EVOLUTION
TAPERED BONE LEVEL
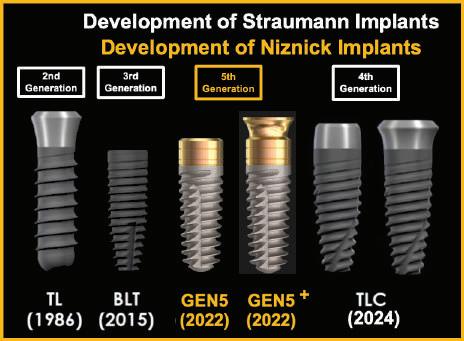
Straumann’s hollow basket system had a narrow post leaving an opening at the crest of the ridge that allowed down growth of soft tissue. This post was designed for screw-retained restorations with no ability to change the angle. By 1980, Straumann introduced a cylindrical, hollow basket implant with a flared smooth neck for tissue-level placement. Without threads, this implant lacked adequate initial stability.
The 1982 Core-Vent hollow basket, screw implant developed by Dr. Gerald Niznick solved the surgical and prosthetic limitations by adding threads for stability and an internal hex for a variety of cemented abutment options. By 1986, Straumann added threads to its hollow basket implants. After a 1 week trial, Straumann was found to infringe on Dr. Niznick’s Core-Vent patent and was required to pay a licensing fee. Straumann’s introduction of this implant with a smooth, flared neck, specifically for 1-stage surgery, was referred to as the TL (Tissue Level) implant. It proved to be as successful as 2-stage, buried implants from the standpoint of achieving osseointegration. It was even more successful from the standpoint of soft-tissue health. This was due to its smooth trans-gingival neck and positioning of the implant-abutment junction supra-crestal. This implant did not have an internal wrench-engaging feature to screw-in the implant. Instead, the insertion tool engaged the internal threads. To remove it without unscrewing the implant, required one handle to provide counter torque on the implant and another handle unscrewed the insertion tool.
Around 1995, Straumann introduced the ITI Bonefit®*, a solid straight, bullet shaped implant with minimal threads and no vertical self-tapping groove. It had a 15 degree (as measured from the horizontal) lead-in bevel and an internal octagon. Since an octagon is closer to being round than a hex, at higher torque levels the octagon tool could rotate within the implant, locking the components together. This combination of a lead-in bevel and internal wrench-engaging surface infringed Niznick’s internal conical connection patent and litigation ensued. In 2000, Straumann took another license on the Niznick patent that was limited to an octagon, precluding the preferred use of an internal hex. In 2008, Implant Direct launched the SwishPlusTM with a compatible internal octagon, added a square for ratcheting the implant into place. The SwishPlusTM improved the body compared to Straumann’s Tissue Level (“TL”) implant by making it tapered, adding progressively deeper threads and a vertical self-tapping cutting groove. It also had a very light texture and micro-grooves to the top few millimeters for vertical flexibility in placement.
Finally, in 2007, after the Niznick Conical Connection patent expired, Straumann introduced its first bone-level implant (“BL”). It had the same straight body, minimal threads, lack of a self-tapping vertical cutting groove and 15 degree, lead-in bevel as its TL implant. The differences between the TL and BL implants were the elimination of the smooth flared neck and the introduction of a slotted, wrench engaging surface called “CrossfitTM*”. It was not until 2014 that Straumann offered a tapered bone-level implant which it called BLT.
*Trademarks of Straumann
Straumann introduced its first 2-stage, bone level implant in 2007. It took Straumann until 2015 to launch a tapered implant. In 2019 Straumann introduced the BLX implant and the TLX in 2021. These implants are straight for the top 12mm with an extremely tapered inner core. Pictures of Straumann’s next “implant innovation” called TLC, have been shown on the internet by Dr. Buser. It is a combination of the BLT body, which is tapered in only its apical third. This limits the ability to increase stability by inserting the implant into an undersized socket to compress bone for increased initial stability. The BLT and TLC implants have very shallow, single lead threads that remain the same depth over the majority of their length.
