




By Matthew Battles
And I have felt
A presence that disturbs me with the joy
Of elevated thoughts; a sense sublime Of something far more deeply interfused…
—William Wordsworth, “Lines Written a Few Miles Above Tintern Abbey” (1798)
Walking along the meadow to my office, I lately have been stopping where a few blackberry canes intrude on the path, their dark, heavy drupelets perched among the thorns. These plants are unaccessioned, and so it might not be counted a harm to the collection were one of these clusters to find its way into my hand and thence to my mouth, gently to be crushed between the teeth. You can imagine it as I do now: the soft crack of fruity skin, the breaking of the barriers, the fruit-seep going syrupy, and the granular swarm of seed among teeth; the contraction of the swallow pulsing through palate and gum as inner surfaces register this encounter with the outer, this mingling, this interfusion.
I’ve been fascinated with this word, interfusion, since I re-encountered Wordsworth’s great poem earlier this year. The lines thrill me— the presence of this “something far more deeply interfused” that “rolls through all things”—and yet I am caught short by the ring of electric modernity I hear in it, which gives the lines an uncanny tilt, an awkward prescience. It’s the “fuse” that gets me; I can’t help seeing a switchboard, a server, a tangle of cables.
I’ve gone looking for earlier uses of the word. Before Wordsworth, it shows up perhaps most prominently in Milton’s Paradise Lost (1667), when Adam implores the angel Raphael to disclose the secrets of creation—to reveal “(h)ow first began this Heaven which we behold/ …and this which yields or fills/All space, the ambient air wide interfused/ Embracing round this florid Earth...” The ambient air wide interfused. Clearly, we’re not talking about wires and cables, but a kind of soft solution that buoys a flowering planet.
Today, fuses are fundamental components of the electrified world. They take their name from the fusibility of their metal
elements—fusibility from the Latin fundere, to pour. Before electrical systems, fusion was much more a matter of the liquid world—diffusion, perfusion, solutions mixing and combining. Robert Boyle’s Skeptical Chymist (1661), published six years before Paradise Lost, is full of fusion, perfusion, and affusion (which is the pouring of liquid onto a body), of rinsing and stirring and swishing to discover nature’s liquid intimacies. The emerging science of the seventeenth and eighteenth centuries is full of fusion and combination; its method is to separate that which is mixed by nature. We might recall that “confusion” is part of this family of terms, too.
Back to the meadow. Looking out from the blackberry bramble into the foliage, over the woodland sunflowers and fringing dock into the marshy expanse of cattail and reed, sensing the movement of water through this landscape, moving through the roots, beneath the ragged regatta of willows dipping and tacking in a wind-tossed sea of blade and flower. And rising from it all, a hum that crests the wave of traffic from the Arborway, the swish and stridulation, the creak and crackle, the rustle and the roll, of a great, entangled orchestra.
What about “entanglement”? It’s a term in current favor to name the essential interdependence that is the condition of living things. One imagines the tangle of roots and mycelia, of hawthorn and blackcurrant in meadow and hedge, the Green Man’s eyes peering out amid a leafy mane. Entanglement’s modern currency emerges not from botany, however, but from quantum physics, from the discovery of particles that somehow maintain a connection however far they wander from each other. “Spooky action at a distance,” borrowing a phrase Einstein used for the collapse of the wave function. “Entanglement,” then, describes a state of separation: however strong the effects of connection in the quantum realm, the emphasis lands on distance. Not fusion but entanglement is the motif for our hyperconnected age, with its fraught hope of distances to be overcome.
There’s a Zen koan that comes to mind:
right now, stop the distant temple bell. And arising in response, I sense somehow, no— treasure this distance.
What is it instead to come together in solution? I think of the horticultural practice of grafting, in which branches are joined more intimately than in entanglement. In the graft, it’s essential to bring the living tissues of the two plants into contact. And then what happens? As wounded plant tissues release hormones of sealing and healing into the liquid that bathes the cut, cells reach for cells, unite to form new passages. Interfusion. Maybe this is why the architect and Graduate School of Design professor Jeanne Gang uses “grafting” as a metaphor for an architecture informed by, and in a gentler relation with, the living world. Rather than tearing down and bulldozing, rather than starting with a blank slate that never can be clean, Gang explores the joining of new buildings to old, like scion to rootstock. Can we imagine an architecture, a way of living together, which nurtures interfusion? And what does it mean that grafting begins in woundedness?
Crush of blackberry and the light of setting suns, and the round ocean and the living air, and the blue sky. And the mind? There’s something that happens here in the swarming, fluid intimacy. The trees, the trees are tapped into it. Water in the cattail, water in the willow, and the blackberry, water in me, and then, the sea.
matthew battles is editor of Arnoldia.

—Orville Daniels, Arnold Arboretum Member









Two men stand beside a Larix gmelinii var. principis-rupprechtii in what today is North Korea’s North Hamyong Province in August 1917.
by E. H. Wilson
Madeline Schill recounts what it’s like as the boots-on-the-ground archivist for a large plant expedition; Miles Schwartz Sax climbs Mount Monadnock with students from Zhejiang and Beijing Forestry Universities; Alaina Bisson presents her research on root-microbial symbioses and leaf herbivory in the Arnold Arboretum collections.
Brett J. Chedzoy, Peter J. Smallidge, Paul D. Curtis, Suzanne Treyger, Kass Urban-Mead, Kristi L. Sullivan, and Andrew Hubbard
Lisa Pearson
Kathleen McDermott
Editor
Matthew Battles
Designer Andy Winther
Editorial & Production Assistant
Claire Neid
Editorial Committee
Anthony S. Aiello
Yota Batsaki
Peter Del Tredici
Michael S. Dosmann
Rosetta Elkin
William (Ned) Friedman
Jon Hetman
Jonathan Shaw
Creative Consultants
Point Five
Arnoldia (ISSN 0004–2633; USPS 866–100) is published quarterly by the Arnold Arboretum of Harvard University. Periodicals postage paid at Boston, Massachusetts. Subscribe to Arnoldia by becoming a member of the Arnold Arboretum. For more information, visit arboretum.harvard.edu/support/membership or contact the membership department at 617.384.5766 or membership@arnarb.harvard.edu. Send all other Arnoldia communications to Circulation Manager, Arnoldia, Arnold Arboretum, 125 Arborway, Boston, MA 02130-3500. Telephone 617.524.1718; fax 617.524.1418; email arnoldia@arnarb.harvard.edu.
Postmaster: Send address changes to Arnoldia Circulation Manager The Arnold Arboretum 125 Arborway Boston, MA 02130–3500
Copyright © 2025. The President and Fellows of Harvard College

Arnoldia welcomes your ideas, questions, and feedback. Letters should be sent with the writer’s name, address, and phone number via email to arnoldia@arnarb. harvard.edu. Letters may be edited for length and clarity.
Find us online
ArnoldiaMag ArnoldArboretum ArnoldArboretum. Harvard
Having recently read the summer 2025 publication of Arnoldia, I was struck by the various articles related to the demise of the accessioned silver maple that grew adjacent to Meadow Road. I was particularly taken by the article by Michael Dosmann, “Trees for the Public Good.” He brought the history and importance of this silver maple to life. Having lived in Cambridge over 50 years ago, I would often walk through the arboretum to test my nomenclature and taxonomy of trees and shrubs. During that time, I never particularly took notice of this grand old tree. I now recognize its significance. While genetically not a particularly important species, it has clearly left a massive hole in the sky, but more importantly a hole in people’s hearts.
The saving grace is that its demise has left an opportunity for other plantings. A time for promise and hope.
Peter Trowbridge emeritus professor and chair Department of Landscape Architecture Cornell University

This year marks the centennial of renowned paleobotanist and plant taxonomist H. H. Hu’s (1894–1968) reception of his doctorate in applied biology from Harvard University. Hu Hsen-Hsu (胡先骕 , courtesy name 步曾 ) is most famous for his seismic discovery of Metasequoia glyptostroboides with Cheng Wan-Chun (郑万钧) in 1948, which prompted the subsequent expedition led by E. D. Merrill to collect and plant Metasequoia in the Arnold Arboretum. But his work’s influence began in 1925 with the publication of dissertation, Synopsis of Chinese Genera of Phanerogams. As the first monograph that surveyed and systematized phanerogam plants in China, Hu’s dissertation spurred his decadeslong career in advancing botanical taxonomy and classification systems in China, founding multiple teaching and research institutions and scientific periodicals, and establishing botanical communities around the world. The 100-year mark of Hu’s doctoral achievement offers an opportunity to reflect on his academic entrepreneurship, scientific prowess, and significant contributions to botany that have endured political, national, and institutional histories.







Madeline Schill collects the stories of plant exploration that spill forth on the road.
It was May 7th, 2025, and I was on a plane to Shanghai, China for the 2025 North America-China Plant Exploration Consortium (NACPEC) expedition with my colleagues Michael S. Dosmann (Keeper of the Living Collections) and Miles Schwartz Sax (Assistant Curator of Living Collections). I had been working as a Collections Fellow at the Arnold Arboretum for less than six months on an archival project for NACPEC.
Founded in 1991 by the U.S. National Arboretum, the Holden Arboretum (now
Holden Forests and Gardens), and the Morris Arboretum, this group consists of nine member botanical gardens and arboreta in North America and partners in China who collaborate to facilitate the collection of plant germplasm and vouchers in the field, share germplasm across gardens for ex situ conservation, and exchange personnel, research, and botanical knowledge across international boundaries.
The Arnold Arboretum Horticultural Library and Archives was designated the
official NACPEC archival repository in 2012, and I was hired to ensure that these archives were processed, organized, digitized, and accessible to anyone who seeks to use them. I was invited to join the NACPEC 2025 expedition along with Michael, Miles, and five other participants from the Morton Arboretum, Holden Forests and Gardens, Longwood Gardens, and the UBC Botanical Garden. Miles and I tested out a novel system of documenting the trip by assigning roles to participants each day to generate photographs, daily journal entries, plant observation lists, and plant photos. It was my role as the trip archivist to consolidate those materials and process them into the archives after the trip. Between the eight of us, we were hoping to have one of the most comprehensively documented expeditions yet.
I also had a goal to record interviews and oral histories of the expedition team. As NACPEC approaches its thirty-fifth year, an initiative to plan for its future has prompted an inspection of its past. Many original NACPEC participants have retired
Oral histories allow us to hear firstperson accounts in the speaker’s own voice.
or, in some cases, passed away. While archival papers and correspondence can unveil stories from the early years of the consortium, oral histories allow us to hear first-person accounts in the speaker’s own voice. With an assembly of veteran and first-time NACPEC participants representing gardens from both sides of the Pacific, the 2025 expedition was a rare opportunity to record these perspectives in the field for the first time.
The first few days of the expedition flew by. By May 14th we had traveled 200 miles from Shanghai to the Tianmu Shan (shan, 山, meaning mountain in Mandarin) Grand Canyon. I had been searching for a moment to record an interview or oral history since the trip began, but the reality of botanizing in the field is that the focus is on the plants: taking notes, keying out species, snapping photographs, and—with NACPEC’s documentation standards in practice—snapping photographs of the people focused on the plants. There was an ease with which the group interacted with each other and the environment. Interrupting that focus felt
In their most recent report, the “State of the UK’s Woods and Trees,” the Woodlands Trust underscored the value of understanding, inventorying, and protecting ancient woodlands across UK landscapes. Ancient woodlands have developed over centuries—some since 1600—making them some of the most biodiverse terrestrial ecosystems on the planet. The Ancient Tree Inventory, an initiative that began in 2019, seeks to record where these remnant woodlands and ancient individuals are holding on, using Light Detection and Ranging (LiDAR) charts, geo-referenced old maps, ecological site surveys, and local knowledge. Such mapping is imperative as developers, policy makers, landowners, foresters, conservationists, and landscape historians make sense of and operate among ancient ecosystems. Coordinated efforts to identify and protect centenarian oaks, yews, ashes, beeches, alders, chestnuts, and more signify the means relationships between ligneous giants and their ecological and human communities.

