

We make discoveries that help us understand human biology and improve lifelong health




We make discoveries that help us understand human biology and improve lifelong health


Welcome to this overview of the Institute’s achievements across 20232024. This period saw the Institute undergo a quinquennial review (QQR) by BBSRC in which we reported on the achievements from our 20172024 funding round and presented our future research vision and plans for 2024-2028. This is summarised in the following section: Life sciences research for lifelong health. You will also find feature articles on our three strategic research programmes, sharing perspectives from Gavin Kelsey (Epigenetics), Martin Turner (Immunology) and myself (Signalling).
The QQR was a huge effort from teams across the Institute and I am immensely proud of the outcomes. Our research programmes were all ranked as ‘high international’ or 'internationally leading’ and we received an award of £48m in strategic funding from BBSRC to support our research from 2024-20281. I am excited about our overarching theme of ‘proteostasis across the life course’, which connects our three research programmes and shapes our evolving global collaborations.
Beyond the QQR, this review also features more personal stories from across the Institute, sharing
our big ambitions, reflecting on going further, doing more and our commitment to support success and make a difference. These demonstrate the achievements, excellence and dedication from teams within our community
2023 and 2024 were also significant milestone years. 2023 marked the 75th anniversary of the creation of the Institute (then the Institute of Animal Physiology) in 1948 and 30 years since the Institute’s reshaping as the Babraham Institute in 1993. 2024 marked the 30th anniversary of the Institute’s annual Schools’ Day – more on that in the feature on page 37.
People
During 2023 and 2024 I was delighted to welcome two new appointments to the Immunology research programme. Professor Yiliang Ding became our fifth honorary group leader2. Professor Ding, based at the John Innes Centre in Norwich, brings expertise on the dynamics of RNA structure and this partnership is shaping how we study gene regulation across each of the research programmes.
Professor Kai-Michael Toellner joined us in 2024 as a senior group leader3. Kai’s interests in immune cell differentiation, antibody production and long-term immune memory interface strongly with several of our Immunology groups and are stimulating exciting new areas of discovery. Learn more about Kai and his research in the feature on page 35.
Signalling group leader, Dr Hayley Sharpe, received tenure with unanimous recognition of her transformative research on protein tyrosine phosphatases and how they might be targeted for therapeutic use. As Chair of equity4success Hayley also led the successful renewal of the Institute’s Silver Athena Swan award for 2024-20294.
Professor Dame Linda Partridge became Chair of the Institute Board in February 2023 and we welcomed six new Trustees to the Board in September 2023, bringing scientific expertise aligned to the Institute’s research and operational expertise in personnel strategy and financial management5. I would like to express my immense appreciation for the contributions from our Trustees as a whole and share my grateful thanks to the Trustees who stepped down in this period for their service and dedication: outgoing Chair Professor Peter Rigby, and Trustees Professor Peter Parker, Professor Nic Jones, Dr Lynne Gailey and Mr Geoff Braham.
Our Science
At the Institute we are united by a passion for discovery and innovation to bring benefit. These attributes were demonstrated by our success in securing four BBSRC Pioneer Awards in 2023. Awarded to Dr Ian McGough (Signalling), Dr Teresa Rayon, Dr Jon Houseley and Dr Maria Christophorou (all Epigenetics group leaders), these ambitious research projects promise paradigm-shifting progress to potentially shape therapeutic strategies to prevent protein
aggregation (McGough lab), reveal the fundamental molecular mechanisms that control developmental tempo (Rayon lab), develop approaches to beat the acquisition of drug resistance in pathogenic fungi (Houseley lab) and explore the function of histone proteins as signalling messengers (Christophorou lab)6.
Within our Immunology programme, Dr Michelle Linterman’s research continues to uncover critical insights into the causes of diminished immune responses with age. With a BBSRC International Partnering Award, she is collaborating with the Malaghan Institute of Medical Research in New Zealand to create more effective mRNA vaccines for older people7. Michelle also joined the global IMMPROVE project to explore how current and future vaccines can be enhanced to provide greater protection from respiratory pathogens, such as SARS-CoV-2 virus, ‘flu and respiratory syncytial virus.
Our Signalling research programme saw discoveries that provide vital underpinning knowledge for future therapeutic approaches. The David lab identified a mechanism preventing the toxic effects of protein aggregation in specific tissues of C. elegans when normal methods of molecular monitoring fail8. Building on this finding may inform therapeutic interventions for diseases of protein aggregation as well as ways to prevent undesirable protein aggregation that occurs with age.
A long legacy in PI3K signalling continued with a paper by Dr Tamara Chessa (Stephens-Hawkins lab), identifying a new driver of PI3K signalling and cancer growth in a mouse model of prostate cancer9. Tamara was awarded the 2023 Sir Michael Berridge Prize for this work, which provides a potential new avenue for therapeutic targeting of the PI3K signalling pathway in human cancers with minimal predicted toxicity.
In the Epigenetics research programme, the Houseley lab made exciting new discoveries about the link between diet and ageing, showing that in yeast, healthy ageing can be achieved by dietary change without caloric restriction10.
In preparing for the Institute’s QQR we had the opportunity to reflect on our vision for a culture to enable research excellence, inclusivity and wellbeing for all. We have defined this vision as a commitment to sustain an inclusive community, in a positive environment that values dignity, inclusion, openness and integrity, for everyone to thrive and take pride in their contribution to the delivery of world-class bioscience research that benefits society.
As part of this work we undertook a culture consultation which has created a roadmap to place team science at the heart of our strategy and culture. This work underpins our commitment to provide an equitable environment, where everyone can thrive, be themselves and do their best work.
The Institute’s five strategic initiatives; equity4success, research integrity, technician commitment, wellbeing, Green Labs, are leading the way. I was very proud of the renewal of our Silver Athena Swan award and our new Technician Commitment action plan, which sets out our ambitions for 20242027. The Institute’s Green Labs team, drawn from across the Institute, have been instrumental in ensuring that our science and operations are more sustainable, and this was recognised by a Platinum Green Impact award in 2024.
The QQR was a crucial opportunity to reflect on the impact delivered by the Institute’s fundamental research in the last cycle of strategic investment from BBSRC (2017-2024). This included:
- 745 research papers, many describing ground-breaking research and technological advances; these papers have received 48,000 citations.
- establishing a transient cellular reprogramming method that can rejuvenate cells without affecting cell identity.
- a better understanding of the cellular process of autophagy, helping researchers accurately monitor and distinguish between distinct autophagy processes in fundamental and translational research.
- significant advance in our understanding of the effects of age on the immune system and how to improve vaccines and vaccination
8 Researchers discover tissue-specific protection against protein aggregation
strategies to confer better protection of the older population. This work included validation of the vaccination strategy for the Oxford – AstraZeneca COVID-19 vaccine.
- three spin-outs arising from 21 patent families: Enhanc3D Genomics, Aila Biotech and Elithium Bio.
- 73 researchers trained to PhD level and equipped to contribute to the UK’s bioeconomy through research and innovation.
In 2023-2024 we were pleased to launch the UK Proteostasis Network, bringing together researchers across the UK for exchange and collaboration. The Network’s inaugural conference was held at Babraham in May 2024 and we are excited to see the Network continue to grow and take shape.
Peter Rugg-Gunn, a senior group leader in the Epigenetics research programme and Head of Public Engagement (PE), and our PE team led a foundational public dialogue on the use of human embryos in research as part of the Human Developmental Biology Initiative (HDBI)11. The PE team also did a fantastic job in coordinating our annual summer Research Access Programme and delivering the Institute’s 30th Schools’ Day. Both activities focus on reaching underserved audiences to support equitable access to science.
The Institute is the academic heart of the Babraham Research Campus (BRC). A recent Impact study on the Campus highlighted the many ways the Institute supports the commercial success of campus companies12. Beyond engaging with campus companies through consultancy, collaborations and access to our cutting-edge research facilities, there has been a fruitful shared endeavour between the Institute’s Knowledge Exchange and Commercialisation (KEC) team, Babraham Institute Enterprise (BIE Ltd., the commercialisation arm of Babraham Institute) and Babraham Research Campus Ltd. to establish a thriving ecosystem of entrepreneurship, innovation and knowledge exchange. In these ways, we support innovation, accelerate translation, nurture enterprise and entrepreneurship with wider impacts for UK bioscience.
10 Yeast studies show that diet in early life matters for lifelong health
11 Public support for extending the 14-day rule on human embryo research indicated by foundational dialogue project
12 Babraham Research Campus impact report illustrates Campus’s supportive ecosystem as key to commercial success











2023 was an important year of reflection, both internally and externally. The Institute Assessment Exercise, where we outline our progress and future ambitions to our strategic funder BBSRC, provides the structure for this regular quinquennial review; a sort of science stock-taking exercise.
The overarching aim of our 2024-2028 research plans is to understand the cellular mechanisms that promote resilience across the life course. Lifelong health reflects successful adaptation to a lifetime of challenges (resilience) whilst the breakdown in physiology with age reflects failures in this adaption and/or maladaptive responses that can promote ageing, such as chronic inflammation.
Our future plans build on progress in uncovering the biological basis of ageing, sets out new areas of discovery and defines how our work is relevant to the global challenge of an ageing population. It specifically aligns with the BBSRC’s Strategic Priorities of Ageing, Health across the Life Course and Bioscience for an Integrated Understanding of Health as well as wider government priorities for health.
How we operate to achieve this mission our research culture is equally important. Delivery of excellent science depends on the
development and support for our people, a culture that supports innovation and collaboration, an outward-looking mindset and an inclusive community. Our vision for the Institute is to create a supportive and dynamic environment where people, ideas and research thrive.
Discovery research to underpin health innovations
Our three strategically-funded research programmes will leverage world-leading expertise and technical capabilities to make the discoveries that will ultimately feed forward into drug and vaccine development.
Research in our Signalling programme will identify new adaptive mechanisms to promote resilience to the collapse in proteostasis, damage to lysosomes and dysregulation of oxidant signalling; these stresses are all recognised 'drivers' of age-related functional decline and associatedchronic illness.
Our Epigenetics research programme will define the role of intracellular metabolites in epigenetic resilience and adaptability, the factors controlling establishment and robustness of epigenetic states at key stages in development, how they adapt over the life course, and the potential for interventions that safeguard chromatin states.
Our Immunology programme will study the germinal centre response and the formation of B and T cell memory to shed new light on the decline in immunity across the life course and ways to mitigate this. A unique focus on RNA regulation in immune cell biology will enable innovations relevant to emerging therapeutics in this field.
Proteostasis across the life course – a unifying theme
One essential aspect of resilience is proteostasis: how the integrity of the cell’s protein production, regulation and disposal processes is maintained. Proteostasis breakdown is a lifelong challenge, a hallmark of ageing, a driver of age-related physiological decline and of age-related disease. Our 2023-2024 research plans incorporate proteostasis as a common, over-arching theme of the Institute’s three research programmes.
Our Signalling programme will define links between age-associated declines in poly-ubiquitylation and increases in protein aggregation at a proteomewide level across organisms, tissues, sexes, and cell types, aiming to identify vulnerabilities in key protein quality control steps and signalling pathways that drive proteostatic breakdown. Our Epigenetics programme will define how altered cellular metabolism impacts ageing phenotypes, in particular proteostasis,

and whether developmental delay and metabolic disruption in early life predispose to loss of proteostasis in aged mouse tissues. Our Immunology programme will study regulated mRNA translation and its adaptation to specific differentiated cell states such as antibody secreting plasma cells, part of a wider study of factors affecting resilience to withstand infection and the molecular mechanisms underlying inflammation.
Partnerships are key to our mission and consequently collaboration and knowledge exchange are central to our approach. The Institute remains integral to the success of the Babraham Research Campus (BRC), one of Europe’s most successful Innovation Campuses. Through a diverse range of interactions—collaborations, scientific services access, consultancies and training—the expertise of Institute staff supports commercial research on the Campus. In several instances we also lead culture change, such as through the Green Labs initiative and our LGBTQ+ Network.
The Institute is also active in bringing together and mobilising researchers across themes. In 2024 we led the creation of the UK Proteostasis Network; this makes connections that will accelerate our understanding of how proteostasis supports health but is also relevant to plant proteostasis and resilience of crops.
With our plans for 2024 to 2028 we are excited by the opportunity to expand our global collaborations, both with academic partners and commercially in the biotech and pharma sectors to accelerate the exchange and translation of our discovery research for public benefit.
OUR COLLABORATIVE ACADEMIC PROJECTS
UK AND WORLDWIDE
68
ACTIVE PROJECTS
21 COUNTRIES
114 ORGANISATIONS
Working with commercial partners
IP AGREEMENTS
PATENTS CONSULTANCIES
COLLABORATIONS
INNOVATION OPPORTUNITIES DISCLOSED
People we’ve trained in our scientific facilities this year
Core ISP grants & BBSRC non-grant income
Income from services provided by the Institute
Competitively awarded grant income in 2023
BBSRC additional income (including business cases, equipment etc.)
Value of all grants awarded in 2023
UK funders
International grants*
Value of UK grants awarded in 2023
£27.5M £6.4M £6.7M
Wellcome Trust
BBSRC
MRC
UKRI
Other
Milner Therapeutics Institute
8
29
ONLINE PUBLIC ENGAGEMENT EVENTS IN PERSON PUBLIC ENGAGEMENT EVENTS
158 PEOPLE ENGAGED
6
1,600
81 INVOLVING STAFF AND STUDENTS
95
14
PhDs COMPLETED

