






N F I D E N T

The Next Generation Of Contamination Monitoring Technology by
For the cleaning verification of surgical instruments, endoscopes, and surfaces, Clean Read Handheld by Empire Bio Diagnostics is a cloudbased cleaning monitoring system used to help hospitals and other healthcare organizations achieve optimal standardized cleaning levels.






















AUGUST 2025

Sourcing & Logistics
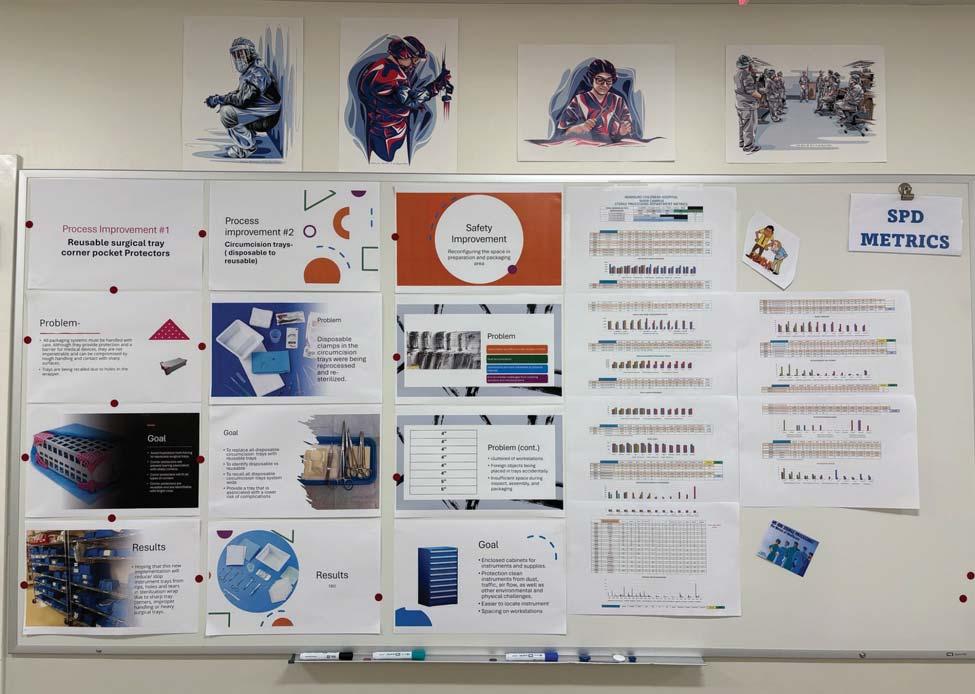
8 > 2025 Supply Chain Department of the Year: Stanford Medicine
JANETTE WIDER
34 > Tackling Tariffs: What Providers and Suppliers Can Do
KAREN CONWAY
Infection Prevention
14 > Addressing the Rise of Antimicrobial Resistance
MATT MACKENZIE
Sterile Processing
18 > Evolving Education in Sterile Processing: Modernizing Learning, Elevating Confidence
KARA NADEAU
24 > Education Nation: Understanding the Factors That Shape Sterile Processing
SARAH B. CRUZ
28 > Flexible Float Pools Can Fill SPD Staffing Needs
DAVID TAYLOR
30 > Demagnetization
ADAM OKADA
32 > What is Sterile Processing?
DEWEY BARKER

Departments
6 > I Still Love Supply Chain
7 > What’s on the Web, Advertiser Index

























BY JANETTE WIDER
Last year for our September edition, featuring 2024’s Supply Chain Department of the Year, I wrote about my dad:
“...I was thrilled when the opportunity to come over to the supply chain side was presented to me.
“Why, you ask? Because of my dad.
“My father has worked in purchasing and supply chain for his entire career. I grew up hearing him talk about how he ‘managed procurement cycles from requisition to payment’ and ‘established and developed viable, sustainable relationships with suppliers.’ He’s worked in various industries, including spending several years at PerkinElmer…”
I also celebrated that year’s winner, University of Utah Health. You can read that piece here: https://www.hpnonline.com/sourcing-logistics/article/55131196/ why-i-love-supply-chain.
To say this year has been interesting from a healthcare supply chain perspective is an understatement. The challenges posed from the back and forth between tariffs, supply chain disruptions, and well, just the general challenges that come with working in healthcare supply chain.
This year, I’m writing to celebrate 2025’s winner: Stanford Medicine.
I wrote, “Stanford Medicine showed immense success and we’re very pleased to present this honor to the team.

“It’s known that healthcare supply chains are under immense pressure to do more with less and Stanford Medicine emerged as an innovator in 2024, showcasing an unmatched commitment to digital efficiency and innovation. Although many organizations are on digital transformation journeys, Stanford tackled three deployments all at once:
• AI enabled Contract Lifecycle Management (CLM),
• Enterprise Resource Planning (ERP) tool in the cloud, and
• A proprietary Procedural Analytics tool”
During this time of uncertainty, Stanford not only implemented these three but showed true passion for healthcare supply chain. Amanda Chawla, chief supply chain officer put it well. She said, “Have the courage to not only have a vision but take the risks to action on it; get your hands dirty in creating something that is so beautiful. Be intentional in your work, think about the scaffolding early on and design it—and continue to refine it as that will be the key to deliver results in a world and environment that is ever changing. Embrace change, differing viewpoints, and take things from the bench, from an idea, to the bedside. And lastly, your team is essential—they are the key— trust, support, challenge, listen, and empower them!”
August 2025, Vol. 49, No. X
VP & Market Leader
Healthcare and Dental
Chris Driscoll cdriscoll@endeavorb2b.com | 978-880-8345
Editor-in-Chief
Janette Wider jwider@hpnonline.com
Associate Editor Matt MacKenzie mmackenzie@endeavorb2b.com
Senior Contributing Editor
Kara Nadeau knadeau@hpnonline.com
Advertising Sales
East & West Coast
Kristen Hoffman khoffman@endeavorb2b.com | 603-891-9122
Midwest & Central
Brian Rosebrook brosebrook@endeavorb2b.com | 918-728-5321
Advertising & Art Production
Production Manager | Ed Bartlett
Art Director | Kelli Mylchreest
Advertising Services
Karen Runion | krunion@endeavorb2b.com
Audience Development Laura Moulton | lmoulton@endeavorb2b.com
Endeavor Business Media, LLC
CEO Chris Ferrell
COO Patrick Rains
CRO Paul Andrews
CDO Jacquie Niemiec
CALO Tracy Kane
CMO Amanda Landsaw
EVP Medical & Healthcare Technology Kylie Hirko
EVP Endeavor Business Intelligence Paul Mattioli
Healthcare Purchasing News USPS Permit 362710, ISSN 1098-3716 print, ISSN 2771-6716 online is published 11 times annually - Jan, Feb, Mar, Apr, Jun, Jul, Aug, Sep, Oct, Nov/Dec, Nov/Dec IBG, by Endeavor Business Media, LLC. 201 N Main St 5th Floor, Fort Atkinson, WI 53538. Periodicals postage paid at Fort Atkinson, WI, and additional mailing offices. POSTMASTER: Send address changes to Healthcare Purchasing News, PO Box 3257, Northbrook, IL 600653257. SUBSCRIPTIONS: Publisher reserves the right to reject non-qualified subscriptions. Subscription prices: U.S. $160.00 per year; Canada/Mexico $193.75 per year; All other countries $276.25 per year. All subscriptions are payable in U.S. funds. Send subscription inquiries to Healthcare Purchasing News, PO Box 3257, Northbrook, IL 60065-3257. Customer service can be reached toll-free at 877-382-9187 or at HPN@omeda.com for magazine subscription assistance or questions.
Printed in the USA. Copyright 2025 Endeavor Business Media, LLC. All rights reserved. No part of this publication should be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopies, recordings, or any information storage or retrieval system without permission from the publisher. Endeavor Business Media, LLC does not assume and hereby disclaims any liability to any person or company for any loss or damage caused by errors or omissions in the material herein, regardless of whether such errors result from negligence, accident, or any other cause whatsoever. The views and opinions in the articles herein are not to be taken as official expressions of the publishers, unless so stated. The publishers do not warrant either expressly or by implication, the factual accuracy of the articles herein, nor do they so warrant any views or opinions by the authors of said articles.
Jimmy Chung, MD, MBA, FACS, FABQAURP, CMRP, Chief Medical Officer, Advantus Health Partners and Bon Secours Mercy Health, Cincinnati, OH
Joe Colonna, Chief Supply Chain and Project Management Officer, Piedmont Healthcare, Atlanta, GA; Karen Conway, Vice President, Healthcare Value, GHX, Louisville, CO
Dee Donatelli, RN, BSN, MBA, Senior Director Spend symplr and Principal Dee Donatelli Consulting LLC, Austin, TX
J. Hudson Garrett Jr., PhD, FNAP, FSHEA, FIDSA, Adjunct Assistant Professor of Medicine, Infectious Diseases, University of Louisville School of Medicine
Melanie Miller, RN, CVAHP, CNOR, CSPDM, Value Analysis Consultant, Healthcare Value Management Experts Inc. (HVME) Los Angeles, CA
Dennis Orthman, Consulting, Braintree, MA
Janet Pate, Nurse Consultant and Educator, Ruhof Corp.
Richard Perrin, CEO, Active Innovations LLC, Annapolis, MD
Jean Sargent, CMRP, FAHRMM, FCS, Principal, Sargent Healthcare Strategies, Port Charlotte, FL
Richard W. Schule, MBA, BS, FAST, CST, FCS, CRCST, CHMMC, CIS, CHL, AGTS, Senior Director Enterprise Reprocessing, Cleveland Clinic, Cleveland, OH
Barbara Strain, MA, CVAHP, Principal, Barbara Strain Consulting LLC, Charlottesville, VA
Deborah Petretich Templeton, RPh, MHA,Chief Administrative Officer (Ret.), System Support Services, Geisinger Health, Danville, PA
Ray Taurasi, Principal, Healthcare CS Solutions, Washington, DC

