



















Combined and Co-ordinated Sciences for Cambridge IGCSE™ MULTI-COMPONENT SAMPLE Executive Preview We are working with Cambridge Assessment International Education towards endorsement of these titles. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Dear Cambridge Teacher,
Our new edition of Cambridge IGCSE™ Combined and Co-ordinated Sciences will publish in spring 2023, updated for the revised Cambridge IGCSE Combined Science (0653) and the double award Cambridge IGCSE and IGCSE (9–1) Co-ordinated Sciences (0654/0973) for examination from 2025.
This Executive Preview contains sample content from the series, including:
• a guide explaining how to use the series
• a guide explaining how to use each resource
• the table of contents from each resource
• sample chapters from each resource, including the coursebook, each workbook and the teacher’s resource.
Our new edition of this popular series has been designed after extensive research interviews and lesson observations with teachers and students around the world. As a result, we have a fully integrated approach to differentiation across the series to ensure all learners in your classroom are engaged and supported.
All three of our workbooks employ a three-tier approach, which progresses from ‘focus’, to ‘practice’ and finally to ‘challenge’. This approach has been received warmly in our Cambridge IGCSE™ sciences series. We hope that you find this useful in tracking learner progress, and that your learners find this structured approach beneficial.
Our coursebook includes a new ‘Getting started’ feature, which enables you to evaluate learners’ prior knowledge and standardise it where possible before starting a new topic. Differentiation by outcome is also present throughout the coursebook, through questions, projects and much more.
The teacher’s resource has also been extensively updated to include differentiation opportunities. Each teaching activity includes suggestions for differentiation support, alongside differentiated worksheets and tests. The resource also includes teaching activity, assessment and homework ideas, tests for learners, guidance on how to tackle common misconceptions in each topic and a new feature developing your own teaching skills. We hope this time-saving resource will provide inspiration for your teaching and help you provide support for all your learners.
Visit our website to view the full series or speak to your local sales representative.
cambridge.org/education
With best wishes from the Cambridge team,
Carys Morley
Commissioning Editor for Cambridge IGCSE Combined and Co-ordinated Sciences Cambridge University Press
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Preview contents How to use this series 4 Coursebook sample 6 Contents 7 Introduction 10 How to use this book 12 B1 Biology 14 C7 Chemistry 22 P2 Physics 32 Biology Workbook sample 44 Contents 45 How to use this book 46 B1 Cells and organisms 47 Chemistry Workbook sample 52 Contents 53 How to use this book 54 C7 Acids, bases and salts 55 Physics Workbook sample 61 Contents 62 How to use this book 63 P2 Energy, work and power 64 Teacher’s Resource sample 71 Contents 72 How to use this Teacher’s Resource 74 B1 Biology 78 C7 Chemistry 97 P2 Physics 110 Preview contents 3 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
How to use this series
We offer a comprehensive, flexible array of resources for the Cambridge IGCSE™ Combined and Co-ordinated syllabuses. We provide targeted support and practice for the specific challenges we’ve heard that students face: learning science with English as a second language; structured learning for all; and developing practical skills.
The coursebook provides coverage of the full Cambridge IGCSE™ Combined and Co-ordinated syllabuses. Each chapter explains facts and concepts, and uses relevant realworld examples of scientific principles to bring the subject to life. Together with a focus on practical work and plenty of active learning opportunities, the coursebook prepares learners for all aspects of their scientific study. Questions and practice questions in every chapter help learners to consolidate their understanding and provide practice opportunities to apply their learning.


The teacher’s resource contains detailed guidance for all topics of the syllabuses, including common misconceptions identifying areas where learners might need extra support, as well as an engaging bank of lesson ideas for each syllabus topic. Differentiation is emphasised with advice for identification of different learner needs and suggestions of appropriate interventions to support and stretch learners. The teacher’s resource also contains support for preparing and carrying out all the investigations, including a set of sample results for when practicals aren’t possible.
The teacher’s resource also contains scaffolded worksheets and unit tests for each chapter. Answers for all components are accessible to teachers for free on the Cambridge GO platform.
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: COURSEBOOK 4
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Cambridge Assessment



Education towards endorsement of these resources.
We are working with SAMPLE
How to use this series 5
International
The skills-focused workbooks have been carefully constructed to help learners develop the skills that they need as they progress through their Cambridge IGCSE™ Combined and Co-ordinated Sciences course, providing further practice of some of the topics in the coursebook, each science with its own separate workbook. A three-tier, scaffolded approach to skills development enables students to gradually progress through ‘focus’, ‘practice’ and ‘challenge’ exercises, ensuring that every learner is supported. The workbooks enable independent learning and are ideal for use in class or as homework. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication.

We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Contents 7 Contents Acknowledgements viii Introduction x How to use this series xii How to use this book xiv Biology 1 B1 Cells and organisms B1.01 Characteristics of living organisms 2 B1.02 Cell structure 3 B1.03 Specialised cells 9 B1.04 Sizes of specimens 10 B2 Movement into and out of cells B2.01 Diffusion 17 B2.02 Osmosis 20 B2.03 Active transport 25 B3 Biological molecules B3.01 Carbohydrates, fats and proteins 31 B4 Enzymes B4.01 Biological catalysts 42 B4.02 Factors that affect enzymes 43 B5 Plant nutrition B5.01 Photosynthesis 54 B5.02 Leaves 58 B5.03 Factors affecting photosynthesis 61 B6 Human nutrition B6.01 Diet 76 B6.02 Digestive system 79 B6.03 Digestion 82 B7 Transport B7.01 Transport in plants 88 B7.02 Transport in animals 98 B8 Diseases and immunity B8.01 Pathogens and transmissible diseases 119 B8.02 The immune response 125 B9 Gas exchange and respiration B9.01 Gas exchange in humans 137 B9.02 Respiration 142 B10 Coordination and response B10.01 Coordination and response 150 B10.02 Hormones 154 B10.03 Homeostasis 156 B11 Reproduction B11.01 Asexual and sexual reproduction 169 B11.02 Sexual reproduction in plants 173 B11.03 Sexual reproduction in humans 178 B11.04 Sexually transmitted infections 183 B12 Inheritance B12.01 Chromosomes and genes 194 B12.02 Cell division 196 B12.03 Monohybrid inheritance 199 B13 Variation and selection B13.01 Variation 210 B13.02 Selection 212 B13.03 Drugs 217 B14 Organisms and their environment B14.01 Energy flow 225 B14.02 Food chains and food webs 225 B14.03 Carbon cycle 230 B15 Human influences on ecosystems B15.01 Habitat destruction 238 B15.02 Conservation 242 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: COURSEBOOK 8 Chemistry 251 C1 States of matter C1.01 Solids, liquids and gases 253 C1.02 Diffusion 262 C2 Atoms, elements and compounds C2.01 Elements, compounds and mixtures 275 C2.02 Atomic structure and the Periodic Table 276 C2.03 Isotopes 283 C2.04 Chemical bonding 284 C2.05 Ions and ionic bonds 285 C2.06 Simple molecules and covalent bonds 287 C2.07 Giant covalent structures 293 C2.08 Metallic bonding 294 C3 Stoichiometry C3.01 Chemical formulae and equations 303 C3.02 Relative masses of atoms and molecules 311 C3.03 The mole and the Avogadro constant 315 C4 Electrochemistry C4.01 Electrolysis 328 C4.02 Hydrogen–oxygen fuel cells 335 C5 Chemical energetics C5.01 Exothermic and endothermic reactions 345 C6 Chemical reactions C6.01 Physical and chemical changes 355 C6.02 Rate of reaction 356 C6.03 Redox reactions 365 C7 Acids, bases and salts C7.01 The characteristic properties of acids and bases 377 C7.02 Oxides 383 C7.03 Preparation of salts 385 C8 The Periodic Table C8.01 Arrangement of elements 399 C8.02 Group I properties 402 C8.03 Group VII properties 403 C8.04 Transition elements 405 C8.05 The noble gases 406 C9 Metals C9.01 Properties of metals 412 C9.02 Uses of metals 415 C9.03 Alloys and their properties 417 C9.04 Reactivity series 419 C9.05 Corrosion of metals 424 C9.06 Extraction of metals 427 C10 Chemistry of the environment C10.01 Air quality and climate 439 C10.02 Water 444 C11 Organic chemistry C11.01 Formulas and technology 453 C11.02 Naming organic compounds 455 C11.03 Alkanes 456 C11.04 Alkenes 457 C11.05 Alcohols 462 C11.06 Fuels 464 C11.07 Polymers 467 C12 Experimental techniques and chemical analysis C12.01 Experimental design 480 C12.02 Separation and purification 484 C12.03 Chromatography 490 C12.04 Identification of ions and gases 494 C12.05 Acid–base titrations 501 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Contents 9 Physics 509 P1 Motion P1.01 Measuring length and volume 511 P1.02 Density 514 P1.03 Measuring time 517 P1.04 Understanding speed 519 P1.05 Understanding acceleration 524 P1.06 Mass, weight and gravity 529 P1.07 Forces 531 P1.08 Force, mass and acceleration 534 P1.09 Stretching springs 536 P1.10 Turning forces 539 P1.11 Pressure 546 P2 Energy, work and power P2.01 Energy stores, transfers and conservation 556 P2.02 Energy calculations 560 P2.03 Energy resources 562 P2.04 Doing work 570 P2.05 Power 574 P3 Thermal physics P3.01 Kinetic particle model of matter 584 P3.02 Pressure changes 588 P3.03 Matter and thermal properties 589 P3.04 Thermal processes 595 P4 Properties of waves P4.01 General wave properties 613 P4.02 Light 619 P4.03 Electromagnetic spectrum 636 P4.04 Sound 640 P5 Electricity and magnetism P5.01 Simple phenomena of magnetism 656 P5.02 Electric charge 659 P5.03 Current, voltage and resistance 663 P5.04 Electrical energy and electrical power 671 P6 Electric circuits P6.01 Describing circuits 681 P6.02 Resistors 683 P6.03 Circuit calculations 685 P6.04 Electrical safety 688 P7 Electromagnetic effects P7.01 Magnetic effect of current 700 P7.02 Force on a currentcarrying conductor 701 P7.03 The d.c. motor 702 P7.04 Electromagnetic induction 704 P7.05 The a.c. generator 706 P7.06 The transformer 708 P8 Nuclear physics P8.01 The nuclear atom 723 P8.02 Radioactivity 726 P9 Space physics P9.01 The Solar System 743 P9.02 The Sun as a star 743 P9.03 The life cycle of stars 746 P9.04 Galaxies and the Universe 748 Glossary 753 Index 775
Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
We are working with Cambridge Assessment International Education towards endorsement of these resources.
This book covers two syllabuses: Cambridge IGCSE Combined Science (0653) and the double award Cambridge IGCSE (9-1) Co-ordinated Sciences (0654/0973). We hope that you enjoy using it.
All the biology topics come first, followed by chemistry and then physics. However, you almost certainly won’t follow this sequence in your lessons. Where possible, the book follows the order of topics in the syllabus. Some topics have been merged or moved where concepts are closely related. You will probably find that you study biology, chemistry and physics alongside each other, so you will use different parts of the book in different lessons.
Core and Supplement
Your teacher will tell you whether you are studying:
• Cambridge IGCSE Combined Science (0653) or Cambridge IGCSE (9-1) Co-ordinated Sciences (Double Award) (0654/0973)
• only the Core part of the syllabus, or the Supplement as well.
Cambridge IGCSE Combined Science (0653) is a single award syllabus. This means that your final papers are the equivalent of one IGCSE subject. Cambridge IGCSE (9-1) Co-ordinated Sciences (0654/0973) is a double award syllabus. In this case, your final papers are the equivalent of two IGCSE subjects.
If you study 0654 Core only, you will be entered for Papers 1 (Multiple Choice (Core)) and 3 (Theory (Core)) and either Paper 5 (Practical Test) or 6 (Alternative to Practical). If you also study the Supplement, you may be entered for Papers 2 (Multiple Choice (Extended)) and 4 (Theory (Extended)), and either Paper 5 (Practical Test) or 6 (Alternative to Practical).
There are sidebars in the margins of the coursebook to show which material relates to each syllabus and paper. If there is no sidebar, it means that everyone will study this material. Use this table to ensure that you study the right material for your syllabus and paper:
You will study the material:
Without a sidebar
You will study the material:
Without a sidebar
With a dashed blue sidebar
With a dashed black sidebar
You will not study material with a solid blue sidebar or a solid black sidebar.
You will study the material:
Without a sidebar
With a solid blue sidebar
With a dashed black sidebar
You will not study material with a solid black sidebar or a dashed blue sidebar.
You will study everything
You do not need to pay attention to sidebars.
A simplified table has also been included on the inside back flap of this coursebook to open out and view alongside the exercises.
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: COURSEBOOK 10
Introduction
Cambridge IGCSE Combined Science (0653)
Cambridge IGCSE Co-ordinated Sciences (0654) Core Supplement Core Supplement
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Questions
Each chapter has several sets of questions within it. Most of these require quite short answers and simply test if you have understood what you have just read or what you have just been taught.
At the end of each chapter, there are some longer questions testing a range of material from the chapter. Some of these are written by the authors and are similar in style to Cambridge questions.
Activities
Just learning your work and remembering it is not enough to make sure that you achieve your best result in your exam. You also need to be able to use what you’ve learned in unfamiliar contexts (AO2) and to demonstrate your experimental skills (AO3).
Each chapter contains activities. These will help you to develop the practical skills you will need in your course.
There are two possible papers aimed at testing your practical skills, Paper 5 and Paper 6 (Practical Test and Alternative to Practical, respectively). Your teacher will tell you which of these you will be entered for. You should try to do the activities in this coursebook no matter which of these papers you are entered for.
Summary
At the end of each chapter, there is a short list of the main points covered in the chapter. Remember, though, that these are only very short summaries and you will need to know more detail than this for your course.
Projects
You will find a project at the end of every chapter, which gives you the opportunity to work in groups, exercise your creativity, and develop your research and critical thinking skills.
Workbooks
There are three workbooks to go with this coursebook – one for each science. If you have the workbooks, you will find them really helpful in developing your skills, such as handling information and solving problems, as well as some of the practical skills.
Introduction 11
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
We are working with Cambridge Assessment International Education towards endorsement of these resources.
How to use this book
Throughout this book, you will notice lots of different features that will help your learning. These are explained below.
LEARNING INTENTIONS
These set the scene for each chapter, help with navigation through the coursebook and indicate the important concepts in each topic. They will begin with the header ‘In this chapter you will:’ and will list the key topics of the chapter for all students.
IN THIS CHAPTER YOU WILL:
• learn about the seven characteristics of living organisms
• find out how the binomial system is used to name organisms
BEFORE YOU START
This contains questions and activities on subject knowledge you will need before starting this chapter.
EXPERIMENTAL SKILLS
This feature focuses on developing your practical skills. They include lists of equipment required and any safety issues, step-by-step instructions so you can carry out the experiment, and questions to help you think about what you have learnt.
ACTIVITY
Activities give you an opportunity to check and develop your understanding throughout the text in a more active way, for example by creating presentations, posters or role plays. Where activities have answers, teachers can find these for free on the Cambridge GO site.
SELF/PEER ASSESSMENT
At the end of some activities and experimental skills boxes, you will find opportunities to help you assess your own work, or that of your classmates, and consider how you can improve the way you learn.
Questions
Appearing throughout the text, questions give you a chance to check that you have understood the topic you have just read about. The answers to these questions are accessible to teachers for free on the Cambridge GO site.
KEY WORDS
Key vocabulary is highlighted in the text when it is first introduced, and definitions are given in boxes near the vocabulary. You will also find definitions of these words in the Glossary at the back of this book.
COMMAND WORDS
Command words that appear in the syllabus and might be used in exams are highlighted in the practice questions. In the margin, you will find the Cambridge International definition. You will also find these definitions in the Glossary at the back of the book with some further explanation on the meaning of these words.
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: COURSEBOOK 12
Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
TIP
These contain advice to help you avoid common misconceptions and provide support for answering questions.
REFLECTION
These activities ask you to think about the approach that you take to your work, and how you might improve this in the future.
SUMMARY
There is a summary of key points at the end of each chapter.
PRACTICE QUESTIONS
WORKED EXAMPLES
Worked examples are used to demonstrate the steps you should take to answer a specific type of question. They are followed by opportunities for you to practise the techniques for yourself.
These boxes tell you where information in the book is extension content, and is not part of the syllabus.
Questions at the end of each chapter provide more demanding practice questions, some of which may require use of knowledge from previous chapters. The answers to these questions are accessible to teachers for free on the Cambridge GO site.
SELF-EVALUATION CHECKLIST
The summary checklists are followed by ‘I can’ statements which relate to the Learning intentions at the beginning of the chapter. You might find it helpful to rate how confident you are for each of these statements when you are revising. You should revisit any topics that you rated ‘Needs more work’ or ‘Almost there’.
13
I can See topic . . . Needs more work Almost there Ready to move on
How to use this book We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Cells and organisms

IN THIS CHAPTER YOU WILL:
• learn about the seven characteristics of living organisms
• find out about the structure of the cells of bacteria, plants and animals
• learn about the functions of each of the cells of bacteria, plants and animals
• identify cell structures in diagrams
• describe how the structures of some specialised cells are related to their functions state that new cells are produced by division of existing cells describe the meaning of the terms cell, tissue, organ, organ system and organism practise using the magnification equation
• convert measurements between millimetres (mm) and micrometres (μm).
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
BEFORE YOU START

Different types of organisms have different kinds of cells. With a partner, think about the answers to these questions:

1 The list below contains some features of living organisms. With a partner, discuss which of these features are found in all living organisms.










breathing excretion a blood system a nervous system sensitivity growth reproduction movement nutrition respiration

























2 If you have a microscope, how can you distinguish between a cell from a plant, and a cell from an animal?





























B1.01 Characteristics of living organisms









Biology is the study of organisms. An organism is a complete living thing – such as yourself, a platypus, a bacterium or a mango tree. There are very many different kinds of organism










Growth: All organisms begin small and get larger, by the growth of their cells and by adding new cells to their bodies. Growth can be defined as a permanent increase in size or dry mass. Measuring dry mass involves killing and drying the organism (so this method is more often used for plants than for animals). The mass of its body without any water is then found.
on Earth, but all of them share seven characteristics (Figure B1.01). Some non-living things have some of these characteristics, but no non-living thing has all of them.



Movement: All organisms are able to move to some extent. Most animals can move their whole body from place to place, and plants can slowly move parts of themselves.


Sensitivity: All organisms pick up information about changes in their environment, and react to the changes. These changes may be in the internal environment (such as the temperature of the blood) or the external environment (such as the intensity of sunlight).


















































































































Excretion: All organisms produce unwanted or toxic waste products as a result of their metabolic reactions, and these must be removed from the body.
Reproduction: Organisms are able to make new organisms of the same species as themselves.


Nutrition: Organisms take substances from their environment and use them to provide energy or materials to make new cells.
Respiration: All organisms break down glucose and other substances inside their cells, to release energy that they can use. Organisms use the energy that they obtain from respiration to make other chemical reactions in their cells happen. All of these chemical reactions together –including respiration – are called metabolism.

Figure B1.01: Characteristics of living organisms.






























