



Volume 1: Issue 3 - Dec 2022 woundmasterclass.com All Evidence Is Not Created Equal: What Makes Good Clinical Data? The Future of 3D Printed Biofilms for In Vitro and In Vivo Wound Infection Models The Safe Guide to Debridement in the Challenging Clinical Setting Offloading: The Comprehensive Guide Alligator-Derived Hyaluronic Acid: Bacteriostatic and Fungastatic Properties Against Pathogens Mixed Aetiology Lower Extremity Ulcers: The Role of Omega-3 Products Masterclass GUIDES Topical Cyclical Oxygen Therapy Surgical Site Infection Lymphedema in Clinical Practice Emerging Technologies in Clinical Practice Anhydrous Omega-3, 6, 9 Therapy Topical External Haemostat Hydro-Responsive Wound Dressing Open Access | Peer Reviewed | International | Quarterly ISSN 2753-6963 Official Journal of the Association for the Advancement of Wound Care®

































United Kingdom & Europe North America South & Central America Africa Prof Dimitri Beeckman Professor of Nursing Science, Ghent University (Belgium) and Vice-Head of the School of Health Sciences, Örebro University (Sweden) Ghent, Belgium Prof Dr C. Can Cedidi Clinic Director for Plastic, Reconstructive & Aesthetic Surgery Bremen, Germany Dr Paul Chadwick National Clinical Director, Royal College of Podiatry Manchester, United Kingdom Dr Przemysław Lipiński Wound Surgeon, National Representative of Poland in D-Foot International Łódź, Poland Prof Declan Patton Director of Nursing and Midwifery Research and Deputy Director of SWaT Research Center, RCSI University of Medicine and Health Sciences Dublin, Ireland Mr Harm Jaap Smit Wound Biologist, Erasmus MC Academy Rotterdam Rotterdam, Netherlands Ms Lian Stoeldraaijers President, Dutch Association of Diabetes Podiatrists Valkenswaard, Netherlands Mr Frank Aviles Wound Care Clinical Coordinator, Natchitoches Regional Medical Center Natchitoches LA, United States Dr Windy Cole Director of Wound Care Research, Kent State University of Podiatric Medicine National Director of Clinical Safety, Quality and Education, Woundtech Streetsboro OH, United States Ms Kara Couch President-Elect, Association for the Advancement of Wound Care Associate Research Professor of Surgery, School of Medicine and Health Studies George Washington University Director, Wound Care Services, The George Washington University Hospital Arlington VA, United States Dr Kenneth Burhop Life Sciences Advisor and Consultant San Diego CA, United States Mr Tobe Madu Data Scientist, Net Health Atlanta GA, United States Dr M. Mark Melin Medical Director of the M Health Wound Healing Institute Adjunct Associate Professor, University of Minnesota Surgical Department Mineapolis MN, United States Dr Leo Nherera Director, Global Head of Health Economics & Outcomes Research Fort Worth TX, United States Dr Mitch Sanders CSO and EVP Alira Health. CEO of WoundForce Inc. and Firefly Innovations LLC. Boston MA, United States Dr Joon Pio Hong Professor of Plastic and Reconstructive Surgery at the University of Ulsan College of Medicine and Asan Medical Center Seoul, South Korea Dr Luis Alejandro Boccalatte Head and Neck Surgeon, Associate Professor Instituto Universitario Hospital Italiano Buenos Aires, Argentina Sr Trish Idensohn Wound Nurse Specialist, Consultant and Educator Durban, South Africa Prof Dr Harikrishna K. R. Nair President Elect, WUWHS - World Union of Wound Healing Societies President, Asia Pacific Association of Diabetic Limb Problems Kuala Lumpur, Malaysia Dr Brandon Bosque Foot and Ankle Surgeon Philadelphia PA, United States Australia Dr Ross D Farhadieh Cosmetic Plastic & Reconstructive Surgeon Sydney, Australia Editorial Board Dr Eduardo Camacho Plastic and Reconstructive Surgeon Mexico City, Mexico Miss Negin Shamsian Consultant Plastic & Reconstructive Surgeon (Locum) Chief Editor of Wound Masterclass London, United Kingdom East Asia Prof David Armstrong Professor of Surgery and Director, Southwestern Academic Limb Salvage Alliance (SALSA), Keck School of Medicine of USC Los Angeles CA, United States Prof Jan Kottner Professor of Nursing Science, Charité - Berlin University of Medicine Berlin, Germany Dr Aliza Lee Clinical Research Investigator, Department of Veterans Affairs Salem VA, United States Prof Dr Luca Dalla Paola Specialist in Endocrinology, Metabolic Diseases and Diabetology Expert in medical and surgical treatment of Diabetic Foot Ferrara, Italy Dr Alton R. Johnson Podiatric Surgeon Wound Care Physician Ann Arbor MI, United States Dr Jonathan Johnson Surgical Director, Comprehensive Wound Care Services Washington DC, United States Dr David Alper Trustee - Board of Trustees, American Podiatric Mddical Association Board Member - American Duabetes Association(New England) Surgical staff (Emeritas) Mount Auburn Hispital Cambridge, MA, United States Boston MA, United States Dr Sebastian Probst EWMA President Professor of Tissue Viability and Wound Care at the School of Health Sciences, University of Applied Sciences and Arts Western Switzerland, Geneva Genf, Switzerland Ms Terry Swanson Vice Chair, International Wound Infection Institute Victoria, Australia
Chief Editor
Miss Negin Shamsian
Commercial Director
Mr Alec Wright
Contact Editor editor@woundmasterclass.com

Commercial Inquiries
commercial@woundmasterclass.com Article Submissions submissions@woundmasterclass.com
The Road Less Travelled | Miss Negin Shamsian
Accelerated Healing and Advanced Wound Care: What is the Role of Wireless, Closed-Loop, Smart Bandages With Integrated Sensors and Stimulators? | Mr Artem Trotsyuk
Optimizing Management of Lymphedema | Dr M. Mark Melin
All Evidence Is Not Created Equal: What Makes Good Clinical Data? | Ms Kara Couch
The Safe Guide to Debridement in the Challenging Clinical Setting | Dr Aliza Lee
Closing the Gap of Health Disparity in the Wound Care Industry With the Use Long Wave Infrared Thermography in Dark Skin Individuals | Mr Frank Aviles
Mixed Aetiology Lower Extremity Ulcers: The Role of Omega-3 Products | Dr Windy Cole
Offloading: The Comprehensive Guide | Dr Anthony Tickner
Diabetic Foot Ulceration: The Triad of Treatment Evaluation | Dr Alton R. Johnson, Dr Brennen O’Dell
Alligator-Derived Hyaluronic Acid: Bacteriostatic and Fungastatic Properties Against Pathogens | Dr Mitch Sanders
The Future of 3D Printed Biofilms for In Vitro and In Vivo Wound Infection Models | Dr Mitch Sanders, Ms Mia Hanna
Wound Biopsies of Atypical Wound Presentations Lead to the Diagnosis of Rare Disease States: A Case Series | Dr Windy Cole
Minimizing the Legal Issues Around Pressure Injury Care | Ms Kathleen Martin
Reducing Pain and Accelerating Healing in New Technologies | Ms Liz Ovens, Ms Claire Allan
Biosurgery: Indications, Contraindications, Interactions and Side-effects | Dr Ronald A. Sherman, Dr Frank Stadler
Biosurgery: Application and Dressing Technology | Dr Ronald A. Sherman, Dr Frank Stadler
of Tendon Disorders | Prof Anand Pillai
Published
©
Ltd. This
is intended for
distribution and this issue is
form
December 2022
by Clarus Communications Ltd., Oxford, United Kingdom No part of this issue is to be copied or reproduced without permission of the publisher
Clarus Communications
publication
online
not suitable for print in this
To inquire about obtaining a printable version of this issue or any article therein, please contact the editor
3 4 - 8 10 - 14 16 - 20 22 - 26 34 - 38 40 - 41 48 - 55 56 - 58 60 - 67 68 - 72 74 - 78 86 - 88 90 - 96 97 - 103 105 - 112 113 - 115 Masterclass GUIDES Topical External Haemostat Anhydrous Omega-3, 6, 9 Therapy 28 - 31 42 - 45 Cover image: Licenced from Adobe Stock Credit: Vink Fan
Terminology
EWMA 2023
THE 33RD CONFERENCE OF THE EUROPEAN WOUND MANAGEMENT ASSOCIATION
WOUND CARE – FROM ART TO SCIENCE

DALL’ARTE ALLA SCIENZA: L’EVOLUZIONE DELLA CURA DELLE FERITE
OTHER COLLABORATORS:
MILAN, ITALY 3
5 MAY 2023
–
The Road Less Travelled
As we find ourselves in the winter of this unique year, we reflect with fondness on the end of one chapter and the very happy memories and the great friendships, partnerships and collaborative links we’ve fostered. At the start of a new year we look forward to all our new projects and plans for the upcoming year. Esteemed American poet Robert Frost highlighted motifs of seasons and in the autumn of his life he wrote what is considered one of his best pieces; he wrote about choices in life and their consequences and the beauty of taking a road less travelled.
Every author that has contributed to this packed issue of Wound Masterclass has taken the road less travelled and it has made all the difference.
In this winter issue we look at the role of Wireless Closed-Loop Smart Bandages with Integrated Sensors and its role in advancing healing, and Dr Mark Melin explores the Optimisation of Lymphedema, Kara Couch takes an indepth look at What makes Good Clinical Data, Dr Lee takes us on a journey of Safe Debridement, Dr Tickner highlights a comprehensive guide to Offloading, Dr Alton Johnson looks at the Triad of Treatment for Diabetic Foot Ulceration, and Saunders and Goodeve look at the role of TLCNOSF Dressings for Diabetic Foot Ulceration. With a nod to futuristic trends in technology in wound care we have two excellent articles one on The Future of 3D Printed Biofilm for In Vitro and In Vivo Infection Models and a look at Alligator-derived Hyaluronic Acid. Dr Cole looks at A Case Series of Atypical Wound Presentations. Ms Hayley Ryan comments on Addressing the Challenge of Pressure Injuries, and Ms Kathleen Martin advises on how to Minimise the Legal Issues around Pressure Injury Care, Dr Sherman and Dr Stadler explore
the role of Biosurgery in Clinical Wound Care. Prof Anand Pillai provides a useful guide for wound care clinicians to Tendon Disorders.
We have an excellent series of Masterclass Guides looking at in-depth topics in a concise collectible format. This issue has a Masterclass Guide on: Topical External Hemostats, Anhydrous Omega-3,6,9 Therapy, Hydro-Responsive Wound Dressings, Lymphedema, and the MasterSeries on Topical Cyclical Oxygen Therapy, and Surgical Site Infection Prevention. As always we are deeply appreciative of your support of Wound Masterclass. We are fortunate to have some of the world’s most innovative wound care leaders on our global editorial board who share in our quest to provide free wound care education.

We have great appreciation for all the time and effort made by our authors and editorial board this year. Thanks to all of you for reading our journal, providing content, interacting with us, watching our MasterSeries 60 minutes interactive, a fully immersive experience.
On behalf of the Wound Masterclass team we hope you enjoy this issue and we wish you all a very happy new year ahead!
Wound Masterclass - Vol 1 - December 2022 3 Miss Negin Shamsian Consultant Plastic & Reconstructive Surgeon (Locum) Chief Editor of Wound Masterclass London, United Kingdom
Two roads diverged in a yellow wood, And sorry I could not travel both And be one traveler, long I stood And looked down one as far as I could To where it bent in the undergrowth;Then took the other, as just as fair, And having perhaps the better claim, Because it was grassy and wanted wear; Though as for that the passing there Had worn them really about the same, And both that morning equally lay
In leaves no step had trodden black. Oh, I kept the first for another day! Yet knowing how way leads on to way, I doubted if I should ever come back. I shall be telling this with a sigh Somewhere ages and ages hence: Two roads diverged in a wood, and I— I took the one less traveled by, And that has made all the difference.
© Copyright. Wound Masterclass. 2023
Robert Frost
Accelerated Healing and Advanced Wound Care:
What is the Role of Wireless, Closed-Loop, Smart Bandages With Integrated Sensors and Stimulators?
Editorial Summary
Current standard-of-care wound dressings are passive and do not actively respond to variations in the wound environment. Smart bandage technologies address these challenges, with their ability to integrate multi-modal sensors and stimulators for real-time monitoring and active wound care treatment with little intervention from medical providers required. This article provides an overview of the role of wireless, closed-loop, smart bandages with integrated sensors and stimulators.
Introduction
Chronic non-healing wounds are a major healthcare burden, with approximately 2.2 million people with a chronic wound in the UK, with DFUs and PUs costing £1 billion and £531 million, respectively.1 In the United States, the total number of prevalent cases of DFU was 4,551,498 cases in the year 2020. Unhealed ulcers and foot infections are the leading cause of diabetes related amputations, with diabetic foot ulcers preceding 85% of amputations.2 In the United States, DFU patients are twice as costly to US Medicare as those with diabetes alone. These wounds are associated with loss of function and mobility, increased social stress and isolation, depression and anxiety, prolonged hospitalization and overall increased morbidity and mortality.2
Current standard-of-care wound dressings are passive and do not actively respond to variations in the wound environment.2 Smart bandage technologies address these challenges, with their ability to integrate multi-modal sensors and stimulators for real-time monitoring and active wound care treatment with little intervention from medical providers required.3-9 As a wound heals, skin impedance increases, but when a wound becomes infected wound impedance decreases due to the development of biofilm.10-11 Further development of infection causes an increase in wound temperature through local inflammation.12 These signals can be captured by low-cost sensors embedded in a wearable device to act as a sentinel for impending wound infection.2 These biophysical signals provide rapid, robust and accurate information about
wound conditions in real time, creating an opportunity to diagnose and monitor a nonhealing wound quickly and autonomously in a closed-loop fashion.2
Current smart bandage technologies can detect a variety of physiological changes including pH,1,3-4, temperature,15-18 oxygenation,19 impedance,10,20-1 motion22-3 and enzymatic fluctuations,18, 24-5 of the wound.
Electrical stimulation can reduce bacterial colonization and biofilm infection and restore normal wound healing.26 It also improves tissue perfusion, stimulates immune cell function and accelerates keratinocyte migration through a process known as galvanotaxis.27-9
We developed a battery-free flexible bioelectronic system consisting of wirelessly powered sensing and stimulation circuits with tissue-interfacing tough hydrogel electrodes using a biocompatible conducting polymer.2 We designed a miniaturized flexible printed circuit board (FPCB) containing an energy-harvesting antenna, a microcontroller unit, a crystal

4 Wound Masterclass - Vol 1 - December 2022 Authors and Contributors Yuanwen Jiang1,9 Artem A. Trotsyuk2,7,9 Simiao Niu1,9 Dominic Henn2 Kellen Chen2,7 Chien-Chung Shih1 Madelyn R. Larson2 Alana M. Mermin-Bunnell2 Smiti Mittal2 Jian-Cheng Lai1 Aref Saberi1 Ethan Beard2 Serena Jing2 Donglai Zhong1 Sydney R. Steele2 Kefan Sun1 Tanish Jain2 Eric Zhao1 Christopher R. Neimeth2 Willian G. Viana3 Jing Tang1,8 Dharshan Sivaraj2,7 Jagannath Padmanabhan2 Melanie Rodrigues2 David P. Perrault2 Arhana Chattopadhyay2 Zeshaan N. Maan2 Melissa C. Leeolou2 Clark A. Bonham2 Sun Hyung Kwon2 Hudson C. Kussie2,7 Katharina S. Fischer2,7 Gurupranav Gurusankar1 Kui Liang4 Kailiang Zhang4 Ronjon Nag5 Michael P. Snyder6 Michael Januszyk2 Geoffrey C. Gurtner2,7 Zhenan Bao1
© Copyright. Wound Masterclass. 2023 Image licenced from Adobe Stock. Credit: HYUNGKEUN
oscillator and filter circuits for dual-channel continuous sensing of wound impedance and temperature, and a parallel stimulation circuit to deliver programmed electrical cues for accelerated wound healing.2To ensure efficient signal exchange and energy delivery between the circuits and the soft skin tissue, a lowimpedance and adhesive hydrogel electrode was used, which has lower impedance across the entire frequency domain compared to wellestablished ionically conducting hydrogels which results in more efficient charge injection during stimulation.30-1 To mitigate secondary skin damage when peeling off the adhesive electrodes we used a thermally controlled reversible phase transition mechanism to the hydrogel backbone and achieved two orders of magnitude lower adhesion at elevated temperature when compared with normal skin temperature.2 Multiple preclinical animal models demonstrated that their smart bandage could continuously monitor skin physiological signals and deliver directional electrical cues, leading to accelerated wound closure, increased neovascularization and enhanced dermal recovery.2 The wireless nature of their smart bandage also allowed us to use complex animal models, such as parabiosis, to investigate the possible underlying mechanisms behind the observed effect of electrical stimulation, which suggested that the beneficial wound-healing outcomes could be attributed to the activation of proregenerative genes in the monocyte and macrophage cell populations.2
The wound management system that we designed was a battery free, wirelessly powered FPCB for simultaneous wound treatment and monitoring and a tissue-interfacing conductive adhesive hydrogel interface for robust and gentle skin integration.2 The smart bandage was flexible and can be comfortably attached to wound surfaces.2 It can be inductively coupled with a radio frequency identification (RFID) reader.2 Through the radiofrequency (RF) energy-harvesting process, the antenna
can provide power to apply electric bias across the wound for programmed treatment and, at the same time, drive the microcontroller unit (MCU)and other integrated circuits (for example, oscillator and filter) for continuous monitoring of wound impedance and temperature via a near-field communication (NFC) transponder in the MCU.2
We first confirmed that mice wearing their wireless devices were able to move freely, with a distance travelled similar to that of mice with no device attached, demonstrating an ideal therapeutic modality for patient use, ie., lightweight and untethered with cables.2 The temperature and impedance sensors were able to monitor wound state continuously as the mice moved freely in the cage.2 The hydrogel was biocompatible and did not initiate any sensitization or irritation after continuous contact with the skin over 15 days, demonstrating absence of adverse reactivity signs compared with normal skin.2 To test the functionality of the platform in a biological system, a splinted excisional wound mouse model was used in which stimulated mice were treated with continuous electrical pulses, while control mice received standard sterile wound dressings without electrical stimulation.2 We found that stimulation resulted in accelerated wound closure and a significant increase in wound impedance to attain a faster impedance plateau, signifying a return to an unwounded state.2,10,20 Stimulation of wounds also improved functional tensile recovery with increased dermal thickness, collagen deposition and overall dermal appendage count.2 Compared with a wired modality, our smart bandage allowed for longer and potentially continuous treatment durations, which have been linked to accelerated wound closure.2, 26 Stimulated wounds also showed an increase in collagen fibre heterogeneity, resulting in more random, shorter and less aligned fibre orientations.2
We observed a significant increase in
Accelerated Healing and Advanced Wound Care: What is the Role of Wireless, Closed-Loop,
With
and Stimulators? Wound Masterclass - Vol 1 - December 2022 5 © Copyright. Wound Masterclass. 2023
“The wound management system that we designed was a battery free, wirelessly powered FPCB for simultaneous wound treatment and monitoring and a tissueinterfacing conductive adhesive hydrogel interface for robust and gentle skin integration.2”
Smart Bandages
Integrated Sensors
neovascularization among stimulated wounds, with increased microvessel count and higher expression of CD31 and α-smooth muscle actin (α-SMA), with similar results observed in a murine burn wound healing model.2 The smart bandage significantly reduced infection in the wound, decreasing overall bacterial colony count and by continuous monitoring of wound impedance and temperature, their wireless smart bandage could detect early onset of infection and modulate treatment in a closed-loop manner to avoid further wound complications.2
In current clinical practice, doctors still rely on qualitative markers such as swelling or erythema to identify wound infections, which are often difficult to judge in the early stages of biofilm development, but with quantitative biophysical signals recorded by our smart bandage, treatment can be provided when clinically used markers are still ambiguous, enabling timely treatment of chronic wounds, reduction in hospital readmissions and medical cost and improvement in patient woundhealing outcomes.2,32 We also validated their system in a streptozotocin (STZ)-induced diabetic excisional wound model, which most closely resembles type 1 diabetes in patients, also observing an accelerated time to wound closure, improved dermal collagen fiber heterogeneity and increased vascularization.33-4 On the cellular level, we demonstrated the expected ability of our device to prompt cell alignment and migration, inducible with a directional electric field.2
Although the beneficial effects of electrical stimulation have been reported, the cellular and molecular mechanisms for this remained unclear.27 In our work, due to the lightweight and untethered nature of our smart bandage, we were able to evaluate the long-term effects of electrical stimulation on circulating cells involved in wound repair using a complex parabiosis model.2,35 This would not have
been possible previously because, with a conventional wired modality, parabiosis under a long anaesthesia regimen would not survive.2 To do this, we performed parabiosis of five green fluorescence protein (GFP)-positive mice to wild-type (WT) mice. WT mice were wounded and either subjected to electrical stimulation or left untreated.2 Wound tissues from both groups were explanted on day 5 and their transcriptional profiles analysed by singlecell RNA sequencing (scRNA-seq) using the 10X Genomics Chromium platform (figure 1).2 Of all the circulating inflammatory cells activated by our smart bandage, monocytes and macrophages had the highest number of differentially expressed genes in both electrically stimulated and untreated wounds. Even with many neutrophils present, the magnitude of differentially expressed genes did not reach statistical significance.2 Similarly, while there were higher numbers of B and T cells in the stimulated group, signifying greater recruitment of these cells from the circulation, the overall number of cells was low and the amount of differentially expressed genes was nominal.2

Accelerated Healing and Advanced Wound Care: What is the Role of Wireless, Closed-Loop, Smart Bandages With Integrated Sensors and Stimulators? 6 Wound Masterclass - Vol 1 - December 2022
"The smart bandage significantly reduced infection in the wound, decreasing overall bacterial colony count and by continuous monitoring of wound impedance and temperature, their wireless smart bandage could detect early onset of infection and modulate treatment in a closed-loop manner to avoid further wound complications." 2
© Copyright. Wound Masterclass. 2023
Figure 2: Schematic diagram illustrating the experimental flow for scRNA-seq. Tissues from an excisional wound of a WT mouse paired with a GFP-positive mouse, subjected to either treatment (that is, stimulation) or not (that is, control), were sorted for GFP-positive cells using FACS and analyzed by 10X sequencing.
To specifically investigate macrophages and monocytes, we performed a series of evaluations to validate and define the high number of differentially expressed genes observed.2 First, we re-embedded our macrophages and monocytes and used cellular trajectory reconstruction analysis using gene counts and expression (CytoTRACE) to confirm that our defined monocytes possessed less differentiated cell states based on the distribution of unique messenger RNA transcripts.2 We then overlaid the stimulated and unstimulated macrophages and monocytes and performed RNA velocity and pseudo time analyses using scVelo and Monocle 3, respectively, to combine RNA velocity information with trajectory inference to compute a map of potential fates that the macrophages and monocytes can undergo in response to electrical stimulation.2 We first used scVelo to infer our root node and transcriptional directionality across the manifold, based on mRNA splicing of macrophages and monocytes. We found three general transcriptional vector paths in which mRNA splicing could occur within individual cells, with a relatively higher amount of differentiated individual cells found on the left of the embedding and less differentiated cells on the right, further confirming CytoTRACE.2 We then performed pseudo time analysis with Monocle 3,using a root node identified with scVelo to infer terminal cell states.2 Our analysis once again revealed three distinct transcriptional trajectories.2 To further understand why macrophages and monocytes had a higher amount of differentially expressed genes activated by our smart bandage, we performed uniform manifold approximation and projection (UMAP)-based clustering, which revealed five transcriptionally distinct clusters.2 Of the five clusters, cluster 0, consisting of both macrophages and unstimulated control cells, had a higher expression of genes such as JUN and FN1,60,61 which have previously been associated with wound healing, whereas clusters 1, 2 and 3, consisting predominantly of stimulated monocytes and macrophages,
demonstrated elevated expression of genes involved in the wound repair process, such as CD74, SELENOP, APOE, MRC1, CD163 and FABP5.2,36-9 When we looked at the stimulated and control feature plots of highly expressed genes in macrophages and monocytes, we saw that cells with a strong enrichment for previously reported proregenerative markers, notably CD163 and MRC1 (CD206), as well as SELENOP and APOE, all localized around Seurat cluster 2 and trajectory 2 (middle), which primarily contained stimulated macrophages.2 CD163 and MRC1 (CD206) have previously been described as M2 anti-inflammatory macrophage markers40 while SELENOP has been found to be anti-inflammatory, regulating macrophage invasiveness and other inflammatory mediators responsible for pathogen clearance and tissue repair, and is linked to M2 macrophage markers such as STAB1, SEPP1 and ARG1.2 APOE has been also shown to enhance in vitro phagocytosis of macrophages,increasing muscle and soft tissue regeneration.2, 41-2 We further confirmed these transcriptional changes at the protein level, performing flow cytometry on GFP-positive cells circulating to wounds in our parabiosis model. We identified a higher percentage of CD163-positive cells in stimulated wounds as compared with controls.2 This was further confirmed by immunofluorescent staining of healed tissue, with significantly higher CD163 and CD206 expression observed in stimulated as compared with untreated wounds.2
These data suggest that electrical stimulation may drive macrophages towards a more regenerative phenotype, and could underlie the accelerated wound healing observed in our preclinical studies.2 The high predominance of regenerative macrophages could, in part, be due to macrophages responding to local micro environmental stimuli. Modulation of the cell membrane electric potential with electrical stimuli could activate more ATP-sensitive potassium ion channels, which has previously
Accelerated Healing and Advanced Wound Care: What is the Role of Wireless, Closed-Loop, Smart
With Integrated Sensors and Stimulators? Wound Masterclass - Vol 1 - December 2022 7
Bandages
© Copyright. Wound Masterclass. 2023
“To specifically investigate macrophages and monocytes, we performed a series of evaluations to validate and define the high number of differentially expressed genes observed.” 2
been shown to affect macrophage differentiation plasticity and function.2,43-4 Taken together, our pre-clinical studies attribute one mechanism by which electrical stimulation may coordinate and regulate macrophage functions, including those essential for microbial clearance and wound healing. Our smart bandage, in turn, will enable further biological discovery and allow for researchers to explore hypotheses previously less well studied due to current treatment modality limitations and animal model complexities.2
References
1. https://www.bjfm.co.uk/spotlight-on-managing-chronic-wounds-diabetic-foot-ulcersand-pressure-ulcers#:~:text=There%20are%20approximately%202.2%20million,and%20 %C2%A3531%20million%2C%20respectively. Chadwick P. Spotlight on managing chronic wounds, diabetic foot ulcers and pressure ulcers. British Journal of Family Medicine, 25/6/20
2. Jiang Y et al. Wireless, closed-loop, smart bandage with integrated sensors and stimulators for advanced wound care and accelerated healing. Nat Biotechnol (2022).
3. McLister A et al.New developments in smart bandage technologies for wound diagnostics. Adv. Mater. 28, 5732–5737 (2016).
4. Derakhshandeh H et al. Smart bandages: the future of wound care. Trends Biotechnol. 36, 1259–1274 (2018)
5. Long Y. et al. Effective wound healing enabled by discrete alternative electric fields from wearable nanogenerators. ACS Nano 12, 12533–12540 (2018).
6. Liu A et al. Accelerated complete human skin architecture restoration after wounding by nanogenerator-driven electrostimulation. J. Nanobiotechnol. 19, 280 (2021).
7. Farahani M, Shafiee A. Wound healing: from passive to smart dressings. Adv. Healthc. Mater. 10, e2100477 (2021)
8. Dincer, C. et al. Disposable sensors in diagnostics, food, and environmental monitoring. Adv. Mater. 31, e1806739 (2019)
9. Barros Almeida I et al. Smart dressings for wound healing: a review. Adv. Skin Wound Care 34, 1–8 (2021).
0. Kekonen A. et al. Bioimpedance sensor array for long-termmonitoring of wound healing from beneath the primary dressingsand controlled formation of H2O2 using low-intensity directcurrent. Sensors 19, 2505 (2019).
11. Lukaski HC, Moore M. Bioelectrical impedance assessment ofwound healing. J. Diabetes Sci. Technol. 6, 209–212 (2012).
12. Chanmugam, A. et al. Relative temperature maximum in woundinfection and inflammation as compared with a control subjectusing long-wave infrared thermography. Adv. Skin Wound Care30, 406–414 (2017).
13. Tamayol A. et al. Flexible pH-sensing hydrogel fibers forepidermal applications. Adv. Healthc. Mater. 5, 711–719 (2016).
14. Xu G et al. Battery‐free and wireless smart wound dressingfor wound infection monitoring and electrically controlled on‐demand drug delivery. Adv. Funct. Mater. 31, 2100852 (2021).
15. Trung TQ et al. Anall-elastomeric transparent and stretchable temperature sensorfor body-attachable wearable electronics. Adv. Mater. 28,502–509 (2016).
16. Hattori, Y. et al. Multifunctional skin-like electronics forquantitative, clinical monitoring of cutaneous wound healing.Adv. Healthc. Mater. 3, 1597–1607 (2014).
17. ShiX, Wu P. A smart patch with on-demand detachableadhesion for bioelectronics. Small
17, e2101220 (2021).
18. Pang, Q. et al. Smart flexible electronics-integrated wounddressing for real-time monitoring and on-demand treatment ofinfected wounds. Adv. Sci. 7, 1902673 (2020).
19. Marks H. et al. A paintable phosphorescent bandagefor postoperative tissue oxygen assessment in DIEP flapreconstruction. Sci. Adv. 6, eabd1061 (2020).
20. Swisher SLet al. Impedance sensing device enables earlydetection of pressure ulcers in vivo. Nat. Commun. 6, 6575 (2015).
21. McCafrey C et al. Flexible bioimpedancespectroscopy system for wound care monitoring.
In 2019 IEEEBiomedical Circuits and Systems Conference (BioCAS) 1–4 (IEEE,2019).
Affiliations
1. Department of Chemical Engineering, Stanford University, Stanford, CA, USA.
2. Department of Surgery, Division of Plastic and Reconstructive Surgery, Stanford University School of Medicine, Stanford, CA, USA.
3. Department of Biology, Stanford University, Stanford, CA, USA.
4. BOE Technology Center, BOE Technology Group Co., Ltd, Beijing, China.
5. Stanford Distinguished Careers Institute, Stanford University, Stanford, CA, USA.
6. Department of Genetics, Stanford University School of Medicine, Stanford, CA, USA.
7. Department of Surgery, University of Arizona College of Medicine, Tucson, AZ, USA.
8. Department of Materials Science and Engineering, Stanford University, Stanford, CA, USA.
9. These authors contributed equally: Yuanwen Jiang, Artem A. Trotsyuk, Simiao Niu.
Our preclinical work showed proof of concept, but future work must extend their smart bandage to a human-sized form factor and running preliminary tests in large-animal models followed by human trials.2 The manufacturing cost needs reduced to make this device affordable.2 The technology used in this device platform may be transferred and adapted for use in other diseases in bioelectronic medicine.2
22. Kalidasan V. et al. Wirelessly operated bioelectronic sutures forthe monitoring of deep surgical wounds. Nat. Biomed. Eng. 5,1217–1227 (2021).
23. Zhao Yet al. Skin‐Inspired antibacterial conductive hydrogels forepidermal sensors and diabetic foot wound dressings. Adv. Funct.Mater. 29, 1901474 (2019).
24. Ciani I et al. Development of immunosensors for direct detectionof three wound infection biomarkers at point of care usingelectrochemical impedance spectroscopy. Biosens. Bioelectron.31, 413–418 (2012).
25. Gao Y et al. A flexible multiplexed immunosensor forpoint-of-care in situ wound monitoring. Sci. Adv. 7, eabg9614(2021).
26. Thakral Get al. Electrical stimulation to accelerate woundhealing. Diabet. Foot Ankle 4, 22081 (2013).
27. Kloth LC Electrical stimulation technologies for wound healing.Adv. Wound Care 3, 81–90 (2014).
28. Zhao M et al. Electrical signals control wound healing throughphosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 442,457–460 (2006).
29. Cohen DJ, Nelson WJ,Maharbiz MM. Galvanotactic controlof collective cell migration in epithelial monolayers. Nat. Mater.13, 409–417 (2014).
30. Liu Y et al. Soft and elastic hydrogel-based microelectronicsfor localized low-voltage neuromodulation. Nat. Biomed. Eng. 3,58–68 (2019).
31. Jiang Y et al. Topological supramolecular network enabledhigh-conductivity, stretchable organic bioelectronics. Science375, 1411–1417 (2022).
32. Negut I et al. Treatmentstrategies for infected wounds. Molecules 23, 2392 (2018)
33. Chen H. et al. Dissolved oxygen from microalgae-gel patchpromotes chronic wound healing in diabetes. Sci. Adv. 6,eaba4311 (2020).
34.Wu, J. & Yan, L. J. Streptozotocin-induced type 1 diabetes inrodents as a model for studying mitochondrial mechanisms ofdiabetic cell glucotoxicity. Diabetes Metab. Syndr. Obes. 8,181–188 (2015).
35. Duyverman AM et al. A transient parabiosis skin transplantation model in mice. Nat. Protoc. 7, 763–770 (2012).
36. Farr L, GhoshS, MoonahS. Role of MIF cytokine/CD74receptor pathway in protecting against injury and promotingrepair. Front. Immunol. 11, 1273 (2020).
37. Carlson, B. A. et al. Selenoproteins regulate macrophageinvasiveness and extracellular matrix-related gene expression.BMC Immunol. 10, 57 (2009).
38. Lin JD et al.Single-cell analysis of fate-mapped macrophagesreveals heterogeneity, including stem-like properties, duringatherosclerosis progression and regression. JCI Insight 4,e124574 (2019).
39. Huang ZH, Reardon CA, Mazzone T. Endogenous ApoEexpression modulates adipocyte triglyceride content andturnover. Diabetes 55, 3394–3402 (2006).
40. Martinez FO, Gordon S. The M1 and M2 paradigm ofmacrophage activation: time for reassessment. F1000Prime Rep.6, 13 (2014).
41. Arnold Let al. CX3CR1 deficiency promotes muscle repair andregeneration by enhancing macrophage ApoE production. Nat.Commun. 6, 8972 (2015).
42. Wang, Y. et al. Tissue-resident macrophages promoteextracellular matrix homeostasis in the mammary gland stroma ofnulliparous mice. eLife 9, e57438 (2020).
43. Li C, Levin M, Kaplan DL. Bioelectric modulation ofmacrophage polarization. Sci. Rep.
6, 21044 (2016).
44. Hoare JI et al. Electric fields are novel determinants of human macrophagefunctions. J. Leukoc. Biol. 99, 1141–1151 (2016).
Accelerated Healing and Advanced Wound Care: What is the Role of Wireless, Closed-Loop, Smart Bandages With Integrated Sensors and Stimulators? 8 Wound Masterclass - Vol 1 - December 2022
“The technology used in this device platform may be transferred and adapted for use in other diseases in bioelectronic medicine.” 2
© Copyright. Wound Masterclass. 2023
foam


H&R H 3 Redc T: + 44
When it comes to consistency in quality, care and savings look no further than Kliniderm foam silicone. A trusted choice whatever your supply route – savings of up to 47%*.
patients
hurting
*Drug Tariff prices correct from January 2022, based on 10 x 10cm dressing size.
silicone Heal your
without
your budgets www.kliniderm.co.uk
Dr M. Mark Melin
 M Health Fairview Wound Healing Institute, University of Minnesota Physicians Minneapolis MN, United States
M Health Fairview Wound Healing Institute, University of Minnesota Physicians Minneapolis MN, United States
Optimizing Management of Lymphedema
Editorial Summary
Lymphedema is a major global condition defined as progressive swelling of the body part which is in part due to disruption of the lymphatic system. This article is going to discuss the pathophysiology of lymphoedema, the demographics of the condition and its management. There will be a deep overview of the importance of the endothelial or Calix layer as well as the importance of the starling model of capillary fluid change. A detailed look at the lymph circulation including the capillaries and lymphatic endothelial cells and its relevance to lymphedema. There will also be a focus on hyperglycaemia and diabetes in lymphoedema In this article. The role of albumin and glycocalyx as well as sodium are explored. The clinical characteristics and management will also be analysed.
Introduction
Lymphedema affects 140-300million patients worldwide. It is defined as the progressive swelling of a body part, usually an extremity following developmental (primary lymphedema) or acquired (secondary lymphedema) disruption of the lymphatic system resulting in lymph accumulating in the interstitial space.1 This article discusses the pathophysiology of lymphedema, demographics of this condition and its management.
Endothelial Glycocalyx Layer
In 1894, Starling proposed a model of capillary fluid exchange, based on hydrostatic and oncotic pressures in the blood capillaries and interstitium, with the capillary acting as a semiporous membrane, through which fluid moves freely in and out.2 In 1940, Danielli introduced the concept of a protein-based lining of vessels which played a vital role in fluid filtration, and in 1966, Luft visualised this layer using electron microscopy.2 The “endothelial glycocalyx layer” (EGL) was then recognised as controlling the movement of proteins and fluid across the blood capillary wall, through dynamic and complex processes.2
The endothelial glycocalyx is a complex carbohydrate-rich gel-like layer lining the luminal surface of blood vessels functioning as a barrier between the blood and vessel wall.3 The glycocalyx layer is composed of membrane-bound proteoglycans, secreted glycosaminoglycans (GAGs), sialic acidcontaining glycoproteins, and glycolipids
associated with the endothelial surface.3 The main proteoglycans of the endothelial glycocalyx are membrane-spanning syndecans and glycosylphosphatidylinositol-linked glypicans which carry the two main GAGs, heparan sulphate and chondroitin sulfate.3
In 2010, it was demonstrated that there was no net resorption of fluid back to the venous side of the blood capillaries and there is only diminishing net filtration across the capillary bed.2 Capillaries and venules can only resorb fluid in extreme situations.2 An acute reduction of transendothelial pressure, for example caused by precapillary vasoconstriction, post-capillary vasodilation, haemorrhage or hypovolaemia will allow transient venous absorption preserve blood volume.2 This challenges the previously accepted view regarding Starling forces.2
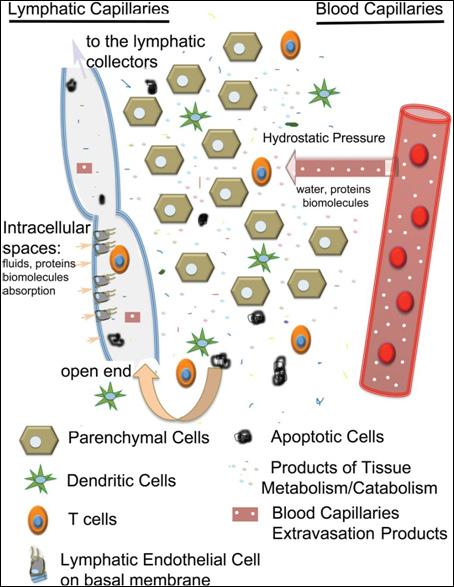
Acting as a complex molecular sieve, the EGL precisely regulates fluid and protein movement through the capillary wall into the tissues and prevents movement of proteins and fluid back into the venous side of the capillaries, even when interstitial tissue hydrostatic pressure is increased, or capillary oncotic pressure is higher than the tissue oncotic pressure (Figure 1).2,4 All fluid and blood proteins moving into the interstitium each day must be removed via reabsorption through the lymphatic capillaries alone.2 Thus, all oedemas fall on a continuum of lymphedema, and can lead to chronic inflammation and tissue thickening caused by accumulation and degradation of proteins.2
Glycocalyx and endothelial cell damage occur in several clinical situations including ischemia–reperfusion injury, hypoxia/reoxygenation,
10 Wound Masterclass - Vol 1 - December 2022
© Copyright. Wound Masterclass. 2023
inflammation, sepsis, haemorrhagic shock, hypervolemia, hyperglycemia, excessive shear stress and coronary artery bypass surgery.3 These injuries determine pathological changes in the endothelial glycocalyx such as impaired mechanotransduction, increased egress of leukocytes, loss of coagulation control, loss of anti-oxidant defence, loss of deposited growth factors and increased vascular permeability, which is of vital importance in lymphedema.3
entry of proteins, fluids, macromolecules, small molecules and immune cells.4 The lymphatic capillaries coalesce into progressively larger lymphatic collectors which are formed by one layer of lymphatic endothelial cells supported by a more organized basal membrane containing lymphatic muscle cells, connective tissue and fibroblasts.4 The directional flow of lymph is maintained through a series of unidirectional valves, positioned along the collectors, which open and close in synchrony with the vessel contraction.4 Contraction from the more distal lymphangion toward the one closer to the lymph node, in synchrony with directional valve closure, allows unidirectional lymph transport and prevents backflow, enabling the collectors to work as pumps.4
Increased lymphangiogenesis has been observed in primary and secondary lymphedema, acute and chronic inflammation and cancer, related to the increased production of different vascular endothelial growth factors released by immune and stromal cells and nuclear factor- B up-regulates the transcription factor Prox1 that promotes lymphatic endothelial cell proliferation.4
Hyperglycaemia and Diabetes in Lymphedema
Lymph Circulation
All parenchymal organs, with the exception of the brain, contain a network of openended lymphatic capillaries, which collect the interstitial fluid.4 The capillaries are formed by a single layer of lymphatic endothelial cells which function as one-way valves to facilitate
In response to hyperglycaemia, the thickness of the glycocalyx on blood vessel endothelia is significantly reduced, leading to loss of protective functions and other deleterious changes, including increase in risk of lympoedema.5 A study showed that acute hyperglycaemia in healthy subjects was associated with a ~50% reduction in glycocalyx volume which was likely to be due to damage by reactive oxygen species (ROS) generated under hyperglycaemic conditions, because infusion of the antioxidant N-acetylcysteine (NAC) could prevent this reduction.5-6 Both the systemic glycocalyx volume and the directly measured glycocalyx thickness are reduced by 50–86%
Optimizing Management of Lymphedema Wound Masterclass - Vol 1 - December 2022 11
“In response to hyperglycaemia, the thickness of the glycocalyx on blood vessel endothelia is significantly reduced, leading to loss of protective functions and other deleterious changes, including increase in risk of lympoedema.” 5
Figure 1: Schematic of lymph formation.4
© Copyright. Wound Masterclass. 2023
in type 1 diabetic patients relative to normal controls.5 Hyaluronan (HA) is an important component of the glycocalyx and is affected in diabetes - hyaluronidase activity and circulating levels of HA are both elevated in the serum of diabetic patients, suggesting that hyaluronidase activity may contribute to the degradation of the endothelial glycocalyx in diabetes.5
Albumin and the Glycocalyx
Albumin has a net negative charge, but its amphoteric nature promotes tight binding to the glycocalyx which results in reduced hydraulic conductivity across the vascular barrier, resisting glycocalyx degradation (i.e., protecting against shedding) and thereby contributing to maintenance of vascular integrity and normal capillary permeability, and facilitating transmission of shear stress.3 Under physiological conditions, the concentration of intravascular albumin is the major determinant of plasma colloid osmotic pressure.3
Exposed thiol groups on the albumin molecule act as a scavenger for reactive oxygen species (ROS) such as superoxide and hydroxyl radicals and reactive nitrogen species, e.g., peroxynitrite radicals.3 Albumin has an additional antioxidant effect through binding to free copper ions (Cu2+) which accelerate the production of free radicals.3
Studies illustrate the multifunctional nature of albumin including maintaining glycocalyx integrity and partially restoring impaired vascular permeability via release of sphingosine-1-phosphate (S1P) from red blood cells, anti-inflammatory and anti-oxidative effects, improvement of the microcirculation and hemodynamics following hemorrhagic shock or endotoxemia, and acting as an effective plasma volume expander.3 Compared with saline, albumin improves skin endothelial cell function, improving microcirculatory blood flow, and this maybe independent of the oncotic
properties of albumin as neither cardiac output nor skin blood flow differed between albuminand saline-treated patients in these studies.3
Sodium and the Glycocalyx
The glycocalyx covering the luminal surface of the vascular endothelium also plays an essential role in the regulation of sodium homeostasis.7 When the volume of the endothelial glycocalyx is reduced or its integrity is impaired, its capacity to bind and buffer sodium diminishes.7 As previously discussed, several clinical conditions are known to damage the endothelial glycocalyx, but with respect to sodium homeostasis, the acute sodium/volume loading appears to be the most relevant.7 In response to salt loading, the barrier function of the glycocalyx diminishes and more sodium reaches the luminal surface of endothelial cells, where it induces and activates epithelial sodium channels, which results in increased sodium uptake by the cells, stiffening of the cortex of the cells and a reduction in the generation of endothelial NO which elevates vascular tone.7 As a mechanotransductor, the glycocalyx mediates flow-dependent vasorelaxation by stretching the glycocalyxlipid bilayer cytoskeleton system and increases NO production though the activation of transient receptor potential (TRP) channels.7
The endothelial glycocalyx functions as a sodium buffer and first-line barrier to protect endothelial cells against increased sodium influx when exposed to excess circulating sodium.7 In clinical conditions characterized by a decreased sodium binding capacity of the glycocalyx, more sodium enters into the cells, causing impaired NO generation, elevated vascular resistance, and hypertension.7 The sodium load would increase unbound, osmotically active sodium, resulting in water retention, volume expansion, and a increase in blood pressure.7
Optimizing Management of Lymphedema 12 Wound Masterclass - Vol 1 - December 2022 © Copyright. Wound Masterclass. 2023
“The glycocalyx covering the luminal surface of the vascular endothelium also plays an essential role in the regulation of sodium homeostasis.” 7
Clinical Characteristics
The most common cause of lower extremity lymphedema is chronic venous insufficiency (CVI), followed by cancer-related lymphedema, primary lymphedema and lipedema with secondary lymphedema.8 Patients are most commonly female, white, obese with bilateral involvement.5 Surgery, particularly total knee replacements and trauma are associated with worsening lymnphoedema.8
Management
There is no definitive cure for lymphedema, but management options that have been used include physical therapy, drugs and surgical options.9 Physical treatments include massages, lymphatic drainages, the application of different kind of compression garments, and intermittent pneumatic compression.9 Natural compression can be simulated by intermitted pneumatic compression devices, which use a sequential airflow to inflate special hoses and, therefore, applying positive pressure on the tissue, and these have been shown to be an effective treatment in those with secondary lymphedema.9 A novel therapeutic approach that uses the application of negative pressure (representing a pulling/opening force) has been described and whilst this approach has already been used in the field of wound healing, its use in lymphedema has not yet been fully investigated.9 Another form of negative pressure uses kinesiology tapes which are used to decongest lymphatic fluid that accumulates under the skin.9 Kinesiological methods have been shown to improve quality of life and a reduction in volume, but these are not seen as superior compared to other treatments.9
Diuretics, benzopyrones, ketoprofen and tacrolimus have all been suggested for the management of lymphedema but are not generally recommended.9 Surgical methods have been investigated, which can be divided
in to physiological and excisional procedures.9 Physiological procedures are aimed at promoting fluid flow properties, either by redirecting the lymphatic flow directly into the venous system or by providing new pathways.9 Excisional procedures involve the removal of affected tissue parts.9 In lymphvenous anastomosis (LVA) surgery, a connection between the lymphatic structures and blood vessels is established.9 Patients report improvements following this surgery, which is more effective in upper than lower limbs, but compression garments are still required post-surgery.9 Vascularized lymph node transfer (VLNT) is performed in more advanced stages in lymphedema patients in which lymphatic vessels are dysfunctional and/ or lymph nodes are not present.9 The proposed mechanism of action of this is that accumulated fluid in the close area is absorbed by the nodes and VEGF-C induced lymph angiogenesis by vascularized lymph nodes.9 LVA and LVNT have both shown promising outcomes in clinical studies in terms of limb volume reduction and reduced episodes of cellulitis but no beneficial effect in reducing fibrosis has been observed.9 In cases of fibroadipose hypertrophy, as can often be found in chronic lymphedema, suction assisted lipectomy (SAL) can be the surgery of choice, but lifelong compression bandages are required to prevent recurrence.9
The standard therapy of choice is a form of physical therapy that is known as complete/ complex decongestive (physio-)therapy (CDT).9 This form of treatment is not curative for lymphedema, but mainly aims at reducing fluid volume as well as preventing the disease from progression.9 It can generally be separated into two phases: Phase one involves manual lymphatic drainage (MLD), usage of multilayered compression bandages, carrying out physical exercises, and meticulous skin care.9 Phase two mainly focuses on self-care via elastic sleeves or compression stocks application as well as continuous exercise.9
Optimizing Management of Lymphedema Wound Masterclass - Vol 1 - December 2022 13 © Copyright. Wound Masterclass. 2023
“There is no definitive cure for lymphedema, but management options that have been used include physical therapy, drugs and surgical options.” 9
Main Components of CDT:
• Manual Lymphatic Drainage (MLD) is performed to enhance lymphatic outflow. Lymph therapists use specific hand movements (rhythmic, flowing or stirring) in a frequency that mimics the intrinsic frequency of the lymphangion, starting in the area of healthy tissue and then expanded into adjacent areas where the obstructed vessels are located
• Compression bandages are applied, aiming at increasing interstitial pressure and therefore, to decrease capillary filtration leading to a decrease in accumulated fluid/ volume
• Physical exercise such as ergometry, aerobic exerciseand/ or resistance exercise as well as associated respiratory movements which are believed to assist in increasing lymphatic flow, in reducing swelling and in improving muscle strength as well as quality of life in lymphedema patients
• Skin care and skin restauration
• Psychological support
• Educational seminars on skin care or nutrition.9
Micronized Purified Flavonoid Fraction (MPFF) in Lymphoedema
MPFF has been demonstrated in rats to have dose dependent anti-oedema properties°. In humans, a study demonstrated that treatment with MPFF resulted in an increase in the number of functional lymphatics and reduction of the diameter of lympathic capillaries and of the intralymphatic pressure. A recent study found a significant improvement in
lymphoscintigraphic parameters and also observed a tendency in favour of MPFF in patients with more severe lymphedema°. A Cochrane review of benzopyrones (including MPFF) for lymphedema acknowledged the difficulties about studies in this area, especially as lymphedema is frequently distributed unevenly, so volume calculation based on circumference measurement becomes inaccurate and chronic lymphedema needs long periods of treatment, long follow-up, and standardising decongestive therapy is difficult.
Conclusion
An understanding of the pathophysiology of lymphedema, and in particular the glycocalyx, is important when considering the management of this condition. Glycocalyx and endothelial damage occur in a number of clinical situations, and an understanding of the role of sodium, albumin and hyperglycaemia in the function of the glycocalyx is vital. There is no cure for lymphedema, but a multifaceted approach is used in its management.
References
1. Lymphedema. BMJ Best Practice. Last updated 8/2/22 https://bestpractice.bmj.com/ topics/en-gb/610
2. Bjork R, Hettrick H. Endothelial glycocalyx layer and interdependence of lymphatic and integumentary systems. Wounds International 2018. 9 (2): 50-55
3. Aldecoa Cet al. Role of albumin in the preservation of endothelial glycocalyx integrity and the microcirculation: a review. Ann Intensive Care. 2020 Jun 22;10(1):85.
4. Kirk Cet al. Lymph formation, composition and circulation: a proteomics perspective, International Immunology. 2015. 27(5): 219–227
5. Shakya S et al.Hyperglycemia-Induced Changes in Hyaluronan Contribute to Impaired Skin Wound Healing in Diabetes: Review and Perspective. International Journal of Cell Biology. 2015
6. Nieuwdorp Met al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes.2006. 55 (2): 480–486
7. Sulyok Eet al.Tissue Sodium Accumulation: Pathophysiology and Clinical Implications. Antioxidants (Basel). 2022. 11(4):750
8. Dean SMet al. The clinical characteristics of lower extremity lymphedema in 440 patients. J VascSurg Venous LymphatDisord. 2020 8(5):851-859.
9. Brix, Bet al. Biology of Lymphedema. Biology 2021, 10, 261.
Optimizing Management of Lymphedema 14 Wound Masterclass - Vol 1 - December 2022
“There is no cure for lymphedema, but a multifaceted approach is used in its management.”
© Copyright. Wound Masterclass. 2023
Biodegradable Temporising Matrix
Moving forward with a robust foundation
NovoSorb® BTM is a synthetic, bioabsorbable scaffold that enables generation of a vascularised neodermis, to provide a robust foundation for reconstruction over deep structures, including exposed bone and tendons.1,2

