
20 A Year in Profiles Interview highlights




At the Charing Cross (CX) Symposium 2024 (23–25 April, London, UK), Andrew Bradbury (University of Birmingham, Birmingham, UK) and the BASIL-3 team of trialists will address the pressing question of which endovascular strategy wins in the disputed femoropopliteal segment. The investigators will be presenting—for the very first time—the results of this long-awaited, only completed, fully publicly funded randomised controlled trial (RCT) in the space.
The BASIL-3 team will reveal clinical and cost-effectiveness data comparing three alternative femoropopliteal endovascular revascularisation strategies—plain balloon angioplasty with or without bailout bare metal stent, drug-coated balloon with or without bail-out bare metal stent, and drug-eluting stent— for the
[CLTI]). “They’re actually very different technologies,” Bradbury points out to Vascular News ahead of CX 2024.
“When we set up BASIL-3, we thought it was very important to have a threearm trial so that we could compare these three different endovascular strategies. And, we’ve been able to do that; we’ve recruited to target, observed more than the required number of primary endpoints and that gives us at least 90% power.


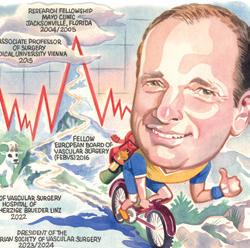
We have very complete, virtually 100%, follow-up data on the primary outcome, which is amputation-free survival. So, we think they’re good-quality data.”
“It’s a very controversial area,” Bradbury opines, making the BASIL-3 first-time data presentations an apt centrepiece for this year’s controversiesfocused meeting. “Although these drug devices have been in practice for probably 15 years now and certainly the last 10 years, it’s been a rocky road for them,” Bradbury explains, citing the paclitaxel mortality debate sparked by the 2018 meta-analysis from Katsanos as some important context to the trial. The publication directly affected the progress of BASIL-3, halting proceedings for many months.
“There were concerns around what appeared to be excess mortality across a wide range of industry-funded trials at two and five years, and it’s taken a longtime to resolve that,” Bradbury remarks, providing a brief summary of the six-year saga that a separate session at CX 2024 will address in detail.
He references recent reversals of paclitaxel safety warnings by both the US Food and Drug Administration (FDA) and subsequently the UK Medicines and Healthcare products Regulatory Agency (MHRA) that have, “to a great extent”, put any concerns “in the rearview mirror”.
Some of the data being presented at CX 2024 will illuminate the cost effectiveness of the three strategies being compared. Bradbury underlines the “very complicated” nature of health economics and details what the investigators have looked for in BASIL-3. “There’s a close relationship between number of days spent in hospital and overall cost,” he says. “So, if you have a procedure that’s more expensive but reduces the length of the index admission and also subsequent readmissions—because a lot of these patients come back into hospital—that saving, in days in hospital and further interventions, could easily wipe out even quite a large excess of increased cost from the procedure, which in this case





being three endovascular strategies would be the device costs.”
Bradbury also considers the global landscape of endovascular care. He underlines variation and unpredictability in endovascular practice across the world, highlighting a need for concrete data in the field. “Sometimes what you hear at meetings is not really what happens day to day in various vascular centres,” Bradbury points out. The BEST-CLI study, for example, showed a benefit of vein bypass over endovascular in cohort one. “But if you look at the data,” he says, “only half of the patients who were put into the endovascular group actually received a drug device.” This, Bradbury points out, is much lower than might be expected for USA-based practice.
In BASIL-2, on the other hand, around a third of the patients in the endovascular group had a drug device. “That’s higher than I expected,” he comments.
“It’s really interesting to look at what is custom and practice at the moment in different countries and I think it’s quite difficult to know what that it,” Bradbury highlights.
“We think you’ll find the data that we present on clinical effectiveness and
We thought it was very important to have a three-arm trial.”
also cost-effectiveness very interesting, whatever your views at the moment on the role of these drug devices in the management of CLTI,” Bradbury remarks. “We very much hope that these new Level 1 data will be of great interest to the Charing Cross audience and help people make decisions in their everyday practice.”
The BASIL-3 podium first is set to take place at 16:10 on Tuesday 23 April as part of the peripheral arterial controversies programme’s inaugural



Welcome to the early Spring edition of Vascular News. I hope that you have had a good winter period since the last edition. It has been raining more or less nonstop in the UK, so everything is waterlogged including the populace. I hope that your weather has been a little more clement than ours.


As we enter the Spring congress season, many of you have already enjoyed the European Vascular Course (EVC) in Maastricht, The Netherlands, and the Society of Interventional Radiology (SIR) meeting in Salt Lake City, USA, has just concluded.
We now look forward to the forthcoming Charing Cross (CX) Symposium (23–25 April, London, UK). This will be the first CX Symposium since the passing of its founder and chair Professor Roger Greenhalgh. This will be an emotional time for Lord Stephen Greenhalgh and his family, so my thoughts go out to them as I am sure also do your good wishes.
This edition of Vascular News is a special edition that highlights CX 2024, which moves to a new venue at the ExCeL in East London. The new trans-London Elizabeth line adds to the many transport links connecting this international congress venue to central London and to London airports.
Most of you will be already aware that in January, the UK Medicines and Healthcare products Regulatory Agency (MHRA) released an updated document on paclitaxel that aligned the MHRA with the US Food and Drug Administration (FDA) statement from 2023. This new MHRA document confirms the removal of the mortality signal for paclitaxel-coated devices, enabling vascular practitioners to use these devices without concern for patient safety or regulatory disapproval. This event will be featured during the CX 2024, and the announcement and a discussion thereof is also featured in this issue.
Several other peripheral arterial topics are covered. A new multi-society public awareness campaign has just been launched entitled ‘Get a Pulse on PAD’. There are also interviews with Michel Reijnen and Majorie van Helvert on their research regarding ultrafast contrast-enhanced ultrasound blood flow quantification in peripheral arterial disease (PAD) patients after endovascular treatment that was presented at the VEITHsymposium 2023.
In the aortic space, Bijan Modarai discusses his recent

presentation at EVC—‘Beyond aortic repair: are our patients living long enough to benefit from aortic repair?’. There is also a description of Philips’ Fiber Optic RealShape (FORS)powered LumiGuide 3D imaging technology, which is being introduced at selected vascular centres in Europe and the USA.
Artificial intelligence (AI) initiatives are increasing and may eventually affect all our work practices. An interesting piece in this issue describes the adoption of AI into workflows to facilitate the assessment of patients with pulmonary emboli.
This edition of Vascular News is a special edition that highlights CX 2024, which moves to a new venue at the ExCeL in East London.”
Finally, there is a featured article that covers the last four profiles of vascular specialists in Vascular News. Extracts from the interviews that were conducted for these profiles, which involved Rachel Bell, Jürgen Falkensammer, Peter Schneider, and Daniel Clair will be included in the article.
In addition to the above, there are many other articles that encompass all aspects of the vascular world. I hope that you enjoy this issue of Vascular News
Happy reading!
ROBERT MORGAN is professor of interventional radiology at St George’s University Hospitals NHS Foundation Trust (London, UK) and the president of the British Society of Interventional Radiology.
Editors-in-chief: Robert Morgan (European Edition) and Ross Milner (North American Edition)
Content director: Urmila Kerslake | Global commercial director: Sean Langer
Editor: Jocelyn Hudson Jocelyn@bibamedical.com | Editorial contribution: George Barker, Jamie Bell, Will Date, Bryan Kay, Éva Malpass, Brian McHugh | Design: Terry Hawes, Josh Lyon and David Reekie
Advertising: Rav Pankhania Rav@bibamedical.com and Jamia Trigiani Jamia@bibamedical.com
Subscriptions: subscriptions@bibamedical.com
Published by: BIBA News, which is a subsidiary of BIBA Medical Ltd
BIBA Medical, Europe, 526 Fulham Road, Fulham, London, SW6 5NR, United Kingdom
BIBA Medical, North America, 155 North Wacker Drive, Suite 4250, Chicago, IL 60606, United States
Printed by: Buxton Press. Reprint requests and all correspondence regarding the newspaper should be addressed to the editor at the United Kingdom address. © BIBA Medical Ltd, 2024. All rights reserved.
n FLOW IMAGING:

Majorie van Helvert (Enschede, The Netherlands) and Michel Reijnen (Arnhem, The Netherlands) are currently conducting research on ultrafast contrast-enhanced ultrasound blood flow quantification in peripheral arterial disease (PAD) patients after endovascular treatment. Early findings show that the new technique could offer an innovative imaging alternative. Vascular News recently spoke with both van Helvert and Reijnen, and they went into more detail about their findings.
For more on this story go to page 22.
n CAROTID STENTING:

Data from a new study that maps out the levels at which adverse postoperative events decrease following transfemoral carotid artery stenting (TfCAS) based on depth of physician experience with the procedure represent an “absolute minimum threshold of learning,” lead author Marc Schermerhorn (Boston, USA) says. The analysis comes in the wake of the US Centers for Medicare & Medicaid Services (CMS) decision to expand carotid stenting coverage, which loosened restrictions on the use of TfCAS.
For more on this story go to page 24.
n VASCULAR ACCESS:
Alik Farber (Boston, USA) presented the results of a multicentre US study at the 2024 Society for Clinical Vascular Surgery (SCVS) annual meeting (16–20 March, Scottsdale, USA) that showed creating arteriovenous fistulas with a vascular external support system, VasQ (Laminate Medical), decreases the median time to achieve two-needle cannulation by reducing the need to perform maturation procedures.

For more on this story go to page 32.
If you have comments on this issue or suggestions for upcoming editions write to jocelyn@bibamedical.com
• BASIL-3 Podium First Presentation: Including clinical and health economic results
Tuesday, 23 April
• Late Breaker:
BEST-CLI vs BASIL 2 - outcomes when comparing apples with apples
Thursday, 25 April
• Great Debate:
Best medical treatment remains the standard for uncomplicated type B aortic dissection
Wednesday, 24 April




New data and heated debates set to spark controversy in
“These will be important, salutary lessons. Should a controversy arise again involving a proven efficacious therapy, we now know that stopping access to that therapy may result in unwanted and unexpected negative effects on patient care.” These are the preluding thoughts of Andrew Holden (Auckland City Hospital; University of Auckland, Auckland, New Zealand)—one of the CX Symposium co-chairs—ahead of this year’s meeting, referencing the paclitaxel mortality controversy. Here, Holden sets sights on the unmissable peripheral arterial programme, which will tackle global controversies and host heated debates.
“THE PERIPHERAL ARTERIAL programme is jam-packed with controversy this year—controversy being the theme for CX 2024,” says Holden, highlighting this fact by noting that the first session will address paclitaxel mortality. The paclitaxel-mortality controversy in peripheral arterial disease (PAD) will be punctuated by a late-breaking presentation from Eric Secemsky (Beth Israel Deaconess Medical Center, Boston, USA), who plans to examine whether the meta-analysis and subsequent regulatory body restrictions on paclitaxel caused harm.
“Although the original paclitaxel mortality controversy did not make sense in terms of causation theories, it was appropriate to take this report very seriously. However, in many geographies this resulted in stopping a therapy that had been shown to be more effective and durable than our nondrug-coated strategies,” details Holden. “An important question that hasn’t been addressed to date is when depriving patients of a more effective modality, were we causing patients harm? With the benefit of hindsight, can we learn important lessons regarding this?”
“Hopefully we will provide the final chapter of this controversy, we have key opinion leaders presenting new data, and some input from regulators such as the US Food and Drug Administration (FDA) and the UK Medicines and Healthcare products Regulatory Agency (MHRA),” Holden says. “I’m very grateful to Eric and his group for providing additional data to allow us, for the first time, to dive into this issue.”
Holden notes that during this session, speakers and audience members will have the opportunity to ask the regulators what their thoughts are with regard to the removal of access to paclitaxel-coated devices and the duration of time it has taken to have these therapies reinstated.
Paclitaxel-coated devices also feature in later sessions, Holden contributes, commenting that the ‘Paclitaxel and sirolimus-coated balloons in the femoropopliteal arteries’ session will end with a debate, which asks if
“following the FDA announcement, do paclitaxel-coated devices now beat limus-coated devices”. He adds that drug-coated technologies will be of “great interest” at this years’ CX Symposium and will provide delegates with the opportunity to share their thoughts and experiences.
“Promising” future for bioresorbable scaffolds in the tibial arteries
Later in the peripheral programme, speakers will turn to bioresorbable scaffolds in the tibial arteries. Brian DeRubertis (Weill Cornell Medicine, New York, USA) will present lakebreaking data in the LIFE-BTK clinical trial evaluating the safety and efficacy of the everolimus-eluting Esprit below-theknee (BTK) system (Abbott) in chronic limb-threatening ischaemia (CLTI) patients with infrapopliteal artery disease.

a statistically significant benefit for primary bioresorbable scaffolds, Holden believes this year’s CX is positioned well for a “timely deep dive” into the subject and “promising” emergent technologies.
On both Tuesday and Wednesday at CX 2024 the inaugural Roger M Greenhalgh late-breaking trials sessions will take place. “I believe Professor Greenhalgh would be very proud of the latebreaking trial sessions we are hosting in the peripheral arterial space at CX 2024,” says Holden.
on the device’s “effectiveness” at the trial’s UK centre.
Elsewhere in the peripheral programme, Holden highlights the comprehensive coverage of CLTI trials—including updates from BEST-CLI and the BASIL-3 podium first—with a focus on the imaging modalities session.
“Bioresorbable scaffolds have been the holy grail for lower limb arterial interventions for many years,” Holden details. He draws attention to the high percentage of cases that require a scaffold due to issues such as dissection and recoil after angioplasty, but also the long-term negative effects of metal stents when placed in the tibial arteries.
“We’ve looked for a bioresorbable scaffold that works in the acute phase, but then dissolves with time and allows arterial structure and physiology to be restored. Many trials have failed both above and below the knee,” he says.
However, following the LIFE-BTK trial results in 2023, which showed
At a glance, he highlights that deep venous arterialisation is a topic of “growing interest” that will feature in this session. He states the session will provide an opportunity for attendees to evaluate where this new technology may sit within physicians’ current armamentarium, particularly in patients with advanced CLTI. New data from the CLARITY trial will be presented by Anahita Dua (Massachusetts General Hospital, Boston, USA), evaluating transcatheter arterialisation of deep veins for nooption CLTI.
Holden also places focus on the sixmonth SHOCC study data, which will be presented by Athanasios Saratzis (University of Leicester, Leicester, UK). The trial analysed the Shockwave lithotripsy device (Shockwave Medical) for patients with PAD and will produce new data during Wednesday’s Roger M Greenhalgh late-breaking trials session. This data, Holden shares, will shed light
“One of the big challenges with imaging and CLTI is trying to identify the patients who are going to respond to revascularisation. We know that CLTI patients often have a multifactorial cause for their disease in addition to their macrovascular arterial disease. In many cases, we can spend a lot of time revascularising patients who don’t benefit so we need to get a better idea of favourable responders,” says Holden.
Holden restates the importance of pre-procedural and intra-procedural imaging, which will be explored during this session, enabling physicians to get to grips with new technologies to assist in measuring when a therapeutic endpoint has been reached during a revascularisation procedure”.
“Imaging and CLTI is an expanding and very important topic that we’re going to certainly delving into on the Thursday of CX 2024,” he adds.
The CX 2024 peripheral programme will also play host to a variety of controversial debates including paclitaxel versus limus-coated devices, and bypass versus endovascular surgery, affording CX delegates the opportunity to cast their votes. Aptly devised, the debates in the peripheral arterial programme are centred on controversial topics and are hoped to spark lively discussion. To this end, Holden hopes that they will “drive consensus and effectively further the conversation” surrounding these complex treatment modalities.



Debates will shed new light on controversies in the treatment of aortic disease at the 2024 CX Symposium, alongside practical sessions with a focus on techniques and technologies in both aortic endovascular and open surgical repair.
RUNNING OVER THE FULL THREE days of the symposium, the comprehensive aortic programme at CX 2024 opens with aortic techniques and technologies on day one, followed by a full day focused on aortic arch and thoracic aortic controversies, and finishing with sessions covering juxtarenal and abdominal aortic aneurysm controversies, alongside advanced imaging and radiation reduction on the final day.
CX has a three-year cycle of raising vascular and endovascular controversies in order to challenge
the available evidence and to be able to reach a consensus after discussion with an expert audience.
“It is a controversies year, so the focus will be on debate,” says CX Executive Board member Tilo Kölbel (University Heart Center Hamburg, Hamburg, Germany).
The treatment of uncomplicated acute type B aortic dissection will be the subject of a great debate during Wednesday’s thoracic aortic controversies session, during which Kevin Mani (Uppsala University, Uppsala, Sweden) and Hence Verhagen (Erasmus Medical Center, Rotterdam, The Netherlands) will put forward their case that best medical treatment remains the standard of care, whilst Christoph Nienaber (Royal Brompton & Harefield Hospitals, London, UK) and Firas Mussa (McGovern Medical School, Houston, Texas) put forward the counter argument.
Gustavo Oderich (University of Texas Health, Houston, USA) and Eric Verhoeven (Paracelsus Medical University, Nuremberg, Germany) will debate the use of self-expandable covered stents versus balloon-expandable stent grafts in branched endovascular aortic repair later that day.
“It is of great interest to the community to understand the pros and cons for the use of balloonversus self-expandable stents in branched repair,” says Kölbel of the significance of this debate. “Branched aortic repair is being done in increasing numbers, with more off-the-shelf devices being available for use in North America and in Europe. There are basically two different schools in vascular surgery. One uses balloon-expandable stents, which may be less flexible, and one uses self-expandable stents, which are more difficult to use because they need more profile.”
This session will also see Martin Austermann (St Franziskus-Hospital, Münster, Germany) and Nikolaos Tsilimparis (Ludwig-Maximilians
The topics of acute stroke care, carotid disease and neurointerventional surgery return to the CX Symposium with renewed vigour.
THE PROGRAMME WILL KICK OFF WITH ROSS NAYLOR’S (Leicester Vascular Institute, Leicester, UK) presentation tackling “unanswered questions” arising from the European Society for Vascular Surgery (ESVS)’s 2023 guidelines. Following this will be a debate on the motion, ‘Patients with symptomatic near occlusion in the internal carotid artery (ICA) should only be treated medically’—the first of five debates on the programme.
This year’s programme will also play host to a number of case presentations within an acute stroke controversies carousel—CX Executive Board member Barbara Rantner (Ludwig-Maximilians-University of Munich, Munich, Germany) is set to deliver the first of these cases, discussing considerations following the treatment of a post-dissection ICA aneurysm. Levansri Makalanda (Barts Health NHS Trust, London, UK), Gert J de Borst (UMC Utrecht, Utrecht, The Netherlands), and CX Executive Board member Domenico Valenti (King’s College Hospital, London, UK), will share their experiences here as well, presenting “very difficult cases for which there is not yet a standardised treatment defined by guidelines”, according to Valenti.
The latter part of the programme is set to see an increased focus on CAS— initially, with a debate entitled, ‘CAS is the treatment of choice for underlying ICA stenosis after mechanical thrombectomy’.
In addition, the audience will be treated to the first ever European presentation of data from PERFORMANCE II, with Ralf Langhoff (Hospital Sankt Gertrauden, Berlin, Germany) detailing findings on a novel carotid stent system observed within this prospective, randomised study.
University Hospital, Munich, Germany) trade views on the justification for prophylactic spinal drainage for thoracoabdominal endovascular repair.
“Spinal cord ischaemia is the most devastating outcome of complex aortic repair,” comments Kölbel. “One of the best ways to prevent that from happening is spinal drainage and it has been a recommended part of guidelines for many years. Recent guidelines have downgraded that recommendation, indicating that there is more controversy over whether the downsides and complications of spinal drainage still allow for prophylactic use.”
Polling at the end of each debate will give a clear indication as to where the CX community stands on each of these issues.
Alongside these sessions, Tuesday’s techniques and technologies session will focus on specific techniques in open and endovascular repair. Highlights include an edited case, presented by Oderich, which will cover transcatheter septotomy in chronic aortic dissection.
A podium first presentation during Thursday’s advanced imaging and reducing radiation session will explore how operator position influences radiation dose.
It is of great interest to the community to understand the pros and cons for the use of balloon- versus selfexpandable stents in branched repair.”
Tilo Kölbel

programme set to
The vascular trauma programme at CX 2024 will involve vascular surgery trainees from across Europe, showcase data from the Vascular Interventions and Surgery in Trauma Audit (VISTA), and address controversy surrounding resuscitative endovascular balloon occlusion of the aorta (REBOA).
member Ross Davenport (Barts Health NHS Trust; Queen Mary University of London, London, UK) points out that there will be significant involvement from vascular trainees in this year’s programme. Indeed, the opening session is hosted by CX in partnership with the European Vascular Surgeons in Training (EVST) and will focus on some “really difficult, challenging vascular cases”.
Results from the VISTA, which was conducted by a trainee collaborative, is also a programme highlight. Rachel Bell (Freeman Hospital, Newcastle, UK) will be delivering a talk on the Vascular Society and the quality-improvement arm of the national society, and how to turn these data from VISTA into “real quality
improvement,” Davenport adds.
CX Executive Board member Chris Aylwin (Imperial College Healthcare NHS Trust, London, UK) is also keen to highlight the “controversy” of REBOA that will feature on the programme. A debate will focus on whether there is a role for REBOA after the UK-REBOA trial, which, Aylwin notes, “in itself, has been quite controversial”.
An exploration of which conduit to use for vascular peripheral trauma is another of Aylwin’s programme highlights, with multiple talks on the programme comparing the use of veins to prosthetic conduits.
“We very much look forward to seeing as many vascular traumainterested attendees as possible at the 2024 CX meeting,” says Aylwin.


