

SOUTHWESTRETORT

SEVENTY-EIGHTH YEAR September 2025
Published for the advancement of Chemists, Chemical Engineers and Chemistry in this area published by
The Dallas-Fort Worth Section, with the cooperation of five other local sections of the American Chemical Society in the Southwest Region.
Vol. 78 (1) September 2025
Editorial and Business Offices: Contact the Editor for subscription and advertisement information.
Editor: Connie Hendrickson: retort@acsdfw.org
Copy and Layout Editor: Lance Hughes: hugla64@gmail.com
Business Manager: Martha Gilchrist: Martha.Gilchrist@tccd.edu
The Southwest Retort is published monthly, September through May, by the Dallas-Ft. Worth Section of the American Chemical Society, Inc., for the ACS Sections of the Southwest Region.






Data Entry and Editing Specializes in:
• Company newsletters
• Confidential Data Entry
• Free Quotes
Contact Lance at hugla64@gmail.com Or 214-356-9002
2025 ACS DFW Executive Committee
Chair: Denise Lynn Merkle, PhD
Chair-elect: Jonathan Dannatt, PhD
Past Chair: Rajani Srinivasan, PhD
Treasurer: Martha Gilchrist, MS
Secretary: Trey Putnam, PhD
Councilors:
MaryAnderson, PhD
Kirby Drake, JD
Linda Schultz, PhD
Rebecca Weber, PhD
Alternate Councilors:
Daniela Hutanu, PhD
Danny Tran, PhD
Yunxiang Li, PhD

Letter from the Chair
Welcome to the September 2025 issue of the Southwest Retort!
In case you don't know, I'm the 2025 Chair of the Dallas-Fort Worth Local Section of the ACS (ACSDFW). Since July 2023, when I was appointed Chair-Elect of the local section, it's been my pleasure to join the other Executive Committee (ExCom) members to ensure the local section fulfills its obligations to its members and to our parent organization, ACS.
You've all heard - and probably know first-hand - that member involvement is the backbone of the American Chemical Society. Although ACS National has paid staff and some divisions and sections have enough members to support a paid support person or two, the vast majority of what gets the job done in the ACS is volunteerism.
Some contributions, such as those in governance, are via elected positions. Each year officers are elected to the Executive Committee, on which they serve 2- or 3- year terms. This year's election will select the Secretary, Alternate Councilor, Councilor, and Chair-Elect whose terms will begin on January 1, 2026. Other opportunities to work with your local section and promote chemistry abound: National Chemistry week programs (NCW), newsletter support, website support, hosting of meetings... The possibilities are as varied as the inherent time commitments. So, what exactly is involved in all these potential activities?
The ACSDFW ExCom recognizes that there's not a huge amount of detail available for those who want to become more involved. So - to give our members more information on how to interact with ACS - including your ACSDFW local section - and what the different roles entail, ACSDFW will host a recorded ZOOM meeting, 'Volunteer Like a Boss' on Tuesday, September 30, 2025 from 7 - 9.30p ct. Members of the ExCom and Chemistry Community will give short talks to inform attendees about what their volunteer activities require and how volunteerism has impacted their careers. Meeting video will be posted on ACSDFW.ORG (&/or on ACSDFW's youtube.com channel). Register via Eventbrite to find out how to participate in ACS activities and enhance your life.
Elections will be held in November - please vote - especially if you're a candidate! For more info, get in touch via DFWChemists@gmail.com.
Thanks, everyone, for your dedication to science.
Chem Happy! Looking forward to ZOOM-seeing everyone on September 30!
Denise Lynn Merkle, PhD, ACSF ChairACSDFW President, SciConsult, Inc. Director, Badderloch
Woad, Inc.