• Robust in the presence of infection3

• Designed to minimise scarring and contracture4

Discover more: polynovo.com
Indicated for full or deep partial thickness burns, traumatic wounds, surgical and reconstructive wounds. Refer to the Instructions For Use for full device details. References:
 1. Greenwood JE, et al. Eplasty. 2016. 2. Damkat-Thomas L, et al. PRS – Global Open. 2019. 3. Greenwood JE, et al. Burns Open. 2018. 4. Wagstaff MJD, et al. Burns Open. 2019. ® PolyNovo and NovoSorb are registered trademarks of PolyNovo Biomaterials Pty Ltd.
Pictured: Alan – Necrotising fasciitis survivor.
Complex wound from necrotising fasciitis
BTM fully integrated 3 months post treatment
Ms Kara Couch
President-Elect, Association for the Advancement of Wound Care Associate Research Professor of Surgery, School of Medicine and Health Studies, George Washington University. Director, Wound Care Services, The George Washington University Hospital Arlington VA, United States
1. Greenwood JE, et al. Eplasty. 2016. 2. Damkat-Thomas L, et al. PRS – Global Open. 2019. 3. Greenwood JE, et al. Burns Open. 2018. 4. Wagstaff MJD, et al. Burns Open. 2019. ® PolyNovo and NovoSorb are registered trademarks of PolyNovo Biomaterials Pty Ltd.
Pictured: Alan – Necrotising fasciitis survivor.
Complex wound from necrotising fasciitis
BTM fully integrated 3 months post treatment
Ms Kara Couch
President-Elect, Association for the Advancement of Wound Care Associate Research Professor of Surgery, School of Medicine and Health Studies, George Washington University. Director, Wound Care Services, The George Washington University Hospital Arlington VA, United States

All Evidence Is Not Created Equal: What Makes Good Clinical Data?
Editorial Summary
There were an estimated 3.8 million patients with a wound managed by the NHS in 2017/ 2018, of which 70% healed in the study year.1 The annual NHS cost of wound management was £8.3 billion, of which £2.7 billion and £5.6 billion were associated with managing healed and unhealed wounds, respectively.1 The annual prevalence of wounds increased by 71% between 2012/2013 and 2017/ 2018. This article explores clinical data in wound care.
Introduction
One cause of chronic wounds are diabetic foot ulcers (DFUs) - there were an estimated 326, 000 diabetic foot ulcers, which equates to 9% of all adult diabetic patients having a foot ulcer in the study year 2017/ 2018. Additionally, many of those patients with other causes of chronic wounds (e.g. non-healing venous leg ulcers) are also diabetic.1 In the United States, the total number of prevalent cases of DFU was 4,551,498 cases in the year 2020. Unhealed ulcers and foot infections are the leading cause of diabetes related amputations, with diabetic foot ulcers preceding 85% of amputations.2 In the United States, DFU patients are twice as costly to US Medicare as those with diabetes alone. The rate at which major amputations occur in a population with diabetes can be used as a good overall proxy measure of the effectiveness of health care and the foot care system for patients with diabetes.3 Survival rates have been found to be poor following a major amputation –the five year mortality for a diabetic patient following major amputation is 68% (compared to only 15% for breast cancer).4-5 There were 7,957 major lower-limb amputation procedures for patients with diabetes in England between 2017/ 2018 and 2019/ 2020 – this made up 51% of all lower limb amputations in England in this period.3 There were 21,738 minor lowerlimb amputation procedures for patients with diabetes in England between in the same period, with diabetic patients representing 69% of patients in this group.3
The cost of health care for ulceration and amputation in diabetes in 2014-2015 is
estimated at between £837 million and £962 million; 0.8% to 0.9% of the National Health Service (NHS) budget for England.6 More than 90% of expenditure was related to ulceration.6
How Is Evidence Graded?
The evidence pyramid is a useful visual representation of the internal validity of different study designs; designs of low internal validity are at the base of the pyramid and designs of high internal validity are at the top (Figure 1).6 While the evidence pyramid is a useful guide, it is important to recognise it has limitations.6 When considering evidence in the context of the evidence pyramid, it is important to consider the goal of the research project: to understand the effects of treatment where high internal validity is a key requirement or to seek to make new discoveries and find explanations for the causes of disease.6 Where the goal is to understand the disease aetiology, the traditional research pyramid may be reversed, with case reports and case series providing useful data to start an exploration of disease causation.6 This may be especially valid in the case of rare diseases or harms where there are few patients with the condition available for recruitment into high internal validity studies, such as randomised controlled trials (RCTs).6 However, the evidence pyramid provides a simple overview of study designs that may have high internal validity and, as such, may impact or change clinical practice where a clear relationship is found between a treatment and clinical outcome.6
16 Wound
Vol
Masterclass -
1 - December 2022
© Copyright. Wound Masterclass. 2023
Individual randomised controlled trials
Non-randomised controlled trials
Systematic reviews (with homogeneity) of cohort studies
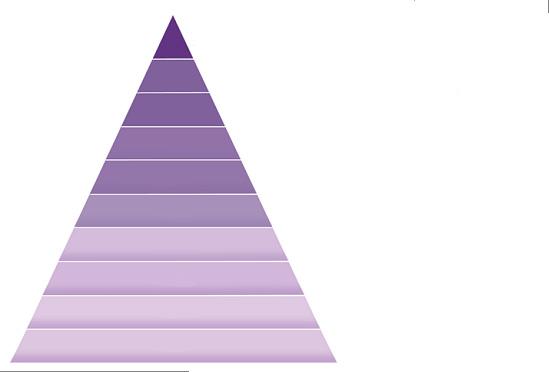
Individual cohort studies, low-quality randomised controlled trials (e.g., <80% follow up) and non-comparative, uncontrolled studies
“Outcomes” research
Systematic reviews (with homogeneity) of case-control studies
Individual case-control studies
Case-series (and poor-quality cohort and case-control studies)
Expert opinion without explicit critical appraisal, or based on physiology, bench research or ‘first principles’
Randomised Controlled Trials
The design of the randomised controlled trial (RCT) compared to other research methodologies offers the clearest understanding of the relationship between an intervention and clinical outcome.6 The RCT, given its methodological rigour, is generally preferable over non-randomised or observational study designs, and forms the main source upon which systematic reviews of interventions are based.6
Conduct bias refers to methodological flaws in a study design and conduct that lead to bias6. While the RCT offers methodological rigour, failure to adhere to the study protocol can introduce bias and reduce the confidence of clinicians in the trial results and conclusions. It is generally believed that there are four key sources of bias that could be reduced by details of design of RCTs: selection, performance, attrition or exclusion and detection bias (Table 1).
Reporting bias describes the bias that arises due to selective reporting of only the statistically significant study findings.6 Reporting bias can occur if authors overemphasise differences of marginal statistical significance (with perhaps limited clinical significance) and/or positive results of secondary analyses. Adverse outcomes of an intervention may be reported selectively by researchers, which will also exaggerate the beneficial outcome of the intervention.6
Publication bias can occur when the outcome of a study influences the decision to publish and typically results in negative findings not being published as they are less likely to be of interest to journals seeking to maintain a high impact factor.6 There are three forms of negative findings:
• Conclusive negative results: derived from well designed and conducted studies that show clear evidence of a neutral or negative effect (i.e., intervention is as good as the control, or even less effective than the control)
Exploratory negative results: derived from well designed and conducted studies, with exploratory data analysis suggesting the intervention was less effective than the control
Inconclusive negative results: poorly designed and conducted study, which is often too small to show the effect of the intervention.6
What Makes for Good Clinical Data in Wound Care? Wound Masterclass - Vol 1 - December 2022 17 Systematic reviews and meta-analyses
Figure
in
care6 LoE 1a LoE 1b LoE 1c LoE 2a LoE 2b LoE 2c LoE 3a LoE 3b LoE 4 LoE 5
Type of bias Stage of RCT How it occurs Selection bias Group selection Randomisation and allocation to treatment groups are flawed Performance bias Exposed or not exposed to the intervention Blinding to treatment allocation does not occur A change in treatment occurs as current intervention is not considered to be working Attrition or exclusion bias Follow-up period Number of patients lost to follow up are high or different between the treatment arms Detection bias Assessment of outcomes Outcome in one treatment arm is measured in a different way to the other arm © Copyright. Wound Masterclass. 2023
1: Levels of Evidence for studies on therapy, prevention, aetiology and harm
wound
Table 1: Sources of bias in RCTs6
Validity Scoring
Some organisations specialising in the promotion of evidence-based practice offer simple validity checklists to assess internal and external validity (e.g., in the UK, the Centre for Evidence Based Medicine, the Joanna Briggs Institute, and the Scottish Intercollegiate Guidelines Network).A group representing both the European Wound Management Association and the International Working Group on the Diabetic Foot published a 21-point score designed to assess the validity of intervention studies relating to diabetic foot ulcers (DFUs; Figure 2).6
When assessing a study about the prevention and management of diseases of the foot in diabetes, the participants need to be appropriately selected – i.e., the participants are patients with diabetes who are at risk of developing a diabetic foot ulcer or whose disease is complicated by a diabetic foot ulcer.7 If more than one foot ulcer is present, only one (a specified index ulcer) should be included per participant.7
The type of ulcer chosen should be appropriate for the type of intervention – for example some trials of new interventions are carried out on those with uncomplicated neuropathic ulcers, for which cheap and effective treatments already exist.7 New treatments should be targeted to those ulcers that have failed to heal despite administration of good standard care in expert centres.7 When an intervention is administered for the prevention or treatment of diabetic foot ulcers, it will inevitably be given in conjunction with other aspects of care, and these other components must be described.7 Many participants in studies about DFU are lost to follow up, and the higher the loss to follow-up, the greater the likelihood of bias in any observations made.7 There is no consensus on the rate of retention or attrition that is acceptable in this population, although a figure of <25% loss to follow up with an intervention phase of 20 weeks or more is generally
studied in the trial based on an appropriate sample size calculation?
9. Was the chosen primary outcome of direct clinical relevance?
10. Was the person who assessed the primary outcome or outcomes blinded to group allocation?
11. Was either the clinical researcher who cared for the wound at research visits or the participant blinded to group allocation?
Study conduct
12. Did the study complete recruitment?
13. Was it possible to document the primary outcome in 75% or more of those recruited?
14. Were the results analysed primarily by intention to treat?
15. Were the appropriate statistical methods used throughout?
Outcomes
16. Was the performance of the control group of the order that would be expected in routine clinical practice?
17. Are the results from all participating centres comparable?
Answer 'Yes' if the study was done in only one centre.
Rationale: study design
The intervention should be the only difference between study groups, there should be no difference between the baseline characteristics of the participants, other than those that may be the result of chance. It is also important that all participants otherwise receive defined good standard case. The importance of this is to ensure that any intervention being studied is the only difference between groups, which could account for any observed difference. The method of randomisation (ideally by an independent agency) should be described, together with a sample size calculation, blinding/ masking (especially of the outcome observer) and a choice of an outcome measure that is clinically relevant.
Rationale: study conduct
The four questions relate to completion of recruitment and follow-up, as well as to statistical analysis.
Rationale: study outcomes
Question 16 checks that the differences observed between groups are not the result of unusually poor performance in the control group, as has been the case in a number of published trials reporting apparent benefit of an intervention. For question 17, as many multicentre studies have a core of high-recruiting centres and a majority in which recruitment was either moderate or low, it is important to ensure that the aggregate outcomes are not dominated by performance in a small number of high recruiting centres. For example, if usual care is different in different centres, any benefit could be by chance, but if randomisation is drafted by centre, then this could have less of an influence. While this can be minimised by randomising seperately by study centre, this can increase the total number of participants needed.
Study reporting
18. Is the report free from errors of reporting, e.g. discrepancies between data reported in different parts of the same report?
19. Are the important strengths and weaknesses of the study discussed in a balanced way?
20. Are the conclusions supported by the findings?
21. Is the report free from any suggestion that the analyses or the conclusions could have been substantially influenced by people with commercial or other personal interests in the findings?
considered acceptable.7
Rationale: study reporting
The four questions are designed to explore the possibility of reporting bias. Questions 19 - 21 aim to expose aspects of the report that reflect intentional or unintentional choice of words, which could either exaggerate or obscure some aspects of the findings.
Some expensive interventions have been widely used as a result of studies that would now be viewed as flawed.7 In some cases, the apparent benefit was based on a significant difference from the comparator group when the difference could be accounted for by poor performance in the comparator group receiving usual care.7 Therefore the outcome in the comparator group must be scrutinised to check that performance is similar to that used as the basis for the sample size calculation.7
What Makes for Good Clinical Data in Wound Care? 18 Wound Masterclass - Vol 1 - December 2022
“When assessing a study about the prevention and management of diseases of the foot in diabetes, the participants need to be appropriately selected.”
Study design 1. Are adequate definitions includes for the terms 'ulcer', 'healing', and all other required aspects of the population and the outcomes? 2. Was the choice of study population appropriate for the chosen intervention and the stated outcomes? 3. Was the control population managed at the same time as those in the intervention group? 4. Is the intervention sufficiently well described to enable another researcher to replicate the study? 5. Are the components of other aspects of care described for the intervention and comparator groups? 6. Were the participants randomised into intervention and comparator groups? 7. Were the participants randomised by an independent person or agency? 8. Was the number of participants
Figure 2: Required rationale and markers of quality: the 21-point scoring system for reports on clinical trials for the prevention and management of diseases of the foot in patients with diabetes6,7
© Copyright. Wound Masterclass. 2023
Example: Leucopatch System for Management of Hard-To-Heal Diabetic Foot Ulcers
This was a multicentre, observer-blinded RCT.8 There was a 4-week run in period, and 326 patients (out of 595 consented) were excluded during that run-in period.8 The primary outcome was the proportion of ulcers that healed within 20 weeks assessed in the intention-to-treat population (all participants with post-randomisation data collected), defined as complete epithelialisation (confirmed by an observer who was masked to randomisation group), and remained healed for 4 weeks.8 The randomisation process (computer-generated, web-based) resulted in well matched groups (leucopatch + standard care v standard care) in terms of age, sex, T2DM, mean duration of diabetes, diabetes related complications, mean baseline haemoglobin, estimated GFR and foot ulcer characteristics and related complications (ABPI, loss of sensation at two or more sites, area of ulcer, depth of ulcer, affected foot position and type of offloading).8 The authors found a statistically significant effect of the intervention.8
This can be viewed as particularly valuable information for a number of reasons. The study population was well chosen. The ulcer must have been non-responsive to treatment for more than four weeks, as those whose ulcers healed in the run-in period were excluded. Grade 3 wounds and patients with a ABPI down to 0.5, indicating severe disease were included, indicating that this study chose the important group – those with hard to treat DFUs which are unlikely to respond to standard care that is already available, therefore targeting those most likely to benefit from novel treatment. Both groups received defined best standard of care including debridement, offloading, NPWT and protease inhibitors.
Groups exist to review evidence relating to the management of DFU. As a result of the Leucopatch II trial, the International Working Group for the Management of the Diabetic Foot Ulcer recommended that clinicians should
consider the use of autologous combined leucocyte, platelet and fibrin as an adjunctive treatment, in addition to best standard of care, in non-infected diabetic foot ulcers that are difficult to heal, but with a weak strength of recommendation and moderate grading of the quality of evidence to support this recommendation.9 They felt that whilst the quality of this one available study was strong, the lack of cost effectiveness, applicability in daily practice and the importantly, the absence of additional supportive studies meant that the strength of their recommendation was weak9. Similarly, the National Institute for Clinical Excellence, NICE, does not recommend their use in the NHS as there are uncertainties around whether the evidence would generalise to current NHS practive because of how and when the treatment would be used.10 Cost analysis also showed that the clinical benefits seen in the trial are unlikely to lead to a cost saving in practice.10
Summary
Chronic ulcers, particularly DFUs, are a major economic burden to the NHS and often lead to amputation which is associated with a high mortality. Therefore, when assessing evidence it is important to assess if that evidence is of high quality and can therefore be implemented in to practice. Randomised control trials are the best quality of evidence, after systematic reviews and meta-analyses in the pyramid of evidence. It is important to ensure than an RCT is free from conduct, reporting and publication bias and scoring systems have been developed to help assess this. Groups exist which assess these studies to enable them to make recommendations about clinical practice based on their assessment of the quality of the study conducted – it is therefore important for investigators to make their study design as free from bias as possible.
What Makes for Good Clinical Data in Wound Care? Wound Masterclass - Vol 1 - December 2022 19 © Copyright. Wound Masterclass. 2023
“When assessing evidence it is important to assess if that evidence is of high quality and can therefore be implemented in to practice.”
What Makes for Good Clinical Data in Wound Care?

1. Guest JF, Fuller GW, Vowden P. Cohort study evaluating the burden of wounds to the UK’s National Health Service in 2017/2018: update from 2012/2013. BMJ Open 2020;10:e045253.
doi: 10.1136/bmjopen-2020-045253
2. Edmonds M, Manu C, Vas P. The current burden of diabetic foot disease. J Clin Orthop Trauma. 2021 Jun; 17: 88–93.Published online 2021 Feb 8. doi: 10.1016/j.jcot.2021.01.017
3. Office for Health Disparities and Improvement (above): National Diabetes Foot Care Report. 2022.https://fingertips.phe.org.uk/static-reports/diabetes-footcare/national-diabeticfootcare-report.html
4. Icks A et al. Time-Dependent Impact of Diabetes on Mortality in Patients After Major Lower Extremity Amputation: Survival in a population-based 5-year cohort in Germany. Diabetes Care.34(6). 1 June 2011
5. Office of National Statistics: Cancer survival in England – adults diagnosed.12/8/19 https:// www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/ datasets/cancersurvivalratescancersurvivalinenglandadultsdiagnosed
6. World Union of Wound Healing Societies (2020) Evidence in wound care. London: Wounds International.
7. Jeffcoat W et al on behalf of the International Working Group on the Diabetic Foot and the European Wound Management Association.Reporting standards of studies and papers on the prevention and management of foot ulcers in diabetes: required details and markers of good quality.Lancet Diabetes Endocrinol 2016; 4: 781–88
8. Game F et al for the Leucopatch II trial team. Leucopatch system for the management of hard-to-heal diabetic foot ulcers in the UK, Denmark and Sweden: an observer-masked, randomised controlled trial. The Lancet Diabetes and Endocrinology. 6(11): 870-8. 1/11/18
9. IWGDF Guidelines on the Prevention and Management of Diabetic Foot Disease Chapter: IWGDF Guideline on interventions to enhance healing of foot ulcers in persons with diabeteshttps://iwgdfguidelines.org/wp-content/uploads/2021/03/IWGDF-2019-final.pdf
10. National Institute for Clinical Excellence. 3D patch for treating diabetic foot ulcers.
7/3/22 https://www.nice.org.uk/guidance/mtg66/chapter/1-Recommendations

20 Wound Masterclass - Vol 1 - December 2022
woundmasterclass.com/Register Register for full access to the journal, educational resources, information about upcoming events and more woundmasterclass.com © Copyright. Wound Masterclass. 2023 Image licenced from Adobe Stock. Credit: Dragana Gordic
References
largerBrandnew size To know more about our Debrisoft® family and other L&R products visit lohmann-rauscher.co.uk Debrisoft is registered to L&R. ® 2019. The whole Debrisoft® Family is now
NICE
Debrisoft®
recommended by
NICE concluded that
is more clinically and cost effective than other debridement methods
Dr Aliza Lee Clinical Research Investigator, Department of Veterans Affairs Salem VA, United States

The Safe Guide to Debridement in the Challenging Clinical Setting
Editorial Summary
Debridement should be carried out as part of standard of care treatment. Safety and minimising of complications are essential when carrying out debridement in various clinical settings. Standard of care for debridement involves initial debridement to healthy tissue and any further weekly debridements as required. Debridement methods can be generalized into five broad categories, autolytic, enzymatic, biologic, mechanical, and sharp debridement.1-4 This article explores how wound debridement in anticoagulated patients can be achieved safely with use of a chitosan-based hemostatic agent.
Introduction
Several wound care guidelines strongly recommend debridement be performed as part of the standard of care in the treatment of wounds.1-6 This recommendation has been in place through several iterations of wound care guidelines as wound debridement has been shown to reduce time to complete healing and increase the number of healed wounds.1,7 The recommended standard of care for debridement involves initial debridement followed by routine debridement to maintain a healthy wound base to promote wound resolution..2,3,8 Debridement methods can be generalized into five broad categories, autolytic, enzymatic, biologic, mechanical, and sharp debridement.1-4 While limited evidence exists to support the use of one debridement modality over another, ease of access, cost effectiveness, time efficiency in removal of nonviable and infected tissue, the ability to remove large amounts of tissue quickly and significantly reduce the number of microorganisms in the wound bed, make sharp debridement the preferred technique.1,3,7 Despite these recommendations, the use of sharp debridement may be limited due to patient factors, such as pain and insufficient arterial supply, and provider factors, such as limited experience, skill, scope of practice and licensing restrictions, resource access, and the concern that sharp debridement may cause the patient harm.1,3,4,6,8 One of these latter concerns that providers may have with sharp debridement is its relative contraindication in patients with bleeding disorders or those on anticoagulant therapy.3 Multiple modalities exist for achieving hemostasis following
wound debridement. The most common modalities utilized in the outpatient, inpatient, and operating room setting include holding pressure, use of various topical hemostatic agents, chemical cautery with silver nitrate, and electrocautery.9,10 Disadvantages of these methods of achieving hemostasis include the time required to achieve hemostasis, the potential for tissue injury, expense, lack of access to necessary supplies, and the time required by the provider for appropriate follow up.3,11 Providers may choose to perform limited wound debridement or avoid it altogether in this patient population for these reasons, potentially contributing to delayed wound healing.8
Chitosan is a naturally occurring biocompatible, biodegradable, and non-toxic hemostatic agent with antimicrobial and antifungal properties that has been shown to promote wound healing.12 Presented here are the results of use of a chitosanbased hemostatic agent (OMNI-STAT®, Omnistat Medical Inc, New York, NY) following wound debridement in anticoagulated patients.
Methods
A review of a series of patients on anticoagulant therapy with wounds requiring debridement, in the outpatient and operating room settings, in which a chitosan-based hemostatic agent was used to achieve hemostasis was performed. Sharp debridement was performed in all patients. After sharp debridement to bleeding tissue, chitosan-based granules and/ or impregnated gauze were applied to the wound following the protocol outlined in Table 1. The incidence of rebleed following sharp debridement was collected.
22 Wound Masterclass - Vol 1 - December 2022
© Copyright. Wound Masterclass. 2023
Results
Twelve patients on anticoagulant therapy underwent sharp debridement of their wound. All the patients were male. The average patient age was 69.9 ± 7.4 years. Patients were on an average of 2 anticoagulant medications (range: 1 – 3), including Apixaban, Aspirin, Clopidogrel, Heparin, Lovenox, and Warfarin. (Table 2) Wound etiologies included acute and chronic wounds due to pressure, diabetes, peripheral arterial disease, mixed arterial disease and venous insufficiency, and trauma. Sharp and/ or hydrosurgical debridement was performed in all patients in either the outpatient or operative setting. The level of debridement ranged from subcutaneous tissue to bone. Eight patients had coagulation labs drawn, two (25.0%) of these patients had elevated or near critical INR levels. Only one patient had occurrence of a rebleed following intraoperative surgical debridement. Rebleed did not occur in the two patients with elevated or near critical INR levels who underwent surgical debridement.
Discussion
Debridement is a central component in the standard of care for treatment of acute and chronic wounds. A 28-day reduction in time to healing for diabetic foot ulcerations has been reported when debridement is included in the treatment protocol compared to when debridement is not a part of the treatment protocol.7 Given the accelerated time to healing and incidence of healed wounds, multiple guidelines provide a strong recommendation that the standard of care for wounds include initial and routine sharp debridement.1-7 Sharp debridement is recommended to such an extent that while one guideline states that sharp debridement is a relative contraindication in patients with bleeding disorders or an anticoagulant therapy, as it must be performed “to bleeding tissue”, the same guideline also recommends the use of clean, dry dressings
for the first 8-24 hours for wounds associated with bleeding with subsequent use of moist dressings once hemostasis is obtained.3,6,9
Despite the existence of multiple modalities to achieve hemostasis, no “gold standard” exists.11 Currently available hemostatic modalities used in the outpatient, inpatient, and operating room settings are limited by provider preference, the time required to achieve hemostasis, resource availability, cost, and the potential for causing the patient harm.1,3,4,6,8 In a survey of 75 Australian podiatrists and wound care nurses, lack of staff time and resource access and requirements were cited as one of the top three reasons for less frequent wound debridement.8 The time required for holding pressure for bleeding cessation in patients on anticoagulant therapy can be longer than the normal bleeding time of <3 minutes to <8 minutes.13 Less frequent wound debridement was also noted by providers practicing in rural areas compared to those practicing in urban areas.8 While the reason for this difference was not stated, one can speculate that providers in urban areas have greater access to the time and resources required should a complication with debridement occur. Topical hemostatic agents, such as flowables, gelatins, thrombins, and oxidized regenerated celluloses, are most often used in the operating room setting. Costs of these hemostatic agents range from $50 to >$500.14 While some may argue that the cost of the product itself is inconsequential, the total cost related to operating room time, staff, equipment and necessary supplies, and a potential need for return to the operating room must be considered. A study comparing use of a chitosan-based hemostatic agent to electrocautery following sharp debridement in the operating room setting found use of the chitosan-based hemostatic agent resulted in less time to achieve hemostasis and reduced preprocedure and procedure times.15 The study included 112 patients, 59 of which were treated with the chitosan-based hemostatic agent and
The Safe Guide to Debridement in the Challenging Clinical Setting Wound Masterclass - Vol 1 - December 2022 23 © Copyright. Wound Masterclass. 2023
“Despite the existence of multiple modalities to achieve hemostasis, no “gold standard” exists.”
89 of which were treated with electrocautery following sharp debridement. Patients on anticoagulant therapy comprised 60.7% of patients treated with electrocautery and 42.3% of patients treated with the chitosan-based hemostatic agent. The time required to achieve hemostasis following sharp debridement was ~10 minutes less with the chitosan-based hemostatic agent than with electrocautery (38:58 vs 48:11, p<0.001), a significant reduction in time. A significant reduction in pre-procedure (20:37 vs 24:53, p<0.001) and procedure times (10:10 vs 14:37, p<0.001), ~4 minutes less for patients treated with the chitosanbased hemostatic agent compared to those treated with electrocautery, was also reported. These significant differences in times may be related to the need for several large pieces of equipment for electrocautery and the time for individualized cautery of each bleeding vessel. Use of the chitosan-based hemostatic agent does not require any additional equipment and provides rapid hemostasis of the entire wound at one time. The reduction in perioperative and operative times with use of the chitosanbased hemostatic agent compared to the use of electrocautery has the potential to reduce healthcare resource utilization as one minute of operating room time, excluding the costs associated with anesthesia, implants, radiology, pathology, and physician services, has been estimated to be $46.04.16 Electrocautery, as well as the use of silver nitrate for chemical cautery, also cause tissue damage that the patient’s body must heal in addition to the initial tissue breakdown related to the wound.9 The disadvantages associated with current methods of achieving hemostasis following wound debridement may result in limited or no wound debridement being performed. Suboptimal wound debridement, or the lack of wound debridement altogether, can increase the patient’s risk for delayed wound healing and its associated complications.
An unmet need exists for a cost-effective, rapid, effective, non-caustic hemostatic agent that can be utilized in the surgical, inpatient, and outpatient settings. Use of a chitosan-based hemostatic agent and/or chitosan impregnated gauze may fill this unmet need. In addition to the potential cost savings mentioned above, this agent could increase provider confidence to provide the standard of care for wound treatment for patients with bleeding disorders or those on anticoagulant therapies, which
includes optimal sharp debridement to bleeding tissue to accelerate wound healing. A study that evaluated the use of a chitosanbased gelling fiber dressing (Opticell, Medline Industries, Inc., Northfield, IL) following wound debridement in the outpatient setting reported reduced times to achieving hemostasis and reduction in wound size at one week.12 The study included 20 patients with chronic wounds of various etiologies. Seventeen (85%) patients had sharp debridement performed, the remaining three (15%) patients had electrocautery alone or sharp debridement followed by electrocautery. Bleeding following debridement was described as moderate to severe in 11 (55%) patients. Hemostasis was achieved in all patients at ~1-minute. Of the 18 patients evaluated 1-week following initial treatment, 9 (50%) had a significant reduction in wound size.
The cases presented here further support the ability of a chitosan-based hemostatic agent to achieve rapid hemostasis in acute and chronic wounds in patients on anticoagulant therapy. In addition to achieving rapid hemostasis, use of the chitosan-based hemostatic agent in this case series suggests that its use may also support healing in a variety of ways. First, the ability to achieve rapid hemostasis allowed for the early use of other advanced wound care products to aid in accelerated wound healing. Second, the biocompatible, innate, and nontoxic properties of chitosan avoid the additional tissue damage associated with the use of chemical cautery or electrocautery, negating further stress on the patient’s natural reparative mechanisms.17 Third, the inherent properties of chitosan may also aid in wound healing. Chitosan has been demonstrated to provide a 3-dimensional extracellular matrix, and stimulate macrophages, fibroblast proliferation, and keratinocyte delivery, key components of the proliferative phase of wound healing.12,17,18 Chitosan may also increase collagen deposition and organization, potentially increasing the tensile strength of wounds once they are healed.18 One patient in this case series achieved wound resolution following the discontinuation of all advanced wound care products and treatment with debridement and application of the chitosanbased hemostatic agent alone. Chitosan has also been shown to have inherent bacteriostatic and bactericidal effects which may assist in infection prevention.17,18
The Safe Guide to Debridement in the Challenging Clinical Setting 24 Wound Masterclass - Vol 1 - December 2022 © Copyright. Wound Masterclass. 2023
Results of the case series presented here support the use of the chitosan-based hemostatic agent following guideline recommended sharp debridement to bleeding tissue in patients on anticoagulant therapy given its ability to achieve rapid hemostasis. In addition to the ability to achieve rapid hemostasis in this patient population, use of the chitosan-based hemostatic agent may help expedite wound resolution due to its ability to promote early advanced wound care product use and the potentially beneficial inherent properties of chitosan.
and incidence of intraoperative re-bleed
Conclusions
The Safe Guide to Debridement in the Challenging Clinical Setting Age Laterality Ulcer Type Anticoagulation Medication Level of Debridement Hemostatic Agents Used Adjunctive Advanced Wound Care Products Utilized Occurrence of Rebleed Occurrence of Rebleed 59 Right Pressure Aspirin, Heparin Bone Chitosan impregnated gauze, Hemostatic Matrix Kit with Thrombin NPWT Yes 61 Right DFU Clopidogrel Bone Chitosan impregnated gauze None No 63a Right Mixed Warfarin Bone Chitosan impregnated gauze NPWT No 64 Right DFU Aspirin, Clopidogrel Tendon Chitosan granules Antibiotic coated collagen, Resorbable boratebased glass fiber matrix No 65 Right Traumatic Apixaban Subcutaneous tissue Chitosan granules Antibiotic coated collagen No 71a Left Ischemic Aspirin, Clopidogrel Tendon Chitosan impregnated gauze NPWT No 72 Left PAD Clopidogrel Subcutaneous tissue Chitosan granules Antibiotic coated collagen dressing silver impregnated collagen dressing two types of placental allografts No 73 Left Traumatic Enoxaparin. Warfarin Subcutaneous tissue Chitosan granules Silver-impregnated collagen No 74 Left DFU Apixaban, Aspirin Tendon Chitosan granules Antibiotic coated collagen. bilayer xenograft, NPWT No 76 Left Ischemic Apixaban Tendon Chitosan impregnated gauze Antibiotic coated collagen. bilayer xenograft No 79 Left Pressure Apixaban, Aspirin, Lovenox Bone Chitosan impregnated gauze Bilayer xenograft No 82 Left DFU Aspirin, Apixaban, Lovenox Tendon Chitosan impregnated gauze Bilayer xenograft, Antibiotic coated collagen, Collagenase, Gentian Violet and Methylene Blue Antibacterial Dressing No DFU, diabetic foot ulceration; NPWT,
aElevated INR levels; Patient age 71 – PT: 31.4, INR: 3.1; Patient age 63 – PT: 39.8, INR: 4
negative pressure wound therapy
Chitosan-based hemostatic granules Chitosan-based hemostatic gauze POUR Granules to cover the wound bed PACK Granules into the wound to ensure contact The wound with the gauze PRESS Holding pressure for 1 to 3 minutes Holding pressure for 1 to 3 minutes
Figure 2: Patient demographics, use of anticoagulants,
Wound Masterclass - Vol 1 - December 2022 25 © Copyright. Wound Masterclass. 2023
Figure 1:
References
1. Rayman G, Vas P, Dhatariya K, et al. Guidelines on use of interventions to enhance healing of chronic foot ulcers in diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020;36 Suppl 1:e3283. doi:10.1002/dmrr.3283
2. Marston W, Tang J, Kirsner RS, Ennis W. Wound Healing Society 2015 update on guidelines for venous ulcers. Wound Repair Regen. 2016;24(1):136-144. doi:10.1111/wrr.12394

3. Gould L, Stuntz M, Giovannelli M, et al. Wound Healing Society 2015 update on guidelines for pressure ulcers. Wound Repair Regen. 2016;24(1):145-162. doi:10.1111/wrr.12396
4. Lavery LA, Davis KE, Berriman SJ, et al. WHS guidelines update: Diabetic foot ulcer treatment guidelines. Wound Repair Regen. 2016;24(1):112-126. doi:10.1111/wrr.12391
5. Federman DG, Ladiiznski B, Dardik A, et al. Wound Healing Society 2014 update on guidelines for arterial ulcers. Wound Repair Regen. 2016;24(1):127-135. doi:10.1111/wrr.12395
6. Sibbald RG, Elliott JA, Persaud-Jaimangal R, et al. Wound bed preparation 2021. Adv Skin Wound Care. 2021;34(4):183-195. doi:10.1097/01.ASW.0000733724.87630.d6
7. Dayya D, O’Neill O, Habib N, Moore J, Iyer K, Huedo-Medina TB. Debridement of diabetic foot ulcers: public health and clinical implications - a systematic review, metaanalysis, and meta-regression. BMJ Surg Interv Health Technol. 2022;4(1):e000081. Published 2022 May 30. doi:10.1136/bmjsit-2021-000081
8. Nube VL, Alison JA, Twigg SM. Frequency of sharp wound debridement in the management of diabetes-related foot ulcers: exploring current practice. J Foot Ankle Res. 2021;14(1):52. Published 2021 Aug 12. doi:10.1186/s13047-021-00489-1
9. Ogawa C, Sato Y, Suzuki C, Mano A, Tashiro A, Niwa T, Hamazaki S, Tanahashi Y, Suzumura M, Hayano S, Hayakawa M, Tsuji T, Hoshino S, Sugiyama Y, Kidokoro H, Kawada JI, Muramatsu H, Hirakawa A, Ando M, Natsume J, Kojima S. Treatment with silver nitrate versus topical steroid treatment for umbilical granuloma: A non-inferiority randomized control trial. PLoS One. 2018 Feb 13;13(2):e0192688.
10. Martyn D, Meckley LM, Miyasato G, et al. Variation in hospital resource use and cost among surgical procedures using topical absorbable hemostats. Clinicoecon Outcomes Res. 2015;7:567-574. Published 2015 Nov 6. doi:10.2147/CEOR.S88698
11. Groenewold MD, Gribnau AJ, Ubbink DT. Topical haemostatic agents for skin wounds: a systematic review. BMC Surg. 2011 Jul 12;11:15.
12. Keast DH, Janmohammad A. The Hemostatic and Wound Healing Effect of Chitosan Following Debridement of Chronic Ulcers. Wounds. 2021;33(10):263-270.
13. Russeau AP, Vall H, Manna B. Bleeding Time. In: StatPearls. Treasure Island (FL): StatPearls Publishing; August 8, 2022.
14. Allotey JK, King AH, Kumins NH, et al. Systematic review of hemostatic agents used in vascular surgery. J Vasc Surg. 2021;73(6):2189-2197. doi:10.1016/j.jvs.2020.10.081
15. Thibodeaux KT, Speyrer MS, Thibodeaux RP, Rogers AA, Rippon MG. Management of postoperative bleeding in surgically debrided wounds: topical haemostat versus electrocautery. J Wound Care. 2020;29(8):444-451. doi:10.12968/jowc.2020.29.8.444

16. Smith TS, Evans J, Moriel K, Tihista M, Bacak C, Dunn J, Rajani R, Childs B. Cost of operating room time is $46.04 dollars per minute. J Ortho Business. 2022; 2(4):10-13.
17. Matica MA, Aachmann FL, Tøndervik A, Sletta H, Ostafe V. Chitosan as a Wound Dressing
Starting Material: Antimicrobial Properties and Mode of Action. Int J Mol Sci. 2019;20(23):5889.
Published 2019 Nov 24. doi:10.3390/ijms20235889
18. Dai T, Tanaka M, Huang YY, Hamblin MR. Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects [published correction appears in Expert Rev Anti Infect Ther. 2013 Aug;11(8):866]. Expert Rev Anti Infect Ther. 2011;9(7):857-879. doi:10.1586/ eri.11.59
Guide to Debridement in the
26 Wound Masterclass - Vol 1 - December 2022 woundmasterclass.com Submit Your Research to Our Journal Case reports, randomized controlled trials, clinical reviews, audits, and research projects submissions@woundmasterclass.com © Copyright. Wound Masterclass. 2023 Image licenced from Adobe Stock. Credit: Rawpixel.com
The Safe
Challenging Clinical Setting











woundmasterclass.com/Events Live & On Demand woundmasterclass.com/Register MasterSeries 60 Minutes Interactive Better wound care. Better content. Better clinical articles. Get accredited. January 24th: 1pm EST | 6pm GMT Supported by Getting the Best Patient Outcomes in Chronic Venous Disease; From Micro to Macro Moderator Miss Negin Shamsian Consultant Plastic & Reconstructive Surgeon (Locum) London, United Kingdom Global expert Dr Abigail Chaffin Plastic and Reconstructive Surgeon at Tulane University Medical Director, MedCentris Wound Healing Institute at Tulane New Orleans LA, United States Global expert Co-chairperson Dr M. Mark Melin Medical Director of the M Health Wound Healing Institute Mineapolis MN, United States Global expert Co-chairperson Dr Monika Gloviczki Scientist and Artist at Vascular Science & Art Scottsdale AZ, United States Global expert Dr Peter Gloviczki Editor-in-Chief of the Journal of Vascular Surgery Publications. Professor of Surgery (Emeritus), Mayo Clinic, Rochester, Minnesota Scottsdale AZ, United States Global expert Dr Lee Ruotsi Medical Director at the Saratoga Hospital Center for Wound Healing and Hyperbaric Medicine Saratoga Springs NY, United States
GUIDES
Introduction
This Masterclass Guide is a concise overview aimed at exploring the use of a temporary topical external hemostat, and how to incorporate this in to your clinical practice.
What Is OMNI-STAT
?
■ Hemostasis is the first stage of wound healing process and data supports that chitosan and its derivatives have been shown to a positive effect1
■ Bleeding control is essential in any clinical setting, and safety in performing tasks such as sharp debridement in the OR and in the Wound Clinic is essential2
■ Rapid bleeding control enables the clinician to quickly advance to the next stage of wound healing2
■ OMNI-STAT® is a fast and effective solution for bleeding control intended for temporary topical external use for minor, moderate and severe bleeding1,3

■ OMNI-STAT® is a unique and proprietary hemostatic agent derived from chitosan, an organic polysaccharide3

■ A highly purified form of chitin from shrimp is selected and deproteinized which undergoes partial deacetylation converting chitin to chitosan3
■ OMNI-STAT® may improve quality of granulation tissue at the wound site. It is safe and easy to use with no adverse events reported4,5
Topical External Hemostat: OMNI-STAT®
Keywords
■ Wound repair
■ Rapid bleeding control
■ Hemostatic agents
■ Temporary Topical External Hemostat
■ Surgical and non surgical wounds
■ Chitin
■ Chitosan
■ Blood clotting mechanism
How Does OMNI-STAT® Work?
■ The granules are comprised of high surface area granular flakes3
■ When they come into contact with blood, they absorb fluids and concentrate platelets to form a gel like clot3
■ The gel like clot plugs the bleeding source, and seals the wound3
■ This newly created physical barrier, as well as the activation of platelets via platelet concentration, enable OMNI-STAT® to stop the bleeding, and reduces chances of a rebleed6
■ Mechanism of action is independent of the body's natural clotting cascade6
■ Works in hypothermic conditions3
■ May offer benefits that aid patients with impaired coagulation3
■ Effective Product Range for minor wounds to severe arterial bleeding7
■ Effective in the presence of common anticoagulants, and clotting dysfunction8
■ Does not damage healthy tissue4
28 Wound Masterclass - Vol 1 - December 2022
Masterclass
Figure 1: OMNI-STAT® Gauze, left, and Granules, right
Figure 2: OMNI-STAT® products are chitosan derived hemostats.
®
Method and instructions for OMNI-STAT® and CELOX™
OMNI-STAT® and CELOX™ are cleared for use under the supervision of a healthcare professional.
Omni-stat Medical Inc offers a complete product line, including Celox technology that can be used for bleeding control beyond wound care and throughout the hospital.

OMNI-STAT® gauze and granules may be used for:
• Diabetic Foot Ulcers
• Venous Stasis Ulcers
• Pressure Ulcers
• The properties of OMNI-STAT® may enable the specialist to perform more aggressive sharp debridement on highrisk anticoagulated patients, in an outpatient or inpatient setting9
CELOX Z-Fold Gauze may be used In the setting of:
• Arterial injuries
• Road traffic accidents
CELOX™ applicator may be used for:
• Knife trauma
• Bullet or shrapnel wounds
• Complex or irregularly shaped injuries
Granules can be used for:
• Hairy areas
• Skin tears
• Lacerations
• Avulsions
• Punctures
OMNI-STAT® Granules (3g)
The technology can also be used for:
• Bleeding that’s hard to control
• Anticoagulant patients
• Vascular access sites
• Bleeding fistulas
• Areas that could be difficult or Impossible to pack with standard gauze
Products may be used:
• To control external bleeding

• Exudate from sutures
• Surgical procedures such as amputations
• Post sharp debridement Split thickness skin grafts, donor sites
• Cath lab, interventional radiology, dialysis centres
CELOX Vascular can be used for:
• Local management and control of surface bleeding from vascular access sites Percutaneous catheters or tubes utilizing introducer sheaths up to 16 French
How to Use/ Recommendations for Removal
Peel open OMNI-STAT outer pouch and remove inner pouch. 1
Tear open OMNI-STAT inner pouch. Blot away any excess blood or exudate from the wound. 2

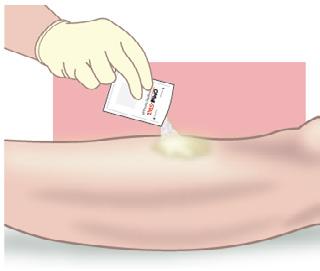
3
Pour enough OMNI-STAT onto the wound to cover the entire bleeding area. OMNI-STAT should be applied directly to the source of bleeding.
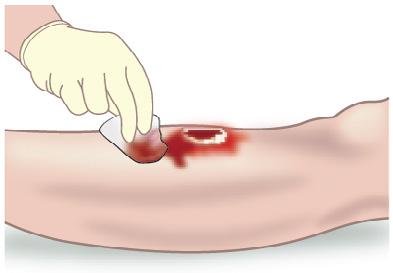

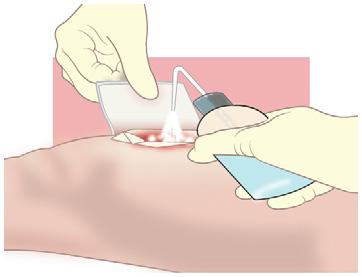
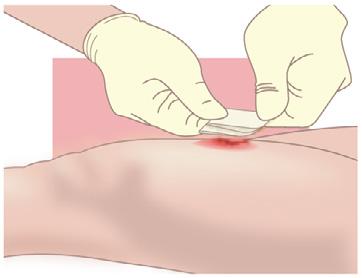

Quickly cover with gauze and apply firm pressure for 1 to 3 minutes depending on the severity of the bleeding. If bleeding continues apply pressure for 3 more minutes. If further OMNI-STAT


Removal Instructions:
Five minutes after bleeding has stopped, gently remove any excess OMNI-STAT . Use saline if required. Discard any unused OMNI-STAT
At first dressing change after use, OMNI-STAT should be cleansed and removed from the wound using standard wound cleansing protocols. If required OMNI-STAT should be soaked with salone prior to removal (physically) and then any residual irritated awayy with water or saline.
Wound Masterclass - Vol 3 - December 2022 29 Topical
Hemostat: OMNI-STAT® Masterclass
External
GUIDES
OMNI-STAT® Gauze (4in x 4in) How to Use/ Recommendations for Removal Tear open OMNI-STAT gauze pack. Blot away any excess blood or exudate from the wound. Apply pre-folded OMNI-STAT onto the wound to cover the entire bleeding area. OMNI-STAT should be applied directly to the source of bleeding. 1 2 If bleeding source is below the wound surface, then OMNI-STAT gauze can be unfolded and tightly packed into wound. Apply firm pressure for 1 to 3 minutes depending on the severity of the bleeding. If bleeding continues apply pressure for 3 more minutes. If OMNI-STAT® gauze becomes saturated then use an additional OMNI-STAT gauze pack.
Instructions:
first dressing change after use, OMNISTAT should be cleansed and removed from the wound using standard wound cleansing protocols. If required OMNI-STAT should be soaked with salone prior to removal (physically) and then any residual irritated awayy with water or saline.
3 4 Removal
At
additional pouch. 4
is required use an
5
Wound Care Trauma and Critical Situations Emergency Departments Hard to Control Bleeding Areas Operating Rooms
OMNI-STAT is a registered trademark of Omni-stat Medical Inc. All Rights Reserved. MTO-20-230 OMNI-STAT is a registered trademark of Omni-stat Medical Inc. All Rights Reserved. MTO-20-220
What Is the Evidence?
Various studies and trials highlight the efficacy and reliability of OMNI -STAT® and CELOX™ products. OMNI-STAT® and CELOXTM are brand names of equivalent proprietary chitosan technology.
Hemorrhage Control
■ In an animal study conducted by Kozen et al., a standard industry model of a complex groin injury with transection of the femoral vessels and 3 minutes of uncontrolled bleeding was created in 48 swine10

■ Results show that CELOX™ significantly improved hemorrhage control and survival10
■ Rall et al. report that in independent invivo test conducted, subjects treated with CELOX™ (OMNI-STAT®) Gauze demonstrated the highest rate of observed survival with 90% when compared to the current standard of care, Combat Gauze demonstrated a 60% survival rate. CELOX™ (OMNI-STAT®) also has a substantial history of use on the battlefield (with conventional and special forces) and has repeatedly proven itself in austere settings11
Vascular Access
■ Eason et al. found that CELOXTM Vascular resulted in 100% hemostasis following 5 minutes compression. It performed considerably better than both the standard gauze negative control (57% n=7) and the D-Stat Dry positive control (67% n=9)12
■ Johnson et al. assessed CELOXTM in two in vivo wound models, a lethal wound model of arterial bleeding in 6 subjects and a vascular closure site. Results showed 100% survivability and 0 occurrence of re-bleed. CELOXTM was effective in both stopping the major arterial bleeding and in sealing the simulated vascular closure site13
Wound Care
■ Snyder et al. report that OMNI-STAT® statistically significantly reduced time to hemostasis vs standard gauze. Mean time to hemostasis for OMNI-STAT® was 1min 19sec. The quality of the granulation tissue of the wound after 1 week was significantly improved (90%) and pain scores evaluated showed virtually no pain upon application or removal4
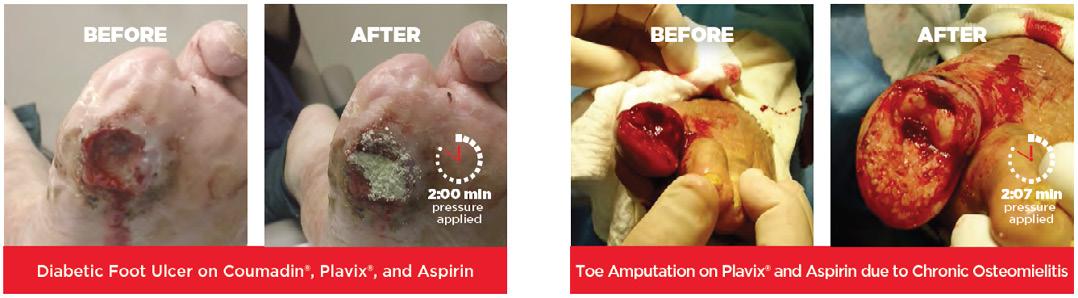
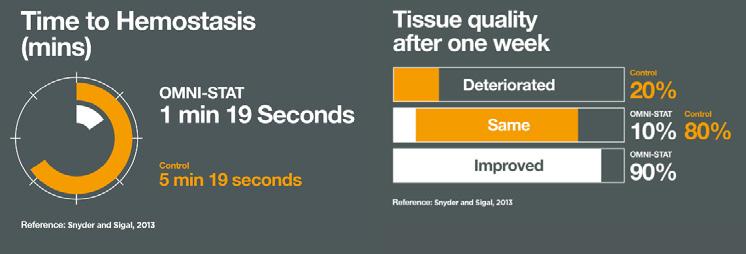
■ A prospective evaluation of OMNI-STAT® was conducted in the OR (52 patients) to compare the effectiveness of OMNI-STAT® Granules vs the retrospective use of electrocautery (89 patients) for bleeding control in patients undergoing surgical wound debridement14
■ OMNI-STAT® was shown to be as effective as electrocautery in achieving hemostasis. Significant time savings in the OR was also found, reducing mean total OR time by 19.1%. The results also showed that wounds treated with OMNI-STAT® demonstrated a more advanced stage of healing, which may be a result of the lack of tissue damage seen relative to electrocautery. The study concluded that the improved OR times may translate into increased cost effectiveness, relative to electrocautery, by increasing the number of surgical cases per day and/or using resources more effectively to treat a greater number of patients. It may also allow for bleeding control in an outpatient clinic or bedside, which could free up OR time and enable more effective management of healthcare resources14

OMNI-STAT® Masterclass
30 Wound Masterclass - Vol 1 - December 2022
Topical External Hemostat:
GUIDES
Figure 3: OMNI-STAT® : Time to Hemostasis.
Figure 4: OMNI-STAT® Case images.
Key
“...the
“Use of a chitosan-based hemostatic agent was able to achieve hemostasis in patients on anticoagulant therapy who required aggressive surgical debridement, including debridement of bone, and in two patients who had an elevated or near critical INR level.” 16
References
1. MT-18-240 Omni-stat Clinical and Scientific Product Monograph Dec 2018.
2. OMNI-STAT® MT-21-110. July 2021
3. OMNI-STAT omni-stat.com. 2023. Accessed 10-01-2023.