Erin Murphy (Atrium Health’s Sanger Heart and Vascular Institute, Charlotte, USA), one of the CX Symposium cochairs and a CX Executive Board member, along with fellow CX Executive Board members Stephen Black (Guy’s and St Thomas’ Hospital; King’s College London, London, UK) and Manj Gohel (Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK; Imperial College London, London, UK), highlight what delegates can expect from the venous programme at CX 2024.
The mission of CX is to raise vascular and endovascular controversies to world-class faculty in order to challenge the available evidence and ultimately agree on steps forward after lengthy discussion.
“This year, we’re really looking forward to another spin of the controversies cycle,” Black tells Vascular News. “There is absolutely no shortage of controversies in the field of venous disease and treatment to help underpin a cutting-edge venous programme.”
“We have a broad venous programme designed to really cover all of the different challenging areas that an international venous audience will encounter, whether you work in the USA, the UK, Europe, the Middle East, or beyond,” Gohel adds.
Murphy says,“Charing Cross isn’t just another conference. It’s a dynamic force that’s been shaping the global vascular community across specialties for years. The venous programme is a cornerstone of the gathering.”
What to expect
Black is counting down to Steven Abramowitz’s (MedStar Health, Washington, DC, USA) presentation scheduled for day two (Wednesday, April 24).
The focus of the presentation will be on appropriate follow-up in clinical trials. Black emphasised the significance of this topic, stating that addressing appropriate follow-up is crucial for the successful execution of studies, particularly in the context of venous disease trials.
“We’re excited to continue our tradition of discussing the appropriateness of venous interventions with a great debate,” Black states. “Steve Elias [Englewood Hospital, Englewood, USA] will debate Gerry O’Sullivan [University Hospital Galway, Galway,
Ireland] on the need for stenting in NIVL [non-thrombotic iliac vein lesion] patients, particularly in patients with a lower level of venous disease.”
“Elias will argue that the evidence supports treating CEAP 3 patients with varicose veins and swelling, all the way up to CEAP 6 patients with ulceration, while O’Sullivan will argue that the evidence is not supportive of this approach,” Black says.
“In many debates, participants may find themselves advocating for a position they don’t necessarily agree with. However, in this particular debate, there is a clear division in the data and evidence. Therefore, it promises to be a compelling discussion based on the existing disparities in evidence.”
As for why this debate is happening in the first place, Black states: “We do see complications from venous stenting. Stent migrations in particular, have been reported more frequently in America, where NIVL patients are treated more frequently. So, if we’re going to do procedures that carry risks for patients, we need to ensure that the necessity of the treatment is unequivocally established beforehand.”
“I want you to keep an ear out for Gloria Salazar [University of North Carolina, Chapel Hill, USA],” Murphy tells Vascular News. “She’ll be introducing the groundbreaking EMBOLIZE trial. This is the first randomised controlled trial looking
at the success of embolisation for pelvic congestion.
Murphy continues, explaining why this trial is so important. “We are currently facing challenges in obtaining insurance coverage for these patients,” she informs this newspaper. “Insurance denials often cite a lack of objective data and label the procedures as experimental.”
Murphy anticipates that this trial will help shift the situation and influence coverage plans to permit broader and enhanced treatment options for these patients. “At present, many women undergo procedures, such as hysterectomies, to address issues that could potentially be resolved with endovascular embolisation,” Murphy explains. “The associated risks with hysterectomy are certainly higher.”
The results from the VenaSeal Spectrum programme are some of the most highly anticipated data to be revealed this year. Sponsored by Medtronic, the VenaSeal Spectrum programme’s goal is to assess cyanoacrylate glue across three studies, one comparing it with endothermal ablation, one comparing it with surgical stripping, and one testing the device on venous leg ulcers.
“It’s been an incredible achievement for the investigators around the world to complete this series of three studies,” Gohel says. “The real goal of the Spectrum programme is to identify the positives, the effectiveness, the safety, and also the overall role of cyanoacrylate glue in what is quite a complex current landscape of superficial venous interventions.”
Another study that will be releasing data is SYNCHRONOUS which determined the impact of a synchronous prophylactic treatment of the anterior accessory saphenous vein on the recurrent varicose vein rate in patients undergoing thermal ablation of an insufficient great saphenous vein.
“An unbreakable rule in superficial venous treatment is not to treat a vein that is not refluxing,” Gohel says. “There are specific reasons why prophylactic treatment of the anterior accessory saphenous vein has been considered a good idea. The concern would be encouraging treatment,


and potentially overtreatment of non-refluxing veins.”
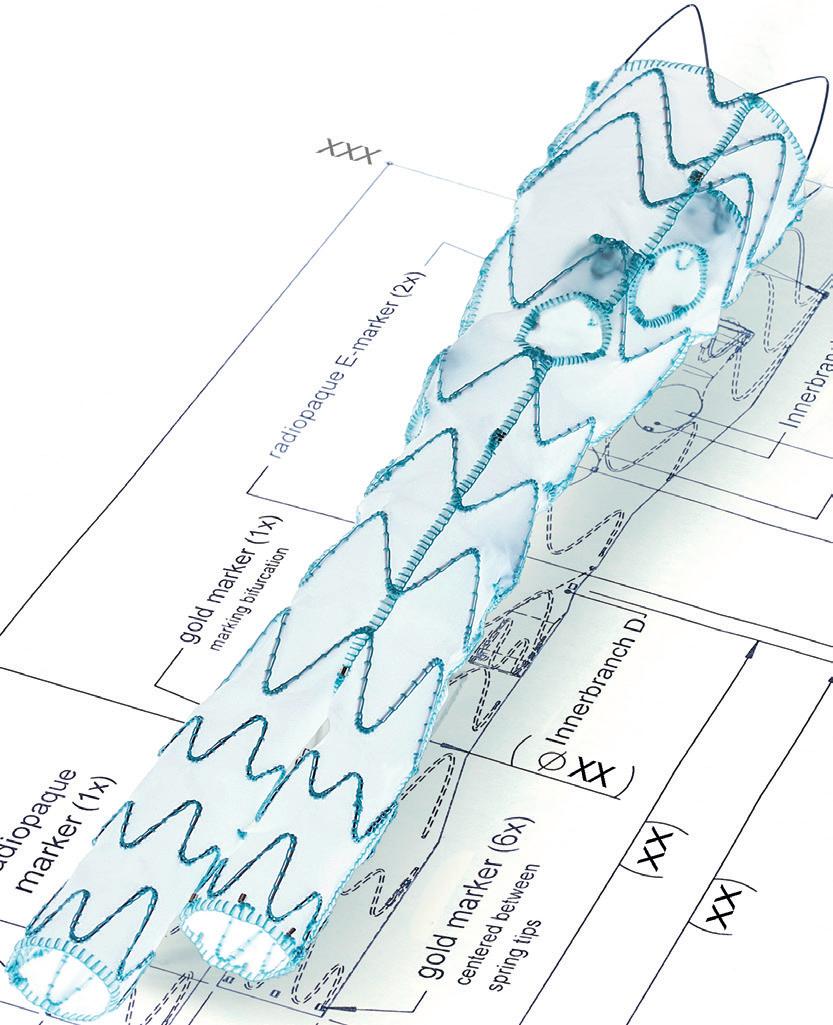
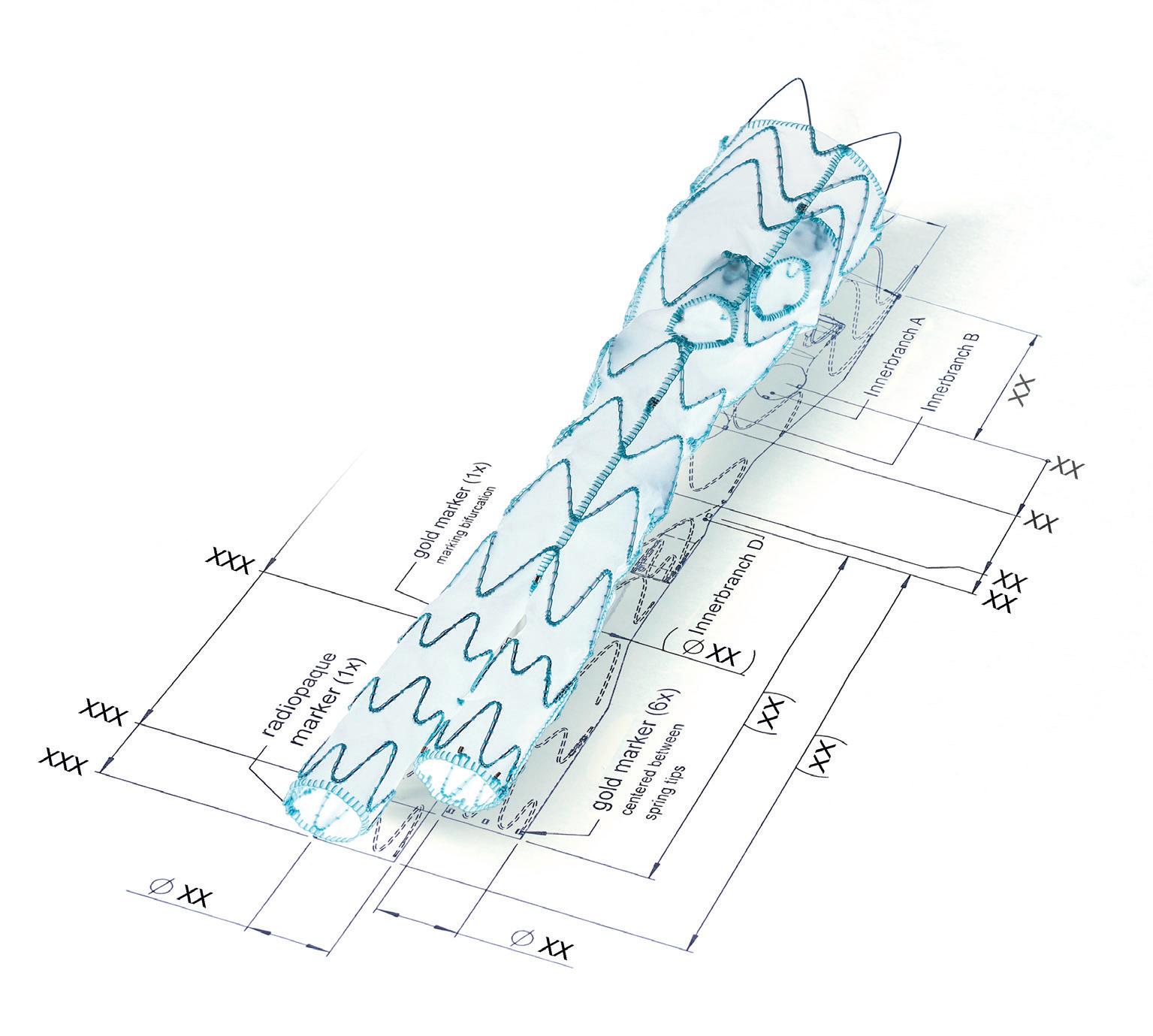
Stephen Black will be giving a podium-first presentation at CX 2024 on the need for a dedicated inferior vena cava (IVC) stent.
“There are several venous stents available, but none of them are onlabel for use in the inferior vena cava,” Black notes. “Gore has developed a stent, Viafort, specifically designed for use in the IVC, making it the first of its kind.”
The Viafort IVC study is evaluating the performance, safety and efficacy of using the investigational Viafort stent for the treatment of symptomatic IVC obstruction with or without combined iliofemoral venous obstruction. It is a prospective, multicentre, nonrandomised, single-arm study.
Ahead of his presentation, Black explains why the Viafort stent is interesting to watch. Importantly, this pertains to the combination of stent size and its ability to withstand the movements of the diaphragm. Viafort utilises the Gore expanded polytetrafluoroethylene (ePTFE) technology in conjunction with a single-wire, sinusoidal-wound nitinol frame.
“The reason why we’re presenting this as a podium first is that the FDA [US Food and Drug Administration] allowed breakthrough device designation for this study, where we identify, and the FDA agrees, there is a significant clinical need and a significant need for innovation.”
CX 2024 workshop
Gohel also speaks about the venous workshop at this year’s CX.
“The workshop at Charing Cross is something that’s been going on for many years, and it’s an excellent opportunity for delegates to come and meet expert faculty, [participate in] interactive case discussions, and also to try the latest technology and equipment supported by our industry partners,” Gohel explains.
The workshops will take place in the middle of the industry space, which is an easily accessible area. “I certainly encourage people to visit the area for lots of interesting discussions and technological advances,” Gohel said.
Murphy also spoke about the workshop, urging symposium participants to explore it.

“We’re committed to offering a comprehensive venous workshop on the Tuesday of the programme,”
The workshop is “designed to take you through procedural steps, providing hands-on access to the most cutting-edge technologies available. It’s an unparalleled opportunity to elevate your clinical skills and stay ahead of the curve.”



“not only the complex, the new, the innovative, but also the everyday” in vascular access and renal interventions
Vascular News spoke to CX Executive Board members Nick Inston (University Hospitals Birmingham, Birmingham, UK) and Kate Steiner (East and North Herts NHS Trust, Stevenage, UK) about the vascular access and renal interventions programmes at this year’s meeting.
ONE THING THAT BOTH INSTON AND Steiner made sure to highlight was the fact that the programme this year will be heavily focussed on controversies, and the fact that the issues that arise in vascular access do so on a global scale. When speaking on what she felt merited close attention, one of the first things that Steiner spoke of was the Great
Debate, which will be taking place the morning of day one. The topic—endovascular arteriovenous fistulas (endoAVFs) are a failed experiment—will be hosted by Inston and moderated by Narayan Karunanithy (Guy’s and St Thomas’ NHS Trust, London, UK), and is a debate that Steiner says she is sure “will be controversial”. She also said that, whilst she is “very much against the motion”, she feels that it will “be really interesting to see if that changes at the end of the debate”.
Steiner is not alone in her belief that this will be a programme highlight, as Inston also highlighted the importance of addressing endoAVFs and their role in vascular access.
“This technology has been around for about 10 years,” Inston averred, “first in an experimental setting, but now, fully into the clinical area”. According to Inston, however, the issue arises from the fact that “we don’t really know yet where we should be using these fistulas. Should these be for everybody? Should these be for a select few? Should there be actually certain indications in certain patients? Or actually, are we not using them in a wide enough application?” This, he states, will be the focus of the Great Debate, with input from, as Inston puts it, “the experts who’ve done some of the highest volumes of these cases in the world”.
Another feature of the programme that both Steiner and Inston highlighted was the focus on drug-coated balloons (DCBs).
Whilst addressing how variations in reimbursement can lead to differences in standard of care on a global level, Steiner referred to DCBs, stating that “in the UK, these drug-coated technologies are reimbursed via the high-cost device tariff. This doesn’t happen globally, and they can therefore add a significant expense to a procedure, which is then reimbursed, and the reimbursement may not be reflected in the tariff for that procedure. So again, we see this can create
Hans-Henning Eckstein, the vascular surgeon who played a leading role in the SPACE and SPACE 2 randomised controlled trials on the treatment of carotid stenosis and founder of the Munich Vascular Conference (MAC), died on 24 February at the age of 68. Colleagues have paid tribute to an “excellent doctor, outstanding researcher and university professor” as well as a “bridge-builder between vascular surgery and neurology”.
ECKSTEIN HAD BEEN CHAIR
and professor of the Department of Vascular and Endovascular Surgery at the University Hospital “Rechts der Isar” of the Technical University of Munich (Munich, Germany) and was widely recognised for advancing research and knowledge in the field of vascular surgery. He was awarded an honorary doctorate from the Medical Faculty of the University of Larissa (Larissa, Greece) in 2017 and, since 2019, had also been a visiting professor at the Medical School of Pittsburgh (Pittsburgh, USA) and Stanford University (Stanford, USA).
After studying medicine at Ruprecht-Karls University Heidelberg (Heidelberg, Germany), Eckstein completed his PhD in 1986 and two
years later acquired his postdoctoral teaching qualification in Heidelberg.
From 1999 until 2003, Eckstein was medical director of the Clinic for Vascular Surgery at Ludwigsburg Hospital (Ludwigsburg, Germany).
In 2004, he became director of the Department of Vascular Surgery at the University Hospital “Rechts der Isar” of the Technical University of Munich and five years later was appointed the first holder of the newly created chair for vascular and endovascular surgery at the university—a role he held until his retirement in 2023.
A statement on the Technical University of Munich’s website mourning the loss of Eckstein reflects on his impact at the institution: “Prof Eckstein made the clinic known far
differences in pathways and can lead to inequalities in care globally.” The afternoon of day one will give several opportunities to address these drug-coated technologies, with a session addressing whether or not we know which DCB to use and the ABISS trial, as well as a podium-first session dedicated solely to the topic of which DCB to use.
Another aspect of the programme that Inston highlighted was the fact that it is not just vascular access that will be the topic of discussion, but also renal interventions. “This session is not just about vascular access,” Inston stated. “This is also about management of the patient with renal failure. And for a lot of us who do intervention and surgery, we will be presented with patients with kidney problems, particularly in the vasculature, and this might include nutcracker syndrome, this might include renal arteries, but also the issue of renal denervation, particularly for hypertension, is coming back into the clinical arena. We’re going to discuss all of these things and present the cutting-edge up-to-date research on this.”
Another aspect of the programme that is vital to Inston is making sure to address “not only the complex, the new, the innovative, but also the everyday”. To do so, there is a session in which Inston will be presenting cases to international experts and asking them “what would you do?”
Steiner also made sure to highlight the wider coverage of the programme: “The other sessions that we have which are going to be great,” she stated, “are the case presentations and discussions in the presentation zone that will be a really good opportunity for people to, in a smaller forum, ask the experts, and we’ve got a really good programme for that.”
As well as this, she also put focus onto a workshop examining the maturation of surgical and endoAVFs, with vascular access nurses, sonographers, and patients present for the hands-on workshop.
beyond the borders of Munich and developed it into a leading centre and attraction for patients with vascular diseases.”
Eckstein played a leading role in research including randomised controlled trials on symptomatic and asymptomatic carotid stenosis, which—a statement on the European Stroke Organisation (ESO) website reads—made him “well-known” in vascular neurology. He was co-chair of the steering committee of SPACE and SPACE 2, representing vascular surgery in both multicentre trials.
vascular education and founded the Munich Vascular Conference (MAC). The 12th iteration of this event took place in December 2023 and received international attention.
Several colleagues have paid tribute to Eckstein and highlighted his legacy in the fields of vascular surgery and neurology.

