Featured in this issue:
T.J. Miller discusses life as an AVM survivor
page 7


Featured in this issue:
T.J. Miller discusses life as an AVM survivor
page 7

Post-hoc analyses of a large, randomised clinical trial have revealed the potential advantages associated with electronic informed consent (eConsent) in acute ischaemic stroke studies, including increased enrolment rates and improved adherence to consent documentation.
These findings were recently published in the journal Stroke by lead author Iris Davis (University of Cincinnati, Cincinnati, USA), corresponding author Christopher Streib (University of Minnesota, Minneapolis, USA), and colleagues.
“EConsent […] was associated with higher individual site enrolment, higher remote consent rates, and improved consent documentation adherence, over paper consent,” Davis et al write, concluding their paper. “Our study outlines the potential advantages of eConsent adoption in future acute ischaemic stroke clinical trials and stroke research networks.”
Davis et al begin their paper by noting that obtaining timely informed consent represents a “key barrier” in acute ischaemic stroke clinical trial recruitment—and, while eConsent may have the capacity to optimise recruitment by enabling electronic delivery and documentation of the informed consent process, it remains both “limited and understudied” in these types of trials. The researchers therefore set out to conduct a post-hoc
Profile Johanna Fifi

Is combining embolisation and drainage the next step in cSDH
www.neuronewsinternational.com
Endarterectomy retains favour with CX audience
analysis of eConsent adoption within the MOST trial, a Phase 3 trial evaluating the potential benefits of two different bloodthinning medications alongside standard-of-care intravenous thrombolysis (IVT) in adult ischaemic stroke patients. Late-breaking data from MOST were presented at last year’s International Stroke Conference (ISC) and have been published in full in the New England Journal of Medicine, ultimately revealing no significant 90-day clinical benefits with either argatroban or eptifibatide as compared to placebo treatments.


For their subsequent analyses of MOST, Davis et al reviewed study databases to ascertain whether participants were enrolled using conventional paper consent forms or eConsent. Additionally, they determined whether the participant or their legally authorised representative provided informed consent in person, or if they were located remotely. Participants were then assigned to one of four informed consent modalities: paper (in person), paper (remote), eConsent (in person), and eConsent (remote). The authors also note in their Stroke publication that study sites had the freedom to use paper consent or eConsent for each enrolment. Outlining the results of their research, Davis et al state that eConsent was utilised for 173 (93 in person,
Continued on page 2
page 19
AS PER DATA FROM THE Stroke Services Tracker (SST)— a monitoring tool evaluating progress towards Stroke Action Plan for Europe (SAP-E) targets—improvements are being made with regard to stroke care planning and treatment access, but persistent disparities requiring “urgent attention” remain at play.
Data launched on European Stroke Awareness Day (13 May) indicate “encouraging progress” coupled with “serious inequalities” across the continent, say the European Stroke Organisation (ESO) and the Stroke Alliance for Europe (SAFE).
A total of 47 countries contributed to the latest dataset, providing the “most comprehensive picture” to date of stroke care across Europe. The findings—based on data from 2023— show improvements in national planning, emergency treatment and prevention, but also indicate that patients still face “major challenges” in accessing the care, rehabilitation and follow-up they need.
“It is unacceptable that [...] we still see such wide disparities in access to stroke unit care, early rehabilitation and life-after-stroke support,” said SAFE director general Arlene Wilkie. “Stroke survivors need holistic and continuous care. We call on all national governments and policymakers to do better in providing the essential services required for all those affected by stroke.”

Electronic consent demonstrates potential to overcome “key barrier” in stroke trial recruitment
Continued from page 1
80 remote) of a total of 514 participants (33.7%), while the remaining 341 (337 in person, four remote) involved more traditional, paper-based consent. Of the 57 sites enrolling patients in the MOST trial, 32 (56.1%) utilised eConsent at least once—and those sites enrolled a higher number of study participants as compared to non-eConsent sites (median 7.5 vs 3, respectively; p<0.001). Overall, eConsent was also completed remotely on a significantly more frequent basis than paper consent (46.2% vs 1.2%, respectively; p<0.001). Thus, in the authors’ view, this difference in recruitment may be explained, in part, by increased enrolment opportunities for remote patients when eConsent is used.
“It is worth emphasising that fewer than 1% of participants were consented remotely using paper,” Davis commented, speaking exclusively to NeuroNews “Examples of this scenario would be delivering a consent form to, and back from, a potential participant or their surrogate via fax machine, or by scanning it and sending via email. Before eConsent, this was the only method of obtaining consent from a patient or their surrogate when they were not physically in the same location as the researcher—which happens often in the acute research setting. The small number of consents obtained remotely using paper compared to using eConsent highlights the fact that electronic management and delivery of informed consent forms is a more practical and simple solution.”
According to the authors, findings on demographic diversity of participants were similar between the in-person eConsent and in-person paper consent groups, as were median baseline neuroimaging-to-randomisation times (58.5 vs 55 minutes, respectively). And, “importantly”, consent documentation adherence was superior in the analysis’ inperson eConsent cohort as compared to its in-person paper consent cohort with decreased rates of data clarification requests (44 vs 81, respectively, per 100 participants) and a notable reduction in reportable unanticipated events (six vs 25, respectively, per 100 participants).
“The three most common reportable, consent-related issues in MOST were missing HIPAA [Health Insurance Portability and Accountability Act] documentation, using the incorrect version of the consent form, and using the incorrect person during the consent process—for example, not using an impartial witness when required,” Davis explained. “These types of issues where the informed consent process is incomplete or inadequate—according to human subjects research regulations—must be reported to the overseeing institutional review board [IRB], because they have the potential to impact the rights of participants.” When asked to comment on the possibility of eConsent holding comparable benefits in other types of stroke trials—
for example, those evaluating thrombectomy procedures, thrombolytic drug treatments, triage protocols, or artificial intelligence (AI) solutions—Streib affirmed that its advantages are likely to be “generally applicable”.
“The ability to connect clinicians, research teams, and patients or their legally authorised representatives in order to rapidly obtain informed consent addresses a common barrier in clinical trials studying time-sensitive interventions,” he told NeuroNews
Streib’s view is that, while multiple remaining hurdles need to be overcome to achieve broader integration of eConsent into today’s stroke trials—and stroke trials of the future—the benefits of doing so could be significant.
“Centralised eConsent requires additional research infrastructure to manage the eConsent system and that can be considerable,” he stated. “There are also additional regulatory layers, including those within IRBs, who must approve the use of eConsent for a trial. Some institutions’ IRBs still either do not allow eConsent or considerably restrict its use.
These processes and barriers are still being navigated to determine optimal eConsent implementation, Streib noted.
“EConsent may be especially important to facilitate the participation of rural stroke patients in clinical trials,” he added. “This is a huge priority in stroke clinical research and something we are actively working to address.
“Rural hospitals typically lack onsite clinical research teams, making trial enrolment nearly impossible. This is problematic because rural stroke patients may have different risk factors and different treatment needs. For example, a neuroprotectant medication that slows the progression of stroke would be of huge benefit to rural patients whose definitive treatment is delayed until they can be transferred to a larger, urban stroke centre.”
“Today, electronic approaches commonly replace conventional methods of paper documentation in healthcare, and in clinical research, to better manage medical data maintenance and access. EConsent provides a contemporary solution to documenting informed consent in clinical trials for patients, researchers, and healthcare systems,” Davis commented. “As discussed in our paper,
EConsent may be especially important to facilitate the participation of rural stroke patients in clinical trials.”
Christopher Streib
we found key advantages of implementing eConsent to be increased enrolment opportunities and improved regulatory compliance. A shift towards using eConsent over paper consent could improve the efficiency of stroke trials by providing more patients the opportunity to participate, and by decreasing the regulatory reporting burden on research teams.”
n MEDTRONIC AND Q’APEL ISSUE URGENT DEVICE RECALLS:

Medtronic Neurovascular and Q’Apel Medical have both announced urgent Class I recalls of notable products within their respective neurovascular device portfolios, with the former advising physicians not to deploy unused Pipeline Vantage 027 devices, and the latter recalling and ultimately discontinuing its 072 aspiration system. Both instances have been categorised by the US Food and Drug Administration (FDA) as the most serious type of medical device recall.
For more on this story go to page 4.
n “WE CAN’T AFFORD TO WAIT”:
The “world’s first” multisector advocacy movement dedicated to stroke—the Global Stroke Action Coalition—launched earlier this year on 9 April, and has issued a call to action to address growing inequities in stroke.

For more on this story go to page 5.
n STUDY HIGHLIGHTS RESOURCE-EFFICIENT SUBDURAL HAEMATOMA TREATMENT: Performing a middle meningeal artery (MMA) embolisation procedure during the same anaesthesia session as a burr hole evacuation surgery represents a safe and potentially resource-efficient approach to the management of chronic subdural haematoma (cSDH), as per a retrospective review published by Charles Matouk (New Haven, USA) and colleagues. in the Journal of NeuroInterventional Surgery
For more on this story go to page 9.

Editor-in-chief: Prof Philip M Meyers | Publisher: Stephen Greenhalgh
Editor-in-chief: M Stephen
Content director: Urmila Kerslake | Global commercial director: Sean Langer
Editor: Jamie Bell jamie@bibamedical.com | Editorial contribution: Jocelyn Hudson, Will Date and Bryan Kay
Editor: Jamie Bell jamie@bibamedical.com | Editorial contribution: Jocelyn Hudson and Bryan Kay
Design: Terry Hawes, Josh Lyon and David Reekie
Design: Terry Hawes, Wes Mitchell and David Reekie
Advertising: Abbie Richardson abbie@bibamedical.com
Subscribe here
Advertising: Michael Broughton michael@bibamedical.com
Subscriptions: subscriptions@bibamedical.com
Subscriptions: subscriptions@bibamedical.com
Published by: BIBA News, which is a subsidiary of BIBA Medical Ltd
BIBA Medical, Europe, 526 Fulham Road, Fulham, London, SW6 5NR, United Kingdom Tel: +44 (0) 20 7736 8788
Published by: BIBA News, which is a subsidiary of BIBA Medical Ltd
BIBA Medical, Europe, 526 Fulham Road, Fulham, London, SW6 5NR, United Kingdom Tel: +44 (0) 20 7736 8788 BIBA Medical, North America, 155 North Wacker Drive, Suite 4250, Chicago, IL 60606, United States Tel: +1 708-770-7323
BIBA Medical, North America, 155 North Wacker Drive, Suite 4250, Chicago, IL 60606, United States Tel: +1 708-770-7323
Printed by: Buxton Press. Reprint requests and all correspondence regarding the newspaper should be addressed to the editor at the United Kingdom address. © BIBA Medical Ltd, 2025. All rights reserved.
Printed by: Buxton Press. Reprint requests and all correspondence regarding the newspaper should be addressed to the editor at the United Kingdom address. © BIBA Medical Ltd, 2024. All rights reserved.
have comments this suggestions for
If you have comments on this issue or suggestions for upcoming editions write to jamie@bibamedical.com




A systematic review and meta-analysis published recently in the Journal of NeuroInterventional Surgery has synthesised the existing data on intracranial aneurysm treatments utilising the novel Contour neurovascular system (Stryker), ultimately finding the device to be a safe and effective therapeutic option.
STUDY AUTHORS AHMET GÜNKAN (Istanbul, Turkey), Pascal Jabbour (Philadelphia, USA) et al initially posit that—while Contour has a “unique design” intended to address various aneurysm morphologies, including wide-necked, irregular and shallow-shaped lesions—evidence on the device’s clinical safety and efficacy “remains limited”.
“As this is a new device, there haven’t been big series or trials published,” Günkan said, speaking to NeuroNews. “That’s why an extensive review like this one is really important at this stage—to try to summarise the findings of all the small series published so far and to shed light on this new device, its efficacy, and its safety profile.”
Medtronic and Q’Apel
As such, the researchers conducted a comprehensive search, guided by Preferred reporting items for systematic reviews and meta-analyses (PRISMA) standards, across three major repositories of medical research: PubMed, Embase and Web of Science. Their search included studies reporting on Contour device cases with a minimum of five patients.
Key efficacy outcomes considered in their analysis include rates of immediate and final follow-up adequate aneurysm occlusion, as well as technical success, while notable safety outcomes include ‘good’ functional outcomes (modified Rankin scale [mRS] 0–2) at final follow-up; procedure-related morbidity, measured via permanent neurological deficits; procedure-related mortality; and intraoperative and postoperative complications. Pooled analyses with 95% confidence intervals (CIs) were conducted, with heterogeneity assessed using I² statistics, and a random-effects model was applied, the authors relay.
Data from nine studies—including 483 patients (58.8% female; mean age, 59.3 years) with 484 aneurysms—were analysed, and the majority of patients in whom information on rupture status was available presented with unruptured (81.8%) as opposed to ruptured (18.2%) aneurysms.
Regarding their efficacy-related outcomes of interest, Günkan, Jabbour and colleagues report an immediate adequate aneurysm occlusion rate of 53% (95% CI, 1–100%), with the final follow-up adequate occlusion rate rising to 93% (95% CI, 88–97%), and the technical success rate being observed as 98% (95% CI, 95–100%).
Safety outcomes in their analysis revealed intraoperative and postoperative complication rates of 3% (95% CI, 0–7%) and 7% (95% CI, 3–12%),
MEDTRONIC NEUROVASCULAR
and Q’Apel Medical have both announced urgent recalls of notable products within their respective neurovascular device portfolios. In late March, a US Food and Drug Administration (FDA) statement reported that Medtronic had advised physicians not to deploy unused Pipeline Vantage 027 devices—and, the following month, Q’Apel issued a similar recall notice relating to its 072 aspiration system.
Both instances have been categorised by the US FDA as Class I, the most serious type of medical device recall.
Medtronic’s voluntary recall letter to affected customers advised physicians against implanting any unused 027-compatible Pipeline Vantage embolisation devices with Shield technology, owing to concerns over an increased risk of incomplete wall apposition and braid deformation both during and after endovascular brain aneurysm procedures. Affected customers were also told to remove and quarantine all affected unused products and return them to Medtronic.
Simultaneously, the company issued an update to the instructions for use (IFU) for its 021-compatible Pipeline Vantage embolisation device with Shield technology, recommending that healthcare providers read and apply this
updated labelling when completing any future procedures with these devices. These updates are intended to help achieve “optimal device size selection and stent braid deployment to reduce the risk of complications and patient harms by lowering the incidence of incomplete wall apposition and/or braid deformation”.