From the ACS Press Room
Removing yellow stains from fabric with blue light
“Environmentally Friendly Photobleaching Method Using Visible Light for Removing Natural Stains from Clothing”
ACS Sustainable Chemistry & Engineering
Sweat and food stains can ruin your favorite clothes. But bleaching agents such as hydrogen peroxide or dry-cleaning solvents that remove stains aren’t options for all fabrics, especially delicate ones. Now, researchers in ACS Sustainable Chemistry & Engineering report a simple way to remove yellow stains using a high-intensity blue LED light. They demonstrate the method’s effectiveness at removing stains from orange juice, tomato juice and sweat-like substances on multiple fabrics, including silk.
“Our method utilizes visible blue light in combination with ambient oxygen, which acts as the oxidizing agent to drive the photobleaching process,” says Tomohiro Sugahara, the study’s corresponding author. “This approach avoids the use of harsh chemical oxidants typically required in conventional bleaching methods, making it inherently more sustainable.”
Yellow clothing stains are caused by squalene and oleic acid from skin oils and sweat, as well as natural pigments like beta carotene and lycopene, present in oranges, tomatoes and other foods. UV light is a potential stainremoving alternative to chemical oxidizers like bleach and hydrogen peroxide, but it can damage delicate fabrics. Sugahara and Hisanari Yoneda previously determined that a high-intensity blue LED light could remove yellow color from aged resin polymers, and
they wanted to see whether blue light could also break down yellow stains on fabric without causing damage.
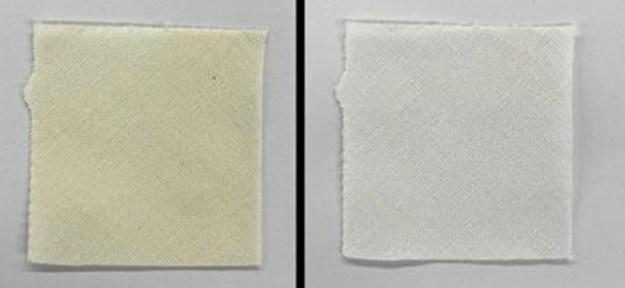
Initially, they exposed vials of beta-carotene, lycopene and squalene to high-intensity blue LED light for three hours. All the samples lost color, and spectroscopic analyses indicated that oxygen in the air helped the photobleaching process by breaking bonds to produce colorless compounds. Next, the team applied squalene onto cotton fabric swatches. After heating the swatches to simulate aging, they treated the samples for 10 minutes, by soaking them in a hydrogen peroxide solution or exposing them to the blue LED or UV light. The blue light reduced the yellow stain substantially more than the hydrogen peroxide or UV exposure. In fact, UV exposure generated some new yellow-colored compounds. Additional tests showed that the blue LED treatment lightened squalene stains on silk and polyester without damaging the fabrics. The method also reduced the color of other stain-causing substances, including aged oleic acid, orange juice and tomato
Continued on page 15
Exposing a sweat-like stain on cotton (left image) to a blue LED light for 10 minutes significantly removed the yellow color (right image).
Tomohiro Sugahara
From the ACS Press Room
Bacteria that ‘shine a light’ on microplastic pollution
“Detection of Microplastics Pollution Using a Green Fluorescent Protein-Based Microbial Biosensor Coupled with Raman Spectroscopy”
ACS Sensors
Microplastics are tiny, plastic fragments many too small to see found in the air, soil and water. Measuring their abundance in nature can direct cleanup resources, but current detection methods are slow, expensive or highly technical. Now, researchers publishing in ACS Sensors have developed a living sensor that attaches to plastic and produces green fluorescence. In an initial test on realworld water samples, the biosensor could easily detect environmentally relevant levels of microplastics.
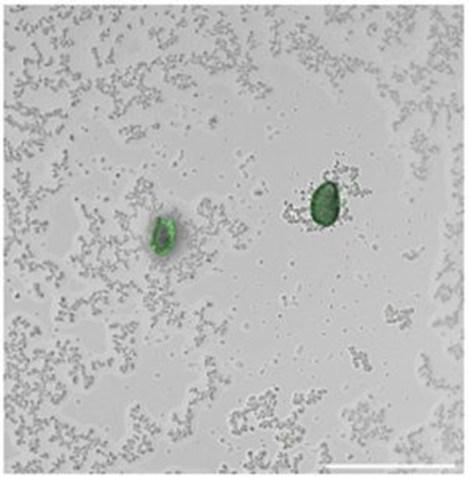
Engineered microbes produce bioluminescence in the presence of microplastics, creating a living sensor seen here as a detectable green fluorescence signal, which is laid over a scanning electron microscope image.
Song Lin Chua
Currently, scientists detect microplastics in water samples using microscopes or analytical tools, such as infrared or Raman spectroscopy. While these techniques are accurate,
they require multiple steps to prepare samples before analysis and can be expensive and time-consuming. In a step toward a simpler method, Song Lin Chua and colleagues created a living microplastics sensor from the bacterium Pseudomonas aeruginosa. This bacterium is commonly found in the environment and can naturally establish biofilms on plastic materials, though some strains are opportunistic human pathogens. The team wanted to modify the bacterium slightly to create a living sensor that easily detects microplastics in water samples.
The researchers added two genes to a noninfectious laboratory strain of P. aeruginosa to make the sensor. One gene produces a protein that activates when bacterial cells contact plastic, and the other gene produces a green-fluorescent protein in response. In lab tests, the engineered bacteria fluoresced in vials containing plastic pieces and a growth medium, but not in separate vials of other materials such as glass and sand. A measurable fluorescence was produced within 3 hours for various plastics, including polyethylene terephthalate (recycling symbol 1) and polystyrene (recycling symbol 6). Additionally, the modified bacterial cells stayed active for up to 3 days in the refrigerator (39 degrees Fahrenheit, 4 degrees Celsius), which the researchers say indicates it could be transported to field locations.
To test the living microplastics sensor as an environmental monitoring tool, the researchers added the engineered P. aeruginosa to seawater from a city waterway. The seawater was first filtered and then treated to remove
Continued on Page 15
ACS Fall Meeting ChemLuminaryAwards
ACS ChemLuminary Awards honor the best examples of programming, outreach, and operations from local sections, divisions, regional meetings, and international chemical sciences chapters.
Video:
https://players.brightcove.net/2698740430001/default_default/index.html? videoId=6341115342112