Furthermore, Eckstein was heavily involved in the creation of international guidelines in his field, including the German S3 guideline for the diagnosis, therapy, and follow-up care of patients with carotid stenosis and the ESO guideline for carotid stenosis.
Eckstein’s involvement in professional organisations included his 2009–2010 presidency of the German Society of Vascular Surgery and Vascular Medicine (DGG), and his editorship of the journal Gefässchirurgie (Vascular Surgery).
Eckstein was also involved in
“We are deeply saddened by the death of Prof Eckstein,” a statement on the Technical University of Munich’s website reads. “With Prof Eckstein we are losing an extremely committed colleague and excellent doctor, outstanding researcher and university professor.”
Eckstein is also remembered in a statement on the ESO website, with Werner Hacke, senior professor of neurology at Ruprecht-Karls University of Heidelberg, and Peter Ringleb, academic researcher at the University Hospital Heidelberg (Heidelberg, Germany) stating: “In Hans-Henning Eckstein we have lost an extremely dedicated colleague and excellent physician, an outstanding researcher, university lecturer, bridgebuilder between vascular surgery and neurology, and a friend.”
“This is a milestone for arch repair,” Dittmar Böckler (Heidelberg, Germany) says of the first ever implantation of the Gore TAG thoracic branch endoprosthesis (TBE) in Europe. Böckler, who is chief of the Department of Vascular Surgery and Endovascular Surgery at University Hospital Heidelberg, performed the procedure on 30 January this year and here speaks with Vascular News about the wider significance of the device for patients requiring aortic arch treatment.
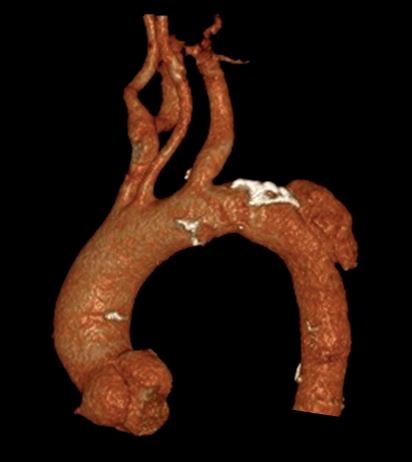
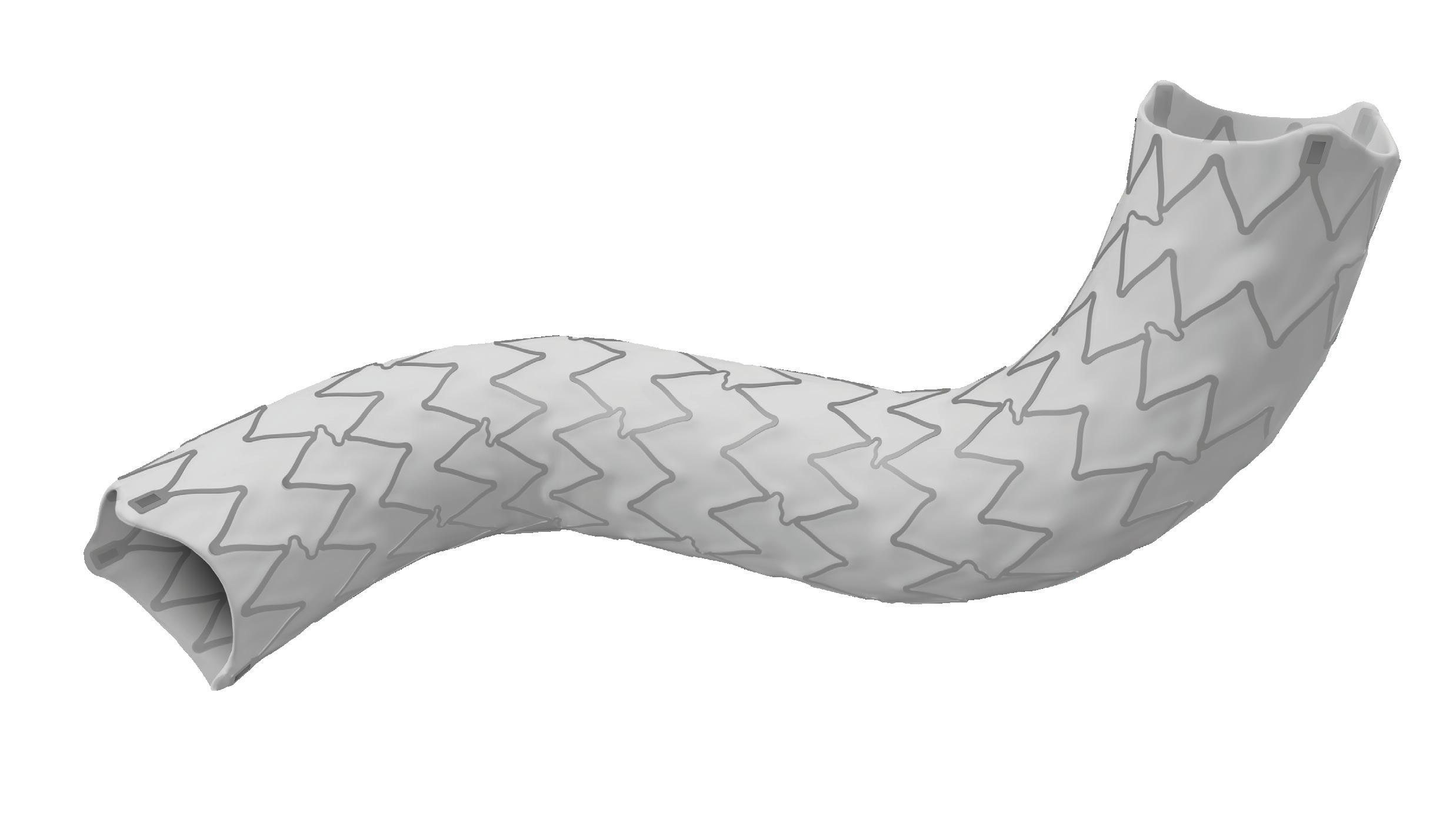
The Gore TAG thoracic branch endoprosthesis is indicated for endovascular repair of lesions in the descending thoracic aorta of patients with the appropriate anatomy, including isolated lesions, such as aneurysms, traumatic transections, and type B dissections, while maintaining flow into the left subclavian artery. “This is an off-the-shelf device, so there are no waiting times, for emergencies, within IFU [instructions for use],” Böckler states, underlining one of the key benefits of the Gore TAG TBE at a time when the majority of comparable devices are still custom made.
Rigorous in vitro and in vivo testing and extensive clinical trials have demonstrated the safety and performance of the device, paving the way for it to become the first endovascular graft for the aortic arch zone 2 to be granted both US Food and Drug Administration (FDA) and Medical Device Regulation (MDR) CE-mark approval.
“Treating aortic arch disease has traditionally posed challenges,” Böckler comments, by way of background. He states that current options involve procedures like open surgery, hybrid approaches with surgical revascularisation, or those that use non-CE mark devices. As a complete system including a dedicated arch-branch component, Böckler explains that the Gore TAG TBE “simplifies the treatment of zone 2 revascularisation by eliminating the need for surgical left subclavian artery debranching”. He continues: “The ability to endovascularly perfuse the left subclavian artery plays a key role in minimising surgical procedures and related risks.”
Giving a broad overview of the first European TBE case, Böckler shares that it was an elective procedure to treat an aneurysm of the distal arch. “Normally, we would treat a patient such as this either by overstenting the left subclavian artery or by
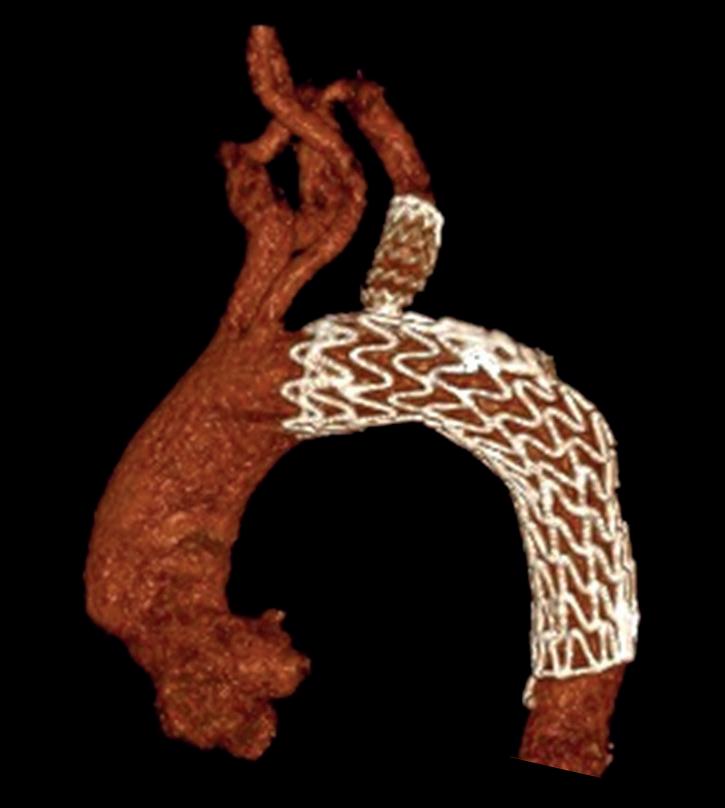
doing a left carotid subclavian bypass,” he details. The Gore TAG TBE, however, offers a minimally invasive alternative for maintaining left subclavian artery patency. Böckler recalls that the procedure was “really very quick,” involving the creation of two percutaneous accesses—one brachial and the other transfemoral—followed by use of the “intuitive” Gore device. The whole process was completed within 60 minutes and the patient was back on the peripheral ward after four hours.
The ability to endovascularly perfuse the left subclavian artery plays a key role in minimising surgical procedures and related risks.”
Böckler reports an “excellent” outcome, detailing that the procedure was “uneventful,” performed with minimal radiation, and completed in just one stage. “If you imagine that this case would have been performed in two stages, with a bypass and then in a second stage—or simultaneously—with a normal stent graft, that’s a much longer procedure, with longer anaesthesia time and longer postoperative surveillance time on ICU [intensive care unit],” he explains.
Böckler summarises the benefits this device might have on the treatment of aortic arch patients, including less anaesthesia, shorter procedure time, shorter recovery room time, shorter overall hospital stay, and fewer access problems. On this last point, he
elaborates: “When you do carotid bypasses, you do a cutdown, you may end up with a nerve injury, you may end up with lymphatic problems, and these are gone with the Gore TAG TBE.” Böckler also predicts this off-the-shelf availability to be a “big advantage” in the future. “You will be able to use the device in emergencies when patients are unstable, and the device won’t cause additional access trauma or any other open surgery trauma,” he said.
In terms of clinical data, technical feasibility has already been proven. The pivotal study conducted in the USA enrolled 238 patients requiring treatment, including the left subclavian artery, across multiple aortic pathologies. All patients were enrolled with a technical success rate of 95.8%, reintervention rate of 2.9%, left subclavian artery branch patency of 99.2% and disabling stroke rate of 3.4% through 12
months of follow-up. Böckler does stress, however, that further data are needed. “Stroke rate is still an issue for any kind of endovascular procedure and of course also open surgery in the arch, so we will need prospective safety data,” he emphasises, for example. In addition to data on the associated stroke rate, Böckler adds that additional data on branch patency and the cost-effectiveness of the device would prove beneficial moving forward.
Long-term follow-up data are important too, Böckler points out. “Long-term durability of course cannot be answered in the next one or two years,” he says, “but we need long-term follow-up data to address the typical Achilles’ heel that is reintervention rate.”
Taking a step back from the specifics, Böckler underlines the fact that there is a “big demand” for the TBE device that is now closer than ever to being met. “There’s a big patient cohort out there that would really benefit from this device,” he says, which is one that offers an alternative for all the patients who might otherwise have to wait up to eight weeks for a customised device.
Böckler looks ahead to a forthcoming case that is planned at his centre. “The patient came in last week with some chest pain. We considered them to be symptomatic and they are now scheduled for treatment with the TBE device tomorrow, and I think he will benefit because he’s old—85 years old—so would not really be a good candidate for open repair and would not be able to wait six or eight weeks for a custom device,” Böckler shares.
“The Gore TAG TBE will fill a gap,” he summarises. “It will play a big role in our armamentarium and be complementary to the customised solutions. Also, I think it will reduce the need for physician-modified solutions.” Overall, Böckler is confident that the Gore TAG TBE “will increase the number of patients who are able to be treated—patients who would otherwise be refused treatment.”

