



























•
•
•










































































•
•
•













































Group Publisher Chris Driscoll cdriscoll@endeavorb2b.com

 Janette Wider Editor-in-Chief
Janette Wider Editor-in-Chief
This month, we are featuring a topic that hasn’t been covered very much for Healthcare Purchasing News cybersecurity. Cybersecurity issues are prominent in many industries, including healthcare. The big difference between other industries and healthcare is that patient lives are literally on the line.
In October of 2021, I reported for HPN’s sister publication, Healthcare Innovation, that the first credible public claim that a death was caused, at least in part, by ransomware was reported by The Wall Street Journal on Sept. 30. An Alabama woman, whose 9-month-old child died, had filed a lawsuit against Springhill Medical Center (located in Mobile, Ala.), where her daughter was born.
According to a CBS News article, “Springhill Medical Center was besieged by a ransomware attack when Nicko Silar was born July 17, 2019. The resulting failure of electronic devices meant a doctor could not properly monitor the child’s condition during delivery, according to the lawsuit by Teiranni Kidd, the child’s mother.”
Further, “‘The number of healthcare providers who would normally monitor her labor and delivery was substantially reduced and important safety-critical layers of redundancy were eliminated,’ the suit claims.”
The baby had severe brain injuries, among other issues, and died in 2020 at another hospital after months of intensive care.
This story is incredibly alarming and if you search the web, you can find similar stories of health systems or hospitals that were affected by ransomware attacks, sometimes even having to revert back to paper charts.
Cybersecurity needs to be taken seriously by everyone at a healthcare organization. IT professionals are not the only ones who need cybersecurity training; all individuals who are employed by healthcare facilities should be trained on best practices—like not leaving login information on a sticky note under one’s keyboard or how to identify a phishing email.
Our story this month focuses mostly on medical devices and the internet of medical things (IoMT). Cyber Expert Richard Staynings of Cylera and the University of Denver spoke with Editor-in-Chief, Janette Wider and Associate Editor, Matt MacKenzie about a number of cybersecurity things, including the increasing prevalence of artificial intelligence (AI) and machine learning (ML) in healthcare settings. You can read it on page 12.
I’ll admit that I have a passion for healthcare cybersecurity, as that’s the “beat” I covered most extensively when I started as a journalist. And I guess my interest probably goes back to when I saw “WarGames” on cable when I was a kid. (“Shall we play a game?”)
I thought it only appropriate for HPN to cover this topic now that we’re officially a few months into 2024, as threats from hackers are not slowing down. On Jan. 31, The Record reported that Chicago-based Saint Anthony Hospital was the victim of a cyberattack claimed by the LockBit ransomware gang late last year. The gang posted the hospital to its “leak site” and gave the organization two days to pay approximately $900,000 in ransom. LockBit also took credit for an incident in November where multiple facilities in New Jersey and Pennsylvania had to cancel appointments and operate without patient files.
The Cybersecurity and Infrastructure Security Agency (CISA) has more information available on LockBit here: https://www.cisa.gov/news-events/ cybersecurity-advisories/aa23-165a.
Later this year, we’ll be covering ransomware in depth. Stay vigilant out there, readers.
facebook.com/hpnonline
twitter.com/hpn_online
linkedin.com/company/ healthcare-purchasing-news/
Editor-in-Chief Janette Wider jwider@hpnonline.com
Associate Editor Matt MacKenzie mmackenzie@endeavorb2b.com
Senior Contributing Editor Kara Nadeau knadeau@hpnonline.com
East & West Coast Kristen Hoffman khoffman@endeavorb2b.com
603-891-9122
Midwest & Central April Bruffy abruffy@hpnonline.com
713-992-0381
Strategic Accounts Sales Chris Driscoll cdriscoll@endeavorb2b.com
978-880-8345
Production Manager Ed Bartlett
Art Director Tracy Arendt
Advertising Services Karen Runion krunion@endeavorb2b.com
Audience Development Laura Moulton lmoulton@endeavorb2b.com
Jimmy Chung, MD, MBA, FACS, FABQAURP, CMRP, Chief Medical Officer, Advantus Health Partners and Bon Secours Mercy Health, Cincinnati, OH; Joe Colonna, Chief Supply Chain and Project Management Officer, Piedmont Healthcare, Atlanta, GA; Karen Conway, Vice President, Healthcare Value, GHX, Louisville, CO; Dee Donatelli, RN, BSN, MBA, Senior Director Spend symplr and Principal Dee Donatelli Consulting LLC, Austin, TX; Hudson Garrett Jr., PhD, FNAP, FSHEA, FIDSA, Adjunct Assistant Professor of Medicine, Infectious Diseases, University of Louisville School of Medicine; Melanie Miller, RN, CVAHP, CNOR, CSPDM, Value Analysis Consultant, Healthcare Value Management Experts Inc. (HVME) Los Angeles, CA; Dennis Orthman, Consulting, Braintree, MA; Janet Pate, Nurse Consultant and Educator, Ruhof Corp.; Richard Perrin, CEO, Active Innovations LLC, Annapolis, MD; Jean Sargent, CMRP, FAHRMM, FCS, Principal, Sargent Healthcare Strategies, Port Charlotte, FL; Richard W. Schule, MBA, BS, FAST, CST, FCS, CRCST, CHMMC, CIS, CHL, AGTS, Senior Director Enterprise Reprocessing, Cleveland Clinic, Cleveland, OH; Barbara Strain, MA, CVAHP, Principal, Barbara Strain Consulting LLC, Charlottesville, VA; Deborah Petretich Templeton, RPh, MHA,Chief Administrative Officer (Ret.), System Support Services, Geisinger Health, Danville, PA; Ray Taurasi, Principal, Healthcare CS Solutions, Washington, DC area
CEO Chris Ferrell | President June Griffin
COO Patrick Rains | CRO Reggie Lawrence
Chief Digital Officer Jacquie Niemiec
Chief Administrative and Legal Officer Tracy Kane
EVP Medical & Healthcare Technology Kylie Hirko
EVP Endeavor Business Intelligence Paul Mattioli
Healthcare Purchasing News USPS Permit 362710, ISSN 1098-3716 print, ISSN 2771-6716 online is published 12 times annually with an additional issue in December - Jan, Feb, Mar, Apr, June, Jun, Jul, Aug, Sep, Oct, Nov, Nov IBG, Dec, by Endeavor Business Media, LLC. 201 N Main St 5th Floor, Fort Atkinson, WI 53538. Periodicals postage paid at Fort Atkinson, WI, and additional mailing offices. POSTMASTER: Send address changes to Healthcare Purchasing News, PO Box 3257, Northbrook, IL 60065-3257. SUBSCRIPTIONS: Publisher reserves the right to reject non-qualified subscriptions. Subscription prices: U.S. $160.00 per year; Canada/Mexico $193.75 per year; All other countries $276.25 per year. All subscriptions are payable in U.S. funds. Send subscription inquiries to Healthcare Purchasing News, PO Box 3257, Northbrook, IL 60065-3257. Customer service can be reached toll-free at 877-382-9187 or at HPN@omeda. com for magazine subscription assistance or questions.
Printed in the USA. Copyright 2024 Endeavor Business Media, LLC. All rights reserved. No part of this publication June be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopies, recordings, or any information storage or retrieval system without permission from the publisher. Endeavor Business Media, LLC does not assume and hereby disclaims any liability to any person or company for any loss or damage caused by errors or omissions in the material herein, regardless of whether such errors result from negligence, accident, or any other cause whatsoever. The views and opinions in the articles herein are not to be taken as official expressions of the publishers, unless so stated. The publishers do not warrant either expressly or by implication, the factual accuracy of the articles herein, nor do they so warrant any views or opinions by the authors of said articles.
Printed in USA
Paper manufactured in USA Soy ink made in USA
Booth 2217

Designed to visually inspect internal channels of potentially soiled or damaged items, this borescope provides enhanced light, vision & magnification. Visit our booth for a hands-on experience!




Detect and locate defects such as pinholes, cracks and bare spots in the jacket or coating of laparoscopic & bipolar electrosurgical instruments. Visit our booth for a hands-on experience!



for In-Booth Education Sessions
Earn Continuing Education Units while learning about topics critical to infection prevention, sterile processing, endoscopy & more from our team of experienced industry professionals. Visit our team in booth 2217 during AORN Expo to learn more!

There was a time not too long ago when supply chain operators were hospital basement dwellers, emerging from their lairs only to cycle count and replenish. But what a transformation has taken place over the past decade! The hospital supply chain has evolved into a strategic powerhouse, with warehouses and storerooms emerging as linchpins in the efficient management of healthcare supplies. Let’s explore the transformative journey of these spaces, once considered mere storage areas but now pivotal in optimizing healthcare logistics and, by extension, patient care quality.
Historically, hospital storage areas were simple and functional. They served as basic repositories for medical supplies, with minimal emphasis on technology or strategic management. Fundamentally, of course, this is still what they are. But there was a bit of a collective light bulb that went on when hospital after hospital, supply chain leader after supply chain leader, started paying attention to loss and expiry, recalls and reconciliation. Supplies are the second highest expense in healthcare delivery, following labor. This is a massive expense line that demanded more strategic oversight.
And so, healthcare supply chain ascended from the basement and took a seat at the table. The very function of warehouses, storerooms, and distribution centers would evolve over the next
decade to that of a clinically integrated networked operation. Today, driven by the need for effi ciency and accuracy, accelerated by the adoption of advanced technologies such as artificial intelligence (AI), robotics, and data analytics, these spaces have both transformed the physicality of warehouses and storerooms and redefined their operational ethos.
Nearly every health system has a combination of warehouses and storerooms. Rather than working as siloed operations, these facilities are now almost always part of a larger, more integrated system that relies on centralized management.
Currently, a growing trend in hospital logistics is the operation of a consolidated service center (CSC) at the warehouse level, which feeds supplies into the technologically equipped storerooms. This approach has become increasingly common and significantly enhances how supplies are managed across the entire healthcare facility’s supply chain. The ecosystem comprises storerooms within the hospital and an offsite CSC, working in tandem to manage supplies more effectively with the aid of advanced technology.
This integration has transformed the nature of inventories and the management of materials, aiming to create a