The Joint Commission Announces Accreditation 360: The
According to a June 30 press release, The Joint Commission announced, “Accreditation 360: The New Standard,” a comprehensive redesign of its healthcare accreditation process aimed at streamlining requirements and focusing more on outcomes and collaboration.
“Healthcare organizations today are navigating historic complexity, and the pressures are enormous,” said Jonathan B. Perlin, MD, PhD, president and CEO of the Joint Commission. “Accreditation 360 directly responds to what this moment demands.”
Read on: hpnonline.com/55300426
The first study found that “adults hospitalized for RSV infection had a significantly higher risk of in-patient mortality and adverse respiratory and kidney outcomes than those hospitalized for flu.” During the study period, 41,206 and 3,565 patients were hospitalized for flu and RSV respectively. Mortality was higher in the RSV cohort, with a “10.1% death rate, compared to 5.5% for flu. RSV infection showed a 52% higher risk of death during hospitalization.” Secondary bacterial pneumonia rates were also significantly higher in the RSV group.
Read on: hpnonline.com/55299306
Christopher Salkowitz, Director of Sales, RST Automation LLC, joins Editor-in-Chief Janette Wider on this episode to discuss AI.
Salkowitz has been the Director of Sales for a year tasked with penetrating the market and expanding the outreach and visibility of the transformative innovations of their founders, Russell Baker, Braun Kiess, Paul Seid, and Larry Zelner who had realized more than a decade ago the need for this technology.
Listen at: hpnonline.com/55296117


BY JANETTE WIDER
It’s that time of year again when Healthcare Purchasing News celebrates the Supply Chain Department of the Year. 2025’s winner is Stanford Medicine. Stanford Medicine showed immense success and we’re very pleased to present this honor to the team.
It’s known that healthcare supply chains are under immense pressure to do more with less and Stanford Medicine emerged as an innovator in 2024, showcasing an unmatched commitment to digital efficiency and innovation. Although many organizations are on digital transformation journeys, Stanford tackled three deployments all at once:
• AI enabled Contract Lifecycle Management (CLM),
• Enterprise Resource Planning (ERP) tool in the cloud, and
• A proprietary Procedural Analytics tool
As Stanford Medicine expanded its operations, the need for a more effective contract management system became increasingly urgent. The cumbersome legacy systems in place led to lengthy contract drafting times and a lack of visibility into contract performance. With over 4,300 contracts to manage, the organization recognized that a streamlined approach was essential for enhancing operational efficiency and driving significant cost savings.
The strategic decision to implement a CLM was a gamechanger. However, the journey was not without its challenges. Ensuring that all stakeholders were comfortable with the new platform was paramount. Many team members were accustomed to outdated systems, making the transition to a more automated and integrated solution

daunting. To facilitate this change, the Supply Chain team took on the Change Management with our 1,300 customers and rolled out a comprehensive training program, complete with hands-on workshops and ongoing support, empowering staff to embrace the new CLM tool with confidence.
Integration posed another significant challenge. The team worked tirelessly to ensure that the CLM connected seamlessly with existing workflows and systems, requiring meticulous planning and collaboration across departments. The result? A smoother integration experience that has transformed contract management at Stanford Medicine.
The benefits of Icertis have been nothing short of remarkable. Contract drafting times have plummeted from an average of eight days to just one day, enabling faster turnaround and improved responsiveness. With advanced features that leverage artificial intelligence, Icertis has streamlined the entire contract lifecycle, minimized errors, and ensured compliance with organizational standards. The tool’s AI integration that ensures supplier compliance with contractual terms is projected to generate an astounding $11.6 million in savings through rebate optimization and enhanced service credit realization.
When asked about the desire to use an AI enabled platform for the CLM, Sree Duggineni, vice president of Strategic Sourcing and Supplier Relations, and Omar Devlin, executive director of Supply Chain Systems and Analytics, said, “The true return on investment of our new CLM system comes from the seamless integration between the AI enabled CLM and ERP. The tool actively monitors contract performance in real time through tracking and
Front Row: Allan Pilapil, Lindsey “Reggie” Black, Amanda-Victoria Lee, Chris Mitchell, Mayra Gomez, Jennifer Rodriguiez, Mona Biswas, Joyce Caburao, Jane Rumbaoa, Michael del Giudice, Gino Varela, Victoria Tran, Janae Silacci, Ace Manzano, Michael Truman Cavanaugh, Will Udo, Zach Vaughn Middle Row: Amanda Chawla, Steve Carhart, Sam Ayana, Clara Jiminez Tran, Robert Ignacio, Michael Smith, Michelle Sherman, Joe Gonzales, Almeda McLarin, Tiffany Lash, Neil Prasad, Jeff Javier, Chris McCall, Masai Sung, Nick Trzeciak, Katie Dean, Christy Richie, Omar Devlin, Meridith Baker, Prashanth Soundarajan, Pedro Martin, Ronald AllieBack Row: Jamie Anderson, Melissa Mapa, Jose “Neto” Orellana, Rob Hatkins, DaRice Bracy, Meredith Edwards, Paulyna Renggli, Jim Booker, CJ Tovar Bedolla, Robert Harris, Marc Lowenthal, Ty Lafont, Nanda Garber, Sree Duggineni, Eligio Ladringan, Joey Miller, Habtamu Getasew, Omar Evora, James Hill, Rachell Corsbie, Jose Pluguez, Doug Obogo, Olu Lijadu, Mengfan Zhang, Andrew Miller, Kim Lockett, Ben Lee, Nick Musumeci, Adam Gutierrez, Adam Littlejohn, Lin Ni, Young Seo, Will Sulva, Brian Bunce
verifying obligations, tiered pricing, rebates, and KPIs, without the need for extensive staffing. By automating the review process, we can efficiently manage thousands of contracts across multiple Stanford Medicine entities, each containing several clauses related to rebates, price breaks, early payment discounts, and performancebased incentives.
“The AI-enabled CLM technology not only addresses this complexity but also reduces the necessity for a large workforce to oversee these activities. Furthermore, it enhances the accuracy of fulfilling contractual obligations, primarily through the transformation of contract data into actionable insights.
“Our CLM leverages AI to facilitate this transformation, converting contract data into business rules that optimize contract management. Their AI-driven analytics empower users with valuable insights, fostering improved decisionmaking and ultimately driving better business outcomes and stronger supplier partnerships.”
Amanda Chawla, Chief Supply Chain Officer, commented, “Stanford Medicine Supply Chain has been on a multi-year transformation journey, with technology enablement as one of its core pillars. We have been intentional in our governance, design, and selection of solutions and partners, as well as in the advanced analytics development work. Artificial Intelligence is a pivotal tool in our toolbox, one that we are leveraging to bring forth automation and insights, thereby advancing our operations and enhancing efficiency. AI is a critical component to our sourcing and contract compliance strategy bringing forward visibility to allow for better controls, monitoring, and providing insights. By embracing advanced technologies, we are not just improving our current operations; we are evolving the way in which healthcare supply chain operates. This journey is about more than just technology; it is about transforming our approach to supply chain management and driving excellence in delivering all the ‘rights’ to the healing hands that care for our patients.”
Duggineni noted challenges during the implementation of the CLM by saying that “The implementation of the AI-enabled Contract Lifecycle Management (CLM) system presented several key challenges. One of the primary hurdles was achieving stakeholder alignment and securing buy-in regarding the benefits of the new system, particularly in demonstrating the anticipated return on investment (ROI). It was essential to convey the value of the CLM to various stakeholders, which required a clear articulation of its advantages and potential impact on operational efficiency.”
Further, she added, “Driving consensus among multiple operational groups regarding standardized workflows and business requirements proved to be a complex task. Each group had its own established processes, and harmonizing these disparate workflows into a cohesive system necessitated extensive collaboration and negotiation.
“The design, implementation, and training of the AI module also posed significant challenges. This aspect of the project was particularly unique, as it required the Supply Chain team to effectively communicate their requirements while also quantifying the value derived from the AI integration. The learning curve associated with this process was steep, yet it provided invaluable insights into the intricacies of AI-enabled platforms and their application within contract management.
“Throughout the implementation,” she said, “the Supply Chain team worked closely with Stanford’s Information Technology department, specifically the Technology and Digital Solutions (TDS) team. This collaboration was crucial in navigating the complexities of a system designed to meet the diverse needs of three distinct hospital entities: the Adult Academic Medical Center (AMC), the Children’s AMC, and a
community hospital. Despite these initial challenges, which we call the ’growing pains,’ the organization stands to benefit significantly from the visibility and insights the CLM provides.”
Stanford Medicine’s Supply Chain team worked in two different onprem ERP systems for decades. When Stanford made the decision to change
the needs of each individual entity. With the guidance of their system integrator, Supply Chain took a leadership role within the project, in collaboration with Finance, HR, and IT, facilitating a unifying change management team to bring all the stakeholders together with a comprehensive change management strategy that included extensive training programs and ongoing support. They hosted an astounding 32 change champion events and