4. Snyder RJ, Sigal BD.The importance of hemostasis in chronic wound care: an open-label controlled clinical study of OMNI-STAT (chitosan)versus standard of care in post-debridement treatment of patients with chronic wounds with or without concomitant use of anticoagulants.WoundCareHyperbOxygen2013;4(2):9-16
5. MT-20-310 Allergenicity Potential of CELOX™.OMNI-STAT Hemostatic Devices Position Statement.
6. In Vitro and In Vivo Data on file at Omni-stat Medical Inc.
7. OMNI-STAT and CELOX™ (product packaging).
8. Millner R, Lockhart AS, Marr R.Chitosan arrests bleeding in major hepatic injuries with clotting dysfunction: an in vivo experimental study in a model of hepatic injury in thepresenceofmoderatesystemicheparinisation.AnnRCollSurgEngl2010;92(7):559-561
9. Snyder RJ, Sigal BD. Evaluation of hemostatic gauze versus standard of care for the treatment of chronic wounds in the presence of anticoagulants. Presented as a poster at SAWC 2013
10. Kozen BG, Kircher SJ, Henao J, Godinez FS, Johnson AS. An alternative hemostatic dressing: comparison of CELOX, HemCon, and QuikClot. Acad Emerg Med. 2008 Jan;15(1):74-81. doi: 10.1111/j.1553-2712.2007.00009.x. PMID: 18211317.
11. Rall JM, Cox JM, Songer AG, Cestero RF, Ross JD. Comparison of novel hemostatic dressings with QuikClot combat gauze in a standardized swine model of uncontrolled hemorrhage. J Trauma Acute Care Surg. 2013 Aug;75(2 Suppl 2):S150-6. doi: 10.1097/TA.0b013e318299d909. PMID: 23883900.
12. Reduced Application Time with a Rapid Packing Gauze Hemostat. Hoggarth A, Hardy C, Eason G, Marsden C. ATACCC, FL 2011.
13. Burgert JM, Gegel BT, Austin R 3rd, Davila A, Deeds J, Hodges L, Hover A, Lockhart C, Roy J, Simpson G, Weaver S, Wolfe W, Johnson D. Effects of arterial blood pressure on rebleeding using CELOX™ and TraumaDEX in a porcine model of lethal femoral injury. AANA J. 2010 Jun;78(3):230-6. PMID: 20572410.
14. Thibodeaux, KT, Speyrer, MS, Thibodeaux, RP, Rogers, AA, Rippon, MG. Management of postoperative bleeding in surgically debrided wounds: topical Hemostat versus electrocautery. Journal of Wound Care 2020; 29(8): 444-451.
15. Millner R, Lockhart AS, Marr R. Chitosan arrests bleeding in major hepatic injuries with clotting dysfunction: an in vivo experimental study in a model of hepatic injury in the presence of moderate systemic heparinisation. Ann R Coll Surg Engl. 2010 Oct;92(7):559-61. doi: 10.1308/003588410X12699663903593. Epub 2010 Jun 1. PMID:
Useful
20522310; PMCID: PMC3229344. 16. Lee, A. DFCon 2021.Hemostasis in Limb Salvage Surgery Achieved with a Chitosan Based Hemostatic Agent in an Anticoagulated Population with Chronic Wounds. 2021. 17. Cannon, J. (2018, January 25). Hemorrhagic Shock. The New England Journal of Medicine, 378, 370-9 Sponsored by OMNI-STAT®. All production resources provided by OMNI-STAT®. MTO.23.100 Masterclass Guides: Wound Masterclass. Chitosan Based Hemostat: OMNI-STAT® Volume 1. No 3. December 2022
Points
Fast, safe, easy to use and effective ■ Proven to control bleeding on a broad variety of wounds
Controls bleeding even in the presence of common anticoagulants ■ Shows many of the characteristics of an ideal hemostat ■ Cost-effective4,13,15
■
■
Links
to Cite this Article Masterclass GUIDES Wound Masterclass - Vol 1 - December 2022 31
your device to scan this QR code for more information about OMNI-STAT® Visit the OMNI-STAT® website Topical External Hemostat: OMNI-STAT®
How
Use
clots
an extra margin of safety in the presence of elevated
13
are stronger compared to clots formed in the control group and may provide
blood pressures.”


For more information contact info@omni-stat.com visit www.omni-stat.com 1. In Vitro and In Vivo Data on file at Omni-stat Medical Inc. 2. Millner R, Lockhart AS, Marr R.Chitosan arrests bleeding in major hepatic injuries with clotting dysfunction: an in vivo experimental study in a model of hepatic injury in the presence of moderate systemic heparinisation.Ann R Coll Surg Engl 2010; 92(7):559-561 3. Snyder RJ, Sigal BD.The importance of hemostasis in chronic wound care: an open-label controlled clinical study of OMNI-STAT (chitosan) versus standard of care in post-debridement treatment of patients with chronic wounds with or without concomitant use of anticoagulants.Wound Care Hyperb Oxygen 2013; 4(2):9-16 MTO.21.110 STOP THE BLEEDING START THE HEALING Rapid bleeding control for surgical and non surgical wounds A fast acting, safe and easy to use temporary topical external hemostat Controls minor, moderate and severe bleeding1 Stops bleeding in as little as 1 minute1 Effective even in the presence of common anticoagulants and clotting dysfunction2 Does not damage healthy tissue3 R Powered by Celox™ Technology
 Mr Frank Aviles
Wound Care Clinical Coordinator, Natchitoches Regional Medical Center
Natchitoches LA, United States
Mr Frank Aviles
Wound Care Clinical Coordinator, Natchitoches Regional Medical Center
Natchitoches LA, United States
Closing the Gap of Health Disparity in the Wound Care Industry With the Use Long Wave Infrared Thermography in Dark Skin Individuals
Editorial Summary
In healthcare, skin color presents its own set of challenges where inequity is evident. Studies have indicated that black and brown people are twice as likely to die from diabetes than white people,3 black and brown people account for 12.1% of people with diabetes, compared to 7.4% of non-hispanic white people,4 darker pigment skin have a higher occurrence of pressure injuries (PI) 5,6,75.5% of black men and 75.7% of black women developed hypertension by the age of 55 compared to 54.5% of white men and 40% of white women,7 and blacks had 1.5 to 2 times higher risk of hypertension.7 This article outlines how these issues can be addressed.
Introduction
Health disparity in the United States has been defined as differences that exist among specific population groups in the attainment of full health potential that can be measured by differences in incidence, prevalence, mortality, burden of disease, and other adverse health conditions.1 These disparities can negatively impact outcomes on a specific group of people creating a downward spiral in their health, their quality of life and burden the health system affecting future generations.
In healthcare, skin color presents its own set of challenges where inequity is evident. Studies have indicated that black and brown people are twice as likely to die from diabetes than white people,3 black and brown people account for 12.1% of people with diabetes, compared to 7.4% of non-hispanic white people,4 darker pigment skin have a higher occurrence of pressure injuries (PI),5,6 75.5% of black men and 75.7% of black women developed hypertension by the age of 55 compared to 54.5% of white men and 40% of white women,7 and blacks had 1.5 to 2 times higher risk of hypertension.7

In the wound care field, especially in the outpatient clinics, our chronic wounds are typically stuck in the inflammatory phase and our aim is to progress them along the healing cascade. While in the inpatient settings, patients are at risk of developing pressure injuries, admitted for management of a diabetic foot ulcer infection, and surgical site infections or dehiscence. The common denominator in both settings is the fact that the
wound/ periwound tissue may be in the proverbial prolonged inflammatory stage.
Skin has been an indicator of our health. As healthcare workers, we assess each individual patient’s skin to unfold the untold story. Do they have scars to determine history of prior wounds and/ or surgeries, any healed or unhealed areas over bony prominences, visual and tactile changes assessing signs of inflammatory abnormalities, eliciting a blanchable response, presence of callouses, and diabetic foot ulcer complications. These signs can be helpful in preventative and treatment programs.
A key successful element to any pressure injury prevention program is a skin assessment. The National Pressure Injury Advisory Panel (NPIAP) recommends that an assessment should include noting localized heat, edema or induration. The visual skin assessment may be easier to observe on a light pigmented individual, but it becomes a challenge and a disadvantage on darker pigmented skin. Baumgarten et al.8 and then Rosen et al.9 found darker pigmented residents were more likely to have a grade 2 - 4 ulcers than lighter counterparts suggesting failure to identify early signs based on skin pigmentation.
In 1995, the National Pressure Ulcer Advisory Panel (NPUAP. Currently known as the National Pressure Injury Advisory Panel –NPIAP) developed a task force to determine the best method to detect early signs of pressure damage on dark pigmented skin.10,11
34 Wound Masterclass - Vol 1 - Month 2022
© Copyright. Wound Masterclass. 2023
“We can harness the power of technology to help improve our skin assessment. Long wave infrared thermography (LWIT) is a powerful assessment tool to aid our visual assessments where skin pigmentation is not a factor.”
Their recommendations were as follows: Then in 2016, the NPIAP stated that pain and temperature changes often precede skin color changes in deep tissue injury (DTPI) and that discoloration could appear differently in darker pigmented skin.12 The NPIAP clinical practice guideline recommends considering skin temperature as an adjunct assessment strategy for patients with darker pigment skin.13
• The color of intact dark pigmented skin may remain unchanged (does not blanch) when pressure is applied over a bony prominence
• Localized skin color changes can occur where pressure is applied. These changes may differ from the individual’s usual skin color.
• Local areas of intact skin subject to pressure may feel either warm or cool when touched. This assessment should be performed without gloves to make it easier to distinguish differences in temperature. It is important to clean the skin of any body fluids before this direct contact.
• If patients have had a previous pressure ulcer, the healed area may be lighter in color.
• Areas of skin subjected to pressure may be purplish/ bluish/violet in color. This can be compared with the erythema seen in people with lighter skin tones.
• Edema may occur with an induration (area of skin hardness) more than 15mm in diameter. The skin may be taut and shiny.
• Patients may complain of or indicate current or recent pain or discomfort at body sites where pressure has been applied.
The utilization of specialized cameras to determine patients at risk of deep tissue injuries, surgical dehiscence, high levels of inflammation disturbing the wound healing progression, infection, and levels of hypoperfusion continues to benefit patients especially darker pigmented patients. Our visual assessments when looking for deep tissue injuries and the classic signs of inflammation are difficult to assess on our patients with a higher level of melanin.
Sprigle et al. utilized technology to identify early skin changes on darker pigmented patients with a 90% accurate rate for identifying erythema for light and dark pigmented volunteers.14
We can harness the power of technology to help improve our skin assessment. Long wave infrared thermography (LWIT) is a powerful assessment tool to aid our visual assessments where skin pigmentation is not a factor. LWIT does not discriminate skin color, and it is useful in pressure injury preventative programs. This author has utilized this technology for approximately the past 36 months and has found many applications such as in a preventative fashion, assessment assistance, intervention selection, assessing interventions, just to name a few. I will highlight this technology in the following case studies to understand the usefulness of this technology.
LWIT’s specialized cameras capture the amount of radiation (thermal energy) that a body is emitting in the nonvisible range of the electromagnetic spectrum. This thermal energy is not viewed by the human eye and
Closing the Gap of Health Disparity in the Wound Care Industry Wound Masterclass - Vol 1 - Month 2022 35
© Copyright. Wound Masterclass. 2023
and once captured, it is translated into a relative temperature pattern viewed on a device. The viewed pattern is not related to core temperature but rather about the thermal energy being produced due to an abnormal metabolic or physiological process occurring in the site being inspected. An increase of thermal energy or increased temperature indicates an inflammatory response, an infectious process, or results from increased circulation to the area. A decrease in thermal energy or decrease in relative temperature could indicate hypoperfusion, edema, or an ischemic response. Below you will find images to describe normal and abnormal responses on both light and dark skin pigmented individuals. After seeing the images below, one will note how this technology can be our assistance in various applications especially when assessing dark pigmented skin. I do admit that more research is needed, but seeing is truly believing.
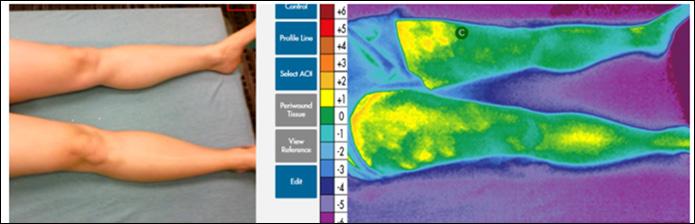
LWIT Images
The device used is the Scout by WoundVision (Indianapolis, In) which offers a digital camera to capture the picture of the area in question and an infrared camera capturing thermal images that are at the invisible end of the electromagnetic spectrum. The software system converts the image into a color scale of relative degrees Celsius found in between the picture and thermal image. Temperatures above 0 degrees indicate an increased thermal response whereas below 0 degrees indicates a decreased thermal response.
1a: Normal thermal image on light skin.
1b: Abnormal thermal image on light skin (note the presence of periwound redness on picture and infection).
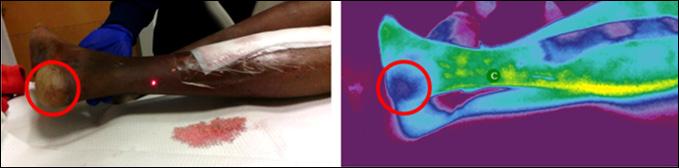
1c: Normal thermal image on dark pigmented skin.
1d: Deep Tissue Injury on dark pigmented skin.
Institutionalized elderly patient at risk for pressure injuries was imaged with LWIT device. Image review revealed no abnormal thermal findings-within normal limits.



Closing the Gap of Health Disparity in the Wound Care Industry 36 Wound Masterclass - Vol 1 - Month 2022
“A decrease in thermal energy or decrease in relative temperature could indicate hypoperfusion, edema, or an ischemic response.”
Figure 1:
Case 1: Normal Visual and Thermographic Images
© Copyright. Wound Masterclass. 2023 1a 1b 1c 1d
Case
49-year old patient with type 1 Diabetes Mellitus (DM), a hard-to-heal wound, LWIT showed increased thermal energy/ pattern which led to additional testing including a positive culture biopsy which resulted in an atypical vascular ulcer diagnosis.

Case
78-year-old patient with non-healing leg wound, history of type 2 DM, end stage renal disease (ESRD), and PAD referred for revascularization. LWIT showed increased thermal energy/ pattern in combination with clinical and objective testing led to a diagnosis of PG with subsequent prednisone therapy.
57-year-old patient with Type 2 DM, nonhealing foot wound complicated by infection. LWIT showed increased thermal energy/ pattern resulting in additional diagnostic tests which revealed gas gangrene.



Patient imaged one-week post-prednisone treatment showed a decrease in thermal energy/ pattern.
Case
45-year-old patient with a dehisced abdominal wound that became infected. Readmitted to hospital for Incision & Drainage (I & D) and antibiotics. LWIT demonstrated increased thermal energy/ pattern. Extensive induration palpated.
Patient reimaged 6 days later noting a decrease in thermal pattern. Induration decreased noted by palpation.



Beyond Prediction
This technology continues to prove beneficial in multiple areas in addition to detecting patterns of early inflammation/infection and pressure injury prevention on dark pigmented skin. There are various applications in both the inpatient and outpatient settings but since this is beyond the scope of this article, be in the lookout for upcoming articles as well as published research using this device.
Closing the Gap of Health Disparity in the Wound Care Industry Wound Masterclass - Vol 1 - Month 2022 37
2: Atypical and Infection
Case 3: Gas Gangrene
6: Pyoderma Gangrenosum (PG)
Case 5: Infection Post-Amputation
7: Infection and Dehisced Incision
© Copyright. Wound Masterclass. 2023
Figure 2:
2a: This is a patient with an autoimmune condition on IV antibiotics for an infected wound on medial mid distal leg to ankle. Appreciate the absence of visual signs of inflammation on the picture and the intense level of inflammation on the thermal image. This image led to the selection of immediate advanced treatments to control the level of inflammation/ infection. (Right great toe does not have redness but was painted with betadine at time of picture).

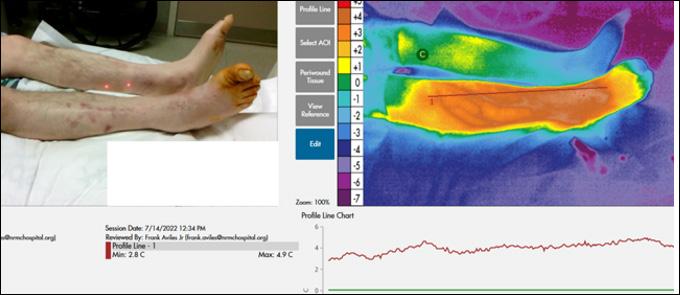
2b: Serial image at 4 weeks with significant resolution of abnormal physiological inflammation.
Conclusion
LWIT is proving useful in preventative programs to detect early signs of skin damage, providing us with real time point of care objective assessment validation for treatment decision making, intervention validation, earlier recognition of inflammatory/ infectious conditions, decrease overutilization of antibiotics, amongst other applications. It is a powerful objective tool allowing us to advocate for patients, especially ones with darker skin.
References
1. NIH (National Institutes of Health). Health disparities. 2014. [November 2, 2016]. http:// www.nhlbi.nih.gov/health/educational/healthdisp.
2. 3. U.S. Department of Health and Human Services Office of Minority Health. Diabetes and African Americans.
4.
5.
6.
7. Thomas S J et al. Cumulative Incidence of Hypertension by 55 Years of Age in Blacks and Whites: The CARDIA Study. J Am Heart Assoc. 2018;7:e007988.
8. Baumgarten M et al (2004) Black/white differences in pressure ulcer incidence in nursing home residents. Journal of the American GeriatricsSociety; 52: 8, 1293-8.
9. Rosen J et al (2006) Pressure ulcer prevention in black and white nursing home residents: A QI initiative of enhanced ability, incentives and management feedback. Advances in Skin and Wound Care; 19: 5, 262-8.

10. Bennett MA (1995) Report of the task force on the implications for darkly pigmented intact skin in the prediction and prevention of pressure ulcers. Advances in Wound Care; 8:
6, 34-35.
11. Clark M (2010) Skin assessment in dark pigmented skin: a challenge in pressure ulcer prevention. Nursing Times; 106: 30, early online publication

12. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel. NPUAP Announces a change in terminology from pressure ulcer to pressure injury and updates the stages of pressure injury. 2016. Available online: www.woundsource.com/blog/nationalpressure-ulcer-advisory-panel-npuap-announces-change-in-terminology-pressure-ulcer
13. Kottner J, Cuddigan J, Carville K, Haesler E. A closer look at the 2019 international guideline on the prevention and treatment of pressure ulcers/injuries. J Tissue Viability. 2020;29(4):225–6.
14. Sprigle S et al (2009) Detection of skin erythema in darkly pigmented skin using multispectral images. Advances in Skin and Wound Care; 22: 4, 172-79.
Closing the Gap of Health Disparity in the Wound Care Industry 38 Wound Masterclass - Vol 1 - December 2022
Have an innovative product in the field of wound care? Feature it in our commercial@woundmasterclass.com Masterclass GUIDES © Copyright. Wound Masterclass. 2023
 Dr Windy Cole College of Podiatric Medicine, Kent State University
Dr Windy Cole College of Podiatric Medicine, Kent State University

Kent OH, United States
Mixed Aetiology Lower Extremity Ulcers: The Role of Omega-3 Products
Editorial Summary
total wound area reduction in subjects treated with Omeza® Combination Therapy (OCT) and tracked bacterial burden via fluorescence images and PCR cultures throughout the 12-week treatment period. This is a single-site, open label case series. 3 subjects >18 years and having open wounds of the lower extremity were enrolled. This case series included one diabetic foot ulcer and two venous leg ulcers. The mean baseline wound age was 24 weeks with a mean baseline wound size of 8.61 cm2. The mean wound area reduction of all wounds combined was 82%. The two VLUs healed during the study period. The TWAR of the DFU was 53% at 6-weeks when the patient was lost to follow-up due to a geographic relocation. Fluorescence imaging showed clearance of pathologic levels of bacterial contamination over the course of the study for all subjects. The use of OCT proved to be effective and safe. The wounds included in this study were stalled in the inflammatory stage with no signs of healing. OCT supported wound healing and reduced bacterial loads in this patient cohort.
Introduction
Wound healing is optimized by utilizing therapies that control bacterial burden, decrease tissue inflammation, support the extracellular matrix and establish a balanced healing environment. The Omeza® products are formulated to combat the underlying pathophysiologies contributing to wound chronicity. All products contain an anhydrous formulation of Omega-3, 6 and 9 oils designed to deliver nutrients for balanced wound healing.
Objective
This pilot study evaluated the total wound area reduction in subjects treated with the Omeza® products and tracked bacterial burden via fluorescence images and PCR cultures throughout the 12-week treatment period.
Methods
This is a single-site, open label, case series. 3 subjects >18 years and having open wounds of the lower extremity were enrolled. All subjects signed the informed consent and successfully completed the screening visit prior to entering the study. Fluorescence and standard wound photographs and measurement were obtained weekly via a fluorescence imaging device. PCR wound cultures were obtained per protocol. Wound debridement was performed per discretion of the investigator followed by application of Omeza® products used in combination therapy plus SOC dressings. Patients were seen weekly throughout the course of the study.
• This case series included one diabetic foot ulcer and two venous leg ulcers
• The mean baseline wound age was 24 weeks with a mean baseline wound size of 8.61 cm2
• The mean wound area reduction of all wounds combined was 82%
• The two VLUs healed during the study period
• The TWAR of the DFU was 53% at 6-weeks when the patient was lost to follow-up due to a geographic relocation
• Fluorescence imaging showed clearance of pathologic levels of bacterial contamination over the course of the study for all subjects
• Apply Omeza® Lidocaine Lavage to periwound, wait 5 minutes
• Perform appropriate sharp debridement as indicated
• Apply additional Omeza® Lidocaine Lavage to periwound
• Apply Omeza® Collagen Matrix
• Apply Omeza® Skin Protectant to periwound
• Apply absorbent non-adhesive foam dressing
40 Wound
- Vol 1 - December 2022
Masterclass
Wound healing is optimized by utilizing therapies that control bacterial burden, decrease tissue inflammation, support the extracellular matrix, and establish a balanced healing environment. This pilot study evaluated the
© Copyright. Wound Masterclass. 2023
Results Protocol
Conclusion
The use of Omeza® products in combination proved to be effective and safe. The wounds included in this study were stalled in the inflammatory stage with no signs of healing. The Omeza® products supported wound healing and reduced bacterial loads in this patient cohort.
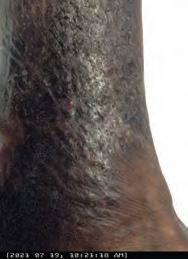

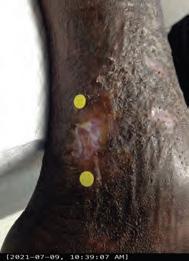


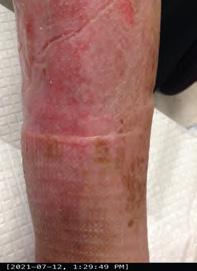


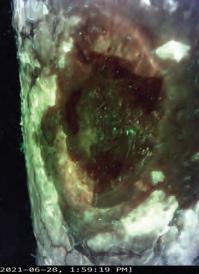

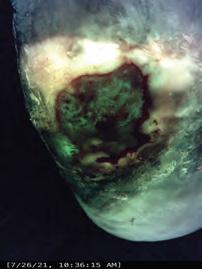



References 1. Frykberg R, Banks J. Challenges in the Treatment of Chronic Wounds. Adv Wound Care. 2015 Sep1: 4(9):560-582. 2. Calder PC Nutrients. 2010 Mar; 2(3): 355–374. 3. Omega-3 Fatty Acid and Inflammatory Process. Published online 2010 Mar 18. doi: 10.3390/ nu2030355 4. Innis SM. Brain Res. Dietary omega-3 fatty acids and the developing brain. 2008 Oct 27;1237:35-43. doi: 10.1016/j.brainres.2008.08.078. Epub 2008 Sep 9 5. Stark A, Crawford M, Reifen R. Nutr Rev. Update on alpha-lineoleic acid. 2008 Jun;66(6):32632. doi: 10.1111/j.1753-4887.2008.00040.x.
of Omega 3 Products, Correlating Biomarkers, and
of Bacterial Contamination
a Novel Violet-Light Camera Wound Masterclass - Vol 1 - December 2022 41
The Role
Evaluation
Using
Subject 1, Visit 1: Post Debridement Subject 1, Visit 6: Post Debridement Subject 2: Visit 1: Post Debridement Subject 2, Visit 2: Pre Debridement Subject 2, Visit 3: Healed Subject 3, Visit 1: Post Debridement Subject 3, Visit 2: Pre Debridement Subject 3, Visit 2: Healed Subject 3, Visit 2: Post Debridement © Copyright. Wound Masterclass. 2023
Omeza® Treatments: Anhydrous Omega Therapies Masterclass GUIDES
Introduction
This Masterclass Guide is a concise overview aimed at exploring the use of Omeza® treatments and incorporating this into your clinical practice.
Wound healing is optimized by utilizing therapies that control bacterial burden, decrease tissue inflammation, support the extracellular matrix and establish a balanced healing environment.
Omeza® products are oil-based and contain no water (anhydrous). They were created to address the pathophysiologies associated with the development of chronic wounds.
What are Omeza® Treatments?
■ All Omeza® products contain an anhydrous formulation of Omega-3, 6 and 9 oils designed to deliver nutrients for balanced wound healing
■ The Omeza® Lidocaine Lavage is a topical analgesic periwound prep designed to help manage debris and biofilm and reduce irritation
■ The Omeza® Collagen Matrix is a drug-device combination product comprised of hydrolyzed fish collagen, infused with cod liver oil and other plant derived oils

■ The Omeza® Skin Protectant is designed to help strengthen fragile skin, reduce inflammation, and promote remodeling when used daily on intact skin
Keywords
■ Wound healing
■ Bacterial contamination
■ Diabetic foot ulcer
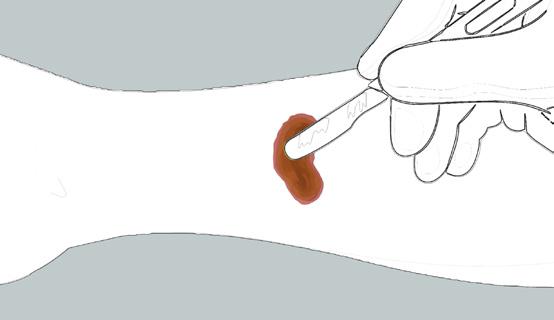
■ Venous leg ulcer
■ Omega fatty acids
■ Anti Inflammatory
■ Wound
■ Pressure Ulcer
■ Periwound
■ Anti-inflammatory
How Omeza® Treatments Work:
■ A drug-delivery device that delivers hydrolyzed fish collagen infused with cod liver oil, as well as other nutrients into the wound bed
■ When applied to a warm wound bed it softens, migrating into tunnels and crevices and conforming to the irregular wound topography
■ It is designed to promote the natural wound healing process via a bio-compatible structure that is naturally incorporated into the wound bed
• Always handle Omeza® Collagen Matrix using aseptic techniques
• Only content is sterile
• Prior to application of Omeza® Collagen Matrix, prepare the wound bed using standard methods to ensure wound is free of debris and necrotic tissue
• Snap or cut open the tip of the Omeza® Collagen Matrix vial
• Slowly squeeze vial to apply directly to wound bed in stripes, or onto a suitable applicator (e.g. a tongue depressor)
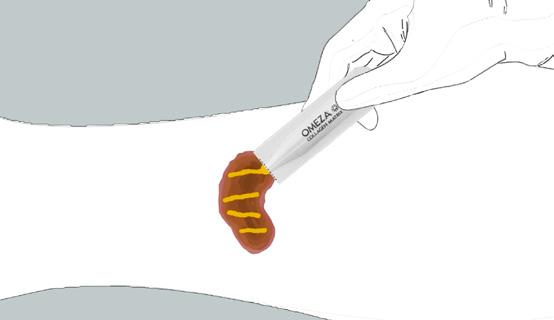
• After application, cover with the appropriate dressing to maintain matrix adherence and protect the wound area
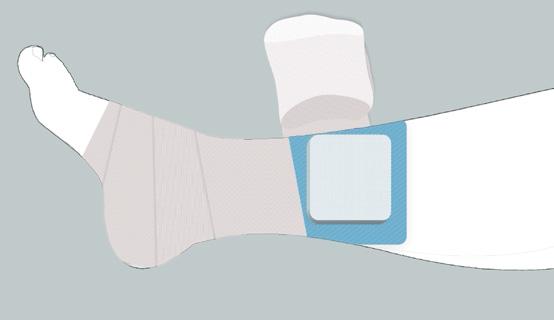
• The optimum dressing is determined by wound location, size, depth, and volume of exudate
Reduces pain and irritation
■ Low dose of rapidly absorbing lidocaine reduces pain and irritation to aid in patient experience
■ Anhydrous formula designed to help inhibit growth of microorganisms
■ Peri wound prep oil rich in omegas known to decrease inflammation
Strengthen and protect skin
■ Designed to improve skin elasticity and turgor of vulnerable skin
■ Soothing gel formula rich in omegas known to decrease inflammation
■ Designed to provide a low perfusion pulse during each application
42 Wound Masterclass - Vol 1 - December 2022
Omeza® Lidocaine Lavage Omeza® Skin Protectant
Omeza® Collagen Matrix
Application of Omeza® Collagen Matrix
Omeza® Treatments: Anhydrous Omega Therapies Masterclass GUIDES
Wounds That Are Suitable (Omeza® Collagen Matrix)
■ Partial, full-thickness wounds

■ Pressure ulcers
■ Venous ulcers






■ Diabetic ulcers
■ Chronic vascular ulcers
Wounds That Are Not Suitable (Omeza® Collagen Matrix)






■ Undermined wounds
■ Surgical wounds
■ Trauma wounds
■ Draining wounds
■ Known sensitivity to fish collagen or cod liver oil
■ Third degree burns


Scope of Usage
A case series to evaluate Omeza® products in combination therapy for the treatment of lower extremity ulcers of mixed etiologies exhibiting bacterial contamination as determined by a novel violet-light camera system and correlating biomarkers. Fluorescence images were acquired to assess the presence of bacteria at moderateto-heavy loads. Fluorescence imaging was implemented into the assessment to determine appropriate debridement level and location based on bacterial fluorescence signals.
Wound Masterclass - Vol 1 - December 2022 43
Patient 1, Visit 1: Post Debridement Patient 1, Visit 6: Post Debridement Patient 2: Visit 1: Post Debridement Patient 2, Visit 2: Pre Debridement Patient 2,
3: Healed Patient 3, Visit 1: Post Debridement Patient 3, Visit 2: Healed
Visit
LOWER EXTREMITY ULCERATION
3 Subject 3, Visit 2: Pre Debridement Subject 3, Visit 2: Post Debridement
Figure
Omeza® Treatments: Anhydrous Omega Therapies
Evidence
The challenge of hard-to-heal wounds are a significant burden on the patient and the healthcare system. Omega-based wound treatments offer a treatment option for the management of partial, full-thickness wounds; pressure ulcers, venous ulcers; diabetic ulcers, chronic vascular ulcers; undermined wounds, surgical wounds; trauma wounds, and draining wounds.
A summary of the evidence of this innovative wound therapy is below.
■ A pilot study was conducted by Seo et al., into the Antibacterial Activity of Topical Fatty Acids
■ An ex vivo porcine skin explant model was used to establish a functional S. aureus biofilm for 5 days. The wounds were then treated with either an oil or with moistened gauze. The wounds were re-treated daily with either oil or more saline. Six explants per day from each group were harvested and viable total bioburden and biofilm were assessed
■ After the initial results, the experiment was run again with the added step of first soaking the explant in phosphate buffered saline with 0.05% Tween® 20 in order to see if the oil was masking the bacteria
■ At Day 0, the average bioburden was 4.8 log10 CFU/ml with 3.64 log10 CFU/ml of biofilm. After Day 1, total bacterial levels in the oil treated sample were reduced to 1.8 log10 CFU/ml while the control increased to 9.0 log10 CFU/ml. The levels of the oil-treated samples remained low and the control remained high every day thereafter
■ The results show the potential for a nutritive pro-healing oil to also have an antibacterial activity1
■ A 54-year-old male presented with a five-month history of Venous Leg Ulcer (VLU) to the left lower extremity

■ An omega-based protocol focused on wound and periwound preparation and care (protocol listed) performed once weekly
■ Pain and necessity for narcotic analgesics rapidly resolved.
■ Wound resolution was achieved at 11 weeks
■ The use of an omega-based complete care protocol resulted in expedited resolution of a chronic venous leg ulceration in a patient that had failed other advanced treatment modalities5,6,7,8,9,10,11,12
■ This patient (Figure 4) presented to the wound clinic with a hard-to-heal ulcer which has been present for four months, prior to commencing Omeza® therapy. The patient has a diabetic foot ulcer and Charcot foot arthropathy


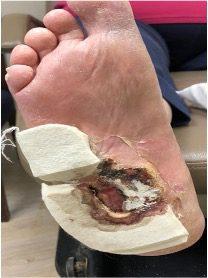
Charcot foot (Charcot arthropathy) is a serious condition affecting diabetic foot patients. Diabetic Charcot foot occurs in people who have neuropathy and an injury occurs which causes the bone to fracture or dislocate in the foot. Often the injury occurs without the knowledge of the patient. The symptoms that occur include swelling, heat, and occasionally some redness to the foot. Some patients experience some pain while some patients experience no pain at all.
■ At commencement of Omeza® therapy his ulcer measured 3.0cm x 1.0cm and 0.2cm. Within 8 weeks of weekly therapy with Omeza® products he was completely healed
Biofilm Study
Venous Leg Ulcer Study
Diabetic Foot Ulcer with Charcot Foot
Masterclass
44 Wound Masterclass - Vol 1 - December 2022
GUIDES
Figure 4a: Commencing Omeza® treatment.
Figure 4b: Week 2 of Omeza® treatment.
Figure 4c: Week 6 of Omeza® treatment.
Figure 4d: Week 8 of Omeza® treatment.
Omeza® Treatments: Anhydrous Omega Therapies
Key Points
■ No preparation needed
■ Easy to apply - snap and squeeze
■ Readily available for patients
■ Easy to store
■ Increases practice efficiency
■ Off-the-shelf, single use vials
“After 37 years of wound care experience, having tried everything in a wound care center setting I can only suggest... drop what you are using and try Omeza. The results are astounding. The simplicity of application and alleviation of patient responsibility cannot be overstated. Recommend highly!”
Dr Gregory A Black, DPM Sylvania, OH Sylvania Podiatry
“I went into our case study looking to improve skin and wounds on long term, “non-healable” patients that reside in nursing homes my company serves. The response to the Omeza treatment protocol was greater than expected and healed 3 wounds that were present for greater than a year without having to change the behavior of the patient or the facility. I also introduced it to my clinic and some skeptical nurses are now demanding it for our tough stasis dermatitis patients.”
Carmen Hudson, MD, FACS, CWSP Medical Director – United Wound Healing
References
1. Seo S, Jeong S, Gibson DJ. A pilot study of the antibacterial activity of topical fatty acids. Poster presented at the Symposium on Advanced Wound Care Spring/Wound Healing Society meeting. San Antonio, TX. May 7–11, 2019.
2. Laboratory testing University of Florida. Data on file.
3. Independent clinical and consumer use testing performed by Princeton Consumer Research. Data on file.
4. Independent testing performed by Augustine Scientific. Data on file
5. Mostow EN, et al; OASIS Venus Ulcer Study Group. Effectiveness of an extracellular matrix graft (OASIS Wound Matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J Vasc Surg. 2005 May;41(5):837-43.
6. Raffetto JD, et al; Why Venous Leg Ulcers Have Difficulty Healing: Overview on Pathophysiology, ClinicaConsequences, and Treatment. J Clin Med. 2020 Dec 24;10(1):29.
7. Calistru AM, Baudrier T, Gonçalves L, Azevedo F. Thrombophilia in venous leg ulcers: a comparative study in early and later onset. Indian J Dermatol Venereol Leprol. 2012 May-Jun;78(3):406.
8. Zutt M, et al; Thrombophilia in patients with chronic venous leg ulcers-a study on patients with or without post-thrombotic syndrome. J Eur Acad Dermatol Venereol. 2011 Dec;25(12):1432-9.
9. Mackenzie RK, Ludlam CA, Ruckley CV, Allan PL, Burns P, Bradbury AW. The prevalence of thrombophilia in patients with chronic venous leg ulceration. J Vasc Surg. 2002 Apr;35(4):718-22.
10. Falanga V, Sabolinski M. A bilayered living skin construct (APLIGRAF) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen. 1999 Jul-Aug;7(4):201-7.
11. Jia YC, Qiu S, Xu J, Kang QL, Chai YM. Docosahexaenoic Acid Improves Diabetic Wound Healing in a Rat Model by Restoring Impaired Plasticity of Macrophage Progenitor Cells. Plast Reconstr Surg. 2020 May;145(5):942e-950e.
12. Babaei S, et al; Omegaven Improves Skin Morphometric Indices in Diabetic Rat Model Wound Healing. J Am Coll Clin Wound Spec. 2018 Apr 28;9(1-3):39-45
Useful Links
Use
QR code for more information about Omeza® treatments

How
Click here to request more information from
Masterclass Guide: Wound Masterclass. Omeza® Treatments: Anhydrous Omega Therapies. Volume 1. No 3. Dec 2022
Article Masterclass
Wound Masterclass - Vol 1 - December 2022 45
to Cite this
GUIDES
your device to scan this
Omeza®
Sponsored by Omeza®. All production resources provided by Omeza®
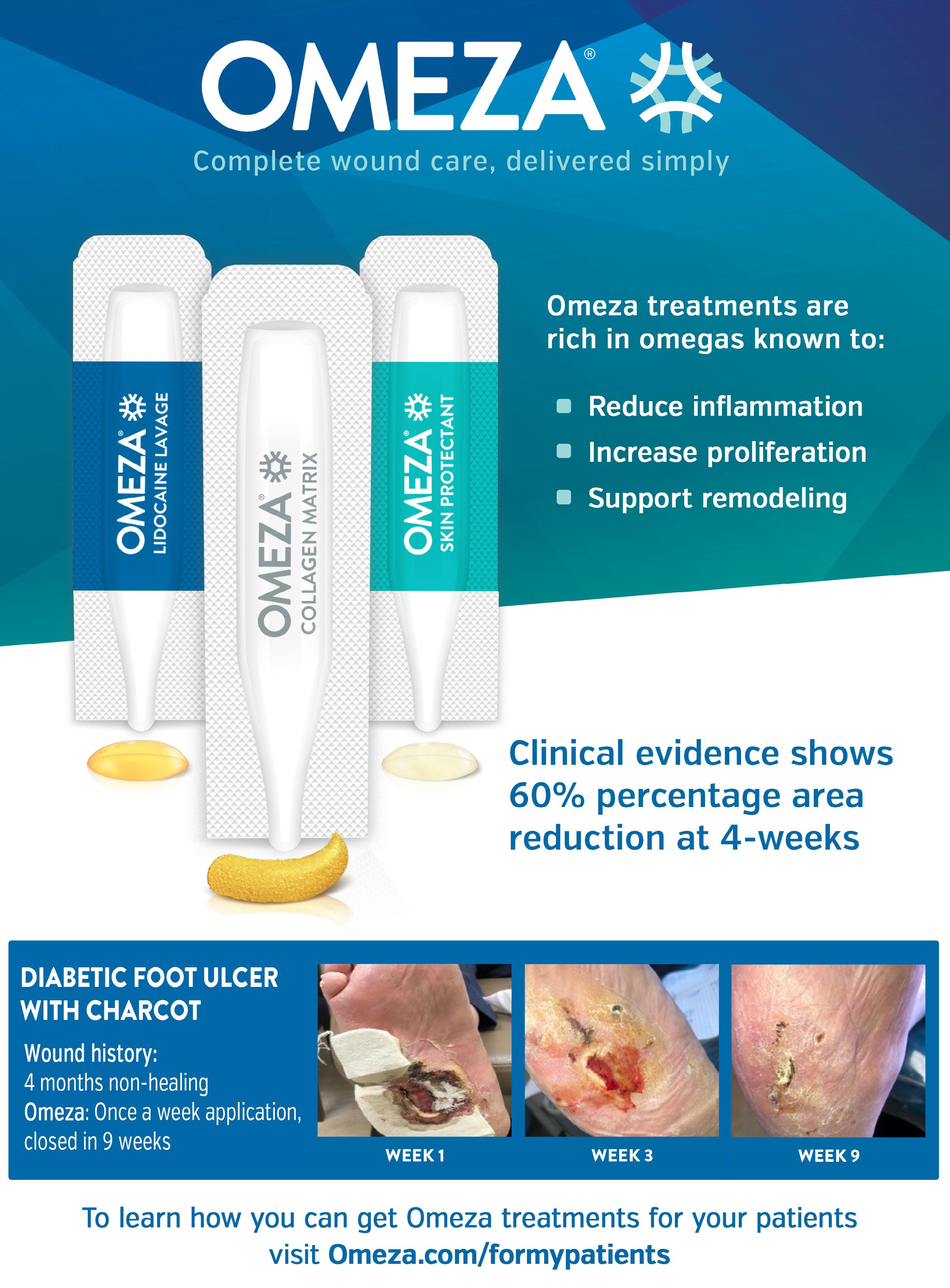



If you think... ...have a look at the VACOcast Diabetic Boot Contact us at sales@opedmedical.com or call (800) 334-1906. . ● to the innovative Vacuum Technology*1-5 ● Fast, safe & easy to apply ● Non-removable, but removable for dressings ● as TCC*2-4 *1 Biomechanics Study by BASiS Biomechanics Institute at Munich University J. Mitternacht et.al 08/09 1994 *2 *3 Evaluating a removable knee high cast walker within the diabetic foot pathway G Bowen P Spruce 2019 Diabetic Foot Journal 22(3): 52–9 *4 *5 Vacuum cushioned removable cast walkers reducing foot loading in patients with diabetes mellitus A Nagel D Rosenbaum; Gait & Posture Volume 30 Issue 1, July 2009, p11-15. opedmedical.com
Dr Anthony Tickner
Medical Director, Saint Vincent Hospital Wound Healing Center Vice President, Massachusetts Foot & Ankle Society President & CEO-Associated Foot Specialists, P.C. Westford MA, United States


Offloading: The Comprehensive Guide
Editorial Summary
implication. Early detection and active management can delay or even prevent the onset of adverse outcomes. Protocols reflect the need for regular foot evaluations in every diabetic patient should have foot evaluation at yearly intervals. The cornerstone of the management are a multidisciplinary approach to prevention, including prevention of infection, appropriate wound care and management of non-viable tissue, and pressure reduction. The Triad of peripheral neuropathy, foot deformity and minor trauma have been found as risk factors in majority of cases of diabetic foot ulcers. Offloading the affected foot is a pivotal management strategy dispersing pressure away from the affected part. This article provides a concise overview of a variety of offloading techniques and their pros and cons.
Introduction
In patients with diabetes, 12-25% develop a diabetic foot ulceration (DFU) in their lifetime. This is a leading cause of diabetesrelated hospitalization and non-traumatic lower extremity amputation. DFU’s may lead to an increase in global morbidity, mortality, healthcare costs from hospitalizations, surgical and nonsurgical interventions, and amputations. Excessive pressure contributes delayed healing in up to 94% of these ulcerations. Pressure offloading can arguably be the most important principle implemented in treating diabetic foot ulcerations.1,2,3,4,5
Offloading Around the World
There are many different offloading techniques used around the world. Although Total contact casting (TCC) is the gold standard for offloading, it requires resources that may not be available in various countries. Fortunately, physicians are innovative and resourceful when it comes to caring for their patients and alternatives have been found.
Bohler Iron Walking Cast
Dr Lee Rogers
Chief-Podiatric Services, UT Health San Antonio
President, American Board of Podiatric Medicine
Associate Editor, Journal of The American Podiatric Medical Association San Antonio TX, United States
In India, the Bohler iron walking cast has been discussed in recent literature as a more effective offloading method because the entire plantar surface is off the ground. In this method, a lower leg cast is applied followed by an iron rod with a plantar platform. The iron rod is then applied with a clamp proximally and secured with more casting material. A modification is also utilized with roads and a distally placed hinge, to allow for a posterior platform for heel ulcerations.6


Mandakini Offloading System
The Mandakini offloading system is also discussed in Indian literature as an effective and cost-effective offloading technique. First, a pair of gloves is rolled together. The number of gloves used is dependent on the patient size. The gloves are then placed in an adhesive dynoplast, which is then applied to the foot. For distal lesions, it is applied proximally, and for rearfoot lesions, it is applied distal to the lesion. The device is then secured with a dressing. This technique is recommended to be changed every week for 4-6 weeks and authors noted complete healing without patient complaints with over 2 years of regular uses of the technique.7

48 Wound
- Vol 1 - December 2022
Masterclass
Foot complications are one of the most serious and debilitating consequences of Diabetes Mellitus. With global predictions of the number of diabetic patients near doubling by 2030, this condition is likely to be at the forefront of our clinical caseload. Aside from the morbidity and global healthcare burden in a diabetic patient with foot ulcers and amputations there is a significant financial
Figure 1: Bohler Iron Walking Cast. 6
Figure 2: Mandakini Offloading System. 31
© Copyright. Wound Masterclass. 2023 Images available from open access article: Venkatakrishnan S, Zachariah K, et al. A Description of a Modified Bohler Iron Walking Cast in the Management of Plantar Ulcers. J Foot Ankle Surg (Asia-Pacific) 2019;6(1):10–12. Images available from open access article: Kari SV. The economical way to off-load diabetic foot ulcers [Mandakini off-loading device]. Indian Surg. 2010 Apr;72(2):133-4. doi: 10.1007/s12262-010-0042-3. Epub 2010 Jul 1. PMID: 23133224; PMCID: PMC3452512.
The Samadhan Offloading System
The Samadhan is another instant offloading technique discussed in Indian literature. It is described as easy to apply, affordable, and effective. In this technique, the foam is painted with an adhesive liquid and rolled into a cylinder. The cylinder is then cut to fit the size of the ulcer and is applied proximal to the ulcer. The cylinder is then secured with coban or other dressing materials. In oral abstracts, this technique allowed for 73% healing compared to 13% in footwear. This technique can also be used with the patient’s footwear, as demonstrated in the picture.8




The Suvidha Offloading System
For the Suvidha system, the Gamgee pad pillows are applied first. The Gamgee pad is made of foam and folded in the shape of a “pillow” with a 2.5cm thickness and 1cm wound overlap. Paper tape is then used to wrap the “pillow”. This is done 2-3 times to make individual offloading components. The pillows are then placed around the wound with overlapping of 2cm at each junction. This is then secured using paper tape. Elastic plaster is then used to secure the pillows with 5cm of overlap. An elastic bandage is then used for additional security. A small prospective study examined this technique and found a 2-week healing time for 1x1cm hallux ulcerations and 12 weeks for 4x4cm midfoot ulcerations. Patients had high satisfactions with this technique. Overall, authors concluded that the Suvidha offloading footwear is a cost effective, easily replicable, and efficient dressing requiring only the readily available dressing materials. The results were thought to be comparable with other methods of offloading practiced worldwide.9


Offloading: The Comprehensive Guide Wound Masterclass - Vol 1 - December 2022 49
“Overall, authors concluded that the Suvidha offloading footwear is a cost effective, easily replicable, and efficient dressing requiring only the readily available dressing materials.”
Figure 3: Samadhan Offloading System. 8
© Copyright. Wound Masterclass. 2023 Images available from open access article: Shankhdhar LK, Shankhdhar K, Shankhdhar U, Shankhdhar S (2016) Instant Offloading of a Diabetic Foot Ulcer. Clin Res Foot Ankle 4:207. doi: 10.4172/2329-910X.1000207. Images available from open access article: Bhat, Sunay & Vinoth, D.. (2020). Studying the impact of the cost-effective Suvidha off-loading dressing in healing neuropathic ulcers in diabetic foot: a case series of 83 cases from South India. International Surgery Journal. 7. 2371. 10.18203/2349-2902.isj20202851.
Figure 4: Suvidha Offloading System. 9
The Importance of Offloading
So, with limited materials, increased clinic time, and other considerations, why is offloading so important? Here, we can see what havoc can occur when offloading isn’t a part of patient care. New ulcerations can develop. These new ulcerations or chronic ulcerations are always at risk for infection and/or amputation. If offloading isn’t implemented with every ulceration care plan, our patients are at an increased risk for morbidity and mortality.

