

@ UT Southwestern DISCOVERY













Snapshot of Twist2+ cells differentiating to form muscle. A team from the Eric Olson laboratory discovered this novel stem cell in adult skeletal muscle, critical to maintaining muscle mass throughout aging. “This image is really special to me,” says Glynnis Garry, M.D., Assistant Professor of Internal Medicine and Molecular Biology, about the photo depicting one of her earliest discoveries at UT Southwestern, which happened while she was a medical student in the Olson lab. She recalls that upon learning about the Twist2+ cells, Dr. Olson told her: “Magic happens in this lab. Everyone is capable of discovery here.”
5,300+
Letter From Leadership


The latest edition of Discovery at UT Southwestern features the stories of scientists who are working across the spectrum of biomedical and clinical research against the backdrop of the collaborative scientific culture that sets our institution apart.
As one of the nation’s premier academic medical centers, UT Southwestern is known for impactful discoveries. Our faculty approach their work with curiosity and collegiality, often partnering with colleagues in other disciplines and nurturing the careers of rising stars. They are pushing the boundaries of what we know and inspiring what can be.
For example, a multidisciplinary team of researchers has upended the traditional view
Sincerely,
W. P. ANDREW LEE, M.D.
Executive Vice President for Academic Affairs, Provost, and Dean, UT Southwestern Medical School


of how healthy and cancerous cells acquire their supplies of purines – vital components of DNA, RNA, and other cellular systems. A story on cancer metabolism explains how the impact of their findings ranges from showing how chemotherapy resistance occurs to identifying metabolic dysfunction in other diseases.
Another research team of biomedical engineers, biochemists, oncologists, and pharmacologists developed a system in which nanocarriers direct genetic medicines to desired organs.
A pilot program that introduced the OR Black Box to William P. Clements Jr. University Hospital revealed this technology improved safety, outcomes,
JOAN W. CONAWAY, PH.D.
Vice Provost and Dean for Basic Science
ALYSSA H. HASTY, PH.D.
Vice Provost and Senior Associate Dean for Faculty Affairs and Career Development


and efficiencies, and strengthened surgical teams.
UT Southwestern’s wealth of research infrastructure and clinical resources is enhanced by robust connections with partner institutions that make conducting large-scale, pragmatic clinical trials and quality improvement initiatives possible.
We are committed to fostering an environment where faculty, trainees, students, and staff feel supported and empowered to actively participate in our mission to promote health and a healthy society that enables individuals to achieve their full potential.
SHERRY C. HUANG, M.D.
Vice Provost and Senior Associate Dean for Education
ERIC PETERSON, M.D., M.P.H.
Vice Provost and Senior Associate Dean for Clinical Research
From left: Drs. Huang, Hasty, Lee, Conaway, and Peterson photo credit : Mei - chun Jau / ut southwestern Medical center