seamless process that reliably and efficiently services the storerooms. It is the synergy between the warehousing facilities and the in-hospital storerooms that is key to an optimized supply chain, ensuring it can support immediate patient care needs effectively. This interplay is vital in maintaining a smooth, responsive supply chain that is essential for high-quality healthcare delivery.
In the past, supply chain management in healthcare was heavily reliant on manual processes. Supply technicians would count inventory levels and reorder supplies using paper forms. This method, while straightforward, was time consuming and prone to errors.
Hospitals and healthcare facilities prioritize quality outcomes alongside financial considerations, which tamps down accelerated technology adoption. This strong dedication to patient care, coupled with an inherent risk aversion, means that healthcare supply chain leaders are typically more cautious about adopting the latest technologies, often a step or two behind other industries. Instead, they focus on implementing technologies and processes that enhance patient care while ensuring safety and reliability.
Despite that conservatism, there are several proven and reliable technologies that are finding their way into more healthcare organizations today. Whether a health network is operating a CSC or a more traditional siloed supply chain operation, it has become a necessity in order to operate at the level and efficiency that the industry demands.
Over time, the supply chains have integrated more sophisticated systems at the application level, significantly enhancing efficiency in the storeroom. In warehouses, automation has evolved to include industry-standard systems that are continually advancing.
Now, the healthcare supply chain makes extensive use of barcodes, radio frequency identification devices (RFID), and advanced technologies like voice
activation, artificial intelligence, predictive demand planning, and robotics. These innovations have revolutionized storerooms, enabling precise tracking of expiration dates, serial numbers, and lot numbers.
Given the pressure on hospital systems to manage financial resources wisely, the ROI calculation for automation becomes increasingly favorable as the cost of these technologies decreases and the cost of labor increases. With more vendors and options entering the automation market, the costs are becoming more manageable, making automation an appealing alternative to hiring additional staff.
In warehouses where robots are deployed, they play a vital role in packing and palletizing goods, streamlining the distribution process significantly. The precision and speed of these robots ensure that orders are prepared accurately and dispatched promptly, enhancing the overall responsiveness of the supply chain. More traditional warehouse automation systems like automated sortation and conveyors can drive higher efficiencies. Space utilization can be maximized with goods-to-person systems like ASRS and vertical lifts. Automatic guided vehicles (AGVs) or autonomous mobile robots (AMRs) can help in picking, packing, sorting, and storing. The world of automation is coming to life, and it promises to change the look of healthcare warehouses and storerooms in the future.
Healthcare supply chain leaders face a weighty decision: whether to invest in more staffing or to shift towards a setup that includes more robotics and automation. Looking to the future, decision makers will need to carefully weigh the financial implications, workforce availability, and operational efficiency to determine the optimal balance between technology integration and human capital. This balance is key to deciding which approach is easier and more effective to manage in the ever-evolving landscape of healthcare.
Warehouses and storerooms, serving as the backbone of the healthcare supply chain, are responsible for maintaining adequate inventory levels and ensuring the integrity and quality of medical supplies. Effective warehouse management, facilitated by advancements in

technology and automation, ensures that supplies are not only stored correctly but are also distributed efficiently and accurately to various storerooms across healthcare facilities. This careful orchestration helps to prevent issues such as stockouts, expired supplies, and inventory mismanagement, all of which can critically impact patient care.
Looking ahead at the future of storerooms and warehouses in healthcare, it appears evident that integrating physical spaces with technology is key to creating an efficient supply chain ecosystem. The operational paradigm shift brought about centralization and the CSC model. The maturation of various technologies is now providing compelling use cases around data-driven optimization and system-driven processes. The ongoing labor challenges across the country present interesting ROI trade-offs between investment in automation and talent shortages.
The role of warehouses and storerooms in healthcare goes well beyond storage and inventory management. The future will see physical warehousing and storerooms seamlessly linked with software systems to enhance efficiency, but every health system will chart very different courses to get there and travel at very different speeds. As healthcare continues to evolve, the strategic management of these facilities will remain crucial in upholding and enhancing the
integrated supply chain practice and, in turn, care delivery. HPN
Cory Turner CMRP leads Healthcare Strategy for Tecsys, one of the largest supply chain IT organizations in the healthcare industry. He has two decades of experience in healthcare supply chain operations and solutioning, having earned his credentials at Greenville Health System, (now PRISMA Health), the largest IDN in South Carolina. He has since built his career with experience with Infor, Omnicell and Workday solutions. Cory’s insights into the healthcare supply chain market are distinctively informed by his tenure as both an operator and provider of SCE software.


ospitals face a challenging situation when it comes to managing their supply chain. In many cases, they are caught between using complex and expensive inventory systems that are not designed to identify expired and recalled items and a manual inventory count process that is outdated, time consuming, and prone to errors. Unfortunately, this has resulted in an alarming number of expired and recalled items being found in hospital supply rooms, which can have serious consequences.
Recall events in the U.S. medical device industry increased by 8.8 percent in 2022, up from 837 events in 2021 to 911 in 2022. On average, 1 percent of items currently in hospital supply rooms have been recalled—many of those items are implants at high risk of being used in a patient procedure.
Meanwhile, a Cardinal Health study found that nearly a quarter (24 percent) of healthcare providers say they have seen or heard of an expired product being used on a patient. What is shocking is that it has become accepted by the industry that 8 to 10 percent of all disposables expire on hospital shelves annually. The reason is understandable: Resources are tight, and freeing up the time and effort needed to stay on top of recalls and inventory means losing those resources elsewhere. However, with recalls on the rise and more single-use devices on the shelves, hospitals have to look at new ways to manage these processes.
Although hospitals have processes to look for expired and recalled products, they are challenged by the fact that these are often manual processes. Most hospitals rely on the supply chain team or clinicians to physically look at every item in the hospital—every month—to remove products that have expired.
The same Cardinal Health study cited above found that nearly 20 percent of clinicians’ work weeks are spent on supply chain management tasks and that 66 percent of respondents wished they did
not have to perform supply, inventory, and administrative tasks. Likewise, 78 percent of respondents still manually count inventory in some areas of their supply chain.
Nearly one-third of respondents said it’s been six years or more since their hospital has implemented a new inventory management system. Another 25 percent of respondents did not know if an inventory management system had ever been implemented or updated.
Manual inventory counts can take one or two staff members up to two days a month to complete, and one operating room acknowledged that this manual method was not efficient, as the OR area suffered from $80,000 a year in wastage costs due to expired products. Keep in mind that is just what was recorded.
Again, this isn’t the reality that hospitals desire. They’re simply handcuffed by legacy processes and a lack of appropriate systems.
Many hospitals have advanced inventory management systems. These are very expensive, complex systems for tracking devices and instruments in a digital system. Theoretically, with an inventory management system, the hospital knows what’s on its shelves in the supply rooms. However, the information is incomplete and doesn’t include important information like expiration dates and lot numbers.
Neither inventory management systems nor manual counts create the levels of inventory control needed to eliminate the use of expired or recalled products. Meanwhile, devices are expiring on the shelves, recalled products aren’t being removed, and PAR [“periodic automatic replenishment] levels are driving excessive numbers of unused devices.
Inventory management systems provide on-hand inventory levels; they don’t provide inventory control. Control looks like having visibility into expiration dates and lot numbers in a cloud-based system. It would empower the clinician looking for the recalled or expired products to do a query to
confirm the lot numbers are on the shelf and, more importantly, where those items are—even when there is not a PAR location.
Hospitals need to move beyond standard inventory management systems to employ technologies that can help them truly understand the supplies they have on hand, where they are, and whether they’re still safe to use on patients. One such solution comes in the form of unique device identifier (UDI) scanning. Since 2017, all manufacturers have been required to use standardized barcodes, or UDIs, which contain information about expiration dates, lot numbers, and more. With the right scanning technology, capturing this additional data can be done in both PAR and nonPAR locations.
The consequences of not getting a better handle on hospitals’ inventories are critical, as this need goes straight to the heart of patient safety. Today’s hospitals must take the steps necessary to de-risk their supply chains, and that begins with exploring the latest advances in UDI scanning. In the process, they’ll unlock much more than greater financial sustainability; they’ll unlock the ability to better care for their patients.
We can all agree that expired and recalled items should never be found in a hospital supply room. When they are, there is a good chance they will be used on a patient. As an industry, we must take concrete steps to de-risk the healthcare supply chain for the benefit of patients and hospitals alike. HPN
Ashlea Souffrou is a highly experienced medical device industry veteran with a passion for developing sustainable and cost-effective healthcare solutions. As the founder and CEO of SxanPro, she has successfully digitized inventory processes throughout the hospital supply chain. Souffrou is a certified materials and resource professional, an active member of the Association for Healthcare Resource and Materials Management, and a UDI implementation expert for the industry-leading podcast, Power Supply. She holds two patents for her mobile application technology that extracts product data from medical devices.
Healthcare Purchasing News asked a couple of solutions providers some questions on the current state of storeroom/ warehouse technologies. Here’s what they had to say.