■ Gore & Associates P01
With more than 55 million medical devices implanted over the course of more than 45 years, Gore builds on its legacy of improving patient outcomes through research, education, and quality initiatives. Gore is joined in service with clinicians and through this collaboration we are improving lives. goremedical.com/eu
■ Medtronic PO2
We lead global healthcare technology, boldly attacking the most challenging problems. Our mission—to alleviate pain, restore health, and extend life—unites a global team of 90,000+ people, and our technologies transform the lives of two people every second, every hour, every day. Expect more from us. Medtronic. Engineering the extraordinary. medtronic.com
■ Cook Medical 125
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we are combining medical devices, biologic materials, and cellular therapies to help the world’s healthcare systems deliver better outcomes more efficiently. We have always remained family owned so that we have the freedom to focus on what we care about: our patients, our employees, and our communities. For the latest news, follow us on Twitter, Facebook and LinkedIn. cookmedical.eu
■ Terumo Aortic 309
At Terumo Aortic, we partner with our customers to revolutionise aortic care. We deliver innovation, versatility, and precision with the broadest range of solutions that can be personalised for every patient. We are further complementing our implantable device portfolio through the development of digital technologies. terumoaortic.com
■ Inari Medical
107
Inari Medical Inc is a medical device company focused on developing
products to treat and transform the lives of patients suffering from venous diseases. Inari has developed two minimally invasive, novel catheterbased mechanical thrombectomy devices—FlowTriever (pulmonary embolism) and ClotTriever (deep vein thrombosis [DVT]). The company purpose-built its products for the specific characteristics of the venous system and the treatment of the two distinct manifestations of venous thromboembolism, or VTE: DVT and pulmonary embolism. inarimedical.com/int
■ Lombard Medical/ Endovastec 307
Lombard Medical Limited is focused solely on the minimally invasive treatment of aortic disease. In partnership with MicroPort Endovastec, we offer a broad portfolio covering thoracic endovascular aortic repair (TEVAR), fenestrated endovascular aneurysm repair (FEVAR) and endovascular aneurysm repair (EVAR) anatomy, adding even more treatment options to the physician’s armamentarium.
To find out more, come and visit us in the Exhibition Hall lombardmedical.com
■ Penumbra 121
Penumbra Inc, headquartered in Alameda, California, is a global healthcare company focused on innovative therapies. Penumbra designs, develops, manufactures, and markets novel products and has a broad portfolio that addresses challenging medical conditions in markets with significant unmet need. Penumbra supports healthcare providers, hospitals, and clinics in more than 100 countries. Connect on Twitter and LinkedIn. penumbrainc.com
■ Shockwave Medical
305
Shockwave Medical is a company focused on developing and commercialising products intended to transform the way calcified cardiovascular disease is treated. We aim to establish a new standard of care for medical device treatment of atherosclerotic cardiovascular disease through our differentiated and proprietary local delivery of sonic pressure waves for the treatment of calcified plaque, which we refer to as ‘Intravascular Lithotripsy.’
shockwavemedical.com
■ Siemens Healthineers
119
We pioneer breakthroughs in healthcare. For everyone. Everywhere. Sustainably. The innovative healthcare solutions offered by Siemens Healthineers are crucial for making high-quality healthcare accessible for everyone, for clinical decision-making, and for treatment pathways. We are a team of more than 71,000 Healthineers in over 70 countries passionately pushing the boundaries of what is possible in healthcare to help improve the lives of people around the world. siemens-healthineers.com
■ Teleflex
109
Teleflex is a global provider of medical technologies designed to improve the health and quality of people’s lives. We apply purpose driven innovation—a relentless pursuit of identifying unmet clinical needs—to benefit patients and healthcare providers. Our portfolio is diverse, with solutions in the fields of vascular and interventional access, surgical, anaesthesia, cardiac care, urology, emergency medicine and respiratory care. Over 14,000 Teleflex employees worldwide are united in the understanding that what we do every day makes a difference. teleflex.com
■ AOTI (Advanced Oxygen Therapy Inc)
229
AOTI’s TWO2 therapy is clinically proven to heal chronic wounds, reducing hospitalisations and amputations while improving quality of life and reducing costs. As an at-home therapy, TWO2 improves patient access and compliance. Our Nexa negative pressure wound therapy (NPWT) system offers simplicity, performance and affordability. aotinc.net
■ APR Medtech Limited
TT
APR Medtech is a UK-based medical technology company specialising in the supply of innovative products and solutions for use within a range of clinical settings, including vascular intervention. With a focus on patient care and safety, we work with several global manufacturing partners to help achieve our goal of contributing towards improving treatment and health outcomes for patients. aprmedtech.com
■ Artivion
237
Artivion Inc is a medical device company focused on developing simple, elegant solutions that address cardiac and vascular surgeons’
most difficult challenges in treating patients with aortic diseases. Artivion’s four major product groups include: aortic stents, stent grafts, surgical sealants, mechanical heart valves, and implantable cardiac/vascular human tissues. artivion.com
■ BD
105
BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. BD and its 75,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians’ care delivery process, enable laboratory scientifsts to accurately detect disease and advance researchers’ capabilities to develop the next generation of diagnostics and therapeutics. bd.com
■ Cordis
227
Cordis is a worldwide leader in the development and manufacture of interventional vascular technology, with a reputation for clinical acumen, training, and services. For more than 60 years, Cordis has delivered revolutionary products to treat millions of patients. Our focus is on cardiology and endovascular platforms, with highquality products such as diagnostic and interventional catheters, drugeluting balloons, and vascular closure devices. cordis.com
301
We are thrilled to announce the launch of our state-of-the-art in-house studio, CX Vascular, revolutionising the way we deliver educational content. With our new TV-style recording capabilities, we can provide year-round dynamic and immersive learning experiences for healthcare professionals worldwide. Insights carries out research globally in the vascular and cardiovascular fields, with an extensive panel of health care professionals built from our proprietary work, news publications and educational conferences as well as a presence at all main symposia. Specialising in both quantitative and qualitative insights, we offer a diverse portfolio of research solutions including syndicated monitors, custom surveys, brand tracking and focus groups, to name a few. We pride ourselves on delivering vital feedback, directly from physicians, to drive key decisions.
BIBA News and Media is a multimedia, multichannel news publisher of nine print, digital and social titles with unparalleled reach into global medical communities. Vascular News is our flagship title, going out to over 19,000 vascular and endovascular specialists in print
in North America and Europe and 23,000 users on our website, globally. Research carried out by our in-house Insights division finds that 8/10 readers surveyed are satisfied or very satisfied with Vascular News and 91% likely to recommend the publication to a colleague. Nearly 80% say that their practice has changed as a result of reading Vascular News bibamedical.com
■ Cydar Medical
235
Cydar Medical is a global cloud-based software company that provides an end-to-end integrated solution to improve outcomes in minimally invasive image guided surgical procedures. The product, Cydar Maps, supports pre-procedure planning, interoperative image guidance and postoperative analysis of the procedure outcome. It uses the power of artificial intelligence (AI) to advance surgical visualisation and decision-making in theatre, and across the surgical pathway. Cydar AI continuously learns from procedures conducted globally to provide analytics to assist clinicians, enabling them to make faster, easier, and safer decisions. Cydar’s platform will continue to deliver new products and capabilities to improve the delivery of healthcare. Cydar Medical is headquartered in Cambridge, UK, and is working towards its vision of a world where all surgery conducted works exactly as planned. cydarmedical.com
■ Endologix
217
Endologix LLC is a California-based, global medical device company dedicated to improving patients’ lives by providing innovative therapies for the interventional treatment of vascular disease. Endologix’s therapeutic portfolio includes a variety of products in various stages of development that are designed to treat diseases that currently have clinically relevant unmet needs. Endologix’s commercial products, including the AFX2 endovascular abdominal aortic aneurysm (AAA) system, Alto abdominal stent graft system, and the Detour system, are designed to treat a range of vascular diseases, from AAAs to lower limb peripheral vascular disease. The company has offices and manufacturing sites in Irvine, Milpitas and Santa Rosa, USA. endologix.com
■ Envveno Medical Corporation
TT Venous workshop
Envveno Medical is a medical device company involved with finding solutions for deep venous reflux in patients with chronic venous insufficiency.
envveno.com
■ GE HealthCare
115
As a standalone company, GE HealthCare is a leader in precision care, infusing innovation with patientfocused technologies to enable better care. We’re dedicated to providing integrated solutions that make hospitals more efficient, clinicians more effective, therapies more precise, and patients healthier. Together our Imaging, Ultrasound, Patient Care Solutions, and Pharmaceutical Diagnostics businesses help improve patient care from prevention and screening, to diagnosis, treatment, therapy, and monitoring.
gehealthcare.com
■ InspireMD
219
InspireMD is a commercial-stage medical device company developing innovative stent designs with integrated embolic prevention systems (EPS) delivering neurovascular protection and stroke prevention. InspireMD seeks to utilise its proprietary MicroNet technology to make its products the industry standard for carotid stenting by providing outstanding acute results and durable, stroke-free, long-term outcomes.
inspiremd.com
■ iVascular
113
iVascular is a fast-growing company founded in 2010 in Barcelona (Spain) with the aim of developing medical devices and therapies to improve patients’ quality of life. It is empowering the value of technology and innovation in the vascular field. Today, iVascular has fulfilled the quality standards of more than 70 countries.
ivascular.global
■ Kreussler Pharma (STD & Kora)
TT
The long-established and family-owned sclerosant companies, Kreussler Pharma and STD Pharmaceuticals joined forces in 2023. With the synergies this provides for production, regulatory compliance and marketing, they will be strengthening the position of sclerotherapy with the two leading sclerosants worldwide, Aethoxysklerol and Fibrovein. kreussler.com
■ Laminate Medical
211
Laminate Medical Technologies is committed to advancing arteriovenous fistula (AVF) function for haemodialysis patients globally. Laminate’s flagship product, VasQ, is an external support
device designed to improve surgical fistula outcomes by providing permanent reinforcement to the arteryvein connection, promoting stability against wall tension, and decreasing turbulent flow.
laminatemedical.com
■ LeMaitre
211
LeMaitre is a leading global provider of devices for the treatment of cardiovascular and peripheral vascular disease and for arteriovenous access. We develop, manufacture and market a broad range of unique disposable as well as biologic and biosynthetic implantable devices to address the needs of vascular and cardiac surgeons and interventionalists. lemaitre.com
■ LifeTech Scientific
104
Established in 1999, LifeTech Scientific Corporation is committed to the research and development, manufacture, and sales of minimally invasive interventional medical devices for cardio-cerebrovascular and peripheral vascular diseases. Providing patients with innovative device solutions in the treatment of structural heart diseases, peripheral vascular diseases, and bradycardia, the company also expands its business scope in respiratory interventional business, neurointerventional business and interventional oncology business in the world marketplace. Over 1,900 high-quality patents have been filed by the company, and its sales network has penetrated more than 100 countries and regions around the world.
lifetechmed.com/en
■ LSO Medical
225
LSO Medical—expert of the vascular laser. Driver of the innovation in the treatment of venous pathology, LSO Medical is a French company specialised in the design of vascular lasers for over 20 years. Serving patients is a privilege, for which we demand the highest standards of quality and ethics. These requirements are applied to all of the company’s activities: design, manufacture and sale of our products. LSO Medical improves the quality of life of patients, by constantly optimising existing treatments. lsomedical.com
■ Merit Medical
TT Vascular Access workshop
Merit Medical offers an integrated portfolio of products designed to support your vascular surgery procedures, dialysis, peripheral vascular interventions, interventional radiology, and cardiology. We bring you innovative solutions for vascular challenges. Join our educational session to learn more. merit.com
201
As a German manufacturer for selfexpanding nitinol stents with a patientcentric mindset, we stand for high quality and innovation in stents. Our deep, expert knowledge in vascular surgery and great partnerships with surgeons worldwide help us to create unique products such as life-saving ductus stents for infants or special venous stents, like the Sinus-Obliquus. Our latest addition to the wide portfolio is the Tentos 4F, a COFadjusted arterial stent for infrapopliteal and superficial femoral artery (SFA) applications, with the largest stent variety in the 4F segment. optimed.com
■ Philips 203
Philips is a leading health technology company focused on improving people’s health and enabling better outcomes—from healthy living and prevention, to diagnosis, treatment and home care. philips.com
■ PrediSurge
221
PrediSurge develops predictive software solutions for cardiovascular interventions that allow physicians and MedTech companies to improve procedure planning and medical devices designs. Based on preoperative imaging, their technology enables the creation of patientspecific digital twins: numerical models simulating arteries and valves’ biomechanical behaviour. This solution allows physicians to optimise intervention strategy, by choosing a graft adapted to each patient and anticipating potential operative complications. predisurge.com
■ Scanlan International 207
Celebrating over 100 years and our journey continues. The world’s finest surgical instruments designed and manufactured by the Scanlan family since 1921. Experience the Scanlan difference at exhibit #207.
scanlaninternational.com
■ Scitech 102
Scitech Medical is a minimally invasive medical device company that was founded over 20 years ago and is currently present in more than 45 countries. Its 6950m2 state-of-the-art CE 13485-certified facility is located in Brazil. Currently the company develops a wide portfolio of products for peripheral vascular, interventional cardiology etc.
scitechmed.com
■ Shanghai INT Medical 223
Established in 2006, Shanghai INT




Medical Instruments Co Ltd specialises in innovative medical devices across various intervention fields. With three production bases in China, INT leads a complete industrial chain from product design to sterilisation. Products with CE mark and US Food and Drug Administration (FDA) approval have been exported to over 60 countries and areas.
kdl-int.com
■ Shape Memory Medical
205
Shape Memory Medical is reshaping clinical success through the science of smart polymer. Smart polymer upgrades device performance and redefines embolisation possibilities. Our conformable smart polymer delivers unparalleled volume, returns imaging clarity, and promotes healing as the material absorbs. We continue to drive a cross-specialty portfolio to meet procedural demands.
shapemem.com
■ Syndeo Medical
101
Syndeo Medical is a Belgium-based, global designer and developer of highly customised endovascular procedure packs and systems which incorporate everyday-use devices into a single, convenient and innovative solution. We are committed to elevating patient experience through innovation, delivering meaningful value, and empowering outcomes for patients throughout the world. syndeomedical.be
■ The University of Edinburgh
TT
Study part-time for a Master of Surgery degree in Vascular & Endovascular Surgery completely online and gain extensive knowledge of the specialty. Improve your clinical decisionmaking skills through learning how to evaluate and apply evidence in your practice. Provides structured learning for national board exams including Fellowship of the Royal College of
Surgeons (FRCS) and Fellowship of the European Board of Vascular Surgery (FEBVS). ed.ac.uk
■ Vascular Technology
231
We are a medical device company specialising in the development, manufacture and sale of surgical devices including intraoperative Doppler systems and surgical suction irrigation systems (ROSI). We have served multiple surgical specialties including vascular surgery, reconstructive surgery, urology, neurosurgery, and robotic surgery for over 30 years. vti-online.com
■ Veryan Medical
233
Veryan has developed innovative technology to improve the performance of vascular stents by adopting the principle of biomimicry; developing structures that imitate those occurring naturally. Veryan’s vascular biomimetic stent technology involves adapting a straight stent design to a threedimensional helical shape, which more
closely mimics the natural geometry of the human vascular system. veryanmed.com
■ Wisepress Medical Bookshop
103
Wisepress.com, Europe’s leading conference bookseller, attend around 200 conferences every year. We have an extensive range of books and journals relevant to the themes of this conference available at our booth. We also have a comprehensive range of scientific, technical and medical (STM) titles available on our online bookshop. Follow us on X @WisepressBooks. wisepress.com
■ Ziehm Imaging
209
Ziehm Imaging is specialised in the development and manufacture of mobile C-arms. Since 1972, we have produced technologies that enhance imaging and streamline clinical workflows. Our devices’ exceptional image quality and flexibility in the operating room serve as an important basis for treatment success.
ziehm.com

CX Symposium attendees are invited to this year’s faculty dinner and reception. The event will take place in the Painted Hall at the Old Royal Naval College in Greenwich. Tickets are £125 per person.
Guests will be able to enjoy a drinks reception followed by a three-course meal surrounded by baroque walls and ceilings covered in striking images depicting kings, queens, and mythological creatures.
A coach service will be provided to transport guests from ExCeL to the venue, included in the ticket price. A guest list will be used on the night, there will be no need to print or email a ticket.
Note that tickets are available for CX Symposium attendees only. The admission of guests may be permitted subject to numbers.
For more information visit cxsymposium.com.
Are you able to achieve ultra-low-dose DAP values in your standard EVAR procedures?
With ARTIS pheno, you can.

A Dose Mindset:
Strategies to Reduce Radiation Dose in Endovascular Aortic procedures
Prof. Maani Hakimi, MD, Kantonsspital Luzern, Switzerland
Prof. Pekka Aho, MD, Helsinki University Central Hospital, Finland
Theatre 1
Thursday 25th of April, 12:35 p.m. – 01:05 p.m.
The last year of Vascular News profiles has seen key leaders in the field share important lessons and advice based on notable careers. This overview of their individual in-depth interviews is an annual look back at their insights and experiences.

A specialist in complex open aortic surgery, Rachel Bell (Freeman Hospital, Newcastle-upon-Tyne, UK) spoke to Vascular News last summer about her career so far. She was, at the time, president of the Vascular Society of Great Britain and Ireland (VSGBI).
“My mum was a nurse—I grew up listening to all the stories from her training and it piqued my interest,” Bell said of her decision to pursue a career in medicine. “I chose to go to medical school in London because I had lived in Yorkshire for almost all of my childhood and, aged 18, I just needed to experience life outside of Yorkshire. I started at Guy’s and St Thomas’ medical school in 1989 and had pretty much determined by the time I had finished that I was more likely to be a surgeon than a medic.”
Bell also considered the field of vascular surgery more widely, sharing her thoughts on some of the significant developments she has witnessed since starting her career. “There has been a paradigm shift from open surgery to endovascular treatment, and it has really broadened the specialty,” she remarked. “You have to be multiskilled, and that has changed everything. It has changed how we treat our patients, how we work within the hospital, how we train our juniors, and it has changed vascular research.

Jürgen Falkensammer (Wilhelminen Hospital Vienna, Vienna, Austria) spoke to Vascular News in September about his career so far.
Falkensammer recalled that, as a school pupil, he was interested in science and, within this broad subject area, had to make a choice between studying two very different options: one was archaeology, and the other was medicine. “I considered medicine as the more future-proof option,” he shared. In addition, he noted that there are several physicians in his family and “it felt like everybody liked his or her job”. “Early on,” he said, “I learned that medicine could be a dedication, not just a job.”
Falkensammer spoke about his current research, sharing that this is focused on the treatment of patients with complex aortic pathologies. “Custom-made fenestrated branched endografts have enabled us to treat older and sicker patients,” he commented, before underlining some key unanswered questions about their use that his research aims to address, including how patient selection can be improved and durability optimised.
Recalling the details of a particularly memorable case, Falkensammer took Vascular News readers back to a
From an endovascular point of view there are questions about durability, but we are now able to offer very complex surgery to a much larger population and that has been the biggest and the best change.”
Despite the positives of this shift, Bell was keen to stress that there is a role for both endovascular and open surgery. “Nowadays, I see younger vascular surgeons with a total passion for the endovascular side who are a little bit scared of open surgery, particularly in the abdomen. Training has changed now to encompass the endovascular requirements, but the need for open surgeons has not gone away.”
Training has changed now to encompass the endovascular requirements, but the need for open surgeons has not gone away.”
On one of the biggest challenges facing the specialty, Bell stated: “The NHS [National Health Service] is currently under incredible pressure and the combination of trying to get on top of the waiting lists whilst simultaneously coping with the problems of staff retention and burnout, the financial crisis and industrial action puts the NHS in the most perilous position that I have witnessed in the last 30 years.”
Bell was the second woman to be president of the VSGBI, after Professor Averil Mansfield. “It never crossed my mind that one day I would follow in the footsteps of some of my vascular surgical heroes and heroine,” she said of her appointment to the role. “I would like to make the Vascular Society more inclusive and less elitist,” Bell noted, sharing one of her main goals for the presidency, adding that she also wanted to continue the drive to ensure safe national pathways for patients with aortic dissection.
Outside of medicine, Bell shared a love for singing among other hobbies and interests. “If I had not been a vascular surgeon, I probably would have been a singer. I sing all the time— to patients, in theatre, and I am a member of a choir—it brings me joy.”
How can we provide a sufficient caseload for open as well as endovascular surgery to ensure that our team of tomorrow is able to perform either at the highest level?”
couple of days after he had started working at the Wilhelminen Hospital in Vienna. “A patient with a ruptured AAA [abdominal aortic aneurysm] was transferred from the ER [emergency toom] to our operating suite. A fit octogenarian in stable condition. I expected the usual stressful situation from intubation until the placement of the aortic clamp. Far from it! Slight sedation, local anaesthesia in the groin, small oblique groin incisions, blocking balloon, etc. Ninety minutes later, the guy was awake in intensive care. I knew I was in the right place!”
Falkensammer also shared some advice for anyone looking to start a career in medicine. For those wanting to study medicine to be a doctor, Falkensammer advised them to “go for it!” emphasising a “wide spectrum of opportunities” that are available. “Medicine can truly be a mission rather than a job, if you let it be,” he said. On starting a career in the sense of “climbing the ladder” towards management, he commented that “there is probably an inherent discrepancy between a healthy work-life balance and carving out a career, independent of the profession” while acknowledging that “there is a great need for physicians with good management abilities, at all levels”.
Falkensammer listed hiking, mountain biking and running as some of his hobbies and interests outside of medicine, and also considers himself “a family man”.
Trained during the era of open surgery only, Peter Schneider (University of California San Francisco, San Francisco, USA) saw the benefits of endovascular intervention early on, writing Endovascular Skills in the early 1990s and unlocking “one of the most fun aspects” of his career so far, with doctors from around the world sharing with him the importance of the book in their catheter experience.
Speaking to Vascular News last November, he shared his reasons for pursuing a career in vascular surgery: “Work on the vascular system caught my attention because of the unique combination of multifaceted, multiorgan system care in patients with little margin for error. Vascular surgery gives the opportunity to provide full spectrum care, to make long-term relationships, and to make a difference in the lives of the most vulnerable. The goals are practical, dilemmas are intellectually stimulating, the procedures are anatomically based, aesthetically pleasing, and technically challenging, and there are numerous opportunities for innovation.”
Schneider was chief of Division of Vascular and Endovascular Surgery at Kaiser Permanente in Honolulu, USA, of which he was a founding member, from 1994 to 2018. He recalled that over this 24-year period, one of the things he is most proud of is the senior endovascular fellowship that was offered between 1999 and 2011.
“This permitted trained vascular surgeons who had the content knowledge, the practice, and the open experience to perform endovascular techniques with us for three months or more and then return to their respective practices,” Schneider shared. “We trained 40 physicians in endovascular care.”
Schneider has served as national principal investigator for numerous clinical trials and told Vascular News that out of all of them, there are two he feels had a particularly significant impact on vascular surgery. “IN.PACT SFA was a landmark femoral popliteal drug-coated balloon (DCB) randomised controlled trial (RCT) study that

“The future of vascular surgery is incredibly bright,” Daniel Clair (Vanderbilt University Medical Center [VUMC], Nashville, USA) opined in an interview with Vascular News earlier this year.
“I honestly cannot remember a time I did not envision myself as a physician,” he began. “I planned to pursue a career in family practice and return to my home as a family medicine doctor, until I completed a surgery rotation in medical school at the University of Virginia (UVA). Long known for its training of primary care physicians, UVA was my first choice for medical school because of this history, but I truly enjoyed surgery and providing options to reverse urgent problems—a part of medicine I would not have experienced had I pursued a career in family practice.”
According to Clair, vascular surgery faces several significant challenges, including the training paradigm and the need to address the current method of training, the lack of adequate numbers of vascular surgeons, the
Issue 100/ November 2023

I believe that we are on the precipice of the next major acceleration in advancements.”
Issue 101/ February 2024
While not all amputations are preventable, it is clear we can provide more limb salvage than we are currently.”
immediately established a standard for ‘best in class’ which continues today, more than 10 years later,” he said. “The other study of which I am quite proud is ROADSTER 2. In this prospective, neurologically adjudicated, US Food and Drug Administration (FDA)-mandated study of transcarotid artery revascularisation (TCAR), the majority of cases were performed by new users, as opposed to previous carotid stent trials which required substantial experience of the operating physicians. The stroke and death risk in ROADSTER 2 was quite low and I believe also established a standard which subsequent carotid trials aimed to meet.”
On his hobbies and interests outside of medicine, Schneider detailed that his “lifelong passion” is surfing and his personal relationships with family and friends.
increasing need for vascular care as the population ages, and the amputation “crisis” facing the global community. “While these challenges will not be easy to address,” he admitted, “there are a variety of approaches that can be taken to improve each of these, and therein lie the opportunities for the community of vascular surgeons.” Clair noted that vascular surgery is a relatively young specialty and, as one that is maturing, “there are areas where we will need to look to other specialties for insight into methods to deal with these challenges”. Orthopaedic surgery, ophthalmology, and neurosurgery as well as cardiology, gastroenterology, and oncology, he specified, “are all areas that have matured as specialties in ways that vascular surgery can take lessons from”.
Clair is co-principal investigator of the PROMISE trials, which he commented “have documented an ability to salvage limbs for patients previously relegated to amputation”. “My hope is that this will enhance options for patients and will continue to ‘move the needle’ toward limb salvage for more patients,” he remarked. “While not all amputations are preventable, it is clear we can provide more limb salvage than we are currently. Any practice that really wants to provide limb salvage options needs to have this method available and the hope would be that this would reduce the number and rate of amputations from vascular disease. In addition, as with other technologies, I believe we are just seeing the first steps in the use of this approach and there will be increases in the application of and use of this approach.”
Beyond medicine, Clair noted that he has always had an interest in physical activity and tries to work out at least four to six days per week. “I also enjoy playing golf and tennis with my wife Patty who has supported me through my career,” he shared.

