DIRECT STROKE TRIAGE: Recent data “affirm” benefits page 7

Michihiro Tanaka page 16

DIRECT STROKE TRIAGE: Recent data “affirm” benefits page 7

Michihiro Tanaka page 16

www.neuronewsinternational.com
Rebecca Middleton page 18

Three recently presented clinical trials have demonstrated positive outcomes associated with middle meningeal artery (MMA) embolisation in chronic subdural haematoma (SDH) patients, representing a “landmark moment” and “major shift” in the treatment of this condition.
Findings from the EMBOLISE, MAGIC-MT and STEM trials—all of which were disclosed for the first time at the 2024 International Stroke Conference (ISC; 7–9 February, Phoenix, USA)—evaluated the addition of minimally invasive MMA embolisation to standard medical care, as compared to standard care alone, and found that the procedure was linked to reduced failure rates without an increased occurrence of serious complications.
“This is a landmark moment in the treatment of SDHs,” said ISC 2024 chair Tudor Jovin (Cooper University Health Care, Camden, USA). “This is a moment to which I draw parallels with the thrombectomy trials in 2015. It’s a similar situation—this technique has been in practice at certain centres, inconsistently—but, now, we have level-one evidence from not one but three trials of unequivocal benefit, both in patients who require surgery and those receiving medical management only.”
The EMBOLISE randomised controlled trial (RCT) was the
first of these studies to be delivered at ISC, with leading investigators Jason Davies (State University of New York, Buffalo, USA) and Jared Knopman (Weill Cornell Medical College, New York, USA) taking to the podium to outline first-time data. The trial enrolled 400 adults—all of whom were about to undergo surgery for a subacute or chronic SDH, and considered able to care for themselves and likely to survive for at least one year—across 39 US hospitals. Patients were randomly assigned to receive either surgery alone, or surgery plus MMA embolisation using the Onyx liquid embolic system (Medtronic), to help reduce SDH progression or recurrence.
EMBOLISE’s primary endpoint was the rate of SDH recurrence requiring surgical drainage within 90 days, which was observed in 4.1% of patients who underwent surgery plus MMA embolisation, compared to 11.3% with surgery alone— a “highly significant” difference. In addition, at 90 days after surgery, increasing disability and neurological dysfunction was found to be statistically comparable between these two groups, occurring in 11.9% of surgery-plus-embolisation patients and 9.8% of surgery-only patients. As per a 30-day safety endpoint in the trial, serious adverse events attributed to MMA
Continued on page 2
Stimwave CEO charged with fraud over ‘fake, non-functional’ device parts page 25
A “HISTORICAL MILESTONE” for stroke patients in Brazil was achieved on 24 November 2023, with the country’s Ministry of Health (MOH) publishing Order 1.996/2023 to add mechanical thrombectomy to the list of procedures covered by the Brazilian public health system. According to the World Stroke Organization (WSO), “this achievement crowns the efforts of a tireless network of stroke neurologists and neurointerventionists”.
Much of the basis for the approval came from the RESILIENT trial, which was initiated by Raul Nogueira (University of Pittsburgh Medical Center, Pittsburgh, USA) and Sheila Martins (Federal University of Rio Grande do Sul, Porto Alegre, Brazil) more than a decade ago to evaluate the procedure in large vessel occlusion (LVO) acute ischaemic stroke.
Having essentially been “demanded” by the Brazilian MOH, RESILIENT ultimately replicated the findings of the five major stroke thrombectomy trials from 2015, producing results “overwhelmingly favouring” the procedure versus the existing standard of care in lower- and middle-income countries. Subsequent analyses established the procedure’s costeffectiveness as well.
Presentation and publication of these data were followed by a decision from the MOH Secretary of Science, Technology, Innovation and Strategic Health Supplies to make the intervention available up to 24 hours from symptom onset in LVO acute ischaemic stroke patients.

embolisation trials represent “major shift” in subdural haematoma treatment
Continued from page 1
embolisation occurred in 2% of patients who underwent the procedure, while 90-day stroke and neurological death rates were also similar between groups.
Based on these findings, the investigators concluded their ISC presentation by stating that MMA embolisation “should be considered” in patients presenting with symptomatic subacute/chronic SDHs requiring surgery.
“The EMBOLISE trial showed that there was a nearly three-fold reduction in reoperation for patients that were treated with surgery plus embolisation,” said Davies. “Fewer trips to the operating room mean less potential for pain, complications and expense, and better recovery, for the patient.”
The second MMA embolisation RCT featured at ISC, also assessing the Onyx system, was presented by Ying Mao (Huashan Hospital/Fudan University, Shanghai, China), who detailed 90-day outcomes from the prospective MAGIC-MT study. A total of 722 symptomatic, non-acute SDH patients from 31 centres across China—randomised to receive either standard care (surgical drainage and/or non-surgical management) plus embolisation, or standard care alone— were ultimately included in the trial’s analysis.
As per MAGIC-MT’s primary endpoint, 7.2% of patients in the MMA embolisation group died, or experienced symptomatic SDH recurrence or progression, within 90 days post-randomisation, comparing favourably to 12.2% in the control group. All-cause mortality at 90 days was also lower in the embolisation group, with just two deaths occurring—although the between-group difference was not deemed statistically significant (0.6% vs. 2.2%). In addition, while overall equipoise between MMA embolisation and standard care was indicated across a number of clinical and radiological outcome measures in the trial, a statistically significant difference favouring the former was found regarding the rate of serious adverse events (6.7% vs. 11.6%).
The third and final RCT presented at ISC, the STEM study, evaluated the Squid liquid embolic system (Balt), and was delivered by co-principal investigator Adam Arthur (University of Tennessee Health Science Center/SemmesMurphey Clinic, Memphis, USA). This pivotal trial took place across 25 US sites and a handful of European centres. It saw a total of 310 patients allocated to either surgical or non-surgical management for their chronic SDH, with patients in each group then being randomised to MMA embolisation plus their allocated management strategy, or their allocated management strategy only.
As per STEM’s primary effectiveness endpoint, there were three distinct modes of failure in the trial: residual or reaccumulated SDH (≥10mm) at 180 days postintervention; reoperation after index procedure or surgical rescue within 180 days of randomisation; and a new, major disabling stroke, myocardial infarction or death from any
neurological cause within 180 days of randomisation. Preliminary results outlined by Arthur indicated a failure rate of 15.2% with MMA embolisation compared to 39.2% without—data ultimately associated with an odds ratio of 3.6 favouring the intervention. Further subgroup analyses also revealed that this discrepancy was more pronounced when specifically comparing MMA embolisation plus nonsurgical management against non-surgical management. The trial’s 30-day safety endpoints found similar rates of allcause mortality (2.7% vs. 3.1%) and major disabling stroke (0% vs. 0.6%) between its treatment and control arms, respectively, with none of the deaths in the treatment arm being attributed to MMA embolisation or Squid itself.
“Chronic SDH is an exceedingly common problem, and these findings will be relevant to a large population of patients,” said David Fiorella (Stony Brook Cerebrovascular Center, Stony Brook, USA), co-principal investigator for STEM. “The presented data indicate that this novel, adjunctive procedure represents an important advance, as it reduces the failure rates of standard management strategies with a high margin of procedural safety.”
In an official statement following these three presentations, the Society of Neurolnterventional Surgery (SNIS) acknowledged MMA embolisation as a beneficial adjunctive treatment for chronic SDH, describing the procedure as a “major shift” in the management of this disease. The SNIS’ statement noted that the magnitude of the effect of adjunctive MMA embolisation in MAGICMT and STEM’s non-surgical arms indicate many patients “may be able to avoid open surgery completely”, while EMBOLISE suggests adjunctive MMA embolisation can significantly reduce reoperation rates for patients who do require surgical drainage.
This is a moment to which I draw parallels with the thrombectomy trials in 2015.”
Tudor Jovin
“I believe there is going to be enough of a body of evidence to justify a complete change in practice, having MMA embolisation becoming the standard of care for this condition,” Jovin concluded. “And, yes, it’s going to be a big boost for the endovascular field in general, because it will increase the requirement for a higher number of practitioners and the infrastructure to match.”
“In many ways, these data are really just the beginning— there will be a tremendous amount of additional insight coming very soon,” Fiorella added, alluding to the recently completed MEMBRANE RCT evaluating the Trufill n-BCA liquid embolic system (Cerenovus/J&J), as well as a planned, combined analysis of all four of these major MMA embolisation studies. Additional insights are also expected, including comparisons of embolisation versus non-surgical management in EMBOLISE, and the presentation of further, clinical endpoints from STEM.
n ZERO-DEGREE HEAD POSITIONING LINKED TO SHORT-TERM CLINICAL IMPROVEMENT:

Findings from the randomised ZODIAC trial have indicated the potential benefits associated with zerodegree head positioning, rather than a more traditional 30-degree angle, prior to a mechanical thrombectomy for ischaemic stroke. These data were delivered by Anne Alexandrov (Memphis, USA) at the International Stroke Conference (ISC 2024; 7–9 February, Phoenix, USA).
For more on this story go to page 5.
n NO BENEFIT TO ADDING BLOOD THINNERS TO STANDARD ISCHAEMIC STROKE CARE:

The MOST trial, which was halted early, has found that the addition of blood thinners to clotbusting medications does not produce improved outcomes in ischaemic stroke patients, as per a late-breaking presentation from Opeolu Adeoye (St Louis, USA) and Andrew Barreto (Houston, USA) at ISC 2024.
For more on this story go to page 9.
n DUAL-STENT RETRIEVER STRATEGY DEMONSTRATES PROMISE IN MECHANICAL THROMBECTOMY:
The potential benefits of performing a mechanical thrombectomy procedure using two stent-retriever devices simultaneously have been elucidated by the recently presented TWIN2WIN trial. Marc Ribo (Barcelona, Spain) disclosed these results at ISC 2024, reporting improved recanalisation and no major safety concerns—as compared to the more established approach involving a single stent retriever.

For more on this story go to page 13.
Editor-in-chief: Prof Philip M Meyers | Publisher: Stephen Greenhalgh
Editor-in-chief: Prof
Content director: Urmila Kerslake | Head of Global News: Sean Langer
Content Kerslake Global commercial director: Sean Langer
Editor: Jamie Bell jamie@bibamedical.com | Editorial contribution: Jocelyn Hudson and Anthony Strzalek
Editor: Jamie Bell jamie@bibamedical.com Editorial contribution:
Design: Terry Hawes, Wes Mitchell and David Reekie
Design: Terry
Subscribe here
Subscribe here
Advertising: Michael Broughton michael@bibamedical.com
Advertising: michael@bibamedical.com
Subscriptions: subscriptions@bibamedical.com
Subscriptions: subscriptions@bibamedical.com
Published by: BIBA News, which is a subsidiary of BIBA Medical Ltd
Published News, a subsidiary
BIBA Medical, Europe, 526 Fulham Road, Fulham, London, SW6 5NR, United Kingdom Tel: +44 (0) 20 7736 8788
BIBA Europe, Fulham Road, SW6 United Kingdom Tel: 7736
BIBA Medical, North America, 155 North Wacker Drive, Suite 4250, Chicago, IL 60606, United States Tel: +1 708-770-7323
BIBA North America, 155 North Wacker Drive, States +1 708-770-7323
Printed by: Buxton Press. Reprint requests and all correspondence regarding the newspaper should be addressed to the editor at the United Kingdom address. © BIBA Medical Ltd, 2024. All rights reserved.
Printed regarding newspaper should to the at the address. © Medical 2024.
If you have comments on this issue or suggestions for upcoming editions write to jamie@bibamedical.com
If you have comments on this issue or suggestions for upcoming editions write to jamie@bibamedical.com
neuronews linkedin.com/company/neuronews/ @NN_publishing
neuronews linkedin.com/company/neuronews/
• Debate:
Carotid artery stenting is the treatment of choice for underlying ICA stenosis after mechanical thrombectomy
Wednesday, 24 April
• Podium First:
First release of PERFORMANCE II data in Europe
Wednesday, 24 April
• New Carotid Workshop: Including open and endovascular hands-on training
Thursday, 25 April




Conrad Yiu, a pioneer and visionary in interventional ischaemic stroke therapy, passed away in the final weeks of 2023, succumbing to the very disease for which he so fervently sought to enable and advance treatment. In a guest piece for NeuroNews, Chloe Brown pays tribute to her long-time colleague and friend, and highlights the fact that Yiu’s death is a saddening yet poignant reminder that “we are all vulnerable” to ischaemic stroke.
In the realm of interventional ischaemic stroke therapy, the global medical community mourns the loss of Conrad Yiu—a pioneering force whose indelible contributions have left an enduring mark on the landscape of neurovascular care. Conrad passed away recently, succumbing to an ischaemic stroke; the very disease he dedicated his life to combatting. His departure leaves a void that resonates across continents and disciplines.
Born with a brilliant mind and an incredible vision, Conrad’s impact on the medical device industry— particularly in the field of endovascular stroke therapy—was nothing short of revolutionary. His involvement in the treatment of acute ischaemic stroke began at Ev3, and he later became a vital part of Covidien and Medtronic before moving on to Viz.ai, where he emerged as a key figure in shaping the trajectory of thrombectomy and its transformative role in ischaemic stroke treatment.
Conrad first witnessed the use of the Solitaire stent-retriever device in the treatment of acute stroke in Hôpitaux Universitaires de Genève (HUG; Geneva, Switzerland) with Vitor Mendes Pereira (University of Toronto, Toronto, Canada). Captivated by the potential of what lay ahead,
our meetings with Jan Gralla and Stacey Pugh in designing the STAR trial, and how it influenced my whole career after. We will miss his passion, enthusiasm, wisdom, and his smile! Rest in peace, Conrad—we will keep fighting against this terrible disease in your honour.”
However, Conrad’s impact extended far beyond the confines of clinical trials. His relentless pursuit of improving stroke treatment globally was characterised by his involvement in numerous projects, studies and initiatives aimed at reaching underserved populations. His tireless efforts exemplified his commitment to the mantra that “no patient should be left behind” in the fight against this debilitating disease. It is both ironic, and overwhelmingly sad, that this mantra unfortunately did not ultimately extend to his own life.
As a mentor, Conrad had a profound impact on my own career. He brought me to the Ev3 headquarters in Paris in 2011 to develop stroke educational and referral programmes with Vitor Mendes Pereira in Geneva; Tommy Andersson in Stockholm, Sweden; and Jan Gralla in Bern, Switzerland. Conrad inspired creativity, encouraged big thinking, and helped transform ideas into reality.
“Conrad has been an inspiring and energetic person, and a driving force especially to get endovascular stroke treatment established,” said Jan Gralla (Inselspital, University of Bern, Switzerland). “He has left a major impact on neurovascular treatment, and the way of cooperation between physicians and industry. Our community will miss him.”
and David Golan in the development of their diagnostic platform for acute stroke, which is intended to automate the diagnosis of this condition, providing support for less experienced physicians and enabling faster treatment for patients.
Chris Mansi, co-founder and chief executive officer of Viz.ai, said: “Conrad joined Viz.ai in January 2018, helping the company pioneer the first AI US FDA [Food and Drug Administration] approval via the de-novo pathway, aimed at improving outcomes in stroke. He brought his relentless passion to Silicon Valley, ensuring that what was at the time a nascent technology could be directed to improve patient outcomes in a disease he cared passionately about.”
Manoj Ramachandran, Viz.ai co-founder, added: “Witnessing first hand Conrad’s ability to build and nurture transformational, collaborative relationships with the people he came into contact with was simultaneously humbling and inspirational. He was a warm, kind-hearted, and considerate friend and colleague who will be sorely missed.”
Conrad’s infectious smile and boundless energy were emblematic of his enthusiasm for the field. He played a pivotal role in demystifying mechanical thrombectomy, ultimately helping to change the landscape of ischaemic stroke treatment. Colleagues fondly remember him as a beautiful person who was always smiling, and an optimist who leaves an enduring legacy in the neurovascular community.
he took swift action in the following weeks. Behind the scenes, Conrad worked diligently, connecting key individuals and orchestrating the collaboration that would propel the field forward at a remarkable pace. This visionary initiative marked the genesis of the STAR trial—a groundbreaking endeavour that would underpin the future of ischaemic stroke trials.
Colleagues and friends remember Conrad as a force multiplier; a visionary with an infectious laugh that could light up any room. His commitment to pushing boundaries and achieving tangible results was evident in his advocacy for groundbreaking trials like SWIFT Prime, ESCAPE, EXTEND-IA, and REVASCAT, all of which played a pivotal role in altering the standard of care for ischaemic stroke patients, ushering in a new era of therapeutic possibilities.
“Conrad has helped us shape the field of endovascular stroke therapy,” remarked Vitor Mendes Pereira. “It was his vision that connected many of us in the early days of mechanical thrombectomy and resulted in the early trials, stroke networks, and groundbreaking papers. He was the driving force behind many of the great achievements that built the foundation of our field today. I still remember
Born with a brilliant mind and an incredible vision, Conrad’s impact on the medical device industry— particularly in the field of endovascular stroke therapy— was nothing short of revolutionary.”
Conrad’s pursuit of enabling treatment for acute ischaemic stroke extended beyond treatment with devices. He knew that a big gap remained in the ability for patients to be quickly diagnosed with a treatable large vessel occlusion. Seeing the potential benefits artificial intelligence (AI) could bring to help tackle this problem, he joined Viz.ai in 2018 to aid Chris Mansi, Manoj Ramachandran
Brett Wall, executive vice president and president of Medtronic’s Neuroscience portfolio, wrote on LinkedIn: “Conrad made the world a better place for so many and it is important that his significant contributions are not lost in this great story of a revolution in therapy.”
Conrad Yiu’s foundational contributions to ischaemic stroke treatment have undoubtedly made the world a better place. His passion, drive and unwavering belief in building a greater good for all will continue to inspire generations of medical professionals. The neurovascular community has lost a giant, but Conrad’s legacy will endure, reminding us all to continue the fight against this devastating disease. In the words of Conrad himself, “never assume anything”.

