. If anticoagulants have been added, the fluid phase is different and is called plasma. Clotting factors are no longer in the serum because they have joined the clot, but these factors remain in plasma because the blood was not allowed to clot. These internal components of the innate immune system categorized into the cellular components and the humoral components will be discussed separately.
the cells of the innate immune system
The cells of the innate immune system are all white blood cells and include granulocytes (neutrophils, eosinophils, and basophils), monocytes/macrophages, mast cells, dendritic cells, natural killer (NK) cells, and lymphokine-activated killer (LAK) cells. Surface proteins of white blood cells are used along with the cells’ microscopic appearance to characterize and differentiate the cells. These surface protein markers were identified using monoclonal antibodies and are numbered beginning with the designation CD for cluster of differentiation. An example of the use of CD markers is the commonly used term, the CD4/CD8 ratio, to discuss an AIDs diagnosis (9, 10, 11).
All white blood cells express CD45. Granulocytes, which express CD45 and CD15, have granules in their cytoplasm
■ Figure 1.4 This image shows the spread of droplets from an uncovered sneeze. Source: © CDC/Brian Judd.
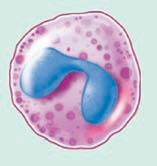
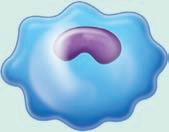
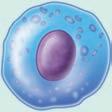
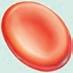
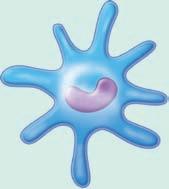
■ Figure 1.5 The blood cells are shown in (a)–(f) and the tissue white blood cells are shown in (g), (h), and (i). The 3 types of granulocytes: (a) neutrophils, (b) eosinophils, and (c) basophils. As well as a (d) monocyte, (e) lymphocyte, and (f) red blood cell. Next are white blood cells of tissue: (g) macrophage, (h) mast cells, and (i) dendritic cells. Source: GOODENOUGH, JUDITH; MCGUIRE, BETTY A., BIOLOGY OF HUMANS: CONCEPTS, APPLICATIONS, AND ISSUES WITH MASTERINGBIOLOGY®, 4th, ©N/A. Printed and Electronically reproduced by permission of Pearson Education, Inc., Upper Saddle River, New Jersey.
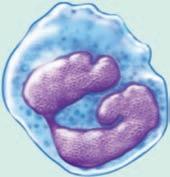
(see Figure 1.5 ■ for the three types of granulocytes (neutrophils, eosinophils, and basophils) as well as monocytes, mast cells, and macrophages, which are shown with a lymphocyte and a red blood cell for relative size comparisons). The three types of granulocytes are named for their characteristic staining pattern when using Wright’s stain. All granulocytes can be recruited from the blood by chemotaxic factors to enter the tissues that stimulate them to move to the site of an infection. Neutrophils are the most abundant type of granulocyte, and they contain neutrally staining granules, that is, their granules do not stain when Wright’s stain is utilized. Fifty to 70% of white blood cells in the blood are neutrophils. The nucleus of a neutrophil is irregular in shape with multiple lobes, which gives the neutrophil the alternative name polymorphonuclear leukocyte with the shortened names of either polys or PMNs. Neutrophils are the first cells that enter the site of an acute infection. They reside only about 12 hours in the circulation or one to two days after migration to the tissue. These cells are in the pus of an infected area, and eventually macrophages come into the infected area as well. Neutrophils are very active in phagocytosis (engulfment and digestion) of foreign cells and particles. They have recently been found to be involved in the presentation of the antigen to T cells, which is one type of cell of the acquired immune system. Antigen
presentation, is a process in which a cell of the innate immune system shows or presents the antigen to the cells (lymphocytes) of the acquired immune system. The numbers of neutrophils can increase in an acute infection or inflammation. The eosinophil contains red-stained granules after Wright’s staining, which indicates that the granules are acidic. These cells are involved in antiparasitic responses and allergic reactions. The numbers of eosinophils can increase during an allergic reaction, parasitic infection, and skin inflammation. In blood, 1 to 3% of the white blood cells are eosinophils. The basophil, the rarest of the granulocytes, composes only 0.4 to 1% of white blood cells. The numbers of basophils can be elevated in leukemia, in some allergic responses, in patients with chronic inflammation, and in patients following radiation therapy (9, 10, 11). After Wright’s staining, basophils have blue-black-stained granules, indicating that the granules are basic. The function of basophils is not completely defined, but we do know that they play a role in inflammation and allergy. Mast cells are very similar in appearance to basophils but come from a different lineage. Mast cells have a surface receptor that binds IgE (the antibody molecule involved in allergy) with a high affinity, and this relates to their primary role in allergic and antiparasitic reactions. Mast cells contain granules of histamine and heparin and, when bound to IgE,
Tissue
are responsible for most of the effects in allergic reactions. Mast cells are found in tissues and connective tissues and near mucosal surfaces (9, 10, 11).
Macrophages express CD14 and are the largest white blood cell. They are called monocytes while they are in the blood, and when they travel to tissues, they are called macrophages. Macrophages are called Kupfer cells in the liver, microglial cells in neural tissue, histiocytes in connective tissue, osteoclasts in the bone, mesangial cells in the kidney, and either alveolar macrophages or dust cells when they are in the lungs. When macrophages have accumulated lipids in an arterial wall in a plaque of atherosclerosis in coronary artery disease, they are called foam cells. Of the white blood cells in blood, 4 to 6% are monocytes. The number of monocytes in the blood can increase in infection, inflammation, and certain cancers. The life span of a macrophage can be several months. These cells, like neutrophils, are very important in phagocytosis of pathogens. Macrophages are more important in antigen presentation than neutrophils (9, 10, 11).
