

SOUTHWESTRETORT

SEVENTY-EIGHTH YEAR October 2025
Published for the advancement of Chemists, Chemical Engineers and Chemistry in this area published by
The Dallas-Fort Worth Section, with the cooperation of five other local sections of the American Chemical Society in the Southwest Region.
Vol. 78 (2) October 2025
Editorial and Business Offices: Contact the Editor for subscription and advertisement information.
Editor: Connie Hendrickson: retort@acsdfw.org
Copy and Layout Editor: Lance Hughes: hugla64@gmail.com
Business Manager: Martha Gilchrist: Martha.Gilchrist@tccd.edu
The Southwest Retort is published monthly, September through May, by the Dallas-Ft. Worth Section of the American Chemical Society, Inc., for the ACS Sections of the Southwest Region.







Data Entry and Editing Specializes in:
• Company newsletters
• Confidential Data Entry
• Free Quotes
Contact Lance at hugla64@gmail.com Or 214-356-9002
2025 ACS DFW Executive Committee
Chair: Denise Lynn Merkle, PhD
Chair-elect: Jonathan Dannatt, PhD
Past Chair: Rajani Srinivasan, PhD
Treasurer: Martha Gilchrist, MS
Secretary: Trey Putnam, PhD
Councilors:
MaryAnderson, PhD
Kirby Drake, JD
Linda Schultz, PhD
Rebecca Weber, PhD
Alternate Councilors:
Daniela Hutanu, PhD
Danny Tran, PhD
Yunxiang Li, PhD

October Letter from the Chair
Hello ACSDFW Members-
I hope autumn is starting out well and whatever you're working on is rewarding. It's seriously difficult to slog through the current situations we're all in - the fear is real- so it's uplifting when even small things work. While there's no need for me to encourage everyone to do what they can to counter the anti-science, anti-education, anti- humanitarian onslaught we're facing, I hope we all will be able to speak up and act as much as possible protect the important aspects of our personal and chemistry-focused lives. For those who remember the TV cartoon the Jetsons, I again quote George Jetson, 'Jane! Stop this crazy thing!'
On a local section note, regardless of what rebellious activity in which our members engage, ACSDFW is still here, and is still working to promote chemistry and support chemists in many different ways. Last week's 'Volunteer Like a Boss' Zoom gave attendees a lot of information pertinent to participating in the ACSDFW local section and in ACS National committees. Many thanks to those who logged on and to those who used their time to share their duties and their experiences. We have a Local Section election coming up and are seeking candidates for Chair-Elect, Councilor, Alternate Councilor, and Secretary. To hear more about the positions, contact DFWChemists@gmail.com. Using the subject line 'Candidate Info Requested' will lower the Activation energy required to send your e- to the correct chemist.
You'll hear a lot more about this in the coming months (and I hope you already know) that next year's ACS Southwest Regional Meeting (SWRM 2026) will be hosted by our ACSDFW local section. Numerous volunteers are needed to help plan the meeting and also assist at the meeting itself, which will likely be in early November 2026. To receive more info on the intriguing opportunities available to those who wish to participate in SWRM 2026, contact DFWChemists@gmail.com with 'SWRM 2026' as the subject. The last three SWRMs hosted by ACSDFW: 1994, 2004, 2014 were wildly successful and critically acclaimed - please work with your local section to maintain its cred.
You'll probably hear a lot more about this, too - my last day as Chair will be 12/31/2025. It's been great to work with the ExCom and local section scientists to govern ACSDFW, but 2026 will be the year for Dr. Jonathan Dannatt of UD, current Chair-Elect to take over, and for me to fade into the advisory role of Past Chair. Please sign up to volunteer and assist Dr. Dannatt to further develop our local section and enhance its usefulness to members.
Many thanks to everyone who volunteers - or even considers it. Remember National Chemistry Week, by the way! Plan an event to celebrate the Chemistry of Spices - or join in someone else's - and inspire those nascent scientists!
Chem Happy, Everyone-

