Cambridge International AS Level Biology
Molecular and structural formulae
Ring structures
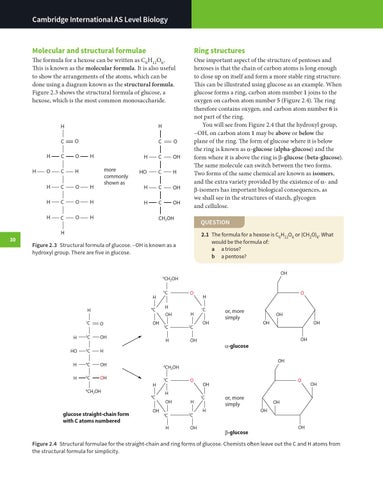
The formula for a hexose can be written as C6H12O6. This is known as the molecular formula. It is also useful to show the arrangements of the atoms, which can be done using a diagram known as the structural formula. Figure 2.3 shows the structural formula of glucose, a hexose, which is the most common monosaccharide.
H
H
H
One important aspect of the structure of pentoses and hexoses is that the chain of carbon atoms is long enough to close up on itself and form a more stable ring structure. This can be illustrated using glucose as an example. When glucose forms a ring, carbon atom number 1 joins to the oxygen on carbon atom number 5 (Figure 2.4). The ring therefore contains oxygen, and carbon atom number 6 is not part of the ring. You will see from Figure 2.4 that the hydroxyl group, –OH, on carbon atom 1 may be above or below the plane of the ring. The form of glucose where it is below the ring is known as α-glucose (alpha-glucose) and the form where it is above the ring is β -glucose (beta-glucose). The same molecule can switch between the two forms. Two forms of the same chemical are known as isomers, and the extra variety provided by the existence of α- and β -isomers has important biological consequences, as we shall see in the structures of starch, glycogen and cellulose.
C
O
H
C
O
O
C
H
H
C
O
H
H
C
O
H
H
C
O
H
H more commonly shown as
C
O
H
C
OH
HO
C
H
H
C
OH
H
C
OH
CH2OH
QUESTION
H 30
2.1 The formula for a hexose is C6H12O6 or (CH2O)6. What
Figure 2.3 Structural formula of glucose. –OH is known as a hydroxyl group. There are five in glucose.
would be the formula of: a a triose? b a pentose?
OH
CH2OH
6
C
O
H OH
H
5
H C
4
H C
O
C
OH
1
H
2
C
H
C
OH
C
OH
HO
3
H
4
H
5
OH
H
OH
C
2
or, more simply
OH OH
OH OH
OH
α-glucose OH
CH2OH
C
5
6
OH
O
H
C
4
glucose straight-chain form with C atoms numbered
C
1
6
H CH2OH
C
3
O
H
C
3
H
C
1
H
OH
C
2
OH
O
OH
H
or, more simply
OH
OH OH
β-glucose
OH
Figure 2.4 Structural formulae for the straight-chain and ring forms of glucose. Chemists often leave out the C and H atoms from the structural formula for simplicity.