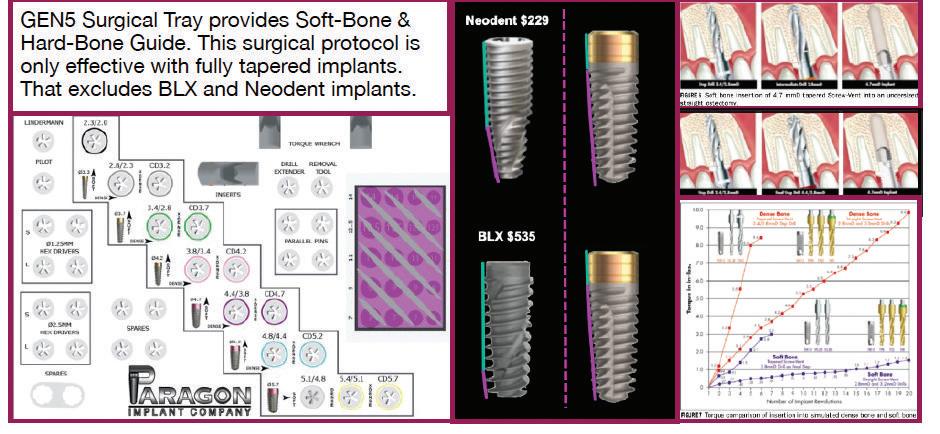
The GEN5™ implant from Paragon has a fully tapered body and a 2.5mm smooth, anodized neck. Score lines are added at 1 mm, 2mm and 2.5mm for the top to aid in positioning the implant at or above the crest of the ridge. The GEN5™ implant body has double lead for faster insertion and progressively deeper threads for increased surface area and stability The GEN5+™ implant is the same as the GEN5™, but has a 2mm friction-fit healing collar/Extender attached to facilitate 1-stage surgery. The Extender has a 4.8 mm flat platform that converts to a standard MUA with the attachment of a prosthetic screw, that is available in 3 heights. The US list prices for the Straumann’s BLT implant is $439 with the SLA® surface and $499 with the SLActive® surface, the later packaged in sterile saline for increased hydrophilic properties. An animal study showed no increase in stability with Hydrophilic vs Hydrophobic surfaces.


The US list price for Straumann’s BLX/TLX implants with SLA® surface is $535. Shortcomings in their design should also be considered. By contrast, the US list price for Paragon’s GEN5™ implant is $125 and the GEN5+™ is $150 This represents a 72% to 77% savings compared to the US list price for Straumann’s implants.
SHORTCOMINGS OF STRAUMANN’S BLX AND TLX IMPLANTS
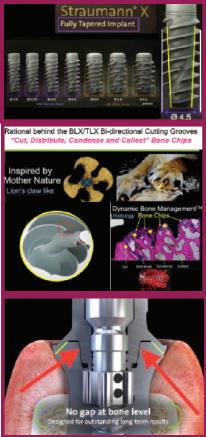
Straumann claims BLX/TLX implants are “Fully Tapered,” but the top 12mm of any length are basically straight making implants 12mm and shorter straight implants. Inserting a straight implant into an undersized socket increases the chance of bone fracture or failure to fully seat the implant.
2. BLX/TLX implants have spiral, bi-directional “claw like” grooves. The aggressive threads and bi-directional cutting grooves of the BLX/TLX turn the soft bone in to chips rather than compressing the bone for stability.
3.Straumann claims that by using only one connection for all the diameters of BLX and TLX implants, they have “Simplified Workflow [and] Reduced inventory.” While it is true that only having one connection would reduce the inventory of abutment and transfer components, this comes at the expense of creating more esthetic and hygienic emergence profiles and precludes wider based abutments that may be needed to prevent fractures in posterior restorations. Straumann’s solution is an abutment that overlaps the shoulder of the wider implants, but metal cannot make contact on both the shoulder and the internal conical connection, thus sacrificing stability.
1.
IMPLANT TAPER, THREAD DESIGN AND SELF-TAPPING FEATURES DETERMINE INITIAL STABILITY
Important differences exist in implant design: Read Shortcomings of Implant Systems
•Tapered implants for insertion into undersized sockets (Tapered Screw-Vent, Legacy, GEN5);
•Progressively deeper threads increase contact with bone (Legacy & GEN5);
•Reverse buttress threads most favorable for stress distribution (Legacy & GEN5);
•Self-tapping cutting grooves need to extend up full taper (Legacy & GEN5);
•Bi-directional, spiral cutting grooves generate bone chips rather than condense bone (BLX);
•Smooth neck provides vertical flexibility in placement & reduces peri-implantitis (GEN5).
Reverse Buttress Threads found to distribute stress better in finite element study. This study also showed less stress at the crest with a smooth neck. “On vertical loading, maximum stress was seen at Buttress thread design within the cortical bone which is not favorable for the bone. At the cancellous bone, maximum stress was seen at Vthread design. Reverse buttress thread design transferring the stresses along the long axis of the implant.