B1 Cells and organisms 15
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
SCIENCE IN CONTEXT B1.01
How many cells in a human body?
In some organisms, it is possible to count the number of cells in their bodies. In organisms such as humans, we can only estimate numbers. It would be impossible to count them all. Cells differ in their volume, mass and how closely or loosely they are packed together.
Scientists recently calculated the numbers of different cells and cell types in different organs and added them up.
KEY WORD
organism: a living thing.
ACTIVITY B1.01
Matching the characteristics of living things with their descriptions
Work in a group of four or five for this activity.
You will need:
• 14 pieces of blank card, all exactly the same.
1 Write the seven characteristics of living things on seven of the pieces of card.
2 Write descriptions of each of the seven characteristics on the other seven cards.
3 Shuffle each set of cards. Place them face down in
This latest estimate suggests that the body of an adult human contains around 37 trillion cells – that is 37 000 000 000 000.
Discussion question
1 Can you think of any advantages for larger organisms of consisting of multiple cells, rather than just one larger cell?
Questions
B1.01 A student claimed that plants show fewer of the characteristics of living things than animals. Explain why this claim is wrong.
B1.02 Consider two organisms. For each, identify:
a the characteristics of living things that the organism carries out all the time

b the characteristics of living things that only happen at certain times.
B1.02 Cell structure
All organisms are made of cells. Cells are very small, so large organisms contain millions of cells. They are multicellular. Some organisms are unicellular, which means that they are made of just a single cell. Bacteria and yeast are examples of single-celled organisms.
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: COURSEBOOK
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Microscopes


To see cells clearly, you need to use a microscope (Figure B1.02). The kind of microscope used in a school laboratory is called a light microscope. This is because it shines light through the piece of animal or plant you are looking at. It uses glass lenses to magnify and focus the image. A very good light microscope can magnify about
1500 times, so that all the structures in Figures B1.03 and B1.04 can be seen.
A photograph taken using a light microscope is called a photomicrograph. Figure B1.05 is a photomicrograph of some animal cells, and Figure B1.06 is a photomicrograph of some plant cells.

eyepiece focusing knob stage, where the specimen is placed
objective lenses
mirror to reflect light up through the specimen
B1 Cells and organisms 17
cell membrane cytoplasm nucleus ribosomes small
or vesicle mitochondria
Figure B1.02: A light microscope.
vacuole
cell
cell
mitochondria
Figure B1.03: An animal cell as it appears through a light microscope.
cytoplasm
wall
membrane nucleus
chloroplast vacuole ribosomes
cell
nucleus
Figure B1.04: A plant cell as it appears through a light microscope.
membrane cytoplasm
Figure B1.05: These are cells from the trachea (windpipe) of a mammal. They have been stained (coloured) with a dye that makes the nucleus look darker than the cytoplasm.
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
ACTIVITY B1.02
Comparing animal cells and plant cells
Work in a group of three or four for this activity. You are going to make a display to compare the structures of animal cells and plant cells. Decide how you will do this. You could perhaps use annotated drawings, construct a large comparison table, or make a presentation.
You can use the information in this chapter to make your comparison. You might also like to look for some more pictures on the internet.
Bacterial cells
Bacteria (singular: bacterium) are unicellular organisms. Bacterial cells are rather different from the cells of animals and plants. Figure B1.12 is a diagram of a bacterium.
cell wall made of peptidoglycan, not cellulose
cytoplasm
Figure B1.12: A bacterial cell.
Bacterial cells always have a cell wall. Unlike plant cells, this cell wall is not made of cellulose.
But the function is the same as in plant cells – the bacterial cell wall helps to support the cell, and prevents it from bursting if the cell takes up a lot of water.
A partially permeable cell membrane is pressed tightly against the inside of the bacterial cell wall.
As in plant and animal cells, the cell membrane controls what enters and leaves the cell.
Bacterial cells have cytoplasm and ribosomes.
These have the same functions as in animal and plant cells.
KEY WORD
bacteria: unicellular organisms whose cells do not contain a nucleus.
Bacterial cells do not have mitochondria or chloroplasts. The most important difference between a bacterial cell and animal or plant cells is that bacteria do not have a nucleus. Bacterial cells are also known as prokaryotic cells. ‘Pro’ means ‘before’, and ‘karyotic’ means ‘nucleus’. Prokaryotic cells appeared on Earth millions of years before cells with nuclei appeared.
Instead of chromosomes inside a nucleus, bacteria have a circle of DNA. This is sometimes called a bacterial chromosome.
The DNA has exactly the same function as in other cells – it provides instructions for making proteins.
Bacterial cells often have one or more smaller circles of DNA, called plasmids
Scientists can use plasmids in the genetic modification of cells and organisms.
KEY WORDS
prokaryotic cells: cells with no nucleus; bacteria have prokaryotic cells. plasmids: small, circular molecules of DNA, found in many prokaryotic cells in addition to the main, much larger circle of DNA.
Question
B1.07 Construct a table to compare the structure of a bacterial cell with animal and plant cells. Remember to include similarities as well as differences.
REFLECTION
How will you try to learn the names of the parts of animal, plant and bacterial cells, and their functions? Think about which of these ideas might work for you:
• looking at diagrams and reading about the structures
• practising drawing your own diagrams and labelling them
• getting a friend to test you by asking questions
• making some revision cards for yourself, with the name of a structure on one side and its function on the other side.
What other ideas might you try?
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: COURSEBOOK 18
circular DNA
plasmids cell
ribosomes
membrane
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CONTINUED
2 Make a magnified drawing of your objects. Calculate the magnification of each one and write it next to your drawings. Try to use different magnifications for each object. (You could even try drawing some objects smaller than they really are, so that the magnification is less than 1.)
Part 2
Work with a partner.
3 Exchange your drawings with a partner. Each of you now uses the drawings and magnifications to work out the size of the actual object.
4 Check your answers with your partner. Did you correctly calculate the actual sizes of the objects they had drawn? Did your partner calculate the actual sizes of the objects that you had drawn?
Self assessment
Did you calculate the magnifications of your drawings correctly, so that your partner could work out the actual size of each drawing?
Reflection
If not, where did you go wrong? Were you able to calculate the actual size of the objects your partner had drawn? If not, where did you (or they) go wrong?
When you carried out your calculations, did you make any mistakes? What could you do to avoid making those mistakes next time? What can you do to help you to remember the equation and how to use it?
PROJECT B1.01 THE CHARACTERISTICS OF A NEW SPECIES
Each year, biologists discover new species. Some of these are small (e.g. insects, small plants) while others are surprisingly large (e.g. mammals, trees).


Work in a group of three or four. Use the internet to search for some examples of newly discovered species and select one to research in detail. Try to find one that has unusual features, or which was discovered in an extreme or hard-to-reach environment.
Decide how you will share the results of your research with others. For example, you could give an illustrated talk, or produce a poster. Decide how you will share out the tasks between you.
Try to find information about some or all of these issues:
• How did the scientists know that the species represents a living organism?
How are some of the features of the seven characteristics of life of these species unusual compared to previously identified species?
Where and how was the new species discovered?
Why had it not been discovered before?
Is anything known about the structure of its cells and how they are specialised?
Biologists will want to find out more about the new species. However, if it is rare they will not want to take many specimens from the wild, or disturb it in its habitat. How have these conflicts been resolved?
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Figure B1.14: The tree Dinizia jueirana-facao is a species that was recently discovered in Brazil.
SUMMARY
All organisms show seven characteristics: movement, respiration, sensitivity, growth, reproduction, excretion and nutrition.
All organisms are made of cells. New cells are always made by the division of existing cells.
Animal and plant cells contain cytoplasm, a cell membrane, ribosomes, mitochondria and a nucleus containing DNA in the form of chromosomes. Plant cells also contain a cell wall, a large vacuole and sometimes chloroplasts.
Bacterial cells have cytoplasm, a cell membrane, a cell wall and ribosomes. They do not have a nucleus. Their DNA is circular. They may have extra, small circles of DNA called plasmids.
The cell membrane of all cells is partially permeable and controls what enters and leaves the cell. The cell wall is fully permeable and allows all molecules and ions to pass through it.
Ribosomes are the site of protein synthesis in a cell.
Mitochondria release energy from glucose and other nutrients, by aerobic respiration.
Chloroplasts are the site of photosynthesis.
Cells may be specialised for specific functions.
Magnification can be calculated using the equation magnification= size of image size of actual object
Conversion from millimeters, to micrometers, μm.
PRACTICE QUESTIONS
1 Identify the characteristic which is not shown by all living organisms.
A excretion
B movement
C photosynthesis
D respiration [1]
2 The tongue-eating louse is a parasite of large fish. As its name suggests, it has an unusual way of gaining nutrition. It detects chemicals on the gills of a fish. From the gills it crawls into the fish’s mouth. First, the louse sucks blood from the tongue. This causes the tongue to shrink and eventually fall off. Next, the louse takes the place of the tongue. Here the louse remains, feeding on food that the fish takes into its mouth.
a Define nutrition. [2]
b Apart from nutrition, explain how two features of the louse are characteristics of all living organisms. [2]
c The tongue-eating louse can reproduce.
i Define reproduction. [2]
ii Suggest one reason why scientists know very little about the process of reproduction in this species. [1]
[Total: 7]
COMMAND WORDS
identify: name/select/ recognise.
define: give a precise meaning.
explain: set out purposes or reasons/make the relationships between things clear/say why and/ or how and support with relevant evidence.
TIP
The mark allocation for a question will often indicate the number of points that you should make, in order to fully answer that question.
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: COURSEBOOK 20
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CONTINUED
6 This is an electron micrograph of a small part of a cell from the pancreas. This cell makes large quantities of protein molecules, which are stored in vesicles before being exported from the cell.

vesicle containing protein molecules made by the cell
a Structure A contains molecules that determine the type of proteins made in the cell. Identify structure A. [1]
b Use the information above to explain why the cell has large numbers of structures B and C. [6]
c State one way you can tell that these are not bacterial cells. [1]
[Total: 8]
SELF-EVALUATION CHECKLIST
After studying this chapter, think about how confident you are with the different topics. This will help you to see any gaps in your knowledge and help you to learn more effectively.
I can See topic . . . Needs more work Almost there Confident to move on define the seven characteristics of living organisms B1.01 describe how all organisms are made of cells, and new cells are always made by the division of existing cells B1.02
describe and compare the structure of a bacterial cell, an animal cell, and a plant cell B1.02
describe the functions of each of the structures found in an animal cell and a plant cell B1.02
B1 Cells and organisms 21
B A C
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
C7 Acids, bases and salts
IN THIS CHAPTER YOU WILL:
• describe acids and bases in terms of their effect on indicators
• describe the characteristic reactions of acids
• compare the relative acidity or alkalinity or pH of a solution using universal indicator
• describe how acids and alkalis react together in neutralisation reactions
• learn that all metal oxides and hydroxides can act as bases, while many oxides of non-metals can be classified as acidic oxides
• learn that some metal oxides (amphoteric oxides) can react with both acids and alkalis
• understand that salts are an important group of ionic compounds
• learn that some salts are soluble in water, while others are insoluble
• describe the preparation, separation and purification of soluble salts by reaction of the parent acid with either excess metal, excess insoluble base or excess insoluble carbonate
• describe the preparation, separation and purification of a soluble salt by titration of an acid with an alkali

• describe the preparation of an insoluble salt by precipitation
• describe a hydrated substance as a substance that is chemically combined with water and an anhydrous substance as a substance containing no water.
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
C7.01 The characteristic properties of acids and bases
What is an acid?
Vinegar, lemon juice, grapefruit juice and spoilt milk are all sour tasting because of the presence of acids (Figure C7.02). Carbonic acid from carbon dioxide dissolved in water is present in soft fizzy drinks. The acids present in these circumstances are weak and dilute. But taste is not a test that should be tried. Some acids would be dangerous, even deadly, to taste!
colour if they are put into an acid or alkaline solution. Three commonly used indicators are litmus, methyl orange and universal indicator.

Litmus and methyl orange
Litmus is extracted from lichens and is purple in neutral solution. When it is added to an acidic solution, litmus turns red. This colour change is the result of a chemical reaction. Substances with the opposite chemical effect to acids are needed to reverse the change, and these are called bases. Bases turn litmus solution blue.
You can also use litmus paper. This is paper that has been soaked in litmus solution. It is available in blue and red forms.
KEY WORDS
acid: a solution of an acid turns litmus red and has a pH below 7.
corrosive: a corrosive substance (e.g. an acid) is one that can dissolve or ‘eat away’ at other materials (e.g. wood, metals or human skin).
indicator: a substance that changes colour when added to acidic or alkaline solutions, e.g. litmus or phenolphthalein.
litmus: the most common indicator; turns red in acid and blue in alkali.
A number of acids are also corrosive. They can eat their way through clothing, are dangerous on the skin, and some are able to attack stonework and metals. Table C7.01 shows how common acids occur.
Indicators
The easiest way to detect whether a solution is acidic or not is to use an indicator. Indicators are substances that change
methyl orange: an indicator which is red in acid and yellow in alkali.
universal indicator: a mixture of indicators that has different colours in solutions of different pH. base: a substance that neutralises an acid, producing a salt and water as the only products.
Table
C7 Acids, bases and salts 23
Figure C7.02: Citrus fruits have an ‘acidic’ sharp taste.
Type Name Formula Strong or weak? Where found or used Organic acids ethanoic acid CH3COOH weak in vinegar methanoic acid HCOOH weak in ant and nettle stings; used in kettle descaler
acid CH3CH(OH)CO2H weak in sour milk
acid C6H8O7 weak in lemons, oranges and other citrus fruits
acids carbonic acid H2CO3 weak in fizzy soft drinks
acid HCl strong used in cleaning metal surfaces; found as the dilute acid in the stomach
acid HNO3 strong used in making fertilisers and explosives
acid H2SO4 strong in car batteries; used in making fertilisers, paints and detergents
acid H3PO4 strong in anti-rust paint; used in making fertilisers
lactic
citric
Mineral
hydrochloric
nitric
sulfuric
phosphoric
C7.01:
common acids. We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Some
The blue form of litmus paper changes colour to red when dipped into acid solutions. Red litmus paper turns blue in alkaline solutions. Alkalis are soluble bases. Note that litmus only gives a single colour change.
Figure C7.03 shows a simple visual memory aid to help you to remember the colour change that litmus shows for acids and bases.
The presence of water is very important in the action of acids and bases. One practical consequence of this is that, when we use litmus paper to test gases, it must always be damp. The gas needs to dissolve in the moisture to bring about the colour change. This is important in your practical work.
Another frequently used indicator is methyl orange. This gives a different colour change from litmus (Table C7.02) and the colour changes are sometimes easier to detect than for litmus.
Indicator Colour in acid Neutral colour Colour in alkali

litmus red purple blue
methyl orange red orange yellow
Table C7.02: Some common indicator colour changes.
Universal indicator
KEY WORDS
alkali: a soluble base; a solution of an alkali turns litmus blue and has a pH above 7. soluble: a solute that dissolves in a particular solvent. pH
Another commonly used indicator is universal indicator (or full-range indicator). It is a mixture of indicator dyes. Such an indicator is useful because it gives a range of colours (a ‘spectrum’) depending on the relative strength of the acid or alkali added (Figure C7.04). When you use universal indicator paper, you see that solutions of different acids produce different colours depending on their relative acidity. Solutions of the same acid with different concentrations will also give different colours.
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: COURSEBOOK 24
ac i d / b ase r e l u e
Figure C7.03: The colour change of litmus in acid (red) and base (blue).
14131211109876543210 redorangeyellowgreenblue violet strongly acidicweakly acidicneutralweakly alkalinestrongly alkaline
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Figure C7.04: How the colour of universal indicator changes in solutions of different pH values.
The more acidic solutions (e.g. battery acid) turn universal indicator bright red. A less acidic solution (e.g. vinegar) will only turn universal indicator orange–yellow. There are also colour differences produced with different alkali solutions. The most alkaline solutions give a violet colour.
The pH scale
The most useful measure of the relative strength of an acid or alkaline solution is the pH scale. The scale runs from 1 to 14 (Figure C7.04), and the following general rules apply:
• Acids have a pH less than 7; the more acidic a solution, the lower the pH.
• Neutral substances, such as pure water, have a pH of 7.
• Bases have a pH greater than 7; the more basic a solution the higher the pH.
The pH of a solution can be measured in several ways including using universal indicator or a pH meter.
It is very important to remember that the ‘reference point’ when measuring pH is neutrality, pH 7 – the mid-point of the scale. Therefore:
• As we move down from pH 7, the solution is getting more acidic.
• Moving up from pH 7, the solution is getting more alkaline (Table C7.03).
Questions
C7.01 a What do you understand by the word corrosive?
b What acid is present in orange or lemon juice?
c What acid is present in vinegar?
C7.02 a Methyl orange is an indicator. What does this mean?
b Is a solution acidic, alkaline or neutral if its pH is:
i 11?
ii 7?
iii 8?
iv 3?
C7.03 Which solution is more acidic: an acid with a pH of 4 or an acid with a pH of 1?
Bases and neutralisation
When investigated, it was found that all metal oxides and hydroxides would neutralise acids. These substances are known as bases. These bases all react in the same way with acids. A base will neutralise an acid, and in the process a salt is formed. This type of reaction is known as a neutralisation reaction. It can be summed up in a general equation:
acid + base → salt + water
Most bases are insoluble in water. This makes the few bases that do dissolve in water more significant. They are given a special name: alkalis. The common alkalis are shown in Table C7.04.
KEY WORDS
pH scale: a scale running from 0 to 14, used for expressing the acidity or alkalinity of a solution; a neutral solution has a pH of 7.
salt: ionic compound made by the neutralisation of an acid with a base (or alkali), e.g. copper(II) sulfate and potassium nitrate.
neutralisation: a chemical reaction between an acid and a base to produce a salt and water only.
insoluble: a substance that does not dissolve in a particular solvent.
Table C7.03: The pH values of some common solutions.
C7 Acids, bases and salts 25
Substance pH strongly acidic hydrochloric acid (HCl) 0.0 gastric juices 1.0 lemon juice 2.5 vinegar 3.0 acid rain 4.4 rainwater 5.6 urine 6.0 weakly acidic milk 6.5 NEUTRAL pure water, sugar solution 7.0 weakly alkaline blood 7.4 baking soda solution 7.5 toothpaste 9.0 limewater 12.4 household ammonia 13.0
alkaline sodium hydroxide (NaOH) 14.0
strongly
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
yellow
red methyl orange
blue thymolphthalein colourless
This titration method is very useful not only for preparing salts but also for finding the concentration of a particular acid or alkali solution (see Chapter C12).

EXPERIMENTAL SKILLS C7.02
Quick and easy copper(II) sulfate crystals
This activity is an adaptation of the larger-scale method of preparing a soluble salt (see Figure C7.14). The essential feature of this method is that it is easier to carry out than the method involving multiple stages. The method uses a reaction between a dilute solution of the parent acid (sulfuric acid in this case) and an excess of an insoluble base.
You will need:
• measuring cylinder (25 cm3)
• boiling tube
• sulfuric acid (2 mol/dm3)
• beaker (250 cm3)
• copper(II) oxide
• filter funnel and filter paper
• conical flask (100 cm3)
• crystallising dish
• Bunsen burner
• tripod and gauze
• heat-resistant mat
• kettle
• balance.
Safety
Wear eye protection throughout. Be careful with chemicals. Never ingest them and always wash your hands after handling them. Note that sulfuric acid is corrosive and an irritant at the concentration used.
Getting started
It is important that you are well organised and have all the apparatus and chemicals that you need before starting the experiment. You should be familiar with the techniques of filtration and crystallisation (Chapter C12). You may find it helpful to set up the filtration apparatus before starting.
Method
1 Pour 15 cm3 of 2 mol/dm3 sulfuric acid into a boiling tube.
2 Place the tube in a beaker half-filled with boiling water from a kettle.
3 Weigh out between 1.8 g and 2.0 g of copper(II) oxide.
4 Add half the copper(II) oxide to the acid in the boiling tube. Shake the boiling tube and return it to the hot water.
5 When the solid has dissolved, add the remaining portion of copper(II) oxide.
6 Keep the tube in the hot water for five more minutes, taking it out occasionally to shake the tube.
7 Filter off the unreacted solid, collecting the clear blue solution in a 100 cm3 conical flask. A fluted filter paper can be used to speed up the filtration.
8 Set up the Bunsen burner and boil the solution for two to three minutes.
9 Pour the hot solution into a clean, dry crystallising dish and watch the crystals grow!
Questions
1 Write word and balanced chemical equations for the reaction taking place.
2 What does the fact that there is some unreacted solid left after the reaction tell you about the proportions of reactants used? Why is it useful that the reaction is carried out with these proportions?
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: COURSEBOOK 26
alkaliacid
Add acid until the colour just changes. a b
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Figure C7.18 a: Colour changes for the indicators methyl orange and thymolphthalein during the titration. b: Actual colours of methyl orange in acid and alkali.
CONTINUED: C7.02
Peer assessment
The practical work to prepare copper(II) sulfate crystals requires careful and coordinated work with your partner. When you have completed the experiment, discuss with your partner how efficiently you worked together. Think about the following questions:
Reflection
Do you find carrying out practical work a useful way of learning aspects of chemistry?
Questions
C7.12 Name the salts formed when:
a dilute hydrochloric acid reacts with magnesium
b calcium oxide reacts with dilute nitric acid
c zinc carbonate reacts with dilute sulfuric acid.
C7.13 a In the methods of preparing a salt using a solid metal, base or carbonate, why is the solid used in excess?
b In such methods, how is the excess solid removed once the reaction has finished?
c Name the two important pieces of graduated glassware used in the titration method of preparing a salt.
d What colour is the indicator methyl orange in alkali?
e Why should the crystals prepared at the end of experiments to prepare salts not be heated too strongly when drying them?
C7.14 There are two methods of preparing soluble salts depending on the solubility of the reagent reacted with the acid: method A (titration using a burette and an indicator) and method B (addition of an excess of a base or a metal to a dilute acid and removal of the excess solid).
a Which method, A or B, would you use to prepare the soluble salt, zinc sulfate, from the insoluble base, zinc oxide?
b Write down the reagent to use.
c Write the word equation.
• Were you able to handle the manipulation of the reaction, filtration and crystallisation stages confidently?
• Did you share the work equally between you?
• Did carrying out the experiment help you to understand the importance of the different steps involved in preparing a soluble salt?
Is there a way in which you could gain more from performing experiments?
Preparing insoluble salts
The reaction between marble chips (calcium carbonate) and sulfuric acid would be expected to produce a strong reaction, with large amounts of carbon dioxide being given off. However, the reaction quickly stops after a very short time. This is caused by the fact that calcium sulfate is insoluble. This insoluble calcium sulfate soon forms a layer on the surface of the marble chips, stopping any further reaction. This reaction emphasises that some salts are insoluble in water, e.g. silver chloride and barium sulfate. Such salts cannot be made by the crystallisation methods we have described earlier. They are generally made by precipitation.
For example, barium sulfate can be made by taking a solution of a soluble sulfate (e.g. sodium sulfate). This is added to a solution of a soluble barium salt (e.g. barium chloride). The insoluble white solid, barium sulfate, is formed immediately. This solid ‘falls’ to the bottom of the tube or beaker as a precipitate (Figure C7.19). The precipitate can be filtered off. It is then washed with distilled water and dried in a warm oven.
The equation for this reaction is:
barium chloride + sodium sulfate → barium sulfate + sodium chloride
BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl(aq)
This equation shows the importance of state symbols. It is the only way we can tell that this equation shows a precipitation.
KEY WORD
precipitation: the sudden formation of a solid when either two solutions are mixed or a gas is bubbled into a solution.
C7 Acids, bases and salts 27
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Questions
C7.15 What do the following terms mean in connection with chemical reactions to produce salts?
barium chloride solution
Cl– Cl–
Na+ Na+ BaSO4(s)
Ba2+ SO42–Na+ Na+ Cl–Cl–
sodium sulfate solution
Ba2+ and SO42– ions combine to form a precipitate of BaSO4; the Na+ and Cl–ions stay in solution.
a precipitation b titration
c an ionic equation
C7.16 Which of these salts can be prepared by precipitation?
A silver iodide
B magnesium nitrate
C lead(II) chloride
C7.17 The insoluble salt, barium sulfate, can be prepared from a solution of barium nitrate by precipitation.
a Name a soluble salt that could be added to the barium nitrate solution to give a precipitate of barium sulfate.
b What colour is the precipitate of barium sulfate?
PROJECT C7.01 STOP-START GO: WHEN IONS COLLIDE!
‘Stop-motion’ animations are a popular tool for helping to explain concepts that involve movement. The animations are created by linking a series of individually photographed images. In this project, you are going to create a stop-motion animation of a precipitation reaction.
In a precipitation reaction, solutions containing ions are mixed. The ions are moving in the solutions but when certain ions meet, they combine and fall together out of solution (Figure C7.19). The reaction lends itself to animation as we cannot see the individual ions in solution and how they come together.
In a pair or small group, choose a precipitation reaction to focus on. Figure C7.20 shows the precipitation of silver chloride, but you can choose one of several different reactions (think about the analysis tests in Chapter C12).
You need to produce a storyboard to help you visualise the final animation. This should explain the movement of the ions during the mixing of the two solutions. Your storyboard must include:
1 an image of the two separate solutions before mixing
2 some images of one solution being poured into the other
3 images of the precipitate settling; possibly of it being stirred up and allowed to settle again. Having produced your storyboard, you should then produce your animation. This can be done in various ways. For example, you could draw some illustrations similar to Figure C7.19 to show diagrammatically how the precipitate forms. Include some pictures of how an ionic equation for the reaction is constructed. Then take a series of photographs, perhaps adding callouts which you can assemble together as a set of slides. You could use a stop-start animation app that will allow you to put together a series of photographs and add a voiceover to describe the process. In both cases, the more photographs you take and the less the movement between them, the better it will look.
If you do not have access to a camera/laptop then you could produce a flipbook with lots of little images showing a precipitation reaction.

CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: COURSEBOOK
Figure C7.19: Precipitation of barium sulfate. The solid can be collected by filtration or centrifugation.
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Figure C7.20: Precipitation of silver chloride.
SUMMARY
Acids turn litmus and methyl orange red. Alkalis turn red litmus paper blue and methyl orange yellow.
Bases are the oxides and hydroxides of metals, and soluble bases are also referred to as alkalis.
The relative acidity or alkalinity or pH of a solution can be assessed using the colour observed using universal indicator.
Acids and bases react together in a neutralisation reaction to produce a salt and water only.
Most metal oxides and hydroxides are basic (e.g. CuO and CaO), while many oxides of non-metals can be classified as acidic oxides (e.g. CO2 and SO2).
Some metal oxides, such as aluminium oxide and zinc oxide, can react with both acids and alkalis, and are therefore classified as amphoteric oxides.
Acids take part in certain characteristic reactions, such as those with bases (neutralisation), with metals to produce hydrogen and with metal carbonates to form carbon dioxide.
Salts are formed by the replacement of the hydrogen in an acid with a metal.
Some salts are soluble in water while other salts are insoluble. There are general solubility rules that apply to the various different types of salt.
A hydrated substance is a substance that is chemically combined with water. An anhydrous substance is a substance containing no water
Methods are available for the preparation, separation and purification of soluble salts by the reaction of the parent acid with either excess metal, excess insoluble base or excess insoluble carbonate.
A soluble salt can be prepared by titration of an acid with an alkali followed by separation and purification.
An insoluble salt can be prepared by precipitation followed by filtration.
PRACTICE QUESTIONS
1 Metals and non-metals generally produce different types of oxides when reacted with air or oxygen. Identify which row in the table correctly defines the nature of the oxides of the elements listed. Forms
A phosphorus sulfur
B magnesium sulfur
C sulfur phosphorus
D sulfur magnesium
COMMAND WORD
Identify: name/select/ recognise.
C7 Acids, bases and salts 29
an acidic oxide
a basic oxide
Forms
[1]
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CONTINUED
2 A student tested 50 cm3 of hydrochloric acid with methyl orange indicator. She then added the hydrochloric acid to powdered calcium carbonate to make carbon dioxide.
a Give the colour that the indicator turned. [1]
b Give a word equation for the reaction of hydrochloric acid with calcium carbonate; include state symbols. [2]
c When carbon dioxide is tested with moist universal indicator paper, the paper turns orange. Suggest what this shows about carbon dioxide. [1]
d pH is a measure of how acidic or alkaline a substance is. Describe how you could use an indicator to find the pH of a solution of calcium hydroxide. [3]
[Total: 7]
COMMAND WORDS
3 The method of preparing a soluble salt in the laboratory has certain clear stages. Identify the three steps in the table that are needed to make sodium sulfate crystals from sodium hydroxide solution and dilute sulfuric acid.
First step Second step Third step
A evaporation crystallisation neutralisation
B neutralisation evaporation crystallisation
C neutralisation crystallisation evaporation
D evaporation neutralisation crystallisation
4 A list of salts is shown below:
barium sulfate copper sulfate potassium carbonate sodium chloride zinc nitrate
Identify the salt that:
a cannot safely be made by reacting acid with a metal [1]
b is made by titration [1]
c is insoluble [1]
d reacts with acid to produce a gas. [1]
[Total: 4]
give: produce an answer from a given source or recall/memory. suggest: apply knowledge and understanding to situations where there are a range of valid responses in order to make proposals/put forward considerations. describe: state the points of a topic/give characteristics and main features.
5 Excess magnesium carbonate was added to dilute sulfuric acid to make a sample of magnesium sulfate.
a Suggest an observation that would be made as the reaction took place. [1]
b Give a reason for why excess magnesium carbonate was used. [1]
c State how you would know when the reaction was complete. [1]
d Explain how you would separate a pure sample of magnesium sulfate from the mixture. [3]
[Total: 6]
COMMAND WORDS
state: express in clear terms.
explain: set out purposes or reasons/make the relationships between things clear/say why and/ or how and support with relevant evidence.
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: COURSEBOOK 30
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CONTINUED
6 A list of copper salts is shown below:
copper carbonate copper chloride copper nitrate copper sulfate
Identify the salt that could be made by the following method.
• mix the solutions of two salts
• filter the mixture
• wash the residue
• dry the residue [1]
7 A student wanted to make a sample of the insoluble salt lead(II) chloride.
a Identify two substances which could be used to form this salt. [2]
b Give the steps the student should use to produce a pure dry sample of lead(II) chloride from these substances. [4]
[Total: 6]
SELF-EVALUATION CHECKLIST
After studying this chapter, think about how confident you are with the different topics. This will help you see any gaps in your knowledge and help you to learn more effectively.
I can
describe acids and alkalis in terms of their effect on indicators such as litmus and methyl orange
state that bases are the oxides and hydroxides of metals and that alkalis are soluble bases
compare the relative acidity or alkalinity or pH of solutions using the colour observed using universal indicator
describe how acids and bases react together in a neutralisation reaction
describe the characteristic reactions of acids with bases; and with metals to produce hydrogen; and with metal carbonates to form carbon dioxide
classify metal oxides and hydroxides as basic while many non-metal oxides are acidic oxides
classify some metal oxides such as ZnO and Al2O3, as amphoteric as they can react with both acids and alkalis
C7 Acids, bases and salts 31
See topic . . . Needs more work Almost there Confident to move on
C7.01
C7.01
C7.01
C7.01
C7.01
C7.02
C7.02 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
P2 Energy, work and power
All learners study some content in this chapter
IN THIS CHAPTER YOU WILL:
• identify changes in different energy stores

• recognise different energy transfers
• understand the meaning of energy efficiency
• calculate efficiency
• apply the principle of conservation of energy
• calculate potential energy and kinetic energy
• distinguish between renewable and non-renewable energy sources
• describe how electricity or other useful stores of energy may be obtained from different energy resources
• consider the advantages and disadvantages of each energy resource
• understand that the Sun is the source of energy for all our energy resources except geothermal, nuclear and tidal
• understand that energy is released by nuclear fusion in the Sun
• understand that work is done when a force causes movement
• relate power to work done and time taken
• use the equation W = Fd = ΔE to calculate work done
• use the equations P = ΔE t and P = W t to calculate power.
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
BEFORE YOU START
Work with a classmate to describe the energy transfers that are taking place in each diagram of Figure P2.01. What do you already know about energy?
With a classmate, draw an energy mind map to include everything you know about this topic, including the principle of conservation of energy.
KEY WORD
energy: quantity that must be changed or transferred to make something happen.
SCIENCE IN CONTEXT P2.01
Is thorium the perfect fuel?

Kirk Sorensen worked for NASA to come up with a reliable source of energy for a Moon base. None of the energy resources that are used on Earth were suitable. But then he found a book about liquid fluoride thorium reactors (or ‘lifters’), an environmentally friendly and safe version of nuclear power (Figure P2.02). They were being developed by the USA so that aircraft carrying nuclear bombs would only have to land to change crews and take on supplies. But the experiment was abandoned in 1956 because missiles could more easily send nuclear bombs over great distances.
Nuclear power stations need water, but a lifter would not. This would make it suitable for the Moon. But Sorenson thought, ‘Why not have them here on Earth?’ There are huge reserves of thorium fuel available, they produce tiny amounts of radioactive waste, an accidental meltdown would be impossible, and it would be extremely difficult to make a nuclear bomb using a lifter.
Discussion questions
1 Explain why energy resources used on Earth would not be suitable for the Moon.
2 Would you be in favour of nuclear power based on a lifter? Explain your answer.
P2 Energy, work and power 33
e f b a d c g h
Figure P2.01 a: Flashlight switched on. b: Wound up toy. c: Moving radio-controlled car. d: Bunsen burner.
e: Loudspeaker in use. f: Ringing bicycle bell. g: Solar-powered battery. h: Hair dryer in use.
Figure P2.02: Pellets of thorium.
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
P2.01 Energy stores, transfers and conservation
Energy is hard to define. It is easier to describe it using examples. Energy is the quantity which is transferred whenever we observe a change, such as a change of motion or temperature, a lamp shining or a power station producing electricity.
Energy is measured in joules.
Energy stores
Energy can be stored in many different ways. Table P2.01 shows some of the main energy stores. Many situations involve lots of different stores of energy as shown in Figure P2.03.
Store Examples
Kinetic energy Cars, moving water, runners. Gravitational potential energy Aeroplanes, divers.
Chemical energy Food, fuel, batteries. Elastic (strain) energy Catapults, stretched elastic bands.
Nuclear energy Nuclear power stations, the Sun.
Electrostatic energy Thunderclouds.
Internal (thermal) energy Hot water, human bodies.
KEY WORDS
joule: the SI unit for energy transferred or work done. kinetic energy: the energy store of a moving object. gravitational potential energy: the energy store of an object raised up against the force of gravity; more generally, it is the distance between particles or bodies. chemical energy: energy stored in bonds between atoms that can be released when chemical reactions take place.
elastic (strain) energy: energy stored in the changed shape of an object.
nuclear energy: energy stored in the nucleus of an atom. electrostatic energy: the energy stored when charges are separated or squashed together.
internal (thermal) energy: the energy of an object; the total kinetic and potential energies of its particles.
Questions
P2.01 What name is given to the energy of a moving object?
P2.02 How can the gravitational potential energy of an object be increased?
P2.03 What energy is stored in a stretched spring?
P2.04 Give examples of objects or materials which store:
a kinetic energy
b gravitational potential energy
c elastic energy
d internal energy.
Energy transfers
Energy stores are potential energy. This is energy which is available but is not being used. Energy can also transfer between stores, but the total amount of energy never changes. So, energy can be stored or it can be transferred in an event or a process (Table P2.02).
KEY WORDS
event: something that happens or takes place, often at a specific time and place. process: a series of actions or steps, often taking place over a long period of time.
Figure
uphill has several stores of energy: kinetic as it is moving, gravitational potential as it has driven uphill, chemical in its fuel tank and internal as the engine will have heated up.

CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: COURSEBOOK 34
P2.03: This car driving
Table P2.01: The different ways energy can be stored.
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Energy transfers Example events and processes
Forces (mechanical work)
Electrical currents
Kicking a football increases its store of kinetic energy causing it to move. When a force makes something move, we say it is doing work
An electrical current transfers energy from the chemical energy store of a flashlight battery to the internal energy of a bulb.
Heating Heating water with a Bunsen burner flame transfers energy from the chemical store of the gas to the internal energy store of the water.
Waves Infrared (heat) radiation from the Sun is transferred through space by waves and can be used to increase the thermal energy store of water in a solar panel
KEY WORDS
doing work: transferring energy by means of a force. solar panel: used to collect energy that is transferred by light from the Sun.
Energy can be transferred from one store to another, even within the same object.
For example, when you climb a hill, you are transferring energy from your chemical store to your gravitational potential energy store. Figures P2.04 and P2.05 illustrate some energy transfers. At a major rock concert, giant loudspeakers transfer sound to the audience. Extra generators may have to be brought on to the site to act as a source of energy to power the speaker systems. Much of the energy supplied is wasted as thermal energy, because only a fraction of the energy is transferred by sound.

When a catapult fires a ball, energy is transferred from the elastic store of the catapult to the kinetic store of the ball. If the ball is fired vertically upwards, energy from the kinetic energy store is transferred to the gravitational potential energy store, until there is nothing left in the kinetic energy store. The ball stops moving upwards and starts falling. Now, energy is transferred from the gravitational store back to the kinetic store.

Questions
P2.05 Look at the physical clues in the left column of Table P2.03. For each, write down which energy store is changing.
Physical clue Which energy store is changing?
material changing shape
object changing speed
chemical reaction
change of temperature
nuclear fission or fusion
Table P2.03: Changing energy stores.
P2.06 What energy transfers are going on in the following?
a Coal is burnt to heat a room and to provide a supply of hot water.
b A student uses an electric lamp while she is doing her homework.
c A hair dryer is connected to the mains electricity supply. It blows hot air at the user’s wet hair. It whirrs as it does so.
P2 Energy, work and power 35
Table P2.02: Energy can be transferred between stores.
Figure P2.04: Light and sound energy make this concert impressive.
Figure P2.05: When the boy lets go, the store of elastic energy is transferred to the ball’s store of kinetic energy.
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
ACTIVITY P2.01
Energy changes
Examine some devices that transfer energy. Some ideas are shown in Figure P2.01 in the ‘Before you start’ box.
• In pairs, examine each of the devices you are provided with. For each of them, describe what energy transfers are going on in the device.
• With your partner, decide how to record and present the energy transfers you have described for each device.
• Compare your answers with the answers of other members of the class and correct or add to your own answers.
Conservation of energy
When energy is transferred from one store to another, it is often the case that some of the energy ends up as unwanted energy. For example, a light bulb transfers light (which we want) and heat (which is not wanted).
This is an example of a very important idea: the principle of conservation of energy:
In any energy transfer, the total amount of energy before and after the transfer is constant.
This tells us something very important about energy: it cannot be created or destroyed. The total amount of energy is constant. If we measure or calculate the amount of energy before a transfer, and again afterwards, we will always get the same result. If we find any difference, we must look for places where energy may be entering or escaping unnoticed.
KEY WORDS
principle of conservation of energy: energy cannot be created or destroyed; it can only be stored or transferred.
WORKED EXAMPLE P2.01
A car burns 3 × 105 J of fuel (chemical energy) per second. It has 1.3 × 105 J of kinetic energy and gains 0.7 × 105 J of gravitational potential energy as it goes up a slope. How much energy transfers away from the car through thermal energy transfer?
Step 1: Write down what you know, and what you want to know.
input energy:
chemical energy = 3 × 105 J
output energy:
kinetic energy = 1.3 × 105 J
gravitational potential energy = 0.7 × 105 J thermal energy transferred = ?
Step 2: Write down any equations or useful principles. According to the principle of conservation of energy, the total input energy should equal the total output energy.
Step 3: Apply the principle to this problem and substitute known values to solve the problem.
input energy = output energy
chemical energy = kinetic energy + gravitational potential energy + thermal energy
3 × 105 J = 1.3 × 105 J + 0.7 × 105 J + thermal energy = 1.0 × 105 J transfers away from the car through thermal energy transfer.
Answer
1.0 × 105 J transfers away from the car as thermal energy.
Question
P2.07 A light bulb is supplied with 60 J of energy each second.
a How many joules of energy are transferred from the bulb each second?
b 4 J of energy are transferred from the lamp each second as light. How many joules of energy are transferred each second by heating?
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: COURSEBOOK 36
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Figure P2.21: Three examples of forces doing work. In each case, the force moves as it transfers energy. a: Pushing a shopping trolley to start it moving. The pushing force does work. It transfers energy to the trolley, and the trolley’s kinetic energy increases. b: An apple falling from a tree. Gravity pulls the apple downwards. Gravity does work, and the apple’s kinetic energy increases. c: Braking to stop a bicycle. The brakes produce a force of friction, which slows down the bicycle. The friction does work, and the bicycle’s kinetic energy is transferred to the internal energy of the brakes, which get hot.
How much work?
Think about lifting a heavy object, as shown in Figure P2.20. A heavy object needs a big force to lift it. The heavier the object is, and the higher it is lifted, the more its gravitational potential energy increases. This suggests that the amount of energy transferred by a force depends on two things:
• the size of the force: the greater the force, the more work it does
• the distance moved in the direction of the force: the further it moves, the more work it does.

So a big force moving through a big distance does more work than a small force moving through a small distance.
Words in physics
You will by now understand that ‘work’ is a word that has a specialised meaning in physics, different from its meaning in everyday life. When physicists think about the idea of work, they think about forces causing movement. Consider the students in Figure P2.22. The older student is reading and thinking, but no forces are involved (until she lifts a book or starts writing). She is not doing work. The younger girl has lifted the books up. She has done work against gravity.
Figure P2.22: Who is doing more work? The older student has not used a force to move anything. According to the physics definition of work, she has done no work against gravity. The younger student has lifted the books against the pull of gravity, so has done work.


