A PICTURE-PERFECT RESPONSE
How data snapshots from a Weil Institute predictive analytic are helping care teams at U-M Health stop patient deterioration events before they start. p.10

MORE STORIES FROM THE WEIL INSTITUTE, OUR MEMBERS AND PARTNERS
Four Midwest centers unite to advance new therapies for acute respiratory distress syndrome, sepsis and pneumonia. p.26
Meet the inductees of the inaugural Acute and Critical Care Engineering (ACCE) Training Program. p.28
Tourniquets developed at the Weil Institute help support emergency medical response efforts in Ukraine. p.31
ISSUE 1 | SPRING 2024

FROM THE DESK OF DR. WARD
It is our pleasure to provide you with the very first edition of MAX, a magazine dedicated to showcasing the team science at the University of Michigan that is transforming the field of critical care.
Critical illness and injury is a group of diseases and conditions that can take a life in seconds to minutes to days and include conditions such as sepsis, cardiac arrest, traumatic brain injury, respiratory failure, trauma, and others. This can impact populations ranging from premature neonates to wounded soldiers on the battlefield.
Named after one of the fathers and founders of critical care medicine, the Max Harry Weil Institute for Critical Care Research and Innovation is a wholly unique research enterprise in academic medicine dedicated to transforming a field through innovation, integration, and entrepreneurship. A University of Michigan Biomedical Engineering graduate, Dr. Weil was one of the first leaders and innovators in medicine to adopt a multidisciplinary and convergence science approach to solving complex challenges that bridged the span from discovery to translation to commercialization of knowledge. We are proud to continue his legacy and to create the next generation of innovators in the field.
Launched in 2014 as the Michigan Center for Integrative Research in Critical Care, we have come a long way in leveraging the vast intellectual capital and creativity that exists across the University of Michigan. We are especially proud of the deep engagement the Institute has developed with

U-M Weil Institute 2800 Plymouth Road NCRC 10-A106 Ann Arbor, MI 48109
weilinstitute.med.umich.edu
Kate Murphy Design Megan VanStratt Editor

schools, colleges and units across the University such as the Colleges of Engineering and Pharmacy, Precision Health, Innovation Partnerships, and Fast Forward Medical Innovation.
The Team, the Team, the Team is a frequent research mantra at the University. However, real team science can be challenging. The Weil Institute’s unique infrastructure and programming was strategically developed to lower the energy barrier to team science that results in true impact. This includes inviting and engaging those with a heart for philanthropy to stand alongside the Institute as members.
We invite you to dive into MAX and explore how the Institute is enabling its members to tackle problems and develop lifesaving knowledge and solutions. These innovations range from artificial intelligence to devices, diagnostics, and therapeutics, across the spectrum of critical care. We hope our work excites you and motivates you to become a member of Team Weil!
Contributing Writers
Kevin Ward, MD Executive Director, Max Harry Weil Institute Follow Us
Jennifer Baker
Sherman Fan, PhD
Cindy Hsu, MD, PhD, MS, FCCM
Kenn Oldham, PhD
Gene Parunak
Robert Neumar, MD, PhD


@UMichWeil Weil-Institute
1


PICTURE Score ISSUE FOCUS: PRECISION MEDICINE Featured Weil Institute Member Research • A Closed-Loop Control System for Optimizing Fluid Management in Pediatric Heart Surgery Patients • Physician-Assisted AI Could Improve ARDS Detection • Using Continuous Glucose Monitors to Study Traumatic Brain Injury Outcomes Meet Micro-Gas Innovation in Focus: AHI COVER STORY: A PICTURE-Perfect Response WEIL MEMBER PERSPECTIVES Reimagining Critical Care Delivery in Emergencies By: Cindy Hsu, MD, PhD, MS, FCCM Collaborating with Industry: Navigating the Complex Landscape By: Gene Parunak Engineering Solutions for the Future of Critical Care By: Kenn Oldham, PhD 5 14 16 18 6 8 10 2




MORE STORIES FROM THE WEIL INSTITUTE Filling in the Blanks By: Jennifer Baker Four Midwest Centers Unite to Advance Novel Therapeutics Weil Institute Establishes Training Program for Critical Care Engineers An Investment in the Future of Translational Research “Turn-i-Kits” for Ukraine LEARN ABOUT THE WEIL INSTITUTE Our History The Weil Institute Today Grand Challenges at the Weil Institute Meet our Members Our Products • Available for Licensing • Emerging Products 3 20 34 35 36 40 43 26 28 30 31
WE ASKED A WEIL INSTITUTE MEMBER...

What does the history of innovation in critical care medicine tell us about the discipline’s future?
As with any medical specialty, innovation is the driving force that advances the quality of patient care and improves patient outcomes.
ROBERT NEUMAR, MD, PHD Professor and Chair, Emergency Medicine

As a clinician, what is on your critical care innovation wish list?
One of our greatest challenges in critical care is understanding the physiologic state of the injured brain. Although we have made important advances in imaging, electrophysiologic monitoring, multimodal invasive physiologic monitoring, and blood biomarkers, these all have significant limitations. Real-time non-invasive bedside monitoring of the adequacy of cerebral oxygen delivery would be transformative.
What words of advice do you have for future researchers and clinicians in critical care?
Clinicians caring for critically ill patients are in the best position to identify the knowledge gaps that are barriers to optimizing care and improving outcomes. A subset of those clinicians must acquire the right skills and capabilities, collaborate with others, and be prepared to do research to find solutions to fill those knowledge gaps. Those who are providing care have a great responsibility to prioritize this patient population.
4
FEATURED MEMBER RESEARCH



Weil Team’s Closed-Loop System Optimizes Fluid Management in Pediatric Heart Surgery Patients
Researchers led by Daniel Ehrmann, MD, and Kayvan Najarian, PhD, are developing a closed-loop control system powered by an advanced neural network to optimize treatment of fluid overload—a common yet deadly complication of heart surgery. Since debuting at Weil’s inaugural Kahn Pediatric Critical Care Grand Challenge in 2023, the project has gone on to receive additional support, including a $350,000 grant from the Gerber Foundation as well as winning one of two grand prizes during the 2023 Frankel Cardiovascular Center Innovation Challenge.
Weil Core Teams Utilized Funding Mechanisms Engaged
Proposal Development

Data Science

• Gerber Foundation
• Frankel Innovation Challenge

Weil Team Finds Physician-aided AI Could Improve Detection of Acute Respiratory Distress Syndrome
A team led by Negar Farzaneh, PhD, a Research Investigator and Data Scientist at the Weil Institute, compared the strengths and weaknesses of an artifical intelligence (AI) model with those of human expert physicians in the detection of ARDS findings on chest X-rays. They found that the physicians outperformed the model when interpreting X-rays classified as “difficult”, whereas the model outperfomed the physicians when interpreting X-rays that were not as difficult. Additionally, when the physicians lacked confidence in interpreting a chest X-ray, the AI model provided more accurate results, and vice versa. While further study will be needed, these initial findings suggest that AI and clinician expertise could potentially complement each other to improve ARDS detection and diagnosis.
Weil Core Teams Utilized Publication


Weil Team Uses Continuous Glucose Monitors to Study TBI Outcomes
Glycemic variability (changes in blood sugar) are commonly present following traumatic brain injury (TBI) and can contribute to secondary brain injury and worse clinical outcomes.
Continuous glucose monitors (CGMs) allow real-time, minimally invasive monitoring via a temporarily implanted sensor in the skin of the abdomen or arm.
Several commercially available CGMs are used frequently for outpatients with diabetes, given their convenience, simplicity, and autonomous continuous collection of glucose readings (often every five minutes).
By applying CGMs to Emergency Department patients during the golden hours following TBI, the team aims to generate highly granular data of glycemic variability, and associate these findings with outcomes.
Funding Mechanisms Engaged
• Caswell Diabetes Institute 2024 Research Career Development Award
• Massey TBI Grand Challenge
npj Digital Medicine doi: 10.1038/s41746-023-00797-9
DANIEL EHRMANN, MD
NEGAR FARZANEH, PHD
KAYVAN NAJARIAN, PHD
Data Science
5
KATHERINE SEAGLY, PHD NATHAN HAAS, MD
How U-M and Weil Institute researchers turned the breathalyzer into a tool for rapid, point-of-care disease detection and diagnosis.
Exhaled breath contains hundreds of volatile organic compounds (VOCs) that carry important information about our physiology. Diseases can impact VOC combinations and create unique patterns called “breathomic signatures.”
In 2015, Dr. Xudong (Sherman) Fan partnered with Dr. Kevin Ward to develop a device that could both recognize and analyze breathomic signatures indicative of various disease states.
While the technology, known as gas chromatography (GC), had existed for years, contemporary GC analyzers were too large and impractical for use at the bedside. Ward and Fan envisioned a portable device similar to a breathalyzer that would enable rapid diagnosis and the ability to monitor disease trajectory in real-time.
Together with a team of University of Michigan engineers, clinicians and data scientists, Fan and Ward built their technology to analyze vapor molecules on a parts-per-billion scale. The device is portable, fully automated, and weighs only nine pounds.
Patients simply breathe into the collection module, and the advanced algorithm powering the device will generate diagnostic results in less than twenty minutes. It can even be attached to ventillators for patients who cannot breathe on their own.
The team would call their technology “Micro-Gas Chromatography” or Micro-GC .
6
Micro-GC Detects ARDS and Asthma
In 2019, the Micro-GC team published a study in Analytical and Bioanalytical Chemistry demonstrating how they used human breath to differentiate between Acute Respiratory Distress Syndrome (ARDS) and nonARDS causes of respiratory failure.
Compared to the Berlin criteria (the gold standard method of diagnosing ARDS), the Micro-GC device and algorithm achieved an overall accuracy of 87.1% with 94.1% positive predictive value (PPV) and 82.4% negative predictive value. The high overall accuracy and high PPV suggested that this method could accurately diagnose ARDS.
The team would then examine the device in a setting of asthma in 2021, through which they determined nine VOCs that distinguished between asthma and nonasthma/non-atopic (allergies) subjects, along with sets of two and four VOCs, respectively, that further distinguished asthmatic from atopic controls, and between atopic and non-atopic controls.
They also indentified other unique VOCs that distinguished subjects by blood eosinophil (a type of white blood cell) levels, obesity status, inhaled corticosteroid treatment, and also acute upper respiratory illnesses within asthmatic groups.
This work demonstrated that breath VOC profiling can also be a clinically accessible tool for asthma diagnosis and phenotyping and that a portable GC system could be a viable option for rapid assessment in asthma.
Rapid breath analysis for acute respiratory distress syndrome diagnostics using a portable two-dimensional gas chromatography device. Anal Bioanal Chem 411, 6435–6447 (2019). https://doi.org/10.1007/s00216-019-02024-5
Real Time Breath Analysis Using Portable Gas Chromatography for Adult Asthma Phenotypes. Metabolites, 11(5), 265. https://doi.org/10.3390/metabo11050265
Micro-GC Distinguishes Between COVID Variants
During the COVID-19 pandemic, the tentative reopening of many states led to a marked increase in COVID cases and contributed to the rise of new COVID variants. During this time, the Micro-GC team was awarded a $2 million grant from the National Institutes of Health (NIH) to test their device’s ability to detect and monitor COVID-19 breath markers.
They defined four sets of VOCs that were able to distinguish between COVID-19 and non-COVID illness. However, when the team applied the same VOCs in a setting of presumed Omicron, sensitivity decreased drastically. They undertook additional biomarker searches and defined new VOCs to discern between Omicron and Delta, Omicron and non-COVID illness, and between patients with COVID-19 and non-COVID illness regardless of variants.
The combined analysis resulted in the ability to detect COVID-19 infected patients (regardless of variant) from non-COVID patients with a sensitivity of 89.4%, a specificity of 91.0% and an accuracy of 90.2%. This performance was shown to be close to that of RT-PCR tests (the gold standard) and better than many rapid antigen tests!
Portable Breath-Based Volatile Organic Compound Monitoring for the Detection of COVID-19 During the Circulation of the SARS-CoV-2 Delta Variant and the Transition to the SARS-CoV-2 Omicron Variant JAMA Network Open. DOI: 10.1001/jamanetworkopen.2023.0982