Inserting Tapered Implant into an Undersized Socket increases Stability
Achieving Osseontegration in Soft Bone: The Search for Improved Results (Niznick 2000)
Achieving initial stability in soft bone, needed for immediate loading, is best achieved by inserting a fully tapered implant into an undersized socket to compress soft bone.
Paragon’s GEN5™ implants utilize the proven tapered body of ZimVie’s TSV implants (Niznick 1999) and the progressively deeper, reverse buttress threads of Implant Direct’s Legacy implants (Niznick 2008).
GEN5™ IMPLANT NECK: Based on the proven clinical success of the Straumann’s hybrid surface tissue level implants in resisting peri-implantitis, the GEN5™ implants have a 2.5mm smooth, anodized neck. This differentiates the GEN5™ from most other implants with threads, microthreads, micro-grooves, laser lines and/or blasted surface to top.
GEN5™ IMPLANT LENGTHS: To facilitate 1-or 2-stage surgery, and to accommodate uneven ridges, an additional 1mm of length has been added to the standard lengths of the Screw-Vent and Legacy implants. The implants have gauge lines at 1mm, 2mm and 2.5mm from the top of the implant for vertical flexibility during placement. By placing the implant 1mm supra-crestal, the soft tissue attachment remains undisturbed during the entire restorative workflow and there is no interference when seating flared prosthetic components. The remaining 1.5mm of the smooth neck can be either (a) subcrestal to accommodate for future resorption or (b) exposed with uneven ridges and extraction sockets, thereby eliminating the need for bone grafting to cover exposed blasted surfaces.
The GEN5™ was designed to follow Dr. Buser's protocol for hybrid implant insertion. The GEN5™, with its 2.5mm smooth, anodized neck and score lines, facilitates vertical flexibility in placement. This Extender becomes the neck of an MUA by the attachment of a prosthetic screw, which is available in 3 heights. The GEN5+™ offers even greater vertical flexibility by adding a 2mm friction-fit Extender that accepts a number of abutments for conventional crown & bridge procedures, similar to Nobel’s ON-1 healing collar. The Extender can be removed to lower the platform to establish a subgingival margin for esthetics.
Simulated 1mm HeightSupra-crestalt Su
GEN5 $125
GEN5 $125
Lecture and interview of Dr. Daniel Buser, Prof. Emeritus, University of Bern, explaining why implants with hybrid design surfaces reduce the incidence of peri-implantitis.
Dr. Niznick Article: AO News Vol.33 No. 2. 2022: Hybrid Surface - "Design of the Future"
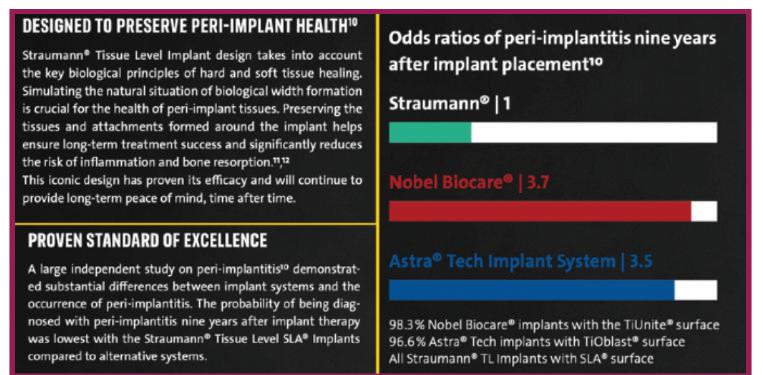
“Dr. Buser cites a Swedish 10-year study comparing three implants: Astra, NobelBiocare™ and Straumann’s Tissue Level implant, claiming the latter exhibited significantly less peri-implantitis.
3D Video explaining the cause, treatment and prevention of peri-implantitis
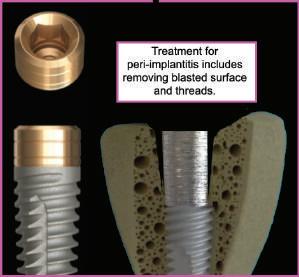
While roughened surfaces are advantageous for osseointegration, they are also very prone to bacterial colonization. Bone remodeling causing the lower endosseous rough part of the implant to be exposed to the oral cavity, creates an environment that may lead to peri-implantitis.