Denise Lynn Merkle, PhD, ACSF 2025 Chair, ACSDFW

From the ACS Press Room
A prototype LED as thin as wallpaper that glows like the sun
“Sunlike Full-Spectrum Electroluminescent White Light-Emitting Diodes Based on Cu (In,Ga)S2 Quantum Dots Coated with Multiple ZnS Shells”
ACS Applied Materials & Interfaces
Light bulbs come in many shapes and styles: globes, twists, flame-like candle tips and long tubes. But there aren’t many thin options. Now, researchers report in ACS Applied Materials & Interfaces that they have created a paper-thin LED that gives off a warm, sun-like glow. The LEDs could light up the next generation of phone and computer screens and other light sources while helping users avoid disruptions to their sleep patterns.
“This work demonstrates the feasibility of ultra-thin, large-area quantum dot LEDs that closely match the solar spectrum,” says Xianghua Wang, a corresponding author of the study. “These devices could enable nextgeneration eye-friendly displays, adaptive indoor lighting, and even wavelength-tunable sources for horticulture or well-being applications.”
People want indoor lighting that looks natural and creates a comfortable atmosphere. Previous researchers accomplished this with flexible LEDs containing red and yellow phosphorescent dyes that produced a candle-like glow. Alternatives to lightemitting dyes are quantum dots that convert electric energy into colored light. Other teams have used quantum dots to create
white LEDs, but they have struggled to match the full spectrum of colors that comprise the sun’s white light, especially in the yellow and green wavelengths where it shines most strongly. So, Lei Chen and colleagues wanted to develop quantum dots that would mimic the desired natural glow when incorporated in a thin, white quantum dot LED (QLED). And in collaboration, a research group led by Wang suggested a strategy for slim electrically conductive materials that operates at modestly low voltage.
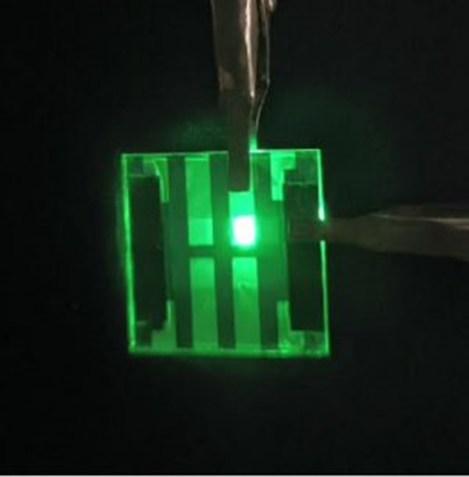
A paper-thin device uses quantum dots, similar to those described in this work, to light up LEDs. Lin Zhou, Xianghua Wang
The researchers first synthesized red, yellowgreen and blue quantum dots wrapped in zinc -sulfur shells and found the ratio of the three colors that produced an emission spectrum closest to sunlight. Then they built a QLED on top of an indium tin oxide glass substrate, depositing layers of electrically conductive polymers, the quantum dot mixture, metal oxide particles, and finally a layer of aluminum or silver. The quantum-dot layer was only tens of nanometers thick significant-
Continued on page 17
From the ACS Press Room
A high-performance supercapacitor made from upcycled water bottles
“All-Plastic Supercapacitors from Poly (ethylene terephthalate) Waste”
Energy & Fuels
Lots of single-use water bottles made from poly(ethylene terephthalate) (PET) end up in landfills, but there’s a growing interest in upcycling them instead. Researchers in ACS’ Energy & Fuels report on new heatbased fabrication methods to transform PET into supercapacitor electrodes and separator films for upcycled energy storage devices. In demonstrations, an all-plastic supercapacitor made from discarded water bottles outperformed a similar design that used a traditional glass fiber separator.
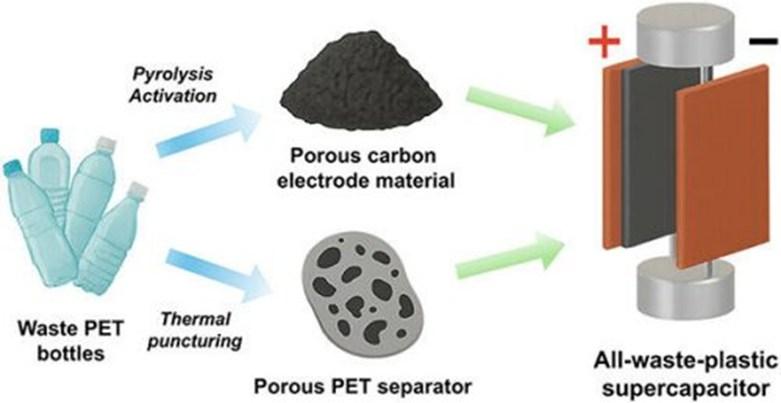
To keep single-use plastics out of landfills, researchers developed a heat-based strategy to upcycle waste PET bottles into high-performance supercapacitor components. Adapted from Energy & Fuels 2025, DOI: 10.1021/ acs.energyfuels.5c03370
“PET is used to produce over 500 billion single-use beverage bottles each year, which generates a significant amount of plastic waste and poses a major environmental challenge,” says lead researcher Yun Hang Hu.
“PET-derived supercapacitors hold great po-
tential for diverse applications in transportation and automotive systems, electronics and consumer devices, as well as industrial and specialized sectors.”
Converting waste plastics like PET into carbon-based materials, especially ones that are electrically conductive, is an attractive way to manufacture more cost-effective and sustainable energy storage devices like supercapacitors. These devices use highly conductive carbon electrodes to store and release a large amount of energy quickly and repeatedly. So, Hu and colleagues wanted to upcycle old water bottles into components for a type of supercapacitor called an electrical doublelayer capacitor (EDLC). This device is characterized by two porous carbon-based electrodes separated by a thin, perforated film immersed in a liquid electrolyte.
Hu’s team developed two processes to turn used PET water bottles into components for the upcycled device:
• For the electrodes, the researchers cut the plastic bottles into tiny, couscoussized grains. They added calcium hydroxide and heated the mixture to nearly 1300 degrees Fahrenheit (700 degrees Celsius) in a vacuum. This process converted the plastic into a porous, electrically conductive carbon powder. The researchers combined the carbon powder, carbon black and a polymer binder, and then dried it into thin layers.
• For the separator, the researchers flatContinued on Page 18