ERPs, they did so by consolidating into one technology system, having all 6 entities on one technology for the first time. This monumental shift, planned as early as 2021, aimed to create a more integrated approach to managing financial, supply chain, and human resources functions.
The implementation of a single cloud-based ERP was a significant undertaking, fraught with challenges. Aligning stakeholders, processes, and policies across various entities was crucial, as many team members were accustomed to their own ways of working, and the ability to customize their workflows to
held a user experience testing day that included 14 modules, created 33 trainings and 289 job aids, and hosted 135 hours of office hours to ensure a smooth transition.
Data migration was another critical hurdle. The team meticulously ensured that all relevant data from both legacy systems was accurately transferred to the single system, maintaining data integrity and enabling the organization to fully leverage the new ERP system. The results have been transformative: improved data governance, streamlined Procure to Pay processes, and enhanced operational efficiency.
The new cloud-based ERP provides a user-friendly interface and robust reporting capabilities, empowering staff to access critical information quickly, facilitating better tracking of inventory, procurement, and financial data. The integration of the Supplier Portal has further enhanced supplier engagement, simplified vendor onboarding, and improved communication. This digital platform has significantly reduced the administrative workload on the Data Management team, allowing for more efficient management of supplier relationships.
Katie Dean, vice president of Strategic Programs and Business, commented, “Coordination was one of the most complex parts of the project. There were many structures surrounding the project to ensure all teams and workflows were in lock step. We applied foundational principles: definition of ownership
of activities, open and honest communication, frequent collaborative meetings, and a strong sponsor group consisting of our C-suite leaders. At the end of the day, we had to be able to work out any differences. It might sound simple, but when we ran into barriers, we put the right people together in a room to determine the best path forward.”
When asked about governance, Omar Devlin, executive director of Supply Chain Systems and Analytics, said, “Data governance controls and ongoing monitoring are essential to ensure the accuracy and completeness of data. This ongoing work enables the data to be useful and effective for the business. Maintenance of the data is an iterative process that requires constant review of ‘Data Health’ and an understanding of the business processes that use this data to support decision making.”


In its mission to improve clinical efficiency and reduce supply costs, Stanford Medicine recognized the need for a specialized analytics tool. Variations in supply usage during surgical procedures could lead to unnecessary expenses and impact patient care. To tackle this challenge, the organization developed the Procedural Analytics tool, distinctively tailored to meet its specific needs and enhance decision-making across clinical teams.
The creation of the tool required close collaboration between clinical staff, data analysts, and IT professionals to ensure effective integration of data from various sources, including electronic medical records (EMR) and supply chain systems. Gaining buy-in from clinical stakeholders












































was essential, and the project team conducted workshops to demonstrate the tool’s capabilities and the potential benefits of data-driven decision-making.
The insights gained from the Procedural Analytics tool have been transformative. By allowing for a detailed examination of surgical procedures, the tool has identified substantial differences in supply costs among surgeons performing similar operations. This data-driven approach has enabled the Supply Chain team to identify opportunities for standardization and cost reduction, ultimately enhancing patient care by ensuring that clinical teams have access to the most effective and cost-efficient supplies.
Targeting $8M in savings, Procedural Analytics can yield multi-disciplinary insights leading to financial benefit, democratizing quality and patient outcome insights, unlocking supply and implant standardization, and shedding light on potential contract negotiation opportunities. For one specific SKU for one surgeon alone, about $700 of savings per procedure was identified from reducing unwarranted variation via procedural analytics pilot work, and a savings of $360,000 has already been realized from identifying contract pricing improvements alone with only 5 months from implementation.
When asked about the coordination for the procedural analytics tool, Chawla and Devlin said, “The coordination for the procedural analytics tool involved a comprehensive and collaborative effort that spanned over 18 months, engaging multiple stakeholders, including clinical staff, data analysts, IT professionals, hospitals, and supply chain experts. Integration with the clinical teams was not only critical to the development of the procedural analytics but also essential for a successful launch.
“This year, we have made significant advancements in this initiative, as our clinicians have come to trust the data. By partnering closely and being present at every step of the process, we have fostered trust and buy-in, leading to measurable results. This project marked the first time that clinical and non-clinical datasets were combined across Stanford Medicine’s organization, highlighting the importance of cross-collaboration
outcomes, and cost reduction. The purpose is to align all clinical utilization work to generate the best possible patient outcomes. Standing firm on the pillar of quality over quantity lets our clinicians engage in a healthier debate around documented data sets across multiple service lines and business units for a single holistic approach to patient care. The initial insights gained have been significant revelations into the variation
“This project marked the first time that clinical and non-clinical datasets were combined across Stanford Medicine’s organization, highlighting the importance of cross-collaboration in the ideation, build, exploration, and results phases.” --AMANDA CHAWLA AND OMAR DEVLIN
in the ideation, build, exploration, and results phases. The engaged and supportive multidisciplinary team laid a solid foundation for navigating complexities, from service line viewpoints to interoperability and procedural practices, as well as the integration of utilization variation, sourcing, and contracting.
“This constant learning cycle, characterized by iteration and stakeholder feedback, enabled us to rapidly review which insights were most valuable to explore further, helping to prioritize the data team’s focus on developing meaningful data relationships. Overall, while we have only scratched the surface of integrating supply chain optimization logic with clinical data insights, the journey has set the stage for continued progress and innovation.”
As for the insights gained, Nick Trzeciak, vice president of Supply Chain Operations and Logistics and Devlin, said, “Procedural analytics offers a unique view of the triad of metrics required to make the best clinical decisions; standards/volume of items used, quality of patient
of item use, the reasons behind that variation, and the collective willingness to solve macro level problems together rather than individually. Many hands make light work; or as we like to say at Stanford, One Team, One Mission, One Dream.”
When asked what makes the team at Stanford Medicine so successful, Chawla asserted, “People are at the heart of our strategy and are the core enabler of our success. At Stanford Medicine Supply Chain, we believe that our greatest asset is our team. Our approach requires us to operate as one unified supply chain network, rather than multiple disparate departments. This philosophy fosters a culture of mutual support and collaboration, where each area leans on and supports the others. By working together seamlessly, we harness the collective power of our diverse expertise to achieve extraordinary results.”
Indeed, she added, “Collaboration is not negotiable; it’s required. Gaining buy-in upfront might feel like it slows
you down, but it pays dividends in the end and even more so when everyone is aligned towards the common mission, objectives, and value proposition. Our team is successful at collaboration because we actively work at being a team; for example, years ago we mandated a collaboration day where all leaders at all levels regardless of where they physically work come together onsite one day a month to make real connections and work through intra-operational workflows. The emphasis on collaboration has led to the dismantling of silos and fostered teamwork, not only on major initiatives but also in our daily tasks. We encounter challenges, but our leaders are empowered to work together to overcome barriers.”
“We are deliberate about our communications, learning from both our mistakes and our achievements – whether they are small or significant,” she continued. “Celebrating milestones not only boosts morale but also reinforces our collective achievements and progress. Although our team is occasionally pushed to its limits, we acknowledge and celebrate that it is our people who drive success. By fostering a culture of teamwork and mutual support, we have built a resilient and dynamic team capable of achieving remarkable outcomes.”
As for words of wisdom for supply chain leaders, Chawla chimed in and said, “Any leader understands that the journey toward excellence is centered on self-awareness, emphasizing that it is less about the ‘what’ and more about the ‘how’ and the impact one has on others. Understand yourself – what motivates you to get out of bed in the morning, what energizes you, and what drains your energy. Recognize what you bring to the team (because it is, and always will be, a team effort) and how you can positively contribute to the overall goals and mission, especially since the work is not easy.
“Have the courage to not only have a vision but take the risks to take action on it; get your hands dirty in creating something that is so beautiful. Be intentional in your work, think about the scaffolding early on and design it – and continue to refine it as that will be the key to
deliver results in a world and environment that is ever changing. Embrace change, differing viewpoints, and take things from the bench, from an idea, to the bedside. And lastly, your team is essential – they are the key –trust, support, challenge, listen, and empower them!” HPN







BY MATT MACKENZIE
Antimicrobial resistance (AMR) is on the rise, and projections for how rates will grow from here are not promising.
A study published in The Lancet in September 2024 sounded the alarm on how antimicrobial resistance is becoming even more of a problem worldwide and projected how much of an impact it will have all the way through 2050. The authors looked at data across 204 countries and territories from 1990 to 2021 and eventually ended up collecting 520 million individual records or isolates.
In 2021 alone, the authors estimated 4.71 million deaths were associated with bacterial AMR and 1.14

million were directly attributable. Those numbers are expected to rise precipitously according to the researchers’ calculations; they estimate about 8.22 million deaths associated with AMR and 1.91 million attributed to it in 2050. Providing some optimism is the notion that nearly 92 million deaths could be averted between 2025 and 2050 through “better care of severe infections and improved access to antibiotics.” An additional complicating wrinkle is that rates of deaths associated with AMR rose particularly quickly in adults over age 70, which represents a concerning trend only made more frightening by the rapidly aging world population.1

Another study, available to view on the NIH website’s PubMed Central, aimed to categorize some broad spectrum facts about antibiotic resistance specifically and the drivers of its rise in prevalence. The researchers laid out that the transmission and acquisition of AMR occurs “primarily via the human-human interface both within and outside of healthcare facilities. Humans, animals, water, and the environment are found to be reservoirs, and antimicrobial-resistance genes can be transmitted between and within these reservoirs.”
They also explain several specific factors that make antimicrobial resistance happen in the first place. Among these factors include the misuse and overuse of antibiotics; an increase in gross domestic product around the globe, leading to an elevation in global antibiotic use; inappropriate prescribing patterns; a lack of new novel antibiotics to counteract existing antibiotic resistance; the use of antibiotics in agriculture; easy travel routes facilitating more human movement; and knowledge gaps among healthcare workers and members of the public.2

Healthcare Purchasing News spoke with Rodney Rohde, PhD, MS, SM(ASCP)CM, SVCM, MBCM, FACSc, Regents’ Professor at Texas State University System, on the rise of antimicrobial resistance and what clinicians can do to play a part in reducing the burden as well as general trends concerning AMR in general.
What causes antibiotics to be prescribed in situations that don’t necessarily call for them?