OUR COLLABORATIVE ACADEMIC PROJECTS
Working with commercial partners
IP AGREEMENTS
PATENTS CONSULTANCIES
COLLABORATIONS
INNOVATION OPPORTUNITIES DISCLOSED
People we’ve trained in our scientific facilities this year
Core ISP grants & BBSRC non-grant income Income from services provided by the Institute BBSRC additional income (including business cases, equipment etc.)
Competitively awarded grant income in 2024 £32.6M
Value of all grants awarded in 2024
15 ONLINE PUBLIC ENGAGEMENT EVENTS
33 IN PERSON PUBLIC ENGAGEMENT EVENTS
2,500 INVOLVING
155 PEOPLE ENGAGED
Innovate UK £4M UK funders
International grants*
Value of UK grants awarded in 2024
BBSRC
MRC
Milner Therapeutics Institute
8
79
56 STAFF AND STUDENTS
23





Institute receives £48m strategic investment from BBSRC
Strategic BBSRC funding supports the Institute to undertake three core programmes of work: epigenetic control across the life course, cellular responses to stress, and immunity, resilience and repair. All three programmes have a strong focus on the mechanisms that drive ageing and will provide new insights into age-related disease that the Institute will progress with biotech, pharma and clinicians.

Public dialogue first to collect views on 14-day rule
As part of the Human Developmental Biology Initiative, the Institute’s Public Engagement team and members of the Epigenetics research programme participated in public dialogues to collect opinions and attitudes towards embryo research. This foundational piece of work was an initial step towards wider UK public engagement on this topic and provides direction to future public consultations and research.
Yeast study shows ageing trajectory set in early life
The Houseley lab showed that the content of diet in yeast, rather than caloric intake, influences yeast health in later stages of their lifecycle. While their results cannot be directly translated into humans, these findings show that healthy ageing can be achieved by optimising diet if changes are made at an early stage in life.
Athena Swan Silver Award renewed
In 2024, the Institute received a renewal of its Silver Athena Swan Award. Activities at the Institute include establishing the UK’s first Roving Researcher position, continued provision of excellent childcare, promotion of cultural understanding in collaboration with Muslim staff, and training around the impact of inclusive language.




The Institute and the Malaghan Institute of Medical Research in New Zealand are collaborating to investigate how ageing affects the germinal centre response to mRNA vaccination, supported by a BBSRC International Partnering Award. By combining expertise from the Linterman and Turner labs with the Malaghan Institute’s vaccine platform, the project aims to improve mRNA vaccine effectiveness for older people.

Public engagement joins forces with YouthSTEMM
The Public Engagement team have expanded their ‘BioInspire’ programme by partnering with the YouthSTEMM Award, sponsoring 60 young people and providing webinars, workshops and in-person experiences to support them to achieve their award.
LGBTQ+ Network established to support colleagues and allies
Established by staff in 2023, the LGBTQ+ Network aims to raise awareness and the profile of LGBTQ+ issues and staff in the workplace, help remove barriers to inclusion and ensure that there are visible role models at different levels of the organisation. The network has organised coffee mornings and events for LGBTQ+ staff and allies.
The Institute’s Nursery team were recognised in the Cambridge Regional College’s Apprenticeship Awards 2024 as Employer of the Year (Care, Health and Early Years category) for their support of apprentices who join the Nursery team as part of their training. The Nursery has supported a total of eight apprentices from CRC and other training providers since welcoming their first apprentice in 2016.




The Institute undertook a series of activities, workshops and surveys to agree an organisational definition of ‘team science’, evaluate workplace culture and set a vision for how the Institute can be a place for everyone to thrive. The Institute Culture Consultation project culminated with the production of a roadmap for team science with ‘quick win’ activities and longer-term deliverables spanning the next five years.

Scientists contribute to stem cell-based embryo models guidelines
Peter Rugg-Gunn, group leader and Head of Public Engagement, and Kathy Niakan, honorary group leader, helped develop the first ever UK guidance and an oversight process for the generation and use of stem cell-based embryo models in research. The guidelines were developed by a working group of experts from a range of institutions across the UK, representing world-leading expertise in developmental biology.
30th Schools’ Day sees record number of students experience lab life
The Institute celebrated its 30th Schools’ Day in 2024 – an annual event where researchers guide secondary and sixth-form students through hands-on lab projects and showcase the variety of careers in science. The 30th year saw the most schools and students attending from the event’s history, and the highest proportion of students from schools in areas with historically lower access to similar opportunities.
The Institute achieved a Platinum Award from the SOS-UK Green Impact programme in 2023 and 2024 in recognition of activities across the Babraham Research Campus including revamping the bike repair station, upgrading inefficient ultra-low freezers, sending over 100 items of equipment for reuse via the UniGreen scheme, and planting hedges and sowing wildflower seeds on campus.


Researchers discover tissue-specific protection against protein aggregation
Della David and her team discovered a safety mechanism that acts to lower levels of protein aggregation in C. elegans pharyngeal muscles, but is not active in body-wall muscles. By further understanding how some tissues employ protective protein control pathways, the research may help develop future strategies that prevent protein aggregation in vulnerable tissues during ageing.
A new Campus impact study found that the powerful combination of the Institute’s strategic research focus, state-of-the art facilities and prowess in innovative methodology development, together with the Campus’s translational culture and collaboration opportunities is a defining factor in what the Campus can offer to companies.

Institute receives Technician Commitment Impact Award
The Institute received a Technician Commitment Impact Award, recognising three years of work to increase the visibility, recognition, career development and sustainability for technician skills and roles. The Technician Commitment steering group and cohort members have published the action plan for the next three years at the Institute.

Institute launches Proteostasis Network UK
The Proteostasis Network was launched by the Institute at an inaugural conference in May 2024. With over 170 attendees, representing a diversity of research areas and roles, the event was a chance for the new community of UK-based researchers working on proteostasis-related areas to exchange research news, expertise and knowledge.


Hayley Sharpe, a group leader in the Signalling research programme, was awarded tenure and received an ERC Consolidator Grant. The five-year project will focus on uncovering how T cell receptor binding initiates the signalling events that lead to various T cell behaviours. Understanding this mechanism is important for T cell-based therapies in cancer as well as extending our understanding of cell-cell communication more widely.



Martin Turner, Head of the Immunology programme, received a Wellcome Discovery Award to study T cell regulation by RNA binding proteins. The funding will support the creation of new research tools and approaches to define the dynamics of protein and RNA interactions, with the aim of providing deeper insight into the molecular regulation of the immune system.

Michelle Linterman, senior group leader in the Immunology programme, joined the GSK Immunology Network to connect the lab’s knowledge about long-lived immunity with GSK’s longstanding interest in understanding how to harness the power of the immune system to improve health. The Network embeds academics in GSK’s UK research and development hub to exchange ideas and expand knowledge.

Jo Durgan, senior research associate in the Signalling research programme and chair of the Green Labs Steering Group, was a runner up in the Green Sustainability Hero award from SOS-UK (Students Organising for Sustainability). Jo was recognised for her dedication in championing sustainability and leadership in spearheading sustainability initiatives on the Babraham Research Campus and within the local community.
Rachael Walker, Head of the Flow Cytometry facility won a Papin Prize for her contribution to knowledge exchange. The Papin Prizes celebrate the achievements of technical staff and recognise the best technical talent across the UK. Rachael’s nomination noted her passion and effort in promoting new flow cytometry technologies and techniques, her leadership in the adoption of full spectrum cytometry and her commitment to flow cytometry training to develop the skills of cytometry users.
Two animal technicians, Jess and Abbie, received the Outstanding Collaborator Award at the 2023 Research Institute Technician Awards (the RITAs) for their achievement in devising and leading the Institute’s first Animal Technician Conference. Since its inception, the event runs annually to bring together animal technicians from the Institute and from research institutions, universities and industry from across the UK.
Aimee Paterson, an animal technician and supervisor within the Biological Support Unit, was the recipient of the inaugural Sir Colin Blakemore Award from Understanding Animal Research. Aimee received the award in recognition of her passion and bravery in talking about her work and that of the unit more widely in the Institute’s takeover of UAR’s Instagram account as part of Mice in Research week in May 2023.

Ian McGough, Maria Christophorou, Jon Houseley and Teresa Rayon were all awarded BBSRC Pioneer Awards, created to support frontier bioscience research with the potential to transform our understanding of life. These innovative research projects are widening our understanding of how toxic protein aggregates form and how developmental time is set in different species, looking for novel ways to tackle drug-resistant fungal pathogens and investigating the possibility that cells talk to each other using an unknown signalling pathway.

Postdoctoral fellow Theresa Pankhurst joined the Linterman lab at the Institute through a unique fellowship between the Institute and the Malaghan Institute of Medical Research in Wellington, New Zealand. The Te Urungi Churchill College By-Fellowship supports Theresa to undertake research that strives for equitable health outcomes for Māori.

The following individuals were recognised by Institute prizes recognising achievements in research excellence, knowledge exchange and commercialisation, public engagement, supporting equality, diversity and inclusion, and creating an engaging scientific image.
• The 2023 Sir Michael Berridge Prize was awarded to postdoc Tamara Chessa for her contribution to the research published in the paper: PLEKHS1 drives PI3Ks and remodels pathway homeostasis in PTEN-null prostate.
• The Knowledge Exchange and Commercialisation Prize was presented to Rachael Walker for leadership and enthusiasm for activities across many aspects of knowledge exchange and commercialisation –from founding a spin-out company to organising the Spectral Flow Cytometry Conference.
• Amy Wilkinson won the Public Engagement Prize for work bringing together artists, scientists and the public in a SciArt project and for contributions to a public dialogue project, both associated with the Human Developmental Biology Initiative.
• The Award for Contributions to Research Integrity was given to three members of staff, each recognised as an exemplar in demonstrating and supporting best practice in research integrity. These were: Richard Acton, Data Outputs Manager; Emily Watson, Research Assistant; Cass Flowers, Chief Information Officer.
• The equity4success Award went to PhD student Oishee Rahman for her work with One Million Mentors and Close the Gap to support first generation university students and postgraduates from minority backgrounds, and her role as the Ethnic Minority Welfare Officer at the Jesus College MCR.
• The Image Prize was awarded to Aurora Xu, PhD student, for her image ‘Gut’s feelings’.

Continuing the Institute’s history of hosting Royal Society Entrepreneurs in Residence, Louise Jopling joined the Knowledge Exchange and Commercialisation team for two years as our latest Entrepreneur in Residence. Louise brings more than 25 years of experience in drug discovery, development and commercialisation within academia, biotech and pharmaceutical organisations to assist with identifying translational opportunities for our discovery research.

Hanane Hadj-Moussa, a Marie Skłodowska-Curie Fellow in the Houseley lab, received the Korenchevsky Prize from the British Society for Research on Ageing for the best presentation by an early-career researcher. Hanane spoke about her work identifying ageing mechanisms in yeast and as the prize recipient will speak at the 53rd annual meeting of the American Aging Association in Alaska, USA.


Teruhito Ishikara from the Kelsey lab was awarded a Marie Skłodowska-Curie Fellowship to study genomic imprinting and the acquisition of differentially methylated regions in mammalian oocytes. This research has the potential not only to advance our understanding of the evolutionary mechanisms of genomic imprinting, but also to provide clues to address why such a phenomenon exists, and has evolved, only in marsupial and eutherian mammals.

