
May 2023 | Issue 69 www.cardiovascularnews.com





Join the CX Vascular platform today to connect and engage with the global vascular community, share expertise and experiences, and stay up-to-date with the latest education and news in the vascular world. Members will also have access to exclusive content including live discussions on the latest advances in the vascular field and interviews with key thought leaders in the space. Register now Education, News, Insights Join the community at https://cxvascular.com
All cardiac cath labs should have the capability for intravascular imaging to augment conventional angiography, experts from the American College of Cardiology (ACC) Interventional Council have recommended in a state-of-the-art review paper published in the Journal of the American College of Cardiology (JACC)
The statement adds growing weight to the calls from proponents of the technology, who say its use optimises outcomes for PCI, and it comes as new data, presented at the American College of Cardiology (ACC) 2023 Scientific Sessions (4–6 March, New Orleans, USA), point to its growing use as a beneficial adjunct to angiography.
Attendees of ACC 2023 also heard the findings of one of the largest studies to date on the use of intravascular imaging in patients with complex coronary artery lesions, pointing towards a benefit of using the technology in these patients compared to procedures using angiography guidance alone.
Use of intravascular imaging systems such as intravascular ultrasound (IVUS) or optical coherence tomography (OCT) addresses the “inherent limitations of imaging a threedimensional structure using a two-dimensional lumenogram”, Alexander Truesdell (Virginia Heart/Inova Heart and Vascular Institute, Falls Church, USA) and colleagues write in their JACC paper, in which they analyse all currently available intravascular imaging techniques, and review the data supporting the use of adjunctive imaging for PCI optimisation.
Based upon their review, Truesdell and colleagues recommend the routine use of intravascular imaging as an essential adjunct to conventional angiography during PCI. While observing that current evidence supports intravascular imaging of specific lesion subsets including left main and proximal left anterior descending (LAD) lesions, complex lesions, or any scenario where angiography may not be sufficient to fully elucidate anatomy, they further note that there is increasing evidence of benefit for intravascular imaging for all-comer lesions.
Offering his view as to why the uptake of intracoronary

Statins save lives after aortic repair regardless of dose
Statin treatment after aortic repair is associated with improved long-term survival, while dose does not matter. This was the key message from a first-to-podium presentation delivered by Kevin Mani (Uppsala University, Uppsala, Sweden) at the 2023 Charing Cross (CX) International Symposium (25–27 April, London, UK). The CX audience showed their support for this conclusion, with 89% agreeing with the statement ‘Statins save lives’ during discussion time.
imaging has, so far, been slow, Truesdell told Cardiovascular News that there are a variety of reasons driving this reluctance from operators. “Some of it is likely to be habit,” he said. “In an extremely rapidly evolving field like interventional cardiology I think it is difficult for many to repeatedly and continuously evolve over the course of a multi-decade career. I think this may be even harder for lower volume operators in the USA who may not get enough annual experience to ensure proficiency.”
According to Truesdell, these points may be addressed by focused online and in-person training, spearheaded by national and international societies, as well as side-by-side training amongst colleagues.
Whilst latest data confirm that the pace of change is slow, there is a suggestion that adoption is growing—similar to the recent experience with other contemporary PCI tools and techniques such as transradial access.
At the ACC meeting, Reza Fazel (Beth Israel Deaconess Medical Center, Boston, USA) presented data on temporal trends and clinical outcomes of PCI procedures performed using intravascular imaging guidance, measuring the uptake of the technology in the USA between 2013 to 2019. The research, conducted alongside Eric Secemsky (Beth Israel Deaconess Medical Center, Boston, USA), found that overall usage of the technology had risen from 9.5% in 2013 to 15.4% in 2019.
MANI BEGAN BY UNDERLINING the fact that abdominal aortic aneurysm (AAA) is a cardiovascular disease that shares risk factors with atherosclerotic cardiovascular disease (ASCVD). According to the American Heart Association (AHA), the presenter detailed, AAA is in fact classified as one of the ASCVDs.
“AAA patients have a higher mortality than the general population due to cardiovascular disease,” Mani noted, adding that statin treatment is associated with improved survival in patients with ASCVD.

The presenter detailed that current European Society for Vascular Surgery (ESVS) guidelines on the management of abdominal aortoiliac artery aneurysms, published in 2019, suggest that patients with AAA should have blood pressure control, statins and antiplatelet therapy. “This is a class IIa recommendation with level b evidence,” the presenter specified, which he said indicates that “probably all patients” with AAA should have statin treatment.

The AHA guidelines, Mani highlighted, split statin treatment into high dose and moderate to low dose. “The suggestion is that patients with ASCVD including those with AAA should have high-dose statin treatment,” the presenter shared with the CX
audience. “However,”
Continued on page 9
Continued on page 7
Featured in this issue: Profile:
Paulis
EU agrees timeline delay
26 May 2023 | Issue 69 6 Cardiac surgery: UK MINI MITRAL Trial www.cardiovascularnews.com
Ruggero De
MDR:
page
2023
Intracoronary imaging “at a turning point”: Consensus statement and trial data tip balance towards imaging-guided PCI
We all need to overcome our own barriers to use— whether it is perceived added procedural time or discomfort with imaging interpretation— to provide the best care for our patients.”
CX 2023
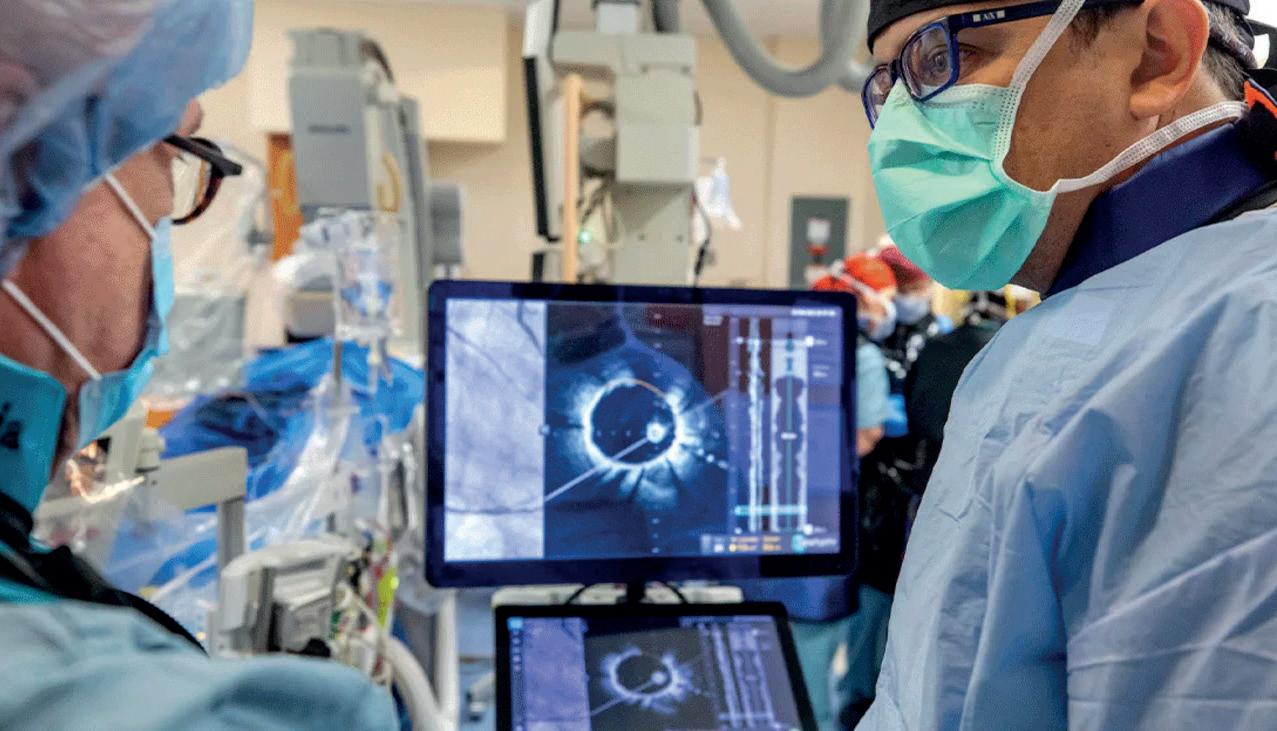
First-in-human highfrequency optical coherence tomography (HF-OCT) imaging performed at Tampa General Hospital (Tampa, USA) on 22 February 2021.
Hiram Bezerra and Michael Jones review images on the HF-OCT console.
Behind the headlines: Clinical trialists deserve praise
Welcome to the May 2022 edition of Cardiovascular News, bringing you the latest news and updates from the fields of cardiovascular health, encompassing interventional cardiology and cardiac surgery.
Regular readers of this publication will already be aware of the results from the REVIVED trial, which were initially presented at the European Society of Cardiology (ESC) congress last August (26–29 August, Barcelona, Spain) with a simultaneous publication in the New England Journal of Medicine. The results, which were covered in detail in the November 2022 issue of Cardiovascular News, surprisingly showed no clear benefit of percutaneous revascularisation in patients with severe left ventricular impairment and severe coronary artery disease. In this edition, our editorial team report on more data from the trial, which suggested that viability testing, long been thought to be a useful clinical tool, does not clearly identify which patients may benefit from revascularisation.
When we read these headline results, we often fail to think about the huge amount of work that goes on behind the scenes, succeeding in delivering a trial such as this. As Divaka Perera—the trial’s lead investigator—is one of my colleagues at St Thomas’ Hospital in London, I had a glimpse into this process. The concept for the trial went back to well before 2010, with funding obtained from National Institute for Health and Care Research (NIHR) in 2013 (having been rejected by the British Heart Foundation [BHF] in 2011) and the first patient enrolled in August 2013, completing enrolment over six years later in March 2020. The phenomenal amount of work by Divaka and colleagues, as well as the London School of Hygiene and Tropical Medicine, needs to be recognised.
Also highlighted in this issue is the importance of intracoronary imaging to guide percutaneous revascularisation. There is now very good evidence for improved outcomes when routine intravascular imaging is performed. This
is emphasised by the recently reported and published Renovate-Complex-PCI trial which showed a significant reduction in events associated with intravascular imaging for complex PCI. There is also growing evidence that it may benefit all patients undergoing PCI, not just those with complex lesions, which is summarised in a state-of-the-art review published in the Journal of the American College of Cardiology (JACC)

Transcatheter aortic valve implantation (TAVI) continues to expand across the globe, with a rapid movement to lower-risk, and younger, patients. As this occurs, the importance of durability and the potential for repeat procedures assumes greater importance, as highlighted in an interview with Nicolas Van Mieghem. He highlights that TAVI appears to show less valve dysfunction than surgical aortic valve replacement. Although these are sub-group analyses and more data are accumulating, it does appear to throw into debate the long-held belief that conventional surgery remains the gold standard. A further important consideration, which is likely to increase in significance as we treat younger patients, is the issue of future coronary access, which may be an important factor in initial valve selection. Guiseppe Tarantini gives an in-depth interview on the factors which need to be taken into consideration as we plan for re-do TAVI procedures, again a topic likely to assume greater importance in the years to come. We eagerly await longer-term followup of the many trials and registries highlighted. Finally, there is a detailed section on the importance and role of renal denervation for the management of hypertension, and an interview with Ruggero de Paulis, the inventor of the Valsalva graft, together with the usual sections on industry news.


We hope you enjoy this issue and as always welcome comments and suggestions for future articles.
Editor-in-chief: Simon Redwood | Publisher: Roger Greenhalgh | Content Director: Urmila Kerslake
Senior editor: Will Date will@bibamedical.com | Editorial contribution: Jamie Bell, Jocelyn Hudson, Éva Malpass, Clare Tierney and Benjamin Roche
Design: Terry Hawes, Wes Mitchell and David Reekie
Advertising: Melanie Stevenson melanie@bibamedical.com
Subscriptions: subscriptions@bibamedical.com
If you have comments on this issue or suggestions for upcoming editions write
4 cardiovascularnews linkedin.com/company/cardiovascular-news @cn_publishing
| News or advertising queries Tel: +44 (0)20 7736 8788 Published by: BIBA News, which is a subsidiary of BIBA Medical Ltd | BIBA Medical, Europe, 526 Fulham Road, Fulham, London, SW6 5NR, United Kingdom Tel: +44 (0) 20 7736 8788 BIBA Medical, North America, 155 North Wacker Drive, Suite 4250, Chicago, IL 60606, United States Tel: +1 708-770-7323 Printed by: Buxton Press Reprint requests and all correspondence regarding the newspaper should be addressed to the editor at the United Kingdom address. © BIBA Medical Ltd, 2023. All rights reserved.
to will@bibamedical.com
When we read these headline results, we often fail to think about the huge amount of work that goes on behind the scenes, succeeding in delivering a trial such as this.”
LETTER
EDITOR'S
Simon Redwood



Orsiro®
Stent System PK
Mission Drug-Eluting
Papyrus® Covered Coronary Stent System
Orsiro Mission and PK Papyrus are trademarks or registered trademarks of BIOTRONIK Group of Companies. ReCross is a product of IMDS. Distributed by BIOTRONIK in selected countries. © 2023 BIOTRONIK AG – All rights reserved. Specifications are subject to modification, revision and improvement. Conquering the Complex From everyday complexity to the toughest 1% Find out more info.biotronik.com/ctc
Dual Lumen OTW Microcatheter
Equivalent recovery times shown between minimally invasive and conventional surgery for mitral valve repair
The largest randomised controlled trial to date to compare minimally invasive and conventional mitral valve surgery—UK Mini Mitral—has found outcomes and quality of life to be similar in patients who received either technique for at 12 weeks.
Presented at the American College of Cardiology (ACC) annual Scientific Session (4–6 March, New Orleans, USA), lead author Enoch Akowuah (Newcastle University, Newcastle upon Tyne, UK) investigated patients with severe degenerative mitral valve regurgitation to assess recovery and subsequent complications.
The study included 330 patients treated at 10 centres across the UK—the cohort’s average age was 67 years—30% of whom were women. Participants were randomly assigned to undergo mitral valve repair by either sternotomy or mini-thoracotomy.
Expertise randomisation was a focal area of the study—28 surgeons were approved by the Trial Steering Committee and were required to have performed at least 50 procedures—and Akowuah stated that this was based on patient feedback prior to the study’s design to “remove the learning curve” and assure patients would receive a “high-quality procedure” regardless of group designation.
The researchers outlined their primary endpoint as the change in patients’ physical functioning and ability to carry out day-to-day activities at 12 weeks post-procedure. This was measured by changes in SF-36v2 quality-of-life and physical functioning scale from baseline. Akowuah et al assessed changes through periodic questionnaires and updates via an accelerometer that patients wore on their wrists.
Defining their secondary endpoint, the
researchers included physical function at six weeks, physical activity and sleep efficiency measure via accelerometery at both six and 12 weeks, MVr rates, quality of mitral valve repair and adverse events, such as death, stroke, heart failure and repeat intervention.
When assessed at 12 weeks, Akowuah and colleagues found physical function levels pre- and post-surgery were similar in both groups. Although at six weeks, they noted, who underwent minithoracotomy had recovered physical function compared to pre-surgery, whereas patients who received a sternotomy had not.

