Shrimp hatcheries in India
Hatchery shrimp feeds
Tilapia genetics
Editorial:
editor@hatcheryFM.com
Editor/Publisher: Lucía Barreiro
Consulting Editor: Suzi Dominy
Assistant Editor: Marissa Yanaga
Conferences and webinars: info@hatcheryFM.com
Advertising enquiries/request media pack: sales@hatcheryFM.com
Accounts & all other enquiries: info@hatcheryfm.com
SUBSCRIBE
Digital editions are free to industry subscribers. You may also purchase print copies. Subscribe at HatcheryFM.com to receive your own digital copy of our publications.
https://bit.ly/HATCHERYSUBSCRIBE




We are grateful to the following companies for sponsoring this issue of the magazine. Their support allows us to make our publications available without charge.
Aquafeed Media, S.L.U., Ames, 15220 A Coruña, Spain.


MOVING TOWARDS PRECISION BREEDING 13
The expansion of the genotyping services to improve operations sustainably and efficiently for all farmed species.

SHRIMP FEED SUPPLEMENTATION WITH PROBIOTICS 16
An effective strategy against mass mortalities caused by Vibrio harveyi and Vibrio parahaemolyticus infections in cultured Penaeus vannamei.
SHAPING THE FUTURE OF THE TILAPIA INDUSTRY 36

Recent advances and implemented innovations in tilapia breeding to maximize performance.

HFM: How many hatcheries are currently operating in India?
RKY: In India, there are 550-560 hatcheries, the majority on the east coast and a few on the west coast. Of all those hatcheries, only 50-60% are currently operational. Business is not good and farmers are not stocking shrimp PLs. So hatcheries are on and off. That is how it has been for the past year and a half. Each hatchery cycle takes 30-35 days and in a normal situation, hatcheries do nine cycles per year. Currently, they are going for five to six cycles per year.
HFM: What are the current main bottlenecks for hatcheries in India?
RKY: Right now, the major bottleneck is business. Farmers are not stocking shrimp due to low shrimp prices and diseases. Those who are stocking shrimp
have decided to go with lower densities to overcome the disease pressure. Therefore, PL demand has been reduced, so most of the times the supply is higher than the demand.
Hatcheries are struggling at this moment but they haven’t suspended operations. If the seed demand improves and there are just a few hatcheries operating, prices go up and everybody jumps in again. Then, the supply increases and the price falls again with some hatcheries closing again. It is almost like a sine curve; continuously going up and down.
Hatcheries are at the beginning of the value chain and if something hits the industry, we are the first to get the hit. The wars in the Middle East and Ukraine, stagnant demand for shrimp in Europe and the US due to the macroeconomic situation, glut of shrimp supply from Ecuador, etc. have a huge impact on our business.


HFM: How many shrimp species are being bred in the country? Would diversification help during difficult times?
RKY: The predominant shrimp species in the country is vannamei and is stocked throughout the year. India also raises black tiger shrimp with around 10-12 hatcheries that operate seasonally and are stocked mainly during summer.
In normal course, India produces 100 billion PLs per year, but in the current context is producing 60 billion PLs, of which only 1.5 billion are black tiger (97% of vannamei and 3% of black tiger).
India also has other shrimp species such as the Indian white prawn (Fenneropenaeus indicus) which can be easily domesticated since it reaches maturity in 4-5 months. The government has started a domestication program and probably in two years, we will have another species to farm. Another candidate is the banana prawn, which is also easy to domesticate and, in fact, Australians have already done so.
I think we need more species because it would make it easier to deal with disease outbreaks. We have these two candidate species and through domestication we could have them as a backup in the future, in case vannamei has any issues.
HFM: There is a wide variety of hatchery feeds. What are the main feed procedures in India? Is there room for improvement in this sense?
RKY: All commercial larval feeds in India are imported from reputed feed companies abroad. We don’t have any local manufacturing facilities. Even polychaetes are imported, two shipments per week from Europe. Apart from that, we also use wild polychaetes and blood worms but we pasteurize them. This process ensures they are free of pathogens and maintain the original nutritional profile at the same time. Moreover, hatchery operators screen their broodstock periodically to know the disease status. However, polychaete production within a shrimp farming country has its own risks. You can come up with a SPF facility but the disease could find its way through. Therefore, there is room for all feed companies to work on much-improved artificial feeds to replace live feeds.
HFM: In terms of hatchery infrastructure and husbandry, what are the main trends in the country? Is there room for improvement?
RKY: Indian hatcheries are the most sophisticated in the world. They are very biosecure, use SPF broodstock and are modular in operations with smaller tanks and with much better control of the operation as well.

However, there is always room for improvement. Hatcheries have a window of three days to sell PLs from PL9 to PL12. Once it crosses PL13-14, you will have size variation and farmers will not be interested in purchasing them. Hatcheries need to go for fast stocking to reduce the length of the stocking and thereby keep the bacterial loads under control and get better survivals. However, when the seed demand is not good, you can’t go for fast paced stocking because you have to sell again the seed at the same pace. So it’s a kind of a catch-22 situation. Hatcheries are realizing that and not stocking all the tanks.
One of the key challenges for hatcheries is size variation after PL13. Any technology in terms of feed, feeding systems, aeration, husbandry, etc. that would allow to keep PL length uniform and keep them for a little longer period at the hatchery would be a breakthrough and it would allow farmers to get bigger PLs of uniform size and enable hatcheries to have a longer window to sell.
HFM: India’s shrimp industry is facing some challenges and one of the most important is diseases. From your perspective, should the industry change the approach in which SPF broodstock is being used?
RKY: In the past few years, genetics have allowed us to improve the business, but not now. The environment has changed, both biotic and abiotic. Nowadays, we have a host of diseases; viral, bacterial and fungal making the life of the farmer difficult. Broodstock
which used to perform very well five years ago is not doing well today. Some industry stakeholders are blaming breeding companies but it is not all their fault. Having said that, they also have the responsibility to change their stock to fit this changed environment in the mutual interest.
India used to buy around 250,000260,000 broodstock per year and it even went up to 280,000, but today it has dropped to 150,000-160,000 and could be reduced further. If the situation continues to be like this, everybody could lose. Their lines would do very well under biosecure conditions, but foolproof biosecurity cannot be achieved in the Indian industry. We have a lot of small farmers that can’t go for indoor/semi-indoor facilities. Even in Vietnam where they have gone for indoor/semiindoor and multi-phase culture systems, the results are not that encouraging.
Breeding companies should work on disease resistance. It is not just enough being disease-free because there is also horizontal transmission. Breeding companies need to develop tolerant and resistant lines, like in South America, where these lines are giving good results. These strains combined with low densities would allow us to improve.
We had a dialogue with all the breeding companies and now they understand our requirements, not only in India but throughout Asia. A few companies are coming up with sentinel trials. One company has sent several of their families in a batch of broodstock and PLs are produced from that batch and distributed to farmers. The results are going to be followed up to select the families that are best suited for Indian conditions.
HFM: 2023 was a difficult year for the shrimp industry. How do you see 2024 in India?
RKY: My prediction is that this year’s production in India is going down. The export figures in January and February were higher than last year, but March was down 13-14%. I think that is going to be the trend this year, with a reduction of 13-15% and some months could go down by 20% also.
In late 2022, a coalition of industry players, investors, and academic resources partnered up to create a high-quality supply option for Atlantic Salmon eggs in the US and as a result, SalmoGen Company Inc. was founded.
SalmoGen is an investment partnership between Cuna Del Mar, Builders Vision, the Penobscot Indian Nation, and Xcelerate Aqua. The company is collaborating with some of the most experienced resources in the US and beyond in breeding program development. US partners include resources from the USDA, the University of Maine, the University of Idaho, and the University of Maryland. The Center for Aquaculture Technologies, Innovasea, and Xcelerate Aqua are strategic development partners. The company has had broodstock under development since early 2023 in two partner locations. The company’s broodstock facility is being developed on Penobscot Trust Land in Maine and the first product in the market is scheduled for 2027.
BCWD-resistant trout
are now available year-round

Troutlodge achieved a milestone in year-round availability of Bacterial Cold Water Disease (BCWD) resistant trout eggs for customers all over the world. This development is the culmination of over ten years of research and investment to combat this damaging disease. Troutlodge, in collaboration with the USDA, initiated research over ten years ago to help solve this issue. Through the development of an SNP chip, the use of genomic selection and laboratory challenges, the company has focused on increased BCWD resistance in its breeding program, with outstanding results.
The FAO publication on brine shrimp is a manual for all those who are using Artemia or have an interest in this organism, whether as a source of live food in the hatchery, as a model organism in research, or for other purposes. It is intended for those who wish to update their knowledge on its biology, production or use, but also for those who want to learn about Artemia for the first time. A team of leading Artemia experts from around the globe with diverse backgrounds, expertise and working in research, education and/or the industry have jointly contributed to its writing.


Gustavo Bozano has been appointed as new CEO of GenoMar Genetics Group. “I am very excited about joining GenoMar to further develop its position as the leading tilapia genetics supplier worldwide. I have experienced the value of genetics and efficient distribution of genetic products to be a key contributor to a healthy and profitable tilapia industry,” said Bozano. Alejandro Tola Alvarez, who has served as CEO of GenoMar Genetics Group for the past seven years, was appointed as executive director for Non-Salmonids in Blue Future Holding.

Marcell Boaventura has stepped down as CEO of hatchery feed innovator Molofeed.
Boaventura held the position for the past three years. During his lead, the company developed new processes and products, scaleup production, delivered an R&D aquaculture facility in Brazil, succeeded in the import/ export regulatory environment, achieved brand recognition, delivered against commercial goals, launched new products in the market and met sales targets in Asia and Latin America, Boaventura said. In 2019, Aqua-Spark added Molofeed to its investment portfolio.
A new project to breed “super” snapper that is more resistant to disease, grows faster, and can thrive in warmer water could help drive more economic growth through aquaculture, New Zealand’s Oceans and Fisheries Minister, Shane Jones, announced.
“The potential here goes far beyond growing a better and more resilient breed of fish, it also supports our efforts to grow and future-proof New Zealand’s aquaculture. Climate change is affecting the condition of our oceans, and this project is a practical response by a key industry to that change,” Jones said.


Microbiome profiling company, Luminis Water Technologies, has optimized both water and shrimp gut microbiomes, resulting in 56% growth enhancement and a 30% reduction in pathogen loads. The optimization process involved a multifaceted approach, integrating precision microbiome engineering techniques with Lumins’ AquaGENius microbiome collection kit together with their Next Gen Precision Probiotics. By modulating the microbiome in both water and shrimp gut, Luminis achieved an optimal balance that facilitated enhanced nutrient uptake, improved digestive efficiency, and a fortified immune system in the shrimp population.
Nutreco opened a state-of-the-art fish and poultry feed production facility in Ibadan, Oyo State, Nigeria, through its operating company trading under the names Skretting and Trouw Nutrition. The new EUR 25 million facility was built on 170,000 square meters of land and has the capacity to manufacture 125,000 metric tons of extruded fish and animal feeds per year. Skretting Nigeria Limited, launched by Nutreco in 2014, produces catfish superior feeds, broodstock feeds and tilapia catering for the entire fish life cycle.