Dendritic cells, which express CD11c, are found in an immature state in the bloodstream and in a mature state in tissues. The concentration of these cells is very low, but they are very active and efficient in immune processes including phagocytosis and antigen presentation. Dendritic cells are named for the long branching processes that they project (9, 10, 11).
Natural killer (NK) cells are lymphocytes similar to the T and B lymphocytes of the acquired immune system. Unlike B or T cells, however, NK cells do not have the epitope-specific surface receptors of the B cells (immunoglobulin) or T cells (the T-cell receptor), so they are not antigen specific. NK cells are larger and more granular than T or B cells and make up about 10 to 15% of the peripheral blood lymphocytes or about 2 to 3% of the white blood cells of the peripheral blood. NK
✪ Table 1.2
The Cells of the innate response
Granulocytes
Granulocytes
eosinophils
Granulocytes basophils
Monocytes
Macrophages
Mast cells
1–3%
0.4–1%
4–6% Found in tissues
Found in tissues
Dendritic cells Found in tissues
Natural killer (NK) cells
Lymphokine-activated killer (LAK) cells
2–3% also 10–15% of the lymphocytes
Present only when NK cells are activated
cells are recognized for their ability to kill tumor cells and virally infected cells but can also respond to bacterial and protozoal infections. NK cells make direct contact with their target and secrete cytotoxic proteins, including perforins and granzymes, which kill the target cell. Perforins function just as their name suggests, by perforating the target cell, causing leakage and lysis. The markers used to differentiate NK cells from other white blood cells are that NK cells are CD3 negative and CD56 and CD16 positive. Because CD16 is a molecule that binds antibody by its Fc region, its presence enables NK cells to bind antibody-coated target cells and kill them by a process called antibody directed cellular cytotoxicity (ADCC). This is one of many places in which the innate immune system works with the adaptive immune system to remove pathogens. A 2009 paper suggests that in addition, NK cells may have memory, thus having some components of the innate immune response and some components of the acquired immune system (12). NK cells also have a surface receptor for a cytokine called IL-2, and once they interact with this cytokine, they change and become LAK cells. These cells are much more efficient at killing their target, than NK cells, and partially successful trials have involved the use of LAK cells in cancer therapy. LAK cells also produce cytokines, which increase the immune reaction of other cells. See Table 1.2 ✪ for a summary of information about the cells of the innate immune system (9, 10, 11).
✓Checkpoint! 1.2
Which is the largest white blood cell? [Hint: This type of cell has a different name for every tissue it is in.]
Phagocytosis—first cell at an infection
Antigen presentation
Allergic and antiparasitic responses
Not completely defined but have a role in allergy and antiparasitic responses
Phagocytosis
Antigen presentation
Primary cell in allergy and antiparasitic responses
Phagocytosis and antigen presentation
Killing of tumor cells, virally infected cells, some bacteria and protozoan
Improved killing of tumor cells, virally infected cells, some bacteria and protozoan
+
CD15 +
CD15 +
CD14 +
Receptor for IgE
CD11c +
CD56 + CD16 + CD3 -
CD56 + CD16 + CD3 -
the molecules of the innate immune system
Three classes of molecules can be discussed in relation to the innate immune system: (1) recognition molecules, termed pattern recognition receptors, (2) molecules produced in response to an infection, which include cytokines, antimicrobial peptides, and acute phase reactants, and (3) the complement proteins (1, 3, 6, 7, 13, 14).
The innate system is not specific in its reaction to a particular pathogen, so how then can the response be against foreign particles rather than self? The answer to this lies in the pattern recognition molecules that are involved in the innate response. The innate immune system recognizes certain surface molecules that are expressed in groups of microorganisms. The patterns that are recognized are called pathogen-associated molecular patterns (PAMPs). Common PAMPs include:
1. Bacterial and viral unmethylated DNA containing increased levels of cytosine with a phosphodiester bound to guanine (CpG dinucleotides) (this is reminiscent of the primitive immunity of some bacteria in the introduction section of this chapter).
2. Surface expression of the terminal sugar (saccharide) mannose that is a common feature on microbial glycolipids and glycoproteins but is not terminal on human structures (this is reminiscent of the self-recognition of sponges in the introduction section).
3. Other fungal-associated saccharides.
4. Lipopolysaccharides (LPS) of the gram-negative cell wall.
5. Peptidoglycans and lipotechoic acids of the gram-positive cell wall.
6. Bacterial flagellin.
7. The amino acid N-formylmethionine found in bacteria but not in mammals.
8. Double-stranded and single-stranded viral RNA.
The molecules of the innate immune system that recognize these PAMPs are called pattern recognition receptors (PRRs), PRRs can be either on the cell surface or in solution in the serum. Cell surface PRRs can aid in the phagocytosis of a foreign particle. The cell surface binding of PAMPs to PRRs can also cause the release of effector molecules called cytokines, which are secreted by cells and interact with receptors on the surface of cells to create a response. Cytokines can act on the cell that produced them (autocrine response), on nearby cells (paracrine response), or on distant cells (endocrine response) (1, 3, 6, 7, 13, 14). Cytokines will be discussed in more depth in Chapter 4, Cellular Immunity.
