
campbell.edu
Medicine



campbell.edu

CAMPBELL MEDICINE
As the first and only osteopathic medical school established in the state of North Carolina, Campbell University School of Osteopathic Medicine is cultivating the next generation of physicians.
ACADEMICS
We emphasize intellectual achievement, compassion and mind-body-spirit centered patient care.
STUDENT EXPERIENCE
We are a diverse community bound together by common interests and strong relationships.
campbell.edu
The development of research programs serves as part of our mission. Our experienced faculty drive efforts towards finding solutions to the most current medical problems while advancing medicine and science. At Campbell Medicine, our research includes biomedical, translational, clinical research, educational research and more.
Dear Readers,
It is with great pride and enthusiasm that we introduce The Journal of Campbell University Medicine [JCAM], Campbell University’s inaugural peer-reviewed, medical research journal. This premiere publication was imagined and propelled by the students and faculty of Campbell University School of Osteopathic Medicine, all of whom are thrilled to contribute to the advancement of medical knowledge and practice.
The first issue of JCAM comes at an exciting time for medicine in North Carolina, and our mission is clear: to push the boundaries of medicine forward for our community and beyond. Through rigorous research, insightful analyses, and thought-provoking discussions, we aim to provide a platform where innovative ideas thrive and the next generation of medical professionals can make a significant impact.
With a commitment to excellence, integrity, and inclusivity, our journal seeks to showcase the diverse voices and perspectives within the Campbell medical community. We invite you to join us in this endeavor as we strive to shape the future of healthcare, particularly for our rural and underserved communities.
The articles presented in this edition of JCAM are original scientific articles addressing various pertinent topics and scientific innovations relevant to the medical field. Our vision for future editions is to include content related to any and all areas of healthcare, highlighting original work in clinical and academic medicine, novel medical devices, pharmaceuticals, healthcare disparities, and alternative treatments - whatever it is that drives you in our shared goal of moving medicine forward.
We have been honored in our tenure as the founding editorial team, and are excited to see the tangible results of JCAM’s reach. To all our readers, enjoy!
Sincerely,
The Founding Editorial Team
PRESIDENT
J. Bradley Creed, PhD
DEAN
Brian Kessler, DO
ASSOCIATE DEANS
Michael Mahalik, PharmD
Eric Gish, DO
Terri Hamrick, PhD
Victoria Kaprielian, MD
Robin King-Thiele, DO
James Powers, DO
Robert Terreberry, PhD
David Tolentino, DO
Daniel Marlowe, PhD
Mark Hammond, PhD
CONTRIBUTORS
Shelley Hobbs
Billy Liggett
Evan Budrovich
Bennett Scarborough
ALUMNI
We welcome alumni of any Campbell University health programs to contribute to future publications.
DESIGN/PUBLISHER
Adam Fish
Vincent Benbenek
John Trump
Marketing & Communications at Campbell University School of Osteopathic Medicine medicine@campbell.edu
Hallie Dunning
EDITOR-IN-CHIEF
h_dunning1208@email.campbell.edu
Reach out for:
Questions regarding the Editorial Policy, partnerships, or professional inquiries.
Helen Paglia
EDITOR & REVIEW BOARD DIRECTOR
h_paglia0103@email.campbell.edu
Reach out for:
Connecting with a principal investigator, or acquiring a role on the Review Board.
Ariela Christian
EDITOR & SUBMISSIONS DIRECTOR
abchristian1128@email.campbell.edu
Reach out for:
Guidance regarding the submission process, or acquiring a role on the Editorial Team.
ASSOCIATE EDITORS
Special thanks to these individuals that contributed directly to this edition of JCAM:
Kian Bagheri
Emily Carletto
Jagroop Doad
Peter Sells
Additional Thanks to: Alexander Caudill, Aimee Dubin, Zvi Harris, Clayton Moore, Brady Pickett, Brittany Smith, Katherine Zhou
Journal of Campbell Medicine FALL 2024
Volume 01 Issue 01
Development of Novel Transformation Procedure to Modify the Mitochondrial Genome
Hunderup, S., Jawad, R., Lushia, W.
SNAPSHOT:
A novel mitochondrial transformation technique may pave the way for future mitochondrial gene therapy
A Brief Review of Psychological Flexibility and the Well-Being of Medical Students
Maffey, M., Kaiser, M. S.
SNAPSHOT:
How can psychological flexibility be wielded to protect against burnout and depression in medical students?
Bladder Cancer: Case Report and Review of Diagnostic Advancements
Clemson, L., Karabinus, M., Sandoval, T., Brenseke, B., Proia, A.
SNAPSHOT:
Innovative technology provides physicians with a less-invasive tool for diagnosing bladder carcinoma.
Sarah Hunderup*, Rayyanoor Jawad*, Dr. Warren Lushia*
* Biochemistry and Genetics, Campbell University School of Medicine
ABSTRACT: Mitochondrial DNA mutations and dysfunction have been implicated in many cancers and mitochondrial disorders, yet the mitochondrial genome has been manipulated with limited success. Our project aims to develop a novel mitochondrial transformation technique utilizing the cotransport of tRNA to import exogenous DNA into the mitochondria of S. cerevisiae cells.
Previous work on this project created novel tRNA-DNA hybrid vectors, optimized transformation protocols, and analyzed putative S. cerevisiae mitochondrial transformants. We continued this investigation with the optimization of spheroplast and polymerase chain reaction (PCR) procedures to verify the successful insertion of the Arg8 gene into the mitochondrial genome of Arg8 knockout yeast cells. We also explored the stability and lifespan of post-transformation yeast cells by analyzing the mitochondrial gene, ATP6. Posttransformation yeast cells appear to show instability in the mtDNA; however, further research is needed to confirm this.
If the cotransport of tRNA-Arg8 is successful, our findings could be applied to human mitochondria, providing a tool to better study human diseases and a foundation for future mitochondrial gene therapy.
KEYWORDS: spheroplast, mtDNA editing
Cancer is the second leading cause of death after heart disease worldwide and the number of new cancer cases per year is expected to rise to 30 million by 2040 [1]. Thus far, cancer research has focused on nuclear DNA mutations in the search for effective targets for therapies. Mitochondrial DNA (mtDNA) mutations have been less explored even though most cancers undergo mitochondrial dysfunction. Otto Warburg first uncovered that tumor cells partake in increased glycolysis for energy production and reduced oxidative phosphorylation, even in the presence of oxygen. According to one study where nearly 859 patients with 20 different types of cancer were analyzed, 66% of the cancers were found to carry at least one somatic point mutation of mtDNA [2]. This finding, like many other reports, suggests that mtDNA mutations are common in cancer progression. MtDNA mutations have been known to affect the redox reactions in the mitochondria, with different mutations having varying significance in their effects. It can affect electron transport, oxygen use, cellular metabolism, and apoptosis. It is believed that if severe, it will lead to apoptosis and cell death, but minor dysfunction leads to cell proliferation and tumorigenesis [3]. By creating a method to edit the mtDNA, we hope to open up an entirely new way to look at cancer treatments and allow previously untreatable cancers to have more options.
Despite the evidence for mitochondria’s significant role in cancer and other diseases, there has been little success in mtDNA editing. Notable previous attempts include a technique called microprojectile bombardment in which DNA and gold particles are aimed at cells and expected to penetrate the cell and mitochondrial membranes. The metal particles deliver the DNA to edit the mtDNA, however, with major
limitations. The technique has only been used successfully in a few cells containing cell walls, and notably has never been used successfully in human cells. The technique is also very inefficient in that only a few microprojectiles successfully penetrate the mitochondria to deliver the DNA while leaving the cell in a living state [4].
Looking for other ways to edit the mtDNA, vector-based therapy has been considered. Past attempts to deliver DNA have included mitochondrially-targeted proteins, lipids, and other chemicals. Notably, nucleic acids in the form of RNA have been successfully introduced into mitochondria using specific sequences found in naturally imported RNAs. Our group has hypothesized that attaching these specific RNA sequences to DNA molecules may allow the cotransport into mitochondria and subsequent transformation of the mitochondrial DNA. It is proposed that the structure of an RNA is similar enough to DNA that the transport mechanisms would allow the passage or DNA into the mitochondria. Our group has focused on using a specific transfer RNA (tRNA) whose transport into mitochondria has been well-studied in both yeast and human systems [5]. The goal is for the system to be developed initially in yeast cells and then adapted for use in human cells once studies have proven to show accurate editing of the mtDNA without affecting the stability.
Previous work on this project has developed a technique to ligate tRNA to DNA and optimized transformation conditions using a yeast model. Saccharomyces cerevisiae (S. cerevisiae) is a good eukaryotic model because it has a relatively small genome, and it shares many of the same important cellular processes as higher eukaryotes such as protein translocation and secretion [6]. Yeast is also an ideal model for mitochondrial transformation because of several factors. Most notably,
there is an available selectable marker (Arg8m) and the ability to survive with mitochondrial gene mutations [4]. These characteristics allow us to edit the mitochondrial freely to see if the technique is successful. Our project incorporates two strains of S. cerevisiae, BY4741 and 7711. BY4741 is the parent strain of 7711, which contains the nuclear Arg8 gene. 7711 has had its nuclear Arg8 gene knocked out and replaced with a kanamycin resistance gene and can be selected for in the presence of geneticin, or G418 antibiotic. Notably, 7711 cannot grow on minimal media that does not contain arginine. In our experiments, we attempt to insert a mitochondrial version of Arg8 (Arg8m) into 7711 which, if successful, would allow growth on minimal media lacking arginine. The mitochondrial version of Arg8 is optimized for the genetic code of the yeast mitochondria and contains several sequences that would be stop codons in the nuclear genetic code, thereby eliminating the possibility of an accidental nuclear transformation.