P2 Energy, work and power 37
F F F F F F a b c
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Many words have specialised meanings in science. It is important for physicists to agree on the terms they are using.
In Chapter P1, we saw that mass and weight are different, though in everyday language they are used to mean the same thing – how heavy something is. This is not a problem, provided you know whether you are using a particular word in its scientific sense or in a more everyday sense.
Questions
P2.29 Which of the following involve work being done?
a A weightlifter holds a weight stationary for a minute.
b A boy pushes as hard as he can against a door that will not open.
c A baby lifts up a spoon.
P2.30 A girl carries a box up a flight of stairs. Name two factors which affect the amount of work she does.
Calculating work done
When a force does work, it transfers energy to the object it is acting on. The amount of energy transferred is equal to the amount of work done. We can write this as a simple equation:
W = ΔE
In this equation, we use the symbol Δ (Greek capital letter delta) to mean ‘amount of’ or ‘change in’. So,
ΔE = change in energy
How can we calculate the work done by a force? The work done depends on two things:
• the size of the force, F
• the distance, d, moved by the force.
KEY EQUATION
Mechanical work done =force distance moved by by a force t × h he force in the directio n of the force
W = Fd = ΔE
The phrase ‘in the direction of the force’ will be explained shortly. As the amount of work done is the same as the amount of energy transferred, it is measured in joules (J), the SI unit of energy.
Joules and newtons
The equation for the work done by a force (W = F × d) shows us the relationship between joules and newtons. If we replace each quantity in the equation by its SI unit, we get 1 J = 1 N × 1 m = 1 Nm. So, a joule is a newton metre.
WORKED EXAMPLE P2.04
A crane lifts a crate upwards through a height of 20 metres. The lifting force provided by the crane is 5.0 kN, as shown in Figure P2.23.
a How much work is done by the force?
b How much energy is transferred to the crate?
Step 1: Write down what you know, and what you want to know.
F = 5.0 kN = 5000 N
d = 20 m
W = ?
Step 2: Write down the equation for work done, substitute values and solve.
W = F × d = 5000 N × 20 m
= 100 000 Nm ≡ 100 000 J
Answer
a The work done by the force is 100 000 J, or 100 kJ.
b Since work done = energy transferred, 100 kJ of energy is transferred to the crate.
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: COURSEBOOK 38
5.0 kN 20 m
Figure P2.23: A crane lifts a crate upwards.
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Work done and mgh
Worked example P2.04 illustrates an important idea. The force provided by the crane to lift the crate must equal the crate’s weight, mg. It lifts the crate through a height, h. Then the work it does is force × distance, or mg × h The gain in gravitational potential energy of the crate is mgh. This explains where the equation for gravitational potential energy comes from.
In Figure P2.24, the child slides down the ramp. Gravity pulls her downwards and makes her speed up. To calculate the work done by gravity, we need to know the vertical distance, h, because this is the distance moved in the direction of the force. If we calculated the work done as weight × distance moved down the ramp, we would get an answer that was too large. Now you should understand why we write the definition of work done like this:
KEY EQUATION
work done = force × distance moved in the direction of the force
Figure P2.25: When you sit still in a chair, there are two forces acting on you. Neither transfers energy to you.
WORKED EXAMPLE P2.05
A girl can provide a maximum pushing force of 200 N. To move a box weighing 400 N onto a platform, she uses a plank as a ramp, as shown in Figure P2.26.
a How much work does she do in raising the box?
b How much gravitational potential energy does the box gain?
Figure P2.24: It is important to use the correct distance when calculating work done by a force. Gravity makes the child slide down the slope. However, to calculate the energy transferred by gravity, we must use the vertical height moved.
Forces doing no work
If you sit still on a chair (Figure P2.25), there are two forces acting on you. These are your weight, mg, acting downwards, and the upward contact force, C, of the chair, which stops you from falling through the bottom of the chair.
Neither of these forces is doing any work on you. The reason is that neither of the forces is causing movement, so you do not move through any distance, d
From W = F × d, the amount of work done by each force is zero. When you sit still on a chair, your energy does not increase or decrease as a result of the forces acting on you.
Step 1: Write down what you know, and what you want to know.
pushing force along the ramp, F = 200 N distance moved along ramp, d = 2.5 m weight of box downwards, mg = 400 N vertical distance moved, h = 0.75 m work done along the ramp, W = ? gravitational energy gained, ΔE p = ?
Step 2: Calculate the work done, W, by the pushing force along the ramp.
W = pushing force × distance moved along ramp along ramp
= F × d
= 200 N × 2.5 m
= 500 J
P2 Energy, work and power 39
h
contact force of chair, C weight, mg
400N 200N 0.75m 2.5m
Figure P2.26: Doing work against gravity.
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
PROJECT P2.01 IMPROVING LIVES WITH ENERGY
Choose one of the options below and either produce a short report (fewer than 500 words) along with relevant illustrations or produce a short presentation (two or three minutes), with suitable visual aids.
Option 1: Inventions for remote places

Research an invention that provides useful energy in a location without an obvious or reliable energy supply. If you cannot track down another invention, focus on one of the following examples.
• PlayPump is a system that uses a children’s roundabout to power a water pump.
• Trevor Baylis invented the wind-up radio, (Figure P2.29) which worked without batteries or access to an electrical power source.
Option 2: Efficiency
It is important to increase efficiency to reduce waste, reduce environmental damage, and save money. Investigate efforts to improve the efficiency of one device (for example, a light bulb, or a car) or create better insulation for homes.
SUMMARY
Energy can be stored in different ways and can be transferred between stores.
A moving object has a store of kinetic energy.
Heating a body will increase its internal energy.
Changing the shape of a body will change its elastic (strain) energy.
Lifting a body will increase its gravitational potential energy.
Food and fuels are stores of chemical energy.
Mechanical work can transfer gravitational potential energy to an object, by lifting it.
Electric currents transfer energy electrically.
Energy is conserved. It cannot be created or destroyed; it can only transfer from one store to another.
Efficiency is the fraction of the total energy that is useful.
When a process is not 100% efficient, the wasted energy spreads out (usually increasing the thermal energy store of the surroundings) and is not useful.
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: COURSEBOOK 40
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Figure P2.29: A wind-up radio.
CONTINUED
change in gravitational potential energy = weight × change in height or
change in gravitational potential energy = mass × gravitational field strength × change in height or
ΔE p = mgΔh.
Kinetic energy is Ek = 1 2 mv2
The energy we use can come from a variety of resources: fossil fuels, biofuels, water, geothermal, nuclear, solar and wind.
Some energy resources are used directly, others transfer energy to electrical energy using turbines and generators. Solar cells (also known as photocells) generate electricity using energy from the Sun.
Oil, coal and natural gas are all examples of fossil fuels.
Non-renewable energy resources will run out. This includes fossil fuels and nuclear fuel, but not biofuel.
Biofuels are renewable, reliable, cheap to set up and use, but are diffuse.
Geothermal energy is harvested (collected) where hot rock is close to the Earth’s surface.
Wind power, wave power and solar power are renewable but unreliable and dilute energy resources. Running costs are low but they are expensive to set up.
Hydroelectric power, tidal power and geothermal power are renewable, reliable and concentrated energy resources but suitable locations are limited, and they are expensive to set up.
Nuclear power stations use nuclear fuel, which produces thermal energy by nuclear fission when heavy nuclei break apart.
The Sun is the origin of all our energy resources except geothermal, nuclear and tidal.
The source of the Sun’s energy is nuclear fusion, when hydrogen fuses (joins) together to form helium.
The Moon is the main source of tidal energy. Work is done when a force causes something to move.
The amount of work done depends on the distance moved in the direction of the force; the further it moves, the more work is done.
The amount of work done is equal to the amount of energy transferred. work done = force × distance moved in the direction of the force power = energy transferred ÷ time taken = work done ÷ time taken
P2 Energy, work and power 41
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
PRACTICE QUESTIONS
Use this diagram for questions 1 and 2
This diagram shows an amusement park roller coaster ride (not drawn to scale).
1 Identify which store of energy is greatest at point P.
A gravitational potential energy
B kinetic energy
C elastic energy
D chemical energy [1]
2 Identify which store of energy increases as the cart moves from point P to point Q.
A gravitational potential energy
B kinetic energy
C elastic energy
D chemical energy [1]
3 State the principle of conservation of energy. [1]
4 Give one renewable and one non-renewable energy resource. [2]
5 Copy and complete these sentences. Choose words from this list. work energy more less
a When a force makes an object move, ______ is done. [1]
b The greater the distance an object is moved by the force, the ______ work it does. [1]
c Power is the rate at which ______ is transferred. [1]
d A low-powered motor will transfer ____ energy each second than a more powerful motor. [1]
[Total: 4]
COMMAND WORD
identify: name/select/ recognise.
COMMAND WORDS
state: express in clear terms. give: produce an answer from a given source or recall/memory.
6 State the SI units for:
a energy [1]
b work [1]
c power [1]
[Total: 3]
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: COURSEBOOK 42
50 m ? P Q R S
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
SELF-EVALUATION CHECKLIST
After studying this chapter, think about how confident you are with the different topics. This will help you to see any gaps in your knowledge and help you to learn more effectively.
describe the advantages and disadvantages of different energy resources
describe how most of our energy resources depend on the energy supplied by the Sun and name the process by which the Sun releases this energy
three energy resources which do not rely on the Sun
describe what is meant in physics by the word ‘work’
P2 Energy, work and power 43
I can See topic . . . Needs more work Almost there Confident to move on identify different energy stores P2.01 describe how energy is transferred between stores P2.01 describe what is meant by efficiency P2.02 calculate efficiency P2.02 state and explain the principle of conservation of energy P2.02 recall and use the equation to calculate kinetic energy P2.02 recall and use the equation to calculate gravitational potential energy P2.02 describe energy resources P2.03 describe
P2.03
P2.03
how energy resources can be used to generate electricity
P2.03
P2.03
P2.04 recall
P2.04
P2.05 recall
P2.05 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
name
and use the equation to calculate work done
explain how the rate at which work is done is related to power
and use the equation to calculate power.

We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication.
SAMPLE
Contents v Contents How to use this series vi How to use this book viii Introduction ix 1 Cells and organisms Characteristics of living organisms 1 Cell structure 3 Specialised cells and sizes of specimens 6 2 Movement into and out of cells Diffusion 10 Osmosis 13 Active transport 17 3 Biological molecules Carbohydrates, fats and proteins 19 4 Enzymes Biological catalysts 27 Factors that affect enzymes 29 5 Plant nutrition Photosynthesis 38 Leaves 41 Factors affecting photosynthesis 43 6 Human nutrition Diet 48 The digestive system 50 Digestion 54 7 Transport Transport in plants 58 Transport in animals 66 8 Diseases and immunity Pathogens and transmissible diseases 78 The immune response 87 9 Gas exchange and respiration Gas exchange 90 Respiration 95 10 Coordination and response Coordination and response 101 Hormones 104 Homeostasis 106 11 Reproduction Asexual and sexual reproduction 109 Sexual reproduction in plants 111 Sexual reproduction in humans 115 Sexually transmitted infections 120 12 Inheritance Chromosomes and genes 122 Cell division 124 Monohybrid inheritance 125 13 Variation and selection Variation 131 Selection 133 Drugs 136 14 Organisms and their environment Energy flow 140 Food chains and food webs 142 Carbon cycle 145 15 Human influences on ecosystems Habitat destruction 147 Conservation 150 Glossary 153 Acknowledgements 158 45 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
How to use this book
Throughout this book, you will notice lots of different features that will help your learning. These are explained below.
LEARNING INTENTIONS
These set the scene for each exercise and indicate the important concepts.
KEY WORDS
Definitions for useful vocabulary are given at the start of each section. You will also find definitions for these words in the Glossary at the back of this book.
TIP
The information in these boxes will help you complete the exercises, and give you support in areas that you might find difficult.
Exercises
These help you to practise skills that are important for studying IGCSE Biology. Questions within exercises fall into one of three types:
• Focus questions will help build your basic skills.
• Practice questions provide more opportunities for practice, pushing your skills further.
• Challenge questions will stretch and challenge you even further.
SELF/PEER ASSESSMENT
At the end of some exercises, you will find opportunities to help you assess your own work, or that of your classmates, and consider how you can improve the way you learn.
viii CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: BIOLOGY WORKBOOK
46 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Chapter 1 Cells and organisms Characteristics of living organisms
Exercise 1.1
IN THIS EXERCISE YOU WILL:
• practise naming and describing the characteristics of living things.
Focus
1 Draw lines to match each term with its description.
Term Description
nutrition
making more of the same kind of organism
respiration removing waste products of metabolism
growth a permanent increase in size and dry mass
excretion taking in materials for energy, growth and development
reproduction chemical reactions that release energy from nutrient molecules
KEY WORDS
excretion: the removal of waste products of metabolism and substances in excess of requirements.
growth: a permanent increase in size and dry mass.
metabolic reactions: chemical reactions that take place in living organisms.
movement: an action by an organism or part of an organism causing a change of position or place.
nutrition: the taking in of materials for energy, growth and development.
organism: a living thing.
reproduction: the processes that make more of the same kind of organism.
respiration: the chemical reactions in cells that break down nutrient molecules and release energy for metabolism.
1
47 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Practice
2 Figure 1.1 shows a plant, growing towards the light. Inside its leaves, photosynthesis is taking place. Photosynthesis uses carbon dioxide to make glucose, and releases oxygen.
Add labels to Figure 1.1. Your labels should include short descriptions stating how the plant is showing these characteristics of living things:
• reproduction
• growth
• sensitivity
• excretion.
Challenge
3 Imagine that someone from another planet is visiting Earth. They see aeroplanes and birds moving through the sky.
Explain to the visitor why birds are alive and aeroplanes are not alive, even though they seem to share some of the characteristics of living things.
KEY WORD
sensitivity: the ability to detect and respond to changes in the internal or external environment.
2
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: BIOLOGY WORKBOOK
Figure 1.1: A plant growing towards the light.
48 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Cell structure
Exercise 1.2
IN THIS EXERCISE YOU WILL:
• practise drawing and labelling animal and plant cells
• outline the functions of some of the parts of cells
• use information to explain some of the features of a specialised cell.
Focus
1 On the animal cell diagram, label these parts: cell membrane cytoplasm mitochondrion nucleus
2 Complete the diagram of the plant cell, and then label these parts: cell membrane cell wall chloroplast vacuole containing cell sap cytoplasm mitochondrion nucleus membrane around vacuole
KEY WORDS
aerobic respiration: a chemical reaction that happens in mitochondria, where oxygen is used to release energy from glucose.
bacteria: unicellular organisms whose cells do not contain a nucleus.
cell: the smallest unit from which all organisms are made.
cell membrane: a very thin layer surrounding the cytoplasm of every cell; it controls what enters and leaves the cell.
cell sap: the fluid that fills the large vacuoles in plant cells.
cell wall: a tough layer outside the cell membrane; found in the cells of plants, fungi and bacteria.
cellulose: a carbohydrate that forms long fibres, and makes up the cell walls of plants.
chromosome: a length of DNA, found in the nucleus of a cell; it contains genetic information in the form of many different genes.
cytoplasm: the jellylike material that fills a cell.
1 Cells and organisms 3
a b
Figure 1.2 shows an animal cell and the outline of a plant cell.
Figure 1.2 a: An animal cell. b: A plant cell.
49 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
SELF ASSESSMENT
How confident do you feel about drawing a plant cell? Give yourself a mark for each of the points in the checklist. Award yourself:
2 marks if you did it well
1 mark if you made a good attempt at it and partly succeeded
0 marks if you did not try to do it, or did not succeed
Checklist
I used a sharp pencil for drawing.
I drew single, clean lines; the lines are not broken or fuzzy.
I did not use any shading or colours.
I drew the parts of the cell in the right place.
I drew label lines with a ruler.
Each label line touches the part it is labelling.
Total (out of 12):
Practice
3 a Describe the function of each of these parts in a plant cell. Cell membrane
Marks awarded
Mitochondrion
KEY WORDS
DNA: a molecule that contains genetic information, in the form of genes, that controls the proteins that are made in the cell.
fully permeable: allows all molecules and ions to pass through it.
4 CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: BIOLOGY WORKBOOK
50 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Challenge
6 Neurones are cells that transmit electrical signals throughout the body. This requires a lot of energy. They also synthesise (make) proteins, which help them to communicate with other neurones nearby. Use this information to explain why neurones contain many mitochondria and many ribosomes.
Specialised cells and sizes of specimens
Exercise 1.3
IN THIS EXERCISE YOU WILL:
• use the magnification equation
• practise giving answers to a required number of decimal places
• practise rearranging the magnification equation
• convert from millimetres to micrometres (µm) when using the magnification equation.
Focus
1 Complete the equation that we can use to calculate magnification. magnification =
KEY WORD
magnification: how many times larger an image is than the actual object.
6 CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES:
WORKBOOK
BIOLOGY
51 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
SAMPLE

We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication.
Contents v Contents How to use this series vi How to use this book viii Introduction ix 1 States of matter Solids, liquids and gases 1 Diffusion 11 2 Atoms, elements and compounds Elements, compounds and mixtures 15 Atomic structure and the Periodic Table, and isotopes 18 Chemical bonding, ions and ionic bonds, and simple molecules and covalent bonds 26 Giant covalent structures and metallic bonding 32 3 Stoichiometry Chemical formulae and equations 35 Relative masses of atoms and molecules 41 The mole and the Avogadro Constant 46 4 Electrochemistry Electrolysis 54 Hydrogen–oxygen fuel cells 62 5 Chemical energetics Exothermic and endothermic reactions 65 6 Chemical reactions Physical and chemical changes 72 Rate of reaction 74 Redox reactions 90 7 Acids, bases and salts The characteristic properties of acids, bases, and oxides 97 Preparation of salts 104 8 The Periodic Table Arrangements of elements 110 Group I properties 113 Group VII properties 115 Transition elements and the noble gases 118 9 Metals Properties and uses of metals 121 Alloys and their properties 123 Reactivity series 127 Corrosion of metals 135 Extraction of metals 139 10 Chemistry of the environment Air quality and climate 144 Water 153 11 Organic chemistry Formulae, functional groups and terminology, and naming organic compounds 155 Alkanes, alkenes and alcohols 160 Fuels 166 Polymers 170 12 Experimental techniques and chemical analysis Experimental design 173 Separation and purification 177 Chromatography 180 Identification of ions and gases 182 Acid–base titrations 188 Glossary 191 Periodic Table 196 Acknowledgements 197 53 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
How to use this book
Throughout this book, you will notice lots of different features that will help your learning. These are explained below.
LEARNING INTENTIONS
These set the scene for each exercise and indicate the important concepts.
KEY WORDS
KEY EQUATIONS
These boxes remind learners of important equations that are required to answer questions in a topic or exercise.
Definitions for useful vocabulary are given at the start of each section. You will also find definitions for these words in the glossary at the back of this book. TIP
The information in these boxes will help you complete the exercises, and give you support in areas that you might find difficult.
Exercises
These help you to practise skills that are important for studying IGCSE Chemistry. Questions within exercises fall into one of three types:
• Focus questions will help build your basic skills.
• Practice questions provide more opportunities for practice, pushing your skills further.
• Challenge questions will stretch and challenge you even further.
SELF/PEER ASSESSMENT
At the end of some exercises, you will find opportunities to help you assess your own work, or that of your classmates, and consider how you can improve the way you learn.
viii CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: CHEMISTRY WORKBOOK
54 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Acids, bases and salts
The characteristic properties of acids and bases, and oxides
Exercise 7.1
IN THIS EXERCISE YOU WILL:
• focus on the differences between acids and alkalis in terms of the pH values of their solutions
• learn the formulae of some common laboratory acids and alkalis
• consider how the pH of a solution will tell you how acidic or how alkaline the solution is
• describe the colours of different indicators under acid, neutral and alkaline conditions
• relate the colours of universal indicator to the pH value of a solution.
Focus
1 Use the words provided to complete the following paragraph. Some words are used more than once. (Note: this activity continues on the next page.)
acids alkalis greater hydrochloric hydrogen hydroxide insoluble less nitric oxides potassium
sodium soluble sulfuric water
Acids are substances that dissolve in ____________ to give a solution with a pH ____________ than 7. ____________ acid has the formula HCl and is a strong acid. ____________ acid (formula H2SO4) and ____________ acid (formula HNO3) are also strong ____________. In acidic solutions, the concentration of ____________ ions is greater than the concentration of ____________ ions.
KEY WORDS
acid: a substance that dissolves in water, producing H+(aq) ions. A solution of an acid turns litmus red and has a pH below 7. Acids act as proton donors.
alkali: a soluble base that produces OH (aq) ions in water – a solution of an alkali turns litmus blue and has a pH above 7.
antacids: compounds used medically to treat indigestion by neutralising excess stomach acid.
base: a substance that neutralises an acid, producing a salt and water as the only products. Bases act as proton acceptors.
indicator: a substance which changes colour when added to acidic or alkaline solutions, e.g. litmus or thymolphthalein.
97 Chapter
7
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Bases are the ____________ and hydroxides of metals and ammonia. A base will neutralise an acid to form a salt and ____________. The solutions of bases have pH values ____________ than 7. Most bases are ____________ in water but alkalis are bases that are ____________ in water. KOH (____________ hydroxide) and NaOH (____________ hydroxide) are both strong ____________. In alkaline solutions, the concentration of ____________ ions is greater than the concentration of ____________ ions.
Practice
2 Look at the statements in Table 7.1. Each statement describes an acid, a base or an alkali. Put a tick (3) in the correct column for each statement. One row of the table has been completed as an example.
Practical observation
A solution of the substance has a pH of 8.
A solution of the substance turns litmus paper blue. ✓
A solution of the substance turns litmus paper red.
A substance that neutralises an acid but is insoluble in water.
A substance that neutralises an acid and is soluble in water.
A substance that is an insoluble oxide or hydroxide of a metal.
A substance that is a soluble hydroxide of a metal.
A solution with a pH of 9 that is produced when ammonia is dissolved in water.
A solution of the substance has a pH of 3.
A solution of the substance has a pH of 13.
KEY WORDS
litmus: the most common indicator; turns red in acid and blue in alkali.
methyl orange: an acid–base indicator that is red in acidic solutions and yellow in alkaline solutions.
pH scale: a scale running from below 0 to 14; use to express the acidity or alkalinity of a solution; a neutral solution has a pH of 7.
salts: ionic compounds made by the neutralisation of an acid with a base (or alkali), e.g. copper(II) sulfate and potassium nitrate.
thymolphthalein: an acid–base indicator that is colourless in acidic solutions and blue in alkaline solutions.
universal indicator: a mixture of indicators that has different colours in solutions of different pH.
98 CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: CHEMISTRY WORKBOOK
AcidBaseAlkali
Table 7.1: Statements about acids, bases and alkalis.
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication.
SAMPLE
Challenge
3 The graph in Figure 7.1 shows what happens to the pH values if sodium hydroxide solution (an alkali) is added to a solution of hydrochloric acid. At points A, B and C, samples of the mixture are removed on a glass rod and spotted onto universal indicator paper.
Figure 7.1: pH when different volumes of alkali are added to hydrochloric acid.
a What is the colour of the universal indicator paper with samples A, B and C?
colour with sample A:
colour with sample B:
colour with sample C:
b What would be the colour of methyl orange at pH 12?
c On the graph, mark with an N where the acid is neutralised by the alkali.
d What volume of alkali is needed to neutralise the acid?
e What is the best apparatus to use to add the alkali accurately to the acid?
f What is the pH of the solution when 15 cm3 of alkali have been added?
7 Acids, bases and salts 99
pH Volume of alkali added / cm3 2.00 4.00 6.00 8.00 10.00 12.00 14.00 0 10 20 30 40 50 60 A B C
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Exercise 7.2
IN THIS EXERCISE YOU WILL:
• reinforce your knowledge of acids
• classify oxides according to their acid–base character
• consider the reactions of acids with reactive metals, metal oxides, metal hydroxides and metal carbonates
• write the word and balanced symbol equations for these reactions
• predict the products of the reactions of acids
• interpret observations of the reactions of acids.
Focus
1 The boxes on the left describe the reactions of acids with different substances. The boxes on the right contain statements about these reactions. Draw lines to connect each type of reaction with the statements that are true about that reaction. You can draw more than one line from each type of reaction.
Type of reaction
acid + metal carbonate
Statements about these reactions
gives H2 gas as a product
gives a salt + water + a gas as the products
acid + reactive metal
acid + insoluble metal oxide
gives CO2 as a product
produces effervescence (fizzing)
gives a salt and water as the only products
acid + alkali gives a salt and gas only
100 CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: CHEMISTRY WORKBOOK
58 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
2 Oxides of metals and non-metals have different acid–base characteristics. Complete Table 7.2 to show whether the oxides of each element are acidic or basic.
Element carboncopperphosphorussulfur calcium
Acidic or basic oxide?
3 a Some metal oxides are amphoteric. Complete the following sentence to explain what this means.
An amphoteric oxide
b Write balanced symbol equations to show how Al2O3 reacts with HCl and NaOH.
Practice
4 Table 7.3 gives the formulae of some different acids, bases, reactive metals, reactive metal carbonates and salts. Use this information to write the word and balanced symbol equations for reactions a–f.
7 Acids, bases and salts 101
Table 7.2: Acid–base characteristics of oxides.
Acid Base/ reactive metal CarbonateSalt HNO3 NaOH CaCO3 CaCl2 HCl Ca(OH)2 Na2CO3 NaCl H2SO4 CaO NaNO3 Mg Na2SO4 Ca(NO3)2 MgCl2
59 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Table 7.3: Formulae of different acids, bases, reactive metal carbonates and salts.
5
a magnesium + hydrochloric acid
word equation:
symbol equation:
b calcium hydroxide + hydrochloric acid
word equation:
symbol equation:
c calcium oxide + nitric acid
word equation:
symbol equation:
d sodium hydroxide + sulfuric acid
word equation:
symbol equation:
e sodium carbonate + nitric acid
word equation:
symbol equation:
f magnesium + nitric acid
word equation:
symbol equation:
Cross out the incorrect words to complete the text. Then complete the neutralisation equation.
When an acid reacts with water / a base, a neutralisation / precipitation reaction occurs. In this reaction, the pH of the acid is lower / higher than that of the neutral product.
This reaction is shown by the general equation:
acid + base → _______________ + ____________________
102
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: CHEMISTRY WORKBOOK
60 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE

We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication.
SAMPLE
v Contents Contents How to use this series vi How to use this book viii Introduction ix 1 Motion Measuring length and volume 1 Density 2 Measuring time 5 Understanding speed 8 Understanding acceleration 21 Mass, weight and gravity 27 Forces 30 Force, mass and acceleration 36 Stretching springs 38 Turning forces 42 Pressure 49 2 Energy, work and power Energy stores, transfers and conservation 54 Energy calculations 58 Energy resources 60 Doing work 66 Power 72 3 Thermal physics Simple kinetic particle model of matter 75 Pressure changes 80 Matter and thermal properties 82 Thermal processes 85 4 Properties of waves General wave properties 100 Light 105 The electromagnetic spectrum 117 Sound 119 5 Electricity and magnetism Simple phenomena of magnetism 123 Electric charge 127 Current, voltage and resistance 133 Electrical energy and electrical power 139 6 Electrical circuits Describing circuits 143 Resistors 152 Circuit calculations 155 Electrical safety 158 7 Electromagnetic effects Magnetic effect of current 160 Force on a current-carrying conductor 162 The d.c. motor 164 Electromagnetic induction 166 The a.c. generator 167 The transformer 169 8 Nuclear physics The nuclear atom 172 Radioactivity 176 9 Space physics The Solar System 189 The Sun as a star 191 The life cycle of stars 195 Galaxies and the Universe 197 Glossary 199 Acknowledgements 204 62 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
How to use this book
Throughout this book, you will notice lots of different features that will help your learning. These are explained below.
LEARNING INTENTIONS
These set the scene for each exercise and indicate the important concepts.
KEY WORDS
Definitions for useful vocabulary are given at the start of each section. You will also find definitions for these words in the Glossary at the back of this book.
KEY EQUATIONS
These boxes remind learners of important equations that are required to answer questions in a topic or exercise.
TIP
The information in these boxes will help you complete the exercises, and give you support in areas that you might find difficult.
Exercises
These help you to practise skills that are important for studying Cambridge IGCSE Physics. Questions within exercises fall into one of three types:
• Focus questions will help build your basic skills.
• Practice questions provide more opportunities to test your knowledge, pushing your skills further.
• Challenge questions will stretch and challenge you even further.
SELF/PEER ASSESSMENT
At the end of some exercises, you will find opportunities to help you assess your own work, or that of your classmates, and consider how you can improve the way you learn.
viii CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: PHYSICS WORKBOOK
63 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
2 Energy, work and power Energy stores, transfers and conservation
Exercise 2.1
IN THIS EXERCISE YOU WILL:
• check that you know what energy stores and transfers are
• practise identifying energy stores and transfers in everyday situations
• apply the principle of conservation of energy
• check that you understand the concept of efficiency.
KEY WORDS
chemical potential energy: energy stored in chemical substances and which can be released in a chemical reaction.
efficiency: the fraction of energy that is transferred to a useful form.
elastic (strain) energy: energy stored in the changed shape of an object.
TIP
Efficiency is the proportion (not the amount) of total input that is transferred usefully.
You will see this expressed in terms of energy, work and power, but work and energy are the same thing and power is the rate of doing work. To decide what is useful, ask yourself: ‘What is the purpose of this device?’ For example, a light bulb exists to produce light so the thermal energy it produces is not generally considered useful.
energy: quantity that must be changed or transferred to make something happen.
gravitational potential energy: energy stored in an object that is raised up against the force of gravity.
internal energy: the energy of an object; the total kinetic and potential energies of its particles.
54
efficiency of adevice useful energy output totalenergyinput =× 100%% efficiency of adevice useful power output total power input =× 100%
KEY EQUATIONS
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Chapter
Focus
1 Complete Table 2.1 to show the energy stored in each situation. The first example has been done for you.
KEY WORDS
Description

Store of energy energy in a stretched spring/elastic elastic potential energy in the nucleus of a uranium atom energy in diesel fuel energy of a ball held above your head energy of a hot cup of coffee
Table 2.1
transferred as sound energy
chemical energy (stored in fuel and oxygen)
transferred as light energy
thermal energy
gravitational potential energy
kinetic energy
kinetic energy: energy stored in a moving object.
nuclear potential energy: energy stored in the nucleus of an atom.
principle of conservation of energy: the total energy of interacting objects is constant provided no net external force acts.
thermal energy: energy transferred from a hotter place to a colder place because of the temperature difference between them.
2 Energy, work and power 55
2 Figure 2.1 shows a rocket being launched into space, and the energy transfers that are involved.
Figure 2.1: A rocket being launched.
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
In Table 2.2, explain how you know that each of these energy changes is happening. The first one has been done for you.
Energy transfer: chemical energy to … How we can tell
sound
light
thermal energy
gravitational potential energy
The rocket launch is very noisy.
kinetic energy
Table 2.2
Practice
3 A child eats their breakfast, then takes the bus to school. At school, they climb the stairs to their classroom on the top floor.
a Identify two stores of chemical energy.
b Identify the energy store the child increases as they climb the stairs.
c Describe the energy transfers which occur as the bus is travelling.
4 A washing machine has a motor that turns the drum. In a particular washing machine, the motor is supplied with 1500 J of energy each second. Of this, 1200 J of energy is used to turn the drum. The rest is wasted as thermal energy. Calculate the amount of energy wasted by the motor as heat each second.
56 CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED
WORKBOOK
SCIENCES: PHYSICS
We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Energy resources
Exercise 2.3
IN THIS EXERCISE YOU WILL:
• recall which resources are renewable and non-renewable
• discuss the advantages and disadvantages of different types of energy resources.
Focus
1 Complete Table 2.3 as follows:
• In the second column, write the name of the type of energy resource.
• In the third column, indicate whether the resource is renewable or non-renewable.
The first example has been done for you.
wood biofuelrenewable
natural gas
coal
splitting of uranium nuclei
hydrogen nuclei combine to release energy
sunlight captured to make electricity or heat water
hot rocks underground used to heat water moving air turns a turbine
water running downhill turns a turbine
Table 2.3
KEY WORDS
biomass fuel: a material, recently living, used as a fuel.
fossil fuel: a material formed from longdead material, used as a fuel.
geothermal: the energy stored in hot rocks underground. non-renewable: energy resource which, once used, is gone forever.
nuclear fission: the process by which energy is released by the splitting of a large heavy nucleus into two or more lighter nuclei.
nuclear fusion: the process by which energy is released by the joining together of two small light nuclei to form a new, heavier nucleus.
renewable: energy resource which, when used, will be replenished naturally.
Renewable is about being able to replace something at least as fast as we use it. It is not about being able to re-use it.
60 CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: PHYSICS WORKBOOK
Description Energy resource Renewable or non-renewable?
TIP
67 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Practice
2 List two advantages and two disadvantages of using solar power.
Challenge
3 Compare the energy resources by completing Table 2.4. You will need to conduct research to find much of the missing information.
ResourceRenewable?Cost per MWh of electricity Environmental impact Reliability
fission
2 Energy, work and power 61
wind wave tidal
nuclear
solar geothermal hydroelectric
68 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Table 2.4
Exercise 2.4
IN THIS EXERCISE YOU WILL:
• consider wind power in some detail.
Focus
1 Figure 2.3 shows how much electricity was generated worldwide from the wind from 1996 to 2018. (The units of energy are GWh or gigawatt-hours. One gigawatt is 109 watts.) Table 2.5 shows the ten countries that contributed most to this total in 2018.
50100150200250300350400450500550600
Cumulative capacity / GWh
62
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: PHYSICS WORKBOOK
1996 1998 2000 2002 2004 2006 2008 2010 2012 2014 2016 2018 0 6.1 7.6 10.2 13.6 17.4 23.9 31.1 39.4 47.6 59.1 74.0 93.9 120.7 159.1 198.0 238.1 282.9 318.7 369.9 432.7 487.3 539.1 591
Year
CountryGlobal wind power 2018 / % China 44.3 United States 14.7 Germany6.1 India 4.2 United Kingdom 4 CountryGlobal wind power 2018 / % Brazil 3.7 France 3 Mexico1.8 Sweden1.4 Canada1.1
Figure 2.3: Graph of global wind power capacity.
69 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Table 2.5 Source: Global Wind Energy Council
Study Figure 2.3 and Table 2.5, then read each of the statements below. Decide whether each statement is TRUE or FALSE. If a statement is FALSE, cross out the incorrect word(s) and write the correct word(s) in the space below. Here is an example to help you:
The amount of electricity generated from the wind reached 50 GWh in 2001 2005
i The amount of electricity generated from the wind has increased every year since 1996.
ii The amount of electricity generated from the wind exceeded 100 GWh in 2006.
iii The amount of electricity generated from the wind doubled between 2002 and 2005.
iv The top three countries generate more than 50% of the world’s wind energy.
v The UK makes less use of wind energy than France.
Practice
2 Think about the area where you live. Suggest a good place to put a wind turbine to generate as much electricity as possible. Give reasons for your suggestion.
Challenge
3 State objections that might be raised to using wind power as a major source of our electricity.
TRUE/FALSE
TRUE/FALSE
TRUE/FALSE
TRUE/FALSE
TRUE/FALSE
TRUE/FALSE
2 Energy, work and power 63
70 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE

We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication.
SAMPLE
Introduction
About the authors
How to use this series
How to use this Teacher’s Resource
How to use this Teacher’s Resource as CPD
About the syllabus
About the assessment
Biology
B1 Cells and organisms
B2 Movement into and out of cells
B3 Biological molecules
B4 Enzymes
B5 Plant nutrition
B6 Human nutrition
B7 Transport
B8 Diseases and immunity
B9 Gas exchange and respiration
B10 Coordination and response
B11 Reproduction
B12 Inheritance
B13 Variation and selection
B14 Organisms and their environment
B15 Human influences on ecosystems
Chemistry
C1 States of matter
C2 Atoms, elements and compounds
C3 Stoichiometry
C4 Electrochemistry
C5 Chemical energetics
C6 Chemical reactions
1
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
72 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Contents
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
C7 Acids, bases and salts
C8 The Periodic Table
C9 Metals
C10 Chemistry of the environment
C11 Organic chemistry
C12 Experimental techniques and chemical analysis
Physics
P1 Motion
P2 Energy, work and power
P3 Thermal physics
P4 Properties of waves
P5 Electricity and magnetism
P6 Electric circuits
P7 Electromagnetic effects
P8 Nuclear physics
P9 Space physics
Additional downloadable resources
The following items are available on Cambridge GO.
Syllabus correlation grid
Lesson plan template
Letter to parents
Approaches to learning and teaching
Active learning
Assessment for learning
Developing language skills
Differentiation
Language awareness
Metacognition
Skills for Life
Glossary
Coursebook answers
Core Practice Book answers
Extended Practice Book answers
2
73 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
How to use this Teacher’s Resource
This Teacher’s Resource contains both general guidance and teaching notes that help you to deliver the content in our Cambridge resources.
There are teaching notes for each chapter of the Coursebook. Each set of teaching notes contains the following features to help you deliver the chapter.
At the start of each chapter there is a teaching plan (see below). This summarises the topics covered in the chapter, including the number of learning hours recommended for each topic, an outline of the learning content, and the Cambridge resources from this series that can be used to deliver the topic.
Coursebook:
Describe the characteristics of living organisms by describing:
• movement as an action by an organism or part of an organism causing a change of position or place
• respiration as the chemical reactions in cells that break down nutrient molecules and release energy for metabolism
• sensitivity as the ability to detect and respond to changes in the internal or external environment
• growth as a permanent increase in size and dry mass
• reproduction as the processes that make more of the same kind of organism
• excretion as removal of the waste products of metabolism and substances in excess of requirements
• nutrition as taking in of materials for energy, growth and development.
Exam-style questions 1 and 2
Workbook:
Exercise 1.1: Characteristics of living organisms
Exercise 1.2: The biological classification system
Exercise 1.3: Keys
vi CAMBRIDGE IGCSE™ BIOLOGY: TEACHER’S RESOURCE
Topic Approximate number of learning hours Learning content Resources 1.1 Characteristics of living organisms 0.5 Core:
74 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CAMBRIDGE IGCSE™ BIOLOGY: TEACHER’S RESOURCE
1.2 Concept and uses of a classification system
2 Core:
State that organisms can be classified into groups by the features that they share.
Describe a species as a group of organisms that can reproduce to produce fertile offspring. Describe the binomial system of naming species as an internationally agreed system in which the scientific name of an organism is made up of two parts showing the genus and species. Construct and use dichotomous keys based on identifiable features.
Supplement:
Explain that classification systems aim to reflect evolutionary relationships.
Explain that the sequences of bases in DNA are used as a means of classification. Explain that groups of organisms which share a more recent ancestor (are more closely related) have base sequences in DNA that are more similar than those that share only a distant ancestor.
Practical Workbook: Practical investigation
1.1: Construct a dichotomous key
Coursebook: Exam-style questions 3 and 6
Workbook: Exercises 1.4 and 1.5: Keys
The topic order generally follows the same sequences as the topics in the syllabus with some exceptions where appropriate.
Content that is aimed at learners who are studying the Supplement is shown in blue in the teaching plan, and there is an arrow to the left of the table.
Each chapter also includes information on any background knowledge that learners should have before studying this chapter, advice on helpful language support, and a teaching skills focus that will help you develop your skills across a number of key pedagogical areas.
These boxes tell you where information in the book is extension content, and is not part of the syllabus.
At the beginning of the teaching notes for the individual topics there is an outline of the learning objectives (see Learning Plan shown) for that topic, as well as any common misconceptions that learners may have about the topic and how you can overcome these. Syllabus learning objectives for learners who are studying the Supplement subject content are indicated in the table in a darker blue colour, with an arrow on the left.
vii
Topic Approximate number of learning hours Learning content Resources
75 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CAMBRIDGE IGCSE™ BIOLOGY: TEACHER’S RESOURCE
LEARNING PLAN
Syllabus learning objectives
Core:
State that organisms can be classified into groups by the features that they share.
Describe a species as a group of organisms that can reproduce to produce fertile offspring.
Describe the binomial system of naming species as an internationally agreed system in which the scientific name of an organism is made up of two parts showing the genus and species.
Construct and use dichotomous keys based on identifiable features.
Supplement:
Explain that classification systems aim to reflect evolutionary relationships.
Explain that the sequences of bases in DNA are used as a means of classification.
Explain that groups of organisms which share a more recent ancestor (are more closely related) have base sequences in DNA that are more similar than those that share only a distant ancestor.
Learning intentionsSuccess criteria
In this topic, learners will:
• find out how the binomial system is used to name organisms discover the relationship between DNA and classification.
Learners will be able to determine how an unfamiliar species is classified and apply knowledge to explain this process.
For each topic, there is a selection of starter ideas, main activities and plenary ideas. You can pick out individual ideas and mix and match them depending on the needs of your class. The activities include suggestions for how they can be differentiated or used for assessment. Many of the challenge ideas focus on allowing learners to apply syllabus concepts to new situations.
Homework ideas give suggestions for tasks, along with advice for how to assess learners’ work.
At the end of the teaching notes for each chapter are a selection of useful links to digital resources, information on cross-curricular links with other subjects, and project guidance to support you in teaching the end-of-chapter project in the Coursebook.
You will find answers to the Coursebook and Workbook questions and exercises at the end of each chapter in this Teacher’s Resource and answers to the Practical Workbook questions at the end of this resource.
This Teacher’s Resource includes a diagnostic test, a mid-point test, three end-of-course practice tests, and end-of-chapter tests. It also includes a differentiation worksheet pack for each chapter. The types of worksheet pack include single worksheets with accompanying help sheets and extra stretch sheets, worksheets that require learners to take on different roles to accomplish a group task, single worksheets that allow for differentiation by outcome, and three different worksheets with varied levels of scaffolding support.
This Teacher’s Resource also contains practical work guidance to support you in teaching the exercises in the Practical Workbook, and any exemplar data that accompany the Practical Workbook chapter. Tests and sample answers have been written by the authors. In examinations, the way marks are awarded may be different.
viii
76 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CAMBRIDGE IGCSE™ BIOLOGY: TEACHER’S RESOURCE
ACTIVITY CONTRIBUTED BY TEACHERS AROUND THE WORLD
This feature consists of activities written by science teachers around the world. They provide you with additional teaching ideas, tried and tested in classrooms.
WHAT OUR CAMBRIDGE PANEL SAYS
This feature is a quote from teachers on our online research community, the Cambridge Panel, showing you what they think of the activity and how they might use it in their classroom.
If you’d like to join the Panel, apply here: https://thepanel.cambridge.org
ix
77 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
B1 Cells and organisms
Teaching plan
B1.01
1 Coursebook: Before you start Question 1
Questions B1.01–B1.02
Practice questions 1–2
Workbook: Exercise 1.1
Describe the characteristics of living organisms by defining:
a movement as an action by an organism or part of an organism causing a change of position or place
b respiration as the chemical reactions in cells that break down nutrient molecules and release energy for metabolism
c sensitivity as the ability to detect and respond to changes in the internal or external environment
d growth as a permanent increase in size and dry mass
e reproduction as the processes that make more of the same kind of organism
f excretion as the removal of waste products of metabolism and substances in excess of requirements
g nutrition as the taking in of materials for energy, growth and development.
B1.02 Cell structure
B1.03 Specialised cells
B1.04 Sizes of specimens
2 Coursebook: Before you start Question 2.
Questions B1.03–B1.09.
Practice questions 3–6.
Workbook: Exercises 1.2–1.3
Describe and compare the structure of a plant cell with an animal cell, limited to: cell wall, cell membrane, nucleus, cytoplasm, chloroplasts, vacuoles.
Identify the cell structures listed above in diagrams and images of plant and animal cells.
Describe the structure of a bacterial cell, limited to: cell wall, cell membrane, cytoplasm, ribosomes, circular DNA, plasmids.
Identify the structures listed above from diagrams of plant, animal or bacterial cells.
Describe the functions of the structures listed above.
1
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
Topic Suggested learning hours Resources Learning content
Characteristics of living organisms
(continued) 78 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CAMBRIDGE
BACKGROUND KNOWLEDGE
• Learners will have some knowledge of the characteristics of living organisms. Even if learners have not followed the Cambridge Lower Secondary course, they will have an understanding of the difference between the terms living and non-living.
• Learners will have some knowledge of cell structure. Even if learners have not followed the Cambridge Lower Secondary course, they will know that living organisms consist of cells and will be familiar with the use of light
State that new cells are produced by division of existing cells.
Describe the meaning of the terms cell, tissue, organ, organ system and organism as illustrated by examples given in the syllabus.
State that specialised cells have specific functions, limited to:
a ciliated cells – movement of mucus in the trachea and bronchi
b root hair cells – absorption
c palisade mesophyll cells – photosynthesis
d neurones – conduction of electrical impulses
e red blood cells – transport of oxygen
f sperm and egg cells (gametes) –reproduction.
State that specialised cells have specific functions, limited to:
a root hair cells – absorption
b palisade mesophyll cells – photosynthesis
c red blood cells – transport of oxygen.
State and use the formula:
magnification image size actual size =
Calculate the size or magnification of biological materials using millimetres as length units.
Convert measurements between millimetres (mm) and micrometres (μm).
microscopes to view cells. They should also be familiar with some subcellular structures, and the similarities and differences between plant and animal cells.
• Learners may know that different types of cell exist in multicellular organisms, and may have heard of red blood cells, sperm cells and so on. If they have not, inform them of the fact that there are trillions of cells in a human body, that they are of different types and have different roles.
2
Topic Suggested learning hours Resources Learning content
IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
79 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
CONTINUED
• Learners may have seen microscopes in use in their prior education, but this is likely to have been heavily led by the teacher. Very early on in this sequence of lessons, show a microscope or a picture of a microscope, and ask learners what they know about this piece of equipment.
TEACHING SKILLS FOCUS
Area of focus: In this chapter, there are many opportunities to formatively assess learners. This is sometimes called ‘assessment for learning’. Its focus is to use learners’ responses to inform the subsequent direction of teaching and learning. Specific focus: As a specific focus, prepare a ‘question plan’ for at least one question in this chapter. This should contain a list of ‘hinge questions’ that you ask at predetermined times during the lesson. These questions are based on just one important concept that is vital to understand before the lesson moves on. All learners must be able to answer it quickly (by labelling, selecting a multiple-choice answer or completing a missing-word passage) and simultaneously (using one of the whole-class response systems described throughout this chapter). The lesson then progresses in one of two ways dependent on their responses. You could also ask this type of question at the very start of a lesson: an initial assessment can be useful to find out what learners know before you begin. They are also important at the end, to help with planning of the next lesson,
LANGUAGE SUPPORT
There are many important terms in this chapter, often describing structures in the cell. This provides an opportunity for learners to develop good habits in note-taking, such as underlining and highlighting key terms or building a glossary as they go.
In each lesson, it is a good idea to write key words on the board. Crosswords, anagrams and missing-word exercises can help learners get to know how to spell and make meaning of the key terms.
• Learners will be familiar with the idea of magnification, as they may have used a magnifying glass previously. Ask learners to think about the actual sizes of biological specimens as a way to introduce the use of units smaller than millimetres and the importance of calculating and using magnification factors.
perhaps in the form of an ‘exit card’ as they leave the room.
Benefits: The benefits of providing opportunities to formatively assess learners using hinge questions are that their progress is fully supported, and learning is more effective. You will also be able to determine who finds what more challenging, empowering you to target the support and challenge activities, listed in this chapter, to the appropriate learners. Think of the purpose of this assessment as ‘feeding forward’, rather than as typical ‘feedback’ that is traditionally offered in response to learners’ answers.
Reflect: To consider how effectively you respond to the challenge, write a short reflection on the effectiveness of the formative assessment opportunities you incorporate into your question plan. You should consider how your approach improved the development of learners’ knowledge and understanding, and which hinge questions were the most effective – and why. How might this result in a change to the wider approach you use in subsequent lessons?
Techniques such as matching words can be useful in this topic. Provide learners with a series of terms in boxes (such as structures found in cells) that must be matched with their descriptions. This activity could be made more active by providing learners with individual printed boxes and challenging them to find a peer with the matching term or description. For descriptions of key words please refer to the Glossary.
3
80 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
B1.01 Characteristics of living organisms
LEARNING PLAN
Syllabus learning objectives
• Describe the characteristics of living organisms by defining:
a movement as an action by an organism or part of an organism causing a change of position or place
b respiration as the chemical reactions in cells that break down nutrient molecules and release energy for metabolism
c sensitivity as the ability to detect and respond to changes in the internal or external environment
d growth as a permanent increase in size and dry mass
e reproduction as the processes that make more of the same kind of organism
f excretion as the removal of waste products of metabolism and substances in excess of requirements
g nutrition as the taking in of materials for energy, growth and development.
Learning intentions
In this topic, learners will:
• learn about the seven characteristics of living organisms.
Common misconceptions
Success criteria
• Learners will be able to decide whether an organism is living or non-living and explain the difficulty in determining whether or not some characteristics apply in some cases.
Misconception
How to identify
How to overcome Learners often perceive plants as organisms that are somehow ‘less alive’ than animals.
Ask learners a ‘trick question’ –ask them to rank order a human, a plant and a bacterium, but do not specify on which terms. Ask one or two learners to explain their choice.
Emphasise that all seven characteristics of living organisms apply to all living organisms; they are just more visible in some organisms than others.
Respiration is often mistaken for breathing or gas exchange.
Egestion is often thought of as a type of excretion. Related to this, learners also assume that plants do not excrete.
Ask learners whether a plant (or another organism that does not ventilate) respires.
Challenge learners to list the materials that they consider to be waste, and then decide if removing each of these can be considered an example of excretion.
Emphasise that the term respiration refers to metabolism; explore the meaning of this term.
Underline the term ‘metabolic’ in the definition of excretion and build an understanding that the contents of faeces have never been a part of the body. Also draw attention to the carbon dioxide that organisms excrete; emphasise that this is invisible.
4 CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
81 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
Starter ideas
Searching for similarities (15 minutes)
Resources: Coursebook: Before you start activity; a potted plant and a desk lamp.
Description and purpose: Learners read and undertake the Before you start activity in the Coursebook. Extend this activity by holding a potted plant in one hand and a desk lamp in the other. Ask learners to think of features that you (as a human) and the potted plant have in common, which are not shared with the desk lamp. Engage learners in a ‘think, pair, share’ activity in which they have 10 seconds to think by themselves, then another 30 seconds to share their ideas with their partner. Then, select a number of learners at random from the class to share their ideas and build a common understanding of the characteristics of life.
What to do next: If learners found the activity difficult, recap the common features of animals and plants with them.
Main teaching ideas
1 Converting definitions into drawings (30 minutes)
Learning intention: Learners describe the characteristics of living organisms by defining the seven characteristics of life.
Resources: Sheets of card that can be cut into small pieces (20 × 20 cm approx.).
Description and purpose: Provide learners with a sheet of card and a pair of scissors. Ask them to produce a series of seven flashcards that each show the name of a characteristic of life and its definition on one side. On the other side learners draw a sketch that represents the characteristic in a visual form. At the end of the task, shuffle all the cards (there will be seven times the number of learners) and hand out seven at random to each learner, face down on their desks. Learners should rank order the cards in terms of quality of communication–whether the sketch accurately reflects the characteristic–rather than drawing quality. This places an emphasis on helping learners understand the meaning of the characteristics, which can be difficult to consider from just the terminology.
Safety: Learners need to be careful when using the scissors.
Differentiation ideas:
Support – During the activity, allow learners who find it difficult to get started with ‘clue words’ to help. For example, a learner may find it difficult to illustrate the concept of respiration. Provide them with a clue word such as ‘gas’, ‘waste’ or ‘energy’.
Challenge – If any learners complete their sketches before the others, ask them to write a very short story about ‘a day in the life of an animal or plant’ and include the seven characteristics of life.
Assessment ideas: Provide learners with a series of unfinished sentences that are written to reinforce their knowledge of this learning. Ask for learners to read out their ideas and ask for comments from other pairs. An example could be: ‘All organisms undertake respiration, which is . . .
2 Characteristics card sort (30 minutes)
Learning intention: Learners describe the characteristics of living organisms by defining the seven characteristics of life.
Resources: Cards or small sheets of paper that have the characteristics of life written on some of them (e.g. ‘can reproduce’, ‘can grow’) and a wide range of other characteristics written on others (e.g. ‘has a brain’ and ‘can carry blood in vessels’).
Description and purpose: Before you start this activity, remove the characteristics of life if you had written them on the board. Give one pack of cards/paper sheets to each pair of learners. Encourage learners to sort through the cards and place them into two piles – one pile that contains features that apply to all living organisms, and one pile that applies to only some organisms. Then ask pairs of learners to swap seats and explore the decisions made by another pair of learners. Hold a class discussion to arrive at a common understanding.
5
82 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
Differentiation ideas:
Support – While learners are sorting their cards, periodically ask them to stop and reconsider. Ask questions to correct misconceptions if you spot incorrect decisions, and encourage learners’ selfreflection. For example, some learners may consider respiration to be the same as breathing. Help learners to overcome this by informing them that plants also respire, but do not breathe.
Challenge – Challenge learners to discuss with each other how the seven characteristics of life can be applied to organisms in different ways, and whether groupings can be made. For example, with regard to reproduction, female humans, mice and bats give birth to live young; (most) reptiles, amphibians and fish lay eggs. Although it should not be acknowledged in this lesson, this activity is good preparation for subsequent lessons on classification.
Assessment ideas: Ask learners to think of a mnemonic for the first letter of each of the seven characteristics of life. The class could then vote for their favourite. ‘MRS GREN’ is a very common option, but are there others?
Plenary ideas
Living laboratory equipment? (15 minutes)
Resources: Laboratory equipment, balance, pH probes, thermometers and so on.
Description and purpose: Ask learners to consider how items of laboratory equipment or other items, such as a moving car, do satisfy some of the characteristics of life. For example, a thermometer is able to sense a change in the environment and the liquid inside it ‘grows’ in response.
Homework ideas
1 Summary questions
Questions related to this topic are Practice questions 1 and 2 in the Coursebook and Exercise 1.1 (Characteristics of living organisms) in the Workbook.
2 Create a characteristics crossword
Challenge learners to design a crossword (either with a pencil and paper or on a computer). The seven words should be the seven characteristics of life; they must write clues for another learner to find them. The choice of which homework task to provide learners may depend on the time that they have available to devote to this. The Coursebook questions will require a greater time commitment. The crossword activity provides an opportunity for learners to be more creative. The follow-up assessment of either task will provide an opportunity for formative assessment: determining if learners have mastered these topics before moving on.
6
83 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
B1.02 Cell structure; B1.03 Specialised cells; B1.04 Sizes of specimens
LEARNING PLAN
Syllabus learning objectives
• Describe and compare the structure of a plant cell with an animal cell, limited to: cell wall, cell membrane, nucleus, cytoplasm, chloroplasts, vacuoles.
• Identify the cell structures listed above in diagrams and images of plant and animal cells.
• Describe the structure of a bacterial cell, limited to: cell wall, cell membrane, cytoplasm, ribosomes, circular DNA, plasmids.
• Identify the structures listed above from diagrams of plant, animal or bacterial cells.
• Describe the functions of the structures listed above.
• State that new cells are produced by division of existing cells.
• Describe the meaning of the terms cell, tissue, organ, organ system and organism as illustrated by examples given in the syllabus.
• State that specialised cells have specific functions, limited to:
a ciliated cells – movement of mucus in the trachea and bronchi
b root hair cells – absorption
c palisade mesophyll cells – photosynthesis
d neurones – conduction of electrical impulses
e red blood cells – transport of oxygen
f sperm and egg cells (gametes) – reproduction.
• State that specialised cells have specific functions, limited to:
a root hair cells – absorption
b palisade mesophyll cells – photosynthesis
c red blood cells – transport of oxygen.
• State and use the formula
magnification image size actual size =
• Calculate the size or magnification of biological materials using millimetres as length units.
• Convert measurements between millimetres (mm) and micrometres (μm).
Learning intentions
In this topic, learners will:
• find out about the structure of the cells of bacteria, plants and animals
• learn about the functions of each of the parts of the cells of bacteria, plants and animals
• discover how some specialised cells are adapted for their functions
• practice using the magnification equation.
Success criteria
• Learners will be able to describe the structure of cells, including animal, plant and bacterial cells, and explain the functions of different components.
• Learners will be able to explain how the structural features of a specialised cell are related to the role of that cell.
• Learners will be able to correctly calculate the size or magnification of biological images.
7 CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
84 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
Common misconceptions
Misconception How to identify How to overcome
Learners sometimes think of cells as rigid or solid, like bricks–probably because they are referred to as ‘building blocks’. Related to this, many learners incorrectly assume that they are flat, two-dimensional objects.
Learners often use inappropriate terms to describe the function of the nucleus (e.g. the ‘brain’ of the cell).
Learners can assume that the vacuole in plant cells is a vacuum, or an empty space.
Starter ideas
What is a cell? (10 minutes)
Show an image of a cell to learners and ask them to describe its shape.
The activities in this chapter that require learners to model cells help to resolve this misconception.
Ask learners for one or two words to describe the function of the nucleus.
Ask learners to suggest how the vacuole was given its name.
Refer to the importance of using appropriate scientific terms in descriptions of cell features. Instead of using the term ‘brain’, use ‘control’.
The term vacuole is derived from the Latin word vacuus which means ‘empty’. Emphasise that this name was given before vacuolar contents could be explored. We now know differently.
Resources: Coursebook: Before you start activity
Description and purpose: Ask learners to read the Before you start feature in the Coursebook and discuss the answers to the questions with a partner. Extend the activity by projecting a large image of some cells onto a screen. Challenge learners to discuss with their partner what is meant by a ‘cell’. What to do next: It is important to ensure that learners understand the terms relevant to the definition of a cell and how they relate to one another. Write these terms, which may include ‘unit’, ‘tissue’ and ‘smallest’, on the board. These will serve as a reminder for learners to refer to as they undertake the subsequent activities.
Main teaching ideas
1 Modelling cells (45 minutes)
Learning intention: Learners make a simple three-dimensional model of a cell to show the structures found inside.
Resources: Coursebook: Figures B1.03–1.06; Sheets of A4 paper that can be easily crumpled up. If coloured paper is used, avoid green.
Description and purpose: Before you start, ask learners to refer to Figures B1.03–B1.06 in the Coursebook. They show a number of photomicrographs of different types of animal and plant cell. Learners draw small pictures of the features of cells on both sides of a piece of A4 paper, ensuring that they only draw one nucleus, but a number of other structures such as chloroplasts and a vacuole. They then crumple their paper into a sphere. Learners swap their models and open them up to explore the various structures inside and provide feedback on each other’s work. Provide prompts to help learners in their discussions: for example, which organelles are found in animal cells and which in plant cells? Are some organelles found only once inside cells, whereas others are found many times? Hold a class discussion to arrive at a common understanding of cell structure.
8
85 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
Differentiation ideas:
Support – Ensure that each learner has a ‘go-to buddy’ during the activity, with whom they can exchange ideas and seek help if necessary. This will help scaffold the learning experience for all.
Challenge – Ask learners to consider how they could convert their model from an animal cell into a plant cell. Suggestions may include wrapping another layer of paper around the outside to represent the cell wall, or include a small additional structure to represent the vacuole. An extra extension could be to produce a bacterial cell using a similar approach.
Assessment ideas: Provide learners with a series of unfinished sentences that are written to reinforce their knowledge of this learning. Ask for learners to read out their ideas and ask for comments from other pairs. An example could include: ‘The job of the cell membrane is . . .’
2 Practical microscopy (50 minutes)
Learning intention: Learners explore how to use a light microscope to view animal and plant cells in the laboratory.
Resources: Light microscope, Samples of animal and plant tissue, Screen projector, Images of drawings by scientists such as Robert Hooke or Charles Darwin.
Description and purpose: Learners should, if possible, view samples of animal and plant tissues under a microscope themselves. If you have suitable equipment, you could project images from a microscope onto a screen. Include in the lesson images of drawings by scientists such as Robert Hooke or Charles Darwin. Ask learners to discuss the importance of drawing a scientific drawing accurately. Ask the learners what they think makes a good scientific drawing and then show them examples of some learners’ drawings and get them to discuss what is good and what could be improved. Ensure there is an opportunity for learners to draw a specimen they see using their microscope. Challenge learners to swap their drawing with others and peer assess their work. What do they think they have done well, and what could be improved?
Differentiation ideas:
Support – Provide learners with an opportunity to seek support. This can be done by producing a series of ‘clue cards’, available on request. If a learner feels they need support, they can request a card from you. Each card provides a ‘hint’ that is intended to give the learner just enough information to help them move on with their work (e.g. ‘Look at your microscope from the side and ensure the objective is positioned just above the slide, before moving it upwards as you look through the eyepiece’ or ‘Use the coarse focus to find the object, and then the fine focus to bring it into a clear focus’). Be aware that many learners find it difficult to exercise the fine motor skills required when using a microscope. Be patient and give encouragement.
Challenge – Look at the photomicrographs in Figures B1.05 and B1.06 in the Coursebook. Suggest why a stain has been added to the animal cells, but we can easily see the plant cells without using a stain.
Assessment ideas: Learners write a short guide for a younger learner to explain how to use a light microscope. This could be accompanied by some brief sketches in the style of a step-by-step cartoon strip or an animated flow diagram on the computer with statements separated by arrows. Select the best two or three creative guides and ask all learners to appraise them, identifying ‘what went well’, and ‘even better if’ to give feedback. An alternative is the ‘two stars and a wish’ approach, whereby learners identify two points of positive feedback and one point of constructive criticism. The very best examples can be bound in a book or put onto the school’s virtual platform, for learners to refer to throughout the course.
Plenary ideas
Activity B1.02 Comparing animal cells and plant cells (10 minutes)
Resources: Coursebook: Activity B1.02
Description and purpose: Learners work on their own or in pairs to make a drawing of a plant cell. They then summarise the functions of three to four structural features of the cell–for example, the cell wall, the vacuole and the chloroplast. Provide an opportunity for learners to peer assess each other’s work.
9
86 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Homework ideas
1 Workbook Exercise 1.2 Organelles and cells
In this exercise, learners practise drawing and labelling animal and plant cells and outline the functions of some of the parts of cells.
2 Create a cell structure concept map
Challenge learners to design a concept map. The various terms associated with cell structure should be included and they must write details regarding their functions in their concept map.
The choice of which homework task to give learners may depend on the time that they have available. The concept map task will take longer but may suit creative learners more, or learners who need encouragement to engage with the content more deeply. The follow-up assessment of either task offers an opportunity for formative assessment: determining if learners have mastered these topics before moving on.
Links to digital resources
Here are some links to a variety of online resources that may be relevant for learners:
• Microscope images 1
• Microscope images 2
• Cells alive website
• Cells from the human body
• Skill development programme website
CROSS-CURRICULAR LINKS
Link the topics learners encounter in this chapter with the curricula of other subjects and collaborate with other teachers from other departments in your school. Here are some examples:
• Compare the specialisation of cells with the specialisation of different tools or cooking utensils used in the technology department. This can be expanded as an analogy as appropriate.
• Refer to the techniques used to rearrange equations in mathematics to help learners rearrange the magnification formula. An example includes the use of a triangle to help determine which factors should be multiplied and which should be divided.
• Liaise with teachers of English to consider the derivation of words used to describe structures found within cells. These include terms such as vacuole, which have both been mentioned earlier in this chapter. Other examples include cytoplasm (derived from ‘cyto-’ which is the Ancient Greek for ‘container’) and nucleus (derived from the Latin for ‘seed’).
With regard to the rest of their biology course, encourage learners to consider how their study of this topic is important in topics such as inheritance (Chapter B12). The skills that learners develop using a microscope are directly required for learners’ observations of blood smears and the gross structure of blood vessels in animals, as well as observing root hair cells and locating the position of xylem and phloem in plants.
Project guidance
A new species
During the project, provide roles to learners during the group work to ensure that all members are engaged. Roles could include the decision maker, the scribe and the internet researcher. This can also be used to differentiate learning: provide a more challenging role for a more confident learner (answering the final question in the project details, for example).
10
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
87 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
To assess the work that learners produce, provide the opportunity to ‘showcase’ their work. This could be in the form of a ten-minute poster marketplace, where one member of each group remains with their poster to explain its contents to other groups as they circulate. Alternatively, short presentations could be given, with learners in the audience encouraged to actively listen: give them a checklist to complete, or challenge them to think of one question to ask at the end of each talk. Provide feedback on their work, and emphasise in your comments that the most successful submissions are those that cover the content of the syllabus, but which use new examples of animals and plants that are unfamiliar to other members of the class.
11
88 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
B1: Cells
B1.1 Specialised cells circus
Worksheet linked to the lesson on specialised cells that provides prompts for learners to determine the difference in structure and function between specialised cells. This task allows for differentiation by outcome: learners produce work of differing levels.
B1.2 Cells–unanswered questions

Structured worksheet that focuses on some of the unanswered questions related to cells, with a help sheet and an extra-stretch sheet to meet the demands of all learners in a class.

CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: BIOLOGY WORKSHEET B1 Cambridge IGCSE™ Combined and Co-ordinated Sciences – Smyth © Cambridge University Press 2023 1
89 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Name
Date B1.1: Specialised cell circus
Cells have a remarkable variety of sizes and shapes. In this activity, you will work in pairs and move around your classroom to complete a research task organised by your teacher. Task 1. In your pair, decide on a one-sentence definition of the term ‘specialised’. Once you have done this, let your teacher know so that they can check your sentence. Task 2. Move around the six stations that your teacher has organised around the room. At each station:
• Draw a diagram of the cell. Remember that your diag ram should be drawn with a sharp pencil and no shading.
• Summarise the function of the cell by using the key words listed in Chapter B1.
• List the structures that are found in this cell tha t adapt it to this function.


CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCE S: BIOLOGY WORKSHEET B1.1 Cambridge IGCSE™ Combined and Co-ordinated Science s –Smyth © Cambridge University Press 2023 1
90 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Name Date


B1.2: Cells–unanswered questions
Although biologists now have a good understanding of the structure and function of cells, many questions still remain. In this worksheet, you will consider some of them.
1 Chloroplasts and mitochondria are structures that share similar features with prokaryotic cells. This has led some scientists to develop the theory that they developed from smaller cells that were absorbed by larger cells a very long time ago. Use the following missing words to explain some of the evidence for this theory.
ATP chlorophyll energy glucose protection respiration
Chloroplasts contain _______________, a pigment that absorbs light energy. Mitochondria are the site of aerobic _______________, a process that produces _______________ that provides cells with _______________. This theory suggests that the larger cell may have provided the smaller cell with _______________, in exchange for _______________.
2 Viruses are non-living particles that invade animal and plant cells. New viruses often emerge, and research often focuses on how they enter cells and use structures inside these cells.
Suggest why the following features of host cells are required by viruses to reproduce.
a Ribosomes
b Mitochondria
CAMBRIDGE IGCSE™
AND
SCIENCES: BIOLOGY
Cambridge IGCSE™ Combined and Co-ordinated Sciences – Smyth © Cambridge University Press 2023 1
COMBINED
CO-ORDINATED
WORKSHEET B1.2
91 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Name Date
Help sheet for Worksheet B1.2: Cells–unanswered questions
Use the information and activities on this sheet to help you answer the questions on the corresponding worksheet.
Question 1
Use Figure 1.1 to help you complete the missing word exercise.

Question 2
Draw lines between the boxes on the left and the boxes on the right to match the features of cells with their descriptions.
nucleus
cell wall
mitochondria
vacuole

cytoplasm
ribosomes
cell membrane
Question 3
A very thin layer surrounding the cytoplasm of every cell; it controls what enters and leaves the cell.
Tiny structures found in animal cells and plant cells. They are so small that we can only see them with an electron microscope.
A tough layer outside the cell membrane; found in the cells of plants, fungi and bacteria.
Small structures in a cell, where aerobic respiration releases energy from glucose.
A structure containing DNA in the form of chromosomes.

A fluid-filled space inside a cell, separated from the cytoplasm by a membrane.
The jelly-like substance that forms the ‘background material’ of all cells.
The number of bacteria in and on a typical human body is around 112 trillion, or around 1.1 × 1014
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: BIOLOGY WORKSHEET B1.2 Cambridge IGCSE™ Combined and Co-ordinated Sciences – Smyth © Cambridge University Press 2023 1
Figure 1.1
92 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Name Date
Extra-stretch sheet for Worksheet B1.2: Cells–unanswered questions
1 The idea that some of the structures in today’s animal and plant cells were once free-living bacteria. This is called the endosymbiotic theory. Research the meaning of the terms endoand -symbiotic
2 Some medicinal drugs have been developed to prevent viruses causing damage to human cells. Carry out some internet research to find out which medicines prevent a virus from performing the following actions.
a Attaching to a cell membrane.
b Carrying out protein synthesis at the ribosome.
c Using the ATP produced by the mitochondria.
3 A typical cell in a human has a diameter of 10 μm. Assuming that all cells are the same size, calculate the distance that would be covered if all of the human cells in a human body were lined up next to each other. Give your answer in kilometres.


CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: BIOLOGY WORKSHEET B1.2 Cambridge IGCSE™ Combined and Co-ordinated Sciences – Smyth © Cambridge University Press 2023 1
93 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Name Date
Diagnostic test

1 Some information about a type of animal is provided below.
Animals have hair on their bodies.
Females give birth and feed their young with milk.
The characteristics given above best describe which type of animal? Choose and circle the answer you consider correct.
a fish
a bird
a mammal
an amphibian
2 Movement is a characteristic common to all organisms. List three other characteristics.
i ____________________
iii ____________________
3 Draw a circle around two cell structures that are found in both plant cells and animal cells. nucleus cell wall cytoplasm cell membrane chloroplast
4 Humans are composed of cells, tissues, organs and systems. Give one example of each.
a Cell ____________________
b Tissue ____________________
c Organ ____________________
d Organ system ____________________
5 Put a tick () in the box by each statement to show whether the statement is true or false. True False
a Digestion occurs in the mouth.
b Fat is an important component of a healthy diet.

c Amino acids are produced when carbohydrates are digested.
d Glycerol reacts with oxygen in aerobic respiration.
e The equation for respiration is the reverse of the equation for photosynthesis.


CAMBRIDGE
TEST Cambridge IGCSE™ Combined and Co-ordinated Sciences – Smyth © Cambridge University Press 2023 1
IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: DIAGNOSTIC
ii
94 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Exam-style questions and sample answers have been written by the authors. In examinations, the way marks are awarded may be different. References to assessment and/or assessment preparation are the publisher's interpretation of the syllabus requirements and may not fully reflect the approach of Cambridge Assessment International Education.
Mid-point test
1 Figure 1.1 shows three types of cell found in some humans.
a Identify two cells that are free to move. Write the letters ______ and ________ [2]
b Give one way in which cell R is adapted to its function. [1]


[Total: 3]
2 Figure 1.2 shows four plant cells. The cytoplasm is shown in grey.

Which cell was placed into a solution with the lowest water potential? Choose and circle the answer you consider correct.
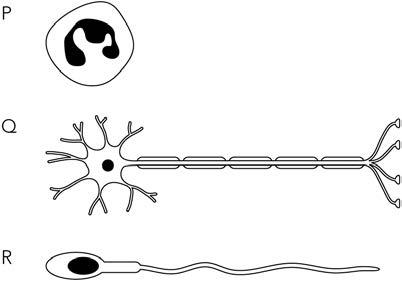
Cambridge IGCSE™
and Co-ordinated Sciences –
© Cambridge University Press 2023 1
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: MID-POINT TEST
Combined
Smyth
Figure 1.1
Figure 1.2
95 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
[1]
Name Date


End-of-chapter B1 test
1 Figure 1.1 is a diagram of a type of virus.

This virus consists only of a protein coat and genetic material. No chemical reactions occur inside the virus. No substances go into or out of the virus.
In order to reproduce, the virus must get inside a cell.
Once it is formed, the size of the virus stays the same.
Explain why some scientists use these facts to class viruses as non-living. [6] [Total: 6]
Cambridge
1
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: END-OF-CHAPTER TEST B1
IGCSE™ Combined and Co-ordinated Sciences – Smyth © Cambridge University Press 2023
Figure 1.1
96 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
C7 Acids, bases and salts
Teaching plan
C7.01
2 Coursebook: Questions C7.01–C7.08
Practice questions 1, 2
Workbook: Exercises 7.1–7.2
• Describe the characteristic properties of acids in terms of their reactions with:
a metals
b bases
c carbonates.
• Describe acids in terms of their effect on the indicators:
a litmus
b methyl orange.
• State that bases are oxides or hydroxides of metals and that alkalis are soluble bases.
• Describe the characteristic properties of bases in terms of their reactions with acids.
• Describe alkalis in terms of their effect on the indicators:
a litmus
b methyl orange.
• Describe how to compare neutrality, relative acidity and relative alkalinity in terms of colour and pH (using universal indicator).
• Describe the neutralisation reaction between an acid and an alkali to produce a salt and water (The ionic equation for this reaction is not required.)
• Classify oxides as either acidic, including SO2 and CO2, or basic, including CuO and CaO, related to metallic and nonmetallic character.
• Describe amphoteric oxides as oxides that react with acids and with bases to produce a salt and water.
• Classify Al2O3 and ZnO as amphoteric oxides.
2
Topic Suggested learning hours Resources Learning content
The characteristic properties of acids and bases
C7.02 Oxides
(continued) 97 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
Questions C7.09–C7.17
Practice questions
3–7
Workbook: Exercises 7.3-7.4
• Describe the preparation, separation and purification of soluble salts by reaction of acid with:
a an alkali by titration
b excess metal
c excess insoluble base
d excess insoluble carbonate. (students do not need to know general solubility rules for salts.)
• Define a hydrated substance as a substance that is chemically combined with water and an anhydrous substance as a substance containing no water.
• Describe the preparation of insoluble salts by precipitation. (students do not need to know general solubility rules for salts.)
BACKGROUND KNOWLEDGE
• Learners should already be familiar with the use of a litmus indicator to distinguish an acid from an alkali and may have previously used universal indicator to determine pH. It is important that learners are confident with these ideas before moving on. If necessary, pause and consolidate the vocabulary and ideas.
• Learners need to be familiar with the idea of concentration as a measure of the amount of a substance dissolved in a given volume of water. Of more importance in this chapter is a qualitative understanding that a higher concentration of hydrogen ions means that
there are more hydrogen ions in the same volume of water. Learners also need to understand that dilution reduces concentration.
• Learners are likely to have been introduced to soluble and insoluble substances before but may not be confident in their understanding of the term ‘solubility’ so ensure learners understand this.
• Learners will need to recall previous learning about ionic bonding and the properties of ionic substances. It may help to revisit ideas from Coursebook Chapter C2.
3
Topic Suggested learning hours Resources Learning content
Preparation of salts 2 Coursebook:
C7.03
98 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
TEACHING SKILLS FOCUS
Area of focus: Active learning.
Specific focus: Reduction of cognitive load in practical work.
Benefits: Often in science we ask a lot of learners in terms of the cognitive load we place upon them. This is particularly true in practical work where we often require learners to process information from many sources at once.
Thus, if we can reduce the cognitive load in the instruction-giving section of such tasks, learners will have more capacity to think about the outcomes of their practical work.
Develop: Firstly, if cognitive load theory is not something that you are familiar with, it would be worth doing some reading around this. There are lots of resources that you can access online; a suggestion is given in the Links to digital resources section.
Showing them a demonstration of what they need to do will support in the interpretation of
LANGUAGE SUPPORT
In this chapter you will find that for some key words an everyday familiarity will help learners to recall the scientific term. Discuss the everyday meaning of the word with learners using a sentence as an example. Then link this everyday meaning to the scientific term. For example, an indicator light on a car is a signal. It tells other road users whether the car is turning left or right. In chemistry an indicator also gives information. It tells us if a solution is an acid or alkali. If something is universal it applies everywhere, e.g., universal indicators work for all pH values.
For other key words, the everyday meaning and alternative meaning in science may cause misunderstanding. Make learners aware of these areas of confusion.
written instructions. You could also consider try using instructions integrated with the diagrams needed for an experiment or even using a video or simulation prior to the learners undertaking the practical to ensure they are familiar with what is required of them.
Reflect:
Before you start teaching this chapter, it would be worth considering the following:
• How easy do the learners in your class normally find it to access practical instructions? It might be worth talking to the learners about what they think.
• How are you going to try and decrease the cognitive load on learners in the practicals in this chapter?
During the exercise design:
• How are you thinking differently about this task to try and reduce cognitive load?
• Is this more difficult for you?
In everyday English, basic means simple. In chemistry basic is the adjective that is used to describe a base. A basic oxide is an oxide that is a base. It is not a simple oxide.
Learners are likely to have met the word neutral in relation to a lack of positive or negative charge. It is important that neutralisation is linked to the neutral pH. Confusingly, if learners continue their studies in chemistry, learners will discover that neutralisation does not always produce a neutral solution.
In day-to-day life, the word salt refers to common salt (sodium chloride). In this chapter learners will need to use the word salt to mean a range of ionic compounds.
For descriptions of key words please refer to the Glossary.
4
99 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
C7.01 The characteristic properties of acids and bases and C7.02 Oxides
LEARNING PLAN
Syllabus learning objectives
• Describe the characteristic properties of acids in terms of their reactions with:
a metals
b bases
c carbonates.
• Describe acids in terms of their effect on the indicators:
a litmus
b methyl orange.
• State that bases are oxides or hydroxides of metals and that alkalis are soluble bases.
• Describe the characteristic properties of bases in terms of their reactions with acids.
• Describe alkalis in terms of their effect on the indicators:
a litmus
b methyl orange.
• Describe how to compare neutrality, relative acidity and relative alkalinity in terms of colour and pH (using universal indicator).
Describe the neutralisation reaction between an acid and an alkali to produce a salt and water (The ionic equation for this reaction is not required.)
• Classify oxides as either acidic, including SO2 and CO2, or basic, including CuO and CaO, related to metallic and non-metallic character.
• Describe amphoteric oxides as oxides that react with acids and with bases to produce a salt and water.
• Classify Al2O3 and ZnO as amphoteric oxides.
Learning intentions
In this topic, learners will:
• describe acids and bases in terms of their effect on indicators
• describe the characteristic reactions of acids
• compare the relative acidity or alkalinity or pH of a solution using universal indicator
• describe how acids and alkalis react together in neutralisation reactions, and that bases displace ammonia from ammonium salts
Success criteria
• Learners will be able to describe acids and alkalis by the effect they have on litmus and methyl orange.
• Learners will be able to state that bases are the oxides and hydroxides of metals, and that soluble bases are referred to as alkalis.
• Learners will be able to compare the relative acidity or alkalinity, or pH of solutions by observing the colour of universal indicator paper.
• Learners will be able to describe neutralisation reactions between acids and alkalis.
5 CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES:
RESOURCE
TEACHER’S
100 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CONTINUED
Learning intentions
• learn that all metal oxides and hydroxides can act as bases, while many oxides of nonmetals can be classified as acidic oxides
• learn that some metal oxides (amphoteric oxides) can react with both acids and alkalis.
Common misconceptions
Success criteria
• Learners will be able to classify metal oxides and hydroxides as bases and recognise that many non-metal oxides are acidic.
• Learners will be able to classify some metal oxides and hydroxides as amphoteric as they can react with both acids and alkalis.
Misconception How to identify How to overcome
A higher pH value means a higher concentration of hydrogen ions.
Show learners three beakers containing acid labelled with a letter and pH.
Fix a piece of string across the classroom. Use pegs to attach paper labels to show pH 1, pH 7 and pH 14. Check that learners recall that acids will have pHs on the left (pH 0–6) and alkalis on the right (pH 8–14). Write paper labels with the letters X, Y and Z and ask a volunteer to peg these in a suitable place along the string.
Ask learners to give the letter of a solution that has a higher concentration of hydrogen ions than Z.
Starter idea
Coursebook: Before you start (10–15 minutes)
Resources: Coursebook (Before you start)
Encourage learners to create sentences that correctly compare the acidity of the three solutions. For example, ‘Solution X is more acidic than solutions Y and Z’. Make sure that learners recall that a more strongly acidic solution has a higher hydrogen ion concentration. Then ask learners to create more sentences that compare the hydrogen ion concentration of the three solutions.
Description and purpose: Arrange learners into small groups and ask learners to read and undertake the Before you start feature in Chapter C7 of the Coursebook. When learners have written their list of everyday acids and alkalis ask them to answer Question 1 and record what each is used for. Ask learners if there are any similarities in the uses, for example alkalis used for cleaning or acids used for flavouring food.
What to do next: Question 2 asks learners to think about how safe the acids and alkalis on their list are. Learners should notice that we eat some acids, so these are safe. Make sure learners know that no other acids should be tasted and that nothing should ever be tasted in their school laboratory. Sometimes learners think that everyday chemicals are safer than laboratory chemicals. You may wish to show the warning labels on some cleaning products to make it clear that household chemicals can also be dangerous. Also remind learners that both acids and alkalis can be unsafe.
6
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
Y
SolutionpH X 1
6 Z 4
101 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
Main teaching idea
Practical investigation: Indicators (10–15 minutes)
Learning intention: Learners will observe the colour change of two different indicators when added to an acid, alkali and neutral solution.
Resources: Spotting tile, pipettes, suitable solutions and indicators.
Description and purpose: Ask learners to create a 3 × 2 grid on the spotting tile. Add a few drops of acid to each spot in the first column. Add a few drops of a neutral solution to column 2 and an alkali to column 3. Learners will then be able to compare the colour of two different indicators (litmus and methyl orange) by adding a few drops along one row.
Safety: Eye protection should be worn throughout. Methyl orange indicator is corrosive, flammable, a health and moderate hazard, hazardous to the aquatic environment, and is acutely toxic. Check hazard warnings for any acids, alkalis or indicators used and take appropriate precautions.
Answers: See Table C7.02 in the Coursebook for the expected colour changes.
Differentiation ideas:
Support – Provide learners with a diagram to show what solutions and indicators should be added where.
Challenge – Ask learners to evaluate the indicators. Ask learners questions such as ‘Will all the indicators tell you whether a solution is an acid or alkali?’, ‘In what way do you think universal indicator is a better indicator?’ or ‘In what way is it not so good?’
Assessment ideas: Add another solution (acid or alkali) to three beakers. Ask learners to predict the colour change for each indicator. Add drops of each indicator to allow learners to check if they were correct.
Plenary idea
Venn diagrams (5 minutes)
Resources: A copy of a Venn diagram with one circle on the left, labelled ‘acid’. A second circle on the right, labelled ‘base’, with a smaller circle inside it labelled ‘alkali’.
Description and purpose: Give each learner a blank copy of the Venn diagram. Ask learners to write the following substances in the correct place on the diagram to help them to understand how it works:
HCl (acid), CaO (base), KOH (alkali), water (neutral)
Then ask learners to write the following oxides in the diagram. Acidic oxides should be written in the acid circle, basic oxides in the base circle, and neutral or amphoteric (Supplement) oxides in neither. This exercise will check learners’ understanding of the pH of metal and non-metal oxides, and the difference between dissolved bases (alkalis) and bases.
CO2 (acidic), H2O (neutral), CaO (basic), MgO (basic), SO2 (acidic), Al2O3 (amphoteric)
Assessment ideas: Collect in learners’ completed diagrams to check their responses.
Homework ideas
1 Workbook exercises 7.1 and 7.2
Ask learners to answer selected questions from these exercises.
2 Acids and alkalis in the home
Ask learners to list a range of substances in their home that contain acids or alkalis. You can find lists of these online or use the link provided in the Links to digital resources. If resources allow you could give learners a few strips of indicator paper (e.g., red and blue litmus indicator) to take home to test some of the items–you would need to discuss which types of items would be safe to test, such as food-related items or toothpaste.
7
102 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
C7: Acids, bases and salts
C7.1: Acids, bases and salts


