Cambridge International AS Level Chemistry
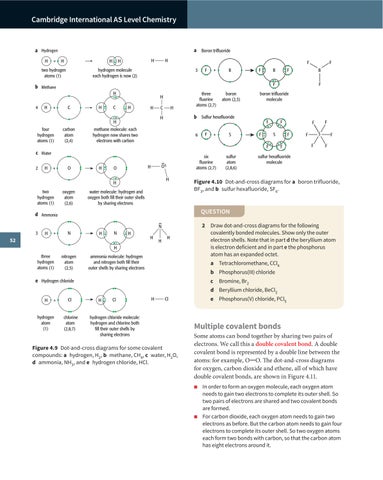
a Hydrogen H
+
a Boron trifluoride H
H
two hydrogen atoms (1)
H
H
H
F
hydrogen molecule each hydrogen is now (2)
3
F
+
B
F
B
H H
+
C
H
C
H H
H
carbon atom (2,4)
C
H
three fluorine atoms (2,7)
boron atom (2,3)
methane molecule: each hydrogen now shares two electrons with carbon
6
F
+
S
F
F S
F F
c Water 2
H
+
O
H
oxygen atom (2,6)
six fluorine atoms (2,7)
O
H
O
H
H two hydrogen atoms (1)
water molecule: hydrogen and oxygen both fill their outer shells by sharing electrons
+
N
H
N
H
52
F
S
F F
F F
sulfur hexafluoride molecule
2 Draw dot-and-cross diagrams for the following covalently bonded molecules. Show only the outer electron shells. Note that in part d the beryllium atom is electron deficient and in part e the phosphorus atom has an expanded octet.
H
H
H
H three hydrogen atoms (1)
F
QUESTION N
H
sulfur atom (2,8,6)
F
F
Figure 4.10 Dot-and-cross diagrams for a boron trifluoride, BF3, and b sulfur hexafluoride, SF6.
d Ammonia 3
F
boron trifluoride molecule
b Sulfur hexafluoride
H
H four hydrogen atoms (1)
B
F
b Methane
4
F
F
nitrogen atom (2,5)
ammonia molecule: hydrogen and nitrogen both fill their outer shells by sharing electrons
a Tetrachloromethane, CCl4 b Phosphorus(III) chloride c
e Hydrogen chloride
Bromine, Br2
d Beryllium chloride, BeCl2 H
+
hydrogen atom (1)
Cl
chlorine atom (2,8,7)
H
Cl
H
e Phosphorus(V) chloride, PCl5
Cl
hydrogen chloride molecule: hydrogen and chlorine both fill their outer shells by sharing electrons
Figure 4.9 Dot-and-cross diagrams for some covalent compounds: a hydrogen, H2, b methane, CH4, c water, H2O, d ammonia, NH3, and e hydrogen chloride, HCl.
Multiple covalent bonds
Some atoms can bond together by sharing two pairs of electrons. We call this a double covalent bond. A double covalent bond is represented by a double line between the atoms: for example, O O. The dot-and-cross diagrams for oxygen, carbon dioxide and ethene, all of which have double covalent bonds, are shown in Figure 4.11. ■
■
In order to form an oxygen molecule, each oxygen atom needs to gain two electrons to complete its outer shell. So two pairs of electrons are shared and two covalent bonds are formed. For carbon dioxide, each oxygen atom needs to gain two electrons as before. But the carbon atom needs to gain four electrons to complete its outer shell. So two oxygen atoms each form two bonds with carbon, so that the carbon atom has eight electrons around it.