This guest article was sourced and written by Chloe Brown, who is currently the chief executive officer of Ceroflo, and holds senior roles at Synchron, Tegus Medical and Port Ceres Consulting. Brown hopes that the death of Conrad Yiu—and this tribute to him—will remind the acute stroke community to keep fighting for better care and treatment.
Laying large vessel occlusion (LVO) ischaemic stroke patients flat with their heads at a zero-degree angle prior to a mechanical thrombectomy has resulted in significant short-term improvements in neurological function, as compared to patients whose heads were elevated at a 30-degree angle, in the ZODIAC trial. Researchers are keen to impress, however, that zero-degree head positioning is “a rescue manoeuvre, not a treatment” in stroke, serving as a way to preserve brain function by optimising blood flow until the thrombectomy can be performed.
“BY THREE MONTHS FOLLOWING SURGERY, there was no difference in outcomes for patients in either group; however, it’s exciting to see that we were able to discharge patients from the hospital with less disability requiring rehabilitation,” said Anne Alexandrov (University of Tennessee Health Science Center, Memphis, USA), who presented first-time data from ZODIAC at the 2024 International Stroke Conference (ISC; 7–9 February, Phoenix, USA). “Our findings suggest that gravitational force can play an important role in improving blood flow temporarily while patients are waiting for surgery. Zero-degree head positioning is a safe and effective strategy to optimise
blood flow to the brain until the thrombectomy can be performed, and it should be considered the standard of care for stroke patients prior to thrombectomy.”
Currently, hospital beds for stroke patients awaiting thrombectomy are typically set with the head of the bed at a slight incline of 30 degrees. However, pilot studies conducted previously by her and her team showed that, when the head of the bed is flat, thrombectomy patients benefit from increased gravitational blood flow through the occluded vessel, as well as having more open collateral arteries for the procedure.
“Many thrombectomy patients have delays until the procedure can be started, whether due to slow internal hospital processes, multiple patients arriving at the same time, or if the patient needs to be transferred to another hospital,” Alexandrov noted. “Optimising blood flow to the brain while patients are waiting for surgery is essential to minimise the risk of neurological deficits and ultimately disability.”
In the prospective, randomised ZODIAC trial, which included 92 LVO acute ischaemic stroke patients from 12 US centres, researchers used the National Institutes of Health stroke scale (NIHSS) to evaluate patients’ consciousness, vision, speech, motor strength, and sensory loss. Their primary endpoint looked at whether patients’ conditions remained stable or worsened depending on if they were set with zero-degree or 30-degree head positioning before thrombectomy. Stroke patients’ baseline NIHSS scores were measured at zero degrees immediately after neuroimaging, and they were then randomly assigned to head positioning at either zero or 30 degrees. Patients underwent repeated NIHSS scoring every 10 minutes until the thrombectomy was performed (or until more than two hours passed), with a final NIHSS score being assessed immediately before the procedure.
An interim analysis of the trial found that—based on these repeated NIHSS scores—zero-degree head positioning before thrombectomy resulted in greater
Final results from the PROST randomised controlled trial (RCT) have been published in JAMA Neurology and—according to the study’s leading investigators—provide evidence that thrombectomy with the Preset stent retriever (Wallaby/Phenox) is a safe and effective option in large vessel occlusion (LVO) stroke patients, having demonstrated non-inferiority to the Solitaire revascularisation device (Medtronic).
EARLY LAST YEAR, INITIAL results from PROST were presented for the first time, with investigators noting that primary and secondary endpoints had been successfully achieved. In a press release, Wallaby/ Phenox also reported that data from the trial supported the US Food and Drug Administration’s (FDA) decision to clear the device for use in the USA.
The full publication of these results, dated 2 January 2024, details that many stent-retriever designs are currently available in thrombectomy, but “comparison of these technologies in well-conducted studies is lacking”.
Against this backdrop, coprincipal investigators Raul Nogueira (University of Pittsburgh Medical Center, Pittsburgh, USA) and Ricardo Hanel (Baptist Neurological Institute, Jacksonville, USA), and colleagues, conducted the multicentre, prospective,
open-label, adaptive PROST RCT. Via blinded primary endpoint evaluations, the study set out to determine whether LVO stroke therapy with Preset is noninferior to treatment with Solitaire—a device long considered the gold standard in stent-retriever thrombectomy.
Between October 2019 and February 2022, multicentre participation in the trial occurred at 19 research hospitals and/or universities across the USA, as well as five in Germany. LVO stroke patients were enrolled and included up to eight hours after symptom onset, undergoing 1:1 randomisation to thrombectomy with either Preset or Solitaire. PROST’s
stability and/or clinical improvement prior to surgery compared with 30-degree head positioning. Due to this demonstration of potential efficacy, ZODIAC’s data and safety monitoring board (DSMB) stopped enrolment in the trial on 1 November 2023. The trial ultimately found a significant between-group difference favouring zero-degree positioning, as per its primary outcome measure of an NIHSS score worsening ≥2, as well as an estimated number needed to harm of 1.88 with an elevated pre-thrombectomy head position.
In addition to their preprocedural assessments, the investigators also explored possible differences in NIHSS scores at 24 hours following surgery, and at seven days post-surgery or discharge—whichever came first. Alexandrov said they did not expect to find a significant difference on this front due to the wellknown and dramatic improvements in patient outcomes associated with thrombectomy. As such, it came as a “pleasant surprise” to find that, at both 24 hours and seven days/discharge, patients in the zero-degree group had reduced disability and fewer neurological deficits on NIHSS versus those in the 30-degree group.
Alexandrov concluded her ISC presentation by stating that zero-degree head positioning “may be one
Many thrombectomy patients have delays until the procedure can be started.”
of the most important first steps” in managing an LVO stroke patient who is a candidate for thrombectomy— also averring that this is “critically important” when immediate access to thrombectomy is unavailable, and particularly in LVO cases requiring hospital-to-hospital transfer for the procedure.
primary outcome was the difference in the rate of 90-day functional independence across these groups, using a −12.5% non-inferiority margin for the lower bound of the one-sided 95% confidence interval (CI) of the difference between the two devices.
A total of 340 patients (median age, 73 years; 50% female) were randomised, and the study procedure was completed in 322 of these patients. The PROST investigators report that— as per intention-to-treat analysis—the trial’s primary endpoint of 90-day functional independence was achieved by 95 patients (54.9%) in the Preset group and 96 (57.5%) in the Solitaire group, giving rise to an absolute difference of −2.57% (95% CI, −11.42 to 6.28).
“As the lower bound of the 95% CI was greater than −12.5%, the Preset retriever was deemed non-inferior to the Solitaire retriever,” the authors write in JAMA Neurology. They go on to note that the non-inferiority of Preset versus Solitaire was also observed in the secondary clinical endpoint of overall disability, measured as a 90-day shift in modified Rankin scale (mRS) scores.
Similar parity was seen in both of the PROST trial’s angiographic endpoint
assessments: successful reperfusion within three thrombectomy passes, and at the first pass. An expanded treatment in cerebral infarction (eTICI) score of 2b50 or greater within three passes was achieved in 146 of 173 patients (84.4%) with Preset versus 149 of 167 (89.2%) with Solitaire (absolute difference, −4.83%; 95% CI, −10.84 to 1.19), while an eTICI score of 2c or greater following the first pass was achieved in 76 patients (43.7%) and 74 patients (44.3%) with Preset and Solitaire, respectively (absolute difference, −0.63%; 95% CI, −9.48 to 8.21).
Outlining results from safety endpoint analyses in the trial, the investigators report that symptomatic intracranial haemorrhage occurred in zero patients in the Preset group and two (1.2%) in the Solitaire group. Mortality occurred in 25 Preset patients (14.5%) and 24 Solitaire patients (14.4%) at 90 days. Furthermore, findings from PROST’s per-protocol and as-treated analyses were in concordance with these results from intention-to-treat analyses.
Speaking to NeuroNews, the authors highlighted the fact that, “in large part due to historical regulatory pathways and financial constraints”, many recent thrombectomy device trials have opted for a single-arm design. However, the PROST trial stands out by “establishing a new scientific benchmark for stroke device trials”, with the goal of ensuring “not only recanalisation, but also a clinical benefit”, they added.
Late last year, on 24 November, a decade’s work from a passionate group of physicians bore fruit, with the Brazilian Ministry of Health adding mechanical thrombectomy for stroke to the list of procedures covered by the country’s public health system. Current World Stroke Organization (WSO) president Sheila Martins (Federal University of Rio Grande do Sul, Porto Alegre, Brazil) recently told NeuroNews about the role played by the RESILIENT trial, and Medtronic’s contributions to it, in achieving this historic milestone.
Low- and middle-income countries (LMICs) have very different patient populations compared to their high-income counterparts. As Martins says, in LMICs, emergency rooms are often overcrowded; ambulances and hospital care systems in general are limited; and angio suites tend to be shared with several other specialties, ultimately increasing procedure timelines and leaving acute ischaemic stroke patients more vulnerable. Martins also relays that, in her home country of Brazil, neurointerventionists are trained in private practices but are usually less familiar with working in public hospitals. Hospitals in many other Latin American countries can only deploy monoplane angiography systems in lieu of access to more specialised imaging modalities, and stroke rehabilitation methods are less advanced than in higher-income geographies as well.
“Everything is more difficult and, because of this, it was so important to prove that it is feasible to implement [thrombectomy], with the same results that we have seen internationally, and show that it is cost effective so it is affordable for patients,” Martins says.
While a number of large clinical trials published in 2015 helped to establish thrombectomy as a safe, effective and cost-efficient treatment approach for stroke, Martins states that these studies were conducted predominantly in developed countries across North America and Europe, and thus bore “minimal” relevance to poorer regions. The RESILIENT trial was therefore set up to assess the procedure within realworld practice in a middle-income country like Brazil “for the first time”, according to Martins.
“I think ‘RESILIENT’ is the perfect name for everything we’ve been doing for years here in Brazil, because it is difficult to create a system to treat acute stroke patients,” she comments. “For the trial, we needed to rebuild stroke care in Brazil—organising the prehospital [settings] to evaluate patients for large vessel occlusion [LVO], discussing with health managers to create comprehensive stroke centres, and setting up a network to make it possible to transfer patients from primary to comprehensive centres.”
Conducted at 12 stroke centres across Brazil, the multicentre, prospective RESILIENT randomised controlled trial enrolled nearly 221 patients with LVO acute ischaemic stroke, presenting no more than eight hours from symptom onset, to receive thrombectomy plus medical management, or medical management alone. However, RESILIENT’s data and safety monitoring board recommended the trial should be terminated early, having clearly crossed the prespecified effectiveness boundary at its first interim analysis—at which point 174 patients had available 90-day follow-up data.
Across the 221 patients ultimately enrolled in RESILIENT, thrombectomy displayed superiority to medical management in terms of its primary endpoint of the 90-day ordinal shift in modified Rankin scale (mRS) scores, but also favourable outcomes regarding
functional independence (mRS ≤2), and comparable rates of symptomatic intracranial haemorrhage and mortality. Presenting these data back in 2019 at the European Stroke Organisation Conference (ESOC; 22–24 May, Milan, Italy), Martins averred that “the overwhelming efficacy of mechanical thrombectomy persists despite the many limitations encountered in the public healthcare system of a developing country”.
Nearly five years on from the first presentation of these momentous findings—now published in the New England Journal of Medicine—Martins reflects on factors additive to the strength of the intervention itself that enabled RESILIENT to produce such positive outcomes with thrombectomy. Firstly, she hails the significance of training and education, and the contributions of experienced operators like her co-principal investigator Raul Nogueira (University of Pittsburgh Medical Center, Pittsburgh, USA) towards mentoring Brazil’s neurointerventionists. These proctors helped make decisions over whether patients were suitable candidates for stroke thrombectomy, and assisted less experienced operators with more difficult cases, often in real time, in the trial’s early stages.
“One important thing is that we showed it was possible to train the neurointerventionists, specifically for mechanical thrombectomy, in a short space of time,” Martins adds, noting that RESILIENT was able to produce “great results” on par with many other countries. She opines that providing thrombectomy training for all relevant staff—not just neurointerventionists, but also neurologists, nurses, technicians and emergency room staff—was also key.
Martins also keenly emphasises the fact that, unlike many high-income countries, Brazil has a healthcare system that covers its entire patient population—and, thus, the criticality of financial analyses at a handful of large, high-volume centres in RESILIENT that showed thrombectomy care was “highly cost effective”. These findings were published in 2021, and revealed estimated incremental costs and quality-adjusted life years gained with thrombectomy plus medical management to be $7,440 (international dollars) and 1.04, respectively, versus medical management alone. Overall, some 1,000 simulations revealed that most of the treatments produced results below a prespecified costeffectiveness threshold.
“This is wonderful for the patient, and for society, and it also saves money—all of the financial benefits of the treatment are much higher than the initial cost of the devices, the equipment, and the stroke team,” Martins says. “And RESILIENT was a landmark publication, increasing implementation of the treatment in low- and middle-income countries across the globe, and showing thrombectomy to be very strong even in poorer populations with generally less organised healthcare systems.”
The ideal partner
“Public-private partnership is also very important, because we cannot do anything alone,” Martins continues, alluding to the commitment Medtronic has made towards supporting thrombectomy research in developing countries. “We need partners to have ideas for implementation, to create initiatives for training and educating, and also to negotiate better costs in, for example, LMICs that need a lot more help.”
Prior to the inception of RESILIENT, Medtronic was the first company to sponsor a Brazilian Ministry of Health thrombectomy teaching course launched as part of new national stroke policies in 2012. And, having contributed to RESILIENT itself by supporting training and providing tools like its SolitaireTM revascularisation device for many of the thrombectomies in the trial, Medtronic has since sponsored WSO-led education across Latin America to help train more interventionists in the procedure.
“That is so important, because the procedure is wonderful, but it really requires training and skills, and the organisation of services—and Medtronic has supported these efforts,” Martins notes. “Medtronic has also supported the certification of stroke centres, firstly in Latin America and now in India, which is fundamental to ensuring all evidence-based strategies are implemented in clinical practice. All of this makes Medtronic a very important partner of the WSO, and [for] increasing the assessment of the best stroke treatments for patients across the globe.”

Finally, she commends Medtronic’s contributions to the WSO’s Global Stroke Alliance (GSA) meetings over the past few years. These meetings are intended to pull together specialist physicians, researchers, healthcare managers, stroke support organisations, public and private hospitals, and industry, to discuss the best strategies for policy implementation. “Here, it is not about the results of clinical trials,” Martins says. “It is about how to implement what we already know, and what we have shown to be [clinically] effective and cost effective.”
Medtronic is the leading sponsor of these efforts, having supported the earliest GSA meeting iterations in 2020 and 2022—both held in Brazil—and the 2023 edition in New Delhi, India, which brought together nine countries from across Asia. According to Martins, Medtronic has played a major part in these endeavours and many others, including a conference hosted in Uruguay that drew hundreds of attendees from 13 Latin American countries in November last year.
Martins cites these cost-utility outcomes as a key reason for stroke treatment via mechanical thrombectomy ultimately gaining approval from the country’s health ministry, and thus being covered within the Brazilian public health sector. She further highlights the fact that initial costs of establishing thrombectomy services are outweighed by decreased rates of hospitalisation, complications, and severe long-term disability. Thrombectomy procedures also enable patients to regain functional independence and return to work more quickly, she adds.
“Medtronic is proud of its partnership with the WSO and the GSA,” said Signe Haughton, senior director, Neurovascular Medical and Government Affairs, Medtronic. “We are focused on driving education and training, and supporting policy initiatives that will ensure the best stroke treatments are accessible to all patients around the world. Medtronic Neurovascular’s strategy—centred on doubling the number of people we help treat to 500,000—requires us to not only optimise care for those who have access but, as importantly, increase access for those who don’t.”
In an editor’s column published recently in the Journal of NeuroInterventional Surgery (JNIS), leading members of the Society of NeuroInterventional Surgery (SNIS) have argued that the entire body of research, including new evidence from two prospective clinical trials, “clearly confirms” that most patients with emergent large vessel occlusions (LVOs) will benefit from triage directly to comprehensive stroke centres (CSCs).
AN SNIS PRESS RELEASE
details that JNIS editors are “urging hospital stakeholders to understand” that data from two recent, concurrent and seemingly negative trials—
TRIAGE-STROKE and RACECAT— “actually both provide confirmatory evidence” that a direct triage programme is beneficial for patients in most geographies in the USA, and also worldwide.
Direct triage approaches, whereby patients with emergent LVOs are transported directly to CSCs to undergo interventional therapy, have been endorsed by the National Association of State Emergency Medical Services (EMS) Officials. Equivalents to CSCs in the USA are commonly referred to as thrombectomy-capable centres (TCCs) in Europe.
SNIS leaders state that, since the release of prospective data from TRIAGE-STROKE and RACECAT throughout 2023, “trade media and
select hospital systems have issued messaging proclaiming, without qualification, that direct triage programmes are ineffective”. This has caused concerns about risks to patient care and outcomes in the neurointerventional community, the SNIS release adds.
“Misinterpreting both studies— which were stopped early—poses a grave risk to our patients in the USA, and much of the international community,” said David Fiorella (Stony Brook Cerebrovascular Center, Stony Brook, USA), lead author of the JNIS commentary and senior member of the SNIS. “We’ve begun to see the effects, with some primary stroke centres [PSCs] and hospital systems leveraging perceived financial incentives to interrupt lifesaving, direct-to-CSC triage policies.”
The JNIS commentary’s authors aver that RACECAT studied a “very large, diffusely populated, non-urban
One-year follow-up data from the TESLA randomised controlled trial (RCT) evaluating mechanical thrombectomy in patients with ischaemic strokes caused by large-core infarct volumes have demonstrated the more long-term clinical benefits of the procedure—when coupled with standard medical management (MM). The results were presented at this year’s International Stroke Conference (ISC; 7–9 February, Phoenix, USA) by Osama Zaidat (Mercy Hospital, Toledo, USA) and Albert Yoo (Texas Stroke Institute, DallasFort Worth, USA).
ZAIDAT INITIALLY GAVE A BRIEF RECAP of 90-day data from the TESLA trial, which demonstrated a “strong suggestion” favouring thrombectomy plus MM over MM alone but ultimately fell short of showing statistical superiority.
Zaidat also commented on several notable features of TESLA, including the fact it covered a broader range of stroke severity than many other large-core trials to date, enrolling patients with Alberta stroke programme early computed tomography score (ASPECTS) 2–5 rather than just 3–5, in addition to employing a pragmatic design with only non-contrast computed tomography (CT)-based selection up to 24 hours.
geography in Catalonia, Spain, where all TCCs are clustered in Barcelona”. They describe this system structure as “vastly different” to much of the USA. In addition, they argue, the majority of patients (56%) triaged directly to a TCC required more than 60 minutes of transport time, having “little relevance” to most of the US population, who live within one hour of a CSC. And, unlike USA-based EMS providers, the EMS providers in Catalonia “were not capable of administering treatment”, which “exacerbated the detrimental effects of
We must continue to apply triage and transport models that are proven to save lives.”
Mahesh Jayaraman
prolonged transport times” to facilities located further away.
The authors of TRIAGE-STROKE reported data “overwhelmingly in favour” of direct triage to a CSC for emergent LVOs—but, “given its small study population, it failed to reach statistical significance”, the JNIS commentators continue. The data on intravenous lytic administration from the trial “also supported previous studies” demonstrating that excess transportation time being required to get to a CSC
This prospective RCT—for which the primary endpoint was the difference in 90-day utility-weighted modified Rankin scale (UW-mRS) scores between groups—saw 300 patients randomised to either thrombectomy plus MM, or MM alone, across 44 US sites.
Taking to the ISC 2024 podium, Yoo reported a loss to follow-up of roughly 8%, with 144 thrombectomygroup patients and 133 controlgroup patients ultimately being available for intention-to-treat (ITT) analyses at one year.
He went on to relay oneyear mean UW-mRS scores of 3.654 with thrombectomy and 2.776 with controls, resulting in a mean difference of 1.175 favouring the treatment group.

Yoo said that, with a Bayesian probability of superiority of 0.999—analogous to a one-sided p-value of 0.001—thrombectomy was therefore associated with “a more pronounced effect” at one year, versus 90 days, and surpassed the 0.975 superiority threshold used in that original primary endpoint analysis. However, he continued, “it is important to note that this was not a prespecified hypothesis test, and it wasn’t adjusted for multiplicity”.
Among key secondary endpoint findings from TESLA at one year were a rate of mRS 0–2 of 22% with thrombectomy and 6% with controls (p=0.0001); a rate of mRS 0–3 of 34% and 16% in the two groups, respectively (p=0.0009); and an mRS shift analysis that also favoured thrombectomy, with an odds ratio (OR) of 1.82. In addition, there was a mean difference of more than 10 in terms of EQ-5D-5L quality-of-life scores—60.3 with thrombectomy and 49.3 controls.
Regarding safety endpoints, Yoo reported
“does not diminish access to lytic therapy”. Finally, the authors note that TRIAGE-STROKE found evidence that the risk of dependence or death was higher for stroke patients triaged to a PSC first, rather than directly to a CSC, whether they had an emergent LVO or not.
“Globally, mechanical thrombectomy is not only considered effective for eligible patients, but firmly established as the standard of care for emergent LVO patients,” the SNIS’ recent release states. “However, the efficacy of such stroke therapies is largely dependent on time to treatment, and not all stroke centres are equipped to treat these patients—further emphasising the need for quick and appropriate protocols.”
“Much of the neurointerventional field is alarmed that these studies are being aggressively and inappropriately applied to interrupt or prevent direct transport programmes from being implemented,” added SNIS president Mahesh Jayaraman (Rhode Island Hospital, Providence, USA). “We must continue to apply triage and transport models that are proven to save lives. These are life and death decisions, and it is our duty as care providers to prioritise patient outcomes over additional profits.”
Discussing their results with NeuroNews previously, leading authors on both the TRIAGE-STROKE and RACECAT studies agreed that—despite producing mixed signals regarding direct transfer protocols in acute ischaemic stroke—their findings highlight the need for further research.
comparable mortality rates—roughly 45%—across the two groups at one year, also relaying that there was a non-statistically significantly greater rate of mortality specifically from 90 days to one year in the control group (thrombectomy, 5.5% vs. controls, 9.8%). One-year per-protocol group analyses produced very similar findings that also favoured thrombectomy at one year, including across the primary endpoint and several secondary endpoints, according to Yoo.

“Regarding mechanisms, one potential explanation for the greater benefit we see here at one year versus 90 days is continued recovery beyond 90 days in largecore patients,” he concluded, also noting that both followup and rehabilitation may be required for a longer time period in these stroke populations. “And, although this is uncertain and should be investigated, [thrombectomy] may promote long-term brain plasticity in these large-core infarcts.”
One potential explanation for the greater benefit we see here at one year versus 90 days is continued recovery beyond 90 days in large-core patients.”
Albert Yoo
A new guidance document from the UK National Institute of Health and Care Excellence (NICE) has endorsed National Health Service (NHS) deployment of two artificial intelligence (AI)-derived software technologies to support the review and reporting of computed tomography (CT) brain scans in patients with suspected stroke.
BASED ON EXISTING DATA, RAPIDAI (RapidAI; formerly iSchemaView) and e-Stroke (now known as Brainomix 360; Brainomix) can be used in the UK healthcare system “while more evidence is generated”, but only once they have gained appropriate Digital Technology Assessment Criteria (DTAC) approval.
According to the latest diagnostic guidance from NICE on stroke and transient ischaemic attack (DG57), “the software should only be used with healthcare professional review, and centres should maintain existing scan reporting protocols to reduce the risk of incorrect results”. The document notes that “centres should ensure that images shared between different stroke centres can be remotely reviewed to help with decision making by healthcare professionals at a different site”.
“We welcome this latest guidance from NICE, which reflects the clinical value that Brainomix 360 brings to stroke care—which we have been seeing since we first implemented the software in March 2020,” said Kiruba Nagaratnam (Royal Berkshire Hospital, Reading, UK), as quoted in a recent Brainomix press release. “Our recently published study showed that the implementation of Brainomix 360 not only improved our speed of treatment but, most notably, improved patient outcomes, with the number of patients achieving functional independence rising from 16% to 48%.”
“AI will never replace the clinical expertise that our doctors and consultants have, but harnessing this latest technology is allowing us to make very quick decisions based on the experiences of thousands of other stroke patients,” stated Jenny Vernel (Hereford Hospitals NHS Trust, Hereford, UK), speaking in December 2023 after her hospital became the first in the UK West Midlands to roll out RapidAI software for stroke assessments. “The system is linked to the comprehensive stroke centre at University Hospitals Birmingham NHS Foundation Trust [Birmingham, UK], meaning that clinical teams and experts at both sites can make faster and more informed clinical decisions. This is essential when treating stroke patients.”
NICE’s recent guidance—published online on 23 January—also lists a number of technologies that “can only be used in research” currently, including Accipio (MaxQ AI), Aidoc (Aidoc Medical), BioMind (BioMind AI), BrainScan CT (Brainscan AI), Cercare Perfusion (Cercare Medical), CINA Head (Avicenna AI), CT Perfusion 4D (GE Healthcare), icobrain CT (Icometrix), Neuro Solution (Nanox AI), qER (Qure AI), and Viz (Viz AI).
The guidance states that “more research is needed” on all of these AI-derived software to support review and reporting of CT brain scans in patients with suspected stroke, and that access to these technologies “should be through company or research funding” i.e. non-core NHS funding.
ISCHAEMIC STROKE SURVIVORS who received care recommendations from an artificial intelligence (AI)based system experienced fewer recurrent strokes and heart attacks, and a lower rate of vascular death, within three months—as compared to people whose stroke treatment was not guided by AI tools—in the recently presented GOLDEN BRIDGE II study. Lead study author Zixiao Li (Beijing Tiantan Hospital/Capital Medical University, Beijing, China) delivered findings from the trial in a late-breaking science session at the International Stroke Conference (ISC; 7–9 February, Phoenix, USA).
“This research showed that an AIbased clinical decision support system for stroke care was effective and feasible in clinical settings in China, and improved patient outcomes,” Li said. “This type of technology aids neurologists by facilitating the sharing of information between humans and AI, using their combined strengths.”
In the GOLDEN BRIDGE II clinical trial, 77 hospitals across various regions of China were randomly assigned to deliver diagnosis and treatment for
ischaemic stroke patients based on either recommendations from the AI technology system, or assessments and recommendations by the hospitals’ stroke care teams. The AI system integrated participants’ brain imaging scans—interpreted by AI—with established clinical knowledge for stroke diagnosis, stroke classification, and guideline-recommended treatment and strategies to prevent secondary stroke.
Across 21,603 hospitalised, adult acute ischaemic stroke patients included in the study, researchers then measured the number of vascular events—ischaemic and haemorrhagic strokes, heart attacks, or death due to a vascular event—among all patients after their initial ischaemic stroke during a three-month follow-up period.
Some 11,054 patients received AI evaluation and treatment, while 10,549 received standard stroke care, and the vast majority of patients (21,579) were included in the final data evaluation after completing the three-month follow-up.
The analysis found that using an AIbased clinical decision support system reduced the chances of new vascular events by 25.6% during this initial
Additionally, NICE advises that further evidence generation and more research are needed on: the impact of the addition of AI-derived software on a healthcare professional’s ability to identify people for whom thrombolysis and thrombectomy is suitable; how often the software is unable to analyse CT brain scans, with reasons for this; the impact of using the software on time to thrombolysis or thrombectomy; and the impact of using the software on how many people have thrombolysis or thrombectomy.
Alongside this guidance, NICE has released an evidence generation plan providing further information on prioritised evidence gaps and outcomes, ongoing studies, and potential real-world data sources.
NICE also details that the UK Department of Health and Social Care (DHSC) and NHS England have launched several initiatives, such as the AI Diagnostic Fund and the AI Deployment Platform, that will help generate more evidence on the use of AI technologies.
“Clinical evidence on the software is limited in quality,” the NICE guidance further states. “There is no evidence on their diagnostic accuracy when used alongside healthcare professional review that met review inclusion criteria. Some studies on two technologies (e-Stroke and RapidAI) in clinical practice suggest that people had faster or greater access to treatment after using the software, but it is unclear to what extent this is an effect of the software. In the economic model, a small increase in the number of people having thrombectomies because of AIderived software would likely make the software cost effective.”
According to NICE, e-Stroke and RapidAI are already “widely used” in the UK NHS, and can continue to be deployed while further evidence is generated to help better determine their cost effectiveness—but the other aforementioned technologies, for which there is “no evidence on how they impact time or access to treatment”, should only be used in research.
three-month period, and also improved stroke care quality, with patients more likely to be treated with guidelinedirected medical therapy. At three months, participants treated at hospitals using AI support also experienced fewer total vascular events compared to people receiving standard poststroke evaluation and treatment (2.9% vs. 3.9%). Li and colleagues observed no statistically significant differences in physical disability levels between patients in either of the two groups at three months, as assessed using modified Rankin scale (mRS) scores.
We hope AI applications can be broadened to apply to other health conditions.”
“The reduction in new vascular events is a significant finding, because it shows that AI has the potential to make a real difference in stroke care and benefit this large population of stroke survivors,”
Li added. “In the future, we hope to have more AI applications validated through clinical research and hope that the clinical decision support system can be expanded to include more aspects of stroke care, including reperfusion therapy and long-term secondary prevention, rehabilitation, and so on. At the same time, we also hope that AI applications can be broadened to apply to other health conditions.”
Notable limitations of GOLDEN BRIDGE II include the fact that entire hospitals were randomised to the AIbased strategy or standard care, rather than individual patients. Differences in care patterns and outcomes between hospitals, and subsequent outpatient care, may have impacted the results too.
The researchers believe that questions remain over whether or not these AI-led improvements outcomes can be sustained, thus requiring further evaluation, and the functionality of the clinical decision support system may need to be constantly updated to keep pace with evidence-based clinical guideline revisions. More extensive and sustainable clinical application models of the system for health conditions beyond stroke, and for use in other countries, need to be explored too.
Zixiao Li