A: There are many reasons for this issue and sometimes it is a complex set of variables. Some of the most common reasons are an uncertain diagnosis. This can be really difficult if a patient is in danger from something like sepsis and it is life threatening. In these cases, time may not be available to wait for a laboratory confirmation via culture and antibiotic susceptibility testing or even a rapid test. In these cases, a broad-spectrum antibiotic may be utilized as a “cover our bases” for sepsis or other dangerous conditions. Patient pressure/expectations are always an issue, and we all need to be able to “educate” our patients about why antibiotics should not just be handed out. Physician fatigue, lack of patient education, limited diagnostic tools, and perception of risk can also play into this issue.
How can clinicians ensure antibiotics are not being over-prescribed?
A: The quick answer is education surrounding adherence to antibiotic (or other antimicrobial drugs) stewardship



principles and practices. “How to” elements are now available for clinicians via CDC and other entities for creating an Antibiotic Stewardship Program [ASP] to implement guidelines. Clinicians and others can also advocate for better diagnostic tools for a more accurate diagnosis and treatment plan. ASP teams should be collaborating with the medical laboratory, the ID pharmacist, and others on the ASP team to optimize prescription practices: selection of best antibiotic, correct dose/duration, delayed prescribing for conditions that might resolve without antibiotics, and optimized delivery (oral versus IV).
ASP teams should be working with clinicians to enhance education and expertise to create “positive peer pressure” to not prescribe unnecessarily. They should also be working to build better tracking and reporting systems for prescribing to provide feedback. Lastly, ASP teams and all involved should work to engage patients in education and expectations for the use of antimicrobials, including the global issue of AMR.
What specific concerns exist around antimicrobial resistance right now?
tremendous amount of effort, education, and global willingness to work on the issues stated.
What steps can be taken by clinicians in the fight against rising antimicrobial resistance?
A: Clinicians, infection preventionists, pharmacy, nursing, patients, and many in the healthcare arena must work together to optimize antimicrobial prescribing practices.

A: There are so many concerns on AMR. The ongoing and increasing difficulty in treating infections due to “superbugs” has led to reduced effectiveness of existing drugs. This has led to more threats and concerns while performing typical surgeries, transplants, and other procedures. The rise of specific resistant (including pan-resistant) pathogens like MRSA, CRE, TB, fungi (like Candida auris), malaria, and many others is an ongoing threat for all treatment.
The drivers of AMR are many and it’s a global concern that must be addressed via “One Health” frameworks. Misuse, overuse, inappropriate use (like agriculture), lack of regulation in some countries coupled with globalization making travel of pathogens “easy” now are all examples of factors contributing to AMR.
How do you see the topic of antimicrobial resistance changing over the next 10 or so years?
A: In my professional opinion, AMR is going to continue to become an even more urgent and pressing global problem in the next decade and even beyond. It will take a
This is currently supported by research. For example, clinical “nudging” and the use of accurate antibiograms has been adopted by many who are in ASP teams. The enhancement of infection prevention and control measures can reduce the risk of infections and thus reduce the use of antimicrobial measures (hand hygiene, vaccination, isolation precautions, proper sterilization and disinfection of surfaces, for example). Education, collaboration, and advocacy are critical to building an environment that helps reduce pressure to use antibiotics or other antimicrobials without proper reasoning.
A: As AMR grows, we can expect any number of infections to become increasingly more difficult or impossible to treat. It may become more expensive to treat them, which could impact a patient’s ability to even address infections. This will in turn put a greater strain on healthcare systems with economic and healthcare equity consequences. Longer hospital stays with increased costs, higher morbidity and mortality, and reduced productivity which may
“AMR could very likely be the #1 killer, outpacing things like cancer, in the coming decades
to global health security.”
lead to higher poverty are all possible outcomes. AMR could very likely be the #1 killer, outpacing things like cancer, in the coming decades which will be a threat to global health security. Simple infections like strep throat or a “staph” skin infection may become things we can’t take for granted any longer.
Have you observed anything encouraging or troubling with regards to antibiotic prescription lately?
A: If you look at the literature and reporting, there is increased awareness and action by many global health organizations and governments. CDC and WHO have invested significant funding for infrastructure like surveillance tools, diagnostic tools, and education materials. In some settings, we have seen some declining AMR rates such as long-term care pharmacies coupled with improved
prescription adherence practices. ASP teams are increasing in healthcare systems, and more clinicians are collaborating with these teams to tackle specific resistant infections like MRSA bloodstream infections and surgical-site infections in the U.S. The Global Antimicrobial Resistance and Use Surveillance System [GLASS] and others have shown the global burden of AMR deaths among the pediatric population under 5 has declined by 50% between 1990 and 2021.

Rodney Rohde
Any final thoughts for our readers?
A: I am a passionate advocate of Science Communication and Health Literacy in the framework of a One Health approach. We must all work to educate our population about AMR being a significant global threat. We need more support for antimicrobial research and development, better and more accurate diagnostic testing and tools for all nations, stronger surveillance systems, and overall promotion and funding support for the experts and professionals needed to combat this global trend. HPN




BY KARA NADEAU
The sterile processing department (SPD) sits at the heart of safe surgical care, yet its educational needs have often been sidelined. Today, increasingly complex devices, rising case volumes, and evolving standards are driving a transformation in how SPD professionals are educated and trained. SPD leaders, educators, and vendors are responding with accessible, tech-enabled, and highly targeted
learning programs. As a result, SPD education is shifting from occasional and burdensome to intentional and empowering.
HPN interviewed SPD education experts across these groups to gain best practices in SPD education, including what works, what doesn’t work, and how to drive advancements in this area forward. We share success stories and lessons learned from three U.S. healthcare organizations:
The MetroHealth System, Nemours Children’s Hospital, and OhioHealth.
The rise of dedicated SPD educators
With the complexities of sterile processing and the ever-changing guidance and standards, it would seem the role of SPD educator would be prevalent throughout U.S. health systems and hospitals. But historically, many SPDs have lacked somebody in this role to guide staff education and training.
A certified sterilization technician from a health system in the Northeastern U.S. commented on challenges technicians face when pursuing continuing education opportunities, stating:
“I feel that the challenges to SP techs sadly often come from within the facilities where they are employed. Even just completing the basic
required CRCST certification sometimes results in a lot of red tape to be reimbursed. Techs desiring to go further don’t always have the support unless they do it on their own.”
She expressed the need for healthcare facilities to support other types of SPD education as well, “such as funding for attending seminars, conferences, and activities to promote networking with other techs.”
Within some health systems and hospitals, the tide is turning toward greater support for SPD education. As more healthcare organizations place greater value on sterile processing and its role in patient care and safety, they are establishing full-time educator positions. The following are stories from two U.S. healthcare organizations.
Expanding the scope of learning: Continuing education at MetroHealth System
Becoming an SPD educator has long been part of the career plan for Janene McGlynn, CRCST, CIS, CER, CHL. After spending nearly a decade in the sterile processing profession, she finally saw her opportunity when The MetroHealth System in Cleveland created its first SPD educator position.
As the first person in this role, McGlynn faced understandable skepticism. Staff weren’t sure what to expect. “There was a lot of, ‘What’s your function? What do you actually do?’” she said. “And that was understandable because no one had done this here before.”
“In some places, the educator just managed onboarding paperwork and relied on vendors to handle continuing education,” McGlynn explained.
resources so staff can engage with training on their own time. She also distributes vendor-provided in-service information to maximize flexibility.
“SPD education is about being flexible and understanding your department—what they’re willing to do, what they’re not going to do, and customizing a program for them.” - JANENE MCGLYNN, CRCST, CIS, CER, CHL
“In others, the educator created custom in-services and secured certification for continuing education units (CEU). And I’ve been in places where the educator existed in name only— no one really saw them. It goes to show how there’s no handbook telling you what an SPD educator should do. It’s up to you to figure it out.”
At The MetroHealth System, continuing education had traditionally been managed by SPD leadership who selected vendor-led in-services a year in advance. McGlynn has taken a different approach. Her goal has been to expand CEU access while keeping learning manageable.
“I bring in vendors once a month who offer CEUs that are accredited by the Healthcare Sterile Processing Association (HSPA) and CBSPD,” said McGlynn. “That way, I know staff are covered on all fronts.”
McGlynn is a firm believer that education should reflect how people actually learn.