Study showed more bone loss with sub-crestal compared to supra-crestal placement
Study shows sub-crestal implant placement reduces primary stability
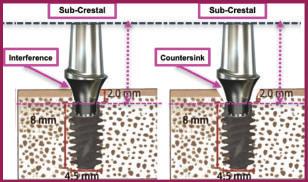
Countersinking needed to seat flared abutment in sub-crestal implant
Study shows no difference between 45 and 78 degree lead-in bevel
Straummann
1. GEN5™ designed for 1-or 2-stage surgery;
2. GEN5+™ designed for 1-stage surgery;
3. GEN5™, GEN5+™ and NizPlant™ provide vertical flexibility to accommodate uneven ridges and immediate extraction sites;
4. GEN5™, GEN5+™ and NizPlant™ minimize the need for different abutment heights by varying the implant’s insertion depth;
5. GEN5™, GEN5+™ and NizPlant™ reduce the need for bone grafting and minimizes exposure of rough surfaces & threads that contribute to peri-implantitis;
6. GEN5™ and GEN5+™ have friction-fit connections to stabilize implant-abutment junctions;
7. NizPlant™ 1-piece implant has a Dual-Function platform that serves as an overdenture (Locator compatible) attachment and as a MUA abutment.
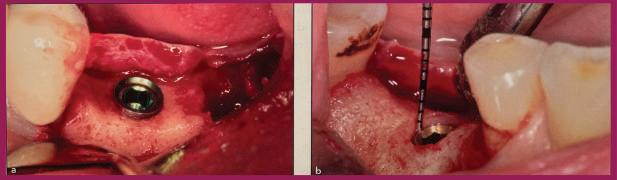
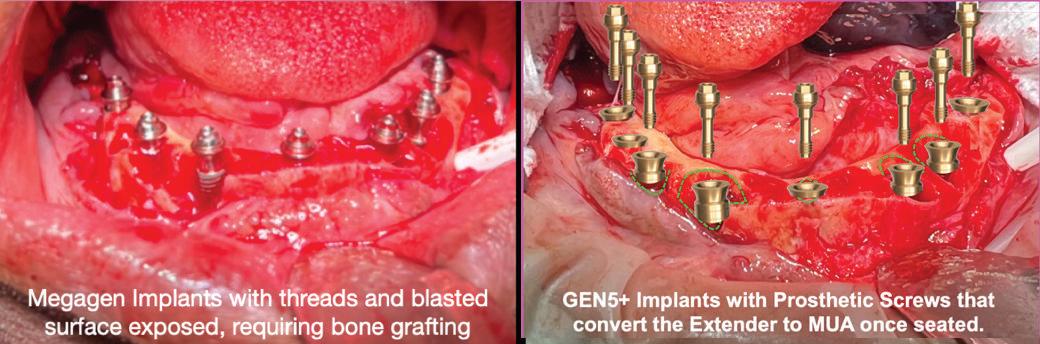
(L) The picture on the left shows a typical placement of Megagen implants in extraction sockets. The exposed threads and blasted surfaces require bone grafting to minimize exposure. Ultimately, resorption and/or remodeling will occur, again exposing the threaded and blasted surfaces.
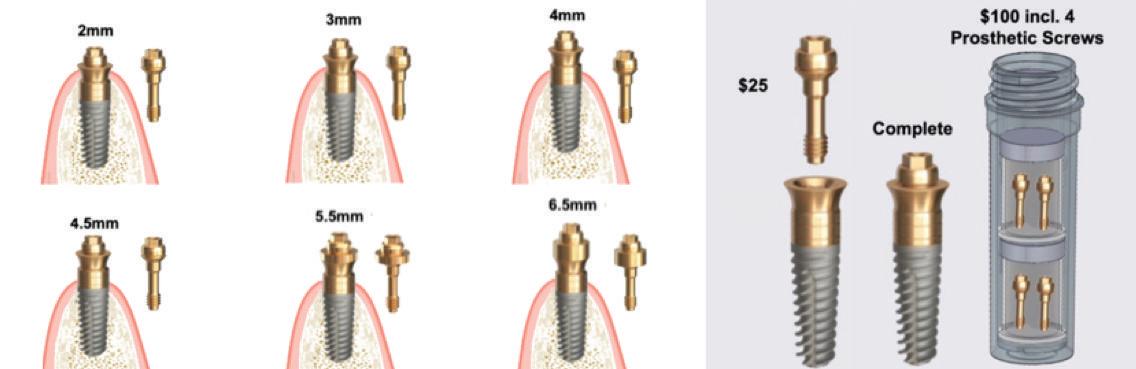
(R) The picture on the right shows the same extraction sockets with simulated GEN5+™ implant placement. GEN5+™ has a 2.5mm machined, anodized neck, that, combined with its 2mm friction-fit Extender, provides the ultimate in vertical flexibility in placement. Its 4.5mm smooth surface eliminates the need for bone grafting and minimizing the risk of peri-implantitis. The implant-Extender junction is sealed and secured with its combination of a 45 degree lead-in bevels and internal friction-fit connection. It requires 15-30Ncm to seat prior to packaging. Up to 25 degrees angle correction can be accommodated with the Angled Screw Channel titanium sleeve or the channel trajectory can be created in the restoration. A retrieval screw or tool is required to separate the Extender should that become necessary for connect a 30 degree angled MUA to the implant.