Reteplase demonstrates superiority to alteplase in acute ischaemic stroke trial
Reteplase holds potential as an alternative to alteplase in ischaemic stroke patients requiring intravenous thrombolysis (IVT), as per RAISE trial findings presented by Yongjun Wang (Beijing Tiantan Hospital, Beijing, China) at the 2024 International Stroke Conference (ISC; 7–9 February, Phoenix, USA).
HAVING ASSESSED RETEPLASE
against alteplase across hundreds of Chinese stroke patients, RAISE trial investigators found that the former produced superior efficacy, and observed no significant difference between the fatal safety profiles of the two drugs. As such, Wang stated that “these overall findings should encourage administration of reteplase in appropriate patients with acute ischaemic stroke”.
The presenter began by positing that, while alteplase has been the gold standard in IVT for some time, new thrombolytic candidates including tenecteplase, lanoteplase and reteplase have now entered the fray, and are designed to be more convenient and effective alternatives. Reteplase itself was approved as a myocardial infarction treatment in Europe and the USA in the 1990s, but has more recently garnered interest as a potential new player in ischaemic stroke.
Wang and his team recently evaluated reteplase in a Phase 2,
multicentre randomised controlled trial (RCT) whereby stroke patients were randomised to a lower reteplase dose (12mg+12mg), a higher reteplase dose (18mg+18mg), or a standard dose of alteplase, within 4.5 hours of symptom onset. In the study, reteplase was sufficiently tolerated by patients, displaying a similar efficacy profile and comparable safety outcomes versus alteplase, with the 18mg+18mg dose trending towards an improvement in clinical efficacy.
Therefore, the researchers progressed to the Phase 3 RAISE study—another multicentre RCT with the primary goal of proving reteplase’s non-inferiority compared to alteplase. The RAISE trial involved acute ischaemic stroke patients at ≤4.5 hours of symptom onset, with 707 patients being randomly allocated to receive an 18mg+18mg dose of reteplase, and 705 being allocated to receive a 0.9mg/kg dose of alteplase. The primary endpoint pertained to modified Rankin scale (mRS) scores of 0–1 at 90 days; secondary efficacy
endpoints included 90-day mRS 0–2, mRS ordinal distribution, and changes in National Institutes of Health stroke scale (NIHSS) scores; and symptomatic intracranial haemorrhage (sICH), allcause death and other adverse events at 90 days were among safety endpoints.
Between 21 March 2022 and 22 June 2023, a total of 1,412 IVT-eligible acute ischaemic stroke patients gave informed consent and were enrolled across 62 clinical sites in China. Wang reported that baseline characteristics were well balanced between the reteplase and alteplase groups—average age was 63 years in both, and roughly 30% of the population was female in each. Some 1,370 patients—exactly 685 in each group—were available for RAISE’s final 90-day follow-up analyses.
Rate
and an ‘ordinary’ 90-day mRS score of 0 (reteplase, 54.5% vs. alteplase, 41.9%; common odds ratio [OR], 0.61). Improvements in NIHSS scores of ≥4 points/an overall score ≤1 at both 24 hours (reteplase, 58.4% vs. alteplase, 48.5%; RR, 1.21) and seven days (reteplase, 74.1% vs. alteplase, 66.8%; RR, 1.11) also saw reteplase reach noninferiority in relation to alteplase, and favoured the former.
Wang went on to report no statistically significant differences between subgroups—including those stratified by age, sex, NIHSS at admission, onset-to-needle time, and bridging thrombectomy—when it came to the study’s primary efficacy endpoint.
80.1%
Reteplase ultimately achieved not only its prespecified non-inferiority goal, but also superiority over alteplase, with investigators observing an 80.1% rate of mRS 0–1 at 90 days posttreatment in the reteplase group versus 71.1% in the alteplase group (relative risk [RR], 1.13). “In other words, the efficacy of reteplase is better than that of alteplase,” Wang noted.
71.1%
Regarding key secondary efficacy endpoints, the more novel of the two drugs also demonstrated non-inferiority in terms of 90-day mRS 0–2 (reteplase, 85.8% vs. alteplase, 80.4%; RR, 1.07),
Giving blood thinners in addition to clot-busting medications does not improve ischaemic stroke patients’ outcomes at 90 days, according to findings from the MOST trial, which were revealed in a late-breaking science presentation at the 2024 International Stroke Conference (ISC; 7–9 February, Phoenix, USA).
“WHEN WE BEGAN THE TRIAL, WE believed the medications would improve outcomes, so we were surprised with the negative results,” said Opeolu Adeoye (Washington University School of Medicine, St Louis, USA), lead author of the study, who presented these results alongside Andrew Barreto (UTHealth Houston, Houston, USA) at ISC. “However, we designed the trial to allow us to efficiently answer the question for two blood-thinning medications in one trial. We have definitely done that and are pleased with the ability to answer this question.”
The MOST trial—conducted at 57 US centres—was halted in July 2023 after an independent data and safety monitoring board (DSMB) analysed results on the first 500 patients out of a planned 1,200 participants, and determined it “highly unlikely” that a benefit would be found if the research was completed.
The study enrolled adult patients whose ischaemic strokes were severe enough that rehabilitation would likely be needed (National Institutes of Health stroke severity [NIHSS] score ≥6), all of whom received a standard clot-busting medication to dissolve their blood clot—intravenous thrombolysis (IVT)—within three hours of symptom onset or time last seen well.
Participants were then randomised to one of three
groups for additional treatment. Some 59 patients received the blood thinner argatroban within 75 minutes of IVT, followed by a 12-hour infusion of argatroban; 228 patients received an initial dose of the blood thinner eptifibatide within 75 minutes of IVT, followed by a two-hour infusion of eptifibatide and a 10-hour infusion of saline placebo; and, in the control group, 227 patients were given IVT and a placebo treatment (12hour infusion of intravenous saline solution containing neither of the blood-thinning medications). Across all three groups, some 44% of patients also underwent a mechanical thrombectomy procedure.

“A lot of our approaches in stroke treatment were learned from how we treat heart attacks,” Adeoye noted. “In previous trials, we first tested to make sure these medications were safe for use in stroke, and then launched MOST to confirm their safety, and test whether they would work to improve functional outcomes and reduce disability after stroke.”
The primary outcome in MOST was the level of physical function at 90 days after ischaemic stroke, assessed using the modified Rankin scale (mRS). MRS scores were translated into a utility-weighted mRS
Finally, he outlined findings regarding RAISE’s key safety endpoint of sICH within 36 hours, revealing “no clear difference” between the two, with sICH occurring at a rate of 2.4% in the reteplase group and 2% in the alteplase group (RR, 1.21; p=0.64). Additional safety endpoints also showed comparable rates of sICH within seven days, death, and serious/ non-serious adverse events. And, while Wang did relay that there was a statistically significantly higher incidence of any ICH within 90 days in the reteplase group compared to the alteplase group, no significant difference was observed regarding the fatal safety profile of each.
score using validated ratings of functional outcomes by patients and physicians, resulting in a scale of 0–10 on which a higher score indicated a greater treatment benefit. The study’s primary safety measure was the occurrence of symptomatic bleeding in the brain, known as intracranial haemorrhage (ICH), within 36 hours of receiving one of the two blood thinners, with this outcome being reviewed by the DSMB after every 30 patients enrolled. Medical professionals providing care in the trial were aware of whether a blood thinner or placebo had been given to each patient, but neither patients nor clinicians rating their outcomes—independent reviewers using videotaped assessments—were aware of which treatment patients had received.
The DSMB’s interim analysis— which was planned from the start of the study—ultimately included data from 514 enrolled patients (average age, 68 years; 50% women), and found that the two blood-thinning medications used did not significantly increase the risk of ICH. However, neither drug improved functional outcomes in the trial either. Patients in the control group, who received IVT plus placebo, had an average utility-weighted mRS score of 6.8, compared to 5.2 in those who received argatroban and 6.3 with eptifibatide.
“In addition, we were not able to address the possible benefit of giving these or similar blood thinners directly into an artery in the area of the stroke, rather than giving the medications systemically through a vein, as done in this trial,” Adeoye concluded.
On this front, multiple studies involving patients receiving a thrombectomy to remove their strokecausing clots, intended to determine whether delivering blood thinners into the affected artery may improve outcomes, are currently underway.
In light of a recent publication detailing the safe and effective use of the Solitaire X 3mm (Medtronic) stent retriever in medium- and distal-vessel occlusion (MeVO/DVO) stroke, Marios Psychogios (University Hospital Basel, Basel, Switzerland) discusses the relevance of having a dedicated device for these cases, as well as providing insight on what he feels is the “next frontier” in mechanical thrombectomy.
“We were one of the first clinics that used the device in Europe, and we are happy with it,” Psychogios says, highlighting navigability and radial force as being among key characteristics of the Solitaire X 3mm. “Generally, I like these aspects—the navigability for those superiortrunk M2 and M3 occlusions is really good, and it still has enough radial force to actually be able to capture clots [in those locations].”
He attributes these abilities, at least in part, to the device’s parametric design, which helps it to better accommodate the clot while also providing a strong balance of navigability and radial force, as well as the right level of retrievability and “softness” for use in more distal vessels.
“What [Medtronic] has done nicely here is find the ‘sweet spot’ of combining all these things,” Psychogios adds.
Writing in the Journal of Clinical Medicine, Psychogios et al recently published findings from a retrospective study involving 68 consecutive primary and secondary MeVO/DVO stroke cases treated via thrombectomy across 12 European centres.1 Psychogios is keen to emphasise “really good” reperfusion results reported in the paper—as per the study’s primary endpoint, the Solitaire X 3mm achieved a first-pass rate of complete or nearcomplete reperfusion (modified treatment in cerebral infarction [mTICI] 2c–3) of 32.3%.
Other key statistics he highlights are a final-pass mTICI 2c–3 rate of 67.6%, a final mTICI 2b–3 rate close to 90%, and—regarding safety endpoints—a 13% rate of intracranial haemorrhage (ICH), with none of these complications being symptomatic or resulting in subsequent neurological deterioration. The researchers also observed no device malfunctions in the study. Psychogios acknowledges that retrospective analyses such as this do inevitably carry limitations and some inherent biases, but that the findings outlined here compare favourably to prior data on other stent-retriever devices.
One salient piece of advice Psychogios offers to his neurointerventional peers is to make use of these smaller, specialised stent retrievers like the Solitaire X 3mm when treating ischaemic strokes caused by MeVO/DVOs, if they have access to them at their centres. He advises against the deployment of regular (4–6mm) stent retrievers in said cases, stressing the importance of “dedicated” thrombectomy devices for tackling distal locations where more tortuous vessels are found and a greater risk of bleeding complications—most notably, clinically relevant ICHs—exists.
Psychogios says that, thanks to this newer generation of more specialised devices, his centre has the ability to treat distal occlusions more effectively, but also go after secondary distal occlusions more safely, as they can switch from using a larger stent retriever for an initial large vessel occlusion (LVO) to a smaller device like the Solitaire X 3mm within the same case if necessary.
having a dedicated device for those secondary distal occlusions leads to better safety and then probably better outcomes for the patient,” he states. “This is something that we always do and, if you have the means, I would recommend it—don’t just stay with your LVO setup! It’s good to have a ‘standard’—and we always have that—but, if you [encounter] a new condition in the angio suite, you have to adapt.”
“Another important thing is that most of the cases [78%] were done with a combined approach, with a dedicated aspiration catheter, and this is something we have been promoting and using in our patients,” Psychogios also says of the study. “With the Quattro technique, we are seeing fewer complications [at our centre], so we promote this combined approach.2 In my opinion, it is not just about the device—you need the whole setup and, usually, we combine [the stent retriever] with an aspiration catheter—but it’s also crucial that the device is correctly chosen and, for those more curved segments, the Solitaire X 3mm is a very good device.”
Psychogios goes on to state that it is often underestimated how vital a standardised setup—like the one he and his colleagues in Basel use consistently—can be in helping to reduce complications, as it gives operators access to the best materials for any given
I strongly believe that, in a few years’ time, we’re going to go after those distal occlusions.”
“It comes back to the safety aspect, and the fact that
 Marios Psychogios
Marios Psychogios
occlusion type. According to Psychogios, while it has been suggested that aspiration alone may be sufficient for more distal occlusions, his own experiences as well as meta-analysis data from some 2,500 thrombectomy cases point towards a higher rate of clinically relevant bleeding, and the fact “you probably don’t open the vessels as [effectively] as with a combined approach”.
While the Solitaire X 3mm is tailormade to fit into this combined setup, one problem Psychogios does encounter when implementing a combined approach is the paucity of dedicated aspiration catheters that are well-suited to the treatment of distal occlusions. He notes that this is something he often emphasises in conversations with industry, adding, “having smaller stent retrievers is a good first step, and we can see in the paper that this leads in the right direction, but I think we can achieve even more if we have the whole setup regarding distal occlusions”.
Distal “truth” moves closer Psychogios also provides a brief update on the progress of the global DISTAL trial evaluating thrombectomy in primary MeVO/DVO stroke, for which he is the principal investigator: as of early February 2024, the study has enrolled 370 patients out of a targeted 530, with Psychogios anticipating finalised recruitment later in the year and, “hopefully”, initial data presentations at the start of 2025. He goes on to disclose that DISTAL very recently received approval from its data and safety monitoring board (DSMB) to continue, following a planned interim analysis that revealed no safety- or futility-related concerns.
“We will have data from randomised trials showing what is best for these occlusions next year,” he continues. “We also plan to join forces with the other trials—ESCAPE MEVO, DISTALS, DISCOUNT—to pool data. What we have at the moment is just a glimpse of the truth, and I think next year we are going to have a better overview of the whole truth. I strongly believe that, in a few years’ time, we’re going to go after those distal occlusions. We’ve seen this with the LVOs, then ‘late-window’ LVOs, and now the largecore patients, and I think this is going to be the next frontier.”
Psychogios posits that the emergence of thrombectomy for more distal stroke cases is “a process”, as, within his own department, he is observing far fewer complications when treating a MeVO today compared to even 3–4 years ago. He cites improved techniques and training, a greater wealth of experience accrued over time, and the introduction of new devices, as likely drivers of this change.
“That is the beauty of neurointervention,” Psychogios concludes. “We are always improving, and looking at ways of making things safer and making things better—again, I think the Solitaire X 3mm helps a lot in this direction—and, maybe, in the future, we will be able to achieve even better outcomes.”
References:
DISCLAIMER:
The
and
RESULTS FROM A LARGE-SCALE randomised controlled trial (RCT) have indicated that mechanical thrombectomy is effective and safe in the treatment of ischaemic stroke patients with large-core infarcts—a finding consistent with the study’s initial, positive outcomes at three month. Amrou Sarraj (Case Western Reserve University, Cleveland, USA) delivered these long-term data from SELECT2—a prospective RCT conducted across 31 sites in the USA, Canada, Europe, Australia and New Zealand—at this year’s International Stroke Conference (ISC; 7–9 February, Phoenix, USA).
Sarraj initially recapped that the study was halted early due to efficacy signals favouring thrombectomy, and ultimately met its primary endpoint of improved functional outcomes at 90 days. From 352 originally randomised in the trial, 170 patients in the thrombectomy-plus-medical management (MM) group and 159 in the MM-only group had follow-up data available at one year. For the intention-to-treat (ITT) analysis, all patients (178 thrombectomy and 174 MM) were included, with multiple imputation being used to
compensate for missing data.
Based around the primary endpoint of modified Rankin scale (mRS) score distribution/ordinal shift, with mRS 5 and 6 merged, the SELECT2 investigators’ one-year ITT analysis revealed a Wilcoxon-Mann-Whitney (WMW) measure of a probability of superiority of 0.59, and a general OR of 1.43 (p=0.0019), favouring thrombectomy over MM. In addition to this statistically significant shift indicating better functional outcomes, Sarraj reported an estimated number needed to treat of six to achieve these improved outcomes. The presenter went on to detail positive and statistically significant improvements in mRS 0–2 (thrombectomy, 24% vs. MM, 6%) and mRS 0–3 (thrombectomy, 37% vs. MM, 18%) rates—without an increase in mortality, as 45% of patients died in the thrombectomy arm versus 52% in the MM arm (risk ratio, 0.89).
“I bring your attention to the fact that the difference in mortality is more apparent now, at one year, compared to what we had at three months—but it was not statistically significant,” Sarraj noted.
Regarding quality-of-life outcomes, and Neuro-QoL scores specifically, SELECT2 found that thrombectomy led to statistically significant improvements in quality of life across all four of the categories assessed— mobility, social, cognitive, and depression—at oneyear follow-up. The trial’s as-treated and per-protocol analyses also produced comparable findings to those seen in its ITT population. In addition, functional

Philipp Dammann
Point of View
As per an observational study published in the journal Neurology last year, female hormone therapy use may be associated with a higher risk of intracranial haemorrhage from cerebral cavernous malformations (CCMs)—raising questions about the safety of these therapies for CCM patients in clinical practice. Here, study authors Alejandro Santos and Philipp Dammann (both Essen, Germany) attempt to elucidate the wider implications of their findings.
CCMs can lead to seizures and neurological deficits due to intracranial haemorrhage, or nonhaemorrhagic focal neurological injury. The pathophysiology leading to stroke due to intracranial haemorrhage in patients with a CCM is still unclear, but some theories exist based on previous research. It is believed that the latter might be triggered by clot formation in the dilated vessels of the CCM through which the blood flows very slowly.
outcome improvements favouring thrombectomy were consistent across subgroups such as age, clot location, and time to randomisation, as well as imaging-related variables including different core extents on Alberta stroke programme early computed tomography score (ASPECTS), according to Sarraj.
“It’s important to highlight that most of the stroke trials look at outcomes at 90 days, and this is an opportunity to start reconsidering that, since patients continue to improve up to one year,” he added, also averring that larger-core patients may require more time to achieve optimal outcomes with thrombectomy.
As well as highlighting considerations relating to future clinical trial design, Sarraj said SELECT2’s latest analysis indicates it is “plausible” there is a need for more long-term rehabilitation in these particularly severe strokes, and could lead to additional explorations of the potential role for neuroprotection as well as other alternate mechanisms of recovery.
It’s important to highlight that most of the stroke trials look at outcomes at 90 days, and this is an opportunity to start reconsidering that.”
To this end, the University Hospital of Essen in Germany recently performed an observational cohort study with longterm follow-up in collaboration with the Mayo Clinic (Rochester, USA) to investigate such associations.
Both clinics included consecutive patients with a CCM being followed in their respective departments, and compared the association between use of female hormone therapy and the occurrence of intracranial haemorrhage due to the CCM across up to five years of prospective follow-up. Out of a cohort of 722 female patients, aged 10 years or older at time of CCM diagnosis, 137 used female hormone therapy at any point during follow-up.
This study showed that female hormone therapy use was associated with an increased risk of subsequent intracranial haemorrhage and, moreover, that the use of oral contraceptives in female patients aged 10–44 years was associated with an even higher risk of subsequent intracranial haemorrhage.
unfortunately still insufficient to warrant change in the known guidelines, as well as clinical practice for this disease. Such findings are, however, a starting point to drive further research on the matter.
In pursuance of answering these critical points for such a rare disease, future research should motivate an international collaboration between centres specialised in this area to investigate the association between female hormone therapy and intracranial haemorrhage in females suffering from a CCM.
Through such wide collaboration— gathering data from thousands of patients—the aim will be to better understand this unknown disease, as well as assess haemorrhagic risk in all female CCM patients under female hormone therapy.
Future study results could significantly impact the future of female CCM patients and modify patient counselling.
Therefore, the rationale of having increased blots clots in the caverns of CCMs in patients under female hormone therapy—which might subsequently lead to higher rates of bleeding—is reasonable.
Unfortunately, the lack of studies assessing this possible effect leave guidelines unable to make recommendations for patients under female hormone therapy suffering from a CCM.
Female hormone therapy—oral contraception in females of reproductive age and menopausal hormone therapy in postmenopausal females—has been shown to increase the risk of stroke and venous thrombosis. Yet, the latter is not withheld from patients with CCMs. The effects of these drugs on the risk of intracranial haemorrhage are also unknown.
Being the first piece of collaborative group research showing these unprecedented results, our findings raise questions about the safety of female hormone therapy in clinical practice in patients with CCM. This constitutes the clinical importance and relevance of our study in terms of addressing the following:
Establishing an association between female hormone therapy intake and intracranial haemorrhage in females with CCM
Segregating different types of available female hormone therapy and assessing their individual risk leading to intracranial haemorrhage in terms of dose, method of administration, mode of mechanism etc. Coming from a study with Class III evidence, these data alone are
By performing large, international, multicentre studies addressing this matter, we might be able to advise females suffering from CCM under female hormone therapy in terms of which medications (including type of medication and method of administration) have been shown to have a harmful association, and which have not.
Alejandro Santos is a medical doctor in the Clinic for Neurosurgery at Essen University Hospital in Essen, Germany.
Philipp Dammann is a senior physician and consultant in the Clinic for Neurosurgery at the Essen University Hospital. He specialises in neurosurgery and intensive care medicine.
The authors declared no relevant disclosures.
The recent Barts Research and Advanced Interventional Neuroradiology (BRAIN) conference (4–6 December 2023, London, UK) saw Tommy Andersson (Karolinska University Hospital, Stockholm, Sweden) put forward the case for post-thrombectomy rescue stenting in ischaemic strokes caused by challenging, neutrophil extracellular trap (NET)-rich clots.
“NETS INTERACT WITH PLATELETS, AND are specifically abundant in red blood cell-poor and platelet-rich clots,” Andersson informed the BRAIN audience, also stating it is “definitely” possible that failed revascularisation post-thrombectomy could be caused by the clot being rich in NETS. “And, stenting is probably the best bailout option for difficult, NETrich clots—but maybe not for ICAD [intracranial atherosclerotic disease], at least with the materials we have available today, because then we see risks of snow-ploughing or cheese-grating. It can, however, sometimes be very difficult to differentiate a difficult clot from ICAD.”
Initially covering potential reasons for a mechanical thrombectomy failing to achieve successful revascularisation, Andersson relayed that ICAD is “fairly common”, but mainly observed in Asian populations, while intracranial dissections are “very uncommon”. However, challenging clots—which tend to be rich in platelets, dense fibrin, von Willebrand factor, and/or NETs—are more prevalent and represent a “big problem”, particularly in Europe, due to their resistance to both thrombolytic drug therapies and thrombectomy procedures.
“We have long said that dense fibrin is the bad guy,” Andersson noted. “But, maybe it’s not so much the dense fibrin—maybe it’s the platelets. And, maybe it’s the platelets in combination with NETs. [Another] problem is that the clots that we can analyse are the ones that we can get out, but the clots that we cannot take out are unable to be analysed.”
Here, Andersson referenced a national registry in Sweden that has gathered data on “every single” stroke thrombectomy in the country since 2013, including collecting and analysing clot information from some