“Urgent” action needed to improve outcomes in women undergoing coronary artery bypass surgery
Women have been found to have significantly higher risk of operative mortality and postoperative complications after isolated coronary artery bypass (CABG) when compared with men. Results from a retrospective cohort study of over a million US patients were released today, revealing the “essentially unchanged” excess operative risk for women between 2011 and 2020.
PUBLISHED ONLINE IN JAMA
Surgery, the investigators assert theirs is the first to provide “contemporary nationwide analysis” in operative mortality and morbidity trends for women undergoing CABG in the US. Women, the authors preface, are more commonly older and have a higher prevalence of cardiovascular risk factors when presenting for CABG. However, despite a national upward trend in CABG outcomes over the past decades, it is “unclear” why this improvement has remained static for
women, the researchers state.
Led by Mario Gaudino (Weill Medical College, New York, USA)
the authors reviewed data from the Society of Thoracic Surgeons Adult Cardiac Surgery Database (STSACSD), comparing outcomes between men (979,488 [75.5%]) and women (317,716 [24.5%]). Spanning 110 participating centres, the STS-ACSD represents over 95% of the US cardiac surgical volume, and was evaluated by the authors using the primary analytic method to estimate the association
At one year follow-up, Akowuah et al recorded that all secondary outcomes were not significantly different between the two groups. Despite one mini-thoracotomy patient requiring a secondary operation due to bleeding, the researchers found mini-thoracotomy patients typically spent a median of five days in hospital—compared to six days for sternotomy patients—and were more likely to be discharged early.
Reflecting on the significance of their findings, Akowuah highlighted that speed of recovery to ultimately regain physical function and return to normal activities is important for patients. “Our results show that at three months, physical recovery is equivalent in both groups of patients,” he said. “In addition, we show that when both surgical procedures are performed by expert surgeons, minimally invasive mitral valve surgery is as safe and effective as conventional surgery.”
Answering focal questions about the effectiveness of approaches, the authors state their study confirms the valve repair rate and the quality and durability of valve repair when using mini-thoracotomy. Akowuah asserted: “Valve repair rates were excellent [at 96%] and similar to those obtained with sternotomy. Moreover, at one year after surgery more than 92% of patients in both groups had no or mild valve leakage.”
of female sex with CABG operative outcomes over time.
Asserting their primary and secondary endpoints as operative mortality and combined mortality and morbidity respectively, Gaudino et al found their primary endpoint revealed significantly higher unadjusted mortality when compared with men (2.8% vs 1.7%; p<0.001). Their secondary endpoint also yielded significant results, showing the overall incidence of the composite of operative mortality and morbidity to be 22.9% for women (95% CI, 22.7–23.0) and 16.7% for men (95% CI, 16.6–16.8) (p<0.001).
Regarding trends over time, Gaudino and colleagues report that unadjusted mortality in women increased from 2.9% in 2011 to 3.3% in 2020, while adding the operative risk attributable to female sex varied from 1.28% in 2011 to 1.41% in 2020, showing no improvement over time.
“The reason for the lack of improvement in outcomes for women in the last decade is unclear,” the authors write. However, they recognise
there are clear differences in baseline anatomical and clinical characteristics between men and women—such as the pattern of ischaemic heart disease— alluding to revascularisation being less beneficial in some cases.
The authors point out, however, that current diagnostic and therapeutic protocols for coronary revascularisation, including studies comparing coronary artery bypass with percutaneous coronary intervention (PCI) are “all informed by data derived from studies performed prevalently in men”, and so provide “inadequate” generalisability to women.
Addressing the larger significance of their results, the authors believe a “multifactorial” approach is required to reduce mortality in women after CABG. They affirm that it is important that sex disparities are evaluated in basic science research and women enrolled in clinical trials, and Gaudino et al conclude that “further investigation in the determinants of operative outcomes in women is urgently needed”.
Enoch Akowuah
May 2023 | Issue69 6
We hope that the results of this trial will give confidence to both clinicians and patients and drive uptake of the mini approach.”
News, reflecting that there are now various trials supporting the use of intravascular imaging, coupled with the consensus statement from ACC. “Overall, intravascular imaging offers the ability to perform more optimal and precise PCI, reduce short and long-term outcomes and preserve a safety profile that places the patient at little risk.
Medical Centre, Sungkyunkwan University School of Medicine, Seoul, South Korea).
Continued from page 3
The researchers also reported that intracoronary imaging was associated with lower rates of one-year mortality, myocardial infarction (MI), repeat PCI procedures, and major adverse cardiac events (MACE).
Research from outside of the USA paints a picture of similar trends elsewhere. A paper from the UK, authored by Mohamed O Mohamed (Keele University, Keele, UK), published in the Journal of the American Heart Association (JAHA) in late 2022, points to a more than doubling of intracoronary imaging usage in England and Wales between April 2014 and March 2020, with better rates of in-hospital survival for intracoronary imaging-guided PCI than angiographyguided PCI for specific indications. Despite this trend, the authors of the study noted that intracoronary imaging remains underused with fewer than one in five cases using these modalities nationally in 2020.

“I do think we are at a turning point,” Secemsky told Cardiovascular

“We all need to overcome our own personal barriers to use—whether its perceived added procedural time or discomfort with imaging interpretation—to provide the best care for our patients. I think that we are approaching a moment where greater adoption will follow, similar to what we saw with radial artery access, and continued investment in this technology and improving procedural workflow will position the PCI field for improved outcomes guided by intravascular imaging.”
Truesdell also drew the parallel with the uptake of radial access amongst the interventional cardiology community, adding that the change may have been driven by a younger generation.

“Radial uptake lagged years behind the safety and outcomes data for many of the same reasons—habit, training, ability and willingness to train and evolve mid-career—but ultimately arrived at an inflection point as more and more younger interventionalists exited the training pipeline into practice with familiarity and comfort with radial access and helped raise up their peers,” Truesdell commented. “I think the same will occur over the next few years with intravascular imaging.”
Among the latest studies in the field is RENOVATE-COMPLEX-PCI, results from which were presented at the ACC meeting by Joo-Yong Hahn (Samsung
Quality of life improvements drive benefit for transcatheter therapy in TRILUMINATE pivotal trial
Results of the randomised TRILUMINATE pivotal trial indicate that transcatheter repair in symptomatic tricuspid regurgitation (TR) patients using the Triclip (Abbott) system was effective in reducing TR and led to improvements in quality of life at one year.
HOWEVER, THE RESULTS DID NOT SHOW any significant difference in survival or heart failure hospitalisation between patients treated with the interventional approach or with medical therapy, the study’s control arm, with the superiority of the device in meeting its composite primary endpoint primarily driven by improvements in quality of life for patients. Presented during the opening late-breaking trial session of the American College of Cardiology (ACC) annual scientific session (4–6 March, New Orleans, USA), by Paul Sorajja (Minneapolis Heart Institute Foundation, Minneapolis, USA) and published
Eric Secemsky
The prospective, multicentre, openlabel trial, was conducted in 20 sites throughout South Korea, where use of intravascular imaging modalities is more routine. Findings were simultaneously published in the New England Journal of Medicine (NEJM)
The study was set up with the intention to investigate whether intravascular imaging-guided PCI would improve outcomes as compared with
(n=1,092) or angiography-guided PCI (n=547), assessing the outcome of each approach against the composite primary endpoint of cardiovascular death, targetvessel MI, or clinically driven targetvessel revascularisation, as well as the safety of the procedures.
Hahn reported that at a median follow-up of 2.1 years, a primary endpoint event occurred in 76 patients (cumulative incidence 7.7%) in the intravascular imaging group and in 60 patients (cumulative incidence 12.3%) in the angiography group (hazard ratio [HR] 0.64; 95 confidence interval [CI] 0.45 to 0.89; p=0.008).
Death from cardiac causes occurred in 16 patients (cumulative incidence 1.7%)
angiography-guided PCI in patients with complex coronary artery lesions. Complex lesions were defined as true bifurcation lesions, with a side branch diameter of at least 2.5mm, a chronic total occlusion, unprotected left main coronary artery disease, long coronary artery lesions, multivessel PCI, a lesion involving in-stent restenosis, a severely calcified lesion, or ostial lesions of a major epicardial coronary artery.
A total of 1,639 patients were randomised in a 2:1 ratio to undergo either intravascular imaging-guided PCI
simultaneously in the New England Journal of Medicine (NEJM), the results from 350 patients are “very meaningful for a highly symptomatic population whose quality of life is impacted by TR”, investigators have suggested.
TRILUMINATE Pivotal saw patients considered to be at intermediate or greater risk for tricuspid valve surgery randomised 1:1 to receive either transcatheter repair using the Triclip device or medical therapy at centres throughout North America and Europe. Patients in Sorajja’s one-year report had a median age of 78 years and the population consisted of 55% women.
All patients enrolled in the trial had severe tricuspid regurgitation and heart failure symptoms despite receiving medical therapy, and 51% had torrential tricuspid regurgitation. Most patients had either atrial fibrillation (AF), high blood pressure, or both. Patients who had severe left ventricular heart failure, untreated other valvular disease or severe pulmonary hypertension were not eligible to enrol.
Headline findings at 12 months presented by Sorajja indicate that the trial met the primary endpoint, a composite of mortality or tricuspid valve surgery, heart failure hospitalisation, and quality of life improvement ≥15 points assessed using the Kansas City Cardiomyopathy Questionnaire (KCCQ), evaluated in a hierarchical fashion using the Finkelstein Schoenfeld methodology favour transcatheter therapy (win ratio 1.48; 95% confidence interval 1.06 to 2.13; p=0.02).
Sorajja reported that the trial saw a significant reduction to moderate or less TR (grade <2) achieved in 87% of patients in the device arm at 30
in the intravascular imaging group and in 17 patients (cumulative incidence 3.8%) in the angiography group; target vessel-related myocardial infarction occurred in 38 (cumulative incidence 3.7%) and 30 (cumulative incidence 5.6%), respectively; and clinically driven target vessel revascularisation in 32 (cumulative incidence, 3.4%) and 25 (cumulative incidence, 5.5%), respectively. There were no apparent between-group differences in the incidence of procedure-related safety events.
days, compared to 4.8% in the control group. TR reduction was sustained at one year, according to the investigators.
Added to this, patients receiving the device saw a significant improvement in quality of life, with 50% of the investigational group reporting at least a 15-point improvement in KCCQ score at one year, next to 26% in the control group. The rate of hospitalisation for heart failure did not appear to differ between the groups.
“Patients with TR transcatheter edge-to-edge repair (TEER) with the TriClip device, experienced significant improvements in quality of life,” said Sorajja, the lead author of the study. “In a patient population with a high symptom burden, this is a meaningful benefit.”
Allied to the efficacy findings, investigators also reported that three patients (1.7%) had a major adverse event at 30 days (one death due to a cardiovascular cause and two cases of new kidney failure). Nine patients treated with TEER (5.2%) experienced a major bleeding event within one year. Five patients in the TEER group (2.9%) and five in the control group (2.9%) needed surgery to implant a permanent pacemaker or defibrillator within one year.
One limitation of the study is that it was unblinded, according to Sorajja—who relayed that patients and their clinicians knew who had received TEER and who had not. However, independent experts who were blinded to patient treatment assessed the hospitalisations, deaths and other adverse events that occurred in the study.
Issue69 | May 2023 7
Conference Coverage
Intracoronary imaging “at a turning point”: Consensus statement and trial data tip balance towards imagingguided PCI
COVER STORY continued INTRACORONARY IMAGING USAGE DURING PCI 2019 2020 USA ENGLAND 15.4% 17.5% 2013 2014 9.5% 7.8%

Statins save lives after aortic repair regardless of dose
Continued from page 3
he said, “the evidence for what dose should be given to AAA patients is non-existent, and the vascular surgical guidelines do not recommend a specific dose for AAA patients.”
To address this gap in the literature, Mani and colleagues conducted a national study assessing the potential benefit of statin treatment in AAA patients and whether dose has an effect. The team used four national registries and then cross-matched these to identify all AAA repairs performed in Sweden between the years of 2006 and 2018, the presenter explained. The team then assessed statin treatment by combining patient data and the national prescribed drug registry, looking at outcomes in terms of death, cause of death and rehospitalisation, also using national registries.
Mani detailed that the researchers performed three analyses, the first looking at 90-day mortality for patients who had statin treatment preoperatively, the second assessing statin versus no statin treatment postoperatively, and the final one examining high-dose versus low-to-moderate-dose statin treatment postoperatively, considering overall survival, aortic-related survival and freedom from cardiovascular events. Propensity score matching was used to ensure that the groups were comparable in terms of baseline comorbidities and characteristics.
The presenter revealed that approximately 60% of the 11,000 patients who underwent AAA repair in the national study had statin treatment prior to undergoing repair. Postoperatively, he added, half of the patients had continuous statin treatment 80% of the time after the operation. In both instances, the rates were higher among men.
Looking at perioperative mortality, Mani reported that this was the same in the group on statin treatment versus no statin treatment, with an overall 90-day mortality of under 3%.
The presenter also shared the finding that patients on statin treatment had an improved rate of survival in the long term, and that overall survival was “significantly improved” in a propensity score-matched group of patients with or without continuous statin treatment after AAA repair. Aorticrelated survival was improved with statin treatment, he stated, as was freedom from cardiovascular events, if the patients were on statin treatment.
Finally, the team assessed the high-dose statin group versus the low-to-moderatedose group. “There was no effect of the dose of statins, neither on overall survival nor on aortic-related survival or cardiovascular events,” he communicated. “These were equal, irrespective of dose.”
results challenge “long-held beliefs” on myocardial viability testing prior to PCI
Assessing myocardial viability does not aid the selection of patients who will benefit from percutaneous coronary intervention (PCI) for the treatment of severe ischaemic cardiomyopathy. This is according to the findings of an analysis of the REVIVEDBCIS-2 trial, in which investigators assessed the effect of myocardial viability, functional recovery and PCI on clinical outcomes.
Divaka Perera (King’s College London, London, UK) delivered the findings at the American College of Cardiology (ACC) 2023 Scientific Session (4–6 March, New Orleans, USA), where he commented that the results suggest there is not a “Goldilocks zone” to identify patients suitable for PCI based upon their myocardial viability characteristics.
Perera had previously presented the primary results of REVIVED-BCIS-2 at the annual congress of the European Society of Cardiology (ESC) in 2022 (26–29 August, Barcelona, Spain), where he revealed that there was no reduction in all-cause mortality or heart failure hospitalisation gained through PCI compared to medical therapy among 700 patients with severe left ventricular (LV) dysfunction and extensive coronary artery disease randomised to either approach.
Through their latest analysis, the REVIVED investigators sought to determine the extent to which viability on cardiac magnetic resonance (CMR) imaging or Dobutamine Stress echocardiography (DSE) determines the impact of PCI on clinical outcomes and whether reverse LV remodelling affects clinical outcomes.
Observational data from the latter half of the 20th century have led to the view that viability assessment is a useful tool for picking patients that would benefit from revascularisation, Perera told attendees of ACC 2023, adding that this pointed to the idea that, “if you had viable myocardium classified in a binary way, you do much better with revascularisation than with medical therapy alone”.
Data from the STICH trial, which compared coronary artery bypass graft (CABG) surgery and medical therapy in which around half of patients had discretionary viability testing, appeared to challenge this notion, Perera added. “When those patients were classified once again in a binary way as having sufficient or insufficient viability, those investigators found that it did not seem to predict the benefit of coronary artery bypass surgery, which challenged the prior data,” he said, adding that the STICH investigators also found in a smaller subgroup of that study that those patients that had improvement in LV function did just as well or badly, as those who did not.
This led the REVIVED investigators to test four hypotheses, namely that viability characterisation predicts event-free survival among these patients, that it predicts LV recovery, response to PCI or medical therapy, or that LV recovery predicts event-free survival.
of relationship between dysfunctional but viable myocardium or hibernating myocardium and clinical outcomes.
Contrastingly, he said, looking at all viable myocardium, including all the normal segments, there was an interaction, with a hazard ratio of 0.93, with a 7% reduction for every 10% increase in viable myocardium.
Looking at the relationship between scar burden and clinical outcome, Perera said that for every 10% increase in scar volume, there was an 18% increase in the risk of meeting the primary clinical outcome, which he described as a highly significant result.
Dissecting the data by treatment assignment, Perera said that there was no impact of viability assessment in terms of hibernating myocardium or scar burden on the effect of PCI versus medical therapy versus alone.
On ventricular recovery, Perera said that there was a median change of 4.7%. “What we see is that if you look at all viable myocardium, it does predict recovery of LV function, and if you look at scar burden it does the reverse: the more scar you have the less likely you are to recover,” he commented. Hibernating myocardium does not predict LV recovery, he added, describing this as an interesting result.
“We have shown that characterisation by viability assessment does not allow us to select a group of patients who benefit from PCI over medical therapy,” Perera remarked during the concluding comments of his presentation.