An important set of cell surface PRR is called the Toll-like receptor (TLR) family of molecules. Each of the 12 different types of Toll-like receptors binds a different PAMP. Binding to any of these molecules causes inflammation, immune cell proliferation, and chemotaxis. The PRRs in solution include
■ Figure 1.6 There is a species specificity to some infections, which involves cell surface molecule recognition. But do not be too complacent, some diseases can be transmitted from other species. This photo is of an aquarium water frog. In 2009, the CDC investigated diarrhea caused by Salmonella associated with contact with water frogs. Source: © CDC/Christine Prue.
an acute phase protein called C-reactive protein. This protein binds PAMPs and triggers complement binding and phagocytosis of the particle.
Because the ability of a pathogen to infect the host cells is also regulated by recognition of certain surface molecules, a bacterium or a virus has the ability to react with cells bearing certain molecules on their surfaces and will not infect cells that do not express these molecules. The combination of the rapid sequestering of certain pathogens by the innate immune system and the fact that pathogens can infect only cells with certain surface molecules leads to the fact that many infections are species specific, so you will not get the same diseases as your goldfish—no ick (a common goldfish disease) for us. However, do not be too complacent, some diseases can spread from animals to humans. Shown in Figure 1.6 ■ is an aquarium water frog, and such animals have been linked by the CDC to the spread of diarrhea caused by Salmonella (1, 3, 6 , 7, 13, 14)!
The antimicrobial peptides produced by the innate immune system in response to infection are usually less than 100 amino acids in length. Similar peptides are found as far back in evolution as prokaryotes. Antimicrobial peptides bind to the cell wall of the microbe and increase the membrane permeability to ultimately cause the pathogen’s death. Two major families of antimicrobial peptides in humans are defensins and cathelicidins. These peptides have very different secondary structures but have a similar function. Both are produced by epithelial cells and phagocytes and provide protection from outside attacks at all epithelial surfaces from the mouth to the anus (1, 3, 6, 7, 13, 14).
The acute phase proteins are proteins whose concentrations change with an inflammation, and the levels of these proteins can either be increased or decreased by the inflammation. The elevation of these proteins can cause the increase in
erythrocyte sedimentation rate (ESR) that is seen with inflammation. The cytokine IL-6 stimulates the production of acute phase proteins, which include C-reactive protein, alpha-1 acid glycoprotein, haptoglobin, fibrinogen, serum amyloid A, and complement proteins (which are discussed separately). An acute phase reactant that is frequently used to assess inflammation is C-reactive protein, the PRR mentioned earlier.
C-reactive protein (CRP) was so named because it reacts with the C-polysaccharide of Streptococcus pneumonia. Sensitive assays for increases in this marker have recently been approved to measure risk of cardiovascular disease. CRP is a sensitive indicator of inflammation, rising up to 1000 fold quickly after inflammation and rapidly falling when the inflammation resolves. This marker is involved in the immune system at many levels: It activates complement, is an opsonin, and this enhances cell-mediated cytotoxic effects on the pathogen. Alpha-1 acid glycoprotein is found to be elevated in some autoimmune disorders. Like many of the acute phase proteins, the liver produces alpha-1 acid glycoprotein. Its primary function may be the inhibition of progesterone and other drugs. It has also been called orosomucoid. Haptoglobin is an acute phase protein that removes free hemoglobin that has been released through injury and red blood cell lysis. The haptoglobin molecule acts as an antioxidant. Fibrinogen is an acute phase protein molecule that is involved in the coagulation pathway. It is converted to fibrin and then fibrin is cross-linked to form a clot. Serum amyloid A is an apolipoprotein, which is associated with highdensity lipoprotein (HDL) in the blood stream. Serum amyloid A is involved with the transport of cholesterol to the liver and in the induction of extracellular matrix degrading enzymes that are involved in repair after infection-induced tissue damage. Additionally, it is a chemoattractant, bringing cells of the
✪ Table 1.3
The Molecules of the innate immune response
Molecules of the innate immune System
Recognition molecules
Pattern recognition receptors
Effector molecules
Cytokines
Effector molecules
Antimicrobial peptides
Effector molecules
Acute phase proteins
Effector molecules
Complement
Targets or Molecules recognized
Pathogen-associated molecular patterns
Immune cells
Bacterial cell walls
Produced in response to infection
3 pathways, recognize (1) surfaces of bacteria, fungi, viruses, tumor cells or (2) mannose (3) antibody bound to antigen
innate and acquired immune systems to the site of the infection (1, 3, 6, 7, 13, 14).
The complement system contains about 25 proteins; with the origin of some of these proteins found back as far as insects in evolution. Within this system, the roles of the innate and the acquired immune system are truly blended. Three pathways of activation of complement proteins exist: the classical pathway, the alternative pathway and the lectin pathway. These pathways differ in the way that the C3 convertase is formed, and after this step, the pathways are the same with a cascade of proteins each in turn activating other proteins until pathogen lysis occurs. The classical pathway functions only with antibody bound to antigen at the onset, so this pathway is linked to the acquired immune system. The alternative pathway begins with the spontaneous hydrolysis of some of the C3 that is in the serum, which may bind to surfaces of bacteria, fungi, viruses, or tumor cells and subsequently bind the next molecules of the pathway. The lectin pathway begins with the binding of a mannose binding lectin (a PRR) to the mannose on the surface of the pathogen. The mannose-binding lectin is associated with enzymes that bind and cleave the complement component C4. The cleaved C4 subsequently binds to C3 and, thus, complement is activated. Complement will be discussed further in Chapter 4 (1, 3, 6, 7, 13, 14). See Table 1.3 ✪ for a summary of the information about the molecules of the innate immune response (1, 3, 6, 7, 13, 14) and Table 1.4 ✪ for a summary of the information about acute phase proteins (1, 3, 6, 7, 13, 14).