Learn More about the Micro-GC Device
7
Innovation in Focus: AHI System
“Most vital signs measurements are static, subject to human error, and require validation and interpretation. AHI is the opposite of all of that: it’s dynamic , gives a binary output of ‘stable’ or ‘unstable’, and may enable early marshaling of resources to patients who may not have been on a clinician’s radar.”
- Ben Bassin, MD, Clinical Associate Professor of Emergency Medicine
Principal Investigators
Ashwin Belle, PhD
Kevin Ward, MD
Licensed To Milestones
• FDA De Novo (2021)
• FDA 510k (2022)
• Incorporated into Sickbay™ Platform (2023)
Website
fiftheye.com/AHI
Shifts in vital signs such as hemodynamic instability (low vital organ blood flow) occur prior to adverse health events, but they are often recognized too late to avoid patient deterioration.
Based on technologies developed and tested at the Weil Institute, AHI, the Analytic for Hemodynamic Instability, continuously detects changes in hemodynamic status in real-time using data from a single electrocardiogram (ECG) lead. It can then automatically and continuously predict the likelihood of future episodes of hemodynamic instability ahead of traditional vital signs.
AHI can also leverage the same data from several FDA-approved wearable ECG patches, meaning the potential exists to provide an almost ICU level of monitoring in patients on the general ward who traditionally only have their vital signs measured and recorded every 4-6 hours.
AHI is currently begin trialed in several major academic medical centers across the country.
Detection of Hemodynamic Status Using an Analytic Based on an Electrocardiogram Lead Waveform. doi: 10.1097/CCE.0000000000000693
Prediction of episode of hemodynamic instability using an electrocardiogram based analytic: a retrospective cohort study. doi: 10.1186/s12871-023-02283-x
8
IN PREVENTING SUDDEN CARDIAC ARREST FATALITIES IN ATHLETES
The Weil Institute has become a new member of the expanded Smart Heart Sports Coalition of the National Football League (NFL). This partnership unites the Weil Institute with 36 other leading healthcare, advocacy and professional sports organizations under the shared goal of preventing sudden cardiac arrest (SCA) fatalities in athletes.
Founded after the life-saving emergency care provided to Buffalo Bills safety Damar Hamlin, who experienced a cardiac arrest on the field in January of 2023, the Smart Heart Sports coalition advocates for the nationwide adoption of evidence-based policies to help improve athletics-related SCA response efforts and outcomes. These policies include implementing Emergency Action Plans at athletic venues, installing clearly marked automated external defibrillators (AEDs) at or near where practices or competitions are held, and educating coaches in cardiopulmonary resuscitation (CPR) and AED use.
The partnership serves as a natural extension of the Weil Institute’s continued efforts to transform management of cardiac emergencies no matter where they occur. Research from Weil members has enabled the development of multiple lifesaving innovations such as a mobile health technology that can predict severe
cardiac events on the road to prevent vehicle crashes, and a portable oxygen delivery device that can pair with an AED to help bridge the critical time gap between the initial response to a cardiac event and the arrival of emergency medical services.
Further exemplifying its commitment to transforming care and outcomes for cardiac emergencies, the Institute served as host for the seventeenth iteration of the Wolf Creek Conference --a historic tradition that brings together world leaders in resuscitation science with the aim of determining the research priorities that will drive the future direction of the field. Two of the featured speakers at this event were none other than Dr. Jim Ellis, Chief Medical Officer of the United States Football League, and Denny Kellington, Assistant Athletic Trainer for the Buffalo Bills and the very specialist who delivered CPR to Damar Hamelin. Dr. Robert Neumar, Professor and Chair of Emergency Medicine at Michigan Medicine, led 2023 Wolf Creek Conference and also championed the Weil Institute’s new partnership with the NFL.
“[We are] delighted to partner with the Smart Heart Sports Coalition,” he said. “We envision a future state where it is the expectation that athletes will survive sudden cardiac arrest, and believe this can be achieved through advocacy, education, training, research, and innovation. It will take a team effort, and we are excited to be on the team.”
In May of 2024, Weil and Smart Heart pushed new Michigan legislation to strengthen AED and CPR training requirements in schools!

THE NFL AND THE MAX HARRY WEIL INSTITUTE TEAM UP TO SCORE A
Scan the QR code to the right to read the full story! 9

A PICTURE-PERFECT RESPONSE
By providing continuous, real-time snapshots of patients’ clinical status, a predictive analytic developed by the Weil Institute helps the U-M Health Adult Rapid Response Team stop deterioration events before they start.
COVER STORY 10
While in the hospital, a patient’s condition may deteriorate due to various factors such as heart rate abnormalities or an unexpected drop in blood pressure. During deterioration events, early intervention is key to a positive outcome, yet their unanticipated and often sudden onset makes them difficult to identify in advance. At U-M Health, a predictive analytic tool developed by the University of Michigan Max Harry Weil Institute for Critical Care Research and Innovation is helping members of the hospital’s Rapid Response Team (RRT) stop the spark of deterioration before it starts.
Comprised of nurses from the surgical intensive care unit (SICU), the RRT functions like an ambulance inside the hospital, quickly bringing lifesaving interventions to patients whenever bedside teams make the call. RRT members Tiffany Watts, RN, and Ernie Saxton, BSN, RN, CCRN have been working alongside the Weil Institute’s Data Science core to deploy Weil’s “PICTURE” analytic within the RRT. Short for “Predicting Intensive Care Transfers and other UnfoReseen Events,” PICTURE uses electronic health record data to passively and accurately predict a patient’s risk of deteriorating up to an average of 30 hours in advance of symptoms. While it has only been a few months since the official launch, the team is already reporting improved response times thanks, in part, to these predictions.
“PICTURE’s alerts have been very consistent. It seems like we’re getting to these patients faster now,” said Watts, who serves as Clinical Lead for the Adult RRT. “It’s still early, but we’re seeing exciting things.”
In addition to responding to emergencies when they happen, a chief goal of the RRT is intervening before these emergencies can begin. To help them get ahead of potential events, the RRT members will round at the start of their shifts, meeting with care teams and patients on each floor to learn in advance where they may be needed and why.
“Rounding can be a time-consuming process,” said Brandon Cummings, Senior Data Scientist at the Weil Institute and a lead on the PICTURE team. “The RRT might get a range of different feedback based on the experiences of who is on staff that day. Having a system like PICTURE—something that can prioritize patients for them—saves a lot of time and legwork.”
“With PICTURE, we’re getting the knowledge we need earlier in the day so we can go to the patients that it flags as highrisk and potentially catch them before a situation elevates,” said Saxton, who serves as Educational Nurse Coordinator.
According to Saxton, PICTURE’s advanced warnings also provide key information once the team is at the bedside. “It can be difficult to focus while we’re also listening to the bedside nurse and the physician, while we’re putting a fluid IV in or putting some oxygen on. PICTURE helps us know what the situation is before we even get there.”

“Prior to having an early warning system, we would talk to the nurses in charge on each floor to see which patients might need an extra set of eyes on them. And we had to do that manually, going unit to unit. ”
11
Ernie Saxton, BSN, RN, CCRN

Tiffany Watts, RN Clinical Lead, Adult RRT

“As a floor nurse before joining the Rapid Response Team, all I ever wanted was to be able to give the reason for my gut feeling when everything about a patient looked technically fine but something ‘felt bad’.”
PICTURE is designed to model patient physiology as opposed to clinician behavior to ensure it always provides novel information. To generate its predictions, the system automatically and continuously processes an array of patient data including waveforms, vital signs and lab results. It takes this information and consolidates it into a number—a risk score—that allows care teams to see and understand a patient’s status at a glance. PICTURE also provides a list of the factors that go into each score to help guide clinical decision-making. To the RRT, such explanations are invaluable.
“As a floor nurse before rapid response, all I ever wanted was to be able to give the reason to my gut feeling when everything about a patient looked technically fine but something ‘felt bad’.” said Watts. “PICTURE provides a concrete number that we can use with our own critical thinking to determine how these patients are really doing.”
“Because we have this list of potential events, and because we can see what exactly is going into each score, we can consult ahead of time with the floor nurses and staff as well as within the RRT,” said Saxton. “If we notice a patient who initially had a lower risk score start trending higher, we can bring that up.”
Over the next year, the Weil Institute Data Science Team will be working with Saxton, Watts and the RRT as a whole to further optimize PICTURE. The system currently includes versions for Adult and Pediatric All-Cause Deterioration Prediction with additional models in development.
“We used to say rapid response systems generally would stop a spark from becoming a fire,” said Saxton. “Now, with PICTURE, it seems like we’re able to look at cases where someone was getting ready to strike the match, and we’re able to stop the spark on some of them.”
more of the RRT’s experiences with PICTURE, scan the QR code above to watch our highlight reel! 12
To hear

THE PICTURE SUITE
Found to significantly outperform the Epic Systems Deterioration Index*and is actively applied in real-time to over 1,000 adult and pediatric beds at U-M Health.



Learn More and Explore Licensing Opportunities
*See page 47 for studies referenced
13