What are the current challenges healthcare organizations are facing when it comes to storeroom/warehouse?
Space is at a premium in healthcare organizations, and teams are facing the significant challenge of truly understanding where mobile medical equipment (MME) needs to be, how it’s utilized, and its status. Emerging technologies such RealTime Location Systems (RTLS) address these uncertainties and help maximize the strategic distribution and allocation of MME, which frees up storage areas to better utilize space and save time for staff and patients.
What is RTLS? How can it help?
RTLS is designed to provide current and historical visibility into asset locations by automating the processes of moving equipment in and out of warehouse and storage spaces. RTLS includes uses such as asset management where users can identify and monitor the equipment in any storage location through advanced software and IoT-enabled tags that can streamline processes around who needs what, where, when, and how often. It is imperative for hospitals and IDNs to
understand where the location of mobile medical equipment (MME) at every point in time. This also all-ows cost savings in regard to purchases and rental decisions.
RTLS is an important component in helping to free up storage space within hospitals and healthcare organizations. Throughout a hospital, whether in the ED, the PACU, or on critical care floors, leveraging smaller storeroom locations or clean equipment rooms instead of utilizing one large communal location can ensure organizations are not over capitalizing on central storage. RTLS informs on the current location of assets, but more importantly, shares insights on where it should be located to better leverage space. These location insights create actionable context and meaning. This is how we work to educate decision-makers on how to find medical equipment and where to store it to produce the best ROI.
The future of RTLS and storeroom/warehousing needs to focus on continuous process improvement. It’s all about leveraging lean methodology to constantly do more with less.
We’re also moving in the direction of using AI responsibly with RTLS, leveraging it to be more predictive about what items are available, where items need to be and when. If you implement RTLS and know exactly where an IV pump is when you need it, your team saves time on non-value-added tasks and storage space. This real-time technology can accomplish so much when utilized to its full extent.
 — Todd Stewart, VP, Solution Sales, CenTrak
— Todd Stewart, VP, Solution Sales, CenTrak
Why are there dedicated supply cores to each procedure area in a hospital?
Storerooms and warehouses in healthcare facilities serve to ensure that hospitals always have the medical devices they need for procedures and patient care. Having the right supplies at hand can mean the difference between life or death for a patient, so stocking ample supplies is critical to the quality of care provided. At the same time, overstocking or stocking the wrong products is a major challenge for U.S. healthcare facilities – financially and in terms of risk management. Supply chain professionals are trained to handle inventory efficiently to reduce the risk of stockouts while reducing the amount of over-stocked products. This ensures that procedural areas have the necessary supplies when needed, minimizing disruptions.
Do all hospitals have OR supply cores?
All hospitals have centralized store locations as well as “local” storage locations like OR rooms or specialty carts. Supplies can float between these locations, but it remains a challenge for most hospitals to keep track of “what is where” and minimize overstock and remove expired products from storage locations, for example.
What are the current challenges healthcare organizations are facing when it comes to managing inventory in an OR supply core and operating room non-stock locations?
The key challenges in managing inventory are stagnant PAR locations, returns from unused products from the OR, and outdated expiration date (and product recall) checks: Hospitals spend millions of dollars every year storing products they no longer use and ordering products in excess of their needs. This happens in spite of the fact that lots of hospitals have very advanced inventory management systems that supposedly track their inventory.
The root of the problem can always be traced back to manual processes. Ultimately – inventory management system or not – hospitals rely on staff to conduct manual counts, select products, and make decisions about what and how much is needed. It all comes down to resources, particularly people’s time. Proper inventory management is often dependent on knowledge of the right product, for the right procedure, for the right physician, and this increases dependency on people and manual labor.
People will never be removed from this equation, but there are manual parts of this process that can be replaced or enhanced with easily accessible technology, and this is where hospitals today have an opportunity to reduce supply costs significantly, while also reducing risk and maintaining inventories adequate for their patient care operations.
According to Medline and Cardinal data, 8-10% of inventory expires annually. Hospitals have to deploy significant
resources to look for outdated inventory, as JCHAO requires an expired inventory process to be documented and proven. This is a manual process. Think about that: There are more than 30,000 items in an OR procedure area in an average 500bed hospital. Using a manual process for this inventory check adds up to a lot of time and resources that could otherwise be deployed caring for patients.
Are there any new technology innovations that are assisting in these challenges?
Many hospitals are using advanced inventory management software to streamline and automate the inventory management process. These are expensive, complex systems for tracking devices and instruments in a digital system that has to be integrated with other hospital IT systems. Theoretically, with an inventory management system, the hospital knows what’s on its shelves in the supply rooms. However, the information is incomplete and doesn’t include important information like expiration dates and lot numbers.
Hospitals need to employ technologies that can help them truly understand the supplies they have on hand, where they are, and whether they’re still safe to use on patients. This is the difference between inventory management and inventory control. One such solution comes in the form of unique device identifier (UDI) scanning. Since 2017, all manufacturers have been required to use standardized barcodes, or UDIs, which contain information about expiration dates, lot numbers, and more. With the right scanning technology, capturing this additional data can be done in both PAR and non-PAR locations.
Hospitals understandably want to integrate all of their technologies, but that process is slowing them down right now. There needs to be shared knowledge between systems. However, solving a problem with an easy-to-use technology that is department-specific and solves department-specific problems needs to be considered as part of the road map for a long-term strategy.
What does the future of this space look like?
An integrated system that leverages UDI as the source of truth will solve the everyday supply chain problem.
Integrating the GTIN into the master data file has to be a priority for all health systems. A good analogy can be found in the retail industry. The way hospitals are operating today is like the days of the local grocery store owner using a price tag gun. That was the reality 20 years ago, and look at how transacting in retail has changed! Retailers have been able to aggregate and organize their data to make it actionable by putting mobile technology in the hands of their people and leveraging a non-integrated model.
If billion-dollar retail stores can trust mobile app technology, why can’t hospitals? Standard worries about HIPPA don’t apply when it comes to using mobile scanning technology to manage and control inventory at a more granular level. In these cases, we are dealing with product data versus personal data, and systems that scan UDI can eliminate the manual processes currently plaguing hospital inventory management.
Any final thoughts?
If manufacturers would include an RFID tag on all of their products that included the info that they submitted to the FDA for the UDI, it would revolutionize how hospitals use supply data in supply chain, finance and clinical outcomes.
—Ashlea Souffrou, Founder and President of SxanProDETECTO manufactures more unique models for each clinical product category than any other medical scale company. Giving you better choices means you never have to settle for a product that isn’t
DETECTO: A true vertically-integrated medical scale manufacturer – family owned and privately held – with factory and headquarters in Webb City, MO.





Healthcare is a frequently targeted field for nation-state hackers across the world. The profusion of protected information that healthcare entities hold makes it a hot target for hackers who want to disrupt societies at large and specific individuals within them, or even just to bilk money out of hospitals or patients by holding their information ransom.
Hospital staff are often unaware of just how many vendors’ services they are employing in the day to day running of their organizations. Cybersecurity expert Richard Staynings recalls a situation where, when assessing a large hospital, he found an enormous discrepancy between the number of vendors a hospital thought they were working with and how many they were actually working with. The procurement department believed they had sought the services of about 500 vendors, but in reality, they had over 10,000 contracts with different vendors, which introduces an enormous security risk. When even the procurement department does not seem to have any record of who they are working with, it leads to the possibility that they will not be monitored or managed -- much less patched -- to fit the ever-changing needs in the space. Healthcare Purchasing News had the pleasure of speaking with Staynings, a healthcare technology and cybersecurity strategist, affiliated with both cybersecurity firm Cylera and with the University of Denver, about cybersecurity, specifically as it pertains to medical and surgical devices.
What constitutes a medical device?
A medical device is any device that aids in the treatment of a patient, like a tongue depressor. However, from a cybersecurity perspective, connected medical devices are usually what we’re talking about – O2 saturation, blood oxygenation systems, patient telemetry systems, and blood pressure cuffs. All kinds of other systems such as nurse call systems, wearables, and devices that connect wireless devices to the hospital network are also included. This is all generally referred to as the “internet of medical things.”


How do these ecosystems need to be refined to fit cybersecurity standards?
A lot of building management systems, elevators, HVAC, CCTV, cameras, proximity door locks to keep patients out of the OR, are all owned by different groups. They’re owned by facilities, physical security guards, and building management companies. Sometimes, they may be managed by a third party from hundreds of miles away because it’s cheaper to have your elevators managed by someone off-site than on-site. All these different systems need to communicate, which is really where the risks lie.
Most medical devices were not designed with security in mind, but rather for clinical functionality. They were tested by FDA for clinical safety and functionality, and if they pass that test, they were approved for use. Pre-market and post-market guidance about cybersecurity, updates, and patching have begun to happen under the auspices of patient safety, as cyberattacks can create a patient safety risk. A cyberattack that causes, say, an infusion pump to be hacked and rendered inoperable could be devastating to a patient, causing the

drug flow to be interrupted or accelerated in a way that could become life-threatening. Even software failures can cause these devices to stop working. More than that, a lot of these devices run Windows embedded, which is a shrunk down version of Windows, which is not very reliable even in its modern flavors. However, some of these devices are still running Windows 95, which is highly unreliable.
Hackers can get into these devices to hold a patient’s life to ransom, or, alternatively, use that medical device as an ingress point as a foothold on the medical network to go after the EMR (electronic medical records) in order to encrypt them for ransomware or exfiltrate higher value information.
What federal regulations have been put in place to secure these devices?


The Patch Act was passed last year [in 2022], which legislates the optional guidance that FDA was pushing before. It’s put the onus back on medical device manufacturers to disclose security vulnerabilities in the medical devices to make patches available in a timely manner. Then, at the end of December of that year, the Appropriations Act gave money to FDA and gave them the go ahead to write new rules. These new rules went into effect midway through 2023, and they culminated in the refusal to accept rule when went into effect as of October 1, 2023. That means FDA is now able to refuse to accept a medical device for approval for use in the United States. It’s probably 10 years later than it should have happened, but it’s a great step in the right direction.
The biggest concern with it, however, is that it isn’t retroactive, so it doesn’t impact the devices that anyone bought before October 1. So you have a whole load, millions of medical devices out there, which are now considered legacy devices, which are unsecure, unpatchable, and highly vulnerable. That makes them easily compromised. We need to adopt a different security approach to those devices, including using compensating security controls like network micro-segmentation and better zoning of systems.
What issues do hospitals face in securing their medical devices?
The biggest issue in healthcare is that hospitals don’t know what connects to their networks – there are millions of endpoints. Medical devices make up about 75% of connected assets in your typical hospital today, and IT only manages and only has an asset inventory of about 25%, which are the servers, workstations, laptops, etc. That 25% is managed by IT, running traditional Windows operating systems.
Medical devices have a half-life similar to plutonium: they don’t go away. Your average medical device probably has a usable lifespan of eight years, and bigger equipment like x-ray machines will be in use for 20 years or so. It’s almost impossible to tell the CFO of a hospital that an x-ray machine they have can no longer need to be patched and they need to buy a new one. Say it costs $35 million for a new x-ray machine and the existing, unpatchable x-ray machine with seven years left on the amortization schedule cost $25 million in the first place. This is where micro-segmentation is important to minimize the risks so that devices can only communicate using a zero-trust framework right on the ports, destination IP addresses and protocols that we’ve specifically authorized.
Another big concern is that we don’t train doctors to look for indicators of compromise of systems. We don’t train nurses either, so they’re operating blind. We need to have better annual
cybersecurity training, not computer-based training programs that everyone sleeps through, but a multifaceted, multimodal, educational awareness program that talks about specific threat vectors and gives specific examples of what happens when medical devices fail. For example, at Intermountain Healthcare, they have a system where they use different colored dinosaur stickers to remind the staff of different risks. A green dinosaur inside of an elevator indicates to staff to not talk about patients in public; meanwhile, the patients just see a green dinosaur and say, “Oh, that’s cute.” It serves to both make the hospital less scary to kids and sends a subliminal message to doctors and nurses to not have a conversation about a patient while you’re in the elevator.
How can we ensure medical device manufacturers are taking proper cybersecurity measures?
What we need to do with medical device manufacturers is hold them accountable. We need to do this via purchasing contracts, and we need to ensure that medical device manufacturers patch systems on a regular basis and disclose vulnerabilities on a regular basis. Certain manufacturers will say “unfortunately we don’t have a patch for that” about certain things hospitals identify as needing a patch, and that response needs to be logged by procurement so you know which medical devices are the most compliant of all the medical device manufacturers they work with, which can then form the basis for a purchase list and an exclusion list for those who have the worst security.
Annual audits for medical device vendors and better management of third-party vendors that manage medical devices for hospitals are other important steps. We need tools that monitor our risk profile in order to tell us what assets are connected to the hospitals’ networks, and then analyze the risks to tell us where they lie so that hospitals can hold the vendors to account.
How do artificial intelligence (AI) and machine learning (ML) increase risk factors in medical devices rather than stabilize them?
AI models can be poisoned or corrupted, data sets can be mislabeled or corrupted, and AI can either be purposely manipulated into making mistakes or it can inadvertently make mistakes on its own. At this stage, it’s important that humans remain the final decision maker when it comes to AI’s recommendations. For instance, in medical radiological imaging, AI will analyze an image and it will make recommendations, but the professional radiologist will then validate or dispute that information.
Where it may not need to be a partnership is in fields like defensive AI for security. We need to invest in defensive AI tools in order to recognize an AI-tipped attack, tools that can respond in a nanosecond without human intervention in order to block that attack. It needs to identify when things are behaving anomalously and kick those things off the network so that a technician can then come and validate that the AI is correct.
As far as Terminator-type self-awareness, that’s generations away, more than likely. We’re going to see continued evolution of various forms of AI, particularly in the healthcare space, because we need to improve efficiency here. There’s a lot of things that we can do on the medical side that AI can assist with. If we can recognize the onset of cellular mass changes that are indicative of a tumor growth months or years before that tumor starts to develop, we can treat that cellular mass change before cancer erupts in the body and spreads to lymph nodes or becomes malignant. HPN