Medtronic’s recall letter states that, for patients who have already been implanted with Pipeline Vantage 027 and 021 devices, the treating physician should determine the need for followup imaging or changes to medical management based on the patient’s overall health.
“Incomplete wall apposition and braid deformation, also sometimes called fish-mouthing, braid narrowing, or braid collapse, are known risks of these devices,” the US FDA’s
respectively. In addition, procedure-related morbidity was found to be 2% (95% CI, 0–3%), with no procedure-related mortality reported, and 98% (95% CI, 95–100%) of 378 patients with clinical follow-up available achieved a good functional outcome— leading the researchers to affirm the safety and efficacy of the Contour device across currently available data.
“There are definitely some indications for this new device that separate it from the existing intrasaccular devices,” Günkan stated, commenting on the unmet need Contour may resolve in the neurovascular space. “Matching the right patient with the right tool is crucial and, hopefully, more studies in the future will enable us to answer this question more accurately. For example, shallow aneurysms could be a good—and unique— indication for the Contour device.”
“At this point, we just finished enrolment in the NECC trial in the USA evaluating the Contour device,” Jabbour added, discussing additional data that will become available in the future. “We will wait for the results of this trial to be able, scientifically, to assess the evidence on efficacy and safety of the device.”
There are definitely some indications for this new device that separate it from the existing intrasaccular devices.”
Ahmet Günkan
announcement details. “The risks were higher in females, especially those younger than 45 years of age.
“The use of affected products may cause serious adverse health consequences, including thrombosis, stroke, or death. There have been 13 reported injuries and four reports of death related to the 027-compatible Pipeline Vantage embolisation device. There have been four reported injuries and no reports of death related to the 021-compatible Pipeline Vantage embolisation device.”
Q’Apel’s Class I voluntary recall has seen the company request the return of 1,617 units of its 072 aspiration system, which includes the Hippo catheter and Cheetah delivery tool, and is intended for the treatment of acute ischaemic stroke via a thrombectomy procedure. Q’Apel first initiated a discontinuation and recall of these units on 26 February, with the US FDA Class I categorisation coming on 7 April.
The recall was initiated after the company received a warning letter from the US FDA that “raised questions about whether the features and characteristics of the distal tip of the Hippo aspiration catheter were within the scope of its 510(k) clearance”, according to a Q’Apel press release. The company’s release notes that, rather than pursuing a new regulatory pathway, it has voluntarily removed all affected product lots and discontinued the
072 aspiration system line.
Q’Apel states that it has submitted three ‘medical device reportable’ adverse events for the Hippo product to date—including a reported tip detachment, retrieved without patient injury; a vessel rupture; and a vasospasm. The company describes all of these event types as “known risks” associated with any aspiration catheter and claims that, based on its own investigations, factors other than the device’s distal tip likely contributed to the reported adverse events. However, Q’Apel also highlights the potential consequences of these events, which include flow-limiting dissections or vessel perforations in cases of vascular injury, and blockage of blood vessels, ischaemia of end organs, or death, in cases of an unretrieved detached tip.
Q’Apel notes that the relevant Hippo product configurations have been discontinued in all markets— and, having proactively notified all customers and distributors, the company will “continue to monitor the situation closely”.
Last year, Route 92 Medical also announced a medical device recall categorised as Class I by the US FDA. The company issued a voluntary recall of specific lots of products featuring its Tenzing 7 delivery catheters in March 2024, citing multiple instances of distal-tip separation at the device’s proximal marker band.

“We can’t afford to wait”—new coalition calls for urgent action to address stroke crisis
The “world’s first” multisector advocacy movement dedicated to stroke—the Global Stroke Action Coalition—launched on 9 April, and has issued an urgent call to action to address growing inequities in stroke. A press release notes that stroke is already a leading cause of death and disability—and, without intervention, the global burden of the disease is projected to rise by a further 50% over the next 25 years, claiming 100 million lives and costing US$1.6 trillion each year.
AT THE LAUNCH, HEALTH and economic policy specialists, clinical experts, and people with lived experience of stroke, highlighted key data and recommendations for action to address human and financial impacts of stroke.
Drawing on both data and stroke patients’ experiences, the coalition will share its inaugural policy document, ‘Stroke Action Now’, which sets out evidence-based examples of interventions that can significantly advance progress on a disease that is largely “preventable, treatable, and recoverable”.
The release details that some 80% of the current stroke burden is linked to 10 modifiable risk factors—and identifying and managing hypertension alone could cut the rate of stroke in
half. In addition, advances in clot removal and clot-busting technologies— thrombectomy and thrombolysis—can dramatically improve patient health outcomes, minimising disability and reducing the economic impact of stroke, and access to rehabilitation helps people regain independence, and reduces the risk and cost of long-term disability.
Despite these “clear opportunities”, the release goes on to state that prevention, treatment and rehabilitation services are only available to a fraction of stroke patients. For example, just 3% of medically eligible patients currently receive thrombectomy, and 20–40% of healthcare settings worldwide are yet to implement basic stroke rehabilitation services.
“The global burden of stroke has doubled in the past 30 years,” said
coalition co-chair Bo Norrving (Lund University, Lund, Sweden). “During that same period, huge advances have been made across the care pathway that offer us an incredible opportunity to reduce inequitable health outcomes, and make significant progress towards global health and development targets. Committing to the development of national stroke plans should be a key priority for governments as part of their forward strategy for prevention and control of NCDs [non-communicable diseases]. We can’t afford to wait another 30 years to turn this around. Millions of lives depend on governments taking action now.”
Coalition leaders are calling on governments attending the fourth highlevel United Nations (UN) meeting on NCDs in September 2025 to commit to five actions:
● Making stroke a priority in NCD prevention and control strategies so that it becomes an explicit—and integral—part of national and internal health agendas
● Developing national stroke action plans that address the entire care
Millions of lives depend on governments taking action now.”
A prospective study has established the safety of venous sinus stenting (VSS) using a novel device in idiopathic intracranial hypertension (IIH) patients who have failed standard medical therapy, as well as suggesting the potential efficacy of this approach.
THE RIVER STUDY—FROM WHICH ONEyear data were recently published in the Journal of NeuroInterventional Surgery (JNIS)—was performed in order to obtain humanitarian device exemption approval from the US Food and Drug Administration (FDA) for the River stent (Serenity Medical). Writing in JNIS, principal investigator Athos Patsalides (North Shore University Hospital, Manhasset, USA) and colleagues describe this device as “the first stent specifically designed for intracranial venous sinuses”.
“The River stent significantly enhances the treatment of patients suffering from debilitating symptoms of IIH,” Patsalides told NeuroNews. “It provides several advantages over traditional stents, including being the first stent specifically created for patients with IIH, and tailored to the anatomy and function of the cerebral venous system.
“For over 15 years, I have recognised that patients suffering from cerebral venous disorders, such as IIH and pulsatile tinnitus, are often underdiagnosed and treated with suboptimal devices. In the next decade, we will be able to diagnose and manage various conditions using stents and other endovascular implants designed explicitly for the cerebral venous system. The findings from the RIVER study will drive further advancements in the evolving field of cerebral venous disorders and significantly improve patient care.”
Via a prospective, multicentre, single-arm, open-
label trial, Patsalides et al enrolled 39 participants across five US centres in order to evaluate the River stent among patients with a clinical diagnosis of IIH who had severe headaches or visual field loss, and in whom conventional medical therapy had been unsuccessful.
Their primary safety endpoint was the one-year rate of major adverse events when compared to historical controls involving cerebrospinal fluid (CSF) shunting, while their primary endpoint in terms of efficacy-related benefits was a composite of clinical improvement and absence of VSS at one year.
Secondary endpoints in the RIVER study included improvements in terms of pulsatile tinnitus, visual symptoms, quality of life (QoL) scores, and medication usage.
The authors ultimately report that, in the study, all procedures were technically successful. There was, however, one serious adverse event—a gastrointestinal haemorrhage observed at two months following the procedure while the patient in question was still on dual antiplatelet therapy.
RIVER’s primary safety endpoint
pathway and include measurable targets that respond to specific in-country needs
● Committing to funding evidencebased interventions and exploring innovative financing models, such as taxing harmful substances, to build domestic resources
● Implementing robust stroke monitoring systems that measure changes in stroke burden, and the provision and outcomes of stroke care
● Including stroke survivors and caregivers in policy development, and prioritising meaningful representation at all levels of decision-making
The coalition’s call comes at a “defining moment”, the release continues, as the upcoming UN high-level meeting is a “rare opportunity” for world leaders to shape the next 25 years of action on NCDs. The coalition’s view is that stroke “must not be overlooked”, and it is urging governments to seize this moment and ensure that stroke is “recognised, prioritised, and acted upon”.
Key members of the coalition include the American Stroke Association (ASA), Asia Pacific Stroke Organization (APSO), Bayer, Boehringer Ingelheim, European Stroke Organisation (ESO), Heart & Stroke Foundation South Africa, Ipsen, March of Dimes Canada, Medtronic, Philips, Stroke Association UK, and Society of Vascular and Interventional Neurology (SVIN).
was met, with Patsalides et al observing a 5.4% rate of major adverse events with the novel stent device compared to 51.7% in patients who received CSF shunts. The trial’s primary efficacy endpoint was achieved in 60% of trial participants too, with additional improvements also being seen regarding opening CSF pressure, headaches, papilloedema, pulsatile tinnitus, visual symptoms and QoL scores.
Post-hoc analyses relayed by the authors in JNIS suggest that enrolled patients with minimal or absent papilloedema at baseline demonstrated similar levels of clinical improvement versus those who had baseline papilloedema—in terms of headaches, pulsatile tinnitus, and QoL.
These findings lead Patsalides et al to conclude that one-year results from the RIVER study “establish safety and suggest efficacy” with VSS in IIH patients who have failed medical therapy.

“We are highly encouraged by the promising results of this first-of-its-kind study with a stent specifically designed for intracranial venous sinuses,” commented Adnan Siddiqui (Jacobs Institute, Buffalo, USA), senior author on the paper. “Stents that are currently used in venous sinus stenting were not specifically developed for this purpose. As a dedicated venous device, the length, diameter, radial force and flexibility of the River stent are optimised for the structure and unique mechanical properties of the area we are addressing. We are hopeful that it will offer the opportunity to benefit patients whose lives are disrupted by IIH and who have no options for treatment.”
17th Congress 2025

“When you want something, all the universe conspires to help you achieve it”
Paulo Coelho
Congress Presidents: Ivan Vukasinovic, Serbia
Kamil Zelenak, Slovakia
Congress Vice-Presidents: Alessandra Biondi, France
Elisa Ciceri, Italy
Christian Taschner, Germany
3 - 5 September 2025
Palais du Pharo, Marseille, France

“Everyone just thought I was crazy”—T.J. Miller discusses life as a brain
“Imagine if you saw construction workers building a house, and they were using a hammer or a saw, and you were like, ‘what the f*** is that?’”
This is the perspective comedian and actor T.J. Miller puts forward to explain his recent fascination with the devices and processes involved in treating arteriovenous malformations (AVMs). Miller underwent a successful embolisation procedure to remove a ruptured AVM from the right frontal lobe of his brain in 2010, but it wasn’t until a representative from leading neurovascular firm Balt approached him nearly 15 years later that he truly began to take an interest in how the condition is managed surgically.
“It had never been a part of my identity, and I think that’s really interesting,” he tells NeuroNews. “So, in working with Balt, I’ve been able to learn all about the equipment that’s used—which I think is fascinating— and I think it’s really helpful to better understand your injury, or disability.”
This quest for knowledge is what led Miller to the 2024 Society of Vascular and Interventional Neurology (SVIN) meeting (20–22 November, San Diego, USA), where he sat down with NeuroNews before giving a guest talk outlining his experiences as someone who has suffered from, survived and come to terms with a brain AVM.
From realisation to embolisation
Miller was in the middle of filming Yogi Bear 3D in the late 2000s when, in his own words, he started becoming “crazy”. Some lighthearted ‘for examples’ he gives here include becoming obsessed with disentanglement puzzles, and starting to narrate his own life and actions in real time. On a more serious note, he recalls having major difficulty sleeping, and using a combination of heavy drinking, medications and actively monotonous podcasts in an attempt to remedy this.
“I [later] asked my manager what he thought was going on and he was like, ‘I don’t know, I just thought you were on drugs’,” Miller recounts.
Following a period of increasingly erratic behaviour, Miller experienced two seizures—one during a meeting with his managers, and another while in hospital. He then came to in the neurocritical care unit at Cedars-Sinai Medical Center in Los Angeles, USA, and found that all of the other patients in
the room were intubated and/ or comatose.
“It was really heavy-duty stuff,” he says. “They told me I had an AVM and I, of course, didn’t know what that was. Then I found out they were usually [discovered] during autopsies— especially at the time—and that they’re very, very rare.”
Miller also recalls being informed of the embolisation procedure through which his AVM would be treated and removed. However, the fact this surgery was technically elective created a dilemma the like of which very few people under the age of 30 are ever faced with. Below is a rough transcript based on Miller’s memories of how this conversation went.
> Miller: “What happens if I don’t do it?”
> The intern tasked with explaining this situation: “You’ll probably die in your mid-30s.”
> Miller: “Well, after the surgery, will I still be funny?”
> Intern: “…what?”
Somewhat understandably, the neurological doctors at Cedars-Sinai had placed saving and prolonging Miller’s life at the top of their priority list. But Miller, whose performances typically rely on improvisation and standup ability, initially held reservations over the surgery if doctors couldn’t guarantee his comedic wit would be retained post-procedure.
“That’s what I do—it’s my purpose, and it’s sort of everything to me,” he states. “So, if I’m not going to be funny, then I think I’ll just take a pass. I’d had a good life and would’ve been fine to do another few years of being hilarious. This guy [Miller gestures to the aforementioned Balt representative, who also helped organise our interview] is going to live until he’s 90 but he hasn’t even said one funny thing in the last 48 hours. It’s a toss-up!”
Having been assured that the AVM was actually located in a part of the brain with little bearing on his award-winning drollery, Miller ultimately opted to “roll the dice” on the 10% fatality risk associated with this
complex procedure.
The operation was successful, which Miller attributes to him being treated at one of the best hospitals in the world where they perform roughly three AVM embolisations every day. One detail he found particularly surprising was the fact that, despite having just undergone brain surgery, “they didn’t need me to do anything” in terms of rehabilitation—he was allowed to walk out of the hospital within a few days of the procedure.
Reflecting on the surgical and postsurgical care he received, Miller notes that the initial focus was on his functional outcomes, as opposed to any potential psychological repercussions of having part of his brain removed. He was referred to a world-class neurologist, prescribed an anti-seizure medication (levetiracetam) and— outwardly, at least—appeared to be doing well.
Five years after the surgery, Miller stopped taking levetiracetam and subsequently had another seizure—the only one he has experienced since his AVM ruptured. Then, in 2018, Miller suffered a manic episode, leading to a since-resolved incident that he describes as a “misunderstanding with the US federal government”. Looking back, Miller sees this moment as a blessing in disguise because it resulted in his attorney Stacey Richman introducing him to a neuropsychologist, Sejal Vyas (New York, USA), to participate in a cognitive remediation programme.
Tests done by his neuropsychologist indicated that Miller held certain characteristics commonly associated with being bipolar or autistic, ultimately revealing the true extent of the cognitive side-effects—the “invisible disability”— stemming from his brain injury.
“She works with a lot of people with traumatic brain injuries [TBIs], and she told me, ‘your intelligence meant no one could’ve ever imagined that you had any cognitive deficits’,” Miller explains. “This actually made it more difficult for me, because people just think I’m forgetting their names, I don’t care about the names of their spouses or their dogs, I keep interrupting them, I talk for long periods of time—all those things just make me seem like an a**hole. But it’s been fascinating to learn about my own mind, and undergo this cognitive remediation where she begins to help me get better at managing and overcoming these challenges.”