From the ACS Press Room
A step toward diagnosing the flu with your tongue
“A Viral Neuraminidase-Specific Sensor for Taste-Based Detection of Influenza”
ACS Central Science
Flu season is fast approaching in the northern hemisphere. And a taste-based influenza test could someday have you swapping nasal swabs for chewing gum. A new molecular sensor has been designed to release a thyme flavor when it encounters the influenza virus. Researchers reporting in ACS Central Science say that they plan to incorporate this type of low-tech sensor into gum or lozenges to increase at-home screenings and potentially prevent pre-symptomatic transmission of the disease.
Staying home is critical to preventing the spread of infectious diseases like influenza; however, people with the flu are contagious before they develop symptoms. Current flu diagnostics like nasal swab-based PCR tests are accurate, but they are slow and expensive. At-home lateral flow tests, akin to those used to test for COVID-19, are convenient and generally low-cost, but don’t catch presymptomatic infections.
As written in their published study, Lorenz Meinel and colleagues address these flu detection shortcomings “by switching away from complex detectors and machinery and toward a detector that is available for anyone, everywhere and anytime: the tongue.” The team developed a molecular sensor that releases a flavor that human tongues can detect thymol, found in the spice thyme. The sensor is based on a substrate of the influenza virus glycoprotein called neuramini-
dase (the “N” in H1N1). Influenza viruses use neuraminidase to break certain bonds on the host’s cell to infect it. So, the researchers synthesized a neuraminidase substrate and attached a thymol molecule to it. Thymol registers as a strong herbal taste on the tongue. Theoretically, when the synthesized sensor is in the mouth of someone infected with the flu, the viruses lob off the thymol molecules, and their flavor is detected by the tongue.

A taste-based test that releases a thyme-like flavor in the presence of influenza viruses could be incorporated into chewing gum or lozenges for easy, at-home screening. Lordn/ Shutterstock.com
After developing their molecular sensor, the researchers conducted lab tests with it. In vials with human saliva from people diagnosed with the flu, the sensor released free thymol within 30 minutes. When they tested the sensor on human and mouse cells, it didn’t change the cells’ functioning. Next, Meinel and team hope to start human clinical trials in about two years to confirm the sensor’s thymol taste sensations in people with pre- and post-symptomatic influenza.
If incorporated into chewing gums or lozenges, “this sensor could be a rapid and accessible first-line screening tool to help protect people in high-risk environments,” says Meinel.
Continued on page 18
From the ACS Press Room
Pathogenic yeast strains found in urban air but not along the coast
“Public Health Implications of Airborne Candida: Viability, Drug Resistance, and Genetic Links to Clinical Strains”
Environmental Science & Technology Letters
As city dwellers may know, escaping to the beach can provide a much-needed change of scenery or a mental reset. Historically, some doctors even prescribed trips to the sea to treat diseases. And now, research published in ACS’ Environmental Science & Technology Letters provides another reason to visit the coast. A pilot study found that urban air contained pathogenic strains of Candida yeast that were absent in coastal air samples, revealing a potential transmission method.