Reimagining Critical Care Delivery in Emergencies
BY CINDY HSU, MD, PHD, MS, FCCM
Division Chief of Critical Care, Associate Professor of Emergency Medicine and Surgery, University of Michigan
I worked a busy shift in the emergency department last week. Constant stream of critically ill patients from local communities and other hospitals filled our resuscitation bays. They were victims of out-of-hospital cardiac arrest, septic shock, severe burn, intracranial hemorrhage, pneumonia, multi-organ failure, traumatic injuries, and metastatic cancer. My team tried our best to provide timely care for these patients, but we were constantly interrupted by phone calls and pages. My conversations with patients and their loved ones felt rushed, as did the time I spent reviewing their diagnostic results. I also worried about the 20 other patients who were already under my team’s care and the over 50 patients in the waiting room.
As an emergency physician and intensivist, I get to care for patients who come to the emergency department when they most need help. I became an emergency physician so that I can care for patients regardless of their illnesses and socioeconomic status. I became an intensivist so that I can better care for critically ill patients from the moment they arrive in the emergency department through their hospital stay. I am grateful I get to do this for a living.
Yet, I have become acutely aware of the fragilities in our healthcare system. The COVID-19 pandemic has exposed and perpetuated the existing inequities and strains of our society. The patients who come to the emergency department are the most vulnerable, many uninsured or underinsured,1–3 all with limited access to primary and subspecialty care especially during evenings and weekends.2 They tend to present later in their clinical course, with higher severity of illness.4,5 The emergency department serves as their portal to healthcare. Yet, the bottleneck at this portal has hindered and fragmented critical care delivery6,7 to those who most need it, when they most need it. Furthermore, our frontline healthcare providers are battered and
exhausted. In a time where boundary setting is not only necessary but required to sustain our workforce, emergency medicine has become the specialty without boundaries.
Different strategies have been developed to care for critically ill patients within the strains of our healthcare system. Specialized teams can allocate more time and resources to care for critically ill patients in dedicated spaces. This concept of intensive care began in the 1850s, when Florence Nightingale placed the most injured soldiers closest to the nursing station during the Crimean War.8 In 1959, Drs. Max Harry Weil and Herbert Shubin established the Shock Ward at the University of Southern California/Los Angeles County General Hospital9 while Dr. Peter Safar created the first modern intensive care unit at the Baltimore City Hospital.10 Recent models of resuscitative care units11 include University of Michigan Emergency Department’s Emergency Critical Care Center12,13 and University of Maryland’s Critical Care Resuscitation Unit.14 These units can expedite critical care access and therapeutic interventions in the emergency department12,15 and inpatient setting, respectively.14,16 Stanford University17 and Henry Ford Hospital18 have established critical care consult teams to help care for critically ill patients in their emergency departments. Other medical centers provide critical care telehealth support for their emergency departments.19
Critical illnesses are unscheduled, and their therapies are time-sensitive. Therefore, critical care delivery has also evolved from dedicated units within hospitals to system of care models that incorporate prehospital care. Life-saving therapies such as cardiopulmonary resuscitation, defibrillation, intranasal naloxone, and hemorrhage control are most effective when initiated early by lay rescuers, first responders, or paramedics. Unmanned aerial vehicles can even deliver
14
page 46 for the complete list of publications referenced in this article. PERSPECTIVES
See
essential supplies to patients and rescuers in more remote areas.20,21
Emergency department crowding is a manifestation of hospital crowding. The emergency department serves as the ailing canary for healthcare system dysfunction.22 Hospital crowding is perpetuated by high inpatient census due to misaligned incentives,22–24 insufficient healthcare capacities,25 and strained post-hospitalization resources.26 Until we can dismantle the structural inefficiencies of hospital crowding, critical care delivery will remain inequitable, ineffective, and costly.6,7,17
Innovative solutions to hospital crowding and critical care delivery are unlikely to come from any single person or team, but from collaborative engagements across different disciplines with diverse viewpoints. They are our patients and their caretakers, healthcare workers, researchers, designers, federal and private sponsors, insurance payers, administrators, politicians and policymakers, military, nonprofit organizations, and sectors in business, technology, art, and athletics.
Innovation emerges from creative plays in brave spaces. We cultivate brave spaces to empower those who have been historically marginalized to speak and be heard. We have meaningful conversations with patients and their loved ones to provide care best aligned with their values. We listen to them early in the creative process to identify the pertinent challenges and impactful strategies for critical care delivery. We differentiate disease phenotypes of critical illnesses so that we can tailor therapies for each patient. We harness big data analytics, artificial intelligence, and extended reality to create safe and just systems of care while lowering burden on patients, caretakers, and healthcare workers. We diversify our ideas, pipeline, and funding. We train and retain our healthcare workforce through sustainable and engaging strategies.
Just as the coal miners have evolved from bringing caged canaries into the coal mines to detect poisonous gases, it is time for our healthcare system to evolve as well. It will take courage, humility, and adaptability. It will require investment from the highest levels.
Let’s set the canary free.

“Until we can dismantle the structural inefficiencies of hospital crowding, critical care delivery will remain inequitable, ineffective, and costly.”
 Photo: Dr. Cindy Hsu
Photo: Dr. Cindy Hsu
15

Collaborating with Industry: Navigating the Complex Landscape
BY GENE PARUNAK Managing Director, in2Being, LLC.
In the intricate realm of medical device development, successful collaboration between industry and academia has become paramount. As the Managing Director of in2being, LLC, a company that partners with innovators to bring medical devices from concept to commercialization, I have had the privilege of insight into the challenges and opportunities within this dynamic landscape. In this article, we will explore the complexities of collaborating with industry, shedding light on the pitfalls to avoid on the pathway to success.
Before exploring the pitfalls, let’s address a common question: when should you start to reach out to industry for collaboration? Can you reach out too early?
There is never really a “too early”, so long as you are prepared to hear that your partner needs more information before they can be of use to you.
You may not yet be speaking their language. You may not yet know what you need to provide to your partners to enable them to achieve success for you. But sincere, curious, early engagement with industry is critical to your learning process as an entrepreneur. Approach this unfamiliar domain with good questions and a little caution against things that are too-good-to-be-true, and you’ll do well. Keep in mind that having something you can show, whether a concept, a prototype, or at least some research results will help you communicate with potential partners. Finally, never forget to establish a non-disclosure agreement before revealing inventions not yet patented—your tech transfer department will thank me.
Some of the areas where researchers typically find value in interacting with industry include:
• Regulatory strategy: pre-sub, testing plans, documentation, FDA submissions
• Design: mechanical, electrical, software, labeling
• Prototype iterations, concept, feasibility, alpha, beta, for clinical use, for animal use, Depending on the use case, prototypes may need to be manufactured in a way that closely represents final commercial manufacturing, for example when a
device needs to be terminally sterilized. Recognizing nuances such as these comes only with experience—and a few bruises along the way. Many a startup has paid a handsome sum price shopping only to rediscover that apples and oranges aren’t the same thing.
Pitfalls
#1 – Assuming that manufacturers are designers
While some manufacturers can credibly do design work, most contract manufacturers are there to build what you tell them to build. They simply will not (and cannot) tell you what to you need to build. A good designer will already speak the language of manufacturing and will be able to create a bid package that you can shop around so you aren’t dependent on any one manufacturer. There’s nothing worse than not owning and controlling the design you thought was yours.
#2 – Assuming you need clinical data to submit to the FDA
Many devices do not require clinical data to receive clearance (510K). However, clinical trials are a way to get user and performance feedback prior to commercialization. Working with business and regulatory strategists can help layout the most efficient path to YOUR goals. How you write a study in academia vs how you write a study for FDA vs how you write a study for your market may be three different things.
#3 – Assuming that FDA clearance means you are all ready to sell
There can be a massive abyss that lives between clearance and commercial distribution of your new device. We like to say that “510(k) is halfway” to commercial use of the new device. Halfway in time. Halfway in money. At clearance you still often have 100% more effort to go before you can start selling your device. There are times where clinical data is more value add than a clearance because clinical data gets you to early market validation prior to clearance.
16 PERSPECTIVES
Market validation after clearance takes a lot more money and sales. Working with business and regulatory strategists can help layout the most efficient path to YOUR goals based on the type of device you are developing.
#4 – Assuming the iteration in the clinical trial is the commercial device
Devices for first-in-human use are often quite different than their final commercial cousins, but this point is often overlooked. We’ve received the request over and over for a “design freeze” so that the innovator can go all the way from proof of concept through clinical trial, through FDA clearance, and get onto the market. In reality, this may be 3 to 5 iterations of increasing complexity. If this is hard to see, consider for example that what it takes to get FDA clearance and what it takes to get IRB approval (especially for non-significant risk devices) can be VERY different. It likely makes little sense to go all the way to a commercial launch device if you know full well that that device is going to change based on patient and user feedback received in your first-in-human work.
in2being’s Collaboration with the Weil Institute
Over the last decade, in2being and the Weil Institute have fostered a fruitful partnership. This collaboration has extended across various areas, ranging from educational endeavors and presentations to hands-on prototyping efforts. Our collaboration has involved working closely with Weil Institute researchers to develop and field new medical device prototypes that have the potential to revolutionize critical care.
We look forward to many more years of successful collaborations with the Weil Institute, driven by the know-how of our friends and partners. Together, we aim to shape the future of medical devices for critical care, making a meaningful impact on healthcare outcomes.
With a commitment to innovation and a deep understanding of the collaborative nature of the medical device industry we’re excited to see the Michigan ecosystem at the forefront of advancing healthcare solutions.
“...Sincere, curious, early engagement with industry is critical to your learning process as an entrepreneur.“

 Photo: Gene Parunak speaks at the 2023 Massey TBI Regional Conference
Photo: Gene Parunak speaks at the 2023 Massey TBI Regional Conference
17
Engineering Solutions for the Future of Critical Care
BY KENN OLDHAM, PHD
Associate Director, Weil Institute; Professor of Mechanical Engineering, University of Michigan
Emergency and critical care medicine place demands on technology that require novel solutions from engineering research and product development. Among medical specialties, response to acute injury and illness involves an unusually wide range of treatment providers and points of care, from first responders through emergency departments and into rehabilitation and treatment. Treatment is highly time-sensitive, and regularly involves multiple physiological systems. Engineering and computer science provide many potential tools that align with these needs. Microsystems technologies, from microelectronics through microelectromechanical systems (MEMS) and microfluidics offer dramatic improvements in size, weight, and speed of diagnostic and monitoring devices. Signal processing and machine learning techniques continue to accelerate the speed and complexity with which data can be synthesized and transformed into actionable information by clinicians. And humancentered design processes help ensure that tools are robust and readily usable across care providers with disparate backgrounds and resources.
WE ASKED A WEIL INSTITUTE MEMBER...
 Xudong (Sherman) Fan, PhD
Richard A. Auhll Endowed Professor of Engineering; Professor of Biomedical Engineering; Associate Director, Weil Institute
Xudong (Sherman) Fan, PhD
Richard A. Auhll Endowed Professor of Engineering; Professor of Biomedical Engineering; Associate Director, Weil Institute

However, demanding settings also pose major challenges for technology development. Fast-moving, complex events create a substantially more challenging environment for validation than may be available to other biomedical research, and case heterogeneity further complicates the scientific and practical evaluation of new tools. Meanwhile, heterogeneous, complex patient states are difficult if not impossible to replicate in benchtop testbeds or with healthy subjects. These challenges can be a high barrier to entry for engineers interested in testing new ideas such that they can successfully produce research publications, technology translation, or both.
For engineers, the Weil Institute serves as an indispensable resource for moving ideas to implementation in settings that can genuinely prove an impact on emergency care. The Weil preclinical testing core has developed multiple models for complex disease and injury processes, much closer to human testing conditions than can be achieved with benchtop or biological phantom experiments, while maintaining a comparatively controlled setting. For example, the
How can engineering play a role in the innovation of critical care solutions?
Before I joined the Weil Institute, I had very little knowledge about critical care medicine including the variety of diseases it includes, the number and types of patients impacted and how great the need is to develop new technologies to improve care and outcomes. Because of its complexity and the need for rapid diagnosis and treatment, critical care poses some unique challenges in medicine for which I believe the discipline of engineering can offer real value in carefully developing meaningful solutions. Engineering, like medicine, has many specializations such as biomedical, chemical, computer science, electrical, mechanical, materials science, and robotics. It’s natural to believe that under the right conditions, we could create novel technologies and approaches to save lives.
PERSPECTIVES
early development of cardiovascular monitoring tools in my laboratory was able to first piggyback—pun somewhat intended—off ongoing experiments with swine models for trauma.
With later seed funding through Weil and affiliated UM offices, Weil researchers helped us develop dedicated experiments in which specific cardiovascular parameters could be perturbed according to our needs. At later stages of development, Weil clinical coordinators can help navigate human subject testing and patient recruitment. Again, this has provided critical support to my group as we progress to sensor testing in real-time with critical care patients.
The network of clinicians, engineers, and professional staff already engaged with the Weil Institute has created a setting for technology to move from concept to real-world testing in an exceptionally accessible way. I encourage any of our engineering researchers to look for opportunities to link their innovations with critical care needs. Ready points-of-entry include the Institute’s Grand Challenge and Seminar events. We have also initiated an NIH-supported T32 predoctoral training program in Acute and Critical Care Engineering, which can provide novel clinical and technology translation training to early-stage graduate students. Critical care and emergency medicine can only benefit from tools that are smaller, faster, and more capable, and few institutions can meet those needs as effectively as our College of Engineering.
“For engineers, the Weil Institute can serve as an indispensable resource for moving ideas to implementation in settings that can genuinely prove an impact on emergency care.”
How has the Weil Institute impacted your career and lab?
It has brought so many opportunities which range from developing new diagnostic technologies like breath and body odor monitors to diagnose and track ARDS, sepsis, and other diseases to training graduate and post graduate students. This has led to the opportunity to create new intellectual property and even develop new start-up companies to license and commercialize these discoveries. Everything about the Weil Institute is specifically designed and tuned to encourage collaboration and quickly move ideas to clinical impact. It is one of the most unique programs I have ever been a part of and one reason why I am so pleased to be an Associate Director.