Isis Lamphier, MPH, CIC, is a board-certified infection preventionist. Currently, she is the Manager of Infection Prevention and Control at Tampa, Fla.-based Moffitt Cancer Center. Her passion for infection prevention and control was ignited after successfully attaining a Bachelor of Science in Health Education and a Master of Public Health in Epidemiology from the University of Florida. Prior to her current role in the hospital setting, Lamphier gained valuable experience working at both the county health department and a long-term care facility.
Healthcare Purchasing News had the opportunity to speak with Lamphier about all things personal protective equipment (PPE), including the current state of COVID and education when it comes to reusing PPE.

We are still seeing an increase in COVID, and the trend is we’re seeing consistent cases of COVID. It hasn’t declined as much as the public might think. The only thing that has decreased is our active detecting at the beginning of the pandemic. We saw a lot of testing centers that offer testing and now testing isn’t as readily available. The primary method that individuals are getting tested is through home test or if they go to the hospital instead.
I could get a COVID PCR through a hospital, so we’re still seeing COVID cases happening as well as a mix of respiratory viruses as well. We’re seeing flu AB, RSV, and some of our other viruses, like parent influenza as well.
We are seeing an increase of flu and it’s important that if a patient tests positive for COVID or any respiratory virus that they wear a surgical mask. If they have to go out to the public or if they have to go to a hospital or clinic visit, it is very important that they wear one because we know our respiratory viruses and COVID are spread through droplet particles and that way they can protect other healthcare workers or other individuals around them.
We haven’t had any PPE shortages recently that I’m aware of, especially in the state of Florida, and one thing that I think the PPE shortages affected when we started the pandemic is we were reusing PPE. That’s something that I’ve been working a lot to educate the staff on, is that we don’t need to reuse PPE since we’re not in a shortage so we don’t need to reuse our single use surgical mask or our single use KN95s because it’s also important that we have a clean mask on as well when
we’re taking care of patients or if we’re putting it on ourselves if we’re sick.
What I have seen instead is a shortage of medical supplies or medical equipment that are non-PPE. I know right now we were notified that there was a shortage of our temperature-sensing foley catheters, and we’ve also had some alcohol wipes that we use in the hospital that we had a shortage of. So we’re seeing a shortage instead of other items due to manufacturing delays, but I haven’t seen any PPE shortages, and I think the reason why we haven’t seen a shortage of PPE is because so many individuals during the pandemic started making PPE and their companies previously didn’t make PPE and now they do.
Has education on not reusing PPE been a challenge for you?
Yes, definitely. Staff tend to think it’s wasteful to use it only after one use. For example, let’s say a nurse is to go into a room where a patient is in isolation for respiratory virus. They have to put on that PPE, and they were so used to wearing that one mask that whole day and that’s not the recommendation—they should put on a clean mask every time they go into that isolation room.
They think it’s wasteful because they were used to prolonged use of PPE and wearing that one surgical mask that whole day or unfortunately sometimes even a week, they would wear that one surgical mask. But once they exit that patient’s isolation room, they can remove that mask and then don a new one if they need to go to another isolation room or wear it for another purpose.
What are some other best practices surrounding PPE?
Another best practice regarding PPE is to ensure that you perform hand hygiene before putting on PPE, and that’s because we don’t want to contaminate the PPE that we’re putting on, or even the packaging. If you’re reaching into a glove box, you don’t want to contaminate all those gloves for other people.
That’s something that’s very important that we educate our staff on and we try to do education at my facility during orientation and also continuous education that any time we put on PPE, we should perform hand hygiene as well as when we remove it, so we don’t contaminate ourselves or contaminate that clean PPE when we’re putting it on.
And again, keeping PPE single use if that’s its intent. If the instructions for use say that it is a single use item, ensuring that we’re doing single use. Also, making sure that we establish a clean environment, it’s important where we store our PPE in our healthcare facilities. Sometimes we’ll see nurses that
might put a bunch of gloves in their pockets or on their cart and remove them out of the glove box and just leave them in like a caddy or something similar in order to make it easier to put on that PPE. It’s very important that we have that PPE in a clean environment and keep it in its packaging because, if not, we could contaminate the PPE further by taking it out of its box and putting it somewhere else just for ease of use and convenience.
What are some important aspects that individuals employed at hospitals should be aware of when evaluating purchasing PPE?
It’s important that, depending on the PPE, it is certified. For example, N95 masks should be the NIOSH [National Institute for Occupational Safety and Health] approved ones through OSHA [Occupational Safety and Health Administration].
Maybe someone that’s ordering might not be familiar with the different levels of protection. Level 1 is less protective than a Level 3 gown or mask, and depending on its intended use, is what you should look for.
Some surgical masks should be splash resistant; that’s an added feature in certain surgical masks. In level, the surgical mask and those masks would be beneficial for someone that is at risk for splashes—for example, technicians and sterile processing.
It’s important that you’re evaluating what the healthcare worker is doing because that will determine what level of PPE they need. My biggest recommendation for anyone that is in supply chain and purchasing is that they’re working with their infection prevention departments as well as occupational health in order to protect their healthcare workers. These workers should put in their input as well as nursing on the level that’s needed and the type of PPE that’s needed because we want to make sure that they’re protected from pathogens and splashes and body fluids.
Also, we want to make sure that it’s comfortable as well. Sometimes we might want to offset costs and choose something that might be more beneficial to our organization’s costs, but we also want to make sure it’s good quality and that it is not going to break because when things start breaking, you’ll need to get more, and so that changes costs. HPN
Jackie Stegemann, vice president, US Product Marketing for Cardinal Health’s Medical Product & Distribution business shared her insights with Healthcare Purchasing News from a solutions providers perspective.
Has anything changed regarding PPE over the past few years?
Increased focus on protection and clinical needs: Exam gloves have seen a shift back to clinical needs and protection. During the pandemic, healthcare facilities were heavily focused on securing any exam gloves they could. Now that facilities have moved through pandemic storage and inventory, there is a renewed focus on protection and clinical preference. Similarly for isolation gowns, there is an increased focus around higher levels of protection and AAMI rated gowns.
Customer buying patterns have shifted back to normal pre-covid trends, especially related to cold and flu season: Demand spiked during the pandemic for earloop procedure masks, as they were used by clinical staff and patients. They remained elevated through the pandemic until masking mandates were lifted last spring. We are seeing increased demand due to respiratory season.
Customers stocked up on isolation gowns and exam gloves during the pandemic and worked through their extensive stockpiles. Over the past few months, we have experienced increased requests for stockpile replenishments.
Were there any new innovations to note?
Facial Protection:
Cardinal Health launched a sensitive skin line of surgical masks with ties: Cardinal Health™ Sensitive Skin Surgical Mask Portfolio
We’ve also just launched a new anti-fog surgical mask with hydrogel, the first of its kind in the anti-fog mask space. More on this NEW mask is in the attached – feel free to expand on this. We can also connect you to an expert if you want to dive into this a bit more in a separate piece.
Infection Control Apparel:
Cardinal Health carries a comprehensive line of chemotherapy gowns that protect healthcare personnel from exposure to bodily
fluids through AAMI Level 4 protection and has been tested with commonly used chemotherapy drugs, from preparation and handling to administration and disposal. More information in the attached PDF.
Was COVID a factor in this?
These innovations were based upon customer feedback and meeting needs of the market.
What current challenges are healthcare professionals facing regarding PPE?
Maintaining Protection:
Healthcare professionals are facing a myriad of challenges regarding PPE. There are new concerns around fentanyl testing, enhanced protection against certain chemotherapy drugs and chemical agents. At the end of the day, the focus remains on protection and making clinicians’ jobs safer and easier.
Customers are increasingly more concerned with levels of protection associated with PPE products. They are seeking education to understand what level of protection is needed to help keep healthcare professionals safe.
Supply Efficiency:
Customers purchased from a multitude of suppliers during the pandemic and are actively working to simplify their supply chains through SKU consolidation. Customers are identifying how to maintain a diverse supplier base while ensuring they aren’t overstocked on product.
We know supply chain issues were abundant during the pandemic, can you comment on the current situation?
Supply constraints were prevalent during the pandemic due to the macro environment. At Cardinal Health, we have prioritized increasing inventory levels and diversifying our supplier base to mitigate challenges in the future.