Miller was subsequently placed on two further antiseizure medications (lamotrigine and lacosamide) to regulate his brain activity, and began taking lithium to better control his mood swings. While these medications
have had a positive effect, Miller also emphasises the profound impact of his neuropsychology appointments, which have enabled him to join the dots between his day-to-day struggles— forgetfulness, episodes of erratic behaviour, and dependence on alcohol and drugs in order to achieve sleep— and the surgery he underwent in 2010.
“If you don’t want to talk about it, then no one knows about it,” he says. “Everyone just thought I was crazy. And, in Hollywood, and in the arts in general, people just go, ‘well, he’s an actor’. It’s that tortured genius type of thing.”
Miller did address his experiences as an AVM survivor in those years following the surgery—initially in conversation with fellow comic Pete Holmes on the You Made It Weird podcast in 2011, and then more prominently via a standup routine on an episode of the TV show This Is Not Happening in 2013. However, he only discussed his experiences publicly on a handful of occasions and, due to the rareness of the condition, even opportunities to speak to other people who have had a brain AVM are few and far between.
“Whenever I see an AVM survivor or somebody who has one, I’m the highestprofile person they know of and they’ve never even met anybody else with an AVM,” Miller says. “I’m part of a TBI support group, but none of those people have an AVM or know anybody who has an AVM. It is so incredibly rare.”
Miller credits the relationship he has developed alongside Balt with him being able to “absorb” the repercussions of the brain injury as part of his identity, leading to his attendance at SVIN 2024 as well as the Society of NeuroInterventional Surgery (SNIS) meeting earlier in the year.
“In connecting with these types of conferences—and with people like yourself—I’m able to increase my visibility in this space and, hopefully, help raise awareness and share my story,” he adds. “The best thing in terms of my relationship with Balt is, the more you understand the procedure and the equipment that goes into this, the more that knowledge empowers you. And I never thought that’d be the case, but it was.
“I visited a facility yesterday and went into the room where they make all the catheters and coils and delivery systems, and it just helps you understand more of what they’re doing. I felt that, in speaking to Balt and getting involved with them, it made it possible for me to feel closer to what the surgery was and how it led to me being alive today.”
Moving forward, Miller is looking to further improve his own understanding of brain AVMs in addition to raising awareness of the condition for the benefit of fellow TBI patients; a group whose disabilities are often unseen, but whose voices should not go unheard.







Performing a middle meningeal artery (MMA) embolisation procedure during the same anaesthesia session as a burr hole evacuation surgery represents a safe and potentially resourceefficient approach within the management of chronic subdural haematoma (cSDH).
THIS IS THE KEY FINDING OF A retrospective review published recently in the Journal of NeuroInterventional Surgery (JNIS) by Charles Matouk (Yale School of Medicine, New Haven, USA) and colleagues.
“The development and evaluation of new approaches with the potential to improve the safety and efficiency of MMA embolisation in cSDH management is essential,” Matouk told NeuroNews. “The field is still in its infancy, and we have a lot of work to do to understand who will benefit most and how to streamline the patient experience.”
In order to evaluate preliminary
experiences with this combined approach, Matouk and colleagues performed a retrospective review of all patients who underwent MMA embolisation and burr hole surgery during the same admission at a major academic institution in the US state of Connecticut between 2019 and 2024. Patients were dichotomised by those undergoing both procedures during a single anaesthesia session
(combined) or across two separate sessions (separate). The authors relay in JNIS that baseline demographics, comorbidities and complications were compared, with primary outcomes of the study being in-hospital and 90-day rates of complications and reoperations.
A total of 103 patients (median age, 74 years) were included in the study, with 33.9% of these patients being part of the combined cohort.
Demographics, comorbidities and radiographic characteristics were ultimately found to be similar between the two study cohorts.
Matouk and colleagues relay that, while cumulative procedure times were similar between the combined (96 [82–127] minutes) and separate (85 [71–110]


Following her presentation on a similar subject at this year’s American Academy of Neurology (AAN) annual meeting (5–9 April, San Diego, USA), Mary O’Neal (Brigham and Women’s Hospital, Boston, USA) speaks to NeuroNews about the fruits potentially borne out of successful collaboration between specialists and primary care providers (PCPs) in neurological care.
Why is there a need for improved collaboration between primary care and more specialised neurological centres?
Access to neurologists is currently limited, with several months of delay for patient evaluations. This challenge arises from the growing demand for neurological care, which can outpace the number of specialists available. As a result, primary care physicians often take on the responsibility of managing neurological conditions. By improving education around neurological care, PCPs could manage more cases independently, allowing neurologists to focus on more complex cases. This would also ensure that patients needing a neurological evaluation receive enhanced care before their consultation.
Do you think that, across the USA generally, there is a lack of effective collaboration between these two types of entities—and, if so, what are the likely causes of this?
I believe that, across the USA, collaboration is often limited due to siloed workflows and the demands of busy schedules. There are also breakdowns and inefficiencies in all components of the referral process stemming from incomplete transfer of information, insufficient data available for informed medical
decision-making, and disagreements among different specialists over their respective roles.
How would you describe the current collaborative approach between your own hospital and nearby primary care centres?
As a neurologist at an academic centre, we typically collaborate through electronic medical records (EMR), online consultations, and emails. We also have some neurologists embedded in PCP practices, which enhances collaboration and relationship building. The effectiveness of this approach is reflected in various metrics, including reduced time to specialist visits and recommendations, decreased hospitalisations and emergency room (ER) visits, lower annual cost of care, improved patient satisfaction scores, and better provider retention and wellbeing.
minutes) groups, the total anaesthesia time was “significantly longer” for patients undergoing separate versus combined procedures (225 [193–264] vs 165 [145–183] minutes, respectively; p<0.001).
The researchers found that there were no differences in the rates of in-hospital access site complications, reoperation, stroke or mortality between the cohorts—and, while the combined study cohort trended towards having a shorter length of stay compared to the separate procedure group (5 [4–7] vs 6 [5–8] days, respectively; p=0.058), there were no differences in complication or reoperation rates within 90 days either.
“We were high enrollers in one of the MMA embolisation trials,” Matouk commented.


“As a result, I got to see firsthand how awkward it is to submit a patient for two procedures— and two rounds of anaesthesia—during the same admission.
“Now, we know that it is safe to combine the procedures. Whether it is equally as effective, or more effective, in preventing recurrences or reoperations compared to the standard approach remains to be determined.”
the neurologist—whether it’s a one-time consultation to assist with diagnosis and management, or a full transfer of care for the patient’s neurological disorder. In turn, we must clearly communicate our expectations to the PCP, including specific questions that need to be addressed and key information from examinations, imaging, and labs—ideally through a standardised referral form. We also need to understand what the patient or their caregiver wants; for example, ease of scheduling appointments, communication among all healthcare team members, and access to care without the need for ER or hospital visits.

What are the prevailing factors that may represent a barrier to the implementation of more effective collaboration?
Potential challenges to navigate with a view to improving collaboration include securing support from hospitals and healthcare systems, gaining acceptance from all stakeholders, addressing reimbursement issues, and managing time constraints.
What do you think are the major benefits of better collaborative efforts between specialised and primary care centres?
What are the most important factors that more specialised physicians need to consider in attempting to improve how they work and communicate with primary care centres?
It’s important to understand what PCPs expect from
Both neurologists and PCPs want to deliver highquality treatment to our patients. Improving subspecialist access can be addressed in several ways, with education being a key avenue. Better education around neurological care would mean PCPs could confidently manage simple neurological disorders and reserve referrals for more complex conditions. This would reduce unnecessary demand and allow patients who most need specialist care to be seen more promptly.
“Aspiration used to be something straightforward, almost like a ‘no-brainer’, whereas now it is more technical,” says Vincent Costalat (University Hospital of Montpellier, Montpellier, France), speaking shortly after giving a presentation on ‘expanding the options to open the vessel’ at the 2024 European Society of Minimally Invasive Neurological Therapy (ESMINT) congress (4–6 September, Marseille, France). He goes on to state that “we should adapt the ADAPT [direct aspiration] technique” to specific vessel sizes, with the goal of providing a more bespoke solution dependent on the anatomy of the individual acute ischaemic stroke patient being treated.
“Ithink we are moving from a concept of oversimplification of the aspiration process […] to a concept of aspiration being more optimised, and being tailored to the target vessel,” Costalat tells NeuroNews. “And, when you dive deeper into the science of aspiration— as Penumbra has promoted—it’s interesting to see that you cannot reach the same effect if you do not follow appropriate sizing.”

On this topic, Costalat is not simply speaking from atop an ivory tower; he recalls his own tendency, earlier on in his career, to essentially take the largest-diameter catheter available to him and track it as far as possible into the neurovasculature during mechanical thrombectomy procedures.
“I thought that, the larger the lumen [of the catheter], the more efficient the aspiration of the target clot would be,” he says. “But, when you dive into the physics of it, you understand that this is not the case.”
He goes on to describe recent progress and neurointerventionists’ improved understanding of the science behind aspiration thrombectomy as a “paradigm shift”, leading to a greatly increased focus on more precise catheter sizing as opposed to trying to go as distal as possible with an oversized device.
The relevance of precise sizing
The major technical advantage of appropriate sizing that Costalat highlights is the preservation of blood flow around the outside of the catheter itself during a procedure—a concept that, during benchtop testing, has been shown to boost the efficacy of aspiration.
“This concept of aspiration has to be understood [in terms of how it relates to] ingestion,” he notes. “If you want to bring something very big into the vessel, the diameter of the catheter may be beyond the diameter of the target vessel—then, you are enlarging the vessel yourself with the catheter. And you can still apply force to the thrombus but, after this, you are more prone to get a collapse of the artery because there is no flow surrounding the catheter. You vacuum the rest of the blood, squeezing the vessel.”
In Costalat’s view, this raises a tricky question, because an oversizing approach “may work, but may also harm”.
“We have all had cases where we oversized and got some dissection of the vessel,” he adds. “However, when you are just below the diameter of the target vessel—in terms of the OD [outer diameter] of your catheter—you allow systolic flow to surround the device, and this helps to push the thrombus inside the catheter because you increase the gradient of pressure between the outside of the catheter and the inside of the catheter, in addition to the arterial pressure. “Secondly, [with the undersizing approach], if you
have not ingested the whole thrombus in one attempt, but you still have a pressure gradient, then you can still pull in distant clots [or emboli] that were not initially captured by the catheter because the aspiration is still happening. But, if you are blocking the vessel [with oversizing] after the first part of the thrombus being ingested, then you cannot vacuum anything else because there is no reverse flow induced by the technique.”
Another key area within which Costalat sees promise—beyond the present discussion of catheter sizing—pertains to what he dubs “the intelligence of aspiration”, as enhanced control of the aspiration itself could represent an additional avenue to improving the efficacy of thrombectomy procedures. Penumbra’s investigational computer-assisted vacuum thrombectomy (CAVT) technology is currently being evaluated within the US THUNDER study, and Costalat comments that he and his colleagues will be “very attentive” to its results.
Extended portfolio, extended value With the company having worked to expand and improve its offering to European neurointerventionists over the past few months, physicians across the continent now have access to a total of five Penumbra catheters intended for use in aspiration thrombectomy: the RED 43, RED 62, RED 68, RED 72 with SENDit technology and RED 78 reperfusion catheters. These devices are compatible with the latest advancements in neurovascular access with the company’s stainless steel hypotube technology—BMX81 and BMX96.
“When you see the different sizes of the vessels in the brain, the margins in between them are quite small,” Costalat explains. “It’s not 3mm or 4mm of difference, it’s more like 0.5mm or maybe 1mm. So, if we want to promote the level of technicity and this concept of preserving flow around the catheter to get the maximum benefit of aspiration, then you need to size before selecting the catheter. We used to have two sizes and now we have five sizes, so the extension of the portfolio allows us to think differently about aspiration.”
Having a broader range of catheter sizes available, according to Costalat, allows operators to be “more precise and more efficient” when it comes to aspiration thrombectomy—and
means they are less likely to have to compromise on device selection.
“Penumbra extending its portfolio is extending the capability for us to reproduce the concept of ‘full aspiration’, with a good safety level,” he adds.
“The compatibility is just essential,” Costalat continues, alluding to the fact that all of Penumbra’s RED and BMX devices are designed to work harmoniously and interchangeably alongside one another. “It means that you can use one catheter and, if you find that the size was not really appropriate, you can bring a larger-diameter device over the first one and complete the treatment—or, conversely, if you use a bigger size during the first pass and then there is a distal migration, you can complete the treatment without changing anything other than adding a longer catheter to reach the distality.”
When asked to reflect on the slogan accompanying Penumbra’s portfolio of ischaemic stroke products in Europe—which is, ‘Versatile. Compatible.
ADAPTable’—Costalat comments that he would describe the company’s current offering as “a fully comprehensive solution for all occlusion types and sizes, and for all situations”.
Delivering a concluding message on this topic, Costalat reiterates that the benchtop studies indicating potential benefits with more precise catheter sizing are “extremely exciting”, adding that he anticipates more extensive evaluations prospectively evaluating the safety and clinical impact of the approach in the near future.
Many of the insights Costalat provides here tie in closely with the notion of Science-Based Aspiration Thrombectomy (S-BAT), which was also recently discussed in detail by Ian Kaminsky (Radiology Imaging Associates, Denver, USA) and Penumbra engineer emeritus Dave Barry for the 2024 SLICE symposium (8–9 November, virtual).
For additional information on this, visit Penumbra’s S-BAT webpage: https://www.penumbrainc.com/ science-based-aspiration-thrombectomy-center/.
Vincent Costalat is a professor and head of the Department of Neuroradiology at the University Hospital of Montpellier in Montpellier, France, and course director for the SLICE symposium.