Offloading Consensus
The International Working Group (IWGDF) on the Diabetic Foot has published evidencebased guidelines on the prevention and management of diabetic foot disease since 1999. In 2019, the IWGDF published a list of guidelines specifically for the offloading of the active diabetic foot ulcer.11


First Line of Offloading
The Science Behind Offloading
The thought behind offloading is to relieve the abnormal stresses that the foot experiences from the loss of protective sensation and biomechanical malfunction. The combination of loss of protective sensation and elevated mechanical stress can lead to tissue damage. The mechanical stress is thought to be due to increased compressive, frictional, and shearing forces during the gait cycle, causing repetitive microtrauma. This is the mechanism that is thought to cause many diabetic foot ulcerations. So, the goal of offloading is to distribute pressure away from the area of ulceration or pre-ulceration.10
The first recommendation from the IWGDF for a diabetic patient with a neuropathic forefoot or midfoot ulceration is the use of a nonremovable, knee-high offloading device with appropriate foot to device interface, such as TCC and nonremovable knee-high walking boots. When comparing these two non-removable devices, one study found that plantar pressure had greater reduction in a nonremovable knee-high walker device compared to the total contact cast. The total contact cast reduced forefoot ulcer pressures 84% whereas the nonremovable knee-high walker devices reduced the pressure by 92%. In the midfoot, nonremovable kneehigh walker devices reduced pressure by 77% compared to the 63% in the total contact cast. However, literature comparing these two nonremovable devices is limited.11

Offloading: The Comprehensive Guide 50 Wound Masterclass - Vol 1 - December 2022
“If offloading isn’t implemented with every ulceration care plan, our patients are at an increased risk for morbidity and mortality.”
Figure 5a: New ulceration. 5b: Infection. 5c: Amputation.
5a 5b 5c © Copyright. Wound Masterclass. 2023
Figure 6: A Non-Removable, Knee-High Offloading Device with an Appropriate Foot to Device Interface.
Total Contact Casting
Total contact casts are the gold standard for offloading, and they were first developed for leprosy patients by Dr. Paul Brand. They are indicated for forefoot and midfoot plantar ulcerations and early Charcot immobilization. For offloading Charcot patients, the TCC was recommended for an average immobilization time of 4 months in recent literature with cast changes approximately every 14 days. Unfortunately, there are some contraindications to TCC’s, which have also been applied to nonremovable devices. Ischemia, heavy exudating ulcers, infection, and patient intolerance are considered contraindications for non-removable offloading devices. Additionally, caution should be used in bedridden patients and patients with peripheral artery disease. In bedridden patients, caution is used as total contact casts can transfer pressure to the heel and could cause more harm and potentially limb-threatening situations. In patients with peripheral vascular disease, it is typically cautioned that the patient should have palpable or dopplerable pedal pulses prior to cast application. However, one systematic review did find that an ankle-brachial index of greater than 0.55 is considered acceptable to total contact cast consideration.

Finally, adjunct procedures to total contact casting have been noted to be effective in reduce time to healing. Achilles lengthening in combination with total contact casting has been shown to rapidly increase healing in forefoot ulcers and lead to a 2-fold decrease in recurrence.12,13,14,15,16
What Does a TCC Do?
TCCs have been shown to bear approximately 30% of the plantar pressure load and are aimed to redistribute these pressures on the foot. The cast is also designed to have increased padding, which creates a cavern for the foot to sit in and allow for less pressure to prominent areas. Additionally, TCCs can be cumbersome, which can decrease walking, stride length, and ultimately reduce the forces applied to the foot.17,18,19,20
Examining the Gold Standard
Offloading Effects on Foot Areas
Because patients can ambulate in TCCs, it is important to know the benefit of TCCs in each area of the foot. Overall, TCCs lead to an 8492% reduction in plantar pressures. If this is broken down into the individual areas of the foot, there is a 32% reduction at the 5th MPJ, a 63% reduction at the 4th MPJ, a 69% reduction at the 1st MPJ. There is a 65% reduction of pressure at the hallux, and 45% reduction at the heel. However, as previously stated, pressure reduction at the heel is debated within the literature.21,22,23,24
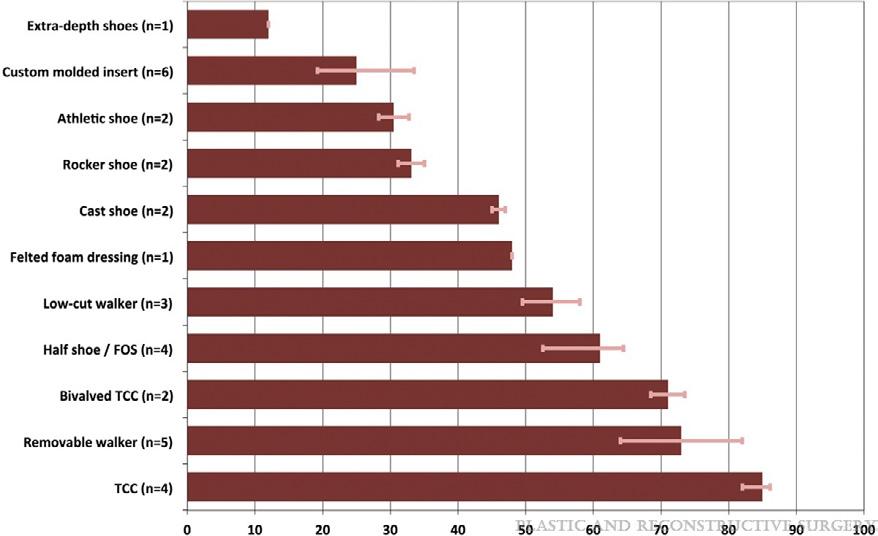
Percentage Offloaded at Ulcer Site
In a literature review, Bus examined the offloading capacities for various devices based on the patient’s peak plantar pressures. He found that total contact cases had the highest average of offloading power by having the highest peak pressure reduction percentage (87%), followed by removable devices, and so on down the line. It was noted that nonischemic, noninfected ulcerations should heal within 6-8 weeks with proper offloading and wound care.25
Non-removable Device Cautions
When assessing if a patient is a good candidate for a non-removable offloading device, it is important to consider a few patient aspects. Even though complication rates are noted to be around 5% in literature, clinical judgement and evaluation is needed to minimize any risk of
Offloading: The Comprehensive Guide Wound Masterclass - Vol 1 - December 2022 51
“Achilles lengthening in combination with total contact casting has been shown to rapidly increase healing in forefoot ulcers and lead to a 2-fold decrease in recurrence.”
12,13,14,15,16
65% 32% 64% 69% 45%
© Copyright. Wound Masterclass. 2023
Figure 7: Offloading Effects on Foot Areas.
complication to the patient. Complications were noted to be 1.55 times higher in patients with a BMI of 30 kg/m2 or more, 1.27 times higher in patients with moderate glycemic control, and 1.48 times higher in patients with poor glycemic control.
One of the main concerns for diabetic patients is their increased fall risk and gait alterations. Due to the neuropathy, their proprioception and muscle strength are altered. This can lead to mediolateral instability, especially in the ankle, and falls. Studies have also shown gait alterations with unilateral offloading in diabetic patients leading to slower gait, unsteadiness, limp, and decreased stride length. So, patients with peripheral neuropathy may need gait training or a contralateral shoe lift if a total contact cast or non-removable weightbearing device is being considered. Total contact casts and non-removable devices can also cause proximal joint pain or new ulcerations due to rubbing and different force distributions. Finally, some patients may not want a nonremovable device. This may be due to several different factors such as scratches from the device, anxiety, general intolerance, etc. For any number of reasons, the patient can simply prefer to have a removable device over a nonremovable device.26,27,28,29
How Do TCC’s Work?
They act as an exoskeleton, removing plantar pressure and transferring it to the tibia. They immobilize the ankle, reducing the plantarflexory forces at the midfoot and forefoot. TCC’s reduce the contact time of the affected foot on the ground. They’re irremovable, forcing adherence and also cumbersome and cause patients to take fewer steps per day.

Total Contact Cast Applications
Total contact casting (TCC) is a bespoke
designed cast which as its mechanism works by taking the weight off the foot (off-loading) in patients with diabetic foot ulcers (DFUs). Reducing pressure on the wound by taking weight off the foot has proven to be very effective in DFU treatment. Since the 1960’s TCC has been used for offloading DFUs in the US, with many clinicians regarding it as the "reference standard" for off-loading the sole of the foot.
The general principles of total contact cast application include minimal amount of padding should be used on the leg, a single layer of cotton padding is sufficient during application. Folds and creases should be avoided in the stockinette to avoid sources of friction. Bony prominences should also be well padded to avoid any friction or unwanted pressure. Padding can be added between the toes to avoid rubbing of the digits and toe caps can be used to avoid friction on toes in casts. And finally, the duration of the cast should be determined by patient characteristics, such as wound characteristics, patient activity level, etc. could determine if a patient needs a cast change sooner versus later. Typically, the initial cast is changed in approximately 3 days of its application. This is to ensure that no issues are arising, as there is a high potential for reduced oedema or change in limb shape. Total contact casts are then typically changed every 1-2 weeks to monitor the wound and managed swelling.27

Offloading: The Comprehensive Guide 52 Wound Masterclass - Vol 1 - December 2022
“One of the main concerns for diabetic patients is their increased fall risk and gait alterations. Due to the neuropathy, their proprioception and muscle strength are altered. This can lead to mediolateral instability, especially in the ankle, and falls.”
© Copyright. Wound Masterclass. 2023
Figure 8: How do TCCs work?
Second Line of Offloading
If a patient does not qualify or does not tolerate a non-removable offloading device, a removable knee-high device with an appropriate foot to device interface is recommended as the second line choice of treatment. Some studies found that this device is effective because of the reduced walking done in these devices and that the removable offloading device should distribute the peak pressures in a very similar fashion to the non-removable devices. However, one caveat is that patients are to ideally to wear the device constantly and rarely off the devices. One study found that patients only wore their removable offloading device during 28% of their daily activity. Another study found that patients wore their removable cast walker during 55% of their daily activity. A third study compared a removable device with and without cohesive bandaging. The cohesive bandaging kept patients from removing the device, and patients who could not remove their device had a higher portion of healed ulcers at 12 weeks of treatment. This study concluded that the modification of cohesive bandages increased patient compliance to allow for increased ulcer healing. Because of compliance concerns, the selection of the removable offloading device is important, and the factors of the removable device should be carefully considered for each patient. Patient compliance, design of the device, and its ability to maintain a good foot to device interface are key components in removable offloading devices.26,27,28,29



Third Line of Offloading
For the patient who cannot tolerate or is contraindicated to wear a knee-high removeable offloading device, the third line choice of offloading is an ankle-high offloading device. Traditionally, literature has also found that a kneehigh device is more effective than an ankle-high device. However, recent literature has contradicted these findings by concluding that there was no difference between a knee-high and an ankle-high removable offloading device.
One study compared custom-make knee-high removable devices, custom-made ankle-high devices, and prefabricated ankle-high devices. No significant differences were found between ulcer healing rates between any of the devices. Literature has claimed that the key component for knee and ankle-high devices is the fixation of the ankle joint. Because of this, ankle-high devices have shown a reasonable time to healing if the devices are used regularly, and ankle offloading devices more effectively reduced plantar pressures than custom-made or standard footwear. Anklehigh devices are also noted to be 20% lighter than knee-high walkers, which may lead to increased patient compliance and facilitate gait better than a knee-high walker. It has also been noted that these devices have a low adverse event rate and comfort was shown to be equivalent to therapeutic footwear.
Offloading: The Comprehensive Guide Wound Masterclass - Vol 1 - December 2022 53
“For the patient who cannot tolerate or is contraindicated to wear a knee-high removeable offloading device, the third line choice of offloading is an ankle-high offloading device.”
Figure 9: Knee-high removable offloading device with appropriate foot-device interface.
© Copyright. Wound Masterclass. 2023
Figure 10: Ankle-high removable offloading device with appropriate foot-device interface.
Fourth Line of Offloading
Some patients are still either unable or unwilling to tolerate an ankle-high offloading device.

In this case, a felted foam in an appropriately fitting shoe can be a lower fourth line alternative for knee or ankle high devices. Studies have found that when felted foam was applied to an ulceration in a surgical shoe, planter pressures were reduced and lead to shorter healing time than in a half shoe alone. However, there has not been any difference found between felted foam applied directly to the foot and felted foam fitted to a post-operative shoe. Some authors have made note that adhering the felted foam to the foot may increase compliance and provide offloading when the patient does not wear the shoe.
Even still, evidence for the use of felted foam to offload diabetic foot ulcerations is low, and more studies are needed in this area.

Heel Ulceration Offloading
For heel ulcerations in the diabetic patient, the offloading device needs to be carefully considered and it is often a difficult task to undertake. Studies have not found significant differences between TCCs and therapeutic footwear in heel ulceration closure. The literature is mixed at this time as to the effects of total contact casts compared to removable knee-high walkers on heel ulceration outcomes, and no known studies has tested the efficacy of heel pressure relief in specific heel offloading shoes. Crutches to prevent heel strike have been found to be effective. In patients that are non-ambulatory, pressurerelieving mattresses, heel-offloading shoes, or pillows should be employed. Overall, some evidence had found that using knee-high offloading devices may be more effective in time-to-healing and reducing plantar pressures at the heel, but the evidence is low and heel ulceration relapse is the most difficult to prevent.
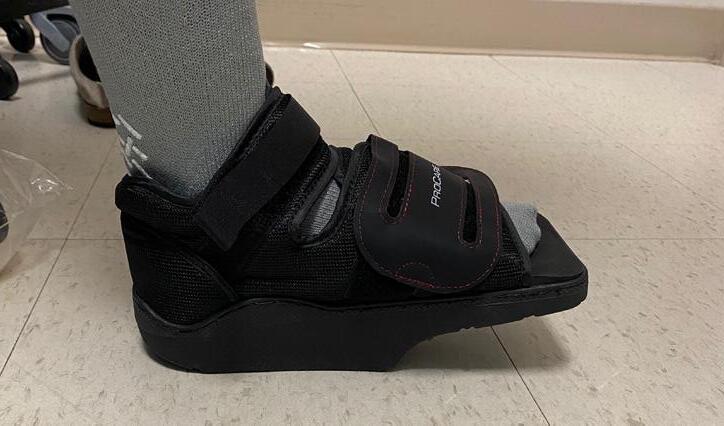
Conclusion
Ideally, offloading devices should be tailored to each patient as much as possible. If this is not possible, standard offloading options can be entertained to provide some plantar pressure relief. The first line of treatment is a knee-high, nonremovable device, followed by a removable knee-high device, an ankle high removable device, and finally felted foam in therapeutic shoes. While offloading can be a tricky aspect of wound care to master, it is a key to the healing and prevention of diabetic neuropathic ulcerations.
Offloading: The Comprehensive Guide 54 Wound Masterclass - Vol 1 - December 2022
“Evidence for the use of felted foam to offload diabetic foot ulcerations is low, and more studies are needed in this area.”
© Copyright. Wound Masterclass. 2023
Figure 11: Felted foam padding in combination with therapeutic footwear or post-operative shoe.
1. Hoffstad O, Mitra N, Walsh J, Margolis DJ. Diabetes, lower-extremity amputation, and death. Diabetes Care. 2015 Oct;38(10):1852-7.
2. Fleischli JG, Lavery LA, Vela SA, Ashry H, Lavery DC. 1997 William J. Stickel Bronze Award. Comparison of strategies for reducing pressure at the site of neuropathic ulcers. J Am Podiatr Med Assoc. 1997 Oct;87(10):466-72.
3. Armstrong DG, Boulton AJM, Bus SA. Diabetic Foot Ulcers and Their Recurrence. New England Journal of Medicine. 2017;376(24):2367-75.
4. Lazzarini PA, Pacella RE, Armstrong DG, Van Netten JJ. Diabetes-related lower-extremity complications are a leading cause of the global burden of disability. Diabetic Medicine. 2018;35:1297-9.
5. Walsh, J. W., Hoffstad, O. J., Sullivan, M. O., & Margolis, D. J. (2016). Association of diabetic foot ulcer and death in a population‐based cohort from the United Kingdom. Diabetic Medicine, 33(11), 1493-1498
6. Venkatakrishnan, S., Zachariah, K., Chinnappan, P., & Rawat, N. (2019). A description of a modified Bohler iron walking cast in the management of plantar ulcers. J Foot Ankle Surg (Asia-Pacific), 6(1), 10-12.

7. Shankhdhar, L. K., Shankhdhar, K., Shankhdhar, U., & Shankhdhar, S. (2015). Offloading a diabetic foot ulcer in the developing world. Podiatry Today, 28(10), 18-24 Agrawal, V. P. (2012). Are we forgetting to offload the Diabetic foot Ulcers in Rural setup?. J Clin Biomed Sci, 2(4), 206.
8. Shankhdhar, L. K., Shankhdhar, K., Shankhdhar, U., & Shankhdhar, S. (2016). Instant Offloading of a diabetic foot ulcer. Clin Res Foot Ankle, 4(3).
9. Bhat, S. N., & Vinoth, D. (2020). Retracted: Studying the impact of the cost-effective Suvidha off-loading dressing in healing neuropathic ulcers in diabetic foot: a case series of 83 cases from South India. International Surgery Journal, 7(7), 2371-2377.

10. Armstrong DG, Boulton AJM, Bus SA. Diabetic Foot Ulcers and Their Recurrence. New England Journal of Medicine. 2017;376(24):2367-75.
11. Bus, S., Armstrong, D., Gooday, C., Jarl, G., Caravaggi, C., Viswanathan, V., &Lazzarini, P. (2019). IWGDF guideline on offloading foot ulcers in persons with diabetes
12. Guyton, G. P. (2004). The total contact cast: indications and technique. Techniques in Foot & Ankle Surgery, 3(3), 186-191.
13. Lin, S. S., Lee, T. H., & Wapner, K. L. (1996). Plantar forefoot ulceration with equinus deformity of the ankle in diabetic patients: the effect of tendo-Achilles lengthening and total contact casting.
14. Mueller, M. J., Sinacore, D. R., Hastings, M. K., Strube, M. J., & Johnson, J. E. (2003). Effect of Achilles Tendon Lengthening on Neuropathic Plantar Ulcers*: A Randomized Clinical Trial. JBJS, 85(8), 1436-1445.
15. Griffiths, D. A., & Kaminski, M. R. (2021). Duration of total contact casting for resolution of acute Charcot foot: a retrospective cohort study. Journal of foot and ankle research, 14(1), 1-12.
16. Riopelle, A., LeDuc, R., Wesolowski, M., Schiff, A. P., &Pinzur, M. S. (2021). Risk of Complications With the Total Contact Cast in Diabetic Foot Disorders. Foot & ankle specialist, 14(1), 25-31
https://www.aofas.org/news/press-releases/2020/11/10/tips-for-diabetic-foot-care
17. Myerson M, Papa J, Eaton K, Wilson K. The total-contact cast for management of neuropathic plantar ulceration of the foot. J Bone Joint Surg Am. 1992 Feb;74(2):261-9.
18. Armstrong DG, Nguyen HC, Lavery LA, van Schie CH, Boulton AJ, Harkless LB. Off-loading the diabetic foot wound: a randomized clinical trial. Diabetes Care. 2001 Jun;24(6):1019-22.
19. Armstrong DG, Lavery LA, Wu S, Boulton AJ. Evaluation of removable and irremovable cast walkers in the healing of diabetic foot wounds: a randomized controlled trial. Diabetes Care. 2005 Mar;28(3):551-4.
20. Snyder RJ, Frykberg RG, Rogers LC, Applewhite AJ, Bell D, Bohn G, Fife CE, Jensen
J, Wilcox J. The management of diabetic foot ulcers through optimal off-loading: building consensus guidelines and practical recommendations to improve outcomes. J Am Podiatr Med Assoc. 2014 Nov;104(6):555-67.
21. Myerson M, Papa J, Eaton K, Wilson K. The total-contact cast for management of neuropathic plantar ulceration of the foot. J Bone Joint Surg Am. 1992 Feb;74(2):261-9.
22. Armstrong DG, Nguyen HC, Lavery LA, van Schie CH, Boulton AJ, Harkless LB. Off-loading the diabetic foot wound: a randomized clinical trial. Diabetes Care. 2001 Jun;24(6):1019-22.
23. Armstrong DG, Lavery LA, Wu S, Boulton AJ. Evaluation of removable and irremovable cast walkers in the healing of diabetic foot wounds: a randomized controlled trial. Diabetes Care. 2005 Mar;28(3):551-4.
24. Snyder RJ, Frykberg RG, Rogers LC, Applewhite AJ, Bell D, Bohn G, Fife CE, Jensen J, Wilcox J. The management of diabetic foot ulcers through optimal off-loading: building consensus guidelines and practical recommendations to improve outcomes. J Am Podiatr Med Assoc. 2014 Nov;104(6):555-67.
25. Bus, Sicco A. PhD The Role of Pressure Offloading on Diabetic Foot Ulcer Healing and Prevention of Recurrence, Plastic and Reconstructive Surgery: September 2016 - Volume 138 - Issue 3S - p 179S-187S
26. Bus, S., Armstrong, D., Gooday, C., Jarl, G., Caravaggi, C., Viswanathan, V., &Lazzarini, P. (2019). IWGDF guideline on offloading foot ulcers in persons with diabetes.
27. Guyton, G. P. (2004). The total contact cast: indications and technique. Techniques in Foot & Ankle Surgery, 3(3), 186-191.
28. Hingorani, A., LaMuraglia, G. M., Henke, P., Meissner, M. H., Loretz, L., Zinszer, K. M., ... & Murad, M. H. (2016). The management of diabetic foot: a clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. Journal of vascular surgery, 63(2), 3S-21S.
29. Hochlenert, D., & Fischer, C. (2020). Ventral Windowed Total Contact Casts Safely Offload Diabetic Feet and Allow Access to the Foot. Journal of Diabetes Science and Technology, 1932296820964069
30. Singh S, Yoong M, Kaur A . Offloading techniques for diabetic foot. J Diabetes Metab Disord Control. 2017;4(3):84-88. DOI: 10.15406/jdmdc.2017.04.00112
31. Kari SV. The economical way to off-load diabetic foot ulcers [Mandakini off-loading device]. Indian J Surg. 2010 Apr;72(2):133-4. doi: 10.1007/s12262-010-0042-3. Epub 2010 Jul 1. PMID: 23133224; PMCID: PMC3452512.
Offloading: The Comprehensive Guide Wound Masterclass - Vol 1 - December 2022 55 References woundmasterclass.com Introducing Wound Masterclass Video woundmasterclass.com/Video © Copyright. Wound Masterclass. 2023 Image licenced from Adobe Stock. Credit: BillionPhotos.com
Use of an Advanced Collagen Powder to Close Non-healing Post-amputation Wounds



Editorial Summary
Treatment strategies and new technologies are being developed to meet the need for the signififcant problem in healthcare systems of chronic wounds; designed to enhance the healing rates of stalled wounds, they have the potential to lessen the burden that chronic wounds place on patients and healthcare providers as well as reducing healthcare costs. This article features a case using an advanced collagen powder.
Introduction

Chronic wounds are a significant health issue that impact millions of patients each year by adding to overall patient morbidity as well as to reducing quality of life. Treatment strategies and new technologies that can enhance the healing rates of stalled wounds have the potential to lessen the burden that chronic wounds place on patients and healthcare providers as well as reducing healthcare costs.
Patient History
An 83-year-old male with a history of peripheral arterial disease was referred on April 16th 2021 for the management of gangrenous digits on the right foot following the repair of a dissecting aneurysm of the ascending thoracic aorta. A diagnosis of Blue Toe Syndrome or occlusive vasculopathy secondary to shower emboli was made and conservative management of the gangrenous digits continues. Due to his peripheral arterial disease, the patient was closely monitored for the need for a transmetatarsal amputation. The right 3rd digit was partially amputated one month later, followed by the 2nd and 4th digits approximately 3 weeks later (Figure 2). All partial amputations resulted in non-healing wounds.


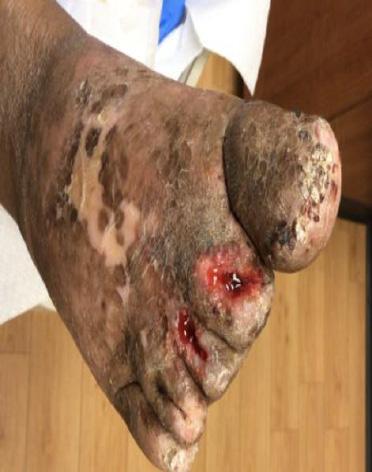

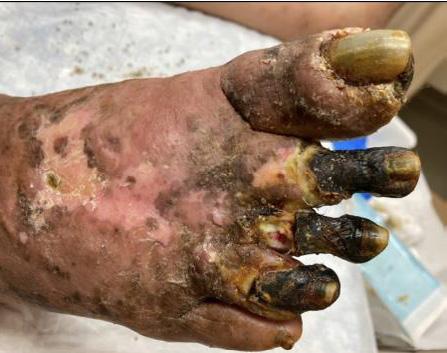
1b:
1c: Delayed healing
1d:
1e:
56 Wound Masterclass - Vol 1 - December 2022
of
MA, United States
Dr Alton R. Johnson
University
Michigan Michigan
Podiatry
Michigan
United
Dr Brennen O’Dell
Diabetic Limb Preservation and Research Fellow
MA,
States
Figure 6: ColActive Plus® (Covalon Technologies LTD., Mississauga, ON, Canda), in individual packaging.
Figure 1:
1a: Initial presentation. ~1 month conservative management/ amputation
Following partial amputation.
wounds.
Delayed healing, cont. 3 months non-healing, treatment with Collagen/ ORC dressing
1a 1b 1c 1d 1e © Copyright. Wound Masterclass. 2023
Wound closure achieved. Wound closure using Pulse Lavage and ColActive Plus Powder
Treatment

Following amputation, healing of the 2nd and 3rd digit sites failed to progress over several months with the use of a collagen/ ORC (Oxidized Regenerated Cellulose) dressing (Figure 3). Following a failed delayed primary closure attempt, the wounds were treated weekly with pulse lavage therapy and weekly application of a novel collagen/ ethylenendiaminetetraacetic (EDTA) powder product. Patient’s wounds were closed 28 days later and have remained healing to date. ColActive Plus Powder (Figure 6) is a formulation of fast acting hydrolyzed collagen, including EDTA for protease inhibition, in addition to carboxymethylcellulose (CMC) and alginate which enhance absorbency and promote moisture balance.
Conclusion
For this patient, the combination of pulse lavage cleansing and debridement, and the application of novel collagen/ EDTA powder product initiated healing and lead to the cessation of chronicity, allowing for wound closure. Despite the challenging nature of this case and failed previous attempts at wound closure, use of the advanced collagen/ EDTA formulation progressed healing rapidly. The product was simple to apply and implement as part of the patient’s existing treatment schedule.

References
1. Olsson M, Jarbrink K, Divakar U, Bajpai R, Upton Z, Schmidtchen A, Car J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019 Jan;27(1):114-125. Use of an Advanced Collagen Powder to Close Non-healing Post-amputation Wounds Wound Masterclass - Vol 1 - December 2022 57 woundmasterclass.com/Register Register for full access to the journal, educational resources, information about upcoming events and more woundmasterclass.com © Copyright. Wound Masterclass. 2023 Image licenced from Adobe Stock. Credit: Dragana Gordic
A
FORWARD
HEALING NATROX® Oxygen Wound Therapy RCT results on non-healing diabetic foot ulcers1 NEW greater healing rate* greater average % reduction in wound size* 52% 71% 73% *Compared to control healed AD_05/21 (V3) Ground Floor Unit 7340, Building 7300 | Cambridge Research Park | Waterbeach | Cambridge | Cambridgeshire | CB25 9PD | United Kingdom How can you achieve the same results? Visit: http://bit.ly/clinicians-natrox NATROX® is a registered trade mark of Inotec AMD Limited in EU, China, USA & UK 1. Serena TE, Bullock NM, Cole W et al. Topical oxygen therapy in the treatment of diabetic foot ulcers: a multicentre, open, randomised controlled trial. J Wound Care 2021; 30: Suppl.5 S7-14.
STEP
IN WOUND



Alligator-Derived Hyaluronic Acid: Bacteriostatic and Fungastatic Properties Against Pathogens
Editorial Summary
Chronic wounds, also known as hard-to-heal wounds, have a significant economic and health burden globally. Hyaluronic acid has a number of advantageous properties in wound healing. These include properties of protection of stem cells, growth factors, and cytokines. Alligator tissues have been considered to have bacteriostatic and fungistatic properties. Chronic skin wounds are often infected with pathogenic microorganisms that may contribute to delayed wound healing. Antimicrobial-based advanced wound care products are widely used to treat microbially contaminated wounds. However, toxicology and wound healing studies have shown antimicrobials can delay wound healing and are cytotoxic at high levels. Furthermore, many of these products are not safe for the environment, as such there is a trend in the United States and Europe to utilize natural ingredients that are ecofriendly, reduce the bioburden, and accelerate wound healing. Since it has previously been suggested that American alligator-derived tissues exhibit bacteriostatic and fungistatic properties, we compared the bacteriostatic and fungistatic properties, biofilm eradication capability and cytotoxicity of American alligator-derived hyaluronic acid (aHA) with that of commercially available streptococcal-derived HA (sHA).
Introduction
Worldwide, chronic wounds are a substantial burden to the quality of life (QoL) of the patients as well as a significant economic burden to our health care system with an estimated annual cost of $50B in the US alone.1,2 Nonhealing wounds are often stalled in the inflammatory phase (see Figure 1) and considered hard-to-heal or chronic wounds when healing is delayed in the phase of wound healing for greater that 4 weeks and up to more than three months.3 Some wounds remain chronic for years. Thus, the longer time it takes these wounds to heal provides a severe risk for a polymicrobial infection which can stall the wound even further in the inflammatory phase and is concomitant with a higher incidence of lower extremity amputation (LEA) and mortality.4
Hyaluronic acid matrices have been utilized in wound care to replenish and stabilize the extracellular matrix in a chronic wound bed.5,6 Hyaluronic acid has also been shown to protect stem cells, growth factors, and cytokines required for cell proliferation and angiogenesis.7,8,9 Additional evidence suggests that hyaluronic acid may also inhibit bacterial growth and could possibly bind host and bacterial proteases that are hyperactivated in the stalled inflammatory phase of a diabetic wound.10,11







Since Alligator tissues have been implicated as having bacteriostatic and fungistatic properties, our hypothesis was that this source of aHA may play a critical role in reducing the bioburden to promote chronic wound healing.12,13
 Ms Nikole Siegmund
Mr Ted Bundrick
Ms Nikole Siegmund
Mr Ted Bundrick
60 Wound Masterclass - Vol 1 - December 2022
Dr Mitchell Sanders
Ms Mia Hanna
Dr Swathi Balaji
Dr Da Wei Ms Lindsay Poland
Scientist III/ Lab Operations Manager, Alira Health Boston MA, United States Vice President, Preclinical Development, Alira Health Boston MA, United States Project Manager/ Research Associate III, Alira Health Boston MA, United States Assistant Professor, Baylor College of Medicine and Texas Children’s Hospital Houston TX, United States Chief Scientific Officer, Alira Health Boston MA, United States Vice President of Operations, Lacerta Life Sciences Lafayette LA, United States Scientist II/ Project Manager, Alira Health Boston MA, United States
Founder and CEO SerenaGroup Inc. Cambridge MA, United States © Copyright. Wound Masterclass. 2023
Dr Thomas Serena
Figure 1: Normal wounds progress swiftly through the inflammatory phase to granulation tissue formation and the tissue remodeling phase of wound healing. Chronic wounds are often infected and exhibit an extended and hyperproliferative inflammatory phase (redline). Bacterial toxins, specifically proteases such as V8, Endo Glu-C in Staphylococcus aureus play an important role in delaying the healing process by hyperactivation of host metalloproteases (MMPs) and human neutrophil elastase causing matrix degradation, leading to senescent fibroblasts and recruitment macrophages stalling the inflammatory phenotype (M1).

antimicrobial resistance isolate bank: Candida auris (C. auris) AR-0383.
Purification of Hyaluronic Acid From American Alligator
In vitro, we tested the effectives of aHA at microbial stasis with common bacterial and fungal pathogens including Staphylococcus aureus, Pseudomonas aeruginosa, Streptococcus pyogenes, Enterococcus faecalis, Escherichia coli, Candida albicans, and Candida auris. In vivo, we utilized a delayed wound healing diabetic mouse model to characterize the wound healing parameters, gene expression and immunohistochemistry with a novel high molecular weight aHA matrix, named LacertaMatrix™, that activates NF-kB activation to quell the inflammatory phase and accelerate wound healing. Here we provide evidence to suggest that aHA can act on the inflammatory phase of wound healing either by:
1. Reducing the microbial bioburden
2. Through the quelling of the inflammatory phase through NF-kb signaling and macrophage plasticity
Materials and Methods
Chemicals, Antibodies, and Cell Culture
Microbes were either purchased from ATCC, CDC, or were clinical ID wound isolates from our clinical wound isolate library. Specifically, Staphylococcus aureus (S. aureus) ATCC 6538, Pseudomonas aeruginosa (P. aeruginosa)
ATCC 15692, Enterococcus faecalis (E. faecalis) ATCC11823, Escherichia coli (E. coli)
ATCC BAA 1427. Streptococcus pyogenes (S. pyogenes) UCO012-and Candida albicans (C. albicans) 509-8-2 clinical wound strains from our clinical wound isolate library. A Candida strain was purchased from CDC
Hyaluronic acid was purified using a method describe by Sanders.14 Briefly we utilized a 4-step process grinding, extraction, anion exchange chromatography, and tangential flow filtration. The ground alligator backstrap was provided by Lacerta Life Sciences for overnight extraction at 4°C in a buffer containing; Tris, EDTA, and SDS (10 mM Tris, 10mM EDTA, 0.1% SDS. The extract was pH adjusted to 6.2 with 3M Na Acetate buffer and then clarified by depth filtration using a combination 5.0 - 0.2 μm filters (Pall Life Sciences). Next, anion exchange chromatography (STIC PA, Sartorius) was utilized with a 50m M- 3M NaCl step gradient to purify the hyaluronic acid from proteins and nucleic acids. Most protein eluted in the flow through, and the low salt step gradient (500-800 mM), while the purified hyaluronic acid eluded in a 2-3M step gradient of NaCl. The hyaluronic acid was desalted and concentrated using a 100K MWCO tangential flow filtration membrane. We utilized the Purple Jelly Kit (Biocolor LTD) to quantitate the concentration of hyaluronic and a DNA kit (ThermoFisher) to measure the impurities of each prep.
Bacteriostasis and Fungistasis Studies
Bacteria (S. aureus, P. aeruginosa, S. pyogenes, E. faecalis, E. coli) were cultured in Tryptic Soy Broth (TSB) at 37°C for overnight. C. auris and C. albicans were grown in Yeast ExtractPeptone-Dextrose (YPD) overnight at 30°C Microbe concentrations were determined the following morning in Phosphate Buffer Saline (PBS) dilutions, and then the cultures were diluted to 106 cells/mL. 100 μl of each bacteria was added in pentuplicate to a 96 well plate with different concentration of HA (Plate 1) and then incubated at 37°C. 100 μl of C. albicans or C. auris was added in pentuplicate to a separate plate with different concentrations of HA (Plate 2) and incubated at 30°C. Bacteriostatic and fungistatic properties were measured at 600nm and the background was subtracted from the media + HA alone controls at each concentration of HA. Two HA incubation concentrations at 3 and 1.5 mg/mL were used for S. aureus, S. pyogenes, E. faecalis and C.
Pathogens Wound Masterclass - Vol 1 - December 2022 61
Alligator-Derived Hyaluronic Acid: Bacteriostatic and Fungastatic Properties Against
© Copyright. Wound Masterclass. 2023
was used for E. coli, P. aeruginosa, and C. auris.
Formation of Hyaluronic Acid Matrices
We developed a method for forming hyaluronic acid matrices using serine benzyl ester and zero length crosslinkers N-Hydroxysuccinimide (NHS) and ethylene dichloride (EDC) as described previously (Sanders, 2019). Briefly the hyaluronic acid is diluted to 1.2 mg/mL and reacted with an equal amount w/w of serine benzyl ester and NHS follow by a 1-5fold molar excess of EDC. The material was mixed for 2 - 5 minutes with a high-speed mixer and then poured in metal trays lined with parchment paper to set overnight at 37°C with 30-35% relative humidity.14
Wound Healing Studies
85 male diabetic (BKS.Cg-Dock7m +/+ Leprdb/J) mice, 9 weeks old were purchased and received from Jackson Laboratories. We utilize male mice because they have a stronger disease phenotype and are profoundly obese, insulin resistant, and glucose intolerant.15,16
Animal wound healing studies were carried out at Woodland Biosciences and Alpha Preclinical at the Tufts Cummings School of Veterinary Medicine AAALAC-accredited laboratories in North Grafton, MA. The IACUC protocols for the study were G2019-96 and G2021-28.
Animal health was accessed daily to check for general health, mortality, and moribundity. Animals were housed two per cage in 100% PET plastic, BPA-free caging with corncob bedding (Innovive, San Diego, CA). Environmental controls included a 12-hour light-dark cycle (0700 - 1900) at 20 – 26°C and 30 - 70% relative humidity. Animals were provided water and rodent chow ad libitum.
All procedures were approved by the Tufts University Cummings School of Veterinary Medicine Institutional Animal Care & Use Committee. Mice were anesthetized by inhalation of isoflurane (1 - 4%) with oxygen (O2). Each animal received Buprenorphine-SR (1.0 mg/kg) subcutaneously one hour prior to wounding for analgesia.
The dorsal skin was shaved and was cleaned with isopropanol wipes prior to wounding the skin with a sterile 8 mm punch biopsy. Animals were treated weekly with aHA or sHA and then each wound was covered with a sterile Tegaderm™ dressing. The Negative controls consisted of Tegaderm™ alone, and positive controls PDGF-β (10 μg/ml) and TGF-α (1 μg/ml) prepared in 0.5% hydroxypropyl methylcellulose (HPMC) and applied topically for the first seven (7) days of the study. Animals were anesthetized every three days post wound creation (Days 3, 6, 9, 12, 15, 18 and day 20 prior to termination). The Tegaderm™ film dressing was removed for taking images of the wounds measured with Tissue Analytics imaging software of the wound and a new Tegaderm™ film dressing applied. Topical treatments were applied on Days 0-6 for controls (anesthetized each day) and Days 0, 3, 6, 9, 12, 15, 18 for aHA and sHA treatments (Figure 2). Animals were observed daily from start through termination and bodyweights were recorded at least three times per week.
Photographs and Digital Measurements of the Wound Sites
Wound healing was assessed over a 20-day period in terms of:
i. Initiation of neo-dermal repair responses
ii. Wound closure
Alligator-Derived Hyaluronic Acid: Bacteriostatic and Fungastatic Properties Against Pathogens 62 Wound Masterclass - Vol 1 - December 2022
Figure 2: Wound biopsies were performed on Day 1 and wound healing measurements were made on day 0, 3, 6, 9,1 2, 15, and 18. qPCR was performed on day 3 and 6, while histology was performed on day 4.
© Copyright. Wound Masterclass. 2023
Alligator-Derived Hyaluronic Acid: Bacteriostatic and Fungastatic Properties Against Pathogens
Table 1: Genes expression, by qPCR, as performed on wounds harvested from study animals on day 6 of wound healing. Genes involved in the proliferative phase of wound healing were chosen and are listed along with the forward and reverse primers, and the thermal cycling parameters.
qPCR Genes and Primers
IL6
IRAK1
CXCL9
TACCACTTCACAAGTCGGAGGC CTGCAAGTGCATCATCGTTGTTC
CGACTGGCTGTGGACACCGATAC
CCTAGTGATAAGGAATGCACGATG
CCTGGGCTACTCCTCACACTTGATTC
CTAGGCAGGTTTGATCTCCGTTC
MIP2 CATCCAGAGCTTGAGTGTGACG GGCTTCAGGGTCAAGGCAAACT
NFκB-1
TNF-α
NGF
KI-67
Arg1
GAAATTCCTGATCCAGACAAAAAC ATCACTTCAATGGCCTCTGTGTAG
GGTGCCTATGTCTCAGCCTCTT GCCATAGAACTGATGAGAGGGAG
GTTTTGCCAAGGACGCAGCTTTC GTTCTGCCTGTACGCCGATCAA
ATCATTGACCGCTCCTTTAGGT GCTCGCCTTGATGGTTCCT
AACACGGCAGTGGCTTTAACC GGTTTTCATGTGGCGCATTC
CD163 GGCTAGACGAAGTCATCTGCAC CTTCGTTGGTCAGCCTCAGAGA
GAPDH* CATCACTGCCACCCAGAAGACTG ATGCCAGTGAGCTTCCCGTTCAG
HPRT1* CTGGTGAAAAGGACCTCTCGAAG CCAGTTTCACTAATGACACAAACG
* Housekeeping Gene
Initiation of neo-dermal tissue formation were expressed as the number of wounds responding in each group at each time point. Wound closure was considered in both overall terms and in terms of its components, wound contraction and wound re-epithelialization. Wound closure (contraction and reepithelialization) was determined from digital images taken on Days 0, 3, 6, 9, 12, 15, 18, and 20. Images were taken before the treatment started. Any adverse effects of treatment were fully documented and managed by the preclinical site, according to IACUC guidelines, in consultation with the attending veterinarian, up to and including euthanasia.
Wound Healing and Histopathology Measurements
Wound Healing measurements were taken every 3 days either with a digital caliper and/or Tissue Analytics software which allows the measurements of the wound volume as well as the color of the wound which complements the visual assessment of the wound bed.
qPCR and Gene Expression Studies
To quantify the level of mRNA expression on Day 3 and Day 6 of the target genes with the four treatment groups, wounds were isolated and quickly incubated in tissue extraction buffer (Qiagen Catalogue number 76106) followed by snap freezing in liquid nitrogen, and storing at -80°C until processed for RNA extraction. Total
1. 50°C - 10 minutes
2. 95°C - 1 minute
3. 95°C - 10 seconds
4. 55°C - 30 seconds
5. Plate read
6. Repeat steps 3, 4, 5 35X
7. 4°C infinity
Total RNA was extracted by a Qiagen kit according to manufacturer’s instructions. About 2–5 μg of RNA was used for reverse transcription with the CFX384™ Real-Time PCR thermocycler. The resultant cDNA was used for qPCR with the SYBR Green qPCR mix (Bio-Rad) according to manufacturer’s instructions. qPCR was carried out using CFX384™ Real-Time PCR thermocycler (Bio-Rad Laboratories, Hercules, CA) with fluorescence detection and set of reagents iTaq Universal SYBR Green 1- Step Kit (BioRad #172-5151). The primers used for qPCR are found in Table 1 which includes the thermal cycling parameters. The CFX Maestro software 3.1 was used to quantitate the relative gene expression and the p values for statistical significance (day 3 and day 6).
Immunohistochemistry
The wound tissue was harvested on day 4, and were fixed in 4% paraformaldehyde in PHEM Buffer (60 mm PIPES, 25 mm HEPES, 10 mm EGTA, and 2 mm MgCl2 at pH 6.9) at 4°C for 24 hours. The samples were rinsed with PBS, followed successive dehydration steps with ethanol (70%, 90%, 100%), the tissue was embedded in paraffin, and sectioned at 5m for histopathology. Paraffin sections were dewaxed and were rehydrated by xylene and gradient alcohols and were stained with hematoxylin and eosin (H&E) for microscopic observation. Histology was performed by HistoWiz, Inc. (Brooklyn, NY) using standard operating procedures and fully automated
Wound Masterclass - Vol 1 - December 2022 63
Forward Primer Reverse Primer Thermal Cycling Parameters
Gene
© Copyright. Wound Masterclass. 2023
workflow. Multiplex immunohistochemistry was performed on a Bond Rx auto-stainer (Leica Biosystems, Nussloch, Germany) with heat-mediated epitope retrieval by using standard protocols. The primary antibody that was used is Arg1 (Cell Signaling Technologies, Danvers, MA; CAT# CST93668), at a dilution of 1/100. Wholeslide scanning (40x and 60x) was performed with an Aperio AT2 (Leica Biosystems, Nussloch, Germany). A secondary fluorescent antibody was used for the indirect immunofluorescence staining with Arg1.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 9.2 (San Diego, CA) with the differences between the negative control, positive control and the treatment groups analyzed by ANOVA (p <0.05 considered as statistically significant). Results were expressed as mean +/- standard deviation.
Results
This study assessed the bacteriostatic and fungistatic properties of hyaluronic acid derived from America Alligator (aHA) and a Streptococcus source (sHA) and the wound healing properties of LacertaMatrix™ a novel aHA derived matrix versus HyaloMatrix® derived from sHA.
Our findings indicate that aHA is more effective at bacteriostatsis than sHA for both gram positive (+) and gram negative (-) bacteria (Figure 3 and 4). Low concentrations aHA were required for microbial stasis of Gram (+) bacteria and C. albicans

In contrast, C. auris, a multidrug resistant and emerging pathogen, was resistant to the fungistatic properties of aHA and Gram (-) bacteria and required a higher concentration of sHA (5 mg/mL) to be effective.
In a non-diabetic mouse, the phases of wound healing occur over a 18-20 day period. The overlapping wound healing process including hemostasis (0–several hours after injury), inflammation (1–3 days), proliferation (4–21 days) and tissue remodeling (21 days–1 year).
In contrast, in a diabetic mouse model the early stages of wound healing are profoundly
Properties Against Pathogens
delayed in the inflammatory (1-6 days) and the proliferative (7-31 day) phases. Our findings indicate that LacertaMatrix™ quells the inflammatory phase by 3 days as judged by the reduced wound size and significant reduction in red color by days by 5-6 vs. the untreated control (p <0.05).
Over 24 hours. Bacteriostasis was proportional to the concentration of HA. In general, higher concentrations of aHA were required for treating gram negative bacteria (5 mg/mL) vs gram positive bacteria (1.5 mg/mL). Five (5) mg/mL of aHA totally inhibited the bacterial growth of the E. coli as compared to the streptococcalderived HA. Surprisingly, LacertaMatrix™ was less effective at inhibiting P. aeruginosa even at 5 mg/ ml and the streptococcal-derived HA enhanced the growth of the bacteria.
Figure 4: Fungal Growth Inhibition Over 24 hours. Fungistasis was profound at low concentrations (1.5 and 3 mg/mL) of aHA. Streptococcal-derived (sHA) also inhibited C. albicans but the growth inhibition was not as effective as aHA. p-values of statistical significance are all noted above the respective bars. Limited activity was seen against C. auris which is a hard pathogen to kill.