Ultrafast contrast-enhanced ultrasound for PAD patients shows promise in early feasibility research
Majorie van Helvert (University of Twente, Enschede, The Netherlands) and Michel Reijnen (Rijnstate Hospital, Arnhem, The Netherlands) are currently conducting research on ultrafast contrast-enhanced ultrasound blood flow quantification in peripheral arterial disease (PAD) patients after endovascular treatment. Early findings show that the new technique could offer an innovative imaging alternative.
VASCULAR
RECENTLY SPOKE WITH both van Helvert and Reijnen, and they went into more detail about their findings. Traditionally, conventional ultrasound systems are used in the diagnostic work-flow of PAD. These systems have limited temporal resolution which is typically capped out at 10–100 frames per second. However, with the novel technique that Reijnen and van Helvert used, they were able to increase that framerate to thousands or even tens of thousands of frames per second. This may be necessary to adequately quantify very high velocities and short-lived flow disturbances, for instance, in and around stenoses.
“Within that technique we use a new type of ultrasound transmissions, which is called plane wave ultrasound,” Reijnen explained. “When you look at regular ultrasound, and its image reconstruction, it’s done line-by-line. So, they make the image in separate lines, and all these lines are combined into one image. With plane wave, all transducer elements are simultaneously active in transmit and receive, thus you construct the image in one go. That enables you to get much more frames per second.”
Besides the increased framerate, the team also used contrast microbubbles. For this purpose an IV [intravenous drip] needs to be inserted to allow for contrast administration prior to the ultrasound acquisitions. “Once we use the ultrasound, these contrast microbubbles make sure that the echogenicity of the blood is enhanced,” van Helvert said. “Next to that, they can be used as tracer particles; that is really the basis for this technique in deeper structures, like the iliac arteries. When we’re looking at more superficial arteries, we don’t really need the contrast as we can perform our analysis based on the bloodspeckle signal itself.”
Reijnen explained that, when you look at anatomy, you are going to mostly use computed tomography (CT) angiography or magnetic resonance
imaging (MRI). “Those are the two most important modalities,” Reijnen said. “When we look at functional imaging, there’s mostly duplex ultrasound. A duplex ultrasound is a regular ultrasound combined with doppler. It gives us flow velocities in one direction, generally in the middle of the vessel.”
He continued by saying that there are some drawbacks to duplex ultrasound, next to the aforementioned temporal resolution. “One of them is that it’s only in one directional. Also it requires a beam-to-flow angle correction which makes it highly operator dependent.”
While MRI technologies offer acceptable imaging, there are still some drawbacks to that as well. Namely, Reijnen explains, it’s time consuming, expensive, and the fact that multiple cardiac cycles are combined during image reconstruction may filter out important diagnostic information. “Another important drawback for example,” Reijnen said, “when there are stents, metal and MR is not a good combination. So, there may be disturbances because of the material of the stent.”
Once the images from the ultrafast contrastenhanced ultrasound have been compiled, the more technical aspect comes into play. In order to determine blood flow patterns, the recorded images from the contrast-enhanced ultrasound are divided into different sub-windows. An algorithm then registered how the microbubbles moved across the images.
“We calculate a cross correlation, and the peak in this cross correlation defines the most probable movement that these microbubbles made between two consecutive images that we captured,” van Helvert stated. “Combined with the time in between the frames, we can calculate a velocity vector per sub-window.”
After the images are captured, they must be filtered to see the microbubble contrast compared to the tissue clutter. The thousands of images that the team


captures through ultrasound and thereafter analyse, allows them to build a velocity vector field over time. However, one unfortunate downside of this is that the images and the velocity vector field are still twodimensional compared to the three-dimensional flow characteristics.
“But, the upside of this technique compared, for instance, to MRI-based flow imaging, is that we measure a couple of cardiac cycles and its proven feasible inside stents.. So, it does provide added information in that sense,” van Helvert explained.
Reijnen went into more detail. “We can really track these bubbles during the cardiac cycle, and we can assess different patterns from that. So, we can see recirculations, we can see stasis of blood, we can see forward flow and backflow. We can also see that there is difference in blood flow at the anterior wall and the dorsal wall of the blood vessel where there a re bifurcations.”
“What we’re aiming for is, at least my view on things, that in an early disease stage you can do such measurements reliably and minimally invasive to assess if patients have favorable or unfavorable blood flow with respect to disease progression,” van Helvert said.
As for the next steps, Reijnen noted: “At this stage we’re looking at the technique itself, so we’re looking at which parameters can we reliably derive. How can we standardise them and how can we quantify them? And when we’ve done that, then we can do clinical studies in which we start to look at the predictive value of each of them.”
“There’s a lot of steps ahead of us,” van Helvert told us. “We’re in the very first stage.”
This year marks a significant milestone as BD celebrates 25 years of its RotarexS rotational atherothrombectomy system, a journey that started in the Swiss mountains with Immanuel Straub, and has today touched the lives of over 250,000 patients. In this article, Vascular News outlines the heritage and history of the device and presents a case report highlighting its continued real-world application and outcomes.
The story of RotarexS begins in the early 1990s, when Immanuel Straub, a Swiss engineer and founder of Straub Medical, teamed up with a medical doctor to create a tool to treat peripheral arterial disease (PAD). Straub combined knowledge from Swiss watchmakers (specialists in micro engineering) and Swiss mountain tunnellers (with their ability to break through the toughest materials) and along with his own expertise in creating high-quality and highdurability springs for the car industry, they came up with a concept for the treatment of arterial blockages. This concept became RotarexS.
Mark Portou (Royal Free London NHS Foundation Trust, London, UK) has extensive clinical experience with the RotarexS and here shares with Vascular News a case report demonstrating the effective use of the device. Specifically, the consultant vascular surgeon, along with vascular specialty registrar Katherine Hurndall, outline utilisation of the RotarexS rotational atherothrombectomy catheter in an occluded deep-vein reconstructed aorto-bifemoral bypass graft.
Procedural overview
A 41-year-old female patient presented with a fourday history of sudden onset severe right leg pain, worsening paralysis, and progressive paraesthesia. She had undergone an autologous deep venous aortoiliac reconstruction 18 months prior, following the explantation of an infected dacron aorto-bifemoral bypass. This was performed for symptomatic occlusive disease. In addition, an emergency right common femoral (CFA) to superficial femoral artery (SFA) long saphenous vein (LSV) jump graft and fasciotomies were performed in the days following the explant and deep vein reconstruction, following an acute graft occlusion. She had continued to smoke.

The RotarexS rotational atherothrombectomy system is designed to remove both thrombus and plaque in acute to chronic arterial occlusions. It is indicated for use in peripheral arteries for native blood vessels or vessels fitted with stents, stent grafts or native or artificial bypasses. With four functions, the device is designed to detach occluding material from the vessel, aspirate detached material into the catheter head, fragment the aspirated material and transport it out of the patient’s body. The device is available in three size options: 6F, 8F and 10F.
She presented tachycardic with fixed mottling below the knee. There was no motor or sensory function and no pulses throughout the right leg. All pulses were palpable on the left side. A computed tomography (CT) angiogram demonstrated an occlusion from the origin of the right neo-iliac venous conduit at the anastomosis with the native aorta, throughout the length of the graft, in the LSV jump graft conduit, the native SFA throughout its length, with contrast reconstituted in the right profunda femoris (PFA), just distal to the origin (Figures 1, 2 and 3).
The patient was counselled regarding the likely unsalvageable nature of the right lower leg, and concerns regarding the ability of the remaining limb to heal post-amputation were discussed. Open
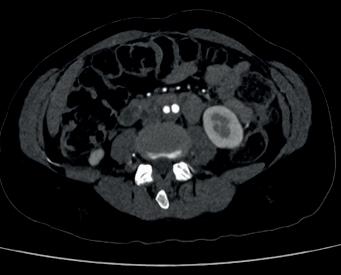
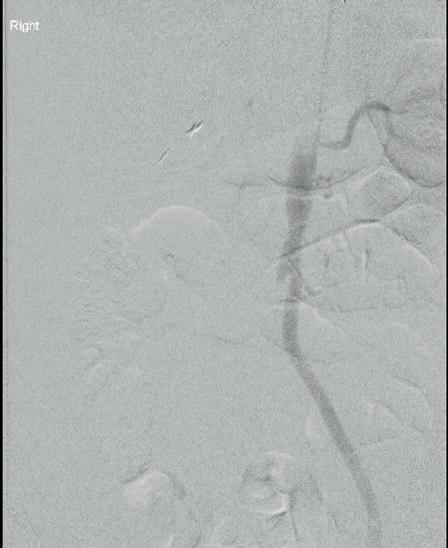
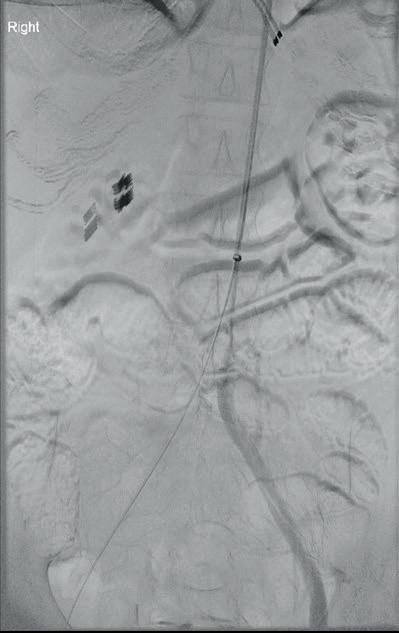
surgical options to restore inflow and enable a successful amputation were considered, but avoided due to extensive scarring, previous infection, lack of covering tissue and the presence of an underlying vein jump graft.
An open left brachial cut down for 6Fr-sheath access was performed, along with an ultrasoundguided percutaneous retrograde right popliteal puncture using a micropuncture sheath. Through-andthrough access was obtained following wire passage from the antegrade left brachial sheath (Figure 4) through the soft thrombotic occlusion and the LSV conduit, with rendezvous in the proximal native SFA. A through-and-through approach was taken in case of perforation of either vein graft or disruption of the surgical anastomosis, to enable balloon occlusion or rapid covered stent repair (Figure 5).
The V18 wire (Boston Scientific) was exchanged for the 0.018 Rotarex wire through a micro catheter. The 6F RotarexS rotational atherothrombectomy catheter was deployed from the antegrade left brachial sheath with a pressurised heparinised saline flush. A full mechanical thrombectomy of the neo-aorta iliac graft, the CFA jump graft, the PFA and SFA was performed (Figure 6). Bolus doses of intra-arterial catheter directed thrombolysis were targeted down the PFA and SFA.
Conclusion
Flow into the PFA and SFA was achieved, with restoration of the right femoral pulse. Patency of the right deep vein and LSV jump grafts was confirmed on angiography (Figure 7). She subsequently underwent a successful amputation, which healed without complication and after rehabilitation is now able to walk up and down stairs independently with a prosthetic limb.
Visit the BD website to find out more about the evolution of the RotarexS system and register to get exclusive access to RotarexS workshops, designed to deepen your understanding and skills with rotational atherothrombectomy technology.

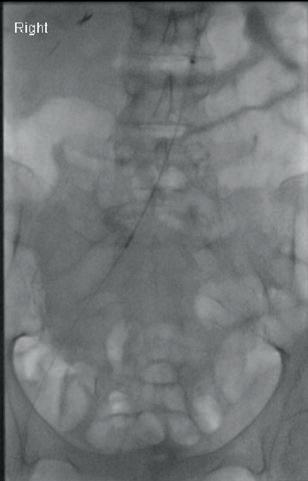
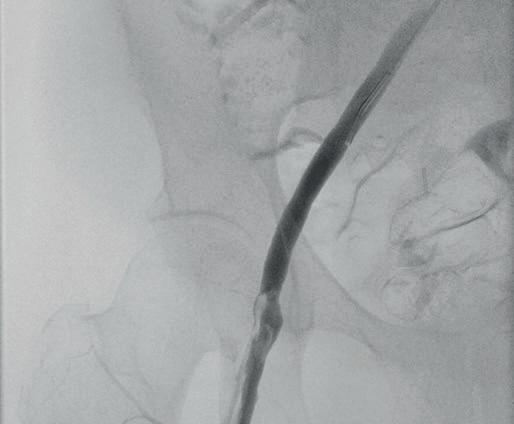

New data underscore physician experience levels required to derive benefit from transfemoral carotid artery stenting
Findings from an analysis of the Vascular Quality Initiative (VQI), presented at the 2024 Society for Clinical Vascular Surgery (SCVS) annual meeting (16–20 March, Scottsdale, USA), showed that the number of procedures a physician had to perform in order to get an in-hospital stroke and death rate below 4% in symptomatic patients was 235.
SIMILARLY, TO ACHIEVE A STROKE AND death rate below 2% in asymptomatic patients, the threshold physicians had to reach to derive benefit was 13 procedures. Rates were based on the fact the best available VQI registry data relate to in-hospital stroke and death, not the 30-day stroke and death thresholds of 6% and 3% for symptomatic and asymptomatic patients, respectively.
Marc Schermerhorn (Beth Israel Deaconess Medical Center, Boston, USA), the study’s senior author, raised concerns over how the coverage expansion will interplay with data reporting and, ultimately, the tracking of stroke and death rates going forward.
“Now that CMS [US Centers for Medicare &
Medicaid Services] has done away with all the regulations and restrictions on who can do [TfCAS] procedures, where, etc., people may not submit data to VQI anymore, unfortunately,” he said.
“There have been a lot of other studies that have shown transfemoral stenting outcomes are not as good as TCAR [transcarotid artery revascularisation] and carotid endarterectomy, but I think the concern now is, by opening up the doors for anybody to do carotid stenting, there could be a lot of people who have never done carotid stenting before who now decide to get into the game.
“I think that the way things were before, when you had to be an approved centre and you had to have credentialing committees that had to collect data, monitor that local data and submit it to Medicare, that prevented a lot of people from doing the procedure. So it concentrated in the people who have the most experience.”
But the respective thresholds for symptomatic and asymptomatic cases represent the basement of learning, continued Schermerhorn. “You have to remember that transfemoral stenting has been around since the turn of the century—and even before that,” he said. “The VQI didn’t start collecting data on carotid stenting until 2005, and that was only in New England. It wasn’t until 2011 that we started to collect national data. So, most people who were in the carotid stent registries in the VQI had experience before they started submitting data, so we actually don’t think this is a full representation of the learning curve.
“We know almost certainly most people had been doing cases prior to entry of data into the VQI, so this is an absolute minimum threshold of learning curve, and the true learning curve is likely larger. I don’t know how much, but it’s higher numbers.”
 Marc Schermerhorn
Marc Schermerhorn
Schermerhorn said that with the possibility of “a lot more novices” performing TfCAS in the wake of CMS coverage expansion, his team’s latest study clearly demonstrates “results are highly dependent on experience.” Even supposing a lot of TfCAS experience, he added, “the results don’t seem to be as good as with either TCAR or endarterectomy.”
Despite the potential for TfCAS entries into the VQI registry to decrease, Schermerhorn expects monitoring to continue, including via administrative databases, albeit with a lower level of clinical detail available, but expressed hope enough interest would persist in submitting data to the VQI. “That’s my other concern,” he said, “The people who are interested in submitting data are probably the ones who are more committed to quality improvement and striving toward knowing what their outcomes are—and improving them.”
That raises another dimension of the study presented at SCVS 2024, noted Schermerhorn: the research team also looked at whether those who had bad outcomes when they first started doing procedures were less likely to continue performing TfCAS. They found that this was indeed the case.
“The people who went on to do more than 25 procedures had better outcomes than the people who quit at up to 25 and then didn’t do anymore,” he said. “We presume that there is some feedback to people and that, if they’re finding that their own results are not that good, then they don’t continue to do the procedure. If people don’t track their outcomes somewhere, then they may not know.



This year’s International Stroke Conference (ISC; 7–9 February, Phoenix, USA) hosted discussions on the current state of play in carotid stenosis treatment—and, most notably, advances that may erode the dominance of long-established carotid revascularisation procedures in the USA.
SEEMANT CHATURVEDI
(University of Maryland School of Medicine, Baltimore, USA), who began by stating that things have “dramatically changed” since the advent of the NASCET and ECST trials in the 1990s. The succeeding decades have seen well-established medical therapies like antiplatelets, statins, blood-pressure control, physical activity, and dietary modification joined by numerous modern options, including proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, inclisiran, bempedoic acid, semaglutide, and combination antithrombotic therapy.
Here, Chaturvedi touched on the various studies that have demonstrated the ability of these newer medications to safely reduce low-density lipoprotein (LDL) levels and ultimately minimise cardiovascular events. Evolocumab— an example of a PCSK9 inhibitor— and inclisiran have been tested and performed well in large-scale trials, while bempedoic acid serves as an alternative for patients who are unable or unwilling to take highpotency statins.
Chaturvedi then somewhat facetiously summarised “all of the data” evidencing the superiority of surgical revascularisation over intensive medical therapy for asymptomatic carotid stenosis—presenting the audience with a completely blank slide.
“But, we do have some emerging evidence that, maybe, revascularisation is not as good,” he continued, alluding to the fact that carotid endarterectomy (CEA) and medical therapy produced similar outcomes in the SPACE-2 trial, and highlighting the importance of data from the CREST-2 trial, which are expected in early 2026.
intensive medical therapy,” he summarised, also stating there is “no excuse” not to aim for lowering LDL levels to at least <70mg/dl in carotid stenosis patients—as per the target in CREST-2. However, he did note that he aims for LDL levels of <55mg/dl, if not lower, in his own practice. Starting medication alone is “not enough” in modern-day care, Chaturvedi continued, emphasising the importance of administering treatment with certain goals in mind.
The third mode
Following this, Caitlin Hicks (Johns Hopkins Medicine, Baltimore, USA) discussed another frontier that appears to have moved alongside CEA and carotid artery stenting (CAS) over the past decade: transcarotid artery revascularisation (TCAR). Pioneered by Silk Road Medical via the Enroute stent system, TCAR’s intended benefits include avoiding the aortic arch, providing proximal protection, and improving particle capture.
TCAR received US Food and Drug Administration (FDA) approval in 2015 for use solely in high-risk carotid stenosis patients, but with the caveat that physicians had to contribute clinical data—including perioperative and one-year outcomes—to the Vascular Quality Initiative TCAR Surveillance Project (VQI-TSP) in order to actually gain reimbursement for the procedure.