The Bangladeshi government has approved to importation of more than five million pieces of non-native vannamei shrimp seed, aiming to start commercial farming in the country, local news reported. The company Green Bio Tech (BD) Corporation will import the shrimp seed called shrimp PL (10-12) from India. The permission has been given on condition that such seed will be used only for production of new seed.



Current situation
In today’s highly competitive global market, genetic improvement emerges as a crucial tool to enhance shrimp production efficiency and maintain market competitiveness, especially in larval production. The shrimp industry continues to face significant challenges due to the high supply and pressure on market prices. Countries in the Americas and Asia have increased shrimp production without a corresponding rise in demand, resulting in the lowest prices in decades. Genetic improvement can significantly boost shrimp production efficiency by selecting shrimp lines with faster growth rates and better resistance to diseases and environmental conditions. These improvements
accumulate generation after generation, increasing efficiency per unit area and maintaining market competitiveness by reducing production costs.
Impact of genetic selection
Genetic improvement in shrimp extends beyond growth and grow-out phases to reproductive parameters such as the number of spawns per cycle and the number of eggs or nauplii per female. This leads to more efficient broodstock, cost reduction, and increased profitability over the long term.
Recent research indicates that improvements in spawning frequency can be inherited at rates ranging from 15% to 37%. Similarly, enhancements in the
number of eggs and nauplii per female are heritable at rates of approximately 17-26% and 18%, respectively. This implies that genetic selection programs can effectively boost these parameters, increasing productivity and significantly reducing hatchery costs.
Animal welfare is another critical aspect. Traditionally, many hatcheries use eyestalk ablation (removal of an eyestalk) to increase female productivity. However, this practice negatively impacts shrimp by shortening their productive lifespan, increasing mortality, and raising disease prevalence. Genetic selection offers a nonintrusive alternative to improve female productivity without such adverse effects. Moreover, genetic improvement programs focused on growth can indirectly enhance female productivity, positively impacting hatchery efficiency. This is supported by a strong positive correlation (>90%) between female weight and the number of eggs and nauplii produced per spawning.
Genetic information also helps manage inbreeding in broodstock, which can negatively affect growth and survival during larval development and grow-out stages. Although the effect of inbreeding on reproductive parameters has not been extensively studied, it is significant. A 10% increase in female inbreeding has been observed to reduce egg fertilization rates by 3.0% to 26.0% and decrease nauplii numbers by 2.9% to 24.6%. Managing inbreeding through genetic monitoring and strategic crosses is essential.
Implementation of genetic selection programs
The Center for Aquaculture Technologies (CAT) specializes in implementing genetic improvement programs tailored to the characteristics and needs of clients and the market. These programs use genetic information (genetic markers) to generate data for mass selection, pedigree, or genomic selection programs, aiming to improve key phenotypic traits for the client. Selecting productive traits such as growth and survival during grow-out is indispensable for adding value for those using the produced larvae. However, selecting reproductive traits can be crucial to reducing costs and increasing productivity. A genetic selection program should focus on generating data associated with these traits to include them in selection models. This will allow for improvements in multiple productive traits by optimizing the design of crosses and selecting
animals with the best genetic and phenotypic potential. Proper management will ensure the control of genetic diversity and inbreeding within the population, in addition to generating improvements in multiple traits of interest.
Steps
1. Define Objectives: Determine the traits to improve (e.g., growth rate, disease resistance, reproductive efficiency).
2. Baseline Assessment: Conduct a Genetic Overview (GO) to characterize current genetic diversity, inbreeding levels, and relationships within the shrimp population.
3. Select Genetic Markers: Choose appropriate genetic markers (e.g., SNP panels) for tracking genetic variations and guiding selection.
4. Design the Genetic Selection Program: Develop a breeding strategy that includes mass selection, familybased breeding, or genomic selection based on your objectives and resources.
5. Implementation: Start selecting and breeding shrimp based on the identified markers and desired traits.
6. Monitoring and Adjustment: Continuously monitor genetic progress and adjust the breeding program as needed to ensure ongoing improvement and sustainability.
Measurable improvements in productivity and cost reduction can vary based on species, breeding program, and initial genetic diversity. Generally, initial improvements can be seen within one to three generations. Continuous monitoring and adjustments can further enhance these gains over time.
The initial costs of implementing a genetic improvement program include:
Genetic analysis: Costs for conducting a genetic overview and developing SNP panels.
Program design: Expenses for designing and establishing the genetic program.
Operational costs: Ongoing costs for maintaining the breeding program, including data collection, analysis, and monitoring.
While there are costs to set up and run the program, the return on investment (ROI) in the medium to long term is significant due to improved productivity and reduced production costs.
Ongoing research and collaborations
Ongoing research projects and collaborations at CAT continue to push the boundaries of shrimp genetics. For example, high-density SNP panels for shrimp genotyping have been developed, integrating data from over 20 shrimp populations worldwide. These panels provide precise genetic analysis and support sophisticated breeding programs.
Research also includes exploring genomic selection and genome editing technologies to further enhance shrimp breeding. By focusing on traits like growth, disease resistance, and reproductive efficiency, these efforts aim to make significant strides in improving shrimp production.
One promising area is genome editing, which allows for precise modifications of shrimp DNA to enhance desirable traits. Research labs, such as those at CAT in San Diego, are at the forefront of developing these technologies, with the potential to revolutionize shrimp farming in the near future.
Conclusion
Genetic improvement is vital for increasing production efficiency and maintaining competitiveness in the shrimp industry. By focusing on producing robust, disease-resistant shrimp with better reproductive parameters, the industry can tackle current challenges and secure a sustainable future. CAT can help establish a comprehensive genetic improvement approach, benefiting producers by reducing costs, increasing profitability, and promoting sustainable and ethical shrimp production.

By leveraging genetic technologies and ongoing research, shrimp farming can evolve to meet the demands of a growing market while ensuring sustainability and economic viability. Genetic improvement programs, when correctly implemented, offer a powerful tool for achieving these goals, paving the way for a more efficient and resilient shrimp farming industry.
More information: Carlos Pulgarin
Breeding Scientist
The Center for Aquaculture Technologies

Marcos De Donato Breeding Scientist
The Center for Aquaculture Technologies

Alejandro Gutierrez Director of Genetic Services
The Center for Aquaculture Technologies E: info@aquatechcenter.com

The Hydrotech Value Drum Filter series focuses on reduced maintenance, increased component quality and simplified operation – all to give your plant maximum filtration performance at a minimum operational cost. There are more than 50 technological improvements in the new series, compared to the previous series, that make these filters excellent value for money.
Let us help you! Call +46 (0)40 42 95 30, or visit www.hydrotech.se

Credits: Benchmark Plc
Genetic improvement is a cornerstone of improved sustainability and efficiency in global aquaculture. The recent expansion of genetic support services by Benchmark Genetics is a significant step, not just for the company as a leading provider of genetic improvement services, but for the entire aquaculture industry. This includes making novel genomic tools available and accessible to global breeders, hatcheries, and producers. This development opens new doors to innovation, accessibility, and sustainable practices for global aquaculture. So, what does this mean for the people at the heart of aquaculture? Let’s explore the importance of this expansion from the perspective of those who produce.
For any managing independent hatcheries or involved in larger integrated aquaculture operations, implementation of cutting-edge genetic tools in applied breeding programs has often seemed beyond reach. With Benchmark Genetics expanding its service offerings, these advanced technologies have become more accessible and affordable, marking a significant shift for the industry. Now, smaller operations have the opportunity to tap into genetic improvements previously only feasible to larger entities. This development is about leveling the playing field, allowing for widespread enhancements in stock quality and operational efficiency across multiple species.

Sustainability isn’t just a goal, it’s crucial for the future of aquaculture. The latest technologies and services from Benchmark Genetics focus on making fish farming more sustainable, profitable, and invested in animal welfare. By providing improved tools for improving complex traits like disease resistance and enhanced feed efficiency, these advancements enable direct action against environmental challenges and promote better resource utilization. This commitment to innovative, sustainable practices ensures a more productive and financially viable future for aquaculture, accessible and understandable to everyone in the field.
The benefits of genetic improvements in aquaculture are supported by solid evidence, not just theory. The initiatives led by experts, including Benchmark’s own renowned team of geneticists, have resulted in significant, measurable improvements across a range of economically important traits, including growth
rate and resource efficiency, product quality and traits like disease resistance critical of improving animal health and animal welfare. This progress is crucial for producers, indicating a leap towards not only higher productivity and profitability but also more sustainability. Applied selection for traits like improved growth performance and disease resistance which is extensively scientifically and commercially documented, clearly shows the shift towards practices that are both economically beneficial and environmentally responsible.
Benchmark Genetics expanding its service offerings and becoming a one-stop provider for all genetic technical support services including access to genomic tools and genotyping is a big step forward for wider uptake of cost-effective genetic improvement across the aquaculture sector. It makes sustainable implementation of effective selection into daily operations much easier, letting producers focus on their main job: farming. Now, everyone can
access a wide range of services and world-leading expertise for the full range from optimized broodstock management to planning and integration of state-ofart multi-trait selection without needing to be experts themselves. This now includes access to genotyping tools for many species at market-leading prices. These changes are significant, making things easier for many in the industry.
application:
Genetic improvement in tilapia farming
Although examples to illustrate the practical application of genomics tools can be found in several species, let’s consider the impact on tilapia farming. Tilapia is one of the most widely farmed fish species globally, valued for its adaptability, rapid growth, and good taste profile. However, tilapia farming faces challenges, such as disease susceptibility and variable growth rates.
With the newly accessible genetic tools, tilapia producers can now integrate advanced genomic selection techniques into their breeding programs. For example, by utilizing genotyping services, hatcheries can identify and select broodstock with superior traits, such as increased growth rates and enhanced disease resistance. This targeted selection helps in developing a more robust and efficient stock, ultimately leading to higher yields and better resource utilization.
One specific application is the selection for resistance against Streptococcus, a common bacterial infection in tilapia. By applying genetic markers associated with disease resistance, producers can breed tilapia that are less prone to infections, reducing mortality rates and the need for antibiotics. This not only improves animal welfare but also contributes to more sustainable farming practices.
Additionally, advancements in feed efficiency through genetic improvement allow tilapia to convert feed more effectively, lowering production costs and minimizing environmental impact. This is particularly important in regions where feed resources are limited and expensive.
In summary, the use of Benchmark Genetics’ tools in tilapia farming exemplifies how genetic advancements can directly enhance productivity, sustainability, and profitability. These practical applications demonstrate the tangible benefits of integrating cutting-edge genetic technologies in everyday aquaculture operations.
A collective step towards a sustainable future
The growth of genetic support services is not only about a company’s achievements; it is a common journey toward sustainability within the aquaculture sector. By refining our available genetic resources, we are building a stronger industry together. This joint effort towards better sustainability not only improves our own operations but also supports the health of our industry, playing a crucial role in maintaining environmental quality and ensuring food security worldwide.
Promoting innovation through partnership
Benchmark Genetics’ approach is built on collaboration. By joining forces with industry partners, research organizations, and tech creators, they make sure their developments really make a difference. This lets the producers be part of a bigger network of new ideas, where their needs and the problems they face are taken into account. It is about working together to make aquaculture better for everyone.
The expansion of Benchmark Genetics’ and genotyping services is more than a milestone for the company, it’s a milestone for the aquaculture industry. Producers are first in line to benefit from these advancements, which aim to improve their operations sustainably and efficiently. Making the most of these new opportunities, we are adapting to change and participating in shaping the future of aquaculture.
More information: Morten
Rye Director, External Services and International Strategies Benchmark Genetics Ross Houston Director, Genetics, and Innovation Benchmark Genetics
Ross Houston Director, Genetics, and Innovation Benchmark Genetics