Solving the Mystery of the Body’s Defenses
IDiscoveries by Zhijian “James” Chen, Ph.D., illuminate how innate immunity is triggered, inspiring new approaches to treat many diseases, including cancer.
nnate immunity serves as the body’s first line of defense, fighting off invaders such as microbial infections, inflammatory diseases, and even cancerous tumors. But exactly how immunity gets triggered and why it sometimes fails has long baffled scientists.
Thanks to a series of significant discoveries by biochemist Zhijian “James” Chen, Ph.D., Professor of Molecular Biology and Director of the Center for Inflammation Research at UT Southwestern Medical Center, this mystery is being solved. Dr. Chen’s groundbreaking discovery of the cGAS enzyme, which he describes as the body’s “burglar alarm” that sets off immune and inflammatory responses, has opened up new approaches to the treatment of cancer, autoimmune diseases, and more.
Award-Winning Research
In 2024, Dr. Chen received the prestigious Albert Lasker Basic Medical Research Award, known as “America’s Nobel,” in recognition of the cGAS discovery. His work on innate immunity has also been honored with the Paul Ehrlich and Ludwig Darmstaedter Prize, Germany’s highest honor in the field of medicine (2024); the Louisa Gross Horwitz Prize (2023); the William B. Coley Award
for Distinguished Research in Basic and Tumor Immunology (2020); the Breakthrough Prize in Life Sciences (2019); the Switzer Prize (2019); the Lurie Prize in Biomedical Sciences (2018); and the National Academy of Sciences Award in Molecular Biology (2012).
“To be able to discover something that nobody has ever discovered before is extremely exciting,” says Dr. Chen, a Howard Hughes Medical Institute Investigator since 2005 and an elected member of the National Academy of Sciences (2014) and the National Academy of Medicine (2022).
His findings on the inner workings of innate immunity have been recognized not only for their originality and elegance but also for their significance among the scientific community around the world.
“Dr. Chen’s creative and meticulous work exemplifies our commitment at UT Southwestern to fundamental research that has the potential to lead to new and better ways to treat disease,” says W. P. Andrew Lee, M.D., Executive Vice President for Academic Affairs, Provost, and Dean of UT Southwestern Medical School. “His discovery of the cGAS-cGAMP-STING pathway is a product of the UT Southwestern scientific environment, where talent is actively recruited, nurtured, and promoted.”
Zhijian “James” Chen, Ph.D., addresses the crowd at a reception celebrating his Albert Lasker Basic Medical Research Award.
“I vividly remember how awestruck my colleagues and I were when James first presented this work in a faculty meeting.”
A Legacy of Discoveries
Originally from rural China, Dr. Chen earned an undergraduate degree in biology from Fujian Normal University and a Ph.D. in biochemistry from State University of New York at Buffalo. After a brief stint in the biotech industry, he joined UT Southwestern in 1997.
Early in his career, Dr. Chen identified key functions of a small protein called ubiquitin that activates other proteins key to regulating immune and inflammatory responses. This led, in 2005, to the detection of a new protein that showcases the role of mitochondria in immunity. While other misfolded proteins are known for causing disease, the mitochondrial antiviral signaling (MAVS) protein that Dr. Chen discovered was the first prion in humans to show a benefit, in this case fighting off RNA viruses such as influenza, West Nile virus, SARS-CoV-2, and Ebola. Dr. Chen created an acronym from the protein’s function, dubbing it MAVS after his favorite basketball team, the Dallas Mavericks.
Then, in 2012, Dr. Chen discovered the cyclic GMP-AMP synthase enzyme now known as cGAS. He and his colleagues found that cGAS produces a small molecule called cGAMP, which in turn acts as a secondary messenger to activate STING (stimulator of interferon genes) and trigger an inflammatory response. This research into how a cell communicates with its surroundings and responds to foreign stressors containing DNA is fundamental to human health.
“James Chen is a true wizard of biochemistry,” says Eric Olson, Ph.D., Professor and Chair of Molecular Biology, who sought out Dr. Chen as his first recruit when he launched the department in 1996. “His ingenious biochemical strategy to unveil the signaling system that senses rogue DNA in the cytoplasm is truly breathtaking. I vividly remember how awestruck my colleagues and I were when James first presented this work in a faculty meeting.”
Lasting Impact
Dr. Chen found that cGAS works as a sensor to identify pathogens containing DNA. And because cGAS can be activated by any double-stranded DNA, it plays an essential role in a variety of physiological and pathological processes:
• Autoimmune and inflammatory diseases, including a rare disease called AicardiGoutières syndrome (AGS), lupus, arthritis, and severe COVID-19 infections
• Neurodegenerative diseases, such as Parkinson’s, Alzheimer’s, and amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease
• Antimicrobial infections caused by bacteria, viruses, and parasites
• Cellular senescence, which acts as a defense mechanism by halting cell division
• Autophagy, which plays a key role in antiaging principles as cells remove damaged or dysfunctional parts and repair themselves
The goal now, Dr. Chen says, is to understand how to harness the power of this cGAS-cGAMPSTING pathway for the treatment of human diseases, whether by inhibiting cGAS or using cGAMP and its derivatives as adjuvants for vaccines or cancer immunotherapies.
“Immunity is all about balance,” explains Dr. Chen, member of the UT Southwestern Harold C. Simmons Comprehensive Cancer Center and Professor in the Center for the Genetics of Host Defense. “If the immune system is not active enough, infectious diseases or cancers can take hold. An overactive immune system, on the other hand, can cause autoimmune diseases like lupus, and neurodegeneration such as Parkinson’s disease and Alzheimer’s disease.”
Dr. Olson says his colleague’s research will have a lasting impact as its applications in medicine are developed.
“Dr. Chen’s discoveries of the mechanisms whereby cells detect viral RNA and misplaced DNA in the cytoplasm of cells will stand for all time as a cornerstone of immunology,” he says. “We are only beginning to comprehend the range of biomedical implications of his discoveries, which connect virology, autoimmune disorders, cancer, immunotherapy, and other areas yet to be discovered.”

“James” Chen, Ph.D., Professor of Molecular Biology and Director of the Center for Inflammation Research at UT Southwestern Medical Center

P. Andrew Lee, M.D., Executive Vice President for Academic Affairs, Provost, and Dean of UT Southwestern Medical School

and
Zhijian
W.
Eric Olson, Ph.D., Professor
Chair of Molecular Biology

helped her team uncover targets for chemo-resistant cancers.
Gerta Hoxhaj, Ph.D., uses a microscope alongside her laboratory members. A multidisciplinary approach has
photo courtesy of the vilcek foundation (photographer stewart cohen )