McGlynn brought experience from multiple hospitals, including those with and without SPD educators. She knew that an educator’s role could look very different depending on the department’s culture, leadership, and expectations.
“My team isn’t into games or team-building-style sessions. If I did that, they’d just stare at me,” she joked. “SPD education is about being flexible and understanding your department—what they’re willing to do, what they’re not going to do, and customizing a program for them.”
She surveyed her staff to understand their learning preferences and gaps, creating QR code-based CEU
“It’s all about meeting people where they are,” she added.
Empowering education through ownership: GEMBA rounds at Nemours
“When a hospital creates a sterile processing educator position, it demonstrates the value of the SPD to the organization, underscoring what our profession stands for and the level of competency in our work,” said Nemours Children’s Hospital’s sterile processing supervisor of education Jean-Philippe Grunderson, MHA, CRCST, CIS, CHL, CER.
In collaboration with the hospital’s assistant vice president (AVP) of perioperative services & SPD, DV, Edna Gilliam, DNP, MBA, RN, CNOR, NEA-BC, and sterile processing department manager Kwame Addomah Gyabaah, MHA, Mini MBA, BS, ACHE, CHL, CER, CIS, CRCST, Grunderson is redefining SPD excellence through Lean thinking and frontline ownership.
To educate SPD team members on industry best practices and spark meaningful improvements, Gilliam, Grunderson, and Gyabaah instituted GEMBA rounds—an approach rooted in Lean and Six Sigma methodologies. Instead of leadership selecting which improvements to pursue in the SPD, they empowered a group of technicians to take ownership— tasking them to identify two process issues and one safety issue they wanted to address.
Gyabaah noted some skepticism to the GEMBA approach at first, but as they progressed through the chosen improvements, the benefits became clear. He stated:

“A lot of people in the SPD, even myself, questioned, ‘How is this going to work?” But it’s so exciting once you get into it. It just flows. We can’t wait to finish whatever we’re doing for improvements, because there are more in the pipeline for us to put on the GEMBA board.”
One early win for the SPD team was resolving delays in first-case surgical trays. Technicians created a separate “first-case” rack, ensuring clear prioritization and readiness. The result: Smoother OR starts and improved shift-to-shift communication.
A survey by The Joint Commission (TJC) in May/June 2024 prompted another initiative, as Gilliam explained.
“When the TJC inspectors conducted their survey, they spent hours in SPD and could not find anything wrong. And then they went to one of the clinics for which the
SPD processes instruments. They discovered disposable instruments used by the clinic that were going back to SPD for reprocessing.”
“That’s a huge problem, because SPD was not aware of what these clinics were ordering for instruments,” Gilliam continued. “And the clinic wasn’t aware of the fact that they’re ordering single use instruments and then sending them back to SPD.”
To address this issue, Gilliam, Grunderson, and Gyabaah implemented KeyDot from Steris, a 2D data matrix barcode label for tracking individual instruments.

“In the SPD, we put them on the reusable instruments we are reprocessing. If something comes to SPD without a KeyDot, then we know that we need to investigate that item.”
As the SPD team works toward their improvement goals, the executive leadership team, including Gilliam, rounds with them once a week to remove any barriers and help them progress.
“I think one of the secrets to our continuous improvement success is that the SPD technicians choose what they feel is important to address and then own these initiatives,” said Gilliam. “Another is the engagement and support of the executive leadership team, including myself as the AVP, along with the chief nursing officer (CNO), and other OR leadership members.”
“When the SPD team corrects an issue—we’ve already retired a couple of these GEMBA initiatives—it’s empowering, especially when they can showcase their success to leadership,” she continued. “This, in turn, drives leadership to invest in more of these improvement initiatives.”
The role of vendors in SPD education: Standardized paths and data-driven improvements
Manufacturers of surgical instrumentation/devices, sterile processing equipment and supplies, and technology solutions offer a wealth of continuing education resources for SPD educators and their team members. Vendors in this space shared their insights on SPD education best practices and trends.
Out of the box SPD education: The Incision/OhioHealth story
When Incision, a perioperative education company, first exhibited at the Association of periOperative Registered Nurses (AORN) conference four years ago, the team didn’t expect the flood of questions about sterile processing education.
At the time, Incision had just one SPD course. With dual experience in the OR and SPD, Aimee Space, BSN, RN, Product Owner, Medical Content for Incision, saw an opportunity for more structured, digital, and accessible education in the SPD.
With input from past HSPA presidents, educators from both clinical and academic settings, and working SPD professionals, Incision built a comprehensive education curriculum.
“It was designed to complement onthe-job preceptorships,” Space explained. “Training is how you do something in your department. Education is why you do it that way and this is where we often fall short. You need both to be competent.”
In 2024, Incision partnered with OhioHealth to implement its standardized, “out of the box” SPD learning program across 13 hospitals. Facing growing surgical demand and limited educator bandwidth, the health system needed a scalable way to educate and support SPD staff.
Between August 2024 and March 2025:
•216 users enrolled in the 22-course program
•Over 3,500 certificates were issued
• 1,714 continuing education credits were awarded (HSPA and CBSPD-approved)
•More than 70% of users completed the full program
Survey results six months after implementation showed marked gains:
•87% of staff felt knowledgeable or very knowledgeable (up from 46%)
•88% reported feeling confident or very confident (up from 59%)
“Investing in this program was a great decision for our system,” said Dr. Josh Bowles, CRCST, system director, sterile processing, OhioHealth. “Not only has it significantly enhanced knowledge and skill development; it has also dramatically boosted the confidence of associates. The returns in both personal and professional growth have been extraordinary.”
These improvements held true across all experience levels, highlighting the program’s relevance to both novice and veteran staff, as Space explained: “It’s not just new staff who benefit from this kind of education. Experienced staff need to know the ‘why’ behind what they do, especially as they grow into the next generation of SPD leaders.”
Today, Incision’s SPD curriculum includes not only “back to the basics” fundamentals but also specialty areas like flexible endoscope reprocessing and (upcoming) specialty instrumentation. Learn more about Incision’s SPD education programs at incision.care.
Motivating the modern SPD workforce: Data-driven success
When asked about SPD education in a LinkedIn post, one SPD leader responded, “The standard standing around and talking is not keeping the staff motivated.”
Jennifer Greisen, principal of Strategic Solutions, Surgical Asset Management at Aesculap, emphasized the importance of engagement through transparency.
“Effective strategies include publishing metrics to share performance data regularly, providing incentives to reward achievements and boost morale, and connecting their work to patient outcomes to highlight the impact of their efforts,” said Greisen. “Also, using quality tracking tools to implement real-time productivity data via dashboards on monitors or on handheld devices ensures consistent communication.”
Interactive in-services and ondemand learning modules are emerging as valuable tools in SPD education, as Greisen explained:

Jennifer Greisen
“A quality tracking platform should be cloud-based with the ability to embed videos for ondemand training, while offering access to documents and/or websites with detailed instructions (e.g., IFU/DFU instructions and manufacturer’s recommendations).”
Tips for advancing SPD education
HPN asked those interviewed for this article to provide advice to other SPD leaders and educators on how to advance their departments’ education initiatives. Culture and collaboration were two best practices that rose to the top of the list.
Embody a culture of broad learning
In Nemours Children’s Hospital’s SPD, staff are urged to read standards, ask questions, and stay informed through professional organizations like HSPA. As sterile processing supervisor of education, Grunderson not only talks the talk when it comes to education, he also walks the walk:
“I read a lot. I’m a nerd when it comes to ANSI/AAMI ST79 and HSPA literature. I also frequently reach out to my former colleagues, managers, supervisors, and infection preventionists for advice. Just because I’m used to doing something one way doesn’t make it the right way. I turn to them to determine if there’s a better way. I attend conferences, and when I can’t, I seek feedback from colleagues who have attended.”
Gyabaah added how LinkedIn is a great tool for information sharing across the SPD community. He pointed out how industry associations (e.g., AAMI, HSPA) and SP professionals frequently post valuable insights on standards and individual learnings related to the field.
Grunderson encourages rotation of SPD technicians across tasks to build well-rounded expertise. He stated, “The best way to keep yourself up to date is being in a department where you’re rotated every day on
different assignments because you retain more knowledge that way.”
Engage in cross-functional collaboration “We think we all struggle with unique challenges, but at the end of the day, the OR and SPD often face the same issues, such as staff turnover, time constraints, and nonstandardized education,” said Space. “Education tailored to these departments not only benefits the growth and learning needs of periop professionals, but ultimately the patients they serve.”
From cross-department collaboration to surgeon tours of the SPD, the team at Nemours is fostering a culture of collaboration and transparency. “We’re educating our staff, executives, and even our surgeons. That kind of awareness elevates the whole department,” said Gyabaah.
“Kwame and Jean-Phillipe have opened up the SPD from an education standpoint,” said Gilliam. “One surgeon who recently took a tour of the department responded with ‘Wow. I mean, just wow.” She noted how the surgeon had previously underestimated the complexity of sterile processing.
Recognizing the critical connection between SPD and the OR, McGlynn proactively invites MetroHealth System’s OR service line leads to present to her team about instrumentation/tray issues. “That’s been huge,” she said. “Now our teams know each other’s faces. It’s been a real success.”
McGlynn is one of six educators at MetroHealth, alongside peers in the OR, Cath/IR lab, and endoscopy. Collaboration is a cornerstone of their success, as she explained:
“We regularly meet and if there are any problems in any instrument reprocessing we talk about them. The OR educators and I collaboratively hold table-talk sessions with the OR team. Topics are focused on overlapping OR/ SPD topics, such as sterility or indicator education. Then I present the same information to the CSPD department to keep us in loop.”
As surgical instrumentation and devices become more complex, an aging population drives demand for higher procedural volumes, and safety rises to the forefront of patient consciousness and payer reimbursements, the need for well-educated, well-trained, and empowered SPD professionals becomes even more critical.
From dedicated educator roles and standardized programs to Lean-driven improvement efforts and datainformed training strategies, forward-thinking SPD leaders are proving that when health system and hospital executives invest in SPD education, they invest in patient care. HPN

HPN’s podcast features interviews with special guests and article reads on our many verticals including supply chain, sterile processing, surgical and critical care, infection prevention, and more.

