Transforming below-the-knee interventions with IVL technology
Maria Antonella Ruffino (Lugano, Switzerland) explains how the Shockwave E8 and Shockwave Javelin peripheral intravascular lithotripsy (IVL) technologies are reshaping outcomes for patients with chronic limb-threatening ischaemia (CLTI).
PERIPHERAL ARTERIAL DISEASE
(PAD) involving below-the-knee (BTK) arteries remains a formidable clinical challenge, particularly in patients with CLTI. This subset of very frail patients frequently presents with heavily calcified, long-segment lesions that are difficult to cross and dilate. The presence of medial arterial calcification (MAC) contributes to vessel rigidity and poor response to conventional balloon angioplasty. Traditional endovascular techniques often fall short in delivering optimal results, contributing to high rates of restenosis, vessel recoil, and incomplete revascularisation. Thus, the risk of vessel dissection, perforation, and spasm is significantly higher, especially when using high-pressure balloon angioplasty or atherectomy devices. In addition, the fragile microcirculation in these distal territories places patients at increased risk of ischaemic complications during interventions. Attempts to forcibly expand calcified vessels often lead to vessel trauma rather than meaningful luminal gain leading to significant vessel recoil post-treatment, undermining procedural durability. The complexity of BTK interventions is further amplified by involvement of below-theankle (BTA) vessel disease that necessitates devices with extremely low profiles, excellent trackability, and the ability to modify calcified lesions without causing injury. In this scenario, IVL offers a new therapeutic strategy for managing calcified arterial disease by delivering pulsatile sonic pressure waves through a low-pressure balloon. This mechanism allows for controlled, circumferential calcium fracture without causing significant trauma to surrounding soft tissue. Early clinical experiences with IVL in CLTI patients suggest high technical success rates, excellent safety profiles, and minimal need for bailout stenting.1 These benefits are particularly evident in small-calibre, calcified vessels where traditional devices have underperformed.
The introduction of Shockwave E8 and Shockwave Javelin marks a paradigm shift in the management of calcified BTK and BTA disease as dedicated IVL platforms for the treatment of these vessels. The Shockwave E8 catheter offers a purpose-built solution for the small, highly calcified BTK arteries.
Designed with an integrated balloon length of 80mm, and delivering 400 pulses per treatment, the catheter facilitates treatment of longer lesions continually encountered in BTK intervention. The catheter is available in diameters ranging from 2.5–6mm enabling optimal sizing in all BTK vessel. The enhanced tip configuration of the Shockwave E8 and the 0.014” guidewire compatibility make the catheter an ideal tool for BTK and BTA work where crossing support and trackability are essential.
The newest catheter to arrive in Shockwave’s IVL peripheral portfolio is Shockwave Javelin; this catheter features a single distal emitter that creates up to 120 shockwave pulses. Each pulse creates a spherical energy field that extends beyond the tip of the catheter. This novel design delivers lithotripsy closer to calcium than the balloonbased platform, enhancing lesion crossing in complex, calcified infrapopliteal arteries and targeting lesions previously deemed uncrossable and in anatomies that were traditionally too challenging.
Freedom from clinically driven target lesion revascularisation (CD-TLR) was 90.7% and freedom from amputation/death was 86.4%. Also noticeable is the significant change in Rutherford classification in a cohort where 80% of patients had CLTI at baseline compared to only 28.3% at six months. The results demonstrate the durability and effectiveness of IVL in challenging BTK disease.1

Early outcomes with Shockwave IVL in BTK vessels are promising. Real-world case series have demonstrated high technical success rates, low complication profiles, and improved long-term patency. Operators report significant reductions in residual stenosis post-IVL treatment, with minimal use of bailout stents. In particular, the ability of IVL to treat concentric and eccentric calcification without relying on mechanical debulking reduces the need for adjunctive procedures.
Recently, six-month outcomes from the DISRUPT BTK II observational study—a prospective, non-randomised, multicentre, single-arm study evaluating the real-world safety and effectiveness of the Shockwave M5+ and S4 catheters for calcified, stenotic lesions in BTK arteries—have been presented. In total, 250 patients (57% Rutherford Category 5, 85% with moderate to severe calcification) with 268 lesions were treated with an IVL catheter, with two dissections (0.7%), no distal embolisation, and a 4.9% rate of stent implantation, supporting the safety of this technology.