14,000 patients. A closer look at histopathological data from “unusual” clots considered to be outliers versus more common clot compositions in the registry indicated that these were all “very difficult” to remove. This exercise also revealed higher-than-average levels of extracellular DNA—many times, in conjunction with microcalcification—in these outlier clots.
“So, no wonder we had problems removing them,” he quipped, going on to cite multiple studies seen in an earlier presentation from Simon de Meyer (Catholic University of Leuven, Kortrijk, Belgium) suggesting that NET-rich clots often take “a lot of time and effort” to remove.
Offering possible solutions to the challenge posed by NET-rich clots, Andersson went on: “Once you have tried—and failed—using your favourite first-line strategy, you could attempt more advanced materials or techniques, like two stent retrievers, or the Nimbus [Cerenovus/J&J].”
The latter device—described by Cerenovus as a geometric clot extractor that is “designed to remove tough clots”—offers one “pretty good” alternative to
RESCUE STENT PLACEMENT
following a failed thrombectomy procedure has demonstrated “good outcomes” and a low risk of clinically significant bleeding in a multiethnic cohort of acute ischaemic stroke patients from the Middle East, Africa and Asia. Writing in the Journal of Vascular and Interventional Radiology, researchers conclude that their results are “similar” to those seen with postthrombectomy rescue stenting in the published literature.
Yahia Imam (Hamad Medical Corporation, Doha, Qatar) and colleagues initially note that, while mechanical thrombectomy has “revolutionised” stroke therapy in recent years, rescue stent placement following an
unsuccessful thrombectomy “remains controversial”—particularly for Asian patients, in whom intracranial atherosclerotic disease is more prevalent and believed to contribute more significantly to re-occlusion versus in Western populations.
As such, they conducted a retrospective, observational review of a multiethnic stroke database to evaluate the safety and effectiveness of rescue stenting in acute stroke patients with a large vessel occlusion (LVO) refractory to a thrombectomy procedure. Their primary outcomes included incidence of intracranial haemorrhage (ICH), recanalisation score, and favourable prognosis (modified Rankin scale [mRS] score ≤2), at 90 days. Imam and colleagues also detail that, after
more traditional tools that “may actually work” in more difficult, NET-rich clots, according to Andersson. However, this technology and double stent-retriever methods would “basically be useless” in ICAD cases, he added.
Andersson continued: “But, if we still fail, what should we do? Should we stent—and, if so, when, and after how many attempts? For me, the question is not ‘if’, but ‘when’, we should stent, because that’s the bailout for a difficult clot.”
The speaker then averred that, while existing data indicate an increased number of thrombectomy attempts being linked to worsening outcomes, the “worst outcome” is to leave the vessel occluded. “That is never, ever good,” he added.
Moving on to the pros and cons of balloon angioplasty as a post-thrombectomy rescue strategy, Andersson noted that this adjunctive technique does mean dual antiplatelet therapy (DAPT) can be avoided. However, balloon angioplasty in ICAD cases creates the potential problem of ‘snow-ploughing’, he posited, also citing challenges around balloon grading, which can lead to the operator overinflating the device; target-vessel re-occlusion due to recoil at the end of the procedure; and the “very real” risk of dissections and ruptures, potentially causing “catastrophic events”.
After warning neurointerventionists to “be careful with balloons”, Andersson disclosed that his own paradigm for tackling difficult clots is to try a given approach once or, at most, twice—for example, with two stent retrievers or the Nimbus device—before reverting to stenting.
Andersson rounded out his talk by stating that there are “quite a few” published papers supporting rescue stenting, specifically highlighting one of several metaanalyses on this topic that was published in 2020 in Interventional Neuroradiology by the Salpêtrière group from Paris, France. The paper concluded that permanent intracranial stenting after failed thrombectomy “appears” to improve clinical outcomes, before adding that further trials are needed to confirm this.
According to Andersson, a randomised trial—the “holy grail” for many—is currently being conducted by the same group, led by Kévin Premat (Charles Foix Hospital, Paris, France), and, as such, “we will see” what the most appropriate thrombectomy rescue strategy is in due course.
stent placement, an “aggressive” antiplatelet protocol was followed with glycoprotein IIb/IIIa infusion in all patients included in the analysis.
The review compared patients from the Middle East and North Africa (MENA) region, and those from other regions. Fifty-five patients (87% men; mean age, 51.3 years) were ultimately included; 32 were from South Asia (58%), 12 from the MENA region (22%), nine from Southeast Asia (16%), and two from elsewhere (4%; West Africa and East Asia).
The authors report that successful recanalisation (modified thrombolysis in cerebral infarction [mTICI] score
2b/3) was achieved in 43 patients (78%), while symptomatic ICH occurred in two patients (4%). A favourable outcome at 90 days was also seen in 26 of the 55 patients (47%). In addition—apart from a significantly higher average age (mean, 62.8 years vs. 48.1 years) and greater coronary artery disease burden (four [33%] vs. one [2%], p<0.05)—MENA patients demonstrated risk factors, stroke severity, recanalisation rates, ICH rates, and 90-day outcomes, that were all similar to those seen in patients from South and Southeast Asia.
Their findings within a multiethnic cohort of MENA and South Asian patients are therefore “comparable” to prior reports from East Asia, the authors aver, and constitute level-three evidence that rescue stent placement may be deemed a “viable option” in cases of refractory LVO due to probable atherosclerosis in acute ischaemic stroke patients. Utilisation of aggressive post-stent placement platelet inhibition was comparably safe and effective as well, Imam and colleagues state.
Late-breaking findings from the TWIN2WIN trial—presented recently at the International Stroke Conference (ISC; 7–9 February, Phoenix, USA)—have demonstrated the potential benefits of performing mechanical thrombectomy procedures using two stent retrievers (SRs) simultaneously. The trial compared a first-line dual-SR strategy to a traditional single-SR technique across more than 100 acute ischaemic stroke patients, and found improved recanalisation rates as well as no safety concerns with the more novel of the two approaches.
PRIOR TO DELIVERING THESE data for the first time at ISC 2024, Marc Ribo (Vall d’Hebron University Hospital, Barcelona, Spain) informed the audience that dual-SR techniques have been used for many years as a rescue strategy following initially unsuccessful thrombectomy attempts. He cited the fact that the approach can “dramatically reduce” direct friction between the clot and the arterial wall— instead “sandwiching” the clot between the two device structures—as one of the key benefits of using two SRs.
Recent research has indicated that the concurrent deployment of two SRs could also carry advantages as a first-line thrombectomy technique, particularly in certain middle cerebral artery (MCA) or terminal internal carotid artery (TICA) occlusions, leading to growing interest in this area.
In 2022, Ribo and his colleagues completed an animal study that ultimately suggested one dual-SR thrombectomy pass may induce slightly less cumulative histological damage to the endothelium compared to two passes with a single SR. A later study from Ribo et al involving in-vitro models then revealed positive findings regarding first-pass recanalisation with a dual- versus single-SR approach—a phenomenon
found to be more pronounced in MCA occlusions and, in particular, ‘saddle thrombi’ that are sitting across two codominant M2 branches.
Considering these two positive signals in combination, Ribo and his colleagues set out to establish a more direct comparison of dual- versus single-SR treatment strategies via the TWIN2WIN clinical trial. This prospective trial enrolled acute stroke patients undergoing thrombectomy—specifically those whose primary occlusion site was a bifurcation located in the TICA, the distal M1 segment of the MCA, or the top of the basilar artery—and randomised them to thrombectomy with either one or two SR devices as the first-line approach. Its primary endpoint was the rate of perfect/near-perfect recanalisation (thrombolysis in cerebral infarction [TICI] 2c–3) at the first pass.
TWIN2WIN was conducted across
five clinical sites in Spain, and initially planned to enrol 212 patients. However, according to Ribo, the trial was subject to a preplanned interim analysis after reaching 50% of its intended total patient population in November 2023—and, just a matter of days prior to the deadline for ISC 2024 abstract submissions, received a recommendation from its data and safety monitoring board (DSMB) to stop enrolment due to positive efficacy signals favouring dual-SR thrombectomy in that interim analysis.
Ribo reported that—across the 108 patients who were ultimately analysed—the distribution of occlusion locations was “well balanced”, and there were no major differences in baseline characteristics, between the dual- and single-SR study arms.
As per TWIN2WIN’s primary efficacy endpoint, the first-pass TICI 2c–3 rate was “substantially higher” in the study’s dual-SR arm (46.6%) versus the single-SR arm (24%), with Ribo reporting a statistically significant difference between the two (p=0.015).
Regarding key secondary endpoints, the presenter went on to note “more equal” outcomes in terms of final-pass TICI rates between groups—although there was still a numerically higher rate of TICI 2c–3 recanalisation with the dual-SR approach. He also relayed that, while overall numbers here were low, subanalyses revealed improved rates of successful recanalisation with a dual-SR strategy across different initial occlusion locations, consistent with the primary endpoint finding. Analysis of another secondary endpoint revealed that the mean number of thrombectomy passes was 1.58 in the dual-SR arm and 2.02 in the single-SR arm, while
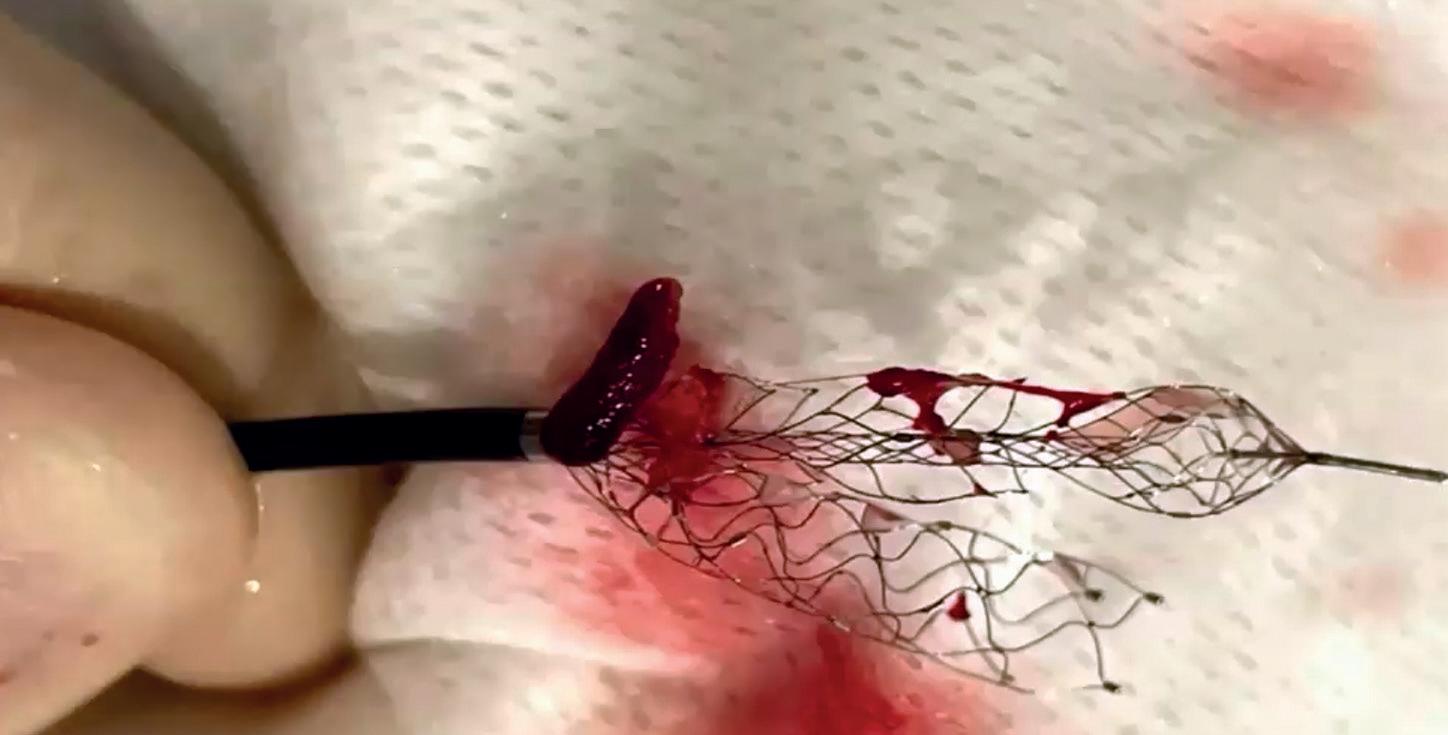
A SUBGROUP ANALYSIS OF THE ASTER2 clinical trial has found that a first-line combined thrombectomy approach, which involves using contact aspiration and a stent retriever in tandem, may produce better reperfusion outcomes compared to use of a stent retriever alone in patients whose ischaemic stroke is caused by an internal carotid artery (ICA) terminus occlusion with or without an M1-middle cerebral artery (MCA) occlusion.
Writing in the journal Stroke, Arturo Consoli (Foch Hospital, Suresnes, France) and colleagues do note, however, that the same superiority was not observed in stroke patients with isolated M1-MCA occlusions.
For this analysis, researchers included patients enrolled in ASTER2—a randomised trial comparing
the effects of combined versus stent retriever-only thrombectomy in large vessel occlusion (LVO) stroke patients. More specifically, they included patients with an ICA terminus occlusion with or without an M1-MCA occlusion, as well as those with an isolated M1-MCA occlusion.
Consoli and colleagues evaluated the effects of combination therapy against stent retriever-only thrombectomy as first-line approaches via angiographic and clinico-radiological outcomes. The former meant looking at the rate of first-pass effect, expanded treatment in cerebral infarction (eTICI) score ≥2b50, and eTICI ≥2c, at the end of the first-line strategy and at the end of the procedure. The latter consisted of 24hour National Institutes of Health stroke scale (NIHSS) scores, European cooperative acute stroke study (ECASS-III) grades, and three-month modified Rankin scale (mRS) scores.
A total of 362 patients were included in the postsubgroup analysis—299 had an isolated M1-MCA occlusion, 150 of whom were treated with a first-line combined approach, and 63 had an ICA terminus±M1MCA occlusion, with 30 undergoing a first-line combined approach.
Rates of eTICI ≥2b50 after the first-line technique
the median numbers of passes were 1 and 2, respectively, in the two groups (p=0.026).
Ribo went on to detail “absolutely no concerns” regarding safety, relaying symptomatic intracranial haemorrhage (sICH) rates of 6.4% in the dualSR arm and 4.3% in the single-SR arm—both of which, he averred, are “within normal ranges”. He then noted a non-statistically significant trend towards better clinical outcomes in the dual- versus single-SR arm, indicated by average rates of 90-day modified Rankin scale (mRS) scores of 0–1 (12.5% vs. 21.6%, respectively), and also median
I would conclude that these data show efficacy in terms of angiographic outcomes, and we are already planning further phases to confirm the clinical impact of these results.”
National Institutes of Health stroke scale (NIHSS) scores at discharge (11 vs. 6.5, respectively).
Finally, Ribo posited that, based on observations in the TWIN2WIN trial, “the duration is not longer with two stents”, owing to average procedure times that were “basically exactly the same”—roughly 45 minutes—in both groups.
“I would conclude that these data show efficacy in terms of angiographic outcomes, and we are already planning further phases to confirm the clinical impact of these results,” he added.
were significantly higher with the combined approach (100%) compared to the stent retriever-only approach (75.8%) in patients with ICA terminus±M1-MCA occlusions (odds ratio [OR], 11.83; 95% confidence interval [CI], 2.32–60.12), Consoli and colleagues report. They add that the rate of eTICI ≥2c in the combined group (66.7%) was roughly double that of the stent-retriever group (33.3%; OR, 4.09; 95% CI, 1.39–11.94). However, the same trend was not present in patients with isolated M1-MCA occlusions.
Across the 63 patients with ICA terminus±M1-MCA occlusions included in the analysis, the authors also relay that they found a modified first-pass effect rate of 33% with the combined thrombectomy approach, compared to 21.2% with use of a stent retriever only. The need for rescue treatments was distinctly higher in the stent retriever-only group (42.4%) versus the combined-thrombectomy group (20%).
However, positive clinical outcomes were more even between the two—as per three-month rates of mRS scores of 0–2, which were 37.9% in the stent-retriever group and 40.6% in the combined group. Decreases in NIHSS scores at 24 hours were also similar across groups, at −0.2 points (95% CI, −3.5–3.2) in the former and –0.7 points (95% CI, –4.2–2.9) in the latter.











AN INTERNATIONAL PANEL OF clinical researchers has published a report in the journal Stroke in which they advocate “immediate change” regarding treatment protocols for intracranial haemorrhage (ICH), citing the fact that haemorrhagic stroke management “lags far behind” that seen in more common ischaemic strokes.
ICH is caused by the spontaneous rupture of a small artery in the brain that results in bleeding into the brain. And, although ICH accounts for only 15–20% of all strokes, it is “by far the deadliest and most disabling form of stroke”, with a mortality rate of approximately 30%. That is according to a press release announcing the panel’s publication.
The same release details that, while a highly standardised and optimised
workflow has been adopted worldwide for treating acute ischaemic stroke, no such time-based emergency protocols are in widespread use for ICH.
Ischaemic stroke treatment places an emphasis on the restoration of blood flow as quickly as possible via clotbusting medications or a mechanical thrombectomy procedure to remove the clot. As such, stroke centres employ standardised protocols, and are required to report how many patients receive reperfusion therapy, how quickly it is given, and how often it is successful.
However—according to the present publication—no such quality measures are universally applied for ICH. The paper, entitled “Code ICH: A call to action”, is intended to help correct this disparity. It reviews the latest scientific
A new scientific statement from the American Heart Association (AHA) emphasises the need to boost patient and physician awareness of cerebral venous thrombosis (CVT), with a view to improving recognition of this condition and initiation of prompt medical treatment. The statement was published on 29 January in the AHA/American Stroke Association’s peer-reviewed journal Stroke
THIS RECENT STATEMENT REVIEWED THE latest evidence to update the AHA’s 2011 guideline outlining recommendations for diagnosing and managing CVT, focusing on advancements in treatment approaches including anticoagulation, catheter-based endovascular therapies, and surgery.
“Medical decisions undergo a thoughtful process, commencing with the suspicion of a diagnosis followed by the pursuit of relevant investigations to facilitate the implementation of optimal treatment. CVT serves as a relevant example in this regard,” Gustavo Saposnik (University of Toronto, Toronto, Canada), a leading author of the scientific statement, told NeuroNews. “Brain imaging, biomarkers, endovascular interventions and pharmacological therapies are rapidly advancing. It is anticipated that,
evidence supporting the effectiveness of various strategies for treating ICH, including lowering of elevated blood pressure, reversal of blood thinners, treatment for brain swelling, and surgical haematoma removal.
Based on current evidence, the authors advocate for the “immediate and widespread adoption” of a ‘care bundle’ designed to reduce blood pressure and reverse the effects of blood thinners within one hour of hospital arrival.
“Neurologists typically cite the phrase ‘time is brain’ to educate the public about the importance of acting quickly when someone is suspected of having a stroke,” said Stephan Mayer (New York Medical College, Valhalla, USA), co-senior author of the paper. “The fact of the matter is that this principle is unevenly applied. Hospitals are required to treat ischaemic stroke urgently and report their performance, but are under no obligation to do the same for ICH— even though it’s a more deadly disease. This disconnect has to change.”
“ICH is an emergency and should be treated as one,” added Joshua Goldstein (Harvard Medical School, Boston, USA), co-senior author of the paper. “We know that, during the first hours after a brain haemorrhage, there is active bleeding that causes continued damage in up to 40% of patients. Stroke
in the next decade, we will witness a transformation in the approach to diagnosing and treating CVT.”
The statement initially details that CVT is a rare condition accounting for fewer than 3% of all strokes, and that it often affects younger patients; women who are pregnant, postpartum, or using oral contraceptives; and people with a tendency to form blood clots. It also notes that—in the years since the AHA’s 2011 statement—obesity and active COVID-19 infection have been identified as additional risk factors associated with CVT.
The statement goes on to report that the diagnosis of CVT is “challenging”, because the most common symptoms are persistent headaches and seizures, which may mimic other neurological conditions like migraines or epilepsy.
Doctors typically use brain imaging, such as a magnetic resonance (MR) or computed tomography (CT) scan of the venous system, to identify CVT, but these tests “may not be routinely ordered” for patients presenting with headaches or seizures.
centres regularly treat hypertension and reverse anticoagulation, but there are currently no standards or requirements to give these treatments as quickly as possible.”
“Care bundles that emphasise ultra-early intervention for ICH have been studied; they dramatically reduce treatment times and improve outcome,” noted Qi Li (The Second Affiliated Hospital of Anhui Medical University, Hefei, China), first author of the paper. “Evidence-based guidelines
ICH is an emergency and should be treated as one.”
Joshua Goldstein
from professional organisations are used to codify best practices, but they can take years to develop. We wrote this consensus statement because our patients can’t wait that long. ICH is a life and death situation, and the time to act is now.”
The author group includes 18 experts in ICH care representing the USA, UK, Canada, China, Australia, Italy, and Germany.
appears to be a safe and effective alternative to vitamin K antagonists (VKAs) like warfarin.
However, according to the statement, “there is a need to identify other non-invasive treatments that are able to quickly dissolve blood clots and reduce pressure in the veins”.
It goes on to detail that endovascular procedures are typically reserved for patients with neurological deterioration despite medical therapy, or for those who cannot take blood-thinning medications.