Alligator-Derived Hyaluronic
64 Wound Masterclass - Vol 1 - December 2022
Acid: Bacteriostatic and Fungastatic
Figure 3: Growth Inhibition
4 3 © Copyright. Wound Masterclass. 2023
In addition to dramatic reduction in the inflammatory phase, hyaluronic acid treated animals completed healing by 17 days, as if they were normal animals (Figure 5).
These findings are quite different from the treatment of these diabetic wound with human amnion chorion matrices (HACM) which seem to only accelerate the proliferative and tissue remodeling phases of wound healing (Sanders et al., submitted).
H&E Staining indicates that the LacertaMatrix™ healed wound have a mature and repaired epidermal and dermal layer with marked tissue granulation but no apparent
inflammation or necrosis. In contrast the negative control wounds had incomplete reepithelialization with a higher inflammatory response (not shown). The LacertaMatrix™ dressing derived from aHA had statistically significant healing vs. the untreated control (Figure 6).

Based on the reduction in the inflammatory phase in wound healing, we wanted to examine gene expression levels on day 3 and Day 6 to study biomarkers that are thought to be involved in quelling the inflammatory phase and stimulating the early proliferative phase of wound healing (Figure 7


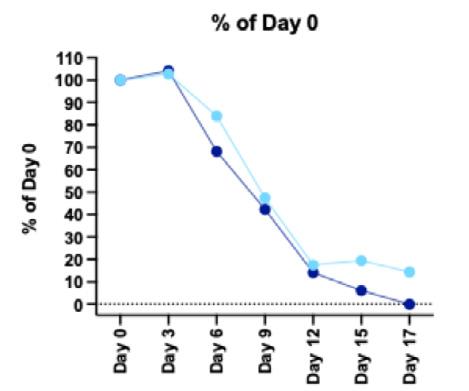
Alligator-Derived Hyaluronic Acid: Bacteriostatic and Fungastatic Properties Against Pathogens Wound Masterclass - Vol 1 - December 2022 65
Figure 5: The LacertaMatrix™ dressing derived from aHA was more rapid at healing the inflammatory phase of wound healing vs the untreated control. The untreated controls also had higher score for edema and erythema (not shown).
Figure 6: Percent (%) of Days 0 for the four (4) Treatment groups. The Lacerta dressing derived from aHA had statistically significant healing vs. the untreated control. The was no statistical difference between LacertaMatrix and sHA (HyaloMatrix).
and 8).
Figure 7: Percent (%) of Days 0 for the four (4) Treatment groups. The Lacerta™ dressing derived from aHA had statistically significant healing vs. the untreated control. The was no statistical difference between LacertaMatrix™ and sHA (HyaloMatrix).
© Copyright. Wound Masterclass. 2023
Figure 8. Attenuation of NF-kB results in the attenuation of CXC9 and K167 and the induction of TNF-α and Arg1. Arginase 1 is a key effector and marker of M2a macrophages. M2a macrophages are anti-inflammatory and lead to fibroblast proliferation and tissue repair. TNF-a produced by M2a macrophages promotes the TNF-a promotes the formation of ECM.
By day 3 there was an induction of transcription factor NF-kB but by day 6 it was dramatically attenuated. NF-kB is an important transcription factor that functions as a pivotal mediator of the inflammatory response in wound healing. Attenuation of NF-kB occurred at the same time as a reduction of CXC9 and K167 and the over expression of TNF-α and Arginase 1 (Arg1). Arg1 is a key effector and marker of anti-inflammatory M2a macrophages. M2a macrophages stimulate fibroblast proliferation and tissue repair. TNF-α produced by M2a macrophages promotes the formation of new extra cellular matrix (ECM) to accelerate tissue proliferation. Immunofluorescence also indicates that Arg1 has a higher level of staining than the untreated control which is consistent with what was observed by qPCR (Figure 8). This induction of Arg1 by day 6 is somewhat unanticipated since previous reports have shown that the transition of inflammatory M1 macrophages to the M2a anti-inflammatory macrophages usually occurs much later in the proliferative phase of wound healing (Sanders et al., submitted).17
Discussion and Conclusions
Our findings indicate that the source of HA (aHA, Alligator vs. sHA Streptococcal) has profound differences in microbial stasis properties and to a lesser extent slightly improved wound healing in a diabetic mouse delayed healing model. By STIC PA anion exchange chromatography, we determined that aHA is more anionic and binds to a STIC PA anion exchange column more strongly with 2-3M NaCl elution profile, while sHA elutes from the same column at 500mM NaCl. The strong anionic character of the aHA likely is modulating the improved bacteriostatic and fungistatic properties (as described in Figures 3 and 4). Higher concentrations of aHA were required for gram negative bacteria, which makes sense because gram negative bacteria have three membranes (inner cytoplasmic membrane (CM), a peptide glycan wall (PGW), and a closely attached outer membrane (OM)). In contrast gram positive bacteria only have a CM and a PGW, while lack the outer membrane. Findings by Erickson et al., (2021)18 suggest that these bacteria are under a great amount of turgor pressure and we suspect that the aHA treated bacteria are experiencing osmotic shock due to the strong
anionic nature of the hyaluronic acid material which inhibits the growth at 37°C. In 2011, Kang et al. demonstrated that low molecular weight (1630 kDa) sHA had modest fungistatic properties and the activity was concentration dependent.19 However, there was only a 1020 hr inhibition that C. albicans was able to recover from by 24 hours. Our findings suggest that aHA is much more potent than sHA, even after 3 days of incubation. C. auris is a multidrug resistant and emerging pathogen in hospitals and we suspected that this organism would be more resistant to aHA, and our findings suggest that to be case. We observed a statistical reduction in OD600 for C. auris but it was modest compared to that of the C. albicans data. It is well understood that the transition from M1- to M2-like macrophage phenotypes is impaired in diabetic wounds resulting in increased accumulation of M1-like macrophage phenotypes (Figure 1). Our findings indicate that the novel hyaluronic acid matrix is able to quell the inflammatory phase of wound healing based on clinical signs, wound healing and gene expression (qPCR). Regulation of NF-kB is imperative for an appropriate inflammatory response.20,21 HA has been shown to modulate the inflammatory response through the proposed mechanism of binding CD44 to activate NFkB pro-inflammatory pathways or through activation of antiinflammatory pathways, dependent upon the molecular weight (MW) range of HA fragments.22,23 LacertaMatrix™ contains HA fragments in a range of MWs that is crosslinked into a high MW, therefore the HA present in this novel aHA-derived matrix may aid in correcting chronic inflammation while simultaneously contributing to cellular proliferation. In contrast low MW HA can be internalized by cells by a CD44 mediated endocytosis process which can exacerbate the inflammatory phase of wound healing.24 Our results suggest that LacertaMatrix™ has a dual role to quell the inflammatory phase through microbial stasis and while modulating anti-inflammatory pathways and macrophage plasticity (M1 to M2 switching) to aid in the closure of chronic wounds. These findings collectively suggests that LacertaMatrix™ may be a beneficial early intervention for chronic wounds stalled in the inflammatory state.
Disclosures
This project was funded by Lacerta Life Sciences.
Alligator-Derived Hyaluronic Acid: Bacteriostatic and Fungastatic Properties Against Pathogens 66 Wound Masterclass - Vol 1 - December 2022 © Copyright. Wound Masterclass. 2023
1. Olsson M, Järbrink K, Divakar U, Bajpai R, Upton Z, Schmidtchen A, Car J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019 Jan;27(1):114-125. doi: 10.1111/wrr.12683. Epub 2018 Dec
2. PMID: 30362646.
2. Sen CK. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv Wound Care (New Rochelle). 2019 Feb 1;8(2):39-48. doi: 10.1089/wound.2019.0946. Epub 2019 Feb 13. PMID: 30809421; PMCID: PMC6389759.

3. Eriksson E, Liu PY, Schultz GS, Martins-Green MM, Tanaka R, Weir D, Gould LJ, Armstrong DG, Gibbons GW, Wolcott R, Olutoye OO, Kirsner RS, Gurtner GC. Chronic wounds: Treatment consensus. Wound Repair Regen. 2022 Mar;30(2):156-171. doi: 10.1111/wrr.12994. Epub 2022 Feb 7. Erratum in: Wound Repair Regen. 2022 Jul;30(4):536. PMID: 35130362; PMCID: PMC9305950.
4. Armstrong DG, Swerdlow MA, Armstrong AA, Conte MS, Padula WV, Bus SA. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020 Mar 24;13(1):16. doi: 10.1186/ s13047-020-00383-2. PMID: 32209136; PMCID: PMC7092527.
5. Caravaggi C, Grigoletto F, Scuderi N. Wound Bed Preparation With a Dermal Substitute (Hyalomatrix® PA) Facilitates Reepithelialization and Healing: Results of a Multicenter, Prospective, Observational Study on Complex Chronic Ulcers (The FAST Study). Wounds. 2011 Aug;23(8):228-35. PMID: 25879233.
6. Motolese A, Vignati F, Brambilla R, Cerati M, Passi A. Interaction between a regenerative matrix and wound bed in nonhealing ulcers: results with 16 cases. Biomed Res Int. 2013;2013:849321. doi: 10.1155/2013/849321. Epub 2013 Jul 18. PMID: 23971047; PMCID: PMC3732600.
7. Ren Y, Zhang H, Wang Y, Du B, Yang J, Liu L, Zhang Q. Hyaluronic Acid Hydrogel with Adjustable Stiffness for Mesenchymal Stem Cell 3D Culture via Related Molecular Mechanisms to Maintain Stemness and Induce Cartilage Differentiation. ACS Appl Bio Mater. 2021 Mar 15;4(3):2601-2613. doi: 10.1021/acsabm.0c01591. Epub 2021 Feb 15. PMID: 35014377.
8. Kim H, Kong WH, Seong KY, Sung DK, Jeong H, Kim JK, Yang SY, Hahn SK. Hyaluronate- Epidermal Growth Factor Conjugate for Skin Wound Healing and Regeneration. Biomacromolecules. 2016 Nov 14;17(11):3694-3705. doi: 10.1021/acs. biomac.6b01216. Epub 2016 Nov 3. PMID: 27775884.

9. Shahbazi MA, Sedighi M, Bauleth-Ramos T, Kant K, Correia A, Poursina N, Sarmento B, Hirvonen J, Santos HA. Targeted Reinforcement of Macrophage Reprogramming Toward M2 Polarization by IL-4-Loaded Hyaluronic Acid Particles. ACS Omega. 2018 Dec 27;3(12):18444- 18455. doi: 10.1021/acsomega.8b03182. Erratum in: ACS Omega. 2019 Mar 28;4(3):5931. PMID: 31458417; PMCID: PMC6711357.
10. Carlson GA, Dragoo JL, Samimi B, Bruckner DA, Bernard GW, Hedrick M, Benhaim P. Bacteriostatic properties of biomatrices against common orthopaedic pathogens. Biochem Biophys Res Commun. 2004 Aug 20;321(2):472-8. doi: 10.1016/j.bbrc.2004.06.165. PMID: 15358200.
11. Gao Z, Yang X, Jones E, Bingham PA, Scrimshire A, Thornton PD, Tronci G. An injectable, self-healing and MMPinhibiting hyaluronic acid gel via iron coordination. Int J Biol Macromol. 2020 Dec 15;165(Pt B):2022-2029. doi: 10.1016/j. ijbiomac.2020.10.079. Epub 2020 Oct 17. PMID: 33080264.
12. Santana FL, Arenas I, Haney EF, Estrada K, Hancock REW, Corzo G. Identification of a crocodylian -defensin variant from Alligator mississippiensis with antimicrobial and
antibiofilm activity. Peptides. 2021 Jul;141:170549. doi: 10.1016/j.peptides.2021.170549. Epub 2021 Apr 15. PMID: 33865931.
13. Barksdale SM, Hrifko EJ, van Hoek ML. Cathelicidin antimicrobial peptide from Alligator mississippiensis has antibacterial activity against multi-drug resistant Acinetobacter baumanii and Klebsiella pneumoniae. Dev Comp Immunol. 2017 May;70:135-144. doi: 10.1016/j.dci.2017.01.011. Epub 2017 Jan 13. PMID: 28089718.
14. Purification of reptilian hyaluronic acid and its use for soft and hard tissue repair. WO WO2021108790A1 Mitchell C. Sanders Lacerta Life Sciences, LLC Priority 2019-11-30 • Filed 2020-11-30 • Published 2021-06-03
15. Huynh P, Phie J, Krishna SM, Golledge J. Systematic review and meta-analysis of mouse models of diabetes-associated ulcers. BMJ Open Diabetes Res Care. 2020 May;8(1):e000982. doi: 10.1136/bmjdrc-2019-000982. PMID: 32467222; PMCID: PMC7259859.
16. Gilliver SC, Emmerson E, Campbell L, Chambon P, Hardman MJ, Ashcroft GS. 17betaestradiol inhibits wound healing in male mice via estrogen receptor-alpha. Am J Pathol. 2010 Jun;176(6):2707-21. doi: 10.2353/ajpath.2010.090432. Epub 2010 May 6. PMID: 20448060; PMCID: PMC2877833.
17. Sanders MC, S Balaji, WB Martin, N Siegmund, L Poland, MS Hanna, H Kaliada, S Littlejohn, and T Ganey, Protecting human amnion and chorion matrices (HACM) during processing: Performance enhancement in a Diabetic Mouse Model. Wound Repair and Regeneration (In Revision, Wound Repair and Regeneration).
18. Erickson HP. How Teichoic Acids Could Support a Periplasm in Gram-Positive Bacteria, and Let Cell Division Cheat Turgor Pressure. Front Microbiol. 2021 May 10;12:664704. doi: 10.3389/fmicb.2021.664704. PMID: 34040598; PMCID: PMC8141598.
19. Kang JH, Kim YY, Chang JY, Kho HS. Influences of hyaluronic acid on the anticandidal activities of lysozyme and the peroxidase system. Oral Dis. 2011 Sep;17(6):577-83. doi: 10.1111/j.1601-0825.2011.01807.x. Epub 2011 Apr 8. PMID: 21477181.
20. Müller S, Sindikubwabo F, Cañeque T, Lafon A, Versini A, Lombard B, Loew D, Wu TD, Ginestier C, Charafe-Jauffret E, Durand A, Vallot C, Baulande S, Servant N, Rodriguez R. CD44 regulates epigenetic plasticity by mediating iron endocytosis. Nat Chem. 2020 Oct;12(10):929-938. doi: 10.1038/s41557-020-0513-5. Epub 2020 Aug 3. PMID: 32747755; PMCID: PMC7612580.
21. Jaskuła K, Sacharczuk M, Gaciong Z, Skiba DS. Cardiovascular Effects Mediated by HMMR and CD44. Mediators Inflamm. 2021 Jul 10;2021:4977209. doi: 10.1155/2021/4977209. PMID: 34335086; PMCID: PMC8286199.
22. Noble PW, McKee CM, Cowman M, Shin HS. Hyaluronan fragments activate an NF-kappa B/I-kappa B alpha autoregulatory loop in murine macrophages. J Exp Med. 1996;183(5):2373- 2378. doi:10.1084/jem.183.5.2373
23. Pandey MS, Baggenstoss BA, Washburn J, Harris EN, Weigel PH. The hyaluronan receptor for endocytosis (HARE) activates NF- B-mediated gene expression in response to 40-400- kDa, but not smaller or larger, hyaluronans. J Biol Chem. 2013;288(20):14068-14079. doi:10.1074/jbc.M112.442889
24. Müller S, Sindikubwabo F, Cañeque T, Lafon A, Versini A, Lombard B, Loew D, Wu TD, Ginestier C, Charafe-Jauffret E, Durand A, Vallot C, Baulande S, Servant N, Rodriguez R. CD44 regulates epigenetic plasticity by mediating iron endocytosis. Nat Chem. 2020 Oct;12(10):929-938. doi: 10.1038/s41557-020-0513-5. Epub 2020 Aug 3. PMID: 32747755; PMCID: PMC7612580.
Alligator-Derived Hyaluronic Acid: Bacteriostatic and Fungastatic Properties Against Pathogens Wound Masterclass - Vol 1 - December 2022 67 References woundmasterclass.com Introducing Wound Masterclass Video woundmasterclass.com/Video © Copyright. Wound Masterclass. 2023 Image licenced from Adobe Stock. Credit: BillionPhotos.com
The Future of 3D Printed Biofilms for In Vitro and In Vivo Wound Infection Models



Editorial Summary
This paper provides an overview of the future of three dimensional printed biofilm for in vitro and in vivo wound infection models. Biofilm is an essential component of understanding the mechanism of stalling of wound healing. Biofilm removal remains a contentious area of wound care. Very few antimicrobial rinses and dressings on the market have been developed to target biofilms as there still remains to be an established ‘ideal’ assay to study mature biofilms in a high throughput screen that is reproducible. We describe a novel 3D printed biofilm model that can be utilized in vitro as well as in vivo
Introduction
Chronic wounds are a significant financial burden ($25B) to our health care system.1-3 Chronic hard-to-heal wounds are also a significant burden to the quality of life (QoL) of the patients. One of the major risk factors associated with lower extremity amputations and patient mortality is infected wounds, that are often stalled in the inflammatory phase of wound healing.
Wound infections are resistant to antibiotics and antimicrobial therapies because the bacteria are deep in the wound bed in a polymicrobial biofilm with a dense layer of polysaccharides, proteins, nucleic acids, and lipids referred to as the exopolymeric substance, or EPS. The biofilm is resistant to mechanical shear, the microbes deep in the biofilm are senescent and are resistant to antibiotics.
Although there are countless antimicrobial wound care rinses and dressings on the market, many have limited efficacy for removing biofilms because these antimicrobials and antibiotics were developed using minimal inhibitory concentration (MIC) assays with planktonic bacteria, which are easier to kill compared to complex biofilms.4-7 In addition, very few of these products have been developed to target biofilms because there hasn’t been an ideal assay to study mature biofilms in a high throughput screen (HTS), that is reproducible and has a high signal to noise ratio.
Boston MA, United States
One of the best characterized ex vivo models to study biofilms was developed in Greg Schultz’s laboratory.8,9 This model system can generate mature biofilms that are more reflective of a hard to kill biofilm in a chronic wound, but the model system requires several replicates due to the high standard deviation. Although recent improvements in the sterilization of the explants with immersion in 70% ethanol followed by 10% bleach reduced this standard deviation to some extent, there is still quite a lot of variability from lot to lot of the porcine skin due to the potential contamination of the explants with B. subtilis spores.
Here we report the development of the first of a kind 3D printed
 Ms Vanessa Vu
Research Associate I, Alira Health
Boston MA, United States
Ms Mia Hanna
Project Manager/ Research Associate III, Alira Health
Boston MA, United States
Dr Mitchell Sanders
Chief Scientific Officer, Alira Health
Ms Lindsay Poland
Scientist III, Lab Operations Manager, Alira Health
Ms Vanessa Vu
Research Associate I, Alira Health
Boston MA, United States
Ms Mia Hanna
Project Manager/ Research Associate III, Alira Health
Boston MA, United States
Dr Mitchell Sanders
Chief Scientific Officer, Alira Health
Ms Lindsay Poland
Scientist III, Lab Operations Manager, Alira Health
68 Wound Masterclass - Vol 1 - December 2022 © Copyright. Wound Masterclass. 2023
Boston MA, United States
“To develop an assay for a drug high throughput screen for biofilms, we had to develop methods for measuring the integrity of the biofilms and the ratio of living to dead bacteria within the 3D biofilm.”
collagen impregnated polymicrobial mature biofilms that have the ideal properties of high signal to noise, and low standard deviation that make each replicate reproducible enough for microplate assay (96 and 384 well) formats for drug and antimicrobial assays.
biofilm formation and then rinsed with water prior to storage in a sterile 24 well plate at -80°C for further use.
3D Printed Biofilms
Materials and Methods
Bacterial Strains and Reagents
Staphylococcus aureus (6538), Pseudomonas aeruginosa GFP (10145), and unlabeled P. aeruginosa (15442) were purchased from ATCC. Bacteria were grown overnight in tryptic soy broth (TSB) at 37°C prior to seeding into the collagen 3D printed biofilms. Shaved pig skin was obtained from Sierra for Medical Science.
Pig explants were sterilized as described by Yang et al. (2017), with the following modifications: The shaved porcine skin was sterilized with a combination of detergent (0.1% MACAT LHS) and a commercially available antimicrobial rinse (undiluted Oxivir 1) to reduce the starting bacterial bioburden. Following rinsing with water and air drying in the biosafety cabinet, the porcine explants were isolated with an 8 or 12 mm sterile biopsy punch. We then treated the biopsies with a 1M solution of CaCl2 for 20 minutes to mimic a calcium burn to improve
We used a Cellink 3D BioX Printer to print methylacrylated collagen (LifeLink 200) loaded with either GFP-Pseudomonas aeruginosa or Staphylococcus aureus and Pseudomonas aeruginosa and then photo-activated the collagen with lithium phenyl-2,4,6-trimethylbenzoyl phosphinate (LAP) dye and polymerized with a near-UV light source (365 nm). Briefly, we set up the Cellink 3D Bio X printer to print 12, 5 x 1 mm biofilm collagen disks at a pressure of 12 kPa, at a speed of 4 mm/sec, and an infiltration rate of 75%. An amber syringe containing a suspension of 2 ml of Lifelink 200 Collagen and a 250 μl of an overnight culture of each microbe was loaded into the printer in a dark room to avoid premature curing of the collagen with ambient light. After printing all the layers of the collagen-microbial disk, the UV light was utilized to cure the 3D print.
Following printing, the biofilm disks were exposed to natural light for 30 min to allow for complete curing and then incubated overnight at 37°C. The disks were then incubated for another 24 hours at 37°C in a proprietary biofilm binding buffer and were either used immediately or stored in tryptic soy broth with 2% sucrose for lyophilization followed by ambient storage or stored at -80°C for future use.
Imaging of the Biofilms
To develop an assay for a drug high throughput screen for biofilms, we had to develop methods for measuring the integrity of the biofilms and the ratio of living to dead bacteria within the 3D biofilm. Biofilms were imaged using a Keyence BZX-810 Fluorescence Microscope with DAPI, Texas Red, and GFP filter sets and a CFI
The Future of 3D Printed Biofilms for In Vitro and In Vivo Wound Infection Models Abbreviations AVG Average CFU Colony-forming unit CHG Chlorohexidine CN Cinnamon EPS Exopolymeric substance GFP Green fluorescent protein HTS High Throughput Screen LAP Lithium phenyl-2,4,6-trimethylbenzoylphosphinate LG Lemongrass MIC Minimal inhibitory concentration PI Propidium iodide PNAG Poly-N-acetyl-glucosamine PBS Phosphate buffered saline STDEV Standard deviation TSB Tryptic soy broth WGA Wheat germ agglutinin
Wound Masterclass - Vol 1 - December 2022 69 © Copyright. Wound Masterclass. 2023
PLAN FLUOR DL 4X or 20x Nikon Lens. Biofilm integrity assays were performed by staining the poly-N-acetyl-glucosamine, (PNAG) with Alexa Fluor 488 labeled wheat germ agglutinin as describe by Skogman et al. (2006).10 For our Live/Dead Analysis of the bacteria in the biofilms, we utilized LIVE/ DEAD™ BacLight™ Bacterial Viability Kit which utilizes SYTO 9 to stain live cells with green fluorescence and propidium iodide (PI) to stain dead cells red.
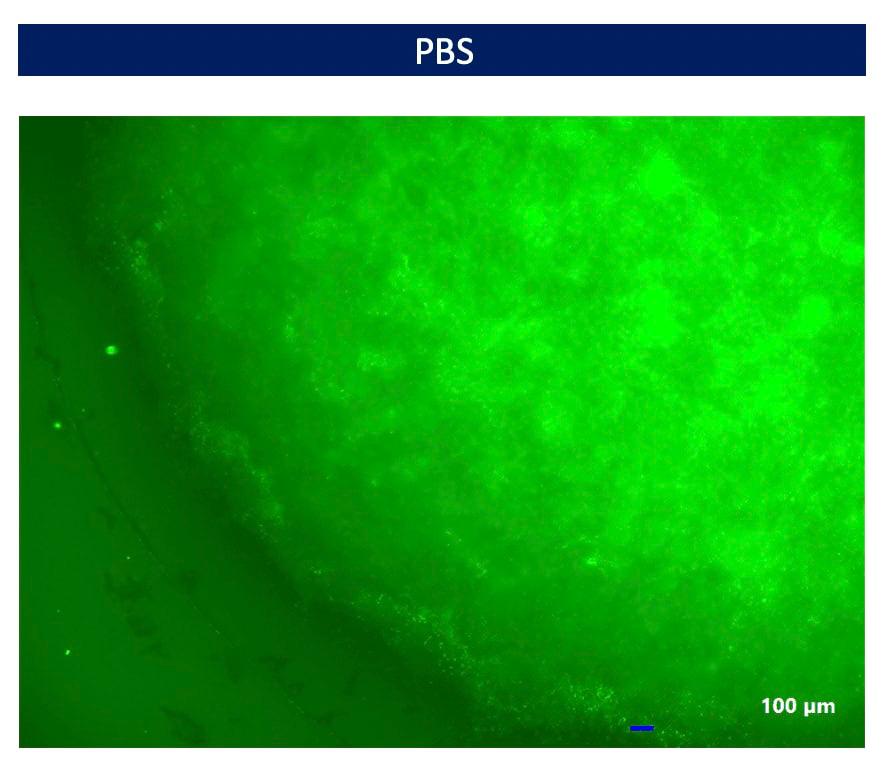
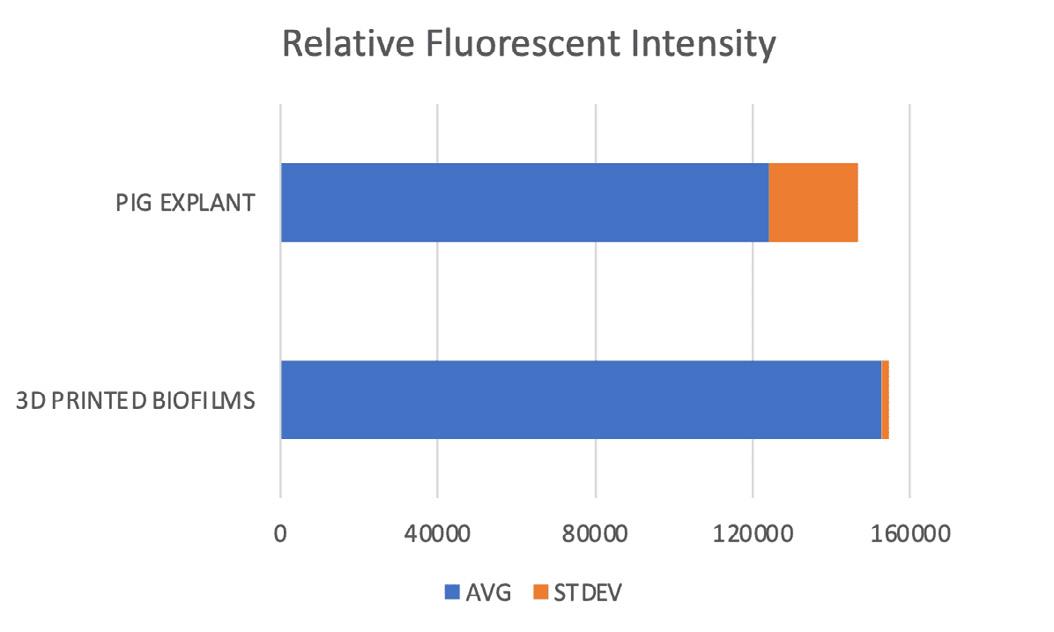
Results
We used a handheld 365nm UV light source and a digital phone camera to show the integrity of 3D printed GFP P. aeruginosa biofilms as shown in Figure 1.
Comparison of 3D Printed Biofilms vs. Pig Explant Biofilms
One of the inherent issues with the classical
and well recognized pig explant biofilms developed in Greg Schutz’s Lab is that each replicate has a high standard deviation requiring many replicates to achieve statistical significance. The second major inherent issue with the pig explant model is that it is often contaminated with Bacillus subtilis spores which makes each lot of porcine material less reproducible. We used P. aeruginosa labeled with GFP to compare the intensity and the standard deviation of biofilms formed in pig explants or the 3D printed collagen biofilms as shown in Figure 2.
Figure 4 illustrates the Live/Dead staining of the 3D printed biofilms at 20X magnification. Most of the bacteria in the biofilm stain is Green in the PBS control with a low intensity of dead bacteria in the Red image.
Figure 3 shows the diffuse PNAG fluorescence of the 3D printed biofilm at 20x magnification after incubation for 48 hours to form mature biofilms of S. aureus and P. aeruginosa and then incubated overnight in PBS.

The Future of 3D Printed Biofilms for In Vitro and In Vivo
Wound Infection Models
Figure 1: P. aeruginosa expressing GFP in 3D printed biofilms to demonstrate the uniformity of the replicates.
Figure 2: Relative fluorescent intensity of GFP P. aeruginosa biofilms grown on pig explants or 3D printed in bovine collagen. Pig explant biofilms have good signal with inherent variability even from the same lot (18.2%,124,011+ 22,604). 3D printed biofilmshave much better signal to noise ratio (152,826 + 1,752). The 3D printed biofilms have a p value of 0.0259 using a two-sample unequal variance t-test suggesting that the new model system is superior to the classic pig explant model.
70 Wound Masterclass - Vol 1 - December 2022 © Copyright. Wound Masterclass. 2023
Figure 3: PNAG Staining of 3D Printed Biofilms.
Drug and Antimicrobial Screening
To validate and test the efficacy of the 3D printed biofilm model, we treated the biofilms with chlorohexidine (CHG) or an essential oil (lemongrass (LG) and cinnamon (CN)) at 4% overnight at 37°C. Figure 5 shows that CHG had a profound reduction in the PNAG staining suggesting that it can affect the integrity of the biofilms. In contrast the PNAG staining was less affected by LG and CN.
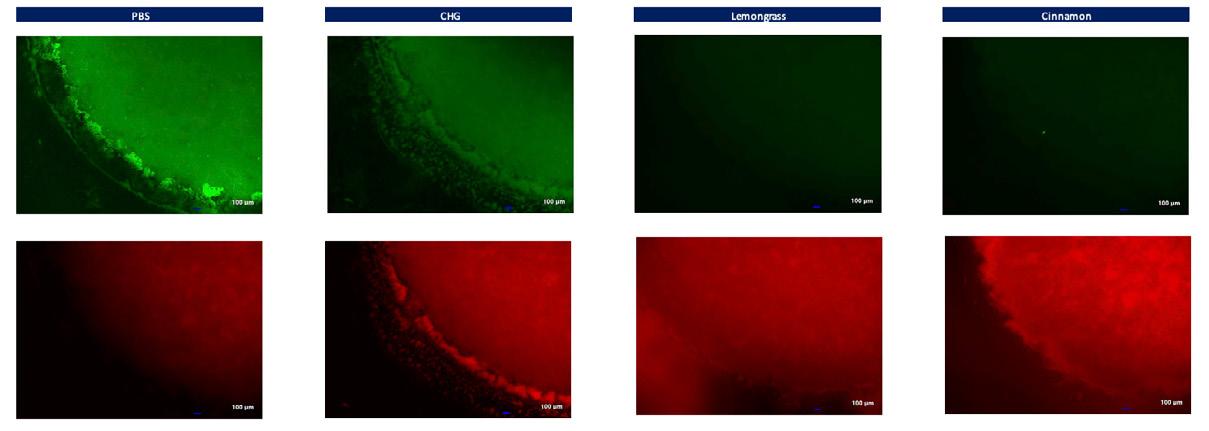
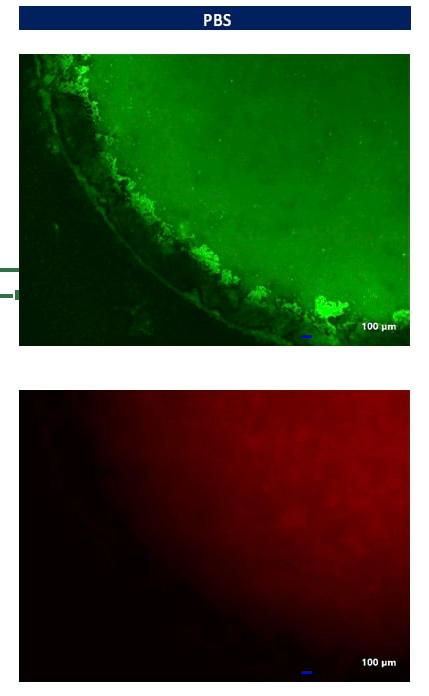
Following imaging of the biofilms we were able to sonicate three replicates of each treatment group and determine the CFU level of the bacteria in the polymicrobial biofilms (P. aeruginosa and S. aureus). Figure 7 shows that the treatment groups of CHG, LG and CN reduced the bacterial bioburden of the 3D printed biofilms but did not kill all the bacteria suggesting that 3D printed biofilms are hard to kill and robust enough for a high throughput
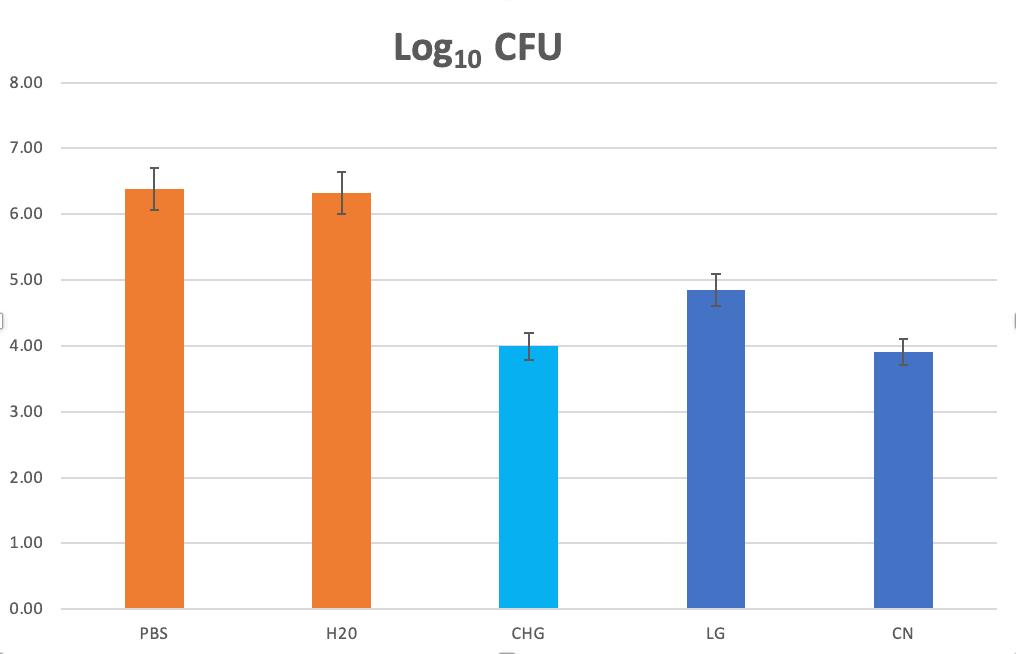
screen.
Discussions and Conclusions
In this paper, we describe the development of a standard HTS assay for mature polymicrobial biofilms using a state-of-the-art 3D Bioprinter to make consistent and reproducible replicates. The 3D printed mature polymicrobial biofilm have very high signal with limited standard deviation (<2%) among individual replicates. We are currently optimizing the lyophilization conditions so that plates can be shipped to collaborators for wound biofilm studies in vitro as well as in vivo
Our findings indicate that the 3D printed biofilms are robust and more reproducible than the pig explant model system described previously by Yang et al.1,2 The 3D printed biofilms can be stained with fluorescent probes to measure both the integrity of the biofilm
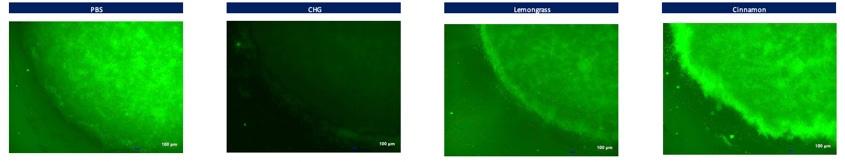
The Future of 3D Printed Biofilms for In Vitro and In Vivo Wound Infection Models
Figure 4: Live/Dead Staining of 3D Biofilms.
Figure 7: Log reduction of Polymicrobial Biofilms.
Figure 5: PNAG Staining of 3D Biofilms Treated with PBS, CHG, LG, and CN.
Wound Masterclass - Vol 1 - December 2022 71 © Copyright. Wound Masterclass. 2023
Figure 6: Live/Dead Staining of 3D Biofilms Treated with CHG, LG and CN.
The Future of 3D Printed Biofilms for In Vitro and In Vivo Wound Infection Models
Disclosures
We have no conflicts of interest to disclose.
References
1. Sen CK, Roy S, Mathew-Steiner SS, Gordillo GM. Biofilm Management in Wound Care. Plast Reconstr Surg. 2021 Aug 1;148(2):275e-288e. doi: 10.1097/PRS.0000000000008142. PMID: 34398099; PMCID: PMC8439557.
2. Olsson M, Järbrink K, Divakar U, Bajpai R, Upton Z, Schmidtchen A, Car J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019 Jan;27(1):114-125. doi: 10.1111/wrr.12683. Epub 2018 Dec 2. PMID: 30362646.
3. Sen CK. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv Wound Care (New Rochelle). 2019 Feb 1;8(2):39-48. doi: 10.1089/wound.2019.0946. Epub 2019 Feb 13. PMID: 30809421; PMCID: PMC6389759.

4. Percival SL. Restoring balance: biofilms and wound dressings. J Wound Care. 2018 Feb 2;27(2):102-113. doi: 10.12968/jowc.2018.27.2.102. PMID: 29424642.
5. Daeschlein G. Antimicrobial and antiseptic strategies in wound management. Int Wound J. 2013 Dec;10 Suppl 1(Suppl 1):9-14. doi: 10.1111/iwj.12175. PMID: 24251838; PMCID: PMC7950476.
6. Clinton A, Carter T. Chronic Wound Biofilms: Pathogenesis and Potential Therapies. Lab
Med. 2015 Fall;46(4):277-84. doi: 10.1309/LMBNSWKUI4JPN7SO. PMID: 26489671.
7. Mancl KA, Kirsner RS, Ajdic D. Wound biofilms: lessons learned from oral biofilms. Wound Repair Regen. 2013 May-Jun;21(3):352-62. doi: 10.1111/wrr.12034. Epub 2013 Apr 1. PMID: 23551419; PMCID: PMC3648594.
8. Yang Q, Phillips PL, Sampson EM, Progulske-Fox A, Jin S, Antonelli P, Schultz GS. Development of a novel ex vivo porcine skin explant model for the assessment of mature bacterial biofilms. Wound Repair Regen. 2013 Sep-Oct;21(5):704-14.

(PNAG) or the live/dead staining which correlates well with the fold log reduction of the CFU. We are currently validating this model system in a Diabetic mouse model to show that 3D printed biofilms have utility both in vitro as well as in vivo
doi: 10.1111/wrr.12074. Epub 2013 Aug 8. PMID: 23927831. 9. Yang Q, Larose C, Della Porta AC, Schultz GS, Gibson DJ. A surfactant-based wound dressing can reduce bacterial biofilms in a porcine skin explant model. Int Wound J. 2017 Apr;14(2):408-413. doi: 10.1111/ iwj.12619. Epub 2016 May 22. PMID: 27212453; PMCID: PMC7950006. 10. Skogman ME, Vuorela PM, Fallarero A. A Platform of Anti-biofilm Assays Suited to the Exploration of Natural Compound Libraries. J Vis Exp. 2016 Dec 27;(118):54829. doi: 10.3791/54829.PMID:28060302;PMCID:PMC522 72 Wound Masterclass - Vol 1 - December 2022 woundmasterclass.com Submit Your Research to Our Journal Case reports, randomized controlled trials, clinical reviews, audits, and research projects submissions@woundmasterclass.com © Copyright. Wound Masterclass. 2023 Image licenced from Adobe Stock. Credit: Rawpixel.com









Flexible solutions for complex wound reconstruction Native collagen fibers Elastin No chemical cross-linking
Atypical Wound
Presentations Lead to the Diagnosis of Rare Disease States: A Case Series

Editorial Summary
Therefore, clinicians should have a high index of suspicion for atypical wound etiologies and skin condition in wounds that fail to heal with standard of care. This article details the clinical and histological appearance of 3 atypical wound recently encountered by the author: Granular Parakeratosis, Bullous Disseminated Herpes Zoster, and Pancreatic Panniculitis. Identifying atypical wounds can be a difficult undertaking. If a chronic wound persists despite appropriate wound care treatments, then typical wound etiologies should be ruled out. Atypical wounds are rare, and their pathophysiology is not well understood. The diagnosis and management of these ulcer types is a real challenge to physicians. Skin biopsy plays a pivotal role in making the diagnosis and should be performed in all cases of refractory wounds.
Introduction
The prevalence of atypical wounds has been estimated that 20% of all chronic wounds are due to unusual causes.1,2 As our population ages clinicians are caring for patients with increased numbers of comorbidities and pathological processes that can contribute to the development of hard-toheal wounds. The negative impact of chronic wounds is well recognized in the literature. It is not uncommon for wound patients to suffer daily with pain, malodor, exudate management and reduced physical mobility. Patients dealing with chronic wounds often relate feelings of isolation and depression. Therefore, ability to identify and treat chronic wounds caused by uncommon etiologies is an important skill. Unfortunately, it can take years of clinical experience to master. To this argument, it is imperative that all wound care clinicians are knowledgeable about uncommon wound etiologies. Wound care providers are encouraged to be proactive when faced with hard-to-heal wounds of the lower extremity.
This case series details the clinical and histological appearance of 3 atypical wounds: Porphyria Cutanea Tarda, Bullous Disseminated Herpes Zoster and Pancreatic Panniculitis. A detailed history and physical exam including medical, travel, recreational and occupational histories were obtained to assure an accurate diagnosis was made. In addition, a complete physical exam and wound assessment including wound measurement, location, staging, tissue character and color, odor, exudate quality and amount, peri-wound tissue appearance, and pain were noted for each patient. In each case,
Case 1: Porphyria Cutanea Tarda
An 88-year-old female presented with multiple partial thickness wounds on the extremities. No history of trauma or other inciting event. PMH consists of Diverticulosis, CKD stage II, polyneuropathy, NIDDM, DJD, asthma, breast cancer, HTN and heart failure. Wound appearance is as follows: partial thickness tissue loss with irregular borders. Wound base is mixed of fibrotic and pink granulation tissue with scant serosanguinous exudate. The periwound skin appearance is friable with normal temperature. (Figure 1).
Previous therapy included cleansing the wound using an antibacterial wound cleanser, protecting the periwound skin with barrier prep and applying an antimicrobial moisture managing dressing changed twice weekly.
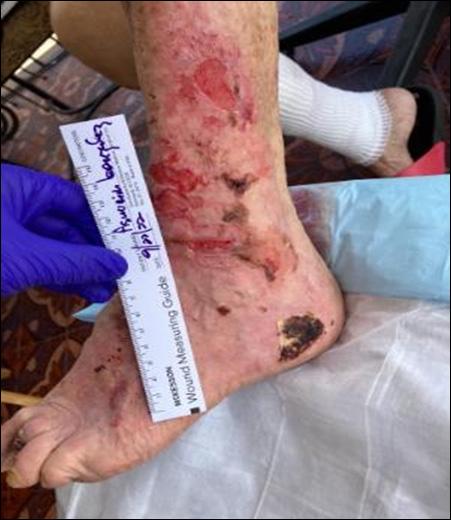
Wound Biopsies of
74 Wound Masterclass - Vol 1 - December 2022
Kent OH, United
Dr Windy Cole College of Podiatric Medicine, Kent State University
States
The prevalence of atypical wounds has not been studied extensively, but it has been estimated that 20% of all chronic wounds are due to unusual causes. As our population ages, clinicians are caring for patients with increased numbers of comorbidities and pathological processes that can contribute to the development of hard-to-heal wounds.
Figure 1: Clinical appearance of Porphyria Cutanea Tarda wounds
© Copyright. Wound Masterclass. 2023
“Cutaneous lesions are a result of a phototoxic reaction occurring in the upper dermis therefore lesions are commonly found in light-exposed areas of the body.3 Additionally minor skin trauma can result in sharply marginated eroded wounds.”
Biopsy was performed to rule out atypical wound etiologies including bullous disorders. Histology results were consistent with Porphyria Cutanea Tarda (Figure 2).

disorder with secondary effects on the skin, and liver disease is a major concern in the management. In almost all patients, liver biopsy reveals increased stainable iron, fatty change and intracellular porphyrin crystals.3 Roughly 15% of PCT patients will eventually develop hepatocellular carcinoma over the decade after presentation.3
Biopsy guided diagnosis is key. Histology samples display characteristic “caterpillar bodies”. Caterpiller bodiesare eosinophilic, periodic acid-Schiff (PAS)-positive globules arranged in a linear fashion in the epidermis overlying subepidermal blisters of PCT.4
Case 2: Bullous Disseminated Herpes Zoster
Porphyria cutanea tarda (PCT) is characterized by blisters of skin in light-exposed areas. Cutaneous lesions are a result of a phototoxic reaction occurring in the upper dermis therefore lesions are commonly found in light-exposed areas of the body.3 Additionally minor skin trauma can result in sharply marginated eroded wounds.
PCT is usually an acquired condition caused by inhibition of the uroporphyrinogen decarboxylase enzyme in the liver.3 Risk factors include hereditary haemochromatosis, hepatitis C virus infection, alcohol, estrogens, and a family history of PCT.(3) It is common to also have a history ofliver disease, including hepatocellular carcinoma, in patients with PCT. Low dose chloroquine administered twice weekly is the current best treatment.3 In patients with severe iron overload, venesection is a helpful adjunctive therapy.
In short, PCT can be thought of as a liver
A 74-year-old male was initially evaluated in his home for assessment of a chronic nonhealing wound on the sacrum. Past medical history included hemiplegia following a spinal cord injury, GERD, arthritis, BPH, COPD, hypertension, PVD and obesity. The wound has been present for over 4 months. Patient was noted to be non-ambulatory and bed bound. Upon initial assessment, the wound the wound base extends through subcutaneous tissue and is red and granular. The periwound tissue displays mild maceration and there is epibole of the tissue appreciated at the wound edge. No clinical signs and symptoms of infection were noted. The diagnosis of a Stage 4 pressure injury was made. Treatment consisted of sharp debridement, followed by application of collagen to the wound base covered with boarded gauze. Offloading of the area was achieved with an alternating pressure mattress, ROHO cushion and turning education provided to the caregiver. Patient was continuously followed for wound assessment and evaluation, but the wound continued to deteriorate despite offloading and advanced wound care, (Figure 3).
Wound Biopsies of Atypical Wound Presentations Lead to the Diagnosis of Rare Disease States: A Case Series Wound Masterclass - Vol 1 - December 2022 75
Figure 2: Linear segments of degenerated keratinocytes called ‘caterpillar bodies’ seen in the basal layer.
© Copyright. Wound Masterclass. 2023
The patient was eventually hospitalized where he was taken to surgery for a debridement and biopsy of the wound. The histology report noted that the tissue specimen contained herpes viral inclusions. Immunohistochemical stains for VZV were positive. AFB, GMS and gram stains were negative for infectious organisms, (Figure 4).
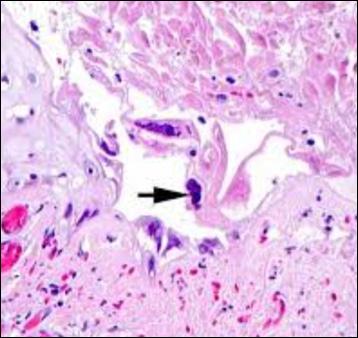
The Herpes zoster virus usually affects the thoracic and lumbar vertebra (T3–L3), while sacral herpes zoster is rare and has only been reported in 4-8% of cases.5 An atypical wound presentation such as this is a rare occurrence The incidence of VZV increases in patients over the age of 60.6 Decreases in cellular immunity is
a well-defined risk factor in the development of active cases of shingles and chronic nonhealing wounds.6 It stands to reason that patient with multiple underlying chronic disease states would be a greater risk of developing more severe cases of shingles. Consequently, it should be kept mind that VZV, which develops in geriatric patients and individuals with weaker immune systems, may also appear with sacral region involvement, which is rarer, in addition to its typical regions of involvement.