Timeline
This project aims to develop an mtDNA editing procedure using a yeast model in hopes that it will be applied to humans for the mitigation of various cancers and mitochondrial diseases. Previous work has established the spheroplast (yeast cell lacking a cell wall) and electroporation protocols, which are involved in creating new transformants, and optimized polymerase chain reaction (PCR) conditions for DNA analysis. The latest contributions continued the optimization of the procedures in place, tested for transformation success, and explored the post-transformation stability of yeast.
Mitochondrial DNA (mtDNA) in S. cerevisiae is successfully edited using the novel transformation procedure, however, the process of subculturing transformants destabilizes the cell resulting in the knockout of all mtDNA. We hypothesize that a lack of subculturing will result in the preservation of all or some of the mtDNA after the spheroplast experiments.

FIGURE 1. Timeline: Weekly, a liquid culture of 7711 yeast strain was initiated and maintained for making new spheroplasts towards the end of the week, and previous years’ transformants were analyzed using PCR. The growth of 7711 was monitored daily using the BioTex Synergy/HTX – Multimode Reader and Gen5 3.03 Microplate Reader application.
Subcultures
Maintenance of the 7711 strain of yeast required weekly plate-to-plate inoculations. To obtain specific volumes of culture for spheroplast dilutions, a liquid subculture was maintained daily.
Plate to Plate
Inoculate a new YPD G418 plate with a 7711-strain colony from the previous week’s plate in 10 µL of YPD.
Plate to Liquid
A liquid solution of YPD/G418 was inoculated with a colony from a 7711 plate and was placed in a 30°C incubator with shaking overnight.
A liquid solution of YPD/G418 and the previous day’s liquid culture was mixed at a dilution of 1:100 in a tube and placed in a 30°C incubator overnight with shaking. This process was repeated daily until the spheroplast protocol.
Vector synthesis involves the novel preparation of the tRNA that will transport DNA into the mitochondria via ligation. This protocol and the vector preparations for each spheroplast experiment were developed and carried out by previous work in our laboratory (Lushia, manuscript in preparation).
The spheroplast protocol refined by our lab allows yeast to be utilized at optimal growth. We used a 1:4000 dilution (YPD + G418 + culture) at 20 hours after inoculation. The culture underwent a series of washes, centrifugations, and resuspensions. After each centrifugation, the supernatant was poured off. The culture was pelleted by centrifugation at 1000g for 5 min. (2) The pellet was washed with water and resuspended in Buffer A (0.5M tris [pH 7.5], 2M sorbitol, 1M MgCl2, H2O, 1M dithiothreitol (DTT)). (4) The culture was transferred to a petri dish, mixed immediately with 100 µL Zymolyase-100T, and placed on an incubator rocker at 30°C°C for 60 min. Then, the culture was pelleted by centrifugation at 500g for 5 min, resuspended in Buffer A (2x), and then resuspended in YPD sorbitol (1:1 of 2x YPD broth and 2M sorbitol) to be poured onto a petri dish. The dish was placed on an incubator rocker at 30°C for 90 min. Next, the culture was pelleted by centrifugation at 500g for 5 min, resuspended in 1M sorbitol, and pelleted again at 500g for 5 min (2x). Lastly, the culture was resuspended in 1M sorbitol and placed on ice.
Electroporation introduces an electric field to cells, making their cell membrane more permeable for the uptake of any added molecules. A mixed sample of 180 µL of yeast cells from the end of the spheroplast protocol and 5 µL of corresponding DNA was transferred to electroporation cuvettes using large orifice pipettes. The samples were electroporated at 0.8 kV. Then, 1µL of iced 1M sorbitol was added to the cuvette, the contents were mixed with their corresponding dropout + sorbitol regeneration media (-URA or -ARG) in 50 mL conical tubes, gently mixed, and poured onto a corresponding plate. The plates were allowed to cool and placed in a 30°C incubator.
DNA extraction is a process used to purify DNA. This protocol was used to purify genetic material from previous years’ transformants. Cells were grown in 10 mL of appropriate growth medium in 50 mL conical tubes at 30°C, rotating to aerate. The cells were then pelleted by centrifugation at 1000g for 5 min, resuspended in ddH20, and transferred to a microcentrifuge tube. The cells were centrifuged at 17,000g for 1 min and the pellet was resuspended in Buffer A (0.5M tris pH 7.5, 2M sorbitol, 1M MgCl2, H2O, DTT), and zymolyase. The cell mixture was placed in an incubator at 30°C for 1 hr, centrifuged at 17,000g for 1 min, and resuspended in 5x TE. Next, the cells were opened by mixing in 10% SDS and incubated for 5 min at RT. Then, 5M potassium acetate was mixed in the sample to precipitate cellular debris and incubated on ice for 10 min. Afterwards, the sample was centrifuged at max speed for 10 min and the supernatant was mixed with isopropanol in a fresh microcentrifuge tube to precipitate DNA. The mixture was centrifuged again at max speed for 10 min and the DNA pellet was washed with 70% ethanol. Then, the pellet was air dried in a hood. Lastly, to prepare the DNA for PCR, it was resuspended
in 200 ul ddH2O containing 10mg/mL RNase and incubated for 15 min. at 37C.
The Veriti 96-well Thermal Cycler was used to analyze previous years’ transformants with various primers including primers to amplify the following genes: ACT1, kanamycin resistance, Cox3, ATP6, and Arg8. The PCR gel was run with a 100bp ladder and H2O control between 120-130V until the DNA ladder in the region of the expected PCR product was separated sufficiently. Each PCR sample contained a master mix (FWD and REV primers, dNTPs, Phusion enzyme, H2O, and 5X Phys Buffer), well 2 contained water with no DNA as a negative control, wells 3 and 4 contained BY4741 and 7711 DNA, respectively, and wells 5-10 contained the DNA of different putative transformants. Before PCR analysis, each sample was mixed in with a 6X loading buffer (ThermoFisher Scientific).
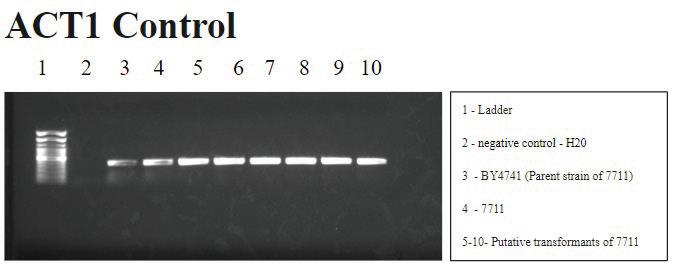
2. ACT1 PCR: The gel correctly shows the actin gene present in wells 3-10 as it is a nuclear gene which should be present in all the
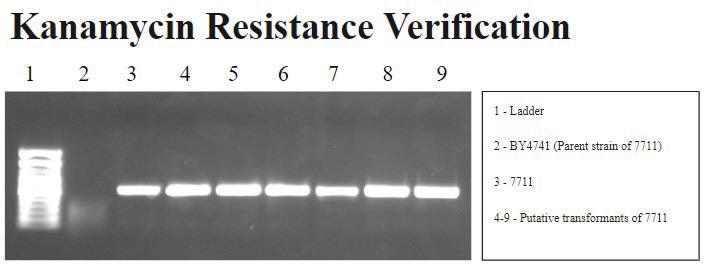
FIGURE 3A. Kanamycin resistance gene PCR: Signals are correctly observed in wells 3-9, showing that BY4741 (well 2) does not have the kanamycin resistance gene, while 7711 (well 3) and its putative transformants do. Note, this experiment does not have a water control in Lane 2 as in the other figures.
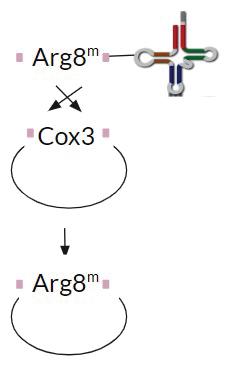
FIGURE 3B. A schematic representing homologous recombination of Cox3 gene with the exogenous Arg8m gene.
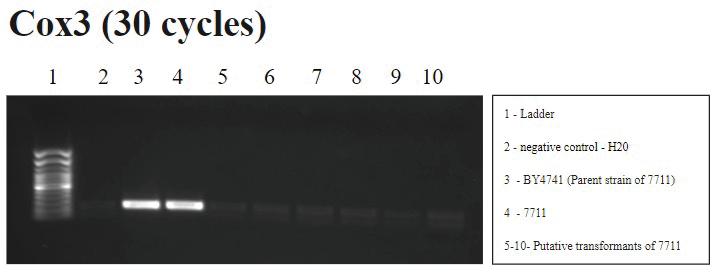
FIGURE 4. Cox3 PCR (30 cycles): A signal is correctly shown in wells 3 and 4, BY4741 and 7711, respectively, which did not undergo the transformation procedure. Water contamination is shown in well 2 and the same signal is present in wells 5-10, the putative transformants, suggesting a successful transformation. Background bands are also observed, though they are less visible due to a reduction in PCR cycles.