What words of advice do you have for future researchers & engineers interested in critical care?
First of all, join the Weil Institute. I would be very surprised if there is not an opportunity for you to find collaborators in medicine, engineering and other disciplines and for you to make an impact. Second, don’t be afraid that you don’t know enough about critical care. I have been able to learn a lot in a short period of time through the great clinical collaborations that the Weil Institute makes possible. Third, don’t be shy about putting an idea forth even if it is very early.Weil is all about engaging in high-risk, high-reward team science. I think no matter where you are in your path to develop a solution, the Weil Institute will have something to offer to move your idea forward.
 Photo: Dr. Kenn Oldham presents at the Massey TBI Grand Challenge kickoff
Photo: Dr. Kenn Oldham presents at the Massey TBI Grand Challenge kickoff
19
FILLING IN THE
How COVID is shaping the future of critical care medicine
BY JENNIFER BAKER
“You should really talk to my wife. You have to understand, I basically have no memory of the forty-some days I was on the ventilator.”
I was caught off-guard, as my purpose for talking with David O’Brien, the voice at the other end of the call, was to learn about his experience as a COVID patient who experienced critical care. After recovering from my surprise and reassuring him, I wondered just how convincing I had been. Would an article featuring a patient with major memory gaps be complete enough to be compelling? As we talked, I found the answer to be a resounding yes.
Like Dave’s family piecing together his memories so he can move forward with recovery, the field of critical care can only advance by taking a detailed, retrospective look at the experiences that shaped it. The COVID pandemic, with its waves of critically ill and recovering
patients, is one of these experiences, as multiple aspects of critical care medicine – including emergency services, intensive care, and rehab – are involved in restoring these patients back to health.
Because of its massive demand on the field, the pandemic undoubtedly has been a pivotal time in critical care. While major improvements were made, giant gaps are also left to fill. To bring about the envisioned future – one that improves outcomes for all patients using lessons from COVID ¬– the challenge is finding the right solutions for these gaps. And that’s just what members of the Weil Institute for Critical Care Research and Innovation at the University of Michigan are working to achieve.
20
The
COVID-19 crisis brought critical care medicine into the global spotlight and re-exposed systemic gaps in patient care.
Four years after the initial surge of cases, the Max Harry Weil Institute for Critical Care Research and Innovation at the University of Michigan is asking the question: where do we go
from here?
In December 2021, David, a self-described “early retired” former General Motors engineer, and his wife Michele, a social worker in the local school district, contracted COVID-19. While Michele was able to recover at home, Dave was admitted to the University of Michigan Hospital in Ann Arbor on December 18th with low blood oxygen.
“It’s a fuzzy memory, but I remember them putting me in a wheelchair and taking me down the hallway and that’s about it,” he recalled during an interview at his home in Gaines, Michigan. “I was out for a long, long time.”
For 41 days, Dave fought for his life on a ventilator, diagnosed with acute respiratory distress syndrome from severe COVID pneumonia. But even armed with a year of experience in pandemic patient management and a new technique called prone positioning that helped patients breathe, doctors were reluctant to give Dave any promise of recovery.
It was an emotional sequel for Michele and children Isabella, Olivia, and Grant, who just two months before Dave’s illness, lost Davis, their 22-year-old brother and son, to hydrocephalus after years of frequent hospitalizations.
Despite the odds, Dave woke up over a month later, requiring his wife and a nurse to convince him of the date as he insisted on making plans for Christmas. After three weeks of in-patient rehab, Dave arrived home on March 9th, 82 days after his initial hospital admission. His doctors now call him “one of the lucky ones.”
with assistance from University of Michigan hospital staff. Image by Isabella O’Brien and courtesy of David O’Brien.
21
Moving beyond supportive care
One prominent aspect of the early pandemic was the lack of any available treatments, let alone personalized ones, for COVID patients dealing with their illness at home. O’Brien recalls a conversation with his family doctor prior to hospitalization, wishing there was something available to prevent worsening symptoms besides rest and fluids.
Practitioners of critical care medicine share Dave’s frustration. Weil Deputy Director and Associate Professor of Pulmonary and Critical Care Medicine Dr. Robert Dickson put it this way: “Critical care in 2023 is still basically supportive care. We provide life support with the ventilator, with other devices and medications to support failing organs, but we’re not actually tailoring our therapies molecularly to the patient in front of us. I think that’s where we clearly need to go, but there’s some major barriers to get there.”
One of these major barriers to tailored treatments is the lack of accurate, real-time data to guide decisions about patient care. If available, information on key health parameters, especially those related to biological processes that make patients distinct, could be used to make better decisions on a case-by-case basis and guide molecular therapies when they become available.
But even without inventing new tests or devices, the amount of data generated for every critically ill patient is staggering: up to 100,000 data points per second, making it easy to overlook details during interpretation and time-consuming to find the right information after patient transfers. As Weil Executive Director and Professor of Emergency Medicine and Biomedical Engineering Dr. Kevin Ward summarized, “There is no bigger big data problem than critical care.”
This makes the critical care data problem a multi-faceted one with simultaneous needs, including a push to ensure the accuracy of medical devices for all patients. The inaccuracy of pulse oximeters on patients with darkly pigmented
skin is one such design flaw highlighted by work from Weil Associate Director and Professor of Pulmonary and Critical Care Medicine Dr. Michael Sjoding. Pulse oximeter bias entered the public spotlight during the pandemic since these finger clips devices are used to measure blood oxygen levels, a key metric used to decide whether medical care should be escalated for COVID patients. As the result of a study led by Dr. Sjoding recapitulating this issue, health policy discussions regarding device bias have been renewed and regulatory action by the FDA is ongoing.
Developers at Weil are also moving toward devices and tests that are rapid, non-invasive, and portable. Wearable devices may be particularly useful during recovery, a period when real-time data is limited. “I would love to have a smart watch where I can look at it and see how good or bad I was doing [during] a small activity,” says O’Brien, referencing the inconvenience of carrying a pulse oximeter to track his progress. Rapid, noninvasive devices like electronic nose technology, which senses volatile markers of disease, are also a growing trend in medical device design. Weil Associate Director Dr. Xudong “Sherman” Fan has already shown success applying this approach to analyze the breath of COVID patients and is currently developing a wearable device for a wider range of diseases.
Even as methods of collecting health data improve, the information gathered is useless without reliable systems to streamline and analyze the information. Though artificial intelligence is not yet safe to use as the sole basis for making clinical decisions, it has potential to assist healthcare providers as they evaluate patients. Several AI-based methods for data interpretation are being developed by Weil members, including those that can predict patient decline from medical records, analyze chest images to detect acute respiratory distress syndrome, and determine causes
22
of sepsis to guide antibiotic dosing.
As new solutions for data management and decision making emerge, the most useful methods will focus on clearing, not clouding, the healthcare team’s judgement.
“All of the exquisite molecular science in the world is useless from the physician’s perspective if it isn’t delivered to them in actionable form,” Dr. Dickson concluded. “We need to rise to the challenge of not just generating complex, highdimensional data, but synthesizing it and turning it into actionable, accessible information in a way that physicians can use.”
Playing the long game
For Dave, getting back to his regular activities has been key for recovery after his hospitalization. A true outdoorsman, he is getting back to hunting and adding to the piles of firewood at the end of his driveway, while slowly chipping away at the oxygen tanks lined neatly inside his front door.
Dave especially credits physical therapy during in-patient rehab as important for his recovery. “It was monumental taking steps, Like three or four, and then 15, and then the hallway. It was three weeks of progress,” O’Brien recalled. “I wanted to stay. I was realizing how damaged I was.”
For survivors of critical illness, continuing care is just as important as the interventions that saved their lives in the ICU. Patients with COVID and other conditions like sepsis face a host of health challenges during recovery, including the inability to perform daily tasks and increased risk of recurring critical illness. Collectively termed post-intensive care syndrome, PICS is receiving increased attention in the aftermath of the pandemic emergency, as practitioners care for a growing population of survivors.
Dave recalls thinking a lot about the need to move his body as soon as he was able.

Dr. Madeline Lagina and Dr. Rachel Hechtman began their pulmonary and critical care fellowship training at the University of Michigan in the middle of the pandemic, and their practice will continue to be shaped by innovations inspired by the collective COVID experience. Photo courtesy of Madeline Lagina and Rachel Hechtman.
“During recovery, I wanted physical therapy people to work me,” he said. “Mechanically, I knew I was getting all weak. I just remember thinking I wish those services were more often.”
As Dave sensed, there is increasing evidence that early movement, even while patients are on ventilators, improves long-term mobility. In the future, therapeutic vibration devices like one pioneered by Weil researchers may serve a vital role for increasing rehab services in the ICU and reducing weakness and mobility issues associated with PICS, while alleviating the burden on peopleand time-intensive services like physical therapy.
“The original goal of ‘just keeping them alive today’ feels shortsighted now,” explained Dr. Rachel Hechtman, a pulmonary and critical care fellow at the University of Michigan.
23
“It’s like a balancing act at all times to keep [patients] going but also minimize any kind of harm from being incredibly ill. I think that’s a systems problem, because there is no way that even the best critical care doctor in the world could keep all of that in their mind effectively for every patient.”
Beyond physical rehabilitation, meeting the holistic needs of patients is also important during recovery. Post-ICU clinics like the one at the University of Michigan, which offers social support and pharmacy consults alongside physical exams, is one way to connect recovering patients with holistic care. Billed as a “one-stop shop” for multiple forms of care, post-ICU clinics are popping up globally and are showing promise for alleviating the impact of PICS and helping patients work towards health in a holistic sense.
“Health is multi-faceted. Health includes financial stability, it includes housing, it includes socioeconomic health, it includes access to medication, emotional health and wellness, and physical health,” said Dr. Madeline Lagina, a University of Michigan pulmonary and critical care fellow who also holds a master’s degree in public health. “I think we’re just starting to understand how every single relationship with everything in your life sums to some form of wellness.”
Improving holistic services for recovering patients, among other challenges in critical care, will take a large network of collaborators with diverse expertise outside of critical care. This approach, called convergent science, emphasizes deep education of individual experts in multiple disciplines, rather than multiple experts working individually within their discipline on the same problem.
“The Weil Institute is trying to broaden the family of disciplines to get interested in critical care as a problem at any level: the cellular level, the microbiologic level, the physiologic level, the therapeutic level, digital health, devices, or diagnostics,” Dr. Ward explained. “You may have the perfect solution for a problem you’ve never heard of.”
We’re not in a post-critical illness world.
As critical care moves beyond crisis mode, it is clear that the future of the field is already –unavoidably – shaped by our collective COVID experience. Though the pandemic emergency has subsided, “we’re not in a post-critical illness world,” reminds Dr. Dickson. If there is any value to be found in a devastating viral outbreak, it is that patients far into the future will continue to benefit from molecular therapies, methods of real-time data analysis, and resources for holistic care ¬– engineered to fill in the blanks exposed by recent experience and pioneered, in part, by the University of Michigan Weil Institute.
Dave’s recovery is likewise far from over. He regularly follows up at the University of Michigan post-ICU clinic to monitor his improvements in mobility and heart and lung function. At his most recent appointment, Dave completed a six-minute walk down the hallway with no oxygen for the first time since his hospitalization. He never goes anywhere without his camo print backpack that holds his oxygen cylinder, and daily tasks like taking the dog out or taking the stairs still induce lots of heavy breathing. Even so, Dave is feeling stronger every day.
“I feel like I’m still somewhat improving, at least I certainly hope I am.”
Jennifer Baker is a PhD candidate in Microbiology and Immunology at the University of Michigan, where her dissertation research in Dr. Robert Dickson’s lab focuses on the relationship between the microbiome and acute lung injury. This article was written as part of a collaboration between the Weil Institute and Michigan Science Writers, an organization at the University of Michigan that trains graduate students and post-doctoral fellows in science writing and editing.
24

