













Peter Rugg-Gunn, Head of Public Engagement Babraham Institute

"This booklet showcases the entries to the Babraham Institute Proteostasis Design Competition. The range of posters and artwork developed by students is fantastic to see.
The thought, creativity, and research that has gone into all of them is evident to see. A huge thank you to all everyone who has shared their work!"





ByRomaisaH,WestcliffHighSchoolforGirls

Introduction
Proteostasis,orproteinhomeostasis,referstothedelicatebalanceofproteinsynthesis,foldingand degradationnecessarytomaintaincellularfunctionandhealth.Proteostasisisessentialforcellsurvival,as evenminordisruptionscanleadtoproteinaggregation,cellularstress,andultimately,disease.Autophagyis acellularrecyclingprocessandakeypartoftheproteostasisnetwork,breakingdowndamagedproteinsand organellestopreventtoxicityandsupportcellularresilience.Thisprocessiscarefullyregulatedby nutrient-sensingpathwayslikemTORandAMPK,whichrespondtonutrientandenergylevelsinthecell, adjustingautophagyasneeded.Targetingthesepathwayscouldenabletherapiestoboostautophagyin diseaseswhereproteostasisfails,suchasinneurodegenerativedisorders,includingAlzheimer’sdisease.
Autophagy,meaning“self-eating”inGreek,describes theprocessbywhichcellsengulfandbreakdown theirowncomponents.Duringautophagy,unwanted componentsareengulfedinvesicles,degradedby lysosomes,andrepurposed,whichsupports proteostasisbypreventingtoxicbuildup.
Macroautophagy:Themostcommontype,wherelarge cellularcomponentsareenclosedinadouble-membrane vesicle(autophagosome)anddeliveredtothelysosomefor degradation
Chaperone-MediatedAutophagy(CMA):Selectivetype wherespecificdamagedormisfoldedproteinsare identifiedbychaperoneproteinsanddirectlytransported acrossthelysosomalmembranefordegradation

Nutrient-SensingPathwaysandTheir ImpactonAutophagy
Severalnutrient-sensingpathwaysregulate autophagyinresponsetothecell’smetabolicneeds. Thisdynamicregulationensurescellscanadaptto changingenvironmentalconditions,preserving proteinhealthandoverallcellularfunction.
mTOR:Activatedinnutrient-richconditions,suppresses autophagytoallowforcellgrowthandproteinsynthesis
AMPK:Activatedduringlowenergyornutrientstress, promotesautophagytoconserveresourcesandmaintain energybalance
SIRT1:Respondstocellularstressandenergylevels, enhancingautophagytosupportproteinqualitycontrol andcellsurvival


AnimportantstudyontheroleofautophagyinADbyNixon etal.(2005)foundincreasedautophagosomesinthe frontoparietalcortexofpatientsusingimmuno-electron microscopy(Figure1).AstudybyPickfordetal.(2008) showedthatBeclin-1,aproteinthatplaysacentralrolein autophagy,issignificantlyreducedinADbrains.Additionally, autophagy-regulatinggeneslikePICALMarelinkedtotau accumulation,formingneurofibrillarytanglesinmodel organismssuchaszebrafishandDrosophila(Moreauetal., 2014).OveractivationofmTORC1signaling,anaminoacid sensingpathway,isalsoassociatedwithincreased amyloid-betaaccumulation,whileinhibitingmTORreduces amyloid-betalevels(Kioussisetal.,2021;Caccamoetal, 2010).Thesefindingsunderscorethepotentialoftargeting autophagyandnutrient-sensingpathwaysasstrategiesto mitigateproteinaggregationandslowADprogression.

Conclusion:Howdoesthisinformfuture directionsinproteostasisresearch?
Figure1-“Ultrastructural appearanceofautophagic vacuolesinADbrainandhighly purifiedsubcellularfractionsfrom mouseliver”byNixonetal. (2005)
mTORinhibition: Rapamycinhasshown promiseinanimalmodelsof AD.ByinhibitingmTOR, rapamycinactivates autophagy,leadingto enhancedclearanceof amyloid-beta.
AMPKactivation:

AMPKpromotesautophagy under low-energy conditions. Metformin has beenstudiedforitsabilityto enhance clearance of protein aggregates in neurodegenerativemodels
Nutrient-sensingpathways,particularlymTORandAMPK,areessentialfor regulatingautophagy,acellularprocesscriticaltoproteostasis,whichisoften disruptedinneurodegenerativediseases.InconditionslikeAlzheimer’s,impaired autophagyleadstotoxicproteinbuildupandcellularstress,butmodulatingthese pathwaysoffersapromisingstrategytomitigatetheseeffects.Overall,thefieldhas madesignificantprogressinuncoveringthedynamicsbetweenautophagyand proteostasisbuttherearestillmanyunansweredquestionsworthyoffurtherstudy.