Placing implants subcrestal is necessitated by desire to bury the rough surface and, with systems having only 1 narrow abutment platform, and to create a transition to an acceptable abutment width. Neither are necessary with the GEN5™ and GEN5+™ System.
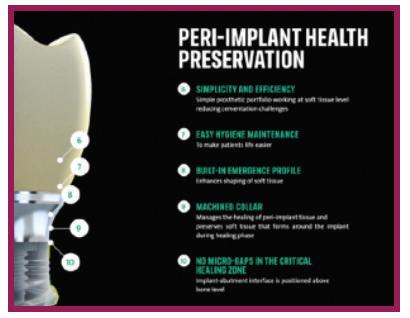
Straumann’s Tissue Level implants are available with a 1.8mm and 2.8mm machined neck. The GEN5™ has a 2.5mm machined neck with score lines, giving flexibility to position some or all of its smooth neck above the bone. Straumann attributes peri-implant health preservation with its Tissue Level implants to:
1. Supra-crestal positioning of the implant-abutment junction; 2. Its machined, smooth neck and ideal emergence profile; 3.Hygiene maintenance due to its contour and smoothness.
GEN5™ offers the same benefits while the GEN5+™ offers the additional flexibility of a 2mm friction-fit collar that can serve as the trans-mucosal collar of an abutment or be removed for abutment connection directly to the top of the implant for unprecedented vertical flexibility.
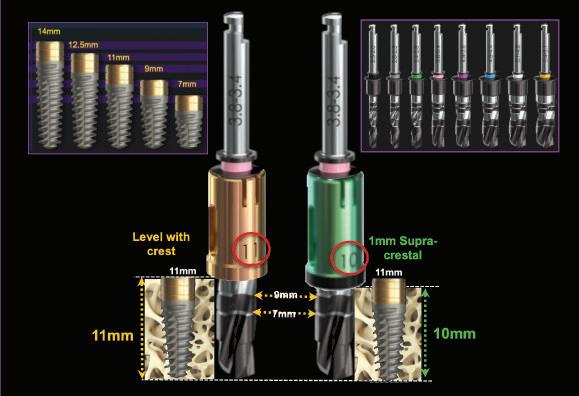
Paragon’s GEN5™ , GEN5+™ and NizPlant™ implants have the same implant body with a 2.5mm machined, anodized neck. Depth gauge lines at 1mm, 2mm and 2.5mm from the top (Pat. Pend.), along with drill stops facilitate placement level with or 1mm above the crest of the ridge. The insertion depth control, in conjunction with the ability to vary the height of the prosthetic screw, minimizes the need and cost of maintaining an inventory of abutment heights. Penetrating to the 13mm score line on a ZimVie Screw-Vent or Implant Direct Legacy drill, will position the GEN5™ implant 1mm above the crest of the ridge.
The GEN5+™ provides further vertical flexibility with a 2mm friction-fit Extender that converts to a standard MUA by attaching a prosthetic screw available in 3 heights. Like Nobel’s ON1 prosthetic system, this raises the platform for simplified abutment attachment.
Each Paragon implant is 1mm longer than the standard lengths of the respective Screw-Vent and Legacy implants. Paragon’s surgical system includes two options of drill stops. One is for placement 1mm supra-crestal, which moves the implant-abutment junction away from the bone and and creates a 1mm supra-crestal zone of titanium, for undisturbed soft tissue attachment when prosthetic components are attached and removed from the implant. The other drill stop positions the implant level with the highest point on the the ridge, usually on the lingual, leaving the smooth neck exposed if there is bone recession on the labial/buccal.