Theresa Pankhurst, Te Urungi Churchill College By-Fellow in the Linterman lab, was awarded a Kia Niwha Leader Fellowship from the Te Niwha Infectious Diseases Research Platform which provides access to an intensive 12-month leadership programme. The aims of the programme are to develop and equip researchers working in infectious disease with the ability to operate as research leaders in ways that align with the Te Niwha framework of integrity and accountability, relationships, ability to partner for impact, and leadership.

Knowledge Exchange and Commercialisation Officer, Maha Ashraf, was awarded a Knowledge Transfer Innovation Fellowship at LifeArc, a self-funded, not-for-profit medical research organisation that aims to turn promising scientific breakthroughs into new test, treatments and devices for patients. The year long programme provides fellows with the skills and knowledge to transition into careers in technology transfer through a curriculum that combines formal training with networking, mentoring and practical experience in the field.

Alyssa Silva-Cayetano, a former postdoc in the Linterman lab, was awarded the 2024 Sir Michael Berridge Prize by the Institute for her leading contribution to the research described in the paper: Spatial dysregulation of T follicular helper cells impairs vaccine responses in aging. This work uncovered key changes to germinal centres that occur with age and lessen the immune response to vaccination.

The Knowledge Exchange and Commercialisation team welcomed Aljona Kolmogorova as its first Entrepreneurial Lead, supported by the Innovate UK’s Innovation-to-Commercialisation of University Research (ICURe) Explore programme. Aljona worked as a postdoctoral researcher with Maria Rostovskaya and Peter Rugg-Gunn in the Epigenetics research programme and as Entrepreneurial Lead applied her in-depth subject knowledge to advance the market development of Maria and Peter’s patented amniotic epithelial stem cell technology.



Juliet Emery, Head of Science Technical Services, Laura Durrant, Genomics Specialist, and Megan Hamilton, Head of our Genomics facility all completed the Herschel Programme for Women in Technical Leadership. This national six-month programme is designed to elevate and advance opportunities for women who are current or aspiring leaders in technical roles.

The Institute’s annual prizes for 2024:
• The Knowledge Exchange and Commercialisation Prize was awarded jointly to Richard Berks, Named Information Manager, and Sarah Drummond, Experienced Animal Technician, for their leadership and work organising the Institute’s 2024 Animal Technician Conference.
• The Public Engagement Prize was presented jointly to PhD students Ellie Griffiths and Jake Cross for their work developing and delivering the Big Autophagy Obstacle Course. Amongst other exceptional nominations, this project was recognised as a great example of researcher-led engagement sharing the Institute’s research in a novel way.
• The equity4success Award was given to Stephane Guillaume, PhD student in the Linterman lab, and Honor Pollard, Communications Officer. Stephane and Honor are Chair and Co-Chair of the Institute’s LGTBQ+ Network, Pride@Babraham, and were recognised for their work establishing the Network and creating a visibly inclusive and supportive community.
• The Award for Contributions to Research Integrity was awarded to Trevor Smith, Health and Safety and Quality Assurance Manager, for his long-term commitment to supporting research integrity, especially recognising that his knowledge and expertise were instrumental to embedding research integrity at the Institute, not least with undertaking reviews of research integrity to secure continued progress towards excellence in research integrity practices.
• The Image Prize was awarded to Pavi Manivannan from the David lab for her image ‘Luminous lysosomes’, showing the pharynx of the model organism C. elegans with fluorescently tagged proteins. The image was captured as part of research using C. elegans to explore protein degradation (lysosomes are vital organelles responsible for protein degradation in cells) and protein aggregation.
By working on mouse and human immunology and developing new tools, technologies and conceptual frameworks in which to understand immunity, we hope to contribute to improved health for all. Immunology
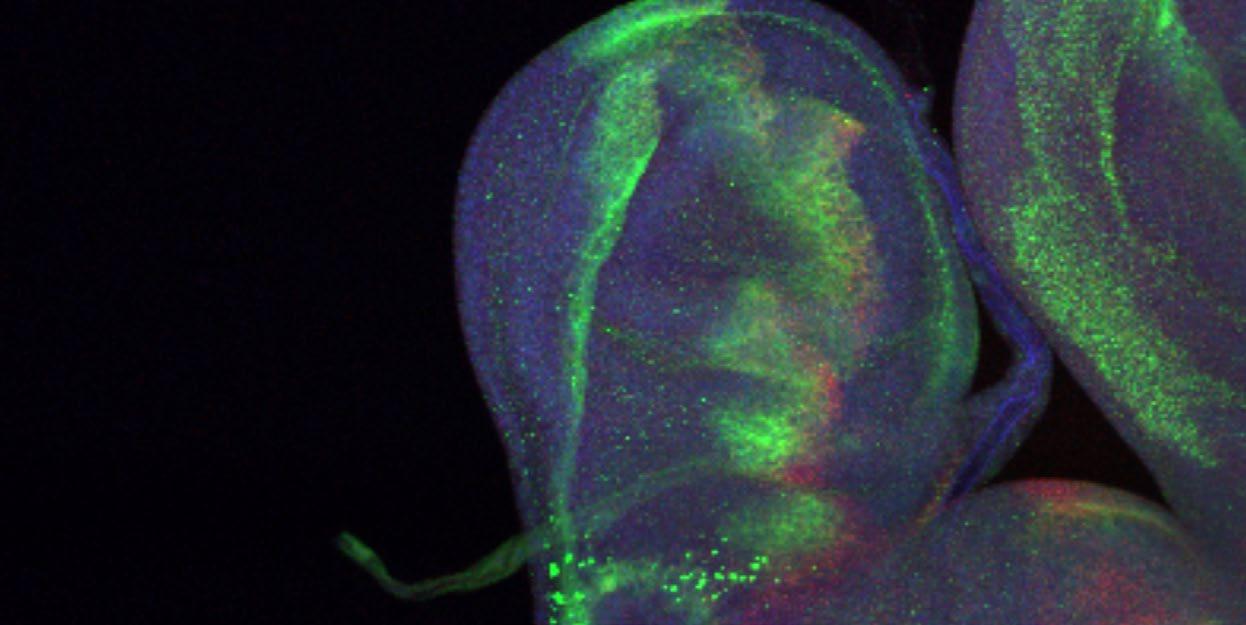
By working in model cell systems and organisms (worms, flies and mice) we hope to understand how signalling controls cellular and organismal resilience and how this can be applied to improve human health. Signalling






















Our research explores how epigenetic tags are set up during development and how they are modified by what we eat and as we age, with a view to understanding how defining and maintaining the epigenome promotes health across the life course.


Facilities
Bioinformatics
Biological Chemistry
Biological Support Unit
Flow Cytometry
Gene Targeting
Genomics
Imaging
Mass Spectrometry

Gavin Kelsey Programme leader

Martin Howard Honorary Group Leader

Maria Christophorou

Kathy Niakan Honorary Group Leader

Jon Houseley

Wolf Reik Honorary Group Leader

Teresa Rayon Jointly appointed to Epigenetics and Signalling

Peter Rugg-Gunn

Philipp Voigt
Facilities

Simon Andrews Head of Bioinformatics
Paul Symonds
Co-facility Head, Biological Support Unit

Jonathan Clark Head of Biological Chemistry
Marc Wiltshire
Co-facility Head, Biological Support Unit

Megan Hamilton Head of Genomics

Asif Nakhuda Head of Gene Targeting

David Oxley Head of Mass Spectrometry

Rachael Walker Head of Flow Cytometry

Simon Walker Head of Imaging

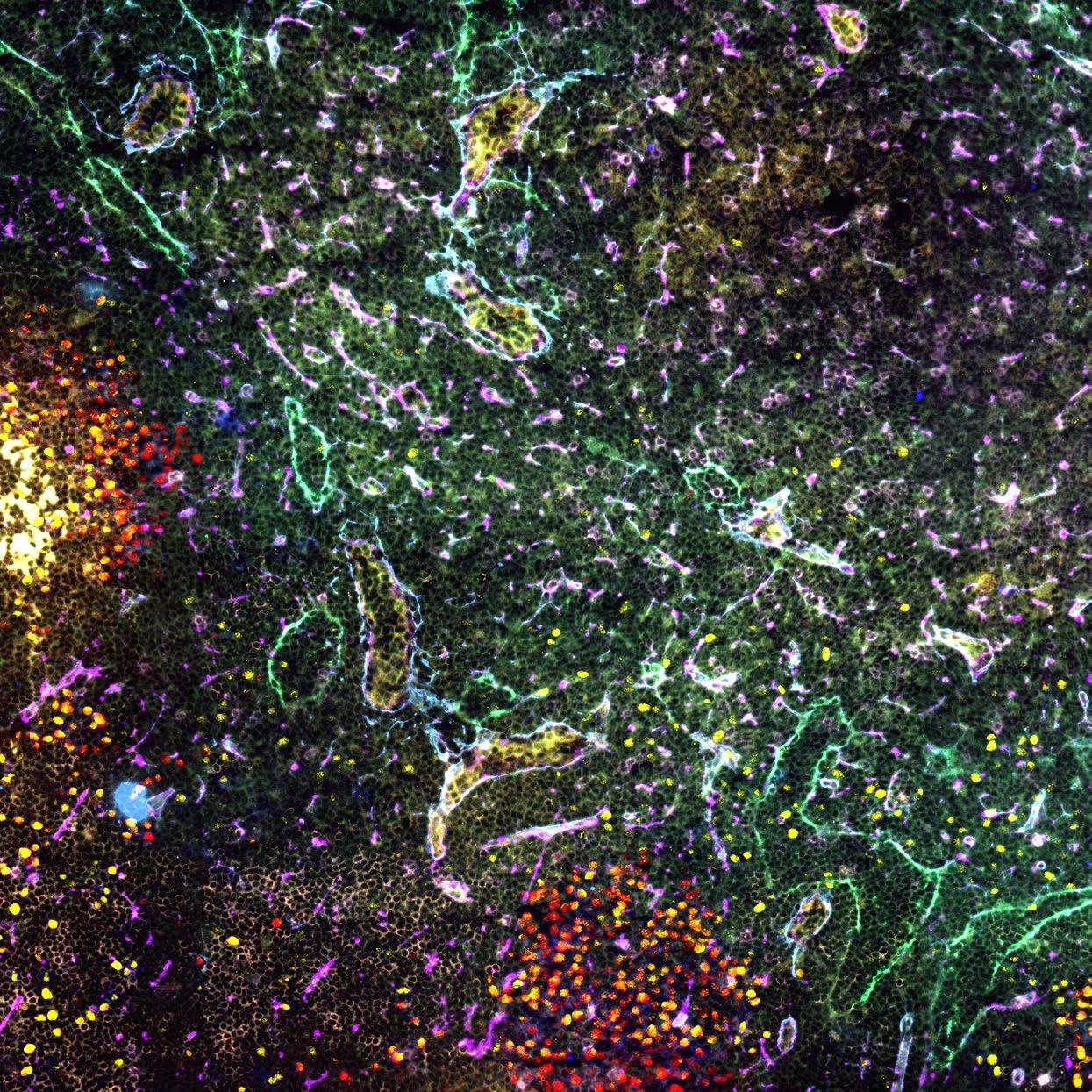
Our research aims to generate a biological understanding of the molecular and cellular events responsible for the decline in the function of the immune system seen with age. By using mouse models and human samples combined with innovative technologies we can uncover the detail of the molecular pathways, cellular interactions and microenvironment effects that together govern an effective immune system.
The combined purpose of our work is to enable the development of approaches, such as vaccines, small molecules and biological/cellular therapeutics, to maximise immune protection and utilise the power of the immune system. In this way, our research aims to support health throughout the life course.
At the heart of the immunological response to vaccination is the germinal centre – hubs of intensive cell communication and coordination in secondary lymphoid tissue. The germinal centre response generates long-lived antibody secreting plasma cells and memory B cells.
With advanced age the formation of germinal centres is delayed, their size reduced and the quality of the antibody response impaired. Work published by the Linterman lab (Silva-Cayetano et al. 2023) showed that the spatial organisation of the germinal centre is changed in ageing. Through a combination of mouse research, computer modelling and analysis of human vaccination data, they showed that changes to key interactors of B cells in the light zone of the germinal centre, T follicular helper cells (Tfh), and also to light-zone specific cells – follicular dendritic cells (FDCs) –were at the heart of the diminished vaccination response.
Parallel work (Lee et al. 2023 showed that B cell extrinsic factors are also responsible for the poor-quality germinal centre response in ageing, highlighting the significance of the germinal centre microenvironment. These findings implicate the lymph node microenvironment and Tfh cells as targets for vaccine formulations that promote antibody mediated immunity in the later years of life.
We were delighted to expand our expertise in germinal centre biology with the recruitment of Professor Kai-Michael Toellner. Kai’s expertise in immune cell differentiation, antibody production and how long-term immune memory is formed is highly synergistic to the work of the programme with several opportunities for combined approaches.
The germinal centre-focused work is complemented by investigating the molecular pathways that act within T and B cells to regulate the formation, persistence and responses of lymphocytes. Work in the Turner lab continued to investigate the mechanisms, mediated by RNA binding proteins of the ZFP36-family, that govern T cell effector function. They identified a role for ZFP36L1 in sensing antigen affinity and promoting the expansion of high‐affinity T‐cell clones by regulating sensitivity to cytokine signals (Petkau et al. 2023).
The programme welcomed Professor Yiliang Ding as an honorary group leader, building on a research collaboration with the Ribeiro de Almeida lab and opening up the application of Yiliang’s innovative approaches to investigate RNA structure and RNA–protein interactions to the work of several groups across the Institute.
Our technology-driven approach and growing expertise in proteomics methods has delivered insights into the proteome
changes during B cell maturation, advancing what we know about how cell identity and specialisms are established in B cell subsets (Salerno et al. 2023). Excitingly, this work suggested that the immune system might prime for rapid action using mRNAs to enable expediated B cell responses.
In October 2023, Michelle Linterman joined the GSK Immunology Network. Made up of internationally recognised scientists, the programme bolsters connections by embedding academics in GSK laboratories. As a member, Michelle advised on the development of immunology programmes, accelerating the application of our discovery research to support health.
An international partnership between the Institute and the Malaghan Institute of Medical Research in New Zealand, was supported through a BBSRC International Partnering Award. The partnership unites expertise in the Linterman and Turner labs with the Malaghan Institute’s novel mRNA vaccine development platform and expertise in fullspectrum flow cytometry. Part of the work will be carried out by Theresa Pankhurst, Linterman lab postdoctoral researcher and holder of the inaugural Te Urungi Churchill By-Fellowship from the Malaghan Institute.
The data collected by Salerno et al. was published as an open-access data resource via the Immunological Proteomic Resource, contributing to this detailed map of the immune cell proteomes as a resource for the research community. The ImmPRes resource integrates data derived from high-resolution mass spectrometry analysis of mouse hematopoietic populations to allow the rapid exploration of the protein landscape of key immune cells.
A Wellcome Discovery Award is supporting work in the Turner lab to generate approaches to define how the dynamics of protein and RNA interactions affect immunity and immunological memory.
We will also continue to develop our capabilities in human immunology, so that we can translate our findings from mouse models.
In a cross-programme endeavour, we will investigate RNA binding proteins in the context of the regulation of proteotoxic stress using immune cells as models. This has important consequences for the extended lifespans of antibodysecreting cells.
Publications
Silva-Cayetano, A. et al. (2023). Spatial dysregulation of T follicular helper cells impairs vaccine responses in ageing. Nat Imm 24(7):1124–1137
Lee, J.L. et al. (2023). B cells from aged mice do not have intrinsic defects in affinity maturation in response to immunization. J Immunol 211(10):1506-1515
Petkau, G. et al. (2023). Zfp36l1 establishes the high‐affinity CD8 T‐cell response by directly linking TCR affinity to cytokine sensing. Eur J Immunol 54(2):2350700
Salerno. F. et al. (2023). An integrated proteome and transcriptome of B cell maturation defines poised activation states of transitional and mature B cells. Nat Commun 14(1):5116
Mielczarek, O. et al. (2023). Intra- and interchromosomal contact mapping reveals the Igh locus has extensive conformational heterogeneity and interacts with B-lineage genes. Cell Rep 42(9):113074
Muir, A. et al. (2024). Single-cell analysis reveals lasting immunological consequences of influenza infection and respiratory immunisation in the pig lung. Plos Pathog 20: e1011910