From the ACS Press Room
In-mouth hydrogel releases artificial saliva to treat dry mouth
“Poly(hydroxyethyl methacrylate) SalivaGel: A Polymer-Based Solution for Xerostomia Treatment”
ACS
Applied Polymer Materials
Saliva is more than spit. It helps with chewing and swallowing, protects teeth and gums, and even has antimicrobial and digestive properties. However, certain conditions or medical treatments, such as hemodialysis, chemotherapy and radiation therapy, reduce natural saliva production. Now, researchers publishing in ACS Applied Polymer Materials have created a reusable hydrogel that releases artificial saliva over time, which could help provide sustained relief from dry mouth.
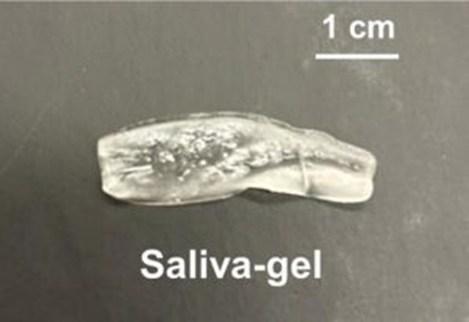
This little blob absorbs and releases artificial saliva, making it a potential treatment for dry mouth.
Adapted from ACS Applied Polymer Materials 2025, DOI: 10.1021/acsapm.5c00881
Dry mouth is a condition whereby the salivary glands do not produce enough saliva. Many medications to treat dry mouth boost natural saliva production, but they either provide temporary relief or have unwanted side
effects, including mouth irritation and tooth erosion. Previous research identified that artificial saliva, often used in laboratory studies, could be a promising alternative to current medications. Like natural saliva, artificial saliva is mostly water and contains mucins a class of compounds that lubricate the mouth and have antimicrobial properties. So, Suman Debnath, Georgia Malandraki, Bryan Boudouris and colleagues wanted to develop an artificial saliva reservoir that could be placed in the mouth and regulate the release of saliva over time.
To create the reservoir, the researchers mixed artificial saliva with a biocompatible polymer poly(hydroxyethyl methacrylate), which is commonly used in medical applications such as contact lenses. The resulting hydrogel is a clear blob roughly the size of a U.S. quarter and small enough to fit in the inner cheek or below the tongue. To determine how much artificial saliva a single reservoir could absorb, the researchers submerged it in artificial saliva for 6 hours. The saliva gel expanded up to 400% of its original volume, indicating that the gel has a high saliva storage capacity.
Next, the researchers tested the reservoir’s ability to release stored saliva. The gel released all stored saliva over a 4-hour time span at 98.6 degrees Fahrenheit (37 degrees Celsius), starting off a little faster and then slowing over time. This demonstrates that human body temperature helps initiate saliva release from the hydrogel. The researchers
Continued on page 16
From the ACS Press Room
An innovative system that dehydrates fruit without heat
“Dehydration of Mango and Apple through Atmospheric Water Harvesting under Vacuum”
ACS Food Science & Technology
Dried fruit is a tasty snack or sweet addition to recipes, but the water removal process often requires heat and energy. In a step toward more sustainable food preservation, researchers reporting in ACS Food Science & Technology have developed a method to dry food at room temperature by adjusting air pressure conditions and using food-safe calcium chloride. In a proof-of-concept, the system successfully dried mango and apple slices to commercial levels.
Dehydrating food turns perishable items such as fruit into long-lasting pantry staples. Most tabletop and industrial-scale food dehydrators use circulated hot air to remove moisture, which is simple and effective but requires a lot of energy. Sun-drying foods uses mostly solar energy but is slow and darkens the final products. So, Luis Bastarrachea is developing food preservation processes that don't require heat. In this recent study, the moisture-adsorbing salt calcium chloride, an ingredient used in cheese and molecular gastronomy applications, was incorporated into a room-temperature dehydration method and tested to see if it would impact the drying fruit’s color.
The researchers built a no-heat dehydrating chamber with three sets of screens above a container of calcium chloride solution. They placed mango and apple slices on the screens and then compared two room-temperature
drying methods: one with the chamber at standard air pressure and the other under a slight vacuum.