Despite the challenging nature of the calcified lesions studied, the clinical outcomes from the FORWARD PAD IDE trial demonstrated that Shockwave Javelin shows an excellent safety and efficacy profile.2 In light of these data, both Shockwave E8 and Shockwave Javelin promise to provide modification of both intimal and, importantly, medial calcium, which is prevalent in challenging CLTI BTK and BTA disease. At the same time, they offer a synergy. Shockwave E8 acts as a workhorse tool to meet the needs of most BTK interventions, preparing the lesion bed in a way that optimises outcomes for definitive therapy, like angioplasty, drug-coated balloon (DCB), or stenting. Shockwave Javelin enables lesion crossing that previously would have required more aggressive and risky techniques such as subintimal tracking or re-entry devices. This streamlined strategy minimises procedure time, contrast usage, and overall risk—key considerations for patients with renal dysfunction or other comorbidities typical of CLTI patients. Despite the need for an increased evidence base to validate IVL technology below the knee, this dual-technique approach might become a new standard in infrapopliteal revascularisation. As devices like Shockwave E8 and Javelin continue to evolve, the future of BTK intervention appears increasingly precise, safe, and effective. The cases that follow will illustrate how the Shockwave E8 and Shockwave Javelin devices could change current practice and set a new standard for managing BTK and BTA occlusive disease.
References:
1. BTK II Study Investigators. 6-month outcomes from the BTK II IVL registry. Presented at: CX Symposium; 2025.
2. Corl J, Clair D, Shammas N, et al. First clinical use of a novel forward-shifted peripheral intravascular lithotripsy system: primary outcomes of the mini S/FORWARD PAD IDE Study. JACC 2024;84 (18) Suppl B.
Maria Antonella Ruffino is head of service of interventional radiology at Ente Ospedaliero Cantonale in Lugano, Switzerland and a paid Shockwave Medical consultant. The views expressed in this article are those of the physician and may not reflect the views of Shockwave Medical.
Maria Antonella Ruffino
Expanding the boundaries of calcified CLTI treatment below the knee with Shockwave E8