An effective strategy against mass mortalities caused by Vibrio harveyi and Vibrio parahaemolyticus infections in cultured Penaeus vannamei
The farming of Penaeus vannamei is a billion USD industry. However, with the intensification of culture systems to meet a growing market demand, farming is challenged by emerging pathogens, threatening the economic viability and sustainability of this industry. To date, the industry has been stricken with serious economic losses due to the outbreaks of bacterial pathogens that include Vibrio parahaemolyticus and luminous Vibrio harveyi Vibrio parahaemolyticus is known as the causative agent of the early mortality syndrome (EMS) disease, technically known as acute hepatopancreatic necrosis disease (AHPND). This disease is caused by a strain of V. parahaemolyticus (VpAHPND) that harbors insect neurotoxins that are highly pathogenic to shrimp. It has been associated with mass mortalities in shrimp farms across the Asia-Pacific region. Luminous vibriosis disease caused by V. harveyi is an important bacterial pathogen in cultured shrimp and is known to cause shrimp mortalities of up to 100% in hatcheries and grow-out operations. The efficient control of bacterial fish pathogens with the application of probiotics is well established. Similarly, there have been some reports on the application of lactic acid probiotic bacteria to control APHND infections in shrimp. However, information on probiotics that are effective in controlling both pathogenic luminous V. harveyi and VpAPHND is very limited or non-existent. Also, the effectiveness of an applied probiotic consortia to control disease-causing Vibrio in shrimp has not been fully evaluated.
This article describes work conducted to evaluate the effectiveness of mixed-species Bacillus probiotics to prevent infection caused by luminous V. harveyi and VpAPHND in cultured juvenile P. vannamei
To evaluate the efficacy of the mixed Bacillus species probiotics, a feeding trial was conducted using groups of P. vannamei that were maintained with diets supplemented with the test probiotics and a control that received no probiotic supplementation. The probiotic blend evaluated in this study was a selected mixed Bacillus species probiotic (RescueTMZeigler USA) that is known to exhibit inhibitory properties against Vibrio.
The test juveniles (mean weight, 1.0g) were obtained from a local hatchery in Panay Island, Philippines. The trial had five treatments in triplicates. Experimental shrimps were randomly distributed in 15 plastic tanks of 135L capacity at a density of 80 individuals per tank. The treatment group includes the negative control (one group), the positive controls (two groups, one for V. harveyi exposure and one for V. parahaemolyticus exposure), and the two treated groups receiving the Rescue supplemented diets (one for V. harveyi exposure and the other for V. parahaemolyticus exposure).
The negative control group was maintained with diets without probiotic supplementation and was not exposed to the pathogens. The positive control was maintained with diets without probiotics supplements
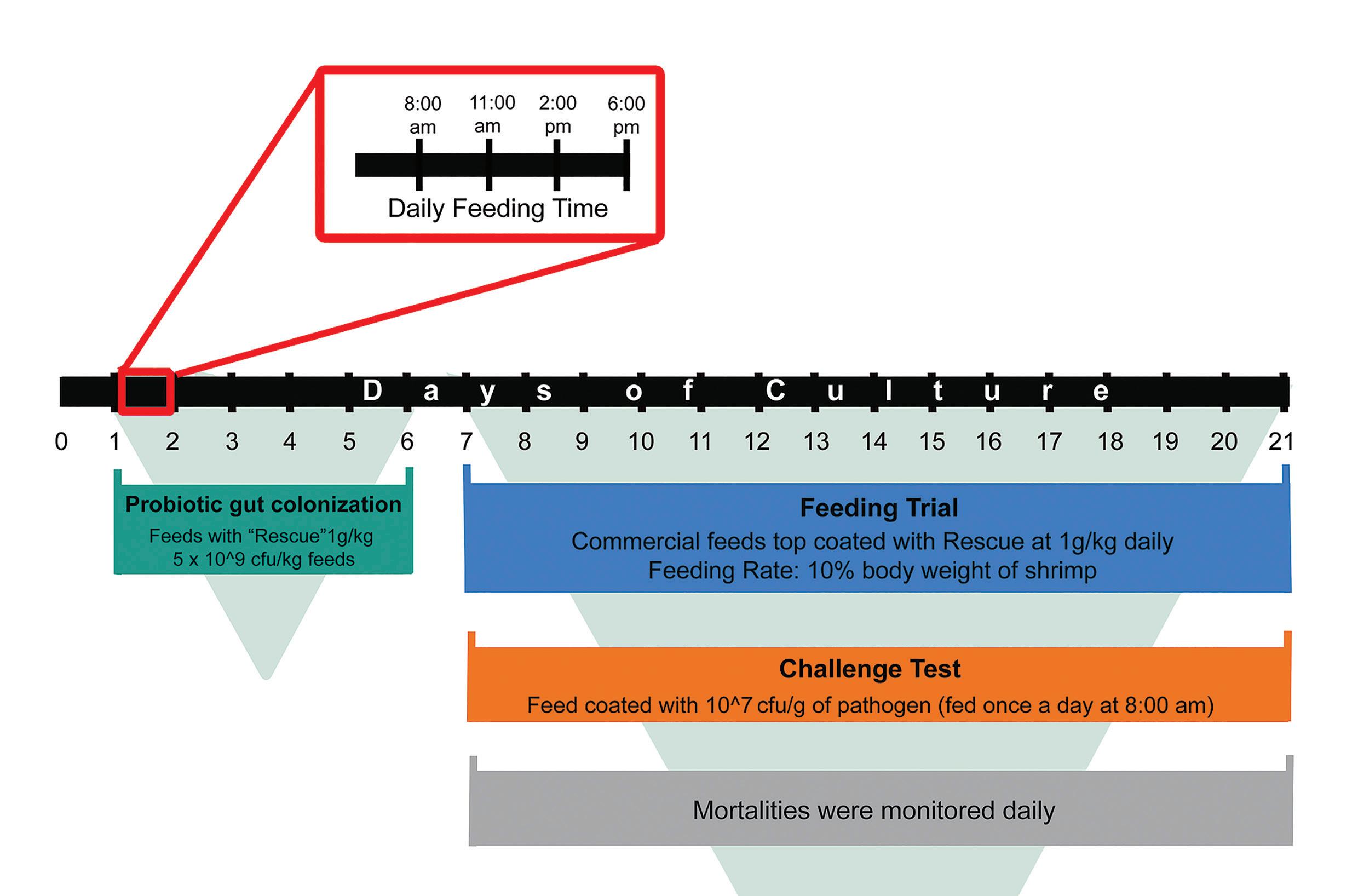
but was exposed to V. harveyi and V. parahaemolyticus pathogens. The treated group was maintained with probiotic-supplemented diets and was exposed to the test bacterial pathogens.
The experimental animals were fed daily at 10% of their body weight. The feed allocation was divided equally into four feed rations given at 8:00 AM., 11:00 AM, 2:00 PM and 6:00 PM. Before the start of the pathogen exposure test, the treated groups (two groups) were fed with probiotics-supplemented diets (Rescue, 1g/kg, 109 CFU/kg feeds) for 6 days to initiate probiotic gut colonization. Following the 6-day prefeeding period, the pathogen-exposed groups were challenged during the 8:00 am feeding only, with diets top coated with V. harveyi, and V. parahaemolyticus at an LC50 concentration of 107 CFU/g feed.
During the trial, dead shrimp were recorded and collected daily in each experimental group and the mortality rates between the control groups and the experimental groups were calculated. The trial was terminated on day 21, 15 days after pathogen exposure. To confirm the mechanism of Vibrio inhibitory activity of the probiotic, an in vitro plate inhibition assay was also performed to evaluate the bactericidal activity against V. parahaemolyticus and V. harveyi. Direct bactericidal activity of the probiotic was evaluated as a clear zone of
inhibition when the probiotic was spotted in the lawn of the pathogens plated in a solid media. Results were analyzed using SPSS16 with a significance level set at 0.05. Bacterial counts were log-transformed before being subjected to a one-way analysis of variance (ANOVA) to determine if there were differences among the treatments for each sampling time. The results with significant differences were subjected to post-hoc analysis using Tukey’s Test.
Inhibition of pathogenic Vibrio
Results from this trial confirmed that Rescue probiotics exhibited potent bactericidal activity against the test pathogens, V. parahaemolyticus, and V. harveyi. Direct bactericidal activity of the probiotics manifested as a clear zone of inhibition when the probiotic was spotted in the lawn of the pathogens plated in a solid media (Fig. 1 A, 1B).
Results indicated that dietary supplementation of the mixed Bacillus probiotics influenced the total gut Bacillus counts in the treated shrimp group as compared to the control groups (Fig. 2). Total Bacillus counts in shrimp gut in the Rescue treatment group showed a significant increase starting from day 3. The significant increase


in gut Bacillus contents during the early phase of the rearing indicated colonization of the applied probiotics on the shrimp gut. Bacillus species are known to be surface colonizers and are also known to effectively colonize the digestive tract of aquatic animals. However, in the present work, the level of gut Bacillus counts was found to be similar in all the treatment groups in the last weeks of the trial. The
data suggested that endogenous Bacillus were also present in shrimp gut specifically in the negative control group. Bacillus species are known to comprise the natural gut microflora of aquatic animals and have been documented to be also present in shrimp gut. The increase in the control group gut Bacillus counts could be attributed to the growth of this normal bacterial flora in response to the nutrients coming from the feeds.