Our cells are constantly exposed to environmental challenges. Some of these are ‘normal’; for example, insulin responds to changes in nutrient status. But others are ‘stressful’, causing damage to DNA, proteins and lipids. Healthy ageing requires that cells are resilient, able to detect and adapt to these challenges to remain viable and functional. Failure to adapt can undermine cell, tissue and organ function in later life.
Signalling refers to the network of biochemical pathways inside cells that are activated by growth factors and hormones (normal) or by the accumulation of damaged or misfolded proteins (stressful) and orchestrate the cell’s response. We are studying how these pathways are regulated and how they change cell behaviour (growth, division, death). Notably, these pathways harbour drug targets for a range of age-related diseases.
One key signalling pathway that is activated by growth factors receptor tyrosine kinases (RTKs) and nutrients is the phosphoinositide 3-kinase (PI3K) pathway, which controls cell metabolism and growth. In healthy tissues this pathway is controlled by insulin and related growth factors. This pathway is deregulated in cancer. Using a mouse model of prostate cancer we have used mass spectrometry to understand how the components of the PI3K pathway change in cancer. We find that the IRS proteins, major components of the PI3K pathway, are downregulated and replaced by a protein called PLEKHS1 that sustains PI3K signalling in prostate cancer (Chessa et al., 2023).
Activation of RTKs leads to the activation of the protein kinases ERK1 and ERK2 and promotes cell survival and cell division. This pathway is frequently activated in cancer due to mutation of ERK1/2 pathway components, making ERK1/2 attractive drug targets. We have discovered nearly all ERK inhibitors (ERKi) not only inhibit these protein kinases but also drive the degradation of the most abundant ERK2 enzyme. Since ERK2 has been proposed to have kinase-independent, non-catalytic functions, the ability to degrade the enzyme may impart such ERKi with superior drug-like properties (Balmanno et al., 2023).
Whilst RTKs phosphorylate proteins on tyrosine residues to propagate signals for cell division, growth and motility, the receptor protein tyrosine phosphatases (RPTPs) remove phosphate from tyrosine and have been suggested to simply act in opposition to RTKs. We have shown that Protein Tyrosine Phosphatase Receptor Type K (PTPRK) suppresses invasive behaviour in colorectal cancer cells. In vivo PTPRK supports recovery from inflammation-induced colitis and functions as a tumour suppressor in the mouse colon and colorectal cancer xenografts. Remarkably, and contrary to the prevailing dogma, PTPRK suppresses cancer phenotypes independent of its catalytic function suggesting that it acts as a signalling scaffold (Young et al., 2024).
Proteins control every aspect of cellular life and the timely synthesis, folding, stabilisation, trafficking and degradation of proteins (proteostasis) is critical for lifelong health. The evolution of our research programme now provides critical mass for proteostasis research.
Using advanced mass spectrometry techniques, we have identified proteins which are stabilised by the molecular chaperone heat shock protein 90 (HSP90). This study reveals the selective effect of HSP90 inhibitors, identifies new components of the HSP90dependent proteome and provide
a rich data resource for the HSP90 and proteostasis communities (Samant et al., 2023).
Lysosomes are key sites of degradation and recycling of damaged or unwanted proteins and foreign organisms. Maintenance of lysosome function is critical for lifelong health and we have recently described a novel pathway, Conjugation of ATG8s to Single Membranes (or CASM), that responds to lysosome damage by recruiting a protein called ATG2, a lipid transfer protein central to lysosome repair (Cross et al., 2023).
Aggregation of proteins is a driver of normal age-related decline and certain diseases. Both in disease and during ageing, proteins selectively aggregate in certain tissues and not others. We have described a novel safety mechanism that selectively targets newly synthesised proteins to suppress their aggregation and associated proteotoxicity (Jung et al., 2023).
During 2023-24 the Signalling programme has continued its close work with the biotech and pharmaceutical sector. Studies on PI3K remodelling in cancer suggest that PLEKHS1 and related proteins may be potential drug targets in prostate cancer and this will be explored in collaboration with AstraZeneca. Understanding how ERKi drive the degradation of ERK2 and the consequences of this is
being progressed in collaboration with PhoreMost. In addition to PTPRK, studies from the Sharpe lab are rewriting the PTP rule book, suggesting new opportunities to understand and drug this enzyme class; these are being progressed with AstraZeneca, Cancer Research Horizons and Oppilotech.
Our work on proteostasis, ubiquitylomics, senescence and ageing is also attracting industrial collaborations including AstraZeneca, PhoreMost and Stemnovate. Furthermore, we have led the launch of the UK Proteostasis Network hosting the first very successful conference here at Babraham.
We will study how signalling mechanisms sense and adapt to different types of stress across the life course. A major focus will be on pathways which regulate 'proteostasis across the life course' - with colleagues in our Epigenetics and Immunology programmes. We will also reveal new molecular understanding of how reactive oxygen species and PTPases regulate epithelial barrier integrity, how the PI3K network is rewired during ageing, how the V-ATPase transduces lysosomal stress and the role of phase-separation in the induction of autophagy. We will use this knowledge to identify new opportunities for therapeutic intervention to mitigate age-related physiological decline.
Publications
Chessa, T.A.M. et al. (2023) PLEKHS1 drives PI3Ks and remodels pathway homeostasis in PTEN-null prostate. Mol Cell. 83:2991-3009
Balmanno, K. et al. (2023) ERK1/2 inhibitors act as monovalent degraders inducing ubiquitylation and proteasome-dependent turnover of ERK2, but not ERK1. Biochem J. 480:587
Young, K.A. et al. (2024) The receptor protein tyrosine phosphatase PTPRK promotes intestinal repair and catalysis-independent tumour suppression. J Cell Sci. 137:jcs261914
Samant, R.S. et al. (2023) Native size-exclusion chromatography -based mass spectrometry reveals new components of the early heat shock protein 90 inhibition response among limited global changes. Mol Cell Proteomics. 22(2):100485
Cross, J. et al. (2023) Lysosome damage triggers direct ATG8 conjugation and ATG2 engagement via non-canonical autophagy. J Cell Biol. 222:e202303078
Jung, R. et al. (2023) A safety mechanism enables tissue-specific resistance to protein aggregation during aging in C. elegans. PLoS Biol 21:e3002284
Publications