Bruno Migliara (Peschiera del Garda, Italy) views the Shockwave E8 as an essential part of his treatment algorithm for complex calcified lesions in patients with below-the-knee (BTK) chronic limb-threatening ischaemia (CLTI), here sharing a case report demonstrating his clinical experience with the device.
Clinical case
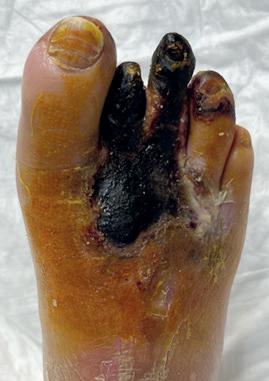
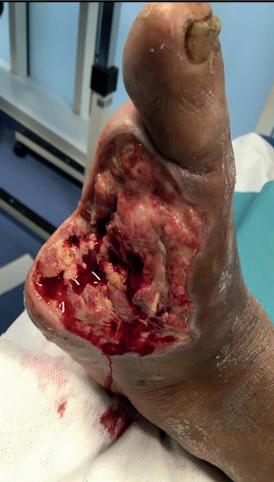
A 76-year-old male with arterial hypertension, diabetes, severe heart disease (previous coronary bypass and aortic valve stenosis) and chronic renal disease (Stage IV with eGFR=27) presented in the out-clinic department with an extensive dry gangrene of the second and third toes and distal gangrene of the fourth (Figure 1). Duplex scan showed diffuse calcification of the superficial femoral artery (SFA) and popliteal artery, but no haemodynamic stenosis. The scan also showed occlusion of the BTK arteries, with indirect flow at the
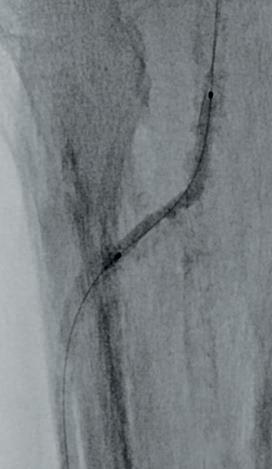

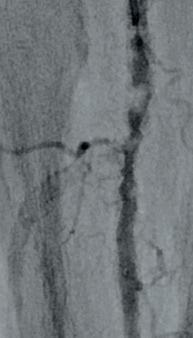
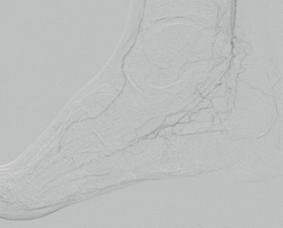
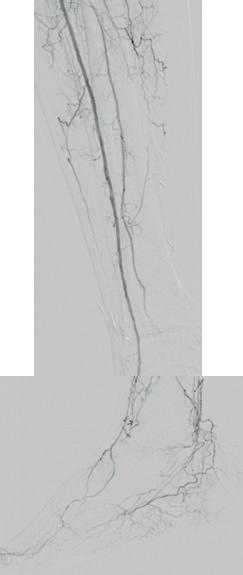
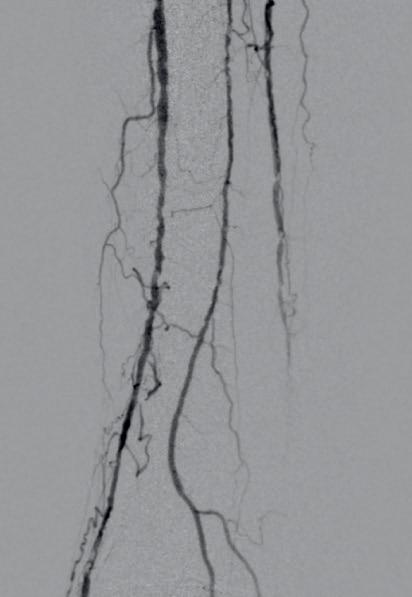
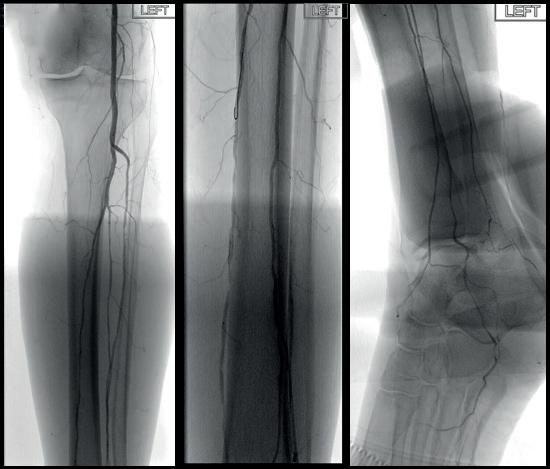
dorsalis pedis (pedal acceleration time [PAT]=223msec, stage 4). Transcutaneous oxygen pressure (TcPO2) was 15mmHg and the Wound, Ischemia, and foot Infection (WIfI) class was 3-3-2. Based on the clinical and noninvasive evaluation, we performed an angiographic study. This showed diffuse calcification, with short sub-occlusion of the distal popliteal artery (P3), total flush occlusion of the anterior tibial artery (ATA), total occlusion of the posterior tibial artery (PTA), and a patent but extensively diseased peroneal artery supplying the flow to the foot (Figures 2–4).
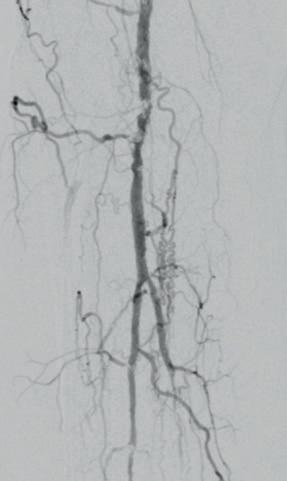
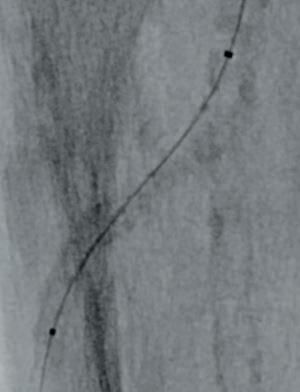
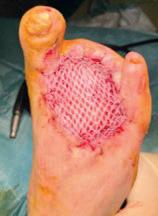
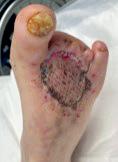
Figure 1. Wound at baseline
Figures 2–4. Baseline angiograms

Figures 5–7. Attempted treatment with Shockwave S4
Figures 8–10. Treatment with Shockwave E8
Figure 11. Final angiogram
Figure 12. Wound at around 35 days postrevascularisation
Figure 13. Wound at 65 days post-revascularisation
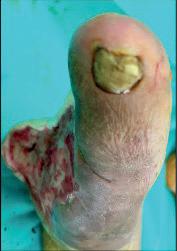
Figure 14. Healed wound at three months
Treatment
Based on the location of the lesions and on the angiography, we decided to try to cross and treat the ATA in order to obtain a direct flow into the forefoot, improving oxygen and nutrition in the dorsum, where the lesion was more extensive.