Air samples collected using this device, shown overlooking the South China Sea, lacked the pathogens found in air from an urban area of Hong Kong.
Yolanda Wang, adapted from Environmental Science & Technology Letters 2025, DOI: 10.1021/ acs.estlett.5c00795
Candida yeasts are a group of common microbes that exist harmlessly on people’s skin and in the lining of internal organs. Yet in certain conditions, they can overgrow and cause vaginal yeast infections or oral thrush. These infections are known to spread
through direct contact or bodily fluids. However, previous research found Candida DNA in the air, suggesting that the yeast is capable of airborne transmission. So, Ling Nathanael Jin and colleagues looked for infectious strains of live Candida in urban and coastal air samples.
The researchers collected air samples in Hong Kong and in a nearby less populated location overlooking the South China Sea once a month for an entire year. In 12 of the urban air samples, they found three species of Candida classified by the World Health Organization as fungal pathogens: C. albicans, C. parapsilosis, and C. tropicalis. Conversely, the samples collected at the coastal site did not have detectable levels of Candida. This difference between the locations suggested to the researchers that the airborne yeast has industrial or urban origins, such as wastewater treatment plants.
Additionally, a few of the urban air samples contained pathogenic Candida species that are resistant to common anti-fungal drugs. The researchers say that overuse of antifungal drugs, pollutants such as heavy metals in urban environments, or rising air temperatures may contribute to this resistance. Finally, the genetic makeup of one of these airborne Candida strains was closely related to samples previously taken from Candidainfected individuals, suggesting that the airborne strains could be infectious.
Continued on page 18
Punkin'
Chunkin' 10/8/14 - updated 10/12/2025
White glue does not prevent your Jack-o'-lantern from molding. This must be true; I read it on the internet. The Google reveals all sorts of Jack-o-Lantern-saving treatments, from dunkin' your punkin' in dilute bleach to make it microbe resistant to using a carving technique that doesn't involve cutting the lid off the top. In the past 10 years the dangers of 'chemicals' for pumpkin eaters have led to recommendations of vinegar as a preservative. A 'vinegar is chemical free' article is not cited herein, although the Farmer's Almanac recommendations for saving your squash are1. Having been raised in a family that accepted the transitory nature of fruit, I can't say that I ever fretted over how fast a carved pumpkin would mold. I once contemplated the overnight disappearance of a carefully whittled apple, however, the thoughts were more toward the identity of the entity that devoured everything but the candle inside than how to prevent the consumption of more vegetable votive holders. Sodium Hypochlorite or some other microbicide would probably protect subsequent works of ghostly talent from hungry creatures, but really, it's a Jack-o'-lantern, not a permanent art installation.
Why pumpkins?And When? Pumpkins and other squash are of course native to North America, so that answers the Why. When? Depending on which of the 3,280,000 links offered up by The Google takes your fancy, the practice is ancient - or not. Carved gourds have been around for approximately 10,000 years, and fruit-as-lanterns have been known for 700 years2. North American pumpkin stories appear in the mid-1800s3, but it's likely there's some history there, as well.
While it's interesting to think that originally, carved turnips were meant to simulate Will-o'-the -Wisps, that eerie swamp-gassy emanation from bogs and marshes, it's also fun to think of all the excitement the humble pumpkin experiences. Aknife, a design, and a candle make the pumpkin a flickering lantern on a Halloween porch. A hankering after a Guinness world record led to the massing of a Jack-o-lantern and more than 30,000 of its decorated buddies - twice. That's a lot of Swamp Gas, to be sure. No doubt - passive pumpkins enjoy their place in the autumn world, but for the real thrill, adrenaline-junkies go Chunkin'. No measly porch flickering for these gourds. Atrebuchet and a wide-open field are their delight (until they land, of course). Punkin' Chunkin' is a momentous activity for humans, too. Scholarships are raised, science is taught, and happiness and defeat are imparted by the flights of Cucurbita pepo4 . 'What is the point of all this?’ you ask. Happiness is a Chunked Punkin'. That's all. Now get ready to raid that plastic Jack-o-lantern and eat some of that candy.
1 https://www.farmersalmanac.com/5-ways-keep-jack-o-lantern-fresher-longer
2 https://en.wikipedia.org/wiki/Jack-o'-lantern
3 https://www.yahoo.com/lifestyle/did-jack-o-lantern-become-221503773.html
4 https://science.howstuffworks.com/transport/engines-equipment/question502.htm
From the ACS Press Room
Sea foam could contain more ‘forever chemicals’ than water below, study finds
“Detection and Quantitation of Per- and Polyfluoroalkyl Substances in North Carolina Sea Foam and the Corresponding Sea Water”
Environmental Science & Technology
Sea foam is a common sight along the coastline as breaking waves churn up air and algae. Now, a study in ACS’ Environmental Science & Technology reports that sea foam from several beaches along North Carolina’s coast contain higher levels of per- and polyfluoroalkyl substances (PFAS) compared to the water below. Some foam samples had more PFAS than what is allowed in drinking water, highlighting the need to clean up and reduce environmental PFAS pollution.

