
11 minute read
WHY IS HEROIN SO ADDICTIVE?
Abstract
Heroin is an illicit drug that has instigated a worldwide crisis which has led to a steep rise in drug related deaths since its discovery in the 1900’s. Its tendency to be addictive has caused scientists to look at the pharmacology of the drug to devise treatments such as the drug methadone. Heroin has a structure likened to morphine however has more potent effects. Described as an opioid; it is a semi-synthetic drug that can be harvested from poppy seeds but can be synthesised in labs. Heroin has a history embedded in pain relief however exploitation of this has led to a drastic influx of addiction over the last century.
Heroin’s action as an opioid
The term opioid refers to all compounds that bind and exert their pharmacological actions through three opioid receptors (μ, κ, and δ) at pre-synaptic neurons.[1] Receptors can be defined as ‘molecules that recognise a specific second small molecule whose binding brings about the regulation of a cellular process.’ [2] Overall, these neurons make up the opioid system which controls the feelings of pain and reward. The opioid system, within the central and peripheral nervous systems, are stimulated by the body’s naturally synthesised endogenous opioids such as met-enkephalin. These are considered natural pain killers and regulate the body’s response to pain.[1]
Heroin, also known as diamorphine, is considered an exogenous opioid. In comparison to endogenous opioids, these are not naturally synthesised by the body and are introduced into the body externally. However, exogenous opioids such as heroin have very similar chemical structures to endogenous opioids such as metenkephalin (Figure 1). This feature allows heroin to mimic and deceive the body into thinking it is an endogenous opioid, enabling it to effectively engage and stimulate the body’s opioid system. As well as this, heroin is described as a full agonist as it stimulates many more opioid receptors compared to endogenous opioids resulting in a massive amplification in opioid receptor activity.[3] Once entering the body heroin is broken down into 2 main metabolites, depending on how it enters the body. If ingested, heroin breaks down into morphine. If taken via intravenous administration heroin metabolises into 6acetylmorphine (6-AM) in the central nervous system. Both metabolites are able to bind and activate μ receptors.[4]
Heroin’s mechanism of action is located at synapses. A synapse is a tiny gap between adjoining neurons where neurotransmitters, e.g. dopamine, are released to transmit chemical messages across the nervous system. When an electrical impulse reaches the end of a presynaptic neuron it triggers the release of neurotransmitters from tiny sacs called vesicles. Once released into the synapse they diffuse across to the post-synaptic neuron. Once enough neurotransmitters bind to the specific postsynaptic receptor, the neuron fires an electrical impulse.[5]
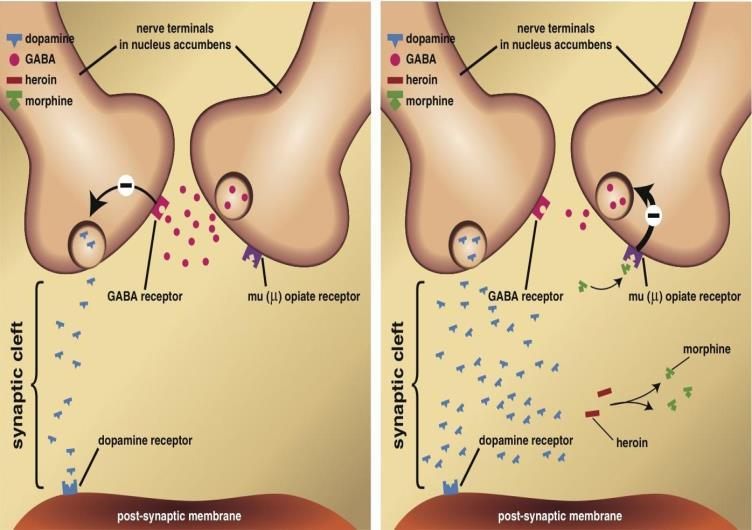
With heroin involved, its metabolites bind to μ receptors on the pre-synaptic neuron. This sends an electrical impulse to a dopamine terminal.
Met-enkephalin
(an endogenous opioid)
Morphine
(a metabolite of heroin)
Figure 1: Structural correlation between metenkephalin and morphine.
Dopamine terminals are pathways that control the release of dopamine – the neurotransmitter that plays a vital role in how the brain processes feelings of reward and motivation.[6] They are found at dopamine-producing neurons which are mostly concentrated at the brain’s Ventral Tegmental Area (VTA) [7] (Figure 2). The impulse from the metabolites instructs the terminal to release significantly higher levels of dopamine than it normally would. Causing extremely high levels of dopamine to be released into the synapse and bind to receptors at the post-synaptic neuron (Figure 3). Dopamine is an excitatory neurotransmitter and so it increases the likelihood that the post-synaptic neuron will fire. Resulting in increased electrical activity as many more postsynaptic neurons are firing to the nucleus accumbens and the prefrontal cortex (Figure 2). These are areas of the brain where feelings of reward and motivation are processed.[6]
The metabolites of heroin also have another mechanism of action. μ receptor activation decreases the release of the neurotransmitter GABA. GABA is an inhibitory neurotransmitter which is also found at the VTA, responsible for regulating levels of dopamine in order to stabilise mood. When the release of GABA is reduced, it is unable to stabilise dopamine levels effectively. Resulting in the already high levels of dopamine, caused by an over-release at dopamine terminals, unable to be reduced and controlled (Figure 3).[8] The excessive levels of dopamine are why heroin users experience an unstable sense of intense excitement and euphoria. Repeated high levels of dopamine also enhance the reward-related memories heroin brings. It does this by strengthening synapses at various sections of the brain: the hippocampus – an area of the brain focused on learning and memory, the amygdala –regions focused on planning and reasoning and the prefrontal cortex – an area focused on emotional associations with rewards. The formation of these memories is why heroin is so addictive, and why relapse is so extremely common.[9] The relapse rate for all substance abuse disorders ranges from 40% to 60%, but for heroin specifically it is around 90% or more.[10]
Pharmacokinetics is a term that refers to the absorption, distribution and metabolism of a compound within the body. Derived from the Latin for drug, pharmakon, the distribution of heroin can vary the effects from pain relief to instigating a sensation of euphoria. The rapid effects on the brain, due to effective delivery, has been linked to the highly addictive properties of this opiate. Similar to the effects of morphine, heroin also works on mediating rewards in the brain thus giving the user a sense of euphoria.[11]

