Purification of novel antibody formats: A toolbox approach

Antibody-based therapies offer targeted treatment options for a cancer to autoimmune disorders. As the field continues to evolve bispecific and fragment antibodies have emerged as important new modalities, enabling novel mechanisms of action—while also introducing new challenges in purification.
Antibody fragments, such as Fab, scFv, and individual light chain (VL) or heavy chain (VH) variable domain fragments, are an attractive therapeutic modality due to their smaller size, ease of production, potential for better tissue penetration and reduced immunogenicity compared to full-size mAbs.
Bispecific antibodies (BsAbs) represent another breakthrough in therapeutic design, with their ability to simultaneously engage different antigens. By simultaneously binding to a cancer cell and a T cell, bispecific antibodies draw immune cells closer to tumors, amplifying the body’s immune response beyond the capabilities of traditional mAbs. Other forms of bispecifics engage targets to block aberrant signaling pathways that drive a range of pathological conditions. According to the United States Food and Drug Administration (FDA), more than 100 BsAbs are in clinical development. Since 2014, the FDA has approved nine BsAb marketing applications to treat cancer, as well as hematologic and ocular diseases.i
Novel modalities present new purification challenges
The unique properties of antibody fragments and BsAbs not only expand the potential of biologic therapies, but also pose different challenges in purification and manufacturing that must be addressed for successful clinical and commercial development.
Standard mAb purification workflows typically rely on Protein A affinity chromatography, which efficiently binds to the Fc region of antibodies, enabling a straightforward and high-yield purification process. However, many antibody fragments lack
the Fc region, making Protein A ineffective. While Protein L is useful for capturing antibody fragments lacking an Fc region, it has several limitations. It selectively binds kappa light chains, making it ineffective for lambda or light chain-free fragments. The lower binding capacity and pH sensitivity can affect efficiency and purity, while its high cost and reduced stability limit reusability. Additionally, Protein L may exhibit non-specific binding, necessitating further polishing steps.
For bispecific antibodies, purification is complicated by the potential for mispaired chains, unwanted byproducts, and the formation of aggregates. Mispairing of antibody chains occurs during production when the two heavy and two light chains fail to pair correctly, leading to product-related impurities such as homodimers, half-antibodies, and mixed light chain antibodies. Mispaired chains can lead to misfolding, exposing hydrophobic regions that promote protein aggregation.
The complexity of purification workflows for BsAbs and fragments makes process development a critical aspect of manufacturing, requiring customized approaches tailored to the specific structure and properties of each therapeutic candidate.
The value of a toolbox approach for purification
A flexible, multifaceted purification strategy is essential for novel antibody formats. By utilizing a diverse selection of resins and purification techniques, as shown in Figure 1, process developers can tailor their approach based on key factors such as impurity profiles, protein properties, and process parameters. This adaptable methodology enhances the purification efficiency of both the capture and polishing steps, ensuring scalability and robustness while accommodating the specific needs of different antibody formats.
To address the challenges of purifying novel antibody formats, selecting the appropriate chromatography resins is crucial. The following studies highlight different resins that can be used with a range of antibody formats to optimize downstream processing.



Antibody fragment capture using an alternative to Protein L
MiMode™ PuraBead® HX2 has a unique synthetic ligand that demonstrates broad specificity for the capture and purification of mAbs and their derivatives, with affinity for both kappa and lambda chains. The synthetic nature allows for robust base-stability, and the PuraBead® 6HF base matrix offers high flow properties even for large-scale manufacturing. These attributes make it an appealing alternative to Protein L as a platform technology.
MiMode™ PuraBead® HX2 was compared to a competitor Protein L resin for antibody fragment capture and purification using an E. coli derived variable kappa light chain (Vk) fragment as a model feedstock.
Q PuraBead® Edge SP PuraBead® Edge
Working with a tricky mAb molecule?
Astrea Bioseparations has a toolbox of purification strategies available for a broad range of antibody challenges, including:
• Host cell protein reduction
• Viral clearance
• Aggregate removal
• Host cell DNA reduction
• Protein A leachates
• Endotoxin removal
• Light chain separation
• Glycoprotein separation




The buffer conditions selected for Vk fragment purification on the 1 mL pre-packed MiMode™ PuraBead® HX2 column was a pH 9.0 equilibration followed by an elution step in acetate buffer at pH 5.0. MiMode™ PuraBead® HX2 elution steps were performed at milder pH conditions (pH 5) than the competitor Protein L (pH 2.5) and assessed for Vk recovery and purity.
