Below a summary of the updates:
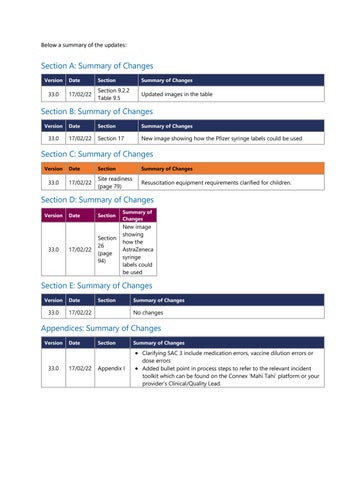
Section A: Summary of Changes Version
33.0
Date
Section
Summary of Changes
17/02/22
Section 9.2.2 Table 9.5
Updated images in the table
Section B: Summary of Changes Version
33.0
Date
Section
Summary of Changes
17/02/22
Section 17
New image showing how the Pfizer syringe labels could be used
Section C: Summary of Changes Version
33.0
Date
Section
Summary of Changes
17/02/22
Site readiness (page 79)
Resuscitation equipment requirements clarified for children.
Section D: Summary of Changes Version
33.0
Date
17/02/22
Section
Summary of Changes
Section 26 (page 94)
New image showing how the AstraZeneca syringe labels could be used
Section E: Summary of Changes Version
33.0
Date
Section
Summary of Changes
No changes
17/02/22
Appendices: Summary of Changes Version
Date
Section
Summary of Changes
• Clarifying SAC 3 include medication errors, vaccine dilution errors or dose errors 33.0
17/02/22
Appendix I
• Added bullet point in process steps to refer to the relevant incident toolkit which can be found on the Connex ‘Mahi Tahi’ platform or your provider’s Clinical/Quality Lead.