Hybrid Implant Designs - Tissue specific manipulation of the host response. David Cochran, DDS, MS, PhD, MMS; AO Academy News Guest
“I was asked to write a commentary of three articles published in the Academy News about hybrid implant designs by Drs. Daniel Buser, Gerald A. Niznick and Dennis P Tarnow. Both Drs. Buser and Tarnow point out the historical development of hybrid designed implants and then relate these to the development of peri-implantitis. Dr. Niznick draws an important distinction …a surface created by a subtractive process is better than an additive process since roughness is created without porosity. Porosity allows for the creation of microbial niches that are difficult to eradicate. Dr. Niznick also brings up an even more significant issue, which the creation of implant component interfaces and their location relative to the bone level. …It is crystal clear that a microbial niche is created in these interfaces which harbors bacterial growth 360 degrees around the implant. The host cannot eliminate the bacteria and their associated inflammation, so the host resorbs bone and uses epithelium to try to isolate the host insult.”
The job to be done with biofilm and titanium implants : Ole T. Jensen et al.
“The job to be done remains: to inhibit or arrest the development of microbial biofilms on titanium surfaces of an intra-oral implant.”
“Continual exposure of an implant surface to the oral environment is speculated to be from 2% to 30% by ten years in function. Causes are often proposed to be physiologic, but more often are related to practitioner error such as poor surgical placement or inadequate prosthetic management such as use of misfit components, poorly executed restorations, or restorations designed with compromised clean-ability and other health-related changes can occur over time as well, including physiological, pharmacological or ingestion-related insults (e.g., tobacco that lead to catabolic bone changes at the bone-implant interface) ”
“In certain patients, once an implant surface is exposed, biofilm appears to accelerate further implant surface exposure over time. Therefore, device surface modification …mitigate implant failure risks that, for a myriad of reasons, become exposed and biofilm-colonized in order to curtail or even arrest progression of peri-implant disease.”
SCIENCE SUPPORTS CLINICAL BENEFITS OF A HYBRID DESIGN BUT NOT A HYDROPHILIC SURFACE
Discussions in the Academy of Osseointegration News, Vol. 34, #2, 2023 overwhelmingly supported hybrid surfaces and supra-crestal positioning of the implant-abutment junction to reduce the risk of peri-implantitis. The Swedish Derks 9 year study compared Straumann’s Tissue Level, hybrid surface implants to NobelBiocare’s and Astra’s bone level implants. Straumann’s TL implants experienced about 1/3 the incidence of peri-implantitis documented with the two other implants with textured surfaces to the top of the implant. In spite of this finding, Straumann still sells the BL and BLX implants and recently announced “Straumann taps into peri-implantitis treatment by a full acquisition of GalvoSurg Dental AG," a company that makes a device for cleaning the exposed rough surfaces of implants.
The GEN5™ and GEN5+™ implants incorporate all the clinical advantages claimed by NobelBiocare for its *On1TM with none of its shortcomings Healing Collar: Video interview of Drs. Rompen & Touati
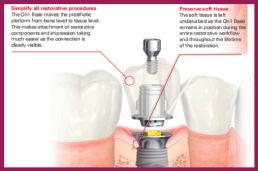
1. “Simplify all restorative procedures”:
[GEN5] “moves the prosthetic platform from bone level to tissue level. This makes attachment of restorative components and impression taking much easier as the connection is clearly visible”
2. “Preserves soft tissue attachment”:
“The soft tissue is left undisturbed as the [GEN5] smooth neck remains in position during the entire restorative workflow and throughout the lifetime of the restoration.”
NobelActive ($538) + On1TM Base ($76) = $614
Paragon’s GEN5™ Implant at $125 = SAVINGS: $489
Paragon’s GEN5+™ Implant at $150 = SAVINGS: $464
Attaching the flared On1TM Extender after the implant is seated may require bone reduction

GEN5+™ is the GEN5™ implant but packaged with a 2mm high friction-fit Extender. The Extender has a flat 4.8mmD platform with a 45 degree lead-in bevel and internal hex, just like the implant. This provides the ultimate in surgical and prosthetic flexibility. The platform can be 2.0mm to 5.5mm supra-crestal by varying the depth of implant placement. Several heights of prosthetic screws can be attached to the Extender to convert it to a standard MUA. A cover screw is also available. Similar to NobelBiocare’s On1TM Healing Collar, a variety of straight, angled, cad milled and ASC abutments can be attached to the platform for conventional crown & bridge applications. If soft tissue recedes in the esthetic zone after osseointegration has been established, the 2mm Extender can be removed to allow abutment connect directly to the implant platform. With the use of two implant platforms (3.5mmD and 4.5mmD), a hygienic and esthetic emergence profile can be created.

