











Takes effect on 13 Feb 2023

Covid funding is confirmed until June 2023, anticipate unlikely further significant adjustments between Feb and June.
Targeted approach to support those most in need of care
Those not identified as most in need of care may need to access care through usual care mechanisms, with co-payment











Ø Removed:
• Chart reviews for all cases
• Higher rate of funding for high needs/unenrolled patients
• Post-hospital discharge follow-up within 6 weeks of diagnosis
• Patient-initiated follow-up after acute illness but within 6 weeks of diagnosis
• Phone advice/triage with no RAT/PCR test done
Ø Changed:
• Level of funding for other services has changed
Ø Added:
• Fee for primary care prescriber support to pharmacy initiated antivirals

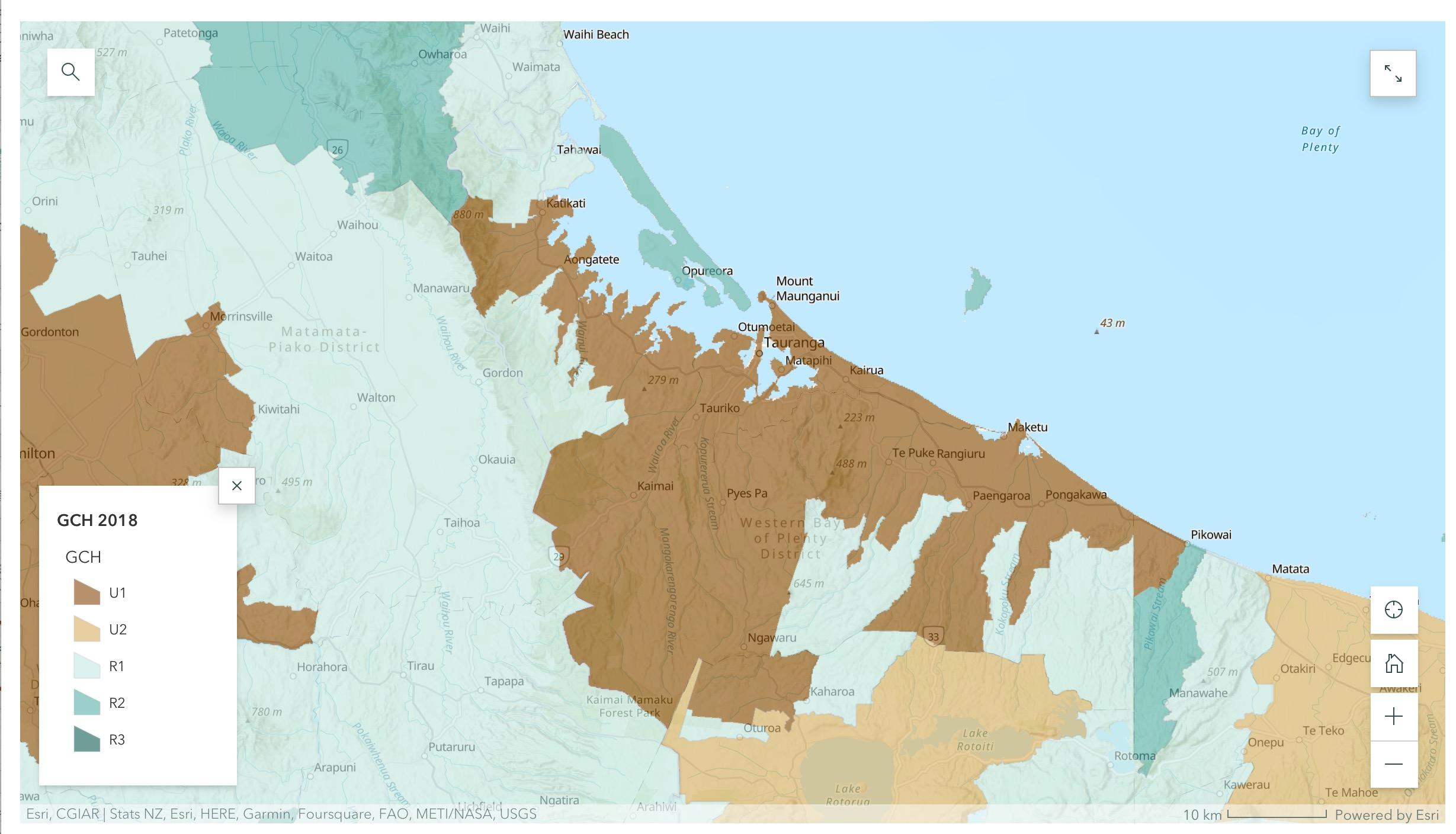
Q - How is eligibility for funding ‘rural and remote’ defined?
ØA Rurality is defined according to the Geographic Classification of Healthcare and based on location of the patient’s home address.
ØThose in locations designated R2 and R3 will be eligible for funding.
The Geographic Classification for Health (arcgis.com)
https://storymaps.arcgis.com/stories/da035e374dbb4ea0ae3b31b6777924ad




