
9 minute read
HOW IS CIS-PLATIN USED TO TREAT CANCERS?
Taran Dhillon and Max Newman
Abstract
Cis-diamminedichloroplatinum (II) is a cytotoxic drug used in chemotherapy. Classified as an alkylating agent, it binds to cell DNA and interferes with mechanisms like transcription and repair. Its mode of action promotes apoptosis, or cell death, by binding to the molecule. Whilst effective against several human cancers, patients can demonstrate resistance to the drug, such as an upsurge in restoration to the damaged DNA. In this literature project, a number of interactions are given: detailing the structure of the complex and how this helps its uptake into cells; how it is hydrolysed in order to approach the nucleus; and how it binds to DNA, causing a cytotoxic effect.
Introduction
Synthesised by Italian chemist Michele Peyrone, cisplatin was long known as Peyrone’s salt. Little was known about the compound until Alfred Werner clarified the structure of the yellow precipitate in 1893. Containing the same two chlorine atoms as the white-blood-cell-killing mustard gas, it was when in 1965 biophysical chemist Barnett Rosenberg discovered its use as a cancer treatment, that it gained media awareness. 50 years ago, Rosenberg and microbiologist Loretta van Camp had devised an experiment to ascertain whether an electric field could affect cell division. As the current was pushed through the culture of E. coli, the bacterial cells grew up to 300x their normal size. However, the lack of cell division observed was not due to the electric current alone, but by a platinum compound released by the electrodes. This discovery was made 4 years after the original experiment but its impact on medicine would be ground-breaking.
In the 5 years prior to the discovery, male and female survival rates of colon cancer were 43% and 47% respectively. At the time, the diagnosis were for many as good as a death sentence. The mainstays of treatment for the disease were surgery and radiotherapy; both of which produced destructive side effects.
Hearing about Rosenberg’s discovery that platinumcontaining compounds can kill bacteria and shrink tumours in mice, researchers across the globe embarked on a hunt for novel cancer-treating drugs.
In particular, a British physicist, Alexander Haddow, along with a young, determined pharmacologist Tom Conners, set out on examining numerous platinumbased contenders as a result. Connors employed an exhaustive approach until he struck the necessary balance between toxicity and effectiveness and by 1971, cis-platin was taken forward into clinical trials. At the Royal Marsden Hospital, a team led by Eve Wiltshaw gave cis-platin to patients for the first time in the UK. Whilst other platinum-based drugs with a similar structure to cis-platin have been developed since, it has never been replaced.
Structure of cis-platin
As shown in the title picture, cis-platin has square planar geometry with a heavy metal centre. It is a platinum complex with chloride leaving groups and inert carrier NH3 ligands. A coordinate complex is the product of a Lewis acid-base reaction in which neutral molecules or anions (negative ions) called ligands are bonded to a central atom or ion by coordinate bonds. In cis-platin, ammonia molecules and chloride anions are coordinated to a central platinum (II) ion. A leaving group is a molecular fragment that departs with a pair of electrons in heterolytic bond cleavage. The ammonia molecules form a strong interaction with the platinum ion and the chloride ions act as leaving groups, allowing the platinum ion to form bonds with DNA bases.
How this helps its uptake into cells?
Cis-platin is administered intravenously as a sterile solution. The high concentration of chloride ions in our bloodstream, around 97-107 mmol/L , means that the complex remains whole and does not break apart. Upon approach to the cell, the molecule passes through the plasma membrane by passive diffusion. This is assisted by the fact that the two chloride ligands cancel out the charge on the platinum ion, making cis-platin neutral. Active transport is also responsible for the uptake of cis-platin into cells. High-affinity copper transporters, encoded by genes like CTR1, have been found to raise accumulation of the drug in the cell. S. Ishida et al. demonstrated this by deleting the CTR1 gene in yeast but proposed that cis-platin uptake is also facilitated via this way in mammals. Mouse cell lines deficient in one or both mouse Ctr1 (mCtr1) alleles displayed increased cisplatin resistance and diminished cis-platin accumulation in parallel with mCtr1 gene dosage.12 Little is known about how copper transporter’s function, but it is evident that without them cisplatin’s uptake into cells would be greatly reduced.
How is cis-platin hydrolysed in the cell?
