





• Biocompatible acellular human placental extracellular matrix (ECM)
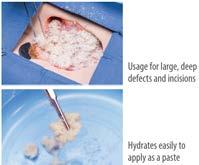
• Suitable for deep, tunneling, or irregular soft tissue deficits and surgical incision management
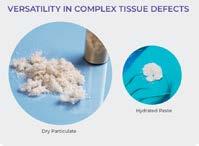
• Can be applied as a dry particulate or a hydrated paste
AXIOFILL is an acellular human extracellular matrix (ECM) derived from the placental disc. It preserves multiple ECM components and other matrix-bound proteins. AXIOFILL is designed for the replacement or supplementation of damaged or inadequate integumental tissue. It is compatible with adjunctive therapies such as Negative Pressure Wound Therapy (NPWT) and Hyperbaric Oxygen Therapy (HBOT).
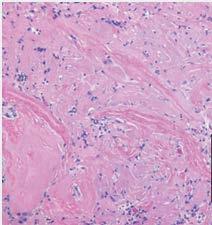
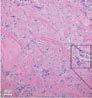
MIMEDX’s PURION patented process preserves extracellular matrix components, including regulatory factors and other matrix-associated proteins. Immunohistochemistry (IHC) staining identifies specific ECM molecules in the final AXIOFILL product, with brown coloration indicating reactivity with the antibody probe.

Dry applications
Hydrated, paste applications
Surgical debridement Tunnel/undermined wounds
Partial and full thickness tissue deficits Fistulae
Pressure ulcers Deep, irregular wounds
Venous ulcers Incisions
Diabetic ulcers
Surgical wounds (donor sites/ grafts, post-Mohs surgery, post-laser surgery, podiatric, and dehiscence)
Chronic vascular ulcers Other procedures possible
Trauma wounds (abrasions, lacerations, second-degree burns, and skin tears)
Draining wounds

• Versatile: Can be applied dry or hydrated into a paste
• Scientifically backed: Preserves ECM components, matrix-associated proteins, and regulatory factors while supporting cellular attachment
• Cost-effective: An economic alternative for large soft tissue defects
• Long shelf life: 5 years when stored at 2-30°C
• Therapy compatibility: Works with NPWT and HBOT
AXIOFILL restores site-appropriate and functional tissue by fostering an environment conducive to cellular integration and remodeling. Clusters of dark pink-colored cells indicate neovascularization throughout the implant site.

• The outer peel pouch is NOT sterile; only the inner vial is sterile unless compromised.
• Using aseptic technique, slowly peel a corner of the pouch and remove the sterile vial.
•Avoid tapping the glass vial with hard or metal instruments.
•Post-debridement, disperse the product from the vial onto the treatment area or transfer it to a sterile basin before applying.
•Optional: Hydrate by adding a few drops of sterile saline solution. (figure 1)
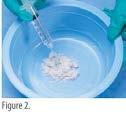
• To achieve a paste consistency, mix 1.5 mL of sterile saline per 250 mg of AXIOFILL in a separate sterile bowl. (figure 2)
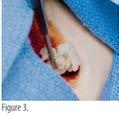
• Once hydrated, promptly apply the product to the treatment area. (figure 3)