“We also found—and this is perhaps surprising as it challenges the theory of hibernation—that the abundance of dysfunctional yet viable segments is not yet associated with prognosis or even with LV recovery,” he said. Characterising myocardium in terms of scar or the extent of non-viable myocardium, is highly predictive of prognosis and the likelihood of LV recovery, Perera added, noting that this is independent of LV ejection fraction. “In fact, if you were to correct LV ejection fraction for scar, it is no longer associated with outcome or LV recovery,” he noted.
“Similar to the initial REVIVED trial [this] challenges many long-held beliefs in our profession,” Sunil Rao (NYU Langone Health, New York, USA) said in discussion that followed the presentation at ACC. Rao asked Perera to expand on how the data inform understanding definition of hibernating myocardium.
“I think it challenges it,” Perera said in response to this question. “We had come to use viability tests as a prospective marker of hibernation and that is predicated on an assumption that we can pick the parts of the ventricle that recover, or on a whole patient level, the patients that are going to have LV recovery.
“Using those very metrics that are currently used, we have shown that that does not predict LV recovery. I think we need to challenge the paradigm and hibernation as we have known and used it does not seem to be valuable in clinical practice anymore.”
Rao also asked whether, in patients with LV dysfunction who have concomitant coronary artery disease, the decision to revascularise should viability testing continue, and if not how the data should inform decision-making as to who should get revascularisation?
“Unequivocally, no,” was Perera’s response to the question of continuing viability testing to guide patient selection for revascularisation. “There is absolutely no evidence from this or from the STICH viability data that viability testing predicts a group of patients who benefit, and that challenges what we would do in our multidisciplinary team meetings week on week.”
Kevin Mani
Viability characterisation was assessed in two ways, Perera said, the first based on the potential for recovery— looking at dysfunctional segments—as well as the amount of scar in the heart. All analyses accounted for potential confounders, such as the modality of viability testing and baseline LV function.
Detailing the results, Perera said there was a lack
But, Perera said he did not see this as justification to “throw out” viability testing altogether. “We need to change the way in which we use the viability tests, it should not be focused on dysfunctional segments with the prospect of recovery, and we should not link recovery as the only mechanism of benefit, but instead in those very viability tests that we do is embedded really useful information—scar and non-viable myocardium.” Whether this can be used to stratify patients better will need to be tested in future trials, he said.
Issue69 | May 2023 9 Conference Coverage REVIVED-BCIS-2
CX 2023
Ongoing studies add to evolving understanding of TAVI valve durability
New data offer greater perspective on the long-term performance of transcatheter aortic valve implantation (TAVI) devices, but more insight into longer-term follow-up is still needed before interventionalists can gain the full picture of the optimal treatment for patients with aortic stenosis. This is among the messages of Nicolas van Mieghem (Thoraxcenter, Erasmus University Medical Center, Rotterdam, The Netherlands), speaking to Cardiovascular News about the latest research shaping understanding on long-term TAVI outcomes.
“We now have randomised controlled trials comparing TAVI with surgery across the entire risk spectrum,” Van Mieghem comments. “With the the Evolut Low Risk and PARTNER 3 trials, the durability issue becomes more and more relevant, because the patient age is creeping down from 80 or more, to now 75 years or less, meaning that on top of the lower risk profile of the patients, their life expectancy exceeds 10 or 15 years,” he says, detailing why valve durability is now an issue of particular focus.
Ongoing research continues to add to the TAVI knowledge base, and two recent data releases are seen as providing important insights to the field both in terms of valve durability and of the benefits of the treatment in patients at low surgical risk.
At the 2023 Cardiovascular Research Technologies (CRT) conference (25–28 February, Washington DC, USA) Steven Yakubov (Riverside Methodist Hospital, Columbus, USA) presented analysis of five-year data on bioprosthetic valve dysfunction for Medtronic’s CoreValve™ and later-generation Evolut™ TAVI platforms from the CoreValve US High Risk and SURTAVI randomised trials.
Taking in data from 1,128 TAVI patients and 971 surgery patients, the pooled analysis evaluated valve performance and durability of the surgical transcatheter valves, assessed by the incidence of overall bioprosthetic valve dysfunction, including structural valve deterioration, non-structural valve dysfunction, thrombosis and endocarditis.
In the study, the CoreValve, Evolut R and Evolut PRO (Medtronic) transcatheter valves exhibited a significantly lower rate of bioprosthetic valve dysfunction compared to surgery (7.8% vs. 14.2%, p<0.001), and five-year rates of structural valve deterioration and non-structural valve dysfunction were significantly lower after TAVI compared to surgery (2.2% vs. 4.4%, p 0.004), and non-structural valve dysfunction (4.3% vs. surgical aortic valve replacement [SAVR] 8.8%, p<0.001).

“That is an important insight in the discussion on durability, and I think we are coming to a point where you can no longer make a claim that the surgical valve is the standard reference,” says Van Mieghem of these data. “There is no difference in the low rates of endocarditis and valve thrombosis, but there is clearly more structural and non-structural valve degeneration after surgery.”
According to Yakubov’s analysis, patients in both groups who developed bioprosthetic valve dysfunction through five years had around a 1.5-fold greater risk of death or hospitalisation for valverelated disease or heart failure than patients who did not develop bioprosthetic valve dysfunction.

Coming shortly after the presentation of the results of the analysis from the CoreValve US High Risk and SURTAVI trials, were three-year data from the Evolut Low Risk trial shared at the 2023 American College of Cardiology (ACC) annual scientific session (4–6 March, New Orleans, USA) by John Forrest (Yale School of Medicine, New Haven, USA) and published simultaneously in the Journal of the American
College of Cardiology (JACC)
The Evolut Low Risk trial is a randomised, non-inferiority study, comparing TAVI with a self-expanding supra-annular bioprosthetic valve (CoreValve, Evolut R, or Evolut Pro) with SAVR in patients with severe aortic stenosis at low surgical risk.
The analysis presented by Forrest at ACC 2023 included 1,468 patients with a median age of 74 years. All of the patients enrolled in the trial had severe symptomatic aortic stenosis and were deemed to be at low risk from surgery, defined as having no more than a predicted 3% risk of death within 30 days of the procedure. Patients were randomly assigned to receive either TAVI or SAVR.
The results detailed by Forrest showed that 7.4% of those treated with TAVI had died or experienced a disabling stroke, compared with 10.4% of those treated with SAVR, a difference that fell just short of statistical significance (log-rank p=0.051).
in terms of the composite endpoint of mortality and disabling stroke out to three years.”
“That is impressive because some people were alluding to a separation of the curves in terms of clinical outcomes in favour of surgery, and that definitely is not present in Evolut Low Risk, which is reassuring.
“There were more patients with mild paravalvular leak (PVL) or who required a pacemaker in the TAVI arm, but that did not affect the outcome at three years because still there was a strong trend in favour of TAVI in low-risk patients. That was an important observation,” he adds.
Though these data offer some reassurance over the durability of currently available TAVI platforms, one important discussion point for Van Mieghem is that there appears to be no class effect when it comes to valve durability according to the randomised trial data, meaning that not every transcatheter valve will behave in the same way. “Five-year data of intermediate-risk randomised controlled trials clearly suggest that self-expanding, supra-annular functioning devices behave differently than the balloon-expandable devices and also in comparison to surgery,” Van Mieghem says. And, while he comments that there is a “clear difference” in the behaviour of supra-annular valves relative to balloonexpandable platforms, datasets up to 10 years—as is planned through the Evolut Low Risk trial—will be needed before the claim can be made conclusively.
Occurrence of the secondary combined endpoint of all-cause mortality, disabling stroke, or aortic valve rehospitalisation was significantly lower in the TAVI group (13.2%) than in the SAVR group (16.8%, p=0.050). The results did show that mild paravalvular regurgitation was more frequent in the TAVI group (20.3% vs. 2.5%), but at three years there was no significant difference in the presence of moderate or greater paravalvular regurgitation (0.9% TAVI vs. 0.2% surgery).
In presenting the results at ACC, Forrest commented that the consistent benefit of TAVI at three years is not something that has been observed in prior studies, “and provides further evidence that TAVI deserves to be the dominant treatment modality for patients with aortic stenosis undergoing valve replacement.”
Van Mieghem comments: “What was relevant in that study was that TAVI with an Evolut system outperformed surgical valve replacement
When considering the use of a supra-annular valve, Van Mieghem cautions that it is important to be mindful of facilitating future coronary access. “It is important to evaluate the coronary height, the width of the sinuses of Valsalva, and the ascending aorta to determine what the risk is for immediate coronary obstruction,” he advises. “In that regard, commissural alignment becomes very important, and the more extensive the coronary artery disease, the higher the likelihood for a future need for coronary interventions,” he adds, noting that this may influence device selection.
Looking to the future of TAVI trials, Van Mieghem says the question of durability will continue to be an important topic for research, particularly as the treatment is made available to younger patients.
“If we have good knowledge of durability and that the durability of a transcatheter valve will be similar to a surgical valve, then I think the uptake of TAVI will increase even further and then the discussion will shift to lifetime management,” he concludes.
10 Advertorial May 2023 | Issue69
THIS ADVERTORIAL IS SPONSORED BY MEDTRONIC
Five-year data of intermediate-risk randomised controlled trials clearly suggest that self-expanding, supraannular functioning devices behave differently than the balloonexpandable devices and also in comparison to surgery.”
Nicolas van Mieghem
Evolut PRO+

Structural Heart Interventions
What to know when planning for a second TAVI valve
Recent guidelines on valvular heart disease in Europe and the USA have expanded the indications for transcatheter aortic valve implantation (TAVI) to younger patients and those at lower surgical risk with severe symptomatic aortic stenosis—a change that is likely to herald a substantial increase in TAVI procedures worldwide, including “redo” procedures. Giuseppe Tarantini (University of Padua Medical School, Padua, Italy) tells Cardiovascular News why this topic is increasing focus, and details the fundamentals of planning for TAVI reintervention.
With TAVI being performed in younger patients, can we expect to see more redo procedures in future?

This is an extremely relevant topic, as now the mean age of TAVI patients, particularly in the USA, is around 70 years of age. For most of these subjects, one transcatheter heart valve will not be enough. In fact, if we consider the patients’ life expectancy and the “supposed” prosthesis durability of about 10 years, many of these subjects will outlive their transcatheter heart valve, meaning that they will need reintervention. At that point, they will be likely too old to consider TAVI explant and redo TAVI—if feasible—will be the preferable option.

What do data tell us about the outcomes of redo TAVI?
Available data on redo TAVI come from two large international registries, with patients treated at high volume (i.e. expert) centres. First of all, redo TAVI represents only 0.33% of the aortic transcatheter procedures. Survival rate at 30 days was around 98%, with low rates of stroke of 1‒2%, coronary obstruction (1%) and pacemaker implantation (10%). But, we should keep in mind that these are highly selected patients treated at tertiary care hospitals and thus we: 1) do not know how many patients were denied redo TAVI because it was believed to be unfeasible based on preprocedural computed tomography (CT) planning, 2) do not know if these results are reproducible in lower volume centres.
Are there studies currently ongoing and what do we hope to learn?
There are many registries ongoing on this topic, promoted by major transcathether heart valve manufacturers. I am the principal investigator of a European multicentre prospective registry looking at redo-TAVI with a balloon-expandable valve. From these studies we expect to learn the timing and failing mechanism of transcatheter aortic valves, the incidence of redo TAVI unfeasibility and the real world outcomes of this procedure. Moreover, we will try to understand what is the best operative practice in doing these procedures, for which I have written, together with a group of international experts, an operative manual. Finally, these studies will provide us with the real world short and mid-term outcomes of redo TAVI and, for instance, the clinical impact of leaflets’ overhanging.
How does planning and executing a redo procedure differ from a first implant?
Planning redo TAVI is more complex than planning TAVI in native aortic valves. In fact, operators need
Study highlights disparity in TAVI outcomes based on procedural availability
Research has highlighted significant regional variations in the availability of transcatheter aortic valve implantation (TAVI) and patient outcomes between Ontario, Canada and New York State, USA.
PATIENTS IN NEW YORK
enjoyed better TAVI accessibility and outcomes compared to those in Ontario. Furthermore, statistical analysis suggested that if the same New York residents were treated in Ontario, they would have experienced poorer outcomes. The findings are published in the Canadian Journal of Cardiology
According to current clinical guidelines, TAVI is preferred for patients who are considered high-risk or ineligible for surgical aortic valve replacement (SAVR) and a viable option for those at intermediate or low risk. Despite this, access to TAVI varies significantly across regions.
Lead investigator Harindra Wijeysundera (University of Toronto, Toronto, Canada), stated that the potential benefits of centralising TAVI
procedures to a fewer number of specialised centers with potentially higher procedural volumes must be weighed against possible patient harms.
“In areas such as New York, there has been a rapid expansion of new TAVI centres, which has increased the capacity but resulted in relatively low volumes at some facilities. Because low operator-hospital volume is linked to poorer TAVI outcomes, this raises concerns about the potential for poorer post-procedural outcomes as a possible clinical consequence if TAVI availability becomes more widespread,” he said.
As there is limited knowledge on how these contrasting scenarios compare (potentially sicker patients before the procedure, but with better post-procedural outcomes due to
to consider not only the native aortic valve anatomy, but also the metrics of the failing transcatheter heart valve (i.e. leaflets high, stent dimensions, skirt height etc.) and its mode of failure. The relation between the failing transcatheter heart valve and the aortic root anatomy will determine the risk of coronary obstruction, and thus guide the way we will implant the second valve (lower versus higher) and the degree of leaflets overhanging that we will accept.
How important is valve selection in redo TAVI? Do different features benefit the procedure?
More than the second valve, is perhaps the choice of the first valve that will influence the feasibility and the outcome of redo TAVI procedure. In fact the main difficulty of redo TAVI comes from the risk of coronary obstruction, which is highest when the first valve implanted is a supra-annular transcatheter heart valve. In fact, with the implantation of the second prosthesis inside the first transcatheter heart valve, the degenerated leaflets will be tilted up, thereby creating a “covered stent” which might impair coronary flow, particularly in the presence of a small aortic root. The higher the leaflet’s position of the first THV, the higher the risk of coronary flow impairment after redo TAVI. In this sense, if the first prosthesis implanted was a short frame THV, the redo TAVI procedure might be less cumbersome (independently from the choice of the second prosthesis, provided the latter is implanted with commissural alignment). On the other hand, treatment of a failing supra-annular transcatheter heart valve is likely to be best approached with the implantation of a short frame balloon-expandable valve, which can be implanted lower or higher based on the risk of coronary obstruction and the expected haemodynamic result of leaflets overhanging (more detrimental if the failing mechanism is a stenosis rather than regurgitation).
higher operator-hospital experience, versus less sick patients with shorter wait times, but potentially poorer post-procedural outcomes due to lower operator-volume experience), researchers conducted an observational, retrospective cohort study that compared outcomes between the two regions as a natural experiment. They aimed to examine whether differences in healthcare delivery in regions with high versus low access to TAVI translated to differences in post-
procedural mortality and readmissions. All Ontario and New York State residents aged 18 years or older who underwent TAVI between January 2012, and December 2018 were identified. The primary outcomes were post-TAVI 30-day in-hospital mortality and all-cause readmissions.
The study found significant differences in TAVI access rates between the two jurisdictions. Although there was no significant difference in the rate of readmission at 30 days between the two jurisdictions (14.6% in Ontario and 14.1% in New York State), the 30-day in-hospital mortality rate was higher in Ontario (3.1%) than in New York State (2.5%). To determine the potential impact of treatment in Ontario on New York patients, the investigators calculated the observed versus expected outcomes for New York patients had they been treated in Ontario.
Wijeysundera noted that the study results suggest that greater access to TAVI is linked with better outcomes, possibly due to early intervention in the disease trajectory.
May 2023 | Issue69 12
This raises concerns about the potential for poorer postprocedural outcomes as a possible clinical consequence if TAVI availability becomes more widespread.”
Giuseppe Tarantini
Harindra Wijeysundera

ESC and EAPCI publish renal denervation consensus statement
Renal denervation represents another treatment option in patients with uncontrolled resistant hypertension and may be used in selected patients deemed intolerant to antihypertensive drugs.