Where can digital technologies, analytics, and artificial intelligence deliver value?
The majority of healthcare executives surveyed by Deloitte “expect the accelerated adoption of digital tools will impact their strategy in 2024,” and “agree that generative AI has the potential to address many of the sector’s most vexing issues.” 1
“Almost every part of healthcare has been touched by digital, AI, and innovative technologies,” noted the authors of the January 2024 report, “Transforming Healthcare: Navigating Digital Health with a Value-Driven Approach,” from the World Economic Forum and Boston Consulting Group. 2
So, how have these advanced technologies touched sterile processing (SP) departments?
The workflows around instrument management, including the building and reprocessing of case carts, kits, and trays, are ripe for technology solutions that can make the SP professional’s role easier, more efficient, and more effective, enabling them to focus not on non-value-added tasks but rather clinically relevant activities at the top of their licensure.
SP professionals and technology solutions vendors offer their insights on the current and future state of advanced technologies for the SP

department, including digital applications, analytics, and AI.
Dr. Marjorie Wall, EDBA, CSSBB, Associate Director, Sterile Processing, Cedars-Sinai, Los Angeles, is enrolled in a Harvard Medical School degree program along with 59 other healthcare professionals, 30 of them international, where they have been discussing SP topics. Her realization – SP challenges are global.
“One topic that keeps coming up in the group is preference card accuracy,” said Dr. Wall. “With the expansion of AI across healthcare applications, I am seeing the development of solutions that employ AI to monitor instrument and supply usage in the OR and build bots to automate preference card maintenance. These solutions will provide better data and flow of information so SPD [sterile processing departments] can pull what surgeons need rather than extra trays that go unused and must be reprocessed.”
There are challenges not only knowing which instruments and supplies to pick and pack based on preference cards, but also where to find them based on the order and accuracy of
pick sheets. This is another area where advanced technologies can help.
Carol Malone, AAB, CSPDT, CRCST, CIS, CHL, System Supervisor, and Amanda Wilcox, BA, CSPDT, CRCST, Education Coordinator, Sterile Processing Education, University Hospitals, Cleveland, spoke to the challenges of picking and packing.
“For new SP team members, and even experienced ones, the picking process can be frustrating when pick sheets are incorrect or out of order,” Wilcox explained. “The sheets should list items in the order of where they are stored in sterile storage, for example, from the front to the back of the space, to make the process easier and faster to complete. When the pick sheets list items out of order, we find ourselves running all over sterile storage trying to fi nd what we need.”
One issue that contributes to inaccurate pick sheets is lack of integration between a healthcare organization’s electronic health record (EHR) and instrument











“Following the instructions for use (IFU) is crucial for maintaining a high standard of infection control in case cart handling,” said Lawayne Perkins, CHL, CRCST, COO of Dr. SPD, a consulting company that specializes in sterile processing (SP). He offers the following ways in which IFU adherence contributes to infection control:
1. Proper Cleaning and Disinfection: The IFU provides specific instructions on how to clean and disinfect case carts effectively. Following these guidelines ensures that all surfaces, including handles, wheels, and interiors, are thoroughly cleaned and disinfected, removing any potential contaminants that could contribute to the spread of infections.
2. Preventing Cross-Contamination: The IFU outlines proper loading and unloading techniques, including the segregation of clean and dirty instruments. By following these instructions, healthcare professionals can minimize the risk of cross-contamination between contaminated instruments and clean equipment or surfaces. This helps prevent the transfer of pathogens and reduces the likelihood of infections.
3. Equipment Maintenance: The IFU often includes recommendations for regular maintenance and inspection of case carts. By adhering to these instructions, healthcare facilities can ensure that case carts are in good working condition, free from any defects or damage that could compromise their functionality or pose a risk for contamination. Regular maintenance helps maintain the integrity of case carts

and reduces the potential for infection transmission.
4. Staff Training and Education: The IFU provides valuable information and guidelines for case cart handling. By following the instructions and providing proper training to healthcare staff, they can gain a better understanding of the importance of infection control practices and the specific steps required to maintain a high standard of care. Staff education ensures that everyone involved in case cart handling is knowledgeable and compliant with the necessary protocols.
5. Standardization and Consistency: Following the IFU promotes standardization and consistency in case cart handling practices across healthcare facilities. Consistent adherence to the recommended procedures helps minimize variations in infection control practices, ensuring that all case carts are handled in a uniform and safe manner. This consistency contributes to a higher level of infection control and reduces the risk of errors or oversights that could compromise patient safety.
In summary, following the Instructions for Use (IFU) plays a crucial role in maintaining a high standard of infection control in case cart handling. Proper cleaning and disinfection, prevention of cross-contamination, equipment maintenance, staff training, and standardization are all key elements that the IFU addresses. By adhering to these guidelines, healthcare facilities can minimize the risk of infections, promote patient safety, and maintain a hygienic healthcare environment.
tracking systems. According to Malone and Wilcox, when these two systems cannot seamlessly share data, the team must manually intervene to correct the gaps.
“There are a lot of pieces that must fall into place for electronic systems to work properly,” Malone noted. “With our old EHR system, none of the tray ID numbers transferred over to our instrument tracking system and that is what allows the trays to show up on our pick sheets. There is a lot of backtracking work to manually add those numbers.”
“If we could put the data in one system – for both instruments and disposables – and have it autogenerate in multiple systems, that would ease a lot of pain,” Wilcox added.
One company that is applying intelligent algorithms to solve instrument tracking/EHR system integration challenges and facilitate data integrity in perioperative workfl ows is Ascendco Health. The company’s Co-Founder & CEO Brian Reed commented on its surgical asset management platform, stating:
“A perioperative team may believe they have an interface between their EHR and instrument tracking systems, but when the SP team generates a pick list, there is no match between what the physician preference card is calling for and the actual inventory that needed to be picked.”
According to Reed, Ascendco Health’s platform works around its own instrument tracking system and legacy tracking systems to “fix that complicated data problem that hospitals can’t fix on their own.” They collect and clean a hospital’s surgical data, leverage sophisticated algorithms in their relational database to
match and pair instrument IDs and names in the EHR and tracking systems, and correct any errors, such as missing, inaccurate, or duplicate information.
“Another area where I have seen AI in SPD is with sterile processing technician assist systems or co-pilots, which are technology solutions that help guide technicians in reprocessing workflows,” said Dr. Wall.
One such solution is ScalpelAI, which leverages computer vision (CV) and machine learning (ML) to identify surgical instruments without the need for tags. Non-invasive sensors provide real time information to the platform throughout the perioperative pathway. CV and ML algorithms identify patterns in clinical actions, inventory use, and safety incidents. The platform integrates data from the sensors and clinical actions to generate actionable insights for perioperative teams.
“Our technology has been a huge value-add in SPD, particularly for early stage and semiskilled SP techs,” said ScalpelAI founder & CEO Yeshwanth Pulijala, PhD. “It’s quite daunting when they are tasked with learning hundreds of surgical instruments, especially today with the huge burden of staffing issues and turnover. ScalpelAI helps by reducing stress levels and giving them peace of mind through on-the-job learning.”

AI assist in building trays is one ScalpelAI application where Dr. Pulijala says SP teams have gained great value, stating:
“If a technician is unsure whether a specific instrument belongs in a tray, they place the instrument in front of the cameras and the platform tells them what it is and whether it belongs in the tray they are assembling. It provides them with the tools to help them get to the source of truth quickly and make sure they are packing trays correctly. We’ve seen techs pack a tray accurately in half the time.”
While manufacturer instructions for use (IFU) are a necessary part of sterile processing, SP professionals are vocal about their struggles to access them, understand them, and put them to use in a real-world environment (more on this topic in the March 2024 HPN article, IFU Confusion).
Hannah Schroeder, BSHA, CRCST, CIS, CHL, CER, Clinical Education Specialist, Pure Processing, speaks to the potential for AI to help address IFU challenges.
“In taking an operational spin on IFU review, how could AI give us the opportunity to change how we configure our trays and case carts and prepare them for the OR? For example, maybe the IFUs for items sharing space in a set conflict in some way. Could AI help us identify and resolve those discrepancies?”
Xcelrate UDI has developed a point of use unique device identification (UDI) barcode scanning application that leverages AI technology to not only provide SP teams immediate access (less than three seconds) to latest manufacturer IFUs and medical device data but also help them better understand them and resolve conflicting and confusing information.
“The interactive AI capabilities of IFUVitals are indeed transformative,” said Joan Melendez, President & CEO, Xcelrate UDI. “Staff can quickly obtain answers to crucial questions such as ‘Is this medical device IUSS

compatible?’ ‘What are the exact sterilization temperatures?’, ‘Can lumens be processed in this peel pack?’”
An alarming post from researcher Cori Ofstead, CEO at Ofstead & Associates, on LinkedIn called out how there were 8,573 reports of endoscope-related adverse events to the U.S. Food and Drug Administration (FDA) in Q4 2023, which “eclipsed the previous record set in Q3.”3
Because endoscopes are so challenging to clean, solutions providers have developed advanced technologies that aid with scope inspection.
“We are already starting to see artificial intelligence making its debut in the sterile processing industry by way of borescope software,” said Bobby Hutchinson, Marketing Manager, Pure Processing. “Its use aids in the identification and action against bioburden and damaged equipment making its way back to the point of care.”
Performing sterilization according to manufacturer IFUs and monitoring cycles for safety and compliance can be streamlined through the application of advanced technologies.
“In the context of sterilization, it’s about assisting compliance and analyzing the instruments themselves to make sure they were properly sterilized – there are a lot of areas where artificial intelligence can help the process,” said Henry Schein’s Chief Innovation Officer Bruce Lieberthal.