As per the statement, recent evidence suggests that vascular imaging of the venous system using new MRI sequences or techniques could improve the likelihood of CVT diagnosis too.
The progression of CVT “may be difficult to predict”, according to the statement; while 80–90% of individuals with the condition achieve functional independence, many experience a variety of residual symptoms and—despite receiving intensive medical treatment—some 10–15% of patients experience severe outcomes, including death, disability, or loss of independence.
Standard treatment involves anticoagulation therapy to prevent growth of the blood clot causing CVT, and to ultimately facilitate reopening of the blood vessel and prevent recurrent clots.
Recent studies, the statement continues, have shown that the use of direct oral anticoagulants (DOACs)
As per the statement, decompressive craniectomy involving surgical removal of a portion of the skull is a “lifesaving procedure” to be considered for patients with severe CVT or progressive neurological deterioration.
The statement adds that,
“although there are no randomised controlled trials of this surgical approach, a meta-analysis of 51 studies found that surgery within 48 hours of admission may decrease mortality, and result in improved functional outcomes, in patients with severe or progressive disease”. The analysis in question—published in the European Journal of Clinical Investigation in late 2022—included a total of 483 CVT cases treated via decompressive craniectomy, and also concluded that further prospective studies with appropriate control arms will be required to confirm the superior efficacy of surgery versus medical management.
Finally, the statement details that, in 2021, the US Centers for Disease Control and Prevention (CDC) and Food and Drug Administration (FDA) found that adenovirus-based SARS-CoV-2 vaccines were associated with vaccine-induced thrombotic thrombocytopaenia (VITT)—a condition that causes low blood platelet count and affects clotting.
That said, CVT in VITT is “rare”, and there is “no evidence” of VITT in adults who received either of the two FDA-authorised mRNA COVID-19 vaccines from Pfizer and Moderna, the statement avers.
Michihiro Tanaka, director of Neurosurgery and Neuroendovascular Surgery at Kameda Medical Center (Chiba, Japan), speaks to NeuroNews to discuss the factors that have shaped his career as a neurosurgeon, his tenure as World Federation of Interventional and Therapeutic Neuroradiology (WFITN) president, and the current landscape of neurosurgical care.
What initially drew you to medicine, and the field of neurosurgery specifically?
Ever since I can remember, I’ve been interested in where the human body came from and what our ancestors looked like, and eventually became interested in palaeontology, and the study of dinosaurs, ammonites, and other things. As I studied mathematics, physics, chemistry and biology in junior high and high school, I became increasingly interested in the origins of living things and the history of vertebrates. If I was going to study vertebrates, I wanted to learn about the anatomy of Homo sapiens—which have a large brain—so I entered medical school without any doubts.
Who have your key mentors been and how have they impacted your career?
I have three people whom I consider to be my lifelong mentors. The first one is Isao Yamamoto, a professor in the Department of Neurosurgery at Yokohama City University School of Medicine (Yokohama, Japan) who worked on microanatomy under Albert Rhoton Jr at the University of Florida (Gainesville, USA) during his younger years. He was the mentor who taught me the importance and joy of microanatomy. The second one is Nobuo Hashimoto, who was the chief of the Department of Neurosurgery at the National Cardiovascular Center (Osaka, Japan) when I was a resident, and later became a professor at Kyoto University (Kyoto, Japan). He taught me the basics of microneurosurgery. The third is Anton Valavanis, who was a professor in the Department of Neuroradiology at the University Hospital of Zürich (Zürich, Switzerland). He taught me the fundamentals and clinical aspects of endovascular treatment in the brain, as well as embryological perspectives on brain arteriovenous malformations (AVMs). Regrettably, he passed away last May. He is the most important mentor in my life.
How did your time at the University Hospital of Zürich help shape your neurosurgical career?
When I initially took up my position in 1998, I was scheduled for a one-year clinical fellowship. However, as I had been studying German as a second language, Professor Valavanis offered me a salaried position (assistant professor) in 1999. By 2002, he promoted me to the position of Oberarzt (associate professor). In total, I had the opportunity to work at the University of Zürich for nearly six years. The university, founded in the 16th century, has produced many notable neuroscientists. It is famously associated with pioneers like Constantin von Monakow and Walter Rudolf Hess—and, in recent times, Mohamed Gazi Yaşargil, the founder of
microneurosurgery, served as the university’s second professor of neurosurgery. My mentor, Professor Valavanis, was a radiologist who studied under Yaşargil and became the first professor of neuroradiology. Working at such a historic institution for nearly six years was a significant asset for me. Experiencing endovascular treatment of the brain in the mecca of microneurosurgeons was extremely important. It allowed me to learn surgical techniques but also gain an understanding of cerebrovascular disorders and the basics of clinical research within an interdisciplinary approach, which was very meaningful.
Could you outline your priorities as WFITN president?
The WFITN is a scientific society established in 1990 in Val d’Isère, France by Luc Piccard, Alejandro Berenstein, Pierre Lasjaunias, and others. It is a very historic association in this field and, as president, I have three main missions. The first is to consolidate neurointervention societies from around the world into a federation assembly. Currently, 18 societies from 16 countries participate in this federation assembly. One of my missions is to expand this assembly further, forming a community that transcends national and racial boundaries, and to provide multinational clinical research and educational programmes. The second mission involves managing the WFITN Educational Fund. This system uses funds collected from companies in various countries to assist young doctors from lowincome countries who aspire to specialise in neurointervention. In 2023 specifically, seven doctors were able to participate in training programmes in Europe using this educational fund. The third mission is to organise WFITN anatomy courses. Knowledge of functional anatomy is essential for safe endovascular treatment of the brain. We are committed to holding training courses on anatomy relevant to endovascular treatment in various countries worldwide, helping young doctors in this field to perform surgeries safely.
What are the most notable ways in which neurosurgery has changed over the course of your career?
The Guglielmi detachable coil (GDC), introduced in 1997 in Japan, was indeed a revolutionary development. Until then, the cornerstone of cerebral aneurysm treatment had been clipping surgery via craniotomy, but the advent of this new device marked a significant shift. Clipping surgeries for aneurysms—particularly those in the posterior circulation like the basilar artery—were challenging areas for microneurosurgeons. The ability to treat aneurysms deep within the skull without craniotomy represented a major revolution in neurosurgical
therapeutics. Encountering the GDC was a pivotal moment for me. It was then that I first became convinced that the future of cerebral aneurysm treatment would inevitably shift to endovascular methods, and this realisation was the impetus for my transition from being a microneurosurgeon to a neurointerventionist.
In the future, how is the relationship between open and endovascular neurosurgical techniques likely to shift?
2022–2024: President, WFITN
2023–present: Director of Neurocenter, Neurosurgery and Neuroendovascular Surgery, Kameda Medical Center
2016–present: Advisory committee, Ministry of Health Labor and Welfare, Pharmaceuticals and Medical Devices Agency (PMDA)
Visiting professor at Tokushima University (2022–present), Tokyo Jikei University School of Medicine (2022–present), Fukuoka University Chikushi Hospital (2016–present), and Showa University of Medicine (2016–present)
EDUCATION
2006: PhD, medical science, Yokohama City University School of Medicine
1998–1999: Clinical fellowship, Institute of Neuroradiology, University Hospital of Zürich
1994–1996: Residency, Neurovascular Surgery, National Cardiovascular Center
1985–1991: MD, National Yamanashi University School of Medicine (Kofu, Japan)
Intracranial aneurysms, AVMs, dAVFs, head and neck tumours, spinal cord vascular malformations, and carotid artery revascularisation
Discussing gastric cancer as an example, the advancement of preventive medicine, such as the eradication of Helicobacter pylori—along with progress in endoscopic examination and treatment—has led to a situation where open gastrectomy for gastric cancer is now rarely performed. This trend suggests that the indications for open craniotomy will undoubtedly continue to diminish. In our hospital, traditional open craniotomies under the microscope have significantly decreased, being replaced by surgeries performed with neuroendoscopes and neuro-exoscopes.
Surgical treatment is evolving towards an increasingly minimally invasive style. Regarding the treatment of cerebrovascular disorders, the role of craniotomies is expected to reduce further, with endovascular treatments becoming the mainstream. However, craniotomies using the next generation of operative imaging will still remain an important treatment modality for trauma and benign tumours at the skull base.
What are the biggest challenges facing neurointerventional care in Japan today?
Subspecialty dispersion among neurosurgeons is a significant challenge; Japanese neurosurgeons tend to be generalists, covering a wide range of responsibilities from surgery to postoperative management. This broad scope often leads to a dispersion of subspecialties. For instance, while cerebrovascular disorders are a primary focus for many, neurotrauma and neurocritical care are less emphasised. One survey indicated a decline in neurosurgeons specialising in neurotrauma and even fewer choosing neurocritical care as their subspecialty. The rate of older patients (octogenarians) undergoing neuroendovascular therapy has also increased significantly. This shift is likely due to Japan’s ageing population, which presents unique challenges in treatment approaches and outcomes.
Which piece of research you have been involved in has had the most significant impact within the neurosurgical space?
I contributed a couple of articles regarding the embryological consideration of dural arteriovenous fistulas (dAVFs)—vascular abnormalities that can be influenced by their embryological origin. In particular, dAVFs located in the falcotentorial area, which derive from the neural crest, have been identified as a risk factor for a more aggressive clinical course. This includes more significant clinical presentations, and a higher likelihood of cortical and spinal venous reflux. The neural crest origin of these dAVFs—particularly in areas like the olfactory groove, falx, tentorium of the cerebellum, and nerve sleeves of the spinal cord—contributes to their unique clinical characteristics and challenges in treatment. This highlights the importance of considering the embryological origins of dAVFs in their diagnosis and management.
Which areas are likely to have the greatest impact on neurosurgery over the next 10 years?
Enhanced imaging and visualisation techniques; the integration of robotics and artificial intelligence (AI); research in nerve regeneration and neuroplasticity; genomics and personalised medicine; neuroprosthetics, including brain-computer interfaces; and new neuro-oncology therapies and techniques in treating brain tumours, such as immunotherapy and targeted drug delivery. These areas reflect the convergence of technology, personalised medicine, and innovative surgical techniques, driving forward the field of neurosurgery.
Could you describe one memorable case and the effect it had on you as a surgeon?
In 1996, I encountered a remarkable case that profoundly influenced my career choice: a baby girl born with severe heart failure due to Vein of Galen aneurysm malformation. We successfully treated her with an endovascular approach on the third day and again at one month after birth. This intervention effectively
resolved her heart failure and she experienced normal neurological development. Now, she is 27 years old, in good health, and gainfully employed. This unforgettable experience solidified my decision to specialise in neuroendovascular surgery.
What advice would you give to people embarking on a career in neurosurgery?
Embarking on a career in neurosurgery is a challenging yet immensely rewarding journey. It is as demanding as it is fulfilling, requiring a blend of intellectual capability, technical skill, emotional strength, and a profound commitment to patient care. As such, there is plenty of advice to consider. Ensure you have a strong grasp of the basic sciences, particularly anatomy, physiology and pathology. Be prepared to continually update and refine your knowledge, and surgical skills, to stay abreast of the latest techniques and technologies. Be prepared for long hours and demanding work schedules—resilience, perseverance and a strong work ethic are essential. Exercise excellent communication
"Embarking on a career in neurosurgery is a challenging yet immensely rewarding journey.”
skills and empathy, as these are vital when dealing with patients and their families. Engage in research and academic pursuits that contribute to the field, but also enhance your understanding and open opportunities for career advancement. Build a network of peers and mentors, as guidance and support from experienced neurosurgeons is invaluable. Balancing work and personal life is crucial for long-term success and wellbeing, so take care of your mental and physical health. And, always prioritise ethical practice—the patient should be at the heart of all your decisions.
What are your interests outside of medicine?
A passion for wine, conducting genetic research on grapes, engaging in tea ceremonies influenced by Zen teachings, and playing Bach’s piano compositions.
What would you be doing for a living if you had not chosen a career in medicine?
Working as a farmer of grapes, especially pinot noir, and producing my own brand of wine.

Intracranial aneurysms are a largely underestimated condition for which global awareness and clinical research are currently lacking—and these sentiments apply even more pertinently to hereditary brain aneurysms. For NeuroNews, Rebecca Middleton (Leicester, UK) discusses how her own diagnosis led to the inception of Hereditary Brain Aneurysm (HBA) Support, and outlines the organisation’s achievements to date as well as future goals.
In the winter of 2015, my life took an unforeseen turn when I received a life-altering diagnosis: a brain aneurysm coupled with familial intracranial aneurysm syndrome. This moment signalled the onset of a journey marked by uncertainty and an enormous desire for understanding. Every patient seeks solace in a community of understanding. But, despite the gravity of my condition—one that had claimed the lives of my mother, grandmother and others in my family—I grappled with the realities of the diagnosis alone, with little specialist support. However, as vice chair for rare conditions on the participant panel at Genomics England, I understood I was unlikely to be truly alone and that there would be hundreds of families living with the complexities of hereditary brain aneurysms. Knowing this spurred me to launch HBA Support in 2022. We’re now a growing and busy patient organisation with a clear mission to build a community of people who share experience and insight, supporting families and together advancing our understanding of the condition as a genetic, life-changing disease.
A hereditary brain aneurysm refers to a specific type of intracranial aneurysm that exhibits a familial clustering pattern within families. Unlike sporadic cases, where aneurysms occur due to environmental or other risk factors, hereditary brain aneurysms are characterised by a genetic predisposition that increases the likelihood of developing aneurysms among close relatives. Diagnosed individuals often have a confirmed family history of the condition, indicating a genetic component. This clustering of aneurysms within families can pose unique challenges in diagnosis, management and treatment.
One of the distinguishing features
population patterns. The TLR also evidences an absence of comprehensive guidelines for the management and treatment of hereditary brain aneurysms, and underscores the need for tailored, evidence-based recommendations. By identifying areas for improvement, the TLR serves as a catalyst for collaboration, advocacy and progress. HBA Support recently connected with the ROAR study, led by Diederik Bulters (Southampton University, Southampton, UK), and hopes this ambitious research will shed more light on knowledge gaps identified in the TLR.
of hereditary brain aneurysms is their heightened risk of rupture, which can lead to potentially severe consequences. Research suggests that a person with a strong family history of intracranial aneurysms is up to three times more likely to have the condition compared to someone in the general population. This increased risk underscores the importance of early detection, vigilant monitoring and tailored treatment strategies for individuals and families affected by hereditary brain aneurysms.
To drive a better understanding and help unravel the mysteries surrounding the condition for all stakeholders but, in particular, patients and their families, HBA Support commissioned a targeted literature review (TLR), executed on a pro-bono basis by Costello Medical. Previously, families had limited access to easy-to-understand resources on the condition. They often find themselves trying to piece together information from multiple sources, which can be difficult to understand and navigate, and tend to be found behind paywalls.
The TLR unpacks the pattern and distribution of hereditary brain aneurysms, as well as their genetic causes, and the UK and global guidelines for diagnosis, management and treatment. It also highlights existing gaps in research and knowledge. While much remains to be interpreted and discovered about the genetic markers underlying hereditary brain aneurysms, ongoing research efforts offer hope for better understanding and management of this complex condition.
It is reported in the TLR that the global prevalence of unruptured intracranial aneurysms in individuals with a family history is 2.3–29.4%, compared to 0.02–8.8% in the general population—pointing to the need for more research, and an improved understanding of familial and general
At HBA Support, we’re dedicated to providing vital assistance to those living with hereditary brain aneurysms in a clear, understandable way. Our approach is simple: we listen, connect and empower. Our active and growing online community of around 400 people is supported by a part-time team of engagement and community specialists, and experienced and clinical volunteers. During our first year, we launched a series of user-friendly patient guides. So far, the guides—developed by UK National Health Service (NHS) genetic counselling associates—have been downloaded more than 400 times. We’ve also started hosting webinars with expert groups, such as the Brain Aneurysm Foundation in the USA as well as members of our patient and advisory panels, tackling key topics like familial risk factors and screening. And we’re not stopping there. Soon, we’ll be launching a peer-to-peer Facebook community to sit alongside our information page, providing a safe space where people can connect.
With a brain aneurysm comes fear and, often, loss. We’re keen to redress the balance by outlining the care options that offer hope and choice.”
I had my growing aneurysm coiled and stented in 2018 after close monitoring for a number of years. I now undergo regular scans and continue to have my care from specialists at Queen’s Medical Centre (Nottingham, UK). In 2015, I also joined the world-first 100,000 Genomes Project, delivered by the NHS and Genomics England with the aim of whole-genome sequencing 100,000 babies. While I continue to wait for my own genetics diagnosis, the project did lead me to the participant panel at Genomics England in 2016, where I now sit as vice chair for rare conditions. Here, I learnt the power of patient
involvement, lived experience and collaboration in patient advocacy.
This has led to HBA Support playing an active role in a number of networks aligned with our organisational values and goals. We’re proud members of Genetic Alliance, Eurordis, Gene People Partnership Network, and Neuro Alliance; we’re also an active member of Rare Disease Research Network, and work closely with the NHS Genomic Medicine Service and Genomics Education colleagues. We presented a poster at the Barts Research and Advanced Interventional Neuroradiology (BRAIN) conference (4–6 December 2023, London, UK) as well. With this experience and knowledge, we’re keen to collaborate further in brain aneurysm research, and are delighted to have two MSc genetic counselling students from Cardiff University (Cardiff, UK) working with us soon to look at the emotional and psychological impact of this disease.
We also have a busy year ahead in terms of building awareness of our mission within rare disease and research communities. We recently visited the UK House of Commons for Rare Disease Day 2024 (29 February). We’ll also be speaking at the Economist’s Cell and Gene Therapy Summit in Brussels, Belgium in April; heading to Barcelona, Spain for the Eurordis Summer School in June; building our campaign ready for brain aneurysm awareness month in September; and planning our first fundraising event in Leicester, UK in October to bring all aspects of our community together.
With a brain aneurysm comes fear and, often, loss. We’re keen to redress the balance by outlining the care options that offer hope and choice. My family was blighted with tragedy but, through screening and surgery, I now have some peace of mind and there is light in the darkness. For my family and for the whole community, we have a duty to balance the fear with accessible information, fact-based conversations, and informed pathways, ensuring an equitable and supported patient journey for all. By actively engaging with patients, participating in collaborative research efforts, and advocating for improved screening and treatment guidelines, the medical and patient community can make a profound impact—providing hope and light in often dark and risk-filled journeys. Together, through collective action and unwavering dedication, we can drive positive change, improve patient outcomes and, ultimately, save lives.
Rebecca Middleton is the founder and chief executive officer of HBA Support. She is also a founding member of the participant panel at Genomics England, for which she is vice chair of rare conditions, and sits on the patient and public voice (PPV) panel for the East Genomic Medicine Service.
The author declared no relevant disclosures.
“The third way” in intrasaccular aneurysm treatment demonstrates positive one-year outcomes
A device labelled “the third way” to potentially treat intracranial aneurysms via an intrasaccular approach has demonstrated positive effectiveness and safety results at one year in a first-inhuman (FIH) study, as per a presentation at the Barts Research and Advanced Interventional Neuroradiology (BRAIN) conference (4–6 December 2023, London, UK).
ON THE OPENING DAY OF THE conference, Boris Pabon (AngioTeam, Medellin, Colombia) shared one-year follow-up data from the PRE-SEAL IT trial, which is being conducted to assess the feasibility, effectiveness and safety of the novel Saccular Endovascular Aneurysm Lattice (SEAL) embolisation system (Galaxy Therapeutics).
“This kind of innovation is designed for those interventionists who are dissatisfied with the current intrasaccular technologies,” Pabon added, also detailing that the SEAL device is made from nitinol and has a dual-braided structure, offering a high mesh density at the level of the neck.
Pabon initially averred that, in wide-neck bifurcation aneurysms, coiling rates are currently going down, with usage of flow diverters and intrasaccular systems increasing—but that, “probably, no one […] has the right answer” when it comes to the best tool for treating these cases. Two key technologies exist right now in the intrasaccular space: Woven EndoBridge (WEB; Microvention/Terumo) and, more recently introduced, Contour (Cerus/Stryker).
“I think we have a gap, in terms of effectiveness
between intrasaccular technologies and endoluminal technologies, and this gap represents a true opportunity—an opportunity that requires movement,” Pabon stated. “We really need something new. But, not just new. We need something different, and better, that helps us to reduce this gap.”
This was the context within which the Galaxy team began to conceptualise and develop its SEAL technology. Recounting an “exciting conversation” that took place back in 2021 with Osama Zaidat (Mercy Health, Toledo, USA)—now president and chief executive officer of Galaxy—Pabon recalled thinking: “Wow, I really want to be a part of this!” He also briefly outlined the device’s journey from being “a drawing on a napkin” to benchtop testing, animal models, and finally the FIH PRE-SEAL IT trial that started in early 2022.
The primary endpoints of the PRE-SEAL IT trial were feasibility, as per technical procedural success; effectiveness, as per complete occlusion rates; and safety outcomes. The trial was conducted in Medellin, Colombia, and saw a total of 33 non-consecutive, “highly selected” intracranial aneurysms treated across 29 patients. The majority of these aneurysms were located in the anterior circulation, and the “vast majority” were electively treated, Pabon detailed. Both configurations of the device—the SEAL Arc, designed to treat elongated, deep, saccular aneurysms, and the SEAL Base, better suited for irregular-shaped, shallow, non-saccular aneurysms—were used in the trial.
Arriving at PRE-SEAL IT’s key results, Pabon reported a technical success rate of 100%, with most of the cases—all but 10—being performed “without previous medication”. In addition, he relayed that no adjunctive therapies, such as assistive stents or balloons, were required in any of the cases.
Pabon went on to note that complete occlusion was achieved “immediately” after deployment in 20% of cases, and adequate occlusion was accomplished in two thirds of cases at the same timepoint. Twentyfour hours later, the investigators observed complete and adequate occlusion rates of 51.5% and 72.2%, respectively. And, at six months, they found that progressive complete occlusion had been reached in roughly 73% of cases, while adequate occlusion rates were closer to 90%.
“But, most importantly, at the one-year follow-
A NEW STUDY, PUBLISHED IN the Journal of NeuroInterventional Surgery (JNIS), has assessed the safety of the latest iteration of the Pipeline Vantage embolisation device (Medtronic) in treating unruptured intracranial aneurysm patients—ultimately producing “acceptable outcomes” with the technology.
The PEDVU study’s authors—led by Thomas Booth (King’s College Hospital NHS Foundation Trust, London, UK)— further report that this second, updated version of the Pipeline Vantage appears to boast superior efficacy and a similar safety profile in treating unruptured aneurysms, as compared to previous generations of the device.
“It is always vital to assess new devices in the clinic to assess and compare how effective they are in treating the disease, and how safe they are for the patients,” Booth said.
“Overall, these are acceptable outcomes in this pragmatic and non-industrysponsored study.”
Using data from eight hospitals, researchers based across the UK were able to show that the second version of the Pipeline Vantage could effectively and safely stop blood flow into patients’ unruptured aneurysms—as well as demonstrating that prior challenges regarding device placement had been overcome.
The first version of Medtronic’s Pipeline Vantage was introduced via a limited, premarket release. However, operators experienced technical difficulties when placing the device into an artery, leading to its withdrawal from the market. The second version was released globally
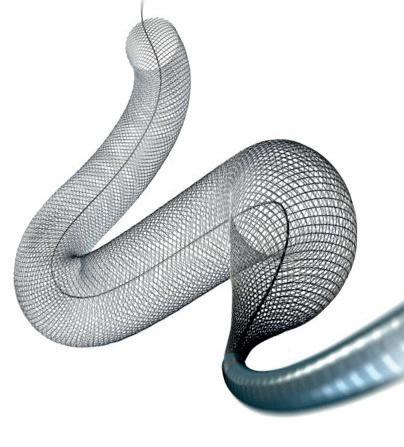

up—in 26 of the 33 aneurysms [78.7%] in the series for which we had angiographic [data]—the complete occlusion rate was 76.9%, and adequate occlusion was seen in 84.6% of the cases,” Pabon said, also detailing that the PRE-SEAL IT investigators are expecting to have oneyear follow-up data on all the aneurysms treated in the trial by early 2024. The presenter further noted, at one year, regrowth occurred in two aneurysms (8%), while all 29 patients (100%) with clinical follow-up data achieved a modified Rankin scale (mRS) score of 0–2.
Here, Pabon also posited that the complete occlusion outcome seen here provides evidence of a superior occlusion rate compared to the “famous number” of 50–60% typically reported with other intrasaccular technologies.
Regarding safety outcomes, Pabon stated that there were no haemorrhagic adverse events, including instances of ‘re-bleeding’; minor and transient thromboembolic adverse events were seen in three of the 33 treated patients (9%); and no major or definitive thromboembolic adverse events, nor definitive morbidities or procedural mortalities, were observed.
Pabon concluded his presentation by noting that, within these one-year experiences, the SEAL appears to be a feasible treatment option for intracranial aneurysms owing to its technical success rate of 100%. Furthermore, the device demonstrated effectiveness, via a 76.9% rate of progressive complete occlusion at one year, and a “really good safety profile”, with no observed morbidity or mortality, and a 0% retreatment rate.
In addition, the presenter speculated that the SEAL device may offer a “good solution” across a range of challenging scenarios—including in aneurysms that are ruptured, shallow, or greater than 10mm in size, but also as a means for avoiding dual antiplatelet therapy in some “highly selected” sidewall aneurysms.
following modifications to address these technical difficulties but, until now, had not been assessed clinically to confirm that said difficulties had been remedied. Researchers therefore undertook a multicentre study, with retrospective and prospective elements, using core laboratory assessments. They evaluated 30-day and ≥3-month mortality and morbidity rates, and six- and 18-month radiographic aneurysm occlusion rates, for procedures performed between July 2021 and March 2023. Primary and secondary objectives of their study were to determine outcomes for unruptured and ruptured aneurysm cohorts, respectively.
“We included 121 consecutive patients with 131 aneurysms,” the researchers write in JNIS. “The adequate occlusion rate for the unruptured cohort at shortterm and medium-term followup, and also for the ruptured cohort at short-term follow-up, was >90%. Two aneurysms (1.5%) underwent retreatment. When mortality attributed
to a palliative case in the unruptured cohort or subarachnoid haemorrhage in the ruptured cohort was excluded, then the overall major adverse event rate in respective cohorts was 7.5% and 23.5%, with 0% mortality rates for each. When all event causes were included on an intention-to-treat basis, the major adverse event rates in respective cohorts were 8.3% and 40.9%, with 0.9% and 22.7% mortality rates.”
They further conclude that this second version of the Pipeline Vantage appears easier to deploy compared to its predecessor, in addition to suggestions of superior efficacy and an equivalent safety profile for unruptured aneurysms.
While their deductions regarding ruptured aneurysms are “limited” by a small cohort size within the study, the researchers report observations of efficacy that are similar to previous studies and “may be acceptable”. However, they add that there appears to be a high rate of adverse events in these acute and typically riskier cases— and, as such, evidence from further, prospective studies is needed to justify the routine use of Pipeline Vantage devices in ruptured aneurysms.
CAROTID ARTERY STENTING (CAS) delivered via transradial artery access has demonstrated no increased risk of stroke, death, myocardial infarction, transient ischaemic attack, or access site complications, as compared to the more traditional transfemoral approach. That is the concluding finding of a recent Stroke: Vascular and Interventional Neurology publication from Santiago Ortega-Gutierrez (University of Iowa Hospitals and Clinics, Iowa City, USA) and colleagues.
“The transradial approach shows promise as an alternative method for CAS, offering potential benefits without increased risk of complications,” the authors write. “However, further studies are needed to confirm these findings.”
Ortega-Gutierrez and colleagues initially aver that transfemoral arterial access, while still the most widely used and preferred method for CAS, is associated with “inherent limitations and potential complications”, primarily related to access. The authors further state that positive results and experiences within interventional cardiology have seen transradial displace transfemoral access, becoming the primary approach for many cardiovascular procedures.
“Consequently, exploring transradial artery access as a potential option [due to innovative advances in large bore catheters] becomes crucial in optimising patient outcomes and procedural success rates,” they add, also noting that there are limited data comparing the approach and its outcomes to transfemoral access in CAS.
As such, the authors conducted a systematic review and meta-analysis of the existing literature in this space. From an electronic search of four databases, randomised and non-randomised studies alike involving CAS via transradial or transfemoral approaches were included. Overall, six studies comprising a total of 6,917 patients featured in their analyses. Some 602 of these patients (8.7%) underwent transradial access for the procedure, while 6,315 (91.3%) received transfemoral access.
Ortega-Gutierrez and colleagues’ subsequent meta-analysis showed “no significant difference” between the two access approaches in terms of stroke occurrence, with strokes being observed in 1.7% of patients in the transradial group and 1.9% in the transfemoral group (odds ratio [OR], 0.98). Furthermore, no significant differences were detected regarding death (1% vs. 0.9%, respectively; OR,
A large, single-centre analysis has found that, despite parity between the two procedures in terms of perioperative clinical outcomes, carotid endarterectomy (CEA) was associated with a reduced rate of late stroke/death compared to transcarotid artery revascularisation (TCAR).
WRITING IN THE JOURNAL of Vascular Surgery (JVS), Ali AbuRahma (Charleston Area Medical Center/West Virginia University, Charleston, USA) and colleagues also concluded that CEA produced a marginally lower rate of restenosis ≥80% at two years—although this difference did not reach statistical significance.
The authors initially note that multiple registry studies—primarily ones conducted within the Society for Vascular Surgery’s (SVS) Vascular Quality Initiative (VQI)—have compared early outcomes, and outcomes up to one year, between TCAR, CEA and carotid artery stenting (CAS).
However, no large, single-centre studies have reported on late clinical outcomes between these procedures and, as such, they conducted a retrospective analysis comparing intermediate outcomes with TCAR versus CEA.
Their study analysed data collected from the TCAR Surveillance Project on patients at the Charleston Area
Medical Center, with these data being compared and subsequently propensity matched against patients undergoing CEA. Patients in both groups were treated by the same providers across the same time period, the authors note.
The primary outcome of the analysis was a combination of perioperative stroke/death and late stroke/death rates, while secondary outcomes included combined stroke/death/ myocardial infarction (MI), cranial nerve injury (CNI), and bleeding. Kaplan-Meier analyses were used to estimate patients’ freedom from stroke, stroke/death, and also rates of restenosis ≥50% and ≥80%.
Across 637 patients, a total of 646 procedures (404 CEAs and 242 TCARs) were analysed, with AbuRahma and colleagues finding no significant differences in indications for carotid intervention between these two groups—although TCAR patients had more high-risk criteria including hypertension, coronary artery disease, congestive heart failure, and renal failure.
The authors report that there was no
0.95), myocardial infarction (0.2% vs. 0.3%; OR, 1.53), transient ischaemic attack (0.4% vs. 1%; OR, 0.46), or access site complications (2.2% vs. 1%; OR, 0.97).
These findings align with other, previously published meta-analyses of the data in this area, according to the authors. However, in contrast to those analyses, the present study employed a Grading of recommendation, assessment, development, and evaluation (GRADE) approach to assess the certainty of the evidence—with the hope of providing “a more comprehensive and robust evaluation of the safety of CAS to better inform decision-making”.
“Although these results indicate the potential viability of the transradial approach as an alternative to the traditional transfemoral access for CAS, the relatively small number of studies, considerable heterogeneity [in outcome definitions] among them, and the low certainty of evidence, highlight the need for caution in relying on these findings,” Ortega-Gutierrez and colleagues write. “The clinical decision-making process should therefore be guided by a personalised approach that considers individual patient characteristics and anatomical considerations. Further studies, including RCTs [randomised controlled trials] with larger sample sizes, are warranted to confirm and refine these findings, and to guide clinical decision-making in the selection of the optimal access approach for CAS.”
significant difference between CEA and TCAR in terms of the rates of 30-day perioperative stroke (1% vs. 2%, respectively), stroke/death (1% vs. 3%), or major haematoma (2% vs. 2%). However, the rate of early CNI was found to be significantly higher with CEA (5%) versus TCAR (1%; p=0.0138).
In addition, late follow-up analyses at two years revealed results favouring CEA over TCAR, as the former produced lower rates of stroke (1% vs. 4%, respectively; p=0.0273), stroke/death (8% vs. 15%; p=0.008), and restenosis ≥80% (0.5% vs. 3%; p=0.0139).
The type of intervention, CEA versus TCAR, should depend on risk stratification— anatomical and/or physiological.”
AbuRahma and colleagues’ propensity-matched analysis, under which 242 CEAs and 242 TCARs were included, found broadly similar perioperative outcomes regarding stroke rate (CEA, 1% vs. TCAR, 2%), stroke/ death rate (CEA, 2% vs. TCAR, 3%) and CNI rate (CEA, 3%
vs. TCAR, 1%). Once again, however, their later follow-up analysis generally favoured CEA, as demonstrated by a rate of stroke of 1%—compared to 4% with TCAR (p=0.0615)—and a rate of stroke/death of 8%—compared to 15% with TCAR (p=0.0345).
Finally, the authors also note a marginally smaller difference in the occurrence of restenosis ≥80% following propensity matching, as compared to their regular analysis, with rates of 0.9% associated with CEA versus 3% with TCAR (p=0.099). This difference was not deemed statistically significant, they add, and rates of restenosis ≥50% were not statistically significantly different between the two procedures either.
“In propensity-matched analysis, both CEA and TCAR have similar perioperative clinical outcomes. However, CEA was superior to TCAR for the rates of late stroke/death and had somewhat lower rates of ≥80% restenosis at two years,” AbuRahma told NeuroNews, outlining the salient message from the paper. “The type of intervention, CEA versus TCAR, should depend on risk stratification—anatomical and/