Figure 2: Brain structures involved in the reward system. Figure 3: Effects of heroin on the synaptic transmission of dopamine and GABA.
Pharmacokinetics and Addictiveness
Heroin can also be further subclassified in the group of opioids as a phenanthrene since it causes greater incidences of nausea and hallucinations due to the presence of a hydroxyl group on the sixth carbon. Intravenous methods of taking heroin lead to it being metabolised into 6acetylmorphine and consequently morphine.[12]
Heroin is a central nervous system (CNS) depressant that slows down the activity in the brain. How heroin breaks down in the body depends on the route of administration and how you use the drug. If heroin is taken orally, it passes through the liver and breaks down into morphine before it reaches the brain. If heroin is injected, smoked or snorted, the drug does not pass through the liver. Instead, the heroin goes straight to the brain, where it immediately breaks down into morphine and other chemicals, 6-AM and 3-AM, before it binds to the brain’s opioid receptors. When morphine and these chemicals bind to the opioid receptors, a euphoric high is felt.[13]
Heroin works on binding to μ opioid receptors (MORs) in the brain. These are specific for the regulation of pain and wellbeing. The activation of this means they stimulate the neurotransmitter dopamine. Dopamine promotes motivation and is involved in the brains rewards system – this pleasurable feeling is often a cause for addiction to the opioid. Over time if a person takes an opioid repeatedly, the brain does not naturally produce dopamine as it once did as it is reliant upon the stimulation of a drug such as heroin to provide the means of the neurotransmitter.[14] Heroin is also described as a full agonist due to its effects of activating opioid receptors in the brain which fully result in the full opioid effect since it has high efficacy and high activity in the body – triggering sensitive mu and kappa opioid receptors.

Figure 4: The structures of heroin and morphine The addition of two acetyl groups makes heroin more lipid soluble resulting in a rapid onset of action. These acetyl positions are on both the 2and 6- positions meaning that the effects of heroin are more potent than morphine.[15] This acetylation is thought to enhance the permeability of the drug into the blood barrier membrane thus resulting in a greater penetration to the brain even at a lower dose. The effectiveness is enhanced since they dissolve in the phospholipid membrane of cells and easily enter the brain. Once within the brain, the acetyl groups on heroin are removed enzymatically to produce morphine, which leaves the brain only slowly. [16] This deacetylation is thought to produce 6-AM. This form is an intermediate that subsequently converts into morphine.
Morphine and heroin can be distinguished through how they drugs are metabolised and excreted. As mentioned, 6-AM and heroin do not remain in the blood as these forms as they are converted into morphine. After an injection of morphine, morphine is the dominant active series and is excreted rapidly as a glucuronide. The formation of a glucuronide occurs when the drug binds to a glucuronic acid via a glycosidic bond through a condensation reaction where one water molecule is removed. In contrast, once heroin is injected the presence of 6-AM in urine distinguishes the drug as heroin from morphine.[17] Heroin can be analysed through electron impact mass spectroscopy to demonstrate accurate levels of heroin and metabolites present in a blood matrix.
Panel A (Figure 5) demonstrates the initial characterisation of heroin and B demonstrates the levels of 6-AM and morphine as a standard. Thus, panel C and E shows the presence of an underived 6-AM as shown by the peak with the arrow forming at the expense of heroin depletion. Showing how morphine can further be derived from this intermediate.