FIGURE 5. Arg8m PCR (Primer Group 1): There is a signal in the negative control well 2 that suggests contamination as well as in well 4 that should not be expected, however the signal intensity (brightness) is lower than in the other wells, suggesting the background could be differentiated from a positive signal. Well 3 has a brighter signal than wells 2 and 4, suggesting that Arg8m is a part of the mtDNA rather than contamination. Wells 5-10 are the putative transformants of 7711 and have a signal similar in intensity to well 3, also suggesting
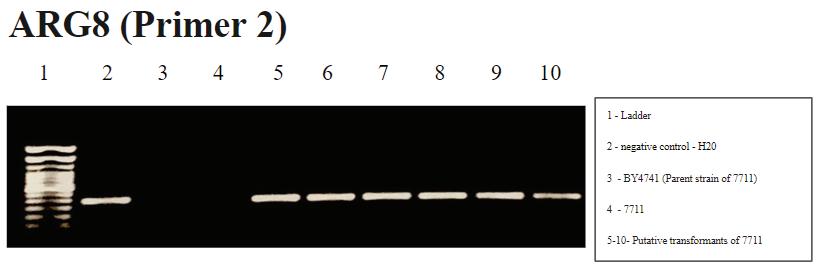
FIGURE 6. Arg8m PCR (Primer Group 2): Well 2 shows water contamination and since wells 5-10 also have the same signal, results are inconclusive using this group of primers. It is unclear why the water contamination is absent in wells 3 and 4 despite the use of the same water as in well 2 in those samples.
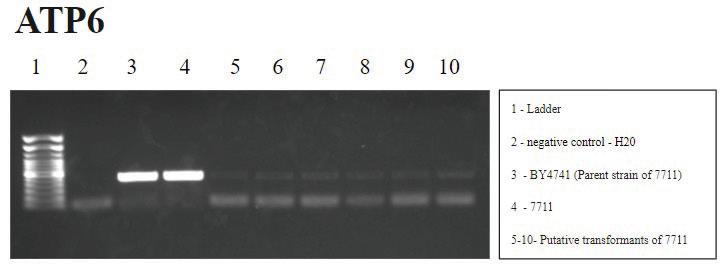
FIGURE 7. ATP6 PCR: An absence of signal in well 2 confirms a lack of water contamination. Signals in wells 3 and 4 accurately correspond to the presence of ATP6 gene in BY4741 and 7711. The faint signal in wells 5-10 suggest more of ATP6 gene is retained but is lower than controls, suggesting some mitochondrial instability post-transformation.
The control and experimental PCR results suggest the successful, but unstable mitochondrial transformation of S. cerevisiae yeast cells. The mtDNA appears to degrade over time with subsequent subcultures following the spheroplast protocol. The control PCR results are shown in Figures 2 and 3a. Figure 2 demonstrates that ACT1, a nuclear gene that encodes the actin protein, is accurately observed in all wells except the negative control (well 2). This signal pattern confirms the presence of S. cerevisiae and suggests that there is no contamination with other yeast. More specifically, Figure 3a demonstrates that we are working with the 7711 strain of S. cerevisiae, which has the Arg8 gene knocked out and not the parent strain, BY4741.
The transformation procedure requires that to have successful homologous recombination in yeast mitochondria, the Cox3 gene will undergo homologous recombination with the exogenous Arg8m gene resulting in the observed absence of Cox3 and the presence of Arg8m during analysis as shown in Figure 3b. In Figure 4, the absence of Cox3 in the putative transformants and the presence of the gene in the yeast strains suggests successful homologous recombination.
To confirm the addition of Arg8m and the excision of Cox3, Arg8m was also observed using PCR analysis as shown in Figures 5 and 6. The results from Figure 5 suggest successful mitochondrial transformation, although due to the possibility of loading error, repetition with primer group 1 (primers 148 & 149) is required. Figure 6 demonstrates mostly inconclusive results contrary to the anticipated signal pattern - which would be to see the signal in wells 3 plus wells 5-10 and absent in wells 2 and 4. With the positive results in well 2, the water control, it can be extrapolated that there was contamination of the water. This would lead us to assume that all of the wells would show a positive result; however, wells 3 and 4 showed no signal, although the same water is present with the addition of yeast strains BY4741 and 7711, respectively. Water contamination is present in the putative transformants and further analysis is
required to confirm the presence of Arg8m due to mitochondrial transformation rather than contamination. These results could be due to potential degradation of the contamination when mixed with the yeast strains. Potentially to reduce this risk in the future, having different lab benches, with different water sources, for the spheroplast and for the PCR.
Lastly, a peripheral gene that could provide information about the rest of the mitochondrial genome post-transformation was also analyzed, as shown in Figure 7. The faint signal of ATP6 in the putative transformants was not observed in previous years’ suggesting that the more Arg8m is selected for with subculturing, the greater the destabilization of the yeast mitochondrial genome.
Mitochondrial DNA mutations and dysfunction have been implicated in most cancers and mitochondrial disorders, yet the mitochondrial genome has been manipulated with limited success. Our project aims to develop a novel mitochondrial transformation technique utilizing the cotransport of tRNA to import exogenous DNA into the mitochondria of S. cerevisiae cells. We continued previous investigations by optimizing spheroplast and PCR procedures. We also explored the stability of post-transformation yeast cells by analyzing a peripheral mitochondrial gene, ATP6. Our results suggest that mitochondrial transformation is taking place, but the resulting transformants are unstable. Future research could explore whether time and the number of subcultures play a role in mitochondrial transformation stability. If the cotransport of tRNA-Arg8m is successful, our findings could be applied to human mitochondria, providing a tool to better study human diseases and a foundation for future mitochondrial gene therapy.
The author(s) declare no competing interests.
A great deal of appreciation is due to the following individuals/ institutions: Dillon Pham & Ashley Stewart (Previous years’ scholars), CUSOM, and the Summer Scholars funding.
[1] American Cancer Society. Global Cancer Facts & Figures. Cancer. org. Published 2018. https://www.cancer.org/research/cancerfacts-statistics/global.html
[2] Hsu, C. C., Tseng, L. M., & Lee, H. C. (2016). Role of mitochondrial dysfunction in cancer progression. Experimental biology and medicine (Maywood, N.J.), 241(12), 1281–1295. https://doi. org/10.1177/1535370216641787
[3] Luo, Y., Ma, J., & Lu, W. (2020). The significance of mitochondrial dysfunction in cancer. International Journal of Molecular Sciences, 21(16), 5598.
[4] Bonnefoy, N., & Fox, T. D. (2007). Directed alteration of Saccharomyces cerevisiae mitochondrial DNA by biolistic transformation and homologous recombination. In Mitochondria (pp. 153-166). Humana Press.
[5] Schneider, A. (2011). Mitochondrial tRNA import and its consequences for mitochondrial translation. Annual review of biochemistry, 80(1), 1033.
[6] Nielsen, J. (2019). Yeast systems biology: model organism and cell factory. Biotechnology journal, 14(9), 1800421.
Matthew Maffey* Mark Stewart Kaiser*
Dr. Jeffrey Krepps, Faculty Mentor
* Behavioral Health, Campbell University School of Osteopathic Medicine
ABSTRACT: Medical students encounter high levels of stress related to their education experience. Consequently, these students suffer from burnout and depression at higher rates than their age-matched peers. In recent years university interventions have increased access to mental health counseling; however, the rates of burnout and depression among medical students have remained unchanged for nearly 40 years. Psychologists practicing Acceptance and Commitment therapy propose that psychological flexibility is central to well-being and protective against psychopathology. Research has proven that individuals with greater psychological flexibility have greater mental health outcomes, and that psychological rigidity is associated with psychopathology. This review was conducted to explore literature on the relationship between psychological flexibility, burnout, and depression specifically in medical students. This brief literature review demonstrates that psychological flexibility benefits students by allowing them to build self-perspective, accept uncomfortable emotions, and remain engaged in meaningful parts of their personal lives in spite of the challenges of medical education. There is a need for more research on this topic and these results reinforce the promise that psychological flexibility plays an important role in the wellbeing of medical students.
KEYWORDS: psychological flexibility, burnout, depression, medical students, students
Addressing mental health problems among medical students is a priority for medical universities. The incidences of depression and burnout among the future leaders of our healthcare system are well documented and are known to be related to their education experience [1]. All medical students must endure the intense workload, interpersonal competition, financial burden, and exposure to death and sickness that are inherent to their early career training [5]. Thus, medical universities are bolstering their resources to address mental health problems by increasing access to mental health care, educating students about mental health problems, and attempting to reduce the stigma associated with psychopathology. These university programs should be adopted universally as they have demonstrated a mild decrease in depressive symptoms and reported suicidal ideology at individual universities [9]. However, these approaches alone have not proven adequate to address the epidemic of mental health problems across US medical schools.
In 2003 researchers at the University of Hawaii John A. Burns School of Medicine implemented such changes; “... increased individual counseling for students, faculty education, and a specialized curriculum including lectures and a student handbook” [10]. While they were successful in reducing the total burden of depression across students, at the end of their intervention the recorded prevalence of depression at their university remained at 24% [10]. A recent meta-analysis from 2022 demonstrated that across 34 years (1984-2016), the US national prevalence of depression among medical students has remained steady at about 27%. In addition, of the students
struggling with mental health problems only about 15% access help [9]. These findings can be compared to data from 2014 collected in the National Survey on Drug Use and Mental Health in the United States. Compared to medical students, the prevalence of depression among age-matched peers (18-25-year-olds and 26-49-year olds) was recorded as 9.3% and 7.2% respectively [2].
Research continues to describe the negative impact of depression and burnout on empathy, thoughts of dropping out of medical school, suicidal ideation, and academic performance [1,5]. One survey, spanning multiple medical schools, reported burnout affecting 49.6% of students, with 11.2% of students revealing thoughts of suicide within the past year [3]. This data comes even as typical university campaigns have largely failed to improve the mental health crisis at medical universities. Researchers, students, and medical institutions are justifiably wondering - how can we best address the mental health crisis of medical students now?