RubinszteinDC,CodognoP,LevineB.Autophagy modulationasapotentialtherapeutictargetfor diversediseases.NaturereviewsDrugdiscovery 2012;11(9):709-730. doi:https://doi.org/10.1038/nrd3802OndaroJ, HaizeaHernandez-Eguiazu,MaddiGarciandiaArcelus,etal.DefectsofNutrientSignalingand AutophagyinNeurodegeneration.Frontiersin CellandDevelopmentalBiology.2022;10. doi:https://doi.org/10.3389/fcell.2022.836196



proteins across the kingdom
Animals don’t have cell walls. We rely on proteins to keep our body shapes, from skeletons to butterfly wings.

Iona , Beaulieu Convent School
The protein elastin is essential across our body, from allowing arteries to stretch as blood surges through them to allowing our skin to move as our musculoskeletal systems does. However this protein and its connections can be affected by illnesses as well as genetics. We often see the external effects first as skin sags alerting healthcare professionals to the dysregulation of elastin function.


1.Background


Proteostasisisthemechanismthatmaintainsthebalanceof proteinsynthesis,foldinganddegradationincells[1] Itiscrucialtocellularfunctionandoverallhealth.
Studieshavesuggestedthatcancercellsthriveonproteostasis [3][1]:
Theymodifyproteostasispathwaystosupportrapidgrowth andsurvival
Upregulationofmolecularchaperonestoenhanceprotein foldingandpreventaggregation
Activationoftheunfoldedproteinresponse
Therpeutictargeting[5]:
Increasedproteasomeinhibitorslikebortezomibinmultiple myelomacanselectivelytargetcancercellshighlighting theirvulnerabilitytoproteostasisdistruption
AIhasbeenfoundtobeausefultoolinresearchrelatedto proteostasisandcancer,forexample[4]:
Ithasrevolunionisedthepredictionofproteinstructures withhighaccuracy
Helpidentifymutationsthatalterfoldingandhowthese changescandrivecancer


2.Researchquestions
RQ1:Howcanproteostasisimpactthedevelopmentof cancer?
RQ2:Towhatextentcancancerbeeliminatedbymeans oftherapeutictargetingusingproteasomeinhibitor Bortezomib?
RQ3:TowhatextentisAIusefulintrackingtheeffects ofproteasomeinhibitorBortezomibincancer?
3.Methodology
Participantsaged18-40 100+participantsineachcategory

References:



Mainexperiment
Part1:Allparticipantswillbeexposedtotheproteosomeinhibitor-Bortezomib
Proteasomeinhibitor:Bortezomib
Part2:DatacollectedfromthetherapywillbefedintothesupervisedMLmodel
Supervisedmachinelearningmodel

Figure1:AItrackingproteostasisdifferencesinhealthyvscancerouscells

4.ExpectedOutcomesandImplications
Anticipatedfindings:
Somesensitivityincancerpatientswithregularproteostasis duetothedistruptionofnormalproteindegradation
Higherefficacyisexpectedincancerpatientswithenhanced proteostasisduetoincreasedrelianceonproteosomal degradation regularproteostasis morepronouncedthaninpatientswith potentiallyleadingtocelldeath

Minimalormildresponseinthecontrolgroup
Impact:
Significantefficacy cancertherapy validatesproteostasispathwaysfor developmentofmoredrugs

Morepersonalisedtreatmentstrategies
[1]Labbadia,J.,&Morimoto,R.I.(2015).Proteostasisandcancer:emergingconceptsandtherapeutictargets.JournalofBiologicalChemistry,290(32),18703–18711.https://www.jbc.org/article/S00219258%2822%2900370-2/fulltext[2]Hipp,M.S.,Park,S.-H.,&Hartl,F.U.(2014).Theproteostasisnetworkanditsdeclineinageing.Nature,509(7501),451–460.https://www.nature.com/articles/nature20144[3]Davenport,A.,&Torres,P.(2020).Proteostasisincancer:emergingtherapeuticopportunities.InCancerTherapeutics:Challengesand Opportunities(pp.50–67).Springer.https://link.springer.com/chapter/10.1007/978-3-030-38266-7_4[4]DeepMind.(2020).AlphaFold:Asolutiontoa50-year-oldgrandchallengeinbiology.Nature Retrievedfromhttps://www.nature.com/articles/s41586-020-03163-7[5]Adams,J.(2004).Thedevelopmentofproteasomeinhibitorsasanticancerdrugs.CancerCell,5(5),417-421.