like disrupting something sacred—a flow that would have stiffened with the formality of a recorded interview.
The audiophile in me wanted to capture all of these sounds with the equipment I had spent hours researching and carried with me across thousands of miles: a brand new RØDE WirelessPRO. But when the time came to take my first recording, I found myself pulling out my cell phone instead. Tony Aiello (associate director of collections at Longwood Gardens and longtime NACPEC participant) and I had just sat down at a rest point overlooking the stairs we had just climbed up the Tianmu Grand Canyon gorge. Despite being a bit out of breath, he agreed to speak. Our conversation lasted only the three-and-a-half minutes it took for the rest of the group to catch up. I only conducted one other interview this way: a brief chat with Miles Schwartz Sax (assistant curator at the Arnold Arboretum) and Andy Hill (curator-horticulturist at UBC Botanical Garden) as we sat down for lunch fifteen minutes later. I was happy to collect some content, but it was becoming clear this format was not going to be the most productive. Our rest breaks were needed after hiking in 93° F heat in altitudes
The audiophile in me wanted to capture all of these sounds with the equipment I had spent hours researching and carried with me over thousands of miles.
over 6,000 feet, and we didn’t have enough time or energy to get into the meat of the topics I hoped to cover.
That evening, I pored over options for capturing oral histories. Rest-stop interviews were more or less out; they were too rushed and intrusive. Walking interviews were also out, since the majority of our botanizing was at high elevations with frequent stops to look at plants and trees. Meals were typically on the go or coupled with more rest. I even tried to add oral histories to the schedule for the day, but when the time came, everyone was too exhausted to take part. I was, to be honest, at a loss.
After a travel day on May 15th leaving Hangzhou for Yichang, May 16th took us up into the Shennongjia mountains. We gawked out the van windows at Abies fargesii (Farges’ fir), Betula albosinensis (Chinese red birch), and over a dozen species of oaks. We spent the day climbing to scenic overlooks, botanizing roadsides, and piling in and out of the vans for many short drives in between. After nearly ten hours, the group was headed back to Muyu (木鱼) town, a twohour drive. The sun had fallen behind the mountains and left us shrouded in a low blue light. We were passing the time with
a steady flow of conversation between Kim Shearer (director of collections and curator at the Morton Arboretum), Kang Wang (head of education at the National Botanical Garden, Beijing), Zhang Xuteng (a graduate student from Zhejiang University), Liu Qun (Kunming Institute of Botany), Tony Aiello, and myself. Kang and Tony were counting the NACPEC trips they’d been on to see who held the record (Kang won, eight to seven). Tony, reflecting on his NACPEC career, casually made a comment about how his first trip should have been in 2001 but had to be postponed a year because his and Kris Bachtell’s flights were cancelled on 9/11. I had been hoping to ask Tony about his experience during a future visit to Longwood Gardens, but here in a van in the Shennongjia mountains with a captive audience and nowhere else to be, the opportunity arose to learn his side of the story. As soon as he mentioned where he was on September 11th, 2001, I pulled out my phone, and he agreed to share the story for the archives.
Tony told us he had flown from Philadelphia to Chicago in early September 2001 a few days before his inaugural NACPEC expedition to Shanxi Province. The morning of his flight to Beijing on September 11, he got to O’Hare Airport early to try to upgrade his seat to business class. When he approached the counter, the TV behind it showed that the first tower had been hit. The woman helping him at the counter didn’t seem to know if the events would affect his flight or not, so Tony was able to secure an upgrade. He checked his luggage and arrived at his gate just as the second tower was hit. At this point people in the airport were beginning to panic, so airport staff came and turned off all the TVs. All flights were grounded, and everybody at the airport was stranded for hours. Tony remembered the Salvation Army arrived with sandwiches for passengers and airport staff. After waiting all day to retrieve his checked bag, Tony was able to get to Kris Bachtell’s (NACPEC participant from the Morton Arboretum) home where he stayed for several nights. The trip cancellation was communicated to their partners awaiting
The car interview became the most reliable format for recording audio.
them in China. A few days later, Tony rented a car and drove all the way back to Philadelphia. One year later, Tony, Kris, Carole Bordelon of the U. S. National Arboretum, and Peter Bristol of the Chicago Botanic Garden left for Shanxi. The itinerary was identical to the one planned for 2001, except they made a point not to schedule their departing flights on September 11th.
Following this impromptu recording, the car interview became the most reliable format for recording audio. During our last group dinner in Xi’an ( 西安 ) on May 21st before folks began heading their separate ways, the group passed my phone around and shared their highlights from the NACPEC 2025 trip. The recording begins with my own voice and the chatter of the table and waitstaff. A roaring stove in the kitchen bellowed like a dragon every few minutes. As we passed the phone around, stewing in 95-degree heat and full of lamb pao mo ( 羊肉泡饃 ), one sentiment was shared by all twelve participants: gratitude.
As I edited my recordings back in Jamaica Plain, the deep hum of the road lifted the chorus of voices and brought me back to those long hours spent winding through the mountains of China and, more importantly, learning from the people that have shaped NACPEC into the exemplary consortium it has become. NACPEC has persisted through decades of political and social change between nations, with its focus remaining on collaboration and the shared love of plants. This is clear through the boxes of archival correspondence where introductions were made, agreements secured, research shared, and excitement expressed at the prospect of working together to connect and share knowledge and germplasm between multiple countries. Every person involved with NACPEC loves plants and people—something that breaks down the barriers our countries are consistently putting up. Documenting and digitizing these interactions through the NACPEC archival project is crucial to the future of the consortium and the effort it puts towards plant conservation, research, and above all, collaboration.
Miles Schwartz Sax helps a group of visiting Chinese students experience New England’s floristic biodiversity

We hiked up the rocky slopes of Mount Monadnock on a sunny, clear day in mid-July, the wind picking up speed as we neared the summit. We were climbing through incrementally shorter stands of red spruce (Picea rubens) and catching glimpses of alpine flora such as mountain cranberry (Vaccinium vitis-idaea), threetoothed-cinquefoil ( Sibbaldiopsis tridentata), and Rand’s goldenrod (Solidago simplex) growing in cracks in the rock. Rising in elevation, these changes became legible as a gradient of floristic communities across elevation. This included seeing red-spruce krummholz (as the short, tangled thickets of treeline conifers are called), balsam fir (Abies balsimia), mountain ash (Sorbus americana), and alpine flora which, without the effects of altitude, would require driving significantly further north and undertaking longer climbs. This diversity of floristic communities was exactly what we had come here to see, with students who traveled halfway across the world to be here.
A group of fourteen students and four instructors hailing from Zhejiang University and Beijing Forestry University were visiting to participate in a six-day field course exploring New England’s unique ecosystems. The trip was hosted by Arnold Arboretum staff Michael Dosmann (keeper of living collections), Madeline Schill (collections fellow), and myself (assistant curator), and together, we were their instructors and guides. These students had come here experience the region’s ecology, floristic biodiversity, and botanical institutions. Within a few weeks’ time, they would visit sand-dune communities, salt marshes, bogs, mountain tops, hardwood forests, meadows, museums, and cultivated landscapes. They would learn to read the landscape, uncovering the region’s geological, anthropological, and natural histories.
As we found our way to the top of the monadnock, the wind howled as came into clear views. We could make out Boston’s skyline and the peaks of the Green and White Mountains in the distance. The hike was worth the views alone, and we shared a feeling of triumph reaching the top. Hunkering down below the summit, we took shelter from the wind as we ate snacks, took selfies, and explored the tiniest of plants that dwell there.
Born from a desire to experience and understand ecology, the field course allowed a group of people from across cultures and continents to come together to appreciate and deepen our connection with nature. Just as disjunct genera bind common evolutionary ancestry across disparate corners of the globe, so does the human desire to understand and find our place in the ecology of our planet. While the majors of the students varied across fields, I felt assured that this experience would strengthen their bond to nature. I hope that as the students looked out at the seemingly endless expanse of the New England Acadian forests from the top of Mount Monadnock, they saw what I saw: glimpses of the strands of evolutionary history that bind all living organisms together on this planet we call our home.
PhD student Alaina Bisson sets out in the collection to discover how root-microbial symbioses shape leaf herbivory
This summer saw the culmination of a two-year-long research project in the Living Collections at the Arnold Arboretum that uniquely combined two perspectives on plant relationships in terrestrial ecosystems. Beginning in the summer of 2023, I teamed up with DaRin Butz intern Maya Akazawa and my advisor Benton Taylor to research plant-microbe symbioses. Maya was passionate about insects and plants, but the Taylor lab largely studies below-ground dynamics between plants and soil microbes and how they are shaped by global change. How could we bridge above-ground plant-insect interactions with below-ground plant-soil microbe


Alaina Bisson is a PhD student in Dr. Benton Taylor’s lab at Harvard University studying how plants mediate carbon and nutrient cycling in terrestrial ecosystems.
relationships? And how could we generate a research project combining these two perspectives?
The symbiotic partnerships that plants form with root-associated microbes are a key mechanism by which carbon and nutrients, such as nitrogen (N), are exchanged between the plant and surrounding soil. These below-ground dynamics can
TREES OF LIFE
On the night of September 28, 2023, two men felled Britain’s most iconic tree, the Gap Sycamore, which stood next to the ancient Roman fortification known as Hadrian’s Wall. For some 150 years, the tree (an Acer platanoides or Norway maple, called sycamore in Britain) had stood in a dip in the Whin Sill, a geological feature the Wall follows in sections across Northumberland. Evidence in court suggested that Daniel Graham and Adam Carruthers had used a storm as cover to make their cut, later exchanging text messages celebrating news coverage of the deed and its aftermath. In July of this year, High Court judge Christina Lambert sentenced the men to four years three months in prison. Their act has spurred calls for improved protection of heritage trees throughout Britain. Meanwhile, the National Trust reports that new shoots have sprung up from the stump; with care, the tree may rise again to grace the Sycamore Gap in another century.
influence plant leaf chemistry, which shapes how insect herbivores select leaves to eat. Reaching outside the box, we built a research idea: interactions below ground (the Taylor lab’s specialty) could be linked to patterns of insect herbivory above ground (Maya’s focus) through differences in how nutrients are allocated within leaves. We sought to explore why some leaves experience more insect herbivory than others— why they “taste” the way they do—and what this might indicate about their belowground symbioses. That is, how does the stuff we can’t see (the chemistry and nutrient acquisition in root-microbe symbioses) drive the stuff we can see (leaf herbivory)?
We set out for the collections with two central questions: (1) Do insect herbivory rates differ among below-ground symbiotic groups? and, (2) Are insect herbivory rates related to leaf nitrogen?
We randomly selected 138 individual woody plants both native (e.g., Acer pensylvanicum, Quercus alba, Amorpha fruticosa) and nonnative (e.g., Acer, yui, Fagus japonica, Caragana boisii) representing our three root-microbe symbioses: arbuscular mycorrhizae (AM), ectomycorrhizae (EcM), and N-fixing bacteria. Each of these three root-microbe relationships offer different forms of nutrients and leaf chemistry. Trees that associate with AM fungi often have thinner leaves made up of inorganic N and
“Humans may write with landscapes less by extraction and more by planting seeds, by leaning toward sunlight.”
Gretchen Ernster Henderson in “Withness Trees,” page 66.
How does the stuff we can’t see (the chemistry and nutrient acquisition in rootmicrobe symbioses) drive the stuff we can see (leaf herbivory)?
phosphorus. In contrast, trees that associate with EcM fungi have thicker, tougher leaves more resistant to decomposition. Trees that form partnerships with N-fixing bacteria house the bacteria in root nodules where they can convert atmospheric N into bioavailable forms for the plant to utilize. Trees engaging in symbiotic N-fixation often have leaves high in nitrogen, making them potentially very attractive to insect herbivores.
From June 2023 to July 2023, we collected one sun-exposed leaf from a lateral shoot in each cardinal and secondary direction for every individual plant, resulting in a total of eight leaves per plant. After amassing hundreds of leaves, we calculated the percent leaf area consumed using LeafByte—a mobile app that measures total and consumed leaf area—and the rate of herbivory for each leaf by dividing the percent leaf area consumed by the number of days the leaf had been out (around May first for all plants). We then dried the leaves and ground them into a fine, uniform powder to analyze their nitrogen content N content using a FlashSmart NC Soil Elemental Analyzer.
In our many hours of collecting leaves, we found that EcM trees experienced the highest rates of herbivory, AM trees showed intermediate rates, and N-fixing trees experienced the lowest—an unexpected outcome, given that N-fixing trees have significantly higher leaf nitrogen than both AM- and EcM-associated trees. We saw no differences in herbivory rates between native and non-native species, suggesting that the patterns were driven more by leaf chemistry than by a plant’s geographic origin. If having higher leaf nitrogen wasn’t leading to more herbivory, what was?
This question carried us into the summer of 2024 with another DaRin Butz intern, Oscar de la Torre. Herbivore feeding is influenced not only by leaf nutrient concentrations, but also by chemical defenses. Plants can produce phenolics, carbon-based secondary compounds that can reduce leaf consumption by insects. Might insect herbivory rates be related to leaf phenolic

Along the banks of the Colorado River and the tributaries that flow into it, research and low-technology restoration efforts are helping boost the presence of generations of cottonwood trees (Populus deltoides, P. angustifolia, and P. deltoides ssp. wislizenii). As reported by NPR and Utah Public Radio, decades of damming and drought conditions have exacerbated threats to cottonwoods—a keystone species in riparian ecosystems that rely on periodic flooding and perennial water. Recently, efforts to construct earthen dams—a technology used by southwestern Indigenous peoples such as the Zuni, not to mention millennia of beavers—have begun, wherein weeds are pulled and replaced with native grasses, trenches are dug and filled with wooden posts and rocks to help water spread laterally and slowly outward from the river, allowing cottonwoods crucial water access. In some areas, drones have measured tributaries and creeks expanding in width from two to twenty feet where willows and young cottonwoods have begun to populate.
concentrations?
Using our leaf samples collected in 2023, we employed colorimetric methods to measure total phenolic concentration by mixing the leaf samples with a reagent wherein the intensity of color indicated the phenolic concentration.
Our hours of toiling at the lab bench resulted in another intriguing finding: N-fixing species had significantly lower leaf phenolic concentrations than AMand EcM-associated species. How was it that N-fixing species, the group that experienced the lowest rate of herbivory, also have the lowest concentrations of phenolics, compounds that are thought to deter herbivores?
It’s possible that the plants increased their phenolic production in response to herbivory stress or that the leaf nitrogen in N-fixing plants takes the form of N-based defense compounds, rather than carbon-based defense compounds (e.g., phenolics).
The two summers of intern-based research strongly suggested that N-based defense compounds may be the dominant drivers of plant-herbivore interactions. Uniquely, and fortunately, because the living collections within the Arnold Arboretum grow under relatively similar field conditions, we could control for environmental variables and attribute our findings to root-microbe symbioses. In other words, the interactions we can’t see, especially those hidden below ground—and often as a result, underestimated—are often influencing the interactions we can see.
William (Ned) Friedman delights in the diversity of inflorescences produced by this showy hydrangea.
Driving around New England right now, you can see big blue heads of showy hydrangea flowers dominating many a front yard. The wild progenitor species, Hydrangea macrophylla (bigleaf hydrangea), has very different looking inflorescences. Fortunately, at the Arnold Arboretum we have this endemic Japanese species in bloom right now. It’s a real beauty.
Bigleaf hydrangeas, like all hydrangeas, have two forms of flowers: one showy and typically at the periphery of the inflorescence and the other small, towards the interior of the inflorescence, and less prominent (at least to an insect pollinator). This can be seen for example with 405-2007*D, one of the bigleaf hydrangeas in the Explorers Garden, whose small flowers are mostly pentamerous (five parted); in the upper left image you can see two open flowers with five wonderful blue petals (the rest of the yellow-turning-to-blue flower buds have yet to open). The sepals (the outermost whorl of the flower) are very small and not visible. The showy flowers however (upper right image; 405-2007*D) are tetramerous, but here is where things get interesting. The showy parts of these flowers are the sepals! As you can see in the upper right photograph, there are four large light blue sepals and four small, intensely blue petals in the middle of the flower that are very similar to the petals of the small pentamerous flowers.
Most of our cultivated hydrangeas are derived from a small set of mutations that convert most of the small flowers into large, showy flowers. This year, a team of scientists in Japan figured out which genes are involved (these natural mutations occurred in wild plants that then were used to breed new cultivars). The flat-topped inflorescence of the wild species is referred to as a “lacecap” form and the huge, spherical mounds of the showy inflorescences found in our yards, gardens, and many temples in Japan are called a “mophead” form. Personally, I love the subtlety of the natural or wild species, the floating heads of flowers delicately set off against the deep green leaves, with just a sprinkling of the showy flowers on the perimeter of each inflorescence. This can be seen nearby with another bigleaf hydrangea in the Explorers Garden, 405-2007*A (lower image). But, for sheer over-the-top floral displays, you gotta go with the mopheads!