✓
Checkpoint! 1.3
These molecules of the innate immune system can bind to PAMPs.
Primary Function example
Recognition
Enhance or decrease immune response
Protect at epithelial cell surface
Different proteins, different functions
Include activating complement to removing free hemoglobin
Target cell lysis, improve phagocytosis, increase vascular permeability
Toll-like receptors (TLR)
Interleukins
Interferons
Defensins
Cathelicidins
C-reactive protein
Haptoglobin
Alternative Lectin or classical pathways
✪ Table 1.4
Acute Phase Proteins
Acute Phase reactant Primary Function
C-reactive protein Activates complement Is an opsonin
Enhances cell-mediated cytotoxic effects on the pathogen
Alpha-1 acid glycoprotein Binds drugs and hormones and inhibits their function
Haptoglobin Clears free hemoglobin
Fibrinogen Forms clot
Serum amyloid A Binds cholesterol for clearance
Recruits enzymes to digest the extracellular matrix
Chemotactic
the processes of the innate immune system
Inflammation is the result of the responses to harmful stimuli, which can be due to physical (heat, cold, pressure), chemical (acids, bases, other irritants), or microbial factors. Inflammation helps bring the response to the site of the infection, helps eliminate it, repairs the damage, and removes any debris caused by the infection or the response. The hallmarks of inflammation are redness, pain, heat, swelling, and sometimes loss of function. Increased vascular permeability brought about by the cells and soluble substances of the innate response can lead to the heat, redness, and swelling, which in turn can cause pain and sometimes loss of function (Figure 1.7 ■).
To describe inflammation in a particular area, the suffix -itis is often added to the name of that area, so dermatitis, meningitis, tonsillitis, and carditis indicate inflammation of the skin, meninges of the brain, tonsils, and heart, respectively. With inflammation, there is an increased blood supply to the area and migration of first neutrophils and then macrophages to the area. The soluble mediators start and stop the inflammation with factors in the coagulation pathway amplifying the reaction. Neutrophils arrive to the site of the inflammation within 30 to 60 minutes; macrophages arrive between 16 to 18 hours later. The cells come to the site as the result of attraction to chemotactic factors at the site. Chemotactic factors include those released by microbes and from the coagulation pathway, the complement pathway, cytokines, and other cellular products. After the neutrophils come to the site, they can phagocytize the microbe (1, 3, 6, 7, 13, 14).
Monocytes, macrophages, and neutrophils can move out of the circulation and into the infected tissues by squeezing through the cells of the intact blood vessel walls by a process called diapedesis. Once at the site of infection, phagocytosis is the process by which the leukocyte engulfs and digests the microbe or other particle. The cell first becomes attached to the particle, either by PRRs binding to PAMPS, or through
■ Figure 1.7 Inflammation associated with a new tattoo. Note the redness and swelling. Source: Kellee Rogers.
the coating of the particle with opsonins, which are immune factors that enhance phagocytosis. The term opsonin is Greek and means to prepare food for. These factors, which include breakdown products of complement, C-reactive protein, and antibody molecules, bind to the pathogen and then to receptors on the phagocytic cell in a manner that increases phagocytosis of the particle.
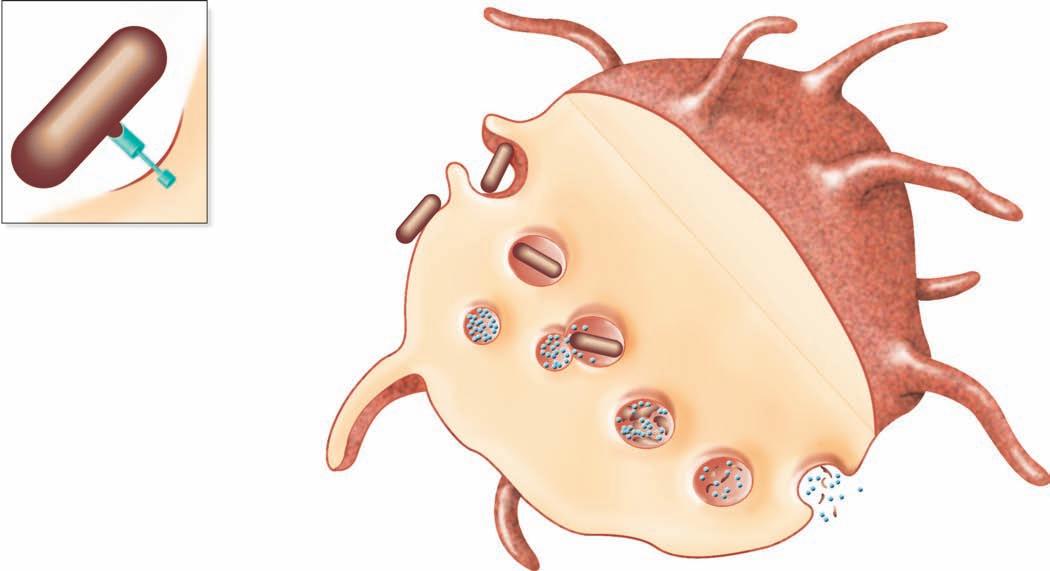