“On the symptomatic side, we also don’t really have much evidence that revascularisation is much better than modern medical therapy [either]—there hasn’t been a large-scale study since 1991, and so there is a void in the current data,” Chaturvedi added. Once again, he drew attention to the relevance of ongoing studies in the area, including the SCORE registry.
“In 2024, we need to have very
This has created a greater wealth of both comparative and non-comparative data on TCAR, and studies evaluating it have “exploded” over the past few years as a result, Hicks relayed. Broadly, these studies have suggested TCAR produces stroke/death rates, comparable to those seen with CEA out to one year, across asymptomatic and symptomatic patients alike.
Figures suggest TCAR has “taken off” in the USA over the past few years, with CEA and CAS declining slightly—a trend that is especially pronounced for high-risk carotid stenosis patients, in whom TCAR is now the predominant revascularisation approach nationally. Hicks did also
acknowledge that the 2023 Centers for Medicare and Medicaid Services (CMS) coverage expansion, under which CAS is no longer limited to certain lower-risk patients, will likely lead to an increased stenting uptake and TCAR “losing ground” in more high-risk patients. However, “on the flipside”, with TCAR also being covered by this expansion, physicians are no longer mandated to contribute to the VQI-TSP database, potentially making the procedure less expensive to accommodate and thus boosting uptake at smaller community hospitals in the USA.

Hicks then noted that “there has been very close oversight of the use of TCAR by industry”—although she feels this is likely to be “unsustainable” in the long term, especially if the procedure continues to expand and is
taken up by multiple other specialties. She also highlighted the fact that data on TCAR remain limited, overall, as the majority come from the industrysponsored ROADSTER studies as well as the VQI-TSP registry, which are helpful indicators of realworld patient outcomes, but certainly not equivalent to a randomised controlled trial (RCT).
“People always call for an RCT comparing TCAR to CEA—and/ or transfemoral stenting,” she continued. “As much as I would love to see that happen, it almost certainly will not. It’s not financially viable. Silk Road Medical has seen a large market-share increase, and there’s really no reason for them to fund a study like that. So, unless something like that is funded by the government or another format, this will likely not be in our future.”
People always call for an RCT comparing TCAR to CEA— and/or transfemoral stenting. As much as I would love to see that happen, it almost certainly will not. It’s not financially viable.”
—Caitlin Hicks
For the reconstruction of diabetic foot wounds and venous leg ulcers.
BTM supports cellular migration and formation of neodermis.1,2
• It provides a porous framework that bioabsorbs, leaving a robust vascularized dermal layer.3
• A temporary sealing membrane protects the wound while the body heals.4 Indicated for full or deep partial thickness burns,traumatic wounds, complex vascular and surgical and reconstructive wounds.
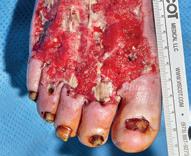
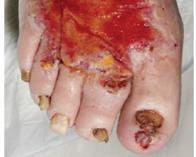
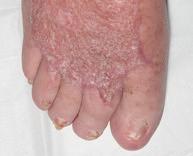

In this article, Victoria Bristow (Cambridge, UK) discusses the NovoSorb Biodegradable Temporising Matrix (BTM) from PolyNovo and shares a case report demonstrating its use in the treatment of a patient with chronic limb-threatening ischaemia (CLTI) and extensive tissue loss. According to Bristow, “BTM provides a useful adjunct to aid wound healing in revascularised CLTI patients with extensive tissue loss, especially when exposed tendons and bones are present”.
NovoSorb BTM has been studied reasonably extensively in patients with burns, necrotising fasciitis and, more recently, in diabetic foot disease. However, there is a paucity of literature in the setting of tissue loss secondary to CLTI.
Dermal templates are indicated for the use of deep dermal or full thickness wounds. The gold standard treatment for these wounds is autologous split thickness skin grafting, which is not often possible due to contraindications for use of active bleeding and/or infection, known malignancy, or exposed underlying structures including bone, tendon, nerves, or vessels.1
BTM is a dermal matrix that is commonly used in extensive and hardto-heal wounds. The dermal matrix adjunct can cover important structures to maintain structural function such as vessels, joint capsules, bone, and tendons. This is important in vascular patients, enabling them to continue to be mobile and avoid major limb amputation, which could lead to a longer hospital admission and, for some, loss of independent living. The matrix is initially placed over the defect to create a neodermis.
This is where dermal grafts come into their own. Another positive of using dermal matrix grafts is that they can be applied in both acute and chronic stages of wound healing.
Novosorb BTM is a completely synthetic matrix that is made of 2mm polyurethane bilayer dermal template, which consists of both a porous matrix and a sealing membrane. A benefit of BTM being synthetic allows for the graft to be placed without worrying about immunity rejection or disease transmission. It is also inexpensive to manufacture and can be placed in those with cultural objections to using animalderived products.2
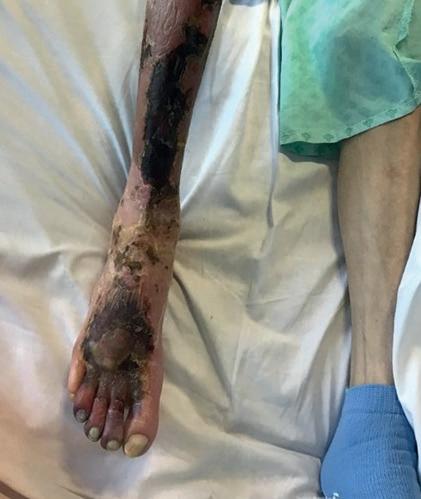
over 18 years old, had a wound distal to the malleolus and were treated with BTM. They used the wound, ischaemia, and foot infection (WIFI) score to assess amputation risk. In total they applied BTM to 22 patients with 23 wounds. Complete wound healing— which they defined as 100% epithelialisation with no exudate—was seen in 15 patients; three wounds failed to go on to fully heal; two patients required major amputations and three patients underwent minor amputations. All patients who had BTM applied underwent revascularisation and surgical debridement. However, no objective level of perfusion was documented. Within this study they placed all patients in negative pressure wound therapy (NPWT) post BTM application for 14 days at a reduced level of 75mmhg.
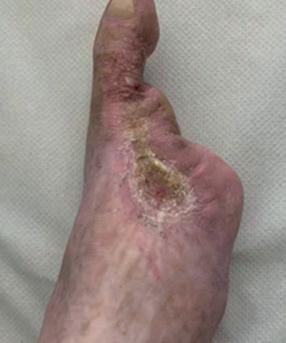
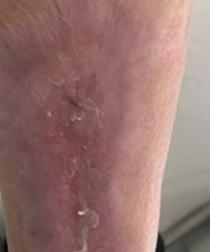
Most recently, an Australian study by Guerriero et al looked at barriers to wound healing in the neuropathic and neuro-ischaemic diabetic foot cohort using a biodegradable synthetic matrix.3 This study was a single-centre, prospective observational study, the aim of the which was to look at complete wound healing as well as amputation outcomes. The inclusion criteria within the study included anyone who had a diabetic foot ulcer treated with BTM between December 2019 and October 2021. All the participants recruited had to have a confirmed diagnosis of diabetes with a HBA1c of 48mmols or more, were
Our first case using BTM was a 62-yearold male who presented with CLTI and extensive tissue loss involving the dorsum of the foot, multiple toes, and the calf. On presentation to the emergency department, he had extensive necrosis and lower limb ischaemia with only a femoral pulse palpable on the right leg (Figure 1). On discussion with the patient and his daughter, it was revealed that he had presented three times at a local district hospital and was misdiagnosed.
He was admitted to the vascular ward for further investigations and revascularisation by the means of a leftto-right femoral crossover graft using a polytetrafluoroethylene (PTFE) graft and his inflow was improved with an iliac angioplasty.
Debridement of the foot was required including amputation of the second to fifth toes. BTM was applied to the dorsum of the foot and the calf. Delamination was carried out at eight weeks. His wounds were fully healed at 24 weeks.
Discussions then began with the team whether his leg was salvageable due to the large area of tissue loss. It was decided that the patient would be discharged home to continue to allow the leg to demarcate. He was reviewed regularly in the outpatient clinic. Sharp debridement was carried out but there was concern that this eschar was covering and protecting important structures including tendons, bone, and blood vessels.
A consultant colleague and I had recently attended a talk showcasing BTM in burns patients from
Australia. These case studies had large areas of tissue loss and it was felt this product would be suitable to the vascular patient group.
The patient was counselled as to this being the first time this product was being used by our centre but was aware due to the large amount of tissue coverage needed the only other option would have been a below-knee amputation and the patient was keen to try and salvage his limb in the first instance.
The matrix was applied in theatre under general anaesthesia in full sterile conditions. With the BTM the wound needs to be able to bleed through the matrix to encourage angiogenesis and create a neodermis. It is secured using staples and negative pressure wound therapy (NPWT) is placed on top this was used for protection and as well as exudate control.
Interestingly, not all studies looking at dermal templates recommend application of NPWT postsurgical application of BTM. NPWT has been used in varying wounds in both acute and hard-to-heal wounds since the 1990s. However, an interesting recent article by Austin in the Journal of Wound Care looked at a case series in which patients receiving NPWT in conjunction with BTM increases the rate of integration.4
The case series was conducted using a retrospective approach of reviewing the clinical records of 28 patients. A total of 23 patients had successful integration, 16 of the patients received both BTM and NWPT and 12 patients received BTM alone. The NWPT group had a P score of (0.046) making it statistically significant. Limitations of this study include that it was non-blinded and a non-randomised chart review. It was also a very small sample size and only from one centre.
In our case, dressings were done weekly by the specialist nurse for seven weeks, wound photos were taken at every visit and the NPWT was replaced on both the forefoot and posterior calf. Contraction of the wound was visible but very limited.
After seven weeks the graft was delaminated: the staples were removed, and the sealing membrane was peeled back. Once removed some slough was present but there was granulation tissue present with islands of epithelisation occurring.
Simple dressings were then applied from this point onwards with silver as the primary dressing, as per the instructions for use from Polynovo. This was carried out by district nurses until week 24 at which the wound had fully healed (Figures 2a and 2b).
To conclude, we have found that BTM provides a useful adjunct to aid wound healing in revascularised CLTI patients with extensive tissue loss, especially when exposed tendons and bones are present. Furthermore, the patient in this case experienced minimal pain throughout the duration of treatment. We have also found that NPWT adds specific benefits of removing excess exudate, promoting healing, and accelerating angiogenesis.
References
1. Braza M and Fahrenkopf M. Split skin grafts. Florida: Treasure Island 2023.
2. Shahroki S, Arno A and Jeschke M. The use of dermal substitutes in burn surgery: acute phase. Wound Healing Society. 2014;22:14–22.
3. Guerriero F, Clark R, Miller M and Delaney C. Overcoming barriers to wound healing in a neuropathic and neuro-ischaemic diabetic foot cohort using a novel bilayer biodegradable synthetic matrix. Biomedicines 2023;11(3):721.
4. Austin C. Biodegradable temporising matrix: use of negative pressure wound therapy shows a significantly higher success rate. Journal of Wound Care. 2023;3(32):159–166.
VICTORIA BRISTOW is a vascular specialist nurse at Cambridge University Hospitals in Cambridge, UK.
Consent was gained from the patient to share his photos in this case study.


Following a review, the UK Medicines and Healthcare Products Regulatory Agency (MHRA) has updated guidance on the use of paclitaxel-coated devices, stating that such devices can be considered for the treatment of peripheral arterial disease (PAD), including intermittent claudication and chronic limb-threatening ischaemia (CLTI).
THE MHRA HAD PREVIOUSLY issued a statement in April 2022 on the use of paclitaxel drug-coated-balloons or drug-eluting stents in patients with CLTI, which stated that they should only be used in patients where ‘the benefits may outweigh the risks’. The parameters for use outlined that exposure should be kept to a minimum, which referred to using the lowest dose device available and avoiding/ reducing repeated exposure to a device. Furthermore, the 2022 guidelines noted that paclitaxel devices should not be used in the routine treatment of patients with intermittent claudication due to the
reported risk of longer-term increased mortality.
The updated guidance has been issued following an extensive review of the most recent published literature alongside the MHRA’s request for the advice of the Interim Devices Working Group (IDWG) and other invited experts. The IDWG advised that the new studies did not support a statistically significant increased risk of harm associated with the use of paclitaxel-coated devices in patients with PAD, irrespective of disease type or severity.
In this update, the MHRA refers
to the 2023 evaluation of numerous randomised controlled trials and real-world studies which compared paclitaxel-coated devices versus control devices in a patient-level pooled analysis.
Led by Sahil A Parikh (Columbia University Irving Medical Center, New York, USA), the analysis included a total of 2,666 participants with a median follow-up of 4.9 years. Their
results showed that no significant increase in deaths were observed for patients treated with paclitaxel-coated devices, providing reassurance to patients, physicians, and regulators on the safety of said devices.
Subsequently, the MHRA has removed its previous restrictions on indication, dose, and repeated exposure for paclitaxel-coated devices for both intermitted claudication and CLTI.

The multi-society PAD Pulse Alliance is taking part in a peripheral arterial disease (PAD) public awareness campaign called “Get a Pulse on PAD”.
THE GROUP—FORMED OF THE SOCIETY for Vascular Surgery (SVS), the Association of Black Cardiologists (ABC), the Society for Cardiovascular Angiography & Interventions (SCAI) and Society of Interventional Radiology (SIR)—aims to educate people on PAD risk factors and potential symptoms, as well as to encourage patients to advocate for their own health with their doctors.
The awareness campaign, taglined “Kick Off the Conversation,” kicked off as the PAD Pulse Alliance released survey data showing that 70% of Americans are unaware of the disease, contributing to 400 amputations performed each day in the USA. The top risk factors for PAD are common chronic health conditions that disproportionately impact underserved communities. Yet among Black and Hispanic adults, nearly 80% report never having a doctor or healthcare provider discuss PAD with them.
“These new insights are particularly concerning among those most at risk and come at a time when a staggering one in 20 Americans over 50 years of age experience PAD,” said SCAI President George D Dangas (Mount Sinai Medical Center, New York City, USA). “The survey confirms what we feared: millions don’t have the tools they need to help start a conversation with their healthcare providers because patients are unaware of their risks and the common signs and symptoms. That’s why we’re encouraging
anyone with leading risk factors, diabetes, high blood pressure and use of tobacco products, to know your ‘three for PAD’ and talk to your doctor.”
Data from the PAD Pulse Alliance survey highlights a disconnect among people between the risk factors and their personal perceived risk of PAD. Nearly 75% of Black and Hispanic adults surveyed report having or knowing someone who has diabetes, high blood pressure, or is a smoker, but only 30% believe they could be at risk. This is in stark contrast to real-world impact. Black people are twice as likely to suffer from PAD and up to four times more likely to undergo an amputation compared to white people. Hispanics present with more progressive PAD leading to worse outcomes including greater risk of amputation.
“The disturbing variations in PAD prevalence, treatments and outcomes underscore another perilous consequence of the health equity gap in managing chronic conditions,” said Foluso Fakorede (Cardiovascular Solutions of Central Mississippi, Cleveland, USA), co-chair of the Association of Black Cardiologists PAD Initiative. “It’s critical to increase awareness among racial and ethnically marginalised communities and the providers who serve them to close the gap. This campaign is one way we are doing just that, but it will take an ongoing conversation to ensure we’re meeting patients where they are with the tools and
resources they need.”
“Screening for PAD is easy, quick, and noninvasive. Yet, this survey confirmed that critical patient-provider conversations addressing common symptoms aren’t happening,” said SIR President Alda L Tam (University of Texas MD Anderson Cancer Center, Houston, USA). “If we can educate more people on the risk factors and early warning signs associated with PAD, it’s our hope we can foster dialogue earlier between providers and patients to kick off screening and treatment—ultimately preventing amputations and saving lives.”
“The collaboration among these medical societies is a testament to the devastating impact PAD can have on people, families and whole communities if not diagnosed and treated early, and, importantly, early treatment usually consists of medication and lifestyle changes,” said SVS President Joseph L Mills (Baylor College of Medicine, Houston, USA). “We hope that care teams will continue to collaborate to ensure early and proper diagnosis with the ultimate goal of improving outcomes for patients.”
It’s critical to increase awareness among racial and ethnically marginalised communities and the providers who serve them to close the gap.”
—Foluso Fakorede
An abstract presented at the 2024 American Venous Forum (AVF) annual meeting (3–6 March, Tampa, USA) revealed that, in the treatment of patients with pulmonary embolism (PE), the implementation of artificial intelligence (AI) software has been linked to a shorter time to assessment.
JACOB SHAPIRO, A RESIDENT at TriHealth in Cincinnati, USA, shared this and other key findings from a review of patients diagnosed with PE at a single centre over a six-year period.
Shapiro and colleagues outline in their abstract that the serious and potentially life-threatening nature of PE necessitates a “streamlined PE workflow with timely assessment and initiation of treatment” to potentially improve a patient’s chance of survival. They add that AI has been increasingly used in healthcare to improve clinical efficiency.
automatically detect and triage patients with suspected PE. The aim of the study, they share, was to evaluate the clinical impact of AI software on time to assessment, time to anticoagulation, and patient outcomes at the institution.
150 Shapiro et al reviewed patients diagnosed with PE between January 2017 and July 2023
The authors detail that, in October 2022, TriHealth implemented an AI-powered parallel workflow tool designed to
Shapiro et al reviewed 150 patients diagnosed with PE between January 2017 and July 2023, retrospectively collecting data on these patients prior to AI implementation and comparing it against those of PE patients following AI implementation. The researchers looked at scan-to-assessment time, scan-to-alert time—which they note was used as a surrogate for scan-to-assessment time following AI implementation assuming
best practice—time to anticoagulation administration, Pulmonary Embolism Response Team (PERT) activations, and in-hospital mortalities.
Shapiro reported at AVF that scanto-alert time in the post-AI group of 45 patients was “significantly faster” than scan-to-assessment time in the pre-AI group of 113 patients. He added that anticoagulants were administered faster for post-AI cases with PERT activation compared to cases without PERT activation, and that in-hospital mortalities decreased from 8.4% (pre-AI) to 2.2% (post-AI), with all mortalities occurring in cases without PERT activation.
“Adoption of AI into our workflow was associated with faster time to assessment of PE patients,” Shapiro and colleagues conclude in their abstract. They elaborate that, with an average AI alert time of under six minutes, AI “optimises standard of care by promoting quicker triage.”
Furthermore, they note that the combined benefit of AI and PERT activation was highlighted by faster anticoagulation administration and decreased mortality in their sample. “These findings suggest a link between earlier anticoagulation and reduced risk of mortality,” they write.
Speaking ahead of AVF, Shapiro stated that “further research is needed to determine if long-term patient outcomes are impacted by
this technology.”
On the learning curve with this AI software, Shapiro remarked that it is “very minimal.” He explains: “The interface for reviewing CT [computed tomography] scans is very intuitive and the chat feature is easy to navigate. The patient’s lab values are also automatically imported and obviously displayed.”
Shapiro commented that overreliance on AI could be a drawback of the technology. However, he emphasised his overall opinion that, as an adjunct to an expert reviewer, there are “no real downsides.” Specifically, he gives the example of the ability to look at scans in views other than axial (i.e. coronal or sagittal) as a benefit for reviewers.
Further research is needed to determine if longterm patient outcomes are impacted by this technology.”
—Jacob Shapiro
OF
VERSUS
sinus-Venous
Predicting longevity in patients who may be candidates for aortic repair is challenging. This, coupled with a high and unchanging long-term postoperative mortality rate, begs the question: are patients living long enough to benefit from aortic repair?
BIJAN MODARAI (KING’S
College London and Guy’s and St Thomas’ NHS Foundation Trust, London, UK) set out and addressed this conundrum at the 27th European Vascular Course (EVC; 3–5 March, Maastricht, The Netherlands). He argued that a lack of evidence for predicting longevity—a key factor in deciding whether a patient should undergo aortic aneurysm repair— necessitates a future move towards precision medicine and personalised treatment to avoid operating on patients unnecessarily.
“Our assessment of patients has got better, but we still get surprised,” was Modarai’s opening message, sharing with the EVC audience three cases to illustrate his point that predicting