Revalesio has announced new data from its completed Phase 2 RESCUE clinical trial of RNS60 in acute ischaemic stroke patients, demonstrating that patients were—on average—discharged 4.8 days sooner from the hospital following treatment with RNS60 plus standard-of-care endovascular therapy (EVT) compared to patients who only received EVT (p=0.022). A recent press release details that patients who received RNS60 also experienced a >50% reduction in brain infarct volume growth post-EVT (p<0.05).
THESE RESULTS WERE delivered during an oral presentation at the 2025 American Academy of Neurology (AAN) annual meeting (5–9 April, San Diego, USA).
“Revalesio’s choice to capture infarct growth post-EVT is a great example of how Phase 2 clinical trials should be designed to properly evaluate cytoprotective drugs with the use of imaging to confirm results,” said David Liebeskind (University of California Los Angeles [UCLA], Los Angeles, USA). “I congratulate the investigators and Revalesio on their results, and look forward to seeing RNS60 in a Phase 3 trial.”
In addition to the aforementioned findings suggesting reduced infarct
growth and statistically significantly earlier hospital discharge with a high dose of RNS60, highlights from the oral presentation include 55% of patients treated with high-dose RNS60 being discharged to their home compared to 21% of those treated with placebo; 72% of patients on high-dose RNS60 being independent compared to 37% on placebo, based on dichotomised modified Rankin scale (mRS) scores of 0–2 at day 90; and RNS60 being safe and well tolerated overall.
“Our Phase 2 trial results sum up a decade’s worth of research where the consistency in results has been remarkable. In preclinical models of stroke and traumatic brain injury, RNS60 reduced brain loss by up to
50%,” said Greg Archambeau, president of Revalesio and co-inventor of RNS60. “Upon a potential FDA [Food and Drug Administration] approval, we will be ready to rapidly scale our specialised US manufacturing capability for RNS60 so patients won’t have to wait.”
In RESCUE—a multicentre, doubleblinded, placebo-controlled, randomised Phase 2 clinical trial—Revalesio evaluated the safety and initial efficacy of RNS60. Eighty-two participants with acute ischaemic stroke eligible for EVT were enrolled and received intravenous RNS60 0.5mL/kg/h (low dose), RNS60 1mL/kg/h (high dose), or placebo, starting before completion of the EVT and continuing for 48 hours.
The trial had two primary endpoints: safety and mortality. Secondary endpoints for the study evaluated disability based on mRS, change in the extent of stroke as measured by magnetic resonance imaging (MRI) at 48 hours, and additional endpoints including Barthel index, National Institutes of Health stroke scale (NIHSS), and EQ-5D-5L.
“There is a lot of discussion currently taking place on improving human health while lowering the cost of healthcare,” commented Bert van den Bergh, Revalesio’s executive chairman of the board of directors and former president of neuroscience products at Eli Lilly and Company. “Given RNS60’s Phase 2 results showing reduced brain tissue loss, improved function, and reductions
Anaconda Biomed has announced that results of the ANAIS study, which evaluated the safety and performance of its Ana funnel catheter in the endovascular treatment of large vessel occlusion acute ischaemic stroke, have been published in the American Journal of Neuroradiology. The data demonstrate high rates of reperfusion and first-pass success with a strong safety profile, supporting the Ana device as an adjunct to stent retriever thrombectomy, according to Anaconda.
“THE ANAIS RESULTS REPRESENT A significant step forward in refining mechanical thrombectomy interventions. By reducing the need for multiple passes, these advancements may ultimately translate into improved neurological outcomes after stroke,” said principal investigator Alejandro Tomasello (Vall d’Hebron Hospital, Barcelona, Spain).
The ANAIS study evaluated the safety and performance of Anaconda’s Ana funnel catheter, a novel device designed to work in conjunction with stent retrievers to arrest blood flow and reduce the risk for clot fragmentation during mechanical thrombectomy.
Conducted prospectively across three centres in Spain, ANAIS was a single-arm feasibility study with blinded-outcome assessment by an independent imaging core lab and oversight from an independent data safety monitoring board. Forty-three patients were enrolled to receive thrombectomy within 24 hours of symptom onset, with a median National Institutes of Health stroke scale (NIHSS) score of 16 at admission. The primary efficacy endpoint—successful reperfusion (expanded thrombolysis in cerebral infarction [eTICI] 2b50–3) within three passes—was achieved in 70% of patients in the intention-to-treat
(ITT) population and 81% in the per-protocol (PP) population. No severe device-related adverse events or symptomatic intracranial haemorrhages were observed at 24 hours.
In addition, the study allowed investigators to explore optimal device deployment strategies. When the Ana device was deployed in the internal carotid artery (ICA) C2–C3 segment and continuous aspiration was applied, first-pass effect (FPE; eTICI 2c–3) was achieved in 83% of cases, with successful reperfusion
in both hospital stay and the need for long-term care, RNS60 has the potential to greatly benefit patients and their families while lowering the economic burden of stroke.”
RNS60 is an investigational therapeutic drug being developed to provide disease-modifying and potentially restorative treatments for neurological conditions, with Revalesio’s lead clinical programme currently focusing on the area of ischaemic stroke.
Revalesio’s choice to capture infarct growth postEVT is a great example of how Phase 2 clinical trials should be designed to properly evaluate cytoprotective drugs with the use of imaging to confirm results.”
David Liebeskind
within three passes in 100% of this population.
“We are grateful to the investigators, centres and patients who contributed to this study. The high rates of FPE are encouraging, and the optimal deployment techniques identified during ANAIS are now being implemented in the ongoing ATHENA clinical trial— the first randomised stroke trial to include FPE as a primary outcome measure,” said Hendrik Lambert, chief medical officer at Anaconda.
Earlier this year, in April, Anaconda also announced the enrolment and treatment of the first US patient by Shahram Majidi (Mount Sinai Health System, New York, USA) in its ATHENA clinical trial—a global, randomised, 327-patient pivotal study evaluating the safety and effectiveness of the Ana funnel catheter.
“We have long recognised the impact of fast and complete reperfusion in stroke patients, and we are delighted to have initiated this important clinical trial for examining the role that flow restriction with a funnel catheter can play in enhancing endovascular thrombectomy and potentially improving outcomes for stroke patients,” Majidi commented.
Anaconda intends to complete enrolment in the ATHENA IDE study in the first half of 2026.
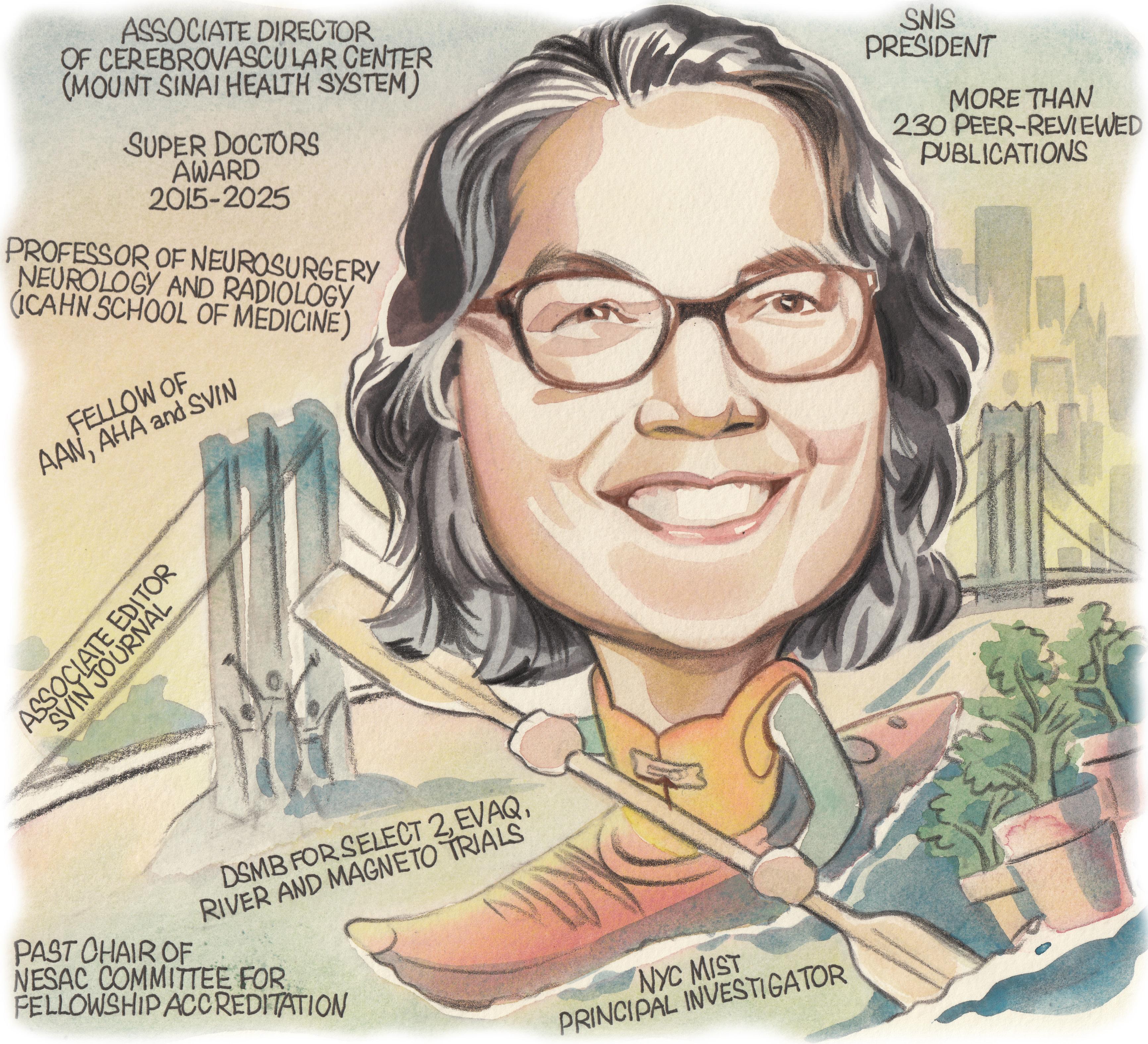
Following in the footsteps of many of her neurointerventional mentors as president of the Society of NeuroInterventional Surgery (SNIS), Johanna Fifi (New York, USA) has proudly led the society’s mission to expand and enhance the cerebrovascular field throughout the past year. In her current roles as associate director of the Cerebrovascular Center and director of the Pediatric Cerebrovascular Disorders Program at Mount Sinai Health System, she devotes her time and expertise to furthering treatments for adults and children alike with neurovascular diseases. Here, Fifi—a professor of neurosurgery, neurology and radiology—discusses her career to date in addition to key areas of promise in the neurointerventional field.
What initially attracted you to medicine, and the cerebrovascular field specifically?
What initially drew me to medicine was a love of the sciences and a deep sense of responsibility and empathy—I wanted to be the kind of person others could trust in their most vulnerable moments. Medicine offered a career where I could not only help people physically but see them fully, and help allay their fears, give them appropriate hope, and support their dignity.
The cerebrovascular field specifically appealed to me because of that combination of humanity, precision, and also urgency. With stroke and everything else that we treat, you’re not just treating a condition but often treating to preserve someone’s ability to speak, to move, to think—all that makes them who they are. When I was introduced to the concept of neurointervention, I could not believe how much of a meaningful combination of knowledge, your hands, engineering and creativity it was to do this work.
Which clinicians and researchers have been your most valued mentors, and in what ways have they impacted your career?
There are many, and I feel incredibly lucky for that. I have had mentors at many stages and in several domains, including clinical, leadership and scientific.
For clinical care, I have had mentors throughout my career that have influenced me. Stroke neurologists Rodney Bell, Steven Warach and Chelsea Kidwell have taught me how to be an empathetic clinician and to treat based on the science.
In my neurointerventional training, I am grateful to William O Bank for showing me the ropes in the transition to a proceduralist and for many other life lessons. This continued with Philip M Meyers, John PileSpellman and Sean Lavine.
Alejandro Berenstein has been my most longstanding professional mentor, friend and partner. I consider him as family. Finally, J Mocco has been in inspiration as a partner, and has really been a shining example of how to lead from the heart.
What are your main reflections from your tenure as SNIS president?
My tenure as SNIS president has been one of the most rewarding and enlightening chapters of my career. It has been truly remarkable
to follow in the footsteps of prior leaders of our field, dedicated to the mission of improving the lives of our patients afflicted with cerebrovascular disease, working with our dedicated board and members as well as other global societies around the world to enhance science, education and knowledge through networking, and access to care for our patients. The SNIS staff, board and membership stand out as truly remarkable for their dedication to our mission.
How would you say the SNIS has evolved in the years since you first joined the society?
I became a senior member of the society in 2010, but I attended my first SNIS meeting back in 2004. Since then, I think that the SNIS has evolved into a multidisciplinary society where neurointerventionists from all of our specialties can find a welcome home. Neurologists, radiologists and neurosurgeons alike are welcome. And I think the perspective of each are truly welcome as an additive value to our society. This is unique, and it’s what strengthens and positions our society to lead this field.
Of all the clinical studies and registries you are currently involved with, which do you feel has the potential to make the greatest impact within the neurointerventional space?
This is difficult to answer. There have been so many stroke trials I’ve been involved with in the past that have changed our field. Currently, I’m excited about technologies that are expanding the orbit of our field.
Firstly, Stentrode (Synchron)—this has the potential to bring neuroendovascular into the electrophysiology of our brain. Going back to what I said in the beginning, this ability to intermingle with the essence of our humanity, to restore our most human functions, is groundbreaking.
Less philosophical but no less impactful, I think the current STRIDE registry (Cerevasc) that we are performing to look at cerebrospinal fluid (CSF) diversion has the potential to impact numerous lives across the world.
What do you feel have been the three most important developments in the cerebrovascular field during your career?
Well, the most impactful without a doubt, in terms of lives touched, has been the evolution of endovascular treatment for stroke. The
2023–present: Professor of Neurosurgery, Neurology and Radiology, Mount Sinai (New York)
2021–present: Director, Pediatric Cerebrovascular Disorders Program, Mount Sinai
2018–present: Associate director, Cerebrovascular Center, Mount Sinai
EDUCATION
2007–2008: Senior fellow in Neurointerventional Radiology, Columbia University (New York)
2006–2007: Junior fellow in Neurointerventional Radiology, Washington Hospital Center (Washington DC)
2004–2006: Fellow in Vascular Neurology and Stroke MRI, National Institutes of Health (Bethesda)
2001–2004: Resident in Neurology, Thomas Jefferson University Hospital (Philadelphia)
1996–2000: Doctor of Medicine, Boston University School of Medicine (Boston)
HONOURS (SELECTED)
2015–2025: Super Doctors award
2015–2025: Top Doctors award
2010: Young Hearts Award for Achievement in Neurovascular Science and Medicine (AHA/ASA)
validation of stroke thrombectomy as a proven and highly efficacious treatment for large vessel occlusion has revolutionised—for many—the landscape of our practice.
I am so grateful to have been involved in many of the trials over the last 20 years or so that have shaped this space, including MR RESCUE as a fellow, as well as SWIFT, THERAPY, COMPASS, DEFUSE 3, SELECT2 and DISTALS, to name a few. The coming together of physicians and scientific minds drawn by the possibilities of saving patients’ lives and humanity to prove this therapy is really not surpassed by anything I’ve witnessed in medicine.
I have to mention a disease I hold near to my heart: paediatric arteriovenous shunts. Working with children has been a passion of mine from fellowship, and it has led me to pursue working with Alejandro Berenstein, which I have done for the past 17 years. Progress in the technological achievements we can perform to save these young children’s lives has been revolutionary.
I believe that we currently stand at a precipice in terms of understanding the molecular and genetic underpinnings of the disease, and of technological ability, and I am looking forward to unravelling how we can make sure these patients live long and productive lives.
How would you assess the role for thrombectomy in more distal-occlusion strokes following the recently presented trial data in this area?
The role of thrombectomy in more distalocclusion stroke beyond M2, A1 or P1 locations has been a special interest of mine as well. I am serving a role on the nationwide National Institutes of Health (NIH)-funded STEP platform trial on distal occlusions to clarify the benefit of thrombectomy in these more distal occlusions—often termed medium-vessel occlusions (MeVOs). While recent studies like DISTAL and ESCAPE-MeVO have planted doubts about the treatment, I believe that patient selection is crucial. We will need the upcoming data to elucidate the parameters of benefit.
Besides your own work, what is the most interesting piece of neurointerventional research you have seen over the course of the past year?
I think that the conglomerate evidence that has amassed for middle meningeal artery (MMA) embolisation for subdural haematoma over the
past couple of years via multiple randomised trials (MEMBRANE, STEM, EMBOLISE and MAGIC-MT) has created a real shift in treatment paradigms. This is interesting to me based on the sheer number of patients affected and helped.
What advice would you give to people embarking on a career in the cerebrovascular field?
The sky is the limit. Think creatively but respect the knowledge gained from the past. This is a field that is maturing—learn from your mentors but keep an open mind for the future.
Last but not least, what are your hobbies and interests outside the field of medicine?
Despite or perhaps because of a demanding work schedule, my interests outside of work are varied. They largely lie with my
immediate family—my wife, and two kids aged 12 and 8. Though it may not seem intuitive, living in New York City, we enjoy spending time outdoors together. I love getting out cycling or on the water kayaking, or going upstate in the mountains, hiking and visiting farms on weekends.
Recently, I’ve taken the outdoors inside by setting up a little indoor garden with flowers and herbs that we can use for cooking, which I enjoy. Living in the city also gives me the opportunity to indulge—usually with friends—in musicals and plays, including seeing my daughter in a production of Frozen Junior earlier this year!
Lastly, I really enjoy travelling, which we do often to visit extended family, but I also get to travel a bit for work. I love exploring new places and learning about history and culture.
"While recent studies like DISTAL and ESCAPE-MeVO have planted doubts about the treatment, I believe that patient selection is crucial. We will need the upcoming data to elucidate the parameters of benefit."