Case 3: Pancreatic Panniculitis Pancreatic Panniculitis
A 38-year-old female presented to the emergency department with fatigue and right lower extremity pain. The patient’s past medical history was significant for systemic lupus nephritis (SLE), end stage renal disease (ESRD) on hemodialysis, on anticoagulation with coumadin due to mitral valve replacement, cholecystectomy, chronic gastroesophageal reflux, colitis, venous insufficiency, anemia, and opioid abuse on methadone. The lower extremity examination revealed multiple ill-defined full thickness ulcers with yellow purulent drainage, and erythematous and macerated borders throughout the medial and posterior aspects of the right lower leg (Figure 5). The left leg showed no edema or open wounds. The patient’s hemodialysis catheter site was intact and nontender.
Wound Biopsies of Atypical Wound Presentations Lead to the Diagnosis of Rare Disease States: A Case Series 76 Wound Masterclass - Vol 1 - December 2022
“The Herpes zoster virus usually affects the thoracic and lumbar vertebra (T3–L3), while sacral herpes zoster is rare and has only been reported in 4-8% of cases.” 5
Figure 3: Clinical appearance of disseminated herpes zoster wounds.
Figure 4: Large intraepidermal vesicle surrounded by inflammatory cell infiltrate.
© Copyright. Wound Masterclass. 2023
Figure 5: Clinical appearance of Pancreatic Panniculitis wounds.
A punch biopsy of one of the right lower leg wounds was obtained. Histopathological examination revealed tissue necrosis, gram positive bacterial cocci, hemosiderin deposition, possible thrombosis of blood vessels, numerous neutrophils and ghost cells (Figure 6). Stains were negative for fungus and subcutaneous vascular calcification.

Discussion
One of the first indications that a chronic wound may be atypical is that it lacks a history of an acute trauma and/or it does not fit into a known clinical category. Common wound presentations include arterial wounds caused as a result of poor blood supply. Arterial ulcers often present as necrotic, well defined wounds that are most often localized on the dorsum of the foot or distal toes.9 In cases of arterial wounds, pain typically occurs with leg elevation. Venous leg ulcers (VLUs) are due to venous insufficiency. Most VLUs occur around the medial malleolus.9 These wounds are highly exudative and display irregular borders and tend to be covered by a layer of fibrin. Prolonged pressure can lead to pressure injuries. Pressure ulcers often occur in areas of boney prominences due to increases in stress and shearing forces.9 Diabetic foot ulcers (DFUs) occur in a patient with long-standing diabetes, neuropathy and/or peripheral arterial disease.9 If a wound does not seem to fit into any of these categories and fails to respond to standardized wound therapies, clinicians should dig deeper in order to ascertain the correct diagnosis and begin to provide the appropriate wound care.
Pancreatic panniculitis is a rare disorder caused by the release of pancreatic enzymes into the bloodstream resulting in subcutaneous fat necrosis. This condition can be difficult to diagnose. The cutaneousmanifestation of PPresults in painful or painless subcutaneous nodules on the legs. Clinically, pancreatic panniculitis lesions appear as subcutaneous focal necrosis. Histologically, the presence of “ghost cells,” which are anucleate necrotic adipocytes with thick, obscure walls is pathognomonic.7
Current management for wounds caused by pancreatic panniculitis include the utilization of fluid therapy, antibiotics, pain analgesia, systemic anti-inflammatories, and antimalarials upon initial presentation of the lesions.8 Effective wound management for pancreatic panniculitis remains poorly described in theliterature due to the paucity ofreported cases of this condition.
There are several wound characteristics that can alert the clinician that a wound my have an atypical etiology.10
• Unusual location: A wound that appears to bevenous in nature, but it does not appear on the typical locationfor a VLU.
• Asymmetry: Wounds with irregular edges should be closely monitored.
• Excessive or friable granulation tissue: When granulation tissue has disproportionate cell growth or bleeds very easily, this may be an indication of an underlying pathologic process.
Wound Biopsies of Atypical Wound Presentations Lead to the Diagnosis of Rare Disease States: A Case Series Wound Masterclass - Vol 1 - December 2022 77
“One of the first indications that a chronic wound may be atypical is that it lacks a history of an acute trauma and/or it does not fit into a known clinical category.”
Figure 6: Ghost cells.
© Copyright. Wound Masterclass. 2023
Wound Biopsies of Atypical Wound Presentations Lead to the Diagnosis of Rare Disease States: A Case Series
• Patient age: It would be very unusual for a young patient to present with PAD and gangrene of a toe.
• Radiation to the area: Radiation can lead to cell death and tissue necrosis.
• Remote history of trauma: Repetitive trauma can lead to pathological changes in the skin and surrounding tissues.
• Pain: When patients present with pain out of proportion to clinical appearance, an atypical wound should be ruled out.
• Pigmented lesions: This can indicate an inflammatory or malignant process is occurring.
• Vegatative growth: Fungating tissue growth can indicate an infective process or be a sign of malignancy.
Conclusion
Identifying an atypical wound can be a difficult undertaking. A detailed history and physical exam are the two most critical factors in assuring an accurate diagnosis is made. Obtaining in-depth medical, travel, recreational and occupational histories should be gathered from the patient. A complete physical exam and wound assessment including wound measurement, location, staging, tissue character and color, odor, exudate quality and amount, peri-wound tissue appearance, and pain are important details to notate. If a chronic wound persists despite appropriate wound care treatments and typical wound etiologies are ruled out based on these findings additional diagnostic testing is appropriate.
Atypical ulcers exhibit random clinical features, histology, and location. Resistance to standard therapies and difficulty in diagnosing often results in delayed treatment. Tissue biopsies should be considered in all wounds not
responding to standard of care and/or presenting with an atypical appearance. In many cases, the histopathological features found in the tissues are pathognomonic for specific disease states. There are distinct differences seen between wounds caused by malignancies, vasculopathies, infections, metabolic disorders and other inflammatory processes. Site selection is of paramount importance. Perilesional tissue biopsies are frequently the key to confirming a diagnosis. Best practices include obtaining a punch or incisional biopsy of the ulcer border.11 Multiple biopsy samples are helpful to aid in difficult diagnoses.11 If possible, an additional sample should be collected from the center of the wound.11 The biopsy specimen should contain the epidermis, dermis and subcutaneous tissue. It is preferable to engage a dermatopathologist for histological examination of specimens. Routine histology specimens can be placed in 10% formalin solution. If direct immunofluorescence or microbiology studies are indicated, specimens should be placed in saline solution with the sample reaching the lab is less than 24 hours. Correctly marking the tissue for orientation in cases of suspected malignancy can be achieved with permanent marker or suture material. The orientation should be clearly marked, and the information conveyed to the pathologist. Specimen containers labeled with the name of the patient, date and time specimen was collected, and tissue site is essential. Additionally, providing a brief history and clinical description of the lesion may be helpful in developing a diagnosis.
References
1. Shanmugam VK, Angra D, Rahimi H, McNish S. Vasculitic and autoimmune wounds. J Vasc Surg Venous LymphatDisord. 2017 Mar;5(2):280-292.
2. Shanmugam VK, Schilling A, Germinario A, Mete M, Kim P, Steinberg J, Attinger CE. Prevalence of immune disease in patients with wounds presenting to a tertiary wound healing centre. Int Wound J. 2012 Aug;9(4):403-11.
3. Sarkany, R.P.E. (2001), The management of porphyria cutanea tarda. Clinical and Experimental Dermatology, 26: 225-232.
4. Egbert BM, LeBoit PE, McCalmont T, Hu CH, Austin C. Caterpillar bodies: distinctive, basement membrane-containing structures in blisters of porphyria. Am J Dermatopathol. 1993 Jun;15(3):199-202.
5. Hur J. Sacral herpes zoster associated with voiding dysfunction in a young patient with scrub typhus. Infect Chemother. 2015;47(2):133–136.)
6. Dworkin RH, Johnson RW, Breuer J et al. Recommendations for the management of herpes zoster, ClinInfectDis2007;44(Suppl 1):S1-26. http://dx.doi.org/10.1086/510206 PMid:17143845
7. Montes de Oca MK, Jamison RA. Pancreatic panniculitis as the initial presentation of intrahepatic cholangiocarcinoma. JAAD Case Rep. 2018 Jun 2;4(6):528-530.
8. Kapoor, A., Hayat, M. H., Yousaf, A., & Tariq, R. (2021). Abdominal pain and leg lesions: Is there a connection? BMJ Case Reports, 14(3). https://doi.org/10.1136/bcr-2021-242422
9. Martinengo L, Olsson M, Bajpai R, et al. Prevalence of chronic wounds in the general population: systematic review and meta-analysis of observational studies. Ann Epidemiol. 2019;29:8–15.
10. Hamm RL, Shah JB. Atypical Wounds. Text and Atlas of Wound Diagnosis and Treatment: 2nd edition. New York: McGraw Hill Education. 2019,235-268.
11. Stevenson P, Rodins K. Improving diagnostic accuracy of skin biopsies. Aust J Gen Pract. 2018 Apr;47(4):216-220.
78 Wound Masterclass - Vol 1 - December 2022
© Copyright. Wound Masterclass. 2023
RELIEVE PAIN STIMULATE HEALING
Chronic wounds can also be extremely painful –between 50% and 60% of patients with a chronic wound experience persistent pain.[1,2] One major issue is that pain can make gold standard treatments, such as compression, unbearable.[3]
Electrical stimulation has been used globally in specialist clinics for more than a decade to relieve pain and accelerate healing in chronic wounds. With nine meta analyses and over 35 randomised controlled trials published, electrical stimulation is one of the most evidence-based technologies in wound management.
Electrical stimulation therapy is now available in a simple to use, single use, wearable device, enabling its more widespread use across different care settings. Accel-Heal Solo electrical stimulation wound therapy is a one-off, interventional, 12-day treatment which is applied alongside existing care to relieve pain [4] and accelerate healing [4,5]. Accel-Heal Solo improves the quality of life for the patient [5] and helps facilitate the use of compression therapy where wound pain has been an issue.[6]
To find out more about Accel-Heal Solo, please contact: customerservices@accelheal.com

References. 1. Price P, et al. Managing painful chronic wounds: the Wound Pain Management Model. Int Wound J. 2007;4(1). 2. Hellström A, et al. Leg ulcers in older people: a national study addressing variation in diagnosis, pain and sleep disturbance. BMC Geriatr. 2016;16(1):25. 3. Atkin L, et al. Accel-Heal Made Easy. Wounds UK. 2019. https://www.wounds-uk.com/resources/details/made-easy-accel-heal-electrical-stimulation 4. Guest J, et al. Cost-effectiveness of an electroceutical device in treating non-healing venous leg ulcers: results of an RCT. J Wound Care. 2018;27(4):230-243. 5. Turner N, L. Ovens. Clinical outcome results and quality of life improvements using electroceutical treatment* - Patient perspectives. Presented at: EWMA 2018. 6. Ovens L. Presented at: Wounds UK. ; 2014.
There have been significant advances in chronic wound management treatments over the last 30 years, however, hard-to-heal wounds are an increasing problem.
Ms Hayley Ryan
Director, WoundRescue Pty Ltd. Wound Clinical Nurse Consultant
Newcastle NSW, Australia
Addressing the Challenge of Pressure Injuries

Editorial Summary
This article provides a concise overview of the significant problem of pressure ulcers and how the prevalence of this avoidable public health issue can be reduced. The various factors that increase risk of pressure ulcers are explored, as well as some of the standards and guidelines that can help healthcare practitioners identify symptoms early and prevent pressure ulcers from developing.
Introduction
Pressure injury (PI) is a significantly under recognised public health issue. The approximate total cost of pressure injury in the United States is $26.8 billion per year.1 In Australia, this number is approximately $1.8 billion.2 Clearly, we have a significant issue; we also know that in the United States around 60,000 patients will die every year specifically related to a pressure injury.3 At Wounds Australia we think it is a lot higher than that, but since we don’t actually collect data in Australia, it’s very difficult to make an estimation.
When answering these questions it is vital to review the latest evidence based practice.
Understanding the Skin
In order to treat a pressure injury, or prevent them, it really starts in understanding the skin. When it comes to understanding the skin in relation to wound healing, there are two principle layers: The epidermis, which functions to resurface wounds and restore the barrier against bacteria; and the dermis, which functions to restore structural integrity, collagen and physical properties of the skin.
When it comes to pressure injury, it is crucial to know where it affects to skin, since this is the part which helps us diagnose the correct stage.
Stages of Pressure Injury
• Stage 1: Quite superficial; top layer of skin
• Stage 2: Visible breaks in the skin
• Stage 3: Visible fatty tissue
• Stage 4: Visible bone, tendon or muscle
Importance of Skin Ph
We know that skin pH tends to be between 4 and 6.5. Optimal skin pH, however, is 5.5; this is because the acid mantle, which protects the skin against microorganisms, is protected.
One focus for maintaining skin integrity is to consider things like soap-free cleansers, and pH neutral emollients; no matter what area of healthcare you are working in, you should always encourage patients to use emollients and soaps which are soap-free, fragrance-free, and pH neutral; this is key to preventing the skin from breaking down.
Nutrition
In wound management, nutrition is pivotal. Changes in nutritional status can alter skin structure and function if there is a wound present. It is important to screen people for nutritional status.
There is debate over whether to focus on prealbumin or serum albumin levels. I request serum albumin levels when I’m considering someone who may be malnourished; if results are <30g/L, this will delay healing. This tells us that the focus we need to have is on high
80 Wound Masterclass - Vol 1 - December 2022
© Copyright. Wound Masterclass. 2023
“If we pre-emptively protect the skin, we are less likely to see a breakdown into pressure injuries.”
protein diet, and extra fluids.
The micronutrients that you should also focus on include iron, zinc, vitamins A and C; these will support wound healing. Deficiencies in vitamins and proteins are common in the elderly, by definition, over the age of 65.
Tips for Maintaining Skin Integrity
• Use a soap free substitute
• Apply a sunscreen, even in winter
• Avoid hot and frequent showers
• Pat skin dry
• Inspect skin every shift
• Consider the need for support surfaces
• Maintain optimal nutrition and hydration
• Moisturize skin twice daily
Skin Moisturising
Carville et al., in a randomized controlled trial which was conducted in aged care homes, showed that twice-daily moisturizers on the limbs showed a reduction of skin tears to up to 50%.
The theme here is, if we pre-emptively protect the skin, we are less likely to see a breakdown into pressure injuries.
PI in the Aged Person
The skin changes as we age. We start to get decreased sensation, we get increasing dryness; skin starts to thin, there is decreased vitamin D synthesis; we get reduced immune response, and a decrease in the ability to control body temperature. The skin wrinkles, there is breakdown of collagen; the skin is more prone to susceptible to breaking down.
Risk Factors for Pressure Ulcer Development
1. Inability to perceive pressure
2. Incontinence/ moisture
3. Decreased activity level
4. Inability to reposition
5. Poor nutritional intake
6. Friction and shear
Medical Risk Factors
Disease
Those living with dementia, have had a stroke, or are living with Parkinson’s disease, unrepaired hip fractures, frailty and sepsis; heart failure, or calcium channel antagonists and alpha blockers, are at higher risk of developing PI.
Aetiology
Factors putting patients at greater risk of PI include hypomania/ hypermania, mobility/ contractures; mobility/ tone, nutrition/ Catabolic state, or poor healing reserve; localised oedema and pedal oedema.
Intervention
Intervention can also lead to a higher risk of PI; over-sedation, thrombolyses and dopamine issues, and diuretics are examples here.
These factors will all impact and increase the person’s risk of PI. There are also major gaps and issues in the home and community space when people are independently looking after themselves.
Addressing the Challenge of Pressure Injuries Wound Masterclass - Vol 1 - December 2022 81
© Copyright. Wound Masterclass. 2023
“The 2019 guidelines include excellent visual guides to the stages and types of PI, and very importantly, in darkly pigmented skin, which can look very different than in lighter skin tones.”
What is a PI?
From the 2019 guidelines, a pressure injury is defined as:
“Localised damage to the skin and/ or underlying tissue, as a result of pressure or pressure in combination with shear. Pressure injuries usually occur over a bony prominence but may also be related to a medical device or other object.”
• The 2019 guidelines include excellent visual guides to the stages and types of PI, and very importantly, in darkly pigmented skin, which can look very different than in lighter skin tones:
https://npiap.com/page/PressureInjuryStages
Recognising Incontinence Associated Dermatitis
Incontinence Associated Dermatitis (IAD) is defined as “...a type of irritant contact dermatitis found in patients with faecal and/ or urinary incontinence.”
• Initially erythema that can be pink to red, darker skin tones can be paler, darker, purple
• Poor defined edges/ patchy
• May feel warmer
• Lesions: vesicles or bullae, papules or pustules
• Varying depths
• Discomfort, pain, burning, itching or tingling
• Susceptible to skin infections
IAD needs to be categorized. Naturally, if there is no redness and the skin is intact, it is not IAD. However, if there is visible redness and the skin is intact, it is category 1. If the skin is red, and broken down, it is a category 2. Most importantly, if the patient is not incontinent, then the condition is not IAD.
The Impact of the COVID-19 Pandemic
Medical Device Related Pressure Injuries
Multiple pressure injuries can be caused by common medical devices, such as oxygen delivery/ Nebuliser masks; intubation devices to ventilate, and catheters. Common locations of these are the cheeks, nose/ nostrils, neck, ears, lips, and tongue.
In terms of the COVID-19 pandemic, we have had to alter our work environments; some of us closed wound clinics, we had to merge and change wards to ‘COVID wards’ Home and Community (HCC) has seen an increase of services being cancelled, Aged Care has seen shifts in work flows and visitor limitations which added to environmental concerns, GPs seeing an increase of patients presenting with advanced symptoms, and the setting up of drive through PCR testing centers. We had redirection of resources to the COVID-19 response, and changes to wound care delivery, e.g., remote care/ Telehealth.
Wound Care in the COVID-19 Era and Beyond
The Wound Care Learning Network have done a fantastic job in putting together a proposed new focus during the COVID-19 era, which is not over; maybe we need to shift our thinking when it’s so difficult to get to patients during COVID-19 outbreaks. Rather than saying wound healing, we should maybe be focusing on wound maintenance. What can we do to stop that wound from breaking down while we can’t get to that patient?
82 Wound Masterclass - Vol 1 - December 2022
Addressing the Challenge of Pressure Injuries
© Copyright. Wound Masterclass. 2023
“Prevention works, but ongoing preventative programs work better. Systematic reviews show that pressure injury prevention programs result in statistically and clinically significant reductions in pressure injury rates, from 50% to 100%..”
During the COVID-19 pandemic we have also seen damage to skin from intense, extended use of PPE by clinicians and those working in healthcare. This is pressure injury; from friction, pressure and shear. The main areas affected are the nasal bridge, forehead, cheeks and ears.
How do we prevent this safely? Dressings under PPE can disrupt their purpose and use, so thin dressings are suggested to avoid seal issues. The integrity of dressings should be monitored during work shifts and at each PPE change. The correct size of mask should be chosen (fit testing is important), adjusting to the anatomy of the face to optimize the seal, and of course, the manufacturer requirements must be followed. The skin can be protected by washing the face with pH balanced cleanser and drying it well. Apply moisturiser, 1 - 2 hours before donning PPE, ensuring it is fragrance free and is pH neutral. We can aim to reduce the friction/ shear caused by PPE; again, consider the fit. Protect the skin from moisture trapped underneath the PPE. Avoid wearing makeup. Finally, limit the length of time that PPE is worn.
PI Prevention Programs
Why should we invest time in PI Prevention Programs (PIPP)? It’s a community expectation not to develop PI; prevention avoids poor practice; depending on geographic location, PIs can be a reportable complication. It’s more cost effective to prevent PI considering the cost involved in treatment. Realistically, it’s the right thing to do.
Prevention works, but ongoing preventative programs work better. Systematic reviews show that pressure injury prevention programs result in statistically and clinically significant reductions in pressure injury rates, from 50% to 100%. Other benefits include optimal client care and avoiding the cost of treating Stage 3 and above injuries.
So where do we begin? Firstly, risk assessments. There are differences in opinion in the literature about which one is best, but use one, as it provides a guide; remember, every assessment is only as good as the person doing the
assessment; if risk assessment is rushed, nonmethodical, it may not give the right result. Secondly, brainstorm together and talk to one another; discuss at meetings and handoverscollaborate! Consider manual handling; are you repositioning the person with the right devices, like a slide sheet, for example? Think about nutrition and hydration. Manage moisture. Protect the skin, and check it often. Crucially, start making referrals early. Collect data.
Surface: Make sure your patients have the right support
Skin inspection: Early inspection means early detection. Show patients and carers what to look for
Keep your patients moving
Incontinence/ Moisture: Your patients need to be clean and dry
Nutrition/ Hydration: Help patients have the right diet and plenty of fluids
Preventative Tips
• Daily Skin Inspections: consider temperature/ skin tone
• Avoid massaging bony areas
• Complete risk assessments
• Moisturize twice a day: consider barrier creams to manage moisture
• Monitor those that are incontinent: use pH neutral soaps
• Check equipment daily: e.g., mattresses
• Reposition based on the individual assessment
• Consider nutrition
Make prevention a priority and share knowledge!
Addressing the Challenge of Pressure Injuries Wound Masterclass - Vol 1 - December 2022 83
SSKIN
© Copyright. Wound Masterclass. 2023
Conclusion
To conclude, let’s consider a few take home messages. Firstly, the skin; use logical and systematic approaches to manage and prevent skin breakdown. Remember, it is the largest organ on the body. Secondly, the team; think about a constant, accurate and multidirectional flow of information sharing with everybody who works in that area, including the patient, which brings us to the third point; put the patient at the core of all decision making. If we put these three points into play, we are certainly on the right path to preventing pressure injuries.
References
1. Padula, WV, Delarmente, BA. The national cost of hospital-acquired pressure injuries in the United States. Int Wound J. 2019; 16: 634– 640. https://doi.org/10.1111/iwj.13071

2. Nguyen KH, Chaboyer W, Whitty JA. Pressure injury in Australian public hospitals: a cost-of-illness study. Aust Health Rev. 2015 Jun;39(3):329-336. doi: 10.1071/AH14088. PMID: 25725696.
3. Bauer K, Rock K, Nazzal M, Jones O, Qu W. Pressure Ulcers in the United States' Inpatient Population From 2008 to 2012: Results of a Retrospective Nationwide Study. Ostomy Wound Manage. 2016 Nov;62(11):30-38. PMID: 27861135.
4. Carville K, Smith J. A report on the effectiveness of comprehensive wound assessment and documentation in the community. Prim Intent. 2014;12(1):41‐49.
5. Issue paper: Chronic wounds in Australia, http://www.aushsi.org.au/wp-content/ uploads/2017/08/Chronic-Wounds-Solutions-Forum-Issues-Paper-final.pdf
6. Matthew Malone, Saskia Schwarzer, Michael Radzieta, Thomas Jeffries, Annie Walsh, Hugh G. Dickson, Grace Micali and Slade O. Jensen, Effect on total microbial load and community composition with two vs six‐week topical Cadexomer Iodine for treating chronic biofilm infections in diabetic foot ulcers, International Wound Journal, , (2019).
7. Young C, Stoker F. A four‐year review of pressure ulcer prevalence. Prim Intent. 2017; 8( 1): 6‐ 12.
8. Vowden P. Hard-to-heal wounds Made Easy. Wounds International 2011; 2(4): Available from http://www. woundsinternational.com

9. International Wound Infection Institute (IWII) Wound infection in clinical practice. Wounds International 2016
10. European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. The International Guideline. Emily Haesler (Ed.). EPUAP/ NPIAP/PPPIA; 2019.
11. Peko, L., Barakat-Johnson, M., Gefen, A. (2020). Protecting prone positioned patients from facial pressure ulcers using prophylactic dressings: A timely biomechanical analysis in the context of the COVID-19 pandemic. International Wound Journal, 17(6), 1595-1606.
12. Beeckman D et al. Proceedings of the Global IAD Expert Panel. Incontinence-associated dermatitis: Moving prevention forward. Wounds International 2015.
Addressing the Challenge of Pressure Injuries 84 Wound Masterclass - Vol 1 - December 2022 woundmasterclass.com/Register Register for full access to the journal, educational resources, information about upcoming events and more woundmasterclass.com © Copyright. Wound Masterclass. 2023 Image licenced from Adobe Stock. Credit: Dragana Gordic
Ms Kathleen Martin Legal Nurse Consultant Cave Creek AZ, United States

Minimizing the Legal Issues Around Pressure Injury Care
Editorial Summary
Introduction
It is estimated that each year between 1 and 3 million people in the United States develop pressure injuries.1 In skilled nursing facilities, it is estimated that up to 28% of patients suffer pressure injuries.1 The numbers increase dramatically with patients in high-risk groups such as quadriplegics suffering pressure ulcer injuries.2 Because some pressure injuries may be preventable with proper nursing care, these cases are often ripe for litigation.2
The Legal Nurse Consultant (LNC) can play an invaluable role in reviewing these cases and assisting throughout litigation. The LNC must have an understanding of the federal nursing home regulations, the various practices behind prevention and treatment of pressure injuries, and nursing home documentation. These federal regulations require unique charting that differs from hospital records, including the Minimum Data Sets (MDS) and Resident Assessment Protocols (RAP sheets). In addition, to understanding the medical, nursing, and charting issues, the LNC should have a basic understanding of the legal aspects of a pressure ulcer/injury case.
A pressure injury case, at the most basic level, requires the plaintiff to prove these elements:
Federal and State Regulations
Nursing homes are among the most heavily regulated businesses in the country. In 1987, Congress passed the Omnibus Budget and Reconciliation Act (OBRA) also called the Nursing Home Reform Act. The regulations both generally and specifically address a nursing home’s duties to prevent and treat pressure injuries.
The regulations impose a high standard on nursing facilities regarding the prevention and proper treatment of pressure injuries. The facility providers have a duty to ensure:
A resident who enters a facility without pressure injuries does not develop pressure injuries unless the individual’s clinical condition demonstrates that they were unavoidable; and
A resident having pressure injuries receives necessary treatment and services to promote healing, prevent infection and prevent new injuries from developing.
In skilled nursing facilities, it is estimated that up to 28% of patients suffer pressure injuries.1 The numbers increase dramatically with patients in high-risk groups such as quadriplegics suffering pressure ulcer injuries.2 Because some pressure injuries may be preventable with proper nursing care, these cases are often ripe for litigation.2 This article is an overview of how legal issues can be minimized around pressure injury care. •
It might be important that when investigating a pressure injury case against a nursing home that the LNC review at least three years of surveys by the state’s health department. These surveys may reveal a pattern of neglect. Such surveys, if not provided to the LNC/Expert may not use them in the report or other testimony developed by the LNC.
The survey reports may be the foundation for building a punitive damage claim. Punitive damages are an additional recovery for the plaintiff. They are intended to punish the
86 Wound Masterclass - Vol 1 - December 2022
© Copyright. Wound Masterclass. 2023
The health care provider owed a duty of care to the patient • The duty was breached. The provider was negligent or committed malpractice • The patient suffered an injury • The injury was caused by the provider’s breach of the duty * * Prosser,
Keeton, Dobbs, & Keeton, 1984; Morrison v. MacNamara, 1979; Weimer v. Hetrick, 1987; Mitchell v. Parker, 1984.
defendant for egregious conduct and to deter similar conduct in the future (Kemezy v. Peters, 1996). In many states, to recover punitive damages the plaintiff must show that the defendant had knowledge of the employee’s pattern of wrongful conduct or management ratified the wrongful conduct. A LNC can assist the lawyer in proving notice by tracking a pattern of preventable and inadequately treated pressure injuries.
Damages in Pressure Ulcer/injury Cases
Like most medical malpractice actions, these cases are expensive to pursue and defend. From the plaintiff’s perspective, the LNC can be very useful in assessing whether there are sufficient damages to warrant pursuing the case. From the defendant’s view, the LNC can refute allegations made if present.
The starting point is assessing the physical harm to the patient. The defendant is liable for all harm proximately caused by the breaches of the standard of care. A stage IV pressure injury is of course a serious injury. However, if the patient arrived from the community with a stage III ulcer/injury, the damages probably are insufficient to support an adequate award. This is because the defendant is only responsible for the worsening of the condition. Likewise, damages may be insufficient if the patient recovered from the pressure ulcer/injury promptly without additional hospitalizations or surgery. In some cases, damages may not be sufficient if the patient had a very limited life expectancy due to cancer, advanced Alzheimer’s disease, etc. While there is no rule of thumb, if the patient was already on hospice care, damages probably are not sufficient to pursue the case.
Many states now impose caps or limits on recovery regardless of the severity of the injuries as part of tort reform. Some states have a global cap limiting the plaintiff’s recovery regardless
of the severity of the injury.
For the elderly, economic damages are usually limited to medical bills and funeral expenses. Particularly in those states with caps on noneconomic damages, but no cap on economic damages, it is especially important to calculate all related bills. Without substantial medical bills, the case may not make economic sense to pursue.
According to reports from the Center for Medicare and Medicaid Services the average hospital cost for treating pressure injuries is $43,180 .1
Non-economic damages for pressure ulcer/ injury patients can be tremendous. Pressure injuries are painful in themselves. They increase a patient’s nutritional demands, often require surgical treatment, and may lead to loss of mobility and independence. Proving non-economic damages to skeptical jurors is challenging especially if the patient is deceased or unable to testify. While lay witnesses can be helpful, a LNC can find the hard data in the medical records to prove these damages. A detailed flow chart of all complaints of pain, administration of pain meds, and non-verbal signs of pain like grimacing can be used to effectively prove the severity of the injury. Similar charts can graphically show changes in activity level, signs of depression, and other consequences of pressure injuries.
Examples of Verdicts and Settlements
In the 7 years following the enactment of OBRA the average award in nursing home negligence cases nearly doubled to approximately $525,000.3
While there are no comprehensive studies assessing the percentage of preventable pressures injuries resulting in litigation, six and seven figure settlements and verdicts are not
Minimising the Legal Issues Around Pressure Injury Care Wound Masterclass - Vol 1 - December 2022 87 © Copyright. Wound Masterclass. 2023
“In the 7 years following the enactment of OBRA the average award in nursing home negligence cases nearly doubled to approximately $525,000.” 3
Wound care nurses have tremendous experience and expertise. If you are interested in reviewing fascinating cases for attorneys-defense if you prefer, go to:
6figurelegnurse.com
For more information and a free webinar.
unusual. The following are examples of notable settlements and verdicts:
In a 2008 Georgia case, the jury awarded $1.25 million to the estate of a 67 year old nursing home patient who developed a stage IV pressure ulcer/injury on his left buttock and became malnourished and dehydrated. The plaintiff alleged that the nursing home staff failed to prevent and treat the pressure injury by failing to turn and reposition him, failing to keep him clean and dry, and by the nursing home’s failure to provide adequate staffing (Mosby v. Tucker Nursing Ctr. Inc., 2008).
In a 2007 case filed in Cook County, IL, a quadriplegic patient developed multiple Stage IV pressure injuries on his coccyx, hips, and heels after being admitted to the nursing home for rehabilitation. The suit alleged that the nursing home was understaffed, and the staff failed to turn him at appropriate intervals, to keep his skin clean and dry, and to appropriately assess his condition. The parties settled for $1 million (Wazydrag v. Alden N. Shore Rehab. & Health Care Ctr., Inc., 2007).
A Virginia jury returned an $850,000 verdict against a nursing home finding the nursing home negligently caused or contributed to the patient’s death due to pressure injuries, malnutrition and dehydration. The plaintiff introduced evidence that the nursing home staff charted care on the patient when he was not in the facility and even after he died. In a Texas trial, the jury awarded the plaintiff $83 million including $70 million in punitive damages against a nursing home after an 83 year old resident who entered the facility alert but unable to walk allegedly died from infected pressure injuries. The plaintiff also asserted that the nursing home failed to provide water due to insufficient staffing causing the decedent to suffer severe dehydration. The plaintiff introduced evidence of other medical problems at the facility and evidence of 18 other residents
who were hospitalized during the weeks before the decedent’s death. Perhaps most damaging to the nursing home, the plaintiff alleged that the facility fraudulently concealed that the staff was not licensed and the staffing was inadequate (Holder v. Beverly Enterprises Texas, Inc., 1995).
While most pressure injury lawsuits appear to arise in nursing homes, a Las Vegas, N.M. jury recently awarded $10.3 million to the estate of a patient who developed bed injuries at a regional medical center following hip surgery with $595,000 designated as compensatory damages and $9.75 million as punitive damages. According to the plaintiff’s lawyer, the hospital failed to follow its own protocols for screening and preventing pressure ulcers (Haywood, 2011).
Conclusion
The staggering number of preventable pressures injuries has numerous health care and legal implications. As the vast majority of pressure injuries are preventable, health care providers who fail to acquaint themselves with developments in the prevention and treatment of pressure injuries subject their patients to unnecessary risk of serious injury or premature death. When these cases result in litigation, a well-informed LNC can play a critical role in ferreting out the meritorious from the defensible and preparing the case for a successful trial.
References
1. Donner, B., Posthauer, M .E., & Thomas, D. (2009). The role of nutrition in pressure ulcer prevention and treatment: The National Pressure Ulcer Advisory Panel White Paper. Retrieved from http://www.npuap.org/Nutrition White Paper Website Version.pdf
2. National Pressure Ulcer Advisory Panel, 2009.
3. Felsenthal, E. (1995, Sept. 5). Jury awards rise for improper care of elderly. Wall Street Journal, p. B1.
4. Federal Rules of Evidence, Rule 702. (2011).
5. Edwards v. Jackson, 210 Va. 450, 453, 171 S.E.2d 854, 856 (1970).
6. Haywood, P. (2011, Feb. 18), Christus St. Vincent Regional Medical Center slapped with $10.3m penalty in bed ulcer/injury lawsuit. The New Mexican.
7. Black, J. M., Edsberg, L. E., Baharestani, M. M., Langemo, D., Goldberg, M., McNichol, L. Npuapv, N.P. (2011). Pressure ulcers: Avoidable or unavoidable? Results of the National Pressure Ulcer Advisory Panel Consensus Conference. Ostomy Wound Management, 57(2), 24-37.
Minimising the Legal Issues
Pressure Injury Care 88 Wound Masterclass - Vol 1 - December 2022
“A well-informed LNC can play a critical role in ferreting out the meritorious from the defensible and preparing the case for a successful trial.”
Around
© Copyright. Wound Masterclass. 2023
Virtual Reality innovates Wound Care Training
Three-dimensional VR training modules provide healthcare professionals with an interactive and realistic clinical learning experience that allows collaborative learning without the involvement of the patient.
hartmann.info
 Ms Liz Ovens
Independent Tissue Viability Specialist Nurse
London, United Kingdom
Ms Liz Ovens
Independent Tissue Viability Specialist Nurse
London, United Kingdom
Improving Quality of Life Using a Novel Electrical Stimulation Therapy Device to Reduce Pain and Accelerate Healing in Two Patients With Very Different Underlying Aetiologies
Editorial Summary
Despite following best practice, many wounds fail to heal, due to the underlying pathology, with prevalence of wounds increasing year on year,1 and recalcitrant wounds creating the greatest resource challenges in nursing time and dressing costs. Interventional therapies such as electrical stimulation therapy (EST), can lessen this burden of wounds and improve the patient’s quality of life by reducing the pain and kick-starting the wound healing cascade, thus preventing exacerbation and the risk of complications in this patient group. A case series using a cost-effective, novel, compact EST (Accel-Heal Solo), demonstrated the benefits to patients and clinicians of having an easy to use device available in all clinical settings, for patients with hard-to heal wounds.
Introduction
Ahard-to-heal wound has been defined as one that fails to heal with standard therapy in an orderly and timely manner,2 which is applicable to both acute and chronic wounds and is independent of the wound type and aetiology.
Hard-to-heal wounds have an enormous effect on the quality of life for patients,3,4,5,6 and their family, who often have to endure living without any hope of improvement. Social isolation, psychosocial factors, malodour, pain, infection and sepsis, all contribute to reduced well-being for this patient group.
The prevalence of wounds in the UK is estimated to be 3.8 million among the adult population, which is increasing annually,1 at a cost of £8.3bn per annum, with 51% of chronic wounds remaining un-healed in the study year. The financial impact of this is significant, in an already depleted NHS workforce and resources.


Patients with complex co-morbidities, such as lupus erythematosus, and underlying pathologies related to arterial stenosis, invariably cause challenges in wound healing for clinicians, but also have a huge impact on quality of life, with pain being a huge factor. Most people with lupus experience skin involvement during the course of their disease, with a range of presentations and complications, including inflammation, photosensitivity, alopecia (hair loss), Reynaud’s phenomenon and cutaneous vasculitis.7 Invariably, these wounds can be very painful and result in slow healing,8,9,10 affecting patient’s well-being and life-style
factors.
Pain Management
Evidence has demonstrated that pain is particularly distressing for patients with hardto heal wounds,11,12 with wound pain reported to be the worse thing about having a leg ulcer.13 Presence of pain also prevents patients’ tolerance to effective treatment, such as compression therapy, wound debridement and exercise, which ultimately have an effect on healing.14 Pain management options are often limited, due to the type of pain, ageing, side effects and reluctance by patients to adhere to them, and they can often be ineffective.14 Patients often request priority for their pain to be removed over wound healing, but this can prove challenging for health care professionals.
A widely alternative non-pharmacological therapy for pain management is transcutaneous electrical nerve stimulation (TENS), involving the use of milliamp current to reduce pain and muscle spasms, which is used across a range of co-morbidities and pain types, to interfere with/block the gate control for pain signals in nerve fibres. It has been demonstrated to cause tingling, throbbing and even discomfort.15 Historically, it was not designed for wound care, and is not a cure for the pain, often only providing short-term relief while the TENS machine is being used.
Recent innovations in wound care have seen the development of sub-sensory EST designed to act at a cellular level to reduce pain and accelerate wound healing.
90 Wound
Vol 1 - December 2022
Masterclass -
Ms Claire Allan
© Copyright. Wound Masterclass. 2023
District Nursing Clinical Lead for Stafford South PCN Stafford, United Kingdom
Wound Healing
Wound healing is a complex and carefully coordinated process, the barriers to which include co-morbidities, wound aetiology, patient age, pharmacological effects and wound related factors such as chronic inflammation, biofilm and infection.
Human physiology is electrochemical in nature, and normal wound healing relies on electrical energy,16 to stimulate cellular activity such as macrophages, endothelial cells, fibroblasts and keratinocytes.17 Chronic wounds have been demonstrated to lack electrical energy (known as the current of injury),18 which will inhibit the normal wound healing cascade.16 The magnitude of the electrical field near the wound, has also been demonstrated to dissipate by 48% in the over 65 year olds,19 potentially increasing the risk of non-healing in these patients, many of whom may require surgical interventions, develop pressure ulcers and vascular problems, due to the mere ageing process (Click for previous article, 'What is the Role of Electrical Stimulation Therapy')
Wound Healing Using Electrical Stimulation Therapy
In order to restore the reduced or absent electrical energy, which has become “exhausted” due to the chronicity of the wound, external EST can be applied to mimic the current of injury, to “switch on” the wound healing process. Electric fields are established as part of the normal fundamental mechanisms of cell and tissue growth and control.20 Some clinicians have described this as “waking up the wound”. EST is one of the most evidence based modalities in wound management with nine meta-analyis,21,22,23,24,25,26,27,28,29 eight systematic reviews and over 35 randomised controlled trials, and has been recognised for use internationally,30,31 for a range of chronic wounds (Click for previous article, 'What is the Role of Electrical Stimulation Therapy').
Reduction in Pain Using EST
EST has been demonstrated to reduce pain in chronic wounds.17,32,33,34,35,36,37 The exact mechanism of pain reduction using EST is yet to be established, but pain reduction is often noted well before wound healing is achieved,36,38,39 signifying a possible active and specific effect,14 related to a reduction in inflammation40 (Click for previous article, 'What is the Role of Electrical Stimulation Therapy')
Accel-Heal Solo Electrical Stimulation Therapy
Accel-Heal Solo is a class IIa medical device, which delivers a one-off sub-sensory 12-day pre-programmed, low voltage pulsed current (LVPC) to the wound. It is a small discreet device, which can be worn unobtrusively by the patient in all settings, without the need to attend specialist clinics. Accel Heal Solo is worn continuously over the 12-days and is be used alongside standard therapy, to ensure wound management is delivered according to best practice,31,41,42,43,44,45 such as moist wound healing, anti-biofilm management and compression therapy, as clinically indicated (Click for previous article, 'What is the Role of Electrical Stimulation Therapy')
Evidence has demonstrated the benefits of using Accel-Heal (original device) for pain reduction and wound healing,46 with a mean pain score reduction of 61.5% (n=71), and wound size reduction of 25.8% (n=120) noted within 12 days. Quality of life is improved,36 with many patients reducing or discontinuing their analgesia.38
A case series of two patients was undertaken to demonstrate the benefits of using AccelHeal Solo, in pain reduction and healing on extremely challenging hard-to-heal wounds.
Improving Quality of Life Using a Novel Electrical Stimulation Therapy Device to Reduce Pain and Accelerate Healing in Two Patients With Very Different Underlying Aetiologies Wound Masterclass - Vol 1 - December 2022 91 © Copyright. Wound Masterclass. 2023
“Human physiology is electrochemical in nature, and normal wound healing relies on electrical energy,16 to stimulate cellular activity such as macrophages, endothelial cells, fibroblasts and keratinocytes.” 17
Case Study 1
A case study was undertaken to determine the effectiveness of using Accel-Heal Solo on a complex patient with a Lupus erythematosus rash.
Assessment
A 47 year old female presented to the district nursing team (DN) in June 2021 with multiple wounds to her abdomen due to a Lupus rash, which she reported had been present for several years, and was extremely painful. She had been using hot water bottles applied to her abdomen to reduce her pain. The patient (known as Dorothy) had recently moved into the area and the previous clinical records were unavailable to the team. Several topical treatments were applied including wound bed preparation strategies and anti-biofilm cleansers, but there was no improvement to the wounds, and dressing changes became challenging due to intolerability of adhesive dressings and pain during dressing removal (see Figure 1).
Relevant past medical history included:chronic disease related malnutrition; femoral artery occlusion; Amaurosis fugax; Lupus erythematosus; Depressive disorder; Anorexia Nervosa; irritable bowel syndrome; Chronic liver disease; Hyperthyroidism; Graves disease.
Dorothy lived with her husband and older son, who were the main carers, having no outside care support. She was self-caring for transfers and used a wheelchair during the day. Dorothy was under the dietician for prescribing of nasogastric feeding and her anorexia. She was seen regularly by the occupational therapist for support regarding equipment and mobility.
The tissue viability team requested the Clinical Lead for the DN team, to refer Dorothy to the dermatologist and rheumatologist regarding the abdominal wounds but has not yet been seen by them. On-going wound management was undertaken by Dorothy’s husband, under a shared care agreement with the DN team as the family requested limited visits due to the COVID-19 pandemic.
On 26/5/22, prior to commencing Accel-Heal Solo, measurement of the whole area was approximately 15 cm x 15 cm, with 16 open abdominal wounds, which varied in size. No photos were undertaken, but the district nurses reported there had been no change in the wounds since March 2022, apart from very slight less inflammation (see Figure 1). Exudate levels were low to moderate. However, due to Dorothy being aware of wound odour, her husband changed her dressings every 2-3 days. Peri-wound skin was very tender to touch and occasionally developed maceration, which was managed with a skin protectant. Pain score was between 7-10/10 (Visual Analogue Score (VAS)) all day, despite taking Morphine 10 mgs twice daily, Paracetamol 1000 mgs four times daily, and Pregabalin, 300 mgs twice daily. She was also applying heat pads topically.
Improving Quality of Life Using a Novel Electrical Stimulation Therapy Device to Reduce Pain and Accelerate Healing in Two Patients With Very Different Underlying Aetiologies 92 Wound Masterclass - Vol 1 - December 2022
“The surrounding skin (was) significantly improved with no maceration, reduced inflammation and (showed) a reduction in the wound sizes.”
© Copyright. Wound Masterclass. 2023
Figure 1: Abdominal wounds. 01/03/22.
Treatment with Accel-Heal Solo commenced on 26/05/2022, together with an antimicrobial cleansing solution, skin protectant, emollient, and silicone foam border dressings. Dorothy’s husband undertook all dressing changes, every 2 days, during the treatment with Accel heal Solo, and took photos (see Figure 2), which the family provided consent to share for this article.
On 01/06/22 (day 6) her pain score reduced to 5/10 (VAS), and her husband reported that one of the wounds had already started to dry up and her skin did not appear to be as red (see Figure 2b). The DN team reviewed on 14/6/22 (day 19), and noted the surrounding skin to be significantly improved with no maceration, reduced inflammation and a reduction in the wound sizes (see Figure 2c). Her pain score was 0/10 (VAS).
On 24/06/22 (day 29) there were only two open wounds remaining, which each measured 1.5 cm x 1 cm. The pain score reduced to 3/10, which was only occasional and mainly attributable to issues with constipation. Her wound pain was 0/10. Wound management continued by her husband.
The DN clinical Lead reviewed Dorothy on 08/08/22, when all wounds were noted to be
healed (see Figure 2d), with no exudate. Her pain score remained 0/10 and she no longer required the use of any hot water bottles and she managed to discontinue all her analgesia.
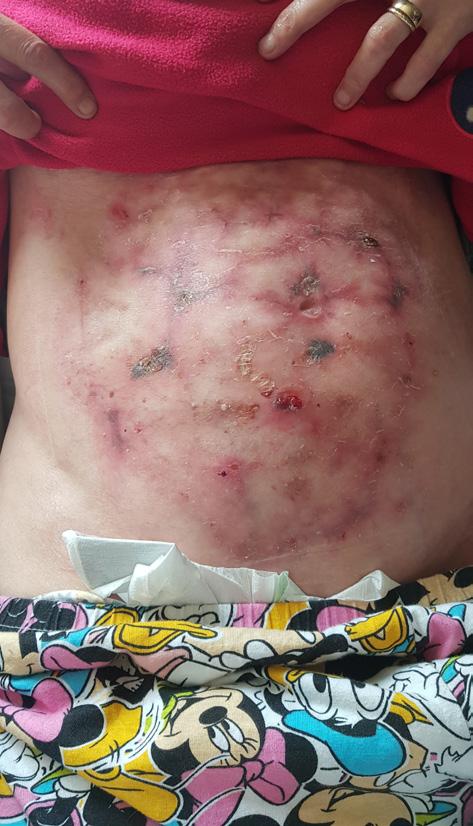
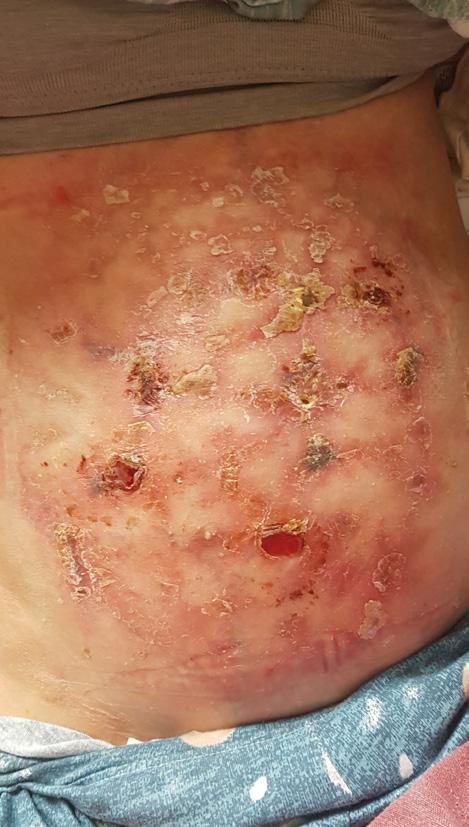
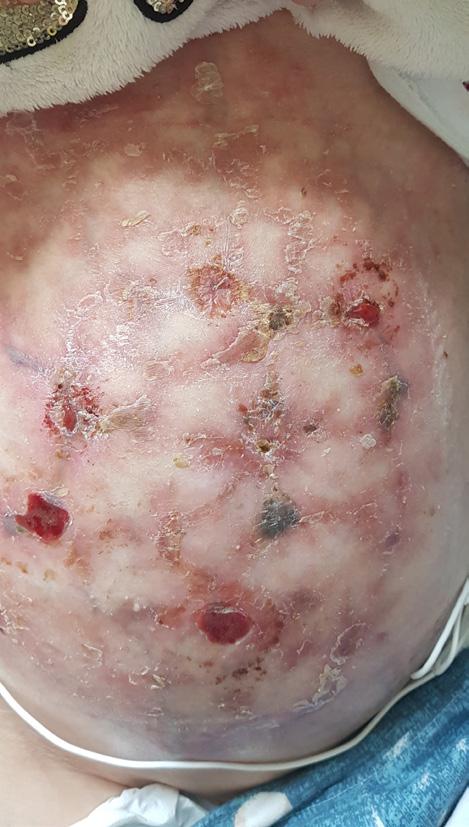
Patient Feedback
Dorothy reported to the team that the device was amazing, and that it had improved her quality of life dramatically.
Clinician Feedback
“Having used Accel heal previously the new Accel-Heal Solo is just as easy to use and to also teach her husband how to use. The instructions provided are easy to follow. Dorothy was made aware that Accel-Heal Solo may not improve her lupus rash/ open wounds but hopefully would improve her pain and in turn improve her quality of life. However, Accel-Heal Solo not only improved her pain from 7/10 to 0 it also improved her lupus rash and dried all the wounds within 2 months of commencing on the treatment, it has given Dorothy independence again and improved her quality of life. As clinical lead for the district nurse team this product has shown that it is versatile and has given confidence to the district nurse team to consider using this again for patients who may have similar pain scores with their wounds.”
Improving Quality of Life Using a Novel Electrical Stimulation Therapy Device to Reduce Pain and Accelerate Healing in Two Patients With Very Different Underlying Aetiologies Wound Masterclass - Vol 1 - December 2022 93 Figure 2: 2a: Day 2. Abdominal wounds on 28/05/22. 2b: Day 6 Abdominal wounds on 01/06/22. 2c: Day 19 Abdominal wounds on 14/06/22. 2d: Abdominal wounds healed on 08/08/22 (74 days after commencing Accel-Heal Solo therapy). 2a 2b 2c 2d © Copyright. Wound Masterclass. 2023
Case Study 2
A case study was undertaken to demonstrate the effectiveness of using Accel-Heal Solo on a painful, recalcitrant arterial foot ulcer to the bunion. The primary objective of the treatment was to reduce the pain, and the secondary aim was to facilitate wound size reduction.
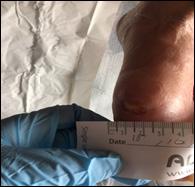

Assessment
An 89 year old female (known as Beryl) presented for assessment by the tissue viability team in May 2021, with a recalcitrant wound to her left bunion, which had developed spontaneously approximately 12 months previously. Past medical history included chronic obstructive airways disease, myocardial infarction, ischaemic heart disease, intermittent claudication, and malignant neoplasm of the bladder. She lived alone in a warden controlled flat and had regular carers. She also had some short term memory loss. She had a low body mass index, although her appetite and nutrition were well balanced. She declined referral to the dietician and was encouraged to have a high protein diet and milky drinks.