Binding to multiple sites can bring the cell membrane of the phagocytic cell around the particle so that it is completely engulfed. In this way, when the particle is inside the cell, it is surrounded by the cell membrane in a bag called a phagosome, which is joined by a second membrane-enclosed bag, this one called a lysosome, which is filled with digestive enzymes (Figure 1.8 ■ ). The new structure formed by the fusion of these two membrane-enclosed bags is called the
Chemotaxis and adherence of microbe to phagocyte
Ingestion of microbe by phagocyte
Formation of a phagosome
Fusion of the phagosome with a lysosome to form a phagolysosome
Digestion of ingested microbe by enzymes
Phases of phagocytosis
Formation of residual body containing indigestible material
Discharge of waste materials
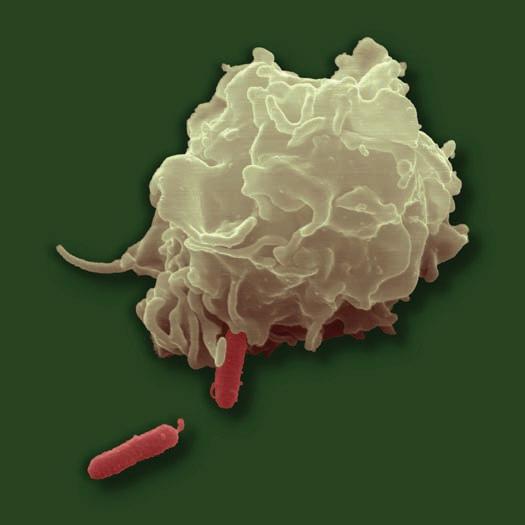
■ Figure 1.8 (a) The phagocytic process, beginning in the upper left with bacterial attachment and phagocytic cell pseudopod formation, followed by ingestion with the bacteria in a phagosome. The phagosome is joined by a lysosome to create a phagolysosome, the microbe is digested, and waste products are excreted. Some processed peptides from the pathogen may be presented to T cells by this antigen presenting cell. Source: TORTORA, GERARD J.; FUNKE, BERDELL R.; CASE, CHRISTINE L., MICROBIOLOGY: AN INTRODUCTION, 10th, ©2010. Printed and Electronically reproduced by permission of Pearson Education, Inc., Upper Saddle River, New Jersey. (b) Scanning electron microscopy showing a phagocytic cell in the process of phagocytizing bacteria. Source: © Juergen Berger/Photo Researchers, Inc.
phagolysosome. In this the pathogen is usually killed and broken down by cellular enzymes and the reaction with peroxide anions, hydroxyl radicals, and singlet oxygen produced by a respiratory burst by the phagocytic cell. Some microbes can survive phagocytosis and can actually spread throughout the body while riding in these phagocytic cells (1, 3, 6, 7, 13, 14).
This concludes the discussion of the innate immune system as a separate entity. All further discussions of these cells and molecules will be as they interact with the acquired immune system.
the acquired immune system
To begin this section, please remember that the hallmarks of the acquired immune system are that it is specific, has a large scope, can discriminate, and has a memory. See Table 1.1 for a summary of the differences between the innate and the acquired immune response.
the ceLLs Of the acquired immune system
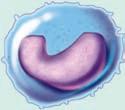
The key cells involved in the acquired immune response are two types of lymphocytes, the T lymphocyte and the B lymphocyte, which are also called the T and the B cells. Lymphocytes are about 20% of the circulating white blood cells and are composed of very little cytoplasm with the resting cell almost full of the nucleus alone. The nucleus can have a slight dent, making it look kidney shaped. These cells get their names from their location of maturation; although they are both derived from the same hematopoietic progenitor, T cells have matured to become T cells in the thymus, while in mammals the B cells have matured to become B cells in the bone marrow and in birds B cells mature in the bursa of Fabricius. This
distinction is important because this easily removable organ in chickens led to the understanding that B cells produce antibody (see Box 1.1 ✪). The acquired immune system is said to have two arms, the humoral and the cellular. The humoral arm is antibody-mediated immunity, and the cellular arm is T-cell-mediated immunity. Both B and T cells recognize antigens through a specific molecule on their surfaces; for the B cell, this is surface immunoglobulin; for T cells, it is called the T-cell receptor (2, 4, 5, 6, 7, 8, 10, 15, 16).