These are among the messages of a new consensus statement published in EuroIntervention following a review of evidence by the European Society of Cardiology (ESC) Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI).
Initial excitement over the potential for renal denervation as a treatment for hypertension was dampened following the release of initial results of the SYMPLICITY HTN-3 trial in 2014 in which it was shown that at six months, renal denervation did not elicit significant incremental blood pressure lowering benefit compared with a sham procedure.
Subsequently, joint ESC and European Society of Hypertension (ESH) guidelines on the management of arterial hypertension, published in 2018, advocated against the routine use of device-based therapies for hypertension, until further evidence of their safety and efficacy came to light. However, newer sham-controlled trials have revitalised hope in the potential of renal denervation, with a number showing statistically significant and clinically meaningful reductions in blood pressure.
In producing the latest consensus statement, the ESC and EAPCI expert panel have reviewed evidence from several “second-generation” randomised, sham-controlled trials, which, they say, demonstrate the safety and the blood-pressure lowering efficacy of radiofrequency and ultrasound renal denervation.
The second generation of trials have involved either the Symplicity Spyral (Medtronic) multi-electrode radiofrequency device or the Paradise (ReCor Medical) ultrasound system.
“Since the publication of the 2018 ESC/ ESH Guidelines for the Management of Arterial Hypertension, several high-quality, randomised, sham-controlled trials have been published, demonstrating a blood-pressure-lowering efficacy over 24 hours for both radiofrequency and ultrasound renal denervation in a broad spectrum of patients whose hypertension ranges from mild-to-moderate to severe and resistant,” Emanuele Barbato (University of Rome, Rome, Italy) and colleagues write in EuroIntervention
“This expert group proposes that renal denervation is an adjunct treatment option in uncontrolled resistant hypertension, confirmed by ambulatory blood pressure measurements, despite best efforts at lifestyle and pharmacological interventions,” the statement notes. Renal denervation may also be used in patients who are unable to tolerate antihypertensive medications in the long term, the writing committee has concluded.

The authors of the paper suggest that a shared decision-making process, taking into account the patient’s global cardiovascular risk and the presence of hypertension-mediated organ damage, should be followed when considering renal denervation as a
Ultrasound renal denervation shows “consistent” results in twin studies
ReCor Medical has announced that primary endpoint results from the RADIANCE II pivotal trial were published in the Journal of the American Medical Association (JAMA). Study results showed that the Paradise ultrasound renal denervation (uRDN) system successfully reduced blood pressure compared to sham.
IN ADDITION, POOLED ANALYSIS results from the combined primary efficacy endpoint and safety data from RADIANCE SOLO, RADIANCE TRIO, and RADIANCE II were concurrently published in JAMA
Cardiology. Results of the pooled analysis showed a consistent blood pressure lowering effect across a broad range of hypertension, including mild to moderate and resistant hypertension.
RADIANCE II is a randomised, sham-controlled US Food and Drug Administration (FDA) investigational device exemption (IDE) pivotal trial of the Paradise uRDN system in the treatment of patients with uncontrolled hypertension. Conducted as an
international multicentre study at more than 60 study centres in eight countries, 224 patients with uncontrolled hypertension were randomised 2:1 to uRDN or a sham.
Patients were to remain off antihypertensive medications throughout the two months of followup unless specified blood pressure criteria were exceeded. At the twomonth primary efficacy endpoint, patients treated with the Paradise uRDN system had a mean reduction in daytime ambulatory systolic blood pressure of -7.9mmHg, compared to a reduction of -1.8mmHg in the sham arm, corresponding to a statistically significant and clinically relevant
treatment option.
Furthermore, they state that interventionalists require expertise in renal interventions and specific training in renal denervation procedures. “Centres performing these procedures require the skills and resources to deal with potential complications,” Barbato et al state.
Presently both the Symplicity Spyral and Paradise system carry a CE mark, and Medtronic and ReCor both filed premarket approval applications to the US Food and Drug Administration (FDA) in late 2022 for their respective devices.
“To date, there are at least 18 societal and/or expert consensus documents published, and the increasing number of citations seems to parallel the mounting evidence for renal denervation therapy,” David Kandzari (Piedmont Heart Institute and Cardiovascular Services, Atlanta, USA), a member of the writing committee for the ESC/EAPCI consensus statement and prinicipal investigator in the SPYRAL HTN-ON MED trial, told Cardiovascular News. “In all, these documents are important for providing clinicians with guidance regarding the evidence basis for renal denervation safety and effectiveness, patient selection, and procedural technique. Many of the documents also underscore the need for shared-decision making and accounting for patient preference.
“The ESC/EAPCI document offers the most contemporary evidence and informed clinical considerations for renal denervation, including recommendations for not only patient selection but also for institutions related to renal denervation programme development, patient selection, and operator proficiency.”
between-group difference of -6.3mmHg (p<0.0001). The study also achieved its primary safety composite outcome with no major adverse events observed.
Concurrently published in JAMA Cardiology, the RADIANCE pooled analysis includes data from more than 500 patients randomised in the three studies from ReCor’s RADIANCE global programme: RADIANCEHTN TRIO, which studied patients with resistant hypertension, and RADIANCE-HTN SOLO and RADIANCE II, which studied patients with mild-moderate hypertension.
The combined dataset showed an overall reduction in daytime ambulatory systolic blood pressure in the uRDN group of -8.5mmHg with a difference between treatment and sham at two months of -5.9mmHg (p<0.0001), favouring uRDN. Blood pressure results were similarly positive in the 24-hour, night-time, home, and office measures. A favourable safety profile was consistently observed following uRDN treatment across the studies.
“The results of the RADIANCE clinical trials are meaningful in that they solidify the role of the Paradise uRDN system as an adjunctive
therapy for hypertension treatment, in addition to medications and lifestyle modification. Having three consistent sham-controlled clinical trials demonstrating that the Paradise uRDN system can safely lower blood pressure across a range of patients is a very high bar to have met,” said co-principal investigator Ajay Kirtane (New York-Presbyterian Hospital, New York, USA).
Co-principal investigator Michel Azizi (Université Paris Cité, Hôpital Européen Georges Pompidou, Paris, France) added: “The pooled analysis of RADIANCE SOLO, TRIO, and RADIANCE II shows a remarkable consistency of effect in patients with mild to moderate hypertension and those with resistant hypertension. These results are in line with the new 2023 consensus statement of the European Society of Cardiology (ESC) Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI).
The publication of these results in JAMA and JAMA Cardiology will bring the evidence of the performance of uRDN in the treatment of hypertension to a broad audience of physicians.”
May 2023 | Issue69 14 Renal Denervation
The ESC/EAPCI document offers the most contemporary evidence and informed clinical considerations for renal denervation.”
Renal denervation
The publication of a consensus statement from the European Society of Cardiology (ESC) Council on Hypertension and the European Association of Percutaneous Cardiovascular Interventions (EAPCI) is among the final steps in the evaluation of renal denervation as a device-based treatment for hypertension. This is according to Felix Mahfoud (Saarland University Hospital, Homburg, Germany), a member of the expert committee behind the paper, and one of the foremost investigators of the technique. He discusses patient selection and future indications for the therapy with Cardiovascular News

What is the background to the ESC/EAPCI consensus statement on renal denervation?
This consensus document was deemed necessary because a significant amount of new sham-controlled trial evidence has become available. In the 2018 guidelines of the ESC and the European Society of Hypertension (ESH), devicebased hypertension treatment was graded with a class 3 recommendation, not to be used routinely in clinical practice. There was a sentence added to the statement that until further evidence regarding the safety and efficacy becomes available, these devices should not be used outside of clinical trials. We felt it was important to re-evaluate the evidence that has aggregated after the publication of the guidelines, and by now we have five sham-controlled clinical trials that have indeed proven the efficacy and safety of renal denervation in the presence and absence of antihypertensive drugs.
Renal denervation: A recent history
What have the recent trials shown?
We know it works in patients with and without antihypertensive medication, but this is a very broad potential patient population. We felt it is also important to provide guidance on where denervation may be used in clinical practice and felt this should be reserved as a treatment option for patients with so-called “resistant” hypertension, meaning despite treatment with three antihypertensive drugs, they still have uncontrolled blood pressure values, as confirmed by office and ambulatory blood pressure.
Despite having evidence that this works in a very broad population, we nailed it down, first and foremost, to patients with resistant hypertension. There is another potential indication for renal denervation and that is in patients where drugs are not tolerated, those not willing or able to take antihypertensive drugs, and those with a preference to be treated with a device-based approach.
How significant a development is this?
It is not the intent of the consensus statement to change people’s perception. The perception needs to be adapted according to the published trial evidence. This is really more to inform clinical practice. What we did is reach consensus on different statements. The challenge is who to treat within clinical practice, and this is the overall objective of such consensus statements. Which patients in which centres? How should the centres be trained? What are the potential complications of the procedure that interventionalists need to be informed about?
Are there “ideal” patients for renal denervation?
What we have to accept is that this is a treatment possibility for patients with uncontrolled blood pressure. It is not replacing drugs, it is not replacing lifestyle modification—this is another approach available in our armamentarium to lower blood pressure. But, it is not exclusive. It is not renal denervation or nothing. Most patients we treat have undergone lifestyle modification, but it was unsuccessful. They have been treated with several drugs, are still uncontrolled and have high blood pressure and have high cardiovascular risk. In these patients it is another treatment that may bring blood pressure down.
How excited are you by this treatment?
We started the scientific evaluation of this approach 15 years ago. It is among the very few device-based treatments that has beaten sham control. This is something we have to keep in mind. There has been a very rigorous evaluation of the procedure, which is not available for other techniques that we use every day. This is something we have to acknowledge, [and] that we have a lot of clinical data, a lot of robust methodologically defined and properly designed studies conducted around the world and they have proven that this technology lowers blood pressure. You cannot question whether or not it works—it works—that is a statement. Now it is our responsibility to offer this to certain, but not all, patients at risk.
2019
I am excited, of course, because it is about science, but this is probably among the last steps in the evaluation of this technology. We are working on a US Food and Drug Administration (FDA) submission so hopefully this will become available in the USA soon. I think overall we are in good shape moving forward with this technology. We are now looking into new indications such as heart failure, atrial fibrillation and ventricular tachycardia. We have registry data confirming that in those populations it is safe, and now we are heading off to new shores and among those are heart failure, certainly and arrhythmias are also very interesting.
Where are the gaps in our knowledge?
Identification of responders is the unmet need. It is something that we are investigating in clinical studies to get further insights, and has never been available, even for antihypertensive drugs. I am not sure if we will succeed in this with renal denervation, but we are still trying. The second question is whether or not this blood pressure lowering translates into improvements in outcomes. We know that blood pressure as LDL [low-density lipoprotein] cholesterol closely associates with cardiovascular morbidity and mortality, and when you lower blood pressure or LDL cholesterol, it is believed that it lowers morbidity and mortality too, so I am pretty confident that this will translate into improved outcomes—but it has not yet been shown.
Medical) gains CE mark
2014 Results of SYMPLICITY HTN-3 bring renal denervation train “to a grinding halt”
2017 SPYRAL HTN-OFF MED, first seen at ESC 2017, provides “proof of principle” that renal denervation works
2018
Positive data from SPYRAL HTN-ON MED and RADIANCE SOLO trials, shared at EuroPCR 2018 “reignite interest in the field”
Six-month onmedication RADIANCEHTN SOLO trial results find patients treated with ReCor ultrasound system were prescribed fewer medications than those treated with a sham procedure
2020
At virtual ACC meeting, three-month SPYRAL HTN-OFF MED trial data show renal denervation bests sham treatment to lower blood pressure in untreated hypertension
2021 EuroPCR 2021 hears registry data indicating that renal denervation with the Symplicity system saw significant and sustained blood pressure reductions in a real-world population through three years
2022
Late-breaking trial data presented at EuroPCR 2022 underscore the potential of renal denervation as an adjunctive therapy to treat hypertension, experts say
2023 ESC and EAPCI publish renal denervation consensus statement, indicating that renal denervation represents another treatment option in patients with uncontrolled resistant hypertension
15 Issue69 | May 2023 Renal Denervation
2011 SYMPLICITY HTN-3 begins enrolment 2012 Paradise catheter (ReCor
You cannot question whether or not it works. It works— that is a statement. Now it is our responsibility as physicians to offer this to certain, but not all, patients at risk.”
Renal denervation “can reach new shores”
Felix Mahfoud
Point of View
Ruggero De Paulis
Inventor of the Valsalva aortic graft Ruggero De Paulis (European Hospital Unicamillus University, Rome, Italy) tells Cardiovascular News about his unique inspiration for the device. A past president of the European Association of Cardio-Thoracic Surgery (EACTS), he turns back the clock on his career in cardiac surgery.
Why did you choose to become a doctor and cardiac surgeon?
I began medical school in my hometown, L’ Aquila, at the age of 17. At the time, I was not really thinking of my future or professional life. My father was a doctor, and I think part of my choice was the desire to follow in his steps—although he did not try to influence my decision, but I always thought that healing and helping people is one of the most rewarding activities for a human being.
Once you start studying medicine, it is common to fall in love with every organ or disease you get to know better. Towards the end of my studies, I realised that I wanted to do something with my hands, and surgery, more than clinical medicine, was the first choice. I also had a passion for the cardiovascular system. Putting this together pointed to cardiac surgery. I applied for residency at the University of Torino where I moved, driving all the way in my old Citroen 2CV. After the first month in the operating room, I knew this was the place I wanted to be.
dilatation. The most important aspect remained a matter of debate.
Who were the
biggest influences on your earlier career?
Mario Morea, the director of the cardiac surgery department at the University of Torino, could be considered an early pioneer, especially in the field of valve surgery at the time when bioprostheses first appeared. Besides his huge experience in all aspects of cardiac surgery, he had a genuine interest for all innovations and an innate sense of curiosity. Since our first encounter, he pushed me to concentrate on education and research more than pure manual surgical activity. He used to say: “With six intensive months you can learn how to move your hands—it takes much longer to understand what the right thing to do is”. In a more practical way, one of the staff surgeons, Gianmaria Ottino, helped me to enter the field of scientific reading and publication as well as managing databases and statistical calculation. For both I feel a profound sense of affection and gratitude.
What is the story of how and why the Valsalva graft came in to being?