longevity remains challenging and uncertain.
Despite showing two examples of aortic patients living longer than anticipated, Modarai highlighted that the totality of the data shows the opposite trend—a high long-term mortality rate. “When you go out to three to five years, there is a significant mortality rate associated with patients who have had an aneurysm repair,” he said, highlighting a 30–50% all-cause mortality rate at five years. “It’s evident that fixing the aneurysm is only one part of what we need to do for our patients.”
Against this backdrop, Modarai then turned his attention to cardiovascular risk factors and malignant transformation, specifically aortic stiffness and aneurysm sac biology, and how both could be managed better.
Regarding aortic stiffness, Modarai outlined some research on the topic: “When you place a stiff stent into the aorta, you change its compliance immediately. That has ramifications for perfusion of organs, particularly for the heart, and there is quite good evidence to show that pulse wave velocity—an indication of increased aortic stiffness—is a cardiovascular risk marker.” He then showed data illustrating that patients with high pulse wave velocity have poor cardiovascular
outcomes. “Is that having an impact on our patients after endovascular aneurysm repair [EVAR]?” he asked. While there are currently few data on the topic, Modarai believes this is a “rich area for research”.
The presenter also spoke on whether radiation influences malignant transformation, referring in this part of his talk to the late results of the EVAR trial. “When you get out to beyond eight years,” he said, “there is a signal for higher malignancy rate in the EVAR group compared to the open group.” He also shared the outcomes of some exploratory work showing fourfold higher numbers of dicentric chromosomes in EVAR patients compared to those in a control group. “Dicentric chromosomes are a marker of genomic instability, caused by radiation damage, and may be a reason why there are more malignancies in this group, but there’s more we need to understand,” he said.
This led Modarai to make one of the key points of his talk. “The guidelines tell us you’ve got to predict longevity in patients when you decide which way to go, but I’m not sure there’s a lot of evidence or robust risk scoring to allow that,” he said.
With risk scoring not available, Modarai outlined how he and his team approach things now. “We’ve made our multidisciplinary meeting
We’re still operating on too many patients with aortic aneurysms, I’m convinced of that.”
FORS-powered LumiGuide 3D imaging technology is rolled out at specialised centres in Europe and USA
Andres Schanzer (UMass Memorial Medical Center, Worcester, USA) has hailed it “one of the most exciting changes” seen in imaging during the course of his career. Philips’ LumiGuide “human GPS” technology—which uses light reflected along an optical fibre inside a guidewire to generate three-dimensional (3D), high-resolution, colour images of devices inside a patient’s body in real-time powered by Fiber Optic RealShape (FORS)—is now available to specialised hospitals in Europe and the USA, the company has announced.
THE LUMIGUIDE SYSTEM ENABLES doctors to navigate through blood vessels during endovascular procedures using light, instead of X-ray, and was first used to treat patients in late 2023 at Maastricht University Medical Center in Maastricht, The Netherlands, closely followed by the University of Alabama at Birmingham in the USA.
LumiGuide initially has been made available to aortic centres of excellence that perform complex aortic repairs in Europe and the USA, a Philips press
release states. The radiation-free technology enables physicians to reduce their reliance on X-ray during complex aortic procedures that can take significantly more time, resulting in a higher radiation dose for patients and clinicians. LumiGuide can be used to see devices including off-the-shelf catheters from any angle and in multiple views, the company adds.
Following a limited release of FORS to nine aortic centres, more than 900 patients have undergone procedures using the technology, with one site
structure a lot more inclusive in the past five years,” he said, noting that it now includes a POPS (perioperative medicine for older people having surgery) team.
He referred to two colleagues who run this service at Guy’s and St Thomas’ and have “transformed perioperative assessment and treatment of our patients”. Despite initiatives such as this, Modarai stressed that “we’ve still got a long way to go in predicting longevity”.
Moving in the right direction
“We need to have a precision approach to how we manage these patients,” Modarai emphasised. “There’s a lot of heterogeneity in the aortic group that we deal with and there is a lack of predictability. It’s all about developing these precision measures and personalised treatment of each patient using multimodal data.”
In terms of progress, Modarai believes “we’re starting to move in that direction”. He referenced a paper showing the use of advanced artificial intelligence (AI)-powered neural multi-task logistic regression techniques to perform patient-specific estimation of survival after elective aneurysm treatment.
He also stressed the importance of AI in the future of precision medicine, opining that “we need to get on this wave early”.
“We’re still operating on too many patients with aortic aneurysms, I’m convinced of that,” Modarai said in his concluding remarks.
The importance of multidisciplinary working was another of Modarai’s take-home messages. “I think medical collaboration and having an open mind about hearing from our medical colleagues is important,” he commented.
conducting a historic cohort comparison showing a 37% reduction in complex aortic procedure time, and a 56% reduction in radiation exposure compared to X-ray.
As detailed in a presentation at the 2023 VEITHsymposium in New York City, USA, last November by Joost van Herwaarden (University Medical Center Utrecht, Utrecht, The Netherlands), the limited edition FORS technology—US Food and Drug Administration (FDA) 501(k)-cleared in 2020—saw more than 800 completed cases by October of last year. The newly released LumiGuide includes workflow enhancements such as artificial intelligence (AI)based automatic registration, along with visualisation of a wider array of catheters, van Herwaarden pointed out
Geert Willem Schurink, from Maastricht University Medical Center, who performed the first procedure with LumiGuide, said: “This artificial intelligence-based semi-automatic registration is very quick and accurate, even in the presence of stent grafts. Especially, if there is a need to re-register the device being guided in the patient’s body during the procedure, it is extremely helpful.”
A randomised controlled trial (RCT) of open repair versus endovascular aneurysm repair (EVAR) for the treatment of abdominal aortic aneurysms (AAAs), as well as head-to-head trials for additional information on EVAR and complex repairs, are warranted. This was the main conclusion of Ross Milner (Chicago, USA) during a presentation he delivered as part of an ‘EVAR Developments’ session at the recent Critical Issues America (CIA) annual meeting (2–4 February, Miami, USA).
THE PROFESSOR OF SURGERY AND CHIEF of the Section of Vascular Surgery at the University of Chicago Medicine in Chicago opened his talk with reference to a 2020 Journal of Vascular Surgery paper by Konstantinos Spanos (Hamburg, Germany) and colleagues titled ‘A new randomised controlled trial on abdominal aortic repair is needed’.
In response to this need, Milner highlighted that Medtronic has launched three trials—HERCULES, ADVANCE and SOCRATES—the latter being the main focus of Milner’s talk at the CIA meeting. He homed in first on HERCULES. The purpose of this multicentre, global, randomised controlled postmarket trial, he shared, is to compare endosuture aneurysm
repair (ESAR) to standard EVAR in the treatment of AAAs with wide proximal necks, and will be the first comparative trial to do so. He noted an enrolment goal of 300 patients at 40 sites across Europe and the USA.
Milner then turned his attention to ADVANCE, which he said is the first EVAR head-to-head RCT looking at aneurysm sac regression outcomes between two devices. “The ADVANCE clinical study will progress the work of sac regression as a key early indicator of long-term outcomes,” he remarked. The study will “bring EVAR evidence into the current decade,” Milner added, and “empower physicians to make precise, evidence-based clinical decisions that improve patient outcomes.”
SOCRATES, meanwhile, will focus on treatment modalities for an especially complex group of AAA patients. Its purpose, Milner elaborated, is to compare the safety and performance of ESAR versus fenestrated EVAR (FEVAR) for the treatment of AAA patients with infrarenal aortic proximal neck lengths of 4–15mm and minimal proximal sealing zone lengths of 8mm.
“Hostile aortic necks can lead to a loss of proximal seal over time,” he said by way of background to the study, detailing that short necks are associated with

increased risk of type Ia endoleak and secondary procedures. Milner noted that there are two treatment options available here: extending the sealing zone proximally or reinforcing the sealing zone.
SOCRATES will compare the two.
The trial will be conducted at up to 40 sites in the USA and Europe, with Milner listing Austria, Belgium, France, Germany, Italy, The Netherlands, Spain and Switzerland as the European countries participating in the trial.
In terms of patient selection, Milner relayed that at least 204 patients are due to be randomised (1:1) and evaluated for non-inferiority. Patients will be treated by one of two methods: ESAR with Medtronic’s Endurant II/IIs with Heli-FX EndoAnchor implants, or FEVAR with either Cook’s Zenith fenestrated stent graft system or—in Europe only—Terumo’s fenestrated Anaconda equivalent.
“[SOCRATES] will be the first comparative trial of ESAR and FEVAR in the treatment of patients ineligible for standard EVAR due to challenging anatomical criteria but within the IFUs [instructions for use] of the two treatment modalities,” Milner summarised. He added that the trial is actively enrolling.
The latest update from the trial, Milner shared, is from August of last year, when the first US patient was treated in the study by co-principal investigator Brant W Ullery in Portland, USA.
“We need more evidence,” Milner said in his closing statement, looking at the field of AAA repair as a whole. Looking forward, he specified that an RCT of open repair as compared to EVAR—as well as FEVAR and branched EVAR (BEVAR)—is needed, and that head-to-head trials “will ideally provide additional safety and efficacy information for EVAR and complex repairs.”









New guidelines from the European Association for CardioThoracic Surgery (EACTS) and the Society of Thoracic Surgeons (STS) have, for the first time, recognised the aorta as “an organ in its own right”.
“RECOGNISING THE AORTA as an organ puts it on a par with the heart, lungs or brain. This is a big step,” said Martin Czerny (University of Freiburg, Freiburg, Germany), chair of the writing committee responsible for putting the new guidelines together.
“The new guidelines clearly recommend bundling the treatment of the aorta in a separate specialty, naturally in close coordination with other specialties. We have been practising this integrative approach at the Medical Center-University of Freiburg for a long time and I am delighted that our work is now also being recognised internationally,” Czerny added. “I am certain that this will improve the treatment of patients with aortic rupture and other serious diseases.”
Published simultaneously in the European Journal of CardioThoracic Surgery and The Annals of Thoracic Surgery, the guidelines take into consideration the impact and risk of different diagnostic or therapeutic methods for the treatment of aortic disease, and are designed to serve as a support tool for physician decisionmaking in the treatment of diseases such as aortic aneurysm.
The aorta is responsible for transporting oxygen-rich blood from the heart to the rest of the body. In recent years, it has become increasingly clear that it also plays an important role in regulating blood pressure and blood flow velocity. In addition, the aorta is involved in the production of certain hormones and has its own layer of smooth muscle cells that help to
Vascular external support system use found to boost key functional outcomes for surgical
Alik Farber (Boston University, Boston, USA) presented the results of a multicentre US study at the 2024 Society for Clinical Vascular Surgery (SCVS) annual meeting (16–20 March, Scottsdale, USA) that showed creating arteriovenous fistulas (AVFs) with a vascular external support system, VasQ (Laminate Medical), decreases the median time to achieve twoneedle cannulation by reducing the need to perform maturation procedures.
THE STUDY SET OUT TO ASSESS THE functional outcomes of fistulas created with an external vascular support device as compared with rigorously matched controls of unsupported fistulas for end-stage kidney disease (ESKD) patients by the same surgeons.
Farber focused on the unreliable trajectory of unsupported fistula functional success that surgeons grapple with: “Arteriovenous fistulas, when they work, are much more durable and much better for patients [than central venous catheters or arteriovenous grafts],” he said. “But to work, they require maturation, which means that the vein that is plugged into the artery needs to grow in diameter and thickness. We are asking the body to do this, but the problem is, this
Recognising the aorta as an organ puts it on a par with the heart, lungs or brain. This is a big step.”
—Martin Czerny
doesn’t work all the time. Sometimes the vein may grow in diameter but not in thickness, or it may not grow in diameter and simply grow in thickness. So, the corollary to that is many surgically created fistulas do not mature; some fistulas require procedures to help with maturation, and these may or may not be successful.
“In the ideal world, the best thing for the ESKD patient is the creation of a fistula that, after a certain period of time, say six weeks, is usable 100% of the time without any other interventions. That’s the predictable ideal, but doesn’t happen very often. So, anything that improves outcomes to get us closer to that ideal is going to be a very good thing for our patients,” he added.
One approach to overcoming this quandary is the concept of external support during surgical fistula creation. The VasQ device is a nitinol-based permanent vascular reinforcement implanted around the artery-vein connection. It is designed to reduce both haemodynamic and mechanical stress to create more functional fistulas. The device, implanted during the surgical creation of either upper arm or forearm fistulas, guides the remodelling in the juxta-anastomotic region to promote outward wall thickening by maintaining the optimal angle, reducing wall tension and facilitating a more stable, laminar flow profile.
maintain its structure and function.
“Our understanding of the aortic organ is continually evolving, especially in regard to its pathophysiology, the timing for treatment, and the application of current and the development of new therapeutic strategies. Aortic disease has emerged as a specialty with significant health economic relevance. Several components of this guideline already establish the foundational structure necessary to meet the needs of treating the aortic organ within a specialised centre by a dedicated interdisciplinary aortic team,” the document states.
and 52 in the VasQ group. Both groups were wellmatched with no significant differences in baseline parameters, including vessel diameter. The researchers evaluated the functional outcomes of time and intervention rate prior to first use, as well as functional success, which was defined as the proportion of fistulas used for haemodialysis at three and six months after AVF creation.
Farber presented the results showing a nearly 30-day reduction in median time to first use, driven by a significantly lower need for maturation procedures in the VasQ group as compared to the unsupported cohort. Additionally, significantly more fistulas in the VasQ cohort achieved functional success by the threemonth timepoint. The VasQ cohort also showed a trend toward higher functional success rate at six months, but that finding did not reach statistical significance.

“[We presented] that the immediate [number of] days to first use is typically shorter with VasQ fistulas. The ratio of interventions to first use is also significantly less in the VasQ-supported fistulas and the functional success is higher in this group,” commented Farber, adding that the device is relatively easy to place and doesn’t add significant time to the procedure.
“It appears that externally supporting the fistula and making the fistula take an improved angle has promise in increasing functional patency and decreasing the number of interventions to get to maturation,” Farber told Vascular News
Six US sites that participated in the VasQ US pivotal study and enrolled more than six patients were eligible to participate in a retrospective chart review of unsupported AVFs to compare them to fistulas that had VasQ support. The cohort for this analysis included 104 ESKD patients with 52 in the unsupported group
Commenting on the results, he said: “The study offers encouraging evidence for the efficacy of an external vascular support of fistulas, especially in lowering intervention rates and shortening the time to achieving functional success.
“I think it’s exciting that there is new clinical science in this space. There’s a huge opportunity that we have with these patients because the maturation rates are currently 50–60%, at best. And that’s a pretty small number. So, there’s a considerable need to improve the maturation rates, and I applaud Laminate Medical and other companies that are trying to develop ways to address this issue.”



Endologix initiates postmarket study of Detour system
Endologix has announced the initiation of the Percutaneous transmural arterial bypass (PTAB)1 postmarket study. This study marks the beginning of a comprehensive postmarket study aimed at evaluating the real-world performance of the Detour system in patients undergoing treatment for long complex superficial femoral artery (SFA) disease.
The study will leverage the Vascular Quality Initiative (VQI) registry infrastructure developed and supported by the Society for Vascular Surgery Patient Safety Organization (SVS PSO), a company press release notes.
The PTAB1 postmarket study evaluates the Detour system’s performance in patients with very long (TASC D) SFA lesions. The study plans to enrol up to 450 patients, with a focus on including at least 200 women and also features an imaging substudy. Recruitment will involve up to 200
sites, with five-year follow-up.
“The initiation of the PTAB1 postmarket study represents a pivotal moment in our ongoing commitment to advance the treatment of complex peripheral arterial disease [PAD]. Through this study, we aim to further validate the Detour system’s innovative approach to overcoming complex PAD challenges,” expressed Matt Thompson, president and chief executive officer of Endologix.
Thomas S Maldonado, the SchwartzBuckley endowed professor of surgery in the Vascular Division at New York University Langone Medical Center (New York City, USA), underscored the significance of being the inaugural site to enrol a PTAB1 patient: “Our privilege of enrolling the first patient in the PTAB1 postmarket study underscores our commitment to advancing patient care in complex PAD. The Detour system offers a glimpse into a future where minimally invasive treatments could redefine our approach to extensive SFA lesions. We are optimistic about the contributions this study will make towards evolving patient treatment paradigms.”
Sensome initiates trial assessing tissue microsensor technology in peripheral arterial disease
Sensome has announced enrolment of the first patients into a feasibility clinical study using the Clotild smart guidewire in peripheral arterial disease (PAD). Clotild was designated a breakthrough medical device for use in brain arteries by the US Food and Drug Administration (FDA) in 2021.
The clinical trial, named SEPARATE, is designed to assess the artificial intelligence (AI)-powered Clotild sensor’s capability to detect various characteristics of blood vessel blockages in PAD patients. The first five patients have been enrolled at AZ Sint-Blasius Hospital in Dendermonde, Belgium, and preliminary results are anticipated in mid-2024.
A key focus of the SEPARATE clinical trial—according to a Sensome press release—is to evaluate the Clotild sensor’s capacity in differentiating between soft and friable “fresh” clots, and organised “old” clots. This critical information empowers physicians to select the most suitable endovascular therapeutic approach, thereby mitigating complications, avoiding embolisation, and enhancing long-term treatment outcomes, the release adds.
“Understanding the makeup of a total occlusion in peripheral artery disease is essential to choose an adequate treatment approach to ensure lower complication rates and more durable long-term outcomes in this complex group of vascular patients,” said Koen Deloose (AZ Sint-Blasius Hospital, Dendermonde, Belgium), principal investigator of the SEPARATE clinical trial. “Sensome’s tissue microsensor technology could become a novel tool to characterise the total occlusion in an objective and simple-to-use way that integrates perfectly with our current existing workflow.”
Franz Bozsak, chief executive officer and co-founder of Sensome, added: “After successfully applying our technology to the treatment of ischaemic stroke patients, we are excited about the opportunity to build on this work to potentially help millions of patients around the world whose lives have been impacted by PAD.”
Study of TIVUS renal denervation system for hypertension treatment completes enrolment
The REDUCED-1 study, a US Food and Drug Administration (FDA) investigational device exemption (IDE)-approved pilot study of the treatment of hypertension with the proprietary Therapeutic Intra-Vascular Ultrasound (TIVUS) system (SoniVie), has completed enrolment.
“Initial results from the ongoing REDUCED-1 study are encouraging and we continue to diligently follow
the enrolled patients,” said Christian Spaulding, chief medical officer of SoniVie, in a press release announcing the study milestone.
The trial had two enrolment cohorts that were conducted under an identical protocol in the USA and in Israel. Twenty-five patients were enrolled in the USA and 15 patients were enrolled in Israel. All patients (n=40) are now in the follow-up phase of the study where primary efficacy (change in daytime systolic ambulatory blood pressure) will be analysed at three months, and safety will be analysed at one month and 12 months follow-up respectively.
Renal denervation with TIVUS is a
minimally invasive procedure that uses high-frequency non-focused ultrasound energy to ablate nerves in the renal arteries. This causes a reduction in the nerve activity, which may decrease blood pressure. This procedure is designed for patients who suffer from resistant hypertension.
“Our next commitment towards patients, physicians and regulators is now to clinically validate the TIVUS system in a global pivotal trial and expand its use under the pivotal study with radial access procedures,” said Tomaso Zambelli, chief executive officer, SoniVie.
Cook Medical reports first patient treated in ZFEN+ fenestrated endovascular graft study
Cook Medical has announced the first patient treated in the clinical study of the ZENITH FENESTRATED+ endovascular graft (ZFEN+) in the USA. The procedure was performed on January 29, 2024 at The University of Texas Health (UTHealth) in Houston, USA, by Gustavo Oderich, global principal investigator.
“We are ecstatic at UTHealth Houston to have treated this first ZFEN+ patient. This is a huge step forward in getting the first patientspecific device for complex abdominal and extent IV thoracoabdominal aneurysms with fenestrations. Rather than a one-size-fits-all approach, the ZFEN+ device design aims to optimise patient needs,” said Oderich.
The ZFEN+ clinical study is being conducted under an investigational device exemption (IDE) from the US Food and Drug Administration (FDA). This clinical study will assess the safety and effectiveness of the ZFEN+ used in combination with the investigational Zenith Universal Distal Body 2.0 graft (Unibody2), the investigational Bentley BeGraft balloon-expandable fenestrated endovascular aortic repair (FEVAR) bridging stent graft system and the
commercially available Zenith Spiral-Z abdominal aortic aneurysm (AAA) iliac leg graft (ZSLE).
The ZFEN+ clinical study will enrol patients in need of treatment of aortic aneurysms involving one or more of the major visceral arteries. Patients with juxtarenal, pararenal and extent IV thoracoabdominal aneurysms meeting specific anatomical requirements may be included in the study. The ZFEN+ device is designed with fenestrations aligned to a patient’s unique anatomy and to extend the proximal margin of aneurysmal disease that can be treated endovascularly.
Endospan releases early TRIOMPHE IDE study results at Society of Thoracic Surgeons annual meeting Endospan shared 30-day results from the first 22 patients enrolled in the TRIOMPHE investigational device exemption (IDE) study in a late-breaking presentation at the 60th annual meeting of the Society of Thoracic Surgeons (27–29 January, San Antonio, USA).
TRIOMPHE is a multi-arm, multicentre, non-randomised, prospective clinical study to evaluate the safety and effectiveness of Nexus in treating thoracic aortic lesions involving the aortic arch. The study will enrol 110 patients at up to 31 sites.
“We were pleased to see that in this high-risk patient population, the 30-day data for these first 22 patients showed a low mortality rate, no disabling strokes, paraplegia, or renal failure and short ICU [intensive care unit] and hospital lengths of stay,” said Bradley Leshnower, director of thoracic aortic surgery at Emory University School of Medicine (Atlanta, USA). “One year data are pending.”
A press release details that more than 120,000 patients suffer thoracic aortic