INSPIRE-S demonstrated that the Solitaire™ revascularization device & React™ catheter deliver excellent real-world outcomes, with consistent results and a strong safety record.5
802 patients
Combination technique, first pass effect, eTICI ≥2c 47.5%5
Combination technique, final revascularisation, eTICI ≥2c 72.0%5

33% of Aspiration cases need a bail out1-3 3x More likely to achieve TICI 2c-3 switching to Combination on 2nd pass4
References
1. Diana F et al. Comparison of aspiration versus combined technique as first-line approach in terminal internal carotid artery occlusion: a multicenter experience. J Neurointerv Surg. 2022 Jul;14(7):666-671.
2. Lapergue et al. Effect of Endovascular Contact Aspiration vs Stent Retriever on Revascularization in Patients With Acute Ischemic Stroke and Large Vessel Occlusion (the ASTER Randomized Clinical Trial). JAMA. 2017;318(5): 443–452.
3. Gupta R, et al. Technique and impact on first pass effect primary results of the ASSIST global registry. J NeurointervSurg. 2024.
4. Martins PN, Nogueira RG, Tarek MA, et al. Early technique switch following failed passes during mechanical thrombectomy for ischemic stroke: Should the approach change and when? J Neurointerv Surg. Published online April 4, 2024.
5. Data on file: 2024 09 19 NTF_INSPIRE S EMEA Primary Results Data Release † Includes Solitaire™ X revascularization device, primarily, with Solitaire™ Platinum revascularization device. Limitations: This data is being presented in advance of publication in a peer-reviewed

Despite the popularity that aspiration-first stroke thrombectomy has gained in recent years, the approach has its limitations—and, on occasions where it proves unsuccessful, neurointerventional physicians should be prepared to pivot towards a different strategy as early as possible in order to maximise their chances of achieving a positive outcome. This is a key message imparted by Manuel Moreu (Hospital Clínico San Carlos, Madrid, Spain), who recently spoke to NeuroNews about the vital role for ‘early technique switches’ following a failed first attempt at recanalisation.
While Moreu and many other neurointerventionists are in agreement that a direct aspiration first pass technique (ADAPT) is appropriate in certain cases, the current literature shows that the approach does not always prove to be sufficient, often leading to stent retriever (SR) deployment being required in order to achieve adequate clot removal. Findings from multiple studies—including the randomised ASTER trial and global ASSIST registry—indicate that approximately 33% of aspiration-first cases will be unsuccessful, thus requiring a rescue or ‘bailout’ strategy in order to boost the likelihood of recanalisation being achieved.
“I am not an ‘aspiration-first guy’ but, if I believe I’m going to be able to remove the clot with aspiration as a first approach, I will use it,” Moreu explains. “It is faster and more straightforward [than other approaches].”
“The question right now,” he continues, “is when to change the technique, and why. We know that, the more passes you perform, the lower the [chance of successful] recanalisation and the lower the probability of a good clinical outcome. The problem is that all of these papers showing this relate to SR passes. We don’t really know what happens with aspiration; we believe it’s similar, but we don’t have the same amount of information as with an SR-first approach.”
According to Moreu, there are two major challenges that face aspiration-first thrombectomy approaches: navigating the catheter smoothly past the ophthalmic segment and through the neurovasculature to the location of the clot, and maximising the area of contact and optimising the angulation between the clot and the catheter’s distal tip.
As such, clots located in more distal locations or in curved segments of the patient’s neurovasculature are among those generally considered less amenable to aspiration alone. An aspiration-only strategy may also be less likely to achieve success in cases presenting with a high clot burden.
Moreu notes that an SR can be deployed to act as an ‘anchor’ in some of these cases, overcoming two of the main drivers of aspiration-first failure by facilitating smoother, safer navigation of the catheter and getting closer to the thrombus interface.
“If you are not able to ascend the aspiration catheter, then anchoring is more than just helpful—it is the only option. It’s what needs to be done to get the catheter high enough,” Moreu avers. “People don’t always count these instances as a failure of aspiration, because there has not been an actual attempt with aspiration alone. But, I do think we should count them as failures, and as rescue techniques, as the aspiration catheter was not able to get to the clot. The most important part is the idea you had from the beginning, so that—if you randomise this in a clinical trial—it counts as an aspiration-first technique with a combined approach as the rescue strategy.”
Regardless of whether this anchoring strategy is universally considered as a bailout or not, the latest evidence suggests that a shift from aspiration alone to a ‘traditional’ combined approach following a failed first pass is beneficial. A US study that analysed almost 3,000 patients and was published within the last year indicates that switching between the two leads to second-pass thrombolysis in cerebral infarction (TICI) 2c–3 recanalisation being three times more likely, with a similar trend observed upon switching between the second and third passes.
“We need to understand that changing the technique may be something we have to do, and it may be something that’s going to be good for the patient,” Moreu says. “ADAPT is a really good technique but, if it has not worked, we need to start to think about changing the technique to try to improve their TICI score. And, it seems [based on the aforementioned study] that, the faster you change from aspiration to a combined technique, the better the results will be after the procedure itself.”
lower the recanalisation rate of each pass, and the lower the rate of good clinical outcomes,” Moreu elaborates. “Several publications have all agreed that [there is a reduction of benefit after each pass], so a good clinical outcome is rare, and you also increase the chance of a symptomatic intracranial haemorrhage [sICH]. You are decreasing the benefits and increasing the risks.”
However, while the first-pass effect is widely accepted as optimal, Moreu believes it is also important to recognise the fact that success at the first attempt is not always possible—hence the need for an appropriate bailout strategy.
“If you start with aspiration and are unable to remove the clot at the first pass, I believe we need to push to change the technique in order to improve the ‘second-pass effect’,” he quips.
A notable reason why neurointerventionists might be reluctant to switch away from aspiration alone pertains to speed and efficiency—as the mantra goes, ‘time is brain’, and ascending an SR will take more time while multiple aspiration attempts can be performed within a relatively short period.

On the flipside, ASTER trial data suggest comparable sICH rates between aspiration and combined-approach thrombectomy, potentially allaying concerns over the latter’s safety. And, given the established reasons to target successful recanalisation within the smallest possible number of passes, Moreu’s view is that switching between these approaches—from aspiration alone or, indeed, an SR alone, to combination thrombectomy—should be considered an option at the outset of any case.
The direct relevance of first-pass success with thrombectomy treatments was initially outlined by Osama Zaidat (Toledo, USA) et al in a 2018 paper,
It seems that, the faster you change from aspiration to a combined technique, the better the results will be after the procedure itself.”
and subsequent studies have produced similar findings—not only in terms of clinical outcomes like modified Rankin scale (mRS) scores, but also from a healthcare economics perspective.
The cost savings enabled by the first-pass effect (FPE) were elucidated in a 2022 paper from Eva González Diaz (Barakaldo, Spain) et al, with researchers demonstrating that successful first-pass recanalisation (modified TICI 0–3) can reduce a patient’s stroke-related lifetime costs by €44,289 compared to those who do not achieve a final modified TICI score of 0–3. The study also shows that a clinical outcome of mRS 2 can save healthcare systems in Spain approximately €63,000, €50,000, and €30,000 in annual long-term costs per patient, as compared to mRS scores of 5, 4 and 3, respectively.
“The greater the number of passes you do, the
“The first-pass technique needs to be the one you are more comfortable with,” he explains. “So, if you’re an aspiration guy [or girl], that’s perfect— COMPASS and ASTER have shown that it is not inferior, so what you are doing is okay. It’s also important to note that we sometimes do a first pass that was not a perfect attempt and we know the technique has not reached its full potential. However, if you believe that the first pass was done perfectly, then changing the technique will improve the recanalisation results, and that will improve clinical outcomes.”
Medtronic’s neurovascular portfolio provides physicians with options across the multiple different approaches to stroke thrombectomy; recent INSPIRE-S registry data from 802 patients demonstrated excellent real-world safety and efficacy outcomes using the company’s SolitaireTM revascularisation device and ReactTM aspiration catheter. The registry is core lab- and clinical events committee-adjudicated, and endorsed by the European Society of Minimally Invasive Neurological Therapy (ESMINT). Combined results from all treatment groups included 55.1% mRS 0–2 at 90 days, 46.5% first-pass expanded TICI ≥2c, 73.5% final successful revascularisation (expanded TICI ≥2c), and 1.5% sICH.
References for this article are available online at neuronewsinternational. com/medtronic-acute-ischaemic-stroke-early-technique-switch
DISCLAIMER: The data and content included in this article express only the clinical perspective of the presenter. They are completely independent and do not necessarily reflect the opinions of Medtronic.
The past 18 months have seen a radical shift in clinical evidence supporting the role of middle meningeal artery embolisation (MMAe) within subdural haematoma (SDH) treatment, with no fewer than four randomised controlled trials—three of which were first presented at the 2024 International Stroke Conference (ISC)—demonstrating its benefits alongside the current, surgical drainage-based standard of care. A key limitation that endures, however, is the fact that haematoma drainage and embolisation are still widely performed as two separate procedures. Against this backdrop, Luis Savastano (University of California San Francisco [UCSF], San Francisco, USA) and his colleagues have been working to develop a novel technology that could enable these two operations to be completed via a fully endovascular, ‘two-in-one’ approach. With initial findings on the technology having been disclosed at this year’s ISC (5–7 February 2025, Los Angeles, USA), Savastano speaks to NeuroNews alongside Pedro Lylyk (Clinica la Sagrada Familia, Buenos Aires, Argentina)—who performed the first-ever minimally invasive surgeries incorporating this novel approach in human patients—to discuss the “quantum leap” it could represent in SDH care.
What is currently the greatest unmet need in the interventional treatment of subdural haematomas?
SAVASTANO: A non-acute or ‘chronic’ SDH (cSDH) is a recurring collection of blood on the brain’s surface, which has an in-hospital mortality rate of 16.7% and a one-year mortality rate of 32%.
Surgical drainage or ‘clot evacuation’ of cSDH— which is the primary treatment for this condition and one of the most common neurosurgical procedures in the USA—is associated with high recurrence rates, greater than 20% in some patients, and complications. MMAe is an emerging treatment for cSDH in which embolic agents are injected into the MMA to decrease the blood supply to the haematoma and prevent re-accumulation. Multiple randomised clinical trials have recently demonstrated a reduction of SDH recurrences in patients undergoing MMAe in addition to surgical drainage. With the rise of MMAe, the ‘two-step’ management that combines surgical clot evacuation for rapid brain decompression alongside endovascular MMAe to prevent rebleeding as a surgical adjunct is rapidly becoming the leading paradigm in symptomatic cSDH treatment. Although effective in preventing recurrences—and rapidly becoming popular—the double-intervention strategy of surgery plus MMAe has several fundamental issues, such as:
1. It carries all the risks and problems of surgery, including postoperative infection, bleeding, seizures, pain, intensive care unit (ICU) stay (typically two days or more when drains are used), more extended hospitalisations, high recurrence, the need to discontinue antiplatelet and anticoagulant agents (potentially subjecting patients to serious ischaemic complications), and the social stigma of brain surgery.
2. It requires two different procedures, typically done by two different teams in two different places; neurosurgeons draining the SDH in the operating room (OR) and endovascular neurointerventionists embolising the MMA in an angiography suite—both frequently done under general anaesthesia, which is detrimental in elderly and fragile patients.
3. Increased levels of costs, healthcare utilisation, and hospital stay.