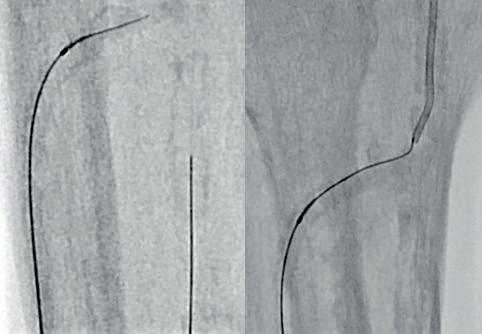
Due to the heavily calcified, flush occlusion of the ATA, it was not crossable with antegrade access. Instead, we performed a retrograde puncture of the distal ATA using the Astato 20 0.014 guidewire (Asahi Intecc Medical). The occlusion was crossed in retrograde fashion, regaining the true lumen in the distal popliteal artery (Figure 5).
After that, the guidewire was externalised



Bruno Migliara
Case report
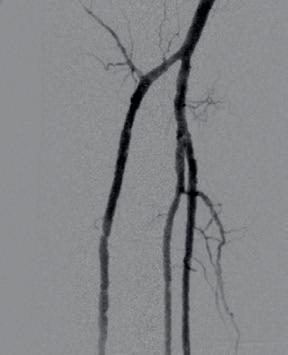
through the proximal sheath and a 2mm low-profile balloon was pulled through the occlusion utilising the BadForm technique (Figure 6). After pre-dilatation of the occlusion, we chose to treat with the 3mm Shockwave S4 catheter. The catheter was introduced and an attempt was made to cross the lesion and treat it in the ATA; however, the Shockwave S4 stopped in the proximal part of the ATA and would not advance across the lesion, highlighting a limitation of the Shockwave S4 catheter (Figure 7). At this time, the new 3.5mm Shockwave E8 catheter had entered the market. We decided to proceed with the Shockwave E8, as this case would provide a good test following the inability to cross with Shockwave S4. As you can see, Shockwave E8 was able to cross the length of occluded ATA from the origin to the distal third, treating a long and heavily calcified occlusion with the 400 pulses available on the new device (Figures 8–10). The final angiography showed complete recanalisation of the ATA, with significant improvement of the flow into the foot (Figure 11). At the end of the procedure, PAT was 84msec. After removal of the necrotic area, reconstruction was performed using autologous skin and complete healing was
The Shockwave E8 now provides a first-line tool to optimise the treatment of complex calcium in an extremely safe and effective way.”
achieved in around three months (Figures 12–14).
Conclusion
In CLTI patients with heavily calcified BTK arteries, after crossing the lesion, it’s mandatory to perform an effective treatment to modify not only the superficial but also the deep calcium. The Shockwave E8’s mechanism of action enables calcium to be modified while preserving soft tissue, improving compliance of the artery wall, and attaining luminal gain with low barotrauma and minimal risk of dissection.
This case illustrated the significant
Shockwave E8: Redefining below-the-knee and below-the-ankle calcium management in CLTI
The Shockwave E8 catheter “significantly broadens the therapeutic landscape” for patients with diffuse below-the-knee (BTK) and below-the-ankle (BTA) disease. This is according to Marta Lobato and August Ysa (Barakaldo, Spain), who here present a case in which the Shockwave E8 played a key role in treatment success.
A 76-YEAR-OLD MALE PRESENTED to our department with signs of chronic limbthreatening ischaemia (CLTI) and a rapidly worsening diabetic foot infection following prior debridement at another centre. He was a former smoker with a history of hypertension, diabetes mellitus, dyslipidaemia and chronic kidney disease. He was under regular treatment with metformin, enalapril, acetylsalicylic acid (ASA) and atorvastatin.
Initial presentation and clinical evaluation
The patient was referred urgently due to
the appearance of an infected forefoot ulcer with signs of local sepsis. He was febrile, with a white blood cell count of 15,000cells/ mm³, and had no palpable pedal pulses. The patient also had acute renal failure, further complicating his clinical status. The ulcer was extensive, with surrounding cellulitis and necrotic tissue. A bedside ankle–brachial index (ABI) was non-compressible, likely due to arterial calcification, and transcutaneous oxygen pressure (TcPO2) on the dorsum of the foot was critically low at 12mmHg.