William (Ned) Friedman is the eighth director of the Arnold Arboretum and the Arnold Professor of Organismic and Evolutionary Biology at Harvard. This portrait first appeared in his newsletter on July 19, 2025.
Small pentamerous flowers (top left) and blooms with showy sepals (upper right) come together beautifully in hydrangea’s “lacecap” inflorescences. Photograph by William (Ned) Friedman





Brett
J. Chedzoy, Peter J. Smallidge, Paul D. Curtis, Suzanne Treyger, Kass Urban-Mead, Kristi L. Sullivan, and Andrew Hubbard
During our formative forestry-school years more than 30 years ago, white-tailed deer ( Odocoileus virginianus ) and invasive plants were not yet acknowledged as barriers to sustaining healthy and productive woodlands. Since then, the forestry community has gradually recognized that these are driving and interactive forces that can create major impediments to forest regeneration (Miller and McGill 2019). Without new trees of diverse species and genetics to periodically replace forests changed by pests, harvesting, storms and age, our vast and beautiful deciduous forests have a limited future. Forestry needed a new practice to limit these barriers,

Figure 1. Current specifications for slash walls are a height of 10 feet, a 2-inch branch diameter, and width of 20 feet. Width maintains effectiveness as the slash wall slumps. Photograph by P. Smallidge

a practice that yields multiple benefits. We think we’ve found one.
The early 2000s management at Cornell University’s Arnot Teaching and Research Forest (www. arnotforest.info) focused on strategies to overcome deer impacts and interfering vegetation. The forestry practices we used were something we’d be proud to show our forestry professors, and consistent with the standards of practice. The deer impacts were addressed with Earn-a-Buck, an aggressive hunting program whereby hunters became eligible to shoot a buck after they harvested two female deer. We controlled interfering vegetation, low-stature native and non-native woody species such as American beech (Fagus grandifolia) or bush honeysuckle (Lonicera spp.) with mechanical or chemical treatments. However, the collective efforts of good forestry practices, reduced deer herd size, and reduced abundance of interfering vegetation still were not sufficient for us to successfully regenerate forests.
By 2010, we conceded that these approaches would continue to be ineffective until we resolved the selective and excessive browsing by deer of
valued woody and herbaceous woodland flora, which was leaving an increasingly degraded vegetative condition in the woods (Figure 2). Consequently, we resigned ourselves to the reality that we would have to start building deer-proof fences that carried significant costs for installation, maintenance, and removal. In the stands we managed, we wanted to leave behind a legacy of something more than the disease-prone beech and non-native shrubs that dominated the lower strata of our aging oak-maple woods.
Our College of Agriculture and Life Sciences maintains multiple forested lands representing a spectrum from nature preserve to operational-scale forest. Because the Arnot Forest is fully managed as a research and teaching forest, we test and demonstrate sustainable practices that can be used to inform management—including regeneration—of New York’s almost 15 million acres of private forests. Most forests of NY and in the Northeast struggle to regenerate hardwoods (Shirer and Zimmerman 2010, Miller and McGill 2019), in large part attributable to selective browsing by white-tailed deer. Numerous remedies to “the deer problem” were being discussed

(e.g., introduce wolves and mountain lions, or implement market hunting), but we didn’t have years or decades to wait for any of the array of possible solutions to emerge. We needed to work within our mission to support the managers of today who work in NY and northeastern forests.
On a particularly stormy day in the spring of 2016, as we finalized plans to build our first sizeable deer fence, the sounds of falling trees and limbs outside helped us to realize that maintaining such a fence just wasn’t within our capabilities. Shortly afterwards, we began talking through the concept of using mechanized logging equipment to pile firewood-quality trees and logging debris, known as “slash,” into windrow-like barriers around the perimeter of harvested areas to prevent browsing pressure on desired seedlings. We could also use the mechanized harvesting equipment to efficiently remove “interfering” understory vegetation such as American beech, eastern hophornbeam (Ostrya virginiana), and striped maple (Acer pensylvanicum).
Because the task of a research forest is to test the ideas that seem a bit risky for the real world,
we set course for building what would become the first “slash wall” (www.slashwall.info). The process started by talking with numerous forester and logger colleagues to solicit their often-blunt opinions—none of which proved discouraging. One source of those opinions came from Zooks Logging Partnership, an Amish contractor from a nearby community. The Zooks were intrigued by the idea of using slash to make formidable barriers for the purpose of protecting vulnerable seedlings. They too were frustrated to see their good management be lost to over browsing by deer.
In March 2017, Zooks started work to implement our novel concept of “slash wall” to protect a 75-acre regeneration harvest. The initial, rather nebulous contract specifications for a slash wall “ten feet high by ten feet wide and dense enough to exclude deer” were soon refined by the ingenious loggers who completed the 7,500-foot-long perimeter of deer-excluding slash wall by July (Figure 1). The loggers drew from prior experiences; ultimately, they built slash walls with a 5-foot tall foundation of 6-inch diameter and larger stems that prevent deer from crawling

under the slash wall, and coarse branches on the top to prevent crossing over even when there was significant snowfall. Lingering deer were pushed out, the gates closed, and effectively no deer activity occurred inside the slash wall for six to seven growing seasons, by which time the promising new forest had grown to 5 feet or more, beyond the deer browsing height. The initial slash walls had plastic mesh fence gates that were too wide and vulnerable to deer, but subsequent slash walls have resolved that design flaw.
Zooks built three other slash walls that year, ranging from nine to thirteen acres, to demonstrate the concept at smaller scales in different forest conditions, which provided additional insights and knowledge (Figure 3). In all cases, Zooks Logging used a feller-buncher. This is specialized logging equipment the size of a large excavator, but the scoop head is replaced with a head having large hydraulic arms and a horizontally mounted circular saw 42 inches in diameter. The feller-buncher can cut trees and brush (like a large weedwhacker) and also pick up trees, logs, and slash for piling.
As part of our Cornell Cooperative Extension
roles, we hosted webinars and field tours before, during, and after the early slash walls were built. This helped spark adoption of this practice, first in Rhode Island and subsequently in other parts of New England and the Midwest (Figure 4).
We satisfactorily answered three key questions with the initial walls:
• could logging equipment build a slash wall with materials at hand? (yes)
• how much did it cost to build the slash walls? ($1.47 per linear foot, plus about $100/acre to brush the understory in 2017 dollars)
• would the slash walls exclude deer? (yes, if built to the current specifications of 10 feet tall to a 2” diameter branch and 20 feet wide. Smallidge et al. 2021)
We then turned our attention to new research questions, starting with an evaluation of the natural regeneration inside and outside of slash walls. It took only a single growing season to begin to see dramatic differences between the slash wall interiors and adjacent control areas, a future versus a failure.

Intensive sampling and monitoring efforts began in 2019.
After the first few growing seasons, clear patterns inside slash walls included the establishment and growth of desired woody species and greater diversity of woody and herbaceous species (see sidebar). We also noted that brushing the understory with the feller-buncher, obviated the need to use herbicides to control native interfering vegetation (e.g., American beech and eastern hophornbeam). We learned that slash walls slump at a rate of about 12 inches per year (Smallidge et al. 2021), so the race was on to grow seedlings above the height of deer before the slash wall’s integrity was lost.
After five to seven growing seasons, the vegetative disparities were even greater (Smallidge, Chedzoy and Ward, in preparation). Commercial tree species occur on 55 percent of sample points inside slash walls versus only 25 percent outside slash walls. By the fourth growing season, commercial and diversity tree species greater than 4.5 feet tall may be 2 to 10 times more abundant inside than outside slash walls. Finally, our colleague David Weinstein conducted
preliminary tree growth modeling that forecasts some of the harvested areas may have 40 percent more carbon sequestered inside versus outside the slash walls after 50 years. On a cost comparison basis, slash walls were about one-third the cost of the fenced exclosures we investigated, required practically no maintenance, and involved no need for removal. The only attention we provide after the slash wall is built is to walk the interior perimeter after a snowfall to make sure there are no gaps that deer have exploited. Over the years, we also periodically keep track of the gate and the slash wall’s integrity, and if a thin spot starts to develop we plug it with slash or cover with a bit of mesh fence. This low-intensity effort contrasts with the twice monthly inspections we invest in a fenced area that may require repairs every month or two. Unlike plastic mesh and wire deer fences, slash walls can be left in place to gradually decompose back into the soil.
The number of woody species, known as “species richness,” was greater inside than outside the slash walls. Species richness depended on the location, how many growing seasons passed since the harvest,
We are grateful for access to an accomplished botanist, F. Robert Wesley of the Cornell Natural Areas. Robert has spent considerable time observing the vegetation of slash walls. Robert has been particularly responsive when we would send pictures of “something we haven’t seen before.” Over the years, Robert has identified many herbaceous species that are, as he describes, “not commonly found” outside of slash walls.
Actaea racemosa (Cimicifuga racemosa) black cohosh, bugbane
Anaphalis margaritacea pearly everlasting
Aralia hispida bristly sarsaparilla
Aralia spinosa Devil’s walking stick
Blitum capitatum (Chenopodium capitatum) (not seen in 40 years) strawberry blite
Chenopodium simplex maple-leaved goosefoot
Galium lanceolatum wild licorice
Gentiana clausa bottle gentian
Gentians clausa closed gentian
Geranium carolinianum Carolina geranium
Gnaphalium uliginosum Everlasting, rabbit-tobacco
Hedeoma pulegioides American pennyroyal
Hieracium scabrum rough hawkweed
Lilium philadelphicum wood lily
Polygala sanguinea old field milkwort
Pseudognaphalium obtusifolium Cudweed
and the size class of woody plants. Woody species richness inside slash walls is 25 to 30 percent higher by the 7th growing season as compared to outside slash walls. For example, in a recent slash wall after 3 growing seasons there were 17 woody species greater than 4 inches tall outside the slash wall and 19 woody species inside the wall, which included American hornbeam (Carpinus caroliniana) and black cherry (Prunus serotina). In one of the first slash walls, after 7 growing seasons, there were 9 woody species greater than 5 feet tall outside the slash wall and 17 inside the slash wall, including for example black cherry, cucumber magnolia (Magnolia acuminata), northern red oak (Quercus rubra), staghorn sumac (Rhus typhina), and willow (Salix spp.)
At other locations around the Arnot Forest, eight new slash walls were built between 2018 to 2020 and ranged from one to over 150 acres in size. This range of sizes helped us assess the lower practical limit for slash walls, which is about 4 to 5 acres during commercial logging. We then turned our attention to
new investigations that included the influences of regulating shade at the canopy and ground levels. Over the winter of 2021-2022, we built a new series of mostly 4-acre slash walls across a 300-acre basin that included multiple forest types and conditions to evaluate the regeneration response to different silvicultural treatments both in the presence and absence of deer. An early result was verification of the amount of wood in a slash wall (Cranmer et al. 2024) (Figure 5). The results from that Climate and Applied Forest Research Institute project (CAFRI, https://www.esf.edu/cafri-ny/index.php) are forthcoming. What we consistently see across the range of slash walls is that the temporary exclusion of deer provides the necessary opportunity for us to establish and grow a diversity and abundance of desired species. These species reflect the species found in the canopy and allow us to replace aging woods with similar but rejuvenated woods. Without restricting deer access, key species like the oaks, red and sugar maple, black cherry, and basswood are browsed by deer and

gradually engulfed by competing plants to the point that they will ultimately disappear from the mix and future forest.
Our next slash-wall project will start in late summer of 2025. This slash wall will focus on the rehabilitation of degraded forest through the incorporation of enrichment plantings. Another aspect of that study will be to evaluate the influences of Beech Leaf Disease, as the dominant beech-brush understory appears to succumb to the rapidly spreading condition. Concurrently, research within the perimeters of older slash walls will assess tactics to ensure that the seedlings and saplings of key species like oak and maple (the cornerstones of our mixed species forests) will successfully be recruited into the main canopy and remain competitive with other tree species. This will be the focus of our annual field practicum, which is always held on the last Thursday in September (details available at www.cornellforestconnect.ning.com).
Perhaps the most satisfying result and validation
of the concept to date has been the widespread adoption of slash walls. Kass Urban-Mead of the Xerces Society documented additional slash walls in RI, NH, NY, CT, MA, VT, PA, and MI on federal state, county, municipal, industry, academic, private, and NGO ownerships (Figures 6 and 7). These experiences have demonstrated the effectiveness of slash walls under a broad range of conditions, and have shown the adaptability of contractors and partners, who have built slash walls with most types of common farm and logging equipment that can move and pile woody material. Most of these slash walls were built with a feller-buncher, sometimes including a forwarder which is a logging-sized motorized “wagon” that can transport the slash around the harvest. On the less mechanized end of the equipment spectrum, for example, one logger built a vertically oriented grill of railroad rails, mounted on his bulldozer, to push the slash into position.
Just as the Arnot Forest seeks to better understand the wide range of benefits and services

provided by forests, so do the owners and managers of private and public forests of the Northeast. Having recognized the potential merits of slash walls, several colleagues and partners have explored them from a variety of perspectives. Wildlife, pollinators, and sustainable water-quality management are just a few of the other areas where slash walls are showing value. The following contributions share some of these perspectives.
Paul Curtis
Hardwood forests of the northeastern U.S. face browsing pressures from white-tailed deer (Curtis et al. 2021, Miller et al. 2019, Lesser et al. 2019, Blossey et al. 2019). Successful regeneration is often impaired by deer, resulting in a “regeneration debt” (Miller and McGill 2019), or an insufficient number of juvenile stems to replace older generations. The primary methods used to reduce deer impacts and enhance forest regeneration include recreational hunting and
wire fencing (Smallidge et al. 2021, Curtis et al. 1994). Although hunting has been used for decades to manage deer populations, Williams et al. (2013) showed that hunting alone was insufficient to reduce deer populations to <10 deer/km2 in New Jersey and Pennsylvania. Wire fencing, while not frequently used in forest settings, can effectively protect seedlings from deer. However, it can be costly to construct, operate, and maintain (Curtis et al. 1994). Perimeter slash walls (Smallidge et al. 2021, Chedzoy and Smallidge 2023) have the potential to cost-effectively exclude deer from regeneration harvests.
Slash describes the coarse woody debris (CWD) and low-value trees remaining after logging practices have concluded, and it is natural barrier for deer. Grisez (1960) reported that 36 percent of seedlings in slash were browsed, compared to 51 percent of seedlings in open sites. Smallidge et al. (2021) found that tagged seedlings inside slash walls at the Arnot Teaching and Research Forest near Van Etten, New York, were taller than seedlings in adjacent control plots after 4 years. Construction of slash walls was also less expensive than wire fencing. On average,
deployment of slash walls cost $4.82 per meter (2017 dollars), compared to wire fencing at $6.50-$12.20 per meter (Smallidge et al. 2021, Curtis et al. 1994).
Slash walls may benefit wildlife, as many taxa use CWD, including mammals, birds, and invertebrates (Loeb 1996, Sullivan et al. 2012). Red foxes (Vulpes vulpes) and American martens (Martes americana) denned under logs (Loeb 1996, Sullivan et al. 2012). A study in Pennsylvania showed that cottontail rabbits (Sylvilagus floridanus), mice (Peromyscus spp.), voles (Microtus spp.), and birds used brush piles as winter refuges (Goguen and Caccese 2011).
Sullivan et al. (2012) examined small mammal diversity (voles, mice, and shrews (Blarina brevicauta) between forest clear cuts with and without CWD in British Columbia, Canada. The overall abundance of mammals in clearcuts was unaffected. However, species richness and diversity were either similar or higher in cuts with CWD, and the constructed debris piles had more winter mammal activity (Sullivan et al. 2012).
In the southeast, cotton mice (Peromyscus gossypinus) used rotting stumps, root boles, and logs as refuges and burrows (Loeb 1996, Fritts et al. 2016). The fungi that grow on decaying logs were eaten by rodents enhancing recycling of CWD (Loeb 1996). Invertebrates also used CWD for food, refuge from extreme temperatures and low moisture, and shelter (Grodsky 2016, Grodsky et al. 2018). CWD served as shelter, refuge from predators, and a food source.
Avian Affirmation of Slash Walls
Suzanne Treyger
The decline of avifauna across all habitat types has been well documented in recent research (Rosenberg et al. 2019; State of the Birds Report 2022 and 2025). Eastern forest birds have declined by 17 percent since 1970, due to several factors, most notably the loss of habitat quantity and quality (Rosenberg et al. 2019). When assessing habitat quality for nearly 100 eastern forest bird species with varying forest habitat needs, multiple forest characteristics/conditions must be considered. Bird species that use mature forest habitat have varied, but specific requirements for places to nest, forage, and perch that are maximized with high vertical structural diversity, or the layering of vegetation from the forest floor to the tall tree canopy, including an understory (the vegetation that occupies the lowest 5 feet of the forest)
Young or regenerating forest within mature