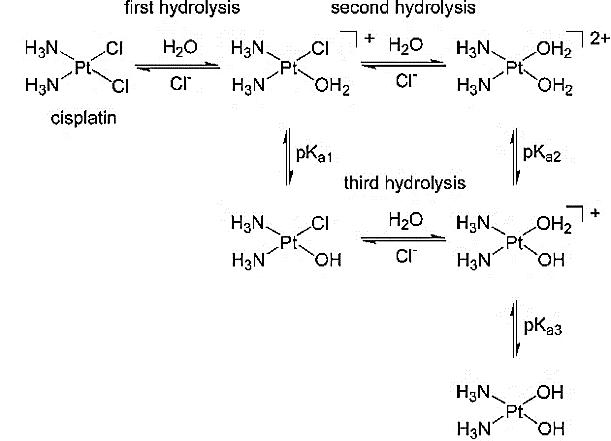
Once the cis-platin has entered the cell, it is broken down by hydrolysis, replacing the chloride groups for water molecules. This is essential for the drug to have an effect within the nucleus and interact with the DNA; it is the key activation step before the drug can reach its specific target. The major reason why the hydrolysis takes place is because of the particularly low concentrations of chloride ions in the surrounding cytoplasm, allowing the chloride groups to be substituted by the water molecules due to water’s relatively high abundance. The mechanism by which this reaction takes place is a two-step nucleophilic substitution.
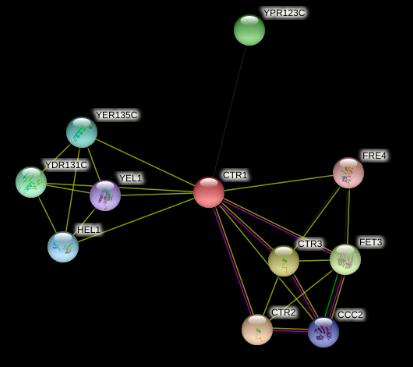
Figure 2. Hydrolysis of cis-platin.
As seen in Figure 2, the reactions between the two molecules are reversible. The hydrolysis most commonly occurs twice on each cis-platin molecule but does have tendencies to occur once.
The Pt-Cl bond has high kinetic lability and greater negative charge compared to the Pt-NH3’s inert and neutral characteristics. A high kinetic lability means that the chloride ligand can undergo a fast substitution reaction. This means that the chloride ligands act better as a leaving group as the Pt-Cl bond is more easily broken. Because water is a good nucleophile, it therefore targets the more vulnerable chloride ligand at a much faster rate, creating [Pt(H2O)2(NH3)2]2+. The hydrolysis of the cis-platin post cell entry is crucial as it could easily pass through the phospholipid bilayer previously, due to it being a non-charged molecule.
How does cis-platin bind to DNA?
Cis-platin is most commonly known for its extreme cytotoxic effects on DNA which ultimately leads to cell death as a result of the distorting effects upon the double helix. After its uptake into the cell, it is now within the nucleus and the positively charged cisplatin ion [Pt(H2O)2(NH3)2]2+ is attracted electrostatically to the partially negative nitrogen on the DNA bases and coordinated to the bases on the DNA nucleotide.
The platinum ion binds to the N7 site on the guanine (Figure 3), forming a mono addition compound. It is described like this due to the singular platinum bonding to the DNA. The cis-platin can bond more tightly with the N7 on guanine as it balances the platinum ion charge much more effectively than chloride. This is because the strength of attraction between the positively charged complex and the negatively charged backbone of DNA would be greater than with the chloride ion.
Figure 3. Structure of guanine.
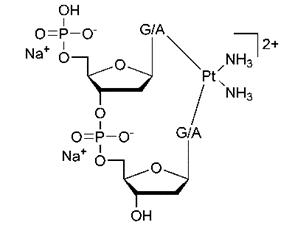
The other chloride ligand also undergoes hydrolysis leading to the formation of a bifunctional adductplatinum bonded to two adjacent nucleobases. The chloride ligand is then essentially substituted with another guanine base on the DNA.
Figure 4. Bifunctional adduct- platinum bonded to two adjacent nucleobases.