A Breakthrough Discovery Into How Cancers Grow
Gerta Hoxhaj, Ph.D., and her lab have challenged a long-held belief about how tumors acquire purine nucleotides.
Cancer Metabolism
When looking for a new place to live, you could use an empty lot and build a structure from the ground up, or you might take an existing house and renovate some rooms or add on. In biology, cells do a similar accounting. To make a particular molecule, they can build it efficiently by assembling with salvaged components – if the parts are lying around. If not, cells have to spend a lot of energy creating the molecule from scratch.
For a class of molecules called purine nucleotides, a collaborative group of UT Southwestern researchers has uncovered how cells create and maintain their supplies of purines, vital components of DNA, RNA, and other cellular systems.
Chemotherapy drugs that target purine nucleotide synthesis have been a cornerstone of cancer treatments for more than 70 years. Recent findings from UT Southwestern scientists have upended the traditional view of how healthy and cancerous cells acquire purines, showing how some chemotherapy resistance occurs and connecting metabolic dysfunction in other diseases.
Exploring Purine Nucleotides
Gerta Hoxhaj, Ph.D., Assistant Professor in Children’s Medical Center Research Institute at UT Southwestern (CRI), began looking in 2020 at how cells generate their purine nucleotides. Despite scientists knowing these molecules were important to many metabolic processes, “we still didn’t know the fundamentals of how normal or cancer cells acquire their purines,” Dr. Hoxhaj says.
Purine nucleotides are composed of a purine base attached to a sugar molecule – ribose or deoxyribose – and one or more phosphate groups. These nucleotides are essential for cell growth and function because they serve as nucleic acid building blocks, signaling molecules, and energy carriers.
Building purine nucleotides from scratch – called de novo synthesis – costs the cell a lot of energy. A more efficient process of recycling, the salvage pathway, takes much less energy but requires a supply of pre-assembled subunits.
Historically, scientists thought normal adult tissues relied on the salvage pathway, while proliferating cells, such as those in cancerous tissues, produced most of their purines through de novo synthesis.
Dr. Hoxhaj, also a member of UT Southwestern’s Harold C. Simmons Comprehensive Cancer Center, wanted to look at both pathways broadly in an unbiased way – not just in a specific organ, tissue, or tumor type – to determine how much each pathway contributed to purine pools.
An Interdisciplinary Approach
Taking an interdisciplinary approach, Dr. Hoxhaj started by conferring with Ralph J. DeBerardinis, M.D., Ph.D., Professor and Director of the Eugene McDermott Center for Human Growth and Development, as well as in CRI, whose lab was also on to its own purine metabolism discovery.
As part of his research, Dr. DeBerardinis studies babies born with genetic disorders called inborn errors of metabolism. In a recent study, his team cultured cells from 10 patients with various genetic mitochondrial diseases and measured hundreds of metabolites to find abnormalities common to all the diseases.
“We were specifically asking: When the mitochondria don’t work properly and energy production is slowed, which other metabolic pathways also don’t work?” says Dr. DeBerardinis, a Howard Hughes Medical Institute Investigator since 2018, as well as Director of CRI’s Genetic and Metabolic Disease Program. “It turned out purine metabolism was the most consistently altered pathway, even more than pathways classically associated with mitochondria.”
The result surprised them because purine metabolism takes place in the cell’s cytoplasm, but the mutations affected the interior workings of the mitochondria. His lab eventually found that mitochondrial dysfunction causes cells to activate purine salvage, presumably allowing them to conserve energy due to impaired energy production.
The research brought in other physician-scientists, such as Kemp Kernstine, M.D., Ph.D., Professor of Cardiovascular & Thoracic Surgery, with whom Dr. DeBerardinis had built a database of altered metabolism in lung cancer.
“Why is a pediatrician working on lung cancer?” Dr. DeBerardinis asks of himself. “Lung cancer also involves altered metabolism, and we thought insights from metabolic diseases in children might help us understand metabolism in these tumors.”
Drs. DeBerardinis and Kernstine quickly discovered lung tumors with low mitochondrial activity activated the purine salvage pathway, both in mouse models and patients.
Collaboration and Discovery
Meanwhile, Dr. Hoxhaj’s team was tracing purine synthesis by infusing mice with a variety of nutrients, including amino acids and purine bases such as adenine and guanine, all tagged with heavy isotopes in order to track how purines were metabolized in both healthy and cancerous mouse tissues.
“We noticed that different organs have unique preferences for using the de novo and salvage pathways,” Dr. Hoxhaj says. “The small intestine, for instance, depended on de novo purine synthesis, while kidneys relied on salvage.”
“Our findings about the kidneys led us to Jim Brugarolas, who was so open to sharing his research and expertise,” she adds.
James Brugarolas, M.D., Ph.D., Professor of Internal Medicine and Director of UTSW’s Kidney Cancer Program, provided mouse models of tumors, allowing Dr. Hoxhaj’s
“The magic often happens at the interface between fields or traditional disciplines. One of our main goals is to create an environment in which people can make discoveries together that they wouldn’t have been able to make on their own.”
team to study purine metabolism in kidney cancer. Additionally, other CRI labs contributed to the purine research: Hao Zhu, M.D., provided a liver cancer model, and Sean Morrison, Ph.D., offered specialized expertise in experimental design and data analysis.
“The magic often happens at the interface between fields or traditional disciplines,” says Dr. Morrison, Director and Professor in CRI, and Professor of Pediatrics. “One of our main goals is to create an environment in which people can make discoveries together that they wouldn’t have been able to make on their own.”
Dr. Hoxhaj’s study ultimately showed tumors are just as effective at using the salvage pathway as they are using the de novo pathway, challenging the traditional view that cancer cells primarily rely on the de novo pathway.
The Hoxhaj lab also discovered that blocking the salvage pathway reduced tumor growth, as well as that feeding mice a nucleotide-rich diet accelerated tumor growth.
“Although nucleotides are present in all foods, they are especially abundant in animal products like meat,” Dr. Hoxhaj says. “Our research suggests nucleotides act as nutrient sources for tumors. These insights could prompt doctors to consider dietary interventions, such as reducing nucleotide intake, especially for patients undergoing chemotherapy or as part of broader cancer treatment strategies.”
Gaining Recognition
Since she joined UTSW, Dr. Hoxhaj’s research has been widely recognized. Already a Cancer Prevention and Research Institute of Texas (CPRIT) Scholar, she was the recipient of the Texas Academy of Medicine, Engineering, Science and Technology’s 2025 Mary Beth Maddox Award and Lectureship. She was awarded the Vilcek Prize for “Creative Promise in Biomedical Science” in 2024, named a Pew-Stewart Scholar for Cancer Research in 2023, an American Cancer Society Scholar in 2022, and a V Foundation Scholar in 2021.
“We were this new lab in town, and we knew we were on to something important,” Dr. Hoxhaj says. “Having the support of the CRI and UTSW communities made it all possible. I’m grateful to be at an institution where people are so open and generous.”