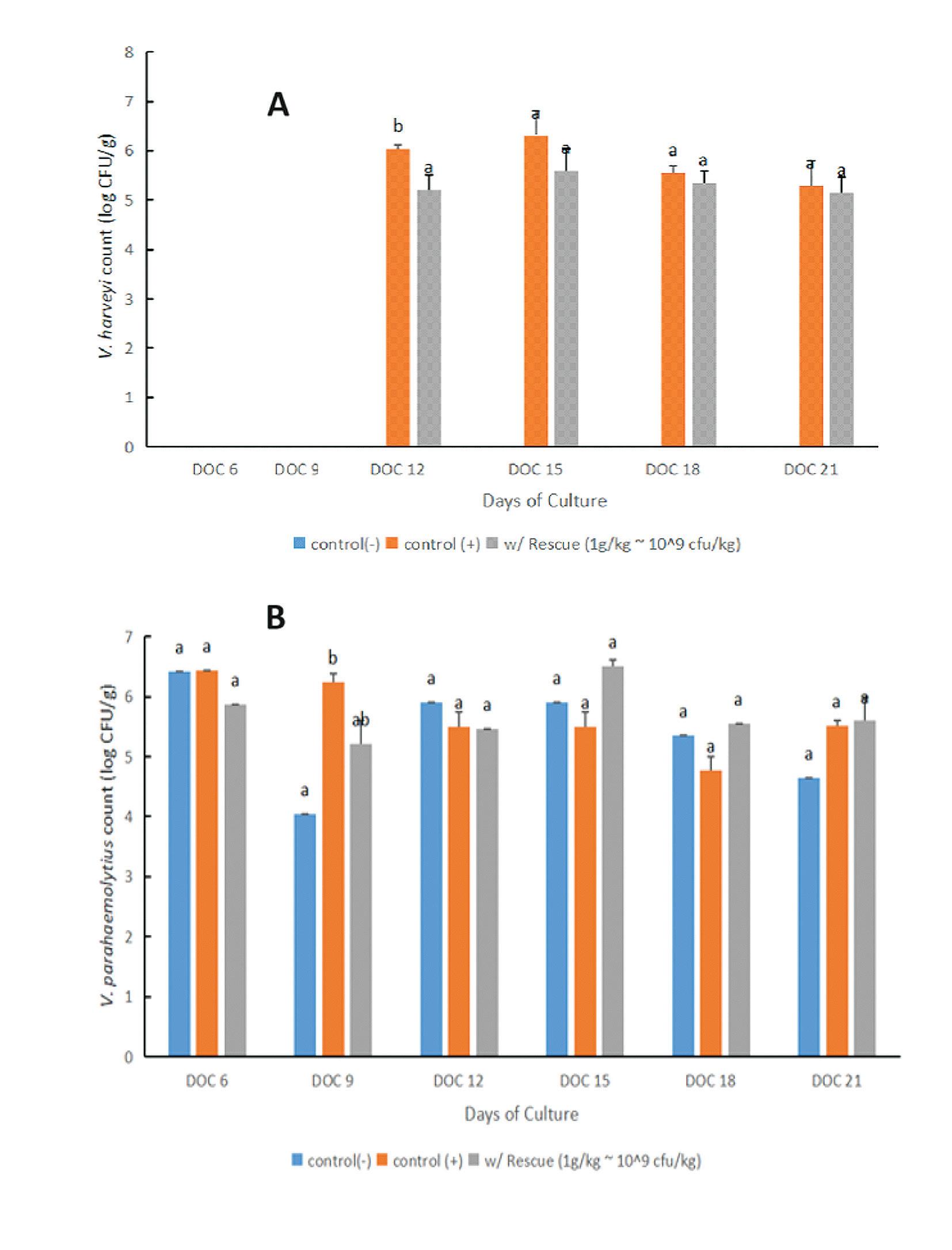
The total V. harveyi colony counts in the gut of the probiotics-treated group were lower than the counts in the positive control group in all the sampling periods (Fig. 3A). However, the difference in counts was not statistically significant among the treatments. The enhanced Bacillus gut colonization in the group receiving the mixed probiotic strains could account for the trends toward lower counts of luminous V. harveyi in the shrimp gut. No V. harveyi was observed in the gut of the negative control group.
In contrast to V. harveyi gut colony counts, V. parahaemolyticus colony counts in the shrimp gut were similar in all the treatments and no clear influence of the probiotic addition could be observed (Fig. 3B). However, the V. parahaemolyticus colony counts in the hepatopancreas suggested that the probiotic-treated group had significantly lower colony counts of V. parahaemolyticus in almost all the sampling periods. These data indicated that dietary application of the probiotic blend promoted the active colonization of the Bacillus probiotics in the shrimp gut and in effect might have inhibited populations of pathogenic V. harveyi and V. parahemolyticus in the shrimp gut.
Efficacy of mixed Bacillus probiotics in preventing mortality
The infection challenge test results indicated that the treated group fed Rescue probiotic Bacillus blend exhibited a 33% improvement (Treated, 60%) in survival as compared to the control group (Control, 46%) when exposed to infectious luminous V. harveyi. Similarly, supplementation of the mixed Bacillus probiotics showed a 40% improvement in survival (Treated, 66%) as compared to the control group (Control, 47%, Fig. 4) following exposure to pathogenic V. parahaemolyticus This improvement in survival could be attributed to the efficient gut colonization of the Bacillus strains in the probiotic blend that might have inhibited the gut tissue entry, infectivity and or pathogenicity of luminous V. harveyi and the APHND-causing V. parahaemolyticus


The hepatopancreas is considered the main target tissue of V. harveyi and V. parahaemolyticus It has been known that V. parahaemolyticus manifests its pathogenicity by infecting and damaging the hepatopancreatic cells of shrimp. The efficient colonization of Rescue probiotics might have prevented the entry of these pathogens in the hepatopancreas and or reduced their pathogenicity, hence resulting in significantly higher survival in the treatment group as compared to the positive control group.
Collectively, the present findings showed that dietary supplementation with the mixed Bacillus probiotics could reduce the populations of pathogenic V. harveyi and V. parahaemolyticus, lessen the virulence of the pathogens, and or enhance the resistance of P. vannamei against these bacterial infectious agents. Additional research will be needed to further elucidate the mode of action of the observed reduction in mortality. Dietary supplementation of Rescue probiotics was shown to be an effective biocontrol approach to minimize mortality and associated economic losses caused by luminous vibriosis and APHND in the commercial farming of P. vannamei
Published first at Aqua Culture Asia Pacific magazine Jan/Feb issue 2024
The significantly higher survival observed in the treated groups could probably be associated with the lower numerical counts of the bacterial pathogens in the shrimp gut. These results might further support the hypothesis that suppression of the pathogenicity of V. harveyi and V. parahaemolyticus due to Rescue supplementation could be linked to the high gut colonization activity, indicated by higher Bacillus counts in shrimp gut. The efficient colonization of Rescue Bacillus could have reduced V. harveyi and V. parahaemolyticus gut populations inhibiting the infection and/or the pathogenicity in the hepatopancreas.



The world's #1 selling Artemia replacement product. Through extensive research and development and improved manufacturing methods, we've identified innovative ways to enhance product performance and bring even better results while continuing to deliver a biosecure substitute for live Artemia.

+ Higher particle nutrient density & digestibility.
+ Enhanced microencapsulation.
+ Improved buoyancy.
+ Demonstrated improved larval performance in trials.
+ Upgraded probiotics for better water quality & gut health.
Researched and tested at Zeigler Aquaculture Research Center







barb@freshflo.com www.freshflo.com
More information: Rex F. Traifalgar Professor and Director Institute of Aquaculture

Rowena E. Cadiz and Fredson H. Huervana Instructors Institute of Aquaculture


Emelyn Joy G. Mameloco Research Associate Institute of Aquaculture

Carmelo del Castillo Assistant Professor and Director National Institute of Molecular Biology and Biotechnology

The above authors are with the College of Fisheries and Ocean Sciences of the University of the Philippines Visayas.



Proper nutrition is essential for a strong start and lifelong development in many animal species, from humans to fish and shrimp. Feed quality for early life stages affects not only growth and performance in the larvae to juvenile phases but also throughout grow-out stages to harvest.
The feeding and nutrition of shrimp larvae are based on supplying feeds which can be live feed (algae, zooplankton, Artemia) and/or formulated diets including microbound feeds, flakes, granulated feeds, agglomerated feeds and liquid feeds. Feed uptake and nutritional requirements vary because of biological differences between larval stages and a knowledge of shrimp larval biology helps in successful feeding.
Life cycle of shrimp as an essential driver to optimizing diets and feeding protocol
Shrimp have two phases in their life cycle: estuarine and marine (Fig. 1). The post larvae migrate to the estuaries, where they grow into juveniles/adults and return to the sea. Here, they mature and spawn and the cycle is repeated. The eggs, larvae and post larvae have pelagic existence, and the juveniles/subadults and adults are benthic (Dall & al, 1990).
The cycle of shrimp from the larval stage to post larvae (PL) is a complex process (Fig. 1) and consists of a succession of molts and metamorphoses. It happens in the wild during migration to “in-shore” brackish estuaries. Therefore, to better answer nutritional needs of larval and PL shrimp, feeding habits (herbivorous, omnivorous, carnivorous), gut maturation and type and quantity of digestive
enzymes produced by the different stages of larval development must be considered.
For instance, a trend of decreasing levels of trypsin exists as the feeding habits of shrimp larvae change from herbivorous to carnivorous for different species and through different stages of larval development within a species (Le Vay & al, 2001).
An adapted diet for each stage
After hatching, there are a few days at Naupliar stages where feeding is only endogenous with yolk as the main energy source. During Zoea stages, larvae become filter-feeders, predominantly herbivorous and with a high rate of consumption. Retention time in the gut is relatively low but enzyme activity is high (e.g., trypsin, lipase). Diets for this stage may not need to be high in protein content if it is highly digestible and of the proper quality (amino acid profile). The use of microalgae as a source of vegetable protein during these first days of

shrimp life can be an option to reach good quality PL. Algae can also be mixed with adapted formulated feed (co-feeding) with particle sizes ranging from 5 to 50 µm. The second stage is Mysis, where larvae are still pelagic filter-feeders but with feeding habits moving to omnivorous/carnivorous. Carnivorous larvae consume less food per unit of time and, therefore, have comparatively longer gut retention times. The feed, exposed to a lower enzyme activity, needs to be highly digestible with a correspondingly high energy content.
During the Mysis stage, the hunting behavior of larvae is still relatively low, so co-feeding of formulated feed and decapsulated artemia cyst can be advised.
Post larval stages are split into two phases: Early post larval from PL1 to PL5 and Post larval from PL 6 to upper PL 15. During the first phases, PL is still mainly carnivorous. Enzyme activity is relatively low with a gut transit time of around 15 minutes. Hence, highly digestible feed rich in protein and with a moderate level of fat (55/15) is required. Due to PL’s hunting capacity, co-feeding with Artemia is possible.
During the second PL phase (> PL6), a formulated diet only can be used with the same nutritional characteristics as during the first phase.
Feed physical properties and feeding protocol
Shrimp size homogeneity at PL 15 is one of the key parameters for hatchery/farm success and it is highly dependent of:
• Water quality
• Feed physical quality
• Feeding management
Effective provision of nutrients to larvae and PL is critically dependent upon the physical form of the microparticulate diet. The shape and the size of diets designed for filter-feeding crustacean larvae is generally confined to particles that are small enough to be entirely consumed without the assistance of a mouth part (NRC, 2011). Only high-technological feed processing systems can achieve optimal physical quality knowing that particle buoyancy and sinking speed also need to be adapted to each stage.
The successful production of robust post larvae for stocking in grow-out ponds also depends on the feeding schedule and the amount that is applied in the hatchery. Underfeeding can generate size heterogeneity between animals and potentially cause loss of production.
On the other hand, overfeeding will cause a wastage of feed and is additionally a potential cause of water pollution, a condition resulting in pathologies, gills fouling, loss of animals or requiring expensive corrective measures. Thus, both overfeeding and underfeeding have serious economic consequences which affect the viability of the farm. Several criteria can be controlled by the farmer to follow the quality of post larvae and to assess the efficiency of the feeding (Table 2).
During recent decades, innovations through feed processing technology have been developed to better