A field study observed higher levels of forever chemicals in sea foam than in water along part of North Carolina’s coast.
Emily Donovan, adapted from Environmental Science & Technology 2025, DOI: 10.1021/acs.est.5c03600
“Our research shows that sea foam along North Carolina’s coast can concentrate PFAS to levels thousands of times higher than the surrounding seawater,” says Jeffrey R. Enders, the study’s corresponding author. “Monitoring these coastal environments is critical because contact with contaminated foam could pose unexpected risks to people and ecosystems.”
Depending on the day, ocean waves may release large bursts of sea spray or create a sudsy, floating foam. Past studies have shown that sea spray can carry PFAS into the air, if these forever chemicals are in the water, exposing people to contaminants that can lead to adverse health effects. But the transfer of forever chemicals into sea foam hasn’t been widely studied. Therefore, a grassroots community group (Clean Cape Fear) in North Carolina collected sea foam from coastal beaches and performed preliminary testing. The initial work found that some foam samples contained PFAS at significantly higher concentrations than the current parts-per-trillion (ppt) drinking water limits regulated by the U.S. Environmental Protection Agency (EPA). This result prompted Enders and colleagues to expand the investigation to 13 places along the mouth of the Cape Fear River and the nearby Atlantic coastline.
The researchers collected water and sea foam samples at each location, screened them for 49 common PFAS, and detected 35 individual PFAS across the samples. Each water sample exceeded 1 ppt total PFAS, with some reaching nearly a thousand times higher. Several foam samples had individual forever chemicals present at more than 1 million ppt, including perfluorooctane sulfonic acid (PFOS) one of six PFAS explicitly regulated by the EPA which was present at 8 million ppt. Additionally, most PFAS were more concentrated in foam than in the water,
Continued on page 17
A
From the ACS Press Room
new bone substitute made out of … 3D-printed glass?
“Rational Design of Purely Inorganic Self-
Healing Colloidal Hydrogels To Enable
“Green” 3D Printing of Bioglass-Based
Bone Substitutes”
ACS
Nano
You might think that glass has no business acting as a replacement for bone, but it turns out the two materials have many similarities. Researchers reporting in ACS Nano developed a 3D printable bio-active glass that served as an effective bone replacement material. In rabbits, it sustained bone cell growth better than regular glass and a commercially available bone substitute.
Both bone and glass can bear weight better than they can withstand being stretched because of the crystalline structures of the molecules and minerals forming them. But unlike bone, the main ingredient in glass silica can exist in a liquid form and can be 3D printed into any desired shape, such as a perfect match to a missing section of bone. However, most 3D-printable glass requires toxic plasticizing agents, or the glass needs to be fused at temperatures higher than 2,000 degrees Fahrenheit (1,100 degrees Celsius). So, Jianru Xiao, Tao Chen, Huanan Wang and colleagues wanted to develop a 3Dprintable glass that didn’t require plasticizers or extremely high temperatures to serve as a scaffold for bone-forming cells.
The researchers combined oppositely charged silica particles as well as calcium and phosphate ions both known to induce bone cell formation to form a printable,
bio-active glass gel. After the glass was shaped with a 3D printer, it was hardened into its final shape in a furnace at a relatively cool 1,300 F (700 C). Next, they tested the new bio-glass against a 3D printed plain silica glass gel and a commercially available dental bone substitute by repairing skull damage in living rabbits.
This 3D-printable bio-active glass (shown in pink) could one day be used as a bone substitute.
Adapted fromACS Nano 2025, DOI:10.1021/ acsnano.5c06377

Although the commercial product grew bone faster, the bio-glass sustained growth longer; after 8 weeks, most bone cells present had grown on the bio-glass scaffold. The plain glass had barely any bone cell growth. The researchers say that this work demonstrates an easy, low-cost way to 3D print a bio-glass bone substitute, which could have wideranging applications across medicine and engineering.
The authors acknowledge funding from the National Natural Science Foundation of China, the Fundamental Research Funds for the Central Universities, the National Science Fund for Excellent Young Scholars, and the China Postdoctoral Science Foundation Special Support.
From the ACS Press Room
Simple color-changing sensor quickly identifies poisonous gases
“Simple and Cost-Effective Fabrication of Embossed Colorimetric Sensor Array for an Optoelectronic Nose via Integration of a Self-Adhesive Paper and Mesoporous Colorimetric Silica Microparticles”
ACS Sensors
Not all poisonous gases have a smell or a color. But a tiny grid of pastel- and candycolored squares that effectively “sniffs” out hazardous chemicals in the air such as chlorosarin a highly toxic nerve agent could help detect them. Researchers report in ACS Sensors that the colorful patterns in their inexpensive and durable paper-based sensor array changed in the presence of poisonous gases, allowing for quick and accurate measurements within minutes.

