Below a summary of the updates:
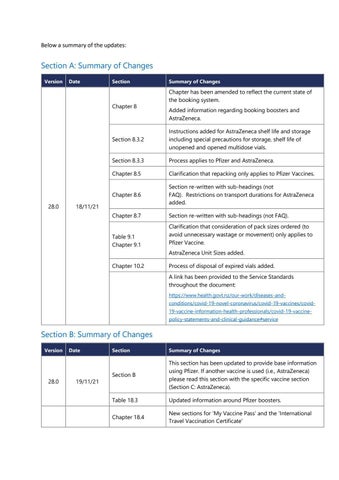
Section A: Summary of Changes Version
Date
Section
Chapter 8
28.0
Summary of Changes
Chapter has been amended to reflect the current state of the booking system. Added information regarding booking boosters and AstraZeneca.
Section 8.3.2
Instructions added for AstraZeneca shelf life and storage including special precautions for storage, shelf life of unopened and opened multidose vials.
Section 8.3.3
Process applies to Pfizer and AstraZeneca.
Chapter 8.5
Clarification that repacking only applies to Pfizer Vaccines.
Chapter 8.6
Section re-written with sub-headings (not FAQ). Restrictions on transport durations for AstraZeneca added.
Chapter 8.7
Section re-written with sub-headings (not FAQ).
Table 9.1 Chapter 9.1
Clarification that consideration of pack sizes ordered (to avoid unnecessary wastage or movement) only applies to Pfizer Vaccine.
18/11/21
AstraZeneca Unit Sizes added. Chapter 10.2
Process of disposal of expired vials added. A link has been provided to the Service Standards throughout the document: https://www.health.govt.nz/our-work/diseases-andconditions/covid-19-novel-coronavirus/covid-19-vaccines/covid19-vaccine-information-health-professionals/covid-19-vaccinepolicy-statements-and-clinical-guidance#service
Section B: Summary of Changes Version
28.0
Date
19/11/21
Section
Summary of Changes
Section B
This section has been updated to provide base information using Pfizer. If another vaccine is used (i.e., AstraZeneca) please read this section with the specific vaccine section (Section C: AstraZeneca).
Table 18.3
Updated information around Pfizer boosters.
Chapter 18.4
New sections for ‘My Vaccine Pass’ and the ‘International Travel Vaccination Certificate’