25
The O’Brien’s 10-year-old beagle, Duke, keeps Dave moving during recovery. Image by Jennifer Baker.
Four Midwest Centers Unite to Advance New Therapeutics in ARDS, Pneumonia & Sepsis
Critical care takes a team—in research as well as in practice. For the first time ever, the University of Michigan, Henry Ford Hospital, the University of Cincinnati and the University of Chicago are teaming up to study and combat life-threatening critical illnesses. Funded by the National Institutes of Health (NIH) and led by members of the University of Michigan Max Harry Weil Institute for Critical Care Research and Innovation, the new collective named the Great Lakes Clinical Center (GLCC) for the ARDS, Pneumonia and Sepsis (APS) Phenotyping Consortium will be intensely focused on examining the various biologic mechanisms underpinning APS conditions to better understand their impact on disease development, trajectory and outcomes.
Dr. Robert Dickson, Deputy Director of the Weil Institute and Associate Professor of Pulmonary and Critical Care Medicine, is one of the principal investigators leading the GLCC’s University of Michigan site, together with Dr. Robert Hyzy and Dr. Hallie Prescott—both Weil Institute members and Professor and Associate Professor, respectively, of Pulmonary and Critical Care Medicine. Over the next six years, their group will use the $3 million NIH grant to collaborate with a nationwide observational study of 5,000 adult patients hospitalized with APS. The four sites within the GLCC will partner with five other clinical sites to use novel data collection approaches to target key research questions, such as how a patient’s body composition impacts survival and how the relationship between skin color and pulse oximetry affects APS outcomes.
ARDS, pneumonia and sepsis are life-threatening conditions that are also known to overlap: pneumonia is a common cause of sepsis, and both sepsis and
pneumonia can contribute to ARDS. While progress has been made to reduce mortality across each, this overlap combined with the illnesses’ heterogeneity— the significant variations in their root causes—has constrained the development of new therapeutics.
“Imagine trying to cure a patient with a fever before knowing that fever can be caused by completely distinct disease processes: infection, cancer, or autoimmune disease,” said Dr. Dickson.
The center’s logo represents the participating institutions’ home states and the five Great Lakes.
“You’d be stuck working with symptomatic treatments like acetaminophen, with no hope of resolving the underlying problem. In a real way, that’s where we are with APS conditions: providing supportive care without a working knowledge of the underlying biology.”
The summer of 2023 marked the Great Lakes Clinical Center’s official start. The four sites met with the nationwide network to harmonize the research and data collection protocols that will guide the consortium for the next six years.
“We are not only combining our clinical and scientific expertise with our GLCC partners; we are also combining our patient populations,” said Dr. Hyzy. “Our service area represents over 3 million people and includes major urban centers such as downtown
Imagine trying to cure a patient with a fever before knowing that fever can be caused by completely distinct disease processes. You’d be stuck working with symptomatic treatments ... with no hope of resolving the underlying problem. In a real way, that’s where we are with APS conditions.”
Robert Dickson, MD
Chicago, Detroit, and Cincinnati. We will be studying a patient cohort far more demographically diverse than what we would see at Michigan Medicine alone. I’m proud and excited that we’ll be studying these conditions in a such a diverse patient population.”
To better understand outcomes and recovery after APS, the consortium will also collect follow-up data from patients at three, six, and twelve months postdischarge.
“Many patients do not experience full recovery when they leave the ICU,” said Dr. Prescott. “They might plateau or decline in the year after discharge, and many get rehospitalized. This consortium will be an amazing opportunity to study the biological and clinical trajectories experienced by our patients after they leave the hospital.”
In tandem with the consortium-wide study, each GLCC site will conduct its own center-specific research. The University of Michigan team and its partners will study the microbiome—the bacterial communities that live in our digestive and respiratory tracts—to determine the role it plays in the development, trajectory, and recovery of APS conditions.
“The microbiome is an important yet poorly understood source of biologic heterogeneity, and it represents a highly promising treatment target,” said Dr. Dickson. “Our team will use cutting-edge molecular techniques to identify the pathways by which gut and respiratory microbiota participate in the acute development and trajectory of APS conditions. This will be an important step toward developing microbiometargeted therapies to prevent and treat these conditions.”
The GLCC is supported by a U01 “ARDS, Pneumonia, and Sepsis Phenotyping Consortium Clinical Centers” grant provided by the NIH Heart, Lung, and Blood Institute (NHLBI) and National Institute of General Medical Sciences (NIGMS).
“We pulled in a lot of expertise to make this grant happen,” said Dr. Dickson. “Our strengths at Michigan Medicine are a big part of what made us so competitive. We have a rich tradition of clinical research in the intensive care unit as well as expertise in long-term outcomes and the microbiome. We also have the largest research institute in the country dedicated to the study of critical care. I can’t imagine a network like this not wanting to involve the Weil Institute.”
“We are thrilled to be partnering with Michigan Medicine and the Weil Institute on this project,” said Dr. Bhakti Patel, Assistant Professor of Medicine at the University of Chicago. “This will be an extraordinary opportunity to bridge our institutions’ strengths in clinical research and microbiome science, all in the interest of providing better care for our sickest patients.”
Ultimately, the GLCC and the nationwide network aims to make its collection of clinical and imaging data and biospecimens publicly available, providing investigators around the world with a wealth of opportunities for further study into clinical disease trajectories; blood cytokines, DNA, RNA, and metabolites; gastrointestinal and respiratory microbiota; and chest and body imaging. This, in turn, will help inform future clinical trials and further guide the development of new and improved therapeutics.
NIH T-32 Acute and Critical Care Engineering Training
New training program at the Weil Institute will equip future generations of engineers to address the unique design and technical considerations of the acute care environment.
Every second counts in critical care. Given the complex and time-sensitive nature of acute and critical illness and injury, technologies used to manage these conditions must work rapidly, be highly accurate, and be easy to use by various medical personnel both in and out of the hospital. However, there are currently no structured opportunities that teach engineers how to address the distinct design and technical needs of the acute care space.
To fill this gap, the University of Michigan Max Harry Weil Institute for Critical Care Research and Innovation has established the Acute and Critical Care Engineering (ACCE) Training Program—a two-year course that will equip doctoral engineering students to not only develop these highly specialized innovations but to also move them from the bench to real applications in patient care.
A core component of the ACCE program is its focus on interdisciplinary mentorship. The program is run jointly by the U-M College of Engineering and Michigan Medicine and features a mentor cohort comprised of over 40 faculty members from multiple departments across both schools. Students will learn from a primary engineering mentor and a secondary clinical mentor who will guide them in their research, involve them in ongoing collaborative projects, and help them develop the skills needed to communicate effectively across medical and engineering fields. All ACCE mentors have expertise in the program’s core technology focus areas as well as extensive experience collaborating across acute and critical care. They are also connected through of a cohesive network of common research interests united within the Weil Institute.

“Acute and critical care is truly a unique umbrella within medicine, but technology development in this area is lagging to the detriment of millions of patients annually,” said Dr. Kenn Oldham, U-M Professor of Mechanical Engineering, Associate Director at the Weil Institute and Director of the ACCE program. “Our goal is to provide students with an understanding of the clinical and technological issues present in this field, as well as exposure and formal mentorship in clinical environments, and education in technology transfer to ensure new devices are appropriately designed and will successfully make their way into the hands of

Unique to ACCE training is its Clinical Immersion aspect which will give students broader exposure to operations across acute and critical care by allowing them to shadow clinicians working in their own environment. Students will be able to conduct their observations in multiple intensive care units (ICUs) in Michigan Medicine’s University Hospital and Mott Children’s Hospital including the Emergency Critical Care Center (EC3), the nation’s first emergency department-based ICU.
“Much of engineering is centered around understanding the needs of the users and stakeholders—and we are generally getting this information second hand—so we want to put the students right there to shadow and do their own observations,” said Oldham. “They can then bring their observations back into talks with other students and faculty and can keep
“Through this program, trainees will be ... equipped to develop systems that are seamlessly integrated and relevant to clinical needs across acute and critical care, propelling their careers in a way that is not currently possible.”
– Kenn Oldham, PhD | ACCE Program Director
returning to these contacts to make their future innovations more likely to be successful in eventual patient care.”
ACCE training also includes a twice-monthly design studio that will provide additional education in biomedical device design and commercialization not covered in existing coursework through collaborations with U-M expert groups such as Fast Forward Medical Innovation (FFMI) and the Office of Innovation Partnerships; a monthly journal club for peer-to-peer discussions about current literature in acute and critical care engineering; and an annual symposium where ACCE trainees will be able to present their research and network with field leaders and experts. ACCE trainees will also have access to various Weil Institute resources, such as its lecture series, Data Science Core, Clinical Research Unit, and Preclinical Critical Care Laboratory.
The ACCE program is supported by a $1.3 million grant from the National Institute of Biomedical Imaging and Bioengineering (NIBIB). Dr. Oldham and his team began developing the program in 2021 and worked closely with the Weil Institute’s Proposal Development Unit (PDU) throughout.
“The T32 mechanism requires significant effort beyond a standard research grant, including collecting biosketches and training table information from dozens of mentors,” said Meagan Ramsey, Proposal Development Director at the Weil Institute. “We managed this effort in collaboration with Lynn Kujawa, who is the Research Administrator in the Department of Mechanical Engineering. This allowed the ACCE team to focus more energy on program design and


proposal writing.”
“Developing this program and getting all of these moving parts into place would not have been possible without the assistance we got through the PDU and Lynn,” said Oldham. “They did a tremendous amount of work putting this together.”
The grant was co-written with Dr. Kevin Ward, Executive Director of the Weil Institute and Professor of Emergency Medicine and Biomedical Engineering, and Dr. Katsuo Kurabayashi, Professor of Mechanical Engineering, who also serve as the ACCE program’s Clinical Associate Director and Engineering Associate Director, respectively.
“The potential to transform the field of critical care by training the next generation of engineers through initiatives like the ACCE program cannot be overstated,” said Dr. Ward. “Graduates of this program will become future leaders in the field”.
The ACCE program recruited its inaugural group of students in the fall of 2023.
“Through this program, trainees will learn to communicate and collaborate across engineering and biomedical disciplines, ensuring that they are prepared to actively participate in and lead interdisciplinary research teams,” said Oldham. “Ultimately, they will be equipped to develop systems that are seamlessly integrated and relevant to the clinical needs across acute and critical care, propelling their careers in a way that is not currently possible.”
ACCETP Inaugural Class