The tiny squares in this colorful patchwork contain silica nanoparticles that change color in the presence of toxic gases.
Adapted fromACS Sensors 2025, DOI: 10.1021/ acssensors.5c01026
Electronic noses, or e-noses, are devices that detect harmful chemical vapors. But their electronic components can be pricey and aren’t practical for humid environments. Optoelectronic noses may address these limitations by replacing the electronic components with dye molecules that change color when they react with certain chemicals. Vijay Tak
and his colleagues created and tested a new optoelectronic nose design: an array of sensors that detect and measure deadly gases. Each sensor within the array is a tiny paper square containing microscopic silica particles coated with dyes that change color and intensity after interacting with specific molecules or ions.
As a proof-of-concept, Tak and the research team created the sensors by soaking silica microparticles in 36 different color-changing dye solutions. After air drying them, they created a 12x3 array of color-changing sensors by placing the dried particles into a microwell plate, laying a piece of adhesive paper on top, and then flipping the plate so that the dye-containing silica particles are embossed onto the paper. To provide structural support for the array, a thin metal sheet is stuck to the sticky underside of the paper.
To test the array’s accuracy, the researchers exposed it to 12 poisonous gases at two concentrations each. The team compared photos of the squares’ color and intensity before and after five minutes of gas exposure. This produced a pattern that they could use to identify the type and concentration of gas present. In repeated experiments, the color-changing sensors achieved 99% accuracy for identifying the type of chemical threat and 96% accuracy for measuring the concentration in the gas samples. Additionally, another demonstration confirmed that the array’s accuracy was not affected by humidity.
Continued on page 19
From the ACS Press Room
Planting bush basil near green beans naturally repels certain pests
“Bush Basil Companion Plants Act as Plant Defense Potentiators for Cultivated Plants”
Journal of Agricultural and Food Chemistry
In the middle of summer, garden vegetables like green beans are proliferating, but so are pests that like to chew and suck on them. Now, a study in ACS’ Journal of Agricultural and Food Chemistry suggests growing bush basil near bean plants could offer a cost -effective, natural (and tasty!) alternative to chemical repellants. The fragrant herb not only helped the beans develop their own defenses against spider mites but also attracted the pests’ natural enemies.
“We undertook this research with the aim of harnessing ‘talking plants’ those that emit volatile organic compounds (VOCs) to enhance agricultural practices, advance scientific understanding of interplant communication, and establish a foundation for the future application of this technology in agricultural settings,” says lead researcher Genichiro Arimura, describing the motivation behind the present study.
Some fragrant plants help save neighboring crops from harmful leaf-eating insects. For example, the strong odor of mint contains VOCs that indirectly activate self-defense genes in nearby plants, protecting them from tobacco cutworms (Spodoptera litura) and spider mites (Tetranychus urticae). Previously, Arimura and colleagues found that growing mint near soybeans and Japanese mustard
spinach increased the two crops’ activity in a defense-related gene called pathogenesisrelated 1 (PR1). Arimura and a new group wanted to see whether basil and its strong, sweet, peppery smell could also help nearby plants keep pests away.

Greek or Bush Basil (Ocimum basilicum var. minimum) produces aromatic, volatile organic compounds that act as natural pest repellants for nearby bean plants.
Peter Turner Photography/Shutterstock.com
Out of six basil types the researchers tested (sweet, holey, Thai, cinnamon, lemon and bush), only bush basil activated the PR1 gene in green beans, soybeans and tomatoes, helping nearby plants defend themselves. Focusing on green beans, in lab tests plants grown close to bush basil had less damage from spider mites (a plant-sucking herbivore) than beans with no nearby basil. However, tobacco cutworms (a chewing herbivore) were unaffected by the nearby basil.
In field trials, green bean plants within slightly more than 1 yard (1 meter) of bush basil plants had substantially fewer pests and less damage to their leaves than green beans that
Continued on page 19
UTDRemembersAccomplishedChemistry Researcher,Mentor,Leader
SWRM 2026
Next Autumn, Your Local Section will host SWRM 2026!
By: Rick Vacek | June 12, 2025
Sign Up to helpACSDFW put on a fabulous regional meeting!
TheACS DFW local section is looking for volunteers for the SWRM 2026 meeting which will take place in Fort Worth Nov. 08-Nov. 11.
Volunteer Commitment:
Volunteers are asked to commit to 4-hour time blocks. You may sign up for multiple shifts across different times and days.
Volunteer Roles:

Volunteers will assist in a variety of capacities to ensure the conference runs smoothly. Possible assignments include:
• Registration: Welcome attendees at the registration booth, provide directions, and distribute pre-made maps for the conference symposia as well as information on local restaurants and attractions.
Dr. John Ferraris joined UT Dallas in 1975 and helped shape the early years of the chemistry department. During his tenure, he served as chair from 1995 to 2017 and as interim dean of the School of Natural Sciences and Mathematics from 2003 to 2006.
• Tech Support: While on-site tech support will be available, techsavvy volunteers may be stationed at symposium venues to help resolve audio-visual issues or quickly contact the tech team as needed.
Dr. John Ferraris, an accomplished scientist and longtime faculty member at The University of Texas at Dallas who was known for his research achievements in organic electronics and his leadership in teaching and mentoring, died May 5 at the age of 78.
John Ferraris, a professor of chemistry and biochemistry, arrived at UTD in 1975, six years after the University was founded. In addition to helping shape the early years of the chemistry department, he served as chair from 1995 to 2017 and as interim dean of the School of Natural Sciences and Mathematics (NSM) from 2003 to 2006. He held the Cecil H. and Ida Green Chair in Systems Biology Science from 2006 to 2009.
General Support: Assist with various logistical tasks such as making copies, ensuring venues are stocked and supported by hotel staff, delivering messages to participants, reporting issues that need ACS staff attention, and addressing other unanticipated needs as they arise.
Dr. John Ferraris worked with students in the George A. Jeffrey NanoExplorers Program. .
Contacts:
Jonathan Dannatt jdannatt@udallas.edu
Denise Merkle dmerkle@sciconsult.com
Mihaela C"Stefan mihaela@utdallas.edu
Kimberly Savage" k_savage@acs.org
Connie Hendrickson hendrickson@arkonconsultants.com DFWchemists@gmail.com>