Vascular assessment determined a reduced ankle brachial pressure index of 0.5, with monophasic sounds. Her pain score was 7/10 (VAS), despite taking regular prescribed analgesia, which was predominant at night, likely due to likely presence of arterial disease. Due to the longevity of the wound and pain level, osteomyelitis was suspected.
The wound measured 2cm x 1.5 cm, with rolled, un-healthy edges and leaking thick puslike fluid, which had been treated with several courses of antibiotics. Following discussion with the GP, she was referred to the vascular team and a foot X-ray was arranged to eliminate osteomyelitis.
Further assessment in early September 2021 determined no improvement. The vascular appointment had been delayed due to the COVID-19 pandemic, and she was due to see them in November 2021. The foot X-ray showed no signs of osteomyelitis. Beryl was feeling very depressed due to sleep deprivation, and she reported that she wanted to go into a care home, as she felt she could no longer manage at home. Discussions were undertaken with Beryl regarding Accel-Heal Solo, and the possible
benefits this intervention could offer, in terms of pain reduction and sleep improvement. Beryl agreed to having the treatment and was excited at the prospect of trying a new therapy, which she hoped might improve her quality of life.
Treatment with Accel-Heal Solo commenced on 04/10/21 (see Figure 3a). The wound measured 2 x 1.5 cm with rolled un-healthy edges and under-mining, and was leaking thick pus-like fluid (see Figures 3b and 3c). Her pain score remained at 7/10 (VAS), and she reported continued sleeping difficulties.
Improving Quality of Life Using a Novel Electrical Stimulation Therapy Device to Reduce Pain and Accelerate Healing in Two Patients With Very Different Underlying Aetiologies 94 Wound Masterclass - Vol 1 - December 2022
Figure 3:
3a: Accel-Heal Solo in-situ
3b, 3c: Accel-Heal Solo commenced on 04/10/21
3d, 3e: Wound following completion of Accel-Heal Solo on 18/10/21
3a 3b 3c 3d 3e 3f 3g © Copyright. Wound Masterclass. 2023
3f, 3g: Wound on 15/11/21 (42 days after commencing Accel-Heal Solo therapy).
Within 48 hours of commencing the Accel-Heal Solo therapy, Beryl reported no pain, and she had managed to sleep without being awoken in extreme discomfort. Less exudate was noted on 06/10/21. At the end of the 12-day treatment, the wound had reduced by 33%, to 2 x 1 cm with healthier wound edges and had lost some depth (see figures 3d and 3e).The exudate reduction continued and it had lost it’s thick consistency, and some reduction in peri wound oedema was noted. A 100% reduction in pain was noted during the 12-day therapy.
Beryl was smiling again, and reported that she no longer wanted to go into a care home. Her mobility improved, and she became hopeful that the wound may continue to heal. Her appetite increased, and there was a general change in her whole persona over the 12-day period.
Following completion of the Accel-Heal Solo therapy, she had developed occasional pain, but reported this to be 3/10 and was well controlled with over-the-counter analgesia.
Beryl was seen by the vascular team on 11/11/21, who planned to regularly review her to determine any plans for intervention. By 15/11/21, the wound showed minimal size reduction, measuring 2 cm x 0.75 cm, with light slough (see Figures 3f and 3g). Unfortunately following light slough debridement, some bone was noted in the middle of the wound base. The wound had moderate thick exudate. Further liaison was undertaken to the GP, to request a referral to the orthopaedic team to eliminate osteomyelitis . The pain was described as an occasional “niggle” at approximately 2.5/10 with no complaints of pain during the night.
The plan was to consider the use of a second Accel-Heal Solo therapy once she was reviewed by the orthopaedic team and osteomyelitis was either eliminated or treated. However, sadly Beryl died of other co-morbidities in March 2022. Her carer reported how grateful she and Beryl were that her last few months of life were relatively pain free.
Patient Perspective
Beryl stated: “The pain and wound have been so much better since using the treatment, I haven’t looked back and I am walking much better”
Clinician Perspective
“The new Accel-Heal Solo device, offers the advantages of Accel-Heal electrical stimulation therapy, in that it facilitates healing and reduces pain for patients with recalcitrant wounds, with the added benefits of being a continuous 12-day therapy. My patient lives alone and has some short-term memory loss, and although she has carers, it was likely to be challenging to change the device every 48 hours, due to staff capacity and irregular visit times. So, knowing that I could apply the treatment and only disturb it during dressing changes, twice a week was a real bonus. I was able to demonstrate the new LED light to her carer who kept an eye that it was working when she visited. The clip and strap made application much easier rather than using tape to secure it.”
Discussion
Evidence suggests that chronic wounds are “stuck” in a chronic inflammatory state,47 and are therefore unable to move forward in a normal wound healing trajectory. This chronic inflammation causes elevated inflammatory markers, high levels of proteases, including MMPs, diminished growth factor activity, reduced fibroblast production and impaired angiogenesis, compared to an acute wound (see Figure 4).47
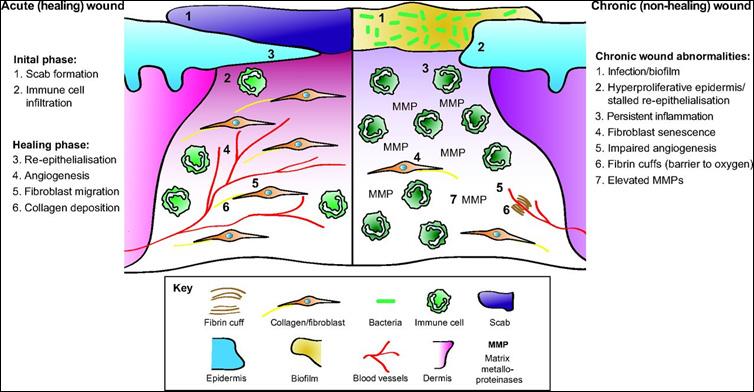
Results
Improving Quality of Life Using a Novel Electrical Stimulation Therapy Device to Reduce Pain and Accelerate Healing in Two Patients With Very Different Underlying Aetiologies Wound Masterclass - Vol 1 - December 2022 95
© Copyright. Wound Masterclass. 2023
Figure 4: Chronic inflammation. Acute vs chronic wound healing.47
Current approaches to reduce the MMPs and biofilm, include wound bed preparation strategies such as debridement, moisture management, topical anti-microbials and protease modulators.42 However, despite following best practice, many wounds still fail to heal and invariably cause pain, which is likely due to the chronic inflammation, together with the absent or diminished electrical energy.
A randomised gene expression analysis,40 demonstrated that Genes linked to inflammation, which are usually increased during wound healing, were down regulated in the skin of the healthy volunteers using Accel-Heal, but not where the volunteers were wearing the placebo devices. This provides evidence that Accel-Heal causes some specific responses in the skin to reduce inflammation, which is likely to have an effect on pain reduction. Delivery of EST to the wound can “kick-start” or “wake-up” the wound back into an “acute” phase by reducing the chronic inflammation.
Due to Accel-Heal Solo being such a compact and pre-programmed device, it is very easy to use in all settings by patients, carers and clinicians, and does not interfere with the patient’s standard wound therapy.
With increasing financial pressures on the NHS, it is imperative that clinicians consider new approaches to facilitate healing of chronic wounds. Guest et al., determined the costs of wound management are increasing year on year,1 and are in excess of managing other complex conditions, such as obesity. Cost effectiveness has been demonstrated for using Accel-Heal,48,49,50,51 which may reduce the cost of treating venous leg ulcers in the NHS by 11%.50 Healing wounds results in fewer nursing interventions, dressings49 and hospital admission.
Conclusion
Using Accel-Heal Solo for these two patients completely transformed their mental health. The fast and significant pain reduction noted for both patients improved their well-being together with wound improvements.
EST needs to be considered for use by clinicians, in order to replenish the diminished/ absent electrical energy in chronic wounds, which is so vital in the complex healing cascade. Accel-Heal Solo has demonstrated improved quality of life for patients, many of whom have given up any hope of their wound/s healing and suffer endless un-managed pain.
Accel-Heal Solo is a cost effective easy to use EST, which is readily available for use in the UK. The unique compact device is ideal for use in a variety of settings, and the ease of use enables it to be accessible for patients, carers and clinicians.
References
1. Guest JF., Fuller GW., Vowden P. (2020) Cohort study evaluating the burden of wounds to the UK’s National Health Service in 2017/2018: update from 2012/2013. BMJ open pages 1-15.
2. Troxler M, Vowden K, Vowden P. Integrating adjunctive therapy into practice: the importance of recognising ‘hard-to-heal’ wounds. World wide wounds 2006. Available from: http://www. worldwidewounds.com/2006/december/ Troxler/Integrating-Adjunctive-TherapyInto Practice.html
3. Arshad M, Arshad S, Arshad S et al. The quality of life in patients with diabetic foot ulcers. J DiabetesMetab. 2020; 11(2):1–2. https://doi.org/10.35248/2155-6156.20.11.e101
4. González-Consuegra RV, Verdú J. Quality of life in people with venous leg ulcers: an integrative review. J Adv Nurs. 2011; 67(5):926–944. https://doi.org/10.1111/j.13652648.2010.05568.x
5. Herber OR, Schnepp W, Rieger MA. A systematic review on the impact of leg ulceration on patients’ quality of life. Health Qual Life Outcomes. 2007; 5(1):44. https://doi. org/10.1186/1477-7525-5-44
6. Upton D, Solowiej K, Hender C, Woo KY. Stress and pain associated with dressing change in patients with chronic wounds. J Wound Care. 2012; 21(2):53–61
7. John Hopkins Lupus Centre (2022). Lupus-Specific Skin Disease and Skin Problems. Available on-lin at:- https://www.hopkinslupus.org/lupus-info/lupus-affects-body/skin-lupus/
8. Avishai E, Yeghiazaryan K, Golubnitschaja O (2017). Impaired wound healing: facts and hypotheses for multi-professional consideration in predictive, preventive and personalised medicine. EPMA J 8 (1): 23-33 doi: 10.1007/s13167-017-0081-y
9. DoerschKM and Newell-Rogers MK (2016). Wound healing in a Model of Systemic Lupus Erythematosus may be Delayed by a Lack of Interleukin -2. Federation of American Societies for Experimental Biology. Available on-line at https://doi.org/10.1096/fasebj.30.1_ supplement.922.6
10. Victoria K. Shanmugam V,Angra D,Rahimi H et al (2017). Vasculitic and autoimmune wounds. J Vasc Surg Venous Lymphat Disord 5 (2): 280-292
11. Wilson A (2013). Quality of life and leg ulceration from the patient’s perspective. British Journal of Nursing 13 (2). Available from:- https://doi.org/10.12968/bjon.2004.13.Sup2.13235
12. Renner R, Seikiwski K, Simon J (2014). Association of pain level, health and wound status in patients with chronic leg ulcers. Acta DermVenereol; 94 91): 50-53
13. Hofman D, Ryan TJ, Arnold F, et al. Pain in venous leg ulcers. J Wound Care. 1997;6(5):222-224. doi:10.4254/wjh.v5.i4.196
14. Milne J, Searle R, Styche T. The characteristics and impact of hard-to-heal wounds: results of a standardised survey. J Wound Care. 2020 May 2;29(5):282-288. doi: 10.12968/ jowc.2020.29.5.282. PMID: 32421485.
15. Atkinson M (un-published). Pain ease microcurrent therapy treatment in subjects with period pain (dysmenorrhoea).A pilot study. Available on line at http://www.painmasterhellas. gr/pdfs/clinical_studies/Clinical%20Trial%20Period%20Pain.pdf(accessed 22/04/22)
16. Kambouris ME, Zagoriti Z, Lagoumintzis G Poulas K (2014) From therapeutic Electrotherapy to Electroceuticals: Formats, Applications and Prospects of Electrostimulation. Ann Res Rev Biol4(20): 3054–70
17. Milne J., Swift A., Smith J., Martin R.(2021) Electrical Stimulation for Pain Reduction in Chronic Wound Healing.Journal of Wound Care30 (7) 2-13
18. Kloth L (2014). Electrical stimulation technologies for wound healing. Adv Wound Care
3(2): 81–90
19. Nuccitelli R., Nuccitelli P., Li C et al (2011). The electric fields near human skin wounds declines with age and provides a noninvasive indicator of wound healing. Wound Rep Reg;
19; 645-655
20. Ovens L (2019). Application of Accel-Heal for patients with chronic venous leg ulcers: an evaluation in a community UK NHS trust. Wounds UK 15 (2) 110-116
21. AroraM, Harvey L, Glinsky J et al (2020). Electrical stimulation for treating pressure ulcers. Database Syst Rev; 1 (1): CD012196. Cochrane review
22. Avendano-Coy J, Lopez-Munoz P, Serrano-Munoz D et al (2021). Electrical microcurrent stimulation therapy for wound healing: A meta-analysis of randomized clinical trials. J Tissue Viability S0965-206X(21)00132-7
23. Barnes R, Shanin Y, Gohil R, Chetter I (2014). Electrical stimulation vs. standard care for chronic ulcer healing: A systematic review and meta-analysis of randomised controlled trials.
Eur J Clin Invest; 44(4):429-440
24. Chen Z, Chen Z Y, Li G (2020). Electrical stimulation as an Effective Adjunctive Therapy for Diabetic Foot Ulcer: A Meta-analysis of Randomized Controlled Trials. Adv Skin Wounds
Care 33 (11) 608-612
25. Gardner S, Frantz R, Schmidt F (1999). Effect of electrical stimulation on chronic wound healing: a meta-analysis. Wound Repair Regen; 7(6):495-503.
26. Girgis B and Duarte J (2018). High Voltage Monophasic Pulsed Current (HVMPC) for stage II-IV pressure ulcer healing. A systematic review and meta-analysis. J Tissue Viability;
Improving Quality of Life Using a Novel Electrical Stimulation Therapy Device to Reduce Pain and Accelerate Healing in Two Patients With Very Different Underlying Aetiologies 96 Wound Masterclass - Vol 1 - December 2022 © Copyright. Wound Masterclass. 2023
27(4):274-284
27. Lala D, Spaulding S, Burke S, Houghton P (2016). Electrical stimulation therapy for the treatment of pressure ulcers in individuals with spinal cord injury: a systematic review and meta-analysis. Int Wound J; 13(6): 1214-1226.
28. Liu L, Moody J, Gall A (2016). A Quantitative, Pooled Analysis and Systematic Review of Controlled Trials on the Impact of Electrical Stimulation Settings and Placement on Pressure Ulcer Healing Rates in Persons With Spinal Cord Injuries.
29. Khouri C, Kotzki S, Roustit M et al (2017) Hierarchical evaluation of electrical stimulation protocols for chronic wound healing: An effect size meta-analysis. Wound Repair Regen 25(5) :883-91
30. Piaggesi A, Läuchli S, Bassetto F et al. EWMA document: advanced therapies in wound management: cell and tissue based therapies, physical and bio-physical therapies smart and IT based technologies J Wound Care, 2018; 27(6), Suppl 6.
31. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, Pan-Pacific Pressure Injury Alliance (2014) Prevention and Treatment of Pressure Ulcers: Quick Reference Guide
32. Leloup P, Toussaint P, Lembelembe J, Phillipe C (2014) The analgesic effect of electrostimulation (WoundEL) in the treatment of leg ulcers. Int Wound Journal 12 (6) 706-9
33. MagnoniC., Rossi E., Fiorentini C et al (2014) Electrical stimulation as adjuvant treatment for chronic leg ulcers of different aetiology: an RCT. Journal of Wound Care 22 (10): 523-33
34. Janković A, Binić I (2008). Frequency rhythmic electrical modulation system in the treatment of chronic painful leg ulcers. Arch. Dermatol. Res. 300:377–383. doi: 10.1007/s00403-008-0875-9
35. Nair HKR. Microcurrent as an adjunct therapy to accelerate chronic wound healing and reduce patient pain. J Wound Care. 2018 May 2;27(5):296-306. doi: 10.12968/jowc.2018.27.5.296. PMID: 29738296.
36. Turner N. and Ovens L. (2018) Clinical outcome results and quality of life improvements using electroceutical treatment* - patients’ perspectives. Poster and presentation at EWMA annual conference 2018.
37. Santamato A., Panza F., Fortunato F et al (2012) Effectiveness of the frequency rhythmic electrical modulation system for the treatment of chronic and painful venous leg ulcers in older adults. Rejuvenation Res 15 (3) 281-7.
38. Nair H (2022). Powering the progression of hard-to-heal wounds with electrical stimulation: an observational analysis of
wounds treated with Accel-Heal. Wounds Asia; (5); 2; 38-47
39. Terrill P and Ovens L (2022). An innovative approach to manage pain and stimulate healing in arterial ulcers using electrical stimulation therapy. Poster accepted to EWMA
40. Lallyett, C, PhD; Yeung., Nielson R., et al (2018) Changes in S100 Proteins Identified in Healthy Skin following Electrical Stimulation: Relevance for Wound Healing. Advances in skin and wound care (3) 7; 322-327
41. Scottish Intercollegiate Guidelines Network (SIGN) (2010). Management of chronic venous leg ulcers. A national clinical guideline.
42. International Wound Infection Institute (IWII) (2022). Wound infection in clinical practice international consensus update. Wounds International.

43. Strohal R, Dissemond J, Jordan O’Brien J et al. Debridement: an updated overview and clarification of the principle role of debridement. J Wound Care. 2013; 22(S1):S1–S49 https://doi.org/10.12968/jowc.2013.22.sup1.s1
44. Wounds UK (2022) Best Practice Statement: Holistic management of Venous Leg Ulceration (second ediction).
45. Wounds UK (2018) Best Practice Statement: Improving Holistic Assessment of Chronic Wounds
46. Ovens L (2022). Stimulating healing in the Challenging Wound. What is the role of electrical stimulation therapy?
Published on-line and available on:- www.woundmasterclass.com

47. Nunan R., Harding KG., Martin P. (2014) Disease models and mechanisms 7, 1205 -1213
48. Turner N., Ovens L., (2017) The results of a clinical evaluation of Accel-Heal Electroceutical treatment in a large NHS Trust. Wounds UK 13 (4) 92-99
49. PosnettJ,Smith
J, McKenna P(2020).Cost-effectiveness of a single-use, portable electrical stimulation device* in the management of venous leg ulcers. EWMA poster 2020. 50. Guest JF., Ayoub N., Greaves T.( 2015) Clinical outcomes and cost-effectiveness of an externally applied electroceutical device in managing venous leg ulcers in clinical practice in the UK. Journal Wound Care2015; 24(12):574–580. 51. Guest J., Singh H., Rana K., et al (2018) Cost effectiveness of an electroceutical device in treating non-healing venous leg ulcers: results of an RCT. J Wound Care. 2018;27(4):230-243. Improving Quality of Life Using a Novel Electrical Stimulation Therapy Device to Reduce Pain and Accelerate Healing in Two Patients With Very Different Underlying Aetiologies Wound Masterclass - Vol 1 - December 2022 97 woundmasterclass.com Introducing Wound Masterclass Video woundmasterclass.com/Video © Copyright. Wound Masterclass. 2023 Image licenced from Adobe Stock. Credit: BillionPhotos.com
Dr Frank Stadler Director, MedMagLabs Sydney, Australia


Biosurgery: Indications, Contraindications, Interactions and Side-effects
Editorial Summary

Maggot therapy is not regulated in most countries, but in those countries in which it is regulated, indications authorised by regulating bodies are the law of the land. Irrespective of particular jurisdictional limitations, this chapter describes when maggot therapy can be used, when it can’t be used, potential adverse events, and when treatment can proceed with caution. The chapter also examines how medicinal maggots interact with concomitant treatments such as systemic antibiotics, anaesthetics and narcotics, or hyperbaric oxygen therapy. Evidence and expert advice suggest that maggot therapy is a safe and widely applicable wound care modality with few side-effects, most of which can be avoided or successfully managed.
Introduction
The terms “indications” and “contraindications” are short-hand for: when should maggot therapy be used and when should it not be used? When deciding whether or not a medical product should be used, it is critical to keep in mind who is issuing the recommendations. For the purposes of the following discussion, two groups of authorities will be referenced: regulators and experts. Maggot therapy is not regulated in most countries, but in those countries in which it is regulated (primarily North America, much of Europe, and several countries in the Middle East and Asia), indications authorised by regulating bodies are the law of the land. Indications issued by regulatory agencies tend to be more restrictive, and the use of maggot therapy for anything other than what is specifically authorised is considered “offlabel”. Off-label use of a product by a licensed care provider is not illegal, but it does put one at legal or professional risk if it is not in keeping with standard-of-care practice by one’s peers and licensing boards. In countries where maggot therapy is not formally regulated, therapists are free to follow the advice of experts. In this chapter, indications recommended by experts but not approved by regulatory agencies will be clearly identified.
Indications for Maggot Therapy
Medicinal maggots have been found to debride wounds, kill microbes, and stimulate wound healing.1-3 Therefore, many experts around the world use maggot therapy for any or all of those purposes. In those countries where maggot
therapy is regulated by health ministries, maggot therapy is approved only for wound debridement. Specific examples will follow.
Debridement
Wound debridement is the removal of dead (necrotic) tissue and debris from the wound. Sharp debridement with scalpels removes dead tissue mechanically, by cutting it out. Enzymatic debriding ointments remove the dead tissue by enzymatically dissolving it.4 Necrotic tissue can also be removed by blasting it with a jet of water (hydrosurgery)5 or with pulses of ultrasound.6 Autolytic debridement involves dressings which potentiate the body’s own mechanisms (mostly enzymatic) to dissolve and discharge the dead tissue.7 Maggot therapy rids the body of necrotic tissue and debris by both mechanical pathways (the physical action of the maggots’ cuticular spines and mouth hooks) and enzymatic pathways (liquefaction of the necrotic tissue by the maggots’ secreted and excreted digestive enzymes).
In the U.S., where medicinal maggots are regulated by the Food and Drug Administration (FDA), cleared indications are for “debridement of non-healing necrotic skin and soft-tissue wounds such as pressure ulcers, neuropathic foot ulcers, chronic leg ulcers, or non-healing traumatic or post-operative wounds.” These indications broadly cover a variety of necrotic and non-healing wounds, such as those described in Chapter 3.8 FDAcleared indications noticeably exclude the debridement of “hard tissue” (bone) as well as the use of maggots for anything otherthan debridement. Yet, many experts will sometimes
98 Wound Masterclass - Vol 1 - December 2022
Dr Ronald Sherman
Director, BioTherapeutics, Education & Research (BTER) Foundation
Irvine CA, United States
Published with the kind permission of the authors; taken from: ‘A Complete Guide to Maggot Therapy’ Sherman, Ronald A. (2022). Chapter 4: ‘Indications, Contraindications, Interactions, and Side-effects of Maggot Therapy’. Open Book Publishers.
© Copyright. Wound Masterclass. 2023
DOI: https://doi.org/10.11647/ obp.0300
“For nearly 100 years, maggot therapy has been observed to enhance wound healing, even in apparently clean but stagnant wounds. Clinicians and researchers have described the rapid proliferation of granulation tissue and hastened closing of the wound margins in previously stagnant, non-healing wounds.19–23
resort to maggot therapy when certain modalities fail to achieve adequate disinfection or wound healing, whether or not debridement is also a major goal.
Disinfection
Microbial infection is a common feature of chronic wounds. If not already infected, most non-healing wounds will eventually become infected by invading bacteria or fungi. After all, necrotic tissue, by definition, has no circulation and no defence against microbial invaders, and it provides a moist, nutrient-rich substrate for microbial growth. Medicinal maggots kill a wide variety of microbes through ingestion and through the secretion of antimicrobial compounds, which is why maggot therapy is commonly used to debride infected necrotic wounds, and to treat chronic wounds whose primary problem is non-gangrenous infection. Chronically infected wounds are typically characterised by drainage, pain, and bad odour. When treated with maggot therapy, all three of these characteristics substantially decrease or resolve.
Examples of infectious complications that are not easily treated with antibiotics include wounds populated by multi-resistant organisms or biofilm. There are now multiple laboratory studies of maggots’ antimicrobial activity, but relatively few controlled clinical studies. Still, a few controlled studies and several case series demonstrate clinically relevant antimicrobial effects of maggot therapy.9–18
Growth Stimulation
For nearly 100 years, maggot therapy has been observed to enhance wound healing, even in apparently clean but stagnant wounds. Clinicians and researchers have described the rapid proliferation of granulation tissue and hastened closing of the wound margins in previously stagnant, non-healing wounds.19–23
As a result, some therapists consider nonhealing wounds—even those without necrotic tissue or obvious infection—to be appropriate candidates for maggot therapy.
Additional Situations
Maggot therapy is also useful for situations that may not fit neatly into the specific categories of debridement, disinfection and wound healing. Maggot therapy may be used for two or three of these indications simultaneously, and it may be used for non-healing wounds with no obvious infection or gangrene. Often therapists turn to maggot therapy only after conventional therapy has failed or is unavailable. In published studies of patients who failed conventional wound care and were scheduled for amputation but given a trial of maggot therapy instead, at least 50% of those patients healed their wounds and avoided amputation.24 This has led many therapists to believe that a non-emergency amputation due to a non-healing wound may itself be an appropriate indication for a trial of maggot therapy. Of course, outcomes are even better when wounds are treated with maggot therapy before they progress to the point (and the underlying circulatory status has regressed to the point) that they are earmarked for amputation.
Maggot therapy is useful in treating wounds that are undermined, difficult to visualise, or connected to inner-body cavities.22 Ordinarily, such wounds might be opened widely (“surgically filleted”) to view them completely before cleaning them out. When that is not feasible without significant damage to nearby vital structures, some therapists apply medicinal maggots to the wound entrance so that they will explore the entire inner cavity, looking for and dissolving infected, necrotic tissue. Since medicinal maggots are obligate air breathers and since their natural instinct is to leave the host when satiated or when there is nothing more to eat, they can be considered
Biosurgery: Indications, Contraindications, Interactions and Side-effects Wound Masterclass - Vol 1 - December 2022 99
© Copyright. Wound Masterclass. 2023
self-extracting.
Maggot therapy is also indicated for patients who would benefit from surgical debridement but are too frail.22 Even patients at the end of life or with wounds unlikely to heal can benefit from maggot therapy, if they are suffering from chronic wounds that are painful, draining, malodorous, or require resource-intense treatments.25 Since maggot therapy can be applied by non-professionals and outside of medical facilities, many patients with nonhealing wounds who lack access to such resources are appropriate candidates for maggot therapy.26–28
Contraindications and Relative Contraindications for Maggot Therapy
There are relatively few absolute contraindications for maggot therapy. One softtissue wound to which they must not be applied is a corneal ulcer, because the maggots’ cuticle and mouth hooks are likely to scratch and damage the corneal surface. Maggots are obligate air breathers, so they cannot be placed within a closed cavity, such as an abscess. However, if that abscess is opened and allowed to drain, the maggots could be placed on the surface of the drained abscess, if desired. Maggot therapy is generally contraindicated for sterile cavities, but most sterile cavities should have no reason to undergo maggot debridement.
The anatomy and location of the wound can affect dressing selection, but generally is not itself a contraindication, except as described above. For example, a toe or anterior foot wound cannot easily be covered with a sheet of net fabric to confine the maggots because affixing it to the surrounding tissue would result in multiple wrinkles and tunnels through which the maggots might escape. In this case, a stocking-like dressing might be used instead, and affixed proximally to the foot or ankle. Similarly, a wound very near to an orifice might
be of concern if the maggots posed a danger to that particular orifice (mouth or tracheostomy, for example). Again, this may be a contraindication to a dressing that requires a sizeable border to adhere to the periphery of the wound, but need not be a contraindication to maggot therapy in general, because a different dressing could be used: maggots contained within a bag.29
Rapidly advancing, life- or limb-threatening infections are not appropriate for maggot therapy. In these cases, the standard of care is surgical resection and broad-spectrum antibiotics. Maggot therapy is not appropriate, even in combination with first-line surgical and medical therapy, if it will interfere with the critical close and frequent observation of the wound. Once stabilised, maggot therapy may be appropriate to debride the necrotic tissue without harming nearby vital structures.22, 30–34
Maggot therapy is generally reserved for skin and soft-tissue wounds; maggot secretions do not dissolve tendons, fascia or bone efficiently. That said, when surgical resection is not feasible because of patient frailty, or lack of surgical expertise in compromised healthcare settings, maggot debridement of wounds that include necrotic harder tissues can be quite useful.22, 35–37
Because maggots are aerobic creatures, they are susceptible to drowning and suffocation. Maggot dressings must be highly permeable to air, and allow the efflux of purulent drainage. Medicinal maggots must not be covered by occlusive dressings (i.e., “semi-permeable” transparent membranes, hydrocolloid and hydrogel pads, etc.) or else they will die. They may also suffocate if applied to wounds along with ointments (petroleum jelly, zinc oxide, silver sulfadiazine, triple antibiotic ointments, etc.), if the oily substance covers the maggots’ breathing holes (spiracles).
Biosurgery: Indications, Contraindications, Interactions and Side-effects 100 Wound Masterclass - Vol 1 - December 2022 © Copyright. Wound Masterclass. 2023
“There are relatively few absolute contraindications for maggot therapy.”
Interactions between Maggot Therapy and Other Treatment Modalities
The viability and debridement capacity of medicinal maggots have been tested under a wide variety of conditions. Under controlled laboratory conditions, medicinal maggots fed increasing concentrations of antibiotics were found not to be affected by pharmacologic doses of any of the tested antimicrobials.23, 38 Insecticides, however, can be lethal and should not be used during or within two halflives prior to application of medicinal maggots. Hyperbaric oxygen therapy (HBOT) has been shown to be lethal to very young larvae, but not to older larvae.39 Therefore, for patients receiving HBOT, maggot therapy should be administered during the non-diving days or else the larvae should be late-second or early-third instars before the patient re-enters the HBO chamber. As discussed in more detail elsewhere in this chapter, bleeding can sometimes occur with maggot therapy. Blood thinning medication puts those patients at increased risk for more significant blood loss (haemorrhage).
Some drugs—especially drugs of abuse—may be present in the blood or tissue without the awareness of the therapist and maybe even without the complete awareness of the patient. Maggot growth and survival has been assessed in the presence of a variety of narcotics and anaesthetics.40–42 While most of these will have some effect on the larvae, they are generally not lethal in the doses tested, and medical maggots should still do their job.
Adverse Event or Complication
Pain
Infection
Hepatic encephalopathy or other mental status changes
Excessive bleeding
Contributing Causes
Most other contraindications are really “relative contraindications”. In other words, the benefits of maggot therapy must be weighed against the risks (which we call “adverse events”). Those risks may be greater in some patients than in others… so great as to be considered a contraindication to maggot therapy. We will discuss such relative contraindications within the context of the underlying adverse effects.
Warnings and Adverse Events Associated with Maggot Therapy
All things considered, there are relatively few serious complications associated with maggot therapy (Table 1), and they are certainly not as serious nor as numerous as those that can result from the wounds themselves, in the absence of maggot therapy. The best way to avoid these adverse events is to understand the patient and their medical history, understand the nature of maggot therapy, and to read all package inserts carefully before using the products. In this way, adverse events can often be avoided or their risks minimised. For example, by reading the package insert it might be discovered that one of the ingredients is something to which the patient may be allergic. This could be a contraindication to therapy. But if the therapist identifies the problem in advance, a special preparation can probably be made to avoid that ingredient, thereby eliminating the contraindication.
Treatment
Pre-existing wound pain; large larvae. Liberal analgaesics; remove the larvae for immediate pain relief.
Pre-existing wound infection; inadequate maggot dosage; inadequate disinfection of larvae.
Underlying hepatic insufficiency; high maggot burden; sepsis.
Suppress Pseudomonas aeruginosa with topical antiseptics prior to maggot therapy; concurrent antibiotics; check adequacy of larval disinfection.
Check for bacteraemia, serum ammonia; remove maggots.
Coagulopathy; necrosis involving major vessel. Remove maggots; find and stop source of bleeding.
Hypersensitivity reaction (local or systemic) Allergy to medicinal maggots (i.e., their media) or the dressings.
Tissue invasion
Check beforehand; remove immediately and treat reaction.
Inappropriate species. Remove maggots; check species.
Biosurgery: Indications, Contraindications, Interactions and Side-effects Wound Masterclass - Vol 1 - December 2022 101
© Copyright. Wound Masterclass. 2023
Table 1: Adverse events and contributing factors (after Sherman). 43
The most common adverse event associated with medicinal maggots is wound pain. Discomfort or pain has been reported in anywhere from 5–30% of patients already experiencing wound pain.21,43–47 Maggotassociated pain or discomfort usually does not manifest until about 24 hours into the therapy, but then increases as the larvae grow larger. Patients that are at risk of experiencing pain can easily be identified (and prepared) in advance, because they are the patients with painful wounds who already receive analgaesics during dressing changes or maybe even constantly. Patients should be given liberal access to analgaesics during maggot therapy and offered the opportunity for early removal of dressings upon their request. If systemic analgaesics no longer control the pain, remove the maggot dressing to achieve immediate relief. With these two provisions, patients often cope much better with therapy-related pain and are more satisfied with their experience, even if they do have pain (personal experience).
Patients may be allergic to fly larvae, their media, or the accompanying dressing components. Patients allergic to the maggots or dressing materials may manifest contact dermatitis or more serious immunologic reactions. Patients known to be allergic to media ingredients should not be treated with those constituents; alternatives usually can be substituted. When in doubt, communicate with the manufacturer.
Pseudomonas aeruginosa and some other hardy gram-negative organisms appear to be more resistant to maggot therapy than other microbes.48 Situations have been reported in which a P. aeruginosa infection has actually spread through the wound during maggot therapy. Some experts believe this may occur as a result of maggot-induced killing of the other microbes, leaving the P. aeruginosa to grow without competition. When treating wounds with P. aeruginosa infection or colonisation, it is recommended that topical anti-pseudomonal antiseptics (i.e., acetic acid, sodium hypochlorite, etc.) be applied for a day or two before maggot debridement, to decrease the P. aeruginosa population. Also, a greater density of maggots in the wound is more effective in killing P. aeruginosa.48 Therefore, a high dose of maggots (10+ larvae/cm2) should be applied when treating wounds suspected of harbouring
this organism.
Mild bleeding is common during maggot debridement, and it is common that the wound drainage is blood-tinged. However, patients with coagulopathy (inability to form blood clots and halt bleeding) are at risk of more substantial bleeding. Maggot therapy in such individuals should only be performed under close supervision.22, 49 Maggot debridement of, or around, necrotic blood vessels may also lead to life-threatening blood loss if and when those vessels dissolve under the influence of maggot excretions and secretions.50 If maggot therapy is attempted for wounds with uncertain vascular integrity, the patient must receive close and continuous observation for bleeding, infection, or thrombosis.
Large maggot burdens in blowfly-infested sheep (>60,000 maggots per animal) are associated with serious complications (“blowfly strike”), including elevated serum ammonia levels (presumably due to the large protein breakdown in the wound) and encephalopathy. Similar complications in humans were predicted43 but not seen until 20,000 larvae were applied (against verbal and written advice) to wounds in a patient with underlying alcoholic hepatic insufficiency.51 Maggot loads over 6,000 should probably be avoided, even in otherwise healthy individuals. Too many maggots in a tight dressing, especially in a patient with insensate wounds, may exert pressure sufficient to compromise circulation and cause further pressure-related necrosis.43
Patients with fever or changes in mental status should be evaluated for spread of infection (i.e., cellulitis, bacteraemia, sepsis) or hepatic encephalopathy (check for elevated serum ammonia level). Maggot dressings may need to be removed immediately, even if just to facilitate wound inspection. Patients with infected wounds—especially those with deep or extensive wounds, and those at increased risk of bacteraemia, should receive systemic antibiotic coverage during maggot therapy to prevent sepsis or cellulitis.
The use of maggots that have not been disinfected or were inadequately disinfected has also been found to pose a risk of local and systemic infection.52
Biosurgery: Indications, Contraindications, Interactions and Side-effects 102 Wound Masterclass - Vol 1 - December 2022 © Copyright. Wound Masterclass. 2023
“Maggot therapy is indicated for the debridement of a wide variety of chronic wounds. Many experts also recommend medicinal maggots for controlling wound infections and the promotion of healthy granulation tissue and reepithelialisation in non-healing wounds.”
Larvae supplied in a single, primary packaging container are intended for single-use only;53 they are not to be multi-dosed nor saved for retreatment of the same patient. Firstly, opening and retrieving maggots may contaminate the internal environment and content of the primary packaging container, and secondly, the remaining maggots will have deteriorated beyond therapeutic effectiveness by the time the patient requires a follow-up treatment (usually 48–72 hours later). Medicinal maggots should not be used on more than one patient, nor be allowed to wander away from their host patient. Once they have been applied to a patient, they are contaminated with the patient’s wound flora, and must be discarded as infectious (“biohazardous”) medical waste.
Summary
Maggot therapy is indicated for the debridement of a wide variety of chronic wounds. Many experts also recommend medicinal maggots for controlling wound infections and the promotion of healthy granulation tissue and reepithelialisation in non-healing wounds. Adverse events are very uncommon, with the exception of increased pain in patients with painful wounds. However, a variety of precautions should be taken when treating patients at risk of bleeding, infection, or the complications of liver disease. Patients with inadequate blood flow may never heal their wounds, even if completely debrided by maggot therapy; but we do not yet know how much blood flow is too little because even patients with so little blood flow that they are scheduled for amputation often heal with a trial of maggot therapy.
References
1. Nigam, Y. and M.R. Wilson, Maggot Debridement, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 143–152, https://doi.org/10.11647/OBP.0300.08.
2. Nigam, Y. and M.R. Wilson, The Antimicrobial Activity of Medicinal Maggots, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 153–174, https://doi. org/10.11647/OBP.0300.09.
3. Nigam, Y. and M.R. Wilson, Maggot-assisted Wound Healing, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 175–194, https://doi. org/10.11647/OBP.0300.10.
4. Ramundo, J. and M. Gray, Enzymatic Wound Debridement. Journal of Wound, Ostomy, and Continence Nursing, 2008. 35(3): pp. 273–280, https://doi. org/10.1097/01. WON.0000319125.21854.78.
5. Kakagia, D.D. and E.J. Karadimas, The Efficacy of Versajet™ Hydrosurgery System in Burn Surgery. A Systematic Review. Journal of burn care & research, 2018. 39(2): pp. 188–200, https:// doi.org/10.1097/BCR.0000000000000561.
6. Messa, C.A., et al., Ultrasonic Debridement Management of Lower Extremity Wounds: Retrospective Analysis of Clinical Outcomes and Cost. Journal of Wound Care, 2019. 28(Sup5): pp. S30-S40, https://doi.org/10.12968/ jowc.2019.28.Sup5.S30.
7. Atkin, L. and M. Rippon, Autolysis: Mechanisms of Action in the Removal of Devitalised Tissue. British Journal of Nursing, 2016. 25(20): pp. S40-S47, https://doi.org/10.12968/ bjon.2016.25.20.S40.
8. Sherman, R. and F. Stadler, Wound Aetiologies, Patient Characteristics, and Healthcare Settings Amenable to Maggot Therapy, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 39–62, https://doi.org/10.11647/OBP.0300.03.
9. Armstrong, D.G., et al., Maggot Therapy in “Lower-extremity Hospice” Wound Care: Fewer Amputations and More Antibiotic-free Days. Journal of the American Podiatric Medical Association, 2005. 95(3): pp. 254–257, https:// doi.org/10.7547/0950254.
10. Blueman, D. and C. Bousfield, The Use of Larval Therapy to Reduce the Bacterial Load in Chronic Wounds. Journal of Wound Care, 2012. 21(5): pp. 244–253, https://doi.org/10.12968/ jowc.2012.21.5.244.
11. Bohac, M., et al., Maggot Therapy in Treatment of a Complex Hand Injury Complicated by Mycotic Infection. Bratislava Medical Journal, 2015. 116(11): pp. 671–673, https://doi. org/10.4149/bll_2015_128.
12. Bowling, F.L., E.V. Salgami, and A.J.M. Boulton, Larval Therapy: A Novel Treatment in Eliminating Methicillin-Resistant Staphylococcus aureus from Diabetic Foot Ulcers. Diabetes Care, 2007. 30(2): pp. 370–371, https://doi.org/10.2337/dc06-2348.
13. Contreras-Ruiz, J., et al., [Comparative Study of the Efficacy of Larva Therapy for Debridement and Control of Bacterial Burden Compared to Surgical Debridement and Topical Application of an Antimicrobial]. Gaceta médica de México, 2016. 152(Suppl 2): pp. 78–87 http://www.anmm.org.mx/GMM/2016/s2/GMM_152_2016_S2_78-87.pdf.
14. Dissemond, J., et al., Treatment of Methicillin-resistant Staphylococcus aureus (MRSA) as Part of Biosurgical Management of a Chronic Leg Ulcer. [German] Hautarzt, 2002. 53(9): pp. 608–612, https://doi.org/10.1007/s00105-002-0336-x.
15. Kaplun, O., M. Pupiales, and G. Psevdos, Adjuvant Maggot Debridement Therapy for
Deep Wound Infection Due to Methicillin-resistant Staphylococcus aureus. Journal of Global Infectious Diseases, 2019. 11(4): pp. 165–167, https://doi.org/10.4103/jgid.jgid_30_19.
16. Malekian, A., et al., Efficacy of Maggot Therapy on Staphylococcus aureus and Pseudomonas aeruginosa in Diabetic Foot Ulcers: A Randomized Controlled Trial. Journal of Wound, Ostomy and Continence Nursing, 2019. 46(1): pp. 25–29, https://doi.org/10.1097/ WON.0000000000000496.
17. Tantawi, T.I., et al., Clinical and Microbiological Efficacy of MDT in the Treatment of Diabetic Foot Ulcers. Journal of Wound Care, 2007. 16(9): pp. 379–383, https://doi.org/10.12968/ jowc.2007.16.9.27868.
18. Wolff, H. and C. Hansson, Larval Therapy for a Leg Ulcer with Methicillin-resistant Staphylococcus aureus. Acta Dermato-Venereologica, 1999. 79(4): pp. 320–321, https://doi.or g/10.1080/000155599750010751.
19. Markevich, Y.O., et al., Maggot Therapy for Diabetic Neuropathic Foot Wounds — a Randomized Study, in EASD Annual Conference. 2000: Jerusalem, Abstract 0059.
20. Sherman, R.A., Maggot Therapy for Foot and Leg Wounds. International Journal of Lower Extremity Wounds, 2002. 1(2): pp. 135–142, https://doi.org/10.1177/1534734602001002009.
21. Sherman, R.A., Maggot Therapy for Treating Diabetic Foot Ulcers Unresponsive to Conventional Therapy. Diabetes Care, 2003. 26(2): pp. 446–451, https://doi.org/10.2337/ diacare.26.2.446.
22. Sherman, R.A., C.E. Shapiro, and R.M. Yang, Maggot Therapy for Problematic Wounds: Uncommon and Off-label Applications. Advances in Skin & Wound Care, 2007. 20(11): pp. 602–610, https://doi.org/10.1097/01. ASW.0000284943.70825.a8.
23. Sherman, R.A., F.A. Wyle, and L. Thrupp, Effects of Seven Antibiotics on the Growth and Development of Phaenicia sericata (Diptera: Calliphoridae) Larvae. Journal of Medical Entomology, 1995. 32(5): pp. 646–649, https://doi. org/10.1093/jmedent/32.5.646.
24. Sherman, R.A., et al., Maggot Therapy, in Biotherapy — History, Principles and Practice, M. Grassberger, et al. (eds). 2013, Springer: Dordrecht; New York. pp. 5–29.
25. Steenvoorde, P., et al., Maggot Debridement Therapy in the Palliative Setting. American Journal of Hospice & Palliative Medicine, 2007. 24(4): pp. 308– 310, https://doi. org/10.1177/1049909107302300.
26. Mirabzadeh, A., et al., Maggot Therapy for Wound Care in Iran: A Case Series of the First 28 Patients. Journal of Wound Care, 2017. 26(3): pp. 137–143, https://doi.org/10.12968/ jowc.2017.26.3.137.
27. Sherman, R.A. and M.R. Hetzler, Maggot Therapy for Wound Care in Austere Environments. Journal of Special Operations Medicine, 2017. 17(2): pp. 154–162.
28. Stadler, F., R.Z. Shaban, and P. Tatham, Maggot Debridement Therapy in Disaster Medicine. Prehospital and Disaster Medicine, 2016. 31(1): pp. 79–84, https://doi.org/10.1017/ S1049023X15005427.
29. Grassberger, M. and W. Fleischmann, The Biobag — A New Device for the Application of Medicinal Maggots. Dermatology, 2002. 204(4): p. 306, https:// doi.org/10.1159/000063369.
30. Dunn, C., U. Raghavan, and A.G. Pfleiderer, The Use of Maggots in Head and Neck Necrotizing Fasciitis. The Journal of Laryngology & Otology, 2002. 116(1): pp. 70–72, https:// doi.org/10.1258/0022215021910212.
31. Fonseca‐Muñoz, A., et al., Clinical Study of Maggot Therapy for Fournier’s Gangrene. International Wound Journal, 2020. 17(6): pp. 1642–1649, https://doi.org/10.1111/iwj.13444.
32. Preuss, S.F., M.J. Stenzel, and A. Esriti, The Successful Use of Maggots in Necrotizing Fasciitis of the Neck: A Case Report. Head & Neck, 2004. 26(8): pp. 747–750,
Biosurgery:
Wound Masterclass - Vol 1 - December 2022 103
Indications, Contraindications, Interactions and Side-effects
© Copyright. Wound Masterclass. 2023
Biosurgery: Indications, Contraindications, Interactions and Side-effects

https://doi.org/10.1002/hed.20092.
33. Steenvoorde, P., et al., Maggot Debridement Therapy of Infected Ulcers: Patient and Wound Factors Influencing Outcome a Study on 101 Patients with 117 Wounds. Annals of the Royal College of Surgeons of England, 2007. 89(6): pp. 596–602, https://doi.org/10.1308/003588407X205404.
34. Teich, S. and R.A.M. Myers, Maggot Therapy for Severe Skin Infections. Southern Medical Journal, 1986. 79(9): pp. 1153–1155.
35. Baer, W.S., The Treatment of Chronic Osteomyelitis with the Maggot (Larva of the Blow Fly). The Journal of Bone and Joint Surgery. American Volume, 1931. 13: pp. 438–475, https://doi.org/10.1007/s11999-010-1416-3.
36. El-Tawdy, A.H.F., E.A.H. Ibrahim, and T.A. Morsy, An Overview of Osteomyelitis with Reference to Treatment in Particular Maggot Debridement Therapy (MDT). Journal of the Egyptian Society of Parasitology, 2016. 46(3): pp. 613–624.
37. Mumcuoglu, K.Y., et al., [Maggot Therapy for Gangrene and Osteomyelitis]. Harefuah 1997. 132: pp. 323–325, 382.
38. Peck, G.W. and B.C. Kirkup, Biocompatibility of Antimicrobials to Maggot Debridement Therapy: Medical Maggots Lucilia sericata (Diptera: Calliphoridae) Exhibit Tolerance to Clinical Maximum Doses of Antimicrobials. Journal of Medical Entomology, 2012. 49(5): pp. 1137–1143, https://doi.org/10.1603/ME12066.
39. Sherman, R.A., B. Khavari, and D. Werner, Effect of Hyperbaric Oxygen on the Growth and Development of Medicinal Maggots. Undersea and Hyperbaric Medicine, 2013. 40(5): pp. 377–380.
40. Gosselin, M., et al., Methadone Determination in Puparia and Its Effect on the Development of Lucilia sericata (Diptera, Calliphoridae). Forensic Science International, 2011. 209(1): pp. 154–159, https://doi.org/10.1016/j. forsciint.2011.01.020.
41. Kharbouche, H., et al., Codeine Accumulation and Elimination in Larvae, Pupae, and Imago of the Blowfly Lucilia sericata and Effects on Its Development. International Journal of Legal Medicine, 2008. 122(3): pp. 205–211, https://doi. org/10.1007/s00414-007-0217-z.
42. Zou, Y., et al., Effect of Ketamine on the Development of Lucilia sericata (Meigen) (Diptera: Calliphoridae) and Preliminary Pathological Observation of Larvae. Forensic Science International, 2013. 226(1): pp. 273–281, https://doi. org/10.1016/j.forsciint.2013.01.042.
43. Sherman, R.A., Maggot versus Conservative Debridement Therapy for the Treatment of Pressure Ulcers. Wound Repair and Regeneration, 2002. 10(4): pp. 208–214, https://doi.org/10.1046/j.1524-475X.2002.10403.x.
44. Dumville, J.C., et al., Larval Therapy for Leg Ulcers (VenUS II): Randomised Controlled Trial. BMJ, 2009. 338(7702): pp. 1047–1050, https://doi.org/10.1136/bmj.b773.
45. Mumcuoglu, K.Y., et al., Pain Related to Maggot Debridement Therapy. Journal of Wound Care, 2012. 21(8): pp. 400–405, https://doi.org/10.12968/jowc.2012.21.8.400.
46. Steenvoorde, P., T. Budding, and J. Oskam, Determining Pain Levels in Patients Treated with Maggot Debridement Therapy. Journal of Wound Care, 2005. 14(10): pp. 485–488, https://doi.org/10.12968/jowc.2005.14.10.26846.
47. Steenvoorde, P., et al., Maggot Debridement Therapy in Necrotizing Fasciitis Reduces the Number of Surgical Debridements. Wounds, 2007. 19(3): pp. 73–78.
48. Andersen, A.S., et al., Quorum-sensing-regulated Virulence Factors in Pseudomonas aeruginosa Are Toxic to Lucilia sericata Maggots. Microbiology (Society for General Microbiology), 2010. 156(2): pp. 400–407, https://doi. org/10.1099/ mic.0.032730-0.
49. Rojo, S. and S. Geraghty, Hemophilia and Maggots: From Hospital Admission to Healed Wound. Ostomy Wound Management, 2004. 50(4): pp. 30, 32, 34.
50. Steenvoorde, P. and L.P. Van Doorn, Maggot Debridement Therapy: Serious Bleeding Can Occur: Report of a Case. Journal of Wound, Ostomy, and Continence Nursing, 2008. 35(4): pp. 412–414, https://doi.org/10.1097/01. WON.0000326662.32390.72.