Cai Computational Medicine and Bioinformatics Lucy Spicher Mechanical Engineering Zixiao Zhang Mechanical Engineering
Lingrui
29
AN INVESTMENT IN THE FUTURE
OF CRITICAL CARE
U-M groups invested over $2M to support development and refinement of new preclinical models at the Weil Institute.
Conditions requiring critical care, such as sepsis and traumatic brain injury (TBI), are difficult to study in humans due to their unpredictability and the absence of translatable preclinical models that accurately recreate the evolution of the body’s responses to these conditions.
In 2022, nine U-M groups united to invest over $2 million in the Weil Institute’s Preclinical Critical Care Laboratory to support the development and refinement of long-term outcome models of critical illness/injury.
Now in the second year of the investment, Weil has made major advancements in the development of such models that continue to be used to develop and test new technologies both at U-M and around the world. Today, the Preclinical Critical Care Lab at the Weil Institute is one of the only labs in the country with models of Cardiac Arrest, ARDS, Sepsis, TBI, and Polytrauma.
POST-TBI BREATH ANALYSIS USING PORTABLE GAS CHROMATOGRAPHY
Researchers in the Weil Preclinical Critical Care Lab are now studying the potential of using a portable gas chromatography device developed at U-M and the Weil Institute to detect breath signatures that indicate the presence and severity of a traumatic brain injury.
While further research will need to be done, this model and technology could have a profound impact on the care and management of TBI, such as enabling investigators to non-invasively monitor and assess TBI directly at the point of care.
BLUNT IMPACT TRAUMATIC BRAIN INJURY
A blunt impact TBI causes the brain to bounce around or twist inside of the skull. Weil’s preclinical model of blunt impact TBI gives investigators a unique window into the dangeous cascade of effects that follow this unique trauma, informing the development of therapies targeting mitochondrial dysfunction, secondary brain injury, and more.
IMPELLA CP CPR WITH BALLOON AORTIC OCCLUSION
Studies have shown that using the Impella CP® left ventricle assist device during CPR provides more effective circulatory support and an enhanced rate of ROSC over standard CPR. Weil’s preclinical model builds upon this method by incorporating a technique called “temporary aortic occlusion” in which the part of the aorta that delivers blood to the lower body is pinched shut, creating a smaller circuit that diverts blood flow to the heart and brain.
SEPSIS DRIVEN ACUTE KIDNEY INJURY
Sepsis is a dysfunctional immune response to an infection that causes widespread, inflammation leading to multi-organ failure and death. The kidney is one of the earliest injured organs during sepsis. Weil’s model of sepsis-driven acute kidney allows investigators to study these effects and glean insights needed to develop targeted therapeutics.
CARDIAC ARREST, RESUSCITATION AND 24-HOUR CARE
This model allows investigators to study the moment-by-moment occurences that take place during a cardiac arrest; to gauge the effects of different methods ofresuscitation, and to assess how cardiac arrest affects neurologic function and outcomes--all with sophisticated, around-the-clock monitoring capabilities found in the Weil Preclinical Lab.


TURN-I-KITS FOR UKRAINE
Precision Trauma LLC, a partner of the University of Michigan’s Max Harry Weil Institute for Critical Care Research and Innovation, announced it has donated 780 “Turn-i-Kit” external hemorrhage control devices to support emergency care providers in Ukraine.
“Hemorrhage control at the point of injury represents the greatest need in any conflict,” said Precision Trauma CEO Ethan Miles, MD. “As a veteran-owned and operated company, we have many connections to those who are supporting medical response efforts in war-torn areas, and we have noticed a high demand for tourniquets, specifically. In Ukraine, this demand has led to an influx of counterfeit tourniquets with inferior quality as commercial entities struggle to keep up.”
In 2019, Precision Trauma partnered with the Weil Institute to develop and commercialize a series of trauma and combat casualty care innovations including the Turn-i-Kit—a reimagining of the modern tourniquet designed to be inherently simple for untrained first responders to apply under stressful conditions. Originally developed at Weil in response to the United States government’s “Stop the Bleed” campaign, the device combines a specialized tightening mechanism with a wider band to deliver greater pressure and stop bleeding faster while reducing discomfort.
Kevin Ward, MD, who is the Executive Director of the Weil Institute and a veteran himself with combat deployment experience said, “Hemorrhage is the leading cause of preventable death on the battlefield. In the case of Ukraine, major civilian population centers are becoming a part of the battle landscape. As such, effective and simple to use products like the Turn-i-Kit can add precious hours to allow both civilian and military casualties to get to definitive care.”
“The Weil Institute has been a tremendous partner in our efforts to save lives,” said Dr. Miles. “Their technical expertise in the initial development allowed us to take this unique design to end users around the world. Our collaboration resulted in a tourniquet that presents many advantages over existing devices and is well suited for prolonged casualty care as well as transportation of those with life threatening extremity hemorrhage.”
Other Weil Institute innovations licensed to Precision Trauma include GROA (Gastroesophageal Resuscitative Occlusion of the Aorta) the IPHD (Intra-Peritoneal Hemostasis Device), and external pelvic junctional and abdominal aortic occlusion tourniquets. These technologies are designed to control noncompressible hemorrhage occurring in the abdomen and pelvis.
Precision Trauma also announced it has donated an additional 50 Turn-i-Kits to a game reserve in Kenya to support training and response efforts for anti-poaching teams.
31
A collection of Weil Institute highlights and happenings from the past year.
SHOWCASING INNOVATION EXCELLENCE
Below: Jay Semerad, Weil Institute Senior Product Manager, presents Model Performance Diagnostics to Dr. Santa Ono (left), President of the University of Michigan, during the 2023 Celebrate Invention event hosted by U-M Innovation Partnerships.
This was the third year the Weil Institute was invited to showcase one of its technologies at Celebrate Invention. Conference attendees included members of the U-M executive leadership team as well as researchers, innovators, entrepreneurs and experts in product development and commercialization from across the University community and the state of Michigan.



DATA SCIENCE SETS SAIL
Above: Dr. Sardar Ansari, Director of the Weil Institute Data Science Team, speaks at the 2024 Symposium on Artificial Intelligence in Learning Health Systems (SAIL) Conference in Puerto Rico. Dr. Ansari would also give a Spotlight Talk titled “Monitoring Dataset Shift in Clinical AI/ML Models during the PostDeployment Phase”.
This year, every member of the Data Science Team had an abstract accepted to be displayed at SAIL.

32

INHERITING A LEGENDARY TRADITION
Above: Weil staff and world leaders in resuscitation science pose for a group photo during the 2023 Wolf Creek Conference. Historically hosted by the Weil Institute—and newly inherited by the Weil Institute at U-M—Wolf Creek meetings foster the robust exchange of ideas with the goal of stimulating laboratory and clinical research to inform and enhance the future of cardiac arrest care.



The three-day event was filled with fierce debate and discussions as attendees proposed what they thought should be considered the top knowledge gaps, barriers to translation and research priorities in six key areas of cardiac arrest and resuscitation science. Dr. Max Harry Weil, who often chaired Wolf Creek during its early years, was also in attendance as a guest of honor (bottom center portrait).
A UNITED FRONT TO IMPROVE TBI CARE
Left: The 2023 Massey TBI Regional Conference brought together multidisciplinary experts from across the Midwest to lay the foundation for a new research network poised to transform the diagnosis and management of traumatic brain injuries (TBI).
Dr. Susan Rowell (left), Professor of Surgery, Trauma and Acute Care Surgery at the University of Chicago, gave the keynote presentation. Dr. Frederick Korley (right), Scientific Director of the Massey TBI Grand Challenge and Professor of Emergency Medicine at the University of Michigan led the conference proceedings.
33
THE WEIL INSTITUTE’S
HISTORY


Major Milestones on the Path to Weil

2014 2014 2015
The University of Michigan Medical School invests in the creation of a new research center to be housed in its North Campus Research Complex: the Michigan Center for Integrative Research in Critical Care (MCIRCC).

MCIRCC holds a Grand Challenge for sepsis research. As the first of its kind, this event would serve as the blueprint for future iterations including the Massey and Kahn Grand Challenges of today.
MCIRCC receives a $7M investment from the Joyce and Don Massey Family Foundation to launch the Massey TBI Grand Challenge, Regional Conference and the Joyce Massey TBI Summit.

2015 2016 2017
Development begins on MCIRCC’s Big Data platform for critical care research through a collaboration between the MCIRCC Data Science Team, the U-M Department of Emergency Medicine and external partners.

MCIRCC completes its Big Data platform and begins collecting waveform data from over 400 beds across University of Michigan Health. Over the years, this platform will empower dozens of MCIRCC member research projects & grants.
MCIRCC begins a strategic campaign to compete for large team science grants from foundations including the National Institutes of Health, the National Science Foundation and the Department of Defense (DoD).

2018 2021 2021
MCIRCC develops its first Big Data predictive analytic: the Analytic for Hemodynamic Instability (AHI). AHI would go on to be licensed to FifthEye, Inc., which would raise over $10M for FDA approval and commercialization.
MCIRCC meets with the family of Dr. Max Harry Weil, a cardiologist and U-M alumnus considered a key founder of the field of critical care. The family sees Dr. Weil’s vision magnified in MCIRCC’s work and begins discussions with MCIRCC leadership about the center’s future.
The Weil Family invests $10M in MCIRCC, formally establishing the new Max Harry Weil Institute for Critical Care Research and Innovation at the University of Michigan.
34
Fueling Research from Bench to Bedside TODAY

Institute Members Depts. & Disciplines Represented
Licensed Products In Venture Funding for Spin-off Companies
In total research support provided to our members through resources such as our Proposal Development team and Grand Challenge events.
Weil Institute Services Empower Research Successes
Proposal Development
Provides consulting and support services for multi-disciplinary teams pursuing research awards.
Preclinical Laboratory
Produces high-fidelity models of multisystem disease and injury such as sepsis and ARDS.
Product Commercialization
Collaborates with PIs and teams to propel products to commercial viability.
Clinical Research Team
Assists investigators with patient recruitment, IRB submissions, and data collection/processing.
Data Science Team
Provides data modeling, analysis and analytics used to develop clinical decision support tools.
Special Events
Hosts member events to promote networking, training, education and funding opportunities.
THE WEIL INSTITUTE
35

“Our family’s investment in Michigan Medicine through the Weil Institute was an investment in the lives of millions of patients around the world. Together, we are creating a brighter future for emergency and critical care through bold research, unparalleled education, and exceptional patient care.”
Brenda Massey President, The Joyce and Don Massey Family Foundation
36

Breaking traditional funding barriers to accelerate transformational research.
Weil Institute Grand Challenges are powerful funding avenues for multidisciplinary critical care research teams developing device, diagnostic, therapeutic and health IT solutions. The intensive process includes education sessions, two rounds of proposal submissions, project reviews, and collective feedback from experts across the University of Michigan, industry, and the Department of Defense.

Massey Traumatic Brain Injury (TBI) Grand Challenge
Funds research and innovations targeting the golden hours following severe TBI.
Supported by the Joyce and Don Massey Family Foundation
Kahn Pediatric Critical Care Grand Challenge
Funds research and innovations targeting gaps in the diagnosis, monitoring and treatment of critically ill and injured children.
Supported by philanthropist Mark Kahn
Support future Grand Challenges
Weil Institute Grand Challenges provide a unique and effective opportunity to support innovative research and solutions that will save countless lives.
Supported by philanthropist Mark Kahn
For more information contact Emergency Medicine Assistant Director of Development Mark Clark at markclar@umich.edu or 734-763-1749.
Every gift is critical.
37

WEIL INSTITUTE EXECUTIVE LEADERSHIP TEAM













 Kevin Ward, MD Executive Director
Xudong (Sherman) Fan, PhD Associate Director
Lena Napolitano, MD, FACS, FCCP, FCCM Associate Director
Robert Dickson, MD Deputy Director Deputy Director
Phil Jacokes, MBA Managing Director
Heidi Flori, MD, FAAP Associate Director
Kenn Oldham, PhD Associate Director
Rodney Daniels, MD Scientific Director, Kahn Pediatric Critical Care Grand Challenge
Frederick Korley, MD, PhD Scientific Director, Massey TBI Grand Challenge
Michael Sjoding, MD Associate Director
J. Scott VanEpps, MD, PhD Associate Director
Michael Maile, MD Associate Director
Kayvan Najarian, PhD Associate Director
Kevin Ward, MD Executive Director
Xudong (Sherman) Fan, PhD Associate Director
Lena Napolitano, MD, FACS, FCCP, FCCM Associate Director
Robert Dickson, MD Deputy Director Deputy Director
Phil Jacokes, MBA Managing Director
Heidi Flori, MD, FAAP Associate Director
Kenn Oldham, PhD Associate Director
Rodney Daniels, MD Scientific Director, Kahn Pediatric Critical Care Grand Challenge
Frederick Korley, MD, PhD Scientific Director, Massey TBI Grand Challenge
Michael Sjoding, MD Associate Director
J. Scott VanEpps, MD, PhD Associate Director
Michael Maile, MD Associate Director
Kayvan Najarian, PhD Associate Director
38
Kathleen Stringer, PHARMD

INTERNAL ADVISORY BOARD











Departments and Disciplines Represented
Christopher Shoemaker, MEd, MBA, CFRE
Robert Neumar, MD, PhD
MeiLan Han, MD, MS
Ryan Barbaro, MD, MS
Steven Kunkel, PhD
Lena Napolitano, MD, FACS, FCCP, FCCM
Robert Bartlett, MD
Kelly Sexton, PhD
Mark Burns, PhD
Biomedical Engineering Anesthesiology Bioinformatics Chemical Engineering Emergency Medicine Clinical Pharmacy Innovation Partnerships Development Mechanical Engineering Neurosurgery Microbiology Pathology Pediatrics Pharmacology Pulmonary Surgery Medical School Leadership Research
Mary-Ann Mycek, PhD
39
UPCOMING EVENTS
TBISUMMITV JOYCE MASSEY
FALL 2024
Information about the 5th Joyce Massey TBI Summit will be available later this year. Visit our website or join our mailing list to stay up to date!