At the end of 1980s when stentless valves were developed I was prompted by the chief of my residency programme to study the physiology of the aortic root to better understand the potential for this approach. I embarked on reviewing a series of old in vitro and in vivo studies, and learned that the aortic root, differently from the rest of the ascending aorta, expands almost exclusively on the horizontal plane while its longitudinal compliance is almost zero.
Two types of valve-sparing operations were introduced by Magdi Yacoub and Tirone David at the beginning of 1990, but they were rarely performed by other surgeons. At major cardiac surgical meetings, I witnessed the two surgeons confronting the peculiarity of their techniques many times. The critical points were always the same: Yacoub claimed his technique was more physiological because it re-established the sinuses of Valsalva while David claimed his technique was more durable because it addressed the annular
I was intrigued and obsessed with finding a solution that could somehow combine the two options. One day, when assisting on a Bentall operation, I started thinking of a material that would stretch horizontally to fill the space previously occupied by the root aneurysm. Such material still does not exist, but on the way home I kept thinking that a cylindrical conduit was certainly not the best solution for substituting a pear-shaped piece of anatomy. Once I entered my house, the solution was simply hanging in front of me! My wife, a fashion designer, had been working on a peculiar dress completely made by pleated tissue material. The upper part had horizontal pleats while the lower part, corresponding to the skirt, had vertical pleats that would naturally open and close. I remained speechless for a long period while suddenly visualising the perfect shape for a Bentall procedure and the potential for providing sinuses to a David procedure. I immediately went back to collect some spare aortic grafts and, on the kitchen table with the help of my wife, cut open a corrugated Dacron graft, rotated it by 90 degrees and sewed it back on. The Valsalva graft was born.
In the following days I worked on lengths and diameters to find the optimal solution that would respect anatomical and physiological parameters—dimensions and proportions that have not been changed ever since.
What lessons can you impart on budding surgeon innovators?
The first challenge was finding a company that might be interested in producing a commercial version of my prototype. After six months of discussion with a major manufacturer of Dacron grafts, I was turned down because they had other priorities. A little depressed after this experience, with the help of a friend I was put into contact with a second company, where I met Roshan Maini, who had the patience to listen to all my explanations about anatomy and physiology, and the wisdom to see a future where aortic surgery and valve sparing operations would flourish. After the first prototypes were implanted and imaging showed excellent root reconstruction in all different techniques, the Valsalva graft was introduced into clinical practice.
At this point the challenge was to convince the surgical community of the potential benefits of a graft designed for the aortic root. Bench experiments, postoperative imaging and clinical follow-up had been used to show the benefit of an easier procedure, coupled with an optimal anatomical root reconstruction yielding good, long-lasting results. The lesson is that a good idea is not always enough—a lot of work needs to be done afterwards. In simple terms, the formula is 10% genius and 90% perspiration.
How has the Valsalva graft changed the face of aortic root replacement?
My initial hope was to change the standard of care for patients where a root replacement was needed, no matter whether it was associated with aortic valve replacement or a valve sparing operation. After 20 years I feel that this has been fulfilled and, in the
near future, I envisage that the great majority of root surgeries will be performed using a Valsalva graft. By providing neo-sinuses in a simple and standardised fashion, the Valsalva has two main benefits. From one side it facilitates surgery, making coronary button reimplantation easier in all the various anatomical presentations. From another side it provides vortex inside the neo-sinuses, guaranteeing a physiological cusp motion with larger orifice area in systole and smooth leaflet closure during diastole. Altogether it should improve durability and longevity both for bioprostheses and natural valves.
16 Interview May 2023 | Issue69
Andy Watt/NB Illustration
PROFILE
The formula is 10% genius and 90% perspiration.”
Appointments
Director and Chief, Cardiac Surgery, European Hospital, Rome Italy
EACTS President 2018–2019
Associate Editor ICTVS
2017–2020
STS/AATS Member
Chair, EACTS Aortic Forum
Education
University of L’Aquila
University of Torino
University of Utah
VI and XII University of Paris Inventor
Valsalva graft
Physioflex mitral band
From a research point of view the Valsalva graft has been demonstrated to be a formidable tool to study the anatomy and physiology of the root, the effect of suboptimal root reconstruction on the aortic cusp movement, as well as the effect of the sinuses on coronary flow, among many other experimental studies on flow turbulence or wall stress, for example.
You also played a part in the development of the Physioflex ring for mitral repair. What has this innovation added to the field?
Alongside the design of the Valsalva graft, this device shares the desire to always mimic normal human anatomy and physiology. Mitral rings have been in use for many years, both in the form of complete rings or open bands, and changes and improvements have happened in small steps.
The design of the Physioflex ring starts from the belief that the anterior portion of the mitral curtain does not need to be immobilised by a ring but left free to move during the cardiac cycle. During systole the root needs to expand and wedge into this region. Leaving this anatomical region free from prosthetic material offers the opportunity for a larger effective orifice area in the left ventricular outflow tract (LVOT) during systole and a larger effective orifice in the mitral during diastole. On top of this, the Physioflex has a complete saddle shape to offer the advantages of a good annuloplasty while maintaining a proper ring shape necessary to guarantee optimal leaflet apposition and coaptation, with reduced stress for the various elements of the mitral valve complex.
What are your current
research interests?
At present we are working on a better understanding of the pathology of the bicuspid valve and the potential for standardisation of aortic valve repair in different anatomical presentation. Overall, the ability to repair the majority of purely regurgitant aortic valves is certainly appealing and it is important to popularise and diffuse these techniques.
On a different level we are interested in better understanding intraventricular flow and how flow is modified depending on specific pathologies, the type of surgery or the type of devices utilised.
How important has your involvement in EACTS been for your career and research?
My time at the EACTS, first as a chair of the vascular domain, and then as president, has contributed to a personal boost of visibility and increased my professional connections across the world. The time spent in shaping many annual meeting programmes, coupled with contribution in the general governance of the Association, has been an extremely rewarding experience that continues today. In fact, I now have the honour of chairing the Francis Fontan Fund (FFF) that provides scholarships to young colleagues with specific interests in various aspects of our profession. Education is certainly the main focus of an association like EACTS and the FFF represents one of its best expressions.

Outside of medicine, what are your hobbies and interest?
During my studies, I used to play rugby and basketball for my hometown teams. After entering the cardiac surgery residency, I had less time for team sports. Nowadays, I like outdoor activity like running, swimming, or cycling to keep in a good shape. However, my great passion has always been alpine skiing. I think I will try to maintain this activity as long as possible. I spend my free time in Puglia with my wife, even during the winter, in close contact with the sea and nature.
17 Interview Issue69 | May 2023
Fact file
Ready for the primetime? The future role of drug-coated balloons in PCI


“The evolution of PCI [percutaneous coronary intervention] has always been a continuum, with new devices addressing the limitations of previous devices,” Marie-Claude Morice (Institute Hospitalier Jacques Cartier, Massy, France) tells Cardiovascular News, discussing the growing interest in the use of drug-coated balloons (DCBs) in coronary interventions.

In the history of coronary interventions, this evolution has taken in coronary artery bypass graft (CABG) surgery, then balloon angioplasty, followed by bare metal stents, and later first- and secondgeneration drug-eluting stent (DES) platforms. “Now the drug-coated balloons are coming,” says Morice, commenting that these devices may address the limitations of even the best second-generation DES platforms. “It is now obvious that there are a few but unavoidable events that are occurring in the long term,” says Morice of the current-generation devices. Potential advantages of the DCB platform arise from the fact that there is no permanent implant left behind in the vessel after implantation, which can avoid some of the potential drawbacks of placing a DES, such as late stent thrombosis or in-stent restenosis.
Presently, DCBs are favoured in a subset of PCI indications, including the treatment of instent restenosis, where there are a wide set of data underscoring the safety and efficacy of such devices. The use of a DCB in this context carries a class I recommendation—with the level of evidence A—in guidelines from the European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery (EACTS). This recommendation is formed solely upon data from the PACCOCATH ISR I and II or PEPCAD trials which were performed in the majority using the SeQuent® (B Braun) family of devices. A key statement in the guidelines is that “a class effect for all DCBs cannot be assumed”— underscoring the importance of device selection based upon robust data.
“There is less but relatively strong evidence in small vessel native coronary artery disease,” adds Morice, discussing applications of the technology. “We all know that having those long lesions in small arteries treated by a full metal jacket leads to complications,” she adds. In this context, the BASKET SMALL-2 study is one such trial to have proven DCBs to be non-inferior to DES in native coronary lesions with a diameter less than 3mm, with the trial showing major adverse cardiovascular events (MACE) to be comparable between the two groups (7.5% for the DCB group vs. 7.3% for the DES cohort) after one year. However, larger trials
are needed to confirm such finding, and they are ongoing.
Furthermore, Morice highlights bifurcation lesions as another indication in which the use of DCBs has shown to be a promising strategy. “We know that having two stents across a bifurcation is not ideal so interventional cardiologists are exploring drug-coated balloons in the side branch, or even the complete treatment of the bifurcation, in both branches,” she explains, caveating that this particular usage still requires further studies before it can be firmly incorporated into routine clinical practice.
Emerging indications
Similarly, patients with a high risk of bleeding may also benefit from a DCB-only strategy, Morice comments, noting that this approach can facilitate a shorter course of dual-antiplatelet therapy (DAPT), a factor that was considered in the aforementioned BASKET SMALL-2, where patients with stable coronary artery disease received one month of DAPT, compared to six months for those patients who received stents. Further data on this topic come from the DEBUT trial, a single-blind, randomised, noninferiority trial examining the treatment of de novo coronary lesions in patients with high-bleeding risk using the SeQuent® Please DCB. Results from the trial, which were published in The Lancet in 2019, showed that the DCB was superior to stenting against a primary endpoint of MACE at nine months, with MACE having occurred in one patient (1%) in the DCB group versus 15 (14%) in the stent group.
To date, much of the evidence surrounding the safety and efficacy of DCBs centres on the use of paclitaxel-coated devices, but advances in sirolimusbased technology suggest that it holds promise as an alternative to paclitaxel for the treatment of both in-stent restenosis and de novo lesions. There is some evidence suggesting equivalency coming from 101 patients with in-stent restenosis randomised to SeQuent SCB or to SeQuent Please Neo, and another 70 patients with de novo lesions from a randomised
trial conducted in Malaysia. Although the results are promising they must be confirmed in larger trials, Morice comments, adding that demonstrating the efficacy and safety of sirolimus-based platforms will be an important future area of study. “The evidence that we have at the moment is mostly on paclitaxel,” she states.
The step further is to compare each type of DCB (as there is no class effect) with the latest generation DES in de novo lesions in clinical trials sufficiently powered for a clinical primary endpoint. This is currently ongoing in the REVERSE trial for the B Braun paclitaxel coated balloon (large vessels ≥3mm),
TRANSFORM 2 with the Concept Medical sirolimus-eluting balloon (small vessels ≤3mm) and Selution DeNovo (a large DCB study in de novo lesions, any size of the vessel) for the Medalliance Sirolimus-eluting balloon.











Turning to the question of whether DCBs can be considered as moving into the “primetime” for PCI, Morice says that the signs are positive. “The rationale of the drug-coated balloon is that it is a new paradigm,” she comments. “It preserves the arterial physiology, allows positive remodelling of the vessel, avoids the metallic scaffold complications and allows short DAPT therapy in stable coronary artery disease patients.”
bbraun.com/dcb
bbraun.com/dcb










Contingent on positive trials comparing DCBs to stents in de novo lesions, the devices may play an “important role” in PCI procedures in years to come, she predicts, commenting that this change in perception is likely not to be far away when the results of the trials cited above will be available, if they have a positive outcome.
“We know all the success we have obtained with drug-eluting stents, but we also know that they have some limitations,” she adds. “DCBs may play a big role, complementary to drug-eluting stents, to treat our patients better according to the nature of the disease, the size of the artery, the location of the lesion, the quality of predilatation, or the grade of residual calcification. The more efficient and safer devices we have the better we can treat our patients.”



Research continues
While new indications are explored through ongoing trials and further evidence mounts on the indications for which DCB are now seen as established therapy, there is likely to be a continuing growth in the interest in this device technology into the future. Hinging on the results of future research in de novo large vessels, DCB-usage could increase to 30–40% of cases, Morice speculates.
As the field evolves, emerging data will shape the future role of DCBs in PCI. Morice comments that the continuation of research into the utility of DCBs is among the most eagerly anticipated fields of research in coronary interventions currently underway. “For coronary disease this is the most exciting research that is going on. We now know that there are some limits of the DES, and, if DCBs work as expected, restoring the vessel with nothing left behind is a dream.”
18 Advertorial May 2023 | Issue69
THIS
ADVERTORIAL
IS SPONSORED BY B BRAUN MELSUNGEN AG
Backing up cardiologists with unparalleled clinical data and proven performance for both paclitaxel and sirolimus coated balloons.
SeQuent® DCBs
The proven performers in coronary angioplasty
SeQuent® DCBs. The choice is yours.
Marie-Claude Morice
For coronary disease this is the most exciting research that is going on.”
Marie-Claude Morice
Preclinical model could aid future research of heart attack therapies
Researchers have detailed a new preclinical model for non-ST elevation myocardial infarction (NSTEMI), which they hope will aid in the development of future strategies for the treatment of NSTEMI patients.
Published in Nature Communications in February, the preclinical model is the output of a research collaboration between CÚRAM SFI Research Centre for Medical Devices at the National University of Ireland Galway (Galway, Ireland) alongside University of Milano-Bicocca (Milan, Italy), University of Paris Est Créteil (Paris, France), University of Gothenburg (Gothenburg, Sweden) and the Lithuanian University of Health Sciences (Kaunas, Lithuania).
Patients who survive a heart attack have variable degrees of damage to their cardiac tissue, which can lead to heart failure. In the last two decades, NSTEMIs have markedly risen in hospitalised patients. NSTEMI results in a smaller amount of tissue damage compared to ST segment elevation

myocardial infarction (STEMI), though recent clinical registry data show that NSTEMIs are associated with higher long-term mortality than STEMIs.
Currently, preclinical models of heart attack mimic only full-thickness STEMI and hence cater only for an investigation into therapeutics and interventions directed at the NSTEMI subset of heart attack.
Paolo Contessotto and Renza Spelat (both National University of Ireland Galway, Galway, Ireland), who were co-authors on the study, comment: “Advanced analyses on the affected heart tissue highlighted a distinctive pattern of alterations in the tissue, especially in the sugar moieties (glycans) which compose cardiac cell membranes and extracellular matrix (the network of proteins and other molecules that surround, support, and give structure to cells and tissues in the body). Identifying such changes in molecular elements that can be accessed and treated with injectable drugs sheds light on how we can develop targeted pharmacological solutions to correct these changes.”
In this new study, researchers have developed a preclinical model of NSTEMI by adopting a novel surgical procedure in an ovine model that closely resembles the complexity of clinical cases in humans. Researchers validated the presented model by comparing it with an established method to achieve STEMIs. They performed a detailed
analysis at the main acute and late time points after the induction of NSTEMI, at seven and 28 days, respectively.
Adds Abhay Pandit, CÚRAM Scientific Director and senior author of the study: “There is a need in the field to adopt clinically relevant models to study NSTEMI pathophysiology and reveal its functional differences with STEMI induction. This new model will facilitate the translation of future research in the field, enabling the discovery of new clinically relevant treatments for patients.”
Mark Da Costa, clinical investigator at CÚRAM and senior author of the study, said: “Currently, NSTEMI is the most common presentation of acute heart attack. The concern is that NSTEMI patients have lower in-patient (during their admission for the primary NSTEMI) and short-term mortality rates but significantly higher longterm mortality than those of STEMI patients. A Danish registry study of 8,889 patients showed that the five-year mortality after NSTEMI was 16%, and another registry study highlighted a 10year survival rate of only around 50%. To the best of our knowledge, there are currently no models that can reproduce
Finding the roots of the disease before treating it: Modelling non-ST elevation myocardial infarction
Paolo Contessotto (National University of Ireland Galway, Galway, Ireland) outlines his research developing a preclinical model for non-ST elevation myocardial infarction (NSTEMI) and discusses how this could aid in the future development of strategies to treat patients with this condition.