Figure 5
Pain relief of Morphine to the addiction of Heroin
Although diacetylmorphine was not prescribed as a medicine much before 1900 its preparation had already been reported in 1874 by C. R. Wright at St. Mary's Hospital in London. The main purpose of his work was to determine the constitution of some natural and purified alkaloids.[18] By boiling anhydrous morphine alkaloid for several hours with acetic anhydride he was able to isolate acetylated morphine derivatives. This compound corresponds to diacetylmorphine according to our present nomenclature.[19]
Opioids have had a long run of being able to widely treat pain and cure an opium addiction. Throughout last century, Morphine has been used as a pain killer such as in the American civil war it was used to manage pain from battle wounds. German pharmaceutical company ‘The Bayer Co,.’ used heroin as a cough medicine and a treatment that worked against tuberculosis and pneumonia. However, physicians noticed that patients experienced an unhappy side effect of becoming dependent on the medication. Later in 1912, it emerged to be used recreationally by young men in New York City. In the UK a heroin user could get treatment from the NHS through seeing a local GP or contacting a local drug treatment service. They would be asked a series of questions to gauge the extent of their drug use and a urine sample is taken to confirm the use of heroin. Proceeding this the individual would be asked to choose between two different approaches of treating heroin: Maintenance therapy or detoxification (detox). Maintenance therapy - switches from heroin to a heroin substitute such as methadone (Figure 6) or buprenorphine and then stay on a stable dose of the substitute. Detoxification switches from heroin to a heroin substitute but this time the individual is gradually withdrawn from the substitute until they are completely free from both.[21]
In the UK, a heroin user could get treatment from the NHS through seeing a local GP or contacting a local drug treatment service. They would be asked a series of questions to gauge the extent of drug use including a pee sample to confirm the use of heroin [11]. Proceeding this the individual would be asked to choose between two different approaches of treating heroin: Maintenance or detoxification (detox). Maintenance therapyswitches from heroin to a heroin substitute such as methadone or buprenorphine and then stay on a stable dose of the substitute. How does methadone have similar mode of action but slower (for treatment of addiction) Addicts emerged years later knocking on the doors of hospitals for treatment. This spiralling addiction led to 1/5th of the population of America to become addicted to heroin.[20]
How heroin addiction is treated
Methadone, also called physeptone mixture linctus, is a synthetic opiate used as an alternative to heroin in the detoxing process. It has similar effects to morphine and heroin. It is administered by mouth in the form of liquid, pill or sublingual tablet. Tests were carried out in the 1960s and 1970s to test the effectiveness of methadone as a substitute. When morphine was tried and given to patients it gave euphoria to opiate addicts but only lasted 4 – 6 hours of which patients would then need another dose.
However, methadone seemed to provide full analgesia in contrast to the 4-6 hours experienced with other drugs. When repeated doses of methadone was given over 24 hours opiate side effects were observed. It was inferred that there was an accumulation of methadone which resulted in a much longer half-life of the medication. When methadone was given to patients addicted to heroin it prevented signs and symptoms of withdrawal for 24 hours.[22]
Its main mechanism of action is as an agonist at opioid receptors whilst also acting as an antagonist which blocks NMDA (glutamate) receptors which is thought as to be the cause of

pain reliving effects as shown in Figure 7. In addition to this, the mechanism involves the binding of methadone in body tissues (primarily the liver) with subsequent release into the circulation, and extensive plasma protein binding, which limits plasma total and unbound concentrations of methadone and prolongs the pharmacological actions of methadone in patients receiving a daily maintenance dose.[23] Therefore, methadone is well suited to daily interval dosing due it is ability of amassing a reservoir which has its own timed release unlike other short acting opiates like heroin and morphine.

Figure 6: The structure of methadone Figure 7: The mechanism of methadone at opioid receptors