Figure 8. In areas where deer densities are high, Indigo Bunting abundance is reduced, or they are absent altogether. Richard Benjamin/Audubon Photography Awards
forested landscapes is also important for birds as it provides nesting and post-fledging habitat (Figure 8). However, the availability of quality young forest and understory is often impaired or absent in areas where white-tailed deer are overabundant due to prolific browsing of small trees and other vegetation.
Research shows that deer densities influence bird populations, and bird species that use the forest floor or understory layer for nesting or foraging are more adversely affected (deCalesta 1994; McShea and Rappole 1999; Tymkiw et al. 2013; Rushing et al. 2000). Indeed, research indicates that bird species richness and abundance were negatively impacted by deer densities as low as 7.9 – 14.9 deer/km2 (deCalesta 1994). Multiple species like wood thrush (Hylocichla mustelina), ovenbird (Seiurus aurocapilla), great crested flycatcher (Myiarchus crinitus), Acadian flycatcher (Empidonax virescens), yellow-billed cuckoo (Coccyzus americanus), indigo bunting (Passerina cyanea), American robin (Turdus migratorius), and hooded warbler ( Setophaga citrina ) experienced reduced abundance or were entirely absent in areas with high deer densities as noted by these studies.
Excluding deer by installing fencing or slash walls can be effective in reducing negative impacts to birds. These exclusion methods have shown positive results to vegetation growth and avian richness and abundance. For example, the Rheinstrom Hill Audubon Sanctuary in eastern New York was managed to increase young forest through two patch cuts (ranging from 6-6.5 acres in size); but had a local deer population estimated to be 55 deer per square mile. To protect regenerating trees and shrubs and the habitat they provide to birds, deer fence was installed around half of the cut areas. Breeding bird surveys conducted annually for four years after forest management show that fenced areas had increases of 154 percent of young forest nesting species, 24 percent increase of mature forest nesting species, and 209 percent increase in generalist species (i.e. birds that may use both young and mature forest) by comparison to unfenced areas. Vegetation surveys at these same sites show higher native plant diversity within fenced areas. Deer typically do not browse most non-native (and usually invasive) plants like Japanese barberry (Berberis thunbergii), European buckthorn (Rhamnus cathartica), glossy buckthorn
(Frangula alnus), multi-flora rose (Rosa multiflora), garlic mustard (Alliaria petiolata), Japanese stiltgrass (Microstegium vimineum), etc. American beech and hay-scented fern (Dennstaedtia punctilobula) are native species that can become invasive, and deer typically do not browse them, either. Native trees and shrubs support far more insects compared to non-native plants, which deer typically do not browse (Tallamy 2007, Tallamy and Shropshire 2009). Since forest-bird nestlings require a diet of insects, this boost in food availability from within slash walls may aid in long-term survival rates and increased fitness ahead of migration (Figure 9).
At Cornell University’s Arnot Teaching and Research Forest, where multiple slash walls are protecting regenerating forest, birding walks conducted inside the slash walls are overwhelming at times due to the number of singing individuals. Here, chestnut-sided warbler (Setophaga pensylvanica), eastern towhee (Pipilo erythrophthalmus), common yellowthroat (Geothlypis trichas), yellow-billed (Coccyzus americanus) and black-billed cuckoo (Coccyzus erythropthalmus), indigo bunting ( Passerina cyanea), and many more birds sing prolifically while

Figure 9. Deer browse regenerating understory forest plants, preventing the development of vertical structural diversity, a necessary habitat component for breeding wood thrush. Photograph by Will Stuart
establishing their breeding territories in the abundant young forest made possible by the protective slash walls. As the young forest within the slash wall matures, the bird species community will shift from young forest nesting obligates to mature forest nesting obligates and generalists. Where deer densities are high, exclusion methods such as slash walls are essential for successful forest regeneration, which provides critical breeding habitat for eastern forest birds and helps support positive population trends.
Kass Urban-Mead and Kristi L. Sullivan
Pollinators are crucial for seed and fruit production of our crop and wild plants, and we often think of showy pollinators visiting our gardens. Yet research shows up to 2/3 of our northeastern bee species rely on forested habitats for at least part of their lives, and up to 1/3 use them for their full life cycles (Harrison et al. 2018, Smith et al. 2021). Healthy forests are complex, and different insects use different parts of a forest—including many important wild bees and
other pollinators (Ulyshen et al. 2021, Mola et al. 2021, Urban-Mead et al. 2023). Caterpillars develop on leaves of native trees (Narango et al. 2020, 2025); bees collect pollen to provide for their larvae; bumblebees (Bombus spp.) seek out leaf litter and cavities for safe hibernation; leaf-cutter bees (Megachile spp.) cut ovals from leaves; still others nest in tunnels in dead stems, underground in the soil, or in dead wood. These species vary in vulnerability to challenges facing our forests, including the loss of plant diversity due to excessive deer browse (e.g. Schweitzer et al. 2014).
Once slash walls exclude deer, some pollination-dependent plant species are more likely to survive and mature, with cascading indirect effects for the pollinators that use those plants. In the young slash wall exclosures we have visited, the increased light from the recent harvest and the absence of deer browse creates a profusion of flowers. Research shows that following a storm, fire, or timber harvest, ground-level flower density is highest in the first 10 years, then declines once saplings begin to densely compete and shade the forest floor (Mathis et al. 2021, Mathis et al. 2022; Cunningham-Minnick 2024).


Figure 10. A queen of the Common Eastern Bumble Bee, Bombus impatiens, flying to forage on Polygala paucifolia. Queen bumblebees overwinter alone. When they emerge in the spring they must quickly gather enough pollen and nectar to found a brand new colony. Forest understory flowers are a crucial source of this early spring nutrition. Photograph by K. Urban-Mead. Figure 11. The forest-associated shiny green sweat bee, Augochlora pura, peeking out of its nest in a piece of woody debris. Inside the log, which is soft enough to excavate but still structurally sound, are a series of tunnels and chambers where the green bee creates one large pollen provision for each egg. Photograph by H. Holm
Once a stand matures, adult trees and shrubs provide many resources to pollinators. Hundreds of caterpillars each use one or more of our native trees—like oak (Quercus spp.), black cherry (Prunus serotina), maples (Acer spp.), ash (Fraxinus spp.), poplars ( Populus spp.), hickory (Carya spp.), and birches (Betula spp.)—and then grow to be beloved moths and butterflies. The pollen and nectar provided by a mature tree is like a “meadow in the sky,” because of the enormous amounts of pollen and nectar a single flowering tree makes.
Bumblebee queens emerging from hibernation rely on spring flowers as they begin new colonies (Figure 10) (Mola et al. 2021a, Mola et al. 2021b). Also, some species of solitary bees fly for just a few weeks in spring and specialize on particular spring ephemerals such as bellwort (Uvularia spp.), geranium (Geranium maculatum), trout lily (Erythronium americanum), and spring beauty (Claytonia virginica) (e.g. Davis et al. 1975). Each of these species benefits from
protection from excessive deer browse and invasive species management (e.g. McKinney et al. 2010).
Delightfully, slash walls also provide a nesting element in abundance: woody debris, of course (Cane et al 2007, Eckerter et al 2021, Requier & Leonhardt 2021). Stone walls and brush piles are good sites for bumble bee nests. And, like a few other mason bees (Osmia spp.) and the sweat bee family Halictidae, one particularly charismatic wood-nester is the iridescent green sweat bee, Augochlora pura (Figure 11) (Stockhammer et al 1966). This sweat bee seems to prefer hardwood over softwood logs, and needs logs which are dry and structurally sound, but soft enough to excavate into tunnels and chambers. It collects pollen to create one large ball of pollen per chamber, onto which it lays an egg. Once logs or snags get too old, they aren’t structurally sound—so the descendants of the bees in today’s slash wall will have to find a new ones. Those softer logs in turn will provide oviposition sites for many beneficial flies, homes for beetles,

salamanders, and fungi.
Landowners who would like to support high diversity of pollinators in their woods can focus on reducing deer impacts and controlling the dense layers of invasive species that often dominate an overbrowsed understory. With deer browse at healthier levels, efforts will improve to restore canopy, shrub, and forest floor plant diversity, and to establish the diverse canopy trees of the future needed for the diverse pollinators which use and prefer different species (Batra 1980, Saunder 2018, Urban-Mead et al 2023, Traylor et al 2024).
Andrew Hubbard
In September of 2019 I attended a tour at the Arnot Teaching and Research Forest. This was my first introduction to the concept of slash walls. Within an hour of seeing a slash wall I was determined to construct one on the watershed forest lands I manage in Northwestern Connecticut.
I identified 75 acres of oak-hickory forest that had
been defoliated by spongy moth (Lymantria dispar) caterpillars. This overmature oak stand was suffering significant mortality due to defoliation events. I prescribed to salvage dead and declining oaks for lumber and firewood, retaining oak and other hardwoods with adequate crown vigor, and particularly focus on retaining white oak (Figure 12).
Despite being actively hunted as part of the deer management program, the deer still were browsing too impactfully to allow the property to regenerate a mix of desirable native hardwoods. My employer was in favor of the slash-wall concept because it was viewed as cheaper than building deer fences. As context, in 2019 we contracted for the construction of a 50-acre, eight-foot-high woven wire deer fence that cost $28,000. We found that while fences are effective at preventing browsing, there are significant added costs in the form of continued maintenance and eventual removal. With this in mind, I decided to construct the slash wall around 40 of the approximately 70 acres of the next harvest area to provide a protected and control area for research by Dr. Jeff Ward of the Connecticut Agricultural Experiment Station (Figure 13).

13. The MDC Water Company for Hartford CT was an early adopter to ensure forests were sustained in their watersheds. Photograph by A.
Hubbard