Epigenetic tags are essential in regulating gene activity. They provide a memory of decisions during development, safeguard cell states, and prepare cells for future events. Although epigenetic programmes are remarkably stable in most cells, erosion of epigenetic states can undermine cellular identity, causing errors in development or loss of tissue integrity in later life.
Our research explores how epigenetic tags are set up during development and how they are modified by what we eat and as we age.
The metabolites within our cells are likely to be a major factor determining epigenetic states. This is because these metabolites work with the enzymes that add or remove epigenetic tags on our genes. Changes in metabolite levels in relation to age, nutrition and other environmental factors could profoundly impact the epigenome. We aim to harness knowledge of the metabolic inputs into epigenetic tags to identify interventions that may benefit lifelong health.
Progress and research highlights in 2023 and 2024
One of our key aims is to understand how nutrients affect how well we age. Calorie restriction has been shown in many organisms to improve ageing health but, as a long-term intervention, is likely to be undesirable in human populations. Working in yeast, we have found that we can enhance ageing health by changing diet without restricting calories (Horkai et al., 2023). By exploring the underlying cellular metabolic pathways, we have been able to create mutations in two sensor proteins, allowing yeast to ageing healthily even on an unlimited, rich diet. Because these are highly-conserved sensors, we are exploring whether we can similarly improve ageing health in more complex animals: fruit flies (working with colleagues in the Signalling programme), and ultimately in mouse models.
One of the key cellular metabolites is acetyl-CoA, which serves many purposes in the cell in response to the cell’s energy status, as well as being required for placing epigenetic tags – acetylation – on the histone proteins that DNA is complexed with in the nucleosome units of chromosomes. In this way, the level of acetylation on histones can respond to nutrients; conversely, histones could constitute a reservoir for acetyl-CoA for other cellular functions. We have explored this by detailed metabolic analysis in mouse cells (Soaita et
al., 2023), confirming that histone acetylation is indeed nutrient responsive, but has a limited capacity to feed into the metabolic demands of the cell.
Just which and how many epigenetic tags histones carry is important in determining whether genes are active, silent, or poised for activity when developmental cues induce new differentiation events. The poised state is typified by ‘bivalent nucleosomes’, in which histones paradoxically carry both ‘activating’ and ‘repressive’ epigenetic tags. Until now, we have limited understanding of what gene regulatory proteins recognise bivalent nucleosomes, but by developing new techniques to construct bivalent nucleosomes, we have for the first time been able to identify proteins uniquely binding to the combination of active and repressive marks (Bryan et al., 2025). Unexpectedly, we find that these dual binders include proteins that add acetylation to histones, providing a potential link to cellular energy status, and the shifts in metabolism that occur as cells differentiate.
How embryonic cells coordinate development into different lineages in the early human embryo is poorly understood, as we are unable to access and manipulate embryos at these stages. One such important lineage is the amnion, a thin membrane that encloses the embryo. Previous work has used human pluripotent stem cells (hPSCs) as a model to investigate
how the amnion develops but found that the signals required are shared with other cells, such as surface ectoderm which gives rise to tissues like the skin. Further optimisation of this culture system now reveals that cell density can be decisive in determining whether they become amnion or ectoderm (Nakanoh et al., 2024). This advance will help facilitate development of methods to produce others tissues from hPSCs.
Impact highlights
Supported by grants from Wellcome and ScienceWise, and together with the Human Developmental Biology Initiative, members of the programme supported a public dialogue project exploring the future of human embryo research, and to understand public views on the 14-day rule. The findings attracted substantial interest from groups such as the International Society for Stem Cell Research (ISSCR), and the media. Peter Rugg-Gunn gave evidence to the Human Embryology and Fertilisation Authority (HFEA) and has been appointed to the HFEA’s Scientific and Clinical Advances Advisory Committee.
There has been rapid development in our ability to derive human ‘embryo-like’ models from stem cells (SCBEMs), but they raise ethical questions and their legal status is undefined. Epigenetic researchers contributed to a dialogue and policy project on SCBEMs with
Cambridge Reproduction, and Peter Rugg-Gunn reviewed a Parliamentary Office of Science and Technology research briefing (POSTnote). Peter serves on UK and committees including ISSCR that are developing guidelines for research using these models. The UK group published a Code of Practice in July 2024 which gained widespread attention in scientific and public media.
We have been translating outcomes of our work, with patent filings in nutrient sensing in ageing (Houseley); embryo implantation models (Rugg-Gunn); cell reprogramming activators and methods (Christophorou); and single-cell multi-omics methods (Rugg-Gunn).
Looking ahead
We expect to make progress in understanding early events in human embryogenesis, with the development of new culture systems that mimic the process of embryo implantation. We shall apply powerful new methods we are developing that enable us to profile multiple epigenetic tags in individual cells, to understand how epigenetic processes help define the first lineages in the human embryo, and set up epigenetic states of lifelong importance. Reprogramming of cell fate is pivotal to tissue regeneration; we expect to uncover mechanisms by which histones operating outside of the cell can promote regeneration of damaged tissues.
Publications
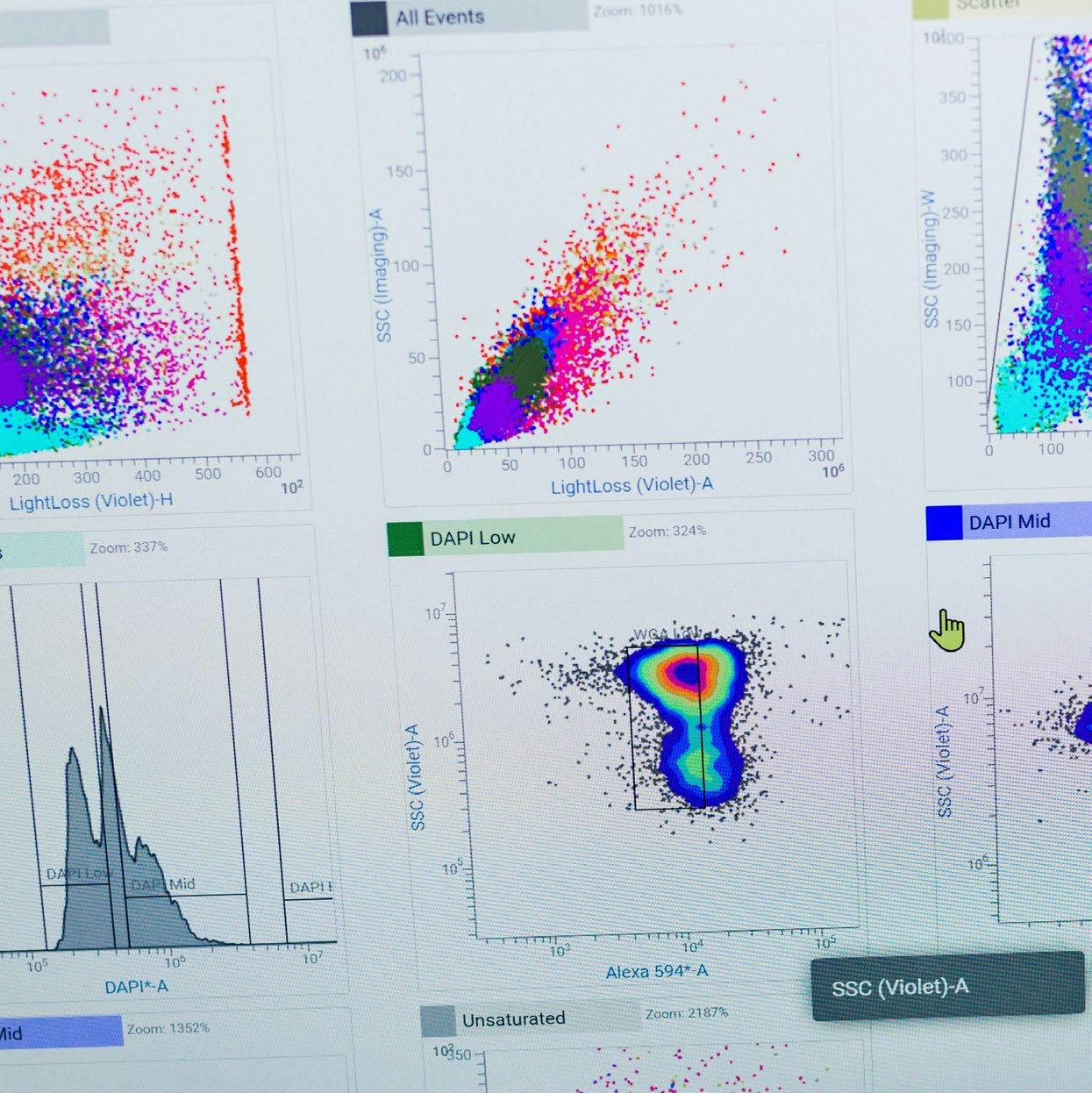
In 2023 and 2024 developments continued within the Institute’s eight facilities that underpin the Institute’s research and also support commercial companies on the Babraham Research Campus and beyond.
The Imaging, Flow Cytometry and Genomics facilities are spotlighted here to illustrate how they continue to follow leading innovations in their fields.
In addition to advancing research through their technical capabilities, several of the facility teams provide unparalleled training tailored to the needs of the external research community in the UK and internationally. In this way they strengthen the skills of researchers and technical professionals across academic and commercial life science.
BBSRC investment in the Genomics facility in 2023 and 2024 allows the team to provide ‘the whole package’ for Institute teams and companies. The expanded library preparation services and new equipment mean Facility head, Megan Hamilton, and her team are now able to support more projects with comprehensive sample-to-sequence pipelines.
The facility has upgraded its sequencing platform to the AVITI. As the first UK core facility to install this platform, the Genomics facility can now deliver faster turnaround times and higher
The Flow Cytometry facility has expanded its capabilities with the addition of a spectral and imaging BD FACSDiscover S8 Spectral Sorter, funded through a BBSRC Alert grant. This upgrade enables researchers to sort cells using cell imaging, allowing researchers to identify and obtain cell populations that could not previously be isolated. The new capabilities allow direct visualisation of cells which can then be sorted based on spatial and morphological features, adding an additional layer of data to experiments.
These enhancements support a range of research applications, including the Rayon lab’s fused cell experiments and the Houseley lab’s work on yeast
throughput sequencing. With enhanced depth and data quality, it supports a broad range of applications, from small genome sequencing to high-throughput RNA sequencing.
The team supports projects by enabling in-depth analysis of gene expression and methylation patterns. The facility’s capabilities allow researchers to generate high-quality sequencing data, providing detailed insights at the single-cell level for immune cell profiling and differential gene expression in C. elegans
This investment strengthens in-house sequencing capabilities and provides a complete sequencing workflow for internal and external research projects, including drug discovery and antibody development.
ageing, where imaging is used to study budding scars as markers of replicative age.
The facility has also installed a Sony ID7000 spectral analyser, featuring seven lasers and allowing detection of over 50 parameters per cell. This significantly increases the amount of biological information extracted from each sample, reducing the need for multiple experiments. Researchers can now build more complex panels, improving the resolution of immune profiling and cell state characterisation.
Since 2024, with support from Innovate UK, the facility has been collaborating with ChromaTwist
Continued strategic investment from BBSRC has allowed the Institute’s Imaging facility to expand its capabilities. One area of focus has been provision of new sample preparation tools for electron microscopy (EM), including a high-pressure freezer, freeze substitution unit, ultramicrotome and critical point drier.
These have significantly enhanced the facility’s EM services by providing a new volumetric EM method called serial array tomography along with optimal sample preservation and enhanced ease of use.
Additionally, a new Leica Thunder light microscope has been integrated into the EM suite to enable a full correlative imaging workflow, where samples can be imaged using fluorescence microscopy and then rapidly transferred into the high-pressure freezer in preparation for EM.
The facility has also replaced its high content imaging platform with a Molecular Devices HT.ai and acquired a new Sartorius Incucyte for long term live cell imaging experiments.
on a project testing novel fluorescent dyes designed for extended spectral detection. These new dyes, coupled with an additional laser line, expand the number of detectable colours, enhancing multicolour flow cytometry and enabling deeper analysis across diverse cell populations. This external partnership is just one example of the sharing of expertise by the facility which also includes events, training and publications.
These systems are supporting a number of Institute projects across all research programmes but are also highly sought after by commercial users. Expanding the facility’s range of services in ways that benefit both the Institute and the regional research community helps maximise the benefit of the investment and promotes regional growth.
The Babraham Institute plays a pivotal role in fostering innovation at the Babraham Research Campus by driving translational research and entrepreneurial initiatives, supporting early-stage companies through access to state-of-the-art facilities, and building impact-driven collaborations with industry.
Institute strategy
The Institute is committed to embedding an entrepreneurial culture across the Institute and the wider Babraham Research Campus. By leveraging its research strengths, the Institute aims to build capacity, foster cross-sector engagement, and maximise the societal and economic impact of its research. This commitment extends to identifying and protecting innovations, developing tailored translational strategies, and managing intellectual property in collaboration with a diverse range of stakeholders, including industry, R&D partners, charities, and funders. The Institute’s unique combination of strategic focus, state-of-the-art research facilities, and close collaboration with the Campus underpins a thriving translational culture that drives impactful research toward lifelong health benefits.
Team changes and strategic appointments
The 2023-24 period has seen enhanced collaboration between the Institute’s Knowledge Exchange and Commercialisation (KEC) team and Campus teams. This approach has fostered entrepreneurial skill development, strengthened
translational efforts, and enhanced career mentoring across the Institute and Campus. Notably, the Commercialisation Manager and the Campus Director of Science and Entrepreneurship/Chief Innovation and Science Officer have engaged in cross-institutional roles, supported by a Royal Society Entrepreneur-inResidence fellowship for 2024-2026.
The close collaboration has significantly benefited initiatives such as Accelerate@Babraham, the Collaborative Training Partnership (CTP) PhD Programme, enhanced Campus translational training, and new academic-industry collaborations under the BBSRC Campus Innovation and Accelerator Award (CIAA) grant.
Access to scientific facilities and collaborations
The Institute’s facilities continue to provide crucial support to early-stage commercial companies, offering research collaboration opportunities, staff training, and access to cuttingedge scientific resources. 33 Campus companies (75% of all researchactive Babraham Research Campus companies) utilise Institute facilities. Additionally, the Institute has engaged in 32 R&D collaborations with Campus companies between 2019 and 2024,

demonstrating its significant role in fostering industry partnerships.
Launched in 2022, the BBSRC Campus CTP programme has successfully supported 22 collaborative industry PhD studentships, further strengthened by the integration of the Accelerate@Babraham programme for second-year students. The CTP programme sets a new benchmark in bioscience education by integrating industry placements, translational skills development, and exposure to entrepreneurial training through Accelerate@Babraham.
The CTP programme has been a key enabler of collaborations between the Institute and industry, supporting training of the next generation of innovators, while seeding new relationships between the Institute and industry researchers. Industrial supervisors provide extensive industry engagement, ensuring students have continuous access to a unique set of expertise and resources. Industry partners have acknowledged the programme’s value, emphasising its significant industry engagement and entrepreneurship opportunities, as well as the strong collaboration between the Institute and companies.
“75% of all research-active Campus companies utilise Institute facilities”