Want to know what’s next at Weil? Use the QR code above to see upcoming Events!


40
Emergency Medicine (Continued)
Steven Kronick, MD, MS
Mark Lowell, MD
Prashant Mahajan, MD, MPH, MBA
Ronald Maio, DO, MS
Richard P. Medlin Jr. MD, MSIS
William Meurer, MD, MS
Takahiro Nakashima, MD, PhD
Kayvan Najarian, PhD
Robert Neumar, MD, PhD
Thomas Sanderson, PhD
Phillip Scott, MD, MBA
Graham Smith, MD
Florian Schmitzberger, MD, MS
Nik Theyyunni, MD
M. Hakam Tiba, MD, MS
J. Scott VanEpps, MD, PhD
Kevin R. Ward, MD
Joseph Wider, PhD
Douglas Wiebe, PhD
Internal Medicine
Andrew Admon, MD, MPH
Lawrence An, MD
Owen Albin, MD
Y. Eugene Chen, MD
Colin R. Cooke, MD, MSc, MS
Scott Denstaedt, MD
Robert P. Dickson, MD
Scott A. Flanders, MD

Internal Medicine (Continued)
Hamid Ghanbari, MD, MPH
Jessica Golbus, MD, MS
Hitinder S. Gurm, MD
Meilan Han, MD, MS
Michael Heung, MD, MS
H. David Humes, MD
Scott L. Hummel, MD, MS
Robert C. Hyzy, MD
Theodore J. Iwashyna, MD, PhD
Daniel A. Lawrence, PhD
Liandi Lou, MD
Nikhilesh Mazumder, MD, MPH
James Morrissey, PhD
Elizabeth Munroe, MD, MS
Brahmajee K. Nallamothu, MD, MPH
Roomi Nusrat, MD
Christopher Pino, PhD
Hallie C. Prescott, MD, MSc
Karthik Ramani, MD, MHA
Mohammed Saeed, MD, PhD
Benjamin H. Singer, MD, PhD
Michael W. Sjoding, MD
Theodore J. Standiford, MD
Balazs Szamosfalvi, MD
Thomas S. Valley, MD
Lenar Tatios Yessayan, MD
Mechanical Engineering
Lei Chen, PhD
Erin Donnelly
Bogdan Epureanu, PhD, MS
Jianping Fu, PhD
Miguel Fuñes-Lora, PhD
Xun Huan, PhD
Sridhar Kota, PhD
Grant H. Kruger, PhD
Katsuo Kurabayashi, PhD
Xiaogan Liang, PhD
Lauro Ojeda, MS
Kenn R. Oldham, PhD
Young Geun Park, PhD
Jeff Plott, MS
Chengzhi Shi, PhD
Yujing Song, PhD
Medical Students
Erkin Ötles, MSE
Helly Patel
Sidney Perkins, MSc
Molecular and Integrative Physiology
Daniel Beard, PhD
Jimo Borjigin, PhD
Brian Carlson, PhD
Geoffrey G. Murphy, PhD
Santiago Schnell, PhD
Neurosurgery/ Neurology
Steven Broglio, PhD
Jeffrey Fletcher, MD
Hugh J.L. Garton, MD, MHSc
Teresa Jacobs, MD
Jacob Joseph, MD
Richard F. Keep, PhD
Aditya S. Pandey, MD
Venkatakrishna Rajajee, MBBS
Kyle Sheehan, MD
Craig A. Williamson, MD
Guohua Xi, MD
Nursing
Patricia A. Abbott, PhD, RN, FAAN
Deena Costa, PhD, RN
Ivo D. Dinov, PhD
Andrew Heiler, MBA, RN
Fadi Islim, RN, MSN, DNP (c)
Pediatrics
Ryan P. Barbaro, MD, MSc
Giulia Benedetti, MD
John Charpie, MD, PhD
Mary Dahmer, PhD
Rodney Daniels, MD
Karl Desch, MD
Daniel Ehrmann, MD, MS
Michael Gaies, MD
Rachel Gottlieb-Smith, MD, MHPE
Beau Hunsinger, MD
41
MEET OUR MEMBERS
Pediatrics (Continued)
Joseph Kohne, MD, MSc
Andrea Les, PhD
Rebecca Lombel, MD
Frank W. Moler, MD, MS
Gabe Owens, MD, PhD
Mike Quasney, MD, PhD
Nathaniel Sznycer-Taub, MD
Pharmacy
Mike Dorsch, PharmD, MS
Michael Kenes, PharmD
James J. Moon, PhD
Manjunath (Amit) Pai, PharmD
Leslie Satin, PhD
Anna Shenderova Schwendeman, PhD
Steve Schwendeman, PhD
Kathleen A. Stringer, PharmD
Peter Tessier, PhD
Surgery
Anne Cain-Nielsen, MS
Eliason, Jonathan L. MD
Mark R. Hemmila, MD

Surgery (Continued)
Jill Jakubus, MHSA, MS
Yongqing Li, MD
Lena M. Napolitano, MD, FACS, FCCP, FCCM
Pauline K. Park, MD, FACS, FCCM
Krishnan Raghavendran, MD
Alvaro Rojas-Peña, MD
Nicole L. Werner, MD, MS
Aaron Williams, MD
Undergraduates
Shreya Kashyap
Sid Lakhani
Shakira Woods
Other
Omar Ahmed, PhD
Brian Athey, PhD
Maryam Bagherian, PhD
Cagri G. Besirli, MD, PhD
Ryan C. Bailey, PhD
Pete Bodary, PhD
David T. Burke, PhD
Sung Won Choi, MD, MS
Other (Continued)
Zoey Chopra, MD/PhD Candidate
Amy Cohn, PhD
Dalis Collins, DVM
Douglas Craig, MS, MFA
Louis G. D’Alecy, DMD, PhD
Robertson D. Davenport, MD
Patricia de Assis, PhD
Harm Derksen, PhD
Richard Gonzalez, PhD
Peter F. Green, PhD
Myron Hepner
Gerald Higgins, MD, PhD
Sangchoul Im, PhD
Jeanette Jackson, MBA
Henry Jewell
Kimberly Johnston, VMD
Ioulia Kovelman, PhD
Steven L. Kunkel, PhD
Kenichi Kuroda, PhD
Elham Mahmoudi, PhD
Brendan McCracken, BS
Brandon McNaughton, PhD
Mark Meyerhoff, PhD
Other (Continued)
Joseph Myers, OD, FAAO
Jean A. Nemzek, DVM, MS, DACVS
Yannis Paulus, MD, FACS
Carleen Penoza, MHSA, BSN, RN
Zachary Pickell
Vitaliy Popov, PhD
Deborah M. Rooney, PhD
Elyas Sabeti, PhD
Sara E. Samborn, RN, MSN
Katharine Seagly, PhD
Satinder Singh, PhD
Cristiane Squarize, DDS, MS, PhD
Emily Wittrup, MS
Interested in becoming a member? (It’s free!)
open
member,
gain access to innovation, financial, business
commercialization guidance
resources that will help push your research from the bench to the bedside. weilinstitute.med.umich.edu/become-a-member 42
Membership at the Weil Institute is
to all U-M faculty and staff. As a
you will
and
and
PRODUCTS AVAILABLE FOR LICENSING
Reach out to U-M Innovation Partnerships to begin a conversation about any of the Weil Institute’s Available to License products listed below!

Desktop Digital Biomarker Analysis System
A point-of-care precision device for rapid diagnosis of up to 14 blood biomarkers and diseases.
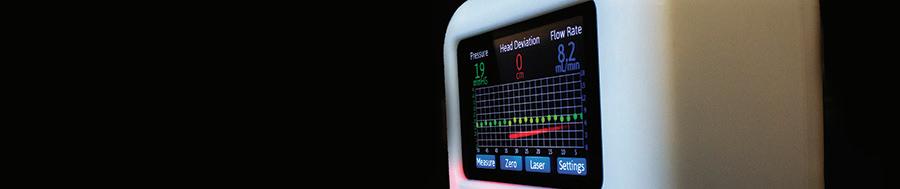
Digital Extraventricular Drain (DEVD) with Data Analytics Integration
Improves accuracy of intracranial pressure (ICP) measurements and control, while monitoring patient position and providing real-time alerts.

Environment for Model Maintenance, Integration and Tuning (EMMIT)
Software platform that provides a framework for the hosting, integration, testing, and monitoring of machine learning models in a healthcare setting.
PICTURE: Adult & Pediatric All-Cause Deterioration Prediction
Suite of machine learning algorithms that utilizes electronic health record data to predict patient deterioration in hospital settings.


DETECT-ARDS - Analytic for Detecting Acute Respiratory Distress Syndrome
Analytic that identifies ARDS findings on chest x-ray images with human-level accuracy.
Electrocardio-matrix (ECM)
An analytic that improves accuracy and efficiency in cardiac arrhythmia & ischemia detection through a new electrocardiogram (ECG) signal analysis technique.

Model Performance Diagnostics Suite (MPD)
Tools for assessing the performance of predictive models post-deployment. Compatible with the EMMIT platform.

Therapeutic Vibration Device (TVD)
A full-body device helping ICU patients avoid Post Intensive Care Syndrome (PICS) by applying vibration to activate their muscles.
43
EMERGING PRODUCTS
The products listed below have completed initial testing and proof-of-concept validation. They have high confidence for commercial viability and will be ready to license in 1-2 years.
PICTURE - Adult Rehabilitation
Deterioration Prediction
A model of Weil’s PICTURE analytic modeled specifically for inpatient rehabilitation units.
Portable Disease and Pathogen
Detection System
Battery-powered, handheld device for rapid detection and monitoring of critical conditions.
VAP Prevention Mouthpiece with Novel Essential Oil
Specialized mouthguard with antimicrobial coating that prevents Ventilator-associated Pneumonia (VAP) in mechanically ventilated patients.
Point-of-Care Microfluidic Platform for Detecting Traumatic Brain Injury (TBI)
Portable device that measures FDA-approved biomarker concentrations in whole blood and plasma to detect TBI.
Redox Point-of-Care Platform
Microfluidic chip platform that captures real-time measurements of oxidation reduction (redox) in whole blood and other biologic fluids at the point of care.

AMPLIFYING THE IMPACT OF RESEARCH
Innovation Partnerships is the central hub for research commercialization activity at the University of Michigan. We are inspired to redefine how world-class university research can fuel a region and solve the world’s great challenges.