7 ADDITIONAL CLINICAL ADVANTAGES WITH GEN5+™ VS. ON1TM FROM NOBEL BIOCARE
1. With GEN5™, the implant-abutment junction is 0mm to 2.5mm above crest of the ridge, whereas the connection between the On1TM Healing Collar and the implant is at the level of the bone.
2. With GEN5™, the abutment engages the implant’s conical connection for stability whereas the junction between the On1TM abutment and the top of the On1TM Healing Collar is a butt joint connection.
3.With GEN5™, the fixation screw fastens the abutment to the implant, whereas with the On1TM assembly its abutment fixation screw connects to the top of the On1TM Collar fixation screw.
4. With GEN5™, there is no need to select between the 1.75mm and 2.5mm high On1TM collars. Varying the depth of the drill penetration determines the length of smooth neck projecting above the crest. Recommended to drill full depth of implant and determine platform height at insertion.
5. With GEN5™, there is no need to attach the On1TM Collar and tighten its fixation screw to a prescribed torque level, before torquing in the abutment screw.
6. With GEN5™, there is no need to attach the Bone Mill Guide and utilize the Bone Mill trephine drill to remove bone for attaching the Healing Collar, thus preserving bone.
7. With GEN5™, the per tooth replacement cost is 84% less than a NobelActive & On1TM Healing Collar.
Paragon’s GEN5™ implant (Pat. Pend.) represents a paradigm shift in design and surgical protocol. It has a 2.5mm smooth, anodized neck with depth-gauge score lines and has an additional 1mm added to the length of each implant. The additional 1mm allows the implant to be placed level with the highest point of the ridge and/or slightly supra-crestal. The 1mm in extra length provides vertical flexibility in positioning the implant in uneven ridges and the 2.5mm smooth neck minimizes bone grafting to cover exposed rough surfaces. Other advantages include (1) Implant-abutment junction above the bone with soft tissue attachment not disturbed by prosthetic procedures and (2) simplifies attachment of flared healing collars & abutments — no need to countersink the socket to prevent interference.
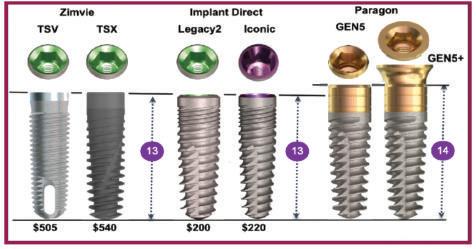
A study comparing the 78 degree lead-in bevel of the NobelActive, InterActive and Iconic implants, to the 45 degree lead-in bevel of the Screw-Vent, Legacy and GEN5™ implants demonstrated no difference clinically. Many implant companies introduce implants with new and often untested features to create marketing buzz and justify price increases. The new, untested designs often fall short of their original designs with years of documented success. Two examples are the TSX from Zimvie and the Iconic from Implant Direct.
1. The TSX Implant from Zimvie claiming to offer improvements on the Tapered Screw-Vent Implant (Niznick 1999).
2. The Iconic Implant from Implant Direct, claiming to offer improvements on the Legacy Implant (Niznick 2008).
3. The GEN5™ implant from Paragon, is set for launch in late 2024. GEN5™ is surgically and prosthetically compatible with Legacy and TSV A smooth, anodized 2.5mm Neck with score lines for depth control replaces the micro-threads of the Legacy.
The GEN5+™ is the GEN5™ with a pre-packaged 2mm friction-fit Extender that serves as a healing collar and the trans-mucosal portion of a variety of abutment options. The friction-fit extender (Niznick US Patent # 5,334,024) can only be removed with the use of a retrieval screw.The advantages of these friction-fit connections were evaluated in an article by Paul P Binon: The Evolution and Evaluation of Two Interference-Fit Implant Interfaces. Published in Postgraduate Dentistry 1996.
GEN5+™ HAS UNPRECEDENTED SURGICAL AND PROSTHETIC SIMPLICITY, VERSATILITY AND VALUE
GEN5+™ HAS A FRICTION-FIT 2MM EXTENDER WITH A 4.8MMD FLAT PLATFORM AND INTERNAL HEX/BEVEL CONNECTION. IT SERVES AS A HEALING COLLAR, A STANDARD MUA AND ACCEPTS A VARIETY OF ABUTMENTS
GEN5+™ WITH ITS 3 PROSTHETIC SCREWS INCLUDES 6 HEIGHT OPTIONS: 2MM, 3MM, 4MM, 4.5MM, 5.5MM & 6MM
The case below (left) shows 8 Megagen implants placed into extraction sockets with threads & blasted surfaces exposed. Bone grafting needed to cover rough surfaces but will become exposed from inevitable remodeling, increasing risk of peri-implantitis. Simulated case below (right) shows 8 GEN5+™ implants. Little or no bone grafting needed because only smooth surfaces exposed. Attaching a Prosthetic Screw converts platform to standard MUA.