After four days under standard air pressure, the calcium chloride solutions drew out and adsorbed less moisture from the fruits than those placed under vacuum. The fruit slices at standard air pressure also dried inconsistently. Slices on the top screen contained 5070% water (by weight) and those on the bottom had 20-30% water after the dehydration process. In contrast, the vacuum-assisted method produced consistently dried mango and apple pieces made up of about 30% moisture, which is similar to the amount in commercially available dried fruit, and represented a removal of approximately 95% of
Continued on page 16
A pilot test of this system using vacuum and calcium chloride successfully dried apple and mango, matching moisture levels in commercial products.
Luis Bastarrachea
From the ACS Press Room
Simple color-changing sensor quickly identifies poisonous gases
“Simple and Cost-Effective Fabrication of Embossed Colorimetric Sensor Array for an Optoelectronic Nose via Integration of a Self-Adhesive Paper and Mesoporous Colorimetric Silica Microparticles”
ACS Sensors
Not all poisonous gases have a smell or a color. But a tiny grid of pastel- and candycolored squares that effectively “sniffs” out hazardous chemicals in the air such as chlorosarin a highly toxic nerve agent could help detect them. Researchers report in ACS Sensors that the colorful patterns in their inexpensive and durable paper-based sensor array changed in the presence of poisonous gases, allowing for quick and accurate measurements within minutes.
Electronic noses, or e-noses, are devices that detect harmful chemical vapors. But their electronic components can be pricey and aren’t practical for humid environments. Optoelectronic noses may address these limitations by replacing the electronic components with dye molecules that change color when they react with certain chemicals. Vijay Tak and his colleagues created and tested a new optoelectronic nose design: an array of sensors that detect and measure deadly gases. Each sensor within the array is a tiny paper square containing microscopic silica particles coated with dyes that change color and intensity after interacting with specific molecules or ions.
As a proof-of-concept, Tak and the research team created the sensors by soaking silica
microparticles in 36 different color-changing dye solutions. After air drying them, they created a 12x3 array of color-changing sensors by placing the dried particles into a microwell plate, laying a piece of adhesive paper on top, and then flipping the plate so that the dye-containing silica particles are embossed onto the paper. To provide structural support for the array, a thin metal sheet is stuck to the sticky underside of the paper.
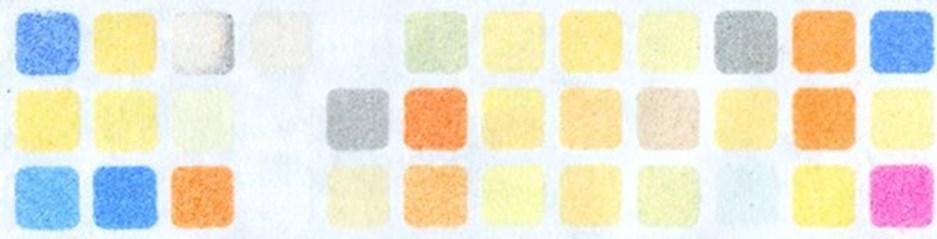
The tiny squares in this colorful patchwork contain silica nanoparticles that change color in the presence of toxic gases.
Adapted from ACS Sensors 2025, DOI: 10.1021/ acssensors.5c01026
To test the array’s accuracy, the researchers exposed it to 12 poisonous gases at two concentrations each. The team compared photos of the squares’ color and intensity before and after five minutes of gas exposure. This produced a pattern that they could use to identify the type and concentration of gas present. In repeated experiments, the color-changing sensors achieved 99% accuracy for identifying the type of chemical threat and 96% accuracy for measuring the concentration in the gas samples. Additionally, another demonstration confirmed that the array’s accuracy was not affected by humidity.
With an estimated fabrication cost of 20 cents USD per array, the researchers say their
Continued on page 17
From the ACS Press Room
Nanoparticles for rheumatoid arthritis prevention, flare control
”Immunomodulatory Nanoparticles Enable Combination Therapies To Enhance Disease Prevention and Flare Control in Rheumatoid Arthritis”
ACS Central Science
As a chronic condition, rheumatoid arthritis (RA) can’t be cured, so treatment focuses on managing the disease and controlling its progression. Although current treatments help control RA symptoms in most people, they cannot prevent the onset of RA or painful flare-ups. Now, researchers publishing in ACS Central Science have developed nanoparticles that could slow disease progression and reduce flare severity, based on results from tests with human blood and mice models with RA-like disease.
For a person diagnosed with RA, their immune system attacks tissue that makes up the joints, causing inflammation, swelling and pain. However, as the disease progresses, serious cartilage and bone damage can occur if left uncontrolled. Disease-modifying antirheumatic drugs (DMARDs) such as abatacept reduce disease activity and slow progression of symptoms, but most people taking DMARDs still experience symptom flare-ups. And for people with pre-RA, who have detectable levels of RA autoantibodies but don’t have symptoms, there are no approved treatments to prevent disease onset.
In previous research published in ACS Nano, a research team led by Nisarg Shah and Nunzio Bottini reported that calcitriolloaded nanoparticles, termed CLNP, help
regulate immune responses and decrease inflammation for autoimmune diseases in joints. The nanoparticles were made of a polymer containing calcitriol, the active form of vitamin D3. In addition, the researchers attached a small protein fragment to the CLNP. The fragment is derived from aggrecan (Agg), a protein in the joints that the immune system can mistakenly attack in RA. To expand on their previous work, the researchers wanted to see if the modified nanoparticles could treat RA flares and pre-RA.