The lesion was classified using the Wound, Ischemia, and foot Infection (WIfI) system
benefits of the Shockwave E8 catheter versus the previous generation of tibial catheter: Shockwave S4. Indeed, the new generation of this catheter has significantly better trackability due to the improved tip configuration and hydrophilic coating, allowing it to cross complex lesions. The Shockwave E8 also enables treatment of longer lesions with an integrated balloon length of 80mm and 400 available pulses versus the Shockwave S4, with a 40mm integrated balloon length and 160 pulses, which reduced the device’s ability to treat complex BTK disease. The Shockwave E8 now provides a first-line tool to optimise the treatment of complex calcium in an extremely safe and effective way.
Bruno Migliara is chief of the Vascular and Endovascular Unit at Pederzoli Hospital in Peschiera del Garda, Italy and a paid Shockwave Medical consultant. The views expressed in this article are those of the physician and may not reflect the views of Shockwave Medical.
Scan code to link to corresponding video interview


as stage 3-3-3, reflecting high risk of limb loss. An urgent surgical debridement was performed, followed by initiation of broadspectrum intravenous antibiotics.
Imaging and decision-making
Subsequent diagnostic angiography revealed severe multilevel calcific arterial disease, with diffuse narrowing and dense calcification throughout the tibial vessels. The anterior tibial artery (ATA) was patent in its proximal and mid segments with significant lesions, but showed distal occlusion with no reconstitution at the level of the foot. The posterior tibial artery (PTA) exhibited multiple stenoses with heavy calcification, distal third occlusion, and reconstitution at the level of the plantar artery (Figures 1–3). Based on these angiographic findings, a decision was made to proceed with an endovascular-first strategy, with the main
Marta Lobato
August Ysa
goal of re-establishing direct in-line flow to the ischaemic wound site via the PTA.
Endovascular procedure: Leveraging Shockwave E8
An antegrade femoral approach under ultrasound guidance was perfromed. A long sheath was advanced and positioned in the popliteal artery to provide stable support. After crossing the PTA occlusion with a 0.014” guidewire, the decision was made to use the Shockwave E8 catheter as the primary tool for vessel preparation. This decision was based on several key factors. The lesion’s morphology showed extensive concentric calcification, which limited the effectiveness of standard angioplasty and carried a high risk of dissection or recoil. The Shockwave E8 catheter’s enhanced pushability and trackability made it ideal for navigating the tortuous, narrow tibial anatomy. Furthermore, the longer integrated balloon of the Shockwave E8 allowed the entire diseased segment to be treated with a single device, reducing the need for multiple exchanges and improving procedural efficiency.
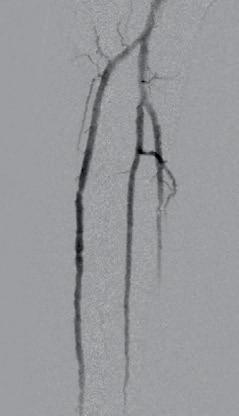

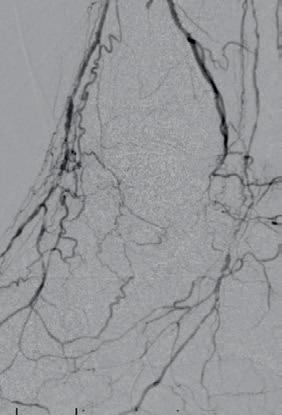

Figures 1–3. Baseline angiograms
Figures 4–5. Treatment with Shockwave E8
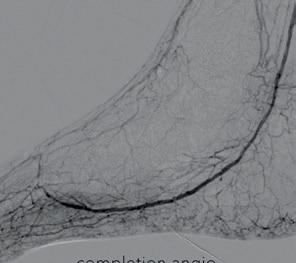
Figures 6–8. Completion angiograms


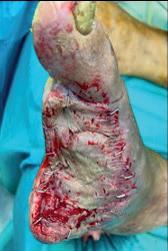
Figures 9–12. Clinical evolution of wound at seven, 14, 15 and 21 days
The Shockwave E8 catheter was advanced across the lesion and activated, delivering 400 pulses at low inflation pressures of 2–4atm. This achieved improved compliance through modification of the calcified plaque and enabled subsequent balloon expansion without complications. The procedure was completed with a non-compliant balloon angioplasty to optimise luminal gain (Figures 4–5).