antibOdy intrOductiOn—GeneraL structure
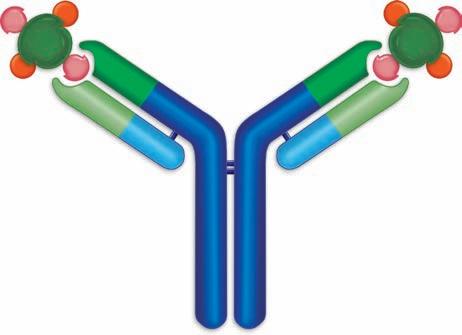
Antibody molecules are also called immunoglobulin molecules and gamma globulins. The derivation of the first name is obvious because they have an immune function and are globular proteins, but the second name—gamma globulin—takes a little explanation. In serum protein electrophoresis, serum is placed in an electric field in agarose at pH 8.6, and the proteins in the serum, mostly with a negative charge at this pH, are separated into five groups. Albumin is the most anionic protein and thus is the fastest-moving protein toward the anode. Next are the alpha 1 globulins, alpha 2 globulins, then the beta globulins, and finally the gamma globulins (see Figure 1.9 ■). The antibody activity is found in the gamma region, hence, the name gamma globulins. The serum protein electrophoresis assay is performed to yield information about certain clinical diseases, such as decreased antibody production (hypogammaglobulinemia) and increased antibody production due to the cancer of antibody-producing cells (myeloma), so this assay will be discussed again later. The 5 types of antibody molecules are IgG, IgM, IgA, IgE, and IgD. There are some similarities between these molecules and some physical differences with the physical differences resulting in biological differences as
The Accidental Discovery of the role of the Bursa in B-Cell Development
The discovery of the role of the bursa for B cell development in birds, was one of the many serendipitous moments in which pivotally important information was discovered accidentally, and not immediately respected. Bruce Glick in 1952 was a scientist in the field of poultry science and was trying to discover the role of the bursa in birds. He had bursectomized several chickens so that he could study the physiologic function of the bursa. A graduate student asked if he could have a few of these chickens to show an undergraduate class that vaccination results in the production of antibodies that can agglutinate Salmonella. A week after this vaccination, much to the hilarity of the undergraduates, when the graduate student mixed the drop of blood with the bacteria nothing happened in the first few samples tried. However, as the graduate student continued, samples from other chickens did agglutinate the bacteria.
The graduate student, Tim Chang, went to talk to Bruce Glick about this and they realized that the animals whose sera did not agglutinate had been bursectomized when they were very young. Further studies showed a link of the bursa to antibody production. Science magazine did not accept this manuscript because they wanted the mechanism explained. This data was subsequently published in the Journal of Poultry Science, and has become a Citation Classic (13).
✪ box 1.1
■ Figure 1.9 Serum protein electrophoresis is a technique in which serum components separate into 5 different major groups based on their mobility in an electric field. The sample was applied on the right side of the figure. The peak farthest from the origin is albumin, the next contains the alpha 1 globulins, and then the alpha 2, followed by the beta globulins, and finally the gamma globulin peak, which contains the immunoglobulin.
well (Figure 1.10 ■). These will be discussed in Chapter 2. B cells produce antibody in response to the antigen specifically binding to their surface immunoglobulin and can respond to soluble antigen alone. B cells are characterized by this surface immunoglobulin and by the presence of the surface molecules CD19, CD20, and CD21 (2, 4, 5, 6, 7, 8, 10, 15, 16).
Checkpoint! 1.4
Serum has been taken from a patient and analyzed by serum protein electrophoresis. The patient is immunized with a number of vaccines to prepare for travel and to meet the requirements for college entrance. Blood is drawn 2 weeks later, giving the patient enough time to make antibody. Which peak in the serum protein electrophoresis will be increased?
the ceLLuLar arm
The cellular arm of the immune response is due to the functions of T cells. T cells respond to antigens that specifically bind to their T-cell receptor (TCR) and that are presented by an antigen-presenting cell in either a major histocompatibility
■ Figure 1.10 The basic structure of an antibody molecule. Two heavy chains are joined by two light chains. The antibody contains two binding sites, each of which is formed by a heavy and a light chain. The constant portion of each chain is shown in blue and the variable region in green.
complex (MHC) class I molecule or MHC class II molecule. Additional signals of costimulation through binding of other molecules on the antigen-presenting cells (APCs) and cytokines are also required. The major histocompatibility complex was so named because these antigens were first discovered as a result of their role in the rejection or acceptance (compatibility) of tissue (histo) grafts. These genetically inherited molecules have been found to be important in antigen presentation and in the immune response.
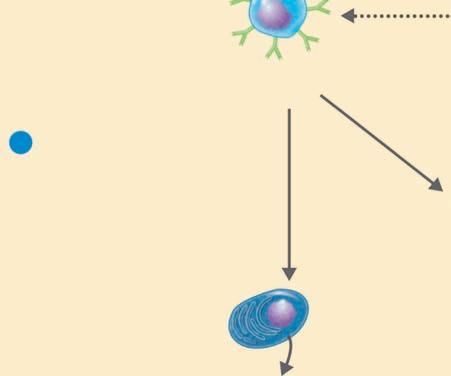
T cells can be divided into helper T cells, cytotoxic T cells, and regulatory T cells. The products of helper T cells are cytokines that can upregulate the immune response; the product of cytotoxic T cells is their direct cytotoxicity (cell killing) of cells bearing the antigen; and the products of regulatory T cells are cytokines that downregulate the immune response when the pathogen is cleared and help prevent autoimmunity. Helper T cells respond to a specific antigen that binds to their TCR in association with the MHC class II molecule, cytotoxic T cells respond to a specific antigen that binds to their TCR in association with the MHC class I molecule, and regulatory T cells bind to their specific antigen through their TCR usually in association with MHC class II molecules but sometimes in association with MHC class I molecules. T cells can be identified by the CD3 marker on their surface, which is part of the T-cell receptor. In addition, helper T cells are CD4 + , cytotoxic T cells are CD8 + , and regulatory T cells are usually CD4 + . CD8 + regulatory cells can also be found with Foxp3 + serving as the marker that characterizes these cells (Figure 1.11 ■) (2, 4, 5, 6, 7, 8, 10, 15, 16). More about these cells and their functions and controls will be described in Chapter 4.