Ali AbuRahma
This year’s International Stroke Conference (ISC; 7–9 February, Phoenix, USA) hosted discussions on the current state of play in carotid stenosis treatment—and, most notably, advances that may erode the dominance of long-established carotid revascularisation procedures in the USA.
Session moderator Larry Goldstein (University of Kentucky, Lexington, USA) provided a fitting initial comment on the lack of up-to-date trial data evaluating different carotid disease treatments: “Looking around the room, some of you weren’t even born when these trials were done!”
As Goldstein noted, the trials in question—NASCET in the USA and ECST in Europe—were conducted and published some 30 years ago, and only included patients with symptomatic carotid artery stenosis.
“For some of you, this is almost ancient history—but, if you go to our national guidelines, this is all the grade A, level one evidence that we have,” he continued. “When the studies were done, they were internally valid, but time marches on, and things that we thought were true 40 years ago may not be true today.”
Dwindling need for revascularisation?
This proved to be an appropriate segue into a presentation from Seemant Chaturvedi (University of Maryland School of Medicine, Baltimore, USA), who began by stating that things have “dramatically changed” since the advent of those original trials in the 1990s. The succeeding decades have seen well-established medical therapies like antiplatelets, statins, blood-pressure control, physical activity and dietary modification joined by numerous modern options, including proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, inclisiran, bempedoic acid, semaglutide, and combination antithrombotic therapy.
Here, Chaturvedi touched on the various studies that have demonstrated the ability of these newer medications to safely reduce low-density lipoprotein (LDL) levels and ultimately minimise cardiovascular events. Evolocumab— an example of a PCSK9 inhibitor— and inclisiran have been tested and performed well in large-scale trials, while bempedoic acid serves as an alternative for patients who are unable or unwilling to take high-potency statins. Chaturvedi then somewhat facetiously summarised “all of the data” evidencing the superiority of surgical revascularisation over intensive medical therapy for asymptomatic carotid stenosis—presenting the audience with a
completely blank slide.
“But, we do have some emerging evidence that, maybe, revascularisation is not as good,” he continued, alluding to the fact that carotid endarterectomy (CEA) and medical therapy produced similar outcomes in the SPACE-2 trial, and highlighting the importance of data from the CREST-2 trial, which are expected in early 2026.
“On the symptomatic side, we also don’t really have much evidence that revascularisation is much better than modern medical therapy [either]—there hasn’t been a large-scale study since 1991, and so there is a void in the current data,” Chaturvedi added. Once again, he drew attention to the relevance of ongoing studies in the area, including the SCORE registry.
discussed another frontier that appears to have moved alongside CEA and carotid artery stenting (CAS) over the past decade: transcarotid artery revascularisation (TCAR). Pioneered by Silk Road Medical via the Enroute stent system, TCAR’s intended benefits include avoiding the aortic arch, providing proximal protection, and improving particle capture.
TCAR received US Food and Drug Administration (FDA) approval in 2015 for use solely in high-risk carotid stenosis patients, but with the caveat that physicians had to contribute clinical data—including perioperative and oneyear outcomes—to the Vascular Quality Initiative TCAR Surveillance Project (VQI-TSP) in order to actually gain reimbursement for the procedure.


“In 2024, we need to have very intensive medical therapy,” he summarised, also stating there is “no excuse” not to aim for lowering LDL levels to at least <70mg/ dl in carotid stenosis patients—as per the target in CREST-2. However, he did note that he aims for LDL levels of <55mg/dl, if not lower, in his own practice. Starting medication alone is “not enough” in modern-day care, Chaturvedi continued, emphasising the importance of administering treatment with certain goals in mind.
Lipoprotein (a), factor XIa inhibitors, colchicine, and agents that target vascular inflammation, are among additional medications “on the horizon” in carotid disease, according to Chaturvedi, who speculated that the neurovascular community is likely to hear more about each of these over the next 1–2 years.
“There is a plethora of new treatment options which have been proven to be associated with cardiovascular event reduction and, if we’re able to optimally use all of these agents, that should decrease the need for carotid revascularisation.”
Following this, Caitlin Hicks (Johns Hopkins Medicine, Baltimore, USA)
This has created a greater wealth of both comparative and non-comparative data on TCAR, and studies evaluating it have “exploded” over the past few years as a result, Hicks relayed. Broadly, these studies have suggested TCAR produces stroke/death rates, comparable to those seen with CEA out to one year, across asymptomatic and symptomatic patients alike. Additional studies demonstrating nonsignificant differences in rates of stroke/death/myocardial infarction between the two— specifically those involving standard-risk patients— ultimately supported an expanded indication of the procedure from the US FDA in 2022.
Figures suggest TCAR has “taken off” in the USA over the past few years, with CEA and CAS declining slightly—a trend that is especially pronounced for high-risk carotid stenosis patients, in whom TCAR is now the predominant revascularisation approach nationally. Hicks did also acknowledge that the 2023 Centers for Medicare and Medicaid Services (CMS) coverage expansion, under which CAS is no longer limited to certain lower-risk patients, will likely lead to an increased stenting uptake and TCAR “losing ground” in more high-risk patients. However, “on the flipside”, with TCAR also being covered by this expansion, physicians are no longer mandated to contribute to the VQI-TSP database, potentially making the procedure less expensive to accommodate and thus boosting uptake at smaller community hospitals in the USA.
Hicks then noted that “there has been very close oversight of the use of TCAR by industry”—although she feels this is likely to be “unsustainable” in the long term, especially if the procedure continues to expand and is taken up by multiple other specialties. She also highlighted the fact that data on TCAR remain limited, overall, as the majority come from the industry-
sponsored ROADSTER studies as well as the VQI-TSP registry, which are helpful indicators of real-world patient outcomes, but certainly not equivalent to a randomised controlled trial (RCT).
“People always call for an RCT comparing TCAR to CEA—and/or transfemoral stenting,” she continued. “As much as I would love to see that happen, it almost certainly will not. It’s not financially viable. Silk Road Medical has seen a large market-share increase, and there’s really no reason for them to fund a study like that. So, unless something like that is funded by the government or another format, this will likely not be in our future.”
Nevertheless, Hicks feels data on longer-term outcomes are needed to help understand “where TCAR fits into our carotid revascularisation armamentarium”.
“Ultimately, I think having patients see physicians that are able to perform all three procedures is probably the best bet, because we can really try to weigh up the risks and benefits, and have patient-specific decision-making,” Hicks commented.
In a subsequent discussion, Thomas Ford (UMass Memorial Health, Worcester, USA) said that, while an “ideal world” would see a single operator offer all three of the aforementioned procedures, there is a certain “division of labour” in many centres whereby vascular surgeons often perform most CEAs and TCARs, while interventionists perform a lot of noninvasive procedures like CAS.
There hasn’t been a largescale study since 1991, and so there is a void in the current data.”
Responding to Ford’s question on how best to navigate this, Hicks admitted it is “difficult, for sure”, but that the keys to success are the conversations had between vascular surgeons, interventional neuroradiologists, and stroke neurologists, where necessary.
“I certainly don’t think that there’s a perfect way,” she added. “The new carotid NCD [National Coverage Determination] calls for shared decisionmaking, with a discussion of all three carotid revascularisation options prior to choosing one. There is no current, validated shared decision-making tool to use with patients, so that’s a little bit misaligned with what we have available in the clinical realm—but, I think, as that develops moving forward, at least being able to have a conversation about the three choices and then trying to steer the patient in the right direction is what we all need to aim for.”
Extolling the benefits of evidence-based practice, timely surgical intervention and intensive medical therapy, Dominic PJ Howard (Oxford, UK) spoke at the recent Vascular Society of Great Britain and Ireland’s (VSGBI) annual scientific meeting (VSASM 2023; 22–24 November, Dublin, Ireland) on what needs to be known regarding carotid disease management.
THE CONSULTANT VASCULAR surgeon at Oxford University Hospitals NHS Foundation Trust first addressed whether clinicians should be operating on any patients with carotid disease.
“We know from the NASCET, ECST and Veterans Affairs trials that early intervention for patients with high-grade symptomatic stenosis is very effective at preventing strokes,” Howard said. The presenter did point out, however, that these trials are over 30 years old.
Howard stressed that advances in medical therapy over the last two decades have been “significant”, highlighting that some studies have suggested intensive medical therapy can reduce stroke risk by up to 80% in patients presenting with minor events.
The presenter also highlighted interim results from ECST-2, presented earlier last year, which found that at two years there was no obvious benefit of revascularisation on top of medical therapy for carotid patients.
However, he urged audience members to be aware that ECST-2 did struggle with funding, recruiting only 400 out of a planned 2,000 patients, and only randomised low-risk patients— both symptomatic and asymptomatic— with moderate stenosis.
Sharing some data supporting the notion that medical therapy alone may be adequate to treat symptomatic carotid patients, Howard pointed to a pooled analysis of older versus newer randomised trials looking at intervention. The data showed that, for patients on medical therapy whilst awaiting intervention, there is a “reasonably high” stroke risk over 120 days; in the newer trials, on the other hand, the stroke risk on medical therapy whilst awaiting intervention “appears to be very low, at around 2%”. Howard did urge caution with interpreting these data, however. “These events were only those that were collected after randomisation, and the majority of patients in these trials were randomised beyond two weeks of index event,” he shared with the VSASM audience.
Howard then referenced an adhoc analysis of the POINT trial, which looked at dual antiplatelets versus aspirin for prevention of recurrent stroke. “We can see that dual antiplatelet therapy does reduce the risk of recurrent events in patients without carotid disease, and also in patients with symptomatic disease,” he said.
The presenter did identify an “elephant in the room” in this analysis, however, which is the fact that patients with symptomatic carotid disease, despite dual antiplatelet therapy, have a much higher recurrent stroke risk than those without carotid disease.
“We also have to be aware that our surgical risks have come down,” Howard stressed. Over the last two decades, he specified that procedural risks have more than halved with regard to stroke or death in the perioperative period. “We might say it’s simply because we got better at operating; I think it’s probably because of better medical therapy before and after operating, and also possibly due to higher-volume centres performing more operations,” the presenter opined.
The lack of patient compliance with various therapies was also addressed. Howard detailed: “Intensive medical therapy, intensive exercise and a Mediterranean diet are fantastic for our patients, but patient compliance with intensive medical therapy is less than 50%, one-third of our patients are found to have antiplatelet resistance in studies—and yet we don’t test for this, so we don’t know whether we’re giving them optimal medical therapy or not—and compliance with lifestyle and exercise, which are arguably the most important interventions, is less than 10%.”
At this point, Howard acknowledged that trial participants may not necessarily represent the typical patient
If we’re going to treat people and give them maximum benefit from urgent intervention, we have to prioritise carotid surgery for symptomatic patients as an emergency procedure.”
seen in the clinic. Here, the presenter referenced the 20-year OxVasc study. In 100,000 patients, Howard relayed that he and his colleagues identified a “dramatic” fall in the rate of recurrent events. He suggested that, while this will be due partly to improvements in medical therapy, the results will also stem from the fact that patients are receiving treatment sooner.
“This brings us on to the fact that we are too slow,” Howard stated. “In fact, we’re getting slower.” In light of this, Howard urged: “If we’re going to treat people and give them maximum benefit from urgent intervention, we have to prioritise carotid surgery for symptomatic patients as an emergency procedure and put them on the next operating list.”
Here, Howard called attention to a “very exciting” trial in the pipeline— COMET—which will be running from Leicester, UK, and will look at randomising patients with symptomatic stenosis to carotid endarterectomy versus best medical therapy.
Howard then turned his focus to the timing of intervention after symptom onset. He reported that, when this topic was first looked at, it was Swedish registry data that suggested operating within the first few days of an event was “very risky”. In the data, he specified, stroke or death was over 10% for those operated on within 48 hours.
Despite this, the presenter relayed that larger datasets from the UK and Germany have now quelled concerns. “Actually, the event rates appear to be pretty similar to those patients operated on beyond 48 hours,” he communicated.
Conversely, Howard stated that early stenting, meaning stenting within seven days of an event, “appears to be quite dangerous”. Referencing a pooled analysis of interventional trials, the presenter shared that patients receiving a stent within seven days have an 8.4% risk of stroke or death compared to 1.3% for those undergoing urgent endarterectomy.
The presenter then moved on to a “more common problem” faced by clinicians in day-to-day practice: the question of whether to operate following thrombolysis or mechanical

thrombectomy. He noted that Ross Naylor (Leicester Vascular Institute, Leicester, UK) and colleagues conducted a meta-regression analysis on the topic, concluding that the safest time to operate on these patients appears to be at around six days post-thrombolysis.
Looking at what the guidelines say, Howard shared that the new European recommendations continue with their advice that symptomatic patients who have greater than 50% stenosis should be considered for intervention (endarterectomy, not stenting) as soon as possible. There is a new recommendation in these guidelines, however, which says that a clinician should consider operating on patients receiving thrombolysis, but should wait six days before initiating surgery.
Finally, Howard looked at whether there is any way of selecting asymptomatic patients for intervention. He mentioned the ACST trial, which did show that patients on triple therapy— antithrombotics, blood pressure control and statins—benefitted from intervention over a five- and 10-year period. The European guidelines, the presenter noted, suggest using a variety of criteria to try and help select patients who may benefit from intervention.
“The problem is that most of us don’t have these investigations available to us in our normal practice,” he remarked.
In order to try and get a definitive answer to this question, Howard pointed to a study he and his colleagues recently had published in The Lancet, which looked at whether degree of stenosis may be predictive and could help with decision making.
“We found that, over the last 20 years, patients with high-grade asymptomatic stenosis have always had a two-to-three-fold increased risk of stroke compared to those with moderate stenosis,” he reported. “And this would appear to be a linear increase—as the degree of stenosis increases, so does the ipsilateral stroke risk.”
In view of this study, the presenter noted that the American guidelines have changed their recommendation to now suggest that asymptomatic patients with greater than 70% stenosis should be considered for intervention provided they have a low surgical risk and a good life expectancy.
Summarising, Howard put forward that intervention for symptomatic carotid disease is “under scrutiny” in view of improvements in medical therapy. However, one of his key messages was thus: “If we’re going to operate on these patients, we should still be evidence based.”
Dominic PJ Howard
The presenter continued that, at the moment, operating as soon as possible is important, yet time from event to intervention is currently too slow. “Urgent endarterectomy is safe but urgent stenting is not”, was another of Howard’s key messages, and he closed with the statement that “all patients require intensive medical therapy and lifestyle changes”.
The use of a relatively novel scoring system, referred to as Symptomatic carotid atheroma inflammation lumen-stenosis (SCAIL), has produced favourable predictive qualities versus the Oxford carotid stenosis tool (OCST) and Essen stroke risk score (ESRS). According to researchers, the SCAIL score led to superior recurrent stroke predictions after minor stroke/transient ischaemic attack (TIA) and symptomatic carotid stenosis before revascularisation—as compared to these more established approaches—in a recent study involving three pooled, prospective cohorts of patients.
WRITING IN THE EUROPEAN
Stroke Journal, Sarah Gorey (Stroke Clinical Trials Network Ireland/ University College Dublin, Dublin, Ireland) et al note that SCAIL led to discrimination “better than chance”, and provided added value for prognosis when used in conjunction with the clinically based ESRS system.
These findings indicate that a combined assessment of stenosis and plaque inflammation on fluorodeoxyglucose positron-emission tomography (FDG-PET) imaging like the one utilised in SCAIL “may improve risk stratification in carotid stenosis”.
The authors are also keen to stress the need for further studies to fully determine the role FDG-PET could play in evaluating plaque inflammation as a means for patient selection within randomised carotid revascularisation
trials of the future.
Gorey and colleagues pooled three prospective cohort studies of patients with recent (<30 days), non-severe ischaemic stroke/TIA (modified Rankin scale [mRS] ≤3) and internal carotid artery stenosis ≥50%, and used these data to compare SCAIL— and its ability to predict ipsilateral stroke recurrence in symptomatic carotid stenosis—with both OCST and ESRS.
The researchers detail that all of the included patients had carotid FDG-PET/computed tomography (CT) angiography and late followup data, with censoring at carotid revascularisation.
Across a total of 212 included patients, 16 post-PET ipsilateral recurrent strokes occurred in 343 patient years of follow-up—and occurred at a median timepoint of
42 days. Relaying their results, the authors state that baseline SCAIL predicted recurrent stroke with an unadjusted hazard ratio (HR) of 1.96 (confidence interval [CI] 1.2–3.22, p=0.007) and an adjusted HR of 2.37 (CI 1.31–4.29, p=0.004). In contrast, the HR for OCST was 0.996 (CI 0.987–1.006, p=0.49) and for ESRS was 1.26 (CI 0.87–1.82, p=0.23).
The concordance (c-statistic) for each of the scoring systems evaluated in the study—with higher numbers indicating better discriminatory power—was 0.66 (CI 0.51–0.80) with SCAIL, 0.52 (CI 0.4–0.64) with OCST, and 0.61 (CI 0.48–0.74) with ESRS.
As per comparisons with ESRS alone, the addition of plaque
inflammation measures (maximum standardised uptake value [SUVmax]) to ESRS gave rise to improved risk prediction outcomes when analysed both continuously (HR 1.51, CI 1.05–2.16, p=0.03) and categorically (p=0.005 for risk increase across groups; HR 3.31, CI 1.42–7.72, p=0.006; net reclassification improvement 10%). SUVmax plus ESRS was found to carry an adjusted HR of 1.57 (CI 1.04–2.36, p=0.03), and stratified recurrent stroke risks were low in 2.6% of patients, moderate in 5%, and high in 17.3%.
However, Gorey et al report that their recurrent stroke prediction findings were unchanged by the further addition of carotid lumen stenosis measures—the other metric taken into consideration, alongside plaque inflammation, by SCAIL.
“The SCAIL score has demonstrated validity to predict early and late recurrent stroke,” the authors add. “In our study, SCAIL improved identification of patients with recurrent stroke when directly compared to established clinical risk scores. However, SCAIL correctly classified just over half of [the] patients in our study, and the addition of clinical variables in the ESRS provided only modest further improvements.
“Prediction models including measures of vascular inflammation— either alone or in combination with clinical risk factors—need to be further refined and validated before they can be applied in clinical practice for patient selection for carotid revascularisation.”
THE FINAL PUBLICATION OF MIDTERM results from the observational CARAS study suggest that cerebral ischaemic event (CIE) incidence in asymptomatic carotid stenosis patients “should not be underestimated”, so say authors Rodolfo Pini (University of Bologna, Italy) and colleagues, relaying these findings in the Journal of Stroke and Cerebrovascular Diseases.
The authors further conclude that plaque progression and contralateral stenosis are among factors that may serve as primary predictors of CIEs, as per the results of their midterm analyses—which were initially presented at last year’s Vascular Annual Meeting (VAM; 14–17 June 2023, National Harbor, USA).
“Carotid endarterectomy [CEA] in patients with asymptomatic carotid stenosis remains a subject of debate,” Pini and colleagues initially state. “Current recommendations are based on randomised trials conducted over 20 years ago and improvements in medical therapies may have reduced the risk of CIEs. This study presents a midterm analysis of results from an ongoing, prospective, observational study of [asymptomatic carotid stenosis] patients to assess their CIE risk in a real-world setting.”
CARAS is a prospective, observational study of a cohort of asymptomatic patients with >60% carotid stenosis—as per criteria from the original NASCET trial—identified in a single duplex ultrasonography vascular laboratory. Short life expectancy and absence of signs of plaque vulnerability are among the factors cited by the authors for patients in the study not being considered for CEA.
The study’s primary endpoint is to assess CIEs ipsilateral to asymptomatic carotid stenosis, including strokes, transient ischaemic attacks (TIAs), and amaurosis-fugax, along with plaque progression rate and patient survival.
Patient enrolment took place from January 2019 to March 2020, with a sample size of 300 patients being targeted and a five-year follow-up scheduled. CARAS ultimately included a total of 307 patients (average age, 80 years; 55% male), of whom 61 (20%) had contralateral stenosis >60% and some 77% were on best medical management.
At a mean follow-up of 41 months, seven ipsilateral strokes and nine TIAs occurred, resulting in a total of 14 CIEs, Pini and colleagues report. They also note that two patients experienced both stroke and TIA during the follow-up period. And, as
per a Kaplan-Meier analysis, the four-year CIE rate was 6%, with an annual CIE rate of 1.5%.
Disclosing further detail on their midterm results, the authors relay that 58 patients (19%) had a stenosis progression that was associated with a higher four-year estimated CIE rate compared to patients with stable plaque (10.3% vs. 3.2%, p=0.01). Similarly, contralateral carotid stenosis >60% was associated with a higher four-year estimated CIE rate (11.7% vs. 2.9%, p=0.002).
A multivariate cox analysis further revealed that these factors were independently associated with a high CIE risk, with hazard ratios of 3.2 and 3.6, respectively.
“The midterm results of this prospective study suggest that the incidence of CIE in [asymptomatic carotid stenosis] patients should not be underestimated, with plaque progression and contralateral stenosis serving as primary predictors of CIEs,” Pini and colleagues conclude.
Carotid endarterectomy in patients with asymptomatic carotid stenosis remains a subject of debate.”