The slash-wall project area is located on water utility lands and is closed to the public, except for participants in the lottery-based deer hunt program. There was no significant public opposition to the project.
The timber was marked and put out to bid for a lump-sum sale through a sealed bid process. Bidders were required to attend a showing of the harvest site where the project was explained in detail. Bidders were also required to watch Cornell’s Slash Wall video from the Arnot Forest (see references for the link).
A major takeaway from this project was the importance of the layout. The slash wall needs to be designed and sited to maximize the acreage inside per linear foot of slash wall constructed. In the future I will also clearcut a 100-foot-wide strip at the perimeter where the slash wall will be built. Within this footprint, all trees (except high quality sawtimber designated with paint) will be required to be used in the slash wall. The number of trees to be cut in this area could be tallied separately for the bidders’ reference, but tree volume will not be included in the sale. This process deviates slightly from typical practice where the timber to be cut (or retained) is marked by
the forester with paint, tallied, and the summary of volume is shared with loggers and sawmills who bid to purchase and cut the trees. The seller and buyer sign a legal contract that stipulates the details of the harvest. If the buyer pays in full before the harvest begins, the sale is known as a “lump sum up front” sale. Commonly the “seller” includes the owner who is represented by the forester and the “buyer” might be a sawmill and/or their logging crew. Sometimes the logger buys the trees and sells the logs after harvesting to a sawmill, and sometimes the forester works for the sawmill. The loggers cut, skid (i.e., drag), and sort the logs into their highest-value products such as sawlogs (for lumber), scrag logs for railroad ties, firewood, and more. There may be five to seven products sorted by the loggers. Log trucks transport the various products to the mills buying them. Specific to a slash wall harvest, any high quality sawtimber present in proximity to the slash wall will be marked and tallied separately from the other trees in the harvest, but trees less than highest value will be used to build the slash wall. Approximately every 100 feet along the perimeter, a low-value tree in or near the slash wall will be retained and marked at a height
of 10 feet where it is visible from all sides. This reference mark will aid both the logger who builds the slash wall builder and and the forester who inspects the slash wall during construction.
The logging contractor who built the slash wall recommended that in the future we avoid retaining sawtimber sized trees near the slash wall (Figure 14). These trees became an obstacle that slowed production. It’s also best to avoid sharp turns in the slash wall as gradual angle changes are easier for equipment and operators. For this system to work, it is best if the contractor is mechanized. It’s also important to require the slash wall to be built concurrently with the harvest. Do not let slash wall construction fall behind logging operations. A typical harvest has a performance deposit or “surety bond” that ensures the contract is executed, or pays for required practices, such as, perhaps, smoothing skid trails, that aren’t completed. For a slash-wall harvest, a higher-value bond would help cover any costs associated with incomplete slash wall building.
Any site you are considering for a slash wall must be densely stocked to provide enough material. Areas that have previously been thinned may lack sufficient material proximate to the location of the slash wall. Using only the tops of sawtimber trees will not be adequate. Lastly, timing the harvest to coincide with robust seed crop helps ensure regeneration establishment.
To date, our slash wall has been a tremendous success! In my approximately 30 years as a forester, it is by far the best regeneration of any of my projects. There is a nice mix of native hardwoods inside the slash wall. Outside the slash wall, the forest is dominated by ferns, black birch, and heavily browsed oaks and red maple stump sprouts. The slash wall has successfully excluded deer and moose. This area has a transient moose population. There was a small bull moose hanging around while the wall was under construction. We have trail cameras located inside the fence year-round, and have pictures of rabbits, bears, coyotes, bobcats and turkeys. We even had a bear build a den in the slash wall.
Slash walls provide what may become an important new forestry practice to avoid the impacts of deer browsing on forest regeneration. Slash walls have particular merit in regeneration harvest patches greater than about five acres with ample low-grade trees, especially if loggers use mechanized equipment.
While slash walls are new within the scope of professional forestry, a profession more than 125 years old, their initial indications are favorable to ensure greater species diversity, higher abundance of young trees, and reduced costs as compared to the installation and maintenance of fencing. Their rapid and widespread adoption suggests that numerous foresters also see their potential merit. Our work has focused primarily on the response of the plant community. Future research will need to quantify the abundance and duration of the carbon stored in the wall, the geographic patterns of herbaceous species diversity and abundance inside slash walls, how a variety of wildlife respond to the slash wall, potential changes to soil parameters such as moisture and stored carbon, the moisture content and flammability of slash walls if prescribe fire is used, and if different slash wall dimensions are acceptable for various sizes of harvests.
brett j. chedzoy is regional extension forester and Arnot Forest manager , Cornell Cooperative Extension of Schuyler County ; peter j. smallidge is New York State extension forester and Arnot Forest director, Cornell University School of Environment and Sustainable Development ; paul d. curtis is a New York State Extension wildlife specialist, Cornell University School of Environment and Sustainable Development (retired) suzanne treyger is senior manager of forests, Audubon Connecticut and New York ; kass urbanmead is with the Xerces Society for Invertebrate Conservation and Forest Pollinators ; kristi l. sullivan is with Cornell University School of Environment and Sustainable Development ; andrew hubbard is forester for the Hartford Metropolitan District Commission, CT Licensed Forester #010
acknowledgements
This work was supported by a joint research and extension program funded by the Cornell University Agricultural Experiment Station (McIntire Stennis funds, Accession number 1020776) and Cornell Cooperative Extension (Renewable Resources Extension Act and Smith Lever funds proposal number 2019-20-208) received from the National Institute for Food and Agriculture (NIFA,) U.S. Department of Agriculture. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture. Additional funds were provided by NYS Department of Environmental Conservation, NYS Department of Agriculture and Markets, NYS Wood Products Development Council, and CAFRI, the Climate and Applied Forest Research Institute. Mike Ashdown and Mike Mobilio collected field data. Jeff Ward and J.P. Barsky provided suggestions for study design. Dozens of foresters, loggers, and woodland owners shared their opinions about slash walls.
references (forestry)
Chedzoy, B. J., and P. J. Smallidge. 2023. Slash Walls: Concepts and Applications for the Control of Deer Impacts to Forest Vegetation. ForestConnect, Cornell University, Department of Natural Resources and the Environment. https://bpb-us-e1. wpmucdn.com/blogs.cornell.edu/dist/b/5769/files/2023/09/ Slash-Wall-Layout-Final.pdf
Cornell ForestConnect extension program. Slash walls exclude deer, encourage regeneration, and improve forest diversity. Video, https://www.youtube.com/watch?v=k3_ aDNURj_8 accessed August 4 2025.
Cranmer, N., T. Han, B. Chedzoy, P. J. Smallidge, C. Beier, L. Johnson, and X. Xu. 2024. Estimating Merchantable and NonMerchantable Wood Volume in Slash Walls Using Terrestrial and Airborne LiDAR. Forest Ecology and Management 569: 122211. https://doi.org/10.1016/j.foreco.2024.122211
Miller, K. M., and B. J. McGill. 2019. (duplicated in wildlife section) Compounding Human Stressors Cause Major Regeneration Debt in Over Half of Eastern US Forests. Journal of Applied Ecology 56: 1355–1366. https://doi. org/10.1111/1365-2664.13343
Shirer, R., and C. Zimmerman. 2010. Forest Regeneration in New York State. 25 p. The Nature Conservancy.
references (for bird section)
deCalesta, D. S. 1994. Effect of WhiteTailed Deer on Songbirds within Managed Forests in Pennsylvania. The Journal of Wildlife Management 58 (4): 711–718. https://doi. org/10.2307/3809829
McShea, W. J., and J. H. Rappole. 2000. Managing the Abundance and Diversity of Breeding Bird Populations through Manipulation of Deer Populations. Conservation Biology 14 (4): 1161–1170. https://doi. org/10.1046/j.1523-1739.2000.99094.x
North American Bird Conservation Initiative. 2022. State of the Birds Report. Washington, DC: The State of the Birds, United States of America. StateoftheBirds.org.
North American Bird Conservation Initiative. 2025. State of the Birds Report. Washington, DC: The State of the Birds, United States of America. StateoftheBirds.org.
Rosenberg, K. V., et al. 2019. Decline of the North American Avifauna. Science 366: 120–124. https://doi.org/10.1126/science.aax1313
Rushing, C. S., et al. 2020. LongTerm Variation in WhiteTailed Deer Abundance Shapes LandscapeScale Population Dynamics of ForestBreeding Birds. Forest Ecology and Management 456: 117629. https://doi.org/10.1016/j.foreco.2019.117629
Tymkiw, E. L., J. L. Bowman, and W. G. Shriver. 2013. The Effect of WhiteTailed Deer Density on Breeding Songbirds in Delaware. Wildlife Society Bulletin 37 (4): 714–724. https://doi. org/10.1002/wsb.339
deer – wildlife references (p. curtis)
Blossey, B., P. D. Curtis, J. R. Boulanger, and A. Dávalos. 2019. Red Oak Seedlings as Bioindicators to Assess Browsing Pressure and Efficacy of WhiteTailed Deer Management. Ecology and Evolution 9: 13085–13103. https://doi.org/10.1002/ ece3.5860
Curtis, P., M. Fargione, and M. Richmond. 1994. Preventing Deer Damage with Barrier, Electrical, and Behavioral Fencing Systems. Vertebrate Pest Conference 16: 223–227.
Curtis, P., K. Sullivan, P. Smallidge, and J. Hurst. 2021. AVID: A Rapid Method for Assessing Deer Browsing of Hardwood Regeneration. Forest Ecology and Management 497: 119534. https://doi.org/10.1016/j.foreco.2021.119534
Fritts, S. R., et al. 2017. Rodent Response to Harvesting Woody Biomass for Bioenergy Production. Journal of Wildlife Management 81: 1170–1178. https://doi.org/10.1002/jwmg.21241
Goguen, C., and R. Caccese. 2011. Winter Use of Brush Piles by Wildlife in OldField Habitats of Northeastern Pennsylvania. Journal of the Pennsylvania Academy of Science 85: 71–75.
Grisez, T. 1960. Slash Helps Protect Seedlings from Deer Browsing. Journal of Forestry 58 (5): 385–387.
Grodsky, S. M., et al. 2018. Invertebrate Community Response to Coarse Woody Debris Removal for Bioenergy Production from Intensively Managed Forests. Ecological Applications 28: 135–148. https://doi.org/10.1002/eap.1655
Grodsky, S. M. 2016. How Good Is Downed Wood? Avian and Invertebrate Use of Harvest Residues and the Implications of ForestBased Bioenergy. PhD diss., North Carolina State University.
Lesser, M. R., et al. 2019. Modelling WhiteTailed Deer Impacts on Forest Regeneration to Inform Deer Management Options at Landscape Scales. Forest Ecology and Management 448. https://doi.org/10.1016/j.foreco.2019.116427
Loeb, S. 1996. The Role of Coarse Woody Debris in the Ecology of Southeastern Mammals. US Forest Service Technical Report SE94. Washington, DC.
Miller, K. M., and B. J. McGill. 2019. Compounding Human Stressors Cause Major Regeneration Debt in Over Half of Eastern US Forests. Journal of Applied Ecology 56: 1355–1366. https://doi.org/10.1111/1365-2664.13343
Smallidge, P., B. Chedzoy, P. Curtis, and K. Sims. 2021. Evaluating the Construction and Effectiveness of Slash Walls at the Perimeter of Regeneration Harvests to Exclude Deer. Forest Ecology and Management 497: 119529. https://doi. org/10.1016/j.foreco.2021.119529
Sullivan, T., D. S. Sullivan, P. Lindgren, and D. B. Ransome. 2012. If We Build Habitat, Will They Come? Woody Debris Structures and Conservation of Forest Mammals. Journal of Mammalogy 93 (6): 1456–1468. https://doi. org/10.1644/11-MAMM-A-292.1
Williams, S. C., A. J. Denicola, T. Almendinger, and J. Maddock. 2013. Evaluation of Organized Hunting as a Management Technique for Overabundant WhiteTailed Deer in Suburban Landscapes. Wildlife Society Bulletin 37: 137–145. https://doi.org/10.1002/wsb.249
pollinator references
Batra, S. W. 1980. Ecology, Behavior, Pheromones, Parasites and Management of the Sympatric Vernal Bees Colletes inaequalis, C. thoracicus and C. validus. Journal of the Kansas Entomological Society: 509–538.
Cane, J. H., T. Griswold, and F. D. Parker. 2007. Substrates and Materials Used for Nesting by North American Osmia Bees (Hymenoptera: Apiformes: Megachilidae). Annals of the
Entomological Society of America 100 (3): 350–358. https:// doi.org/10.1603/0013-8746(2007)100[350:SAMFUN]2.0.CO;2
CunninghamMinnick, M. J., J. Milam, A. Fassler, and D. I. King. 2024. Best Management Practices for Bee Conservation in Forest Openings. Conservation Science and Practice 6 (11): e13231. https://doi.org/10.1111/csp2.13231
Davis Jr., L. R., and W. E. LaBerge. 1975. The Nest Biology of the Bee Andrena (Ptilandrena) erigeniae Robertson (Hymenoptera: Andrenidae). Biological Notes no. 95: 1–30. (No DOI found)
Dobson, A., and B. Blossey. 2015. Earthworm Invasion, WhiteTailed Deer and Seedling Establishment in Deciduous Forests of NorthEastern North America. Journal of Ecology 103 (1): 153–164. https://doi.org/10.1111/1365-2745.12305
Dobson, A., et al. 2024. Individual and Combined Effects of NonNative Earthworms and Native WhiteTailed Deer on Understorey Plant Survival, Growth and Reproduction. Journal of Ecology 112 (5): 1039–1057. https://doi. org/10.1111/1365-2745.14224
Eckerter, T., J. Buse, J. Bauhus, M. I. Förschler, and A. M. Klein. 2021. Wild Bees Benefit from Structural Complexity Enhancement in a Forest Restoration Experiment. Forest Ecology and Management 496: 119412. https://doi. org/10.1016/j.foreco.2021.119412
Harrison, T., J. Gibbs, and R. Winfree. 2018. Forest Bees Are Replaced in Agricultural and Urban Landscapes by Native Species with Different Phenologies and LifeHistory Traits. Global Change Biology 24 (1): 287–296. https://doi.org/10.1111/ gcb.13921
Mathis, C. L., D. J. McNeil Jr., M. R. Lee, C. M. Grozinger, D. I. King, C. R. Otto, and J. L. Larkin. 2021. Pollinator Communities Vary with Vegetation Structure and Time Since Management within Regenerating Timber Harvests of the Central Appalachian Mountains. Forest Ecology and Management 496: 119373. https://doi.org/10.1016/j.foreco.2021.119373
Mathis, C. L., D. J. McNeil Jr., M. R. Lee, C. M. Grozinger, D. I. King, C. R. Otto, and J. L. Larkin. 2022. Can’t See the Flowers for the Trees: Factors Driving Floral Abundance within EarlySuccessional Forests in the Central Appalachian Mountains. Canadian Journal of Forest Research 52 (7): 1002–1013. https:// doi.org/10.1139/cjfr-2021-0359
McKinney, A. M., and K. Goodell. 2010. Shading by Invasive Shrub Reduces Seed Production and Pollinator Services in a Native Herb. Biological Invasions 12: 2751–2763. https://doi. org/10.1007/s10530-009-9556-6
Mola, J. M., J. Hemberger, J. Kochanski, L. L. Richardson, and I. S. Pearse. 2021. The Importance of Forests in Bumble Bee Biology and Conservation. Bioscience 71 (12): 1234–1248. https://doi.org/10.1093/biosci/biab138
Mola, J. M., L. L. Richardson, G. Spyreas, D. N. Zaya, and I. S. Pearse. 2021. LongTerm Surveys Support Declines in Early Season Forest Plants Used by Bumblebees. Journal of Applied Ecology 58 (7): 1431–1441. https://doi. org/10.1111/1365-2664.13760
Nuttle, T., T. E. Ristau, and A. A. Royo. 2014. LongTerm Biological Legacies of Herbivore Density in a LandscapeScale Experiment: Forest Understoreys Reflect Past Deer Density Treatments for at Least 20 Years. Journal of Ecology 102 (1): 221–228. https://doi.org/10.1111/1365-2745.12160
Requier, F., and S. D. Leonhardt. 2020. Beyond Flowers: Including NonFloral Resources in Bee Conservation Schemes. Journal of Insect Conservation 24 (1): 5–16. https://doi. org/10.1007/s10841-019-00211-6
Saunders, M. E. 2018. Insect Pollinators Collect Pollen from WindPollinated Plants: Implications for Pollination Ecology and Sustainable Agriculture. Insect Conservation and Diversity 11 (1): 13–31. https://doi.org/10.1111/icad.12209
Schweitzer, D., J. R. Garris, A. E. McBride, and J. A. Smith. 2014. The Current Status of Forest Macrolepidoptera in Northern New Jersey: Evidence for the Decline of Understory Specialists. Journal of Insect Conservation 18 (4): 561–571. https://doi.org/10.1007/s10841-014-9695-y
Smith, C., T. Harrison, J. Gardner, and R. Winfree. 2021. ForestAssociated Bee Species Persist amid Forest Loss and Regrowth in Eastern North America. Biological Conservation 260: 109202. https://doi.org/10.1016/j.biocon.2021.109202
Stockhammer, K. A. 1966. Nesting Habits and Life Cycle of a Sweat Bee, Augochlora pura (Hymenoptera: Halictidae). Journal of the Kansas Entomological Society: 157–192.
Traylor, C. R., M. D. Ulyshen, D. C. Bragg, and J. V. McHugh. 2024. Forest Bees Benefit from Compositionally Diverse Broadleaf Canopies. Forest Ecology and Management 566: 122051. https://doi.org/10.1016/j.foreco.2023.122051
UrbanMead, K. R., M. Van Dyke, P. Muñiz, A. D. Young, B. N. Danforth, and S. H. McArt. 2023. Early Spring Orchard Pollinators Spill Over from ResourceRich Adjacent Forest Patches. Journal of Applied Ecology 60 (3): 553–564. https:// doi.org/10.1111/1365-2664.14213