Sean J. Morrison, Ph.D., Professor and Director of Children’s Medical Center Research Institute at UT Southwestern

Hao Zhu, M.D., Professor in Children’s Medical Center Research Institute at UT Southwestern
James Brugarolas, M.D., Ph.D., Professor of Internal Medicine and Director of the Kidney Cancer Program at the Harold C. Simmons Comprehensive Cancer Center
Gerta Hoxhaj, Ph.D., Assistant Professor in Children’s Medical Center Research Institute at UT Southwestern
Ralph J. DeBerardinis, M.D., Ph.D., Professor and Director of the Eugene McDermott Center for Human Growth and Development
Using Nanotechnology to Deliver Genetic Medicines
FSynthetic lipid nanoparticles developed at UT Southwestern show promise in delivering therapies to the lungs, bone marrow, and beyond.
or decades, scientists have strived to harness the potential of genetic medicines – nucleic acid therapies that treat diseases by prompting cells to produce therapeutic proteins or correcting genetic defects. However, effectively delivering genetic medicines where they’re needed has proved challenging, says Daniel Siegwart, Ph.D., Professor of Biomedical Engineering, of Biochemistry, and in the Harold C. Simmons Comprehensive Cancer Center.
As a materials chemist housed within an academic medical center, Dr. Siegwart is using his expertise to solve this problem. He and his colleagues have developed synthetic lipid nanoparticles to serve as delivery vehicles, showing in a series of studies that adjusting the composition of these nanocarriers can direct genetic medicines to the lungs, spleen, bone marrow, liver, and other organs of animal models. Different formulations of these nanocarriers can also be utilized for imaging,
suggesting that this strategy could be used for “theranostics,” which combines therapy and diagnostics into a single modality.
“Dr. Siegwart’s discoveries have made great strides in bringing nucleic acid therapies for diseases including cystic fibrosis and cancer closer to clinical use,” says Carlos L. Arteaga, M.D., Director of the Harold C. Simmons Comprehensive Cancer Center and Professor of Internal Medicine. “His approaches for drug targeting and development are some of the most novel in the field and likely to have high impact.”
Unlocking Entry Into Cells
The nucleic acids DNA and RNA hold instructions that cells use to make proteins for performing every vital function in the body. However, their properties make it difficult for them to enter cells. Bare nucleic acids have a highly negative charge that repels them from negatively charged cell

Daniel Siegwart, Ph.D. (center), Lukas Farbiak, Ph.D., and Richard Wang, M.D., Ph.D., review experimental plans. Their work has the potential to create transformative treatments for conditions like cancer, sickle cell anemia, and cystic fibrosis.
membranes, and the immune system quickly clears this genetic material from circulation since it views standalone nucleic acids as a threat.
Researchers have used several strategies over the years to usher nucleic acids into cells, but each has had significant drawbacks, Dr. Siegwart explains. For example, packaging DNA or RNA into viruses can be effective, but they can’t be mass-produced, and viruses can stimulate an undesirable immune response. Researchers have also had success with packaging nucleic acids inside lipid nanoparticles with formulations similar to cholesterol particles. But the body treats these nanocarriers as it would natural cholesterol and directs them only to the liver. Although these lipid nanocarriers are currently being investigated for genetic medicines to treat liver diseases, they aren’t effective delivery vehicles for diseases that affect other organs.
To solve this problem, Dr. Siegwart and his colleagues – including biochemists, biomedical engineers, physicians, and pharmaceutical scientists – developed nanoparticles made from a mix of synthetic lipids. Through trial and error and rational design, these researchers have developed a system they named SORT – short for Selective Organ Targeting – in which these synthetic lipid nanocarriers direct genetic medicines to desired organs by changing the lipid mix.
“My team and I are excited to wake up in the morning and go to work because our research has such extraordinary potential to help patients.”
“At UT Southwestern, our researchers’ work is not siloed. Discoveries like SORT require extensive collaborations with different labs, diverse departments, and multiple investigators,” says Samuel Achilefu, Ph.D., Professor and Chair of Biomedical Engineering, Professor of Radiology and in the Harold C. Simmons Comprehensive Cancer Center. “The interdisciplinary research environment here allows this integration to happen organically.”
Extraordinary Potential
Dr. Siegwart and his colleagues have recently published a series of papers that show SORT’s broad potential. In one study, the researchers packaged messenger RNA (mRNA) inside SORT nanoparticles that contained instructions to convert immune cells called T cells into cancer-fighting agents called chimeric antigen receptor T cells (CAR T cells).
Although CAR T cells have shown significant promise in fighting malignancies, the conventional process to produce this therapy includes extracting the patient’s immune cells and inducing them to become CAR T cells in cell culture. Then, the cancerfighting cells are reintroduced to the body. This process costs hundreds of thousands of dollars and can take weeks or months to complete successfully, time that many cancer patients don’t have. The study showed that SORT particles prompted cells in the spleen of lab animals to produce their own CAR T cells that could kill cancer cells and extend survival.
In another study, Dr. Siegwart’s team used a similar strategy to direct genetic medicine to the bone marrow of animal models of sickle cell anemia. These bone marrow-homing nanoparticles contained geneediting machinery that corrected the genetic defect that causes this condition, offering a potential cure.
The researchers also used SORT to direct genetic medicines to the lungs. Using this approach, they were able to prompt cells there to produce proteins to replace defective ones in animal models of a condition called primary ciliary dyskinesia (in which hair-like protrusions in the lungs called cilia stay stationary, rather than beating rhythmically to move contaminants out of the lungs) and cystic fibrosis. In addition, the researchers used gene-editing machinery to repair the genetic defect that causes cystic fibrosis, a strategy that could eventually offer patients a lifelong cure.
“My team and I are excited to wake up in the morning and go to work because our research has such extraordinary potential to help patients,” Dr. Siegwart says. “We are contributing the basic knowledge needed to make a whole new category of medicine.”