HOW MANY TIMES DO WE READ A scientific article which starts with a mere numbering of the worldwide clinical cases of a certain pathology, and how many times are those data actually reversed by the entry in the scene of the proposed—mostly preclinically promising—therapy described in such articles? Indeed, several clinical fields currently fight with the lack of suitable animal preclinical models which can accurately reproduce the disease of interest.
Nowadays, in the cardiovascular field, a clear rise in non-full thickness infarcts, also known as NSTEMI, is affecting a significant portion of both hospitalised and non-hospitalised patients, therefore representing a major socioeconomic issue. Patients affected by NSTEMIs differ from STEMIs since they can present with more subtle symptoms, and a marked decrease in ejection fraction (EF) is not usually shared among all these patients. Nonetheless, so far preclinical studies aiming to discover solutions to tackle MI were addressing full-thickness infarcts and therefore did not specifically target the other subtype of MI. Moreover, advances in high-throughput analyses which are able to provide us with details on the
complexity of the pathological environment following cardiac ischaemia, basically the death of the main cells composing the heart muscle, were only partially applied and exploited.
In this study recently published in Nature Communications, resulting from a collaboration across multiple prestigious academic institutions in Europe, the researchers develop and fully characterise a preclinical model of NSTEMI and pave the way towards possible specific therapeutic interventions to treat the worst adverse long-term outcome of MI— heart failure. Through a meticulous experimental design which took into consideration the key phases of post-ischaemia cardiac remodelling, care was taken to investigate the early (seven days postNSTEMI) and progressive (28 days) steps from when the pathology was induced in sheep. Notably, the employment of a really limited (21) pool of these animals under stringent European regulations, allowed a reasoned significant decrease in the usually much higher use adopted in studies published in prestigious journals using other types of animals (mainly mice and rats), which do not possess similar anatomical
both the functional and histological characteristics of NSTEMIs. This novel model may specifically serve as a preclinical foundation to study interventions that could combat the short and long-term effects of NSTEMI.”
proportions and as complex physiological response when compared to humans or large animals.
A precise procedure based on multiple ligations lateral and parallel to the left anterior descending coronary artery (LAD) was adopted and consistently reproduced to achieve focal, non-full thickness infarcts in the left ventricle. Researchers observed a progressive deterioration of the affected ischaemic tissue from the first week until the endpoint of the study by looking at the deposition of a fibrotic scar to replace the dead cardiac cells as well as the invasion of immune cells during the immediate inflammatory phase post-MI. Here, most interestingly, omics analyses looking at gene expression and protein data suggested a clear role of other moieties which heavily characterise the inflammatory cell populations which invade the region. Indeed, glycans, which represent one of the most abundant post-translational modifications in the cells present in our body, switched their profile depending on the cell types which were present at the specific moment evaluated in the study. Therefore, by the identification of such molecular targets to be modulated within a certain time window post-MI, researchers introduce in the field the relevance of such potential therapeutic strategy to contrast adverse fibrotic remodelling, and by consequence, heart failure.
Issue69 | May 2023 19 NSTEMI Research
This new model will facilitate the translation of future research in the field, enabling the discovery of new clinically relevant treatments for patients.”
VIEWPOINT
Paolo Contessotto







*Available for US and EU readers only **Available worldwide Visit renalinterventions.net and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription** Subscribe today Available in print and digital formats and through our social channels A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the field of renal disease management Editorially independent
PCI can be performed as safely without on-site access to surgery, says SCAI
Percutaneous coronary intervention (PCI) without onsite surgical support is as safe as PCI at centres that have access to surgical support, according to a new consensus statement from the Society for Cardiovascular Angiography & Interventions (SCAI).
THE RECOMMENDATION comes after a review of data from across randomised controlled trials, observational studies, and international experience. The statement, published in the Journal of the Society for Cardiovascular Angiography & Interventions (JSCAI) and JACC: Cardiovascular Interventions, explains that adequate operator experience, appropriate clinical judgment and case selection, and facility preparation are essential to a safe and successful PCI programme with no-SOS—surgery on site.
PCI with no surgical support was once considered high risk, SCAI notes in a press release,
despite that it has been performed with “acceptable” outcomes for nearly four decades. In 2007, SCAI published an initial consensus statement on the procedure within this setting, with the last update in 2014.
“Since we released the last consensus statement in 2014, same-day discharge after elective PCI has increased to 28.6% of all PCIs and 39.7% of radial PCIs in the USA,” said Cindy Grines (Northside Hospital Cardiovascular Institute, Atlanta, USA), chair of the writing group and SCAI past president. “Elective PCI in no-SOS settings have increased in volume and complexity. Concurrently, there have been

Immediate revascularisation non-inferior to staged procedure in acute coronary syndrome and multivessel disease
Immediate, complete revascularisation with percutaneous coronary intervention (PCI) in patients presenting with acute coronary syndrome and multivessel disease was non-inferior to staged complete revascularisation both in terms of safety and efficacy, findings of the BIOVASC randomised trial have shown.
ROBERTO DILETTI (ERASMUS MEDICAL Center, Rotterdam, The Netherlands) delivered insights from the trial at the American College of Cardiology (ACC) 2023 Scientific Session (4–6 March, New Orleans, USA), with the findings published simultaneously in The Lancet

The trial—conducted in 29 hospitals across Belgium, Italy, The Netherlands and Spain—randomised patients to undergo either an immediate complete revascularisation, whereby PCI of the culprit lesion was performed first, followed by other non-culprit lesions deemed to be clinically significant by the operator during the procedure, or they underwent a staged procedure, whereby the non-culprit lesions were treated within six weeks of the index procedure.
“A large proportion of patients with acute coronary syndrome are presenting with multivessel coronary artery disease, and there are a number of papers already demonstrating that a complete coronary revascularisation is probably the best way to treat these patients compared with a culprit-only lesion strategy,”
operators performing PCI in officebased laboratories (OBLs) and ambulatory surgery centres (ASCs) with positive outcomes. Thanks to improvement in PCI safety and several global studies in recent years, we now know that PCI at ASCs may improve access, patient satisfaction, and reduce costs.”
This expert consensus statement was endorsed by the American College of Cardiology (ACC), American Heart Association (AHA), British Cardiovascular Intervention Society (BCIS), Canadian Association of Interventional Cardiologists (CAIC), and Outpatient Endovascular and Interventional Society (OEIS).
According to SCAI:
● Elective PCI in settings with no-SOS has increased in volume and complexity (extending beyond the simple lesion recommendations in the 2014 document). In addition, PCI is now being performed outside of the hospital setting, in office-based laboratories and ambulatory surgery centres.
● Several new studies in the USA and abroad have demonstrated that PCIs performed at no-SOS centres have very low rates of complications and similar outcomes to PCIs performed at surgical centres.
● Despite increase in age, comorbidities, and lesion complexity, the rate of periprocedural complications has remained constant, or declined, with rates of emergency surgery as low as 0.1% in many series.
● Complex PCI, including unprotected left main, is being performed in some no-SOS centres, with no increase in major adverse cardiovascular events or emergency coronary artery bypass graft surgery (CABG) compared with PCI at surgical centres. The SCAI writing group proposes a new PCI treatment algorithm that expands the type of cases that can be performed with noSOS compared with its 2014 document, with consideration of the patients’ clinical and lesion risk, the operator experience (both recent and accumulated), and the experience and rescue capabilities of the site. In the USA, there are considerable financial savings (to insurers and Medicare) for PCI to be performed in ASC and OBL settings, thus out-migration of procedures from hospitals should be anticipated.
Diletti commented in his presentation, noting that the optimal timing for treatment remains unknown.
Investigators randomised 1,525 patients, with 764 undergoing the immediate revascularisation strategy, and 761 the staged approach. Patients had a mean age of 65 years and there was a majority male population in both groups. Endpoints were assessed at one year after the index procedure.
Among 1,506 patients at one year of follow-up, 7.6% of patients in the immediate complete revascularisation group had a primary endpoint event compared with 9.4% of those who received a staged procedure, Diletti reported (hazard ratio [HR] 0.78, 95% confidence interval [CI] 0.55–1.11, p-non-inferiority=0·0011).
There was no difference in all-cause death between the two groups (1.9% vs. 1.2%, HR 1.56, 95% CI 0.68–3.61, p=0.30), though more than twice as many patients in the staged treatment group (4.5%) had myocardial infarction (MI) than in the immediate treatment group (1.9%, HR 0.41, 95% CI 0.22–0.76, p=0.0045). Over 40% of the MIs in patients in the staged treatment group occurred during the interval before their second stenting procedure, Diletti said. The median interval between procedures for patients in the staged treatment group was 15 days.
Furthermore, unplanned additional stenting procedures were more frequent among patients in the staged treatment group (6.7%) compared with the immediate treatment group (4.2%), a significant difference (HR 0.61, 95% CI 0.39–0.95, p=0.030). The rate of cerebrovascular events was similar in the two groups (1.5% vs. 1.6%, HR 0.91, 95% CI 0.40–2.07, p=0.83). The median hospital stay was one
day shorter for patients in the immediate complete revascularisation group than for those whose procedure was staged.
“In patients with acute coronary syndrome and multivessel disease, an immediate complete revascularisation strategy was non-inferior to a staged complete revascularisation strategy in terms of the primary endpoint and was associated with a reduction in MIs and unplanned ischaemia-driven revascularisation,” Diletti told attendees of ACC in the concluding remarks of his presentation.
Limitations of the trial highlighted by the authors in their Lancet paper include that the patient population was overwhelmingly male, and that the trial was conducted in countries with populations that are “predominantly white”.
“Therefore, our findings might not pertain to different demographic environments with a higher representation of females and people of different races and ethnicities,” the investigators note.
The latter point was picked up by Diletti during discussion that followed his presentation at ACC, where he was asked by Dipti Itchhaporia (Hoag Hospital Newport Beach, Newport Beach, USA). “In my opinion this is reducing our ability to correctly detect the culprit lesions and even to correctly treat the lesion with optimisation,” said Diletti of the adoption of functional and imaging assessment in Europe, after Itchhaporia had picked up on the low utilisation in the trial.
Describing the findings of the BIOVASC trial as “very important”, Itchhaporia asked Diletti to reflect on the disparity in rates of MI reported in the two arms, to which Diletti offered up two explanations. “It might be possible that the operator misjudged the culprit lesion—so treated a lesion that was not the actual culprit, and the actual culprit gave a second event in the very early phase,” he commented. His second potential explanation was that “there are multiple unstable plaques during acute coronary syndrome, and then treating only the culprit does not complete the job”.
Issue69 | May 2023 21 Coronary Intervention
A large proportion of patients with acute coronary syndrome are presenting with multivessel coronary artery disease.”
Roberto Diletti
Cindy Grines
ACC, AHA and SCAI lay roadmap for interventional cardiology training
The American College of Cardiology (ACC), the American Heart Association (AHA) and the Society for Cardiovascular Angiography and Interventions (SCAI) have jointly issued a clinical document outlining competency-based training requirements for interventional cardiology trainees.
In a joint press release, the organisations said that this is the first document of its kind to define the training requirements for the full breadth of interventional cardiology for adults, which lay the foundation for coronary interventions, peripheral vascular and structural heart interventions.
The training pathway for cardiovascular fellows to gain the necessary experience in interventional cardiology includes:
1) A three-year general cardiovascular disease fellowship (successful completion consists of Level I competency in all aspects of cardiovascular medicine and Level II competency in diagnostic cardiac catheterisation to pursue interventional c ardiology training);
2) A one-year accredited interventional cardiology fellowship, the focus of which is coronary intervention
with the opportunity to gain procedural experience in various aspects of peripheral or structural heart (Level III competency); and
3) An option for additional postfellowship training based on the trainee’s career goals.
Level III training aims to give interventional cardiology trainees a well-rounded, competency-based education, including didactic instruction, clinical experience in the diagnosis and care of patients, and hands-on procedural experience.
Competency requirements are defined using the Accreditation Council for Graduate Medical Education’s six competency domains:
Medical Knowledge
Patient Care and Procedural Skills
Practice-Based Learning and Improvement
Systems-Based Practice
Interpersonal and Communication Skills
Professionalism
These competencies are essential for all interventional cardiology trainees, as well as additional select competencies in peripheral and structural interventions for trainees based on career focus, the statement adds. To support the attainment of competencies, the writing committee recommends a minimum of 250 interventional cardiology procedures. Of the 250 procedures, 200 should be coronary procedures, with the remaining 50 specialised in coronary, peripheral or structural heart interventions, which allows the fellows to customise training based on their
Guidelines set flexible framework to help interventional cardiology trainees meet career “benchmarks”
Theodore Bass (University of Florida, Jacksonville, USA), chair of the joint committee tasked with producing the ACC and AHA joint training guidelines tells Cardiovascular News about the process of drafting of the document, its intended scope, and the current state of training for interventional cardiologists.
“WE WANT TO CREATE A roadmap for training to produce not only cognitively but also procedurally competent interventional cardiologists, regardless of where they trained”, outlined Bass. Aware that the expanding speciality of interventional cardiology has bled into adjacent fields such as peripheral, congenital heart and structural interventions, Bass underlines that the presiding importance of the guidelines is to ensure trainees are “well-trained”, to enable “comfortable, portable and individual cardiology”.
The document elects three main areas, namely coronary, peripheral, and structural heart interventions. In brief, their proposed training pathway to gain the required amount of experience includes a three-year general cardiovascular disease fellowship, a year of accredited interventional cardiology fellowship, and a final option to participate in post-fellowship
training, based on the individual’s desired career trajectory.
The writing committee emphasises that Level III training aims to provide trainees with a well-rounded, competency-based education, including didactic instruction, clinical experience in the diagnosis and hands-on care of patients.

The writing committee also recommends a minimum of 250 interventional cardiology procedures. Of the 250 procedures, 200 should be coronary, with the remaining 50 specialised in coronary, peripheral or structural interventions.
“It is an issue—how can you designate competency in the surgical or procedural field? It centres around your exposure and experiences, however it is not a one-size-fits-all situation”, Bass explains. He states that some trainees may need more procedural experience, noting that the figures themselves
career goals. Adjunctive procedures related to physiologic assessment and intracoronary imaging are also required (25 of each). These minimum numbers are meant to provide trainees with exposure to a variety and spectrum of complexity of clinical case material and give supervising faculty sufficient opportunity to evaluate trainees’ competency.
Trainees must also acquire experience working as part of a multidisciplinary team to provide a holistic approach to patient care. The document also highlights the importance of cardiovascular health equity, mentorship and lifelong learning.
The “2023 ACC/AHA/SCAI Advanced Training Statement on Interventional Cardiology (Coronary, Peripheral Vascular, and Structural Heart Interventions)” is published simultaneously in the Journal of the American College of Cardiology, Circulation: Cardiovascular Interventions, and the Journal of the Society of Cardiovascular Angiography and Interventions
The statement was developed in collaboration with and endorsed by the American Association for Thoracic Surgery, the American Society of Echocardiography, the Heart Failure Society of America, the Heart Rhythm Society, the Society of Cardiovascular Anesthesiologists, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, the Society of Thoracic Surgeons and the Society for Vascular Medicine.
were meant to provide programmatic, procedural training guidance following in-depth discussions involving a diverse group of interventional cardiologists, interventional cardiology training directors and trainees and representatives from various professional societies and specialties involved in the cardiovascular field: “One number or another number might be uncomfortable for some, but the numbers are a means of guidance. I am very comfortable that we have come out with some thoughtful and very reasonable recommendations.”
Taking an almost “holistic approach” to patient care, the guidelines require trainees to work within a multidisciplinary team and emphasises the importance of cardiovascular health equity, mentorship and lifelong learning.
Echoing this, Bass reiterates that training is a “lifelong process”, observing that, despite entering the latestages of his interventional career, he is “learning things all the time”, including patient-facing communication and interpersonal skills.
Bass makes clear that pastoral skills
and system-based practices are also at the core of the proposed guidelines, making for a “better professional, no matter what field they enter”. He accentuates that for patients that perhaps speak another language, ensuring they “know what their choices are, and that they know they are in charge of their healthcare, so that they feel comfortable and not forced into agreement—we are not selling cars, we are here to educate them on very important aspects of their healthcare”.
Returning to the foundations of the guidelines, Bass reiterates the potential this roadmap has in providing a flexible framework to help trainees reach “benchmarks” and set “objectives and goals” in their career. He adds that interventional cardiology is a heavily subscribed specialty today, which has included an increasing number of female professionals entering the field. Overall, Bass concludes that the field is heading in the “right direction”, and in providing these guidelines they hope to improve the training pathway to produce well-rounded, multidisciplinary interventional cardiologists.
May 2023 | Issue69 22 Cardiology Training
We want to create a roadmap for training to produce not only cognitively but also procedurally competent interventional cardiologists, regardless of where they trained.”
VIEWPOINT







*Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the vascular arena Editorially independent Visit vascularnews.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription** Subscribe today Available in print and digital formats and through our social channels
Statin use associated with lower risk of stroke in patients with atrial fibrillation