By Lisa Pearson
This article inaugurates a new department, From the Archives, in which authors reflect on a significant article from Arnoldia’s backfile. To begin, we’re revisiting Sheila Connor’s “The Nature of Eastern Asia: Botanical and Cultural Images from the Arnold Arboretum Archives” (Arnoldia 63:3, 2005). Lisa Pearson uses Connor’s article as a springboard to reflect on digitization and its legacy of scholarship—a legacy which continues, as Pearson and her team in the Arboretum’s Horticultural Library rethink the collection for improved access and insight. Connor’s text is indicated in italics throughout.—Editors
Twenty years ago, as we approached the conclusion of our second Harvard Library Digital Initiative (LDI) grant, Sheila Connor, Horticultural Research Archivist and head of the Arboretum Library at that time, wrote in this publication about our efforts to catalog and digitize our collection of historical eastern Asian photographs taken by Arnold Arboretum collectors in the first quarter of the 20th century. These large projects cataloged over 5,200 historical images and a complementary collection of over 1,900 images taken by modern collectors from 1984 to 2000 in the same region of China explored by Joseph Rock from 1924–1927. These projects were major undertakings at a time when large-scale digitization initiatives were new territory. I had the honor of working on both as a young image cataloger, and over the past two decades, all of these photographs have become old friends whom I visit and revisit almost daily as I assist scholars with their research or fulfill image use requests. By digitizing these materials, we provided the catalyst to generate more scholarship about these photographs, which in turn has generated more scholarship, and so on. I feel like we have succeeded beyond our wildest dreams, and I look forward to the coming decades and what might be created in the future.
The Arnold Arboretum’s collection of eastern Asian photographs represents the work of several intrepid plant explorers who traveled to exotic lands in the early years of the twentieth century and returned with not only seeds, live plants, and dried herbarium specimens, but with stunning images of plants, people, and landscapes as well. We owe these images to the foresight of Charles Sprague Sargent, the director of the Arboretum during its first fifty years. In December 1906, when E. H. Wilson signed an agreement to collect in China for the Arboretum, Sargent set the precedent of asking all his explorers
Ernest Wilson photographed this mixed stand of conifers on the slopes of Mount Fuji 富士山, Japan, in May 1914. Note the startlingly sharp resolution provided by the large-format glass-plate negative.
to document their expeditions with photographs: “A good set of photographs are really about as important as anything you can bring back with you,” he wrote. He would later urge William Purdom to “take views of villages and other striking and interesting objects as the world knows little of the appearance of those parts of China which you are to visit.” And to Joseph Rock, the last of the great explorers who would work for him, Sargent wrote, somewhat peevishly, “I don’t know how you got the idea that we didn’t want scenery. These are always important and interesting additions to our collection, and you may be sure you cannot send us too many of them.”
As an institution, we have long recognized the value of photographs for botanical research, which along with our living plants and dried plant specimens, form a “three-legged stool” of our collections, with each leg being of equal importance for scholarship. The Library began to collect and house dendrological photos long before Sargent directed Wilson to get a camera for his trip. The photos had been taken by staff members such as Alfred Rehder or John G. Jack to record plants on our grounds, or received, sometimes with plant material or correspondence, from collectors or botanists outside of the Arboretum community.
When we first proposed our participation in the LDI program, digitization was a brave new world, and institutions were more used to, and interested, in digitizing humanities related collections. We were told point blank that our photographs were “just trees” with the implication that they had less value than the more familiar fine arts collections that were being proposed for digitization. Part of our motivation for embarking on these two projects was the realization that the photographs actually included far more than “just trees.” They showed little-known landscapes of the far-flung locations, cultural objects—so many of which had been lost in the political and social upheavals of the mid-20th century, objects of daily life and their uses by the Indigenous people, and the people themselves in regions our collectors explored. All these visual riches had been trapped in their filing cabinets since they were created, only accessible onsite in the Horticultural Library reading room. Our historical filing system for these photos was by genus and species. If you wanted to see images of Abies koreana taken in Korea, for example, you went to the drawer and rifled through the contents until
you came to that species. There were typed lists of the eastern Asian pictures but there was no card index (and certainly not an electronic spreadsheet or a database!) on which could have been noted potentially interesting details in the images.
Our first LDI project digitized a collection of photographs taken by Joseph Charles Francis Rock during his explorations in Gansu and Tibet from 1924-1927. We contributed over 600 photos from the Arboretum’s collection, which was augmented by an additional 246 ethnographic images from the Harvard Yenching Library. These were paired with a collection of 1,900 modern photographs from the same botanically diverse region of west central China taken by Arboretum collectors David Boufford, Susan Kelley, and Richard Ree from 1984-2000. The project also digitized a very large hand drawn map made by Rock of the region, digitized the type specimens Rock collected on this expedition, and created a gazetteer that correlated historical place names with their modern equivalents. Rock kept a detailed journal of his travels, and we extracted the relevant portions of his entries which describe the images. These entries flesh out the sometimes-laconic descriptions on the photos themselves.
The second LDI grant was even more ambitious in its scope and sought to digitize all our remaining eastern Asian photographs—about 4,600 in all. They included all of Ernest Wilson’s images from China, Japan, Korea, and Taiwan; an additional collection of photos by Jospeh Rock taken in 1922; a large collection of photographs by USDA (and Arboretum) collector Frank Meyer; a small but early collection of images taken by John Jack in Japan, Korea, China, and Taiwan in 1905; another small but ethnographically interesting collection of photos by William Purdom taken primarily in Mongolia and Gansu from 1909-1911; a collection of images by contract collector Joseph Hers; and a group of photographs by unknown and miscellaneous photographers.
John George Jack (1861–1949)
167 images (1905)
G. Jack was already experienced in plant exploration when he became the first staff member after Sargent to visit Asia. Jack had joined the Arboretum in 1886 and almost immediately began collecting and photographing plants in the U.S. and abroad. Between 1898 and 1900 he spent summers working for the U.S. Geological Survey,


Top, Jack photographed a stand of Larix kaempferi near Chuzenji Lake 中禅寺 湖 in Tochigi Prefecture 栃 木 県 in August, 1905, with Mount Nantai 男体山 in the background. Below, Jack was given photographs of forestry and lumbering operations by his Japanese hosts. Here men in Yoshino 吉野町 in Nara Prefecture 奈良県, guide carts of Cryptomeria japonica down a denuded hillside in 1904.
exploring and photographing the forests of Colorado and the Big Horn Mountains of Wyoming. In 1891, he visited botanic gardens and nurseries in England and on the continent, and in 1904, he and Arboretum taxonomist Alfred Rehder collected specimens in the western United States and in Canada. It is likely that his yearlong Asian journey was self-financed; although Sargent’s Annual Report for the Year Ending July 31, 1905, states that “Mr. J. G. Jack has started on a journey to the East to obtain material for the Arboretum in Japan, Korea, and northern China,” no record of the Arboretum underwriting this expedition appears in the archives. Jack’s introduction to an undated, unpublished manuscript entitled “Notes on Some Recently Introduced Trees and Shrubs” may explain why. ...
“On the first of July, 1905, I left Boston for Japan[.] The object of my trip was primarily rest and recreation for three or four months, combined with a desire to observe some of the interesting arborescent flora of central and northeastern Japan ... A short visit was also made to Korea and to Peking in China....”
Forestry was a lifelong interest for Jack. Covering some of the ground that E. H. Wilson would later visit, Jack photographed the forest preserves around Mt. Fuji and elsewhere in Japan, as well as the forests of Taiwan and Korea. The scenes he captured in Beijing include formal portraits of people in traditional costumes.
In addition to herbarium specimens representing 258 plants, Jack returned with 171 images, many of them in a format especially useful for him - lantern slides. Jack had been appointed Harvard University lecturer on arboriculture in 1890 (the title was changed to lecturer in forestry in 1903) and he continued teaching throughout his career. In the fall and spring of each year he taught courses in dendrology to students and teachers using the Arboretum’s living collections as his classroom, and he taught forestry both at Harvard ... and at the Massachusetts Institute of Technology, where he also held a lectureship.
When we embark on digitization projects, we really have no idea what uses people might make of the images we place online, but by placing those
images out there for anyone to see, we increase the awareness of a particular subject. The John Jack photographs, which we digitized in our second LDI grant project, are a case in point. In the early 2010s Jack’s granddaughter contacted us to say that she had a number of items that had belonged to her grandfather and would we like them. I unhesitatingly answered yes, and soon, we were in possession of a variety of items, including several versions of an autobiography, pocket diaries, and, extremely exciting for our photographic collection, an additional 200 photographs from his 1905 trip. The trip images, which Jack had donated to the Arboretum in 1935, and which we had digitized as part of the grant, could be characterized as being very businesslike and were almost exclusively of Japan. There were photographs of forestry stations and men engaged in forestry activities, those of lumber storage and processing, and of trees “at home,” to use Wilson’s words, in their native habitats. The later donation rounded out Jack’s trip and allowed us to see some of fun that occurred in between those work-related visits. Excitingly the new collection contained more photos from the other legs of Jack’s trip, including his visits to Korea and China, as well as some sights on his return voyage through the Suez Canal and a stop in Italy.
With the exception of an article I wrote for this publication in 2014, no additional scholarship has been generated around John Jack. This is unfortunate, partially because of his extensive contributions to the early history of the Arboretum in public education, for his plant exploration in the American West, and perhaps most important of all, his instruction of a generation of Asian botanists, including H.H. Hu, widely considered the father of Western botany in China.
Born in England in 1876, Wilson received his training in horticulture at the Birmingham Technical College and at the Royal Botanic Gardens, Kew. His career as an explorer began in 1899 when he traveled to China seeking the dove tree, Davidia involucrata, for the Veitch Nursery in England. A visit to the Arnold Arboretum on his way to China initiated a lifelong collaboration with Charles Sargent. As Wilson was preparing for his first Arboretum journey, Sargent insisted that he take along a
In 2008, the Arnold Arboretum Archives was approached by Professor Yin Kaipu to provide publication quality copies of about 250 of Ernest Wilson’s 1907-1911 photographs of Western China for a book1 that was to accompany a re-photography project he had embarked upon several years previously.
Yin, a retired professor of plant ecology from the Chengdu Institute of Biology, Chinese Academy of Sciences, is a staunch advocate of nature conservation who believes in the sustainable use of natural resources, and is working to preserve the biodiversity and ethnic culture of Western China.
His extensive travels in the region brought him to the same places that Ernest Wilson explored over 100 years ago, many of which he recognized when studying Wilson’s images! Upon his retirement, he began to travel the region to relocate more places that Wilson had photographed, eventually identifying over 250 separate locations. Yin, then conceived a project to re-photograph the places to provide a georeferenced record of 100 years of change in the area.
Yin Kaipu not only found over 250 of Wilson’s photography locations but also tracked down descendants of some of the people pictured, including several of Ernest Wilson’s collecting team, men who had assisted him since his expeditions for Veitch Nurseries from 1899-1905. Professor Yin is hopeful that several nationally broadcast television programs featuring his work will encourage more people to come forward who might recognize their ancestors in the photos.
Yin’s photographs show a landscape which has modernized and become more populated but also revealed areas which have benefited from recent reforestation programs. Perhaps more importantly, he also created a pictorial record of the landscape prior to the devastating earthquakes in the region in 2008 and 2011 which radically reshaped the landscape in many places. Professor Yin has since returned and re-photographed these locations, adding GPS positioning, so that in another 100 years, scientists can return and again re-photograph the exact locations.
large format, Sanderson whole-plate field camera capable of recording both great detail and broad perspectives without distortion. The rest of his camera gear included a cumbersome wooden tripod and crates of heavy, fragile, 6 1/2-x-8 1/2inch glass-plate negatives.
For three years Wilson explored western Hupeh [Hubei] and western Szechuan [Sichuan]. He returned to Boston in 1909 via ... London, where he spent several months developing the glassplate negatives and seeing for the first time his 720 images.... [H]is second Arboretum expedition, which began in 1910, was to collect cones and conifer seeds in the central and southwestern parts of China. In September of that year, while he was traveling between Sungpan and Chengdu [Sichuan], a landslide hit the expedition group, crushing Wilson’s leg. After several months in a hospital at Chengdu, Wilson returned to Boston in March 1911.... Before the accident, however, he had managed to take 374 images....
In January 1914..., Wilson sailed for Japan, where he would focus his attention on horticulture and cultivated plants including conifers, Kurume azaleas, and Japanese cherries. By the time the Wilsons returned to Boston at the beginning of 1915, there were 619 new images to add to the photograph collection. Wilson next undertook a “systematic exploration” of Korea. Beginning in 1917 with the Japanese islands and Taiwan, he then traveled along the Yalu River into the far northern reaches of Korea, returning to Boston in 1919 with seeds, living plants, 30,000 herbarium specimens, and 700 photographs. His last expedition, a tour of the gardens of the world, took place from 1920 to 1922 and included a stop at the Singapore Botanical Garden in June of 1921. Of the 250 images he shot during this journey, 15 were taken in Asia.
Wilson is rightly revered as the greatest Arnold Arboretum plant collector of our early years. His industry and productiveness are astounding, especially when we consider that much of his traveling in


Top, Wilson photographed this large Populus suaveolens in Xintangguan 新塘关村 in Songpan County 松潘县 , Sichuan 四 川 in August 1910. The tree was still standing when I visited in 2017. Below, Wilson’s collecting team in February 1911: “My Chinese collectors, all faithful and true.”
country in China and Korea was on foot in extremely rough country. In China, he relied on a tight knit team of trained collectors, men from Yichang, Hubei Province, and its environs whom he had mentored. Some of the men might also have collected previously for Augustine Henry, the Irish botanist, who was attached to the customs service in China and directed Wilson in finding the Davidia tree during his first expedition for Veitch Nursery. Wilson relates the incident in 1907, upon his return to China, that having sent word ahead to Yichang of his impending arrival, one of his men, who had collected for him during his expeditions for Veitch, met him on the gangplank of his ship to say that his men had been reassembled. In 1910, he might well have died in the avalanche mentioned above had it not been for the efforts of the men of his team. They stabilized his injuries and splinted his badly broken leg with his camera tripod. Then they carried him for three days down from the mountains, even encountering a 100-animal mule train coming the opposite way, to the Friends Mission in Chengdu for medical treatment. While Wilson convalesced, his men returned to the field and collected seeds and bulbs from locations that had been identified during scouting earlier in the season. It is safe to say that this expedition would not have succeeded at all had it not been for the dedication of Wilson’s collecting team. In February 1911, just before he left western China for the last time, he photographed the men who had saved his life and his expedition. I think it is very telling the high regard in which he held them that he captioned the photograph, “My Chinese collectors, all faithful and true.” That said however, he didn’t provide their names! It would take another century and research by Professor Yin Kaipu as part of his Wilson rephotography project, to name two of the men. The bearded man third from the right was named Wang Tianguan, and the man fourth from the right had the family name Yin.
The injury Wilson sustained in Sichuan was severe. When he left China in February 1911, he was still on crutches and the trauma had cause his right leg to heal one inch shorter than his left leg. His next expedition would not come until 1914, and to save excessive strain it was decided that he should explore in Japan where the well-developed rail system allowed for much easier travel. One of his tasks on this journey was to document flowering cherries and try to answer some lingering taxonomic questions about the plants. Starting on the south islands, Wilson followed the blooming trees north. The results
Wilson is rightly revered as the greatest Arnold Arboretum plant collector of our early years.
of his observations were published in 1916 in The Cherries of Japan.
Wilson’s 1917-1919 trip to eastern Asia was his most productive, concentrated primarily on exploring Korea, with sojourns in Japan and Taiwan. He spent the bulk of 1917 and half of 1918 traversing the length and breadth of the country and documented his travels with about 300 photographs taken with his Sanderson camera. He visited locations of great natural beauty such as the Geumgengsan or Diamond Mountains which today lies just over the North Korean border of the Demilitarized Zone. These photographs are particularly poignant because most of the religious institutions there were destroyed by bombing in the Korean War. His most rigorous time in the field was an extended exploration in far northern Korea in August and September 1917. During this time in the field, he traveled about 500 miles and much of that on foot. This area today is in North Korea and inaccessible to botanical exploration. Sargent’s admonition to William Purdom that he should photograph the landscape, “as the world knows little of the appearance of those parts of China (or in this

Wilson photographed this spectacularly craggy landscape of the Manmulsang in the Geumgang-san 금강산 or Diamond Mountains, in what is today southern North Korea, in October 1917.