Megagen Implants with threads and blasted surface exposed requiring bone grafting.
GEN5+™ Implants with prosthetic screws that convert the Extender to an MUA once seated.
EVOLUTION AND INNOVATION: THREE PATENTED FEATURES OF THE
GEN5™ IMPLANT WITH 2.5MM MACHINED, ANODIZED NECK AND 1 MM, 2 MM, AND 2.5MM GAUGE LINES FOR 1- OR 2- STAGE SURGERY
AND
PATENT ON IMPLANT WITH SMOOTH NECK AND SCORE LINES FOR DEPTH CONTROL
ABSTRACT:
Dental Implants include a threaded body portion having a textured surface, and an externally smooth neck portion having one or more depth gauge lines to guide insertion into a patient’s jawbone, with the neck portion projecting above the jawbone, allowing the gingival tissue to be sutured over the implant, or with the neck portion projecting through the gingival tissue.
GEN5™ IMPLANT WITH 2MM FRICTION-FIT EXTENDER THAT CONVERTS TO A STANDARD MUA WITH THE ATTACHMENT OF A PROSTHETIC SCREW AVAILABLE IN 3 HEIGHTS.
PATENT ON FRICTION-FIT IMPLANT EXTENDER WITH FLAT TOP TO CONVERT TO MUA
ABSTRACT:
A screw-type dental implant assembly includes two parts: an endosseous dental implant with an internally threaded shaft and an internal wrench-engaging surface, located inside the body of the implant, for inserting the implant into a patient’s jawbone; and a separate Extender/post/abutment (the “Extender”) for frictional insertion into, and for attachment to the implant’s internal shaft, which includes a flat top surface with a diameter of about 4mm to 6mm in diameter, where the flat top has a circular profile.
PATENTED FEATURES OF THE 1-PIECE NIZPLANT™ IMPLANT WITH ITS DUAL-FUNCTION PLATFORM
NIZPLANT™ 1-PIECE IMPLANT WITH DUAL-FUNCTION PLATFORM FUNCTIONS AS AN OVERDENTURE AND A MULTI-UNIT ABUTMENT
NIZPLANT™ 1-PIECE LOCATOR-COMPATIBLE IMPLANT WITH INTERNAL THREADS
ABSTRACT:
A screw-type endosseous dental implant includes, near the top of the implant’s external surface, a ridge projecting laterally, and an internally threaded shaft with a lead-in, beveled opening, an internal wrench-engaging surface located below said lead-in, beveled opening, and, below said internal wrench-engaging surface and above said internal threads, an internal undercut/groove forming a chamber configured to receive a snap attachment for the retention of an over-denture.
NizLoc™ Attachments Engage both outside and inside of the NizPlant implant. The male projection can be removed to reduce the degree of retention.
SHORTCOMINGS OF THE ZEST IMPLANT SYSTEM - A 2-PIECE IMPLANT WITH 90 PRE-PACKAGED OPTIONS WITH IMPLANT, ABUTMENT AND CAP AT $220 VERSUS ONLY 30 NEEDED FOR NIZPLANT™ AT $150
Zest Locator 2-Piece
Implant
NizPlant™ 1-Piece Implant
with Over-denture Attachments at $220, Includes Cap Attachment Components with Dual-Function Platform at $150, Includes Cap Attachment Components
•Made in U.S.A by Dr. Gerald Niznick
•Designed for 1- or 2-stage
•2 platforms with
•Designed
•1mm added to each implant for 2.5mm smooth anodized neck
•Optional positioning of implant platform for 1 or 2-stage surgery
•1mm supra-crestal eliminates countersinking to seat flared abutment, and provides an undisturbed surface for soft tissue attachment
•2.5mm smooth neck for uneven ridges
•GEN5+™ provides a 2mm friction-fit extender that converts to MUA by attaching a prosthetic screw
•NizPlant™ 1-piece implant with a Locator® compatible platform that also serves as MUA. The 2.5mm smooth neck provides vertical flexibility, providing a range from 2mm to 5.5mm of abutment height.