Pathology diagnosis Negative to WSSV, (PCR/histology) EHO, IHHNV, NHP, TSV, etc.
cope with the morphological capacity of shrimp to ingest feed and nutrients. As mentioned above, feed particles for larval shrimp must be very small, nutritionally complete, homogenous and water-stable, yet attractive and digestible to larvae (Hardy & Barrows, 2002). Small feed particles have a large surface areato-volume ratio that accelerates leaching rates of water-soluble nutrients, so this fact adds a degree of complexity to the development of microdiets.
The two general categories of microdiets are crumbled and on-size feeds, terms that relate to the processes by which they are produced. Crumbled feeds are made from conventional extruded pellets that are fractured and then passed through screens to separate particles into different size ranges.
On-size feeds, in contrast, are small pellet-like particles produced using other technologies, such as particle assist rotational agglomeration (PARA process; Fig. 2: Royal Caviar).
By using microencapsulation with “PARA” process, it is possible to produce complex feed particles in which small, water-stable, microencapsulated particles are embedded inside larger particles. Agglomeration meets the nutritional needs of young larvae. This process neither denatures proteins nor damages microcapsules. Commercial shrimp larvae production requires environmental parameters such as temperature, salinity, pH and dissolved oxygen to be carefully

controlled to guarantee the ideal development of the larvae. Furthermore, the diets need to be formulated considering high digestibility which is primarily achieved with good selection and control of ingredients. This selection aims to minimize the excess of nitrogen and phosphorus aiming to reduce water discharge and risk related to accumulation of organic material. Shrimp health in hatcheries may be affected by various living or non-living agents in their environment (Table 2). Many infectious organisms such as bacteria, fungi, viruses or parasites may cause disease to farmed shrimp larvae, and organic waste in water can serve as a carrier to these pathogenic organisms and increase their development.
Innovations have been developed to better control the environment where shrimp is evolving. There is

increasing evidence that the use of microorganisms such as Bacillus spp. may play an important role in improving water quality (Banerjee & al, 2010) and increasing the growth and survival of shrimp. Rapid degradation of accumulated organic wastes and overall improvement in the health and yield of cultured organisms have been recorded upon introducing the active cells of selected microorganisms into ponds or tanks (Gatesoupe, 1999). This external seeding of useful microorganisms, popularly known as bioremediation, is gaining impetus, especially in environmental management. Bioremediation is a process of reducing hazardous organic wastes to environmentally safe levels using microorganisms.
Ingredients available in LATAM for shrimp larvae feeds
The refinement and selection of local ingredients can bring a competitive advantage for the formulation and production of larvae diets associated with reducing the dependence of the industry on imports. South American aquaculture features a diversity of local marine protein sources with good potential for shrimp larvae nutrition. In Peru and Chile, premium and superpremium options for oils and fishmeals are sourced from sardines, anchovies and mackerel. Another premium source is the hydrolyzed meals from squid (Dosidicus gigas) and byproducts from fish and shrimp. Uruguay has access to krill oils (Euphausia superba) that originated in Antarctica. Brazil has a large availability
of soybean concentrate products and the production of liquid and dry DHA from microalgae. Brazil has also grown in a variety of unicellular sources of proteins and yeast, which are normally produced from corn and sugar cane fermentation. Additionally, both Brazil and Argentina have access to a variety of hydrolyzed proteins originated from the swine and poultry industry. Also fundamental is the processing and formulation of diets that are adapted to the local industry, considering aspects like climate, water availability and production infrastructure. The availability of quality ingredients may be limited and technologies to manufacture them may not be available locally. However, opportunities are linked to the growth of the shrimp industry in countries like Ecuador, Brazil, Peru and Venezuela. Investments in research and development in the shrimp nutrition field also have the potential to bring significant results, diversifying ingredient sources and improving diet efficiency associated with a reduction in formulation cost.
References available on request.
More information:
Frederic Baron
Aquaculture Nutritionist
Creation & Development
ADM

A fundamental change in the aquaculture industry has occurred in recent years due to an increasing awareness that nutrition is intrinsically connected to immune responses and disease resistance. Traditionally, aquafeeds were designed to support growth and avoid any nutritional deficiencies of the farmed animals, while, currently, feed manufacturers prioritize the development of diets with functional properties, granting beneficial effects beyond growth. Functional feeds are extremely important for sectors like shrimp farming, which commonly employ production methodologies (namely, high stocking densities, feeding frequencies and dissolved nutrient loads) that can suppress the shrimp’s immune system, making them more susceptible to pathogenic outbreaks. In fact, the results of the shrimp sector have been inconsistent throughout the years, and these fluctuations in yields have often been associated with severe pathogenic episodes. Moreover, the use of antibiotics is increasingly more restricted, and shrimp cannot be vaccinated due to the lack of acquired immunity. Consequently, nutritional strategies that can help improve the health status of shrimp and make them more resilient in challenging husbandry situations are deemed critical for the success of shrimp farming in years to come. This applies especially to the hatchery and nursery phases, as events in early life events have a tremendous impact on the posterior stages of production.
Joint effort to develop a functional microdiet for whiteleg shrimp post-larvae
Over the past seven years, SPAROS Lda, RIASEARCH Lda and CIIMAR/University of Porto have collaboratively conducted a substantial research effort to develop a microdiet for whiteleg shrimp (Penaeus vannamei) post-larvae (PL) tailored for production in clearwater recirculating aquaculture systems (RAS), an emerging technique used in the industry in several geographies. Yet, this functional diet may also have the potential to be used in other production techniques employed worldwide due to its ability to improve the PL health status.
A series of experimental trials were performed at RIASEARCH Lda research facilities focusing on whiteleg shrimp PL nutrition, functional properties of the microdiets and their commercial competitiveness, allowing to lay the foundations necessary for beginning the development of a novel functional microdiet to be commercialized by SPAROS Lda. CIIMAR had a crucial role in the analytical effort to assess functionality.
Protein requirements in clear-water RAS production
Although commercial feeds for the larval/postlarval phases of whiteleg shrimp are currently widely available, formulations can still be optimized, as these are often not tailored specifically for each given production technique and can lead to an under/ overestimation of the nutritional requirements of the animals. This is particularly relevant for dietary protein,
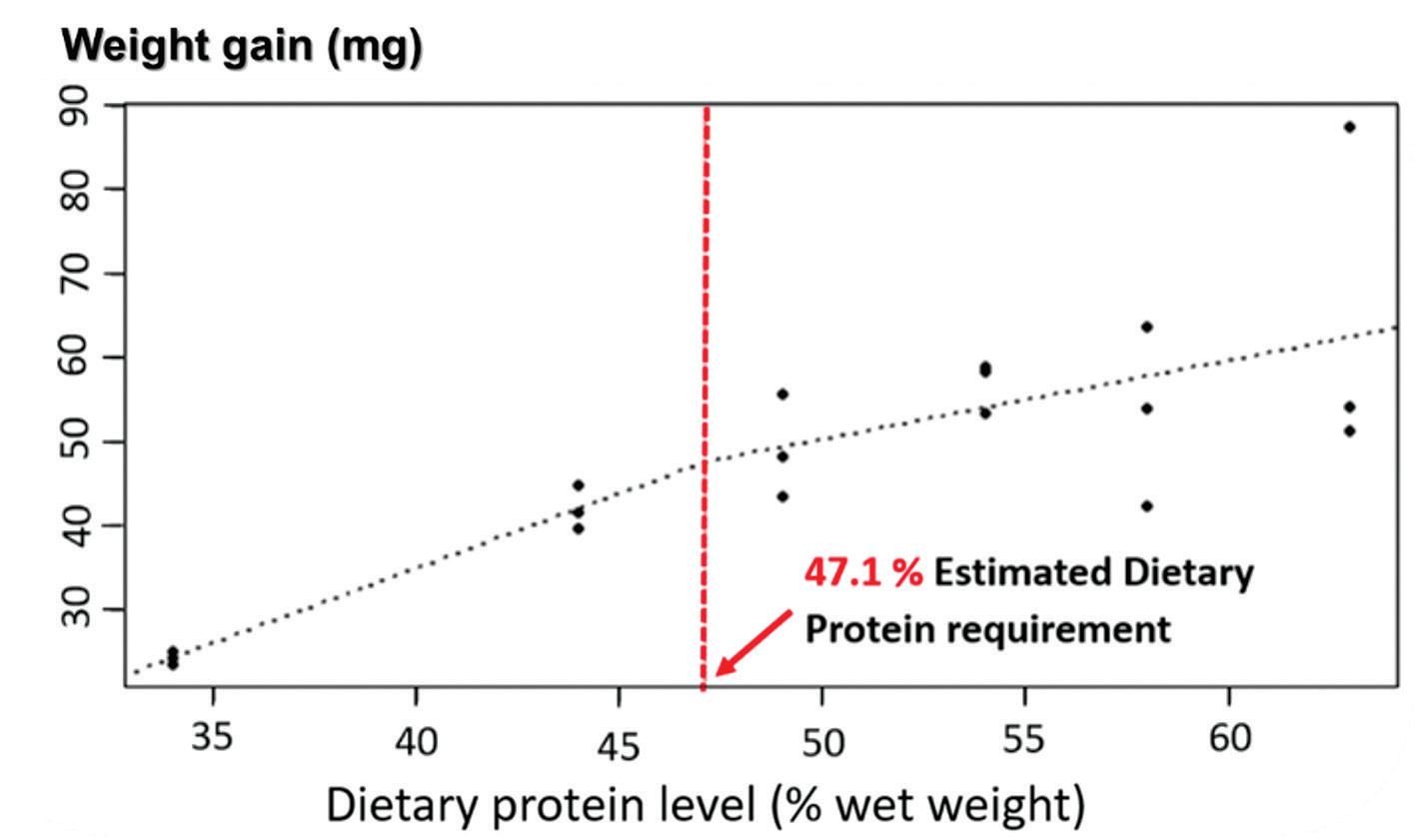
as it is the most crucial nutrient for growth but also the costliest, strongly impacting feed prices. Consequently, determining the optimal protein levels to incorporate in microdiets for whiteleg shrimp PL produced in clear-water RAS was considered vital and the starting point of the experimental work conducted in the scope of this project.
Six microdiets with graded levels of protein ranging from 34 to 63% were tested with whiteleg shrimp PL (around 3 mg of wet weight) for 21 days. Results from this study indicated that protein contents around 47% were optimal for weight gain (Fig. 1), feed conversion and survival, and even greater inclusion levels, up to 54%, seemed to be beneficial to the antioxidant status of PL. Such levels increased the activity of catalase, superoxidase dismutase and total glutathione, and are probably also beneficial for other non-growth purposes. In traditional production methods performed in outdoor systems, protein contents in inert diets can be as low as 25%, or 35% in biofloc systems since the natural productivity in the tanks serves as a supplementary feed source for the shrimp. Conversely, this project showed the importance of using a high-protein aquafeed in the nursery phase when whiteleg shrimp PL are grown in a clear-water RAS devoid of natural productivity, as inert diets are the exclusive source of nutrients in this type of production system (Barreto et al., 2023).
Protein sources impact the functional properties of the microdiets
The source of dietary protein has been proven to be equally as important as having adequate levels of the latter in microdiets. Conventionally, most aquafeeds intended for hatchery/nursery applications include fishmeal as the main ingredient due to its substantial protein content, balanced amino acid profile, nutrient bioavailability, high palatability and constant commercial availability. However, the aquaculture industry has collectively been making a conscious effort to reduce the use of fishmeal as it is foreseen that supplies may not be sufficient to cover the expanding necessities of the sector. Other marine protein sources commercially available such as squid and krill meals have relatively comparable nutritional characteristics, yet these have not been extensively tested in microdiets for the earlier stages of shrimp development.
Considering the previously determined optimal protein levels, four experimental diets were tested for 21 days in whiteleg shrimp PL (13 mg of wet weight) and formulated to have: a balanced mix of fish, squid and krill meal (BASE); fishmeal (FISH), squid meal (SQUID) or krill meal (KRILL) meals as the main protein sources. Squid and krill meals proved to be good sources of protein for the early development of whiteleg shrimp, as well a mixture of these two with a high-quality fishmeal. Growth rates above 15% per day and a feed conversion ratio below 0.9 were obtained with all experimental diets, yet, using either a balanced mix of fish, squid and krill meal or krill meal as main ingredients seemed to promote higher survival values (Fig. 2).
Despite the good results previously obtained utilizing fish, squid and krill meals as the main protein sources of the microdiets, a subsequent experimental trial was designed to evaluate the potential of using soy protein concentrate (SPC) to reduce the preponderance of marine ingredients in the composition of the