Results and postoperative course
Post-Shockwave E8 angiography showed excellent luminal gain, with no flowlimiting dissections or residual stenosis requiring further intervention. The final result demonstrated significantly increased flow through the PTA into the plantar arch, confirming technical success (Figures 6–8).
Within hours of the procedure, the posterior tibial pulse returned, and follow-up TcPO2 increased dramatically to 61mmHg, correlating with successful reperfusion. The patient continued antibiotic therapy (ciprofloxacin and clindamycin) and underwent vacuum-assisted closure (VAC) therapy to promote wound healing.
The Shockwave E8 catheter’s enhanced pushability and trackability made it ideal for navigating the tortuous, narrow tibial anatomy.”
He was discharged 10 days later, under close home-care monitoring. A split-thickness skin graft was performed in the following weeks, and progressive wound closure was observed. By the end of follow-up, the wound had completely healed, and the patient had recovered full ambulatory function (Figures 9–12).
Discussion
This case highlights the increasing role of Shockwave intravascular lithotripsy (IVL) as a frontline tool in the treatment of complex, calcified BTK disease, particularly in patients with CLTI and limited revascularisation options. Calcium modification is essential
in these scenarios, not only to achieve immediate luminal gain but also to minimise trauma and avoid stenting, particularly in the distal BTK and BTA segments, where vessels are small, mobile, and at high risk of fracture or occlusion.
While the Shockwave S4 catheter has played a central role for IVL in small BTK vessels, in our practice the introduction of the Shockwave E8 offers several practical and clinical advantages. The device has enhanced pushability and flexibility, allowing it to cross tight, calcified, and tortuous segments more efficiently, particularly in distal tibial and foot territories. Its extended treatment segment permits operators to treat long, calcified lesions with a single device, improving efficiency, reducing procedure time, and minimising the number of catheter exchanges. Treating a full-length tibial lesion with one catheter also reduces the overall procedural cost and simplifies logistics. In addition, the uniform energy delivery and low-pressure inflation reduce barotrauma, improving safety and reducing postprocedural complications.
In conclusion, the Shockwave E8 catheter significantly broadens the therapeutic landscape for patients with diffuse BTK and BTA disease by enabling safe, effective, and comprehensive treatment of long, calcified arterial segments. In the setting of CLTI, preserving native arterial anatomy and maintaining future options is crucial. The Shockwave E8 represents a true paradigm shift in the management of infrapopliteal calcification and represents a new valuable tool in BTK and BTA endovascular care.
Marta Lobato and August Ysa are both vascular and endovascular surgeons in the BTK-BTA Unit at Hospital de Cruces in Barakaldo, Spain and paid Shockwave Medical consultants
The views expressed in this article are those of the physicians and may not reflect the views of Shockwave Medical.
Case report
Shockwave Javelin: A new tool to overcome challenging tibial and below-the-ankle lesions
The Shockwave Javelin peripheral intravascular lithotripsy (IVL) catheter “bridges a critical gap” in the management of chronic limb-threatening ischaemia (CLTI) in patients with complex multilevel disease. This is according to Ashish Patel and Narayanan Thulasidasan (London, UK), who here outline a case where the Shockwave Javelin offered a solution for “device-uncrossable” calcium.
OUR EARLY EXPERIENCE WITH THE Shockwave Javelin peripheral IVL catheter suggests that, when applied in appropriately selected patients, it can provide a safe and reliable means of bridging the gap between wire crossing and vessel preparation, restoring straight-line flow to the foot and broadening treatment options for patients with multilevel disease and advanced tissue loss.
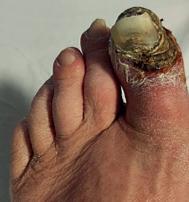
A 71-year-old woman was referred with a three-month history of progressive discolouration of the left hallux, which had become globally dusky with dry gangrene affecting the distal phalanx (Rutherford Category 5; Figure 1). She also had severe nocturnal rest pain that had been disrupting her sleep for several months. She continued to smoke and had a history of hypertension and hyperlipidaemia, but no other significant comorbidities. Examination revealed the left foot to be cooler than the right, with a weak popliteal pulse and absent pedal pulses. Doppler assessment demonstrated monophasic dorsalis pedis and posterior tibial signals, with an ankle–brachial pressure index (ABPI) of 0.3. Arterial duplex ultrasonography showed significant stenotic disease within the mid-segment of the superficial femoral artery (SFA) and multilevel tibial disease involving both her posterior and anterior tibial arteries. During our multidisciplinary team (MDT) discussion, it was noted that both her posterior tibial and lateral plantar arteries contained multiple focal calcific steno-occlusive lesions. The extent and distribution of this calcification was anticipated to present a considerable technical challenge, particularly in tracking catheters or advancing angioplasty balloons into the target vessels beyond these lesions. After reviewing the imaging and clinical context, the consensus opinion of the MDT was that an endovascular-first approach would be the most appropriate treatment strategy, proceeding to amputation of the


left hallux during the same procedure if the planned recanalisation was successful. It was also agreed that to maximise the chance of durable healing post-amputation, revascularisation should establish direct ‘straight-line’ flow into the foot, with specific attention to reconstituting the lateral plantar artery and the plantar arch.