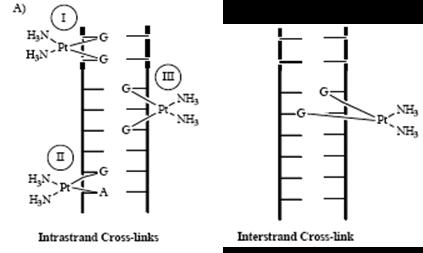
The binding between the cis-platin and the DNA results in the many crosslinks that are either intrastrand or interstrand (Figure 5). Figure 5. Diagram showing both types of cross-links.
Interstrand crosslinks are the links between the opposite strands within the DNA and the intrastrand crosslinks are the links between the same strand of DNA. This is highlighted in Figure 5, detailing the crosslinks the cis-platin has caused within the DNA. The crosslinks shown have detrimental effects on the cancerous cells’ DNA such as: the unwinding of the DNA itself; bending the DNA and even breaking strands off it.
The formation of a particular type of intrastrand crosslinks, of the 1,2 intrastrand, results in significant distortion to the DNA. The preferability of platinum to bind to two adjacent guanines on the same strand makes up for 60% of the bound platinum resulting in a bending of 45 degrees towards the major groove. This results in the DNA unwinding entirely by up to 20%, therefore prohibiting DNA mechanisms for asexual reproduction, a crucial process for the replication of the cancerous cell.
Side effects of cis-platin
Although there are many positives surrounding cisplatin, there are some considerable drawbacks which will be experienced by almost all patients when going through chemotherapy. This is because cis-platin attacks both cancerous and non-cancerous cells. Although cis-platin does specifically target cells that reproduce at a faster rate, such as cancer cells, they also affect non-cancerous cells that also reproduce at a fast rate. This is the causation behind effects such as vomiting and anaemia. Similarly, the cis-platin molecules have a negative effect upon the digestive system and is able to irritate specific parts of the tract, leading to side effects such as diarrhoea and a loss of appetite.


Figure 6. Structure of carboplatin (top) and oxaliplatin (bottom).
Cis-platin is also seen to have some close relatives as seen in Figure 6, carboplatin and oxaliplatin. With regards to structure, the carboplatin differs ever so slightly from cis-platin in that it has a bidentate dicarboxylate in substitute for the two chloride ligands in cis-platin. It takes up a very similar role of cis-platin and ultimately distorts the DNA through the formation of interstrand crosslinks, prohibiting it from replicating.
However, carboplatin exhibits lower reactivity, resulting in a slower DNA binding speed. It is also seen to cause different morphological changes in MCF-7, a breast cancer cell, while exerting their destructive behaviours on the double helix. Furthermore, the lower reactivity leads to a lower excretion rate meaning the body retains more carboplatin, causing longer lasting side effects such as a low red cell blood count and vomiting.
The other platinum-based compound that is commonly used as an anti-cancer drug is oxaliplatin. This drug consists of a cyclohexane structure combined with an ethanedioate ion to form a molecule with IUPAC name of [1R,2R]cyclohexane-1,2-diamine](ethanedioate0,0)platinum(II). The chemotherapy drug effectively treats cancers within the digestive system such as stomach cancer, pancreatic cancer and bowel cancer. Its mode of action is similar to cis-platin and it also prohibits the synthesis of RNA and DNA within the nucleus, leading to cell death. Due to the similar structure, the side effects of the platinum compound include vomiting, loss of hearing and Neutropenia (low number of white blood cells). Oxaliplatin has less ototoxicity (being toxic to the ear, specifically the cochlea and the auditory nerve) compared to cis-platin and carboplatin.
Conclusion
First synthesised in a 19th century Italian laboratory, cis-diamminedichloroplatinum (II), has over time, become a household name. The use of mustard gas in WW1 prompted many researchers to investigate further into these chlorine-containing compounds.
It was not until Rosenberg’s accidental discovery in 1965, however, that the potential of cis-platin to treat cancer was unleashed. Its mechanism of action allows the drug to bind to cell DNA and interfere with maintenance, eventually killing the cell.
Between 2010 and 2016, colon cancer survival rates were at a high of 63%. Whilst this 18% increase in survival rates may also be contributed to societal factors, the lower demand for surgery or radiotherapy is sure to improve the quality of life of survivors as well as cancer patients: a valuable yet immeasurable benefit.