microdiets and reduce their overall cost. There is evidence in the scientific literature of successful replacements of fishmeal with SPC in diets for whiteleg shrimp juveniles (Chen et al., 2017; Guo et al., 2020; Ray et al., 2020; Xie et al., 2016; Zhu et al., 2021), yet knowledge of the potential of using SPC in diets for earlier developmental stages is limited. Furthermore, this trial also aimed to assess how impactful the inclusion of krill meal in the microdiets was, as it is an expensive raw material and, like fishmeal, requires the harvesting of wild stocks.
Hence, four experimental diets were tested for 21 days in whiteleg shrimp PL (5 mg of wet weight) and formulated to: have a balanced mixture of fishmeal, soy protein concentrate and krill meal (BASE); have a blend of marine and vegetal protein sources, using krill meal as main ingredient (KRILL); and exclude krill meal (NoKRILL) or SPC (NoSOY) from the diet composition. A further improvement in the diets formulations was achieved as at the end of the feeding period relative growth rates were above 18%/day, feed conversion ratio was around 0.6 and

survival was between 79-91%. The results were similar for all diets tested, which suggests SPC can be used to partially replace marine ingredients in the formulation of diets for whiteleg shrimp PL reared in clear water RAS with no adverse effects on zootechnical parameters. Moreover, PL were exposed to Vibrio harveyi after the feeding period and their capacity to resist infection was apparently compromised, resulting in higher mortalities (around 6x higher than BASE and KRILL diets), when SPC was not included in the diet formulation (Fig. 3).
The complete removal of krill meal from the diet composition in NoKRILL was also detrimental to the PL capacity to resist infection, as mortalities were more than 8x higher than those fed the BASE and KRILL diets (Fig. 3). These results can probably be related to the lower relative expression of several genes associated to antioxidant and immune mechanisms before the challenge when compared to those that were fed diets containing krill meal (Fig. 4). Still, krill meal is a costly commodity and there seems to be no benefits in utilizing it as the main protein source in microdiets for whiteleg shrimp PL when compared to lower proportions, as the ones used in diet BASE.
Health-promoting additives have potential to add value to microdiets
The use of functional dietary additives in aquafeeds to enhance the shrimp’s health status has been studied as a prophylactic alternative to interventional chemotherapeutic methodologies and is regarded as an extremely important strategy to overcome the constraints of intensive shrimp farming. Aiming to further strengthen the functional properties of the developed diets, the potential beneficial effects of several health-promoting nutrients/additives when supplemented in microdiets for whiteleg shrimp post larvae (initial weight: 9 mg wet weight) were evaluated. Four experimental diets were tested for 18 days: a premium diet for whiteleg shrimp PL (PC) and three experimental variants based on the PC, differing only in the ingredient formulation by lower vitamin C and E contents (NC); the supplementation of taurine and methionine (AO); and the supplementation of Saccharomyces cerevisiae β-(1, 3)/(1, 6)-glucans (BG). The additives tested had no effect on PL growth (15%/day), feed conversion ratio (0.9) and survival (86 %). However, these had

4. Relative expression of target immune and antioxidant related genes of whiteleg shrimp PL fed the different microdiets for 21 days, and before being exposed to Vibrio harveyi . Results expressed as mean ± standard deviation (n = 3 experimental units). Different superscript letters indicate significant statistical differences (p < 0.05) between dietary treatments.

modulatory effects on several antioxidant and immune biomarkers that were evaluated. The inclusion of β-glucans reduced lipid peroxidation when compared to the standard diet and promoted the upregulation of the PvHm117 crustin P (crus) and penaeidin-3a (pen-3) genes, both associated with the shrimp innate immune response, when compared to the NC (Fig. 5). Moreover, the supplementation with taurine and methionine resulted in a higher relative expression of the gene crus when compared to the NC, and increased vitamin C and E levels in the PC resulted in an upregulation of the pen-3 gene when compared to the NC, where the level of these vitamins was reduced, which suggests a putative improvement in the PL resistance against pathogenic agents (Fig. 5). Although, these were not tested in particularly challenging husbandry conditions and shrimp were
not exposed to pathogenic agents, the additives tested seem to have potential to add value to microdiets for whiteleg shrimp PL.
Assessing the cost-benefit of functional microdiets
Considering feed is a substantial portion of the overall production costs, is there any advantage in utilizing a functional microdiet with a higher cost in the early stages of whiteleg shrimp development? Despite being high-performing, the developed microdiets can be deemed premium alternatives when compared to what is available commercially for the whiteleg shrimp PL nursery phase. To assess the cost-benefit relationship of utilizing a costlier microdiet in this stage of production, three different formulations were tested: a standard diet (A); a premium diet with a cost around 53% higher than diet A (B); and an ultra-premium diet


± standard deviation (n = 3 experimental units). Different superscript letters indicate significant statistical differences (p < 0.05) between dietary treatments.
with a cost 144% higher than diet A (C). After 18 days of feeding, whiteleg shrimp PL (initial wet weight of 5 mg) fed the ultra-premium diet grew significantly faster than those fed the remaining diets (Fig. 6), while survival values were high and not affected by diet (75-87%). No significant differences were found in the economical conversion ratio (ECR - € spent in feed per Kg of shrimp biomass produced). Furthermore, the ultra-premium diet seems to have enhanced the whiteleg shrimp PL immune and antioxidant mechanisms when exposed to a virulent pathogen when compared to the remaining diets, through the upregulation of the penaeidin-3a, PvHm117 crustin P, c-type lectin and glutathione s-transferase genes (Fig. 7). These results suggest that a higher quality functional microdiet can be advantageous in early phases of production, potentially reducing the time shrimp take to reach a commercial size, which may lead to a higher number of production cycles per year and savings in operational costs. Additionally, these seem to confer benefits beyond faster growth, as evidenced by the apparent improvements in the PL overall health status, which can be an indication that shrimp may be more resilient in potentially challenging husbandry situations.
Is there a place for functional microdiets in whiteleg shrimp hatcheries and nurseries? As our comprehension grows on whiteleg shrimp nutrition and how it can be used to modulate healthrelated factors, functional microdiets for the initial phases of production will become increasingly more advantageous and cost-effective. Investing in a highquality microdiet can potentiate faster growth rates with less feed while contributing to the robustness of the shrimp. What at first glance may seem like an extra expense for producers can translate into savings in operational costs due to shorter production cycles and greater and higher-quality yields. Functional microdiets are increasingly more prevalent in the market and, as in many other species, will eventually become a first choice for whiteleg shrimp hatcheries and nurseries.
More information:
André Barreto Project coordinator RIASEARCH Lda E: andrebarreto@riasearch.pt
Fatty acids (FAs) are essential for bivalve larval growth. In particular, essential fatty acids such as docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA) and arachidonic acid (ARA) are critical for development. A complete absence of these can lead to improper development and failed larval cohorts. Additionally, other fatty acids contribute to the total energy content of algae, which impacts overall larval growth and survival. Shellfish hatcheries typically use algal species known to have high fatty acid levels, including a significant proportion of one or more essential fatty acids.
The fatty acid and lipid content of algae is known to shift in response to the growth phase of the culture, with plateauing, slower-growing or even crashing cultures tending towards a higher proportion of polyunsaturated fatty acids (PUFAs). As PUFAs are constituent molecules for cell membrane fluidity, this



increased proportion of PUFAs may simply be a result of the algae conserving these important molecules when stressed. Different culture regimes have been proposed to enhance the proportion of PUFAs, which may trade off with algal productivity.
To evaluate the tradeoffs between algal quantity and quality, Mallet Oysters, of Shippagan, New Brunswick, Canada, grew Chaetoceros muelleri (CHGRA) with different steady-state harvest rates using Industrial Plankton’s automated PBR 1250Ls while measuring fatty acid composition in harvested cells.
Two paired CHGRA cultures were scaled up in two Industrial Plankton PBR 1250L photobioreactors. Once scaled up, automated harvests were scheduled daily for 200 L or 400 L. Samples were collected for fatty acid analysis at the start of harvesting, and then again two weeks later. Cell densities were estimated on a daily basis using fluorescence. Then, the two PBRs were re-started, and the experiment was repeated to increase replication (4 runs total, 2x per harvest treatment).
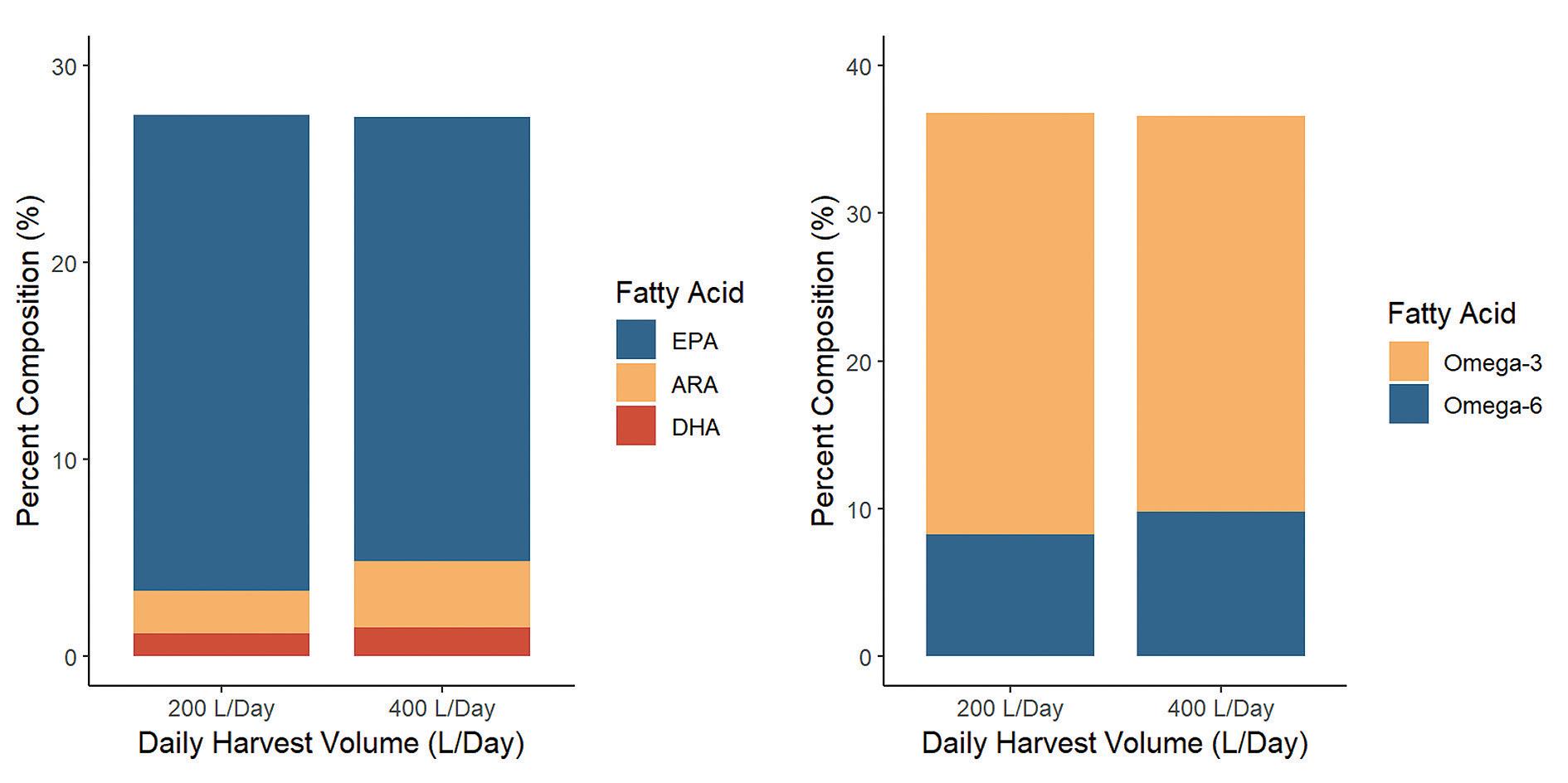