We thought that a technology capable of draining the SDH and embolising the MMA in a single, fully endovascular approach was urgently needed and could result in a paradigm shift in treating this condition. The problem was that this ‘moonshot’ required a purpose-built technology to obtain safe, easy and consistent trans-arterial access into the intracranial space, which had never been attempted before.
Could you briefly describe the technology you and your team have been working on to meet this need?
SAVASTANO: We have been working on the first minimally invasive technology for ‘trans-vascular’ surgery that enables MMAe and cSDH drainage in a single, fully endovascular intervention. In a typical case use, the procedure starts by navigating catheters over wires into the MMA from peripheral arterial access using conventional endovascular techniques, followed by delivery of embolisation agents to occlude the distal vasculature. At this point, a proprietary wire is delivered through the catheter into the MMA lumen, and actuated to perforate through the arterial wall and dura. The wire has unique features that allow it to self-orient and control perforation depth. The advancement of the wire creates a corridor from the MMA to the subdural space, through which a catheter is guided into the subdural haematoma and used to drain the fluid. After drainage, the system is pulled back into the MMA, the trans-vascular access is occluded, and the whole system is removed from the patient’s vasculature, concluding the procedure. Hence, MMAe and SDH drainage are seamlessly integrated into a single, minimally invasive endovascular procedure.
Could you also summarise where this idea came from, and how long you’ve been working on it and refining it up to now?
SAVASTANO: We started to work on this technology intensively about five years ago, and we were immediately faced with many challenges that we had to overcome to turn the idea into reality. The premise of navigating natural vascular corridors in our bodies into the head, gaining trans-vascular access to the brain surface, and treating non-vascular conditions, was fascinating—but, how to do it was completely uncharted. Since the pioneers of our specialty started delving intravascularly with wires and catheters a few decades ago, the neuroendovascular field has reached a remarkable degree of technical and technological sophistication. However, this development has mostly evolved within the intravascular boundaries and focused on solving vascular problems, such as aneurysms and arterial blockages. We wanted to break through the vascular walls’ limits, and open up a new frontier to access the brain and treat nonvascular conditions; something that we call the ‘trans-vascular’ field.
We ran two demanding programmes in parallel to start conquering this new horizon (from which the company’s name, Endovascular Horizons, is derived). On one side, we had to develop the first-inclass technology to accomplish something deemed impossible and full of engineering oxymorons, for which we recruited the absolute top wire and catheter engineers into the company. On the other side, with the support of the National Institutes of Health (NIH)—through the Blueprint MedTech Program—we partnered with the UCSF Transcatheter Neurosurgical Technologies Lab to make use of their best-in-class preclinical testing platforms including 3D phantoms, large animals and human cadaveric models. These parallel efforts supported extensive prototyping and iterative testing, leading to the maturation of an optimised technology that recently provided very positive clinical results.
What stage of clinical evaluation is the technology at right now, and how has it performed in testing and studies to date?
SAVASTANO: The system is currently under evaluation in a prospective, single-arm, first-in-human study (EMBODRAIN) with Pedro Lylyk and his team at Clinica la Sagrada Familia. This is a safety and feasibility study to evaluate the technical and clinical success of trans-vascular drainage of SDH plus MMAe with long-term follow-up. We recently presented results from the first five patients treated with the technology at the 2025 American Association of Neurological Surgeons (AANS) annual scientific meeting (25–28 April, Boston, USA).
We are happy to report that this study shows that our technology consistently enables a safe transvascular access to the subdural space, achieving a
Despite thousands of years of medical and technological improvements, we continue to open holes in patients’ heads for most neurosurgical problems.”
Luis Savastano
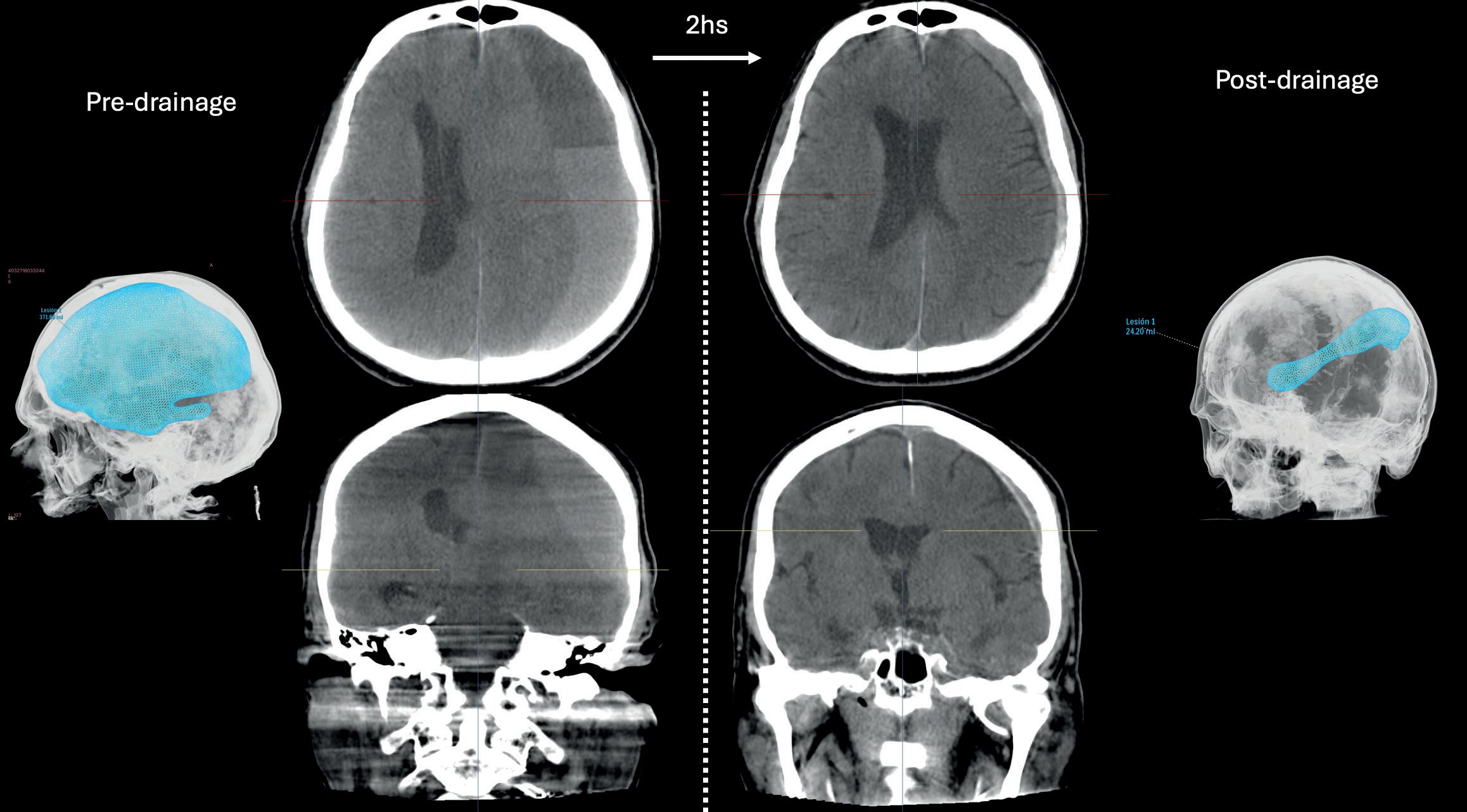
major volumetric reduction of subdural haematomas with a dramatic midline shift and rapid clinical improvement. Acute technical success—defined as creation of a leak-proof transvascular passageway and access to the subdural space with a microcatheter, along with drainage of the SDH and occlusion of the MMA—was achieved in all five cases. No serious adverse events were reported. Moreover, these patients are doing remarkably well at a long-term follow-up of six months, with no SDH recurrences nor progressions requiring surgery observed to date. We are currently continuing enrolment in this study, with 12 patients treated as of May 2025.
What is your eventual goal for this technology—and how do you believe this approach will fit in alongside traditional MMA embolisation, given the recent results from randomised clinical trials?
SAVASTANO: The immediate goal of this technology is to radically improve the care and outcomes of patients with SDH, most of whom are elderly and vulnerable, and we are actively working with the US Food and Drug Administration (FDA) to make this therapy available in the USA as soon as possible. This goal is monumental for patients—since most are septuagenarians or octogenarians—as well as patients’ families, healthcare providers, and payers. However, the technology we are developing is fundamentally a way to access the intradural space and brain without opening the head. Trepanation, which involves drilling a hole in the skull, is the oldest surgical procedure known to humanity, extending back over 5,000 years. Despite thousands of years of medical and technological improvements, we continue to open holes in patients’ heads for most neurosurgical problems. The ultimate goal for Endovascular Horizon’s technology is to enable a new era in medicine where the natural vascular corridors in our bodies are used to access the brain ‘trans-vascularly’ to treat non-vascular conditions (for example, draining SDH), deliver drugs and therapeutics, and implant devices like neuroprostheses and brain-computer interfaces.
Very recently, randomised controlled clinical trials have demonstrated that adjunctive MMAe, in addition to surgery, is associated with a significant reduction in non-acute SDH recurrences requiring surgical drainage compared to surgery alone. This is great, but the tradeoff is the need for two interventions
with associated risks and discomfort, prolonged ICU and hospital stay, and drastically increased medical care costs. Our technology was built to enable SDH drainage by gaining intradural access through the MMA itself following embolisation in the same session. This is a quantum leap compared to the current standard of care that demands the surgical creation of holes in the head—sometimes with temporary implantation of drains—or other potential approaches that could rely on a trans-venous route and
I think that all neuroendovascular surgeons will be able to rapidly learn and incorporate this technology in their practices, especially now that MMAe has become a routine procedure in most places.”
Pedro Lylyk
end up doubling the amount of required work, risks, procedural time and devices needed.
All of the discussed benefits of this technology translate, in turn, into better outcomes and accelerated recovery for patients. Furthermore, all the procedures performed to date have been done with patients fully heparinised, suggesting that the endovascular SDH drainage during MMAe could be done without interrupting anticoagulation and antiplatelets, thus minimising perioperative cardiovascular events. Finally, there is a large and growing neurointerventional workforce capable of safely performing MMAe that could easily incorporate the endovascular drainage of SDH in their practice. By providing a new treatment option to a large group of physicians, we expect to decrease the need to transfer patients to tertiary centres and improve timely access to a lifesaving intervention for communities that have historically suffered from a lack of readily available, specialised neurosurgical care—including underserved rural populations, people with lower
socioeconomic status, and racial and ethnic minority groups.
If this technology continues to demonstrate promise and produces benefit in patients, what do you envisage as the role for open neurosurgical approaches in nonacute SDH moving forward?
SAVASTANO: In the limited clinical experience to date, we have intentionally been very inclusive to capture all types of non-acute SDH, and the results have been very promising—even in those cases that would have conventionally required craniotomies, such as in multiloculated SDH, very large SDH and acute-on-chronic SDH. We are therefore bullish in our projections, and we anticipate that most nonacute SDHs will be subject to endovascular drainage as the first-line treatment. We envision that open approaches for non-acute SDH will be valuable as a rescue procedure when endovascular drainage and MMAe are not successful in achieving sufficient brain decompression to resolve the patient’s symptoms rapidly; in the rare circumstance of SDH recurrence after endovascular treatment; or when the anatomy of a particular patient means the embolisation is not technically possible or is contraindicated.
Having become the first physician in the world to use this device in human cases, what are your initial thoughts on its performance and characteristics?
LYLYK:The use of the system to perforate and drain the SDH is very intuitive and straightforward, and only requires basic endovascular skills—basically, embolising the distal MMA branches, then pushing a wire through the dura, and then advancing a microcatheter co-axially over the trans-vascular wire for drainage. The components of the system are compatible with most guide catheters and microwires currently used for MMAe, and the drainage itself only adds a few minutes to the overall procedure (but saves a trip to the OR for surgery and prevents ICU days). I think that all neuroendovascular surgeons will be able to rapidly learn and incorporate this technology in their practices, especially now that MMAe has become a routine procedure in most places.
Further down the line, if this technology demonstrates positive results in clinical trials and is used more widely, how much of an impact could it have on SDH care?
LYLYK: It will be transformational. I expect that, within the next decade, most SDH will be treated endovascularly with Endovascular Horizons’ technology. Why would you do two different procedures when you can fix the problem with one solution? When we discuss the possibility of participating in the trial with our patients and their families in Buenos Aires, they immediately understand the benefits of avoiding a second procedure—especially when it involves drilling holes in the head. In addition, the clinical outcomes so far have been outstanding. We see patients rapidly improving to their baselines, spending fewer days in the hospital, and not returning with SDH recurrences or other problems after discharge.
This technology is opening a new era in minimally invasive neurosurgery, and treating SDH is the first step. I have been involved in first-in-human clinical experiences of neuroendovascular devices for more than 30 years. I was fortunate to witness those breakthrough innovations that have radically transformed the field; this is one of them.




The established ‘gold-standard’ approach to carotid revascularisation remained enshrined as the go-to operation as far as the 2025 Charing Cross (CX) Symposium (23–25 April, London, UK) audience gathered for a carotid and acute stroke challenges great debate was concerned.
AN OVERWHELMING 76% OF those who voted in the post-debate poll sided with Barbara Rantner (Ludwig Maximilians University Munich, Munich, Germany) after she made the case for carotid endarterectomy (CEA), long established as the gold-plated method of tackling carotid disease requiring intervention. Transcarotid artery revascularisation (TCAR) and transfemoral carotid artery stenting (CAS) each drew 12% of the audience share apiece, with the proposition for TCAR put forward by Michael Stoner (University of Rochester, Rochester, USA) and that of CAS made by Christopher Metzger (Harvard Medical School, Columbus, USA).
Rantner, a co-chair of the 2023 European Society for Vascular Surgery (ESVS) carotid guidelines, looked at CEA through the prisms of gender, age and timing of procedure. “There is no gender issue, there is no age issue, there are no limitations in timing of treatment,” she declared, pointing out the absence of a superiority trial showing an alternative revascularisation option that has been proven to perform better than CEA. “Is endarterectomy the gold standard? Yes.”
Among the articles she invoked in support, she referenced 10-year ACST-1 trial results that showed

surgery equally benefitted male and female asymptomatic patients. Symptomatic females undergoing CAS in CREST, Rantner said, “suffered from significantly higher periprocedural complications” when compared to CEA. Focusing on age, trial analysis showed that CAS was not of benefit for patients aged 65 years or older. “Implementing endarterectomy remains standard of care, and also in the elderly population of 80 years and older,” Rantner added. In terms of timing, data demonstrated CAS “very significantly increased risk when we looked at early treatment onset”, she said.
The case for TCAR, meanwhile, rests in its minimal-access benefits for highrisk patients, the fact the procedure takes embolic risk in the aortic arch off the table, its safety profile, which is “as good—or better—than CEA”, the
fact that it is “highly reproducible and scalable”, and its “rapid learning curve for contemporary vascular surgeons”, argued Stoner. He led attendees through the three ROADSTER studies which produced data in support of TCAR, and looked at how the procedure compares to CAS. “TCAR is safer than CAS in any trial that has looked at this,” Stoner said. “How does it compare to CEA, the ‘gold standard’? Well, the gold standard doesn’t fare as well as TCAR. We have equivalent stroke/death rates, improved myocardial infarction, pre- and postprocedural hypotension and length of stay of less than one day, and you can see a sustained benefit for TCAR.”
“Percutaneous CAS is plagued by procedural embolic risk and worse outcomes over time in at-risk patients,” Stoner summarised. “TCAR solves the
We should individualise our approach and, in 2025, with patient selection and good technique, all of these therapies are phenomenal.”
Christopher Metzger
CAS embolic problem. Hard outcomes are equivalent to CEA or even better, and there is a strong patient preference toward minimal-access surgery. As such, I’ll submit that TCAR is now the contemporary gold standard for carotid revascularisation.”
Taking up the case for CAS, Metzger reasoned that all of the carotid revascularisation strategies
“are excellent” and that there is no single gold standard for the procedure. Clear-cut gains for transfemoral stenting include no general anaesthesia and less stress on older or sicker patients, he said. Disadvantages, on the other hand, include the fact that not all patient anatomies are suitable for CAS, Metzger noted. The case for CAS includes four randomised trials in which the strategy went up against CEA and, in one, medical therapy. “The last four randomised trials of carotid stenting versus endarterectomy have produced outstanding results for both therapies and their equivalent,” he said. With newer carotid stents emerging and contemporary data in CREST-2, “the results keep getting better”.
The three strategies are seemingly complementary rather than competitive, Metzger concluded. “We should individualise our approach and, in 2025, with patient selection and good technique, all of these therapies are phenomenal.”
In post-debate discussion, a comment from the audience took issue with Stoner’s description of the ROADSTER studies as “trials”. “None of the data you have shown are from trials,” the audience member observed. “You need to be careful about terminology and you need to be careful about head-tohead comparisons” based on nonrandomised, observational data, a point conceded by Stoner.
Another commenter from the floor challenged both Stoner and Metzger to offer the situation in which they go against the grain and, respectively, proceed with transfemoral CAS in the case of the former and with CEA in the case of the latter. “Transfemoral versus TCAR? Based on the common carotid artery, I do think you can cheat somewhat on the length, but you can’t cheat on the quality of artery,” Stoner responded.

A retrospective, nationwide cohort study has revealed carotid endarterectomy (CEA) performed better than carotid artery stenting (CAS) in France over the course of a decade, while also highlighting the overuse of both procedures in the same period. Based on their findings, the research team calls for the suspension of interventions for carotid artery disease until appropriate studies are conducted.
THE INVESTIGATION—WHICH AUTHORS
Eric Steinmetz (CHU Dijon Bourgogne, Dijon, France) and colleagues note involved the largest cohort of asymptomatic carotid procedures ever
studied—was recently published as an Editor’s Choice paper in the European Journal of Vascular and Endovascular Surgery (EJVES)
Steinmetz et al begin by stating that it was their objective to compare periprocedural stroke or death within 30 days of a procedure in patients who underwent either CEA or CAS.
The researchers used data from the Plan de financement à l’acte (PMSI) hospital database, including in their study all patients who underwent either CEA or CAS between 2010 and 2019 in France. Steinmetz and colleagues share that, between 2010 and 2019, 164,248 patients underwent a carotid artery procedure in France, comprising 156,561 CEA and 7,687 CAS procedures. They note that asymptomatic women made up around 25% of the total cohort and high-risk patients, 40%.
The authors report in EJVES that the rate of periprocedural stroke or death within 30 days was 1.5% overall, 1.3% in asymptomatic patients, and 3.3% in symptomatic patients. They detail that, after matching and adjustment, the risk of periprocedural stroke or death within 30 days was statistically significantly greater in patients who underwent CAS than in patients who underwent CEA.
In their conclusion, Steinmetz et al stress
that many patients in the study—specifically asymptomatic women, high-surgical-risk patients, and patients undergoing CAS—received procedures that were more likely to be harmful than beneficial, according to the results of past randomised trials.
In addition, the authors write that, “despite the huge number of asymptomatic carotids treated in the present sample, there is currently no proven benefit for asymptomatic carotid intervention compared with best medical treatment alone, or for screening for carotid artery disease”.
Based on these conclusions, Steinmetz and colleagues propose there “should be a moratorium on procedures for carotid artery disease until appropriate studies are conducted to determine whether [these procedures are] beneficial and for whom,” adding that studies are warranted to assess whether screening to detect carotid stenosis improves outcomes compared with non-invasive care directed only by clinical risk factors and without arterial imaging.
Furthermore, Steinmetz et al highlight a “tremendous overuse” of carotid procedures and an “urgent need to reconsider guidelines that encourage carotid artery procedures when there is no evidence of procedural benefit, and concurrent ongoing proof of procedural harm and significant cost”.
Whether or not patients implanted with spinal cord stimulation (SCS) technologies can undergo a magnetic resonance imaging (MRI) scan—in other words, whether such devices can be considered ‘MR conditional’—relates to the safety and overall applicability of the given system, and therefore represents a nuanced yet vital issue. Here, clinical scientist Peter Wright (Plymouth, UK) and consultant pain specialist Cormac Mullins (Cork, Ireland) discuss how technical considerations, nomenclature and guidelines relate to these debates.
“MRI is increasingly becoming the gold standard across the board for diagnosis and detection of diseases like cancers and stroke—it’s hugely beneficial as an imaging modality,” Wright explains. “And, for those patients with any implant—let alone neurostimulators or other electronic devices like cardiac pacemakers—there is always a risk with undergoing an MRI, to the point that patients with certain medical devices are completely contraindicated. Patients with an ‘MR-conditional’ device can undergo these procedures but under very safe, strict conditions that must be followed and are defined by the device manufacturer.
“The importance of being able to offer that relates to equity—it’s being able to say that we can provide the same imaging and diagnostic opportunities to all patients, whether or not they have an implant, such as a neurostimulator.”