51. Borst, G.M., et al., Maggot Therapy for Elephantiasis Nostras Verrucosa Reveals New Applications and New Complications: A Case Report. International Journal of Lower Extremity Wounds, 2014. 13(2): pp. 135–139, https://doi. org/10.1177/1534734614536036.
52. Nuesch, R., et al., Clustering of Bloodstream Infections during Maggot Debridement Therapy Using Contaminated Larvae of Protophormia terraenovae. Infection, 2002. 30(5): pp. 306–309, https://doi.org/10.1007/ s15010-002-3067-0.
53. Stadler, F., Packaging Technology, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 349–362, https://doi. org/10.11647/OBP.0300.16.
54. Takáč, P., et al., and F. Stadler, Establishment of a medicinal maggot production facility and treatment programme in Kenya in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 331–346, https://doi.org/10.11647/OBP.0300.15.
104 Wound Masterclass - Vol 1 - December 2022
Submit Your Research to Our Journal Case reports, randomized controlled trials, clinical reviews, audits, and research projects submissions@woundmasterclass.com © Copyright. Wound Masterclass. 2023 Image licenced from Adobe Stock. Credit: Rawpixel.com
woundmasterclass.com
Pre-meshed 2:1
fish-skin graft
Expands to cover wounds over 100 cm²
A novel solution for chronic wounds that are larger than 100cm2 and treated in the outpatient setting
Scan the QR code for more information
 Dr Frank Stadler Director, MedMagLabs Sydney, Australia
Dr Frank Stadler Director, MedMagLabs Sydney, Australia


Biosurgery: Application and Dressing Technology
Editorial Summary

Maggot therapy dressings are intended to keep maggots on the wound during treatment. Some therapists make their own dressings, others use commercially produced dressings, and many use both, depending on their patients’ wounds. There are two basic designs for maggot dressings. Maggot confinement dressings confine the maggots to the wound bed and allow them complete access to the wound, while maggot containment dressings totally contain the maggots within a net bag that facilitates easy handling but does not allow full access to the wound. This chapter describes the basic principles and goals of the ideal maggot dressing, and provides examples of how that ideal dressing can be achieved.
Introduction
The first question most people ask about maggot therapy is: “How do you get the maggots off?” In fact, removing the maggots is not difficult because the species employed for maggot therapy are “self-extracting”—their instinctive behaviour is to leave the host as soon as they are satiated or as soon as there is no more nutritious food (necrotic tissue or exudates) left in the wound. Since some of the maggots will become satiated earlier than others, the real problem is how to keep the maggots corralled in one spot until the therapist is ready to remove them all. The solution is the maggot therapy dressing.
There is no one single correct or best maggot dressing. Several techniques exist, each with their advantages and disadvantages. Several different commercial dressings are available, but many therapists fashion their own dressings at the bedside. This chapter will describe the basic principles or goals of the ideal maggot dressing, and then provide examples of how that ideal dressing can be achieved.
Maggot Therapy Dressings - Past and Present
The minimal requirements for a maggot dressing are that it be of a porous fabric that allows air to enter and fluid to drain out. This is to prevent the maggots from suffocating or drowning. Also, the dressing should be constructed in such a way that it keeps the maggots from wandering off the wound. Ideally, the dressing should also be comfortable, affordable, and simple to apply, maintain, and remove.
When maggot therapy was commonly used for osteomyelitis and pus-forming wounds in the 1930s, dressings were frequently constructed out of metal screens and/or cloth. Looseknit gauze does not make an effective barrier because the larvae can easily escape through the large spaces between the woven fibres. The dressing was usually kept in place with an adhesive tape (plaster) and sometimes foam padding was placed between the skin and the maggot dressing.1 Some dressings were even made to be re-used for repeat applications of maggots to the same wound, with a port to put the young maggots in and take the satiated maggots out.
During the 1990s renaissance of maggot therapy, there was a push to construct maggot dressings from materials readily available on a medical ward.2 Additionally, many of the patients now receiving therapy are old and frail and have thin sensitive skin prone to tearing. Therefore, efforts were made to identify materials less traumatic to the peri-wound skin, which led to the use of hydrocolloid pads as foundations to which the maggot dressings were then affixed.3 This and related dressing designs are still commonly used today. Because these dressings confine the maggots to the wound but still provide them with free access to the wound bed and all of its nooks and crannies, they are sometimes called “free-range” or “confined” maggot dressings (Figures 1A and 1B).
106 Wound Masterclass - Vol 1 - December 2022
Dr Ronald Sherman
Irvine
Published with the
of
from: ‘A
Guide
DOI:
© Copyright. Wound Masterclass. 2023
Director, BioTherapeutics, Education & Research (BTER) Foundation
CA, United States
kind permission
the authors; taken
Complete
to
Maggot
Therapy’ Sherman,
Ronald A. (2022). 5. ‘Medicinal Maggot Application and Maggot Therapy Dressing Technology’. Open Book Publishers.
https://doi.org/10.11647/ obp.0300
“For nearly 100 years, maggot therapy has been observed to enhance wound healing, even in apparently clean but stagnant wounds. Clinicians and researchers have described the rapid proliferation of granulation tissue and hastened closing of the wound margins in previously stagnant, non-healing wounds.” 19–23
Figure 1: Three types of maggot dressings. A) Polyester net fabric was glued to a hydrocolloid pad, the centre of which was cut out to match the wound margins before placing maggot-impregnated gauze over the wound bed. B) Here, a strip of hydrocolloid was placed all around the anterior foot, just proximal to the non-healing toe amputation stump wound. After placing maggot-impregnated gauze over the wound, a nylon stocking (net) was pulled over the anterior foot and glued over the hydrocolloid strip. After covering the adhesive border with water-resistant tape, the excess nylon stocking will be cut off. Many therapists now use only water-resistant tape, not liquid adhesive, to hold the net in place. (Pictures by R.A. Sherman, courtesy of the BioTherapeutics, Education & Research Foundation). C) Maggots are contained within a netted bag. Photos by R. Sherman, Monarch Labs, CC BY.

Characteristic
Efficacy
Debridement
Efficiency/Dressing weartime
Wound pain
Confinement Dressing Containment Dressing
Able to access and more efficiently debride undermined areas, sinus tracks, and other crevices
Fast, 48–72 hours
Valuable for wounds near eyes, mouth, or other sensitive sites where it is imperative to avoid escapes
Slower, 96 hours (4 days)
Most analyses are in patients with confinement dressings; pain occurring in 5%-30%. In the few comparative reports, there has been no significant difference in the frequency or severity of pain between confinement and containment dressings5–8
Applying Maggot Dressings
The method of dressing application depends on several factors, including the type and location of the wound, the type of dressings to be used (i.e., confinement or containment dressings), and the availability of supplies. Here are descriptions of some common dressing methods, followed by increasingly more complex methods, each intended to address a typical problem or complication.
In its simplest iteration, the maggot dressing might be constructed with a simple breathable or net fabric covering a maggot-laden wound (Figures 2, 3 and 4). The net can be affixed to the peri-wound skin with water-resistant tape. When available, a polyester net fabric is very durable, and provides a known pore size, which is optimally 100 um–160 um: small enough that the larvae cannot escape, but large enough to allow the thick, purulent drainage to drain easily. A fixed-weave fabric prevents these pores from expanding. Other fabrics with similar pore sizes are often described as having a mesh size of approximately 80 to 140. If using a stretchable fabric, the pore size may need to be within the smaller range to prevent the
Escaping maggots
Aesthetics
Cost
Inexperienced therapists report more escapes from confinement dressings. Experienced therapists report no more escapes from confinement dressings than from containment dressings7
Less acceptable
Maggots are less costly to produce, but additional dressing supplies may add cost
More acceptable
Contained maggots are more costly to produce, but few other dressings are required beyond a gauze wrap
Confined Maggots Contained Maggots
Disadvantages
• Maggots have direct contact with the entire wound bed, including undermined areas, sinus tracts, etc. As a result, they are more efficient
• Less expensive (less costly to produce)
• Therapist may need to see or touch the maggots (gloved, of course); less aesthetically pleasing
• Requires a “cagedressing” to secure the maggots on the wound
• Need at least 1cm peri-wound skin to support (adhere) the cage-dressing
• Therapist does not touch maggots directly; more aesthetically acceptable to patients and therapists
• Faster application
• Do not need periwound skin to support the dressing
•
• Maggots have limited direct contact with the necrotic tissue; cannot directly access all areas of the wound bed. As a result, they are less efficient
• More expensive (more labourintensive to produce)
Biosurgery: Application and Dressing Technology Wound Masterclass - Vol 1 - December 2022 107
Table 1: Characteristics of confinement and containment dressings.
Table 2: Advantages and disadvantages associated with maggot confinement dressings compared to containment dressings.
© Copyright. Wound Masterclass. 2023
maggots from squeezing through and escaping. If such fabrics are not available, one could use appropriate items of clothing such as a T-shirt, blouse, or shirt (Figure 4). But beware: if the pores are too small, the thick purulent drainage that accumulates during therapy may not be able to exit through the fabric. This could lead to a fluid build-up and drowning of the maggots, or it could block the pores, which in turn would suffocate the maggots.
The optimal dose of maggots is considered to be 5–10 per cm2 (the upper range is used for wounds with more necrotic or infected tissue). Medicinal larvae are supplied in primary packaging containers, with or without gauze [9]. Larvae in tubes can be rinsed out with sterile saline or clean water and poured onto a piece of gauze or directly onto the polyester net fabric, which is then inverted and placed over the wound. When using maggot-impregnated gauze, it is not necessary to count individual larvae if the gauze is labelled with the concentration of maggots. Simply apply to the wound bed the amount of gauze that contains the approximate number of maggots needed.
Since the maggots will liquefy the necrotic tissue, one should expect a fair amount of wound drainage. To prevent that drainage from settling on healthy tissue as it flows out of the wound and through the net, the net should be covered with an absorptive material such as cotton gauze.
The absorptive gauze should not be too thick or else it could obstruct airflow to the maggots. Wet gauze is not permeable to oxygen, so the absorptive dressings should be changed when soiled. This dressing design is sometimes called a “two-layer” maggot dressing: the “cage layer” on the bottom, and the “absorptive layer” on top.
In order to minimise the risk of peri-wound skin becoming macerated by the drainage, the peri-wound skin can be coated with a skin protectant (liquid, fast-drying). Some therapists coat the peri-wound skin with zinc oxide, being careful to avoid applying it in areas where the adhesive will need to stick to the skin.
Patients with skin integrity problems (i.e., elderly or malnourished patients) may develop skin tears when removing strongly adhered tape (less tacky tapes do not hold the maggot cage layer securely enough to prevent maggots from escaping). Therefore, many therapists do not tape the net directly to the skin. Rather, they first place strips of hydrocolloid, hydrogel, or tissue-friendly tape on the skin, and then tape or glue the net to those strips. Alternatively, a hole is cut in a hydrocolloid pad such that the pad now surrounds the wound and completely covers the skin around the wound, such that the larvae are not able to crawl out of the wound and onto the normally innervated skin (Figure 1A). This will prevent the itching, tickling, or pain that sometimes occurs when no
Figure 2: How to make a free-range maggot dressing at the bedside using adhesive glue. 1) Place a hydrocolloid pad over the peri-wound skin, with a hole cut out to correspond precisely to the wound perimeter. Alternatively, tape or another simple adhesive could be used to surround the wound. 2) Apply liquid adhesive to the surface of the hydrocolloid pad. 3) As the liquid adhesive sets and becomes tacky, place maggot-impregnated gauze over the wound bed. To prevent escape, apply the net over the wound and hydrocolloid pad immediately thereafter. Apply a second layer of adhesive over the hydrocolloid and net, such that the first and second layers of glue bond through the pores in the net. A layer of water-resistant tape may then be applied over the still sticky hydrocolloid-glue-net-glue “sandwich”, but not on the skin (not shown). 4) Finally, after securing the medicinal maggots within their “cage”, place a layer of absorbent gauze on top to collect the wound drainage (liquefied necrotic tissue and wound exudates). Change the outer gauze dressing whenever it becomes soiled with drainage (about every 8 hours) so that fluid does not leak onto the patient’s skin, and air can continue to reach the maggots through mostly dry gauze. Photos by R. Sherman, BioTherapeutics, Education & Research Foundation, CC BY.

Biosurgery: Application and Dressing Technology 108 Wound Masterclass - Vol 1 - December 2022
© Copyright. Wound Masterclass. 2023
Figure 3: Application guidance for a confinement dressing without adhesive glue. 1) Clean the wound and peri-wound area with potable water or saline. 2) Cut hydrocolloid sheets into 2–3 cm-wide strips, perpendicular to the two pieces of plastic film covering the adhesive side of the hydrocolloid. 3) Place hydrocolloid strips around the wound and as close to the wound edge as possible. 4) Cut fine-mesh medical nylon or polyester netting to size. 5) Attach one side of the netting to the hydrocolloid border and flip it out of the way. 6) Apply zinc crème to protect the skin that is not covered by the hydrocolloid. 7) Apply loose medicinal maggots. If the maggots are supplied without a gauze pad, then use some water or saline to wash them out of their primary packaging onto a gauze pad which you apply directly to the wound. 8) Close the netting and secure it on the hydrocolloid strips using waterproof adhesive strips. Alternatively, you can use fast-curing glue to attach the netting to the hydrocolloid. 9) Place a moistened gauze pad on top of the netting. 10) Secure the gauze pad loosely with a bandage. 11) Place dry absorbing gauze pads on the bandage above the wound to absorb any exudate during treatment. Secure them loosely with another bandage. 12) The wound and dressing must be off-loaded during treatment to protect the medicinal maggots. Replace the outer dressings daily or when heavily soiled with exudate. 13) Removal of dressings and maggots is best done over a large, plastic waste bag to easily capture fast-moving maggots, dressing materials and water/ saline you may use to rinse the wound. 14) Wash, wipe, suck or pick maggots off the wound. The most convenient method is determined by the wound morphology, the body region and experience of the clinician. Clean the wound and surrounding skin carefully. 15–16) If the wound is free of necrotic tissue, continue regular wound care, or else repeat maggot therapy. Courtesy MedMagLabs and Creating Hope in Conflict: A Humanitarian Grand Challenge, CC BY.


Figure 4: Application guidance for a standard confinement dressing in low-resource healthcare settings. 1) Clean the wound and peri-wound area with potable water. You may boil some water for 20 minutes and let it cool before use. 2) Apply loose medicinal maggots. If the maggots are supplied without a gauze pad, you can use some water or saline to wash them out of their primary packaging onto a gauze pad which you apply directly to the wound. Place some larger moistened gauze pads on top of the wound. 4) Secure the gauze pads loosely with a bandage. 5) Use the legs or sleeves of suitable clothing items to confine the maggots on the wound. Make sure the clothing is finely woven to keep maggots in. 6) Cut the leg or sleeve section to size. 7) Tape the fabric tube at the upper and lower end to the leg, making sure there are no gaps for maggots to escape. 8) Place dry absorbing gauze pads on the confinement fabric above the wound to absorb any exudate during treatment and secure them loosely with another bandage. The wound and dressing must be off-loaded during treatment to protect the medicinal maggots. Replace the outer dressing daily or when heavily soiled with exudate. 9) Removal of dressings and maggots is best done over a large, plastic waste bag to easily capture fast-moving maggots, dressing materials, and water/saline that you may use to rinse the wound. 10) Wash, wipe, or pick maggots off the wound. The most convenient method is determined by the wound morphology, the body region and experience of the clinician. 11) Clean the wound and surrounding skin carefully. 12) If the wound is free of necrotic tissue, continue regular wound care, or else repeat maggot therapy. Courtesy MedMagLabs and Creating Hope in Conflict: A Humanitarian Grand Challenge, CC BY.
such barrier blocks the maggots from accessing healthy skin. Though pre-manufactured maggot therapy confinement dressings can be purchased commercially, they are relatively simple and often less costly to construct at the bedside with locally available materials2 (see also Figures 2, 3 and 4). While this system works well for flat wounds, the flat fabric does not conform well to circumferential leg wounds or stump and foot wounds. For such threedimensionally challenging dressings, alternative net fabrics such as net bags (performing a sock
or glove function) or nylon stockings (Figure 1B) may be used3, or even clothing items (Figure 4). In rare instances, non-permeable dressings may be used10,11 as long as a source of fresh oxygen can be passively or actively circulated through the dressing.
Since maggots within a containment bag (bagged maggots) do not require additional confinement, they are faster and simpler to apply. The bags of maggots are simply laid over the wound bed and then held in place with
Biosurgery: Application and Dressing Technology Wound Masterclass - Vol 1 - December 2022 109
© Copyright. Wound Masterclass. 2023
gauze wrap or taped gauze pads. The larvae still require plenty of air, and an absorptive dressing layer will need to be changed daily and whenever soiled. In addition, it is recommended that the larvae be provided with water or saline to prevent dehydration, especially during the first day or two. This hydration can be provided by spraying the bags of larvae during the dressing or re-dressing procedure, or by covering them with moist gauze instead of dry gauze.12
Dressing Changes, Maintenance & Repair
Guidance for optimal dressing maintenance should always be sought in the package insert. In general, the goal of maintenance is to ensure that the maggots are healthy, active, and contained or confined to the wound. To that end, many authorities recommend dressing inspection at least once daily, though that inspection need not be done by a health professional as long as the observer knows what s/he is looking for.
If there is a lot of drainage or soiling of the outer absorbent gauze dressing, it should be changed in order to optimise aeration of the maggot dressing and minimise the accumulation or spread of fluids and microorganisms. It is not uncommon for wound exudate to increase during maggot therapy and require 2–6 changes or more per day of the outer gauze dressings. If there is a lot of drainage, the fresh gauze dressings may be reapplied dry. If there was not a lot of drainage—say only one or two dressing changes are required per day—the fresh outer gauze should be moist, in order to keep the maggots hydrated. The outer gauze dressing should be changed at least once daily.
When the outer absorbent dressing is changed, also inspect the netted dressings for signs of loosening. Loosened borders can allow the maggots to escape on their own. Reinforce the dressing with extra tape, or extend the border with transparent membrane dressing (such as negative pressure dressing waste). If a hole in the dressing is discovered and maggots are not seen—especially in a dressing over 48 hours old—assume that the maggots have escaped. Open the net dressings to check. If maggots are found, and if they appear not to be satiated (not full size), then the dressing can be repaired with tape or a new net, and left for another day or
two longer.
At the time of the dressing change, check on the status of the maggots. If the dressing is only 24 hours old or less, the larvae may not be visible if they are feeding down on the wound bed. If the dressing is 48 hours old, however, then it should be possible to see movement (undulations) of the maggot dressings, if not the maggots themselves. If no movement or live maggots are visible between 48 and 72 hours, the maggots may have escaped or they may be dead. Look for a break in the netting and repair it, as described in detail, above. If the maggots are found to be dead, take down the dressing and dispose of the maggots, as described in the next section. Leaving non-viable maggots in place serves no benefit and may even increase risks. For example, wound infection may worsen under a dressing of dead maggots. If the dressing is opened and the larvae are found to be alive and healthy, then the dressing can be re-mounted, if desired.
Dressing Duration and Removal
Free-range (confined) maggots mature more quickly than bagged (contained) maggots. Over 50% of free-range maggots will likely be satiated and ready to leave the wound by 48 hours. At that point, they will be near the surface and margins of the maggot dressing, looking for a way out. Many therapists remove the dressings at that point, because leaving them in place for longer increases the risk of pain and escapes. Once satiated, the larvae will spend their time attempting to escape from under the dressings until they are finally removed. In patients without wound pain, some therapists chose to leave the maggot dressings in place for up to 72 hours so that the remaining maggots will provide additional debridement. After 72 hours, all of the free-range maggots should have matured, and will be at the surface trying to escape.
When it is time to remove free-range maggots, remember that the larvae are already lined up at the edges of the dressing, ready and eager to crawl far away. Therefore, have all needed materials at the bedside, including wet gauze to wipe up the larvae, and a receptacle for discarding the larvae. Place a barrier (rubbish bag, incontinence pad, etc.) under the work
Biosurgery: Application and Dressing Technology 110 Wound Masterclass - Vol 1 - December 2022 © Copyright. Wound Masterclass. 2023
area (wound) in order to catch maggots that may fall off. Remove the outer absorbent gauze and then gently peel back the netted dressing as though it were a banana peel. Meanwhile, wipe the wound with a water- or salinemoistened gauze pad, closely following the peeled net dressing, sandwiching the maggots between the wet pad and the dressing that is being removed. Then drop the dressing and sandwiched maggots into a biohazardous waste bin. If there are any maggots left behind, they can be removed with the aid of another wet gauze pad, a swab or water irrigation. If the remaining maggots are small and appear to be still working to remove more necrotic tissue, then it would be reasonable to replace a gauze pad over the wound and allow the last few maggots to continue working for another day. The gauze dressing can be removed the following day, by which time the last remaining maggots should be satiated and ready to leave the wound. Thoroughly rinse the wound with sterile water or saline once all of the maggots are removed.
Contained maggots grow more slowly, and typically are not satiated until about 96 hours. Since they are contained, the risk of escape is very low; but the risk of pain still increases with the duration of therapy.5 There is no benefit to leaving the maggots in place longer than the time that they are feeding, since they will stop secreting their digestive enzymes when they are satiated. Contained maggots are easy to remove because they are not running loose. Simply unwrap the outer absorbent gauze wraps and then remove each sachet of bagged maggots, placing them in a biohazard bag. Thoroughly rinse the wound with sterile water or saline.
When the dressings are opened, if the larvae are found to be dead—or not found at all— pay close attention to the wound bed and the condition of the dressings, for they can reveal what went wrong. If few or no maggots are found and there was a breach in the netted
dressing layer, then the maggots likely escaped. If there was no breach in the maggot dressing, look for evidence of dead maggots—either dead bodies or remnants of their mouth hooks (little black dots scattered among the wound bed). If either is found, it is highly likely that most or all of the maggots died all at about the same time, by drowning or suffocation. Crushing is also a possibility, but does not commonly kill all the maggots at the same time.
If very few or no maggot bodies are found—and, again, assuming that no maggots escaped— then the maggots may have died slowly, over the course of 24 hours or more. The causes for this may be more difficult to pinpoint. Again, the first place to start the investigation is with a careful examination of the dressing materials to ensure that the maggots did not suffocate or escape. A clean wound suggests that starvation may have played a role, and this is not an uncommon occurrence when maggots are placed on a relatively clean wound for the purpose of maintenance debridement or growth promotion.13 The live maggots can survive on the decomposing bodies of the dead ones, but soon some of the survivors may, themselves, starve and die. Starvation is not likely to cause maggots to die unless they are very young. Older maggots, when starved, can survive, but they do not grow as quickly or as large. Hostile environmental factors (chemical or physical) are sometimes invoked to explain mass loss of larvae. A few drugs and wound treatments may be harmful to the maggots.13 Specific bacterial or viral microbes within the wound might be a cause. This has not been demonstrated clinically, but there is laboratory evidence that certain microbes—at least Pseudomonas aeruginosa—can produce maggot-lethal virulence factors.14 Another possible cause is that the maggots may have been unhealthy to begin with: too old, too starved, exposed to temperature extremes, or contaminated. If poor maggot quality is suspected, look for supporting evidence such as other maggots in the same
Biosurgery: Application and Dressing Technology Wound Masterclass - Vol 1 - December 2022 111
© Copyright. Wound Masterclass. 2023
“Maggot therapy is indicated for the debridement of a wide variety of chronic wounds. Many experts also recommend medicinal maggots for controlling wound infections and the promotion of healthy granulation tissue and reepithelialisation in non-healing wounds.”
batch with similar problems.
Often, a definitive cause for widespread maggot death cannot be found. Fortunately, mass maggot death is a rare event when dressings are properly applied, and under these circumstances, a repeat course of therapy will usually proceed without any problem. Some therapists and researchers have speculated that mass maggot death may be due to the host’s immunological response. While allergic reactions are known to exist in animals, such reactions should repeat themselves in a maggotimmune wound. As it happens, the rare instance of massive maggot death even more rarely repeats itself in the same patient, except when one of the causes listed above goes uncorrected.
Reapplication or Follow-up Treatment?
Once the maggots have been removed and the wound rinsed, it is time to determine the next course of action. If necrotic tissue still remains, it is usually desirable to reapply another course of maggot therapy. A subsequent course of maggots may be applied straight away (a useful strategy for out-patients, in order to minimise the number and inconvenience of visits to the clinic), or the maggots may be re-applied according to the schedule that best fits the therapist and/or the maggot lab (for example, every “maggot therapy day” on Monday and Thursday, or once weekly). Keep in mind that the sooner the wound is completely debrided, the better the chances for attaining total debridement and, ultimately, wound-healing. Still, sometimes a patient can benefit from a break for a day or two, especially when anxiety or discomfort causes sleepless nights.
It is difficult to define an “average” number of maggot treatments needed to completely debride a wound because the number of treatments is highly dependent on the wound itself. The thicker or deeper the necrotic tissue and the drier that tissue, the greater number of treatments will be required; the greater the number of healthy maggots that are placed on the wound at one time, the fewer will be the number of treatments required. Most wounds can be completely debrided with one or two applications of maggots; some may require four or more applications. One of the most effective ways to hasten the debridement (decrease the
number of treatment cycles required) is to remove as much dry necrotic tissue (eschar) as possible before applying the maggots. This can be done with a scalpel, just prior to application of the maggot dressing, or it can be achieved by softening the existing dry tissue with an autolytic dressing technique overnight (occlusive dressing, hydrocolloid, etc.). This process should be fast and simple, done on the day of or the day before applying the maggots. Spending more effort and time than necessary for a very crude thinning or softening of the necrotic tissue is a waste of time and effort, once the decision has already been made that maggot therapy is the best course of action. The maggots will debride the tissue themselves, dry or not, within a few days.
If the wound bed is now well-debrided, it is time to begin the treatment of choice to effect wound closure. Depending on the therapist, patient and resources, treatment choices can vary widely, and are beyond the scope of this article. The important thing to note is that there is no need to delay definitive medical or surgical wound closure after maggot therapy. Maggot therapy does not increase the risk of infection or dehiscence, even if wound closure follows immediately.15 If a decision about the method of closure cannot be made straight away, it is fine to apply a tissue-supportive dressing (i.e., saline-moistened gauze, non-toxic ointments, honey, hydrogels, etc.) until the definitive decision can be made.
Summary
Maggot therapy dressings are intended to keep the maggots on the wound until most are satiated and have stopped feeding. Some therapists make their own dressings; others use commercially produced dressings. Many therapists use both, depending on their patients’ wounds. All maggot dressings have at least the following characteristics in common: they prevent the maggots from escaping, they allow adequate amounts of oxygenated air to reach the maggots, and they facilitate the drainage of accumulating liquids (liquefied necrotic tissue and exudates). There are two basic designs for maggot dressings: maggot confinement dressings, which confine the maggots to the wound bed but allow them complete access to the wound; and maggot containment dressings,
Biosurgery: Application and Dressing Technology 112 Wound Masterclass - Vol 1 - December 2022 © Copyright. Wound Masterclass. 2023
which totally contain the maggots within a net bag that facilitates easy handling but prevents the maggots from direct contact with all the nooks and crannies of the wound. Each has its advantages and disadvantages. Therapists should use the type of dressing that best meets their needs and their patients’ wounds. Once maggot debridement is complete, the appropriate and definitive medical or surgical treatment for closing the wound may begin.
References
1. Fine, A. and H. Alexander, Maggot Therapy: Technique and Clinical Application. The Journal of Bone and Joint Surgery, 1934. 16(3): pp. 572–582.
2. Sherman, R.A., A New Dressing Design for Use with Maggot Therapy. Plastic and Reconstructive Surgery, 1997. 100(2): pp. 451–456, https://doi.org/10.1097/00006534199708000-00029.
3. Sherman, R.A., J.M. Tran, and R. Sullivan, Maggot Therapy for Venous Stasis Ulcers. Archives of Dermatology, 1996. 132(3): pp. 254–256 https://jamanetwork.com/journals/ jamadermatology/fullarticle/vol/132/pg/254.
4. Grassberger, M. and W. Fleischmann, The Biobag — A New Device for the Application of Medicinal Maggots. Dermatology, 2002. 204(4): p. 306, https://doi.org/10.1159/000063369.
5. Dumville, J.C., et al., Larval Therapy for Leg Ulcers (VenUS II): Randomised Controlled Trial. BMJ, 2009. 338(7702): pp. 1047–1050, https://doi.org/10.1136/bmj.b773.
6. Steenvoorde, P., T. Budding, and J. Oskam, Determining Pain Levels in Patients Treated with Maggot Debridement Therapy. Journal of Wound Care, 2005. 14(10): pp. 485–488, https://doi.org/10.12968/jowc.2005.14.10.26846.

7. Steenvoorde, P., C.E. Jacobi, and J. Oskam, Maggot Debridement Therapy: Free-Range or Contained? An in-vivo Study. Advances in Skin & Wound Care, 2005. 18(8): pp. 430–435, https://doi.org/10.1097/00129334-200510000-00010.
8. Steenvoorde, P., et al., Maggot Debridement Therapy of Infected Ulcers: Patient and Wound Factors Influencing Outcome — A Study on 101 Patients with 117 Wounds. Annals of the Royal College of Surgeons of England, 2007. 89(6): pp. 596–602, https://doi. org/10.1308/003588407x205404.
9. Stadler, F., Packaging Technology, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 349–362, https://doi.org/10.11647/OBP.0300.16.
10. DeFazio, M.V., et al., Home Improvement in Maggot Therapy: Designing a Simple, CostEffective, Closed-System Habitat to Facilitate Biodébridement of Complex Distal Lower Extremity Wounds. Plastic and Reconstructive Surgery, 2015. 136(5): pp. 722e-723e, https:// doi.org/10.1097/prs.0000000000001685.
11. Felder, J.M., 3rd, et al., Increasing the Options for Management of Large and Complex Chronic Wounds with a Scalable, Closed-system Dressing for Maggot Therapy. Journal of Burn Care & Research, 2012. 33(3): pp. e169–175, https://doi.org/10.1097/ BCR.0b013e318233570d.
12. All Wales Tissue Viability Nurse Forum. The All Wales Guidance for the Use of Larval Debridement Therapy (LDT). 2013. https://www.wounds-uk.com/download/resource/5850.

13. Sherman, R., Indications, Contraindications, Interactions, and Side-effects of Maggot Therapy, in A Complete Guide to Maggot Therapy: Clinical Practice, Therapeutic Principles, Production, Distribution, and Ethics, F. Stadler (ed.). 2022, Cambridge: Open Book Publishers, pp. 63–78, https://doi.org/10.11647/OBP.0300.04.
14. Andersen, A.S., et al., Quorum-sensing-regulated Virulence Factors in Pseudomonas aeruginosa Are Toxic to Lucilia sericata Maggots. Microbiology (Society for General Microbiology), 2010. 156(2): pp. 400–407, https://doi.org/10.1099/mic.0.032730-0.
15. Sherman, R.A. and K.J. Shimoda, Presurgical Maggot Debridement of Soft Tissue Wounds Is Associated with Decreased Rates of Postoperative Infection. Clinical Infectious Diseases, 2004. 39(7): pp. 1067–1070, https://doi.org/10.1086/423806.
Biosurgery: Application and Dressing Technology
Wound Masterclass - Vol 1 - December 2022 113 woundmasterclass.com Introducing Wound Masterclass Video woundmasterclass.com/Video © Copyright. Wound Masterclass. 2023 Image licenced from Adobe Stock. Credit: BillionPhotos.com
Prof Anand Pillai Consultant Orthopaedic Foot & Ankle and Adult Reconstruction Surgeon Manchester, United Kingdom


Terminology of Tendon Disorders
Editorial Summary
Often, we come across random terms such as Haglund’s deformity, Pump Bump, Cucumber Heel, Knobbly Heels and high prow heels; etc. As clinicians, we tend to use such terms as tendonsis, paratendinitis, tenosynovitis, peritendinitis or tendonosis in different circumstances quite interchangeably. The suffix ‘itis’ is traditionally supposed to imply an underlying inflammatory pathology. Most currently practicing general practitioners were told and many still believe that patients who present with overuse syndromes have a largely inflammatory cause, and will benefit from anti-inflammatory medication. This dogma seems to be deep rooted. We now know that inflammation and inflammatory cytokines are more important in the acute injuries and are not universally prominent across the spectrum of some other tendon disorders.2 This article is a concise exploration of these themes.
Introduction
The ambiguity of the nomenclature appears to stem from the lack of standard terminology and the large pool of confusing terms and definitions often used interchangeably.
In this article, we highlight the use of uniform terminology which may help promote more effective communication and documentation and also could go a long way in information sharing and affording clarity to our patients. This can possibly aid progress in the management of these conditions.
Anatomy
It is important to understand the anatomy of a tendon before we talk about pathology. Anatomically, a tendon has 3 coverings named epitenon, endotenon and paratenon. The epitenon having vascular, lymphatic and nerve supply extends into the tendon between tertiary bundles as endotenon. The epitenon is covered by the paratenon (loose type 1 and 3 collagen). Together, the paratenon and epitenon are known as the peritenon.
Discussion
Historically there have been attempts to tackle this variability Hippocratic first described a ‘Tendo Magnus of Hippocrates’. In 1693, Philip Verheyen attempted to further clarify this and in 1883 Raynal’s description of ‘Cellulite Peritendineuse’, also tries to shed some light on this problem.
The first modern classification of tendon pathologies has to be credited to Lipscomb (1950) who brought in new definitions such as:
a. Paratendinitis: inflammation of tendons/ parts of tendons without sheath.
b. Tenosynovits : inflammation of tendons within a sheath.
c. Peritendinitis : general descriptor could be either a/b.
In the 1970s Perugia and in 1998 Puddu increased the focus on histology and described the terms:
a. Peritendinitis: inflammation in peritendinous sheaths, no tendon pathology.
b. Tendinosis : tendon degeneration without histological inflammation.
c. Peritendinitis associated with Tendinosis
Clain & Baxter (1992) tried to subdivide this based on anatomic location with the introduction of the terms insertional and noninsertional to further focus the pathology.
More recently, Maffulli (1998) suggested
114 Wound Masterclass - Vol 1 - December 2022
Dr Naeem Jagani
© Copyright. Wound Masterclass. 2023
Specialist Registrar in Trauma and Orthopaedics, Manchester University NHS Foundation Trust Manchester, United Kingdom
Tendinosis
Tendinitis/ Partial rupture
Paratenonitis
Paratenonitis with tendinosis
Intratendinous degeneration (commonly due to ageing, microtrauma, vascular compromise)
Symptomatic degeneration of tendon with vascular disruption and inflammatory repair response
‘inflammation’ of the outer layer of the tendon (paratenon) alone, whether or not the paratenon is lined by synovium
Paratenonitis associated with intratendinous degeneration.
that the diagnosis of overuse pathology should be only histological; therefore, an excision biopsy is required before any definitive nomenclature can be attributed.
Bonar in 1999 modified Clancy’s classification of tendonopathies and suggested use of terminologies like tendinosis, tendinitis/partial rupture, paratenonitis and paratenonitis with tendinosis (Table 1).2
This reiterates that overuse tendon conditions have a non-inflammatory pathology. The knowledge of the continuum of physiology to pathology makes it unambiguous that overuse and cumulative microtrauma, micro ruptures or overload have several stages / pathogenic cascades with a common pathway of neural ingrowth and neo vascularisation. It reaffirms that inflammation and degeneration are not mutually exclusive.3
Collagen disorientation, disorganisation and fibre separation by an increase in mucoid ground substance, increased prominence of cells and vascular spaces with or without neovascularization and focal necrosis or calcification.
Degenerative changes as noted above with superimposed evidence of tear, including fibroblastic and myofibroblastic proliferation, haemorrhage and organising granulation tissue.
Mucoid degeneration in the areolar tissue is seen. A scattered mild mononuclear infiltrate with or without focal fibrin deposition and fibrinous exudate.
Degenerative changes as noted in tendinosis with mucoid degeneration with or without fibrosis and scattered inflammatory cells in paratenon alveolar tissue.
The ‘Pathology continuum’ model of Cook et. al embraces the transition from normal through to degenerative tendinopathy and highlights the potential for reversibility early in this continuum. This reversibility is unlikely in these latter degenerative stages (Figure 1).4
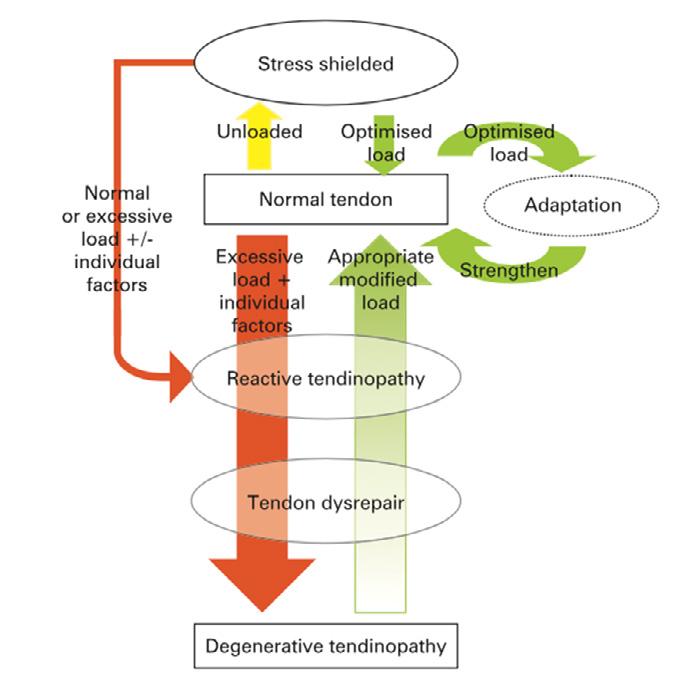
This work also factors in the differences in pharmacological and physical management in early versus late tendon disrepair (Table 2).
Stage
Reactive tendinopathy/early tendon dysrepair
Late tendon dysrepair/degeneration
Pharmacological management
Tenocyte inhibitors (ibuprofen, celecoxib, corticosteroids), aggrecan inhibitors (ibuprofen, naproxen, indomethacin)
Prolotherapy (including blood), aprotinin, sclerosing therapy, glyceryl trinitrate
Physical management
Load management. Reduction in frequency +/- intensity of tendon load
Exercise with eccentric component, Extracorporeal shock wave therapy, frictions, ultrasound.
Terminology of Tendon Disorders Wound Masterclass - Vol 1 - December 2022 115 Pathological diagnosis Concept (macroscopic pathology) Histologic finding
Table 1: Bonar’s modification of Clancy’s classification.
Figure 1: ‘Pathology Contiuum’ model of Cook et. al. 4
© Copyright. Wound Masterclass. 2023
Table 2: Clinical and pharmacological treatments placed in the model.
Adding or removing load is the primary stimulus that drives the tendon forward or back along the continuum, especially in the early stages. Within the constraints of recovery proposed in the model, reducing load may allow the tendon to return to a previous level of structure and capacity within the continuum.5 Therefore, there is a long term therapeutic advantage in understanding and clarifying terminology.
Van Dijk et. al (2011) attempted rationalisation of the nomenclature by using modern imaging (Table 3).6 Thus, by using terms such as midportion tendonopathy, para tendonopathy (acute or chronic), insertional tendonopathy, retrocalcaneal bursitis and superficial bursitis localised the area of pathology. There is now more clarity along with an insight into the findings associated with these tendon disorders.
Therefore, a clear understanding of the anatomical, histological, imaging and clinical findings could go a long way in helping us correctly label conditions and possibly effectively direct the necessary therapeutic measures.
Conclusion
We would recommend that it is time to abandon the ‘tendinitis’ myth. Physicians should acknowledge that painful overuse tendon conditions have a non-inflammatory pathology.
We suggest that the nomenclature be simplified and communications be streamlined.
We suggest to use a more unified classification like that of Van Dijk . Nomenclature for the clinical presentation of tendon disorders should reflect the true histopathological basis underlying clinical presentation.
Furthermore, by allowing time for collagen turnover and remodelling inherent in the pathology of tendonosis we can provide patients with a more realistic prognosis that better reflects the findings of clinical studies.
References
1. Klatte-Schulz F, Minkwitz S, Schmock A, Bormann N, Kurtoglu A, Tsitsilonis S, Manegold
S, Wildemann B. Different Achilles Tendon Pathologies Show Distinct Histological and Molecular Characteristics. Int J Mol Sci. 2018 Jan 30;19(2):404. doi: 10.3390/ijms19020404.
2. Khan KM, Cook JL, Bonar F, et al. Histopathology of common tendinopathies: update and implications for clinical management. Sports Med. 1999;27:393-408
3. Abate M, Silbernagel KG, Siljeholm C, Di Iorio A, De Amicis D, Salini V, Werner S, Paganelli R. Pathogenesis of tendinopathies: inflammation or degeneration? Arthritis Res Ther. 2009;11(3):235. doi: 10.1186/ar2723. Epub 2009 Jun 30.
4. Cook JL, Purdam CR . Is tendon pathology a continuum? A pathology model to explain the clinical presentation of load-induced tendinopathy British Journal of Sports Medicine 2009;43:409-416.
5.Cook JL, Khan KM,Kiss ZS, et al Asymptomatic hypoechoic regions on patellar tendon ultrasound: A 4-year clinical and ultrasound followup of 46 tendons. Scand J Med Sci Sports 2001;11:321–7.
6. Van Dijk CN, van Sterkenburg MN, Wiegerinck JI, Karlsson J, Maffulli N. Terminology for Achilles tendon related disorders. Knee Surg Sports TraumatolArthrosc. 2011 May;19(5):835-41. doi: 10.1007/s00167-010-1374-z. Epub
Terminology of Tendon
116 Wound Masterclass - Vol 1 - December 2022
Disorders
2011 Jan 11. Location Symptoms Clinical Findings Histology X-Rays Ultrasound scan CT MRI Mid portion tendinopathy 5cm from insertion Pain, swelling, impaired function Diffuse/local swelling Degeneration No inflammation Failed healing Soft tissue deviation Larger in cross section Disruption of pattern Increased vascularity x Fusiform expansion Intratendinous neovascularity Paratendinopathy Acute Midportion Edema, hyperemia Swelling/Crepitus Inflammatory infiltration Exudate x Hypo echogenic halo x Peripheral enhancement Chronic Midportion Exercise induced pain Swelling/Crepitus Thickened paratenon Adhesions x Thickened paratenon Adhesions x Same Insertional Tendinopathy Insertional Pain solid swelling Painful insertion Ossification of Enthesial fibrocartilage Ossification x Bone formation Bone formation Small tears Retrocalcaneal Bursitis Insertional Distal Painful swelling Pain swelling either side Synovial hypertrophy inflammation Posterosuperior prominence Fluid in bursa Prominence Hyperintense on T2 Superficial Bursitis Distal Swelling Shoe impingement Skin discolouration Visible swelling Acquired adventitious bursa Soft tissue deviation Fluid between skin and tendon x Hyperintense subcutaneous
© Copyright. Wound Masterclass. 2023
Table 3: Classification by Van Dijk et. al.
Biodegradable Temporising Matrix
Moving forward with a robust foundation
NovoSorb® BTM is a synthetic, bioabsorbable scaffold that enables generation of a vascularised neodermis, to provide a robust foundation for reconstruction over deep structures, including exposed bone and tendons.1,2

• Robust in the presence of infection3

• Designed to minimise scarring and contracture4

Discover more: polynovo.com
Indicated for full or deep partial thickness burns, traumatic wounds, surgical and reconstructive wounds. Refer to the Instructions For Use for full device details. References:
 1. Greenwood JE, et al. Eplasty. 2016. 2. Damkat-Thomas L, et al. PRS – Global Open. 2019. 3. Greenwood JE, et al. Burns Open. 2018. 4. Wagstaff MJD, et al. Burns Open. 2019. ® PolyNovo and NovoSorb are registered trademarks of PolyNovo Biomaterials Pty Ltd.
Pictured: Alan – Necrotising fasciitis survivor.
Complex wound from necrotising fasciitis
BTM fully integrated 3 months post treatment
1. Greenwood JE, et al. Eplasty. 2016. 2. Damkat-Thomas L, et al. PRS – Global Open. 2019. 3. Greenwood JE, et al. Burns Open. 2018. 4. Wagstaff MJD, et al. Burns Open. 2019. ® PolyNovo and NovoSorb are registered trademarks of PolyNovo Biomaterials Pty Ltd.
Pictured: Alan – Necrotising fasciitis survivor.
Complex wound from necrotising fasciitis
BTM fully integrated 3 months post treatment















































 M Health Fairview Wound Healing Institute, University of Minnesota Physicians Minneapolis MN, United States
M Health Fairview Wound Healing Institute, University of Minnesota Physicians Minneapolis MN, United States




 1. Greenwood JE, et al. Eplasty. 2016. 2. Damkat-Thomas L, et al. PRS – Global Open. 2019. 3. Greenwood JE, et al. Burns Open. 2018. 4. Wagstaff MJD, et al. Burns Open. 2019. ® PolyNovo and NovoSorb are registered trademarks of PolyNovo Biomaterials Pty Ltd.
Pictured: Alan – Necrotising fasciitis survivor.
Complex wound from necrotising fasciitis
BTM fully integrated 3 months post treatment
Ms Kara Couch
President-Elect, Association for the Advancement of Wound Care Associate Research Professor of Surgery, School of Medicine and Health Studies, George Washington University. Director, Wound Care Services, The George Washington University Hospital Arlington VA, United States
1. Greenwood JE, et al. Eplasty. 2016. 2. Damkat-Thomas L, et al. PRS – Global Open. 2019. 3. Greenwood JE, et al. Burns Open. 2018. 4. Wagstaff MJD, et al. Burns Open. 2019. ® PolyNovo and NovoSorb are registered trademarks of PolyNovo Biomaterials Pty Ltd.
Pictured: Alan – Necrotising fasciitis survivor.
Complex wound from necrotising fasciitis
BTM fully integrated 3 months post treatment
Ms Kara Couch
President-Elect, Association for the Advancement of Wound Care Associate Research Professor of Surgery, School of Medicine and Health Studies, George Washington University. Director, Wound Care Services, The George Washington University Hospital Arlington VA, United States








































 Mr Frank Aviles
Wound Care Clinical Coordinator, Natchitoches Regional Medical Center
Natchitoches LA, United States
Mr Frank Aviles
Wound Care Clinical Coordinator, Natchitoches Regional Medical Center
Natchitoches LA, United States


















 Dr Windy Cole College of Podiatric Medicine, Kent State University
Dr Windy Cole College of Podiatric Medicine, Kent State University













































































 Ms Nikole Siegmund
Mr Ted Bundrick
Ms Nikole Siegmund
Mr Ted Bundrick













 Ms Vanessa Vu
Research Associate I, Alira Health
Boston MA, United States
Ms Mia Hanna
Project Manager/ Research Associate III, Alira Health
Boston MA, United States
Dr Mitchell Sanders
Chief Scientific Officer, Alira Health
Ms Lindsay Poland
Scientist III, Lab Operations Manager, Alira Health
Ms Vanessa Vu
Research Associate I, Alira Health
Boston MA, United States
Ms Mia Hanna
Project Manager/ Research Associate III, Alira Health
Boston MA, United States
Dr Mitchell Sanders
Chief Scientific Officer, Alira Health
Ms Lindsay Poland
Scientist III, Lab Operations Manager, Alira Health

























 Ms Liz Ovens
Independent Tissue Viability Specialist Nurse
London, United Kingdom
Ms Liz Ovens
Independent Tissue Viability Specialist Nurse
London, United Kingdom


















 Dr Frank Stadler Director, MedMagLabs Sydney, Australia
Dr Frank Stadler Director, MedMagLabs Sydney, Australia