Scan the QR code so you don’t miss an episode!



BY SARAH B. CRUZ
1.Review the various entities and documents that shape sterile processing practices
2.Assess how these expectations are integrated into the healthcare facility
3.Identify the ways in which hospital policy and practice is upheld in department procedures
Sponsored by:

Sterile Processing (SP) is a critical component of patient safety, where outcomes are affected by the adherence to or deviation from established protocols. For those new to the field, it’s easy to assume that processes happen seamlessly, almost automatically. For seasoned technicians, years of experience can sometimes lead to unconscious procedural shortcuts. For others who may never have been taught, the actions of SP technicians are influenced by a carefully structured framework of external and internal components.
Review the various entities and documents that shape sterile processing practices
SP is governed by a web of external requirements that create a foundation for safe practices. At the top of this framework are regulations. Regulations are the rules and requirements that include words like
The complexity of SP extends beyond the department assignments of decontamination, sterilization, and assembly. When regulations, standards, and instructions for use (IFUs) combine with facility policy, procedure, and standard works, the expected outcomes of SP professionals are created. Mastering the “why” behind this framework of patient safety is essential for delivering consistent, high-quality care.
“must”, “will”, and so on. Failure to comply with regulations can result in serious legal and financial consequences for healthcare facilities. In the United States, regulations are enforced by government agencies like the Occupational Safety and Health Administration (OSHA), the Centers for Medicare & Medicaid Services (CMS), and the Food and Drug Administration (FDA). These regulations protect both patients and healthcare workers by establishing minimum safety standards for infection prevention, device reprocessing, and workplace safety.
Industry standards provide evidence-based best practices developed by subject matter experts and professional organizations, such as the American National Standards Institute (ANSI) and Association for the Advancement of Medical Instrumentation (AAMI). For SP professionals, the ANSI/AAMI ST:79 standard details comprehensive guidelines for steam sterilization and sterility assurance. Equally important is AAMI ST:90, which focuses on quality management systems and reinforces the need for continuous improvement within SP departments. These documents are revised and updated consistently. New standards are introduced as the SP industry evolves, such as ANSI/AAMI ST108:2023, which provides guidelines for the quality of water used in the processing of medical devices. This standard evolved from a technical information report (TIR), AAMI TIR34:2014/ (R)2021, that a workgroup of professionals contributed to and revised into the document it is today. A TIR provides additional context on technical issues and often serves as the connection between standards and guidelines.
Yet even the most carefully developed standards are meaningless without comprehensive IFUs provided by device manufacturers. IFUs are legally binding documents that dictate how
instruments and devices should be used, cleaned, disinfected, and sterilized. IFUs outline essential steps that, if ignored, can compromise device integrity and patient safety. To assist in document consistency, all manufacturing IFUs are also guided by their own AAMI standard.
Assess how these expectations are integrated into the healthcare facility These complex regulatory requirements are translated into hospital policies. While regulatory requirements are created by governing bodies, policies are created by healthcare facilities to define what must be done to ensure compliance with said governance. Moreover, policies define expectations and establish accountability throughout all areas of the hospital. They serve as the framework for the implementation of various regulations. Consider a common example: A technician is reprocessing a flexible endoscope. The IFU specifies manual cleaning followed by high-level disinfection using a specific chemical. If the technician skips the manual cleaning step or substitutes a different disinfectant, the result may be an improperly processed device, potentially putting a patient at risk for infection. Understanding and respecting the nuances of IFUs is not just a matter of compliance, it’s a safeguard for patient safety.
To achieve departmental compliance, policies are translated into documents known as Standard Operating Procedures (SOPs). SOPs provide broad overviews that outline the goals and expectations of specific processes. To adhere to SOPs, SP leadership can create standard works. Standard works break down complex processes into step-by-step instructions for an SP technician to follow. SOPs and standard works minimize variability, increase precision, and reduce the risk of error via process standardization.

August 2025
This lesson was developed by Solventum. Lessons are administered by Endeavor Business Media.
A er careful study of the lesson, complete the examination online at educationhub.hpnonline.com. You must have a passing score of 80% or higher to receive a certificate of completion.
The Certification Board for Sterile Processing and Distribution has preapproved this in-service unit for one (1) contact hour for a period of five (5) years from the date of original publication. Successful completion of the lesson and post-test must be documented by facility management and those records maintained by the individual until recertification is required. DO NOT SEND LESSON OR TEST TO CBSPD. www.cbspd.net


Healthcare Sterile Processing Association, myhspa.org, has pre-approved this in-service for 1.0 Continuing Education Credits for a period of three years, until June 02, 2027.
For more information, direct any questions to Healthcare Purchasing News editor@hpnonline.com.

Identify the ways in which hospital policy and practice is upheld in department procedures
A policy is only as strong as its execution, and that’s where competency assessments and training programs come into play. Competencies go beyond written tests or quizzes; they assess whether a technician can apply knowledge effectively in real-world situations. They involve hands-on practice, visual demonstrations, and scenario-based learning that accommodate different learning styles. Some technicians absorb information best by seeing a process demonstrated, while others may benefit from performing the task themselves or studying detailed written guides. By tailoring training methods to individual needs, department leadership can cultivate a workforce that not only understands policies but can consistently execute them with confidence.
Compliance in SP isn’t a one-time achievement; it’s an ongoing process that requires validation and verification at multiple levels. Validation ensures that processes consistently produce the desired outcome, while verification checks that processes are being followed as intended.
In real-world practice, this means sterile processing professionals must remain vigilant. For example, if a sterilizer consistently produces wet loads or a washer leaves residual bioburden on instruments, it’s up to the SP team to investigate, report, and resolve the issue. This isn’t just the responsibility of educators or managers; it’s a shared duty in which every technician plays a critical role.
Frontline professionals often have the first opportunity to identify and address process deviations. When something isn’t working, they are in the best position to flag the issue and propose adjustments. Consider a situation where a recently updated SOP for preparing trays for low-temperature

sterilization omits a critical drying step. A seasoned technician notices the oversight and reports the discrepancy, leading to a correction that prevents potential patient harm. This type of proactive problem-solving is not just encouraged, it’s essential for maintaining compliance and improving outcomes.
SP professionals, regardless of their position, have the power to drive change from the ground up. What happens in individual departments can influence hospital policies, shape industry standards, and even inform national regulations. When technicians provide feedback, report inconsistencies, and propose solutions, they contribute to a continuous improvement process that resonates beyond their department.
updates to internal policies or industry standards.

For example, if a department identifies a recurring issue with instrument reprocessing due to unclear IFU language, that feedback can be escalated to manufacturers and potentially lead to clearer, more user-friendly instructions. Similarly, persistent challenges with process consistency may inspire
Whether you are just starting your career in SP or have years of experience, understanding the relationship between regulations, standards, policies, and procedures empowers you to excel in your role. New technicians gain confidence by understanding the “why” behind their work, seasoned professionals reconnect with the foundation of best practices, and those who may have missed these connections earlier in their careers can realign their work with the highest standards. Your role goes beyond simply following instructions. By mastering this framework, you become an advocate for patient safety, a guardian of compliance, and a catalyst for positive change. Every tray you assemble, every instrument you inspect, and every load you prepare directly impacts the safety and wellbeing of the patients you serve.
SP is more than just a job: it’s a responsibility that requires vigilance, precision, and a commitment to

continuous learning. By understanding how regulations, standards, and policies shape your work, you elevate your practice and contribute to a culture of safety and excellence. Whether you are a new technician laying the foundation, a seasoned professional refining your approach, or someone eager to reconnect with best practices, you play a vital role in ensuring that every patient receives the highest standard of care. HPN
1.Cruz, Sarah B. 2025. Procedural Relationships: The Importance of IFUs, Guidelines, & Policies. Webinar, hosted by Solventum, March 16th, 2024. https://www.solventum.com/en-us/ home/medical/education/.
2.Cruz, Sarah B. Education Nation: Curriculum Based In-Services. Healthcare Purchasing News. Accessed March 30, 2025. https://educationhub.hpnonline.com/courses/educationnation-curriculum-based-in-services/.
3.Cruz, Sarah B. Education Nation: Creating Sterile Processing Department SOPs. Healthcare Purchasing News. Accessed March 30, 2025. https://educationhub.hpnonline.com/courses/ education-nation/.
Sarah B. Cruz , CSPDT, CRCST, CHL, CIS is president and founder of PRETREAT CSS, where she and Sterile Processing professionals work together to create objective-driven goals to advance their professional development. She is an industry speaker, published writer, social media enthusiast, mentor, and CTSPA Chapter President and HSPA Director. Sarah is a Sterile Processing instructor at her local community college and a CS Quality Education Program Development Coordinator in her full-time role.
1.What is a regulation?
A.A legally binding rule or requirement
B.A voluntary guideline to improve procedures
C.A suggestion from the healthcare facility None of the above
2.True or False: Compliance with regulations is optional as long as patient safety is maintained.
A.True
B.False
3.What does IFU stand for?
A.Instructions For Use Infection-Free
B.Use Internal Functional Unit
C.None of the above
4.Which of the following documents outlines essential steps for cleaning, disinfecting, and sterilizing medical devices?
A.Policy
B.Standard Operating Procedure (SOP) Instructions For Use (IFU)
C.Best Practice
5.True or False: Policies in healthcare facilities are designed to ensure that staff follow rules but may not be directly connected to external regulations.
A.True
B.False
6.What is the primary purpose of a Standard Operating Procedure (SOP) in Sterile Processing?
A.To provide general guidelines for reprocessing devices
B.To outline broad expectations and end goals of a process
C.To outline detailed, specific instructions for technicians to follow
D.None of the above
All CEU quizzes must be taken online at: educationhub.hpnonline.com The cost to take the quiz is $10.
7.Which of the following best defines “Standards” in the context of Sterile Processing?
A.Voluntary guidelines created by healthcare facilities
B.Evidence-based practices developed by industry experts to ensure patient safety
C.A series of written rules imposed by government agencies
D.None of the above
8.“Best Practice” in Sterile Processing refers to the set of most effective techniques derived from ongoing research and expert consensus, aimed at ensuring safety and quality.
A.True
B.False
9.A technician skips the manual cleaning step for an endoscope, instead directly applying a disinfectant. According to the IFU, this is a deviation from the proper procedure. What potential risk could this pose?
A.The disinfectant could be more effective, ensuring better sterilization.
B.The endoscope could be improperly cleaned, posing a risk for patient infection.
C.The technician can decide whether to follow the IFU or not, as it’s not legally binding.
D.None of the above
10.Which of the following is NOT part of the foundational framework of Sterile Processing?
A.Regulations
B.Standards
C.Personal preferences of technicians Policies