Mode’, Medtronic’s SureScan technology ensures that the device can be scanned without any impedancerelated restrictions or risks of thermal tissue damage created by lead fractures. Additional benefits of this technology include the ability to perform a scan regardless of the SCS device’s charge levels, and with the patient in the prone position, as is required for an MRI intended to detect breast cancers. Wright and Mullins are in total agreement over the fact that, as the latter puts it, “some devices are more MR conditional than others”.
“And, MR conditionality is not a binary label,” Wright notes, suggesting that the concept of an SCS device being amenable to MRI is more of a broad spectrum than a simple ‘yes-or-no’ answer.

The criteria under which certain implanted devices may be deemed ‘MR conditional’ could involve controlling or limiting the rate at which the patient’s body absorbs energy during the MRI scan, the presence of a specialist team to programme the device in order to reduce safety risks, or specialised equipment capable of providing adequate physiological monitoring, during a scan. However, as Wright avers, many smaller or mobile imaging facilities will not be able to accommodate these additional requirements, limiting patient access to MRI scans at the ‘point of care’.
Alluding to relevant examples where this restricted access can be particularly problematic, Mullins highlights two time-sensitive contexts—cauda equina syndrome, for which an MRI scan is needed to diagnose the disorder and facilitate urgent surgery, and acute stroke, an emergent condition for which diagnostic MRI is the gold standard. “Unfortunately, that’s where issues over MRI access come into play,” he adds. “Some centres are bigger and more used to dealing with spinal cord stimulators, but others aren’t—and, as soon as they hear ‘SCS’, they get scared.”
MRI and SCS
“One device’s MR conditions won’t necessarily be the same as another’s, and you can see that in spinal cord stimulators, with different manufacturers having different MR conditions in order to be able to safely scan their device,” Wright states.
On this front, a highly relevant characteristic of Medtronic’s Inceptiv family of SCS systems pertains to lead impedance. Wright comments that, while the MR conditions of some stimulators stipulate that impedance must be measured immediately before scans to detect potentially dangerous lead fractures, Medtronic’s tantalum lead-shielding feature disperses excess heat and allows ‘out-of-range’ electrode impedances to be scanned safely—removing the need for these burdensome tests. This is a key factor in maintaining a patient’s MRI access as, once they have switched their spinal cord stimulator to ‘MRI
“‘MR conditional’ is a catch-all term, but there are so many conditions that you can have,” Mullins adds. “The one I’ve touched on in this article is impedance— if impedances are elevated, that is a potential hurdle to undergoing an MRI. And, in something like an emergency setting, it becomes a major hurdle.”
The research Mullins alludes to here was published in Pain Practice in late 2023, and—based on a single-centre, retrospective review of 363 UK patients implanted with SCS systems from Abbott, Boston Scientific and Nevro—concludes that these MRconditional devices may ultimately become ineligible for an MRI due to impedance-related issues. In the study, 18.5% of patients implanted with Nevro, Boston Scientific or Abbott devices had leads with at least one contact impedance >10,000Ω at an average of 2.25 years follow-up, while the risk of lead impedance failure increased by 35.4% with each successive year and peaked at 43% of patients with impedance failure at five years post-implant. Conversely, Medtronic’s SureScan technology carries a 0% risk of MRI access being lost over time due to high electrode impedances.
Due to the fact that elevated impedance in certain MR-conditional SCS devices results in a potential loss of eligibility to undergo an MRI scan, Mullins and his co-authors conclude that, “if future MRI is likely, patients and clinicians should consider implanting impedance-independent [Medtronic SureScan] MR-conditional systems, and possible failure of MR conditionality should be routinely incorporated into patient consent prior to implant”.
“The consequences of [elevated lead impedances],” Mullins tells NeuroNews, “are delayed diagnosis— potentially in an emergency setting—and treatment delays, which could make a huge difference to the clinical outcome, and also a potential device explant to facilitate an MRI in less urgent settings like cancer or progressive neurological deterioration.”
Here, Mullins reiterates his description of Medtronic’s SCS technologies as ‘impedanceindependent’, adding that they boast MRI access that is “effectively guaranteed” to the patient, therefore also alleviating many concerns clinicians may have over MR conditionality with other systems.
Shifting guidelines
Another point on which Wright and Mullins are aligned is the flawed notion of ‘MR compatibility’—with Wright describing this phrase as “one of the banes of my life”, as it implies, incorrectly, that these delineations are binary between ‘compatible’ and ‘incompatible’. “MR compatible doesn’t exist in my language, and it shouldn’t really exist in anyone’s language,” he says. “There are only three statuses: ‘MR safe’, ‘MR conditional’ and ‘MR unsafe’. But, I still hear ‘MR compatible’ being bandied around and, if I could remove that from the English language, I would.”
On this matter, Mullins makes reference to a 2020 paper published in Neuromodulation, entitled, ‘A comprehensive practice guideline for magnetic resonance imaging compatibility in implanted neuromodulation devices’, echoing Wright’s views on the problematic nature of defining SCS systems as ‘MR compatible’, and noting that the aforementioned guideline does not specify “many of the things that we have discussed”, nor does it define or even mention the relevance of impedance measures. “We have both recommended that there should be a more up-to-date guideline that includes specific guidance on delineating between the different devices that are conditional—and what ‘conditional’ really means,” Mullins says.
Returning to Wright’s assertions on MRI evolving into the gold-standard modality across many pathologies, Mullins adds that rates of these scans are “going through the roof”, before also commenting that the proportion of SCS-implanted patients who will require an MRI in the future is “close to 100%”.
The data he references here stem from a 2015 analysis published in Spine, which projects that up to 84% of SCS patients can be expected to need at least one MRI scan within five years post-implantation—a figure that rises to 98% after 10 years. And, when these decade-old findings are considered in conjunction with National Health Service (NHS) England data cited by Wright suggesting that MRIs across the UK increased by 73.6% from 2012–13 to 2022–23, with an annual growth of roughly 6.5%, the ever-growing significance of MRconditional implants becomes even more apparent.
“I became involved in this area because, in clinical practice, we observed that this was becoming a big issue: patients implanted with MR-conditional devices had their device interrogated, and their impedances were elevated and they could no longer have an MRI,” Mullins explains. “We saw so many of these patients that could not have an MRI and, when we looked at the literature, there was nothing published on it.”
Mullins states that, despite a handful of more recent publications, this remains an “underrepresented” area within the current literature—placing an onus on clinicians and scientists to produce a greater body of research, and on device manufacturers to improve the quality of their MRI access. Wright and Mullins both ultimately feel that, moving forward, updated guidelines should give healthcare providers and patients alike improved clarity on the definitions and importance of MR conditionality when it comes to SCS devices.
“Ideally, we would go through the literature to profile the safety studies that are out there and try to provide clinicians with a practice algorithm for this—a common guideline that isn’t specific to the manufacturer—and join up all of the evidence to create clearer criteria on circumstances in which it’s safe, or high-risk, or unsafe, to undergo an MRI,” Mullins concludes.

Microtransponder has announced the publication of new findings in the journal Stroke validating the beneficial long-term outcomes of paired vagus nerve stimulation (VNS) therapy via its Vivistim system for chronic stroke recovery. The findings demonstrate that stroke survivors treated with paired VNS for upper-extremity deficits post-stroke had improvements in symptoms of motor impairment, as well as activity, participation in daily life and quality of life, for at least one year after completing therapy.
‘LONG-TERM OUTCOMES OF vagus nerve stimulation paired with upper-extremity rehabilitation after stroke’, as published in Stroke, is a longterm analysis of VNS-REHAB— a pivotal, multicentre, triple-blinded randomised controlled clinical trial previously published in The Lancet that served as the basis for US Food and Drug Administration (FDA) premarket approval of the Vivistim paired VNS system in 2021.
One year after completing paired VNS therapy alongside post-stroke upper-extremity rehabilitation, participants maintained significant and clinically meaningful improvements in motor impairment and functional activity as measured by the Fugl-Meyer assessment—upper extremity (FMAUE) and the Wolf motor function test (WMFT). Average improvements from baseline were 5.23 and 0.5, respectively. Microtransponder also notes in a press release that 66.2% of 74 participants responded positively to paired VNS therapy.
Additional patient-reported outcomes demonstrated improvements in activity, participation in daily life and quality of life across multiple validated measures, including motor activity log; stroke impact scale—activities of daily living; stroke impact scale— hand; stroke-specific quality-of-life scale; and overall health-related quality of life (EQ-5D).
“These long-term clinical and qualityof-life outcomes of paired VNS therapy are particularly notable because sustained, comprehensive benefits are rarely shown in chronic stroke recovery,” said Teresa Jacobson Kimberley (Massachusetts General Hospital [MGH] Institute of Health Professions, Boston, USA), the lead researcher on the study. “Most stroke survivors need improved hand and arm function because it is essential for nearly every task in daily life, and
Vivistim may be the boost that helps get them there.”
Microtransponder’s press release states that, in current clinical practice, the Vivistim device is implanted in stroke survivors, enabling therapists to use a wireless remote that communicates with the device to pair VNS with highrepetition, goal-oriented functional activities to increase neuroplasticity. The standard protocol is for stroke survivors to participate in in-clinic Vivistim therapy during 90-minute sessions three times per week for six weeks. In-clinic paired VNS therapy is complemented by self-initiated Vivistim therapy, which allows stroke survivors to swipe a magnet across the implant to activate VNS while doing daily activities at home or in the community.
The company further claims that this one-year study analysis demonstrates that self-initiated paired VNS therapy may facilitate the continued refinement and consolidation of behaviourally relevant activities, resulting in long-term, persistent improvements.
5.23 0.5 Average improvement from baseline
The pivotal VNS-REHAB trial was conducted between October 2017 and June 2022.
Researchers note that some therapy and assessments occurred during the COVID-19 pandemic, but improvements were sustained in spite of the potentially unfavourable impact the pandemic may have had on participants’ lives. Sex, age, side of stroke, and time since stroke, did not significantly impact FMA-UE or WMFT outcomes, and no long-term serious adverse events related to therapy or stimulation were reported.
Researchers from Carnegie Mellon University and Allegheny Health Network in Pittsburgh, USA have developed a new method for deep brain stimulation (DBS). The technique, referred to as ‘DeepFocus’, uses trans-nasal stimulation to achieve more accurate electrical stimulation in the brain.
DEEPFOCUS USES CLOSE PROXIMITY AND highly conductive pathways offered by thin bones between the nasal cavity and brain to create larger, more accurate electric fields in deep brain regions, as compared to traditional scalp electrode configurations. This method enables more efficient and lower risk targeting of deep brain structures to treat multiple neural conditions, including depression, post-traumatic stress disorder (PTSD), obsessive compulsive disorder (OCD), and substance abuse disorder.
The researchers believe DeepFocus could provide both short- and long-term treatments. Chronic treatments that require persistent stimulation could be delivered through an implant, while acute applications could be delivered in short sessions with endoscopic insertion and removal of the device, they add.
There is a precedent for treating neurological conditions with implanted electrodes in the deep brain, but this requires a sophisticated, highly invasive surgical procedure that carries risks of intracranial
haemorrhage and infection. Also, this traditional method is not steerable, which means the stimulation target cannot be changed once the electrodes are implanted inside the brain.
“Early results of invasive deep brain stimulation in
The USA is facing a severe mental health crisis, with PTSD, depression and substance abuse disorders among the most pressing societal challenges.”
Study details “largest” long-term report of real-world data on permanent PNS device
NALU MEDICAL HAS announced the publication of realworld data in the journal Chronic Pain and Management, describing this as a “significant milestone” that establishes Nalu peripheral nerve stimulation (PNS) as a medically necessary treatment for chronic pain. The analysis includes data from 2,273 patients implanted with the Nalu micro-implantable pulse generator (IPG) PNS system, making it the largest published real-world dataset on a permanent PNS device, according to the company. The large sample size and a responder rate of 94% (Patient global impression of change [PGIC]) constitute “solid evidence of reliable, exceptional effectiveness”, Nalu claims.
“The consistent improvements observed across a large, diverse sample of real-world clinic patients treated in multiple anatomic regions and nerve combinations emphasises the broad applicability of PNS therapy delivered by the Nalu micro-IPG PNS system,” said John Hatheway (Franciscan Pain Management Clinic at St Anthony, Gig Harbor, USA), lead author of the paper. These real-world data are claimed by Nalu to confirm the published one-year findings of the COMFORT randomised controlled trial, with the magnitude of the present sample size now supporting generalisation of those findings to the population at large, and previously published data also reporting a 50% reduction in total healthcare costs among patients receiving Nalu PNS therapy.
treating neuropsychiatric conditions have been very promising,” said Pulkit Grover (Carnegie Mellon University, Pittsburgh, USA), the senior author of this study. “But, the sophisticated surgery required for invasive deep brain stimulation technology makes it unlikely to be widely adopted.”
According to the researchers, non-invasive techniques—such as transcranial magnetic stimulation (TMS), transcranial focused ultrasound stimulation (tFUS), and transcranial electrical stimulation (TES)— have reduced risks and are steerable, but they are not as effective as implanted electrodes due to limited depth and resolution. TMS and TES can also cause high scalp pain because of more intense electric currents.
DeepFocus offers a minimally invasive solution that is more accurate, less painful, and steerable, the researchers claim.
“The USA is facing a severe mental health crisis, with PTSD, depression and substance abuse disorders among the most pressing societal challenges,” Grover added. “While surgically implanted deep brain stimulation has shown promise, it lacks both widespread acceptance and the necessary US FDA [Food and Drug Administration] approvals for these conditions. Our minimally invasive, low-risk approach, which can be implemented in an outpatient setting, presents a scalable and widely applicable solution.”
Artedrone demonstrates autonomous thrombectomy capabilities with Sasha solution Artedrone, a Truffle Capital medtech portfolio company, has announced the successful completion of various in vitro and animal studies demonstrating the ability of its Sasha microrobotic solution to autonomously perform mechanical thrombectomies in stroke treatment. Results from the study are being submitted for publication in a research journal, an Artedrone press release notes.
“Mechanical thrombectomy is by far the most efficient treatment for large vessel stroke but is currently highly underused due to the fact that it is often only performed at specialised stroke centres, which denies life-saving treatment access to millions of patients,”
said Frédéric Clarençon (Pitié Salpêtrière Hospital, Paris, France), a principal investigator on the study. “We’re encouraged by these preclinical results and will be conducting additional studies to advance Sasha as a means to expand access to mechanical thrombectomy for stroke patients around the world.”
perfusion (CTP) and magnetic resonance imaging (MRI)-derived core volume assessments. According to a Brainomix press release, this addresses a “longstanding unmet need” in stroke triage, enabling physicians across stroke networks to access expert-level insights to improve decision-making for the treatment and transfer of patients using routinely available brain scans.
The release goes on to state that Brainomix 360 is the only stroke artificial intelligence (AI) imaging tool with a demonstrated impact on stroke treatment rates.
Artedrone’s release goes on to detail that the preclinical programme for Sasha is evaluating mechanical thrombectomy performance in a variety of settings—including in vitro and in vivo porcine cerebral anatomies, as well as in vitro human cerebral vasculature. In each of the previous models, pre- and post-procedure vessel perfusion was analysed to confirm the effectiveness of the microrobot design. Most notably, Artedrone claims, the latest successful preclinical milestone examined both biological analogue clot and native clot retrieval from in vivo porcine cerebral anatomy.
Artedrone also recently announced Liane Teplitsky as its chief executive officer (CEO), in addition to appointing Nnamdi Njoku and Dan Raffi to its board of directors.
Brainomix gains US FDA clearance for new core volume assessment feature Brainomix has announced the US Food and Drug Administration (FDA) clearance of a novel and patented feature that enables physicians to assess ischaemic core volume from universally available non-contrast computed tomography (NCCT) images through the Brainomix 360 Stroke solution.
The core volume feature has been studied and validated by leading US stroke centres, with results demonstrating equivalency to CT
“The ability of Brainomix 360 to estimate ischaemic core volumes in a reliable and reproducible manner with a similar performance to CTP represents an attractive alternative in centres without ready access to either advanced imaging modalities or stroke neurologists and/or neuroradiologists for imaging interpretation, and has the potential to make endovascular therapy more widely available,” said Mehdi Bouslama (Broward Health, Fort Lauderdale, USA), who co-authored a recent study published in the journal Stroke validating Brainomix’s NCCT core volume feature.
Brainomix’s release details that a separate study published in the Journal of NeuroInterventional Surgery has suggested that Brainomix NCCT core volumes were comparable to those obtained from concurrent CTP.
“Our results demonstrate the ability of this algorithm to estimate ischaemic core volumes from NCCT with similar performance to CTP and may be an attractive alternative in centres without ready access to advanced imaging modalities,” the study concludes.
Imperative launches dual aspiration thrombectomy catheter following M2 occlusion stroke trial announcement Imperative Care has announced that the Zoom stroke system has been further expanded with the launch of its Zoom DuoPort technology, which allows physicians to use the novel continuous dual aspiration technique (CDAT) on two Zoom aspiration catheters simultaneously from a single vacuum source.
Earlier this year, Imperative received US Food and Drug Administration (FDA) 510(k) clearance for its Zoom system—a comprehensive stroke thrombectomy system including largebore 0.088-inch catheters indicated for both access and aspiration when used with a Zoom catheter. In a press
release, the company states that clinical data from the Imperative trial supporting clearance of the Zoom system showed that dual aspiration with Zoom is fast, effective and safe in patients with a range of complex anatomies.
With the introduction of CDAT and Zoom DuoPort, the Zoom system is now the first and only system to enable continuous dual aspiration for stroke thrombectomy, according to Imperative.
One of the first cases utilising CDAT and Zoom DuoPort was performed by Hakeem Shakir (Oklahoma University Health, Oklahoma City, USA), and saw a patient presenting with an internal carotid artery (ICA) terminus occlusion ultimately achieve first-pass thrombolysis in cerebral infarction (TICI) 3 revascularisation with clot captured in the Zoom 88 catheter.
“In this case, a first-pass TICI 3 result was made possible because of the control I was able to achieve with continuous dual aspiration. Without CDAT, an additional pass would likely have been required,” said Shakir. “In my experience, CDAT is a more common-sense approach to aspiration thrombectomy that streamlines the process, optimises efficiencies and brings me immediate confidence that the stroke-causing clot has been captured.”
In April, Imperative also announced plans to fund an investigator-initiated, multicentre randomised controlled clinical trial comparing aspiration thrombectomy with the Zoom stroke system plus best medical treatment versus best medical treatment alone in patients suffering from acute ischaemic stroke due to a primary occlusion of the M2 segment of the middle cerebral artery (MCA) within eight hours of symptom onset.
more than 180 patients across multiple centres in the USA and Europe “ahead of schedule”, according to Perfuze.
The study is evaluating the safety and effectiveness of the company’s Millipede 088 super-bore aspiration catheter, which is designed for rapid and complete clot removal in patients suffering from large vessel occlusion (LVO) acute ischaemic strokes, and aims to improve stroke treatment outcomes and reduce procedural complexity.
“The data generated from MARRS will provide important insights that have the potential to meaningfully impact the future of stroke treatment,” said Raul Nogueira (University of Pittsburgh School of Medicine, Pittsburgh, USA), the study’s principal investigator.
Announcing receipt of US FDA 510(k) clearance for its Zipline access catheters, Perfuze noted that this regulatory milestone will strengthen its growing portfolio of innovative neurovascular devices, empowering physicians to treat acute ischaemic stroke with greater speed, ease, and precision.
The Zipline access catheters are engineered to enhance the trackability and delivery of large (070) and super-bore (088) aspiration catheters, simplifying neurointerventional procedures and enabling faster, more efficient stroke treatment, by providing increased support and navigational ease, the company states in a press release.