The Institute continues to develop and expand its facility offerings to respond to researcher needs, exemplified by investments in cutting-edge technologies such as the Flow Cytometry facility’s new image-based sorting capability (BD FACSDiscover S8 Spectral Sorter), with pilot use supported by a CIAA grant. This facility provides an unmatched provision of technology and expertise to researchers at the Institute, on the Babraham Research Campus and to the wider R&D sector. The addition of combined cell sorting and cell imaging capability enables the facility to support cutting-edge research, benefiting both academic and industry stakeholders and helping progress innovative science along the R&D pathway, getting novel technologies closer to patients.
Through these initiatives, the Institute continues to strengthen its position as a key driver of scientific and economic impact, fostering a thriving innovation ecosystem that bridges fundamental research with real-world application.
Proteostasis, the myriad mechanisms that ensure our proteins work correctly, is key to healthy ageing. We speak to Dr Della David and Dr Rahul Samant about their cutting-edge research, why proteostasis is such a growing area of strength at the Institute, and how the new UK Proteostasis Network will help accelerate research in the field.
In understanding health and disease, it’s impossible to overstate the importance of proteins.
The smooth working of a plethora of proteins—the so-called proteome —ensures that our cells function correctly and can adapt to a changing environment. That’s why proteostasis —the quality control mechanisms that keep proteins working properly—is a burgeoning field of research.
Many diseases—particularly those of ageing—stem from problems in proteostasis and the breakdown of proteostasis is recognised as a hallmark of ageing itself. Dr Della David, one of several group leaders at the Institute who focuses on proteostasis, uses the tiny nematode Caenorhabditis elegans to discover mechanisms to help combat agerelated diseases and promote healthy ageing.
“Keeping the integrity of your proteome is really challenging but so important for life; every organism needs these mechanisms that protect the proteome. These mechanisms look after the proteins, make sure
“We are at a critical moment to bring the community together and realise its potential”
they function at the correct location, and ensure their disposal when they become damaged or aggregate, so proteostasis is absolutely fundamental and poor proteostasis accelerates the ageing process,” she explains.
Dr Rahul Samant, also a group leader in the Signalling programme, works on cellular senescence, another hallmark of ageing. As we age, accumulation of damage—including through proteostasis faults—causes a build up of large cells that have lost their intended function in our tissues. No longer able to divide or regenerate but persistent and hard to kill, these so-called zombie cells accumulate in ageing tissues causing chronic inflammation.
“Fifteen years ago it was unclear whether these cells were a cause of ageing or just correlated with it. Then, researchers genetically engineered a mouse with no zombie cells and found they not only lived longer but were healthier,” he says. The same has since been found in other species and
researchers are working to unravel the fundamental biology to explain why.
Using a range of different human cell types, Samant is looking at how the same senescence stress drives diverse responses in proteostasis. “At first, our question was highly focused: if you introduce this stress, what happens to the molecular circuitry? In fact, there are diverse responses, so we are looking at whether there is more commonality in all types of senescent cells in our body rather than in senescent and non-senescent cells of the same type.”
This understanding will be crucial to drug discovery, he says. “We always look for magic bullets – drugs that will kill every senescent cell in the body. Some think we’ll be able to find this if we look harder. Others think there is no unifying factor and that multiple treatment options will be needed depending on the exact type of senescence driving that disease.”


“Diversity is important for understanding ageing and proteostasis better, that’s one of the Institute’s great strengths”
Answering such challenging questions demands different perspectives and solid technical capabilities, he says. “Diversity is important for understanding ageing and proteostasis better, that’s one of the Institute’s great strengths. I don’t know anywhere else working on proteostasis across such a diverse set of organisms, including yeast, worms, flies, mice and human cells. The other key strength is the breadth of our expertise, both scientifically in our research groups, but also of the technical staff in our facilities.”
“For example, we work really closely with the mass spectrometry facility at the Institute. For us, the facility is not simply a service, they are partners we actively collaborate with. The same is true with bioinformatics. It’s an iterative process built on two-way communication. It's one of the main reasons I joined the Institute,” explains Samant.
Another example of the Institute’s leadership role and collaborative ethos is the UK Proteostasis Network, established in 2024 with David and colleague Dr Oliver Florey as two of its cofounders. “The aim is to bring the
UK proteostasis community together, hold events and empower early career researchers because although the UK has great strengths in proteostasis research, we could achieve even more by sharing ideas and expertise,” David explains.
During its first 12 months, the Network has gone from strength to strength. Its first two-day meeting in Spring 2024 attracted 170 attendees from academia and industry and a second will follow in 2025 — evidence of the community’s huge enthusiasm to work together and build the Network.
“Individual groups are doing amazing work and I’m super excited about animal and plant scientists working together to cross pollinate ideas,” she concludes. “And now is absolutely the right time. We have a clear view of what we need to discover. We are at a critical moment to bring the community together and realise its potential.”
In 2024, Professor Kai-Michael Toellner joined the Institute’s Immunology programme after 30 years at the University of Birmingham’s Institute of Immunology and Immunotherapy. We find out what drew him from the UK’s second-largest city to the Babraham Research Campus, the pressing questions he wants to answer, and why it’s vital we understand more about how our immune system works.
Immunisation is a global health success story. It saves millions of lives every year, helps control outbreaks of infectious diseases such as Covid, and is a vital defence against microbial resistance. Despite this and a history dating back hundreds of years much of how our immune system works at a molecular and cellular level remains to be discovered.
Many gaps in our knowledge exist because the immune system is extraordinarily complex, involving myriad communications between multiple components. But deepening our understanding is crucial if we are to develop more and better vaccines, including against diseases such as cancer, tackle autoimmune diseases, and find out why our immune system works less well as we age.
Professor Kai-Michael Toellner has studied the biology of the immune system and how it responds to vaccination and infection for over 30 years. “We want to understand how our bodies react to infection and vaccination,” he says. “Vaccination essentially mimics an infection in
“
The B cell response is essentially Darwinian evolution on a cellular level”
a non-dangerous way. And we’re interested in how the body makes antibodies to that, and ultimately how the body remembers exposure, something we call immune memory.”
Toellner and his team work on B cells, part of the so-called adaptive arm of the immune system. Some B cells produce antibodies in response to active infection while others, known as memory B cells and plasma cells, act as our immunological memory. These long-lived cells remember past infections or immune priming through vaccination so they can respond rapidly and robustly the next time they encounter the same infection.
“It’s a very complex process,” Toellner explains. “The main focus of our research is germinal centres. These are structures in lymphoid tissues where clusters of dividing B cells are found. When the immune system encounters an infection or vaccination, it’s here that B cells respond by dividing, mutating, and then selecting the fittest
variants, a process that is essentially Darwinian evolution on a cellular level.”
Several other cell types help regulate this process, including follicular dendritic cells, which keep antigens in place. This provides a training ground for B cell selection. Also present in the germinal centre are T cells which stimulate the B cells and give them the right signals, and macrophages which clean up the many unsuitable cells that are not fit for selection and do not survive.
Teasing apart this complex system requires a top-notch toolkit, which is one of the Institute’s key strengths. “One of the things that attracted me to the Institute is its excellent facilities, and the huge amount of experience here in the mouse models I use,” he says. “Doing cutting-edge research requires the best equipment, from flow cytometry to cell sorting and genomics. Being here really enhances my toolkit.”


“Our research could open up more affordable treatments for cancer patients in the global south”
He is also enjoying other aspects of his new environment: the Institute’s positive and collaborative nature, its proximity to existing academic and biotech collaborators in Cambridge and Granta Park, and the prospect of building new partnerships. “The strength of the biotech community here is a huge plus, so I’m keen on setting up new collaborations,” he says.
While Toellner’s focus is on fundamental science, advances in the field will feed into far-reaching practical applications, including new treatments for cancer and autoimmune diseases, and new strategies for healthier ageing.
New vaccines that trigger our immune system to produce its own antigens against cancer, for example, are of major interest and could bring many benefits. The team have had a longstanding interest in using the immune response to eliminate cancer cells in the same way as it identifies and attacks infected cells. “We are looking at certain antigens that are expressed in tumours and tumour vessels and working out ways of triggering antibody responses by the host itself,” explains Toellner. “If we can develop vaccines to elicit an immune response against the tumour, this would be cheaper than cellular
therapies, opening up more affordable treatments for cancer patients in the global south.”
Another potential application stems from a chance discovery by Toellner and his biotech collaborators. In an unexpected outcome, they produced a mouse in which all B cells are switched off. B cells recognising our body’s own cells must be inactivated to protect the body from its own immune system and prevent autoimmune diseases, but with age this control can fail. “Interestingly, as these mice age they spontaneously start to make autoimmune responses, so this may offer us a way of studying autoimmunity in ageing,” he says.
While his focus remains firmly on the fundamentals, his research illustrates how crucial fundamental research is in the pipeline linking new ideas, new knowledge and ultimately new applications to promote healthier ageing. “Our focus is on the fundamental question of how the body reacts to foreign antigens. Without understanding this you can’t understand vaccination or autoimmunity,” Toellner concludes. “Only when you start understanding this process better, can you start trying to improve it.”
Last year the Institute’s Schools’ Day celebrated its 30th birthday. To mark this milestone, Dr Peter Rugg-Gunn, head of public engagement and Dr Mike Norman, public engagement manager reflect on the changing face of public engagement, the Institute’s new public engagement strategy and why being a leader in public engagement is a vital component of the Institute’s world-class science.
2024 was a landmark year for the public engagement team at the Institute. As well as its 30th annual Schools’ Day, the Institute published its new public engagement strategy. Building on the Institute’s rich history of public engagement, the strategy aims to widen and deepen dialogue between scientists and public audiences to build trust and transparency in scientific research alongside inspiring a new generation of scientists.
“The new strategy embodies the Institute’s vision of being an open, transparent and accountable organisation,” says Dr Peter RuggGunn, senior group leader and head of public engagement. “A major focus is on engaging underserved communities. Another key element is promoting researcher-led engagement, empowering and supporting our scientists and staff to devise new ways of engaging target audiences.”
Over the past 30 years, public engagement has changed. “Like research, public engagement is constantly evolving. Methods improve, new opportunities for
“It’s hugely exciting to see our approach revolutionising how we undertake public
engagement
and seeing the benefit this brings”
engaging different groups open up, so it's important the Institute stays at the forefront,” he explains.
A demonstration of this is the Institute’s shift in prioritising underserved schools and communities under the banner of the Institute’s BioInspire programme, a move initiated by Dr Mike Norman since he joined the team as public engagement manager in 2020.
Whereas in the past the Institute often engaged audiences with ‘high science capital’, staff are using socio-economic data to build relationships with schools and communities out of reach of most opportunities to understand and remove barriers to engagement.
“It’s about having a considered approach to how we engage with these communities,” Norman says.
“I want to ensure public perspectives are heard in research and enable them to have informed conversations with scientists. It’s crucial those voices
come from all areas of society because our research impacts everyone.”
Together with a more targeted approach, Institute scientists and staff are in the driving seat when it comes to generating new ways to engage. Scientists and staff are empowered to deliver by seed funding, training and support, backed by strong buy-in from senior leadership, and celebrated through reward and recognition practices. It’s a collaborative approach that works for everyone.
An impactful demonstration of researcher-led engagement is the pivotal contribution of the Institute’s Epigenetics researchers at all levels in the public dialogue project of the Wellcome-funded Human Developmental Biology Initiative (HDBI). This public dialogue project, coordinated by Norman, was one of the first of its kind to bring the public, scientists, law makers and ethicists together for two-way,