The Guryong Waterfall 구룡폭포 was one of the sights that enchanted Wilson in October 1917 in the Geumgang-san 금강산. The water drops 243 feet (74 m.) to the Nine Dragon Pool below.
case Korea) which you are to visit,” is particularly apt, because between war and the decades-long closure of North Korean society this area is terra incognita.
We included 15 photographs from Wilson’s 1920–1922 “Tour of the Gardens of the World” that were taken at the Singapore Botanic Garden because they were taken in temperate Asia. We did not include any other images from this trip because Wilson primarily visited tropical areas. The title of this trip was rather misleading. Margaret Grose, who has studied and written about this expedition extensively, characterizes it as a good will tour to the British Dominion countries which (with the exception of Canada), it was. Her research revealed Wilson concerns with forestry and conservation in the countries he visited and has given us a far greater and more nuanced understanding of this journey.
Wilson’s photographic legacy has generated the largest amount of scholarship by far since we engaged in our two LDI projects. They prompted Yin Kaipu to begin his rephotography project which has identified about 300 Chinese locations that Wilson photographed 100 years earlier (see sidebar). The photographs have also led to an increased awareness of Wilson’s published works amongst Chinese scholars, in particular the monumental China Mother of Gardens, which has led to at least three translations2 or partial translations of the work. Wilson’s explorations in Sichuan have generated much local interest in the region. In Wilson’s beloved Songpan, the city has erected a large, engraved-stone mural of his panoramic photographs of the town that he took in 1910. I had the honor to visit there in 2017 to lecture on his photographs and had the pleasure of seeing this striking landscape for myself. I recognized a number sites, including the imposing tree illustrated on page 44.
1,310 images
In 1905, the United States Department of Agriculture’s Office of Seed and Plant Introduction recruited Frank Meyer, a native of Holland who had immigrated to America in 1901, to gather economically useful plants in China. Through an arrangement worked out between Sargent and David Fairchild of the USDA, Meyer was to send to the Arboretum trees and shrubs of ornamental value along with images of his travels. The photographs in the Meyer Collection document his four expeditions to western China and
“Meyer’s images have an immediate and spontaneous quality, perhaps because they document daily life.”
Manchuria. Unlike Wilson’s highly composed photographs, Meyer’s images have an immediate and spontaneous quality, perhaps because they document daily life in this remote region: farmers and other people going about their work, manufacturing techniques, and markets were all captured through his lens. Even his images of plants often include local people or architectural backgrounds.
Meyer and Wilson corresponded occasionally, trading information on routes, travel conditions, and collecting strategies. Occasionally their letters touched on personal matters. In a letter from Beijing in 1907 Meyer wrote, “This roaming about, always alone, takes lots of energy away from a fellow, don’t you agree with me too, in this respect?” On June 2, 1918, Meyer disappeared from a steamer and although his body was eventually recovered, the circumstances of his death remain a mystery.
Frank Meyer’s photographs primarily reflect his main assignment in Asia for the United States



Department of Agriculture, to collect plants of economic value that could be grown profitably in America. He understood that many of the plants were entirely new to Western audiences and as such, he devoted much effort to document the uses that the local people had for a variety of food products and building materials. He took pains to write detailed descriptions of the preparation of different dishes, such this one for mung bean sprouts that accompanied the lower photograph on page 49, “A cart full of tubs of bean sprouts, produced by the humble mung bean. These bean sprouts are eaten as a vegetable, when scalded, and they are a very tasteful product when served cold as salad with some soy-bean, vinegar and oil sprinkled over them. Sixteen or seventeen catties of dry beans supply from fifty to sixty catties of sprouts.” Meyer’s photos were taken with an inexpensive snapshot camera which allowed him to be spontaneous and very much in the moment. His image of boatmen on the Huang Ho clad only in loincloths has an almost sculptural quality as the men lean into their work. He describes the
scene thus, “On the Huangho, near Chao yi, Shansi. Incidents of travel. On the Yellow River, being in the deep water now where the boats float down with the current. Only paddling is practised now in the shallow places the boat is pulled by 8 or 10 men. These hard working fellows earn only two taels per month (U.S. gold $1.70) and receive board and lodging, but what a board and lodging!”
William Purdom (1880–1921)
161 images (1909–11)
In 1909, with Wilson about to return from southern China and the agreement with the USDA in place to ensure that Frank Meyer’s Asian collections would be shared with the Arboretum, Sargent was eager to dispatch yet another plant collector to the largely unexplored northeastern provinces of China. Hoping, in Sargent’s words, to “bring into our gardens Chinese plants from regions with climates even more severe than those of New England,” William Purdom-the most


1926.
inexperienced of Arboretum explorers embarked on his first expedition in February of that year....
For three years the shy and retiring novice followed the Yellow River north, his work always overshadowed by, and his meager results compared to, the successful exploits of the gregarious, prolific Wilson. His collection techniques improved, however, and he is now known for his later successes, when he worked with Reginald Farrer. Eventually he accepted a post as inspector of forests for the Chinese government. He must have been glad to be relieved of Sargent’s exacting photographic demands, for although he never complained to Sargent himself, in 1909 he wrote to Veitch: I am not a specialist at photography and do not wish to infer that my camera is not a good one but I do now believe that a camera to carry on one’s back with films is the most serviceable thing out here on these rough roads for it is nearly impossible to carry the plates. No end of mine got smashed.
Much to Charles Sargent’s annoyance, William Purdom made fewer plant collections than Ernest
Wilson, but it wasn’t for lack of trying; he initially was instructed to collect in northern China, an area that Sargent was convinced was covered in trees but had been largely denuded of forests. He found success in his collecting in Shaanxi Province however, locating a wild form of tree peony, later followed by a period of botanizing in Gansu Province. The second leg of his expedition was hampered by political unrest however and he had a narrow escape from Gansu.
Although he complained about breakage of his plates, from an ethnographic perspective, Purdom’s expedition was extremely successful. He proved a gifted portraitist, capturing for posterity a rich record of the peoples of Tibetan border region. Unlike Wilson, he had taught himself some Chinese so he might have been able to put his subjects more at ease for their photos. The Arboretum holds 173 prints of his photographs in our collection. About 35 of the original glass plate negatives from his Arboretum expedition are held in the British Library, along with over 200 nitrate film negatives from this expedition and possibly also from his expedition with Reginald Farrar.
When we digitize images and place them online, it is with the hope that they will generate scholarship and thus increase our knowledge of our own holdings. Purdom’s photographs have done that beyond our wildest dreams. He has been the subject of three full length biographies, one by botanist and plant collector Alistair Watt,3 one by independent researcher Francois Gordon,4 and the third by independent researcher O.V. Presant.5 All three biographies dovetail well, Watt’s concentrates on a botanical perspective, Gordon’s on the socio-political world of Purdom’s expeditions, with Presant’s giving a good overview of his career.
1923–24)
It was Hers, a railroad engineer and administrator of the Lung-Hai and Pien-Lo railways, who approached Sargent with a proposal to collect specimens for the Arboretum. Stationed in Chengchow, a city on the Huang Ho River that had become an important railroad center thanks to its position at the junction of the Longhai (east-west) and the Beijing-Guangzhou (north-south) lines, Hers was superbly situated, with a job that enabled him to range far and wide collecting plants.
... Hers offered to send “seeds, or cuttings, or photos.” After he had done so, Sargent wrote him that “this is one of the most important collections of Chinese plants which has been sent to the Arboretum and I am extremely obliged to you for sending it to us.” Although we have only 63 of his images, Hers went on to collect seeds and specimens of more than 2,000 species, most of them sent to the Arnold Arboretum.
It is probably appropriate that Joseph Hers should follow William Purdom in this review of Arboretum plant collectors because he and Hers were colleagues in the Chinese rail service when Purdom was working on reforestation along the lines in the later 19-teens. Unfortunately, there has been only a small amount to scholarship generated from our placing Hers photographs online, but his living legacy at the Arnold Arboretum is still strong. There are 59 plants growing on the grounds that were grown directly from seed received from Hers or from repropagations of original Hers plants. Perhaps a scholar in the future will take inspiration from our images and take up Hers cause.
Botanist, anthropologist, explorer, linguist, and author, Rock was the last of the great plant hunters employed by Sargent, who by then was elderly. Rock had immigrated to the United States from his native Austria in 1905, but between 1920 and 1949 he lived in China for extended periods, exploring, collecting plants and animals, and taking pictures for various United States agencies and other institutions, including The National Geographic Society, the U.S. Department of Agriculture, and the Arnold Arboretum. He is still remembered by the older villagers in the city of Lijiang, which was his home base for many years. The 653 photographs that Rock took during the Arboretum’s 1924-27 expedition have already been digitized and are available on the VIA website; the remaining 320 photographs document his 1920-22 expedition to Thailand, Myanmar, and the Yunnan Province of China and include images of plants, landscapes, villages, architecture, and the ethnic minority peoples of the region.
Joseph Rock was a larger-than-life figure in a field and place that seemed to attract such people. He always seemed to have multiple irons in the fire, for multiple employers, an activity that came to annoy Charles Sargent no end. Rock was a dedicated diarist and for our first LDI grant, we paired his photos with relevant extracts from his journals. He also left a diary from his 1922 expedition to Yunnan, and we hope in the future to add quotes from that journal to that collection of images.
Professor Yin has begun another rephotography project, this time to seek out and rephotograph locations from Rock photos as he did for Ernest Wilson. This is not the first project of this kind for Joseph Rock images, however. Robert K. Moseley, a conservation ecologist formerly from the Nature Conservancy, tackled the subject two decades ago. His book Revisiting Shangri-La 6 documents changes in the land in Yunnan and Gansu in the country Rock explored in the 1920s.
Tangential, because the project was not directly driven by our digitization projects, is Hartmut Walravens’ program to publish Joseph Rock’s correspondence and other writings. I am hopeful that Walravens’ most recent volume, which concerns Rock’s 1922 expedition in search of chaulmoogra oil (used


Top, Rock’s yaks at Dzang (Saizong 赛宗寺 ) Monastery in Qinghai 青海 Province in May 1926; Rock notes that his men were reapportioning the loads. Below, the natural gate or shi-men in Yiwa Gorge near Yiwaxiang 益哇镇 , Têwo County 迭部县
, Gannan Tibetan Autonomous Prefecture 甘南州
, Gansu 甘肃 photographed by Joseph Rock in July 1926.
for the treatment of Hansen’s Disease), will be helpful for enhancing the catalog records for this expedition.7
In light of the extensive use all these photographs have received by scholars worldwide over the past several decades since we placed them online, we have come to the realization that we need to recatalog this valuable collection to further increase its visibility and utility. Last year, I wrote a National Endowment for the Humanities (NEH) grant which received excellent reviews but because of governmental cuts was not funded. We then approached a donor who funded a portion of the project.
Recataloging this collection will incorporate scholarly discoveries such as those mentioned above. Professor Yin’s research will be particularly helpful, especially for identifying geographical locations for the Wilson photos from China, as will the biographies of William Purdom, and the collected writings of Joseph Rock which are being published by Hartmut Walravens.
Through content that will contextualize the times and places, we will be able to publicly confront and explain our institutional role in colonialism of the period and in particular our engagement with Japanese occupation forces in Korea and Taiwan. It will also allow us a platform to formally acknowledge the contributions of the indigenous and local people our explorers encountered, interacted with, and employed on their expeditions. In some cases, it gives us a chance to put a newly learned name with a face.
In the decades since our materials were first placed online, botanical nomenclature has evolved and our institutional interest in conservation status has increased. We will update the former and include for the first time the latter, as well as recording any crop wild relatives for the plants. To prevent these data becoming outdated, we will also create procedures for conducting periodic updates to the records, as we do for all of the plants in our living collections.
The Arboretum Library staff receives requests for use of these images on at least a weekly basis and sometimes daily. They have been featured in books and online, but they have also been exhibited in museums across Asia. Recently, we provided 60 image files to the Xie Zilong Photography Museum in Changsha, Hunan for their exhibition, “Between Nakhi and Tibet: Joseph Rock’s Ethnographic Photography in China.” Since its opening in November 2024, the show has attracted over 70,000 visitors and its run has been extended until 2027.
We did not know what might become of these
photographs when we placed them online. They might have just sat there, unappreciated, but instead they have been discovered by scholars, scientists, historians, and others and used to increase knowledge about an eventful period in Asian history. I have been amazed by the variety of uses they have received and have personally grown from my interactions with their users. I hope that our upcoming recataloging project will prepare this popular collection for another quarter or even half a century of intensive use and I look forward to all the new knowledge that will be generated.
notes
1. Yin, Kaipu. 2010. Tracing one hundred years of change: Illustrating the environmental changes in Western China Beijing: Encyclopedia of China Publishing House.
2. Wilson, Ernest Henry, and Zhiyi Bao. 2017. Zhongguo nai shi jie hua yuan zhi mu = China, mother of gardens 第 1 版 Beijing: Zhongguo qing nian chu ban she. Wilson, Ernest Henry, and Qiming Hu. 2015. Zhongguo, yuan lin zhi mu = China, mother of gardens. Guangzhou: Guangdong ke ji chu ban she. Wilson, Ernest Henry, Yin Hong, and Wenqing Gan. 2009. Weierxun Zai Aba : 100 Nian Qian Weierxun Zai Sichuan Xi Bei Bu Wenchuan Maoxian Songpan Xiaojin Lu Xing You Ji. 第 1 版. Chengdu: Sichuan min zu chu ban she.
3. Watt, Alistair, and Seamus O’Brien. 2019. Purdom and Farrer: Plant Hunters on the Eaves of China. [Place of publication not identified]: Published by the author.
4. Gordon, Francois. 2021. Will Purdom: Agitator, PlantHunter, Forester. Edinburgh: Royal Botanic Garden Edinburgh.
5. Presant, O. V. 2019. A perfect friend: the life of Cumbrian plant hunter William Purdom / O.V. Presant. Hayloft Publishing Ltd.
6 Moseley, Robert K. 2011. Revisiting Shangri-La: Photographing a Century of Environmental and Cultural Change in the Mountains of Southwest China. 第 1 版. Beijing]: China Intercontinental Press.
7. This book is coming in the mail and is not yet in Harvard collections.
by Kathleen McDermott