Research in psychology has revealed that psychological flexibility, which describes a group of helpful psychological skills, protects individuals from various psychological difficulties - including burnout and depression. Moreover, the inverse of psychological flexibility, termed psychological rigidity, is an independent risk factor for depression, anxiety, and psychological distress [8]. With this information in mind, the authors of this review sought to explore the current literature on psychological flexibility, depression, and burnout in medical students.
Stephen Hayes, a well-known practitioner of Acceptance and Commitment Therapy (ACT) describes psychological flexibility as “ the ability to contact the present moment more fully as a conscious human being, and to change or persist in behavior such that one continues to behave in a way that is consistent with their pre-established and identified values ” [4]. Acceptance and Commitment Therapy is a cognitive behavioral modality of psychotherapy aimed to increase psychological flexibility. ACT practitioners can break down psychological flexibility into a collection of six processes or positive psychological skills useful in adapting to situations.
▶ Acceptance: observe private experiences without judgment (observe frustration or fear rather than avoid such difficulties)
▶ Cognitive defusion: reduce believability of negative, false thoughts
▶ Being present: awareness in each moment
▶ Self as context: to see the context in which private experiences occur
▶ Values: consistency with personal values
▶ Committed action: choosing behaviors that will lead to desired outcomes and core values [4]
The sub-processes of psychological flexibility are interconnected in our everyday lives. They allow individuals to respond more effectively to the problems that life inevitably brings. Practitioners of ACT advocate that psychological flexibility makes a major contribution to daily well-being and lasting psychological health [6]. While psychological flexibility does not change a particular situation or event, flexibility is crucial in changing how medical students relate to the challenges inherent to the medical school experience [8]. This can empower students to connect their thought processes, chosen behaviors, and outcomes. Flexibility frees students to choose behaviors that reflect their values. The inverse of flexibility is psychological inflexibility or rigidity, and it influences behavior through the utilization of maladaptive habits and spontaneous reactions [6, 7]. The sub-processes of psychological flexibility combine over the long term to provide individuals with powerful tools to move towards meaningful goals, like maintaining personal well-being and advancing through medical school despite its inherent challenges [6].
For this brief literature review, Pubmed and the Journal of Contextual Behavioral Sciences were searched for peerreviewed publications (2012-2022). Pubmed was selected for its wide scope of publication content and the Journal of Contextual Behavioral Sciences was selected as a known hub for publications related to Acceptance and Commitment Therapy. Searches included Medical Subject Heading Phrases:
(medical students OR students) AND (depression OR burnout OR psychological flexibility OR acceptance and commitment therapy). The papers were objectively reviewed and included as applicable based on the data presentation, quality of the methodology used, and relevance to the population of interest. Due to the limited data available on the population of medical students, the decision was made to include studies that included graduate and undergraduate students.
The articles chosen present results comparing psychological flexibility to depression or burnout, or a combination of these outcomes among undergraduate students, graduate students, and medical students. All studies included were completed in the United States. One study evaluating psychological flexibility and mental health outcomes in underrepresented minority graduate students was included to highlight the need for future research among minority students.
Yadavaia, Hayes, and Vilardago reported that psychological flexibility mediated changes in depressive symptoms and psychological stress among undergraduate students [12]. These authors performed a mediation analysis to determine how psychological flexibility impacted changes in depression and psychological stress after a 6-hour ACT workshop intervention. Their workshop intervention focused on addressing cognitive defusion and acceptance in the students. At the start of the study 225 students met inclusion criteria, and only 38% (78), attended the informed consent session. Ultimately, 73 students completed the trial.
Participants identified self-critical thoughts and selfconceptualizations and worked through them by decreasing fusion with false thoughts, building self-perspective, and strengthening self-kindness through acceptance [12]. The team surveyed students with the Acceptance and Action Questionnaire-II (AAQ-II), General Health Questionnaire (GHQ), and Depression and Anxiety Scale-21 (DASS-21) 1 week before the intervention, 1-2 weeks after the intervention, and 8-9 weeks after the intervention.
The experimental intervention transformed exercises from an ACT workbook to a group setting, which was previously described by Lillis, Hayes, and Bunting in 2009 [12]. Ultimately, this intervention demonstrated that levels of psychological flexibility mediated a difference of 59% in depressive symptoms across the student sample [12]. Greater psychological flexibility appeared protective against developing depression. Furthermore, after the intervention, the undergraduate students reported higher levels of self-compassion and lower levels of general psychological stress [12].
Kroska et. al captured an ideal research study for measuring psychological flexibility, burnout, and depression in medical
students [7]. In this study, 256 medical students were surveyed about depressive symptoms, burnout symptoms, avoidance behaviors, and value-based behaviors. Avoidance behaviors and disengagement with value-based behaviors served as a proxy measurement for psychological inflexibility in this population. Avoidance was measured using the Revised Acceptance and Avoidance Questionnaire (AAQ-II) - a demonstrated reliable index of psychological inflexibility. Engagement in values-based actions was measured by the Valued Living Questionnaire (VLQ) which examines values in domains of life including relationships, family, career, and leisure [7]. Burnout was further divided into clinically relevant subcomponents of emotional exhaustion, depersonalization, and a lower sense of personal accomplishment. The authors measured burnout by Maslach Burnout Inventory (MBI) and depression by the Inventory for Depression and Anxiety Symptoms (IDAS).
The results from the survey of second, third, and fourthyear medical students showed that higher levels of avoidance behavior were associated with higher levels of depression (B = 0.56). Lower levels of value-based behaviors mediated levels of depression (R² = 0.46) and burnout. Subtypes of burnout included in this study were Emotional Exhaustion (R² = 0.26), Depersonalization (R² = 0.22), and Personal Accomplishment (R² = 0.13) [7].
This research might serve as a model for future research on psychological flexibility in medical students. Their report on mediation analysis is essential for discussion of the processes which lead to burnout and depression. In this study, avoidance and decreased engagement in valued-based behaviorsmeasures of psychological inflexibility - mediated scores of burnout and depression. In their conclusion, the authors suggested that guiding medical students in clarifying their values and engaging in value-based behaviors - improving on components of psychological flexibility - are essential to enhance the well-being of medical students [7].
Miller and Orsillo measured psychological flexibility and levels of depression in underrepresented minority graduate students [9]. These researchers concluded that psychological flexibility mediated a greater effect on depressive symptoms (R²=0.23) than negative racial experiences alone [9]. The results of this study suggest that interventions to increase psychological flexibility, such as an ACT workshop, may be especially beneficial to underrepresented minority students. This research brings into question whether underrepresented minority students in medical school may also benefit from interventions to increase psychological flexibility to help cope with negative racial experiences which may contribute to psychopathology [9].
A brief overview of the current literature on psychological flexibility and students suggests that psychological inflexibility is related to burnout and depression among medical students
[7]. Meanwhile, psychological flexibility seems to be negatively associated with psychopathology. Similar findings among undergraduate students and minority students reinforce the need for more research on these topics.
Medical students take on many stressors unique to medical training. In these circumstances, they must balance their academics, relationships, sleep, exercise, personal hygiene, and hobbies under time pressure. To successfully navigate through medical school, medical students can leverage positive psychological skills, summarized as psychological flexibility, to adapt to their ever-changing circumstances.
This brief review validates the need for more research on the topics of psychological flexibility, burnout, and depression among medical students. The available research from Kroska et. al strengthens the hypothesis that psychological flexibility is an important mediator of burnout and depression in medical students [7]. A similar research endeavor to Yadavia et. al should be reproduced in a population of medical students to contribute to the current evidence base. Utilizing a standard protocol, as in this study, could be crucial in standardizing cause-andeffect relationships. Lastly, Miller and Orsillo demonstrate the value of psychological flexibility to help build resilience against negative racial experiences that minority students encounter during their education experiences [9].
Together, this brief review supports the need for future investigations surrounding psychological flexibility, burnout, and depression in medical students. A project of greater scope is forthcoming to expand this body of work.
DECLARATIONS OF INTEREST
The authors declare no competing interests at this time.
The authors would like to acknowledge Jeffrey Krepps Ph.D., Director of Behavioral Health Education and Research at Campbell University School of Osteopathic Medicine, for his contributions as supervisor of this review article and for providing unwavering support towards exploring this topic.
[1] Brazeau, Chantal M.L.R., Tait Shanafelt, Steven J. Durning, F. Stanford Massie, Anne Eacker, Christine Moutier, Daniel V. Satele, Jeff A. Sloan, and Liselotte N. Dyrbye. “Distress Among Matriculating Medical Students Relative to the General Population:” Academic Medicine 89, no. 11 (November 2014): 1520–25. https://doi.org/10.1097/ACM.0000000000000482.