“ Public engagement is embedded in our DNA”
deliberative discussion about human embryo research and the 14-day rule. Its report, published in 2023, will be invaluable in informing future research and engagement programmes, whilst also feeding into national policy discussions.
The team can also see the effects of its shift towards prioritising underserved audiences. Four years ago, only 4% of Schools’ Day participants came from underserved areas. Through targeting, relationship building, and reducing barriers by providing travel bursaries, that figure reached 40% in 2024 and is just one example of how BioInspire is broadening the Institute’s reach in underserved communities.
Recognising additional barriers to participation in research opportunities, the Institute’s Research Access Programme provides eight-week placements for undergraduate students from lower-income families, minority ethnic communities, disabled students and students with caring responsibilities. Of the 30 students that have taken part so far, 13 are currently undertaking or applying for PhDs, and others have gone on to Masters courses or to jobs in the science sector. Rugg-Gunn reflects: “Providing paid,
eight-week research placements enables undergraduates to gain their first research experience. It makes a real difference to them and their confidence, and the host groups at Babraham also find it very rewarding to be part of this programme; it has been a huge success.”
When considering the factor at the heart of the Institute’s success in engagement, both Rugg-Gunn and Norman emphasise the importance of top-to-bottom support. “Public engagement is embedded in the Institute’s DNA and this is reflected in the widespread enthusiasm to take part,” comments Norman. “– It’s genuinely researcher-led and it’s an integral part of their work.”
“We’re an outward-looking institute, and our senior leadership is behind public engagement all the way, which means we’ve been able to build a talented team, invest in them and develop a long-term vision,” adds Rugg-Gunn. “It’s hugely exciting to see our approach revolutionising how we undertake public engagement and seeing the benefit this brings.”
Understanding how things work underpins the Institute’s past, present and future. Dr Simon Cook, Institute Director and head of the Signalling programme, explains why he’s an evangelist for discovery research, how this quest for understanding is reflected in the Institute’s science, its people and the ecosystem of the Babraham Research Campus, and looks ahead to the next four years’ work.
In building his case for discovery science, Dr Simon Cook need look no further than the Signalling programme he heads at the Institute he now leads. “If you look at advances over the last 40 years in understanding the molecular basis of cancer, they have closely parelleled our understanding of the molecular basis of signalling pathways: one has often led to discoveries in the other,” he says.
Signalling refers to the processes which enable our cells to respond to changes in their environment, from hormones and growth factors to radiation and ageing. At the cellular, tissue and organism level, we are constantly adapting to change and challenge. That’s why understanding how signalling controls cell behaviour is critical to understanding lifelong health – and how to improve it.
Cancer is a case in point. “Signalling pathways, or the genes that encode proteins making up these pathways, are often mutated in cancer. These mutations lead to pathways being switched on at the wrong time.
“I’m an evangelist for discovery research”
As a result, cells receive signals telling them to grow and divide when they shouldn't, which can lead to the initiation of cancer,” says Cook. “Understanding how this drives cancer and recognising how particular components are mutated underpins our ability to develop drugs to inhibit these pathways.”
Since 2019, Cook has overseen a strategic shift in the Signalling programme, introducing new faces including Dr Hayley Sharpe working on signalling by reactive oxygen species and Drs Rahul Samant, Ian McGough and Della David to strengthen the Institute’s ageing and proteostasis research. “We have recruited group leaders with a forte in ageing, cell senescence and proteostasis, and they have brought with them a wider range of model organisms such as worms and flies, short-lived species which are important in ageing research,” he explains.
This re-engineering of the Signalling programme will drive discoveries in proteostasis and feed into practical, translational benefits, including new drug targets, something that the Institute is adept at. Today, the Institute is the hub of the Babraham Research Campus which with more than 60 companies and 2,000 employees creates the right conditions for discovery research and business to come together, accelerating innovation and strengthening links between academia and the commercial life sciences sector.
The Campus began life 25 years ago with the retrofitting of an old barn. “The director then, Dr Richard Dyer, had this vision,” Cook recalls. “He looked at other science parks and saw they were dominated by large, established companies, but there was nowhere nurturing small startups, so he made that happen. The Campus has been a stunning success because the Institute is behind it.


“There is real synergy between the Institute and Campus companies – it’s a delight to see”
There's real synergy of effort between the Institute and Campus companies, which is a delight to see.”
The Signalling programme has a history of working with pharmaceutical companies, and the benefits are mutual. Industry empowers the Institute’s work through access to earlystage probe compounds from drug discovery. And by sharing knowledge of signalling pathways with pharma giants including AstraZeneca and GlaxoSmithKline, the programme’s discovery research paves the way for new drugs. “By giving early insights into the impact of inhibiting particular pathways, our research has supported drug-discovery programmes in pharma and biotech,” says Cook.
The same happens on Campus, where daily interactions between Institute and biotech strengthen the science, the scientists and the community. “The Babraham Research Campus is unique. Many science parks exist, but ours has a world-leading research institute at its heart,” he says. “We have a community where group leaders in the Institute have collaborative PhD projects with Campus companies, they use our science facilities, they rub shoulders with our scientists in the coffee queue, and that leads to grant applications. It makes for a very special place.”
All these ingredients from new directions, tools, faces and collaborations fuel Cook’s excitement for the future. “Recognising our existing strength in proteostasis provided an opportunity to build on a strategically important area in ageing research,” he says. “Understanding the mechanisms of proteostasis and how this fails in ageing and neurodegenerative diseases remains fundamental to finding new drug targets. I think there’s a real chance to understand the defects that drive protein aggregation in ageing and then identify ways to address this.”
The Institute’s core facilities are future focused too. “The mass spectrometry capability here is giving us a proteome-wide understanding of how proteostasis declines with age. Every time you open a journal there’s a new technique being developed to look at proteostasis in even more detail. It’s a really exciting time,” Cook concludes. “It’s easy to think discovery has all been done, we just need to translate. But when you stop doing discovery bioscience, the pipeline stops. So I remain an evangelist for discovery research.”
As we age, our bodies become more prone to infection and disease, and vaccination becomes less effective. Dr Martin Turner, head of the Immunology programme, talks about why a deeper understanding of the immune system is key to lifelong health and why—after almost 30 years at the Institute—the programme’s research continues to excite.
Wrinkles and grey hair may be the most visible outward signs of passing years, but inwardly, a decline in adaptive immunity is one of the most significant hallmarks of ageing, making us more susceptible to infection and disease, and poorer at responding to vaccination.
As we get older, our immune system changes. It becomes less adept at fending off illness and infection and less able to promote tissue homeostasis and repair. This is why older people are more prone to flu and may also explain higher cancer rates among older people. But although we can see the impact of the ageing immune system, we need to know much more about how it works in order to improve it.
Today, Dr Martin Turner is head of the Immunology programme. When he joined the Institute in 1997, the human genome had still to be sequenced, the Higgs Boson particle had yet to be discovered and Google did not exist, but his excitement for research endures.
“What’s so exciting is that there is still so much to discover”
“We are 25 years past sequencing the human genome. Some 20,000 protein encoding genes have been identified, a similar number to those in the nematode worm. That’s worth reflecting on, because humans are considerably more complex than C. elegans,” he says. “Very little is known about many of these 20,000 genes. What is even more challenging to understand is that each gene produces many distinct RNAs or alternative transcripts to form a transcriptome and these drive the complex behaviour of different cells in the body. You can think of the genome as a zip file for the transcriptome. What’s so exciting is that there’s still so much to discover.”
Over the next four years of the Institute’s immunology research, the focus is to discover more about the fundamental biology of our immune system, knowledge we need in order to improve vaccines, immunisation strategies and cell-based therapies. There are many questions still to answer, including how long-term immunological memory is generated
and persists, and why, as we age, we become less resilient to respiratory infection.
Our immunological memory describes the way our immune system recognises and reacts to pathogens it has previously encountered, thanks to long-lived memory B and T cells created during the initial response which can persist for years and even decades. In their work in this area, the programme’s research teams want to understand the molecular mechanisms which regulate the formation of longlived B cells which secrete antibodies and so-called CD8 memory T cells which can recognise cells infected with viruses or bacteria.
“This kind of immunological memory is very important, particularly in older people with diminished antibody responses, or in situations where viruses have undergone changes in their antigenic makeup so that they're no longer efficiently recognised by antibodies, and has significant practical applications,” says Turner.


“Our technical specialists are extraordinary, it’s impossible to overstate how important they are to the world-class science we do”
Just like the complex interactions and interplay needed to shape an effective immune response, Turner is proud of the complementary expertise held by the research groups in the programme, dovetailing to advance key facets of the fundamental biology of the immune response: how different B and T cell types are produced and activated, B cell training in pathogen recognition, immune cell proteomics and how genes are switched on and off during these processes.
There are wider reasons why Turner believes the Institute is perfectly placed to answer these important questions, from the synergies between its Immunology, Signalling and Epigenetics programmes and the expertise of its technical staff, to its collaborations with biotech companies on the Babraham Research Campus and beyond.
“The Institute’s technical specialists are extraordinary. We absolutely rely on the expertise of the technical staff who support our use of mouse models to understand immune responses, and on the staff who are experts in the high-dimensional flow cytometry, sequencing and imaging technologies we use,” he says. “It’s impossible to overstate how important they are in delivering the world-class science we do here.”
Beyond generating new scientific knowledge in fundamental biology, Turner is also passionate about the development of researchers from the start of their careers onwards. Together, the Institute and Campus provide an innovative training environment for the next generation of researchers. Many studentships are shared between the Institute and Campus biotech firms, giving young researchers unrivalled experience in both fundamental research and the biotech industry.
“We have trained and continue to train many students and postdocs who go on to successful careers elsewhere, either as research group leaders or in industry in start-ups and scale-ups as well as big pharma leaders like AstraZeneca and GSK,” Turner concludes. “Many of the young researchers joining the Institute now will be the future leaders in the field, in both academia and industry. Their success speaks volumes about the Institute’s reputation this is a fantastic training environment and the quality of the young people we are training.”
For some, living longer brings opportunities, but for others it heralds ill health. Ameliorating deficits in healthspan requires deeper understanding of the complex changes in biological functions that lead to ageing. Dr Gavin Kelsey, head of the Epigenetics programme, talks about the programme’s science, ambitions for the next four years, and shares a small secret about what helps fuel his research.
Epigenetics describes the systems that control our genes’ activity throughout our lives. Key epigenetic ‘marks’ are established early in development, defining the body’s different cell types and keeping cells working correctly. As we age, epigenetic information can change and deteriorate, partly due to lifestyle factors, affecting cell function and renewal. The Institute’s Epigenetics programme focuses on how the epigenetic control of gene expression helps maintain and support lifelong health, opening up new ways to promote healthy ageing.
“The big theme of the programme is to link two areas of biology, metabolism and epigenetics, so we're trying to investigate how much some aspects of our metabolism and our diet affect how our genes are controlled,” says Dr Gavin Kelsey.” This is important because we know that all of the processes that go on around the DNA to ensure that genes are regulated correctly depend upon metabolites within cells, and these metabolites are also shared by other processes in the cell, as part of the cell’s normal metabolism.”
“The big theme of the programme is to link two areas of biology – metabolism and epigenetics”
DNA is not a simple molecule. Within the nucleus of our cells, DNA is wrapped around histone proteins, organised into chromatin and packaged to form chromosomes. The DNA itself is modified or ‘decorated’ with chemical tags, and the histone proteins are also highly modified by chemical tags made available by the pool of metabolites within in the cell. These reversible modifications control subsequent gene expression, locking away some genes while leaving others accessible to the gene reading machinery at the appropriate time.
“Although part of this is probably hardwired and unlikely to be affected by changes in nutrition, some metabolites may be helping genes respond to environmental or nutritional change,” he explains. “This is the area we're particularly interested in, and how the interface between metabolism and epigenetics changes as we age.”
One key question is how these epigenetic changes the sum of the various modifications to particular
genes are set up early in development and affect how our genes are programmed throughout our lives.
As a result, the programme does much work at this development stage and is learning much from early human embryos.
“We have wonderful model systems, but they don’t always perfectly recapitulate exactly what goes on during human development,” says Kelsey. “That’s why it's vital we translate what we know from models, such as mouse, into human systems; looking at the epigenetic processes happening in the very early embryo, as different cell types start to emerge from what is otherwise a ball of identical cells, is really exciting and transforming what we know about early human development.”
Answering these questions over the next four years depends on the best scientists – research and technical, a bold vision, and the capability to realise it, which is what makes the Institute world leading in the field.