Samuel Achilefu, Ph.D., Professor and Chair of Biomedical Engineering, Professor of Radiology and in the Harold C. Simmons Comprehensive Cancer Center

Carlos L. Arteaga, M.D., Director of the Harold C. Simmons Comprehensive Cancer Center and Professor of Internal Medicine

Daniel Siegwart, Ph.D., Professor of Biomedical Engineering, of Biochemistry, and in the Harold C. Simmons Comprehensive Cancer Center




A Comprehensive Approach to Improving Patient Care
UTSW scientists are implementing studies to provide clinicians with evidence-based strategies to enhance patient care.
Discovering the most effective ways to treat patients with multiple chronic conditions is a critical yet challenging goal for clinicians, who often face barriers to providing therapies based on clinical guidelines. At UT Southwestern, a wealth of clinical resources and research infrastructure has helped build a foundation for the development of treatments that are decreasing hospitalizations and saving lives.
“That’s one of the advantages of UT Southwestern – this is a special place where there is a significant amount of trust across different health systems, which facilitates important research and clinical collaborations,” says Miguel Vazquez, M.D., Professor of Internal Medicine and Clinical Director of the Division of Nephrology. “When it comes to research that’s ultimate goal is to find effective therapies for our patients, we can answer the really big questions.”


Orchestrating a Massive Multi-Center Trial
One area of focus in Dr. Vazquez’s research has been investigating how to support physicians with the latest technology as they care for their patients. In a recently published national study in The New England Journal of Medicine, he and his colleagues revealed a robust model for testing the efficacy of interventions for chronic health conditions.
The research team designed a large-scale pragmatic trial testing the effectiveness of guideline-directed therapies for patients with the kidney-dysfunction triad of co-occurring chronic kidney disease, Type 2 diabetes, and hypertension. Therapies can help patients control their disorders, but Dr. Vazquez was unsure if patients were consistently receiving them, or how to best administer them.
The team tested whether having additional personnel to support practitioners by delivering guideline-directed therapies would improve patient health. They leaned on UT Southwestern’s robust connections with partner institutions in the DallasFort Worth area, including Parkland Health, Texas Health Resources, and the VA North Texas Health Care System, enrolling 11,000 adults from diverse racial and socioeconomic backgrounds at 141 primary care practices.
Miguel Vazquez, M.D. (center), led a clinical trial that included multiple health systems in the Dallas-Fort Worth area.
“UT Southwestern has developed mechanisms to support researchers in conducting trials of this scale, from training younger scholars and pairing them with experienced investigators to providing tools and resources.”


“When patients want to know which treatments are best, we need to have a way to test that,” says Dr. Vazquez, who is also Chief of Nephrology at Parkland Memorial Hospital.
Valuable Lessons
Dr. Vazquez collaborated with experts in informatics, electronic health records, computer software, data management, and institutional leadership, including Robert Toto, M.D., Professor of Internal Medicine, Associate Dean for Clinical and Translational Research, and co-leader of the study.
The researchers used a computerized algorithm to analyze patients’ electronic health records and uncover cases of undiagnosed kidney disease, and randomly selected patients to receive therapies from support staff in addition to their doctors during appointments. The findings revealed that these interventions led to only modest differences in patient care at one year. However, the study provided valuable lessons on how to enhance collaboration across diverse settings, health conditions, and patient populations, and will serve as a model for future studies to improve care for patients.
Eric Peterson, M.D., M.P.H., Vice Provost and Senior Associate Dean for Clinical Research, says it’s important to prioritize the infrastructure needed to improve clinician and patient adoption of proven therapies.
“Despite advances in medicine, clinicians do not always deliver consistent treatment,” Dr. Peterson says. “It’s vital to rigorously evaluate whether treatments are effective in the real world. UT Southwestern has developed mechanisms
to support researchers in conducting trials of this scale, from training younger scholars and pairing them with experienced investigators to providing tools and resources.”
Saad Omer, M.B.B.S., M.P.H., Ph.D., Dean of the Peter O’Donnell Jr. School of Public Health, notes that the study’s implications go beyond clinical care.
“The trial was very much in line with the public health paradigm of research to improve people’s lives,” Dr. Omer says. “This kind of high-quality science is generalizable to broader populations and has the potential to impact public health and clinical guidelines.”
On the Horizon
Future research directions include analyzing electronic health records to identify patients who need therapies but aren’t receiving them, and training health care providers to support doctors. Dr. Vazquez, in collaboration with Wanpen Vongpatanasin, M.D., Professor of Internal Medicine and Director of the Hypertension Section in the Division of Cardiology, is currently conducting a large-scale pragmatic trial to determine whether lowering blood pressure can reduce cognitive decline.
“It’s an incredible privilege to be the beneficiaries of our patients’ trust,” Dr. Vazquez says. “It’s our responsibility to care for them and advance knowledge about the best treatments. We are so fortunate to work with patients who want to help us learn more.”