A new nanoparticle treatment for rheumatoid arthritis could help prevent disease progression and reduce flareups.
Zay Nyi Nyi/Shutterstock.com
First, the researchers improved the nanoparticle formulation, focusing on size and stability. This helped ensure that the nanoparticles were free from any contaminants and could be frozen for a month without any damage.
The researchers then confirmed that the nanoparticles regulate dendritic cell activity, a type of immune cell responsible for initiating
Continued on page 17
UTDRemembersAccomplishedChemistry
Researcher,Mentor,Leader
By: Rick Vacek | June 12, 2025

Dr. John Ferraris joined UT Dallas in 1975 and helped shape the early years of the chemistry department. During his tenure, he served as chair from 1995 to 2017 and as interim dean of the School of Natural Sciences and Mathematics from 2003 to 2006.
Dr. John Ferraris, an accomplished scientist and longtime faculty member at The University of Texas at Dallas who was known for his research achievements in organic electronics and his leadership in teaching and mentoring, died May 5 at the age of 78.
John Ferraris, a professor of chemistry and biochemistry, arrived at UTD in 1975, six years after the University was founded. In addition to helping shape the early years of the chemistry department, he served as chair from 1995 to 2017 and as interim dean of the School of Natural Sciences and Mathematics (NSM) from 2003 to 2006. He held the Cecil H. and Ida Green Chair in Systems Biology Science from 2006 to 2009.