“Looking at the epigenetic processes in the very early embryo is transforming what we know about early human development”
“What's really exciting, particularly in Peter Rugg-Gunn's group, is the development of single-cell technologies and these amazingly sensitive methods that allow us to map epigenetic marks over genes in individual cells. Until now, we've only been able to do this for individual epigenetic marks, but his team has developed a technique to look at multiple different modifications in a single cell, which will be a game changer,” he explains.
The Epigenetics programme has also been strengthened by strategic appointments in 2022 when Dr Sophie Trefely and Dr Teresa Rayon joined the Institute. Rayon’s group is interested in the rate at which organisms develop and how this might be linked to lifespan. The mechanisms underlying biological timing remain largely unknown so, by comparing what happens during development in mice versus humans, she is working on what controls biological timing and whether we can modulate developmental timing and extend lifespan.
Trefely is interested in metabolism and the fact that within the cell, metabolites are not uniformly distributed. Some are produced
in particular organelles, such as mitochondria, but to impact DNA regulation, metabolites must act in the cell’s nucleus. “She is using elegant methods to examine the metabolic profile of a nucleus, altering the cell’s metabolic regime to discover how metabolites change in the nucleus. Using these new technologies, we can discover what happens at the interface between metabolites and gene regulation," explains Kelsey.
Trefely and Rayon are joint appointments, bridging the Epigenetic and Signalling programmes, and an example of how the synergies between the Institute’s three programmes mean advances in one translate into advances across the others.
On a personal level, Kelsey who joined the Institute in 1995 is trying to age healthily. “I’m going to the gym regularly for the first time in my life and enjoying it. I try to eat healthily and maintain a healthy weight. I try to be less sedentary, hence my standing desk. But I’ve never tried intermittent fasting,” he concludes. “I think I’d find fasting quite difficult. I'm already begging for a croissant at 9am, despite having had breakfast. It’s habit, rather than necessity!”
Thirty years on from the Institute’s official renaming, former director Richard Dyer (1994-2005) and current director Simon Cook reflect on shared history, the essence of Babraham and where another thirty years might take us.
The moment it changed: why did we become the Babraham Institute?
RD: The reason it became called the Babraham Institute was because everywhere I went in the world people would say “how are things at Babraham?". They wouldn't say how is “the Institute of Animal Physiology and Genetics and ...” that would be ridiculous, they would just say “how are things at Babraham?”. And then I realised, later, that that was a master stroke. Call it Babraham because then you’re not defined, you can change where you're going. If you're the institute for something, then you can be accused; ‘Oh, you're not doing that.’ And so after that we were just the Babraham Institute.
Richard, could you describe the early tasks and challenges of stepping into the director role?
RD: There were too many isolated people, and I fundamentally believe in teamwork. I believed then that the day of the lone scientist was more or less over. And I also believed in working together, with a shared vision and shared achievement. One of the things
“ I knew what it was like to stop what I was doing, run down the corridor, and be excited about somebody else’s achievement and that's what I wanted to create here.” Richard
about being an electrophysiologist, which is my background, is that actually if someone's having a successful experiment, you can see it in real time. So I knew what it was like to stop what I was doing, run down the corridor, and be excited about somebody else’s achievement and that's what I wanted to create here.
The first thing that was required, which was a huge battle after I became director, was to actually make lab groups. There were some obvious ones, and then there were some complete surprises, ‘you three are going to work together’! At the time of the 2000 review report, all of that was behind us. We’d successfully redefined the Institute and it all looked very positive into the future. I was happy to read the report again and I was happy to be reminded about the journey, as they say, and I think the outcome was good. The really nice thing was that after the

labs were made, progressively everybody pulled together. That's why I felt so happy because it had been a privilege to do all this, it was a very creative job. Now, of course, you Simon will have different sorts of challenges, but I used to say two things, to myself mostly, but to those who would listen occasionally: the status quo is unsatisfactory by definition, it implies perfection. So there is no status quo. And I used to say, even Mozart had to satisfy the Archbishop of Salzburg.
Simon, do you remember this sort of time at the Institute? And how does it make you think about your early days in the director seat?
SC: I've often felt that I arrived at a very interesting time (in 1997). There were still farm animal projects going on but they very quickly closed and we quickly evolved a more focused, strategic vision.


1999.

We have a remarkable alumni now; people who have gone out into the world and are achieving incredible things in all spheres of life. And that, if anything, is the legacy of the Institute, sustained and influenced through successive directors. There are so many things you say that still resonate more than 30 years on. You said that you found it very insular in the beginning. I think the Institute in that sense has changed beyond all recognition and this also speaks to the move towards team science. You're absolutely right that the days of the lone researcher are long gone. Partly, this reflects the expectations placed upon us from society and governments, which really drives the need for team science to deliver ambitious projects, addressing global problems. But it also reflects the nature of the science we do; so much of it is impossible without a team.
I think that you're absolutely right that there is no such thing as a status quo in science. I have always felt that you are only as good as the last piece of work you published so we’re all looking to innovate and break new ground and we’re inspired along that path by the combined progress of the scientific community. Despite the challenges, it's still a job that gives me great joy as a researcher. And as a director, I get immense joy out of the success of my colleagues. I think one of the standout things about being a director is actually the brilliant people we work with.
What do you each consider to be the most rewarding aspect of leading the Babraham Institute, and how has it shaped your views on your professional legacy?
RD: I think it's one of the best things to happen to me in my life from a professional perspective, better than my Nature papers for example. I think of it like this: with research, you put a brick on the wall but all you’ve done is put a brick in the wall. In leading a community of people and shaping an organisation’s vision your creativity lasts longer, I mean it absolutely, than most of your published papers.
SC: Stewardship is a very relevant word. For me personally, when people ask me what I do, I first of all say scientist, and this is the first and the last thing I will be, but I think, like Richard, that the things that I have derived most value from is not the papers published, but my PhD students and postdocs who have gone on to bigger and better things, which I find very rewarding. And that, I think, speaks to something that Babraham is all about, which is supporting people and nurturing people. That was the case 27 years ago when I arrived here and I benefited from that, and it continues to this day.
Shifting from leadership to day-to day experiences, what are your hopes for the staff who support the science behind the scenes?
RD: What I think about Babraham, is that it is a metropolitan, international activity taking place in the countryside. One of the things I felt very strongly about is that I wanted everybody to be proud to work here, even if they had a very humble job. And to be proud to work here means they had to be able to answer the first questions at the pub – “where do you work?” I work at Babraham. “Oh, what do they do there?”
SC: I couldn't agree more. First and foremost the Institute exists to do great science. But that can only happen if we have a whole host of people behind the scientists keeping everything running. As soon as one part of that fails, then quite a lot of things very quickly fall down. Recognising that and understanding that I think, is critical to the success of the Institute, and is something that the Institute has done well in the 27 years I’ve been here.
I felt valued from a very early point when I arrived here. You took me out for lunch not long after I arrived and you wanted my opinion on things. I think the fact that I was asked, and my opinions were sought when I had been here just a few months, showed it was important for the Institute and I felt like my voice was heard.
Richard, how does the Campus measure up to your imagined future for it?
RD: Is it where I thought it might be?
The answer to that is no, which may surprise you. The reason I say that is because I’m the one who started it; I suppose I saw the opportunity and ignored the naysayers. In planting the seed of this Campus we stepped into the gap and realised the value in supporting early-stage life science companies and that widened the base of what the Institute could offer. The Campus today has exceeded my expectations. I’m delighted to see the synergy and close interweaving of the Institute with the commercial campus community.
SC: I can't put myself in your shoes and imagine what the aspirations were, but I think the vision you had was remarkable and has been an incredible success to see it realised. You know, it is one of the most successful research campuses in Europe. And in terms of an innovation campus that has both academic and commercial bioscience it's essentially been the model for BBSRC’s innovation campuses around the country. If I'm asked what the Institute’s USP is, I say two things, our great science and the campus, and the Babraham Research Campus’ USP is the Institute. Without the Institute the campus is a science park like every other; that is the difference between ‘place’ and ‘space’.
Simon, if you looked ahead as far as Richard is looking back, what might you see in a crystal ball?
SC: I’m not sure I can think that far into the future but something that Richard has said I think is very relevant here, which is this widening of the base and I think we need to be thinking about finding diverse funding sources to build a new research programme to broaden our research portfolio. We have three fantastic programmes funded by BBSRC but we need to grow. One way is to explore more flexible funding models with other research councils this was one of the goals of UKRI after all but also with other funders as our fundamental bioscience has many applications.
Another way to grow is to engage with the commercial science sector in a much more cheek-by-jowl approach, closer even than we have currently on the Babraham Research Campus. I am excited about the idea of an ‘innovation hub’ where research scientists can do perhaps more translational work, side-by-side with scientists working for companies. And this will be much more of a blurring of the academic-industry boundary, but that interface is where some of the most exciting biology is being done now.




“ If you first understand how a process works, you can then identify where to intervene. And that is something that the Institute has always had a reputation for doing.” Simon
I also see us continuing to embrace new technologies, some of which are coming out of the commercial sector. There's an old-fashioned view that knowledge exchange is unidirectional; an academic has an idea and it's translated into companies. But the reality is, it's very much a circular, iterative process where ideas come across from the commercial sector. We are very familiar with this due to our history of commercial collaborations.
What about the future of the Institute in the context of the changing landscape of bioscience?
SC: My view is that the Institute will continue to be internationally known for its ability to interrogate molecular mechanisms; that is key to understanding how biology works. If we think about societal impacts and economic impacts, particularly drugs for treatment of disease, you are only going to understand and deliver those drugs if you first understand how a process works, because you can then identify where to intervene to inhibit or activate that process. But we also know that systems are dynamic and resilient, so we also need to understand how they adapt to such interventions.
And that is something that the Institute has developed a reputation for doing in the last 50 years; it is why I moved here to start my research group. Moving forward we will need to embrace computational biology and AI in support of this. I am also excited by the opportunities afforded by engineering biology approaches, especially in the area of proteostasis which in recent years has emerged as major focus at Babraham.
So we are back to the start of our conversation and the need to keep moving forwards and embracing change!

After setting our science course for the next four years we look to make strides in uncovering new knowledge that will pave the way for the development of therapeutics to support healthy ageing. Starting from discovery, we will continue to apply innovation, creativity and leadership in capitalising on this knowledge. In doing this we will not only maximise the translational impacts of our research but also train the next generation of researchers contributing to a thriving research and innovation ecosystem and wider UK bioscience.
During the past two years several of our facilities have received significant investment by the BBSRC to allow them to maintain their position at the cuttingedge, adopting new technologies and underpinning our capacity to deliver new biological insights. These enhanced capabilities will not only support the innovation of our discovery research, but also that of the companies on the Babraham Research Campus and beyond.
With the core themes of resilience and proteostasis our goal of understanding mechanisms that promote health across the life course will benefit from the coalescence of expertise across our research programmes and facilities. Having created the UK Proteostasis Network we look forward to being an active supporter of the Network as it grows to connect proteostasis researchers across specialisms and kingdoms of life.
With the efforts of our Knowledge Exchange and Commercialisation team we have built a strong pipeline of entrepreneurship and commercial awareness and expect to see steady growth in activities related to research translation.

For our growth as an effective, sustainable and inclusive organisation, we will continue our work in key areas with the implementation of new actions plans for our equal4success and the Technician Commitment strategic initiatives, continued leadership in driving the adoption of sustainable science approaches and the implementation of our Roadmap for Team Science.
We look forward to detailing progress in each of these areas in the next report presenting activities in 2025-26.
Babraham Institute
Babraham Research Campus
Cambridge CB22 3AT
UK
www.babraham.ac.uk
Tel: +44 (0)1223 496000
The Babraham Institute
@babrahaminst.bsky.social
The Babraham Institute
Registered office: Babraham Hall, Babraham, Cambridge, CB22 3AT
Registered in England and Wales No. 3011737 as a company limited by guarantee
Registered Charity No. 1053902
receives strategic funding from the BBSRC.