Cell densities were stable within a given run over the two-week experiment, with one exception: a single run in which cell density decreased somewhat after harvesting began due to too high a starting density.
As expected, the PUFA percent composition of algal biomass increased with the higher density culture maintained while harvesting 200L/day. Though the expected trends were evident in the changing proportions of PUFA/SFA/MUFA, omega-3/omega-6, and EPA% composition with harvest cell density, these shifts were marginal compared to the more interesting increase in biomass yield.
The PBRs with the higher harvest rate (400L) had lower steady-state densities than those with the lower harvest rate (200L). However, interestingly production from the higher harvest rate yielded 19% more algae per day.
Conclusions
The amount of shellfish a hatchery can produce is directly proportional to the amount of algae it can produce. Hatcheries are generally limited by the availability of algal production units, labor, and time, so it is imperative for them to make the most of the resources available to them. In light of this limited study, it is clear that using Industrial Plankton’s PBR 1250Ls to produce algae is more efficient at higher harvest rates as it significantly increases food supply without
a significant decrease in food quality. Here, we tested harvest rates of 16.6% and 33.3%, but the logistic growth rates of algae can certainly support higher harvest rates. It will certainly be interesting to continue this research by harvesting higher daily volumes to see if we can further optimize daily production from these automated bioreactors.
More information:
Martin Mallet Co-owner and Hatchery Manager Mallet Oysters Industrialplankton.com


HFM: With more than 30 years in the business, how did it all start?
RJ: The journey of modern tilapia breeding began over 30 years ago, in the late 1980s with the development of the Genetically Improved Farmed Tilapia (GIFT) strain through a collaboration between Norwegian and Philippine partners under UNDP/CGIAR. Despite initial skepticism, Norwegian researchers spearheaded this initiative, inspired by successful breeding principles used in Norwegian salmon farming. Over the years, various global technologies were developed and implemented in tilapia aquaculture practices. In 1999, GenoMar
acquired the ninth generation of the GIFT strain and was granted commercial rights and the responsibility to continue the breeding program. Under GenoMar, we have since continued our selective breeding program, incorporating various key traits and advanced technologies, making it a high-performing tilapia brand.
HFM: Where is the company today in terms of breeding lines, centers and markets served?
RJ: Before the acquisition by EW Group in 2017, we primarily operated a single breeding program serving the Filipino and Malaysian markets. The group expanded

by acquiring the brands Aquabel and AquaAmerica and distribution centers. Currently, we operate two state-of-the-art breeding nuclei in the Philippines and Brazil, running distinct breeding programs through various breeding lines. We distribute products via our hatcheries in five countries: the Philippines, Malaysia, Vietnam, Brazil, and Colombia. We deliver commercial fingerlings or juveniles directly to farmers using our fully owned hatcheries or through joint ventures in new markets.
HFM: What breeding technologies and tools has the company been using to develop these lines?
RJ: Our primary focus remains on growth, although we have evolved methods to address production system complexities and market demands. We now use a comprehensive growth index based on data from various locations worldwide. Additionally, we have long incorporated traits like fillet yield, general robustness, and specific disease resistance into our breeding programs. We have included resistance against diseases like streptococcosis, columnaris (Flavobacterium), Aeromonas infection, and francisellosis. In terms of technology, our advancements include the completion of the initial R&D stage of using CT scan and ultrasound (USG) for precise measurements of specific traits.
HFM: What about technologies like using genomics and/or DNA information for selection? How does this compare with other aquaculture species like salmon?
RJ: We began using DNA information for pedigree reconstruction in breeding as early as 2000 and pioneered the first genotyping tool (high-density SNP array) of 50K SNPs (single nucleotide polymorphism) for Nile tilapia in 2016. After nearly six years and testing of around 300,000 fish, we refined our array to 70K SNPs, enhancing the coverage. To provide a comparison with other aquaculture species, let’s consider the density of the 70K SNPs array in relation to the Atlantic salmon array. While the tilapia genome spans approximately 1 Gb, the salmon genome is about 3 Gb – three times larger than the tilapia genome. Consequently, the SNP coverage provided by the 70K SNPs array for the tilapia genome is equivalent to that of a high-density array for Atlantic salmon, containing 210K SNPs.
While very few salmon breeding companies use these kind of high-density SNP array with >200K SNPs in routine genomic evaluation, we utilize the 70K SNPs array in our breeding program for genomic selection across all traits. This approach significantly enhances prediction accuracy, enabling us to deliver fast-growing, robust, disease-tolerant, and highly productive tilapia strains to the industry.
Compared to other tilapia breeding programs, I believe we stand out as the sole adopter of genomic selection across all our traits in the tilapia breeding world, owing to the substantial resources needed for each generation. This enables us to achieve fast genetic progress for many traits and deliver significant benefits to farmers more rapidly than tilapia bred with simpler methods.
HFM: Why are there just a few genetic markers in tilapia?
RJ: We have identified genetic markers (Quantitative Trait Loci (QTLs)) in tilapia, but not large QTLs influencing the breeding strategy for tilapia, like those for IPN in Atlantic salmon. Not using the identified small to medium QTLs for product formation is partly due to the reproductive nature of tilapia, where selecting breeders based on specific QTLs is economically impractical due to the low offspring numbers and their relatively lower value compared to salmon. Moreover, our experience has shown that genomic selection provides higher prediction accuracy for desirable traits than markerbased selection, even when QTLs are identified. Therefore, we prioritize genomic selection to maximize genetic gain, which consistently delivers a superior product to farmers compared to relying solely on markers in tilapia.
HFM: What is your position in terms of gene editing?
RJ: All genetic material from our breeding centers and contracted hatcheries throughout the breeding, production, and distribution processes, have not undergone any form of genetic modification by means of transgenic methods or gene editing methods. Selection is based on classical breeding and genomics to select the best animals. The genomic information utilized in our breeding processes is derived from natural variations within the species.
Gene editing, particularly for health, survival and welfare traits rather than production traits, presents exciting possibilities for genetic advancements in tilapia breeding. Techniques like CRISPR-Cas9 are promising for enhancing disease resistance, sterility and color. However, any exploration of gene editing must be approached responsibly, considering ethical standards, environmental impacts, and potential unintended consequences. We prioritize the well-being of tilapia and their ecosystems, engaging stakeholders and adhering to rigorous, sustainable practices.
HFM: How different are the current tilapia lines in terms of growth than the original ones? Is there still room for more growth?
RJ: The current GenoMar tilapia lines have demonstrated substantial growth improvements over the original lines, thanks to 34 generations of advanced
breeding techniques. Quantifying these growth differences accurately can be challenging. However, during pre-launch tests conducted under commercial farming conditions in Brazilian production systems, our product GenoMar 1000 showed significant growth rates, increasing from a body weight of approximately 20 grams to 1,000 grams in just 114 days in cages and 121 days in ponds. The fish was compared with a high performing Brazilian strain, and GenoMar 1000 exhibited 30% faster growth.
Despite these advancements, there is still potential for further growth improvements in tilapia lines. The integration of genomic selection and line breeding together could further accelerate the pace of genetic gains, allowing for even more rapid growth rates.
HFM: How far is it for tilapia to be the “aquatic chicken”?
RJ: Like chickens, tilapia are renowned for their productivity and their ability to thrive under diverse environmental conditions globally. However, elevating tilapia to the level of modern commercial poultry
involves focusing on areas such as accelerating growth rates, improving fillet yield, and increasing survival rates. Although progress is being made, there is still a significant journey ahead to achieve the status of the “aquatic modern chicken”. To hasten genetic gains, we are adopting line breeding techniques used in the poultry industry as a foundational blueprint. These efforts are aimed at systematically optimizing and advancing tilapia breeding, with the ultimate goal for GenoMar to become the “aquatic chicken.”
Specifically, we have developed multiple breeding lines, each aligned with our product portfolios. Each line operates under a separate breeding program, concentrating on specific traits tailored to meet market demands. Within each breeding line, the breeding goals are balanced, but the emphasis on targeted traits is increased compared to what could be achieved with a single breeding program. To produce commercial fingerlings, we implement a strategic mating scheme that pairs males and females from different breeding lines. This strategic cross-breeding is designed to