ARE YOU READY TO AMPLIFY THE IMPACT OF YOUR RESEARCH? Connect with us today to get started. Alliances Licensing Ventures innovationpartnerships.umich.edu | innovationpartnerships@umich.edu | 734-763-0614 | @INNOVPARTNER | \ @Innovation Partnerships Our Corporate Research Alliances team provides faculty-facing support to convert industry relationships into successful sponsored research collaborations. Our Licensing team brings business, scientific and legal expertise to amplify the impact of U-M research. Services include invention intake support and funding, intellectual property strategy and license agreement negotiation. Our Ventures team supports the launch of startups based on U-M technologies and research discoveries by providing a full suite of resources, including business mentorship and venture capital connections.
Learn More weilinstitute.med.umich.edu/products-about











Commercialization Education Business Development Mentorship & Funding Michigan Biomedical Venture Fund What’s Your Innovation Path? THE
DOOR FOR CUTTING-EDGE BIOMEDICAL RESEARCH AT THE UNIVERSITY OF MICHIGAN AND AROUND THE WORLD 45
FRONT

APPENDIX
Reimagining Critical Care Delivery in Emergencies
1. Singer AJ, Thode HC Jr, Pines JM. US Emergency Department Visits and Hospital Discharges Among Uninsured Patients Before and After Implementation of the Affordable Care Act. JAMA Netw Open 2019;2(4):e192662.
2. Newton MF, Keirns CC, Cunningham R, Hayward RA, Stanley R. Uninsured adults presenting to US emergency departments: assumptions vs data. JAMA 2008;300(16):1914–24.
3. Scott KW, Scott JW, Sabbatini AK, et al. Assessing Catastrophic Health Expenditures Among Uninsured People Who Seek Care in US Hospital-Based Emergency Departments. JAMA Health Forum 2021;2(12):e214359.
4. Castaneda MA, Saygili M. The health conditions and the health care consumption of the uninsured. Health Econ Rev 2016;6(1):55.
5. Rodriguez RM, Passanante M, Phelps MA, et al. Delayed emergency department presentation in critically ill patients. Crit Care Med 2001;29(12):2318–21.
6. Chalfin DB, Trzeciak S, Likourezos A, Baumann BM, Dellinger RP, DELAY-ED study group. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med 2007;35(6):1477–83.
7. Peltan ID, Bledsoe JR, Oniki TA, et al. Emergency Department Crowding Is Associated With Delayed Antibiotics for Sepsis. Ann Emerg Med 2019;73(4):345–55.
8. Weil MH, Shoemaker WC. Pioneering contributions of Peter Safar to intensive care and the founding of the Society of Critical Care Medicine. Crit Care Med 2004;32(2 Suppl):S8–10.
9. Weil MH, Shubin H, Rosoff L. FLUID REPLETION IN CIRCULATORY SHOCK: CENTRAL VENOUS PRESSURE AND OTHER PRACTICAL GUIDES. JAMA 1965;192:668–74.
10. Safar P, Dekornfeld TJ, Pearson JW, Redding JS. The intensive care unit. A three year experience at Baltimore city hospitals. Anaesthesia 1961;16:275–84.
11. Leibner E, Spiegel R, Hsu CH, et al. Anatomy of resuscitative care unit: expanding the borders of traditional intensive care units. Emerg Med J 2019;36(6):364–8.
12. Gunnerson KJ, Bassin BS, Havey RA, et al. Association of an Emergency Department–Based Intensive Care Unit With Survival and Inpatient Intensive Care Unit Admissions. JAMA Netw Open 2019;2(7):e197584–e197584.
13. Bassin BS, Haas NL, Sefa N, et al. Cost-effectiveness of an emergency department-based intensive care unit. JAMA Netw Open 2022;5(9):e2233649.
14. Scalea TM, Rubinson L, Tran Q, et al. Critical Care Resuscitation Unit: An Innovative Solution to Expedite Transfer of Patients with Time-Sensitive Critical Illness. J Am Coll Surg 2016;222(4):614–21.
15. Tran QK, O’Connor J, Vesselinov R, et al. The Critical Care Resuscitation Unit Transfers More Patients From Emergency Departments Faster and Is Associated With Improved Outcomes. J Emerg Med 2020;58(2):280–9.
16. Powell E, Sahadzic I, Najafali D, et al. Is the Critical Care Resuscitation Unit Sustainable: A 5-Year Experience of a Beneficial and Novel Model. Crit Care Res Pract 2022;2022:6171598.
17. Mitarai T, Gordon AJ, Nudelman MJR, et al. Association of an Emergency Critical Care Program With Survival and Early Downgrade Among Critically Ill Medical Patients in the Emergency Department. Crit Care Med 2023;51(6):731–41.
18. Howard M, Pflaum-Carlson J, Hurst G, et al. A roadmap for developing an emergency department based critical care consultation service: Building the early intervention team (EIT). Am J Emerg Med 2023;66:81–4.
19. Kadar RB, Amici DR, Hesse K, Bonder A, Ries M. Impact of Telemonitoring of Critically Ill Emergency Department Patients Awaiting ICU Transfer. Crit Care Med 2019;47(9):1201–7.
20. Schierbeck S, Svensson L, Claesson A. Use of a DroneDelivered Automated External Defibrillator in an Out-ofHospital Cardiac Arrest. N Engl J Med 2022;386(20):1953–4.
21. Roberts NB, Ager E, Leith T, et al. Current summary of the evidence in drone-based emergency medical services care. Resusc Plus 2023;13:100347.
22. Kelen GD, Wolfe R, D’Onofrio G, Mills AM. Emergency department crowding: the canary in the health care system. Health Care Innov [Internet] Available from: https://catalyst. nejm.org/doi/abs/10.1056/CAT.21.0217
23. Forster AJ, Stiell I, Wells G, Lee AJ, van Walraven C. The effect of hospital occupancy on emergency department length of stay and patient disposition. Acad Emerg Med 2003;10(2):127–33.
24. Institute of Medicine, Board on Health Care Services, Committee on the Future of Emergency Care in the United States Health System. Hospital-Based Emergency Care: At the Breaking Point. National Academies Press; 2007.
25. American Hospital Association. TrendWatch Chartbook 2018 [Internet]. [cited 2022 Feb 16]. Available from: https:// www.aha.org/system/files/2018-07/2018-aha-chartbook.pdf.
26. Ragavan MV, Svec D, Shieh L. Barriers to timely discharge from the general medicine service at an academic teaching hospital. Postgrad Med J 2017;93(1103):528–33.

Innovation in Focus: AHI
1. Schmitzberger FF, Hall AE, Hughes ME, Belle A, Benson B, Ward KR, Bassin BS. Detection of Hemodynamic Status Using an Analytic Based on an Electrocardiogram Lead Waveform. Crit Care Explor. 2022 May 17;4(5):e0693. doi: 10.1097/ CCE.0000000000000693. PMID: 35620767; PMCID: PMC9116956.
2. Benson B, Belle A, Lee S, Bassin BS, Medlin RP, Sjoding MW, Ward KR. Prediction of episode of hemodynamic instability using an electrocardiogram based analytic: a retrospective cohort study. BMC Anesthesiol. 2023 Sep 22;23(1):324. doi: 10.1186/s12871-023-02283-x. PMID: 37737164; PMCID: PMC10515416
A PICTURE-Perfect Response
1. Gillies CE, Taylor DF, Cummings BC, Ansari S, Islim F, Kronick SL, Medlin RP Jr, Ward KR. Demonstrating the consequences of learning missingness patterns in early warning systems for preventative health care: A novel simulation and solution. J Biomed Inform. 2020 Oct;110:103528. doi: 10.1016/j.jbi.2020.103528. Epub 2020 Aug 11. PMID: 32795506.
2. Cummings BC, Ansari S, Motyka JR, Wang G, Medlin RP Jr, Kronick SL, Singh K, Park PK, Napolitano LM, Dickson RP, Mathis MR, Sjoding MW, Admon AJ, Blank R, McSparron JI, Ward KR, Gillies CE. Predicting Intensive Care Transfers and Other Unforeseen Events: Analytic Model Validation Study and Comparison to Existing Methods. JMIR Med Inform. 2021 Apr 21;9(4):e25066. doi: 10.2196/25066. PMID: 33818393; PMCID: PMC8061893.
3. Cummings BC, Blackmer JM, Motyka JR, Farzaneh N, Cao L, Bisco EL, Glassbrook JD, Roebuck MD, Gillies CE, Admon AJ, Medlin RP Jr, Singh K, Sjoding MW, Ward KR, Ansari S. External Validation and Comparison of a General Ward Deterioration Index Between Diversely Different Health Systems. Crit Care Med. 2023 Jun 1;51(6):775-786. doi: 10.1097/CCM.0000000000005837. Epub 2023 Mar 16. PMID: 36927631; PMCID: PMC10187626.
47

Executive Officers of Michigan Medicine
Marschall S. Runge, M.D., Ph.D.
Executive Vice President for Medical Affairs, Dean, University of Michigan Medical School, C.E.O., Michigan Medicine
David C Miller, M.D., M.P.H
President, University of Michigan Health System, Executive Vice Dean for Clinical Affairs, Medical School
Debra F. Weinstein, M.D.
Executive Vice Dean for Academic Affairs, Medical School Chief Academic Officer for Michigan Medicine
Steven L. Kunkel, Ph.D.
Executive Vice Dean for Research, Medical School Chief Scientific Officer, Michigan Medicine
The Regents of the University of Michigan
Jordan B. Acker, Michael J. Behm, Mark J. Bernstein, Paul W. Brown, Sarah Hubbard, Denise Ilitch, Ron Weiser, Katherine E. White, Santa J. Ono (ex officio)
© 2024 Regents of the University of Michigan A Non-discriminatory, Affirmative Action Employer
48

www.weilinstitute.med.umich.edu Learn More MAX HAR RY WEIL INSTITUTE






































 Photo: Dr. Cindy Hsu
Photo: Dr. Cindy Hsu


 Photo: Gene Parunak speaks at the 2023 Massey TBI Regional Conference
Photo: Gene Parunak speaks at the 2023 Massey TBI Regional Conference
 Xudong (Sherman) Fan, PhD
Richard A. Auhll Endowed Professor of Engineering; Professor of Biomedical Engineering; Associate Director, Weil Institute
Xudong (Sherman) Fan, PhD
Richard A. Auhll Endowed Professor of Engineering; Professor of Biomedical Engineering; Associate Director, Weil Institute




















































 Kevin Ward, MD Executive Director
Xudong (Sherman) Fan, PhD Associate Director
Lena Napolitano, MD, FACS, FCCP, FCCM Associate Director
Robert Dickson, MD Deputy Director Deputy Director
Phil Jacokes, MBA Managing Director
Heidi Flori, MD, FAAP Associate Director
Kenn Oldham, PhD Associate Director
Rodney Daniels, MD Scientific Director, Kahn Pediatric Critical Care Grand Challenge
Frederick Korley, MD, PhD Scientific Director, Massey TBI Grand Challenge
Michael Sjoding, MD Associate Director
J. Scott VanEpps, MD, PhD Associate Director
Michael Maile, MD Associate Director
Kayvan Najarian, PhD Associate Director
Kevin Ward, MD Executive Director
Xudong (Sherman) Fan, PhD Associate Director
Lena Napolitano, MD, FACS, FCCP, FCCM Associate Director
Robert Dickson, MD Deputy Director Deputy Director
Phil Jacokes, MBA Managing Director
Heidi Flori, MD, FAAP Associate Director
Kenn Oldham, PhD Associate Director
Rodney Daniels, MD Scientific Director, Kahn Pediatric Critical Care Grand Challenge
Frederick Korley, MD, PhD Scientific Director, Massey TBI Grand Challenge
Michael Sjoding, MD Associate Director
J. Scott VanEpps, MD, PhD Associate Director
Michael Maile, MD Associate Director
Kayvan Najarian, PhD Associate Director