[2] Center for Behavioral Health Statistics and Quality. (2015). Behavioral health trends in the United States: Results from the 2014 National Survey on Drug Use and Health (HHS Publication No. SMA 15-4927, NSDUH Series H-50). Retrieved from http:// www.samhsa.gov/data/
[3] Dyrbye LN, Thomas MR, Massie FS, Power DV, Eacker A, Harper W, Durning S, Moutier C, Szydlo DW, Novotny PJ, Sloan JA, Shanafelt TD. Burnout and suicidal ideation among U.S. medical students. Ann Intern Med. 2008 Sep 2;149(5):334-41. doi: 10.7326/0003-4819-149-5-200809020-00008. PMID: 18765703
[4] Hayes S. C., Wilson K. G., Gifford E. V., Follette V. M., Strosahl K. (1996). Experiential avoidance and behavioral disorders: a functional dimensional approach to diagnosis and treatment. J. Consult. Clin. Psychol. 64 1152–1168. 10.1037/0022-006X.64.6.1152
[5] Hill, Monica R., Shelby Goicochea, and Lisa J. Merlo. “In Their Own Words: Stressors Facing Medical Students in the Millennial Generation.” Medical Education Online 23, no. 1 (January 2018): 1530558. https://doi.org/10.1080/10872981.2018.1530558
[6] Kashdan, T. B., & Rottenberg, J. (2010). Psychological flexibility as a fundamental aspect of health. Clinical Psychology Review, 30(7), 865-878. https://doi.org/10.1016/j.cpr.2010.03.001
[7] Kroska, E. B., Calarge, C., O’Hara, M. W., Deumic, E., & Dindo, L. (2017). Burnout and depression in medical students: Relations with avoidance and disengagement. Journal of Contextual Behavioral Science, 6(4), 404-408. https://doi.org/10.1016/j. jcbs.2017.08.003
[8] Lappalainen, Päivi, Sitwat Langrial, Harri Oinas-Kukkonen, Asko Tolvanen, and Raimo Lappalainen. “Web-Based Acceptance and Commitment Therapy for Depressive Symptoms With Minimal Support: A Randomized Controlled Trial.” Behavior Modification 39, no. 6 (November 2015): 805–34. https://doi. org/10.1177/0145445515598142.
[9] Miller, A. N., & Orsillo, S. M. (2020). Values, acceptance, and belongingness in graduate school: Perspectives from underrepresented minority students. Journal of Contextual Behavioral Science, 15, 197-206. https://doi.org/10.1016/j. jcbs.2020.01.002
[10] Rotenstein, Lisa S., Marco A. Ramos, Matthew Torre, J. Bradley Segal, Michael J. Peluso, Constance Guille, Srijan Sen, and Douglas A. Mata. “Prevalence of Depression, Depressive Symptoms, and Suicidal Ideation Among Medical Students: A Systematic Review and Meta-Analysis.” JAMA 316, no. 21 (December 6, 2016): 2214. https://doi.org/10.1001/jama.2016.17324.
[11] Slavin, Stuart J., Debra L. Schindler, and John T. Chibnall. “Medical Student Mental Health 3.0: Improving Student Wellness Through Curricular Changes.” Academic Medicine 89, no. 4 (April 2014): 573–77. https://doi.org/10.1097/ACM.0000000000000166.
[12] Yadavaia, J. E., Hayes, S. C., & Vilardaga, R. (2014). Using acceptance and commitment therapy to increase selfcompassion: A randomized controlled trial. Journal of Contextual Behavioral Science, 3(4), 248-257. https://doi.org/10.1016/j. jcbs.2014.09.002
Lindsey Clemson*, Madison Karabinus*, Thomas Sandoval*, Bonnie Brenseke**, Alan Proia**
* Medical Students, Campbell University School of Osteopathic Medicine ** Department of Pathology, Campbell University School of Osteopathic Medicine
ABSTRACT This case details urothelial carcinoma of the urinary bladder in an 86-year-old-female cadaver. During cadaveric dissection, the urinary bladder was lined by markedly thickened and irregular epithelium which was sampled for gross and histological evaluation, revealing papillary urothelial carcinoma. Comprehensive review of the literature demonstrated new testing modalities for earlier diagnosis of bladder cancer, including the UroVysion bladder cancer kit which is utilized in patients with hematuria under suspicion of bladder carcinoma who opt out of invasive diagnostic modalities, such as cystoscopy. This technological innovation provides the practicing clinician with a less invasive bladder carcinoma diagnostic tool.
KEYWORDS: urothelial carcinoma, Urovysion, end stage renal disease
Worldwide, bladder cancer is the ninth most common malignancy. Specifically within the United States, the incidence of bladder cancer is 80,000 new cases per year. Within the same year span, bladder cancer leads to 17,000 deaths [1]. Urothelial carcinoma is the dominating subtype in North America and Europe [2]. Distribution of bladder cancer cases varies by sex, race, and age. While white males have a higher incidence of bladder cancer, females and African Americans are more likely to present with tumors of a more advanced stage [3]. A majority of those diagnosed with bladder cancer are older in age, with approximately 73% being over age 65 [4, 5].
In Western countries, the most important environmental factor affecting the incidence of bladder cancer is cigarette smoking [6-8]. Research has shown that the onset and aggressiveness of bladder malignancy is related to the magnitude of cigarette smoking. Those who smoke heavily are more likely to present with a high-grade tumor that has invaded into the muscle of the bladder [9]. Smoking is also an irreversible risk factor, as cessation can decrease but not eradicate bladder cancer risk [7, 8]. Studies have also shown that secondhand smoke exposure can be a risk factor for bladder cancer in females [10, 11]. Certain occupational exposures also pose a greater risk of bladder cancer than others. Heavy machinery operators exposed to diesel exhaust, painters working with polycyclic aromatic hydrocarbons, and hairdressers handling certain hair dyes are all at increased risk [12-14]. Those involved in the production of carpet, plastics, and industrial chemicals are also at risk [15, 16]. Aside from environmental exposures, additional aspects of a patient’s medical history may reveal risk factors. The long-standing irritation associated with chronic cystitis may induce metaplasia, dysplasia, and eventual carcinoma [17]. High-risk subtypes of HPV, such as 16 and 18, have also been associated with bladder cancer [18, 19]. Pelvic radiation and cyclophosphamide’s production of acrolein have been implicated in bladder cancer risk assessment [20-23].
Patients with bladder cancer may initially present with painless hematuria, visible or microscopic, or with lower urinary tract symptoms, such as dysuria, frequency, and urgency. Hematuria is the most common symptom at presentation. Gross hematuria is defined as the visible discoloration of urine to a red or brown pigment and is heme (hemoglobin or myoglobin) positive. Microscopic hematuria is defined as three or more red blood cells (RBCs) per high-power field (HPF) in a spun urine sediment [24].
In patients with microscopic hematuria, the American Urological Association (AUA) and the Society of Urodynamics, Female Pelvic Medicine & Urogenital Reconstruction (SUFU) have outlined a preferred evaluation algorithm that is further detailed (Appendix A) [25, 26]. Voiding symptoms or lower urinary tract symptoms are another common presenting symptom in patients with bladder carcinoma [27]. These symptoms may be the sequelae of a decrease in bladder capacity, detrusor muscle hyperactivity, trigone invasion, or physical obstruction of the bladder neck and urethra. Patients who present with pain or constitutional symptoms should be immediately evaluated due to the increased likelihood that these are associated with advanced or metastatic tumors.
Bladder tumor staging is the most important factor in predicting prognosis of the cancer and determining the recurrence risk and expected progression of the disease. The tumor, node, metastasis (TNM) system, devised by the American Joint Committee on Cancer (AJCC), is the preferred method to determine prognosis, with the depth of invasion of the primary tumor being the most important prognostic indicator (Appendix B) [28].
Currently, cystoscopy remains the most reliable method for diagnosing bladder cancer. Cystoscopy for the purpose of diagnosing urothelial carcinoma involves an endoscopic examination of the urinary bladder using a cystoscope advanced through the patient’s urethra. This allows physicians
to examine the tissue and contents of the urinary bladder, and provides a mildly invasive means to biopsy and treat potential urinary pathology. Local anesthetic can be administered to avoid pain during a rigid endoscopy procedure, but flexible cystoscopy can be performed without anesthesia [29]. If any suspicious lesions are found during the procedure, a transurethral bladder tumor resection (TURBT) can be performed, in which a small amount of tissue is sampled for histological examination by a pathologist, who will aid in the staging and grading of a potential malignancy [30]. However, cystoscopy can fail to visualize certain cancers, especially carcinoma in situ. One recent improvement to cystoscopy has been the use of blue light fluorescence, in which hexyl aminolevulinate hydrochloride is administered into the bladder and accumulates in cancerous cells, which then fluoresce when exposed to blue light [31]. There are risks to cystoscopy, including dysuria, hematuria, and even urinary incontinence [29]. Due to the risk of discomfort and potential urinary adverse effects of cystoscopy, less invasive diagnostic tools have been pursued regarding the early detection of bladder cancer. Imaging is commonly used for the recognition of potential cancerous masses, staging of a known cancer, or determining metastasis of a malignancy. Computerized tomography (CT) with oral or intravenous contrast medium, magnetic resonance imaging (MRI), positron emission tomography (PET), and US imaging can all be used to aid in the visualization of cancerous lesions and identify potential spread [32]. Still, even simpler and less expensive early detection screening tools are being used in patients who have shown signs of a potential malignancy. Urinary cytology is a helpful additive diagnostic tool in which microscopy is performed on a random urine sample. Epithelial cells that are normally shed in the urinary tract are collected from the urine and microscopically examined for cancerous cells, inflammation, or signs of infection. This procedure can be helpful in detecting malignancies that lie along the entire urinary tract, including the bladder, urethra, kidney, and ureters. Cytology is usually ordered if a patient presents with mild, painless hematuria. However, concerns revolve around relying on only cytology as a means to diagnose bladder cancer, as cytology has a low sensitivity for low-grade bladder tumors [33, 34]. This lack of sensitivity, along with a variable symptom presentation, makes bladder carcinoma difficult to diagnose [35, 36].