Lieberthal noted how Henry Schein recently entered into an agreement with a provider of a technology solution for sterilization compliance and reporting.
“Using automation, the solution can connect with virtually all sterilizers to monitor in real-time the cycle and whether it was successful. It can also link back the instruments that were sterilized to a particular run of the sterilization,” Lieberthal explained. “It monitors the compliance of the team with sterilization protocols, including biological monitoring. It’s an interesting solution.”
“In my opinion, emerging technologies will be a game changer,” said Erica Smith, Surgical Solutions Manager, Henry Schein Medical. “It will help clinicians minimize the time spent on administrative tasks, which will allow for more time to focus on patient care.”
Education and training

Research has shown how standard training programs can decrease quality errors in surgical instrument reprocessing.4 But in today’s SP environment of staffing shortages, high turnover rates, and an ever-increasing volume of surgical instruments and devices, finding the time and the resources to train technicians is a tremendous challenge.
In response, SP professionals and solutions providers have been looking for ways to leverage technology for efficient and effective on-the-job education and training.
In the perioperative space, Marian Scheer, Chief Medical Officer for Incision, and her team have developed a digital training app called Assist. Through the mobile app, OR team


members can digitally access protocols including surgeons’ preferences, materials and draping requirements, room setup in 3D, surgical steps, and postoperative instructions.
The Incision team is now exploring a version of the Assist app for the SPD, as Aimee Space, BSN, RN, Incision Product Owner, U.S. Content, explained:
“As we have been implementing Assist in U.S. ORs, we are getting feedback from SPDs as well because both functions are so closely intertwined with their workflows. Specifically, SP teams have told us that the app could help with picking cases. These are applications we are exploring for the future.”
To support SP professional education today, Incision offers eLearning courses aligned with industry guidelines (including HSPA, AAMI, AORN) and certification programs (CNOR, CRCST).
Based on SP department supervisors’ requests, Melendez says her company is currently focused on the association of AI-driven employee continuing education (CE) validation for IFU use and adapting facility-specific standard operating procedures (SOPs) for IFU limitations.
“For example, addressing unavailable specific detergents or incompatible processing instructions provided by manufacturers,” Melendez commented.
The ability to analyze the vast quantities of SP data and surface insights to improve SP workflows and reprocessing quality and safety is perhaps one of the most promising and impactful for the application of advanced technologies.
“An arguable game changer in SP departments would be AI integration into instrument tracking software,” said Hutchinson. “With an ability to provide unbiased narrative of data, trends, and patterns, we can expand our horizons and opportunities as to the way we manage and facilitate the work in SPD. Opportunities could include analyzing peak processing times and changing the way we schedule work hours, measure productivity, and overall efficiency in our space.”
“To quote Peter Drucker, ‘You can’t improve what you don’t measure,’” said Joseph Avila, MBA, BHA, CRCST, Regional Director, Memorial Hermann, Houston. “The SPD could be very rich in the data it provides, but having worked in multiple hospitals, not every department has the feasibility to capture and track it. For example, some teams are still using Word documents for count sheets or handwriting their sterilization loads.”
Avila noted how many instrument tracking systems have advanced analytics capabilities to help SP teams identify patterns and trends. For Memorial Hermann, the largest trauma hospital in the U.S., Avila uses the “rich analytics” in their tracking system to build staffing schedules, evaluate tray utilization, identify missing inventory, and forecast inventory needs, among other applications. Avila presented examples of how they leverage this intelligence in “smart purchasing” decisions:
“If we look at the tray utilization data and determine a tray is heavily used, which necessitates same day turnover, which is something we try to avoid, we use these metrics to justify inventory increases.”
As Reed pointed out, the lifeblood of AI is accurate, clean, complete data. He stated:
“The SPD sits on a goldmine of information that could not only benefit them specifically but also the OR, infection prevention, quality management, and supply chain. It had been challenging to use this data because of distractions –those edge cases – that pollute the information. We’ve built an engine around filtering out those edge cases so they can get to the real story. Our information has become very actionable for those leaders that lean into it.”
HPN asked those interviewed where they see future applications of advanced technology in SP departments. Here were some of their responses.
“I see some of the biggest changes happening in decontamination, which is a notoriously complex place to work,” said Dr. Pulijala. “There is a lot of support that AI can bring with manual labor to help SP teams perform their tasks.
“I would say the biggest game changer in SPD will be an AI assisted technician,” Dr. Pulijala added. “But it is important to make the distinction that this would be augmented intelligence, not artificial intelligence because there is still the need for people. You cannot replace the human element in processes that require critical thinking. But there is a lot of work you can take off their plates. I see a combination of humans and AI as the future of SPD.”
“The potential in SPD is tremendous,” said Melendez. “Future applications might involve predictive maintenance of equipment, optimizing sterilization workflows, and improving resource allocation.”
“Vendors and manufacturers will most likely spearhead the pathway of AI into sterile processing as they integrate it for product development, industry conditions, and forecasting,” said Hutchinson. “Vendors will have to lead the way for more advanced applications because it requires significant time and investment, which most hospitals don’t have available. Hospitals will want turnkey solutions as opposed to having to muddle through how to develop and apply these technologies.”HPN
References:
1. 2024 Outlook for Health Care Planning for the Future of Health: Top trends for 2024, Deloitte, December 6, 2023, https://www2.deloitte.com/us/en/blog/health-careblog/2023/outlook-for-health-care.html
2. Transforming Healthcare: Navigating Digital Health with a Value-Driven Approach, the World Economic Forum and Boston Consulting Group, January 2024
3. 2023 ended with a bang in terms of endoscope-related adverse events! Cori Ofstead LinkedIn post, January 10, 2024, https://www.linkedin.com/feed/update/urn:li:activity:7150526303866109952/
4. Angela M. Salmen, Justin Fordeck, Crystal Heishman, Error Reduction in Sterile Processing Through Standardization of Operations and Training, American Journal of Infection Control, Volume 50, Issue 7, Supplement, 2022, Pages S16-S17, ISSN 0196-6553, https://doi.org/10.1016/j.ajic.2022.03.090


This self-study lesson was developed by STERIS. Lessons are administered by Endeavor Healthcare Media.
Earn CEUs
After careful study of the lesson, complete the examination online at educationhub. hpnonline.com. You must have a passing score of 80% or higher to receive a certificate of completion.

The CBSPD (Certification Board for Sterile Processing and Distribution) has preapproved this in-service for one (1) contact hour for a period of five (5) years from the date of original publication. Successful completion of the lesson and post-test must be documented by facility management and those records maintained by the individual until recertification is required. DO NOT SEND LESSON OR TEST TO CBSPD. For additional information regarding certification, contact CBSPD–148 Main Street, Suite C-1, Lebanon, NJ 08833 • www.cbspd.net.
HSPA (Healthcare Sterile Processing Association, https://myhspa.org) has pre-approved this in-service for 1.0 Continuing Education Credits for a period of three years, until January 29, 2027.
For more information, direct any questions to Healthcare Purchasing News editor@ hpnonline.com.
1. Identify aspects of ANSI/AAMI ST108 that apply to processing technicians
2. Describe the test methods that a processing technician would perform Sponsored by:
What is used most when processing reusable medical devices? Water. Yet for years, the quality of water was not important unless there was staining, scaling, or pitting. Water has a broad impact on processing. It can damage instrumentation, cause cleaning equipment to fail, and transfer bacteria to medical devices resulting in infectious outbreaks. The knowledge of water in medical device processing is rising and with it there are quality expectations. Processing technicians now have new responsibilities to ensure the quality of water.
In the past, water quality became a concern after medical device processing issues arose. Facilities would spend thousands of dollars each year in repair costs before realizing it was poor water quality. Though the Association for the Advancement of Medical Instrumentation provided information on key components of water impurities in its technical document TIR34, it was not a standard. This changed in 2023 as the first water quality standard for processing medical devices was published, ANSI/AAMI ST108 Water Quality for Medical Device Processing. ANSI/AAMI ST108 builds on the former technical information report TIR34.
The new standard identifies three types of water (utility, critical, and steam), sets quality specifications for sixteen impurities commonly found in water, and defines four properties of water that can be detrimental to medical devices, cleaning equipment, steam sterilizers, and patient health. The specific quality expectations for water will depend upon the stage of medical device processing and the potential risks.
Utility Water is general purpose water used during the first rinse, cleaning, and intermediate rinses of medical device processing, unless otherwise directed by the manufacturer’s instruction for use.
Utility water may be used to create point of use treatment solutions for flushing endoscopes. Utility and Tap water are different types of water. Tap water may not meet the requirements of utility water and require added water treatment steps before it can be used as utility water.
Critical Water is highly treated water that removes ionic impurities and keeps bacteria levels low. Critical water is not sterile water. The final rinse of the cleaning process and all rinses for manually high level disinfected medical devices use critical water.
Steam, or rather its condensate, is the last type of water specified within ANSI/ AAMI ST108. Steam is the most common sterilant used in sterile processing.
With quality specifications comes the need to test. First, quality is proven through validation testing after which routine testing shows the water still meets the necessary requirements to be safe for medical device processing.
Validation testing requires frequent testing of all impurities and water properties after water treatment and at each location that the water is used. This can last an entire year as incoming water quality changes throughout the year.
After validation is complete, routine testing reduces the frequency and location of testing. The types and frequency of routine testing are based upon the results of the validation. ANSI/AAMI ST108 gives minimum recommendations for both. See tables 1 and 2.
As with any equipment, maintenance is an important part of good water quality. The water treatment system, remote water treatment equipment, and the water delivery system, including all storage tanks, must be maintained per manufacturer’s instructions for use. Parts that could harbor bacterial biofilms are regularly disinfected. This could include storage tanks, water lines, and certain point of use filters. Disinfection frequency will be dependent upon the facility’s unique water conditions and systems.
The frequency is established during the validation of the water treatment system.
Developing the water management program and ensuring compliance is the role of the water management committee. The diverse backgrounds of the multidisciplinary committee supply the expertise needed to develop and oversee the water management program. Medical device processing technicians may be asked to join the committee. Considering device processing occurs in multiple areas, the committee may encompass technicians from endoscope processing, emergency room, satellite clinics, ambulatory surgery, and gastroenterology centers. As committee members, they help to ensure that all water use locations for medical device processing is listed in the water management program.
Routine water monitoring uses many types of tests. Some tests require specialized equipment to be utilized in order to complete the test. Others use test strips or readers. Technicians may be asked to collect water samples, read and interpret meters, and perform testing. Facility policies and procedures will dictate the technician’s role, responsibility, and required training.
Water treatment systems use a variety of filters to remove water impurities. Systems are maintained and tested by facilities or the water treatment system’s vendor. Technicians may be asked to check gauges, read conductivity meters, or look for warning lights or messages each day of operation. All performance checks should be documented with a quality system in place to manage alerts and failing measurements.
Of the three water types, technicians may be involved in utility and critical water testing at the point of water use. The points of water use are locations where the water is dispensed for immediate use. This
includes utility sinks, washer disinfectors, and cart washers, for example.
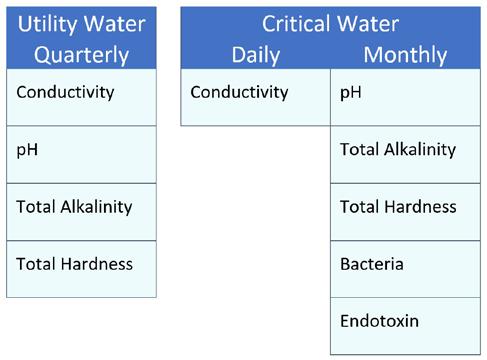
Visual evaluation is completed by looking at the water without cleaning chemistries or high-level disinfectants. The water should be clear, not cloudy, and colorless. Each sink faucet, washer disinfector, cart washer, and automated endoscope reprocessor should be observed. It may be necessary to observe the water used in mechanical cleaning and highlevel disinfection processors during first fill, intermediate rinse, and/or final rinse depending upon the timing of cleaning agent and disinfectant dispensing.
Conductivity and pH testing may also be performed by technicians at the points of water use. Water samples are collected from the point of water use into a glass or polypropylene bottle. The conductivity meter’s probe is placed within the bottle and the measure taken. It is especially important to rinse the probe with deionized water prior to placing it in the water sample. Residual water from earlier testing or any other ionic contamination can be carried into the water sample and create a failing test result.
After conductivity testing is complete, a pH test strip can be used to measure the pH of the water. Always do this after the
conductivity measurement. The pH strips can introduce ionic contaminants that can alter the conductivity reading. Follow the pH strip’s instructions for use for the test requirements and interpretation.
Typically, a test laboratory completes alkalinity and water hardness testing. However, test strips are available for routine monitoring. As before, the water sample used for conductivity measurements can be used for alkalinity and water hardness test strip testing. Follow the test strip’s instruction for use. Test strips are available for routine monitoring.
Bacterial count and endotoxin testing requires specialized skill and laboratory equipment to perform. Technicians will not be asked to do this type of testing but may be asked to collect water samples for the test. Proper water sample collection and handling is critical. Always wear fresh, clean gloves when collecting water samples. Change gloves if they become contaminated. Additionally, be sure to