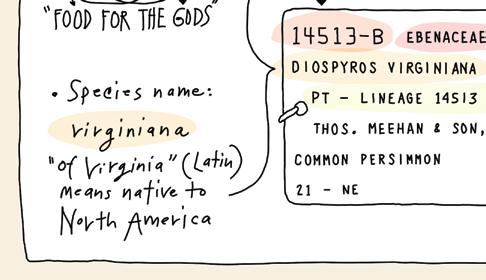













sources
Page 57:
Briand, C.H, “The Common Persimmon, (Diospyros virginiana L): The History of an Underutilized Fruit Tree (16th-19th centuries),” Huntia (Department of Biological Sciences, Salisbury University, Salisbury, MD.) 12/1 2005, 71- 89. https:// faculty.salisbury.edu/~chbriand/pdfs/huntia05.pdf
“Diospyros virginiana L.” Southern Research Station—USDA https://www.srs. fs.usda.gov/pubs/misc/ag_654/volume_2/diospyros/virginiana.htm
Jack, J. G. “Persimmons.” Bulletin of Popular Information (Arnold Arboretum, Harvard University) 5, no. 11 (1931): 41–44. http://www.jstor.org/stable/42962054
Quinnett, M. P. “Diospyros virginiana: The American Persimmon” (Masters Thesis), 1982. https://thekeep.eiu.edu/theses/2904/
Page 58:
Barlow, C. “Anachronistic fruits and the ghosts who haunt them.” Arnoldia, 61(2), 2001: 14–21. https://arboretum.harvard.edu/stories/anachronistic-fruits-and-theghosts-who-haunt-them/ and Barlow, C. The Ghosts of Evolution: Nonsensical Fruit, Missing Partners, and Other Ecological Anachronisms. New York: Basic Books, 2002.
Boone, M., et al. “A Test of Potential Pleistocene Mammal Seed Dispersal in Anachronistic Fruits using Extant Ecological and Physiological Analogs.” 14 Southeastern Naturalist, 2025: 22-32. 10.1656/058.014.0109.
“Diospyros virginiana,” in Arnold Arboretum Explorer: “About This Tree” and “About This Plant,” https://arboretum.harvard.edu/explorer/ Lescaze, Z. Paleo Art: Visions of the Prehistoric Past. Taschen: Koln, Germany: 2017. Newsom, L. & Mihlbachler, M. (2006). “Mastodon (Mammut americanum) diet and foraging patterns based on analysis of dung deposits.” 10.1007/978-1-4020-46940_10. (From: First Floridians and Last Mastodons: The Page-Ladson Site in the Aucilla River (pp.263-331) Springer Verlag: 2006.
Rebein, M., et al. “Seed dispersal of Diospyros virginiana in the past and the present: Evidence for a generalist evolutionary strategy.” Ecology and Evolution, Vol. 7,11 4035-4043. 4 May 2017, doi:10.1002/ece3.3008
Aiello, A. S. “Thomas Meehan: the horticultural popularizer.” Arnoldia, 78 (5-6), 2021: 50–61. https://arboretum.harvard.edu/stories/ thomas-meehan-the-horticultural-popularizer/
Briand, C.H, “The Common Persimmon, (Diospyros virginiana L): The History of an Underutilized Fruit Tree (16th-19th centuries),” Huntia (Department of Biological Sciences, Salisbury University, Salisbury, MD.) 12/1 2005, 71- 89. https:// faculty.salisbury.edu/~chbriand/pdfs/huntia05.pdf
“Diospyros virginiana L.” Southern Research Station—USDA. https://www.srs. fs.usda.gov/pubs/misc/ag_654/volume_2/diospyros/virginiana.htm
Jack, J. G. “Persimmons.” Bulletin of Popular Information (Arnold Arboretum, Harvard University) 5, no. 11 (1931): 41–44. http://www.jstor.org/stable/42962054
Quinnett, M. P. “Diospyros virginiana: The American Persimmon” (Masters Thesis), 1982. https://thekeep.eiu.edu/theses/2904/
Twitty, M. The Cooking Gene: A Journey Through African American Culinary History in the Old South. New York: Amistad, 2017: 284-296. “The seeds of Diospyros virginiana have been found in the quarters of the enslaved across the entire South …” 290.
kathleen mcdermott loves to learn and share from the full range of Arnold Arboretum programs, building on 40 years as an historian, artist, and urban cyclist (see ww. hautehistory.com).

By Gretchen Ernster Henderson
Imagine encountering a tree with a yellow metal tag marked BEARING. You meet the tagged trunk while scrambling over rocky knobs outside a one-stop town in the Carson National Forest in New Mexico. The namesake stones of Tres Piedras rise from high sage plains at the threshold of forested mountains. They appear like fossilized scat from primordial giants, melted mini-mountains, rounded anomalies. The transitional rocks harbor niches for habitations of limbs, roots and shoots of scrub oaks, junipers, pines, and this BEARING TREE whose puzzle bark suggests ponderosa—before the slope slants toward aspens, greening around alpine meadows toward timberline where snow meets sky. Without a destination, learning these rocks by steps, you lose yourself in this landscape that happily disorients your bearings from human language to your own moving limbs. Each foothold demands attention, reorienting you to dirt and stone, bark and leaf, wildflower and pine needle, slanting sun and storm clouds, electrifying the horizon. The bright yellow tag startles you with its humanness, an inaudible shout like a ventriloquist’s wish, messaging meaning. You wonder less about the human marker of BEARING than the weight this tree bears.
Now, imagine a different tree: green-barked, gnarled yet limber as a dancer in the Sonoran Desert. Upon first meeting this tree with green bark, you were startled by her hardy delicacy. In the harsh climate of Arizona, months of summer span into triple digits and, in winter nights, can drop below freezing. The trees in your yard are not labeled, but each spring, these “green sticks” bud into golden blooms. Reading this tree’s classification as Parkinsonia startles you in a different way than finding a tree marked BEARING. You think the classification derives from a geologist turned neurologist whose name is now linked with disease. You are mistaken. The palo verde doesn’t hide her scars but grows a sheath to protect them, woodening around scabs like the Japanese practice of kintsugi, painting
gold in ceramic cracks: to stay strong in the broken places. This goldening tree bears her wounds by concentrating energy into living limbs. She is nicknamed a nurse plant, including nursing herself by rooting in earth and branching in sky. Among other arboreal strongholds, from ironwoods to mesquites, among towering saguaro cacti, palo verdes petal your yard with feathers and nests, growing from ancient volcanic rocks and beckoning by example, doing more with less, thriving where you least expect.
Now, imagine a climbable tree with creamy blossoms as big as your head. The waxy green leaves fit the size of your cupped hands. This magnolia in the nation’s capital is different than a pole of ponderosa that witnessed your marriage beside a slivered lake in the Sierras. Or a majestic oak in Virginia blown down by a storm, leaving a stump that makes you grieve. Think of all the trees you have loved: eucalyptus glowing under a full moon and cypress silhouetted at sunset by the sea, a sycamore bending like a hammock beside a river, a cedar snag where ospreys land in the Cascades. In a redwood grove in California, you first brought students to listen to water and woods and grope toward language as a landscape. From magnolias to oaks, ponderosa pines to palo verdes, redwood to cedar to maple and ash: you meet more and more trees who you cannot imagine your life






































Gretchen Ernster Henderson is the Spence L. Wilson Distinguished Professor in Humanities at Rhodes College in Memphis, TN. Her work includes five books across genres, along with intermedia arts and opera libretti. She founded the Dear Body of Water project (dearbodyofwater.poetsforscience.org) with the University of Arizona Poetry Center to support renewing relationships with bodies of water. Gretchen was recently awarded the 2025-2026 Woodberry Poetry Room Creative Fellowship at Harvard University. This essay on “Withness Trees” is dedicated to her father.
What if time were not linear but ringed? Can a story be told as circles within circles, rooted enough to communicate beyond words?
without. Stories of birchbark peel into handmade postcards on which your forebears wrote love letters a century ago. Your carpenter ancestors survive in your great-uncle’s hand-carved ducks and turned bowls, your grandfather’s redwood bookcases, his grave by acacias, and your father’s crafted furniture: walnut nightstands, a cedar pump lamp, and a maple rocking chair. You have gathered with family and friends around dinner tables of mesquite, cherry, and ash. When you look back and all around you, trees move from edges to center, ringed like sapwood and heartwood that measure more than a single life, more than mere witnesses. Being with. Withness trees.
What does it mean if withness comes as arboreal afterlife: in a chair or bookcase, floorboard or bench? Can we trace the plank back to the woodshop, beam to the lumbermill, or kindling to the axe in hand? Does firewood warm us differently than the likeness of logs lit by gas? I once went in search of witness trees and found them peddled as splinters in pens by battlefields, in metes-and-bounds of property deeds, “deeply wounded” and “not unbounded” in poetry (by Robert Frost). Imagining what trees may have seen and heard and felt, I followed the Witness Tree Protection Program, sat on clustered stumps listening to Janet Cardiff and George Bures Miller’s composed FOREST (for a thousand years), and visited a lab in the desert that birthed the field of dendrochronology: the study of tree rings. Search your memories with mine, evacuated from a forest fire amid scorching pines, cedars, manzanitas, madrones, and firs. Smell the smoke. Deep in a redwood forest on a cooler day, miles from the mountains and close to the coast, I made pilgrimage to a furrowed stump whose slow-growth lifespan was recorded in rings, where pinned signs counted back centuries from bark to pith as number one—“Modern calendar begins”—in the redwood’s heart. If you read between the lines, tree rings record

tales of fire and flood and blight, weathers and climates that shape any life, reminders of how we are always interrelated with the more-than-human world.
What if time were not linear but ringed? Can a story be told as circles within circles, rooted enough to communicate beyond words? What if “ancestor” and “descendant” branched beyond family trees to beget meanings for future forests, beyond any mete or bound or lifespan that a human thinks to measure? Stories bear overstories and understories, where forests think, and Mother Trees grow saplings. Who will current seedlings become with or without us? Withstanding the limits of language, humans may write with landscapes less by extraction and more by planting seeds, by leaning toward sunlight, by being present to lean upon, and by providing shade and support for more than ourselves. Do you have a tree whom you love, grieve, and cannot imagine your life without? You may not need to imagine the tree already rooting and branching inside you, your heartwood coursing with life. Do you feel your treeness?
By Jenny L. Davis
1. nassapi’ shokhalanchapi’ (red oak tree)
Those early anthropologists got it wrong—our traditional tales weren’t myths. They are prophesies.
2. naksish filammi’ (branch)
Do you know the stories about how Cedar and Willow started out as humans and became trees? Even today they continue to care for us and we treat them as kin.
3. nasi’ (acorn)
Oak trees are great caregivers. Each acorn is protected by a capule that acts as packed lunch car seat and knitted blanket all-in-one. Ingenious.
Darling acorns, I can’t pass up their roundness, the stripes on Quercus palustris, the absolute largeness of Quercus macrocarpa, the way Quercus rubra fits perfectly in my palm. I almost always have one in my pocket, sometimes two.
4. itti’ hakshish (root)
Our words are a way of staying connected of knowing ourselves even in faraway places of becoming rooted.
5. itti hobak (tree that will not bear fruit)
If I ever go missing as Indigenous women often do look for an oak tree in an unexpected place a canopy growing wide on denim, adipose, and ditch water
jenny l. davis is a citizen of the Chickasaw Nation and an associate professor of anthropology and American Indian studies at the University of Illinois, Urbana-Champaign, where she is the director of the American Indian Studies Program and the co-director of the Center for Indigenous Science. Her 2022 poetry manuscript, Trickster Academy, was published in the University of Arizona Press Sun Tracks Series. This poem was first published in Meridians: feminism, race, transnationalism. Vol 24, No. 1: (2) (Spring), and will appear in Davis’s forthcoming second poetry collection Extant (Michigan State University Press).

By Scott Phillips, Assistant Manager of Horticulture
F1800 pounds of fallen leaves are added to compost annually.
15–20 yards of leaf mold are generated from collected leaves. Photograph
all at the Arboretum is an incredible time of year to witness tree foliage ignite with a spectrum of new colors. Horticulturists and gardeners in the landscape employ three different strategies for leaf clean up. Our primary strategy is to leave leaves wherever possible. Leaves act as natural mulch, suppress weeds, retain moisture, and insulate and enrich the soil. The second method uses mowers to mulch leaves into organic material that rapidly decomposes and releases essential nitrogen, phosphorus, and potassium for soil microbes. Visitors may see and hear our third mode of leaf collection: leaf vacuuming. Using rakes and leaf blowers, we push leaves to the road where they are vacuumed and carried to our compost site to break down. About 1800 lbs. of leaves are put in a large bin, to add to our compost. Collected leaves are also used as a natural soil amendment, by creating leaf mold. 15 to 20 yards of leaf mold are generated from collected leaves. A “yard” refers to one cubic yard, which is a volume measurement equal to 27 cubic feet (3 feet long × 3 feet wide × 3 feet high).



“Do you have a tree whom you love, grieve, and cannot imagine your life without?” — p. 62