Miguel
and Clinical Director of the Division of Nephrology

Wanpen Vongpatanasin,
, Professor of Internal Medicine and Director of the Hypertension Section in the Division of Cardiology
Saad Omer, M.B.B.S., M.P.H., Ph.D., Dean of the O’Donnell School of Public Health
Eric Peterson, M.D., M.P.H., Vice Provost and Senior Associate Dean for Clinical Research
Robert Toto, M.D., Professor of Internal Medicine and Associate Dean for Clinical and Translational Research
Vazquez, M.D., Professor of Internal Medicine
M.D.

Operating
Room Black Box Increases Patient Safety
Pilot of surgical recording system at UTSW has exceeded expectations, providing an unprecedented level of data.
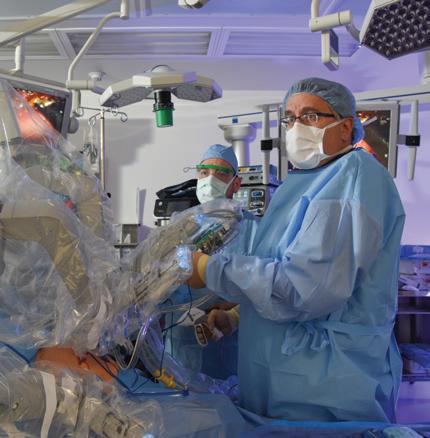
Herbert Zeh, M.D., operates a surgical robot. The ‘Black Box’ program aims to improve efficiency and quality control in surgeries by analyzing recordings from the OR.
UT Southwestern aimed to enhance safety, surgical outcomes, and efficiency when it introduced the OR Black Box to William P. Clements Jr. University Hospital in Dallas in 2020. The system, which captures data on multiple aspects of a surgery, has far exceeded expectations.
“Not only has it improved safety, survival, and length of stay for patients, it has strengthened surgical teams,” says William Daniel, M.D., Vice President and Chief Quality Officer. “It has done a remarkable job.”
The OR Black Box, developed by Surgical Safety Technologies Inc. (SST) in Toronto, was originally installed in five UT Southwestern operating rooms as part of a three-year pilot. It was so effective that today the system is in 38 ORs at Clements University Hospital, where it has recorded almost 70,000 hours of surgical cases. This has given UT Southwestern more experience with the OR Black Box than anywhere else in the world.
“With the OR Black Box in every room, the amount of data we are getting is unprecedented,” says Herbert Zeh, M.D., Professor and Chair of Surgery. “It allows us to measure things that we were never able to measure accurately before.”
Improving Performance
Data from the OR Black Box is fed into computer servers, then electronically relayed to SST, which uses artificial intelligence and trained analysts to evaluate issues such as the proper use of safety checklists. Each month, SST relays its findings to UT Southwestern. That data is then used to conduct quality-improvement meetings at which OR team members review the reports.
“It is almost like a game film,” Dr. Zeh says, referencing how professional sports teams study video of games to improve their strategies.
“One of the things we realized early on was that when people were counting instruments and needles, they were getting interrupted,” Dr. Daniel says. “Now we make sure we perform ‘timeouts’ during every case, when we pause and make sure the entire team is engaged.”
Tags and Flags
The system’s patient identity-protected video clips have provided insight into surgical “tagged and flagged” events. Tagging is used to raise awareness of something that is atypical, such as a longer procedure. Flagging highlights potential problems or outstanding achievements.
“The most transformational items have been the tags and flags,” Dr. Zeh says.
Drs. Zeh and Daniel stress that the goal of the system is to identify areas of continued improvement and recommend best practices.
“From the beginning, we made it abundantly clear that this was not being used punitively,” Dr. Daniel says. “The reason we have it is to help ourselves get better.”
While avoiding patient harm is paramount, recognizing a job well done is also important.
“It gives people a chance to celebrate each other,” Dr. Daniel says. “When you see someone make a great catch, you see that esprit de corps.”
“Not only has [the OR Black Box] improved safety, survival, and length of stay for patients, it has strengthened surgical teams.”
Leading the Way
The OR Black Box has been well-received by patients. During the pilot, 97% of patients agreed to its use.
“We are a leading academic medical center, and our patients expect that we would be innovative and lead the way in safety practices,” Dr. Daniel says.
In line with high-reliability organizations such as NASA, UT Southwestern is dedicated to quality improvement. The OR Black Box is another way to further that commitment to safety.
“We are already leaders,” Dr. Zeh says. “I think that with time, some devices like this will be in every OR in the country.”