Nowadays,peoplearemoreawareofthefactthatstressmightleadtoaging,butareweawareofthereason behind?Today,wewillbeexploringoneofthecausesofaging-proteostasis. Proteostasis,orproteinhomeostasis,isacomplexsystemofourbodythatmaintainsthebalanceofproteins intermsofsynthesis,folding,transport,anddegradation.Adysfunctioninproteostasiscanleadtoprotein aggregation(whichisthemisfoldingofproteins),whichthencancontributetomanyage-relateddiseases suchascancer,AlzheimerdiseaseandParkinsondisease.
Theheatstressresponseistriggeredbytheexposureof stressineukaryoticcellswithintheenvironment.
ResearchhasshownthatwhenHSRistriggered,heat shockproteins(HSPs)whichactasmolecular chaperonsaresynthesised.HSPprimarilyservesa cytoprotectivefunctionastheyhelpprotectcellsfrom theharmfuleffectsofstressbyhelpingotherproteins tofoldintotheircorrectshape,allowingthemtocarry outtheirfunction.Incasesofproteinaggregation, HSPsbindtothesemisfoldedproteins,helpingthemto refoldandthereforeprotectingcellsfromthetoxic effectsofaggregatedproteins.However,whenthese stressesbuildup,itcanleadtoproteindefect,leading towidevarietyofdisordersrangingfrom neurodegenerativediseasestoischemia-reperfusion injury.

Thisfigureshowsgenetically modifiedC.elegansworms thatexpressesafluorescent reporter for heat shock protein70(HSP70).
Theunfoldedproteinresponse(UPR)isthemaintenanceof balancedproteinfoldingintheendoplasmicreticulum(ER) whichisresponsibleforthesynthesisandtransportof proteinstootherorganelles.TheUPRisactivatedwhen unfoldedproteinsaccumulateintheER.Theactivationof theUPRinvolvesthreesignalingpathways,IRE1,PERK andATF6.
IRE1 - supports cellular health by reducing stress through adaptiveresponses,includingmRNAsplicinganddegradation. PERK-reducingproteinsynthesis,promotingstressresponse genes,andtriggeringapoptosisifstresscontinuestobuildup ATF6-enhancesthecell'sabilitytocopewithERstressby increasing the production of chaperones, promoting protein degradation

However,iftheUPRfailstoreduceunfoldedproteinsandthe UPRsignalremainshigh,itcanpossiblyleadtocelldeath.A studyin“ProteostasisandAging”showsthatstress-induced changesintheendoplasmicreticulum(ER)cancausea30% increaseinproteinmisfolding,leadingtoneurodegeneration.

Oxidativestressiscausedbytheimbalancebetween freeradicalsandantioxidantswhichcansometimesbe triggeredbyinfectionsandover-excercising,etc.With aging,oxidativestressalsotendstoincrease.Asa result,theexcessfreeradicalsarelikelytodamage bodycellsandtissues,specificallylipidsandproteins, resultinginthebuildupofmisfoldedproteins,which arecharacteristicsinage-relateddiseasessuchas AlzheimerdiseaseandParkinsondisease.Astudyin “OxidativeStress,ProteostasisandAging”showsthat increasedoxidativemodificationshavebeenshownto correlatewitha40%declineinproteostasiscapacityin agedcells.ItcanalsocausedamagetoDNAinhealthy cells,increasingthechancesofcancer.
Thefigureaboveshowsthatascellsage,theefficacyofautopagyandproteostasisdecreases, accompaniedbytheincreaseinoxidativestress.Whentheseprocessesdecline,damagedproteins canaccumulateincells,disruptingcellularfunction,increasingchancesinvariousdiseases.In conclusion,apositivecorrelanceisshownbetweenstressandage-linkeddiseasesandthereforeis importantforustostayawareofthesituationandtakeactionearlyon.Italsohighlightsthe importanceofproteostasisinourbodiesandsignificanceofhealthyagingbytakingaproactive approachtoyourhealth.
https://www.nature.com/articles/s41580019-0101-y https://www.sciencedirect.com/topics/me dicine-and-dentistry/heat-shockresponse#:~:text=The%20heat%20shock %20response%20(HSR,the%20proteome %20(Figure%20268.1). https://journals.plos.org/plosgenetics/arti cle?id=10.1371/journal.pgen.1003466 https://pmc.ncbi.nlm.nih.gov/articles/PM C9345296/ https://pmc.ncbi.nlm.nih.gov/articles/PM C8146082/ https://www.researchgate.net/figure/Aut ophagy-decreases-with-age-leading-toloss-of-proteostasis-accumulation-ofprotein_fig3_338582840 https://my.clevelandclinic.org/health/arti cles/oxidative-stress
Stress and its impact on age-related diseases
Jasmine T, Hills Road Sixth Form College