There are many treatment options for management of carcinoma in situ of the bladder, including administration of Bacillus Calmette-Guérin, numerous immunotherapies, and fibroblast growth factor receptor inhibitors (Appendix C) [3741]. However, for muscle-invasive bladder carcinomas, mainstay management includes partial or radical cystectomy, which includes removal of part or the entire bladder, depending on the grading and spread of the cancer. Cystectomy usually involves pelvic node dissection and may be accompanied by prostate removal in males or removal of the ovaries, oviducts, uterus, and cervix in females [42]. Due to the invasive nature of a cystectomy and removal of a vital organ, reconstructive surgery is often required following bladder removal to help divert urine
out of the body. An incontinent diversion requires placement of an extracorporeal urostomy bag, while a continent diversion involves the formation of an intraabdominal bladder-like pouch that can store urine without an external bag [43]. While there are many potential options for treatment of bladder cancer depending on the given grade, stage, and metastases, many risks are associated with these treatment therapies, including infection, incontinence, and sexual dysfunction.
This case details urothelial carcinoma of the urinary bladder in an 86-year-old-female with the suspected cause of death being end-stage renal disease. The gross and microscopic findings are provided and diagnostic advancements for this malignancy are discussed. Cadaveric dissection performed by medical students at Campbell University Jerry M. Wallace School of Osteopathic Medicine revealed a urinary bladder that was lined by markedly thickened and irregular transitional epithelium. A bladder sample approximately 2 x 1 cm in size was collected and stored in 10% neutral buffered formalin at room temperature, a process referred to as fixation which helps to prevent decay and autolysis in sampled tissue. Following storage in the buffered formalin for 1 week, the tissue was trimmed to be fit into a tissue cassette and sent to the University of North Carolina at Chapel Hill to be processed, embedded, sectioned, and stained. Processing of the sample involved dehydration in an alcohol bath and clearing the alcohol with xylene to solidify the tissue. The sample was then embedded in paraffin wax and sectioned into samples that were retrieved and dried on a glass slide. A standard hematoxylin and eosin (H&E) stain was used, which involves a basophilic (hematoxophilic) stain that reacts with acidic nuclei and an acidophilic (eosinophilic) stain that reacts with basic proteins and cytoplasm. The glass slide was then scanned to allow for virtual microscopy capabilities. The histological sample was examined thoroughly by pathologist Dr. Alan Proia for any histological abnormalities.
Gross examination revealed markedly thickened urinary bladder mucosa arranged in numerous exophytic papillary projections (figure 1).
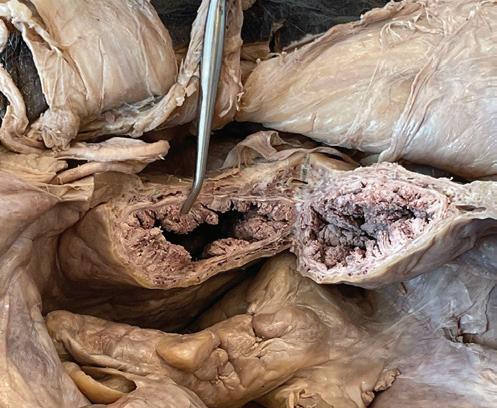
1. Cross section of the urinary bladder.
Additionally, the uterus was removed antemortem, multiple adhesions were observed on the sigmoid colon, and both kidneys were shrunken (figure 2).
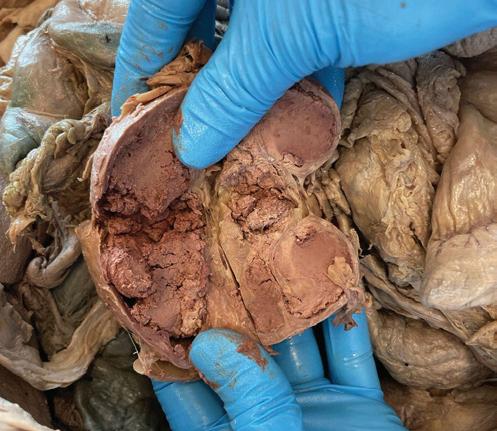
2. Cross section of right kidney. Note the shrunken appearance with nodular cysts within.
Histologic examination revealed that the normal urothelial lining was replaced by papillary projections of neoplastic urothelium supported by fibrovascular cores (figure 3) with deep penetration into the lamina propria of the tissue (figure 4).

3. Urinary bladder tumor. Note the papillary architecture with extensive invasion of the mucosa lamina propria and vasculature. H&E, 10x.

FIGURE 4. Urinary bladder tumor. Carcinoma has penetrated the lamina propria lymphatics. H&E, 400x.
Given that this patient’s cause of death was ESRD, it is inferred that she was previously on dialysis prior to her death. A recent retrospective cohort study examined the incidence of specific cancers in patients with ESRD receiving dialysis treatment. Within this population, researchers found a cumulative incidence of cancer of 9.48% during the 5 years post-dialysis initiation. Specifically, the risk was raised the most regarding cancers of the kidney and bladder. Based on this retrospective study, statistics indicate that those with ESRD on dialysis have a potentially increased risk of developing cancer. A study conducted on chronic dialysis patients in Taiwan yielded similar results. Young patients and those in their first year of dialysis had an increased incidence of cancer, specifically bladder cancer with a frequency of 21.2%. The overall incidence of cancers in patients on chronic dialysis was higher in females than males [44]. Several proposed mechanisms exist to explain this discrepancy in cancer incidence in patients on dialysis. It is hypothesized that nutritional problems and ESRD-associated
immunodeficiency may play a role. Additionally, there is a possible interaction between risk factors such as alcohol and tobacco use and immune dysfunction related to dialysis and uremia. The combination of these stressors on the body is thought to have an association with cancer development and burden [45].
Further retrospective study has demonstrated that urothelial carcinomas are typically high-grade and diagnosed at a more advanced stage in dialysis patients [46]. Diagnosed as noninvasive or invasive, this determination is crucial in developing a treatment plan and prognosis. For this reason, diagnostic improvements allowing for earlier detection are essential. A sole reliance on asymptomatic gross hematuria in the determination of bladder cancer is not possible in dialysis patients, as they frequently display anuria or oliguria. In this case, a lack of typical symptom presentation can lead to a delayed diagnosis and advanced tumor stage by the time treatment is initiated. The Taiwan study discussed above describes barriers to screening patients, such as the costly and invasive nature of a cystoscopy. For this reason, the researchers recommended a more selective screening of patients solely at dialysis induction or during the initial stages [46].
As these recent studies have brought to light the associations between bladder cancer and ESRD/dialysis, it is crucial that better surveillance techniques are utilized in monitoring these high-risk patients for cancer development. While cystoscopy has become the standard diagnostic test, the costly and invasive nature of this procedure has made it difficult to apply as a broad screening test.
As previously discussed, cytology is more commonly utilized as a diagnostic method for urothelial carcinoma due to its noninvasive and inexpensive nature. Advancements in urinary cytology have made this process highly sensitive and specific in diagnosing high-grade urothelial carcinoma of the bladder and kidney. However, cytology often fails to identify low-grade cancers or renal cell carcinoma, making this testing modality less reliable when used alone compared to traditional diagnostic methods, such as cystoscopy and biopsy [47]. Still, many noninvasive, rapid examinations for bladder cancers are beginning to emerge. The nuclear matrix protein 22 (NMP22) assay is a marker of mitotic activity of cells within a urine sample, which may be suggestive of malignancy, and has been shown to be more sensitive in detecting bladder cancer than cytology alone [48]. The bladder tumor antigen (BTA) test is another rapid test used to detect human complement factor H related protein (CFHRs) in the urine sample of patients with suspected bladder cancer. These CFHRs bind complement C3b and may contribute to multiple diseases, including bladder cancer, in poorly understood mechanisms [49, 50].
One of the most promising diagnostic methods for urothelial carcinoma being researched and pursued is the use of fluorescence in situ hybridization (FISH) to detect loss of 9p21, as well as aneuploidy of chromosomes 3, 7, and 17, an assay referred to as UroVysion [51]. UroVysion has recently been FDA approved for the purpose of assisting in the diagnosis
of recurrent bladder cancer in patients with a past medical history of this disease, as well as the diagnosis of bladder cancer in patients presenting with hematuria without a history of cancer. This kit utilizes three alpha-satellite repeat sequence probes, in addition to another unique probe, that bind to the centromere regions of specific chromosomes of interest. CEP 3 SpectrumRed hybridizes to chromosome 3, CEP 7 SpectrumGreen hybridizes to chromosome 7, and CEP 17 SpectrumAqua hybridizes to chromosome 17, while LSI p16 (9p21) SpectrumGold hybridizes to the CDKN2A region on the chromosome 9p21 locus, which encodes the p16 tumor suppressor gene [52, 53]. The lack of these chromosomes or presence of mutations can disrupt normal functioning of the cell-division cycle, resulting in improper progression, uncontrolled and dysregulated cell growth, and potential cancer formation [54]. Although UroVysion has been shown to be more sensitive in detecting bladder cancer when compared to cytology, the Urovysion kit is intended to be used in adjunct with other traditional diagnostic methods as a means to support a diagnosis of bladder cancer. Urovysion is often applied in conjunction with cytology in patients with suspected bladder cancer who wish to avoid more invasive procedures, such as cystoscopy [51]. This kit is also being used to monitor bladder cancer progression following the administration of the BCG vaccine, and as a means to screen cancer potential in patients with a past medical history of bladder cancer [55, 56]. UroVysion tests have been positive in patients who were diagnosed with or later developed other urinary cancers, such as renal cancer [52]. Use of this Urovysion kit is being considered as the next step in research regarding noninvasive diagnostic methods for detecting and monitoring urothelial cancers.