BY DAVID TAYLOR
Ashortage of skilled personnel can place excessive strain on core staff, leading to burnout and diminished performance. To address these issues, hospitals can implement a flexible float pool system. This approach not only helps maintain consistent operations but also improves staff morale, retention, and satisfaction, especially in today’s environment, which increasingly values flexibility over traditional scheduling models. The concept of float pools to address staffing variations was first introduced in 1981 and has since become recognized as a long-term solution for managing fluctuating staffing needs across different specialties, clinical environments, and patient populations.1
Most healthcare organizations understand that effective management, employee engagement, job satisfaction, and retention are critical to building cost-efficient, outcomes-driven, and motivated teams, and float pools are no exception. A well-structured float pool can deliver measurable benefits such as improved staff satisfaction and retention due to greater flexibility; long-term cost savings for the organization due to reduced staffing gaps and improved coverage that drives operational improvements and efficiencies; and the ability to more readily shift staffing resources where they are needed most to meet evolving demands.
Implementing a float pool system during peak staffing periods requires not
only logistical coordination but also strategic foresight, transparent communication, and a culture that values flexibility and fairness. These steps can drive success:
Use data to assess staffing needs –Begin by analyzing historical staffing data to identify patterns in holiday absenteeism, shift coverage gaps, and patient census trends. Go beyond anecdotal knowledge, using workforce analytics to determine which departments and shifts are consistently under-resourced. This data helps forecast the number of float pool participants required and highlights areas that are high-risk and need attention. Ensure a transparent and fair scheduling process – A scheduling platform should allow for open bidding or volunteering on holiday shifts. A system that clearly shows who has signed up, the incentives being offered, and shifts that remain unfilled reduces confusion and builds trust. From an HR perspective, involving staff in scheduling decisions promotes a sense of agency and reduces the perception of topdown mandates.
Incentivize with meaningful rewards – Offering time and a half may not be enough. Think creatively and layer incentives that reflect the difficulty of the shift. Examples include extra paid time off (such as a floating holiday for those who work on major holidays), gift cards or small bonuses for night or double shifts, and public recognition or thank-you notes from leadership. The shortterm cost of incentives can be far outweighed by the long-term gains

in retention, morale, and reduced agency reliance.
Communicate early, clearly, and often – Announce the holiday float-pool program several months in advance, outlining expectations, incentives, and timelines. Clarity and consistency in communication provide staff with sufficient time to manage their personal commitments. Consider using multiple communication channels, such as email, team huddles, and HR newsletters to ensure maximum reach and engagement. Early buy-in is essential, particularly in organizations that have historically struggled with last-minute holiday staffing.
Evaluate and evolve as needed – Solicit feedback about the float pool program through anonymous surveys and team debriefs. Assess what worked, what did not, and what could be improved. Key metrics to review can include shift fill rates, staff satisfaction scores, and overtime costs.
Adopting a well-planned float pool is a forward-thinking workforce strategy that supports clinical quality, financial health, and organizational resiliency. The program’s success depends on operational mechanics and a shift from reactive scheduling to proactive workforce planning. In a competitive labor market, flexibility and employee-centric practices are strategic imperatives. HPN
REFERENCES:
1.






BY ADAM OKADA
Q: “Is it still recommended to demagnetize instrumentation that has become magnetized?”
A:In a word, “Yes.” End of article. But . . . since we have some confusion in the industry about how and/or why:
• Instruments can become magnetized.
• This can be a problem.
• We need to fix it.
Let’s dive into more details!
How does a surgical instrument become magnetized?
The shortest and simplest answer is that our instruments can become magnetized when exposed to a magnetic field (e.g., a magnetic mat is sometimes used on the surgical field).
According to e-MagnetUK, “Magnets work because of tiny particles called electrons. These electrons spin and create small magnetic fields.
In most things, these fields cancel out. However, in certain materials, like iron, the tiny magnetic fields align in the same direction.”1
A magnetized item like a magnetic pad (Fig. 1) will have an aligned magnetic field, or domain. When a metallic item (e.g., surgical needle holder) touches the magnetic field, it can rearrange the atoms and electrons to magnetize your surgical instrument to align with the magnetic domain.
This process of magnetizing surgical instruments is more easily accomplished with smaller instruments and needle holders, like those used in cardiac or ophthalmic procedures, though it can happen on larger, steel instruments as well. Instruments can also magnetize each other through rubbing and friction.
Why is magnetization of surgical instruments a problem?
When a surgical instrument becomes magnetized, metallic items will be attracted to its magnetic field.

In my experience, the most common instruments to become magnetized are small needle holders (i.e., Castro-Viejo needle holders) used in cardiac or ophthalmic procedures.
Imagine you’re observing in a cardiac or ophthalmic surgical case, as Tiffany Dailey (ST, CSPM, CSPDT, HACP-IC, and Interim Sterile Processing Leader) has many times. Tiffany scrubbed surgical cases for years, including many heart procedures.
Dailey (personal communication, June 17, 2025) stated that “A magnetized surgical instrument, such as a needle holder, can adversely impact the course of a surgical procedure in several significant ways. One primary concern is the unintended attraction of small metallic objects, such as needles, clips, or metal fragments within the operative field.
“Furthermore, the presence of magnetization can interfere with the surgeon’s ability to maintain the delicate control necessary during suturing. When a magnetized needle holder interacts with nearby metallic materials, it may jerk or pull unpredictably, diminishing the surgeon’s tactile feedback. This loss of subtle control increases the risk of inadvertent tissue trauma or improper suture placement, both of which can affect patient outcomes.
“From a sterility and safety standpoint, magnetized instruments pose additional risks. By attracting microscopic metallic debris from the operating room environment or
Figure 1: Magnetic Pad - Photo source: Healthmark, A Getinge company.1 Healthmark. (Dec 2024). Magnetic Pad. Healthmark, A Getinge company. https://www.hmark.com/product/magnetic-mat/
instrument trays, this can inadvertently introduce contaminants into the sterile field. Such contamination elevates the risk of postoperative infections, jeopardizing patient recovery.
“Finally, magnetization can have a detrimental effect on the mechanical performance of surgical instruments. Components such as locking mecha-
“From a sterility and safety standpoint, magnetized instruments pose additional risks ... of postoperative infections, jeopardizing patient recovery.”
nisms or hinges may stick or function inconsistently due to magnetic interference.
“To mitigate these risks, it is essential to implement routine inspection and demagnetization protocols, ensuring that instruments remain in optimal condition to support successful surgical outcomes and uphold the highest standards of patient care.”
How can you test and/or fix a magnetized instrument?
A common informal quality test in sterile processing can be placing a
small metallic item, often an office product like a staple or paper clip, next to or against the instrument to see if it sticks to it. The problem with this type of test is that it can create other problems. In a past Healthcare Purchasing News (HPN ) article by Stephen M. Kovach ( HPN, March 2024)2 ,
Stephen relayed a story where the facility was testing their instruments for magnetization and a paper clip was left inside of the surgical tray.