Elution recovery of Vk fragments was comparable between the two adsorbents, at approximately 77%, however, the elution purity for MiMode™ PuraBead® HX2 was significantly higher than that of the competitor Protein L product, as shown in Figure 2. The non-reduced SDS-PAGE was also used to compare the Vk elution profiles, as shown in Figure 3, showing effective purification using MiMode™ PuraBead® HX2.
cell protein (PPM log clearance)
The optimized workflow for purifying E. coli-derived Vk fragments using MiMode™ PuraBead® HX2 offers a potential alternative to Protein L, demonstrating significant improvements in HCP clearance under milder pH conditions. Beyond Vk fragments, this workflow also highlights the potential of MiMode™ PuraBead® HX2 for purifying other antibody formats, such as Fab, scFv, and bispecific antibodies, making it a versatile solution across diverse therapeutic modalities.
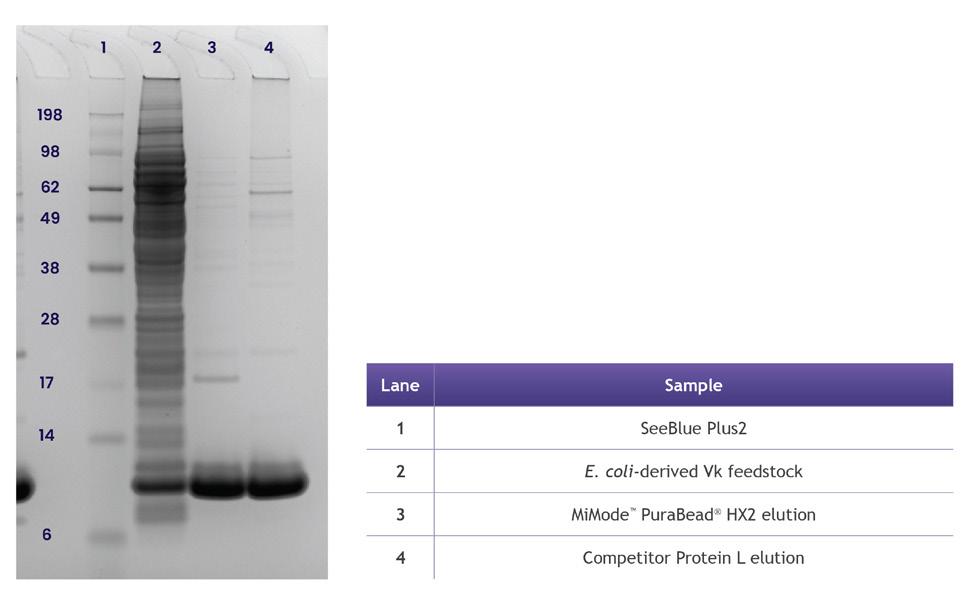
3: Non-reduced SDS-PAGE used to compare the Vk elution profiles.
Exploring unique ligand chemistry
The removal of HCPs is a universal challenge across all antibody and antibody derivatives. MiMode™ PuraBead® HL4 is a mixed-mode resin with a different operational space compared to other commercially available products and can provide alternative strategies for problematic HCPs. The following study evaluated the impact of pH and conductivity on IgG purification using MiMode™ PuraBead® HL4 resin against another market adsorbent.
When using a toolbox approach, a DOE study is an invaluable, systematic method for efficiently exploring process conditions to identify the optimal purification parameters for polishing of novel antibody formats. MiMode™ PuraBead® HL4 was evaluated using DOE methodology for the impact of pH and conductivity on IgG purification versus another commercially available adsorbent. Heat maps, a predictive modeling tool used to visualize results, identify the most effective purification conditions, which, for MiMode™ PuraBead® HL4 showed consistent performance across a broad pH range. The importance of a toolbox approach and screening various resins is highlighted in the DOE study results summarized in Figure 4. The heat maps display HCP clearance and IgG yield, with the red regions illustrating the most optimal performance.
The MiMode™ PuraBead® HL4 resin also demonstrated consistent performance across a broad conductivity range, maintaining high HCP clearance and IgG yield with minimal variation. In contrast, the competitor resin showed strong pH dependence, with optimal performance at pH 6, but significant yield drops at higher pH values (highlighted by the blue regions in Figure 4) suggesting a different interaction between the ligands chemistry and IgG. While MiMode™ PuraBead® HL4 maintains high IgG recovery across various pH levels, increasing the pH in the competitor’s product caused the antibody to bind to a greater degree, reducing recovery in the flow-through fraction and ultimately lowering yield.