Iona , Beaulieu Convent School
The same virus that causes chickenpox can lie dormant in neuronal tissue due to a transcript stopping the expression of a gene needed for viral replication. This self-governing proteostasis mechanism on a viral scale can allow the virus to lie dormant for decades, and then blossom as flowers in a garden when conditions are just right.

Danielle , Beaulieu Convent School
Different cells have different roles to play. They need to be able to recognise each other as safe so not to trigger an immune response, but sometimes the proteins on surfaces can be altered or over-expressed leading to a loss of function or an autoimmune response.


Alexa M
Proteostasis, or protein homeostasis, refers to the cellular processes that maintain protein function, health and stability. It’s essential for cellular function, as proteins play a role in a variety of functions, from providing structural support to catalysing metabolic events.
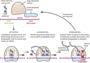
The idea of proteostasis covers protein synthesis, folding, trafficking and degradation (destruction) to prevent disruption to cellular functions.

A cell’s ribosomes link amino acids in an order specified by the genetic code to translate mRNA into proteins. Errors may occur during the translation process, producing proteins that are incomplete or incorrect for a cell’s function. Under stress, the cell’s ability to regulate the rate and timing of protein synthesis is essential. Certain proteins are synthesised more quickly in response to stress to control/lessen damage, while other synthesis processes may be stopped temporarily to conserve a cells resources. Cells have quality control mechanisms, during synthesis, that recognise and tag incorrectly synthesised proteins for degradation.


Synthesised proteins are needed to fold into specific shapes to function correctly. Proteins, called Chaperone proteins, bind to partially folded proteins to prevent incorrect folding or aggregation (when misfolded/damaged proteins clumps together to form aggregates).
Certain proteins require enzymatic modifications or cofactors to fold correctly. Some proteins require a complex environment to fold, for example Chaperonin complexes in humans, which provide an isolated environment for folding.

modifications happen in the Endoplasmic Reticulum (ER). The proteins are checked here for quality before the proteins proceed to the Golgi Apparatus. Properly modified and folded proteins are packaged in transport vesicles and are sent to the Golgi Apparatus for further soring and modifications, after which are directed to their function e.g. lysosomes, cell surface etc.
In the ER, there is quality control,
where only correctly modified and folded proteins are allowed to move forward in the trafficking pathway. The proteasome tags and retro translocates misfolded proteins or aggregates out of the ER for destruction.






Ubiquitin-Proteasome System (UPS) is the main cell breakdown process. Ubiquitin molecules bind to proteins that have been tagged to be broken down, guide them to the proteasome, where proteins are hydrolysed into peptides. The UPS is essential to prevent buildup by breaking down damaged or misfolded proteins.
Autophagy occurs when larger aggregates are needed to be broken down. This process involves the packaging of damaged or large aggregates in autophagosomes, which then merge with lysosomes.
Aggresomes are structures that act as short-term storage locations for misfolded proteins that can’t be broken down right away, to reduce the harmful consequences of protein aggregates.

Proteostasis is the foundation for cellular longevity and health. Proteostasis lowers the risk of disorders linked to protein aggregation and misfolding, promotes resistance against stress, and slows down the ageing process by controlling the quality and function of proteins


Leona , Beaulieu Convent School
Our ability to control our emotions pulls on the genetic code and how the environment leads to proteins being made or not. Dysregulation shows how our minds are not always controllable.