The US Securities and Exchange Commission (SEC) has charged Laura Tyler Perryman—the former chief executive officer (CEO) and cofounder of Florida-based medical device startup Stimwave Technologies—with defrauding investors out of approximately US$41 million by making “false and misleading statements” about one of the company’s key medical device products.
ACCORDING TO THE SEC’S COMPLAINT, THE medical device comprised several components, one of which was a fake, non-functional component that was implanted into patients’ bodies. As per a press release from 19 December 2023 summarising the complaint, the SEC alleges that, during capital fundraising events from 2018 through 2019, Perryman made “material misrepresentations” about Stimwave’s peripheral nerve stimulation (PNS) device, which purported to treat chronic nerve pain by delivering electrical signals to targeted nerves.
The device in question consisted of three key components: a transmitter, a receiver, and an electrode array. The transmitter was worn by patients in a pouch outside the body and sent a wireless signal into the body. A receiver and electrode array were implanted inside patients’ bodies and, used in combination, were supposed to receive the signal and convert it into electrical currents that stimulated target nerves.
Office. “Investors are entitled to know material information about the products of the companies in which they invest. The SEC is committed to holding bad actors accountable.”
The SEC’s complaint—filed in the US District Court for the Southern District of New York—charges Perryman with violating the antifraud provisions of the federal securities laws. The SEC is seeking permanent injunctions, including a conduct-based injunction, disgorgement plus prejudgment interest, a civil penalty, and an officer and director bar.
In a parallel action, also announced on 19 December, the US Attorney’s Office for the Southern District of New York filed a superseding indictment against Perryman that added criminal securities fraud charges to the fraud charges levelled at her by the SEC.
Perryman was originally arrested on the morning of 9 March 2023, and presented to the US District Court for the Southern District of Florida later that same day. A press release issued by the US Attorney’s Office for the Southern District of New York at the time detailed the filing of a twocount indictment charging Perryman in connection with “a scheme to create and sell a non-functioning dummy medical device for implantation into patients suffering from chronic pain, resulting in millions of dollars in losses to federal healthcare programmes”.
The release states that Perryman had been charged with one count of conspiracy to commit wire fraud and healthcare fraud, which carries a maximum potential sentence of 20 years in prison, and one count of healthcare fraud, which carries a maximum potential sentence of 10 years in prison.

As alleged, Stimwave included two receivers of different sizes with its PNS device—the smaller of which was designed to be used when the larger receiver was too big to implant. The SEC’s complaint alleges that Perryman “knew, or was reckless in not knowing”, that the smaller receiver was, in reality, “fake and nothing more than a piece of plastic”.
According to the complaint, Perryman misrepresented to investors that the PNS device was approved by the US Food and Drug Administration (FDA) and was the only effective device of its kind on the market. The complaint also alleges that Perryman made false and misleading statements to investors about Stimwave’s historical revenues, revenue projections, and business model.
After Perryman’s alleged fraud unravelled in the second half of 2019, Stimwave voluntarily recalled its PNS devices and eventually filed for bankruptcy, announcing the sale of its business in June 2022.
“We allege that Perryman touted a supposedly innovative medical pain-relief device while concealing that a primary component of the device was fake, and that patients were unwittingly undergoing unnecessary surgeries to implant the non-functional component into their bodies,” said Monique C Winkler, director of the SEC’s San Francisco Regional
In addition, Damian Williams—US Attorney for the Southern District of New York—announced details from a non-prosecution agreement with Stimwave, which had been entered into on 29 October 2022.
Under the terms of this previously sealed agreement, the company accepted responsibility for its conduct by, among other things: making admissions and stipulating to the accuracy of an extensive Statement of Facts; paying a US$10 million monetary penalty; and maintaining an adequate compliance programme going forward, as well as agreeing to cooperate fully with the US government.
“As alleged, at the direction of its founder and CEO
Laura Perryman, Stimwave created a dummy medical device component—made entirely of plastic—designed to be implanted in patients for the sole purpose of causing doctors to unwittingly bill Medicare and private insurance companies more than US$16,000 for each implantation of the piece of plastic,” said Williams.
“The defendant and Stimwave did this so that they could charge medical providers many thousands of dollars for purchasing their medical device. Our Office will continue to do everything in its power to bring to justice anyone responsible for perpetuating healthcare fraud—which, in this case, led to patients being used as nothing more than tools for financial enrichment.”
We allege that Perryman touted a supposedly innovative medical pain-relief device while concealing that a primary component of the device was fake.”
Monique C Winkler
COMBINING VAGUS NERVE stimulation (VNS) with intense physical rehabilitation helped stroke survivors recover movement in their arms and hands, and maintain these improvements for one year, within a study presented at the 2024 International Stroke Conference (ISC; 7–9 February, Phoenix, USA).
“The pairing of rehabilitation therapy with VNS likely helps the brain strengthen new neural pathways—like building a bridge to bypass a damaged area,” said the study’s lead author Teresa Kimberley (Massachusetts General Hospital [MGH] Institute of Health Professions, Boston, USA). “These long-term, pivotal results mirror our long-term results from an earlier pilot study where we found that patients continue to improve or maintain their gains up to three years after starting VNS therapy paired with rehabilitation.”
In the VNS-REHAB trial, a total of 108 patients who had a stroke resulting in moderate-to-severe upperextremity impairment were divided into experimental and control groups, and completed six weeks of in-clinic, intense rehabilitation paired with active or sham VNS. All participants were implanted with a stimulation device—specifically, the Vivistim system (MicroTransponder)—and then randomised to receive either ‘real’ or sham stimulation.
The researchers’ analysis of 74 patients with available data found that, at one year, upper-limb function improved by 5.3 points on the FuglMeyer Assessment-Upper Extremity (FMA-UE) index and by 0.51 points in the Wolf Motor Function Test when compared to baseline. In addition, VNS therapy improved hand and arm function by 2–3 times more than intense rehabilitation alone.
The present study is, however, limited by its small sample size and variability of the rehabilitation regimens followed by each patient over the one-year period, with future research and an ongoing clinical registry set to explore the long-term impact of active VNS in real-world settings.

A team of physicians, neuroscientists and engineers at Duke University (Durham, USA) have reported multiple new strategies that use deep brain stimulation (DBS) to improve the symptoms of Parkinson’s disease. By simultaneously targeting two key brain structures and using a novel, self-adjusting device in an experimental study model, the team showed that they can efficiently target and improve disruptive Parkinson’s symptoms—as per a paper published in the journal Brain
DBS HAS PROVED TO BE AN EFFECTIVE yet imperfect therapy for addressing advanced Parkinsonian symptoms like tremors and stiffness, particularly in cases where medication does not provide sufficient treatment. Research groups like the Duke University team have continually sought to explore ways of improving DBS therapy, and overcoming the limitations associated with traditional targeted stimulation.
“Physicians place the electrodes for DBS in either the subthalamic nucleus or the globus pallidus, which are two structures in the brain closely associated with movement,” said Dennis Turner (Duke University School of Medicine [DUSM], Durham, USA), senior author of the recent Brain publication. “There are benefits to both locations on their own depending on the patient’s symptoms, but we believed placing the electrodes at both locations could be complementary, and [may] help reduce medication doses and sideeffects, as well as [implementing] a completely new approach to adaptive DBS.”
Beyond just increasing the area of stimulation, the team wanted to explore whether a technique called adaptive DBS could make their system more efficient. In traditional DBS, a physician sets key electrical parameters—such as the amplitude, pulse frequency and pulse duration—to best treat symptoms while minimising side-effects.
Those parameters may stay the same for days, weeks, months and even years, depending on the patient’s response. However, according to Warren Grill (Duke Biomedical Engineering, Durham, USA), these unchanging parameters are far from optimal.
“The amount of stimulation a person living with
Parkinson’s needs changes, depending on their medications or activity levels,” he explained. “A patient will need more stimulation if they are walking their daughter down the aisle at her wedding than if they are just watching TV. An adaptive system is like a smart thermostat in your office that makes adjustments based on the temperature outside.”
To implement their bespoke approach, the team worked with experimental technology provided
The amount of stimulation a person living with Parkinson’s needs changes, depending on their medications or activity levels.”
Warren Grill
by Medtronic to create their own adaptive DBS techniques. By programming the device to sense and record key biomarkers and brain activity in the patient, the researchers developed a system that can adjust the parameters of stimulation automatically to provide optimal symptom relief throughout the day. The team tested their strategies in a clinical trial involving six patients aged 55–65 years, each of whom had varying symptoms of Parkinson’s disease.
Initially, the researchers spent two years observing and testing the efficacy of stimulating both the
THE UNIVERSITY OF Cincinnati’s Center for Addiction Research and Spark Biomedical have jointly announced that they are conducting a clinical trial to help patients with opioid use disorder (OUD) and post-traumatic stress disorder (PTSD) stick with medication treatment while finding the right dose.
The planned multi-year trial—funded by a US$2.1 million National Institute on Drug Abuse grant—will attempt to address the high dropout rate of roughly 50% for patients taking buprenorphine, a medication for maintenance treatment of opioid dependence.
“Opioid withdrawal brings symptoms like anxiety and sweating, while PTSD adds layers of hypervigilance, sleep
problems, and exaggerated reactivity,” said principal investigator Joel Sprunger (University of Cincinnati College of Medicine, Cincinnati, USA). “These overlapping issues significantly complicate the treatment process.”
The trial will utilise Spark Biomedical’s Sparrow Ascent technology—a patient-administered wearable device that delivers mild electrical stimulation to the cranial branches of the vagus and trigeminal nerves on and around the ear. This method, known as transcutaneous auricular neurostimulation (tAN), is US Food and Drug Administration (FDA)-cleared for relieving opioid withdrawal symptoms and may also aid in alleviating PTSD symptoms.
subthalamic nucleus and the globus pallidus with standard, continuous DBS. The results were measured using a combination of patient feedback, tracking the amount of time a patient could move without experiencing involuntary movements, and recording how much a patient could reduce their medication without experiencing symptoms.
During this period, the team also ran experiments to establish the parameters for an adaptable DBS system. They studied a specific frequency of brain activity, called beta oscillations, in the subthalamic nucleus. Previous research had shown that a high level of beta oscillations is linked to the slow, halting movement seen in most cases of Parkinson’s.
“We were able to test different levels of stimulation to determine the optimal levels of beta oscillations that would improve symptoms under different circumstances,” said Stephen Schmidt (Duke University, Durham, USA). “This helped us establish the initial settings for the adaptive DBS, and allowed us to compare how the adaptive and standard DBS operated in a home setting.”
After two years of study with the adaptive system, the team found that targeting the subthalamic nucleus and the globus pallidus at the same time led to a greater degree of improved motor symptoms as compared to targeting either region alone. The team also found that the adaptive DBS applied less stimulation but was just as effective as dual-target, continuous DBS in both clinical and home settings.
“Clinically, the patients are doing phenomenally,” noted Kyle Mitchell (DUSM, Durham, USA).
“Looking at their rating scales, they are doing better than the average DBS patient when both target areas are stimulated. We’re not only seeing excellent clinical responses to dual-target stimulation, but we’re also able to integrate this adaptive, smart tool into the brain that can at least match this clinical response. It’s very exciting.”
Spurred on by these initial successes, the team is planning to further optimise adaptive DBS and pursue additional testing for the next stage of their clinical trials.
“This tool has great potential down the road for making DBS a more tailored and elegant therapy,” Grill went on to conclude, also describing the present study as “very promising research” for the field of DBS.
A pilot clinical trial will evaluate the feasibility, safety and effectiveness of tAN in 20 patients with co-occurring OUD and PTSD who are beginning buprenorphine therapy as part of their treatment at the Gibson Center for Behavioral Change in Cape Girardeau, USA. Participants will be randomly assigned to receive either active tAN therapy using Sparrow Ascent or active sham stimulation at a level that will not activate the targeted nerves.
The trial’s primary goal is to increase buprenorphine retention rates over a three-month period. It will also seek to learn how patients perceive and react to the use of Sparrow Ascent as part of their treatment of OUD and PTSD, alongside standard buprenorphine therapy.
“Autonomic imbalance is a common occurrence in PTSD, which is characterised by experiencing high sympathetic drive (fight or flight) and low parasympathetic drive (rest and digest),” said Navid Khodaparast, chief science officer at Spark Biomedical.
“Additionally, PTSD sufferers experience lower levels of endorphin production, which can lead to emotional distress. In this study, we believe tAN therapy will improve these PTSD symptoms, and ultimately help maintain long-term buprenorphine treatment.”
In addition to its effects on physical symptoms, Sparrow Ascent is intended to give patients direct control over a part of their recovery.
Sparrow Ascent device
NALU MEDICAL PRESENTED
interim data from the “landmark” COMFORT randomised controlled trial (RCT)—indicating long-term and holistic pain management outcomes with its peripheral nerve stimulation (PNS) system—at the recent North American Neuromodulation Society (NANS) annual meeting (18–21 January 2024, Las Vegas, USA).
The ongoing COMFORT RCT is a post-market, open-label, “minimalrisk”, multicentre clinical study focused on evaluating the effectiveness of PNS therapy to treat chronic pain in the shoulder, low back, knee, or ankle/ foot, with the Nalu neurostimulation system. As per a Nalu press release, the study is designed to develop three-year data to demonstrate the sustained pain relief and quality-of-life improvements achievable with the Nalu PNS system.
Interim 12-month pain relief data from a total of 15 patients showed that 87% of subjects were responders
Latest study findings shed light on utility of differential target multiplexed
THIS YEAR’S NORTH American Neuromodulation Society (NANS) annual meeting (18–21 January, Las Vegas, USA) witnessed new data demonstrating the long-term superiority of Medtronic’s differential target multiplexed (DTM) SCS versus conventional medical management (CMM) for indicated chronic back pain patients who are ineligible for spine surgery.
The 24-month data in question came from a post-market randomised controlled trial (RCT) conducted across 12 sites in four different European countries.
As per two-year outcomes, Medtronic’s proprietary DTM SCS waveform was associated with a superior back pain responder rate in patients with chronic low-back pain (CLBP) at 12, 18 and 24 months, as compared to CMM. At
(≥50% pain relief), and found an average pain reduction of 73%.
In addition, holistic outcomes from 72 patients at three months revealed that 98% of subjects using Nalu PNS experienced ≥50% pain relief and/or reported improvement, compared with 21% of subjects who did not use Nalu PNS (p<0.001). Some 93% of subjects using Nalu PNS also experienced ≥50% pain relief and/or reduced disability compared with 35% in those who did not use Nalu PNS (p<0.001).
“Clinicians recognise the value of combining pain relief with functional outcomes to provide a more comprehensive assessment of device effectiveness in treating chronic, intractable pain,” said John Hatheway (Northwest Pain Care, Spokane, USA), first author and presenter of this oral report from COMFORT. “In the COMFORT RCT, we are measuring variables that present this broader picture. Results show that the Nalu
24 months, patients using DTM SCS also experienced a 77% reduction in CLBP, a 93% leg pain responder rate (subjects with ≥50% leg pain relief), and a significant reduction in extent of disability as measured by the Oswestry disability index (ODI; >26 point average ODI reduction).
Additionally, some 95% of patients were “satisfied or very satisfied” with DTM SCS treatment at 24 months, Medtronic claims in a recent release.
“As the first DTM SCS RCT in Europe, these results provide additional evidence of the clinical and quality-of-life benefits that DTM SCS offers this patient population, which has few available treatment options,” said Jan Willem Kallewaard (Rijnstate Hospital, Arnhem, The Netherlands), the study’s lead investigator.

PNS system is truly unique. For the first time, clinicians have access to advanced technology that enables us to offer effective, long-term therapy to a broader range of patients.”
“We are thrilled to see such promising results from our advanced micro-IPG [implantable pulse generator] in a rigorous RCT setting,” added Nalu chief executive officer Tom West. “The COMFORT RCT is intended to provide reliable evidence of the sustained effectiveness and impact on quality of life provided by Nalu PNS therapy, and to expand the use of Nalu PNS to patients for whom physicians have limited options. Our approach to this research underscores our commitment to take a leadership role in generating evidence for continued and extended application of effective PNS therapy for chronic pain sufferers.”
Early this year, Nalu also announced the closing of an incremental US$20 million in equity financing from new investor B Capital, bringing its total Series E round of equity investment to US$85 million. The round was led by Novo Holdings, with some US$65 million of the total funding having been closed in December 2023.
Nalu has stated that this funding will to used to support the expansion of its neurostimulation system for the treatment of chronic neuropathic pain. The acceleration of commercial growth, expansion of clinical and health-economic evidence, continuation of product development, and scaling of operations, are among additional areas the company has said it plans to focus on following this funding boost.
For the first time, clinicians have access to advanced technology that enables us to offer effective, longterm therapy to a broader range of patients.”
John Hatheway

BOSTON SCIENTIFIC CORPORATION HAS ANNOUNCED POSITIVE one-year results from the SOLIS randomised controlled trial, demonstrating sustained pain relief using its WaveWriter Alpha spinal cord stimulation (SCS) systems for the treatment of non-surgical back pain (NSBP), presented at the 2024 North American Neuromodulation Society (NANS) annual meeting (18–21 January, Las Vegas, USA).
Outcomes from the 128-patient SOLIS study, which had met its primary endpoint, were highlighted in a late-breaking data presentation by James North (Carolinas Pain Institute and Center for Clinical Research, Winston-Salem, USA).
Key findings from the study at its initial primary endpoint interval of three months include 90% of patients treated with WaveWriter systems reporting significant pain relief of 50% or greater without an increase in opioids, compared with 8% of patients treated with conventional medical management (CMM) alone (p<0.0001).
In addition, at one year, 84% of patients treated with WaveWriter systems reported significant pain relief of 50% or greater and sustained improvement in their ability to participate in activities of daily living, with a mean 25-point improvement in disability, as measured by the Oswestry Disability Index (ODI).
CMM patients who subsequently chose to ‘cross over’ and receive SCS therapy also achieved significant improvements in pain and disability at a one-year interval, consistent with subjects in the SCS arm. Some 85% of crossover patients reported a 50% or greater reduction in pain, with a mean 30-point improvement in disability on ODI.
“Continued positive results from the SOLIS study illustrate the need for early and effective pain treatment when just the standard of care is not enough,” said Jim Cassidy, president, Neuromodulation, Boston Scientific.
“Backed by consistently strong clinical evidence, our transformative pain management solutions help individualise care and improve the quality of life for the many people living with chronic pain today.”
These data played a key role in Boston’s WaveWriter SCS systems gaining an expanded NSBP indication from the US Food and Drug Administration (FDA),
Abbott launches
Liberta RC DBS system with remote programming capabilities
Abbott recently announced that it has received approval from the US Food and Drug Administration (FDA) to launch the Liberta RC deep brain stimulation (DBS) system—the “world’s smallest” rechargeable DBS device with remote programming, according to the company—to treat people living with movement disorders. Abbott claims that the Liberta RC DBS system also requires the fewest recharges of any FDA-approved DBS system, needing only 10 recharge sessions a year for most people.