The trial is set to enrol and randomise patients 1:1, and will be conducted independently through an academic research organisation. Its primary efficacy endpoint will measure clinical outcomes using 90-day modified Rankin scale (mRS) scores. Patients randomised to the arm evaluating the Zoom stroke system are set to undergo treatments utilising CDAT and Imperative’s novel DuoPort technology.
Perfuze completes MARRS study enrolment, gains US FDA clearance for Zipline catheters Perfuze has announced the completion of enrolment in its US pivotal investigational device exemption (IDE) clinical trial, MARRS—an announcement that comes shortly after US Food and Drug Administration (FDA) 510(k) clearance of the company’s Zipline access catheters in March.
The MARRS pivotal study— conducted under an IDE from the US FDA—has successfully enrolled
“The Zipline catheters represent an innovative technology that I believe will simplify stroke intervention, reduce costs and accelerate reperfusion,” commented Jay Dolia (Emory University School of Medicine, Atlanta, USA). “In my initial experience, they have enabled rapid clot access and aspiration, even in complex anatomy.”
Perfuze also recently secured €22 million in follow-on funding in a round led by existing investors including Earlybird, EQT Life Sciences, Seroba and SV Health, in addition to welcoming medtech executive Joe Rotger as the company’s new executive vice president of sales.
Kandu Health and Neurolutions merge to form “new leader” in stroke recovery space
Kandu Health and Neurolutions have merged to form Kandu Inc—a company that “expects to set a new standard of care for stroke recovery” via a “first-in-kind”, end-to-end solution for stroke survivors that combines brain-computer interface (BCI) technology with personalised telehealth services.
As part of the merger, Kandu Inc completed the first close of a US$30 million financing round, which was coled by Ally Bridge Group and AMED Ventures, with participation from other
existing investors. Kandu Inc will use proceeds from the financing to support ongoing commercialisation and continued execution of its mission to enable stroke recovery that is accessible to patients and caregivers at home.
Kandu Health has made its name as a technology-enabled healthcare services company specialising in stroke recovery, providing remote, multidisciplinary support to stroke survivors and care partners in outpatient settings through a team of licensed clinicians, and an easy-to-use app. Neurolutions primarily focuses on the use of non-invasive BCI technology for post-stroke therapy via its IpsiHand system—which, the company claims, is the only non-invasive US Food and Drug Administration (FDA)-cleared breakthrough device that can accelerate motor recovery of the affected upper limb after chronic stroke.
“Despite years of improvements in stroke treatment technology and acute intervention, our system of care has not yet produced meaningful improvements in patients’ functional outcomes following hospital discharge,” said Demetrius Lopes (Advocate Aurora Health, Chicago, USA). “Kandu offers a new approach to post-acute care that I believe will continue to improve functional outcomes for stroke survivors.”
Neurolutions chief executive officer (CEO) Leo Petrossian, who is set to lead the newly formed Kandu Inc as CEO, commented that this merger positions Kandu Inc as a “leader” in the stroke recovery space, and will allow the company to offer a “seamless continuum of care”, from the immediate post-acute phase through chronic rehabilitation and recovery.
Terumo Neuro receives US FDA approval for carotid stent system Terumo Neuro has announced that its carotid stent system has received premarket approval (PMA) from the US Food and Drug Administration (FDA).
This milestone marks the first duallayer micromesh carotid stent approved in the USA, offering physicians a clinically proven option to improve patient outcomes in carotid artery disease treatment, as per a Terumo Neuro press release.
The company’s carotid stent system
29–30 May
ARISE III Roundtable
Discussion with Industry and Stroke Experts
Washington DC, USA
W: ariseconsensus.com
2–4 June
LINNC Paris Paris, France
W: linnc.com/Course-information/ LINNC-Paris-2025
is indicated for the treatment of carotid artery stenosis in patients at increased risk for adverse events following a carotid endarterectomy procedure.
The device is intended to treat patients with de novo atherosclerotic or post-endarterectomy restenotic lesions in the internal carotid arteries, or at the carotid bifurcation, with ≥50% stenosis in symptomatic patients or ≥80% stenosis in asymptomatic patients— as determined by angiography.
According to Terumo Neuro’s recent release, the device also accommodates vessel reference diameters between 3.5mm and 9mm at the target lesion.
Route 92 secures Australian TGA approval for neurovascular interventional portfolio
Route 92 Medical has announced approval from the Australian Therapeutic Goods Administration (TGA) for its portfolio of innovative neurovascular interventional products intended to treat patients with acute ischaemic stroke.
Within this portfolio are the first 0.088-inch super bore reperfusion systems to be approved for use in Australia, including the FreeClimb 88 catheter powered by Tenzing technology and the Monopoint-based HiPoint 88 super-bore catheter, according to a Route 92 press release.
The company further notes that its products are covered by a global portfolio of more than 140 patents, with this TGA regulatory authorisation spanning the majority of its neurovascular devices, including the HiPoint 70/Tenzing 7 reperfusion system; HiPoint 88/Tenzing 8 reperfusion system; FreeClimb 70/ Tenzing 7 reperfusion system; FreeClimb 88/Tenzing 8 reperfusion system; and Base Camp sheath system.
“We’ve had great success in our initial evaluation of Route 92 Medical’s stroke treatment portfolio,” said Hal Rice (Gold Coast University Hospital, Gold Coast, Australia). “It offers superb, enhanced delivery with the Tenzing system to navigate easily in the most tortuous of neurovasculature to quickly and efficiently reach the site of stroke vessel occlusion. The system offers robust capabilities that address a broad range of
4–7 June
Vascular Annual Meeting (VAM25) New Orleans, USA W: vascular.org/vam-2025
18–20 June
British Neuro-Oncology Society (BNOS) Annual Meeting London, UK
W: bnosconference.co.uk/bnosconference
neurovascular intervention challenges, including efficiently navigating to and effectively aspirating strokecausing clots to deliver rapid cerebral reperfusion and a high proportion of first-pass effect in cases we have recently treated.”
Route 92 recently showcased its technology at the 2025 Australian and New Zealand Society of Neuroradiology (ANZSNR) annual scientific meeting (27–29 March, Perth, Australia).
TG Medical concludes patient enrolment in study evaluating novel ICAD stroke treatment TG Medical has announced the successful completion of patient enrolment in its approval study for the TG dilator—a novel device designed to treat intracranial atherosclerotic disease (ICAD) in patients presenting with acute ischaemic stroke due to large vessel occlusion.
“Acute ischaemic strokes caused by intracranial atherosclerosis remain a significant treatment challenge,” said Nobuyuki Sakai (Kobe City Medical Center General Hospital, Kobe, Japan), the principal investigator of the trial. “The TG dilator represents a promising solution to improve recanalisation success and patient outcomes in this difficult-to-treat population.”
This milestone follows promising results from a first-in-human (FIH) trial, published in the Journal of NeuroInterventional Surgery, which demonstrated the TG dilator’s safety and potential efficacy in treating ICAD-related acute ischaemic strokes.
A TG Medical press release states that, in the study, all 10 patients achieved significant recanalisation without any device-related adverse events, and 80% attained favourable functional outcomes at 90 days.
The company’s multicentre approval study is being conducted across leading stroke centres in Japan, and has reached full enrolment ahead of schedule, marking what TG Medical describes as a “significant step” toward regulatory approval.

The TG dilator is specifically engineered to address the unique challenges of reopening occluded vessels in the setting of underlying atherosclerotic disease—a common cause of acute
ischaemic stroke in Asian populations. TG Medical claims that its TG dilator features a unique design intended to facilitate safe and effective vessel dilation, improving outcomes where conventional thrombectomy devices “may be insufficient”.
Following final data collection and analysis, the company plans to submit results from these studies to Japanese regulatory authorities.
Penumbra announces Red 72 Silver Label product featuring company’s latest engineering enhancements
Penumbra recently announced a significant update to its flagship large-bore reperfusion catheter, Red 72. Developed for speed and ease of navigation, the new Red 72 Silver Label features “meticulously blended” transition zones to better accommodate the natural distal anatomy of the brain, according to the company.
As per a recent Penumbra press release, the Red 72 Silver Label has been adapted from the Red 43 and Red 62 catheters, and features the company’s latest engineering enhancements. The new Red 72 Silver Label with Sendit technology—a configuration intended to minimise the ledge effect, simplify preparation and make delivery easier—has shown a 50% increase in trackability over the original Red 72 with Sendit technology, the release adds.
“I had the opportunity to try the new Red 72 Silver Label during a recent procedure,” commented Daniel Vela Duarte (Palm Beach Neuroscience Institute, West Palm Beach, USA).
“The change and overall performance are radically better.”
The release goes on to state that the design of Red 72 reflects Penumbra’s commitment to the principles of Science-Based Aspiration Thrombectomy (S-BAT).
“Red 72 Silver Label tracked with ease to an M1 occlusion in a patient in their 90s with extreme cervical carotid tortuosity and underlying atherosclerotic disease,” Ian Kaminsky (Radiology Imaging Associates [RIA] Neurovascular, Englewood, USA) said after completing the first case with the catheter. “We are really looking forward to implementing this incredible technology into our practice.”
21–24 June
European Academy of Neurology (EAN) Congress
Helsinki, Finland W: ean.org/meet/congresses/eancongress-2025-helsinki
23–27 June
The European Course in Minimally Invasive Neurological Therapy (ECMINT) 6.1 Oxford, UK W: esmint.eu/education/ecmint
14–18 July
Society of NeuroInterventional Surgery (SNIS) Annual Meeting and Fellows Course Nashville, USA W: snisannualmeeting.org
21–23 August
Asian-Australasian Federation of Interventional and Therapeutic Neuroradiology (AAFITN) Biennial Congress Bangkok, Thailand W: aafitn.com/meeting
3–5 September
European Society of Minimally Invasive Neurological Therapy (ESMINT) Congress
Marseille, France
W: esmint.eu/congress
17–21 September
European Society of Neuroradiology (ESNR) Annual Meeting Istanbul, Turkey W: esnr.org/event-details/6890
The philosophy that guides product development at Penumbra, incorporating basic scientific principles with the latest clinical evidence to develop technologies that give the greatest number of acute ischaemic stroke patients their best chance at an optimal outcome.

Recognising energy flows and what actually occurs when catheter meets clot


Understanding average vessel sizing and the optimal catheterto-vessel ratio for effective aspiration
Evaluating the consequences of excessively restricting blood flow during a thrombectomy procedure
Analysing procedure time in relation to patient outcomes, and key opportunities to reduce time


Every case is different, and the RED family of catheters provides a full range of products designed to optimise lumen size and trackability for the situation.














































































































