A region-wide study in more than 50,000 patients with atrial fibrillation (AF) has found reduced risks of stroke and transient ischaemic attack in those who started statins within a year of diagnosis compared with those who did not. The findings were presented at the annual congress of the European Heart Rhythm Association (EHRA 2023, 16–18 April, Barcelona, Spain).
“Our study indicates that taking statins for many years was even more protective against stroke than short-term use,” said study author Jiayi Huang (University of Hong Kong, Hong Kong).
AF is the most common heart rhythm disorder, affecting more than 40 million people worldwide. Patients with the condition have a five times greater risk of stroke than their peers. Anticoagulant medication is recommended to prevent strokes in those with AF but does not completely eliminate risk. Statin therapy is widely prescribed to lower blood cholesterol and reduce the likelihood of heart attack and stroke. However, the benefit of statins for stroke prevention in patients with AF has been unclear.
This study evaluated the association between statin use and the incidence of stroke and transient ischaemic attack in patients with AF. The researchers used the Hong Kong Clinical Data Analysis and Reporting System to identify all patients with a new diagnosis of AF between 2010 and 2018. Participants were divided into two groups: statin users and non-users. Users had received statins for at least 90 consecutive days during the year after being
diagnosed with atrial fibrillation.
The primary outcomes were the combined endpoint of ischaemic stroke or systemic embolism; haemorrhagic stroke; and transient ischaemic attack. Patients were followed until the occurrence of the primary outcomes, death or the end of the study on 31 October 2022.
A total of 51,472 patients with a new diagnosis of AF were included, of which 11,866 were classified as statin users and 39,606 were non-users. The median age of participants was 75 years and 48% were women. During a median follow-up of five years, statin users had a significantly lower risk of all primary outcomes compared to non-users. Statin use was associated with a 17% reduced risk of ischaemic stroke or systemic embolism (hazard ratio [HR] 0.83; 95% confidence interval [CI] 0.78–0.89), a 7% reduced risk of haemorrhagic stroke (HR 0.93; 95% CI 0.89–
Novel score predicts heart failure following atrial fibrillation ablation
A SCORE BASED ON FOUR clinical and imaging parameters identifies the heart failure patients who benefit most from atrial fibrillation (AF) ablation, late-breaking data presented at EHRA 2023 have shown.
Medium-sized randomised trials have produced mixed evidence on the benefit of ablation in patients with heart failure, with outcomes depending on the selection and characteristics of patients. Therefore, there is uncertainty about which heart failure patients should be referred for ablation.
The European Society for Cardiology (ESC) Guidelines on AF recommend the procedure to reverse left ventricular dysfunction in AF patients when tachycardia-induced cardiomyopathy is highly probable. In addition, catheter ablation should be considered in selected AF patients with heart failure and reduced ejection fraction to improve survival and reduce heart failure hospitalisation.
“The tools to help clinicians determine who exactly these selected patients are and which patients have tachycardia-mediated cardiomyopathy are elusive and often subjective,” said study investigator Marco Bergonti
(Istituto Cardiocentro Ticino, Lugano, Switzerland). “Further evidence is needed to help stratify and identify those patients who will most likely benefit from AF ablation. The Antwerp score was developed to predict the response to ablation in heart failure patients with impaired (below 50%) ejection fraction.”
The score is based on four parameters: QRS width above 120 milliseconds (two points), known aetiology (two points), paroxysmal AF (one point) and severe atrial dilation (one point). Scores range from zero to six, with zero indicating a greater likelihood of recovery. A previous single-centre study showed that the score estimated the probability of left ventricular ejection fraction (LVEF) recovery after ablation.
The latest study aimed to externally validate the Antwerp score in a large European multicentre cohort. The researchers retrospectively identified patients with heart failure, impaired LVEF and AF who had an ablation procedure at eight centres in Europe. Participants underwent echocardiography to assess LVEF before ablation and 12 months
0.98) and a 15% reduced risk of transient ischaemic attack (HR 0.85; 95% CI 0.80–0.90).
The researchers also found that long-term statin use was associated with greater protection than shortterm use. Compared to those taking the medication for between three months and two years, patients using statins for six years or longer had a 43% lower risk of ischaemic stroke or systemic embolism (HR 0.57; 95% CI 0.54–0.61), 44% reduced likelihood of haemorrhagic stroke (HR 0.56; 95% CI 0.53–0.60) and 42% reduced risk of transient ischaemic attack (HR 0.58; 95% CI 0.52–0.64). These associations were consistent regardless of whether or not patients used anticoagulant medication and the type of anticoagulant.
Huang said: “These data support the use of statins to prevent stroke and transient ischaemic attack in patients with new-onset AF. The findings have important clinical implications particularly given
afterwards. The primary endpoint was sufficient improvement in ejection fraction at the 12-month echocardiography to be considered a “responder” to treatment. Responders were defined according to the 2021 universal definition of heart failure in patients with a baseline LVEF of 40–50%, an LVEF increase to 50% or more, in those with a baseline LVEF of 40% or below, an increase in LVEF at least 10% from baseline, and a second measurement of LVEF above 40%.
The study included 605 patients,
some 427 of whom (70%) were classified as responders and more likely to have positive ventricular remodelling (odds ratio [OR] 8.9, p<0.001), fewer heart failure hospitalisations (OR 0.09, p<0.001) and lower mortality (OR 0.11, p<0.001) compared to non-responders. The Antwerp score predicted LVEF improvement after ablation with an area under the curve of 0.86 (95% confidence interval [CI] 0.82–0.89; p<0.001). For total scores of zero, one, two, three, four, and five to six, the proportion of responders was 94%, 92%, 82%, 51%, 40% and 17%, respectively.
Bergonti said: “Based on our findings, patients with a low score (two or less) may benefit from early referral for catheter ablation, with a more than 90% chance of recovery. Patients with a high score (five or higher) have a very low expected recovery rate (below 20%) and hence may benefit more from alternative strategies such as aggressive rate control. Those in the intermediate zone (score three to four, expected recovery rate 47%) may benefit from further diagnostic tests such as cardiac magnetic resonance to improve their diagnostic assessment, as the presence of late gadolinium enhancement has been associated with less LVEF improvement.”
May 2023 | Issue69 24 Arrhythmia Updates
EHRA 2023
The tools to help clinicians determine who exactly these selected patients are and which patients have tachycardiamediated cardiomyopathy are elusive and often subjective.”








A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the cardiac rhythm field Editorially independent Visit cardiacrhythmnews.com and click ‘Subscriptions’ for e-newsletter subscription Subscribe today Available in digital format and through our social channels
Handheld ECG device scoops CX 2023 Innovation
Showcase prize
Judges of the CX 2023 Dragon’s Den-style contest—the finale of the Charing Cross (CX) Symposium (25–27 April, London, UK) Innovation Showcase programme—described the field of entrants to this year’s edition of the competition as the strongest line-up in its history.
The judging panel of physician-innovators selected HeartEye, a Netherlands-based developer of handheld electrocardiogram (ECG) devices, as the overall winner of the innovation prize, which comes with a £1,000 award. Honourable mention was given to four other entrants from the field of 12.

Peter Doevendans (UMC Utrecht, Utrecht, The Netherlands) gave an overview of the HeartEye technology in a short presentation entitled ‘ECG anytime, anywhere in 60 seconds’, describing it as a “digital transformation” for ECG acquisition. HeartEye is a pocket-sized device that can take clinical-standard ECG readings wirelessly, negating the need for a large, stationary ECG unit.
The technology, which has been developed with the
support of an Innovative Medical Devices Initiative (IMDI) grant from the Netherlands Organisation for Health Research and Development (ZonMw) is patented, and could be marketed to healthcare providers or direct to consumers within two years, Doevendans explained.
“We have been doing this for a number of years, and this was the toughest by far,” said judging panellist Robert Mitchell (Park City, USA), before the announcement of HeartEye as the winner of the prize. Euphrates Vascular, the developer a nano-scale endovascular system to address microvascular occlusion and no reflow, was among those singled out by the judges as being of particular interest. Presenter David Deaton (Medstar Georgetown University
Medtech sector welcomes extension of EU MDR deadlines
The European Union’s (EU) Council of Ministers has adopted a resolution to extend the deadline for the certification of medical devices under the Medical Devices Regulation (MDR).
PRODUCERS OF MEDICAL devices will have until 31 December 2027 for higher-risk devices and until 31 December 2028 for medium and lower-risk devices to meet the legal requirements.
The extension of the transition period will be granted under certain conditions, ensuring that only devices that are safe and that only manufacturers have already started the certification procedure will benefit from the additional time.
The regulation—which changes the way that medical devices are certified for use in the European market—first came into effect in 2021 with an initial three-year transition period, having been delayed by one year in 2020 due to the onset of the COVID-19 pandemic. However, challenges in the implementation of the legislation led to concerns about a potential shortfall in the availability of certain

devices, which prompted a rethink in the timetable for the regulation as proposed by the European Commission in December.
Adoption of the resolution by EU Council members, comprising ministers from each of the EU’s member states, means that the decision to extend the implementation period will enter into force on the day of its publication in the Official Journal of the EU.
“Today we have agreed on measures that will allow the industry to continue bringing essential medical devices to the market and ensure that patients
Hospital, Washington, DC, USA) told the judges that the Pulse NanoMed device extends the reach of current therapies, and has been given approval to begin a US Food and Drug Administration (FDA) investigational device exemption (IDE) trial in patients with acute ischaemic stroke.
Tilo Kölbel (University of Hamburg, Hamburg, Germany) introduced Mokita Medical, which has developed a technology to address air embolisation during procedures such as thoracic endovascular aneurysm repair (TEVAR) and transcatheter aortic valve implantation (TAVI), a cause of stroke and cognitive decline. The Mokita technology uses a gas-soluble fluid to eliminate air from devices, and is being developed into a disposable device that can be connected to the delivery system for a transcatheter procedure. The company is planning a first-in-man study for 2024, and anticipates commercialisation from 2026 onwards.
Mitchell praised Kölbel’s work in this area as being “really important and impactful”, and said that the entry had been one of the strongly considered options for the prize.
Another technology featured in the session included a non-invasive, wearable monitoring system for arteriovenous fistulas (AVFs), intended to aid early identification of failing AVFs. The innovation, presented by Ali Kordzadeh (Anglia Ruskin University, Braintree, UK) is worn like a wristwatch by patients to monitor venous outflow. Novel device coating materials were also exhibited, with Tony Simula (Mawson Lakes, Australia) detailing Bioinvisible, a drug-free polymer coating that could replace existing drug coatings in devices such as stents, vascular grafts and heart valves.
have safe access to medical devices,” Acko Ankarberg Johansson, Sweden’s minister for healthcare, was quoted as saying when the changes were confirmed in March.
MedTech Europe, which represents the continent’s device manufacturers, has welcomed the adoption of the amended transitional provisions, which it said will help mitigate the immediate risk that medical devices across all areas of medicine, which are still on the EU market, would no longer be available after May 2024.
“The amendment of the Medical Devices Regulations’ transitional provisions is a needed step forward to help ensure that more medical devices remain available to patients and healthcare systems across Europe.
This decision grants Notified Bodies more time to complete certification of more than 500.000 medical devices and accelerates efforts to certify innovative devices in the pipeline,” says Oliver Bisazza, CEO of MedTech Europe.
As soon as the amendment comes into force, MedTech Europe said that alongside its members it will work toward its implementation according to the new provisions and extended deadlines.
“In that regard, it is important that all stakeholders have an aligned and clear interpretation of the amendment, including the process for submitting applications to Notified Bodies, and how the extended validity of certificates can be concretely demonstrated,” the organisation’s statement adds.
May 2023 | Issue69 26 Medtech News
We have agreed on measures that will allow the industry to continue bringing essential medical devices to the market and ensure that patients have safe access to medical devices.”
We have been doing this for a number of years, and this was the toughest by far.”
Acko Ankarberg Johansson
Winner Peter Doevandans (third from left)







*Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the neuro interventional arena Editorially independent Visit neuronewsinternational.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription** Subscribe today Available in print and digital formats and through our social channels
Product News
Smart glasses guide remote support for pioneering TAVI procedure
The UK’s first remotely-supported transcatheter aortic valve implantation (TAVI) using smart glasses for remote guidance has been performed at the Essex Cardiothoracic Centre (CTC) at Basildon Hospital (Basildon, UK).
During the procedure, nurses who were wearing the smart glasses are guided remotely through a complex multi-step procedure by experts seeing exactly what they see. The glasses enable heart staff to receive real-time advice from anywhere in the world as they share a live feed from the operating theatre through a highresolution camera on the smart glasses.

Previously, staff were advised by a clinical expert from Boston Scientific present in the operating theatre, but they can now be remotely guided by the expert via the smart glasses from anywhere in the world, helping save time for patients and staff.
Rohan Jagathesan, medical director at the Essex CTC, said: “This new innovative equipment allows us to be more independent and to carry out more procedures, and means we can treat patients more quickly as we do not need a specialist to come to the hospital.We can also use the smart glasses to train our staff during a simulated procedure and further develop their skills, helping to improve the service we give to our patients.”
Abbott’s Epic Max stented tissue aortic valve gains US FDA approval
Abbott has announced that the US Food and Drug Administration (FDA) has approved the company’s Epic Max stented tissue valve to treat people with aortic regurgitation or stenosis.
Epic Max is designed to achieve excellent haemodynamics, or blood flow, and its low-profile frame facilitates potential future transcatheter
interventions for patients, Abbott said in a press release. This new valve is built on the Epic surgical valve platform, leveraging its long-term performance and durability.
“The aortic valve is one of the heart valves most commonly impacted by cardiovascular disease, frequently requiring replacement,” said Joseph Bavaria (University of Pennsylvania, Philadelphia, USA). “Abbott’s Epic Max design optimises blood flow for patients and has a low profile that makes future cardiac interventions, if necessary, easier.”
Adept launches Adducted Arm Scoop product for use during image-guided procedures
Adept Medical has launched the Adducted Arm Scoop—an improved solution for supporting the adducted arms of supine patients during imageguided procedures.
Adept’s new, “extremely durable” Adducted Arm Scoops are purposedesigned, providing security and comfort on narrow imaging tables, as per a press release.
Moulded with gentle, curved edging to reduce the likelihood of pressure injury and improve handling, the ergonomic design allows patients to comfortably rest their arms at their side with assurance. The ideal height, extending just above the arm, allows access and ease of cable and line management during procedures, Adept

also claims.
“After 10 years developing solutions for interventional cardiology/ radiology teams, listening to their needs, and observing how they care for their patients, it became obvious that existing arm supports could be improved immensely,” said Mike Oxborough, lead product designer at Adept. “Typically made from folded plastic sheet, they appeared to have very little thought put into design and were almost an afterthought, which is strange given they are an essential component for achieving patient comfort and safety on narrow imaging tables.
“Common complaints from nurses and MRTs [medical response teams] were that the arm boards would crack or break too easily, were expensive to replace, or were simply too flexible to support the heavy arms of larger patients. Improving durability was key, so selecting a material with superior chemical resistance was critical to prevent stress-cracks resulting from exposure to disinfectants used between patients.
“We wanted to create a product that would be more rigid, more durable, would look and feel like it had been designed for the job, and could be purchased for the same price or less than existing boards. With the Adept Medical Adducted Arm Scoop, we have achieved that.”
The Adducted Arm Scoops are quick and easy to install, according to the release. They simply slide under the mattress and are secured by the patient’s weight. Designed for use with existing lab and imaging equipment, they are compatible with any magnetic resonance (MR), computed tomography (CT) or C-Arm imaging tables.
OPN NC “super-highpressure” coronary
balloon launches in USA
SIS Medical AG has announced the launch of its OPN NC percutaneous transluminal coronary angioplasty (PTCA) dilatation catheter with ‘TwinWall’ technology in the USA.
“The product extends treatment options by high performance and safety as well as providing success rates where other balloons fail,” SIS Medical said of the device in a press release. The catheter is to be distributed by Worldwide Innovations & Technologies, which is headquartered in Lenexa, USA.
SIS Medical AG has developed the Twin-Wall technology, which it says provides a super-high-pressure resistance with a rated burst pressure of 35atm with a very low compliance. These features allow for preparation of challenging lesions prior to stenting without over-dilating of the blood vessel, the company says. The device can also be applied for post-dilatation of under-expanded stents.
OPN NC coronary balloon is the only super-high-pressure coronary balloon rated with 35atm which has
received 510(k) clearance from the US Food & Drug Administration (FDA), SIS Medical claims.
The first US procedure using the device was undertaken by Philippe Genereux (Morristown Medical Center, Morristown, USA). “I was extremely satisfied with the performance of the OPN NC balloon,” said Genereux. “We were successful in efficiently treating multiple patients with very complex and calcified lesions. The OPN NC balloon is an essential tool for operators treating resistant lesions, restenosis, or to treat acutely under-expanded stent, and is a true cost-effective technology compared to other devices.”
First US implant of LuxValve Plus transcatheter tricuspid valve performed in Detroit
The first transcatheter tricuspid valve replacement procedure in the USA using the LuX-Valve Plus (Jenscare) device for the treatment of symptomatic tricuspid valve disease has been performed by interventional cardiologists at Henry Ford Hospital, Detroit, USA.
Pedro Villablanca and Brian O’Neill successfully performed the first US procedure for Norma O’Connor, an 80-year-old Detroit-area woman with severe tricuspid regurgitation, when it became evident that no other means would work. Traditional valve replacement was not medically viable for the patient, who was deemed to be a high risk for surgery.
“These are patients with severe tricuspid regurgitation who have no other options available to them in the USA, based on the anatomy of their native valve and medical complexities,” said Villablanca.
Merit Medical expands SwiftNinja steerable microcatheter offering
Merit Medical has announced the expansion of its SwiftNinja steerable microcatheter product line. New sizes include a low-profile 2.4Fr distal diameter option in 125cm and new longer 150cm lengths.
The 180-degree articulating microcatheter is designed to provide access to challenging peripheral and coronary vasculature without the use of a guidewire. It is part of the broader Merit Vascular portfolio that includes sheath introducers, inflation devices and embolics.
“Unlike conventional microcatheters that are limited to a set shape, the steerable SwiftNinja is really a gamechanger,” said Jason Hoffmann (New York University, New York, USA), and consultant for Merit Medical. “The tip shape and angle can be changed in real time while inside a patient, allowing for shorter procedures with less radiation exposure. Without the need for a guidewire, there is the potential for significant cost savings as well—all of which benefit the patient. The SwiftNinja has paved the way for better embolization care.”
28 Market Watch May 2023 | Issue69
Adducted Arm Scoop
The Essex Cardiothoracic Centre team using a remote guidance headset for a TAVI procedure
Clinical News
February, Washington DC, USA), have demonstrated a low in-scaffold late lumen loss (LLL) rate and a good safety profile with no scaffold thrombosis at six months.
OpSens completes European enrolment in SAFE-TAVI study
OpSens has announced the successful completion of enrolment in the SAFE–TAVI clinical study, studying left ventricular rapid pacing using SavvyWire in transcatheter aortic valve implantation (TAVI), in Europe.