Deep within the central nervous system, coordinating neurones need to be able to receive chemical signals from adjacent cells and then send chemical messages out to other cells across synapses. If receptors are down-regulated through over-exposure to stimuli, these cells may become desensitised and not coordinate a response. This piece highlights the zonal expression of different proteins within a bipolar cell. Our ability to learn depends on neurones forming spatial connections with those around them, being held in the right places and in the correct orientation. This requires new proteins to be expressed with polarity, whether these are structural or involved in signalling. As we get older, some of these connections are lost and we struggle to learn new things and remember what we previously knew.

The key to cellular stability
Cambridge

Hannah, Beaulieu Convent School
Following damage our bodies must repair themselves to prevent infection or further damage. Cells close to the injury need to upregulate the proteins involved in repair which as often fibrous proteins. These proteins may be strong, but they put down in such quantities that the tissue changes, it will look different, it will feel different, it is scar tissue.

The right proteins in the right place
Danielle , Beaulieu Convent School
Our bodies are full of trillions of cells, each specialised for a particular role. Cells must be able to express only the correct proteins in order to support our bodies to survive and thrive. If some proteins are over- or under-expressed it could lead to a loss of function or perhaps the loss of regulation leading to cancerous cells dividing uncontrollably.


Vincent C
Cardiff Sixth Form College Cambridge


Proteostasisisaregulatorycellularmechanismthathelpsmaintainthestability,foldingand degradationofproteinsinourcells.Proteostasisiscrucialinthepreventionofdiseasessuchas cancer,neurodegenerativedisorders,andmetabolicsyndromes.
Proteostasisplaysadynamicroleintumourgrowthinhibitionand progression.Disruptingproteostasiscancauseanaccumulationof misfoldedproteins,whichisassociatedwiththemutationofnormal cellsintocancercells.However,heatshockandunfoldedprotein responses(HSR&UPR),whichareproteostasisstressresponses tomisfoldedproteinaccumulation,canpromotetumourgrowth. Thisposterwillmainlyfocusontherelationshipbetweenproteostasis stressresponsesandtumourgrowth.


The Endoplasmic Reticulum (ER) is an organelle in human cells responsible for protein synthesis, folding, modification and quality control. ER stress is incurred when the ER becomes overwhelmed and unable to properly fold and process proteins. This can lead to a buildup of misfoldedorunfoldedproteins,thelongerthemisfoldedproteinsandERstressispresent,the higherthechanceoftumourigenesis,theformationofmalignanttumours.
In humans, the unfolded protein response (UPR) signal pathway is initiated in response to ER stress to maintain proteostasis by orchestrating proper protein folding and degradation of unfolded/misfolded proteins. Surprisingly, UPR has also been discovered to be linked with cancer development, as the UPR is often found to be upregulated in cancer, suggesting that UPRs may serve a tumorigenic-promoting role by increasing the protein folding capacity and prolonging cancer cells’ resistance to anticancer drugs.
Like UPRs, heat shock responses (HSRs) assist in proper protein folding and prevent the accumulation of misfoldedproteins,maintainingcellularproteostasisand reducingtheriskoftumorigenesis.However,thereisalso overwhelming evidence that HSR is linked to tumourigenesis, with heat shock proteins (HSP) being expressed at high levels in cancers to form fostering environmentsthatareessentialfortumourdevelopment. In addition, HSPs also participate in the evolution of cancertreatmentresistance.

1. Ojha R, Amaravadi RK. Targeting the unfolded protein response in cancer. Pharmacol Res. 2017 Jun;120:258-266. doi: 10.1016/j.phrs.2017.04.003. Epub 2017 Apr 8. PMID: 28396092;PMCID:PMC5542584.
2. Calderwood SK, Gong J. Heat Shock Proteins Promote Cancer: It's a Protection Racket. Trends Biochem Sci. 2016 Apr;41(4):311-323. doi: 10.1016/j.tibs.2016.01.003. Epub 2016 Feb 11. PMID: 26874923; PMCID:PMC4911230.



For many years we have understood how DNA is transcribed into mRNA which is translated into proteins, but we have only scratched the surface. We are now developing our understanding of how genes are regulated, how mRNA polynucleotides are altered and how polypeptides are twisted and glycolated in different specialised cells.
Thank you to all those involved in judging the Design Competition :
Ian McGough
Becky Gilley
Amar Soory
Ellie Griffiths
Sophie Sanford
Thank you to Babraham Research Campus for providing the venue for the Babraham Institute Sixth Form Conference, where these design competition entries were displayed.
Thank you to all the guest speakers at this year’s conference:
Nick Ktistakis
Yoon Hee Choi
Nicola Watts
Khashayar Khoshrou