Assessment and testing is now only funded for those who meet antiviral access criteria


OR are in a priority or vulnerable or population group

Eligible patients with negative in-consultation RAT
Eligible patients with positive RAT
Eligible patients with negative RAT, Clinical reason for PCR (e.g. severe immune suppression)
Consult Not Funded co-payment applies
Consult Funded $90 in hours
$135 after hours/weekends
Consult Funded $90 in hours
$135 after hours/weekends
Symptomatic patient
Negative test at home
Not a household contact of known positive case
Symptomatic patient
Positive test at home (or in surgery)
Single claim per person/ per day



- Meets antiviral criteria?
- In priority group?

- In vulnerable group?
Co-payment applies
If all 3 above are NO,
Co-payment for care applies
If YES to any of the 3, Qualifies for $90 claim
With the exception of “clinical escalation”

Ø When a definitive test result will change management

Ø Personal risk factors:
• Clinical conditions that mean prompt initiation of antivirals is important (e.g. immune suppression)
Ø Situational risk factors:
• Whānau with significant medical conditions or immune suppression where a definitive result may change site of isolation or decision to issue advance prescription antivirals to other household members
Ø For low-risk people with negative RAT, isolating and repeating RAT in 1224 hours is suggested
ØYes, if:

• They are a household contact of a confirmed case
• They are symptomatic
• They meet the other antiviral access criteria
Ø Consultations for advance scripts only funded for those who meet antiviral access criteria:
• Consultation and initial prescription - $90


• Consultation for eligible patient that does not result in prescription - $60
• Repeat advance prescription where initial script has expired - $45
Ø Primary Care prescriber support for pharmacist -initiated antivirals:
• Those that meet anti-viral criteria OR, eligibility review that doesn’t meet criteria - $37.50*
*Please note this claim is found under “Community Care” not “Advanced Prescriptions” claims within Halcyon

When you log into Halcyon you should now see the following claim forms:
This form will be de-activated shortly. It is currently live for PRE-13th FEB CLAIMS ONLY
We are seeking clarity on this form. We have not been advised of any national changes at this stage.
This form will be de-activated shortly. It is currently live for PRE-13th FEB CLAIMS ONLY
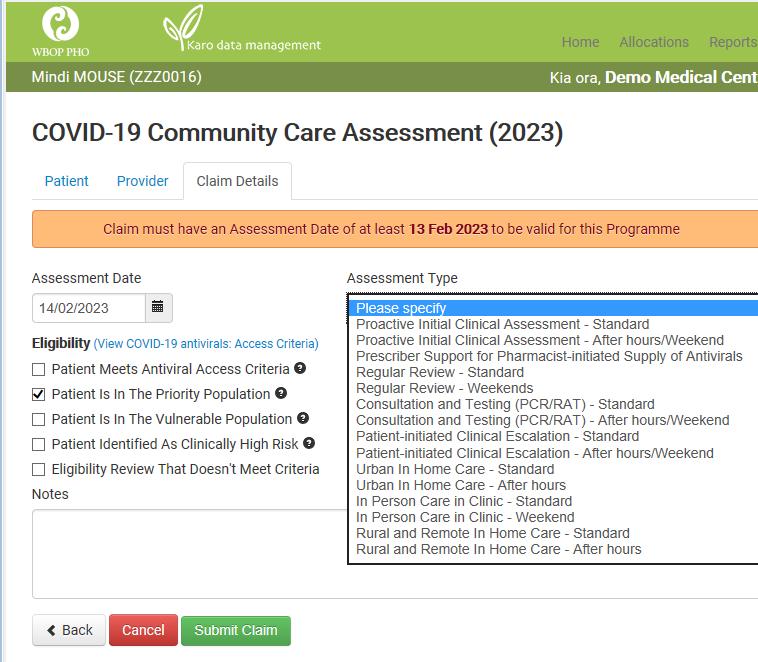
This is the new form to use for all Covid Community Management Claims – including Assessment & Testing (Post-Feb 13th claims)

This form remains active, with some recent updates to “outcomes” options
This form remains active, with no changes
New (v.3) form for Community Management:

To be used for all Covid-19 Community Management and Testing Claims from 13th
February 2023


https://portal.wboppho.org.nz/covid-19/

Ø For use in children with complex chronic medical conditions (including neurodevelopmental conditions), severe immunocompromise, e.g. undergoing treatment for cancer or transplant, or risk factors for severe illness from COVID19.

Ø The vaccine is a modified version of the vaccine used in children aged 5 to 11 years. Three dose regime – second dose is given after three weeks third dose given minimum of eight or more weeks later.
Ø Not recommended for children aged six months to four years who are not considered at risk for severe illness from COVID-19
Ø Available at Rangiora Hub in WBOP