William Daniel, M.D., Vice President and Chief Quality Officer

Herbert Zeh, M.D., Professor and Chair of Surgery
Gut Reaction: Understanding the Microbiome
New insights from the Hooper lab could harness the power of commensal gut bacteria.
Living harmoniously inside the intestines of humans and other mammals are hundreds of trillions of microbial cells. Decades ago, scientists discovered that these microbes improve the efficiency of digestion. Over the last 20 years, research led by Lora Hooper, Ph.D., Professor and Chair of Immunology at UT Southwestern Medical Center, has shown that this vast population of commensal organisms does far more than assist in digestion. Her research has revealed that these microscopic assistants also play key roles in regulating the immune system and the circadian clock.
Dr. Hooper, who also serves as Professor of Microbiology and is part of the Center for Genetics of Host Defense, explains that these findings answer fundamental questions about how mammalian biology has come to depend on microbes.
Pivotal Insights
Dr. Hooper joined UT Southwestern after a postdoctoral fellowship at Washington University School of Medicine in St. Louis with Jeffrey Gordon, M.D., a professor of pathology and immunology who is widely considered the father of the field of gut microbiome research. There, she became intrigued with the precarious balance that gut microbes have with their host organisms.
After she established her lab at UT Southwestern in 2003, Dr. Hooper’s initial experiments focused on how the gut microbiome affected gene expression in intestinal cells. In 2008, she was named a Howard Hughes Medical Institute Investigator.
One pivotal finding stemming from this early work is that gut microbes interact with intestinal cells to regulate the circadian clock. In 2021, she and her colleagues reported in Cell that the amount of natural compounds produced by the intestines to fight foodborne infection changes depending on the time of day, a phenomenon driven by an interplay between intestinal cells and gut bacteria.
Another finding spurred by these initial experiments is that gut microbes regulate the absorption of dietary lipids. In a recent paper on this topic, published in Science in 2023, she and her colleagues showed that a gene called Snhg9 becomes less active in intestinal cells in the presence of



Dr. Hooper’s research has revealed that the gut microbiome plays key roles in regulating the immune system and the circadian clock.
intestinal bacteria, a process that increases the amount of lipids taken up by the intestines.
“Lora is a very big-picture thinker,” says Gabriella Quinn, a graduate student researcher in the Hooper lab and in UTSW’s Perot Family Scholars Medical Scientist Training Program. “She encourages us to think about things like, why would this system have evolved this way? What advantages are there to the host?”
Today, the Hooper lab is investigating how the microbiome might be involved in extracting the lipid vitamin A from food and delivering it to immune cells that need this nutrient to develop.
Looking Ahead
Collaborations are taking the Hooper lab in new directions, says Andrew Y. Koh, M.D., Professor of Pediatrics, of Microbiology and in the Harold C. Simmons Comprehensive Cancer Center. Drs. Koh and Hooper have three ongoing collaborative projects: one to investigate how Candida yeast colonizes the gut, a phenomenon that can cause fatal yeast infections in cancer and stem cell transplant recipients; one to study how vitamin A influences adaptive immune regulation; and one examining how the circadian clock modulates the response to cancer immunotherapy.
Every piece of research adds new insight into the gut microbiome, a field that has taken off over the course of Dr. Hooper’s career.
“The microbiome is incredibly complex,” says Dr. Hooper, member of the National Academy of Sciences (2015) and the National Academy of Medicine (2022). “It’s different in every single person, and it’s incredibly difficult to study. But our understanding of it has enormous potential to promote human health.”



Lora Hooper, Ph.D., Professor and Chair of Immunology, Professor of Microbiology and in the Center for the Genetics of Host Defense
Andrew Y. Koh, M.D., Professor of Pediatrics, of Microbiology and in the Harold C. Simmons Comprehensive Cancer Center
Gabriella Quinn, graduate student in the Hooper lab and in UTSW’s Perot Family Scholars Medical Scientist Training Program
Lora Hooper, Ph.D., and Andrew Y. Koh, M.D., gather insights about the gut microbiome. Their thoughtful collaboration has uncovered pivotal discoveries in how the microbiome can impact human health.