Around the Area

UT-Dallas
The Department of Chemistry and Biochemistry congratulates Drs. Michael C. Biewer, Jeremiah J. Gassensmith, and Ronald A. Smaldone for being promoted to Full Professors, and Drs. Christina Thompson and Nimali Abeykoon for being promoted to Full Professor of Instruction and Associate Professor of Instruction, respectively. Professor Gassensmith was also selected as a Fellow of the Alexander von Humboldt Foundation. Associate Professor Sheena D’Arcy was awarded a $2.4M grant from the NIH entitled The Role of Dynamics in Regulating Multi-Activity Protein Complexes and Chromatin, Associate Professor Mario Wriedt was awarded a $61K sponsored research agreement from Estee Lauder entitled Characterization of Coreshell Boundaries in Polymer-coated Sunscreen Microparticles, and Robert A. Welch Distinguished Chair in Chemistry Vladimir Gevorgyan was awarded a $2.2M grant from the NIH entitled Novel Transition Metal Catalysis for Chemical Synthesis. Chemistry doctoral students Ikeda and Orikeda Trashi (Gassensmith Lab) earned first place and a $12K prize in the student competition of the Big Idea Competition for Biodelivera, a platform designed to deliver cancer therapy directly to tumors using virus-like particles. Harish Suryadevara, an undergraduate researcher in the lab of CPRIT Scholar Filo Romiti, was awarded an NSF Graduate Research Fellowship.
From the ACS Press Room
A prototype LED
page 6
ly thinner than conventional color conversion layers giving the final white QLED a thickness similar to that of wallpaper.
In initial tests, the thin QLED performed best under a 11.5-volt (V) power supply, giving off the maxmium bright, warm white light. The emitted light had more intensity in red wavelengths and less intensity in blue wavelengths, which is better for sleep and eye health, according to the researchers. Objects illuminated by the QLED should appear close to their true colors, scoring over 92% on the color rendering index.
In further experiments, the researchers made 26 white QLED devices, using the same quantum dots but different electrically conductive materials to optimize the operating voltage. These light sources required only 8 V to reach maximum light output, and about 80% exceeded the target brightness for computer monitors.
The authors acknowledge funding from the National Natural Science Foundation of China, the Natural Science Foundation of Anhui Province, and the Major Science and Technology Special Project of Zhongshan City.
From the ACS Press Room
continued
A high-performance supercapacitor
Continued from page 7
ened small plastic pieces about the size of postage stamps and poked holes in them using hot needles. The holes' pattern optimized the passage of current through the electrolyte.
To assemble their PET-based supercapacitor, the researchers submerged two porous carbon electrodes in a liquid potassium hydroxide electrolyte and separated them with the perforated PET film. In demonstrations, the upcycled supercapacitor retained 79% of its capacitance (storage ability), while a similar device with a glass fiber separator retained 78%.
Hu and colleagues say this research introduces a potential strategy for transforming PET waste into supercapacitor components,
“opening new opportunities for circular energy storage technologies.” In addition, they say the upcycled EDLC is less expensive to produce than devices made with glass fiber and is itself fully recyclable.
“With further optimization, PET-derived supercapacitors might realistically transition from laboratory prototypes to market-ready devices within the next five to 10 years,” says Hu, “especially as demand grows for sustainable, recyclable energy storage technologies.”
The authors acknowledge funding from the Charles and Carroll McArthur Fund.
diagnosing the flu with your tongue
Continued from page 9
The authors acknowledge funding from the Federal Ministry of Research and Education (now called the Federal Ministry of Research, Technology and Space) and have registered a patent with the European Patent Office on this technology.
Pathogenic yeast strains
Continued from page 10
The researchers say that this work challenges the long-standing assumption that Candida is primarily transmitted through direct contact, instead presenting it as an emerging airborne pathogen. However, more studies are needed to investigate where urban Candida originates and to understand exactly how infectious these airborne particles may be.
The authors acknowledge funding from the Research Grants Council of Hong Kong, the National Natural Science Foundation of China, the Presidential Young Scholar Scheme, the Research Institute for Sustainable Urban Development Joint Research Fund, the Research Centre for Nature-based Urban Infrastructure Solutions, and HuaJun Metal Products (Hong Kong) Co. Limited.
From the ACS Press Room
continued
Sea foam could contain more ‘forever chemicals’
Continued from page 12
reaching levels that were tens to thousands of times higher in many locations. The researchers also identified new PFAS in the samples, which they say likely came from nearby manufacturing facilities.
These findings show that PFAS concentrations in sea foam are higher than in regular sea water, potentially impacting human and animal exposures. The researchers conclude that this work underscores the need to reduce regional PFAS pollution and expand coastal monitoring for contamination.
The authors acknowledge funding from the National Institute of Environmental Health Sciences of the National Institutes of Health.
Simple color-changing sensor
Continued from page 14
With an estimated fabrication cost of 20 cents USD per array, the researchers say their designs could offer a cost-effective and customizable approach for environmental monitoring in real-world conditions. Next, they plan to develop a handheld optoelectronic-nose prototype to test for hazardous chemicals outdoors.
The authors acknowledge funding from India’s Defence Research & Development Organization.
Planting bush basil
Continued from page 15
were around 4 yards (4 meters) away from the herbs. The researchers identified linalool and eugenol as the primary VOCs emitted by bush basil and found that eugenol alone enhanced the defensive responses of green bean plants. In addition, the VOCs attracted the natural enemies of spider mites in laboratory experiments.
By helping nearby green beans up their selfdefenses and by attracting pests away from them, bush basil could be a natural solution to pest management and an effective way to protect crops from damage, the researchers say. The authors acknowledge funding from the Japan Society for the Promotion of Science Grant-in-Aid for Transformative Research Areas; Tokyo University of Science Research Grants; and the Ministry of Education, Culture, Sports, Science and Technology as part of the Joint Research Program implemented at the Institute of Plant Science and Resources, Okayama University.
From the Editor
It’s a whole year until the next SWRM, for goodness sake. Why are we calling for volunteers NOW?! That is because it is ONLY A YEAR! Read the ad and decide what you would like to do and be ready to commit to it.
Best articles? I usually favor an article from J.Ag.Food Chem., as that is where my dissertation research was published. The one this month shows that “...growing bush basil near bean plants could offer a cost-effective, natural (and tasty!) alternative to chemical repellants. The fragrant herb not only helped the beans develop their own defenses against spider mites but also attracted the pests’ natural enemies…”
Glass as a replacement for bone? Researchers reporting in ACS Nano have developed a 3D printable bio-active glass that served as an effective bone replacement material. Glass and bone have similar crystalline structures that can bear weight. But unlike bone, the main ingredient in glass silica can exist in a liquid form and can be 3D printed into any desired shape,
Remember: Volunteer! The ACS needs you.