There are magnetic field testers (Fig. 2) now on the market that can test your instrumentation for a magnetic field. These tests are inexpensive, easy to use, and reusable.
Once you’ve identified that your surgical instrument is magnetized, what do you do about it?
To demagnetize your instrument or needle holder, you’ll need a demagnetizer (Fig. 3) or degausser. Demagnetizers work by using electromagnets to create intense, highfrequency, alternating current (AC) magnetic fields. This shifts the magnetic field/domain randomly so that electrons are no longer aligning in the same direction.
After using the demagnetizer, test your instrument again to make sure the magnetic field is gone.
There you have it! We should be testing for magnetization of surgical instruments, which can be a
Figure 2: Magnetic Field Tester - Photo source: Healthmark, A Getinge company. HEALTHMARK. (DEC 2024). MAGNETIC FIELD TESTER. HEALTHMARK, A GETINGE COMPANY. HTTPS://WWW. HMARK.COM/PRODUCT/MAGNETIC-FIELD-TESTER/
Figure 3: Demagnetizer - Photo source: Healthmark, A Getinge company. HEALTHMARK. (DEC 2024). DEMAGNETIZER. HEALTHMARK, A GETINGE COMPANY. HTTPS://WWW HMARK.COM/PRODUCT/ DEMAGNETIZER/
problem during surgery. If magnetization is identified with a quality test, then we have tools available to fix the problem. HPN
REFERENCES:
1. Marketing. (March 1, 2024). How Does Magnetism Work & Do Magnets Lose Their Strength? e-Magnets UK. https://emagnetsuk.com/how-does-magnetism-work-do-magnetslose-their-strength/
2.Kovach, S.M. (Feb 24, 2024). Magnetized Surgical Instruments. Healthcare Purchasing News (HPN ). https:// www.hpnonline.com/sterile-processing/article/53083591/ magnetized-surgical-instruments
Adam Okada has 18+ years of experience in Sterile Processing and is passionate about helping improve the quality of patient care by giving SPD professionals and their partners greater access to education and information. He has worked in just about every position in the Sterile Processing Department, including Case Cart Builder, SPD Tech I, II, and III, Lead Tech, Tracking System Analyst, Supervisor of both SPD and HLD, Manager, and now as an Educator. Adam is the owner of Sterile Education, the world’s first mobile application dedicated to sterile processing education, and a former Clinical Manager at Beyond Clean. He has published articles for HSPA’s Process magazine, is a co-chair on AAMI WG45 as well as co-project manager for the KiiP “Last 100 Yards” group, and is the former President for the Central California Chapter of HSPA. Adam is currently a Clinical Education Specialist at Healthmark, A Getinge company, where he works on Healthmark webinars, hybrid events, and educational videos, as well as the “Ask the Educator” Podcast with Kevin Anderson.

BY DEWEY BARKER, MS, RN, BSN, CRCST, CIS
“St erile Processing” is both a place and a process. Today, we will focus on the process. The primary goal of sterile processing is to eliminate all forms of microbial life from the surfaces of surgical devices and instruments. This includes, but is not limited to bacteria, viruses, fungi, and many other microorganisms. Sterile processing is the decontamination, cleaning, rinsing, inspection, repair or replacement of damaged instruments, packaging, sterilization, and preparation of surgical instruments,
devices and equipment for safe use in the care of patients in hospitals, ambulatory surgery centers (ASCs), and other healthcare facilities. These processes are carried out by Sterile Processing Technicians, the frontline warriors, hidden in a behindthe-scenes lair that is often ignored and unseen until there is a problem identified in the operating room. The critical role of sterile processing in healthcare is to ensure patient safety and infection prevention and control, through attention to detail throughout every step of the reprocessing cycle. Sterile processing employs the
use of a variety of chemical agents including, detergents, disinfectants, sterilants, and other cleaners. The use of the correct chemical for the job is critical for removing all bioburden and microorganisms before using the instruments on or in another patient. They must also use the correct dosage of each chemical, so as to not leave residue on any surfaces or do damage to the device they are cleaning. Adhering to the established industry standards and following the instructions for use (IFUs) to the letter protects patients and staff and leads to a longer useful life for the
instruments. Taking shortcuts can lead to negative patient outcomes and early deterioration of surgical instruments and containers. The critical tasks of sterile processing are carried out in four strategically defined areas of the Sterile Processing Department (SPD).
The first of these task areas is Decontamination . Cleaning is the critical first step and should actually begin at the point of use, in the operating room. Gross soil should be removed at the point of use and a multi-enzymatic pre-soak spray should be applied, to keep instruments moist until processed in the decontamination area. This will prevent bioburden from drying on the instruments and biofilms from forming. The quicker the contaminated instrumentation can be cleaned, following the surgical procedure, the better. The use of a liquid multi-enzymatic pre-soak cleaner can speed up the cleaning process by beginning the breakdown of bioburden at the point of use, increasing productivity in Decontamination and reducing turnaround time. Only detergents, meeting all the ANSI/ AAMI: ST79 guidelines for a quality detergent, should be used. For additional safety, Decontamination staff should also watch for the “Safer Choice” label, for cleaners certified by the U.S. EPA. The decontamination process involves disassembling all instruments for cleaning in accordance with the manufacturer’s instructions. Proper disassembly is essential to ensure thorough cleaning, whether performed manually at the sink using sponges and brushes, or with ultrasonic cleaners and automated washers. Failure to disassemble instruments correctly can result in incomplete cleaning, which may compromise subsequent disinfection or sterilization procedures. This will interfere with subsequent disinfection or sterilization. Whether
cleaning manually or mechanically, a thorough rinse under the flow of water is critical for removing bioburden and leaving no detergent residue. There should always be a thorough rinse after every step of the cleaning and decontamination process.
After Decontamination , the next area in sterile processing is Inspection, Assembly and Packaging. Every instrument should be inspected after each use, during the set assembly process. They should be inspected for proper function, damage, missing parts, and any bioburden or detergent residue. Rigid sterilization containers are also inspected for physical and surface damage. The lid and base should fit securely, gaskets should be intact, latches should be fully functional, and filter retention plates should be flat, lock onto the positioning pins, and hold filters in place over vents in the containers. Physical properties such as surface integrity should also be inspected. Following Inspection, each instrument is placed into the proper set by using a count sheet of instruments for each set. Completing this process from memory, without a countsheet, leads to errors. After Assembly, the basket of set contents is packaged, with the appropriate filters and chemical indicators, into appropriately sized rigid sterilization containers or are wrapped in polypropylene wraps.

the same time not doing any damage to the device itself. The most commonly used method of sterilization is by the use of steam in an autoclave. Another commonly used method includes low temperature sterilizers, like STERRAD and V-Pro, which use hydrogen peroxide instead of steam. Other sterilization methods include Ethylene Oxide (EtO), Nitrogen Oxide (NO2) and dry heat. Non-steam sterilization is used for instruments and devices that are heat sensitive and/or are extremely delicate in construction. High-level disinfection is used for items that don’t need to be sterilized or when it is impractical because of how the devices are used. Some instruments can only be high-level disinfected, through the use of liquid sterilants.
The last task area in sterile processing is Storage and Distribution After the instrument sets are sterilized and properly cooled, they are placed on shelving or in carts in a sterile storage area. They are stored in this area until needed for the next surgical procedure. The instrument sets are kept in this area, where sterility can be monitored and maintained. They are not removed from this area until needed for patient care.
From the Packaging area, the instrument set moves to the Sterilization area where a variety of sterilants and equipment are used to sterilize the instruments, depending on the instruments or devices being sterilized. The instrument manufacturer always provides written instruction for use that must be followed exactly, so instruments are properly and fully cleaned, while at
Sterile processing is the science of reprocessing surgical instruments and other medical devices for safe reuse in healthcare facilities. It is the process of rendering surgical instruments functional, safe and sterilized or high level disinfected for use on the next patient. Sterile processing is an extremely detailed scientific process that intentionally strives to provide patient safety and infection prevention and control, while maintaining the longest possible life for the instruments and devices they process. HPN
BY KAREN CONWAY
More than 80 percent of hospital and healthcare system executives expect the Trump Administration’s tariffs to increase the costs they pay for medical products by at least 15 percent, with an even greater percentage planning to pass those costs on to payors and patients, that according to Black Book Research. At the same time, a variety of supply chain strategies are being pursued by both providers and suppliers to minimize the impacts on a health system that is facing product shortages and anticipated cuts to Medicare and Medicaid funding.
Some of these strategies you would expect, but others may surprise you. Let’s start with some of the more obvious strategies:
Both manufacturer and provider advocacy organizations, including Advamed and the American Hospital Association, are actively lobbying the administration for an exemption for medical products.
According to AHRMM Executive Director Mike Schiller, many supply chain professionals are seeking alternative products that can be sourced from countries with lower tariffs or, when possible, from domestic manufacturers. This is especially true for products made in China, which face some of the highest and most volatile tariffs.
Some manufacturers, meanwhile, are planning on or at least considering moving more manufacturing to the United States, but those efforts will likely take years. Other manufacturers say the demands of a global market and the uncertainty of what will happen in the next U.S. administration make it more feasible to move manufacturing from China to countries like Vietnam, Costa Rica, and the Philippines that have lower tariffs. Changes in where products and their components are manufactured can also trigger costly regulatory reviews, especially for higher risk products, which is why most of these changes have been for lower cost, lower risk products.

Provider supply chain organizations are actively reviewing contracts and renegotiating when possible to garner protections from tariff-induced price increases. Others, according to Schiller, are simply sending letters to suppliers rejecting any tariff price increases or surcharges, the success of which is as yet unknown.
Some healthcare systems are tying their tariff strategies to sustainability initiatives. By moving to reusable products when possible, they can minimize the impact of tariffs on future purchases as well as the waste generated by disposable and single use devices.
Many medical products are produced by multi-stage supply chains in which the final product goes through a series of interconnected steps, often involving different companies and countries, before being delivered to the end consumer. As an example, producing an IV pump might first require acquisition of electronic parts from Germany and plastic parts molded in Mexico, with the production of each of those components also going through a series of steps. Those parts, in turn, may be assembled in Costa Rica, with the final product packaged and distributed in the United States. A new paper by researchers from the Johns Hopkins School of Public Health explains how each of the parties in a multi-stage supply chain can absorb a small portion of the tariffs, thereby insulating the final purchaser — the hospital in this case — from the total tariff sticker price.
This final strategy may be the most effective for providers and suppliers alike. Rather than arguing over who pays the higher costs incurred as a result of tariffs, collaboration across the supply chain can soften the blow for all. It can also strengthen long term relationships among organizations that work together to address supply chain challenges. HPN
Streamline ordering across product categories, help control rogue spending and rely on uninterrupted service from our nationwide delivery network.