wear proper personal protective equipment as defined for the work area.
Water samples used to measure water properties and chemical impurities should be a representation of the water delivered to the point of use. For faucets, the water is collected from the water faucet after it has been allowed to run for a specified amount of time. Running the water clears the faucet of environmental contamination that may have collected in and on the faucet during use. It also clears the line of stagnant water that could create a false result. Spray heads may require an added step that cleans and rinses the surface with a specific water type prior to flushing.
After flushing, the water’s flow should be reduced to the width of a pencil. This reduces the force of the water, preventing splashing during water collection. Use a fresh glass or polypropylene bottle. If the collection vessel is reusable, clean and rinse three times with deionized water prior to each use. While holding the base of the bottle, carefully remove the lid as to not touch the sides of the bottle. Place the bottle base beneath the flow of water. Fill the bottle to allow sufficient coverage of the conductivity probes and test strips then place the lid onto the bottle. If more than one bottle is needed, collect the next water sample.
Testing should be done right after sample collection. If the sample needs to be shipped to a test laboratory, it should be labeled and stored refrigerated until shipped to the test lab. Follow the test lab’s instructions for collection volumes and ship times. Many require sample shipment on ice and within a specific time.
Mechanical equipment may have specialized adapters or require the use
of the emergency stop to collect water samples. Consult the equipment manufacturer for collection procedures.
Bacterial and endotoxin samples require a little more. Bacterial and endotoxin testing is sensitive to contamination from the environmental surfaces, technician hands, the sample bottle, and many other potential sources. Use meticulous care to collect samples.
Faucets, spray heads, and sample ports may require external disinfection followed by sterile water rinsing prior to water flushing to prevent sample contamination from environmental bacteria. Mechanical equipment may have additional needs. Consult the bacterial test lab and mechanical equipment manufacturer for specific requirements.
Samples are collected into sterile bottles specifically designed for bacterial and endotoxin testing. Using a nonsterile bottle or one that is not suited for endotoxin sample collection could
negatively affect the results. Bacterial testing often requires larger volumes of water than other water test samples with a typical sample collection size of 100 ml or approximately 0.4 cups. Care should be taken when collecting samples to avoid touching the bottle lip during collection and when reapplying the lid. Do not place the lid on any surfaces. Contact with surfaces could transfer contaminants to the lid.
All samples should be labeled and refrigerated. Testing should begin as soon as possible. Endotoxin levels decline due to degradation over time, but bacteria levels increase as bacteria replicate within the sample. Often samples are shipped overnight to the test laboratory.
Processing technicians play a vital role in water quality management. Their knowledge gives a comprehensive look into water usage during medical device processing. They work the most with water in their day-to-day job. They are invaluable partners ensuring the quality of processing water in every healthcare facility. HPN
References:
1.American Society of Heating, Refrigeration and AirConditioning Engineers (ASHRAE) (2018). ANSI/ASHRAE 188-2018 Legionellosis: Risk Management for Building Water Systems. American National Standards Institute
2.Association for the Advancement of Medical Instrumentation (AAMI) (2023) ANSI/AAMI ST108:2023 Water for the processing of medical devices. AAMI
3.Centers for Disease Control and Prevention (2021) Developing a Water Management Program to Reduce Legionella Growth & Spread in Buildings. CDC. https:// www.cdc.gov/legionella/downloads/toolkit.pdf

Sponsored by
Circle the one correct answer:
1. Which standard provides recommendations for water used to process medical devices?
A. ASNI/AAMI ST108
B. ANSI/AAMI ST79
C. ANSI/AAMI ST41
D. ANSI/AAMI ST58
2. Which type of water is used to make cleaning solutions?
A. Critical Water
B. Potable Water
C. Steam
D. Utility Water
3. How long can validation testing last?
A. 1 day
B. 1 week
C. 2-3 months
D. 1 year
4. What happens to water testing after the validation is complete?
A. It increases
B. It decreases
C. It stops
D. It is only needed for problems
5. What is point of water use?
A. It is the end of the water treatment system
B. It is the exit of the storage tank
C. It is the location where water is dispensed for use
D. It is precleaning of endoscopes
6. What is used to measure water conductivity?
A. pH meter
B. Conductivity meter
C. Test strip
D. Visual inspection
7. Which test uses a test strip?
A. Bacterial
B. Conductivity
C. Endotoxin
D. pH
8. What is the technician’s role in the water management committee?
A. Write policies and procedures
B. Perform water treatment system testing
C. Lists all processing water use locations
D. Manage the entire water program
9. How should water samples be kept after sampling?
A. Refrigerated
B. Room temperature
C. Incubated at 55-60°C
D. Boiled for 3 minutes
10. Who should be consulted when developing water sampling procedures for mechanical equipment?
A. Mechanical equipment manufacturer
B. Cleaning chemistry manufacturer
C. Water treatment system vendor
D. Infection preventionist


HPN now offers all CEU quizzes online by scanning the QR code on the right or visiting: https://educationhub.hpnonline.com. The cost to take the quiz online remains at $10.



As of December 1st 2023 all exams must be taken online.
Mailed in tests will no longer be accepted.
Approval codes are printed on successfully completed certificates at the end of each online course at https://educationhub.hpnonline.com


he beginning months of a new year are an opportune time to turn a new page professionally. Although I’ve never been one to set resolutions, I do find it helpful to reflect upon my work habits to stay grounded and energized in the long term. Our purpose and goals can be nurtured by focusing on habits to drive success.
It is important to recognize that what works for one person may not always work for another. Therefore, when reading this article, I encourage you to consider what resonates with you on a personal level. Take what speaks to you and let the rest go. My goal is to provide a springboard to launch yourself with renewed energy through 2024 and beyond.
Prioritize your non-negotiables –Successful individuals identify the essential habits they cannot do without. Time for non-negotiables should be created in the calendar first. From a personal perspective, this may include a daily walk, meditation, or exercise. For work, I schedule my employee rounding, education, and management tasks throughout the calendar year. This gives me structure around the time I have available and the work I can accommodate.
Embrace empathy – Practice self-compassion. Many healthcare workers are facing burnout, and the last few years have been especially arduous. Sterile processing (SP) leaders are struggling to maintain their energy, but how much of that fatigue is due to the voice in our heads? Often, we impose our own limits, and this can manifest itself physically. Be kind to yourself to find your inner strength and grace.
Put goals in writing – Writing down our goals helps set them in motion. When we articulate our goals, this provides clarity on what we wish to accomplish and provides motivation and focus to achieve that goal. I would also
recommend adopting SMART goal formats (Specific, Measurable, Achievable, Realistic, Timebound). When SMART goals are set, we create the destination and the timeline for achievement. We must invest in ourselves and remember that we get what we give.
Find your Zen – Simplifying life whenever possible is prudent, as is focusing on effective time management. Managing our time effectively can help reduce stress, and planning our days or weeks in advance helps us maintain control and reduce overwhelm. Prioritization of tasks and schedules and breaking activities down into more manageable pieces are some small ways to reduce mental clutter.
If inspired, consider decluttering your workspace, organizing the environment, and working toward creating systems to make life more efficient and streamlined.
Honor promises – Fulfilling our commitments helps us achieve our ambitions, while also forging trust and more effective relationships with colleagues and customers. This may seem like an easy concept; however, it requires practice because meeting our commitments can be challenging. It is acceptable to decline and not over-commit ourselves if time and resources simply cannot allow. For commitments that are accepted, consider writing them down, defining the need, and communicating with the appropriate parties to ensure that the request is understood.
Don’t wait for inspiration, create it –It is acceptable to take a mental break to allow time to refocus. Feel free to look to others for sources of inspiration. If you’ve run out of ideas, team up with a friend or colleague and bounce ideas off one another. Break your inspirations down into smaller, more manageable pieces. Reading is a particularly effective way to refresh your ideas or generate new ones.
Believe in yourself – This has been a game changer for me, leading to greater
emotional self-control and resiliency. Every great leader uses belief in some context, be it practical, spiritual, or emotional. Our faith and beliefs require action, and when combined with our personal and professional values, such as maintaining a positive attitude and committing to hard work and professional development, they help build the framework for success. If you are personally struggling with this, I encourage you to find some quiet time to ponder how self-belief and positive self-talk may help put you on the path to becoming the best SP leader possible.
Keep the passion – Effective, inspirational leaders show genuine passion for their work and the goals they aim to meet. Passion is contagious, and if an SP leader is passionate about their work, they can help others feel the same energy. When work becomes especially challenging, reconnecting with our purpose to serve our team, customers, patients, and community can be invigorating. Passion brings that positive energy that inspires us to keep going and persevere, regardless of the circumstances.
Reflecting each year (and throughout each month) helps us set, prioritize, and adjust our goals and can serve as a powerful catalyst for personal and professional transformation. Our goals needn’t be complex or intimidating, and considering each of the suggestions in this article can serve as a first step toward achieving them. HPN
Marie Brewer, CST, CRCST, CIS, CHL, CER, GTS, CLSSBB, serves as sterile processing manager for St. Luke’s Hospital, Finley Hospital and Jones Regional Medical Center in Iowa, and is a subject matter expert and columnist for the Healthcare Sterile Processing Association.