Given the severity of her rest pain and the anticipated technical challenges, the procedure was carried out under general anaesthesia for both patient comfort and procedural control. Under ultrasound guidance, antegrade left common femoral artery (CFA) access was obtained using a micropuncture kit and a 6Fr sheath then advanced into the proximal SFA. Heparinisation was commenced with a target activated clotting time >200 seconds. Intra-arterial digital subtraction angiography confirmed the duplex findings, with mid-SFA lesions in addition to multilevel occlusive lesions in both the posterior tibial and anterior tibial arteries (Figure 2). Angiographic runs of the foot revealed a heavily diseased lateral plantar artery (the main target vessel in the foot for this case) and an incomplete foot arch (Figure 2). Intravascular ultrasound (IVUS; Visions PV .014P RX, Philips) was used to accurately define the extent and morphology of the SFA lesions, as well as to obtain precise vessel diameter measurements. The SFA was then treated with 5mm plain
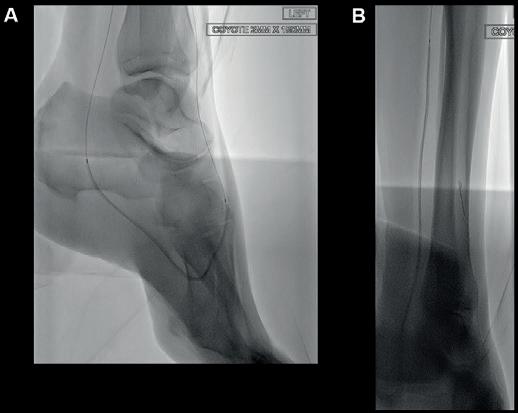
balloon angioplasty (Sterling, Boston Scientific), followed by a 5x200mm drugcoated balloon (Ranger, Boston Scientific). Attention was then turned to the posterior tibial artery, which was crossed using an 0.014” Asahi Gladius MG ES wire (Asahi Intecc) supported by an angled 0.014” support catheter (CXI, Cook Medical). Given the difficulty in tracking the CXI through the distal posterior tibial vessels and the presence of the focal lateral plantar artery lesions seen on angiography, we decided that the Shockwave Javelin would be ideally suited for calcium modification in this scenario.
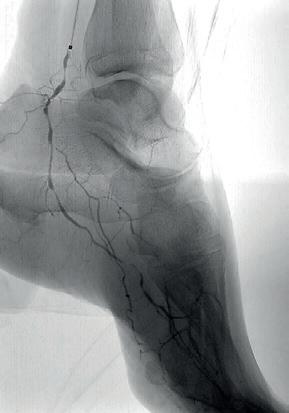
The Shockwave Javelin tracked without difficulty to the mid-posterior tibial artery, where three treatment cycles (30 pulses in total) were delivered to the mid and distal segments (Figure 3). This calcium modification allowed the catheter to easily pass into the ankle. At this point, a selective angiogram of the pedal arch was performed through the Shockwave Javelin catheter to delineate the anatomy of the foot vasculature in greater detail (Figure 4). Using a combination of 0.014” Asahi Halberd and Gladius MG ES wires, the lateral plantar artery and plantar arch were successfully crossed. Seven further cycles of the Shockwave Javelin (70 pulses) were used to treat the lateral plantar artery, plantar arch and the dorsalis pedis artery (Figure 5). Following this, both the IVUS catheter (Figure 6) and a low-profile 0.014” balloon (Coyote, Boston Scientific) could now be advanced without resistance into the foot vessels, reflecting the marked improvement in vessel compliance after successful calcium modification with the Shockwave Javelin. The plantar arch and lateral plantar artery were treated with a 2x150mm angioplasty balloon (Figure 7A). Following sizing with IVUS, the posterior tibial artery was treated (Figure 7B) with a 2.5x220mm balloon (Coyote, Boston Scientific). Notably, in the distal posterior tibial artery and plantar vessels, balloons expanded to their nominal diameter at just 6atm, highlighting the significant compliance
The successful outcome underscores the unique value of the Shockwave Javelin in CLTI.”