Worksheet, help sheet and extra-stretch sheet
This worksheet pack comprises three worksheets. The first worksheet is aimed at all learners, which covers the main aspects of Chapter 7. The second worksheet is designed as an extra stretch and challenge, and explores the reactions of acids and bases in greater detail. The third help worksheet is designed to support learners.
Allow learners to self-select an appropriate worksheet but they can use another sheet to help support them if needed. Learners can swap and Peer mark using the answers provided.
C7.2: A video on preparation of salts
The nature of this activity is open-ended–a single worksheet gives all learners the same task but is designed to allow learners to achieve the outcome in different ways, depending on their individual level and confidence. Learners’ submitted work must contain key points from Chapter C7 of the Coursebook. The success criteria shown in the learner sheet is a useful document to refer to when marking.
The accuracy of the submitted work should be checked. However, you should also consider the effort and degree of originality and creativity of this piece of work. You could help learners by guiding them to specific parts of the Coursebook, e.g., providing page numbers. As well as this, you could give key words that should be included for each point. You could make available the necessary practical equipment so that learners are aware of the equipment needed for the practicals. You may wish to consider grouping learners together to reduce the demand of the activity. If learners work in groups, then consider group-working skills when marking their work. As a challenge, learners could complete this activity before you teach them the content. This approach is known as flipped learning (see the Teaching skills focus in Chapter C7 for more details).
In terms of producing a video, the following devices could be used:
• smart phones/cameras
• tablets
• laptop/desktop with presentation software and microphone
• visualiser/webcam.
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: CHEMISTRY WORKSHEET C7 Cambridge IGCSE™ Combined and Co-ordinated Sciences – Smyth © Cambridge University Press 2023 1
103 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Name Date
Extra-stretch sheet for Worksheet C7.1: Acids, bases and salts
1 Many of the pyramids constructed by the Ancient Egyptians still stand today because of the lime cycle. This process involved applying a solution, known as slaked lime, Ca(OH)2, which then hardened as it reacted to reform solid calcium carbonate.
a Use this information and your own understanding to complete the cycle in Figure 7.1.
b Write a balanced symbol equation to show the reaction of slaked lime with hydrochloric acid.
2 This question explores the reaction of magnesium ribbon with hydrochloric acid (HCl) and ethanoic acid (CH3COOH). Ethanoic acid is the acid found in vinegar.
a Write a balanced symbol equation for the reaction of magnesium with HCl.


b A student wanted to compare the difference in reactivity between hydrochloric acid with magnesium ribbon and ethanoic acid with magnesium ribbon. They were supplied with the following equipment:
• Measuring cylinder
• Ruler
• Conical flask
• Gas syringe
• Timer
• Hydrochloric acid
• Ethanoic acid

• Magnesium ribbon
i Write a method to explain how the difference in reactivity can be investigated.
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: CHEMISTRY WORKSHEET C7.1 Cambridge IGCSE™ Combined and Co-ordinated Sciences – Smyth © Cambridge University Press 2023 1
Figure 7.1
104 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Name Date
Help sheet for C7.1: Acids, bases and salts
1 Complete these word equations to show the substances, or types of substances that are formed.
a acid + base → + water
b acid + __________ → salt + hydrogen
c + → salt + water + carbon dioxide
In the first equation, sodium chloride would be an example of this type of substance.


In the second equation, magnesium would be an example.
In the third equation, one substance is a type that reacts with bases. The other contains the elements from which carbon dioxide is formed.
1 This question relates to the formation and reaction of oxides.
a Complete the table.
b What is the link between the type of oxide and solution formed?
c Why are the oxides of zinc and aluminium considered to be unusual? They are called amphoteric oxides, but what does that mean?
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: CHEMISTRY WORKSHEET C7.1 Cambridge IGCSE™ Combined and Co-ordinated Sciences – Smyth © Cambridge University Press 2023 1
Element Oxide Reaction with oxygen Solution Reaction with further oxygen and/or water Li + → ____ Li2O Li2O + H2O → ____LiOH Na + → ____ + H2O → ____NaOH Ca + → ____ CaO CaO + H2O → ____ ____Mg + → + H2O → Mg(OH)2 + → SO2 SO2 + O2 + → ____H2SO4 N ____ + ____ → ____NO2 ____NO2 + O2 + ____ → ____HNO3
105 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
2 This question relates to the use of indicators.


a Match the indicator to the correct observations.
b Give an advantage and disadvantage of the use of universal indicator. Think of the range of pH values and how many numbers there are on the scale. How many colours are possible with universal indicator?
3 Describe the difference between a base and an alkali. This is about solubility in a common solvent.
CAMBRIDGE
Cambridge IGCSE™
©
University Press 2023 2
IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: CHEMISTRY WORKSHEET C7.1
Combined and Co-ordinated Sciences – Smyth
Cambridge
Indicator Colour in acid Neutral Colour in alkali Universal indicator Red Purple Blue Litmus Red Orange Yellow Methyl orange Red Green Purple
106 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Name Date
Diagnostic test
1 Use the words to answer the questions below.
acid copper melting salt condensation liquid metal solution
Each word may be used once, more than once or not at all.
a Which word is shiny silver substance that conducts electricity?
b Which word describes the change that takes place when a gas changes to a liquid?
c Which word describes a substance that turns litmus paper red?
d Which substance is a solid dissolved in a liquid? [1]


e Which substance is an element?
2 When lumps of calcium carbonate are added to acid, bubbles are produced as the reaction proceeds:
calcium carbonate + hydrochloric acid → calcium chloride + water + carbon dioxide
a Which substance produces the bubbles?
b How could calcium chloride be separated from the mixture?
CAMBRIDGE IGCSE™
TEST Cambridge IGCSE™ Combined and Co-ordinated Sciences – Smyth © Cambridge University Press 2023 1
COMBINED AND CO-ORDINATED SCIENCES: DIAGNOSTIC
[1]
[1]
[1]
[Total: 5]
[1]
[1]
[1] 107 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Exam-style questions and sample answers have been written by the authors. In examinations, the way marks are awarded may be different. References to assessment and/or assessment preparation are the publisher's interpretation of the syllabus requirements and may not fully reflect the approach of Cambridge Assessment International Education.
Mid-point test
1 The list shows seven words related to atomic structure and bonding:
A anion
B cation
C covalent
D ionisation
Answer the following questions.
E isotopes
F nucleons
G sharing
Each letter may be used once, more than once or not at all.
Give the letter A, B, C, D, E, F or G that:
a describes an ion with a positive charge


b describes protons and neutrons
c describes what happens to electrons when carbon reacts with oxygen
d describes atoms with the same number of electrons but different numbers of neutrons
e describes what happens to a sodium ion when it reacts with chlorine
f describes an atom that has lost electrons.
Cambridge
1
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: MID-POINT TEST
IGCSE™ Combined and Co-ordinated Sciences – Smyth © Cambridge University Press 2023
[1]
[1]
[1]
[1]
[1]
[1] [Total: 6] 108 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Name Date
End-of-chapter C7 test
1 The formulae of several compounds are shown.
CO2 NaCl H2O HCl
CaCO3
Na2SO4
H2SO4 Write the letters of the three compounds that are salts. ________________ and ______________ and _______________ [3]


2 Acids are important compounds with many uses. They react with metal and metal carbonates.
a Name a metal that will not react with hydrochloric acid. [1]
b Write a word equation to show the reaction between zinc carbonate and sulfuric acid. [2]
c Acids also react with bases.
i State which types of oxide are bases. [1]

ii State the name given to a base that is dissolved in water. [1]
d What name is given to the reaction between an acid and a base that results in neutral products [1] [Total: 6]



Cambridge IGCSE™
and Co-ordinated Sciences –
© Cambridge University Press 2023 1
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: END-OF-CHAPTER TEST C7
Combined
Smyth
109 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
P2 Energy, work and power
Teaching plan
P2.01–P2.12
Practice questions
1–3
Workbook: Exercise 2.1
• State that energy may be stored as kinetic, gravitational potential, chemical, elastic (strain), nuclear, electrostatic and internal (thermal).
• Describe how energy is transferred between stores during events and processes, including examples of transfer by forces (mechanical work done), electrical currents (electrical work done), heating, and by electromagnetic, sound and other waves.
• Know the principle of conservation of energy and apply this principle to simple examples including the interpretation of simple flow diagrams (Sankey diagrams are not required).
• Understand, qualitatively, the concept of efficiency of energy transfer.
• Define efficiency as:
a efficiency useful energy output energy input =× 100%
b efficiency useful power output total powe r input =× 100% recall and use the equations. P2.02
Coursebook: Questions
P2.13–P2.17
Practice question 7
Workbook: Exercise 2.2
• Recall and use the equation for kinetic energy Emv k 1 2 = 2
• Recall and use the equation for the change in gravitational potential energy
ΔE p = mgΔh
2
Topic Suggested learning hours Resources Learning content
Energy stores, transfers and conservation 1 hour Coursebook:
P2.01
Questions
hour
Energy calculations 1
(continued) 110 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
P2.04 Doing
work
0.5
P2.18–P2.28
Practice questions
8, 9
Workbook:
Exercises 2.3, 2.4, 2.5
• Describe how useful energy may be obtained, or electrical power generated, from:
a fossil fuels
b biofuels
c water, including waves, tides, and hydroelectric dams
d geothermal resources
e nuclear fission
f light from the Sun (solar cells)
g infrared and other electromagnetic waves from the Sun to heat water (solar thermal collectors)
h wind (wind turbines)
including references to a boiler, turbine and generator where they are used.
• Give advantages and disadvantages of each method in terms of renewability, availability, reliability, scale and environmental impact.
• Know that radiation from the Sun is the main source of energy for all our energy resources except geothermal, nuclear and tidal.
• Know that energy is released by nuclear fusion in the Sun (detailed knowledge of the process of fusion is not required).
• Know that energy is released by nuclear fission in nuclear reactors (detailed knowledge of the process of fission is not required).
2.05 Power 0.5
Coursebook: Questions
P2.29–P2.32
Practice questions
5, 6 b, 9 a, b, c
Workbook:
Exercises 2.6, 2.7
Coursebook: Questions
P2.33–P2.37
Practice questions
6 c, 9 d, e
Workbook: Exercise 2.8
• Understand that mechanical or electrical work done is equal to the energy transferred.
• Recall and use the equation for mechanical working W = Fd = ΔE
• Define power as work done per unit time and also as energy transferred per unit time; recall and use the equations
a P W t =
3
Topic Suggested learning hours Resources Learning content
2 hours Coursebook:
P2.03 Energy resources
Questions
t
111 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
b P E
= ∆
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
BACKGROUND KNOWLEDGE
• Learners should know that energy is a quantity that must be changed or transferred in order to make something happen.
• Learners should know that energy can be stored, and be able to recall the names of some energy stores.
• Learners should know that energy can be transferred, and be able to recall some of the ways in which energy transfer occurs.
• Learners should recall the principle of conservation of energy.
• Learners should understand that energy can be transferred between stores from previous courses and be able to name some of these methods.
• Learners should understand that not all energy is changed or transferred usefully.
TEACHING SKILLS FOCUS
Area of focus: Metacognition
Specific focus: Trying to understand energy
Teachers of physics have a doubly difficult role.
First, they are trying to convey understanding of a conceptually challenging subject that contains many abstract ideas. Second, they are trying to convey understanding of some concepts that have not been fully worked out, even by researchers at the highest level.
To quote Nobel Prize-winning physicist Richard Feynman: ‘It is important to realise that in physics today, we have no knowledge of what energy is’.
Benefits: How, therefore, do we expect learners to understand energy?
The solution to this problem is to present energy in a simplified way, within the context of what learners actually need to know. How the learners process this knowledge to gain understanding can be greatly helped with metacognition.
Develop: Metacognition can be thought of in two parts (sometimes called dimensions!): metacognitive knowledge and metacognitive regulation.
Metacognitive knowledge describes what learners know about their own learning, for example: ‘I find it difficult to remember the
• Learners should understand that wasted energy is dissipated.
• Learners should be familiar with the idea that the gravitational effects of the Moon and Sun cause tides on Earth, and that these cause sea levels to rise and fall daily.
• Learners should recall that energy sources can be classed as renewable or non renewable and be able to give examples of each.
• Learners should recall information about climate change and the effects of combustion on the carbon cycle.
• Learners should understand how to convert a fraction or decimal number less than 1 to a percentage.
• Learners should know about forces and their effects from Chapter P4, and from previous courses.
names of the energy stores.’ It also describes their perception of tasks, for example: ‘I know I’m going to find the ideas in this topic to be complex.’
Metacognitive regulation describes what learners will do about their own learning, for example ‘I have used this strategy before, but it’s not working this time, so I’m going to try X instead.’ Putting this into practice involves the teacher doing certain things:
1 Setting out and sharing clear learning outcomes for the topic or lesson. This does not always need be done at the start of the lesson, but may appear mid-way through a task: ‘This is the part you need to understand.’
2 Fostering a supportive learning environment. Learners are not worried about making mistakes, showing lack of understanding or asking for help. No matter what they say, they will not be made to feel silly.
3 Allowing learners to add to their own notes. This can be done as a learning blog where learners have their more formal notes and, maybe in the margin, their own personal comments reflecting on how they think about different parts of the topic.
4
112 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CONTINUED
4 Thinking aloud. Teachers think that a learner talking about what they are thinking during a task is an annoying distraction. However, research has shown that saying thoughts out loud can help learners to focus on the task and improve cognitive processing.
5 Allowing learners to develop their own strategies. Mnemonic devices are a wellknown example of this, but developing visual or imaginary models can also be useful.
LANGUAGE SUPPORT
For meanings of key words, please see the glossary.
In the past, teachers would sometimes refer to ‘forms’ or ‘types’ of energy. We do not use these terms any more because they imply that the energy is somehow a different type of quantity. Instead now, we refer to the names of the stores or the methods of transfer. This revised terminology will help learners better understand the topic.
Helping learners to make these connections across subjects will consolidate their learning, and make your task easier!
Reflect:
Remember:
• we need to create opportunities for learners to develop and practise new learning strategies
• we need to help learners to learn how to think, rather than telling them what to think.
Care should be taken with the symbol for work done, which is printed as the italic upper-case W, and the unit of power, which is printed as uppercase but not italic W. When hand-written, or written on a board for teaching, these will look the same.
Learners should be aware that in physics, power is an active term and only applies when an event or process is occurring. In some areas of everyday speech, power can have passive uses, such as a country or political leader described as having power.
P2.01 Energy stores, transfers and conservation
LEARNING PLAN
Syllabus learning objectives
• State that energy may be stored as kinetic, gravitational potential, chemical, elastic (strain), nuclear, electrostatic and internal (thermal).
• Describe how energy is transferred between stores during events and processes, including examples of transfer by forces (mechanical work done), electrical currents (electrical work done), heating, and by electromagnetic, sound and other waves.
• Know the principle of conservation of energy and apply this principle to simple examples including the interpretation of simple flow diagrams (Sankey diagrams are not required).
• Understand, qualitatively, the concept of efficiency of energy transfer.
5 CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
113 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: TEACHER’S RESOURCE
Links to digital resources
Here are some links to a variety of online resources that may be relevant for learners:
• How a wind turbine works
• Tidal power
• GCSE revision: work and power
• GCSE revision: work done and energy transfer
• Work done and power
CROSS-CURRICULAR LINKS
Link the topics learners encounter in this chapter with the curricula of other subjects and collaborate with other teachers from other departments in your school. Here are some examples:
• Maths: calculating work, power, kinetic energy and gravitational potential energy.
• Biology: energy available from aerobic and anaerobic respiration.
Project guidance
For Option 1, learners could start by researching the wind-up radio that works without batteries, although the generator concept will be covered later, in Chapter P5.
Learners could also research the play pump. This was a device invented as a horizontal wheel, approximately 3 m in diameter, for children to push and then ride upon, like a roundabout. The axle of the wheel was connected to a water pump.
Some water pumps are powered by windmills. These are very efficient ways of raising water. A windmill with a 2.5 m diameter fan in a 30 km/h wind can raise over 500 litres of water through a height of 50 m in one hour.
Windmills and water mills, including tide mills, have been used for centuries before the discovery of electricity generation.
Option 2 can trace the development first of filament lamps followed by compact fluorescent tube (CFT) ‘energysaving’ bulbs and then light-emitting diode (LED) lamps.
The easiest way for learners to investigate the efficiency of car engines is to research the improvements in fuel economy, usually measured in litres per 100 km. Here, the lower the number, the greater the efficiency.
16
114 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
P2: Energy, work and power


P2.1 Energy stores and transfers
Worksheets P2.1A, B and C contain differentiated questions on energy stores and transfers. Worksheet P2.1A requires only qualitative answers whereas Worksheet P2.1C also contains calculations. Consideration should be given to what syllabus route the learners are taking before allocating these worksheets.
P2.2 Work and power
A set of differentiated worksheets on the topics of work and power. Learners can find understanding of these concepts to be challenging, so the worksheets are designed to assess understanding rather than just recall. Worksheet P2.2C requires greater understanding than P2.2A or P2.2B.
CAMBRIDGE IGCSE™
AND
SCIENCES:
P2 Cambridge IGCSE™ Combined and Co-ordinated Sciences – Smyth © Cambridge University Press 2023 1
COMBINED
CO-ORDINATED
PHYSICS WORKSHEETS
115 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Name Date
P2.1A: Energy stores and transfers
In this worksheet you will think about energy stores and transfers.
1 The list contains some energy stores and transfers. Write each one into the correct column of the table electric current kinetic electrostatic chemical gravitational potential light sound nuclear

2 Name the energy store used in each of these. The first one has been done for you. Stretched rubber band elastic

CAMBRIDGE IGCSE™
AND CO-ORDINATED SCIENCES:
Cambridge IGCSE™ Combined and Co-ordinated Sciences – Smyth © Cambridge University Press 2023 1
COMBINED
PHYSICS WORKSHEET P2.1A
store
transfer
Hot water _______________ Sugar _______________ Book on a shelf _______________ 116 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
3 Figure 2.1 shows a simple circuit.


Describe the energy stores and transfers that happen in this circuit. Include where each store or transfer occurs.
4 a State the principle of conservation of energy.
b The engine of a truck converts 40% of the energy from the fuel into kinetic energy. Use the principle of conservation of energy to suggest what else is happening in the engine.

CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: PHYSICS WORKSHEET P2.1A
2
Cambridge IGCSE™ Combined and Co-ordinated Sciences – Smyth © Cambridge University Press 2023
Figure 2 1
117 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Name Date
Diagnostic test
1 Write the unit for each of these quantities. Choose a unit from the list. You do not have to use all the units.
cm3 kg W J N m V N/m2
a weight _______________
b mass _______________
c volume _______________


d pressure _______________
e energy _______________
2 A long spring is attached to a wall. A student holds the other end of the spring, as shown in Figure 1.

CAMBRIDGE IGCSE™
AND
SCIENCES:
TEST Cambridge IGCSE™ Combined and Co-ordinated Sciences – Smyth © Cambridge University Press 2023 1
COMBINED
CO-ORDINATED
DIAGNOSTIC
Figure 1
118 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Draw arrows on Figure 1 to show how the student’s hand should move to model a sound wave in the spring.
Exam-style questions and sample answers have been written by the authors. In examinations, the way marks are awarded may be different. References to assessment and/or assessment preparation are the publisher's interpretation of the syllabus requirements and may not fully reflect the approach of Cambridge Assessment International Education.
Mid-point test
You will need a protractor.
1 A student has a book and a ruler. The book has 122 pages. Describe how the student can use the ruler to measure the thickness of one page. [2]
[Total: 2]
2 Some quantities are scalars and others are vectors.
a Complete the table by putting a tick () in the correct column to show whether each quantity is scalar or vector. The first one has been done for you. [2]
b Figure 1 shows two forces acting on a point P. The forces act in opposite directions. The figure is not to scale.


Cambridge IGCSE™
and Co-ordinated Sciences –
© Cambridge University Press 2023 1
CAMBRIDGE IGCSE™ COMBINED AND CO-ORDINATED SCIENCES: MID-POINT TEST
Combined
Smyth
Quantity Scalar Vector force mass acceleration velocity kinetic energy
1 119 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
Figure
Name Date


End-of-chapter P2 test
1 Figure 2.1 shows a water tower. The pump works using electricity. The pump moves water from underground up through the pipe.

The water is stored in the storage tank. State the energy changes that happen in:
a the pump [2]
b the water in the pipe. [2]
CAMBRIDGE
1
IGCSE™
COMBINED
AND CO-ORDINATED SCIENCES: END-OF-CHAPTER TEST P2
Cambridge IGCSE™ Combined and Co-ordinated Sciences – Smyth © Cambridge University Press 2023
Figure 2.1
120 We are working with Cambridge Assessment International Education towards endorsement of these resources. Original material © Cambridge University Press & Assessment 2023. This material is not final and is subject to further changes prior to publication. SAMPLE
[Total: 4]











































































