Each of these noninvasive tests for bladder cancer are promising alternatives to the standard screening regiment involving the more invasive method, cystoscopy. With more widespread use of UroVysion, individuals such as the patient in this case report may have been diagnosed earlier. UroVysion utilization in patients with ESRD on dialysis is a promising method to screen for bladder cancer, as sole reliance on asymptomatic gross hematuria is not possible. Applying noninvasive screening regiments in patients with bladder cancer risk factors may allow for close monitoring with earlier diagnosis and treatment. As the most important prognostic factor of bladder cancer is tumor staging, an earlier diagnosis at a lower stage with decreased metastasis and lymphatic invasion could potentially lead to more favorable treatment outcomes.
As the ninth most common malignancy worldwide, bladder carcinoma typically presents with a painless hematuria. This symptom may be a diagnostic aid in many patients, however individuals with end stage renal disease on dialysis add another layer of complexity. While studies have shown that those on dialysis display an increased incidence of bladder cancer, the principal sign of painless hematuria may be more difficult to detect due to the use of dialysis. The increased association with cancer and lack of a classic symptom presentation alludes to
the necessity of frequent, noninvasive screening techniques for bladder cancer in high-risk populations. While cystoscopy is considered the gold standard for bladder carcinoma diagnosis, this procedure can be costly and invasive. A promising new method for diagnosis and follow-up of bladder carcinoma is Urovysion. This diagnostic approach involves collecting a patient’s urine and processing the sample through fluorescence in situ hybridization to detect genetic defects commonly associated with bladder cancer. Currently the Urovysion kit is primarily utilized to monitor bladder cancer progression during treatment. However, this non-invasive technique is being considered a promising diagnostic adjunct in the early detection of bladder cancer, especially in high-risk patients such as those with ESRD on dialysis.
The authors declare no competing interests.
We thank the donor and the family for their contribution to the benefit of our medical education. We also recognize Chelsea Hudson, MS-2 for notifying the team of this pathological finding, as well as Ms. Ashley Valley, BS for her assistance in photography. This project was made possible through the internal support and funding provided through CUSOM and the efforts of the CUSOM pathology department, Dr. Bonnie Brenseke, DVM, PhD, DACVP, and Dr. Alan Proia, MD, PhD.
[1] Ebrahimi H, Amini E, Pishgar F, et al. Global, regional and national burden of bladder cancer, 1990 to 2016: results from the GBD study 2016. J Urol. 2019;201(5):893-901. doi:10.1097/ JU.0000000000000025
[2] Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: a cancer journal for clinicians. 2022;72(1):7-33. doi:10.3322/ caac.21708
[3] Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer. 2009;115(1):68-74. doi:10.1002/cncr.23986
[4] Burger M, Catto JW, Dalbagni G, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63(2):234-241. doi:10.1016/j.eururo.2012.07.033
[5] Lynch CF, Cohen MB. Urinary system. Cancer. 1995;75(1 Suppl):316-329. doi:10.1002/1097-0142(19950101)75:1+<316::aidcncr2820751314>3.0.co;2-t
[6] Hinotsu S, Akaza H, Miki T, et al. Bladder cancer develops 6 years earlier in current smokers: analysis of bladder cancer registry data collected by the cancer registration committee of the Japanese Urological Association. Int J Urol. 2009;16(1):64-69. doi:10.1111/j.1442-2042.2008.02194.x
[7] Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women [published correction appears in JAMA. 2011 Nov 23;306(20):2220]. JAMA. 2011;306(7):737-745. doi:10.1001/ jama.2011.1142
[8] Cumberbatch MG, Rota M, Catto JW, La Vecchia C. The role of tobacco smoke in bladder and kidney carcinogenesis: a comparison of exposures and meta-analysis of incidence and mortality risks. Eur Urol. 2016;70(3):458-466. doi:10.1016/j. eururo.2015.06.042
[9] Pietzak EJ, Mucksavage P, Guzzo TJ, Malkowicz SB. Heavy cigarette smoking and aggressive bladder cancer at initial presentation. Urology. 2015;86(5):968-972. doi:10.1016/j. urology.2015.05.040
[10] Skipper PL, Tannenbaum SR, Ross RK, Yu MC. Nonsmokingrelated arylamine exposure and bladder cancer risk. Cancer Epidemiol Biomarkers Prev. 2003;12(6):503-507.
[11] Jiang X, Yuan JM, Skipper PL, Tannenbaum SR, Yu MC. Environmental tobacco smoke and bladder cancer risk in never smokers of Los Angeles County. Cancer Res. 2007;67(15):75407545. doi:10.1158/0008-5472.CAN-07-0048
[12] Smailyte G, Kurtinaitis J, Andersen A. Mortality and cancer incidence among Lithuanian cement producing workers. Occup Environ Med. 2004;61(6):529-534. doi:10.1136/oem.2003.009936
[13] Zeegers MP, Swaen GM, Kant I, Goldbohm RA, van den Brandt PA. Occupational risk factors for male bladder cancer: results from a population based case cohort study in the Netherlands. Occup Environ Med. 2001;58(9):590-596. doi:10.1136/oem.58.9.590
[14] Ames BN, Kammen HO, Yamasaki E. Hair dyes are mutagenic: identification of a variety of mutagenic ingredients. Proc Natl Acad Sci U S A. 1975;72(6):2423-2427. doi:10.1073/pnas.72.6.2423
[15] Kogevinas M, ‘t Mannetje A, Cordier S, et al. Occupation and bladder cancer among men in Western Europe. Cancer Causes Control. 2003;14(10):907-914. doi:10.1023/ b:caco.0000007962.19066.9c
[16] Gaertner RR, Trpeski L, Johnson KC; Canadian Cancer Registries Epidemiology Research Group. A case-control study of occupational risk factors for bladder cancer in Canada. Cancer Causes Control. 2004;15(10):1007-1019. doi:10.1007/s10552-0041448-7
[17] Delnay KM, Stonehill WH, Goldman H, Jukkola AF, Dmochowski RR. Bladder histological changes associated with chronic indwelling urinary catheter. J Urol. 1999;161(4):1106-1109.
[18] Kawanishi S, Ohnishi S, Ma N, Hiraku Y, Oikawa S, Murata M. Nitrative and oxidative DNA damage in infection-related carcinogenesis in relation to cancer stem cells. Genes Environ. 2017;38:26. Published 2017 Jan 1. doi:10.1186/s41021-016-0055-7
[19] Li N, Yang L, Zhang Y, Zhao P, Zheng T, Dai M. Human papillomavirus infection and bladder cancer risk: a meta-analysis. J Infect Dis. 2011;204(2):217-223. doi:10.1093/infdis/jir248
[20] Kleinerman RA, Boice JD Jr, Storm HH, et al. Second primary cancer after treatment for cervical cancer. An international cancer registries study. Cancer. 1995;76(3):442452. doi:10.1002/1097-0142(19950801)76:3<442::aidcncr2820760315>3.0.co;2-l
[21] Travis LB, Curtis RE, Storm H, et al. Risk of second malignant neoplasms among long-term survivors of testicular cancer. J Natl Cancer Inst. 1997;89(19):1429-1439. doi:10.1093/jnci/89.19.1429
[22] Pedersen-Bjergaard J, Ersbøll J, Hansen VL, et al. Carcinoma of the urinary bladder after treatment with cyclophosphamide for non-Hodgkin’s lymphoma. N Engl J Med. 1988;318(16):1028-1032. doi:10.1056/NEJM198804213181604
[23] Rabbani F, Perrotti M, Russo P, Herr HW. Upper-tract tumors after an initial diagnosis of bladder cancer: argument for longterm surveillance. J Clin Oncol. 2001;19(1):94-100. doi:10.1200/ JCO.2001.19.1.94
[24] Khadra MH, Pickard RS, Charlton M, Powell PH, Neal DE. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol. 2000;163(2):524-527.
[25] Barocas DA, Boorjian SA, Alvarez RD, et al. Microhematuria: AUA/SUFU guideline. J Urol. 2020;204(4):778-786. doi:10.1097/ JU.0000000000001297
[26] Committee opinion no.703: asymptomatic microscopic hematuria in women. Obstet Gynecol. 2017;129(6):e168-e172. doi:10.1097/ AOG.0000000000002059
[27] DeGeorge KC, Holt HR, Hodges SC. Bladder cancer: diagnosis and treatment. Am Fam Physician. 2017;96(8):507-514.
[28] Edge SB. AJCC Cancer Staging Manual. Eighth edition. Springer; 2017.