The study enrolled 120 patients with severe aortic valve stenosis and other conditions requiring a TAVI procedure in which left ventricular rapid pacing was considered necessary.
The SAFE-TAVI study was conducted in nine hospitals including eight centres across Spain and one in Canada, at Quebec Heart and Lung Institute—Laval University (IUCPQ). As the global principal investigator of the SAFE-TAVI study, Josep RodésCabau from IUCPQ oversaw and coordinated the principal investigators in their respective Spanish hospitals.
OpSens’ SavvyWire was used for left ventricular pacing to evaluate the potential benefits of eliminating the need for venous access, reducing procedure time, and avoiding potential complications associated with right ventricular pacing.

“We successfully used the SavvyWire in a large variety of anatomies, implanting valves from all major companies, including Edwards Lifesciences, Medtronic, Abbott, and Boston Scientific,” said RodésCabau. “Left ventricular rapid pacing is necessary to restrict the range of motion of the heart muscle during TAVI for optimal valve placement. The rapid pacing feature is important in SavvyWire’s performance in addition to providing real-time, accurate haemodynamic measurement during the TAVI procedure.”
OpSens received Health Canada and US Food and Drug Administration (FDA) clearance for SavvyWire in 2022. The SAFE-TAVI clinical study is being conducted as part of OpSens CE mark clinical strategy for the commercialisation of SavvyWire BIOMAG-I study
demonstrates low rate of in-scaffold late lumen loss at six months
Angiographic and clinical data from the BIOMAG-I clinical study, presented at the 2023 Cardiovascular Research Technologies (CRT) conference (25–28
Michael Haude (Rheinland Klinikum, Neuss, Germany), BIOMAG-I coordinating clinical investigator, presented the results of the study, a prospective singlearm study assessing the safety and clinical performance of the Dreams 3G (Biotronik) resorbable magnesium scaffold (RMS).
The trial showed a low proportion of malapposed struts after implantation, and at six months struts were no longer discernable. The intravascular imaging documented a preservation of the scaffold area with a low mean neointimal area.
Bioresorbable scaffolds have been developed to provide temporary mechanical support, while controlling neointimal proliferation over the vascular healing period and preventing long-term stent-related adverse events. Dreams 3G, Biotronik’s next-generation drug-eluting RMS, is comprised of a proprietary magnesium alloy and maintains a resorption time of 12 months, the company said in a press release. Compared to its predecessor Dreams 2G, it offers benefits such as reduced strut thickness and a higher radial strength, the press release adds.
“Dreams 3G did not only show a low in-scaffold late lumen loss rate, we could also see the preservation of the scaffold area with mostly wellembedded and resorbing scaffold struts,” said Haude. “This thirdgeneration scaffold is on the level of contemporary drug-eluting stents while providing the benefits of a resorbable scaffold.”
The prospective BIOMAG-I clinical trial assesses the angiographic, clinical and safety performance of Dreams 3G of 116 patients with de novo coronary artery lesions. Fourteen clinics in eight European countries are taking part in the BIOMAG-I clinical trial with 20% of the patients presented with nonST-elevation myocardial infarction (NSTEMI) and more than 75% with B2/C lesions.
Xeltis secures funding to progress clinical trials
Xeltis has raised €32 million in a Series D2 equity fundraise, backed by a
syndicate of current and new investors, which the company says will enable it to progress its clinical programmes into pivotal trials.
Investors include Grand Pharma, DaVita Venture Group, EQT Life Sciences, Invest-NL and others.
Xeltis’ proprietary endogenous tissue restoration (ETR) platform utilises an advanced polymer-based material which triggers the body’s natural healing response to regenerate the patient’s own tissue around it, forming new, living and long-lasting vessels and valves.
The company’s most advanced programme, aXess, is a vascular access graft for patients with chronic kidney disease (CKD) requiring haemodialysis. Xeltis is also pursuing clinical programmes in pulmonary valve replacement and coronary artery bypass grafts.
Alongside the equity fundraise, Xeltis and Grand Pharma have completed a license deal, covering Greater China, for aXess and other potential haemodialysis products developed under the same technology platform. Under the agreement, Grand Pharma will have exclusive rights to develop, produce and commercialise these products in Greater China.
Enrolment target met in ECLIPSE coronary atherectomy trial
Cardiovascular Systems has announced the complete enrolment of its 2,000-patient ECLIPSE coronary trial.
ECLIPSE is a prospective, multicentre, randomised clinical trial of approximately 2,000 patients with severely calcified coronary lesions in the USA. Half of the participants received orbital atherectomy prior to drug-eluting stent implantation, while the other half received conventional angioplasty, including specialty balloons, followed by drug-eluting stent (DES) implantation.
The trial is powered to demonstrate differences in the primary endpoints of post-procedural in-stent minimal crosssectional area (assessed by optical coherence tomography [OCT] imaging in a subset of approximately 400 patients), and in the clinical outcome of target vessel failure at one year.
Thousandth patient enrolled in SELUTION DeNovo coronary study
MedAlliance has announced enrolment of over 1,000 patients in its SELUTION DeNovo coronary randomised study. Recruitment is now a third of the way towards the target of 3,326 patients. SELUTION DeNovo compares the treatment strategy using a novel sirolimus drug-eluting balloon (DEB), Selution, versus a limus drugeluting stent (DES).
SELUTION DeNovo involves up to 70 participating sites across 15 countries. Patients are randomised before any vessel preparation to reflect current medical practice and to reduce bias. The objectives of the study are to
demonstrate non-inferiority at both one and five years, and superiority for target vessel failure (TVF) at five years.
Selution SLR consists of an angioplasty balloon coated with MicroReservoirs containing a mixture of biodegradable polymer and the antirestenotic drug sirolimus. The microreservoiris are intended to provide controlled and sustained release of the drug for over 90 days, similar to a DES, but without leaving behind a metal scaffold.

“This is a major milestone for the SELUTION DeNovo trial, as it is now the largest DEB study ever conducted,” said co-principal investigator Christian Spaulding (Paris Cité University, Paris, France). “The study is performed in a true all-comers population and is not just looking at small vessel artery disease. The results will have a major impact on clinical practice.”
Selution SLR was awarded a CE mark for the treatment of coronary artery disease in May 2020.
MedAlliance was the first drug-eluting balloon company to receive US Food and Drug Administration (FDA) breakthrough designation status. In addition to the below-the-knee (BTK) and superficial femoral artery (SFA) indications for which the company received FDA IDE approval in May and August 2022, MedAlliance received coronary in-stent restenosis (ISR) IDE approval in October 2022 and de novo coronary artery lesions approval on 6 January 2023.
CONFORMAL EFS study results presented at CRT 2023
Results from the CONFORMAL early feasibility study (EFS), evaluating the use of angiography compared to transoesophageal echocardiogram (TEE) for left atrial appendage (LAA) assessment have been presented at the 2023 Cardiovascular Research Technologies (CRT) conference (25–28 February, Washington DC, USA).
William Gray, co-director of the Lankenau Heart Institute and professor of medicine at Thomas Jefferson University (Philadelphia, USA) during the Left Atrial Appendage Closure Forum at CRT.
The CLAAS system is designed to seal the LAA in patients with nonvalvular AF to reduce the risk of stroke without the need for anticoagulants. Featuring a proprietary foam-based architecture, the implant addresses a wide spectrum of LAA anatomies with only two sizes.
29 Market Watch Issue69 | May 2023
SavvyWire
Selution SLR
aXess graft
Industry News
requested that the Nasdaq Stock Market LLC file a delisting application on Form 25 to report the delisting of the Common Shares of Neovasc from Nasdaq.
Abbott to acquire Cardiovascular Systems
Azeem Latib joins Supira Medical as medical director Supira Medical has announced the appointment of Azeem Latib (Montefiore Health Systems, New York, USA) as medical director.

Shockwave Medical completes Neovasc acquisition
Shockwave Medical has announced the completion of its previously announced acquisition of Neovasc.
The Neovasc Reducer system is a first-of-its-kind technology to address refractory angina. Refractory angina is a chronic condition in which a patient suffers chest pain that cannot be controlled by conventional therapies.
It is estimated that each year, in the USA and the European Union (EU) alone, up to 300,000 new patients with obstructive coronary disease who are ineligible for conventional revascularisation experience refractory angina, despite guideline-directed medical therapy.
In addition, it is estimated that up to another 500,000 new patients present with angina and non-obstructive coronary artery disease in the USA and the EU each year.
The Reducer has been granted Breakthrough Device designation by the US Food and Drug Administration (FDA), is CE-marked and is currently enrolling patients in the COSIRA-II study, a randomised clinical trial being conducted under an investigation device exemption (IDE) intended to support FDA approval for patients with coronary obstructive refractory angina.
Shockwave has acquired all of the outstanding common shares of Neovasc upfront by way of a statutory plan of arrangement.
As a result of the completion of the arrangement, Neovasc’s common shares will be delisted from the Toronto Stock Exchange. Neovasc has also
Conference calendar
16–19 May
EuroPCR 2023 Paris, France pcronline.com
18–20 May
Society for Cardiovascular Angiography and Interventions (SCAI) 2023 Scientific Sessions Phoenix, USA scai.org/education-and-events
Abbott and Cardiovascular Systems (CSI) have announced a definitive agreement for Abbott to acquire CSI. Under terms of the agreement, CSI stockholders will receive US$20 per common share at a total expected equity value of approximately US$890 million.
CSI is a leader in devices for atherectomy, the procedural use of which can help maximise the benefits of standard balloon angioplasty or stent treatments in restoring blood flow in complex arterial disease. CSI also has an early-stage pipeline of complementary vascular intervention devices in development.

“The acquisition of CSI will add new, complementary technologies to Abbott’s leading vascular device offerings,” said Lisa Earnhardt, executive vice president, Medical Devices, Abbott.“We are pleased to have reached an agreement with a leading global company that shares our passion for the development and commercialisation of innovative solutions for treating complex peripheral vascular disease and coronary artery disease,” said Scott Ward, CSI’s chairman, president, and chief executive officer.
Upon closing, the transaction is expected to be neutral to Abbott’s recently issued 2023 ongoing earnings per share guidance.
The transaction, which has been approved by the boards of directors of CSI and Abbott, is subject to the approval of CSI stockholders and the satisfaction of customary closing conditions, including applicable regulatory approvals.
JP Morgan Securities LLC is serving as financial advisor to CSI.
7–10 June The Structural Heart Summit (TVT) Phoenix, USA tvt.crfconnect.com
14 – 15 July Interventional Complications 2023 Seattle, USA complications2023.crfconnect.com
25–28 August European Society of Cardiology (ESC) Congress Amsterdam, The Netherlands escardio.org
The company credits Latib with having played a key role in the development of its novel percutaneous ventricular assist device (pVAD) technology, previously serving as a clinical advisor and attending the initial first-in-human cases. Latib will retain his current position as the section head of Interventional Cardiology and director of Structural Heart Interventions at Montefiore Health Systems.
“I am very excited to join the Supira team and believe it has an excellent offering with one system designed to support cardiovascular haemodynamics for both high-risk percutaneous coronary intervention (HRPCI) and cardiogenic shock (CS) patients,” stated Latib. “The unique pump is designed to achieve the required high continuous flow without the risk of bleeding often associated with these types of devices. This will advance clinical practice and enable more physicians to confidently perform these procedures.”
Latib’s professional expertise focuses on complex coronary interventions and structural heart disease, as well as a passion for new device innovation, Supira Medical said in a press release. He has authored over 700 peerreviewed articles and serves on the editorial board for several publications including the Journal of American College of Cardiology (JACC), Cardiovascular Revascularization Medicine and EuroIntervention. Latib is a member of several professional
organisations including the American College of Cardiology (ACC) and the European Society of Cardiology (ESC).
HeartBeam acquires LIVMOR assets
HeartBeam, a cardiac technology company that has developed the three-dimensional (3D)-vector electrocardiogram (VECG) platform for heart attack detection has announced the strategic acquisition of all assets from LIVMOR, a digital health solutions company providing a patientengaging remote monitoring system of critical physiological biomarkers. The acquisition extends HeartBeam’s reach in remote monitoring and detection with full ownership of an existing US Food and Drug Administration (FDA)cleared product.
Founded in 2016, LIVMOR developed the Halo+ atrial fibrillation (AF) detection system, which they believe to be the first FDA-cleared prescription wearable for continuous cardiac rhythm monitoring. The Halo system provides continuous monitoring of pulse rhythms for the detection of AF on-demand during the day and automatically overnight. LIVMOR’s technology was commercially deployed within the Veteran Affairs Healthcare System in Dallas, USA.
In February 2022, HeartBeam partnered with LIVMOR to build a HeartBeam-branded version of LIVMOR’s FDA-cleared Halo cloudbased software platform to connect physicians and patients. Utilising the Halo cloud-based platform allowed HeartBeam to meet its schedule for FDA submission of the HeartBeam AIMI software for acute care settings that provides a VECG comparison of baseline and symptomatic 12-lead electrocardiogram (ECG) to more accurately identify a heart attack.
Under the terms of the acquisition agreement, HeartBeam purchased LIVMOR’s intellectual property for an undisclosed amount, including three issued USA patents, LIVMOR technology, including the Halo AF detection system, LIVMORs business contracts and relationships as well as all Samsung watch and tablet inventory. Additionally, HeartBeam hired LIVMOR’s key technical employees.
7–10 November Cardiovascular Interventions La Jolla, USA cvinterventions.com
27–29 January 2024 Society of Thoracic Surgery (STS) Annual Meeting San Antonio, USA sts.org/meetings
4–7 October European Association for Cardio-Thoracic Surgery (EACTS) Annual Meeting Vienna, Austria eacts.org/annual-meeting
23–26 October TCT 2023 San Francisco, USA tct2023.crfconnect.com
11–13 November
American Heart Association (AHA) 2023 Scientific Sessions Philadelphia, USA professional.heart.org
19–21 November PCR London Valves 2023 London, UK pcronline.com/courses
9–12 March 2024
Cardiovascular Research Technologies (CRT) 2024 Washington DC, USA crtmeeting.org
30 Market Watch May 2023 | Issue69
2–4 October CX Aortic Vienna Vienna, Austria cxaortic.com
Neovasc Reducer
Azeem Latib

















*Available for US and EU readers only **Available worldwide A trusted provider of latest news, review of cutting-edge research, congress coverage and opinion from thought leaders A specialised news source in the cardiovascular field Editorially independent Visit cardiovascularnews.com and click ‘Subscriptions’ for complimentary print subscription* and e-newsletter subscription** Subscribe today Available in print and digital formats and through our social channels
CLASSICAL OPEN AND ENDOVASCULAR SOLUTIONS


CARDIAC, VASCULAR AND ENDOVASCULAR AORTIC ADVANCES

HOLD THE DATES
MONDAY–WEDNESDAY

2–4 OCTOBER 2023
IN PERSON AND VIRTUAL ANDAZ VIENNA AM BELVEDERE, VIENNA, AUSTRIA









AORTIC
cxaortic.com
VIENNA































































































