Building a Legacy of Transformative Science
The Endowed Scholars Program has served as a key stepping stone in the careers of pioneering researchers, including nine Howard Hughes Medical Institute Investigators and three National Academy of Sciences members.
Nearly three decades ago, leaders at UT Southwestern Medical Center invested $60 million in philanthropic funds to establish a program to help young scientists launch their research careers. Since 1998, the Endowed Scholars Program in Medical Science has provided support to over 140 newly appointed tenure-track assistant professors, recruiting some of the best and brightest minds to UT Southwestern and laying the foundation for continued excellence in biomedical research.
“The selected candidates demonstrate exceptional skill and promise as independent researchers,” says Joan W. Conaway, Ph.D., Vice Provost and Dean for Basic Research. “UT Southwestern provides them with rich financial support and the latest tools, equipment, and resources they need to succeed.”
Supporting Crucial Research
To understand the program’s far-reaching impact, consider the career of Vincent Tagliabracci, Ph.D., Associate Professor of Molecular Biology, who joined UT Southwestern as a Michael L. Rosenberg
Scholar in Medical Research. His study of enzymes rewrote the textbooks on pseudokinases, a novel branch of the protein kinase family that was previously thought to be catalytically inactive. He discovered that these so-called “zombie enzymes” can carry out different chemical transformations than their classical counterparts, which could shape our understanding of bacterial infections and diseases.
In 2018, more than a year before COVID19 plunged the world into a global health crisis, Dr. Tagliabracci and his team decoded the secrets of one obscure pseudokinase, selenoprotein-O (SelO). Soon afterward, they realized it was similar to an important protein called NiRAN in SARS-CoV-1, the virus that caused an outbreak of SARS in the early

Joan W. Conaway, Ph.D., Vice Provost and Dean for Basic Research
2000s. When its genetic cousin, SARS-CoV-2, the virus that caused COVID-19, emerged, they were ahead of the curve and building on knowledge already at hand. Subsequent studies during the pandemic allowed them to identify a key step in the viral life cycle that the virus uses to camouflage its genetic material.
“A significant number of proteins are often overlooked in research due to a lack of direct association with human diseases, resulting in their underappreciation. Our goal is to focus on studying these underexplored proteins that others tend to ignore,” says Dr. Tagliabracci, who credits the Endowed Scholars Program with enabling this and other groundbreaking discoveries. “Without this support, we might never have had the opportunity to pursue this kind of curiosity-driven investigation.”
Dr. Tagliabracci’s work has gained national recognition. He is a Howard Hughes Medical Institute Investigator and was the recipient of the 2020 Norman Hackerman Award in Chemical Research – the eighth UT Southwestern researcher to receive the coveted prize. More recently, he received the 2024 Edith and Peter O’Donnell Award from the Texas Academy of Medicine, Engineering, Science and Technology for “potentially lifesaving research” and the 2024 Earl and Thressa Stadtman Young Scholar Award from the American Society for Biochemistry and Molecular Biology, recognizing his promise as an emerging scientist.
His achievements exemplify the potential of the Endowed Scholars Program to serve as a runway to take researchers to new heights.
“Our donors have helped us build this platform to invest in faculty members who are true scientific leaders,” Dr. Conaway says. “An academic institution without talented people is just a building, but an environment with highcaliber researchers – that is a place where great science is born.”

Vincent Tagliabracci, Ph.D., Associate Professor of Molecular Biology
Vincent Tagliabracci, Ph.D., credits the Endowed Scholars Program with supporting his groundbreaking, curiosity-driven research.
New Pediatric Campus Breaks Ground in Dallas

In February 2024, UT Southwestern Medical Center and Children’s Health℠ broke ground on a new $5 billion pediatric health campus in Dallas’ Southwestern Medical District.
Encompassing more than 4.7 million square feet – including a pediatric hospital as its centerpiece – the new pediatric campus will draw upon the extensive academic resources and

James J. Collins III, Ph.D., Associate Professor of Pharmacology at UT Southwestern Medical Center who leads groundbreaking research
collaborative, leading-edge research underway at UTSW. The innovative design of facilities – next door to UTSW’s globally ranked research hub – will help recruit leading pediatric clinicians, established and emerging researchers, residents, fellows, medical students, and the most talented individuals in nursing, medical technology, and related health professions.


Drs. Arteaga and Mangelsdorf Elected to National Academy of Medicine
Carlos L. Arteaga, M.D., and David Mangelsdorf, Ph.D., were formally elected to the National Academy of Medicine, one of the highest honors in health and medicine.
Dr. Arteaga, Director of the Harold C. Simmons Comprehensive Cancer Center and Associate Dean of Oncology Programs, is widely recognized for innovative breast cancer research that has led to the development of molecularly targeted therapies, including PI3K inhibitors for patients with breast cancer.
Dr. Mangelsdorf, Chair of Pharmacology and Professor of Biochemistry, has made significant contributions to lipid biology, with discoveries that could lead to new therapies for diseases including diabetes, obesity, cancer, and parasitism.
Pharmacologist Named Howard Hughes Medical Institute Investigator
into the parasitic disease schistosomiasis, has been named a Howard Hughes Medical Institute Investigator. Second only to malaria as the most devastating parasitic disease, schistosomiasis progresses as female parasitic worms lay millions of eggs inside the host, causing debilitating inflammatory responses and scarring as
eggs get trapped in the liver, intestines, or even the brain.
Dr. Collins, who joined UT Southwestern in 2014 as an Endowed Scholar, was the first to set up the culture conditions to monitor the reproductive cycle of the worms without having to pass them through a host. In doing so, he has transformed the understanding of schistosomes by
discovering and isolating the pheromone, or signal, that male worms use to control female sexual development and egg production. Experts think that understanding and isolating this signal provides a great new direction for the field and may bring relief to the millions of people the tropical disease affects each year in developing nations.
UT Southwestern Medical Center and Children’s Health’s new pediatric health campus marks a new chapter in their more than 60-year partnership.