Dr. John Ferraris worked with students in the George A. Jeffrey NanoExplorers Program.
Councilor Report onACS Spring, 2025, National Meeting
March 23-27, 2025 – San Diego, CA, by
Linda Schultz
If I had to sum up this meeting in only one word. It would be “Enjoyable”. It literally had something for everyone. One could attend events either in person (15,332) or virtually (1,081), but somehow it never seemed crowded. Registrants included 17 countries outside of the US. Keynote events focused on innovations in Chemistry, Industry events featured networking, and the Technical Program featured more than 12,723 presentations (7,677 oral and 6,087 posters). The Expo always seemed to have free food, and the Sci-Mix was awesome.
On a more serious note, the various governance groups went out of their way to inform attendees about ACS operations and encourage member participation. ACS is always seeking volunteers for committees to shape ACS policy at all levels, so contact your Local Sections about opportunities (and do not forget to vote this fall when your ballots arrive). Candidates for President-Elect will be Christina Bodurow and Dawn Mason. Candidates for Director of Region IV (us) will be Lisa Houston and Milagros Degado. All seem to be strong candidates. Reports on their activities were also given by the President of the Board of Directors, the past and current ACS Presidents, and the President Elect, among others.
Some of the interesting factoids that I picked up are as follows: ACS is in the process of developing a new Strategic Plan and Mission Statement. Overall Society Membership remains steady at about 100,000. Dues are scheduled to remain constant for next year.
The main source of income for ACS is Information Services and publications, and both are currently running above the 2024 level, which was “in compliance” with all 5 established financial guidelines, so the ACS is currently in good financial health.
ACS identified over 900 counties and nearly 1k members within the United States that are currently unassigned to a Local ACS Section and are working to assign all unassigned counties (and individuals) to individual local sections. Results will be presented at the Fall 2025 ACS National Meeting, which will be in Washington DC August 17 – 21.
Around the Area

UT-Dallas
The Chemistry Department welcomes Assistant Professor Ziwen Jiang to the faculty as a CPRIT Scholar in Cancer Research following a NIH/Ruth L. Kirschstein Postdoctoral Fellowship in Pharmaceutical Chemistry at UCSF. The Chemistry Department also congratulates Dr. Sheel C. Dodani for being named a Eugene McDermott Distinguished Professor, and Dr. G. Andrés Cisneros for being named a Fellow of the Royal Society of Chemistry. Graduate student Tejas Shah (Torabifard and Stefan Groups), for received an outstanding award for experimental and computational work on designing new polymeric micelles for drug delivery applications from the ACS Division of Polymer Chemistry.