arch disease every year in the USA and Europe, with only about 25% diagnosed or treated. Despite significant advancements, open surgical aortic arch repair maintains high mortality and morbidity. Patients with excessive perioperative risk or anatomical factors are not indicated for surgery, yet anatomical complexity and lack of approved devices for the arch has often prohibited endovascular repair. This makes the choice of treatment difficult or even impossible for some patients. Providing the alternative of minimally invasive repair decreases the requirement for extra corporal circulation and possibility of hypothermia, translating into reduced procedure and hospitalisation time.

Endovascular excellence starts here. Enhance your knowledge at the premier meeting.
“
The education shared, knowledge gained, faculty interacted with, and handson opportunities with new devices and technologies are unmatched at any other meeting in the space.”
— Past Attendee









US FDA Breakthrough Device designation granted for Biotronik’s Freesolve BTK resorbable magnesium scaffold Biotronik has been granted Breakthrough Device designation (BDD) from the US Food and Drug Administration (FDA) for the Freesolve below-the-knee (BTK) resorbable magnesium scaffold (RMS).
The Freesolve BTK RMS is designed for individuals suffering from chronic limb-threatening ischaemia (CLTI).
A press release notes that the newly CE-launched Freesolve RMS for coronary artery lesions, based on the Biomag magnesium alloy and the proven Orsiro drug-eluting stent (DES) coating technology, provides safety, improved deliverability, and optimal
performance and vessel support during and after implantation. It has shown 99.6% degradation of magnesium observed 12 months after implantation in coronary arteries, the release details.
These characteristics of the Freesolve RMS may be of particular value in BTK interventions, where vessel scaffolding is desired in the short-term to resist vessel recoil, yet ultimately leave the vessel implant-free.
“Biotronik’s focus on vascular interventional excellence is evident in our strategic investments and persistent dedication to innovation,” said Jörg Pochert, president of Vascular Intervention at Biotronik. “Our efforts to expand therapeutic possibilities, underlined by the introduction of the Freesolve RMS for coronary artery disease treatment, will continue in the BTK indication with this groundbreaking innovation.”
“This Breakthrough Device designation for the Freesolve RMS for BTK treatment is a significant milestone in advancing treatment options. Biotronik is committed to design our products to enhance the lives of patients,” stated Ryan Walters, US president at Biotronik. “Our next-generation RMS represents a leap forward over existing resorbable technology, incorporating technical innovations intended to address physicians’ needs and optimise outcomes for patients suffering from CLTI.”
Baylis Medical Technologies announces launch of PowerWire Pro RF guidewire
Baylis Medical Technologies has announced the 510(k) clearance and launch of the PowerWire Pro radiofrequency (RF) guidewire in the USA, facilitating venous stent recanalisation for total occlusions.
Patients with stenotic or occluded peripheral vessels often undergo stent placement to restore vessel patency and normal blood flow. Unfortunately, stents can re-occlude, requiring additional interventions. A press release notes that the PowerWire
Pro RF guidewire has been designed to address this challenge by employing radiofrequency technology to cross totally occluded stents in peripheral vessels. Its design allows selective application of RF energy to cross segments of the occlusion that cannot be traversed mechanically, the company notes. When contacting metal, the RF energy terminates, reducing the potential for vessel extravasation when crossing in-stent occlusions.
The addition of the PowerWire Pro RF guidewire to Baylis Medical Technologies’ portfolio of radiofrequency wires expands its clinical solutions in radiology and vascular surgery.
Frank Baylis, executive chairman of Baylis Medical Technologies, expressed enthusiasm about the PowerWire Pro RF guidewire’s potential impact, stating: “We are very excited to announce new additions to our family of PowerWire RF guidewires. With these new tools, healthcare professionals will now be able to treat patients suffering from both native and in-stent peripheral occlusions using our radiofrequency puncture technology. The PowerWire Pro RF guidewire broadens the doctors’ capabilities in treating such occlusions, where traditional methods have previously fallen short.”
Silk Road expands TCAR portfolio with launch of tapered Enroute system
Silk Road Medical has announced the launch of its tapered Enroute transcarotid stent system to hospitals in the USA, expanding upon the company’s prior Enroute transcarotid stent system and offering additional configurations to better tailor the transcarotid artery revascularisation (TCAR) procedure to patient anatomy.
“The new tapered stent is a welcome addition to my TCAR toolkit, further differentiating the company’s core product offering,” said Joseph Lombardi (Cooper University Health Care, Camden, USA). “The Enroute stent has always provided comprehensive lesion coverage and durable procedural outcomes, but I now have more options to customise treatment to each patient’s anatomy.”
“As pioneers in stroke prevention, our product development efforts are focused on extending our lead in the
minimally invasive treatment of carotid artery disease,” said Chas McKhann, chief executive officer of Silk Road. “New tapered configurations for our Enroute transcarotid stent system build upon the robust portfolio of Silk Road’s carotid solutions. We are pleased to bring this portfolio expansion to market as part of our commitment to offering a diverse toolkit for physicians, allowing them to address individual patient anatomy.”
TCAR is a minimally invasive surgical procedure designed to provide stroke protection while minimising adverse events in patients requiring treatment for carotid artery stenosis.
The Enroute transcarotid stent system is the only commercially available transcarotid stent system indicated for patients at high risk and standard risk for adverse events from carotid endarterectomy (CEA), as per a Silk Road press release. It features an optimised cell design balancing lesion coverage and anatomical conformability for long-term plaque stabilisation—and was purpose-built for TCAR, with a short delivery s ystem for ergonomic and precise stent delivery.
Philips launches new motorised mobile C-arm system
Royal Philips has announced the launch of its image-guided therapy mobile C-arm system 9000—Zenition 90 Motorized, designed to help surgeons deliver high-quality care to more patients at the European Congress of Radiology (ECR) annual meeting (28 February–3 March 2024, Vienna, Austria).
The new mobile C-arm with expanded capabilities is designed to meet complex vascular needs and a range of clinical procedures such as cardiac interventions, pain management and urology. Zenition 90 Motorized will be commercially available from the second quarter of 2024.
In a recent press release, Philips has stated that the Zenition 90 Motorized is an intuitive C-arm that allows the surgeon to control it from the tableside with user-friendly controls and time-saving features, giving them greater flexibility and independence. It delivers state-of-the-art image quality for the most challenging procedures
and is designed to meet complex vascular needs. The system allows greater clinical efficiency thanks to its automated workflows, the image controls via the Touch Screen Module and the advanced software solutions.
“During complex procedures, it’s vital to be able to rely on surgical imaging systems. As surgeons
navigate their way through challenging vasculature, the priority is to quickly visualise small anatomical details while limiting contrast use and X-ray dose,” said Mark Stoffels, business leader, Philips image-guided therapy systems. “The new Zenition 90 Motorized empowers medical teams to confidently perform a wide range of interventions while achieving the best possible outcome for their patients.”
The launch of the Zenition 90 Motorized follows the recent launch of the Philips Zenition 30 C-arm, and is the latest device in the renewed portfolio of the company’s Zenition C-arm systems.
Philips has added that data collected from usability studies from clinicians in Europe and the USA show that the Zenition 90 Motorized performs beneficially in simulated environments. Results showed that 100% of users found that, with the table-side operator function, they have complete control over C-arm movements, and 97% reported that workflow features such as automatic vascular outlining will help save time during procedures.
As part of its stated commitment to sustainability and providing customers with responsible choices, Philips has also leveraged its EcoDesign process for the Zenition 90 Motorized to improve product life by 25% and power efficiency by 13%.
Efemoral Medical granted
Breakthrough Device designation
Efemoral Medical has announced that the US Food and Drug Administration (FDA) has granted its novel Efemoral vascular scaffold system (EVSS) Breakthrough Device status for the treatment of de novo or restenotic lesions of the infrapopliteal arteries in patients with chronic limb-threatening ischaemia (CLTI).
A press release states the EVSS offers a new approach to treating peripheral arterial occlusive disease by addressing the specific anatomic challenges and complex biomechanics of patients with athero-occlusive disease in the leg. Using multiple, serial, intravascular scaffolds, the patented FlexStep technology combines flexibility with support to open clogged vessels and sustain healthy blood flow while accommodating tortuosity and skeletal movement.
Formulated with sirolimus antiproliferative drug elution, the bioresorbable scaffolds restore normal vessel diameter at the time of the procedure, deliver therapeutic benefits across all lesion lengths and morphologies, and maintain durable patency while leaving no permanent implant behind. The device designed for femoropopliteal intervention is currently being tested in a firstin-human trial, EFEMORAL I, in investigative sites in New Zealand and Australia. Encouraged by the early clinical results, Efemoral Medical is now developing an additional device for treating infrapopliteal arteries in patients with CLTI.
Getinge and Cook Medical enter US commercial distribution agreement for iCast covered stent Getinge and Cook Medical today announced an exclusive sales and distribution agreement for the iCast covered stent system, which recently received US Food and Drug Administration premarket approval for the treatment of symptomatic iliac arterial occlusive disease.
Cook Medical will assume sales, marketing and distribution rights for the product in the USA over the coming months. The iCast covered stent system will continue to be manufactured by Atrium Medical Corporation (Merrimack, USA), which is part of Getinge.

senior vice president of Cook Medical’s vascular division.
Cook and Getinge continue to collaborate in other strategic areas, also partnering to complete the PRESERVE-Zenith branch endovascular graft-iliac bifurcation investigational device exemption (IDE) study with five-year follow-up. The purpose of this study was to evaluate the safety and effectiveness of the Zenith device in combination with the iCast covered stent in patients requiring treatment of aortoiliac and iliac aneurysms.
while providing dynamic support to restore function and maintain an open lumen, according to a company press release. The DynamX BTK System for the treatment of BTK vessels impacted by CLTI broadens the use of the technology beyond the treatment of coronary artery disease, the press release stated.
“The bioadaptor platform was developed to transform treatment of coronary and peripheral artery disease [PAD],” said Motasim Sirhan, Elixir Medical CEO. “We appreciate the FDA recognition of our innovation for treating the CLTI population with BTK disease and its potential impact on the patients suffering from vascular disease.”
The DynamX BTK System is not available for sale in the USA.
Martijn Nugteren, director of sales and marketing at Bentley. “Thanks to this collaboration with Cook, we will now have a better commercial footprint in the USA with even more opportunities to treat patients with debilitating arterial and venous disease.”
Cook and Bentley have a long history of collaboration aimed at advancing patient care, A Bentely press release noted.
“We are excited to welcome
Bentley’s BeBack crossing catheter to our peripheral intervention portfolio,” said Alec Cerchiari, Cook’s director of product management for peripheral arterial disease (PAD) and venous.
“The BeBack crossing catheter is a unique tool for crossing heavily calcified lesions in antegrade or retrograde fashion.”
“This agreement with Cook Medical ensures that the iCast covered stent system will reach the optimum number of patients who will benefit from it in the USA,” said Patricia Fitch, president of Getinge in North America.
“This product fills the need of a covered stent in our vascular portfolio with a proven technology. iCast has five-year data aligned with our commitment to long-term clinical evidence and predictable results for [peripheral arterial disease] therapies,” said Mark Breedlove,
US FDA Breakthrough Device designation granted for DynamX BTK for use in CLTI treatment below the knee Elixir Medical has announced Breakthrough Device designation by the US Food and Drug Administration (FDA) for its novel DynamX BTK System, an implant for use in the treatment of narrowed or blocked vessels below-the-knee (BTK) in patients with chronic limb-threatening ischaemia (CLTI).
The DynamX bioadaptor platform is a metallic device designed to support vessels during the healing phase, after which it unlocks and “uncages” them
Bentley and Cook Medical enter US distribution agreement for BeBack crossing catheter Cook Medical and Bentley today announced a distribution agreement for the BeBack crossing catheter in the USA, with Cook assuming commercial responsibilities for the Bentley product in the coming months.
The BeBack device is designed for steering through chronic total occlusions (CTO) and provides targeted re-entry options.
“The BeBack crossing catheter is an effective approach for successful recanalisation and should be available in every lab across the USA,” said


Healthcare Inroads announces global launch
Healthcare Inroads (HCIR)—which aims to deliver value-driven solutions to medical technology companies, healthcare delivery systems, and investors worldwide—has announced its global launch.
HCIR was formed by the merger of Healthcare Insights and Inroad Medical and is a partnership of clinicians and allied specialists dedicated to accelerating the goals and milestones of medical device companies, investors, and healthcare systems. A press release details that HCIR partners use their deep experience as clinicians, investigators, inventors, entrepreneurs, and healthcare system leaders to develop the most efficient process possible to achieve their clients’ goals.
HCIR’s founding partners are Gary Ansel, David Deaton, Michael R Jaff, Peter Schneider, Steve Cagnetta, and Craig McChesney..
Frank J Veith joins ViTAA Medical Solutions’ board of directors
ViTAA Medical Solutions has announced the appointment of Frank J Veith, professor of surgery at the
23–25 April
Charing Cross (CX) Symposium London, UK cxsymposium.com
28 April
5th Annual Endovascular Interventions Symposium Palm Springs, USA na.eventscloud.com/website/53519/home
23–24 May
Pacific Northwest Endovascular Conference (PNEC) Seattle, USA pnec-seattle.org
Cleveland Clinic Lerner College of Medicine of Case Western Reserve University (Cleveland, USA) and New York University Medical Center (New York City, USA), to its board of directors.
With a career spanning several decades, Veith has earned international acclaim for his contributions to vascular surgery, particularly in the areas of limb salvage, lung transplantation, and endovascular treatments. A press release details that his work includes performing the first endovascular aortic repair in North America, a milestone that has significantly influenced the direction and capabilities of modern vascular surgery.
His leadership and expertise have also been instrumental in managing VEITHsymposium, one of the largest annual conferences regrouping vascular surgeons, interventional radiologists, interventional cardiologists and other vascular specialists to learn the most current information about what is new and important in the treatment of vascular disease.
“We are honoured to welcome Dr Veith to our board of directors,” said
 Frank J Veith
Frank J Veith
Mitchel Benovoy, chief executive officer of ViTAA Medical Solutions.
“His unparalleled expertise, visionary leadership, and commitment to improving patient care align perfectly with ViTAA’s mission. We are confident that Dr Veith’s guidance will be invaluable as we continue to innovate in the vascular health industry.”
Cagent Vascular raises US$30 million series C financing
Cagent Vascular has announced a series C financing close in excess of US$30 million. US Venture Partners (USVP) led the round. Participation included new investor Blue Ridge Medical and existing investors, including Sectoral Asset Management.
“We are pleased with the significant investment from US Venture Partners and other new and existing investors. To date, we estimate that over 10,000 Serranator PTA [percutaneous transluminal angioplasty] serration balloon catheters have been used to treat those suffering from peripheral arterial disease (PAD). This infusion of capital will increase our commercial reach, helping to provide greater access for healthcare providers and their
patients,” stated Carol A Burns, CEO. USVP is a Silicon Valley venture capital firm based in Menlo Park, USA. “At US Venture Partners, we understand the challenges facing PAD patients and are keenly aware of the need for innovation for this vulnerable patient population. As we reviewed the impressive clinical data and spoke to physicians about their patient outcomes, it was clear that serration angioplasty is truly innovative. This was further underscored by the strong early commercial success driven by the exceptional team at Cagent Vascular,” said Casey Tansey, general partner, US Venture Partners.
28–31 May
Leipzig Interventional Course (LINC) Leipzig, Germany leipzig-interventional-course.com/visitors/ linc-2024
19–22 June
Vascular Annual Meeting (VAM24) Chicago, USA vascular.org/2024-vascular-annual-meeting
27–28 June
British Society of Endovascular Therapy (BSET) annual meeting South Gloucestershire, UK bset.co.uk/meetings/bset-annualmeeting-2024
27–29 June
24th European Venous Forum (EVF) annual meeting Athens, Greece europeanvenousforum.org



A press release details that the Serranator has received US Food and Drug Administration (FDA) 510k clearance and is intended for dilatation of lesions in the iliac, femoral, iliofemoral, popliteal and infrapopliteal arteries for the treatment of obstructive lesions of native or synthetic arteriovenous dialysis fistulae. The product is currently being sold in the USA. The Serranator device also has CE mark and limited distribution in Europe.
14–18 September
Cardiovascular and Interventional Radiological Society of Europe (CIRSE) annual congress Lisbon, Portugal cirsecongress.cirse.org/about/ theannualcongress
24–27 September
European Society for Vascular Surgery (ESVS) annual meeting Kraków, Poland esvs.org/events/annual-meeting



Ability to axially and radially reposition graft while partially expanded
90%
technical success 1
98%
Radiopaque markers for improved visualisation and alignment accuracy