HUMORAL
(ANTIBODY-MEDIATED) IMMUNE SYSTEM
Control of freely circulating pathogens
Extracellular antigens
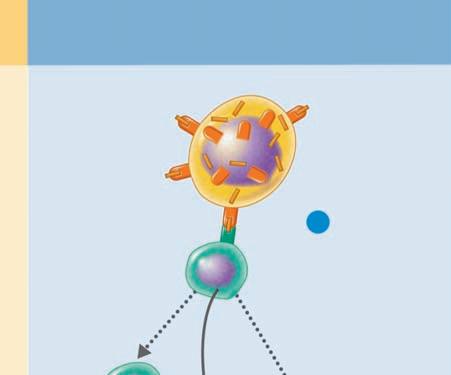
A B cell binds to the antigen for which it is specific. A T-dependent B cell requires cooperation with a T helper (TH) cell.
The B cell, often with stimulation by cytokines from a TH cell, differentiates into a plasma cell. Some become memory cells.
Cytokines activate T helper (TH) cell
CELLULAR (CELL-MEDIATED) IMMUNE SYSTEM
Control of intracellular pathogens
Exposure to a processed intracellular antigen: intracellular antigens expressed on the surface of a cell infected by a virus, bacterium, or parasite (also may be expressed on the surface of an APC).
1 A T cell binds to MHC–antigen complexes on the surface of the infected cell, activating the T cell (with its cytokine receptors).
Cytokines
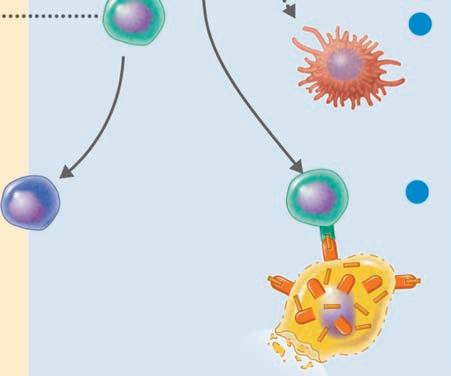
Cytokines from the TH cell transform B cells into antibody-producing plasma cells.
Plasma cell
Plasma cells proliferate and produce antibodies against the antigen.
Memory cell
Some T and B cells differentiate into memory cells that respond rapidly to any secondary encounter with an antigen.
Cytotoxic T lymphocyte
Cytokines
Lysed target cell
2 Activation of macrophage (enhanced phagocytic activity).
3 The CD8+ T cell becomes a cytotoxic T lymphocyte (CTL) able to induce apoptosis of the target cell.
Regulatory T cell
■ Figure 1.11 The humoral and cellular immune response. At the left is the humoral (or B-cell mediated); at the right is the cellular (or T-cell mediated) immune system. B cells become antibody secreting plasma cells or memory cells with antigen stimulation. T cells are of three general types: (1) helper T cells that secrete cytokines, which upregulate the immune response, (2) cytotoxic T cells that kill target cells after making direct contact with their target, and (3) regulatory T cells, which serve to downregulate the immune response.
Source: TORTORA, GERARD J.; FUNKE, BERDELL R.; CASE, CHRISTINE L., MICROBIOLOGY: AN INTRODUCTION, 10th, ©2010. Printed and Electronically reproduced by permission of Pearson Education, Inc., Upper Saddle River, New Jersey.
Checkpoint! 1.5
Which T cells seem to be on opposite sides of a battle?
the LymphOid OrGans
The primary lymphoid organs, the bone marrow and the thymus, are where the lymphocytes mature into either T or B cells. Organs in which these white blood cells meet antigens, respond, proliferate, and interact with other lymphocytes are
called secondary lymphatic organs. The secondary lymphatic organs include lymph nodes, the spleen, tonsils, mucosalassociated lymphoid tissue (MALT), and skin-associated lymphoid tissue (SALT). MALT includes Peyer’s patches in the intestine, tonsils, and the appendix (2, 4, 5, 6, 7, 8, 10, 15, 16).
the primary Lymphatic OrGans
The primary lymphoid organs are those in which lymphocytes are generated and the initial differentiation of the lymphoid cells occurs to form mature T cells, B cells, and NK cells. Antigen contact in primary lymphatic organs results in cell
B cell
death via apoptosis (a form of cell suicide), and this process eliminates autoreactive cells (2, 4, 5, 6, 7, 8, 10, 15, 16).
the bone marrow
The bone marrow is the major site of hematopoiesis after gestation; prior to birth the major sites are the fetal liver and the spleen. Hematopoietic stem cells (HSC) reside in the bone marrow and can become any blood cell type. It is interesting to note that as few as 10 of the HSC can reconstitute all hematopoietic cell types in a lethally irradiated mouse. The bone marrow is the major lymphoid organ, filling the central cavity of all bones. Many cells reside in the bone marrow, including the HSC and all hematopoietic cells, macrophages, stromal cells, connective tissue, and adipocytes. In mammals, the bone marrow is the place for the differentiation of both B cells and NK cells. In birds, the bursa of Fabricius is involved in B cell differentiation. Self-reactive B cells are deleted in the bone marrow by apoptosis (2, 4, 5, 6, 7, 8, 10, 15, 16).
the thymus
The thymus is a bilobed organ that is below the thyroid and over the heart. It is about 22 grams at birth and increases to about 35 grams at puberty after which it decreases in size.