From the ACS Press Room
continued
Removing yellow stains
page 6
juice, on cotton swatches.
High-intensity blue LED light is a promising way to remove clothing stains, but the researchers say they want to do additional colorfastness and safety testing before commercializing a light system for home and industrial use.
The authors do not have an external funding source for this work. They are employed by Asahi Kasei Corporation, a company that develops fiber products, chemicals and electronic materials.
Bacteria that ‘shine a light
’
Continued from page 7
organic matter before the bacteria were added. Based on the fluorescence intensity values, the water samples contained up to 100 parts per million of microplastics. Further water analysis with Raman microspectroscopy revealed that the microplastics were primarily biodegradable types, such as polyacrylamide, polycaprolactone and methyl cellulose, which the biosensor detected despite the initial tests being done on traditional polymers
“Our biosensor offers a fast, affordable and sensitive way to detect microplastics in environmental samples within hours,” says Chua. “By acting as a rapid screening tool, it could transform large-scale monitoring efforts and help pinpoint pollution hotspots for more de-
tailed analysis.”
From the ACS Press Room
continued
The authors acknowledge funding from the Environment and Conservation Fund, Health and Medical Research Fund, Research Centre for Deep Space Explorations, and Pneumoconiosis Compensation Fund Board.
In-mouth hydrogel
Continued from page 9
also report consistent saliva release rates across five consecutive tests with a single reservoir, demonstrating its potential as a reusable treatment option. Lastly, cultured cells in contact with the gel had no change to their survival or growth rates, indicating the reservoir’s biocompatibility.
“In future work, we plan to continue refining this saliva-gel in terms of durability and the amount of artificial saliva it can release with a single use. We also intend to test additional materials that would make it fully dissolvable,” say Malandraki and Debnath. “Our goal is to develop an easy and affordable solution for dry mouth for the millions who suffer from this frustrating condition.”
The authors acknowledge funding from the Henson Program in Soft Materials in the Davidson School of Chemical Engineering at Purdue University and the University of Wisconsin Comprehensive Cancer Center Support Grant.
An innovative system
Continued from page 10
the initial water mass. Vacuum-dried mango pieces kept the raw fruit’s attractive bright yellow color; however, the two dehydration methods darkened the apples by similar amounts. In addition, scanning electron microscopy images showed breakdown of starch granules in all the samples, but more of them broke down under standard pressure, suggesting that the vacuum-assisted method slows down deterioration mechanisms and retains freshness.
And the water pulled out of the fruit could potentially be reused. Bastarrachea says that “the collected water in the calcium chloride solution can be removed by evaporation, and the reconcentrated calcium chloride solution can be reutilized in more dehydration cycles.” Ultimately, the recovered water could be used in industrial applications or further treated for human consumption.
The authors acknowledge funding from the U.S. Department of Agriculture, National Institute of Food and Agriculture, Agriculture and Food Research Initiative.

From the ACS Press Room
continued
Simple color-changing sensor
Continued from page 11
designs could offer a cost-effective and customizable approach for environmental monitoring in real-world conditions. Next, they plan to develop a hand-held optoelectronic-nose prototype to test for hazardous chemicals outdoors.
The authors acknowledge funding from India’s Defence Research & Development Organization.
Nanoparticles for rheumatoid arthritis Prevention
Continued from page 12
inflammation and flare-ups in RA. To test the nanoparticles’ effectiveness, the researchers took blood samples from people with and without RA and treated the samples with Agg-CLNP. Agg-CLNP reduced dendritic cell activity which, in turn, reduced the cell’s immune response. By suppressing the immune response, AggCLNP could help alleviate RA symptoms such as inflammation and swelling.
The researchers also tested Agg-CLNP in a mouse model for RA. Agg-CLNP delayed inflammation and swelling when administered as a preventative treatment, but it had little effect when administered after the onset of RA. In a subsequent study,
when the researchers gave both abatacept and Agg-CLNP to the mice, the combination delayed disease onset and reduced joint inflammation, swelling and bone damage. Additional tests in mice also showed that Agg-CLNP reduced future RA flare severity when administered after corticosteroid treatment, which is often used to provide symptomatic relief. The researchers say that these results highlight Agg -CLNP as a potential therapeutic to address current limitations in RAtreatments.
The authors acknowledge funding from the National Institutes of Health, the Arthritis National Research Foundation, a Hellman Fellowship, the Swedish Society for Medical Research, the Swedish Research Council, the Foundation of King Gustaf V’ s 80th Anniversary, and the IngaBritt and Arne Lundberg Research Foundation.
The study’s experimental approach was approved by the Internal Review Board at Cedars -Sinai Medical Center.

From the Editor
Welcome back to the Southwest Retort; I hope you are all enjoying the new school year.
Congratulations to the DFW Section for receiving a ChemLuminary Award! ACS ChemLuminary Awards honor the best examples of programming, outreach, and operations from local sections, divisions, regional meetings, and international chemical sciences chapters.
Favorite article...artificial saliva gel for dry mouth syndromes. Dry mouth may sound trivial but if you got it it’s not!
DFW Section Zoom meeting is scheduled at the end of September; look for an email for you to sign in for it.