Ashish Patel Narayanan Thulasidasan


gain post-IVL. The anterior tibial artery was then crossed and treated using the same balloon at 12atm.
Completion angiography demonstrated significant luminal gain in the posterior tibial, lateral plantar, plantar arch and dorsalis pedis vessels, with brisk preferential flow into the posterior tibial and lateral plantar vessels and a widely patent pedal arch (Figure 8). There was some vasospasm in the proximal anterior tibial artery. IVUS also confirmed good luminal gain and the restoration of visible pulsatility within the lateral plantar artery, which had not been present prior to treatment with the Shockwave Javelin.
A closure device (MynxControl, Cordis) was used for closure of the CFA access and check ultrasonography confirmed that the CFA remained widely patent. The patient then underwent ray amputation of her left hallux during the same procedure, where it was noted that there was pulsatile bleeding of the wound bed. The amputation site was left open to heal by secondary intention (Figure 9) and she had an uneventful postoperative recovery. The patient was reviewed regularly in our dedicated vascular foot clinic following discharge, with dressing changes performed by community nursing teams under close communication with our service. At her two-week follow-up, the amputation site was healthy, with evidence of granulation tissue and no signs of infection. She also reported complete resolution of her rest pain. At eight weeks, the amputation site had fully


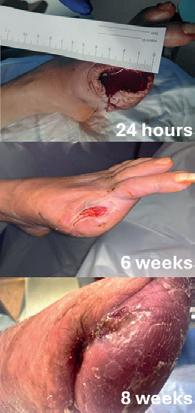
healed (Figure 9) and the patient’s ABPI had improved from a preoperative value of 0.3 to 0.8. She had resumed independent ambulation and was engaging with smoking cessation services.
This case illustrates the unique capability of the Shockwave Javelin to address one of the most persistent technical barriers in complex tibial and pedal revascularisation— the ‘device-uncrossable’ calcified lesion. In this patient, both the posterior tibial and lateral plantar arteries contained multifocal heavily calcified steno-occlusive disease that was expected to prevent the passage of even low-profile angioplasty balloons. The successful outcome underscores the unique value of the Shockwave Javelin in CLTI, particularly in the setting of diffuse, heavily calcified tibial and below-the-ankle (BTA) disease. By safely and predictably modifying calcific plaque, the Shockwave Javelin enables delivery of definitive therapies, such as balloon angioplasty, to distal target vessels that are often otherwise inaccessible, restoring straight-line flow to the foot and reestablishing an intact pedal arch. In this case, achieving luminal gain in the lateral plantar artery and plantar arch was essential for wound healing following hallux amputation and would have been extremely challenging without calcium modification.
A key procedural finding was the marked improvement in vessel compliance following IVL, demonstrated by low-pressure balloon expansion to nominal diameter in the distal
tibial and pedal arteries and the absence of recoil on IVUS. These changes were achieved without flow-limiting dissection, perforation, or distal embolisation, complications that remain a concern during vessel preparation in this territory. As an enabling technology for effective endovascular treatment of tibial and BTA vessels (territories where few reliable options have previously existed) the Shockwave Javelin bridges a critical gap in the management of CLTI in patients with complex multilevel disease.
Ashish Patel is a consultant vascular and endovascular surgeon at Guy’s and St Thomas’ NHS Foundation Trust and a reader in vascular surgery and sciences at King’s College London in London, UK. He is a paid Shockwave Medical consultant
Narayanan Thulasidasan is a consultant interventional radiologist at Guy’s and St Thomas’ NHS Foundation Trust and a paid Shockwave Medical consultant
The views expressed in this article are those of the physicians and may not reflect the views of Shockwave Medical.
Figure 1. Clinical presentation
Figure 2. Baseline angiograms
Figure 3: Treatment with Shockwave Javelin
Figure 4: Foot angiogram
Figure 5: Treatment with Shockwave Javelin
Figure 6: Intraoperative IVUS
Figure 7. Treatment with balloon angioplasty
Figure 8: Completion angiograms
Figure 9: Wound healing at 24 hours, six weeks, and eight weeks