balance performance in the offspring by combining complementary traits. As a result, this method not only fulfills multiple criteria, such as growth, disease resistance, and overall robustness, but also the commercial fingerlings are expected to exhibit hybrid vigor, or heterosis, enhancing productivity beyond that of the parental lines.
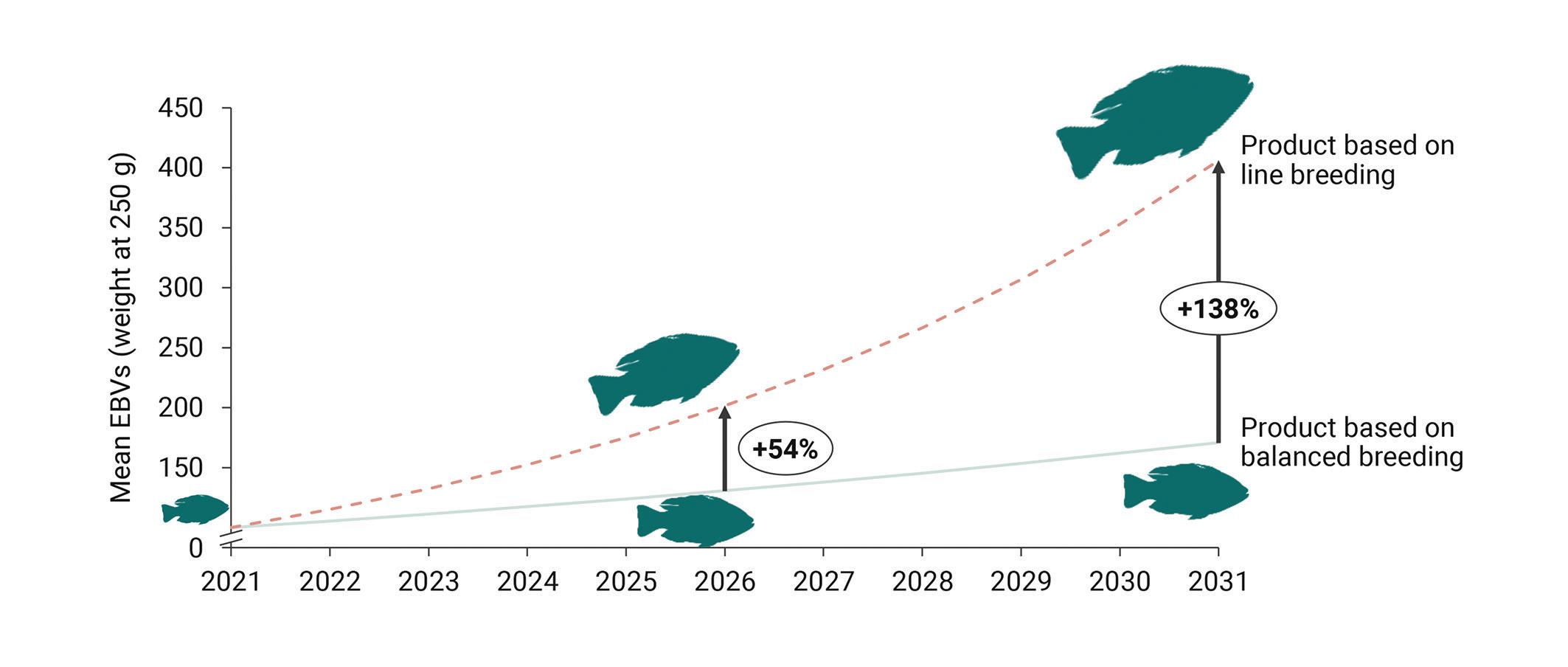
Simulations show that engaging in line breeding will yield an additional 138% genetic gain for GenoMar 250 over the next ten years, which is a very significant difference and the pathway for GenoMar to become the “aquatic chicken”.
HFM: One of the main issues that tilapia farmers are facing is diseases. How can genetics help out?
RJ: Diseases pose a significant risk not only to the substantial investments of commercial farmers, but also to the livelihoods and food security of millions of small-scale farmers. Data from the Philippines demonstrates that farmers can achieve an additional 1.29% net profit for every 1% increase in fish survival at harvest. At GenoMar, we are addressing this challenge through genetic innovation. We have developed a product, GenoMar STRONG, which is a robust fish known for its resilience and superior survival under farming conditions.
Initially launched with resistance to Streptococcosis, GenoMar STRONG has evolved to provide a robust
defense against a variety of major bacterial diseases affecting tilapia in aquaculture, such as infections caused by Streptococcus, Flavobacterium, and Aeromonas; with relative percentage survival compared to our GenoMar 1000 product ranging from 12-22%. Choosing GenoMar STRONG allows farmers to benefit from higher survival rates, thus promoting better animal welfare, sustainability, and profitability within the tilapia production system.
HFM: How do you ensure the health status of your fingerlings to customers?
RJ: Our commitment to biosecurity and health standards is foundational. Our Philippine breeding center (both nucleus and multiplication unit) is the world’s only SPF (specific pathogen free)-certified tilapia facility, reflecting our dedication to preventing pathogen entry into the food chain. We are also pursuing similar certifications for our facility in Brazil, conducting regular surveillance to maintain disease-free status. Since 2021, Brazil’s Ministry of Agriculture (MAPA) has conducted biannual surveillance checks at our Brazilian facilities to achieve SPF and disease-free compartment certifications, testing nearly 1,000 fish with consistently negative results for all major bacterial and viral diseases. These rigorous standards assure our customers the highest quality in fish health and safety.
HFM: Being a species with low margins, especially in developing countries, what can farmers expect in this sense when deciding to go for improved genetics?
RJ: Although our fingerlings come at a premium, the overall cost relative to total production expenses is minimal. Yet the benefits, including improved feed efficiency, growth rate, and overall productivity, are substantial. Trials in Brazil and Malaysia have shown that using our genetically enhanced strains can significantly increase profitability, even during disease outbreaks, proving the strategic value of choosing improved genetics.
For example, a pre-launch trial of GenoMar 1000 in Brazil demonstrated that a fish farm stocking 200,000 fish could see an annual profit increase of 36% in cages and 31% in ponds by switching to GenoMar 1000 genetics (with the price premium). Similarly, a field trial in Malaysia in 2020 highlighted the economic advantages of GenoMar STRONG, showing that even with a 100% premium cost for these genetically enhanced fingerlings (which is not the actual premium price), the return on investment remains significantly higher, especially during disease outbreaks.
HFM: How do you see the future of this industry?
RJ: We are optimistic about the future, with the potential to significantly impact the industry through genetic improvements. Given the annual global tilapia production of 6.5-7 million tonnes, the demand for tilapia fingerlings is expected to be around 35-40 billion per year. This global demand for tilapia fingerlings, comparable to that for day-old chicks in the poultry industry (30-40 billion birds), highlights the scope for expansion and innovation.
Results from field trials have demonstrated that GenoMar fingerlings can help farmers increase both production and profitability by over 30%. This presents a substantial opportunity not just for farmers, but also for primary breeding companies like GenoMar.
Dr. Rajesh Joshi serves as the Senior Researcher at GenoMar Genetics Group, based in the R&D department in Ås, Norway. His role encompasses overseeing the planning and execution of various activities within the breeding nucleus and multiplication facilities of tilapia, located in both the Philippines and Brazil. Additionally, Dr. Joshi is also responsible for product planning and coordinates benchmarking, documentation and performance evaluation efforts across various countries in Asia and Latin America.














In the dynamic world of aquaculture, managing a hatchery demands precision, efficiency, and adaptability. Every aspect, from broodstock management to larval rearing, plays a critical role in ensuring one’s success and sustainability. Recognizing these intricate needs, aquaManager offers a dedicated module engineered to streamline hatchery operations and elevate productivity to unprecedented levels.
aquaManager Hatchery isn’t just another management system; it’s a dedicated platform tailored exclusively to the unique demands of hatchery management. With its intuitive interface and comprehensive feature set, aquaManager Hatchery simplifies the complexities
of aquaculture management, empowering hatchery operators to focus on what matters most: delivering superior results.
Broodstock management
One of the critical pillars of hatchery operations is broodstock management. From stocking to hatching, aquaManager Hatchery facilitates seamless management of all broodstock-related activities. Whether it’s monitoring health parameters, tracking transactions, or recording environmental conditions, aquaManager Hatchery offers unparalleled configurability and precision. This system ensures individualized management and egg collection, allowing
operators to maintain detailed records and optimize breeding programs effectively.
Effective incubator management is essential for maintaining high hatch rates and healthy larvae. aquaManager Hatchery excels in this area by facilitating seamless egg transfers, treatments, and measurements, ensuring precise monitoring at every stage. The software’s ability to streamline sales processes further enhances operational efficiency and profitability. By integrating all aspects of incubator management, aquaManager Hatchery provides a comprehensive solution that ensures the health and quality of the hatchery’s output.
Achieving optimal algae quality is crucial for hatchery success as it serves as a primary feed for larvae. aquaManager Hatchery gives users instant control over the algae production process, ensuring high-quality feed for larvae while minimizing costs. Efficient parameter control and streamlined transaction support mean that operators can focus on maintaining the best possible conditions for algae growth, directly impacting the health and growth rates of the hatchery’s larvae.
Meeting the demands of rotifer and Artemia production is made straightforward with aquaManager’s dedicated modules. These tools provide real-time information, user-defined measurements, and batch code traceability, empowering operators to enhance productivity, eliminate quality issues, and reduce costs. This feature ensures that the live feed production process is as efficient and effective as possible, providing the necessary nutrients to the developing larvae.
Larval rearing and weaning are critical stages in hatchery operations, and aquaManager Hatchery provides comprehensive tools to manage these processes effectively. From environmental monitoring to feed consumption tracking, aquaManager provides proactive insights to optimize production and resource utilization. This ensures that larvae receive the best
possible care and conditions, promoting healthy growth and improving survival rates.
Maximizing output while minimizing costs is a constant challenge in hatchery management. aquaManager’s advanced planning and scheduling tools help achieve this by allowing for precise planning tailored to the hatchery’s needs. User-defined parameters, capacity restrictions, and production targets enable operators to develop detailed and accurate production plans. This capability ensures that all resources are used efficiently, and production goals are met consistently.
Effective inventory control is essential to avoid costly mistakes and ensure efficient resource utilization. aquaManager’s robust inventory management features, including batch code tracking, complete traceability, and automatic notifications, ensure optimal stock levels. This allows hatchery operators to manage supplies efficiently and avoid disruptions in production due to inventory shortages or overstocking.
Transparency and accuracy in cost tracking are vital for any business, and hatcheries are no exception. aquaManager’s comprehensive cost accounting capabilities allow operators to identify trends, pinpoint inefficiencies, and make informed decisions to enhance profitability. This feature ensures that all costs are tracked accurately, providing a clear picture of the financial health of the hatchery.
In today’s fast-paced world, having real-time data capture and access is crucial. aquaManager’s mobile applications empower hatchery teams with on-the-go data registration and insights, ensuring seamless communication and productivity. These mobile solutions enable operators to stay connected and informed, making it easier to manage operations from anywhere.
In today’s competitive aquaculture landscape, aquaManager Hatchery stands as an indispensable

partner in achieving operational excellence. With its unmatched features and unwavering commitment to innovation, aquaManager Hatchery paves the way for a new era of hatchery management. By addressing every critical aspect of hatchery operations – from broodstock and incubator management to algae production and cost accounting –aquaManager Hatchery empowers producers to unlock the full potential of their operations. Experience the difference – choose aquaManager Hatchery for
your aquaculture enterprise and take your hatchery operations to new heights.
More information: Eleftheria Bikou Brand Manager aquaManager E: ebikou@aqua-manager.com


2024
JUNE
27 - 29: Shimp Summit, India responsibleseafood.org
JULY
2 - 5: Asian Pacific Aquaculture 2024, Indonesia www.was.org
15 - 18: 9th International Workshop on the Biology of Fish Gamete www.fgulem.unileon.es
AUGUST
25 - 29: AQUA 2024, Denmark was.org
SEPTEMBER
3 - 5: Global Shrimp Forum, The Netherlands www.shrimp-forum.com
4 - 6: Seafood Expo Asia, Singapore www.seafoodexpo.com
9 - 12: Larvi 2024, Belgium www.aquaculture.ugent.be/larvi
11 - 13: 10th International Aquaculture & Fisheries Expo Taiwan taiwanagriweek.com
24 - 27: LAQUA 2024, Colombia www.was.org
OCTOBER
9 - 11: Aquaculture Vietnam 2024 www.aquafisheriesexpo.com
16 - 17: Smolt production in the future, Norway nofima.com
21 - 24: AQUA EXPO Guayaquil, Ecuador aquaexpo.com.ec
2025
FEBRUARY
3 - 5: Simec Expo, Saudi Arabia simec-expo.com
MARCH
6 - 10: Aquaculture 2025 New Orleans, USA was.org
26 - 28: VietShrimp, Vietnam vietshrimp.net
APRIL
24 - 27: World Aquaculture 2025, China was.org
SEPTEMBER
22 - 25: Aquaculture Europe 2025, Spain was.org
Grand City Surabaya, Indonesia
& Exposition of World Aquaculture Society and Asian Pacific Aquaculture 2024 Annual
of Asian Pacific Chapter,