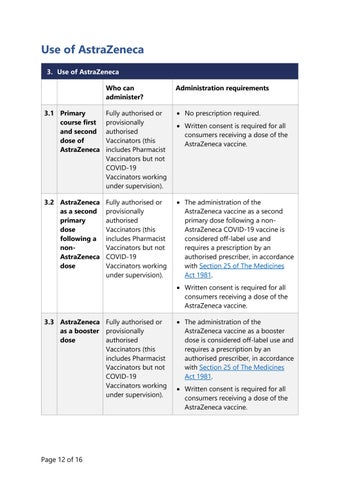
Use of AstraZeneca 3. Use of AstraZeneca Who can administer?
Administration requirements
3.1 Primary course first and second dose of AstraZeneca
Fully authorised or provisionally authorised Vaccinators (this includes Pharmacist Vaccinators but not COVID-19 Vaccinators working under supervision).
• No prescription required.
3.2 AstraZeneca as a second primary dose following a nonAstraZeneca dose
Fully authorised or provisionally authorised Vaccinators (this includes Pharmacist Vaccinators but not COVID-19 Vaccinators working under supervision).
• The administration of the AstraZeneca vaccine as a second primary dose following a nonAstraZeneca COVID-19 vaccine is considered off-label use and requires a prescription by an authorised prescriber, in accordance with Section 25 of The Medicines Act 1981.
• Written consent is required for all consumers receiving a dose of the AstraZeneca vaccine.
• Written consent is required for all consumers receiving a dose of the AstraZeneca vaccine. 3.3 AstraZeneca Fully authorised or as a booster provisionally dose authorised Vaccinators (this includes Pharmacist Vaccinators but not COVID-19 Vaccinators working under supervision).
Page 12 of 16
• The administration of the AstraZeneca vaccine as a booster dose is considered off-label use and requires a prescription by an authorised prescriber, in accordance with Section 25 of The Medicines Act 1981. • Written consent is required for all consumers receiving a dose of the AstraZeneca vaccine.