non-invasive, magnetic peripheral nerve stimulation (mPNS) treatment for PDN, as per a company press release.
Neuralace claims that Axon therapy utilises the “pioneering approach” of mPNS to deliver a quick, painless, nonpharmacological and non-invasive treatment. Each session with the technology lasts just 13.5 minutes, and harnesses the power of magnetic pulses to provide relief, representing—in the company’s view—a “significant advancement” in pain management.
full-body MRI-compatible sacral neuromodulation devices, or invest in creating public awareness of advanced therapies for people with incontinence.
“Axonics took a different path and created a renaissance in sacral neuromodulation therapy by developing long-lived implantable devices and introducing full-body MRI compatibility in this category for the first time. Axonics refuses to be intimidated by Medtronic and intends to defend itself vigorously. In the meantime, we remain focused on innovation for improving the lives of patients with incontinence and continuing on our path to market leadership.”
Medtronic also currently has a separate infringement suit pending in the US District Court for the Central District of California, through which it asserts that Axonics has infringed additional technologies developed by and belonging to Medtronic—as detailed in a press release.
approval of its Percept RC deep brain stimulation (DBS) system. This small, rechargeable neurostimulator is the latest innovation in the Medtronic Percept family, which includes the Percept PC neurostimulator, BrainSense technology and SenSight directional leads.
According to Medtronic, the Percept family is the only sensing-enabled DBS system on the market, allowing the physician to personalise treatment for patients with movement disorders like Parkinson’s disease, essential tremor, and dystonia, as well as epilepsy.
Percept RC is equipped with BrainSense technology that captures and records brain signals to provide insights that enable a healthcare provider to adapt and personalise therapy to a patient’s evolving needs. Unlike other rechargeable devices, Medtronic claims, the Percept RC battery also offers at least 15 years of service life with consistent and fast recharge performance.
“When our patients choose a rechargeable DBS system, it is often based on the smaller size of the device, but the trade-off has always been how recharge frequency affects their lifestyle,” said Paul Larson (University of Arizona, Tucson, USA). “The Liberta RC DBS system excels in both areas as a compact, rechargeable device with the lowest recharge requirement of any FDA-approved DBS system. This achievement, coupled with the integration of remote programming capabilities, is a significant advancement for patients.”
At approximately the height and width of a smartwatch face, the Liberta RC DBS system is about 31% smaller than other commonly used, implantable, rechargeable DBS devices currently available in the USA, as per a recent Abbott press release. Liberta RC can also be controlled on an Abbott-supplied patient controller, or a compatible and secure iOS device, and offers users helpful notifications and customisable settings for a “personalised charging experience”, the company further claims.
Additionally, Abbott announced earlier this year that the US FDA has approved expanded magnetic resonance imaging (MRI) labelling for its dorsal root ganglion (DRG) stimulation therapy, the Proclaim DRG neurostimulation system, which allows patients to receive full-body MRI scans while implanted with the device.
Neuralace Medical has announced the US Food and Drug Administration (FDA) clearance of its Axon therapy for the treatment of chronic painful diabetic neuropathy (PDN)—a “landmark approval” that constitutes the first ever FDA clearance of a
In the AT-PDN study—a doubleblind, multicentre randomised controlled trial (RCT) involving 71 patients—Axon therapy demonstrated “remarkable” efficacy, as per a press release. The study saw patients allocated to either sham or active mPNS treatment, with sham-group patients given an opportunity to cross over at 30 days. Key findings within AT-PDN’s treatment group at day 30 included a 72.3% responder rate, 57.6% average reduction in 30-day visual analogue scale (VAS) pain score, 35% average reduction in numbness, and 20% average reduction in quality of life-diabetic neuropathy (QOL-DN) total score.
“These results not only demonstrate the effectiveness of Axon therapy in reducing pain and numbness associated with PDN, but also highlight its role in significantly enhancing the quality of life for patients,” stated AT-PDN principal investigator Lora Brown (TruWell Health, St Petersburg, USA).
Axonics responds to complaint alleging “unauthorised use” of Medtronic innovations
Axonics has announced in a press release that it has received notice of Medtronic’s recently filed complaint to the US International Trade Commission (ITC), as well as the parallel action in US District Court for the District of Delaware, attempting to block Axonics from “improperly importing and selling products” that infringe two Medtronic patents related to the magnetic resonance imaging (MRI) compatibility of implantable medical devices.
“We believe Medtronic’s claims are designed to stifle competition, limit patient and physician choice, and protect the incumbent’s market position,” said Raymond W Cohen, chief executive officer of Axonics. “For over 20 years, Medtronic took advantage of its monopoly position in this category and chose not to innovate, develop
“Medtronic is continuing our efforts to stop Axonics from profiting off of their unauthorised use of our innovations and intellectual property,” said Mira Sahney, president of the pelvic health business in the neuroscience portfolio at Medtronic. “The pattern is clear: Axonics uses Medtronic technologies to improperly compete in the market. It is time for Axonics to be held accountable for these unlawful acts.”
Earlier in the year, Axonics also announced that it has entered into a definitive agreement to be acquired by Boston Scientific Corporation for US$71 in cash per share, representing an equity value of approximately US$3.7 billion.
Mainstay gains expanded MRI labelling from US FDA for ReActiv8 system
Mainstay Medical has announced that the US Food and Drug Administration (FDA) has approved full-body magnetic resonance imaging (MRI) conditional labelling for the ReActiv8 restorative neurostimulation system— an implantable medical device designed to treat adults with intractable chronic low back pain (CLBP).
According to the company, this approval applies to all current and future ReActiv8 patients in the USA implanted with the current 45cm, commercially available leads.
The approval grants patients implanted with ReActiv8 the ability to undergo 1.5T full-body MRI scans, as per a Mainstay press release. The company also details that specific scan conditions and safety information are provided in the ReActiv8 FDA MRI Guidelines manual.
Medtronic receives US FDA approval for Percept RC DBS system
Medtronic has announced the US Food and Drug Administration (FDA)
“While more data are needed, the sensing capability of this unique deep brain stimulation system allows me the potential to tune stimulation delivery to brain activity, which may be a way to personalise this therapy for Parkinson’s disease in the future,” said Casey Halpern (University of Pennsylvania Health System, Philadelphia, USA).
“This new rechargeable neurostimulator technology provides me with insights into my patients’ symptoms and can capture data even when they’re outside of the clinic,” said Eleni Okeanis Vaou (University of Texas Health [UTHealth], San Antonio, USA). “Now, I have a rechargeable DBS therapy option with sensing technology, allowing me to track a patient’s response to DBS and medications. I use these data to inform how to personalise and optimise patient therapy.”
Tivic Health Systems has announced that enrolment in its pilot clinical study of a proprietary approach to non-invasive vagus nerve stimulation (VNS) has been completed less than six months from initiation of the study.
Tivic is testing a proprietary approach to more precisely influence vagus nerve signals, while enhancing effectiveness and safety, with a noninvasive, bioelectronic treatment, according to a company press release.
The pilot study utilised a new neurostimulation approach and involved 20 individuals, with the data collection and analysis being performed by researchers at the Feinstein Institutes for Medical Research in Manhasset, USA. This work aims to provide more precise targeting strategies for VNS, and more control over the types of physiologic effects that result from stimulation, the release adds.
Arissa enrols initial patients in first-in-human Syntra study
Arissa Medical has announced the enrolment of the first patients in its Syntra study—a first-in-human (FIH), early feasibility study evaluating the safety and efficacy of the company’s Syntra device for the treatment of wide-necked sidewall and wide-necked bifurcation cerebral aneurysms.
Initial cases in the study were successfully performed at Instituto de Neurocirugía Dr Alfonso Asenjo in Santiago, Chile by interventional neuroradiology (INR) principal investigator Juan Gabriel Sordo (Clínica Santa María, Santiago, Chile).
The Syntra device is a novel scaffold endoskeleton frame structure designed to facilitate enhanced intrasaccular neck coverage, reducing neck diameter, and enabling improved adjunctive coiling, to achieve optimal packing density and volume for intrasaccular blood flow-attenuated embolisation treatment in cerebral aneurysm cases.
Arissa claims that, because Syntra is a purely intrasaccular device, the use of dual antiplatelet medication alongside it is not necessary.
Another benefit of the device is its deliverability, Arissa notes. It is designed to “easily” navigate through tortuous vessels via a flexible, monolithic intraaneurysmal implant for facilitating delivery, retraction, and placement, as well as the ability to negotiate acute angulation of the aneurysm from the parent vessel, enabling the treatment of challenging aneurysms.
According to the company, Gary Duckwiler (University of California Los Angeles [UCLA] Medical Center, Los Angeles, USA) and Luis Augusto Lemme (Buenos Aires, Argentina) contributed significantly to preclinical evaluations of the device by providing a “roadmap” to ensure the necessary requirements for achieving safety and efficacy were fulfilled.
“Initial control of the neck of a wide-necked aneurysm is critical to stable and safe aneurysm treatment, and the Syntra is designed expressly for this purpose,” said Duckwiler. “Practitioners familiar with aneurysm coiling will find the transition to using Syntra to be simple. These characteristics—as well as the safety and effectiveness of the device—will
need to be fully examined, but the results from this initial cohort are promising.”
Imperative launches study of device intended to provide DAPTfree aneurysm treatment
Imperative Care has announced the initiation of a first-in-human (FIH) clinical study for its novel stent system designed to only require single antiplatelet therapy for patients undergoing stent-assisted treatment of wide-neck intracranial aneurysms.
The first three patients enrolled were successfully treated by Nobuyuki Sakai (Kobe City Medical Center General Hospital, Kobe City, Japan). All three patients underwent planned procedures for stent-assisted coiling of unruptured aneurysms, and all three were discharged from the hospital within 48 hours on an aspirin-only antiplatelet regimen, and without any device- or procedure-related complications— including clot formation.
“The ability for neurovascular implants to avoid dual antiplatelet drug therapy [DAPT], which carries the risk of serious bleeding complications, will be an important step forward,” said Sakai. “I am encouraged by these positive early results with Imperative Care’s innovative stent technology and look forward to future investigations in a wide range of patients, including those with ruptured aneurysms.”
Imperative’s investigational stent system is a coated, extremely lowprofile nitinol scaffold designed as a platform technology for a range of vascular applications. The initial focus of the company’s clinical development programme is neurovascular disorders, beginning with wide-neck aneurysms. However, the company intends to investigate versions of the stent for a wider scope of haemorrhagic and ischaemic stroke-related conditions too.
“A single antiplatelet stent, which is designed to allow patients to be managed solely on aspirin, will be a gamechanger for patient outcomes and in the practice of neurointerventional surgery,” said Aquilla Turk (Greenville Health System, Greenville, USA), Imperative’s chief medical officer.
“By aiming to eliminate the trade-off between the risk of clot formation without DAPT and bleeding complications with antiplatelet therapy, the Imperative Care stent could represent a platform technology with improved safety and broad applications in neurovascular disorders beyond unruptured aneurysms.”
Medtronic announces first case with CE-marked Rist 6Fr radial access catheter
Medtronic has announced the first neurointerventional case in Europe with its Rist 6Fr radial access catheter, which recently received regulatory approval for use in European Union
(EU) countries and other geographies accepting a CE mark.
According to Riitta Rautio (Turku University Hospital, Turku, Finland), who performed the first case, the 6Fr Rist “expands the number of patients [we can treat] and the variety of neurointerventions we can perform transradially”.
The case in question involved the treatment of a 79-year-old female patient with chronic subdural haematoma (SDH) via a middle meningeal artery (MMA) embolisation procedure. Rautio described the navigation of the 6Fr Rist device to the patient’s right external carotid artery (ECA) as “extremely smooth, trackable and stable”, with the distal

MMA being accessed successfully, and Onyx 18 (Medtronic) being injected to embolise and treat their chronic SDH prior to radial artery closure with a compression band.
“I prefer radial access, especially for older patients, due to fewer femoral access site complications and early ambulation time,” Rautio said, also noting that this patient was discharged home the following day.
The “flexible and hydrophilic” nature of Rist enabled “easy” navigation to the anatomic position in the patient’s ECA, and excluded the need for a distal access catheter, in the case, Rautio added. She further stated that the distal end of the 6Fr Rist catheter is shorter than the 7Fr iteration of the device, resulting in better stability when working close to the aortic arch.
“With smaller devices, we make fewer complications,” she concluded.
With its recent CE-mark approval, Medtronic’s Rist 6Fr radial access catheter joins the company’s Rist 7Fr radial access guide catheter and radial access selective catheter on the European market.
Route 92 completes enrolment in 250-patient SUMMIT MAX trial
Route 92 Medical has announced the completion of enrolment in the 250-patient SUMMIT MAX clinical trial evaluating the safety and effectiveness of its HiPoint 88 and HiPoint 70 reperfusion catheters as part of the Monopoint reperfusion system.
Route 92 claims to have become the first company to complete enrolment in a trial studying the safety and efficacy of a ‘super-bore’ aspiration catheter with at least a 0.088-inch inner diameter for the treatment of a large vessel occlusion—the cause of many acute ischaemic strokes.
“SUMMIT MAX is a pivotal randomised controlled trial [RCT] investigating the safety and efficacy
of a 0.088-inch aspiration catheter, a technology advancement the clinical community has believed important to improve stroke care for many years,” said Thanh N Nguyen (Boston University School of Medicine, Boston, USA), co-national principal investigator for the trial. “In a field where time is brain, systems of devices that could improve the quality of reperfusion, reduce procedure times and simplify the procedure are needed. SUMMIT MAX is the first FDA [Food and Drug Administration]approved aspiration thrombectomy trial which is randomised. RCTs provide the best clinical science and the SUMMIT MAX trial compares the Route 92 Medical Monopoint system to the largest commercially available conventional aspiration system. The data from SUMMIT MAX will provide robust evidence to guide clinical decision-making.”
The HiPoint 88 reperfusion catheter is designed to quickly and efficiently reperfuse occluded vessels to treat patients suffering an acute ischaemic stroke. As part of the Monopoint reperfusion system, the HiPoint catheters are advanced from a single point of control and are delivered by Tenzing 8 or Tenzing 7 catheters to provide a streamlined unit that is designed to track through vascular curvatures without catching side branches, utilising a onepiece advancement technique. The telescoping design of the Monopoint reperfusion system, with tapered components, is designed to reduce ledge effect, enabling atraumatic movement through tortuous anatomy.
Canadian hospital uses “world’s smallest camera” to see inside stroke patient’s blood vessels Physicians at The Ottawa Hospital (TOH) in Ottawa, Canada recently became the first in the world to use a new stroke technology, the MicroAngioscope (Vena Medical), in a patient procedure.
Equipped with “the world’s smallest camera”, the device goes inside veins and arteries, allowing physicians to see inside the blood vessels of the patient’s brain.
On 14 November 2023, Robert Fahed (TOH, Ottawa, Canada) used the MicroAngioscope to treat a patient who suffered from repeated strokes. The device’s advanced and high-resolution imaging enabled Fahed to pinpoint the subtle condition that led to the strokes, allowing for a tailored treatment approach, as per a TOH press release.
“For the first time in the world and history of interventional neurology, we used a new technology that allowed us to visualise the inside of the vessels of a patient,” said Fahed, who was the lead physician during the procedure. “This opens the door to a new way of practising this specialty. This also means that—now that we can finally see the inside of the vessels— everything needs to be re-explored and redefined.”
Basking closes US$55 million financing to support reversible stroke thrombolytic Basking Biosciences has announced the close of US$55 million in financing, which will be used to accelerate clinical development of its novel thrombolytic therapy for acute stroke.
New investor ARCH Venture Partners led the round, with participation from additional new investors Insight Partners, Platanus, Solas BioVentures and RTW Investments, as well as existing investors Longview Ventures, Rev1 Ventures, and The Ohio State University (Columbus, USA). Steven Gillis, managing director of ARCH Venture Partners, will serve as chairman of Basking’s board of directors, as per a company press release.
Basking will utilise the proceeds to accelerate clinical development of BB-031—a first-in-class, reversible ribonucleic acid (RNA) aptamer, targeting von Willebrand factor (vWF), engineered for rapid onset and short duration of effect. In 2023, the company announced positive Phase 1 results demonstrating the safety and tolerability of BB-031 with no serious adverse events reported, and dosedependent inhibition of vWF.
Basking will initiate a Phase 2 proofof-concept study, the RAISE trial, in patients with acute ischaemic stroke in 2024. In addition, the company will use these recently raised funds to advance BB-025—a complementary, rapid-acting reversal oligonucleotide capable of quickly neutralising the pharmacological activity of BB-031— through a Phase 1 clinical programme.
Cerenovus launches Cereglide 71 catheter for stroke revascularisation
Cerenovus, part of Johnson & Johnson MedTech, has announced the launch of its Cereglide 71 device—a nextgeneration intermediate catheter with TruCourse technology indicated for the revascularisation of patients suffering from acute ischaemic stroke. The device is now commercially available in the USA.
The Cereglide 71 intermediate catheter is the latest innovation in a planned Cereglide family of catheters to join the Cerenovus Stroke Solutions portfolio, according to a company press release. The device is optimised for effective direct aspiration as well as delivery of compatible stent retrievers, including the Embotrap III
revascularisation device, into the neurovasculature.
Cereglide 71 is equipped with TruCourse technology to increase flexibility of the device, and is designed to help physicians with improved navigation and access to clots—even in challenging anatomical conditions— during thrombectomy procedures.
“Balancing trackability, support and aspiration efficacy is crucial in overcoming the challenges of swift clot access during endovascular thrombectomy,” said Fawaz Al-Mufti (Westchester Medical Center/New York Medical College, New York, USA). “As the global first user, in initial use, Cereglide 71 intermediate catheter does just that. It impressively navigates, accesses occlusion sites, and engages with effectiveness to aspirate clots, rapidly restoring blood flow in the patient’s brain—potentially a lifesaving intervention.”

Ceroflo raises €6.4 million to aid progress of novel ICAD stroke treatment
Ceroflo recently announced that it has raised €6.4 million in investment-round funding, which will be used to advance development of the company’s SubMax device—a novel stent intended to “revolutionise” intracranial atherosclerotic disease (ICAD) treatments.
In a press release, Ceroflo reports that these funds will help support first-in-human clinical trials of its innovative stroke technology across 30 patients, and provide the company with a platform to support further US and Japanese regulatory studies.
Ceroflo engaged accounting firm DHKN to lead an Employment and Investment Incentive Scheme (EIIS) investment round.
The round raised €5 million in a number of weeks, with the additional €1.4 million being raised from “highly respected Irish MedTech entrepreneurs and leading global stroke key opinion leaders”, according to the company’s recent release.
The company says its SubMax stent represents a “gamechanger” in the treatment of ICAD, as its shape and structure have been developed to suit the unique challenges of this disease. It is designed to gently increase vital blood flow to the brain while reducing the risks associated with first-generation devices, including haemorrhage and stroke.
Evasc announces plans to expand European presence with German subsidiary Evasc Neurovascular has announced new strategic growth initiatives, having recently achieved a milestone of 120 successful cerebral aneurysm cases with its innovative Eclips device—and the company plans to expand its European operations with the establishment of a new subsidiary, Evasc Neurovascular GmbH, headquartered in Germany.
This expansion constitutes “a significant leap forward” in Evasc’s commitment to providing enhanced neurovascular care solutions in Europe, as per a press release. By establishing a direct presence in Germany, Evasc aims to facilitate more efficient services and direct sales, solidifying its position as a “key player” in the European market.
In parallel with this expansion, the company is reporting “noteworthy progress” in the France-based EESIS trial—a “crucial component” of its ongoing efforts to validate the efficacy and safety of its groundbreaking neurovascular treatments. The strategic move into Germany also positions Evasc to contribute even more significantly to the advancement of neurovascular care throughout Europe, the release adds.
Throughout 2023, Evasc successfully treated a total of 80 patients, achieving a “commendable milestone” in the company’s history that underscores its “unwavering dedication” to improving patient outcomes and advancing neurovascular care.
InspireMD receives CE-mark recertification under new EU regulatory framework
InspireMD has announced that it has received CE-mark recertification under the European Union’s (EU) new Medical Device Regulation (MDR) regulatory framework for its CGuard embolic prevention system (EPS), which is a carotid stent device intended to facilitate the prevention of stroke.
In light of this recertification, InspireMD will now continue to work to make CGuard EPS the standard of care for carotid artery revascularisation in its existing commercial territories while also advancing new product pipeline using the pathway provided under MDR. That is according to a company press release.
The release notes that, in parallel, the company’s work on a premarket approval application (PMA) for the C-GUARDIANS US investigational device exemption (IDE) trial “continues to progress nicely”, with primary endpoint results expected in mid-2024, followed by submission of the final module of the PMA application to the FDA in the second half of this year.
In addition, InspireMD says it will continue to “aggressively work” toward multiple value-creating milestones, including the potential US approval of the CGuard Prime EPS stent system in the first half of 2025.
Mentice gains US FDA 510(k) clearance for Ankyras software
Mentice recently announced that Ankyras, the company’s clinical decision support application, has received 510(k) clearance from the US Food and Drug Administration (FDA). The approval of this product by the FDA demonstrates the safety and effectiveness of Ankyras, and confirms Mentice’s commitment to providing innovative and high-quality healthcare solutions, as per a company press release.
The software is designed to assist interventional neuroradiologists in selecting the most suitable flow diverter prior to the interventional treatment of intracranial aneurysms. Ankyras offers an “intuitive and precise solution” for clinical treatment planning, leading to more accurate predictions than traditional methods, the release adds.
It is a platform that provides users with various functionalities to investigate device and treatment options in greater detail. Mentice states that the platform offers unique morphological assessment metrics that provide customers with a comprehensive and interactive analysis of all artery segments, ensuring they are better equipped to handle reallife challenges.
Moving forward, Mentice plans to continue improving the Ankyras solution by incorporating advanced functionalities and more treatment modalities, as well as integrating it with other Mentice solutions—especially the recently acquired Biomodex line of biorealistic haptic simulators.
Perfuze announces US FDA 510(k) clearances for novel aspiration and access catheters
Perfuze has announced US Food and Drug Administration (FDA) clearances for the Millipede 070 aspiration catheter and the second-generation of its Millipede 088 access catheter.
The first of these devices, the Millipede 070 aspiration catheter, is intended to remove blood clots rapidly and safely in the treatment of acute ischaemic stroke, as per a Perfuze press release. This “novel, unique” catheter is designed for “superior deliverability and high procedural efficiency” during endovascular thrombectomy procedures, the release adds.
“In our initial experience with Millipede 070, the system easily delivered to the target occlusion, allowing rapid, effective and safe reperfusion,” said David Fiorella (Stony Brook Cerebrovascular Center, Stony Brook, USA).
Additionally, Perfuze recently received 510(k) clearance for its second-generation Millipede 088 access catheter, which is designed to facilitate the safe insertion and guidance of microcatheters for neurointerventional or diagnostic procedures.
The Millipede 088 access catheter initially received US FDA 510(k) clearance in 2022.
Philips launches Azurion neuro biplane system
Royal Philips has announced major enhancements to its image-guided therapy system—Azurion—with the launch of its new Azurion neuro biplane system.
Featuring enhanced imaging and gantry positioning flexibility, this new angio suite system is designed to streamline neurovascular procedures and help care teams make the right decisions faster, treat more patients, and achieve better outcomes, the company says in a press release. By allowing neurointerventionists to treat more patients, more efficiently, with potentially better outcomes, Philips’ new Azurion neuro biplane system is intended to enhance both staff and patient experience, and contribute to a lower cost of care.
Philips’ Azurion neuro biplane image-guided therapy system is designed to smooth and optimise procedure workflows, where a combination of 2D and 3D imaging is needed for confident diagnosis, and precision treatment. Used with
3–4 April
Stroke Live Course (SLICE) Next Frontiers Virtual masterandfellow.com/slice/nf
13–18 April
American Academy of Neurology (AAN) Annual Meeting Denver, USA aan.com/events/annual-meeting
the company’s latest Neuro Suite software and services, it provides neurointerventionists with a fully integrated solution that combines Philips’ ClarityIQ low-dose imaging with a range of neuro-dedicated tools and value-added services that offer “unprecedented levels” of efficiency, flexibility, and control.
Q’Apel introduces newgeneration Hippo aspiration system for stroke thrombectomy
Q’Apel Medical has launched a newgeneration aspiration technology, the 072 Hippo aspiration system, which has been developed for patients suffering from a stroke due to a large vessel occlusion.
The recently US Food and Drug Administration (FDA)-cleared stroke system—comprised of the 072 Hippo aspiration catheter and its Cheetah companion—represents a “paradigm shift” in emergent neurovascular intervention, as per a Q’Apel press release.
“Our initial experience with the Hippo aspiration system really demonstrated outstanding system performance,” said Raymond Turner (PRISMA Health, Greenville, USA).
“In my opinion, the impact of a highly visible and adaptive catheter tip is superior to other aspiration systems. I can determine when my catheter is engaged with the clot, as I could see the tip conforming to the thrombus. Once the thrombus was removed, the petals
16–19 April
Society of British Neurological Surgeons (SBNS) Spring Meeting Edinburgh, UK sbns.org.uk/index.php/conferences/ edinburgh-2024
23–25 April
Charing Cross (CX) Symposium London, UK cxsymposium.com
3–6 May
returned to their original state, so I knew I had success. This adaptive and visible tip aids physicians in making informed decisions, in real time, that leads to a successful thrombectomy outcomes for patients.”
Rapid announces Japanese Tigertriever approval and Kaneka partnership
Rapid Medical has announced Japanese approval for its Tigertriever revascularisation device. With Pharmaceuticals and Medical Devices Agency (PMDA) approval, Tigertriever serves as “the first device to offer individualised solutions for mechanical thrombectomy”, a Rapid press release claims. Kaneka Corporation has exclusive distribution rights with Rapid in Japan, the release also details.
According to Rapid, Tigertriever technology is inspired by advancements in aerospace engineering, and “transforms” thrombectomy procedures from a passive to an active approach. Contrasting with traditional stent retrievers, Tigertriever offers added user control—something that the company notes is of particular importance in Japan and other Asian countries with a high prevalence of underlying intracranial atherosclerotic disease.
The Tigertriever device can be expanded and reduced “on demand”, activating clot integration and potentially reducing the risk of vessel perforation or damage during device retrieval, according to Rapid.
American Association of Neurological Surgeons (AANS) Annual Scientific Meeting Chicago, USA annualmeeting.aans.org
8–10 May
World Live Neurovascular Conference (WLNC) Istanbul, Turkey wlnc.org/en/WLNC-2024.html




Viz.ai gains US FDA 510(k) clearance for ICH imaging algorithm
Viz.ai has announced receipt of US Food and Drug Administration (FDA) 510(k) clearance for its Viz Intracerebral Haemorrhage (ICH) Plus algorithm, which is intended to automate the process of identifying, labelling and quantifying the volume of segmentable brain structures on non-contrast computed tomography (NCCT) images.
The Viz ICH Plus software is indicated for analysing intracranial hyperdensities, lateral ventricles, and midline shift, providing volume measurements of brain bleeds for timely and informed treatment decisions—according to a company press release.
“The ability and mobility to obtain accurate and quantifiable measurements of intracerebral haemorrhages through Viz ICH Plus significantly enhances our decision-making process,” said Peter Kan (University of Texas Medical Branch, Galveston, USA). “This technology, which marries precision with AI [artificial intelligence], is poised to transform how we approach intracerebral haemorrhage cases.”
Viz.ai notes that radiologists, neurologists and neurosurgeons can incorporate Viz ICH Plus “seamlessly” into their workflows, and automate the manual process of measuring brain bleeds. The software is available on the AI-powered Viz.ai One platform.
11–16 May
International Neuromodulation Society (INS) 16th World Congress Vancouver, Canada ins-congress.com
15–17 May
European Stroke Organisation Conference (ESOC) Basel, Switzerland eso-stroke.org/esoc2024
18–22 May
American Society of Neuroradiology (ASNR) Annual Meeting Las Vegas, USA asnr.org/asnr-annual-meeting-2024
3–5 June
LINNC Paris 2024 Paris, France linnc.com/Course-information/ LINNC-Paris-2024