“Can you explain why surgical instruments get magnetized? We seem to be having more lately. What might be the best way to detect them and demagnetize them once found?”
ALet me simplify these questions:
1. Why do our instruments become magnetized?
2. What can we do about it?
This reminds me of the time when I was called into an operating room because a paper clip was found in a sterilized tray. I told the surgeon I would take care of the issue. More on that later.
Reducing incidence of surgical instruments from becoming magnetized and detecting magnetized instruments before use in surgery is part of any department’s Quality Management System (QMS) for inspection of medical devices.
Remember our mantra: “Clean and functional.”
1. Why do our instruments become magnetized? What is magnetism?
Magnetism is an invisible force that can be produced by magnets, friction, or electrical currents that can be exerted onto specific metals and alloys given the proper conditions. Thus, medical devices like surgical instruments made of a combination of (ferrous) metals such as iron, cobalt, nickel, and other materials get magnetized often.
• Some delicate microsurgical and ophthalmic instruments become magnetized over a period due to simple friction (i.e., contact between instruments/rubbing against each other).
• Some facilities use magnetic mats to hold instruments in place during surgery.
• Not segregating instruments by metal types during the cleaning and the sterilization process can cause instruments to become magnetized.

Detecting magnetized instruments during the assembly process is a vital step in staff and patient safety.
• Tagging: If a medical device is found to be magnetized in the decontamination area, like a needle holder that has a needle already staying on it because it is magnetized, you can use a special tag that says “Magnetized” to identify and alert staff on the assembly side to remove the needle to the appropriate container and have the device demagnetized. The Operating Room might also tag instruments that are magnetized during surgery.
• Separation: Preventing magnetization requires segregation by type of metal (ferrous and non-ferrous) in the cleaning and sterilization process of these instruments. Use separate wrapping or containers and inserts to separate the instruments so they do not become magnetized.
• Method: Have a proven method for detection before anything is placed in a tray or individually peel pouched or wrapped.
How can I detect if an instrument is magnetized?
While magnetic fields cannot be seen, they can be detected by observing their effect on ferrous materials (e.g., paper clip/ needle), causing them to move toward a magnetized medical device. If it does move, then the medical device will be removed from service.
Back to my story. Our process at the time was to use a paper clip to detect if an instrument was magnetized. The staff member simply forgot to take the instrument with the paper clip out of service. In my travels, I have seen some departments use a needle to detect if an instrument is magnetized.
2. What can we do about it?
How do I demagnetize my instruments?
If there is an instrument that is magnetized it must be demagnetized. Demagnetizers (also known as degaussers) are available.
• A degausser uses electromagnets to generate intense, high frequency alternating current (AC) magnetic fields.
• In response to AC magnetic fields, individual domains realign randomly, so their magnetic fields cancel (or nearly cancel), eliminating (or substantially reducing) undesired magnetism.
• After using a demagnetizer, use the same paper clip/needle test to ensure the device is no longer magnetized. If it still attracts, then demagnetize again until there is no attraction; then you can safely place the instrument in the tray set or individual package (i.e., peel pouch).
I am not saying to only use a paper clip or a needle. I have been told there are some other methods available for departments to use. Therefore, your department needs an approved process (as part of your QMS) to reduce the possibility of magnetized instruments showing up in the surgical field (as well as undesired paper clips or needles). HPN








Brayden Bennett, regional sales manager, Northeast, LogiQuip shared his perspectives on workstations with Healthcare Purchasing News. Here’s what he had to say.
What are workstations used for in a hospital setting?
Workstations are used in Sterile Processing Departments for instrument inspection, testing, validation, and for packing wrapped instruments sets.
Who uses these? Is it primarily nursing staff ? SPD?
Users vary but the most common user would be a sterile processing technician.
What current challenges are hospital staff facing when it comes to workstations?
Some of these challenges include poor ergonomics with fixed height workstations which, over time, can cause the Sterile Processing Technician long term back and neck pain. In addition, limited storage capacity for the supplies needed, inefficient workflow design, dimly lit work areas, and smaller departments means limited space for large workstations forcing the SPD to use less effective solutions.
Are there any innovations helping solve these challenges?
Height Adjustable Workstations allow for each technician to set the table to a suitable height, helping to prevent neck

and back pain. The limited storage capacity can be addressed by removing the plastic bins above the table and replacing them with fine mesh wire baskets that eliminate wasted space. Workflow design can be improved by a workflow review to create a more seamless layout/design. Workstations need overhead LED lighting and lighted magnifiers for better visibility of the task at hand. Increasing capacity in sterile processing during new construction will lead to increased throughput and faster turnover in the operating room.
What unique offerings does your organization have?
LogiQuip offers highly customizable, height adjustable Workstations to meet each facility’s unique needs. In addition to a wide variety of accessories to make our Workstations as ergonomic as possible, we also incorporate our ParWall® Fine Mesh Basket Shelves to allow for better space utilization, supply visibility, and less maintenance.
Where do you see this space in the next 5-10 years? Any new tech being added?
I see this space becoming more and more important. In the last 5 years there has been significant attention and training brought to the sterile processing space. Over the next 5-10 years we expect to continue to see this level of education and care increase. HPN
CLINICAL INTELLIGENCE FOR SUPPLY CHAIN LEADERSHIP
How to contact us
Chris Driscoll, Group Publisher, Healthcare Healthcare Innovation (HI)
Healthcare Purchasing News (HPN)
Medical Laboratory Observer (MLO) 561-801-6576 • cdriscoll@endeavorb2b.com
SEND EDITORIAL INQUIRIES & MATERIALS TO Janette Wide, Editor-in-Chief
Healthcare Purchasing News (941) 229-0484 • jwider@hpnonline.com
SEND ADVERTISING MATERIALS TO
Karen Runion
Advertising Services Manager (330) 736-1291 • krunion@endeavorb2b.com
EAST/WEST COAST SALES
Kristen Hoffman 603-891-9122 • khoffman@endeavorb2b.com
MIDWEST SALES
April Bruffy (713) 936-5076 • Cell: (713) 992-0381 abruffy@hpnonline.com



recently reviewed the results of Optum’s survey1 of healthcare executives on the path to a more sustainable health system. While I don’t disagree with what they list as their top areas of importance - “improving health equity” and “data and analytics maturity”-- I do see a disconnect with their responses related to how to get there, including the potential contribution of the supply chain. In this month’s column, I take a supply chain lens to the survey results and offer some recommendations on where and how supply chain professionals can demonstrate their role in supporting the C-suite’s priorities.
Despite ranking #1 when asked what was needed for health system sustainability, the executives surveyed ranked “improving health equity” in the middle of the pack for investment priorities (#4 out of 9 options) and even lower when ranking top challenges (#15 out of 20) and listing areas that partnerships can support (#14 out of 19). The latter is particularly surprising given that numerous industry and academic organizations have promoted hospital and community partnerships as a key strategy to addressing health inequities.
Further, it appears as if the leaders surveyed do not recognize and/or financially support the role of supply chain in addressing health disparities despite a longstanding effort by many of these leaders to direct more of their procurement spending to certified diverse vendors, especially those who hire local individuals from more disadvantaged communities. Hospital supply chain leaders also handle procurement of food and other social determinants of health and are increasingly helping to expand access to care in the communities where disparities are the greatest,
which are both noted in the survey as key strategies. Unfortunately, investing in supply chain ranked lowest in funding priorities for those surveyed, which was mirrored in a recent GHX poll of supply chain leaders who said lack of funding, not interest, was the primary reason they were not able to do more to support health equity.
Beyond reminding executives about the points made above, supply chain leaders can also speak to the role sourcing professionals play in addressing the unique needs of different patient populations. For example, as noted in recent studies on blood pulse oximeters, the color of a patient’s skin can impact performance. Supply chain can also support patient satisfaction by helping select personal grooming products for patient rooms that are more appropriate for racially and ethnically diverse patients.
When it came to data and analytics, there was more fi nancial alignment, with the executives listing it as their top investment priority. Those surveyed also spoke about the progress they believe they have made in preparing data to support the use of artificial intelligence and machine learning. Once again, given the low ranking for supply chain investments, I question if the executives fully appreciate the value of the data captured and managed by supply chain – data related to not only the purchase price of products, but also how and when they are used, on which types of patients and by which clinicians, and to what clinical and financial outcomes. This data is not only used for analysis on the cost and quality of care delivered, but it is also key to generating evidence on which products work best on which patient populations, which helps physicians and value analysis professionals
standardize on those products. This is particularly striking, given reducing unnecessary care variation was ranked second among the activities being pursued by the executives to achieve valuebased healthcare.
In its commentary, Optum speaks to the value of standardizing claims and clinical data to foster the desire by providers and payors to offer more customized products and services. While not addressed specifi cally in the survey, the continued lack of executive support for the adoption of supply chain data standards also makes me question their understanding of the role of unique device identifiers for medical products, which can help standardize both the data captured in claims and medical records, and enable more comparative analytics.
Finally, a note on supply chain disruption, given it was ranked as a top challenge by only 3 percent of the executives, and last among the areas where those responding believe partnerships are important. To me, this clearly points to a lack of understanding among executives about how data sharing between providers and suppliers can improve demand generation and provide more advance notice to address potential and impending disruptions. The Healthcare Industry Resilience Collaborative (HIRC), created in response to the severe supply shortages during the pandemic, is a prime example of how providers and suppliers have partnered together to support more resiliency, which is fundamental to a truly sustainable healthcare system. HPN







Unwrap the future of sterile supply management. With features that help streamline processes and reduce the possibility of wet sets, Aesculap’s next generation rigid container is everything you’d expect from the market leader.
Visit aesculapusa.com/aicon to learn more about how this breakthrough technology can help your SPD Operate With Greater Precision.