[29] Matulewicz RS, DeLancey JO, Meeks JJ. Cystoscopy. JAMA. 2017;317(11):1187. doi:10.1001/jama.2017.0364
[30] Kim LHC, Patel MI. Transurethral resection of bladder tumour (TURBT). Transl Androl Urol. 2020;9(6):3056-3072. doi:10.21037/ tau.2019.09.38
[31] Grossman HB. Improving the management of bladder cancer with fluorescence cystoscopy. J Environ Pathol Toxicol Oncol. 2007;26(2):143-147. doi:10.1615/jenvironpatholtoxicoloncol.v26. i2.90
[32] Mirmomen SM, Shinagare AB, Williams KE, Silverman SG, Malayeri AA. Preoperative imaging for locoregional staging of bladder cancer. Abdom Radiol (NY). 2019;44(12):3843-3857. doi:10.1007/s00261-019-02168-z
[33] Planz B, Jochims E, Deix T, Caspers HP, Jakse G, Boecking A. The role of urinary cytology for detection of bladder cancer. Eur J Surg Oncol. 2005;31(3):304-308. doi:10.1016/j.ejso.2004.12.008
[34] Barak V, Itzkovich D, Einarsson R, Gofrit O, Pode D. Non-invasive detection of bladder cancer by UBC rapid test, ultrasonography and cytology. Anticancer Res. 2020;40(7):3967-3972. doi:10.21873/ anticanres.14389
[35] Lobo N, Shariat SF, Guo CC, et al. What is the significance of variant histology in urothelial carcinoma?. Eur Urol Focus. 2020;6(4):653-663. doi:10.1016/j.euf.2019.09.003
[36] Zibelman M, Asghar AM, Parker DC, et al. Cystoscopy and systematic bladder tissue sampling in predicting pT0 bladder cancer: a prospective trial. J Urol. 2021;205(6):1605-1611. doi:10.1097/JU.0000000000001602
[37] Han J, Gu X, Li Y, Wu Q. Mechanisms of BCG in the treatment of bladder cancer-current understanding and the prospect. Biomed Pharmacother. 2020;129:110393. doi:10.1016/j.biopha.2020.110393
[38] Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder cancer: a review. JAMA. 2020;324(19):1980-1991. doi:10.1001/jama.2020.17598
[39] Lopez-Beltran A, Cimadamore A, Blanca A, et al. Immune checkpoint inhibitors for the treatment of bladder cancer. Cancers (Basel). 2021;13(1):131. Published 2021 Jan 3. doi:10.3390/ cancers13010131
[40] Garje R, An J, Obeidat M, Kumar K, Yasin HA, Zakharia Y. Fibroblast growth factor receptor (FGFR) inhibitors in urothelial cancer. Oncologist. 2020;25(11):e1711-e1719. doi:10.1634/ theoncologist.2020-0334
[41] Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin. 2020;70(5):404-423. doi:10.3322/caac.21631
[42] Hamad J, McCloskey H, Milowsky MI, Royce T, Smith A. Bladder preservation in muscle-invasive bladder cancer: a comprehensive review. Int Braz J Urol. 2020;46(2):169-184. doi:10.1590/S1677-5538. IBJU.2020.99.01
[43] Lee RK, Abol-Enein H, Artibani W, et al. Urinary diversion after radical cystectomy for bladder cancer: options, patient selection, and outcomes. BJU Int. 2014;113(1):11-23. doi:10.1111/bju.12121
[44] Lin HF, Li YH, Wang CH, Chou CL, Kuo DJ, Fang TC. Increased risk of cancer in chronic dialysis patients: a population-based cohort study in Taiwan. Nephrol Dial Transplant. 2012;27(4):1585-1590. doi:10.1093/ndt/gfr464
[45] Butler AM, Olshan AF, Kshirsagar AV, et al. Cancer incidence among US Medicare ESRD patients receiving hemodialysis, 1996-2009. Am J Kidney Dis. 2015;65(5):763-772. doi:10.1053/j. ajkd.2014.12.013
[46] Sato Y, Kondo T, Takagi T, Junpei I, Tanabe K. Treatment strategy for bladder cancer in patients on hemodialysis: a clinical review of 28 cases. Int Urol Nephrol. 2016;48(4):503-509. doi:10.1007/s11255015-1199-2
[47] Bostwick DG. Urine cytology. In: Cheng L, MacLennan GT, Bostwick DG, eds. Urological Surgical Pathology. Fourth edition. Elsevier; 2020: 322-357.
[48] Saint F, Quintens H, Roupret M, et al. Actualités urologiques concernant les tests diagnostiques utilisés pour les tumeurs de vessie: le NMP22 [Diagnostic test for bladder cancer: the NMP22®]. Prog Urol. 2011;21(4):245-249. doi:10.1016/j. purol.2010.09.027
[49] Guo A, Wang X, Gao L, Shi J, Sun C, Wan Z. Bladder tumour antigen (BTA stat) test compared to the urine cytology in the diagnosis of bladder cancer: A meta-analysis. Can Urol Assoc J. 2014;8(5-6):E347-E352. doi:10.5489/cuaj.1668
[50] Cheng ZZ, Corey MJ, Pärepalo M, et al. Complement factor H as a marker for detection of bladder cancer. Clin Chem. 2005;51(5):856-863. doi:10.1373/clinchem.2004.042192
[51] Halling KC, Kipp BR. Bladder cancer detection using FISH (UroVysion assay). Adv Anat Pathol. 2008;15(5):279-286. doi:10.1097/PAP.0b013e3181832320
[52] Abbott Molecular. UroVysion Bladder Cancer Kit. Molecular. Abbot.com. Accessed March 20, 2022. https://www.molecular. abbott/us/en/products/oncology/urovysion-bladder-cancer-kit
[53] Williams SV, Sibley KD, Davies AM, et al. Molecular genetic analysis of chromosome 9 candidate tumor-suppressor loci in bladder cancer cell lines. Genes Chromosomes Cancer. 2002;34(1):86-96. doi:10.1002/gcc.10050
[54] Kar S. Unraveling cell-cycle dynamics in cancer. Cell Syst. 2016;2(1):8-10. doi:10.1016/j.cels.2016.01.007
[55] Kocsmár I, Pajor G, Gyöngyösi B, et al. Development and initial testing of a modified UroVysion-based fluorescence in situ hybridization score for prediction of progression in bladder cancer. Am J Clin Pathol. 2020;153(2):274-284. doi:10.1093/ajcp/ aqz165
[56] Yoder BJ, Skacel M, Hedgepeth R, et al. Reflex UroVysion testing of bladder cancer surveillance patients with equivocal or negative urine cytology: a prospective study with focus on the natural history of anticipatory positive findings. Am J Clin Pathol. 2007;127(2):295-301. doi:10.1309/ADJL7E810U1H42BJ
APPENDIX
Appendix A. The SUFU algorithm categorizes patients into three subgroups based on risk stratification for urothelial cancer. “Low-risk” patients are women less than 50 years old or men less than 40 years old; never-smokers or less than 10 packyears; 3-10 RBC/HPF on one urinalysis (UA); no additional risk factors for urothelial cancer (as outlined in the algorithm); no prior episodes of microhematuria. Low-risk patients should be involved in shared decision-making to decide if they would like to either repeat the UA within 6 months or undergo cystoscopy and renal ultrasound (US). “Intermediate-risk” patients are women 50-59 years old or men 40-59 years old; 10-30 packyears of smoking, 11-25 RBC/HPF on a single UA; any additional risk factor for urothelial cancer; a previously low-risk patient without a prior evaluation and 3-25 RBC/HPF on repeat UA. Intermediate-risk patients should undergo cystoscopy and renal US. “High-risk” patients are women and men 60 years old or greater; more than 30 pack-years of smoking, greater than 25 RBC/HPF on a single UA, history of gross hematuria; a previously low-risk patient without a prior evaluation and 3-25 RBC/HPF on repeat UA. High-risk patients should undergo cystoscopy and computed tomography (CT) scan [27]. As per the American College of Obstetricians and Gynecologists (ACOG) and the American Urogynecologic Society (AUGS), asymptomatic females 35-50 years old, who are low-risk and never-smoking should only undergo evaluation if they have more than 25 RBC/HPF [28].
Appendix B. The American Joint Committee on Cancer TNM staging for cancer categorized primary tumor (T) into six major categories. Ta lesions are noninvasive papillary (exophytic) lesions. Tis lesions are urothelial carcinoma in situ lesions that are high-grade intraepithelial tumors that do not contain subepithelial connective tissue infiltration. T1 lesions invade into the submucosa or lamina propria. T2 lesions invade into the muscularis propria. T3 lesions invade in the perivesical soft tissue. T4 lesions invade adjacent organs such as prostatic
stroma, uterus, pelvic wall, or abdominal wall. Advanced disease is denoted by regional lymph nodes (N) or distant metastasis (M). N1 classification is the involvement of a single lymph node within the true pelvis, whereas N2 classification is multiple lymph nodes within the true pelvis. N3 classification is involvement of the common iliac lymph nodes. Absence of any lymph node involvement in the true pelvis would be classified as N0. Lymph node involvement beyond the common iliac lymph nodes crosses the threshold to be described as metastasis and is denoted by the M1 classification. All prior tumors described that do not involve lymph nodes beyond the common iliac lymph nodes would be described as M0 [28].
Appendix C. Standard treatment for carcinoma in situ of the bladder is the intravesical administration of Bacillus CalmetteGuérin (BCG), a live attenuated strain of Mycobacterium bovis which usually acts as a vaccine against tuberculosis [39, 40]. The mechanism of action of the vaccine preparation against bladder cancer is poorly understood, but it is widely believed to act as an immunotherapy that induces cytokine release. BCG administration involves an induction course, along with a lengthy maintenance therapy lasting up to 3 years, and is accompanied by periodic biopsies of the bladder to monitor response to the treatment. However, for BCG treatment to effectively combat urinary cancer, the patient must be immunocompetent with a small tumor burden [39]. Thus, other bladder-preserving treatments are often considered for more advanced and aggressive cancers. Chemotherapy and radiation regimens are often pursued as monotherapies or adjuncts to other treatments. Recently, immune checkpoint inhibitors have been considered for treatment in patients ineligible for cisplatin therapy and in metastatic urothelial carcinoma of the bladder, especially in patients expressing programmed death-ligand 1 (PD-L1). Anti-PD-L1 antibodies, including pembrolizumab and nivolumab have shown promising results, with increased overall patient survival when compared to standard treatment alone [41]. FGFR inhibitors have also been used recently for the treatment of urothelial cancers, with erdafitinib being recently approved by the US Food and Drug Administration (FDA) in patients with an altered FGFR gene [42, 43].