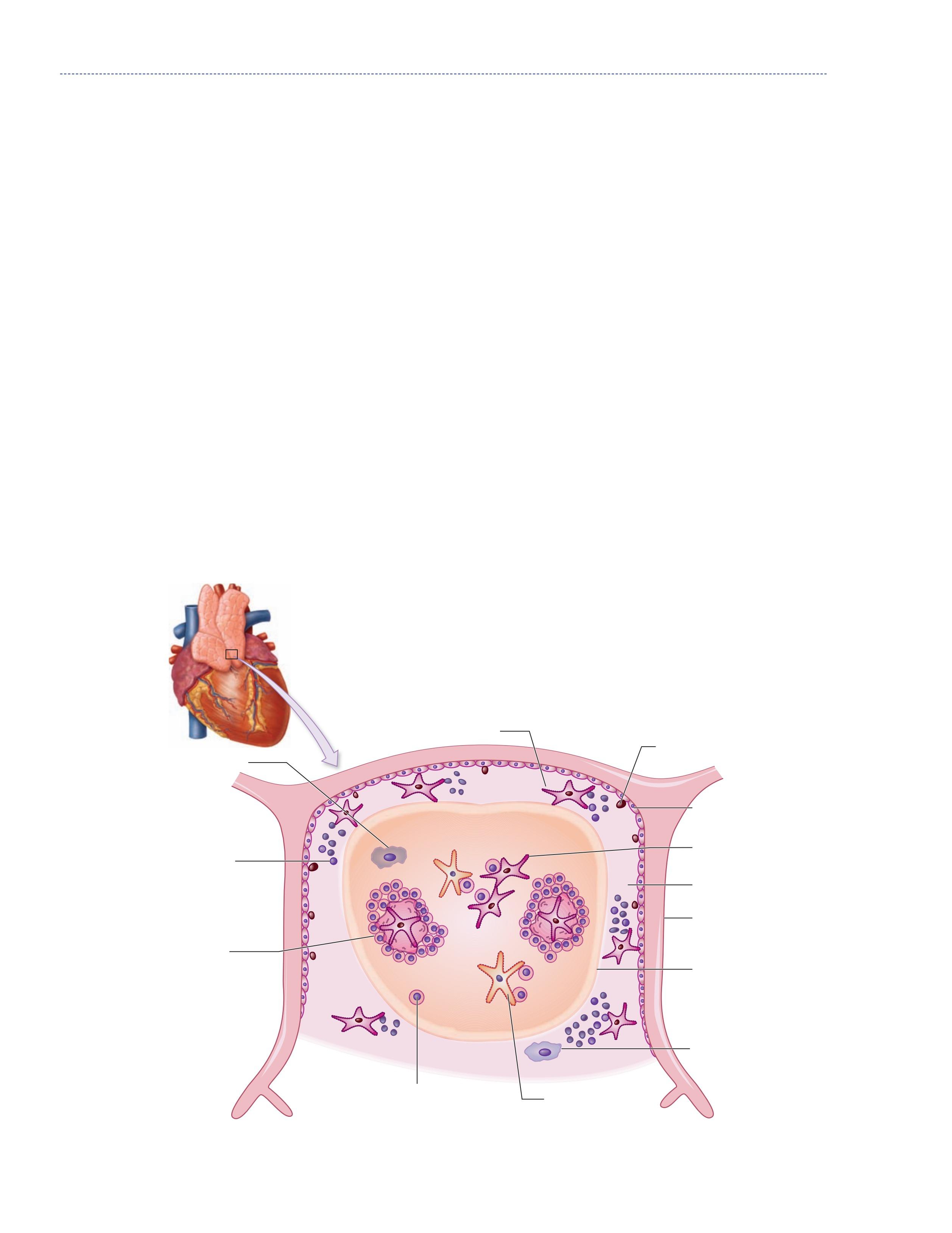
It is largest in proportion to the individual’s mass at birth and largest in absolute size at puberty. After puberty, it involutes, and in the adult, the thymus is composed of mostly fat and fibrous tissue. Even though the thymus in the adult appears this way, it still has significant function. Each of the two thymic lobes is broken into lobules. Each lobule has a cortex and a medulla. Lymphoid progenitor cells from the bone marrow enter the thymus at the cortex, and they become immature thymocytes, cortical epithelial cells, and macrophages. Some of these cortical epithelial cells with long extensions have been dubbed the thymic nurse cells for their role in helping the thymocytes mature. Thymocytes proliferate rapidly in the cortex. Between the cortex and the medulla are interdigitating dendritic cells, which interact with the thymocytes as they travel toward the medulla. The medulla contains mature and almost mature T cells with medullary epithelial cells, dendritic cells, and macrophages (2, 4, 5, 6, 7, 8, 10, 15, 16). Figure 1.12 ■ shows the structure of the thymus.
Thymocytes begin as CD3, CD4, and CD8, and in the first step in their maturation, T-cell receptors that have either αβ chains or γδ chains develop. The γδ T cells represent a minor population of specialized T cells, which eventually will ■
Figure 1.12 The thymus, a primary lymphoid organ where T cells mature.
Subcapsular cortex
Thymocyle precursors
Cortex Medulla
Mature thymocytes
Immature thymocytes
Hassall’s corpuscles
Dendritic cell
Capsule
Cortical epithelial cell
Macrophage
Macrophage
Medullary epithelial cell