MiMode™ PuraBead® HL4 Competitor mixed-mode resin
Figure 5: Chromatogram showing Protein A affinity purified bispecific feed loaded onto a SP PuraBead® Edge column (20 cm bed height) and eluted with step increases of 1 M NaCl elution buffer, conductivities at which peaks eluted are annotated in black text.
Figure 4: Results of a DOE study used to determine optimal pH and conductivity for HCP log reduction and yield for an IgG produced in an HEK cell line. The heat maps demonstrate MiMode™ PuraBead® HL4’s stable performance across a broad pH and conductivity range.
MiMode™ PuraBead® HL4 contains a unique mixed-mode ligand which gives it a broad operational space and stable performance compared to other commercially available products, providing a flexible alternative for hard-to-remove HCPs.
Bispecific antibody polishing
BsAb aggregates can trigger immune responses in patients and must be removed via purification processes, which can impact overall purification yields. While Protein A can still be used in the initial capture step of BsAbs, subsequent polishing steps must efficiently separate correctly assembled bispecifics from mispaired species, aggregates, and other impurities. The heterogeneity introduced by bispecific antibody production requires additional purification strategies, such as orthogonal chromatographic techniques, to ensure a final product with high purity and functionality.
Figure 6: Using SP PuraBead® Edge for BsAb aggregate reduction (post-Protein A bispecific feed), reducing aggregates from 20% to <5% using an optimized step elution. Average percentage of higher molecular weight (MW) impurities (yellow) and antibody (teal) content determined by fluorescence SEC analysis.
SP PuraBead® Edge cation-exchange (CEX) adsorbent leverages the differences in charge interactions on a 65 µm bead, to achieve high resolution separation of the desired BsAb from unwanted mispaired species, aggregates and other impurities. Typically, the target protein binds to the resin in a low-salt buffer at a pH below its isoelectric point (pI), allowing it to interact with negatively charged functional groups. Aggregates often exhibit stronger binding, allowing them to be effectively separated by manipulating the conductivity levels of the elution, with bsAbs eluting first, followed by aggregates at higher salt concentrations. Slight variation in charge density of the product impurities (mispaired variants and fragments) also allows separation by manipulating salt levels in the elution.
Potential mispaired species Higher MW impurities

mispaired species. Banding circled in teal represents higher molecular weight (MW) impurities. The main target product is seen in the 28 mS/cm elution fraction at approximately 125 kDa.
This polishing has been demonstrated using SP PuraBead® Edge, post-Protein A affinity using differing conductivity ranges (Figure 5). The result of this study demonstrated a BsAb aggregate reduction from 20% in the load (post-Protein A bispecific feed) to <5% with the use of an optimized step elution, as shown in Figure 6. SDS-PAGE analysis of the fractions (Figure 7) demonstrates not only the reduction in aggregate content, but also the effective separation of mispaired species and other unwanted byproducts, highlighting the versatility of SP PuraBead® Edge in improving both purity and functionality. The results demonstrate SP PuraBead® Edge’s capability to resolve charge-based variants in bispecific antibody feeds, effectively reducing aggregates and mispaired species. This highlights its value as a high-
resolution polishing step for mAb derivatives, for improved
Novel biologic formats, such as bispecific antibodies and antibody fragments, are redefining the landscape of drug development. These innovative modalities offer distinct advantages in targeting and efficacy, but, also introduce new complexities in purification that require tailored solutions.
A toolbox approach leveraging a diverse array of resins, ligand chemistries, and optimization techniques enables process developers to fine-tune purification workflows for optimal yield, purity, and scalability. Solutions such as MiMode™ HL4, and SP PuraBead® Edge address critical purification challenges, demonstrating improved HCP clearance, enhanced antibody fragment recovery, and superior aggregate removal for bispecific
As the demand for next-generation biologics accelerates, the ability to efficiently purify complex antibody formats will be a key determinant of success in clinical and commercial manufacturing. By embracing innovative purification technologies and methodologies, the industry can ensure the continued advancement of novel antibody therapeutics, bringing more precise, effective, and scalable treatments to patients in need.
Specialized purification technologies will remain integral in improving manufacturing efficiency, enhancing product quality, and overcoming the unique challenges associated with antibody fragments and bispecific antibodies.
Discover how MiMode™ PuraBead® HX2, MiMode™ PuraBead® HL4, and SP PuraBead® Edge can optimize your antibody fragment purification processes. For detailed insights into any of the resins in this paper, or to discuss your specific purification needs, visit our website astreabioseparations.com or reach out to us sales@astrea-bio.com
